Abstract
Recent evidence suggests pulmonary hypertension (PH), a disease of the pulmonary vasculature actually has multiorgan pathophysiology and perhaps etiology. Herein, we demonstrated that fecal matter transplantation from angiotensin‐converting enzyme 2 overexpressing mice counteracted the effects of chronic hypoxia to prevent pulmonary hypertension, neuroinflammation, and gut dysbiosis in wild type recipients.
Keywords: microbiota, neuroinflammation, renin angiotensin system and pathophysiology
1. INTRODUCTION
Pulmonary hypertension (PH) is traditionally considered a pulmonary vascular disease. However, recent evidence of gastrointestinal tract pathology, its microbiota, and activated microglia in the hypothalamic paraventricular brain region suggests that PH is associated with an impaired gut–brain axis. Therefore, it could be considered a multiorgan disease. 1 This novel concept of impaired gut–brain–lung communication in PH provides opportunities for evaluation of new targets for PH treatment. Angiotensin‐converting enzyme 2 (ACE2) is an emerging candidate. Principle evidence includes (i) observation of decreased ACE2 and its enzymatic product, angiotensin‐(1‐7) in animal models of PH. Activation, supplementation, or overexpression of ACE2 ameliorates PH. (ii) Global Ace2 overexpressing (Ace2KI) mice are protected from chronic hypoxia‐induced PH. 2 (iii) In many studies of patients with PH, plasma ACE2 is decreased and recombinant ACE2 has beneficial effects on pulmonary hemodynamics. 3 , 4 These observations prompted our proposition that ACE2 could rebalance the impaired gut–brain–lung axis by influencing the gut microbiota and gut ACE2. Thus, we tested the hypothesis that fecal matter transfer (FMT) from Ace2KI mice that have distinct gut microbiota and protection from hypoxia‐induced PH, would rebalance the gut–brain–lung axis and protect against PH.
2. METHODS
All protocols were approved by the Institutional Animal Care and Use Committee at our University and followed national guidelines for the use of animals. After acclimation for 14 days, the gut microbiota of 10 weeks old C57BL6/J (wild type [WT]) recipient male mice, was depleted with an antibiotic cocktail (streptomycin [2 g/L], gentamicin [0.5 g/L], bacitracin (1 g/L], and ciprofloxacin (0.125 g/L)] in autoclaved drinking water provided ad libitum for 7 days, followed by sterile water to drink for 2 days. Cecal contents of Ace2 overexpressing male mice (Ace2KI) and their controls (WT) (n = 10 and 12, respectively) were collected Pooled cecal contents of each mouse strain(~0.4 g/animal), was mixed and suspended in sterile phosphate‐buffered saline (PBS) at the ratio of 1 g of cecal content/2 ml of PBS. Following centrifugation to remove insoluble debris, the supernatant (150 μl) was used for fecal transfer by oral gavage three times every other day. Following microbiota reconstitution, mice were exposed to either hypoxia or normoxia for 28 days to yield four groups of mice; WT mice receiving WT FMT in normoxia (N) (n = 10) or hypoxia (H) (n = 11) and WT mice receiving Ace2 FMT in normoxia (n = 12) and hypoxia (n = 13) (WT‐WT‐N, WT‐WT‐H, WT‐Ace2‐N, WT‐Ace2‐H). These groups of male mice were used to evaluate the gut–brain–lung axis by studying gut pathology and gut microbiota, neuroinflammation, and cardiopulmonary pathophysiology following 28 days of chronic hypoxia exposure. The hypoxia protocol, heart parameters, and tissue collection were all as previously published. 2 GraphPad Prism 8.0 (La Jolla) software was used to analyze data and for the graph generation. Comparisons among groups were assessed by two‐way analysis of variance (ANOVA) followed by Tukey's multiple comparison post‐hoc test. Group data were expressed as mean ± SEM, and p < 0.05 was considered significant.
3. RESULTS AND DISCUSSION
We have previously demonstrated that WT‐WT‐H mice show increased right ventricular systolic pressure (RVSP, 175% p < 0.001), RV end‐diastolic pressure (158%, p = 0.03), and RV: left ventricular + septum ratio (135%, p = 0.0002) compared with WT‐WT‐N group. While there was no difference in RVSP between WT‐WT‐N and WT‐Ace2‐N, the hypoxia‐induced increase in RVSP was ameliorated in WT‐Ace2‐H group, 2 we show that increased pulmonary vessel medial wall thickness was also attenuated (Figure 1a) indicating that Ace2KI‐FMT was sufficient to attenuate pulmonary pathophysiology. Hypoxia decreased colonic Ace2 mRNA in WT‐WT mice (WT‐WT‐N: 1.0 ± 0.11; WT‐WT‐H: 0.3 ± 0.095, p = 0.0021). As expected, Ace2 mRNA in WT‐Ace2‐N was 2.5‐fold that of WT‐WT‐N mice and reduced by hypoxia, but remained higher during hypoxia than WT‐WT‐N (Figure 1b).
Figure 1.
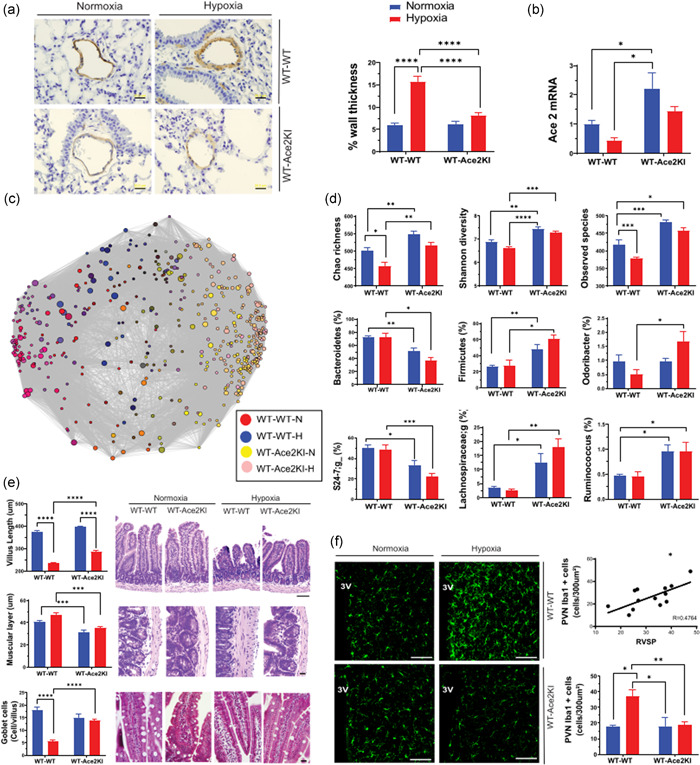
Fecal rransplantation from ACE2 overexpressing mice counteracts effects of chronic hypoxia: Mice were exposed to either hypoxia or normoxia for 28 days to yield four groups of mice; wild type (WT) mice receiving WT fecal matter transfer (FMT) in normoxia (N) (WT‐WT‐N: n = 10) or hypoxia (H) (WT‐WT‐H: n = 11) and WT mice receiving ACE2 FMT in normoxia (WT‐ACE2‐N: n = 12) and hypoxia (WT‐ACE2‐H: n = 13). (a) Smooth muscle α actin staining of pulmonary blood vessels. Representative micrographs of muscular pulmonary arteries stained with α‐smooth muscle actin (SMA) antibody (1:600; Sigma‐Aldrich) (scale bar = 20 μm) for analysis of medial wall thickness of muscularized pulmonary vessels, as described before (2). Data are presented as percent of the wall thickness of the total vessel diameter (n = 4–5/group). (b) Ace2 mRNA expression in colon: quantitative polymerase chain reaction was used to measure Ace2 mRNA in the colon by the relative gene expression method (2 − ΔΔC t) normalized against glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) (GAPDH expression was not altered by hypoxia or ACE2‐FMT). Data are fold‐change relative to WT‐WT‐N. (c) Network analysis of the gut microbiomes of WT‐WT and WT‐ACE2 recipients following hypoxia or normoxia. The variable regions V4‐V5 of 16S ribosome DNA gene were sequenced on Miseq and assigned to bacterial operational taxonomic units (OTU) and (d) α diversity measures of gut microbiome, as previously described (5). (e) Gut histological analysis. Hematoxylin and eosin staining of jejunum, 5 μm sections showing decreased villus length (scale bar = 100 μm) and increased thickness of muscular layer (scale bar = 20 μm) and Masson's trichrome (scale bar = 20 μm) revealing decreased goblet cells in the villi, following hypoxia in WT‐WT mice and the overall improvement of these gut pathologies by ACE2‐FMT. Staining and quantification were previously described (2). (f) WT‐ACE2 FMT prevented the hypoxia‐induced increase in activated microglia in the paraventricular nucleus of the hypothalamus (PVN). Representative 20× confocal microscope images of Iba1 staining of microglia in the PVN. 3V, third ventricle (scale bar = 100 μm). Total number of Iba1 positive cells were quantified in 300 μm2 in the PVN and the number of microglia in the PVN of each animal was correlated against its respective right ventricular systolic pressure (RVSP). Methods used were previously described (6). p value of *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001 was considered significant.
Next, we compared gut microbiota in FMT animals following hypoxia. Network analysis revealed some separation of bacterial genera when WT‐WT mice were subjected to hypoxia (red vs. blue circles, Figure 1c), but little separation when WT‐Ace2 were compared (yellow vs. pink circles); the major separation of bacterial genera was due to WT versus Ace2 FMT (Figure 1c). α diversity scores (Chao richness, Shannon diversity, and Observed species) were greater in WT‐Ace2 than WT‐WT mice (Figure 1d). Hypoxia decreased Chao and Observed Species α diversities in WT‐WT mice but had no significant effect on any of the three α diversity counts in WT‐Ace2 group. We observed a significant decrease in Bacteroidetes and increase in Firmicutes with Ace2‐FMT (Figure 1d). The most pronounced differences were observed in short‐chain fatty acid (SCFA)‐producing bacteria. Odoribacter, Lachnospiraceae, and Ruminococcus (Figure 1d) were increased in WT‐Ace2‐recipient mice compared to WT‐WT, and except Odoribacter that increased with hypoxia in the WT‐Ace2 mice, not altered by hypoxia in either group. The increases in SCFA‐producing bacteria are particularly interesting since increased butyrate has been associated with attenuations of lung and gut inflammation, neuroinflammation, and microglia activation. These observations suggest that FMT from Ace2KI mice significantly alters overall and specific gut microbial communities in WT recipients.
Mucus production by goblet cells, gut immune protection, and tight junctions (TJ) between epithelial cells promote healthy intestinal barrier function. Impaired intestinal barrier function and gut leakiness have been associated with increased infiltration of bacterial products into host circulation leading to a pro‐inflammatory state inducing the release of pro‐inflammatory cytokines. 5 Our observations also support this view. Evaluation of gut histology identified pronounced hypoxia‐induced gut pathology, including shortened villi, thickened muscular layer, and decreased goblet cells/villus in WT‐WT‐H but not in WT‐Ace2‐H mice (Figure 1e). FMT from Ace2KI mice led to increased expression of TJ proteins claudin‐4 and claudin‐5, and abolished the decrease in Tjp1 induced by hypoxia (WT‐WT‐N: 1.03 ± 0.07; WT‐WT‐H: 0.46 ± 0.8*; WT‐Ace2‐N: 0.95 ± 0.08; WT‐Ace2‐H: 0.06; *p < 0.001), likely enhancing protection against hypoxia‐induced gut and systemic problems (data not shown), and reinforcing the potency of FMT from Ace2KI mice alone to influence the gut and consequently pulmonary pathophysiology.
We have shown that animal models of PH exhibit increased microglial cells and neuroinflammation in autonomic brain regions, particularly the hypothalamic paraventricular nucleus (PVN). 1 , 2 , 6 , 7 In this study, hypoxia increased PVN Iba+ microglia in WT‐WT‐H group, which positively correlated with RVSP (R = 0.4764; p = 0.0063), indicating a correlation of increased microglia in the PVN and severity of PH. Ace2 FMT was sufficient to prevent their increase during hypoxia (WT‐WT‐N: 23.3 ± 4.8; WT‐WT‐H: 37 ± 4.2*; WT‐Ace2‐N: 17.8 ± 4.3; WT‐Ace2‐H: 18.8 ± 1.8; *p < 0.05 again correlating with RVSP (Figure 1f). The absence of microglia activation in WT‐Ace2 exposed to hypoxia might be connected to decreased gut leakiness and consequently, decrease of bacteria‐induced pro‐inflammatory cytokines. Another possibility might be the protective effect of butyrate against neuroinflammation.
A recent study from Muller et al. revealed that the enteric associated neurons act as a core sensory system, whereby alterations in microbial composition can activate gut‐projecting neurons. More specifically, SCFA and other gut‐related metabolites were identified as physiological modulators of gut sympathetic neuronal activation. 8 This is specially compelling, since we have previously demonstrated a pronounced increase in gut‐PVN connection in PH animals, where retrograde tracing green fluorescent protein‐pseudorabies virus applied in the gut, leads to a substantial increase in neurons labeled in the PVN of PH animals compared to controls. 9 Sympathetic activity is increased in PH in humans and animal models. This, indicates an increased activation of this neuronal pathway in PH animals, that if attenuated by SCFA, may contribute to the protection observed in WT‐Ace2 groups. The protection induced by SCFA should be evaluated in future studies, nonetheless, these findings support the paradigm‐changing concept that considers PH a systemic disease with important input from gut–brain–lung axis communication.
Together, these observations show that (1) Ace2KI mice exhibit gut microbial communities that benefit gut health; (2) FMT from Ace2KI mice increases colonic Ace2, ameliorates gut pathology, rebalances the bacterial community, attenuates increases in PVN microglia, and protects WT mice from hypoxia‐induced cardiopulmonary pathophysiology. Our study reinforces the beneficial role of ACE2 in gut–brain–lung axis and cardiopulmonary pathophysiology. Importantly, we demonstrated the novel concept that the benefits of Ace2 overexpression are transferable through FMT in the context of PH. This highlights its potential clinical relevance for PH management by FMT from healthy subjects. Additionally, consumption of probiotics overexpressing ACE2 is possible and is being explored. We have shown that overexpression of Lactobacilli Ace2 increases plasma Ace2 and improves diabetic retinopathy 5 and cardiac hypertrophy in monocrotaline‐induced PH (unpublished data). In conclusion, this study rationalizes testing strategies involving gut microbiota and ACE2 for PH treatment.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
ETHICS STATEMENT
All animal experimental procedures were approved by the University of Florida Institute Animal Care and Use Committee.
AUTHORS CONTRIBUTION
Aline C. Oliveira, Tao Yang, Elaine M. Richards, and Mohan K. Raizada, conceived and designed research; Aline C. Oliveira, Tao Yang, Jing Li, Ravindra K. Sharma, Marianthi M. Karas performed experiments; Aline C. Oliveira, Tao Yang, Jing Li, and Ravindra K. Sharma analyzed data; Aline C. Oliveira, Tao Yang, Elaine M. Richard, and Mohan K. Raizada interpreted results of experiments; Aline C. Oliveira prepared figures; Aline C. Oliveira, drafted manuscript; Aline C. Oliveira, Tao Yang, Andrew J. Bryant, Bina Joe, Elaine M. Richards, and Mohan K. Raizada, edited and revised manuscript; Aline C. Oliveira, Tao Yang, Jing Li, Ravindra K. Sharma, Marianthi K. Karas, Andrew J. Bryant, Annette D. de Kloet, Eric G. Krause, Bina Joe, Elaine M. Richards, and Mohan K. Raizada, approved final version of manuscript.
ACKNOWLEDGMENT
The authors acknowledge financial support from the National Institutes of Health National Heart, Lung, and Blood Institute Grants: HL102033, HL110170, HL143082. American Heart Association Postdoctoral Fellowship grant AHA 20POST35210516 (Aline C. Oliveira) and Biocodex Microbiota Foundation (Tao Yang).
Oliveira AC, Yang T, Li J, Sharma RK, Karas MK, Bryant AJ, Kloet AD de, Krause EG, Joe B, Richards EM, Raizada MK. Fecal matter transplant from Ace2 overexpressing mice counteracts chronic hypoxia‐induced pulmonary hypertension. Pulm Circ. 2022;12:e12015. 10.1002/pul2.12015
REFERENCES
- 1. Oliveira AC, Richards EM, Raizada MK. Pulmonary hypertension: pathophysiology beyond the lung. Pharmacol Res. 2020;151:104518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sharma RK, Oliveira AC, Yang T, Karas MM, Li J, Lobaton GO, Aquino VP, Robles‐Vera I, Kloet AD de, Krause EG, Bryant AJ, Verma A, Li Q, Richards EM, Raizada MK. Gut pathology and its rescue by ACE2 (angiotensin‐converting enzyme 2) in hypoxia‐induced pulmonary hypertension. Hypertension. 2020;76:206–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kim S, Rigatto K, Gazzana MB, Knorst MM, Richards EM, Pepine CJ, Raizada MK. Altered gut microbiome profile in patients with pulmonary arterial hypertension. Hypertension. 2020;75:1063–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kleinsasser A, Pircher I, Treml B, Schwienbacher M, Schuster M, Janzek E, Loibner H, Penninger JM, Loeckinger A. Recombinant angiotensin‐converting enzyme 2 suppresses pulmonary vasoconstriction in acute hypoxia. Wilderness Environ Med. 2012;23:24–30. [DOI] [PubMed] [Google Scholar]
- 5. Witkowski M, Weeks TL, Hazen SL. Gut microbiota and cardiovascular disease. Circ Res. 2020;127:553–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oliveira AC, Sharma RK, Aquino V, Lobaton G, Bryant AJ, Harrison JK, Richards EM, Raizada MK. Involvement of microglial cells in hypoxia‐induced pulmonary hypertension. Am J Respir Cell Mol Biol. 2018;59:271–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sharma RK, Oliveira AC, Kim S, Rigatto K, Zubcevic J, Rathinasabapathy A, Kumar A, Lebowitz JJ, Khoshbouei H, Lobaton G, Aquino V, Richards EM, Katovich MJ, Shenoy V, Raizada MK. Involvement of neuroinflammation in the pathogenesis of monocrotaline‐induced pulmonary hypertension. Hypertension. 2018;71:1156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Muller PA, Schneeberger M, Matheis F, Wang P, Kerner Z, Ilanges A, Pellegrino K, Del Mármol J, Castro T, Furuichi M, Perkins M, Han W, Rao A, Pickard AJ, Cross JR, Honda K, de Araujo I, Mucida D. Microbiota modulate sympathetic neurons via a gut‐brain circuit. Nature. 2020;583:441–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sharma RK, Oliveira AC, Yang T, Kim S, Zubcevic J, Aquino V, Lobaton GO, Goel R, Richards EM, Raizada MK. Pulmonary arterial hypertension‐associated changes in gut pathology and microbiota. ERJ Open Res. 2020;6:253–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]


