Key Points
Question
What are the perioperative, safety, and survival outcomes of robot-assisted laparoscopic radical cystectomy (RARC) with intracorporeal urinary diversion compared with open radical cystectomy (ORC)?
Findings
This cohort study included 3169 patients undergoing cystectomy for bladder cancer between 2011 and 2018 in Sweden. After propensity score matching, patients who underwent RARC had lower overall mortality rate, fewer high-grade complications, and more favorable perioperative outcomes compared with those who underwent ORC.
Meaning
These findings suggest that in spite of the technically complex nature of the fully intracorporeal approach, nationwide implementation is feasible, with beneficial outcomes for patients with bladder cancer.
This cohort study compares perioperative, safety, and survival outcomes between robot-assisted radical cystectomy and open radical cystectomy among patients with bladder cancer in Sweden.
Abstract
Importance
Mortality rates resulting from bladder cancer have remained unchanged for more than 30 years. The surgical community has put hope in robot-assisted radical cystectomy (RARC) with intracorporeal urinary diversion (ICUD) in an effort to improve surgical outcomes and bladder cancer survival without strong supporting evidence.
Objective
To evaluate perioperative, safety, and survival outcome differences between RARC with ICUD and open radical cystectomy (ORC).
Design, Setting, and Participants
This nationwide population-based cohort study used data from the Swedish National Register of Urinary Bladder Cancer and population-based Cause of Death Register, which includes clinical information on tumor characteristics, treatment, and survival and covers approximately 97% of patients with urinary bladder cancer in Sweden. All patients who underwent radical cystectomy for bladder cancer in any hospital between January 2011 and December 2018 were included. Follow-up data were collected until December 2019. Data analysis was conducted from June to December 2020.
Exposures
RARC or ORC.
Main Outcomes and Measures
The main outcomes were all-cause and cancer-specific mortality between RARC and ORC, compared using propensity score matching. Secondary outcomes were differences in perioperative outcomes after the different surgical approaches.
Results
Throughout the observation period, 889 patients underwent RARC and 2280 patients underwent ORC at 24 Swedish hospitals. The median (IQR) age was 71 (66-76) years and 2386 patients (75.3%) were men. After a median (IQR) follow-up of 47 (28-71) months, the 5-year cancer-specific mortality rates were 30.2% (variance, 1.59) for ORC and 27.6% (variance, 3.12) for RARC, and the overall survival rates were 57.7% (variance, 2.46) for ORC and 61.4% (variance, 5.11) for RARC. In the propensity score–matched analysis, RARC was associated with a lower all-cause mortality (hazard ratio, 0.71; 95% CI, 0.56-0.89; P = .004). Compared with ORC, RARC was associated with a lower estimated blood loss (median [IQR] 150 [100-300] mL vs 700 [400-1300] mL; P < .001), intraoperative transfusion rate (odds ratio [OR], 0.05; 95% CI, 0.03-0.08; P < .001), and shorter length of stay (median [IQR], 9 [6-13] days vs 13 [10-17] days; P < .001), and with a higher lymph node yield (median [IQR], 20 [15-27] lymph nodes vs 14 [8-24] lymph nodes; P < .001) and 90-day rehospitalization rate (OR, 1.28; 95% CI, 1.02-1.60; P = .03). The RARC group, compared with the ORC group had lower risk of Clavien-Dindo grade III or higher complications (OR, 0.62; 95% CI, 0.43-0.87; P = .009).
Conclusions and Relevance
These findings suggest that compared with ORC, RARC with ICUD was associated with a lower overall mortality rate, fewer high-grade complications, and more favorable perioperative outcomes.
Introduction
Radical cystectomy with pelvic lymph node dissection (PLND) is considered the criterion standard treatment for muscle invasive bladder cancer and selected patients with high-risk nonmuscle invasive bladder cancer1 and is associated with high rates of perioperative morbidity and mortality.2 Minimally invasive strategies have gained popularity because of their potential to reduce surgical morbidity and shorten hospital length of stay (LOS). Since its introduction in 2003, robot-assisted laparoscopic radical cystectomy (RARC) has been gradually adopted as a surgical option with the goal to improve perioperative outcome and survival.3
Available studies investigating the perioperative safety and oncological efficacy of RARC compared with open radical cystectomy (ORC) are primarily small trials at academic institutions with short follow-up time.4 Additionally, the diversion technique has been almost exclusively limited to an extracorporeal hybrid approach (ECUD), leaving the question concerning the risks and benefits of a fully minimal-invasive intracorporeal technique unanswered.
Sweden has been an early adopter of RARC with intracorporeal urinary diversion (ICUD), with introduction of the technique in routine clinical practice in 2004.5 The procedure was implemented at multiple national sites in the subsequent years providing an opportunity to perform a broad-based analysis of its long-term efficacy. In this study, we used nationwide population-based data to compare perioperative and oncological outcomes after RARC vs ORC in Sweden over a period of 8 years.
Methods
This cohort study was approved by the Stockholm research ethics board. Because this study used registry-based data, informed consent was not required, per Swedish regulations.6 This study is reported following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Data Source
We retrieved data from the Swedish National Register of Urinary Bladder Cancer (SNRUBC), which includes clinical information on tumor characteristics and treatment and covers approximately 97% of patients with urinary bladder cancer in Sweden.7,8 In the database, cause and date of death were retrieved from the population-based Cause of Death Register using the national registration number assigned to all Swedish residents.9
Patient Population and Covariates
We included all patients who underwent RC for bladder cancer in any hospital between January 2011 and December 2018 using International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) code C67.9. Patients with metastatic disease before surgery were excluded. Patient covariates included age, sex, body mass index (BMI; calculated as weight in kilograms divided by height in meters squared), American Society of Anesthesiologists (ASA) score, status of prior abdominal or pelvic surgery or radiotherapy, clinical T- and N-stage, and receipt of neoadjuvant chemotherapy (NAC). Hospital volume was calculated as the mean annual caseload of RCs at a given institution during the study period. All procedures were either classified as ORC or RARC.
Outcomes
Our primary aim was to assess all-cause and cancer-specific mortality after RARC and ORC. Secondarily, we aimed to study differences in perioperative parameters, postoperative complications, and length of stay after the different surgical approaches. Intraoperative parameters included the performance of PLND, performance of urethrectomy, diversion type, estimated blood loss (EBL), intraoperative transfusion rate, and operation time. Diversion type was classified as ileum conduit, orthotopic bladder substitution (OBS), continent cutaneous urinary diversion, and others. RARC procedures converted to open surgery remained in the RARC cohort. Postoperative data included 90-day complications by type categorized using the modified Clavien-Dindo (CD) classification,10 reoperation rate, LOS, 90-day rehospitalization rate, pathological T-stage, number of removed lymph nodes (LNs), and nodal status. Death from bladder cancer was defined as ICD-10 code C67.8, C67.9, C68.9, or D41.4 as underlying death cause. Starting date of the study was the date of RC and last date of the study was date of death, emigration, or December 31, 2019, whichever happened first.
Statistical Analysis
First, we compared baseline characteristics of patients undergoing ORC vs RARC. Differences between the groups were statistically assessed using the Pearson χ2 for categorial variables and Mann-Whitney U test for continuous variables.
Second, to account for possible selection bias, observed differences in baseline characteristics between patients who received ORC vs RARC were addressed using propensity score (PS) matching. Specifically, a logistic regression PS model for the type of the surgical approach (ORC vs RARC) was created, and the following variables were included in the model: age, BMI, ASA score, prior surgery or radiotherapy, clinical T- and N-stage, NAC, annual hospital volume, and pathological T-stage. Fixed ratio (1:2) nearest-neighbor matching was performed with a 0.2-width caliper of the SD of the logit of the PS.11
Third, descriptive statistics were used to summarize data for the PS-matched groups and balance between covariates was assessed by using the standardized mean difference (SMD). Primary and secondary outcomes were compared in the PS-matched cohort. Results are presented as median (IQR) or frequency (percentage). Categorical end points were assessed using logistic regression accounting for clustering in a fixed effects model. Cumulative incidence functions for cancer-specific and other-cause mortality were estimated nonparametrically with the Aalen-Johansen estimator and compared between ORC and RARC using the Gray test.12 Overall survival (OS) curves were derived as 1 – all-cause mortality and compared between surgical groups with the log-rank test. We compared cancer-specific mortality, other-cause mortality, and all-cause mortality in the RARC vs the ORC groups by using Cox regression models using robust variance estimation to take into account the correlation of matched individuals. We stratified the estimated Cox regression models by hospital, allowing separate baseline hazard functions for each hospital to further capture between-hospital variability. We evaluated the robustness of study results to possible unmeasured confounders by computing the E-values.13 We further performed 2 different sensitivity analyses: in the first analysis we included year of surgery as matching variable for PS matching. For the second analysis, we excluded all low-volume hospitals (<25 RCs per year) before PS matching to further address the potential confounding bias of surgical experience. For the subgroup analysis of the population not receiving NAC, these patients were excluded from the PS-matched cohort.
Statistical analyses were performed by using SPSS version 26.0 (SAS Institute) and R version 4.1.2 (R Project for Statistical Computing) statistical software. All tests were 2-sided, and P < .05 was considered statistically significant. Data analysis was conducted from June to December 2020.
Results
Baseline Characteristics and PS Matching
Between January 2011 and January 2019, RC for bladder cancer in nonmetastatic disease was performed in 3169 patients at 24 different hospitals in Sweden (RARC at 8 centers, including 52 patients [5.8%] with ECUD and 837 patients [94.2%] with ICUD). The median (IQR) age was 71 (66-76) years, and 2386 (75.3%) were men. The studied population had a median (IQR) BMI of 26 (23-28). A total of 1760 patients (55.5%) were classified as ASA II, and 967 patients (30.5%) were classified as ASA III. Nearly 1 in 5 patients (586 patients [18.5%]) had prior abdominal surgery or radiation to the pelvis. Baseline oncological parameters showed muscle invasive disease (≥T2) in 2294 patients (72.4%), and 1048 patients (33.1%) received NAC. Baseline characteristics of the entire cohort stratified for 2280 patients who underwent ORC (71.9%) and 889 patients who underwent RARC (28.1%) are displayed in Table 1. Survival curves and univariate Cox regression analysis are presented in eFigure 1 and eTable 1 in the Supplement. The PS-matched cohort included 2428 patients (1554 patients [64.0%] in the ORC group and 874 patients [36.0%] in the RARC group). Following matching, all covariates were comparable between the ORC and RARC group (absolute SMD < 0.1) (Table 1). The PS distributions for the groups both before and after matching confirming the adequacy of the model are shown in eFigure 2 in the Supplement.
Table 1. Baseline Patient Characteristics Overall and After Propensity Score Matching.
| Characteristic | Before propensity matching | After propensity matching | ||||||
|---|---|---|---|---|---|---|---|---|
| No. (%) | SMD | P value | No. (%) | SMD | P value | |||
| ORC (n = 2280) | RARC (n = 889) | ORC (n = 1554) | RARC (n = 874) | |||||
| Sex | ||||||||
| Men | 1713 (75.1) | 673 (75.4) | −0.01 | .74 | 1160 (74.6) | 660 (75.5) | −0.02 | .67 |
| Women | 567 (24.9) | 216 (24.3) | 394 (25.4) | 214 (24.5) | ||||
| Age, median (IQR), y | 71 (66 to 76) | 71 (65 to 76) | −0.07 | .12 | 71 (65 to 76) | 71 (65 to 76) | 0.003 | .80 |
| BMI | ||||||||
| Median (IQR) | 26 (23 to 28) | 26 (23 to 28) | 0.21 | .37 | 26 (23 to 28) | 26 (23 to 28) | −0.03 | .56 |
| Missing | 27 (1.2) | 1 (0.1) | NA | NA | 5 (0.3) | 1 (0.1) | NA | NA |
| ASA score | ||||||||
| I | 271 (12.0) | 101 (11.5) | −0.02 | <.001 | 178 (11.5) | 101 (11.6) | 0.004 | .08 |
| II | 1327 (58.9) | 433 (49.1) | −0.19 | 856 (55.5) | 433 (49.9) | −0.02 | ||
| III | 635 (28.2) | 332 (37.6) | 0.2 | 491 (31.8) | 323 (37.3) | 0.03 | ||
| IV | 20 (0.9) | 16 (1.8) | 0.07 | 18 (1.2) | 10 (1.2) | −0.03 | ||
| Missing | 27 (1.2) | 7 (0.8) | NA | 11 (0.7) | 7 (0.8) | NA | ||
| Prior surgery or radiation treatment | ||||||||
| Any | 429 (19.3) | 157 (17.8) | −0.03 | .36 | 285 (18.4) | 156 (18.0) | −0.006 | .78 |
| Missing | 56 (2.5) | 6 (0.7) | NA | NA | 9 (0.6) | 6 (0.7) | NA | NA |
| Clinical T stage | ||||||||
| CIS/Ta/T1 | 591 (25.9) | 244 (27.4) | 0.03 | <.001 | 428 (27.5) | 241 (27.6) | 0.02 | .86 |
| T2 | 1185 (52.0) | 512 (57.6) | 0.11 | 868 (55.9) | 501 (57.3) | 0.01 | ||
| T3 | 336 (14.7) | 86 (9.7) | −0.17 | 172 (11.1) | 85 (9.7) | −0.01 | ||
| T4 | 135 (5.9) | 40 (4.5) | −0.07 | 71 (4.6) | 40 (4.6) | 0.02 | ||
| Unknowna | 33 (1.4) | 7 (0.8) | NA | 15 (1.0) | 7 (0.8) | NA | ||
| cN+ | 226 (9.9) | 62 (7.0) | −0.12 | <.001 | 123 (7.9) | 62 (7.1) | −0.002 | .52 |
| NAC | ||||||||
| Any | 745 (32.9) | 303 (34.2) | 0.03 | .50 | 510 (32.9) | 297 (34.1) | 0.01 | .56 |
| Missing | 17 (0.7) | 3 (0.3) | NA | NA | 5 (0.3) | 3 (0.3) | NA | NA |
| Annual hospital volume, procedures/y | ||||||||
| 1-10 | 287 (12.6) | 35 (3.9) | −0.45 | <.001 | 65 (4.2) | 35 (4.0) | −0.02 | .19 |
| 11-25 | 564 (24.7) | 144 (16.2) | −0.23 | 301 (19.4) | 144 (16.5) | −0.03 | ||
| >25 | 1429 (62.7) | 710 (79.9) | 0.43 | 1188 (76.4) | 695 (79.5) | 0.02 | ||
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CIS, carcinoma in situ; NA, not applicable; NAC, neoadjuvant chemotherapy; ORC, open radical cystectomy; RARC, robot-assisted radical cystectomy; SMD, standardized mean difference.
Pathologist did not or could not classify a pathological stage.
Primary Outcomes
The median (IQR) follow-up time for survivors was 47 (28-71) months (minimum 12 months). Deaths of any cause occurred in 628 patients (40.4%) in the ORC group and 275 patients (31.5%) in the RARC group, and bladder cancer deaths occurred in 439 patients (28.2%) in the ORC group and 209 patients (23.9%) in the RARC group. The estimated cancer-specific mortality rate at 5 years was 30.2% (variance, 1.59) for ORC and 27.6% (variance, 3.12) for RARC, and at 7 years, estimated cancer-specific mortality was 32.3% (variance, 1.91) for ORC and 30.3% (variance, 5.13) for RARC (P = .16) (Figure 1). The estimated other-cause mortality rate at 5 years was 12.1% (variance, 0.875) for ORC and 11.0% (variance, 1.99) for RARC, and at 7 years, estimated other-cause mortality was 16.5% (variance, 1.5) for ORC and 11.4% (variance, 2.20) for RARC (P = .03) (Figure 1). These differences remained for OS, with 5-year estimates of 57.7% (variance, 2.46) for ORC and 61.4% (variance, 5.11) for RARC, and 7-year estimates of 51.2% (variance, 3.41) for ORC and 58.2% (variance, 7.33) for RARC (P = .01) (Figure 1). The 30-day all-cause mortality rate was 1.5% for ORC and 0.9% for RARC (odds ratio [OR], 0.58; 95% CI, 0.15-2.28; P = .44), and the 90-day mortality rate was 4.2% for ORC and 2.7% for RARC (OR, 0.70; 95% CI, 0.32-1.54; P = .38). In Cox regression analysis, all-cause mortality was superior for RARC (hazard ratio [HR], 0.71; 95% CI, 0.56-0.89; P = .004) (eTable 2 in the Supplement). The calculated magnitude of confounding that would be necessary to fully explain the observed risk ratio was E = 2.17 (95% CI, not available to 1.50) (eTable 2 in the Supplement). Other parameters independently associated with all-cause mortality were EBL, blood transfusions, higher LN yield, nodal status, and CD grade III or IV complications
Figure 1. Propensity Score–Matched Survival Analysis.
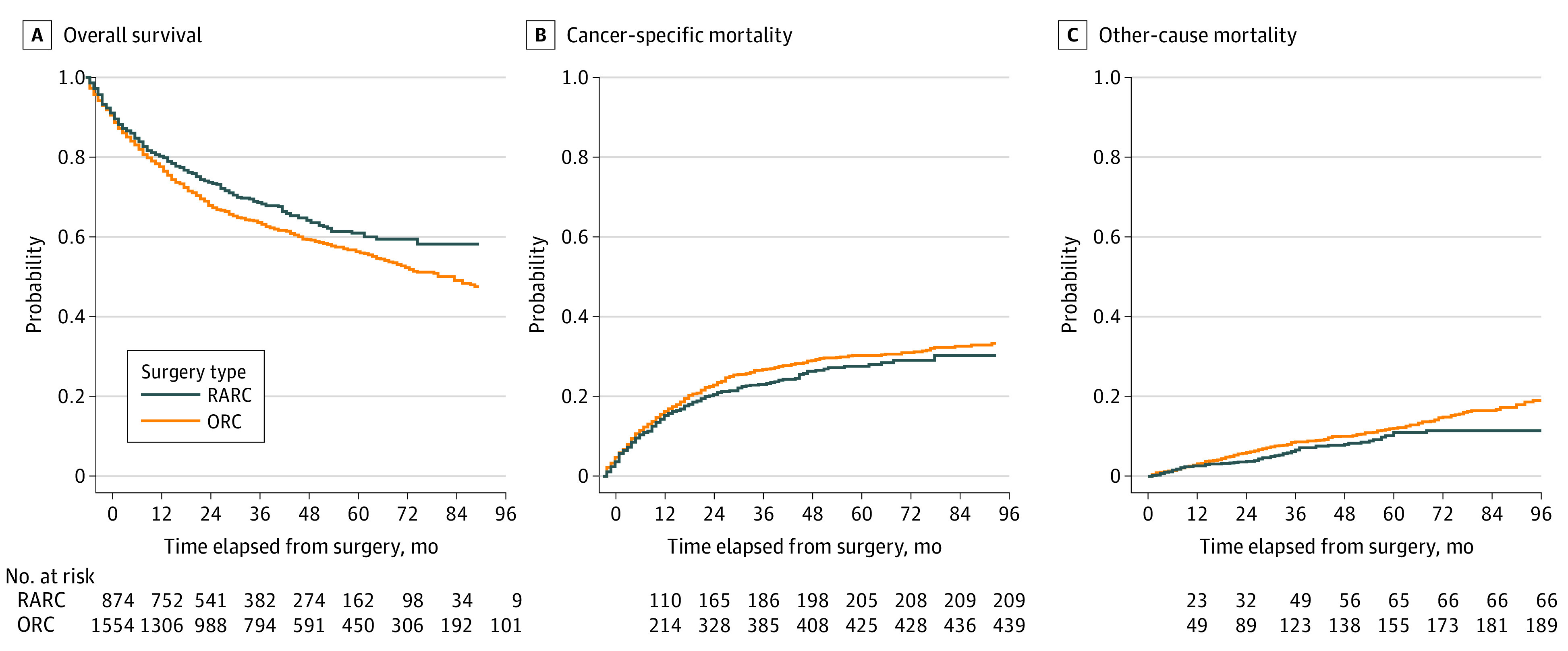
ORC indicates open radical cystectomy; RARC, robot-assisted radical cystectomy.
To rule out a possible bias of differences in local NAC regimen on survival, a subgroup analysis of patients not receiving systemic therapy before surgery in the matched cohort was performed. All-cause mortality for RARC remained superior compared with ORC (HR, 0.66; 95% CI, 0.49-0.87; P = .004).
Secondary Outcomes
RARC, compared with ORC, was associated with a higher rate of OBS (177 patients [20.3%] vs 116 patients [7.5%]; P < .001), lower EBL (median [IQR], 150 [100-300] mL vs 700 [400-1300] mL; P < .001), and lower intraoperative transfusion rate (63 patients [7.7%] vs 572 patients [38.7%]; OR, 0.05; 95% CI, 0.03-0.08; P < .001) (Table 2). With regard to postoperative outcomes, patients undergoing RARC, compared with those who underwent ORC, had a shorter LOS (median [IQR], 9 [6-13] days vs 13 [10-17] days; P < .001) but a higher 90-day rehospitalization rate (294 patients [34.3%] vs 393 patients [26.1%]; OR, 1.28; 95% CI, 1.02-1.60; P = .03). LOS was not associated with readmission (OR, 1.00; 95% CI, 0.99-1.01; P = .15). The number of removed LNs was significantly higher for RARC than ORC (median [IQR], 20 [15-27] LNs vs 14 [8-24] LNs; P < .001).
Table 2. Perioperative and Postoperative Characteristics of the PSM Cohort.
| Characteristic | No. (%) | SMDa | P value | ||
|---|---|---|---|---|---|
| ORC (n = 1554) | RARC (n = 874) | Before PSM | After PSM | ||
| Intraoperative parameters | |||||
| Urethrectomy | |||||
| Yes | 326 (21.1) | 172 (19.8) | NA | NA | .46 |
| Missing | 11 (0.7) | 5 (0.6) | NA | NA | NA |
| Deviation type | |||||
| Ileum conduit | 1386 (89.2) | 692 (79.2) | NA | NA | <.001 |
| OBS | 116 (7.5) | 177 (20.3) | NA | NA | |
| CCUD | 21 (1.4) | 1 (0.1) | NA | NA | |
| Other | 31 (2.0) | 4 (0.5) | NA | NA | |
| Missing | 0 | 0 | NA | NA | |
| Estimated blood loss | |||||
| Median (IQR), mL | 700 (400 to 1300) | 150 (100 to 300) | NA | NA | <.001 |
| Missing | 19 (1.2) | 22 (2.5) | NA | NA | NA |
| Intraoperative blood transfusion | |||||
| Yes | 572 (38.7) | 63 (7.7) | NA | NA | <.001 |
| Missing | 75 (4.8) | 59 (6.8) | NA | NA | NA |
| Perioperative blood transfusionb | |||||
| Yes | 429 (50.8) | 143 (20.7) | NA | NA | <.001 |
| Missing | 137 (14.0) | 12 (1.7) | NA | NA | NA |
| Operation time | |||||
| Median (IQR), min | 323 (250 to 407) | 320 (260 to 380) | NA | NA | .45 |
| Missing | 32 (2.1) | 29 (3.3) | NA | NA | NA |
| Postoperative parameters | |||||
| Clavien-Dindo classification | |||||
| I-II | 404 (27.0) | 286 (33.3) | NA | NA | .001 |
| III | 284 (19.0) | 122 (14.2) | NA | NA | |
| IV | 46 (3.1) | 19 (2.2) | NA | NA | |
| V | 27 (1.8) | 7 (0.8) | NA | NA | |
| Missing | 59 (3.8) | 14 (1.6) | NA | NA | |
| Reoperation | |||||
| Yes | 182 (12.0) | 81 (9.4) | NA | NA | .05 |
| Missing | 41 (2.6) | 11 (1.3) | NA | NA | NA |
| Length of stay | |||||
| Median (IQR), d | 13 (10 to 17) | 9 (6 to 13) | NA | NA | <.001 |
| Missing | 34 (2.2) | 10 (1.1) | NA | NA | NA |
| Rehospitalization in 90 d | |||||
| Yes | 393 (26.1) | 294 (34.3) | NA | NA | <.001 |
| Missing | 48 (3.1) | 17 (1.9) | NA | NA | NA |
| Pathological T-stage | |||||
| T0 | 344 (22.1) | 200 (22.9) | 0.05 | 0.001 | .99 |
| CIS/Ta/T1 | 347 (22.3) | 198 (22.7) | 0.04 | 0.02 | |
| T2 | 283 (18.2) | 161 (18.4) | 0.02 | 0.02 | |
| T3 | 418 (26.9) | 228 (26.1) | −0.02 | −0.01 | |
| T4 | 151 (9.7) | 80 (9.2) | −0.14 | −0.02 | |
| Unknownc | 11 (0.7) | 7 (0.8) | NA | NA | |
| Removed lymph nodesd | |||||
| Median (IQR), No. | 14 (8 to 24) | 20 (15 to 27) | NA | NA | <.001 |
| Missing | 90 (6.6) | 21 (2.6) | NA | NA | NA |
| N+ | |||||
| Yes | 305 (20.2) | 169 (20.4) | NA | NA | .91 |
| Missing | 45 (2.9) | 46 (5.3) | NA | NA | NA |
Abbreviations: CCUD, continent cutaneous urinary diversion; CIS, carcinoma in situ; NA, not applicable; OBS, orthotopic bladder substitution; ORC, open radical cystectomy; PSM, propensity score matching; RARC, robot-assisted radical cystectomy; SMD, standardized mean difference.
Reported for variables used in propensity score matching.
Total number of patients receiving transfusions during hospitalization (including intraoperative transfusions). Variable reported since 2014 (after matching, there were 981 patients in the ORC group and 703 in the RARC group).
Pathologist did not or could not classify a pathological stage.
In patients undergoing pelvic lymph node dissection.
The overall 90-day complication rate was comparable in ORC and RARC groups (761 patients [50.9%] vs 434 patients [50.5%]) (Table 2). However, RARC was associated with a lower high-grade (CD grade ≥III) complication rate compared with ORC (148 patients [17.2%] vs 357 patients [23.9%]; OR, 0.62; 95% CI, 0.43-0.87; P = .009). Infectious complications were more common in the RARC group, while lymphoceles and events concerning the cardiovascular or respiratory system or the abdominal wall or stoma occurred more frequently in the ORC group (Table 3). Differences in categories remained when only high-grade complications were considered, except for infectious complications. The 90-day CD grade V complication rate was 7 patients (0.8%) for the RARC group and 27 patients (1.8%) for the ORC group (OR, 0.73; 95% CI, 0.18-3.00; P = .66).
Table 3. Subclassifications of Complications.
| System/category | No. (%)a | P value | |
|---|---|---|---|
| ORC (n = 1554) | RARC (n = 874) | ||
| Subclassification of any complication reported | |||
| Gastrointestinal | 192 (12.4) | 101 (11.6) | .62 |
| Cardiovascular and respiratory | 99 (6.4) | 36 (4.1) | .02 |
| Infectious | 454 (29.2) | 300 (34.4) | .009 |
| Abdominal wall or stoma | 187 (12.0) | 38 (4.4) | <.001 |
| Genitourinary | 115 (7.4) | 74 (8.5) | .34 |
| Bleeding | 22 (1.4) | 16 (1.8) | .50 |
| Lymphocele | 65 (4.2) | 12 (1.4) | <.001 |
| Other | 191 (12.3) | 117 (13.4) | .45 |
| Subclassification of complications in patients with ≥1 high-grade complication (Clavien-Dindo III-V) | |||
| Gastrointestinal | 113 (7.3) | 51 (5.8) | .18 |
| Cardiovascular and respiratory | 63 (4.1) | 14 (1.6) | .001 |
| Infectious | 179 (11.6) | 87 (10.0) | .22 |
| Abdominal wall or stoma | 118 (7.6) | 26 (3.0) | <.001 |
| Genitourinary | 97 (6.3) | 57 (6.5) | .80 |
| Bleeding | 18 (1.2) | 13 (1.5) | .57 |
| Lymphocele | 29 (1.9) | 7 (0.8) | .04 |
| Other | 72 (4.6) | 33 (3.8) | .35 |
Abbreviations: ORC, open radical cystectomy; RARC, robot-assisted cystectomy.
Patients can be assigned to multiple categories.
To assess a possible bias of the learning curve on postoperative complications, we compared the rate of CD grade III or greater from 2011 to 2014 vs 2015 to 2018. In this comparison, there was no difference in the ORC group (OR, 0.90; 95% CI, 0.71-1.15; P = .36), while for the RARC group, the rate declined significantly (OR, 0.62; 95% CI, 0.43-0.90; P = .01).
Sensitivity Analyses
Our first sensitivity analysis, including year of surgery as matching variable, showed similar results in regards to the primary and secondary outcomes (eTable 3 and eFigure 3 in the Supplement); therefore, we proceeded with the more parsimonious model that maximized sample size. In the second sensitivity analysis only including high-volume hospitals, the survival and complication advantage of RARC compared with ORC remained, with an estimated 7-year OS of 58.2% (variance, 8.40) in RARC vs 53.7% (variance, 3.97) in ORC (HR, 0.83; 95% CI, 0.71-0.97; P = .02) (Figure 2) and a high-grade complications rate (CD grade ≥III) (OR, 0.66; 95% CI, 0.45-0.96; P = .03).
Figure 2. Sensitivity Survival Analysis including High-Volume Hospitals Only.
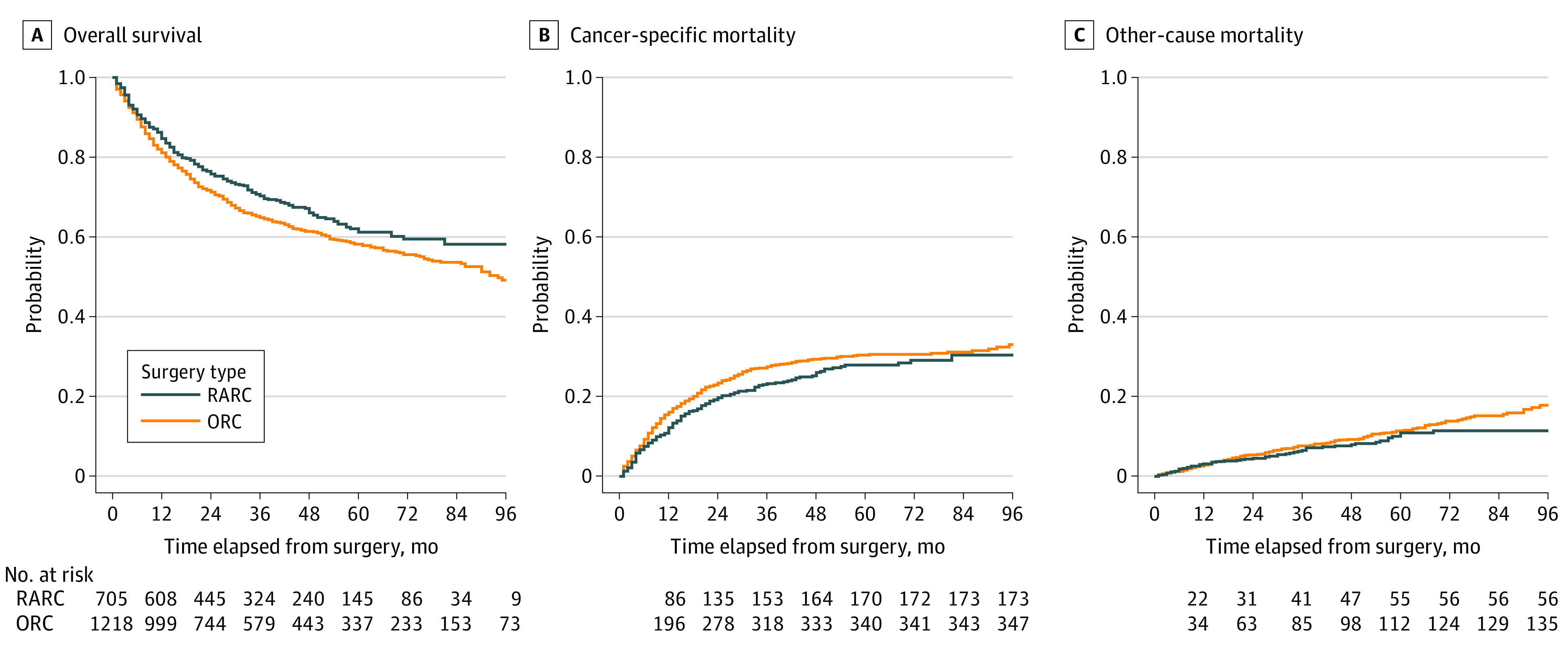
Analysis restricted to patients who received care in hospitals with a yearly volume of more than 25 procedures. ORC indicates open radical cystectomy; RARC, robot-assisted radical cystectomy.
Discussion
In this large cohort study of bladder cancer in Sweden, we found a higher overall mortality after ORC than after RARC. Moreover, the robotic approach had a similar operation time, shorter LOS, and was associated with fewer postoperative high-grade complications. The main strength of this study is the use of a nationwide population-based cohort encompassing nearly all patients with bladder cancer undergoing RC over 8 years, with detailed clinicopathological, perioperative data for long and complete follow-up. We performed a matched analysis to reduce patient selection bias and provide a valid comparison between the 2 techniques. To our knowledge, this is the largest comparative analysis of RARC with ICUD vs ORC controlling for patient, tumor, and hospital characteristics.
Efforts have been made to examine the surgical and oncological safety of RARC compared with the previous standard of care of ORC. Results from 5 prospective randomized clinical trials involving 541 patients have confirmed that RARC and ORC are comparable in terms of complications and oncological outcomes, while RARC reduces the risk of blood transfusions and LOS.14,15,16,17,18,19,20,21 However, participants of these trials almost exclusively underwent ECUD.
It has been proposed that ICUD might have potential benefits in terms of decreased fluid loss, further reduced EBL, and quicker return to bowel function.22,23 In a 3-way comparison of ORC, RARC with ECUD, and ICUD, the fully intracorporeal approach was associated with reduction of EBL, LOS, ileus rate, and major complication rates but longer operation time compared with the other 2 techniques.24 However, studies investigating the potential benefits of ICUD are limited to single-institution series with limited sample sizes.25 Our findings suggest superior perioperative outcomes within the RARC cohort; we found that RARC with ICUD was associated with an 80% lower EBL and blood transfusion rate. While the median EBL of 700 mL for ORC in this study was in line with other reported rates for ORC (median [IQR], 800 [600-1150] mL), the median EBL of 150 mL for RARC with ICUD was significantly lower than reported volumes for RARC with ECUD (median [IQR], 350 [288-453] mL).25 This difference further translated into a significantly lower intraoperative transfusion rate of only 8% vs 39% for ORC.
While the overall complication rate was comparable between groups, we observed a significantly lower high-grade complication rate in the RARC cohort. In line with prior reports17,19,20 the overall infectious complication rate was significantly higher for RARC compared with ORC. However, this difference of 5.2% diminished in the subanalysis of patients with high-grade complications only. At the same time, complications concerning the cardiovascular or respiratory system, the abdominal wall or stoma, and lymphoceles remained more frequent in the ORC group, suggesting that these kind of incidents were more likely to be CD graded as III or greater than infectious complications. While the 23.9% rate of CD grade III or greater in the ORC group was comparable with that of ORC cohorts in other comparative studies (20%-22%) the rate for RARC with ICUD was 17.2%, lower than reported rates for RARC with ECUD (22%-35%).15,17,20 However, owing to the technically more challenging nature of ICUD, a higher complication rate at the beginning of the learning curve, as observed in this study, has to be taken into consideration.26
While LOS was shorter in the RARC group compared with the ORC group, the readmission rate was significantly higher. We investigated if an earlier postoperative discharge, attributed to an assumed broader implementation of enhanced recovery after surgery protocols at RARC centers, may have contributed to increased readmissions. However, this was not the case (OR, 1.00; 95% CI, 0.99-1.01; P = .15). Although the reason for rehospitalization was not available, we hypothesize that the higher rate of infectious complications in the RARC cohort, which in many cases make intravenous antibiotics necessary based on local guidelines, may have attributed to this finding.
A meticulous PLND is an integral part of RC, although the extent of the dissection is controversial.27 Prior studies have reported equal15 or higher28 LN yields for RARC, contradicting concerns regarding the quality of PLND for this technique. In this study, the median LN yield was significantly higher for RARC compared with ORC. Notably, the higher LN yield did not prolong the operation time. Moreover, we observed a comparable operation time in the RARC group, in spite of a higher rate of OBS. Although the SNRUBC database did not contain information on conversion, these findings add more evidence that after moving forward on the learning curve, RARC with high quality PLND and ICUD can be performed in a favorable time frame. The higher rate of OBS in the RARC group remained after adjusting for known factors associated with the choice of urinary diversion, such as age, tumor stage, and prior surgery and radiation therapy. It has been shown that OBS are constructed more frequently at academic centers and even at high-volume centers, this rate varies widely among institutions suggesting a substantial influence of the surgeon or local guidelines.29 Indeed, the largest national hospital predominantly contributing to the RARC cohort was an academic center and had also the highest rate for OBS.
Initially, the question had been raised of whether RARC would be associated with worse survival outcomes, potentially owing to lower LN yields or alteration of recurrence patterns due to tumor seeding linked to the pneumoperitoneum or insufflation.30 Meanwhile, randomized clinical trials have demonstrated the noninferiority of RARC with ECUD in terms of progression-free, cancer-specific, and overall survival.21 However, long-term oncological follow-up data for RARC with ICUD alone in a representative cohort or in comparison with ORC have not been reported, to our knowledge.31 We observed a significantly higher OS rate for patients undergoing RARC compared with ORC, but there was no difference in cancer-specific mortality. The OS benefit was 2% in the first year and increased to around 7% after 7 years. The improved OS rate for RARC remained after adjusting for the year of surgery and excluding low-volume hospitals. We assume that a combination of multiple factors of the minimal invasive approach may have contributed to the OS benefit detected in this study. Besides the observed lower CD V complication rate for RARC, EBL, blood transfusions, higher LN yield, and CD grade III or IV complications were associated with all-cause mortality in our Cox analysis; RARC with ICUD had a beneficial association with all of them (eTable 2 in the Supplement).
A central concern with use of observational data is bias by unmeasured confounding. To address this issue, we calculated E-values for the mortality estimates. Using the E-values, we found that an unmeasured confounder needed to be associated with all-cause mortality and surgical approach by a risk ratio of 2.17 to explain away the observed risk ratio of 0.71 in favor of RARC. While we cannot rule out the presence of such an unmeasured confounder, any uncontrolled confounder sufficient to cancel our results would also have to be independent of the multiple covariates already adjusted for in the analysis, which is unlikely to be the case.
Limitations
This study has several limitations. As in all register-based studies, misclassification and underreporting are potential concerns. PS methods adjust for known confounders but do not adjust for unobserved differences between the groups with potential associations with survival outcome, which is an inherent limitation of a retrospective analysis. Notably, specific data for comorbidities were not available. Nevertheless, age and ASA score were well balanced between the groups after PS matching, with initially more RARC patients in ASA categories III and IV. The variability among the institutions in terms of surgical techniques, perioperative protocols (eg, enhanced recovery after surgery), and pathology reporting represents another limitation. Information on whether cystectomies were performed for curative, salvage, or palliative reasons was not captured in the SNRUBC database. Similarly, data on surgeon experience, conversion rate, histological subtypes, surgical margin status, and detailed information about the type or frequency of NAC are lacking. To overcome some of these limitations, a prospective trial with estimated completion by the end of 2021 is in progress: iROC, a multicenter randomized clinical trial comparing RARC with ICUD vs ORC.32 Although, more follow-up time will be needed to assess survival, iROC will provide level 1 evidence for perioperative outcome and complications.
Conclusions
In this cohort study, we observed a lower high-grade complication rate and a significant OS benefit for RARC compared with ORC. The minimally invasive method was associated with lower EBL, lower intraoperative transfusion rate, higher LN yield, and shorter LOS compared with the open technique. However, the 90-day readmission rate was higher. In spite of the technically complex nature of the fully intracorporeal approach predominantly performed in Sweden, our findings support the feasibility of a nationwide implementation with beneficial outcomes for patients with bladder cancer.
eFigure 1. Survival Analysis Before Propensity Score Matching
eTable 1. Univariate Cox Regression Model Before Propensity Score Matching
eFigure 2. Propensity Score Distributions Before and After Propensity Score Matching
eTable 2. Univariable Cox Regression Analysis and E-Values for Mortality Analysis After Propensity Score Matching
eTable 3. Sensitivity Analysis for Year of Surgery, Baseline Characteristic, Outcomes, Univariable Cox Regression Analysis for All-Cause Mortality
eFigure 3. Sensitivity Survival Analysis for Year of Surgery
References
- 1.Witjes JA, Bruins HM, Cathomas R, et al. European Association of Urology guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2020 guidelines. Eur Urol. 2021;79(1):82-104. doi: 10.1016/j.eururo.2020.03.055 [DOI] [PubMed] [Google Scholar]
- 2.Lowrance WT, Rumohr JA, Chang SS, Clark PE, Smith JA Jr, Cookson MS. Contemporary open radical cystectomy: analysis of perioperative outcomes. J Urol. 2008;179(4):1313-1318. doi: 10.1016/j.juro.2007.11.084 [DOI] [PubMed] [Google Scholar]
- 3.Zamboni S, Soria F, Mathieu R, et al. ; European Association of Urology - Young Academic Urologists (EAU-YAU), Urothelial carcinoma working group . Differences in trends in the use of robot-assisted and open radical cystectomy and changes over time in peri-operative outcomes among selected centres in North America and Europe: an international multicentre collaboration. BJU Int. 2019;124(4):656-664. doi: 10.1111/bju.14791 [DOI] [PubMed] [Google Scholar]
- 4.Sathianathen NJ, Kalapara A, Frydenberg M, et al. Robotic assisted radical cystectomy vs open radical cystectomy: systematic review and meta-analysis. J Urol. 2019;201(4):715-720. doi: 10.1016/j.juro.2018.10.006 [DOI] [PubMed] [Google Scholar]
- 5.Jonsson MN, Adding LC, Hosseini A, et al. Robot-assisted radical cystectomy with intracorporeal urinary diversion in patients with transitional cell carcinoma of the bladder. Eur Urol. 2011;60(5):1066-1073. doi: 10.1016/j.eururo.2011.07.035 [DOI] [PubMed] [Google Scholar]
- 6.Ludvigsson JF, Håberg SE, Knudsen GP, et al. Ethical aspects of registry-based research in the Nordic countries. Clin Epidemiol. 2015;7:491-508. doi: 10.2147/CLEP.S90589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Häggström C, Liedberg F, Hagberg O, et al. Cohort profile: the Swedish National Register of Urinary Bladder Cancer (SNRUBC) and the Bladder Cancer Data Base Sweden (BladderBaSe). BMJ Open. 2017;7(9):e016606. doi: 10.1136/bmjopen-2017-016606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Urinblåse- och urinvägscancer, Nationell kvalitetsrapport för 2018. Accessed March 31, 2022. https://cancercentrum.se/globalassets/cancerdiagnoser/urinvagar/urinblase--och-urinrorscancer/rapporter/urinblasa_arsrapport_2018_final.pdf
- 9.Ludvigsson JF, Almqvist C, Bonamy AK, et al. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol. 2016;31(2):125-136. doi: 10.1007/s10654-016-0117-y [DOI] [PubMed] [Google Scholar]
- 10.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205-213. doi: 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rassen JA, Shelat AA, Myers J, Glynn RJ, Rothman KJ, Schneeweiss S. One-to-many propensity score matching in cohort studies. Pharmacoepidemiol Drug Saf. 2012;21(suppl 2):69-80. doi: 10.1002/pds.3263 [DOI] [PubMed] [Google Scholar]
- 12.Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26(11):2389-2430. doi: 10.1002/sim.2712 [DOI] [PubMed] [Google Scholar]
- 13.Linden A, Mathur MB, VanderWeele TJ. Conducting sensitivity analysis for unmeasured confounding in observational studies using E-values: the evalue package. Stata J. 2020;20(1):162-175. doi: 10.1177/1536867X20909696 [DOI] [Google Scholar]
- 14.Bochner BH, Dalbagni G, Marzouk KH, et al. Randomized trial comparing open radical cystectomy and robot-assisted laparoscopic radical cystectomy: oncologic outcomes. Eur Urol. 2018;74(4):465-471. doi: 10.1016/j.eururo.2018.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bochner BH, Dalbagni G, Sjoberg DD, et al. Comparing open radical cystectomy and robot-assisted laparoscopic radical cystectomy: a randomized clinical trial. Eur Urol. 2015;67(6):1042-1050. doi: 10.1016/j.eururo.2014.11.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parekh DJ, Messer J, Fitzgerald J, Ercole B, Svatek R. Perioperative outcomes and oncologic efficacy from a pilot prospective randomized clinical trial of open versus robotic assisted radical cystectomy. J Urol. 2013;189(2):474-479. doi: 10.1016/j.juro.2012.09.077 [DOI] [PubMed] [Google Scholar]
- 17.Parekh DJ, Reis IM, Castle EP, et al. Robot-assisted radical cystectomy versus open radical cystectomy in patients with bladder cancer (RAZOR): an open-label, randomised, phase 3, non-inferiority trial. Lancet. 2018;391(10139):2525-2536. doi: 10.1016/S0140-6736(18)30996-6 [DOI] [PubMed] [Google Scholar]
- 18.Khan MS, Omar K, Ahmed K, et al. Long-term oncological outcomes from an early phase randomised controlled three-arm trial of open, robotic, and laparoscopic radical cystectomy (CORAL). Eur Urol. 2020;77(1):110-118. doi: 10.1016/j.eururo.2019.10.027 [DOI] [PubMed] [Google Scholar]
- 19.Nix J, Smith A, Kurpad R, Nielsen ME, Wallen EM, Pruthi RS. Prospective randomized controlled trial of robotic versus open radical cystectomy for bladder cancer: perioperative and pathologic results. Eur Urol. 2010;57(2):196-201. doi: 10.1016/j.eururo.2009.10.024 [DOI] [PubMed] [Google Scholar]
- 20.Khan MS, Gan C, Ahmed K, et al. A single-centre early phase randomised controlled three-arm trial of open, robotic, and laparoscopic radical cystectomy (CORAL). Eur Urol. 2016;69(4):613-621. doi: 10.1016/j.eururo.2015.07.038 [DOI] [PubMed] [Google Scholar]
- 21.Rai BP, Bondad J, Vasdev N, et al. Robotic versus open radical cystectomy for bladder cancer in adults. Cochrane Database Syst Rev. 2019;4:CD011903. doi: 10.1002/14651858.CD011903.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson TG, Guru K, Rosen RC, et al. ; Pasadena Consensus Panel . Best practices in robot-assisted radical cystectomy and urinary reconstruction: recommendations of the Pasadena Consensus Panel. Eur Urol. 2015;67(3):363-375. doi: 10.1016/j.eururo.2014.12.009 [DOI] [PubMed] [Google Scholar]
- 23.Hussein AA, Elsayed AS, Aldhaam NA, et al. A comparative propensity score-matched analysis of perioperative outcomes of intracorporeal vs extracorporeal urinary diversion after robot-assisted radical cystectomy: results from the International Robotic Cystectomy Consortium. BJU Int. 2020;126(2):265-272. doi: 10.1111/bju.15083 [DOI] [PubMed] [Google Scholar]
- 24.Zhang JH, Ericson KJ, Thomas LJ, et al. Large single institution comparison of perioperative outcomes and complications of open radical cystectomy, intracorporeal robot-assisted radical cystectomy and robotic extracorporeal approach. J Urol. 2020;203(3):512-521. doi: 10.1097/JU.0000000000000570 [DOI] [PubMed] [Google Scholar]
- 25.Kimura S, Iwata T, Foerster B, et al. Comparison of perioperative complications and health-related quality of life between robot-assisted and open radical cystectomy: a systematic review and meta-analysis. Int J Urol. 2019;26(8):760-774. doi: 10.1111/iju.14005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hussein AA, May PR, Jing Z, et al. ; Collaborators . Outcomes of intracorporeal urinary diversion after robot-assisted radical cystectomy: results from the International Robotic Cystectomy Consortium. J Urol. 2018;199(5):1302-1311. doi: 10.1016/j.juro.2017.12.045 [DOI] [PubMed] [Google Scholar]
- 27.Gschwend JE, Heck MM, Lehmann J, et al. Extended versus limited lymph node dissection in bladder cancer patients undergoing radical cystectomy: survival results from a prospective, randomized trial. Eur Urol. 2019;75(4):604-611. doi: 10.1016/j.eururo.2018.09.047 [DOI] [PubMed] [Google Scholar]
- 28.Hu JC, Chughtai B, O’Malley P, et al. Perioperative outcomes, health care costs, and survival after robotic-assisted versus open radical cystectomy: a national comparative effectiveness study. Eur Urol. 2016;70(1):195-202. doi: 10.1016/j.eururo.2016.03.028 [DOI] [PubMed] [Google Scholar]
- 29.Ashley MS, Daneshmand S. Factors influencing the choice of urinary diversion in patients undergoing radical cystectomy. BJU Int. 2010;106(5):654-657. doi: 10.1111/j.1464-410X.2009.09183.x [DOI] [PubMed] [Google Scholar]
- 30.Nguyen DP, Al Hussein Al Awamlh B, Wu X, et al. Recurrence patterns after open and robot-assisted radical cystectomy for bladder cancer. Eur Urol. 2015;68(3):399-405. doi: 10.1016/j.eururo.2015.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hussein AA, Elsayed AS, Aldhaam NA, et al. Ten-year oncologic outcomes following robot-assisted radical cystectomy: results from the International Robotic Cystectomy Consortium. J Urol. 2019;202(5):927-935. doi: 10.1097/JU.0000000000000386 [DOI] [PubMed] [Google Scholar]
- 32.Catto JWF, Khetrapal P, Ambler G, et al. Robot-assisted radical cystectomy with intracorporeal urinary diversion versus open radical cystectomy (iROC): protocol for a randomised controlled trial with internal feasibility study. BMJ Open. 2018;8(8):e020500. doi: 10.1136/bmjopen-2017-020500 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Survival Analysis Before Propensity Score Matching
eTable 1. Univariate Cox Regression Model Before Propensity Score Matching
eFigure 2. Propensity Score Distributions Before and After Propensity Score Matching
eTable 2. Univariable Cox Regression Analysis and E-Values for Mortality Analysis After Propensity Score Matching
eTable 3. Sensitivity Analysis for Year of Surgery, Baseline Characteristic, Outcomes, Univariable Cox Regression Analysis for All-Cause Mortality
eFigure 3. Sensitivity Survival Analysis for Year of Surgery


