Abstract
Cell division cycle 20 homologue (CDC20) is a well-known regulator of cell cycle, as it controls the correct segregation of chromosomes during mitosis. Many studies have focused on the biological role of CDC20 in cancer development, as alterations of its functionality have been linked to genomic instability and evidence demonstrated that high CDC20 expression levels are associated with poor overall survival in solid cancers. More recently, novel CDC20 functions have been demonstrated or suggested, including the regulation of apoptosis and stemness properties and a correlation with immune cell infiltration. Here, we here summarize and discuss the role of CDC20 inside and outside mitosis, starting from its network of interacting proteins. In the last years, CDC20 has also attracted more interest in the blood cancer field, being overexpressed and showing an association with prognosis both in myeloid and lymphoid malignancies. Preclinical findings showed that selective CDC20 and APC/CCDC20/APC/CCDH1 inhibitors, namely Apcin and proTAME, are effective against lymphoma and multiple myeloma cells, resulting in mitotic arrest and apoptosis and synergizing with clinically-relevant drugs. The evidence and hypothesis presented in this review provide the input for further biological and chemical studies aiming to dissect novel potential CDC20 roles and targeting strategies in hematological malignancies.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13046-022-02363-9.
Keywords: CDC20, Mitotic checkpoint, Apcin, Hematological malignancies
Background
Cancer hallmarks attempt to organize the complexity of tumor biology into major features. To date, cell growth, signaling regulation, apoptosis evasion, uncontrolled replication, neo-angiogenesis, tissue invasion and metastasis are the most known and investigated mechanisms [1]. An increasing number of studies highlighted the central role of cellular replication, DNA repair, apoptosis and senescence regulation in cancer biology [2]. In eukaryotic cells, cell replication is finely tuned through sequential checkpoint steps that ensure the proper duplication and distribution of the genetic material in daughter cells. Indeed, specialized intracellular pathways control DNA replication, chromosomes condensation and twin chromatids segregation in dividing cells [3, 4] and their dysfunctions can lead to an incomplete or damaged genome, generating cells with a putative oncogenic potential. The relationship between cancer development and deregulation of cellular replication has been well established [5] and is supported by the growing list of genetic or transcriptional alterations affecting key components of the cell cycle regulation machinery in malignant cells [6]. Moreover, cell cycle proteins represent a new class of potential therapeutic targets [7, 8].
Many studies focused on the mechanisms underlying the control of the correct separation of twin chromatids during the final step of cell division. It has been shown that the cell division cycle 20 (CDC20) plays a crucial role in the final steps of mitosis. Recently, there is different evidence which suggest that CDC20 contributes to a number of cellular processes that have been partially explored. An overexpression and/or oncogenic role of CDC20 has been already described in a variety of human solid tumors [9, 10] including pancreas [11, 12], breast [13, 14], lung [15, 16], prostate [17–19], gastric, colorectal [16, 18], hepatocellular [20, 21], kidney [22], ovarian cancer [23, 24], osteosarcoma [25–27] and glioblastoma [28, 29]. Moreover, CDC20 expression level has been reported as a putative marker of clinical outcome in many cancer types, being associated with advanced stage, high grade and poor prognosis [9, 10, 30, 31].
The following sections summarize CDC20 functions, including mitosis-related and unrelated ones, and the available data sustaining the oncogenic impact of CDC20 in hematological malignancies in order to evaluate its potential role as prognostic factor and therapeutic target in these neoplasms.
Main text
CDC20 role and interactors during the cell cycle
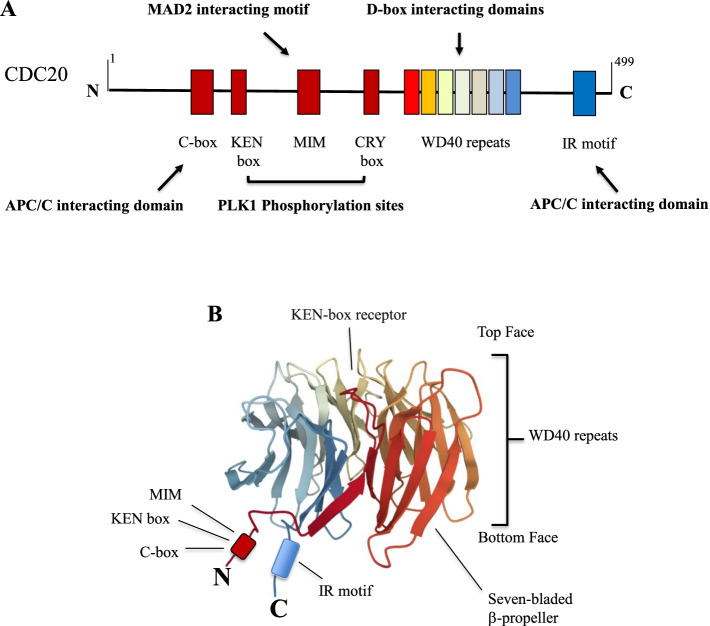
The CDC20 gene localizes on the short arm of chromosome 1 and encodes for a 499-amino acid and a 51 kDa protein (Human Protein Atlas proteinatlas.org). CDC20 is composed by two main segments: the N-terminal region characterized by low structural complexity and the C-terminal region containing the WD40 repeats [32]. The N-terminal regions contain different functional structures such as the C-box, KEN-box and CRY-box motifs. The KEN-box and CRY-box represent two independent degradation signals (degrons) and both are crucial binding sites for APC/C. In particular, the CRY-box includes the residue S170, which is phosphorylated by polo-like kinase-1 (PLK1), leading to the timely ubiquitination and destruction of CDC20 [33]. The C-terminal region contains, as reported above, the WD40 repeats and the Ile-Arg (IR) motif. The WD40 repeats define a seven-bladed β-propeller in which two highly conserved surfaces responsible for APC/C degron recognition can be identified: The KEN-box receptor (on the top side) and the D-box co-receptor lying in a channel between blades 1 and 7 of WD40 domain [34]. The KEN-box receptor is crucial for CDC20 regulation. Indeed, two CDC20 regulators, MAD2 and MAD3/BUBR1 interact with CDC20 through different KEN motifs. Additional regulatory regions fall in the amino- and carboxy-terminal extensions and include the C-box, crucial for CDC20’s co-activator function, the MAD2-intercating motif (MIM) and the IR tail, whose function is to bind CDC20 to APC/C. (Fig. 1A-C). Several phosphorylation sites (S41, S42, S72, S92, S153, T157, and S161) relevant for CDC20 functionality have been identified [35]. Mutations of these residues impair checkpoint arrest in mitosis, presumably due to the loss of BUB1-mediated phosphorylation [36].
Fig. 1.
CDC20 domains and motifs. A Structure of human CDC20 with its C-box, KEN box, MAD2-interacting motif (MIM), CRY box, seven WD40 repeats and IR motif. B 3D structure of CDC20 (https://www.rcsb.org/)
CDC20 was discovered in 1970 by Hartwell’s group for its role in initiating anaphase and chromosome segregation in yeast models [37]. The key role of CDC20 in mitotic progression has been subsequently demonstrated in mouse models, in which the CDC20 loss determined embryonic lethality due to prolonged metaphase arrest caused by securin stabilization [38–41], and also in human embryos [42].
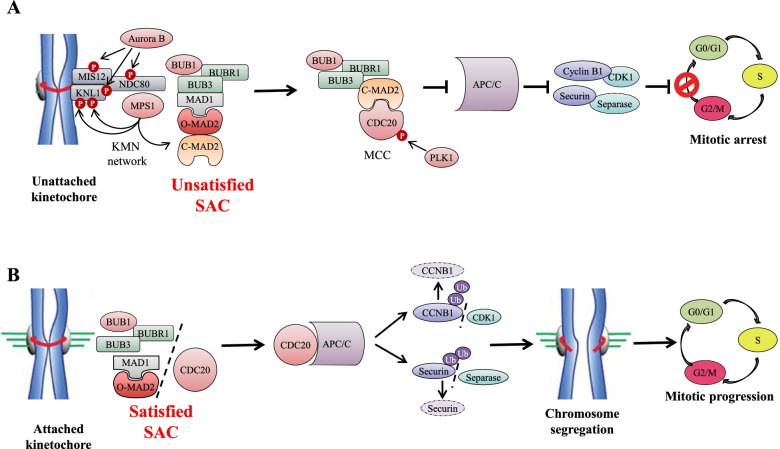
During metaphase, CDC20 complexes with MAD1, MAD2, BUBR1, BUB1, and BUB3, to generate the mitotic checkpoint complex (MCC), a crucial effector of the spindle assembly checkpoint (SAC) [43]. The SAC is a multi-protein complex regulating microtubule attachment to each kinetochore during mitosis, in order to avoid the generation of cells with incomplete or altered genomes [44]. Physiologically, the SAC arrests the transition from metaphase to anaphase in the presence of unattached kinetochores, preventing the activation of APC/C [45]. When the kinetochore lacks spindle fibers attachment (SAC complex “unsatisfied”), Aurora B kinase phosphorylates different kinetochore substrates [46, 47] contributing to the recruitment of MPS1 kinase by the KMN network (composed by the 2-subunit KNL1 complex, the 4-subunit MIS12 complex and the 4-subunit NDC80 complex) [48–50]. In turn, MPS1 phosphorylates the kinetochore KNL1 complex at multiple sites [51–53] (Fig. 2A), promoting the localization of the MCC complex on the surface of kinetochores [54]. In detail, phosphorylated KNL1 recruits the BUB3-BUB1 protein complex [55, 56], which is also phosphorylated by MPS1, enabling the interaction with the heterotetrameric MAD1-MAD2 complex [57]. This mechanism promotes the conversion of inactive cytosolic MAD2 into its active conformation [58], a process supported by MPS1 activity [59, 60]. In the nucleus, active MAD2 forms heterodimers with its inactive forms, in order to recruit them to the kinetochore [58]. Lastly, BUB1/BUB3 and MAD2 provide docking sites for BUBR1 and CDC20, which are recruited to the newly formed MCC [61] through spatially and temporally coordinated conformational changes [62]. PLK1 cooperates with the process by phosphorylating CDC20 and keeping it associated with the MCC until all the kinetochores are properly attached to the mitotic spindle fibers (SAC complex “satisfied”, Fig. 2B) [63, 64]. CDC20 is then released from the MCC and binds the APC/C complex. APC/C is a multi-protein complex with a E3-ubiquitin ligase activity that promotes the ubiquitination and proteasomal degradation of several target proteins required for mitotic exit [65, 66], recognized by a D-Box domain [67, 68], a TEK [69] or ABBA motif [61]. APC/C activation and substrate specificity are regulated by the availability of two cofactors, CDC20 and CDH1 [70]. The association and the activity of the APC/CCDC20 complex is finely regulated. In addition to CDK1 and other mitotic kinases [71, 72], Tank Binding Kinase 1 (TBK1) [73], CCNB3 [74], Apc1 loop domain (Apc1-loop500) [75] and Hematopoietic PBX-interacting protein (HPIP) [76] were also shown to be involved. APC/CCDC20 exerts its function during the metaphase to anaphase transition ensuring proper chromatids segregation [35] (Fig. 2B).
Fig. 2.
Schematic representation of SAC activity. A Unattached or misaligned kinetochores activate the KMN network, leading to recruitment of SAC proteins and conformational change of MAD2 (closed form, C-MAD2). This allows to the formation of the MCC, which sequestrate CDC20 leading to mitotic arrest. B Properly attached kinetochores satisfy the SAC, which allow the onset of anaphase through release of CDC20 from MAD2 (open, form, O-MAD2) the interaction between CDC20 and APC/C. APC/CCDC20 complex promotes Cyclin B and Securin ubiquitination and degradation, starting sister chromatid separation
The key role exerted by CDC20 during mitosis is further supported by the analysis of the CDC20 interactome. Overall, 817 physical interactions with 171 partners have been reported in humans by proteomic analyses, according to The Biological General Repository for Interaction Datasets (BioGRID, https://thebiogrid.org [77], Table 1). The list of CDC20 interactors is enriched for genes involved in different cell cycle phases, from G1/S to G2/M transition and from DNA damage response to anaphase-promoting complex-dependent catabolic process (Table S1, adjusted p < 0.001, KEGG 2021 and Gene Ontology Biological processes pathways 2021, https://amp.pharm.mssm.edu/Enrichr/ [78]). The expression levels of some interactors (BUB1, CCNA2, CCNB1, CDK1, MAD2L1, and PLK1) were positively correlated with CDC20 in cancer cells [10]. Moreover, the list of interactors included some APC/CCDC20 substrates, that are also critical cell cycle regulators, as Securin (PTTG1) [79], CCNB1 [80], CCNA1/2 [81, 82], NEK2 [83, 84], Zwint-1 [85] and p21 [86], indicating an APC/C-mediated role of CDC20 in cell cycle progression and chromosome segregation.
Table 1.
List of CDC20 interactors identified in human cells
| Protein name | |||
|---|---|---|---|
| ANAPC1 | CDR2 | HSPA8 | RASSF1 |
| ANAPC10 | CDT1 | HSPA9 | RAVER1 |
| ANAPC11 | CEP128 | HSPD1 | RNF34 |
| ANAPC13 | CHFR | HUWE1 | RNF4 |
| ANAPC15 | CILP | ID1 | RNH1 |
| ANAPC16 | CKS1B | IST1 | RPL23 |
| ANAPC2 | CKS2 | KDM5B | RPS3 |
| ANAPC4 | CLSPN | KIF18A | SIRT2 |
| ANAPC5 | COPS5 | KRT31 | SKP2 |
| ANAPC7 | CPVL | KRT32 | SPATC1 |
| APP | CREBBP | KRT33A | SPINT2 |
| AURKA | CUEDC2 | KRT85 | SPOP |
| AURKB | CUL1 | KRTAP19–5 | SSSCA1 |
| AXIN2 | CUL3 | KRTAP9–2 | STAU1 |
| BANP | DAXX | KRTAP9–3 | TAS2R13 |
| BTRC | DCPS | LNP1 | TCP1 |
| BUB1 | DSC1 | MAD1L1 | TFDP1 |
| BUB1B | E2F1 | MAD2L1 | TGFB1 |
| BUB3 | EGLN3 | MAD2L1BP | TK1 |
| CAPNS1 | EIF2A | MAD2L2 | TNIP2 |
| CASC5 | EP300 | MDC1 | TP63 |
| CCDC59 | FBXO31 | MOV10 | TP73 |
| CCNA1 | FBXO43 | MXI1 | TRIM33 |
| CCNA2 | FBXO5 | MYC | TRIP13 |
| CCNB1 | FBXW5 | NEK2 | TRRAP |
| CCNF | FGFR1 | NINL | TTK |
| CCT2 | FRY | NUP98 | TUBA1A |
| CCT3 | FZR1 | OTUD7B | TUBA1C |
| CCT4 | GM9174 | PARK2 | TUBA4A |
| CCT5 | GMNN | PAXIP1 | TUBB |
| CCT6A | HAUS1 | PBRM1 | TUBB2A |
| CCT7 | HDAC1 | PCDHAC2 | TUBB4B |
| CCT8 | HDAC2 | PCNA | TUBG1 |
| CDC16 | HDAC6 | PHF8 | UBB |
| CDC23 | HECW2 | PIM1 | UBC |
| CDC25A | HHEX | PLEKHA4 | UBE2C |
| CDC25B | HIF1AN | PLEKHO2 | UBE2S |
| CDC26 | HNRNPF | PLK1 | UBR5 |
| CDC27 | HOXD13 | PPP2CA | USP22 |
| CDC5L | HSF1 | PPP2CB | USP37 |
| CDC6 | HSF2 | PPP2R1A | XPO1 |
| CDK1 | HSPA1A | PPP2R5A | YAP1 |
| CDK2 | HSPA1L | PPP2R5E | YARS2 |
| CDKN1A | HSPA5 | PTTG1 | YBX1 |
CDC20 functions beyond chromosome segregation
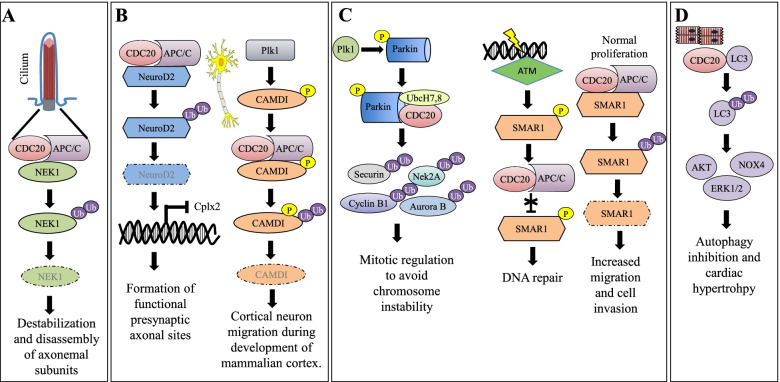
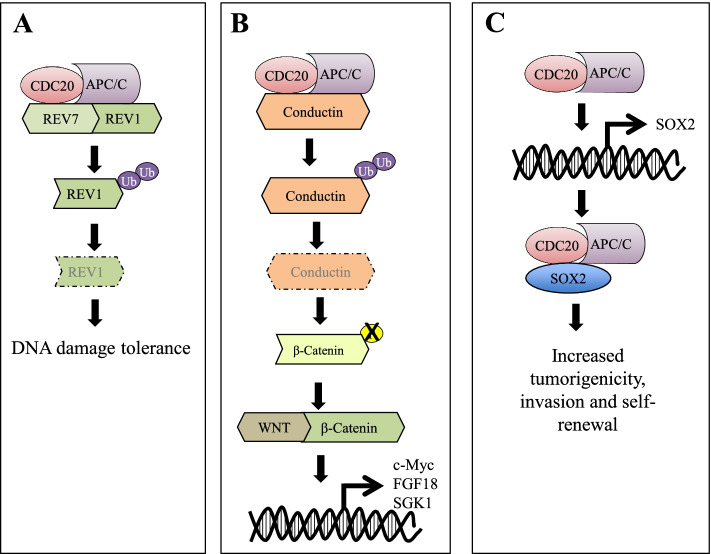
Pathway analysis of CDC20 interacting proteins suggested additional roles of CDC20 outside cell cycle regulation, with a significant enrichment of specific cellular processes, including protein modification, localization and degradation, telomeres regulation, transcription, and other signaling pathways, as Hippo, TGF-β, β-catenin, MAPK (Table S1). Accordingly, it has been recently shown that CDC20 exerts a pivotal role in different cell type-specific biological processes, as ciliary disassembly [87, 88], brain development [89, 90], necrosis suppression in neural stem cells under catastrophic cellular stresses [91], tissue homeostasis and cell fate in human keratinocytes [92, 93], genomic stability [94, 95], aging [96] and autophagy [97, 98] (Fig. 3A-D). Moreover, CDC20 has been associated with cellular processes relevant to tumorigenesis, including the regulation of DNA damage response, by controlling the stability of REV1, a protein involved in the DNA damage-tolerance mechanisms, responsible for the replication after DNA damage [99]. Accordingly, CDC20-knockdown promoted, in association with acidic culture environment, chromosomal instability in normal lung, colon and epithelial models, resulting in increased survival, metabolic reprogramming and the acquisition of an immortal cancer cell phenotype, characterized by suppression of autophagy and p53-induced apoptosis [98]. In addition, in colorectal cancer, the activation of Wnt/β-catenin signaling during G1 phase, is controlled by CDC20-mediated degradation of conduction [100] and a CDC20-APC/SOX2 axis regulates invasiveness and self-renewal of glioblastoma stem-like cells [101](Fig. 4A-C).
Fig. 3.
Schematic representation of the role of CDC20 in cell type-specific biological pathways. A Ciliary disassembly. B Brain development. C Genomic stability and DNA repair. D Autophagy regulation
Fig. 4.
Schematic representation of the role of CDC20 in tumor-associated pathways. A Regulation and tolerance of DNA damages. B Activation of Wnt/β-catenin signaling. C Induction of cell migration and self-renewal
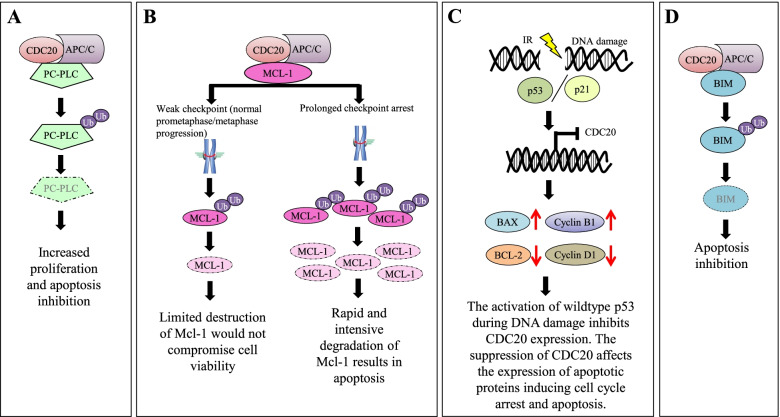
CDC20 has been also involved in the apoptotic response, by regulating phosphatidylcholine (PC) cycle [102, 103] and balancing the activity of anti-apoptotic factors, as MCL-1 [104–108] and BCL-2 [108, 109], and pro-apoptotic factors, as BIM [110] (Fig. 5A-D). Regarding PC cycle, CDC20 overexpression modulated the localization of phosphatidylcholine specific phospholipase C (PC-PLC), causing its degradation by the ubiquitin-proteasome pathway [103]. Chen and colleagues also described a role of CDC20-induced degradation of PC-PLC in inducing apoptosis in hepatocellular carcinoma models [102]. Focusing on pro-apoptotic factors, Wan et al. recently reported that BIM is physiologically reduced during mitosis, when APC/CCDC20 is active, and that CDC20 depletion allows a significant up-regulation of BIM, activating the DNA damage-induced apoptosis of cancer cells [110]. Regarding MCL-1 dynamics, APC/CCDC20 contributes to its degradation during mitosis, and MCL-1 expression levels help distinguish prolonged arrest from normal mitotic events [106, 107]. Sloss and colleagues also described the relationship between MCL-1 and CCNB1 levels, suggesting that MCL-1 competes with CCNB1 for APC/CCDC20 binding, thus affecting the rate of CCNB1 degradation and slowering mitotic slippage [105]. Moreover, in colorectal cancer models CDC20 regulated MCL-1 expression levels and its downregulation increased radiosensitivity and induced apoptosis, while BAK, BAX, PUMA, BCL-2, and BCL-xL levels were not affected by the silencing [108]. Conversely, in the HeLa cervix carcinoma model, CDC20 knockdown resulted in an increased phosphorylation of the anti-apoptotic BCL-2 and BCL-xL proteins and MCL-1 degradation, promoting CDK1 signaling activation and apoptosis [111]. The overexpression of BCL-2 or BCL-xL had a protective role on cell death mediated by CDC20 downregulation. In breast cancer models CDC20-depleted cells underwent mitotic arrest and were primed to die by apoptosis, which was, at least in part, dependent of BCL-xL phosphorylation on serine 62 residue [112]. Therefore, pharmacological or genetic Bcl-xL (but not BCL-2) silencing, during mitotic arrest was able to induce caspase and Bax-dependent apoptosis.
Fig. 5.
Schematic representation of the role of CDC20 in the regulation of apoptosis. A Regulating phosphatidylcholine (PC) cycle. B Regulation of MCL-1 activity. C Regulation of BCL2 and BAX expression induced by CDC20 following DNA damages. D Regulation of BIM activity
Recently, CDC20 expression has been also associated with immune infiltration in cancer. A positive correlation has been demonstrated with the infiltration of cancer-associated fibroblasts and myeloid-derived suppressor cells across several cancer types [10], with an immune risk score based on enrichment of 2 T helper cells, memory B cells and plasmacytoid dendritic cells in lower-grade glioma [113], with the infiltration of CD8+ T cells, monocytes [114, 115], exhausted T cells [114], CD4+ T cells, regulatory T cells, B lymphocytes, and natural killer cells [115] in hepatocellular carcinoma.
In summary, CDC20 is involved in a number of biological and tumor-related functions, that deserve further investigation in the field of hematological malignancies.
Role of CDC20 in hematological malignancies
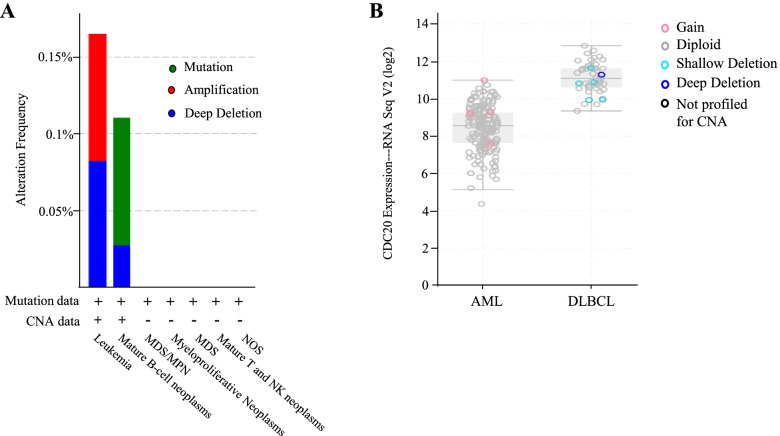
Similarly to other SAC genes, CDC20 is rarely mutated across cancers [9]. Across 6786 onco-hematological cases including pediatric and adult acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML), chronic lymphocytic leukemia (CLL), non-Hodgkin lymphomas, myelodysplastic syndrome (MDS) and myeloproliferative neoplasms (MPN), only 3 missense mutations (G284V, E413G and A128T) have been reported (Fig. 6A, https://www.cbioportal.org). All three mutations reported above have been identified the diffuse large B cell lymphoma (DLBCL) cohort. Moreover, copy number alterations also occur rarely and have been detected in AML and DLBCL, with a frequency lower than 0.2% (https://www.cbioportal.org). They include both amplifications and deletions, with concordant consequences on the gene expression levels in most cases (Fig. 6B). However, copy number losses are quite unexpected based on the pro-tumorigenic role of CDC20, thus suggesting that structural alterations of the gene can be secondary and passenger events with a minor effect on its transcriptional and functional regulation. Despite the low frequency of genetic alterations, CDC20 overexpression and functional deregulation are common events in hematological malignancies, as discussed in the following section (Table 2).
Fig. 6.
CDC20 alterations across hemato-oncological patients. A Frequency and distribution of CDC20 mutations and copy number alterations (CAN) in hematological malignancies. B Effect of CNA on CDC20 mRNA level in AML and DLBCL cases
Table 2.
Deregulated expression of CDC20 and its involvement as prognostic and therapeutic target in hematological malignancies
| Cancer type | Evidence in primary samples | Impact on prognosis | Pre-clinical studies | Ref. |
|---|---|---|---|---|
| ATL |
CDC20 over-expression in ATL samples compared with normal CD4+ T cells. Aberrant activation of APC/CCDC20 induced by Tax |
[116, 117] | ||
| AML | CDC20 over-expression in aneuploid and complex karyotype patients. | [118, 119] | ||
| CLL | CDC20 over-expression in aggressive subtypes (U-CLL and CD38+ CLL). | [120, 121] | ||
| CML | CDC20 stabilization induced by CDH1down-regulation in imatinib-resistance patients | [122] | ||
| DLBCL | CDC20 over-expression | Inferior OS | proTAME induces prolonged metaphase and caspase-dependent apoptosis. Combination of proTAME with Apcin, doxorubicin and venetoclax show synergic effects. | [123, 124] |
| MCL | CDC20 over-expression | Inferior OS | proTAME induces prolonged metaphase and caspase-dependent apoptosis. Combination of proTAME with Apcin, doxorubicin and venetoclax show synergic effects. | [124, 125] |
| MDS | CDC20 over-expression in high-risk patients | Shorter RFS and inferior OS | [126–128] | |
| MM | CDC20 over-expression in cell lines and high-risk patients | Inferior OS | proTAME treatment induced G2/M arrest and increased apoptosis. Combination with etoposide and doxorubicin, vincristine or melphalan potentiated proTAME effect. | [129–132] |
Lymphoid neoplasms
Acute lymphoblastic leukemia
Few data are available on the role of CDC20 in ALL pathogenesis and drug-induced changes of its expression. In the Jurkat T-ALL model, APC/CCDC20 was reported to recognize and bind the DEAD-box sequences on the RUNX1 transcription factor, thus inducing its proteosomal degradation, during G2/M-G1 transition [133]. Likewise, APC/CCDC20 is responsible for CKS1-mediated degradation of MLL [134], a lysine methyltransferase relevant to hematopoiesis, during the late M phase of the cell cycle, by targeting its N-terminal domain [135]. This assures a correct cell cycle execution, together with SCFSkp2 that exerts the same function in the S phase. The expression of the MLL-AF4, MLL-AF9, MLL-ENL and MLL-ELL fusion transcripts, that characterize ALL and/or AML, confers resistance to MLL degradation mediated by the cell cycle ubiquitin/proteasome system. Moreover, ALL cells exhibit synergic inhibition of proliferation and reduction of viability after combined treatment with arsenic trioxide (ATO) and paclitaxel (PTX) that act through the induction of mitotic arrest and activation of the spindle checkpoint [136]. In particular, ATO/PTX treatment increased the activity of CDK1 resulting in higher phosphorylation of BUBR1 and subsequent formation of the inhibitory checkpoint complex BUB1R/CDC20 that prevented the onset of anaphase.
Adult T cell leukemia/lymphoma
Adult T-cell leukemia/lymphoma (ATL) is a CD4+ T-cell malignancy caused by infection from human T-cell leukemia virus type 1 (HTLV-1). The progression from infection to malignant transformation has not been fully described, but it has been linked to the activation of the viral trans-activator/oncoprotein, Tax. Tax mediates activation of viral transcription and alters cellular mechanisms in a pleiotropic manner inducing NF-κB activation, cell cycle perturbation and cell transformation [137]. Liu and colleagues found that Tax activation perturbs mitotic entry and G2/M arrest in S. cerevisiae, rodent, and human cells leading impaired chromosome segregation and causing severe aneuploidy [138]. The study showed evidence that the mitotic defects caused by Tax are associated with a premature and drastic reduction in Securin and Cyclin B1 levels mediated by APC/CCDC20, supporting the idea that Tax promotes aberrant activation of APC/CCDC20 to avoid the block of mitotic exit and progression of aneuploid cells, highly represented in ATL [116]. Accordingly, CDC20 is a hub gene in the protein-protein interaction network of differentially expressed genes between ATL samples and normal CD4+ T cells [117].
Lymphomas
CDC20 was also reported as a hub protein among tumor-associated genes in DLBCL [123]. Indeed, in different studies it has been found that high CDC20 expression correlates with poor overall survival (OS) (P = 0.0058 [124]; P = 0.0247 [139]) and higher risk of death (hazard ratio, HR = 2.4 [124]) in DLBCL patients. Moreover, a superior sensitivity in prognosis prediction was obtained by combining the expression levels of CDC20 and PTGDS2, another hub gene that is downregulated in DLBCL [123]. Deregulated expression of CCNA, CCNB1 and CDC20 conferred to B-cell lymphoma cells the ability to aberrantly bypass the mitotic arrest, as demonstrated in the IgHμ-HOX11 transgenic mouse [140]. CDC20 expression was regulated by the MDM2-p53 pathway in DLBCL [139]. Indeed, MDM2 silencing restored p53 expression and reduced CDC20 protein level in DLBCL cell lines.
Recent studies showed that CDC20 is highly expressed not only in DLBCL but also in mantle cell lymphoma (MCL) [124, 125]. Functional enrichment analysis performed on gene expression data revealed that CDC20 is among the top five altered genes involved in the development of MCL and it is significantly associated with shorter OS (5-year OS around 10 and 60% in CDC20 high and low, respectively; P = 2.623e− 11 [125]).
Chronic lymphocytic leukemia
In CLL, high CDC20 expression has been reported in the high-risk category characterized by unmutated IGHV (U-CLL) [120]. Indeed, compared with IGHV mutated (M-CLL) cases, the more aggressive U-CLL subtype exhibited an increased expression of cell cycle genes, including ATF2, CCNB2, CDC20, CDC25A, CREB1, E2F4, ESR1, FOXM1, MKI67, MYC, POU2F2, RBL2, SP3, TYMS, UBE2C, VRK1. CDC20 was also associated with CD38 expression, another marker of disease aggressiveness [121]. Moreover, primary CD38+ B-CLL samples had an increased level of APC/C subunit 5 (APC/C 5), which controls some regulatory sub-functions of the APC/C complex [121]. As demonstrated in Drosophila models, APC/C 5 mediated the “wait” signal from the SAC and, in presence of mutations disrupting that signal, mitotic cells prematurely advanced through chromatid segregation and anaphase [141]. This evidence suggests that the overexpression of APC/C 5 observed in CD38+ CLL cells could represent an alternative strategy adopted to mimic the effects of CDC20 overexpression.
Multiple myeloma
The genome of multiple myeloma (MM) patients is highly unstable and is characterized by chromosome translocations and aneuploidy, affecting the disease outcome [142]. The high degree of aneuploidies suggested that MM cells exhibit a weakened SAC activity that allows them to tolerate gains or losses of a small number of chromosomes. Indeed, MM cell lines generally expressed lower levels of some SAC components (AURKC, PLK2, PLK3) compared to normal plasma cells and higher levels of others, including CDC20, and were able to bypass the SAC-mediated arrest when challenged with nocodazole [129]. High levels of CDC20 transcript were also confirmed in primary cells from high-risk MM patients [130], that also displayed elevated expression of BUB1B [131] together with reduced levels of CDH1 [143], that sustain MM cell proliferation. Indeed, CDC20 knockdown reduced the viability of MM cell lines, by inducing cell growth arrest and accumulation of the APC/CCDC20 substrate CCNB1 [132]. Moreover, high CDC20 expression was associated with inferior OS both per se (P = 1.08e-05 and P = 0.00619 in TT2-cohort and HM-cohort, respectively [130]) and in combination with BUB1B and CCNB levels (P < 0.05 [131]).
Myeloid neoplasms
Myelodysplastic syndrome
Emerging evidence shows that MDS patients harbor deregulated expression of several components of SAC machinery. In particular, MDS patients with hypercellular and normocellular bone marrow, reflecting a more aggressive disease, had higher expression of SAC components in comparison with those characterized by hypocellular bone marrow [126]. Moreover, high levels of CDC20 and MAD2 characterized MDS patients with severe thrombocytopenia and complex karyotypes [127]. Notably, the higher expression of both genes was associated with a significantly poorer OS in MDS patients (P = 0.013) [127].
Acute myeloid leukemia
More than 20% of AML patients have defects in chromosome segregation, also supported by an altered activity of the SAC components, caused by BUB1 deregulation, BUBR1 repression, or aberrant expression of MAD2 and CDC20 [9, 144, 145], that was further exacerbated by decitabine treatment in AML cell lines [144]. We have recently shown that CDC20 is upregulated both at transcript and protein level in aneuploid compared with euploid AML and a 3-gene signature including high CDC20 and PLK1 and low RAD50 expression was able to discriminate the aneuploid from euploid cases [118]. In addition, complex karyotype AML, which includes a number of aneuploid cases, was enriched for a G2/M checkpoint gene signature, including CDC20 [119]. CDC20 was reported to interact with proteins playing a crucial role in AML pathogenesis, including RUNX1 [146], MEIS1, p21 [147] and NUP98 [148]. CDC20 (and CDH1) can target RUNX1 to degradation by APC/C. Binding of CDC20 (but not CDH1) to RUNX1 was mediated by phosphorylation of the target at serine 276 and 303 residues [146]. Moreover, it has been demonstrated that CDC20-mediated ubiquitination of MEIS1 and p21 participates in the regulation of quiescence in hematopoietic stem cells and leukemia initiating cells [147]. In particular, MEIS1 and p21 degradation was hampered by PPM1K thorough induction of branched chain amino acid catabolism, which in turn resulted in reduced protein ubiquitination by CDC20 and enforced glycolysis and quiescence of AML cells. APC/CCDC20 also showed an aberrant interaction with NUP98 fusion oncoproteins [148], a rare pathogenic mechanism in AML that is, however, overrepresented in high-risk pediatric patients [149]. Wildtype NUP98 is a conditional target of APC/CCDC20 and the physical interaction is dependent on the phosphorylation of a PEST sequence within NUP98 C-terminal domain, which occurs prior to mitotic entry [150]. The peptidyl-prolyl isomerase PIN1 then induces NUP98 conformational changes driving its dissociation from APC/CCDC20 during mitosis. Conversely, Salsi et al. demonstrated that NUP98 fusion oncoproteins bind APC/CCDC20 during mitosis, through the NUP98 GLEBS-like domain in the absence of the RAE1 partner protein. This interaction led to BUBR1 displacement and consequent attenuation of the SAC [148], that could be restored by CDC20 or MAD2 overexpression [150].
Chronic myeloid leukemia
Tyrosine kinase inhibitors, which have changed the management of chronic myeloid leukemia (CML) patients during the last 10 years [151], control cell cycle and apoptosis through several mechanisms, including the regulation of CDH1 levels [122]. It has been shown that CDH1 expression is significantly lower in imatinib-resistant CML blast crisis patients compared with imatinib-sensitive ones and its downregulation induced stabilization of SKP2 and CDC20, resulting in increased proliferation and genomic instability, with the formation of multinucleated cells, suggesting a role of CDC20 in therapy resistance [122].
CDC20 targeting with specific inhibitors in hematological malignancies
Due to the potential oncogenic role of CDC20, different chemical compounds and inhibitors have been developed and tested for their efficacy as antineoplastic agents: tosyl-L-arginine methyl ester (TAME) and its pro-drug (pro-TAME), APC inhibitor (Apcin), Withaferin A, N-alkylated amino acid-derived (NAHA), Ganodermanontriol, Genistein, CARP-1 functional mimetic 4 (CFM-4) and 6-brominated coumarin hydrazide-hydrazone derivative (BCHHD), that have been extensively revised by Wang et al [30]. Among them, Apcin and pro-TAME have been identified as selective CDC20 and APC/CCDC20/APC/CCDH1 inhibitors [152], respectively, and are currently under preclinical investigation for their efficacy against different cancer subtypes, including hematological malignancies.
Apcin
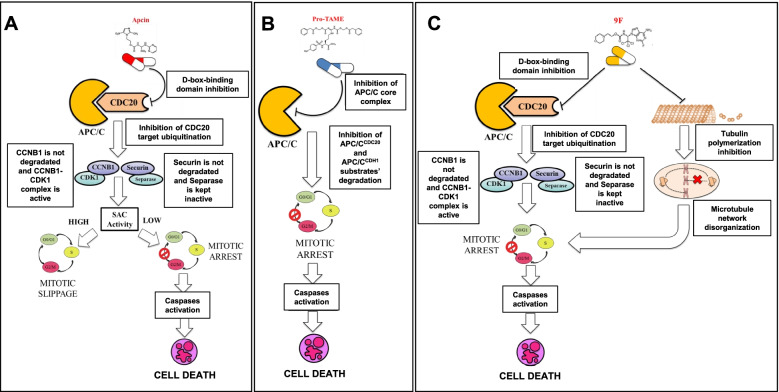
Apcin is a small molecule that binds in a competitive manner the D-box-binding domain of CDC20 thus preventing its substrate recognition capacity and inhibiting the ubiquitination of CDC20 targets [152] (Fig. 7A). Surprisingly, it has been recently observed that Apcin had a paradoxical effect on tumor cell lines: it induces mitotic arrest (which is the predicted effect of an APC/CCDC20 inhibitor) or mitotic slippage depending on the low or high SAC activity, respectively [153]. Regarding the mitotic slippage, Apicin interacts with the D-box-binding domain on CDC20 which is essential for both substrate ubiquitination and mitotic checkpoint complex-dependent APC/C inhibition through BUBR1 interaction. In the field of hematological malignancies, the efficacy of Apcin has been tested in DLBCL and MM models. In DLBCL, Apcin significantly reduced cell viability and proliferation and induced cell cycle arrest in G2/M phase and apoptosis of OCI-Ly3 and OCI-Ly10 lines [139]. In vivo models confirmed that Apcin treatment dampened CDC20 expression and inhibited the tumor growth in NOD/SCID mice bearing OCI-Ly10 xenografts [139]. Apcin also showed an activity in MM models, with minor effects as a single agent, but a higher efficacy in combination with pro-TAME, in terms of apoptosis induction [130].
Fig. 7.
Schematic representation of CDC20 inhibition strategies tested in hematological malignancies. A The small molecule Apcin prevents the substrate recognition capacity of APC/CCDC20 leading to the stabilization of CDC20 substrates that could result in mitotic arrest or mitotic slippage based on intracellular SAC activity. B Pro-Tame binds in a competitive manner the APC/C core complex preventing its association with CDC20 that results in mitotic arrest through stabilization of CCNB1. C The compound 9f, like Apcin, inhibits CDC20 downstream activity leading to mitotic arrest. It has also been shown that 9f inhibits tubulin polymerization and compromises microtubule network organization, causing cell cycle arrest and inducing apoptosis
Pro-TAME
pro-TAME is a small molecule that mimics the IR motif of CDC20 and CDH1 involved in their recruitment to the APC/C. It binds the APC/C core complex in a competitive manner and prevents its association with APC/C activators [154] (Fig. 7B), leading to the inhibition of APC/CCDC20 and APC/CCDH1 substrates’ degradation and mitotic arrest [155]. Pro-TAME has also been tested in combination with Apcin, showing a synergistic effect on the stabilization of CCNB1, securin, CCNA2 and NEK2A together with a significant increase of the mitotic fraction [152].
In MCL and DLBCL cellular models proTAME induced metaphase arrest, resulting in accumulation of the APC/CCDC20 substrate CCNB1, together with phosphorylation-mediated inactivation of the anti-apoptotic factors BCL-2 and BCL-xL, reduction of cell viability and activation of caspase-3 dependent apoptosis [124]. Pro-TAME efficacy was confirmed in primary cells from MCL and DBCL patients. In addition, proTAME strongly synergized with Apcin and clinically relevant drugs, including doxorubicin and venetoclax in lymphoma cellular models.
Consistently with the high CDC20 expression in MM, treatment of cell lines and primary cells with proTAME resulted in the stabilization of CCNB1 and cell cycle arrest at G2/M phase [130, 132]. Moreover, cells treated with proTAME showed cleavage of caspase 3, 8, 9 and PARP, and accumulation of the pro-apoptotic protein BIM, leading to apoptosis [130, 132]. Cell death induction in both MM cell lines and primary samples was further exacerbated by the combinations of proTAME with topoisomerase inhibitors, etoposide and doxorubicin, especially when proTAME treatment was preceding the administration of the other drugs and in association with the microtubule inhibitor vincristine or the chemotherapy agent melphalan [130].
Conclusion
In line with evidence from solid cancer, CDC20 overexpression also plays a critical oncogenic role in hematological malignancies. The available data gathered in this review revealed that the up-regulation of CDC20 is associated with inferior OS in different hematological malignances. In agreement with its potential role as prognostic marker, higher expression of CDC20 was observed in high-risk MM, CLL, MDS and AML patients. Moreover, over-expression of CDC20 has been reported as a biomarker of resistance to TKI therapy in CML patients. In line with these observations, studies performed in DLBCL, MCL and MM cells treated with Apcin or proTAME, or their combination, demonstrated that targeting CDC20 is a promising therapeutic strategy in hemato-oncology. Indeed, it has been shown that CDC20 inhibitors significantly potentiate the efficacy of conventional therapeutic agents in different hematological malignances. In addition, novel therapeutic combinations based on the synthetic lethality mechanisms could be explored. For example, Apcin effectiveness is enhanced in cells carrying defective sister chromatid cohesion, that also characterize a subgroup of AML patients [156], as shown by using cellular models of Warsaw breakage syndrome with defective function of the DNA helicase DDX11 [157]. In addition, the correlation between CDC20 expression and infiltration of immune cells, including those inducing tolerance, led us to hypothesize that targeting CDC20 may reinforce the immune response and also synergize with immunomodulatory drugs in patients expressing high CDC20 levels.
Further development towards clinical application of CDC20 inhibitors is hampered by the poor bioavailability of the compounds, because of the high dosage needed to achieve a therapeutic response. This evidence and the current knowledge provided the rationale for the development of other specific inhibitors. We have synthesized and tested novel tryptamine derivatives bearing aminopyrimidyl- or imidazolyl- moieties, which are also present in Apcin [158]. In particular, compound 9, characterized by 2-aminopyrimidyl- and trichloroethyl- moieties, similarly to those in Apcin, showed a preferential efficacy in hematology compared with solid tumor models, and significantly reduced the growth of AML and ALL cells. Moreover, Huang and colleagues synthesized a series of 2,2,2-trichloro-1-aryl carbamate derivatives starting from the modification of Apcin structure [159]. They identified two compounds, namely 7d and 9f showing a higher efficacy compared with Apcin in terms of mitotic arrest and apoptosis induction, which occurred through stabilization of CCNB1 and activation of caspase-3 and PARP, respectively. Interestingly, the most potent one, compound 9f, also played additional functions, as it inhibited cell migration, invasion and tubulin polymerization and it disorganized the microtubule network. Thus, the increased compound efficacy may be related to the dual activity, in line with a recent study reporting that inhibition of APC/CCDC20 enhances the sensitivity of cancer cells to microtubule interfering agents [160] (Fig. 7C).
Overall, this evidence proves a growing therapeutic interest in CDC20 targeting in hematological malignancies, which will promote novel studies towards the development of better combination strategies, the identification of patients’ cohorts that will mostly benefit of them and the definition of optimal therapeutic windows.
Supplementary Information
Acknowledgements
Not applicable.
Abbreviations
- ABL1
Abelson
- AML
Acute myeloid leukemia
- Apc1-loop500
Apc1 loop domain
- APC/C
Anaphase promoting complex/cyclosome
- ATF2
Activating Transcription Factor 2
- ATL
Adult T-cell leukemia/lymphoma
- Bcl-2
B-cell lymphoma 2
- Bcl-xL
B-cell lymphoma-extra large
- BCR
Breakpoint cluster region
- BIM
Bcl-2-like protein 11
- BM
Bone marrow
- BUB1
BUB1 Mitotic Checkpoint Serine/Threonine Kinase
- BUB3: BUB3
Mitotic Checkpoint Protein
- BUBR1/BUB1B
BUB1 Mitotic Checkpoint Serine/Threonine Kinase B
- CCNB2
Cyclin B2
- CDC20
Cell division cycle 20 homologue
- CDC25A
Cell division cycle 25 A
- CDH1
CDC20 homologue 1
- CDK1
Cyclin-dependent kinase 1
- CEMP-F
Centromere protein F
- CLL
Chronic lymphocytic leukemia
- CML
Chronic myeloid leukemia
- CREB1
CAMP Responsive Element Binding Protein 1
- DLBCL
Diffuse large B-cell lymphoma
- DNA
Deoxyribonucleic acid
- E2F4
E2F Transcription Factor 4
- EMI 1
Early mitotic inhibitor1
- ESR1
Estrogen Receptor 1
- FOXM1
Forkhead Box M1
- HTLV-1
Human T-cell leukemia virus type 1
- HSC
Hematopoietic stem cells
- IGHV
Immunoglobulin heavy chain variable region
- IPSS-R
International Prognostic Scoring System
- JUN
AP-1 Transcription Factor Subunit
- KMN
Knl1 complex, the Mis12 complex and the Ndc80 complex
- KNL1
Kinetochore Scaffold 1
- TKIs
Tyrosine kinase inhibitors
- MAD1/MAD1L1
MAD1 Mitotic Arrest Deficient Like 1
- MAD2/MAD2L1
Mitotic Arrest Deficient 2 Like 1
- MCC
Mitotic checkpoint complex
- MCL
Mantle cell lymphoma
- MCL-1
Myeloid cell leukemia 1
- M-CLL
Mutated-Chronic lymphocytic leukemia
- MDS
Myelodisplastic syndromes
- MIM
MAD2-interacting motif
- MKI67
Marker Of Proliferation Ki-67
- miR
micro RNA
- mRNA
messenger RNA
- MIS12
Kinetochore Complex Component
- MM
Multiple myeloma
- MPS1
TTK Protein Kinase
- MYC
Myelocytomatosis oncogene
- NEK2A
Never in Mitosis (NIMA) Related Kinase 2A
- NDC80
NDC80, Kinetochore Complex Component
- OS
Overall survival
- PARP
Poli ADP-ribosio polimerasi
- PB
Peripheral blood
- PC-PLC
Phosphatidylcholine specific phospholipase C
- PLK1
Polo Like Kinase 1
- POU2F2
POU Class 2 Homeobox 2
- p21
Cyclin-dependent kinase inhibitor 1
- RAD50
Double Strand Break Repair Protein
- RAF
Proto-Oncogene, Serine/Threonine Kinase
- RAS
Rat Sarcoma-oncogene
- RASSF1A
Ras association domain family 1 isoform A
- RBL2
RB Transcriptional Corepressor Like 2
- RFS
Recurrence-free survival
- SAC
Spindle assembly checkpoint
- SP3
Specificity Protein 3
- STAT
Signal Transducer And Activator Of Transcription
- Tax
Trans-activator/oncoprotein
- TBK1
Tank Binding Kinase 1
- TYMS
Thymidylate Synthetase
- TP53/p53
Tumor protein 53
- TRP
Tetratricopeptide repeat
- UBE2C
Ubiquitin Conjugating Enzyme E2 C
- U-CLL
Unmutated-Chronic lymphocytic leukemia
- USP44
Ubiquitin-specific protease 44
- VRK1
Vaccinia-related kinase 1
Authors’ contributions
S.B., A.G.L.D.R, R.N., S.S and G.S. drafted the first version of the manuscript and created the Figs. G.M. contributed to the clinical sections. S.B., A.G.L.D.R and G.S contributed to the final version of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by University of Bologna, Alma Idea Junior Research Grant (to G.S.), TrevisoAIL and by ERA-Per-Med (reference number: ERAPERMED2018–275).
Availability of data and materials
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
G.M. has competing interests with Menarini/Stemline Therapeutics, Pfizer, Astellas, Abbvie, Astrazeneca. G.Ma. has competing interests with Ariad/Incyte, Pfizer, Celgene/BMS, Amgen, Roche, AbbVie, GlaxoSmithKline, Astellas, Daiichi Sankyo, Takeda, Gilead, Astellas, Janssen, Novartis, MSD.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hanahan D. Hallmarks of Cancer: new dimensions. Cancer Discov. 2022;12:31–46. doi: 10.1158/2159-8290.CD-21-1059. [DOI] [PubMed] [Google Scholar]
- 2.Fouad YA, Aanei C. Revisiting the hallmarks of cancer. Am J Cancer Res. 2017;7:1016–1036. [PMC free article] [PubMed] [Google Scholar]
- 3.Rhind N, Russell P. Signaling pathways that regulate cell division. Cold Spring Harb Perspect Biol. 2012;4:a005942. doi: 10.1101/cshperspect.a005942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duronio RJ, Xiong Y. Signaling pathways that control cell proliferation. Cold Spring Harb Perspect Biol. 2013;5:a008904. doi: 10.1101/cshperspect.a008904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbacid M, Ortega S, Sotillo R, Odajima J, Martín A, Santamaría D, et al. Cell cycle and cancer: genetic analysis of the role of cyclin-dependent kinases. Cold Spring Harb Symp Quant Biol. 2005;70:233–240. doi: 10.1101/sqb.2005.70.005. [DOI] [PubMed] [Google Scholar]
- 6.Helsten T, Kato S, Schwaederle M, Tomson BN, Buys TPH, Elkin SK, et al. Cell-cycle gene alterations in 4,864 tumors analyzed by next-generation sequencing: implications for targeted therapeutics. Mol Cancer Ther. 2016;15:1682–1690. doi: 10.1158/1535-7163.MCT-16-0071. [DOI] [PubMed] [Google Scholar]
- 7.Otto T, Sicinski P. Cell cycle proteins as promising targets in cancer therapy. Nat Rev Cancer. 2017(2):93–115. [DOI] [PMC free article] [PubMed]
- 8.Ghelli Luserna Di Rorà A, Martinelli G, Simonetti G. The balance between mitotic death and mitotic slippage in acute leukemia: A new therapeutic window? J Hematol Oncol. 2019;12(1):123. doi: 10.1186/s13045-019-0808-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simonetti G, Bruno S, Padella A, Tenti E, Martinelli G. Aneuploidy: Cancer strength or vulnerability? Int J Cancer. 2019;144(1):8–25. [DOI] [PMC free article] [PubMed]
- 10.Wu F, Sun Y, Chen J, Li H, Yao K, Liu Y, et al. The oncogenic role of APC/C activator protein Cdc20 by an integrated Pan-Cancer analysis in human tumors. Front Oncol. 2021;11:721797. doi: 10.3389/fonc.2021.721797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li D, Zhu J, Firozi PF, Abbruzzese JL, Evans DB, Cleary K, et al. Overexpression of oncogenic STK15/BTAK/Aurora A kinase in human pancreatic cancer. Clin Cancer Res. 2003;9:991–997. [PubMed] [Google Scholar]
- 12.Chang DZ, Ma Y, Ji B, Liu Y, Hwu P, Abbruzzese JL, et al. Increased CDC20 expression is associated with pancreatic ductal adenocarcinoma differentiation and progression. J Hematol Oncol. 2012;5:15. doi: 10.1186/1756-8722-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan B, Xu Y, Woo J-H, Wang Y, Bae YK, Yoon D-S, et al. Increased expression of mitotic checkpoint genes in breast cancer cells with chromosomal instability. Clin Cancer Res. 2006;12:405–410. doi: 10.1158/1078-0432.CCR-05-0903. [DOI] [PubMed] [Google Scholar]
- 14.Karra H, Repo H, Ahonen I, Löyttyniemi E, Pitkänen R, Lintunen M, et al. Cdc20 and securin overexpression predict short-term breast cancer survival. Br J Cancer. 2014;110:2905–2913. doi: 10.1038/bjc.2014.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kato T, Daigo Y, Aragaki M, Ishikawa K, Sato M, Kaji M. Overexpression of CDC20 predicts poor prognosis in primary non-small cell lung cancer patients. J Surg Oncol. 2012;106:423–430. doi: 10.1002/jso.23109. [DOI] [PubMed] [Google Scholar]
- 16.Kidokoro T, Tanikawa C, Furukawa Y, Katagiri T, Nakamura Y, Matsuda K. CDC20, a potential cancer therapeutic target, is negatively regulated by p53. Oncogene. 2008;27:1562–1571. doi: 10.1038/sj.onc.1210799. [DOI] [PubMed] [Google Scholar]
- 17.Kwan PS, Lau CC, Chiu YT, Man C, Liu J, Tang KD, et al. Daxx regulates mitotic progression and prostate cancer predisposition. Carcinogenesis. 2013;34:750–759. doi: 10.1093/carcin/bgs391. [DOI] [PubMed] [Google Scholar]
- 18.Wang Q, Tiffen J, Bailey CG, Lehman ML, Ritchie W, Fazli L, et al. Targeting amino acid transport in metastatic castration-resistant prostate cancer: effects on cell cycle, cell growth, and tumor development. J Natl Cancer Inst. 2013;105:1463–1473. doi: 10.1093/jnci/djt241. [DOI] [PubMed] [Google Scholar]
- 19.Bieniek J, Childress C, Swatski MD, Yang W. COX-2 inhibitors arrest prostate cancer cell cycle progression by down-regulation of kinetochore/centromere proteins. Prostate. 2014;74:999–1011. doi: 10.1002/pros.22815. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Gao J-Z, Du J-L, Huang Z-X, Wei L-X. Increased CDC20 expression is associated with development and progression of hepatocellular carcinoma. Int J Oncol. 2014;45:1547–1555. doi: 10.3892/ijo.2014.2559. [DOI] [PubMed] [Google Scholar]
- 21.Kim H-S, Vassilopoulos A, Wang R-H, Lahusen T, Xiao Z, Xu X, et al. SIRT2 maintains genome integrity and suppresses tumorigenesis through regulating APC/C activity. Cancer Cell. 2011;20:487–499. doi: 10.1016/j.ccr.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gu Y, Lu L, Wu L, Chen H, Zhu W, He Y. Identification of prognostic genes in kidney renal clear cell carcinoma by RNA-seq data analysis. Mol Med Rep. 2017;15:1661–1667. doi: 10.3892/mmr.2017.6194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun Q, Zhao H, Zhang C, Hu T, Wu J, Lin X, et al. Gene co-expression network reveals shared modules predictive of stage and grade in serous ovarian cancers. Oncotarget. 2017;8:42983–42996. doi: 10.18632/oncotarget.17785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang D, He Y, Wu B, Deng Y, Wang N, Li M, et al. Integrated bioinformatics analysis for the screening of hub genes and therapeutic drugs in ovarian cancer. J Ovarian Res. 2020;13(1):10. [DOI] [PMC free article] [PubMed]
- 25.Shang G, Ma X, Lv G. Cell division cycle 20 promotes cell proliferation and invasion and inhibits apoptosis in osteosarcoma cells. Cell Cycle. 2018;17:43–52. doi: 10.1080/15384101.2017.1387700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Si WM, Yu MQ, Dong LD, Juan LX, Juan DL, Li N, et al. CDC20 and its downstream genes: potential prognosis factors of osteosarcoma. Int J Clin Oncol. 2019;24:1479–1489. doi: 10.1007/s10147-019-01500-3. [DOI] [PubMed] [Google Scholar]
- 27.Gao Y, Guo C, Fu S, Cheng Y, Song C. Downregulation of CDC20 suppressed cell proliferation, induced apoptosis, triggered cell cycle arrest in osteosarcoma cells, and enhanced chemosensitivity to cisplatin. Neoplasma. 2021;68:382–390. doi: 10.4149/neo_2020_200614N629. [DOI] [PubMed] [Google Scholar]
- 28.Marucci G, Morandi L, Magrini E, Farnedi A, Franceschi E, Miglio R, et al. Gene expression profiling in glioblastoma and immunohistochemical evaluation of IGFBP-2 and CDC20. Virchows Arch. 2008;453:599–609. doi: 10.1007/s00428-008-0685-7. [DOI] [PubMed] [Google Scholar]
- 29.Ji P, Smith SM, Wang Y, Jiang R, Song SW, Li B, et al. Inhibition of gliomagenesis and attenuation of mitotic transition by MIIP. Oncogene. 2010;29:3501–3508. doi: 10.1038/onc.2010.114. [DOI] [PubMed] [Google Scholar]
- 30.Wang L, Zhang J, Wan L, Zhou X, Wang Z, Wei W. Targeting Cdc20 as a novel cancer therapeutic strategy. Pharmacol Ther. 2015;151:141–151. doi: 10.1016/j.pharmthera.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Z, Wan L, Zhong J, Inuzuka H, Liu P, Sarkar FH, et al. Cdc20: a potential novel therapeutic target for cancer treatment. Curr Pharm Des. 2013;19:3210–3214. doi: 10.2174/1381612811319180005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chao WCH, Kulkarni K, Zhang Z, Kong EH, Barford D. Structure of the mitotic checkpoint complex. Nature. 2012;484:208–213. doi: 10.1038/nature10896. [DOI] [PubMed] [Google Scholar]
- 33.Hyun SY, Sarantuya B, Lee HJ, Jang YJ. APC/CCdh1-dependent degradation of Cdc20 requires a phosphorylation on CRY-box by polo-like kinase-1 during somatic cell cycle. Biochem Biophys Res Commun. 2013;436:12–18. doi: 10.1016/j.bbrc.2013.04.073. [DOI] [PubMed] [Google Scholar]
- 34.Tian W, Li B, Warrington R, Tomchick DR, Yu H, Luo X. Structural analysis of human Cdc20 supports multisite degron recognition by APC/C. Proc Natl Acad Sci U S A. 2012;109:18419–18424. doi: 10.1073/pnas.1213438109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu H. Cdc20: A WD40 activator for a cell cycle degradation machine. Mol Cell. 2007;27:3–16. doi: 10.1016/j.molcel.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 36.Tang Z, Bharadwaj R, Li B, Yu H. Mad2-independent inhibition of APCCdc20 by the mitotic checkpoint protein BubR1. Dev Cell. 2001;1(2):227–37. [DOI] [PubMed]
- 37.Hartwell LH, Culotti J, Reid B. Genetic control of the cell-division cycle in yeast. I. Detection of mutants. Proc Natl Acad Sci U S A. 1970;66:352–359. doi: 10.1073/pnas.66.2.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li M, York JP, Zhang P. Loss of Cdc20 causes a Securin-dependent metaphase arrest in two-cell mouse embryos. Mol Cell Biol. 2007;27:3481–3488. doi: 10.1128/MCB.02088-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li M, Fang X, Wei Z, York JP, Zhang P. Loss of spindle assembly checkpoint-mediated inhibition of Cdc20 promotes tumorigenesis in mice. J Cell Biol. 2009;185:983–994. doi: 10.1083/jcb.200904020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malureanu L, Jeganathan KB, Jin F, Baker DJ, van Ree JH, Gullon O, et al. Cdc20 hypomorphic mice fail to counteract de novo synthesis of cyclin B1 in mitosis. J Cell Biol. 2010;191:313–329. doi: 10.1083/jcb.201003090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo C, Kong F, Lv Y, Gao N, Xiu X, Sun X. CDC20 inhibitor Apcin inhibits embryo implantation in vivo and in vitro. Cell Biochem Funct. 2020;38:810–816. doi: 10.1002/cbf.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sang Q, Zhou Z, Mu J, Wang L. Genetic factors as potential molecular markers of human oocyte and embryo quality. J Assist Reprod Genet. 2021;38(5):993–1002. doi: 10.1007/s10815-021-02196-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lara-Gonzalez P, Westhorpe FG, Taylor SS. The spindle assembly checkpoint. Curr Biol. 2012;22:R966–R980. doi: 10.1016/j.cub.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 44.Ovejero S, Bueno A, Sacristán MP. Working on genomic stability: from the S-phase to mitosis. Genes (Basel). 2020;11(2):225. [DOI] [PMC free article] [PubMed]
- 45.Maresca TJ, Salmon ED. Welcome to a new kind of tension: translating kinetochore mechanics into a wait-anaphase signal. J Cell Sci. 2010;123:825–835. doi: 10.1242/jcs.064790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu D, Vader G, Vromans MJM, Lampson MA, Lens SMA. Sensing chromosome bi-orientation by spatial separation of Aurora B kinase from kinetochore substrates. Science (80- ) 2009;323:1350–1353. doi: 10.1126/science.1167000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Welburn JPI, Vleugel M, Liu D, Yates JR, Lampson MA, Fukagawa T, et al. Aurora B phosphorylates spatially distinct targets to differentially regulate the kinetochore-microtubule Interface. Mol Cell. 2010;38:383–392. doi: 10.1016/j.molcel.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stucke VM, Baumann C, Nigg EA. Kinetochore localization and microtubule interaction of the human spindle checkpoint kinase Mps1. Chromosoma. 2004;113:1–15. doi: 10.1007/s00412-004-0288-2. [DOI] [PubMed] [Google Scholar]
- 49.Nijenhuis W, Von Castelmur E, Littler D, De Marco V, Tromer E, Vleugel M, et al. A TPR domain-containing N-terminal module of MPS1 is required for its kinetochore localization by Aurora B. J Cell Biol. 2013;201:217–231. doi: 10.1083/jcb.201210033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petrovic A, Keller J, Liu Y, Overlack K, John J, Dimitrova YN, et al. Structure of the MIS12 complex and molecular basis of its interaction with CENP-C at human kinetochores. Cell. 2016;167:1028–1040.e15. doi: 10.1016/j.cell.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shepperd LA, Meadows JC, Sochaj AM, Lancaster TC, Zou J, Buttrick GJ, et al. Phosphodependent recruitment of Bub1 and Bub3 to Spc7/KNL1 by Mph1 kinase maintains the spindle checkpoint. Curr Biol. 2012;22:891–899. doi: 10.1016/j.cub.2012.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.London N, Ceto S, Ranish JA, Biggins S. Phosphoregulation of Spc105 by Mps1 and PP1 regulates Bub1 localization to kinetochores. Curr Biol. 2012;22:900–906. doi: 10.1016/j.cub.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vleugel M, Tromer E, Omerzu M, Groenewold V, Nijenhuis W, Snel B, et al. Arrayed BUB recruitment modules in the kinetochore scaffold KNL1 promote accurate chromosome segregation. J Cell Biol. 2013;203:943–955. doi: 10.1083/jcb.201307016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamagishi Y, Yang CH, Tanno Y, Watanabe Y. MPS1/Mph1 phosphorylates the kinetochore protein KNL1/Spc7 to recruit SAC components. Nat Cell Biol. 2012;14:746–752. doi: 10.1038/ncb2515. [DOI] [PubMed] [Google Scholar]
- 55.Primorac I, Weir JR, Chiroli E, Gross F, Hoffmann I, van Gerwen S, et al. Bub3 reads phosphorylated MELT repeats to promote spindle assembly checkpoint signaling. Elife. 2013;2:e01030. doi: 10.7554/eLife.01030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Larsen NA, Al-Bassam J, Wei RR, Harrison SC. Structural analysis of Bub3 interactions in the mitotic spindle checkpoint. Proc Natl Acad Sci U S A. 2007;104:1201–1206. doi: 10.1073/pnas.0610358104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mora-Santos M del M, Hervas-Aguilar A, Sewart K, Lancaster TC, Meadows JC, Millar JBA. Bub3-Bub1 binding to Spc7/KNL1 toggles the spindle checkpoint switch by licensing the interaction of Bub1 with Mad1-Mad2. Curr Biol. 2016;26:2642–2650. doi: 10.1016/j.cub.2016.07.040. [DOI] [PubMed] [Google Scholar]
- 58.Mapelli M, Massimiliano L, Santaguida S, Musacchio A. The Mad2 conformational dimer: structure and implications for the spindle assembly checkpoint. Cell. 2007;131:730–743. doi: 10.1016/j.cell.2007.08.049. [DOI] [PubMed] [Google Scholar]
- 59.Tipton AR, Ji W, Sturt-Gillespie B, Bekier ME, Wang K, Taylor WR, et al. Monopolar spindle 1 (MPS1) kinase promotes production of closed MAD2 (C-MAD2) conformer and assembly of the mitotic checkpoint complex. J Biol Chem. 2013;288:5149–5158. doi: 10.1074/jbc.M113.522375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hewitt L, Tighe A, Santaguida S, White AM, Jones CD, Musacchio A, et al. Sustained Mps1 activity is required in mitosis to recruit O-Mad2 to the Mad1-C-Mad2 core complex. J Cell Biol. 2010;19:25–34. doi: 10.1083/jcb.201002133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.DiFiore B, Davey NE, Hagting A, Izawa D, Mansfeld J, Gibson TJ, et al. The ABBA motif binds APC/C activators and is shared by APC/C substrates and regulators. Dev Cell. 2015. [DOI] [PMC free article] [PubMed]
- 62.Piano V, Alex A, Stege P, Maffini S, Stoppiello GA, Huis In’T Veld PJ, et al. CDC20 assists its catalytic incorporation in the mitotic checkpoint complex. Science (80- ). 2021;371:67–71. doi: 10.1126/science.abc1152. [DOI] [PubMed] [Google Scholar]
- 63.Jia L, Li B, Yu H. The Bub1-Plk1 kinase complex promotes spindle checkpoint signalling through Cdc20 phosphorylation. Nat Commun. 2016;7:10818. doi: 10.1038/ncomms10818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.O’Connor A, Maffini S, Rainey MD, Kaczmarczyk A, Gaboriau D, Musacchio A, et al. Requirement for PLK1 kinase activity in the maintenance of a robust spindle assembly checkpoint. Biol Open. 2015;5:11–19. doi: 10.1242/bio.014969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alfieri C, Chang L, Zhang Z, Yang J, Maslen S, Skehel M, et al. Molecular basis of APC/C regulation by the spindle assembly checkpoint. Nature. 2016;536:431–436. doi: 10.1038/nature19083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Watson ER, Brown NG, Peters J-M, Stark H, Schulman BA. Posing the APC/C E3 ubiquitin ligase to orchestrate cell division. Trends Cell Biol. 2019;29:117–134. doi: 10.1016/j.tcb.2018.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Clute P, Pines J. Temporal and spatial control of cyclin B1 destruction in metaphase. Nat Cell Biol. 1999;1:82–87. doi: 10.1038/10049. [DOI] [PubMed] [Google Scholar]
- 68.Nasmyth K. Disseminating the genome: joining, resolving, and separating sister chromatids during mitosis and meiosis. Annu Rev Genet. 2001;35:673–745. doi: 10.1146/annurev.genet.35.102401.091334. [DOI] [PubMed] [Google Scholar]
- 69.Jin L, Williamson A, Banerjee S, Philipp I, Rape M. Mechanism of ubiquitin-chain formation by the human anaphase-promoting complex. Cell. 2008;133:653–665. doi: 10.1016/j.cell.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Clijsters L, Ogink J, Wolthuis R. The spindle checkpoint, APC/C(Cdc20), and APC/C(Cdh1) play distinct roles in connecting mitosis to S phase. J Cell Biol. 2013;201:1013–1026. doi: 10.1083/jcb.201211019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kramer ER, Scheuringer N, Podtelejnikov AV, Mann M, Peters JM. Mitotic regulation of the APC activator proteins CDC20 and CDH1. Mol Biol Cell. 2000;11:1555–1569. doi: 10.1091/mbc.11.5.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kraft C, Herzog F, Gieffers C, Mechtler K, Hagting A, Pines J, et al. Mitotic regulation of the human anaphase-promoting complex by phosphorylation. EMBO J. 2003;22:6598–6609. doi: 10.1093/emboj/cdg627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maan M, Agrawal NJ, Padmanabhan J, Leitzinger CC, Rivera-Rivera Y, Saavedra HI, et al. Tank binding kinase 1 modulates spindle assembly checkpoint components to regulate mitosis in breast and lung cancer cells. Biochim Biophys Acta Mol Cell Res. 2021;1868:118929. doi: 10.1016/j.bbamcr.2020.118929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Garrido D, Bourouh M, Bonneil É, Thibault P, Swan A, Archambault V. Cyclin B3 activates the anaphase-promoting complex/Cyclosome in meiosis and mitosis. PLoS Genet. 2020;16(11):e10. doi: 10.1371/journal.pgen.1009184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fujimitsu K, Yamano H. PP2A-B56 binds to Apc1 and promotes Cdc20 association with the APC/C ubiquitin ligase in mitosis. EMBO Rep. 2020;21(1):e485. doi: 10.15252/embr.201948503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Khumukcham SS, Samanthapudi VSK, Penugurti V, Kumari A, Kesavan PS, Velatooru LR, et al. Hematopoietic PBX-interacting protein is a substrate and an inhibitor of the APC/C–Cdc20 complex and regulates mitosis by stabilizing cyclin B1. J Biol Chem. 2019;294:10236–10252. doi: 10.1074/jbc.RA118.006733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Oughtred R, Rust J, Chang C, Breitkreutz BJ, Stark C, Willems A, et al. The BioGRID database: A comprehensive biomedical resource of curated protein, genetic, and chemical interactions. Protein Sci. 2021;30:187–200. doi: 10.1002/pro.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xie Z, Bailey A, Kuleshov MV, Clarke DJB, Evangelista JE, Jenkins SL, et al. Gene set knowledge discovery with Enrichr. Curr Protoc. 2021;1:e90. doi: 10.1002/cpz1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zur A, Brandeis M. Securin degradation is mediated by fzy and fzr, and is required for complete chromatid separation but not for cytokinesis. EMBO J. 2001;20:792–801. doi: 10.1093/emboj/20.4.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shirayama M, Tóth A, Gálová M, Nasmyth K. APC(Cdc20) promotes exit from mitosis by destroying the anaphase inhibitor Pds1 and cyclin Clb5. Nature. 1999;402:203–207. doi: 10.1038/46080. [DOI] [PubMed] [Google Scholar]
- 81.Geley S, Kramer E, Gieffers C, Gannon J, Peters JM, Hunt T. Anaphase-promoting complex/cyclosome-dependent proteolysis of human cyclin A starts at the beginning of mitosis and is not subject to the spindle assembly checkpoint. J Cell Biol. 2001;153:137–148. doi: 10.1083/jcb.153.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang S, Tischer T, Barford D. Cyclin A2 degradation during the spindle assembly checkpoint requires multiple binding modes to the APC/C. Nat Commun. 2019;10:3863. doi: 10.1038/s41467-019-11833-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hames RS. APC/C-mediated destruction of the centrosomal kinase Nek2A occurs in early mitosis and depends upon a cyclin A-type D-box. EMBO J. 2001;20:7117–7127. doi: 10.1093/emboj/20.24.7117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nilsson J. Escape from the checkpoint: Nek2A binds a unique conformation of the APC /C- MCC complex. EMBO Rep. 2020;21:e50494. doi: 10.15252/embr.202050494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.He Y, Li R, Gu L, Deng H, Zhao Y, Guo Y, et al. Anaphase-promoting complex/cyclosome-Cdc-20 promotes Zwint-1 degradation. Cell Biochem Funct. 2020;38:451–459. doi: 10.1002/cbf.3499. [DOI] [PubMed] [Google Scholar]
- 86.Amador V, Ge S, Santamaría PG, Guardavaccaro D, Pagano M. APC/CCdc20 controls the ubiquitin-mediated degradation of p21 in Prometaphase. Mol Cell. 2007;27:462–473. doi: 10.1016/j.molcel.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang W, Wu T, Kirschner MW. The master cell cycle regulator APC-Cdc20 regulates ciliary length and disassembly of the primary cilium. Elife. 2014;3. [DOI] [PMC free article] [PubMed]
- 88.Doornbos C, Roepman R. Moonlighting of mitotic regulators in cilium disassembly. Cell Mol Life Sci. 2021;78(11):4955–4972. doi: 10.1007/s00018-021-03827-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang Y, Kim AH, Yamada T, Wu B, Bilimoria PM, Ikeuchi Y, et al. A Cdc20-APC ubiquitin signaling pathway regulates presynaptic differentiation. Science. 2009;326:575–578. doi: 10.1126/science.1177087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Okuda S, Sato M, Kato S, Nagashima S, Inatome R, Yanagi S, et al. Oscillation of Cdc20-APC/C-mediated CAMDI stability is critical for cortical neuron migration. J Biol Chem. 2021;297(2):100. doi: 10.1016/j.jbc.2021.100986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kuang C, Golden KL, Simon CR, Damrath J, Buttitta L, Gamble CE, et al. A novel fizzy/Cdc20-dependent mechanism suppresses necrosis in neural stem cells. Development. 2014;141:1453–1464. doi: 10.1242/dev.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Quek LS, Grasset N, Jasmen JB, Robinson KS, Bellanger S. Dual role of the anaphase promoting complex/Cyclosome in regulating Stemness and differentiation in human primary keratinocytes. J Invest Dermatol. 2018;138:1851–1861. doi: 10.1016/j.jid.2018.02.033. [DOI] [PubMed] [Google Scholar]
- 93.Sanz-Gómez N, de Pedro I, Ortigosa B, Santamaría D, Malumbres M, de Cárcer G, et al. Squamous differentiation requires G2/mitosis slippage to avoid apoptosis. Cell Death Differ. 2020;27:2451–2467. doi: 10.1038/s41418-020-0515-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee SB, Kim JJ, Nam HJ, Gao B, Yin P, Qin B, et al. Parkin regulates mitosis and genomic stability through Cdc20/Cdh1. Mol Cell. 2015;60:21–34. doi: 10.1016/j.molcel.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Paul D, Ghorai S, Dinesh US, Shetty P, Chattopadhyay S, Santra MK. Cdc20 directs proteasome-mediated degradation of the tumor suppressor SMAR1 in higher grades of cancer through the anaphase promoting complex. Cell Death Dis. 2017;8:e2882. doi: 10.1038/cddis.2017.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fujita H, Sasaki T, Miyamoto T, Akutsu SN, Sato S, Mori T, et al. Premature aging syndrome showing random chromosome number instabilities with CDC20 mutation. Aging Cell. 2020;19:e13251. doi: 10.1111/acel.13251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xie YP, Lai S, Lin QY, Xie X, Liao JW, Wang HX, et al. CDC20 regulates cardiac hypertrophy via targeting LC3-dependent autophagy. Theranostics. 2018;8:5995–6007. doi: 10.7150/thno.27706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gu Q, Li F, Ge S, Zhang F, Jia R, Fan X. CDC20 knockdown and acidic microenvironment collaboratively promote tumorigenesis through inhibiting autophagy and apoptosis. Mol Ther Oncolytics. 2020;17:94–106. doi: 10.1016/j.omto.2020.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chun AC-S, Kok K-H, Jin D-Y. REV7 is required for anaphase-promoting complex-dependent ubiquitination and degradation of translesion DNA polymerase REV1. Cell Cycle. 2013;12:365–378. doi: 10.4161/cc.23214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hadjihannas MV, Bernkopf DB, Brückner M, Behrens J. Cell cycle control of Wnt/β-catenin signalling by conductin/axin2 through CDC20. EMBO Rep. 2012;13:347–354. doi: 10.1038/embor.2012.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mao DD, Gujar AD, Mahlokozera T, Chen I, Pan Y, Luo J, et al. A CDC20-APC/SOX2 signaling Axis regulates human Glioblastoma stem-like cells. Cell Rep. 2015;11:1809–1821. doi: 10.1016/j.celrep.2015.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen Z, Yu Y, Fu D, Li Z, Niu X, Liao M, et al. Functional roles of PC-PLC and Cdc20 in the cell cycle, proliferation, and apoptosis. Cell Biochem Funct. 2010;28:249–257. doi: 10.1002/cbf.1634. [DOI] [PubMed] [Google Scholar]
- 103.Fu D, Ma Y, Wu W, Zhu X, Jia C, Zhao Q, et al. Cell-cycle-dependent PC-PLC regulation by APC/CCdc20-mediated ubiquitin-proteasome pathway. J Cell Biochem. 2009;107:686–696. doi: 10.1002/jcb.22163. [DOI] [PubMed] [Google Scholar]
- 104.Harley ME, Allan LA, Sanderson HS, Clarke PR. Phosphorylation of Mcl-1 by CDK1–cyclin B1 initiates its Cdc20-dependent destruction during mitotic arrest. EMBO J. 2010;29:2407–2420. doi: 10.1038/emboj.2010.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sloss O, Topham C, Diez M, Taylor S. Mcl-1 dynamics influence mitotic slippage and death in mitosis. Oncotarget. 2016;7:5176–5192. doi: 10.18632/oncotarget.6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Allan LA, Skowyra A, Rogers KI, Zeller D, Clarke PR. Atypical APC/C-dependent degradation of Mcl-1 provides an apoptotic timer during mitotic arrest. EMBO J. 2018. [DOI] [PMC free article] [PubMed]
- 107.Clarke PR, Allan LA, Skowyra A. Timed degradation of Mcl-1 controls mitotic cell death. Mol Cell Oncol. 2018;5(6):e1516450. [DOI] [PMC free article] [PubMed]
- 108.Gao Y, Wen P, Chen B, Hu G, Wu L, Xu A, et al. Downregulation of cdc20 increases radiosensitivity through mcl-1/p-chk1-mediated DNA damage and apoptosis in tumor cells. Int J Mol Sci. 2020;21:6692. doi: 10.3390/ijms21186692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhao S, Zhang Y, Lu X, Ding H, Han B, Song X, et al. Cdc20 regulates the cell proliferation and radiosensitivity of p53 mutant hcc cells through the bcl-2/bax pathway. Int J Biol Sci. 2021;17:3608–3621. doi: 10.7150/ijbs.64003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wan L, Tan M, Yang J, Inuzuka H, Dai X, Wu T, et al. APC(Cdc20) suppresses apoptosis through targeting Bim for ubiquitination and destruction. Dev Cell. 2014;29:377–391. doi: 10.1016/j.devcel.2014.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Eichhorn JM, Sakurikar N, Alford SE, Chu R, Chambers TC. Critical role of anti-apoptotic Bcl-2 protein phosphorylation in mitotic death. Cell Death Dis. 2013;4:e834. doi: 10.1038/cddis.2013.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bah N, Maillet L, Ryan J, Dubreil S, Gautier F, Letai A, et al. Bcl-xL controls a switch between cell death modes during mitotic arrest. Cell Death Dis. 2014;5:e1429. doi: 10.1038/cddis.2014.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kang K, Xie F, Wu Y, Han C, Bai Y, Long J, et al. Genomic instability in lower-grade glioma: prediction of prognosis based on lncRNA and immune infiltration. Mol Ther Oncolytics. 2021;22:431–443. doi: 10.1016/j.omto.2021.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Xiong C, Wang Z, Wang G, Zhang C, Jin S, Jiang G, et al. Identification of CDC20 as an immune infiltration-correlated prognostic biomarker in hepatocellular carcinoma. Investig New Drugs. 2021;39:1439–1453. doi: 10.1007/s10637-021-01126-1. [DOI] [PubMed] [Google Scholar]
- 115.Lai E, Tai Y, Jiang J, Zhao C, Xiao Y, Quan X, et al. Prognostic evaluation and immune infiltration analysis of five bioinformatic selected genes in hepatocellular carcinoma. J Cell Mol Med. 2021;25:11128–11141. doi: 10.1111/jcmm.17035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu B, Hong S, Tang Z, Yu H, Giam C-Z. HTLV-I tax directly binds the Cdc20-associated anaphase-promoting complex and activates it ahead of schedule. Proc Natl Acad Sci. 2005;102:63–68. doi: 10.1073/pnas.0406424101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jiang GJ, Chen YH, Guo W, Zhang H, Zou L. Screening and verification of key genes in T-cell acute lymphoblastic leukemia. Nan Fang Yi Ke Da Xue Xue Bao. 2018;38:261–267. doi: 10.3969/j.issn.1673-4254.2018.03.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Simonetti G, Padella A, do Valle IF, Fontana MC, Fonzi E, Bruno S, et al. Aneuploid acute myeloid leukemia exhibits a signature of genomic alterations in the cell cycle and protein degradation machinery. Cancer. 2019;125:712–725. doi: 10.1002/cncr.31837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Moison C, Lavallée VP, Thiollier C, Lehnertz B, Boivin I, Mayotte N, et al. Complex karyotype AML displays G2/M signature and hypersensitivity to PLK1 inhibition. Blood Adv. 2019;3:552–563. doi: 10.1182/bloodadvances.2018028480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ecker S, Pancaldi V, Rico D, Valencia A. Higher gene expression variability in the more aggressive subtype of chronic lymphocytic leukemia. Genome Med. 2015;7:8. doi: 10.1186/s13073-014-0125-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pittner BT, Shanafelt TD, Kay NE, Jelinek DF. CD38 expression levels in chronic lymphocytic leukemia B cells are associated with activation marker expression and differential responses to interferon stimulation. Leukemia. 2005;19:2264–2272. doi: 10.1038/sj.leu.2403975. [DOI] [PubMed] [Google Scholar]
- 122.Wang Q, Zhou HS, Huang KK, Jiang XJ, Wu FQ, Cao R, et al. Imatinib and bortezomib induce the expression and distribution of anaphase-promoting complex adaptor protein Cdh1 in blast crisis of chronic myeloid leukemia. Int J Oncol. 2012;40:418–426. doi: 10.3892/ijo.2011.1233. [DOI] [PubMed] [Google Scholar]
- 123.Sun C, Cheng X, Wang C, Wang X, Xia B, Zhang Y. Gene expression profiles analysis identifies a novel two-gene signature to predict overall survival in diffuse large B-cell lymphoma. Biosci Rep. 2019;39:BSR20181293. doi: 10.1042/BSR20181293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Maes A, Maes K, De Raeve H, De Smedt E, Vlummens P, Szablewski V, et al. The anaphase-promoting complex/cyclosome: a new promising target in diffuse large B-cell lymphoma and mantle cell lymphoma. Br J Cancer. 2019;120:1137–1146. doi: 10.1038/s41416-019-0471-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Guo D, Wang H, Sun L, Liu S, Du S, Qiao W, et al. Identification of key gene modules and hub genes of human mantle cell lymphoma by coexpression network analysis. PeerJ. 2020;8:e8843. doi: 10.7717/peerj.8843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Heredia FF, de Sousa JC, Ribeiro Junior HL, Carvalho AF, Magalhaes SMM, Pinheiro RF. Proteins related to the spindle and checkpoint mitotic emphasize the different pathogenesis of hypoplastic MDS. Leuk Res. 2014;38:218–224. doi: 10.1016/j.leukres.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 127.Genga KR, Filho FDR, Ferreira FV de A, de Sousa JC, Studart FS, Magalhães SMM, et al. Proteins of the mitotic checkpoint and spindle are related to chromosomal instability and unfavourable prognosis in patients with myelodysplastic syndrome. J Clin Pathol. 2015;68:381–387. doi: 10.1136/jclinpath-2014-202728. [DOI] [PubMed] [Google Scholar]
- 128.Borges D de P, Dos Santos AWA, Paier CRK, Ribeiro HL, Costa MB, Farias IR, et al. Prognostic importance of Aurora kinases and mitotic spindle genes transcript levels in Myelodysplastic syndrome. Leuk Res. 2018;64:61–70. doi: 10.1016/j.leukres.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 129.Díaz-Rodríguez E, Álvarez-Fernández S, Chen X, Paiva B, López-Pérez R, García-Hernández JL, et al. Deficient spindle assembly checkpoint in multiple myeloma. PLoS One. 2011;6:e27583. doi: 10.1371/journal.pone.0027583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lub S, Maes A, Maes K, De Veirman K, De Bruyne E, Menu E, et al. Inhibiting the anaphase promoting complex/cyclosome induces a metaphase arrest and cell death in multiple myeloma cells. Oncotarget. 2016;7:4062–4076. doi: 10.18632/oncotarget.6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yang Y, Gu C, Luo C, Li F, Wang M. BUB1B promotes multiple myeloma cell proliferation through CDC20/CCNB axis. Med Oncol. 2015;32:81. doi: 10.1007/s12032-015-0542-x. [DOI] [PubMed] [Google Scholar]
- 132.Crawford LJ, Anderson G, Johnston CK, Irvine AE. Identification of the APC/C co-factor FZR1 as a novel therapeutic target for multiple myeloma. Oncotarget. 2016;7:70481–70493. doi: 10.18632/oncotarget.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang S, Zhang Y, Soosairajah J, Kraft AS. Regulation of RUNX1/AML1 during the G2/M transition. Leuk Res. 2007;31:839–851. doi: 10.1016/j.leukres.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 134.Grey W, Ivey A, Milne TA, Haferlach T, Grimwade D, Uhlmann F, et al. The Cks1/Cks2 axis fine-tunes Mll1 expression and is crucial for MLL-rearranged leukaemia cell viability. Biochim Biophys Acta Mol cell Res. 2018;1865:105–116. doi: 10.1016/j.bbamcr.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liu H, Cheng EH-Y, Hsieh JJ-D. Bimodal degradation of MLL by SCFSkp2 and APCCdc20 assures cell cycle execution: a critical regulatory circuit lost in leukemogenic MLL fusions. Genes Dev. 2007;21:2385–2398. doi: 10.1101/gad.1574507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Duan X-F, Wu Y-L, Xu H-Z, Zhao M, Zhuang H-Y, Wang X-D, et al. Synergistic mitosis-arresting effects of arsenic trioxide and paclitaxel on human malignant lymphocytes. Chem Biol Interact. 2010;183:222–230. doi: 10.1016/j.cbi.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 137.Jeang K-T, Giam C, Majone F, Aboud M. Life, death, and tax: role of HTLV-I Oncoprotein in genetic instability and cellular transformation. J Biol Chem. 2004;279:31991–31994. doi: 10.1074/jbc.R400009200. [DOI] [PubMed] [Google Scholar]
- 138.Liu B, Liang M-H, Kuo Y-l, Liao W, Boros I, Kleinberger T, et al. Human T-Lymphotropic virus type 1 Oncoprotein tax promotes unscheduled degradation of Pds1p/Securin and Clb2p/Cyclin B1 and causes chromosomal instability. Mol Cell Biol. 2003;23:5269–5281. doi: 10.1128/MCB.23.15.5269-5281.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sun C, Li M, Feng Y, Sun F, Zhang L, Xu Y, et al. MDM2-P53 signaling pathway-mediated Upregulation of CDC20 promotes progression of human diffuse large B-cell lymphoma. Onco Targets Ther. 2020;13:10475–10487. doi: 10.2147/OTT.S253758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chen E, Lim MS, Rosic-Kablar S, Liu J, Jolicoeur P, Dubé ID, et al. Dysregulated expression of mitotic regulators is associated with B-cell lymphomagenesis in HOX11-transgenic mice. Oncogene. 2006;25:2575–2587. doi: 10.1038/sj.onc.1209285. [DOI] [PubMed] [Google Scholar]
- 141.Bentley AM, Williams BC, Goldberg ML, Andres AJ. Phenotypic characterization of Drosophila ida mutants: defining the role of APC5 in cell cycle progression. J Cell Sci. 2002;115:949–961. doi: 10.1242/jcs.115.5.949. [DOI] [PubMed] [Google Scholar]
- 142.Abdallah N, Rajkumar SV, Greipp P, Kapoor P, Gertz MA, Dispenzieri A, et al. Cytogenetic abnormalities in multiple myeloma: association with disease characteristics and treatment response. Blood Cancer J. 2020;10:82. doi: 10.1038/s41408-020-00348-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lub S, Maes A, Maes K, De Veirman K, Leleu X, Menu E, et al. Targeting the anaphase promoting complex/Cyclosome (APC/C) in multiple myeloma. Blood. 2014;124:2097. doi: 10.1182/blood.V124.21.2097.2097. [DOI] [Google Scholar]
- 144.Wang J, He N, Wang R, Tian T, Han F, Zhong C, et al. Analysis of TET2 and EZH2 gene functions in chromosome instability in acute myeloid leukemia. Sci Rep. 2020;10:2706. doi: 10.1038/s41598-020-59365-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Schnerch D, Schmidts A, Follo M, Udi J, Felthaus J, Pfeifer D, et al. BubR1 is frequently repressed in acute myeloid leukemia and its re-expression sensitizes cells to antimitotic therapy. Haematologica. 2013;98:1886–1895. doi: 10.3324/haematol.2013.087452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Biggs JR, Peterson LF, Zhang Y, Kraft AS, Zhang D-E. AML1/RUNX1 phosphorylation by Cyclin-dependent kinases regulates the degradation of AML1/RUNX1 by the anaphase-promoting complex. Mol Cell Biol. 2006;26:7420–7429. doi: 10.1128/MCB.00597-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Liu X, Zhang F, Zhang Y, Li X, Chen C, Zhou M, et al. PPM1K regulates hematopoiesis and Leukemogenesis through CDC20-mediated Ubiquitination of MEIS1 and p21. Cell Rep. 2018;23:1461–1475. doi: 10.1016/j.celrep.2018.03.140. [DOI] [PubMed] [Google Scholar]
- 148.Salsi V, Ferrari S, Gorello P, Fantini S, Chiavolelli F, Mecucci C, et al. NUP98 fusion oncoproteins promote aneuploidy by attenuating the mitotic spindle checkpoint. Cancer Res. 2014;74:1079–1090. doi: 10.1158/0008-5472.CAN-13-0912. [DOI] [PubMed] [Google Scholar]
- 149.Bolouri H, Farrar JE, Triche T, Ries RE, Lim EL, Alonzo TA, et al. The molecular landscape of pediatric acute myeloid leukemia reveals recurrent structural alterations and age-specific mutational interactions. Nat Med. 2018;25:530. doi: 10.1038/s41591-019-0369-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Salsi V, Fantini S, Zappavigna V. NUP98 fusion oncoproteins interact with the APC/C(Cdc20) as a pseudosubstrate and prevent mitotic checkpoint complex binding. Cell Cycle. 2016;15:2275–2287. doi: 10.1080/15384101.2016.1172156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hochhaus A, Baccarani M, Silver RT, Schiffer C, Apperley JF, Cervantes F, et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 2020;34(4):966–984. doi: 10.1038/s41375-020-0776-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sackton KL, Dimova N, Zeng X, Tian W, Zhang M, Sackton TB, et al. Synergistic blockade of mitotic exit by two chemical inhibitors of the APC/C. Nature. 2014;514:646–649. doi: 10.1038/nature13660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Richeson KV, Bodrug T, Sackton KL, Yamaguchi M, Paulo JA, Gygi SP, et al. Paradoxical mitotic exit induced by a small molecule inhibitor of APC/CCdc20. Nat Chem Biol. 2020;16:546–555. doi: 10.1038/s41589-020-0495-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zeng X, Sigoillot F, Gaur S, Choi S, Pfaff KL, Oh D-C, et al. Pharmacologic inhibition of the anaphase-promoting complex induces a spindle checkpoint-dependent mitotic arrest in the absence of spindle damage. Cancer Cell. 2010;18:382–395. doi: 10.1016/j.ccr.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zeng X, King RW. An APC/C inhibitor stabilizes cyclin B1 by prematurely terminating ubiquitination. Nat Chem Biol. 2012;8:383–392. doi: 10.1038/nchembio.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Thota S, Viny AD, Makishima H, Spitzer B, Radivoyevitch T, Przychodzen B, et al. Genetic alterations of the cohesin complex genes in myeloid malignancies. Blood Am Soc Hematol. 2014;124:1790. doi: 10.1182/blood-2014-04-567057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.De Lange J, Faramarz A, Oostra AB, De Menezes RX, Van Der Meulen IH, Rooimans MA, et al. Defective sister chromatid cohesion is synthetically lethal with impaired APC/C function. Nat Commun. 2015;6:8399. doi: 10.1038/ncomms9399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Simonetti G, Boga C, Durante J, Micheletti G, Telese D, Caruana P, et al. Synthesis of novel tryptamine derivatives and their biological activity as antitumor agents. Molecules. 2021;26:683. doi: 10.3390/molecules26030683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Huang P, Le X, Huang F, Yang J, Yang H, Ma J, et al. Discovery of a dual tubulin polymerization and cell division cycle 20 homologue inhibitor via structural modification on Apcin. J Med Chem. 2020;63:4685–4700. doi: 10.1021/acs.jmedchem.9b02097. [DOI] [PubMed] [Google Scholar]
- 160.Raab M, Kobayashi NF, Becker S, Kurunci-Csacsko E, Krämer A, Strebhardt K, et al. Boosting the apoptotic response of high-grade serous ovarian cancers with CCNE1 amplification to paclitaxel in vitro by targeting APC/C and the pro-survival protein MCL-1. Int J Cancer. 2020;146:1086–1098. doi: 10.1002/ijc.32559. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.