Abstract
Background
Bacillus cereus is a common food poisoning pathogen in humans. This study aimed to investigate the prevalence, molecular typing, antibiogram profile, pathogenicity, dissemination of virulence and antibiotic resistance genes associated with natural B. cereus infection among Mugil seheli.
Methods
Consequently, 120 M. seheli (40 healthy and 80 diseased) were obtained from private fish farms in Port-said Governorate, Egypt. Afterward, samples were processed for clinical, post-mortem, and bacteriological examinations. The recovered isolates were tested for antimicrobial susceptibility, phenotypic assessment of virulence factors, pathogeneicity, and PCR-based detection of virulence and antibiotic resistance genes.
Results
B. cereus was isolated from 30 (25%) examined fish; the highest prevalence was noticed in the liver (50%). The phylogenetic and sequence analyses of the gyrB gene revealed that the tested B. cereus isolate displayed a high genetic similarity with other B. cereus strains from different origins. All the recovered B. cereus isolates (n =60, 100%) exhibited β-hemolytic and lecithinase activities, while 90% (54/60) of the tested isolates were biofilm producers. Using PCR, the tested B. cereus isolates harbor nhe, hbl, cytK, pc-plc, and ces virulence genes with prevalence rates of 91.6%, 86.6%, 83.4%, 50%, and 33.4%, respectively. Moreover, 40% (24/60) of the tested B. cereus isolates were multidrug-resistant (MDR) to six antimicrobial classes and carried the bla1, bla2, tetA, and ermA genes. The experimentally infected fish with B. cereus showed variable mortality in direct proportion to the inoculated doses.
Conclusion
As far as we know, this is the first report that emphasized the existence of MDR B. cereus in M. seheli that reflects a threat to the public health and the aquaculture sector. Newly emerging MDR B. cereus in M. seheli commonly carried virulence genes nhe, hbl, cytK, and pc-plc, as well as resistance genes bla1, bla2, tetA, and ermA.
Keywords: MDR B. cereus, virulence traits, antibiogram, virulence genes, antimicrobial resistance genes
Introduction
Aquaculture is an optimistic sector of food production, providing a source of animal protein to the world’s population and bridging the gap in food shortages due to overpopulation.1 In fact, about 17% of the world’s animal protein sources come from fish, and more than 3.3 million people with 20% of their average per capita animal protein consumption substantially depending on aquatic organisms.2 The world faces serious challenges to feed its growing population. This surpassing increase has been linked to overpopulation, income explosion and urbanization, and the structural development of the aquaculture sectors.3 Therefore, the increase in fish demand must be supported by a concomitant rise in fish-producing sectors in line with the stagnation of fisheries.4 Recently, there has been growing concern about the overuse of freshwater resources, which has prompted many authorities to make stately efforts to maximize fish productivity and profits through the expansion of mariculture.5
Mullets are a significant constituent of Egyptian fisheries and represent the main cash crops of artisanal fisheries in the country’s lagoons and shores.6 Mullets have been grown in the “Hosha” system along the Nile delta as part of a long-established custom for decades.7 Egypt is currently a leading country in mullet farming with a record production of 129,000 million tons in 2015, which represents 10.5% of the aquaculture yield.8 Due to the expansion and intensification of aquaculture, many synthetic chemicals and biologics have widely utilized to control and treat pond water and sediments, causing water and environmental pollution and the emergence of threatened emerging pathogens.9 In fact, the frequent use of chemicals has adverse effects on fish health through impairment of water parameters, alteration of natural flora, and immunosuppression that are predisposing factors for emergence of bacterial diseases.10
Mullets, like other marine organisms, are susceptible to many bacterial diseases, including Gram-positive bacteria.11 Among them, Bacillus cereus was mentioned earlier as a threatening fish pathogen.12 B. cereus is a Gram-positive, motile, facultative anaerobes, and sporogenous ubiquitous bacteria frequently recovered from the water, soil, plants, and various food products.13 It is an opportunistic foodborne pathogen of public health importance; it is initially considered the main cause of gastroenteritis and food poisoning in humans.14 B. cereus possesses various toxins and other virulence traits encoded by several virulence genes, including hemolytic toxins and non-hemolytic enterotoxins, cytotoxin K (cytK), and hemolytic gene encoding phospholipase.15
The produced toxins are responsible for several human illnesses such as emesis and diarrheal syndromes, fulminant septicemia, nervous manifestations (brain abscesses and meningitis), and gas gangrene,16 pneumonia,17 eye infections (endophthalmitis), and neonates bacteremia.18 B. cereus also harbored many antimicrobial-resistance genes and showed different resistance patterns to various antibiotic classes.19 The bacteria are highly resistant to β-lactam antibiotics20 and showed acquired resistance to some antimicrobial agents like erythromycin, ciprofloxacin, streptomycin, and tetracycline.21
A brief study of the whole genome sequence of B. cereus revealed that the bacteria harbored β-lactam resistant genes, macrolide-resistant genes, and tetracycline-resistant genes.19 The regular and indiscriminate use of antibiotics to treat and/or control aquatic diseases, along with bad management, results in the existence of multidrug-resistant strains. Former studies have reported the occurrence of MDR bacterial pathogens in the aquaculture sector that could be transmitted to humans via the consumption of contaminated food.22,23
Bacillus species have been widely used in aquaculture practices as growth promoters, immunostimulants, and probiotics. However, under certain adverse conditions, the bacteria become pathogenic and serious systemic infections occur. Bacillus spp., particularly B. cereus, could induce diseases in several fish species, including common carp (Cyprinus carpi),24 striped bass (Morone saxatilis),12 European seabass (Dicentrarchus labrax),25,26 white seabream (Diplodus sargus),27 and stinging catfish (Heteropneustes fossilis).28
The pathogenicity of B. cereus varies greatly, with the strains being harmless or lethal. This variation may reveal constrains in detecting the dangers of bacteria and often leads to misbelief of the associated risks. Therefore, full awareness about the prospect pathogenicity of bacteria is a main challenge for the fish-producing industries. To our knowledge, this is the first study investigating the prevalence, sequence analysis, and antibiogram pattern of newly emerged B. cereus among cultured Mugil seheli. The study also provides new insights into virulence traits and antibiotic resistance genes typically inherited by bacteria, which contribute significantly to finding decisive solutions to treat or control the disease.
Methods
Animal Ethics
The present study was performed in compliance with the ARRIVE guidelines. All protocols were conducted according to relevant guidelines and regulations. Fish handling and all the experiments were approved by the Animal Ethics Review Committee of Suez Canal University (AERC-SCU), Egypt. The authors have obtained a permission from the farm owners to conduct a research on their fish.
Fish Sampling
One hundred and twenty Mugil seheli (40 healthy and 80 diseased with an average weight 80±15 g) were obtained from private farms in Port-said Governorate, Egypt, from March 2021 to June 2021. The collected fish were transported to the laboratory in aerated sealed plastic bags for further clinical and bacteriological examinations.
Clinical and Post-Mortem Inspection
The collected fish were clinically inspected for detection of gross or internal lesions as previously described by Schäperclaus.29
Isolation and Identification of B. cereus
A 3–5 g of each examined (kidney, liver, and gills) sample was homogenized in 90 mL of buffered peptone water (BPW, Oxoid, UK) for 2 min using a stomacher. Moreover, the obtained samples were subjected to a 15-min heat treatment at 70 °C to eliminate vegetative cells and isolate the bacterial spores.15 To prevent spore germination, the heated samples were immediately placed on ice. Subsequently, 100 µL were inoculated on Mannitol Egg-Yolk Polymyxin (MYP) agar plates and incubated at 37 °C for 24 h. The identification of B. cereus was carried out according to the colonial characters, morphological characteristics, endospore formation, and biochemical reactions: catalase and oxidase activity, H2S test, methyl red Voges–Proskauer test, indole test, and carbohydrate fermentation ability, as described by Maturin and Peeler.30 Furthermore, the isolates were phenotypically identified using the VITEK 2 compact system (bioMérieux, France),31 whereas confirmed genetically using PCR-based detection of the gyrB gene as previously described by Yamada,32 followed by gene sequencing.
B. cereus gyrB Gene Sequencing
The recovered B. cereus isolates were identical in their phenotypic profiles, so the PCR product of one randomly selected strain was sent for direct sequencing in both directions after the purification by QIAquick PCR-Product extraction kit (QIAGEN Sciences Inc., Germantown, MD, USA). The sequencing was performed using the Bigdye Terminator V3.1 cycle sequencing kit (Thermo Fisher Scientific, Waltham, MA, USA). The obtained sequences were deposited in the GenBank with accession number: MZ647998. The BLAST (Basic Local Alignment Search Tool) analyses were conducted to determine the sequence similarity to GenBank accessions. Besides, the phylogenetic analyses were conducted based upon the MegAlign module of Laser gene DNA Star version 12.1 using neighbor-joining in MEGA6.33
Phenotypic Assessment of Virulence Factors of B. cereus Isolated from M. seheli
Biofilm Production
As previously described by Osman,34 the biofilm development was assessed using glass test tubes. Briefly, each B. cereus strain was inoculated into tryptic soy broth (Oxoid, Hampshire, UK) and incubated overnight without shaking at 37 °C. A sterile broth was used as a negative control. After broth removal, the incubated tubes were stained with 1% crystal violet and incubated for 15 min (to examine cells adhering to the test tube). Shortly, the tubes were cleaned thoroughly using sterile distilled water. Each strain was tested three times. The positive result was specified by the production of purple biofilm.
Hemolytic Activity
The tested isolates were streaked onto 5% sheep blood agar (Oxoid, UK) and incubated at 24 °C for 48 h. The appearance of β-hemolysis surrounding the colonies indicates a positive result, as previously described by Wiwat and Thiramanas.35
Lecithinase Production
The tested isolates were inoculated onto Mannitol Egg-Yolk Polymyxin (MYP) agar plates and incubated at 37 °C for 24 h. The lecithinase production was indicated by the formation of a zone of egg yolk precipitation surrounding the colonies, as previously described by Quinn.36
Antibiogram Pattern of the Recovered B. cereus Isolates
The antimicrobial resistance profiles of the retrieved B. cereus isolates were detected on Mueller-Hinton agar (Oxoid, Hampshire, UK) using the Kirby-Bauer disc diffusion method as previously described by Park.37 The following antimicrobial agents (Oxoid, UK) were used (n=14); vancomycin (VAN) (30 μg), penicillin G (PEN) (10 U), amoxicillin (AMX) (30 μg), amoxicillin-clavulanic acid (AMC) (30 μg), meropenem (MEM) (10 µg), cefotaxime (CTX) (30 µg), ceftazidime (CAZ) (30 µg), nalidixic acid (NA) (30 µg), ciprofloxacin (CIP) (5 µg), sulfamethoxazole/trimethoprim (SXT) (25 μg), streptomycin (S) (10 μg), gentamycin (CN) (30 μg), doxycycline (DOX) (30 μg), and erythromycin (E) (15 μg). These antimicrobial agents were used frequently in the fish aquaculture sector in Egypt. The interpretation of results was performed according to the CLSI guidelines.38 B. cereus ATCC 11778 strain was used as a reference strain. The tested B. cereus isolates were categorized according to their resistance patterns into MDR and XDR (Extensively drug-resistance means resistant to one or more antimicrobial agent in all except one or two antimicrobial classes) as previously described by Magiorakos.39 The multiple antibiotic resistance (MAR) index was calculated as previously described by Krumperman40 as the following formula: MAR = The number of antimicrobial agents to which the isolates are resistant/The total number of tested antimicrobial agents.
PCR Detection of Virulence, Enterotoxins and Antibiotic Resistance Genes in the Recovered B. cereus
PCR assay was used to determine the enterotoxins genes; hbl, nhe, cytK, and ces, the hemolytic gene encoding phospholipases; pc-plc, and the antibiotic resistance genes; bla1, bla2, tetA, and ermA in the recovered B. cereus isolates. QIAamp DNA Mini Kit (QIAGEN Sciences Inc., Germantown, MD, USA/Cat. No. ID 51326) was used for genomic DNA extraction. Positive control strains (supplied by The AHRI, Dokki, Giza, Egypt) were used in the PCR assay, while a DNA-free reaction was involved as a negative control. The amplified PCR products were separated on agarose gel (1.5%) stained with ethidium bromide 0.5-μg mL−1, and then photographed. The primers sequences (Thermo Fisher Scientific, Waltham, MA, USA), amplicon size, and the PCR conditions are illustrated in Table 1.
Table 1.
The Oligonucleotides Sequences Used in the PCR Assay
| Primer | Sequence | Amplified Product | Amplification (35 Cycles) | References | ||
|---|---|---|---|---|---|---|
| Denaturation | Annealing | Extension | ||||
| gyrB | F: GAA GTC ATC ATG ACC GTT CTG CAY GCN GGN GGN AAR TTY GA R: AGC AGG ATA CGG ATG TGC GAG CCR TCN ACR TCN GCR TCN GTC AT |
1350 bp | 94°C 1 min. |
66°C 1 min. |
72°C 1 min. |
[32] |
| nhe | F:AAG CIG CTC TTC GIA TTC R: ITI GTT GAA ATA AGC TGT GG |
766 bp | 94°C 30 sec. |
49°C 40 sec. |
72°C 45 sec. |
[41] |
| cytK | F: ACA GAT ATC GGI CAA AAT GC R: CAA GTI ACT TGA CCI GTT GC |
421 bp | 94°C 30 sec. |
49°C 40 sec. |
72°C 45 sec. |
|
| ces | F: GGTGACACATTATCATATAAGGTG R: GTAAGCGAACCTGTCTGTAACAACA |
1271 bp | ||||
| pc-plc | F: GAGTTAGAGAACGGTATTTATGCTGC R: CTACTGCCGCTCCATGAATCC |
411 bp | 94°C 1 min. |
55°C 1 min. |
72°C 1 min. |
[42] |
| hbl | F: TGCACAAGAAACGACCGCTCA R: ATAATTTGCGCCCATTGTATTCCAT |
987 bp | 94°C 30 sec. |
54°C 40 sec. |
72°C 45 sec. |
[43] |
| bla1 | F: CATTGCAAGTTGAAGCGAAA R:TGTCCCGTAACTTCCAGCTC |
680 bp | 94°C 30 sec. |
50°C 40 sec. |
72°C 45 sec. |
[44] |
| bla2 | F: TTGTCGATTCTTCTTGGGATG R: CCCCTACTTCTCCATGACCA |
483 bp | ||||
| tetA | F: GGCGGTCTTCTTCATCATGC R: CGGCAGGCAGAGCAAGTAGA |
502 bp | 95°C 1 min. |
58°C 1 min. |
72°C 1 min. |
[45] |
| ermA | F:TCTAAAAAGCATGTAAAAGAA R:CTTCGATAGTTTATTAATATTAGT | 645 bp | 94°C 30 sec. |
52°C 40 sec. |
72°C 45 sec. |
[46] |
Pathogenicity Assay
Adaptation Period
Approximately 150 apparently healthy Tilapia zillii weighing 36 ± 5 g (with no history of previous infections) were collected from private farms in Ismailia Province, Egypt. The T. zillii was selected as a common model of marine fish due to its ease of handling, while M. seheli is difficult to be handled. To ensure that the collected fish are free from diseases, eight random samples (n=8) were harvested and subjected to bacteriological examination (conventional method of bacterial isolation and identification) as well as parasitological examination (wet mounting, squash preparation, macroscopic and microscopic examinations) as described by Meyers.47 The fish were acclimated in 1000 L fibreglass tanks for 10 days before the experiment. All water parameters values were adjusted at the permissible limits. Only apparently healthy fish with normal responses were harvested for further study.
Inoculum Preparation
The bacteria were basically cultured on MYP agar and left incubated at 37 °C for 24 h. The characteristic pink, lecithinase-producing colony, was selected and streaked on Trypticase Soy Agar (TSA) (Oxoid, UK) at 37 °C for an extra 24 h. Subsequently, the bacterial suspension was adjusted at final concentrations (104–108 CFU mL−1) using McFarland Turbidity Standard.48
Experimental Setup
About 100 acclimated T. zillii were distributed randomly into five groups, each holding two similar subgroups (n= 10). Each subgroup contains ten fish reared in a 120 L glass aquarium. Fish in the first group were injected intraperitoneally (IP) with 200 µL of sterile saline and considered as a negative control (C − ve). The other groups (TI -TV), were injected IP with 200 µL of the overnight culture of B. cereus strain at different concentrations (104–108 CFU mL−1, respectively) to detect the LD50, as there is no published reference study. B. cereus isolate that harbored nhe, hbl, and cytK virulence genes were chosen as a reference virulent strain. The abnormal behaviour and pathological lesions of the treated fish were investigated for two weeks post-inoculation. Cumulative mortalities and LD50 were also recorded and calculated. Moribund and dead fish were regularly harvested and bacteriologically examined to ensure the Koch’s postulates.
Statistical Analyses
The obtained data were analyzed using the Chi-square test (SAS software, version 9.4, SAS Institute, Cary, NC, USA) (p-value< 0.05 indicates a significant difference among the measured parameters). Moreover, the correlation coefficient was performed using R-software (version 4.0.2; https//www.r-project.org/).
Results
Clinical and Post-Mortem Findings
The examined moribound M. seheli exhibited abnormal behavior, skin ulceration, abdominal distention, and hemorrhage on the body surface and gills (Figure 1A), and exophthalmia (Figure 1B). Moreover, the post-mortem examination showed hepatomegaly, congested kidney, and the accumulation of fluids in the abdominal cavity (Figure 2).
Figure 1.
The Naturally infected M. seheli with B. cereus exhibited (A) slight exophthalmia, scales detachment (yellow arrows), (B) abdominal distension (black arrow), (C) hemorrhagic patches on the fin and operculum, and skin ulcerations (red arrows).
Figure 2.
Naturally infected M. seheli with B. cereus showed enlargement and congestion of the kidney and liver (white arrows) with the accumulation of serous fluids in the body activity (S).
Phenotypic Characteristics and the Prevalence of B. cereus Among the Examined Fish
Morphologically, all the retrieved B. cereus isolates were Gram-positive, motile, short rods round which arranged in pairs or chains. The retrieved isolates have a single non-bulging endospore that may be central or terminal. The colonies on Mannitol Egg-Yolk Polymyxin agar plates were pink and surrounded by a zone of precipitation. Biochemically, all the recovered isolates were positive for catalase, citrate utilization, glucose fermentation, Voges-Proskauer, and nitrate reduction tests. Moreover, they were negative for indole, mannitol fermentation, H2S production, oxidase, and methyl red tests. Moreover, the identification of all obtained isolates was confirmed using the VITEK2 compact apparatus and application of GP and BCL cards, where probability reached 100%. The results were estimated after comparing the results of the biochemical tests with the data carried in the VITEK2 software. The results of biochemical identification and VITEK2 were identical.
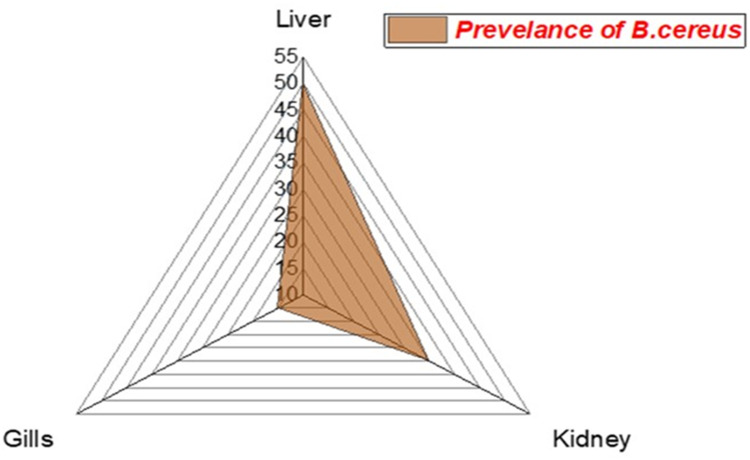
The prevalence of B. cereus among the examined fish was 25% (30/120). Concerning the distribution of B. cereus among different organs of the infected M. seheli, the highest prevalence was observed in the liver (50%), then kidney (35%), and gills (15%), as illustrated in Figure 3. Statistically, there is a significant difference in the prevalence of B. cereus between different internal organs of the examined M. seheli (p< 0.05).
Figure 3.
The radar illustrates the distribution of B. cereus among the internal organs of the examined M. seheli.
Phylogenetic Analyses of the gyrB Gene
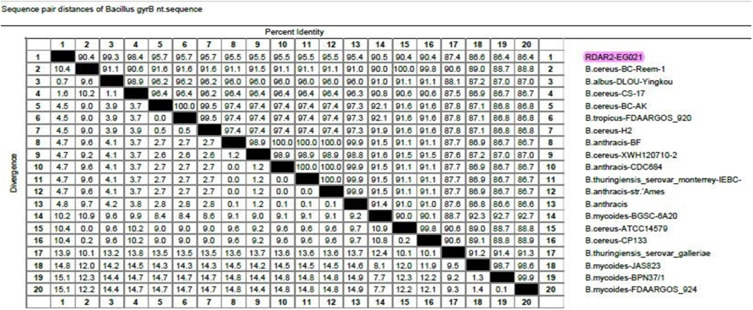
Phylogenetic and sequence analyses of the gyrB gene revealed that the tested B. cereus isolate (Accession No. MZ647998) displayed a remarkable genetic identity to other B. cereus strains from different origins. For example, B. cereus strain CS-17 (98.4%) of China (Accession No. KX346713), B. cereus strain H2 (95.7%) of isolated from rice in Japan (Accession No. AF136388), and B. cereus strain BC-AK (95.7%) of China (Accession No. CP020937), B. cereus strain XWH (95.5%) isolated from catfish in China (Accession No. KF022228), and B. cereus strain BC-Reem-1 (90.4%) isolated from raw milk in Egypt (Accession No. MT802303) as demonstrated in Figures 4 and 5. Besides, the nucleotide frequencies of adenine (A), thymine (T), cytosine (C), and guanine (G) were 36.85% (419), 26.65% (303), 15.57% (177), and 20.93% (238), respectively. Moreover, the nucleotide frequencies of A+T and C+G contents were 63.5% and 36.5%, respectively. The final alignments consisted of 1137 bp, where the sites 1108 and 29 were the most conserved sites and variable sites, respectively. Furthermore, this sequence was found to contain five open reading frames (ORFs): ORF1 (nt 412 to 519), ORF2 (nt 1063 to 1137), ORF3 (nt 237 to 1137), ORF4 (nt 447 to 334), and ORF5 (nt 252 to 172).
Figure 4.
The figure illustrates the phylogenetic analysis of B. cereus gyrB gene sequencing. The tree clarifies the genetic relatedness of the tested B. cereus strain and other B. cereus strains from different origins submitted in the GenBank database. The tested strain in the current study is highlighted (RDAR2-EG021).
Figure 5.
The percentage of B. cereus gyrB nucleotide sequence identity.
Phenotypic Assessment of Virulence Factors of B. cereus Isolated from M. seheli
Biofilm Production
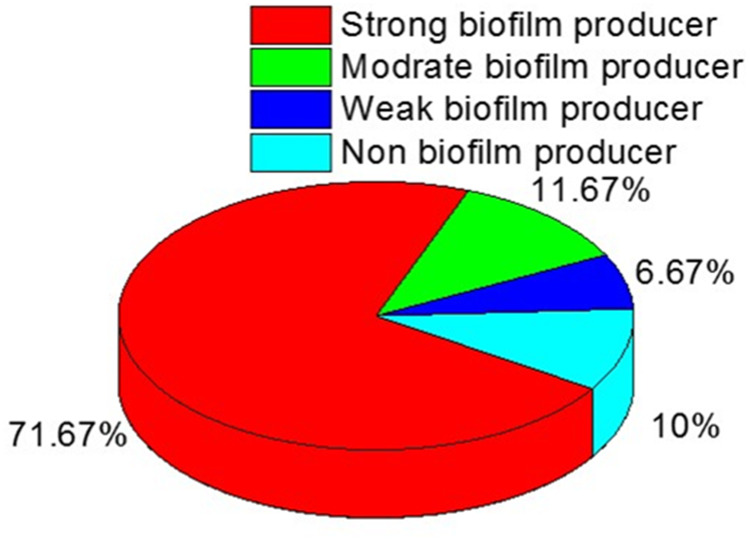
Biofilm production was assessed among the recovered B. cereus isolates, where 90% (54/60) of the tested isolates were positive for biofilm formation. Among the biofilm producers (n=54), four isolates are weak biofilm producers (7.4%), seven isolates (12.96%) are moderate for biofilm producers, and forty-three isolates (79.6%) are strong biofilm producers (Figure 6).
Figure 6.
The distribution of biofilm formation among the isolated B. cereus strains.
Hemolytic Activity
In the present study, all the recovered B. cereus isolates (n=60, 100%) displayed β-hemolysis on sheep blood agar.
Lecithinase Reaction
The lecithinase activity is specified by the production of an opaque zone around colonies on egg yolk agar. All the tested isolates (n=60, 100%) were positive for lecithinase reaction. The statistical analyses revealed a non-significant difference in the phenotypic assessment of virulence factors in the recovered B. cereus strains (p< 0.05).
Antibiogram of the Recovered B. cereus Isolates
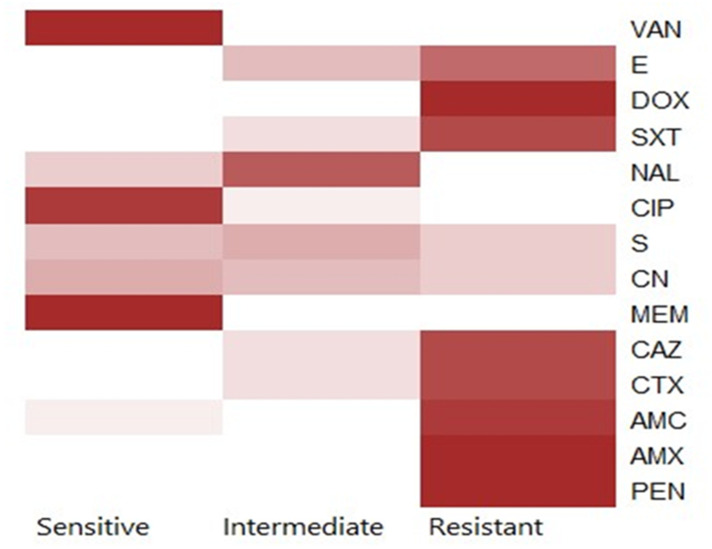
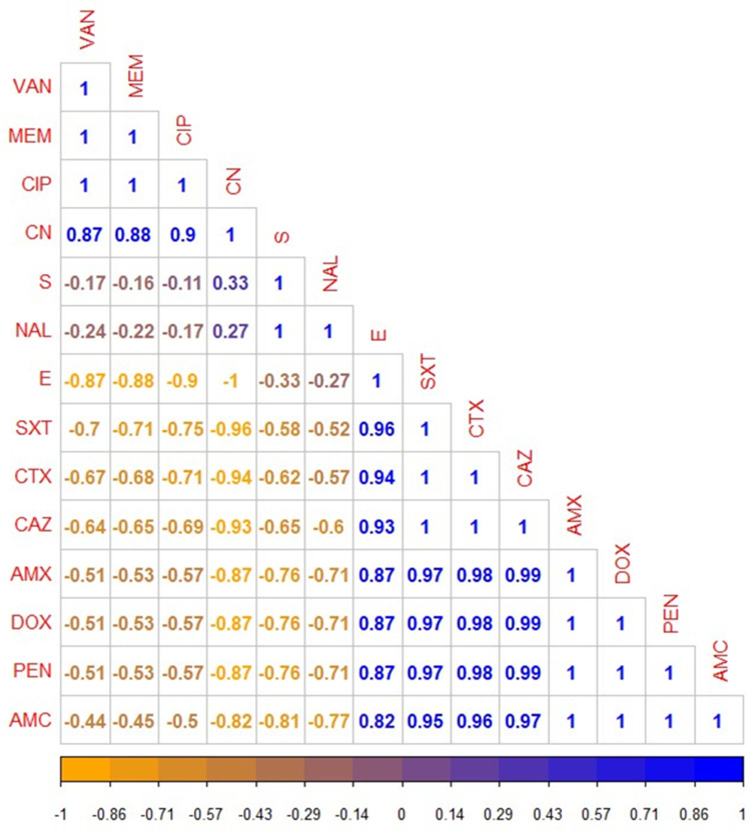
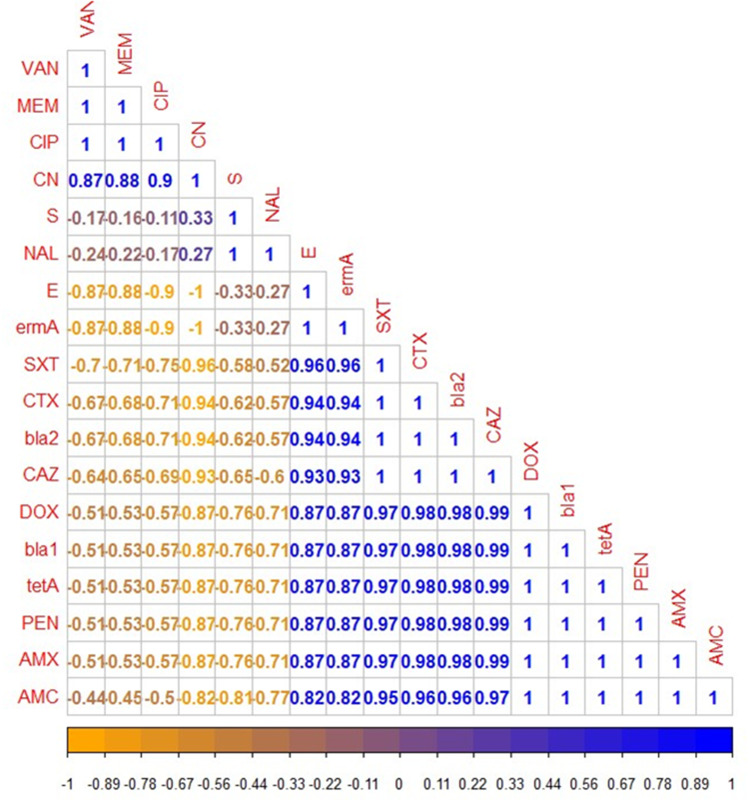
Using the disc diffusion method, vancomycin (98.3%), meropenem (96.6%), and ciprofloxacin (91.6%) exhibited a promising in-vitro antimicrobial activity against the recovered B. cereus isolates. Moreover, the retrieved B. cereus isolates displayed a significant resistance to various antimicrobial classes such as; Penicillins: penicillin and amoxicillin, (100% for each), β-Lactam-β-lactamase-inhibitor combination: amoxicillin-clavulanic acid (91.6%), Cephalosporins: cefotaxime and ceftazidime (83.3%), Sulfonamides: trimethoprim-sulfamethoxazole (100%), and Macrolides: erythromycin (66.6%). Furthermore, the obtained isolates exhibited intermediate resistance to nalidixic acid (78.4%) as described in Table 2 and Figure 7. Statistically, the retrieved B. cereus isolates exhibited a significant difference in their sensitivity to various antibiotics (p<0.05). In addition, the correlation coefficient was determined among various tested antibiotics. Significant positive correlations were observed between PEN, DOX, AMX, and CAZ (r = 0.99); AMX, PEN, DOX, and CTX (r = 0.98); AMX, PEN, DOX, and SXT (r = 0.97); CAZ and AMC (r = 0.97); CTX and AMC (r = 0.96); SXT and E (r = 0.96); SXT and AMC (r = 0.95); CTX and E (r = 0.94); CAZ and E (r = 0.93); CN and CIP (r = 0.90); MEM and CN (r = 0.88); VAN and CN (r = 0.87) (as described in Figure 8).
Table 2.
Antimicrobial Susceptibility Patterns of the Recovered B. cereus Isolates (n=60)
| Antimicrobial Classes | Tested Antimicrobial Agent | Interpretation | |||||
|---|---|---|---|---|---|---|---|
| Sensitive | Intermediate | Resistance | |||||
| n | % | n | % | n | % | ||
| Penicillins | Penicillin | 0 | 0 | 0 | 0 | 60 | 100 |
| Amoxicillin | 0 | 0 | 0 | 0 | 60 | 100 | |
| β -Lactam- β-lactamase inhibitor combination | Amoxicillin-Clavulanic acid | 5 | 8.4 | 0 | 0 | 55 | 91.6 |
| Cephalosporins | Cefotaxime | 0 | 0 | 10 | 16.7 | 50 | 83.3 |
| Ceftazidime | 1 | 1.7 | 9 | 15 | 50 | 83.3 | |
| Carbapenems | Meropenem | 58 | 96.6 | 2 | 3.4 | 0 | 0 |
| Aminoglycosides | Gentamycin | 24 | 40 | 20 | 33.4 | 16 | 26.6 |
| Streptomycin | 19 | 31.7 | 25 | 41.7 | 16 | 26.6 | |
| Fluoroquinolones | Ciprofloxacin | 55 | 91.6 | 5 | 8.4 | 0 | 0 |
| Quinolones | Nalidixic acid | 13 | 21.6 | 47 | 78.4 | 0 | 0 |
| Sulfonamides | Trimethoprim-Sulfamethoxazole | - | - | 12 | 20 | 48 | 80 |
| Tetracyclines | Doxycycline | 0 | 0 | 0 | 0 | 60 | 100 |
| Macrolides | Erythromycin | - | - | 20 | 33.4 | 40 | 66.6 |
| Glycopeptides | Vancomycin | 59 | 98.3 | 1 | 1.7 | 0 | 0 |
| Chi-square | 424.24 | 218.84 | 260.11 | ||||
| p-value | <0.0001 | <0.0001 | <0.0001 | ||||
Figure 7.
The heat-map clarifies the antimicrobial resistance profile of the obtained B. cereus isolates.
Figure 8.
The heat-map clarifies the correlation coefficient (r) among various tested antibiotics in the present study. The intensity of colors indicates the numerical value of the correlation coefficient (r), Orange, and blue color refers to the negative and positive correlations, respectively.
Dissemination of Virulence and Antibiotic Resistance Genes in B. cereus Isolates
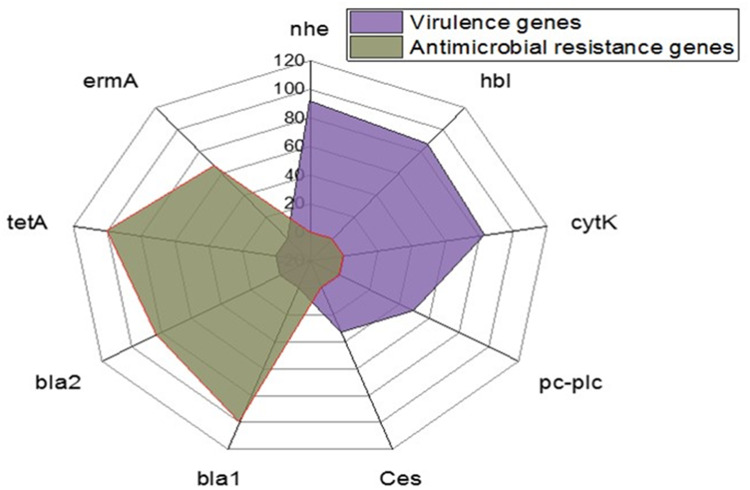
Using PCR, the tested B. cereus isolates commonly harbored the nhe (91.6%), hbl (86.6%), and cytK (83.4%) virulence genes. Moreover, the prevalence of the pc-plc and ces virulence genes was 50% and 33.4%, respectively. Furthermore, the tested isolates harbored the bla1, bla2 (β-lactamase), tetA (tetracycline resistance), and ermA (erythromycin resistance) resistance genes were 100%, 83.3%, 100%, and 66.6%, respectively (Table 3 and Figure 9). The statistical analyses revealed a significant difference (p <0.05) in the existence of virulence genes in the tested B. cereus isolates. However, a non-significant difference (p > 0.05) was noticed in the dissemination of the antibiotic resistance genes among the retrieved B. cereus isolates.
Table 3.
Dissemination of Virulence and Antibiotic Resistance Genes in the Isolated B. cereus (n=60)
| Gene Function | Gene | No. of Positive | % | Chi-Square |
|---|---|---|---|---|
| p-value | ||||
| Virulence genes | nhe | 55 | 91.6 | 23.169 |
| 0.0001171 | ||||
| hbl | 52 | 86.6 | ||
| cytK | 50 | 83.4 | ||
| pc-plc | 30 | 50 | ||
| ces | 20 | 33.4 | ||
| Antibiotic-resistance genes | bla1 | 60 | 100 | 5.2381 |
| 0.1552 | ||||
| bla2 | 50 | 83.3 | ||
| tetA | 60 | 100 | ||
| ermA | 40 | 66.6 |
Figure 9.
The occurrence of virulence and antibiotic resistance genes in the isolated B. cereus strains.
Phenotypic and Genotypic Multidrug-Resistance Profiles in the Recovered B. cereus Isolates
Our findings proved that 40% (24/60) of the recovered B. cereusm isolates are multidrug-resistant (MDR: resistant to one or more antibiotics in three or more different classes) to six antimicrobial classes and carried bla1, bla2, tetA, and ermA genes. Besides, 26.6% (16/60) of the obtained isolates expressed multidrug resistance to seven antimicrobial classes and harbored bla1, bla2, tetA, and ermA genes. In addition, 13.3% (8/60) of the retrieved B. cereus isolates are MDR to five antimicrobial classes and possessed bla1, bla2, and tetA genes, as illustrated in Table 4. The multiple antibiotic resistance (MAR) index values in this study (≥ 0.2) showed multiple resistance patterns indicating that the retrieved isolates were originated from high-risk contamination, as illustrated in Table 4. The correlation coefficient (r) was assessed among the detected resistance genes in B. cereus isolates and various tested antibiotics. The results demonstrated significant positive correlations between: bla1 gene, AMX, PEN, and AMC (r=1); bla2 gene, ctx, and caz (r=1); ermA gene and E (r=1); tetA gene and DOX (r=1); bla1 gene and CAZ (r=0.99); bla1 gene and CTX (r=0.98); bla2 gene, AMX, and PEN (r=0.98); bla2 and AMC (r=0.96) as shown in Figure 10.
Table 4.
The Phenotypic and Genotypic Resistance Profiles of the Isolated B. cereus Strains (n = 60)
| No. of Isolates | % | Type of Resistance | Phenotypic Resistance Profiles | Antibiotic Resistance Genes | MAR Index |
|---|---|---|---|---|---|
| 24 | 40 | MDR |
Penicillins: PEN and AMX β -Lactam- β-lactamase inhibitor combination: AMC Cephalosporins: CTX and CAZ Tetracyclines: DOX Macrolides: E Sulfonamides: SXT |
bla1, bla2, tetA, and ermA | 0.57 |
| 16 | 26.6 | MDR |
Penicillins: PEN and AMX β -Lactam- β-lactamase inhibitor combination: AMC Cephalosporins: CTX and CAZ Tetracyclines: DOX Macrolides: E Sulfonamides: SXT Aminoglycosides: CN and S |
bla1, bla2, tetA, and ermA | 0.71 |
| 8 | 13.3 | MDR |
Penicillins: PEN and AMX β -Lactam- β-lactamase inhibitor combination: AMC Cephalosporins: CTX and CAZ Tetracyclines: DOX Sulfonamides: SXT |
bla1, bla2, and tetA, | 0.50 |
| 7 | 11.6 | MDR |
Penicillins: PEN and AMX β -Lactam- β-lactamase inhibitor combination: AMC Tetracyclines: DOX |
bla1 and tetA, | 0.28 |
| 3 | 5 | Resistant |
Penicillins: PEN and AMX Tetracyclines: DOX |
bla1 and tetA, | 0.21 |
| 2 | 3.33 | MDR |
Penicillins: PEN and AMP Cephalosporins: CTX and CAZ Tetracyclines: DOX |
bla1, bla2, and tetA | 0.35 |
Abbreviations: MDR, multi drug resistant; MAR, multiple antibiotic resistance.
Figure 10.
The heat-map illustrates the correlation coefficient (r) among the antibiotic resistance genes detected in the recovered B. cereus isolates and various tested antibiotics. The intensity of colors indicates the numerical value of the correlation coefficient (r), Orange, and blue color refers to the negative and positive correlations, respectively.
Pathogenicity Test
The cumulative mortality, morbidity, and pathological lesions were regularly observed in the challenged fish for 14 days post-inoculation. Herein, the challenged fish displayed dose-dependent mortalities and characteristic lesions of external hemorrhages, exophthalmia, and ascites, similar to those observed during the natural infection. Surprisingly, none of the control group fish showed any mortalities or pathological lesions. Moreover, the PM examination of the infected fish displayed predictable signs of septicemia manifested by an enlarged liver, congested kidney, and serous fluid exudates in the abdominal cavity.
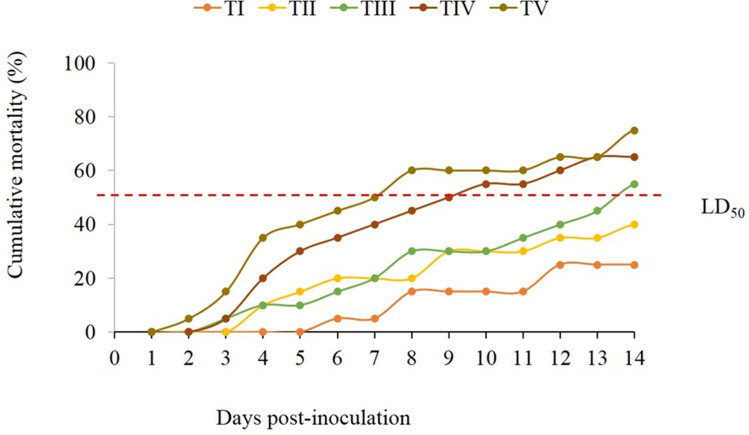
The results also clarified that cumulative deaths were relatively correlated with the inoculated doses and inherited virulence genes, as a higher mortality rate (75%) was recorded in the TV group that received a bacterial dose of 108 CFU mL−1, followed by TIV-TI groups that inoculated by 107–104 CFU mL−1, respectively (Figure 11). The bacterial dose that induces ~50% mortality in the challenged T. zillii (LD50) was 106 CFU mL−1. Bacteriologically, B. cereus was successfully recovered from the pathognomonic lesions of moribund and freshly dead fish. The identification was confirmed based on its biochemical properties and molecular typing.
Figure 11.
Cumulative mortality of Tilapia zillii subjected to intraperitoneal injection with different doses of B. cereus (TI-TV, fish groups received a bacterial dose of 104–108 CFU mL−1, respectively). LD50 is a bacterial dose (106 CFU mL−1) that induces ~50% (exactly 55%) mortality in the challenged fish.
Discussion
Bacillus cereus is a ubiquitous bacterium of public health concern and has recently been categorized as a fish-threatening pathogen, causing huge economic losses in the aquaculture sector.28 In the current study, the results of clinical and post-mortem inspections were congruent with Ali27 who observed deep hemorrhagic skin ulcers, unilateral exophthalmia, tail rot, and congested parenchymatic organs in white seabream (Diplodus sargus) suffered from a mixed infection with Staphylococcus epidermidis and B. cereus. Similar results have been reported in infected stinging catfish,28 and seabass with B. cereus.26 Consistent with the present findings, diffuse necrotizing dermatitis was observed in the naturally infected fish with B. cereus.49
The recorded ante- and post-mortem lesions are a pre-determined consequence of the fish’s immune response to the invading pathogen and may refer to successful colonization and reproduction of the isolated bacteria, in agreement with Aboyadak.26 The variety of lesions also indicates the severity of the current infection and is primarily attributable to the virulence traits encoded by bacteria. B. cereus has been found to be related to the Epizootic Ulcerative Syndrome in Datnioides microlepsis.50 B. cereus possessed several virulence determinants like cytotoxin K, hemolysins, enterotoxins, and phospholipase, which together may lead to disease induction.51 These toxic substances have noxious effects on host tissues through hemagglutination, impaired vascular permeability, apoptosis, and skin necrosis.52
Regarding the bacteriological examination, all recovered isolates displayed the typical phenotypic characteristics of B. cereus, consistent with Osman15 and Gdoura-Ben.53 The isolates also showed harmony in their morphology with identical biochemical profiles. Moreover, the results obtained by the VITEK2 GP and BCL cards were highly comparable and nearly similar to those obtained by conventional biochemical assays. Herein, the VITEK 2 compact system showed a high probability of identifying B. cereus up to 100%. The system was widely acceptable for the detection and distribution of these bacteria and other foodborne pathogens in fish and ready-to-eat products.54
In this study, B. cereus was not isolated from the substantially healthy M. seheli, but the bacteria were recovered from moribund diseased fish with a total prevalence of 25%. Our findings were comparable to those reported by Ali,27 who reported increased mortality (38.33%) in D. sargus broodstock suffered from mixed infection with S. epidermidis and B. cereus. A previous bacteriological study of Chandra28 suggested that B. cereus infection is responsible for 97% of stinging catfish mass mortalities at Burdwan farms in West Bengal, India. B. cereus is occasionally referred to as a pathogen of fish.12 It is a ubiquitous environmental pathogen capable of causing disease in immunocompromised hosts. It is systematically accused of causing severe infection and tissue degeneration in affected fish species.26
Regarding the intensity of B. cereus among different examined organs, the liver was the main affected organ, then the kidney and gills, with no significant difference (p< 0.05). These results are in agreement with those reported by Younes55 who mentioned that hepatopancreas, kidney, and gills were the most predominant infected organs with B. cereus. Differences in prevalence could be attributed to geographic variation, environmental stressors, fish species, and sampling time during the study.
In the current study, the PCR results showed that all the examined isolates harbored a conserved gyrB gene of B. cereus, in agreement with Yamada32 who stated that gyrB was a reliable diagnostic marker and had superior use over the 16S rRNA gene to distinguish B. cereus from other closely related species. Phylogenetic analyses of the amplified amplicon displayed a remarkable genetic identity and cross-lineage to other B. cereus strains from different origins, which may indicate the possible influence of global trade in infection transmission, particularly in the absence of restriction measures for import and export and non-compliance with guidelines on aquaculture advisory services and transport of live fish seeds.56 Herein, the final alignments consisted of 1137 bp, where the sites 1108 and 29 were the most conserved sites and variable sites, respectively. Besides, this sequence was found to contain five open reading frames (ORFs 1–5), which may distinguish these bacteria from other relevant species within the same family.
Concerning the phenotypic assessment of B. cereus virulence factors, 90% of the tested isolates showed different patterns (week-moderate-strong) of biofilm formation, in line with Osman15 who observed the same phenomena in 84.7% of Bacillus species recovered from various meat products. Indeed, most members of the genus Bacillus are firmly attached to the biotic and non-biotic surfaces by forming biofilms.57 Therefore, the reported biofilm activity of the tested isolates indicates their strong association with the host tissue and thus increased disease prevalence and incidence among cultured groups. It also reflects the hazard impact and public health concerns of B. cereus as a foodborne pathogen that may cause severe illness, particularly in people who eat undercooked or raw infected fish.58 Biofilm is most likely to be important because it persists in food processing equipment and protects spores and vegetative fragile cells from inactivation by biocides.59
Surprisingly, all tested isolates of B. cereus showed strong hemolytic activity on sheep blood agar. The present investigation is consistent with the previous findings of Beecher and Wong60 and Hwang and Park.61 Accordingly, the surge in hemolytic activity in this study may be concomitant with some related-virulence genes inherited by the bacteria,62 which still requires further investigation. It was reported that most of the foodborne isolates showed strong hemolytic activity and that most isolates of B. cereus retrieved from different food sources showed hemolysis.63 Likewise, all tested isolates were positive for lecithinase reaction and showed opaque zone around colonies on egg yolk agar, in agreement with Hwang and Park,61 who reported that 26% and 10% of B. cereus isolates retrieved from infant foods showed low and high lecithinase activities, respectively. Lecithinase has been proposed as a presumptive virulence tool in systemic infection with enterotoxin,64 and could often be entangled in hemolysis.65 Bacillus species are known to produce various exoenzymes like hemolysin, gelatinase, lecithinase, enterotoxins, and phospholipase that are responsible for food poisoning and serious human illness.66
Regarding the antibiogram pattern, vancomycin, meropenem, and ciprofloxacin exhibited promising antimicrobial activity against the tested B. cereus isolates. Our results are consistent with those reported by Chandra28 who observed a higher sensitivity of B. cereus isolates to ciprofloxacin. In contrast, the tested B. cereus isolates were resistant to penicillins, sulfonamides, β-Lactam-β-lactamase-inhibitor combination, cephalosporins, nalidixic acid, and macrolides. Nearly similar findings were reported by Savić67 and Mousa.68 The present findings affirm the emergence of MDR isolates of B. cereus in aquaculture sectors and set the fact that the food chain can be a major route of transmission of MDR bacteria between animals and humans.69 The random use of antibiotics to treat emerging diseases has dire consequences in the aquaculture industry, as it may lead to the existence of MDR strains.70–72 Hence, the regimen use of an antibiotic susceptibility testing system is important for selecting specific effective antibiotics and overcoming such problems.22,73
As for the dissemination of virulence genes between B. cereus isolates, there was a significant variation (p< 0.05) in the existence of virulence genes, where the nhe, hbl, and cytK genes were the most prevalent. These results are consistent with those reported by Osman,15 who detected several genes encoding hemolytic (hbl) and non-hemolytic (nhe) enterotoxins and cytotoxin K (cytK) in B. cereus isolates recovered from some poultry and beef meat. The nhe and cytK genes are primarily virulence genes that induce enterotoxins production in B. cereus.74 High prevalence of the nhe and hbl genes complex has been previously reported in B. cereus isolates from environmental and food origins.75 In the current study, all tested isolates of B. cereus contained one or more enteric genes reflecting their public health importance and their potential role in inducing diarrheal disease in humans, in agreement with Smith.76 About 73% of food poisoning strains contained one or more HBL complex genes.77 Interestingly, the nhe gene was the most dominant HBL complex gene inconsistent with previous findings of Tewari.78
Herein, the cytK enterotoxin gene was detected in 83.4% of the recovered B. cereus isolates, in agreement with Ngamwongsatit,79 while others found it in a smaller proportion of their isolates.80 Likewise, the ces virulence gene was determined in 33.4% of B. cereus isolates, which differed greatly from the previous findings of Abdeen81 who detected ces genes in 50% of tested isolates. The ces gene, found on the megaplasmid of B. cereus, is accountable for the production of the cereulide toxin that causes foodborne illness and emesis in humans.13 This toxin is thermostable, active within a wide range of pH (2–11), and abundantly produced during long-term storage of contaminated food.82 Meanwhile, the prevalence of pc-plc gene in this study was 50%, which is quite similar to the results of Abdeen.81 The pc-plc gene is a major virulence determinant for B. cereus toxins which embraces the cytolytic unit cereolysin AB.83 The gene has hemolytic activity and a vital role in toxin production.84 The predominance of both ces and pc-plc genes among the tested isolates emphasizes their virulence and reflects the public health significance of this study.
Concerning the correlation between the genotypic and phenotypic multidrug resistance, most of the retrieved isolates showed multidrug resistance to six or more antimicrobial classes and carried bla1, bla2, tetA, and ermA resistance genes; these results came in harmony with Fiedler19 and Bianco.85 Intriguingly, the MAR index values in this study were ≥ 0.2, alarmingly reflecting that the recovered isolates derived from high-risk origins of contamination. bla1 and bla2 are chromosomal genes of Bacillus species that encoding the penicillinase 2a group and metalloenzyme functional group 3.86 The bla1 possesses penicillinase activity, while bla2 seems to be a cephalosporinase, penicillinase and carbapenem-hydrolysis.87 Herein, both bla1 and bla2 genes are entirely expressed in all isolates, which is nearly similar to those reported by Tahmasebi.88 The resistance of the recovered isolates to amoxicillin-clavulanic acid may be attributed to the synergism of bla1 and bla2 genes. Moreover, several previous studies emphasized the resistance of B. cereus to β-Lactam-β-lactamase-inhibitor combination.19,89,90 In contrast to our findings, Chen91 reported an impairment of bla1 transcription in B. cereus group that harbored bla promoter-lacZ transcriptional fusions and attributed that to the beta-galactosidase activity of the group.
Foreseeably, all recovered strains that harbored tetA and ermA genes showed extensive inhibition regions for tetracycline and erythromycin, proposing a close relationship between tetA resistance gene and phenotypic resistance against tetracycline. The present investigations are congruent with the previous results of Park.37 Overall, antibiotic resistance (AMR) is an inescapable problem worldwide and is closely associated with high mortality and medical crisis.92 AMR is usually caused by the transmission of an antibiotic resistance gene among bacteria, even distantly related ones.93 Since there is no obvious MARindex standard for B. cereus, the assessment of human health risks has become unfeasible due to the newly emergence of MDR isolates of B. cereus in aquaculture sectors. These facts maximize the proper use of antibiotics for aquatic diseases control and reflect the significance of routine antimicrobial susceptibility testing in limiting the spread of MDR isolates.
With respect to the pathogenicity assay, fish infected with B. cereus presented variable mortality in direct proportion to the inoculated doses and displayed the same clinical signs that were found in the naturally infected fish. Our findings have almost resembled a previous study of Younes55 that reported obvious skin lesions, popeyes, abdominal distension, and hemorrhagic patches on the skin and gills of experimentally infected C. gariepinus with B. cereus. Clinical and post-mortem signs observed here were almost similar to the previous findings in white seabream experimentally infected with B. cereus.27 Herein, the mean lethal dose (LD50) for the isolated B. cereus was 106 CFU mL−1, in parallel with a previous study of Younes,55 which emphasized that the LD50 of B. cereus was about 2.7×106 CFU mL−1. Our results were relatively high compared to Chandra28 who demonstrated that a dose of 3.6×105 CFU fish−1 was adjacent to LD50 value and considered suitable for the experimental infection of stinging catfish. Accordingly, affected tissue dissociation could be either related to inherited virulence genes or to the lethal and degenerative effect of bacterial exotoxins and active enzymes, which boost tissue necrosis and cell damage.50,94
In conclusion, as far as we know, this is the first study that highlighted the new emergence of MDR B. cereus in M. seheli that alarmingly reveals a significant threat to both human health and the aquaculture sector. Newly emerging MDR B. cereus in M. seheli commonly harbor nhe, hbl, cytK, and pc-plc virulence genes and bla1, bla2, tetA, and ermA resistance genes. The consistent application of antimicrobial susceptibility testing is essential for screening the newly emerging MDR strains and detecting the antibiotic of choice. Vancomycin and meropenem displayed a hopeful in-vitro antimicrobial activity against the recovered MDR B. cereus isolates from fish. The combination of phenotypic and molecular-based detection techniques is a reliable epidemiological tool for the detection of newly emerging MDR B. cereus strains in fish.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflict of interest.
References
- 1.Abouelmaatti RR, Algammal AM, Li X, Ma J, Abdelnaby EA, Elfeil WM. Experimental immunology cloning and analysis of Nile tilapia Toll-like receptors type-3 mRNA. Central Eur J Immunol. 2013;3(3):277–282. doi: 10.5114/ceji.2013.37740 [DOI] [Google Scholar]
- 2.FAO. STATE of WORLD FISHERIES and AQUACULTURE 2020 (RUSSIAN EDITION): Sustainability in Action. FOOD & AGRICULTURE ORG; 2020. [Google Scholar]
- 3.Boyd C, McNevin A. Aquaculture, Resource Use, and the Environment. John Wiley & Sons; 2015. [Google Scholar]
- 4.Molenaar EJ, Caddell R. International fisheries law: achievements, limitations and challenges. Strengthen Int Fish Law Era Changing Oceans. 2019;3–10. doi: 10.5040/9781509923373 [DOI] [Google Scholar]
- 5.Liu OR, Molina R, Wilson M, Halpern BS. Global opportunities for mariculture development to promote human nutrition. PeerJ. 2018;6:e4733. doi: 10.7717/peerj.4733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saleh M. Capture-based aquaculture of mullets in Egypt. Capture-based aquaculture. Global overview. FAO Fish Tech Paper. 2008;508:109–126. [Google Scholar]
- 7.Eisawy A. Status of aquaculture in the Arab Republic of Egypt. Paper presented at: FAO, Rome (Italy). Fisheries Dept. FAO/CIFA Symposium on Aquaculture in Africa; September 30; 1975; 1975; Accra (Ghana). [Google Scholar]
- 8.Soliman NF. Aquaculture in Egypt Under Changing Climate. Alexandria, Egypt: Alexandria Research Center for Adaptation to Climate Change (ARCA); 2017. [Google Scholar]
- 9.Leung TL, Bates AE, Dulvy N. More rapid and severe disease outbreaks for aquaculture at the tropics: implications for food security. J Appl Ecol. 2013;50(1):215–222. doi: 10.1111/1365-2644.12017 [DOI] [Google Scholar]
- 10.Faruk M, Ali M, Patwary Z. Evaluation of the status of use of chemicals and antibiotics in freshwater aquaculture activities with special emphasis to fish health management. J Bangla Agric Univ. 2008;6(2):381–390. doi: 10.3329/jbau.v6i2.4838 [DOI] [Google Scholar]
- 11.Ovcharenko M. Microparasites of worldwide mullets. Ann Parasitol. 2015;61(4):229–239. doi: 10.17420/ap6104.12 [DOI] [PubMed] [Google Scholar]
- 12.Baya A, Li T, Lupiani B, Hetrick F. Bacillus cereus, a pathogen for striped bass. Paper presented at: Eastern Fish Health and American Fisheries Society Fish Health Section Workshop; 1992. [Google Scholar]
- 13.Stenfors Arnesen LP, Fagerlund A, Granum PE. From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiol Rev. 2008;32(4):579–606. doi: 10.1111/j.1574-6976.2008.00112.x [DOI] [PubMed] [Google Scholar]
- 14.Ramarao N, Tran S-L, Marin M, Vidic J. Advanced methods for detection of Bacillus cereus and its pathogenic factors. Sensors. 2020;20(9):2667. doi: 10.3390/s20092667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osman KM, Kappell AD, Orabi A, et al. Poultry and beef meat as potential seedbeds for antimicrobial resistant enterotoxigenic Bacillus species: a materializing epidemiological and potential severe health hazard. Sci Rep. 2018;8(1):1–15. doi: 10.1038/s41598-018-29932-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bottone EJ. Bacillus cereus, a volatile human pathogen. Clin Microbiol Rev. 2010;23(2):382–398. doi: 10.1128/CMR.00073-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffmaster AR, Hill KK, Gee JE, et al. Characterization of Bacillus cereus isolates associated with fatal pneumonias: strains are closely related to Bacillus anthracis and harbor B. anthracis virulence genes. J Clin Microbiol. 2006;44(9):3352–3360. doi: 10.1128/JCM.00561-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hilliard NJ, Schelonka RL, Waites KB. Bacillus cereus bacteremia in a preterm neonate. J Clin Microbiol. 2003;41(7):3441–3444. doi: 10.1128/JCM.41.7.3441-3444.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fiedler G, Schneider C, Igbinosa EO, et al. Antibiotics resistance and toxin profiles of Bacillus cereus-group isolates from fresh vegetables from German retail markets. BMC Microbiol. 2019;19(1):1–13. doi: 10.1186/s12866-019-1632-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Citron DM, Appleman MD. In vitro activities of daptomycin, ciprofloxacin, and other antimicrobial agents against the cells and spores of clinical isolates of Bacillus species. J Clin Microbiol. 2006;44(10):3814–3818. doi: 10.1128/JCM.00881-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jensen LB, Baloda S, Boye M, Aarestrup FM. Antimicrobial resistance among Pseudomonas spp. and the Bacillus cereus group isolated from Danish agricultural soil. Environ Int. 2001;26(7–8):581–587. doi: 10.1016/S0160-4120(01)00045-9 [DOI] [PubMed] [Google Scholar]
- 22.Algammal AM, Mabrok M, Sivaramasamy E, et al. Emerging MDR-Pseudomonas aeruginosa in fish commonly harbor opr L and tox A virulence genes and bla TEM, bla CTX-M, and tet A antibiotic-resistance genes. Sci Rep. 2020;10(1):1–12. doi: 10.1038/s41598-020-72264-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Algammal AM, Hetta HF, Elkelish A, et al. Methicillin-resistant Staphylococcus aureus (MRSA): one health perspective approach to the bacterium epidemiology, virulence factors, antibiotic-resistance, and zoonotic impact. Infect Drug Resist. 2020;13:3255. doi: 10.2147/IDR.S272733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pychynski T, Malanowska T, Kozlowski M. Bacterial flora in branchionecrosis of carp (particularly Bacillus cereus and Bacillus subtilis). Med Weter. 1981;37(12):742–743. [Google Scholar]
- 25.Yiagnisis M, Athanassopoulou F. Bacteria isolated from diseased wild and farmed marine fish in Greece. Recent Adv Fish Farms. 2011;27(2):61–69. [Google Scholar]
- 26.Aboyadak IM, Sabry NM, Ali NG, El-Sayed HS. Isolation of Staphylococcus epidermidis, Bacillus cereus and Pseudomonas stutzeri from diseased European sea bass (Dicentrarchus labrax) for the first time in Egypt. Egypt J Aquatic Biol Fish. 2016;20(4):103–114. doi: 10.21608/ejabf.2016.11182 [DOI] [Google Scholar]
- 27.Ali NG, Aboyadak IM, El-Sayed HS. Chemotherapeutic control of Gram-positive infection in white sea bream (Diplodus sargus, Linnaeus 1758) broodstock. Vet World. 2019;12(2):316. doi: 10.14202/vetworld.2019.316-324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chandra G, Bhattacharjee I, Chatterjee S. Bacillus cereus infection in stinging catfish, Heteropneustes fossilis (Siluriformes: heteropneustidae) and their recovery by Argemone mexicana seed extract. Iran J Fish Sci. 2015;14(3):741–753. [Google Scholar]
- 29.Schäperclaus W. Fish Diseases. Vol. 1. CRC Press; 1992. [Google Scholar]
- 30.Maturin L, Peeler JT. Bacteriological Analytical Manual. US Food and Drug Administration; 2001. [Google Scholar]
- 31.Navas M, Pincus DH, Wilkey K, et al. Identification of aerobic Gram-positive bacilli by use of Vitek MS. J Clin Microbiol. 2014;52(4):1274–1277. doi: 10.1128/JCM.03483-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamada S, Ohashi E, Agata N, Venkateswaran K. Cloning and nucleotide sequence analysis of gyrB of Bacillus cereus, B. thuringiensis, B. mycoides, and B. anthracis and their application to the detection of B. cereus in rice. Appl Environ Microbiol. 1999;65(4):1483–1490. doi: 10.1128/AEM.65.4.1483-1490.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30(12):2725–2729. doi: 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osman KM, Samir A, Abo-Shama UH, Mohamed EH, Orabi A, Zolnikov T. Determination of virulence and antibiotic resistance pattern of biofilm producing Listeria species isolated from retail raw milk. BMC Microbiol. 2016;16(1):1–13. doi: 10.1186/s12866-016-0880-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wiwat C, Thiramanas R. Detection of hemolysin BL gene of Bacillus cereus isolates. Mahidol Univ J Pharm Sci. 2014;41:22–30. [Google Scholar]
- 36.Quinn P, Markey BK, Carter M, Donnelly W, Leonard F. Veterinary microbiology and microbial disease. Science. 2002;295:1892–1895. doi: 10.1126/science.1068732 [DOI] [PubMed] [Google Scholar]
- 37.Park Y-B, Kim J-B, Shin S-W, et al. Prevalence, genetic diversity, and antibiotic susceptibility of Bacillus cereus strains isolated from rice and cereals collected in Korea. J Food Prot. 2009;72(3):612–617. doi: 10.4315/0362-028X-72.3.612 [DOI] [PubMed] [Google Scholar]
- 38.CLSI. Bacillus. In: Clinical and Laboratory Standards Institute: Performance Standards for Antimicrobial Susceptibility Testing: 27th Ed 424 Informational Supplement. CLSI; 2017. CLSI Doc. M100-S20 (2017). [Google Scholar]
- 39.Magiorakos A-P, Srinivasan A, Carey R, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi: 10.1111/j.1469-0691.2011.03570.x [DOI] [PubMed] [Google Scholar]
- 40.Krumperman PH. Multiple antibiotic resistance indexing of Escherichia coli to identify high-risk sources of fecal contamination of foods. Appl Environ Microbiol. 1983;46(1):165–170. doi: 10.1128/aem.46.1.165-170.1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ehling-Schulz M, Guinebretiere M-H, Monthán A, Berge O, Fricker M, Svensson B. Toxin gene profiling of enterotoxic and emetic Bacillus cereus. FEMS Microbiol Lett. 2006;260(2):232–240. doi: 10.1111/j.1574-6968.2006.00320.x [DOI] [PubMed] [Google Scholar]
- 42.Schraft H, Griffiths M. Specific oligonucleotide primers for detection of lecithinase-positive Bacillus spp. by PCR. Appl Environ Microbiol. 1995;61(1):98–102. doi: 10.1128/aem.61.1.98-102.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sergeev N, Distler M, Vargas M, Chizhikov V, Herold KE, Rasooly A. Microarray analysis of Bacillus cereus group virulence factors. J Microbiol Methods. 2006;65(3):488–502. doi: 10.1016/j.mimet.2005.09.013 [DOI] [PubMed] [Google Scholar]
- 44.Chen Y, Tenover FC, Koehler TM. β-Lactamase gene expression in a penicillin-resistant Bacillus anthracis strain. Antimicrob Agents Chemother. 2004;48(12):4873–4877. doi: 10.1128/AAC.48.12.4873-4877.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rather MA, Aulakh RS, Gill JPS, Mir AQ, Hassan MN. Detection and sequencing of plasmid encoded tetracycline resistance determinants (tetA and tetB) from food–borne Bacillus cereus isolates. Asian Pac J Trop Med. 2012;5(9):709–712. doi: 10.1016/S1995-7645(12)60111-4 [DOI] [PubMed] [Google Scholar]
- 46.Adimpong DB, Sørensen KI, Thorsen L, et al. Antimicrobial susceptibility of Bacillus strains isolated from primary starters for African traditional bread production and characterization of the bacitracin operon and bacitracin biosynthesis. Appl Environ Microbiol. 2012;78(22):7903–7914. doi: 10.1128/AEM.00730-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meyers TR. Fish Pathology Section Laboratory Manual. Alaska Department of Fish and Game, Commercial Fisheries Division; 2000. [Google Scholar]
- 48.Sutton S. Measurement of microbial cells by optical density. J Validation Technol. 2011;17(1):46–49. [Google Scholar]
- 49.Austin B. Emerging bacterial fish pathogens. Bull Eur Assoc Fish Pathol. 1999;19(6):231–234. [Google Scholar]
- 50.Tongyoo N, Thongsaeng P, Kumphakarm R, Moungmai R. Bacillus cereus and B. pumilus associated with epizootic ulcerative syndrome in Siamese Tiger fish (Datnioides microlepsis). Paper presented at: 31st Congress on Science and Technology of Thailand at Suranaree University of Technology; 2005. [Google Scholar]
- 51.Visiello R, Colombo S, Carretto E. Bacillus cereus hemolysins and other virulence factors. In: The Diverse Faces of Bacillus Cereus. Elsevier; 2016:35–44. [Google Scholar]
- 52.Kim M-J, Han J-K, Park J-S, et al. Various enterotoxin and other virulence factor genes widespread among Bacillus cereus and Bacillus thuringiensis strains. J Microbiol Biotechnol. 2015;25(6):872–879. doi: 10.4014/jmb.1502.02003 [DOI] [PubMed] [Google Scholar]
- 53.Gdoura-Ben Amor M, Siala M, Zayani M, et al. Isolation, identification, prevalence, and genetic diversity of Bacillus cereus group bacteria from different foodstuffs in Tunisia. Front Microbiol. 2018;9:447. doi: 10.3389/fmicb.2018.00447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mursyidah R, Zulfa A, Laith A. Isolation and identification bacillus bacteria in tilapia (Oreochromis niloticus) using the Vitek-2 compact. Paper presented at: IOP Conference Series: Earth and Environmental Science; 2021. [Google Scholar]
- 55.Younes AM, Gaafar AY, Alaa Eldeen Z, et al. Isolation and pathogenicity determination of Bacillus cereus associated with ulcer formation in African catfish Clarias gariepinus. Asian J Anim Sci. 2011;15:12–18. [Google Scholar]
- 56.Ababouch L. Assuring fish safety and quality in international fish trade. Mar Pollut Bull. 2006;53(10–12):561–568. doi: 10.1016/j.marpolbul.2006.08.011 [DOI] [PubMed] [Google Scholar]
- 57.Majed R, Faille C, Kallassy M, Gohar M. Bacillus cereus biofilms—same, only different. Front Microbiol. 2016;7:1054. doi: 10.3389/fmicb.2016.01054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cihan AC, Karaca B, Ozel BP, Kilic T. Determination of the biofilm production capacities and characteristics of members belonging to Bacillaceae family. World J Microbiol Biotechnol. 2017;33(6):118. doi: 10.1007/s11274-017-2271-0 [DOI] [PubMed] [Google Scholar]
- 59.Wijman JG, de Leeuw PP, Moezelaar R, Zwietering MH, Abee T. Air-liquid interface biofilms of Bacillus cereus: formation, sporulation, and dispersion. Appl Environ Microbiol. 2007;73(5):1481–1488. doi: 10.1128/AEM.01781-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beecher DJ, Wong A. Identification of hemolysin BL-producing Bacillus cereus isolates by a discontinuous hemolytic pattern in blood agar. Appl Environ Microbiol. 1994;60(5):1646–1651. doi: 10.1128/aem.60.5.1646-1651.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hwang J-Y, Park J-H. Characteristics of enterotoxin distribution, hemolysis, lecithinase, and starch hydrolysis of Bacillus cereus isolated from infant formulas and ready-to-eat foods. J Dairy Sci. 2015;98(3):1652–1660. doi: 10.3168/jds.2014-9042 [DOI] [PubMed] [Google Scholar]
- 62.Prüß BM, Dietrich R, Nibler B, Märtlbauer E, Scherer S. The hemolytic enterotoxin HBL is broadly distributed among species of the Bacillus cereus group. Appl Environ Microbiol. 1999;65(12):5436–5442. doi: 10.1128/AEM.65.12.5436-5442.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pirhonen T, Andersson M, Jääskeläinen EL, Salkinoja-Salonen M, Honkanen-Buzalski T, Johansson T-L. Biochemical and toxic diversity of Bacillus cereus in a pasta and meat dish associated with a food-poisoning case. Food Microbiol. 2005;22(1):87–91. doi: 10.1016/j.fm.2004.04.002 [DOI] [Google Scholar]
- 64.Drobniewski FA. Bacillus cereus and related species. Clin Microbiol Rev. 1993;6(4):324–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Beecher DJ, Olsen TW, Somers EB, Wong AC. Evidence for contribution of tripartite hemolysin BL, phosphatidylcholine-preferring phospholipase C, and collagenase to virulence of Bacillus cereus endophthalmitis. Infect Immun. 2000;68(9):5269–5276. doi: 10.1128/IAI.68.9.5269-5276.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nguyen AT, Tallent SM. Screening food for Bacillus cereus toxins using whole genome sequencing. Food Microbiol. 2019;78:164–170. doi: 10.1016/j.fm.2018.10.008 [DOI] [PubMed] [Google Scholar]
- 67.Savić D, Miljković-Selimović B, Lepšanović Z, et al. Antimicrobial susceptibility and β-lactamase production in Bacillus cereus isolates from stool of patients, food and environment samples. Vojnosanitetski pregled. 2016;73(10):904–909. doi: 10.2298/VSP150415134S [DOI] [PubMed] [Google Scholar]
- 68.Mousa D, Abd El Tawab A, El Hofy F, Maarouf A. Molecular studies on antibiotic resistant Bacillus cereus isolated from meat products and human in Kaliobia, Egypt. Benha Vet Med J. 2020;38(2):125–130. doi: 10.21608/bvmj.2020.25802.1187 [DOI] [Google Scholar]
- 69.Mathur S, Singh R. Antibiotic resistance in food lactic acid bacteria—a review. Int J Food Microbiol. 2005;105(3):281–295. doi: 10.1016/j.ijfoodmicro.2005.03.008 [DOI] [PubMed] [Google Scholar]
- 70.Algammal AM, Mabrok M, Ezzat M, et al. Prevalence, antimicrobial resistance (AMR) pattern, virulence determinant and AMR genes of emerging multi-drug resistant Edwardsiella tarda in Nile tilapia and African catfish. Aquaculture. 2021;2021:737643. [Google Scholar]
- 71.Enany ME, Algammal AM, Shagar GI, Hanora AM, Elfeil WK, Elshaffy NM. Molecular typing and evaluation of Sidr honey inhibitory effect on virulence genes of MRSA strains isolated from catfish in Egypt. Pak J Pharm Sci. 2018;31(5):1865–1870. [PubMed] [Google Scholar]
- 72.El-Sayed M, Algammal A, Abouel-Atta M, Mabrok M, Emam A. Pathogenicity, genetic typing, and antibiotic sensitivity of Vibrio alginolyticus isolated from Oreochromis niloticus and Tilapia zillii. Rev Med Vet. 2019;170:80–86. [Google Scholar]
- 73.Smith P. The performance of antimicrobial susceptibility testing programmes relevant to aquaculture and aquaculture products. 2019.
- 74.Caro-Astorga J, Pérez-García A, de Vicente A, Romero D. A genomic region involved in the formation of adhesin fibers in Bacillus cereus biofilms. Front Microbiol. 2015;5:745. doi: 10.3389/fmicb.2014.00745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Owusu-Kwarteng J, Wuni A, Akabanda F, Tano-Debrah K, Jespersen L. Prevalence, virulence factor genes and antibiotic resistance of Bacillus cereus sensu lato isolated from dairy farms and traditional dairy products. BMC Microbiol. 2017;17(1):1–8. doi: 10.1186/s12866-017-0975-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smith D, Berrang M, Feldner P, Phillips R, Meinersmann R. Detection of Bacillus cereus on selected retail chicken products. J Food Prot. 2004;67(8):1770–1773. doi: 10.4315/0362-028X-67.8.1770 [DOI] [PubMed] [Google Scholar]
- 77.Guinebretière M-H, Broussolle V, Nguyen-The C. Enterotoxigenic profiles of food-poisoning and food-borne Bacillus cereus strains. J Clin Microbiol. 2002;40(8):3053–3056. doi: 10.1128/JCM.40.8.3053-3056.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tewari A, Singh S, Singh R. Incidence and enterotoxigenic profile of Bacillus cereus in meat and meat products of Uttarakhand, India. J Food Sci Technol. 2015;52(3):1796–1801. doi: 10.1007/s13197-013-1162-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ngamwongsatit P, Buasri W, Pianariyanon P, et al. Broad distribution of enterotoxin genes (hblCDA, nheABC, cytK, and entFM) among Bacillus thuringiensis and Bacillus cereus as shown by novel primers. Int J Food Microbiol. 2008;121(3):352–356. doi: 10.1016/j.ijfoodmicro.2007.11.013 [DOI] [PubMed] [Google Scholar]
- 80.Rather M, Aulakh R, Gill J, Ghatak S. Enterotoxin gene profile and antibiogram of Bacillus cereus strains isolated from raw meats and meat products. J Food Saf. 2012;32(1):22–28. doi: 10.1111/j.1745-4565.2011.00340.x [DOI] [Google Scholar]
- 81.Abdeen E, Hussien H, Hadad GAE, Mousa WS. Prevalence of virulence determinants among Bacillus cereus isolated from milk products with potential public health concern. Pak J Biol Sci. 2020;23(3):206–212. doi: 10.3923/pjbs.2020.206.212 [DOI] [PubMed] [Google Scholar]
- 82.Bhunia AK. Bacillus cereus and Bacillus anthracis. In: Foodborne Microbial Pathogens. Springer; 2018:193–207. [Google Scholar]
- 83.Gilmore MS, Cruz-Rodz AL, Leimeister-Wächter M, Kreft J, Goebel W. A Bacillus cereus cytolytic determinant, cereolysin AB, which comprises the phospholipase C and sphingomyelinase genes: nucleotide sequence and genetic linkage. J Bacteriol. 1989;171(2):744–753. doi: 10.1128/jb.171.2.744-753.1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pomerantsev A, Kalnin K, Osorio M, Leppla S. Phosphatidylcholine-specific phospholipase C and sphingomyelinase activities in bacteria of the Bacillus cereus group. Infect Immun. 2003;71(11):6591–6606. doi: 10.1128/IAI.71.11.6591-6606.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bianco A, Capozzi L, Monno MR, et al. Characterization of Bacillus cereus group isolates from human bacteremia by whole-genome sequencing. Front Microbiol. 2020;11. doi: 10.3389/fmicb.2020.599524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Materon IC, Queenan AM, Koehler TM, Bush K, Palzkill T. Biochemical characterization of β-lactamases Bla1 and Bla2 from Bacillus anthracis. Antimicrob Agents Chemother. 2003;47(6):2040–2042. doi: 10.1128/AAC.47.6.2040-2042.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fenselau C, Havey C, Teerakulkittipong N, Swatkoski S, Laine O, Edwards N. Identification of β-lactamase in antibiotic-resistant Bacillus cereus spores. Appl Environ Microbiol. 2008;74(3):904–906. doi: 10.1128/AEM.00788-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tahmasebi H, Talebi R, Zarif B. Isolated of Bacillus cereus in chicken meat and investigation β-lactamase antibiotic-resistant in Bacillus cereus from chicken meat. Adv Life Sci. 2014;4(4):200–206. [Google Scholar]
- 89.Sornchuer P, Saninjuk K, Prathaphan P, Tiengtip R, Wattanaphansak S. Antimicrobial susceptibility profile and whole-genome analysis of a strong biofilm-forming Bacillus Sp. B87 strain isolated from food. Microorganisms. 2022;10(2):252. doi: 10.3390/microorganisms10020252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sornchuer P, Tiengtip R. Prevalence, virulence genes, and antimicrobial resistance of Bacillus cereus isolated from foodstuffs in Pathum Thani Province, Thailand. Pharma Sci Asia. 2021;48(2):194–203. doi: 10.29090/psa.2021.02.19.119 [DOI] [Google Scholar]
- 91.Chen Y, Succi J, Tenover FC, Koehler TM. β-lactamase genes of the penicillin-susceptible Bacillus anthracis Sterne strain. J Bacteriol. 2003;185(3):823–830. doi: 10.1128/JB.185.3.823-830.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Carruth L, Roess AA, Terefe Y, Hosh FM, Salman M. Antimicrobial resistance and food safety in Africa. Lancet Infect Dis. 2017;17:575–576. doi: 10.1016/S1473-3099(17)30273-6 [DOI] [PubMed] [Google Scholar]
- 93.Lerminiaux NA, Cameron AD. Horizontal transfer of antibiotic resistance genes in clinical environments. Can J Microbiol. 2019;65(1):34–44. doi: 10.1139/cjm-2018-0275 [DOI] [PubMed] [Google Scholar]
- 94.Özdemir F, Arslan S. Molecular characterization and toxin profiles of Bacillus spp. isolated from retail fish and ground beef. J Food Sci. 2019;84(3):548–556. doi: 10.1111/1750-3841.14445 [DOI] [PubMed] [Google Scholar]