Abstract
Advances in the integration of wearable devices in our daily life have led to the development of new electrode designs for biopotential monitoring. Historically, the development and testing of wearable electrodes for the acquisition of biopotential signals has been empirical, relying on experiments on human volunteers. However, the lack of explicit control on human variables, the intra-, and inter-subject variability complicates the understanding of the performance of these wearable electrodes. Herein, phantom mimicking the electrical properties of the skin in the low-frequency range (1 Hz-1000 Hz), which has the potential to be used as a platform for controlled benchtop experiments for testing electrode functionality, is demonstrated. The fabricated phantom comprises two layers representing the deeper tissues and stratum corneum. The lower layer of the phantom mimicking deeper tissues was realized using polyvinyl alcohol cryogel (PVA-c) prepared with 0.9% W/W saline solution by a freeze-thaw technique. The properties of the upper layer representing the stratum corneum were simulated using a 100μm thick layer fabricated by spin-coating a mixture of polydimethylsiloxane (PDMS), 2.5% W/W carbon black (CB) for conductance, and 40% W/W barium titanate (BaTiO3) as a dielectric. The hydration of the stratum corneum was modeled in a controlled way by varying porosity of the phantom’s upper layer. Impedance spectroscopy measurements were carried out to investigate the electrical performance of the fabricated phantom and validated against the impedance response obtained across a physiological skin impedance range of five human subjects. The results indicated that the Bode plot depicting the impedance response obtained on the phantom was found to lie in the human skin range. Moreover, it was observed that the change of porosity provides control over the hydration and the phantom can be tuned as per the skin ranges among different individuals. Also, the phantom was able to mimic the impact of dry and hydrated skin on a simulated ECG signal in the time domain. The developed skin phantom is affordable, fairly easy to manufacture, stable over time, and can be used as a platform for benchtop testing of new electrode designs.
Keywords: Biopotential signal, Skin phantom, Impedance spectroscopy, Skin hydration, Wearable electrodes
Graphical Abstract
1. Introduction
Biopotential signals such as Electrocardiogram (ECG), Electromyogram (EMG), and Electroencephalogram (EEG) provide vital information about health (Fu et al., 2020). The use of health-monitoring systems incorporating wearable electrodes has led to the development of new electrode designs, including materials (Peng et al., 2019), geometry (Kaitainen et al., 2014; Maithani et al., 2021), and surface coatings (Chlaihawi et al., 2018), for biopotential monitoring from the skin surface (Acar et al., 2019; Khoshmanesh et al., 2021). However, there is no robust platform to test the performance and functionality of these new electrode designs. Development and testing is typically performed empirically with experiments on human volunteers (Anusha et al., 2018; Bergey et al., 1971; Fink et al., 2021). The properties of the human skin are variable (Beckmann et al., 2010). First, properties and impedance change over time, making it challenging to perform reproducible measurements. Secondly, the properties of human skin differ for each person. A readily manufacturable skin phantom with explicit controlled properties that is stable in time will provide a benchtop platform to aid research and development of skin contact electrodes designed for acquiring biopotential signals.
The tissue anatomy is considered as a stack of layers (muscle, fat, skin) and these layers have different electrical properties that affect the propagation of potential (S. Gabriel et al., 1996). Skin is further comprised of different layers, broadly divided into the epidermis, dermis, and subcutaneous layers. The epidermis further consists of four or five layers; the topmost layer being the stratum corneum (Reicherter, 2015). Each tissue layer has distinct electrical properties, including electrical impedance (Kalvøy et al., 2009), which is frequency dependent (Bera, 2018). The combined skin impedance is of the order of 100Ω at high frequencies and is 10 kΩ–1 MΩ at low (<1 kHz) frequencies (Bîrlea et al., 2014). The increased impedance at lower frequency is thought to be dominated by the stratum corneum (Bîrlea et al., 2014), which has high electrical resistance as it consists of dead cells (Ha et al., 2014; Heikenfeld et al., 2018; Lobodzinski, 2010). The electrical properties of stratum corneum, and subsequently human skin, strongly depend on hydration. Hydration plays an important role in lowering the stratum corneum impedance (Clar et al., 1975), with hydrated skin, the resistance becomes 14 times lower and capacitance is 1.5 times higher than dry skin conditions (Björklund et al., 2013).
One widely used approach for fabricating phantom uses agar, polyethylene powder, sodium chloride, and TX-151 to simulate electrical characteristics of a highly hydrous biological tissue such as muscle in the high-frequency range of 300 MHz-2.5 GHz (Ito et al., 2001). Yamamoto et al. extended this agar approach to mimic the electrical properties (conductivity and relative permittivity) of muscle at 1 MHz (Yamamoto et al., 2013). Beckmann et al. made a skin dummy using agar and saline water for testing biopotential electrodes and simulated the conductance of wet skin tissue in the frequency range of 5 kHz-1 MHz (Beckmann et al., 2010). However, this agar approach is limited to simulating high water content tissues and is unable to mimic skin. Existing phantoms are not able to simulate the impedance of dry and hydrated skin conditions necessary for testing dry wearable electrodes for two reasons. First, biopotentials signals (ECG, EEG, EMG) lie in the low-frequency range (0.1 Hz-1000 Hz). Agar-based methods have only been shown effective at modeling impedance in a much higher frequency range, primarily because they do not include a model of the stratum corneum. There is limited work on the phantoms mimicking electrical properties of the skin in the low-frequency range and has significant gaps. Recently, Kalra et al. developed a skin phantom using oil in gelatin to mimic conductivity and permittivity of the skin in the range of 20 Hz-300 kHz, but the results indicated that the permittivity is off by four orders of magnitude with respect to the reference skin data (Kalra et al., 2020). Owda and Casson improved this by using different gelatin and NaCl concentrations and the contact impedance profile for the phantom was compared with the impedance profile obtained on ex vivo porcine skin over 20 Hz-1000 Hz (Owda and Casson, 2021). However, the impedance of porcine skin is very low in comparison to human skin (Tregear, 1965). Most recently, a two-layered phantom was developed incorporating the effect of the skin layer and was shown to mimic electrical properties in the range 20 Hz-1 MHz (Liu et al., 2019), which is still too high a frequency range to characterize wearable electrodes. Additionally, this recent phantom is complex to fabricate and the skin hydration state cannot be modeled.
Prior efforts to change hydration of artificial skin phantoms include placing the phantom in a humidity chamber (Yao et al., 2017) or leaving it out for drying (Nachman and Franklin, 2016), immersing it in water (Dąbrowska et al., 2017), and covering the phantom with a wet cloth soaked in saline solution to mimic hydrated skin (Chen et al., 2014). All the aforementioned methods lack explicit control and none have demonstrated the ability to mimic the effect of skin hydration on overall tissue impedance. The goal of this work is to develop the phantom that is substantially similar in impedance to the skin across 1 Hz-1000 Hz, crucial for capturing biopotential signals and mimics the effect of skin hydration in a controlled predictive manner. Here, we present a two-layered phantom mimicking electrical properties of deeper tissues and stratum corneum in the frequency range of 1 Hz-1000 Hz. The electrical properties of the deeper tissues have been simulated with polyvinyl alcohol cryogel (15% W/W) prepared with 0.9% W/W saline solution and that of the stratum corneum with a 100μm layer of a PDMS/CB/BaTio3 mixture. Stratum corneum can be modeled electrically by a parallel combination of equivalent capacitor and resistor configuration, here the hydration of the stratum corneum is modeled using a novel approach that integrates fabricated holes on the stratum corneum layer. These holes become filled with saline from the PVA layers, thus affecting resistance. A phantom that can model the hydration in a controlled and consistent way will facilitate in understanding of the role of skin conditions on the electrode performance and benefit electrode testing and development. Further, we demonstrate that known electrophysiological signals can be provided as an input to the phantom, which is not possible for in vivo human skin.
We present here methods for fabrication of the two-layered phantom, followed by experimental techniques used to characterize and evaluate the electrical performance of the phantom with dry electrodes. Conventionally, wet electrodes are the gold standard as they consistently provide clinically acceptable signal quality. However, they are uncomfortable, single use, require skin preparation, and cause skin irritation. Moreover, with recent advancements in remote monitoring technology, wet electrodes are not promising for long-term monitoring due to the gel drying out over time. To overcome the challenges of the wet electrodes, a lot of progress is being made in the field of dry electrodes (contact/non-contact electrodes), as they are comfortable, reusable, require no skin preparation, and generally do not cause skin irritation, albeit with compromised signal quality as compared to wet electrodes. Our phantom is designed to mimic the impedance of the stratum corneum as well as the skin-electrode interface impedance for any dry electrode, and we chose to use stainless steel dry electrodes for evaluating the electrical performance of the fabricated phantom. Currently, dry metal-based stainless steel electrodes are integrated into various in-home monitoring devices such as Kardia Mobile (Alivecor Inc., San Francisco, CA, USA), which is a handheld monitor to capture ECG, and a toilet seat to capture buttocks ECG (bECG) (Conn et al., 2018). As validation, the impedance results obtained from the interaction of the phantom with dry electrodes are compared to that of human skin. Further, we evaluate the time stability and reproducibility of the phantom. Lastly, we demonstrate an active phantom in the time domain using a 0.12 mV (typical bECG amplitude) signal, which is an order of magnitude lower than typical ECG amplitude (1.6 mV). The choice of bECG was made because the combination of dry electrodes and fairly weak ECG signal in the buttocks make the sensing of a high-quality ECG a challenging application representative of applications where a phantom may facilitate electrode design.
2. Materials and Methods
2.1. Phantom Fabrication
Materials:
PDMS (Sylgard 184 silicone elastomer; Electron Microscopy Sciences, Hatfield, PA, USA) was used as bulk materials, but has a high volume resistivity of the order 2.9 × 1014 ohm cm, a dielectric constant of 2.72 @100 Hz and 2.68 @100 kHz (The Dow Chemical Company, 2017), which makes it non-conductive. Conductance was increased by adding carbon black powder (CB; Vulcan XC-72R, Fuel Cell Store, College Station, TX, USA) and dielectric properties were increased by adding barium titanate powder (BaTiO3; Sigma Aldrich, St. Louis, MO, US, product number 208108, mean particle diameter <3μm) (Nawanil et al., 2019). Preliminary analysis and experiments showed that a composite of PDMS doped with 2.5% W/W carbon black and 40% W/W barium titanate resulted in the desired resistance and capacitance of 1MΩ and 10 nF (Chi et al., 2010) for the stratum corneum. PVA (Elvanol 71-30, Chemical Store Inc, Clifton, NJ, USA), a synthetic polymer and soluble in hot water was used to develop the lower layer of the phantom representing deeper tissues, which are hydrous and conductive (Bîrlea et al., 2014). Moreover, the dielectric properties of PVA cryogels can be tuned by freeze-thaw cycling (Getangama et al., 2020).
Fabrication process:
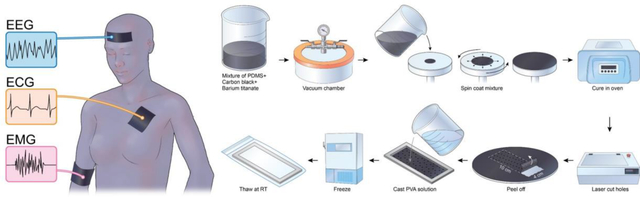
The fabrication process of a two-layered phantom is schematically illustrated in Fig. 1Fig. 1. To fabricate the upper layer of the phantom, 6g of sylgard 184 silicone elastomer was mixed in the ratio of 10:1.5 to obtain PDMS with increased crosslinking, to facilitate mechanical strength and easy handling of the 100μm stratum corneum layer (Jin et al., 2020). We then added 40% W/W BaTio3 and mixed using a stirrer for 10 minutes, followed by 2.5% W/W carbon black powder and 20 minutes mixing. The container was placed in a vacuum chamber for 30 minutes to remove air bubbles. The mixture was spin-coated using a spin coater (Model WS-400-6NPP-LITE, Laurel Technologies Corp., North Wales, PA, USA) onto an acrylic wafer (14 cm diameter, 3/32” thick), at a speed of 1000 rpm for 30 seconds to obtain a thickness of 100μm (Lee et al., 2006). The spin-coated wafer was placed in an oven (Heratherm OGS 100 Thermo Electron LED, GmbH, Thermo Scientific, Waltham, MA, USA) for curing at 80°C for two hours. After curing, a laser cutter (H-Series, 20” ×12” Desktop CO2 laser, Full Spectrum Laser, Las Vegas, NV, USA) was used at a speed of 100, a power of 1, and with 1 pass to cut 0.1 mm-diameter holes and a 10 × 4 cm rectangular piece from the wafer’s center. A mold (of dimension L × W × H = 10 cm × 4 cm × 0.5 cm) was 3D printed using SLA 3D-printing (Form 2, Formlabs Inc., Somerville, MA, USA). The layer representing the stratum corneum was peeled from the acrylic wafer and placed on the bottom of the mold.
Fig. 1.
Steps for the fabrication process of a two-layered phantom. Clockwise from top-left: a mixture of PDMS, Carbon black, barium titanate; followed by removal of air bubbles in the vacuum chamber; Spin coat mixture at 1000 rpm and 30 seconds; Cure in an oven at 80°C for two hours; Laser-cut holes; Peel off the layer; Cast PVA solution; Freeze (12 hours at −20°C); Thaw (24 hours at RT).
For fabricating the deep tissue layers of the phantom, PVA cryogel was developed using a 0.9% W/W saline solution and polymerized by a freeze-thaw technique without any chemical crosslinkers (Stauffer and Peppast, 1992). To prepare the PVA-c solution, 8.8 g of PVA powder was mixed slowly with continuous stirring in 50 ml of 0.9% W/W saline solution (0.9 g of NaCl in 99.1 g of DI water). The solution was microwaved to a boil and stirred. The process of heating was repeated until translucent. The beaker was covered with a parafilm and left to cool to room temperature (RT). Air bubbles at the top of the solution were scraped and discarded. The PVA-c solution was poured on top of the stratum corneum layer in the mold. The mold was subjected to a single freeze (12 hours at −20°C)/thaw (24 hours at room temperature) cycle to cross-link the PVA-c polymer and polymerize the gel (Mix et al., 2018). For thawing, the entire assembly along with mold was stored in a ziplock bag within an environmental chamber (Model 5503, Temperature and Humidity Chamber, Microcontroller 5200, Electro-Tech Systems, Perkasie, PA, USA) at RH of 90% and RT of 22 °C. The ziplock bag was used to prevent the PVA cryogel from drying and losing water. Further, the longer thawing cycle and storage in the humidity chamber facilitated the phantom reach an equilibrium stage.
Hydration control via porosity: Different hydration states of the stratum corneum were modeled in a controlled way on the surface of the phantom by varying porosity. Porosity is defined as a measure of empty spaces in a material. Mathematically, it can be described as the fraction of void space in material over the total volume. Here, the porosity represents the percentage of open area and was calculated by the area fraction as both void and bulk material have the same thickness (eq. 1).
| (eq. 1) |
Where, Apore is the area of the pore, Abulk material is the area of bulk material (SC layer) and Ø represents porosity. It was achieved by cutting out holes from the fabricated upper layer representing stratum corneum with the laser cutter. Different sets of phantom were made and different hydration states were modeled with varying porosity of 0%, 0.16%, 0.28%, and 1.4%. The impedance response across different sets of phantoms was obtained to understand the effect of porosity.
The thickness and uniformity of the fabricated layer were measured using an optical non-contact profilometer (ST400, Nanovea Inc, Irvine, CA, USA). Further, the hole size characterization was performed and the microscopic images were captured using a digital microscope (KH-7700, Hirox-USA, Inc., NJ, USA). The dimensions of the hole were measured along the x-axis and y-axis, and the mean was computed. 10 random holes were selected across the entire phantom and the mean and standard deviation (SD) were obtained. The obtained value of mean was used as the radius of the fabricated hole and Apore was computed, and was also plugged into equation 1 to calculate the porosity.
2.2. Impedance Spectroscopy Measurements
Impedance spectroscopy measurements were performed with a potentiostat (Reference 600, Gamry Instruments Inc., Warminster, PA, USA) in a two-electrode configuration using two stainless steel dry electrodes, each of area 9 cm2 (Fig. 2). The measured impedance includes both the impedance of the phantom and electrode-phantom interface phenomena, which is critical to study the interaction of the phantom with dry electrodes. The potentiostat consists of four probes, namely working (W), counter (C), working sense (WS), and reference (R). The working and counter probe carries the current and the working sense and reference measures the voltage. W and WS were connected to one electrode and C and R were connected to the second electrode. A 25 mV RMS AC voltage signal was generated using a potentiostat over a frequency sweep from 1 Hz-1000 Hz. Bode plot analysis was performed to characterize impedance with respect to frequency.
Fig. 2.
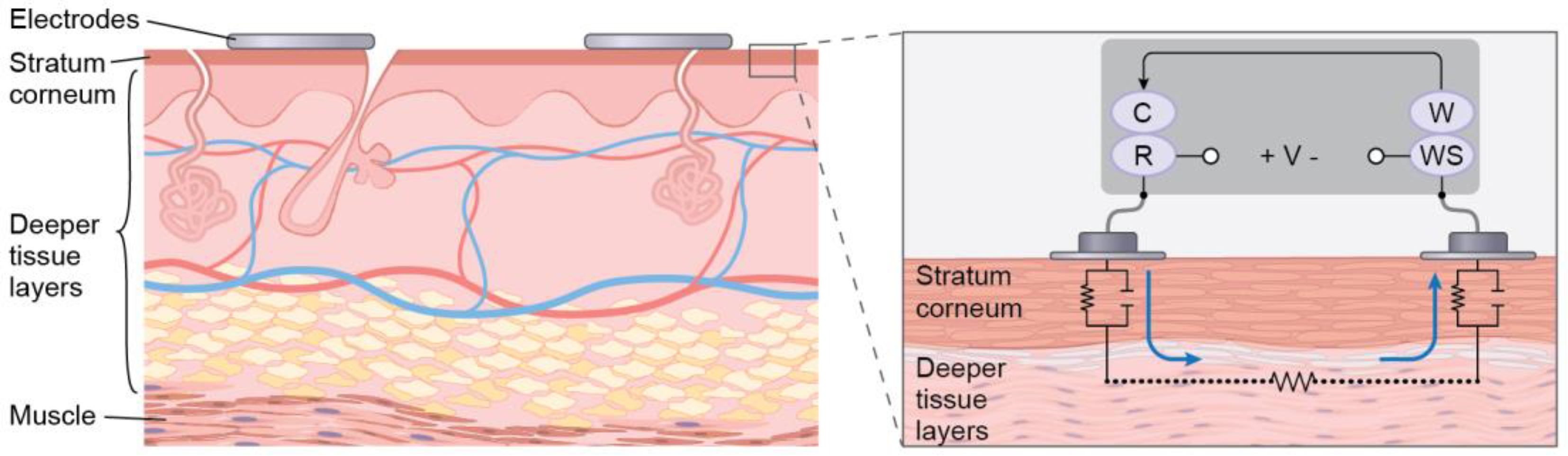
Skin cross-section where stratum corneum represents the outermost layer of the skin and the deeper tissue layers together represent the other layers of the epidermis, dermis, and hypodermis. The zoomed-in view of the skin cross-section showing an electrical equivalent model of the electrode-skin interface and the two-electrode configuration set up where C, R, W, and WS represent counter, reference, working, and working sense electrodes, respectively.
To validate the performance of the developed phantom with the impedance response of human skin, impedance spectroscopy experiments were carried out on the human volar arm for five adult volunteers (four male and one female) belonging to different ethnic groups. Two stainless steel dry electrodes were placed 4 inches apart on the subject’s left volar forearm. Metal snap connectors had previously been integrated into the electrodes and were used to connect the probes of the potentiostat. Medical tape (3M Kind Removal Silicone Tape) was used to attach the electrodes to the arm. A 25 mV RMS AC voltage signal within the current range of 600 nA-600 μA was generated using a potentiostat (Reference 600, Gamry Instruments Inc., Warminster, PA, USA) over a frequency sweep from 1 Hz-1000 Hz, and the data was recorded. The potentiostat was plugged into a medical-grade isolation transformer (ILC-1400MED4, TSI Power Corp., Antigo, WI, USA) to ensure electrical safety.
Experiments were conducted under an informed consent, in a protocol approved by the Rochester Institute of Technology Institutional Review Board for Protection of Human Subjects. The first experiment (“Dry”) was performed with dry electrodes on the untreated skin. Prior to the second (Hydrated) experiment, the subject was asked to soak their volar forearm in a tray partially filled with lukewarm (40°C) water for 30 minutes (Baker and Kligman, 1967; Tagami et al., 1980). The arm of the subject was wiped with the paper towel to remove the excess water from the surface of the skin, and the electrodes were applied and impedance measurements made. The physiological range of skin impedance for untreated and hydrated skin obtained across five subjects was compared to the impedance response on the phantom. To study how accurately the signals are being transmitted through the phantom, group delay values were calculated using the method as in (Owda and Casson, 2021) and compared to the group delay values obtained corresponding to the human skin data.
2.3. Stability and Reproducibility of phantom
To investigate the stability of the phantom over time, and the use of the phantom for multiple days, impedance measurements were carried out several times of the day over consecutive days. The phantom was stored in the environmental chamber between experiments. The weight was measured prior to the impedance measurements. The time required to conduct impedance measurements was around 10 mins which include approximately 5 mins for the frequency sweep (1 Hz–1000Hz) and 5 mins for the setup and application of the electrodes. After the impedance measurements, the phantom was returned to the ziplock bag in humidity chamber at RH 90% and RT 22 °C to reduce drying. This experiment was repeated for four days. To evaluate the reproducibility of the fabricated phantom, four different batches of phantom were made. The impedance measurements were performed across 1 Hz–1000 Hz and at each frequency, mean and SD were calculated across four replicates.
2.4. Active phantom
To demonstrate the behavior of the phantom in the time domain, a simulated ECG signal was injected into the bottom of the phantom and the output signal was recorded from the top surface of the phantom. An ECG signal of 0.12 mV (typical bECG amplitude) with a sampling frequency of 1000 Hz was simulated using LabVIEW Biomedical Toolkit (Simulate ECG express VI), with analog outputs of a DAQ (USB-6215, National Instruments Corp., Austin, TX, USA) connected to wet electrodes (3M Red Dot 2560, 3M, Maplewood, MN, USA) attached to the bottom of the phantom. Stainless steel dry electrodes of area 1 cm2 were held in contact with the phantom’s surface with the micromanipulator (M325, World Precision Instruments, Sarasota, FL, USA). This ensured good contact and consistent pressure between the electrode and the phantom. The acquired ECG signal from a set of phantoms with porosity of 0%, 0.16%, 0.28%, and 1.4% was recorded. The raw signal was processed using a third-order Butterworth bandpass filter with a bandwidth of 0.5 Hz-150 Hz, along with a notch filter at 60 Hz to obtain a denoised ECG signal. The noise component was calculated by subtracting the denoised ECG signal from the raw ECG signal. Power spectral densities of denoised signal and noise component were calculated by Welch’s method (Welch, 1967). The power was computed by approximating the area under the power spectral density curves (Yokus and Jur, 2016). Signal to noise ratio (SNR) was calculated as the ratio of the power of denoised ECG signal to the power of noise component and represented in decibels.
3. Results and Discussion
3.1. Fabricated phantom
The completed integrated phantom with a porosity of 1.4% is shown in Fig. 3. The phantom is 0.5 cm thick and 10 cm × 4 cm. The average thickness of the fabricated stratum corneum measured using an optical profilometer was 99 μm, SD 3 μm, across the surface. All measurements fell within a range of 91 to 104 μm. We do not believe that the PVA-c solution filled these pores, due to the small hole diameter and high viscosity of the PVA-c solution. The hole size was examined to understand the kerf of the laser cutter (Fig. S1. of supplementary information) and holes drawn with a nominal diameter of 0.1 mm and 0.2 mm resulted in actual hole diameters of 0.31 ± 0.020 (mean ± SD) and 0.40 ± 0.021 (mean ± SD) respectively. Moreover, the examination reveals that we have a small variation in the diameters of the actual holes. Unlike previously reported skin phantom (Liu et al., 2019), our developed phantom model and holes are not analogous to the physical skin and the sweat pores, but by using the concept of porosity, the phantom has been modeled to simulate the impedance behavior similar to dry and hydrated human skin. The fabrication process is comprised of standard techniques, such as spin coating and laser cutting, that can be found at most universities and are easily learned. The cost of consumables is a few dollars (< $3) for each fabricated phantom. Thus, our developed phantom is fairly easy to manufacture, affordable and incorporates a simple strategy for modeling the skin hydration state.
Fig. 3.
Photograph of the fabricated two-layered phantom with 1.4% porosity and 2-electrode configuration. The upper layer of the phantom simulates the stratum corneum and the lower layer simulates deeper tissues.
3.2. Electrical performance of the fabricated phantom
The Bode plots depicting the impedance of phantoms shows that the higher porosity phantoms have a lower impedance (Fig. 4). This is likely because the holes in the stratum corneum layer fill with saline, which is more conductive than the polymer. In comparison to the impedance response of human skin, it was found that the phantoms with porosity of 0.16% and 0.28% lie in the physiological range of untreated skin and hydrated skin respectively, and have a similar slope. The similar slope to that of human skin impedance magnitude indicated that the upper layer of the phantom was able to simulate the combination of resistive and capacitive nature, analogous to that of stratum corneum in the actual human skin (Fig. 4a). Moreover, the phase response for 0.16% and 0.28% porosity phantoms was within 15 degrees of one another and within 13 degrees for dry and hydrated skin across the range of frequencies tested (Fig. 4b). The group delay values for 0.16% and 0.28% porosity phantoms are generally <1msec in 10 Hz-1000 Hz (Fig. 4c), which is small as compared to the duration of the electrophysiological signals and comparable to previously reported group delays for wet electrodes (Owda and Casson, 2021). At lower frequencies (1Hz-10Hz), our group delay values are larger, but are the first reported at low frequency and are lower than our measurements on actual human skin. The effect of this mismatch in phase and group delay is investigated in section 3.4, which indicates that there was no signal distortion in the ECG.
Fig. 4.
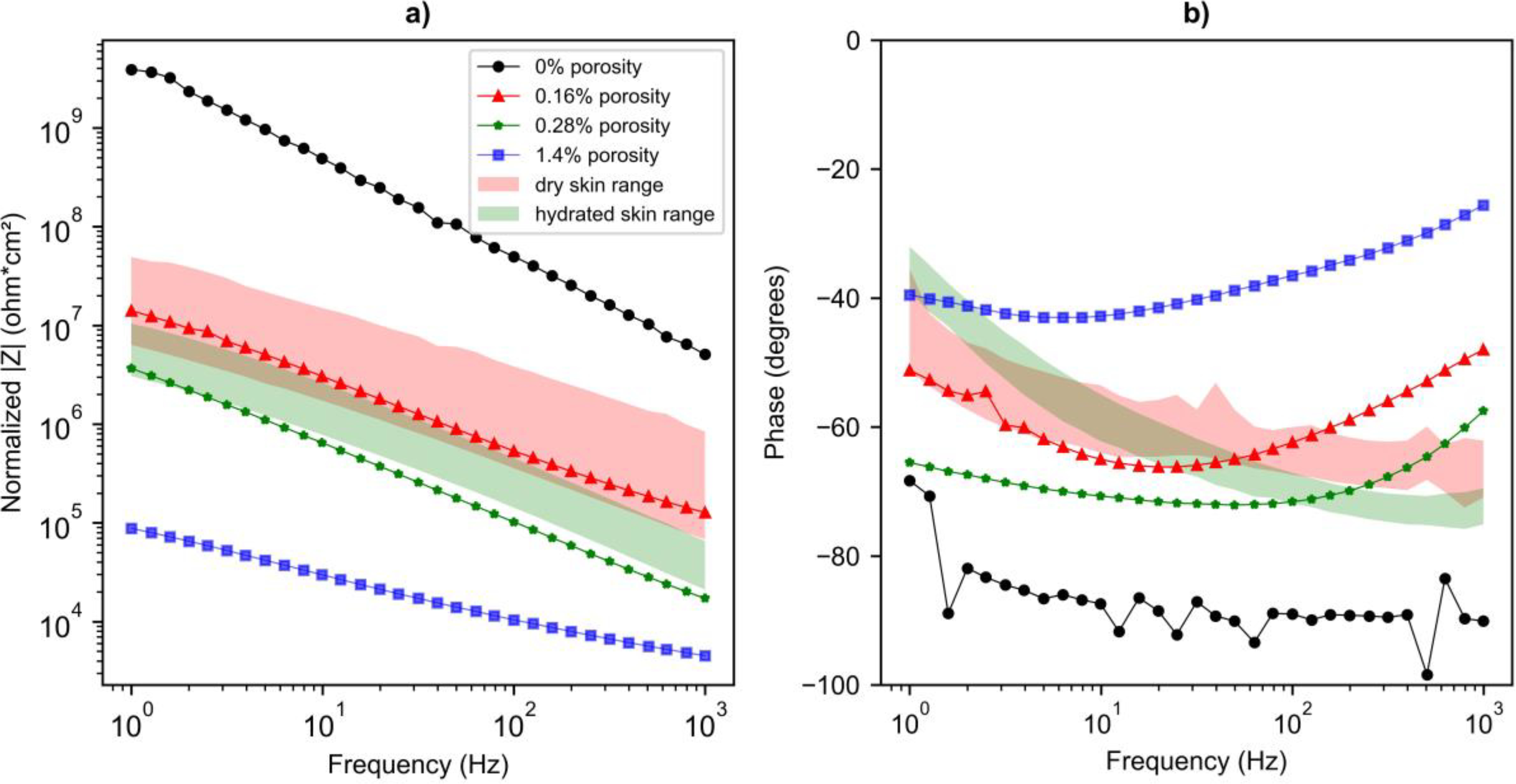

a) Impedance magnitude and b) phase response c) group delay for the phantoms with porosity of 0% (black circle), 0.16 % (red triangle), 0.28% (green star), and 1.4% (blue square). The physiological range of impedance magnitude and phase response obtained on the human arm (five subjects) for untreated and hydrated skin is shown with the red and green background color respectively. Dashed lines in panel c) indicate the curve fit to aid for data visualization. The inset graph shows a zoomed-in view for the group delay corresponding to the frequency range of 10 Hz-1000 Hz.
In comparison to existing phantoms (Beckmann et al., 2010; Kalra et al., 2020), where the phantoms were fabricated as a single homogeneous layer and did not include the stratum corneum impedance, the skin phantom developed in this work incorporates a model to simulate stratum corneum. The impedance of the phantom with 0.28% porosity was found to be lower by half an order of magnitude compared to the 0.16% porosity phantom. This is similar to the hydrated and dry human skin impedance. This indicated that the developed phantom can mimic the effect of skin hydration on the overall impedance. Several factors in the recipe can be varied for decreasing the impedance of the developed phantom to simulate the impedance of hydrated skin. These factors include thickness of the upper layer, concentration of carbon black powder, barium titanate, saline water, and PVA, as well as the freeze-thaw cycles. Preliminary exploration towards deciding onto porosity as the variable to vary the impedance of the phantom is discussed in supplementary information (Fig. S2., Fig. S3., Fig S4.). In addition, porosity can be tuned to mimic different hydration levels and as per different individual skin impedances. Thus, unlike previously reported phantoms (Chen et al., 2014; Owda and Casson, 2021) our phantom includes a mechanism for modeling various skin hydration, which is important for developing and characterizing biopotential electrodes for wearable technologies.
Lastly, the phantom effectively mimics impedance across the entire frequency range (1 Hz-1000 Hz) crucial for capturing various biopotential signals such as ECG, and EMG. Previous research indicated that the frequency content of the ECG waves varies. P wave is characterized by 5–30 Hz, T wave content lies below 10 Hz, and the main power lies in QRS complex, reflected by 5–15 Hz (Tereshchenko and Josephson, 2015; Thakor et al., 1983). The bandwidth of the EMG signal lies between 20 Hz-1000 Hz (Go et al., 2014). Prior phantoms (Kalra et al., 2020; Liu et al., 2019; Owda and Casson, 2021) do not characterize their phantoms in the 1 Hz-20 Hz frequency range. This work is the first comparison of the phantom to in vivo human skin impedance across the frequency range (1 Hz-1000 Hz). A previously reported research study (Kalra et al., 2020), compares the performance of the phantom with respect to relative permittivity and conductivity value of dry and wet skin based upon experiments (S. Gabriel et al., 1996b), however, the database does not provide the dielectric properties below 10 Hz. In this work, we present a comparison of the phantom to in vivo data obtained for dry and hydrated skin across the entire frequency range (1 Hz-1000 Hz) with dry stainless steel electrodes. Our future studies will be using the phantom to characterize different electrode configurations such as electrode size and electrode material.
3.3. Stability and Reproducibility of the fabricated phantom
The results of the 4-day experiment to test the stability of the phantom indicated an upward shift in the impedance response over time (Fig. 5). The weight of the phantom was found to be 24.17 g, 22.50 g, and 22.28 g on Day 1, Day 3, and Day 4 respectively. There was a decline in the weight over time, which indicated that the water loss could be the reason for the observed upward shift in the impedance response of the phantom. This is in complete agreement with the previously reported findings (Owda and Casson, 2021) where the impedance response was studied for seven days and found to increase over time. In our work, we not only investigated the stability of the phantom over time for consecutive days but also the impedance measurements were performed several times each day, and it was found that there was no significant shift in the impedance response on the same day of the experiment. This result indicates that the same phantom can be used to perform experiments multiple times on the same day of the experiment. The results of the impedance measurements obtained for four replicates show that across the range of frequency, the SD was about 25% of the mean, shown in the supplementary information (Fig S5). The phantom developed in this work, uses a quick method for the electrical measurements with the impedance measurement technique. This enables the phantom to be used to evaluate the performance of different electrode configurations fairly quickly.
Fig. 5.

Impedance response for phantom with porosity 0.16% obtained on Day 1 (black circle solid line), Day 1 after three hours (black circle dashed line), Day 1 after six hours (black circle dotted line), Day 2 (red square solid line), Day 3 (cyan triangle solid line), and Day 3 after four hours (cyan triangle dashed line).
3.4. Active phantom
ECG signal recorded on phantoms with different porosities (0%, 0.16%, 0.28%, and 1.4%) was found to be significantly different both qualitatively and quantitatively (Fig. 6). For a 0.12 mV ECG, typical for buttocks (Conn et al., 2018), we saw a low SNR (2.04dB) for the phantom with porosity 0.16% which simulates the dry skin impedance. Hydrated skin, modeled with the 0.28% porosity, corresponds to lower skin-electrode impedance, thus higher SNR (9.08 dB), which is consistent with prior reported work (Albulbul, 2016; Kappenman and Luck, 2010). This is primarily because more noise gets integrated with higher impedance. The difference between the simulated dry and hydrated skin was only evident with the 0.12 mV amplitude ECG signal. Although not shown here, it was observed that the different phantom configurations all had a much higher SNR with either a bigger area electrode (9 cm2) or a higher amplitude ECG signal (1.6 mV), typically obtained from standard locations. This is exactly what would be expected as the increase in the electrode area decreases the skin-electrode contact impedance (Chlaihawi et al., 2018). Hence, the phantom developed in this work can mimic dry and hydrated skin impedance and clearly simulate the impact of dry and hydrated skin conditions on the ECG signal. Therefore, the phantom provides a platform for controlled testing where a known signal can be given and recorded on the surface, which is challenging in the case of human subjects due to the unknown signal and variable skin impedance.
Fig. 6.

ECG recorded from phantoms with different porosities of 0%, 0.16%, 0.28%, and 1.4%. Top to bottom represent injected ECG signal, raw measured ECG superimposed with the denoised signal (bold line) for each phantom respectively. Calculated SNR is shown in text.
4. Conclusion
We developed a first of its kind skin phantom to model the interaction with dry electrodes across the range of frequency necessary for biopotential signals such as ECG, EEG, and EMG. Simple techniques were used for fabrication and properties of the phantom are stable for several days. The phantom mimics the effect of skin hydration in a controlled predictive manner based on the novel approach of varying porosity of a layer simulating the stratum corneum, the uppermost layer of skin. The porosity-based approach provides a method that can be leveraged to tune the phantom to simulate different individual skin impedances and study the impact of different skin types on the wearable electrodes. Spectroscopy measurements performed on the fabricated phantom showed that the impedance response for the phantom is similar to that of the human skin range. We additionally showed that the phantom simulated the impact of actual dry and hydrated skin conditions on the ECG signal quality in the time domain. The phantom could be used to facilitate electrode development, greatly reducing cost and complexity and increasing reproducibility by working with phantoms instead of a human subject.
Supplementary Material
Highlights.
Skin phantom mimics the effect of skin hydration in a controlled predictive manner.
Simulates impedance across 1 Hz-1000 Hz, crucial for capturing biopotential signals.
Includes a model of stratum corneum, necessary for testing wearable electrodes.
Demonstrates an active phantom in the time domain through propagation of ECG signal.
Stable for several days and easy to manufacture with a simple fabrication process.
5. Acknowledgments
This work was supported by the National Institute of Health [grant number R01 NR018301].
Biography
Krittika Goyal is currently pursuing PhD in Microsystems Engineering at RIT (Rochester, NY, USA). She is working on improving the signal quality from dry electrodes to overcome the challenges of in-home physiological monitoring devices. Her research interests include biomedical instrumentation, sensors and transducers, non-invasive physiological measurements, and their computational modeling. She has experience with human subject testing, for IRB-approved studies. She received her Bachelor’s (BE) and Master’s (ME) degree in Electronic Instrumentation and Control from Thapar University, Patiala, India in 2016. She worked as an Assistant Professor in the School of Electronics and Electrical Engineering at Lovely Professional University, Phagwara, India for a year before joining RIT in 2018.
David Borkholder is the Bausch and Lomb Professor of Microsystems Engineering at RIT (Rochester, NY, USA). His laboratory works on a variety of projects in biosensors, MEMS, medical devices, additive manufacturing, and therapies for auditory dysfunction. He founded two spin-out companies, one focused on soldier borne blast dosimetry, and the other on technologies for non-invasive cardiovascular assessment in the home. Prof Borkholder received the BS degree in Microelectronic Engineering from RIT, and the MS and PhD degrees in Electrical Engineering from Stanford University. He has trained at the Marine Biological Laboratory at Woods Hole on the biology of the inner ear.
Steven Day is the Harvey J Palmer Professor of Biomedical Engineering at RIT (Rochester, NY, USA). His research deals with the application of computational mechanics to a range of applied medical and biological problems. This includes collaborations with biologists, medical doctors, and industry partners. Prof. Day hold a BS degree in mechanical engineering and PhD in mechanical and aerospace engineering from the University of Virginia, as well as a diploma from the von Karman Institute for Fluid Dynamics. He is faculty of the Kate Gleason College of Engineering at RIT since 2004, and Department Head of Biomedical Engineering since 2016.
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. References
- Acar G, Ozturk O, Golparvar AJ, Elboshra TA, Böhringer K, Kaya Yapici M, 2019. Wearable and flexible textile electrodes for biopotential signal monitoring: A review. Electronics 8, 1–25. 10.3390/electronics8050479 [DOI] [Google Scholar]
- Albulbul A, 2016. Evaluating major electrode types for idle biological signal measurements for modern medical technology. Bioengineering 3. 10.3390/bioengineering3030020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anusha AS, Preejith SP, Akl TJ, Joseph J, Sivaprakasam M, 2018. Dry Electrode Optimization for Wrist-based Electrodermal Activity Monitoring. MeMeA 2018–2018 IEEE Int. Symp. Med. Meas. Appl. Proc. 1–6. 10.1109/MeMeA.2018.8438595 [DOI] [Google Scholar]
- Baker H, Kligman AM, 1967. Measurement of Transepidermal Water Loss by Electrical Hygrometry. Arch. Dermatol. 96, 441–452. 10.1001/archderm.1967.01610040091018 [DOI] [PubMed] [Google Scholar]
- Beckmann L, Neuhaus C, Medrano G, Jungbecker N, Walter M, Gries T, Leonhardt S, 2010. Characterization of textile electrodes and conductors using standardized measurement setups. Physiol. Meas. 31, 233–247. 10.1088/0967-3334/31/2/009 [DOI] [PubMed] [Google Scholar]
- Bera TK, 2018. Bioelectrical Impedance and the Frequency Dependent Current Conduction Through Biological Tissues: A Short Review. IOP Conf. Ser. Mater. Sci. Eng. 331. 10.1088/1757-899X/331/1/012005 [DOI] [Google Scholar]
- Bergey GE, Squires RD, Sipple WC, 1971. Electrocardiogram Recording with Pasteless Electrodes. IEEE Trans. Biomed. Eng. BME-18, 206–211. 10.1109/TBME.1971.4502833 [DOI] [Google Scholar]
- Bîrlea SI, Breen PP, Corley GJ, Bîrlea NM, Quondamatteo F, Ólaighin G, 2014. Changes in the electrical properties of the electrode-skin-underlying tissue composite during a week-long programme of neuromuscular electrical stimulation. Physiol. Meas. 35, 231–252. 10.1088/0967-3334/35/2/231 [DOI] [PubMed] [Google Scholar]
- Björklund S, Ruzgas T, Nowacka A, Dahi I, Topgaard D, Sparr E, Engblom J, 2013. Skin membrane electrical impedance properties under the influence of a varying water gradient. Biophys. J. 104, 2639–2650. 10.1016/j.bpj.2013.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YH, Op de Beeck M, Vanderheyden L, Carrette E, Mihajlović V, Vanstreels K, Grundlehner B, Gadeyne S, Boon P, van Hoof C, 2014. Soft, comfortable polymer dry electrodes for high quality ECG and EEG recording. Sensors (Switzerland) 14, 23758–23780. 10.3390/s141223758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi YM, Jung TP, Cauwenberghs G, 2010. Dry-contact and noncontact biopotential electrodes: Methodological review. IEEE Rev. Biomed. Eng. 3, 106–119. 10.1109/RBME.2010.2084078 [DOI] [PubMed] [Google Scholar]
- Chlaihawi AA, Narakathu BB, Emamian S, Bazuin BJ, Atashbar MZ, 2018. Development of printed and flexible dry ECG electrodes. Sens. Bio-Sensing Res. 20, 9–15. 10.1016/j.sbsr.2018.05.001 [DOI] [Google Scholar]
- Clar EJ, Her CP, Sturelle CG, 1975. Skin impedance and moisturization. J. Soc. Cosmet. Chem. Japan 26, 337–353. [Google Scholar]
- Conn NJ, Schwarz KQ, Borkholder DA, 2018. Nontraditional electrocardiogram and algorithms for inconspicuous in-home monitoring: Comparative study. JMIR Mhealth Uhealth 6. 10.2196/mhealth.9604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dąbrowska A, Rotaru GM, Spano F, Affolter C, Fortunato G, Lehmann S, Derler S, Spencer ND, Rossi RM, 2017. A water-responsive, gelatine-based human skin model. Tribol. Int. 113, 316–322. 10.1016/j.triboint.2017.01.027 [DOI] [Google Scholar]
- Fink PL, Muhammad Sayem AS, Teay SH, Ahmad F, Shahariar H, Albarbar A, 2021. Development and wearer trial of ECG-garment with textile-based dry electrodes. Sensors Actuators, A Phys. 328, 112784. 10.1016/j.sna.2021.112784 [DOI] [Google Scholar]
- Fu Y, Zhao J, Dong Y, Wang X, 2020. Dry electrodes for human bioelectrical signal monitoring. Sensors (Switzerland) 20, 1–30. 10.3390/s20133651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel S, Lau RW, Gabriel C, 1996a. The dielectric properties of biological tissues: II. Measurements in the frequency range 10 Hz to 20 GHz. Phys. Med. Biol. 41, 2251–2269. 10.1088/0031-9155/41/11/002 [DOI] [PubMed] [Google Scholar]
- Gabriel S, Lau RW, Gabriel C, 1996b. The dielectric properties of biological tissues: III. Parametric models for the dielectric spectrum of tissues. Phys. Med. Biol. Phys. Med. Biol 41, 2271–2293. 10.1088/0031-9155/41/11/003 [DOI] [PubMed] [Google Scholar]
- Getangama NN, De Bruyn JR, Hutter JL, 2020. Dielectric properties of PVA cryogels prepared by freeze-thaw cycling. J. Chem. Phys. 153. 10.1063/5.0007251 [DOI] [PubMed] [Google Scholar]
- Go SA, Coleman-Wood K, Kaufman KR, 2014. Frequency analysis of lower extremity electromyography signals for the quantitative diagnosis of dystonia. J. Electromyogr. Kinesiol. 24, 31–36. 10.1016/j.jelekin.2013.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha S, Kim C, Chi YM, Cauwenberghs G, 2014. Low-Power Integrated Circuit Design for Wearable Biopotential Sensing, in: Sazonov E, Neuman MR (Eds.), Wearable Sensors: Fundamentals, Implementation and Applications. Oxford, U.K.:Academic, pp. 323–352. 10.1016/B978-0-12-418662-0.00018-0 [DOI] [Google Scholar]
- Heikenfeld J, Jajack A, Rogers J, Gutruf P, Tian L, Pan T, Li R, Khine M, Kim J, Wang J, Kim J, 2018. Wearable sensors: Modalities, challenges, and prospects. Lab Chip 18, 217–248. 10.1039/c7lc00914c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Furuya K, Okano Y, Hamada L, 2001. Development and characteristics of a biological tissue-equivalent phantom for microwaves. Electron. Commun. Japan, Part I Commun. (English Transl. Denshi Tsushin Gakkai Ronbunshi) 84, 67–77. [DOI] [Google Scholar]
- Jin C, Ma C, Yang Z, Lin H, 2020. A force measurement method based on flexible PDMS grating. Appl. Sci. 10. 10.3390/app10072296 [DOI] [Google Scholar]
- Kaitainen S, Kutvonen A, Suvanto M, Pakkanen TT, Lappalainen R, Myllymaa S, 2014. Liquid silicone rubber (LSR)-based dry bioelectrodes: The effect of surface micropillar structuring and silver coating on contact impedance. Sensors Actuators, A Phys. 206, 22–29. 10.1016/j.sna.2013.11.020 [DOI] [Google Scholar]
- Kalra A, Lowe A, Anand G, 2020. Bio Phantoms Mimicking the Dielectric and Mechanical Properties of Human Skin Tissue at Low-Frequency Ranges. Mod. Appl. Sci. 14, 1. 10.5539/mas.v14n7p1 [DOI] [Google Scholar]
- Kalvøy H, Frich L, Grimnes S, Martinsen ØG, Hol PK, Stubhaug A, 2009. Impedance-based tissue discrimination for needle guidance. Physiol. Meas. 30, 129–140. 10.1088/0967-3334/30/2/002 [DOI] [PubMed] [Google Scholar]
- Kappenman ES, Luck SJ, 2010. The effects of electrode impedance on data quality and statistical significance in ERP recordings. Psychophysiology 47, 888–904. 10.1111/j.1469-8986.2010.01009.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoshmanesh F, Thurgood P, Pirogova E, Nahavandi S, Baratchi S, 2021. Wearable sensors: At the frontier of personalised health monitoring, smart prosthetics and assistive technologies. Biosens. Bioelectron. 176, 112946. 10.1016/j.bios.2020.112946 [DOI] [PubMed] [Google Scholar]
- Lee HK, Chang S Il, Yoon E, 2006. A flexible polymer tactile sensor: Fabrication and modular expandability for large area deployment. J. Microelectromechanical Syst. 15, 1681–1686. 10.1109/JMEMS.2006.886021 [DOI] [Google Scholar]
- Liu CH, Huang YC, Li SH, Chen YA, Wang WZ, Yu JS, Shih WP, 2019. Microelectromechanical system-based biocompatible artificial skin phantoms. Micro Nano Lett. 14, 333–338. 10.1049/mnl.2018.5112 [DOI] [Google Scholar]
- Lobodzinski SM, 2010. ECG Instrumentation: Application and Design, in: Macfarlane PW, van Oosterom A, Pahlm O, Kligfield P, Janse M, Camm J (Eds), Comprehensive Electrocardiology. Springer, London, pp. 427–480. 10.1007/978-1-84882-046-3_12 [DOI] [Google Scholar]
- Maithani Y, Choudhuri B, Mehta BR, Singh JP, 2021. Self-adhesive, stretchable, and dry silver nanorods embedded polydimethylsiloxane biopotential electrodes for electrocardiography. Sensors Actuators A Phys. 332, 113068. 10.1016/j.sna.2021.113068 [DOI] [Google Scholar]
- Mix DS, Stoner MC, Day SW, Richards MS, 2018. Manufacturing abdominal aorta hydrogel tissue-mimicking phantoms for ultrasound elastography validation. J. Vis. Exp. 2018, 1–9. 10.3791/57984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachman M, Franklin SE, 2016. Artificial Skin Model simulating dry and moist in vivo human skin friction and deformation behaviour. Tribol. Int. 97, 431–439. 10.1016/j.triboint.2016.01.043 [DOI] [Google Scholar]
- Nawanil C, Makcharoen W, Khaosa-Ard K, Maluangnont T, Vittayakorn W, Isarakorn D, Vittayakorn N, 2019. Electrical and dielectric properties of barium titanate–polydimethylsiloxane nanocomposite with 0–3 connectivity modified with carbon nanotube (CNT). Integr. Ferroelectr. 195, 46–57. 10.1080/10584587.2019.1570043 [DOI] [Google Scholar]
- Owda AY, Casson AJ, 2021. Investigating Gelatine Based Head Phantoms for Electroencephalography Compared to Electrical and Ex Vivo Porcine Skin Models. IEEE Access 9, 96722–96738. 10.1109/ACCESS.2021.3095220 [DOI] [Google Scholar]
- Peng S, Xu K, Chen W, 2019. Comparison of active electrode materials for non-contact ECG measurement. Sensors (Switzerland) 19, 1–18. 10.3390/s19163585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reicherter JM, 2015. Anatomy and physiology for polygraph examiners, in: Krapohl DJ, Shaw PK (Eds.), Fundamentals of Polygraph Practice. Elsevier Inc., pp. 29–60. 10.1016/b978-0-12-802924-4.00002-5 [DOI] [Google Scholar]
- Stauffer SR, Peppast NA, 1992. Poly(vinyl alcohol) hydrogels prepared by freezing-thawing cyclic processing. Polymer (Guildf). 33, 3932–3936. 10.1016/0032-3861(92)90385-A [DOI] [Google Scholar]
- Tagami H, Ohi M, Iwatsuki K, Kanamaru Y, Yamada M, Ichijo B, 1980. Evaluation of the skin surface hydration in vivo by electrical measurement. J. Invest. Dermatol. 75, 500–507. 10.1111/1523-1747.ep12524316 [DOI] [PubMed] [Google Scholar]
- Tereshchenko LG, Josephson ME, 2015. Frequency content and characteristics of ventricular conduction. J. Electrocardiol. 48, 933–937. 10.1016/j.jelectrocard.2015.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakor NV, Webster JG, Tompkins WJ, 1983. Optimal QRS detector. Med. Biol. Eng. Comput. 21, 343–350. 10.1007/BF02478504 [DOI] [PubMed] [Google Scholar]
- The Dow Chemical Company, 2017. SYLGARD TM 184 Silicone Elastomer Technical Datasheet. https://consumer.dow.com/content/dam/dcc/documents/en-us/productdatasheet/11/11-31/11-3184-sylgard-184-elastomer.pdf (accessed 9.7.21).
- Tregear R, 1965. Interpretation of Skin Impedance Measurements. Nature 205, 600–601. 10.1038/205600a0 [DOI] [Google Scholar]
- Welch PD, 1967. The Use of Fast Fourier Transform for the Estimation of Power Spectra: A Method Based on Time Averaging Over Short, Modified Periodograms. IEEE Trans. Audio Electroacoust. 15, 70–73. 10.1109/TAU.1967.1161901 [DOI] [Google Scholar]
- Yamamoto T, Sano K, Koshiji K, Chen X, Yang S, Abe M, Fukuda A, 2013. Development of electromagnetic phantom at low-frequency band. Proc. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. EMBS 1887–1890. 10.1109/EMBC.2013.6609893 [DOI] [PubMed] [Google Scholar]
- Yao S, Myers A, Malhotra A, Lin F, Bozkurt A, Muth JF, Zhu Y, 2017. A Wearable Hydration Sensor with Conformal Nanowire Electrodes. Adv. Healthc. Mater. 6, 1–8. 10.1002/adhm.201601159 [DOI] [PubMed] [Google Scholar]
- Yokus MA, Jur JS, 2016. Fabric-based wearable dry electrodes for body surface biopotential recording. IEEE Trans. Biomed. Eng. 63, 423–430. 10.1109/TBME.2015.2462312 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




