Abstract
Treating Veterans with chronic obstructive pulmonary disease complicated by pulmonary hypertension (COPD‐PH) using phosphodiesterase type‐5 inhibitor pharmacotherapy is common, but efficacy data are lacking. To address this further, patients with COPD‐PH from five Department of Veterans Affairs hospitals were randomized (1∶1) to receive placebo or oral tadalafil (40 mg/day) for 12 months. The primary endpoint was changed from baseline in 6‐min walk distance at 12 months. Secondary endpoints included change from baseline in pulmonary vascular resistance, mean pulmonary artery pressure, and symptom burden by the University of California San Diego shortness of breath questionnaire scale at 6 months. A total of 42 subjects (all male; 68 ± 7.6 years old) were randomized to placebo (N = 14) or tadalafil (N = 28). The group imbalance was related to under‐enrollment. Compared to placebo, no significant difference was observed in the tadalafil group for change from the primary endpoint or change in mean pulmonary artery pressure or pulmonary vascular resistance from baseline at 6 months. A clinically meaningful improvement was observed in the secondary endpoint of shortness of breath questionnaire score in the tadalafil versus placebo group at 6 months. There was no significant difference in major adverse events between treatment groups, and tadalafil was well tolerated overall. For Veterans with COPD‐PH enrolled in this study, once‐daily treatment with tadalafil did not improve 6‐min walk distance or cardiopulmonary hemodynamics although a decrease in shortness of breath was observed. Under‐enrollment and imbalanced randomization confound interpreting conclusions from this clinical trial and limit the generalization of our findings.
Keywords: exercise capacity, lung disease, pulmonary vascular disease
INTRODUCTION
Patients with chronic obstructive pulmonary disease complicated by pulmonary hypertension (COPD‐PH) are at elevated risk for increased morbidity, including impaired exercise tolerance and diminished lifespan compared to COPD patients without PH. 1 Decreased bioavailable levels of the vasodilator, anti‐mitogenic, and anti‐fibrotic molecule nitric oxide (NO) are implicated, in part, in the pathogenesis of COPD‐PH. 2 The phosphodiesterase type‐5 inhibitor (PDE‐5i) drug class, in turn, restores NO signaling and is a bona fide therapy to improve cardiopulmonary hemodynamics and outcome in other forms of pulmonary hypertension. 3 This provides a biological rationale for considering treatment with PDE‐5i pharmacotherapy in clinical diseases defined by diminished bioavailable NO and supports our hypothesis that the drug tadalafil is an effective therapy to mitigate morbidity in COPD‐PH.
The Veterans Health Administration is the largest integrated healthcare system in the United States and includes a sizeable population of patients with risk factors for COPD and PH. In the most comprehensive observational right heart catheterization (RHC) cohort study to date, 42% of all Veterans diagnosed with PH had COPD. 4 Furthermore, in line with data from non‐Veterans populations, 5 PDE‐5i therapy use is common in Veterans with COPD‐PH. 6 , 7 However, organized data on the therapeutic effect of PDE‐5i in COPD‐PH Veterans per se are lacking, and a consistent clinical benefit for this drug class across published clinical trials in other COPD‐PH populations has not been observed. 8 , 9 Collectively, these observations suggest that conducting a prospective randomized clinical trial to clarify the clinical efficacy and safety of PDE‐5i in COPD‐PH Veterans is timely and warranted.
METHODS
This study included patients 40–85 years of age with COPD based on the following initial spirometry thresholds: FEV1 79% predicted and FEV1/FVC < 0.7. The initial hemodynamic criteria for PH used to enroll patients in this study were mean pulmonary artery pressure (mPAP) > 30 mmHg, pulmonary vascular resistance (PVR) > 2.5 WU, and pulmonary artery wedge pressure (PAWP) 18 mmHg. Patients with World Symposium Pulmonary Hypertension Group 1, 2, 4, and 5 as the predominant clinical classification of PH were excluded. This determination was based on clinical, hemodynamic data, or echocardiographic data, and was subject to case adjudication by the study team members. Patients requiring supplemental oxygen at a rate of >4 L/min, having diffusion capacity of carbon monoxide <30% predicted, or with baseline 6‐minute walk distance (MWD) < 50 m were excluded. An expanded summary of the study protocol is available in the Supporting Information.
Study design
This study was a prospective, multicenter, placebo‐controlled randomized clinical trial testing the hypothesis that the PDE‐5i inhibitor tadalafil is safe and improves exercise tolerance in COPD‐PH. The study period was March 25, 2013 (Institutional Review Board [IRB] approval)—April 10, 2019 (final patient in the trial completed study). The study enrollment period was June 9, 2014, to February 8, 2018.
The full rationale, study design, and method for screening subjects have been reported previously, 10 and specific inclusion and exclusion criteria are reproduced in Table S1. Briefly, eligible patients at the following Veterans Affairs (VA) institutions were enrolled in the study: VA Boston Healthcare System, VA Medical Center Providence, RI, Greater Los Angeles VA Healthcare System, Atlanta VA Medical Center, and Denver VA Medical Center. The enrollment, randomization, and treatment assignment for each patient in each center are reported in Table S2.
Potential study patients were screened through a search of the VA echocardiography database, chart review via the electronic medical record (computerized patient record system), and in‐clinic referral from pulmonary and cardiology ambulatory programs. Patients with COPD assessed by clinically indicated pulmonary function test (PFT) within 12 months of randomization and PH diagnosed by clinically indicated RHC within 6 months of randomization were considered for enrollment. Patients without a recent nuclear ventilation/perfusion scan or sleep study were referred for those tests before randomization, although completion of these studies was not an enrollment requirement.
Study enrollment and enrollment criteria modifications
The initial PFT inclusion criteria included forced expiratory volume in 1 s (FEV1)/forced vital capacity ratio <70% and FEV1 ≤ 79% predicted. Since PH may accompany mild COPD, the FEV1 ≤ 79% predicted criterion was subsequently eliminated to expand enrollment. For similar reasons, the mPAP threshold of >30 mmHg was adjusted to >25 mmHg, as new empiric information supported a change in the hemodynamic definition of PH. 4 These changes were approved by the IRB and the national VA Data and Safety Monitoring Board (DSMB). The study was discontinued based on the expiration of funding, as designated by the charter for the trial by the sponsor.
Randomization
Patients were randomized using a computer‐generated algorithm in a 1 : 1 ratio to treatment with once‐daily oral placebo or tadalafil 40 mg. To accomplish this end, our research pharmacy generated N = 5 blocks of medication sufficient to treat N = 30 subjects. In this design, each block was assigned to a specific study site. Patients presenting for randomization were assigned a number that was determined chronologically. For example, the first patient within a block was assigned the number “1,” the second patient presenting for randomization within a block was assigned the number “2,” and so forth. Each patient randomization number was pre‐assigned a randomization code (i.e., three‐digit phrase consisting of number and letter characters). We observed an imbalance in placebo versus tadalafil enrollment (14:28, respectively) that was not due to a change in our randomization strategy, which remained 1 : 1 throughout the duration of the study. The randomization scheme is presented in Table S3, which demonstrates that under‐enrollment was the primary driver of randomization imbalance.
Study procedures
The study protocol was approved by the IRB at each participating VA location and the DSMB, both of which oversaw the trial. Informed consent was obtained from each patient before any study procedures were performed. After randomization, but before initiation of the study drug, subjects were administered a test dose of the study medication (20 mg of tadalafil or placebo) and observed for 4 h to ensure that blood pressure and oxyhemoglobin saturation (SaO2) levels were not affected adversely by the medication. Patients were evaluated regularly following the test dose. Medication adherence assessment, clinical status update, and review of potential adverse events and side effects were completed in‐person or by telephone on protocol day 3 and months 1, 3, 6, 9, and 12. Safety assessments were designed to monitor a wide range of potential cardiopulmonary, vascular, ocular, and other off‐target effects. However, based on empiric data in prior studies suggesting that in COPD, pulmonary vasodilation may promote shunt via increased V/Q mismatch 11 , 12 as well as the established effect of tadalafil on systemic blood pressure lowering, 13 we focused in particular on the potential for systemic hypotension or worsening hypoxemia in follow‐up encounters. Additional information on safety monitoring is detailed in Ref. 10.
Study end points
The primary study endpoint was between‐group change in 6‐MWD distance from baseline at 12 months. The secondary endpoints included change from baseline at 6 months for 6‐MWD, mPAP, PVR, dyspnea assessed by the University of California San Diego Shortness of Breath Questionnaire (UCSD SOBQ), and health‐related quality of life assessed by the St. George's Respiratory Questionnaire (SGRQ). The responses to the SGRQ can be aggregated into a total score and three subscores for symptoms, activity, and impact domains. Safety parameters were measured frequently, as described above. Each participating site was responsible for recording serious adverse events, which were tracked through the common VA electronic health record or by contacting patients directly.
Statistical methods
A detailed description of the original power calculation and changes in the power calculations related to adjustments in the study protocol are detailed on pp. 16–17 of the Supporting Information. Briefly, a sample size of 60 subjects in each treatment condition was anticipated to result in a general effect size of 0.60 standard deviation (SD) units corresponding to a change in 6‐MWD of 51 m. 10 The study protocol was revised with the goal of improving trial enrollment, which included a decrease in the mPAP threshold from >30 to >25 mmHg and expanding the clinical profile of Group 3 PH (i.e., the target clinical phenotype of this study) to include eliminating the original FEV1 ≤ 79% enrollment criteria. We also considered alternative clinical trial data with which to base our anticipated change in 6‐MWD. 15 Taken together, these changes led to a predicted enrollment of 25 patients in each group to detect a difference in 6‐MWD mean of 69 m using a two‐group t‐test with a 0.050 two‐sided significance level with 80% power.
All data are presented as mean ± SD if distributed normally or median (interquartile range [IQR]) if non‐normally distributed. Comparison between two groups inclusive of only normally distributed data was performed using the Student's unpaired two‐tailed t test; if data were non‐normally distributed, the exact Wilcoxon nonparametric test was used. For analyses illustrating the change in 6‐MWD for individual patients at different study timepoints, the signed‐rank pair test was used for comparisons between baseline and 6 months, and the Kruska–Wallis analysis of variance (ANOVA) was used for comparisons between baseline, 6 and 12 months. The Pearson r coefficient is reported for correlation analyses. Differences between treatment groups for categorical variables were analyzed using Fisher's exact test. For all analyses, p < 0.05 was considered significant. All available data were included in the analysis. No imputation or other statistical strategy was used to account for missing data, and no adjustments were made for multiple comparisons. All statistical analyses were performed using SAS 9.4 and Origin 9.1.
RESULTS
Clinical profile of the study population
There were N = 3471 patients screened for consideration in this study (Supporting Information for screening details), and the overall enrollment rate was 1.4% (N = 42) (Tables S4 and S5). Among the N = 42 patients randomized to treatment, there were N = 9 and N = 15 patients in the placebo and tadalafil groups, respectively, with 6‐MWD results for analysis at 12 months (Figure 1). The baseline demographic, clinical and hemodynamic profiles of subjects included in analyses at the 12 mo timepoint are presented in Tables 1 and 2. Compared to placebo, no significant difference was observed among patients randomized to tadalafil for FEV‐1 (1.5 0.8 vs. 1.7 0.5 L, p = 0.70), mPAP (33 [27–35] vs. 30 [27–34] mmHg, p = 0.47), PVR (4.5 [2.8–5.9] vs. 3.9 [3.1–5.5] WU, p = 0.41), or echocardiographic parameters including left ventricular ejection fraction (60 [55–65] vs. 60 [55–61] %, p = 0.49), tricuspid annular plan of systolic excursion (2.2 [1.8–2.5] vs. 2.0 [1.6–2.2] cm, p = 0.23), and left atrial diameter (3.4 [3.2–3.8] vs. 4.2 [3.3–4.7] cm, p = 0.14).
Figure 1.
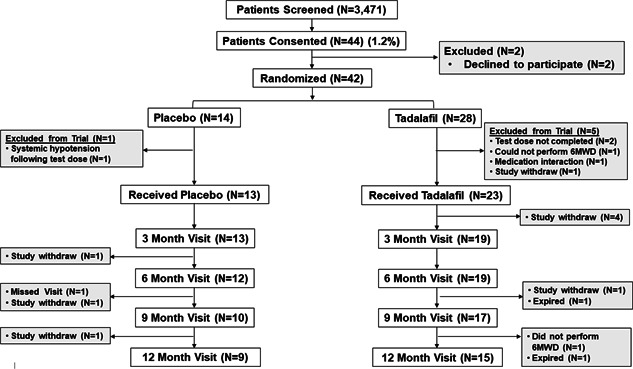
Enrollment, randomization, and follow up. Veterans with chronic obstructive pulmonary disease and pulmonary hypertension diagnosed by right heart catheterization were considered for enrollment in this study randomizing patients to daily treatment with placebo or the phosphodiesterase type 5 inhibitor therapy tadalafil (40 mg daily). The primary endpoint was changed in 6‐min walk distance (6‐MWD) from baseline at 12 months. Secondary endpoints included change in mean pulmonary artery pressure, pulmonary vascular resistance, 6‐MWD, and dyspnea burden from baseline at 6 months
Table 1.
Baseline demographic, hemodynamic, and biochemical characteristics of the study population available for analysis at the 12‐month timepoint
| Placebo | Tadalafil | ||||
|---|---|---|---|---|---|
| N | Result | N | Result | p value | |
| Age (year) | 9 | 66 ± 8.5 | 15 | 69 ± 5.9 | 0.37 |
| Caucasian race | 9 | 8 (89) | 15 | 14 (93) | 1.00 |
| BMI (kg/m2) | 9 | 28.1 (25.0–33.2) | 15 | 30.4 (27.0–31.4) | 0.65 |
| BMI category | 9 | 15 | 0.63 | ||
| Normal (<25) | 2 (22) | 2 (13) | |||
| Overweight (25–30) | 4 (44) | 5 (33) | |||
| Obese (≥30) | 3 (33) | 8 (53) | |||
| 6MWD (m) | 9 | 297 (210–332) | 15 | 254 (174–319) | 0.77 |
| Pulmonary function | |||||
| FEV1 (L) | 9 | 1.6 ± 0.9 | 15 | 1.7 ± 0.5 | 0.70 |
| %‐Predicted FEV1 | 9 | 50 ± 32 | 15 | 52 ± 21 | 0.84 |
| FVC (L) | 9 | 3.2 ± 0.9 | 15 | 3.1 ± 0.7 | 0.81 |
| %‐Predicted FVC | 9 | 75 ± 25 | 15 | 72 ± 25 | 0.71 |
| FEV1/FVC | 9 | 0.45 ± 0.17 | 15 | 0.53 ± 0.08 | 0.25 |
| DLCO (% predicted) | 6 | 36 (24–53) | 15 | 35 (28–46) | 0.79 |
| SaO2 (%) | 9 | 96 (93–97) | 15 | 94 (93–96) | 0.43 |
| Hemodynamics | |||||
| Heart rate (bpm) | 9 | 87 (69–96) | 14 | 71 (61–86) | 0.07 |
| SBp (mmHg) | 9 | 125 (118–140) | 14 | 136 (109–149) | 0.89 |
| DBp (mmHg) | 9 | 80 (71–86) | 14 | 71 (68–83) | 0.43 |
| Systolic PAP (mmHg) | 9 | 46 (42–48) | 15 | 50 (41–57) | 0.28 |
| Diastolic PAP (mmHg) | 9 | 21 (17–27) | 15 | 22 (19–25) | 0.61 |
| Mean PAP (mmHg) | 9 | 33 (27–35) | 15 | 30 (27–34) | 0.47 |
| Mean RAP (mmHg) | 9 | 10 (5–10) | 15 | 9 (6–10) | 0.61 |
| PAWP (mmHg) | 9 | 14 (10–17) | 15 | 15 (11–16) | 0.99 |
| Cardiac output (L/min) | 9 | 5.0 (3.4–5.8) | 15 | 4.6 (3.6–5.5) | 1.00 |
| PVR (WU) | 9 | 4.5 (2.8–5.9) | 15 | 3.9 (3.1–5.5) | 0.41 |
| Quality of life measures | |||||
| SOBQ Score | 9 | 39 (35–51) | 15 | 40 (29–57) | 0.99 |
| SGRQ Summary Score | 9 | 59 (36–65) | 15 | 54 (39–60) | 0.73 |
| Symptoms Domain Score | 9 | 69 (30–78) | 15 | 50 (37–65) | 0.92 |
| Activity Domain Score | 9 | 73 (66–87) | 15 | 79 (60–93) | 0.86 |
| Impact Domain Score | 9 | 47 (19–50) | 15 | 30 (16–50) | 0.82 |
| Biochemical data | |||||
| BNP (ng/mL) | 9 | 33 (23–77) | 15 | 73 (39–100) | 0.41 |
| Creatinine (mg/dL) | 9 | 0.9 (0.9–1.1) | 15 | 1.1 (0.8–1.3) | 0.37 |
| Alanine transaminase (U/L) | 9 | 17 (15–33) | 13 | 18 (15–23) | 0.88 |
| Aspartate aminotransferase (U/L) | 9 | 19 (14–26) | 14 | 23 (18–24) | 0.65 |
| International normalized ratio | 9 | 1.0 (1.0–1.1) | 13 | 1.1 (1.0–1.1) | 0.39 |
| Echocardiographic measurements | |||||
| LVEF (%) | 9 | 60 (55–65) | 15 | 60 (55–61) | 0.49 |
| ePASP (mmHg) | 7 | 42 (24–78) | 13 | 39 (34–51) | 0.88 |
| TAPSE (cm) | 7 | 2.2 (1.8–2.5) | 14 | 2.0 (1.6–2.2) | 0.23 |
| LA diameter (cm) | 8 | 3.4 (3.2–3.8) | 15 | 4.2 (3.3–4.7) | 0.14 |
| IVs (mm) | 9 | 9.2 (9.0–10) | 15 | 9.7 (8.1–11) | 0.67 |
| LVEDD (cm) | 8 | 4.7 (4.1–4.9) | 15 | 4.9 (4.0–5.2) | 0.49 |
Note: N is the total number of patients available for analysis. Data are presented as N (%) for categorical variables, mean ± SD for continuous variables that are distributed normally, or median (IQR) for continuous variables that are non‐normally distributed.
Abbreviations: 6WMD, 6 min walk distance; BMI, body mass index; DBp, diastolic blood pressure; DLCO, diffusing capacity for carbon monoxide; ePASP, estimated pulmonary artery pressure derived from the tricuspid regurgitant jet velocity; ERV, expiratory reserve volume; FEV, forced expiratory volume; FRC, functional residual capacity; FVC, forced vital capacity; IVs, interventricular septum; LA, left atrium; LVEDD, LV end‐diastolic dimension; LVEF, left ventricular ejection fraction; PAP, pulmonary artery pressure; PAWP, pulmonary artery wedge pressure; PVR, pulmonary vascular resistance; RAP, right atrial pressure; RV, residual volume; SaO2, oxyhemoglobin saturation; SBp, systolic blood pressure; SGRQ, shortness of breath questionnaire; SGRQ, St. George's Respiratory Questionnaire; TAPSE, tricuspid annular plane systolic excursion; TLC, total lung capacity.
Table 2.
Baseline clinical characteristics of the study population available for analysis at the 12‐month timepoint
| Placebo | Tadalafil | p value | |||
|---|---|---|---|---|---|
| N | Result | N | Result | ||
| Clinical comorbidities | |||||
| COPD Gold Grade | 9 | 15 | 0.33 | ||
| 1 | 1 (11) | 2 (13) | |||
| 2 | 3 (33) | 6 (40) | |||
| 3 | 1 (11) | 5 (33) | |||
| 4 | 4 (44) | 2 (13) | |||
| Supplemental oxygen use | 9 | 5 (56) | 15 | 5 (33) | 0.40 |
| Obstructive sleep apnea | 9 | 3 (33) | 15 | 4 (27) | 1.00 |
| Systemic hypertension | 9 | 7 (78) | 15 | 11 (73) | 1.00 |
| Diabetes mellitus | 9 | 2 (22) | 15 | 7 (47) | 0.39 |
| Pulmonary embolism | 9 | 0 (0) | 15 | 1 (7) | 1.00 |
| Connective tissue disease | 9 | 0 (0) | 15 | 1 (7) | 1.00 |
| Coronary artery disease | 9 | 1 (11) | 15 | 4 (27) | 0.61 |
| Unstable angina, MI, or stroke | 9 | 0 (0) | 15 | 1 (7) | 1.00 |
| Congestive heart failure | 9 | 1 (11) | 15 | 4 (27) | 0.61 |
| Chronic kidney disease | 9 | 0 (0) | 15 | 2 (13) | 0.51 |
| Diagnostic testing history | |||||
| Sleep study | 9 | 3 (33) | 14 | 6 (43) | 1.00 |
| V/Q scan | 9 | 4 (44) | 15 | 4 (27) | 0.41 |
| COPD medications | |||||
| Long‐acting muscarinic antagonists | 9 | 6 (67) | 15 | 13 (87) | 0.33 |
| Long‐acting ‐agonists | 9 | 5 (56) | 15 | 8 (53) | 1.00 |
| Inhaled corticosteroids | 9 | 5 (56) | 15 | 12 (80) | 0.36 |
| Short‐acting anticholinergics | 9 | 1 (11) | 15 | 0 (0.00) | 0.38 |
| Short‐acting ‐agonists | 9 | 9 (100) | 14 | 12 (86) | 0.50 |
| Oral corticosteroids (chronic) | 9 | 0 (0) | 14 | 0 (0) | 1.00 |
| Other COPD medications | 9 | 1 (11) | 14 | 2 (14) | 1.00 |
| Other medications | |||||
| Digoxin | 9 | 2 (22) | 14 | 2 (13) | 0.61 |
| Loop diuretic | 9 | 5 (56) | 14 | 7 (47) | 1.00 |
| blockers | 9 | 1 (11) | 14 | 6 (40) | 0.19 |
| Warfarin/coumadin | 9 | 1 (11) | 14 | 1 (7) | 1.00 |
| ACE‐I/ARB | 4 | 1 (25) | 12 | 7 (58) | 0.57 |
| Calcium channel antagonist | 5 | 0 (0) | 12 | 3 (25) | 0.51 |
| Aldosterone receptor antagonist | 5 | 1 (20) | 11 | 0 (0) | 0.31 |
Note: N is the total number of patients available for analysis. Data are presented as N (%) for categorical variables, mean ± SD for continuous variables that are distributed normally, or median (IQR) for continuous variables that are non‐normally distributed.
Abbreviations: ACE‐I, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor antagonist; COPD, chronic obstructive pulmonary disease; V/Q, ventilation/perfusion nuclear scintigraphy scan.
The effect of treatment on the outcome measures
Compared to placebo, no significant difference was observed among patients randomized to tadalafil for the primary endpoint of change in 6‐MWD from baseline at 12 months (21 [−9 to 40] vs. 17 [−1.7 to 49] m, p = 0.65). Failure to demonstrate a significant benefit by tadalafil on 6‐MWD was likely hindered by insufficient statistical power but did not appear to be the consequence of individual patient outliers or a specific subgroup associated with attenuated treatment response (Figure 2). Additionally, no significant difference was observed between the placebo and tadalafil treatment groups for the functional or hemodynamic secondary endpoints of change from baseline at 6 months in 6‐MWD (16 [−12 to – 42] vs. 4.9 [−23 to 45] m, p = 0.64), mPAP (0.0 [−3.0 to 5.0] vs. −1.0 [−2.0 to 0.0], p = 0.65), cardiac output (−0.3 [0.8 – 0.8] vs. 0.4 [−0.7 to 0.5] L/min, p = 0.68), PVR (−0.3 [−0.7 to 0.2] vs. −0.5 [−2.1 to 0.4] WU, or PAWP (−0.5 [−4.0 to 2.5] vs. 1.0 [−1.0 to 4.0] mmHg, p = 0.15) (Tables 3, S6, and S7).
Figure 2.
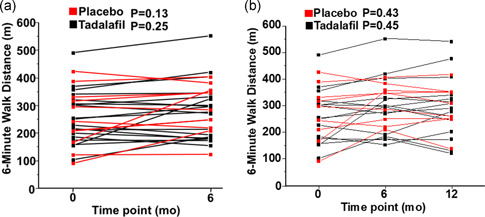
The trajectory of 6‐minute walk distance change from baseline at 6 months and 12 months for individual patients enrolled in the study. (a) Line series illustrating the absolute 6‐minute walk distance (6‐MWD) achieved at baseline and at 6 months and (b) at baseline, 6 and 12 months for patients enrolled throughout the study duration. Patients are stratified by the randomization group: daily placebo or tadalafil 40 mg daily per os
Compared to baseline, patients randomized to tadalafil had within‐patient improvement in health‐related quality of life assessed by the SGRQ total score (−8.7 [−17 to 2.8], p = 0.049) and the SGRQ impact score (−11.2 [−22.4 to 4.89], p = 0.029) at 6 months, whereas parallel findings were not observed in patients randomized to the placebo (SGRQ total score: −1.3 [−9.2 to 7.0], p = 0.791; SGRQ impact score: 4.00 [−9.2 to 10.7], p = 0.910). A similar pattern was observed for dyspnea assessed by the UCSD SOBQ, which improved significantly at 6 months compared to baseline in the tadalafil group compared to the placebo group (−4 [−14 to 7] vs. 12 [−2.0 to 17], p = 0.02) (Table 3). There were no significant changes in SGRQ symptom or activity score in either group at 6 months.
Table 3.
Study endpoints
| Placebo | Tadalafil | ||||
|---|---|---|---|---|---|
| Characteristics | N | Baseline to 12 months | N | Baseline to 12 months | p value |
| 6‐MWD (m) | 9 | 21 (−9 to 40) | 15 | 17 (−1.7 to 49) | 0.65 |
| N | Baseline to 6 months | N | Baseline to 6 months | ||
|---|---|---|---|---|---|
| 6‐MWD (m) | 11 | 16 (−12 to 42) | 19 | 4.9 (−23 to 45) | 0.64 |
| mPAP (mmHg) | 12 | 0.0 (−3.0 to 5.0) | 17 | −1.0  (−2.0 to 0.0) (−2.0 to 0.0) |
0.65 |
| PAWP (mmHg) | 12 | −0.5 (−4.0 to 2.5) | 17 | 1.0 (−1.0 to 4.0) | 0.15 |
| CO (L/min) | 12 | −0.3 (−0.8 to 0.8) | 17 | 0.4 (−0.7 to 0.5) | 0.68 |
| PVR (WU) | 12 | −0.3 (−0.7 to 0.2) | 17 | −0.5 (−2.1 to 0.4) | 0.65 |
| UCSD SOBQ | 12 | 12 (−2.0 to 17) | 19 | −4.0 (14 to 7.0) | 0.02 |
| p = 0.206* | p = 0.199* | ||||
| SGRQ Summary Score | 12 | −1.3 (−9.2 to 7.0) | 19 | −8.7 (−17 to 2.8) | 0.31 |
| p = 0.791* | p = 0.049* | ||||
|
SGRQ Impact Domain Subscore |
12 | 4.0 (−9.2 to 10.7) | 19 | −11.2 (−22.4 to 4.9) | 0.18 |
| p = 0.910* | p = 0.029* | ||||
|
SGRQ Activity Domain Subscore |
12 | −6.5 (−10.9 to 15.1) | 19 | −5.0 (−19.7 to 0.9) | 0.87 |
| p = 0.176* | p = 0.196* | ||||
|
SGRQ Symptoms Domain Subscore |
12 | −0.04 (−14.7 to 9.8) | 19 | −0.02 (−16.3 to 6.5) | 0.74 |
| p = 0.577* | p = 0.284* |
Note: N is the total number of patients available for analysis. Data are presented median (IQR) for continuous variables that are non‐normally distributed.
Abbreviations: 6‐MWD, 6‐min walk distance; CO, cardiac output; mPAP, mean pulmonary artery pressure; PAWP, pulmonary artery wedge pressure; PVR, pulmonary vascular resistance; SGRQ, St. George's Respiratory Questionnaire; UCSD SOBQ, University of California San Diego Shortness of Breath Questionnaire
p values based on within‐group analyses.
Based on prior reports suggesting that the salutary effect of therapies that aim to increase NO bioactivity on pulmonary artery tone may affect the relationship between mPAP and PAWP, 14 we next explored a potential pathophysiological basis by which to explain our findings. A post‐hoc analysis restricted to the tadalafil group showed a positive association between change in mPAP versus the change in PAWP from baseline at 6 months (r = 0.48, p = 0.049), which was not observed in patients randomized to placebo (r = 0.06, p = 0.85) (Figure 3).
Figure 3.
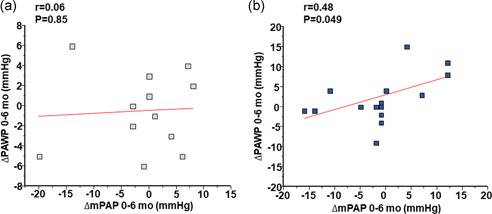
The association between change from baseline at 6 months in mean pulmonary artery pressure versus pulmonary artery wedge pressure in the study cohort. The association between change in mean pulmonary artery pressure (mPAP) versus pulmonary artery wedge pressure (PAWP) at 6 months from baseline for patients randomized to (a) placebo and (b) tadalafil 40 mg daily per os
Study attrition
No significant difference was observed between the tadalafil (N = 19) and placebo (N = 12) groups for time duration between RHC and day of first study dose (4.6 3.8 vs. 3.5 4.0 months, p = 0.22) and time from first study dose to the 6‐month visit (6.3 0.5 vs. 6.9 0.9 months, p = 0.08) or the 12‐month visit (12.3 0.6 vs. 12.4 0.6 months, p = 0.32) (Table S8).
At 6 months, N = 2 and N = 9 patients were excluded or withdrew from the study in the placebo and tadalafil groups, respectively, representing 26% of the original study cohort. Between the 6‐ and 12‐month timepoint, an additional N = 2 and N = 4 patients were excluded, withdrew from the study, or did not have 6‐MWD results available for analysis in the placebo and tadalafil conditions, respectively, representing 38% of the original study cohort in aggregate. An individualized listing of study withdrawal circumstances is provided in Table S9. Subjects that withdrew from the study by 6 months had significantly lower systolic blood pressure (111 [106–119] vs. 133 [114–146] mmHg, p = 0.01) and diastolic blood pressure (70 [58–73] vs. 77 [68–87] mmHg, p = 0.04) compared to the subjects available for analysis at 6 mo (Tables S10 and S11).
Safety endpoints
Study withdrawal after randomization was due to therapy intolerance in N = 3 tadalafil and N = 0 placebo patients (p = 0.067); overall, no significant difference was observed in study attrition at 12 months between‐groups (p = 0.375). Compared to baseline, no significant change in SaO2 level was observed for either group at 6 or 12 months (Table S12). There were N = 6 (14.2%) patients that died during the study. Of these, N = 5 were randomized to tadalafil although N = 3 died before receiving any tadalafil dose. In the placebo group, there was N = 1 death. Overall, there was no significant difference in the mortality rate between patients randomized to placebo versus tadalafil (p = 1.0).
Patients randomized to tadalafil were less likely to experience a COPD exacerbation compared to placebo (0% vs. 43%, p = 0.001). No significant difference was observed in the rate of other major adverse events between treatment groups, including hospitalization for pneumonia or heart failure (Table 4). In the tadalafil group, new or worsened: itch, dyspnea with exertion, and cough were reported in N = 10 (36%), N = 10 (36%), and N = 9 (32%) subjects, respectively. However, these or other adverse events did not occur at a significantly greater rate in subjects randomized to tadalafil compared to placebo (Table S13).
Table 4.
Significant adverse events among randomized patients in this study
| Placebo (N = 14) | Tadalafil (N = 28) | Fisher's exact p value | |
|---|---|---|---|
| Fatalitya | 1 (7.1)a | 5 (18)b | 0.65 |
| Chest pain/myocardial infarction | 1 (7.1) | 1 (14) | 0.65 |
| Hospitalization for pneumonia | 0 (0) | 2 (7.1) | 0.55 |
| Hemoptysis | 0 (0) | 1 (3.6) | 1.0 |
| Heart failure | 0 (0) | 1 (3.6) | 1.0 |
| COPD exacerbation | 6 (43) | 0 (0) | <0.01 |
| Emergency room visit | 3 (21) | 8 (29) | 0.72 |
| Hospitalized overnight for any reason | 4 (29) | 5 (18) | 0.45 |
Abbrevation: COPD, chronic obstructive pulmonary disease.
Sudden death, presumed cardiovascular (N = 1).
Stroke (N = 1), sudden death presumed noncardiovascular (N = 1), deceased before randomization (N = 1), pulmonary embolism (N = 1), respiratory failure (N = 1). Among the N = 5 patients randomized to tadalafil that died, N = 3 patients died before receiving any study drug. Data are presented as N (%).
DISCUSSION
In this multicenter randomized placebo‐controlled clinical trial in Veterans with COPD‐PH, a benefit by tadalafil was not observed for the primary endpoint of change in 6‐MWD at 12 months or the secondary endpoints of change in mPAP and PVR from baseline at 6 months. Patients randomized to tadalafil had a significant within‐patient decrease in the secondary endpoint of dyspnea and an improvement in SGRQ health‐related quality of life, including the impact subscore at 6 mo compared to baseline. These findings were offset somewhat by our observation that no significant differences were reported for these measures between the treatment groups. Randomization to tadalafil was associated with a trend toward increased study withdrawal compared to placebo, although no significant difference was observed for adverse events between study groups. Overall, tadalafil appeared to be well‐tolerated. Interpreting data from this report requires consideration of numerous methodological flaws that could have affected our findings.
Prior work focusing on PDE‐5i pharmacotherapy in PH has yielded mixed results, with some studies suggesting a salutary benefit on pulmonary artery pressure 16 and PVR, 17 while a consistent signal toward improvement in exercise tolerance or quality of life has not yet been demonstrated. 5 Our study expands this experience in several important ways. First, the current trial followed participants for 12 months and included RHC data criteria for enrollment. This, in turn, provided a unique opportunity to monitor the clinical effects by tadalafil in well‐phenotyped patients over a longer time period than reported previously. Second, we did not over‐curate enrollment criteria toward recruiting highly selected (i.e., purely) COPD‐PH patients. Therefore, this study aimed to involve a cross‐sectional subgroup of patients representative of the larger cohort encountered commonly in the VA healthcare systems as a means by which to improve the applicability of our findings to clinical practice. Finally, the VA medical centers participating in the study were geographically dispersed across the United States.
In this study, no significant change in exercise capacity measured using 6‐MWD was observed in the tadalafil group compared to placebo. These findings were consistent with data indicating that tadalafil did not affect individual PH hemodynamic parameters known to be prognostic in PH. 4 Although this study was not designed to monitor the effect of the study interventions on the interaction between hemodynamic variables, a post‐hoc analysis raised this possibility. Specifically, we observed that rising PAWP through the study period was associated with a concomitant rise in mPAP, suggesting that failure of tadalafil to lower mPAP may have been related to changes in left heart filling pressure. This is supported indirectly by baseline echocardiography data indicating a left atrial dimension within the range associated with pulmonary venous hypertension (i.e., Group 2 PH) in most tadalafil patients, 18 as well as the fact that we used a PAWP cut‐off for study enrollment linked to abnormal left heart filling pressure and thus unlikely to be exclusive of precapillary PH. 19 , 20 In turn, these collective findings reinforce accumulating data from cross‐sectional studies implicating greater phenotypic overlap between lung‐ and left heart disease‐associated PH than appreciated previously. 21
Treatment with tadalafil was associated with improvement in the secondary endpoint of dyspnea and health‐related quality of life assessed using two valid scales. Specifically, patients in the tadalafil group reported a significant improvement in dyspnea, as assessed by prespecified analyses using the UCSD SOBQ. The magnitude of this effect is within the range that is identified as clinically meaningful (>5 unit) when considering comparative analyses with other scales that measure dyspnea burden in COPD (e.g., Borg, etc.), 22 and was akin to benefits reported by COPD patients following pulmonary rehabilitation. 23 Additionally, post‐hoc analyses focusing on the individual‐patient change in SGRQ total score and impact domain subscore showed improvement in the tadalafil group, with a clinically meaningful change in SGRQ total score. Although there was no significant difference in overall SGRQ Summary Score between‐groups, nor in subscores, treatments that produce an improvement in SGRQ Summary Score of 4 units are considered significant 22 ; the median score in the tadalafil group improved more than this amount, which appears driven by the Impact Domain subscore. This, in turn, reflects answers to questions relating to how patients feel that they are affected by their disease.
These findings suggest that tadalafil use may be helpful toward achieving a key objective in the management of patients with chronic respiratory disease and PH: mitigation of dyspnea and improvement in health‐related quality of life. However, the mechanisms responsible for this improvement in this study appear unrelated to changes in PVR, mPAP, or 6‐MWD. Further comparisons studying the effect of tadalafil, prescription exercise, or a combination thereof on quality of life in COPD‐PH Veterans seem warranted to clarify these findings.
No significant difference in the rate of major adverse events was observed between treatment groups, including mortality. However, the mortality rate in the tadalafil group was notable and greater than what has been reported for other studies focusing on PH from lung disease. 24 This may reflect the lengthy study duration (1 year), or frailty associated with the study population. Among the five deaths in the tadalafil group, three occurred before randomization, and two deaths were linked to events that appear independent from PDE‐5i therapy (pulmonary embolism and following administration of a drug to treat an unrelated underlying medical condition). Baseline blood pressure emerged as a potential basis for withdrawal from this study. Given the known effect of PDE‐5i on systemic blood pressure, this finding may be useful for stratifying individualized care plans involving the management of COPD‐PH in Veterans. It is notable also that a decrease in episodes of COPD exacerbations, reported as adverse events, was observed in patients randomized to tadalafil compared to placebo. This was an unexpected finding of uncertain significance but is nonetheless consistent with anti‐inflammatory characteristics of PDE‐5i therapies as reported previously in other pulmonary pathophenotypes. 25
Inability to enroll candidate subjects with elevated pulmonary artery systolic pressure estimated by echocardiography and screened through spirometry assessment was common due to comorbid cardiovascular disease, particularly left heart failure syndromes with elevated PAWP discovered on RHC, and underscores a growing view that isolated COPD‐PH is uncommon in “real‐world” populations. 21 , 26 In this regard, the relative and unexpected rarity of isolated COPD‐PH limited our ability to reach our predetermined enrollment goal, despite utilizing 5 PH referral centers. As such, this study was overall underpowered and, therefore, our results are vulnerable to type II statistical errors. Further, the study was not designed to examine diffusing capacity for carbon monoxide (DLCO), hypercapnia, or other PFT measurements shown previously to be important in subphenotyping pulmonary vascular disease patients with COPD. 27
Elevated rate of study attrition, asymmetry in enrollment by study site, randomization imbalance, and lengthy time duration between baseline 6‐MWD and first study dose were major limitations to this study and may have biased our results. A nonpermuted block randomization strategy was selected in this study based on our original enrollment goals; however, under‐enrollment adversely affected randomization balance in addition to our statistical power. Had the study been designed with a drug enrollment goal akin to our actual enrollment population, a permuted block randomization strategy within each stratum would have been a better approach to randomization. We also note missing data above the anticipated rate for some clinical parameters. Although this was not observed for data involving the primary endpoint, we acknowledge that this limitation, like the aforementioned others, must be considered when interpreting our findings. Although the enrollment criteria emphasized COPD‐PH as the dominant clinical pathophenotype, it is possible that patients with mixed PH from lung and left heart disease were ultimately included in the study. Additionally, all patients in the study were male thereby confounding the generalization of our findings to women or nonveteran patient populations.
In conclusion, this study was not powered adequately to assess the effect of tadalafil on 6‐MWD and hemodynamic measurements in COPD‐PH Veterans, and randomization did not successfully produce groups of equal size, limiting the interpretation of our findings. Nonetheless, among the study population, treatment was associated with a within‐patient reduction in the secondary endpoint of dyspnea and improvement in health‐related quality of life that did not translate to between‐group differences. Study withdrawal was associated with lower baseline systemic blood pressure. Tadalafil treatment was not associated with a significant increase in the rate of major adverse events compared to placebo. It is notable that the overall mortality rate (including both treatment conditions) in the study was elevated, likely reflecting the frailty of this population. These data do not support the routine use of tadalafil to improve functional status in COPD‐PH; however, future studies are warranted to assess the effects of treatment on nonhemodynamic endpoints, such as patient‐reported outcomes, which may clarify the implications of the current study
CONFLICTS OF INTEREST
B.A.M. serves as a consultant for Actelion; is the PI on a research project study pulmonary hypertension sponsored by Deerfield; and is coinventor of patents or patent applications that are related to pulmonary hypertension (U.S. Patent #9,605,047; PCT/US2015/029672; Provisional ID: #62475955; Provisional ID: #24624; Provisional ID: #24622).
ETHICS STATEMENT
This study was approved by the Institutional Review Board at each participating VA location, and the Data Safety Monitoring Board, both of which oversaw the trial. Informed consent was obtained for each participant in this study.
AUTHOR CONTRIBUTIONS
Study design, study preparation, patient enrollment, data collection, data analysis, data preparation, manuscript drafting, manuscript editing: Bradley A. Maron, Gaurav Choudhary, Matthew Jankowich, Eric Garshick, Shelley Shapiro, Edward C. Dempsey, Sharon I. Rounds, and Ronald H. Goldstein. Statistical expertise, data analysis, manuscript editing: Ronald H. Goldstein. Data collection, manuscript drafting, manuscript editing: Kathleen A. LaCerda. Patient enrollment, data collection, data analysis, manuscript editing: Brack Hattler, Edward C. Dempsey, and Ruxana T. Sadikot.
Supporting information
Supporting information.
Supporting information.
ACKNOWLEDGMENTS
The investigators wish to acknowledge United Therapeutics for donating the study drug and placebo. The investigators also wish to thank Barbara S. Watson, R.N. at Rocky Mountain Regional VA Medical Center, Aurora, CO for her efforts on this study. This article has an online supplement, which is accessible from this issue's table of contents online. This study was supported by VA Merit Review grant from the Clinical Sciences Research and Development Service of the Department of Veterans Affairs, 5 I01 CX000908‐04. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government. The investigators had full and independent autonomy for the design of this study, data acquisition, data interpretation, and manuscript completion.
Maron BA, Choudhary G, Goldstein RL, Garshick E, Jankowich M, Tucker TJS, LaCerda KA, Hattler B, Dempsey EC, Sadikot RT, Shapiro S, Rounds SI, Goldstein RH. Tadalafil for veterans with chronic obstructive pulmonary disease—pulmonary hypertension: a multicenter, placebo‐controlled randomized trial. Pulm Circ. 2022;12:e12043. 10.1002/pul2.12043
Contributor Information
Sharon I. Rounds, Email: Sharon.rounds@va.gov.
Ronald H. Goldstein, Email: Ronald.goldstein@va.gov.
REFERENCES
- 1. Maron BA, Galiè N. Diagnosis, treatment, and clinical management of pulmonary arterial hypertension in the contemporary era: a review. JAMA Cardiol. 2016;1:1056–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dinh‐Xuan AT, Higenbottam TW, Clelland CA, Pepke‐Zaba J, Cremona G, Butt AY, Large SR, Wells FC, Wallwork J. Impairment of endothelium‐dependent pulmonary‐artery relaxation in chronic obstructive lung disease. N Engl J Med. 1991;324:1539–47. [DOI] [PubMed] [Google Scholar]
- 3. Andersen KH, Iversen M, Kjaergaard J, Mortensen J, Nielsen‐Kudsk JE, Bendstrup E, Videbaek R, Carlsen J. Prevalence, predictors, and survival in pulmonary hypertension related to end‐stage chronic obstructive pulmonary disease. J Heart Lung Transpl. 2012;31:373–80. [DOI] [PubMed] [Google Scholar]
- 4. Maron BA, Brittain EL, Hess E, Waldo SW, Barón AE, Huang S, Goldstein RH, Assad T, Wertheim BM, Alba GA, Leopold JA, Olschewski H, Galiè N, Simonneau G, Kovacs G, Tedford RJ, Humbert M, Choudhary G. The association between pulmonary vascular resistance and clinical outcomes in patients with pulmonary hypertension: a retrospective cohort study. Lancet Respir Med. 2020;S2213‐2600(20):30317–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maron BA, Ryan JJ. A concerning trend for patients with pulmonary hypertension in the era of evidence‐based medicine. Circulation. 2019;139:1861–64. [DOI] [PubMed] [Google Scholar]
- 6. Gillmeyer KR, Rinne ST, Glickman ME, Lee KM, Shao Q, Qian SX, Klings ES, Maron BA, Hanlon JT, Mille DR, Wiener RS. factors associated with potentially inappropriate phosphodiesterase‐5 inhibitor use for pulmonary hypertension in the United States, 2006 to 2015. Circ Cardiovasc Qual Outcomes. 2020;13(5):e005993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nathan SD, Barbera JA, Gaine SP, Harari S, Martinez FJ, Olschewski H, Olsson KM, Peacock AJ, Pepke‐Zaba J, Provencher S, Weissmann N, Seeger W. Pulmonary hypertension in chronic lung disease and hypoxia. Eur Respir J. 2019;53:1801914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen X, Tang S, Liu K, Li Q, Kong H, Zeng X, Xie W, Wang H. Therapy in stable chronic obstructive pulmonarhy disease patietns with pulmonary hypertension: a systematic review and meta‐analysis. J Thorac Dis. 2015;7:309–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Prins KW, Duval S, Markowitz J, Pritzker M, Thenappan T. Chronic use of PAH specific therapy in World Health Orangization Group III pulmonary hypertension: a systematic review and meta‐analysis. Pulmonary Circulation. 2017;7:145–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maron BA, Goldstein RH, Rounds SI, Shapiro S, Jankowich M, Garshick E, Moy ML, Gagnon D, Choudhary G. Study design and rationale for investigating phosphodiesterase type 5 inhibition for the treatment of pulmonary hypertension due to chronic obstructive lung disease: the TADA‐PHiLD (TADAlafil for pulmonary hypertension associated with chronic obstructive lung disease) trial. Pulmonary Circulation. 2013;3(4):889–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Higenbottam T. Pulmonary hypertension and chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005;2:12–9. [DOI] [PubMed] [Google Scholar]
- 12. Patterson D, McInnes GT, Webster J, Mitchell MM, Macdonald TM. Influence of a single dose of 20 mg tadalafil, a phosphodiesterase 5 inhibitor, on ambulatory blood pressure in subjects with hypertension. Br J Clin Pharmacol. 2006;62:280–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Galiè N, Brundage BH, Ghofrani HA, Oudiz RJ, Simonneau G, Safdar Z, Shapiro S, White RJ, Chan M, Beardsworth A, Frumkin L, Barst RJ, Pulmonary Arterial Hypertension and Response to Tadalafil (PHIRST) Study Group . Tadalafil therapy for pulmonary arterial hypertension. Circulation. 2009;119:2894–2903. [DOI] [PubMed] [Google Scholar]
- 14. Hayward CS, Rogers P, Keogh AM, Kelly R, Spratt PM, Macdonald PS. Inhaled nitric oxide in cardiac failure: vascular versus ventricular effects. J Cardiovasc Pharmacol. 1996;27:80–5. [DOI] [PubMed] [Google Scholar]
- 15. Rao RS, Singh S, Sharma BB, Agarwal VV, Singh V. Sildenafil improves six‐minute walk distance in chronic obstructive pulmonary disease: a randomised, double‐blind, placebo‐controlled trial. Indian J Chest Dis Allied Sci. 2011;53:81–5. [PubMed] [Google Scholar]
- 16. Vitulo P, Stanziola A, Confalonieri M, Libertucci D, Oggionni T, Rottoli P, Paciocco G, Tuzzolino F, Martino L, Beretta M, Callari A, Amaducci A, Badagliacca R, Poscia R, Meloni F, Refini RM, Geri P, Baldi S, Ghio S, D'Alto M, Argiento P, Sofia M, Guardamagna M, Pezzuto B, Vizza CD. Sildenafil in severe pulmonary hypertension associated with chronic obstructive pulmonary disease: a randomized controlled multicenter clinical trial. J Heart Lung Transplant. 2017;36:166–74. [DOI] [PubMed] [Google Scholar]
- 17. Goudie AR, Lipworth BJ, Hopkinson PJ, Wei L, Struthers AD. Tadalafil in patients with chronic obstructive pulmonary disease: a randomised, double‐blind, parallel‐group, placebo‐controlled trial. Lancet Respir Med. 2014;2:293–300. [DOI] [PubMed] [Google Scholar]
- 18. Opotowsky AR, Ojeda J, Rogers F, Prasanna V, Clair M, Moko L, Vaidya A, Afilalo J, Forfia PR. A simple echocardiographic prediction rule for hemodynamics in pulmonary hypertension. Circ Cardiovasc Imaging. 2012;5:765–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maron BA, Kovacs G, Vaidya A, Bhatt DL, Nishimura RA, Mak S, Guazzi M, Tedford RJ. Cardiopulmonary hemodynamics in pulmonary hypertension and heart failure: JACC review topic of the week. J Am Coll Cardiol. 2020;76:2671–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oldham WM, Hess E, Waldo SW, Humbert M, Choudhary G, Maron BA. Integrating hemodynamics identifies an extreme pulmonary hypertension phenotype. Eur Respir J. 2021;58:2004625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Horn E. PVDOMICS Study Group. PVDOMICS: World Symposium Classification (WSPH) Group 1 vs. Mixed WSPH Group 1 disease: splitters or lumpers. Am J Respir Crit Care Med. 2019;199:A5586. [Google Scholar]
- 22. Ries AL. Minimally clinically important difference for the UCSD Shortness of Breath Questionnaire, Borg scale, and Visual Change Analog. COPD: J Chron Obstr Pulm Dis. 2005;2:105–10. [DOI] [PubMed] [Google Scholar]
- 23. Ries A, Christensen PJ, Karla S, MacIntyre N, Make B. Pulmonary rehabilitation in the national emphysema treatment trial (NETT): effect of prior rehab experience and use of satellite centers. Am J Respir Crit Care Med. 2001;163:A13. [Google Scholar]
- 24. Nathan S, Behr J, Collard HR, Cottin V, Hoeper MM, Martinez F, Corte T, Keogh A, Leuchte H, Mogulkoc N, Ulrich S, Wuyts W, Malcolm S, Shah S, Yao M, Wells A. RISE‐IIP: riociguat for the treatment of pulmonary hypertension associated with idiopathic interstitial pneumonia. Eur Respir J. 2017;50:OA1985. [DOI] [PubMed] [Google Scholar]
- 25. Laxmi V, Gupta R, Bhattacharya SK, Ray A, Gulati K. Inhibitor effects of sildenafil and tadalafil on inflammation, oxidative stress, and nitrosative stress in animal model of bronchial asthma. Pharmacol Rep. 2019;71:517–21. [DOI] [PubMed] [Google Scholar]
- 26. Hemnes AR, Beck GJ, Newman JH, Abidov A, Aldred MA, Barnard J, Berman Rosenzweig E, Borlaug BA, Chung WK, Comhair S, Erzurum SC, Frantz RP, Gray MP, Grunig G, Hassoun PM, Hill NS, Horn EM, Hu B, Lempel JK, Maron BA, Mathai SC, Olman MA, Rischard FP, Systrom DM, Tang W, Waxman AB, Xiao L, Yuan JX, Leopold JA, PVDOMICS Study Group. PVDOMICS: a Multi‐center study to improve understanding of pulmonary vascular disease through phenomics. Circ Res. 2017;121:1136–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Boerrigter BG, Bogaard HJ, Trip P, Groepenhoff H, Rietema H, Holverda S, Boonstra A, Postmus PE, Westerhof N, Vonk‐Noordegraaf A. Ventilatory and cardiocirculatory exercise profiles in COPD: the role of pulmonary hypertension. Chest. 2012;142:1166–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.


