Abstract
There is increasing evidence of a role for environmental contaminants in disrupting metabolic health in both humans and animals. Despite a growing need for well-understood models for evaluating adipogenic and potential obesogenic contaminants, there has been a reliance on decades-old in vitro models that have not been appropriately managed by cell line providers. There has been a quick rise in available in vitro models in the last ten years, including commercial availability of human mesenchymal stem cell and preadipocyte models; these models require more comprehensive validations but demonstrate real promise in improved translation to human metabolic health. There is also progress in developing three-dimensional and co-culture techniques that allow for the interrogation of a more physiologically relevant state. While diverse rodent models exist for evaluating putative obesogenic and/or adipogenic chemicals in a physiologically relevant context, increasing capabilities have been identified for alternative model organisms such as Drosophila, C. elegans, zebrafish, and medaka in metabolic health testing. These models have several appreciable advantages, most notably the size, rapid development, large brood sizes, and ease of high-resolution lipid accumulation throughout the organisms. They are anticipated to expand the capabilities of metabolic health research, particularly when coupled with emerging obesogen evaluation techniques as described herein.
Graphical Abstract

1. Introduction
Over the last several decades, the global prevalence of metabolic disorders, specifically obesity, has risen at an alarming rate. Despite extensive investments in exploring interventions to address this health trend, the incidence rates continue to rise. In the United States (US), 8.9% of infants and toddlers [1, 2], 18.5% of 2–19 year old’s [1, 2], and 42.4% of adults (20+) [3] are currently classified as obese, with an additional 31.2% of the adult population classified as overweight [4]. Obesity consumes >$200 billion of the US health care expenditure annually and also drives increased risks of various comorbidities (e.g., type II diabetes, cardiovascular disease, hypertension) [5–8]. High societal costs [8, 9] have driven support for research into causal factors, including exposure(s) to environmental contaminants. Previous research estimated extremely high economic costs of obesity, diabetes, and associated health costs reasonably attributable to environmental contaminants in the European Union [9], even when only considering five chemicals for which sufficient epidemiological data were available.
As detailed in the companion review, Obesity II, “obesogens” are environmental chemicals that increase the size of white adipose tissue (WAT) stores in the body as a result of exposure in vivo [10, 11]. Chemicals that can induce adipogenesis in cellular models in vitro but have not yet been shown to increase WAT stores in vivo are designated as potential obesogens [12]. Considering the complexity of human chemical exposures, the increasing reports of obesogens, and the rising incidence of metabolic disorders, it is critical to identify and validate comprehensive models (in silico, in vitro, and in vivo) for the identification and evaluation of obesogens. One of the major challenges in the obesity field is to develop a robust set of tests that can reveal adipogenic and/or obesogenic properties of chemicals and have strong predictive capacity in humans. These tests should be in line with the 3R principles (i.e., reducing the number of animals, refining experiments to minimize the number of animals used, and replacing animal experiments where possible). Practically speaking, the high costs of animal experiments limit the use of mammals in screening for potential obesogens. This supports an urgent need for increased use of lower-order (in silico, in vitro) testing to prioritize higher-order (in vivo) testing. There is also an urgent need for new in vivo models that are less time and cost-intensive to support in vivo testing that is still required for the tens of thousands of chemicals used in commerce. While the number and diversity of cellular models of adipocyte differentiation and metabolic health is increasing, these require comprehensive validation to determine the strengths and weaknesses of each for their relevance to human metabolic health
Despite the potential limitations of available animal models to reproduce human disease fully, they help evaluate exposure pathways, generation of in vivo metabolites, elucidating tissue and/or disease biology, and underlying molecular mechanisms involved in adverse health outcomes. The choice of the animal model should consider the degree to which the outcomes being examined are relevant to humans and the sensitivity of these outcomes to environmental chemicals. The relevance of the model to human health depends considerably on the evolutionary conservation of biological processes impacted by candidate chemical or pharmacological molecules between humans and the animal model used. It is likely that a single test might not reveal all relevant properties and that a battery of tests should be developed. This set of tests should address the following issues: 1) evaluate in vivo obesity according to its different characteristics, including the type and importance of different adipose depots; 2) reveal in vitro and in silico assays/models that reliably predict obesity; 3) identify in vivo biomarkers that are predictive of obesity, and 4) account for delays between exposure(s) to putative obesogens and the appearance of a phenotype.
Mammalian models have been relied on for metabolic health testing due to clear translation of adipose physiology. However, non-mammalian model species are increasingly appropriate for the screening and rapid identification of chemicals and mixtures and the exploration of disease mechanisms. Knowledge acquired from non-mammalian model systems (e.g., vertebrates such as teleost fish and invertebrates such as flies and worms) can provide insights into mechanisms involved in regulating lipid metabolism and transport processes that have been intractable by other approaches [13]. Due to the conservation of lipid metabolism processes among vertebrates, the zebrafish model has become an attractive alternative to rodents, with lower costs and time investments.
2. In vitro assays
The most well-established lower-order testing protocols are the adipogenesis cell assays, although newly developed cell models have allowed an increasing breadth of metabolic disruption assessment (Figure 1). Several in vitro models were developed in various species (primarily human and murine) to identify potential obesogens [14, 15]. These models generally assess three endpoints: commitment to the adipocyte lineage (via multipotent MSC models), preadipocyte proliferation (proliferation of early-stage adipocyte lineage cells), and differentiation into mature adipocytes (adipogenesis; generally determined via quantification of intracellular triglyceride accumulation).
Figure 1:
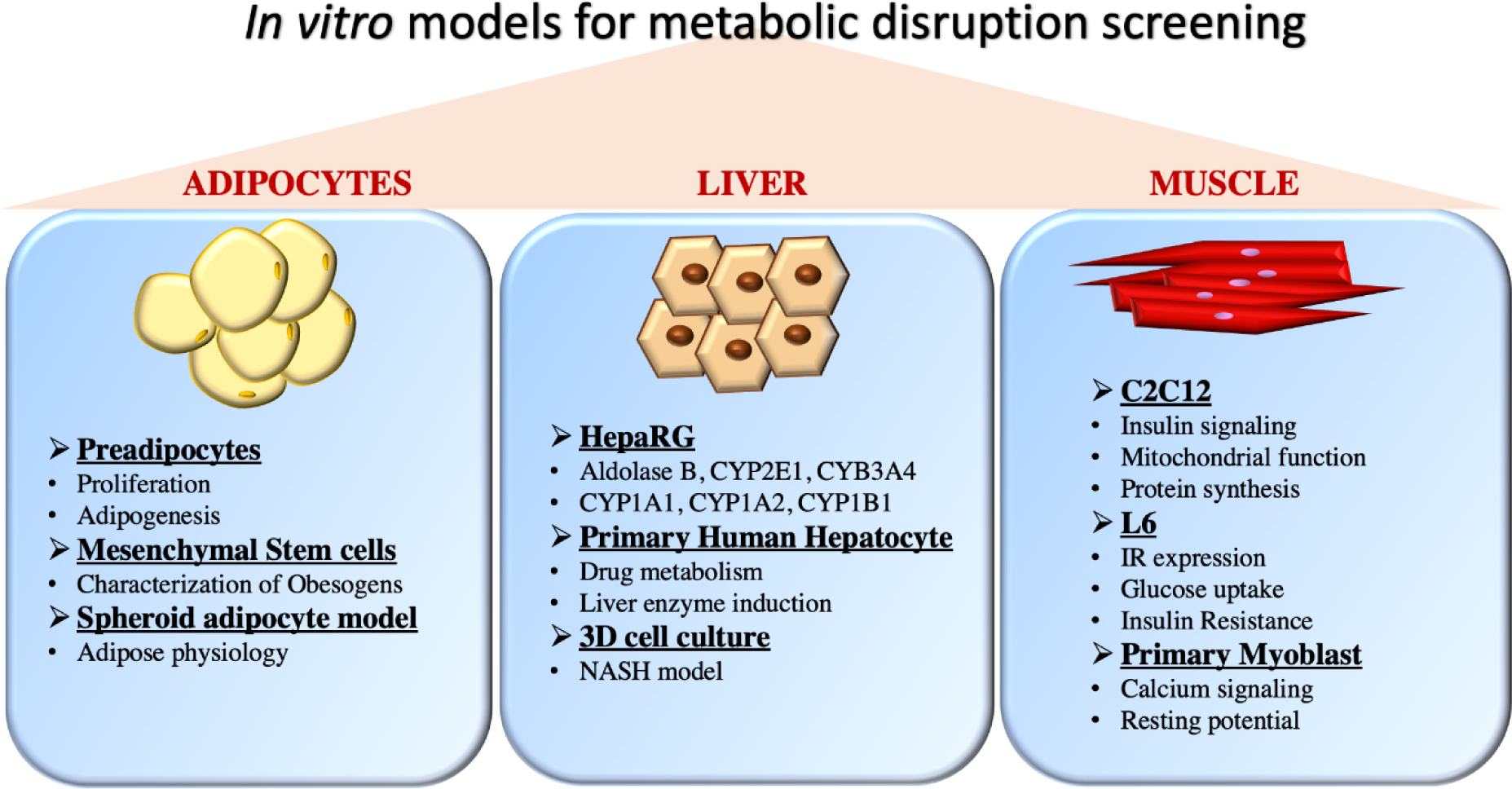
In vitro models used for testing the effect of metabolic disrupting chemicals on various pathways. Common uses of the various cell models are described.
2.1. Preadipocyte models
Preadipocytes are already committed to the adipocyte lineage and thus can be used to examine both proliferation (via nuclear staining) and adipogenesis (via triglyceride quantification). These cells are in an early stage of adipocyte development and require activation of signaling pathways to promote further development/maturation. Adipogenesis can be achieved by treating cells with a “differentiation cocktail” that contains a variety of hormonal and/or growth factors to initiate the process. These factors are often different between laboratories, but generally always include a mixture of fetal bovine serum, insulin, and isobutylmethylxanthine (IBMX); some laboratories also include thyroid hormone and/or glucocorticoids, though the presence of these and concentrations varies widely. Once the cocktail is removed, the relative roles of various test chemicals in the role of differentiation (assessed via triglyceride accumulation) and proliferation (of adipocyte precursor cells) can be assessed [16–19].
The 3T3-L1 mouse cell line was isolated and described in the 1970s and has been utilized for decades as an in vitro screen to examine the mechanisms regulating adipogenesis and evaluate potential adipogenic chemicals [16, 17, 20]. This cell line has been used to carefully explore mechanisms promoting and underlying various stages of adipogenesis [21, 22] and has been shown to appropriately select chemicals for further testing (linking in vitro results to in vivo health outcomes; e.g., bisphenol A and tributyltin) [23–31]. While this line has been well-characterized [21], its sourcing can be unreliable [32, 33]. For example, nuclear receptor expression related to adipogenesis is markedly different between lots and sources of this cell line [32]. These and other cell line integrity issues can contribute to discrepancies in replication efforts between laboratories [34, 35]. We recently undertook an interlaboratory reproducibility effort of 3T3-L1 responses to a positive control chemical (rosiglitazone) and three blinded test chemicals [35]. While the determination of “active” versus “inactive” were consistent across the ten participating laboratories, the potencies and efficacies of the blinded chemical responses varied by orders of magnitude. The cross-over study design allowed for determinations of the sources of variation, and our results demonstrated that inconsistencies of the cell line sources and differentiation protocol differences promoted most of the variation. Thus the harmonization of protocols across laboratories may help support consistent reporting of adipogenic results [35]. Despite these limitations, 3T3-L1 cells remain the most popular model for assessing adipogenic outcomes. Specifically, numerous publications have assessed bisphenols [26, 32, 36], brominated and organophosphate flame retardants [37–39], per and polyfluoroalkyl substances [40, 41], and diverse other environmental contaminants [20, 24, 37] and mixtures [42] using this cell model. There is an emerging interest in determinations of whether environmental contaminant exposures promote the development of normal or abnormal adipocytes, and some preliminary data has begun to evaluate this. For example, BPA enhanced levels of leptin, interleukin-6, and interferon gamma in mature adipocytes, resulting in hypertrophic adipocytes with impared insulin signaling, increased pro-inflammatory cytokine production, and reduced glucose utilization [43].
The OP9 mouse bone marrow-derived stromal cell line is another established preadipocyte model [19, 44] that allows faster differentiation (2–3 versus 10–14 days). This cell line is considered to be a later stage preadipocyte than 3T3-L1 cells because it expressed key adipogenic factors such as CCAAT/enhancer-binding proteins alpha and beta, peroxisome proliferator-activated receptor gamma (PPARγ), sterol-regulatory element-binding protein-1 (SREBP-1), perilipin, and other adipocyte markers that are not expressed in basal 3T3-L1 cells before adipogenic induction [19]. Therefore, OP9 cells can be induced to accumulate triglycerides within two days, differentiation is not diminished by maintenance in culture at high cell density, their adipogenic potential is maintained for >100 passages, and they do not require contact inhibition and reversion to clonal expansion before initiating the differentiation induction [19]. These characteristics suggest a promising model with lower time and cost investments, though this does require careful validation to understand the translation of responses to human health effects. We have reported that these cells do differentially express nuclear receptors relative to 3T3-L1 cells, including PPARγ/α, liver X receptor alpha (LXRα), glucocorticoid receptor (GR), retinoid X receptor-alpha/beta (RXRα/β), and estrogen receptor alpha (ERα) [32]. As a result, responsiveness to adipogenic chemicals in OP9 cells is significantly different from 3T3-L1 cells, characterized by lower responsiveness via activation of GR and greater responsiveness via the RXR pathway [32, 45]. While still an uncommon model for assessing obesogens, OP9 cells have been used to evaluate bisphenols [32], pesticides [45], and other environmental contaminants [45].
More recently, several human preadipocyte models have become available that hold promise for future evaluations of adipogenicity by environmental contaminants. Since the basis for much of our understanding of adipogenesis has been evaluated using the murine 3T3-L1 cells, utilizing these newer human models may help elucidate any species-specific differences that may be present. Many companies now supply primary human preadipocytes (HPAd) isolated from several human subcutaneous depots, visceral depots, and/or adipose surrounding the heart. Moreover, suppliers also provide source-specific HPAd cells, i.e., those sourced from donors with normal, overweight, or obese body mass indices and those with or without diabetes (e.g., see, https://www.zen-bio.com/products/cells/subcutaneous_adipocytes.php). These discrete preadipocyte populations allow more targeted questions and potentially a better molecular understanding of adipogenesis. However, human preadipocyte cell models are cryopreserved at the end of primary culture. They can generally be propagated at most two additional passages before losing their ability to differentiate into mature adipocytes [46, 47]. As such, these models, while potentially more translationally relevant to human health, are extremely costly, as numerous cryopreserved vials are needed to complete any well-designed experiment (e.g., multiple biological replicates). Limitations aside, researchers have begun to utilize human preadipocytes to assess adipogenic and anti-adipogenic effects of botanical and biological mixtures [48–50], bisphenols [51], and flame retardants [38].
The Simpson-Golabi-Behmel syndrome (SGBS) cell line addresses some of these limitations of using primary human preadipocytes. These cells were isolated from an infant with an extremely rare (250 reported cases) metabolic health condition characterized by excess growth; this infant demonstrated expanded subcutaneous fat depots, and a sample of this tissue was obtained postmortem [52]. Profiling these cells suggests that they can be maintained and retain robust differentiation capability over 50 passages [53], a significant advantage over normal human donor preadipocytes, and profiling has suggested morphological, biochemical, and functional similarities to differentiated adipocytes from healthy subjects [52, 54]. These cells also transiently express brown adipocyte markers [55–57], suggesting that this cell line might be useful for assessments of adipocyte browning. Proteomic and transcriptomic analyses of SGBS cells have been used to evaluate the molecular underpinnings of SGBS differentiation, with >1100 proteins and >300 genes differentially expressed in differentiated cells relative to undifferentiated [58]. However, some research comparing this model to existing models has suggested notable differences. Metabolomics and lipidomics profiling revealed a diverse grouping of lipid classes markedly changed throughout the differentiation process, suggesting a radically different metabolite profile than previously observed in 3T3-L1 cells [59]. SGBS cells have been used to evaluate the adipogenic effects of various bisphenols [60], though have not yet seen frequent use in this context. Other human cell lines obtained from tumors or transformed can be differentiated into either white (Lisa, LS-14, AML-1, Chub-S7) or brown (PAZ6) adipocytes [61], but their use in toxicology is rare [60].
2.2. Mesenchymal stem cells (MSCs)
Another option in assessing adipogenesis is the utilization of multipotent mesenchymal stromal stem cells (mesenchymal stem cells, MSCs). MSCs are multipotent cells that can assess adipocyte lineage commitment in addition to adipocyte differentiation [18, 62, 63]. MSCs are isolated from either bone marrow or adipose tissue, and cells from both sources have been used to assess adipogenesis. The use of MSC models has been reviewed previously in the context of obesogens and their potential impacts on cell commitment and subsequent differentiation [64]. Recent work described a novel protocol for separately evaluating adipogenic commitment and subsequent differentiation in primary MSCs [63], previously described for the C3H10T1/2 murine stem cell model [65, 66]. This protocol allows a complete characterization of potential obesogens and their role in disrupting cell commitment and differentiation. While the focus has been on evaluating effects on the adipocyte lineage, a growing body of research has begun to evaluate potential chemical impacts on osteogenic development using these models [67–70]. Some limited research has evaluated chemical impacts on development down the chondrogenic, myogenic, or other cell lineages [64]. Human MSCs are readily available from diverse vendors, although murine models are also routinely used [45, 70–72].
Recent research elegantly described protocols for distinguishing assays to evaluate adipogenic lineage commitment and subsequent adipocyte differentiation [63]; briefly, cells can be pre-treated with test chemicals prior to the differentiation cocktail exposure. These pre-treated cells can be subsequently exposed to the differentiation cocktail and evaluated at the end of the differentiation window. The extent of triglyceride accumulation can be compared with standard adipogenesis plates; chemicals with effects on commitment should have equivalent effects to those differentiated for the full two weeks, whereas cells without effects on commitment should not accumulate more triglycerides than the vehicle control in the commitment assays, regardless of effects in the standard adipogenesis assay [63].
The human MSCs lack the issues inherent in the primary human preadipocyte models; they can be maintained in culture for several more passages, have less variability in sourcing, and are easier to isolate and culture, increasing the utility of this model. This should lead to an increased reliance on human MSCs for adipogenic in vitro testing. However, rigorous reproducibility assessments and comprehensive validation testing are still needed to ensure accurate translation to and/or prediction of in vivo and human health outcomes. Diverse bisphenols [72–74] and their mixtures [75], flame retardants [18], parabens [76], and other environmental contaminants [63, 77–79] have been evaluated using MSC models. Research in female MSCs demonstrated that RXR agonists attenuated glucose uptake; blunted adiponectin expression; promoted a sustained interferon signaling, inhibiting markers of adipocyte browning; and unlike activation of PPARγ, failed to downregulate proinflammatory and profibrotic transcripts [77]. As the authors described, these data implicated RXR agonists in the development of dysfunctional white adipose tissue that could potentially exacerbate obesity and/or diabetes risk in vivo. Future research is needed to evaluate these functional differences in adipocyte physiology to determine more subtle effects of obesogenic contaminants. There has also been some initial research to evaluate the interplay between lineage commitment, suggesting that exposures to certain chemicals can not only commit cells to the adipocyte lineage but can also suppress the osteogenic lineage [45]; this interplay between different cell lineages is an area of research that still requires further investigation and mechanistic assessment.
Human multipotent adipose-derived stem cells (hMADS), obtained from human infant adipose tissue, have also been used to study the effects of aryl hydrocarbon receptor ligands that demonstrated an inflammatory response in pre-and adipocytes, a phenomenon observed in obesity [80]. hMADS were also used to screen 49 contaminants prioritized through ToxCast screening, reporting 26 active chemicals across diverse chemical groups (i.e., pesticides, phenolics, phthalates, etc.) [81].
2.3. Spheroid adipocyte models
Spheroid cell cultures of both MSCs and preadipocytes are being developed and evaluated [82–87]. These culture techniques may allow some inherent benefits over the traditional adherent monolayer cultures. Spheroid culture of adipocyte models may improve differentiation efficiency relative to monolayer cultures [82–86, 88], reducing time and cost investment. The fundamental goal of spheroid models is to maintain greater in vivo or whole tissue-relevant signaling than monolayer models. Indeed, several papers have demonstrated greater adipogenic and osteogenic gene expression relative to monolayer cultures and a down-regulation of stemness markers [82, 83]. Other researchers have demonstrated increased plasticity of spheroid constructs through multiple generations of these cells able to commit to and differentiate into numerous cell lineages [89]. This plasticity might signal a greater variance in these models that requires further investigation. While these models have received no apparent use for the interrogation of putative obesogens, they have been demonstrated to exhibit improved relevance to the in vivo condition [90]. Specifically, researchers have demonstrated that human unilocular vascularized adipocyte spheroids have unilocular morphology and large lipid droplets, and these cells develop key features of adipocyte dysfunction (e.g., insulin resistance, impaired lipolysis, and disrupted adipokine secretion; [90, 91]) and respond to stress (toxin or culture-related) by secreting pro-inflammatory adipokines [92]. These spheroid cultures also maintain expression of markers specific to certain adipocyte types (e.g., brown) for longer than is possible in 2D culture [92]. These 3D cultures also exhibit more physiologically relevant gene expression (>4500 differentially expressed genes relative to 2D culture) and lipid profiles of >1000 lipid species resemble the in vivo condition [93]. As such, these models may allow for a clearer understanding of adipose physiology than was possible with monolayer cultures and hence requires further evaluation and comprehensive validation and testing; this should also include evaluation of known adipogenic and/or obesogenic contaminants to compare responses with existing models.
2.4. Liver cell assays
Obesogens are also known to target liver (either directly or indirectly) and promote metabolic diseases such as toxicant-associated fatty liver diseases (TAFLD) or non-alcoholic fatty liver disease (NAFLD); thus, there is a need to have accurate in vitro hepatocyte models for testing chemicals. Liver cell assays are frequently used as surrogate models to predict in vivo hepatotoxicity related to chemicals and decipher the determinants of NAFLD development and progression. The use of various hepatocyte models for evaluating NAFLD and other metabolic disorders has been covered recently in detail [94–97]. These models have been used to evaluate diverse environmental contaminants, including bisphenols [98, 99], phthalates [99–101], pesticides [102], other environmental contaminants [99, 101], and therapeutics [103] for effects on NAFLD and other metabolic dysfunction.
Among many liver cell lines, HepG2 cells a human hepatoma cell line commonly used for drug metabolism and hepatotoxicity studies. HepG2 cells express certain differentiated hepatic functions like lipoprotein metabolism, triglyceride metabolism, bile acid synthesis, glycogen synthesis, or insulin signaling, making them a useful tool for some studies targeting hepatotoxicity and drug metabolism [104]. HepG2 cells exposed to a low concentration of BPA alter lipid metabolism, mitochondrial function and promote lipid accumulation leading later one to steatosis [105]. Co-incubation of HepG2 with fatty acids palmitic acid and oleic acid, induced lipid accumulation in a dose-dependent manner which will contribute to steatosis [106].
Comparatively, human THLE-2 and murine AML12 cell lines are derived from healthy liver cells and express characteristics of normal adult liver epithelial cells [107]. Insulin receptor expression was low in THLE-2 cells relative to AML12 and HepG2 cells, suggesting disparities in their application to insulin receptor signaling. Gluconeogenesis and hepatokine expression was impaired in both THLE-2 and AML12 cells; while expression of Angiopoietin Like 4 (ANGPTL4) was regulated byδ PPAR activation similarly across THLE-2, AML12, and HepG2 cells, only HepG2 cells reflected the in vivo environment with regulation by cAMP [107]. These models have been utilized to evaluate fatty acid induced lipid droplet accumulation and the presence and causes of heterogeneity in the lipid droplet content [108],
The most prevalent human liver cell line is HepaRG. HepaRG cells can differentiate into hepatocyte-like and biliary-like phenotypes after dimethylsulfoxide (DMSO) (1.75 – 2%) exposure, and possess the ability to stably express several liver-specific genes such as albumin, aldolase B, CYP2E1 and CYP3A4 [109]. Changes in metabolites related to energy metabolism, oxidative stress, and insulin resistance have also been observed in differentiated HepaRG cells supplemented with an oleate/palmitate mixture [110]. These are consistent with alterations observed in the liver tissues of human patients and animal models of NAFLD [111, 112]. Altogether, these data further support the suitability of the fatty acid-supplemented HepaRG model to study the impact of obesogens on steatosis progression towards steatohepatitis in the context of the “two-hit” model [113]. In line with these data, an oleate/stearate mixture is sufficient to decrease the expression of CYP1A1, 1A2, 1B1 and decrease their activity after steatosis induction [114]. These results corroborate data obtained from NAFLD rodent models, especially regarding CYP1A1 and 1A2 [115–117].
In addition, several 3D liver culture models have also been developed to create a cell environment closer to in vivo conditions. In 3D cell cultures, cell growth and interaction with surrounding conditions exhibit higher differentiation and benefit from more extended culture than 2D cultures [118]. When cultured as 3D spheroids, HepaRG cells express genes involved in lipoprotein metabolism, energetic lipid synthesis, gluconeogenesis, glycolysis, and bile acid metabolism, liver-specific functions, and xenobiotic metabolism enzymes [119, 120].
Primary human hepatocytes (PHH) are increasingly used to predict drug metabolism and liver enzyme induction in humans. However, PHH have inherent limitations: scarce and unpredictable availability, limited growth activity and lifespan, and early and variable phenotypic alterations in 2D culture. Moreover, liver-specific functions, particularly cytochrome P450 (CYP) activities and their responsiveness to prototypical inducers, are not maintained with increasing time of culture. Liver-specific functions also usually decrease with time in culture and are differently altered [121, 122]. Cultivated in a 3D collagen matrix, they proliferate, form hollow spheroids, and undergo robust hepatic differentiation. They can be maintained in this state for at least 28 days without decreasing survival rate and cellular polarity and require fewer cells to generate spheroids than 2D cultures [123]. PHH 3D-spheroid models co-cultured with liver sinusoidal endothelial cells, Kupffer cells, hepatic stellate cells, increase human hepatocyte functionality (increased mRNA expression of APOB, CYP3A4, and albumin). Essential factors such as spheroid size, time in culture, and culture media composition affect basal levels of xenobiotic metabolism and liver enzyme inducibility via activators of hepatic receptors such as the aryl hydrocarbon receptor (AhR), constitutive androstane receptor (CAR), and pregnane X receptor (PXR) [124]. Various co-culture techniques have also been developed for liver cell assays to recreate more tissue or disease-relevant environments for the evaluation of disease biology and toxicology [125].
Similarly, primary murine hepatocytes (PMHs) are readily isolated through rapid protocols and thus have improved availability relative to PHH [126]. PMHs have been well-described as a model to assess fat deposition, inflammatory responses, and mechanistic interrogation of fatty acid induced lipid accumulation by diverse contaminants [127–129].
2.5. Muscle cell assays
While skeletal muscle is the main tissue responsible for utilization of glucose and is the main site of the development of insulin resistance, the impact of toxicants on skeletal muscle has not been extensively studied. Detecting effects in vitro can be difficult due to the specific cell culture requirements and stimulation of skeletal muscle fibers required to mimic physiological function. Since skeletal muscle plays a critical role in developing metabolic diseases, any chronic disturbances in muscle cells may contribute to insulin resistance and subsequent obesity.
The most widely used in vitro myocyte model is the murine myoblast cell line, C2C12. These cells can be differentiated into myotubes (immature muscle cells) over several days. BPA and estradiol have been demonstrated to suppress myogenic differentiation by inhibiting Akt signaling in C2C12 cells [130], potentially disrupting ER signaling. Tolylfluanid alters insulin signaling, mitochondrial function, and protein synthesis in C2C12 cells in a manner dependent on fatty acid levels [131]. The rat myoblast cell line, L6, has a longer differentiation time relative to C2C12 cells, as well as appreciable differences in mitochondrial respiration and glucose utilization [132]. In L6 rat myotubes, di(2-ethylhexyl) phthalate (DEHP) exposure was shown to affect insulin receptor expression, GLUT4 expression, as well as glucose uptake and oxidation, indicating that it may negatively influence insulin signaling [133]. The pesticides dichlorodiphenyltrichloroethane (DDT) and lindane impair insulin signaling in L6 myotubes, promoting insulin resistance-like conditions [134].
Human and rodent primary myoblasts are also used. However, they are unsuitable for extended cultures and more extensive screening studies due to relatively low numbers of cells obtained at a relatively high cost. Some polychlorinated biphenyls (PCBs) have been shown to inhibit myogenic differentiation of primary murine myoblasts and L6 cells [135]. In primary murine myoblasts differentiated to myotubes, low micromolar concentrations of BPA and tetrabromobisphenol A (TBBPA) were shown to affect calcium signaling and resting potential. In a similar study, using rabbit skeletal muscle microsomes, BPA and TBBPA were shown to differently affect the function of proteins involved in calcium signaling [136].
Notably, there are distinct differences between mature muscle tissue and myotubes derived from myoblast cell lines or primary myoblasts [132]. Myotubes have lower energy demand, lower oxidative phosphorylation, higher glycolysis, and lower insulin responsiveness [137]. There is a considerable knowledge gap regarding the effects of environmental chemicals in more complex and physiologically relevant skeletal muscle systems, which require additional validations.
3. In vivo assays
While in vitro mechanistic studies are a critical component in environmental chemical research, these studies cannot replace the need for in vivo integrative models, particularly for adverse health outcomes that develop later in life following developmental exposures. Research examining the environmental health consequences of exposure to environmental chemicals using animal models has demonstrated that some adverse health effects of chemical exposures reported in humans are also apparent across other vertebrates [138]. These findings are essential for understanding the impact of environmental chemicals, including obesogens, across all vertebrates [139]. These tests are critical because the classification of obesogens into different classes according to the strength of evidence is highly dependent on the tests used.
Beyond the classical rodent in vivo models used to investigate human obesity, new models have emerged based on alternative model organisms, e.g., bony fishes, worms, and flies [140] (Figure 2). These model organisms, including Danio rerio (zebrafish), Caenorhabditis elegans (C. elegans; roundworm), and Drosophila melanogaster (fruit flies), offer several advantages to accurate discernment of the metabolic processes involved in metabolic diseases such as obesity [141]. These organisms share small size, large numbers of progeny, relatively rapid development, and sequenced genomes. They are well suited to moderate throughput screening of chemicals to study metabolic diseases [142–146]. Moreover, most genes and gene families implicated in metabolic diseases are conserved among flies, worms, zebrafish and humans [144]. Below we present a short overview of the utility of each model and some summarized obesogenic chemical evaluation using these emerging models (Table 1).
Figure 2:

Advantages and disadvantages of in vivo models for metabolic disrupting chemical evaluation. Common or emerging model organisms used in metabolic health research are discussed and various characteristics are described.
Table 1:
Obesogenic chemical testing in emerging in vivo models (zebrafish, medaka, roundworm, fruit fly)
| Species | Mode of action | Representative References |
|---|---|---|
| Danio rerio |
Obesity phenotype Increased weight, adiposity, and/or lipid accumulation |
Cadmium: [177], [270] DDT mixture: [271] Nonylphenol and polyethoxylates: [79] Bisphenols: [272], [27], [165] Phthalates: [176], [273], [174]| PFOS: [169] |
|
NAFLD phenotype
Steatosis, fatty liver changes |
Cadmium: [270] Benzo(a)pyrene: [274], [275] Bisphenols: [276], [277], [278], [279], [280] Phthalates: [281], [282] |
|
|
Metabolism changes Metabolomics, lipids, fatty acids, diabetic phenotype, etc. |
Bisphenols: [283], [278], [165] Phthalates: [176], [284], [174] PFOS: [169] |
|
| Oryzias latipes |
Obesity phenotype Increased weight, adiposity, and/or lipid accumulation |
TBT: [182] TBT/PFOS: [181] |
|
NAFLD phenotype
Steatosis, fatty liver changes |
||
|
Metabolism changes Metabolomics, lipids, fatty acids, diabetic phenotype, etc. |
TBT: [182] Bisphenols: [186] |
|
| C. elegans |
Obesity phenotype Increased weight, adiposity, and/or lipid accumulation |
Bisphenols: [199], [285] Erythromycin: [198] PFOA: [286] |
|
NAFLD phenotype
Steatosis, fatty liver |
||
|
Metabolism changes Metabolomics, lipids, fatty acids, diabetic phenotype, etc. |
Bisphenols: [199] Erythromycin: [198] Methylmercury: [197] PFOA: [286] |
|
| Drosophila melanogaster |
Obesity phenotype Increased weight, adiposity, and/or lipid accumulation |
DEHP: [287] |
|
NAFLD phenotype
Steatosis, fatty liver changes |
||
|
Metabolism changes Metabolomics, lipids, fatty acids, diabetic phenotype, etc. |
PFOA: [288] PFOS: [289] |
Summary table of obesogenic activity testing in the zebrafish, medaka, roundworm, and fruit fly models. Representative obesogenic chemical testing (non-exhaustive) is included to detail the diversity of contaminants examined.
3.1. Danio rerio (Zebrafish)
Zebrafish, a small tropical freshwater fish native to South Asia (e.g., India and Bangladesh), has found wide use in almost all areas of biological research [147, 148]. Zebrafish is one of the most widely used models to study metabolic dysfunction. They have indeed all the critical organs that regulate energy homeostasis and metabolism, including adipose tissue, digestive organs, i.e., pancreas and liver, and skeletal muscles, all physiologically and anatomically like humans [141, 149, 150]. The rapid development of zebrafish promotes metabolically functional organs only a few days post-fertilization (dpf; e.g., pancreas and liver develop around three dpf). Organogenesis and biological processes can be easily monitored due to the extra-uterine development and the semitransparency of the embryo and larva stages that persist until a relatively late stage of development [151].
Zebrafish store excess neutral triglycerides in lipid droplets within white adipocytes similar to mammals [152] and have well-described anatomically, physiologically, and molecularly distinct adipose depots throughout their bodies [153–155]. This contrasts with Drosophila and C. elegans, where fat is stored in non-specialized cells (within the fat body or within the intestine, respectively) that carry out several other functions besides lipid storage [156]. Regulations of body weight, appetite, lipid, and sugar homeostasis share similar mechanisms between humans and zebrafish and are similarly affected by endocrine disrupting chemicals (EDCs) [145, 157, 158]. The development of WAT starts in the pancreatic and abdominal adipose depots, then in various cranial and ocular depots, and finally expands throughout the fish. The appearance correlates with the size rather than the age of the fish [159–161]. The first adipocytes develop from 8–12 dpf or at a minimal larval size of approximately 5 mm [160].
Zebrafish obesity models enable the evaluation of diet, chemical or genetic, phenotypic modifiers through several different techniques [162–165]. Measurement of total body triglycerides may be used as an indicator for evaluating adiposity and/or obesity progression [161]. Adipocytes can also be visualized and quantified by lipid staining with the Oil Red O neutral dye or with various fluorescent lipophilic dyes (e.g., Nile Red, Lipid Green) in live fish, adult zebrafish sections, or fixed zebrafish larvae. Since zebrafish larvae are transparent, live-imaging and fluorescent staining allow ready detection and quantification of intracellular lipid droplets and adipose tissue, including its regional body distribution [166, 167]. These methodological advantages have been exploited for developing a bioassay to evaluate the obesogenic properties of chemicals in zebrafish larvae [161]. Zebrafish models can also help assess specific windows of sensitivity during life as well as transgenerational effects of obesogens [168–170] and can be used to study the interaction between the diet composition and metabolic health effects promoted by subsequent chemical exposures [114, 152, 159, 160, 171]. Interesting recent research demonstrated that long-term dietary vitamin D deficiency promoted stunted growth and increased central adiposity via both adipocyte hypertrophy and hyperplasia in both visceral and subcutaneous depots [172]. Through lipidomics analysis, these fish were demonstrated to have increased bioactive lipids that seemed to be mediated through disrupted endocannabinoid signaling [173].
Zebrafish have been widely applied to obesogenic chemical testing, with expanding capacity for modulation of diverse metabolic disrupting effects [27, 169, 174–177]. Among other obesogenic chemical evaluations, developmental exposure of bisphenol S in combination with overfeeding promoted increased triacylglycerol and visceral adiposity via disrupted lipid metabolism [175], while BPA exposures both transiently and persistently disrupted food intake, increased body weights, and disrupted gene expression related to glucose and lipid metabolism [165]. Halogenated BPA analogs also promoted lipid accumulation in zebrafish larvae in a manner correlated with their activity as zebrafish PPARγ agonists [27]. Developmental exposures to nonylpthenol and nonylphenol polyethoxylates increased body weights and adiposity (in both viscera and subcutaneous adipose depots) and disrupted energy expenditure [79]. Tributyltin exposure has been described to increase body weights, hepatic triglycerides, and hepatosomatic index, along with disrupting genes related to adipogenesis, lipogenesis, and diverse other metabolism and growth-related pathways [178] as well as increasing adiposity [161]. Developmental cadmium exposures have also been demonstrated to increase lipid accumulation, though this effect was transient (observed at one and two months post fertilization but was no longer observed by 3.5 months [177]. Perfluorooctane sulfonate (PFOS) exposures have also been described to increase adiposity and disrupt pancreatic islet morphology and area in developmentally exposed zebrafish, along with increasing fatty acid concentrations and disrupting PPAR gene expression [169].
3.2. Oryzias latipes (Medaka)
The Japanese rice fish, also known as the medaka, are a valuable model for environmental chemical and epigenetic transgenerational research [179]. Similar to zebrafish, this model can be used for estimating adipose tissue volumes and the effects of nutritional factors (dietary soy sauce oil) or various environmental chemicals such as per/polyfluoroalkyl substances and tributyltin chloride [180–182]. However, they lack the thorough characterization of adipose depots and the transparent bodies that zebrafish benefit from. They have also been utilized for determining transgenerational effects on metabolic health outcomes such as lipid metabolism [183]. Research using medaka has also evaluated chemical exposures and effects on bone formation [184], suggesting a potential strength for this model in the evaluation of differential MSC lineage commitment.
Medka have not yet been widely used in obesogenic chemical evaluations, but some preliminary research suggests utility in this model for diverse obesogenic endpoints. Specifically, exposure of medaka to both tributyltin and perfluorooctane sulfonate (PFOS) individually promoted adipose accumulation in larvae, with mixtures of these two obesogens resulting in enhanced effects (even below the individual no-effect concentrations) [181]. In related research, tributyltin exposures disrupted signaling pathways related to PPAR signaling, hormonal metabolism, and genes related to obesity in humans via mRNA-Seq analysis in exposed zebrafish [185]. Similarly, BPA exposure was reported to disrupt genes related to lipid metabolism (cholesterol and lipid synethsis, regulation, and transport, etc.) in a sex-specific manner [186].
3.3. C. elegans (Roundworm)
The roundworm is a small nematode living in temperate soil environments that has been used as a model organism since the 1960s in everything from developmental biology to neurodegenerative disease and aging. Although C. elegans is generally considered genetically and physiologically distant from humans, several studies have shown that the main regulatory pathways of energy homeostasis are shared between mammals and nematodes [144, 187, 188]. These advantages make C. elegans a suitable in vivo model to identify compounds that modulate fat storage and promote obesity [141, 189]. Both simple fluorescence (Nile red or Sudan-black probes) and biochemical (triglyceride assays) techniques can be used to quantify lipid amount and fat storage in these worms [188]. In addition, genetic approaches using mutant or transgenic animals can help evaluate molecular mechanisms underlying metabolic health effects [187, 188]. Moreover, C. elegans can be readily used to measure food intake and energy expenditure [188, 190]; several diets, food-derived or nutraceutical compounds, and fat-increasing compounds have been described to modulate fat accumulation [189–191]. Limitations of this model include lower conservation of biological pathways with humans and a lack of particular organs and circulatory systems [192]. C. elegans also lack PPARγ, though they do express orthologs of both PPARα and δ, and have no identifiable homolog for leptin [193, 194]. Perhaps unsurprisingly, they thus have no cells specifically designed for lipid storage (i.e. adipocytes), though they do still store lipids, primarily in intestinal and epidermal skin-like cells, which are comprised of diverse saturated, monounsatured, and polyunsatured fatty acids [193]. This model has also been used to assess transgenerational effects, with research demonstrating that starvation of the parental generaton promoted disrupted metabolism in the F3 generation, whereas BPA exposures resulted in transgenerational modulation of epigenetic germline silencing through up to five subsequent (non-exposed) generations (reviewed in [195]).
Despite these limitations, this model has been utilized widely in better understanding the genetics of fat accumulation, storage, and obesity [194, 196], and has been applied to obesogenic chemical evaluation successfully. Specifically, methylmercury exposure promotes triglyceride accumulation, lipid storage, and alter feeding behaviors [197], erythromycin promotes increased fat content and triacylglycerol levels as well as promoting overeating, presumably mediated through stimulation of serotonin, dopamine, and acetylcholine and/or disruption of lipogenesis and lipolysis [198]. Recent research demonstrated a non-monotonic increase in overall fat deposition and triglyceride content following bisphenol S exposures, along with modulation of fat synthesis and fatty acid oxidation gene expression [199].
3.4. Drosophila melanogaster (Fruit fly)
The fruit fly is one of the most used model organisms throughout biological research. The small size, short generation time, low cost, ease of breeding, and a large panel of genetic tools have spurred use in genetic and developmental biology research [192, 200]. Many studies have demonstrated the usefulness of this model in nutrition and obesity studies based on the manipulation of diet composition and genes involved in nutrient sensing and regulation of energy balance [201]. Although this model is anatomically different from mammals, many organ systems perform similar functions relative to mammals. For example, the fruit fly fat body covers metabolic functions of liver and adipose tissue (e.g., fat and carbohydrate storage). Instead of a fully differentiated pancreas, there are neurosecretory insulin-producing cells (IPCs), which allow carbohydrate and lipid homeostasis via the production and secretion of an insulin-like peptide [146, 201]. Few studies have utilized this model to evaluate potential obesogens and/or obesity biology, though its suitability for evaluating endocrine impact(s) on development and fertility is well accepted [202]. The efficiency of this model in assessing obesogenic properties of EDCs is highlighted by several studies demonstrating alterations of lipid homeostasis with chemical exposure (e.g., DEHP) and subsequent increase in lipid/adipose accumulation and/or transgenerational effects [203–205].
3.5. Rodents
A critical issue in selecting an animal model is whether the outcomes examined are relevant to human anatomy, physiology, molecular mechanisms and show homology with humans, which has historically driven a reliance on rodent models (e.g., rats and mice). The use of rodents in metabolic health research is well-described and assessed by several previous reviews [206–208]. Here we will address other considerations for in vivo model organism research revealed through comprehensive evaluations in rodent models. Many of these factors have yet to be evaluated or considered for the emerging models described above but will need to be assessed as they are increasingly used.
Dozens of publications have clearly delineated the use of the rodent model in metabolic health research. A number of studies (reviewed in [207, 208]) have explicitly described the use of hypercaloric and/or high fat diets to promote metabolic disorders and the clear translation of this preclinical model to human metabolic syndrome. However, other approaches, such as creating a crowded uterus in pregnant mice due to prior hemiovariectorm, have also been used to generate metabolicly abnormal intrauterine growth restricted (IUGR) and macrosomic offspring in the same litter [209].
There are diverse genetic models of obesity, including db/db mice (leptin receptor mutation that promotes higher body weights, triglycerides, and cholesterol, hyperinsulinemia, and impaired glucose tolerance), ob/ob mice (leptin gene mutation resulting in inactive leptin protein and promoting obesity, hyperinsulinaemia and hyperglycaemia), fa/fa diabetic fatty rats (different leptin receptor mutation promoting hyperinsulinaemia, hypertriglyceridaemia, and increased serum inflammatory markers), and Otsuka Long-Evans Tokushima fatty rats (Pancreatic acini cells insensitive to cholecystokinin, which controls food intake, promoting obesity, hypertriglyceridaemia, impaired glucose tolerance), that have been described in detail previously [206]. Rodents can be robust models for body weight, adiposity, development of specific adipose depots, measurement of diverse lipid classes, glucose and insulin signaling, inflammatory markers, blood pressure, controlled measurement of food and water intake and metabolic activity, as well as NASH and NAFLD, among other metabolic outcomes [206].
3.6. Use of inbred vs. outbred models
Genetic diversity of model organisms (inbred versus outbred) can be an essential design consideration for chemical contaminant studies. Researchers may select an inbred rodent strain without background genetic variation to study the epigenetic basis of phenotypic diversity (e.g., inheritance of an epigenetic trait) [210]. In contrast, a researcher may choose an outbred rodent (e.g., CD-1) for the genetically diverse background to assess toxicant-induced effects more rigorously. However, there are concerns that laboratory outbred rodent strains differ substantially between vendors and relative to bona fide outbred animals. Inbred rodents do not represent the spectrum of sensitivity required to model genetically diverse human populations accurately. For example, males at puberty have considerable heterogeneity in rodent responsiveness to estrogens [211]. The C57BL/6J inbred strain is exquisitely sensitive to estradiol after puberty relative to other mouse strains/stocks and exhibits hyper-estrogenization during fetal life, which becomes apparent in behavioral assays [212]. Interestingly, C57 mice are insensitive to xenoestrogens administered via the dam compared to the outbred, hyper-fertile CD-1 mouse, which exhibits high sensitivity fetal-neonatal response to xenoestrogens [213]. Given this, the choice of strain used can have demonstrable impacts on endpoint measurements.
3.7. Animal feed as a source of variability
Animal feed can be a substantial source of variability in toxins, phytoestrogens, sources of fats, and other components. Open formula feeds provide the proportion of nutrients, which is intended to reduce, but not eliminate, batch-to-batch variability. Closed formula (constant nutrition) feeds just provide information about the amount of protein, fat and fiber, but the sources may vary due to price and availability [214, 215]. Thus, the choice of feed used in animal studies, impacted by price, can be a critical source of variability in outcomes of health-related research and can also be the basis for studies that do not replicate prior results [216]. For example, publications by Thigpen and colleagues reported that a batch of constant nutrition rodent feed (Purina® 5002) containing elevated levels of phytoestrogens (focusing on the soy isoflavones genistein and daidzein) interfered with the ability to see estrogenic effects of a positive control chemical, the potent estrogenic drug diethylstilbestrol (DES). However, DES effects were observed with another batch of 5002 feed that had much lower phytoestrogen levels. The rat strain used also mattered, with Sprague-Dawley rats showing no effect of use of soy feed, while the CD-1 mouse (the model used by the National Toxicology Program), is, as discussed below, very sensitive to components of feed [217].
This observation by Thigpen demonstrated that there can be significant batch-to-batch variability of phytoestrogen levels in laboratory animal feed with presumably the same nutrient profile; a constant level of soy protein in different batches of a feed can have markedly different levels of phytoestrogens, which vary in soy based on many environmental factors [216]. It has been assumed for some time that the only issue of concern with soy-based feeds was variability in the soy phytoestrogens genistein and daidzein, but findings described below suggest other components of soy-based feeds (e.g., contaminated fish meal, source of lipid) may also lead to significant differences in phenotype in mice. Second, the study revealed that specific batches of feed could promote replication failure relative to most prior studies reporting that DES (a known human carcinogen) disrupted development in mice, just as it did in humans [218]. Developmental exposure to DES also promoted obesity during later adulthood in mice maintained on a soy-based (NIH31) open formula feed [219]. This demonstrates that a core issue should be whether the feed used is resulting in an inability to see effects in response to treatments that others are reporting. Not surprising is that industry-funded research on BPA, which claimed to be a replication of findings from multiple laboratories [220], in fact, had used 5002 feed [221, 222]. This led to a failure to demonstrate a BPA-induced effect in both CF-1 mice and Crl:CD Sprague-Dawley (CD-SD) rats. This research also failed to demonstrate effects of DES with this food (included as positive control) [221], suggesting an inappropriate model to detect BPA-induced effects [223].
In other studies, the expected developmental effects of DES were again shown not to occur in CD-1 mice fed 5002 feed, but were found if the mice were fed the constant nutrition, soy-based Purina® 5008/5001 breeder and maintenance feeds, respectively. Specifically, relative to Purina® 5008 fed to pregnant CD-1 mice, the 5002 feed significantly estrogenized and elevated fetal serum estradiol in fetuses. Critically, the 5008 feed had >50% higher total estrogenic activity (detected in a human breast cancer cell bioassay) as well as higher amounts of genistein and daidzein relative to the 5002 feed, substantiating that 5002 feed interfered with finding DES effects, but this was not mediated by elevated genistein and daidzein or total estrogenic activity as initially proposed [224].
In addition to problems related to the use of soy-based 5002 feed, feeding casein-based low phytoestrogen 5K96 feed to pregnant CD-1 mice also elevated endogenous serum estradiol in fetuses compared to CD-1 mice fed Purina® 5008; 5K96 casein feed thus also promoted estrogenization of mouse fetuses, similar to effects in mice exposed as fetuses to xenoestrogens such as DES or BPA [225]. Relevant to this review, the 5K96 feed resulted in morbid obesity in adult CD-1 male mice (all internal organs were encased in fat) compared to Purina 5008/5001 or Harlan Teklad 8604, another soy-based constant nutrition feed [225, 226].
Another example of feed-based impact on a supposed “non-replication” experiment was when prior metabolic effects of BPA and DES were not found is a study in which the control CD-1 mice were morbidly obese and did not show the previously reported effects of fetal exposure to BPA or DES [227] while maintained on the casein-based AIN93G feed [228]. The fetal mice whose mothers were fed casein-based 5K96 or soy-based 5002 feeds potentially had elevated aromatase (estrogen synthetase) activity, thus elevating fetal estradiol levels, compared to other soy-based feeds. Various flavonoids and lignans have been reported to inhibit aromatase activity in a human preadipocyte cell culture assay [229], although the components of the different feeds that caused these effects remain unknown.
There have been many articles published about the issue of non-replication in laboratory research, mostly attempting to sensationalize the problem [230], but clearly, there are issues, such as variability in feed, that are a major contributing factor in non-replication in laboratory animal research. The above findings demonstrate the critical importance of, whenever possible, including a positive control in toxicological or pharmacological studies that will provide information about the sensitivity and validity of the assays and results [223]. The vast diversity of animal feed components, including the casein or soy backbone and multiple sources of protein and lipids, can markedly impact research findings related to metabolic health.
3.8. The role of positive controls in animal model selection
A National Toxicology Program (NTP) panel addressed animal models for EDCs or drug research. It stated: “Because of clear species and strain differences in sensitivity, animal model selection should be based on responsiveness to active endocrine agents of concern (i.e., responsive to positive controls), not on convenience and familiarity.” The rat strain (CRL: CD(SD)) is used by many investigators to examine gestational exposure to estrogenic chemicals and drugs, although this rat strain required over a 15-fold higher dose of ethinylestradiol to show a response relative to women [231]. It is well known that selecting for very high fecundity (CD-SD rats average 14–15 pups per litter), results in low sensitivity to estrogenic drugs and chemicals [232].
It is also possible that the characteristics selected for in the generation of the CD-SD rat strain, with large litter size and accelerated postnatal growth, may make them resistant to contaminant exposures, reducing their future sensitivity and usefulness as a model; this strain is generally used in all FDA and in many commercial laboratory toxicology studies. Some strains have undergone selection for large litter sizes for over 100 generations in commercial laboratories, with the largest 5–10% of litters selected every generation for >100 generations, regardless of whether they were exposed to pesticides (in feed or used in the colony), xenoestrogens in their cage materials, or diseases in the colony, etc. The result is laboratory animal strains that are precocious, excellent breeders and produce large litters. However, the laboratory animal suppliers selected large litter animals not sensitive to environmental chemicals [211, 232]. Thus, before proceeding with experiments using environmental chemicals such as potential obesogens, it is critical to examine the sensitivity of the animal model to appropriate positive controls (e.g., DES for estrogenic testing) for the endpoint examined to ensure that each experimental design is sensitive to the environmental chemical being examined.
3.9. Animal housing
The caging used in an experiment is an additional key factor. This was clearly described in studies of BPA, the monomer used to make polycarbonate cages and bottles. Due to harsh washing of the cages, BPA was found to leach from the polycarbonate cages; this was further shown to expose both control and intervention animals to this xenoestrogen, negatively influencing the experimental determinations of successful meiosis in mouse oocytes [233–235]. It is also worth noting that the vast majority of aquatic housing systems use polycarbonate; there is likely to be leaching of BPA from these and potential recirculation of the chemical throughout the system. While some alternatives do exist [e.g., polysulfone (PS) or glass], they are often cost-prohibitive. Polycarbonate (PC) consists of BPA molecules linked by ester bonds that are subject to hydrolysis under elevated temperature or either high or low pH. PS is a co-polymer of BPA and bisphenol S (BPS) that is linked by ether bonds and is stable under temperature and pH conditions that hydrolyze BPA bonds in polycarbonate, though PS cages are more expensive. It is essential to ascertain the potential impacts of the housing materials (for rodents, also water bottles) on testing estrogenic or other metabolism disrupting chemicals.
3.10. Assays for detecting thermogenic brown fat activity
Beige and brown thermogenic fat produces heat during non-shivering thermogenesis to regulate body temperature by burning calories (i.e., glucose and lipids) [236]. These tissues help regulate glucose and lipid levels, making them high-priority targets for future therapeutics in the treatment and prevention of obesity and other metabolically related diseases [237]. The functionality of beige and brown fat and the discovery that these tissues exist in adults have made the development of reliable assays a critical step to better quantify and harness their therapeutic potential as well as to identify chemicals that promote or inhibit function.
The energy expenditure in beige and brown adipose tissue (BAT) is made possible through the activity of uncoupling protein 1 (UCP1) in brown and beige fat, which uncouples mitochondrial respiration from ATP production, leading to the generation of heat [237]. Reporter systems that focus on UCP1 levels have been developed to measure the activity of thermogenic fat and have been used as a screening tool to identify novel small molecules that can induce thermogenesis within these tissues. Specifically, the ThermoMouse model measures thermogenesis via luciferase activity linked to levels of UCP1 expression in BAT following environmental stimuli (e.g., decreased temperatures) [238], which has also been adapted as an in vitro assay to screen small molecules for luciferase activity [238]. This assay has supported screening of potential drug targets that promote UCP1, and which could provide a foundation for future BAT-mediated drug therapies that could induce thermogenesis and energy expenditure [239–243].
The OLTAM (ODD-Luc based Thermogenic Activity Measurement) system was developed to assay the activity of UCP1 independent thermogenesis in beige and BAT. In this in vivo model, a transgenic mouse that expressed the ODD (oxygen-dependent degradation) domain of hypoxia-inducible factor 1 alpha (HIF1α), tagged with luciferase, was used to measure hypoxia. Hypoxia has been shown to take place during nonshivering thermogenesis in beige and brown fat and is an indicator of thermogenesis [244]. An in vitro system was developed using the stromal vascular fraction of isolated brown adipocytes from these mice to measure cell-based thermogenic activity [244]. These cells could be used to evaluate the action of chemicals on the function of thermogenic beige and brown adipocytes.
Measuring changes in heat generated within BAT offers another tool to assay thermogenic activity. Noninvasive imaging techniques lack sensitivity and specificity due to the distance between the instrument and the tissue, and invasive techniques lack sensitivity due to their inability to directly and safely insert into BAT and their inability to detect more minute temperature fluctuations [245]. Xenon-enhanced computed tomography enabled accurate measurement of BAT within mice due to the lipophilic preference of xenon gas [246], which has been further enhanced through later research [245]. ERthermAC, a small molecule fluorescent dye that responds to changes in intracellular heat, is another tool that has been found to assay chemically stimulated thermogenesis in both rodent and human brown adipocytes [247], and has provided evidence comparable to existing indirect methods of measurement.
Lastly, UCP1-expressing brown adipose cells isolated from supraclavicular depots in humans have revealed that the molecular makeup of these cells more closely resembled mouse beige adipocytes than brown adipocytes [248]. In addition, humans who initially possessed no BAT, were found to create new BAT within the supraclavicular region. This suggests that human BAT is derived from the browning of beige fat. One could develop assays based on these cells to identify chemicals that promote or inhibit the production of these thermogenic adipocytes.
4. In silico tests
Computational strategies offer promising tools for developing animal-free models for human risk assessment of obesogens. Traditional computational methods using structural information of chemicals (quantitative structure-activity relationship (QSAR), Read Across) have already been outlined as a general strategy for non-animal testing approaches, for example, by the US National Research Council (Tox21, Toxicity Testing in the 21st Century) [249] and the Organization for Economic Cooperation and Development (OECD) guidelines. New approach methodologies (NAMs), including silico methods, are increasingly important in toxicant risk assessment [250].
With the recent advance in omics and high throughput screening, the amount of information on gene/protein activity in response to obesogenic chemicals has expanded substantially, thereby enabling the development of innovative approaches such as integrative systems biology/toxicology models. Systems toxicology uses advanced bioinformatics and statistical tools to integrate heterogeneous data types (functional genomic profile of obesogens, protein-protein interactions, protein-tissue associations, disease annotations, etc.) to mimic the complexity of the biological organization, to identify uncharacterized putative associations between an obesogen and its biological targets, and therefore to prioritize further experimental testing, thereby associating these chemicals with the disease [251, 252].
Adverse Outcome Pathways (AOPs) are structured frameworks representing relationships between molecular initiating events, key events, and adverse outcomes. The OECD proposed AOPs to enable robust mechanistic evidence for chemical safety and risk assessment [253]. However, for chemical risk assesssments, a pragmatic approach has been proposed for applying AOP criteria in evaluating the safety of a chemical [254], since a comprehensive understanding of the initiating events and molecular pathways linking chemicals to adverse outcomes is unrealistic; for a chemical such as BPA with over 10,000 publications and clearly understood to result in adverse effects [255], understanding all of the AOPs is still a work in progress. AOPs describe and connect data from various sources, i.e., databases and the scientific literature. Key information used to build AOPs can also be gathered using computational approaches based on artificial intelligence, such as frequent itemset mining and text mining [256]. AOP-helpFinder is a recent hybrid tool that combines text mining and graph theory, helping identify the existing linkages between variables (e.g., an obesogen and a biological event) by automatically screening the available scientific abstracts [257]. Using this tool, it was possible to link exposure to bisphenol S with obesity [258]. Integrative systems toxicology modeling and text mining can also link obesogens to AOPs, as proposed recently for bisphenol F [259].
5. The Future of Screening for Obesogens
A single approach or assay will not yield all the information needed to identify and classify obesogens. Data from epidemiological studies should be integrated with experimental data from animal models to support the evidence for the obesogenic potential of an identified chemical. It is advisable to adopt a tiered approach to identify and characterize EDCs, which can ultimately inform their classification as obesogens, which has been proposed previously [260]. For example, if robust biomarkers such as epigenetic modifications (e.g., DNA methylation), growth factors, or metabolites are identified through in vivo experimental studies, they can be matched with findings from human studies. In vitro methods that assess these changes will support prioritized screening for putative obesogens, which can then be classified accordingly. Structured frameworks, such as the integrated approaches to testing and assessment (IATAs), allow categorization of different tests that support the linkage of a chemical with an adverse outcome and with the different events leading to that outcome. IATAs are expected to be used for large scale obesogen testing and appear to be more time- and cost-effective than current approaches [261]. Additional in vitro tests are needed, including assays that will develop and characterize brown and beige adipocytes to be used to define further the sites and actions of potential and actual obesogens.
Approaches like this have been previously attempted using the ToxCast dataset. The National Institute for Environmental Health Sciences (NIEHS) hosted a workshop in 2011 to develop models for predicting obesogenic and/or diabetogenic outcomes using ToxCast and Tox21 data [262]. Expert panels developed (among others) a model to predict chemicals likely to promote adipocyte differentiation. An early application of this model reported poor performance in predicting both active and inactive adipogenic chemicals and suggested that better validation of primary high throughput screening assays was required before using ToxCast data for this purpose [62]. Later analysis updated the predictive model and reported more promising effects [81]. Computational modeling cannot substitute for experimental (in vitro and in vivo studies) but can help prioritize obesogens, assess human health risks and trigger new epidemiological and experimental studies. To be useful for screening purposes, computational models need to be grounded in real-world data and continually refined such that predicted activities match the results of in vitro and in vivo screening assays.
Indeed, there is consensus regarding the need for standardized testing methods to identify new chemicals that trigger metabolic dysfunction. In this context, initiatives like the French PEPPER (Public-privatE Platform for the Pre-validation of Endocrine disRuptors characterization methods, https://ed-pepper.eu) platform may facilitate development of pre-validated methods and assays in toxicology for identification of novel EDCs [263]. In Europe, a collaborative group of eight projects, named EURION [264], was established in 2019. EURION aimed to develop integrative tests to identify new EDCs. Among EURION’s projects, three projects focus on obesity and metabolic disorders (OBERON [265], GOLIATH [266], and EDCMET [267]), which are expected to deliver standardized batteries of tests for the identification of novel obesogens.
As the field of obesity and adiposity research develops, more research will likely utilize some of the alternative models described above. While historically less utilized than rodents, these models have some advantages that are likely to see increased use in the coming years. Among these are the relatively lower cost and rapid development of assays and models that may allow for superior chemical mixture assessments than using rodent models. In vitro models have also continued to expand, with an anticipated shift to greater use of normal human cell models, three-dimensional culture techniques, and co-cultures techniques that may recreate the physiology present in the tissue microenvironment more accurately. Recent advances in high content analysis provide promising grounds for increased throughput of adipogenesis models, which would enable the screening of larger number of chemicals and their mixtures with increased sensitivity and the possibility to differentiate the changes in adipocyte number as well as size [42, 75]. Predictive models are still early in development but have shown some promise in predicting likely active adipogenic and/or obesogenic chemicals. Predictive models based on key concepts for obesogens (such as those recently described for EDCs and hepatotoxicants [268, 269]) are likely to support determinations of obesogens and their causal mechanisms of action. They should be prioritized on an international level, such as the OECD.
Essential Points.
There are increasing novel capabilities to identify and assess obesogens.
There is still a reliance on using well-defined models with unclear translation to human health.
There is still a need for comprehensive validations of novel metabolic health models.
Computational models show some promise in future predictions and assessments of obesogens.
Acknowledgements:
Graphical abstract produced using Biorender.com.
Publication Support:
Christopher Kassotis, NIH, R00ES030405
Patrick J. Babin, European Union Horizon 2020 Research and Innovation Program, Oberon #825712
Dominique Lagadic-Gossman, European Union Horizon 2020 Research and Innovation Program, Oberon #825712
Sophie Langouet, European Union Horizon 2020 Research and Innovation Program, Oberon #825712
Antoine Legrand, European Union Horizon 2020 Research and Innovation Program, Oberon #825712
Hélène Le Mentec, European Union Horizon 2020 Research and Innovation Program, Oberon #825712
Frederick S. vom Saal, NIH, R02ES02139
Charbel Touma, European Union Horizon 2020 Research and Innovation Program, Oberon #825712
Robert Barouki, European Union Horizon 2020 Research and Innovation Program, Oberon #825712
Amita Bansal, Diabetes Australia #S5610040
Bruce Blumberg, NIH, R01ES023316, R01ES031139
Karine Audouze, European Union Horizon 2020, Research and Innovation Program, Oberon #825712
Vesna Munic Kos, Swedish Research Council for Sustainable Development (FORMAS)#2019–00375
Normand Podechard, European Union Horizon 2020 Research and Innovation Program, Oberon #825712
Abbreviations
- US
United States
- WAT
white adipose tissue
- PPARγ
peroxisome proliferator-activated receptor gamma
- SREBP-1
sterol-regulatory element-binding protein-1
- LXRα
liver X receptor alpha
- GR
glucocorticoid receptor
- RXRα/β
retinoid X receptor-alpha/beta
- ERα
estrogen receptor alpha
- HPAd
human preadipocytes
- SGBS
Simpson-Golabi-Behmel syndrome
- MSCs
mesenchymal stem cells
- hMADS
Human multipotent adipose-derived stem cells
- TAFLD
toxicant-associated fatty liver diseases
- NAFLD
non-alcoholic fatty liver disease
- DMSO
dimethylsulfoxide
- PHH
primary human hepatocytes
- CYP
cytochrome P450
- AhR
aryl hydrocarbon receptor
- CAR
constitutive androstane receptor
- PXR
pregnane X receptor
- DEHP
di(2-ethylhexyl) phthalate
- DDT
dichlorodiphenyltrichloroethane
- BPA
bisphenol A
- PCBs
polychlorinated biphenyls
- TBBPA
tetrabromobisphenol A
- Dpf
days post-fertilization
- EDCs
endocrine disrupting chemicals
- IPCs
insulin-producing cells
- BAT
brown adipose tissue
- UCP1
uncoupling protein 1
- OLTAM
ODD-Luc based Thermogenic Activity Measurement
- ODD
oxygen-dependent degradation
- HIF1α
hypoxia-inducible factor 1 alpha
- QSAR
quantitative structure-activity relationship
- Tox21
Toxicity Testing in the 21st Century
- OECD
Organization for Economic Cooperation and Development
- NAMs
New approach methodologies
- AOPs
Adverse Outcome Pathways
- IATAs
integrated approaches to testing and assessment
- NIEHS
National Institute for Environmental Health Sciences
- PEPPER
Public-privatE Platform for the Pre-validation of Endocrine disRuptors
Footnotes
Credit Author Statement
Conceptualization: JJH; Project Administration: CDK and JJH; Writing- Original draft preparation: all authors - CDK, FSVS, PJB, DL-G, HLM, BB, NM, AL, VMK, CM-C, NP, SL, CT, RB, MJK, KA, MC, NS, AB, SH, and JJH. Writing- Reviewing and Editing: all authors - CDK, FSVS, PJB, DL-G, HLM, BB, NM, AL, VMK, CM-C, NP, SL, CT, RB, MJK, KA, MC, NS, AB, SH, and JJH.
Disclosure Summary: None of the authors have anything to declare regarding writing this review.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Hales CM, Carroll MD, Fryar CD, Ogden CL, Prevalence of Obesity Among Adults and Youth: United States, 2015–2016, National Center for Health Statistics, Hyattsville, MD, 2017. [Google Scholar]
- [2].Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC, Prevalence of Obesity and Severe Obesity in US Children, 1999–2016, Pediatrics (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hales CM, Carroll MD, Fryar CD, CL O, Prevalence of obesity and severe obesity among adults: United States, 2017–2018, NCHS Data Brief, no 360, National Center for Health Statistics, Hyattsville, MD, 2020. [PubMed] [Google Scholar]
- [4].Fryar CD, Carroll MD, Afful J, Prevalence of Overweight, Obesity, and Severe Obesity Among Adults Aged 20 and Over: United States, 1960–1962 Through 2017–2018, in: N.H. E-Stats (Ed.) 2020. [Google Scholar]
- [5].Hammond RA, Levine R, The economic impact of obesity in the United States, Diabetes Metab Syndr Obes 3 (2010) 285–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hamilton D, Dee A, Perry IJ, The lifetime costs of overweight and obesity in childhood and adolescence: a systematic review, Obes Rev 19(4) (2018) 452–463. [DOI] [PubMed] [Google Scholar]
- [7].Goettler A, Grosse A, Sonntag D, Productivity loss due to overweight and obesity: a systematic review of indirect costs BMJ Open 7 (2017) e014632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Biener A, Cawley J, Meyerhoefer C, The Impact of Obesity on Medical Care Costs and Labor Market Outcomes in the US, Clin Chem 64(1) (2018) 108–117. [DOI] [PubMed] [Google Scholar]
- [9].Legler J, Fletcher T, Govarts E, Porta M, Blumberg B, Heindel JJ, Trasande L, Obesity, Diabetes, and Associated Costs of Exposure to Endocrine-Disrupting Chemicals in the European Union, The Journal of clinical endocrinology and metabolism 100(4) (2015) 1278–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Janesick AS, Blumberg B, Obesogens: an emerging threat to public health, American journal of obstetrics and gynecology 214(5) (2016) 559–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Heindel JJ, vom Saal FS, Blumberg B, Bovolin P, Calamandrei G, Ceresini G, Cohn BA, Fabbri E, Gioiosa L, Kassotis C, Legler J, La Merrill M, Rizzir L, Machtinger R, Mantovani A, Mendez MA, Montanini L, Molteni L, Nagel SC, Parmigiani S, Panzica G, Paterlini S, Pomatto V, Ruzzin J, Sartor G, Schug TT, Street ME, Suvorov A, Volpi R, Zoeller RT, Palanza P, Parma consensus statement on metabolic disruptors, Environmental health : a global access science source 14 (2015) 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Heindel JJ, Blumberg B, Cave M, Machtinger R, Mantovani A, Mendez MA, Nadal A, Palanza P, Panzica G, Sargis RM, Vandenberg LN, Vom Saal FS, Metabolism disrupting chemicals and metabolic disorders, Reprod Toxicol 68 (2017) 3–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Babin PJ, Gibbons GF, The evolution of plasma cholesterol: direct utility or a “spandrel” of hepatic lipid metabolism?, Prog Lipid Res 48(2) (2009) 73–91. [DOI] [PubMed] [Google Scholar]
- [14].Poulos SP, Dodson MV, Hausman GJ, Cell line models for differentiation: preadipocytes and adipocytes, Exp Biol Med (Maywood) 235(10) (2010) 1185–93. [DOI] [PubMed] [Google Scholar]
- [15].Ruiz-Ojeda FJ, Ruperez AI, Gomez-Llorente C, Gil A, Aguilera CM, Cell Models and Their Application for Studying Adipogenic Differentiation in Relation to Obesity: A Review, Int J Mol Sci 17(7) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Green H, Kehinde O, An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion, Cell 5(1) (1975) 19–27. [DOI] [PubMed] [Google Scholar]
- [17].Green H, Meuth M, An established pre-adipose cell line and its differentiation in culture, Cell 3(2) (1974) 127–33. [DOI] [PubMed] [Google Scholar]
- [18].Pillai HK, Fang M, Beglov D, Kozakov D, Vajda S, Stapleton HM, Webster TF, Schlezinger JJ, Ligand binding and activation of PPARgamma by Firemaster(R) 550: effects on adipogenesis and osteogenesis in vitro, Environmental health perspectives 122(11) (2014) 1225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wolins NE, Quaynor BK, Skinner JR, Tzekov A, Park C, Choi K, Bickel PE, OP9 mouse stromal cells rapidly differentiate into adipocytes: characterization of a useful new model of adipogenesis, J Lipid Res 47(2) (2006) 450–60. [DOI] [PubMed] [Google Scholar]
- [20].Sargis RM, Johnson DN, Choudhury RA, Brady MJ, Environmental endocrine disruptors promote adipogenesis in the 3T3-L1 cell line through glucocorticoid receptor activation, Obesity (Silver Spring) 18(7) (2010) 1283–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Guo X, Liao K, Analysis of gene expression profile during 3T3-L1 preadipocyte differentiation, Gene 251(1) (2000) 45–53. [DOI] [PubMed] [Google Scholar]
- [22].Fu M, Sun T, Bookout AL, Downes M, Yu RT, Evans RM, Mangelsdorf DJ, A Nuclear Receptor Atlas: 3T3-L1 adipogenesis, Mol Endocrinol 19(10) (2005) 2437–50. [DOI] [PubMed] [Google Scholar]
- [23].Masuno H, Kidani T, Sekiya K, Sakayama K, Shiosaka T, Yamamoto T, Honda K, Bisphenol A in combination with insulin can accelerate the conversion of 3T3-L1 fibroblasts to adipocytes, Journal of Lipid Research 43 (2002) 676–84. [PubMed] [Google Scholar]
- [24].Grun F, Watanabe H, Zamanian Z, Maeda L, Arima K, Cubacha R, Gardiner DM, Kanno J, Iguchi T, Blumberg B, Endocrine-disrupting organotin compounds are potent inducers of adipogenesis in vertebrates, Mol Endocrinol 20(9) (2006) 2141–55. [DOI] [PubMed] [Google Scholar]
- [25].Sargis RM, Johnson DN, Choudhury RA, Brady MJ, Environmental Endocrine Disruptors Promote Adipogenesis in the 3T3-L1 Cell Line through Glucocorticoid Receptor Activation, Obesity (Silver Spring, Md.) 18(7) (2010) 1283–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Riu A, Grimaldi M, le Maire A, Bey G, Phillips K, Boulahtouf A, Perdu E, Zalko D, Bourguet W, Balaguer P, Peroxisome proliferator-activated receptor gamma is a target for halogenated analogs of bisphenol A, Environmental health perspectives 119(9) (2011) 1227–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Riu A, McCollum CW, Pinto CL, Grimaldi M, Hillenweck A, Perdu E, Zalko D, Bernard L, Laudet V, Balaguer P, Bondesson M, Gustafsson JA, Halogenated bisphenol-A analogs act as obesogens in zebrafish larvae (Danio rerio), Toxicological sciences : an official journal of the Society of Toxicology 139(1) (2014) 48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Li X, Ycaza J, Blumberg B, The environmental obesogen tributyltin chloride acts via peroxisome proliferator activated receptor gamma to induce adipogenesis in murine 3T3-L1 preadipocytes, The Journal of Steroid Biochemistry and Molecular Biology 127(1–2) (2011) 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chamorro-Garcia R, Sahu M, Abbey RJ, Laude J, Pham N, Blumberg B, Transgenerational inheritance of increased fat depot size, stem cell reprogramming, and hepatic steatosis elicited by prenatal exposure to the obesogen tributyltin in mice, Environmental health perspectives 121(3) (2013) 359–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Vom Saal FS, Nagel SC, Coe BL, Angle BM, Taylor JA, The estrogenic endocrine disrupting chemical bisphenol A (BPA) and obesity, Molecular and cellular endocrinology 354(1–2) (2012) 74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Angle BM, Do RP, Ponzi D, Stahlhut RW, Drury BE, Nagel SC, Welshons WV, Besch-Williford CL, Palanza P, Parmigiani S, vom Saal FS, Taylor JA, Metabolic disruption in male mice due to fetal exposure to low but not high doses of bisphenol A (BPA): Evidence for effects on body weight, food intake, adipocytes, leptin, adiponectin, insulin and glucose regulation, Reproductive Toxicology 42 (2013) 256–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kassotis CD, Masse L, Kim S, Schlezinger JJ, Webster TF, Stapleton HM, Characterization of adipogenic chemicals in three different cell culture systems: implications for reproducibility based on cell source and handling, Scientific reports 7 (2017) 42104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zebisch K, Voigt V, Wabitsch M, Brandsch M, Protocol for effective differentiation of 3T3-L1 cells to adipocytes, Anal Biochem 425(1) (2012) 88–90. [DOI] [PubMed] [Google Scholar]
- [34].Kleensang A, Vantangoli MM, Odwin-DaCosta S, Andersen ME, Boekelheide K, Bouhifd M, Fornace AJ Jr., Li HH, Livi CB, Madnick S, Maertens A, Rosenberg M, Yager JD, Zhaog L, Hartung T, Genetic variability in a frozen batch of MCF-7 cells invisible in routine authentication affecting cell function, Scientific reports 6 (2016) 28994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kassotis CD, Hoffman K, Volker J, Pu Y, Veiga-Lopez A, Kim SM, Schlezinger JJ, Bovolin P, Cottone E, Saraceni A, Scandiffio R, Atlas E, Leingartner K, Krager S, Tischkau SA, Ermler S, Legler J, Chappell VA, Fenton SE, Mesmar F, Bondesson M, Fernandez MF, Stapleton HM, Reproducibility of adipogenic responses to metabolism disrupting chemicals in the 3T3-L1 pre-adipocyte model system: An interlaboratory study, Toxicology 461 (2021) 152900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ramskov Tetzlaff CN, Svingen T, Vinggaard AM, Rosenmai AK, Taxvig C, Bisphenols B, E, F, and S and 4-cumylphenol induce lipid accumulation in mouse adipocytes similarly to bisphenol A, Environ Toxicol 35(5) (2020) 543–552. [DOI] [PubMed] [Google Scholar]
- [37].Kassotis CD, Hoffman K, Stapleton HM, Characterization of Adipogenic Activity of Semi-volatile Indoor Contaminants and House Dust, Environmental science & technology In 51(15) (2017) 8735–8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Armstrong LE, Akinbo S, Slitt AL, 2,2’,4,4’,5-Pentabromodiphenyl ether induces lipid accumulation throughout differentiation in 3T3-L1 and human preadipocytes in vitro, J Biochem Mol Toxicol 34(6) (2020) e22485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Tung EW, Boudreau A, Wade MG, Atlas E, Induction of adipocyte differentiation by polybrominated diphenyl ethers (PBDEs) in 3T3-L1 cells, PloS one 9(4) (2014) e94583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Xu J, Shimpi P, Armstrong L, Salter D, Slitt AL, PFOS induces adipogenesis and glucose uptake in association with activation of Nrf2 signaling pathway, Toxicology and applied pharmacology 290 (2016) 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Watkins AM, Wood CR, Lin MT, Abbott BD, The effects of perfluorinated chemicals on adipocyte differentiation in vitro, Molecular and cellular endocrinology 400 (2015) 90–101. [DOI] [PubMed] [Google Scholar]
- [42].Johannes Völker Felicity Ashcroft, Vedøy Åsa, Lisa Zimmermann, Wagner M, Adipogenic Activity of Chemicals Used in Plastic Consumer Products, Environmental science & technology 56 (2022) 2487–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ariemma F, D’Esposito V, Liguoro D, Oriente F, Cabaro S, Liotti A, Cimmino I, Longo M, Beguinot F, Formisano P, Valentino R, Low-Dose Bisphenol-A Impairs Adipogenesis and Generates Dysfunctional 3T3-L1 Adipocytes, PloS one 11(3) (2016) e0150762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Lane JM, Doyle JR, Fortin JP, Kopin AS, Ordovas JM, Development of an OP9 derived cell line as a robust model to rapidly study adipocyte differentiation, PloS one 9(11) (2014) e112123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Andrews FV, Kim SM, Edwards L, Schlezinger JJ, Identifying adipogenic chemicals: Disparate effects in 3T3-L1, OP9 and primary mesenchymal multipotent cell models, Toxicology in vitro : an international journal published in association with BIBRA 67 (2020) 104904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zenbio I, Subcutaneous Human Adipocytes Manual, 2015. [Google Scholar]
- [47].Sigma M, Human Preadipocytes (HPAd) Culture Protocol, 2020. https://www.sigmaaldrich.com/technical-documents/protocols/biology/human-preadipocytes.html. (Accessed 4 November 2020.
- [48].You JS, Lee YJ, Kim KS, Kim SH, Chang KJ, Ethanol extract of lotus (Nelumbo nucifera) root exhibits an anti-adipogenic effect in human pre-adipocytes and anti-obesity and anti-oxidant effects in rats fed a high-fat diet, Nutr Res 34(3) (2014) 258–67. [DOI] [PubMed] [Google Scholar]
- [49].You JS, Lee YJ, Kim KS, Kim SH, Chang KJ, Anti-obesity and hypolipidaemic effects of Nelumbo nucifera seed ethanol extract in human pre-adipocytes and rats fed a high-fat diet, J Sci Food Agric 94(3) (2014) 568–75. [DOI] [PubMed] [Google Scholar]
- [50].Li L, Lee SJ, Kook SY, Ahn TG, Lee JY, Hwang JY, Serum from pregnant women with gestational diabetes mellitus increases the expression of FABP4 mRNA in primary subcutaneous human pre-adipocytes, Obstet Gynecol Sci 60(3) (2017) 274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Peshdary V, Styles G, Gagne R, Yauk CL, Sorisky A, Atlas E, Depot-Specific Analysis of Human Adipose Cells and Their Responses to Bisphenol S, Endocrinology 161(6) (2020). [DOI] [PubMed] [Google Scholar]
- [52].Wabitsch M, Brenner RE, Melzner I, Braun M, Moller P, Heinze E, Debatin KM, Hauner H, Characterization of a human preadipocyte cell strain with high capacity for adipose differentiation, Int J Obes Relat Metab Disord 25(1) (2001) 8–15. [DOI] [PubMed] [Google Scholar]
- [53].Fischer-Posovszky P, Newell FS, Wabitsch M, Tornqvist HE, Human SGBS cells - a unique tool for studies of human fat cell biology, Obes Facts 1(4) (2008) 184–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Allott EH, Oliver E, Lysaght J, Gray SG, Reynolds JV, Roche HM, Pidgeon GP, The SGBS cell strain as a model for the in vitro study of obesity and cancer, Clin Transl Oncol 14 (2012) 9. [DOI] [PubMed] [Google Scholar]
- [55].Guennoun A, Kazantzis M, Thomas R, Wabitsch M, Tews D, Seetharama Sastry K, Abdelkarim M, Zilberfarb V, Strosberg AD, Chouchane L, Comprehensive molecular characterization of human adipocytes reveals a transient brown phenotype, J Transl Med 13 (2015) 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Montanari T, Colitti M, Simpson-Golabi-Behmel syndrome human adipocytes reveal a changing phenotype throughout differentiation, Histochem Cell Biol 149(6) (2018) 593–605. [DOI] [PubMed] [Google Scholar]
- [57].Yeo CR, Agrawal M, Hoon S, Shabbir A, Shrivastava MK, Huang S, Khoo CM, Chhay V, Yassin MS, Tai ES, Vidal-Puig A, Toh SA, SGBS cells as a model of human adipocyte browning: A comprehensive comparative study with primary human white subcutaneous adipocytes, Scientific reports 7(1) (2017) 4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Kalkhof S, Krieg L, Buttner P, Wabitsch M, Kuntzel C, Friebe D, Landgraf K, Hanschkow M, Schubert K, Kiess W, Krohn K, Bluher M, von Bergen M, Korner A, In Depth Quantitative Proteomic and Transcriptomic Characterization of Human Adipocyte Differentiation Using the SGBS Cell Line, Proteomics (2020) e1900405. [DOI] [PubMed] [Google Scholar]
- [59].Miehle F, Moller G, Cecil A, Lintelmann J, Wabitsch M, Tokarz J, Adamski J, Haid M, Lipidomic Phenotyping Reveals Extensive Lipid Remodeling during Adipogenesis in Human Adipocytes, Metabolites 10(6) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Schaffert A, Krieg L, Weiner J, Schlichting R, Ueberham E, Karkossa I, Bauer M, Landgraf K, Junge KM, Wabitsch M, Lehmann J, Escher BI, Zenclussen AC, Korner A, Bluher M, Heiker JT, von Bergen M, Schubert K, Alternatives for the worse: Molecular insights into adverse effects of bisphenol a and substitutes during human adipocyte differentiation, Environment international 156 (2021) 106730. [DOI] [PubMed] [Google Scholar]
- [61].Kazantzis M, Takahashi V, Hinkle J, Kota S, Zilberfarb V, Issad T, Abdelkarim M, Chouchane L, Strosberg AD, PAZ6 cells constitute a representative model for human brown pre-adipocytes, Front Endocrinol (Lausanne) 3 (2012) 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Janesick AS, Dimastrogiovanni G, Vanek L, Boulos C, Chamorro-García R, Tang W, Blumberg B, On the Utility of ToxCast and ToxPi as Methods for Identifying New Obesogens, Environmental health perspectives 124(8) (2016) 1214–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Shoucri BM, Martinez ES, Abreo TJ, Hung VT, Moosova Z, Shioda T, Blumberg B, Retinoid X Receptor Activation Alters the Chromatin Landscape To Commit Mesenchymal Stem Cells to the Adipose Lineage, Endocrinology 158(10) (2017) 3109–3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Bateman ME, Strong AL, McLachlan JA, Burow ME, Bunnell BA, The Effects of Endocrine Disruptors on Adipogenesis and Osteogenesis in Mesenchymal Stem Cells: A Review, Front Endocrinol (Lausanne) 7 (2016) 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Tang QQ, Otto TC, Lane MD, Commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage, Proceedings of the National Academy of Sciences of the United States of America 101(26) (2004) 9607–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Katagiri T, Yamaguchi A, Ikeda T, Yoshiki S, Wozney JM, Rosen V, Wang EA, Tanaka H, Omura S, Suda T, The non-osteogenic mouse pluripotent cell line, C3H10T1/2, is induced to differentiate into osteoblastic cells by recombinant human bone morphogenetic protein-2, Biochem Biophys Res Commun 172(1) (1990) 295–9. [DOI] [PubMed] [Google Scholar]
- [67].Muruganandan S, Roman AA, Sinal CJ, Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: cross talk with the osteoblastogenic program, Cellular and molecular life sciences : CMLS 66(2) (2009) 236–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Yue R, Zhou Bo O., Shimada Issei S., Zhao Z, Morrison Sean J., Leptin Receptor Promotes Adipogenesis and Reduces Osteogenesis by Regulating Mesenchymal Stromal Cells in Adult Bone Marrow, Cell Stem Cell 18(6) (2016) 782–796. [DOI] [PubMed] [Google Scholar]
- [69].Csaki C, Matis U, Mobasheri A, Ye H, Shakibaei M, Chondrogenesis, osteogenesis and adipogenesis of canine mesenchymal stem cells: a biochemical, morphological and ultrastructural study, Histochem Cell Biol 128(6) (2007) 507–20. [DOI] [PubMed] [Google Scholar]
- [70].Kim S, Li A, Monti S, Schlezinger JJ, Tributyltin induces a transcriptional response without a brite adipocyte signature in adipocyte models, Archives of toxicology (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Baker AH, Watt J, Huang CK, Gerstenfeld LC, Schlezinger JJ, Tributyltin engages multiple nuclear receptor pathways and suppresses osteogenesis in bone marrow multipotent stromal cells, Chemical research in toxicology 28(6) (2015) 1156–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Chamorro-Garcia R, Kirchner S, Li X, Janesick A, Casey SC, Chow C, Blumberg B, Bisphenol A diglycidyl ether induces adipogenic differentiation of multipotent stromal stem cells through a peroxisome proliferator-activated receptor gamma-independent mechanism, Environmental health perspectives 120(7) (2012) 984–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Reina-Perez I, Olivas-Martinez A, Mustieles V, Ruiz-Ojeda FJ, Molina-Molina JM, Olea N, Fernandez MF, Bisphenol F and bisphenol S promote lipid accumulation and adipogenesis in human adipose-derived stem cells, Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association 152 (2021) 112216. [DOI] [PubMed] [Google Scholar]
- [74].Cohen IC, Cohenour ER, Harnett KG, Schuh SM, BPA BPAF and TMBPF Alter Adipogenesis and Fat Accumulation in Human Mesenchymal Stem Cells, with Implications for Obesity, Int J Mol Sci 22(10) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Norgren K, Tuck A, Vieira Silva A, Burkhardt P, Oberg M, Munic Kos V, High throughput screening of bisphenols and their mixtures under conditions of low-intensity adipogenesis of human mesenchymal stem cells (hMSCs), Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association 161 (2022) 112842. [DOI] [PubMed] [Google Scholar]
- [76].Hu P, Overby H, Heal E, Wang S, Chen J, Shen CL, Zhao L, Methylparaben and butylparaben alter multipotent mesenchymal stem cell fates towards adipocyte lineage, Toxicology and applied pharmacology 329 (2017) 48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Shoucri BM, Hung VT, Chamorro-Garcia R, Shioda T, Blumberg B, Retinoid X Receptor Activation During Adipogenesis of Female Mesenchymal Stem Cells Programs a Dysfunctional Adipocyte, Endocrinology 159(8) (2018) 2863–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Yanik SC, Baker AH, Mann KK, Schlezinger JJ, Organotins are potent activators of PPARgamma and adipocyte differentiation in bone marrow multipotent mesenchymal stromal cells, Toxicological sciences : an official journal of the Society of Toxicology 122(2) (2011) 476–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Kassotis CD, LeFauve M, Chiang Y-TT, Knuth MN, Schkoda S, Kullman SW, Nonylphenol Polyethoxylates Enhance Adipose Deposition in Developmentally Exposed Zebrafish, Toxics 10(99) (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Kim MJ, Pelloux V, Guyot E, Tordjman J, Bui LC, Chevallier A, Forest C, Benelli C, Clement K, Barouki R, Inflammatory pathway genes belong to major targets of persistent organic pollutants in adipose cells, Environmental health perspectives 120(4) (2012) 508–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Foley B, Doheny DL, Black MB, Pendse SN, Wetmore BA, Clewell RA, Andersen ME, Deisenroth C, Screening ToxCast Prioritized Chemicals for PPARG Function in a Human Adipose-Derived Stem Cell Model of Adipogenesis, Toxicological sciences : an official journal of the Society of Toxicology 155(1) (2017) 85–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Wang W, Itaka K, Ohba S, Nishiyama N, Chung UI, Yamasaki Y, Kataoka K, 3D spheroid culture system on micropatterned substrates for improved differentiation efficiency of multipotent mesenchymal stem cells, Biomaterials 30(14) (2009) 2705–15. [DOI] [PubMed] [Google Scholar]
- [83].Baraniak PR, McDevitt TC, Scaffold-free culture of mesenchymal stem cell spheroids in suspension preserves multilineage potential, Cell Tissue Res 347(3) (2012) 701–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Cheng NC, Wang S, Young TH, The influence of spheroid formation of human adipose-derived stem cells on chitosan films on stemness and differentiation capabilities, Biomaterials 33(6) (2012) 1748–58. [DOI] [PubMed] [Google Scholar]
- [85].Turner PA, Gurumurthy B, Bailey JL, Elks CM, Janorkar AV, Adipogenic Differentiation of Human Adipose-Derived Stem Cells Grown as Spheroids, Process Biochem 59 (2017) 312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Turner PA, Harris LM, Purser CA, Baker RC, Janorkar AV, A surface-tethered spheroid model for functional evaluation of 3T3-L1 adipocytes, Biotechnol Bioeng 111(1) (2014) 174–83. [DOI] [PubMed] [Google Scholar]
- [87].Ioannidou A, Alatar S, Schipper R, Baganha F, Ahlander M, Hornell A, Fisher RM, Hagberg CE, Hypertrophied human adipocyte spheroids as in vitro model of weight gain and adipose tissue dysfunction, J Physiol (2021). [DOI] [PubMed] [Google Scholar]
- [88].Miyagawa Y, Okita H, Hiroyama M, Sakamoto R, Kobayashi M, Nakajima H, Katagiri YU, Fujimoto J, Hata J, Umezawa A, Kiyokawa N, A microfabricated scaffold induces the spheroid formation of human bone marrow-derived mesenchymal progenitor cells and promotes efficient adipogenic differentiation, Tissue Eng Part A 17(3–4) (2011) 513–21. [DOI] [PubMed] [Google Scholar]
- [89].Kapur SK, Wang X, Shang H, Yun S, Li X, Feng G, Khurgel M, Katz AJ, Human adipose stem cells maintain proliferative, synthetic and multipotential properties when suspension cultured as self-assembling spheroids, Biofabrication 4(2) (2012) 025004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Ioannidou A, Alatar S, Schipper R, Baganha F, Ahlander M, Hornell A, Fisher RM, Hagberg CE, Hypertrophied human adipocyte spheroids as in vitro model of weight gain and adipose tissue dysfunction, J Physiol 600(4) (2022) 869–883. [DOI] [PubMed] [Google Scholar]
- [91].Turner PA, Tang Y, Weiss SJ, Janorkar AV, Three-dimensional spheroid cell model of in vitro adipocyte inflammation, Tissue Eng Part A 21(11–12) (2015) 1837–47. [DOI] [PubMed] [Google Scholar]
- [92].Klingelhutz AJ, Gourronc FA, Chaly A, Wadkins DA, Burand AJ, Markan KR, Idiga SO, Wu M, Potthoff MJ, Ankrum JA, Scaffold-free generation of uniform adipose spheroids for metabolism research and drug discovery, Scientific reports 8(1) (2018) 523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Shen JX, Couchet M, Dufau J, de Castro Barbosa T, Ulbrich MH, Helmstadter M, Kemas AM, Zandi Shafagh R, Marques MA, Hansen JB, Mejhert N, Langin D, Ryden M, Lauschke VM, 3D Adipose Tissue Culture Links the Organotypic Microenvironment to Improved Adipogenesis, Adv Sci (Weinh) 8(16) (2021) e2100106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Müller FA, Sturla SJ, Human in vitro models of nonalcoholic fatty liver disease, Current Opinion in Toxicology 16 (2019) 9–16. [Google Scholar]
- [95].Chavez-Tapia NC, Rosso N, Tiribelli C, In vitro models for the study of non-alcoholic fatty liver disease, Current medicinal chemistry 18(7) (2011) 1079–84. [DOI] [PubMed] [Google Scholar]
- [96].Kanuri G, Bergheim I, In vitro and in vivo models of non-alcoholic fatty liver disease (NAFLD), Int J Mol Sci 14(6) (2013) 11963–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Soret PA, Magusto J, Housset C, Gautheron J, In Vitro and In Vivo Models of Non-Alcoholic Fatty Liver Disease: A Critical Appraisal, J Clin Med 10(1) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Peyre L, Rouimi P, de Sousa G, Helies-Toussaint C, Carre B, Barcellini S, Chagnon MC, Rahmani R, Comparative study of bisphenol A and its analogue bisphenol S on human hepatic cells: a focus on their potential involvement in nonalcoholic fatty liver disease, Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association 70 (2014) 9–18. [DOI] [PubMed] [Google Scholar]
- [99].Cano R, Perez JL, Davila LA, Ortega A, Gomez Y, Valero-Cedeno NJ, Parra H, Manzano A, Veliz Castro TI, Albornoz MPD, Cano G, Rojas-Quintero J, Chacin M, Bermudez V, Role of Endocrine-Disrupting Chemicals in the Pathogenesis of Non-Alcoholic Fatty Liver Disease: A Comprehensive Review, Int J Mol Sci 22(9) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Chuntao H, Xiao Y, Huiling F, Xi Z, Jing Z, Bixian M, Yang Y. Yunjiang, TPhP Induced Lipid Metabolism Disruption Mediated by miRNA in Hepatocyte, 2008 (2021) 140–150. [Google Scholar]
- [101].Cocci P, Mosconi G, Palermo FA, Changes in expression of microRNA potentially targeting key regulators of lipid metabolism in primary gilthead sea bream hepatocytes exposed to phthalates or flame retardants, Aquat Toxicol 209 (2019) 81–90. [DOI] [PubMed] [Google Scholar]
- [102].Yang JS, Qi W, Farias-Pereira R, Choi S, Clark JM, Kim D, Park Y, Permethrin and ivermectin modulate lipid metabolism in steatosis-induced HepG2 hepatocyte, Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association 125 (2019) 595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Rose S, Cuvellier M, Ezan F, Carteret J, Bruyere A, Legagneux V, Nesslany F, Baffet G, Langouet S, DMSO-free highly differentiated HepaRG spheroids for chronic toxicity, liver functions and genotoxicity studies, Archives of toxicology (2021). [DOI] [PubMed] [Google Scholar]
- [104].Donato MT, Tolosa L, Gomez-Lechon MJ, Culture and Functional Characterization of Human Hepatoma HepG2 Cells, Methods Mol Biol 1250 (2015) 77–93. [DOI] [PubMed] [Google Scholar]
- [105].Huc L, Lemarie A, Gueraud F, Helies-Toussaint C, Low concentrations of bisphenol A induce lipid accumulation mediated by the production of reactive oxygen species in the mitochondria of HepG2 cells, Toxicology in vitro : an international journal published in association with BIBRA 26(5) (2012) 709–17. [DOI] [PubMed] [Google Scholar]
- [106].Yao HR, Liu J, Plumeri D, Cao YB, He T, Lin L, Li Y, Jiang YY, Li J, Shang J, Lipotoxicity in HepG2 cells triggered by free fatty acids, Am J Transl Res 3(3) (2011) 284–91. [PMC free article] [PubMed] [Google Scholar]
- [107].Sefried S, Haring HU, Weigert C, Eckstein SS, Suitability of hepatocyte cell lines HepG2, AML12 and THLE-2 for investigation of insulin signalling and hepatokine gene expression, Open Biol 8(10) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Herms A, Bosch M, Ariotti N, Reddy BJ, Fajardo A, Fernandez-Vidal A, Alvarez-Guaita A, Fernandez-Rojo MA, Rentero C, Tebar F, Enrich C, Geli MI, Parton RG, Gross SP, Pol A, Cell-to-cell heterogeneity in lipid droplets suggests a mechanism to reduce lipotoxicity, Curr Biol 23(15) (2013) 1489–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Aninat C, Piton A, Glaise D, Charpentier TL, Langouët S, Morel F, Guguen-Guillouzo C, Guillouzo A, EXPRESSION OF CYTOCHROMES P450, CONJUGATING ENZYMES AND NUCLEAR RECEPTORS IN HUMAN HEPATOMA HepaRG CELLS, Drug Metabolism and Disposition 34(1) (2006) 75–83 [DOI] [PubMed] [Google Scholar]
- [110].Brown MV, Compton SA, Milburn MV, Lawton KA, Cheatham B, Metabolomic signatures in lipid-loaded HepaRGs reveal pathways involved in steatotic progression, Obesity (Silver Spring, Md.) 21(12) (2013) E561–E570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Wei Y, Wang D, Pagliassotti MJ, Saturated fatty acid-mediated endoplasmic reticulum stress and apoptosis are augmented by trans-10, cis-12-conjugated linoleic acid in liver cells, Molecular and Cellular Biochemistry 303(1) (2007) 105–113 [DOI] [PubMed] [Google Scholar]
- [112].Adams JM, Pratipanawatr T, Berria R, Wang E, DeFronzo RA, Sullards MC, Mandarino LJ, Ceramide content is increased in skeletal muscle from obese insulin-resistant humans, Diabetes 53(1) (2004) 25–31. [DOI] [PubMed] [Google Scholar]
- [113].Wahlang B, Jin J, Beier JI, Hardesty JE, Daly EF, Schnegelberger RD, Falkner KC, Prough RA, Kirpich IA, Cave MC, Mechanisms of Environmental Contributions to Fatty Liver Disease, Current environmental health reports 6(3) (2019) 80–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Bucher S, Tête A, Podechard N, Liamin M, Le Guillou D, Chevanne M, Coulouarn C, Imran M, Gallais I, Fernier M, Hamdaoui Q, Robin M-A, Sergent O, Fromenty B, Lagadic-Gossmann D, Co-exposure to benzo[a]pyrene and ethanol induces a pathological progression of liver steatosis in vitro and in vivo, Scientific reports 8(1) (2018) 5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Roe AL, Howard G, Blouin R, Snawder JE, Characterization of cytochrome P450 and glutathione S-transferase activity and expression in male and female ob/ob mice, International Journal of Obesity and Related Metabolic Disorders: Journal of the International Association for the Study of Obesity 23(1) (1999) 48–53. [DOI] [PubMed] [Google Scholar]
- [116].Zhang D, Liu Z-X, Choi CS, Tian L, Kibbey R, Dong J, Cline GW, Wood PA, Shulman GI, Mitochondrial dysfunction due to long-chain Acyl-CoA dehydrogenase deficiency causes hepatic steatosis and hepatic insulin resistance, Proceedings of the National Academy of Sciences of the United States of America 104(43) (2007) 17075–17080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Fischer J, Koch L, Emmerling C, Vierkotten J, Peters T, Brüning JC, Rüther U, Inactivation of the Fto gene protects from obesity, Nature 458(7240) (2009) 894–898 [DOI] [PubMed] [Google Scholar]
- [118].Zhou Y, Shen JX, Lauschke VM, Comprehensive Evaluation of Organotypic and Microphysiological Liver Models for Prediction of Drug-Induced Liver Injury, Frontiers in pharmacology 10 (2019) 1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Takahashi Y, Hori Y, Yamamoto T, Urashima T, Ohara Y, Tanaka H, 3D spheroid cultures improve the metabolic gene expression profiles of HepaRG cells, Bioscience Reports 35(3) (2015) e00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Rose S, Cuvellier M, Ezan F, Carteret J, Bruyere A, Legagneux V, Nesslany F, Baffet G, Langouet S, DMSO-free highly differentiated HepaRG spheroids for chronic toxicity, liver functions and genotoxicity studies, Archives of toxicology 96(1) (2022) 243–258. [DOI] [PubMed] [Google Scholar]
- [121].Guillouzo A, Morel F, Langouet S, Maheo K, Rissel M, Use of hepatocyte cultures for the study of hepatotoxic compounds, J Hepatol 26 Suppl 2 (1997) 73–80. [DOI] [PubMed] [Google Scholar]
- [122].Guillouzo A, Corlu A, Aninat C, Glaise D, Morel F, Guguen-Guillouzo C, The human hepatoma HepaRG cells: A highly differentiated model for studies of liver metabolism and toxicity of xenobiotics, Chemico-Biological Interactions 168(1) (2007) 66–73 [DOI] [PubMed] [Google Scholar]
- [123].Rose S, Ezan F, Cuvellier M, Bruyère A, Legagneux V, Langouët S, Baffet G, Generation of proliferating human adult hepatocytes using optimized 3D culture conditions, Scientific reports 11 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Ramaiahgari SC, Waidyanatha S, Dixon D, DeVito MJ, Paules RS, Ferguson SS, Three-Dimensional (3D) HepaRG Spheroid Model With Physiologically Relevant Xenobiotic Metabolism Competence and Hepatocyte Functionality for Liver Toxicity Screening, Toxicological Sciences 160(1) (2017) 189–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Duriez M, Jacquet A, Hoet L, Roche S, Bock MD, Rocher C, Haussy G, Vige X, Bocskei Z, Slavnic T, Martin V, Guillemot JC, Didier M, Kannt A, Orsini C, Mikol V, Fevre AL, A 3D Human Liver Model of Nonalcoholic Steatohepatitis, J Clin Transl Hepatol 8(4) (2020) 359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Severgnini M, Sherman J, Sehgal A, Jayaprakash NK, Aubin J, Wang G, Zhang L, Peng CG, Yucius K, Butler J, Fitzgerald K, A rapid two-step method for isolation of functional primary mouseh epatocytes: cell characterization and asialoglycoprotein receptor based assay development, Cytotechnology 64(2) (2012) 187–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Yang S, Zhang A, Li T, Gao R, Peng C, Liu L, Cheng Q, Mei M, Song Y, Xiang X, Wu C, Xiao X, Li Q, Dysregulated Autophagy in Hepatocytes Promotes Bisphenol A-Induced Hepatic Lipid Accumulation in Male Mice, Endocrinology 158(9) (2017) 2799–2812. [DOI] [PubMed] [Google Scholar]
- [128].Guo X, Li H, Xu H, Halim V, Zhang W, Wang H, Ong KT, Woo SL, Walzem RL, Mashek DG, Dong H, Lu F, Wei L, Huo Y, Wu C, Palmitoleate induces hepatic steatosis but suppresses liver inflammatory response in mice, PloS one 7(6) (2012) e39286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Galloway CA, Lee H, Brookes PS, Yoon Y, Decreasing mitochondrial fission alleviates hepatic steatosis in a murine model of nonalcoholic fatty liver disease, Am J Physiol Gastrointest Liver Physiol 307(6) (2014) G632–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Go GY, Lee SJ, Jo A, Lee JR, Kang JS, Yang M, Bae GU, Bisphenol A and estradiol impede myoblast differentiation through down-regulating Akt signaling pathway, Toxicol Lett 292 (2018) 12–19. [DOI] [PubMed] [Google Scholar]
- [131].Davis ALF, Thomas AA, Shorter KS, Brown SL, Baumgarner BL, Cellular fatty acid level regulates the effect of tolylfluanid on mitochondrial dysfunction and insulin sensitivity in C2C12 skeletal myotubes, Biochemical and biophysical research communications 505(2) (2018) 392–398. [DOI] [PubMed] [Google Scholar]
- [132].Abdelmoez AM, Sardón Puig L, Smith JAB, Gabriel BM, Savikj M, Dollet L, Chibalin AV, Krook A, Zierath JR, Pillon NJ, Comparative profiling of skeletal muscle models reveals heterogeneity of transcriptome and metabolism, Am J Physiol Cell Physiol 318(3) (2020) C615–C626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Rajesh P, Balasubramanian K, Di(2-ethylhexyl)phthalate exposure impairs insulin receptor and glucose transporter 4 gene expression in L6 myotubes, Hum Exp Toxicol 33(7) (2014) 685–700. [DOI] [PubMed] [Google Scholar]
- [134].Singh VK, Sarkar SK, Saxena A, Koner BC, Effect of Subtoxic DDT Exposure on Glucose Uptake and Insulin Signaling in Rat L6 Myoblast-Derived Myotubes, International journal of toxicology 38(4) (2019) 303–311. [DOI] [PubMed] [Google Scholar]
- [135].Coletti D, Palleschi S, Silvestroni L, Cannavò A, Vivarelli E, Tomei F, Molinaro M, Adamo S, Polychlorobiphenyls inhibit skeletal muscle differentiation in culture, Toxicol Appl Pharmacol 175(3) (2001) 226–33. [DOI] [PubMed] [Google Scholar]
- [136].Zhang R, Pessah IN, Divergent Mechanisms Leading to Signaling Dysfunction in Embryonic Muscle by Bisphenol A and Tetrabromobisphenol A, Mol Pharmacol 91(4) (2017) 428–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Aas V, Bakke SS, Feng YZ, Kase ET, Jensen J, Bajpeyi S, Thoresen GH, Rustan AC, Are cultured human myotubes far from home?, Cell Tissue Res 354(3) (2013) 671–82. [DOI] [PubMed] [Google Scholar]
- [138].Hayes TB, Anderson LL, Beasley VR, de Solla SR, Iguchi T, Ingraham H, Kestemont P, Kniewald J, Kniewald Z, Langlois VS, Luque EH, McCoy KA, Munoz-de-Toro M, Oka T, Oliveira CA, Orton F, Ruby S, Suzawa M, Tavera-Mendoza LE, Trudeau VL, Victor-Costa AB, Willingham E, Demasculinization and feminization of male gonads by atrazine: consistent effects across vertebrate classes, J Steroid Biochem Mol Biol 127(1–2) (2011) 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Capitão A, Lyssimachou A, Castro LFC, Santos MM, Obesogens in the aquatic environment: an evolutionary and toxicological perspective, Environ Int 106 (2017) 153–169. [DOI] [PubMed] [Google Scholar]
- [140].Kleinert M, Clemmensen C, Hofmann SM, Moore MC, Renner S, Woods SC, Huypens P, Beckers J, de Angelis MH, Schürmann A, Bakhti M, Klingenspor M, Heiman M, Cherrington AD, Ristow M, Lickert H, Wolf E, Havel PJ, Müller TD, Tschöp MH, Animal models of obesity and diabetes mellitus, Nat Rev Endocrinol 14(3) (2018) 140–162. [DOI] [PubMed] [Google Scholar]
- [141].Schlegel A, Stainier DYR, Lessons from “Lower” Organisms: What Worms, Flies, and Zebrafish Can Teach Us about Human Energy Metabolism, PLoS genetics 3(11) (2007) e199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Benchoula K, Khatib A, Jaffar A, Ahmed QU, Sulaiman WMAW, Wahab RA, El-Seedi HR, The promise of zebrafish as a model of metabolic syndrome, Experimental animals 68(4) (2019) 407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, Collins JE, Humphray S, McLaren K, Matthews L, McLaren S, Sealy I, Caccamo M, Churcher C, Scott C, Barrett JC, Koch R, Rauch G-J, White S, Chow W, Kilian B, Quintais LT, Guerra-Assunção JA, Zhou Y, Gu Y, Yen J, Vogel J-H, Eyre T, Redmond S, Banerjee R, Chi J, Fu B, Langley E, Maguire SF, Laird GK, Lloyd D, Kenyon E, Donaldson S, Sehra H, Almeida-King J, Loveland J, Trevanion S, Jones M, Quail M, Willey D, Hunt A, Burton J, Sims S, McLay K, Plumb B, Davis J, Clee C, Oliver K, Clark R, Riddle C, Elliot D, Eliott D, Threadgold G, Harden G, Ware D, Begum S, Mortimore B, Mortimer B, Kerry G, Heath P, Phillimore B, Tracey A, Corby N, Dunn M, Johnson C, Wood J, Clark S, Pelan S, Griffiths G, Smith M, Glithero R, Howden P, Barker N, Lloyd C, Stevens C, Harley J, Holt K, Panagiotidis G, Lovell J, Beasley H, Henderson C, Gordon D, Auger K, Wright D, Collins J, Raisen C, Dyer L, Leung K, Robertson L, Ambridge K, Leongamornlert D, McGuire S, Gilderthorp R, Griffiths C, Manthravadi D, Nichol S, Barker G, Whitehead S, Kay M, Brown J, Murnane C, Gray E, Humphries M, Sycamore N, Barker D, Saunders D, Wallis J, Babbage A, Hammond S, Mashreghi-Mohammadi M, Barr L, Martin S, Wray P, Ellington A, Matthews N, Ellwood M, Woodmansey R, Clark G, Cooper JD, Cooper J, Tromans A, Grafham D, Skuce C, Pandian R, Andrews R, Harrison E, Kimberley A, Garnett J, Fosker N, Hall R, Garner P, Kelly D, Bird C, Palmer S, Gehring I, Berger A, Dooley CM, Ersan-Ürün Z, Eser C, Geiger H, Geisler M, Karotki L, Kirn A, Konantz J, Konantz M, Oberländer M, Rudolph-Geiger S, Teucke M, Lanz C, Raddatz G, Osoegawa K, Zhu B, Rapp A, Widaa S, Langford C, Yang F, Schuster SC, Carter NP, Harrow J, Ning Z, Herrero J, Searle SMJ, Enright A, Geisler R, Plasterk RHA, Lee C, Westerfield M, de Jong PJ, Zon LI, Postlethwait JH, Nüsslein-Volhard C, Hubbard TJP, Roest Crollius H, Rogers J, Stemple DL, The zebrafish reference genome sequence and its relationship to the human genome, Nature 496(7446) (2013) 498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Shen P, Yue Y, Zheng J, Park Y, Caenorhabditis elegans: A Convenient In Vivo Model for Assessing the Impact of Food Bioactive Compounds on Obesity, Aging, and Alzheimer’s Disease, Annu Rev Food Sci Technol 9 (2018) 1–22. [DOI] [PubMed] [Google Scholar]
- [145].Volkoff H, Fish as models for understanding the vertebrate endocrine regulation of feeding and weight, Molecular and Cellular Endocrinology 497 (2019) 110437. [DOI] [PubMed] [Google Scholar]
- [146].Warr CG, Shaw KH, Azim A, Piper MDW, Parsons LM, Using Mouse and Drosophila Models to Investigate the Mechanistic Links between Diet, Obesity, Type II Diabetes, and Cancer, International journal of molecular sciences 19(12) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Lieschke GJ, Currie PD, Animal models of human disease: zebrafish swim into view, Nature Reviews. Genetics 8(5) (2007) 353–367 [DOI] [PubMed] [Google Scholar]
- [148].Teame T, Zhang Z, Ran C, Zhang H, Yang Y, Ding Q, Xie M, Gao C, Ye Y, Duan M, Zhou Z, The use of zebrafish (Danio rerio) as biomedical models, Animal Frontiers 9(3) (2019) 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Goldsmith JR, Jobin C, Think Small: Zebrafish as a Model System of Human Pathology, Journal of Biomedicine and Biotechnology 2012 (2012) 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Menke AL, Spitsbergen JM, Wolterbeek APM, Woutersen RA, Normal Anatomy and Histology of the Adult Zebrafish, Toxicologic Pathology 39(5) (2011) 759–775. [DOI] [PubMed] [Google Scholar]
- [151].Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF, Stages of embryonic development of the zebrafish, Dev Dyn 203(3) (1995) 253–310. [DOI] [PubMed] [Google Scholar]
- [152].Tingaud-Sequeira A, Knoll-Gellida A, André M, Babin PJ, Vitellogenin expression in white adipose tissue in female teleost fish, Biol Reprod 86(2) (2012) 38. [DOI] [PubMed] [Google Scholar]
- [153].Minchin JE, Rawls JF, In vivo imaging and quantification of regional adiposity in zebrafish, Methods Cell Biol 138 (2017) 3–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Flynn EJ 3rd, Trent CM, Rawls JF, Ontogeny and nutritional control of adipogenesis in zebrafish (Danio rerio), J Lipid Res 50(8) (2009) 1641–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Minchin JEN, Rawls JF, A classification system for zebrafish adipose tissues, Dis Model Mech 10(6) (2017) 797–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Seth A, Stemple DL, Barroso I, The emerging use of zebrafish to model metabolic disease, Disease Models & Mechanisms 6(5) (2013) 1080–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Maradonna F, Carnevali O, Lipid Metabolism Alteration by Endocrine Disruptors in Animal Models: An Overview, Front Endocrinol (Lausanne) 9 (2018) 654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Zang L, Maddison LA, Chen W, Zebrafish as a Model for Obesity and Diabetes, Frontiers in cell and developmental biology 6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Flynn EJ, Trent CM, Rawls JF, Ontogeny and nutritional control of adipogenesis in zebrafish (Danio rerio), Journal of Lipid Research 50(8) (2009) 1641–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Imrie D, Sadler KC, White adipose tissue development in zebrafish is regulated by both developmental time and fish size, Developmental Dynamics 239(11) (2010) 3013–3023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Tingaud-Sequeira A, Ouadah N, Babin PJ, Zebrafish obesogenic test: a tool for screening molecules that target adiposity, J Lipid Res 52(9) (2011) 1765–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Ouadah-Boussouf N, Babin PJ, Pharmacological evaluation of the mechanisms involved in increased adiposity in zebrafish triggered by the environmental contaminant tributyltin, Toxicology and applied pharmacology 294 (2016) 32–42. [DOI] [PubMed] [Google Scholar]
- [163].Lutfi E, Babin PJ, Gutierrez J, Capilla E, Navarro I, Caffeic acid and hydroxytyrosol have antio-besogenic properties in zebrafish and rainbow trout models, PloS one 12(6) (2017) e0178833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Zang L, Maddison LA, Chen W, Zebrafish as a Model for Obesity and Diabetes, Front Cell Dev Biol 6 (2018) 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Martinez R, Tu W, Eng T, Allaire-Leung M, Pina B, Navarro-Martin L, Mennigen JA, Acute and long-term metabolic consequences of early developmental Bisphenol A exposure in zebrafish (Danio rerio), Chemosphere 256 (2020) 127080. [DOI] [PubMed] [Google Scholar]
- [166].Minchin JEN, Rawls JF, In vivo Analysis of White Adipose Tissue in Zebrafish, Methods in Cell Biology, Elsevier; 2011, pp. 63–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Minchin JEN, Scahill CM, Staudt N, Busch-Nentwich EM, Rawls JF, Deep phenotyping in zebrafish reveals genetic and diet-induced adiposity changes that may inform disease risk, J Lipid Res 59(8) (2018) 1536–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Mentor A, Brunström B, Mattsson A, Jönsson M, Developmental exposure to a human relevant mixture of endocrine disruptors alters metabolism and adipogenesis in zebrafish (Danio rerio), Chemosphere 238 (2020) 124584. [DOI] [PubMed] [Google Scholar]
- [169].Sant KE, Annunziato K, Conlin S, Teicher G, Chen P, Venezia O, Downes GB, Park Y, Timme-Laragy AR, Developmental exposures to perfluorooctanesulfonic acid (PFOS) impact embryonic nutrition, pancreatic morphology, and adiposity in the zebrafish, Danio rerio, Environ Pollut 275 (2021) 116644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Virote B.d.C.R., Moreira AMS, Souza J.G.d. Silva, Castro TFD, Melo N, Carneiro WF, Drummond CD, Vianna A.R.d.C.B., Murgas LDS, Obesity induction in adult zebrafish leads to negative reproduction and offspring effects, Reproduction 160(6) (2020) 833–842 [DOI] [PubMed] [Google Scholar]
- [171].Tête A, Gallais I, Imran M, Legoff L, Martin-Chouly C, Sparfel L, Bescher M, Sergent O, Podechard N, Lagadic-Gossmann D, MEHP/ethanol co-exposure favors the death of steatotic hepatocytes, possibly through CYP4A and ADH involvement, Food and Chemical Toxicology 146 (2020) 111798. [DOI] [PubMed] [Google Scholar]
- [172].Knuth MM, Mahapatra D, Jima D, Wan D, Hammock BD, Law M, Kullman SW, Vitamin D deficiency serves as a precursor to stunted growth and central adiposity in zebrafish, Scientific reports 10(1) (2020) 16032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Knuth MM, Stutts WL, Ritter MM, Garrard KP, Kullman SW, Vitamin D deficiency promotes accumulation of bioactive lipids and increased endocannabinoid tone in zebrafish, J Lipid Res 62 (2021) 100142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Mentor A, Brunstrom B, Mattsson A, Jonsson M, Developmental exposure to a human relevant mixture of endocrine disruptors alters metabolism and adipogenesis in zebrafish (Danio rerio), Chemosphere 238 (2020) 124584. [DOI] [PubMed] [Google Scholar]
- [175].Wang W, Zhang X, Wang Z, Qin J, Wang W, Tian H, Ru S, Bisphenol S induces obesogenic effects through deregulating lipid metabolism in zebrafish (Danio rerio) larvae, Chemosphere 199 (2018) 286–296. [DOI] [PubMed] [Google Scholar]
- [176].Buerger AN, Schmidt J, Chase A, Paixao C, Patel TN, Brumback BA, Kane AS, Martyniuk CJ, Bisesi JH Jr., Examining the responses of the zebrafish (Danio rerio) gastrointestinal system to the suspected obesogen diethylhexyl phthalate, Environ Pollut 245 (2019) 1086–1094. [DOI] [PubMed] [Google Scholar]
- [177].Green AJ, Hoyo C, Mattingly CJ, Luo Y, Tzeng JY, Murphy SK, Buchwalter DB, Planchart A, Cadmium exposure increases the risk of juvenile obesity: a human and zebrafish comparative study, Int J Obes (Lond) 42(7) (2018) 1285–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Lyssimachou A, Santos JG, Andre A, Soares J, Lima D, Guimaraes L, Almeida CM, Teixeira C, Castro LF, Santos MM, The Mammalian “Obesogen” Tributyltin Targets Hepatic Triglyceride Accumulation and the Transcriptional Regulation of Lipid Metabolism in the Liver and Brain of Zebrafish, PloS one 10(12) (2015) e0143911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Bhandari RK, Medaka as a model for studying environmentally induced epigenetic transgenerational inheritance of phenotypes, Environmental epigenetics 2(1)(1) (2016) 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Tonoyama Y, Tsukada M, Imai Y, Sanada M, Aota S, Oka G, Sugiura S, Hori N, Kawachi H, Shimizu Y, Shimizu N, Establishment of a quantitative in vivo method for estimating adipose tissue volumes and the effects of dietary soy sauce oil on adipogenesis in medaka, Oryzias latipes, PLoS One 13(10) (2018) e0205888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Qiu X, Iwasaki N, Chen K, Shimasaki Y, Oshima Y, Tributyltin and perfluorooctane sulfonate play a synergistic role in promoting excess fat accumulation in Japanese medaka (Oryzias latipes) via in ovo exposure, Chemosphere 220 (2019) 687–695. [DOI] [PubMed] [Google Scholar]
- [182].Chen K, Iwasaki N, Qiu X, Xu H, Takai Y, Tashiro K, Shimasaki Y, Oshima Y, Obesogenic and developmental effects of TBT on the gene expression of juvenile Japanese medaka (Oryzias latipes), Aquat Toxicol 237 (2021) 105907. [DOI] [PubMed] [Google Scholar]
- [183].Kim B-M, Jo YJ, Lee N, Lee N, Woo S, Rhee J-S, Yum S, Bisphenol A Induces a Distinct Transcriptome Profile in the Male Fish of the Marine Medaka Oryzias javanicus, BioChip Journal 12 (2018) 25–37. [Google Scholar]
- [184].Watson AT, Planchart A, Mattingly CJ, Winkler C, Reif DM, Kullman SW, From the Cover: Embryonic Exposure to TCDD Impacts Osteogenesis of the Axial Skeleton in Japanese medaka, Oryzias latipes, Toxicological sciences : an official journal of the Society of Toxicology 155(2) (2017) 485–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Takai Yuki, Takamura Takumi, Enoki Shintaro, Sato Moeko, Yoko Kato-Unoki Xuchun Qiu, Shimasaki Yohei, Oshima Y, Transcriptome analysis of medaka (Oryzias latipes) exposed to tributyltin, Japanese Journal of Environmental Toxicology 23(1) (2020) 10–21. [Google Scholar]
- [186].Kim Bo-Mi, Jo Ye Jin, Nayun Lee, Lee Nayoung, Woo Seonock, Rhee Jae-Sung, Yum S, Bisphenol A Induces a Distinct Transcriptome Profile in the Male Fish of the Marine Medaka Oryzias javanicus, BioChip Journal 12 (2018) 25–37. [Google Scholar]
- [187].Ashrafi K, Obesity and the regulation of fat metabolism, WormBook: The Online Review of C. Elegans Biology (2007) 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Watts JL, Fat synthesis and adiposity regulation in Caenorhabditis elegans, Trends in Endocrinology & Metabolism 20(2) (2009) 58–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Lemieux GA, Liu J, Mayer N, Bainton RJ, Ashrafi K, Werb Z, A whole-organism screen identifies new regulators of fat storage, Nat Chem Biol 7(4) (2011) 206–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Sun Q, Yue Y, Shen P, Yang JJ, Park Y, Cranberry Product Decreases Fat Accumulation in Caenorhabditis elegans, Journal of Medicinal Food 19(4) (2016) 427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Martorell P, Llopis S, González N, Montón F, Ortiz P, Genovés S, Ramón D, Caenorhabditis elegans as a Model To Study the Effectiveness and Metabolic Targets of Dietary Supplements Used for Obesity Treatment: The Specific Case of a Conjugated Linoleic Acid Mixture (Tonalin), Journal of Agricultural and Food Chemistry 60(44) (2012) 11071–11079 [DOI] [PubMed] [Google Scholar]
- [192].Bouyanfif A, Jayarathne S, Koboziev I, Moustaid-Moussa N, The Nematode Caenorhabditis elegans as a Model Organism to Study Metabolic Effects of ω−3 Polyunsaturated Fatty Acids in Obesity, Advances in Nutrition 10(1) (2019) 165–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Mullaney BC, Ashrafi K, C. elegans fat storage and metabolic regulation, Biochim Biophys Acta 1791(6) (2009) 474–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].McKay RM, McKay JP, Avery L, Graff JM, C elegans: a model for exploring the genetics of fat storage, Dev Cell 4(1) (2003) 131–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Mohajer N, Joloya EM, Seo J, Shioda T, Blumberg B, Epigenetic Transgenerational Inheritance of the Effects of Obesogen Exposure, Front Endocrinol (Lausanne) 12 (2021) 787580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Ke W, Reed JN, Yang C, Higgason N, Rayyan L, Wahlby C, Carpenter AE, Civelek M, O’Rourke EJ, Genes in human obesity loci are causal obesity genes in C. elegans, PLoS genetics 17(9) (2021) e1009736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Caito SW, Newell-Caito J, Martell M, Crawford N, Aschner M, Methylmercury Induces Metabolic Alterations in Caenorhabditis elegans: Role for C/EBP Transcription Factor, Toxicological sciences : an official journal of the Society of Toxicology 174(1) (2020) 112–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Luo Z, Yu Z, Yin D, Obesogenic effect of erythromycin on Caenorhabditis elegans through over-eating and lipid metabolism disturbances, Environ Pollut 294 (2022) 118615. [DOI] [PubMed] [Google Scholar]
- [199].Xiao X, Zhang X, Bai J, Li J, Zhang C, Zhao Y, Zhu Y, Zhang J, Zhou X, Bisphenol S increases the obesogenic effects of a high-glucose diet through regulating lipid metabolism in Caenorhabditis elegans, Food chemistry 339 (2021) 127813. [DOI] [PubMed] [Google Scholar]
- [200].Tolwinski N, Introduction: Drosophila—A Model System for Developmental Biology, Journal of Developmental Biology 5(3) (2017) 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Bayliak MM, Abrat OB, Storey JM, Storey KB, Lushchak VI, Interplay between diet-induced obesity and oxidative stress: Comparison between Drosophila and mammals, Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 228 (2019) 18–28 [DOI] [PubMed] [Google Scholar]
- [202].Bovier TF, Cavaliere D, Colombo M, Peluso G, Giordano E, Digilio FA, Methods to Test Endocrine Disruption in Drosophila melanogaster, Journal of Visualized Experiments (149 ) (2019). [DOI] [PubMed] [Google Scholar]
- [203].Cao H, Wiemerslage L, Marttila PSK, Williams MJ, Schiöth HB, Bis-(2-ethylhexyl) Phthalate Increases Insulin Expression and Lipid Levels in Drosophila melanogaster, Basic & Clinical Pharmacology & Toxicology 119(3) (2016) 309–316. [DOI] [PubMed] [Google Scholar]
- [204].Chen M-Y, Liu H-P, Cheng J, Chiang S-Y, Liao W-P, Lin W-Y, Transgenerational impact of DEHP on body weight of Drosophila, Chemosphere 221 (2019) 493–499. [DOI] [PubMed] [Google Scholar]
- [205].Zhang J, Yu Z, Shen J, Vandenberg LN, Yin D, Influences of sex, rhythm and generation on the obesogenic potential of erythromycin to Drosophila melanogaster, The Science of the total environment 771 (2021) 145315. [DOI] [PubMed] [Google Scholar]
- [206].Panchal SK, Brown L, Rodent models for metabolic syndrome research, J Biomed Biotechnol 2011 (2011) 351982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Leonardi BF, Gosmann G, Zimmer AR, Modeling Diet-Induced Metabolic Syndrome in Rodents, Mol Nutr Food Res 64(22) (2020) e2000249. [DOI] [PubMed] [Google Scholar]
- [208].Speakman JR, Use of high-fat diets to study rodent obesity as a model of human obesity, Int J Obes (Lond) 43(8) (2019) 1491–1492. [DOI] [PubMed] [Google Scholar]
- [209].Taylor JA, Coe BL, Shioda T, Vom Saal FS, The Crowded Uterine Horn Mouse Model for Examining Postnatal Metabolic Consequences of Intrauterine Growth Restriction vs. Macrosomia in Siblings, Metabolites 12(2) (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Cropley JE, Dang TH, Martin DI, Suter CM, The penetrance of an epigenetic trait in mice is progressively yet reversibly increased by selection and environment, Proc Biol Sci 279(1737) (2012) 2347–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Spearow JL, Doemeny P, Sera R, Leffler R, Barkley M, Genetic variation in susceptibility to endocrine disruption by estrogen in mice, Science 285(5431) (1999) 1259–1261. [DOI] [PubMed] [Google Scholar]
- [212].Svare B, Kinsley C, Mann M, Broida J, Infanticide: Accounting for genetic variation in mice., Physiol. Behav. 33 (1984) 137–152. [DOI] [PubMed] [Google Scholar]
- [213].Newbold RR, Jefferson WN, Padilla-Banks E, Prenatal exposure to bisphenol a at environmentally relevant doses adversely affects the murine female reproductive tract later in life, Environmental health perspectives 117(6) (2009) 879–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Barnard DE, Lewis SM, Teter BB, Thigpen JE, Open- and closed-formula laboratory animal diets and their importance to research, J Am Assoc Lab Anim Sci 48(6) (2009) 709–13. [PMC free article] [PubMed] [Google Scholar]
- [215].Kick B, Want to know what to feed mice? The Jackson Labs. https://www.jax.org/news-and-insights/jax-blog/2020/january/mouse-diet-guide. Accessed: February 20, 2022, (2020).
- [216].Heindel JJ, vom Saal FS, Meeting report: batch-to-batch variability in estrogenic activity in commercial animal diets--importance and approaches for laboratory animal research, Environmental health perspectives 116(3) (2008) 389–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Thigpen JE, Setchell KD, Padilla-Banks E, Haseman JK, Saunders HE, Caviness GF, Kissling GE, Grant MG, Forsythe DB, Variations in phytoestrogen content between different mill dates of the same diet produces significant differences in the time of vaginal opening in CD-1 mice and F344 rats but not in CD Sprague-Dawley rats, Environmental health perspectives 115( 12) (2007) 1717–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Newbold R, Cellular and molecular effects of developmental exposure to diethylstilbestrol: implications for other environmental estrogens, Environmental health perspectives 103(Suppl 7) (1995) 83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Newbold RR, Padilla-Banks E, Snyder RJ, Jefferson WN, Developmental exposure to estrogenic compounds and obesity, Birth defects research. Part A, Clinical and molecular teratology 73(7) (2005) 478–80. [DOI] [PubMed] [Google Scholar]
- [220].Sheehan DM, Activity of environmentally relevant low doses of endocrine disruptors and the bisphenol A controversy: initial results confirmed, Proc. Soc. Exp. Biol. Med. 224(2) (2000) 57–60. [DOI] [PubMed] [Google Scholar]
- [221].Cagen SZ, Waechter JM, Dimond SS, Breslin WJ, Butala JH, Jekat FW, Joiner RL, Shiotsuka RN, Veenstra GE, Harris LR, Normal reproductive organ development in CF-1 mice following prenatal exposure to Bisphenol A, Tox. Sci. 11 (1999) 15–29. [DOI] [PubMed] [Google Scholar]
- [222].Tyl RW, Myers CB, Marr MC, Thomas BF, Keimowitz AR, Brine DR, Veselica MM, Fail PA, Chang TY, Seely JC, Joiner RL, Butala JH, Dimond SS, Cagen SZ, Shiotsuka RN, Stropp GD, Waechter JM, Three-generation reproductive toxicity study of dietary bisphenol A in CD Sprague-Dawley rats, Toxicol. Sci. 68(1) (2002) 121–46. [DOI] [PubMed] [Google Scholar]
- [223].vom Saal FS, Welshons WV, Large effects from small exposures. II. The importance of positive controls in low-dose research on bisphenol A, Environmental research 100(1) (2006) 50–76. [DOI] [PubMed] [Google Scholar]
- [224].Ruhlen RL, Taylor JA, Mao J, Kirkpatrick J, Welshons WV, vom Saal FS, Choice of animal feed can alter fetal steroid levels and mask developmental effects of endocrine disrupting chemicals, Journal of Developmental Origins of Health and Disease 2(1) (2011) 36–48. [Google Scholar]
- [225].Ruhlen RL, Howdeshell KL, Mao J, Taylor JA, Bronson FH, Newbold RR, Welshons WV, vom Saal FS, Low phytoestrogen levels in feed increase fetal serum estradiol resulting in the “fetal estrogenization syndrome” and obesity in CD-1 mice, Environmental health perspectives 116(3) (2008) 322–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Cederroth CR, Vinciguerra M, Kühne F, Madani R, Klein M, James RW, Doerge DR, Visser TJ, Foti M, Rohner-Jeanrenaud F, Vassalli J-D, Nef S, A phytoestrogen-rich diet increases energy expenditure and decreases adiposity in mice, Environ. Health Perspect. 115(10) (2007) 1467–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Heindel JJ, Blumberg B, Cave M, Machtinger R, Mantovani A, Mendez MA, Nadal A, Palanza P, Panzica G, Sargis R, Vandenberg LN, Vom Saal FS, Metabolism disrupting chemicals and metabolic disorders, Reprod Toxicol 68 (2017) 3–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Ryan KK, Haller AM, Sorrell JE, Woods SC, Jandacek RJ, Seeley RJ, Perinatal exposure to bisphenol-a and the development of metabolic syndrome in CD-1 mice, Endocrinol. 151(6) (2010) 2603–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Wang C, Makela T, Hase T, Adlercreutz H, Kurzer MS, Lignans and flavonoids inhibit aromatase enzyme in human preadipocytes, J Steroid Biochem Mol Biol 50(3–4) (1994) 205–12. [DOI] [PubMed] [Google Scholar]
- [230].Baker M, 1,500 scientists lift the lid on reproducibility, Nature 533(7604) (2016) 452–4. [DOI] [PubMed] [Google Scholar]
- [231].vom Saal FS, Richter CA, Ruhlen RR, Nagel SC, Timms BG, Welshons WV, The importance of appropriate controls, animal feed, and animal models in interpreting results from low-dose studies of bisphenol A, Birth Defects Res (Part A) 73 (2005) 140–145. [DOI] [PubMed] [Google Scholar]
- [232].Spearow JL, Reviewer’s appendix to the EPA White Paper on Secies/Stock/Strain in Endocrine Disruptor Assays. May 26, 2004. https://www.researchgate.net/publication/333639096_Reviewer’s_Appendix_to_the_White_Paper_on_SpeciesStockStrain_in_Endocrine_Disruptor_Assays_Prepared_for_US_EPA_Endocrine_Disruptor_Screening_Program_Washington_DC_Work_Assignment_No_4-5_Task_No16 Accessed: June 6, 2021, (2004).
- [233].Hunt PA, Koehler KE, Susiarjo M, Hodges CA, Ilagan A, Voigt RC, Thomas S, Thomas BF, Hassold TJ, Bisphenol a exposure causes meiotic aneuploidy in the female mouse, Curr Biol 13(7) (2003) 546–53. [DOI] [PubMed] [Google Scholar]
- [234].Koehler KE, Voigt RC, Thomas S, Lamb B, Urban C, Hassold TJ, Hunt PA, When disaster strikes: Rethinking caging materials, Lab animal 32 (2003) 32–35. [DOI] [PubMed] [Google Scholar]
- [235].Howdeshell KL, Peterman PH, Judy BM, Taylor JA, Orazio CE, Ruhlen RL, Vom Saal FS, Welshons WV, Bisphenol A is released from used polycarbonate animal cages into water at room temperature, Environ Health Perspect 111(9) (2003) 1180–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Lizcano F, Arroyave F, Control of Adipose Cell Browning and Its Therapeutic Potential, Metabolites 10(11) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Kajimura S, Spiegelman BM, Seale P, Brown and Beige Fat: Physiological Roles beyond Heat Generation, Cell Metab 22(4) (2015) 546–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Galmozzi A, Sonne SB, Altshuler-Keylin S, Hasegawa Y, Shinoda K, Luijten IHN, Chang JW, Sharp LZ, Cravatt BF, Saez E, Kajimura S, ThermoMouse: an in vivo model to identify modulators of UCP1 expression in brown adipose tissue, Cell Rep 9(5) (2014) 1584–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Kazak L, Chouchani ET, Jedrychowski MP, Erickson BK, Shinoda K, Cohen P, Vetrivelan R, Lu GZ, Laznik-Bogoslavski D, Hasenfuss SC, Kajimura S, Gygi SP, Spiegelman BM, A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat, Cell 163(3) (2015) 643–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Shabalina IG, Kramarova TV, Nedergaard J, Cannon B, Carboxyatractyloside effects on brown-fat mitochondria imply that the adenine nucleotide translocator isoforms ANT1 and ANT2 may be responsible for basal and fatty-acid-induced uncoupling respectively, Biochem J 399(3) (2006) 405–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Long JZ, Svensson KJ, Bateman LA, Lin H, Kamenecka T, Lokurkar IA, Lou J, Rao RR, Chang MR, Jedrychowski MP, Paulo JA, Gygi SP, Griffin PR, Nomura DK, Spiegelman BM, The Secreted Enzyme PM20D1 Regulates Lipidated Amino Acid Uncouplers of Mitochondria, Cell 166(2) (2016) 424–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Park WY, Park J, Lee S, Song G, Nam IK, Ahn KS, Choe SK, Um JY, PEX13 is required for thermogenesis of white adipose tissue in cold-exposed mice, Biochim Biophys Acta Mol Cell Biol Lipids 1867(1) (2022) 159046. [DOI] [PubMed] [Google Scholar]
- [243].Snyder MM, Yue F, Zhang L, Shang R, Qiu J, Chen J, Kim KH, Peng Y, Oprescu SN, Donkin SS, Bi P, Kuang S, LETMD1 is required for mitochondrial structure and thermogenic function of brown adipocytes, FASEB J 35(11) (2021) e21965. [DOI] [PubMed] [Google Scholar]
- [244].Kim DI, Liao J, Emont MP, Park MJ, Jun H, Ramakrishnan SK, Lin JD, Shah YM, Omary MB, Wu J, An OLTAM system for analysis of brown/beige fat thermogenic activity, Int J Obes (Lond) 42(4) (2018) 939–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Antonacci MA, McHugh C, Kelley M, McCallister A, Degan S, Branca RT, Direct detection of brown adipose tissue thermogenesis in UCP1−/− mice by hyperpolarized (129)Xe MR thermometry, Sci Rep 9(1) (2019) 14865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Branca RT, McCallister A, Yuan H, Aghajanian A, Faber JE, Weimer N, Buchanan R, Floyd CS, Antonacci M, Zhang L, Burant A, Accurate quantification of brown adipose tissue mass by xenon-enhanced computed tomography, Proc Natl Acad Sci U S A 115(1) (2018) 174–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Kriszt R, Arai S, Itoh H, Lee MH, Goralczyk AG, Ang XM, Cypess AM, White AP, Shamsi F, Xue R, Lee JY, Lee SC, Hou Y, Kitaguchi T, Sudhaharan T, Ishiwata S, Lane EB, Chang YT, Tseng YH, Suzuki M, Raghunath M, Optical visualisation of thermogenesis in stimulated single-cell brown adipocytes, Sci Rep 7(1) (2017) 1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Kajimura S, Promoting brown and beige adipocyte biogenesis through the PRDM16 pathway, Int J Obes Suppl 5(Suppl 1) (2015) S11–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Knudsen T, Martin M, Chandler K, Kleinstreuer N, Judson R, Sipes N, Predictive models and computational toxicology, Methods in molecular biology (Clifton, N.J.) 947 (2013) 343–74. [DOI] [PubMed] [Google Scholar]
- [250].Bopp SK, Kienzler A, Richarz AN, van der Linden SC, Paini A, Parissis N, Worth AP, Regulatory assessment and risk management of chemical mixtures: challenges and ways forward, Crit Rev Toxicol 49(2) (2019) 174–189. [DOI] [PubMed] [Google Scholar]
- [251].Wu Q, Coumoul X, Grandjean P, Barouki R, Audouze K, Endocrine disrupting chemicals and COVID-19 relationships: a computational systems biology approach, medRxiv (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Audouze K, Brunak S, Grandjean P, A computational approach to chemical etiologies of diabetes, Scientific reports 3 (2013) 2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD, Mount DR, Nichols JW, Russom CL, Schmieder PK, Serrrano JA, Tietge JE, Villeneuve DL, Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment, Environmental toxicology and chemistry / SETAC 29(3) (2010) 730–41. [DOI] [PubMed] [Google Scholar]
- [254].Svingen T, Villeneuve DL, Knapen D, Panagiotou EM, Draskau MK, Damdimopoulou P, O’Brien JM, A Pragmatic Approach to Adverse Outcome Pathway Development and Evaluation, Toxicological sciences : an official journal of the Society of Toxicology 184(2) (2021) 183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Vom Saal FS, Vandenberg LN, Update on the Health Effects of Bisphenol A: Overwhelming Evidence of Harm, Endocrinology 162(3) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256].Oki NO, Edwards SW, An integrative data mining approach to identifying adverse outcome pathway signatures, Toxicology 350–352 (2016) 49–61. [DOI] [PubMed] [Google Scholar]
- [257].Jornod F, Jaylet T, Blaha L, Sarigiannis D, Tamisier L, Audouze K, AOP-helpFinder webserver: a tool for comprehensive analysis of the literature to support adverse outcome pathways development, Bioinformatics (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [258].Carvaillo JC, Barouki R, Coumoul X, Audouze K, Linking Bisphenol S to Adverse Outcome Pathways Using a Combined Text Mining and Systems Biology Approach, Environ Health Perspect 127(4) (2019) 47005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Rugard M, Coumoul X, Carvaillo JC, Barouki R, Audouze K, Deciphering Adverse Outcome Pathway Network Linked to Bisphenol F Using Text Mining and Systems Toxicology Approaches, Toxicological sciences : an official journal of the Society of Toxicology 173(1) (2020) 32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [260].Schug TT, Abagyan R, Blumberg B, Collins TJ, Crews D, DeFur PL, Dickerson SM, Edwards TM, Gore AC, Guillette LJ, Hayes T, Heindel JJ, Moores A, Patisaul HB, Tal TL, Thayer KA, Vandenberg LN, Warner J, Watson CS, vom Saal FS, Zoeller RT, O’Brien KP, Myers JP, Designing Endocrine Disruption Out of the Next Generation of Chemicals, Green chemistry : an international journal and green chemistry resource : GC 15(1) (2013) 181–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [261].Audouze K, Sarigiannis D, Alonso-Magdalena P, Brochot C, Casas M, Vrijheid M, Babin PJ, Karakitsios S, Coumoul X, Barouki R, Integrative Strategy of Testing Systems for Identification of Endocrine Disruptors Inducing Metabolic Disorders-An Introduction to the OBERON Project, International journal of molecular sciences 21(8) (2020) 2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [262].Thayer KA, Heindel JJ, Bucher JR, Gallo MA, Role of environmental chemicals in diabetes and obesity: a National Toxicology Program workshop review, Environmental health perspectives 120(6) (2012) 779–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].Zgheib E, Kim MJ, Jornod F, Bernal K, Tomkiewicz C, Bortoli S, Coumoul X, Barouki R, De Jesus K, Grignard E, Hubert P, Katsanou ES, Busquet F, Audouze K, Identification of non-validated endocrine disrupting chemical characterization methods by screening of the literature using artificial intelligence and by database exploration, Environment international 154 (2021) 106574. [DOI] [PubMed] [Google Scholar]
- [264].Street ME, Audouze K, Legler J, Sone H, Palanza P, Endocrine Disrupting Chemicals: Current Understanding, New Testing Strategies and Future Research Needs, Int J Mol Sci 22(2) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [265].Audouze K, Sarigiannis D, Alonso-Magdalena P, Brochot C, Casas M, Vrijheid M, Babin PJ, Karakitsios S, Coumoul X, Barouki R, Integrative Strategy of Testing Systems for Identification of Endocrine Disruptors Inducing Metabolic Disorders-An Introduction to the OBERON Project, Int J Mol Sci 21(8) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [266].Legler J, Zalko D, Jourdan F, Jacobs M, Fromenty B, Balaguer P, Bourguet W, Munic Kos V, Nadal A, Beausoleil C, Cristobal S, Remy S, Ermler S, Margiotta-Casaluci L, Griffin JL, Blumberg B, Chesne C, Hoffmann S, Andersson PL, Kamstra JH, The GOLIATH Project: Towards an Internationally Harmonised Approach for Testing Metabolism Disrupting Compounds, Int J Mol Sci 21(10) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [267].Kublbeck J, Vuorio T, Niskanen J, Fortino V, Braeuning A, Abass K, Rautio A, Hakkola J, Honkakoski P, Levonen AL, The EDCMET Project: Metabolic Effects of Endocrine Disruptors, Int J Mol Sci 21(8) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [268].La Merrill MA, Vandenberg LN, Smith MT, Goodson W, Browne P, Patisaul HB, Guyton KZ, Kortenkamp A, Cogliano VJ, Woodruff TJ, Rieswijk L, Sone H, Korach KS, Gore AC, Zeise L, Zoeller RT, Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification, Nat Rev Endocrinol 16(1) (2020) 45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [269].Rusyn I, Arzuaga X, Cattley RC, Corton JC, Ferguson SS, Godoy P, Guyton KZ, Kaplowitz N, Khetani SR, Roberts R, Roth RA, Smith MT, Key Characteristics of Human Hepatotoxicants as a Basis for Identification and Characterization of the Causes of Liver Toxicity, Hepatology n/a(n/a) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [270].Kim JY, Kim SJ, Bae MA, Kim JR, Cho KH, Cadmium exposure exacerbates severe hyperlipidemia and fatty liver changes in zebrafish via impairment of high-density lipoproteins functionality, Toxicology in vitro : an international journal published in association with BIBRA 47 (2018) 249–258. [DOI] [PubMed] [Google Scholar]
- [271].Lyche JL, Nourizadeh-Lillabadi R, Almaas C, Stavik B, Berg V, Skare JU, Alestrom P, Ropstad E, Natural mixtures of persistent organic pollutants (POP) increase weight gain, advance puberty, and induce changes in gene expression associated with steroid hormones and obesity in female zebrafish, Journal of toxicology and environmental health. Part A 73(15) (2010) 1032–57. [DOI] [PubMed] [Google Scholar]
- [272].Santangeli S, Notarstefano V, Maradonna F, Giorgini E, Gioacchini G, Forner-Piquer I, Habibi HR, Carnevali O, Effects of diethylene glycol dibenzoate and Bisphenol A on the lipid metabolism of Danio rerio, The Science of the total environment 636 (2018) 641–655. [DOI] [PubMed] [Google Scholar]
- [273].Buerger AN, Dillon DT, Schmidt J, Yang T, Zubcevic J, Martyniuk CJ, Bisesi JH Jr., Gastrointestinal dysbiosis following diethylhexyl phthalate exposure in zebrafish (Danio rerio): Altered microbial diversity, functionality, and network connectivity, Environ Pollut 265(Pt B) (2020) 114496. [DOI] [PubMed] [Google Scholar]
- [274].Bucher S, Tete A, Podechard N, Liamin M, Le Guillou D, Chevanne M, Coulouarn C, Imran M, Gallais I, Fernier M, Hamdaoui Q, Robin MA, Sergent O, Fromenty B, Lagadic-Gossmann D, Co-exposure to benzo[a]pyrene and ethanol induces a pathological progression of liver steatosis in vitro and in vivo, Scientific reports 8(1) (2018) 5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [275].Imran M, Sergent O, Tete A, Gallais I, Chevanne M, Lagadic-Gossmann D, Podechard N, Membrane Remodeling as a Key Player of the Hepatotoxicity Induced by Co-Exposure to Benzo[a]pyrene and Ethanol of Obese Zebrafish Larvae, Biomolecules 8(2) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [276].Sun L, Ling Y, Jiang J, Wang D, Wang J, Li J, Wang X, Wang H, Differential mechanisms regarding triclosan vs. bisphenol A and fluorene-9-bisphenol induced zebrafish lipid-metabolism disorders by RNA-Seq, Chemosphere 251 (2020) 126318. [DOI] [PubMed] [Google Scholar]
- [277].Martella A, Silvestri C, Maradonna F, Gioacchini G, Allara M, Radaelli G, Overby DR, Di Marzo V, Carnevali O, Bisphenol A Induces Fatty Liver by an Endocannabinoid-Mediated Positive Feedback Loop, Endocrinology 157(5) (2016) 1751–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [278].Renaud L, Silveira WAD, Hazard ES, Simpson J, Falcinelli S, Chung D, Carnevali O, Hardiman G, The Plasticizer Bisphenol A Perturbs the Hepatic Epigenome: A Systems Level Analysis of the miRNome, Genes 8(10) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [279].Qin J, Ru S, Wang W, Hao L, Ru Y, Wang J, Zhang X, Long-term bisphenol S exposure aggravates non-alcoholic fatty liver by regulating lipid metabolism and inducing endoplasmic reticulum stress response with activation of unfolded protein response in male zebrafish, Environ Pollut 263(Pt B) (2020) 114535. [DOI] [PubMed] [Google Scholar]
- [280].Wang W, Zhang X, Qin J, Wei P, Jia Y, Wang J, Ru S, Long-term bisphenol S exposure induces fat accumulation in liver of adult male zebrafish (Danio rerio) and slows yolk lipid consumption in F1 offspring, Chemosphere 221 (2019) 500–510. [DOI] [PubMed] [Google Scholar]
- [281].Tete A, Gallais I, Imran M, Legoff L, Martin-Chouly C, Sparfel L, Bescher M, Sergent O, Podechard N, Lagadic-Gossmann D, MEHP/ethanol co-exposure favors the death of steatotic hepatocytes, possibly through CYP4A and ADH involvement, Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association 146 (2020) 111798. [DOI] [PubMed] [Google Scholar]
- [282].Huff M, da Silveira WA, Carnevali O, Renaud L, Hardiman G, Systems Analysis of the Liver Transcriptome in Adult Male Zebrafish Exposed to the Plasticizer (2-Ethylhexyl) Phthalate (DEHP), Scientific reports 8(1) (2018) 2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [283].Qiu W, Liu S, Yang F, Dong P, Yang M, Wong M, Zheng C, Metabolism disruption analysis of zebrafish larvae in response to BPA and BPA analogs based on RNA-Seq technique, Ecotoxicology and environmental safety 174 (2019) 181–188. [DOI] [PubMed] [Google Scholar]
- [284].Jacobs HM, Sant KE, Basnet A, Williams LM, Moss JB, Timme-Laragy AR, Embryonic exposure to Mono(2-ethylhexyl) phthalate (MEHP) disrupts pancreatic organogenesis in zebrafish (Danio rerio), Chemosphere 195 (2018) 498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [285].Zhou X, Li J, Zhang X, Zhang C, Bai J, Zhao Y, Zhu Y, Zhang J, Xiao X, Bisphenol S promotes fat storage in multiple generations of Caenorhabditis elegans in a daf-16/nhr-49 dependent manner, Comparative biochemistry and physiology. Toxicology & pharmacology : CBP 250 (2021) 109175. [DOI] [PubMed] [Google Scholar]
- [286].Li Z, Yu Z, Gao P, Yin D, Multigenerational effects of perfluorooctanoic acid on lipid metabolism of Caenorhabditis elegans and its potential mechanism, The Science of the total environment 703 (2020) 134762. [DOI] [PubMed] [Google Scholar]
- [287].Chen MY, Liu HP, Cheng J, Chiang SY, Liao WP, Lin WY, Transgenerational impact of DEHP on body weight of Drosophila, Chemosphere 221 (2019) 493–499. [DOI] [PubMed] [Google Scholar]
- [288].Wang J, Li Y, Liu Y, Zhang H, Dai J, Disturbance of perfluorooctanoic acid on development and behavior in Drosophila larvae, Environmental toxicology and chemistry / SETAC 29(9) (2010) 2117–22. [DOI] [PubMed] [Google Scholar]
- [289].Kim JH, Barbagallo B, Annunziato K, Farias-Pereira R, Doherty JJ, Lee J, Zina J, Tindal C, McVey C, Aresco R, Johnstone M, Sant KE, Timme-Laragy A, Park Y, Clark JM, Maternal preconception PFOS exposure of Drosophila melanogaster alters reproductive capacity, development, morphology and nutrient regulation, Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association 151 (2021) 112153. [DOI] [PMC free article] [PubMed] [Google Scholar]


