Abstract
Targeted delivery of therapeutics through the use of nanoparticles (NPs) has emerged as a promising method that increases their efficacy and reduces their side effects. NPs can be tailored to localize to selective tissues through conjugation to ligands that bind cell-specific receptors. Although the vast majority of nanodelivery platforms have focused on cancer therapy, efforts have begun to introduce nanotherapeutics to the fields of immunology as well as transplantation. In this article, we provide an overview from a clinician’s perspective of current nanotherapeutic strategies to treat solid organ transplants with NPs during the time interval between organ harvest from the donor and placement into the recipient, an innovative technology that can provide major benefits to transplant patients. The use of ex vivo normothermic machine perfusion (NMP), which is associated with preserving the function of the organ following transplantation, also provides an ideal opportunity for a localized, sustained, and controlled delivery of nanotherapeutics to the organ during this critical time period. Here, we summarize previous endeavors to improve transplantation outcomes by treating the organ with NPs prior to placement in the recipient. Investigations in this burgeoning field of research are promising, but more extensive studies are needed to overcome the physiological challenges to achieving effective nanotherapeutic delivery to transplanted organs discussed in this review.
Keywords: nanotherapeutics, nanoparticles, transplantation, endothelial cells, immunology, machine perfusion, ischemia, ischemia-reperfusion injury, dendritic cells, transplant rejection
Graphical Abstract
Adverse effects that accompany the delivery of systemically administered immunosuppressive agents (ISAs) constitute major obstacles to their use and also hinder long-term success in transplantation.1–4 The emerging method of nanodelivery holds the potential to surmount this barrier and produce a transformative impact on the administration of ISAs by facilitating their transport to specific organs and tissues in a targeted fashion. This targeted method of delivery for ISAs can limit their interactions with unintended sites and the subsequent off-target toxicity.5–7 Other advantages of nanotherapy over conventional systemic medical therapy include a reduction in the required dose of ISAs and a capacity for customization, such as surface modification.8–10
Nanoparticles (NPs) are enclosed bodies of matter with a typical diameter of around 100 nm that can encapsulate a variety of therapeutic agents. The composition of NPs can vary from organic materials, including polymers, liposomes, and proteins, to inorganic materials, such as gold, iron oxide, and quantum dots.8, 11–13 NPs have widespread potential medical applications, due to the principle of active targeting. Active targeting refers to a method in which conjugation to the NP of ligands to receptors that are present in cells of the destination tissue confers the potential capacity of specific delivery of the NP to that tissue.14–18 Successful targeting by NPs relies on physical contact of the NP with the intended target cell and subsequent ligand-mediated retention at the site.14
In clinical practice, successful delivery of NPs to their target faces significant obstacles created by potential interactions with cells in other parts of the body, clearance by the kidney19–21 or mononuclear phagocytic system (MPS) in the liver22–24 and spleen,25–27 as well as trapping and sequestration in the lung capillary bed.28–31
Solid organ transplantation is an ideal clinical scenario in which therapeutic agents can be delivered directly to the organ, during the time period between removal from the donor and placement inside the recipient.32–34 This opportunity for direct access to the organ is a rarity in medicine, a setting that enhances the potential utility of nanotargeting through simplification of the kinetics of administration.35–38
Pertinent physical properties of NPs for intra-organ delivery
A major potential benefit to the use of NPs is optimization of the pharmacokinetics of a drug with a narrow therapeutic index or low bioavailability. These pharmacokinetic properties can be tuned by altering the chemical and physical characteristics of the NP, including its size, surface charge, shape, and surface composition.39–41
Size:
NP size is an important parameter that can be modified to direct the delivery of a drug to a particular organ.42 A significant body of work exists on the importance of NP size variations for applications in cancer.42, 43 Historically, alterations in the size of NPs have been undertaken to increase their systemic circulation. Following intravenous injection, those NPs smaller than 5 nm in diameter are cleared very rapidly by kidneys, while NPs larger than 200 nm are cleared rapidly by the MPS in the liver and spleen.21, 43, 44 The endothelium of the liver is non-continuous with fenestrations of 50–100 nm in diameter, so NPs in this size range will accumulate preferentially in the liver.45 Due to the size of the gap junctions (GJs) between endothelial cells in the spleen, NPs in the 200–500 nm range will accumulate there.46 A diameter between 100 to 150 nm is often considered an ideal NP size in the field of cancer therapeutics, as this range exceeds the size threshold for clearance by the kidneys but also falls short of the size that results in internalization by the MPS.43, 47, 48 Increasing the circulation time of the NP raises the chance that the NP will accumulate in the tumor.43 Nonetheless, the rise in awareness of the importance of lymphoid tissues to mounting an effective anti-cancer immune response may signal a need to revise this size guideline, as the internalization of some NPs that incorporate ISAs by the MPS in the spleen may boost anti-cancer immunity.48
NPs can be injected directly into the arterial blood supply of the organ prior to transplantation, but future experiments are required to determine the proper size for maximizing the intra-organ penetrance of NPs following intraarterial injection.47 As the organ could be perfused by a pump in this scenario, additional studies are required to assess the clearance kinetics of NPs in this setting. Nonetheless, the first layer of cells with which these NPs interact are endothelial cells (ECs). Some NPs traverse passively through the endothelium via intercellular connections called GJs, which are formed between adjacent ECs.49–52 GJs are vital to intercellular transfer of ions, small molecules, nutrients, and secondary messengers.53, 54 GJs also play an important role in vascular inflammation and the stiffness of ECs.50 On the other hand, the surface of NPs could also be coated with various antibodies to produce an active interaction with the ECs (e.g., anti-CD31 antibody).
Cold ischemia time refers to the storage period of transplanted organs in cold solution, immediately following their removal from the donor and prior to the time that they are warmed through restoration of blood supply.32, 55 Ischemia results in the closure of GJs and opening of hemichannels,56 which are comprised of six subunits of a protein called connexin and form one half of the GJ. In the heart, cardiac ischemia can result in closure of GJs between myocytes, decreasing intercellular conductance57–61 and thus also the passive entrance of NPs into the heart tissue. Cold ischemia is a specific cause of hypoxic uncoupling, which results in endothelial GJ damage, a major factor responsible for transplant rejection.62 In fact, addition of the Cx43 mimetic peptide ACT-1 to University of Wisconsin (UW) preservation medium stabilized the endothelial GJs of mouse cardiac tissue in cold storage and notably reduced ischemia-reperfusion injury (IRI).63 Therefore, this tool could be used to increase the targeting efficacy of NPs that rely on GJs to enter transplanted organs.
IRI of the kidney has been associated with disruption of tight junctions, areas of close contact between adjacent epithelial cells in the kidney, through which solutes greater than 1.8 nm typically cannot pass.64, 65 These losses in the integrity of tight junctions suggest that ischemic organs may permit the entrance of NPs of greater size and quantity. However, future studies are required to test this hypothesis.
Surface charge:
NP surface charge, measured as zeta potential (ξ), can also be manipulated to prolong the circulation time and effect targeted delivery.43 NPs with neutral and negative surface charge have decreased serum protein adsorption, thereby extending circulation time.66 However, positively charged NPs undergo more robust non-specific uptake by organs.42, 67 The positive charge can interact specifically with the glycocalyx, a negatively charged layer of polysaccharides that covers the cell membrane of some endothelial cells.44 Thurston et al. showed that cationic liposomes are internalized robustly by tumor-associated endothelial cells.67 Positive surface charge also promotes endosomal release of payloads and limits intracellular drug degradation.68 Thus, NPs with neutral or negative charge have prolonged circulation following intravenous (iv) administration, but a positive surface charge facilitates efficient uptake by some target cells and successful release of the payload inside these cells.42, 69
The kidney, the most commonly transplanted organ, presents particular challenges to effective targeted drug delivery on the basis of charge considerations.70–72 The glomerular basement membrane, a major component of the blood filtration apparatus of the kidney, has a strongly negative charge and thereby functions as a major barrier to the filtration of negatively charged molecules.73 Prior studies have demonstrated that among similarly sized molecules, those that are positively charged cross the filtration barrier faster than neutral molecules, which in turn cross faster than negatively charged molecules.74 Therefore, localization of the NPs to the tubular compartment via passage through the glomerular filtration apparatus in the kidney likely requires the synthesis of neutral or positively charged NPs.75
Shape:
Another important parameter to consider is the shape of NPs, which is also a crucial determinant of their half-life in the circulation.42 Gentile et al. showed that discoidal particles tend to marginate and adhere to endothelium more readily than spherical particles, due to specific tumbling and margination dynamics.76 Geng et al. demonstrated that filo-micelles (filamentous polymer micelles) align naturally with blood flow, resulting in longer circulation time (>1 week) than spherical NPs (2–3 days).77 The shape of NPs also affects cellular internalization, as the initial contact angle of the NP upon macrophage contact determines its rate of phagocytosis.43 Parallel alignment of the short axis of the NP with the cell membrane facilitates more rapid internalization than alignment along the long axis.43, 78 For rod-shaped NPs, internalization is faster when they are perpendicular to the cell axis, i.e. θ = 90°.79 For spherical NPs, NPs with a length of normalized curvature, denoted Ω, of ≤45° undergo faster internalization than particles with a Ω ≥ 45°.42, 80 Park et al. showed that tumors internalized paclitaxel filo-micelles more robustly in comparison to spherical NPs upon IV administration.81
Surface composition:
Many different materials can be harnessed to form NPs used for intra-organ drug delivery, of which the most salient will be highlighted here. Poly(lactic-co-glycolic acid) (PLGA) is a biodegradable organic polymer that is used commonly to form NPs. Drug encapsulation into PLGA NPs leads to stabilization, prolongation of circulation time, and guided release of the drug.82
The surface of PLGA NPs can also be altered through the attachment of different molecules, such as sialic acid or glycolipids. Polyethylene glycol (PEG) provides the PLGA NP with the capacity for evasion of uptake by the mononuclear phagocytic system. For example, cyclosporine, an ISA used commonly for suppression of transplant rejection, can be encapsulated into PEG-PLGA NPs for controlled release, thereby stabilizing the inherent variability in its pharmacokinetic profile as it maximizes its therapeutic efficacy and minimizes its toxicity.83, 84 Additionally, envelopment of the ISAs tacrolimus and sirolimus into liposomes has also yielded similar success.85–88 Active targeting can be achieved through modification of the PEG molecule with monoclonal antibodies or ligands.89–93
High-density lipoproteins (HDLs) can also be used to construct small dynamic NPs that modify the activity of the immune response through their internalization by macrophages.94 NPs formed by HDL target myeloid cells with high specificity, delivering immune therapeutics to APCs readily in vivo.95 Binding by HDL NPs to ATP-binding cassette receptor A1 and scavenger receptor type B-1 on the surface of myeloid cells mediates this interaction.96 Therefore, HDL-NPs represent another efficacious approach to improving ISA therapy in transplant recipients.97
Finally, intracellular adhesion molecule-1 (ICAM-1) antibody-coated nanogels composed of a mixture of dextran and lysozyme have been found to provide efficient delivery of drugs in vitro and in vivo to endothelial cells.98
NP stability:
NP stability refers to the preservation of a specific NP parameter in a given condition.99 One major property that correlates to the integrity of NPs upon their collision with each other is aggregation.99–101 Aggregation must be mitigated to maintain stability of NPs, due to their tendency to cluster following these collisions.102 The creation of this protection can be achieved through adjustment of pH, temperature, and the use of stabilizing agents, such as poly(vinyl pyrrolidone).99, 103 Stability of the chemical composition of the NP is another major factor that must be considered. Oxidation from reactive components in the medium can result in chemical instability.104 Approaches using inert metals,105, 106 graphene oxide,107 or a complex surfactant108 have proven to preserve the chemical composition of NPs.99 Successful strategies to stabilize NP shape correlate to retention of its original structure and radius of curvature.99, 100, 109 Surfactants, such as oleylamine, can be utilized to maintain the shape of NPs by reducing adsorption.110 Stability of NP size refers to conservation of the dimensionality of the NP throughout the course of an experiment or in storage solution.99, 111 Low-density stabilizing agents assist in preserving the stability of large NPs; alteration of surface composition through the use of PEG or EDTA can serve to accomplish this goal.112–114 Finally, stabilization of surface chemistry refers to maintenance of the original surface potential, structure, and composition of the NP.99, 100, 105 Altering the pH of the solution can assist in retention of surface chemistry; for example, silica NPs are stable in a solution with a pH of 5.5 to 6.5 for up to 50 hours.115
Endocytosis of NPs
Equally as important as the forces that govern the extracellular localization of the NP is the intracellular distribution of the NP at its target, as this determines the efficacy and bioavailability of the drug that it carries.116 NPs are internalized into cells via an active transport mechanism called endocytosis.117 During this process, the cell membrane invaginates and engulfs the NP from the extracellular environment, forming an intracellular membrane-bound vesicle called an endosome.117, 118 Then, the endosome fuses with a lysosome, another membrane-bound vesicle that contains hydrolytic enzymes, resulting in degradation of the encapsulated NP and release of the drug.119, 120 Endocytosis can be classified into several different processes, such as phagocytosis, pinocytosis, clathrin-mediated endocytosis, and caveolae-mediated endocytosis.121, 122 Phagocytosis and pinocytosis can be differentiated on the basis of endosome size; large particles are engulfed by large endosomes in the former, whereas small endosomes capture small volumes of fluid in the latter.117, 123, 124
Clathrin-mediated endocytosis is a receptor-mediated pathway, in which a ligand binds to a receptor on the cell membrane in a clathrin-rich area, where the ligand-receptor complex is engulfed subsequently in clathrin-coated vesicles.125–127 These vesicles fuse with endosomes and are degraded via the endosomal-lysosomal pathway.117, 128–131 Caveolae-mediated endocytosis requires caveolae, which are flask-shaped invaginations of the cell membrane.132, 133 Following detachment from the cell membrane, caveolae fuse with caveosomes, thereby avoiding lysosomes.134, 135 Therefore, this internalization pathway does not involve degradation and could be most favorable for the transport of NPs.117, 136, 137
Organ transplantation as a notable medical application for nanotherapy
As discussed earlier, when drug-loaded NPs are administered systemically, many of the NPs accumulate at off-target sites, despite the specificity created by surface conjugation and the principle of active targeting. The dilemma posed by competition with off-target binding sites and interference by the mononuclear phagocytic system during in vivo administration of therapeutics can be circumvented via solid organ transplantation, a clinical scenario in which the organ is temporarily accessible for direct ex vivo treatment. A key feature of organ transplantation is the emergence of ex vivo normothermic machine perfusion (NMP).138
NMP can expand the pool of available organs to include those of marginal initial quality, by enhancing graft assessment, preservation, and resuscitation, through constant supply of oxygen and nutrients as well as clearance of toxic metabolites. Currently, ex vivo kidney perfusion is the most highly developed extracorporeal perfusion technique, although liver, heart, intestine, and lung perfusion have also been introduced into clinical practice.139–143 In addition, machine perfusion of vascularized composite allografts, such as limbs, has created possibilities for transplantation or autologous reimplantation.144
NMP is an ideal process by which therapeutics can be delivered directly to the organ, prior to its implantation in the recipient, In a localized, sustained, and controlled fashion87 (Figure 1). Therefore, this modality can be explored to overcome the limitations of in vivo administration, as discussed earlier. This technology has been associated with a reduction in delayed graft function (DGF) by reducing the cold ischemia time of the organ.145 DGF is an important and common complication of transplantation, affecting around 31% of kidney transplants in the United States.146 DGF is also a significant risk factor for acute rejection and reduced long-term survival of the organ.147–149
Figure 1. Administration of NPs containing anti-inflammatory drugs during ex vivo NMP of heart.
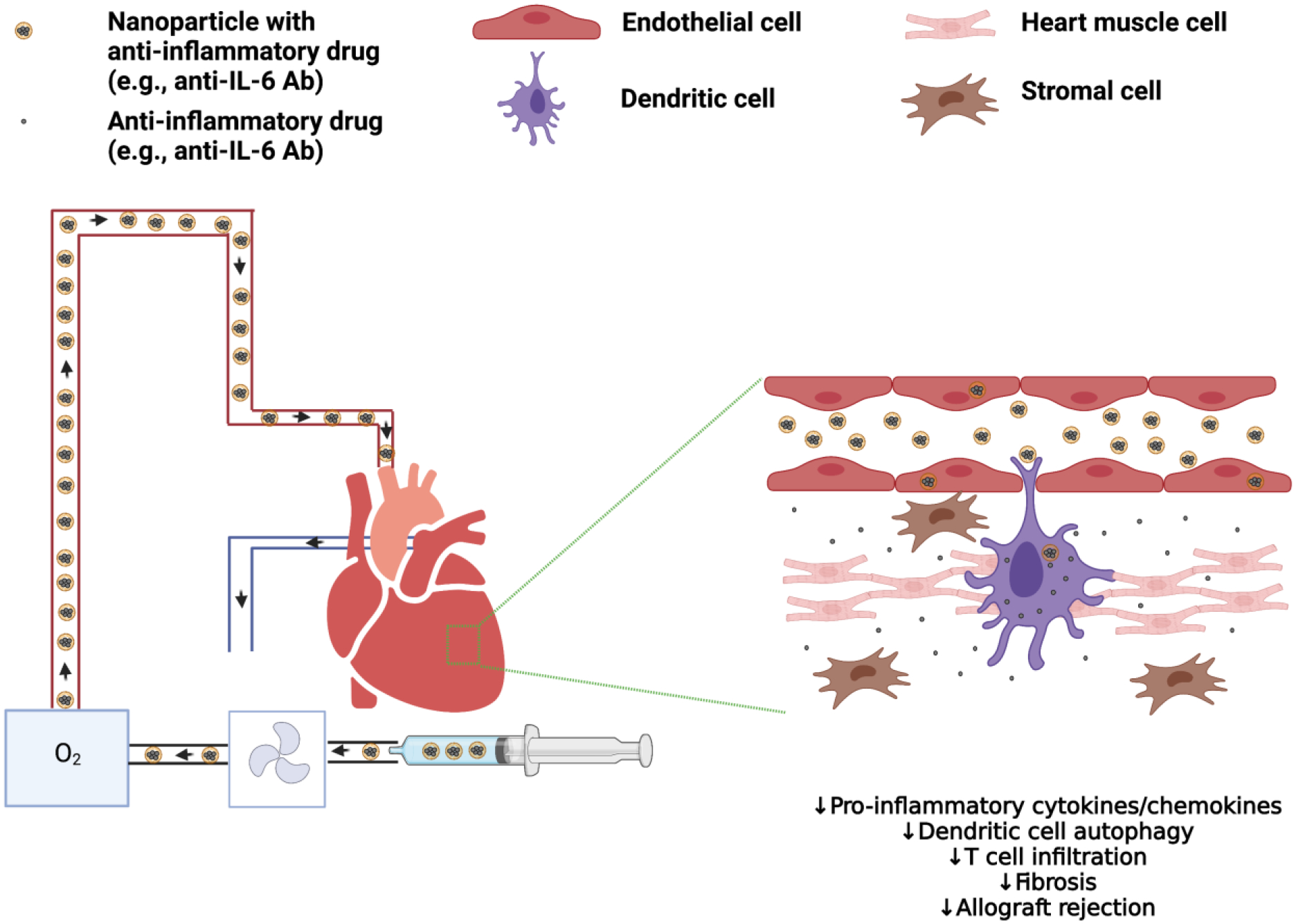
Ex vivo NMP provides an ideal scenario for direct administration of NPs containing anti-inflammatory drugs for intra-organ delivery prior to transplantation of the heart and other solid organs. Anti-IL-6 Ab: antibody against IL-6. Created with BioRender.com.
ISAs can also be delivered via NMP to reduce the total exposure to systemic immunosuppressive medications post-transplantation.8 In addition, machine perfusion also permits ex vivo assessment of isolated kidneys through the analysis of secreted factors in the perfusion fluids,150, 151 which can indicate the quality of the organ prior to its transplantation.152
Application of NPs for Organ Transplantation
Research into the application of NPs for organ transplantation remains in its infancy. However, several recent publications have demonstrated important breakthroughs in this field. ECs, the single layer of cells that line the inside of a blood vessel and regulate the exchange of molecules between blood and tissue, represent the first point of contact for NPs with the organ in both in vivo and ex vivo administration settings. They are also the primary site of damage from both IRI and preformed anti-donor antibodies. Therefore, limiting the perioperative injury to ECs through targeted drug delivery can mitigate the severity of the alloimmune response in the recipient and thereby yield long-term benefits.153
ECs as the first points of contact for NPs position them well for drug targeting. However, several factors must be considered to ensure proper efficacy of the therapeutic platform. First, some target molecules may be shed by ECs under pathological conditions, such as acute inflammation and ischemia. An example is thrombomodulin, which escapes from the lung following acute lung injury, thereby reducing by half the localization of anti-thrombomodulin antibodies to the lung.44, 154, 155 The exact location of the ligand within the EC membrane is important as well. Molecules that are obscured by glycocalyx or located within intercellular junctions will be more difficult to target.156 In addition, proteins that are situated inside membrane invaginations (such as caveolae) will be more challenging to access, as the mouths of the caveolae are typically no greater than 50 nm in diameter.157, 158 Conversely, some pathological conditions that are associated with loss of the glycocalyx may uncover some ligands, such as intracellular adhesion molecule-1 (ICAM-1), and boost their interaction with targeting moieties.159
The authors of an innovative investigation examined the efficacy of a practice that they termed “red blood cell hitchhiking,” in which nanocarriers described above, comprising of a dextran and lysozyme mixture,98 were adsorbed onto red blood cells and injected ex vivo into subsegmental branches of pulmonary arteries of human lungs.160 These red blood cell-adsorbed nanocarriers, which were found earlier to internalize readily to ECs in vitro,98 accumulated in the lungs at 3.7-fold higher density than free nanocarriers.160 However, whether the ECs of the human kidney, the most commonly transplanted organ in clinical practice, could be targeted successfully by NPs was unclear. To answer this question, an important study conducted by Tietjen et al. evaluated whether surface conjugation to polymeric NPs of an Ab reactive with an EC surface molecule could enhance NP targeting to vascular ECs during ex vivo NMP of human kidneys. They adapted an established approach for conjugating a mouse monoclonal Ab to fluorescent dye-loaded poly(lactic acid)–PEG (PLA-PEG) NPs of a consistent diameter and initially used these NPs to analyze attachment to cultured human umbilical vein ECs (HUVECs) under static conditions or in a microfluidic flow chamber under conditions of shear stress that more closely resemble those created during ex vivo NMP. In these initial experiments, the investigators compared anti-CD31-conjugated NPs (CD31-NPs) to NPs conjugated with the relevant isotype control (Control-NP), and they quantified the effects of NP concentration as well as duration of treatment by fluorescence microscopy and flow cytometry on transplant rejection. Then, they assessed the localization of NPs to ECs in 8 human kidneys that had been declined for transplant during ex vivo NMP, under the same conditions as would be applied to organs used for transplantation.153 This study revealed that the attachment of monoclonal anti-CD31 Ab to the surface of PLGA-PEG NPs loaded with a fluorescent dye and administration of these CD31-NPs to isolated human kidneys during ex vivo NMP can lead to enhanced vascular retention, as compared to unconjugated NPs. Using two-color quantitative microscopy on cryosectioned biopsies, the authors observed that CD31-NPs accumulated at a 5- to 10-fold higher rate in the renal vasculature, as compared to the Control-NPs. This approach showed that attachment to NPs of Abs targeted to ECs can augment their accumulation in the ECs of both the glomerular and peritubular capillaries in the kidney during ex vivo NMP.153
Cell-mediated transplant rejection occurs through the recognition of the human leukocyte antigen (HLA) expressed by cells of the donor graft as foreign by the T cells of the recipient. The most accessible HLA molecules in the donor graft to the T cells are located on the surface of the ECs. HLAs are member proteins of the major histocompatibility complex (MHC) that are found in humans. Cui et al. developed small interfering RNA-releasing poly(amine-co-ester) nanoparticles (siRNA-NPs), containing a high content of a hydrophobic lactone. They showed that a single transfection of siRNA-NPs targeting class II transactivator attenuated MHC class II (MHC-II) expression on ECs for at least 4 to 6 weeks after transplantation into immunodeficient mouse hosts. Furthermore, silencing of MHC-II reduced allogeneic T-cell responses in vitro and in vivo. These data suggest that siRNA administered during ex vivo NMP of human organs could be used to modify ECs with a sustained tolerogenic effect following transplantation.161
In one study, a micelle of approximately 10 nm in size that encapsulated the ISA sirolimus was developed, consisting of PEG-PE-amine and N-palmitoyl homocysteine (PHC) modified with a targeting peptide (cRGD) for ECs. These targeted rapamycin (sirolimus) micelles (TRaMs) were internalized successfully by HUVECs in vitro. In addition, treatment with these TRaMs resulted in inhibition of production and release of the pro-inflammatory cytokines Il-6 and IL-8 by HUVECs and mouse cardiac endothelial cells, including in hypoxic conditions, such as those encountered during IRI. The study also demonstrated a dose-dependent uptake of TRaMs by aortic ECs ex vivo.162, 163 In a model of skin transplantation, a visual light-crosslinkable biomaterial composed of gelatin methacryloyl that eluted an antibody against IL-6 receptor (anti-IL-6R) was created and placed between skin allografts and areas of excised skin of mouse recipients.164 This biomaterial doubled the length of survival of the skin allografts by reducing the infiltration of alloreactive T cells and macrophages.164
A key advantage of intra-organ delivery of NPs is to suppress the activation of Toll-like receptors (TLRs) in the antigen presenting cells (APCs). Dendritic cells (DCs) can project cellular extensions into the lumina of blood vessels that can capture NPs from the circulation.165, 166 Thus, coating the surface of NPs with antibodies against the macrophage antigen CD11b and dendritic cell antigen CD11c may increase their uptake into organs through direct interaction with these cells. IRI results in the opening of connexin hemichannels,56 so a greater number of NPs may cross the endothelium between adjacent cells and undergo internalization by APCs of ischemic organs.
One study demonstrated that ex vivo NMP of heart allografts with PLGA nanoparticles encapsulating anti-IL-6 Ab resulted in lower chronic rejection rates of heart allografts in mice, as compared to systemic administration of IL-6. This beneficial effect, which minimized the amount of anti-IL-6 Ab delivered to the animals, was mediated though mitigating the sequelae of IRI-induced autophagy in heart allograft-resident dendritic cells.167 An additional investigation sought to determine whether ex vivo NMP of heart allografts with NPs loaded with the ISA mycophenolate mofetil (MMF) prior to transplantation reduces the rate of transplant rejection in comparison to systemic administration of MMF post-transplant.35 Ex vivo treatment with MMF-loaded NPs prevented the onset of rejection of the heart transplant through the inhibition of pro-inflammatory cytokines and chemokines in the heart graft. The NPs were internalized mainly by CD11b+ macrophages in the organ. Together, these studies show that treatment of transplant organs ex vivo with ISAs prior to transplantation can become a clinically feasible method of reducing the rate of graft rejection post-transplantation through inhibition of APC activity.
Clinical pitfalls and obstacles
Vascular thrombosis:
Aggregation of NPs within the microvasculature of organs can add to the risk of microthrombi, especially in an ischemic organ that might be more predisposed towards microthrombi formation.168, 169 NMP and cold storage can lead to endothelial dysfunction, arterial thrombosis, post-reperfusion syndrome (intraoperative hypotension following reperfusion of liver grafts),170–172 and potential post-transplantation arterial thrombosis.173 Injection of NPs containing heparin could reduce this potential complication of microthrombosis.174 Amongst the various materials used to create NPs, PLGA may represent a safe choice, due to its safety profile with respect to potential endothelial toxicity.175 On the other hand, attachment to the NP surface of antibodies that cross-react with integrins expressed on ECs may constitute additional risk, as these antibodies could potentiate EC activation and vascular thrombosis.
Effect of temperature on NP stability:
The instability of NPs in ex vivo perfusate also presents a major limitation to their widespread clinical use, even though considerable advances have been made to solve this problem, as previously explained. NPs are more stable in a colder environment than a warmer environment.176 After donor death, both hypothermic machine perfusion (HMP) and static cold storage (SCS) are used to maintain the viability of the organ, and several studies have compared the efficacy of these methods in maximizing function. Jochmans et al. advised the use of HMP by demonstrating that it is associated with reduced risk of delayed renal graft rejection and better early post-transplant graft function.177 Jia et al. demonstrated that HMP is superior to SCS for liver allograft preservation, as it improved short-term outcomes and protected against early allograft dysfunction and biliary complications.178 These techniques can also assist in preserving the stability of NPs that are administered during this time period. Future studies are required to compare the stability and efficacy of nanotherapeutics for intra-organ delivery between HMP and NMP.
Temperature affects many properties of NPs, including size, structure, magnetism, aggregation, and stability. Intrinsic temperature-sensitive characteristics of the NP determine how temperature alters its properties. For example, increasing the reaction temperature in the synthesis of cobalt ferrite NPs (CoFe2O4-NPs), augments their size, increases their saturation magnetization, and results in the formation of an equiaxial-shaped, single-phase cubic spinel structure.179 On the other hand, increasing the reaction temperature decreases the size of maghemite NPs (γ-Fe2O3-NPs), lowers their magnetization, and improves their stability.180 Increasing the reaction temperature boosts the aggregation of gold NPs (Au-NPs).181 Finally, intermediate reaction temperatures have been demonstrated to produce smaller silver NPs (Ag-NPs) with narrow size distribution.182 Extensive investigation is required to expand the understanding of the effect of temperature on the characteristics of NPs and its application to machine perfusion of transplanted organs, a process that can occur in normothermic or hypothermic conditions.
Toxicity of payload:
ISAs packaged inside NPs have side effects that must still be considered in their administration. For example, calcineurin inhibitors like cyclosporine and tacrolimus cause endothelial dysfunction due to vasoconstriction, hypertension, and enhanced formation of superoxide.183 Other ISAs like sirolimus and mycophenolate mofetil may be more favorable due to their vascular safety profile. In addition, nanosized drug delivery devices can be developed to control the release of a drug at a concentration within a specific therapeutic range, thereby circumventing the threat of toxicity and overdose, while ensuring consistent efficacy.184, 185
Finally, alternative molecules that dampen the immune response by shutting down the inflammasome like MCC950,186 3,4-Methylenedioxy-β-nitrostyrene (MNS),187 tranilast,188 or oridonin,189, 190 or inhibit nuclear factor-kappa B (NF-κB) like emetine, fluorosalan, sunitinib malate, bithionol, narasin, tribromsalan, or lestaurtinib191 can be placed inside the NPs.
Targeting recipient lymphoid tissue via intra-organ delivery of NPs:
An interesting concept that remains unexplored is targeting the lymphoid tissue with NPs through intra-organ delivery. As systemically administered NPs pass through blood vessels and arrive in the interstitium of the organ, some enter the lymphatic capillaries through solvent drag and arrive to draining lymph nodes (DLNs)—the quintessential sites for the mounting of adaptive immunity--via afferent lymphatic ducts.192, 193 Whether NPs that enter the lymphatic capillaries during intra-organ delivery subsequently home to the LNs following anastomosis in the recipient is unknown.
NPs of higher molecular weight (1000–16,000 kDa) drain through lymphatic channels instead of blood vasculature194, 195 However, NPs of higher size do not diffuse as easily through the interstitium, resulting in a slower drainage rate into lymphatics.196 Ischemia and increased interstitial pressure may enhance the trafficking of these larger NPs from the interstitium to the lymphatics. These NPs could then transport potent immunomodulatory molecules directly to the DLNs, important sites for immune activation.
Conclusion
Nanotherapeutics offer a promising approach to the targeted delivery of ISAs for prevention of solid organ transplant rejection. NPs assist in optimizing pharmacokinetic properties to maximize therapeutic bioavailability, specificity, and efficacy, while minimizing toxicity. However, in vivo application of nanotherapeutics still faces significant physiologic barriers, such as the accumulation of NPs at off-target sites and uptake by mononuclear phagocytes for elimination. Ex vivo NMP permits direct administration of NPs containing ISAs to the solid organ prior to transplantation, circumventing the limitations of in vivo application. An inadequate supply of transplant organs has led to extended wait times, resulting in increased waitlist mortality for chronic organ disease patients. From this perspective, NMP has proven to be a promising advancement, offering opportunities to improve graft preservation and viability at the time of transplantation, as well as to prevent rejection post-transplantation.
A small set of studies have tested the feasibility and efficacy of administering NPs containing immunosuppressive agents during ex vivo NMP to prevent transplant rejection (Table 1), and the data from these preliminary studies have showed promise in prolongation of bioavailability as well as reduction of rejection. Therefore, the use of nanomedicine during preclinical studies of ex vivo NMP has provided a route to potentially groundbreaking progress in the clinical management of solid organ transplant recipients, but extensive investigation remains to overcome the barriers to effective drug delivery imposed by ECs and to translate these promising preliminary findings to significant advances in the prevention of allograft rejection, which remains the largest obstacle to the long-term survival of the transplant.
Table 1.
Summary of preclinical ex vivo nanovehicle administration studies in transplantation.
| Nano-vehicle | Method of Delivery | Therapeutic | Target Organ and Cell population | Results | Reference |
|---|---|---|---|---|---|
| ICAM-1-conjugated dextran-lysozyme nanogel adsorbed onto RBCs | Vascular perfusion | None | Human lung ECs | 3.7-fold higher localization to lung ECs than free nanocarriers | 160 |
| Anti-CD31-conjugated PLA-PEG NP | NMP; whole organ/tissue immersion | None | Human kidney ECs | 5–10-fold higher localization to kidney ECs than isotype control Ab-conjugated NPs | 153 |
| High-lactone poly(amine-co-ester) NP | NMP; whole organ/tissue immersion | siRNA targeting MHC class II transactivator | Human blood vessel ECs | Lowered MHC class II expression by ECs for 4–6 weeks; suppressed allogenic T cell responses | 161 |
| Light-crosslinkable gelatin methacryloyl biomaterial | Whole organ/tissue immersion | Anti-IL-6R Ab | Mouse skin macrophages, T cells | Decreased alloreactive T cell and macrophage infiltration; doubled survival length of skin allografts | 164 |
| PEG-PLGA NP | NMP; whole organ/tissue immersion | Anti-IL-6 Ab | Mouse heart macrophages, T cells | Decreased T cell and macrophage infiltration; inhibited chronic rejection in comparison to ischemic control | 167 |
| PEG-PLGA NP | NMP; whole organ/tissue immersion | MMF | Mouse heart macrophages, T cells | Decreased expression of pro-inflammatory cytokine and chemokines; decreased T cell and macrophage infiltration; inhibited fibrosis and chronic rejection in comparison to free MMF | 35 |
ICAM-1: intercellular adhesion molecule-1; RBC: red blood cell; PLA: poly(lactic acid); NP: nanoparticle; PEG: polyethylene glycol; PLGA poly(lactic-co-glycolic) acid; siRNA: small inhibitory RNA; anti-IL-6R Ab: antibody against IL-6 receptor; anti-IL-6 Ab: antibody against IL-6; MMF: mycophenolate mofetil; EC: endothelial cell; MHC: major histocompatibility complex
VOCABULARY.
Antibodies are molecules in the blood that are produced by B cells in host organisms in response to proteins (antigens) that the immune system of the host recognizes as foreign. Potential sources of these antigens include bacteria, viruses, toxic materials, or any other foreign substance, and the antibodies assist the immune system of the host in eliminating these potentially pathogenic sources.197
Poly(lactic-co-glycolic acid) (PLGA) is a synthetic biodegradable and biocompatible polymer, which is established as the “gold standard” polymer in controlled release systems for prolonged release of drugs.198 It is eliminated via hydrolysis in the body by degradation into lactic and glycolic acid.199, 200
Liposomes are lipid vesicles that can be used as carriers to transport biologically active molecules to their intended sites of action.
High-Density Lipoproteins (HDLs) are heterogenous lipoproteins that are responsible for cholesterol and lipid transport in the body.201 They are involved in reverse cholesterol transport (RCT), which leads to removal of excess cholesterol from blood vessels and uptake by the liver for elimination.202, 203 HDLs also have anti-atherogenic, anti-oxidative, and anti-inflammatory roles in the body.203–206
Antigen-presenting cells (APCs), comprised of macrophages and dendritic cells, are major participants in the innate immune response. Their primary role is to internalize foreign antigens and process them for presentation to T cells, which are the chief arbiters of adaptive immunity. This function positions APCs at the nexus of innate and adaptive immunity.
ACKNOWLEDGMENTS
This work is supported by National Institutes of Health under grants 5K08DK124685 (V.K.), 1P01AI153003 (J.S.B., R.A.), 5R01HL141815 (R.A.), 1R01AI156084 (R.A.), 5R01AI126596 (R.A.), and 5R01HL145813 (R.A.).
Footnotes
The authors declare no competing financial interests.
REFERENCES
- 1.Kerzerho J; Wunsch D; Szely N; Meyer HA; Lurz L; Röse L; Wahn U; Akbari O; Stock P, Effects of Systemic versus Local Administration of Corticosteroids on Mucosal Tolerance. J Immunol 2012, 188 (1), 470–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murgia MG; Jordan S; Kahan BD, The Side Effect Profile of Sirolimus: A Phase I Study in Quiescent Cyclosporine-Prednisone-Treated Renal Transplant Patients. Kidney Int 1996, 49 (1), 209–16. [DOI] [PubMed] [Google Scholar]
- 3.Christians U; Klawitter J; Klawitter J; Brunner N; Schmitz V, Biomarkers of Immunosuppressant Organ Toxicity after Transplantation: Status, Concepts and Misconceptions. Expert Opin Drug Metab Toxicol 2011, 7 (2), 175–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lallana EC; Fadul CE, Toxicities of Immunosuppressive Treatment of Autoimmune Neurologic Diseases. Curr Neuropharmacol 2011, 9 (3), 468–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang Y; Furuno M; Arakawa T; Takizawa S; De Hoon M; Suzuki H; Arner E, A Framework for Identification of On- and Off-Target Transcriptional Responses to Drug Treatment. Scientific Reports 2019, 9 (1), 17603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin A; Giuliano CJ; Palladino A; John KM; Abramowicz C; Yuan ML; Sausville EL; Lukow DA; Liu L; Chait AR; Galluzzo ZC; Tucker C; Sheltzer JM, Off-Target Toxicity Is a Common Mechanism of Action of Cancer Drugs Undergoing Clinical Trials. Sci Transl Med 2019, 11 (509). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rudmann D, On-Target and Off-Target-Based Toxicologic Effects. Toxicologic Pathology 2012, 41. [DOI] [PubMed] [Google Scholar]
- 8.Yao CG; Martins PN, Nanotechnology Applications in Transplantation Medicine. Transplantation 2020, 104 (4), 682–693. [DOI] [PubMed] [Google Scholar]
- 9.Dirito JR; Hosgood SA; Tietjen GT; Nicholson ML, The Future of Marginal Kidney Repair in the Context of Normothermic Machine Perfusion. Am J Transplant 2018, 18 (10), 2400–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordillo-Galeano A; Mora-Huertas CE, Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: A Review Emphasizing on Particle Structure and Drug Release. Eur J Pharm Biopharm 2018, 133, 285–308. [DOI] [PubMed] [Google Scholar]
- 11.Tobias AK; Jones M, Metal-Enhanced Fluorescence from Quantum Dot-Coupled Gold Nanoparticles. The Journal Of Physical Chemistry C 2019, 123 (2), 1389–1397. [Google Scholar]
- 12.Sharma R; Agrawal U; Mody N; Dubey S; Vyas SP Chapter 13 - Engineered Nanoparticles as a Precise Delivery System in Cancer Therapeutics. Engineering Of Nanobiomaterials; William Andrew Publishing: Norfolk, NY, 2016; 397–427. [Google Scholar]
- 13.Shibaev V, Liquid Crystalline Polymers. In Reference Module in Materials Science and Materials Engineering, Elsevier: 2016. [Google Scholar]
- 14.Tietjen GT; Bracaglia LG; Saltzman WM; Pober JS, Focus on Fundamentals: Achieving Effective Nanoparticle Targeting. Trends Mol Med 2018, 24 (7), 598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pearce AK; O’Reilly RK, Insights into Active Targeting of Nanoparticles in Drug Delivery: Advances in Clinical Studies and Design Considerations for Cancer Nanomedicine. Bioconjugate Chemistry 2019, 30 (9), 2300–2311. [DOI] [PubMed] [Google Scholar]
- 16.Clemons TD; Singh R; Sorolla A; Chaudhari N; Hubbard A; Iyer KS, Distinction between Active and Passive Targeting of Nanoparticles Dictate Their Overall Therapeutic Efficacy. Langmuir 2018, 34 (50), 15343–15349. [DOI] [PubMed] [Google Scholar]
- 17.Yoo J; Park C; Yi G; Lee D; Koo H, Active Targeting Strategies Using Biological Ligands for Nanoparticle Drug Delivery Systems. Cancers (Basel) 2019, 11 (5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muhamad N; Plengsuriyakarn T; Na-Bangchang K, Application of Active Targeting Nanoparticle Delivery System for Chemotherapeutic Drugs and Traditional/Herbal Medicines in Cancer Therapy: A Systematic Review. Int J Nanomedicine 2018, 13, 3921–3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Du B; Yu M; Zheng J, Transport and Interactions of Nanoparticles in the Kidneys. Nature Reviews Materials 2018, 3 (10), 358–374. [Google Scholar]
- 20.Choi HS; Liu W; Misra P; Tanaka E; Zimmer JP; Itty Ipe B; Bawendi MG; Frangioni JV, Renal Clearance of Quantum Dots. Nat Biotechnol 2007, 25 (10), 1165–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Longmire M; Choyke PL; Kobayashi H, Clearance Properties of Nano-Sized Particles and Molecules as Imaging Agents: Considerations and Caveats. Nanomedicine (Lond) 2008, 3 (5), 703–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ray K, Clearance of Nanomaterials in the Liver. Nature Reviews Gastroenterology & Hepatology 2016, 13 (10), 560–560. [DOI] [PubMed] [Google Scholar]
- 23.Haute DV; Berlin JM, Challenges in Realizing Selectivity for Nanoparticle Biodistribution and Clearance: Lessons from Gold Nanoparticles. Ther Deliv 2017, 8 (9), 763–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang YN; Poon W; Tavares AJ; Mcgilvray ID; Chan WCW, Nanoparticle-Liver Interactions: Cellular Uptake and Hepatobiliary Elimination. J Control Release 2016, 240, 332–348. [DOI] [PubMed] [Google Scholar]
- 25.Waegeneers N; Brasseur A; Van Doren E; Van Der Heyden S; Serreyn P-J; Pussemier L; Mast J; Schneider Y-J; Ruttens A; Roels S, Short-Term Biodistribution and Clearance of Intravenously Administered Silica Nanoparticles. Toxicology Reports 2018, 5, 632–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feng Q; Liu Y; Huang J; Chen K; Huang J; Xiao K, Uptake, Distribution, Clearance, and Toxicity of Iron Oxide Nanoparticles with Different Sizes and Coatings. Scientific Reports 2018, 8 (1), 2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cataldi M; Vigliotti C; Mosca T; Cammarota M; Capone D, Emerging Role of the Spleen in the Pharmacokinetics of Monoclonal Antibodies, Nanoparticles and Exosomes. Int J Mol Sci 2017, 18 (6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buckley A; Warren J; Hodgson A; Marczylo T; Ignatyev K; Guo C; Smith R, Slow Lung Clearance and Limited Translocation of Four Sizes of Inhaled Iridium Nanoparticles. Particle And Fibre Toxicology 2017, 14 (1), 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi HS; Ashitate Y; Lee JH; Kim SH; Matsui A; Insin N; Bawendi MG; Semmler-Behnke M; Frangioni JV; Tsuda A, Rapid Translocation of Nanoparticles from the Lung Airspaces to the Body. Nat Biotechnol 2010, 28 (12), 1300–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lipka J; Semmler-Behnke M; Sperling RA; Wenk A; Takenaka S; Schleh C; Kissel T; Parak WJ; Kreyling WG, Biodistribution of PEG-Modified Gold Nanoparticles Following Intratracheal Instillation and Intravenous Injection. Biomaterials 2010, 31 (25), 6574–81. [DOI] [PubMed] [Google Scholar]
- 31.Geiser M, Update on Macrophage Clearance of Inhaled Micro- and Nanoparticles. J Aerosol Med Pulm Drug Deliv 2010, 23 (4), 207–17. [DOI] [PubMed] [Google Scholar]
- 32.Postalcioglu M; Kaze AD; Byun BC; Siedlecki A; Tullius SG; Milford EL; Paik JM; Abdi R, Association of Cold Ischemia Time with Acute Renal Transplant Rejection. Transplantation 2018, 102 (7), 1188–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tezza S; Ben Nasr M; Vergani A; Valderrama Vasquez A; Maestroni A; Abdi R; Secchi A; Fiorina P, Novel Immunological Strategies for Islet Transplantation. Pharmacol Res 2015, 98, 69–75. [DOI] [PubMed] [Google Scholar]
- 34.Uehara M; Li X; Sheikhi A; Zandi N; Walker B; Saleh B; Banouni N; Jiang L; Ordikhani F; Dai L; Yonar M; Vohra I; Kasinath V; Orgill DP; Khademhosseini A; Annabi N; Abdi R, Anti-IL-6 Eluting Immunomodulatory Biomaterials Prolong Skin Allograft Survival. Scientific Reports 2019, 9 (1), 6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uehara M; Bahmani B; Jiang L; Jung S; Banouni N; Kasinath V; Solhjou Z; Zhao J; Ordikhani F; Bae M; Annabi N; Mcgrath MM; Abdi R, Nanodelivery of Mycophenolate Mofetil to the Organ Improves Transplant Vasculopathy. ACS Nano 2019, 13 (11), 12393–12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kvietkauskas M; Leber B; Strupas K; Stiegler P; Schemmer P, Machine Perfusion of Extended Criteria Donor Organs: Immunological Aspects. Frontiers In Immunology 2020, 11 (192). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buchwald JE; Xu J; Bozorgzadeh A; Martins PN, Therapeutics Administered During ex Vivo Liver Machine Perfusion: An Overview. World J Transplant 2020, 10 (1), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Angelico R; Perera MT; Ravikumar R; Holroyd D; Coussios C; Mergental H; Isaac JR; Iqbal A; Cilliers H; Muiesan P; Friend PJ; Mirza DF, Normothermic Machine Perfusion of Deceased Donor Liver Grafts Is Associated with Improved Postreperfusion Hemodynamics. Transplant Direct 2016, 2 (9), E97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qie Y; Yuan H; Von Roemeling CA; Chen Y; Liu X; Shih KD; Knight JA; Tun HW; Wharen RE; Jiang W; Kim BYS, Surface Modification of Nanoparticles Enables Selective Evasion of Phagocytic Clearance by Distinct Macrophage Phenotypes. Scientific Reports 2016, 6 (1), 26269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khongkow M; Yata T; Boonrungsiman S; Ruktanonchai UR; Graham D; Namdee K, Surface Modification of Gold Nanoparticles with Neuron-Targeted Exosome for Enhanced Blood–Brain Barrier Penetration. Scientific Reports 2019, 9 (1), 8278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guerrini L; Alvarez-Puebla RA; Pazos-Perez N, Surface Modifications of Nanoparticles for Stability in Biological Fluids. Materials (Basel) 2018, 11 (7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blanco E; Shen H; Ferrari M, Principles of Nanoparticle Design for Overcoming Biological Barriers to Drug Delivery. Nat Biotechnol 2015, 33 (9), 941–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zein R; Sharrouf W; Selting K, Physical Properties of Nanoparticles that Result in Improved Cancer Targeting. Journal Of Oncology 2020, 2020, 5194780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Glassman PM; Myerson JW; Ferguson LT; Kiseleva RY; Shuvaev VV; Brenner JS; Muzykantov VR, Targeting Drug Delivery in the Vascular System: Focus on Endothelium. Adv Drug Deliv Rev 2020, 157, 96–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Braet F; Wisse E; Bomans P; Frederik P; Geerts W; Koster A; Soon L; Ringer S, Contribution of High-Resolution Correlative Imaging Techniques in the Study of the Liver Sieve in Three-Dimensions. Microsc Res Tech 2007, 70 (3), 230–42. [DOI] [PubMed] [Google Scholar]
- 46.Chen LT; Weiss L, The Role of the Sinus Wall in the Passage of Erythrocytes through the Spleen. Blood 1973, 41 (4), 529–37. [PubMed] [Google Scholar]
- 47.Yu W; Liu R; Zhou Y; Gao H, Size-Tunable Strategies for a Tumor Targeted Drug Delivery System. ACS Central Science 2020, 6 (2), 100–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sykes EA; Dai Q; Sarsons CD; Chen J; Rocheleau JV; Hwang DM; Zheng G; Cramb DT; Rinker KD; Chan WCW, Tailoring Nanoparticle Designs to Target Cancer Based on Tumor Pathophysiology. Proceedings Of The National Academy Of Sciences 2016, 113 (9), E1142–E1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Evans WH; Martin PE, Gap Junctions: Structure and Function (Review). Mol Membr Biol 2002, 19 (2), 121–36. [DOI] [PubMed] [Google Scholar]
- 50.Okamoto T; Kawamoto E; Takagi Y; Akita N; Hayashi T; Park EJ; Suzuki K; Shimaoka M, Gap Junction-Mediated Regulation of Endothelial Cellular Stiffness. Scientific Reports 2017, 7 (1), 6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meşe G; Richard G; White TW, Gap Junctions: Basic Structure and Function. J Invest Dermatol 2007, 127 (11), 2516–24. [DOI] [PubMed] [Google Scholar]
- 52.Goodenough DA; Paul DL, Gap Junctions. Cold Spring Harb Perspect Biol 2009, 1 (1), A002576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kumar NM; Gilula NB, The Gap Junction Communication Channel. Cell 1996, 84 (3), 381–8. [DOI] [PubMed] [Google Scholar]
- 54.Saez JC; Berthoud VM; Branes MC; Martinez AD; Beyer EC, Plasma Membrane Channels Formed by Connexins: Their Regulation and Functions. Physiol Rev 2003, 83 (4), 1359–400. [DOI] [PubMed] [Google Scholar]
- 55.Ponticelli CE, The Impact of Cold Ischemia Time on Renal Transplant Outcome. Kidney Int 2015, 87 (2), 272–5. [DOI] [PubMed] [Google Scholar]
- 56.Johansen D; Cruciani V; Sundset R; Ytrehus K; Mikalsen SO, Ischemia Induces Closure of Gap Junctional Channels and Opening of Hemichannels in Heart-Derived Cells and Tissue. Cell Physiol Biochem 2011, 28 (1), 103–14. [DOI] [PubMed] [Google Scholar]
- 57.Kléber AG; Riegger CB; Janse MJ, Electrical Uncoupling and Increase of Extracellular Resistance after Induction of Ischemia in Isolated, Arterially Perfused Rabbit Papillary Muscle. Circ Res 1987, 61 (2), 271–9. [DOI] [PubMed] [Google Scholar]
- 58.Kanno S; Saffitz JE, The Role of Myocardial Gap Junctions in Electrical Conduction and Arrhythmogenesis. Cardiovasc Pathol 2001, 10 (4), 169–77. [DOI] [PubMed] [Google Scholar]
- 59.Severs NJ, Pathophysiology of Gap Junctions in Heart Disease. J Cardiovasc Electrophysiol 1994, 5 (5), 462–75. [DOI] [PubMed] [Google Scholar]
- 60.De Groot JR; Coronel R, Acute Ischemia-Induced Gap Junctional Uncoupling and Arrhythmogenesis. Cardiovascular Research 2004, 62 (2), 323–334. [DOI] [PubMed] [Google Scholar]
- 61.García-Dorado D; Rodríguez-Sinovas A; Ruiz-Meana M, Gap Junction-Mediated Spread of Cell Injury and Death during Myocardial Ischemia–Reperfusion. Cardiovascular Research 2004, 61 (3), 386–401. [DOI] [PubMed] [Google Scholar]
- 62.Peters NS, Myocardial Gap Junction Organization in Ischemia and Infarction. Microsc Res Tech 1995, 31 (5), 375–86. [DOI] [PubMed] [Google Scholar]
- 63.Finnegan R; Zhu P; Stephenson S; Nadig S; Atkinson C, (420) - Cold Storage Stabilization of Gap Junctions Reduces Post Transplant Ischemia Reperfusion Injury. The Journal Of Heart And Lung Transplantation 2016, 35 (4, Supplement), S160–S161. [Google Scholar]
- 64.Lee SY; Shin JA; Kwon HM; Weiner ID; Han KH, Renal Ischemia-Reperfusion Injury Causes Intercalated Cell-Specific Disruption of Occludin in the Collecting Duct. Histochem Cell Biol 2011, 136 (6), 637–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee DBN; Huang E; Ward HJ, Tight Junction Biology and Kidney Dysfunction. American Journal Of Physiology-Renal Physiology 2006, 290 (1), F20–F34. [DOI] [PubMed] [Google Scholar]
- 66.Alexis F; Pridgen E; Molnar LK; Farokhzad OC, Factors Affecting the Clearance and Biodistribution of Polymeric Nanoparticles. Mol Pharm 2008, 5 (4), 505–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thurston G; Mclean JW; Rizen M; Baluk P; Haskell A; Murphy TJ; Hanahan D; Mcdonald DM, Cationic Liposomes Target Angiogenic Endothelial Cells in Tumors and Chronic Inflammation in Mice. J Clin Invest 1998, 101 (7), 1401–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nel AE; Mädler L; Velegol D; Xia T; Hoek EM; Somasundaran P; Klaessig F; Castranova V; Thompson M, Understanding Biophysicochemical Interactions at the Nano-Bio Interface. Nat Mater 2009, 8 (7), 543–57. [DOI] [PubMed] [Google Scholar]
- 69.Yuan YY; Mao CQ; Du XJ; Du JZ; Wang F; Wang J, Surface Charge Switchable Nanoparticles Based on Zwitterionic Polymer for Enhanced Drug Delivery to Tumor. Adv Mater 2012, 24 (40), 5476–80. [DOI] [PubMed] [Google Scholar]
- 70.Bhargava R; Tsokos GC, The Immune Podocyte. Curr Opin Rheumatol 2019, 31 (2), 167–174. [DOI] [PubMed] [Google Scholar]
- 71.Tsokos GC; Tsokos MG, Targeting Targeted Treatment for Immune and Non-Immune Kidney Diseases. Trans Am Clin Climatol Assoc 2019, 130, 88–99. [PMC free article] [PubMed] [Google Scholar]
- 72.Maeda K; Otomo K; Yoshida N; Abu-Asab MS; Ichinose K; Nishino T; Kono M; Ferretti A; Bhargava R; Maruyama S; Bickerton S; Fahmy TM; Tsokos MG; Tsokos GC, Camk4 Compromises Podocyte Function in Autoimmune and Nonautoimmune Kidney Disease. The Journal Of Clinical Investigation 2018, 128 (8), 3445–3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miner JH, The Glomerular Basement Membrane. Exp Cell Res 2012, 318 (9), 973–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bohrer MP; Baylis C; Humes HD; Glassock RJ; Robertson CR; Brenner BM, Permselectivity of the Glomerular Capillary Wall. Facilitated Filtration of Circulating Polycations. J Clin Invest 1978, 61 (1), 72–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ordikhani F; Kasinath V; Uehara M; Akbarzadeh A; Yilmam OA; Dai L; Aksu H; Jung S; Jiang L; Li X; Zhao J; Bahmani B; Ichimura T; Fiorina P; Annabi N; Abdi R, Selective Trafficking of Light Chain-Conjugated Nanoparticles to the Kidney and Renal Cell Carcinoma. Nano Today 2020, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gentile F; Chiappini C; Fine D; Bhavane RC; Peluccio MS; Cheng MM; Liu X; Ferrari M; Decuzzi P, The Effect of Shape on the Margination Dynamics of Non-Neutrally Buoyant Particles in Two-Dimensional Shear Flows. J Biomech 2008, 41 (10), 2312–8. [DOI] [PubMed] [Google Scholar]
- 77.Geng Y; Dalhaimer P; Cai S; Tsai R; Tewari M; Minko T; Discher DE, Shape Effects of Filaments versus Spherical Particles in Flow and Drug Delivery. Nat Nanotechnol 2007, 2 (4), 249–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gabizon A; Papahadjopoulos D, Liposome Formulations with Prolonged Circulation Time in Blood and Enhanced Uptake by Tumors. Proceedings Of The National Academy Of Sciences 1988, 85 (18), 6949–6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Arnida; Janát-Amsbury MM; Ray A; Peterson CM; Ghandehari H, Geometry and Surface Characteristics of Gold Nanoparticles Influence Their Biodistribution and Uptake by Macrophages. European Journal Of Pharmaceutics And Biopharmaceutics 2011, 77 (3), 417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Champion JA; Mitragotri S, Role of Target Geometry in Phagocytosis. Proc Natl Acad Sci U S A 2006, 103 (13), 4930–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Christian DA; Cai S; Garbuzenko OB; Harada T; Zajac AL; Minko T; Discher DE, Flexible Filaments For in Vivo Imaging and Delivery: Persistent Circulation of Filomicelles Opens the Dosage Window for Sustained Tumor Shrinkage. Mol Pharm 2009, 6 (5), 1343–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alshamsan A; Binkhathlan Z; Kalam MA; Qamar W; Kfouri H; Alghonaim M; Lavasanifar A, Mitigation of Tacrolimus-Associated Nephrotoxicity by PLGA Nanoparticulate Delivery Following Multiple Dosing to Mice While Maintaining Its Immunosuppressive Activity. Scientific Reports 2020, 10 (1), 6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tang L; Azzi J; Kwon M; Mounayar M; Tong R; Yin Q; Moore R; Skartsis N; Fan TM; Abdi R; Cheng J, Immunosuppressive Activity of Size-Controlled PEG-PLGA Nanoparticles Containing Encapsulated Cyclosporine A. Journal Of Transplantation 2012, 2012, 896141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ritschel WA, Microemulsion Technology in the Reformulation of Cyclosporine: The Reason behind the Pharmacokinetic Properties of Neoral. Clin Transplant 1996, 10 (4), 364–73. [PubMed] [Google Scholar]
- 85.Mcalister VC; Keshavamurthy M; Lee TD, Oral Delivery of Liposomal Tacrolimus: Increased Efficacy and Reduced Toxicity. Transplant Proc 1999, 31 (1–2), 1110. [DOI] [PubMed] [Google Scholar]
- 86.Alemdar AY; Sadi D; Mcalister VC; Mendez I, Liposomal Formulations of Tacrolimus and Rapamycin Increase Graft Survival and Fiber Outgrowth of Dopaminergic Grafts. Cell Transplant 2004, 13 (3), 263–71. [DOI] [PubMed] [Google Scholar]
- 87.Tasciotti E; Cabrera FJ; Evangelopoulos M; Martinez JO; Thekkedath UR; Kloc M; Ghobrial RM; Li XC; Grattoni A; Ferrari M, The Emerging Role of Nanotechnology in Cell and Organ Transplantation. Transplantation 2016, 100 (8), 1629–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shen LJ; Wu FL, Nanomedicines in Renal Transplant Rejection--Focus on Sirolimus. Int J Nanomedicine 2007, 2 (1), 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Immordino ML; Dosio F; Cattel L, Stealth Liposomes: Review of the Basic Science, Rationale, and Clinical Applications, Existing and Potential. Int J Nanomedicine 2006, 1 (3), 297–315. [PMC free article] [PubMed] [Google Scholar]
- 90.Torchilin VP, Recent Advances with Liposomes as Pharmaceutical Carriers. Nat Rev Drug Discov 2005, 4 (2), 145–60. [DOI] [PubMed] [Google Scholar]
- 91.Hippalgaonkar K; Majumdar S; Kansara V, Injectable Lipid Emulsions-Advancements, Opportunities and Challenges. AAPS Pharmscitech 2010, 11 (4), 1526–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Martinez JO; Evangelopoulos M; Bhavane R; Acciardo S; Salvatore F; Liu X; Ferrari M; Tasciotti E, Multistage Nanovectors Enhance the Delivery of Free and Encapsulated Drugs. Curr Drug Targets 2015, 16 (14), 1582–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Croy SR; Kwon GS, Polymeric Micelles for Drug Delivery. Curr Pharm Des 2006, 12 (36), 4669–84. [DOI] [PubMed] [Google Scholar]
- 94.Duivenvoorden R; Tang J; Cormode DP; Mieszawska AJ; Izquierdo-Garcia D; Ozcan C; Otten MJ; Zaidi N; Lobatto ME; Van Rijs SM; Priem B; Kuan EL; Martel C; Hewing B; Sager H; Nahrendorf M; Randolph GJ; Stroes ES; Fuster V; Fisher EA et al. , A Statin-Loaded Reconstituted High-Density Lipoprotein Nanoparticle Inhibits Atherosclerotic Plaque Inflammation. Nat Commun 2014, 5, 3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kuai R; Li D; Chen YE; Moon JJ; Schwendeman A, High-Density Lipoproteins: Nature’s Multifunctional Nanoparticles. ACS Nano 2016, 10 (3), 3015–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang XP; Amar MJ; Vaisman B; Bocharov AV; Vishnyakova TG; Freeman LA; Kurlander RJ; Patterson AP; Becker LC; Remaley AT, Scavenger Receptor-BI Is a Receptor for Lipoprotein(A). J Lipid Res 2013, 54 (9), 2450–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ochando J; Braza MS, Nanoparticle-Based Modulation and Monitoring of Antigen-Presenting Cells in Organ Transplantation. Front Immunol 2017, 8, 1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ferrer MC; Shuvaev VV; Zern BJ; Composto RJ; Muzykantov VR; Eckmann DM, Icam-1 Targeted Nanogels Loaded with Dexamethasone Alleviate Pulmonary Inflammation. Plos One 2014, 9 (7), E102329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Phan HT; Haes AJ, What Does Nanoparticle Stability Mean? The Journal Of Physical Chemistry C 2019, 123 (27), 16495–16507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xi W; Phan HT; Haes AJ, How to Accurately Predict Solution-Phase Gold Nanostar Stability. Anal Bioanal Chem 2018, 410 (24), 6113–6123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wijenayaka LA; Ivanov MR; Cheatum CM; Haes AJ, Improved Parametrization for Extended Derjaguin, Landau, Verwey, and Overbeek Predictions of Functionalized Gold Nanosphere Stability. The Journal Of Physical Chemistry C 2015, 119 (18), 10064–10075. [Google Scholar]
- 102.Hotze EM; Phenrat T; Lowry GV, Nanoparticle Aggregation: Challenges to Understanding Transport and Reactivity in the Environment. J Environ Qual 2010, 39 (6), 1909–24. [DOI] [PubMed] [Google Scholar]
- 103.Lázár I; Szabó HJ, Prevention of the Aggregation of Nanoparticles during the Synthesis of Nanogold-Containing Silica Aerogels. Gels 2018, 4 (2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yin Y; Li Z-Y; Zhong Z; Gates B; Xia Y; Venkateswaran S, Synthesis and Characterization of Stable Aqueous Dispersions of Silver Nanoparticles through the Tollens Process. Journal Of Materials Chemistry 2002, 12 (3), 522–527. [Google Scholar]
- 105.Phan HT; Haes AJ, Impacts of Ph and Intermolecular Interactions on Surface-Enhanced Raman Scattering Chemical Enhancements. The Journal Of Physical Chemistry C 2018, 122 (26), 14846–14856. [Google Scholar]
- 106.Lu G; Shrestha B; Haes AJ, Importance of Tilt Angles of Adsorbed Aromatic Molecules on Nanoparticle Rattle SERS Substrates. The Journal Of Physical Chemistry C 2016, 120 (37), 20759–20767. [Google Scholar]
- 107.Russ JC, Fundamentals of Energy Dispersive X-Ray Analysis: Butterworths Monographs in Materials; Butterworth-Heinemann: London, 2013. [Google Scholar]
- 108.Li Y; Zhang H; Wu B; Guo Z, Improving the Oxidation Resistance and Stability of Ag Nanoparticles by Coating with Multilayered Reduced Graphene Oxide. Applied Surface Science 2017, 425, 194–200. [Google Scholar]
- 109.Cao G, Nanostructures & Nanomaterials: Synthesis, Properties & Applications. Imperial College Press: London, 2004. [Google Scholar]
- 110.Gao M; Zhu Y; Liu Y; Wu K; Lu H; Tang S; Liu C; Yue H; Liang B; Yan J, The Role of Adsorbed Oleylamine on Gold Catalysts during Synthesis for Highly Selective Electrocatalytic Reduction of CO(2) to CO. Chem Commun (Camb) 2020, 56 (51), 7021–7024. [DOI] [PubMed] [Google Scholar]
- 111.Khan I; Saeed K; Khan I, Nanoparticles: Properties, Applications and Toxicities. Arabian Journal Of Chemistry 2019, 12 (7), 908–931. [Google Scholar]
- 112.Ajitha B; Reddy YA; Reddy P; Jeon H-J; Ahn CW, Role of Capping Agents in Controlling Silver Nanoparticles Size, Antibacterial Activity and Potential Application as Optical Hydrogen Peroxide Sensor. RSC Advances 2016, 6, 36171–36179. [Google Scholar]
- 113.He B; Tan JJ; Liew KY; Liu H, Synthesis of Size Controlled Ag Nanoparticles. Journal Of Molecular Catalysis A: Chemical 2004, 221 (1), 121–126. [Google Scholar]
- 114.Pierre MCS; Mackie PM; Roca M; Haes AJ, Correlating Molecular Surface Coverage and Solution-Phase Nanoparticle Concentration to Surface-Enhanced Raman Scattering Intensities. The Journal Of Physical Chemistry C 2011, 115 (38), 18511–18517. [Google Scholar]
- 115.Kim KM; Kim HM; Lee WJ; Lee CW; Kim TI; Lee JK; Jeong J; Paek SM; Oh JM, Surface Treatment of Silica Nanoparticles for Stable and Charge-Controlled Colloidal Silica. Int J Nanomedicine 2014, 9 Suppl 2 (Suppl 2), 29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li SD; Huang L, Pharmacokinetics and Biodistribution of Nanoparticles. Mol Pharm 2008, 5 (4), 496–504. [DOI] [PubMed] [Google Scholar]
- 117.Foroozandeh P; Aziz AA, Insight into Cellular Uptake and Intracellular Trafficking of Nanoparticles. Nanoscale Res Lett 2018, 13 (1), 339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Makaraci P; Kim K, Trans-Golgi Network-Bound Cargo Traffic. Eur J Cell Biol 2018, 97 (3), 137–149. [DOI] [PubMed] [Google Scholar]
- 119.Chou LY; Ming K; Chan WC, Strategies for the Intracellular Delivery of Nanoparticles. Chem Soc Rev 2011, 40 (1), 233–45. [DOI] [PubMed] [Google Scholar]
- 120.Grant BD; Donaldson JG, Pathways and Mechanisms of Endocytic Recycling. Nat Rev Mol Cell Biol 2009, 10 (9), 597–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Miaczynska M; Stenmark H, Mechanisms and Functions of Endocytosis. J Cell Biol 2008, 180 (1), 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mukherjee S; Ghosh RN; Maxfield FR, Endocytosis. Physiol Rev 1997, 77 (3), 759–803. [DOI] [PubMed] [Google Scholar]
- 123.Hillaireau H; Couvreur P, Nanocarriers’ Entry into the Cell: Relevance to Drug Delivery. Cell Mol Life Sci 2009, 66 (17), 2873–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Panariti A; Miserocchi G; Rivolta I, The Effect of Nanoparticle Uptake on Cellular Behavior: Disrupting or Enabling Functions? Nanotechnol Sci Appl 2012, 5, 87–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kaksonen M; Roux A, Mechanisms of Clathrin-Mediated Endocytosis. Nature Reviews Molecular Cell Biology 2018, 19 (5), 313–326. [DOI] [PubMed] [Google Scholar]
- 126.Mcmahon HT; Boucrot E, Molecular Mechanism and Physiological Functions of Clathrin-Mediated Endocytosis. Nature Reviews Molecular Cell Biology 2011, 12 (8), 517–533. [DOI] [PubMed] [Google Scholar]
- 127.Mettlen M; Chen PH; Srinivasan S; Danuser G; Schmid SL, Regulation of Clathrin-Mediated Endocytosis. Annu Rev Biochem 2018, 87, 871–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rappoport JZ, Focusing on Clathrin-Mediated Endocytosis. Biochem J 2008, 412 (3), 415–23. [DOI] [PubMed] [Google Scholar]
- 129.Soldati T; Schliwa M, Powering Membrane Traffic in Endocytosis and Recycling. Nat Rev Mol Cell Biol 2006, 7 (12), 897–908. [DOI] [PubMed] [Google Scholar]
- 130.Praefcke GJ; Mcmahon HT, The Dynamin Superfamily: Universal Membrane Tubulation and Fission Molecules? Nat Rev Mol Cell Biol 2004, 5 (2), 133–47. [DOI] [PubMed] [Google Scholar]
- 131.Cocucci E; Aguet F; Boulant S; Kirchhausen T, The First Five Seconds in the Life of a Clathrin-Coated Pit. Cell 2012, 150 (3), 495–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Parton RG; Simons K, The Multiple Faces of Caveolae. Nat Rev Mol Cell Biol 2007, 8 (3), 185–94. [DOI] [PubMed] [Google Scholar]
- 133.Thorn H; Stenkula KG; Karlsson M; Ortegren U; Nystrom FH; Gustavsson J; Stralfors P, Cell Surface Orifices of Caveolae and Localization of Caveolin to the Necks of Caveolae in Adipocytes. Mol Biol Cell 2003, 14 (10), 3967–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang Z; Tiruppathi C; Cho J; Minshall RD; Malik AB, Delivery of Nanoparticle: Complexed Drugs across the Vascular Endothelial Barrier via Caveolae. IUBMB Life 2011, 63 (8), 659–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Conner SD; Schmid SL, Regulated Portals of Entry into the Cell. Nature 2003, 422 (6927), 37–44. [DOI] [PubMed] [Google Scholar]
- 136.Rejman J; Conese M; Hoekstra D, Gene Transfer by Means of Lipo- and Polyplexes: Role of Clathrin and Caveolae-Mediated Endocytosis. J Liposome Res 2006, 16 (3), 237–47. [DOI] [PubMed] [Google Scholar]
- 137.Oh P; Borgström P; Witkiewicz H; Li Y; Borgström BJ; Chrastina A; Iwata K; Zinn KR; Baldwin R; Testa JE; Schnitzer JE, Live Dynamic Imaging of Caveolae Pumping Targeted Antibody Rapidly and Specifically across Endothelium in the Lung. Nat Biotechnol 2007, 25 (3), 327–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Patel KJ; Atkinson C; Broome A-M; Mcgillicuddy JW; Chavin KD; Nadig SN, Utilization of Machine Perfusion and Nanotechnology for Liver Transplantation. Current Transplantation Reports 2015, 2 (4), 303–311. [Google Scholar]
- 139.Schlegel A; Kron P; Dutkowski P, Hypothermic Oxygenated Liver Perfusion: Basic Mechanisms and Clinical Application. Curr Transplant Rep 2015, 2 (1), 52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fitton TP; Wei C; Lin R; Bethea BT; Barreiro CJ; Amado L; Gage F; Hare J; Baumgartner WA; Conte JV, Impact of 24 H Continuous Hypothermic Perfusion on Heart Preservation by Assessment of Oxidative Stress. Clin Transplant 2004, 18 Suppl 12, 22–7. [DOI] [PubMed] [Google Scholar]
- 141.Hsin MKY; Iskender I; Nakajima D; Chen M; Kim H; Dos Santos PR; Sakamoto J; Lee J; Hashimoto K; Harmantas C; Hwang D; Waddell T; Liu M; Keshavjee S; Cypel M, Extension of Donor Lung Preservation with Hypothermic Storage after Normothermic ex Vivo Lung Perfusion. J Heart Lung Transplant 2016, 35 (1), 130–136. [DOI] [PubMed] [Google Scholar]
- 142.Weegman BP; Taylor MJ; Baicu SC; Scott WE 3rd; Mueller KR; Kitzmann JD; Rizzari MD; Papas KK, Hypothermic Perfusion Preservation of Pancreas for Islet Grafts: Validation Using a Split Lobe Porcine Model. Cell Med 2012, 2 (3), 105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Muñoz-Abraham AS; Patrón-Lozano R; Narayan RR; Judeeba SS; Alkukhun A; Alfadda TI; Belter JT; Mulligan DC; Morotti R; Zinter JP; Geibel JP; Rodríguez-Dávalos MI, Extracorporeal Hypothermic Perfusion Device for Intestinal Graft Preservation to Decrease Ischemic Injury during Transportation. J Gastrointest Surg 2016, 20 (2), 313–21. [DOI] [PubMed] [Google Scholar]
- 144.Blum MF; Liu Q; Soliman B; Okamoto T; Bassiri-Gharb B; Uso TD; Buccini LD; Quintini C. Machine Perfusion of Organs. Technological Advances in Organ Transplantation; Springer International Publishing: New York City, 2017; 21–62. [Google Scholar]
- 145.Karimian N; Yeh H, Opportunities for Therapeutic Intervention during Machine Perfusion. Curr Transplant Rep 2017, 4 (2), 141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mannon RB, Delayed Graft Function: The AKI of Kidney Transplantation. Nephron 2018, 140 (2), 94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Geddes CC; Woo YM; Jardine AG, The Impact of Delayed Graft Function on the Long-Term Outcome of Renal Transplantation. J Nephrol 2002, 15 (1), 17–21. [PubMed] [Google Scholar]
- 148.Siedlecki A; Irish W; Brennan DC, Delayed Graft Function in the Kidney Transplant. Am J Transplant 2011, 11 (11), 2279–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chen R; Wang H; Song L; Hou J; Peng J; Dai H; Peng L, Predictors and One-Year Outcomes of Patients with Delayed Graft Function after Deceased Donor Kidney Transplantation. BMC Nephrol 2020, 21 (1), 526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Brat A; Pol RA; Leuvenink HG, Novel Preservation Methods to Increase the Quality of Older Kidneys. Curr Opin Organ Transplant 2015, 20 (4), 438–43. [DOI] [PubMed] [Google Scholar]
- 151.Hosgood SA; Saeb-Parsy K; Wilson C; Callaghan C; Collett D; Nicholson ML, Protocol of a Randomised Controlled, Open-Label Trial of ex Vivo Normothermic Perfusion versus Static Cold Storage in Donation after Circulatory Death Renal Transplantation. BMJ Open 2017, 7 (1), E012237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Woud WW; Merino A; Hoogduijn MJ; Boer K; Van Den Hoogen MWF; Baan CC; Minnee RC, Nanoparticle Release by Extended Criteria Donor Kidneys during Normothermic Machine Perfusion. Transplantation 2019, 103 (5), E110–E111. [DOI] [PubMed] [Google Scholar]
- 153.Tietjen GT; Hosgood SA; Dirito J; Cui J; Deep D; Song E; Kraehling JR; Piotrowski-Daspit AS; Kirkiles-Smith NC; Al-Lamki R; Thiru S; Bradley JA; Saeb-Parsy K; Bradley JR; Nicholson ML; Saltzman WM; Pober JS, Nanoparticle Targeting to the Endothelium during Normothermic Machine Perfusion of Human Kidneys. Sci Transl Med 2017, 9 (418). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shuvaev VV; Christofidou-Solomidou M; Scherpereel A; Simone E; Arguiri E; Tliba S; Pick J; Kennel S; Albelda SM; Muzykantov VR, Factors Modulating the Delivery and Effect of Enzymatic Cargo Conjugated with Antibodies Targeted to the Pulmonary Endothelium. J Control Release 2007, 118 (2), 235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Perkowski S; Sun J; Singhal S; Santiago J; Leikauf GD; Albelda SM, Gene Expression Profiling of the Early Pulmonary Response to Hyperoxia in Mice. Am J Respir Cell Mol Biol 2003, 28 (6), 682–96. [DOI] [PubMed] [Google Scholar]
- 156.Garnacho C; Albelda SM; Muzykantov VR; Muro S, Differential Intra-Endothelial Delivery of Polymer Nanocarriers Targeted to Distinct PECAM-1 Epitopes. J Control Release 2008, 130 (3), 226–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Minshall RD; Tiruppathi C; Vogel SM; Niles WD; Gilchrist A; Hamm HE; Malik AB, Endothelial Cell-Surface Gp60 Activates Vesicle Formation and Trafficking Via G(I)-Coupled Src Kinase Signaling Pathway. J Cell Biol 2000, 150 (5), 1057–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Vogel SM; Easington CR; Minshall RD; Niles WD; Tiruppathi C; Hollenberg SM; Parrillo JE; Malik AB, Evidence of Transcellular Permeability Pathway in Microvessels. Microvasc Res 2001, 61 (1), 87–101. [DOI] [PubMed] [Google Scholar]
- 159.Mulivor AW; Lipowsky HH, Role of Glycocalyx in Leukocyte-Endothelial Cell Adhesion. Am J Physiol Heart Circ Physiol 2002, 283 (4), H1282–91. [DOI] [PubMed] [Google Scholar]
- 160.Brenner JS; Pan DC; Myerson JW; Marcos-Contreras OA; Villa CH; Patel P; Hekierski H; Chatterjee S; Tao JQ; Parhiz H; Bhamidipati K; Uhler TG; Hood ED; Kiseleva RY; Shuvaev VS; Shuvaeva T; Khoshnejad M; Johnston I; Gregory JV; Lahann J et al. , Red Blood Cell-Hitchhiking Boosts Delivery of Nanocarriers to Chosen Organs by Orders of Magnitude. Nat Commun 2018, 9 (1), 2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cui J; Qin L; Zhang J; Abrahimi P; Li H; Li G; Tietjen GT; Tellides G; Pober JS; Mark Saltzman W, Ex Vivo Pretreatment of Human Vessels with Sirna Nanoparticles Provides Protein Silencing in Endothelial Cells. Nat Commun 2017, 8 (1), 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Patel K; Atkinson C; Tran D; Nadig SN, Nanotechnological Approaches to Immunosuppression and Tolerance Induction. Current Transplantation Reports 2017, 4 (2), 159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Nadig SN; Dixit SK; Levey N; Esckilsen S; Miller K; Dennis W; Atkinson C; Broome AM, Immunosuppressive Nano-Therapeutic Micelles Downregulate Endothelial Cell Inflammation and Immunogenicity. RSC Adv 2015, 5 (54), 43552–43562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Uehara M; Li X; Sheikhi A; Zandi N; Walker B; Saleh B; Banouni N; Jiang L; Ordikhani F; Dai L; Yonar M; Vohra I; Kasinath V; Orgill DP; Khademhosseini A; Annabi N; Abdi R, Anti-IL-6 Eluting Immunomodulatory Biomaterials Prolong Skin Allograft Survival. Sci Rep 2019, 9 (1), 6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ferenbach D; Hughes J, Macrophages and Dendritic Cells: What Is the Difference? Kidney International 2008, 74 (1), 5–7. [DOI] [PubMed] [Google Scholar]
- 166.Van Leent MMT; Meerwaldt AE; Berchouchi A; Toner YC; Burnett ME; Klein ED; Verschuur AVD; Nauta SA; Munitz J; Prevot G; Van Leeuwen EM; Ordikhani F; Mourits VP; Calcagno C; Robson PM; Soultanidis G; Reiner T; Joosten RRM; Friedrich H; Madsen JC et al. , A Modular Approach toward Producing Nanotherapeutics Targeting the Innate Immune System. Sci Adv 2021, 7 (10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Solhjou Z; Uehara M; Bahmani B; Maarouf OH; Ichimura T; Brooks CR; Xu W; Yilmaz M; Elkhal A; Tullius SG; Guleria I; Mcgrath MM; Abdi R, Novel Application of Localized Nanodelivery of Anti-Interleukin-6 Protects Organ Transplant from Ischemia-Reperfusion Injuries. Am J Transplant 2017, 17 (9), 2326–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ilinskaya AN; Dobrovolskaia MA, Nanoparticles and the Blood Coagulation System. Part II: Safety Concerns. Nanomedicine (Lond) 2013, 8 (6), 969–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cicha I, Thrombosis: Novel Nanomedical Concepts of Diagnosis and Treatment. World J Cardiol 2015, 7 (8), 434–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Jia J; Nie Y; Li J; Xie H; Zhou L; Yu J; Zheng SS, A Systematic Review and Meta-Analysis of Machine Perfusion vs. Static Cold Storage of Liver Allografts on Liver Transplantation Outcomes: The Future Direction of Graft Preservation. Front Med (Lausanne) 2020, 7, 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Parente A; Osei-Bordom D-C; Ronca V; Perera MTPR; Mirza D, Organ Restoration with Normothermic Machine Perfusion and Immune Reaction. Frontiers In Immunology 2020, 11 (2286). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Siniscalchi A; Gamberini L; Laici C; Bardi T; Ercolani G; Lorenzini L; Faenza S, Post Reperfusion Syndrome during Liver Transplantation: From Pathophysiology to Therapy and Preventive Strategies. World J Gastroenterol 2016, 22 (4), 1551–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Peerlinck ID; Barlas A; Karameris A; Papalois VE, The Effect of Machine Perfusion on the Arteries of Porcine Kidneys. Exp Clin Transplant 2005, 3 (2), 375–80. [PubMed] [Google Scholar]
- 174.Rodriguez-Torres MDP; Acosta-Torres LS; Diaz-Torres LA, Heparin-Based Nanoparticles: An Overview of Their Applications. Journal Of Nanomaterials 2018, 2018, 9780489. [Google Scholar]
- 175.Jogala S; Rachamalla SS; Aukunuru J, Development of PEG-PLGA Based Intravenous Low Molecular Weight Heparin (LMWH) Nanoparticles Intended to Treat Venous Thrombosis. Curr Drug Deliv 2016, 13 (5), 698–710. [DOI] [PubMed] [Google Scholar]
- 176.Liu H; Zhang H; Wang J; Wei J, Effect of Temperature on the Size of Biosynthesized Silver Nanoparticle: Deep Insight into Microscopic Kinetics Analysis. Arabian Journal Of Chemistry 2020, 13 (1), 1011–1019. [Google Scholar]
- 177.Jochmans I; Moers C; Smits JM; Leuvenink HG; Treckmann J; Paul A; Rahmel A; Squifflet JP; Van Heurn E; Monbaliu D; Ploeg RJ; Pirenne J, Machine Perfusion versus Cold Storage for the Preservation of Kidneys Donated after Cardiac Death: A Multicenter, Randomized, Controlled Trial. Ann Surg 2010, 252 (5), 756–64. [DOI] [PubMed] [Google Scholar]
- 178.Jia J; Nie Y; Li J; Xie H; Zhou L; Yu J; Zheng S-S, A Systematic Review and Meta-Analysis of Machine Perfusion vs. Static Cold Storage of Liver Allografts on Liver Transplantation Outcomes: The Future Direction of Graft Preservation. Frontiers In Medicine 2020, 7 (135). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Qu Y; Yang H; Yang N; Fan Y; Zhu H; Zou G, The Effect of Reaction Temperature on the Particle Size, Structure and Magnetic Properties of Coprecipitated Cofe2o4 Nanoparticles. Materials Letters 2006, 60 (29), 3548–3552. [Google Scholar]
- 180.Nurdin I, The Effect of Temperature on Synthesis and Stability of Superparamagnetic Maghemite Nanoparticles Suspension. Journal Of Materials Science And Chemical Engineering 2016, 4 (03), 35. [Google Scholar]
- 181.Dutta A; Paul A; Chattopadhyay A, The Effect of Temperature on the Aggregation Kinetics of Partially Bare Gold Nanoparticles. RSC Advances 2016, 6, 82138–82149. [Google Scholar]
- 182.Islam AKMM; Mukherjee M, Effect of Temperature in Synthesis of Silver Nanoparticles in Triblock Copolymer Micellar Solution. Journal Of Experimental Nanoscience 2011, 6 (6), 596–611. [Google Scholar]
- 183.Diederich D; Skopec J; Diederich A; Dai FX, Cyclosporine Produces Endothelial Dysfunction by Increased Production of Superoxide. Hypertension 1994, 23 (6 Pt 2), 957–61. [DOI] [PubMed] [Google Scholar]
- 184.Grattoni A; Tasciotti E; Fine D; Fernandez-Moure JS; Sakamoto J; Hu Y; Weiner B; Ferrari M; Parazynski S, Nanotechnologies and Regenerative Medical Approaches for Space and Terrestrial Medicine. Aviat Space Environ Med 2012, 83 (11), 1025–36. [DOI] [PubMed] [Google Scholar]
- 185.Nicolov E; Ferrati S; Goodall R; Hudson L; Hosali S; Crowley M; Palapattu G; Khera M; Grattoni A, Mp43–20 Nanotechnology-Based Implant for Long Term Testosterone Replacement. Journal Of Urology 2014, 191 (4S), E485–E486. [Google Scholar]
- 186.Perregaux DG; Mcniff P; Laliberte R; Hawryluk N; Peurano H; Stam E; Eggler J; Griffiths R; Dombroski MA; Gabel CA, Identification and Characterization of a Novel Class of Interleukin-1 Post-Translational Processing Inhibitors. J Pharmacol Exp Ther 2001, 299 (1), 187–97. [PubMed] [Google Scholar]
- 187.He Y; Varadarajan S; Muñoz-Planillo R; Burberry A; Nakamura Y; Núñez G, 3,4-Methylenedioxy-B-Nitrostyrene Inhibits NLRP3 Inflammasome Activation by Blocking Assembly of the Inflammasome. J Biol Chem 2014, 289 (2), 1142–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Jiang H; He H; Chen Y; Huang W; Cheng J; Ye J; Wang A; Tao J; Wang C; Liu Q; Jin T; Jiang W; Deng X; Zhou R, Identification of a Selective and Direct NLRP3 Inhibitor to Treat Inflammatory Disorders. J Exp Med 2017, 214 (11), 3219–3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Huang J; Wu L; Tashiro S; Onodera S; Ikejima T, A Comparison of the Signal Pathways between the TNF Alpha- and Oridonin-Induced Murine L929 Fibrosarcoma Cell Death. Acta Med Okayama 2005, 59 (6), 261–70. [DOI] [PubMed] [Google Scholar]
- 190.Zahid A; Li B; Kombe AJK; Jin T; Tao J, Pharmacological Inhibitors of the NLRP3 Inflammasome. Frontiers In Immunology 2019, 10 (2538). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Miller SC; Huang R; Sakamuru S; Shukla SJ; Attene-Ramos MS; Shinn P; Van Leer D; Leister W; Austin CP; Xia M, Identification of Known Drugs that Act as Inhibitors of NF-Kappab Signaling and Their Mechanism of Action. Biochem Pharmacol 2010, 79 (9), 1272–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Thomas SN; Schudel A, Overcoming Transport Barriers for Interstitial-, Lymphatic-, and Lymph Node-Targeted Drug Delivery. Curr Opin Chem Eng 2015, 7, 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Schudel A; Francis DM; Thomas SN, Material Design for Lymph Node Drug Delivery. Nat Rev Mater 2019, 4 (6), 415–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Singh I; Swami R; Khan W; Sistla R, Delivery Systems for Lymphatic Targeting. in Focal Controlled Drug Delivery, Domb AJ; Khan W, Eds. Springer US: Boston, MA, 2014; Pp 429–458. [Google Scholar]
- 195.Supersaxo A; Hein WR; Steffen H, Effect of Molecular Weight on the Lymphatic Absorption of Water-Soluble Compounds Following Subcutaneous Administration. Pharm Res 1990, 7 (2), 167–9. [DOI] [PubMed] [Google Scholar]
- 196.Bettendorf U, Electronmicroscopic Studies on the Peritoneal Resorption of Intraperitoneally Injected Latex Particles via the Diaphragmatic Lymphatics. Lymphology 1979, 12 (2), 66–70. [PubMed] [Google Scholar]
- 197.Britannica, T. E. O. E. Antibody. Https://Www.Britannica.Com/Science/Antibody (Accessed 2/10).
- 198.Kapoor DN; Bhatia A; Kaur R; Sharma R; Kaur G; Dhawan S, PLGA: A Unique Polymer for Drug Delivery. Ther Deliv 2015, 6 (1), 41–58. [DOI] [PubMed] [Google Scholar]
- 199.Makadia HK; Siegel SJ, Poly Lactic-Co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3 (3), 1377–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Gentile P; Chiono V; Carmagnola I; Hatton PV, An Overview of Poly(lactic-Co-Glycolic) Acid (PLGA)-Based Biomaterials for Bone Tissue Engineering. Int J Mol Sci 2014, 15 (3), 3640–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Rajagopal G; Suresh V; Sachan A, High-Density Lipoprotein Cholesterol: How High. Indian J Endocrinol Metab 2012, 16 (Suppl 2), S236–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Toth PP, Reverse Cholesterol Transport: High-Density Lipoprotein’s Magnificent Mile. Curr Atheroscler Rep 2003, 5 (5), 386–93. [DOI] [PubMed] [Google Scholar]
- 203.Kosmas CE; Martinez I; Sourlas A; Bouza KV; Campos FN; Torres V; Montan PD; Guzman E, High-Density Lipoprotein (HDL) Functionality and Its Relevance to Atherosclerotic Cardiovascular Disease. Drugs Context 2018, 7, 212525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kontush A; Chapman MJ, Antiatherogenic Function of HDL Particle Subpopulations: Focus on Antioxidative Activities. Curr Opin Lipidol 2010, 21 (4), 312–8. [DOI] [PubMed] [Google Scholar]
- 205.Barter PJ; Nicholls S; Rye KA; Anantharamaiah GM; Navab M; Fogelman AM, Antiinflammatory Properties of HDL. Circ Res 2004, 95 (8), 764–72. [DOI] [PubMed] [Google Scholar]
- 206.Mineo C; Deguchi H; Griffin JH; Shaul PW, Endothelial and Antithrombotic Actions of HDL. Circ Res 2006, 98 (11), 1352–64. [DOI] [PubMed] [Google Scholar]


