Abstract
Purpose:
Topical, local anesthetic eye drops in conjunction with antibiotics are commonly used to reduce ocular pain and treat patients in emergency clinics; however, their effects on corneal healing are poorly understood. This study examined whether regular or diluted proparacaine eye drops given in combination with common ophthalmic antibiotics affect corneal wound healing parameters using in vitro and in vivo models.
Methods:
Primary human corneal fibroblasts generated from donor corneas and New Zealand white rabbits were used. Regular (0.5%) and diluted (0.05%) proparacaine eye drops, twice daily for 3 days, were applied to cultures and rabbit eyes, with or without ophthalmic antibiotics (polymyxin B sulfate and trimethoprim). Trypan blue, 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT), and scratch wound assays measured cellular viability, proliferation, and migration, respectively, in vitro. Slit lamp biomicroscopy, tonometry, fluorescein eye test, hematoxylin and eosin (H&E) staining, and 4′,6-diamidino-2-phenylindole (DAPI) immunofluorescence were used for in vivo studies.
Results:
Both regular and diluted proparacaine affected wound healing response in the cornea in vitro and in vivo in a time-dependent manner. Adjunct antibiotic treatments had additive effects characterized by reduced corneal fibroblast viability, proliferation, and migration in vitro and corneal epithelial recovery in vivo. Regular proparacaine with antibiotics showed most pronounced effects on corneal wound healing parameters, and diluted proparacaine without antibiotics had minimal negative effects in vitro and in vivo.
Conclusion:
Both methods of regular (0.5%) and diluted (0.05%) proparacaine topical application to the cornea are safe, but impede corneal wound healing in vitro and in vivo. Adjunct antibiotic treatments had additive negative effects on corneal wound repair.
Keywords: proparacaine, corneal epithelium, topical anesthetics, cornea healing
Introduction
The use of topical anesthetic eye drops for pain control after surgery or traumatic injury remains controversial. Numerous reports have documented the dangers of prolonged, and often unsupervised, use of topical anesthetics.1 Most prominently, there were many case reports of corneal ulceration associated with delayed wound healing with topical anesthetic use.1,2 One proposed mechanism of delayed wound healing includes disruption of cytoplasmic filaments that help heal and reepithelialize cells across corneal wounds.3 Despite the known risks of prolonged use and potential for abuse, diluted concentrations used for short duration have been shown to increase patient comfort and decrease use of narcotic medication.
The safety of prescribed short courses of topical anesthetics has been demonstrated in both traumatic injury and postsurgical patients.4–6 Currently, some ophthalmology practices have already sought to reduce postoperative laser-assisted in situ keratomileusis (LASIK) and photoreceptive keratectomy (PRK) pain with diluted 0.05% proparacaine concentrations for a limited duration.7
Proparacaine is one of the most widely used topical ocular anesthetics, therefore we used proparacaine in our study to provide the most widespread application. In addition, we use proparacaine due to it being less potent and having fewer side effects than tetracaine.8 Further studies also conclude that other topical ocular anesthetics can be toxic to the cornea when abused, especially cocaine, which has a high abuse potential with systemic effects.9,10 Additionally, we used polymyxin B sulfate and trimethoprim as our antibiotics since they are commonly used as topical antibiotics as well.
This study was conducted in 2 parts; in the first part, we investigated in vitro effects of regular strength and diluted proparacaine with and without antibiotics on the important cellular functions of human corneal stromal fibroblast cells that mainly facilitate wound healing response in the stroma.
In the second part, we demonstrated the in vivo response of regular and diluted proparacaine administered with or without common ophthalmic antibiotics on rabbit cornea epithelial cells and stromal cell density after epithelial abrasion. The findings of our study seek to elaborate the impact on the safety of prescribing regular or diluted proparacaine for ophthalmic pain relief using in vitro and in vivo evidence-based data and results.
Methods
Proparacaine preparation and administration
Proparacaine hydrochloride, 0.5% (proparacaine hydrochloride ophthalmic solution; Alcon Fort Worth, TX), was termed as regular proparacaine. Balanced salt solution (BSS) (Alcon Laboratories) was used to prepare diluted 0.05% proparacaine by adding a suitable amount of it. BSS served as a vehicle and negative control. One drop of BSS and regular or diluted proparacaine were applied to cell culture and rabbit eyes twice daily for 3 days. A standard ophthalmic antibiotic, polymyxin B sulfate and trimethoprim ophthalmic solution, United States Pharmacopeia, was applied to each treatment group in conjunction with proparacaine, as per the experimental design.
Primary human corneal stromal fibroblast culture
Primary human corneal stromal fibroblasts (hCSFs) were established from donor human corneas procured from an eye bank (Saving Sight, Kansas City, MO), following the reported method.11–13 Briefly, human corneal tissues were washed twice with serum-free, sterile cell culture medium. Epithelial and endothelial layers were gently scraped, and the scraped corneal button was cut into small sections for explant culture. The convex surface of the scraped corneal sections was placed face down on a 10-cm2 tissue culture dish and incubated in a humidified 5% CO2 incubator at 37°C.
Explants were cultured in minimum essential medium (MEM) supplemented with 10% fetal bovine serum (FBS) for ∼20 days with intermittent medium change. Corneal stromal fibroblasts were harvested by trypsinization and seeded at a density of 7.5 × 104 cells/well in 6-well plates. Cultures were allowed to reach 60%–70% confluence for further experiments. Transforming growth factor beta1 (TGF-β1; 5 ng/mL) was added to the medium 12 h before adding any test agent/drug to represent the active stromal wound healing state in vitro.
Trypan blue cell viability assay
Trypan blue is one of the most common stains for measuring cell viability. In this assay, the used azo dye is membrane impermeable in intact and live cells and therefore the dye only enters dead cells with compromised membranes. The assay was performed following the reported method.11–13 Briefly, 3 × 104 hCSFs were plated in a 12-well tissue culture plate (Thermo Fisher Scientific, Waltham, MA), grown for 24 h, and then treated with regular or diluted proparacaine with or without antibiotics for various test time points.
After staining with trypan blue (25-900-Cl; Mediatech, Manassas, VA), as per the vendor's instructions, the stained and nonstained cells were counted in a hemocytometer under a microscope. The cellular viability percentage was calculated by dividing viable cells by total cells multiplied by 100.
3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide cellular proliferation assay
Cellular proliferation of hCSF cells was evaluated by 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay at various times (6–72 h), following the reported method.11–13 The MTT assay (G4100, Promega Corp., Madison, WI) is a colorimetric assay for assessing cell viability by using NAD(P)H-dependent cellular oxidoreductase enzymes that (under defined conditions) can reflect the number of viable cells present.
These enzymes are capable of reducing the tetrazolium dye MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, to its insoluble formazan product, which has a purple color and is then quantified by measuring absorbance using a multiwell spectrophotometer at 500–600 nanometers. Briefly, 5 × 103 hCSFs were plated in a 96-well tissue culture plate (Thermo Fisher, Waltham, MA), grown for 24 h, and then treated with regular and diluted proparacaine with or without antibiotics for various selected times.
After the exposure, the MTT assay was performed as per the vendor's instructional datasheet. Then, 15 μL of the MTT dye solution was added and cells were incubated at 37°C in a CO2 incubator for 4 h. After 4 h, 100 μL of solubilization solution (Stop Mix) was added and the plate was incubated for 1 h. The optical density was measured at 570 nm using an Epoch BioTek plate reader (BioTek Instruments, Winooski, VT).
Scratch wound cell migration assay
Cellular migration for each treatment was gauged by a standard in vitro scratch wound assay.14 In brief, hCSFs were seeded in a 6-well plate with MEM supplemented with 10% FBS and incubated overnight. After overnight incubation, cells were treated with or without TGF-β1 (5 ng/mL) in serum-free media. A linear standardized scratch was created with a p200 pipette tip in a monolayer of cells before addition of BSS and diluted or regular proparacaine with or without antibiotics in accordance with the experimental design.
Phase-contrast microscopy equipped with a Leica DFC290 imaging system (Leica Microsystems, Bannockburn, IL) was utilized to record the proliferation rate and cellular morphology at 24 h. Comparative analyses of cellular migration were performed with phase-contrast images of different treatment groups.
In vivo corneal wounding
Eighteen New Zealand white female rabbits (2.5 to 3.0 kg) were used for the in vivo study. The Institutional Animal Care and Use Committees of the Harry S. Truman Memorial Veterans' Hospital and the University of Missouri, Columbia, approved the animal study. All procedures in animals and hCSFs were conducted in accordance with ethical considerations of the Association for Research in Vision and Ophthalmology and tenets of the Helsinki Declaration.
Anesthesia in rabbits was induced by an intramuscular injection of a cocktail containing ketamine hydrochloride, 50 mg/kg (MWI, Boise, ID), and xylazine hydrochloride, 10 mg/kg (Akorn, Lake Forest, IL). A circular abrasion was made by scraping with a #64 beaver blade. In accordance with the 3R rule animal rule (reduce, replace, and refine), both eyes of all rabbits were used as corneal epithelial abrasion does not cause significant pain, discomfort, and/or distress in rabbits and is routinely performed in humans in ophthalmology clinics.
Thirty-six eyes were divided into 6 groups. Group 1 received BSS without antibiotics (n = 6), Group 2 received BSS with antibiotics (n = 6), Group 3 received regular proparacaine with antibiotics (n = 6), Group 4 received regular proparacaine without antibiotics (n = 6), Group 5 received diluted proparacaine with antibiotics (n = 6), and Group 6 received diluted proparacaine without antibiotics (n = 6). A complete ophthalmic examination under a slit lamp microscope (SL-15 Kowa Company, Ltd., Tokyo, Japan) confirmed the use of healthy eyes.
Clinical evaluation of corneal health
Slit lamp clinical examination, fluorescein eye test, and anterior segment photography were used to determine epithelial wound closure, following the reported method.15–17 Corneas were stained with commercial Flu-Glo fluorescein staining strips (Akorn, Inc., Buffalo Grove, IL) to record levels of corneal reepithelialization. The fluorescein-stained corneas were visualized and photographed under cobalt blue light daily for 3 days.
Intraocular pressure assessment
Administration of drug to the eye has the potential to induce secondary variations in aqueous humor production in the anterior chamber, therefore variations in intraocular pressure (IOP) were recorded with a tonometer (Tono-Pen AVIA; Reichert Technologies, Depew, NY) at regular timed intervals, at 6, 24, 48, and 72 h, as reported previously.15–17 To avoid the normal diurnal variation and operator variability, all measurements were performed between 9 and 11 AM.
Rabbit corneal tissue collection
Rabbits were humanely euthanized by an intravenous injection of pentobarbital, 150 mg/kg (SomnaSol, Henry Schein Animal Health, Dublin, OH), under general anesthesia on day 3, following the reported method.15–17 Corneas were detached from eyes and immediately placed into 24 × 24 × 5-mm molds (Fischer Scientific, Pittsburgh, PA) containing the optimal cutting temperature compound (Tissue Plus O.C.T., Fisher HealthCare, Houston, TX), snap-frozen, and maintained at −80°C until further processing.
Serial corneal sections (8 μm) were made from frozen corneal tissues using a cryostat (HM525 NX UV; Microm GmbH, Walldorf, Germany). Corneal tissue sections were placed on glass microscope slides (Superfrost Plus; Fisher Scientific, Pittsburgh, PA) and stored at −80°C until staining.
Histology and immunofluorescence evaluations
Histological examinations were performed with hematoxylin and eosin (H&E) and immunofluorescence studies with 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Inc., Burlingame, CA), following standard methods.15–17 In brief, hematoxylin stained all nuclei blue–purple and eosin stained the cytoplasm and extracellular matrix with varying degrees of pink.
In brief, immunofluorescence studies were performed by incubating tissue sections at room temperature for 20 min, followed by 3 washes with phosphate-buffered saline (5 min each) and application of an antifade mounting medium containing DAPI (H1200; Vector Laboratories, Inc.).
Statistical analysis
The results of all assays are presented as an average of 3 repeats at least and as mean ± standard error of the mean as applicable. Statistical analysis was conducted with commercial software, GraphPad Prism 9.2 (GraphPad Software, La Jolla, CA). Student's t-test and one-way analysis of variance (ANOVA) with Bonferroni post hoc test were used for statistical tests. Values of P ≤ 0.05 were considered significant.
Results
In vitro cellular viability study
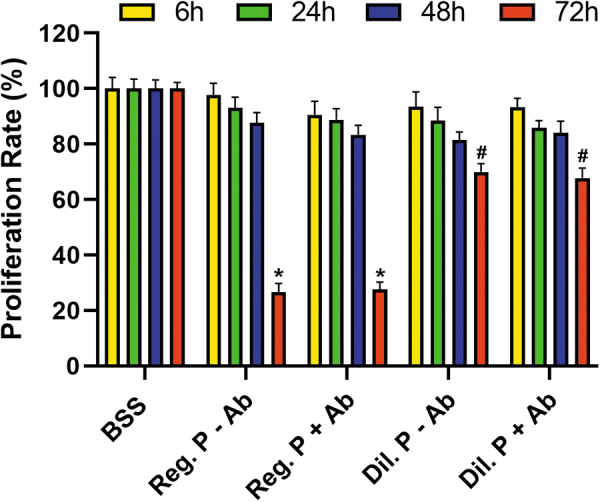
The results and comparisons of the in vitro cellular viability study are summarized in Table 1. The trypan blue cellular viability assay showed 100% cell viability in the control group without any proparacaine or antibiotics (Fig. 1). The cellular viability assay showed that at 6 h, the cell proliferation rate of the control group (BSS) without proparacaine or antibiotics was at 100% (Fig. 1).
Table 1.
In Vitro Cellular Viability Assay
| Treatments | 6 h | 24 h | 48 h | 72 h |
|---|---|---|---|---|
| BSS | 100 ± 8.11 | 100 ± 3.86 | 100 ± 5.12 | 100 ± 8.89 |
| Reg P. –Ab | 97.6 ± 7.78 | 93.1 ± 3.72 | 87.6 ± 4.65 | 26.6 ± 3.11 |
| Reg_P +Ab | 90.5 ± 8.18 | 88.7 ± 4.14 | 83.3 ± 5.17 | 27.6 ± 3.16 |
| Dil_P –Ab | 93.5 ± 6.82 | 88.4 ± 3.76 | 81.4 ± 5.98 | 69.9 ± 6.13 |
| Dil_P +Ab | 93.2 ± 7.98 | 85.9 ± 4.16 | 84.1 ± 6.61 | 67.7 ± 7.23 |
Reg P., regular proparacaine (0.5%); Dil. P, diluted proparacaine (0.05%); +Ab treated with antibiotics; −Ab not treated with antibiotics. The cellular proliferation rate was significantly affected in the Reg. P environment, P < 0.001; whereas the Dil. P environment also affected the cellular proliferation rate, P < 0.01, at the 72-h time point.
BSS, balanced salt solution.
FIG. 1.
Proparacaine impact on corneal stromal fibroblast viability. The trypan blue cellular viability graph showing the effects of regular (0.5%) and diluted (0.05%) proparacaine on hCSF viability with or without the antibiotic environment. The experiments were performed in triplicate and error bars represent ± SEM. *P < 0.001 and #P < 0.01. hCSFs, human corneal stromal fibroblasts; SEM, standard error of the mean. Color images are available online.
At 6, 24, and 48 h, there was no significant change in the viability rate by regular and diluted proparacaine with or without antibiotics (Fig. 1). At 72 h, both regular and diluted proparacaine showed a statistically significant decrease in viability rate with or without antibiotics (P < 0.001 or P < 0.01). However, loss of cellular viability was more significant in regular (P < 0.001) than diluted (P < 0.01) proparacaine at 72 h regardless of the presence or absence of antibiotics.
In vitro cellular proliferation study
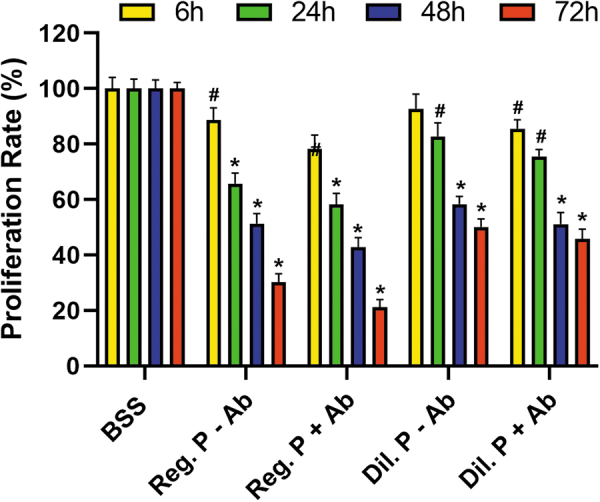
The results and comparisons of the in vitro cellular proliferation assay are shown in Table 2. Cellular proliferation studies with MTT assay showed that at 6 h, the cell proliferation rate of the control group without proparacaine or antibiotics was at 100% (Fig. 2). Treatment with regular proparacaine without polymyxin B and trimethoprim antibiotics reduced cellular proliferation to 88.7% at 6 h, 65.7% at 24 h, 51.3% at 48 h, and 30.1% at 72 h.
Table 2.
In Vitro Cellular Proliferation Study
| Treatments | 6 h | 24 h | 48 h | 72 h |
|---|---|---|---|---|
| BSS | 100 ± 4.06 | 100 ± 3.45 | 100 ± 3.14 | 100 ± 2.25 |
| Reg P. –Ab | 88.7 ± 4.32 | 65.7 ± 3.87 | 51.3 ± 3.65 | 30.1 ± 3.14 |
| Reg_P +Ab | 78.3 ± 4.98 | 58.3 ± 4.01 | 42.7 ± 3.49 | 21.3 ± 2.68 |
| Dil_P –Ab | 92.7 ± 5.32 | 82.7 ± 4.87 | 58.3 ± 2.87 | 50.1 ± 2.98 |
| Dil_P +Ab | 85.5 ± 3.24 | 75.6 ± 2.54 | 51.1 ± 4.21 | 45.7 ± 3.65 |
Reg P., regular proparacaine (0.5%); Dil. P, diluted proparacaine (0.05%); +Ab treated with antibiotics; −Ab not treated with antibiotics. At 6 h, viability was significantly affected, P < 0.01 (in Reg. P); and at 24, 48, and 72 h, the cellular viability was significantly affected, P < 0.001 (in Reg. P and Dil. P).
FIG. 2.
Proparacaine impact on corneal stromal fibroblast proliferation. The MMT proliferation graph showing the effect of regular (0.5%) and diluted (0.05%) proparacaine on the hCSF proliferation rate in the presence or absence of antibiotics. The experiments were performed in triplicate and error bars represent ± SEM. *P < 0.001 and #P < 0.01. Color images are available online.
Treatment with regular proparacaine with polymyxin B and trimethoprim antibiotics further reduced cellular proliferation to 78.3% at 6 h, 58.3.7% at 24 h, 42.7% at 48 h, and 21.3% at 72 h. Conversely, treatment with diluted proparacaine without antibiotics had a much reduced effect on cell proliferation as cellular proliferation was 92.7% at 6 h, 82.7% at 24 h, 58.3% at 48 h, and 50.1% at 72 h. The use of antibiotics in diluted proparacaine negatively impacted cellular proliferation and showed cellular proliferation of 85.5% at 6 h, 75.5% at 24 h, 51.1% at 48 h, and 45.7% at 72 h.
There was a statistically significant decrease in cellular viability of hCSFs at 72 h after adding of regular (P < 0.001) or diluted (P < 0.01) proparacaine. Antibiotic prophylaxis has no significant effect on cellular viability for the diluted and regular proparacaine groups.
In vitro cellular migration study
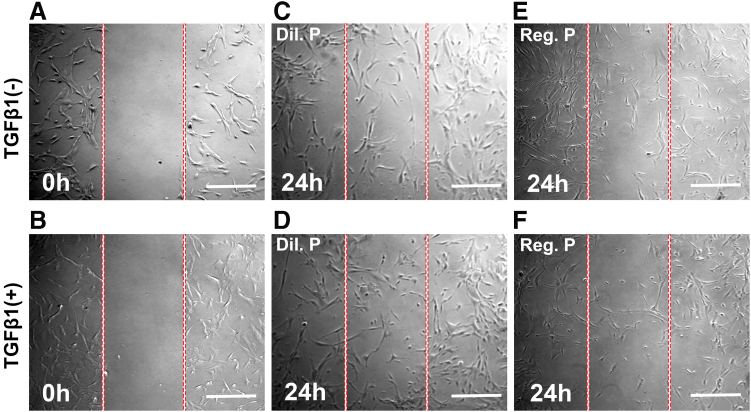
The results and comparisons of regular or diluted proparacaine treatments in the presence or absence of antibiotics on a cellular migration assay are presented in Fig. 3. TGF-β1 in the scratch wound assay was used to represent an active wound healing state. No cells were present at 0 h after creation of a scratch wound in the migration assay (Fig. 3A, B). Regular proparacaine treatment 24 h after scratching demonstrated remarkably reduced cellular migration in both active (+TGF-β1) and inactive (−TGF-β1) wound healing states (Fig. 3E, F).
FIG. 3.
Proparacaine on corneal stromal fibroblast migration. Phase-contrast microscopy images show the effect of regular (0.5%) and diluted (0.05%) proparacaine on the hCSF migration rate with or without an antibiotic environment conducted through scratch assay. The experiments were performed in triplicate. Cultures showing the scratch wound at 0 h in −TGFβ1 (A), scratch wound at 0 h in +TGFβ1 (B), hCSF migration in diluted proparacaine and −TGFβ1 at 24 h (C), hCSF migration in diluted proparacaine and +TGFβ1 at 24 h (D), hCSF migration in regular proparacaine and −TGFβ1 at 24 h (E), and hCSF migration in regular proparacaine and +TGFβ1 at 24 h (F). Scale bar = 100 μm. Color images are available online.
Contrary to this, the diluted proparacaine treatment did not have such drastic reduction in cellular migration 24 h after scratching in both active (+TGF-β1) and inactive (−TGF-β1) wound healing states (Fig. 3C, D). The use of antibiotics had no effect on cellular migration in vitro (data not shown).
In vivo fluorescein staining in live rabbits
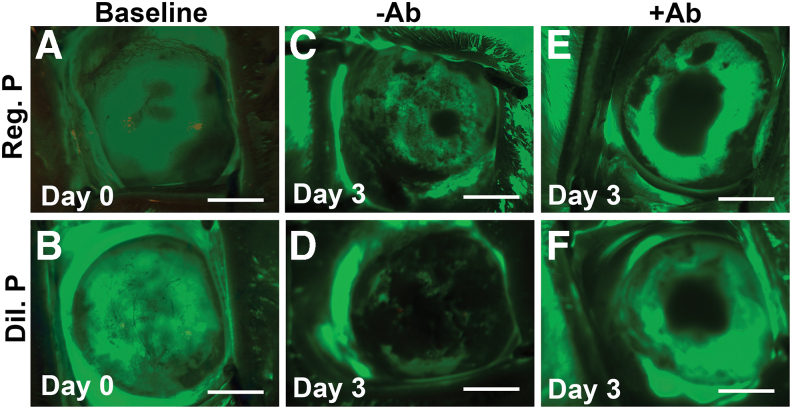
Regular proparacaine treatment on rabbit eyes with a scraped 8-mm abrasion on the corneal epithelium notably delayed corneal reepithelialization compared with the diluted proparacaine treatment on day 3 (Fig. 4). Adjunct antibiotic treatments had an additive impact on corneal reepithelialization in both proparacaine treatment groups (Fig. 4). In rabbit eyes, the abrasion on the corneal epithelium was fully healed by day 3, thus this time point was chosen for investigation in the study.
FIG. 4.
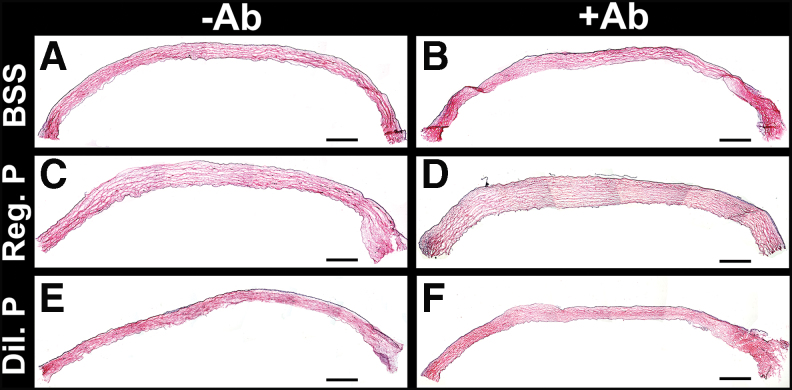
Proparacaine effects on corneal reepithelialization in vivo. Fluorescein staining images show the comparative reepithelialization process in rabbit cornea after addition of regular (0.5%) and diluted (0.05%) proparacaine at day 3. Scale bar = 3.0 mm. Rabbit cornea showing the epithelialization baseline in the groups of regular proparacaine (A), diluted proparacaine (B), regular proparacaine without antibiotics (C), diluted proparacaine without antibiotics (D), regular proparacaine with antibiotics (E), and diluted proparacaine with antibiotics (F). Scale bar = 100 μm. Color images are available online.
A baseline corneal epithelium abrasion on day 0 in rabbit eyes exhibited by a green fluorescein stain on the entire cornea has been presented in Fig. 4A and B. Treatment with regular proparacaine with antibiotics caused the highest delay in corneal reepithelialization (Fig. 4E), followed by diluted proparacaine with antibiotics (Fig. 4F) and regular proparacaine without antibiotics (Fig. 4C). Diluted proparacaine without antibiotics caused no delay in reepithelialization and showed a nearly normal reepithelialization timeline (Fig. 4D).
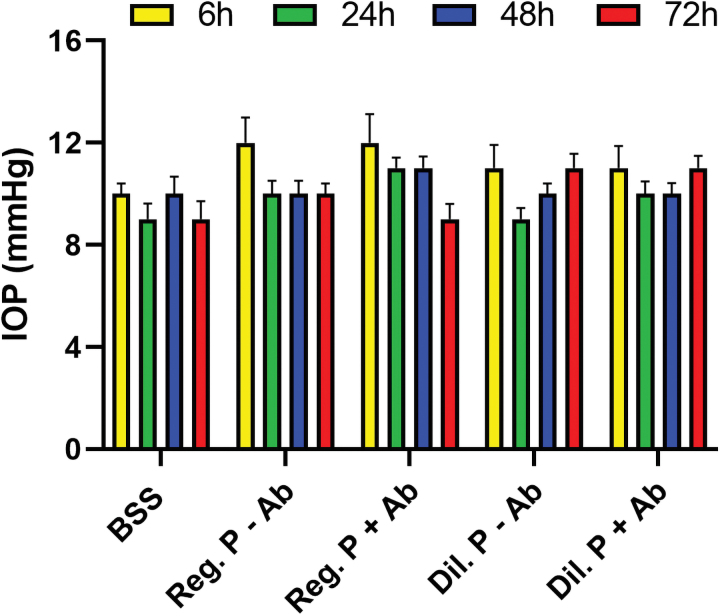
IOP measurement in live rabbits
Neither regular nor diluted proparacaine treatments with or without antibiotics caused any significant change in IOP in rabbit eyes in vivo (Fig. 5).
FIG. 5.
Proparacaine effects on IOP in vivo. The IOP graph shows that the fluid pressure of rabbit eyes in all groups did not deviate from the baseline (BSS control) group animals. The average of 10 readings was recorded in each animal and error bars represent ± SEM. BSS, balanced salt solution; IOP, intraocular pressure. Color images are available online.
DAPI staining in corneal tissue
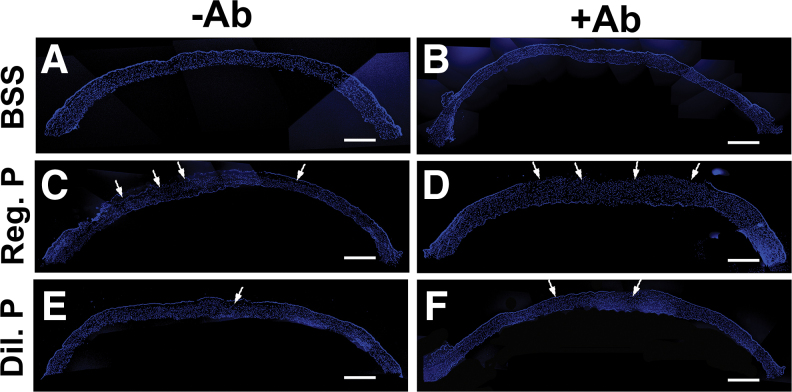
The results and comparisons of DAPI immunofluorescence demonstrated levels of corneal reepithelialization and stromal cell density on day 3 in rabbit corneas treated with BSS and regular or diluted proparacaine with and without antibiotics, which are provided in Fig. 6. All corneal sections treated with BSS with or without antibiotics demonstrated normal epithelium and stromal cell density (Fig. 6A, B).
FIG. 6.
Proparacaine effects on corneal reepithelialization and stromal cell density in vivo. The panoramic images of DAPI immunofluorescence staining of whole tissue sections of rabbit cornea show the reepithelialization pattern with BSS without antibiotics (A), BSS with antibiotics (B), regular proparacaine without antibiotics (C), regular proparacaine with antibiotics (D), diluted proparacaine without antibiotics (E), and diluted proparacaine with antibiotics (F) in corneal tissue sections. Regular strength proparacaine delayed the reepithelialization process compared with diluted strength of proparacaine at day 3. Arrows show the cellular density. Scale bar = 100 μm. DAPI, 4′,6-diamidino-2-phenylindole. Color images are available online.
Regular and diluted proparacaine and antibiotic-treated corneas showed more impeded reepithelialization (Fig. 6D, F) compared with without antibiotic-treated corneas (Fig. 6C, E). No significant differences in stromal cell density were found in any groups: BSS and regular or diluted proparacaine treatments with and without antibiotics (Fig. 6).
H&E histology evaluation
The results and comparisons of gross tissue morphology for all treatment groups with H&E staining are shown in Fig. 7. None of the treatments, BSS and regular or diluted proparacaine with or without antibiotics, caused any clinically relevant pathology except delayed corneal reepithelialization in tissues treated with regular proparacaine with antibiotics (Fig. 7).
FIG. 7.
Proparacaine effects on corneal morphology in vivo. The panoramic images of H&E staining of whole tissue sections of rabbit cornea show the gross tissue morphology, reepithelialization pattern, and presence of macrophages and infiltrating cells with BSS without antibiotics (A), BSS with antibiotics (B), regular proparacaine without antibiotics (C), regular proparacaine with antibiotics (D), diluted proparacaine without antibiotics (E), and diluted proparacaine with antibiotics (F) in corneal tissue sections. Each image, scale bar = 100 μm, in a composite panoramic panel. H&E, hematoxylin and eosin. Color images are available online.
Discussion
The human cornea is a highly innervated tissue and sensitive to various stimuli. The topical local use of anesthetics on the eye is frequent for almost all types of ocular surgeries, but especially during cataract, refractive, and other corneal surgeries, to minimize ocular pain. However, their use has been documented to impair corneal healing. Proparacaine eye drops are known to numb the eye by blocking pain signals at nerve endings.
In the present study, a comparison of corneal wound healing parameters under the influence of regular (0.5%) proparacaine and 10-fold diluted (0.05%) proparacaine eye drops in conjunction with ophthalmic antibiotics was performed using in vitro human and in vivo rabbit models of corneal wound healing. The results of our current study indicate that regular as well as diluted proparacaine treatments are safe for the eye, but negatively affect corneal wound healing in vitro and in vivo to varying levels. Intriguingly, adjunct antibiotic therapy showed additive negative effects by further delaying corneal wound healing.
Our findings further support the idea that adverse effects of proparacaine are dose dependent and cumulative over time8,18 as there is more damage to the cornea seen for a higher dose of proparacaine in our studies, and some of our studies were statistically significant only at day 3 of adding the proparacaine drops. Our studies have shown damage to the corneal epithelium, but other studies have shown damage to corneal stromal tissues in vitro as well.19
In addition, our research reinforces the observation that most adverse events related to anesthetics are related to long-term use of full-strength anesthetics.9,10 Diluted proparacaine (0.5%) is safer than regular proparacaine (0.5%), as evidenced by our study; furthermore, it is strong enough to decrease pain, but is not completely anesthetic.4
From our results, we believe a short-term therapeutic agent in management of small corneal injuries without signs of ulcers may be appropriate, such as in PRK patients. Therefore, a topical anesthetic medication is given after the PRK procedure, and diluted proparacaine is preferred for short-duration treatment. This can provide comfort for patients while limiting ocular anesthetic abuse since PRK patients who are treated with diluted proparacaine have significantly less pain, as seen in other studies.4 However, they should be avoided in PRK patients who have dry eye syndrome or ocular surface disease20 or any psychiatric illness.21–23
Special attention and monitoring should be performed for patients with risk factors for abuse, such as underlying psychiatric illness, exposure to the medical field, and manual laborers who specialize in welding and foundry work.22–24
In another study, both tetracaine and proparacaine were shown to be effective methods of topical anesthesia in LASIK and PRK.25 Tetracaine caused significantly more pain upon instillation in all patients, but resulted in greater analgesia 30 min after surgery in the LASIK group.25 Patients in the LASIK group expressed a preference for tetracaine over proparacaine and there was no significant drop in preference among PRK patients.25 On the other hand, studies have shown that proparacaine is less potent and has fewer side effects than tetracaine.8
Currently, most postoperative treatments use the bandage contact lens (BCL), and alternative treatments use cold balanced saline solution, topical anesthesia, sumatriptan, gabapentin, and morphine.26,27 There have been studies showing that diluted proparacaine and bupivacaine neither delay nor cause stromal damage.26,27 However, if one were to add a topical ocular anesthetic, we still recommend using only diluted proparacaine and for very short duration to prevent complications.
A different study showed that there were no differences regarding pain, persistent symptoms, or corneal healing when comparing short-term use of topical anesthetics with placebo in treatment of corneal abrasions. Data on safety are sparse, and the generalized widespread use of topical anesthetics is currently not supported by evidence.1
The emergency department (ED) studies that do describe anesthetics for corneal abrasions only involved simple abrasions that are not visually significant, large, or in contact lens users.5,6,28,29 These studies also have poor compliance and follow-up with flawed methodology, and ice packs as an alternative have been shown to be effective in corneal epithelial damage.30 It is not yet clear if diluted anesthetics are safe enough to be used for ED treatment of abrasions and further research is needed with more subjects.
We recommend that topical anesthetics should not be encouraged in the ED for general abrasions and should probably only be given for home use in limited quantities and only to those who can manage any potential complications that may arise from their use. This is because there is a difference between a possibly contaminated traumatic wound that is followed by an ED provider and a surgically induced wound that is followed by an ophthalmologist and concomitantly treated with a BCL.
We recommend being vigilant about topical anesthetic abuse as it may be difficult to diagnose. Sometimes signs and symptoms of topical anesthetic abuse align with those of acanthamoeba keratitis, and the correct diagnosis is delayed since the patient may deny topical anesthetic use at first.21,31 A delay in diagnosis can have devastating effects on the cornea. In addition, another risk factor for acanthamoeba is damage to the corneal surface, which may be contributed by late corneal healing from proparacaine.32
Shortcomings of our research study include having limited time points and a large abrasion (size 8 mm) that may not be as generalizable as a smaller abrasion. The rabbit corneas are approximately the same size as human corneas.
Further studies in future can explore the role that preservatives in proparacaine play in corneal healing. Additionally, further studies can compare the corneal effect of topical anesthetic eye drop use on days 1, 2, 3, 5, and 7. The 7-day study is performed to see if the cumulative effects are more pronounced between days 3 and 7. Finally, a future study can assess any damage to the corneal endothelium using photometry.
In conclusion, proparacaine does impact corneal cell viability, proliferation, and migration in vivo and in vitro regardless of the strength. Our studies also show that addition of polymyxin B/trimethoprim has a negative impact on corneal healing. Previous studies and case reports show that anesthetic abuse is difficult to diagnose and can have devastating effects on the cornea, so it is important to be vigilant when using topical anesthetics.
Acknowledgment
Authors thank Ashika Srivastava, an intern from Chamblee High School, Chamblee, GA, for her careful reading of the manuscript.
Authors' Contributions
The authors made substantial contributions to the conception and design of the study (R.R.M. and E.O.); acquisition, analysis, or interpretation of data (S.G., E.Z., P.R.S., and E.O.); and drafting (E.O. and S.G.) or revising (R.R.M. and F.W.F.) the manuscript. All authors agree to be held accountable for all aspects of the work.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This research was supported by the NEI/NIH R01EY030774, NEI/NIH U01EY031650, VA 1I01BX00357, and VA IK6BX005646 grants and the University of Missouri Ruth M. Kraeuchi Missouri Endowed Chair Ophthalmology Fund (to R.R.M.).
References
- 1. Puls, H.A., Cabrera, D., Murad, M.H., et al. . Safety and effectiveness of topical anesthetics in corneal abrasions: systematic review and meta-analysis. J. Emerg. Med. 49:816–824, 2015. [DOI] [PubMed] [Google Scholar]
- 2. Epstein, D.L., and Paton, D.. Keratitis from misuse of corneal anesthetics. N. Engl. J. Med. 279:396–399, 1968. [DOI] [PubMed] [Google Scholar]
- 3. Henkes, H.E., and Waubke, T.N.. Keratitis from abuse of corneal anaesthetics. Br. J. Ophthalmol. 62:62–65, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shahinian Jr., L., Jain, S., Jager, R.D., et al. . Dilute topical proparacaine for pain relief after photorefractive keratectomy. Ophthalmology. 104:1327–1332, 1997. [DOI] [PubMed] [Google Scholar]
- 5. Waldman, N., Densie, I.K., and Herbison, P.. Topical tetracaine used for 24 hours is safe and rated highly effective by patients for the treatment of pain caused by corneal abrasions: a double-blind, randomized clinical trial. Acad. Emerg. Med. 21:374–382, 2014. [DOI] [PubMed] [Google Scholar]
- 6. Waldman, N., Winrow, B., Densie, I., et al. . An observational study to determine whether routinely sending patients home with a 24-hour supply of topical tetracaine from the emergency department for simple corneal abrasion pain is potentially safe. Ann. Emerg. Med. 71:767–778, 2018. [DOI] [PubMed] [Google Scholar]
- 7. Nordan, L. Tetracaine After Keratorefractive surgery. CRS Today. https://crstoday.com/articles/2003-sep/0903_041-html/ (accessed September 9, 2021).
- 8. Moreira, L.B., Kasetsuwan, N., Sanchez, D., et al. . Toxicity of topical anesthetic agents to human keratocytes in vivo. J. Cataract Refract. Surg. 25:975–980, 1999. [DOI] [PubMed] [Google Scholar]
- 9. Mcgee, H.T., and Fraunfelder, F.. Toxicities of topical ophthalmic anesthetics. Expert Opin. Drug Saf. 6:637–640, 2007. [DOI] [PubMed] [Google Scholar]
- 10. Fraunfelder, F.W., and Rich, L.F.. Possible adverse effects of drugs used in refractive surgery. J. Cataract Refract. Surg. 29:170–175, 2003. [DOI] [PubMed] [Google Scholar]
- 11. Balne, P.K., Sinha, N.R., Hofmann, A.C., et al. . Characterization of hydrogen sulfide toxicity to the human corneal stromal fibroblasts. Ann. N. Y. Acad. Sci. 1480:207–218, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gupta, S., Martin, L.M., Sinha, N.R., et al. . Role of inhibitor of differentiation 3 gene in cellular differentiation of human corneal stromal fibroblasts. Mol. Vis. 26:742–756, 2020. [PMC free article] [PubMed] [Google Scholar]
- 13. Rodier, J.T., Tripathi, R., Fink, M.K., et al. . Linear polyethylenimine-DNA nanoconstruct for corneal gene delivery. J. Ocul. Pharmacol. Ther. 35:23–31, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mohan, R.R., Tripathi, R., Sharma, A., et al. . Decorin antagonizes corneal fibroblast migration via caveolae-mediated endocytosis of epidermal growth factor receptor. Exp. Eye Res. 180:200–207, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tripathi, R., Balne, P.K., Sinha, N.R., et al. . A novel topical ophthalmic formulation to mitigate acute mustard gas keratopathy in vivo: a pilot study. Transl. Vis. Sci. Technol. 9:6, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mohan, R.R., Balne, P.K., Muayad, M.S., et al. . Six-month in vivo safety profiling of topical ocular AAV5-decorin gene transfer. Transl. Vis. Sci. Technol. 10:5, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gupta, S., Sinha, N.R., Martin, L.M., et al. . Long-term safety and tolerability of BMP7 and HGF gene overexpression in rabbit cornea. Transl. Vis. Sci. Technol. 10:6, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pang, X., and Fan, T.J.. Cytotoxic effect and possible mechanisms of Tetracaine on human corneal epithelial cells in vitro. Int. J. Ophthalmol. 9:497–504, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Song, Z., and Fan, T.J.. Tetracaine induces apoptosis through a mitochondrion-dependent pathway in human corneal stromal cells in vitro. Cutan. Ocul. Toxicol. 37:350–358, 2018. [DOI] [PubMed] [Google Scholar]
- 20. Chen, H.-T., Chen, K.-H., and Hsu, W.-M.. Toxic keratopathy associated with abuse of low-dose anesthetic. Cornea. 23:527–529, 2004. [DOI] [PubMed] [Google Scholar]
- 21. Patel, M., and Fraunfelder, F.W.. Toxicity of topical ophthalmic anesthetics. Expert Opin. Drug Metab. Toxicol. 9:983–988, 2013. [DOI] [PubMed] [Google Scholar]
- 22. Yeniad, B., Canturk, S., Ozdemir, F.E., et al. . Toxic keratopathy due to abuse of topical anesthetic drugs. Cutan. Ocul. Toxicol. 29:105–109, 2010. [DOI] [PubMed] [Google Scholar]
- 23. Katsimpris, J., Sarantoulakou, M., Kordelou, A., et al. . Clinical findings in patients with topical anaesthetic abuse keratitis: a report of five cases. Klin. Monbl. Augenheilkd. 224:303–308, 2007. [DOI] [PubMed] [Google Scholar]
- 24. Yagci, A., Bozkurt, B., Egrilmez, S., et al. . Topical anesthetic abuse keratopathy: a commonly overlooked health care problem. Cornea. 30:571–575, 2011. [DOI] [PubMed] [Google Scholar]
- 25. Moshirfar, M., Mifflin, M., Mccaughey, M., et al. . Prospective, randomized, contralateral eye comparison of tetracaine and proparacaine for pain control in laser in situ keratomileusis and photorefractive keratectomy. Clin. Ophthalmol. 8:1213–1219, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Garcia, R., Andrade, D.C.D., Teixeira, M.J., et al. . Mechanisms of corneal pain and implications for postoperative pain after laser correction of refractive errors. Clin. J. Pain. 32:450–458, 2016. [DOI] [PubMed] [Google Scholar]
- 27. Golan, O., and Randleman, J.B.. Pain management after photorefractive keratectomy. Curr. Opin. Ophthalmol. 29:306–312, 2018. [DOI] [PubMed] [Google Scholar]
- 28. Ball, I.M., Seabrook, J., Desai, N., et al. . Dilute proparacaine for the management of acute corneal injuries in the emergency department. CJEM. 12:389–394, 2010. [DOI] [PubMed] [Google Scholar]
- 29. Ting, J.Y.S., Barns, K.J., and Holmes, J.L.. Management of Ocular Trauma in Emergency (MOTE) Trial: a pilot randomized double-blinded trial comparing topical amethocaine with saline in the outpatient management of corneal trauma. J. Emerg. Trauma Shock. 2:10–14, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fraser, R., Walland, M., Chan, E., et al. . Topical anaesthetic in the treatment of corneal epithelial defects: what are the risks? Aust. J. Gen. Pract. 48:504–506, 2019. [DOI] [PubMed] [Google Scholar]
- 31. Rosenwasser, G.O., Holland, S., Pflugfelder, S.C., et al. . Topical anesthetic abuse. Ophthalmology. 97:967–972, 1990. [DOI] [PubMed] [Google Scholar]
- 32. Szentmáry, N., Daas, L., Shi, L., et al. . Acanthamoeba keratitis—clinical signs, differential diagnosis and treatment. J. Curr. Ophthalmol. 31:16–23, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]