Abstract
Background/Aims:
Mild cognitive impairment (MCI) lacks a “gold standard” operational definition. The Jak/Bondi actuarial neuropsychological criteria for MCI are associated with improved diagnostic stability and prediction of progression to dementia compared to conventional MCI diagnostic approaches, although its utility in diagnosing MCI in old-old individuals (age 75+) is unknown. Therefore, we investigated the applicability of neuropsychological criteria for MCI among the old-old from the Framingham Heart Study.
Methods:
A total of 347 adults (ages 79–102) were classified as cognitively normal or MCI via both Jak/Bondi and conventional Petersen/Winblad criteria, which differ on cutoffs for cognitive impairment and number of impaired scores required for a diagnosis. Cox models examined MCI status in predicting risk of progression to dementia.
Results:
MCI diagnosed by both the Jak/Bondi and Petersen/Winblad criteria was associated with incident dementia; however, when both criteria were included in the regression model together, only the Jak/Bondi criteria remained statistically significant. At follow-up, the Jak/Bondi criteria had lower MCI-to-normal reversion rates than the Petersen/Winblad criteria.
Conclusions:
Our findings are consistent with previous research on the Jak/Bondi criteria and support the use of a comprehensive neuropsychological diagnostic approach for MCI among old-old individuals.
Keywords: Cognitive deficits, Neuropsychology, Diagnostic criteria, Mild cognitive Impairment, Dementia
Introduction
Mild cognitive impairment (MCI), an intermediate state between normal aging and dementia, is defined by the presence of objective cognitive impairment in the context of intact daily functioning [1, 2]. Characterization of MCI and what constitutes “objective cognitive impairment” varies across definitional schemes, resulting in high variability of MCI prevalence rates and MCI-to-dementia conversion rates [3]. Few studies have examined MCI among the old-old (people age 75+) but estimates of prevalence rates range from 23 to 34% [4–6].
The conventional MCI criterion suggested by Petersen and others relies on impairment on a single neuropsychological test, which is problematic as it has been shown to increase the risk of false positive MCI diagnoses [7]. Neuropsychological criteria for MCI proposed by Jak et al. [8], which require at least two impaired test scores (>1 SD below normative means) within a cognitive domain, have been shown to be more strongly associated with Alzheimer’s disease (AD) biomarkers, higher diagnostic stability, and improved ability to identify individuals who progressed to dementia compared to Petersen/Winblad conventional diagnostic criteria as typified in the Alzheimer’s Disease Neuroimaging Initiative (ADNI) [9]. Examination of neuropsychological MCI criteria in the Framingham Heart Study (FHS) Offspring cohort (mean age = 68.5 years; SD = 5.7) found that the Jak/Bondi criteria and Petersen/Winblad criteria (i.e., –1.5 SD below normative means on a single cognitive test) were both associated with incident dementia; however, only the Jak/Bondi criteria remained statistically significant when both diagnostic approaches were included in the model [10]. The Jak/Bondi criteria identified a third fewer participants with MCI than the Petersen/Winblad criteria yet resulted in a greater proportion of participants who progressed to dementia, suggesting that the Petersen/Winblad criteria may be overinclusive [10].
The Jak/Bondi neuropsychological MCI criteria have not been examined in an old-old sample, although a study by Hong et al. [11] compared a version of the Petersen criteria (–1 SD below norms on prose recall or free recall, or subjective memory complaint) to the neuropsychologically based criteria proposed by Ritchie and colleagues (2001; –1 SD below norms on at least one measure of attention, memory, visuospatial ability, language, or reasoning, or subjective memory complaint) in participants aged 80 years or greater. They found that neither approach predicted dementia, highlighting that there may be a number of reasons beyond incipient dementia for a low cognitive score in the old-old, while emphasizing the need to examine other MCI diagnostic approaches in this population.
Increased variability in neuropsychological performance in the old-old presents a unique challenge when diagnosing MCI, and individuals with early- versus late-onset MCI show differences in neuropsychological predictors of conversion to AD [12]. Furthermore, we have previously shown that the neuropsychological profiles associated with dementia detection in the very-old (mean age >80 years) differ from those in the young-old (mean age <70 years), with the very-old AD group outperforming the young-old AD group when age-adjusted standardized scores were applied, despite the groups achieving comparable raw scores [13]. Research also suggests that differing mechanisms, such as increasing cerebrovascular disease and hippocampal sclerosis, with advancing age likely explains increased prevalence of clinical dementia in the very old despite a tapering of amyloid plaque and neurofibrillary tangle densities after age 95 [14]. Thus, we aimed to compare conventional Petersen/Winblad versus Jak/Bondi neuropsychological criteria for MCI diagnosis in the FHS’ old-old, as well as the modifying effects of vascular risk and apolipoprotein E (APOE) genotype, on diagnosis and prediction of progression to dementia.
Materials and Methods
Participants
The FHS is a longitudinal community-based study that includes serial examinations of the Original cohort since 1948 [15]. A total of 548 Original cohort participants completed a neuropsychological assessment between 1999 and 2008. Each individual’s first post-1998 neuropsychological assessment was used as their baseline data for the purposes of this study. Follow-up neuropsychological evaluations were available for 309 participants. Even if participants did not complete additional neuropsychological evaluations, most were continually followed by the study until death or December 31, 2013, and have available information regarding cognitive status based on dementia review meetings.
Diagnosis of dementia in the FHS has been previously described [16]. Individuals with clear or questionable cognitive deficits are discussed in a dementia diagnostic review meeting during which a team, including at least one neurologist and one neuropsychologist, reviews all available information for the participants to reach a consensus diagnosis. Earliest date of documented dementia is recorded. Participants with prevalent dementia (n = 121) or stroke (n = 31) at or before baseline, missing neuropsychological data (n = 9), missing date of dementia onset (n = 8), and/or lack of follow-up (n = 35) were excluded. The resulting sample consisted of 347 individuals. Participants ranged in age from 79–102 years (mean age = 84.9; SD = 4.0) at their baseline neuropsychological assessment. They were almost entirely Caucasian, 63.4% female, and 78.4% had a high school education or greater. Baseline participant characteristics are presented in Table 1.
Table 1.
Baseline demographic characteristics for all participants in the study (n = 347)
| Age, years | 84.9±4.0 |
| Women | 220 (63.4) |
| Education | |
| <High school degree | 75 (21.6) |
| ≥High school degree | 144 (41.5) |
| ≥Some college | 69 (19.9) |
| ≥College degree | 59 (17.0) |
| APOE ε4 allele | 65 (19.2) |
| Diabetes | 32 (9.7) |
| Hypertension | 258 (77.9) |
| High cholesterol | 14 (4.2) |
| History of cardiovascular disease | 175 (52.9) |
| Current smoker | 11 (3.3) |
Data are presented as n (%) or mean ± SD. Nine participants were missing APOE status; 16 participants were missing vascular risk factors.
Neuropsychological Assessment
Participants underwent a standardized neuropsychological assessment at baseline and follow-up. The battery included five cognitive domains, each comprised of at least two tests per domain, which is necessary for Jak/Bondi MCI classification. The memory domain included delayed recall from the Wechsler Memory Scale (WMS) Logical Memory, Visual Reproduction, and Verbal Paired Associates subtests [17]. Attention/processing speed was measured by Digit Span Forward from the Wechsler Adult Intelligence Scale (WAIS) and Trail Making Test A [18, 19]. Executive functioning tests included WAIS Digit Span Backward and Trail Making Test B [18, 19]. Language was measured by the 30-item Boston Naming Test, WAIS Similarities, and the Controlled Oral Word Association Test (FAS Letter Fluency) [18–20]. Visuospatial functioning tests included the Hooper Visual Organization Test and WAIS Block Design [19, 21]. Standardized residuals (z scores) adjusted for age, education, and sex were based on a sample of old-old Original cohort participants (ages 80–97) who were cognitively normal (CN) and did not progress to MCI or dementia for the duration of their study participation (i.e., “robust” normal controls; n = 40). The beta coefficients derived from the residual calculation at baseline were applied to each participant’s follow-up test scores as well.
MCI Classification
Participants were classified as CN or MCI using the Jak/Bondi and Petersen/Winblad criteria. The conventional Petersen/Winblad criteria define impairment as performance >1.5 SD below normative expectations on a single cognitive test. The comprehensive Jak/Bondi criteria operationalize impairment as performance >1 SD below normative expectations on at least two tests within a cognitive domain or a single impaired test score across all five cognitive domains. For both criteria, participants were also classified as amnestic MCI when memory was impaired and non-amnestic MCI when memory was intact and one or more non-memory domains were impaired. Although subjective memory complaint is typically included in the Petersen/Winblad criteria, it has been associated with elevated misclassification rates, even when considered in the context of neuropsychological performance and clinician judgement [22], and is more likely to be associated with depression and personality traits than cognitive impairment [23]. Therefore, subjective memory/cognitive complaint was not included in the diagnostic criteria in this study.
Vascular Risk Factors and APOE Status
The presence or absence of the following risk factors was determined from clinical interview, physical exam, and laboratory studies: history of cardiovascular disease (myocardial infarction, angina pectoris, coronary insufficiency, intermittent claudication, or congestive heart failure); diabetes (self-report of current treatment for diabetes, or casual blood glucose ≥200 mg/dL); high total cholesterol (≥240 mg/dL); hypertension (use of antihypertensive medications, untreated systolic blood pressure ≥140 mm Hg, or untreated diastolic blood pressure ≥90 mm Hg); and current smoking. APOE ε4 status was collected as well and defined as the presence of at least one ε4 allele.
Statistical Analyses
Descriptive statistics were calculated using means and standard deviations (SDs), frequency counts, and percentages. McNemar’s χ2 tests were conducted to compare diagnostic and stability rates. For comparisons of proportions of participants who progressed from MCI to dementia and from CN to dementia, the two-sided exact version of McNemar’s test was used because of the small sample sizes for those conditions. Cox proportional hazards regression models were used to determine the hazard ratios (HR) and 95% confidence intervals (CI) for each MCI criteria as a predictor of progression to dementia. Time-to-dementia was the number of months from baseline neuropsychological assessment to dementia diagnosis. Participants who did not progress to dementia were censored at their last neuropsychological assessment visit or at death if cognitive status at death was available. The models were adjusted for demographic factors (age, sex, and education group), vascular risk factors (entered individually: history of cardiovascular disease, diabetes, high total cholesterol, hypertension, current smoking), and APOE ε4 status (carrier vs. noncarrier). Model 1 contained these covariates plus the Petersen/Winblad MCI definition; Model 2 contained these covariates plus the Jak/Bondi MCI definition; and Model 3 contained these covariates plus both Petersen/Winblad and Jak/Bondi MCI definitions. Each MCI definition was categorized as overall MCI (CN vs. MCI) and by using indicator variables for amnestic MCI and non-amnestic MCI (with CN as the referent group). The association between vascular risk factors and dementia was also examined separately from models including the MCI criteria. All statistical analysis was performed using SPSS version 24.
Results
Baseline cognitive status diagnoses (n = 347) are presented in Table 2. The Petersen/Winblad criteria classified 144 participants (41.5%) as CN and 203 (58.5%) as MCI, whereas the Jak/Bondi criteria identified 234 participants (67.4%) as CN and 113 (32.6%) as MCI. A McNemar test determined that the proportion of MCI and CN classifications were significantly different between the two sets of criteria (χ2 = 76.16, df = 1, p < 0.001). A total of 106 participants (30.5%) were classified as MCI by both the Petersen/Winblad and Jak/Bondi criteria, and 137 participants (39.5%) were classified as CN by both criteria. Ninety-seven participants (28.0%) were classified as MCI by the Petersen/Winblad definition but CN by the Jak/Bondi criteria. Only 7 participants (2.0%) were classified as MCI by the Jak/Bondi criteria but CN by the Petersen/Winblad criteria.
Table 2.
Baseline diagnoses for the Petersen/Winblad and Jak/Bondi criteria (n = 347)
| Jak/Bondi | Total | |||
|---|---|---|---|---|
| CN | MCI | |||
| Petersen/Winblad | ||||
| CN | 137 | 7 | 144 | |
| MCI | 97 | 106 | 203 | |
| Total | 234 | 113 | 347 |
CN, cognitively normal; MCI, mild cognitive impairment.
The next neuropsychological exam occurred 1.7 ± 1.3 years after baseline testing (n = 309). Results are presented in Table 3. The proportion of participants who remained stable over the follow-up interval (i.e., diagnosed with MCI at both or CN at both time points) was greater when the Jak/Bondi criteria were applied (219, 70.8%) compared to when the Petersen/Winblad criteria were used (193, 62.5%), χ2 = 6.13, df = 1, p = 0.013. Regarding reversion rates, a significantly smaller proportion of participants reverted from MCI to CN for the Jak/Bondi criteria (23, 7.4%) compared to the Petersen/Winblad criteria (42, 13.6%), χ2 = 5.89, df = 1, p = 0.015. The diagnostic approaches did not significantly differ on the proportion of participants who progressed from CN to MCI, χ2 = 0.71, df = 1, p = 0.401. A greater proportion of participants progressed from MCI to dementia using the Petersen/Winblad criteria (p = 0.039, 2 sided), whereas a greater proportion progressed from CN to dementia using the Jak/Bondi criteria (p = 0.039, 2 sided).
Table 3.
Diagnostic stability from baseline to follow-up neuropsychological exam (n = 309)
| Baseline status | Follow-up status | Total | |||
|---|---|---|---|---|---|
| CN | MCI | dementia | |||
| Petersen/Winblad | |||||
| CN | 84 | 42 | 7 | 133 | |
| MCI | 42 | 109 | 25 | 176 | |
| Total | 126 | 151 | 32 | 309 | |
| Jak/Bondi | |||||
| CN | 167 | 35 | 14 | 216 | |
| MCI | 23 | 52 | 18 | 93 | |
| Total | 190 | 87 | 32 | 309 |
CN, cognitively normal; MCI, mild cognitive impairment. The mean time between baseline and the follow-up neuropsychological exam was 1.7 ± 1.3 years. Stable diagnosis = CN at both baseline and follow-up or MCI at both baseline and follow-up; reversion = MCI at baseline and CN at follow-up; progression = CN to MCI or dementia, or MCI to dementia.
Over a mean follow-up period of 5.7 ± 3.7 years, 140 participants (40.3%) developed all-cause dementia (Table 4). For both criteria, participants classified as MCI had greater risk of progression to dementia compared to those classified as CN (Model 1 Petersen/Winblad: HR = 1.91, 95% CI = 1.31–2.79, p = 0.001; Model 2 Jak/Bondi: HR = 2.52, 95% CI = 1.75–3.62, p < 0.001). When the Petersen/Winblad and Jak/Bondi criteria were included in the same model with the covariates (Model 3), the HR remained statistically significant only for the Jak/Bondi definition (Petersen/Winblad: HR = 1.23, 95% CI = 0.78–1.23, p = 0.38; Jak/Bondi: HR = 2.23, 95% CI = 1.42–3.49, p < 0.001). Baseline diagnoses of individuals who eventually progressed to dementia are presented in Table 5.
Table 4.
Hazard ratios of dementia for each MCI definition (n = 347)
| Independent variable | Dementia cases/participants, n | Model 1: multivariablea + Petersen/Winblad | Model 2: multivariablea + Jak/Bondi | Model 3: multivariablea +Petersen/Winblad + Jak/Bondi | |||
|---|---|---|---|---|---|---|---|
| hazard ratio (95% CI) | p value | hazard ratio (95% CI) | p value | hazard ratio (95% CI) | p value | ||
| Overall MCI | |||||||
| Petersen/Winblad | |||||||
| CN | 47/144 | 1.00 (referent) | – | – | – | 1.00 (referent) | – |
| MCI | 93/203 | 1.91 (1.31–2.79) | 0.001 | – | – | 1.23 (0.78–1.97) | 0.38 |
| Jak/Bondi | |||||||
| CN | 74/234 | 1.00 (referent) | – | 1.00 (referent) | |||
| MCI | 66/113 | 2.52 (1.75–3.62) | <0.001 | 2.23 (1.42–3.49) | <0.001 | ||
| MCI subtype category | |||||||
| Petersen/Winblad | |||||||
| CN | 47/144 | 1.00 (referent) | – | – | – | 1.00 (referent) | – |
| Amnestic MCI | 53/97 | 2.43 (1.60–3.69) | 0.001 | – | – | 1.39 (0.82–2.35) | 0.23 |
| Non-amnestic MCI | 40/106 | 1.45 (0.92–2.29) | 0.11 | – | – | 1.11 (0.66–1.86) | 0.70 |
| Jak/Bondi | |||||||
| CN | 74/234 | – | – | 1.00 (referent) | – | 1.00 (referent) | – |
| Amnestic MCI | 41/65 | – | – | 3.77 (2.46–5.76) | <0.001 | 3.10 (1.84–5.25) | <0.001 |
| Non-amnestic MCI | 25/48 | – | – | 1.63 (1.00–2.66) | 0.05 | 1.45 (0.84–2.53) | 0.19 |
CN, cognitively normal; MCI, mild cognitive impairment; CI, confidence interval;
Multivariable = age, sex, education group (<high school degree, high school degree, some college, ≥college), APOE ε4, diabetes, hypertension, high cholesterol, history of cardiovascular disease, and current smoking.
Table 5.
Baseline diagnoses and progression to dementia
| Baseline diagnoses by both criteria | Convert to dementia | |||
|---|---|---|---|---|
| Petersen/Winblad | Jak/Bondi | |||
| MCI | MCI | 106 | 62 (58.5%) | |
| CN | CN | 137 | 43 (31.4%) | |
| MCI | CN | 97 | 31 (32.0%) | |
| CN | MCI | 7 | 04 (57.1%) | |
CN, cognitively normal; MCI, mild cognitive impairment. The mean time between baseline and conversion to dementia or end of study participation was 5.7 ± 3.7 years.
Regarding MCI subtypes, amnestic MCI was significantly associated with incident dementia for both diagnostic definitions (Petersen/Winblad: HR = 2.43, 95% CI = 1.60–3.69, p < 0.001; Jak/Bondi: HR = 3.77, 95% CI = 2.46–5.76, p < 0.001), whereas non-amnestic MCI was not significantly associated with dementia (Petersen/Winblad: HR = 1.45, 95% CI = 0.92–2.99, p = 0.11; Jak/Bondi: HR = 1.63, 95% CI = 1.00–2.66, p = 0.05). Again, when the Petersen/Winblad and Jak/Bondi criteria were included in the same model, the HR for amnestic MCI remained statistically significant only for the Jak/Bondi criteria (Petersen/Winblad: HR = 1.39, 95% CI = 0.82–2.35, p = 0.23; Jak/Bondi: HR = 3.10, 95% CI = 1.84–5.25, p < 0.001).
Of the 140 participants diagnosed with dementia at follow-up, 103 were diagnosed with probable AD (92 AD without stroke, 5 AD with stroke, 6 mixed AD + vascular dementia), 6 were diagnosed with vascular dementia without AD, 1 with frontotemporal dementia, 11 with dementia with Lewy bodies, and 19 had an unspecified dementia diagnosis. When the MCI criteria were examined for prediction of conversion to specifically AD, amnestic MCI was significantly associated with AD whereas non-amnestic MCI was not for both criteria (Petersen/Winblad: amnestic HR = 2.64, 95% CI = 1.61–2.93, p < 0.001, non-amnestic HR = 1.50, 95% CI = 0.88–2.56, p = 0.14; Jak/Bondi: amnestic HR = 4.94, 95% CI = 2.96–8.25, p < 0.001, non-amnestic HR = 1.79, 95% CI = 1.00–3.16, p = 0.05). Progression to the other types of dementia was not examined due to the small numbers in those groups.
Regarding the association between baseline vascular risk factors and incident dementia, diabetes predicted a faster rate of progression to dementia (HR = 2.59, 95% CI = 1.44–4.68, p = 0.002), while history of cardiovascular disease predicted a lower risk of progression (HR = 0.69, 95% CI = 0.48–0.99, p = 0.04). When APOE ε4 status was added to the model, the relationship between cardiovascular disease and dementia was no longer significant (HR = 0.71, 95% CI = 0.50–1.02, p = 0.07), but the relationship between diabetes and dementia did not change. Being an APOE ε4 carrier was significantly associated with dementia at follow-up (HR = 1.77, 95% CI = 1.17–2.67, p = 0.007). The pattern of findings did not change in models examining the relationship between the MCI criteria and dementia with and without inclusion of vascular risk factors as covariates.
Discussion/Conclusion
Our study compared prevalence rates, diagnostic stability, and progression to dementia between two different MCI diagnostic approaches in the Framingham Heart Study’s old-old individuals. The criteria, which differed on impairment cutoffs and number of impaired tests, resulted in discrepancies in baseline diagnostic rates: the Petersen/Winblad criteria classified 58.5% of the sample as MCI and the Jak/Bondi criteria classified 32.6% of the sample as MCI. Rates of MCI diagnosis in this old-old Original cohort were higher than prevalence rates in the young-old Offspring cohort (Petersen/Winblad: 33%, Jak/Bondi: 24% [10]), which is to be expected given the association between cognitive impairment and advancing age. However, compared to MCI prevalence rates in other very old samples, which range from 23.2 to 34% [4–6], the Petersen/Winblad criteria resulted in higher than expected MCI rates, whereas the Jak/Bondi criteria produced rates consistent with these estimates. Examination of diagnostic stability from baseline to follow-up neuropsychological exam also supported the Jak/Bondi criteria, as it showed higher stability and lower MCI-to-normal reversion rate than the Petersen/Winblad criteria.
Despite differences in diagnostic rates, MCI diagnosed via both the Jak/Bondi and Petersen/Winblad criteria was significantly associated with conversion to incident dementia in very old age, even when accounting for baseline demographic factors, vascular risk factors, and APOE ε4 status. Participants diagnosed with MCI via the Petersen/Winblad criteria had approximately twice the risk of progressing to dementia than CN participants and those diagnosed with MCI via the Jak/Bondi criteria were 2.5 times more likely to progress to dementia. When both criteria were included in the model together though, only the Jak/Bondi criteria remained significant, which is consistent with results from the Offspring cohort [10]. Notably, the Jak/Bondi criteria diagnosed a smaller proportion of the sample with MCI, yet performed better in the combined model than the Petersen/Winblad criteria, indicating that the Petersen/Winblad criteria may be overinclusive. In addition, of the 97 individuals who were classified as MCI by the Petersen/Winblad criteria but CN by the Jak/Bondi criteria, only 32% progressed to dementia. This rate is similar to the conversion rate among individuals diagnosed as CN by both criteria (31.4%), whereas 58.5% of individuals diagnosed as MCI by both criteria developed all-cause dementia. These findings also support that the Petersen/Winblad criteria may be overinclusive and increase the chance of having a false positive MCI diagnosis.
When MCI subtypes were examined, amnestic MCI appeared to be driving the relationship between overall MCI and dementia, as non-amnestic MCI was not significantly related to subsequent dementia. Similar findings in The 90+ Study showed that amnestic MCI conferred a greater risk for all-cause dementia than non-amnestic MCI; however, non-amnestic MCI was nevertheless significantly associated with dementia [24]. Approximately 74% of individuals diagnosed with dementia in the current sample were diagnosed with probable AD. Individuals diagnosed with amnestic MCI via the Jak/Bondi criteria had nearly a 5-fold greater risk of progressing to AD than those classified as CN, whereas those diagnosed with amnestic MCI via the Petersen/Winblad criteria had approximately 2.5 times the risk of progressing to AD. Thus, the Jak/Bondi criteria appear to be particularly useful for identifying individuals with amnestic MCI who eventually progress to a clinical diagnosis of probable AD in late life.
Given the potentially important contribution of vascular risk factors to incidence of dementia in the very old [14], we further examined the association between vascular risk and dementia. Baseline diabetes was most strongly associated with subsequent dementia, with a relative risk of 2.59. Both a meta-analysis [25] and mega-analysis [26] of the relationship between diabetes and dementia found that individuals with diabetes had approximately 1.5 times the risk of developing any dementia. We have previously shown that, in the FHS, diabetes is associated with greater cognitive decline in late life [27], and elevated blood glucose – even among nondiabetics – is associated with greater AD pathology (higher density of medial temporal neurofibrillary tangles) at death [28]. However, among the old-old, findings are mixed, with some studies showing increased dementia incidence in individuals with diabetes [29] and others finding that diabetes is not a risk factor for dementia [30]. History of cardiovascular disease actually conferred decreased risk of developing incident dementia (HR = 0.69), but this relationship was attenuated and no longer significant when APOE ε4 status was added to the model. Whereas most studies have found cardiovascular disease to be a risk factor for dementia [31, 32], our finding may be the result of survival bias, in that individuals with cardiovascular disease have increased mortality or they may develop dementia at earlier ages, which would have excluded them from the current sample. Furthermore, Corrada et al. [33] found that late-life onset of hypertension was associated with reduced risk of dementia in The 90+ Study, highlighting that findings of dementia risk in the young-old may not be directly applicable to the oldest-old.
Our study had some limitations that should be noted. One such limitation is that cerebrospinal fluid biomarkers of AD were not available [9]. As the relationship between AD pathology and cognition is different in old-old compared to young-old populations [34], it would be of great interest to examine the association between these biomarkers and MCI diagnosed by neuropsychological criteria in very old age. Progression to probable AD based on clinical consensus diagnosis was examined; however, future research would benefit from incorporating autopsy data to confirm AD diagnosis. It is also important to note that the number of CN participants who progressed to dementia might be somewhat higher than expected for a population-based study. This finding might be explained by the advanced age of participants as well as the length of the follow-up period (5.7 ± 3.7 years). It is likely that many of these participants progressed to MCI at some point during the follow-up period prior to eventually progressing to dementia.
Repeat neuropsychological testing was available for 89% of the baseline sample; thus, the rates of diagnostic stability could be affected by selection bias. Of the 38 participants lost to follow-up, 52.6% were diagnosed with MCI at baseline. It is possible that development of dementia during the follow-up interval contributed to attrition, and in turn, reduced statistical power. Neuropsychological assessment in the very old is complicated by a high prevalence of medical comorbidities and vision and/or hearing loss [35]. Future research may want to consider adapted neuropsychological tools to improve assessment of cognition in this population. Lastly, the sample was almost entirely Caucasian and from the Northeastern region of the US, limiting generalizability to other populations.
Overall, research on neuropsychological criteria for MCI in the old-old is limited, and this study is among the first to examine the comprehensive Jak/Bondi MCI diagnostic criteria in this age group. Hong et al. [11] did not find a significant association between either neuropsychological MCI criteria proposed by Ritchie and colleagues or conventional Petersen/Winblad criteria and dementia in a very old sample; however, the sample was notable for low educational attainment, and neuropsychological test performance was not adjusted for education. The present study created age-, sex-, and education-adjusted residual z scores for the neuropsychological variables, which likely contributed to improved MCI identification. Furthermore, both of the MCI criteria examined by Hong and colleagues required impairment on only one cognitive measure, which differs from the Jak/Bondi neuropsychological MCI criteria that require at least 2 impaired test scores within a cognitive domain in order to balance sensitivity with reliability given the high base rates of one impaired neuropsychological scores within normative samples [8, 36]. Last, the neuropsychological criteria used by Hong et al. [11] were confounded by inclusion of subjective cognitive impairment as a criterion, which has been associated with misdiagnosis of MCI [22].
Our study replicated findings examining MCI criteria in the Offspring cohort of the FHS [10], supporting the use of Jak/Bondi comprehensive neuropsychological MCI criteria in different age cohorts. Although both criteria were similarly associated with subsequent all-cause dementia, the Petersen/Winblad criteria diagnosed almost twice as many participants with MCI compared to the Jak/Bondi criteria. In addition, a relatively low number of participants who were diagnosed as MCI by the Peterson/Winblad criteria but CN by the Jak/Bondi criteria progressed to dementia. These findings, which indicate that the Petersen/Winblad criteria may result in false positive MCI diagnoses, are consistent with the literature demonstrating that operationalization of cognitive impairment based on a single impaired test score tends to inflate MCI prevalence rates [37, 38]. The Jak/Bondi criteria were also found to have a particular strength in identifying individuals with amnestic MCI who eventually progressed to AD.
Overall, the Jak/Bondi criteria demonstrated more advantages compared to the Petersen/Winblad criteria in diagnosing MCI in this very old sample. Clinicians and researchers should consider designing their neuropsychological batteries to allow for application of the Jak/Bondi criteria (i.e., at least two tests per cognitive domain). Early and accurate identification of MCI remains a priority, as MCI is a risk factor for dementia [39], and treatments targeting dementia are likely best implemented early on in the disease process. The old-old represent a unique portion of the population that is rapidly growing. Additional research characterizing cognitive impairment and risk factors for dementia in the old-old is needed to improve our understanding and clinical characterization of pathologic cognitive changes in this understudied age group.
Figure 1.
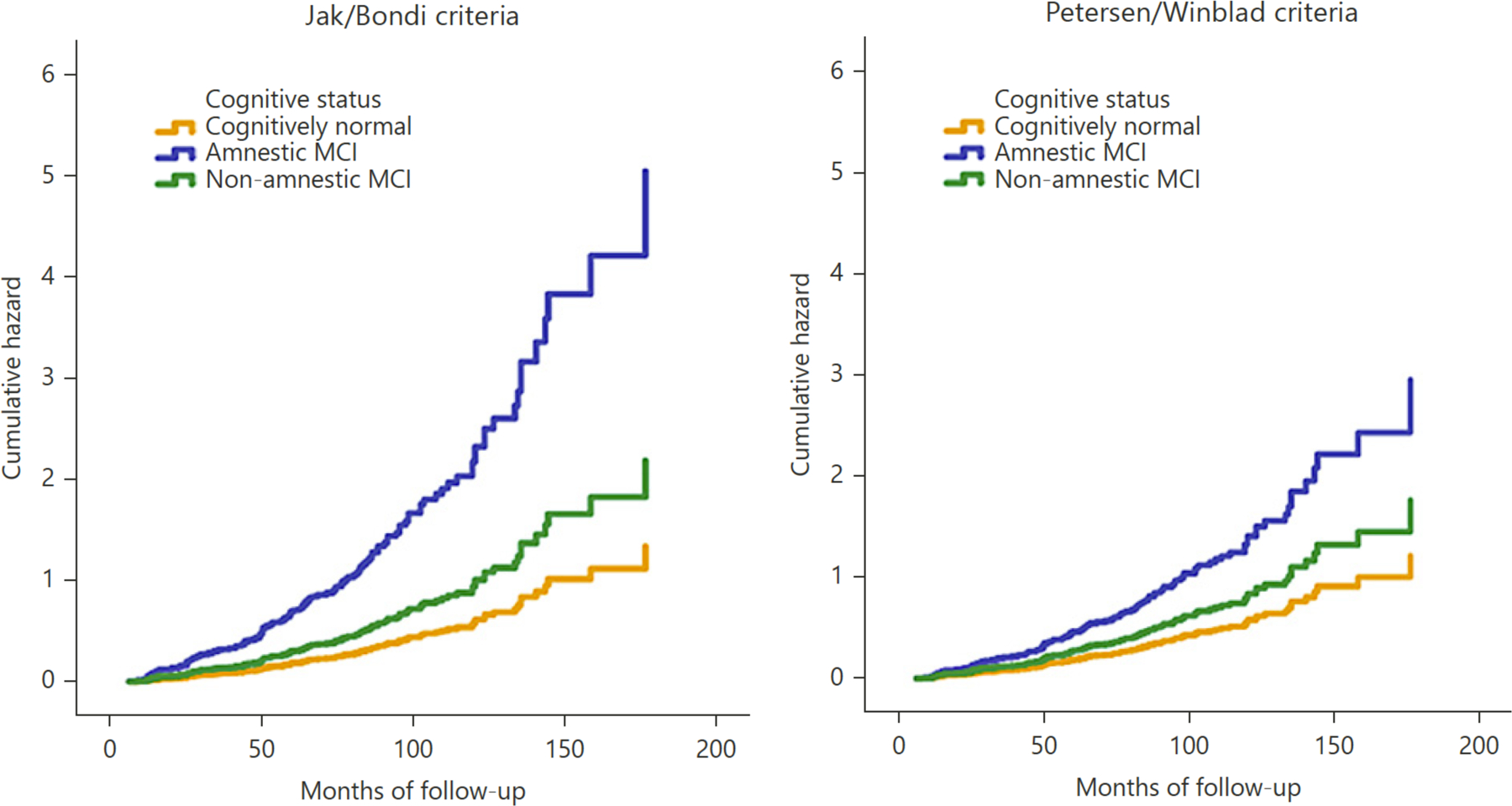
Hazard functions showing risk of progression to dementia across time for MCI subtypes classified by the Jak/Bondi and Petersen/Winblad criteria.
Funding Sources
This work was supported by National Institutes of Health grants R01 AG049810 (M.W.B.); K24 AG026431 (M.W.B.); the Framingham Heart Study’s National Heart, Lung, and Blood Institute contract (N01-HC-25195); National Institute on Aging grants R01-AG008122 (R.A.); R01-AG016495 (R.A.); R01-AG033040 (R.A.); and the US Department of Veterans Affairs Clinical Sciences Research and Development Service (Career Development Award-2 1IK2CX000938 to K.J.B and 1IK2CX001415 to E.C.E.).
Footnotes
Statement of Ethics
This study was approved by the Institutional Review Boards at each of the participating institutions. Written informed consent was obtained from all subjects, and human data included in this article were obtained in compliance with the Helsinki Declaration.
Disclosure Statement
Dr. Bondi is a consulting editor for the Journal of the International Neuropsychological Society, and serves as a paid consultant for Novartis, Eisai, and Roche pharmaceutical companies.
References
- 1.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med 2004. Sep;256(3):183–94. [DOI] [PubMed] [Google Scholar]
- 2.Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, et al. Mild cognitive impairment—beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med 2004. Sep;256(3):240–6. [DOI] [PubMed] [Google Scholar]
- 3.Bondi MW, Jak AJ, Delano-Wood L, Jacobson MW, Delis DC, Salmon DP. Neuropsychological contributions to the early identification of Alzheimer’s disease. Neuropsychol Rev 2008. Mar;18(1):73–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pioggiosi PP, Berardi D, Ferrari B, Quartesan R, De Ronchi D. Occurrence of cognitive impairment after age 90: MCI and other broadly used concepts. Brain Res Bull 2006. Jan;68(4):227–32. [DOI] [PubMed] [Google Scholar]
- 5.Yaffe K, Middleton LE, Lui LY, Spira AP, Stone K, Racine C, et al. Mild cognitive impairment, dementia, and their subtypes in oldest old women. Arch Neurol 2011. May;68(5):631–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peltz CB, Corrada MM, Berlau DJ, Kawas CH. Cognitive impairment in nondemented oldest-old: prevalence and relationship to cardiovascular risk factors. Alzheimers Dement 2012;8(2):87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edmonds EC, Delano-Wood L, Clark LR, Jak AJ, Nation DA, McDonald CR, et al. ; Alzheimer’s Disease Neuroimaging Initiative. Susceptibility of the conventional criteria for mild cognitive impairment to false-positive diagnostic errors. Alzheimers Dement 2015. Apr;11(4):415–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jak AJ, Bondi MW, Delano-Wood L, Wierenga C, Corey-Bloom J, Salmon DP, et al. Quantification of five neuropsychological approaches to defining mild cognitive impairment. Am J Geriatr Psychiatry 2009. May;17(5):368–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bondi MW, Edmonds EC, Jak AJ, Clark LR, Delano-Wood L, McDonald CR, et al. Neuropsychological criteria for mild cognitive impairment improves diagnostic precision, biomarker associations, and progression rates. J Alzheimers Dis 2014;42(1):275–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jak AJ, Preis SR, Beiser AS, Seshadri S, Wolf PA, Bondi MW, et al. Neuropsychological Criteria for Mild Cognitive Impairment and Dementia Risk in the Framingham Heart Study. J Int Neuropsychol Soc 2016. Oct;22(9):937–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong TB, Zarit SH, Johansson B. Mild cognitive impairment in the oldest old: a comparison of two approaches. Aging Ment Health 2003. Jul;7(4):271–6. [DOI] [PubMed] [Google Scholar]
- 12.Ye BS, Seo SW, Lee Y, Kim SY, Choi SH, Lee YM, et al. Neuropsychological performance and conversion to Alzheimer’s disease in early- compared to late-onset amnestic mild cognitive impairment: CREDOS study. Dement Geriatr Cogn Disord 2012;34(3–4):156–66. [DOI] [PubMed] [Google Scholar]
- 13.Bondi MW, Houston WS, Salmon DP, Corey-Bloom J, Katzman R, Thal LJ, et al. Neuropsychological deficits associated with Alzheimer’s disease in the very-old: discrepancies in raw vs. standardized scores. J Int Neuropsychol Soc 2003. Jul;9(5):783–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson PT, Head E, Schmitt FA, Davis PR, Neltner JH, Jicha GA, et al. Alzheimer’s disease is not “brain aging”: neuropathological, genetic, and epidemiological human studies. Acta Neuropathol 2011. May;121(5):571–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dawber TR, Meadors GF, Moore FE Jr. Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health 1951. Mar;41(3):279–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chêne G, Beiser A, Au R, Preis SR, Wolf PA, Dufouil C, et al. Gender and incidence of dementia in the Framingham Heart Study from mid-adult life. Alzheimers Dement 2015. Mar;11(3):310–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wechsler D. Wechsler Memory Scale - Revised New York: Psychological Corporation; 1987. [Google Scholar]
- 18.Reitan RM, Wolfson D. The Halstead Reitan Neuropsychological Test Battery 2nd ed. Tucson (AZ): Neuropsychology Press; 1993. [Google Scholar]
- 19.Wechsler D. Wechsler Adult Intelligence Scale - Revised Manual San Antonio: Psychological Corporation; 1981. [Google Scholar]
- 20.Kaplan EF, Goodglass H, Weintraub S. The Boston Naming Test Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- 21.Hooper HE. Hooper Visual Organization Test Los Angeles: Western Psychological Services; 1983. [Google Scholar]
- 22.Edmonds EC, Delano-Wood L, Galasko DR, Salmon DP, Bondi MW; Alzheimer’s Disease Neuroimaging Initiative. Subjective cognitive complaints contribute to misdiagnosis of mild cognitive impairment. J Int Neuropsychol Soc 2014. Sep;20(8):836–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reid LM, Maclullich AM. Subjective memory complaints and cognitive impairment in older people. Dement Geriatr Cogn Disord 2006;22(5–6):471–85. [DOI] [PubMed] [Google Scholar]
- 24.Peltz CB, Corrada MM, Berlau DJ, Kawas CH. Incidence of dementia in oldest-old with amnestic MCI and other cognitive impairments. Neurology 2011. Nov;77(21):1906–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng G, Huang C, Deng H, Wang H. Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern Med J 2012. May;42(5):484–91. [DOI] [PubMed] [Google Scholar]
- 26.Chatterjee S, Peters SA, Woodward M, Mejia Arango S, Batty GD, Beckett N, et al. Type 2 Diabetes as a Risk Factor for Dementia in Women Compared With Men: A Pooled Analysis of 2.3 Million People Comprising More Than 100,000 Cases of Dementia. Diabetes Care 2016. Feb;39(2):300–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bangen KJ, Beiser A, Delano-Wood L, Nation DA, Lamar M, Libon DJ, et al. APOE genotype modifies the relationship between midlife vascular risk factors and later cognitive decline. J Stroke Cerebrovasc Dis 2013. Nov;22(8):1361–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bangen KJ, Himali JJ, Beiser AS, Nation DA, Libon DJ, Fox CS, et al. Interaction between midlife blood glucose and APOE genotype predicts later Alzheimer’s pathology. J Alzheimers Dis 2016. Jul;53(4):1553–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahtiluoto S, Polvikoski T, Peltonen M, Solomon A, Tuomilehto J, Winblad B, et al. Diabetes, Alzheimer disease, and vascular dementia: a population-based neuropathologic study. Neurology 2010. Sep;75(13):1195–202. [DOI] [PubMed] [Google Scholar]
- 30.Decarli C. Vascular factors in dementia: an overview. J Neurol Sci 2004. Nov;226(1–2):19–23. [DOI] [PubMed] [Google Scholar]
- 31.Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology 2005. Jan;64(2):277–81. [DOI] [PubMed] [Google Scholar]
- 32.Deckers K, Schievink SH, Rodriquez MM, van Oostenbrugge RJ, van Boxtel MP, Verhey FR, et al. Coronary heart disease and risk for cognitive impairment or dementia: systematic review and meta-analysis. PLoS One 2017. Sep;12(9):e0184244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Corrada MM, Hayden KM, Paganini-Hill A, Bullain SS, DeMoss J, Aguirre C, et al. Age of onset of hypertension and risk of dementia in the oldest-old: The 90+ Study. Alzheimers Dement 2017. Feb;13(2):103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balasubramanian AB, Kawas CH, Peltz CB, Brookmeyer R, Corrada MM. Alzheimer disease pathology and longitudinal cognitive performance in the oldest-old with no dementia. Neurology 2012. Aug;79(9):915–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giulioli C, Amieva H. Epidemiology of Cognitive Aging in the Oldest Old. Rev Invest Clin 2016. Jan-Feb;68(1):33–9. [PubMed] [Google Scholar]
- 36.Heaton RK, Miller SW, Taylor MJ, Grant I. Revised Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographically Adjusted Neuropsychological Norms for African American and Caucasian Adults Scoring Program Odessa (FL): Psychological Assessment Resources, Inc.; 2004. [Google Scholar]
- 37.Loewenstein DA, Acevedo A, Small BJ, Agron J, Crocco E, Duara R. Stability of different subtypes of mild cognitive impairment among the elderly over a 2- to 3-year follow-up period. Dement Geriatr Cogn Disord 2009;27(5):418–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trittschuh EH, Crane PK, Larson EB, Cholerton B, McCormick WC, McCurry SM, et al. Effects of varying diagnostic criteria on prevalence of mild cognitive impairment in a community based sample. J Alzheimers Dis 2011;25(1):163–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lopez OL, Kuller LH, Becker JT, Dulberg C, Sweet RA, Gach HM, et al. Incidence of dementia in mild cognitive impairment in the cardiovascular health study cognition study. Arch Neurol 2007. Mar;64(3):416–20. [DOI] [PubMed] [Google Scholar]


