Abstract
The Mycobacterium bovis Bacillus Calmette et Guérin (BCG) vaccine was generated in 1921 with the efforts of a team of investigators, Albert Calmette and Camille Guérin, dedicated to the determination to develop a vaccine against active tuberculosis (TB) disease. Since then, BCG vaccination is used globally for protection against childhood and disseminated TB; however, its efficacy at protecting against pulmonary TB in adult and aging populations is highly variable. Due to the BCG generated immunity, this vaccine later proved to have an antitumor activity; though the standing mechanisms behind are still unclear. Recent studies indicate that both innate and adaptive cell responses may play an important role in BCG eradication and prevention of bladder cancer. Thus, cells such as natural killer (NK) cells, macrophages, dendritic cells, neutrophils but also MHC-restricted CD4 and CD8 T cells and γδ T cells may play an important role and can be one the main effectors in BCG therapy. Here, we discuss the role of BCG therapy in bladder cancer and other cancers, including current strategies and their impact on the generation and sustainability of protective antitumor immunity against bladder cancer.
Keywords: BCG, Bladder cancer, Treatment, Mycobacterium bovis
1. History of BCG
July 18, 2121 will mark the 100th anniversary of the first use of the Bacillus Calmette et Guérin (BCG) vaccine. BCG was first devel oped by Albert Calmette, a bacteriologist, and Camille Guérin, a veterinarian, at the Pasteur Institute by culturing virulent Mycobacterium bovis through 231 passages in glycerinated bile potato medium over a decade (1908–1919) [1]. The serial cultivation attenuated the virulent bacteria making it safe to be used as a vaccine against tuberculosis (TB), which was plaguing Europe at that time. In 1929, it was reported that cancer incidence was lower in patients with TB compared to patients without TB in an autopsy study carried out at the Johns Hopkins Hospital [3]. The mechanistic basis behind this finding was unknown, but it was one of the first studies that suggested an association between cancer and TB [3]. Coley’s toxin, consisting of pooled killed cells of Streptococcus pyogenes and Serratia marcenses, successfully treated different types of cancers; it was the first microorganism-based immunotherapy agent and led to the proposal of using BCG for cancer therapy [4]. Further, in 1939, Dr. Holmgren intravenously injected BCG in cancer patients with some success [5].
The development of novel technologies reinvigorated the exploration of BCG usage. In the 1950s, the development of inbred mice and their use as in vivo cancer models allowed the testing of novel therapies for cancer treatment. Mice inoculated with BCG demonstrated resistance to a subsequent tumor challenge in studies carried out by Lloyd Old at Sloan-Kettering Institute in New York [6]. BCG activated macrophages induced necrosis in murine tumors by inhibiting the growth of cancer cells. Later in the 1970s, a seminal study by Zbar and colleagues at the National Cancer Institute, showed that intradermal injection of BCG into guinea pigs inhibited the growth of intradermal tumors and reduced lymph node metastases [2]. BCG was also was used as adjuvant therapy for acute lymphoblastic leukemia [7]. Morton and colleagues showed that intralesional BCG treatment was capable of reducing malignant melanoma [8]. In the mid-1970s, a case report showed that a cystoscopic injection of live whole BCG eradicated isolated melanoma in a patient’s bladder [9]. These studies provided the foundation for an interest in BCG as an anti-cancer therapy and subsequent clinical trials with BCG in different cancers such as lung, prostate, colon, and kidney cancers were carried out. However, it was in bladder cancer that BCG would become a gold standard treatment.
2. Key trials in BCG immunotherapy in bladder cancer
Dr. Morales in Canada reported the first clinical experience of BCG for bladder cancer, he instilled BCG in the bladder for treating non-muscle invasive bladder cancer (NMIBC). The patient cohort included nine patients and the treatment regimen included 6 weekly intradermal and intravesical injections of BCG [10]. A total of 5 mg of BCG was administered intradermally to the upper thigh with a multiple puncture apparatus and 120 mg of BCG was injected into the bladder via a urethral catheter [10]. These findings were validated in a larger trial by the Southwest Oncology Group (SWOG) [11]. It was the first randomized controlled trial that showed the clinical efficacy of BCG treatments for the regression of bladder cancer [11]. Later, in 1982, Brosnan et al. altered the Morales treatment regimen by removing the intradermal injection step [12]. In the 1990s, the US Federal Drugs Administration (FDA) approved intravesical BCG for the treatment of carcinoma in situ (CIS) of the bladder. A decade later, maintenance therapy of BCG was shown to yield better long-term benefits compared with the same treatment regimen without maintenance therapy [13]. The current standard of care now recommends 6 weekly instillations of BCG as an induction course, followed by 3 weekly treatments at 3, 6, 12, 18, 24, 30, and 36 months post tumor resection as the maintenance course (total maintenance courses = 7, total BCG instillations = 27).
3. Mechanisms of BCG immunotherapy
Though BCG has been used in bladder cancer therapy for a long time, its mechanism of action is not well delineated. It remains unclear to what extent BCG mediates tumor protection through non-specific innate immune mechanisms, direct killing, and antigen-specific immunity, including bacteria-specific or tumor specific immunity. Bevers et al. suggested two possibilities of how BCG attaches to the bladder: 1) via a specific receptor-ligand interaction between the BCG cell envelope surface-exposed antigen 85 (Ag85) and fibronectin of the urothelium, and/or 2) via physiochemical damage to the urothelium glycosaminoglycan layer (protective thick mucus layer of glycoproteins and proteoglycans) [14]. In vivo studies show that the mycobacterial envelope of BCG attaches to fibronectin on the urothelium [15,16]. However, contrary to some of the preclinical murine studies [17,18], clinical studies with patients treated with BCG therapy found no significant effect of fibrin clot inhibitors on mycobacterial attachment [19,20]. These contradictory findings could be explained by the physicochemical properties of the BCG suspension used (e.g. hydrophobicity, overall negative charge), or by the formulation properties in which it is installed [14,21,22] or both could influence BCG interactions with the bladder surface. Further, oncogenic mutations activating certain signaling pathways involved in macropinocytosis – Ras activated Pak1 – are shown to modulate BCG internalization and may explain the differential BCG uptake between BCG resistant and BCG susceptible bladder cancer cells [23].
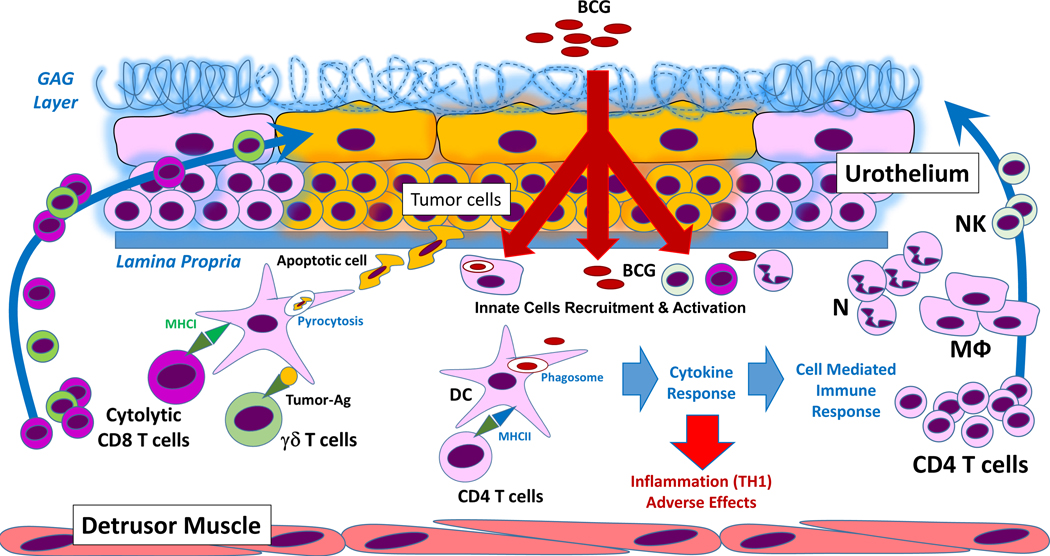
Once BCG is internalized by urothelial cells, it activates both local and systemic immune responses through cytokine release and antigen presentation. Epithelioid and gigantocellular granulomas, consisting of macrophages, dendritic cells (DCs), lymphocytes, neutrophils, and fibroblasts, are detected in the bladder wall after BCG instillation but their functional role in BCG’s antitumor activity is unclear. In vitro, BCG, internalized in bladder tumor cells, induced the production of cytokines such as IL-6, IL-8, and granulocyte–macrophage colony-stimulating factor (GM-CSF) which are involved in the recruitment of immune cells, including tumor protective cytotoxic T cells [25]. Clinical studies confirmed the increase in the levels of IL-8 and GM-CSF levels, IL-1β, IL-15, IL-18, and CXC-chemokine ligand 10 (CXCL10) in blood and/or in the urine of patients after BCG treatment (Fig. 1) [26–28].
Fig. 1.
BCG induced immune response in the tumor. BCG bacilli are internalized by urothelial cells and bladder cancer cells, generating an innate inflammatory response attracting local and peripheral NK cells, macrophages, neutrophils and proliferated/primed CD4 + T cells to the tumor site. In this scenario, BCG bacilli encounter dendritic cells (or DCs). After BCG-phagocytosis, DCs process BCG and present antigens to MHC-II restricted CD4 + T cells and at the same time initiate a Th1-type immune response through inflammatory cytokines required for effective BCG-induced antitumor activity, but simultaneously this inflammatory response could cause adverse effects to the host. At the same time, apoptotic BCG-infected tumor cells will be pyrocytosed by DCs and tumor-specific Ag/BCG-specific Ag will be presented via MHC-I to cytolytic CD8 + T cells. Tumor-Ags can also be directly detected or detected in the context of MHC by T cells, generating anti-tumor cytotoxic responses. DC, Dendritic cells; GAG layer, glycosaminoglycan; MU, Macrophages; N, Neutrophils; NK, Natural Killer cells. Note: Drawing not at scale.
Some of the immune cells found in the urine of patients on BCG therapy or in the bladder cavity of BCG-treated murine bladder cancer models include T cells, neutrophils, macrophages, B cells, and NK cells (Fig. 1, Table 1). BCG therapy did not alter the phenotype and the gene expression of CD4+ and CD8+ T cells in the bladder [29], suggesting that even though BCG is involved in the recruitment of T cells, it may not change their functional activity. Interestingly, BCG instillation was shown to stimulate long-term tumor-specific immunity which was dependent on IFN-γ production by tumor-specific CD4+T cells [30]. Further, TH2 promoting factors such as GATA Binding Protein 3 (GATA3) may be involved in shaping the TH1 response affecting BCG efficacy [31]. Patients who successfully responded to BCG therapy had a higher GATA3/T-bet ratio before the initiation of BCG therapy compared with the patients who failed BCG therapy; smokers with low GATA3 expression also displayed BCG resistance [31].
Table 1.
Experimental strategies used in BCG research in bladder cancer. Use of different strategies using the orthotopic syngeneic (MB49 cells) murine model of bladder and their outcomes. BCG regimen is initiated 1–2 days after tumor induction in all the studies.
| BCG strain | BCG regimen | Strategy | Outcome |
|---|---|---|---|
|
| |||
| BCG Pasteur grown on Middlebrock 7H9 | 107 CFU/dose 5 weekly instilations | T cell depletion using intraperitoneal injections of anti-CD4 or anti-CD8 antibodies | Depletion of either CD4 or CD8 T cells completely abrogates the effect of BCG [30] |
| Commercially available BCG Pasteur strain (Armand Frappier, Quebec) |
107 CFU/dose 1 instillation |
Monoclonal antibodies to thy 1.2, CD8, CD4 injected intravenously to deplete T cell populations | Requirement for T lymphocytes in BCG-mediated antitumor activity. Both CD4 and CD8 subsets are required for BCG efficacy [120] |
| Commercially available BCG Connaught strain (Immucyst, Nippon kayaku, Tokyo, Japan) | 3 × 106 CFU/dose 4 weekly instillations |
IL-17 KO mice and γᵟ T-cell-deficient mice | BCG was ineffective in IL-17 KO mice and γᵟ T-cell-deficient mice [37] |
| Commercially available BCG Connaught, strain (Aventis Pasteur, Canada) in 1 ml of solvent according to the manufacturer’s recommendation | Minimum 6 × 106 CFU/dose 4 weekly instillations |
NK-deficient beige mice and mice treated with anti-NK1.1 monoclonal antibody | Reduced efficacy of BCG on depletion of NK cells and in NK cell deficient mice [121] |
| Commercially available BCG Connaught strain (Aventis Pasteur Ltd, Toronto, Canada) in 09% sodium chloride | 3 × 106 CFU/dose 4 weekly instillations |
IFN-γ knockout (KO), IL-12 KO and IL- 10 KO mice |
BCG treatment was completely ineffective in IFN-c KO and IL-12 KO mice BCG treatment was highly effective in IL-10 KO mice [122] |
| BCG, Connaught substrain, Cytochemia, Ihringen, Germany) at logarithmic growth phase. | 3 × 106 CFU/dose 4 weekly instillations |
Anti-Gr1 antibody | BCG treatment was rendered ineffective on depletion of polymorphonuclear neutrophil granulocytes [32] |
Due to the emphasis on T cells, the role of innate cells in BCG therapy may have been ignored. However, innate immune cells such as NK cells and neutrophils likely play critical roles in BCG therapy (Fig. 1). Murine studies indicate that BCG instillation induces the infiltration of granulocytes into the bladder wall and depletion of granulocytes (e.g. neutrophils, basophils, and/or eosinophils) prevented the infiltration of CD4+ T cells and made BCG ineffective [32]; depletion of NK cells also reduced BCG efficacy [33]. CD56bright NK cells, which are traditionally low cytotoxic and high cytokine producers, were activated upon BCG treatment (Fig. 1). However, a small population of these CD56bright NK cells mediated higher cytotoxicity compared with CD56dim NK cells and correlated with improved survival in bladder cancer [36]. Further, IL-17-producing T cells mediated BCG-induced neutrophil recruitment to the bladder and were critical for the antitumor BCG efficacy in bladder cancer treatments [37]. In vitro studies also found that BCG increases the expression of major histocompatibility complex (MHC) class II on urothelial cells, suggesting that BCG regulates antigen presentation [34,35].
BCG also stimulated “trained immunity” through epigenetic reprogramming of monocytes, mediated by their transcriptional, epigenetic and metabolic rewiring, which leads to increased gene transcription and improved host defence [24]. These epigenetic changes manifest as histone chemical modifications (methylation/acetylation), driving enhanced chromatin accessibility, and subsequently faster and easier transcription of genes involved in antimicrobial responses and improved cell function [38]. Thus, the term “trained immunity” refers to the adaptive memory-like characteristics of innate immune cells [39]. Indeed, BCG vaccination is shown to protect against pneumonia [40] and diseases other than TB [41–44]. Recent studies showed that BCG mediates increased monocyte-derived cytokine secretion (such as IL-1b, TNF, IL-6) in response to unrelated pathogens in healthy individuals [45], where activated CD11b+ monocytes persisted in the circulation 3 months after BCG vaccination [45]. BCG “trained” these monocytes through modulating the NOD2 receptor and increasing histone H3 lysine K4 trimethylation [45].
Recent data showing that subcutaneous BCG priming increases the efficacy of intravesical BCG in a murine model of bladder cancer [46] renewed the interest in using intradermal BCG. Priming was associated with an increase in the T cell infiltration to the bladder tumor and repeated instillations were not required to induce migration of T cells into the bladder [46]. The same authors, in a cohort of 55 bladder cancer patients, found that those patients who displayed BCG immunity [i.e., a positive purified protein derivative (PPD) test due to their childhood M. tuberculosis vaccination] before the BCG instillation, exhibited greater clinical responses to BCG, in terms of recurrence-free survival, than PPD-negative patients [46]. This work contributed to the PRIME clinical trial, which is testing the effect of BCG priming on the efficacy of intravesical BCG therapy [47]. BCG priming was found to be well-tolerated and safe, and it increased the number and activation status of innate immune cells, mainly NK and T cells (Fig. 1) [48]. The cytotoxicity of NK cells and T cells against human bladder cancer cell lines also increased due to BCG priming [48].
In Japan, routine immunization with BCG to prevent TB is common but PPD positivity in bladder cancer patients is variable because BCG immunization status wanes over years. To test if PPD testing could be used to predict BCG therapy responses, Niwa et al. performed PPD skin testing on bladder cancer patients before intravesical BCG instillation [49]. They found that recurrence-free survival is significantly higher in subjects that had a positive PPD test compared with patients with a negative skin test. They concluded that the PPD skin test could be used to predict BCG responses in bladder cancer patients [49]. Currently, the product insert of BCG-TICE suggests PPD testing be employed before intravesical instillation, but PPD test is not commonly applied. PPD testing before BCG therapy requires further consideration, given the ease, safety, and cost-effectiveness of this testing.
4. Strain difference of BCG in immunotherapy
Even though the BCG immunotherapy is dependent on the immune system of the host, the existence of different BCG strains can contribute to the variation of efficacy. From the time BCG was distributed worldwide in 1924, several sub-strains (up to 27) evolved in different countries due to serial passaging [50]. In 1966, a seed-lot system was introduced to prevent further evolution of the different BCG strains. Current BCG strains share many mutations due to the initial 231 serial passage attenuation (1908–1921) made by Albert Calmette and Camille Guérin, and several unique mutations that are specific to the different countries in which BCG was continuously cultured. Commercially available BCG strains are genetically different from each other which results in different immune responses [51–53]. Moreover, there are dosage differences (colony forming units for live BCG), different percentages of dead and live BCG in each commercially available strain that influence the activation of different immune cell populations [54], and even different formulations affect BCG therapy [53,55]. However, there are no studies that definitively conclude that one strain of BCG is better than the other in the treatment of TB [56] or as a bladder cancer therapeutic strategy. Even though evidence from in vitro studies suggests that BCG strains could affect BCG therapy efficacy in bladder cancer [57], there are no adequately powered head-to-head trials that have confirmed that BCG strains influence treatment success in bladder cancer patients [53]. A prospective randomized trial (n = 142) comparing recurrence-free survival in patients given BCG-Connaught vs. BCG-TICE (Organon Teknika Corporation LLC [subsidiary of Merck&Co], Durham, NC, USA) showed that patients receiving BCG-Connaught strain had significantly higher survival than those receiving BCG-TICE (p = 0.01) [58]. However, patients did not receive the BCG maintenance therapy and thus, the higher clinical efficacy of the BGC-Connaught strain over BCG-TICE may be lowered by maintenance BCG treatment [59]. Another systemic review and network meta-analysis comparing 65 clinical trials showed that different BCG strains varied in their clinical efficacy when compared with chemotherapy [53]. BCG-Tokyo-172 displayed higher efficacy among other BCG strains but the lack of appropriate comparative trials could not conclusively predict the superiority of one BCG strain among others [53]. BCG-Tokyo 172 also showed superior efficacy in increasing recurrence-free survival in 129 bladder cancer patients when compared with the BCG-Connaught strain [60], but this trial was underpowered and was interrupted due to supply shortage of the BCG-Connaught strain. Currently, the BCG-Tokyo-172 is being tested in phase III clinical trial (S6102 trial) during a 3-year accrual period to compare its efficacy with BCG-Tice [61].
5. Current challenges in BCG immunotherapy
There are several challenges that BCG therapy faces in bladder cancer. Since Sanofi-Pasteur (Swiftwater, PA, USA or Lyon, France) stopped the production of BCG-Connaught, the United States currently has only one BCG manufacturer (Merck, Whitehouse Station, NJ, USA) producing BCG-TICE only [62]. The problem of this BCG shortage is highlighted further due to an increased global demand for BCG and anticipated shortages announced by companies whose stocks have been depleted [63]. This BCG shortage also increased the cost of BCG and other chemotherapies for NMIBC during the period of restricted BCG supply [64,65]. Even though Merck practically doubled its BCG-TICE production, there still are occasional availability issues [63]. Further, each country uses only one or two BCG strains, thus, comparative data on dose, efficacy, and tolerance of different BCG strains are limited [62].
The combination of BCG therapy with other agents has been unsuccessful. For instance, type I IFNγ was combined with BCG because of its function in innate immunity and possible synergistic function with BCG to activate antitumor protective immune responses [66]. Nevertheless, a significant benefit that would have prompted practice changes was not observed [66–68], rather a higher frequency of undesired side effects were noticed with this combination compared with BCG alone, which dampened enthusiasm in this combination strategy. Other approaches include co-administration of BCG with cytokines (IL-2 or IFN-γ) [69,70]. Some of these approaches are currently being tested or have completed early trial evaluation but, to date, have not boosted BCG efficacy against bladder cancer. Novel therapies which showed promising results in in vitro studies or animal models, such as the combination of BCG with antitumor vaccines [71], mycobacterium species [72], recombinant BCG strains [73–75], or fusion protein of IL-15 and BCG’s immunodominant antigen 85 (Ag85) [76] need to be further investigated. In this regard, PD-L1 is shown to be highly expressed in BCG-induced granulomata in patients who failed to respond to BCG [77]; thus, several clinical trials are currently underway for testing the efficacy of anti-PD1/anti-PDL1 in BCG refractory NMIBC [78]. Early identification of BCG unresponsive bladder cancer patients is needed, which will help in identifying candidates eligible for novel combination therapy studies. One of the barriers to successfully combine BCG with other agents is the initial high response rate to BCG therapy. Thus, clinical trials must be designed taking into account the high response rate of BCG and must include a control group of BCG alone to test the efficacy of the combination of any novel drugs with BCG instillation.
Although the number of serious side effects of BCG is low, adverse events (AE) do occur in a high percentage of BCG-treated patients. Some of the AE are as follows: urinary frequency, urinary urgency, nocturia, bladder pain, low-grade fever, chills, hematuria. Most of these symptoms resolve within 48 h of BCG instillation. More rare AEs include Reiters syndrome (urethral discharge, conjunctivitis, low back pain) [79], parotid gland infection (bilateral swelling of the parotid glands, parotid gland ulcer with continuous pus discharge) [80], arteriocutaneous fistula [81], Psoas abscess, iliac artery rupture [82] and Poncet’s disease (Diffuse arthritis) [83]. BCG infection occurs in a small percentage of bladder cancer patients leading to treat them with antituberculous drugs for six-nine months. Timely diagnosis and treatment of these AEs are critical, but the challenge is to completely understand the antitumoral mechanisms of BCG to enable us to modify or reduce BCG regimens schedule and avoid such AEs.
Another limitation of BCG therapy is the lack of accurate biomarkers that can successfully predict therapy responses, which delay the use of other therapeutic strategies in non-responders. One of the largest screening studies to identify biomarkers for BCG therapy used next-generation DNA sequencing of 105 bladder tumors [84]. However, despite the amount of data generated, only mutations in the chromatin modifier gene, ARID1A, were linked with tumor recurrence in the patient group treated with BCG. In another study, epigenetic alterations such as unmethylated cyclin-dependent kinase inhibitor 2B and MUS81a were associated with BCG failure [85], suggesting that epigenetic changes may also be important in the prediction of the BCG treatment response. Two multicenter studies also showed that fluorescent in situ hybridization (FISH) positivity (4 or more cells with polysomy on at least 2 chromosomes and/or at least 12 cells with a homozygous deletion for 9p21) in urine from bladder cancer patients predicts recurrence after BCG therapy [86,87]. Cytokine levels post BCG treatment have been investigated in several studies to predict BCG failure and tumor recurrence [88–90]. Changes in urinary levels of nine inducible cytokines (IL-2, IL-6, IL-8, IL-18, IL-1ra, TRAIL, IFN-γ, IL-12 [p70], and TNF) measured in 130 patients, 6 weeks after BCG instillation, showed that the patient’s nomogram based on the cytokine panel successfully predicted the patient cancer recurrence [88]. In another study, IL-2 mRNA extracted from peripheral blood during BCG treatment also correlated with remission over a 4 year time span [89]. However, different cytokines have complex regulatory relationships with each other and have different functions in modulating the tumor immunity; thus, pathway analyses may be required to define accurate predictive markers. Differences in urine volume and compositions, and the need for multi-level normalizations further complicate the use of urinary cytokines in predicting BCG treatment responses. Further, urinary cytokines levels may fall below the lower limit of detection before initiation of BCG therapy which complicates obtaining an accurate baseline pre-treatment. To define biomarkers, a retrospective study, using tissues obtained from the Nordic T1 and BCG–mitomycin C (MMC) trials [91], attempted to validate three previously identified biomarkers of BCG treatment response: ezrin, CK20, and Ki-67 [91]. The biomarker ezrin was found to be associated with both progression-free survival and treatment failure-free survival in the BCG treated cohort. However, this association could not be validated in the multivariable analyses [91]. Overall, these studies highlight the difficulty of identifying biomarkers that successfully can predict the BCG therapy response in bladder cancer patients.
6. BCG and other cancers
The involvement of BCG therapy in cancers other than bladder cancer is understudied and controversial [92,93]. Studies showed an increased incidence of lung adenocarcinomas among pulmonary TB patients, suggesting a mycobacteria-induced tumorigenic response [94]. In this context, in vitro studies showed that BCG blocked the TNF-dependent clearance of tumor cells via apoptosis [94], highlighting the controversy about the role of the BCG vaccine in tumor immunity. Conversely, a recent study indicated that BCG therapy drives tumor clearance through the induction of long-term tumor-specific IFN-γ+ CD4+ T cell-dependent responses instead of through BCG-specific driven immunity [30]. This study opens the possibility of using controlled BCG immunotherapy to generate antitumor immunity and treat any kind of cancer.
BCG is reported to be useful in other non-urological cancers [95], including cases of acute myeloid leukemia, lung cancer, and melanomas [8,96,97]. In these cases, both BCG and BCG cell envelope fractions and/or specific components (e.g. trehalose dimycolate, arabinan) are described as antitumor agents [95]. Different whole Mycobacterium spp., including BCG, and mycobacterial derivatives have been used for the treatment of melanoma and lung cancer alone or in combination with chemotherapy or radiotherapy [95]. In human lung cancer clinical trials, BCG is being used in combination with other compounds, such as mAGP (mycolyl-ara binogalactan-peptidoglycan cell wall complex), or together with an anti-idiotypic antibody mimicking the GD3 ganglioside expressed on most small-cell lung cancers [95]. BCG mAGP cell envelope core combined with whole BCG or with Lewis lung carcinoma inactivated antigens showed a TLR2 dependent IFN-γ therapeutic effect in mouse models [98]. However, when translated to humans, these notable therapeutics effects were unclear, with some clinical studies supporting the mouse model results [99,100], and others showing contrary results with no beneficial effects [101,102].
For melanoma treatment, BCG-TICE shows its antitumor benefits by favoring antitumor T cells responses and improving M2 macrophage responses within the melanoma microenvironment [103]. However, synthetic BCG cell envelope component derivatives such as muramyl tripeptide linked to a phospholipid called phosphatidyl-ethanolamine and encapsulated in liposomes showed potential positive effects in treating stage III and IV melanomas [104]. Other BCG-derived compounds alone or in combination with adjuvants have shown limited efficacy against tumorigenesis; these include heat shock proteins, such as HSP65 in the treatment of melanomas [105–107]. In this context, the use of M. tuberculosis complex mycobacterial components such as the mycolyl-transferase Ag85 complex and soluble culture filtrate protein ESAT-6 fused with a GPI-anchor, alone or in combination with B6 tumor antigens seems effective in reducing or dismantling tumors [108,109].
Apart from BCG, attenuated or non-virulent Mycobacterium spp., such as M. smegmatis, M. obuense, and M. vaccae among others have been tested against melanoma formation and treatment but none showed any success [95]. Only M. smegmatis showed some capacity to reduce melanoma tumor growth, but the effect was transient [110].
For other cancers such as gastrointestinal tract cancer, breast cancer, liver cancer, ovarian and cervix tumors, hematopoietic and lymphoid malignancies, and sarcoma and mesothelioma, treatment with Mycobacterium spp. and their cell wall components have been reported to be successful in animal models and some clinical trials when combined with inactivated tumorigenic antigens or with specific adjuvants [95]. Whole M. vaccae and the proprietary heat inactivated M. obuense preparation called IMM-101 showed potential as promising anti-cancer agents. BCG-Moreau in combination with chemotherapy increased gastric cancer patient survival [111].
The potential of BCG has also been studied for pancreatic cancer alone or in combination with chemotherapy [112], showing that compounds such as gemcitabine sensitize pancreatic cells to the antitumor cytotoxic T cell responses generated by BCG. In colon-cancer and hepatoma treatments, few studies showed improvement but none of them directly used live BCG and just used isolated BCG or mixtures of cell envelope soluble components, mainly taking advantage of their adjuvant effects [95]. Conversely, for breast cancer treatment, recombinant live BCG expressing the breast tumor-associated antigen MUC1 and growth factors [113] or TH1/TH2 cytokines [114] is shown to improve outcome in animal models and human clinical trials [115–117]. As in the case of other cancers, treatment of ovarian and cervix tumors with BCG can also provide a benefit [118]. Indeed, the axis composed of “BCG and its associated soluble cell envelope components in combination with tumor-specific Ags - Improved DC Ag presentation – Enhanced NK cytotoxic T cell responses” seem to play a major role in the BCG antitumor effects described for many cancers. Finally, limited studies exist that used whole live BCG for leukemia [106] and sarcoma treatment [119]. Most studies use BCG cell envelope components which increase the local recruitment maturation, Ag-presentation of antigen-presenting cells (both DCs and macrophages), superoxide production, cytotoxic T cell activity among other antitumor beneficial host responses.
7. Conclusions
The impact of BCG on the treatment of bladder cancer has been substantial and it remains one of the successful examples of cancer immunotherapy. Both adaptive and innate immune cells are critical in BCG therapy. However, the mechanism of BCG antitumor response is unclear and likely multifactorial. Many challenges still plague the BCG field in bladder cancer. The use of different strains of BCG in different parts of the world makes comparative data on dose, efficacy, and tolerance of different stains limited and it is not clear whether BCG strain difference holds significant therapeutic relevance. BCG failure, lack of biomarkers to predict BCG response, early identification of BCG non-responders eligible for alternative treatment strategies, and limited success in combining BCG with other agents pose significant therapeutic challenges. Understanding the pathophysiology of BCG resistance and the identification of new mechanistic pathways involved in BCG therapy will be useful in developing predictive signatures and in designing novel patient-adjusted therapy. Ongoing studies in our labs are showing promising results for bladder cancer patients that cannot tolerate BCG treatment. We found that selective temporal delipidation of BCG removing apolar lipids from its cell surface keeps it 100% viable and drives a cellular immune effect akin to the one observed with naïve BCG in a murine model of bladder cancer. Delipidated BCG also does not drive the cytotoxicity and local cytokine storm that generate adverse effects in some bladder cancer patients (data not shown). As the world is also struggling with a scarcity in BCG supply which has resulted in increased cost for BCG, we should also focus on finding improved BCG alternatives for the treatment of bladder cancer and other cancers.
Acknowledgments
(1) the Mays Family Cancer Center at University of Texas Health San Antonio (P30 CA054174), (2) the Roger L. And Laura D. Zeller Charitable Foundation Chair in Urologic Cancer, (3) the Glenda and Gary Woods Distinguished Chair in GU Oncology, (4) The San Antonio Medical Foundation Fund, (5) the Max & Minnie Tomerlin Voelcker Fund, (6) CDMRP CA170270/P1P2, (7) Bladder Cancer Advocacy Network (BCAN), (8) Research Training Award (RP170345) from the Cancer Prevention & Research Institute of Texas, (9) MSTP Program (NIH T32GM113896), and (10) the Robert J. Kleberg Jr. and Helen C. Kleberg Foundation (11) Spanish Ministry of Science, Innovation and Universities - FEDER Funds (RTI2018–098777-B-I00), and (12) the Generalitat of Catalunya (2017SGR-229).
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Robert Svatek reports research support was provided by Japanese BCG Laboratories (JBL). Robert Svatek reports research support was provided by MDx Health. Robert Svatek reports a relationship with FKD Therapies Oy that includes: consulting or advisory.
References
- [1].Calmette AGC, Boquet A, et al. La vaccination préventive contre la tuberculose par le “BCG.”. Paris: Masson et cie; 1927. [Google Scholar]
- [2].Zbar B, Bernstein I, Tanaka T, Rapp HJ. Tumor immunity produced by the intradermal inoculation of living tumor cells and living Mycobacterium bovis (strain BCG). Science 1970;170(3963):1217–8. 10.1126/science:170.3963.1217. [DOI] [PubMed] [Google Scholar]
- [3].Pearl R Cancer and Tuberculosis. Am J Epidemiol 1929;9(1):97–159. [Google Scholar]
- [4].Hoption Cann SA, van Netten JP, van Netten C. Dr William Coley and tumour regression: a place in history or in the future. Postgrad Med J 2003;79 (938):672–80 [published Online First: 2004/01/07]. [PMC free article] [PubMed] [Google Scholar]
- [5].I H. Employment of B. C. G. especially in Intravenous Injection. Acta Medica Scandinavica 1936;90:350–61. [Google Scholar]
- [6].Old LJ, Clarke DA, Benacerraf B. Effect of Bacillus Calmette-Guerin infection on transplanted tumours in the mouse. Nature 1959;184(Suppl 5):291–2. 10.1038/184291a0 [published Online First: 1959/07/25]. [DOI] [PubMed] [Google Scholar]
- [7].Mathe G, Halle-Pannenko O, Bourut C. BCG in cancer immunotherapy: results obtained with various BCG preparations in a screening study for systemic adjuvants applicable to cancer immunoprophylaxis or immunotherapy. Natl Cancer Inst Monogr 1973;39:107–13 [published Online First: 1973/12/01]. [PubMed] [Google Scholar]
- [8].Morton DL, Eilber FR, Holmes EC, et al. BCG immunotherapy of malignant melanoma: summary of a seven-year experience. Ann Surg 1974;180 (4):635–43. 10.1097/00000658-197410000-00029 [published Online First: 1974/10/01]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].deKernion JB, Golub SH, Gupta RK, et al. Successful transurethral intralesional BCG therapy of a bladder melanoma. Cancer 1975;36(5):1662–7. [published Online First: 1975/11/01]. [DOI] [PubMed] [Google Scholar]
- [10].Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-Guerin in the Treatment of Superficial Bladder Tumors. J Urol 2017;197(2S):S142–5. 10.1016/j.juro.2016.10.101 [published Online First: 2016/12/ 26]. [DOI] [PubMed] [Google Scholar]
- [11].Lamm Donald L, Thor Daniel E, Harris Steven C, Reyna Juan A, Stogdill Valerie D, Radwin Howard M. Bacillus Calmette-Guerin immunotherapy of superficial bladder cancer. J Urol 1980;124(1):38–42. 10.1016/S0022-5347(17)55282-9. [DOI] [PubMed] [Google Scholar]
- [12].Brosman SA. Experience with bacillus Calmette-Guerin in patients with superficial bladder carcinoma. J Urol 1982;128(1):27–30. 10.1016/s0022-5347(17)52736-6 [published Online First: 1982/07/01]. [DOI] [PubMed] [Google Scholar]
- [13].Lamm DL, Blumenstein BA, Crissman JD, et al. Maintenance bacillus CalmetteGuerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. J Urol 2000;163(4):1124–9 [published Online First: 2000/03/29]. [PubMed] [Google Scholar]
- [14].Bevers RF, Kurth KH, Schamhart DH. Role of urothelial cells in BCG immunotherapy for superficial bladder cancer. Br J Cancer 2004;91 (4):607–12. 10.1038/sj.bjc.6602026 [published Online First: 2004/07/22]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhao W, Schorey JS, Bong-Mastek M, et al. Role of a bacillus Calmette-Guerin fibronectin attachment protein in BCG-induced antitumor activity. Int J Cancer 2000;86(1):83–8. [published Online First: 2000/03/23]. [DOI] [PubMed] [Google Scholar]
- [16].Ratliff TL, Palmer JO, McGarr JA, et al. Intravesical Bacillus Calmette-Guerin therapy for murine bladder tumors: initiation of the response by fibronectin-mediated attachment of Bacillus Calmette-Guerin. Cancer Res 1987;47 (7):1762–6 [published Online First: 1987/04/01]. [PubMed] [Google Scholar]
- [17].Teppema JS, de Boer EC, Steerenberg PA, et al. Morphological aspects of the interaction of Bacillus Calmette-Guerin with urothelial bladder cells in vivo and in vitro: relevance for antitumor activity? Urol Res 1992;20(3):219–28. 10.1007/BF00299721 [published Online First: 1992/01/01]. [DOI] [PubMed] [Google Scholar]
- [18].Kavoussi LR, Brown EJ, Ritchey JK, et al. Fibronectin-mediated Calmette-Guerin bacillus attachment to murine bladder mucosa. Requirement for the expression of an antitumor response. J Clin Invest 1990;85(1):62–7. 10.1172/JCI114434 [published Online First: 1990/01/01]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Witjes JA, Meijden AP, Doesburg W, et al. Influence of fibrin clot inhibitors on the efficacy of intravesical Bacillus Calmette-Guerin in the treatment of superficial bladder cancer. The Dutch Southeast Cooperative Urological Group [published Online First: 1993/01/01]. Eur Urol 1993;23(3):366–70. 10.1159/000474631. [DOI] [PubMed] [Google Scholar]
- [20].Lipsky MJ, Badalato GM, Motamedinia P, et al. The effect of fibrin clot inhibitors on the immunomodulatory efficacy of Bacillus Calmette-Guerin therapy for non-muscle-invasive bladder cancer. Urology 2013;81 (6):1273–8. 10.1016/j.urology.2012.09.065 [published Online First: 2013/03/27]. [DOI] [PubMed] [Google Scholar]
- [21].Lewis SA. Everything you wanted to know about the bladder epithelium but were afraid to ask. Am J Physiol Renal Physiol 2000;278(6):F867–74. 10.1152/ajprenal.2000.278.6.F867 [published Online First: 2000/06/ 03]. [DOI] [PubMed] [Google Scholar]
- [22].Noguera-Ortega Estela, Blanco-Cabra Núria, Rabanal Rosa Maria, Alejandro SánchezChardi, Roldán Mónica, Guallar-Garrido Sandra, et al. Mycobacteria emulsified in olive oil-in-water trigger a robust immune response in bladder cancer treatment. Sci Rep 2016;6(1). 10.1038/srep27232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gil Redelman-Sidi, Gopa Iyer, Solit David B, Glickman Michael S. Oncogenic activation of Pak1-dependent pathway of macropinocytosis determines BCG entry into bladder cancer cells. Cancer Res 2013;73(3):1156–67. 10.1158/0008-5472.CAN-12-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Buffen K, Oosting M, Quintin J, et al. Autophagy controls BCG-induced trained immunity and the response to intravesical BCG therapy for bladder cancer. PLoS Pathog 2014;10(10):e1004485. 10.1371/journal.ppat.1004485 [published Online First: 2014/10/31]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bevers RF, de Boer EC, Kurth KH, et al. BCG-induced interleukin-6 upregulation and BCG internalization in well and poorly differentiated human bladder cancer cell lines. Eur Cytokine Netw 1998;9(2):181–6 [published Online First: 1998/07/29]. [PubMed] [Google Scholar]
- [26].De Boer EC, De Jong WH, Steerenberg PA, et al. Induction of urinary interleukin-1 (IL-1), IL-2, IL-6, and tumour necrosis factor during intravesical immunotherapy with bacillus Calmette-Guerin in superficial bladder cancer. Cancer Immunol Immunother 1992;34(5):306–12. 10.1007/BF01741551 [published Online First: 1992/01/01]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Luo Y, Chen X, O’Donnell MA. Role of Th1 and Th2 cytokines in BCG-induced IFN-gamma production: cytokine promotion and simulation of BCG effect. Cytokine 2003;21(1):17–26. 10.1016/s1043-4666(02)004908 [published Online First: 2003/04/02]. [DOI] [PubMed] [Google Scholar]
- [28].Bisiaux Aurélie Thiounn Nicolas, Timsit Marc-Olivier Eladaoui Ahmed, Chang Huey-Hsuan Mapes James, et al. Molecular analyte profiling of the early events and tissue conditioning following intravesical bacillus calmette-guerin therapy in patients with superficial bladder cancer. J Urol 2009;181 (4):1571–80. 10.1016/j.juro.2008.11.124. [DOI] [PubMed] [Google Scholar]
- [29].Max Kates, Thomas Nirschl, Sopko Nikolai A, Matsui Hotaka, Kochel Christina M, Reis Leonardo O, et al. Intravesical BCG Induces CD4(+) T-Cell Expansion in an Immune Competent Model of Bladder Cancer. Cancer Immunol Res 2017;5 (7):594–603. 10.1158/2326-6066.CIR-16-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Antonelli AC, Binyamin A, Hohl TM, et al. Bacterial immunotherapy for cancer induces CD4-dependent tumor-specific immunity through tumor-intrinsic interferon-gamma signaling. Proc Natl Acad Sci USA 2020;117(31):18627–37. 10.1073/pnas.2004421117 [published Online First: 2020/07/ 19]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Renate Pichler, Georg Gruenbacher, Zoran Culig, Andrea Brunner, Dietmar Fuchs, Josef Fritz, et al. Intratumoral Th2 predisposition combines with an increased Th1 functional phenotype in clinical response to intravesical BCG in bladder cancer. Cancer Immunol Immunother 2017;66(4):427–40. 10.1007/s00262-016-1945-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Suttmann H, Riemensberger J, Bentien G, et al. Neutrophil granulocytes are required for effective Bacillus Calmette-Guerin immunotherapy of bladder cancer and orchestrate local immune responses. Cancer Res 2006;66 (16):8250–7. 10.1158/0008-5472.CAN-06-1416 [published Online First: 2006/08/17]. [DOI] [PubMed] [Google Scholar]
- [33].Henrik Suttmann, Marc Jacobsen, Karina Reiss, Dieter Jocham, Andreas Böhle, Sven Brandau. Mechanisms of bacillus Calmette-Guerin mediated natural killer cell activation. J Urol 2004;172(4 Part 1):1490–5. 10.1097/01.ju.0000131944.52354.63. [DOI] [PubMed] [Google Scholar]
- [34].Lage Janice M, Bauer Walter C, Kelley David R, Ratliff Timothy L, Catalona William J. Histological parameters and pitfalls in the interpretation of bladder biopsies in bacillus Calmette-Guerin treatment of superficial bladder cancer. J Urol 1986;135(5):916–9. 10.1016/S0022-5347(17)45922-2. [DOI] [PubMed] [Google Scholar]
- [35].De Boer EC, De Jong WH, Van Der Meijden AP, et al. Presence of activated lymphocytes in the urine of patients with superficial bladder cancer after intravesical immunotherapy with bacillus Calmette-Guerin. Cancer Immunol Immunother 1991;33(6):411–6. 10.1007/BF01741603 [published Online First: 1991/01/01]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].García-Cuesta Eva M, Esteso Gloria, Ashiru Omodele, López-Cobo Sheila, Álvarez-Maestro Mario, Linares Ana, et al. Characterization of a human antitumoral NK cell population expanded after BCG treatment of leukocytes. Oncoimmunology 2017;6(4):e1293212. 10.1080/2162402X.2017.1293212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Takeuchi A, Dejima T, Yamada H, et al. IL-17 production by gammadelta T cells is important for the antitumor effect of Mycobacterium bovis bacillus Calmette-Guerin treatment against bladder cancer. Eur J Immunol 2011;41 (1):246–51. 10.1002/eji.201040773 [published Online First: 2010/12/25]. [DOI] [PubMed] [Google Scholar]
- [38].O’Neill LAJ, Netea MG. BCG-induced trained immunity: can it offer protection against COVID-19? Nat Rev Immunol 2020;20(6):335–7. 10.1038/s41577-020-0337-y [published Online First: 2020/05/13]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Netea MG, Quintin J, van der Meer JW. Trained immunity: a memory for innate host defense. Cell Host Microbe 2011;9(5):355–61. 10.1016/j.chom.2011.04.006 [published Online First: 2011/05/18]. [DOI] [PubMed] [Google Scholar]
- [40].Niobey FM, Duchiade MP, Vasconcelos AG, et al. Risk factors for death caused by pneumonia in children younger than 1 year old in a metropolitan region of southeastern Brazil. A case- control study. Rev Saude Publica 1992;26 (4):229–38. 10.1590/s0034-89101992000400004 [published Online First: 1992/08/01]. [DOI] [PubMed] [Google Scholar]
- [41].Velema Johan P, Alihonou Eusèbe M, Gandaho Timothé, Hounye Félicien H Childhood mortality among users and non-users of primary health care in a rural west African community. Int J Epidemiol 1991;20(2):474–9. 10.1093/ije/20.2.474. [DOI] [PubMed] [Google Scholar]
- [42].Vaugelade J, Pinchinat S, Guiella G, Elguero E, Simondon F. Non-specific effects of vaccination on child survival: prospective cohort study in Burkina Faso. BMJ 2004;329(7478):1309. 10.1136/bmj.38261.496366.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kristensen I, Aaby P, Jensen H. Routine vaccinations and child survival: follow up study in Guinea-Bissau, West Africa. BMJ 2000;321(7274):1435–8. 10.1136/bmj.321.7274.1435 [published Online First: 2000/ 12/09]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Moorlag SJCFMRJW, van Crevel RMG. Non-specific effects of BCG vaccine on viral infections. Clin Microbiol Infect 2019;25(12):1473–8. 10.1016/j.cmi.2019.04.020. [DOI] [PubMed] [Google Scholar]
- [45].Kleinnijenhuis J, Quintin J, Preijers F, et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci USA 2012;109 (43):17537–42. 10.1073/pnas.1202870109 [published Online First: 2012/09/19]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Biot C, Rentsch CA, Gsponer JR, et al. Preexisting BCG-specific T cells improve intravesical immunotherapy for bladder cancer. Sci Transl Med 2012;4 (137):137ra72. 10.1126/scitranslmed.3003586 [published Online First: 2012/06/08]. [DOI] [PubMed] [Google Scholar]
- [47].Svatek RS, Tangen C, Delacroix S, et al. Background and Update for S1602 “A Phase III Randomized Trial to Evaluate the Influence of BCG Strain Differences and T Cell Priming with Intradermal BCG Before Intravesical Therapy for BCG-naive High-grade Non-muscle-invasive Bladder Cancer. Eur Urol Focus 2018;4(4):522–4. 10.1016/j.euf.2018.08.015 [published Online First: 2018/09/11]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Niannian Ji, Neelam Mukherjee, Morales Edwin EE, Hurez Vincent, Curiel Tyler J, et al. Percutaneous BCG enhances innate effector antitumor cytotoxicity during treatment of bladder cancer: a translational clinical trial. Oncoimmunology 2019;8(8):e1614857. 10.1080/2162402X.2019.1614857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Niwa N, Kikuchi E, Matsumoto K, et al. Purified protein derivative skin test reactions are associated with clinical outcomes of patients with nonmuscle invasive bladder cancer treated with induction bacillus Calmette-Guerin therapy 77 e15–77 e21. Urol Oncol 2018;36. 10.1016/j.urolonc.2017.10.005. [DOI] [PubMed] [Google Scholar]
- [50].Herr HW, Morales A. History of bacillus Calmette-Guerin and bladder cancer: an immunotherapy success story. J Urol 2008;179(1):53–6. 10.1016/j.juro.2007.08.122 [published Online First: 2007/11/13]. [DOI] [PubMed] [Google Scholar]
- [51].Behr MA, Small PM. A historical and molecular phylogeny of BCG strains. Vaccine 1999;17(7–8):915–22. 10.1016/s0264-410x(98)00277-1 [published Online First: 1999/03/06]. [DOI] [PubMed] [Google Scholar]
- [52].Hayashi D, Takii T, Mukai T, et al. Biochemical characteristics among Mycobacterium bovis BCG substrains. FEMS Microbiol Lett 2010;306 (2):103–9. 10.1111/j.1574-6968.2010.01947.x. [DOI] [PubMed] [Google Scholar]
- [53].Boehm BE, Cornell JE, Wang H, et al. Efficacy of bacillus Calmette-Guerin Strains for Treatment of Nonmuscle Invasive Bladder Cancer: a Systematic Review and Network Meta-Analysis. J Urol 2017;198(3):503–10. 10.1016/j.juro.2017.01.086 [published Online First: 2017/ 03/14]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Gloria Esteso, Nacho Aguiló, Esther Julián, Omodele Ashiru, Ho Mei M, Martín Carlos, et al. Natural killer anti-tumor activity can be achieved by in vitro incubation with heat-killed BCG. Front Immunol 2021;12. 10.3389/fimmu.2021.622995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Guallar-Garrido S, Julian E. Bacillus Calmette-Guerin (BCG) Therapy for Bladder Cancer: An Update. Immunotargets Ther 2020;9:1–11. 10.2147/ITT.S202006 [published Online First: 2020/02/28]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Moliva JI, Turner J, Torrelles JB. Prospects in Mycobacterium bovis Bacille Calmette et Guerin (BCG) vaccine diversity and delivery: why does BCG fail to protect against tuberculosis? Vaccine 2015;33(39):5035–41. 10.1016/j.vaccine.2015.08.033 [published Online First: 2015/09/01]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Secanella-Fandos S, Luquin M, Julian E. Connaught and Russian strains showed the highest direct antitumor effects of different Bacillus CalmetteGuerin substrains. J Urol 2013;189(2):711–8. 10.1016/j.juro.2012.09.049 [published Online First: 2012/09/18]. [DOI] [PubMed] [Google Scholar]
- [58].Rentsch CA, Birkhauser FD, Biot C, et al. Bacillus Calmette-Guerin strain differences have an impact on clinical outcome in bladder cancer immunotherapy. Eur Urol 2014;66(4):677–88. 10.1016/j.eururo.2014.02.061 [published Online First: 2014/03/29]. [DOI] [PubMed] [Google Scholar]
- [59].Witjes JA, Dalbagni G, Karnes RJ, et al. The efficacy of BCG TICE and BCG Connaught in a cohort of 2,099 patients with T1G3 non-muscle-invasive bladder cancer 484 e19–84 e25. Urol Oncol 2016;34(11). 10.1016/j.urolonc.2016.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Sengiku A, Ito M, Miyazaki Y, et al. A prospective comparative study of intravesical bacillus Calmette-Guerin therapy with the Tokyo or Connaught strain for nonmuscle invasive bladder cancer. J Urol 2013;190(1):50–4. 10.1016/j.juro.2013.01.084 [published Online First: 2013/02/ 05]. [DOI] [PubMed] [Google Scholar]
- [61].Meeks JJ, Lerner SP, Svatek RS. Bacillus Calmette-Guerin Manufacturing and SWOG S1602 Intergroup Clinical Trial. J Urol 2017;197(3 Pt 1):538–40. 10.1016/j.juro.2016.12.024 [published Online First: 2016/12/ 20]. [DOI] [PubMed] [Google Scholar]
- [62].Mukherjee N, Wheeler KM, Svatek RS. Bacillus Calmette-Guerin treatment of bladder cancer: a systematic review and commentary on recent publications. Curr Opin Urol 2019;29(3):181–8. 10.1097/MOU.0000000000000595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Messing EM, The BCG. Shortage. Bladder Cancer 2017;3(3):227–8. 10.3233/BLC-179018 [published Online First: 2017/08/22]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Ourfali S, Ohannessian R, Fassi-Fehri H, et al. Recurrence Rate and Cost Consequence of the Shortage of Bacillus Calmette-Guerin Connaught Strain for Bladder Cancer Patients. Eur Urol Focus 2021;7(1):111–6. 10.1016/j.euf.2019.04.002 [published Online First: 2019/04/22]. [DOI] [PubMed] [Google Scholar]
- [65].Davies BJ, Hwang TJ, Kesselheim AS. Ensuring Access to Injectable Generic Drugs - The Case of Intravesical BCG for Bladder Cancer. N Engl J Med 2017;376(15):1401–3. 10.1056/NEJMp1615697. [DOI] [PubMed] [Google Scholar]
- [66].Lamm D, Brausi M, O’Donnell MA, et al. Interferon alfa in the treatment paradigm for non-muscle-invasive bladder cancer 35 e21–30 [published Online First: 2013/05/01]. Urol Oncol 2014;32(1). 10.1016/j.urolonc.2013.02.010. [DOI] [PubMed] [Google Scholar]
- [67].Nepple KG, Lightfoot AJ, Rosevear HM, et al. Bacillus Calmette-Guerin with or without interferon alpha-2b and megadose versus recommended daily allowance vitamins during induction and maintenance intravesical treatment of nonmuscle invasive bladder cancer. J Urol 2010;184 (5):1915–9. 10.1016/j.juro.2010.06.147 [published Online First: 2010/09/18]. [DOI] [PubMed] [Google Scholar]
- [68].Shepherd AR, Shepherd E, Brook NR. Intravesical Bacillus Calmette-Guerin with interferon-alpha versus intravesical Bacillus Calmette-Guerin for treating non-muscle-invasive bladder cancer. Cochrane Database Syst. Rev. 2017;3:112. 10.1002/14651858.CD012112.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Grossman HB, Lamm DL, Kamat AM, et al. Innovation in bladder cancer immunotherapy. J Immunother 2016;39(8):291–7. 10.1097/CJI.0000000000000130. [DOI] [PubMed] [Google Scholar]
- [70].Steinberg Ryan L, Brooks Nathan A, Thomas Lewis J, Mott Sarah L, O’Donnell Michael A. Bacillus Calmette-Guerin strain may not effect recurrence-free survival when used intravesically with interferon-alpha2b for non-muscle-invasive bladder cancer. Urol Oncol 2017;35(5):201–7. 10.1016/j.urolonc.2016.11.016. [DOI] [PubMed] [Google Scholar]
- [71].Laurent Derré, Cesson Valérie Lucca Ilaria, Yannick Cerantola, Massimo Valerio, Urs Fritschi, et al. Intravesical Bacillus Calmette Guerin Combined with a Cancer Vaccine Increases Local T-Cell Responses in Non-muscle-Invasive Bladder Cancer Patients. Clin Cancer Res 2017;23(3):717–25. 10.1158/1078-0432.CCR-16-1189. [DOI] [PubMed] [Google Scholar]
- [72].Julián EN-OaE. Mycobacteria-Derived Agents for the Treatment of Urological and Renal Cancers. Mycobacterium - Research and Development; 2016. doi: 10.5772/intechopen.69659. [DOI] [Google Scholar]
- [73].Sun E, Nian X, Liu C, et al. Construction of recombinant human IFNalpha-2b BCG and its antitumor effects on bladder cancer cells in vitro. Genet Mol Res 2015;14(2):3436–49. 10.4238/2015.April.15.7 [published Online First: 2015/05/13]. [DOI] [PubMed] [Google Scholar]
- [74].Dunia Rodriguez, Cibelly Goulart, Pagliarone Ana C, Silva Eliane P, Cunegundes Priscila S, Nascimento Ivan P, et al. In vitro Evidence of Human Immune Responsiveness Shows the Improved Potential of a Recombinant BCG Strain for Bladder Cancer Treatment. Front Immunol 2019;10. 10.3389/fimmu.2019.0146010.3389/fimmu.2019.01460.s00110.3389/fimmu.2019.01460.s002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Begnini KR, Buss JH, Collares T, et al. Recombinant Mycobacterium bovis BCG for immunotherapy in nonmuscle invasive bladder cancer. Appl Microbiol Biotechnol 2015;99(9):3741–54. 10.1007/s00253-015-64953. [DOI] [PubMed] [Google Scholar]
- [76].Takeuchi A, Eto M, Tatsugami K, et al. Antitumor activity of recombinant Bacille Calmette-Guerin secreting interleukin-15-Ag85B fusion protein against bladder cancer. Int Immunopharmacol 2016;35:327–31. 10.1016/j.intimp.2016.03.007 [published Online First: 2016/04/20]. [DOI] [PubMed] [Google Scholar]
- [77].Inman Brant A, Sebo Thomas J, Frigola Xavier, Dong Haidong, Bergstralh Eric J, Frank Igor, et al. PD-L1 (B7–H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: associations with localized stage progression. Cancer 2007;109(8):1499–505. 10.1002/(ISSN)1097-014210.1002/cncr.v109:810.1002/cncr.22588. [DOI] [PubMed] [Google Scholar]
- [78].Pettenati C, Ingersoll MA. Mechanisms of BCG immunotherapy and its outlook for bladder cancer. Nat Rev Urol 2018;15(10):615–25. 10.1038/s41585-018-0055-4 [published Online First: 2018/ 07/12]. [DOI] [PubMed] [Google Scholar]
- [79].Ng KL, Chua CB. Reiter’s syndrome postintravesical Bacillus Calmette-Guerin instillations. Asian J Surg 2017;40(2):163–5. 10.1016/j.asjsur.2014.01.016. [DOI] [PubMed] [Google Scholar]
- [80].Eviatar Friedlander, Martínez Pascual Paula, Montilla de Mora Pedro, Scola Yurrita Bartolomé. Bilateral parotid glands infection caused by Calmette-Guerin Bacillus after intravesical therapy for recurrent bladder cancer: a case report. Braz J Otorhinolaryngol 2017;83(6):726–9. 10.1016/j.bjorl.2015.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Torres-Blanco A, Gomez-Palones F, Edo-Fleta G. Arteriocutaneous Fistula Associated with Bilateral Femoral Pseudoaneurysms Caused by Bacillus Calmette-Guerin Apropos of a Case and Review of Literature. Ann Vasc Surg 2017;39:2. 10.1016/j.avsg.2016.07.094. [DOI] [PubMed] [Google Scholar]
- [82].Leeman M, Burgers P, Brehm V, et al. Psoas abscess after bacille Calmette-Guerin instillations causing iliac artery contained rupture. J Vasc Surg 2017;66(4):1236–8. 10.1016/j.jvs.2017.02.038 [published Online First: 2017/05/10]. [DOI] [PubMed] [Google Scholar]
- [83].Sampaio PCM, Lira YG, Ribeiro HYU, et al. Poncet’s disease after the intravesical instillation of Bacillus Calmette-Guerin (BCG): a case report. BMC Res Notes 2017;10(1):416. 10.1186/s13104-017-2606-9 [published Online First: 2017/08/20]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Pietzak Eugene J, Bagrodia Aditya, Cha Eugene K, Drill Esther N, Iyer Gopa, Isharwal Sumit, et al. Next-generation Sequencing of Nonmuscle Invasive Bladder Cancer Reveals Potential Biomarkers and Rational Therapeutic Targets. Eur Urol 2017;72(6):952–9. 10.1016/j.eururo.2017.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Husek P, Pacovsky J, Chmelarova M, et al. Methylation status as a predictor of intravesical Bacillus Calmette-Guerin (BCG) immunotherapy response of high grade non-muscle invasive bladder tumor. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2017;161(2):210–6. 10.5507/bp.2017.008 [published Online First: 2017/03/28]. [DOI] [PubMed] [Google Scholar]
- [86].Bao Y, Tu X, Chang T, et al. The role of fluorescence in situ hybridization to predict patient response to intravesical Bacillus Calmette-Guerin therapy for bladder cancer: A diagnostic meta-analysis and systematic review. Medicine (Baltimore) 2018;97(36):. 10.1097/MD.0000000000012227 [published Online First: 2018/09/12]e12227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Liem E, Baard J, Cauberg ECC, et al. Fluorescence in situ hybridization as prognostic predictor of tumor recurrence during treatment with Bacillus Calmette-Guerin therapy for intermediate- and high-risk non-muscle-invasive bladder cancer. Med Oncol 2017;34(10):172. 10.1007/s12032-017-1033-z [published Online First: 2017/09/04]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Kamat AM, Briggman J, Urbauer DL, et al. Cytokine Panel for Response to Intravesical Therapy (CyPRIT): Nomogram of Changes in Urinary Cytokine Levels Predicts Patient Response to Bacillus Calmette-Guerin. Eur Urol 2016;69(2):197–200. 10.1016/j.eururo.2015.06.023 [published Online First: 2015/06/30]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Kaempfer R, Gerez L, Farbstein H, Madar L, Hirschman O, Nussinovich R, et al. Prediction of response to treatment in superficial bladder carcinoma through pattern of interleukin-2 gene expression. J Clin Oncol 1996;14(6):1778–86. 10.1200/JCO.1996.14.6.1778. [DOI] [PubMed] [Google Scholar]
- [90].Amirali Salmasi, Elashoff David A, Guo Rong, Upfill-Brown Alexander, Rosser Charles J, Rose Jason M, et al. Urinary Cytokine Profile to Predict Response to Intravesical BCG with or without HS-410 Therapy in Patients with Non-muscle-invasive Bladder Cancer. Cancer Epidemiol Biomarkers Prev 2019;28 (6):1036–44. 10.1158/1055-9965.EPI-18-0893. [DOI] [PubMed] [Google Scholar]
- [91].Malmstrom PU, Hemdan T, Segersten U. Validation of the ezrin, CK20, and Ki-67 as potential predictive markers for BCG instillation therapy of non-muscle-invasive bladder cancer. Urol Oncol 2017;35(8):2. 10.1016/j.urolonc.2017.03.010. [DOI] [PubMed] [Google Scholar]
- [92].Yu Yang-Hao, Liao Chien-Chang, Hsu Wu-Huei, Chen Hung-Jen, Liao Wei-Chih, Muo Chih-Hsin, et al. Increased lung cancer risk among patients with pulmonary tuberculosis: a population cohort study. J Thorac Oncol 2011;6 (1):32–7. 10.1097/JTO.0b013e3181fb4fcc. [DOI] [PubMed] [Google Scholar]
- [93].Liang Hui-Ying Xue-Lian, Yu Xiao-Song Peng, Yin Zhi-Hua Qin-Cheng, et al. Facts and fiction of the relationship between preexisting tuberculosis and lung cancer risk: a systematic review. Int J Cancer 2009;125 (12):2936–44. 10.1002/(ISSN)1097-021510.1002/ijc.v125:1210.1002/ijc.24636. [DOI] [PubMed] [Google Scholar]
- [94].Holla S, Ghorpade DS, Singh V, et al. Mycobacterium bovis BCG promotes tumor cell survival from tumor necrosis factor-alpha-induced apoptosis. Mol Cancer 2014;13:210. 10.1186/1476-4598-13-210 [published Online First: 2014/09/12]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Noguera-Ortega E, Guallar-Garrido S, Julian E. Mycobacteria-Based Vaccines as Immunotherapy for Non-urological Cancers. Cancers (Basel) 2020;12(7). 10.3390/cancers12071802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Mathe G, Amiel JL, Schwarzenberg L, et al. Active immunotherapy for acute lymphoblastic leukaemia. Lancet 1969;1(7597):697–9. 10.1016/s0140-6736(69)92648-8 [published Online First: 1969/04/05]. [DOI] [PubMed] [Google Scholar]
- [97].Kennedy A, Sahu KK, Cerny J. Role of Immunomodulation of BCG Therapy on AML Remission. Int Med Case Rep J 2021;14:115–9. 10.2147/IMCRJ.S296387 [published Online First: 2021/03/05]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Murata M. Activation of Toll-like receptor 2 by a novel preparation of cell wall skeleton from Mycobacterium bovis BCG Tokyo (SMP-105) sufficiently enhances immune responses against tumors. Cancer Sci 2008;99 (7):1435–40. 10.1111/j.1349-7006.2008.00832.x [published Online First: 2008/05/03]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Nakajima H, Kawasaki K, Oka Y, et al. WT1 peptide vaccination combined with BCG-CWS is more efficient for tumor eradication than WT1 peptide vaccination alone. Cancer Immunol Immunother 2004;53(7):617–24. 10.1007/s00262-003-0498-0 [published Online First: 2004/06/04]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Grant SC, Kris MG, Houghton AN, et al. Long survival of patients with small cell lung cancer after adjuvant treatment with the anti-idiotypic antibody BEC2 plus Bacillus Calmette-Guerin. Clin Cancer Res 1999;5(6):1319–23 [published Online First: 1999/07/02]. [PubMed] [Google Scholar]
- [101].Giaccone G, Debruyne C, Felip E, et al. Phase III study of adjuvant vaccination with Bec2/bacille Calmette-Guerin in responding patients with limited-disease small-cell lung cancer (European Organisation for Research and Treatment of Cancer 08971–08971B; Silva Study). J Clin Oncol 2005;23 (28):6854–64. 10.1200/JCO.2005.17.186 [published Online First: 2005/09/30]. [DOI] [PubMed] [Google Scholar]
- [102].Bottomley A, Debruyne C, Felip E, et al. Symptom and quality of life results of an international randomised phase III study of adjuvant vaccination with Bec2/BCG in responding patients with limited disease small-cell lung cancer. Eur J Cancer 2008;44(15):2178–84. 10.1016/j.ejca.2008.06.036 [published Online First: 2008/08/05]. [DOI] [PubMed] [Google Scholar]
- [103].Lardone RD, Chan AA, Lee AF, et al. Mycobacterium bovis Bacillus Calmette-Guerin Alters Melanoma Microenvironment Favoring Antitumor T Cell Responses and Improving M2 Macrophage Function. Front Immunol 2017;8:965. 10.3389/fimmu.2017.00965 [published Online First: 2017/08/30]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Gianan MA, Kleinerman ES. Liposomal muramyl tripeptide (CGP 19835A lipid) therapy for resectable melanoma in patients who were at high risk for relapse: an update. Cancer Biother Radiopharm 1998;13(5):363–8. 10.1089/cbr.1998.13.363 [published Online First: 2000/06/14]. [DOI] [PubMed] [Google Scholar]
- [105].Yang M, Yan Y, Fang M, et al. MF59 formulated with CpG ODN as a potent adjuvant of recombinant HSP65-MUC1 for inducing anti-MUC1+ tumor immunity in mice. Int Immunopharmacol 2012;13(4):408–16. 10.1016/j.intimp.2012.05.003 [published Online First: 2012/05/19]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Li D, Li H, Zhang P, et al. Heat shock fusion protein induces both specific and nonspecific anti-tumor immunity. Eur J Immunol 2006;36(5):1324–36. 10.1002/eji.200535490 [published Online First: 2006/04/19]. [DOI] [PubMed] [Google Scholar]
- [107].Kim DH, Moon C, Oh SS, et al. Liposome-encapsulated CpG enhances antitumor activity accompanying the changing of lymphocyte populations in tumor via intratumoral administration. Nucleic Acid Ther 2015;25 (2):95–102. 10.1089/nat.2014.0509 [published Online First: 2015/02/19]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].He X, Wang J, Zhao F, et al. Antitumor efficacy of viable tumor vaccine modified by heterogenetic ESAT-6 antigen and cytokine IL-21 in melanomatous mouse. Immunol Res 2012;52(3):240–9. 10.1007/s12026-012-8332-4 [published Online First: 2012/04/06]. [DOI] [PubMed] [Google Scholar]
- [109].He X, Wang J, Zhao F, et al. ESAT-6-gpi DNA vaccine augmented the specific antitumour efficacy induced by the tumour vaccine B16F10-ESAT-6-gpi/IL-21 in a mouse model. Scand J Immunol 2013;78(1):69–78. 10.1111/sji.12074 [published Online First: 2013/05/18]. [DOI] [PubMed] [Google Scholar]
- [110].Kuhn S, Hyde EJ, Yang J, et al. Increased numbers of monocyte-derived dendritic cells during successful tumor immunotherapy with immune-activating agents. J Immunol 2013;191(4):1984–92. 10.4049/jimmunol.1301135 [published Online First: 2013/07/17]. [DOI] [PubMed] [Google Scholar]
- [111].Popiela T, Kulig J, Czupryna A, et al. Efficiency of adjuvant immunochemotherapy following curative resection in patients with locally advanced gastric cancer. Gastric Cancer 2004;7(4):240–5. 10.1007/s10120-004-0299-y [published Online First: 2004/12/24]. [DOI] [PubMed] [Google Scholar]
- [112].Pei Q, Pan J, Ding X, et al. Gemcitabine sensitizes pancreatic cancer cells to the CTLs antitumor response induced by BCG-stimulated dendritic cells via a Fasdependent pathway. Pancreatology 2015;15(3):233–9. 10.1016/j.pan.2015.04.001 [published Online First: 2015/05/06]. [DOI] [PubMed] [Google Scholar]
- [113].Yuan S, Shi C, Ling R, et al. Immunization with two recombinant Bacillus Calmette-Guerin vaccines that combine the expression of multiple tandem repeats of mucin-1 and colony stimulating-factor suppress breast tumor growth in mice. J Cancer Res Clin Oncol 2010;136(9):1359–67. 10.1007/s00432-010-0787-x [published Online First: 2010/02/04]. [DOI] [PubMed] [Google Scholar]
- [114].Chung MA, Luo Y, O’Donnell M, et al. Development and preclinical evaluation of a Bacillus Calmette-Guerin-MUC1-based novel breast cancer vaccine. Cancer Res 2003;63(6):1280–7 [published Online First: 2003/03/22]. [PubMed] [Google Scholar]
- [115].Yuan S, Shi C, Lv Y, et al. A novel Bacillus Calmette-Guerin-based breast cancer vaccine that coexpresses multiple tandem repeats of MUC1 and CD80 breaks the immune tolerance and inhibits MUC1-positive breast cancer growth. Cancer Biother Radiopharm 2009;24(5):607–13. 10.1089/cbr.2009.0622 [published Online First: 2009/11/03]. [DOI] [PubMed] [Google Scholar]
- [116].Wiseman CL. Inflammatory breast cancer: 10-year follow-up of a trial of surgery, chemotherapy, and allogeneic tumor cell/BCG immunotherapy. Cancer Invest 1995;13(3):267–71. 10.3109/07357909509094460 [published Online First: 1995/01/01]. [DOI] [PubMed] [Google Scholar]
- [117].Convit J, Montesinos H, Oviedo H, et al. Autologous tumor lysate/Bacillus Calmette-Guerin immunotherapy as an adjuvant to conventional breast cancer therapy. Clin Transl Oncol 2015;17(11):884–7. 10.1007/s12094-015-1320-0 [published Online First: 2015/06/17]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Kitamura A, Mastumoto S, Asahina I. Growth inhibition of HeLa cell by internalization of Mycobacterium bovis Bacillus Calmette-Guerin (BCG) Tokyo. Cancer Cell Int 2009;9:30. 10.1186/1475-2867-9-30 [published Online First: 2009/12/04]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Frampton JE. Mifamurtide: a review of its use in the treatment of osteosarcoma. Paediatr Drugs 2010;12(3):141–53. 10.2165/11204910-000000000-00000 [published Online First: 2010/05/21]. [DOI] [PubMed] [Google Scholar]
- [120].Ratliff TL, Ritchey JK, Yuan JJ, et al. T-cell subsets required for intravesical BCG immunotherapy for bladder cancer. J Urol 1993;150(3):1018–23. 10.1016/s0022-5347(17)35678-1 [published Online First: 1993/09/01]. [DOI] [PubMed] [Google Scholar]
- [121].Brandau S, Riemensberger J, Jacobsen M, et al. NK cells are essential for effective BCG immunotherapy. Int J Cancer 2001;92(5):697–702. [published Online First: 2001/05/08]. [DOI] [PubMed] [Google Scholar]
- [122].Riemensberger J, Bohle A, Brandau S. IFN-gamma and IL-12 but not IL-10 are required for local tumour surveillance in a syngeneic model of orthotopic bladder cancer. Clin Exp Immunol 2002;127(1):20–6. 10.1046/j.1365-2249.2002.01734.x [published Online First: 2002/03/08]. [DOI] [PMC free article] [PubMed] [Google Scholar]