Version Changes
Revised. Amendments from Version 1
As a result of the reviewer's suggestions, we updated certain sections of the introduction, defined the data gathering process, and questionnaire development.
Abstract
Background: Vaccine apprehension poses a serious threat to global health. While there has been a tremendous global effort to create a vaccine against coronavirus disease 2019 (COVID-19), little is known about its reception in Iraq. Therefore, we sought to examine COVID-19 vaccine acceptance, hesitation, and related elements in the Iraqi population.
Methods: Between the 19th of May and the 22nd of September 2021, a descriptive, cross-sectional web-based survey was conducted employing a quantitative approach. Respondents from both sexes aged 18 years and above who live in Iraq and agreed to participate were included. An anonymized online structured questionnaire was designed based on data from prior research on vaccine hesitation in general, and COVID-19 vaccination reluctance specifically.
Results: A total of 1221 eligible participants from various regions in Iraq actively participated in the short web-based questionnaire. The overall acceptance rate of the COVID-19 vaccine was 56.2%, with a greater acceptance rate among younger male participants (p<0.05). Marital status had no significant association (p=0.834). Urbanization influenced the acceptance rate significantly (p=0.002). The barriers to receiving the COVID-19 vaccine were exemplified by the vaccine not being evaluated for a sufficient period in 51.4% of the responses, as well as concerns about future side effects in 76.6% of the responses and a lack of efficacy in 55.7% of the responses. The Pfizer-BioNTech vaccine received 39.6% preference and participants confidence, followed by the Oxford/AstraZeneca vaccine at 18.1% and the Sinopharm vaccine at 14.6%.
Conclusions: COVID-19 vaccination apprehension was discovered in almost half of the study population. Lack of understanding about vaccination eligibility, anxiety about adverse events and vaccine efficacy, and distrust in the government were independently predictive of vaccine hesitation.
Keywords: Perception, attitudes, Iraq, COVID-19, coronavirus, vaccine acceptance, SARS‑CoV‑2, vaccine hesitancy
Introduction
The coronavirus disease 2019 (COVID-19) pandemic caused by the emerging severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus, is still causing turmoil around the world. This pandemic seems to have had a negative influence on living standards and placed a strain on health care organizations. 1 Diverse chemotherapeutic and biological treatments have already been tested in COVID-19 cases, but none have been shown to provide a definitive therapeutic efficacy. 2 With little effectiveness, many preventive public health interventions such as quarantining, practicing hand hygiene and cough etiquette, as well as physical separation are all measures that were instated. 3
Lots of studies have demonstrated that when a new vaccination is launched, a variety of factors contribute to vaccine social acceptance. These concerns include the new vaccine safety and effectiveness, negative health impacts, misunderstandings about the importance of vaccination, a lack of faith in the health system, and a lack of community information about vaccine-preventable illnesses. When the vaccine was introduced during a prior pandemic, such as the H1N1 influenza A, the acceptancy rate fluctuated between 8% and 67%, as stated in a systematic review by Larson et al., in 2018. 4 As per Xiao and Wong's meta-analysis, the acceptance rate in the United States in 2020 was 64%. 5 Additionally, approximately half of people surveyed in the United Kingdom indicated a willingness to take the H1N1 influenza vaccination, according to a 2015 report by Chan et al. 6 Throughout the 2014 Avian influenza A(H7N9) pandemic in China, 50.5 % of study participants received the A/H7N9 vaccination as soon as it became available. 7 In Beijing, Prc, 59.5% of survey participants who had heard about H7N9 were prepared to receive an influenza A (H7N9) vaccine in the future. 8 Vaccine acceptability and uptake are complicated and context-specific, altering over time, geography, and the community's perceived behavioral nature. 9 According to a survey performed in Ireland, medical personnel ignored seasonal flu immunization mainly because of misapprehension regarding the efficiency and dependability of the vaccine. 10 In China, demographic characteristics and public perceptions are major factors of vaccine uptake. The primary determinants of vaccine acceptance in Hong Kong are anxiety levels and vaccine experience. 6 In the United States, the most important determinants of influenza vaccination adoption were anticipated vaccine efficiency, cultural influence, and insurance coverage. 7 Higher introversion was related to poorer vaccination uptake in studies conducted in the United States, while greater confidence was linked to greater vaccination uptake. 11 A research in the United Arab Emirates examined at parental views regarding childhood vaccines and found that 12% of caregivers were hesitant to get their children vaccinated. According to the research, vaccination safety (17%), side effects (35%), and too frequent injections (28%), are all important reasons in vaccine hesitation. 12 Participant willingness to get the COVID-19 vaccine was higher among those who had previously received a seasonal flu vaccine. 13
Thus far, several vaccines have been created, with some licensed and others continuing in clinical studies. Notably, the Pfizer-BioNTech, Moderna, and the Oxford/AstraZeneca vaccinations have all been licensed for urgent application and are now in use in a number of countries, including Iraq. 14 , 15 Despite significant progress in vaccine research, public acceptability of the COVID-19 vaccine remains a significant barrier. 16 As per the World Health Organization (WHO), vaccination reluctance is among the 10 leading threats to global health, but it is being aggravated mostly by rising conspiracy theories surrounding COVID-19 and vaccines. Few research have been conducted to investigate the prevalence of COVID-19 vaccination acceptance and factors that affect. In compared to the general population, a study of health care workers (HCWs) in China found a high acceptability of COVID-19 immunization among HCWs. Another research in the United States found that just 20% of people plan to refuse the COVID-19 vaccination. Because vaccination uptake varies according to area, culture, and demography. 17 As a result, we sought to examine the acceptance, hesitation and related elements around the COVID-19 vaccine in the Iraqi population.
Methods
Ethical consideration
This study was approved by the Al-Esraa University College Ethics Committee (approval no. 506). The Al-Esraa University College Ethics Committee waived the need for consent for the acquisition, interpretation, and publication of the prospectively collected anonymous data for this non-interventional research. Before participating in the survey, individuals were allowed to decline participation in the questionnaire. Participation in this study was entirely voluntary, free of coercion, and uncompensated. Participants provided informed, written consent as part of the questionnaire. Throughout the study, anonymity and confidentiality were maintained. All data were saved in a secure file, with only the researchers in charge of the questionnaire accessing the information. The study was carried out according to the World Medical Association’s Declaration of Helsinki. 18
Study design
This was a descriptive, cross-sectional web-based survey performed between the 19th of May and the 22nd of September 2021, using a quantitative approach.
Eligibility criteria
Respondents from both sexes aged 18 years and above who live in Iraq and agreed to participate were included. Simple random sampling was the method of selecting participants in which the author chose a subset of participants randomly from a population. Each individual in the population had an equal probability of being considered.
Bias
The survey was disseminated through a variety of electronic means, including social media like (Facebook, Twitter, and Instagram), and e-mail. Therefore, any concerns regarding sample bias and generalizability may be ameliorated since the form could have been filled out by anybody with an internet connection. Furthermore, the author asserts that there are no instances of reporting bias or performance bias in their findings.
Study sample size
The sample size calculation was based on prior reports of COVID-19 vaccination hesitancy or refusal, ranging from 6% in Egypt to 13.9% in Italy, 23% in the United States, 30.5% in Malta, and 51.4% in Iraqi Kurdistan. 19 , 20 This resulted in a sample size of 962 people, equating to the lowest prevalence, relative precision of 25%, and alpha error of 5%. The sample size was determined using the equation N = Zα2P (1 − P)/d2, where = 0.05 and Z = 1.96, and the permissible margin of error for proportion d is calculated to be 0.1. When we received 1221 completed surveys, we decided to end the survey.
Questionnaire description
Using data from prior research on vaccination reluctance in general, and specifically the COVID-19 vaccine, and earlier validated questionnaires 21 – 26 an anonymous online structured questionnaire was developed with a professional translation from English to the Arabic Language. 43 The set of questions was divided into three sections: the first contained basic demographic information as well as an evaluation of understanding and source of knowledge concerning COVID-19 vaccine, the second contained an assessment of attitudes toward the vaccine, and the third contained details regarding vaccination barriers. This questionnaire was made available online using Google Forms.
Study variables
The sociodemographic variables of sex, age, marital status, educational level, permanent resident, and sources of knowledge about coronavirus and COVID-19 vaccinations were included as independent key predictors, while the acceptance, hesitation, trust, and perceptions concerning COVID-19 vaccination were dependent variables.
Data analysis
For cleaning and coding, submitted questionnaire responses were exported from Google Forms to Microsoft Excel 2019 (Microsoft Excel, RRID:SCR_016137). The processed data were transferred to IBM SPSS software for Windows, version 25 (IBM SPSS Statistics, RRID:SCR_016479) for analysis. Means (standard deviations) and percentages were used to summarize numerical data. For parametric and non-parametric data, the median (interquartile range) was used. Categorical data were collected. Frequency and proportions were used to summarize the data. Relationships between independent factors and dependent variables were determined using chi-square test or Fisher’s exact test, and logistic regression analysis was used to evaluate variables. p<0.05 was considered statistically significant. When dealing with missing data, we relied on two primary methods for eliminating data: list-wise and deleting variables. Listwise method: This strategy deletes all data for an observation that includes one or more missing values. Only observations with a full set of data are subjected to the analysis. Dropping variables: If data are missing for over 60% of the samples, it may be best to remove if the variable is insignificant.
Results
A total of 1221 eligible participants (aged 18 and over) from various country regions actively participated in the short web-based questionnaire. The study population was mainly concentrated in urban areas (71.2% live in urban areas). Furthermore, a larger portion of responders within the age range of 18–29 years represented 40% of the study participants, and the ratio of male to female participants was 0.94:1, where the female percentage was 51.5%. A total of 719 (58.9%) were married. Most study participants held an academic degree (677, 55.4%). The overall acceptance rate was 56.2%. This research showed a higher acceptance rate of COVID-19 vaccination in younger, male participants (p<0.05). No significant association was demonstrated regarding marital status (p=0.834). Geographic area significantly impacted the acceptance rate where most of the respondents lived in urban areas (p=0.002). The level of education seemed to have no noticeable effect on vaccination acceptance rate (p=0.238) ( Table 1). 42
Table 1. Sociodemographic backgrounds of the study population and its association with the COVID-19 vaccine acceptance rate.
| Sociodemographic characteristics | n (%) | COVID-19 vaccine acceptance | p-value | ||
|---|---|---|---|---|---|
| Yes | No | ||||
| Age | 18-29 | 488 (40.0) | 254 | 234 | 0.041 |
| 30-49 | 454 (37.2) | 278 | 176 | ||
| 50-64 | 201 (16.5) | 112 | 89 | ||
| >65 | 78 (6.4) | 42 | 36 | ||
| Sex | Male | 592 (48.5) | 349 | 243 | 0.033 |
| Female | 629 (51.5) | 337 | 292 | ||
| Marital status | Single | 395 (32.4) | 226 | 169 | 0.834 |
| Married | 719 (58.9) | 402 | 317 | ||
| Separated or widowed | 107 (8.8) | 58 | 49 | ||
| Geographic areas | Urban | 869 (71.2) | 513 | 356 | 0.002 |
| Rural | 352 (28.8) | 173 | 179 | ||
| Education | Primary school graduate | 167 (13.7) | 93 | 74 | 0.238 |
| Secondary school graduate | 173 (14.2) | 87 | 86 | ||
| Diploma or bachelor’s degree | 677 (55.4) | 382 | 295 | ||
| Higher education (MSc or PhD) | 204 (16.7) | 124 | 80 | ||
COVID-19, coronavirus disease 2019.
Most of the participants did not have an underlying medical condition (798, 65%), 574 (47.0%) participants had been positive for COVID-19 in the past, while 815 (66.7%) reported the occurrence of COVID-19 infection in family members, from the total number of respondents 880 (72.1%) had children. The association of the above parameters with the vaccination acceptance rate showed that healthy people without a chronic illness had a higher acceptance rate of vaccination (p=0.002), patients with a past medical history of COVID-19 infection were accepting the vaccine at a significantly higher rate than patients without a history of infection (p<0.001), also there was a positive relationship between having family members who had been infected with COVID-19 with vaccination acceptance rate (p=0.001), and respondents who had children (<18 years) seemed to show a statistically higher level of vaccination acceptance (p=0.035) ( Table 2).
Table 2. Medical history and its association with COVID-19 vaccine acceptance rate.
| Parameters | n (%) | COVID-19 vaccine acceptance | p-value | ||
|---|---|---|---|---|---|
| Yes | No | ||||
| Underlying medical condition | Positive | 423 (34.6) | 263 | 160 | 0.002 |
| Negative | 798 (65.4) | 423 | 375 | ||
| Personal history of COVID-19 infection | Positive | 574 (47.0) | 375 | 199 | 0.000 |
| Negative | 647 (53.0) | 311 | 336 | ||
| Family members infected with COVID-19 | Positive | 815 (66.7) | 484 | 331 | 0.001 |
| Negative | 406 (33.3) | 202 | 204 | ||
| Underaged children | Yes | 880 (72.1) | 509 | 371 | 0.035 |
| No | 341 (27.9) | 177 | 164 | ||
COVID-19, coronavirus disease 2019.
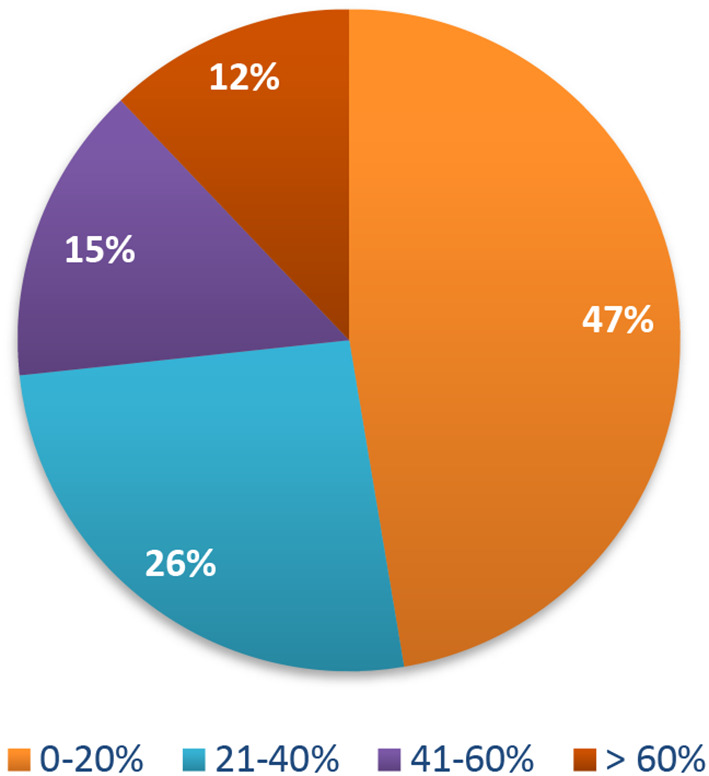
The percentage of perceived risk of getting COVID-19 was very low; the majority of participants (47%) believed their risk ranged from 0 to 20% ( Figure 1).
Figure 1. Risk perception of getting COVID-19.
COVID-19, coronavirus disease 2019.
Table 3 depicts the study population’s understanding of COVID-19 vaccinations; most respondents thought that the priority of vaccination should be focused on health care providers.
Table 3. Public's understanding of COVID-19 vaccinations.
| Factors | Answer | n (%) |
|---|---|---|
| How essential do you think the COVID-19 vaccination is? | Important | 1040 (85.2) |
| Not important | 181 (14.8) | |
| COVID-19 vaccine must always be mandatory for everyone | Agree | 775 (63.5) |
| Disagree | 446 (36.5) | |
| COVID-19 vaccination should always be mandatory for healthcare providers | Agree | 935 (76.6) |
| Disagree | 286 (23.4) | |
| The vaccine's approval ensures its safety | Agree | 930 (76.2) |
| Disagree | 291 (23.8) | |
| Receiving the immunization is the most effective COVID-19 prevention strategy | Agree | 896 (73.4) |
| Disagree | 325 (26.6) |
COVID-19, coronavirus disease 2019.
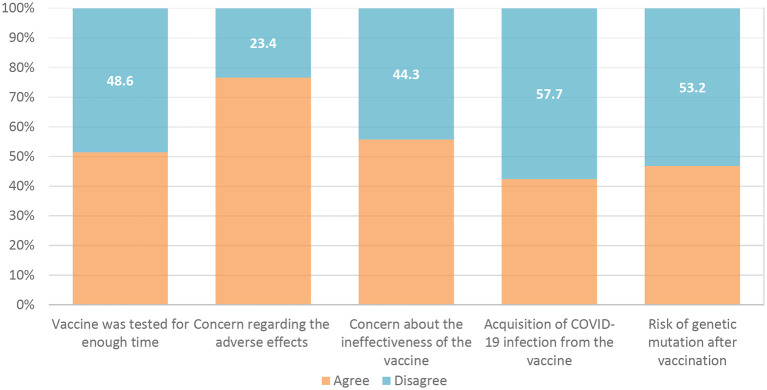
The barriers to receiving the COVID-19 vaccine were exemplified by the vaccine not being evaluated for a sufficient period in 51.4% of the responses, as well as concerns about future side effects in 76.6% of the responses, and a lack of efficacy in 55.7% of the respondents ( Figure 2).
Figure 2. Barriers to receiving COVID-19 vaccine.
COVID-19, coronavirus disease 2019.
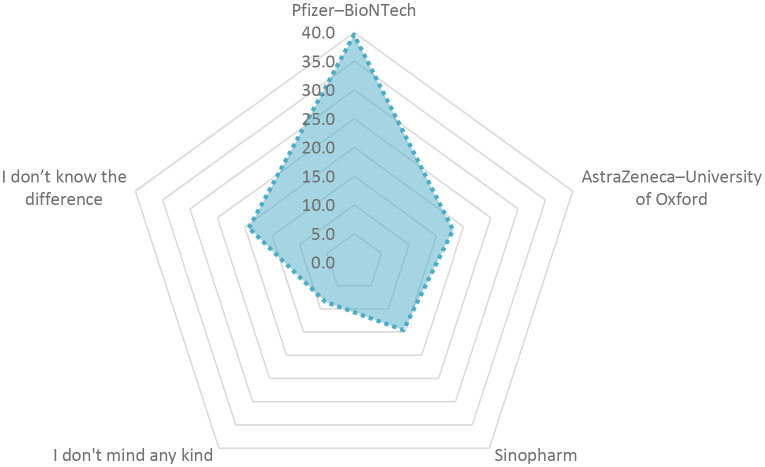
The Pfizer-BioNTech vaccine received 39.6% preference and participants confidence followed by Oxford/AstraZeneca vaccine at 18.1% and the Sinopharm vaccine at 14.6%, as shown in Figure 3.
Figure 3. Vaccine preference.
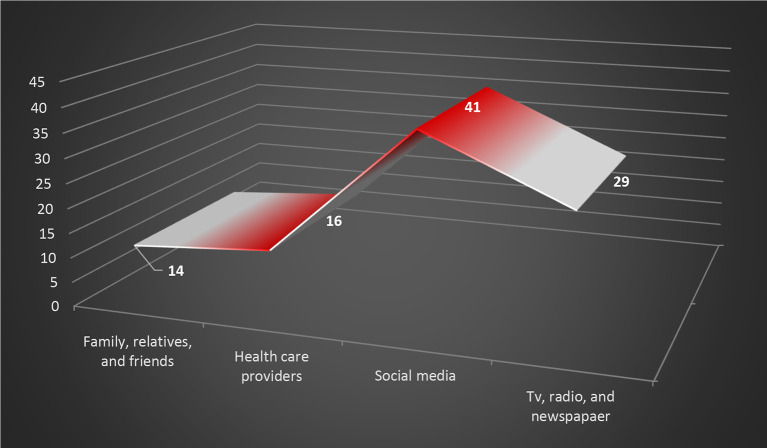
Besides the governmental platforms, social media have become a significant source for COVID-19 information; according to the findings of this study, 41% of respondents obtained medical instruction from social media, as illustrated in Figure 4.
Figure 4. Graphical illustration for the primary source of COVID-19 information.
COVID-19, coronavirus disease 2019.
Discussion
The vaccine is recognized as an important public health innovation of the 21st century. Its acceptability, however, varies with geography, time, socioeconomic class, ethnicity, and human-environmental behavior. 27 Despite the fact that there have been few studies to investigate the intention to use the COVID-19 vaccination during the current crisis in Iraq, our findings contradict those of studies conducted in China and the United States. 28 , 29 According to a study in China, 72.5% of members of the public intended to get the COVID-19 vaccine. 29 In addition, studies conducted in the United States indicated that the COVID-19 vaccine was accepted by 80% of the eligible population. 28 Throughout our research, 56.2% of participants indicated a desire to accept the COVID-19 vaccination. This study discovered there was a significant disparity in vaccination hesitancy frequency between men and women; however, vaccine hesitancy was high in both sexes.
Furthermore, the individuals who were reluctant to get the COVID-19 vaccine were older. According to Robertson et al., in the United Kingdom, 21% of women and 14% of men were apprehensive about getting the COVID-19 vaccine. 30 In contrast to this research, the younger respondents were less likely to refuse the COVID-19 vaccine.
Vaccine reluctance could be caused by a variety of scenarios, including disinformation and conspiracy beliefs. 31 , 32 Furthermore, health inequities, economic constraints, racial profiling, and access hurdles are seen as underlying reasons for poor trust and poor uptake. 33 According to Paul et al., in the United Kingdom, 16% of the study participants showed high levels of skepticism about the COVID-19 vaccines. 34 Minority groups, individuals with lower levels of education, lower yearly income, and a lack of knowledge of COVID-19, and those who do not follow government COVID-19 recommendations have higher levels of distrust. They reported that 14% of respondents had chronic diseases and 23% of this chronically ill population were unsure about receiving the COVID-19 vaccine. Our participants in this study had a greater prevalence of chronic illnesses (34%) and a higher rate of vaccination unwillingness. Meier et al., indicated that people who perceived themselves to be more susceptible to COVID-19 were more likely to have a COVID-19 vaccine in the United States, which is consistent with the results in our research. 35 The majority of study participants said that COVID-19 vaccination was necessary, especially for health care workers, and that it should be made mandatory once it was widely available. Corresponding findings were published by Lucia et al. 36 The Centers for Disease Control and Prevention (CDC) advises that medical practitioners receive the first doses of COVID-19 vaccines because of the higher risk of exposure. 37
The public’s confidence in the COVID-19 vaccine may fluctuate depending on the vaccine type. Research, for example, discovered the greatest degree of confidence in mRNA technology. Compared to AZD1222, both BNT162b and mRNA1273 had a better level of acceptability. 38 Furthermore, cases of bleeding, thrombosis and platelets dysfunction following COVID-19 vaccinations in individuals with preexisting hemodynamic abnormalities or those taking particular drugs have sparked widespread alarm on social media. As a result, the use of the Oxford/AstraZeneca vaccine has been temporarily suspended in various European nations. 39
The evidence shows that it is crucial to concentrate on building confidence in COVID-19 vaccines. This includes negotiating the COVID-19 evidence framework with credible communicators and fostering vaccination confidence via transparency and anticipatory monitoring. Societies must be included early on to respond to complaints, respond to questions, and dispel misconceptions. 40 Because public trust in vaccination is low, COVID-19 immunization programs can only succeed if there is a widespread conviction that the vaccinations offered are safe and effective. 41 Lucia et al., emphasized the importance of transparency to address concerns regarding the efficiency and safety of vaccine development. 36 It is critical to support COVID-19 immunization through public statements and news releases, as well as to monitor and combat false news. 36
Conclusions
COVID-19 vaccination apprehension was discovered in almost half of the study population. Lack of understanding about vaccination eligibility, anxiety about adverse events and vaccine efficacy, and mistrust in the government were all independent predictors of vaccination hesitancy. A heightened risk perception of COVID-19 reduced vaccine hesitancy. Concerns were raised about a lack of vaccination-related information and the vaccine launch before the release of evidence on safety and effectiveness. While vaccination indecision has decreased over time, health education programs designed to raise vaccine awareness and build trust in government authorities would be beneficial.
Limitations
Our study was limited by the fact that it was conducted after COVID-19 vaccination had been initiated. As a consequence, it is probable that it underestimated the initial vaccine rejection among individuals who were vaccinated and then moved to the vaccination accepting group.
Data availability
Underlying data
Zenodo: ‘Questionnaire responses for 1221 participants’. https://doi.org/10.5281/zenodo.6345333. 42
This project contains the following underlying data:
data zenodo.xls (Questionnaire responses for 1221 participants)
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Extended data
Zenodo: ‘Research questionnaire’. https://doi.org/10.5281/zenodo.6345361. 43
This project contains the following extended data:
-
-
Questionnaire.pdf
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 2; peer review: 2 approved]
References
- 1. Bonyan FA, Shareef LG, Al-waily A, et al. : COVID-19 clinical characteristics and outcomes in 60 hospitalized Iraqi patients-Case series. Med. Sci. 2020;2251–2258. [Google Scholar]
- 2. Shareef LG, Abdulwahab SM: Trends in covid-19 therapeutic modalities: A narrative literature. Eur. J. Pharm. Med. Res. 2020;7:757–767. [Google Scholar]
- 3. Looi MK: Covid-19: Is a second wave hitting Europe?. BMJ. 2020 Oct 28;371. 10.1136/bmj.m4113 [DOI] [PubMed] [Google Scholar]
- 4. Larson HJ, Clarke RM, Jarrett C, et al. : Measuring trust in vaccination: A systematic review. Hum. Vaccin. Immunother. 2018 Jul 3;14(7):1599–1609. 10.1080/21645515.2018.1459252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xiao X, Wong RM: Vaccine hesitancy and perceived behavioral control: a meta-analysis. Vaccine. 2020 Jul 14;38(33):5131–5138. 10.1016/j.vaccine.2020.04.076 [DOI] [PubMed] [Google Scholar]
- 6. Chan EY, Cheng CK, Tam GC, et al. : Willingness of future A/H7N9 influenza vaccine uptake: a cross-sectional study of Hong Kong community. Vaccine. 2015 Sep 11;33(38):4737–4740. 10.1016/j.vaccine.2015.07.046 [DOI] [PubMed] [Google Scholar]
- 7. Abbas KM, Kang GJ, Chen D, et al. : Demographics, perceptions, and socioeconomic factors affecting influenza vaccination among adults in the United States. PeerJ. 2018 Jul 13;6:e5171. 10.7717/peerj.5171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu S, Su J, Yang P, et al. : Willingness to accept a future influenza A (H7N9) vaccine in Beijing, China. Vaccine. 2018 Jan 25;36(4):491–497. 10.1016/j.vaccine.2017.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Habersaat KB, Jackson C: Understanding vaccine acceptance and demand—and ways to increase them. Bundesgesundheitsblatt-Gesundheitsforschung-Gesundheitsschutz. 2020 Jan;63(1):32–39. 10.1007/s00103-019-03063-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Halpin C, Reid B: Attitudes and beliefs of healthcare workers about influenza vaccination. Nurs. Older People. 2022 Feb 1;34(1). [DOI] [PubMed] [Google Scholar]
- 11. Brown CC, Young SG, Pro GC: COVID-19 vaccination rates vary by community vulnerability: A county-level analysis. Vaccine. 2021 Jul 13;39(31):4245–4249. 10.1016/j.vaccine.2021.06.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Alsuwaidi AR, Elbarazi I, Al-Hamad S, et al. : Vaccine hesitancy and its determinants among Arab parents: a cross-sectional survey in the United Arab Emirates. Hum. Vaccin. Immunother. 2020 Dec 1;16(12):3163–3169. 10.1080/21645515.2020.1753439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gidengil CA, Parker AM, Zikmund-Fisher BJ: Trends in risk perceptions and vaccination intentions: a longitudinal study of the first year of the H1N1 pandemic. Am. J. Public Health. 2012 Apr;102(4):672–679. 10.2105/AJPH.2011.300407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shareef LG: COVID-19 vaccine coverage and the necessity of its urgent development towards Omicron the new SARS CoV-2 B. 1.1. 529 variant. GSC Biological and Pharmaceutical Sciences. 2021;17(3):058–060. 10.30574/gscbps.2021.17.3.0350 [DOI] [Google Scholar]
- 15. Gasmi A, Srinath S, Dadar M, et al. : A global survey in the developmental landscape of possible vaccination strategies for COVID-19. Clin. Immunol. 2022 Feb 24;108958. 10.1016/j.clim.2022.108958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang P, Fan K, Guan H, et al. : Who is more likely to hesitate to accept COVID-19 vaccine: a cross-sectional survey in China. Expert Rev. Vaccines. 2022 Mar 4(just-accepted);21:397–406. 10.1080/14760584.2022.2019581 [DOI] [PubMed] [Google Scholar]
- 17. Syed Alwi SA, Rafidah E, Zurraini A, et al. : A survey on COVID-19 vaccine acceptance and concern among Malaysians. BMC Public Health. 2021 Dec;21(1):1–2. 10.1186/s12889-021-11071-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. World Medical Association. Declaration of Helsinki. Ethical principles for medical research involving human subjects. Jahrbuch Für Wissenschaft Und Ethik. 2009 Dec 24;14(1):233–238. 10.1515/9783110208856.233 [DOI] [Google Scholar]
- 19. Abdulah DM: Prevalence and correlates of COVID-19 vaccine hesitancy in the general public in Iraqi Kurdistan: A cross-sectional study. J. Med. Virol. 2021 Dec;93(12):6722–6731. 10.1002/jmv.27255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jain J, Saurabh S, Goel AD, et al. : COVID-19 vaccine hesitancy among undergraduate medical students: results from a nationwide survey in India. medRxiv. 2021 Jan 1.
- 21. Salali GD, Uysal MS: COVID-19 vaccine hesitancy is associated with beliefs on the origin of the novel coronavirus in the UK and Turkey. Psychol. Med. 2020 Oct 19;1–3. 10.1017/S0033291720004067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pogue K, Jensen JL, Stancil CK, et al. : Influences on attitudes regarding potential COVID-19 vaccination in the United States. Vaccines. 2020 Dec;8(4):582. 10.3390/vaccines8040582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Graffigna G, Palamenghi L, Boccia S, et al. : Relationship between citizens’ health engagement and intention to take the COVID-19 vaccine in Italy: a mediation analysis. Vaccines. 2020 Dec;8(4):576. 10.3390/vaccines8040576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fisher KA, Bloomstone SJ, Walder J, et al. : Attitudes toward a potential SARS-CoV-2 vaccine: a survey of US adults. Ann. Intern. Med. 2020 Dec 15;173(12):964–973. 10.7326/M20-3569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Detoc M, Bruel S, Frappe P, et al. : Intention to participate in a COVID-19 vaccine clinical trial and to get vaccinated against COVID-19 in France during the pandemic. Vaccine. 2020 Oct 21;38(45):7002–7006. 10.1016/j.vaccine.2020.09.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Geldsetzer P: Knowledge and perceptions of COVID-19 among the general public in the United States and the United Kingdom: a cross-sectional online survey. Ann. Intern. Med. 2020 Jul 21;173(2):157–160. 10.7326/M20-0912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cooper S, Schmidt BM, Sambala EZ, et al. : Factors that influence parents' and informal caregivers' acceptance of routine childhood vaccination: a qualitative evidence synthesis. Cochrane Database Syst. Rev. 2019 Feb;2019(2). 10.1002/14651858.CD013265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thunström L, Ashworth M, Finnoff D, et al. : Hesitancy toward a COVID-19 vaccine. EcoHealth. 2021 Mar;18(1):44–60. 10.1007/s10393-021-01524-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fu C, Pei S, Li S, et al. : Acceptance and preference for COVID-19 vaccination in health-care workers (HCWs). MedRxiv. 2020 Jan 1.
- 30. Robertson E, Reeve KS, Niedzwiedz CL, et al. : Predictors of COVID-19 vaccine hesitancy in the UK household longitudinal study. Brain Behav. Immun. 2021 May 1;94:41–50. 10.1016/j.bbi.2021.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bangalee A, Bangalee V: Fake news and fallacies: Exploring vaccine hesitancy in South Africa. S. Afr. Fam. Pract. 2021;63(1):e1–e3. 10.4102/safp.v63i1.5345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Islam MS, Kamal AH, Kabir A, et al. : COVID-19 vaccine rumors and conspiracy theories: The need for cognitive inoculation against misinformation to improve vaccine adherence. PloS One. 2021 May 12;16(5):e0251605. 10.1371/journal.pone.0251605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Razai MS, Kankam HK, Majeed A, et al. : Mitigating ethnic disparities in covid-19 and beyond. BMJ. 2021 Jan 15;372. 10.1136/bmj.m4921 [DOI] [PubMed] [Google Scholar]
- 34. Paul E, Steptoe A, Fancourt D: Attitudes towards vaccines and intention to vaccinate against COVID-19: Implications for public health communications. The Lancet Regional Health-Europe. 2021 Feb 1;1:100012. 10.1016/j.lanepe.2020.100012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Meier BP, Dillard AJ, Lappas CM: Predictors of the intention to receive a SARS-CoV-2 vaccine. J. Public Health (Oxf.). 2021 Mar 3; 10.1093/pubmed/fdab013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lucia VC, Kelekar A, Afonso NM: COVID-19 vaccine hesitancy among medical students. J. Public Health. 2021 Sep;43(3):445–449. 10.1093/pubmed/fdaa230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mbaeyi S, Oliver SE, Collins JP, et al. : The Advisory Committee on Immunization Practices’ Interim Recommendations for Additional Primary and Booster Doses of COVID-19 Vaccines—United States, 2021. Morb. Mortal. Wkly Rep. 2021 Nov 5;70(44):1545–1552. 10.15585/mmwr.mm7044e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rzymski P, Zeyland J, Poniedziałek B, et al. : The perception and attitudes toward COVID-19 vaccines: a cross-sectional study in Poland. Vaccines. 2021 Apr;9(4):382. 10.3390/vaccines9040382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wise J: Covid-19: European countries suspend use of Oxford-AstraZeneca vaccine after reports of blood clots. BMJ. 2021;372:n699. 10.1136/bmj.n699 [DOI] [PubMed] [Google Scholar]
- 40. Carson SL, Casillas A, Castellon-Lopez Y, et al. : COVID-19 vaccine decision-making factors in racial and ethnic minority communities in Los Angeles, California. JAMA Netw. Open. 2021 Sep 1;4(9):e2127582. 10.1001/jamanetworkopen.2021.27582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wouters OJ, Shadlen KC, Salcher-Konrad M, et al. : Challenges in ensuring global access to COVID-19 vaccines: production, affordability, allocation, and deployment. Lancet. 2021 Mar 13;397(10278):1023–1034. 10.1016/S0140-6736(21)00306-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Alkinani LG: Questionnair responses for 1221 participants [Data set]. Zenodo. 2022. 10.5281/zenodo.6345333 [DOI]
- 43. Alkinani LG: Research questionnaire [Data set]. Zenodo. 2022. 10.5281/zenodo.6345362 [DOI]