Abstract
Cardiovascular disease (CVD) has been showing patterns of extensive rise in prevalence in the contemporary era, affecting the quality of life of millions of people and leading the causes of death worldwide. It has been a provocative challenge for modern medicine to diagnose CVD in its crib, owing to its etiological factors being attributed to a large array of systemic diseases, as well as its non-binary hideous nature that gradually leads to functional disability. Novel echocardiography techniques have enabled the cardiac ultrasound to provide a comprehensive analysis of the heart in an objective, feasible, time- and cost-effective manner. Speckle tracking echocardiography, contrast echocardiography, and 3D echocardiography have shown the highest potential for widespread use. The uses of novel modalities have been elaborately demonstrated in this study as a proof of concept that echocardiography has a place in routine general practice with supportive evidence being as recent as its role in the concurrent COVID-19 pandemic. Despite such evidence, many uses remain off-label and unexploited in practice. Generalization of echocardiography at the point of care can become a much-needed turning point in the clinical approach to case management. To actualize such aspirations, we recommend further prospective and interventional studies to examine the effect of implementing advanced techniques at the point of care on the decision-making process and evaluate their effectiveness in prevention of cardiovascular morbidities and mortalities.
Introduction
Heart failure (HF) has been defined as a global pandemic, affecting 26 million people worldwide and posing a lifetime risk of 20%. In 2012, it accounted for 10% of US health expenses for cardiovascular diseases. These costs are expected to reach a 127% increase by 2030. For a long time, there have been efforts to develop cost-effective methods for primary prevention of HF by tackling its most common risk factors.1 , 2 Systemic diseases are being increasingly sorted among them since their incidence is showing epidemic trends, the most remarkable examples being hypertension (HTN), obesity, and diabetes mellitus (DM) (Table 1 ). Given the modest effects of therapy when cardiac affection is diagnosed late in the disease course, a progressive need arises for a point of care tool. Practitioners of different specialties, not necessarily cardiologists, should be able to use such tool to detect cardiac dysfunction early on in the disease, determine its degree, and monitor disease progression.3
Table 1.
Cardiac burden of systemic diseases.
| Disease | Prevalence | Percentage Of Heart Affection | Risk Of Cardiac Death |
|---|---|---|---|
| Hypertension | 31.5% (low-income countries) 28.5% (high-income countries)7 |
Accounts for 50% of ischemic heart disease cases8 | 13% of total deaths worldwide8 |
| Obesity | 13% in 18 yo and older9 |
40% increased risk for CVD with every 5kg/m2 BMI increase10 | |
| Diabetes mellitus | 8.5%11 | Every 1% increase in HbA1c corresponds with 30% increased risk of HF12 | 65%13 |
| Radiation induced cardiomyopathy | - | 0.5-37% in breast cancer (dose-dependent).14 49.5-54.6% in Hodgkin lymphoma.14 |
1.5 to 3 fold increased fatal myocardial infarction risk compared to unirradiated Hodgkin lymphoma patients.15 62% increased cardiac deaths among breast cancer survivors 16 |
| Chemotherapy-related cardiomyopathy | - | anthracyclines: 5-48% (dose dependent).17 trastazumab: 7-34%18 |
68% 5-year survival rate with cardiac affection as leading cause19 |
| Rheumatic heart disease20 | 30 million people globally | 60% | 305,000 deaths/year |
| Systemic Lupus Erythematosis | 20 to 150 cases per 100,000 in US21 | 50%22 | 32%23 |
| Rheumatoid arthritis | 0.2–1.0%24 | 48% increased risk of developing CVD events25 | 39.6%26 |
Echocardiography is gaining ground in that aspect, being a non-invasive and cost-effective imaging modality that is no longer confined to echocardiography labs but has instead been developed to become more portable attaining commercially available and affordable hand-held devices.4 , 5 Furthermore, increasing evidence is accumulating which supports the use of novel echocardiography techniques and technologies to detect early cardiac injury and proves their advantage over conventional ones in its assessment and management in terms of reproducibility, time consumption, cost-efficiency, and feasibility.6
It is human nature to be enticed by new technology and as physicians, we are no different. However, in order for new technology to realize its full potential, it should be evaluated in light of its real place in the clinical workflow. In this study, we exhibit three relatively nascent echocardiography techniques namely: Speckle Tracking Echocardiography (STE), Contrast Echocardiography, and Three Dimensional Echocardiography (3DE). Showing their advantages, limitations, and promising clinical implications in a variety of secondary cardiac affections caused by a wide spectrum of systemic diseases, as a rationale for their generalization in healthcare service.
Speckle Tracking Echocardiography
Among several new techniques, speckle tracking echocardiography (STE) has enabled the transition of echocardiography from an investigation dependent on subjective image interpretation to an objective set of diagnostic parameters.27 At its core, STE implements 2D or 3D echocardiography to assess ventricular contractility through myocardial deformation imaging widely known as strain imaging.28 Strain is a unitless measurement of deformational change i.e. the relative movement between two points in space during the cardiac cycle, which when related to a timeframe, strain rate is measured. A special software analyzes serial images by precisely tracking the movement of clusters of speckles each of them having an acoustic marker functioning as its unique ultrasonic fingerprint.6 Strain can be evaluated in different planes to image the heart from a longitudinal, circumferential, or radial point of view. The software divides the myocardium into segments individually evaluated for regional strain, then averages the regional values from multiple windows to obtain the global strain. While the majority of cardiac myofibers are circumferentially arranged, longitudinal fibers are the most prone to injury being mostly found in the less perfused sub-endocardium. Thus, global longitudinal strain (GLS) is a very sensitive sign of ventricular injury and an accurate predictor of left ventricular ejection fraction (LVEF) drop.6 It's also the most validated, reproducible, time-efficient, and the most commonly used parameter in clinical trials and practice.29 , 30
Technical Advantages of STE over Tissue Doppler Imaging
Prior to the introduction of STE, strain imaging was based on velocity changes detected by tissue Doppler imaging (TDI) entailing perfect alignment of the probe axis to the vector of contractile motion of interest to the observer; such hurdle is referred to as angle dependency. Significant inter-observer variability and noise artifacts also contributed to the limited use of strain imaging despite its clinical usefulness.31 In addition to angle-independence which is considered one of STE's greatest advantages, shorter analysis time and better reproducibility make it superior to TDI. The technique's functionality is also complemented by its accuracy relative to gold standards as well as its ability to differentiate between the active contraction of each segment and tethering forces from adjacent ones. Additional motion patterns such as torsion, dyssynchrony (segments not reaching peak strain simultaneously) and diastolic parameters can also be assessed.29 Being a semi-automated technique also acts towards minimalizing subjectivity and human error.32
Speckle Tracking Echocardiography in Systemic Diseases
In comparison with conventional parameters, assessment of cardiac strain imaging is capable of detecting cardiac affection in systemic diseases at an earlier stage. This in turn provides the opportunity of earlier intervention and treatment as well as risk assessment and prognostication of such affection's manifestation in the form of cardiac events. Table 2 summarizes the uses of STE in systemic heart diseases.
Table 2.
Uses of STE in systemic heart diseases.
| Toxic cardiomyopathy | Chemotherapy-related cardiac dysfunction(a)(b) Radiotherapy-induced heart disease(b) Septic myocarditis(a) |
| Immune-mediated diseases | Rheumatic heart disease(b) Rheumatoid arthritis Grave's disease Systemic lupus erythematosus(a) Systemic sclerosis |
| Infiltrative diseases | Thalassemia(a)(b) Amyloidosis(b) Sarcoidosis(b) |
| Metabolic and endocrine diseases | Obesity(a) Diabetes mellitus type I&II(b) Hypothyroidism |
| Infective diseases | COVID-19(b) |
| Others | HFpEF(b) |
(a) STE uses having a prognostic role in monitoring response to treatment.
(b) STE uses having a prognostic role in monitoring disease progression.
STE has an early diagnostic value in all uses mentioned.
It's reasonable that STE should acquire an established place in daily practice, given its relative ease and proved potential in improving patient care in diseases across diverse fields of practice. To motivate that claim, we shall present an elaborate outline of available evidence for its uses in systemic heart diseases.
STE in Toxic Cardiomyopathy
Scenarios of cardiac dysfunction caused by sepsis have witnessed the most potential for STE in critical care management where several STE parameters were of benefit. Reduced LVGLS was recorded in patients with normal LVEF and provided valuable prognostic insights. A hypothesis was raised explaining such findings on basis of microvascular ischemia occurring in the pathophysiological cascade of sepsis and affects sub-endocardial layers. RV free wall strain can detect RV dysfunction and surpasses conventional parameters in the decision-making process of device implantation and heart transplantation. Peak torsion and apical rotation were decreased in cases of sepsis when compared against controls in a study where LVEF showed no significant differences.29
STE for Monitoring of Potentially Cardiotoxic Cancer Therapy
Recently, cancer therapy-related cardiac dysfunction (CTRCD) has become more prevalent due to increased survival rates of cancer patients, especially in childhood cancer survivors which has risen from almost 50% to 80 % from 1970 to 2010 due to advanced management techniques.33 Cardiotoxicity is the third most likely cause of morbidity and mortality in childhood cancer survivors, being only preceded by malignancy recurrence or secondaries.34 Furthermore, childhood cancer survivors were found to be at an eightfold increased risk of cardiotoxicity-related death compared to their healthy counterparts.35 This cardiotoxicity, which can limit the use of some chemotherapeutic agents if detected early, will allow agent discontinuation or dose adjustment along with proper management.36 The FDA recommends the interruption of treatment with certain agents whenever LVEF is decreased.37 Recent evidence shows that LVEF decrease is subtle and varies by only 3-5% during and after administration when assessed by standard 2DE. It can be missed during routine assessment as opposed to the early detectable and significant reduction of 2DSTE global parameters. A cut-off value of 14% reduction from pre-administration GLS is a significant sign of cardiotoxicity.38
CTRCD is classified into type 1 dose-dependent irreversible myocardial damage which is mainly attributed to anthracyclines and type 2 dose-independent reversible damage including trastuzumab and other agents. GLS was proved to be helpful in risk stratification and long-term follow up for both types, showing persistent reduction after 6 and 12 months with trastuzumab and after 10 years with anthracyclines in childhood cancer survivors.39 STE parameters decline with preserved or reduced LVEF is also recorded with administration of proteasome inhibitors and immune checkpoint inhibitors.40 Existing recommendation for the use of STE in CTRCD follow up include the SAFE-HEaRt study recommending GLS measurement at baseline and every 3 months in patients on trastazumab.41
Compared to other modalities such as MRI, coronary CT, and positron emission tomography (PET) nuclear imaging, echocardiography is more available, less expensive, and does not involve ionizing radiation. With the downside being its low accuracy.40 Such criteria advocate its use as a first-line investigation to later be confirmed by other modalities.
Multiple STE parameters such as GLS, global circumferential strain (GCS), Longitudinal strain rate (LSR), and circumferential strain rate (CSR) helped track the progress of Angiotensin converting enzyme inhibitors (ACEI) and Angiotensin receptor blockers (ARB) in treatment of CTRCD. The mentioned parameters improved with therapy and were sustained during and after the 1-year follow-up period. Such results were obtained with good reproducibility which provides support for the use of STE in cardiac monitoring of cancer survivors.42
Radiotherapy used for treatment of chest malignancies may also have cardiac complications referred to as radiotherapy-Induced Heart Disease (RIHD), which progresses from inflammation to capillary lesions to a fibrotic end-stage.43 This can potentially lead to cardiac death secondary to coronary lesions and other structural and functional damage.43 , 44 Cardiac morbidity may take place during a variable timeframe, with subjects showing persistent changes for as long as 10 years after therapy.45 Using STE, a dose-dependent LV regional and global longitudinal strain decline was observed in the acute phase of irradiation exposure.46, 47, 48 A cut-off value of GLS decrease > 10% was suggested as a sign of subclinical cardiotoxicity.49 Despite its effectiveness in detecting RIHD -which poses a serious health risk to cancer survivors- with significant and persistent correlation, no recommendations or guidelines were established.50
STE in Immune-Mediated Diseases
In a controlled study involving patients of rheumatoid arthritis free of cardiovascular symptoms, evaluation of left and right ventricular GLS using STE revealed worse results in patients with active disease than those in remission who were still distinguishable from the controls. Meanwhile, the groups were inseparable by conventional echocardiography, which could only detect late diastolic dysfunction in a stage of heart failure. LV wall thickness at end-diastole, EF%, peak mitral and tricuspid E and A wave velocities were among 15 conventional parameters used to assess left and right ventricular function yet turned out to be incapable of differentiating among the three groups.51
Alteration of thyroid hormone levels seen in thyroid diseases has a profound effect on the heart.52 New evidence casts the light on another pathophysiological mechanism leading to cardiac affection. In subjects with Grave's disease, GLS decline was recorded despite treatment and correction of thyroid profile. This suggests the autoimmune component of the disease being responsible for such dysfunction as GLS correlated to TIMP-1 levels, which plays a role in cardiac remodeling. These findings imply the use of STE for detecting subclinical cardiomyopathy as well as ophthalmopathy as they're affected by the same mechanism. Unsuccessful normalization of GLS upon reaching a euthyroid state could suggest Grave's disease as the cause of hyperthyroidism.53
Rheumatic heart disease (RHD) is known to cause severe disability and premature death. While advanced stages of the disease do not directly affect the myocardial function, the acute attack of pancarditis may cause persistent insignificant myocardial changes. Such changes were considered to aid the diagnosis of latent RHD as suggested by a study involving a pediatric cohort with latent RHD who had decreased GLS despite no decline in LVEF or valve lesion.54 STE parameters can also detect mild mitral stenosis and surpass conventional parameters, as well as TDI, in providing prognostic information regarding the optimal timing for surgical correction in subjects with mitral regurgitation to avoid postoperative LV dysfunction.55 , 56
Myocardial involvement in systemic lupus erythematosus (SLE) is caused by accelerated atherosclerosis and poses a 10-fold increase in the risk of cardiovascular morbidity and mortality.57 Despite being among the leading causes of death, early dysfunction is symptomless and undetectable by lab tests or conventional 2DE. STE, on the contrary, shows reduced LVGLS, GLS rate, GCS, GRS, and RV longitudinal strain.58 It's of particular value to patients with juvenile SLE, as it enables preventing long-term cardiovascular complications and monitoring response to therapy. Therefore, routine follow-up with STE is recommended.59
Patients with systemic sclerosis (SSc) suffer from cardiac involvement in the form of pulmonary hypertension and RV dysfunction, which constitutes one of the main causes of morbidity and mortality.60 Cardiac magnetic resonance (CMR) is the current gold standard owing to its ability to detect subtle fibrotic patches.61 Strain parameters including GLS can detect similar abnormalities detected by CMR, along with STE being superior to CMR in regards of cost and availability.62 , 63 STE can also be used in risk stratification concerning cardiovascular events and the need for preventative cardio-protective medication.64
STE in Infiltrative Diseases
Cardiac causes of death in thalassemic patients account for a leading 75%.65 The use of STE has allowed early detection of cardiac affection which occurs due to two main reasons and shows different findings upon imaging: The first is iron deposition within the myocytes through L-Type calcium channels as a result of iron overload, which impairs the excitation-contraction coupling leading to abnormal contractility. The manifestation of iron deposition in the sub-epicardium occurs in the form of abnormal LV GCS. The second is chronic hypoxia which affects the sub-endocardium and influences the LV GLS.66, 67, 68 The utilization of STE in follow up of those patients to detect such signs is of great value since the deposited iron has a half-life of 14 months and earlier intervention to prevent accumulation yields a better outcome.69 Moreover, It allows risk stratification of developing overt systolic dysfunction and helps in tailoring the right chelating agents for each patient.70 , 71 The currently used follow-up technique is T2-weighted cardiac magnetic resonance (CMR T2) which evaluates myocardial and hepatic iron load as a guide for management.67 STE can offer comparable results while being less expensive and has detected abnormal strain in patients with normal T2 CMR.72 , 73 Besides, it takes into consideration other mechanisms of cardiac affection independent of iron overload such as hypoxia, immune-inflammatory, and genetic factors involved in the pathogenesis of the disease and can only be detected by strain imaging. Yet, STE only detects chronic changes and therefore is unable to diagnose acute cardiac changes due to severe iron toxicity or recent therapeutic changes.67
In a prospective study, 150 patients with light chain amyloidosis and normal EF were separated based on clinical evidence of cardiac amyloidosis (CA). 2D STE was found to be an accurate independent prognostic tool and a predictor of all-cause deaths especially in the group free of CA where those with poor GLS, despite otherwise having no evidence of cardiac involvement, had fatal outcomes. Also, GLS is a more reliable parameter in the assessment of CA than wall thickness, which is relatively non-specific, as it detects the apex-sparing characteristic of CA and, thus, can distinguish it from other causes of left ventricular hypertrophy. Relative regional strain ratio (RRSR) is another STE parameter denoting the extent of apical sparing and offers a cut-off value of 1.9 where a mean RRSR of greater value is associated with an increased need for heart transplantation surgery and even death.74
Cardiac sarcoidosis (CS) is a condition carrying poor prognosis with 5-year mortality rate reaching 40%. It starts with subclinical inflammation and fibrosis causing ventricular remodeling that builds up leading to arrhythmia and AV-block.75 GLS decline is a sensitive parameter that detected cardiac affection in subjects with normal-range EF, and predicted hospital admission rate and heart failure. GLS evaluation correlates to more expensive modalities such as PET scans and CMR, signifying its standardization as a screening method when CS is suspected.76 , 77
STE in Metabolic and Endocrine Diseases
Obesity and DM constitute an epidemic, which has a debilitating adverse effect on the cardiovascular system. GLS as measured by STE has established correlation with BMI, the duration of DM, and whether it is controlled or uncontrolled. This provides proof of STE's practicality as a screening method for diabetic and obese patients to recognize patients at risk of cardiovascular disease and initiate prompt treatment.78, 79, 80, 81
Type 2 DM is associated with non-alcoholic fatty liver disease (NAFLD) which contributes to diabetic cardiovascular comorbidity. Through the utilization of 3DSTE, a correlation between the severity of NAFLD (measured by abdominal ultrasound) and subclinical cardiac affection in the form of decreased left ventricular GLS, GCS, GRS, and global area strain (GAS) was established. All cases had a normal LVEF owing to cardiac compensation in the earlier stages. GAS indicates the deformation of the endocardial area and denotes systolic dysfunction. This forms a basis for the importance of cardiac assessment in diabetic patients with moderate to severe NAFLD to allow early clinical intervention and treatment.82 LV remodeling and hypertrophy are complications seen in diabetic patients and were suggested to have a causative contribution to GLS reduction.83
STE also detected systolic dysfunction in a pediatric population with type 1 DM, which corresponded to poor glycemic control of these patients. Type 1 DM is the most prevalent chronic pediatric disorder that can have drastic effects on the quality of life of children owing to its early onset. Being the most common cause of death in diabetic patients, prevention of cardiomyopathy by screening of patients (especially those with poor glycemic control) using STE should be implemented in practice.84
LV GLS was also found to be superior to conventional parameters in management of hypothyroid-caused cardiac dysfunction, detecting affection at earlier stages and following up treatment effectiveness; as such effects are reversible with adequate treatment. Prolonged monitoring by STE is recommended as heart changes require time after correction of thyroid profile to disappear.85
STE in HFpEF
Heart failure with preserved ejection fraction (HFpEF) is a complication seen in hypertensive patients among other chronic diseases. Since EF is preserved, the need for a diagnostic sign is of paramount importance. GLS proved to have an objective role in prognostication of cases where an absolute result of LV GLS below 15.8% independently foresaw hospital admission frequency increase and increased tolls of cardiac arrest and cardiovascular mortality.86 STE should also be integrated into preventative strategies as systolic dysfunction in hypertensive patients could be detected by GLS during the early stages of the disease.87 The European Society of Cardiology (ESC) guidelines also suggested its use for cases with high risk of developing heart failure.88
STE in COVID-19
During the COVID-19 pandemic, multiple studies were conducted to evaluate the prognostic value of STE parameters for the purpose of risk stratification of the numerous patients in need of hospital care. The RV longitudinal strain and LVGLS were found to be independent predictors of mortality.89 , 90 Implementation of STE was able to balance the arduous equation of being vigilant for signs of myocardial dysfunction while striving to minimize the contact between sonographers and ICU patients.91
Limitations
Limitations impeding generalization of STE are categorized into technical and clinical limitations as follows:
Technical Limitations
Inter-vendor variability remains one of the most hindering obstacles as vendors have individual software to calculate strain parameters, which led to results across different vendors to lack cross-compatibility. Such variability demands serial measurements obtained for each patient to be executed on the same equipment and software.92 A joint venture was established for the aim of standardization of software procedures to tackle such issues.93 There's also no available software tailored for assessment of left atrial or right ventricular strain. The current software is dedicated to left ventricular evaluation and should be applied with cautiousness on other chambers.
While STE utilizes images normally acquired for conventional 2D echocardiography (2DE) assessment, additional time is needed for analysis, which varies due to the observer expertise and obtained parameters. GLS requires an average of 64 seconds and more is needed for GCS and GRS. The total time of STE study is estimated to be 5 minutes and will consequently add to the workload of busy echocardiography operators.30
On account of its underlying physical principles, Images must be of adequate quality with minimal artifacts and good viewing of speckles and cardiac borders. Images that fail to track more than one segment should be discarded. Further software-related misinterpretation could also occur despite satisfactory images. It relies on the operator to have a good understanding of the technique's fundamentals, exclude unsatisfactory images, and take into consideration the clinical context to judge the validity of results.92 Such skills require sufficient training, which was estimated to be at least 50 studies to achieve the capability of assessing GLS. Comparable competency to expert echocardiographers in other parameters as GCS and GRS could not, however, be reached by learners even after 100 training studies.6
Clinical Limitations
Clinically-related difficulties can be encountered; such as its inconsistency concerning age, sex, and race. Cardiovascular hemodynamics may also cause variability where increased afterload (as seen in patients with severe aortic stenosis) can yield low strain readings despite normal contractility. Preload decline in patients with severe vomiting and diarrhea can also lead to lower strain values. Such aspects must undergo exceptionally mindful inspection in situations of serial assessment of cases. Drug intake may also play a role in altering the hemodynamic status of patients and must be taken into account as well.83
As previously discussed, GLS reduction is associated with a huge variety of conditions leading to cardiac dysfunction primarily or secondarily (the latter being of concern to our scope of handling systemic conditions). Consequentially, GLS is regarded as a tool of low diagnostic specificity.
Final Remarks and Recommendations
The cost-effectiveness of STE isn't well investigated. With the only exception to our knowledge being its cost-effectiveness in monitoring patients undergoing chemotherapy.94 , 95 More research is required to achieve full comprehension of the logistical aspects of integrating STE into routine practice.
Currently, owing to the large number of cross-sectional studies, STE is more of a diagnostic add-on in echocardiography. With the majority of studies providing evidence of its importance as a sensitive and early sign. Meanwhile, its prognostic value lags behind in need of large prospective studies.
Contrast Echocardiography
Contrast echocardiography involves using intravenous ultrasound enhancement agents (UEA) which is the administration of echogenic gas-filled microbubbles enveloped by albumin, phospholipid, or polymer shell. In opposition to free gas bubbles and hand agitated solutions, whose lifetime in the bloodstream is short, encapsulated microbubbles are compressible and remain intact after passing capillary beds.96 The shell of contrast media is then metabolized and the gas is excreted through respiration.97 UEA grant the cardiac ultrasound advantageous capabilities, not only as a structural imaging modality but also as a functional one.98 The current term UEA is preferable to ultrasound contrast agent (UCA) to avoid confusion regarding iodinated or other radiopaque contrast media.97
Contrast Echocardiography For Enhancement of LV Cavity And Border Delineation
UEA aid in eliminating diagnostic uncertainties encountered during cardiac ultrasound, a major barrier in almost a third of cases referred to echocardiography.98 Recent guidelines recommend contrast administration when more than 20% of the LV endocardium can't be visualized. In suboptimal images, the use of small doses of UEA to increase the opacification of the left ventricle allows better assessment of LV borders and volumes, thus improving reproducibility and diagnosis certainty.99 , 100 The improved feasibility was of value in patients with obesity and chronic obstructive pulmonary disease.101
Contrast echocardiography can diagnose apical hypertrophic cardiomyopathy (ACM), which is a common variant of hypertrophic cardiomyopathy in the east (one in four cases in Japan). It was found to be as diagnostic as LV angiography, showing the characteristic spade-like LV appearance of ACM.102 Contrast enhancement provides border demarcation which aids in detection of LV thrombi showing a filling defect in the LV cavity.103 LV thrombus is a serious complication (can lead to systemic emboli) seen in cases of ischemic heart diseases, dilated cardiomyopathy, peripartum cardiomyopathy, and RHD.104 Most recently, contrast echocardiography was used in COVID-19 patients with severe respiratory distress to feasibly assess the cardiac functions during extracorporeal membrane oxygenation (ECMO).105
Another noteworthy application is enabling better automatic border detection, which in turn led to optimization of otherwise ineligible images for STE.106 , 107 It also improved the feasibility of 3D images; providing accuracy, reproducibility, and short acquisition time.108 Therefore, extensive availability of UEA and the awareness of their benefits and administration protocols is key to unlocking the full potential of emerging echocardiography techniques.
Contrast Echocardiography in Myocardial Perfusion Imaging
Myocardial contrast echocardiography (MCE) denotes the use of UEA for the assessment of myocardial perfusion. Having similar rheology to RBCs,109 the microbubbles are confined to the intravascular compartment and can be used as a marker to obtain both qualitative and quantitative account of myocardial blood flow (MBF) in a non-invasive manner.110 Qualitative assessment depends on a scoring index performed by the operator according to whether contrast flow is absent, heterogeneous, or homogeneous.111 Quantitative evaluation of MBF is done using specific software.112 Since 90% of MBF resides in capillary beds, MCE can visualize the microvasculature of the myocardium and can identify segments with impaired perfusion.113
Cardiovascular involvement in systemic diseases undergoes multiple stages before the appearance of symptoms, each having a corresponding sign. The earliest are metabolic changes and perfusion abnormalities followed by diastolic and systolic dysfunction and lastly ECG changes.110 Taking into consideration the heavy burden of cardiac morbidity and risk of mortality, generalization of MCE for detection of perfusion defects early on in the disease progression will provide insight on both the extent of cardiac affection and prognosis, as well as aid the decision-making process during management.
In order to demonstrate the body of the available evidence for subclinical cardiac hypoperfusion detected in subjects of various systemic disorders, Table 3 shows its high prevalence in several conditions. The results were obtained by a variety of myocardial perfusion imaging modalities such as single-photon emission computer tomography (SPECT), PET scan, and Coronary angiography (CA).
Table 3.
prevalence of abnormal myocardial perfusion in systemic diseases.
| Condition | Imaging modality | Abnormal perfusion% of cases |
|---|---|---|
| Type 2 DM (free of cardiac symptoms) | Technetium-99m sestamibi SPECT | 10.3%114 –15.9%115–25%116 |
| PET scan | 13.5%117 | |
| SLE | Technetium-99m sestamibi SPECT | 33%118 |
| Coronary angiography | 38%118 | |
| Rheumatic heart disease | Coronary angiography | 10.91%119 |
| HIV | 99m Tc-tetrofosmin SPECT | 9.6%120 |
| Sarcoidosis | PET scan | 65.6%121 |
| Amyloidosis | PET scan | 81.9%122 |
| CMR | 100%123 | |
| Sickle beta-thalassemia | Exercise Tc-99m tetrofosmin gated SPECT | 27%124 |
| Emergencies (Acute chest pain with negative ECG and Troponin) | Stress SPECT | 12.1%125 |
Among the mentioned various modalities used for clinical assessment of myocardial perfusion, MCE possesses the following advantages: (1) less invasiveness than CA, PET, and SPECT, as it does not involve using ionizing radiation or catheterization; (2) best temporal resolution allows the acquisition of optimal images; (3) better spatial resolution than SPECT and PET, which allows more accurate representation of sub-endocardial hypoperfusion; (4) its portability enables bedside assessment; (5) its ability to assess absolute MBF; (6) its low cost and wide availability.126, 127, 128
Such advantages do not come at a cost of lower diagnostic accuracy. MCE provides comparable results with nuclear imaging modalities, which are the most commonly used for non-invasive assessment of myocardial perfusion.129 Several studies and meta-analyses concluded MCE's concordance with SPECT130, 131, 132 and CA110 , 112 , 132 in diagnosing coronary artery disease, which is an end-point of many systemic diseases and the leading cause of death worldwide.133 In addition, MCE can be used in diagnosis of coronary microvascular disease (MVD or CMD), a.k.a. small artery disease, which results from systemic atherosclerosis and can lead to cardiac events in younger population even in the absence of coronary obstruction.134 , 135 Such findings vouch for MCE to be generalized as a first-line investigation for suspected myocardial ischemia.110
As for emergency medicine, particularly acute chest pain with non-diagnostic ECG, MCE provides the combined insight of wall motion and MBF, which allows the assessment of the infarction-related artery (IRA). The degree of IRA's patency influences the decision-making process when planning for percutaneous intervention (PCI) as a life-saving procedure in acute myocardial infarction.136 MCE showed superiority over thrombolysis in myocardial infarction (TIMI) score, providing accurate prognostic information regarding the risk of short- and long-term cardiac events.137 Furthermore, intriguing preclinical conclusions that high-mechanical-index flashes during UEA infusion can restore defective microvasculature have motivated the clinical trial of MCE as a supportive therapeutic measure (so-called sonothrombolysis) in ST-elevated myocardial infarction. The addition of sonothrombolysis to PCI yielded sustained improvement in recanalization leading to a smaller infarct when compared to PCI alone.138
Despite the immense amount of evidence on MCE's effectiveness and practicality, as well as the high prevalence of myocardial perfusion defects in systemic diseases, there is a lack of large-scale trials for establishing direct evidence of MCE's role in early diagnosis and intervention in conditions of non-cardiac origin. We consider this as a fundamental obstacle in undertaking an evidence-based approach for generalization of MCE in systemic diseases and encourage further research in that aspect.
Cost-Effectiveness
The cost of UEA and the time they require to be administrated are an investment proven to be clinically profitable, especially in settings of surgical and medical ICUs followed by in- and outpatient assessment for various reasons. The most prominent being reduced technical difficulties involved in image interpretation. In a large cohort of 632 patients, the studies considered as technically difficult decreased from 86.7% before contrast administration to 9.8% (P < 0.0001). In addition, the incomprehensible results dropped from 11.7% to 0.3%.139 In another study adequate visualization was attained in 87% of cases against only 13% before contrast administration. Another important reason is avoiding further imaging procedures such as transesophageal echocardiography -which offered relatively little improvement over MCE- or even nuclear imaging modalities in one of three cases.139, 140 It was also an influential factor in drug choice, altering the previously prescribed drug in 10.4% of cases.139 In many clinical trials a decrease of downstream costs which varied from $122139 to $269140 was attained. Contrast administration eliminated the time spent by the sonographer unsuccessfully attempting to improve suboptimal or uninterpretable images – the so-called "struggle time".141 Also, the use of UEA improved the reproducibility of results and decreased interobserver variability to an agreement rate of 87% when viewed by two independent observers.142
Training
MCE is operator dependent, that's why the MCE training must ensure full understanding of the foundational technology involved to avoid misinterpretation. Despite the availability of automatic gain control, the user must be aware of operational settings and using a suitable mechanical index during the imaging process to avoid artifacts, which may appear as hypoperfused regions of the myocardium.110 Artifacts occur due to unintentional microbubble destruction in the near field, especially with high mechanical index. The opposite can happen, where UEA in the near field shadow deeper segments, causing them to seem ischemic.143 True perfusion defects are, however, typically sub-endocardial and will not disappear by changing the view. Shadowing by the lungs is also encountered and can hinder image interpretation.110
The European association of cardiovascular imaging (EACVI) provides elaborate guidelines and checklists and recommends that operators must be oriented with administration procedures and adverse effects of UEA administration as well as basic life support. They should perform and interpret 25 studies under supervision and uphold their competency by fulfilling a minimum of 50 studies per year.143
Safety of UEA
There are reported cases of immunological reaction to UEA reaching anaphylaxis in rare cases. However, many trials concluded its safety even for the critically ill. The largest trial to our knowledge involved 78,383 administered UEA doses with only 4 patients developing an anaphylactic reaction that resolved with treatment.144 UEA are, however, contraindicated in patients with known allergy to the shell or gas constituents. Yet with multiple commercially available types of UEA, a different agent can simply be used.97 Concerns of ischemic adverse effects, which led to the FDA black-box warning in 2007, have also been addressed providing proof of UEA safety.145
Three-Dimensional Echocardiography
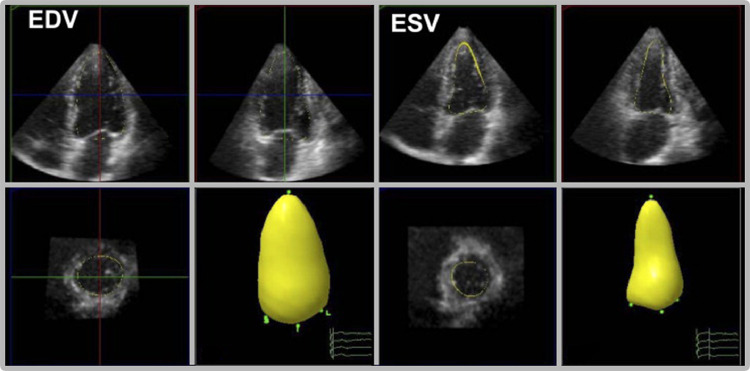
Conventional 2DE includes various geometric assumptions leading to inaccurate volume estimation. To overcome such problem, early 3DE images were constructed by aligning multiple 2D slices, inferring technical difficulties and time consumption. Recent technology has enabled real-time 3DE (RT3DE) through the invention of a probe that can generate and transduce ultrasound waves in a 3D radial manner, acquiring large datasets that allow 3D visualization of the heart, achieving high temporal and spatial resolution without the need for post-imaging processing.146
This technological breakthrough facilitates volumetric evaluation of the heart. Accurate quantification of volumes was of benefit to patients undergoing chemotherapy, being a reproducible parameter for 1 year of follow-up.147 It could also detect left atrial remodeling in a prospective study that concluded the significance of such remodeling as a sign of subclinical cardiac affection due to increased cumulative blood pressure, thereby recommending 3DE as a modality for early detection of cardiovascular disease.148
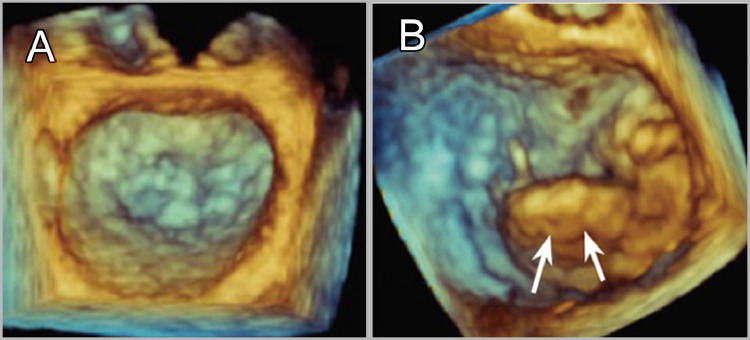
3DE obtains an optimal anatomical representation of the cardiac valves. Assessment of mitral valve area in rheumatic mitral stenosis plays a major role in the decision-making process regarding surgical treatment, the assessment done by 3DE provides comparable measurements to the reference method (Gorlin formula) which entails invasive intervention.149 It was also used for the assessment of aortic valve calcification (AVC), a pathological entity of resemblance to coronary artery disease, providing quantitative evaluation with comparable accuracy to multidetector CT. Being involved in the pathological cascade of aortic stenosis, early detection of AVC in cases at risk of atherosclerosis is of significant relevance to the management plan.150
Utilization of 3DE in STE brings about better tracking of myocardial speckles throughout the cardiac cycle. Since cardiac movement occurs in three planes, speckles can go out of plane when using 2DE. 3DSTE can assess the cardiac strain in one cardiac cycle, overcoming beat-to-beat variability.31 , 151 Its ability to measure all strain parameters from the apical window (as opposed to multiples windows required in 2DSTE) and its shorter analysis time contribute to the overall practicality of 3DSTE by rendering it time-efficient. GAS is an exclusive parameter to 3DSTE, which aided the assessment of diabetic patients among others as mentioned before. Yet, 3DSTE remains limited by 3DE image acquisition being less feasible than 2DE. Overcoming technical difficulties through software and hardware optimization can potentially make 3DSTE the gold standard in the assessment of cardiac functions.152
The Case for Machine Learning in Echocardiography
Implementation of artificial intelligence (AI), particularly machine learning (ML), in medical practice has gained an increasing interest. Automation of echocardiography is already used in techniques as STE, 3DE, and Fourier analysis of 2DE.153 , 154 While automation requires pre-coded fixed algorithms, ML relies on computer-generated algorithms based on existing images that are either pre-labeled (supervised ML) or unlabeled (unsupervised ML) where any emerging patterns could be identified; such algorithms can self-improve over time which is referred to as reinforcement ML.155 ML, therefore, can utilize the huge amount of data being generated and potentially overlooked by manually-coded algorithms.156
ML models provide accuracy that resembles or even surpasses human operators.157 In busy scenarios clinicians resort to visual interpretation, so-called “eye-balling”, which relies on individual expertise.158 Hence, the ability to accurately and almost instantaneously provide quantitative and objective data comprising EF159, 160, 161, wall motion abnormality (WMA)162, 163, 164, mitral regurgitation (MR)165, and GLS159 , 166 among others justifies the need for widespread exploitation of AI's potential aptitudes. Evidence for using ML in medical imaging to diagnose pathological entities is established with the first algorithm for medical decision making without human interference gaining FDA approval in 2018.167 A noteworthy example is its use with CT in hospitalized patients with COVID-19.168 Nonetheless, ML doesn't eliminate the need for diagnostic radiologists but will rather evolve to be an indispensable tool in their arsenal.169
To realize its potential, the most important technical difficulty to be overcome is the establishment of a reliable infrastructure capable of sustainable operation.170 The consequential ethical dilemmas must also be contemplated such as the “black box” problem concerning deep learning algorithms being humanly uninterpretable and will take into consideration factors with no understandable clinical significance. Another example is the legal ownership of the huge corpus of data being generated and the right to obtain a financial profit from it.171
As a matter of fact, the advancements in ML are well beyond the scope of speculative possibility. Its regimentation will allow healthcare professionals with limited (but certainly essential) knowledge of echocardiography to evaluate the heart at the point of care, thereby accrediting the cardiac ultrasound with its widely known promise to become the stethoscope of the future.172
Conclusion
In today's clinical environment, where resources are scarce, the generalization of advanced echocardiography techniques will aid in alleviating the substantial burden of systemic diseases on the heart. STE is a sensitive modality for detection of subclinical systolic dysfunction. LVGLS is the most significant parameter used in STE, providing objective cut-off values to guide the decision-making process. Its superiority to LVEF implies its widespread use as a screening method for subjects at risk of developing secondary cardiac pathology. Its generalization is also encouraged owing to its feasibility and easy trainability. Further research is needed to evaluate STE's cost-effectiveness and logistical hurdles. Contrast enhancement of the cardiac ultrasound eliminates the ambiguity often encountered in echocardiographic imaging, which enables better visualization of the cardiac structure and function; a merit of benefit to patients suffering from obesity or lung diseases. UEA administration is used to opacify the LV cavity, thus detecting intramural lesions. MCE can detect subclinical microvascular perfusion defects that may present with fatal ischemic attacks if remained untreated. Its direct practicality in patients of different systemic diseases must be tested in large-scale trials to obtain indisputable evidence for its generalization. 3DE's ability to visualize the heart in all planes simultaneously and in real time provides accurate quantitative volume analysis without geometrical assumptions, as well as realistic anatomical representations of cardiac valves. The synergistic effect of using multiple modalities at once can overcome technical difficulties and improve feasibility. Combined with the potential of ML, echocardiography enables comprehensive bedside and ambulatory evaluation of the heart. We shed the light on the necessity and urgency of integrating echocardiography into the first ranks of guidelines and management algorithms, as well as making it more available at the point of care and more accessible to general practitioners. The substantial beneficence it grants should not remain contained within echocardiography labs.
Appendix (1)
.
Appendix (2): Figure Legends
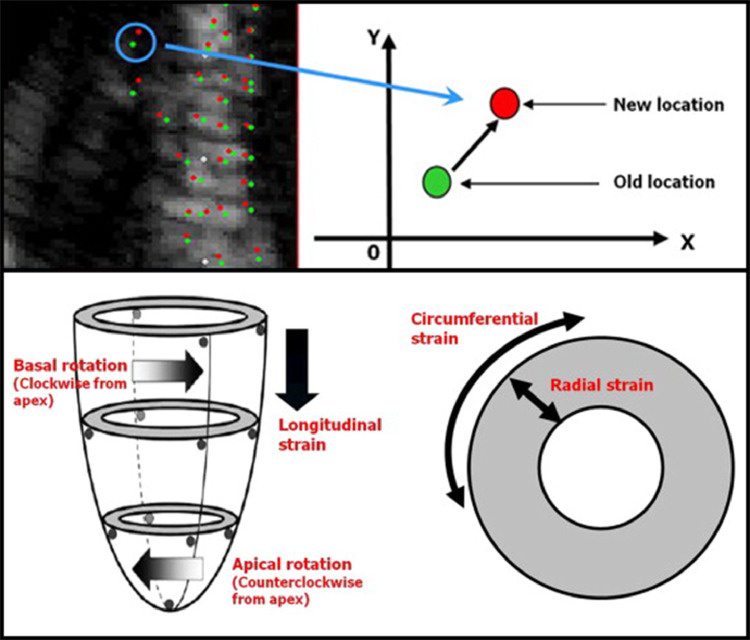
FIG 1: Fundamental Principles of STE.
FIG 1.
Fundamental principles of STE. (Color version of figure is available online.)
Caption: Top: The fundamental principles of speckle-tracking echocardiography. Myocardial speckles are tracked in multiple frames to measure myocardial deformation in different directions. Bottom: Different components of left ventricular myocardial deformation that can be measured by speckle-tracking echocardiography. Image courtesy of Bansal and Kasliwal.173
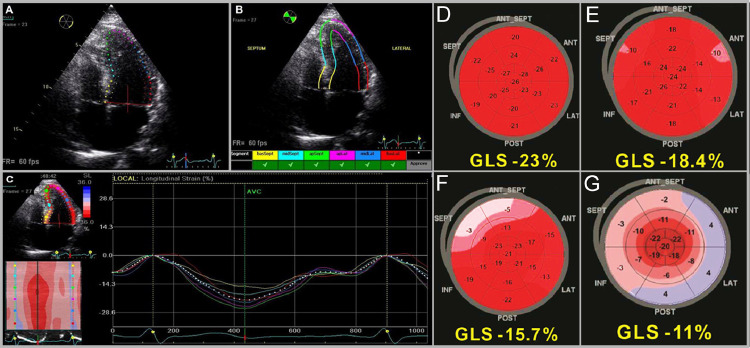
FIG 2.
Steps of performing STE. (Color version of figure is available online.)
Title: Steps Involved in STE.
Caption: (A) Endocardial border is manually traced and the software obtains a region of interest. (B) Reviewing and approving adequate tracking of each segment. (C) Multiple Color-coded strain curves are generated. (courtesy of Bansal and Kasliwal173) A bull's eye map is generated displaying regional and global peak strain in cases of (D) Athlete, (E) HFpEF, (F) Hypertension, and (G) Cardiac amyloidosis with the apical sparing pattern. (courtesy of Zito et al.83)
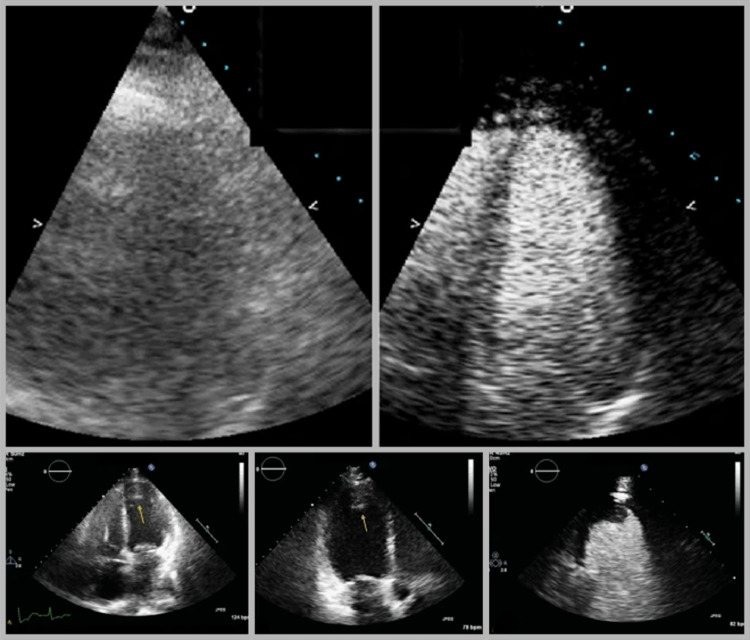
FIG 3.
Contrast echocardiography as a structural imaging modality.
Title: Contrast echocardiography as a structural imaging modality.
Caption: Top: UEA aid in the disambiguation of unclear images. Bottom: Contrast echocardiography clearly detects a cardiac thrombus appearing as a filling defect. (Image courtesy of Eskandari and Monaghan.174)
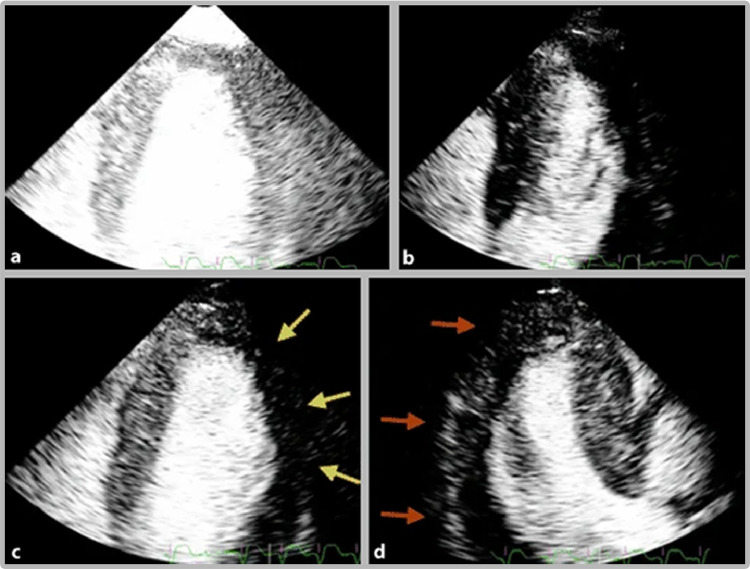
FIG 4.
Contrast echocardiography in myocardial perfusion imaging. (Color version of figure is available online.)
Title: Contrast echocardiography in myocardial perfusion imaging.
Caption: (A, B) The operator uses ultrasound flashes to induce microbubble destruction. (C) Yellow arrows show a perfusion defect in the lateral wall. (D) Red arrows show a perfusion defect in the inferolateral wall. (Image courtesy of Eskandari and Monaghan.174)
FIG 5.
3DE provides quantitative volumetric analysis without geometrical assumptions. (Color version of figure is available online.)
Title: 3DE provides quantitative volumetric analysis without geometrical assumptions.
Caption: Real-time 3DE obtain multiple slices that are used to construct a 3D model of the left ventricle at end-diastolic volume (EDV) and end-systolic volume (ESV). This allows accurate quantitative analysis of the cardiac volume. (Image courtesy of Hung et al.175)
FIG 6.
3DE obtains realistic anatomical representations of the cardiac valves. (Color version of figure is available online.)
Title: 3DE obtains realistic anatomical representations of the cardiac valves.
Caption: (A) Normal mitral valve in systole. (B) Mitral valve prolapse.
Appendix (3): list of abbreviations
2D: 2-dimensional
2DE: 2-dimensional echocardiography
3DE: 3-dimensional echocardiography
ACEI: angiotensin converting enzyme inhibitor
ACM: apical hypertrophic cardiomyopathy
AI: artificial intelligence
ARB: angiotensin receptor blocker
AV: atrioventricular block
AVC: aortic valve calcification
CA: cardiac amyloidosis
CA: Coronary angiography
CMR T2: T2 weighted cardiovascular magnetic resonance
COVID-19: 2019 coronavirus disease
CS: cardiac sarcoidosis
CSR: circumferential strain rate
CT: computer tomography
CTRCD: cancer therapy-related cardiac dysfunction
DM: diabetes mellitus
EACVI: European association of cardiovascular imaging
ECG: electrocardiogram
EF%: ejection fraction
ESC: European Society of Cardiology (USED ONCE)
FDA: Food and drug administration
GAS: global area strain
GCS: global circumferential strain
GLS: global longitudinal strain
GRS: global radial strain
HF: heart failure
HFpEF: Heart failure with preserved ejection fraction
HTN: hypertension
ICI: immune checkpoint inhibitors
IRA: infarction-related artery
LSR: longitudinal strain rate
LV GLS: left ventricular global longitudinal strain
LVEF: left ventricular ejection fraction
MBF: myocardial blood flow
MCE: Myocardial contrast echocardiography
ML: machine learning
MR: mitral regurgitation
MRI: magnetic resonance imaging
MVD or CMD: coronary microvascular disease
NAFLD: non-alcoholic fatty liver disease
PCI: percutaneous intervention
PET: positron emission tomography
RHD: rheumatic heart disease
RIHD: radiotherapy-induced heart disease
RRSR: Relative regional strain ratio
RT3DE: real-time 3-dimensional echocardiography
RV: right ventricle
SLE: systemic lupus erythematosus
SPECT: single-photon emission computer tomography
SSc: systemic sclerosis
STE: speckle tracking echocardiography
TDI: tissue Doppler imaging
TIMI: thrombolysis in myocardial infarction
TIMP-1: tissue inhibitor of metalloproteinase-1
UCA: ultrasound contrast agent
UEA: ultrasound enhancement agents
WMA: wall motion abnormality
Acknowledgments
The authors have no conflicts of interest to declare. All co-authors have seen and agree with the contents of the manuscript. We certify that the submission is original work and is not under review at any other publication.
No grants or financial aid were received for the production of this article.
The figures have been reproduced after acquiring needed permissions as follows: figure (1) is courtesy of Bansal et al 2013 obtained with license number: 4920400141784. license date:2020-10-01. figure 2: (A-C) are courtesy of Bansal et al 2013 obtained with license number: 4920400585555. license date:2020-10-01. (D-G) are courtesy of Zito 2018, open access in terms with the creative commons license: https://creativecommons.org/licenses/by/4.0/ figures 3-4: courtesy of Eskandari and Monaghan 2017, open access in terms with the creative commons license: https://creativecommons.org/licenses/by/4.0/ figure 5: courtesy of Hung et al 2007. Obtained with license number: 4920720641839. license date:2020-10-02. figure 6: courtesy of Shiota 2014, open access in terms with the creative commons license http://creativecommons.org/licenses/by-nc/3.0/
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.cpcardiol.2021.100847.
Appendix. Supplementary materials
References
- 1.Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev. 2017;03:7. doi: 10.15420/cfr.2016:25:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lloyd-Jones DM, Larson MG, Leip EP, et al. Lifetime risk for developing congestive heart failure: the framingham heart study. Circulation. 2002;106:3068–3072. doi: 10.1161/01.CIR.0000039105.49749.6F. [DOI] [PubMed] [Google Scholar]
- 3.Weidemann F, Strotmann JM. Use of tissue Doppler imaging to identify and manage systemic diseases. Clin Res Cardiol. 2008;97:65–73. doi: 10.1007/s00392-007-0566-0. [DOI] [PubMed] [Google Scholar]
- 4.Alpert JS, Mladenovic J, Hellmann DB. Should a hand-carried ultrasound machine become standard equipment for every internist? Am J Med. 2009;122:1–3. doi: 10.1016/j.amjmed.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 5.MARWICK T. Can we Justify the cost of echocardiography? lessons from outcomes research. Eur J Echocardiogr. 2005;6:155–163. doi: 10.1016/j.euje.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Luis SA, Pellikka PA. Is speckle tracking imaging ready for prime time in current echo clinical practice? Prog Cardiovasc Dis. 2018;61:437–445. doi: 10.1016/j.pcad.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. 2020;16:223–237. doi: 10.1038/s41581-019-0244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuroda K. Hypertensive cardiomyopathy: a clinical approach and literature review. World J Hypertens. 2015;5:41. doi: 10.5494/wjh.v5.i2.41. [DOI] [Google Scholar]
- 9.Levesque RJR. Obesity and Overweight. Encyclopedia of Adolescence. doi: 10.1007/978-1-4419-1695-2_447
- 10.Araújo J, Ramos E. Porto biomedical journal paediatric obesity and cardiovascular risk factors-a life course approach. Porto Biomed J. 2017;2:102–110. doi: 10.1016/j.pbj.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarwar N, Gao P, Kondapally Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jia G, Whaley-Connell A, Sowers JR. Diabetic cardiomyopathy: a hyperglycaemia- and insulin-resistance-induced heart disease. Diabetologia. 2018;61:21–28. doi: 10.1007/s00125-017-4390-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee WS, Kim J. Diabetic cardiomyopathy: where we are and where we are going. Korean J Intern Med. 2017;32:404–421. doi: 10.3904/kjim.2016.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang H, Wei J, Zheng Q, et al. Radiation-induced heart disease: a review of classification, mechanism and prevention. Int J Biol Sci. 2019;15:2128–2138. doi: 10.7150/ijbs.35460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yusuf SW, Sami S, Daher IN. Radiation-induced heart disease: a clinical update. Cardiol Res Pract. 2011;1 doi: 10.4061/2011/317659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cuzick J, Stewart H, Rutqvist L, et al. Cause-specific mortality in long-term survivors of breast cancer who participated in trials of radiotherapy. J Clin Oncol. 1994;12:447–453. doi: 10.1200/JCO.1994.12.3.447. [DOI] [PubMed] [Google Scholar]
- 17.Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97:2869–2879. doi: 10.1002/cncr.11407. [DOI] [PubMed] [Google Scholar]
- 18.Moja L, Tagliabue L, Balduzzi S, et al. Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst Rev. 2012;2012 doi: 10.1002/14651858.cd006243.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nolan MT, Lowenthal RM, Venn A, Marwick TH. Chemotherapy-related cardiomyopathy: a neglected aspect of cancer survivorship. Intern Med J. 2014;44:939–950. doi: 10.1111/imj.12532. [DOI] [PubMed] [Google Scholar]
- 20.Albakri A. Rheumatic heart failure: a review of clinical status and meta-analysis of echocardiography diagnosis and efficacy of shorter duration of antibiotic. Intern Med Care. 2018;2 doi: 10.15761/imc.1000123. [DOI] [Google Scholar]
- 21.Epidemiology and pathogenesis of systemic lupus erythematosus - UpToDate. https://www.uptodate.com/contents/epidemiology-and-pathogenesis-of-systemic-lupus-erythematosus. Accessed September 5, 2020.
- 22.Doria A, Iaccarino L, Sarzi-Puttini P, Atzeni F, Turriel M, Petri M. Cardiac involvement in systemic lupus erythematosus. Lupus. 2005;14:683–686. doi: 10.1191/0961203305lu2200oa. [DOI] [PubMed] [Google Scholar]
- 23.Bartels CM, Buhr KA, Goldberg JW, et al. Mortality and cardiovascular burden of systemic lupus erythematosus in a US population-based cohort. J Rheumatol. 2014;41:680–687. doi: 10.3899/jrheum.130874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen CI, Wang L, Wei W, Yuce H, Phillips K. Burden of rheumatoid arthritis among US Medicare population: co-morbidities, health-care resource utilization and costs. Rheumatol Adv Pract. 2018;2:i1–i9. doi: 10.1093/rap/rky005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turesson C. Comorbidity in rheumatoid arthritis. Swiss Med Wkly. 2016;146:w14290. doi: 10.4414/smw.2016.14290. [DOI] [PubMed] [Google Scholar]
- 26.England BR, Thiele GM, Anderson DR, Mikuls TR. Increased cardiovascular risk in rheumatoid arthritis: mechanisms and implications. BMJ. 2018:361. doi: 10.1136/bmj.k1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mor-Avi V, Lang RM, Badano LP, et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications: endorsed by the Japanese Society of Echocardiography. J Am Soc Echocardiogr. 2011;24:277–313. doi: 10.1016/j.echo.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 28.Becker M, Kramann R, Dohmen G, et al. Impact of left ventricular loading conditions on myocardial deformation parameters: analysis of early and late changes of myocardial deformation parameters after aortic valve replacement. J Am Soc Echocardiogr. 2007;20:681–689. doi: 10.1016/j.echo.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Orde S, Huang SJ, McLean AS. Speckle tracking echocardiography in the critically ill: enticing research with minimal clinical practicality or the answer to non-invasive cardiac assessment? Anaesth Intensive Care. 2016;44:542–551. doi: 10.1177/0310057×1604400518. [DOI] [PubMed] [Google Scholar]
- 30.Luis SA, Chan J, Pellikka PA. Echocardiographic assessment of left ventricular systolic function: an overview of contemporary techniques, including speckle-tracking echocardiography. Mayo Clin Proc. 2019;94:125–138. doi: 10.1016/j.mayocp.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 31.Trivedi SJ, Altman M, Stanton T, Thomas L. Echocardiographic strain in clinical practice. Hear Lung Circ. 2019;28:1320–1330. doi: 10.1016/j.hlc.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 32.de Waal K, Phad N. Speckle tracking echocardiography in newborns. in: hemodynamics and cardiology (Third Edition) Content Repository Only! 2019:219–233. doi: 10.1016/B978-0-323-53366-9.00013-2. [DOI] [Google Scholar]
- 33.Bansal N, Amdani S, Lipshultz ER, Lipshultz SE. Chemotherapy-induced cardiotoxicity in children. Expert Opin Drug Metab Toxicol. 2017;13:817–832. doi: 10.1080/17425255.2017.1351547. [DOI] [PubMed] [Google Scholar]
- 34.Mertens AC, Liu Q, Neglia JP, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: the childhood cancer survivor study. J Natl Cancer Inst. 2008;100:1368–1379. doi: 10.1093/jnci/djn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mertens AC, Yasui Y, Neglia JP, et al. Late mortality experience in five-year survivors of childhood and adolescent cancer: the childhood cancer survivor study. J Clin Oncol. 2001;19:3163–3172. doi: 10.1200/JCO.2001.19.13.3163. [DOI] [PubMed] [Google Scholar]
- 36.Brana I, Tabernero J. Cardiotoxicity. Ann Oncol. 2010;21(SUPPL. 7)):173–179. doi: 10.1093/annonc/mdq295. [DOI] [PubMed] [Google Scholar]
- 37.Fda. HIGHLIGHTS OF PRESCRIBING INFORMATION. www.fda.gov/medwatch. Accessed June 30, 2020.
- 38.Gripp E de A, De Oliveira GE, Feijó LA, Garcia MI, Xavier SS, De Sousa AS. Global longitudinal strain accuracy for cardiotoxicity prediction in a cohort of breast cancer patients during anthracycline and/or trastuzumab treatment. Arq Bras Cardiol. 2018;110:140–150. doi: 10.5935/abc.20180021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolf CM, Reiner B, Kühn A, et al. Subclinical cardiac dysfunction in childhood cancer survivors on 10-years follow-up correlates with cumulative anthracycline dose and is best detected by cardiopulmonary exercise testing, circulating serum biomarker, speckle tracking echocardiography, and tissue Doppler Imaging. Front Pediatr. 2020;8:123. doi: 10.3389/fped.2020.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Biersmith MA, Tong MS, Guha A, Simonetti OP, Addison D. Multimodality cardiac imaging in the era of emerging cancer therapies. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.013755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lynce F, Barac A, Geng X, et al. Prospective evaluation of the cardiac safety of HER2-targeted therapies in patients with HER2-positive breast cancer and compromised heart function: the SAFE-HEaRt study. Breast Cancer Res Treat. 2019 doi: 10.1007/s10549-019-05191-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harrington JK, Richmond ME, Fein AW, Kobsa S, Satwani P, Shah A. Two-dimensional speckle tracking echocardiography-derived strain measurements in survivors of childhood cancer on angiotensin converting enzyme inhibition or receptor blockade. Pediatr Cardiol. 2018;39:1404–1412. doi: 10.1007/s00246-018-1910-z. [DOI] [PubMed] [Google Scholar]
- 43.Tuohinen SS, Skyttä T, Poutanen T, et al. Radiotherapy-induced global and regional differences in early-stage left-sided versus right-sided breast cancer patients: speckle tracking echocardiography study. Int J Cardiovasc Imaging. 2017;33:463–472. doi: 10.1007/s10554-016-1021-y. [DOI] [PubMed] [Google Scholar]
- 44.Abe O, Abe R, Enomoto K, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 45.Tuohinen SS, Skytta T, Poutanen T, et al. Radiotherapy-induced global and regional differences in early-stage left-sided versus right-sided breast cancer patients: speckle tracking echocardiography study. Int J Cardiovasc Imaging. 2017;33:463–472. doi: 10.1007/s10554-016-1021-y. [DOI] [PubMed] [Google Scholar]
- 46.Lo Q, Hee L, Batumalai V, et al. Strain imaging detects dose-dependent segmental cardiac dysfunction in the acute phase after breast irradiation. Int J Radiat Oncol Biol Phys. 2017;99:182–190. doi: 10.1016/j.ijrobp.2017.05.030. [DOI] [PubMed] [Google Scholar]
- 47.Erven K, Jurcut R, Weltens C, et al. Acute radiation effects on cardiac function detected by strain rate imaging in breast cancer patients. Int J Radiat Oncol Biol Phys. 2011;79:1444–1451. doi: 10.1016/j.ijrobp.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 48.Tuohinen SS, Skytta T, Huhtala H, Virtanen V, Kellokumpu-Lehtinen P-L, Raatikainen P. Left ventricular speckle tracking echocardiography changes among early-stage breast cancer patients three years after radiotherapy. Anticancer Res. 2019;39:4227–4236. doi: 10.21873/anticanres.13584. [DOI] [PubMed] [Google Scholar]
- 49.Thavendiranathan P, Poulin F, Lim KD, Plana JC, Woo A, Marwick TH. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol. 2014;63(25 PART A):2751–2768. doi: 10.1016/j.jacc.2014.01.073. [DOI] [PubMed] [Google Scholar]
- 50.Trivedi SJ, Choudhary P, Lo Q, et al. Persistent reduction in global longitudinal strain in the longer term after radiation therapy in patients with breast cancer. Radiother Oncol. 2019;132:148–154. doi: 10.1016/j.radonc.2018.10.023. [DOI] [PubMed] [Google Scholar]
- 51.Naseem M, Samir S, Ibrahim IK, Khedr L, Shahba AAE. 2-D speckle-tracking assessment of left and right ventricular function in rheumatoid arthritis patients with and without disease activity. J Saudi Hear Assoc. 2019;31:41–49. doi: 10.1016/j.jsha.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klein I, Danzi S. Thyroid disease and the heart. Circulation. 2007;116:1725-1735. doi: 10.1161/CIRCULATIONAHA.106.678326 [DOI] [PubMed]
- 53.Duzen IV, Tabur S, Ozturk S, et al. Assessment of subclinical left ventricular dysfunction with speckle-tracking echocardiography in hyperthyroid and euthyroid Graves’ disease and its correlation with serum TIMP-1. Acta Cardiol. 2020 doi: 10.1080/00015385.2019.1708598. [DOI] [PubMed] [Google Scholar]
- 54.Beaton A, Richards H, Ploutz M, et al. Cardiac strain findings in children with latent rheumatic heart disease detected by echocardiographic screening. Cardiol Young. 2017;27:1180–1185. doi: 10.1017/S1047951116002778. [DOI] [PubMed] [Google Scholar]
- 55.Poyraz E, Öz TK, Zeren G, et al. Left ventricular mechanics in isolated mild mitral stenosis: a three dimensional speckle tracking study. Int J Cardiovasc Imaging. 2017;33:1323–1330. doi: 10.1007/s10554-017-1109-z. [DOI] [PubMed] [Google Scholar]
- 56.de Isla LP, de Agustin A, Rodrigo JL, et al. Chronic mitral regurgitation: a pilot study to assess preoperative left ventricular contractile function using speckle-tracking echocardiography. J Am Soc Echocardiogr. 2009;22:831–838. doi: 10.1016/j.echo.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 57.Cherin P, Delfraissy JF, Bletry O, Dormont J, Godeau P. Les manifestations pleuropulmonaires du lupus érythémateux systémique. La Rev Med interne. 1991;12:355–362. doi: 10.1016/S0248-8663(05)80846-X. [DOI] [PubMed] [Google Scholar]
- 58.Di Minno MND, Forte F, Tufano A, et al. Speckle tracking echocardiography in patients with systemic lupus erythematosus: a meta-analysis. Eur J Intern Med. 2020;73:16–22. doi: 10.1016/j.ejim.2019.12.033. [DOI] [PubMed] [Google Scholar]
- 59.Dedeoglu R, Şahin S, Koka A, et al. Evaluation of cardiac functions in juvenile systemic lupus erythematosus with two-dimensional speckle tracking echocardiography. Clin Rheumatol. 2016;35:1967–1975. doi: 10.1007/s10067-016-3289-7. [DOI] [PubMed] [Google Scholar]
- 60.Kahan A, Coghlan G, McLaughlin V. Cardiac complications of systemic sclerosis. Rheumatology (Oxford) 2009;48(Suppl 3) doi: 10.1093/rheumatology/kep110. [DOI] [PubMed] [Google Scholar]
- 61.Rodríguez-Reyna TS, Morelos-Guzman M, Hernández-Reyes P, et al. Assessment of myocardial fibrosis and microvascular damage in systemic sclerosis by magnetic resonance imaging and coronary angiotomography. Rheumatol (United Kingdom) 2014;54:647–654. doi: 10.1093/rheumatology/keu350. [DOI] [PubMed] [Google Scholar]
- 62.Ntusi NA, Piechnik SK, Francis JM, et al. Subclinical myocardial inflammation and diffuse fibrosis are common in systemic sclerosis - a clinical study using myocardial T1-mapping and extracellular volume quantification. J Cardiovasc Magn Reson. 2014;16 doi: 10.1186/1532-429X-16-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hromádka M, Seidlerová J, Suchý D, et al. Myocardial fibrosis detected by magnetic resonance in systemic sclerosis patients – relationship with biochemical and echocardiography parameters. Int J Cardiol. 2017;249:448–453. doi: 10.1016/j.ijcard.2017.08.072. [DOI] [PubMed] [Google Scholar]
- 64.Cusmà Piccione M, Zito C, Bagnato G, et al. Role of 2D strain in the early identification of left ventricular dysfunction and in the risk stratification of systemic sclerosis patients. Cardiovasc Ultrasound. 2013;11:6. doi: 10.1186/1476-7120-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Borgna-Pignatti C, Rugolotto S, De Stefano P, et al. Survival and disease complications in thalassemia major. Annals of the New York Academy of Sciences. 1998;850:227–231. doi: 10.1111/j.1749-6632.1998.tb10479.x. In: [DOI] [PubMed] [Google Scholar]
- 66.Geyer H, Caracciolo G, Abe H, et al. Assessment of myocardial mechanics using speckle tracking echocardiography: fundamentals and clinical applications. J Am Soc Echocardiogr. 2010;23:351–369. doi: 10.1016/j.echo.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 67.Parsaee M, Saedi S, Joghataei P, Azarkeivan A. Alizadeh Sani Z. Value of speckle tracking echocardiography for detection of clinically silent left ventricular dysfunction in patients with β-thalassemia. Hematology. 2017;22:554–558. doi: 10.1080/10245332.2017.1312206. [DOI] [PubMed] [Google Scholar]
- 68.Kremastinos DT, Farmakis D. Iron overload cardiomyopathy in clinical practice. Circulation. 2011;124:2253–2263. doi: 10.1161/CIRCULATIONAHA.111.050773. [DOI] [PubMed] [Google Scholar]
- 69.Anderson LJ, Westwood MA, Holden S, et al. Myocardial iron clearance during reversal of siderotic cardiomyopathy with intravenous desferrioxamine: a prospective study using T2* cardiovascular magnetic resonance. Br J Haematol. 2004;127:348–355. doi: 10.1111/j.1365-2141.2004.05202.x. [DOI] [PubMed] [Google Scholar]
- 70.Chen MR, Ko HS, Chao TF, et al. Relation of myocardial systolic mechanics to serum ferritin level as a prognosticator in thalassemia patients undergoing repeated transfusion. Echocardiography. 2015;32:79–88. doi: 10.1111/echo.12590. [DOI] [PubMed] [Google Scholar]
- 71.Bilge AK, Altinkaya E, Ozben B, Pekun F, Adalet K, Yavuz S. Early detection of left ventricular dysfunction with strain imaging in thalassemia patients. Clin Cardiol. 2010;33:E29–E34. doi: 10.1002/clc.20584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mor-Avi V, Lang RM, Badano LP, et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications: endorsed by the Japanese Society of echocardiography. J Am Soc Echocardiogr. 2011;24:277–313. doi: 10.1016/j.echo.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 73.Di Odoardo LAF, Giuditta M, Cassinerio E, et al. Myocardial deformation in iron overload cardiomyopathy: speckle tracking imaging in a beta-thalassemia major population. Intern Emerg Med. 2017;12:799–809. doi: 10.1007/s11739-017-1670-4. [DOI] [PubMed] [Google Scholar]
- 74.Barros-Gomes S, Williams B, Nhola LF, et al. Prognosis of light chain amyloidosis with preserved LVEF: added value of 2D Speckle-Tracking echocardiography to the current prognostic staging system. JACC Cardiovasc Imaging. 2017;10:398–407. doi: 10.1016/j.jcmg.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 75.Ipek E, Demirelli S, Ermis E, Inci S. Sarcoidosis and the heart: a review of the literature. Intractable Rare Dis Res. 2015;4:170–180. doi: 10.5582/irdr.2015.01023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Di Stefano C, Bruno G, Arciniegas Calle MC, et al. Diagnostic and predictive value of speckle tracking echocardiography in cardiac sarcoidosis. BMC Cardiovasc Disord. 2020;20:21. doi: 10.1186/s12872-019-01323-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Joyce E, Ninaber MK, Katsanos S, et al. Subclinical left ventricular dysfunction by echocardiographic speckle-tracking strain analysis relates to outcome in sarcoidosis. Eur J Heart Fail. 2015;17:51–62. doi: 10.1002/ejhf.205. [DOI] [PubMed] [Google Scholar]
- 78.Conte L, Fabiani I, Barletta V, et al. Early detection of left ventricular dysfunction in diabetes mellitus patients with normal ejection fraction, stratified by BMI: a preliminary speckle tracking echocardiography study. J Cardiovasc Echogr. 2013;23:73–80. doi: 10.4103/2211-4122.123953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Struthers AD, Morris AD. Screening for and treating left-ventricular abnormalities in diabetes mellitus: a new way of reducing cardiac deaths. Lancet. 2002;359:1430–1432. doi: 10.1016/S0140-6736(02)08358-7. [DOI] [PubMed] [Google Scholar]
- 80.Khairat I, Khalfallah M, Shaban A, Farag IA, Elkady A. Right ventricular 2D speckle-tracking echocardiography in children with osteosarcoma under chemotherapy. Egypt Hear J. 2019;71 doi: 10.1186/s43044-019-0028-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Elgohary AA, Shalaby MA, Mahfouz RA, Mohamed MG. Effect of diabetic duration on left ventricular global longitudinal strain by speckle tracking imaging. Am J Cardiol. 2017;119:e6. doi: 10.1016/j.amjcard.2017.03.051. [DOI] [Google Scholar]
- 82.Dong Y, Cui H, Sun L, et al. Assessment of left ventricular function in type 2 diabetes mellitus patients with non-alcoholic fatty liver disease using three-dimensional speckle-tracking echocardiography. Anatol J Cardiol. 2020;23:41–48. doi: 10.14744/AnatolJCardiol.2019.66805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zito C, Longobardo L, Citro R, et al. Ten Years of 2D longitudinal strain for early myocardial dysfunction detection: a clinical overview. Biomed Res Int. 2018;2018 doi: 10.1155/2018/8979407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hensel KO, Grimmer F, Roskopf M, Jenke AC, Wirth S, Heusch A. Subclinical alterations of cardiac mechanics present early in the course of pediatric type 1 diabetes mellitus: a prospective blinded speckle tracking stress echocardiography study. J Diabetes Res. 2016;2016 doi: 10.1155/2016/2583747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Erdogan E, Akkaya M, Bacaksiz A, et al. Electrocardiographic and echocardiographic evidence of myocardial impairment in patients with overt hypothyroidism. Ann Endocrinol (Paris) 2013;74:477–482. doi: 10.1016/j.ando.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 86.Stricagnoli M, Cameli M, Incampo E, Lunghetti S, Mondillo S. Speckle tracking echocardiography in cardiac amyloidosis. Heart Fail Rev. 2019;24:701–707. doi: 10.1007/s10741-019-09796-z. [DOI] [PubMed] [Google Scholar]
- 87.Kosmala W, Plaksej R, Strotmann JM, et al. Progression of left ventricular functional abnormalities in hypertensive patients with heart failure: an ultrasonic two-dimensional speckle tracking study. J Am Soc Echocardiogr. 2008;21:1309–1317. doi: 10.1016/j.echo.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 88.P. Ponikowski, A. A. Voors, and S. D. Anker, “2016 ESC Guidelines for the diagnosis and treatment ofacute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (E.
- 89.Li Y, Li H, Zhu S, et al. Prognostic value of right ventricular longitudinal strain in patients with COVID-19. JACC Cardiovasc Imaging. July 2020 doi: 10.1016/j.jcmg.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Baycan OF, Barman HA, Atici A, et al. Evaluation of biventricular function in patients with COVID-19 using speckle tracking echocardiography. Int J Cardiovasc Imaging. August 2020:1–10. doi: 10.1007/s10554-020-01968-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kaminski A, Payne A, Roemer S, Ignatowski D, Khandheria BK. Answering to the call of critically Ill Patients: limiting sonographer exposure to COVID-19 with focused protocols. J Am Soc Echocardiogr. 2020;33:902–903. doi: 10.1016/j.echo.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Collier P, Phelan D, Klein A. A Test in Context: Myocardial Strain Measured by Speckle-Tracking Echocardiography. J Am Coll Cardiol. 2017;69:1043–1056. doi: 10.1016/j.jacc.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 93.Mirea O, Pagourelias ED, Duchenne J, et al. Variability and reproducibility of segmental longitudinal strain measurement: a report from the EACVI-ASE strain standardization task force. JACC Cardiovasc Imaging. 2018;11:15–24. doi: 10.1016/j.jcmg.2017.01.027. [DOI] [PubMed] [Google Scholar]
- 94.Nolan MT, Plana JC, Thavendiranathan P, Shaw L, Si L, Marwick TH. Cost-effectiveness of strain-targeted cardioprotection for prevention of chemotherapy-induced cardiotoxicity. Int J Cardiol. 2016;212:336–345. doi: 10.1016/j.ijcard.2016.02.137. [DOI] [PubMed] [Google Scholar]
- 95.McDonald JP, MacNamara JP, Zaha VG. Challenges in implementing optimal echocardiographic screening in cardio-oncology. Curr Treat Options Cardiovasc Med. 2019;21 doi: 10.1007/s11936-019-0740-5. [DOI] [PubMed] [Google Scholar]
- 96.Kaufmann BA, Wei K, Lindner JR. Contrast Echocardiography. Curr Probl Cardiol. 2007;32:51–96. doi: 10.1016/j.cpcardiol.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 97.Porter TR, Mulvagh SL, Abdelmoneim SS, et al. Clinical applications of ultrasonic enhancing agents in echocardiography: 2018 American Society of Echocardiography guidelines update. J Am Soc Echocardiogr. 2018;31:241–274. doi: 10.1016/j.echo.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 98.Lancellotti Patrizio, Zamorano José Luis, Habib Gilbert BL. The EACVI Textbook of Echocardiography.; 2017. Available at: https://www.oupjapan.co.jp/en/node/16697.
- 99.Nagueh SF.Advancements in Echocardiography.; 2014.
- 100.Olszewski R, Timperley J, Cezary S, et al. The clinical applications of contrast echocardiography. Eur J Echocardiogr. 2007;8 doi: 10.1016/j.euje.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 101.Castello R, Bella JN, Rovner A, Swan J, Smith J, Shaw L. Efficacy and time-efficiency of a “sonographer-driven” contrast echocardiography protocol in a high-volume echocardiography laboratory. Am Heart J. 2003;145:535–541. doi: 10.1067/mhj.2003.164. [DOI] [PubMed] [Google Scholar]
- 102.Soman P, Swinburn J, Callister M, Stephens NG, Senior R. Apical hypertrophic cardiomyopathy: bedside diagnosis by intravenous contrast echocardiography. J Am Soc Echocardiogr. 2001;14:311–313. doi: 10.1067/mje.2001.108475. [DOI] [PubMed] [Google Scholar]
- 103.Thanigaraj S, Schechtman KB, Perez JE. Improved echocardiographic delineation of left ventricular thrombus with the use of intravenous second-generation contrast image enhancement. Journal of the American Society of Echocardiography. 1999;12:1022–1026. doi: 10.1016/S0894-7317(99)70097-0. In: Voldoi: [DOI] [PubMed] [Google Scholar]
- 104.Talle MA, Buba F, CO Anjorin. Prevalence and aetiology of left ventricular thrombus in patients undergoing transthoracic echocardiography at the University of maiduguri teaching hospital. Adv Med. 2014;2014 doi: 10.1155/2014/731936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bleakley C, Smith MR, Garfield B, et al. Contrast echocardiography in VV-ECMO dependent COVID-19 patients. J Am Soc Echocardiogr. July 2020 doi: 10.1016/j.echo.2020.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Medvedofsky D, Lang RM, Kruse E, et al. Feasibility of left ventricular global longitudinal strain measurements from contrast-enhanced echocardiographic images. J Am Soc Echocardiogr. 2018;31:297–303. doi: 10.1016/j.echo.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 107.Karagodin I, Genovese D, Kruse E, et al. Contrast-enhanced echocardiographic measurement of longitudinal strain: accuracy and its relationship with image quality. Int J Cardiovasc Imaging. 2020;36:431–439. doi: 10.1007/s10554-019-01732-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Malm S, Frigstad S, Sagberg E, Steen PA, Skjarpe T. Real-time simultaneous triplane contrast echocardiography gives rapid, accurate, and reproducible assessment of left ventricular volumes and ejection fraction: a comparison with magnetic resonance imaging. J Am Soc Echocardiogr. 2006;19:1494–1501. doi: 10.1016/j.echo.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 109.Lindner JR, Song J, Jayaweera AR, Sklenar J, Kaul S. Microvascular rheology of definity microbubbles after intra-arterial and intravenous administration. J Am Soc Echocardiogr. 2002;15:396–403. doi: 10.1067/mje.2002.117290. [DOI] [PubMed] [Google Scholar]
- 110.Karogiannis N, Senior R. Kontrastechokardiographie zur erkennung von auffälligkeiten der myokardperfusion: eine klinische sichtweise. Herz. 2017;42:287–294. doi: 10.1007/s00059-017-4536-7. [DOI] [PubMed] [Google Scholar]
- 111.Janardhanan R, Swinburn JMA, Greaves K, Senior R. Usefulness of myocardial contrast echocardiography using low-power continuous imaging early after acute myocardial infarction to predict late functional left ventricular recovery. Am J Cardiol. 2003;92:493–497. doi: 10.1016/S0002-9149(03)00713-6. [DOI] [PubMed] [Google Scholar]
- 112.Rakhit DJ, Becher H, Monaghan M, Nihoyannopoulis P, Senior R. The clinical applications of myocardial contrast echocardiography. Eur J Echocardiogr. 2007;8 doi: 10.1016/j.euje.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 113.Seol SH, Lindner JR. A primer on the methods and applications for contrast echocardiography in clinical imaging. J Cardiovasc Ultrasound. 2014;22:101–110. doi: 10.4250/jcu.2014.22.3.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Deepti S, Roy A, Patel CD, et al. Assessment of asymptomatic ischemic heart disease using stress myocardial perfusion imaging in patients with type 2 diabetes mellitus. Indian Heart J. 2018;70:S157–S160. doi: 10.1016/j.ihj.2018.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wackers FJT, Young LH, Inzucchi SE, et al. Detection of silent myocardial ischemia in asymptomatic diabetic subjects: The DIAD study. Diabetes Care. 2004;27:1954–1961. doi: 10.2337/diacare.27.8.1954. [DOI] [PubMed] [Google Scholar]
- 116.Scholte AJHA, Schuijf JD, Kharagjitsingh A.V., et al. Prevalence and predictors of an abnormal stress myocardial perfusion study in asymptomatic patients with type 2 diabetes mellitus. Eur J Nucl Med Mol Imaging. 2009;36:567–575. doi: 10.1007/s00259-008-0967-y. [DOI] [PubMed] [Google Scholar]
- 117.Skali H, Di Carli MF, Blankstein R, et al. Stress PET provides incremental risk prediction in patients with and patients without Diabetes. Radiol Cardiothorac Imaging. 2019;1 doi: 10.1148/ryct.2019180018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sella EMC, Sato EI, Barbieri A. Coronary artery angiography in systemic lupus erythematosus patients with abnormal myocardial perfusion scintigraphy. Arthritis Rheum. 2003;48:3168–3175. doi: 10.1002/art.11260. [DOI] [PubMed] [Google Scholar]
- 119.Bailing Li, Li Li, Xiaolei Hou, et al. Analysis of rheumatic heart disease with coronary heart disease. Chin Med J (Engl) 2007;87:3313–3316. http://rs.yiigle.com/CN112137200747/117999.htm Available at: Accessed April 19, 2020. [Google Scholar]
- 120.Mariano-Goulart D, Jacquet JM, Molinari N, et al. Should HIV-infected patients be screened for silent myocardial ischaemia using gated myocardial perfusion SPECT? Eur J Nucl Med Mol Imaging. 2013;40:271–279. doi: 10.1007/s00259-012-2262-1. [DOI] [PubMed] [Google Scholar]
- 121.Kruse MJ, Kovell L, Kasper EK, et al. Myocardial blood flow and inflammatory cardiac sarcoidosis. JACC Cardiovasc Imaging. 2017;10:157–167. doi: 10.1016/j.jcmg.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 122.Dorbala S, Vangala D, Bruyere J, et al. Coronary microvascular dysfunction is related to abnormalities in myocardial structure and function in cardiac amyloidosis. JACC Hear Fail. 2014;2:358–367. doi: 10.1016/j.jchf.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Martinez-Naharro A, Knight DS, Kotecha T, et al. 028 Routine identification of hypoperfusion in cardiac amyloidosis by myocardial blood flow mapping. Heart. 2017;103(Suppl 1) doi: 10.1136/heartjnl-2017-311399.28. A24-A24doi: [DOI] [Google Scholar]
- 124.Aessopos A, Tsironi M, Vassiliadis I, et al. Exercise-induced myocardial perfusion abnormalities in sickle β-thalassemia: Tc-99m tetrofosmin gated SPECT imaging study. Am J Med. 2001;111:355–360. doi: 10.1016/S0002-9343(01)00835-X. [DOI] [PubMed] [Google Scholar]
- 125.Hickey GW, Nguyen D, Pasupala D, et al. Yield of myocardial perfusion imaging in acute chest pain patients with negative ecg and biomarkers. J Am Coll Cardiol. 2015;65:A1247. doi: 10.1016/s0735-1097(15)61247-5. [DOI] [Google Scholar]
- 126.Ora M, Gambhir S. Myocardial perfusion imaging: A brief review of nuclear and nonnuclear techniques and comparative evaluation of recent advances. Indian J Nucl Med. 2019;34:263–270. doi: 10.4103/ijnm.IJNM_90_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Salerno M, Beller GA. Noninvasive assessment of myocardial perfusion. Circ Cardiovasc Imaging. 2009;2:412–424. doi: 10.1161/CIRCIMAGING.109.854893. [DOI] [PubMed] [Google Scholar]
- 128.Feher A, Sinusas AJ. quantitative assessment of coronary microvascular function: dynamic single-photon emission computed tomography, positron emission tomography, ultrasound, computed tomography, and magnetic resonance imaging. Circ Cardiovasc Imaging. 2017;10 doi: 10.1161/CIRCIMAGING.117.006427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cuocolo A, Acampa W, Imbriaco M, De Luca N, Iovino GL, Salvatore M. The many ways to myocardial perfusion imaging. Q J Nucl Med Mol imaging. 2005;49:4–18. [PubMed] [Google Scholar]
- 130.Senior R, Moreo A, Gaibazzi N, et al. Comparison of sulfur hexafluoride microbubble (SonoVue)-enhanced myocardial contrast echocardiography with gated single-photon emission computed tomography for detection of significant coronary artery disease: a large european multicenter study. J Am Coll Cardiol. 2013;62:1353–1361. doi: 10.1016/j.jacc.2013.04.082. [DOI] [PubMed] [Google Scholar]
- 131.Lipiec P, Wejner-Mik P, Krzemińska-Pakuła M, et al. Accelerated stress real-time myocardial contrast echocardiography for the detection of coronary artery disease: comparison with 99mTc single photon emission computed tomography. J Am Soc Echocardiogr. 2008;21:941–947. doi: 10.1016/j.echo.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 132.Dijkmans PA, Senior R, Becher H, et al. Myocardial contrast echocardiography evolving as a clinically feasible technique for accurate, rapid, and safe assessment of myocardial perfusion. the evidence so far. J Am Coll Cardiol. 2006;48:2168–2177. doi: 10.1016/j.jacc.2006.05.079. [DOI] [PubMed] [Google Scholar]
- 133.Roldan CA. Valvular and coronary heart disease in systemic inflammatory diseases. Heart. 2008;94:1089–1101. doi: 10.1136/hrt.2007.132787. [DOI] [PubMed] [Google Scholar]
- 134.Taqui S, Ferencik M, Davidson BP, et al. Coronary microvascular dysfunction by myocardial contrast echocardiography in nonelderly patients referred for computed tomographic coronary angiography. J Am Soc Echocardiogr. 2019;32:817–825. doi: 10.1016/j.echo.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Crea F, Camici PG, Merz CNB. Coronary microvascular dysfunction: an update. Eur Heart J. 2014;35:1101–1111. doi: 10.1093/eurheartj/eht513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Schnell GB, Kryski AJ, Mann L, Anderson TJ, Belenkie I. Contrast echocardiography accurately predicts myocardial perfusion before angiography during acute myocardial infarction. Can J Cardiol. 2007;23:1043–1048. doi: 10.1016/S0828-282X(07)70871-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tong KL, Kaul S, Wang XQ, et al. Myocardial contrast echocardiography versus thrombolysis in myocardial infarction score in patients presenting to the emergency department with chest pain and a nondiagnostic electrocardiogram. J Am Coll Cardiol. 2005;46:920–927. doi: 10.1016/j.jacc.2005.03.076. [DOI] [PubMed] [Google Scholar]
- 138.Mathias W, Tsutsui JM, Tavares BG, et al. Sonothrombolysis in ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. J Am Coll Cardiol. 2019;73:2832–2842. doi: 10.1016/j.jacc.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 139.Kurt M, Shaikh KA, Peterson L, et al. Impact of contrast echocardiography on evaluation of ventricular function and clinical management in a large prospective cohort. J Am Coll Cardiol. 2009;53:802–810. doi: 10.1016/j.jacc.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 140.Shaw LJ, Gillam L, Feinstein S, Dent J, Plotnick G. AJMC1998SepShawSP169_SP176.pdf. Am J Manag Care. 1998;4 https://www.ajmc.com/journals/issue/1998/1998-09-vol4-n2sp/sep98-1098psp169-sp17 (SPEC. ISS. SEPT.). Available at: Accessed March 20, 2020. [PubMed] [Google Scholar]
- 141.Moccetti F, Lindner JR. Elsevier; 2019. Utilizing Contrast Echocardiography in Practice. [DOI] [Google Scholar]
- 142.Yong Y, Wu D, Fernandes V, et al. Diagnostic accuracy and cost-effectiveness of contrast echocardiography on evaluation of cardiac function in technically very difficult patients in the intensive care unit. Am J Cardiol. 2002;89:711–718. doi: 10.1016/S0002-9149(01)02344-X. [DOI] [PubMed] [Google Scholar]
- 143.Senior R, Becher H, Monaghan M, et al. Clinical practice of contrast echocardiography: recommendation by the european association of cardiovascular imaging (EACVI) 2017. Eur Heart J Cardiovasc Imaging. 2017;18:1205. doi: 10.1093/ehjci/jex182. [DOI] [PubMed] [Google Scholar]
- 144.Wei K, Mulvagh SL, Carson L, et al. the safety of definity and optison for ultrasound image enhancement: a retrospective analysis of 78,383 administered contrast doses. J Am Soc Echocardiogr. 2008;21:1202–1206. doi: 10.1016/j.echo.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 145.Muskula PR, Main ML. Safety with echocardiographic contrast agents. Circ Cardiovasc Imaging. 2017;10 doi: 10.1161/CIRCIMAGING.116.005459. [DOI] [PubMed] [Google Scholar]
- 146.Monaghan MJ. Role of real time 3D echocardiography in evaluating the left ventricle. Heart. 2006;92:131–136. doi: 10.1136/hrt.2004.058388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Thavendiranathan P, Grant AD, Negishi T, Plana JC, Popović ZB, Marwick TH. Reproducibility of echocardiographic techniques for sequential assessment of left ventricular ejection fraction and volumes: application to patients undergoing cancer chemotherapy. J Am Coll Cardiol. 2013;61:77–84. doi: 10.1016/j.jacc.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 148.Vasconcellos HD, Moreira HT, Ciuffo L, et al. Cumulative blood pressure from early adulthood to middle age is associated with left atrial remodelling and subclinical dysfunction assessed by three-dimensional echocardiography: A prospective post hoc analysis from the coronary Artery Risk Development in young adults study. Eur Heart J Cardiovasc Imaging. 2018;19:977–984. doi: 10.1093/ehjci/jey086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pérez de Isla L, Casanova C, Almería C, et al. Which method should be the reference method to evaluate the severity of rheumatic mitral stenosis? Gorlin's method versus 3D-echo. Eur J Echocardiogr. 2007;8:470–473. doi: 10.1016/j.euje.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 150.d'Humières T, Faivre L, Chammous E, et al. A new three-dimensional echocardiography method to quantify aortic valve calcification. J Am Soc Echocardiogr. 2018;31:1073–1079. doi: 10.1016/j.echo.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 151.Saito K, Okura H, Watanabe N, et al. Comprehensive evaluation of left ventricular strain using speckle tracking echocardiography in normal adults: comparison of three-dimensional and two-dimensional approaches. J Am Soc Echocardiogr. 2009;22:1025–1030. doi: 10.1016/j.echo.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 152.Muraru D, Niero A, Rodriguez-Zanella H, Cherata D, Badano L. Three-dimensional speckle-tracking echocardiography: benefits and limitations of integrating myocardial mechanics with three-dimensional imaging. Cardiovasc Diagn Ther. 2018;8:101–117. doi: 10.21037/cdt.2017.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chu WK, Raeside DE. Fourier analysis of the echocardiogram. Phys Med Biol. 1978;23:009. doi: 10.1088/0031-9155/23/1/009. [DOI] [PubMed] [Google Scholar]
- 154.Chu WK, Raeside DE, Chandraratna PAN, Brown RE, Poehlmann H. Echocardiogram analysis in a pattern recognition framework. Med Phys. 1979;6:267–271. doi: 10.1118/1.594579. [DOI] [PubMed] [Google Scholar]
- 155.Alsharqi M, Woodward WJ, Mumith JA, Markham DC, Upton R, Leeson P. Artificial intelligence and echocardiography. Echo Res Pract. 2018;5:R115–R125. doi: 10.1530/erp-18-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Shameer K, Johnson KW, Glicksberg BS, Dudley JT, Sengupta PP. Machine learning in cardiovascular medicine: are we there yet? Heart. 2018;104:1156–1164. doi: 10.1136/heartjnl-2017-311198. [DOI] [PubMed] [Google Scholar]
- 157.Madani A, Arnaout R, Mofrad M, Arnaout R. Fast and accurate view classification of echocardiograms using deep learning. npj Digit Med. 2018;1:1-8. doi: 10.1038/s41746-017-0013-1 [DOI] [PMC free article] [PubMed]
- 158.Gudmundsson P, Rydberg E, Winter R, Willenheimer R. Visually estimated left ventricular ejection fraction by echocardiography is closely correlated with formal quantitative methods. Int J Cardiol. 2005;101:209–212. doi: 10.1016/j.ijcard.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 159.Knackstedt C, Bekkers SCAM, Schummers G, et al. Fully automated versus standard tracking of left ventricular ejection fraction and longitudinal strain the FAST-EFs multicenter study. J Am Coll Cardiol. 2015;66:1456–1466. doi: 10.1016/j.jacc.2015.07.052. [DOI] [PubMed] [Google Scholar]
- 160.Rahmouni HW, Ky B, Plappert T, et al. Clinical utility of automated assessment of left ventricular ejection fraction using artificial intelligence-assisted border detection. Am Heart J. 2008;155:562–570. doi: 10.1016/j.ahj.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 161.Cannesson M, Tanabe M, Suffoletto MS, et al. A novel two-dimensional echocardiographic image analysis system using artificial intelligence-learned pattern recognition for rapid automated ejection fraction. J Am Coll Cardiol. 2007;49:217–226. doi: 10.1016/j.jacc.2006.08.045. [DOI] [PubMed] [Google Scholar]
- 162.Mansor S, Hughes NP, Noble JA. Springer; Berlin, Heidelberg: 2008. Wall Motion Classification of Stress Echocardiography Based on Combined Rest-and-Stress Data; pp. 139–146. In:doi: [DOI] [PubMed] [Google Scholar]
- 163.Chykeyuk K, Clifton DA, Noble JA. Proceedings - International Symposium on Biomedical Imaging. 2011. Feature extraction and wall motion classification of 2D stress echocardiography with relevance vector machines; pp. 677–680. [DOI] [Google Scholar]
- 164.Omar HA, Domingos JS, Patra A, Upton R, Leeson P, Noble JA. Proceedings - International Symposium on Biomedical Imaging. Vol 2018-April. IEEE Computer Society; 2018. Quantification of cardiac bull's-eye map based on principal strain analysis for myocardial wall motion assessment in stress echocardiography; pp. 1195–1198. In: [DOI] [Google Scholar]
- 165.Cobey FC. Intelligent algorithms in perioperative echocardiography: a new era. J Am Soc Echocardiogr. 2017;30:A26–A27. doi: 10.1016/j.echo.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 166.Salte IM, Oestvik A, Smistad E, et al. 545 Deep learning/artificial intelligence for automatic measurement of global longitudinal strain by echocardiography. Eur Hear J - Cardiovasc Imaging. 2020;21(Supplement_1) doi: 10.1093/ehjci/jez319.279. [DOI] [Google Scholar]
- 167.Fatima M, Pasha M. Survey of machine learning algorithms for disease diagnostic. J Intell Learn Syst Appl. 2017;09:1–16. doi: 10.4236/jilsa.2017.91001. [DOI] [Google Scholar]
- 168.Neri E, Miele V, Coppola F, Grassi R. Use of CT and artificial intelligence in suspected or COVID-19 positive patients: statement of the Italian Society of Medical and Interventional Radiology. Radiol Medica. 2020;125:505–508. doi: 10.1007/s11547-020-01197-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Reardon S. Rise of robot radiologists. Nature. 2019;576:S54–S58. doi: 10.1038/d41586-019-03847-z. [DOI] [PubMed] [Google Scholar]
- 170.Narula J. Are we up to speed?: From big data to rich insights in CV imaging for a hyperconnected world. JACC Cardiovasc Imaging. 2013;6:1222–1224. doi: 10.1016/j.jcmg.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 171.Lee JG, Jun S, Cho YW, et al. Deep learning in medical imaging: general overview. Korean J Radiol. 2017;18:570–584. doi: 10.3348/kjr.2017.18.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gandhi S, Mosleh W, Shen J, Chow C-M. Automation, machine learning, and artificial intelligence in echocardiography: a brave new world. Echocardiography. 2018;35:1402–1418. doi: 10.1111/echo.14086. [DOI] [PubMed] [Google Scholar]
- 173.Bansal M, Kasliwal RR. How do i do it? Speckle-tracking echocardiography. Indian Heart J. 2013;65:117–123. doi: 10.1016/j.ihj.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Eskandari M, Monaghan M. Kontrastechokardiographie in der klinischen Praxis. Herz. 2017;42:271–278. doi: 10.1007/s00059-017-4533-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hung J, Lang R, Flachskampf F, et al. 3D Echocardiography: a review of the current status and future directions. J Am Soc Echocardiogr. 2007;20:213–233. doi: 10.1016/j.echo.2007.01.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.