Abstract
Background
the aim of this systematic review and meta-analysis was to update and synthesise the totality of research evidence on the effectiveness of acute geriatric unit (AGU) care for older adults admitted to hospital with acute medical complaints.
Methods
MEDLINE, CINAHL, CENTRAL and Embase databases were systematically searched from 2008 to February 2022. Screening, data extraction and quality grading were undertaken by two reviewers. Only trials with a randomised design comparing AGU care and conventional care units were included. Meta-analyses were performed in Review Manager 5.4 and the Grading of Recommendations, Assessment, Development and Evaluations framework was used to assess the certainty of evidence. The primary outcome was incidence of functional decline between baseline 2-week prehospital admission status and discharge and at follow-up.
Results
11 trials recruiting 7,496 participants across three countries were included. AGU care resulted in a reduction in functional decline at 6-month follow-up (risk ratio (RR) 0.79, 95% confidence interval (CI) 0.66–0.93; moderate certainty evidence) and an increased probability of living at home at 3-month follow-up (RR 1.06, 95% CI 0.99–1.13; high certainty evidence). AGU care resulted in little or no difference in functional decline at hospital discharge or at 3-month follow-up, length of hospital stay, costs, the probability of living at home at discharge, mortality, hospital readmission, cognitive function or patient satisfaction.
Conclusions
AGU care improves clinical and process outcomes for hospitalised older adults with acute medical complaints. Future research should focus on greater inclusion of clinical and patient reported outcome measures.
Keywords: older adults, functional decline, comprehensive geriatric assessment, acute geriatric unit, meta-analysis
Key points
This updated systematic review and meta-analysis included 11, heterogeneous, trials across three countries.
Acute geriatric unit (AGU) care improves clinical and process outcomes for hospitalised older adults with acute medical complaints.
Future research should consider greater inclusion of clinical and patient reported outcome measures.
Clinicians should embed use of frailty criterion, when selecting older adults for admission to an AGU.
Introduction
Population ageing poses a major challenge to health care systems internationally with older adults being the predominant users of inpatient hospital services [1]. Older adults are clinically heterogeneous and are at increased risk of adverse outcomes during hospitalisation due to the presence of multiple comorbid and complex conditions [2, 3]. The most potent intrinsic risk factor in this population group is the clinical condition of frailty [4, 5]. The presence of diminished homeostatic reserves leaves older adults more vulnerable to iatrogenic complications that are not specific to the underlying presentation and occur during the course of care [6]. Hospital-associated disability [HAD], characterised by an acceleration in functional decline with concomitant loss of independence in activities of daily living (ADL) is a common phenomenon [7]. Prevalence of HAD in acute care of older adults was reported at 30% in a recent meta-analysis of 15 studies [8], which highlights the elevated risks this patient population are exposed to during hospitalisation.
Comprehensive Geriatric Assessment (CGA) is considered the gold standard approach to improving a range of outcomes for frail older adults in acute hospitals [9]. A central tenet of CGA delivery are interdisciplinary teams who identify medical, social, and functional needs and develop a coordinated and integrated plan for treatment and follow-up [10]. Several organisational forms of CGA delivery have been proposed to achieve effective and efficient care for older adults with undifferentiated medical complaints; however, the optimal method by which to deliver care hospital wide is unclear [11]. Mobile inpatient geriatric consultation teams and dedicated geriatric evaluation and management units have shown favourable effects on mortality at 6 and 8 months after discharge [12], functional decline at discharge from hospital and institutionalisation at 1 year follow-up [13], respectively. A previous meta-analysis published in 2009 included five randomised controlled trials (RCT) and explored the outcomes of hospitalised older adults by examining the effectiveness of admission to an acute geriatric unit (AGU) when compared to conventional hospital care [14]. The authors defined an AGU as a unit designed with its own physical location and structure, which provide care to older adults during admission to hospital for an acute medical illness including acute exacerbations of chronic diseases. Findings showed that compared with older adults admitted to conventional care units, those admitted to an AGU showed a lower odds of functional decline at discharge from hospital (odds ratio (OR) 0.82, 95% CI 0.68–0.99) and an increased odds of living at home after discharge (OR 1.30, 95% CI 1.11–1.52). Since this review was published, several primary research studies have examined the impact of AGUs on clinical and process outcomes more broadly.
Our review aims to update the totality of research evidence related to the effectiveness of AGU care compared with conventional care units among older adults admitted to hospital with acute medical complaints.
Methods
Registration
The Preferred Reporting Items for Systematic Reviews and Meta-Analysis Protocol (PRISMA-P) [15] was used to prepare this manuscript. The protocol was registered on PROSPERO (CRD 42021237633) and published elsewhere [16]. A completed PRISMA checklist [17] is presented in Supplementary Material A1.
Deviations from the protocol
Since protocol publication, the authors updated their interpretation of heterogeneity and the associated I2 statistic [18]. Due to expected clinical and methodological heterogeneity between intervention and conventional care units across international healthcare systems, we elected to use a random-effects model for all meta-analyses.
Search strategy and study selection
MEDLINE in EBSCO, Cumulative Index of Nursing and Allied Health Literature (CINAHL), Cochrane Central Register of Controlled Trials (CENTRAL) and EMBASE databases were searched with exploded subject headings and relevant keywords, in all languages. Databases were searched from 2008 to 07 February 2022 as this review is an update of a previous meta-analysis [14]. Trials included in the previous version of the review were integrated into the new evidence found. We only included peer-reviewed publications, i.e. grey literature and abstracts were excluded. An example search strategy from MEDLINE in EBSCO is presented in Supplementary Material A2. References generated from the search strategy were exported into Endnote software and duplicates deleted; reference lists of included studies were searched for additional papers.
To ensure scientific rigour when assessing intervention effects, we chose to only include trials with a randomised design. RCTs including cluster-RCTs and quasi-RCTs were also included in this updated systematic review and meta-analysis.
Eligibility criteria
Included trials satisfied the following criteria:
Population—older adults (≥65 years) admitted to an AGU with acute medical complaints.
Intervention—Access to an AGU model of care delivered by interdisciplinary teams during the acute phase of illness to prevent functional decline and related complications in older adults admitted to the acute care setting [14]. The 2017 Cochrane review by Ellis et al. [9] was used as the reference standard when describing the components of CGA across the included trials. In their review, they outline the following components: clinical leadership, structured assessment, multidisciplinary team meetings, goal setting, involving patients and carers in goal setting, outpatient follow-up, ward environment, adequate time, specialty knowledge, experience and competence, and tailoring treatment plans to the individual.
Comparison—usual care, other non-AGU interventions such as admission to acute medical wards.
Exclusion criteria
We excluded trials that involved patients aged <65 years and that evaluated interventions aimed at specific medical or surgical complaints or speciality units such as stroke or orthogeriatric units. To ensure we did not include trials that evaluated interventions in the sub-acute phase, we excluded trials where patients were transferred from other speciality units such as intensive care to an AGU or where patients were admitted to an AGU three or more days after a hospital admission.
Outcome variables
The primary outcome measure was functional decline between baseline 2-week prehospital admission status and discharge and at follow-up. We defined functional decline as a net decrease in the number of ADLs performed independently when compared with prehospital admission baseline [19]. To assess independence in ADLs, we considered any validated measure of functional status, e.g. the Katz index of independence in ADLs [20], Barthel Index [21], ADL Staircase [22] etc.
The secondary outcomes were length of hospital stay, cost of index admission, living at home, mortality, incidence of unscheduled hospital readmission, cognitive function and patient satisfaction with the index admission. Outcomes were recorded at discharge from hospital and at follow-up periods reported in trials.
Data extraction
Two authors (ÍO’S and RG) independently conducted title and abstract screens identifying trials for full-text extraction. Full-text screening was used to identify a final list of included trials. In cases of disagreement, a third author (MO’C) reviewed the trials and agreement was reached by consensus. Relevant data were extracted independently by two authors (ÍO’S and RG) from the included trials into a pre-established Microsoft excel file.
Quality of evidence and risk of bias
Two authors (ÍO’S and RG) independently assessed the risk of bias (RoB) using the Cochrane RoB 2 tool [23] under the following domains: randomisation process, deviation from intended intervention, missing outcome data, outcome measurement, selective reporting and the overall risk of bias. The Grading of Recommendations, Assessment, Development and Evaluations (GRADE) framework was used to assess the quality of evidence for outcomes reported and to summarise data narratively [24].
Data synthesis
For the primary outcome of incidence of functional decline, we calculated risk ratios (RRs) with 95% confidence intervals (CIs) to determine the intervention effect. We dichotomised the overall outcome and considered functional decline to be a deterioration in ADLs; we excluded measures where the direction of change was not presented, i.e. improved, maintained, decreased. RRs with 95% CIs were applied to all other dichotomous secondary outcomes (living at home, mortality, etc.) For continuous outcomes (length of hospital stay and cost of index admission), we calculated the mean difference (MD) between AGU care and conventional care units with a 95% CI. When the standard deviation (SD) was not available, we estimated it from the standard error or 95% CI [25], which were reported in two trials. Data for the meta-analyses were analysed using Review Manager 5.4 software. Due to the expected differences in delivery of AGU interventions across international healthcare system and the date range of included trials, we elected to use a random-effects model for all meta-analyses.
Dealing with missing data
We contacted the authors of included trials to request missing data. The standard deviation for three trials that reported outcomes on length of hospital stay and costs were obtained from a previous systematic review, which examined the effectiveness of AGU care based on the Acute Care for Elders model with acutely ill or injured older adults [26].
Assessment of heterogeneity
We explored heterogeneity across the trials by visually inspecting the forest plots and the associated I2 statistics. We considered I2 > 50% as significant heterogeneity. For outcomes where I2 was >50%, we explored the individual trial characteristics to identify potential sources of heterogeneity, using pre-planned subgroup analyses. Analyses were repeated after removing trials that were conducted in the previous version of the review.
Sensitivity analysis
We applied a fixed-effect model as a form of sensitivity analysis. We explored changes in intervention effect by removing trials with evidence of a high risk of bias in the randomisation process and missing outcome data.
Ethics
Ethical approval was not required for this review.
Results
Trial identification
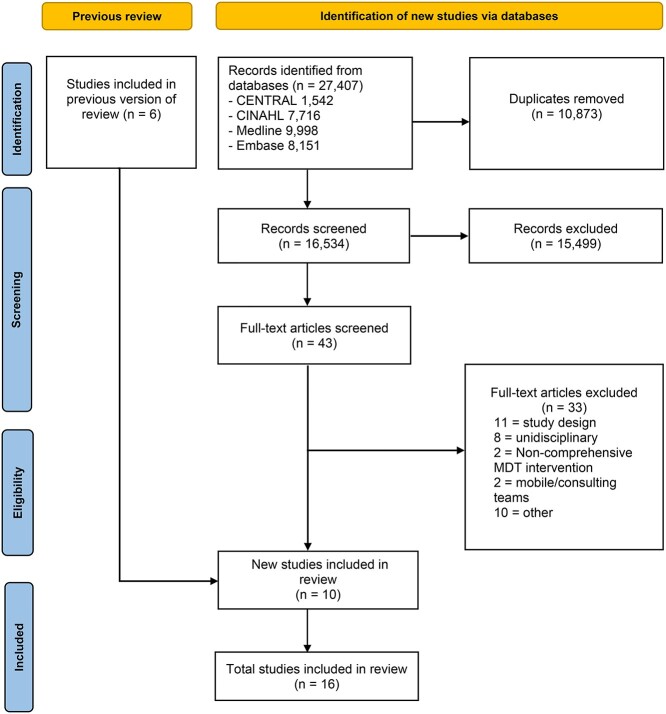
The literature search yielded 16,534 records. Following title and abstract screening, 43 articles were selected for full-text critical reading (Figure 1). Ten studies [27–36] recruiting 3,939 participants, including six original trials and four subsequent papers reporting secondary outcomes related to the original trials by Ekerstad et al. [33–35], and Westgard et al. [36] were included. As this is an updated review, data from five trials [37–41] and one secondary analysis [42] recruiting 3,557 participants from the previous version of the review were integrated into the new evidence found. Therefore, a total of 11 trials recruiting 7,496 older adults were identified.
Figure 1.
PRISMA diagram for updated systematic reviews.
Descriptive characteristics of included trials
The trials dated from 1985 to 2020; seven were conducted in the USA [27–30, 37, 39, 41], three in Sweden [31, 32, 40] and one in Australia [38]. Seven were RCTs [29, 32, 37–41], three were quasi-RCTs [27, 28, 31] and one was a cluster-RCT [30]. Authors of six trials were contacted for additional information [29, 32, 38–41]. A table outlining the descriptive characteristics of included trials is presented in Supplementary Material A3.
Patient selection criteria to the intervention unit was largely based on age and the presence of an acute medical complaint that did not require admission to a speciality unit [27, 28, 30–32, 39, 40]. Three of the 11 trials included patients aged  65 years [27, 30, 37], six aged ≥70 years [28, 29, 38–41] and two trials included patients aged
65 years [27, 30, 37], six aged ≥70 years [28, 29, 38–41] and two trials included patients aged  75 years [31, 32]. Three trials operated frailty criterion through use of screening tools when selecting patients for inclusion [30–32]. Eight trials admitted patients to the intervention unit directly from the emergency department [27, 30, 32, 37–41], one trial admitted patients directly from the ambulance or primary care [31], and two trials did not report source of admission [28, 29]. The characteristics of the intervention units were similar across trials. Bed capacity ranged from 10 beds to 34 beds; three trials did not report this unit [29, 30, 32]. The key components of intervention units are outlined in Figure 2. The core interdisciplinary team comprised of at least one geriatrician and/or primary physician and registered nurses with geriatric training. A physiotherapist was included in all 11 intervention units, a social worker in nine units and an occupational therapist was included in eight units. The presence of a dietitian was reported in four units [30, 32, 39, 40], and a pharmacist was included in two units [28, 30].
75 years [31, 32]. Three trials operated frailty criterion through use of screening tools when selecting patients for inclusion [30–32]. Eight trials admitted patients to the intervention unit directly from the emergency department [27, 30, 32, 37–41], one trial admitted patients directly from the ambulance or primary care [31], and two trials did not report source of admission [28, 29]. The characteristics of the intervention units were similar across trials. Bed capacity ranged from 10 beds to 34 beds; three trials did not report this unit [29, 30, 32]. The key components of intervention units are outlined in Figure 2. The core interdisciplinary team comprised of at least one geriatrician and/or primary physician and registered nurses with geriatric training. A physiotherapist was included in all 11 intervention units, a social worker in nine units and an occupational therapist was included in eight units. The presence of a dietitian was reported in four units [30, 32, 39, 40], and a pharmacist was included in two units [28, 30].
Figure 2.
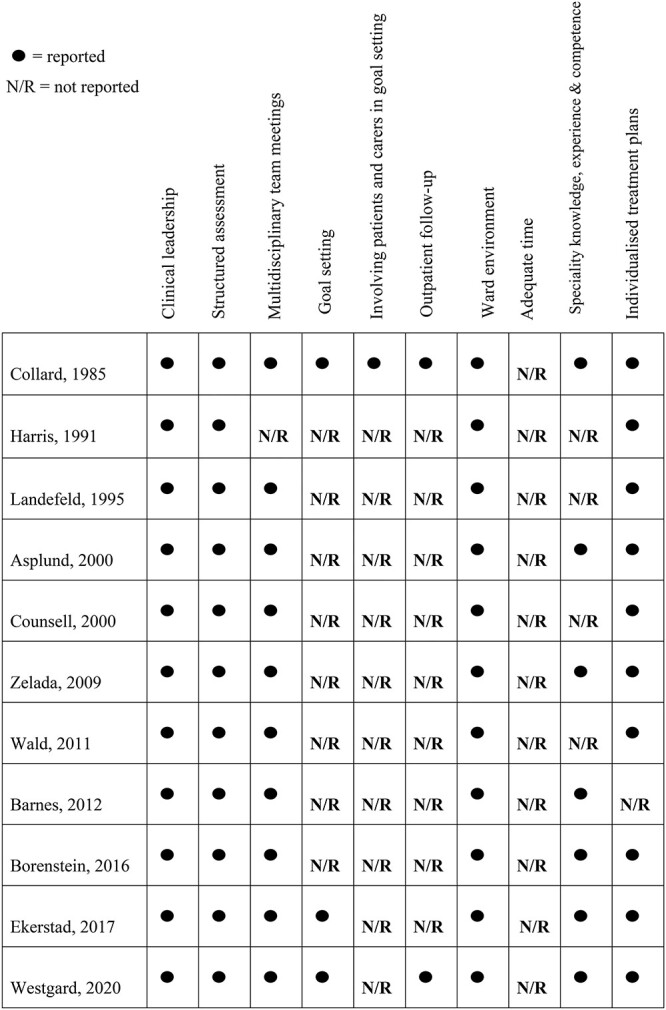
Components of CGA outlined in trials.
The follow-up period varied. Seven of the 11 trials provided data on one or several of the outcome variables at 3 months, at a minimum [29, 31, 32, 38–41]. Six trials reported on the primary outcome of functional decline, five at 3 months [31, 38–41] and three at 6 months [32, 38, 41].
Methodological quality
Assessment of the risk of bias for specific outcomes and endpoints are included in Supplementary Material A4. Four trials were rated as having a high risk of bias in the randomisation process as the sequence was generated based on bed availability in two trials [27, 31] and an open allocation schedule was used in two trials [28, 38]. Use of an appropriate analysis to estimate the effect of assignment to intervention, e.g. intention-to-treat was only reported in five trials, which threatened the deviations from intended intervention [30–32, 40, 41]. Only one trial published a protocol with prespecified outcomes [32]. Supplementary Material A5 summarises the certainty of the evidence for outcomes included in the meta-analyses using the GRADE framework [24].
Functional decline
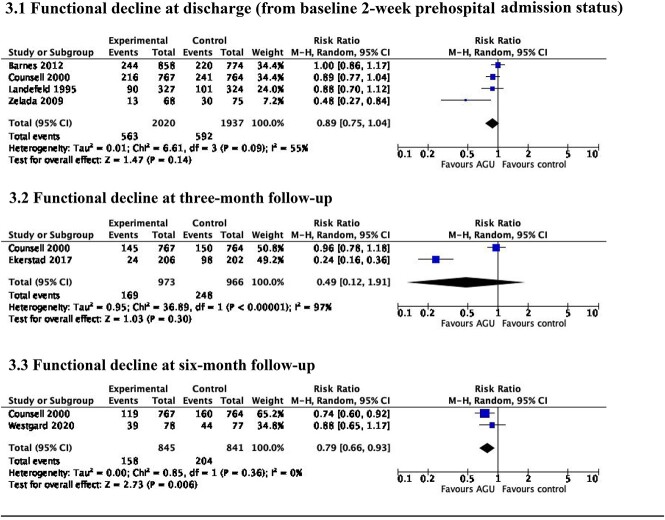
AGU care resulted in little or no difference in functional decline between baseline 2-week prehospital admission status and discharge (RR 0.89, 95% CI 0.75–1.04, I2 = 55%; low certainty evidence) [27, 29, 39, 41] (Figure 3.1), or at 3-month follow-up (RR 0.49, 95% CI 0.12–1.91, I2 = 97%; very low certainty evidence) (Figure 3.2). Five trials [31, 38–41] reported outcomes at three-month follow-up, with complete data in two trials [31, 41]. AGU care was associated with a 21% reduction in functional decline at six-month follow-up (RR 0.79, 95% CI 0.66–0.93, I2 = 0%; moderate certainty evidence) (Figure 3.3). Three trials [32, 38, 41] reported outcomes, with complete data in two trials [32, 41].
Figure 3.
Functional decline at discharge from hospital and at 3- and 6-month follow-up.
Length of hospital stay
Ten of the 11 trials reported data on length of hospital stay, with complete data in nine trials [27–31, 38–41]. Results indicated that there was no benefit from admission to an AGU on length of hospital stay (MD –0.36, 95% CI –0.99 to 0.26, I2 = 77%: low certainty evidence).
Costs
Seven trials reported data on the cost of the index admission, with complete data in six trials [28, 29, 31, 40–42]. Four of the included trials were conducted in the USA [28, 29, 41, 42] and two in Sweden [31, 40]. One of the Swedish trials reported costs in US dollars [31]; costs related to the trial conducted by Asplund et al. [40] were calculated by applying the exchange rate (Swedish krona–US dollars) provided by authors at the time of the trial. AGU care was not associated with cost savings (MD –123.79USD, 95% CI –567.80USD to 320.22USD, I2 = 45%; low certainty evidence).
Living at home
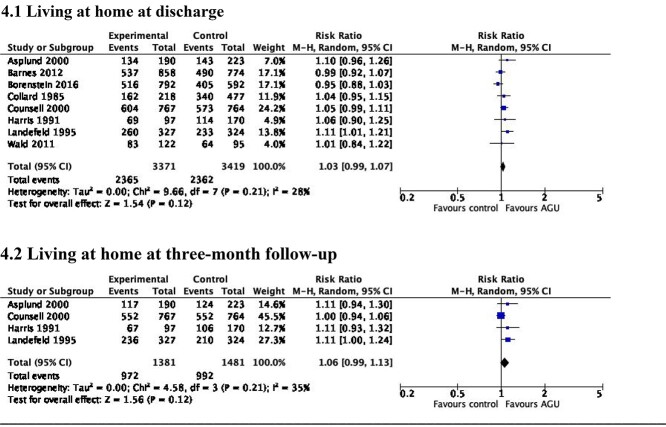
AGU care did not increase the probability of living at home at discharge (RR 1.03, 95% CI 0.99–1.07, I2 = 28%; high certainty evidence) [28–30, 37–41] (Figure 4.1). An increase in the probability of living at home was associated with AGU care at 3-month follow-up (RR 1.06, 95% CI 0.99–1.13, I2 = 35%; high certainty evidence) [38–41] (Figure 4.2).
Figure 4.
Living at home at discharge from hospital and at 3-month follow-up.
Admission to a long-term care institution
AGU care resulted in no difference in the probability of admission to a long-term care institution at discharge (RR 1.01, 95% CI 0.78–1.30, I2 = 72%; low certainty evidence) [28, 30, 37, 39–41], or at 3-month follow-up (RR 0.88, 95% CI 0.72–1.09, I2 = 30%; moderate certainty evidence) [39–41]. Two of the six trials that contributed to the analysis of this measure, referred to long-term care institutions as skilled nursing facilities, rehabilitation hospitals, nursing homes or other institutions assisting with daily activities [30, 39]. Three trials classified them as nursing homes [37, 40, 41] and the remaining trial [28] did not provide detail on the nature of the discharge institution.
Mortality
AGU care resulted in little or no difference in mortality during the index admission (RR 0.89, 95% CI 0.68–1.17, I2 = 27%; low certainty evidence) [29–31, 37–41], or at 3-month follow-up (RR 0.93, 95% CI 0.79–1.09, I2 = 4%; low certainty evidence) [31, 38–41].
Hospital re-admission
AGU care resulted in no difference in the probability of re-admission to hospital at 1-month (RR 1.01, 95% CI 0.80–1.28, I2 = 53%; low certainty evidence) [28, 30, 31, 41], or 3-month follow-up (RR 0.95, 95% CI 0.82–1.09, I2 = 15%; moderate certainty evidence) [29, 31, 39, 41].
Cognitive function
Three trials reported data on cognitive function on discharge [27, 38, 39], with complete data in two trials [27, 39]. It was not feasible to perform a meta-analysis as the outcome was measured differently across trials. Zelada et al. reported a higher incidence of impaired cognitive function as measured by the Mini Mental State Examination (MMSE) [43] in the AGU group. Results reported in Landefeld et al. were similar between groups. Only one trial presented data at 3-month follow-up [40], which found that fewer AGU patients had impaired cognitive function on the MMSE.
Patient satisfaction
Patient satisfaction with hospital care during the index admission was presented in two trials [32, 41]. Westgard et al. reported no differences between groups on patient satisfaction with hospital care (OR 0.94, 95% CI 0.34–2.57). Trialists used eight questions based on the Pyramid Questionnaire [44]; patients were provided with a scale with five responses from agree completely to disagree completely. The results above pertain to the statement: ‘I am satisfied with the hospital care’. Counsell et al. reported higher satisfaction with care among patients cared for in an AGU when compared with conventional care units (75 (mean) ± 16 (SD) vs 72 ± 17; P = 0.012). Likert scales, with a range of 0–100 were used to rate satisfaction with individual items on questionnaires. Both trials measured satisfaction at 1-month follow-up.
Sensitivity analysis
Re-running analyses using a fixed-effects model resulted in a decrease in the probability of functional decline at 3-months (RR 0.68, 95% CI 0.57–0.81; I2 = 97%) and in a reduced length of hospital stay (MD –0.40, 95% CI –0.67 to −0.13; I2 = 77%). Removal of trials with a high RoB in the randomisation process [27, 28, 31, 38] resulted in a reduced length of hospital stay (MD –0.75, 95% CI –1.21 to 0.30; I2 = 47%). Also, re-running the analysis for functional decline between baseline 2-week prehospital admission status and discharge, after removal of trials that had a high RoB in missing outcome data [29] resulted in a decrease in the probability of decline (RR 0.82, 95% CI 0.65–1.03: I2 = 55%). No differences were found after excluding trials that were conducted in the previous version of the review [14].
Discussion
Summary
The results of this updated systematic review and meta-analysis suggest that AGU care decreases the probability that hospitalised older adults with acute medical complaints will experience functional decline at 6-month follow-up and are more likely to be living at home at 3-month follow-up. Findings related to functional decline pertain to two trials, recruiting 1,686 older adults conducted in 2020 and 2000, respectively [32, 41]. Trialists did not report the specific factors associated with this medium-term effect. Maintaining independence in the performance of ADLs is an important determinant of quality of life for older adults [45]; therefore, any intervention that assists in reducing the risk of functional decline must be considered and evaluated across the continuum of care. [46]. Although older adults had a higher probability of living at home at 3 months, no differences were found between groups at hospital discharge. This finding contrasts with those reported in the 2017 Cochrane review [9] and previous meta-analyses [14, 26]. Four of the eight trials that were included in the analysis for discrete wards in the 2017 Cochrane review [9] did not meet our inclusion criteria and dated from 1982 to 1994. Low heterogeneity between trials (I2 = 28%) (Figure 4.1) adds to our confidence in the certainty of evidence for this outcome. The high heterogeneity between trials in length of hospital stay (I2 = 77%) (Supplementary Material A6) is comparable to a recent meta-analysis, which found that various CGA intervention models had no effect on length of hospital stay [47].
Characteristics of an AGU
The included trials provided variable levels of information about the characteristics and organisational form of conventional care units. This is an important consideration given that the effectiveness of AGUs is measured by comparison with these units. Where the core team were described, access to physiotherapy, occupational therapy and social work tended to operate on a referral basis with limited focus on specialisation of care. However, healthcare systems and standards of care have undoubtedly evolved since a number of trials included in this updated review were conducted. CGA is considered a complex intervention [48]; therefore, this review aimed to identify the characteristics and components of CGA within an AGU. Whilst variability in reporting was observed, several distinct components emerged across all 11 trials. Clinical leadership from a geriatrician and/or primary physician and structured comprehensive assessment coupled with individualised intervention plans, delivered by a core team of professionals were consistent across trials. A dedicated ward environment and interdisciplinary team meetings (ranging from daily in six trials to once a week in two trials) were also distinct features. Such features may contribute to the overall effectiveness of AGU care, as has been shown in patients with stroke [49].
Implications for research and clinical practice
It is important to determine the main beneficiaries of admission to an AGU. In our review, the impact of frailty as a determining factor of CGA outcome and/or criterion for admission to an AGU was only examined in three of the 11 trials [30–32]. Although contemporary research findings recommend selecting older adults for CGA interventions based on frailty criteria [11, 50] others found that it was beneficial to use an age-based criterion for selection [51]. Given that frailty status on admission to hospital is predictive of multiple adverse outcomes [4], future trials should include stratification by frailty and the influence of case mix on the effectiveness of AGU care must be examined. Furthermore, the overall RoB across included trials and outcomes was generally ‘high’ or ‘some concerns’ therefore highlighting the need for future methodologically robust trials. Inclusion of process evaluations, in line with the updated Medical Research Council framework for the evaluation of complex interventions should also be considered [48].
CGA is considered a patient-centred process and is both therapeutic and diagnostic [10]; however, clinical and patient reported outcome measures (PROM) were not routinely included across trials. Future research should focus on inclusion of PROMs such as self-rated health status; use of PROMs may assist clinicians in re-focusing care and interventions around older adults’ priorities and preferences [50].
Clinicians may anticipate moderate beneficial effects with respect to preservation of older adults’ functional status and the probability of living at home in the medium term. Given the multifactorial care needs of hospitalised older adults, an interdisciplinary team approach whereby clinicians embed geriatric competencies into their practice is recommended [52].
Strengths and limitations
This review was methodologically robust according to the quality of reporting of meta-analyses and PRISMA reporting guidelines. Most of the trials were conducted in the USA, which may limit generalisability to other health systems. The psychometric limitations imposed by dichotomising the primary outcome of functional decline meant that the interplay of floor or ceiling effects were a factor where there was no change in older adults’ ADL ability at discharge and/or follow-up. The quality and range of outcomes reported was variable, thereby limiting our ability to pool data across trials. Whilst trialists broadly reported the components of CGA within an AGU, they did not provide data on the critical components responsible for the benefits observed. Finally, apart from functional decline, conclusions cannot be drawn on the effectiveness of AGU care on relevant outcomes beyond three months following hospital discharge.
Supplementary Material
Declaration of Sources of Funding
None.
Declaration of Conflicts of Interest
None.
References
- 1. World Health Organization . Integrated Care for Older People: Guidelines on Community-Level Interventions to Manage Declines in Intrinsic Capacity 2017. https://www.who.int/publications/i/item/9789241550109 (17 January 2021, accessed). [PubMed]
- 2. Lim SS, Vos T, Flaxman ADet al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet 2013; 380: 2224–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ruiz M, Bottle A, Long S, Aylin P. Multi-morbidity in hospitalized older patients: who are the complex elderly? PLoS One 2015; 10: e0145372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hubbard RE, Peel NM, Samanta Met al. Frailty status at admission to hospital predicts multiple adverse outcomes. Age Ageing 2017; 46: 801–6. [DOI] [PubMed] [Google Scholar]
- 5. Clegg A, Young J, Iliff S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet 2013; 381: 752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lafont C, Gérard S, Voisin Tet al. Reducing “iatrogenic disability” in the hospitalized frail elderly. J Nutr Health Aging 2011; 15: 645–60. [DOI] [PubMed] [Google Scholar]
- 7. Zisberg A, Shadmi E, Gur-Yaish N, Tonkikh O, Sinoff G. Hospital-associated functional decline: the role of hospitalization processes beyond individual risk factors. J Am Geriatr Soc 2015; 63: 55–62. [DOI] [PubMed] [Google Scholar]
- 8. Loyd C, Markland AD, Zhang Yet al. Prevalence of hospital-associated disability in older adults: a meta-analysis. J Am Med Dir Assoc 2020; 21: 455–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ellis G, Gardner M, Tsiachristas Aet al. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst Rev 2017; 9: CD006211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rubenstein LZ, Stuck AE, Siu ALet al. Impacts of geriatric evaluation and management programs on defined outcomes: overview of the evidence. J Am Geriatr Soc 1991; 39: 8S–16. [DOI] [PubMed] [Google Scholar]
- 11. Conroy SP, Bardsley M, Smith Pet al. Comprehensive Geriatric Assessment for Frail Older People in Acute Hospitals: the HoW-CGA Mixed-Methods Study. Southampton (UK): NIHR Journals Library. 2019. http://www.ncbi.nlm.nih.gov/books/NBK540056/ (3 February 2021, accessed.) [PubMed] [Google Scholar]
- 12. Deschodt M, Flamaing J, Haentjens P, Boonen S, Milisen K. Impact of geriatric consultation teams on clinical outcome in acute hospitals: a systematic review and meta-analysis. BMC Med 2013; 11: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Van Craen K, Braes T, Wellens Net al. The effectiveness of inpatient geriatric evaluation and management units: a systematic review and meta-analysis. J Am Geriatr Soc 2010; 58: 83–92. [DOI] [PubMed] [Google Scholar]
- 14. Baztán JJ, Suárez-García FM, López-Arrieta J, Rodríguez-Manus L, Rodríguez-Artalejo F. Effectiveness of acute geriatric units on functional decline, living at home, and case fatality among older patients admitted to hospital for acute medical disorders: meta-analysis. Br Med J 2009; 338: b50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shamseer L, Moher D, Clarke Met al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. Br Med J 2015; 350: g7647. [DOI] [PubMed] [Google Scholar]
- 16. O’Shaughnessy Í, Robinson K, O’Connor Met al. Effectiveness of acute geriatric unit care on functional decline and process outcomes among older adults admitted to hospital with acute medical complaints: a protocol for a systematic review. BMJ Open 2021; 11: e050524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Page MJ, McKenzie JE, Bossuyt PMet al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J 2021; 372: n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Deeks JJ, JPT Higgins, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: JPT Higgins, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. https://training.cochrane.org/handbooks (21 September 2021, accessed). [Google Scholar]
- 19. Sager MA, Rudberg MA, Jalaluddin Met al. Hospital admission risk profile (HARP): identifying older patients at risk for functional decline following acute medical illness and hospitalization. J Am Geriatr Soc 1996; 44: 251–7. [DOI] [PubMed] [Google Scholar]
- 20. Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of illness in the aged: a standardized measure of biological and psychological function. JAMA 1963; 185: 914–9. [DOI] [PubMed] [Google Scholar]
- 21. Mahoney FI, Barthel DW. Functional evaluation: the Barthel index. Md State Med J 1965; 14: 61–5. [PubMed] [Google Scholar]
- 22. Sonn U. Longitudinal studies of dependence in daily life activities among elderly persons. Scand J Rehabil Med Suppl 1996; 34: 1–35. [PubMed] [Google Scholar]
- 23. Sterne JAC, Savovic J, Page MJet al. RoB 2: a revised tool for assessing risk of bias in randomised trials. Br Med J 2019; 366: 14898. [DOI] [PubMed] [Google Scholar]
- 24. Schunemann HJ, JPT Higgins, Vist GEet al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: JPT Higgins, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. https://training.cochrane.org/handbooks (21 September 2021, accessed). [Google Scholar]
- 25. JPT Higgins, Li T, Deeks JJ. Chapter 6: Choosing effect measures and computing estimates of effect. In: JPT Higgins, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. https://training.cochrane.org/handbooks (25 August 2021, accessed). [Google Scholar]
- 26. Fox MT, Persaud M, Maimets Iet al. Effectiveness of acute geriatric unit care using acute care for elder components: a systematic review and meta-analysis. J Am Geriatr Soc 2012; 60: 2237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zelada MA, Salinas R, Baztán JJ. Reduction of functional deterioration during hospitalization in an acute geriatric unit. Arch Gerentol Geriatr 2009; 48: 35–9. [DOI] [PubMed] [Google Scholar]
- 28. Wald HL, Glasheen JJ, Guerrasio J, Youngwerth JM, Cumbler EU. Evaluation of a hospitalist-run acute care for the elderly service. J Hosp Med 2011; 6: 313–24. [DOI] [PubMed] [Google Scholar]
- 29. Barnes DE, Palmer RM, Kresevic DMet al. Acute care for elders units produced shorter hospital stays at lower costs while maintaining patients' functional status. Health Aff 2012; 31: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Borenstein JE, Aronow HU, Bolton LBet al. Identification and team-based interprofessional management of hospitalized vulnerable older adults. Nurs Outlook 2016; 64: 137–45. [DOI] [PubMed] [Google Scholar]
- 31. Ekerstad N, Karlson BW, Dahlin Ivanoff Set al. Is the acute care of frail elderly patients in a comprehensive geriatric assessment units superior to conventional acute medical care? Clin Interv Aging 2017; 16: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Westgard T, Hammar IA, Dahlin-Ivanoff S, Wilhelmson K. Can comprehensive geriatric assessment meet frail older person’s needs? Results from the randomized controlled study CGA-Swed. Geriatrics 2020; 5: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ekerstad N, Dahlin Ivanoff S, Landahl Set al. Acute care of severely frail elderly patients in a CGA-unit is associated with less functional decline than conventional acute care. Clin Interv Aging 2017; 12: 1239–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Åhlund K, Bäck M, Öberg Bet al. Effects of comprehensive geriatric assessment on physical fitness in an acute medical setting for frail elderly patients. Clin Interv Aging 2017; 12: 1929–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ekerstad N, Karlson BW, Anderson Det al. Short-term resource utilization and cost-effectiveness of comprehensive geriatric assessment in acute hospital care for severely frail elderly patients. J Am Med Dir Assoc 2018; 19: 871. [DOI] [PubMed] [Google Scholar]
- 36. Westgard T, Hammar IA, Holmgren Eet al. Comprehensive geriatric assessment pilot of a randomized controlled study in a Swedish acute hospital: a feasibility study. Pilot Feasibility Stud 2018; 4: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Collard AF, Bachman SS, Beatrice DF. Acute care delivery for the geriatric patient: an innovative approach. Qual Rev Bull 1985; 11: 180–5. [PubMed] [Google Scholar]
- 38. Harris RD, Chalmers JP, Henschke PJet al. A randomised study of outcomes in a defined group of acutely ill elderly patients managed in a geriatric assessment unit or a general medical unit. Aust N Z J Med 1991; 21: 230–4. [DOI] [PubMed] [Google Scholar]
- 39. Landefeld CS, Palmer RM, Kresevic DM, Fortinsky RH, Kowal J. A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients. N Engl J Med 1995; 332: 1338–44. [DOI] [PubMed] [Google Scholar]
- 40. Asplund K, Gustafson Y, Jacobsson Cet al. Geriatric-based versus general wards for older acute medical patients: a randomized comparison of outcomes and use of resources. J Am Geriatr Soc 2000; 48: 1381–8. [DOI] [PubMed] [Google Scholar]
- 41. Counsell SR, Holder CM, Liebenauere LLet al. Effects of a multicomponent intervention on functional outcomes and process of care in hospitalized older patients: a randomized controlled trial of acute care for elders (ACE) in a community hospital. J Am Geriatr Soc 2000; 48: 1572–81. [DOI] [PubMed] [Google Scholar]
- 42. Covinsky KE, King JT, Quinn Let al. Do acute care for elders units increase hospital costs? A cost analysis using the hospital perspective. J Am Geriatr Soc 1997; 45: 729–34. [DOI] [PubMed] [Google Scholar]
- 43. Folstein MF, Folstein SE, McHugh PR. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12: 189–98. [DOI] [PubMed] [Google Scholar]
- 44. Arnetz JE, Arnetz BB. The development and application of a patient satisfaction measurement system for hospital-wide quality improvement. International J Qual Health Care 1996; 8: 555–66. [DOI] [PubMed] [Google Scholar]
- 45. Courtney MD, Edwards HE, Chang AMet al. Improved functional ability and independence in activities of daily living for older adults at high risk of hospital readmission: a randomized controlled trial. J Eval Clin Pract 2012; 18: 128–34. [DOI] [PubMed] [Google Scholar]
- 46. Cuevas-Lara C, Izquierdo M, Gutiérrez-Valencia Met al. Effectiveness of occupational therapy interventions in acute geriatric wards: a systematic review. Maturitas 2019; 127: 43–50. [DOI] [PubMed] [Google Scholar]
- 47. Chen Z, Zhaosheng D, Chen Cet al. Effectiveness of comprehensive geriatric assessment intervention on quality of life, caregiver burden and length of hospital stay: a systematic review and meta-analysis of randomized controlled trials. BMC Geriatr 2021; 21: 377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Skivington K, Matthews L, Simpson SAet al. A new framework for developing and evaluating complex interventions: update of Medical Research Council guidance. Br Med J 2021; 374: n2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Langhorne P, Ramachandra S. Organized inpatient (stroke unit) care for stroke: network meta-analysis. Cochrane Database Syst Rev 2020; 4: CD000197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Parker SG, McCue P, Phelps Ket al. What is comprehensive geriatric assessment (CGA)? An umbrella review Age Ageing 2018; 47: 149–55. [DOI] [PubMed] [Google Scholar]
- 51. Schuurmans H, Steverink N, Lindenberg S, Frieswijk N, Slaets JPJ. Old or frail: what tells us more? J Gerontol A Biol Sci Med Sci 2004; 59: 962–5. [DOI] [PubMed] [Google Scholar]
- 52. Geriatrics Interdisciplinary Advisory Group . Interdisciplinary care for older adults with complex needs: American geriatrics society position statement. J Am Geriatr Soc 2006; 54: 849–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.