Abstract
Background
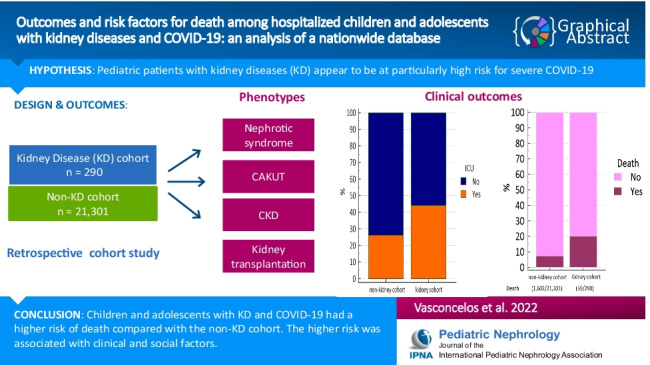
Patients with kidney diseases (KD) appear to be at particularly high risk for severe COVID-19. This study aimed to characterize the clinical outcomes and risk factors for COVID-19-related death in a large cohort of hospitalized pediatric patients with KD.
Methods
We performed an analysis of all pediatric patients with KD and COVID-19 registered in SIVEP-Gripe, a Brazilian nationwide surveillance database, between February 16, 2020, and May 29, 2021. The primary outcome was time to death, which was evaluated considering discharge as a competitive risk by using cumulative incidence function.
Results
Among 21,591 hospitalized patients with COVID-19, 290 cases (1.3%) had KD. Of these, 59 (20.8%) had a fatal outcome compared with 7.5% of the non-KD cohort (P < 0.001). Pediatric patients with KD had an increased hazard of death compared with the non-KD cohort (Hazard ratio [HR] = 2.85, 95% CI 2.21–3.68, P < 0.0001). After adjustment, the factors associated with the death among KD patients were living in Northeast (HR 2.16, 95% CI 1.13–4.31) or North regions (HR 3.50, 95% CI 1.57–7.80), oxygen saturation < 95% at presentation (HR 2.31, 95% CI 1.30–4.10), and presence of two or more associated comorbidities (HR 2.10, 95% CI 1.08–4.04).
Conclusions
Children and adolescents with KD had a higher risk of death compared with the non-KD cohort. The higher risk was associated with low oxygen saturation at admission, living in socioeconomically disadvantaged regions, and presence of other pre-existing comorbidities.
Graphical abstract
A higher resolution version of the Graphical abstract is available as Supplementary information
Supplementary Information
The online version contains a graphical abstract available at 10.1007/s00467-022-05588-0.
Keywords: SARS-CoV-2, COVID-19, Kidney disease, Children, Outcome, Risk factors
Introduction
The pediatric population has classically been viewed as highly vulnerable to infectious diseases. Somewhat unexpectedly, regarding the SARS-CoV-2 infection, there has been mounting evidence that the clinical outcomes in children are much more favorable than in adults [1]. Nevertheless, a relatively small proportion of children might be at risk for severe disease and death [2, 3]. Among the risk factors for unfavorable outcomes in pediatric age, pre-existing chronic conditions have emerged as strongly related with the severe spectrum of the disease [4, 5]. In a previous study, we reported that the presence and the number of pre-existing medical conditions, including kidney diseases (KD), exhibited a step gradient effect significantly increasing the risk of death [6].
Data from the adult population suggested a higher incidence of severe COVID-19 in individuals with chronic kidney disease (CKD) and a poor prognosis of the disease in kidney transplant recipients [7–10]. Nevertheless, information on COVID-19 in children with underlying CKD is limited, with few reports from developed countries [11, 12]. Recently, Krishnasamy et al. [13] reported a comprehensive analysis of the clinical course of SARS-CoV-2 infection in children with CKD in India. The authors have shown that children with CKD presenting with moderate-to-severe COVID-19 are at risk of complications, including severe acute kidney injury (AKI) and death. However, little is known about risk factors for mortality among pediatric patients with KD and COVID-19. Further understanding of these issues may provide important insights and guide development of strategies for target pediatric groups.
We recently analyzed data in children and adolescents hospitalized with COVID-19 from the Influenza Epidemiological Surveillance Information System (SIVEP-Gripe) database, a Brazilian national registry [6, 14]. We described clinical outcomes and risk factors for death related to the first and second waves of COVID-19 in the Brazilian pediatric population. It is important to note that, in these studies, we found a significant additive effect related to the presence of comorbidities. In the present study, using these datasets, we aimed to describe the features and outcomes of pediatric patients with KD and to evaluate the risk factors for COVID-19-related death in this population.
Methods
Study design
We performed a retrospective cohort study including all hospitalized pediatric cases recorded in the SIVEP-Gripe. Detailed information regarding this database, including reporting form and data dictionary, codes, and all de-identified data, are publicly available at https://opendatasus.saude.gov.br/dataset/srag-2020 and at https://opendatasus.saude.gov.br/dataset/srag-2021-e-2022 for data of 2020 and 2021, respectively.
Participants and case-defining
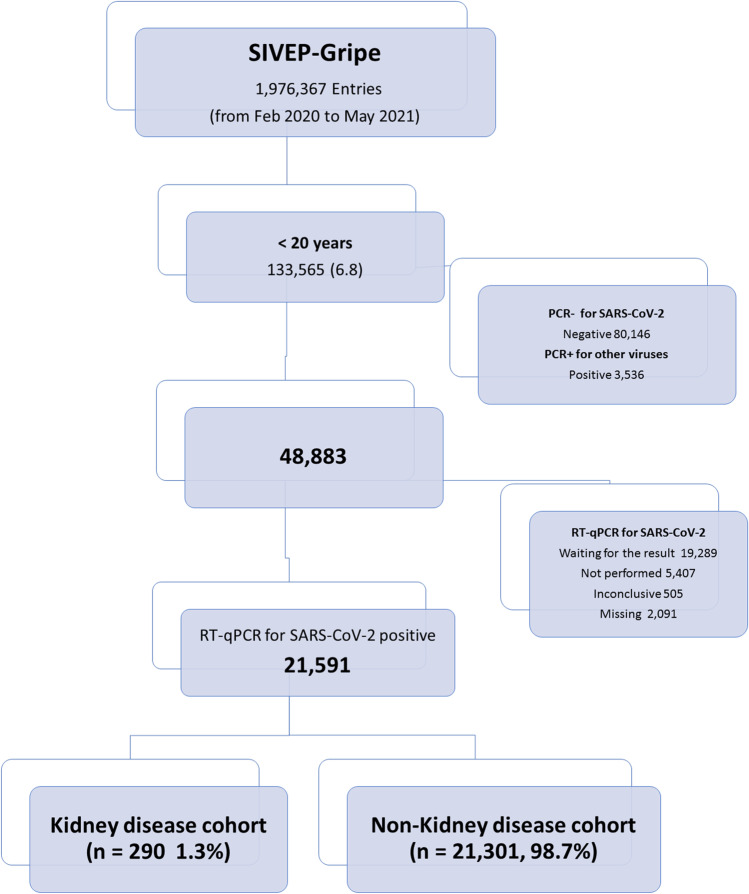
We included all consecutively registered patients, aged less than 20 years, with a positive quantitative RT-PCR (RT-qPCR) test result for SARS-CoV-2 who were admitted to the hospital. For the present study, we integrated two datasets. First, we downloaded the SIVEP-gripe database on January 10, 2021 (corresponding to the cases admitted from February 16, 2020, to December 31, 2020). The second database was downloaded on May 29, 2021 (corresponding to the cases admitted from January 1, 2021, to May 29, 2021). For the purpose of analysis, we merged both datasets into a unique database and the cohort was divided into groups: wave 1 and wave 2. The rationale for separating the sample into two waves was due to the emergence of the Gamma variant identified in January 2021 in the city of Manaus, Brazil [15]. This strain became predominant in Brazil in mid-February 2021 and was characterized by greater transmissibility and a more severe spectrum of the disease in both adult and pediatric populations [14, 16]. Detailed information about included and excluded cases are displayed in the flowchart (Fig. 1). Over the entire period, a total of 1,976,367 hospitalized cases were registered in SIVEP-Gripe database. Of them, 133,565 (6.8%) were younger than 20 years at admission and 21,591 (1% of the total hospitalized cases and 16% of the pediatric age group) had a laboratory-confirmed result for SARS-CoV-2 infection and were included in the analysis.
Fig. 1.
Flow diagram of cohort selection
We identified KD cases in the SIVEP-Gripe database by retrieving data from specific fields for comorbidities. In the database, information about comorbidities is provided in closed-end fields (yes/no). In addition to KD, the dataset provides data on the following comorbidities: asthma, obesity, diabetes, and cardiovascular, lung, kidney, liver, autoimmune, neurologic, and hematologic diseases. However, this information is provided without any detailed clinical data about the underlying condition. In order to minimize this important limitation, we carefully revised the string field “morb_desc,” an open-ended one, which included some relevant clinical information, especially related with comorbidities and other risk factors (for example, identification of a specific disease). Then, we tried to retrieve more information regarding cases with KD by cross-checking the fields “comorbidities” and “morb_desc.” Unfortunately, only about half of the “morb_desc” fields provided more specific information concerning the KD (for example, “urinary tract anomaly,” “kidney transplantation,” and so on). Moreover, we had no access to hospital record data to include information about pharmacotherapies, laboratory results, or detailed clinical course of the patients.
Covariates and definitions
Clinical, demographic, and epidemiological data recorded in SIVEP-Gripe are described elsewhere [6]. The clinical course of the disease was reported in terms of respiratory support (none, non-invasive oxygen support, and invasive ventilation), admission to intensive care unit (ICU), discharge, death, and ongoing clinical situation.
Missing data management
In the SIVEP-Gripe dataset, the following covariates had missing information: gender (0.08%), ethnicity (19.6%), oxygen saturation at admission (24.7%), ICU admission (8%), the use of ventilatory support (5.5%), and primary outcome (0.8%). We used various strategies to partially overcome this problem. We describe these strategies in detail elsewhere [14]. Briefly, patients with missing information about the primary outcome were removed from the survival analyses. For those cases with missing data on a particular symptom or comorbidity, we assumed that the clinical condition was absent. Finally, we performed a multiple imputation using all predictors plus the cumulative incidence function for the primary outcome. Ten imputed data sets were generated using the multiple imputation chain equations (MICE) package from the R software (R Foundation for Statistical Computing, Vienna, Austria. Available on https://cran.r-project.org/web/packages/mice/index.html). We combined the results from analyses on each of the imputed values using Rubin’s rules to produce estimates and confidence intervals that incorporate the uncertainty of imputed values [17, 18].
Outcome
The primary outcome was time until death (in-hospital-mortality). The survival time was defined from the day of admission until the event (death or discharge). In the SIVEP-Gripe database, outcome is classified into four categories: (1) recovered, (2) death, (3) death from other causes, and (4) unknown. For analysis purposes, we considered only the cases registered as COVID-19-related death as the main outcome. Hospital discharge (recovered in the database) and death from other causes were considered concurrent events (using the date of discharge or date of death for other causes). The remaining cases were considered as an ongoing clinical situation and were censored on the last date recorded in the field.
Statistical analysis
We used medians and interquartile ranges (IQRs) or means and standard deviations (SDs) to summarize continuous variables and calculated frequencies and proportions for categorical variables. We compared means and proportions using F test and the chi-squared test. All statistical tests were two tailed, and statistical significance was defined as P < 0.05.
Mortality was evaluated by competing risks analysis, using cumulative incidence function (CIF) [19]. Discharge and death for other causes were analyzed as competing events in the competing-risk analysis. Complete data were not available for all variables, especially ethnicity and oxygen saturation. We carried out multiple imputations using all predictors plus the CIF for the primary outcome. This procedure creates multiple copies of the data and allows imputations of the missing values for each dataset with sensible values randomly selected from their predicted distribution. Ten imputed values were generated using the multiple imputation chain equation (MICE) package of the software R. We combined the results from analyses on each of the imputed values using Rubin’s rules to produce estimates and confidence intervals that incorporate the uncertainty of imputed values [17, 18]. For comorbidities, we assumed missing values as the absence of the clinical condition.
Ethical aspects
We accessed data in SIVEP-Gripe, which are de-identified and publicly available. Following ethically agreed principles on open data, this analysis did not require ethical approval in Brazil. We reported our findings following the guideline STROBE for observational cohort studies [20].
Results
Baseline demographic, clinical characteristics, and outcomes
The entire cohort comprised 21,591 cases. Of them, 290 (1.3%) were reported with KD in the database. The demographic and clinical characteristics of the cohorts according to the presence of KD are shown in Table 1. The comparison of proportions showed that the groups were clinically and demographically distinct. Children and adolescents of the KD cohort were older at admission, were admitted more frequently in the first wave, and had more associated comorbidities than the non-KD cohort. The KD cohort also presented with greater proportion of respiratory symptoms and oxygen saturation < 95% at baseline.
Table 1.
Demographic, clinical characteristics, and outcomes of children and adolescents with laboratory-confirmed COVID-19 according to presence of kidney diseases (n = 21,591)
| Overall (%) 21,591 (100) |
Non-kidney disease cohort (%) 21,301 (98.7) |
Kidney disease cohort (%) 290 (1.3) |
P | |
|---|---|---|---|---|
| Age (years) | ||||
|
Median (Interquartile range) Mean (SD) |
4.7 (0.8–14.6) 7.4 (7.0) |
4.6 (0.8–14.5) 7.4 (7.0) |
10.7 (3.7–16.3) 10.3 (6.4) |
< 0.0001 < 0.0001 |
| Age group (years) | ||||
|
0.0–9.9 10–19.9 |
13,856 (64.2) 7735 (35.8) |
13,719 (64.4) 7582 (35.6) |
137 (47.2) 153 (52.8) |
< 0.0001 |
| Gender (n = 21,573) | ||||
|
Female Male |
10,411 (48.3) 11,162 (51.7) |
10,267 (48.2) 11,016 (51.8) |
144 (49.7) 146 (50.3) |
0.63 |
| Wave | ||||
|
First Second |
11,574 (53.6) 10,017 (46.4) |
11,395 (53.5) 9906 (46.5) |
179 (61.7) 111 (38.3) |
0.005 |
| Region | ||||
|
Southeast South Central-West Northeast North |
8075 (37.4) 2204 (10.2) 2293 (10.6) 5748 (26.6) 3271 (15.1) |
7949 (37.3) 2177 (10.2) 2268 (10.6) 5669 (26.6) 3238 (15.2) |
126 (43.4) 27 (9.3) 25 (8.6) 79 (27.2) 33 (11.4) |
0.13 |
| Ethinicity | ||||
|
White Black/Brown Asian Indigenous |
8047 (37.2) 13,107 (60.7) 181 (0.84) 256 (1.2) |
7933 (37.2) 12,934 (60.7) 179 (0.84) 255 (1.2) |
114 (39.3) 173 (59.7) 2 (0.70) 1 (0.30) |
0.53 |
| Signs and symptoms at baseline | ||||
|
Fever Cough Respiratory distress Oxygen saturation < 95% Dyspnea Odynophagia Diarrhea Vomit Abdominal pain |
14,140 (65.5) 12,971 (60.1) 9733 (45.1) 9580 (44.4) 10,470 (48.5) 3590 (16.6) 3187 (14.8) 3629 (16.8) 1454 (6.7) |
13,966 (65.6) 12,826 (60.2) 6993 (42.5) 6674 (40.6) 7472 (45.4) 2859 (17.4) 2524 (15.4) 2804 (17.1) 1129 (6.9) |
174 (60.0) 145 (50.0) 126 (43.4) 136 (46.9) 136 (46.9) 38 (13.1) 53 (18.3) 52 (17.9) 19 (6.6) |
0.053 0.001 < 0.001 < 0.001 < 0.001 < 0.001 < 0.001 0.15 0.38 |
| Number of comorbidities | ||||
|
None 1 2 > 3 |
16,441 (76.2) 4313 (20.0) 693 (3.2) 144 (0.67) |
16,441 (77.2) 4165 (19.5) 599 (2.8) 96 (0.45) |
0 (0.0) 148 (51.0) 94 (32.4) 48 (16.6) |
< 0.0001 |
| ICU admission (n = 19,867) | ||||
|
Yes No |
5243 (26.4) 14,624 (73.6) |
5123 (24.1) 14,472 (75.9) |
120 (45.1) 152 (55.9) |
< 0.0001 |
| Ventilatory support (n = 20,396) | ||||
|
None Non-invasive Invasive |
11,272 (55.3) 6994 (34.3) 2130 (10;4) |
11,145 (52.3) 6909 (32.4) 2067 (9.7) |
127 (46.2) 85 (30.9) 63 (23.4) |
< 0.0001 |
| Death | ||||
|
Yes No |
1661 (7.7) 19,930 (92.3) |
1602 (7.5) 19,699 (92.5) |
59 (20.3) 231 (79.7) |
< 0.0001 |
The clinical outcomes are also shown in Table 1. Overall, patients with KD had higher prevalence of the severe spectrum of COVID-19, considering ICU admission, invasive ventilation, and death. Regarding critical support, 120 cases with KD (45.1%) were admitted to ICUs compared with 24.1% of patients of the non-KD cohort (P < 0.0001). Of 290 children and adolescents with KD, 63 (23.4%) required invasive ventilatory support as opposed to 9.7% of those without KD (P < 0.0001). Regarding mortality, 59 patients of the KD cohort (20.8%) died as compared with 7.5% of cases without KD (P < 0.0001).
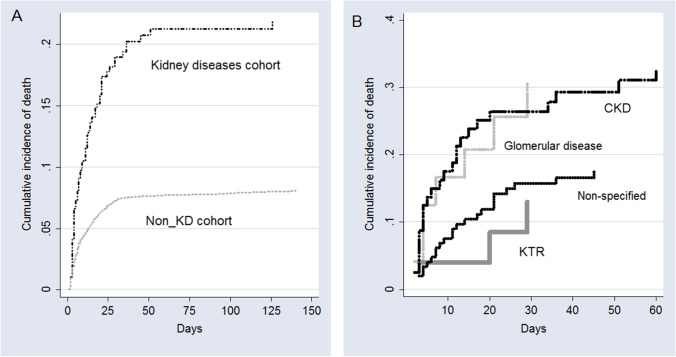
The CIF of death for the groups is shown in Fig. 2A. The estimated probability of fatal outcome for the first 10, 20, 30, and 50 days of hospitalization for pediatric patients without KD was 4.5%, 6.3%, 7.3%, and 7.6% respectively, whereas for cases with KD was 11.2%, 15.9%, 18.9%, and 21.3%, respectively. According to the Fine–Gray model, children and adolescents with KD had a significantly increased hazard of death compared with pediatric patients without KD (HR = 2.85, 95% CI, 2.21–3.68, P < 0.0001).
Fig. 2.
Cumulative incidence function of death according to (A) the overall presence of kidney diseases and (B) specific kidney diseases and non-kidney disease cohorts
Among 290 children and adolescents with KD, data regarding the specific illness were available only for 143 patients (49.3%). The demographic and clinical characteristics of these cases are shown in Table 2. The categories were divided in glomerular disease (8.6%), kidney transplant recipients (KTR, 8.6%), congenital anomalies of kidney and urinary tract (CAKUT, 4.1%), CKD (27.9%), and those cases without information regarding the specific disease (50.7%). The main differences were age, associated comorbidities, and clinical outcomes. KTRs were older and had more associated comorbidities. CAKUT patients had a small proportion of ICU admission and invasive ventilatory support and none died during hospital stay (Table 2). The CIF of death for the KD is shown in Fig. 2B. The estimated probability of in-hospital death for children with CKD was 32.8%, for patients with glomerular disease 30.5%, for non-specified kidney disease 17.5%, and for KTR patients, 13%.
Table 2.
Demographic, clinical characteristics, and outcomes of children and adolescents with kidney diseases and laboratory-confirmed COVID-19 (n = 290)
| Overall (%) 290 (100) |
Nephrotic syndrome 25 (8.6) |
KTRs (%) 25 (8.6) |
CAKUT (%) 12 (4.1) |
CKD (%) 81 (27.9) |
Non-specified (%) 147 (50.7) |
P | |
|---|---|---|---|---|---|---|---|
| Age (years) | |||||||
|
Median (Interquartile range) Mean (SD) |
10.7 (3.7–16.4) 10.3 (6.5) |
10.1 (3.1–16.1) 10.0 (6.5) |
14.4 (10.4–17.5) 14.0 (4.4) |
2.2 (0.9–10.9) 6.1 (6.5) |
7.2 (1.1–15.3) 8.3 (6.9) |
12.0 (6.0–12.5) 11.2 (6.1) |
< 0.001 < 0.001 |
| Age group (years) | |||||||
|
0.0–1.9 2.0–9.9 10–19.9 |
48 (16.6) 89 (30.7) 153 (52.8) |
4 (16.0) 8 (32.0) 13 (52.0) |
0 (0.0) 5 (20.0) 20 (80.0) |
5 (41.7) 4 (33.3) 3 (25.0) |
26 (32.1) 22 (27.2) 33 (40.7) |
13 (8.8) 50 (34.0) 84 (57.1) |
< 0.001 |
| Gender (n = 21,573) | |||||||
|
Female Male |
144 (49.7) 146 (50.3) |
12 (48) 13 (52) |
18 (72) 7 (28) |
3 (25) 9 (75) |
40 (49.4) 41 (50.6) |
71 (48.3) 76 (51.7) |
0.09 |
| Wave | |||||||
|
First Second |
179 (61.7) 111 (38.3) |
14 (56) 11 (44) |
15 (60) 10 (40) |
9 (75) 3 (25) |
48 (59.3) 33 (40.7) |
93 (63.3) 54 (36.7) |
0.80 |
| Region | |||||||
|
Southeast South Central-West Northeast North |
126 (43.4) 27 (9.3) 25 (8.6) 79 (27.2) 33 (11.4) |
9 (36) 3 (12) 3 (12) 6 (24) 4 (16) |
20 (80) 1 (4) 2 (8) 2 (8) 0 (0) |
4 (33.3) 1 (8.3) 2 (16.7) 4 (33.3) 1 (8.3) |
36 (44.4) 3 (3.7) 7 (8.6) 26 (32.0) 9 (11.1) |
57 (38.8) 19 (12.9) 11 (7.5) 41 (27.9) 19 (12.9) |
0.08 |
| Ethnicity | |||||||
|
White Non-White |
114 (39.3) 176 (60.7) |
10 (40.0) 15 (60.0) |
18 (72.0) 7 (28.0) |
8 (66.7) 4 (33.3) |
25 (30.9) 56 (69.1) |
53 (36.0) 94 (63.4) |
0.001 |
| Signs and symptoms at baseline | |||||||
|
Fever Cough Respiratory distress Dyspnea Oxygen saturation < 95% Odynophagia Diarrhea Vomit Abdominal pain |
174 (60.0) 145 (50.0) 126 (43.4) 136 (46.9) 136 (46.9) 38 (13.1) 53 (18.3) 52 (17.9) 19 (6.6) |
15 (60) 15 (60) 9 (36) 11 (44.0) 13 (52.0) 3 (12.0) 5 (20.0) 2 (8.0) 2 (8.0) |
17 (68.0) 9 (36.0) 2 (8.0) 6 (24.0) 9 (36.0) 2 (8.0) 5 (20.0) 6 (24.0) 3 (12.0) |
9 (75.0) 6 (50.0) 5 (41.7) 6 (50.0) 4 (33.3) 1 (8.3) 7 (58.3) 3 (25.0) 0 (0.0) |
59 (72.8) 36 (44.4) 43 (53.1) 40 (49.4) 40 (49.4) 10 (12.3) 11 (13.6) 18 (22.2) 8 (9.9) |
74 (50.3) 79 (53.7) 67(45.6) 73 (49.7) 70 (47.6) 22 (15.0) 25 (17.0) 23 (15.6) 6 (4.1) |
0.011 0.31 0.002 0.196 0.632 0.857 0.006 0.374 0.281 |
| Number of comorbidities | |||||||
|
1 2 3 |
148 (51.0) 94 (32.4) 48 (16.6) |
19 (76.0) 4 (16.0) 2 (8.0) |
1 (4.0) 10 (40.0) 14 (56.0) |
10 (83.3) 1 (8.3) 1 (8.3) |
46 (56.8) 17 (21.0) 18 (22.2) |
72 (49.0) 62 (42.2) 13 (8.8) |
0.001 |
| ICU admission (n = 272) | |||||||
|
Yes No |
120 (44.1) 152 (55.9) |
10 (41.7) 14 (58.3) |
9 (36.0) 16 (64.0) |
3 (27.3) 8 (72.7) |
41 (55.4) 33 (44.6) |
57 (41.3) 81 (58.7) |
0.14 |
| Ventilatory support (n = 275) | |||||||
|
None Non-invasive Invasive |
127 (46.2) 85 (30.9) 63 (22.9) |
7 (31.8) 10 (45.5) 5 (22.7) |
14 (58.3) 7 (29.2) 3 (12.5) |
7 (58.3) 4 (33.3) 1 (8.3) |
27 (35.1) 20 (26.0) 30 (39.0) |
72 (51.4) 44 (31.4) 24 (17.1) |
0.01 |
| Death | |||||||
|
Yes No |
59 (20.3) 231 (79.7) |
7 (28.0) 18 (72.0) |
3 (12.0) 22 (88.0) |
0 (0.0) 12 (100.0) |
25 (30.9) 56 (69.1) |
24 (16.3) 123 (83.7) |
0.017 |
KTR kidney transplant recipients, CAKUT congenital anomalies of kidney and urinary tract, CKD chronic kidney disease
Risk factors of fatal outcome
Risk factors were assessed among the 287 children and adolescents with KD with complete information regarding the primary outcome. In the competing-risk univariate analysis, using the Fine–Gray model-to-model mortality, female sex, patients from the poorest regions of the country (Northeast and North), presence of respiratory symptoms at baseline, oxygen saturation < 95%, and presence of other comorbidities were significantly associated with higher hazard of death.
After adjustment by the competing-risk multivariate regression analysis, three covariates remained significantly associated with the hazard of COVID-19-related death among KD pediatric patients: living in Northeast (HR 2.16, 95% CI 1.13–4.31) or North regions (HR 3.50, 95% CI 1.57–7.80), oxygen saturation < 95% at presentation (HR 2.31, 95% CI 1.30–4.10), and presence of two or more associated comorbidities (HR 2.10, 95% CI 1.08–4.04).
Interestingly, the North region exhibited the higher hazard of death, even greater than any clinical feature. To assess this issue further, we examined the associations between regions, cohorts, and the main outcome. First, stratifying by the cohorts, in the pediatric population without KD, the death rates for North region and other regions were, respectively, 8.1% and 7.4% (P = 0.18). Conversely, in the pediatric patients with KD, the respective values were 36.4% and 16.3% (P < 0.0001). Stratifying by regions, in the North region, the death rate for pediatric patients with KD and those without were, respectively, 8.1% and 36.4% (P < 0.0001). In the other regions, the respective values were 7.4% and 18.5% (P < 0.0001).
Discussion
We describe the outcomes of SARS-CoV-2 infection in 290 pediatric patients with KD included in a large cohort of hospitalized children and adolescents with laboratory-proven diagnosis of COVID-19 in Brazil. Our findings show that children and adolescents with KD had a higher risk of severe COVID-19 and a significantly increased hazard of death compared with the non-KD cohort. Among pediatric patients with KD, after adjustment by the competing-risk survival analysis, the risk factors significantly associated with a higher hazard of death included clinical and social demographic covariates.
In a large systematic review and meta-analysis including 348 studies (382,407 participants with COVID-19 and CKD; 1,139,979 total participants with CKD), Chung et al. [9] found that individuals with CKD are at higher risk of COVID-19 than the general population and are at higher risk of death than patients with CKD but without COVID-19. On the other hand, there is a paucity of data in pediatric patients with CKD. In addition, the data reported are from high- or upper middle-income countries, which may limit the generalizability of the findings. Preliminary data from developed countries have shown that COVID-19 appears to have a benign clinical course in children with underlying KD [21–23]. For instance, Mastrangelo et al. [24] reported findings of the COVID-19 Task Force of the Italian Society of Pediatric Nephrology. Among 1572 children with CKD included in the study, swab tests were performed in 84 patients, mostly symptomatic children. Only three patients (0.19%) tested positive for SARS-CoV-2, all living in the worst affected Italian region (Lombardy), and no patients fulfilled the criteria for the presence of severe COVID-19. Similarly, Plumb et al. [12] reported data from all 13 UK pediatric nephrology centers. Between March 26 and July 15, 2020, five UK children with CKD who tested positive for SARS-CoV-2 infection were reported; none died. Melgosa et al. [11] reported data from 16 children with CKD and COVID-19 in Spain. All patients had a mild spectrum of the disease, none required oxygen therapy, and 7 could be managed as outpatients. Interestingly, Angeletti et al. [25] have shown that patients with nephrotic syndrome under chronic immunosuppression including B cell-depleting therapies seem not to be at increased risk of COVID-19 even in areas of high incidence of SARS-CoV-2 infection.
Data from developing countries instead revealed a more severe spectrum of the disease and worse outcomes. For instance, recently, Krishnasamy et al. [13] reported detailed data of 88 children with CKD and SARS-CoV-2 infection from four pediatric nephrology centers in New Delhi. Seventeen (19.3%) patients developed moderate or severe COVID-19. Systemic complications were observed in 30 patients (34.1%), including AKI (34.2%), COVID-19 pneumonia (15.9%), and shock (4.5%). Nineteen (21.6%) had severe complications (AKI stage 2–3, encephalopathy, respiratory failure, shock). Three (3.4%) patients died due to respiratory failure. Unfortunately, our dataset did not provide detailed information on the clinical course, but the overall mortality rate was much higher in our cohort (20.8%).
We believe that this unforeseen mortality rate should be interpreted in the broad context of the pandemic crisis experienced in Brazil [26–28]. We recently reported an overall mortality rate of 7.7% (1661/21591) for the general pediatric population with COVID-19 in Brazil. Interestingly, a recent study by Nachega et al. [29] corroborated our general findings on the mortality rate in children in developing countries. The authors reported an overall mortality rate of 8.3% (39/469) in pediatric patients with COVID-19 hospitalized in six sub-Saharan African countries. Taken together, the COVID-19 results from low-income regions exhibited a marked difference from data from developed countries that reported an overall mortality rate of 1% or even less in hospitalized pediatric patients [1]. We believe that this disparity in mortality rates between developed and developing countries is largely driven by socioeconomic and environmental factors. Therefore, in this scenario, the high mortality observed in children with KD in our study was certainly associated, in part, with the profound social inequalities and healthcare disparities in Brazil.
In this context, it is important to highlight the significantly different mortality rates among the specific KDs evaluated in our cohort. The mortality rate ranged from no death for patients with CAKUT to about 30% for children with CKD. Interestingly, this finding may reinforce some of the aspects mentioned above. For example, children with CKD who generally require more advanced support had the most unfavorable outcome in our analysis. On the other hand, KTRs had a lower mortality rate (12%) compared to children with nephrotic syndrome (28%) and CKD (30.9%). However, 80% of KTRs are from the Southeast region, which is the most developed region in Brazil. This finding may be partially responsible for the lower mortality rate among patients with KTRs and again highlights the role of socioeconomic characteristics on outcomes in our cohort. Importantly, recent studies on children with nephrotic syndrome and SARS-CoV-2 infection have contributed to our understanding of the results in this scenario. For example, recently Morello et al. [30] published a systematic review of COVID-19 in 43 patients with idiopathic nephrotic syndrome, mostly from developed countries. The authors reported that the disease in this group is generally mild, rarely requiring respiratory support, and with favorable outcomes. On the other hand, in the Indian cohort reported by Krishnasamy et al. [13, 31], among 44 patients with nephrotic syndrome, 10 (22.7%) developed moderate-to-severe COVID-19 during hospitalization, seven (15%) required non-invasive or mechanical ventilation, three (6.8%) required vasopressor support, nine (20.4%) had AKI at presentation, and three later developed AKI. Two (4.5%) patients, both with steroid-resistant nephrotic syndrome who presented during a relapse, succumbed to respiratory failure and shock. In our cohort, there were 25 children with nephrotic syndrome, of which seven had a COVID-19-related death. Again, the lack of detailed clinical data in our epidemiological dataset precludes a more robust comparative analysis. Nevertheless, taken together, these data highlight the need for further studies in low- and middle-income regions to provide us with a comprehensive picture of the prognosis of SARS-CoV-2 infections in children with KD.
We assessed the risk factors of death among pediatric patients with KD by competing-risk survival analysis. In the COVID-19 setting, because patients may recover, die, or are still in an ongoing clinical situation, survival data analysis faces a competing-risk issue [32]. After adjustment by the competing-risk survival analysis, patients from the poorest Brazilian geographic macro-regions (North and Northeast), those with low oxygen saturation at admission, and the presence and number of other associated comorbidities beyond KD exhibited a higher hazard of death. Regarding the regions of the country, Brazil is geopolitically divided into five macro-regions: North, Northeast, Central-West, Southeast, and South. These macro-regions have historical differences in social, economic, and health system capacity and coverage. For instance, recently, Rocha et al. [15] reported, in a comprehensive analysis of the health system in Brazil, that among other social vulnerabilities, the figures of public ICU beds were significantly smaller in North and Northeast regions. It is important to highlight that, in our analysis, the North region exhibited the highest risk of death in the multivariate analysis of survival, even greater than any clinical characteristic. Interestingly, when analyzed separately, the impact of living in the North region was observed only for the cohort of pediatric patients with KD. This finding certainly reflects the huge disparities in health care in Brazil, affecting a vulnerable population that much more frequently needs access to specialized treatment like children with CKD. Of particular interest, in a series of important studies, Koch-Nogueira and colleagues [33–35] highlighted the inequalities in access to adequate treatment for pediatric CKD in Brazil, especially regional disparities with the North and Northeast as the most vulnerable regions. Another covariate independently associated with the main outcome was the presence of comorbidities. Pre-existing medical conditions were strongly related with the prognosis of COVID-19 in the general population of all age groups [36]. However, few studies have evaluated the role of comorbidities in the context of KD and COVID-19. Nevertheless, data from adult KTRs suggest that severity of COVID-19 in this group is mainly related to the associated comorbidities and not to chronic immunosuppression [37, 38].
The strength of this study is the size of the cohort, allowing the analysis of clinical characteristics, risk factors, and outcomes of hospitalized children and adolescents with KD and laboratory-confirmed COVID-19. On the other hand, several limitations must be acknowledged in the current study. First, we should point out that our sample is comprised only of hospitalized patients with certainly a more severe spectrum of the disease. The major limitation of our study is clearly the absence of the fundamental information regarding the specific KD for about half of the cases registered in the dataset. In this sense, we were unable to make a more robust analysis and therefore this fact prevented us from assessing whether this increased risk of a severe course of COVID-19 differs among the heterogeneous group of diseases. Moreover, due to the nature of the database, we were unable to assess some relevant information regarding treatment and management of these patients. Indeed, we had no access to hospital record data to include laboratory results or detailed clinical course of the patients. Therefore, we were not able to include some important issues in the analysis, such as data regarding the use of immunosuppressants or other treatments. Missing data is another inherent issue due to the nature of a registry based on point-of-care case report forms. In this regard, the covariable “ethnicity” had overall 20% of missing information. To try to overcome the limitations of missing variables, we used a multiple imputation technique for relevant predictors.
In conclusion, in this analysis of a large nationwide database of hospitalized patients with laboratory-confirmed COVID-19, we found that children and adolescents with KD had more severe spectrum of the disease and higher risk of death than patients without KD. The higher hazard of death was associated with living in a socioeconomically disadvantaged region, low oxygen saturation at admission, and other associated comorbidities. Our findings support the need of specific preventive measures for pediatric patients with KD, considering the high risk for severe COVID-19 and outcomes.
Role of the funding source
The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We are profoundly grateful and in debt to all frontline healthcare workers for their impressive efforts to tackle the COVID-19 pandemic in Brazil. All data from the SIVEP-Gripe (Influenza Epidemiological Surveillance Information System) were systematically collected in challenging circumstances by these frontline health-care workers.
Funding
This study was supported by the CNPq (National Council for Scientific and Technological Development) and FAPEMIG (Research Support Foundation of Minas Gerais).
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Swann OV, Holden KA, Turtle L, Pollock L, Fairfield CJ, Drake TM, Seth S, Egan C, Hardwick HE, Halpin S, Girvan M, Donohue C, Pritchard M, Patel LB, Ladhani S, Sigfrid L, Sinha IP, Olliaro PL, Nguyen-Van-Tam JS, Horby PW, Merson L, Carson G, Dunning J, Openshaw PJM, Baillie JK, Harrison EM, Docherty AB, Semple MG. Clinical characteristics of children and young people admitted to hospital with covid-19 in United Kingdom: prospective multicentre observational cohort study. BMJ. 2020;370:m3249. doi: 10.1136/bmj.m3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dufort EM, Koumans EH, Chow EJ, Rosenthal EM, Muse A, Rowlands J, Barranco MA, Maxted AM, Rosenberg ES, Easton D, Udo T, Kumar J, Pulver W, Smith L, Hutton B, Blog D, Zucker H. Multisystem inflammatory syndrome in children in New York State. N Engl J Med. 2020;383:347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeBiasi RL, Song X, Delaney M, Bell M, Smith K, Pershad J, Ansusinha E, Hahn A, Hamdy R, Harik N, Hanisch B, Jantausch B, Koay A, Steinhorn R, Newman K, Wessel D. Severe Coronavirus disease-2019 in children and young adults in the Washington, DC. Metropolitan Region J Pediatr. 2020;223(199–203):e191. doi: 10.1016/j.jpeds.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gotzinger F, Santiago-Garcia B, Noguera-Julian A, Lanaspa M, Lancella L, Calo Carducci FI, Gabrovska N, Velizarova S, Prunk P, Osterman V, Krivec U, Lo Vecchio A, Shingadia D, Soriano-Arandes A, Melendo S, Lanari M, Pierantoni L, Wagner N, L'Huillier AG, Heininger U, Ritz N, Bandi S, Krajcar N, Roglic S, Santos M, Christiaens C, Creuven M, Buonsenso D, Welch SB, Bogyi M, Brinkmann F, Tebruegge M. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. 2020;4:653–661. doi: 10.1016/S2352-4642(20)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shekerdemian LS, Mahmood NR, Wolfe KK, Riggs BJ, Ross CE, McKiernan CA, Heidemann SM, Kleinman LC, Sen AI, Hall MW, Priestley MA, McGuire JK, Boukas K, Sharron MP, Burns JP. Characteristics and outcomes of children with coronavirus disease 2019 (covid-19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatr. 2020;174:868–873. doi: 10.1001/jamapediatrics.2020.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oliveira EA, Colosimo EA, Simoes ESAC, Mak RH, Martelli DB, Silva LR, Martelli-Junior H, Oliveira MCL. Clinical characteristics and risk factors for death among hospitalised children and adolescents with COVID-19 in Brazil: an analysis of a nationwide database. Lancet Child Adolesc Health. 2021;5:559–568. doi: 10.1016/S2352-4642(21)00134-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akalin E, Azzi Y, Bartash R, Seethamraju H, Parides M, Hemmige V, Ross M, Forest S, Goldstein YD, Ajaimy M, Liriano-Ward L, Pynadath C, Loarte-Campos P, Nandigam PB, Graham J, Le M, Rocca J, Kinkhabwala M. Covid-19 and kidney transplantation. N Engl J Med. 2020;382:2475–2477. doi: 10.1056/NEJMc2011117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charnaya O, Chiang TP, Wang R, Motter JD, Boyarsky BJ, King EA, Werbel WA, Durand CM, Avery RK, Segev DL, Massie AB, Garonzik-Wang JM. Effects of COVID-19 pandemic on pediatric kidney transplant in the United States. Pediatr Nephrol. 2021;36:143–151. doi: 10.1007/s00467-020-04764-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung EYM, Palmer SC, Natale P, Krishnan A, Cooper TE, Saglimbene VM, Ruospo M, Au E, Jayanti S, Liang A, Jie Deng DJ, Chui J, Higgins GY, Tong A, Wong G, Teixeira-Pinto A, Hodson EM, Craig JC, Strippoli GFM. Incidence and outcomes of COVID-19 in people with CKD: a systematic review and meta-analysis. Am J Kidney Dis. 2021;78:804–815. doi: 10.1053/j.ajkd.2021.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elias M, Pievani D, Randoux C, Louis K, Denis B, Delion A, Le Goff O, Antoine C, Greze C, Pillebout E, Abboud I, Glotz D, Daugas E, Lefaucheur C. COVID-19 infection in kidney transplant recipients: disease incidence and clinical outcomes. J Am Soc Nephrol. 2020;31:2413–2423. doi: 10.1681/ASN.2020050639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melgosa M, Madrid A, Alvárez O, Lumbreras J, Nieto F, Parada E, Perez-Beltrán V (2020) Spanish Pediatric Nephrology Association SARS-CoV-2 infection in Spanish children with chronic kidney pathologies. Pediatr Nephrol 35:1521–1524 [DOI] [PMC free article] [PubMed]
- 12.Plumb L, Benoy-Deeney F, Casula A, Braddon FEM, Tse Y, Inward C, Marks S, Steenkamp R, Medcalf J, Nitsch D. COVID-19 in children with chronic kidney disease: findings from the UK renal registry. Arch Dis Child. 2021;106:e16. doi: 10.1136/archdischild-2020-319903. [DOI] [PubMed] [Google Scholar]
- 13.Krishnasamy S, Mantan M, Mishra K, Kapoor K, Brijwal M, Kumar M, Sharma S, Swarnim S, Gaind R, Khandelwal P, Hari P, Sinha A, Bagga A. SARS-CoV-2 infection in children with chronic kidney disease. Pediatr Nephrol. 2021 doi: 10.1007/s00467-021-05218-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oliveira EA, Simoes ESAC, Oliveira MCL, Colosimo EA, Mak RH, Vasconcelos MA, Miranda DM, Martelli DB, Silva LR, Pinhati CC, Martelli-Junior H. Comparison of the first and second waves of the COVID-19 pandemic in children and adolescents in a middle-income country: Clinical impact associated with SARS-CoV-2 gamma lineage. J Pediatr. 2022 doi: 10.1016/j.jpeds.2022.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faria NR, Mellan TA, Whittaker C, Claro IM, Candido DDS, Mishra S, Crispim MAE, Sales FCS, Hawryluk I, McCrone JT, Hulswit RJG, Franco LAM, Ramundo MS, de Jesus JG, Andrade PS, Coletti TM, Ferreira GM, Silva CAM, Manuli ER, Pereira RHM, Peixoto PS, Kraemer MUG, Gaburo N, Jr, Camilo CDC, Hoeltgebaum H, Souza WM, Rocha EC, de Souza LM, de Pinho MC, Araujo LJT, Malta FSV, de Lima AB, Silva JDP, Zauli DAG, Ferreira ACS, Schnekenberg RP, Laydon DJ, Walker PGT, Schluter HM, Dos Santos ALP, Vidal MS, Del Caro VS, Filho RMF, Dos Santos HM, Aguiar RS, Proenca-Modena JL, Nelson B, Hay JA, Monod M, Miscouridou X, Coupland H, Sonabend R, Vollmer M, Gandy A, Prete CA, Jr, Nascimento VH, Suchard MA, Bowden TA, Pond SLK, Wu CH, Ratmann O, Ferguson NM, Dye C, Loman NJ, Lemey P, Rambaut A, Fraiji NA, Carvalho M, Pybus OG, Flaxman S, Bhatt S, Sabino EC. Genomics and epidemiology of the P.1 SARS-CoV-2 lineage in Manaus. Brazil Science. 2021;372:815–821. doi: 10.1126/science.abh2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bastos LS, Ranzani OT, Souza TML, Hamacher S, Bozza FA. COVID-19 hospital admissions: Brazil's first and second waves compared. Lancet Respir Med. 2021;9:e82–e83. doi: 10.1016/S2213-2600(21)00287-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janssen KJ, Vergouwe Y, Donders AR, Harrell FE, Jr, Chen Q, Grobbee DE, Moons KG. Dealing with missing predictor values when applying clinical prediction models. Clin Chem. 2009;55:994–1001. doi: 10.1373/clinchem.2008.115345. [DOI] [PubMed] [Google Scholar]
- 18.Schafer JL. Multiple imputation: a primer. Stat Methods Med Res. 1999;8:3–15. doi: 10.1177/096228029900800102. [DOI] [PubMed] [Google Scholar]
- 19.Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26:2389–2430. doi: 10.1002/sim.2712. [DOI] [PubMed] [Google Scholar]
- 20.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP (2007) STROBE Initiative the strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 370:1453–1457 [DOI] [PubMed]
- 21.Marlais M, Wlodkowski T, Al-Akash S, Ananin P, Bandi VK, Baudouin V, Boyer O, Vasquez L, Govindan S, Hooman N, Ijaz I, Loza R, Melgosa M, Pande N, Pape L, Saha A, Samsonov D, Schreuder MF, Sharma J, Siddiqui S, Sinha R, Stewart H, Tasic V, Tonshoff B, Twombley K, Upadhyay K, Vivarelli M, Weaver DJ, Woroniecki R, Schaefer F, Tullus K. COVID-19 in children treated with immunosuppressive medication for kidney diseases. Arch Dis Child. 2020;106:798–801. doi: 10.1136/archdischild-2020-320616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marlais M, Wlodkowski T, Vivarelli M, Pape L, Tonshoff B, Schaefer F, Tullus K. The severity of COVID-19 in children on immunosuppressive medication. Lancet Child Adolesc Health. 2020;4:e17–e18. doi: 10.1016/S2352-4642(20)30145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sinha R, Marlais M, Sarkar S, Obukhova V, Lucchetti L, Vasudevan A, Chacon Jaimes DC, Weaver DJ, Jr, Stanczyk M, Lopez-Gonzalez M, Schaefer F, Tullus K. Impact of COVID-19 pandemic on use of rituximab among children with difficult nephrotic syndrome. Pediatr Res. 2021 doi: 10.1038/s41390-021-01744-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mastrangelo A, Morello W, Vidal E, Guzzo I, AnnicchiaricoPetruzzelli L, Benetti E, Materassi M, Giordano M, Pasini A, Corrado C, Puccio G, Chimenz R, Pecoraro C, Massella L, Peruzzi L, Montini G, COVID-19 Task Force of the Italian Society of Pediatric Nephrology; COVID-19 TASK FORCE of the Italian Society of Pediatric Nephrology Impact of COVID-19 Pandemic in Children with CKD or Immunosuppression. Clin J Am Soc Nephrol. 2021;16:449–451. doi: 10.2215/CJN.13120820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Angeletti A, Drovandi S, Sanguineri F, Santaniello M, Ferrando G, Forno R, Cipresso G, Caridi G, Riella LV, Cravedi P, Ghiggeri GM. COVID-19 in children with nephrotic syndrome on Anti-CD20 chronic immunosuppression. Clin J Am Soc Nephrol. 2020;15:1494–1495. doi: 10.2215/CJN.06400420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Editorial COVID-19 in Brazil: "So what?". Lancet. 2020;395:1461. doi: 10.1016/S0140-6736(20)31095-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hallal PC, Victora CG. Overcoming Brazil's monumental COVID-19 failure: an urgent call to action. Nat Med. 2021;27:933. doi: 10.1038/s41591-021-01353-2. [DOI] [PubMed] [Google Scholar]
- 28.Ranzani OT, Bastos LSL, Gelli JGM, Marchesi JF, Baiao F, Hamacher S, Bozza FA. Characterisation of the first 250 000 hospital admissions for COVID-19 in Brazil: a retrospective analysis of nationwide data. Lancet Respir Med. 2021;9:407–418. doi: 10.1016/S2213-2600(20)30560-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nachega JB, Sam-Agudu NA, Machekano RN, Rabie H, van der Zalm MM, Redfern A, Dramowski A, O'Connell N, Pipo MT, Tshilanda MB, Byamungu LN, Masekela R, Jeena PM, Pillay A, Gachuno OW, Kinuthia J, Ishoso DK, Amoako E, Agyare E, Agbeno EK, Martyn-Dickens C, Sylverken J, Enimil A, Jibril AM, Abdullahi AM, Amadi O, Umar UM, Sigwadhi LN, Hermans MP, Otokoye JO, Mbala-Kingebeni P, Muyembe-Tamfum JJ, Zumla A, Sewankambo NK, Aanyu HT, Musoke P, Suleman F, Adejumo P, Noormahomed EV, Deckelbaum RJ, Fowler MG, Tshilolo L, Smith G, Mills EJ, Umar LW, Siedner MJ, Kruger M, Rosenthal PJ, Mellors JW, Mofenson LM, African Forum for Research and Education in Health (AFREhealth) COVID-19 Research Collaboration on Children and Adolescents Assessment of Clinical Outcomes Among Children and Adolescents Hospitalized With COVID-19 in 6 Sub-Saharan African Countries. JAMA Pediatr. 2022 doi: 10.1001/jamapediatrics.2021.6436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morello W, Vianello FA, Proverbio E, Peruzzi L, Pasini A, Montini G. COVID-19 and idiopathic nephrotic syndrome in children: systematic review of the literature and recommendations from a highly affected area. Pediatr Nephrol. 2021 doi: 10.1007/s00467-021-05330-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krishnasamy S, Sinha A, Bagga A. SARS-CoV-2 infection in children with nephrotic syndrome. Pediatr Nephrol. 2022 doi: 10.1007/s00467-021-05399-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCaw ZR, Tian L, Vassy JL, Ritchie CS, Lee CC, Kim DH, Wei LJ. How to Quantify and Interpret Treatment Effects in Comparative Clinical Studies of COVID-19. Ann Intern Med. 2020;173:632–637. doi: 10.7326/M20-4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Konstantyner T, Sesso R, de Camargo MF, de Santis FL, Koch-Nogueira PC (2015) Pediatric chronic dialysis in Brazil: Epidemiology and regional inequalities. PLoS One 10:e0135649 [DOI] [PMC free article] [PubMed]
- 34.Nogueira PC, de Carvalho MF, de Santis FL, Konstantyner T, Sesso R. Inequality in pediatric kidney transplantation in Brazil. Pediatr Nephrol. 2016;31:501–507. doi: 10.1007/s00467-015-3226-z. [DOI] [PubMed] [Google Scholar]
- 35.de Paduapaz I, Konstantyner T, de Castro Cintrasesso R, de Xavier Pinto CC, de Camargo MFC, Nogueira PCK. Access to treatment for chronic kidney disease by children and adolescents in Brazil. Pediatr Nephrol. 2021;36:2827–2835. doi: 10.1007/s00467-021-05009-8. [DOI] [PubMed] [Google Scholar]
- 36.Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, Curtis HJ, Mehrkar A, Evans D, Inglesby P, Cockburn J, McDonald HI, MacKenna B, Tomlinson L, Douglas IJ, Rentsch CT, Mathur R, Wong AYS, Grieve R, Harrison D, Forbes H, Schultze A, Croker R, Parry J, Hester F, Harper S, Perera R, Evans SJW, Smeeth L, Goldacre B. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caillard S, Chavarot N, Francois H, Matignon M, Greze C, Kamar N, Gatault P, Thaunat O, Legris T, Frimat L, Westeel PF, Goutaudier V, Jdidou M, Snanoudj R, Colosio C, Sicard A, Bertrand D, Mousson C, Bamoulid J, Masset C, Thierry A, Couzi L, Chemouny JM, Duveau A, Moal V, Blancho G, Grimbert P, Durrbach A, Moulin B, Anglicheau D, Ruch Y, Kaeuffer C, Benotmane I, Solis M, LeMeur Y, Hazzan M, Danion F. Is COVID-19 infection more severe in kidney transplant recipients? Am J Transplant. 2021;21:1295–1303. doi: 10.1111/ajt.16424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chavarot N, Gueguen J, Bonnet G, Jdidou M, Trimaille A, Burger C, Amrouche L, Weizman O, Pommier T, Aubert O, Celier J, Sberro-Soussan R, Geneste L, Panagides V, Delahousse M, Marsou W, Aguilar C, Deney A, Zuber J, Fauvel C, Legendre C, Mika D, Pezel T, Anglicheau D, Sutter W, Zaidan M, Snanoudj R, Cohen A, Scemla A. COVID-19 severity in kidney transplant recipients is similar to nontransplant patients with similar comorbidities. Am J Transplant. 2021;21:1285–1294. doi: 10.1111/ajt.16416. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.