Abstract
Osteoarthritis (OA) is a progressive degenerative disease resulting in joint deterioration. Synovial inflammation is present in the OA joint and has been associated with radiographic and pain progression. Several OA risk factors, including ageing, obesity, trauma and mechanical loading, play a role in OA pathogenesis, likely by modifying synovial biology. In addition, other factors, such as mitochondrial dysfunction, damage-associated molecular patterns, cytokines, metabolites and crystals in the synovium, activate synovial cells and mediate synovial inflammation. An understanding of the activated pathways that are involved in OA-related synovial inflammation could form the basis for the stratification of patients and the development of novel therapeutics. This Review focuses on the biology of the OA synovium, how the cells residing in or recruited to the synovium interact with each other, how they become activated, how they contribute to OA progression and their interplay with other joint structures.
Osteoarthritis (OA) is the most common form of arthritis, affecting more than 500 million people worldwide (~7% of the global population), with particularly high prevalence in those of advanced age (>65 years of age)1. Epidemiological studies report an increasing incidence of OA in individuals <65 years of age owing to rising obesity, an increasing number of post-traumatic OA (PTOA) cases and diagnosis at an earlier stage2. OA is a complex disease characterized by pathological changes across all the joint tissues, including cartilage, subchondral bone, ligaments, menisci, the joint capsule and the synovial membrane3. The widely accepted hypothesis of OA pathogenesis implicates an initial injury, frequently biomechanical, of any of these structures, which results in the release of mediators that lead to activation of different inflammatory pathways that damage cartilage. However, increasing evidence indicates that low-grade synovial inflammation (synovitis) contributes to radiographic and pain progression in OA.
Baseline synovitis detected by magnetic resonance imaging (MRI) or ultrasonography is associated with radiographic progression of OA, as defined by worsening of Kallgren and Lawrence (KL) grade or narrowing of joint space4-11. Synovitis progression is also associated with more cartilage damage12. Radiographic progression and development of erosions in hand OA13-16 and accelerated knee osteoarthritis (AKOA; defined as a transition from no radiographic knee OA to advanced stage disease within 4 years)17,18 are also associated with synovitis. More than 2 years before onset, patients with AKOA present with more pain, synovitis-effusion of larger volumes and signal alterations in the infrapatellar fat pad (IFP) compared with patients who develop typical knee OA17,18. MRI and ultrasonography have also been used to evaluate associations between synovitis and pain5,19-27, finding that synovitis contributes to pain in OA. Of note, a study found that synovitis partially mediates the association between cartilage damage loss and worsening pain: each 0.1-mm loss of cartilage over 24 months translated to an increase in the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain subscale score of 0.32 (95% CI 0.21–0.44)28.
However, the results of preclinical studies in animal models and of clinical trials have been contradictory29. Although blocking pro-inflammatory mediators secreted by the synovium and cartilage (including IL-6 and IL-1RA) has an analgesic effect and decreases structural progression in several preclinical models of OA30-33, not all of these studies confirmed a protective role of cytokine blocking in animal models34. In addition, in randomized controlled clinical trials in patients with painful erosive hand OA, whose erosive phenotype was associated with the presence of synovitis13-16, inhibition of the inflammatory mediators IL-1β, IL-6 and tumour necrosis factor (TNF) did not improve pain, synovitis or OA progression, as assessed by MRI or ultrasonography35-39. Finally, individuals (n = 18) with knee OA without inflammation (by ultrasonography) experienced a more prolonged benefit from intra-articular corticosteroid treatment than individuals with ultrasonography-identified inflammation (n = 16)40. Taken together, these data raise the question as to whether synovial inflammation is involved in OA pathogenesis, progression or associated joint pain.
In this Review, we describe the current knowledge of synovitis in OA joints, and discuss the pathology (FIG. 1), risk factors (FIG. 2) and cell types associated with synovial inflammation in OA. We focus on the mediators of synovial inflammation (FIG. 3), the crosstalk between synovial cells (FIG. 4) and their clinical relevance (TABLE 1, TABLE 2).
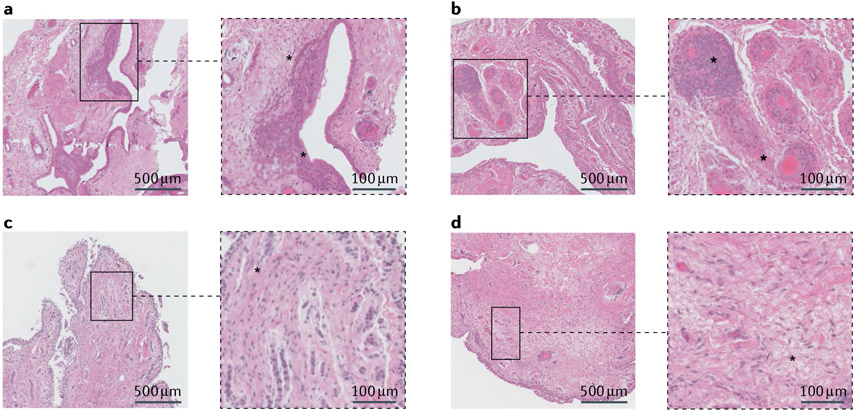
Fig. 1 ∣. Synovial inflammation and fibrosis in osteoarthritis.
Haematoxylin and eosin staining of synovial tissue from patients who underwent total knee replacement. a,b ∣ Features of an inflammatory phenotype are highlighted in the magnified insets, including hyperplasia of the synovial lining (asterisk in part a), and cellular infiltrates and vascularization in the sublining layer (asterisk in part b). c,d ∣ Features of a fibrotic phenotype are highlighted in the magnified insets, including fibrosis in the sublining layer (asterisks in parts c and d).
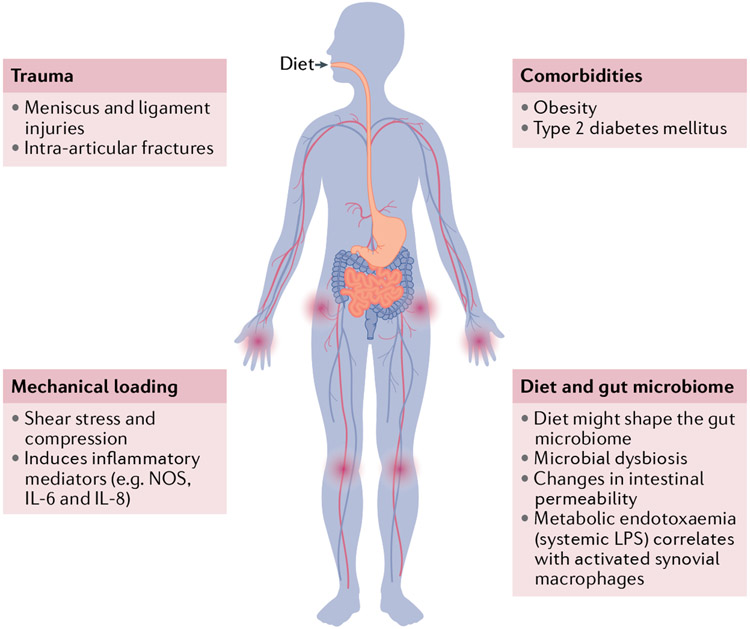
Fig. 2 ∣. Osteoarthritis risk factors and synovitis.
Among the risk factors associated with osteoarthritis (OA) development and progression, trauma, mechanical loading, comorbidities and diet–microbiome interactions are also related to synovitis. Injury to the meniscus or ligaments and intra-articular fractures lead to the development of synovitis. Aberrant and excessive loading is a known risk factor for developing OA, and subseguent shear stress and compression induce production of inflammatory mediators such as nitric oxide synthase (NOS), IL-6 and IL-8, which contribute to OA pathogenesis. Synovitis has also been related to obesity and type 2 diabetes mellitus. Dietary habit, which has been demonstrated to increase pain prevalence in patients with OA, is one of the factors that influence the composition of the gut microbiome. Microbial dysbiosis (that is, alteration in gut microbiome composition) favours inflammation and metabolic syndrome, as well as changes in intestinal permeability and metabolic endotoxaemia, which correlates with recruitment of activated pro-inflammatory synovial macrophages. LPS, lipopolysaccharide.
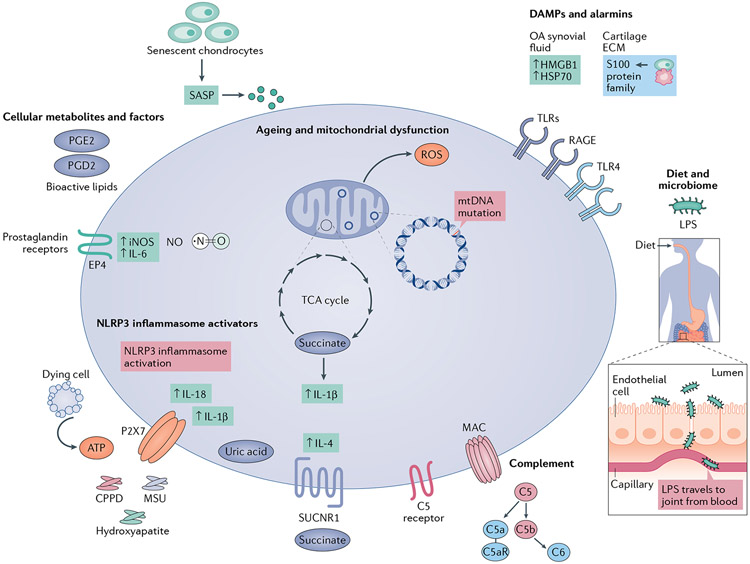
Fig. 3 ∣. Molecular mediators that contribute to synovial inflammation in osteoarthritis.
Ageing and mitochondrial damage increase reactive oxygen species (ROS) production and mitochondrial DNA mutations and can prolong the production of pro-inflammatory cytokines such as IL-1β and IL-6. Senescent cells are associated with age-related pathological conditions such as osteoarthritis (OA), and several senescence-associated secretory phenotype (SASP) factors are inflammatory mediators. Cellular metabolites, such as nitric oxide (NO), succinate and prostaglandins, as well as other bioactive lipids, contribute to inflammation and cartilage damage. NO levels are elevated in chondrocytes and pro-inflammatory macrophages in patients with OA. Succinate accumulates in inflammatory macrophages and supports their pro-inflammatory phenotype. The succinate receptor SUCNR1 is activated by soluble succinate and boosts IL-4 production. Prostaglandin E2 (PGE2) is considered the major contributor to inflammatory pain in the joint and signals through receptors such as EP4, thereby enhancing production of the pro-inflammatory factors NO (by increasing expression of inducible nitric oxide synthase (iNOS)) and IL-6, which also contributes to synovitis and increases hyperalgesia. PGD2 is also enriched in synovial fluid from patients with OA. Damage-associated molecular patterns (DAMPs) and alarmins, in the context of mechanical stress, interact with Toll-like receptors (TLRs), receptor for advanced glycation end products (RAGE) and other pattern recognition receptors to initiate and propagate inflammation. DAMPs such as high mobility group protein B1 (HMGB1) and heat shock proteins (HSPs) are abundant in OA synovial fluid. S100 family proteins are also upregulated in inflamed synovial tissue. Ectopic deposition of hydroxyapatite crystals, calcium pyrophosphate dihydrate (CPPD) microcrystals and monosodium urate (MSU) crystals, which may signal through P2X7 (depicted) or CD11b,CD16 and CD14 (not shown), as well as ATP released from dying cells, are detected by macrophages and trigger NLRP3 inflammasome activation and IL-1β and IL-18 production. MSU crystals correlate with levels of IL-1β and IL-18 in synovial fluid. Complement factors are highly expressed in OA and play a role in OA pathogenesis. Diet is one of the determining factors of microbiome composition. An altered gut microbial composition is associated with increased intestinal permeability and metabolic endotoxaemia (systemic lipopolysaccharide (LPS)), which is associated with recruitment of pro-inflammatory macrophages in the synovium.
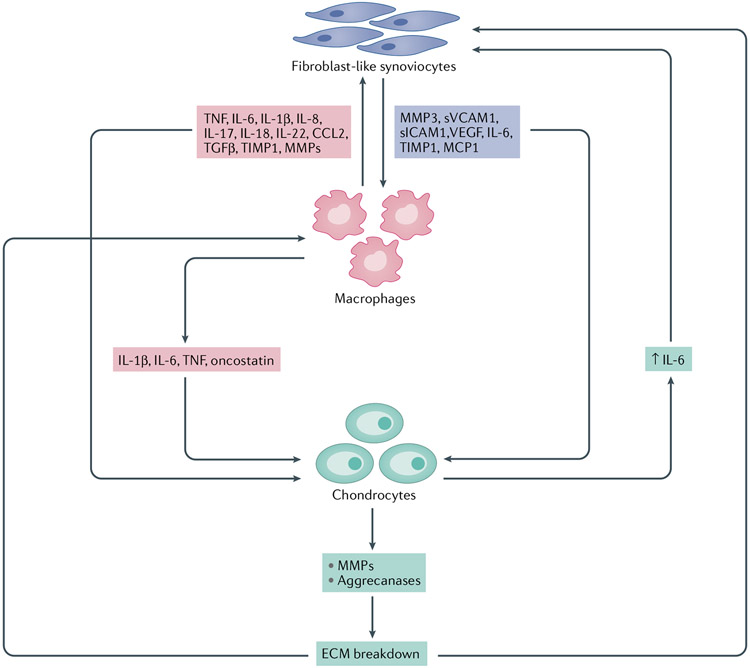
Fig. 4 ∣. Cellular crosstalk in synovitis and OA progression.
Activated fibroblast-Like synoviocytes (FLS) in the osteoarthritis (OA) synovium secrete, among other factors, cytokines, growth factors, matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs), which contribute to macrophage activation and stimulate catabolic pathways in chondrocytes. Similarly, activated macrophages secrete pro-inflammatory mediators that stimulate FLS and chondrocytes, promoting the degradation of extracellular matrix (ECM) components. ECM degradation products further activate both FLS and macrophages, resulting in a repeating cycle of inflammation and cartilagedegradation. CCL2, CC-chemokine ligand 2; MCP1, monocyte chemoattractant protein 1; sICAM1, soluble intercellular adhesion molecule 1; sVCAM1, soluble vascular cell adhesion molecule 1; TGFβ, transforming growth factor-β; TNF, tumour necrosis factor; VEGF, vascular endothelial growth factor.
Table 1 ∣.
Clinical relevance of cell types associated with synovitis in OA
| Cell type | Markers | Clinical relevance | Refs |
|---|---|---|---|
| Macrophages | Folate receptor detected by 99mTc-EC20 SPECT–CT | The quantity of activated macrophages correlated with radiographic OA severity and pain and stiffness | 71 |
| CD14+CD16+ macrophages in synovial fluid express mature macrophage marker 25F9 (indicating activation) | CD14+ macrophages/total macrophages ratio in synovial fluid is a predictor of KOOS and WOMAC scores, regardless of CD16 expression | 63 | |
| CD14 and CD163 in synovial fluid significantly associated with activated macrophages (detected by 99mTc-EC20 SPECT–CT), in the capsule (P = 0.002 and P = 0.005, respectively) and in the synovium (P = 0.0005 and P = 0.002, respectively) | CD14 and CD163 presence in the synovial fluid is associated with osteophyte severity Synovial fluid CD14 and serum CD163 associated with severity of joint space narrowing Severity of self-reported knee joint symptoms associated with both synovial fluid (β = 0.773; P = 0.003) and serum (β = 0.641; P = 0.031) CD14 levels |
71 | |
| CD11c+/CD206+ or CD86+/CD163+ ratio in synovial fluid | Associated with KL grading and severity of knee OA in patients | 72 | |
| Mannose receptors MRC1 and MRC2 | MRC1 and MRC2 recognize collagen, promoting its internalization and lysosomal degradation Resulting improvement in collagen turnover restores ECM homeostasis in the joint and ameliorates cartilage destruction Type II collagen helps to maintain expression of anti-inflammatory macrophage-related genes and pro-chondrogenic cytokines |
77 | |
| SEPP1, FLOR2, STAB1, TXNIP and CD169 | Gene expression profile is indicative of enhanced phagocytic activity and immunosuppressive activity, suggesting an immunoregulatory role | 58 | |
| CCR2+ macrophages | Present in human synovium Invasive cells that are associated with cartilage erosion in OA |
65 | |
| CCL3, CCL4, IL1B and TNF | Pro-inflammatory macrophages | 58 | |
| FLS | CD34−THY1+ FLS | Less abundant in OA synovium than in RA synovium (8% versus 22% of cells, respectively) Perivascular location, proliferative and secrete pro-inflammatory cytokines Proportion of these FLS is correlated with synovitis and synovial hypertrophy assessed by ultrasonography |
90 |
| CD34−THY− FLS | Located in synovial lining Express the osteoblastic bone formation promoter BMP6 (involved in osteophyte formation) More abundant in OA than in RA synovium |
90 | |
| CADM1, COL8A2 and DKK3 | Located in synovial lining DKK3 is a strong inhibitor of cytokine-induced collagen loss |
59,259 | |
| PTGDS, CXCL3, RSPO3, NRN1, NFKBIA, CXCL2, GEM, VCAM1, LIF, IL6 and INHBA | Associated with painful synovial sites in early OA | 52 | |
| HSPA1A, DNAJB1, SLC39A8, HTRA3, ATF3, PTGIS and BNIP3 | Associated with painful synovial sites in end-stage OA | 52 |
ECM, extracellular matrix; FLS, fibroblast-like synoviocytes; KL, Kellgren and Lawrence; KOOS, Knee Injury and Osteoarthritis Outcome Score; OA, osteoarthritis; RA, rheumatoid arthritis; TNF, tumour necrosis factor.
Table 2 ∣.
Risk factors and activators of synovial inflammation in OA
| Risk factor or activator |
Clinical relevance | Ref. |
|---|---|---|
| Obesity | Patients with obesity have a higher prevalence and severity of synovial inflammation assessed by conventional MRI | 25 |
| T2DM | Higher rates of ultrasonography-detected synovitis and effusion in patients with T2DM with end-stage knee OA who underwent arthroplasty compared with patients without T2DM, independent of patient BMI | 139 |
| Metabolic endotoxaemia | The presence of LPS in both plasma and synovial fluid from patients with OA correlates with the presence of activated macrophages in the joint capsule and synovium, radiographic severity (by 99mTc-EC20 SPECT–CT), and total WOMAC score | 158 |
| Microbiome — increased intestinal permeability and endotoxaemia | Pro-inflammatory Streptococcus species are associated with higher effusion on MRI and WOMAC knee pain, independent of BMI | 161 |
| Senescent cells | Positive correlation of the percentage of p16INK4A-expressing synoviocytes and IL-6 concentration in the synovial fluid with the degree of synovitis at the site of biopsy | 168 |
| Bioactive lipids | 11,12-DHET and 14,15-DHET levels are higher in OA knees versus unaffected knees of people with unilateral disease (P < 0.014 and P < 0.003, respectively) and are associated with radiographic progression over 3.3 years | 180 |
| HMGB1 | HMGB1 levels in the synovial fluid higher in patients with KL 4 than in those with KL 2 (P < 0.01) and KL 3 (P < 0.05) | 184 |
| Synovial fluid HMGB1 levels associated with the severity of synovitis and pain | 185 | |
| HSP70 | HSP70 levels higher in both serum and synovial fluid of individuals with knee OA than in healthy controls and both correlate with radiographic severity | 189 |
| MSU crystals | MSU crystals in the joint are associated with increased synovial fluid concentrations of IL-1β (r2 = 0.34, P < 0.0001) and IL-18 (r2 = 0.41, P < 0.0001), OA severity and radiographic progression, and osteophyte formation (P = 0.001 and P < 0.0001, respectively) | 204 |
KL, Kellgren and Lawrence; LPS, lipopolysaccharide; MSU, monosodium urate; OA, osteoarthritis; SPECT, single-photon emission computed tomography; T2DM, type 2 diabetes mellitus; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Synovitis in OA
Synovitis scores based on macroscopic features in the OA joint typically assess the presence and abundance of vascularity, villi, fibrin deposits and hyperplasia assessed by visualization of the synovium during arthroscopy41,42, although other scores use features such as hypertrophy, vascularity and global synovitis43. Synovitis scores based on microscopic histological features have also been developed, such as the histological score developed by Krenn, which includes assessment of synovial hyperplasia, stromal cell activation and inflammatory infiltrate extent; this score was able to discriminate between degenerative and inflammatory diseases44-47. Other synovial OA scores are being developed that include characteristic features of the OA synovium and are based on the predominance of each feature; synovial changes in OA have thus been classified as hyperplastic (villous hyperplasia), fibrotic (capsular fibrosis), detritus-rich (fibrinous exudate and cartilage and bone debris) and inflammatory (diffuse inflammation and aggregates of lymphoplasmacellular infiltrates), despite all of these features usually coexisting48. If present, synovial inflammation (synovitis) is characterized by proliferation of fibroblast-like synoviocytes (FLS) and macrophage recruitment, resulting in hyperplasia of the synovial lining. The synovial sub-lining can also be enriched in macrophages, T cells and, to a lesser extent, mast cells, B cells, plasma cells and endothelial cells (as components of blood vessels)49.
The two main imaging techniques that are used for synovium assessment, MRI (including contrast-enhanced MRI (CE-MRI) and conventional MRI) and ultrasonography, show good correlation with macroscopic and microscopic histological features of inflammation (BOX 1). Imaging studies revealed that synovitis in OA has a patchy distribution in different anatomical sites of the synovium, including in suprapatellar, infrapatellar, lateral and medial parapatellar and subpopliteal locations, as well as adjacent to posterior cruciate ligaments, and the extent of synovitis can also be different across these different locations50. This distribution may be clinically relevant, as different locations and scores of synovial inflammation determined by CE-MRI correlate differently with pain and radiographic OA severity51. Synovitis can be present at any disease stage49, and a study reports a correlation between the patterns of patient-reported knee pain and the location of synovitis; specifically, suprapatellar pain was highly associated with suprapatellar synovitis on MRI52. Joint effusions and synovitis may be detected by MRI in subjects with OA joint pain and normal or very minimal damage by joint radiography, indicating that synovitis is not restricted to late stages of disease11. A post-mortem study reported a prevalence of synovitis of 11% in patients with no OA history or pain, compared with a prevalence of 67% in synovium from end-stage OA joint replacement surgeries53. Interestingly, inflammatory infiltrates coexist with fibrotic changes and angiogenesis in OA, which can be more prevalent in the late stages than in the early stages of the disease48,54 (FIG. 1).
Box 1 ∣. Imaging techniques for synovitis assessment.
Magnetic resonance imaging
Two techniques: conventional MRI and contrast-enhanced MRI (CE-MRI).
Conventional MRI is still the most frequently used technique in OA, despite being unable to distinguish between synovial hypertrophy and joint effusion
Synovial hypertrophy and joint effusion were correlated in a study that identified definite synovitis (synovial thickness ≥2 mm by CE-MRI) in 96.3% of knees with an effusion259
A meta-analysis of 8 studies found that both CE-MRI (6 studies) and conventional MRI (2 studies) findings of synovitis correlated with macroscopic (vascularity, hyperplasia and villi) and microscopic (inflammatory infiltrates, synovial lining cells number, oedema and fibrosis) histological features of inflammation260
Ultrasonography261,262
Ultrasonography can assess and distinguish between synovial hypertrophy and joint effusion
Synovitis appears as thickening of the synovial membrane in grey scale (usually scored on a scale of 0–3)
Power Doppler ultrasonography can detect active synovial inflammation in OA (also scored on a scale of 0–3)
Power Doppler signal correlates with histologically confirmed inflammatory cell infiltrates, increased synovial lining layer thickness and increased vascularity
Power Doppler signal also correlates with MRI findings of synovitis (joint effusion: CI = 0.61; P < 0.001; synovial thickening: CI = 0.45; P = 0.01)263
These techniques could possibly be used to stratify patients with synovial inflammation who could benefit from specific anti-inflammatory treatments.
Cell types in the OA synovium
The inflammatory cell subsets that exist in synovial tissues have been identified by flow cytometry, single-cell transcriptomics and mass cytometry. Evaluation of the synovium from patients with OA undergoing knee replacement showed highly heterogeneous cell populations. Whereas all synovial fibroblasts expressed IL-6, a cytokine independently associated with OA pain and radiographic progression55,56, CD34+CD90+ fibroblasts located in the synovial sub-lining express substantially more IL-6 than CD34−CD90− fibroblasts in the synovial lining57. In addition, study participants categorized into clusters based on a high mesenchymal cell content or IL-6 release in the synovial inflammatory response had a history of prior joint surgery57. Single-cell RNA sequencing (scRNA-seq) detected 12 different expression profiles in cells of the synovium, including (from most to least abundant) synovial sub-intimal fibroblasts, synovial intimal fibroblasts, HLA-DRA+ cells (immune regulatory macrophages and inflammatory macrophages, dendritic cells, activated pro-inflammatory HLA-DRA+ fibroblasts and B cell clusters), smooth muscle cells, endothelial cells, T cells, mast cells and proliferating immune cells58. In addition, OA synovial samples contain more NUPR1+ monocytes than in leukocyte-rich rheumatoid arthritis (RA) synovial samples (P < 0.01), which contain a greater abundance of IL-1β+ (P < 0.001) and IFN-activated monocytes (P < 0.01) than OA synovium. NUPR1+ monocytes express high levels of tissue remodelling factors, such as the receptor tyrosine kinase MERTK and the osteoclast progenitor markers osteoactivin and cathepsin K59. Together, these studies indicate a considerable heterogeneity in cell subtypes and interaction networks in the OA synovium, which requires further characterization and understanding.
Synovial macrophages in OA
Macrophages are the most abundant immune cells in the synovium, comprising 12–40% of synovial immune cells, depending on the surface markers employed58,60,61, and they orchestrate the inflammatory and resolution phases after tissue injury62. Macrophages are also the main leukocyte population in synovial fluid in human OA knee joints (median = 36.5% of leukocytes), followed by T cells (25%)63. In particular, the CD14+CD16+ macrophage subset (35% of the total macrophage population in synovial fluid) expresses the mature macrophage marker 25F9 (17.3% of the CD14+CD16+ macrophages), indicating activation63. Interestingly, linear modelling (adjusted for sex, BMI and age) showed that the ratio of CD14+ macrophages to total macrophages is a predictor of Knee Injury and Osteoarthritis Outcome Score (KOOS) and WOMAC score, regardless of CD16 expression by this subset of macrophages63 (TABLE 1). In OA synovia, scRNA-seq of 10,640 synovial cells from 3 patients revealed that ~12.8 % of these cells were HLA-DRA+, and this subset includes immunoregulatory and pro-inflammatory macrophages, dendritic cells, pro-inflammatory fibroblasts and B cells58. The quantity of activated macrophages in OA knee joints detected by single-photon emission computed tomography (SPECT)–CT with the folate receptor-targeting imaging agent 99mTc-EC20 (etarfolatide) correlated with radiographic OA severity and symptoms, including pain and stiffness (self-reported on a scale from 0 to 3)64.
Consequently, disruption of pro-inflammatory macrophage infiltration into the synovium has been proposed as a potential therapeutic approach. In a mouse model of OA, inhibition of CC-chemokine receptor type 2 (CCR2; the receptor for the monocyte chemoattractant CCL2) impedes blood monocyte recruitment to injured joints and decreases synovitis and cartilage destruction65. In another study, depletion of synovial macrophages by intra-articular injection of anti-CD14-conjugated magnetic beads or clodronate-loaded liposomes decreased production of IL-1β, TNF and matrix metalloproteinases (MMPs) by synovial fibroblasts and reduced cartilage damage and osteophyte formation66,67. By contrast, depletion of joint macrophages in Csf1r–GFP+ macrophage FAS-induced apoptosis transgenic mice resulted in increased synovitis but did not inhibit the development of OA, owing partly to increased infiltration of neutrophils (>eightfold) and CD3+ T cells (>fivefold) into the synovium of injured joints, which cause additional damage68. These results suggest that a better understanding of macrophage subsets and their role in both healthy and injured or inflamed joints is needed. Identification of pathological macrophage subsets might provide a good opportunity to curtail synovitis and tissue damage.
The pro-inflammatory or anti-inflammatory capacity of macrophages is defined based on their effector function, transcription and metabolic programme, and surface marker expression (reviewed elsewhere69,70). Considerable effort has been focused on advancing the identification of macrophage subsets in healthy and inflamed joints and understanding how these populations are associated with clinical outcome. In particular, a study in 184 patients with radiographic knee OA from two different cohorts found that the concentration of the macrophage markers CD14 and CD163 in synovial fluid and blood are associated with OA phenotypic outcomes71. Levels of both macrophage markers in the synovial fluid were significantly associated with activated macrophages in the joint detected by 99mTc-EC20 SPECT–CT, mainly in the capsule (P = 0.002 and P = 0.005, respectively) and the synovium (P = 0.0005 and P = 0.002, respectively), of patients with knee OA71. Interestingly, CD14 and CD163 levels in the synovial fluid were associated with osteophyte severity, whereas synovial fluid CD14 and serum CD163 levels were associated with severity of joint space narrowing. The severity of self-reported knee joint symptoms was associated with CD14 levels in both synovial fluid (β = 0.773, P = 0.003) and serum (β = 0.641, P = 0.031)71. The ratio of CD11c+ to CD206+ macrophages or CD86+ to CD163+ macrophages in synovial fluid was associated with KL grading and severity of knee OA in patients72. Pro-inflammatory macrophages in OA synovium show upregulated production of matrix metalloproteinases (including MMP1, MMP3, MMP13 and MMP9), aggrecanases (including ADAMTS4 and ADAMTS5) and cyclooxygenase 2, leading to articular degeneration73. In addition, secretion of the pro-inflammatory cytokines IL-1β, IL-6, and TNF and oncostatin M stimulates destructive processes in chondrocytes and mesenchymal cells, including downregulating synthesis of type II collagen (an indispensable component of healthy articular cartilage) and aggrecan, limiting chondrogenesis74. Interestingly, CD68+ macrophages also contribute to loss of articular type II collagen by engulfing and presenting collagen fragments to CD4+ T cells75.
Anti-inflammatory macrophages also express the mannose receptors MRC1 and MRC2, which bind to collagen and promote its internalization and lysosomal degradation. The resulting improvement in collagen turnover restores ECM homeostasis in the joint and ameliorates cartilage destruction76. Importantly, type II collagen helps to maintain expression of anti-inflammatory genes in macrophages as well as pro-chondrogenic cytokines77.
Macrophage phenotyping studies have also identified subsets of alternatively activated macrophages (that is, macrophages that are enriched in neither pro-inflammatory nor anti-inflammatory markers), which are more likely to be involved in healing inflammation78. Indeed, OA synovial macrophages do not perfectly align with surface marker expression profiles corresponding to classical pro-inflammatory or anti-inflammatory phenotypes, but have been classified as a population that resembles macrophages in RA based on their expression of proliferation genes, and another population is characterized by expression of cartilage-remodelling genes61. A scRNA-seq study detected heterogeneous macrophage cell types in the OA synovium, including immunoregulatory (expressing SEPP1, FOLR2, STAB1, TXNIP and CD169) and pro-inflammatory (expressing CCL3, CCL4, IL1B and TNF) macrophage subsets58. Interestingly, this immunoregulatory population, which does not align with typical pro-inflammatory or anti-inflammatory phenotypes, exhibits a gene expression profile suggestive of enhanced phagocytic activity and immunosuppressive activity.
These results suggest that shifting macrophages towards phenotypes that might contribute to restoration of the damaged articular cartilage could represent a potential treatment for OA. Several therapeutic interventions have the ability to modify macrophage phenotypes in OA synovium. For example, glucocorticoids increase the proportion of CD163+FRβ+ synovial macrophages, and slightly reduce the proportion of CD68+ macrophages in the synovial lining, in patients with OA, resulting in decreased osteophyte formation79,80. Similarly, a cell-mediated gene therapy that is in phase II trials in patients with OA and allows localized delivery of transforming growth factor β1 (TGFβ1) improved the International Knee Documentation Committee, WOMAC and pain (evaluated on a visual analogue scale from 0 to 10) scores, elevated anti-inflammatory markers in the joints and potentiated IL-10 production81,82. In addition, functional imaging techniques (besides 99mTc-EC20 SPECT–CT) that allow the identification of macrophage subsets will help in stratifying patients with OA. Novel probes that target other macrophage markers, including CD206 (REF.83), formyl peptide receptor 1 (REF.84) or somatostatin subtype receptor 2 (REF.85), will improve understanding of macrophage phenotypes in OA.
Synovial FLS in OA
FLS are specialized mesenchymal cells that lubricate the cartilage by producing synovial fluid rich in lubricin and hyaluronic acid (also known as hyaluronan). The concentration of lubricin and hyaluronic acid is decreased in OA synovial fluid, partly owing to changes in synovial membrane permeability, but it is also associated with a change in hyaluronic acid size86. Synovial fluid viscosity is decreased in OA and may be related to joint pain, as viscosupplementation therapy with intra-articular hyaluronic acid decreases pain in patients with OA87. The transformation of healthy FLS into activated and pathological cells has been extensively studied in RA and less so in OA. Among the many factors that activate FLS in OA, follistatin-like protein 1 (FSTL1) is overexpressed in the OA synovium, and the levels of this protein correlate with OA severity (assessed by KL and WOMAC scores)88. Activated OA FLS secrete pro-inflammatory cytokines, chemokines and proteolytic enzymes (MMPs and aggrecanases), thereby contributing to the propagation of inflammation and destruction of the cartilage matrix89.
The different FLS phenotypes and their roles in OA pathogenesis have been described in studies in the past few years. A study focusing on RA FLS described different functional associated phenotypes in FLS isolated from fresh synovial tissue from patients with OA compared with patients with RA who underwent joint replacement90 (TABLE 1). In a 2021 study, the transcriptomic profiles of synovia and FLS isolated from patients with OA were distinct between patients with early or end-stage OA as well as between patient-reported pain zones and pain-free zones52. The transcriptome of synovium from pain zones in patients with early OA was characterized by upregulated expression of pro-fibrotic and pro-inflammatory genes, whereas the transcriptome of both early and end-stage OA showed upregulation of several nociceptive signalling pathways and neuronal growth genes52. Interestingly, scRNA-seq analysis of synovial explant FLS revealed that the gene expression profile of an FLS cluster representing the end-stage OA pain zone was associated with eicosanoid signalling, and the most active functions in these cells were “migration of cells” and “cell viability”52. Eicosanoid signalling was also associated with a FLS cluster related to early OA pain zone. The end-stage OA FLS had a transcription profile similar to the leukocyte-rich RA FLS described in a previous study59, whereas the early OA FLS resemble the FLS found to be more predominant in OA in this previous study59.
Other synovial cells in OA
Neutrophils are another innate immune cell type that is found in the OA knee joint and are highly abundant in synovial fluid compared with synovial tissue91, although the reason for this distribution is still unknown. The secretion of the key proteolytic enzymes elastase and neutrophil gelatinase-associated lipocalin by activated neutrophils correlates with cartilage damage and radiographic progression91,92. Mast cells are also present in the synovium and are associated with inflammation and cartilage destruction in OA93. Synovial fluid from individuals with OA is enriched in tryptase (2–25 ng/ml), a mast cell-specific enzyme that is released during degranulation, compared with control individuals94. Deficiency of mast cells reduces cartilage loss, osteophyte formation and synovitis in the destabilization of the medial meniscus (DMM) mouse model of OA94. In addition, mast cell-dependent production of prostaglandin D2 in response to elevation in nerve growth factor (NGF) levels leads to an increase in nociceptive signalling in OA joints95.
Endothelial cells are present in joint structures and angiogenesis is implicated in OA pathogenesis96. Histological analysis of established OA synovium detected pericytes in all blood vessels, suggesting that these vessels are fully mature and stable, which might explain the persistent inflammation in OA; by contrast, the synovial vasculature in inflammatory arthritis is characterized by a mixture of mature and immature vessels97. This study also found that blood vessels were distributed throughout the depth of the synovial membrane in OA, without preferential distribution in synovial lining cells97. Endothelial-cell-derived vascular endothelial growth factor (VEGF) seems to play an important part in OA pathogenesis, as the serum and synovial fluid concentration of VEGF correlates positively with WOMAC, radiographic severity (KL score) and the presence of osteophytes and a power Doppler ultrasonography signal of synovitis98. Although VEGF is crucial for cartilage formation, its expression seems to be upregulated in the joint of patients with OA and in surgically induced knee OA in mice; increased VEGF expression is associated with catabolic processes in chondrocytes and synovial cells (related to cartilage destruction)99. Furthermore, conditional knockdown of Vegf attenuated injury-induced OA in mice and intra-articular anti-VEGF antibodies suppressed OA progression and blocked VEGF signalling, as revealed by reduced levels of phosphorylated VEGFR receptors in articular chondrocytes, synovial cells and dorsal root ganglia99. Indeed, oral administration of the VEGFR2 kinase inhibitor vandetanib attenuated OA progression99.
Studies investigating the composition of the synovial membrane also reported the presence of T cells, including T helper (TH) 1 cells, TH2 cells, TH9 cells, TH17 cells, TH22 cells, T regulatory (Treg) cells and cytotoxic T cells100, even in the earliest stages of disease101. Although a change in the profile of T cell subtypes was described to correlate with disease activity and pain102, the role of T cells in the development and progression of OA has yet to be determined100.
The presence of the varied cellular players in the synovial tissue might complicate histological evaluation of the OA synovium. As alteration in the equilibrium and interaction between these cell types shape OA progression and symptomatology, understanding of the mediators of this intricate network is therefore crucial.
OA risk factors and synovitis
Trauma
PTOA (FIG. 2) represents ~12% of all cases of symptomatic OA103. A study found that patients with a 3–10-year history of sport-related intra-articular knee injury developed OA104. Both animal and human studies have demonstrated that joint injuries (to menisci and ligaments, as well as intra-articular fractures) lead to the development of synovitis105-107. For example, data from the Osteoarthritis Initiative showed that injury was associated with accelerated OA development, as assessed by KL grade108,109, whereas other studies found a higher incidence of OA in patients with joint injuries than in those without injuries110. As a surrogate of the presence of joint inflammation, pro-inflammatory cytokines, including IL-1β, IL-2, IL-6, IL-8, IL-12, IFNγ and TNF, as well as the cartilage-degrading markers MMP1, MMP3 and MMP9, are substantially elevated immediately after injury in the synovial fluid of patients with joint injuries111-114, and the elevated cytokine levels persist after bone healing115. As pro-inflammatory factors induce the production of cartilage-degrading enzymes, an association between synovial inflammation and PTOA is a prevalent hypothesis. However, in a study of 113 patients with acute anterior cruciate ligament injury, the levels of inflammatory mediators in the synovial fluid or the presence of moderate-to-severe Hoffa synovitis or of effusion synovitis at 2 years after anterior cruciate ligament injury did not predict structural knee OA at the 5-year follow-up116. More long-term longitudinal studies are needed to evaluate the contribution of synovial inflammation to the initiation and progression of PTOA.
Mechanical loading
Mechanical loading is essential for healthy joint maintenance. Nonetheless, aberrant excessive loading is a known OA risk factor117 and is thought to act through several molecular pathways, including IL-1β, TNF, NF-κB, WNT, microRNA and oxidative stress signalling pathways, which lead to chondrocyte apoptosis and ECM degradation118. However, excessive loading might also affect the synovium. For example, in vitro studies on FLS showed that mechanical loading induced the expression of several mediators involved in OA pathogenesis, such as prolyl-4-hydroxylase-α1 (P4HA1), collagen α2 (I) chain (COL1A2), cyclooxygenase 2 (COX2) and IL-6 (REF.119). In addition, similar studies in human monocytes revealed that mechanical loading, shear stress and compression induce expression of nitric oxide synthase 2 (NOS2), IL-12B, IL-6 and IL-8 (REF.120). Despite all this evidence suggesting that abnormal mechanical loading can facilitate the accumulation of inflammatory mediators in the synovium, the exact mechanism by which aberrant or excessive mechanical loading induces synovitis is still unknown and might involve multiple cellular factors. Of interest, moderate physical activity has been proposed to modulate the immune response by priming circulating monocytes towards an anti-inflammatory macrophage-like differentiation, mediated potentially by peroxisome proliferator-activated receptor-γ (PPARγ) signalling that implicates increased expression of CD36 (1.9 ± 1.5-fold) and liver X receptor-α (LXRα) (5.0 ± 4.7-fold) compared with sedentary individuals121. In a randomized controlled trial in women with knee OA, physical activity increased total intra-articular and perisynovial concentration of the anti-inflammatory cytokine IL-10 (REF.122), which is mainly produced by anti-inflammatory macrophages123 and has a chondroprotective role122.
Obesity and T2DM
Obesity is a well-known risk factor for both OA incidence and progression124, and its role in OA development is different according to sex and also depends on the affected joint125. Incidence of knee, hip and hand OA is higher in women126-128 than in men, and the prevalence of symptomatic OA is higher in women with obesity than in men with obesity2. The contribution of obesity to OA occurs not only through so-called mechanoinflammation but also through systemic low-grade inflammation or meta-inflammation, as obesity is linked to OA in weight-bearing joints such as the knees and in non-weight-bearing joints such as the hands129. The synovium of individuals with obesity displays marked fibrosis, increased macrophage infiltration and elevated expression of the Toll-like receptor 4 (TLR4) gene, but reduced levels of adiponectin and PPARγ130. In addition, abundance of CD14+ and CD206+ macrophages is increased in the synovial tissue of obese individuals130. Furthermore, the synovial fluid levels of the pro-inflammatory adipokine leptin are significantly higher in individuals with obesity than in those without obesity and correlate positively with BMI131. Finally, levels of mast-cell-produced β-tryptase in synovial fluid are also higher in individuals with obesity than in those without obesity132. Studies performed in rat133 and rabbit134 models with diet-induced obesity and surgically induced OA also showed an increase in pro-inflammatory macrophages133 and the pro-inflammatory mediators IL-1β, IL-6 and TNF134 in the synovium, which promote OA. Some studies also report more pain in patients with obesity with OA135,136. FLS isolated from patients with obesity with hip OA who underwent joint replacement surgery secrete higher amounts of IL-6 than FLS from lean patients, which was enhanced by crosstalk with chondrocytes via leptin137. Although patients with obesity have a higher prevalence and severity of synovial inflammation, as assessed by conventional MRI25, improvement in knee pain in patients with obesity with >20% weight loss at 1 year after dietary intervention or bariatric surgery was not mediated by a decrease in synovitis or bone marrow lesions (BMLs), as evaluated by MRI, but was partially explained by improvement in pressure pain threshold (at the patella and wrist) and depression score (CES-D)138. Furthermore, there was no noticeable improvement in BMLs (number and volume on MRI) or synovitis score after weight loss, which is in agreement with results of previous studies139,140. In fact, weight loss had no effect on synovial inflammation, evaluated by both static conventional MRI and dynamic CE-MRI, or on pain, evaluated by KOOS in a Danish study141. The fundamental reasons why obesity seems to facilitate synovitis but weight loss does not reverse this process are still unknown, although it is possible that obesity causes irreversible or long-lasting changes, such as epigenetic modifications or tissue structure alterations, which support OA progression even when individuals lose weight.
Epidemiological studies show a higher prevalence of OA (radiographic and symptomatic) in patients with type 2 diabetes mellitus (T2DM) and a higher rate of arthroplasty142,143, with a meta-analysis reporting a higher risk of OA development in patients with T2DM than in those without T2DM (OR 1.46; 95% CI 1.08–1.96; P = 0.01)144. Although some studies neither confirmed these findings after adjustment for BMI145,146 nor detected an association between T2DM and prevalence or incidence of OA147,148, some of the studies included in the meta-analysis reported the same increased risk after BMI adjustment, suggesting that T2DM is an independent risk factor for OA development143. For example, ultrasonography-detected synovitis and effusion were higher in patients with T2DM and end-stage knee OA who underwent arthroplasty than in those without T2DM, independent of patient BMI143. Several reports have described the effect of hyperglycaemia on synovial inflammation. For example, synovial levels of the pro-inflammatory cytokine TNF were higher in obese patients with OA and T2DM than in those without T2DM149. FLS in patients with diabetes and obesity with OA are also insulin resistant, implying a diminished ability of insulin to decrease production of pro-inflammatory and catabolic mediators that contribute to OA development150. High glucose levels induce VEGF secretion and reactive oxygen species (ROS) production in FLS in OA, increasing angiogenesis, tissue damage and inflammation151. Finally, both diabetes and ageing are associated with the accumulation of advanced glycated end-products, which induce an increase in proMMP1 secretion by FLS and in transcription of bone morphogenetic protein (BMP) genes that are involved in osteophyte formation152.
Diet and the gut microbiome
Although obesity is one of the most important modifiable risk factors to improve outcomes in OA, diet might have a role beyond weight control. A higher dietary inflammatory index score is associated with a higher prevalence of radiographic, symptomatic KOA, independent of patient weight (OR 1.40; 95% CI 1.14–1.72; P = 0.002)153. Interestingly, a randomized controlled trial of vitamin D supplementation slowed the progression of effusion-synovitis volume increase154, supporting the premise that micronutrients might have an effect on chronic pain by modulation of intra-articular inflammation. A subsequent randomized controlled trial showed no effect of vitamin D supplementation on BML volume and synovitis155. In a randomized, controlled trial, Curcuma longa extract, a proposed anti-inflammatory natural product, was superior to placebo in controlling pain but had no effect on knee effusion-synovitis or cartilage damage156. The Mediterranean diet is also believed to have positive effects in patients with OA157, and epidemiological studies from the Osteoarthritis Initiative found that a western diet was associated with progression of OA (higher KL and WOMAC score)158, although no data on its effect on synovitis were provided.
Diet is one of the modifiable factors that influence the composition of the gut microbiome. As germ-free mice have reduced susceptibility to OA from DMM159, and microbial DNA signatures have been detected in the cartilage and synovial tissue of patients with OA160,161, interest in the role of the microbiome in OA development and progression has increased. Western diets lack prebiotic-rich foods, in the form of dietary fibre, other complex carbohydrates and sugar alcohols present in fruits, which might be beneficial in supporting a healthy microbiome. Microbial dysbiosis — adverse alterations of the gut microbiota composition — may favour metabolic syndrome and inflammation. Indeed, obesity is associated with a loss of beneficial Bifidobacterium species and an increased abundance of pro-inflammatory bacterial species, which might increase macrophage recruitment from the gut to the synovium and accelerate knee OA162.
The bacterial endotoxin lipopolysaccharide (LPS) is a known activator of synovial inflammation through TLR4 (REF.163). Metabolic endotoxaemia (that is, the presence of bacterial products such as LPS in the blood) has been linked to changes in intestinal permeability induced by diet164 and has been described in patients with obesity and metabolic syndrome165. Interestingly, the presence of LPS in both plasma and synovial fluid from patients with OA correlates with the presence of activated macrophages in the joint capsule and synovium, radiographic severity (by 99mTc-EC20 SPECT–CT), and total WOMAC score163. Another study in individuals with knee OA found an association of the pro-inflammatory Streptococcus species with higher effusion (on MRI) and WOMAC knee pain, independent of BMI166. Whether changes in the gut microbiota that support inflammation are present in early stages of OA and are a contributing factor in OA radiographic or clinical progression or a consequence, possibly influenced by obesity, needs to be further examined.
Molecular mediators of synovitis in OA
Ageing and mitochondrial damage
Mitochondrial dysfunction (FIG. 3) is characterized by reduced mitochondrial integrity, including decreased mass, number and mitochondrial DNA (mtDNA) content, and impaired mitochondrial respiration, which increases ROS production167. Ageing is postulated to play a role in the mitochondrial dysfunction observed in OA. For example, mice that aged prematurely from accumulation of mtDNA mutations exhibited osteopenia, changes in epiphyseal trabecular bone and the subchondral cortical plate, and elevated numbers of hypertrophic chondrocytes in articular calcified cartilage168. Evaluation of mtDNA single nucleotide polymorphisms has defined mtDNA haplogroups as potential biomarkers for diagnosis or prognosis of OA169. In OA synoviocytes, the frequency of mtDNA mutations is substantially lower than in RA synoviocytes170, but complete characterization and larger population studies are needed to define the involvement of mtDNA mutations in synoviocytes in OA development. Ageing has also been associated with chronic low-grade inflammation, which could promote OA development, although the exact mechanism of the ageing–inflammation link is still unknown3. Mitochondrial dysfunction and deficient ROS scavenging prolong the production of pro-inflammatory cytokines (such as IL-1β and IL-6) and prevent the repolarization of macrophages from a pro-inflammatory to an anti-inflammatory phenotype in other tissues171, all of which could influence the development and progression of OA.
Senescent cells are a feature of various age-related pathological conditions, including OA. In particular, senescent chondrocytes in both PTOA and age-related OA accumulate mostly in the articular cartilage and synovium, and their elimination attenuates the development of OA, reduces pain and increases cartilage formation172. Several senescent-associated secretory phenotype factors are also inflammatory mediators, supporting the hypothesis that senescent cells in OA synovium could play a role in initiating or maintaining synovial inflammation. Studies in ex vivo human OA knee specimens and in a surgically induced OA mouse model have detected senescent synoviocytes in the OA synovium and demonstrated a positive correlation between the degree of synovitis at the biopsy site and the percentage of p16INK4A-expressing synoviocytes and levels of IL-6 in synovial fluid173. Together, these results suggest that ‘aged synovium’, indicated by the presence of senescent cells, is associated with synovitis. Early-stage clinical studies with senolytic agents are now underway, with results forthcoming (NCT04210986, NCT04770064 and NCT04815902).
Metabolites affecting the synovium
The OA synovium is a rich environment containing a wide variety of metabolites and soluble factors that contribute to both inflammation and cartilage damage174.
Nitric oxide.
Synovial fluid from patients with OA contains elevated levels of nitrite and the enzyme responsible for nitric oxide (NO) production, inducible nitric oxide synthase (iNOS; encoded by NOS2)175. NO, which is mainly produced by chondrocytes in the OA joint176, mediates inflammatory mediator production, angiogenesis and cartilage destruction177. Pro-inflammatory macrophages are also an important source of NO, through the metabolic rewiring of arginine metabolism towards NO and l-citrulline production70. Inhibition of iNOS dramatically reduces the production of catabolic and pro-inflammatory factors and prevents OA development in dogs178. Although animal studies support the investigation of iNOS inhibitors as a potential disease-modifying intervention for OA, no successful clinical trials of these agents have been reported179.
Succinate.
Pro-inflammatory macrophages exhibit a dysfunctional Krebs cycle that results in accumulation of succinate, which is shuttled from mitochondria to the cytosol to prevent hydroxylation and degradation of hypoxia inducible factor 1α (HIF1α), a key transcription factor involved in IL-1β production180. Although intracellular succinate supports the pro-inflammatory phenotype of macrophages, activation of succinate receptor 1 (SUCNR1; also known as GPR91) by soluble succinate boosts IL-4 production181, a cytokine that induces macrophage polarization towards an anti-inflammatory phenotype. However, succinate signalling through SUCNR1 in FLS links inflammation with fibrosis and angiogenesis and, indeed, exacerbates RA182. Despite these animal and in vitro data in RA, there are no studies investigating the relationship between succinate levels in the synovium and radiographic progression or clinical symptoms in OA. Indeed, the role of succinate and other intermediate metabolites in glycolysis and the Krebs cycle in FLS in OA is still unknown, and additional metabolic and functional studies are needed to understand the phenotype of FLS in OA.
Prostaglandins and other bioactive lipids.
Both IL-1β and IL-18 substantially increase the production of prostaglandin E2 (PGE2) in the synovium after articular cartilage damage183. In synovial fluid from patients with knee OA, the levels of IL-18 and PGE2 correlate greatly184. PGE2 is considered the major contributor to inflammatory pain in the OA joint. PGE2 signals through multiple receptors that are expressed differentially in both peripheral sensory neurons and the spinal cord. Through the EP4 receptor, PGE2 has been proposed to participate in enhancing the production of aggrecanases and MMP13 induced by pro-inflammatory cytokines185. PGE2 also induces the expression of iNOS and IL-6, which further contributes to maintaining synovitis and increasing hyperalgesia. PGD2 is also enriched in synovial fluid from patients with OA186 and has been suggested to potentiate nociception95. In a study that compared synovial fluid samples from 112 knees of 102 individuals (of whom 58 had knee OA and 44 were healthy controls), including both affected and unaffected knees in those with unilateral OA, increased levels of PGD2, 11,12-dihydroxyeicosatrienoic acid (11,12-DHET) and 14,15-dihydroxyeicosatrienoic acid (14,15-DHET) were associated with the presence of OA186. The levels of 11,12-DHET and 14,15-DHET were higher in affected than in unaffected knees of people with unilateral OA (P < 0.014 and P < 0.003, respectively) and were associated with radiographic progression over 3.3 years of follow-up186.
DAMPs and alarmins
Excess mechanical stress or injury leads to the release of damage-associated molecular patterns (DAMPs), which interact with pattern recognition receptors (PRRs), including TLRs, receptor for advanced glycation end products (RAGE) and others to initiate the innate immune response and propagate inflammation. DAMPs implicated in OA are very heterogeneous and include cartilage fragments, ECM proteins, secreted intracellular proteins, plasma proteins and crystals (extensively reviewed elsewhere187,188).
High mobility group protein B1.
HMGB1 is a non-histone nuclear protein that facilitates transcription factor and nucleosome stability. However, HMGB1 acts as an endogenous danger signal and is released from cytokine-activated cells and damaged or dying cells supporting the inflammatory response189. In OA joints, HMGB1 is secreted by damaged and necrotic chondrocytes189. HMGB1 levels in synovium and synovial fluid are higher in patients with OA than in healthy controls190,191, and they are also higher in the synovial fluid of patients with KL 4 than in those with KL 2 (P < 0.01) or KL 3 (P < 0.05)190. HMGB1 levels in synovial fluid have also been associated with the severity of synovitis, pain and daily activity reduction191. The therapeutic potential of HMGB1 neutralizing antibodies has been evaluated in the DMM mouse model, in which they showed cartilage protective effects192.
Heat shock proteins.
Heat-shock proteins (HSPs) are induced by cellular stress to protect and maintain cellular integrity and function193. In OA synovium, HSP60, HSP70 and HSP90 are the most abundant members of the HSP family194. HSP70 levels in serum and synovial fluid are higher in individuals with knee OA than in healthy controls and correlate with radiographic disease severity195. In the DMM mouse model, overexpression of the HSP70 family member HSPA1A abrogated cartilage erosion while having no effect on DMM-induced osteophyte formation or subchondral bone plate thickening196.
S100 protein family members.
S100 proteins are intracellular calcium-binding proteins that participate in regulating the cytoskeleton and cell migration188. S100A9 is strongly upregulated in inflamed synovial tissue197 and is involved in cartilage matrix degradation and osteophyte formation198. A study in 141 individuals with clinical knee OA showed that serum levels of S100A8 or S100A9 correlated with total WOMAC scores (P = 0.021), weight-bearing pain (P = 0.043) and physical dysfunction (P = 0.010)199. Similar results were obtained in 294 patients with hand OA200.
Other NLRP3 inflammasome activators
Evidence of the participation of the NLRP3 inflammasome, a protein complex involved in processing and maturation of IL-1β and IL-18, in OA onset and progression has led to this complex being proposed as a potential biomarker for OA diagnosis and patient classification201-203. Maturation of IL-1β and IL-18 is a two-step process. First, activation of NF-κB-dependent transcription of NLRP3 and IL1B204, p62 (also known as SQSTM1)205 and SLC44A1 (REF.206) among other genes, and de novo synthesis of mtDNA207. Second, the assembly of the NLRP3 inflammasome, activation of caspase 1 and processing of pro-IL-1β and pro-IL-18 to mature cytokines204. In the OA synovium, ectopic deposition of hydroxyapatite crystals, calcium pyrophosphate dihydrate microcrystals and monosodium urate crystals, and ATP released from dying cells are detected by macrophages and trigger NLRP3 inflammasome activation and IL-1β and IL-18 production208,209. Of note, monosodium urate crystals in the joint are associated with increased synovial fluid levels of IL-1β (r2 = 0.34, P < 0.0001) and IL-18 (r2 = 0.41, P < 0.0001), OA severity and radiographic progression (P = 0.001), and osteophyte formation (P < 0.0001)210. IL-1β induced chondrocyte catabolism, increased MMP and ADAMTS5 activity and suppressed proteoglycan synthesis183. The pathogenetic role of IL-1β in OA synovium might also be due in part to the lack of production of the natural IL-1β antagonist IL-1 receptor antagonist (IL-1Ra) by OA chondrocytes58. Preclinical studies using recombinant IL-1Ra (anakinra) demonstrated a strong protection in OA animal models by improving lubricin expression, preserving cartilage integrity and reducing synovial hyperplasia and inflammatory cell infiltration211. Furthermore, in an exploratory analysis of a randomized controlled trial for the prevention of cardiovascular events, canakinumab (anti-IL-1β) treatment was associated with a lower incidence of hip and knee replacement than placebo212,213, although in a randomized controlled trial, intra-articular injection of anakinra did not improve OA symptoms compared with placebo214. These disparate outcomes have resulted in a lack of consensus regarding the use of IL-1 signalling therapy for OA, concluding that inhibiting the actions of IL-1β alone is not enough to block OA pathogenesis. In this sense, NLRP3 inflammasome inhibitors, which block not only the production of IL-1β but also that of IL-18 and active caspase 1, might be a more potent intervention. In the OA synovium, IL-18 promotes chondrocyte proliferation and expression of COX2, iNOS and MMPs, and boosts IL-6 production, further supporting a procatabolic environment215. Importantly, active caspase 1 also cleaves gasdermin D, releasing the active amino terminal portion that has pyroptotic activity (by pore formation), which could contribute to maintaining persistent inflammation within the synovium216.
Complement
Complement factors are highly expressed in the main tissues involved in OA, including cartilage, bone and synovium, in patients with OA compared with in healthy controls217,218. Furthermore, expression of complement effectors is higher and that of complement inhibitors is lower in the synovium of patients with OA than in healthy donors217. In addition, C5 and C6 deficiency are protective against the development of synovitis and cartilage damage in animal models of OA, and complement activation is associated with increased production of ECM-degrading proteins and inflammatory mediators217. These data suggest a role for complement activation not only in synovitis but also in the development and progression of OA, and it could therefore represent a potential therapeutic target.
Cellular interplay in OA synovium
Interactions between synoviocytes, chondrocytes and osteocytes
In a healthy joint, chondrocytes balance the synthesis and breakdown of the cartilaginous matrix to ensure correct distribution of load across the joint, thereby reducing friction. Both non-mechanical and mechanical factors contribute to OA development, which involves a shift in chondrocyte metabolism to increased proteolytic activity and inflammation and cartilage degradation. Direct and indirect communication between chondrocytes and synoviocytes is thought to contribute to maintaining anabolic and catabolic responses of each cell type219 (FIG. 3). In the inflamed joint, chondrocytes and synoviocytes mutually induce alterations in their transcription programme to favour the production of MMPs220. Cartilage fragments, aggrecan, fibronectin and other DAMPs are sensed by synoviocytes to shift their transcriptomic profile towards chronic inflammatory responses, including cytokine, NLRP3 inflammasome, hypoxia, scavenger receptor and TLR, and integrin pathways221. Importantly, the array of upregulated genes related to maintenance of an inflammatory phenotype are under control of transcription factors that support synovitis, including ATF2, STAT3 and NFKB1 (REF.221). Cartilage fragments can also induce inflammatory responses in synoviocytes by reorganization of the actin cytoskeleton, enhanced production of NO and PGE2 and increased deposition of collagen222 (FIG. 4).
Synovitis can coexist with BML and both can precede the development of radiographic OA223,224. Although it is thought that BMLs result from excessive mechanical loading, it is not known whether synovitis contributes to subchondral bone pathology225, other than by invasion of subchondral bone by pannus-like tissue in the medial compartment226 without producing the marginal erosions typically seen in RA227. The temporal relationship between the synovitis and BMLs is still not known. In a study in patients with end-stage OA before joint replacement, histologically assessed synovitis correlated moderately with the presence of subchondral cysts by MRI (r = 0.350; P = 0.03)228. However, a subsequent analysis of the contribution of different OA pathological processes to pain found no association between subchondral pathology and synovitis, suggesting that subchondral pathology is associated with knee pain independently of cartilage and synovial pathology229.
Synovitis is also associated with osteophyte formation. Hoffa synovitis is significantly associated with osteophyte development in both anterior (P = 0.013, adjusted OR 1.12, 95% CI 1.03–1.23) and medial (P = 0.000, adjusted OR 1.21, 95% CI 1.11–1.31) lesions of the tibia230. In support of the association between Hoffa synovitis and osteophyte development, depletion of macrophages in the collagenase-induced OA model strongly reduced the formation of osteophytes and fibrosis231,232. Macrophages, which together with FLS are the main source of TGFβ in the synovium, contribute to the stimulation of bone formation and production of proteoglycan and type II collagen, and enhance chondrogenesis. TGFβ and the related proteins BMP2 and BMP4 are essential growth factors implicated in osteophyte formation233 and pathological type I collagen deposition during fibrosis234.
Although the relationship between synovitis and OA structural progression is better defined, the role of synovial inflammation in OA pain is not completely understood (BOX 2). Treatment with antibodies against granulocyte–macrophage colony-stimulating factor (GM-CSF), which signals in both the immune and nervous systems, has an analgesic effect in OA without affecting synovitis scores235. Indeed, the number of GM-CSF- and GM-CSFRα-expressing cells per mm2 synovial sub-lining correlated negatively with knee pain235, reinforcing the idea that synovitis and pain are not always associated. Research in OA-related pain has also focused on nerve growth factor (NGF)236, which is a neurotrophin that activates nociceptive neurons to transmit pain signals from the periphery to the central nervous system. NGF is expressed by FLS and macrophages in the synovium237 and by subchondral mononuclear cells, osteoclasts and chondrocytes in the cartilage from patients with knee OA229. Other studies suggest that symptomatic OA is associated with upregulation of MMP1 in the synovium and downregulation of IL-1R1 and VEGF compared with the levels of these molecules in individuals with asymptomatic chondropathy with similar macroscopic joint surface appearances who did not seek total knee replacement18. IL-1β signals through its receptor IL-1R1 and induces the expression and release of MMP1 by FLS. After IL-1–IL-1R1 engagement, IL-1R1 is downregulated, which may explain why therapy with an IL-1R1 antagonist failed in clinical trials238. Angiogenesis in the subchondral bone has been postulated as the initial event in OA pain, as the blood vessels supply nutrients for axonal growth and neo-innervation of the osteochondral junction, likely driven by NGF released from basal articular chondrocytes239. The synovium then contributes to pain by secreting pro-inflammatory factors, such as TRKB (the receptor for brain-derived growth factor), CCL14 and ADAMTS15, angiotensinogen, angiotensin-converting enzyme, netrin 1, CCL2 and CCL8 (REFS18,237,240), either independently or amplifying the process already initiated by NGF.
Box 2 ∣. Gaps in and proposed agenda for synovitis research in OA.
Research gap
To date, the fundamental mechanisms underlying the crosstalk between synovitis and clinical symptoms of osteoarthritis (OA) are not completely identified. Current studies describe histological and molecular characteristics at end-stage OA using imaging but do not capture change over time.
Proposed research agenda
Longitudinal studies that combine cellular and molecular evaluation in combination with histology and imaging at different stages of OA to establish mechanistic links with clinical OA progression.
Research gap
Current imaging techniques cannot capture all histological features of the OA synovium.
Proposed research agenda
Optimization of imaging, including MRI, functional imaging and positron emission tomography to capture the different histological patterns and phenotype subsets in OA.
Research gap
Defining phenotypes that capture the heterogeneous features of OA synovitis.
Proposed research agenda
Personalized medicine for patients with OA by defining phenotypes of OA that capture the inflammatory subtypes through advanced imaging.
Interactions between synovial tissue and the infrapatellar fat pad
Fibrosis may contribute to joint stiffness and pain, which are the main symptoms in OA, but most of the clinical studies relate to postoperative synovial fibrosis. Intra-articular fibrosis can be detected by using MRI scans with advanced metal suppression and with gadolinium contrast241 in patients with stiff and painful arthroplasty. Patients with diagnosed fibrosis242 exhibit thicker tissue (4.4 mm ± 0.2 mm) than patients with a non-fibrotic phenotype (2.5 mm ± 0.4 mm) after total knee arthroplasty (1.9 mm ± 0.2 mm; P < 0.05)241. A promising fibroblast radiotracer for PET, 18F-labelled glycosylated FAPI, demonstrated highly specific uptake in bone structures and joints243 and could aid in improving understanding of the role of fibroblasts and fibrosis in OA and whether or not fibrosis in the synovial tissue contributes to joint pain.
The exact mechanism by which fibrosis occurs in OA synovium is still not entirely clear. It is generally accepted that there is more inflammation, with an increased number of macrophages and T cells in the lining layer, in early OA than in advanced OA, in which inflammation and fibrosis coexist48,244. These observations suggest that the progression of OA is accompanied by a transition from an inflammatory phase to a fibrotic stage and that factors that initiate fibrosis might be induced during synovitis. Several factors that are increased in inflamed OA joints are associated with fibrosis, including TGFβ, procollagen-lysine, 2-oxoglutarate 5-dioxygenase 2 (PLOD2), tissue inhibitor of metalloproteinase 1 (TIMP1), connective tissue growth factor (CTGF), disintegrin and metalloproteinase domain-containing protein 12 (ADAM12) and prostaglandin F2a. For example, ADAM12 mRNA and protein levels in synovium correlate with the severity of histological synovitis244. Both PLOD2 and TIMP1 have been directly implicated in synovial fibrosis and are elevated in the synovium of patients with end-stage OA and mice with experimental OA245.
Another compartment that is involved in the development of synovial fibrosis is the IFP, which is located below the kneecap between the joint capsule and the synovial membrane, protects the knee from mechanical stress and provides a vascular supply. However, during OA development and progression the IFP also undergoes structural changes characterized by increased fibrosis and neovascularization, lymphocyte infiltration in the interlobular septum and smaller fat lobules246-248. Of note, individuals who develop AKOA are more likely to have altered signal intensity in the IFP (30%) than those with no knee OA at 2 years prior to the index visit (OR 2.07, 95% CI 1.14–3.78), and these odds increase twofold at 1 year prior to disease onset and for the next 3 years249. The infiltration of immune cells and increased fibrosis cause the disappearance of adipocytes in the parenchymal region of the IFP250. Part of the contribution of the IFP to synovial fibrosis is mediated by the activation of FLS: PLOD2 expression and collagen production by FLS increases sixfold and 1.8-fold respectively when FLS are co-cultured with fat-conditioned medium from the IFP of patients with OA247. Furthermore, collagen production by FLS correlated with increased levels of prostaglandin F2a in the fat-conditioned medium, whereas no correlation with TGFβ amounts was observed247. In addition, IFP tissue in obese individuals shows increased expression of genes associated with fibrosis and ECM production, but no change in adipocyte size, inflammatory cell infiltration, macrophage polarization, formation of crown-like structures, or expression of genes encoding inflammatory cytokines and chemokines251. In another study, IFP volume was not associated with BMI252. However, macrophages in the IFP from patients with obesity with knee OA were positive for the surface markers CD206 and CD163 (~80% and 40% of all CD14+ macrophages, respectively), and these macrophages produce IL-6 and TNF but not much IL-10; of note, none of these features correlated with BMI252. Animal models of OA also showed an association between synovitis, changes in macrophage polarization (including enrichment of pro-inflammatory macrophage phenotype or increased crown-like structures), and fibrosis in both synovial tissue and the IFP, and treatment that decreased inflammation was associated with changes in macrophage phenotypes and attenuation of fibrosis251-253. Although all these studies showed some contribution of IFP dysfunction to joint inflammation and fibrosis, the mediators of these interactions remain unknown.
Of interest, TGFβ has been proposed as a nexus between fibrosis and pain in OA. In a study of patients with radiographic KL grade 3–4 after total knee arthroplasty, NGF expression in synovial tissue correlated positively with TGFβ expression (P < 0.001) while showing no association with levels of the pro-inflammatory cytokines IL-1β and TNF (P = 0.576 and P = 0.616, respectively). Both TGFβ and NGF colocalized in the lining layer, mainly in the CD45−CD90+ fibroblast population (86.3% of analysed cells in the synovial tissue)254. Similar to findings in articular cartilage255, TGFβ–ALK5 signalling mediates NGF production through the TAK1–p38 pathway in the synovium of patients with knee OA254.
Conclusions
OA is a complex disease in which symptoms and joint function are often dissociated from structural damage. In an effort to identify pathobiological mechanisms in OA, the OA community is intensely investigating synovial inflammation. Consequently, we now know more about the cellular and molecular players in synovitis, although more in-depth studies are needed to evaluate the association of these factors with radiographic progression and contribution to OA symptoms at both early and late stages of disease. Hurdles to be overcome might include the heterogeneous nature of the OA synovium and the complex network of interactions that are involved in synovial inflammation, fibrosis and cartilage damage, processes that often cannot be completely dissociated and evaluated using current imaging techniques. Research advances in phenotype-specific treatment options have provided several novel therapies that could target the inflammatory component of OA256,257. However, further research is needed to determine whether synovial inflammation is relevant for diagnosis, risk stratification or identification of therapeutic targets.
Key points.
Imaging studies suggest that synovial inflammation may be present in both early osteoarthritis (OA) and advanced-stage OA and is involved in the development and progression of OA.
Synovial cells coordinate the production of molecules that initiate and maintain synovial inflammation and contribute to cartilage damage during OA progression.
Diverse stimuli, including bioactive lipids, prostaglandins, tricarboxylic acid cycle intermediates, cytokines and damage-associated molecular patterns, as well as clinical factors such as obesity, ageing, trauma and excessive mechanical loading, regulate the production of pro-inflammatory and anti-inflammatory mediators by synovial cells.
There is a need for functional imaging and cellular and molecular studies, together with a more robust histological interpretation at different stages of OA, to better stratify patients with OA and understand the role of synovitis in OA onset and progression.
Acknowledgements
The authors’ work was supported by the US National Institutes of Health (AR073324 to M.G., 5T32AR064194-07 to R.C., NIH Diversity Supplement to A.T., AG070647 and AR078917 to N.E.L., and K01AR077111 and Resource-based Center for the study of the joint microenvironment in rheumatology UCSD (NIH P30AR073761) to E.S.-L.).
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Hunter DJ, March L & Chew M, Lancet Commission on Osteoarthritis. Osteoarthritis in 2020 and beyond — Authors’ reply. Lancet 397, 1060 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Deshpande BR et al. Number of persons with symptomatic knee osteoarthritis in the US: impact of race and ethnicity, age, sex, and obesity. Arthritis Care Res. 68, 1743–1750 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loeser RF, Collins JA & Diekman BO Ageing and the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol 12, 412–420 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Felson DT et al. Synovitis and the risk of knee osteoarthritis: the MOST Study. Osteoarthritis Cartilage 24, 458–464 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins JE et al. Semiquantitative imaging biomarkers of knee osteoarthritis progression: data from the foundation for the national institutes of health osteoarthritis biomarkers consortium. Arthritis Rheumatol. 68, 2422–2431 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y et al. Knee effusion volume assessed by magnetic resonance imaging and progression of knee osteoarthritis: data from the Osteoarthritis Initiative. Rheumatology 58, 246–253 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atukorala I et al. Synovitis in knee osteoarthritis: a precursor of disease? Ann. Rheum. Dis 75, 390–395 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacFarlane LA et al. Association of changes in effusion-synovitis with progression of cartilage damage over eighteen months in patients with osteoarthritis and meniscal tear. Arthritis Rheumatol. 71, 73–81 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ayral X, Pickering EH, Woodworth TG, Mackillop N & Dougados M Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis–results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthritis Cartilage 13, 361–367 (2005). [DOI] [PubMed] [Google Scholar]
- 10.Roemer FW et al. Can structural joint damage measured with MR imaging be used to predict knee replacement in the following year? Radiology 274, 810–820 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roemer FW et al. Presence of MRI-detected joint effusion and synovitis increases the risk of cartilage loss in knees without osteoarthritis at 30-month follow-up: the MOST study. Ann. Rheum. Dis 70, 1804–1809 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perry TA et al. Association between bone marrow lesions & synovitis and symptoms in symptomatic knee osteoarthritis. Osteoarthritis Cartilage 28, 316–323 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haugen IK et al. Synovitis and radiographic progression in non-erosive and erosive hand osteoarthritis: is erosive hand osteoarthritis a separate inflammatory phenotype? Osteoarthritis Cartilage 24, 647–654 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Haugen IK et al. MRI findings predict radiographic progression and development of erosions in hand osteoarthritis. Ann. Rheum. Dis 75, 117–123 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Mancarella L, Addimanda O, Cavallari C & Meliconi R Synovial inflammation drives structural damage in hand osteoarthritis: a narrative literature review. Curr. Rheumatol. Rev 13, 43–50 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Marshall M, Watt FE, Vincent TL & Dziedzic K Hand osteoarthritis: clinical phenotypes, molecular mechanisms and disease management. Nat. Rev. Rheumatol 14, 641–656 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Driban JB et al. Risk factors and the natural history of accelerated knee osteoarthritis: a narrative review. BMC Musculoskelet. Disord 21, 332 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wyatt LA et al. Molecular expression patterns in the synovium and their association with advanced symptomatic knee osteoarthritis. Osteoarthritis Cartilage 27, 667–675 (2019). [DOI] [PubMed] [Google Scholar]
- 19.Sarmanova A et al. Association between ultrasound-detected synovitis and knee pain: a population-based case-control study with both cross-sectional and follow-up data. Arthritis Res. Ther 19, 281 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hill CL et al. Synovitis detected on magnetic resonance imaging and its relation to pain and cartilage loss in knee osteoarthritis. Ann. Rheum. Dis 66, 1599–1603 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riis RG et al. The association between histological, macroscopic and magnetic resonance imaging assessed synovitis in end-stage knee osteoarthritis: a cross-sectional study. Osteoarthritis Cartilage 25, 272–280 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Abbasi B, Pezeshki-Rad M, Akhavan R & Sahebari M Association between clinical and sonographic synovitis in patients with painful knee osteoarthritis. Int. J. Rheum. Dis 20, 561–566 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Baker K et al. Relation of synovitis to knee pain using contrast-enhanced MRIs. Ann. Rheum. Dis 69, 1779–1783 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ballegaard C et al. Knee pain and inflammation in the infrapatellar fat pad estimated by conventional and dynamic contrast-enhanced magnetic resonance imaging in obese patients with osteoarthritis: a cross-sectional study. Osteoarthritis Cartilage 22, 933–940 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Kanthawang T et al. Obese and overweight individuals have greater knee synovial inflammation and associated structural and cartilage compositional degeneration: data from the osteoarthritis initiative. Skeletal Radiol. 50, 217–229 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guermazi A et al. Assessment of synovitis with contrast-enhanced MRI using a whole-joint semiquantitative scoring system in people with, or at high risk of, knee osteoarthritis: the MOST study. Ann. Rheum. Dis 70, 805–811 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riis RG et al. Synovitis assessed on static and dynamic contrast-enhanced magnetic resonance imaging and its association with pain in knee osteoarthritis: a cross-sectional study. Eur. J. Radiol 85, 1099–1108 (2016). [DOI] [PubMed] [Google Scholar]
- 28.Bacon K, LaValley MP, Jafarzadeh SR & Felson D Does cartilage loss cause pain in osteoarthritis and if so, how much? Ann. Rheum. Dis 79, 1105–1110 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chevalier X, Eymard F & Richette P Biologic agents in osteoarthritis: hopes and disappointments. Nat. Rev. Rheumatol 9, 400–410 (2013). [DOI] [PubMed] [Google Scholar]
- 30.Ansari MY et al. Genetic inactivation of ZCCHC6 suppresses interleukin-6 expression and reduces the severity of experimental osteoarthritis in mice. Arthritis Rheumatol. 71, 583–593 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Attur M et al. Interleukin 1 receptor antagonist (IL1RN) gene variants predict radiographic severity of knee osteoarthritis and risk of incident disease. Ann. Rheum. Dis 79, 400–407 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Latourte A et al. Systemic inhibition of IL-6/Stat3 signalling protects against experimental osteoarthritis. Ann. Rheum. Dis 76, 748–755 (2017). [DOI] [PubMed] [Google Scholar]
- 33.Nasi S, So A, Combes C, Daudon M & Busso N Interleukin-6 and chondrocyte mineralisation act in tandem to promote experimental osteoarthritis. Ann. Rheum. Dis 75, 1372–1379 (2016). [DOI] [PubMed] [Google Scholar]
- 34.Kadri A et al. Osteoprotegerin inhibits cartilage degradation through an effect on trabecular bone in murine experimental osteoarthritis. Arthritis Rheum. 58, 2379–2386 (2008). [DOI] [PubMed] [Google Scholar]
- 35.Chevalier X et al. Adalimumab in patients with hand osteoarthritis refractory to analgesics and NSAIDs: a randomised, multicentre, double-blind, placebo-controlled trial. Ann. Rheum. Dis 74, 1697–1705 (2015). [DOI] [PubMed] [Google Scholar]
- 36.Kloppenburg M et al. Phase IIa, placebo-controlled, randomised study of lutikizumab, an anti-interleukin-1alpha and anti-interleukin-1beta dual variable domain immunoglobulin, in patients with erosive hand osteoarthritis. Ann. Rheum. Dis 78, 413–420 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Persson MSM, Sarmanova A, Doherty M & Zhang W Conventional and biologic disease-modifying anti-rheumatic drugs for osteoarthritis: a meta-analysis of randomized controlled trials. Rheumatology 57, 1830–1837 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richette P et al. Efficacy of tocilizumab in patients with hand osteoarthritis: double blind, randomised, placebo-controlled, multicentre trial. Ann. Rheum. Dis 10.1136/annrheumdis-2020-218547 (2020). [DOI] [PubMed] [Google Scholar]
- 39.Sellam J & Berenbaum F The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat. Rev. Rheumatol 6, 625–635 (2010). [DOI] [PubMed] [Google Scholar]
- 40.Chao J et al. Inflammatory characteristics on ultrasound predict poorer longterm response to intraarticular corticosteroid injections in knee osteoarthritis. J. Rheumatol 37, 650–655 (2010). [DOI] [PubMed] [Google Scholar]
- 41.de Lange-Brokaar BJ et al. Degree of synovitis on MRI by comprehensive whole knee semi-quantitative scoring method correlates with histologic and macroscopic features of synovial tissue inflammation in knee osteoarthritis. Osteoarthritis Cartilage 22, 1606–1613 (2014). [DOI] [PubMed] [Google Scholar]
- 42.Loeuille D et al. Macroscopic and microscopic features of synovial membrane inflammation in the osteoarthritic knee: correlating magnetic resonance imaging findings with disease severity. Arthritis Rheum. 52, 3492–3501 (2005). [DOI] [PubMed] [Google Scholar]
- 43.af Klint E et al. Evaluation of arthroscopy and macroscopic scoring. Arthritis Res. Ther 11, R81 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krenn V et al. Grading of chronic synovitis — a histopathological grading system for molecular and diagnostic pathology. Pathol. Res. Pract 198, 317–325 (2002). [DOI] [PubMed] [Google Scholar]
- 45.Krenn V et al. Synovitis score: discrimination between chronic low-grade and high-grade synovitis. Histopathology 49, 358–364 (2006). [DOI] [PubMed] [Google Scholar]
- 46.Slansky E et al. Quantitative determination of the diagnostic accuracy of the synovitis score and its components. Histopathology 57, 436–443 (2010). [DOI] [PubMed] [Google Scholar]
- 47.Krenn V et al. 15 years of the histopathological synovitis score, further development and review: a diagnostic score for rheumatology and orthopaedics. Pathol. Res. Pract 213, 874–881 (2017). [DOI] [PubMed] [Google Scholar]
- 48.Oehler S, Neureiter D, Meyer-Scholten C & Aigner T Subtyping of osteoarthritic synoviopathy. Clin. Exp. Rheumatol 20, 633–640 (2002). [PubMed] [Google Scholar]
- 49.Mathiessen A & Conaghan PG Synovitis in osteoarthritis: current understanding with therapeutic implications. Arthritis Res. Ther 19, 18 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mussawy H et al. The histopathological synovitis score is influenced by biopsy location in patients with knee osteoarthritis. Arch. Orthop. Trauma. Surg 10.1007/s00402-021-03889-x (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Lange-Brokaar BJ et al. Association of pain in knee osteoarthritis with distinct patterns of synovitis. Arthritis Rheumatol. 67, 733–740 (2015). [DOI] [PubMed] [Google Scholar]
- 52.Nanus DE et al. Synovial tissue from sites of joint pain in knee osteoarthritis patients exhibits a differential phenotype with distinct fibroblast subsets. EBioMedicine 10.1016/j.ebiom.2021.103618 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wyatt LA et al. Histopathological subgroups in knee osteoarthritis. Osteoarthritis Cartilage 25, 14–22 (2017). [DOI] [PubMed] [Google Scholar]
- 54.Benito MJ, Veale DJ, FitzGerald O, van den Berg WB & Bresnihan B Synovial tissue inflammation in early and late osteoarthritis. Ann. Rheum. Dis 64, 1263–1267 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Livshits G et al. Interleukin-6 is a significant predictor of radiographic knee osteoarthritis: the Chingford Study. Arthritis Rheum. 60, 2037–2045 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stannus O et al. Circulating levels of IL-6 and TNF-alpha are associated with knee radiographic osteoarthritis and knee cartilage loss in older adults. Osteoarthritis Cartilage 18, 1441–1447 (2010). [DOI] [PubMed] [Google Scholar]
- 57.Labinsky H et al. Multiparameter analysis identifies heterogeneity in knee osteoarthritis synovial responses. Arthritis Rheumatol. 72, 598–608 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chou CH et al. Synovial cell cross-talk with cartilage plays a major role in the pathogenesis of osteoarthritis. Sci. Rep 10, 10868 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang F et al. Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nat. Immunol 20, 928–942 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Klein-Wieringa IR et al. Inflammatory cells in patients with endstage knee osteoarthritis: a comparison between the synovium and the infrapatellar fat pad. J. Rheumatol 43, 771–778 (2016). [DOI] [PubMed] [Google Scholar]
- 61.Wood MJ et al. Macrophage proliferation distinguishes 2 subgroups of knee osteoarthritis patients. JCI Insight 4, e125325 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Watanabe S, Alexander M, Misharin AV & Budinger GRS The role of macrophages in the resolution of inflammation. J. Clin. Invest 129, 2619–2628 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gomez-Aristizabal A, Gandhi R, Mahomed NN, Marshall KW & Viswanathan S Synovial fluid monocyte/macrophage subsets and their correlation to patient-reported outcomes in osteoarthritic patients: a cohort study. Arthritis Res. Ther 21, 26 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kraus VB et al. Direct in vivo evidence of activated macrophages in human osteoarthritis. Osteoarthritis Cartilage 24, 1613–1621 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Raghu H et al. CCL2/CCR2, but not CCL5/CCR5, mediates monocyte recruitment, inflammation and cartilage destruction in osteoarthritis. Ann. Rheum. Dis 76, 914–922 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bondeson J et al. The role of synovial macrophages and macrophage-produced mediators in driving inflammatory and destructive responses in osteoarthritis. Arthritis Rheum. 62, 647–657 (2010). [DOI] [PubMed] [Google Scholar]
- 67.Bondeson J, Wainwright SD, Lauder S, Amos N & Hughes CE The role of synovial macrophages and macrophage-produced cytokines in driving aggrecanases, matrix metalloproteinases, and other destructive and inflammatory responses in osteoarthritis. Arthritis Res. Ther 8, R187 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu CL et al. Conditional macrophage depletion increases inflammation and does not inhibit the development of osteoarthritis in obese macrophage fas-induced apoptosis-transgenic mice. Arthritis Rheumatol. 69, 1772–1783 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Orecchioni M, Ghosheh Y, Pramod AB & Ley K Macrophage polarization: different gene signatures in M1(LPS+) vs. classically and M2(LPS-) vs. alternatively activated macrophages. Front. Immunol 10, 1084 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Viola A, Munari F, Sanchez-Rodriguez R, Scolaro T & Castegna A The metabolic signature of macrophage responses. Front. Immunol 10, 1462 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Daghestani HN, Pieper CF & Kraus VB Soluble macrophage biomarkers indicate inflammatory phenotypes in patients with knee osteoarthritis. Arthritis Rheumatol. 67, 956–965 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu B, Zhang M, Zhao J, Zheng M & Yang H Imbalance of M1/M2 macrophages is linked to severity level of knee osteoarthritis. Exp. Ther. Med 16, 5009–5014 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Manferdini C et al. From osteoarthritic synovium to synovial-derived cells characterization: synovial macrophages are key effector cells. Arthritis Res. Ther 18, 83 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fahy N et al. Human osteoarthritic synovium impacts chondrogenic differentiation of mesenchymal stem cells via macrophage polarisation state. Osteoarthritis Cartilage 22, 1167–1175 (2014). [DOI] [PubMed] [Google Scholar]
- 75.Saito I, Koshino T, Nakashima K, Uesugi M & Saito T Increased cellular infiltrate in inflammatory synovia of osteoarthritic knees. Osteoarthritis Cartilage 10, 156–162 (2002). [DOI] [PubMed] [Google Scholar]
- 76.Madsen DH et al. M2-like macrophages are responsible for collagen degradation through a mannose receptor-mediated pathway. J. Cell Biol 202, 951–966 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dai M, Sui B, Xue Y, Liu X & Sun J Cartilage repair in degenerative osteoarthritis mediated by squid type II collagen via immunomodulating activation of M2 macrophages, inhibiting apoptosis and hypertrophy of chondrocytes. Biomaterials 180, 91–103 (2018). [DOI] [PubMed] [Google Scholar]
- 78.Hoeksema MA & Glass CK Nature and nurture of tissue-specific macrophage phenotypes. Atherosclerosis 281, 159–167 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Young L et al. Effects of intraarticular glucocorticoids on macrophage infiltration and mediators of joint damage in osteoarthritis synovial membranes: findings in a double-blind, placebo-controlled study. Arthritis Rheum. 44, 343–350 (2001). [DOI] [PubMed] [Google Scholar]
- 80.Siebelt M et al. Triamcinolone acetonide activates an anti-inflammatory and folate receptor-positive macrophage that prevents osteophytosis in vivo. Arthritis Res. Ther 17, 352 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ha CW et al. A multicenter, single-blind, phase IIa clinical trial to evaluate the efficacy and safety of a cell-mediated gene therapy in degenerative knee arthritis patients. Hum. Gene Ther. Clin. Dev 26, 125–130 (2015). [DOI] [PubMed] [Google Scholar]
- 82.Cherian JJ et al. Preliminary results of a phase II randomized study to determine the efficacy and safety of genetically engineered allogeneic human chondrocytes expressing TGF-beta1 in patients with grade 3 chronic degenerative joint disease of the knee. Osteoarthritis Cartilage 23, 2109–2118 (2015). [DOI] [PubMed] [Google Scholar]
- 83.Ting R et al. Fast 18F labeling of a near-infrared fluorophore enables positron emission tomography and optical imaging of sentinel lymph nodes. Bioconjug. Chem 21, 1811–1819 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang X et al. Detection of osteoarthritis inflammation by single-photon emission computed tomography based on an inflammation-targeting peptide cFLFLF. Mol. Imaging Biol 23, 895–904 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Meester EJ et al. Imaging inflammation in atherosclerotic plaques, targeting SST2 with [111In] In-DOTA-JR11. J. Nucl. Cardiol 28, 2506–2513 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Temple-Wong MM et al. Hyaluronan concentration and size distribution in human knee synovial fluid: variations with age and cartilage degeneration. Arthritis Res. Ther 18, 18 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Balazs EA Viscosupplementation for treatment of osteoarthritis: from initial discovery to current status and results. Surg. Technol. Int 12, 278–289 (2004). [PubMed] [Google Scholar]
- 88.Ni S et al. The involvement of follistatin-like protein 1 in osteoarthritis by elevating NF-kappaB-mediated inflammatory cytokines and enhancing fibroblast like synoviocyte proliferation. Arthritis Res. Ther 17, 91 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP & Fahmi H Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol 7, 33–42 (2011). [DOI] [PubMed] [Google Scholar]
- 90.Mizoguchi F et al. Functionally distinct disease-associated fibroblast subsets in rheumatoid arthritis. Nat. Commun 9, 789 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hsueh MF, Zhang X, Wellman SS, Bolognesi MP & Kraus VB Synergistic roles of macrophages and neutrophils in osteoarthritis progression. Arthritis Rheumatol. 73, 89–99 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gupta K, Shukla M, Cowland JB, Malemud CJ & Haqqi TM Neutrophil gelatinase-associated lipocalin is expressed in osteoarthritis and forms a complex with matrix metalloproteinase 9. Arthritis Rheum. 56, 3326–3335 (2007). [DOI] [PubMed] [Google Scholar]
- 93.de Lange-Brokaar BJ et al. Characterization of synovial mast cells in knee osteoarthritis: association with clinical parameters. Osteoarthritis Cartilage 24, 664–671 (2016). [DOI] [PubMed] [Google Scholar]
- 94.Wang Q et al. IgE-mediated mast cell activation promotes inflammation and cartilage destruction in osteoarthritis. eLife 8, e39905 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sousa-Valente J et al. Role of TrkA signalling and mast cells in the initiation of osteoarthritis pain in the monoiodoacetate model. Osteoarthritis Cartilage 26, 84–94 (2018). [DOI] [PubMed] [Google Scholar]
- 96.Enomoto H et al. Vascular endothelial growth factor isoforms and their receptors are expressed in human osteoarthritic cartilage. Am. J. Pathol 162, 171–181 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kennedy A et al. Angiogenesis and blood vessel stability in inflammatory arthritis. Arthritis Rheum. 62, 711–721 (2010). [DOI] [PubMed] [Google Scholar]
- 98.Kim HR, Lee JH, Kim KW, Kim BM & Lee SH The relationship between synovial fluid VEGF and serum leptin with ultrasonographic findings in knee osteoarthritis. Int. J. Rheum. Dis 19, 233–240 (2016). [DOI] [PubMed] [Google Scholar]
- 99.Nagao M et al. Vascular endothelial growth factor in cartilage development and osteoarthritis. Sci. Rep 7, 13027 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li YS, Luo W, Zhu SA & Lei GH T cells in osteoarthritis: alterations and beyond. Front. Immunol 8, 356 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rosshirt N et al. Proinflammatory T cell polarization is already present in patients with early knee osteoarthritis. Arthritis Res. Ther 23, 37 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nees TA et al. T helper cell infiltration in osteoarthritis-related knee pain and disability. J. Clin. Med 9, 2423 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Thomas AC, Hubbard-Turner T, Wikstrom EA & Palmieri-Smith RM Epidemiology of posttraumatic osteoarthritis. J. Athl. Train 52, 491–496 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Whittaker JL et al. Association between MRI-defined osteoarthritis, pain, function and strength 3–10 years following knee joint injury in youth sport. Br. J. Sports Med 52, 934–939 (2018). [DOI] [PubMed] [Google Scholar]
- 105.Scanzello CR et al. Synovial inflammation in patients undergoing arthroscopic meniscectomy: molecular characterization and relationship to symptoms. Arthritis Rheum. 63, 391–400 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pessler F et al. The synovitis of “non-inflammatory” orthopaedic arthropathies: a quantitative histological and immunohistochemical analysis. Ann. Rheum. Dis 67, 1184–1187 (2008). [DOI] [PubMed] [Google Scholar]
- 107.Lieberthal J, Sambamurthy N & Scanzello CR Inflammation in joint injury and post-traumatic osteoarthritis. Osteoarthritis Cartilage 23, 1825–1834 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Davis JE et al. A single recent injury is a potent risk factor for the development of accelerated knee osteoarthritis: data from the osteoarthritis initiative. Rheumatol. Int 37, 1759–1764 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Driban JB et al. Association of knee injuries with accelerated knee osteoarthritis progression: data from the Osteoarthritis Initiative. Arthritis Care Res. 66, 1673–1679 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gelber AC et al. Joint injury in young adults and risk for subsequent knee and hip osteoarthritis. Ann. Intern. Med 133, 321–328 (2000). [DOI] [PubMed] [Google Scholar]
- 111.Sward P, Frobell R, Englund M, Roos H & Struglics A Cartilage and bone markers and inflammatory cytokines are increased in synovial fluid in the acute phase of knee injury (hemarthrosis) — a cross-sectional analysis. Osteoarthritis Cartilage 20, 1302–1308 (2012). [DOI] [PubMed] [Google Scholar]
- 112.Pham TM, Erichsen JL, Kowal JM, Overgaard S & Schmal H Elevation of pro-inflammatory cytokine levels following intra-articular fractures-a systematic review. Cells 10, 902 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pham TM, Frich LH, Lambertsen KL, Overgaard S & Schmal H Elevation of inflammatory cytokines and proteins after intra-articular ankle fracture: a cross-sectional study of 47 ankle fracture patients. Mediators Inflamm. 2021, 8897440 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jacobs CA et al. Dysregulated inflammatory response related to cartilage degradation after ACL injury. Med. Sci. Sports Exerc 52, 535–541 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Adams SB et al. Inflammatory microenvironment persists after bone healing in intra-articular ankle fractures. Foot Ankle Int. 38, 479–484 (2017). [DOI] [PubMed] [Google Scholar]
- 116.Roemer FW et al. Molecular and structural biomarkers of inflammation at two years after acute anterior cruciate ligament injury do not predict structural knee osteoarthritis at five years. Arthritis Rheumatol. 71, 238–243 (2019). [DOI] [PubMed] [Google Scholar]
- 117.Griffin TM & Guilak F The role of mechanical loading in the onset and progression of osteoarthritis. Exerc. Sport. Sci. Rev 33, 195–200 (2005). [DOI] [PubMed] [Google Scholar]
- 118.Fang T, Zhou X, Jin M, Nie J & Li X Molecular mechanisms of mechanical load-induced osteoarthritis. Int. Orthop 45, 1125–1136 (2021). [DOI] [PubMed] [Google Scholar]
- 119.Nazet U et al. Housekeeping gene validation for RT-qPCR studies on synovial fibroblasts derived from healthy and osteoarthritic patients with focus on mechanical loading. PLoS One 14, e0225790 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fahy N, Menzel U, Alini M & Stoddart MJ Shear and dynamic compression modulates the inflammatory phenotype of human monocytes in vitro. Front. Immunol 10, 383 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ruffino JS et al. Moderate-intensity exercise alters markers of alternative activation in circulating monocytes in females: a putative role for PPARgamma. Eur. J. Appl. Physiol 116, 1671–1682 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Helmark IC et al. Exercise increases interleukin-10 levels both intraarticularly and peri-synovially in patients with knee osteoarthritis: a randomized controlled trial. Arthritis Res. Ther 12, R126 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Boonstra A et al. Macrophages and myeloid dendritic cells, but not plasmacytoid dendritic cells, produce IL-10 in response to MyD88- and TRIF-dependent TLR signals, and TLR-independent signals. J. Immunol 177, 7551–7558 (2006). [DOI] [PubMed] [Google Scholar]
- 124.Yoshimura N et al. Accumulation of metabolic risk factors such as overweight, hypertension, dyslipidaemia, and impaired glucose tolerance raises the risk of occurrence and progression of knee osteoarthritis: a 3-year follow-up of the ROAD study. Osteoarthritis Cartilage 20, 1217–1226 (2012). [DOI] [PubMed] [Google Scholar]
- 125.Batushansky A et al. Fundamentals of OA. An initiative of osteoarthritis and cartilage. Chapter 9: obesity and metabolic factors in OA. Osteoarthritis Cartilage 10.1016/j.joca.2021.06.013 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Prieto-Alhambra D et al. Incidence and risk factors for clinically diagnosed knee, hip and hand osteoarthritis: influences of age, gender and osteoarthritis affecting other joints. Ann. Rheum. Dis 73, 1659–1664 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Losina E et al. Lifetime risk and age at diagnosis of symptomatic knee osteoarthritis in the US. Arthritis Care Res. 65, 703–711 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Murphy LB et al. One in four people may develop symptomatic hip osteoarthritis in his or her lifetime. Osteoarthritis Cartilage 18, 1372–1379 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Berenbaum F, Wallace IJ, Lieberman DE & Felson DT Modern-day environmental factors in the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol 14, 674–681 (2018). [DOI] [PubMed] [Google Scholar]
- 130.Harasymowicz NS et al. Regional differences between perisynovial and infrapatellar adipose tissue depots and their response to Class II and Class III obesity in patients with osteoarthritis. Arthritis Rheumatol. 69, 1396–1406 (2017). [DOI] [PubMed] [Google Scholar]
- 131.Duan L et al. Infrapatellar fat pads participate in the development of knee osteoarthritis in obese patients via the activation of the NFkappaB signaling pathway. Int. J. Mol. Med 46, 2260–2270 (2020). [DOI] [PubMed] [Google Scholar]
- 132.Takata K et al. Increase in tryptase and its role in the synovial membrane of overweight and obese patients with osteoarthritis of the knee. Diabetes Metab. Syndr. Obes 13, 1491–1497 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sun AR et al. Obesity-associated metabolic syndrome spontaneously induces infiltration of pro-inflammatory macrophage in synovium and promotes osteoarthritis. PLoS One 12, e0183693 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Larranaga-Vera A et al. Increased synovial lipodystrophy induced by high fat diet aggravates synovitis in experimental osteoarthritis. Arthritis Res. Ther 19, 264 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yusuf E et al. Association between weight or body mass index and hand osteoarthritis: a systematic review. Ann. Rheum. Dis 69, 761–765 (2010). [DOI] [PubMed] [Google Scholar]
- 136.Reyes C et al. Association between overweight and obesity and risk of clinically diagnosed knee, hip, and hand osteoarthritis: a population-based cohort study. Arthritis Rheumatol. 68, 1869–1875 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yang WH et al. Leptin induces IL-6 expression through OBRl receptor signaling pathway in human synovial fibroblasts. PLoS One 8, e75551 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jafarzadeh SR et al. Mediating role of bone marrow lesions, synovitis, pain sensitization, and depressive symptoms on knee pain improvement following substantial weight loss. Arthritis Rheumatol. 72, 420–427 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gudbergsen H et al. Changes in bone marrow lesions in response to weight-loss in obese knee osteoarthritis patients: a prospective cohort study. BMC Musculoskelet. Disord 14, 106 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Henriksen M et al. Structural changes in the knee during weight loss maintenance after a significant weight loss in obese patients with osteoarthritis: a report of secondary outcome analyses from a randomized controlled trial. Osteoarthritis Cartilage 22, 639–646 (2014). [DOI] [PubMed] [Google Scholar]
- 141.Daugaard CL et al. The impact of a significant weight loss on inflammation assessed on DCE-MRI and static MRI in knee osteoarthritis: a prospective cohort study. Osteoarthritis Cartilage 28, 766–773 (2020). [DOI] [PubMed] [Google Scholar]
- 142.Piva SR et al. Links between osteoarthritis and diabetes: implications for management from a physical activity perspective. Clin. Geriatr. Med 31, 67–87 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Schett G et al. Diabetes is an independent predictor for severe osteoarthritis: results from a longitudinal cohort study. Diabetes Care 36, 403–409 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Louati K, Vidal C, Berenbaum F & Sellam J Association between diabetes mellitus and osteoarthritis: systematic literature review and meta-analysis. RMD Open 1, e000077 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Niu J, Clancy M, Aliabadi P, Vasan R & Felson DT Metabolic syndrome, its components, and knee osteoarthritis: the Framingham Osteoarthritis Study. Arthritis Rheumatol. 69, 1194–1203 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Monira Hussain S et al. Incidence of total knee and hip replacement for osteoarthritis in relation to the metabolic syndrome and its components: a prospective cohort study. Semin. Arthritis Rheum 43, 429–436 (2014). [DOI] [PubMed] [Google Scholar]
- 147.Kuusalo L et al. Metabolic osteoarthritis — relation of diabetes and cardiovascular disease with knee osteoarthritis. Osteoarthritis Cartilage 29, 230–234 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rogers-Soeder TS et al. Association of diabetes mellitus and biomarkers of abnormal glucose metabolism with incident radiographic knee osteoarthritis. Arthritis Care Res. 72, 98–106 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hamada D et al. Suppressive effects of insulin on tumor necrosis factor-dependent early osteoarthritic changes associated with obesity and type 2 diabetes mellitus. Arthritis Rheumatol. 68, 1392–1402 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Griffin TM & Huffman KM Editorial: insulin resistance: releasing the brakes on synovial inflammation and osteoarthritis? Arthritis Rheumatol. 68, 1330–1333 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tsai CH et al. High glucose induces vascular endothelial growth factor production in human synovial fibroblasts through reactive oxygen species generation. Biochim. Biophys. Acta 1830, 2649–2658 (2013). [DOI] [PubMed] [Google Scholar]
- 152.Steenvoorden MM et al. Activation of receptor for advanced glycation end products in osteoarthritis leads to increased stimulation of chondrocytes and synoviocytes. Arthritis Rheum. 54, 253–263 (2006). [DOI] [PubMed] [Google Scholar]
- 153.Veronese N et al. The relationship between the dietary inflammatory index and prevalence of radiographic symptomatic osteoarthritis: data from the Osteoarthritis Initiative. Eur. J. Nutr 58, 253–260 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang X et al. Knee effusion-synovitis volume measurement and effects of vitamin D supplementation in patients with knee osteoarthritis. Osteoarthritis Cartilage 25, 1304–1312 (2017). [DOI] [PubMed] [Google Scholar]
- 155.Perry TA et al. Effect of Vitamin D supplementation on synovial tissue volume and subchondral bone marrow lesion volume in symptomatic knee osteoarthritis. BMC Musculoskelet. Disord 20, 76 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang Z et al. Effectiveness of curcuma longa extract for the treatment of symptoms and effusion-synovitis of knee osteoarthritis: a randomized trial. Ann. Intern. Med 173, 861–869 (2020). [DOI] [PubMed] [Google Scholar]
- 157.Veronese N et al. Mediterranean diet and knee osteoarthritis outcomes: a longitudinal cohort study. Clin. Nutr 38, 2735–2739 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Xu C et al. Dietary patterns and progression of knee osteoarthritis: data from the osteoarthritis initiative. Am. J. Clin. Nutr 111, 667–676 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ulici V et al. Osteoarthritis induced by destabilization of the medial meniscus is reduced in germ-free mice. Osteoarthritis Cartilage 26, 1098–1109 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dunn CM et al. Identification of cartilage microbial DNA signatures and associations with knee and hip osteoarthritis. Arthritis Rheumatol. 72, 1111–1122 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhao Y et al. Detection and characterization of bacterial nucleic acids in culture-negative synovial tissue and fluid samples from rheumatoid arthritis or osteoarthritis patients. Sci. Rep 8, 14305 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Schott EM et al. Targeting the gut microbiome to treat the osteoarthritis of obesity. JCI Insight 3, e95997 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Huang ZY, Stabler T, Pei FX & Kraus VB Both systemic and local lipopolysaccharide (LPS) burden are associated with knee OA severity and inflammation. Osteoarthritis Cartilage 24, 1769–1775 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mohammad S & Thiemermann C Role of metabolic endotoxemia in systemic inflammation and potential interventions. Front. Immunol 11, 594150 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Fuke N, Nagata N, Suganuma H & Ota T Regulation of gut microbiota and metabolic endotoxemia with dietary factors. Nutrients 11, 2277 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Boer CG et al. Intestinal microbiome composition and its relation to joint pain and inflammation. Nat. Commun 10, 4881 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mao X, Fu P, Wang L & Xiang C Mitochondria: potential targets for osteoarthritis. Front. Med 7, 581402 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Geurts J et al. Prematurely aging mitochondrial DNA mutator mice display subchondral osteopenia and chondrocyte hypertrophy without further osteoarthritis features. Sci. Rep 10, 1296 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Torroni A et al. Classification of European mtDNAs from an analysis of three European populations. Genetics 144, 1835–1850 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Da Sylva TR, Connor A, Mburu Y, Keystone E & Wu GE Somatic mutations in the mitochondria of rheumatoid arthritis synoviocytes. Arthritis Res. Ther 7, R844–R851 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Van den Bossche J et al. mitochondrial dysfunction prevents repolarization of inflammatory macrophages. Cell Rep. 17, 684–696 (2016). [DOI] [PubMed] [Google Scholar]
- 172.Jeon OH, et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat. Med 23, 775–781 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yohn COB, et al. Senescent synoviocytes in knee osteoarthritis correlate with disease biomarkers, synovitis, and knee pain [abstract]. Arthritis Rheumatol. https://acrabstracts.org/abstract/senescent-synoviocytes-in-knee-osteoarthritis-correlate-with-disease-biomarkers-synovitis-and-knee-pain/ (2019). [Google Scholar]
- 174.Anderson JR et al. 1H NMR metabolomics identifies underlying inflammatory pathology in osteoarthritis and rheumatoid arthritis synovial joints. J. Proteome Res 17, 3780–3790 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Farrell AJ, Blake DR, Palmer RM & Moncada S Increased concentrations of nitrite in synovial fluid and serum samples suggest increased nitric oxide synthesis in rheumatic diseases. Ann. Rheum. Dis 51, 1219–1222 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Melchiorri C et al. Enhanced and coordinated in vivo expression of inflammatory cytokines and nitric oxide synthase by chondrocytes from patients with osteoarthritis. Arthritis Rheum. 41, 2165–2174 (1998). [DOI] [PubMed] [Google Scholar]
- 177.Abramson SB Nitric oxide in inflammation and pain associated with osteoarthritis. Arthritis Res. Ther 10, S2 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Pelletier JP et al. Selective inhibition of inducible nitric oxide synthase in experimental osteoarthritis is associated with reduction in tissue levels of catabolic factors. J. Rheumatol 26, 2002–2014 (1999). [PubMed] [Google Scholar]
- 179.Hellio le Graverand MP et al. A 2-year randomised, double-blind, placebo-controlled, multicentre study of oral selective iNOS inhibitor, cindunistat (SD-6010), in patients with symptomatic osteoarthritis of the knee. Ann. Rheum. Dis 72, 187–195 (2013). [DOI] [PubMed] [Google Scholar]
- 180.Tannahill GM et al. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature 496, 238–242 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Keiran N et al. SUCNR1 controls an anti-inflammatory program in macrophages to regulate the metabolic response to obesity. Nat. Immunol 20, 581–592 (2019). [DOI] [PubMed] [Google Scholar]
- 182.Li Y et al. Succinate induces synovial angiogenesis in rheumatoid arthritis through metabolic remodeling and HIF-1alpha/VEGF axis. Free Radic. Biol. Med 126, 1–14 (2018). [DOI] [PubMed] [Google Scholar]
- 183.Wojdasiewicz P, Poniatowski LA & Szukiewicz D The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014, 561459 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Futani H et al. Relation between interleukin-18 and PGE2 in synovial fluid of osteoarthritis: a potential therapeutic target of cartilage degradation. J. Immunother 25, S61–S64 (2002). [DOI] [PubMed] [Google Scholar]
- 185.Attur M et al. Prostaglandin E2 exerts catabolic effects in osteoarthritis cartilage: evidence for signaling via the EP4 receptor. J. Immunol 181, 5082–5088 (2008). [DOI] [PubMed] [Google Scholar]
- 186.Valdes AM et al. Omega-6 oxylipins generated by soluble epoxide hydrolase are associated with knee osteoarthritis. J. Lipid Res 59, 1763–1770 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lambert C et al. The damage-associated molecular patterns (DAMPs) as potential targets to treat osteoarthritis: perspectives from a review of the literature. Front. Med 7, 607186 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Millerand M, Berenbaum F & Jacques C Danger signals and inflammaging in osteoarthritis. Clin. Exp. Rheumatol 37, 48–56 (2019). [PubMed] [Google Scholar]
- 189.Heinola T et al. High mobility group box-1 (HMGB-1) in osteoarthritic cartilage. Clin. Exp. Rheumatol 28, 511–518 (2010). [PubMed] [Google Scholar]
- 190.Li ZC et al. Correlation of synovial fluid HMGB-1 levels with radiographic severity of knee osteoarthritis. Clin. Invest. Med 34, E298 (2011). [DOI] [PubMed] [Google Scholar]
- 191.Ke X et al. Synovial fluid HMGB-1 levels are associated with osteoarthritis severity. Clin. Lab 61, 809–818 (2015). [DOI] [PubMed] [Google Scholar]
- 192.Aulin C, Lassacher T, Palmblad K & Erlandsson Harris H Early stage blockade of the alarmin HMGB1 reduces cartilage destruction in experimental OA. Osteoarthritis Cartilage 28, 698–707 (2020). [DOI] [PubMed] [Google Scholar]
- 193.Lambrecht S, Juchtmans N & Elewaut D Heat-shock proteins in stromal joint tissues: innocent bystanders or disease-initiating proteins? Rheumatology 53, 223–232 (2014). [DOI] [PubMed] [Google Scholar]
- 194.Takahashi K et al. Localization of heat shock protein in osteoarthritic cartilage. Scand. J. Rheumatol 26, 368–375 (1997). [DOI] [PubMed] [Google Scholar]
- 195.Ngarmukos S, Scaramuzza S, Theerawattanapong N, Tanavalee A & Honsawek S Circulating and synovial fluid heat shock protein 70 are correlated with severity in knee osteoarthritis. Cartilage 11, 323–328 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Son YO, Kim HE, Choi WS, Chun CH & Chun JS RNA-binding protein ZFP36L1 regulates osteoarthritis by modulating members of the heat shock protein 70 family. Nat. Commun 10, 77 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Lambert C et al. Gene expression pattern of cells from inflamed and normal areas of osteoarthritis synovial membrane. Arthritis Rheumatol. 66, 960–968 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Schelbergen RF et al. Alarmins S100A8/S100A9 aggravate osteophyte formation in experimental osteoarthritis and predict osteophyte progression in early human symptomatic osteoarthritis. Ann. Rheum. Dis 75, 218–225 (2016). [DOI] [PubMed] [Google Scholar]
- 199.Ruan G et al. Associations between serum S100A8/S100A9 and knee symptoms, joint structures and cartilage enzymes in patients with knee osteoarthritis. Osteoarthritis Cartilage 27, 99–105 (2019). [DOI] [PubMed] [Google Scholar]
- 200.Addimanda O et al. Elevated serum levels of alarmin S100A8/A9 in patients with hand osteoarthritis. Clin. Exp. Rheumatol 37, 885 (2019). [PubMed] [Google Scholar]
- 201.Daheshia M & Yao JQ The interleukin 1beta pathway in the pathogenesis of osteoarthritis. J. Rheumatol 35, 2306–2312 (2008). [DOI] [PubMed] [Google Scholar]
- 202.Inoue H et al. High levels of serum IL-18 promote cartilage loss through suppression of aggrecan synthesis. Bone 42, 1102–1110 (2008). [DOI] [PubMed] [Google Scholar]
- 203.McAllister MJ, Chemaly M, Eakin AJ, Gibson DS & McGilligan VE NLRP3 as a potentially novel biomarker for the management of osteoarthritis. Osteoarthritis Cartilage 26, 612–619 (2018). [DOI] [PubMed] [Google Scholar]
- 204.Schroder K, Zhou R & Tschopp J The NLRP3 inflammasome: a sensor for metabolic danger? Science 327, 296–300 (2010). [DOI] [PubMed] [Google Scholar]
- 205.Zhong Z et al. NF-kappaB restricts inflammasome activation via elimination of damaged mitochondria. Cell 164, 896–910 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sanchez-Lopez E et al. Choline uptake and metabolism modulate macrophage IL-1beta and IL-18 production. Cell Metab. 29, 1350–1362.e7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zhong Z et al. New mitochondrial DNA synthesis enables NLRP3 inflammasome activation. Nature 560, 198–203 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Jin C et al. NLRP3 inflammasome plays a critical role in the pathogenesis of hydroxyapatite-associated arthropathy. Proc. Natl Acad. Sci. USA 108, 14867–14872 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Martinon F, Petrilli V, Mayor A, Tardivel A & Tschopp J Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241 (2006). [DOI] [PubMed] [Google Scholar]
- 210.Denoble AE et al. Uric acid is a danger signal of increasing risk for osteoarthritis through inflammasome activation. Proc. Natl Acad. Sci. USA 108, 2088–2093 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Elsaid KA et al. The impact of early intra-articular administration of interleukin-1 receptor antagonist on lubricin metabolism and cartilage degeneration in an anterior cruciate ligament transection model. Osteoarthritis Cartilage 23, 114–121 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Schieker M et al. Effects of interleukin-1 beta inhibition on incident hip and knee replacement: exploratory analyses from a randomized, double-blind, placebo-controlled trial. Ann. Intern. Med 173, 509–515 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Lane N & Felson D A promising treatment for osteoarthritis? Ann. Intern. Med 173, 580–581 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Chevalier X et al. Intraarticular injection of anakinra in osteoarthritis of the knee: a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 61, 344–352 (2009). [DOI] [PubMed] [Google Scholar]
- 215.Fu Z et al. Interleukin-18-induced inflammatory responses in synoviocytes and chondrocytes from osteoarthritic patients. Int. J. Mol. Med 30, 805–810 (2012). [DOI] [PubMed] [Google Scholar]
- 216.Shi J, Gao W & Shao F Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem. Sci 42, 245–254 (2017). [DOI] [PubMed] [Google Scholar]
- 217.Wang Q et al. Identification of a central role for complement in osteoarthritis. Nat. Med 17, 1674–1679 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Assirelli E et al. Complement expression and activation in osteoarthritis joint compartments. Front. Immunol 11, 535010 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Li Z, Huang Z & Bai L Cell interplay in osteoarthritis. Front. Cell Dev. Biol 9, 720477 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Dreier R, Wallace S, Fuchs S, Bruckner P & Grassel S Paracrine interactions of chondrocytes and macrophages in cartilage degradation: articular chondrocytes provide factors that activate macrophage-derived pro-gelatinase B (pro-MMP-9). J. Cell Sci 114, 3813–3822 (2001). [DOI] [PubMed] [Google Scholar]
- 221.Hamasaki M et al. Transcriptional profiling of murine macrophages stimulated with cartilage fragments revealed a strategy for treatment of progressive osteoarthritis. Sci. Rep 10, 7558 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Silverstein AM et al. Toward understanding the role of cartilage particulates in synovial inflammation. Osteoarthritis Cartilage 25, 1353–1361 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Roemer FW et al. What comes first? Multitissue involvement leading to radiographic osteoarthritis: magnetic resonance imaging-based trajectory analysis over four years in the osteoarthritis initiative. Arthritis Rheumatol. 67, 2085–2096 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Hugle T & Geurts J What drives osteoarthritis?-synovial versus subchondral bone pathology. Rheumatology 56, 1461–1471 (2017). [DOI] [PubMed] [Google Scholar]
- 225.Hu W, Chen Y, Dou C & Dong S Microenvironment in subchondral bone: predominant regulator for the treatment of osteoarthritis. Ann. Rheum. Dis 80, 413–422 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Yang CC, Lin CY, Wang HS & Lyu SR Matrix metalloproteases and tissue inhibitors of metalloproteinases in medial plica and pannus-like tissue contribute to knee osteoarthritis progression. PLoS One 8, e79662 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Furuzawa-Carballeda J, Macip-Rodriguez PM & Cabral AR Osteoarthritis and rheumatoid arthritis pannus have similar qualitative metabolic characteristics and pro-inflammatory cytokine response. Clin. Exp. Rheumatol 26, 554–560 (2008). [PubMed] [Google Scholar]
- 228.Yusup A et al. Bone marrow lesions, subchondral bone cysts and subchondral bone attrition are associated with histological synovitis in patients with end-stage knee osteoarthritis: a cross-sectional study. Osteoarthritis Cartilage 23, 1858–1864 (2015). [DOI] [PubMed] [Google Scholar]
- 229.Aso K et al. Associations of symptomatic knee osteoarthritis with histopathologic features in subchondral bone. Arthritis Rheumatol. 71, 916–924 (2019). [DOI] [PubMed] [Google Scholar]
- 230.Arepati AI et al. Osteophyte formation is associated with synovitis in osteoarthritis — the Bunkyo Health Study. Osteoarthritis Cartilage 28, S86–S527 (2020). [Google Scholar]
- 231.Blom AB et al. Synovial lining macrophages mediate osteophyte formation during experimental osteoarthritis. Osteoarthritis Cartilage 12, 627–635 (2004). [DOI] [PubMed] [Google Scholar]
- 232.van Lent PL et al. Crucial role of synovial lining macrophages in the promotion of transforming growth factor beta-mediated osteophyte formation. Arthritis Rheum. 50, 103–111 (2004). [DOI] [PubMed] [Google Scholar]
- 233.Blaney Davidson EN et al. Resemblance of osteophytes in experimental osteoarthritis to transforming growth factor beta-induced osteophytes: limited role of bone morphogenetic protein in early osteoarthritic osteophyte formation. Arthritis Rheum. 56, 4065–4073 (2007). [DOI] [PubMed] [Google Scholar]
- 234.Remst DF, Blaney Davidson EN & van der Kraan PM Unravelling osteoarthritis-related synovial fibrosis: a step closer to solving joint stiffness. Rheumatology 54, 1954–1963 (2015). [DOI] [PubMed] [Google Scholar]
- 235.van Helvoort EM, Eijkelkamp N, Lafeber F & Mastbergen SC Expression of granulocyte macrophage-colony stimulating factor and its receptor in the synovium of osteoarthritis patients is negatively correlated with pain. Rheumatology 59, 3452–3457 (2020). [DOI] [PubMed] [Google Scholar]
- 236.Wise BL, Seidel MF & Lane NE The evolution of nerve growth factor inhibition in clinical medicine. Nat. Rev. Rheumatol 17, 34–46 (2021). [DOI] [PubMed] [Google Scholar]
- 237.Stoppiello LA et al. Structural associations of symptomatic knee osteoarthritis. Arthritis Rheumatol. 66, 3018–3027 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Cohen SB et al. A randomized, double-blind study of AMG 108 (a fully human monoclonal antibody to IL-1R1) in patients with osteoarthritis of the knee. Arthritis Res. Ther 13, R125 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Vincent TL Peripheral pain mechanisms in osteoarthritis. Pain 161, S138–S146 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Bratus-Neuenschwander A et al. Pain-associated transcriptome changes in synovium of knee osteoarthritis patients. Genes (Basel) 9, 338 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Attard V et al. Quantification of intra-articular fibrosis in patients with stiff knee arthroplasties using metal-reduction MRI. Bone Jt. J 102-B, 1331–1340 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Kalson NS et al. International consensus on the definition and classification of fibrosis of the knee joint. Bone Jt. J 98-B, 1479–1488 (2016). [DOI] [PubMed] [Google Scholar]
- 243.Toms J et al. Targeting fibroblast activation protein: radiosynthesis and preclinical evaluation of an 18F-labeled FAP inhibitor. J. Nucl. Med 61, 1806–1813 (2020). [DOI] [PubMed] [Google Scholar]
- 244.Kerna I et al. The ADAM12 is upregulated in synovitis and postinflammatory fibrosis of the synovial membrane in patients with early radiographic osteoarthritis. Jt. Bone Spine 81, 51–56 (2014). [DOI] [PubMed] [Google Scholar]
- 245.Remst DF et al. Gene expression analysis of murine and human osteoarthritis synovium reveals elevation of transforming growth factor beta-responsive genes in osteoarthritis-related fibrosis. Arthritis Rheumatol. 66, 647–656 (2014). [DOI] [PubMed] [Google Scholar]
- 246.Rim YA & Ju JH The role of fibrosis in osteoarthritis progression. Life (Basel) 11, 3 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Bastiaansen-Jenniskens YM et al. Stimulation of fibrotic processes by the infrapatellar fat pad in cultured synoviocytes from patients with osteoarthritis: a possible role for prostaglandin f2alpha. Arthritis Rheum. 65, 2070–2080 (2013). [DOI] [PubMed] [Google Scholar]
- 248.Eymard F et al. Knee and hip intra-articular adipose tissues (IAATs) compared with autologous subcutaneous adipose tissue: a specific phenotype for a central player in osteoarthritis. Ann. Rheum. Dis 76, 1142–1148 (2017). [DOI] [PubMed] [Google Scholar]
- 249.Davis JE et al. Effusion-synovitis and infrapatellar fat pad signal intensity alteration differentiate accelerated knee osteoarthritis. Rheumatology 58, 418–426 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Inomata K et al. Time course analyses of structural changes in the infrapatellar fat pad and synovial membrane during inflammation-induced persistent pain development in rat knee joint. BMC Musculoskelet. Disord 20, 8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Barboza E et al. Profibrotic infrapatellar fat pad remodeling without M1 macrophage polarization precedes knee osteoarthritis in mice with diet-induced obesity. Arthritis Rheumatol. 69, 1221–1232 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.de Jong AJ et al. Lack of high BMI-related features in adipocytes and inflammatory cells in the infrapatellar fat pad (IFP). Arthritis Res. Ther 19, 186 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Warmink K et al. High-fat feeding primes the mouse knee joint to develop osteoarthritis and pathologic infrapatellar fat pad changes after surgically induced injury. Osteoarthritis Cartilage 28, 593–602 (2020). [DOI] [PubMed] [Google Scholar]
- 254.Takano S et al. Transforming growth factor-beta stimulates nerve growth factor production in osteoarthritic synovium. BMC Musculoskelet. Disord 20, 204 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Blaney Davidson EN et al. TGF-beta is a potent inducer of nerve growth factor in articular cartilage via the ALK5-Smad2/3 pathway. Potential role in OA related pain? Osteoarthritis Cartilage 3, 478–486 (2015). [DOI] [PubMed] [Google Scholar]
- 256.Grässel S & Muschter D Recent advances in the treatment of osteoarthritis. F1000Res. 9, F1000 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Vincent TL Of mice and men: converging on a common molecular understanding of osteoarthritis. Lancet Rheumatol. 2, e633–e645 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Wang X, Hunter DJ, Jin X & Ding C The importance of synovial inflammation in osteoarthritis: current evidence from imaging assessments and clinical trials. Osteoarthritis Cartilage 26, 165–174 (2018). [DOI] [PubMed] [Google Scholar]
- 259.Roemer FW et al. Anatomical distribution of synovitis in knee osteoarthritis and its association with joint effusion assessed on non-enhanced and contrast-enhanced MRI. Osteoarthritis Cartilage 18, 1269–1274 (2010). [DOI] [PubMed] [Google Scholar]
- 260.Shakoor D et al. Are contrast-enhanced and non-contrast MRI findings reflecting synovial inflammation in knee osteoarthritis: a meta-analysis of observational studies. Osteoarthritis Cartilage 28, 126–136 (2020). [DOI] [PubMed] [Google Scholar]
- 261.Takase K et al. Simultaneous evaluation of long-lasting knee synovitis in patients undergoing arthroplasty by power Doppler ultrasonography and contrast-enhanced MRI in comparison with histopathology. Clin. Exp. Rheumatol 30, 85–92 (2012). [PubMed] [Google Scholar]
- 262.Walther M et al. Correlation of power Doppler sonography with vascularity of the synovial tissue of the knee joint in patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum. 44, 331–338 (2001). [DOI] [PubMed] [Google Scholar]
- 263.Tarhan S & Unlu Z Magnetic resonance imaging and ultrasonographic evaluation of the patients with knee osteoarthritis: a comparative study. Clin. Rheumatol 22, 181–188 (2003). [DOI] [PubMed] [Google Scholar]