Abstract
The effects of neural stem/progenitor cells (NSPCs) have been extensively evaluated by multiple studies in animal models of Parkinson’s disease (PD), but the therapeutic efficacy was inconsistent. Here, we searched 4 databases (PubMed, Embase, Scopus, and Web of Science) and performed a meta-analysis to estimate the therapeutic effects of unmodified NSPCs on neurological deficits in rodent animal models of PD. Data on study quality score, behavioral outcomes (apomorphine or amphetamine-induced rotation and limb function), histological outcome (densitometry of TH+ staining in the SNpc), and cell therapy-related severe adverse events were extracted for meta-analysis and systematic review. Twenty-one studies with a median quality score of 6 (range from 4 to 9) in 11 were examined. Significant improvement was observed in the overall pooled standardized mean difference (SMD) between animals transplanted with NSPCs and with control medium (1.22 for apomorphine-induced rotation, P < .001; 1.50 for amphetamine-induced rotation, P < .001; 0.86 for limb function, P < .001; and –1.96 for the densitometry of TH+ staining, P < .001). Further subgroup analysis, animal gender, NSPCs source, NSPCs dosage, and pretreatment behavioral assessment were closely correlated with apomorphine-induced rotation and amphetamine-induced rotation. In conclusion, unmodified NSPCs therapy attenuated behavioral deficits and increased dopaminergic neurons in rodent PD models, supporting the consideration of early-stage clinical trial of NSPCs in patients with PD.
Keywords: neural stem/progenitor cells, Parkinson’s disease, cell transplantation, meta-analysis, rodent animals
Graphical Abstract
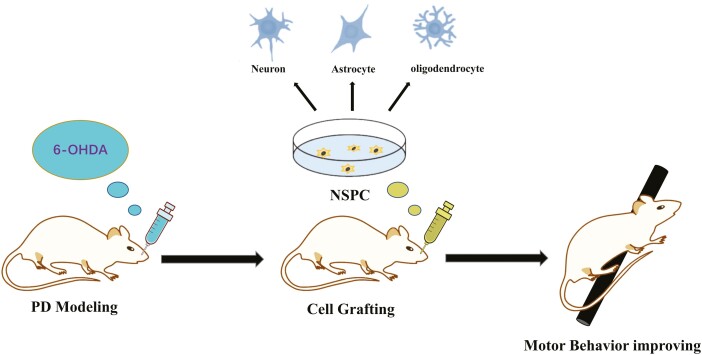
Neural stem progenitor cell (NSPC) is a cell stage capable of differentiation to the main phenotypes of the central nervous system (neuron and glial cells) in vitro and in vivo. After reviewing the investigations with unmodified NSPCs transplantation in rodent Parkinson’s animals, we systematically suggested a potential treatment effect of improving the behavioral motor deficits in Parkinson’s disease.
Introduction
Parkinson’s disease (PD) is one of the most common chronic neurodegenerative diseases, with a reported standardized incidence rate being 8-18 per 100 000 person-year. In industrialized countries, the prevalence of PD is higher, at 0.3% of the entire population and approximately 1% in people over 60 years of age.1-3 Moreover, approximately 90% of cases are sporadic and have no identifiable genetic cause.4 Loss of dopamine-secreting neurons within the substantia nigra pars compacta (SNpc) and the presence of Lewy bodies are significant pathological findings in PD.5 The clinical manifestations of PD are diverse, including both motor and nonmotor features. At present, therapies are available for many nonmotor symptoms, including cholinesterase inhibitors for Parkinson’s disease dementia (PDD),6 antidepressants,7 and pramipexole8 for depression. However, motor symptoms, such as dysphagia, falls, and postural instability, tend to be treatment-resistant. Therefore, patients usually suffer from these motor disorders, which last for the rest of life and significantly reduce the life quality.5
There were many studies to explore the possibility of cell transplantation over 40 years ago. Among the varied cell types for PD cell therapy, neural stem/progenitor cells (NSPCs) and mesenchymal stem cells (MSCs) were both studied widely.9,10 Several published studies indicated that the functional recovery that occurs with MSCs transplanted therapy in animals is far more likely to cause by secreted biological factors that MSCs produce.11,12 In contrast, NSPCs were recognized as a more appropriate source owing to their capabilities of differentiation to the main phenotypes of the central nervous system in vitro and in vivo. Besides, they are also directly able to provide midbrain dopaminergic neurons, whether it derives from fetal tissue, embryonic stem cells (ESCs), or induced pluripotent stem cells (iPSCs).10 Multiple but inconsistent mechanisms about how NSPCs enhance functional recovery were proposed, such as neuroprotection and immunomodulation, and so forth.13,14 Similarly, the clinical curative benefit is conflicting among studies when the following factors are involved: cell source, state, dose, and treatment administration method.
The behavioral test allows insights into the functional benefits of a treatment. To consider the application of NSPCs in a clinical trial involving patients with PD, we performed a meta-analysis to review the preclinical studies and estimate the treatment effect of NSPCs on neurological deficits in rodent animal models of PD.
Materials and Methods
Search Strategy
Two independent investigators searched for correlative studies about NSPCs transplantation in rodent animal models of PD in 4 databases (PubMed, Embase, Scopus, and Web of Science databases, until September 10, 2021). The search strategy was as follows: ((neural stem cell) or (neural progenitor cell) or (neural precursor cell) or NSPC) and (Parkinson or PD or parkinsonian or PD). The default language for all included studies was English. We also searched the reference lists of eligible studies.
Inclusion and Exclusion Criteria
According to the PICOS-scheme (population, intervention, control, outcome, and study design),15 the studies’ eligibility inclusion criteria were set up as follows: (1) PD model (rodent animals); (2) at least 1 experimental group tested the therapeutic effects of NSPCs; (3) sham-controlled (culture medium, or saline) group was set up; (4) providing adequate data on neurobehavioral function assessment or histological assessment; (5) original research studies; (6) published in English. The exclusion criteria were as follows: (1) studies that only evaluated the effects of transfected or modified NSPCs; (2) studies that only tested undifferentiated ESC, iPSC, or differentiated neuron precursor cells; (3) without precise animal numbers for individual comparison.
Study Selection
After removing duplicates, all published articles were conducted by 2 investigators independently. Irrelevant studies were excluded with the agreement of investigators. All relevant articles were retrieved for a comprehensive review, and the criteria outlined above were used to evaluate the articles. Any controversies or uncertainties were judged by a third investigator and resolved through a 100% consensus and when necessary.
Data Extraction
Two investigators abstracted the following information independently: (1) study characteristics (first author, year of publication); (2) features of the included animals (animal species, age, gender, numbers, PD model); (3) cell characteristics (cell source, administration time and site relative to lesion onset, and cell dosage); (4) follow-up period (administration of immunosuppressive drugs, the longest follow-up period of outcomes after NSPCs administration); (5) therapy-related adverse severe events (tumor/teratoma formation, infection, or death); (6) behavioral outcomes at the final time point recorded (amphetamine-induced rotation, apomorphine-induced rotation, and limb function); (7) histological outcome (densitometry of tyrosine hydroxylase-positive (TH+) staining in the SNpc). Limb function was defined as any test that analyzed forepaw use, such as the cylinder test, step test, and adhesive removal test. Get Data Graph Digitizer (version 2.24) was used to quantify the mean value and standard deviation (SD) or standard error (SE) from figures if only graphs were available. While only the standard error was reported, the standard deviation was converted by standard error with the following formula: , where N represents the size of the group. If research contained multiple experimental groups identified by different cell dosage or delivery sites, these groups would be included as independent studies respectively. Only the longest one was extracted when the outcomes were evaluated at different follow-up periods.
Quality Assessment
Collaborative Approach to Meta-Analysis and Review of Animal Data from Experimental Studies (CAMARADES) checklist16 was used to estimate the methodological quality of all included studies, which consist of the following 11 items17,18: (1) publication in a peer-reviewed journal; (2) control of temperature; (3) random allocation to treatment or control; (4) allocation concealment; (5) blinded assessment of outcome; (6) avoidance of neuroprotective anesthetics (such as Ketamine); (7) animal model (aged, diabetic, or hypertensive); (8) sample size calculation; (9) compliance with animal welfare regulations; (10) statement of conflict of interest; (11) pretreatment behavioral assessment. One point for each item, with a total score of 11 points. Two investigators recorded a sum of the quality scores for each study independently. Any differences or uncertainty were resolved by consensus.
Statistical Analysis
All statistical analyseswere performed with Stata (ver. 12.0, Stata Corp). The therapeutic effect size was calculated as standardized mean difference (SMD) by the random effect model and the statistic of Hedges.19 Overall, an effect size lesser than 0.2 represents a small effect, and an effect size greater than 0.8 is defined as a significant effect. Heterogeneity among studies is examined with Cochran’s Q-statistic test and represented by I2, and it was defined as low (25-50%), moderate (50%-75%), or considerable (>75%).20 A P-value of <.1) was considered statistically significant for heterogeneity.21 The statistical significance of the pooled effect size of all studies was performed by t test. A meta-regression analysis was performed while the heterogeneity was moderate or considerable according to several variables. Subgroup analysis with the following characteristics was used to investigate the possible relations with the neurological outcomes22: (1) Animal gender, (2) NSPCs source species (Allogeneic or Xenogeneic), (3) NSPCs state (pluripotent stem cell derivatives or primary cells), (4) NPSCs dosage (≤1E6, >1E6), (5) Administration time postinjury, (6) Administration site, (7) duration of follow-up period, (8) Design of pretreatment behavioral assessment. The interaction of the effects of different subgroups was tested based on random-effects models. Afterward, the potential publication bias was displayed using funnel plots, with an Egger test performed to evaluate the symmetry of the funnel plots.23,24
Results
Study Selection and Characteristics
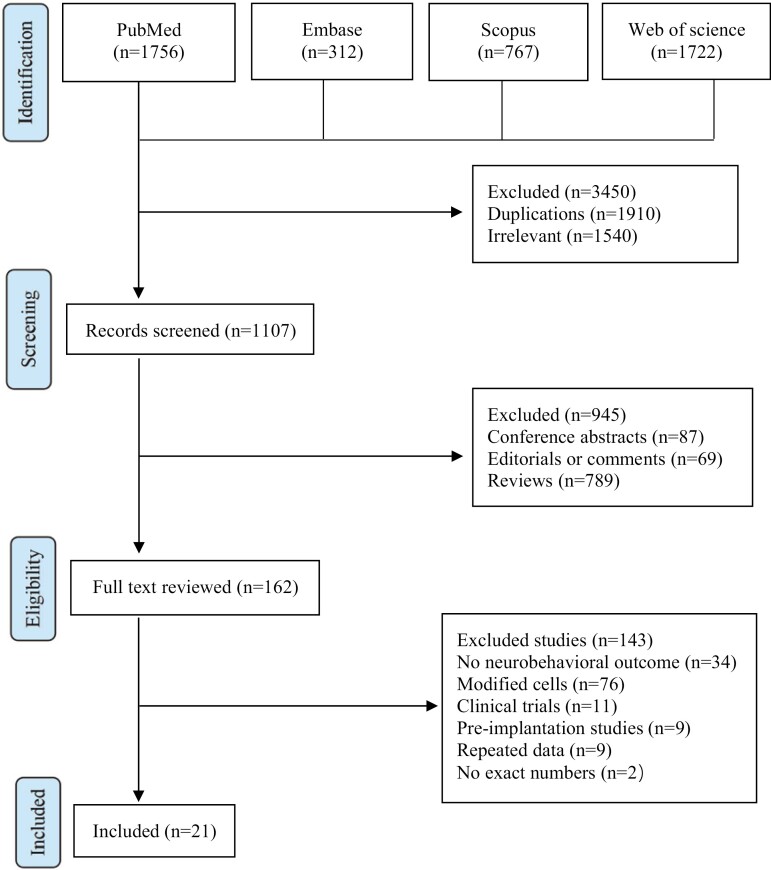
Figure 1 shows the search procedure and strategies. We searched a total of 2647 potential studies from 4 databases. After excluding 1540 irrelevant studies and 945 nonstandard research articles, 162 studies with full text were reviewed. According to the inclusion and exclusion criteria, 143 studies dissatisfying the eligibility criteria were excluded. Finally, a total of 21 studies without duplicate data descriptions were included in this meta-analysis.
Figure 1.
PRISMA flow diagram of included studies for this meta-analysis.
The characteristic proportion of the 21 studies is summarized in Table 1 (see more details for each study in supplementary table 1). All parkinsonian models were from rodents and crated with 6-hydroxydopamine, a widespread PD model. Eleven studies (52.4%) used xenogeneic NPSCs as donors, most derived from humans. Meanwhile, 4 studies (19.0%) used NPSCs derived from pluripotent stem cells (ESC, MSC, or iPSC), while the others used primitive cells. The site of the NPSCs administration in most of the studies (71.4%) was located in the striatum of the brain. Only 6 (28.6%) studies chose other sites or combined with the striatum, such as the substantia nigra. Following 6-OHDA-induced injury, NPSCs were injected over a period varying from 1 to 7 weeks. The dosage of NPSCs ranged from 0.1 to 25 million cells per kilogram, with a median is 1.5 million. The median duration of the follow-up period on neurological assessment was 8 weeks. There were also 12 (57.2%) studies stating the usage of immunosuppression drugs and 9 (42.8%) studies without it.
Table 1.
Characteristic proportion of 21 included studies.
| Characteristics | Summary statistics |
|---|---|
| No. of publications, n (%) | 21 (100%) |
| Animal species, n (%) | |
| Rat | 20 (94.7%) |
| Mouse | 1 (5.3%) |
| Animal gender, n (%) | |
| Male | 12 (57.2%) |
| Female | 7 (33.3%) |
| Unknown | 2 (9.5%) |
| Lesion model, n (%) | |
| 6-OHDA | 21 (100%) |
| NSPCs source specie, n (%) | |
| Allogeneic | 10 (47.6%) |
| Xonogeneic | 11 (52.4%) |
| NSPCs state, n (%) | |
| PC-NSPC | 17 (81.0%) |
| PSC-NSPC | 4 (19.0%) |
| Administration time postinjury, n (%) | |
| ≤2 week | 5 (23.8%) |
| >2 week | 16 (76.2%) |
| Administration site, n (%) | |
| Striatum | 15 (71.4%) |
| Substantia nigra | 6 (28.6%) |
| Cell dosage (cells/kg), median (Q1, Q3) | 1.5E+06 (5.0E+05, 2.5E+06) |
| Follow-up period (weeks), n (%) | |
| <12 weeks | 12 (57.2%) |
| ≥12 weeks | 9 (42.8%) |
| Immunosuppressant, n (%) | |
| No | 9 (42.8%) |
| Yes | 12 (57.2%) |
| Behavioral outcome, n (%) | |
| Amphetamine-induced rotation | 8 (38.1%) |
| Apomorphine-induced rotation | 11 (52.4%) |
| Limb function | 8 (38.1%) |
Abbreviations: 6-OHDA, 6-hydroxydopamine; PC-NSPC, primary cells-neural stem/progenitor cells; PSC-NSPC, pluripotent stem cell-neural stem/progenitor cells; Q1, first quartile; Q3, third quartile.
For any study, if multiple behavioral tests were reported, we considered these different tests as independent experiments within one study. In general, 19 experiments were conducted to evaluate rotational behavior (amphetamine-induced rotation and apomorphine-induced rotation), and 8 experiments evaluated limb function (cylinder test, stepping test) (see Supplementary Table S3). About the histological outcome, the densitometry of TH+ staining in SNpc was assessed in 4 studies. Moreover, the survival rate of the grafted cells was observed in only 2 studies with no more than 5% successful survival rate after 6 weeks post-treatment (data not shown). For severe adverse events related to NSPCs transplantation, tumor formation was reported in 6 studies, and only 2 have reported animal deaths, with the exact number unknown. Considering that rotational behavior is the most common behavioral evaluation used in rodent PD studies, we took it as a primary outcome in this review.
Quality Assessment
The median value of the quality score across all studies was 6, ranging from 3 to 8 (see more details in Supplementary Table S2). The distribution of the quality score meeting with each item is summarized in Table 2. In general, All the articles were published in a peer-reviewed journal, and no study used aged, diabetic, or hypertensive animals. Additionally, most studies claimed compliance with animal welfare regulations (89.5%), temperature control in the whole research (73.3%), as well as the behavioral training process before the treatment (78.9%). Although 11 studies randomly allocated the animals to the treatment or control group, only 4 studies used blinding when assessing the behavioral tests. However, neither the allocation concealment nor the Sample size calculation was performed by any of these studies.
Table 2.
Distribution of the quality score meeting with each CAMARADES item.
| Item | Number of studies | Percentage |
|---|---|---|
| Publication in a peer-reviewed journal | 19 | 100.0% |
| Control of temperature | 14 | 73.7% |
| Random allocation to treatment or control | 11 | 57.9% |
| Allocation concealment | 0 | 0.0% |
| Blinded assessment of outcome | 4 | 21.1% |
| Avoidance of neuroprotective anesthetics | 11 | 57.9% |
| Animal model (without aged, diabetic, or hypertensive) | 19 | 100.0% |
| Sample size calculation | 0 | 0.0% |
| Compliance with animal welfare regulations | 17 | 89.5% |
| Statement of conflict of interest | 12 | 63.2% |
| Pretreatment behavioral assessment | 15 | 78.9% |
Effect Size
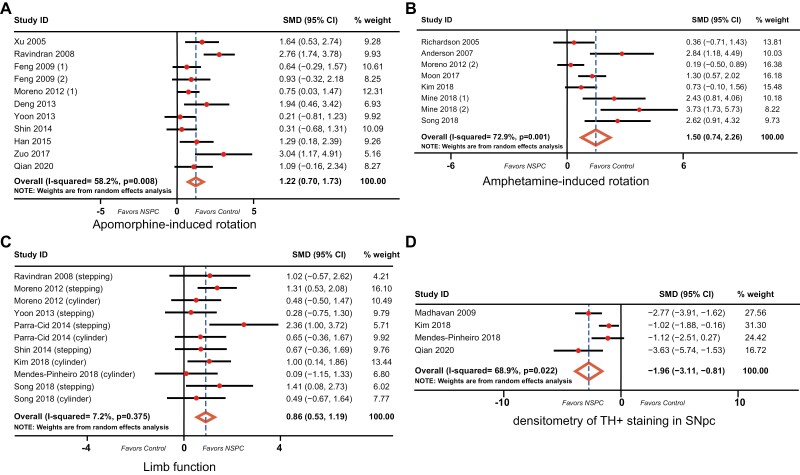
The pooled effect size of NSPCs treatment was estimated based on the random-effects model and the Hedges calculation. Pooling the data of 11 studies that assessed the apomorphine-induced rotation (Fig. 2A) in PD animals showed a significant difference favoring NSPCs treatment (SMD: 1.22, 95% CI: 0.70-1.73, P <.001) with a moderate between-study heterogeneity (χ2 = 23.92, df = 10, P = .008, I2 =58.2%). Additionally, 8 studies assessed amphetamine-induced rotation (Fig. 2B) in 176 animals, and showed a significance difference in behavioral rotation favoring NSPCs group (SMD: 1.50, 95% CI: 0.74-2.26, P < .001) with a considerable between-study heterogeneity (χ2 = 25.85, df = 7, P = .001, I2 = 72.9%). Data on limb function are available in 11 studies. There is also a significant difference between the NSPCs group and control group (SMD: 0.86, 95% CI: 0.53-1.19, P < .001), with a low heterogeneity between studies (χ2 = 10.78, df = 10, P = .375, I2 = 7.2%; Fig. 2C); The densitometry of TH+ staining in SNpc was reported in 4 studies that investigated 71 animals. NSPCs were associated with a higher TH+ density in SNpc than was the control group. The difference is significant (SMD:‒1.96, 95% CI: –3.11 to 0.81, P < .001), with a considerable between-study heterogeneity (χ2 = 9.64, df = 3, P = .022, I2 = 68.9%; Fig. 2D).
Figure 2.
Forest plot of standardized mean difference (SMD) of 4 outcomes between NSPC therapy and control group along with a 95% confidence interval (95% CI). The degree of heterogeneity in the pooled estimates is represented at the I2 statistic. The overall estimate and confidence interval are marked by a diamond. NSPC, neural stem/progenitor cell.
Subgroup Analysis and Meta-Regression Analysis
Following the effect size evaluation and sensitivity analysis, the between-study heterogeneity in apomorphine-induced rotation and amphetamine-induced rotation was still considerable. Therefore, subgroup analysis and meta-regression analysis based on 8 clinically related parameters were performed to investigate their contribution to the significant heterogeneity. Table 3 summarizes the data of apomorphine-induced rotation and amphetamine-induced rotation outcomes, respectively, in diverse subgroups. Generally, the significant therapeutic efficacy of NPSCs treatment was observed in most subgroups. However, some subgroups fail to reach the statistical significance (P < .05), which may be caused by insufficient studies. Additionally, moderate and considerable heterogeneity still could be detected in most subgroups (I2 > 50%). Although there was no significant between-study heterogeneity in several subgroups, we still could not identify the relevant clinical characteristic affecting the heterogeneity. To further investigate the unexplained between-study heterogeneity, univariate meta-regression analysis was used to test the influence of clinical characteristics. For apomorphine-induced rotation, the NPSCs state was the significant resource of the heterogeneity (Adj R2 = 75.90%, P = .058, < 0.01; Table 3). However, the assessment of pretreatment behavior was identified as a significant factor affecting the heterogeneity in amphetamine-induced rotation (R2 = 50.98%, P = .060, < .01; Table 3).
Table 3.
Subgroup analysis and meta-regression of clinical variants correlated with apomorphine-induced rotation and amphetamine-induced rotation.
| Clinical variants | Apomorphine-induced rotation | Amphetamine-induced rotation | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of reports | Pooled estimates (SMD) | 95% conf. Interval | P-value for SMD=0 | I 2 value (%) | P-value for heterogeneity | P-value of interaction | Univariate analysis (Adj R2, P) | No. of reports | Pooled estimates (SMD) | 95% conf. Interval | P-value for SMD=0 | I 2 value (%) | P-value for heterogeneity | P-value of interaction | Univariate analysis (Adj R2, P) | |
| Animal gender | ||||||||||||||||
| Male | 6 | 1.432 | (0.742, 2.121) | <.001 | 53.60% | .056 | .610 | –1.20%, 0.428 | 2 | 2.951 | (1.689, 4.213) | <.001 | 0.00% | .324 | .012 | 47.62%, .102 |
| Female | 3 | 1.083 | (–0.069, 2.226) | .063 | 69.10% | .039 | 6 | 1.104 | (0.389, 1.819) | .002 | 67.50% | .009 | ||||
| NPSCs source species | ||||||||||||||||
| Allogeneic | 5 | 1.148 | (0.629, 1.667) | <.001 | 0.00% | .536 | .810 | –21.00%, 0.957 | 3 | 2.108 | (0.007, 4.210) | .049 | 81.50% | .005 | .459 | –15.76%, .536 |
| Xenogeneic | 6 | 1.274 | (0.402, 2.145) | .004 | 75.90% | .001 | 5 | 1.257 | (0.438, 2.076) | .003 | 71.20% | .008 | ||||
| NPSCs state | ||||||||||||||||
| PC-NSPCs | 8 | 0.874 | (0.398, 1.351) | <.001 | 32.90% | .166 | .043 | 75.90%, 0.058 | 7 | 1.678 | (0.771, 2.603) | <.001 | 76.10% | <.001 | — | –18.08%, .479 |
| PSC-NSPCs | 3 | 1.918 | (1.026, 2.809) | <.001 | 51.60% | .127 | 1 | 0.729 | (–0.103, 1.560) | .086 | NA | NA | ||||
| NPSCs dosage | ||||||||||||||||
| ≤1E6 | 3 | 0.588 | (–0.009, 1.185) | .054 | 0.00% | .743 | .049 | 15.82%, 0.159 | 4 | 0.991 | (0.216, 1.765) | .012 | 66.90% | .028 | .174 | 7.49%, .266 |
| >1E6 | 8 | 1.477 | (0.820, 2.135) | <.001 | 63.30% | .008 | 4 | 2.215 | (0.630, 3.799) | .006 | 76.20% | .006 | ||||
| Administration time | ||||||||||||||||
| ≤2 weeks | 3 | 1.852 | (0.798, 2.907) | .001 | 32.60% | .227 | .187 | 3.63%, 0.233 | 1 | 0.359 | (–0.711, 1.429) | .511 | NA | NA | — | –4.92%, .325 |
| >2 weeks | 8 | 1.047 | (0.476, 1.618) | <.001 | 61.60% | .011 | 7 | 1.703 | (0.845, 2.561) | <.001 | 75.00% | .001 | ||||
| Administration site | ||||||||||||||||
| Striatum | 9 | 1.007 | (0.547, 1.466) | <.001 | 37.40% | .120 | .352 | 34.90%, 0.197 | 7 | 1.325 | (0.562, 2.088) | .001 | 71.60% | .002 | — | 9.05%, .356 |
| Substantia nigra | 2 | 1.884 | (0.094, 3.674) | .039 | 79.70% | .026 | 1 | 2.838 | (1.185, 4.491) | .001 | NA | NA | ||||
| Follow-up period | ||||||||||||||||
| <12 weeks | 5 | 1.25 | (0.312, 2.189) | .009 | 65.50% | .021 | .968 | –20.25%, 0.980 | 4 | 1.573 | (0.297, 2.849) | .016 | 74.70% | .008 | .952 | –26.92%, .982 |
| ≥12 weeks | 7 | 1.227 | (0.579, 1.875) | <.001 | 58.80% | .033 | 4 | 1.523 | (0.387, 2.659) | .009 | 78.50% | .003 | ||||
| Pretreatment behavioral assessment | ||||||||||||||||
| Yes | 8 | 1.439 | (0.745, 2.134) | <.001 | 63.30% | .008 | .119 | 2.96%, 0.276 | 6 | 2.002 | (1.137, 2.868) | <.001 | 63.60% | .017 | <.001 | 50.98%, .060 |
| No | 3 | 0.749 | (0.233, 1.266) | .004 | 0.00% | .439 | 2 | 0.243 | (–0.341, 0.826) | .415 | 0.00% | .800 | ||||
I 2 describes the variation in effect size attributable to heterogeneity. Adj R2 represents the proportion of between-study variance explained.
Abbreviations: APC-NSPC, primary cells-neural stem/progenitor cells; PSC-NSPC, pluripotent stem cell-neural stem/progenitor cells; SMD, standardized mean difference.
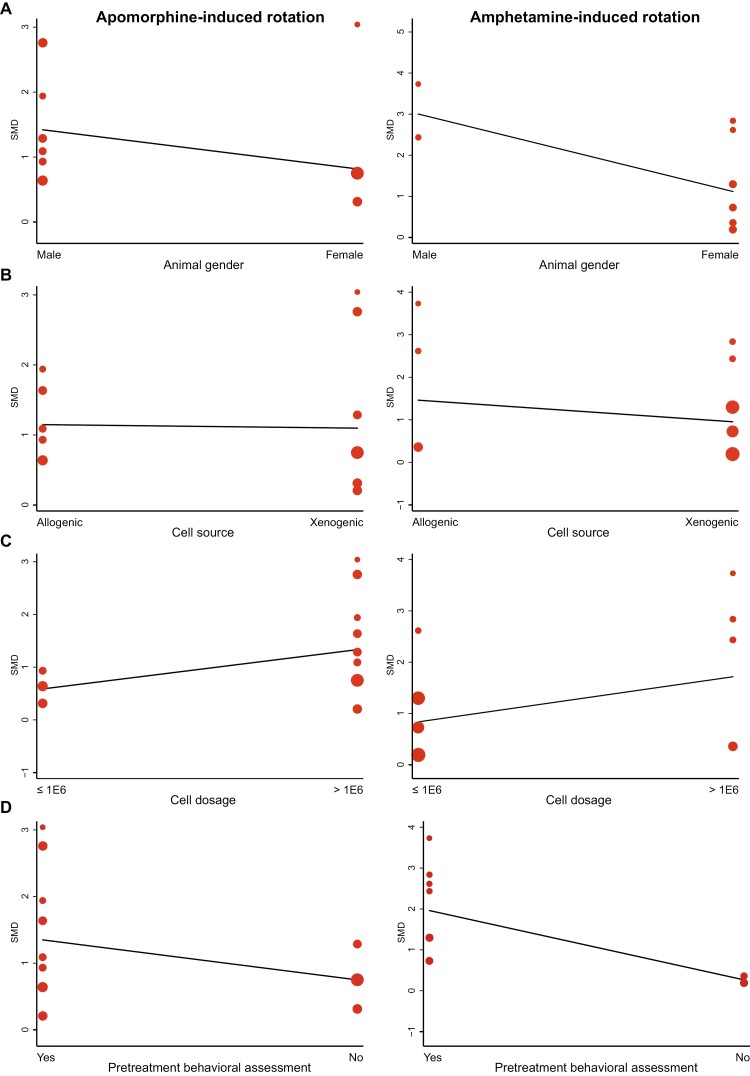
The interaction of diverse subgroups based on each characteristic was calculated with a random effect model. There is no comparative significance when a certain subgroup exists in one study. First of all, animal gender was correlated with the effect size of amphetamine-induced rotation (P = .012) but not on apomorphine-induced rotation (P = .610; Fig. 4A). NSPCs in male animals showed a greater efficacy. Allogenic and xenogeneic cells showed similar beneficial efficacy, although the former increased the amphetamine-induced rotation to a more considerable extent (Fig. 4B). But there was no significance (P = .459). All included studies did not provide a specific dosage of NSPCs. After standardized to animal weight, a more significant effect size with a higher dosage level (> 1 × 106 cells/kg) on both apomorphine (P = .049) and amphetamine-induced rotation (P = .174) was observed (Fig. 4C). No significant correlation was found between administration time, administration site, and follow-up period. At last, the behavioral training before treatment was more beneficial to improve the effect size of both apomorphine (P = .019) and amphetamine-induced rotation (P < .001; Fig. 4D). Overall, since there were so many uncontrollable variables between different studies, the above various subgroup analysis can only generate hypotheses without confirming.
Figure 4.
Meta-regression analysis for related variables and effect sizes of apomorphine-induced rotation and amphetamine-induced rotation. Individual study results are represented by dots, with the y-axis signifying the effect size. The size of the dot is determined by its weight. (A) Animal gender; (B) NPS cells source; (C) NPS cell dosage (cells/kg); (D) pretreatment behavioral assessment.
Publication Bias
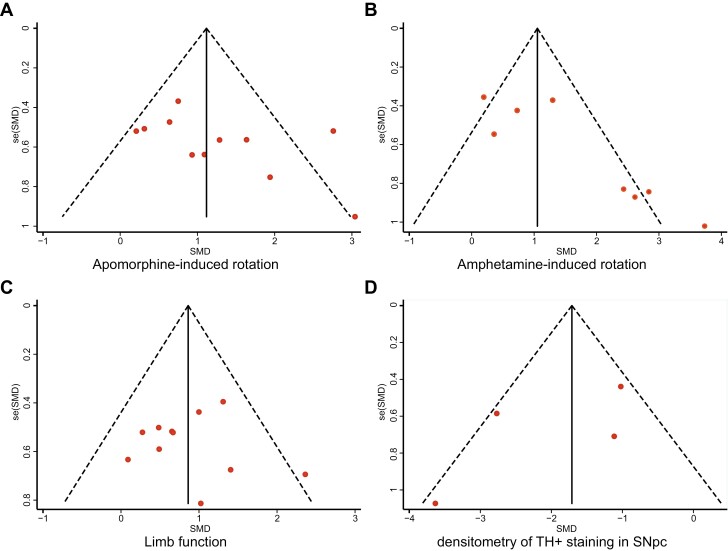
Figure 3 showes the funnel plots for the publication bias of apomorphine-induced rotation, amphetamine-induced rotation, limb function, and the densitometry of TH+ staining. No obvious publication bias was observed by visual observation. However, Egger’s test presented significant publication bias for amphetamine-induced rotation (P = .010). There was no publication bias detected for apomorphine-induced rotation (P = .117), limb function (P = .876), densitometry of TH+ staining (P = .350) by Egger’s test.
Figure 3.
Funnel plot for apomorphine-induced rotation (A), amphetamine-induced rotation (B), limb function (C), and densitometry of TH+ staining (D). Individual study results are represented by dots, with the y-axis signifying study quality and the x-axis showing the study results. The solid vertical line represents the pooled effect size. The dashed diagonal lines represent pseudo-95% confidence limits around the pooled effect size for each standard error (SE) vertical axis. SMD, standardized mean difference.
Discussion
This meta-analysis revealed that treatment with NSPCs significantly improves neurological outcomes in rodent animal PD models. NSPCs treatment showed a significant effect size in either the behavioral deficit or pathology loss since the behavioral indicators could intuitively show the loss and recovery of motor function in animal PD models. At the same time, the densitometry of TH+ staining is publicly recognized as a dopaminergic neuron quantified approach. The extent of the 6-OHDA (an oxidative catecholaminergic toxin) lesion could easily be quantified according to the extent of apomorphine- or amphetamine-induced rotation,25-27 and this approach was widely used to evaluate the functional motor effects of dopaminergic neuron loss in the striatum. Recently, increasing evidence has suggested that limb function, another good indicator of nigrostriatal dopamine depletion, can also provide valuable clinically relevant data.28,29
Until now, studies about cell-based dopamine replacement strategies were initiated to explore the possibility of dopaminergic cell transplantation over 40 years ago. The existence of NSPCs has been known in developing or adult mammalian central nervous system (CNS) tissues, including humans.30-32 Fetal ventral mesencephalic (FVM) tissue grafting was performed to treat PD in the late 1970s.33,34 Although improvement was observed in multiple studies, including clinical trials,35-37 the results were not impressive enough. Moreover, there was growing concern about whether these foreign grafts are effective enough and whether they will cause further damage. Few studies observed that some patients developed severe graft-induced dyskinetic side effects postoperatively.38,39 Recently, ESCs and iPSCs have been widely researched as the most promising cell types because of their multi-directional differentiation potential.40,41 While transplantation of early-stage (undifferentiated) ESCs or iPSCs resulted in the spontaneous development of functional mDA neurons in vivo and significantly restored behavior in parkinsonian rats, it could cause inefficiency, inconsistent and incomplete mDA differentiation, and occasional teratoma formation.41-45 Furthermore, another critical question is whether those gene mutations negatively affect the survival and expansion of ESCs and iPSCs.46,47 To date, the origin of the epigenetic variability and its influence on the differentiation properties of iPSC lines remain controversial.47 In contrast, implantation of later stage, purified mDA neurons eliminated the formation of teratomas, but the transplanted neurons survived very poorly.42-44 Therefore, many investigators have turned to NSPCs, a “middle stage” that has the capacity for self-renewal and multipotent potential to become neurons or glial cells, which may represent a more optimal cell source than fully differentiated cells.43,44,48
As for the possible mechanism of NSPCs therapy on neuroprotective effect, it appears that multiple mechanisms may contribute, although it is still being elucidated. Unlike MSC therapy, NSPC treatment aims to specify neuron replacement, release specific neurotransmitters, and produce factors that promote neuronal growth and regeneration.49 In PD models, transplantation of NSPCs can replace dead and dying dopaminergic neurons due to the relatively focal nature of neuron loss that occurs. And transplanted-NSPCs have also been shown to establish synaptic connections with host neurons and release neurotransmitters or secretions. In addition, NSPCs can express a wide range of neurotrophic factors, such as BDNF, GDNF, and insulin-like growth factor 1 (IGF1), which demonstrate critical roles in the growth and stabilization of dendritic spines, synaptic plasticity, long-term potentiation, survival of neurons and glia, and therefore unsurprisingly motor performance.50-52
Although the effect size may be promising, several limitations still existed in our meta-analysis. First, with only 8 studies reported whether there were teratoma formation or animal death events and no detailed safety test on animals, it is inadequate to evaluate the clinical safety of NPSCs injection in animal PD models. Besides, our approach only covered those studies published in English. Unpublished data may change our results. Moreover, only 4 studies involved the test of the densitometry of TH+ staining in SNpc, indicating that more caution is needed in evaluating NSPCs therapy’s effect on rescuing dopaminergic neuron loss.
Although a considerable mean of the quality score (6.2) across all the studies in this meta-analysis was observed, some points are still worth paying attention to. Most low-quality studies (scoring less than 6.2) were published before 2012. These animal studies are commonly less rigorously designed, which may overestimate treatment effects and potentially influence results. A recognized and extensively applied CAMARADES list was used to evaluate the study quality. We believe that the items on this score list have an essential bearing on study quality. Although some items might be more important than others, and some may have been omitted, it is difficult to identify different items’ weight. The development of a more sophisticated quality score is a curial area for further research. Some items such as publication in a peer-reviewed journal, control of the temperature, randomization, blinded assessment of outcome, compliance with animal welfare regulations, and the statement of conflicts are widely accepted. For the rest, we believed that it is curial to induce blinded to allocation to prevent a bias in the severity of the induced injury. Besides, it is recognized that some anesthetics have much higher intrinsic neuroprotective activity, and their use is relevant to the study quality. Although sample size calculations are uncommon in animal studies, a good study should have an adequate sample size with a formal calculation.53 However, no studies in our meta-analysis performed sample size calculation, which suggested the lack of statistical power to ensure proper estimation of the treatment effects.54 Besides, the pretreatment behavioral training process could prevent a bias of the learning skill within the individuals. Their motor abilities would be utterly different without pretraining.
Moreover, the research models across all studies in this meta-analysis are based exclusively on healthy adult animals. However, the outcomes from rodent models cannot directly extend to humans, and their similarities to humans are limited. In addition, as PD is an age-related disease, a direct proportion between age and the prevalence of PD was stated.55 Moreover, clinically, the majority of PD patients are elderly. Therefore, it is necessary to consider the impact of age in preclinical studies, since the response to therapy may differ extensively in the developing, adult, and elderly brains. It is uncertain that cell therapy may not be able to obtain the same treatment effect in an elderly Parkinson’s animal model.
Another critical limitation in using a meta-analysis to assess potential therapeutic effects is publication bias. Although there is no significant publication bias for apomorphine-induced rotation, limb function, and the densitometry of TH+ staining, significant publication bias is existed in amphetamine-induced rotation, indicating that positive studies related to this behavioral test are more likely to be published compared with negative studies. Furthermore, motor tests in PD animal models do not adequately reflect all aspects of the disease’s neurological abnormalities. As a result of these limitations, our findings must be regarded with caution. Our findings should be validated in more strictly randomized control experiments and carefully interpreted in terms of the design of further animal investigations or clinical translation in the future, given the poor internal and external validity.
Conclusion
This meta-analysis suggested a potential treatment effect of NSPCs over a wide range of doses, improving the behavioral function deficits in rodent animal models of PD, supporting the consideration of early-stage clinical trials of NSPCs in patients with PD.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the financial support of the National Natural Science Foundation of China (NSFC) (81571242 and 81901895) and the China Scholarship Council (CSC).
Contributor Information
Yifeng Zheng, Department of Neurosurgery, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China.
Jun Zhou, Department of Neurosurgery, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China.
Yisai Wang, Department of Electrical and Computer Engineering, Rutgers University-New Brunswick, New Jersey, USA.
Fanfan Fan, Department of Neurosurgery, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China.
Shengwen Liu, Department of Neurosurgery, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China.
Yu Wang, Department of Neurosurgery, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China.
Conflict of Interest
The authors indicated no financial relationships.
Author Contributions
Y.Z.: conception and design, collection or assembly of the data, writing and final approval of the manuscript. J.Z.: collection or assembly of the data, data analysis and interpretation, and final approval of the manuscript. Y.W.: data calculation, analysis, and final approval of the manuscript. F.F.: collection or assembly of the data and final approval of the manuscript. S.L:. administrative support and final approval of the manuscript. Y.W.: conception and design, financial support, data analysis and interpretation, and final approval of the manuscript
Data Availability
All data generated or analyzed during this study are included in this article.
References
- 1. Ascherio A, Schwarzschild MA. The epidemiology of Parkinson’s disease: risk factors and prevention. Lancet Neurol 2016;15(12):1257-1272. [DOI] [PubMed] [Google Scholar]
- 2. de Lau LM, Breteler MM. Epidemiology of Parkinson’s disease. Lancet Neurol 2006;5(6):525-535. [DOI] [PubMed] [Google Scholar]
- 3. Nussbaum RL, Ellis CE. Alzheimer’s disease and Parkinson’s disease. N Engl J Med 2003;348(14):1356-1364. [DOI] [PubMed] [Google Scholar]
- 4. Klein C, Westenberger A. Genetics of Parkinson’s disease. Cold Spring Harb Perspect Med 2012;2(1):a008888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Connolly BS, Lang AE. Pharmacological treatment of Parkinson disease: a review. JAMA 2014;311(16):1670-1683. [DOI] [PubMed] [Google Scholar]
- 6. Mancini M, Chung K, Zajack A, et al. Effects of augmenting cholinergic neurotransmission on balance in Parkinson’s disease. Parkinsonism Relat Disord 2019;69:40-47. [DOI] [PubMed] [Google Scholar]
- 7. Kawada T. Antidepressants and Parkinson’s disease: a causal association. J Neurol Sci 2020;408:116512. [DOI] [PubMed] [Google Scholar]
- 8. Shen T, Ye R, Zhang B. Efficacy and safety of pramipexole extended-release in Parkinson’s disease: a review based on meta-analysis of randomized controlled trials. Eur J Neurol 2017;24(6):835-843. [DOI] [PubMed] [Google Scholar]
- 9. Sonntag KC, Song B, Lee N, et al. Pluripotent stem cell-based therapy for Parkinson’s disease: Current status and future prospects. Prog Neurobiol 2018;168:1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barker RA, Drouin-Ouellet J, Parmar M. Cell-based therapies for Parkinson disease—past insights and future potential. Nat Rev Neurol 2015;11(9):492-503. [DOI] [PubMed] [Google Scholar]
- 11. Riecke J, Johns KM, Cai C, et al. A meta-analysis of mesenchymal stem cells in animal models of Parkinson’s disease. Stem Cells Dev 2015;24(18):2082-2090. [DOI] [PubMed] [Google Scholar]
- 12. Teixeira FG, Carvalho MM, Sousa N, Salgado AJ. Mesenchymal stem cells secretome: a new paradigm for central nervous system regeneration? Cell Mol Life Sci 2013;70(20):3871-3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meyer AK, Maisel M, Hermann A, Stirl K, Storch A. Restorative approaches in Parkinson’s Disease: which cell type wins the race? J Neurol Sci 2010;289(1-2):93-103. [DOI] [PubMed] [Google Scholar]
- 14. Chung S, Moon JI, Leung A, et al. ES cell-derived renewable and functional midbrain dopaminergic progenitors. Proc Natl Acad Sci USA 2011;108(23):9703-9708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Riva JJ, Malik KM, Burnie SJ, Endicott AR, Busse JW. What is your research question? An introduction to the PICOT format for clinicians. J Can Chiropr Assoc 2012;56(3):167-171. [PMC free article] [PubMed] [Google Scholar]
- 16. Macleod MR, O’Collins T, Howells DW, Donnan GA. Pooling of animal experimental data reveals influence of study design and publication bias. Stroke 2004;35(5):1203-1208. [DOI] [PubMed] [Google Scholar]
- 17. Minnerup J, Heidrich J, Wellmann J, Rogalewski A, Schneider A, Schäbitz WR. Meta-analysis of the efficacy of granulocyte-colony stimulating factor in animal models of focal cerebral ischemia. Stroke 2008;39(6):1855-1861. [DOI] [PubMed] [Google Scholar]
- 18. Schmidt A, Wellmann J, Schilling M, et al. Meta-analysis of the efficacy of different training strategies in animal models of ischemic stroke. Stroke 2014;45(1):239-247. [DOI] [PubMed] [Google Scholar]
- 19. Durlak JA. How to select, calculate, and interpret effect sizes. J Pediatr Psychol 2009;34(9):917-928. [DOI] [PubMed] [Google Scholar]
- 20. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ioannidis JP, Patsopoulos NA, Evangelou E. Heterogeneity in meta-analyses of genome-wide association investigations. PLoS One 2007;2(9):e841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21(11):1539-1558. [DOI] [PubMed] [Google Scholar]
- 23. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sterne JA, Egger M, Smith GD. Systematic reviews in health care: Investigating and dealing with publication and other biases in meta-analysis. BMJ 2001;323(7304):101-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hudson JL, van Horne CG, Strömberg I, et al. Correlation of apomorphine- and amphetamine-induced turning with nigrostriatal dopamine content in unilateral 6-hydroxydopamine lesioned rats. Brain Res 1993;626(1-2):167-174. [DOI] [PubMed] [Google Scholar]
- 26. Ungerstedt U, Arbuthnott GW. Quantitative recording of rotational behavior in rats after 6-hydroxy-dopamine lesions of the nigrostriatal dopamine system. Brain Res 1970;24(3):485-493. [DOI] [PubMed] [Google Scholar]
- 27. Cadet JL, Zhu SM. The intrastriatal 6-hydroxydopamine model of hemiparkinsonism: quantitative receptor autoradiographic evidence of correlation between circling behavior and presynaptic as well as postsynaptic nigrostriatal markers in the rat. Brain Res 1992;595(2):316-326. [DOI] [PubMed] [Google Scholar]
- 28. Olsson M, Nikkhah G, Bentlage C, Björklund A. Forelimb akinesia in the rat Parkinson model: differential effects of dopamine agonists and nigral transplants as assessed by a new stepping test. J Neurosci 1995;15(5 Pt 2):3863-3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Emborg ME. Evaluation of animal models of Parkinson’s disease for neuroprotective strategies. J Neurosci Methods 2004;139(2):121-143. [DOI] [PubMed] [Google Scholar]
- 30. Snyder EY. Grafting immortalized neurons to the CNS. Curr Opin Neurobiol 1994;4(5):742-751. [DOI] [PubMed] [Google Scholar]
- 31. Reynolds BA, Weiss S. Clonal and population analyses demonstrate that an EGF-responsive mammalian embryonic CNS precursor is a stem cell. Dev Biol 1996;175(1):1-13. [DOI] [PubMed] [Google Scholar]
- 32. Martínez-Serrano A, Björklund A. Immortalized neural progenitor cells for CNS gene transfer and repair. Trends Neurosci 1997;20(11):530-538. [DOI] [PubMed] [Google Scholar]
- 33. Björklund A, Schmidt RH, Stenevi U. Functional reinnervation of the neostriatum in the adult rat by use of intraparenchymal grafting of dissociated cell suspensions from the substantia nigra. Cell Tissue Res 1980;212(1):39-45. [DOI] [PubMed] [Google Scholar]
- 34. Perlow MJ, Freed WJ, Hoffer BJ, Seiger A, Olson L, Wyatt RJ. Brain grafts reduce motor abnormalities produced by destruction of nigrostriatal dopamine system. Science 1979;204(4393):643-647. [DOI] [PubMed] [Google Scholar]
- 35. Hagell P, Schrag A, Piccini P, et al. Sequential bilateral transplantation in Parkinson’s disease: effects of the second graft. Brain 1999;122 (Pt 6):1121-1132. [DOI] [PubMed] [Google Scholar]
- 36. Widner H, Tetrud J, Rehncrona S, et al. Bilateral fetal mesencephalic grafting in two patients with parkinsonism induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). N Engl J Med 1992;327(22):1556-1563. [DOI] [PubMed] [Google Scholar]
- 37. Kordower JH, Rosenstein JM, Collier TJ, et al. Functional fetal nigral grafts in a patient with Parkinson’s disease: chemoanatomic, ultrastructural, and metabolic studies. J Comp Neurol 1996;370(2):203-230. [DOI] [PubMed] [Google Scholar]
- 38. Kordower JH, et al. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med 2008;14(5):504-6. [DOI] [PubMed] [Google Scholar]
- 39. Rascol O, Lozano A, Stern M, Poewe W. Milestones in Parkinson’s disease therapeutics. Mov Disord 2011;26(6):1072-1082. [DOI] [PubMed] [Google Scholar]
- 40. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126(4):663-676. [DOI] [PubMed] [Google Scholar]
- 41. Bjorklund LM, Sánchez-Pernaute R, Chung S, et al. Embryonic stem cells develop into functional dopaminergic neurons after transplantation in a Parkinson rat model. Proc Natl Acad Sci USA 2002;99(4):2344-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hedlund E, Pruszak J, Lardaro T, et al. Embryonic stem cell-derived Pitx3-enhanced green fluorescent protein midbrain dopamine neurons survive enrichment by fluorescence-activated cell sorting and function in an animal model of Parkinson’s disease. Stem Cells 2008;26(6):1526-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jönsson ME, Ono Y, Björklund A, Thompson LH. Identification of transplantable dopamine neuron precursors at different stages of midbrain neurogenesis. Exp Neurol 2009;219(1):341-354. [DOI] [PubMed] [Google Scholar]
- 44. Moon J, Lee HS, Kang JM, et al. Stem cell grafting improves both motor and cognitive impairments in a genetic model of Parkinson’s disease, the aphakia (ak) mouse. Cell Transplant 2013;22(7):1263-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Arenas E. Towards stem cell replacement therapies for Parkinson’s disease. Biochem Biophys Res Commun 2010;396(1):152-156. [DOI] [PubMed] [Google Scholar]
- 46. Burrows CK, Banovich NE, Pavlovic BJ, et al. Genetic variation, not cell type of origin, underlies the majority of identifiable regulatory differences in iPSCs. PLoS Genet 2016;12(1):e1005793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ortmann D, Vallier L. Variability of human pluripotent stem cell lines. Curr Opin Genet Dev 2017;46:179-185. [DOI] [PubMed] [Google Scholar]
- 48. Kim SU. Genetically engineered human neural stem cells for brain repair in neurological diseases. Brain Dev 2007;29(4):193-201. [DOI] [PubMed] [Google Scholar]
- 49. Ostenfeld T, Tai YT, Martin P, Déglon N, Aebischer P, Svendsen CN. Neurospheres modified to produce glial cell line-derived neurotrophic factor increase the survival of transplanted dopamine neurons. J Neurosci Res 2002;69(6):955-965. [DOI] [PubMed] [Google Scholar]
- 50. Ebert AD, Beres AJ, Barber AE, Svendsen CN. Human neural progenitor cells over-expressing IGF-1 protect dopamine neurons and restore function in a rat model of Parkinson’s disease. Exp Neurol 2008;209(1):213-223. [DOI] [PubMed] [Google Scholar]
- 51. Akerud P, Canals JM, Snyder EY, Arenas E. Neuroprotection through delivery of glial cell line-derived neurotrophic factor by neural stem cells in a mouse model of Parkinson’s disease. J Neurosci 2001;21(20):8108-8118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Goldberg NRS, Caesar J, Park A, et al. Neural stem cells rescue cognitive and motor dysfunction in a transgenic model of dementia with Lewy bodies through a BDNF-dependent mechanism. Stem Cell Reports 2015;5(5):791-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Campbell MJ, Julious SA, Altman DG. Estimating sample sizes for binary, ordered categorical, and continuous outcomes in two group comparisons. BMJ 1995;311(7013):1145-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Schulz KF, Grimes DA. Sample size calculations in randomised trials: mandatory and mystical. Lancet 2005;365(9467):1348-1353. [DOI] [PubMed] [Google Scholar]
- 55. Tysnes OB, Storstein A. Epidemiology of Parkinson’s disease. J Neural Transm (Vienna) 2017;124(8):901-905. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article.