Abstract
Helicobacter pylori, the bacterial pathogen associated with gastritis and peptic ulcers, is highly successful in establishing infection in the human gastric mucosa, a process typically associated with massive infiltration of inflammatory cells. Colonization of the mucosa is suggested to be facilitated by H. pylori-produced cecropin-like peptides with antibacterial properties, giving the microbe a competitive advantage over other bacteria. We show that a cecropin-like antibacterial peptide from H. pylori, Hp(2-20), not only has a potent bactericidal effect but also induces proinflammatory activities in human neutrophils, e.g., upregulation of integrins (Mac-1), induction of chemotaxis, and activation of the oxygen radical producing NADPH-oxidase. Furthermore, we show that these effects are mediated through binding of Hp(2-20) to the promiscuous, G-protein-linked lipoxin A4 receptor–formyl peptide-like receptor 1.
The stomach mucosa infected with Helicobacter pylori, the causative agent of gastritis and peptic ulcers, typically shows massive infiltration of inflammatory cells. It is generally believed that the inflammatory response causes the destruction of the host mucosal tissue, a process that is probably beneficial for H. pylori by promoting a release of nutrients from the epithelial lining and enabling bacterial growth and persistence in the mucosal tissue (2). Proinflammatory proteins and peptides, generated during bacterial growth, may thus play an important role in the pathogenesis of H. pylori-associated disease (20, 22). Since the induced mucosal damage closely correlates with the infiltration of neutrophils (15), proteins and peptides that can activate these inflammatory cells are of particular interest (1, 23).
H. pylori survival in the mucosal lining is dependent on a number of different bacterial virulence features, including production of a vacuolating cytotoxin and an ability to resist phagocytic killing (1, 9). In addition, H. pylori persistence in the mucosa has been suggested to be facilitated also by antibacterial products released from the bacterium. As H. pylori itself is resistant to its antibacterial products, a release of these compounds would give H. pylori a competitive advantage over other microorganisms (21a).
The antibacterial activity of H. pylori has been traced to cecropin-like peptides derived from the amino-terminal part of its ribosomal protein L1 (RpL1) (21a). Cecropins are a group of antibacterial peptides first discovered in the context of insect immunity, later found in higher organisms (e.g., mammals), and recently, also identified in a bacterium (for a review, see reference 3). It has therefore been suggested that cecropins have their evolutionary origin in a bacterial rpl1 gene (21a). The cecropins are composed of two amphipathic α-helices joined by a hinge (3), and one of the most potent of the antibacterial cecropin-like H. pylori peptides, Hp(2-20), is composed of one such cecropin-like helix. The mechanism by which the cecropins exercise their bactericidal effect is not yet fully understood but is thought to involve formation of pore structures, leading to depolarization of the bacterial membrane (12). Whereas the molecular mechanisms behind the resistance of H. pylori to its own antibacterial peptides are unknown, the presence of cholesterol seems to protect eukaryotic membranes against the lytic activity (3).
The aim of the present study was to investigate the effect of the antibacterial peptide Hp(2-20) with respect to its proinflammatory activity on human neutrophil granulocytes, as these cells are key components in the inflammatory response evoked by H. pylori. We found that the peptide is chemotactic for neutrophils, that it induces mobilization of adhesion molecules to the cell surface, and that it activates the NADPH-oxidase. The receptor activated by Hp(2-20) was found to be the lipoxin A4 receptor–formyl peptide-like receptor 1 (LXA4R/FPRL1).
MATERIALS AND METHODS
Peptides.
A cecropin-like peptide, Hp(2-20), with a sequence (AKKVFKRLEKLFSKIQNDK) identical to that of the amino-terminal part of ribosomal protein L1 in H. pylori, was synthesized and purified by high-pressure liquid chromatography (HPLC) by Innovagen (Lund, Sweden). The peptide was dissolved in water and stored at −70°C until use. The hexapeptide Trp–Lys–Tyr–Met–Val–d-Met–NH2 (WKYMVm) was synthesized and purified by HPLC by Alta Bioscience (University of Birmingham, Birmingham, United Kingdom), and the formylated peptides formyl-Met-Leu-Phe (fMLF) and formyl-Met-Leu-Phe-Lys (fMLFK) were from Sigma Chemical Co. (St. Louis, Mo.). These peptides were dissolved in dimethyl sulfoxide to 10 mM and stored at −70°C until use. Further dilutions of all peptides were made in Krebs-Ringer phosphate buffer containing glucose (10 mM), Ca2+ (1 mM), and Mg2+ (1.5 mM) (KRG; pH 7.3). The lipopolysaccharide content in the buffers used was less than 0.15 ng/ml.
Isolation of human neutrophils.
Blood neutrophils were isolated from buffy coats from healthy blood donors by dextran sedimentation and Ficoll-Paque gradient centrifugation (5). The cells were washed and resuspended (107/ml) in KRG and stored on melting ice until use.
Antimicrobial assay.
Escherichia coli bacteria of strain MG1655 were grown overnight in Luria broth at 37°C on a rotary shaker. The bacteria were washed and diluted in phosphate-buffered saline to a density of approximately 105 bacteria/ml. The Hp(2-20) peptide was added to the bacteria at various concentrations, and these samples were then incubated at 37°C for 15 min, after which 10-μl aliquots were diluted and plated onto nutrient agar plates for determination of viable counts.
Mobilization of Mac-1.
Mobilization of subcellular organelles was followed by measurement of the level of exposure of Mac-1 (CD11b/CD18) on the neutrophil surface. Neutrophils were preincubated at 37°C for 5 min, supplemented with the peptides or KRG (control), and incubated for another 10 min at 37°C. After fixation and washing of the cells, the cells were labeled with phycoerythrin-conjugated monoclonal antibodies specific for CD11b (clone 12 catalog no. 347550 [Becton Dickinson, Mountain View, Calif.]; 10 μl to a cell pellet of 106 cell) and examined by FACScan (Becton Dickinson) analysis (10).
Neutrophil chemotaxis.
Neutrophil chemotaxis was determined with ChemoTx multiwell chambers (Neuroprobe Inc., Gaithersburg, Md.) according to the instructions given by the manufacturer. In short, neutrophils were suspended in KRG supplemented with bovine serum albumin (BSA; 0.3% [wt/vol]), and samples (30 μl of 106 cells/ml) were placed on top of 3-μm-pore-size polycarbonate filters. Various concentrations of the different peptides, diluted in KRG-BSA, were applied to the lower reservoir (below the filter). The neutrophils were allowed to migrate through the filters, and the accumulation of cells in the lower compartments was determined after a 90-min incubation period at 37°C. For quantification, the content of myeloperoxidase was assessed in the lysates of transmigrated cells by adding a peroxidase substrate (o-phenylenediamine; Dako A/S, Glostrup, Denmark). The maximal number of cells recovered in the lower compartment (achieved with the highest concentration of attractant) was about 15% of the number added to the top of the filter.
Neutrophil NADPH-oxidase activity.
The NADPH-oxidase activity was determined with luminol- and isoluminol-enhanced chemiluminescence (CL) systems that allow us to measure the released reactive oxygen species (ROS) as well as the ROS generated inside the cells (11). The CL activity was measured in a six-channel Biolumat LB 9505 (Berthold Co., Wildbad, Germany) instrument by using disposable 4-ml polypropylene tubes with a 180- or 360-μl reaction mixture containing 2 × 105 neutrophils. The tubes used for measurement of extracellular ROS release contained horseradish peroxidase (a cell-impermeant peroxidase; 4 U) and isoluminol (a cell-impermeant CL substrate; 2 × 10−5 M). Tubes used for measurement of intracellular ROS generation contained superoxide dismutase (a cell-impermeant scavenger for O2−), catalase (a cell-impermeant scavenger for H2O2), and luminol (a cell-permeant CL substrate). When the NADPH-oxidase inhibitor diphenyleneiodonium (from Sigma) was used, the inhibitor was added to the cells (10−5 M) prior to equilibration. The tubes were equilibrated in the Biolumat instrument for 5 min at 37°C, after which the stimulus (20 or 40 μl) was added. The light emission was recorded continuously.
Changes in cytosolic calcium in HL-60 cells expressing FPRL1 and FPR.
Stable expression of the formyl peptide receptor (FPR) and FPRL1 in undifferentiated HL-60 cells was obtained as described earlier (10), and their interaction with Hp(2-20) was determined by the ability of the peptide to mobilize intracellular calcium. Cells were loaded with 2 μM Fura 2-AM (Molecular Probes, Eugene, Oreg.) for 30 min at 37°C, washed, and resuspended in RPMI without phenol red. The measurements were carried out with a SPEX FluoroMAX fluorescence spectrophotometer with an excitation wavelength of 340 nm and an emission wavelength of 505 nm. Intracellular free calcium concentrations were calculated by the formula Kd(F − Fmin)/(Fmax − F) with a Kd for Fura-2 of 224 nM (7). Fmax is the fluorescence in the presence of 0.04% Triton X-100, and Fmin is the fluorescence obtained after addition of 5 mM EGTA plus 30 mM Tris-HCl (pH 7.4).
RESULTS AND DISCUSSION
Proinflammatory and antibacterial activities of Hp(2-20).
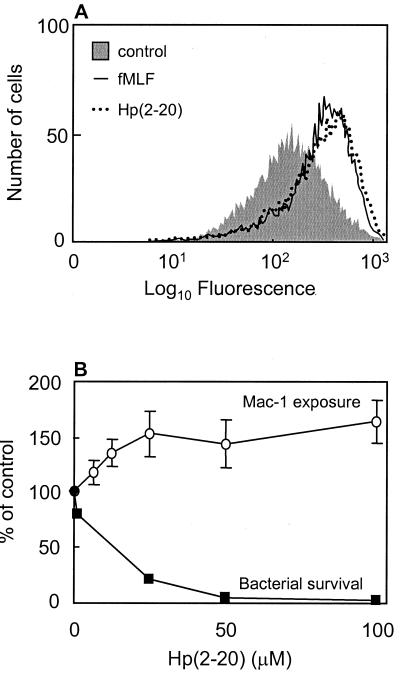
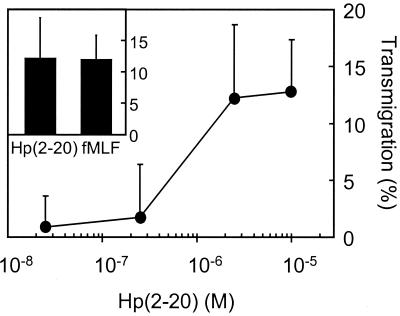
Neutrophil adhesion and endothelial transmigration are, in part, regulated at the level of integrin mobilization to the cell surface; i.e., these adhesion molecules, which are required for firm adhesion to the vessel wall, can be mobilized from intracellular organelles upon cell activation (4). To investigate if Hp(2-20) could induce granule mobilization, we measured the exposure of the integrin Mac-1 on the neutrophil surface after incubation with the peptide. We found that Hp(2-20) induced Mac-1 mobilization (Fig. 1A) in a dose-dependent manner at concentrations similar to those required for a prominent antibacterial effect (Fig. 1B). The maximal level of Mac-1 mobilization was comparable to that induced by the well-characterized neutrophil chemoattractant fMLF (27). The Mac-1 integrins are stored in neutrophil secretory organelles that supplement the plasma membrane not only with novel adhesion molecules required for adhesion to endothelial linings but also with chemoreceptors required for neutrophil extravasation into an infected tissue (26). The effect of Hp(2-20) on neutrophil migration was subsequently investigated. We found that Hp(2-20), in addition to inducing increased integrin expression, also promoted neutrophil chemotaxis (Fig. 2). The maximal migration obtained in response to Hp(2-20) was comparable to the maximal migration achieved with fMLF (Fig. 2, inset).
FIG. 1.
Neutrophil activation and bactericidal effect of Hp(2-20). (A) Hp(2-20) induces Mac-1 mobilization in human neutrophils. Surface exposure of the integrin Mac-1 on neutrophils after stimulation with Hp(2-20) (100 μM; dotted line) and fMLF (50 nM; solid line) are shown as histograms from a representative FACScan analysis. (B) Hp(2-20) induced Mac-1 mobilization in a dose-dependent manner at concentrations similar to those required for a prominent antibacterial effect. The relative increases in Mac-1 surface exposure (open circles) were calculated from the mean fluorescence intensities of cells activated with different concentrations of Hp(2-20) and are expressed as percentages of the value obtained with unstimulated cells. The results are given as means ± standard deviations (n = 4). The surviving fraction of E. coli after 15 min of incubation with the indicated Hp(2-20) concentrations (closed boxes) is also shown (representative experiment), demonstrating the bactericidal effect of the peptide.
FIG. 2.
Hp(2-20)-induced chemotaxis. Hp(2-20) induces chemotactic activity in human neutrophils to a degree comparable to that of fMLF. Neutrophil transmigration after 90 min of incubation at 37°C in response to different Hp(2-20) concentrations is shown. The inset depicts the maximal level of transmigration obtained with Hp(2-20) (100 μM) and fMLF (10 nM). Data are expressed as the percentage of transmigrated cells and are given as the means + standard deviations (n = 3). The spontaneous migration was constantly about 1%.
Hp(2-20)-induced release of ROS.
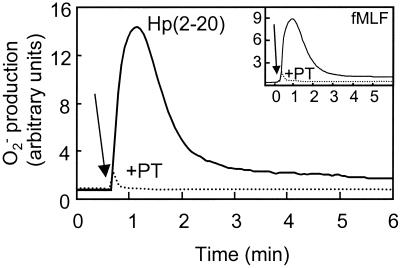
Many neutrophil chemoattractants are also activators of the neutrophil NADPH-oxidase, which transports electrons from cytoplasmic NADPH across the membrane to molecular oxygen, producing toxic oxygen radicals (i.e., superoxide anion and hydrogen peroxide) (8). Challenge of neutrophils with Hp(2-20) induced superoxide anion production (Fig. 3) with kinetics similar to that of an fMLF-induced response (Fig. 3, inset). The site of oxidant production is determined by the nature of the receptor engaged. This is illustrated by the fact that activation through FPR promotes oxidase assembly at the plasma membrane and rapid release of superoxide into the extracellular milieu. In contrast, activation through surface receptors such as CR3 and CD66 promotes oxidase assembly on internal membranes (14, 24), generating ROS inside the phagocyte. The neutrophil NADPH-oxidase activity induced by Hp(2-20) occurred exclusively at the plasma membrane (data not shown), and as a consequence, all the ROS produced was released extracellularly. The superoxide anion production was completely abolished by the addition of superoxide dismutase or by preincubation of the cells with the NADPH-oxidase inhibitor diphenyleneiodonium (data not shown). Such a release of ROS at sites of chronic inflammation not only may exert their toxic effects on infecting microorganisms but also may inflict damage on host cells and tissues (17), resulting in a release of endogenous nutrients, promoting bacterial growth at the inflammatory site (2).
FIG. 3.
Hp(2-20)-induced activation of the neutrophil NADPH-oxidase. Hp(2-20) induces superoxide anion production with kinetics resembling the kinetics of fMLF-induced superoxide anion production. Pertussis toxin completely abolishes activation of the neutrophil NADPH-oxidase, indicating that the activation is dependent on signaling via a G-protein-coupled receptor. Neutrophils were preincubated at 37°C for 60 min in the absence (solid line) or presence (dotted line) of pertussis toxin (PT 500 ng/ml) and were then stimulated with Hp(2-20) (100 μM) or fMLF (50 nM; inset). The superoxide anion release was measured by isoluminol-enhanced chemiluminescence (11). The arrows indicate the addition of peptide.
Characterization of the receptor responsible for Hp(2-20)-induced activation of Neutrophils.
The bactericidal activities of most antibacterial proteins and peptides, including those belonging to the cecropin family, are thought to be dependent on nonspecific electrostatic interactions with bacterial membrane structures such as phosphate residues of bacterial lipopolysaccharides (3, 12). In contrast, the cellular responses evoked in neutrophils are usually dependent on the binding of an agonist to specific receptors present in the plasma membrane of the cell (26). As illustrated in Fig. 3, the oxidative response induced by Hp(2-20) was abolished by preincubation of the cells with pertussis toxin, known to specifically block the signaling induced by 7-transmembrane-spanning receptors linked to Gi-type heterotrimeric G proteins (27). This result indicates that Hp(2-20)-induced activation is dependent on receptor binding.
A number of neutrophil chemoattractant receptors have been identified and characterized (28, 29), including FPR, the LXA4R/FPRL1, the C5a receptor, the CXC chemokine interleukin-8 receptor, the receptor for platelet-activating factor, and the leukotriene B4 receptor. All these receptors have been cloned by the use of exogenous expression or homology hybridization strategies, and all of them belong to the G-protein-linked 7-transmembrane family of receptors. Some of these receptors are highly specific with respect to the activating chemoattractant, whereas others are shared by many different agonists. The cellular responses induced by Hp(2-20) are in many ways similar to those induced by fMLF, suggesting that the receptor engaged by Hp(2-20) should possess similarities with FPR. LXA4R/FPRL1 was originally isolated as an orphan receptor by low-stringency cross-hybridization with FPR cDNA and has 69% sequence identity with FPR (21). The sequences of the two receptors are particularly similar in the transmembrane domains and the intracellular loops, suggesting that they transduce the same signals downstream of the receptor. The differences in the extracellular domains imply that the two receptors should bind to and be activated by different ligands. LXA4R/FPRL1 is a promiscuous receptor that, in addition to the lipoxygenase-derived eicosanoid LXA4 (16), also binds to at least five unrelated peptides and proteins (10, 19, 25, 29), also making it an attractive receptor candidate for Hp(2-20).
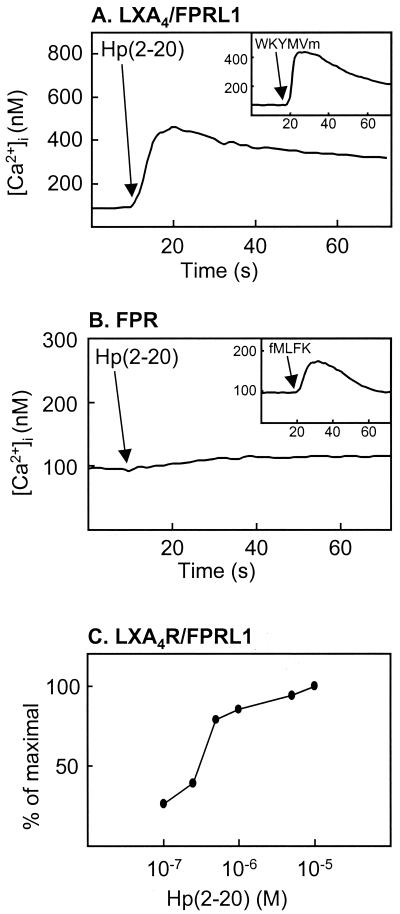
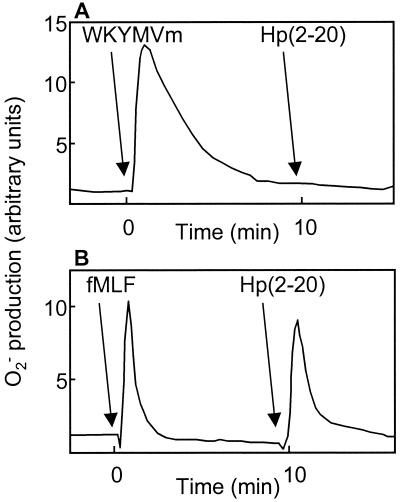
Cells stably transfected with FPR or LXA4R/FPRL1 were investigated with respect to their ability to interact with Hp(2-20). In a previous study, we had stably expressed either FPR or LXA4R/FPRL1 in HL-60 cells, a cell line of myeloid origin that does not express these receptors when the cells are undifferentiated (10). Addition of the newly described LXA4R/FPRL1 agonist WKYMVm (10) to LXA4R/FPRL1-expressing cells induced a calcium concentration rise that peaked at 450 nM (Fig. 4A, inset), and the application of Hp(2-20) at a concentration of 1 μM induced a calcium mobilization with similar kinetics (Fig. 4A). In contrast, stimulation of FPR-expressing cells with Hp(2-20) of concentrations up to 10 μM did not result in a calcium concentration rise (Fig. 4B). The 50% effective concentration of Hp(2-20)-induced calcium mobilization in LXA4R/FPRL1-expressing cells was about 300 nM (Fig. 4C). These results strongly suggest that Hp(2-20) activates neutrophils through LXA4R/FPRL1 but not through FPR. This was confirmed by desensitization (18) experiments with neutrophils performed with the agonist WKYMVm. Neutrophils first activated with WKYMVm were unable to generate a second burst of superoxide when they were challenged 10 min later with Hp(2-20) (Fig. 5A). No such desensitization was obtained with neutrophils first challenged with fMLF at a concentration of 100 nM (Fig. 5B).
FIG. 4.
Hp(2-20)-induced calcium mobilization in transfected HL-60 cells. Changes in cytosolic calcium in undifferentiated HL-60 cells stably transfected with either FPR or LXA4R/FPRL1 were measured by Fura-2 fluorescence. HL-60 cells transfected with LXA4R/FPRL1 (A) and stimulated with Hp(2-20) (1 μM) or WKYMVm (10 nM; inset) responded with a rise in the intracellular calcium concentration. Cells transfected with FPR (B) showed an increase in intracellular calcium concentration when they were stimulated with a formylated peptide (fMLFK, 10 nM; inset) but not when they were stimulated with Hp(2-20) (10 μM). The arrows indicate the addition of agonists. (C) Relative increase in the cytosolic calcium concentration induced by different concentrations of Hp(2-20) in LXA4R/FPRL1-expressing cells. Peak values for the different Hp(2-20) concentrations are given as a percentage of the maximal peak value (obtained at 10 μM).
FIG. 5.
Desensitization of Hp(2-20)-induced NADPH-oxidase activation. Binding of a chemoattractant to its G-protein-linked 7-transmembrane spanning receptor may induce NADPH-oxidase activation. Phosphorylation and cytoskeletal coupling of the ligated receptor terminate the response. This process is known as desensitization and makes the cells unable to generate a second burst of superoxide if they are challenged within 10 min with an activator that uses the same receptor (18). Neutrophils were first activated with WKYMVm (100 nM) (A) or fMLF (100 nM) (B), incubated for 10 min, and then challenged with Hp(2-20) (50 μM). WKYMVm, but not fMLF, was able to desensitize neutrophils against Hp(2-20) activation, implying that Hp(2-20) activates neutrophils via LXA4R/FPRL1 but not FPR. The superoxide anion release was measured by isoluminol-enhanced chemiluminescence (11). The arrows indicate the time of addition of agonists.
Concluding remarks.
Multiple microbial virulence factors have been suggested to affect the inflammatory response during an H. pylori infection. The bacteria have developed a unique ability to modulate neutrophil function, and earlier studies have identified a 150-kDa neutrophil-activating protein (Hp-NAP) as a key player in the inflammatory response induced by H. pylori (1, 13, 23). We now introduce a new proinflammatory H. pylori peptide that shares the basic functional characteristics of Hp-NAP; both Hp(2-20) and Hp-NAP are neutrophil chemoattractants, they activate the phagocyte NADPH-oxidase to produce and release ROS, and they are potentially released from the bacteria after “altruistic” lysis (1, 23).
Furthermore, we add Hp(2-20) to the array of agonists identified by the neutrophil LXA4R/FPRL1. LXA4R/FPRL1 is expressed by a variety of cells of hematopoietic origin as well as in hepatocytes and epithelial cells (28), suggesting that it may play an important role in both inflammatory and adaptive immunological responses. In addition to Hp(2-20), LXA4R/FPRL1 also recognizes WKYMVm, two peptides derived from human immunodeficiency virus type 1 (a leucine zipper-like domain of the human immunodeficiency virus type 1 envelope gp41 and a sequence from the V4-C4 region of gp120, respectively) (19), a necrotactic peptide derived from mitochondria (6), as well as serum amyloid A, an acute-phase protein exhibiting chemotactic activity for both neutrophils and monocytes (25). The Hp(2-20) peptide does not contain any reciprocal sequence homologies to any of the other agonists, a fact that is in agreement with the promiscuous feature of LXA4R/FPRL1.
The presence of a cecropin-like sequence in the H. pylori genome is consistent with the idea that cecropins have their evolutionary origin in a microbial parasite or symbiont (21a). Our data show that the cecropin-like peptide Hp(2-20) not only has a potent antibacterial effect but also possesses proinflammatory activity, linking these two prominent features of innate immunity. This possibly implies that the inflammatory process has evolved in close connection with the more primitive defense mechanism of antibacterial peptides. The bactericidal as well as the proinflammatory activities of cecropin-like H. pylori peptides may be essential for H. pylori virulence, and it will be intriguing to further investigate whether both properties rely on the same structural features and whether the functional dualism displayed by Hp(2-20) is a general feature among cecropin-like peptides.
ACKNOWLEDGMENTS
The work of the Swedish group of investigators was supported by the Swedish Medical Research Council, the King Gustaf V 80-Year Foundation, the Fredrik and Ingrid Thuring Foundation, the Anna-Greta Crafoord Foundation for Rheumatological Research, the Vårdal Foundation, The Swedish Society of Medicine, and the Swedish Foundation for Strategic Research. The Work of the French group of investigators was supported by grants from the Commissariat à l'Energie Atomique, the Centre National de la Recherche Scientifique (CNRS), and the Université Joseph Fourier. Thierry Christophe is the recipient of a fellowship from the Direction Général des Armées.
REFERENCES
- 1.Allen L A. Modulating phagocyte activation. The pros and cons of Helicobacter pylori virulence factors. J Exp Med. 2000;191:1451–1454. doi: 10.1084/jem.191.9.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blaser M J. Helicobacter pylori: microbiology of a ‘slow’ bacterial infection. Trends Microbiol. 1993;1:255–260. doi: 10.1016/0966-842x(93)90047-u. [DOI] [PubMed] [Google Scholar]
- 3.Boman H G. Innate immunity and the normal microflora. Immunol Rev. 2000;173:5–16. doi: 10.1034/j.1600-065x.2000.917301.x. [DOI] [PubMed] [Google Scholar]
- 4.Borregaard N, Cowland J B. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood. 1997;89:3503–3521. [PubMed] [Google Scholar]
- 5.Boyum A, Lovhaug D, Tresland L, Nordlie E M. Separation of leucocytes: improved cell purity by fine adjustments of gradient medium density and osmolality. Scand J Immunol. 1991;34:697–712. doi: 10.1111/j.1365-3083.1991.tb01594.x. [DOI] [PubMed] [Google Scholar]
- 6.Chiang N, Fierro I M, Gronert K, Serhan C N. Activation of lipoxin A(4) receptors by aspirin-triggered lipoxins and select peptides evokes ligand-specific responses in inflammation. J Exp Med. 2000;191:1197–1208. doi: 10.1084/jem.191.7.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christophe T, Rabiet M J, Tardif M, Milcent M D, Boulay F. Human complement 5a (C5a) anaphylatoxin receptor (CD88) phosphorylation sites and their specific role in receptor phosphorylation and attenuation of G protein-mediated responses. Desensitization of C5a receptor controls superoxide production but not receptor sequestration in HL-60 cells. J Biol Chem. 2000;275:1656–1664. doi: 10.1074/jbc.275.3.1656. [DOI] [PubMed] [Google Scholar]
- 8.Clark R A. Activation of the neutrophil respiratory burst oxidase. J Infect Dis. 1999;179(Suppl. 2):S309–S317. doi: 10.1086/513849. [DOI] [PubMed] [Google Scholar]
- 9.Covacci A, Telford J L, Del Giudice G, Parsonnet J, Rappuoli R. Helicobacter pylori virulence and genetic geography. Science. 1999;284:1328–1333. doi: 10.1126/science.284.5418.1328. [DOI] [PubMed] [Google Scholar]
- 10.Dahlgren C, Christophe T, Boulay F, Madianos P N, Rabiet M J, Karlsson A. The synthetic chemoattractant Trp-Lys-Tyr-Met-Val-dMet activates neutrophils preferentially through the lipoxin A(4) receptor. Blood. 2000;95:1810–1818. [PubMed] [Google Scholar]
- 11.Dahlgren C, Karlsson A. Respiratory burst in human neutrophils. J Immunol Methods. 1999;232:3–14. doi: 10.1016/s0022-1759(99)00146-5. [DOI] [PubMed] [Google Scholar]
- 12.Epand R M, Vogel H J. Diversity of antimicrobial peptides and their mechanisms of action. Biochim Biophys Acta. 1999;1462:11–28. doi: 10.1016/s0005-2736(99)00198-4. [DOI] [PubMed] [Google Scholar]
- 13.Evans D J, Jr, Evans D G, Takemura T, Nakano H, Lampert H C, Graham D Y, Granger D N, Kvietys P R. Characterization of a Helicobacter pylori neutrophil-activating protein. Infect Immun. 1995;63:2213–2220. doi: 10.1128/iai.63.6.2213-2220.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feuk-Lagerstedt E, Jordan E T, Leffler H, Dahlgren C, Karlsson A. Identification of CD66a and CD66b as the major galectin-3 receptor candidates in human neutrophils. J Immunol. 1999;163:5592–5598. [PubMed] [Google Scholar]
- 15.Fiocca R, Luinetti O, Villani L, Chiaravalli A M, Capella C, Solcia E. Epithelial cytotoxicity, immune responses, and inflammatory components of Helicobacter pylori gastritis. Scand J Gastroenterol Suppl. 1994;205:11–21. [PubMed] [Google Scholar]
- 16.Fiore S, Maddox J F, Perez H D, Serhan C N. Identification of a human cDNA encoding a functional high affinity lipoxin A4 receptor. J Exp Med. 1994;180:253–260. doi: 10.1084/jem.180.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halliwell B. Free radicals, antioxidants, and human disease: curiosity, cause, or consequence? Lancet. 1994;344:721–724. doi: 10.1016/s0140-6736(94)92211-x. [DOI] [PubMed] [Google Scholar]
- 18.Klotz K N, Krotec K L, Gripentrog J, Jesaitis A J. Regulatory interaction of N-formyl peptide chemoattractant receptors with the membrane skeleton in human neutrophils. J Immunol. 1994;152:801–810. [PubMed] [Google Scholar]
- 19.Le Y, Shen W, Li B, Gong W, Dunlop N M, Wang J M. A new insight into the role of “old” chemotactic peptide receptors FPR and FPRL1: down-regulation of chemokine receptors CCR5 and CXCR4. Forum (Genoa) 1999;9:299–314. [PubMed] [Google Scholar]
- 20.Mai U E, Perez-Perez G I, Allen J B, Wahl S M, Blaser M J, Smith P D. Surface proteins from Helicobacter pylori exhibit chemotactic activity for human leukocytes and are present in gastric mucosa. J Exp Med. 1992;175:517–525. doi: 10.1084/jem.175.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy P M, Ozcelik T, Kenney R T, Tiffany H L, McDermott D, Francke U. A structural homologue of the N-formyl peptide receptor. Characterization and chromosome mapping of a peptide chemoattractant receptor family. J Biol Chem. 1992;267:7637–7643. [PubMed] [Google Scholar]
- 21a.Putsep K, Branden C I, Boman H G, Normark S. Antibacterial peptide from H. pylori. Nature. 1999;398:671–672. doi: 10.1038/19439. [DOI] [PubMed] [Google Scholar]
- 22.Rautelin H, Blomberg B, Fredlund H, Jarnerot G, Danielsson D. Incidence of Helicobacter pylori strains activating neutrophils in patients with peptic ulcer disease. Gut. 1993;34:599–603. doi: 10.1136/gut.34.5.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Satin B, Del Giudice G, Della Bianca V, Dusi S, Laudanna C, Tonello F, Kelleher D, Rappuoli R, Montecucco C, Rossi F. The neutrophil-activating protein (HP-NAP) of Helicobacter pylori is a protective antigen and a major virulence factor. J Exp Med. 2000;191:1467–1476. doi: 10.1084/jem.191.9.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Serrander L, Larsson J, Lundqvist H, Lindmark M, Fallman M, Dahlgren C, Stendahl O. Particles binding beta(2)-integrins mediate intracellular production of oxidative metabolites in human neutrophils independently of phagocytosis. Biochim Biophys Acta. 1999;1452:133–144. doi: 10.1016/s0167-4889(99)00123-8. [DOI] [PubMed] [Google Scholar]
- 25.Su S B, Gong W, Gao J L, Shen W, Murphy P M, Oppenheim J J, Wang J M. A seven-transmembrane, G protein-coupled receptor, FPRL1, mediates the chemotactic activity of serum amyloid A for human phagocytic cells. J Exp Med. 1999;189:395–402. doi: 10.1084/jem.189.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uhing R J, Snyderman R. Chemoattractant stimulus-response coupling. In: Gallin J I, Snyderman R, editors. Inflammation: basic principles and clinical correlates. 3rd ed. New York, N.Y: Raven Press; 1999. pp. 607–626. [Google Scholar]
- 27.West R E, Jr, Moss J, Vaughan M, Liu T, Liu T Y. Pertussis toxin-catalyzed ADP-ribosylation of transducin. Cysteine 347 is the ADP-ribose acceptor site. J Biol Chem. 1985;260:14428–14430. [PubMed] [Google Scholar]
- 28.Ye R D, Boulay F. Structure and function of leukocyte chemoattractant receptors. Adv Pharmacol. 1997;39:221–289. doi: 10.1016/s1054-3589(08)60073-3. [DOI] [PubMed] [Google Scholar]
- 29.Yokomizo T, Izumi T, Chang K, Takuwa Y, Shimizu T. A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature. 1997;387:620–624. doi: 10.1038/42506. [DOI] [PubMed] [Google Scholar]