Supplemental Digital Content is Available in the Text.
Keywords: Spinal fusion, Pain, Analgesics, Pain treatment
Abstract
Patients undergoing spinal surgery are at high risk of acute and persistent postoperative pain. Therefore, adequate pain relief is crucial. This systematic review aimed to provide answers about best-proven postoperative analgesic treatment for patients undergoing lumbar 1- or 2-level fusions for degenerative spine diseases. We performed a search in PubMed, Embase, and The Cochrane Library for randomized controlled trials. The primary outcome was opioid consumption after 24 hours postoperatively. We performed meta-analyses, trial sequential analyses, and Grading of Recommendations assessment to accommodate systematic errors. Forty-four randomized controlled trials were included with 2983 participants. Five subgroups emerged: nonsteroidal anti-inflammatory drugs (NSAIDs), epidural, ketamine, local infiltration analgesia, and intrathecal morphine. The results showed a significant reduction in opioid consumption for treatment with NSAID (P < 0.0008) and epidural (P < 0.0006) (predefined minimal clinical relevance of 10 mg). Concerning secondary outcomes, significant reductions in pain scores were detected after 6 hours at rest (NSAID [P < 0.0001] and intrathecal morphine [P < 0.0001]), 6 hours during mobilization (intrathecal morphine [P = 0.003]), 24 hours at rest (epidural [P < 0.00001] and ketamine [P < 0.00001]), and 24 hours during mobilization (intrathecal morphine [P = 0.03]). The effect of wound infiltration was nonsignificant. The quality of evidence was low to very low for most trials. The results from this systematic review showed that some analgesic interventions have the capability to reduce opioid consumption compared with control groups. However, because of the high risk of bias and low evidence, it was impossible to recommend a “gold standard” for the analgesic treatment after 1- or 2-level spinal fusion surgery.
1. Introduction
Multimodal or balanced analgesia continues to be the leading treatment principle for managing postoperative pain.31 The main concern is to achieve better pain treatment through additive or synergistic effects of several nonopioids, thereby reducing the need for postoperative opioid treatment and opioid-related adverse events such as nausea and vomiting.34,35
Postoperative pain management remains a significant clinical challenge mirroring the lack of knowledge and documentation regarding the effects of most combinations of analgesics.10,17
A commonly performed orthopedic procedure, with increasing rates worldwide (increase of 118% in the United States between 1998 and 2014), is 1- or 2-level spinal fusion surgery.58 Patients undergoing this procedure are at a high risk of acute and persistent postoperative pain, development of postoperative hyperalgesia, and possibly opioid tolerance followed by excessive and continuous use of opioids.4,51 Furthermore, postoperative pain often negatively influences the patients' mobility, resulting in delayed recovery and rehabilitation. These patients often receive preoperative opioid treatment, making postoperative pain treatment difficult to manage.46
Adequate postoperative pain relief improves patient satisfaction and patients' perception of the quality of their hospital stay, and it facilitates early mobilization and optimal rehabilitation.9,35,36 However, there is a lack of consensus regarding the “gold standard” of the postoperative pain treatment strategy in patients undergoing 1- or 2-level lumbar spinal fusion procedures.46,47
Therefore, this systematic review aims to investigate whether the existing literature contains evidence concerning procedure-specific, medication-based interventions for 1- or 2-level spinal fusion surgery.
2. Methods
This review follows the methodology recommended by the Cochrane Collaboration. We performed this systematic review according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses guidelines.49 Before performing the literature search, we registered the protocol at PROSPERO, the international prospective register of systematic reviews on July 26, 2020, registration number: CRD42020192899.
We designed a broad search string, including MeSH and All fields terms, in collaboration with a professional search coordinator to avoid overlooking relevant trials (Appendix 1, available at http://links.lww.com/PR9/A157). Because there was a change in MESH terms after 1988, we only included trials published after 1988. We searched the following databases: PubMed, Embase, and The Cochrane Library (Appendix 1, available at http://links.lww.com/PR9/A157). The last search was on January 18, 2021. We searched published systematic reviews and articles by hand for eligible trials and screened The PROSPECT Database8 and reference lists from relevant reviews. We detected nonindexed journals and their published articles by searching Google Scholar.
We included RCTs comparing the postoperative effect of a perioperative analgesic intervention for 1- or 2-level spinal fusion surgery against a control group. The analgesic intervention had to be initiated in the immediate perioperative period, and trials had to report at least one of the predefined endpoints. Exclusion criteria were abstracts, unpublished observations, quasi-randomized and observational studies, trials not written in English, trials not dealing with spinal fusion surgery, fusions performed on scoliosis, tumors or trauma and more than 2 levels, age <18 years, trials published before 1988, as well as editorials, letters, protocol articles, and comments.
Two authors screened titles and abstracts for eligibility using the predefined inclusion and exclusion criteria.
The primary endpoint was the opioid-sparing effect of the active interventions within 0 to 24 hours postoperatively. Secondary endpoints were pain at rest and during mobilization at 6 and 24 hours postoperatively, opioid-related adverse effects, serious adverse events (SAEs), and length of stay (LOS).
Six authors extracted the data, assessed the full texts independently, and compared their findings afterward. We managed and compared risk of bias using Covidence (Covidence systematic review software; Veritas Health Innovation, Melbourne, Australia). We resolved disagreements by consensus.
We contacted the corresponding author for the trial by email to confirm or obtain data if data were missing, or we classified bias evaluation as unclear in one or more domains. We contacted the authors again after 2 weeks if they had not responded to our initial contact. We used open questions to prevent false confirmation of suggested measures in the answers.
We converted opioid consumption to intravenous (i.v.) morphine equivalents (Appendix 2, available at http://links.lww.com/PR9/A157) and pain scores, such as visual analog scale (VAS) 0 to 10 and numerical rating scale (NRS) 0 to 10, to a 0 to 100 VAS scale. For trials with several treatment arms, we combined mean values and SDs in the intervention groups.26 Furthermore, we converted median and interquartile range values to mean and SDs using the method described by Hozo et al.28 We calculated the risk ratio (RR) with a 95% confidence interval (CI) for dichotomous data.
Two authors performed bias assessment by using Cochrane's 7-step risk of bias tool.29
2.1. Statistical analyses
We performed meta-analyses and sensitivity analyses using Review Manager provided by Cochrane (RevMan version 5.4.1) whenever 3 or more trials reported the preplanned outcomes for continuous data regarding pain, opioid consumption, and postoperative nausea and vomiting (PONV). For the overall assessment of overall significance, we used the procedure suggested by Jakobsen et al.30 We applied the trial sequential analysis (TSA) (computer program) version 0.9.5.10 Beta (Copenhagen Trial Unit, Center for Clinical Intervention Research, Rigshospitalet, Copenhagen, Denmark).70
We assessed the heterogeneity between trials by I2, which quantifies the observed differences and D2 for information size adjustments in the trial sequential analyses.70 Additionally, we inspected the forest plots visually for statistical heterogeneity.
We used sensitivity analyses to explore whether the choice of summary statistics and choices made through the review process, such as selection of event category, were critical for the conclusions of the meta-analysis. To control for random errors, we performed TSA for the primary and secondary outcomes dealing with pain intensity, and we calculated and visualized the diversity-adjusted required information size (DARIS) and the cumulative Z-curve. It was not possible to perform TSA if the accrued information size was <5% or the data were insufficient. We calculated RR for dichotomous data in the presence of interventions of 3 or more trials, with a 95% CI. We considered in both dichotomous and continuous data that, P <0.05 was statistically significant. We performed funnel plots if 10 or more trials were included in the meta-analysis and assessed the presence of heterogeneity by using the magnitude by I2 and forest plots.27
To detect a minimal clinical relevant effect, we chose to detect even a small beneficial effect. Therefore, a mean difference was set to 10 mg morphine i.v. equivalents per 24 hours for opioid consumption and 10 mm on a VAS (0–100 mm) scale for pain scores at 6 and 24 hours.42,50
We used Grading of Recommendations, Assessment, Development, and Evaluation (GRADEpro GDT) to assess the certainty of evidence.23
3. Results
From the literature search, we identified 25,001 trials. First, Covidence removed 4239 duplicates, and after the abstract and full-text screening, we removed 20,080 trials. Furthermore, we excluded trials dealing with spine surgery not related to spinal fusion, 409 trials were full-text screened, ending up with a total exclusion of 364 trials. Hence, 44 trials remained for the final data extraction randomizing 2983 participants1–3,5–7,11–15,18,19,21,22,24,25,29,32,33,38–41,43,44,52,53,55–57,59–62,64–66,68,69,71–74 (Fig. 1).
Figure 1.
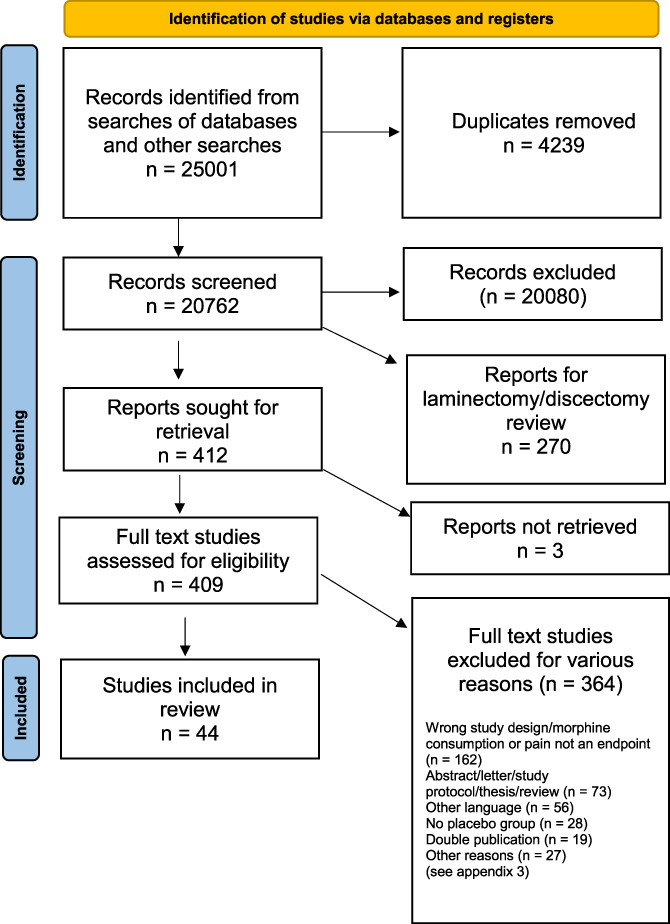
Flow diagram.
For subgroup analyses, we identified 5 groups, which included 3 or more trials: nonsteroidal anti-inflammatory drugs (NSAIDs),3,55,59,62,71 epidural analgesia,2,7,21,32,60 ketamine infusion,1,5,24,41,53,64,66 local infiltration analgesia,6,22,44,61 and intrathecal (i.t.) morphine.12,14,68,74 The remaining studies11,13,15,18,19,25,29,33,38–40,43,52,56,57,65,69,72,73 reported 12 different interventions, including 4 studies that reported on pregabalin but did not have comparable outcomes. For baseline variables, see Table 1.
Table 1.
Study information.
| Author | Basic analgesic regimen all groups | Type of supplemental analgesics | Analgesics in intervention and control groups Type, dose, volume, time points, and type of administration |
|---|---|---|---|
| Abrishamkar, 2012 | Routine analgesic protocol | Morphine s.c. VAS > 4 | 1: (n = 22) ketamine 0.5 mg/kg/h i.v. Control: (n = 23) morphine s.c. |
| Aglio, 2018 | None | Hydromorphone i.v. | 1: (n = 33) hydromorphone 0.5 mg; epidural preoperatively 2: (n = 34) bupivacaine 31.25 mg and hydromorphone 0.5 mg; epidural preoperatively Control: (n = 32) saline 10 mL; epidural preoperatively |
| Aubrun, 2000 | Propacetamol 2 g p.o. every 6 hours. | Morphine i.v. | 1: (n = 25) ketaprofen 100 mg i.v. at the end of surgical procedure Control: (n = 25) dextrose |
| Brinck, 2020 | i.v. paracetamol | PCA oxycodone | 1: (n = 65) ketamine bolus pre-incisional (0.5 mg/kg), followed by S-ketamine infusion of 0.12 mg/kg/h 2: (n = 62) ketamine bolus pre-incisional (0.5 mg/kg), followed by S-ketamine infusion of 0.6 mg/kg/h Control: (n = 62) matching saline pre-incisional |
| Brown, 2018 | PCA morphine | 1: (n = 24) liposomal bupivacaine 266 mg, 60 mL before wound closure; local anaesthetic Control: (n = 26) saline 60 mL before wound closure; local anaesthetic |
|
| Choi, 2014 | Premedicated with acetaminophen 1,000 mg and gabapentin 600 mg PO After surgery, acetaminophen 1,000 mg every 6 hours and oral gabapentin 200 mg every 8 hours. |
PCA hydromorphone | 1: (n = 20) hydromorphone + bupivacaine 0.6 mg bolus (hydromorphone) Bupi + hydromorphone 15 μg 6 mL/h 0.1%; epidural at PACU Control: (n = 18) matching saline; epidural at PACU |
| Dehkordy, 2020 | Paracetamol 1 gr | PCA morphine per demand meperidine 50 mg rescue agent | 1: (n = 40) magnesium i.v. 50 mg/kg bolus followed by a continuous 15 mg/kg/h infusion. Before induction + during surgery Control: (n = 40) matching saline |
| Dhaliwal, 2019 | Acetaminophen, oxycodone, codein, morphine i.v. | PCA morphine | 1: (n = 74) morphine 0.2 mg, 0.4 mL saline before wound closure; spinal Control: (n = 76) matching saline |
| Firouzian, 2018 | None | PCA morphine | 1: (n = 40) naloxone 20 μg + morphine 0.2 mg i.t.; end of surgery Control: (n = 37) morphine 0.2 mg i.t.; end of surgery |
| France, 1997 | None | PCA opioids | 1: (n = 42) duramorph injection 0.011 mg/kg; 30 minutes before surgery Control: (n = 26) matching saline |
| Fujita, 2016 | Indomethacin sup. (50 mg, first choice) pentazocine hydrochloride (15 mg IM, second choice) | PCA morphine | 1: (n = 30) pregabalin 75 mg, 2 hours Prior to surgery 2: (n = 30) pregabalin 150 mg, 2 hours before surgery Control: (n = 29) diazepam 5 mg, 2 hours before surgery |
| Ghabach, 2019 | Paracetamol 1 g every 8 hours and ketoprofen 50 mg every 12 hours i.v. | Sufentanil i.v. 5 mg to reach a VAS score <4 Meperidine 50 mg IM (VAS score 4). |
1: (n = 14) ropivacaine 0.5% 10 mL before wound closure; sponge Control: (n = 16) saline 10 mL before wound closure sponge |
| Ghamry, 2019 | Paracetamol i.v. 1 g per 6 hours, Ketorolac 30 mg loading dose then 15 mg per 8 hours. | Morphine 0.1 mg/kg i.v. (VAS >30) | 1: (n = 30) bupivacaine 0.25%, 20 mL erector spinae block Control: (n = 30) none |
| Gottschalk, 2004 | None | PCA pirimidine | 1: (n = 13) ropivacaine 0.1% 12 mL/hr during surgery; epidural postoperatively Control: (n = 13) matching saline; epidural postoperatively |
| Greze, 2017 | Acetaminophen (1 g x 4 daily), ketoprofen (100 mg x 2 daily) nefopam (20 mg x 4 daily) | PCA morphine | 1: (n = 19) ropivacaine 10 mL bolus + 8 mL/h for 48 hours; end of surgery; wound infiltration Control: matching saline; wound infiltration |
| Hadi, 2010 | None | PCA morphine | 1: (n = 15) ketamine i.v. 1 µg/kg/min; during surgery Control: (n = 15) none |
| Martí/Hernandez-Palazón, 2001 | None | PCA morphine | 1: (n = 21) propacetamol 2 g i.v. every 6 hours; during a period of 72 hours. Control: (n = 21) matching saline |
| Ibrahim, 2018 | Ketorolac 30 mg i.v. and paracetamol 1 g injection for 8 hours | Morphine i.v. VAS was ≥4, or by request | 1: (n = 20) lidocaine i.v. loading before incision then 3 mg/kg/h; during surgery Control: (n = 20) matching saline |
| Kang, 2013 | None | PCA fentanyl | 1: (n = 32) ropivacaine 0.1% 10 mL 20 minutes; before skin incision; epidural Control: (n = 34) matching saline |
| Kawamata, 2005 | Pre-med: 3 mg i.m. midazolam. Post-med: 200 μg i.v. buprenorphine at 1 mL/h rate s.c. | Flurbiprofen 50 mg i.v. | 1: (n = 16) buprenorphine 1.2 + 1 mg droperidol, total 48 mL, 1 mL/h for 48 hours after surgery; continuous s.c. infusion Control: (n = 17) buprenorphine 0.6 mg + droperidol 1 mg, total 48 mL, 1 mL/h for 48 hours after surgery continuous s.c. infusion |
| Kien, 2019 | None | Morphine 2 mg every 3 minutes Until VAS <4 PCA morphine rescue analgesia with fentanyl |
1: (n = 30) pregabalin 150 mg P.O., celecoxib 200 mg P.O., 2 hours before surgery Control: (n = 30) placebo |
| Kim, 2011 | None | PCA fentanyl ketorolac 120 mg, ketorolac 30 mg i.v. VAS >5 | 1: (n = 18) pregabalin 75 mg P.O. 1 hour before surgery 2: (n = 17) pregabalin 50 mg P.O. 1 hour before surgery Control: (n = 17) placebo |
| Kim, 2013 | Ketorolac 30 mg i.v. 10 minutes before skin closure | i.v. morphine | 1: (n = 32) ketamine i.v. infusion of 1 μg/kg/min after bolus 0.5 mg/kg, before skin incision + continued 48 hours postoperatively 2: (n = 32) ketamine 2 μg/kg/min after bolus 0.5 mg/kg before skin incision + continued 48 hours postoperatively Control: (n = 32) matching saline |
| Kim, 2016 | None | PCA morphine | 1: (n = 40) celecoxib 200 mg, pregabalin 75 mg, acetaminophen 500 mg, extended-release oxycodone 10 mg 1 hour preop + twice daily Control: (n = 40) morphine i.v. |
| Levaux, 2003 | Piritramide just before wound closure | PCA piritramide 1 mg piritramide bolus until pain free in emergence |
1: (n = 12) magnesium 50 mg/kg i.v. preoperatively Control: (n = 12) saline i.v. preoperatively |
| Li, 2019 | Ropivacaine 0.5% 20 mL 5 minutes Before incision | PCA morphine | 1: (n = 29) dexmedetomidine 20 mL, 0.5% ropivacaine 1 µg/kg dexmedetomidine 5 minutes before incision Control: (n = 28) 20 mL 0.5% ropivacaine 5 minutes before incision |
| Oh, 2019 | None | PCA fentanyl Hydromorphone 6 mg and nefopam 100 mg |
1: (n = 43) rocuronium 2 mg/mL diluted in 0.9% isotonic saline and started at 15 mL/hr Control: (n = 40) none |
| Pinar, 2017 | Lyrica 150 mg Preop PCM 1 g i.v. per 6 hours |
PCA morphine | 1: (n = 21) pregabalin 150 mg 1 hour preop and ibuprofen 300 mg 30 minutes preoperatively Control: (n = 21) pregabalin 150 mg 1 hour preoperatively |
| Quinlan, 2017 | None | Hydromorphone i.v. | 1: (n = 74) 1 L of crushed ice every 4 hours postoperatively applied to the lower back for 20 minutes Control: (n = 74) none |
| Raja, 2019 | Paracetamol 1 g i.v., dexamethasone 8 mg i.v. after skin incision; postop: paracetamol 1 g i.v. every 6 hours, ketorolac 30 mg every 8 hours, pregabalin P.O. 75 mg | PCA morphine | 1: Paracetamol 1 g, ketorolac 20 mg, pregebalin 75 mg P.O. 4 hours before surgery Control: (n = 50) none |
| Reuben, 2006 | None | PCA, morphine | 1: (n = 20) celecoxib 400 mg + placebo capsule, 1 hour before induction; celecoxib 200 mg + placebo capsules, 12 hours after surgery. 2: (n = 20) pregabalin 150 mg + placebo capsules, 1 hour before induction; pregabalin 150 mg + placebo capsules, 12 hours after surgery 3: (n = 20) celecoxib 400 mg + pregabalin 150 mg 1 hour before induction; celecoxib 200 mg + pregabalin 150 mg, 12 hours after surgery Control: (n = 20) matching placebo capsula |
| Šervicl-kuchler, 2014 | Metamizole 2.5 g per 12 hours | PCA piritramide piritramide 3 mg i.v., VAS >4 | 1: (n = 25) levobupivacaine 0.125% 0.1 mL/kg/h after wound closure; epidural postoperatively Control: (n = 25) matching saline postoperatively |
| Singhatanadgige, 2020 | Celecoxib 400 mg pregabalin 75 mg, paracetamol 500 mg | PCA morphine | 1. (n = 40) bupivacaine 0.5%, 92.5 mg. (18.5 mL), ketorolac 30 mg (1 mL), morphine 5 mg(0.5 mL), and epinephrine 0.5 mg (0.5 mL); end of surgery; wound infiltration Control: (n = 40) bupivacain, ketorolac, epinephrine; end of surgery; wound infiltration |
| Siribumrungwong, 2015 | Paracetamol 500 mg P.O. | i.v. morphine | 1: (n = 32) parecoxib 40 mg i.v. 30 minutes before surgery 2: (n = 32) keterolac 30 mg i.v. 30 minutes before surgery Control: (n = 32) matching saline |
| Song, 2013 | None | Fentanyl 0.5 µg/kg i.v. 20 minutes before wound closure +2 mL/hr; fentanyl i.v. postoperatively; postop: PCA fentanyl (2 mL on demand) postoperatively + 25 mg meperidine i.v. VAS >40 or requested | 1: (n = 24) ketamine 0.3 mg/kg before surgery +3 mg/kg mixed to i.v. PCA on demand in PACU, after induction + postoperatively Control: (n = 25) matching saline |
| Subramaniam, 2011 | None | PCA hydromorphone epidural bupivacaine | 1: (n = 15) ketamine bolus 0.15 mg/kg at induction and continued on 2 mg/kg/min infusion intraoperatively and postoperatively for 24 hours Control: (n = 15) saline bolus at induction and continued as i.v. infusion for 24 hours |
| Urban, 2008 | None | Perop: spinal morphine before wound closure postop: PCA hydromorphone ketamine if NRS = 10 | 1: (n = 12) ketamine i.v. 0.2 mg/kg at induction of GA and 2 μg/kg/h until discharge from PACU Control: (n = 12) none |
| Urban, 2018 | Acetaminophen | PCA hydromorphone | 1: (n = 43) pregabalin 150 mg po, 1hour prior to surgery Control: (n = 43) placebo capsula po, 1 hour prior to surgery |
| Wang, 2020 | Diclofenac 50 mg supp. Parecoxib 50 mg i.v. | PCA sufentanil | 1: (n = 44) 0.2 mg of morphine, 2 mL of saline, 30 minutes before anesthesia induction i.t. |
| Wen, 2016 | None | PCA sufentanil | 1: (n = 20) dezocine 0.1 mg/kg i.v. 5 minutes before suturing the skin 2: (n = 20) dezocine 0.15 mg/kg i.v. 5 minutes before suturing skin 3: (n = 20) dezocine 0.20 mg/kg i.v. 5 minutes before suturing skin Control: (n = 20) matching saline |
| Yamashita, 2006 | None | PCA morphine morphine i.v. 0.1 mg/kg during surgery | 1: (n = 12) flurbiprofen 1 mg/kg i.v. before surgery 2: (n = 12) flurbiprofen 1 mg/kg i.v. after surgery Control: (n = 12) placebo |
| Yeom, 2012 | None | Postop: 1 μg/kg fentanyl i.v. Loading dose + i.v. fentanyl 0.4 μg/kg/mL at 1 mL/h PCA fentanyl |
1: (n = 20) sevoflurane-nitrous oxideoxygen, thiopental sodium 4–5 mg/kg, rocuronium 0.6–0.7 mg/kg maintained with sevoflurane and 50% nitrous oxide in oxygen (3 L/min); before and during surgery; i.v. and inhalation 2: (n = 20) sevoflurane-remifentanil-nitrous oxide-oxygen, thiopental sodium 4–5 mg/kg, rocuronium 0.6–0.7 mg/kg, remifentanil infusion, and sevoflurane inhalation was maintained with sevoflurane, remifentani infusion, and 50% nitrous oxide in oxygen (3 L/min); before and during surgery; i.v. + inhalation 3: (n = 20) propofol-remifentanil-oxygen) propopol and remifentanil infusion, rocuronium 0.6–0.7 mg/kg, anesthesia was maintained with propofol, remifentanil and 50% oxygen (3 L/min); before and during surgery; i.v. + inhalation |
| Zhang, 2020 | Flurbiprofen 1.5 mg/kg at end of surgery | PCA sufentanil NRS > 40 | 1: (n = 30) ropivacaine 0.4% 20 mL, erector spinae block Control: (n = 30) sham block |
| Ziegler, 2008 | Diclofenac 100 mg supp. | PCA piritramide | 1: (n = 23) morphine 0.4 mg before wound closure i.t. Control: (n = 23) matching saline |
i.t., intrathecal; i.v. intravenous; PCA, patient-controlled analgesia; NRS, numerical rating scale; VAS, visual analog scale.
Of the included 44 trials, 38 contained one or more unclear domains, which we addressed by emailing the corresponding authors twice. However, in 6 trials, the corresponding author had left no email address, and 7 email addresses were out of order. Finally, 3 authors answered our questions.
The summarized bias was high in 11, unclear in 26, and low in 7 trials (Fig. 2). Regarding the trial sample size, 32 trials implicated moderate risk of bias and 13 trials implicated high risk of bias.
Figure 2.

Summarized risk of bias.
We changed the original plan to use the most conservative effect estimate regarding random or fixed effect when performing TSA when inspecting the data because considerable heterogeneity was detected between the studies. Therefore, we chose random-effects models to accommodate that.
3.1. Supplemental analgesics
Fifteen trials reported that patients postoperatively were provided with patient-controlled analgesia with morphine, and in 6 cases, the morphine was solely administrated as i.v. or s.c. In 22 cases, patients had a patient-controlled analgesia device with hydromorphone, oxycodone, meperidine, piritramide, sufentanil, pirimidine, or fentanyl. In one study, the patients had flurbiprofen at request. Thirty-five trials reported total opioid consumption but not all after 24 ± 4 hours postoperatively.
Regarding the primary analgesic treatment provided for the patients postoperatively, 14 trials administrated acetaminophen as i.v. or orally, 8 trials administrated different kinds of NSAIDs, 4 studies administrated pregabalin or gabapentin, 3 trials used other analgesics. In 7 trials, they combined analgesics, eg, acetaminophen and ketorolac or pregabalin.
3.2. Pain ratings
The majority of the included studies used NRS (0–10, 0 is no pain, and 10 is worst imaginable pain) or VAS (0–10 cm, or 0–100 mm, where 0 is no pain and 10/100 is the worst imaginable pain. Thirty-one trials reported pain at rest at 6 ±2 hours ranging from VAS 14–63 mm, mean 33 mm for intervention groups, and VAS 15–69 mm, mean 45 mm for control groups. Thirty-eight studies reported pain at rest after 24 ±4 hours ranging from VAS 6–53 mm, mean 31 mm for intervention groups and 14–57 mm, mean 39 mm for control groups. For pain during mobilization at 6 hours, 8 studies reported on VAS outcomes ranging from 17 to 71 mm, mean 46 mm for interventions and VAS 32–79 mm, mean 57 mm for control groups. Pain during mobilization was reported after 24 hours postoperatively by 12 studies, with VAS ranging from 12 to 69 mm, mean 42 mm for intervention groups and 15 to 80 mm, mean 46 mm for control groups (Table 1).
3.3. Adverse events and other outcomes
Twenty-nine trials included patients with chronic pain and daily opioid consumption, 13 trials accepted pain but excluded preoperatively opioid consumption, 2 trials did not mention preoperatively pain or opioid consumption.
Twelve trials reported on LOS. PONV were reported in 20 trials, also separately as nausea (16 trials) and vomiting (7 trials). Dizziness, sedation, and pruritus were reported in 10, 9, and 11 trials, respectively. Furthermore, headache, shivering, paresthesia, hematoma, infection, hallucinations, visual disturbance, confusion, urine retention, and constipation were reported. None of the studies reported SAE.
3.4. Subgroup analysis
3.4.1. Nonsteroidal anti-inflammatory drugs
Eight trials reported on NSAIDs as an intervention,3,38,40,55,57,59,62,71 3 studies in combination with other analgesics.38,40,57 The risk of bias for all trials was low in one trial, unclear in 5 trials, and high in 2 trials (Fig. 2).
3.4.2. Opioid consumption 0 to 24 hours
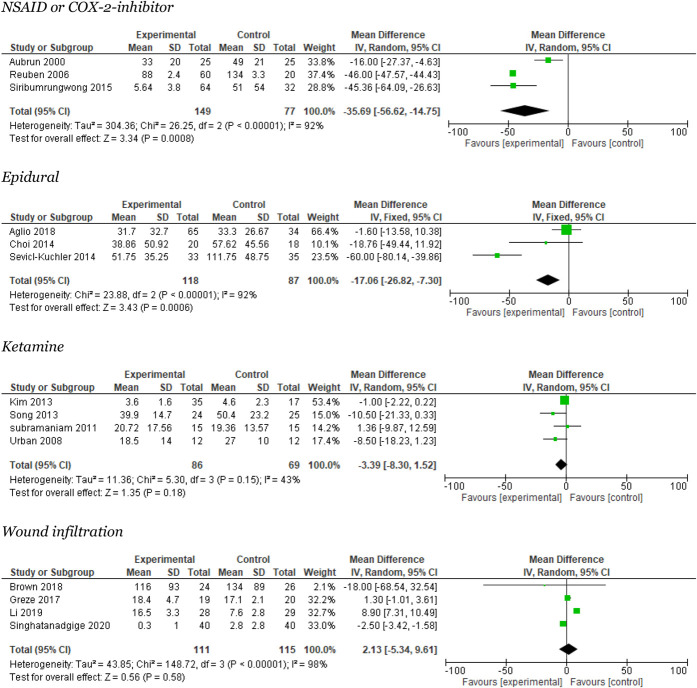
Three trials reported 0- to 24-hour opioid consumption3,59,62 (Fig. 3). The meta-analysis reported a significant reduction in opioid consumption of 35.7 mg i.v. (95% CI: 15–57 mg/24 hours), with large heterogeneity (I2 = 92%). Trial sequential analysis showed that neither the required information size nor the DARIS was crossed or reached (Appendix 3, available at http://links.lww.com/PR9/A157). The quality of evidence (GRADE) was very low (Table 2).
Figure 3.
Meta-analyses for 0 to 24 hours opioid consumption.
Table 2.
Summarized outcomes in Grading of Recommendations Assessment, Development, and Evaluation (GRADE) (mean difference and 95% confidence interval are provided together with quality of evidence).
| NSAID compared with placebo for pain after spinal fusion surgery? | |||||
|
Patient or population: pain after spinal fusion Setting: the immediate postoperative period Intervention: NSAID Comparison: placebo | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with placebo | Risk with NSAID | ||||
| PONV assessed with: numbers of events | 455 per 1.000 | 350 per 1.000 (255–477) | RR 0.77 (0.56–1.05) | 226 (3 RCTs) |
 Moderate |
| Morphine consumption assessed with 0–24 hours postoperatively assessed with: mg | The mean morphine consumption assessed with 0–24 hours postoperatively was 0 | MD 9.05 lower (80.63 lower–62.53 higher) | — | 296 (4 RCTs) |
 Very low |
| Pain score 4–8 hours postoperatively at rest assessed with: VAS 0–100 mm | The mean pain score 4–8 hours postoperatively at rest was 0 | MD 11.29 lower (15.48 lower–7.1 lower) | — | 292 (5 RCTs) |
 Very low |
| Sedation assessed with: number of events | 511 per 1.000 | 302 per 1.000 (194–465) | RR 0.59 (0.38–0.91) | 130 (2 RCTs) |
 Low |
| Pain score 20–24 hours postoperatively at rest assessed with: VAS 0–100 mm | The mean pain score 20–24 hours postoperatively at rest was 0 | MD 7.24 lower (17.15 lower–2.66 higher) | — | 242 (4 RCTs) |
 Very low |
| Dizziness assessed with: number of events | 212 per 1.000 | 186 per 1.000 (99–351) | RR 0.88 (0.47–1.66) | 176 (2 RCTs) |
 Moderate |
| Pruritus | 167 per 1.000 | 180 per 1.000 (93–345) | RR 1.08 (0.56–2.07) | 166 (2 RCTs) |
 Low |
| EPI compared with control for pain after spinal fusion surgery? | |||||
|
Patient or population: pain after spinal fusion Setting: the immediate postoperative period Intervention: EPI Comparison: placebo | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with placebo | Risk with EPI | ||||
| Opioid consumption | The mean opioid consumption was 0 | MD 17.06 lower (26.82 lower–7.3 lower) | — | 205 (3 RCTs) |
 Very low |
| PONV | 296 per 1.000 | 207 per 1.000 (124–337) | RR 0.70 (0.42–1.14) | 198 (4 RCTs) |
 Moderate |
| 24 hours pain at rest | The mean 24 hours pain at rest was 0 | MD 17.19 lower (24.55 lower–9.82 lower) | — | 160 (3 RCTs) |
 Low |
| Pruritus | 667 per 1.000 | 0 per 1.000 (0–0) | Not estimable | 38 (1 RCT) | — |
| Ketamine compared with placebo for pain after spinal fusion surgery? | |||||
|
Patient or population: pain after spinal fusion Setting: the immediate postoperative period Intervention: Ketamine Comparison: placebo | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with placebo | Risk with ketamine | ||||
| Opioid consumption | The mean opioid consumption was 0 | MD 3.39 lower (8.3 lower–1.52 higher) | — | 155 (4 RCTs) |
 Low |
| 6 hours pain at rest | The mean 6 hours pain at rest was 0 | MD 3.19 higher (24.37 lower–30.75 higher) | — | 365 (5 RCTs) |
 Very low |
| 6 hours pain during mob | The mean 6 hours pain during mob was 0 | MD 3.99 lower (11.58 lower–3.6 higher) | — | 131 (3 RCTs) |
 Moderate |
| 24 hours pain at rest | The mean 24 hours pain at rest was 0 | MD 13.32 lower (17.02 lower–9.62 lower) | — | 389 (6 RCTs) |
 Very low |
| 24 pain during mob | The mean 24 pain during mob was 0 | MD 5.16 lower (14.31 lower–3.99 higher) | — | 103 (3 RCTs) |
 Low |
| PONV | 364 per 1.000 | 360 per 1.000 (276–465) | RR 0.99 (0.76–1.28) | 390 (6 RCTs) |
 Very low |
| Dizziness | 140 per 1.000 | 202 per 1.000 (91–446) | RR 1.44 (0.65–3.18) | 115 (3 RCTs) |
 Moderate |
| Wound infiltration compared with placebo for pain after spinal fusion surgery? | |||||
|
Patient or population: pain after spinal fusion Setting: the immediate postoperative period Intervention: Wound infil Comparison: placebo | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with placebo | Risk with wound infil | ||||
| Opioid consumption | The mean opioid consumption was 0 | MD 2.13 higher (5.34 lower–9.61 higher) | — | 226 (4 RCTs) |
 Low |
| PONV | 338 per 1.000 | 0 per 1.000 (0–0) | Not estimable | 137 (2 RCTs) |
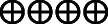 High |
| Pruritus | 0 per 1.000 | 0 per 1.000 (0–0) | Not estimable | 57 (1 RCT) | — |
| 24 hours pain at rest | The mean 24 hours pain at rest was 0 | MD 2.84 higher (5.25 lower–10.93 higher) | — | 146 (3 RCTs) |
 Low |
| Morphine compared with placebo for pain after spinal fusion surgery? | |||||
|
Patient or population: pain after spinal fusion surgery Setting: the immediate postoperative period Intervention: morphine Comparison: placebo | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with placebo | Risk with morphine | ||||
| PONV | 465 per 1.000 | 0 per 1.000 (0–0) | Not estimable | 283 (3 RCTs) |
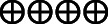 High |
| 6 hours pain at rest | The mean 6 hours pain at rest was 0 | MD 11.82 lower (17.29 lower–6.35 lower) | — | 283 (3 RCTs) |
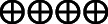 High |
| 6 hours pain during mob | The mean 6 hours pain during mob was 0 | MD 8.98 lower (14.99 lower–2.96 lower) | — | 283 (3 RCTs) |
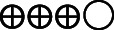 Moderate |
| 24 hours pain at rest | The mean 24 hours pain at rest was 0 | MD 9.58 lower (19.13 lower–0.04 lower) | — | 283 (3 RCTs) |
 Moderate |
| 24 hours pain during mob | The mean 24 hours pain during mob was 0 | MD 9.42 lower (18.09 lower–0.75 lower) | — | 283 (3 RCTs) |
 Moderate |
| Pruritus | 402 per 1.000 | 0 per 1.000 (0–0) | Not estimable | 275 (4 RCTs) |
 Low |
GRADE Working Group grades of evidence: (1) High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. (2) Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. (3) Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. (4) Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect.
The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CI, confidence interval; infil, infiltration; mob, mobilization; NSAID, nonsteroidal anti-inflammatory drug; OR, odds ratio; PONV, postoperative nausea and vomiting; RCT, randomized controlled trials; RR, risk ratio; VAS, visual analog scale.
3.4.3. Pain at rest after 6 hours
Four trials reported on NSAIDs and postoperative pain at rest after 6 ± 2 hours.3,59,62,71 The meta-analysis found a significant reduction of 12 mm in mean VAS score (95% CI: 6–17.5). Heterogeneity was moderate I2 = 65% (Appendix 4, available at http://links.lww.com/PR9/A157). Trial sequential analysis showed that the required information size was not reached, but the DARIS line was crossed (Appendix 3, available at http://links.lww.com/PR9/A157). The quality of evidence (GRADE) was very low (Table 2).
3.4.4. Pain at rest after 24 hours
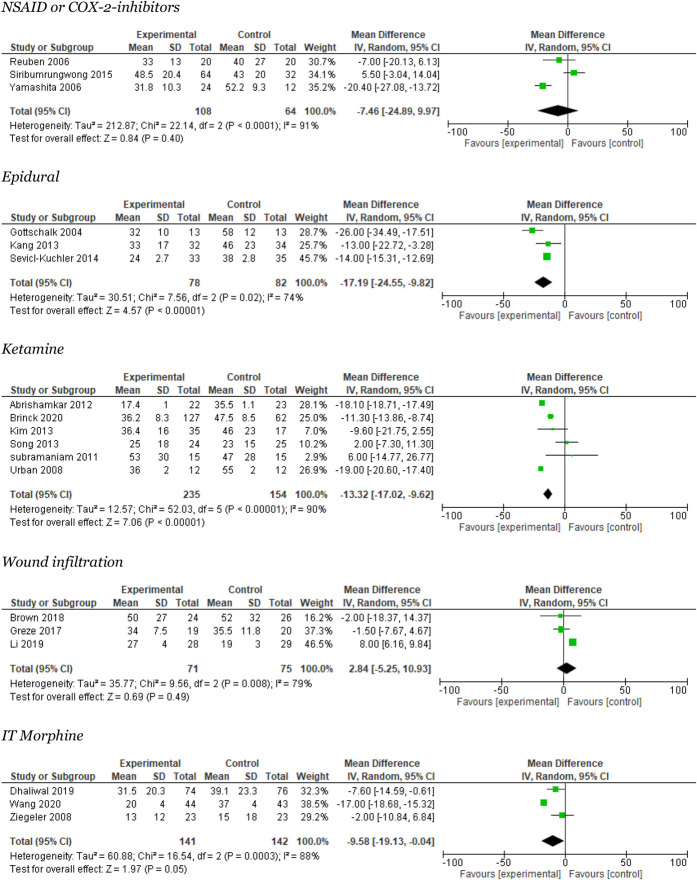
Three trials reported on NSAIDs and postoperative pain at rest after 24 ± 4 hours.59,62,71 The meta-analysis found a nonsignificant reduction of 7.5 mm in VAS score (95% CI: 10–25). The heterogeneity was large, I2 = 91% (Fig. 4). Trial sequential analysis showed that neither was the required information size reached nor was the DARIS line crossed or reached (Appendix 3, available at http://links.lww.com/PR9/A157). The quality of evidence (GRADE) was very low (Table 2).
Figure 4.
Meta-analyses for 24 hours pain rest.
3.4.5. Adverse events
Three trials reported on PONV.3,59,62 The meta-analysis found no significant difference between groups, RR 0.79 (95% CI: 0.54–1.17) with moderate heterogeneity I2 = 58% (Appendix 5, available at http://links.lww.com/PR9/A157). Quality of evidence (GRADE) was moderate. Two trials reported on sedation,3,59 2 on dizziness,59,62 and 1 on pruritus.62
3.5. Epidural
Five trials reported on epidural as an intervention.2,7,21,32,60 Two trials reported on bupivacaine with hydromorphone,2,7 one trial on ropivacaine,21 and 2 trails on levobupivacaine.60 The risk of bias for all trials was unclear in 3 trials, and 2 trials had high risk of bias (Fig. 2).
3.5.1. Opioid consumption 0–24 hours
Three trials reported opioid consumption.21,32,60 The meta-analysis reported a mean reduction of 17 mg i.v. (95% CI: 7–27 mg per 24 hours), with large heterogeneity I2 = 92% (Fig. 3). Trial sequential analysis was not possible to perform. The quality of evidence (GRADE) was very low (Table 2).
3.5.2. Pain at rest after 24 hours
Three trials reported on epidural and postoperative pain at rest after 24 ± 4 hours.21,32,60 The meta-analysis found a significant reduction of −17.2 mm in mean VAS (95% CI: −25 to 10) with moderate heterogeneity of I2 = 74% (Fig. 4). Trial sequential analysis showed that the required information size was not reached, but the DARIS line was crossed (Appendix 4, available at http://links.lww.com/PR9/A157). The quality of evidence (GRADE) was low (Table 2).
No trials reported on pain after 6 hours during rest or mobilization, and no studies were detected dealing with pain during mobilization after 24 hours.
3.5.3. Adverse events
Four trials reported on PONV.7,21,32,60 The meta-analysis found no significant difference between groups, RR 0.70 (95% CI: 0.42–1.14), with moderate heterogeneity I2 = 60% (Appendix 5, available at http://links.lww.com/PR9/A157).
When performing sensitivity analyses, we found a significant difference, P = 0.02 (only in 2 trials). Quality of evidence (GRADE) was moderate (Table 2). One trial reported on pruritus.68
3.6. Ketamine
Seven trials reported on ketamine as an intervention.1,5,24,41,53,64,66 The risk of bias for all trials was low in 2 trials, unclear in 2 trials, and high in 3 trials (Fig. 2).
3.6.1. Opioid consumption 0–24 hours
Four trials reported opioid consumption.41,53,64,66 The meta-analysis reported no significant reduction in opioid consumption 3 mg i.v.. for 24 hours (95% CI: 1.5–8) with moderate heterogeneity I2 = 43% (Fig. 3). Trial sequential analysis showed that the required information size was not reached, and the DARIS line was not crossed (Appendix 7, available at http://links.lww.com/PR9/A157). The quality of evidence (GRADE) was low (Table 2).
3.6.2. Pain at rest after 6 hours
Five trials reported on ketamine and postoperative pain at rest after 6 ± 2 hours.1,7,44,59,72 The meta-analysis showed no significant difference in overall effect in mean VAS 3 mm (95% CI: −24 to 31). The heterogeneity was high, I2 = 99% (Fig. 4). Trial sequential analysis showed that neither was the required information size reached nor was the DARIS line crossed or reached (Appendix 7, available at http://links.lww.com/PR9/A157). Quality of evidence (GRADE) was low (Table 2).
3.6.3. Pain during mobilization after 6 hours
Three trials reported on ketamine and postoperative pain at mobilization 6 ± 2 hours.44,59,73 The meta-analysis showed no significant difference in mean VAS 4 mm (95% CI: 4–12), heterogeneity I2 = 0% (Appendix 8, available at http://links.lww.com/PR9/A157). Trial sequential analysis showed neither was the required information size reached nor was the DARIS line crossed or reached (Appendix 7, available at http://links.lww.com/PR9/A157). The quality of evidence (GRADE) was moderate (Table 2).
3.6.4. Pain at rest after 24 hours
Six trials reported on ketamine and postoperative pain at rest after 24 hours.1,5,41,53,64,66 The meta-analysis showed a significant difference between trials in favor of the experimental group of 13 mm in mean VAS (95% CI: 10–17). When performing sensitivity analyses, the meta-analysis was nonsignificant. We found large heterogeneity I2 = 90% (Fig. 4). The TSA showed that the required information size was not reached, but the DARIS line was crossed (Appendix 7, available at http://links.lww.com/PR9/A157). The quality of evidence (GRADE) was very low (Table 2).
3.6.5. Pain during mobilization after 24 hours
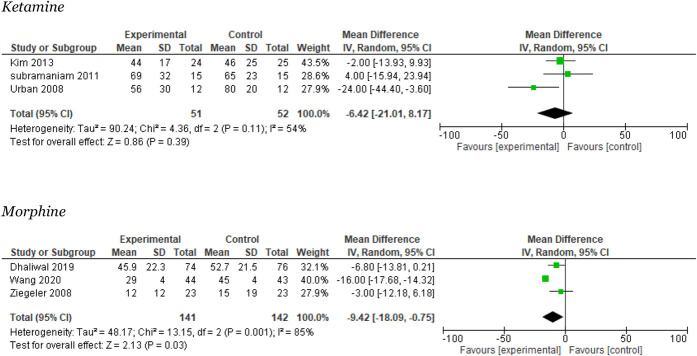
Three trials reported on pain during mobilization after 24 hours.41,64,66 The meta-analysis showed no significant difference between groups in mean VAS −6 mm (95% CI: −21 to 8), moderate heterogeneity I2 = 54% (Fig. 5). The TSA showed that the required information size was not reached, but the DARIS line was crossed (Appendix 7, available at http://links.lww.com/PR9/A157). The quality of evidence (GRADE) was low (Table 2).
Figure 5.
Meta-analyses for 24 hours pain during mobilization.
3.6.6. Adverse events
Six trials reported on PONV.1,5,41,53,64,66 The meta-analysis found no significant difference between groups, RR 0.99 (95% CI: 0.76–1.28) with low heterogeneity I2 = 12% (Appendix 5, available at http://links.lww.com/PR9/A157). The quality of evidence (GRADE) was very low (Table 2). Three trials reported on dizziness.1,41,64
3.7. Wound infiltration
Four trials reported on local infiltration/wound analgesia and opioid consumption.6,22,44,61 The risk of bias for all trials was low in 2 trials, unclear in 1 trial, and high in 1 trial (Fig. 2).
3.7.1. Opioid consumption 0 to 24 hours
Four trials reported on local infiltration/wound analgesia and 24-hour opioid consumption.6,22,44,61 The meta-analysis favored the control group and reported no significant reduction in opioid consumption 2 mg i.v. per 24 hours (95% CI: −5 to 10) with large heterogeneity I2 = 98% (Fig. 3). The TSA showed that the required information size was not reached, but the DARIS line was crossed (Appendix 9, available at http://links.lww.com/PR9/A157). The quality of evidence (GRADE) was low (Table 2).
3.7.2. Pain at rest after 24 hours
Three studies reported on this outcome.6,22,44 The meta-analysis favored the control group and showed no significant difference in the overall effect of 3 mm in mean VAS (95% CI: −5 to 11). The heterogeneity was moderate, I2 = 79% (Fig. 4). The TSA showed that the required information size was not reached, but the DARIS line was crossed (Appendix 9, available at http://links.lww.com/PR9/A157). The quality of evidence (GRADE) was low (Table 2).
No studies reported on pain at rest after 6 hours or pain during mobilization at 6 and 24 hours.
3.7.3. Adverse events
3.8. Intrathecal morphine
Four studies reported on i.t. morphine.12,14,68,74 The risk of bias for all trials was low in one trial, unclear in 2 trials, and high in one trial (Fig. 2).
3.8.1. Pain at rest after 6 hours
Three studies reported on this outcome.12,68,74 The meta-analysis favored the experimental group and showed a significant difference of 12 mm in overall effect mean VAS (95% CI: 6–17). The heterogeneity was moderate, I2 = 52% (Appendix 4, available at http://links.lww.com/PR9/A157). The TSA showed that the required information size was not reached, but the DARIS line crossed (Appendix 10, available at http://links.lww.com/PR9/A157). The quality of evidence (GRADE) was high (Table 2).
3.8.2. Pain during mobilization after 6 hours
Three studies reported on this outcome.12,68,74 The meta-analysis favored the experimental group and showed a significant difference in the overall effect of 9 mm in mean VAS (95% CI: 3–15). The heterogeneity was moderate, I2 = 55% (Appendix 8, available at http://links.lww.com/PR9/A157). The TSA showed that the required information size was not reached, but the DARIS line was crossed (Appendix 10, available at http://links.lww.com/PR9/A157). The quality of evidence (GRADE) was moderate (Table 2).
3.8.3. Pain at rest after 24 hours
Three studies reported on this outcome.12,68,74 The meta-analysis favored the experimental group and showed a significant difference in the overall effect of 10 mm in mean VAS (95% CI: 0.04–19). The heterogeneity was large, I2 = 88% (Fig. 4). The TSA showed that the required information size was not reached, but the DARIS line was crossed (Appendix 10, available at http://links.lww.com/PR9/A157). The quality of evidence (GRADE) was moderate (Table 2).
3.8.4. Pain during mobilization after 24 hours
Three studies reported on this outcome.12,68,74 The meta-analysis favored the experimental group and showed a significant difference in the overall effect of 9 mm in mean VAS (95% CI: 0.75–18). The heterogeneity was large, I2 = 85% (Fig. 5). The TSA showed that the required information size was not reached, but the DARIS line crossed (Appendix 10, available at http://links.lww.com/PR9/A157). The quality of evidence (GRADE) was moderate (Table 2).
3.8.5. Adverse events
Three studies reported on PONV.12,68,74 The meta-analysis favored the experimental group and showed no significant difference in the overall effect RR −0.03 (95% CI: −0.13 to 0.06). The heterogeneity was moderate, I2 = 45% (Appendix 5, available at http://links.lww.com/PR9/A157). The quality of evidence (GRADE) was high (Table 2). Four studies reported on pruritus.12,14,68,74
3.9. Qualitative analyses
Fifteen trials investigated other interventions: buprenorphine s.c.,33 bupivacaine block,19 cold therapy,56 dezocine,69 lidocaine infusion,29 magnesium,11,43 nalaxone,13 pregabalin,15,38,65 propacetamol,25 rocuronium,52 and ropivacaine.18,73 Three trials investigated different analgesic combinations.38,40,57 The risk of bias was low in one trial, unclear in 15 trials, and high in 3 trials.
From those, 10 trials demonstrated a significant effect on opioid consumption/supplemental analgesics11,15,18,19,25,29,33,38,39,73 and 12 studies on pain scores.11,13,15,18,19,25,29,33,38,40,57,73 Four trials demonstrated a significant reduction in opioid-related adverse events.13,39,65,69
4. Discussion
In this systematic review of pain management after 1- or 2-level spinal fusion surgery, we identified 5 significant subgroups dealing with the following analgesic treatment: NSAIDS, epidural, ketamine, wound infiltration, and i.t. morphine.
When applying meta-analyses and TSA, in summary, we found a significant reduction in opioid consumption for NSAIDs and epidural, and both groups achieved the minimal clinical important difference (MCID) of 10 mg. For 6 hours of pain at rest, we found a significant reduction in VAS for NSAID and i.t. morphine. Both groups achieved the MCID of 10 mm. Furthermore, we detected a significant reduction in VAS scores for pain at rest after 24 hours in the following groups: NSAID, epidural, ketamine and wound infiltration. The epidural and ketamine groups achieved MCID. We detected a significant reduction in VAS after 24 hours in pain during mobilization for i.t. morphine. No groups obtained MCID.
For adverse events, it was only possible to perform meta-analysis on PONV because very few studies reported on other types of adverse events, and no trials reported SAEs. Furthermore, it was impossible because of sparse data to report a reduced LOS regarding any analgesic treatment.
Former systematic reviews on postoperative pain and analgesics seem to focus on rare spinal procedures such as complex and major spine surgery, combining different surgery types. Our systematic review is, in our knowledge, the first to investigate the procedure-specific pain treatment for 1- or 2-level spinal fusion, a frequently performed surgical procedure.
Consequently, it was not possible to compare our findings to similar reviews. Reviews of pain treatment in mixed or complex spine surgery indicate that use of paracetamol, NSAIDs, i.v. ketamine infusion, epidural analgesia, and i.t. morphine decrease postoperative pain,45,67 similar to our findings. Unfortunately, they do not investigate opioid consumption. Our results indicate that wound infiltration seemed to favor the control groups for pain levels. That seemed not to be the case in a newer systematic review, which investigates all kinds of lumbar spine surgery. The authors found that the demand for opioids significantly reduced in patients who received wound infiltration.54 Therefore, to further elucidate whether the meta-analyses are relevant for 1- or 2-level spinal fusion patients, several large RCTs are needed.
Our review has several strengths. We performed a broad systematic and stringent search minimizing the risk of missing suitable trials. We published the protocol at PROSPERO in advance. We performed TSAs to reduce type 1 and 2 errors. We assessed all trials for risk of bias and used GRADE to evaluate the certainty of evidence.
This review also has limitations. The majority of the authors we contacted by email to account for the quality assessment did not answer. As a result, we could have rated some of the studies too hard hereby, affecting the GRADE evaluation. Because pain data often per se is nonparametric, it was necessary to perform the meta-analysis by converting median (interquartile range) to mean (SD) values, which could have affected the data. We found considerable heterogeneity between the included studies in sample size and within the analgesic groups such as NSAIDs (including COX-1 and COX-2) and the epidural group (with and without hydromorphone). However, it mirrors the pragmatism in the clinical field. For some regularly used analgesic groups (such as paracetamol), enough studies could not be identified, making it challenging to clarify the evidence on that particular area. According to GRADE, the certainty of evidence was very low or low for the majority of the eligible trials, and bias in most trials was unclear or high, keeping us from recommending any “golden” analgesic treatment.
The principles of multimodal analgesics used for postoperative pain have been the leading principle for years.34 Unfortunately, it is unclear which patients can benefit from which kind of analgesic combination.45,48 Before designating that, studies need to focus on decreasing patients' pain procedure-specific instead of performing RCTs, which primarily aims to demonstrate an effect of an analgesic intervention by using a patient population. Moreover, studies not only need to focus on average pain in groups but also on the individual patient's pain.16
Effective pain treatment aims to ensure a fast recovery for the patients and to provide an acceptable quality of life, the ability of ambulation, few adverse events from the analgesic treatment, and sufficient sleep.20,37,63 Therefore, future RCTs of postoperative pain treatment should measure pain at rest and during mobilization, measure the quality of sleep, the quality of life, and the opioid-related and intervention-specific adverse events.
5. Conclusion
The present systematic review of analgesic treatments for patients undergoing lumbar 1- or 2-level fusion surgery demonstrated that NSAIDs significantly reduce opioid consumption and pain at rest after 6 hours, epidural significantly reduces opioid consumption and pain at rest after 24 hours, i.t. morphine significantly reduces pain levels at 6 and 24 hours during rest and mobilization, and ketamine significantly reduces pain at rest after 24 hours. However, most of the included studies represent an unclear or high risk of bias and low or very low quality of evidence. Therefore, based on the current literature, it is not possible to identify any best-proven analgesic treatment for patients undergoing 1- or 2-level spinal fusion. We suggest that future studies should include large-scale RCTs combined with individual responder analyses to examine relevant clinical analgesic effectiveness.
Disclosures
The authors have no conflicts of interest to declare.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PR9/A157.
Acknowledgements
The authors would like to thank Peter Udby for his contribution in screening trials for eligibility and Mathias Maagaard for providing advice regarding the statistics.
Author contribution: Idea and study concept: A. Geisler; Study design: A. Geisler, J. Zachodnik, R. Bech-Azeddine; Data extraction: A. Geisler, J. Zachodnik, K. Køppen, R. Chakari, R. Bech-Azeddine; Data management: A. Geisler; Project management: A. Geisler, R. Bech-Azeddine; Preparation and submission of the manuscript: A. Geisler, R. Bech-Azeddine; Critical revision of manuscript: all authors.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painrpts.com).
Contributor Information
Josephine Zachodnik, Email: jzc@regionsjaelland.dk.
Kasper Køppen, Email: kasperkoeppen@hotmail.com.
Rehan Chakari, Email: rehan.chakari@gmail.com.
Rachid Bech-Azeddine, Email: rachid.bech-azeddine@regionh.dk.
References
- [1].Abrishamkar S, Eshraghi N, Feizi A, Talakoub R, Rafiei A, Rahmani P. Analgesic effects of ketamine infusion on postoperative pain after fusion and instrumentation of the lumbar spine: a prospective randomized clinical trial. Med Arh 2012;66:107–10. [DOI] [PubMed] [Google Scholar]
- [2].Aglio LS, Abd-El-Barr MM, Orhurhu V, Kim GY, Zhou J, Gugino LD, Crossley LJ, Gosnell JL, Chi JH, Groff MW. Preemptive analgesia for postoperative pain relief in thoracolumbosacral spine operations: a double-blind, placebo-controlled randomized trial. J Neurosurg Spine 2018;29:647–53. [DOI] [PubMed] [Google Scholar]
- [3].Aubrun F, Langeron O, Heitz D, Coriat P, Riou B. Randomised, placebo-controlled study of the postoperative analgesic effects of ketoprofen after spinal fusion surgery. Acta Anaesthesiol Scand 2000;44:934–9. [DOI] [PubMed] [Google Scholar]
- [4].Brill S, Ginosar Y, Davidson EM. Perioperative management of chronic pain patients with opioid dependency. Curr Opin Anaesthesiol 2006;19:325–31. [DOI] [PubMed] [Google Scholar]
- [5].Brinck ECV, Maisniemi K, Kankare J, Tielinen L, Tarkkila P, Kontinen V. Analgesic effect of intraoperative intravenous S-ketamine in opioid-naive patients after major lumbar fusion surgery is temporary and not dose-dependent: a randomized, double-blind, placebo-controlled clinical trial. Anesth Analg 2021;132:69–79. [DOI] [PubMed] [Google Scholar]
- [6].Brown L, Weir T, Shasti M, Yousaf O, Yousaf I, Tannous O, Koh E, Banagan K, Gelb D, Ludwig S. The Efficacy of liposomal bupivacaine in lumbar spine surgery. Int J Spine Surg 2018;12:434–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Choi S, Rampersaud YR, Chan VW, Persaud O, Koshkin A, Tumber P, Brull R. The addition of epidural local anesthetic to systemic multimodal analgesia following lumbar spinal fusion: a randomized controlled trial. Can J Anaesth 2014;61:330–9. [DOI] [PubMed] [Google Scholar]
- [8].Choice Pharma. Prospect. Available at: http://www.postoppain.org/frameset.htm. Accessed January 25, 2021. [Google Scholar]
- [9].Dahl JB, Mathiesen O, Kehlet H. An expert opinion on postoperative pain management, with special reference to new developments. Expert Opin Pharmacother 2010;11:2459–70. [DOI] [PubMed] [Google Scholar]
- [10].Dahl JB, Nielsen RV, Wetterslev J, Nikolajsen L, Hamunen K, Kontinen VK, Hansen MS, Kjer JJ, Mathiesen O, Scandinavian Postoperative Pain Alliance (ScaPAlli). Post-operative analgesic effects of paracetamol, NSAIDs, glucocorticoids, gabapentinoids and their combinations: a topical review. Acta Anaesthesiol Scand 2014;58:1165–81. [DOI] [PubMed] [Google Scholar]
- [11].Dehkordy ME, Tavanaei R, Younesi E, Khorasanizade S, Farsani HA, Oraee-Yazdani S. Effects of perioperative magnesium sulfate infusion on intraoperative blood loss and postoperative analgesia in patients undergoing posterior lumbar spinal fusion surgery: a randomized controlled trial. Clin Neurol Neurosurg 2020;196:105983. [DOI] [PubMed] [Google Scholar]
- [12].Dhaliwal P, Yavin D, Whittaker T, Hawboldt GS, Jewett GAE, Casha S, du Plessis S. Intrathecal morphine following lumbar fusion: a randomized, placebo-controlled trial. Neurosurgery 2019;85:189–98. [DOI] [PubMed] [Google Scholar]
- [13].Firouzian A Gholipour Baradari A Ehteshami S Zamani Kiasari A Shafizad M Shafiei S Younesi Rostami F Alipour A Ala S Darvishi-Khezri H Haddadi K.. The effect of ultra-low-dose intrathecal naloxone on pain intensity after lumbar laminectomy with spinal fusion: a randomized controlled trial. J Neurosurg Anesthesiol 2020;32:70–6. [DOI] [PubMed] [Google Scholar]
- [14].France JC, Jorgenson SS, Lowe TG, Dwyer AP. The use of intrathecal morphine for analgesia after posterolateral lumbar fusion: a prospective, double-blind, randomized study. Spine (Phila Pa 1976) 1997;22:2272–7. [DOI] [PubMed] [Google Scholar]
- [15].Fujita N, Tobe M, Tsukamoto N, Saito S, Obata H. A randomized placebo-controlled study of preoperative pregabalin for postoperative analgesia in patients with spinal surgery. J Clin Anesth 2016;31:149–53. [DOI] [PubMed] [Google Scholar]
- [16].Geisler A, Dahl JB, Karlsen AP, Persson E, Mathiesen O. Low degree of satisfactory individual pain relief in post-operative pain trials. Acta Anaesthesiol Scand 2017;61:83–90. [DOI] [PubMed] [Google Scholar]
- [17].Gerbershagen HJ, Aduckathil S, van Wijck AJM, Peelen LM, Kalkman CJ, Meissner W. Pain intensity on the first day after surgery. Anesthesiology 2013;118:934–44. [DOI] [PubMed] [Google Scholar]
- [18].Ghabach MMB, Mhanna NE, Abou Al Ezz MR, Mezher GN, Chammas MJ, Ghabach MMB. Comparison of effects of hemostatic gelatin sponge impregnated with ropivacaine versus normal saline applied on the transverse process of the operated vertebrae on postoperative pain in patients undergoing spinal instrumentation surgery: a randomized clin. World Neurosurg 2019;128:e1126–30. [DOI] [PubMed] [Google Scholar]
- [19].Ghamry, Elgebaly AS, Anwar AG, Shaddad MN. Ultrasound-guided erector spinae plane block for acute pain management in patients undergoing posterior lumbar interbody fusion under general anaesthesia. South Afr J Anaesth Analg 2019;25:26–31. [Google Scholar]
- [20].Glowacki D. Effective pain management and improvements in patients' outcomes and satisfaction. Crit Care Nurse 2015;35:33–41. [DOI] [PubMed] [Google Scholar]
- [21].Gottschalk A, Freitag M, Tank S, Burmeister MA, Kreil S, Kothe R, Hansen-Algenstedt N, Weisner L, Staude HJ, Standl T. Quality of postoperative pain using an intraoperatively placed epidural catheter after major lumbar spinal surgery. Anesthesiology 2004;101:175–80. [DOI] [PubMed] [Google Scholar]
- [22].Greze J, Vighetti A, Incagnoli P, Quesada JL, Albaladejo P, Palombi O, Tonetti J, Bosson JL, Payen JF. Does continuous wound infiltration enhance baseline intravenous multimodal analgesia after posterior spinal fusion surgery? A randomized, double-blinded, placebo-controlled study. Eur Spine 2017;26:832–9. [DOI] [PubMed] [Google Scholar]
- [23].Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hadi BA, Al Ramadani R, Daas R, Naylor I, Zelkó R. Remifentanil in combination with ketamine versus remifentanil in spinal fusion surgery—a double blind study. Int J Clin Pharmacol Ther 2010;48:542–8. [DOI] [PubMed] [Google Scholar]
- [25].Hernandez-Palazon, Tortosa JA, Martinez-Lage JF, Pérez-Flores D. Intravenous administration of propacetamol reduces morphine consumption after spinal fusion surgery. Anesth Analg 2001;92:1473–6. [DOI] [PubMed] [Google Scholar]
- [26].Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from: www.training.cochrane.org/handbook. [Google Scholar]
- [27].Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses Need for consistency. Bmj 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ibrahim A, Aly M, Farrag W. Effect of intravenous lidocaine infusion on long-term postoperative pain after spinal fusion surgery. Medicine (Baltimore) 2018;97:e0229–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Jakobsen JC, Wetterslev J, Winkel P, Lange T, Gluud C. Thresholds for statistical and clinical significance in systematic reviews with meta-analytic methods. BMC Med Res Methodol 2014, 14:120–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Joshi GP, Schug SA, Kehlet H. Procedure-specific pain management and outcome strategies. Best Pract Res Clin Anaesthesiol 2014;28:191–201. [DOI] [PubMed] [Google Scholar]
- [32].Kang H, Jung HJ, Lee JS, Yang JJ, Shin HY, Song KS. Early postoperative analgesic effects of a single epidural injection of ropivacaine administered preoperatively in posterior lumbar interbody spinal arthrodesis: a pilot randomized controlled trial. J Bone Joint Surg Am 2013;95:393–9. [DOI] [PubMed] [Google Scholar]
- [33].Kawamata T, Sato Y, Niiyama Y, Omote K, Namiki A. Pain management after lumbar spinal fusion surgery using continuous subcutaneous infusion of buprenorphine. J Anesth 2005;19:199–203. [DOI] [PubMed] [Google Scholar]
- [34].Kehlet H, Dahl J. The value of “multimodal” or “balanced analgesia” in postoperative pain treatment. Anesth Analg 1993;77:1048–56. [DOI] [PubMed] [Google Scholar]
- [35].Kehlet H, Dahl JB. Anaesthesia, surgery, and challenges in postoperative recovery. Lancet 2003;362:1921–8. [DOI] [PubMed] [Google Scholar]
- [36].Kehlet H, Wilmore DW. Evidence-based surgical care and the evolution of fast-track surgery. Ann Surg 2008;248:189–98. [DOI] [PubMed] [Google Scholar]
- [37].Kernc D, Strojnik V, Vengust R. Early initiation of a strength training based rehabilitation after lumbar spine fusion improves core muscle strength: a randomized controlled trial. J Orthop Surg Res 2018;13:151–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kien NT, Geiger P, Van Chuong H, Cuong NM, Van Dinh N, Pho DC, Anh VT, Giang NT. Preemptive analgesia after lumbar spine surgery by pregabalin and celecoxib: a prospective study. Drug Des Devel Ther 2019;13:2145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kim JC, Choi YS, Kim KN, Shim JK, Lee JY, Kwak YL. Effective dose of peri-operative oral pregabalin as an adjunct to multimodal analgesic regimen in lumbar spinal fusion surgery. Spine (Phila Pa 1976) 2011;36:428–33. [DOI] [PubMed] [Google Scholar]
- [40].Kim SI, Ha KY, Oh IS. Preemptive multimodal analgesia for postoperative pain management after lumbar fusion surgery: a randomized controlled trial. Eur Spine J 2016;25:1614–19. [DOI] [PubMed] [Google Scholar]
- [41].Kim SH, Kim SI, Ok SY, Park SY, Kim MG, Lee SJ, Noh JI, Chun HR, Suh H. Opioid sparing effect of low dose ketamine in patients with intravenous patient-controlled analgesia using fentanyl after lumbar spinal fusion surgery. Korean J Anesthesiol 2013;64:524–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Laigaard J, Pedersen C, Thea N, Mathiesen O, Peder A. Minimal clinically important differences in randomised clinical trials on pain management after total hip and knee arthroplasty : a systematic review. 2021;126:1029–37. [DOI] [PubMed] [Google Scholar]
- [43].Levaux Ch, Bonhomme V, Dewandre PY, Brichant JF, Hans P. Effect of intra-operative magnesium sulphate on pain relief and patient comfort after major lumbar orthopaedic surgery. Anaesthesia 2003;58:131–5. [DOI] [PubMed] [Google Scholar]
- [44].Li J, Yang JS, Dong BH, Ye JM. The effect of dexmedetomidine added to preemptive ropivacaine infiltration on postoperative pain after lumbar fusion surgery: a randomized controlled trial. Spine (Phila Pa 1976) 2019;44:1333–8. [DOI] [PubMed] [Google Scholar]
- [45].Maheshwari K, Avitsian R, Sessler DI, Makarova N, Tanios M, Raza S, Traul D, Rajan S, Manlapaz M, MacHado S, Krishnaney A, Machado A, Rosenquist R, Kurz A. Multimodal analgesic regimen for spine surgery: a randomized placebo-controlled trial. Anesthesiology 2020, 132:992–1002. [DOI] [PubMed] [Google Scholar]
- [46].Mathiesen O, Dahl B, Thomsen BA, Kitter B, Sonne N, Dahl JB, Kehlet H. A comprehensive multimodal pain treatment reduces opioid consumption after multilevel spine surgery. Eur Spine J 2013;22:2089–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Mathiesen O, Thomsen BA, Kitter B, Dahl JB, Kehlet H. Need for improved treatment of postoperative pain. Dan Med J 2012;59:A4401. [PubMed] [Google Scholar]
- [48].Mathiesen O, Wetterslev J, Kontinen VK, Pommergaard HC, Nikolajsen L, Rosenberg J, Hansen MS, Hamunen K, Kjer JJ, Dahl JB. Adverse effects of perioperative paracetamol, NSAIDs, glucocorticoids, gabapentinoids and their combinations: a topical review. Acta Anaesthesiol Scand 2014;58:1182–98. [DOI] [PubMed] [Google Scholar]
- [49].Moher D. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement David. Syst Rev 2015;207:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Myles PS, Myles DB, Galagher W, Boyd D, Chew C, Macdonald N, Dennis A. Measuring acute postoperative pain using the visual analog scale: the minimal clinically important difference and patient acceptable symptom state. Br J Anaesth 2017;118:424–9. [DOI] [PubMed] [Google Scholar]
- [51].Nielsen RV, Fomsgaard JS, Siegel H, Martusevicius R, Nikolajsen L, Dahl JB, Mathiesen O. Intraoperative ketamine reduces immediate postoperative opioid consumption after spinal fusion surgery in chronic pain patients with opioid dependency: a randomized, blinded trial. PAIN 2017;158:463–70. [DOI] [PubMed] [Google Scholar]
- [52].Oh SK, Kwon WK, Sangwoo, Sul GJ, Joo HK, Youn-Kwan YS, Byung GL. Comparison of operating conditions, postoperative pain and recovery, and overall satisfaction of surgeons with deep vs. No neuromuscular blockade for spinal surgery under general anesthesia: a prospective randomized controlled trial. J Clin Med 2019;8:498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Park SJ, Song JW, Shim JK, Song Y, Kwak YL, Yang SY. Effect of ketamine as an adjunct to intravenous patientcontrolled analgesia, in patients at high risk of postoperative nausea and vomiting undergoing lumbar spinal surgery. Br J Anaesth 2013;111:630–5. [DOI] [PubMed] [Google Scholar]
- [54].Perera AP, Chari A, Kostusiak M, Khan AA, Luoma AM, Casey ATH. Intramuscular local anesthetic infiltration at closure for postoperative analgesia in lumbar spine surgery. Spine (Phila Pa 1976) 2017;42:1088–95. [DOI] [PubMed] [Google Scholar]
- [55].Pinar HU, Karaca Ö, Karakoç F, Doǧan R. Effects of addition of preoperative intravenous ibuprofen to pregabalin on postoperative pain in posterior lumbar interbody fusion surgery. Pain Res Manag 2017;2017:1030491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Quinlan P, Davis J, Fields K, Madamba P, Colman L, Tinca D, Cannon Drake R. Effects of localized cold therapy on pain in postoperative spinal fusion patients: a randomized control trial. Orthop Nurs 2017;36:344–9. [DOI] [PubMed] [Google Scholar]
- [57].Raja SDC, Shetty AP, Subramanian B, Kanna RM, Rajasekaran S. A prospective randomized study to analyze the efficacy of balanced pre-emptive analgesia in spine surgery. Spine J 2019;19:569–77. [DOI] [PubMed] [Google Scholar]
- [58].Reisener M, Pumberger M, Shue J, Girardi FP, Hughes AP. Trends in lumbar spinal fusion—a literature review. J Spine Surg 2020;6:752–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Reuben SS, Buvanendran A, Kroin JS, Raghunathan K. The analgesic efficacy of celecoxib, pregabalin, and their combination for spinal fusion surgery (Retraction in: anesthesia and Analgesia (2009) 108:4 (1350)). Anesth Analg 2006;103:1271–7. [DOI] [PubMed] [Google Scholar]
- [60].Servicl-Kuchler D, Maldini B, Borgeat A, NB, Kosak R, Mavcic B, Novak-Jankovic V. The influence of postoperative epidural analgesia on postoperative pain and stress response after major spine surgery - a randomized controled double blind study. Acta Clin Croat 2014;53:176–83. [PubMed] [Google Scholar]
- [61].Singhatanadgige W, Chancharoenchai T, Honsawek S, Kotheeranurak V, Tanavalee C, Limthongkul W. No difference in pain after spine surgery with local wound filtration of morphine and ketorolac. Clin Orthop Relat Res 2020;478:2823–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Siribumrungwong K, Cheewakidakarn J, Tangtrakulwanich B, Nimmaanrat S. Comparing parecoxib and ketorolac as preemptive analgesia in patients undergoing posterior lumbar spinal fusion: a prospective randomized double-blinded placebo-controlled trial. BMC Musculoskelet Disord 2015;16:59–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Su X, Wang DX. Improve postoperative sleep: what can we do?. Curr Opin Anaesthesiol 2018;31:83–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Subramaniam K, Akhouri V, Glazer PA, Rachlin J, Kunze L, Cronin M, Desilva D, Asdourian CP, Steinbrook RA. Intra- and postoperative very low dose intravenous ketamine infusion does not increase pain relief after major spine surgery in patients with preoperative narcotic analgesic intake. Pain Med 2011;12:1276–83. [DOI] [PubMed] [Google Scholar]
- [65].Urban MK, Labib KM, Reid SC, Goon AK, Rotundo V, Cammisa FP, Girardi FP. Pregabalin did not improve pain management after spinal fusions. HSS J 2018;14:41–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Urban MK, Ya Deau JT, Wukovits B, Lipnitsky JY. Ketamine as an adjunct to postoperative pain management in opioid tolerant patients after spinal fusions: a prospective randomized trial. HSS J 2008;4:62–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Waelkens P, Alsabbagh E, Sauter A, Joshi GP, Beloeil H. Pain management after complex spine surgery: a systematic review and procedure-specific postoperative pain management recommendations. Eur J Anaesthesiol 2021;38:985–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Wang Y, Guo X, Guo Z, Xu M. Preemptive analgesia with a single low dose of intrathecal morphine in multilevel posterior lumbar interbody fusion surgery: a double-blind, randomized, controlled trial. Spine J 2020. [DOI] [PubMed] [Google Scholar]
- [69].Wen X, Huang Y, Chen Y, Fang S, Wu S. Clinical research on postoperative analgesia effect of using dezocine before suturing skin in patients with internal fixation of spine. Int J Clin Exp Med 2016;9:19967–73. [Google Scholar]
- [70].Wetterslev J, Jakobsen JC, Gluud C. Trial Sequential Analysis in systematic reviews with meta-analysis. BMC Med Res Methodol 2017;17:39–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Yamashita K, Fukusaki M, Ando Y, Fujinaga A, Tanabe T, Terao Y, Sumikawa K. Preoperative administration of intravenous flurbiprofen axetil reduces postoperative pain for spinal fusion surgery. J Anesth 2006;20:92–5. [DOI] [PubMed] [Google Scholar]
- [72].Yeom JH, Kim KH, Chon MS, Byun J, Cho SY. Remifentanil used as adjuvant in general anesthesia for spinal fusion does not exhibit acute opioid tolerance. Korean J Anesthesiol 2012;63:103–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Zhang Q, Wu Y, Ren F, Zhang X, Feng Y. Bilateral ultrasound-guided erector spinae plane block in patients undergoing lumbar spinal fusion: a randomized controlled trial. J Clin Anesth 2021;68:110090. [DOI] [PubMed] [Google Scholar]
- [74].Ziegeler S, Fritsch E, Bauer C, Mencke T, Müller BI, Soltesz S, Silomon M. Therapeutic effect of intrathecal morphine after posterior lumbar interbody fusion surgery: a prospective, double-blind, randomized study. Spine (Phila Pa 1976) 2008;33:2379–86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental digital content associated with this article can be found online at http://links.lww.com/PR9/A157.