Summary
In the recent years, the agricultural world has been progressing towards integrated crop protection, in the context of sustainable and reasoned agriculture to improve food security and quality, and to preserve the environment through reduced uses of water, pesticides, fungicides or fertilisers. For this purpose, one possible issue is to cross‐elite varieties widely used in fields for crop productions with exotic or wild genetic resources in order to introduce new diversity for genes or alleles of agronomical interest to accelerate the development of new improved cultivars. However, crossing ability (or crossability) often depends on genetic background of the recipient varieties or of the donor, which hampers a larger use of wild resources in breeding programmes of many crops. In this review, we tried to provide a comprehensive summary of genetic factors controlling crossing ability between Triticeae species with a special focus on the crossability between wheat (Triticum aestivum L.) and rye (Secale cereale), which lead to the creation of Triticale (x Triticosecale Wittm.). We also discussed potential applications of newly identified genes or markers associated with crossability for accelerating wheat and Triticale improvement by application of modern genomics technologies in breeding programmes.
Keywords: crossability, self‐compatibility, self‐incompatibility, Kr/kr gene, Skr/skr gene
Introduction
The Triticeae tribe belongs to the Poaceae family previously known as Gramineae (Soreng et al., 2015). This tribe is of great agronomical importance, as it contains several crops and their close relatives such as wheats (Triticum species), barley (Hordeum), rye (Secale), Triticale (Triticosecale) (Soreng et al., 2015), as well as numerous wild species from genders Aegilops, Agropyron, Amblyopyrum, Dasypyrum, Elymus, Leymus, Pascopyrum, Roegneria and Thinopyrum (Soreng et al., 2015). Triticeae are growing in a wide range of areas and climates allowing them to adapt to very diverse conditions, from cold‐wet to hot‐dry regions. This adaptability is a huge asset as cultivated, or wild species have developed a large reservoir of genes and alleles to improve their resistance to biotic (pathogens, insects, nematodes…) and abiotic (frost/heat and salinity tolerance, drought resistance…) stresses but also to improve agronomic traits such as yield, earliness or protein content, in the context of sustainable and reasoned agriculture to improve food security and quality, and to preserve the environment.
Among the Poaceae, bread wheat (Triticum aestivum L.) is one of the most important crop worldwide and the staple food for one‐third of the world’s population with 220 million hectares and an annual production of ~770 million tons in 2020 (http://www.worldagriculturalproduction.com/crops/wheat.aspx). Today’s agriculture has to face an unprecedented challenge: to keep pace with the human demand in an environmentally and socially sustainable manner (Godfray et al., 2010). To meet this challenge, wheat yield should increase by ~2% per year over the next 30 years while the current yield increase worldwide is only 0.9% per year and even stagnating in the main producing countries [Figure 1 (Le Gouis et al., 2020; Ray et al., 2013)]. This goal would be achievable under the assumption of favourable growing conditions but is less likely under climate change that affects not only yield but also yield stability (Brisson et al., 2010; Porter and Semenov, 2005; Tester and Langridge, 2010). In this context, breeding for wheat varieties that have better resistance to biotic and abiotic stresses is of crucial importance and consequently a priority for agriculture.
Figure 1.
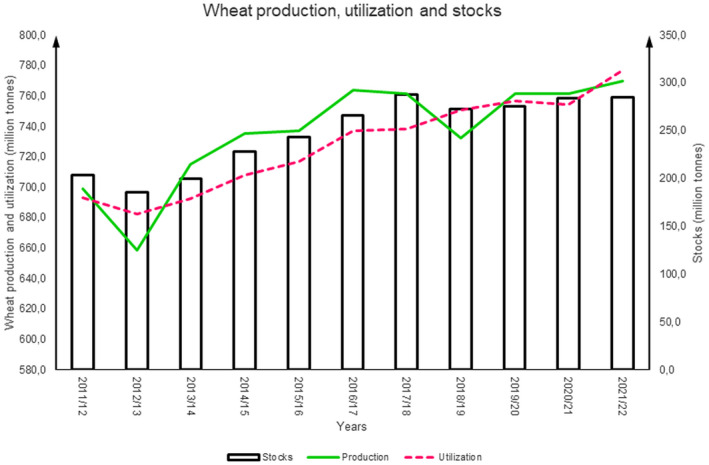
Evolution of wheat production (green line), utilisation (red dashed line) and stocks (black bars) between 2011 and 2021 in the world (in million tons). The data come from the site http://www.fao.org/worldfoodsituation/csdb/en/.
Bread wheat is an allohexaploid species (AABBDD; 2n = 6x = 42) derived from two successive interspecific crosses that involved three related diploid species [for review and details, see The International Wheat Genome Sequence Consortium (Marcussen et al., 2014; IWGSC, 2014, 2018; https://www.wheatgenome.org/)]. The first one occurred about 3‐0.8 million years ago (MYA) and took place between T. urartu (AA genome) and a yet‐unknown species related to the Sitopsis section (Ae. speltoides, Ae. longissima, Ae. sharonensis, Ae. searsii and Ae. bicornis; SS genome related to wheat BB genome). This natural cross gave rise to tetraploid species (T. diccocoides) that further evolved to give T. turgidum, the ancestor of current durum wheat. The second cross arose ~0.4 MYA and involved this newly created tetraploid species and Aegilops tauschii (DD genome) leading to hexaploid bread wheat. This second polyploidisation event occurred when tetraploid wheat started to spread with the migration of the first farmers, just at the time they reached the south of the Caspian Sea, a region that has a rich diversity for Ae. tauschii. Hexaploid wheat started to be widely cultivated 7–10 000 years ago (Balfourier et al., 2019; Preece et al., 2017; Zohary and Hopf, 2000; Zohary et al., 2012), especially because the D‐genome brought adaptation to diverse climates as well as endosperm softness giving a better bread‐making quality (Chantret et al., 2005). Subsequently, wheat evolved with human migration while it was grown by the first farmers in Western Europe and Eastern Asia. This species adapted over the years to the environment of these areas and spread around the world during the 16th century (Balfourier et al., 2019).
The key event for bread wheat domestication is the occurrence of natural mutations leading to favourable characteristics for grain and spike traits compared with wild species. Three loci are essential for that: the brittle rachis locus (Br) and two additional loci, Tg (Tenacious glumes) and Q, causing naked grains (Faris et al., 2003; Jantasuriyarat et al., 2004; Nalam et al., 2006). Moreover, the Q‐locus simultaneously controls the shape of the spike [‘spelt/square‐head’ (Faris et al., 2003)]. These mutants were certainly more attractive to the farmers as their non‐threshing spikes are easier to harvest and the naked‐soft grains easier to grind. Domestication, cultivation and now breeding resulted in current elite cultivars that share only a small fraction of the natural diversity. During the processes of domestication, wheat has undergone important genetic bottlenecks, resulting in a very narrow genetic base, especially when considering the D‐genome. More recently, modern breeding has also participated to reduce the genetic variability especially in winter elite germplasm (Feuillet et al., 2008; Figure 2a). For example, at the beginning of the 20th century in the United States, only five varieties were grown and one (Turkey) covered almost the total acreage (Cox et al., 1986). Therefore, a high strategic priority for wheat improvement worldwide is to enrich the cultivated varieties by incorporating favourable alleles, genes or gene complexes. This can be achieved by introgressing new diversity from the diverse gene pools related to wheat.
Figure 2.
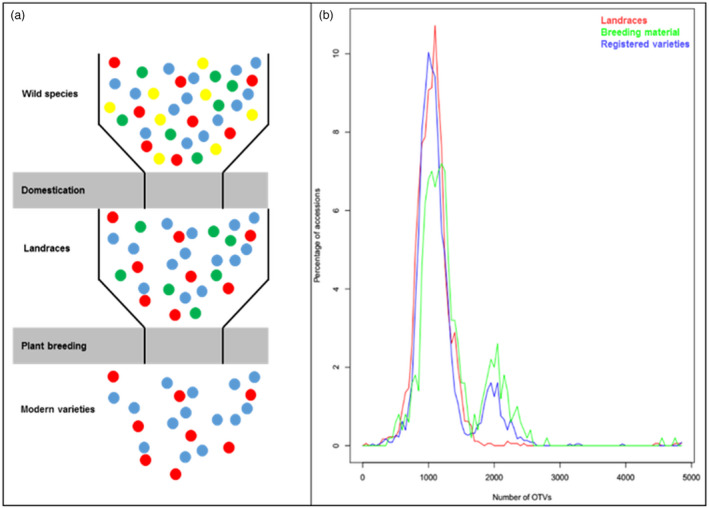
The wheat genetic diversity. (a) Schematic representation of the loss of natural genetic diversity through domestication and breeding in wheat. (b) Introduction of new genetic diversity through alien introgressions during wheat breeding. Percentage of wheat landraces (red), breeding material (green) and registered varieties (blue) as a function of the number of structural variations (OTVs: off‐target variants) observed with a 420K SNP array. The peaks centred on 2000 OTVs illustrate the creation of a new type of diversity based on alien introgressions.
Introgression is defined as the transfer of more or less large portions of alien genomes into a cultivated species (Rieseberg and Wendel, 1993). Introgressions played a major role in the growth of genetic diversity (Wendel et al., 1989), adaptation to novel environmental conditions (Rieseberg et al., 2003), formation of new ecotypes or species (Rieseberg, 1997) or evolution of invasive species (Ellstrand and Schierenbeck, 2000). The use of wild relatives in crop improvement traces back to the early 1940s and gained in prominence during the 1970s and 1980s [for a review, see (Hajjar and Hodgkin, 2007)]. For example, the wild diploid species Aegilops umbellulata (UU) was used to introduce resistance to leaf rust in wheat through X‐irradiation of a T. aestivum addition line (AABBDD + one Ae. umbellulata chromosome; Sears, 1956). This gave 40 translocation lines among which one showed normal pollen transmission and was resistant to leaf rust. The resistant gene was named Lr9, and it remains an essential and efficient gene for resistance to leaf rust (Nocente et al., 2006).
Since the beginning of the 1970s, many other significant successes have been obtained in wheat by the introduction of dwarfing genes (Rht‐B1 and Rht‐D1) derived from Japanese varieties (Norin 10) (Borojevic and Borojevic, 2005) and disease and pest resistances coming from rye (Secale cereale), Agropyron, Aegilops or Thynopyrum species (Muñoz‐Sanz et al., 2020). Numerous Poaceae species have already been successfully crossed with wheat to introgress traits of agronomical importance (for review, see Table 1). Thus, while the natural genetic diversity in wheat elite material is significantly lower than the one observed in landraces, breeding programmes have brought a new type of diversity to wheat cultivars, namely structural variations related to alien introgressions (Figure 2b). To date, novel alleles have been introgressed from more than 50 species from 13 genera, highlighting the importance of these so‐called alien introgressions for wheat breeding (Wulff and Moscou, 2014). The best‐known one is the rye (S. cereale), 1RS translocation that harbours genes involved in multiple disease resistance (Pm8/Sr31/Lr26/Yr9) and yield enhancement. Other examples of introgressions include Sr36/Pm6 from T. timopheevii, Lr28 from Ae. Speltoides, and Pch1 and Sr38/Lr37/Yr17 from Ae. ventricosa. Some of these introgressions have been widely used around the world in commercial lines, e.g. the 1RS.1BL translocation that can be found in 10% of the worldwide genetic wheat diversity (Balfourier et al., 2019; Rabinovich, 1998).
Table 1.
Species from the Poaceae family, which were successfully crossed with hexaploid wheat
| Species | 2n | References |
|---|---|---|
| Aegilops biuncialis | 48 | Knobloch (1968) |
| Aegilops caudata | 14 | Knobloch (1968) |
| Aegilops columnaris | 28 | Kimber and Abubakar (1979) |
| Aegilops comosa | 14 | Kimber and Abubakar (1979) |
| Aegilops crassa | 28 | Jovkova et al. (1977) |
| Aegilops cylindrica | 28 | Kimber and Abubakar (1979) |
| Aegilops dichasians = Triticum dichasians? | 14 | Kimber and Abubakar (1979) |
| Aegilops juvenalis | 42 | Kimber and Abubakar (1979) |
| Aegilops kotschyi | 28 | Knobloch (1968) |
| Aegilops longissima | 14 | Kimber and Abubakar (1979) |
| Aegilops mutica | 14 | Knobloch (1968) |
| Aegilops ovata | 28 | Kimber and Abubakar (1979) |
| Aegilops speltoides | 14 | Chueca et al. (1977) |
| Aegilops squarrosa | 14 | Kimber and Abubakar (1979) |
| Aegilops triaristata | 42 | Kimber and Abubakar (1979) |
| Aegilops tripsaccoides | – | Kimber and Abubakar (1979) |
| Aegilops triuncialis | 28 | Kimber and Abubakar (1979) |
| Aegilops umbellulata | 14 | Kimber and Abubakar (1979) |
| Aegilops variabilis | 28 | Knobloch (1968) |
| Aegilops ventricosa | 28 | Kimber and Abubakar (1979) |
| Agropyron ciliare | 28 | Sharma and Gill (1981a) |
| Agropyron cristatum | 28 | Chen et al. (1989) |
| Agropyron cristatum (L.) | 14 | Limin and Fowler (1990) |
| Agropyron desertorum | 28 | Limin and Fowler (1990) |
| Agropyron desertorum (Fisch. ex Link) Schult. | 28 | Chen et al. (1990); Limin and Fowler (1990) |
| Agropyron distichum | 28 | Pienaar (1981) |
| Agropyron elongatum | 14 | Franke et al. (1992) |
| Agropyron intermedium | 42 | Sharma and Gill (1983) |
| Agropyron michnoi Roshev. | 28 | Chen et al. (1990); Li and Dong (1991) |
| Agropyron podperae | – | Dewey (1981) |
| Agropyron scirpeum | – | Sharma and Gill (1981b) |
| Agropyron trachycaulum | 28 | Sharma and Gill (1981b) |
| Elymus altissimus | 28 | Lu and von Bothmer (1991) |
| Elymus anthosachnoides | 28 | Lu and von Bothmer (1991) |
| Elymus canadensis | 28 | Mujeeb‐Kazi and Bernard (1982, 1985); Yen and Liu (1987) |
| Elymus caninus | 28 | Claesson et al. (1990); Sharma and Baenziger (1986) |
| Elymus caucasicus | 28 | Lu and von Bothmer (1991) |
| Elymus dahuricus | 42 | Mujeeb‐Kazi and Bernard (1982); Yen and Liu (1987) |
| Elymus dolichaterus | 28 | Lu and von Bothmer (1991) |
| Elymus fibrosus | 28 | Mujeeb‐Kazi and Bernard (1982) |
| Elymus giganteus | 28 | Mujeeb‐Kazi and Rodriguez (1981) |
| Elymus parviglumis | 28 | Lu and von Bothmer (1991) |
| Elymus pendulinus | 28 | Lu and von Bothmer (1991) |
| Elymus pseudonutans | 28 | Lu and von Bothmer (1991) |
| Elymus rectisetus | 42 | Liu et al. (1994) |
| Elymus scabrus | 42 | Ahmad and Comeau (1991) |
| Elymus semicostatus | 28 | Lu and von Bothmer (1991) |
| Elymus shandongensis | 28 | Lu and von Bothmer (1991) |
| Elymus tibeticus | 28 | Lu and von Bothmer (1991) |
| Elymus tibeticus | 28 | Lu and von Bothmer (1991) |
| Elymus tshimganicus | 42 | Lu and von Bothmer (1991) |
| Elytrigia acatum | 42 | Mujeeb‐Kazi et al. (1984, 1987) |
| Elytrigia campestre | 56 | Mujeeb‐Kazi et al. (1989) |
| Elytrigia pungens | 56 | Mujeeb‐Kazi et al. (1984, 1989) |
| Elytrigia repens | 42 | Comeau et al. (1985); Mujeeb‐Kazi et al. (1984, 1989) |
| Elytrigia varnese | 42 | Mujeeb‐Kazi et al. (1984, 1987) |
| Haynaldia villosa | 14 | Knobloch (1968) |
| Hordeum bulbosum | 28 | Falk and Kasha (1981) |
| Hordeum bulbosum | 14 | Falk and Kasha (1981) |
| Hordeum californicum | 14 | Gupta and Fedak (1985) |
| Hordeum chilense | 14 | Martin and Chapman (1977) |
| Hordeum claifornicum | 14 | Gupta and Fedak (1985) |
| Hordeum depressum | 28 | Jiang and Liu (1987) |
| Hordeum geniculatum | 28 | Pershina et al. (1988) |
| Hordeum jubatum | 28 | Comeau et al. (1988) |
| Hordeum marinum | 14 | Jiang and Liu (1987) |
| Hordeum pusillum | 14 | Finch and Bennett (1980) |
| Hordeum spontaneum | 14 | Bates et al. (1976) |
| Hordeum vulgare | 14 | Kruse (1976) |
| Leymus angustus | 56 | Plourde et al. (1992) |
| Leymus angustus | 84 | Comeau et al. (1985) |
| Leymus cinereus | 28 | Mujeeb‐Kazi et al. (1984, 1989) |
| Leymus innovatus | 28 | Plourde et al. (1989a) |
| Leymus multicaulis | 28 | Plourde et al. (1989b) |
| Leymus triticoides | 28 | Mujeeb‐Kazi et al. (1984, 1989) |
| Psathyrostachys juncea | 14 | Plourde et al. (1990) |
| Pseudoroegneria geniculata subsp. scythica | 28 | Mujeeb‐Kazi et al. (1984, 1987) |
| Pseudoroegneria stipifolia | 28 | Mujeeb‐Kazi et al. (1984, 1987) |
| Pseudoroegneria strigosa | 28 | Mujeeb‐Kazi et al. (1987) |
| Secale africanum | 14 | Knobloch (1968) |
| Secale ancestrale | 14 | Knobloch (1968) |
| Secale cereale | 14 | Backhouse (1916); Knobloch (1968) |
| Secale montanum | 14 | Knobloch (1968) |
| Secale vavilovii | 14 | Knobloch (1968) |
| Thinopyron curvifolium | 28 | Mujeeb‐Kazi et al. (1984, 1987) |
| Thinopyron gentryi | 42 | Mujeeb‐Kazi et al. (1984, 1987) |
| Thinopyron junceiforme | 28 | Mujeeb‐Kazi et al. (1984, 1989) |
| Thinopyron junceum | 42 | Charpentier et al. (1986); Mujeeb‐Kazi et al. (1984, 1989) |
| Thinopyron sartorii | 28 | Mujeeb‐Kazi et al. (1984, 1987) |
| Thinopyrum bessarabicum | 14 | Sharma and Gill (1983) |
| Thinopyrum ponticum | 70 | Smith (1942) |
| Thinopyrum pulcherrimum | 42 | Mujeeb‐Kazi et al. (1989) |
| Thinopyrum rechingeri(Th. sartori) | 28 | Mujeeb‐Kazi et al. (1987) |
Genetic gene pools to improve wheat diversity
The success rate of gene transfer from wild relatives to cultivated wheat varieties largely depends on relatedness between the species involved. There are three gene pools that have been defined according to this latter point (Table 2; Feuillet et al., 2008). The primary gene pool includes bread wheat itself and closely related species sharing completely homologous genomes with wheat and comprising landraces (including primitives T. spelta, T. macha, T. vavilovi, T. compactum and T. sphaerococcum), tetraploid cultivated (T. turgidum ssp durum AABB) or wild derivatives (T. diccocoides and T. dicoccum) as well as diploid species such as T. monococcum (AA), T. boeoticum (AA), T. urartu (AA) and Ae. tauschii (DD). Genes from this group can easily be transferred to bread wheat through direct hybridization, recombination between homologous chromosomes and selection of the best progenies (Gill et al., 1991; Gill and Raupp, 1987). No cytogenetic manipulation is necessary except embryo rescue in some extreme cases. This gene pool has been widely used, especially the Ae. tauschii accessions, to improve diversity of the D‐genome through the production of synthetic wheats derived from the cross between Ae. tauschii and tetraploid wheats (Fritz et al., 1995; Jiang et al., 1993; McFadden and Sears, 1946; Reader and Miller, 1991). However and despite the synteny of the genomes, chromosomal rearrangements and linkage drag can limit introgression due to the inhibition of recombination, e.g. chromosome 4A of bread wheat, which cannot pair with any of the A‐diploid wheat chromosomes [for a review see (Qi et al., 2007)].
Table 2.
Triticum aestivum genetics gene pools groups
| Species | Ploidy | Genome | |
|---|---|---|---|
| Gene pools | Triticum aestivum | hexaploid | AABBDD |
| Primary gene pool | Aegilops tauschii | diploid | DD |
| Hordeum spontaneum | diploid | HH | |
| Secale vavilovii | diploid | RR | |
| Secale montanum | diploid | RR | |
| Triticum turgidum | tetraploid | AABB | |
| Triticum diccocoides | tetraploid | AABB | |
| Triticum dicoccum | tetraploid | AABB | |
| Triticum monococcum | diploid | AA | |
| Triticum boeoticum | diploid | AA | |
| Triticum urartu | diploid | AA | |
| Triticum spelta | hexaploid | AABBDD | |
| Secondary gene pool | Aegilops ventricosa | tetraploid | DDNN |
| Aegilops speltoides | diploid | SS | |
| Secale sylvestre | diploid | RR | |
| Triticum timopheevii | tetraploid | AAGG | |
| Tertiary gene pool | Agropyron | diploid | PP |
| Hordeum bulbosum | diploid | HH | |
| Hordeum bogdanii | diploid | HH | |
| Thinopyrum | diploid | EE | |
| Secale | diploid | RR |
The secondary gene pool encompasses polyploid species that share at least one homologous genome with the cultivated types (e.g. T. timopheevii, AAGG; Ae. ventricosa, DDNN), as well as Aegilops species of the Sitopsis section (related to the B‐genome donor, e.g. Ae. speltoides¸ SS). In all these cases, pairing between related chromosomes remains possible when the genome is homologous to one of those from wheat (A, B and D), but it is difficult in the other cases, therefore reducing the transfer of alien genes. This requires the use of cytogenetic approaches (ph1 mutation; see further) or irradiation techniques.
Species from the Triticeae tribe that contain genomes other than A, B and D constitute the tertiary gene pool {e.g. genera Secale, RR; Hordeum [including H. bogdanii and H. bulbosum (XX)], HH; Agropyron, PP; Thinopyrum, EE; (Friebe et al., 1996; Harlan and Wet, 1971; Jiang and Gill, 1994)}. Most of these species are perennial but are essential for wheat improvement. In this case, the gene transfer is not possible through classical homologous recombination. However, since they are related (thus called homoeologous), gene transfer is achieved through cytogenetic or irradiation approaches or after in vitro culture. One of the most famous introgression from this tertiary group is the 1RS/1BL translocation where the short arm of wheat chromosome 1B (1BS) is replaced by the short arm of rye chromosome 1R (1RS). There are only four sources corresponding to this translocation (Zarco‐Hernandez et al., 2005; Zhao et al., 2012): two developed in Germany by Salzmunder and Wiehenstephan between 1920 and 1930, one developed in Japan in the 1960s and one developed in the United States in the 1970s. Translocation harbours genes involved in multiple disease resistance (powdery mildew, stem rust, leaf rust, yellow rust, respectively, Pm8/Sr31/Lr26/Yr9) and yield enhancement with a better adaptation and abiotic stress tolerance, a high leaf area and higher grain weight (Moreno‐Sevilla et al., 1995; Zarco‐Hernandez et al., 2005).
Barriers that limit exploitation of wild species in wheat breeding
Exploitation of these three groups relies on three main features: the ability to make the cross between the related species and wheat, the germination capacity and fertility of hybrids and the capability of the homologous/homoeologous chromosomes to recombine properly with those of wheat. Usually, hybrids derived from crosses between species with different ploidy levels are poorly fertile because of imbalanced chromosome number in the F1 individuals, which affects pollen development and subsequent fertilisation (Kihara, 2013). This is due to an increased complexity of the meiotic process where chromosomes search in vain for their partners and remain as univalents or on the contrary form irregular bivalents (or even multivalents) between similar (homoeologous) chromosomes generating unbalanced gametes (Lilienfeld, 1951). Fertilisation with such abnormal gametes thus mainly results in frequent aneuploid descents or new homoeologous‐recombinant chromosomes. For example, fully sterile pentaploid hybrids derived from the cross between T. aestivum and other tetraploid species have been reported (Bhagyalakshmi et al., 2008; Padmanaban et al., 2017). A cross between T. timopheevii (AAGG) and T. aestivum is possible, but the fertility rate is affected (Bhagyalakshmi et al., 2008). Fertility is best achieved when the species with the highest ploidy level is used as female. However, and most of the time, enough seeds are recovered from the backcross between the hybrids and wheat to introduce alien genome fragments and to start the selection process (Padmanaban et al., 2017). For example, crosses between hexaploid Triticum aestivum and tetraploid Triticum turgidum allowed improvement of disease resistance, abiotic tolerance, grain quality and resistance to metal toxicity in the pentaploid hybrids (Han et al., 2016; Lopes and Reynolds, 2010; Padmanaban et al., 2017).
The most problematic process is the capacity of homoeologous chromosomes to recombine with each other. As an allopolyploid species, bread wheat possesses two genetic systems that control recombination: (1) one promoting the strict distribution of cross‐overs (COs) between homologous chromosomes; (2) the second preventing recombination between the homoeologous chromosomes. In allopolyploids, the latter hampers the incorporation of beneficial alleles into crop plants from their wild relatives (Able and Langridge, 2006). It has been known for ~60 years that homoeologous recombination in bread wheat is under the control of a major locus named Ph1 (for pairing homoeologous 1) located on the long arm of chromosome 5B (Riley and Chapman, 1958; Riley et al., 1959). This locus was cloned (Griffiths et al., 2006) and deciphered, and the authors demonstrated that the chromosome 5B copy of ZIP4 (TaZIP4‐B2) suppresses homoeologous COs in wheat‐wild relative hybrids (Rey et al., 2017). ph1 mutation has been largely used to introduce new diversity in wheat [Figure 3; for review see (King et al., 2017)]. In addition to Ph1, the other well‐known locus is Ph2, a gene located on the short arm of chromosome 3D (3DS; Mello‐Sampayo, 1971; Mello‐Sampayo and Canas, 1973; Mello‐Sampayo and Lorente, 1968)). This gene was recently cloned and shown as being TaMSH7‐3D, a protein involved in mismatch repair (Serra et al., 2021). Current evidence suggests that Ph2 does not work in the same way as Ph1. For example, absence of Ph2 leads to a reduction or a delay in synapsis (i.e. intimate connection of chromosome axes along their lengths via the synaptonemal complex), whereas most nuclei complete synapsis in ph1 mutants of wheat (Martinez et al., 2001).
Figure 3.
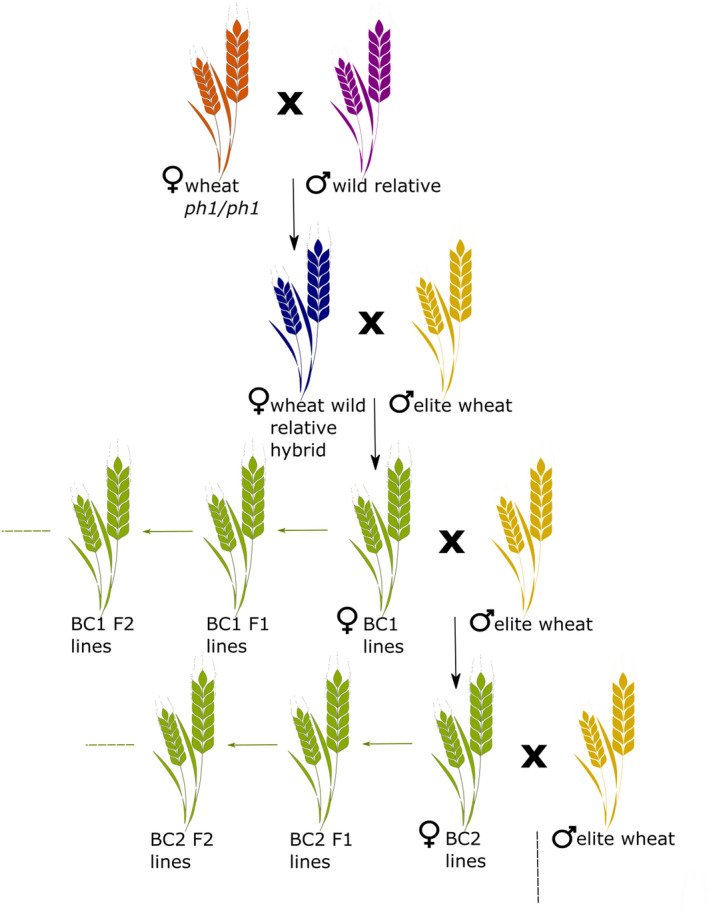
Overview of the approach using ph1 mutant to introgress relevant DNA fragments from wild species in the wheat genome of elite lines. A primary cross is made between the ph1 mutant and the wild species to generate a hybrid. This plant is grown until flowering and homoeologous recombination can occur during meiosis leading to introgressions. This plant is then crossed with elite lines to introduce the alien fragment in their genome. Elite background is recovered either through successive backcrosses (BC; black arrow) or self‐fertilisation (BC F; green arrow). Adapted from Baker et al. (2020).
Most importantly, despite their usefulness to bring new favourable alleles or genes, introgressions suffer from linkage drag, i.e. the reduction in fitness in a cultivar due to deleterious genes introduced along with the beneficial gene (Klindworth et al., 2013). Additionally, the amount of alien chromatin present in the wheat genome is often unacceptable to breeders. Linkage drag arises as an effect of recombination suppression at the introgressed locus (Wulff and Moscou, 2014; Figure 4). Such reduction in recombination was described for tomato (Brouwer and St Clair, 2004; Paterson et al., 1990), barley (Johnston et al., 2013) and lettuce (Den Boer et al., 2013) especially when the introgressed parent species is distantly related to the recurrent parent. Moreover, suppression of recombination becomes stronger as the size of the introgressed segments becomes smaller (Brouwer and St Clair, 2004; Canady et al., 2006; Johnston et al., 2013). Similar results were observed in wheat. One of the best examples is the strong reduction of recombination between the Ae. ventricosa chromosome‐7Dv segment carrying the Pch1 gene conferring eyespot resistance and wheat chromosome 7D (Worland et al., 1988). Reducing the size of the Pch1 introgression through recombination would be of utmost interest since a significant reduction of yield and thousand‐kernel weight is sometimes observed in the absence of the disease (Koen et al., 2002). To overcome this problem, chromosome engineering approaches have been applied to increase chromosomal fragmentation (Endo, 2007; Fedak, 2011, 2011; Feuillet et al., 2008; Qi et al., 2007), but they have not been successful for this fragment to date.
Figure 4.
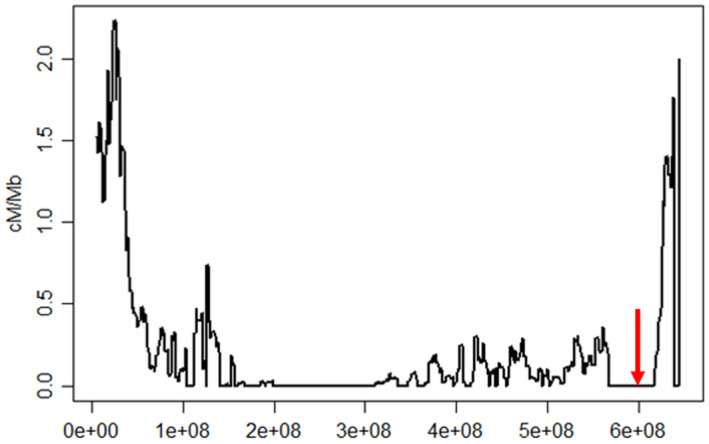
Distribution of recombination events along chromosome 2D in the Chinese Spring x Renan RIL population. The lack of recombination in the distal part of the long arm (red arrow) is due to a ~40‐Mb introgression in the Renan genome likely originating from Aegilops ventricosa.
Interestingly, genes suppressing the repressor effect of Ph1 and, on the contrary, promoting homoeologous chromosome pairing have been reported in several wheat relatives (Li et al., 2017). This suggests that when these species are used as donor, homoeologous recombination is no longer a problem. In 1974, Kimber described a range of variation in Aegilops, which he divided into groups of low, intermediate, high and super‐high pairing (Fernández‐Calvín and Orellana, 1994; Kimber, 1974). When the level of pairing of the Aegilops accession is low, the Ph1 locus is only slightly inactivated and only a few rod bivalents were found in the hybrids between Aegilops and wheat (Fernandez‐Calvin and Orellana, 1992). On the contrary, if the level of pairing of the Aegilops accession is super‐high, the Ph1 locus is strongly inactivated and even some hexavalents were observed in hybrid plants (Fernandez‐Calvin and Orellana, 1992). The most studied loci are Su‐Ph1, derived from Ae. speltoides (Dvorak et al., 2006; Li et al., 2017), and chromosome 5Mg of Ae. geniculata Roth (Koo et al., 2017, 2020); neither of these have been cloned. There are two different loci for Su‐Ph1, which map to Ae. speltoides chromosome arms 3SL (Su1‐Ph1) and 7SL (Su2‐Ph1) (Dvorak et al., 2006). Su1‐Ph1 was successfully introduced into the hexaploid cultivar Chinese Spring and from there into the tetraploid durum wheat cv. Langdon. The Ae. speltoides fragment from chromosome 3S replaced the distal end of the long arm of chromosome 3A in both species (Li et al., 2017). Despite the homoeologous location of TaZip4‐1, the paralogue of TaZip4‐B2 corresponding to Ph1 on chromosome 5B (Rey et al., 2017), the authors suggest that TaZip4‐1 does not correspond to Su1‐Ph1 (Li et al., 2017). Regarding Ae. geniculata, a potent homoeologous pairing promotor locus (Hpp) was identified on chromosome 5Mg, and this chromosome recombined with chromosomes from homoeologous group 5 (5A, 5B or 5D) in the presence of the Ph1 gene, even in proximal chromosome regions where recombination is usually suppressed (Koo et al., 2017). They developed a line that was homozygous for ph1b and heterozygous for 5Mg and an introgression from Thinopyrum intermedium (RobT T7BS.7S#3L; (Danilova et al., 2017)) carrying the wheat streak mosaic virus resistance gene, Wsm3. Homoeologous recombination frequency was increased about 100‐fold compared with the ph1b‐mutant alone suggesting that such a material could help the generation of pre‐breeding materials thereby accelerating wheat crop improvement.
Only about 10% of the wheat diversity has been used for wheat improvement yet. Introgressions from wild relatives have a huge impact all over the world, both in terms of performance and economics, especially with regards to occurrence of diseases and tolerance to climate changes (Rather et al., 2017; Redden et al., 2015; Tadesse et al., 2019).
The last impediment to exploit wild diversity in breeding is the ability to achieve crosses between the species. The barriers preventing interspecific hybridizations play a critical role in the development of new allopolyploids since F1 hybrids can be produced and are occasionally at least partially fertile through hybrid genome doubling. Chromosome doubling in wheat breeding is commonly used as it allows to reach 100% homozygosity at all loci in a single generation. This is commonly done using colchicine treatment after either androgenesis (anther culture and microspore culture) or embryo culture using wheat‐maize wide hybridization (Devaux, 2021). There are two ways to prevent interspecific hybridizations: pre‐zygotic (before fertilisation) and post‐zygotic barriers (after fertilisation). Pre‐zygotic barriers include gamete isolation, a process that occurs after pollen grains fall on stigmas but before fecundation of the ovule. In plants, gamete isolation may result in either self‐incompatibility or competition between con‐ and hetero‐specific pollen for fertilisation, which prevents interspecific hybridization (Heslop‐Harrison and Heslop‐Harrison, 1982). In this review, we present an overview on gamete isolation, especially crossing ability (or crossability, a term that will be used throughout this manuscript) in plants with a special focus on cereals and on the wheat/rye crossability.
Intraspecific self‐incompatibility in plants
Crossability can be defined as the capacity to cross two individuals from the same species or from different species, subspecies, genders etc. to generate embryos or grains that are able to produce F1 plants. These hybrids will be fertile or not according to the genetic pools they are derived from. On the contrary, when a genetic barrier occurs at the fecundation level, the resulting absence of formation of endosperm and/or lethality of the embryo corresponds to crossing inability or non‐crossability.
To achieve fertilisation, a pollen grain must adhere and hydrate on a suitable pistil, germinate and form a pollen tube, which can penetrate the pistil’s transmitting tract. The transmitting tract provides the physiological environment to support pollen tube growth and contains chemotropic substances for tube guidance towards the ovary [for a detailed review on pollen–pistil interaction see (Hiscock and Allen, 2008)]. Upon arrival at the ovary, the two haploid sperm cells are released from the pollen tube to fuse with the egg and central cell for double fertilisation. Thus, in pollen–pistil interactions numerous proteins are required for successful seed formation in cell–cell communication and signalling, as well as for nutritional support of pollen tube growth [reviewed by (Higashiyama and Yang, 2017; Johnson et al., 2019)].
Despite the fact that intraspecific crossability does not directly relates with interspecific crossability, there are some elements suggesting that both could be governed by the same mechanisms (Heslop‐Harrison, 1982, 1982). There can be unilateral incompatibility between taxonomically closely related species. For example, wheat is self‐compatible while rye is self‐incompatible. When rye is pollinated with wheat, the wheat pollen is rejected while, on the contrary, when wheat is pollinated with rye, the pollen is accepted by the wheat pistil and fecundation may sometimes occur (see below). Several mechanisms controlling self‐incompatibility have been described in dicots that may serve as bases to understand crossability in Triticeae.
Control of intraspecific self‐incompatibility
Self‐incompatibility (SI) is a genetically controlled mechanism in angiosperms that prevents self‐fertilisation and promotes outcrossing. SI mechanisms act through inhibition of pollen germination directly on the stigma or pollen tube growth in the style. In many angiosperms, SI is controlled by a single multiallelic locus, the S‐locus. However, system with multiple loci controlling SI have also been identified. Interestingly, SI systems arose several times independently during the evolution of the angiosperms [for a review see (Charlesworth et al., 2005)].
SI is either gametophytically controlled, i.e. the genotype of the pollen grain determines the recognition specificity, or sporophytically, where the paternal genotype of the pollen determines the compatibility phenotype. This is described more in detail below:
Gametophytic self‐incompatibility (GSI)
GSI is widespread throughout the plant kingdom and has been described in diverse families, e.g. Campanulaceae, Fabaceae, Leguminosae, Onagraceae, Papaveraceae, Plantaginaceae, Poaceae, Ranunculaceae, Rosaceae, Scrophulariaceae and Solanaceae (Brewbaker, 1957; Franklin et al., 1985; Igic and Kohn, 2001) [for an exhaustive review, see (McClure and Franklin‐Tong, 2006)]. In a diploid plant with GSI, half of the haploid pollen grains will carry one allele and the other half the other allele of its parent. For example, an S1S2 plant will give rise to 50% S1 and 50% S2 pollen (Figure 5). A self‐incompatible reaction occurs when the S allele of the pollen grain matches one of the alleles of the diploid pistil; hence, this pollen cannot fertilise the corresponding ovule.
Figure 5.
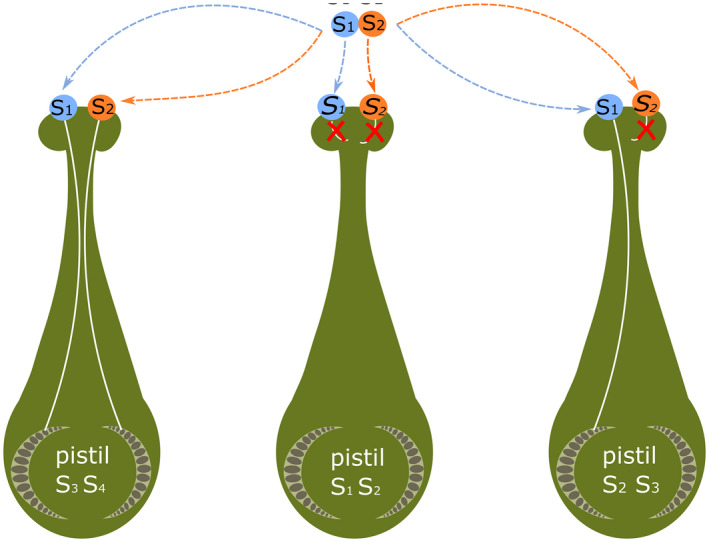
Genetic control of gametophytic SI (GSI). In GSI, the pollen SI phenotype is gametophytically controlled. Thus, half the pollen from an S1S2 plant is phenotypically S1 and the other half is S2. Pollen inhibition occurs on a ‘like‐matches‐like’ basis. When there is a match between the pollen S‐haplotype and either of two haplotypes present in the pistil, an incompatible reaction results and inhibition of that ‘self’ pollen occurs. This results in three classes of reaction: incompatible (all pollen is inhibited), half‐compatible (50% inhibited) or compatible (pollens not inhibited). Adapted from McClure and Franklin‐Tong (2006).
In single locus GSI systems, three levels of compatibility are observed in a cross: fully‐incompatibility (100% of the pollen is inhibited), half‐compatibility (50% of pollen grains grow normally and fertilise the ovules) or full‐compatibility (100% of pollen grains develop normally) (McClure and Franklin‐Tong, 2006) (Figure 5). Two molecular mechanisms for GSI have been described at the molecular level: S‐RNAse and the SI system of Papaver rhoes.
S‐RNAse—S‐locus F‐box gene system
In the S‐RNAse system, which is present in the Plantaginaceae, Rosaceae, Rubiaceae, Rutaceae and Solanaceae, pollen tube growth of incompatible grains is arrested in the transmitting tissue of the style. Molecular investigation over many years resulted in the identification of linked genes at the S‐locus, a polymorphic pistil‐expressed ribonuclease (S‐RNAse) that controls the female specificity (Lee et al., 1994; Murfett et al., 1994) and an S‐locus F‐box gene (SLF or SFB) that controls the pollen specificity [see (Tao and Iezzoni, 2010) for review]. While these genes constitute the sufficient set for a self‐incompatibility reaction in Prunus (Entani et al., 2003; Ushijima et al., 2004) and Antirrhinum (Lai et al., 2002; Qiao et al., 2004), further studies in the Solanaceae and the Maleae subtribe of the Rosaceae showed that rather than having just one SLF/SFB gene, a whole suite of these F‐box genes [S‐locus F‐Box Brothers, SFBB, reviewed by (Sassa, 2016)] are linked to the S‐locus and contribute to the SI interaction. The pistil‐secreted S‐RNAse es are taken up by the growing pollen tube where they exert a cytotoxic effect in case of an incompatible reaction. Kubo and collaborators (Kubo et al., 2010) proposed that the SI reaction is based on a collaborative non‐self‐recognition system in which each SFB/B protein acts as a component of the SCF (Skp1‐Cullin1‐F‐box)‐type E3 ubiquitin ligase complex to mediate the ubiquitination and degradation of a subset of non‐self‐S‐RNases. Self‐RNase fails to be recognised and is hence not degraded (Kubo et al., 2015). This model is supported by gain‐of‐function experiments, and the investigation of CRISPR/Cas9 generated frameshift mutants in Petunia inflata (Hua et al., 2007; Sun et al., 2018). On the other hand, studies of SI in Prunus including results from SFB‐knockout mutants indicate a different mode of action. The proposed model surmises that the non‐self‐S‐RNases that are taken up by the pollen tube are detoxified by a general inhibitor while the SFB protects self‐ S‐RNAse e from degradation. Thereby, the self‐RNase stays active and causes pollen tube arrest (Matsumoto and Tao, 2016a). Possible candidates for the general inhibitor are the S‐locus F‐box‐like genes, since it has been shown that these proteins recognise S‐RNAses and co‐immunoprecipitate (Chen et al., 2018; Matsumoto and Tao, 2016b). We expect that, in the future, these models will be extended to accommodate the various other proteins required in the SI reaction either on the pollen or pistil side, such as the HT‐B protein (McClure et al., 1999) and the 120‐kDa glycoprotein in Solanaceae pistil (Hancock et al., 2005) and the M‐locus glutathione S‐transferase (MGST) (Ono et al., 2018) in Prunus avium pollen.
SI system of Papaver rhoes
In poppy, the site of pollen tube inhibition in an incompatible reaction occurs at the surface of the stigma and is very rapid upon contact or just after germination of the pollen (Matsumoto and Tao, 2016a). SI in poppy is a ‘one‐to‐one’ self‐recognition system. Pollen tube growth arrest is the consequence of the pistil S‐determinant (PrsS), a ~ 14 kDa secreted protein, interacting with the pollen S‐determinant (PrpS), a ~ 20 kDa transmembrane protein, inducing a Ca2+‐dependant signalling cascade (Wheeler et al., 2009). Upon the change of intracellular Ca2+ concentration, several activities take place (Figure 6): (1) Ca2+‐dependent phosphorylation of a 26‐kDa cytosolic protein (Pr‐p26) thus reducing its pyrophosphatase activity, (2) depolymerisation of actin filaments and reorganisation of the microtubule cytoskeleton. Furthermore, the p56 mitogen‐activated protein kinase (p56‐MAPK) is activated, which induces programmed cell death (PCD) of the pollen cell as evidenced by cytochrome c (Cyt c) leakage, activation of caspase‐like activities and, eventually, DNA fragmentation (Eaves et al., 2014; McClure and Franklin‐Tong, 2006).
Figure 6.
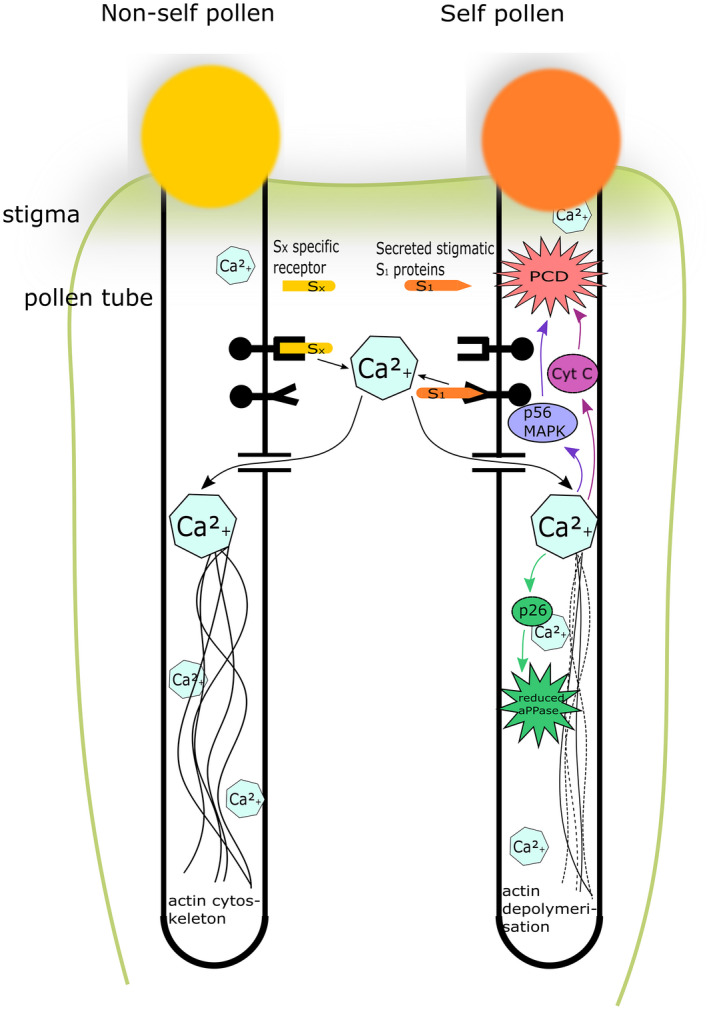
Model of the cellular mechanisms involved in gametophytic self‐incompatibility (GSI) in poppy (Papaver rhoeas). In an incompatible reaction, the pistil S1 protein binds to the pollen S1 receptor triggering an intracellular change in calcium concentration. This causes the rapid modification of two targets: Pr‐p26 shows an increase in phosphorylation leading to inhibition of its sPPase activity, and the actin cytoskeleton is reorganised and depolymerized. Both are predicted to cause rapid arrest of tip growth. Following this growth arrest, p56‐MAPK is activated and emits a signal to the PCD. PCD is linked to cytochrome C caspase activity and DNA fragmentation. This ensure that incompatible pollen do no start growing again. Adapted from McClure and Franklin‐Tong (2006).
Sporophytic self‐incompatibility (SSI)
Sporophytic self‐incompatiblity (SSI) was characterised in Brassicaceae (the family in which the molecular components have been identified; for review, see Doucet et al., 2016; Leducq et al., 2013) but was also identified in Asteraceae, Betulaceae, Caryophyllaceae, Convolvulaceae, Polemoniaceae and Sterculiaceae. In SSI, both male and female cells produce two components, and the SI phenotype of both stigma and pollen is determined by the diploid genotype of the parent plant. As a consequence, sporophytic systems are characterised by the occurrence of dominant‐recessive and co‐dominance effects within pollen and stigma. In some cases, dominance may exist between pairs of alleles, which complicates compatibility/incompatibility relationships. However, this dominance effect results sometimes in the production of double recessive alleles. Contrary to a population for which, all S‐alleles would be co‐dominant, dominance increases the probability of having compatible individuals (Dickinson et al., 2003). Frequency between recessive and dominant S‐alleles reflects the dynamic balance between reproduction (favoured by recessive alleles) and self‐pollination prevention [favoured by dominant alleles; (Ockendon, 1974)].
A pollen grain is incompatible when the dominant allele(s) of the pollen parent matches the dominant allele(s) in the recipient pistil. In the well‐studied SI system of the Brassicaceae, pollen rejection occurs shortly after contact with the stigma, either by blocking hydration of the pollen, or by pollen tube arrest preventing penetration into the stigma. Three tightly linked genes are located at the S‐locus (Figure 7): (1) the S‐locus cysteine‐rich protein/S‐locus protein 11 (SCR/SP11), a small cysteine‐rich protein, which is the male determinant and expressed in the anther tapetum from which it is deposited into the pollen exine (Schopfer and Nasrallah, 2000; Takayama et al., 2000); (2) the S‐receptor kinase (SRK) (Takasaki et al., 2000), a transmembrane protein kinase, which constitutes the female determinant; (3) the S‐locus glycoprotein (SLG), an abundant protein in the stigmatic papillae, which is highly similar to the extracellular domain of the SRK. SLG appears not to be essential for the SI response but may have an accessory role [reviewed in (Kemp and Doughty, 2003)]. In the current model (Figure 7), the pollen SCR/SP11 protein binds to the extracellular domain of SRK leading to autophosphorylation of its intracellular kinase domain (Kachroo et al., 2001; Takayama et al., 2001)). SRK was shown to interact with the M‐locus protein kinase (MLPK), a plasma membrane‐localised serine/threonine kinase (Murase et al., 2004) and the Armadillo repeat‐containing protein 1 (ARC1) with E3 ubiquitin ligase activity, which is specifically expressed in stigmas. The phosphorylated ARC1 in turn ubiquitinates EXO70A1 and glyoxylase 1 (GLO1) thereby leading to their proteasomal degradation. EXO70A1 is part of the exocyst complex, which facilitates transport of vesicles to the plasma membrane. It has been proposed that degradation of this subunit may lead to disruption of vesicular secretion, which is necessary for compatible pollen hydration and tube penetration [reviewed in (Goring, 2018)]. GLO1 degradation could result in an increase in methylglyoxal levels in the stigma, which would have a cytotoxic effect (Jany et al., 2019).
Figure 7.
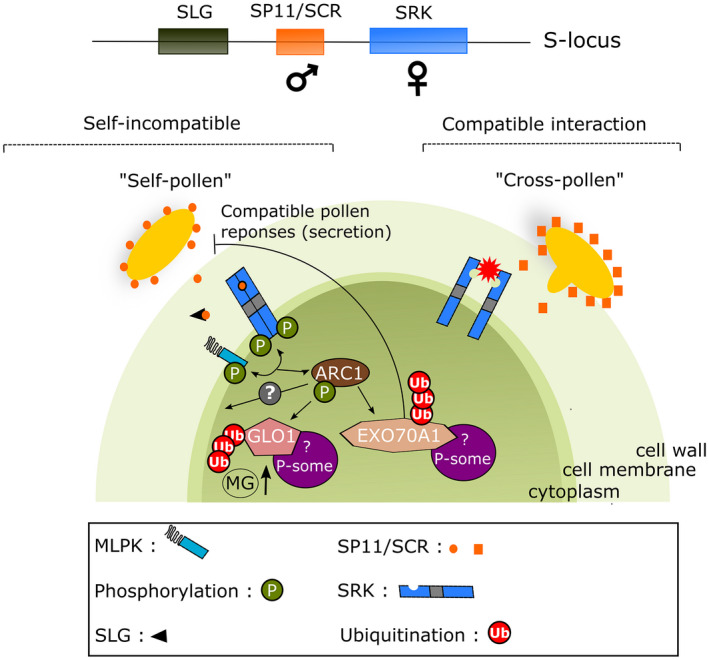
Molecular model of the self‐incompatibility (SI) response in the Brassicaceae. The S‐locus consists of three genes, SRK, SP11 and SLG. The SRK receptor kinase (in blue) is the female factor and covers the plasma membrane of the stigma papilla cell. SP11 (in red) is the male component that is mainly expressed in the anther tapetum where it accumulates in the outer layer of the pollen cell wall during maturation. The pollen SP11/SCR ligand binds to stigma SRK, leading to auto‐phosphorylations of SRK as well as phosphorylation and activation of ARC1. ARC1 ubiquitinates EXO70A1 and GL01. The ubiquitination of EXO70A1 blocks further hydration of the incompatible pollen, whereas the proteasomal degradation of GLO1 is thought to lead to an increased level of the cytotoxic methylglyoxal (MG). Adapted from (Jany et al., 2019; Takayama and Isogai (2005)).
Self‐incompatibility in Poaceae
SI is widespread among the grasses with self‐incompatibile and self‐fertile species often present in the same tribe [Table 3; (Baumann et al., 2000; Connor, 1979; Do Canto et al., 2016)]. Perennial grasses tend to show a higher frequency of SI than that of annuals (Beddows, 1931). Studies in rye (Secale cereale; (Lundqvist, 1954) and in Phalaris coerulescens (Hayman, 1956) have shown that in the Poaceae, SI is gametophytically mediated by two unlinked and multiallelic loci, S and Z. A pollen grain will be incompatible when both its S and Z alleles are present in the pistil (Figure 8). The degree of compatibility ranges from 0%, 50%, 75% or 100%, depending on the genotypes of pollen and stigma, and reciprocal crosses can show different degrees of compatibility. For example, a cross between genotypes S1S2Z1Z2 as the female recipient and S1S1Z1Z2 as the pollen donor is incompatible (pollen genotypes: either S1Z1 or S1Z2 ), whereas the reciprocal cross pollen is 50% compatible (Yang et al., 2008). Pollen tube growth is typically arrested shortly after pollen tube emergence (Shivanna et al., 1982) with Cynodon dactylon being a notable exception where tube growth is inhibited within the style (Thomas and Murray, 1975).
Table 3.
Poaceae subfamilies and tribes with example of self‐compatibility (SC) and self‐incompatibility (SI) species. Clade BOP represent Bambusoideae, Oryzoideae, Pooideae families and clade PACMAD represent Panicoideae, Aristidoideae, Chloridoideae, Micrairoideae and Danthonioideae families. No information for the following subfamilies Aristidoideae, Micrairoideae and tribes Olyreae, Aristideae, Micraireae, Eriachneae and Hubbardieae [extracted from (Baumann et al., 2000; Chen et al., 2017; Connor, 1979; Crain et al., 2020; Do Canto et al., 2016; Lian et al., 2021)]
| Clade | Subfamily | Tribe | Species | SI |
|---|---|---|---|---|
| BOP | Bambusoideae | Arundinarieae | Arundinaria simonii | Yes |
| Bambuseae | Dendrocalamus sinicus | No | ||
| Oryzoideae | Oryzeae | Oryza barthii | Yes | |
| Oryza longistaminata | Yes | |||
| Pooideae | Poeae | Alopecurus myosuroides | Yes | |
| Alopecurus pratensis | Yes | |||
| Anthoxanthum odoratum | Yes | |||
| Arrhenatherum elatius | Yes | |||
| Avena barbata | No | |||
| Briza australis | Yes | |||
| Briza elatior | Yes | |||
| Briza media | Yes | |||
| Briza minor | No | |||
| Bromus inermis | Yes | |||
| Bromus tectorum | No | |||
| Cynosurus cristatus | Yes | |||
| Dactylis aschersoniana | Yes | |||
| Deschampsia flexuosa | Yes | |||
| Festuca pratensis | Yes | |||
| Festuca rubra | Yes | |||
| Holcus lanatus | Yes | |||
| Lolium multiflorum | Yes | |||
| Lolium perenne | Yes | |||
| Lolium rigidum | No | |||
| Lolium temulentum | No | |||
| Phalaris arundinacea | Yes | |||
| Phalaris coerulescens | Yes | |||
| Triticeae | Hordeum bulbosum | Yes | ||
| Hordeum vulgare | No | |||
| Secale cereale | Yes | |||
| Thinopyrum intermedium | Yes | |||
| Triticum aestivum | No | |||
| PACMAD | Panicoideae | Andropogoneae | Miscanthus sinensis | Yes |
| Sorghastrum nutans | Yes | |||
| Themeda australis | No | |||
| Zea mays | No | |||
| Paniceae | Panicum virgatum | Yes | ||
| Chloridoideae | Cynodonteae | Chloris gayana | Yes | |
| Chloris striate | No | |||
| Cynodon dactylon | Yes | |||
| Oryza sativa | No | |||
| Arundinoideae | Molinieae | Molinia caerulea | Yes | |
| Danthonioideae | Danthonieae | Danthonia linkii | No |
Figure 8.
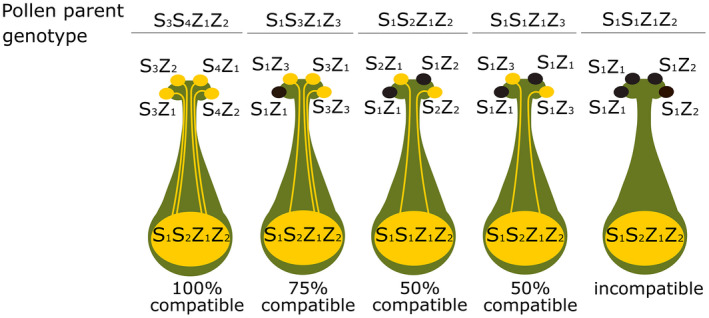
Genetic control of gametophytic self‐incompatibility (GSI) by two multiple‐allelic loci S and Z. When both pollen S and Z alleles are matched in the pistil, incompatibility occurs and pollen growth is inhibited. Adapted from Yang et al. (2008).
In contrast to single locus GSI and SSI in diploids, neither does the two locus system of grasses break down in autotetraploid plants nor is there any dominance or competitive interactions between the alleles of diploid pollen or tetraploid styles (Fearon et al., 1984a, 1984b; Lundqvist, 1957, 2009).
Genes linked to either S or Z will show disturbed segregation in partially compatible crosses (Leach, 1988); hence, distorted segregation analysis provides a means to locate the SI loci in the genome. Initial studies with isozymes demonstrated linkage of the S‐locus to phosphoglycoisomerase (PGI‐2) and a leaf peroxidase (Prx‐7) located on chromosome 1R in Secale cereale (Wricke and Wehling, 1985), and chromosome 6 in Lolium perenne (Cornish et al., 1980). The Z‐locus co‐segregated with the beta‐glucosidase and esterase 4/11 isozymes located on chromosome 2R in rye (Fuong et al., 1993; Gertz and Wricke, 1989). These and further early studies (Leach and Hayman, 1987) suggested that the SI system is conserved across the Poaceae. Detailed molecular mapping experiments carried out in Secale cereale (Hackauf and Wehling, 2005), Phalaris coerulescens (Bian et al., 2004), Hordeum bulbosum (Kakeda et al., 2008) and Lolium perenne (Shinozuka et al., 2010; Yang et al., 2009) confirmed the syntenic chromosomal locations of S and Z [for details see (Klaas et al., 2011)] and thus support a monophyletic origin.
In spite of various attempts to clone the genes over the last decades, the molecular nature of S and Z still remains elusive. However, several promising candidates have been identified. For example, the TC116908 gene, which shows similarity to ubiquitin‐specific proteases, has been put forward as a possible candidate for Z in rye (Hackauf and Wehling, 2005). Based on their map‐based cloning experiment in Lolium perenne, Shinozuka et al. (2010) suggested LpTC116908, the ortholog of the rye gene, and LpDUF247, the male and female determinants. Candidate genes for S were proposed by (Kakeda, 2009) from linkage analyses in Hordeum bulbosum, and more recently by (Manzanares et al., 2016) from their fine‐mapping study in Lolium perenne. Their study provides strong evidence for the LpSDUF247 gene being the pollen S‐determinant, including allelic polymorphism, and mutation or deletion of the gene in self‐compatible species. The fact that both the S and Z candidates contain the same protein domain (DUF247) of unknown function warrants further investigation into the molecular role and evolutionary origin of this domain. Recently, this same team fine mapped a QTL for self‐compatibility (SC) on chromosome 5 of L. perenne (Cropano et al., 2021). They reduced the region to a 3‐Mb segment containing 57 genes among which, seven were relevant candidates.
Interspecific crossability in wheat
Obtaining F1 hybrids after crossing wheat with a related species is a prerequisite to the transfer of alien genes. One of the first study regarding crossability between wheat and wheat relatives was published about a century ago (Backhouse, 1916) and showed that crossability between wheat and rye was a recessive trait. These results were further confirmed (Leighty and Sando, 1928; Meister and Tjumjakoff, 1928; Riley and Chapman, 1967; Taylor and Quisenberry, 1935).
Crossability has mainly been studied with rye. Crosses are achieved when wheat is used as female while the reciprocal cross is almost impossible (Jalani and Moss, 1980). The success rate of hybridization depends on the wheat genotype’s ability used to perform the crosses. Wheats were therefore classified into three classes depending on their crossability (Tozu, 1966): high (>47%), medium (17‐20%) or low (<10%) crossability. (Lange and Wojciechowska, 1976) compared with the crossability rate of 177 wheat varieties originating from diverse countries in the world. They showed that crossable varieties (>25% crossability) came from Argentina, Brazil, China, Iran, Japan, and Yugoslavia and that those with crossability ranging from 20% to 25% came from Mexico and India. Another study evaluating 1400 wheat cultivars exhibited similar results with most of the crossable lines coming from China, Japan, Iran and Siberia and suggesting that crossable lines mainly originate from Asia (Zeven, 1987).
To study whether environmental conditions (light and temperature) could affect crossability, Bertin et al. (2009) used progeny derived from the cross between cv. Hobbit‐sib, a semi‐dwarf winter wheat that is not crossable with rye and has a translocated karyotype with 5BL‐7BL and 5BS‐7BS chromosomes and its nearly‐isogenic line, Hobbit‐sib (CS‐5BL, 7BL), that has a normal karyotype with the 5B and 7B chromosomes that have a short arm from Hobbit‐sib and a long arm from Chinese Spring (Miura et al., 1992). Among the progeny, they selected three crossable and seven non‐crossable lines to produce additional recombinant lines that they assessed during four growing seasons (two winters and two summers). They saw that crossability was higher for some lines in warmer field environments (range temperature: 9–19 °C; mean 14 °C) with large amount of light (150 h and 215 h of sunshine in summer 2007 and 2008 respectively) while their crossability dropped dramatically to a few per cent in winter conditions (range temperature 2–10 °C; mean 6–7 °C; 55–56 h of sunshine), with seed set possibly as low as 3% even for the normally crossable variety Chinese Spring becoming not crossable. However, some lines remained consistently crossable whatever the conditions suggesting that they could be useful for breeders willing to enlarge wheat diversity using related species usually poorly crossable with wheat such as rye (Bertin et al., 2009).
Similarly, the effect of temperature on seed production in hybrids derived from crosses between wheat and diploid cultivated barley Hordeum vulgare or tetraploid wild‐barley Hordeum bulbosum was also studied. Such crosses were initially performed to produce haploid wheat plants (Barclay, 1975). For example, four different temperatures (12, 15, 18 and 21 °C) were evaluated in reciprocal crosses involving the wheat variety Chinese Spring and two spring barley varieties (Betzes, Martonvasari 50; Molnár‐Láng and Sutka, 1994). Like for rye, best results (3.26% of seeds) were observed at high temperature (21 °C) when wheat was used as the female parent, but this was only true for the Chinese Spring x Betzes combination while only 1–2 seeds (~0.1%) were obtained when Chinese Spring was crossed with Martonvasari 50 independent of the temperature. Interestingly, results were opposite in the reciprocal crosses, and the highest amount of seeds (2.36%–4.88%) was observed at 12–15 °C for both barley varieties. This therefore confirmed that temperature plays a major role for interspecific hybrid production and also that the wheat genotype is important.
Physiology of non‐crossability in wheat
Several studies have been conducted to tentatively identify the mode of action of the dominant inhibitor genes of wheat/rye or wheat/H. bulbosum crossability. Results show that the rye (or wild barley) pollen grains germinate on the wheat stigma and that differences between crossable and non‐crossable lines appear later, during pollen growth and just before fecundation (Figure 9). Significant differences are neither observed in pollen grain germination speed (Jalani and Moss, 1980; Lange and Wojciechowska, 1976; Tozu, 1966; Zeven and van Heemert, 1970), nor in the mean number of germinated pollen grains (Jalani and Moss, 1980; Lange and Wojciechowska, 1976) between the wheat × wheat controls and wheat × rye crosses.
Figure 9.
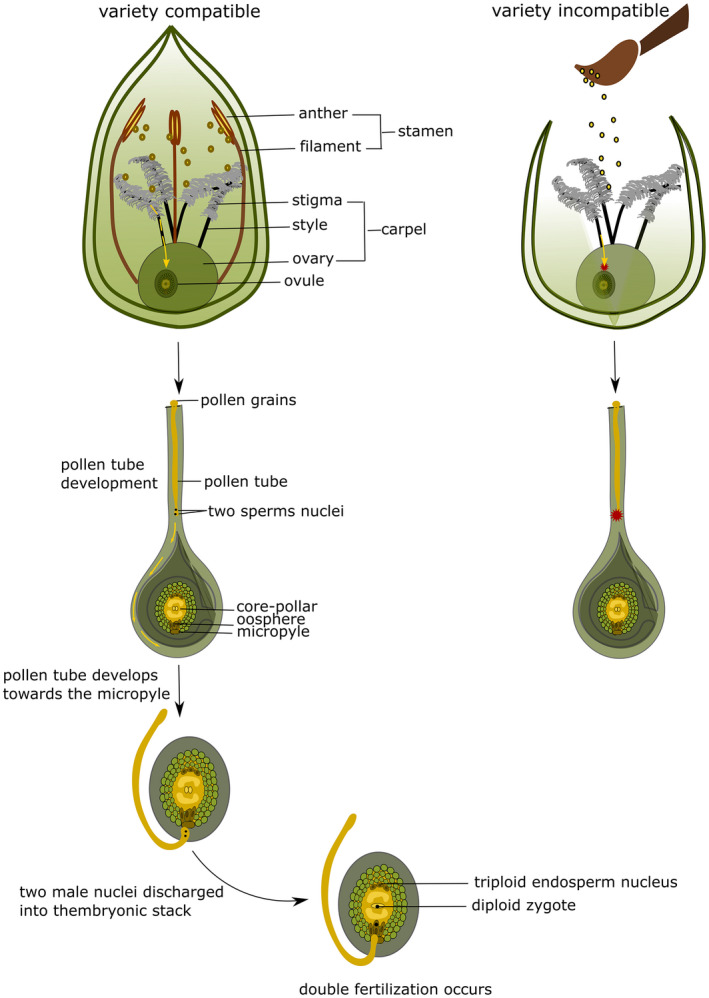
Schematic representation of pollen tube development and fertilisation in wheat, after selfing and after manual cross‐pollination with pollen from an incompatible species. Prior to cross‐pollination, the recipient plant needs to be emasculated.
Jalani and Moss (1980) also observed that the poorly crossable Chinese Spring/Hope 5B substitution line (a line where the pair of chromosomes 5B of the variety Chinese Spring is substituted by the homologous chromosomes from the variety Hope) showed more geminated rye‐pollen grains compared with Chinese Spring itself, which is highly crossable. This confirms that inhibition of crossability does not occur at the pollen grain germination stage. No difference in pollen tube growth speeds between wheat and rye‐pollen grains was observed; development of rye‐pollen tubes appeared even a bit faster.
Jalani and Moss (1980) also compared the number of pollen tubes growing from the stigma to the micropyle, 30 min., 45 min., 1, 2, 5, 6 and 12 h after pollination. Results showed no significant differences in the style and at the base of the style in wheat × wheat controls and in the wheat x rye crosses. However, significant differences depending on the genotypes were observed concerning the time necessary for the pollen tubes to reach the maximum number at the top or in the middle of the embryo sac as well as at the micropyle. The maximum was achieved 1 h after pollination in the control but needed between 2 and 6 h in the wheat × rye crosses. Jalani and Moss (1980) showed a high correlation (r = 0.97, P > 0.01) between the number of micropyles with pollen tubes and the number of grains formed indicating that the difference of crossability between genotypes (i.e. mode of action of inhibitor genes) is probably located at this level. Therefore, when pollen tubes are growing and start to penetrate the style, the development rate of pollen tubes differs between crossable and non‐crossable lines, and the slower pollen tubes never reach the base of the style (Jalani and Moss, 1980; Lange and Wojciechowska, 1976). We can thus conclude that the lack of fecundation is probably the major reason explaining the low number of grains obtained in non‐crossable varieties.
Genetics of wheat/rye crossability
Genetics of gamete isolation in wheat is a well‐documented mechanism especially with regards to the wheat/rye pollination (Matsuoka et al., 2014). In the beginning of the 20th century, Backhouse (1916) noticed that most wheat varieties did not produce seeds when crossed with rye, except Chinese landraces that gave exceptionally high level of viable F1 seeds. During the last five decades, several genes and QTLs involved in wheat/rye crossability have been identified. These genes are distinguished by their effect on inhibition of fecundation, which is usually studied after pollination with regards to endosperm development and number of grains obtained (Krolow, 1970). None of the genes involved in wheat/rye crossability have been isolated so far, which prevents a finer molecular analysis of their mode of action, mechanisms, expression, regulation and partners.
Kr genes (Kr1, Kr2 and Kr3)
First crossability genes (named ‘Kr’ for ‘Kreuzbarkeit’, the German word for ‘crossability’) were evidenced by Lein (1943) who identified two genes, Kr1 and Kr2, involved in crossability between wheat and rye. Lein suggested that the variety ‘Chinese 466’ carried the recessive alleles at these two genes and was therefore kr1kr1kr2kr2 and highly crossable. On the contrary, other lines that were either Kr1Kr1kr2kr2 or Kr1Kr1Kr2Kr2 were only poorly or not crossable respectively. Lein finally suggested that Kr1 had a stronger effect compared with Kr2 regarding wheat/rye hybrid grain production. Mapping of these two genes was achieved more than 20 years later (Riley and Chapman, 1967) using a set of substitution lines developed in the highly crossable variety Chinese Spring in which each pair of homoeologous chromosomes was replace with the homologous one from the variety Hope, a non‐crossable variety. They showed that the lines where chromosomes 5A and 5B from Chinese Spring were substituted by those of Hope presented crossability rates of 26.2% and 6.4% respectively, while Chinese Spring and the other substitution lines exhibited crossability rates rising to 74.3%. It was concluded that Kr1 locates on chromosome 5B and Kr2 on chromosome 5A. Hope has a dominant allele for Kr1 which acts as inhibitor of wheat/rye crossability while Chinese Spring carries recessive alleles (kr1kr2) that rather favour wheat/rye crossability (Riley and Chapman, 1967). A more resolute analysis using ditelosomic lines (lines missing one chromosome arm at a homozygous state) located Kr1 and Kr2 on the long arms of chromosomes 5B and 5A respectively (Lange and Riley, 1973). This study as well as others also confirmed that the effect of Kr1 was stronger than the one of Kr2 (Deng‐Cai et al., 1999; Krolow, 1970; Tixier et al., 1998; Zheng, 1992). A third gene, Kr3, was located in a homoeologous position on chromosome 5D, but this gene had a much lower effect on wheat/rye crossability compared to that of Kr1 and Kr2 (Bertin et al., 2009; Krolow, 1970; Mishina et al., 2009; Tixier et al., 1998). It was hypothesised that the reproduction barrier caused by Kr genes prevents interspecific crossing by inhibiting pollen tube growth therefore blocking pollen from fertilising ovary (Bertin et al., 2009; Romero and Cuadrado, 1992).
The dominant alleles of Kr1 (5.5% of heritability) and Kr2 genes are the sources of the limited crossability between wheat and rye and wheat and wild barley (Mishina et al., 2009; Riley and Chapman, 1967; Tixier et al., 1998). Different levels of crossability were displayed (Lein, 1943; Tixier et al., 1998). These levels were established in wheat‐rye cross: crossability ≤5% means the two Kr genes have dominant alleles (Kr1Kr1/Kr2Kr2; Tixier et al., 1998), crossability reached 10%–30% and 30%–50% the genotypes corresponds to Kr1Kr1/kr2kr2 and kr1kr1/Kr2Kr2 (Riley and Chapman, 1967; Tixier et al., 1998) and to the crossability ≥50%, the two genes must have recessive alleles [kr1kr1/kr2kr2; (Lein, 1943; Romero and Cuadrado, 1992; Tixier et al., 1998)]. The suppression of wheat‐rye crossability by Kr genes is not completely dominant, and some seeds (or no seeds for the crossable lines) may always be obtained (or not) independent of the variety used and the environmental conditions.
Genetic complexity of the crossability trait was confirmed by Bertin et al. (2009) who elaborated an approach to fine‐map Kr1 gene on chromosome arm 5BL. They used three crossable and eight non‐crossable lines, selected from the 71 recombinant substitution lines derived from a cross between Hobbit‐sib and its nearly‐isogenic line, Hobbit‐sib (CS‐5BL,7BL) (Miura et al., 1992). The aim of this cross is to develop additional F1 heterozygotes with segmental recombination on 5BL that were further evaluated for their crossability together with the parental lines. They used a set of 31 markers to molecularly characterise these lines as well as some descendants originating from their crosses and they combined these data with crossability phenotypes. They revealed that two different regions locating on the long arm of chromosome 5B and including Kr1, could be involved in crossability in this cross (Bertin et al., 2009). Moreover, they obtained lines combining these two favourable regions, which exhibited up to 50% of crossability suggesting that they could be useful for breeders to introduce new diversity from alien wheat relatives.
Several additional studies have revealed that the mechanisms governing crossability between wheat and wild barley was probably the same as the one controlling crossability between wheat and rye (Snape et al., 1979). Evaluation of crosses between wheat and Hordeum bulbosum wild barley, using the same set of Chinese Spring/Hope substitution lines (Riley and Chapman, 1967) showed that chromosomes 5B (Kr1), 5A (Kr2) and 5D (Kr3) had the strongest effect with regards to wheat/H. bulbosum crossability, but the crossability rates were lower in this latter case compared to what was achieved with crosses between wheat and rye. The strongest effect observed with substitution of chromosome 5B suggests that both mechanisms are controlled by the same genes. This result was confirmed later with a finer mapping of Kr1 and Kr2 on the long arms of chromosomes 5B and 5A respectively (Falk and Kasha, 1983; Fedak and Jui, 2011; Sitch et al., 1985). Finally, the tiny effect of Kr3 on chromosome 5D (Krolow, 1970) was confirmed, but this gene weakly affects either wheat/rye or wheat/H. bulbosum crossability (Falk and Kasha, 1983; Snape et al., 1979; Zheng, 1992).
Other genes that may affect wheat/rye crossability
Several studies have identified additional loci affecting wheat/rye crossability. It was found that homoeologous chromosomes from group 3 could carry factors affecting crossability between wheat cv. Chinese Spring and H. bulbosum (Miller et al., 1983). It was further confirmed that chromosomes 3D and 3B had the major effect (Romero and Cuadrado, 1992). Similarly, Zheng (1992) identified Kr4 on chromosome 1A. This gene had a stronger effect than Kr2 but a lower effect compared to Kr1 (Deng‐Cai et al., 1999; Luo et al., 1993; Zheng, 1992). Finally, a QTL was located on the long arm of chromosome 7A using a doubled‐haploid (DH) population derived from the cross between the highly crossable variety Chinese Spring and the non‐crossable French variety Courtot (Lamoureux et al., 2002; Tixier et al., 1998). Interestingly, this QTL was found to have a stronger effect than Kr1 in this population.
SKr gene
Initial studies working on mapping of crossability genes used either substitution lines (Riley and Chapman, 1967) or aneuploid stocks missing chromosome arms or even entire chromosomes (Lange and Riley, 1973; Snape et al., 1979). The advent of molecular markers in the early 1990s has allowed the development of high‐density genetic maps that permitted the application of QTL approaches to decipher more precisely the genetic control of wheat/rye crossability. However, to solve the problem of the low polymorphism rate found in wheat, genetic maps were developed using crosses involving synthetic wheats that were not suitable for mapping crossability genes.
The first intervarietal wheat genetic map was obtained from the cross between Chinese Spring and Courtot (CsCt; Cadalen et al., 1997). Fortunately, this cross‐involved two lines that showed opposite behaviour regarding wheat/rye crossability, Chinese Spring being highly crossable with rye (95% of success) while Courtot rarely gives seeds (0‐10%) when crossed with this species. The DH progeny (187 individuals; Felix et al., 1996; Cadalen et al., 1997) derived from this cross was thus suitable for QTL detection for this trait. A QTL analysis was conducted using this segregating population (Tixier et al., 1998). Unexpectedly, the major QTL was detected on the short arm of chromosome 5B, close to the RFLP marker Xfba367‐5B. This locus represented ~17% of the variability of the trait and was named ‘SKr’ (for Suppressor of Kr). This relatively low value was explained either by a large phenotypic variance, mostly due to environmental effects, or by the fact that Xfba367‐5B, although the most distal marker on chromosome 5B, might not be very close to the SKr gene. As expected, the crossable allele was brought by Chinese Spring. Two additional loci were located on chromosome 7A, close to Xtam51‐7A, and on chromosome 5B long arm, close to marker Xwg583‐5B. These two loci explained respectively 5.9% and 3.3% of the additive value. The locus on the long arm of chromosome 5B probably corresponds to Kr1, but interestingly, it had a lower effect than the locus on chromosome 7A. Altogether, these three loci explained 65% of the difference in crossability between the two parents (Tixier et al., 1998). These results were confirmed using a different population derived from the cross between Chinese Spring and a chromosome substitution line of Chinese Spring, which has its chromosome 5B replaced by that of Cheyenne [low crossability; (Mishina et al., 2009)].
Fine mapping of the SKr locus was conducted on the same CsCt population, and marker density was improved using microsatellites as well as AFLP markers (Lamoureux et al., 2002; Sourdille et al., 2003). The closest marker to SKr was found to be an AFLP fragment, E36M49‐287, which was further cloned, sequenced and named DL103. To use the syntenic relationships between wheat and rice to develop additional markers, this clone was mapped on the rice genetic map and locates on short arm of chromosome 11 of rice. However, this approach was unsuccessful, probably because of complexity of the synteny within this region in wheat that is syntenic with segments from chromosomes 5, 6, 11 and 12 of rice separated by undetermined regions (Lamoureux et al., 2002).
To go towards the positional cloning of SKr, a new segregating population was developed (Alfares et al., 2009). The highly crossable CsCt DH line MP98 that carried Chinese Spring allele at the SKr locus was backcrossed with Ct followed by six generations of selfing to generate a SSD population of 618 individuals referred as MP98‐Ct. Collinearity with rice and barley was exploited to develop additional markers. Among the 12 barley ESTs that exhibited a 5B‐specific band, only two were polymorphic between the two parents and were mapped and derived into PCR markers for high‐throughput genotyping assays. Despite the low success rate, this allowed to improve the genetic map as well as to better see the relationships between wheat and barley. Interestingly, the SKr locus co‐segregates with the GSP locus involved in grain softness protein (Chantret et al., 2005), and 14 5B‐specific new markers were developed from this sequence among which, SSR Cfb306 co‐segregated with SKr. A physical map was then generated by anchoring the linked markers to BAC clones from Chinese Spring (Allouis et al., 2003). Positive clones were sequenced representing two contigs of ~300 kb and ~120 kb. This allowed the development of only one additional SSR (Cfb341) that also co‐segregated with SKr confirming the complete linkage of this sequence with the crossability locus. The cfb306 and cfb341 SSR markers are efficient tools for introducing crossability alleles of SKr into breeding programmes (Alfares et al., 2009). These markers can improve the genetic diversity of different species when crossing or producing Triticales (Alfares et al., 2009).
Only five genes were annotated on the collinear rice region (Figure 10), but the sequencing was not complete in wheat, with no certainty that the two contigs actually flank the SKr locus and without any obvious candidate (Alfares et al., 2009). These five genes are determined thanks to markers, one of them located in a gene showing homology to the pentatricopeptide gene Os12g44170 in rice (Alfares et al., 2009). Other markers brought to light the presence of other genes, plasma membrane ATPase1 and N‐acetylglucosaminyltransferase (Alfares et al., 2009). Markers can be useful for further improvement of the physical map as well as for marker‐assisted breeding for wheat/rye crossability.
Figure 10.
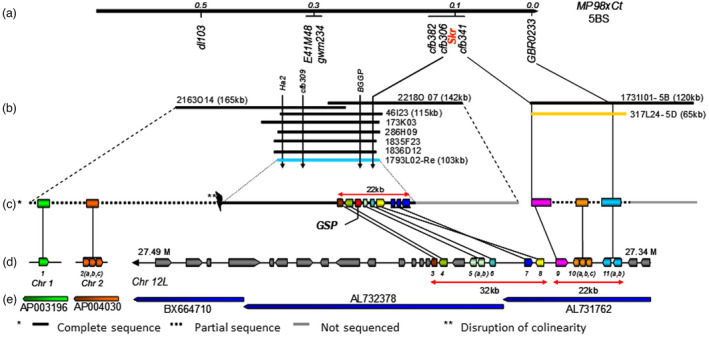
Extended genetic and physical maps at the SKr locus and syntenic relationships with rice. (a) Genetic map of the SKr locus on wheat chromosome arm 5BS. (b) Physical map at the SKr locus on wheat chromosome 5BS. (c) Detailed representation of the BAC clones identified at the 5B homoeologous GSP [1793L02 in blue, (Chantret et al. (2005), Chantret et al. (2008))] and SKr loci. The gene order on partially sequenced BAC 317L24 (in orange) corresponds to the order established on the genetic map for cfb341 and GBR0233. GSP‐1 (grain softness protein). (d) Collinearity with genes located on rice chromosomes 1, 2, and 12L. (e) Rice BAC clones associated with the different wheat orthologous genes on chromosome 5BS. The 11 rice genes on chromosomes 1, 2or 12 are annotated as follows: (1) Os01g14180: Expressed protein; (2 a, b, c) Os02g13990: U2 small nuclear ribonucleoprotein A (U2A); (3) Os12g44250: vesicle‐associated membrane protein; (4) OS12g44240: N‐acetylglucosaminyltransferase; (5 a, b) Os12g44220: ATPase; (6) Os12g44210: ATPase, AAA family domain‐containing protein; (7) Os12g44190: ATPase 3; (8) Os12g44180: nodulin; (9) Os12g44170: pentatricopeptide; (10 a, b, c) Os12g44160: oxidoreductase; and (11 a, b) Os12g44150: plasma membrane ATPase. Grey: other genes present on rice chromosome 12. Adapted from Alfares et al. (2009).
Improving crossability of cultivated wheat varieties
One of the most remarkable realisations derived from wheat/rye hybridization is Triticale (× Triticosecale Wittmack), which is the first man‐made interspecific hybrid species. The initial aim was to combine quality of wheat (especially productivity and bread‐making quality) with the robustness of rye that can grow on less favourable lands. The first haploid wheat/rye hybrid was generated in Scotland at the end of the 19th century [Wilson, 1873, cited by (Leighty, 1916)]. However, the first fertile hybrid was generated 60 years later only, and the production has increased since then with the discovery of colchicine that allows chromosome doubling of haploid hybrids [for a review, see (Oettler, 2005)]. Breeding and production of Triticale started in the 1960s in Poland, and in the 1980s in the rest of Europe. In 2016, world Triticale production has risen to over 20 million tons, half of that coming from Germany and Poland combined showing that Triticale is an important cereal in the European Union and worldwide (Skowrońska et al., 2020). Indeed, Triticale is of agronomic interest for livestock feed production, industrial energy crop and pathogen resistance (Ellis et al., 2014; Sisodia and McGinnis, 1970; Skowrońska et al., 2020). It may be a promising alternative to wheat especially in poor‐land regions (Sisodia and McGinnis, 1970; Skowrońska et al., 2020).
Interestingly, wheat/rye amphiploid hybrids never appeared naturally while hybrid seeds resulting from crosses between hexaploid wheat and rye usually germinate freely and form vigorous and aggressive F1s (Riley and Chapman, 1967). This is because chromosomal stocks remained haploid leading to sterile gametes after meiosis. However, diploid gametes may rarely occur leading to natural polyploid species. This was the case for the creation of tetraploid and hexaploid wheat, but in this case, the diploid and tetraploid species lived in sympatry in the same environment. This was not the same for wheat and rye. Contrary to wheat, rye was a southwest Asian Neolithic crop that became later than wheat, a cultivated plant, and not necessarily in the Fertile Crescent. Rye is an integral part of the ‘secondary crops’, which first evolved as weeds in cultivated habitats (since the origins of agricultural) and was only later established as crops (Preece et al., 2017; Weiss et al., 2012). There are thus no genetic resources for Triticale, and genetic diversity can only be increased by doing new crosses involving different varieties of wheat and rye (Friebe et al., 1996; Jiang and Gill, 1994; Schneider et al., 2008).
Since only a few Asian varieties can easily be crossed with rye (Zeven, 1987), one way to face this challenge is to introduce crossability genes in wheat elite varieties. This can be achieved using maker‐assisted selection (MAS) with the SSRs developed for SKr (Alfares et al., 2009). This approach was evaluated and shown to be successful for six varieties (Alfares et al., 2009). It was further applied at a larger scale for 12 additional lines (Bouguennec et al., 2018) opening the way to enhance Triticale genetic diversity more easily and to improve traits of agronomic interest in Triticale or wheat as well as to study further barriers to intergeneric crosses.
Conclusions
In summary, crossability between wheat and alien species is controlled by plenty of factors among which, Kr1, Kr2, Kr3 and Kr4 play an important role in this crossability as stated above. Concerning other suggested genes, they participate in a minor part of crossing‐compatibility compared with the Kr group. Only the Skr locus seems to be a major player, but it remains poorly understood, and research is necessary to elucidate the function of the different genes present at this locus. Furthermore, the role of Skr gene in crossability remains to be investigated, but this will require the isolation of the best candidate gene. To this day, wheat crossing compatibility with alien species remains unclear, and crosses between wheat and rye are still complex. Several studies are necessary to enrich the cultivated gene pools by incorporating favourable alleles, genes or gene complexes derived from the diverse gene pools of wheat relatives leading to new powerful wheat and Triticale varieties.
Conflict of interest
The authors declare no conflict of interest.
Author contributions
JL, UB and PS followed the literature and shared writing of the manuscript. All authors approved the manuscript.
Acknowledgements
The authors are grateful to Etienne Paux for providing some images from his own unpublished data used in Figures 2 and 4. JL thanks Clermont‐Auvergne Métropole (CAM) for funding her PhD in the frame of I‐Site Cap‐20‐25 Challenge and INRAE‐Transfert (project VACCIN) for funding experiments.
Laugerotte, J. , Baumann, U. and Sourdille, P. (2022) Genetic control of compatibility in crosses between wheat and its wild or cultivated relatives. Plant Biotechnol. J., 10.1111/pbi.13784
References
- Able, J.A. and Langridge, P. (2006) Wild sex in the grasses. Trends Plant Sci. 11, 261–263. [DOI] [PubMed] [Google Scholar]
- Ahmad, F. and Comeau, A. (1991) Production, morphology, and cytogenetics of Triticum aestivum (L.) Thell × Elymus scabrus (R. Br.) Love intergeneric hybrids obtained by in ovulo embryo culture. Theor. Appl. Genet. 81, 833–839. [DOI] [PubMed] [Google Scholar]
- Alfares, W. , Bouguennec, A. , Balfourier, F. , Gay, G. , Bergès, H. , Vautrin, S. , Sourdille, P. et al. (2009) Fine mapping and marker development for the crossability gene SKr on chromosome 5BS of hexaploid wheat (Triticum aestivum L.). Genetics, 183, 469–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allouis, S. , Moore, G. , Bellec, A. et al. (2003) Construction and characterisation of a hexaploid wheat (Triticum aestivum L.) BAC library from the reference germplasm ‘Chinese Spring’. Cereal Res. Comm. 31, 331–338. [Google Scholar]
- Backhouse, W.O. (1916) Note on the inheritance of “Crossability”. J. Gen. 6, 91–94. [Google Scholar]
- Baker, L. , Grewal, S. , Yang, C.Y. , Hubbart‐Edwards, S. , Scholefeld, D. , Ashling, S. , Burridge, A.J. et al. (2020) Exploiting the genome of Thinopyrum elongatum to expand the gene pool of hexaploid wheat. Theor. Appl. Genet. 133, 2213–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balfourier, F. , Bouchet, S. , Robert, S. , De Oliveira, R. , Rimbert, H. , Kitt, J. , Choulet, F. et al. (2019). Worldwide phylogeography and history of wheat genetic diversity. Sci. Adv. 5, eaav0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay, I.R. (1975) High frequencies of haploid production in wheat (Triticum aestivum) by chromosome elimination. Nature, 256, 410–411. [Google Scholar]
- Bates, L.S. , Mujeeb, K.A. and Waters, R.F. (1976) Wheat × barley hybrids problems and potentials. Cereal Res. Comm. 4, 377–386. [Google Scholar]
- Baumann, U. , Juttner, J. , Bian, X. and Langridge, P. (2000) Self‐incompatibility in the Grasses. Ann. Bot. 85, 203–209. [Google Scholar]
- Beddows, A.R. (1931) Seed setting and flowering in varioius grasses. Bull. Welsh Pl. Breed. Stn. Ser. H. 12, 5–99. [Google Scholar]
- Bertin, I. , Fish, L. , Foote, T.N. , Knight, E. , Snape, J. and Moore, G. (2009) Development of consistently crossable wheat genotypes for alien wheat gene transfer through fine‐mapping of the Kr1 locus. Theor. Appl. Genet. 119, 1371–1381. [DOI] [PubMed] [Google Scholar]
- Bhagyalakshmi, K. , Vinod, K.K. , Kumar, M. , Arumugachamy, S. , Joseph, A. and Raveendran, T.S. (2008) Interspecific hybrids from wild x cultivated Triticum crosses ‐ a study on the cytological behaviour and molecular relations. J. Crop Sci. Biotechnol. 7, 257‐262. [Google Scholar]
- Bian, X.‐Y. , Friedrich, A. , Bai, J.‐R. , Baumann, U. , Hayman, D.L. , Barker, S.J. and Langridge, P. (2004) High‐resolution mapping of the S and Z loci of Phalaris coerulescens . Genome, 47, 918–930. [DOI] [PubMed] [Google Scholar]
- Borojevic, K. and Borojevic, K. (2005) The transfer and history of “Reduced Height Genes” (Rht) in Wheat from Japan to Europe. J. Hered. 96, 455–459. [DOI] [PubMed] [Google Scholar]
- Bouguennec, A. , Lesage, V.S. , Gateau, I. , Sourdille, P. , Jahier, J. and Lonnet, P. (2018) Transfer of recessive skr crossability trait into well‐adapted French wheat cultivar Barok through marker‐assisted backcrossing method. Cereal Res. Comm. 46, 604–615. [Google Scholar]
- Brewbaker, J.L. (1957) Pollen cytology and self‐incompatibility systems in plants. J. Hered. 48, 271–277. [Google Scholar]
- Brisson, N. , Gate, P. , Gouache, D. , Charmet, G. , Oury, F.‐X. and Huard, F. (2010) Why are wheat yields stagnating in Europe? A comprehensive data analysis for France. Field. Crop. Res. 119, 201–212. [Google Scholar]
- Brouwer, D.J. and St Clair, D.A. (2004) Fine mapping of three quantitative trait loci for late blight resistance in tomato using near isogenic lines (NILs) and sub‐NILs. Theor. Appl. Genet. 108, 628–638. [DOI] [PubMed] [Google Scholar]
- Cadalen, T. , Boeuf, C. , Bernard, S. and Bernard, M. (1997) An intervarietal molecular marker map in Triticum aestivum L. Em. Thell. and comparison with a map from a wide cross. Theor. Appl. Genet. 94, 367–377. [Google Scholar]
- Canady, M.A. , Ji, Y. and Chetelat, R.T. (2006) Homeologous recombination in Solanum lycopersicoides introgression lines of cultivated tomato. Genetics, 174, 1775–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantret, N. , Salse, J. , Sabot, F. , Bellec, A. , Laubin, B. , Dubois, I. , Dossat, C. et al. (2008) Contrasted microcolinearity and gene evolution within a homoeologous region of wheat and barley species. J Mol Evol, 66, 138–150. [DOI] [PubMed] [Google Scholar]
- Chantret, N. , Salse, J. , Sabot, F. , Rahman, S. , Bellec, A. , Laubin, B. , Dubois, I. et al. (2005) Molecular basis of evolutionary events that shaped the hardness locus in diploid and polyploid wheat species (Triticum and Aegilops). Plant Cell, 17, 1033–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth, D. , Vekemans, X. , Castric, V. and Glémin, S. (2005) Plant self‐incompatibility systems: a molecular evolutionary perspective. New Phytol. 168, 61–69. [DOI] [PubMed] [Google Scholar]
- Charpentier, A. , Feldman, M. and Cauderon, Y. (1986) Chromosomal pairing at meiosis of F1 hybrid and backcross derivatives of Triticum aestivum × hexaploid Agropyron junceum . Can. J. Genet. Cytol. 28, 1–6. [Google Scholar]
- Chen, L.‐N. , Cui, Y.‐Z. , Wong, K.‐M. , Li, D.‐Z. and Yang, H.‐Q. (2017) Breeding system and pollination of two closely related bamboo species. AoB Plants, 9, plx021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Q. , Jahier, J. and Cauderon, Y. (1989) Production and cytogenetical studies of hybrids between Triticum aestivum L. Thell and Agropyron cristatum (L.) faertn. C. r. Acad. Sci., Sér. 3. Sci. Vie. 308, 425–430. [Google Scholar]
- Chen, Q. , Jahier, J. and Cauderon, Y. (1990) Intergeneric hybrids between Triticum aestivum and three crested wheatgrasses: Agropyron mongolicum, A. michnoi, and A. desertorum . Genome, 33, 663–667. [Google Scholar]
- Chen, Q. , Meng, D. , Gu, Z. , Li, W. , Yuan, H. , Duan, X. , Yang, Q. et al. (2018) SLFL genes participate in the ubiquitination and degradation reaction of S‐RNase in self‐compatible peach. Front. Plant Sci. Front. Plant Sci. 9, 227. 10.3389/fpls.2018.00227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chueca, M.C. , Cauderon, Y. and Tempe, J. (1977) In vitro embryo culture technique to obtain wheat X Aegilops hybrids. Annales De L’amelioration Des Plantes, 27, 539–547. [Google Scholar]
- Claesson, L. , Kotimäki, M. and von Bothmer, R. (1990) Production and cytogenetic analysis of the F 1 hybrid, Elymus caninus x Triticum aestivum and the backcross to T. aestivum . Cereal Res. Comm. 18, 315–319. [Google Scholar]
- Comeau, A. , Fedak, G. , St‐Pierre, C.‐A. and Theriault, C. (1985) Intergeneric hybrids between Triticum aestivum and species of Agropyron and Elymus . Cereal Res. Comm. 13, 149–153. [Google Scholar]
- Comeau, A. , Fedak, G. , St‐Pierre, C.A. and Cazeault, R. (1988) Intergeneric hybrids between Hordeum jubatum (4x) and Triticum aestivum (6x). Genome, 30, 245–249. [Google Scholar]
- Connor, H.E. (1979) Breeding systems in the grasses: A survey. NZ J. Bot. 17, 547–574. [Google Scholar]
- Cornish, M.A. , Hayward, M.D. and Lawrence, M.J. (1980) Self‐incompatibility in ryegrass. Heredity, 44, 55–62. [Google Scholar]
- Cox, T.S. , Murphy, J.P. and Rodgers, D.M. (1986) Changes in genetic diversity in the red winter wheat regions of the United States. Proc. Natl. Acad. Sci. USA, 83, 5583–5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crain, J. , Larson, S. , Dorn, K. , Hagedorn, T. , DeHaan, L. and Poland, J. (2020) Sequenced‐based paternity analysis to improve breeding and identify self‐incompatibility loci in intermediate wheatgrass (Thinopyrum intermedium). Theor. Appl. Genet. 133, 3217–3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cropano, C. , Manzanares, C. , Yates, S. , Copetti, D. , Do Canto, J. , Lübberstedt, T. , Koch, M. et al. (2021) Identification of candidate genes for self‐compatibility in perennial ryegrass (Lolium perenne L.). Front. Plant Sci. 12(707901), 10.3389/fpls.2021.707901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilova, T.V. , Zhang, G. , Liu, W. , Friebe, B. and Gill, B.S. (2017) Homoeologous recombination‐based transfer and molecular cytogenetic mapping of a wheat streak mosaic virus and Triticum mosaic virus resistance gene Wsm3 from Thinopyrum intermedium to wheat. Theor. Appl. Genet. 130, 549–556. [DOI] [PubMed] [Google Scholar]
- Den Boer, E. , Zhang, N.W. , Pelgrom, K. , Visser, R.G.F. , Niks, R.E. and Jeuken, M.J.W. (2013) Fine mapping quantitative resistances to downy mildew in lettuce revealed multiple sub‐QTLs with plant stage dependent effects reducing or even promoting the infection. Theor. Appl. Genet. 126, 2995–3007. [DOI] [PubMed] [Google Scholar]
- Deng‐Cai, L. , Chi, Y. , Jun‐Liang, Y. , You‐Liang, Z. and Xiu‐Jin, L. (1999) The chromosomal locations of high crossability genes in tetraploid wheat Triticum turgidum L. cv. Ailanmai native to Sichuan, China. Euphytica, 108, 79–82. [Google Scholar]
- Devaux, P. (2021) Production of wheat doubled haploids through intergeneric hybridization with maize. Methods Mol. Biol. 2287, 267–279. [DOI] [PubMed] [Google Scholar]
- Dewey, D.R. (1981) Cytogenetics of Agropyron ferganense and Its Hybrids with Six Species of Agropyron, Elymus, and Sitanion . Am. J. Bot. 68, 216–225. [Google Scholar]
- Dickinson, H.G. , Hiscock, S.J. , Crane, P.R. , Hiscock, S.J. and Tabah, D.A. (2003) The different mechanisms of sporophytic self–incompatibility. Philos. Trans. R. Soc. Lond. B Biol. Sci. 358, 1037–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do Canto, J. , Studer, B. and Lubberstedt, T. (2016) Overcoming self‐incompatibility in grasses: a pathway to hybrid breeding. Theor. Appl. Genet. 129, 1815–1829. [DOI] [PubMed] [Google Scholar]
- Doucet, J. , Lee, H.K. and Goring, D.R. (2016) Pollen acceptance or rejection: a tale of two pathways. Trends Plant Sci. 21, 1058–1067. [DOI] [PubMed] [Google Scholar]
- Dvorak, J. , Deal, K.R. and Luo, M.‐C. (2006) Discovery and mapping of wheat Ph1 suppressors. Genetics, 174, 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaves, D.J. , Flores‐Ortiz, C. , Haque, T. , Lin, Z. , Teng, N. and Franklin‐Tong, V.E. (2014) Self‐incompatibility in Papaver: advances in integrating the signalling network. Biochem. Soc. Trans. 42, 370–376. [DOI] [PubMed] [Google Scholar]
- Ellis, J.G. , Lagudah, E.S. , Spielmeyer, W. and Dodds, P.N. (2014) The past, present and future of breeding rust resistant wheat. Front. Plant Sci. 5, 641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellstrand, N.C. and Schierenbeck, K.A. (2000) Hybridization as a stimulus for the evolution of invasiveness in plants? Proc. Natl. Acad. Sci. 97, 7043–7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo, T.R. (2007) The gametocidal chromosome as a tool for chromosome manipulation in wheat. Chromosome Res. 15, 67–75. [DOI] [PubMed] [Google Scholar]
- Entani, T. , Iwano, M. , Shiba, H. , Che, F.‐S. , Isogai, A. and Takayama, S. (2003) Comparative analysis of the self‐incompatibility (S‐) locus region of Prunus mume: identification of a pollen‐expressed F‐box gene with allelic diversity. Genes Cells, 8, 203–213. [DOI] [PubMed] [Google Scholar]
- Falk, D.E. and Kasha, K.J. (1981) Comparison of the crossability of rye (Secale cereale) and Hordeum bulbosum onto wheat (Triticum aestivum). Can. J. Genet. Cytol. 23, 81–88. [Google Scholar]
- Falk, D.E. and Kasha, K.J. (1983) Genetic studies of the crossability of hexaploid wheat with rye and Hordeum bulbosum . Theor. Appl. Genet. 64, 303–307. [DOI] [PubMed] [Google Scholar]
- Faris, J.D. , Fellers, J.P. , Brooks, S.A. and Gill, B.S. (2003) A bacterial artificial chromosome contig spanning the major domestication locus Q in wheat and identification of a candidate gene. Genetics, 164, 311–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon, C.H. , Hayward, M.D. and Lawrence, M.J. (1984a) Self‐incompatibility in ryegrass VII. the determination of incompatibility genotypes in autotetraploid families of Lolium perenne L. Heredity, 53, 403–413. [Google Scholar]
- Fearon, C.H. , Hayward, M.D. and Lawrence, M.J. (1984b) Self‐incompatibility in ryegrass VIII. the mode of action of S and Z alleles in the pollen of autotetraploids of Lolium perenne L. Heredity, 53, 415–422. [Google Scholar]
- Fedak, G. (2011) Molecular aids for integration of alien chromatin through wide crosses. Genome, 42, 584–591. [Google Scholar]
- Fedak, G. and Jui, P.Y. (2011) Chromosomes of Chinese Spring wheat carrying genes for crossability with Betzes barley. Can. J. Genet. Cytol. 24, 227‐233. [Google Scholar]
- Felix, I. , Martinant, J.P. , Bernard, M. , Bernard, S. and Branlard, G. (1996) Genetic characterization of storage proteins in a set of F‐1‐derived haploid lines in bread wheat. Theor. Appl. Genet. 92, 340–346. [DOI] [PubMed] [Google Scholar]
- Fernandez‐Calvin, B. and Orellana, J. (1992) Relationship between pairing frequencies and genome affinity estimations in Aegilops ovata × Triticum aestivum hybrid plants. Heredity, 68, 165–172. [DOI] [PubMed] [Google Scholar]
- Fernández‐Calvín, B. and Orellana, J. (1994) Metaphase I‐bound arms frequency and genome analysis in wheat‐Aegilops hybrids. 3. Similar relationships between the B genome of wheat and S or S (l) genomes of Ae. speltoides, Ae. longissima and Ae. sharonensis . Theor. Appl. Genet. 88, 1043–1049. [DOI] [PubMed] [Google Scholar]
- Feuillet, C. , Langridge, P. and Waugh, R. (2008) Cereal breeding takes a walk on the wild side. Trends Genet. 24, 24–32. [DOI] [PubMed] [Google Scholar]
- Finch, R.A. and Bennett, M.D. (1980) Mitotic and meiotic chromosome behaviour in new hybrids of Hordeum with Triticum and Secale . Heredity, 44, 201–209. [Google Scholar]
- Franke, R. , Nestrowicz, R. , Senula, A. and Staat, B. (1992) Intergeneric hybrids between Triticum aestivum L. and wild Triticeae . Hereditas, 116, 225–231. [Google Scholar]
- Franklin, F.C.H. , Lawrence, M.J. and Franklin‐Tong, V.E. (1985) Cell and molecular biology of self‐incompatibility in flowering plants. Int Rev Cytol, 158, 1–64. [Google Scholar]
- Friebe, B. , Jiang, J. , Raupp, W.J. , McIntosh, R.A. and Gill, B.S. (1996) Characterization of wheat‐alien translocations conferring resistance to diseases and pests: current status. Euphytica, 91, 59–87. [Google Scholar]
- Fritz, A.K. , Cox, T.S. , Gill, B.S. and Sears, R.G. (1995) Molecular marker‐facilitated analysis of introgression in winter wheat × Triticum tauschii populations. Crop Sci. 35, 1691–1695. [Google Scholar]
- Fuong, F.T. , Voylokov, A.V. and Smirnov, V.G. (1993) Genetic studies of self‐fertility in rye (Secale cereale L.). 2. The search for isozyme marker genes linked to self‐incompatibility loci. Theor. Appl. Genet. 87, 619–623. [DOI] [PubMed] [Google Scholar]
- Gertz, A. and Wricke, G. (1989) Linkage between the incompatibility locus Z and a β‐glucosidase locus in rye. Plant Breed. 102, 255–259. [Google Scholar]
- Gill, B.S. and Raupp, W.J. (1987) Direct genetic transfers from Aegilops squarrosa L. to hexaploid wheat. Crop Sci. 27, 445–450. [Google Scholar]
- Gill, K.S. , Lubbers, E.L. , Gill, B.S. , Raupp, W.J. and Cox, T.S. (1991) A genetic linkage map of Triticum tauschii (DD) and its relationship to the D genome of bread wheat (AABBDD). Genome, 34, 362–374. [Google Scholar]
- Godfray, H.C.J. , Beddington, J.R. , Crute, I.R. , Haddad, L. , Lawrence, D. , Muir, J.F. , Pretty, J. et al. (2010) Food security: the challenge of feeding 9 billion people. Science, 327, 812–818. [DOI] [PubMed] [Google Scholar]
- Goring, D.R. (2018) Exocyst, exosomes, and autophagy in the regulation of Brassicaceae pollen‐stigma interactions. J. Exp. Bot. 69, 69–78. [DOI] [PubMed] [Google Scholar]
- Griffiths, S. , Sharp, R. , Foote, T.N. , Bertin, I. , Wanous, M. , Reader, S. , Colas, I. et al. (2006) Molecular characterization of Ph1 as a major chromosome pairing locus in polyploid wheat. Nature, 439, 749–752. [DOI] [PubMed] [Google Scholar]
- Gupta, P.K. and Fedak, G. (1985) Intergeneric hybrids between Hordeum californicum and Triticum aestivum . J. Hered. 76, 365–368. [Google Scholar]
- Hackauf, B. and Wehling, P. (2005) Approaching the self‐incompatibility locus Z in rye (Secale cereale L.) via comparative genetics. Theor. Appl. Genet. 110, 832–845. [DOI] [PubMed] [Google Scholar]
- Hajjar, R. and Hodgkin, T. (2007) The use of wild relatives in crop improvement: A survey of developments over the last 20 years. Euphytica, 156, 1–13. [Google Scholar]
- Han, C. , Zhang, P. , Ryan, P.R. , Rathjen, T.M. , Yan, Z. and Delhaize, E. (2016) Introgression of genes from bread wheat enhances the aluminium tolerance of durum wheat. Theor. Appl. Genet. 129, 729–739. [DOI] [PubMed] [Google Scholar]
- Hancock, C.N. , Kent, L. and McClure, B.A. (2005) The stylar 120 kDa glycoprotein is required for S‐specific pollen rejection in Nicotiana . Plant J. 43, 716–723. [DOI] [PubMed] [Google Scholar]
- Harlan, J.R. and de Wet, J.M.J. (1971) Toward a rational classification of cultivated plants. Taxon, 20, 509–517. [Google Scholar]
- Hayman, D.L. (1956) The genetical control of incompatibility in Phalaris Coerulescens desf. Aust. J. Bio. Sci. 9, 321–331. [Google Scholar]
- Heslop‐Harrison, J. (1982) Pollen‐stigma interaction and cross‐incompatibility in the grasses. Science, 215, 1358–1364. [DOI] [PubMed] [Google Scholar]
- Heslop‐Harrison, J. and Heslop‐Harrison, Y. (1982) The pollen‐stigma interaction in the grasses: 4. An interpretation of the self‐incompatibility response. Acta Botanica Neerlandica, 31, 429–439. [Google Scholar]
- Higashiyama, T. and Yang, W. (2017) Gametophytic pollen tube guidance: attractant peptides, gametic controls, and receptors. Plant Physiol. 173, 112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscock, S.J. and Allen, A.M. (2008) Diverse cell signalling pathways regulate pollen‐stigma interactions: the search for consensus. New Phytol. 179, 286–317. [DOI] [PubMed] [Google Scholar]
- Hua, Z. , Meng, X. and Kao, T.‐H. (2007) Comparison of Petunia inflata S‐Locus F‐box protein (Pi SLF) with Pi SLF like proteins reveals its unique function in S‐RNase based self‐incompatibility. Plant Cell, 19, 3593–3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igic, B. and Kohn, J.R. (2001) Evolutionary relationships among self‐incompatibility RNases. Proc. Natl. Acad. Sci. 98, 13167–13171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalani, B. and Moss, J. (1980) The site of action of the crossability genes (Kr1, Kr2) between Triticum and Secale. I. Pollen germination, pollen tube growth, and number of pollen tubes. Euphytica, 29, 571–579. [Google Scholar]
- Jantasuriyarat, C. , Vales, M.I. , Watson, C.J.W. and Riera‐Lizarazu, O. (2004) Identification and mapping of genetic loci affecting the free‐threshing habit and spike compactness in wheat (Triticum aestivum L.). Theor. Appl. Genet. 108, 261–273. [DOI] [PubMed] [Google Scholar]
- Jany, E. , Nelles, H. and Goring, D.R. (2019) Chapter One ‐ the molecular and cellular regulation of Brassicaceae self‐incompatibility and self‐pollen rejection. In International Review of Cell and Molecular Biology, ( Galluzzi, L. ed.), Vol. 343, pp. 1–35. Academic Press. 10.1016/bs.ircmb.2018.05.011 [DOI] [PubMed] [Google Scholar]
- Jiang, J. , Friebe, B. and Gill, B.S. (1993) Recent advances in alien gene transfer in wheat. Euphytica, 73, 199–212. [Google Scholar]
- Jiang, J. and Gill, B.S. (1994) Nonisotopic in situ hybridization and plant genome mapping: the first 10 years. Genome, 37, 717–725. [DOI] [PubMed] [Google Scholar]
- Jiang, J. and Liu, D. (1987) New hordeum‐triticum hybrids. Cereal Res. Comm. 15, 95–99. [Google Scholar]
- Johnson, M.A. , Harper, J.F. and Palanivelu, R. (2019) A fruitful journey: pollen tube navigation from germination to fertilization. Annu. Rev. Plant Biol. 70, 809–837. [DOI] [PubMed] [Google Scholar]
- Johnston, P.A. , Niks, R.E. , Meiyalaghan, V. , Blanchet, E. and Pickering, R. (2013) Rph22: mapping of a novel leaf rust resistance gene introgressed from the non‐host Hordeum bulbosum L. into cultivated barley (Hordeum vulgare L.). Theor. Appl. Genet. 126, 1613–1625. [DOI] [PubMed] [Google Scholar]
- Jovkova, M.E. , Kondeva, E. and Kostova, R. (1977) Biochemical investigations on Aegilops crassa Boiss. x Triticum aestivum L. hybrids. Genet. Sel., 10, 91–98. [Google Scholar]
- Kachroo, A. , Schopfer, C.R. , Nasrallah, M.E. and Nasrallah, J.B. (2001) Allele‐specific receptor‐ligand interactions in Brassica self‐incompatibility. Science, 293, 1824–1826. [DOI] [PubMed] [Google Scholar]
- Kakeda, K. (2009) S locus‐linked F‐box genes expressed in anthers of Hordeum bulbosum . Plant Cell Rep. 28, 1453–1460. [DOI] [PubMed] [Google Scholar]
- Kakeda, K. , Ibuki, T. , Suzuki, J. , Tadano, H. , Kurita, Y. , Hanai, Y. and Kowyama, Y. (2008) Molecular and genetic characterization of the S locus in Hordeum bulbosum L., a wild self‐incompatible species related to cultivated barley. Mol. Genet. Genom. 280, 509–519. [DOI] [PubMed] [Google Scholar]
- Kemp, B.P. and Doughty, J. (2003) Just how complex is the Brassica S‐receptor complex? J. Exp. Bot. 54, 157–168. [DOI] [PubMed] [Google Scholar]
- Kihara, H. (2013) Wheat Studies ‐ Retrospect and Prospects. Elsevier Scientific Publishing Company, 327 pp. [Google Scholar]
- Kimber, G. (1974) Relationships of the S‐genome diploids to polyploid wheats. Wheat Inf. Serv. 38, 1–5. [Google Scholar]
- Kimber, G. and Abubakar, M. (1979) Wheat hybrid information system. Cereal Res. Comm. 7, 257–260. [Google Scholar]
- King, J. , Grewal, S. , Yang, C. , Hubbart, S. , Scholefield, D. , Ashling, S. , Edwards, K.J. et al. (2017) A step change in the transfer of interspecific variation into wheat from Amblyopyrum muticum . Plant Biotechnol. J. 15, 217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaas, M. , Yang, B. , Bosch, M. , Thorogood, D. , Manzanares, C. , Armstead, I.P. , Franklin, F.C.H. et al. (2011) Progress towards elucidating the mechanisms of self‐incompatibility in the grasses: further insights from studies in Lolium . Ann. Bot. 108, 677–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klindworth, D.L. , Hareland, G.A. , Elias, E.M. and Xu, S.S. (2013) Attempted compensation for linkage drag affecting agronomic characteristics of durum wheat 1AS/1DL translocation lines. Crop Sci. 53, 422–429. [Google Scholar]
- Knobloch, I.W. (1968) A check list of crosses in the Gramineae . Department of Botany and Plant Physiology, Michigan State University: East Lansing. [Google Scholar]
- Koen, E. , Labuschagne, M.T. and Viljoen, C.D. (2002) The influence of eyespot resistance genes on baking quality and yield in wheat. J. Sci. Food Agric. 82, 1537–1540. [Google Scholar]
- Koo, D.‐H. , Friebe, B. and Gill, B.S. (2020) Homoeologous recombination: a novel and efficient system for broadening the genetic variability in wheat. Agronomy, 10, 1059. [Google Scholar]
- Koo, D.‐H. , Liu, W. , Friebe, B. and Gill, B.S. (2017) Homoeologous recombination in the presence of Ph1 gene in wheat. Chromosoma, 126, 531–540. [DOI] [PubMed] [Google Scholar]
- Krolow, K.‐D. (1970) Investigations on compatibility between wheat and rye. Zeitschrift Fur Pflanzenzuchtung, 64, 44–72. [Google Scholar]
- Kruse, A. (1976) Reciprocal hybrids between the genera Hordeum, secale and triticum. Hereditas, 84, 244. [Google Scholar]
- Kubo, K. , Entani, T. , Takara, A. , Wang, N. , Fields, A.M. , Hua, Z. , Toyoda, M. et al. (2010) Collaborative non‐self recognition system in S‐RNase‐based self‐incompatibility. Science, 330, 796–799. [DOI] [PubMed] [Google Scholar]
- Kubo, K.‐I. , Paape, T. , Hatakeyama, M. , Entani, T. , Takara, A. , Kajihara, K. , Tsukahara, M. et al. (2015) Gene duplication and genetic exchange drive the evolution of S‐RNase‐based self‐incompatibility in Petunia. Nat. Plants, 1, 14005. [DOI] [PubMed] [Google Scholar]
- Lai, Z. , Ma, W. , Han, B. , Liang, L. , Zhang, Y. , Hong, G. and Xue, Y. (2002) An F‐box gene linked to the self‐incompatibility (S) locus of Antirrhinum is expressed specifically in pollen and tapetum. Plant Mol. Biol. 50, 29–41. [DOI] [PubMed] [Google Scholar]
- Lamoureux, D. , Boeuf, C. , Regad, F. , Garsmeur, O. , Charmet, G. , Sourdille, P. , Lagoda, P. et al. (2002) Comparative mapping of the wheat 5B short chromosome arm distal region with rice, relative to a crossability locus. Theor. Appl. Genet. 105, 759–765. [DOI] [PubMed] [Google Scholar]
- Lange, W. and Riley, R. (1973) The position on chromosome 5B of wheat of the locus determining crossability with rye. Genet. Res. 22, 143–153. [Google Scholar]
- Lange, W. and Wojciechowska, B. (1976) The crossing of common wheat (Triticum aestivum L.) with cultivated rye (Secale cereale L.). I. crossability, pollen grain germination and pollen tube growth. Euphytica, 25, 609–620. [Google Scholar]
- Le Gouis, J. , Oury, F.‐X. and Charmet, G. (2020) How changes in climate and agricultural practices influenced wheat production in Western Europe. J. Cereal Sci. 93, 102960. [Google Scholar]
- Leach, C.R. (1988) Detection and estimation of linkage for a co‐dominant structural gene locus linked to a gametophytic self‐incompatibility locus. Theor. Appl. Genet. 75, 882–888. [Google Scholar]
- Leach, C.R. and Hayman, D.L. (1987) The incompatibility loci as indicators of conserved linkage groups in the Poaceae . Heredity, 58, 303–305. [Google Scholar]
- Leducq, J.‐B. , Gosset, C.C. , Gries, R. , Calin, K. , Schmitt, É. , Castric, V. and Vekemans, X. (2013) Self‐incompatibility in brassicaceae: identification and characterization of SRK‐Like sequences linked to the S‐locus in the tribe biscutelleae. G3 (Bethesda) 4, 983–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, H.S. , Huang, S. and Kao, T. (1994) S proteins control rejection of incompatible pollen in Petunia inflata . Nature, 367, 560–563. [DOI] [PubMed] [Google Scholar]
- Leighty, C.E. (1916) Carman’s wheat‐rye hybrids. J. Hered. 7, 420–427. [Google Scholar]
- Leighty, C.E. and Sando, W.J. (1928) Natural and artificial hybrids of a chinese wheat and rye. J. Hered. 19, 23–27. [Google Scholar]
- Lein, A. (1943) Die genetische grundlage der kreuzbarkeit zwischen weizen und roggen. Z.Ver‐erbungslehre, 81, 28–61. [Google Scholar]
- Li, H. , Deal, K.R. , Luo, M.‐C. , Ji, W. , Distelfeld, A. and Dvorak, J. (2017). Introgression of the Aegilops speltoides Su1‐Ph1 suppressor into wheat. Front. Plant Sci. 8, 10.3389/fpls.2017.02163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, L.H. and Dong, Y.S. (1991) Hybridization between Triticum aestivum L. and Agropyron michnoi Roshev.: 1. Production and cytogenetic study of F1 hybrids. Theor. Appl. Genet. 81, 312–316. [DOI] [PubMed] [Google Scholar]
- Lian, X. , Zhang, S. , Huang, G. , Huang, L. , Zhang, J. and Hu, F. (2021) Confirmation of a gametophytic self‐incompatibility in Oryza longistaminata . Front. Plant Sci. 12, 569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilienfeld, F.A. (1951) H. Kihara: Genome‐analysis in Triticum and Aegilops. X . Cytologia, 16, 101–123. [Google Scholar]
- Limin, A.E. and Fowler, D.B. (1990) An interspecific hybrid and amphiploid produced from Triticum aestivum crosses with Agropyron cristatum and Agropyron desertorum . Genome, 33, 581–584. [Google Scholar]
- Liu, Z.W. , Wang, R.R. and Carman, J.G. (1994) Hybrids and backcross progenies between wheat (Triticum aestivum L.) and apomictic Australian wheatgrass [Elymus rectisetus (Nees in Lehm.) A. Löve and Connor]: karyotypic and genomic analyses. Theor. Appl. Genet. 89, 599–605. [DOI] [PubMed] [Google Scholar]
- Lopes, M.S. and Reynolds, M.P. (2010) Dissecting drought adaptation into its phenotypic and genetic components in wheat. Asp. Appl. Biol. 105, 7–11. [Google Scholar]
- Lu, B.‐R. and von Bothmer, R. (1991) Production and cytogenetic analysis of the intergeneric hybrids between nine Elymus species and common wheat (Triticum aestivum L.). Euphytica, 58, 81–95. [Google Scholar]
- Lundqvist, A. (1954) Studies on self‐sterility in rye, Secale Cereale L. Hereditas, 40, 278–294. [Google Scholar]
- Lundqvist, A. (1957) Self‐incompatibility in rye. Hereditas, 43, 467–511. [Google Scholar]
- Lundqvist, A. (2009) The nature of the two loci incompatibility system in grasses. l. The hypothesis of a duplicative origin. Hereditas, 48, 153–168. [Google Scholar]
- Luo, M.C. , Yen, C. and Yang, J.L. (1993) Crossability percentages of bread wheat collections from Tibet, China with rye. Euphytica, 70, 127–129. [Google Scholar]
- Manzanares, C. , Barth, S. , Thorogood, D. , Byrne, S.L. , Yates, S. , Czaban, A. , Asp, T. et al. (2016) A gene encoding a DUF247 domain protein cosegregates with the S self‐incompatibility locus in perennial ryegrass. Mol. Biol. Evol. 33, 870–884. [DOI] [PubMed] [Google Scholar]
- Marcussen, T. , Sandve, S.R. , Heier, L. , Spannagl, M. , Pfeifer, M. , International Wheat Genome Sequencing Consortium , Jakobsen, K.S. et al. (2014). Ancient hybridizations among the ancestral genomes of bread wheat. Science, 345, 1250092. [DOI] [PubMed] [Google Scholar]
- Martin, A. and Chapman, V. (1977) A hybrid between Hordeum chilense and Triticum aestivum . Cereal Res. Comm. 5, 365–368. [Google Scholar]
- Martinez, M. , Cuñado, N. , Carcelén, N. and Romero, C. (2001) The Ph1 and Ph2 loci play different roles in the synaptic behaviour of hexaploid wheat Triticum aestivum . Theor. Appl. Genet. 103, 398–405. [Google Scholar]
- Matsumoto, D. and Tao, R. (2016a) Distinct self‐recognition in the Prunus S‐RNase‐based gametophytic self‐incompatibility system. Hortic. J. 85, 289–305. [Google Scholar]
- Matsumoto, D. and Tao, R. (2016b) Recognition of a wide‐range of S‐RNases by S locus F‐box like 2, a general‐inhibitor candidate in the Prunus‐specific S‐RNase‐based self‐incompatibility system. Plant Mol. Biol. 91, 459–469. [DOI] [PubMed] [Google Scholar]
- Matsuoka, Y. , Takumi, S. and Nasuda, S. (2014) Genetic mechanisms of allopolyploid speciation through hybrid genome doubling: novel insights from wheat (Triticum and Aegilops) studies. Int. Rev. Cell Mol. Biol. 309, 199–258. [DOI] [PubMed] [Google Scholar]
- McClure, B.A. and Franklin‐Tong, V. (2006) Gametophytic self‐incompatibility: understanding the cellular mechanisms involved in “self” pollen tube inhibition. Planta, 224, 233–245. [DOI] [PubMed] [Google Scholar]
- McClure, B. , Mou, B. , Canevascini, S. and Bernatzky, R. (1999) A small asparagine‐rich protein required for S‐allele‐specific pollen rejection in Nicotiana . Proc. Natl. Acad. Sci. 96, 13548–13553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden, E.S. and Sears, E.R. (1946) The origin of Triticum spelta and its free‐threshing hexaploid relatives. J. Hered. 37(81), 107. [DOI] [PubMed] [Google Scholar]
- Meister, N. and Tjumjakoff, N.A. (1928) Rye‐Wheat hybrids from reciprocal crosses. J. Gen. 20, 233–245. [Google Scholar]
- Mello‐Sampayo, T. (1971) Genetic regulation of meiotic chromosome pairing by chromosome 3D of Triticum aestivum . Nat. New Biol. 230, 22–23. [DOI] [PubMed] [Google Scholar]
- Mello‐Sampayo, T. and Canas, A.P. (1973). Suppressors of meiotic chromosome pairing in common wheat. In: Proc. 4th Int. Wheat Genet. Symp. ( Sears, E.R. and Sears, L.M.S. eds.), Columbia, pp. 709–713. [Google Scholar]
- Mello‐Sampayo, T. and Lorente, R. (1968) The role of chromosome 3D in the regulation of meiotic pairing in hexaploid wheat. EWAC Newsl. 2, 16–24. [Google Scholar]
- Miller, T.E. , Reader, S.M. and Gale, M.D. (1983) The effect of homoeologous group 3 chromosomes on chromosome pairing and crossability in Triticum aestivum . Can. J. Genet. Cytol. 25, 634–641. [Google Scholar]
- Mishina, K. , Sato, H. , Manickavelu, A. , Sassa, H. and Koba, T. (2009) Molecular mapping of SKr for crossability in common wheat. Breed. Sci. 59, 679–684. [Google Scholar]
- Miura, H. , Parker, B.B. and Snape, J.W. (1992) The location of major genes and associated quantitative trait loci on chromosome arm 5BL of wheat. Theor. Appl. Genet. 85, 197–204. [DOI] [PubMed] [Google Scholar]
- Molnár‐Láng, M. and Sutka, J. (1994). The effect of temperature on seed set and embryo development in reciprocal crosses of wheat and barley. Euphytica, 78, 53–58. [Google Scholar]
- Moreno‐Sevilla, B. , Baenziger, P.S. , Peterson, C.J. , Graybosch, R.A. and McVey, D.V. (1995) The 1BL/1RS translocation: agronomic performance of f3‐derived lines from a winter wheat cross. Crop Sci. 35, 1051–1055. [Google Scholar]
- Mujeeb‐Kazi, A. and Bernard, M. (1982) Somatic chromosome variations in backcross I progenies from intergeneric hybrids involving some Triticeaea . Cereal Res. Comm. 10, 41–45. [Google Scholar]
- Mujeeb‐Kazi, A. and Bernard, M. (1985) Cytogenetics of intergeneric Elymus canadensis X Triticum aestivum hybrids (n=5x=35, SHABD) and their backcross progenies with Triticum aestivum . Plant Breed, 95, 50–62. [Google Scholar]
- Mujeeb‐Kazi, A. and Rodriguez, R. (1981) An intergeneric hybrid of Triticum aestivum L. X Elymus giganteus. J. Hered. 72, 253–256. [Google Scholar]
- Mujeeb‐Kazi, A. , Roldan, S. and Miranda, J.L. (1984) Intergeneric hybrids of Triticum aestivum L. with Agropyron and Elymus species. Cereal Res. Comm. 12, 75–79. [Google Scholar]
- Mujeeb‐Kazi, A. , Roldan, S. , Suh, D.Y. , Sitch, L.A. and Farooq, S. (1987) Production and cytogenetic analysis of hybrids between Triticum aestivum and some caespitose Agropyron species. Genome, 29, 537–553. [Google Scholar]
- Mujeeb‐Kazi, A. , Roldan, S. , Suh, D.Y. , Ter‐Kuile, N. and Farooq, S. (1989) Production and cytogenetics of Triticum aestivum L. hybrids with some rhizomatous Agropyron species. Theor. Appl. Genet. 77, 162–168. [DOI] [PubMed] [Google Scholar]
- Muñoz‐Sanz, J.V. , Zuriaga, E. , Cruz‐García, F. , McClure, B. and Romero, C. (2020) Self‐(In)compatibility systems: target traits for crop‐production, plant breeding, and biotechnology. Front. Plant Sci. 11, 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase, K. , Shiba, H. , Iwano, M. , Che, F.‐S. , Watanabe, M. , Isogai, A. and Takayama, S. (2004) A membrane‐anchored protein kinase involved in Brassica self‐incompatibility signaling. Science, 303, 1516–1519. [DOI] [PubMed] [Google Scholar]
- Murfett, J. , Atherton, T.L. , Mou, B. , Gassert, C.S. and McClure, B.A. (1994) S‐RNase expressed in transgenic Nicotiana causes S‐allele‐specific pollen rejection. Nature, 367, 563–566. [DOI] [PubMed] [Google Scholar]
- Nalam, V.J. , Vales, M.I. , Watson, C.J.W. , Kianian, S.F. and Riera‐Lizarazu, O. (2006) Map‐based analysis of genes affecting the brittle rachis character in tetraploid wheat (Triticum turgidum L.). Theor. Appl. Genet. 112, 373–381. [DOI] [PubMed] [Google Scholar]
- Nocente, F. , Gazza, L. and Pasquini, M. (2006) Evaluation of leaf rust resistance genes Lr1, Lr9, Lr24, Lr47 and their introgression into common wheat cultivars by marker‐assisted selection. Euphytica, 155, 329–336. [Google Scholar]
- Ockendon, D.J. (1974) Distribution of self‐incompatibility alleles and breeding structure of open‐pollinated cultivars of Brussels sprouts. Heredity, 33, 159–171. [Google Scholar]
- Oettler, G. (2005) The fortune of a botanical curiosity – Triticale: past, present and future. J. Agric. Sci. 143, 329–346. [Google Scholar]
- Ono, K. , Akagi, T. , Morimoto, T. , Wünsch, A. and Tao, R. (2018) Genome re‐sequencing of diverse sweet cherry (Prunus avium) individuals reveals a modifier gene mutation conferring pollen‐part self‐compatibility. Plant Cell Physiol. 59, 1265–1275. [DOI] [PubMed] [Google Scholar]
- Padmanaban, S. , Zhang, P. , Hare, R.A. , Sutherland, M.W. and Martin, A. (2017) Pentaploid wheat hybrids: applications, characterisation, and challenges. Front. Plant Sci. 8, 10.3389/fpls.2017.00358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson, A.H. , DeVerna, J.W. , Lanini, B. and Tanksley, S.D. (1990) Fine mapping of quantitative trait loci using selected overlapping recombinant chromosomes, in an interspecies cross of tomato. Genetics, 124, 735–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pershina, L.A. , Numerova, O.M. , Belova, L.I. , Devyatkina, E.P. and Shumny, V.K. (1988) Fertility in barley x wheat hybrids H. geniculatum All x T. aestivum L, their regenerants and hybrid progeny of backcrosses to T. aestivum L. Cereal Res. Comm. 16, 157–163. [Google Scholar]
- Pienaar, R. (1981) Genome relationships in wheat x Agropyron distichum (Thunb.) Beauv. Hybrids. Z Pflanzenzücht, 87, 193–212. [Google Scholar]
- Plourde, A. , Comeau, A. , Fedak, G. and St‐Pierre, C.A. (1989a) Production and cytogenetics of hybrids of Triticum aestivum x Leymus innovatus . Theor. Appl. Genet. 78, 436–444. [DOI] [PubMed] [Google Scholar]
- Plourde, A. , Comeau, A. , Fedak, G. and St‐Pierre, C.‐A. (1989b) Intergeneric hybrids of Triticum aestivum × Leymus multicaulis . Genome, 32, 282–287. [Google Scholar]
- Plourde, A. , Comeau, A. and St. Pierre, C.A. (1992) Barley yellow dwarf virus resistance in Triticum aestivum × Leymus angustus hybrids. Plant Breed. 108, 97–103. [Google Scholar]
- Plourde, A. , Fedak, G. , St‐Pierre, C.A. and Comeau, A. (1990) A novel intergeneric hybrid in the Triticeae: Triticum aestivum x Psathyrost achys juncea . Theor. Appl. Genet. 79, 45–48. [DOI] [PubMed] [Google Scholar]
- Porter, J.R. and Semenov, M.A. (2005) Crop responses to climatic variation. Philos. Trans. Royal Society B: Biol. Sci. 360, 2021–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preece, C. , Livarda, A. , Christin, P.‐A. , Wallace, M. , Martin, G. , Charles, M. , Jones, G. et al. (2017) How did the domestication of Fertile Crescent grain crops increase their yields? Funct. Ecol. 31, 387–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi, L. , Friebe, B. , Zhang, P. and Gill, B.S. (2007) Homoeologous recombination, chromosome engineering and crop improvement. Chromosome Res. 15, 3–19. [DOI] [PubMed] [Google Scholar]
- Qiao, H. , Wang, H. , Zhao, L. , Zhou, J. , Huang, J. , Zhang, Y. and Xue, Y. (2004) The F‐Box Protein AhSLF‐S2 Physically Interacts with S‐RNases that may be inhibited by the ubiquitin/26S proteasome pathway of protein degradation during compatible pollination in Antirrhinum . Plant Cell, 16, 582–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovich, S.V. (1998) Importance of wheat‐rye translocations for breeding modern cultivar of Triticum aestivum L. Euphytica, 100, 323–340. [Google Scholar]
- Rather, S. , Sharma, D. , Pandey, I. and Joshi, N. (2017) Alien gene introgression in wheat. In Wheat: a premier food crop ( Kumar, A. , Kumar, A. and Prasad, B. , eds), pp. 91–109. India: Kalyani Publishers. [Google Scholar]
- Ray, D. , Mueller, N. , West, P. and Foley, J. (2013) Yield trends are insufficient to double global crop production by 2050. PLoS One, 8, e66428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reader, S.M. and Miller, T.E. (1991) The introduction into bread wheat of a major gene for resistance to powdery mildew from wild emmer wheat. Euphytica, 53, 57–60. [Google Scholar]
- Redden, R.J. , Yadav, S.S. , Maxted, N. , Dulloo, M.E. , Guarino, L. and Smith, P. (2015) Crop Wild Relatives and Climate Change, p. 357. Blackwell UK: Wiley. [Google Scholar]
- Rey, M.‐D. , Martín, A.C. , Higgins, J. , Swarbreck, D. , Uauy, C. , Shaw, P. and Moore, G. (2017) Exploiting the ZIP4 homologue within the wheat Ph1 locus has identified two lines exhibiting homoeologous crossover in wheat‐wild relative hybrids. Mol. Breed. 37, 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieseberg, L.H. (1997) Hybrid origins of plant species. Annu. Rev. Ecol. Syst. 28, 359–389. [Google Scholar]
- Rieseberg, L.H. , Raymond, O. , Rosenthal, D.M. , Lai, Z. , Livingstone, K. , Nakazato, T. , Durphy, J.L. et al. (2003) Major ecological transitions in wild sunflowers facilitated by hybridization. Science, 301(5637), 1211–1216 [DOI] [PubMed] [Google Scholar]
- Rieseberg, L.H. and Wendel, J.F. (1993). lntrogression and its consequences in plants. In: Hybrid Zones and the Evolutionary Process ( Harrison, R.G. ed.), pp. 70–109. Oxford: Oxford University Press. [Google Scholar]
- Riley, R. and Chapman, V. (1958) Genetic control of the cytologically diploid behaviour of hexaploid wheat. Nature, 182, 713–715. [Google Scholar]
- Riley, R. , Chapman, V. and Kimber, G. (1959) Genetic control of chromosome pairing in intergeneric hybrids with wheat. Nature, 183, 1244–1246. [DOI] [PubMed] [Google Scholar]
- Riley, R. and Chapman, V. (1967) The inheritance in wheat of crossability with rye. Genet. Res. 9, 259–267. [Google Scholar]
- Romero, C. and Cuadrado, C. (1992) Parental genotype influence on seed‐set in different wheat x rye crosses. Cereal Res. Comm. 20, 193–199. [Google Scholar]
- Sassa, H. (2016) Molecular mechanism of the S‐RNase‐based gametophytic self‐incompatibility in fruit trees of Rosaceae. Breed Sci. 66, 116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider, A. , Molnár, I. and Molnár‐Láng, M. (2008) Utilisation of Aegilops (goatgrass) species to widen the genetic diversity of cultivated wheat. Euphytica, 163, 1–19. [Google Scholar]
- Schopfer, C.R. and Nasrallah, J.B. (2000) Self‐incompatibility. Prospects for a novel putative peptide‐signaling molecule. Plant Physiol. 124, 935–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears, E.R. (1956) The transfer of leaf‐rust resistance from Aegilops umbellulata to wheat. Genetics in plant breeding. Brook‐haven Symposia in Biology, 1956, 1–22. [Google Scholar]
- Serra, H. , Svačina, R. , Baumann, U. , Whitford, R. , Sutton, T. , Bartoš, J. and Sourdille, P. (2021) Ph2 encodes the mismatch repair protein MSH7‐3D that inhibits wheat homoeologous recombination. Nat. Commun. 12, 803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, H.C. and Baenziger, P.S. (1986) Production, morphology, and cytogenetic analysis of Elymus caninus (Agropyron caninum) x Triticum aestivum F1 hybrids and backcross‐1 derivatives. Theor. Appl. Genet. 71, 750–756. [DOI] [PubMed] [Google Scholar]
- Sharma, H. and Gill, B.S. (1981a) New hybrids between Agropyron and wheat. I: A. ciliare x wheat and A. Smithii x wheat. Wheat Inf. Serv. 52, 19–22. [Google Scholar]
- Sharma, H.C. and Gill, B.S. (1981b) Wheat hybridization. Ann. Wheat Newsl. 27, 106. [Google Scholar]
- Sharma, H.C. and Gill, B.S. (1983) Current status of wide hybridization in wheat. Euphytica, 32, 17–31. [Google Scholar]
- Shinozuka, H. , Cogan, N.O.I. , Smith, K.F. , Spangenberg, G.C. and Forster, J.W. (2010) Fine‐scale comparative genetic and physical mapping supports map‐based cloning strategies for the self‐incompatibility loci of perennial ryegrass (Lolium perenne L.). Plant Mol. Biol. 72, 343. [DOI] [PubMed] [Google Scholar]
- Shivanna, K.R. , Heslop‐Harrison, Y. and Heslop‐Harrison, J. (1982) The pollen‐stigma interaction in the grasses. 3. Features of the self‐incompatibility response. Acta Botanica Neerlandica, 31, 307–319. [Google Scholar]
- Sisodia, N.S. and McGinnis, R.C. (1970) Importance of hexaploid wheat germ plasm in hexaploid triticale breeding1. Crop Sci. 10, 161–162. [Google Scholar]
- Sitch, L.A. , Snape, J.W. and Firman, S.J. (1985) Intrachromosomal mapping of crossability genes in wheat (Triticum aestivum). Theor. Appl. Genet. 70, 309–314. [DOI] [PubMed] [Google Scholar]
- Skowrońska, R. , Mariańska, M. , Ulaszewski, W. , Tomkowiak, A. , Nawracała, J. and Kwiatek, M.T. (2020) Development of triticale × wheat prebreeding germplasm with loci for slow‐rusting resistance. Front. Plant Sci. 11, 10.3389/fpls.2020.00447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, D.C. (1942) Intergeneric hybridization of cereals and other grasses. J. Agric. Res. 64, 33. [Google Scholar]
- Snape, J.W. , Chapman, V. , Moss, J. , Blanchard, C.E. and Miller, T.E. (1979) The crossabilities of wheat varieties with Hordeum bulbosum . Heredity, 42, 291–298. [Google Scholar]
- Soreng, R. , Peterson, P. , Romaschenko, K. , Davidse, G. , Zuloaga, F. , Judziewicz, E. , Filgueiras, T. et al. (2015) A worldwide phylogenetic classification of the Poaceae (Gramineae). J. Syst. Evol. 53, 117–137. [Google Scholar]
- Sourdille, P. , Cadalen, T. , Guyomarc'h, H. , Snape, J. , Perretant, M. , Charmet, G. , Boeuf, C. et al. (2003) An update of the Courtot × Chinese Spring intervarietal molecular marker linkage map for the QTL detection of agronomic traits in wheat. Theor. Appl. Genet. 106, 530–538. [DOI] [PubMed] [Google Scholar]
- Sun, L. , Williams, J.S. , Li, S. , Wu, L. , Khatri, W.A. , Stone, P.G. , Keebaugh, M.D. et al. (2018) S‐Locus F‐box proteins are solely responsible for S‐RNase‐based self‐incompatibility of petunia pollen. Plant Cell, 30, 2959–2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadesse, W. , Sanchez‐Garcia, M. , Assefa, S.G. , Amri, A. , Bishaw, Z. , Ogbonnaya, F.C. and Baum, M. (2019) Genetic gains in wheat breeding and its role in feeding the world. Crop Breed Genet. Genom. 1, e190005. [Google Scholar]
- Takasaki, T. , Hatakeyama, K. , Suzuki, G. , Watanabe, M. , Isogai, A. and Hinata, K. (2000) The S receptor kinase determines self‐incompatibility in Brassica stigma. Nature, 403, 913–916. [DOI] [PubMed] [Google Scholar]
- Takayama, S. and Isogai, A. (2005) Self‐incompatibility in plants. Annu. Rev. Plant Biol. 56, 467–489. [DOI] [PubMed] [Google Scholar]
- Takayama, S. , Shiba, H. , Iwano, M. , Shimosato, H. , Che, F.‐S. , Kai, N. , Watanabe, M. et al. (2000) The pollen determinant of self‐incompatibility in Brassica campestris . Proc. Natl. Acad. Sci. 97, 1920–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama, S. , Shimosato, H. , Shiba, H. , Funato, M. , Che, F.S. , Watanabe, M. , Iwano, M. et al. (2001) Direct ligand‐receptor complex interaction controls Brassica self‐incompatibility. Nature, 413, 534–538. [DOI] [PubMed] [Google Scholar]
- Tao, R. and Iezzoni, A.F. (2010) The S‐RNase‐based gametophytic self‐incompatibility system in Prunus exhibits distinct genetic and molecular features. Sci. Hortic. 124, 423–433. [Google Scholar]
- Taylor, J.W. and Quisenberry, K.S. (1935) Inheritance of rye crossability in wheat hybrids1. Agron. J. 27, 149–153. [Google Scholar]
- Tester, M. and Langridge, P. (2010) Breeding technologies to increase crop production in a changing world. Science, 327, 818–822. [DOI] [PubMed] [Google Scholar]
- The International Wheat Genome Sequencing Consortium (IWGSC) . (2014) A chromosome‐based draft sequence of the hexaploid bread wheat (Triticum aestivum) genome. Science, 345, 6194. 10.1126/science.1251788 [DOI] [PubMed] [Google Scholar]
- The International Wheat Genome Sequencing Consortium (IWGSC) . (2018) Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science, 361(6403), eaar7191. [DOI] [PubMed] [Google Scholar]
- Thomas, S.M. and Murray, B.G. (1975) A new site for the self‐incompatibility reaction in the Gramineae . Incomp Newslett, 6, 22–23. [Google Scholar]
- Tixier, M.H. , Sourdille, P. , Charmet, G. , Gay, G. , Jaby, C. , Cadalen, T. , Bernard, S. et al. (1998) Detection of QTLs for crossability in wheat using a doubled‐haploid population. Theor. Appl. Genet. 97, 1076–1082. [Google Scholar]
- Tozu, T. (1966) Crossability between wheat and rye. Kihara Institute for Biological Research, Yokohama City University, Annual Report 33–38. [Google Scholar]
- Ushijima, K. , Yamane, H. , Watari, A. , Kakehi, E. , Ikeda, K. , Hauck, N.R. , Iezzoni, A.F. et al. (2004) The S haplotype‐specific F‐box protein gene, SFB, is defective in self‐compatible haplotypes of Prunus avium and P. mume . Plant J. 39, 573–586. [DOI] [PubMed] [Google Scholar]
- Weiss, E. , Zohary, D. and Hopf, M. (2012) Domestication of plants in the old world ‐ the origin and spread of domesticated plants in South‐west Asia, Europe, and the Mediterranean Basin. University Press Scholarship Online, 10.1093/acprof:osobl/9780199549061.001.0001 [DOI] [Google Scholar]
- Wendel, J.F. , Olson, P.D. and Stewart, J.M. (1989) Genetic diversity, introgression, and independent domestication of old world cultivated cottons. Am. J. Bot. 76, 1795–1806. [Google Scholar]
- Wheeler, M.J. , de Graaf, B.H.J. , Hadjiosif, N. , Perry, R.M. , Poulter, N.S. , Osman, K. , Vatovec, S. et al. (2009) Identification of the pollen self‐incompatibility determinant in Papaver rhoeas . Nature, 459, 992–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, S. (1873) II. Wheat and Rye Hybrids. Transactions of the Botanical Society of Edinburgh, 12, 1‐4, 286‐288, 10.1080/03746607309469536 [DOI] [Google Scholar]
- Worland, A.J. , Law, C.N. , Hollins, T.W. , Koebner, R.M.D. and Giura, A. (1988) Location of a gene for resistance to eyespot (Pseudocercosparella herpotrichoides) on chromosome 7D of bread wheat. Plant Breed. 101, 43–51. [Google Scholar]
- Wricke, G. and Wehling, P. (1985) Linkage between an incompatibility locus and a peroxidase isozyme locus (Prx 7) in rye. Theor. Appl. Genet. 71, 289–291. [DOI] [PubMed] [Google Scholar]
- Wulff, B.B.H. and Moscou, M.J. (2014) Strategies for transferring resistance into wheat: from wide crosses to GM cassettes. Front. Plant Sci. 5, 10.3389/fpls.2014.00692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, B. , Thorogood, D. , Armstead, I. and Barth, S. (2008) How far are we from unravelling self‐incompatibility in grasses? New Phytol. 178, 740–753. [DOI] [PubMed] [Google Scholar]
- Yang, B. , Thorogood, D. , Armstead, I.P. , Franklin, F.C.H. and Barth, S. (2009) Identification of genes expressed during the self‐incompatibility response in perennial ryegrass (Lolium perenne L.). Plant Mol. Biol. 70, 709–723. [DOI] [PubMed] [Google Scholar]
- Yen, Y. and Liu, D. (1987) Production, morphology, and cytogenetics of intergeneric hybrids of Elymus L. species with Triticum aestivum L. and their backcross derivatives. Genome, 29, 689–694. [Google Scholar]
- Zarco‐Hernandez, J.A. , Santiveri, F. , Michelena, A. and Javier Peña, R. (2005) Durum wheat (Triticum turgidum, L.) carrying the 1BL/1RS chromosomal translocation: agronomic performance and quality characteristics under Mediterranean conditions. Eur. J. Agron. 22, 33–43. [Google Scholar]
- Zeven, A.C. (1987) Crossability percentages of some 1400 bread wheat varieties and lines with rye. Euphytica, 36, 299–319. [Google Scholar]
- Zeven, A.C. and van Heemert, C. (1970) Germination of pollen of weed rye (Secale segetale L.) on wheat (Triticum aestivum L.) stigmas and the growth of the pollen tubes. Euphytica, 19, 175–179. [Google Scholar]
- Zhao, C. , Cui, F. , Wang, X. , Shan, S. , Li, X. , Bao, Y. and Wang, H. (2012) Effects of 1BL/1RS translocation in wheat on agronomic performance and quality characteristics. Field. Crop. Res. 127, 79–84. [Google Scholar]
- Zheng, Y.L. (1992) Chromosome location of new crossability gene in common wheat. Wheat Inf. Serv. 75, 36–40. [Google Scholar]
- Zohary, D. and Hopf, M. (2000) Domestication of Plants in the Old World: The Origin and Spread of Cultivated Plants in West Asia, Europe and the Nile Valley. 3rd ed. pp 316. UK: Oxford University Press. [Google Scholar]
- Zohary, D. , Hopf, M. and Weiss, E. (2012) Domestication of Plants in the Old World: The origin and spread of domesticated plants in Southwest Asia, Europe, and the Mediterranean Basin. 4th edn (Oxford, 2012; published online May. 2015). Oxford Scholarship Online, 10.1093/acprof:osobl/9780199549061.001.0001 [DOI] [Google Scholar]


