Abstract
Kidney transplant (KT) recipients face post-transplant health issues. Immunosuppressive agents can cause hyperlipidemia, hypertension, post-transplant diabetes, and glomerulopathy. Post-transplant weight gain and decreased activity are associated with poor quality of life, sleep, and cardiometabolic outcomes. This study will test the feasibility and acceptability of a culturally-tailored, diet and exercise intervention for KT patients delivered immediately post-transplant using novel technology. A registered dietitian nutritionist (RDN) and physical rehabilitation therapist will examine participants’ cultural background, preferences, and health-related obstacles (with consultation from the transplant team) to create an individualized exercise and meal plan. The RDN will provide medical nutrition therapy via the nutrition care process throughout the course of the intervention. The Twistle® Patient Engagement Platform will be used to deliver and collect survey data, communicate with participants, and promote retention. Outcomes to be assessed include: intervention feasibility and acceptability, and intervention efficacy on patients’ adherence, medical, quality of life, and occupational outcomes.
Keywords: kidney transplant, post-transplant, lifestyle modification, diet and exercise, medical nutrition therapy
Introduction
Kidney transplantation (KT) is the treatment of choice for patients with kidney failure.(1) Although KT reduces cardiovascular events, cardiac-related death rates in KT recipients remain up to 10 times greater than in the general population.(1–3) In some cases, pre-transplant cardiac risk factors may persist post-transplant, with KT recipients with diabetes-associated kidney failure having higher mortality rates compared to recipients with kidney failure due to other causes.(4) Some transplant-specific factors also increase cardiac risk. Although they are necessary to decrease rejection,(5) post-transplant immunosuppressive agents are associated with cardiometabolic complications (e.g., hyperlipidemia, hypertension, post-transplant diabetes, and glomerulopathy).(6–11) The most common approach to treating these cardiometabolic complications is medication. However, medication effectiveness varies,(3,12,13) and patients may find side effects unacceptable and face contraindications with their complex, costly post-transplant immunosuppression regimen (e.g., calcium channel blockers, angiotensin receptor blockers, ACE inhibitors, antihypertensive agents). (6–10) Given the tremendous resources that go into evaluating and transplanting kidney failure patients, it is important to explore effective, yet potentially less costly, approaches to reducing their cardiac risk post-transplant.(3)
Weight gain in the first year after KT due to immunosuppressive drugs, existing comorbidities, and exercise apprehension, contributes to elevated cardiometabolic risk.(14–18) In addition, low levels of physical activity and reduced physical functioning are common after KT, and are associated with a reduced quality of life (QOL)(19) and poor sleep quality.(20) Reduced sleep quality can result in mental and behavioral problems that further exacerbate KT recipients cardiac risk factors.(20) Behavioral counseling focused on lifestyle modification (changes in diet and exercise) has been the mainstay for initial management of obesity, diabetes, and cardiovascular disease for decades in the general population.(21–23) The U.S. Preventive Service Task Force recommends behavioral interventions centered on nutrition and physical activity for adults at risk for cardiovascular disease.(21) In accordance with these guidelines, an initial short-term investment in enhancing lifestyle modification in KT recipients may yield long-term benefits and cost savings to patients and the healthcare system. However, the presence of both traditional and KT-specific risk factors for cardiovascular events and diabetes after transplantation,(24–26) such as weight gain, hypertension, deconditioning, and hyperlipidemia, makes it critical to establish the feasibility and efficacy of an early post-transplant lifestyle modification intervention specifically for the KT population.(27–29) Even though an approach specifically tailored to KT recipients is an optimal solution,(30,31) seminal reviews of physical activity in the KT population resulted in limited lifestyle management guidelines for KT recipients.(15,16) Additionally, a recent evidence-based clinical practice guideline for nutrition care in CKD and KT recipients from the National Kidney Foundation and the Academy of Nutrition and Dietetics found insufficient evidence to support several dietary recommendations specific to transplant recipients.(32,33) Although a Cochrane review of dietary interventions for CKD(17) highlighted the limitations of current evidence of dietary interventions, it only found four studies that included a total of 168 KT recipients. Thus, interventions for KT patients are limited. It is also important to develop an intervention that accurately and appropriately reflects the current KT population. The latest data from the Organ Procurement and Transplantation Network shows that racial and ethnic minorities comprise 46% of the KT population.(34) However, a meta-analysis of lifestyle interventions(35) found that only 10% were culturally appropriate to the target population. We developed the current protocol to address these gaps.
The Improving Healthcare Outcomes in American Transplant Recipients Using Culturally-Tailored Novel Technology (IMPACT) pilot study is designed to test the feasibility and acceptability of a culturally-tailored, multi-behavior lifestyle intervention using a novel technology for KT recipients. This intervention will be the first of its kind to work with KT patients immediately following transplant. Because of KT recipients’ varied medical status immediately post-transplant, and the need to individually monitor their post-transplant immunosuppression regimen,(2,12–14) a critical component of IMPACT is an individually-tailored exercise and diet plan with a physical therapist/exercise physiologist (PT) and a registered dietitian nutritionist (RDN), who will work closely with the post-transplant team to carefully adjust the patient’s plan to their medical needs. The IMPACT PT and RDN will combine a personalized assessment of patient food and exercise resources, barriers, and cultural and personal preferences with consideration of clinical specifications from the transplant team. This intervention is innovative because it addresses previous limitations (small sample size, limited follow-up, insufficient data collection),(14–17) while adapting its components to meet the needs of the culturally-diverse kidney failure population. Although the sample size for the pilot study is modest, it will amount to approximately 12% of the sample in the Cochrane group meta-analysis noted above, and will serve as the foundation for our large-scale study. Another innovation of IMPACT is the use of the Twistle® Patient Engagement Platform by Health Catalyst(36) to follow-up with participants between their scheduled appointments, promote adherence to the intervention, collect all questionnaire data, and enhance participant retention.
This paper describes the protocol of the IMPACT pilot study. The study aims are to: (1) examine IMPACT’s acceptability and feasibility in KT recipients; and (2) explore the efficacy of IMPACT on proximal outcomes (e.g., weight, lipid profile, HbA1c, sleep quality, QOL) and distal outcomes (e.g., occupational functioning) in KT recipients in line with SONG-Tx core outcomes.(37) Please see Table 1 for an outline of aims, key outcome measures, and their operationalization. The findings of this pilot study will be used to develop a large-scale, pragmatic clinical trial to test the efficacy and cost-effectiveness of IMPACT on KT recipients’ post-transplant outcomes (i.e., obesity, cardiovascular events, diabetes, sleep quality and QOL).
Table 1.
Table of Aims and Outcome Measures
| Aim | Outcomes | Operationalization |
|---|---|---|
|
| ||
| Aim 1: Examine IMPACT’s acceptability and feasibility in KT recipients | ||
| 1a. Assess acceptability by patient ratings of satisfaction with the culturally tailored diet and exercise intervention, as well as the Twistle® patient engagement platform. | Acceptability Feasibility | • Satisfaction with IMPACT Intervention questionnaire • Usability Scale for Twistle® • Usability scale for activPAL • 3 month brief satisfaction interview • 6 month in-depth satisfaction interview |
| 1b. Assess feasibility by determining recruitment and retention rates and assessing participant adherence to the IMPACT intervention | Recruitment Retention | • Eligible patients, number recruited, number retained • Number refused, number withdrawn after consent • Study discontinuation tool and observation log to track reasons for refusal or withdrawal |
| 1c. Determine the ideal personnel hours and number of staff required for the physical therapist/exercise physiologist and registered dietitian nutritionist roles. | Visit frequency Visit duration | • Visit frequency and duration with PT and RDN (including extra visits required to help those patients with greater comorbidities or acute events (infections or other reasons for hospitalization) |
| Aim 2: Test the efficacy of IMPACT on proximal outcomes (e.g., weight, lipid p (e.g., occupational functioning) in KTKT recipients rofile, HbA1c, sleep quality, QOL) and distal outcomes | ||
| 2a. Assess participants’ adherence to the exercise and diet intervention aspects of IMPACT | Adherence | • Diet and exercise adherence questionnaire • Nutrition follow-up visits • PT follow-up visits |
| 2b. Begin to examine changes in patient outcomes and the relationship between adherence to IMPACT and patient outcomes | Quality of life, physical activity, sleep functioning, occupational functioning, biochemical and anthropometric measures | • ActivPAL Data – for physical activity • PROMIS QOL • PROMIS Sleep-Related Impairment and Sleep Disturbance Measures • CHART-SF – occupational functioning • EMR data |
Methods
Overview:
This pilot randomized clinical trial will assess the feasibility and acceptability of initiating a post-transplant diet and exercise intervention for kidney transplant recipients within 24–48 hours of receiving a transplant. KT patients who received a transplant at a state-funded safety-net hospital with a kidney transplant center will be recruited for this study. Also, to facilitate collaboration, the research team will send a periodic email with concise descriptions of the study purpose and procedures to the transplant clinical and administrative staff. In addition to usual post-transplant care, intervention group participants will receive a customized exercise and nutrition plan tailored by a PT and an RDN. Periodic surveys assessing QOL as well as adherence to the diet and exercise plan will be delivered using Twistle®. Participants in usual care (UC) will only receive standard post-transplant care, as well as periodic surveys. Physical activity in all participants will be assessed using the activPAL accelerometer, and follow all participants for 12 months post-transplant. Finally, the study team will conduct open-ended interviews with all intervention participants and clinic staff to assess their impressions and recommendations for the intervention. The study site’s Human Research Protections Office (19–413) approved the protocol.
Target population:
The research team will recruit 20 male or female kidney transplant recipients. To be eligible for the study, patients must be: 18 years of age or older, speak English, mentally competent to make a voluntary decision about trial participation, and receive their kidney transplant from the study site. A majority of kidney failure patients at the study site are Hispanic/Latino/a (HL) (44%) or American Indian (AI) (33%), have a household income of <$25k, are on public insurance, or have a high school or lower-level education. Thus, the study sample will include disadvantaged populations that are underrepresented in past research, and prepare us to conduct a larger study in a diverse population.
Screening and recruitment:
All transplant physicians and transplant staff at the study site will receive a twice weekly email reminder regarding the study inclusion and exclusion criteria, and an inquiry about any upcoming kidney transplants. Clinical team members will notify the research team via email when eligible patients are available for recruitment. The research coordinator will approach eligible patients in the hospital 24–48 hours prior to or after transplant to review the consent form, address any questions, and obtain verbal and written consent from the participant. Once patients consent to study participation, they will be randomized into either the IMPACT intervention or UC study arms.
Random assignment:
Patients who consent to participate in the study, will be randomly assigned either to the IMPACT intervention or the UC arm. Patients will be informed about the different study arms during the consenting process. The research team will not blind participants to their study condition, and participants will be given the option to switch conditions, if they are not satisfied with their assignment. This option was included to assess intervention acceptability during the pilot study; however, this option may be eliminated for the larger-scale study to ensure proper randomization. The study team will collect information about the number of participants that chose to switch study assignment to enhance information regarding intervention feasibility. A blocked stratification randomization approach will be used based on participant’s EMR-extracted racial and ethnic group (non-Hispanic white, HL, non-Hispanic AI/other race). Subsequent patients of each racial and ethnic group will be alternately assigned to a study arm to ensure equal distribution of intervention versus control in each racial and ethnic group.
IMPACT intervention and adaptation:
Participant burden will be minimized by scheduling intervention visits (meetings with the PT and RDN) and questionnaire assessments to commence during the hospital stay and coincide with participants’ standard post-transplant clinic visits (see Figure 1). The transplant team schedules patients for appointments at the post-transplant clinic weekly for the first month, then every two weeks for the next two months, then once a month at 4–6 months post-KT, with a final visit at 12 months. Pairing intervention visits with the post-transplant clinic appointments allows IMPACT participants to have intensive support early on, resulting in approximately 13 total visits over the course of the first year post-transplant. Visit frequency will vary depending on participants’ status and healthcare needs post-transplant, with some participants requiring fewer or more visits. Those in UC will not undergo any diet or exercise intervention, but will wear an activity monitor at the same assessment points as intervention participants (see Section 2.5.2). The study team (including the PT and RDN) will meet on a weekly basis to discuss each participant’s progress and to ensure that the PT and RDN intervention plans do not conflict.
Figure 1.
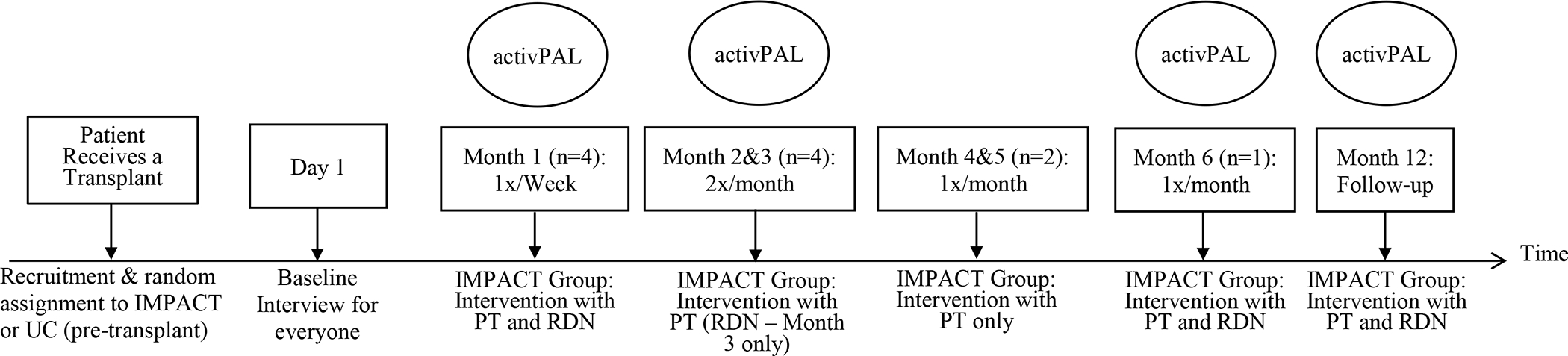
IMPACT and Usual Care Participant Pathway.
Exercise prescription and structure:
Pre and post-KT patients report lower levels of physical activity than the general population due to fatigue, fear of movement, and lack of clinician guidance.(14) Due to heterogeneity among the exercise programs used in previous post-KT studies, it is difficult to reach definitive conclusions regarding the benefit of aerobic versus resistance training.(14,16,27) Thus, for participants in the intervention arm, the study will combine instruction that is professionally guided by a PT, and incorporates a rehabilitation approach to slowly increasing KT recipients’ physical activity(38–40) until they can engage in exercise at levels consistent with the KDIGO guideline (i.e., exercise for 30 minutes, 5 times per week).(41,42) This approach was taken because, in the current era of personalized medicine, it would not be appropriate to prescribe one form of exercise across all participants. A tailored approach to physical rehabilitation with the end goal of a participant’s ability to meet the KDIGO guidelines within their specific built environment is optimal for patient health and outcomes. Participants in the UC arm will not have any contact with our research PT.
Before hospital discharge, the study PT will visit participants to conduct a structured assessment and begin a rehabilitation program. The PT will conduct a standardized assessment of the participant’s history of physical activity using the Global Physical Activity Questionnaire (GPAQ).(43) The PT will then discuss a list of goals with the participant to determine what they would like to gain from the intervention. The domains of participant goals(44) include relationships/family (e.g., participate in activities with family), recreation/physical activity (e.g., increase social life), employment/education (e.g., return to work), household care/maintenance (e.g., engage in care of family or pets), and self-care (e.g., dress, bathe, and complete toileting independently). After determining participants’ goals, the PT will assess participants’ functional mobility and ambulation tolerance. The PT will then prescribe an initial walking program for the participant (e.g., walk 5 min, 3x/day), to be performed until the next PT visit.
At participants’ first outpatient clinic visit, the PT will conduct assessments of their submaximal cardiovascular endurance using the 6 Minute Walk Test (6MWT)(45), functional strength using the 30-second chair stand test(46), and static balance (i.e., feet together, semi-tandem, tandem, and single limb stance).(47–49) The PT will also periodically reassess these measures throughout the intervention to determine participants’ functional progress. In addition, during the clinic visits, the PT will prescribe and modify a physical activity regimen. Physical activity will be focused on rehabilitation (i.e. walking, stationary cycling, and light lower extremity strengthening and endurance exercises) until the participant is surgically cleared for exercise without restriction. Surgical clearance for lifting, abdominal exercises, and activity that is more strenuous generally occurs 6–8 weeks post-transplant, at which point the PT will guide participants to increase the intensity and variety of their exercise program. The PT will assess participants’ tolerance to the intervention by their self-reported subjective comments and the Borg Rate of Perceived Exertion Category-Ratio Scale.(50)
The PT may meet with an intervention participant a total of 13 times throughout the study, because protocol visits will coincide with their other post-transplant visits. However, their visit frequency with the PT may vary depending on their post-transplant clinic visit schedule, which will depend on a specific participant’s post-transplant functional status as assessed by the transplant team. The first two PT visits will be in-person to conduct assessments and set goals. If the study PT is unable to see a participant at their pre-scheduled visit, the PT will conduct subsequent visits over Zoom or telephone to reduce participant burden. Throughout the intervention, the PT will prescribe a combination of aerobic and resistance exercises relating to lower extremity strengthening (e.g., heel raises, lunges, standing hip extensions), upper extremity strengthening (e.g., bicep curls, pushups, rows), core strengthening (e.g., planks, inchworms), balance exercises (e.g., tandem stance, side-stepping), general flexibility (e.g., hamstring stretch, biceps stretch), and cardiovascular exercises (e.g., walking, cycling, jumping jacks). The PT will gradually incorporate and periodically adjust these exercises at subsequent visits to best address the participant’s goals and needs.
Physical activity monitoring:
Within one week post-transplant, the study team will assess a “baseline” test of physical activity, including a measure of time spent sedentary (i.e., sitting/lying), standing, and stepping (and the intensity) during awake hours using the activPAL accelerometer.(51,52) The activPAL is an electronic activity monitor - a small, thin device, similar to a patch, which can be worn on a participant’s mid-thigh during the day and overnight. Regardless of condition, all study participants will wear the device for one week, 4 times throughout the duration of the study (see Figure 1): 4–7 days post-transplant, 6–8 weeks post-transplant, and then at 6- and 12-months post-transplant. Participants will be instructed to mail back the activPAL monitor using a pre-addressed envelope after each use.
Diet prescription and structure:
As in most other transplant centers, post-transplant usual care in our center includes one post-transplant visit with an RDN. Patients only see an RDN again if they have a newly identified condition requiring nutrition counseling, such as uncontrolled or new-onset diabetes, or require a post-transplant diet clarification. In contrast, KT recipients in our IMPACT trial will see the RDN interventionist once per week during the first month of the intervention, and then once at three, six, and 12-months post-transplant.
Based on nutrition care guidelines from KDOQI and other national and international sources(53,54), the first nutrition visit will occur immediately post-transplant, before patients are discharged from the hospital, to emphasize the importance of nutrition in the midst of the intensive medical intervention and management. During this meeting, the RDN will assess participants’ cultural background, food preferences and habits, functional abilities and health, nutrition risks, and food security using an adapted version of the Home and Community Care food service client cultural food preferences assessment tool.(55) At future meetings between the RDN and participants, the RDN will provide medical nutrition therapy via the nutrition care process (NCP), as described by the Academy of Nutrition and Dietetics.(56) The NCP involves conducting a comprehensive nutrition assessment, which includes reviewing participants’ food and nutrition-related history (e.g., meal patterns, food habits), as well as assessing nutrition focused physical findings (e.g., appetite, chewing problems, swallowing problems, gastrointestinal problems). The RDN will make a nutrition diagnosis, work with participants to identify appropriate nutrition intervention(s) and goals and establish a nutrition prescription, and monitor and evaluate progress on the goal and set new goals as appropriate at subsequent sessions. Example nutrition interventions that will be facilitated by the RDN include individualized nutrition education, counseling (e.g., cognitive behavioral therapy and motivational interviewing techniques), skill-building support related to food purchasing and preparation and diet self-monitoring, coordination of care (e.g., referrals to community resources to assist with food security), and overcoming nutrition barriers (e.g., post-transplant dietary knowledge deficit, management of gastrointestinal problems).(53) Dietary prescriptions will consider participants’ history, knowledge, beliefs, and preferences. The RDN will conduct visits either in-clinic, over Zoom, or over the telephone, depending on participant availability and document care into REDCap(57) using standardized Nutrition Care Process Terminology.(56) Although the RDN will use the NCP to assess participants’ dietary preferences, needs, and goals, these visits will also serve as a tool for providing dietary prescriptions based on participants’ history, knowledge, beliefs, and preferences. During these follow-up visits, the RDN will also monitor changes in participants’ dietary intake, anthropometric measures (e.g., body mass index [BMI]), and biochemical values (i.e., electrolytes, glucose, A1c, HDL, LDL, and triglycerides). The continued support participants receive from an RDN at the follow-up visits will support achievement and maintenance of dietary patterns that meet their individualized nutritional needs post-transplant, while also helping to establish nutrition behaviors that reduce the risk of excess weight gain and elevated cardiometabolic risk.
The Twistle® Patient Engagement Platform by Health Catalyst:
The most critical innovation of the IMPACT study is the use of the Twistle®patient engagement platform.(36) Twistle® is a HIPAA-compliant patient engagement platform designed to perform across all clinical specialties, procedures, and chronic conditions. Because Twistle® is a multimodal platform, it can deliver messages and reminders through smartphone apps (Apple and Android), text message (SMS), computer browsers, EHR portal and regular landlines using interactive voice response (IVR) or recorded voice. Twistle® allows participants to receive messages regardless of geographic location, addressing concerns regarding internet and provider access in rural communities.(58,59)
The research team will use Twistle® to create and deploy the periodic questionnaires, and to send personalized reminders and check-in with participants to assess adherence to the intervention and promote maintenance of behaviors (see Section 2.6 Data Collection, for specific measures and timelines). The application allows confirmation of message delivery, participant engagement with the surveys (when a participant views a message versus when they complete a survey, time to survey completion), and triage alert monitoring (automated reminders about surveys and activPALs sent to participants and study staff). Study staff will call participants without smartphones to complete surveys, but will use Twistle® to track survey deployment and capture questionnaire data. All participant communication through Twistle® is automated (i.e., survey deployment, survey reminders) however, study staff can directly communicate with participants through Twistle® in real time, if needed
Data Collection:
Table 2 lists all study assessments and frequency. The primary endpoints for the pilot trial are intervention acceptability and feasibility. The study team will monitor and review study progress on a weekly basis.
Table 2.
Assessment and Intervention Time Points for Usual Care (UC) and Intervention Participants
| Session Frequency |
|||||||
|---|---|---|---|---|---|---|---|
| Variable Name | Baseline | Month 1 Weekly | Month 2 Bi-weekly | Month 3 Bi-weekly | Month 4 & 5 Monthly | Month 6 Once | Month 12 Once |
|
| |||||||
| Predictors | |||||||
| Culturally-tailored diet and exercise baseline assessment | |||||||
| Food Assessment Questionnaire54 | Intervention | ||||||
| GPAQ Questionnaire43 | Intervention | ||||||
| Demographics59 | All | ||||||
| Social Support questionnaire60 | All | ||||||
| Mastery scale61 | All | ||||||
| Kinesiophobia questionnaire62 | All | ||||||
| Outcomes | |||||||
| QOL questionnaire60 | All | Intervention (week 4 only) | Intervention (end of Month 3) | All | All | ||
| Sleep Quality questionnaire60 | All | Intervention (week 4 only) | Intervention (end of Month 3) | All | All | ||
| Satisfaction with IMPACT intervention scale63 | Intervention | Intervention | |||||
| Usability scale for activPAL64 | Intervention | ||||||
| Usability scale for Twistle65 | Intervention (week 4 only) | Intervention | Intervention | ||||
| Occupational functioning questionnaire66 | All | All | All | ||||
| Diet and exercise adherence questionnaire67 | Intervention | Intervention | Intervention | Intervention | Intervention | Intervention | |
| activPAL Data Collection51,52 | All (prerehab) | All (post-rehab) | All | All | |||
| In-depth interview68,69 | Intervention | Intervention | |||||
| EMR data abstraction59,70 | All | ||||||
Aim 1: Examine IMPACT’S acceptability and feasibility in KT recipients
Outcome Variables:
Intervention acceptability we will assessed with participants’ satisfaction with the intervention using a revised version of the Client Satisfaction Questionnaire,(60) where higher satisfaction will indicate greater intervention acceptability, and the System Usability Scale (SUS)(61), where higher scores indicate ease of usability of the Twistle® system. ActivPAL usability and practicality will be assessed with the 11 item activPAL user friendliness questionnaire.(62) Questionnaire acceptability will be assessed using open-ended participant questions. The study team will keep a detailed log of relevant factors that could create barriers to subsequent study completion to provide concrete estimates of the expected rates of missing data and participant attrition.
ActivPAL accelerometers will be used to measure participants time spent sedentary (sitting/lying), standing, and stepping (and the intensity) during awake hours, and these values will be compared between the IMPACT and UC groups. Additionally, feasibility and acceptability of the diet and exercise intervention components will be assessed. The study team will analyze the PT and RDN participant visit documentation and assessments to examine visit attendance and identify patterns in diet and exercise care (e.g. type and duration of exercise prescribed, common nutrition diagnoses and interventions). The study will also use visit documentation to determine key elements of the PT and RDN care, and identify practices to standardize for a larger, multi-site trial. Survey completion times as well as intervention session time estimates will provide data regarding time required for study participation.
Also, the study team will assess the feasibility and acceptability of collecting a number of key participant outcomes in line with SONG-Tx core outcomes:(14–16,18,27) (a) clinical data from participants’ medical records, including weight, lipid profile, HbA1c, BMI, glucose, and medications; and (b) patient reported outcomes,(54,63,64) including the Patient-Reported Outcomes Measurement Information System (PROMIS) Scale v1.2 Global Health Measure, and the PROMIS Sleep-Related Impairment and Sleep Disturbance Measures.(63) PROMIS measures are brief, based on extensive item banks, have been validated in general and kidney disease populations,(65,66) and are favored by the International Consortium for Health Outcomes Measurement (ICHOM) CKD Working Group.(67) Occupational functioning (ability to perform activities of daily living) will be assessed using the occupation subscale of the CHART-SF.(68) The CHART-SF is the most widely used participation measure in rehabilitation research and has been validated with various race/ethnic groups.(69)
Demographic and Health Predictors:
The research team will collect demographic information, health predictors, and other covariates from participants. These items include gender, age, race and ethnicity,(70) marital status, education, occupation, income, and insurance status. The study team will abstract pre-transplant health variables (i.e., dialysis duration, BMI, number of pre-transplant hospitalizations, frailty, medical comorbidities using the Charlson Co-Morbidity Index,(71) and indication for KT) from participants’ medical records.(71) Participants’ level of fear of pain due to movement will be assess with the Tampa Scale for Kinesiophobia.(72) Participants’ evaluation of their available social support will be assessed using the PROMIS Short Form v2.0 item bank.(63) The 7-item Sense of Mastery scale(73) will be used to assess the degree to which participants feel they have personal control over the things that happen to them. The study team will measure adherence to the diet and exercise plan with a modified version of our post-transplant adherence questionnaire.(74,75) The primary goal for this study is to assess the feasibility of collecting this data rather than measuring any changes in these measures.
Intervention Evaluation:
The research team will evaluate the process of implementing the IMPACT intervention using a mixed-methods approach consistent with a Type I Hybrid Intervention/Implementation design.(76) Using the Consolidated Framework for Implementation Research (CFIR)(77) as a theoretical framework, we will assess barriers and facilitators to implementation to provide real-time feedback for the pilot and to inform the future large-scale project. This evaluation will be comprised of a periodic survey with clinical (i.e., PT, RDN) and administrative staff regarding barriers and facilitators of implementation, as well as in-depth interviews with intervention group participants. Given that this is a multicomponent intervention, the survey and in-depth interview approach to intervention evaluation will allow the study team to disentangle staff and participant perceptions of the feasibility and effectiveness of the various components for the participants: individualized prescription of culturally tailored physical activity and diet plan and the Twistle® platform.
Patient Satisfaction Questionnaire and In-Depth Interviews:
A brief questionnaire will be used to survey intervention participants at 3 months post-transplant about their satisfaction with the study. At 6 months, in-depth, open-ended interviews will be conducted with intervention participants to further assess study satisfaction, and examine their approval of the intervention with a focus on their overall experience and recommendations for improvement. All interviews will be recorded and transcribed.
Staff Surveys:
Clinical and administrative staff will be contacted periodically to provide intervention feedback. Staff surveys include their assessment about barriers or facilitators to the intervention they observed or experienced, and suggestions for change, if indicated. Observed barriers and facilitators can be related to direct staff activities or observations of participants interacting with study staff members. We will elicit feedback from the transplant team on the intervention process. We will ask for impressions of the IMPACT intervention and solicit suggestions regarding optimizing the future large-scale study.
Internal Evaluation:
The research coordinator will document all observations and experiences in a Study Observation Log, noting the date and source of the observation. The study team will review the log during research team weekly meetings and identify any process change needs. The research team will then iteratively communicate the problems identified and planned changes to the research, clinical, and administrative teams. The survey data will be quantitatively analyzed, categorizing comments as to which of the major CFIR domains (Intervention, Outer Setting, Inner Setting, Characteristics of Individuals, Process) they refer and assessing counts and frequencies of comments found in each construct.
Participant reimbursement and retention methods:
Participants will be reimbursed for their study participation in $20.00 increments. Participants in the IMPACT intervention arm will receive payments each time they complete a series of questionnaires through Twistle®. Those in the intervention group will also receive an additional $20.00 payment after completing an in-depth interview with study staff at 6 months. Participants in the UC arm will receive payments for completing baseline, 6 month, and 12 month surveys. Thus, intervention participants can receive up to 13 payments maximum ($260.00) for participating in the study in its entirety, and UC participants can receive up to 3 payments ($60.00) for study participation. To facilitate retention and encourage open communication, intervention participants will have frequent contact with the study team via PT and RDN visits and the Twistle® patient engagement platform. Participant retention efforts will be documented using Excel and REDCap,(57,78) and assessed during weekly study team meetings. These meetings will include discussion of reasons for patient refusal and offer opportunities to discuss strategies to improve recruitment and retention rates.
Analysis of Specific Aims
Primary and ancillary analysis:
The participant sample will analyzed with detailed descriptive statistics regarding demographics and health-related characteristics. Missing data points during the study, including attrition and its underlying reasons, will be recorded and summarized as well. The primary analysis will focus on measures of feasibility and acceptability as outlined in Aim 1: Examine IMPACT’s acceptability and feasibility in KT recipients (see Table 1). These analyses will be used to determine endpoints, their variation, plausible intervention effect size, sample size, planned analyses, and accrual expectations. These analyses will guide the design of the subsequent study.(79) Also, these analyses, along with those of Aim 2, will be used to determine a clear rationale to support the next steps in the study process, including “go versus no go” decisions.(79)
For Aim 2, “Test the efficacy of IMPACT on proximal outcomes (e.g., weight, lipid profile, HbA1c, sleep quality, QOL) and distal outcomes (e.g., occupational functioning) in KT recipients,” we will explore the intervention effects on adherence to the diet and exercise intervention, and compare proximal and distal outcomes between groups. This analysis will compare the intervention and UC group on type, frequency, and intensity of exercise, as well as changes in weight and laboratory values, and sleep quality, occupational functioning, and QOL using descriptive statistics and non-parametric methods. The activity in both groups will be compared using the activPAL data. For example, this analysis will answer the questions: what is the plausible range of difference in the proportion of participants engaging in the recommended diet and exercise routine and experiencing positive changes in proximal and distal outcomes? What is the difference in the amount of activity between the two groups? Given the small sample size of this study, we do not expect such differences will be statistically significant, but such differences will inform the planned large-scale study. In addition, analyses will consider participants’ diet and exercise adherence in relation to proximal outcomes, such as weight and lipid profile.
Qualitative data analysis:
The core research team will review all of the interview transcripts to develop an annotated provisional codebook, using qualitative analysis software (Dedoose)(80) to support this analysis. The subsequent interviews will be analyzed iteratively as follows: two primary coders will apply existing codes and will add new codes to the codebook as needed. Disagreement in coding will be resolved through consensus, and inter-rater agreement between coders will be assessed.(81) The study team will maintain an audit trail to track coding decisions, and we will use memos throughout the analysis to identify key emerging concepts. Thematic analysis will be conducted to identify key concepts, including confirmation of existing knowledge and identification of novel themes.
The study team will create a provisional codebook with the barriers and facilitators identified in the brief surveys with clinical team members. Particular attention will be paid to themes that deal with overall ease of use of the intervention and overall satisfaction with implementation. The study team will map results of the qualitative analysis to the major CFIR domains.
Sample size estimates:
Although power estimates are not necessary for a pilot feasibility study, we used data from the last five years of KT recipients at the transplant center to estimate the number of patients we can expect to recruit for the study. The center transplants more than 40 patients per year. Of all eligible patients who receive a KT, we expect that 80% will agree to participate in our study, based on previous intervention research with KT patients,(8,18,28,39,82,83)and our own work.(84,85) Due to the amount of patients transplanted at the transplant center annually, a reasonable recruitment target of 20 KT recipients was set for the pilot study.
Discussion
KT recipients encounter elevated cardiometabolic risks post-transplant.(2,3) This risk is due to the increased likelihood of transplant recipients experiencing weight gain, hyperlipidemia, hypertension, and developing post-transplant diabetes in the first year after transplant.(6–11,28,82) These conditions result in higher mortality rates(4) and reduced QOL(19) for transplant recipients. The IMPACT trial will address this urgent clinical demand in KT by assessing an intervention that may reduce the likelihood of weight gain, uncontrolled diabetes, new onset diabetes, and cardiovascular events in patients post-transplant. This trial will also address the need to culturally-tailor and individualize lifestyle interventions(35) using careful assessment and consideration when prescribing diet and exercise regimens. By providing KT recipients with an intensive, personalized nutrition and exercise regimen that acknowledges and addresses structural barriers and integrates their cultural and other preferences, intervention participants will have the support, knowledge, resources, and self-efficacy to adhere to the program and improve their post-transplant health. Intervention adherence will be promoted through recurrent follow-up by the PT and RDN and use of Twistle®, which will serve as a means to remain in frequent contact with participants. Collecting detailed information on the PT and RDN intervention and using the Twistle® patient engagement platform will provide means to easily standardize, package, and disseminate the intervention to other transplant centers.
A major strength of this pilot trial is that it is being conducted with a transplant center that serves a high proportion of HL and AI patients, who are traditionally underrepresented in KT research and who may experience poorer KT outcomes because of limited resources and infrastructure to address structural barriers and to individualize interventions during usual care. It is essential that a larger trial include underserved KT patients. Thus, feasibility and acceptability information from this pilot trial will help to ensure that this goal is a possibility. Although the small sample size of this study may not allow conclusions on participants’ clinical outcomes, the study will collect descriptive data about weight improvements, changes to lipid panels, and physiological benefits of exercise and diet in kidney transplant recipients. This pilot study will provide invaluable information for the development of a large-scale intervention study. The large-scale trial will be the first to examine early post-transplant behavioral interventions, and will establish a foundation for other transplant centers to implement custom healthy lifestyle interventions for KT recipients. Future research from this study team will examine cost-effectiveness, insurance reimbursement for allied health provider intervention costs, and translation of study materials to other languages.
Practical Application
Given the tremendous resources that go into evaluating and transplanting kidney failure patients, it is important to explore effective, yet less costly, approaches to reducing post-transplant health risks. This intervention is a multi-modal health-system pragmatic clinical intervention designed to improve the health behavior of kidney transplant recipients immediately post-transplant. The research team will use the findings from this pilot trial to develop a large-scale, pragmatic clinical trial to test the efficacy and cost-effectiveness of the intervention on kidney transplant recipients’ post-transplant outcomes in the three largest minority racial/ethnic kidney failure groups: Black Americans, Hispanics, and American Indians.
Acknowledgements
This project was supported by Award Number C-4157 from Dialysis Clinic Inc. (DCI), a national non-profit dialysis provider; and an award from the National Center for Advancing Translational Sciences, National Institutes of Health grant #UL1TR001449. The content is solely the responsibility of the authors and does not necessarily represent the official views of Dialysis Clinic, Inc. DCI had no involvement in the study design; in the collection, analysis and interpretation of data; in the writing of this report; or in the decision to submit this article for publication. CKB is currently supported by an NIH K07 grant CA215937.
List of Abbreviations
- AI
American Indian
- CFIR
Consolidated Framework for Implementation Research
- ESKD
end stage kidney disease
- HL
Hispanic or Latino
- ICHOM
International Consortium for Health Outcomes Measurement
- IVR
interactive voice response
- KT
kidney transplant
- PITT
University of Pittsburgh
- PROMIS
Patient-Reported Outcomes Measurement Information System
- QOL
quality of life
- SMS
text message
- UC
usual car
- UNM
University of New Mexico
- UNMH
University of New Mexico Hospital
- WH
White
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hariharan S, Israni AK, Danovitch G. Long-Term Survival after Kidney Transplantation. N Engl J Med. 2021;385(8):729–43. [DOI] [PubMed] [Google Scholar]
- 2.Neale J, Smith AC. Cardiovascular risk factors following renal transplant. World J Transpl. 2015. Dec 24;5(4):183–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rangaswami J, Mathew RO, Parasuraman R, Tantisattamo E, Lubetzky M, Rao S, et al. Cardiovascular disease in the kidney transplant recipient: epidemiology, diagnosis and management strategies. Nephrol Dial Transplant. 2019;34(5):760–73. [DOI] [PubMed] [Google Scholar]
- 4.Saran R, Robinson B, Abbott KC, Agodoa LY, Albertus P, Ayanian J, et al. US Renal Data System 2016 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2017. Mar;69(3 Suppl 1):A7–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moons P, Vanrenterghem Y, Van Hooff JP, Squifflet JP, Margodt D, Mullens M, et al. Health-related quality of life and symptom experience in tacrolimus-based regimens after renal transplantation: A multicentre study. Transpl Int. 2003;16(9):653–64. [DOI] [PubMed] [Google Scholar]
- 6.Burne-Taney MJ, Yokota N, Rabb H. Persistent renal and extrarenal immune changes after severe ischemic injury. Kidney Int. 2005. Mar;67(3):1002–9. [DOI] [PubMed] [Google Scholar]
- 7.Covic A, Mardare N, Gusbeth-Tatomir P, Buhaescu I, Goldsmith DJ. Acute effect of CyA A (Neoral) on large artery hemodynamics in renal transplant patients. Kidney Int. 2005. Feb;67(2):732–7. [DOI] [PubMed] [Google Scholar]
- 8.Juskowa J, Bartłomiejczyk J, Paczek L, Rowinski W, Szmidt J, Foroncewicz B, et al. Total homocysteine as a risk factor for vascular disease in renal transplant recipients. Transpl Proc. 2002;34(2):576–9. [DOI] [PubMed] [Google Scholar]
- 9.Merkel M Homocysteine as a risk factor of cardiovascular disease. Int Congr Ser. 2004;1262:376–9. [Google Scholar]
- 10.Pavarino-Bertelli EC, Sanches de Alvarenga MP, Goloni-Bertollo EM, Baptista MA, Haddad R, Hoerh NF, et al. Hyperhomocysteinemia and MTHFR C677T and A1298C polymorphisms are associated with chronic allograft nephropathy in renal transplant recipients. Transpl Proc. 2004. Dec;36(10):2979–81. [DOI] [PubMed] [Google Scholar]
- 11.Cosio FG, Pesavento TE, Kim S, Osei K, Henry M, Ferguson RM. Patient survival after renal transplantation: IV. Impact of post-transplant diabetes. Kidney Int. 2002. Oct;62(4):1440–6. [DOI] [PubMed] [Google Scholar]
- 12.Weir MR, Burgess ED, Cooper JE, Fenves AZ, Goldsmith D, McKay D, et al. Assessment and management of hypertension in transplant patients. J Am Soc Nephrol. 2015. Jun;26(6):1248–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shivaswamy V, Boerner B, Larsen J. Post-Transplant Diabetes Mellitus: Causes, Treatment, and Impact on Outcomes. Endocr Rev. 2016. Feb;37(1):37–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takahashi A, Hu SL, Bostom A. Physical Activity in Kidney Transplant Recipients: A Review. Am J Kidney Dis. 2018. Feb 23; [DOI] [PubMed] [Google Scholar]
- 15.Tania Janaudis-Ferreira Sunita Mathur, Deliva Robin, Howes Nancy, Patterson Catherine, Räkel Agnès, et al. Exercise for Solid Organ Transplant Candidates and Recipients: A Joint Position Statement of the Canadian Society of Transplantation and CAN-RESTORE. Transplantation. 2019;103:e220–38. [DOI] [PubMed] [Google Scholar]
- 16.Calella P, Hernández-Sánchez S, Garofalo C, Ruiz JR, Carrero JJ, Bellizzi V. Exercise training in kidney transplant recipients: a systematic review. J Nephrol. 2019;32(4):567–79. [DOI] [PubMed] [Google Scholar]
- 17.Palmer SC, Maggo JK, Campbell KL, Craig JC, Johnson DW, Sutanto B, et al. Dietary interventions for adults with chronic kidney disease. Cochrane Database Syst Rev. 2017;4(Article # CD011998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janaudis-Ferreira T, Mathur S, Konidis S, Tansey CM, Beaurepaire C. Outcomes in randomized controlled trials of exercise interventions in solid organ transplant. World J Transpl. 2016. Dec 24;6(4):774–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klaassen G, Zelle DM, Navis GJ, Dijkema D, Bemelman FJ, Bakker SJL, et al. Lifestyle intervention to improve quality of life and prevent weight gain after renal transplantation: Design of the Active Care after Transplantation (ACT) randomized controlled trial. BMC Nephrol. 2017. Sep 15;18(1):296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pooranfar S, Shakoor E, Shafahi M, Salesi M, Karimi M, Roozbeh J, et al. The effect of exercise training on quality and quantity of sleep and lipid profile in renal transplant patients: a randomized clinical trial. Int J Organ Transpl Med. 2014;5(4):157–65. [PMC free article] [PubMed] [Google Scholar]
- 21.US Preventive Services Task Force, Krist AH, Davidson KW, Mangione CM, Barry MJ, Cabana M, et al. Behavioral Counseling Interventions to Promote a Healthy Diet and Physical Activity for Cardiovascular Disease Prevention in Adults With Cardiovascular Risk Factors: US Preventive Services Task Force Recommendation Statement. JAMA. 2020. Nov 24;324(20):2069. [DOI] [PubMed] [Google Scholar]
- 22.Evert AB, Dennison M, Gardner CD, Garvey WT, Lau KHK, MacLeod J, et al. Nutrition Therapy for Adults With Diabetes or Prediabetes: A Consensus Report. Diabetes Care. 2019. May;42(5):731–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N Engl J Med. 2021. Mar 18;384(11):989–1002. [DOI] [PubMed] [Google Scholar]
- 24.Shah T, Kasravi A, Huang E, Hayashi R, Young B, Cho YW, et al. Risk factors for development of new-onset diabetes mellitus after kidney transplantation. Transplantation. 2006;82(12):1673–6. [DOI] [PubMed] [Google Scholar]
- 25.van Hooff JP, Christiaans MH, van Duijnhoven EM. Evaluating mechanisms of post-transplant diabetes mellitus. Nephrol Dial Transpl. 2004. Dec;19 Suppl 6:vi8–12. [DOI] [PubMed] [Google Scholar]
- 26.Weir MR, Fink JC. Risk for posttransplant diabetes mellitus with current immunosuppressive medications. Am J Kidney Dis. 1999;34(1):1–13. [DOI] [PubMed] [Google Scholar]
- 27.Didsbury M, McGee RG, Tong A, Craig JC, Chapman JR, Chadban S, et al. Exercise training in solid organ transplant recipients: a systematic review and meta-analysis. Transplantation. 2013. Mar 15;95(5):679–87. [DOI] [PubMed] [Google Scholar]
- 28.Painter PL, Hector L, Ray K, Lynes L, Dibble S, Paul SM, et al. A randomized trial of exercise training after renal transplantation. Transplantation. 2002;71(1):42–8. [DOI] [PubMed] [Google Scholar]
- 29.Painter PL, Hector L, Ray K, Lynes L, Paul SM, Dodd M, et al. Effects of exercise training on coronary heart disease risk factors in renal transplant recipients. Am J Kidney Dis. 2003;42(2):362–9. [DOI] [PubMed] [Google Scholar]
- 30.Standards of medical care in diabetes—2006. Diabetes Care. 2006;29(Suppl 1):S4. [PubMed] [Google Scholar]
- 31.Davidson J, Wilkinson A, Dantal J, Dotta F, Haller H, Hernandez D, et al. New-onset diabetes after transplantation: 2003 International consensus guidelines. Proceedings of an international expert panel meeting. Barcelona, Spain, 19 February 2003. Transplantation. 2003. May 27;75(10 Suppl):SS3–24. [DOI] [PubMed] [Google Scholar]
- 32.Ikizler TA, Burrowes JD, Byham-Gray LD, Campbell KL, Carrero J-J, Chan W, et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am J Kidney Dis. 2020. Sep;76(3):S1–107. [DOI] [PubMed] [Google Scholar]
- 33.Academy of Nutrition and Dietetics. CKD Macronutrients Executive Summary of Reccomendations [Internet]. Academy of Nutrition and Dietetics; 2020. [cited 2021 Jul 23]. Available from: https://www.andeal.org/topic.cfm?menu=5303&cat=5558 [Google Scholar]
- 34.Organ Procurement and Transplantation Network and Scientific Registry of Transplant Recipients. Transplants in the U.S. by Recipient Ethnicity. 2018. [DOI] [PubMed]
- 35.Albarracin D, Wilson K, Chan MS, Durantini M, Sanchez F. Action and inaction in multi-behaviour recommendations: a meta-analysis of lifestyle interventions. Health Psychol Rev. 2018. Mar;12(1):1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh Kulmeet, Ross Dave. Twistle Inc., [Internet]. 2018. [cited 2021 Oct 4]. Available from: https://www.twistle.com/
- 37.Sautenet B, Tong A, Manera KE, Chapman JR, Warrens AN, Rosenbloom D, et al. Developing Consensus-Based Priority Outcome Domains for Trials in Kidney Transplantation: A Multinational Delphi Survey With Patients, Caregivers, and Health Professionals. Transplantation. 2017. Aug;101(8):1875–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kastelz A, Tzvetanov IG, Fernhall B, Shetty A, Gallon L, West-Thielke P, et al. Experimental protocol of a randomized controlled clinical trial investigating the effects of personalized exercise rehabilitation on kidney transplant recipients’ outcomes. Contemp Clin Trials. 2015. Nov;45(Pt B):170–6. [DOI] [PubMed] [Google Scholar]
- 39.Tzvetanov I, West-Thielke P, D’Amico G, Johnsen M, Ladik A, Hachaj G, et al. A novel and personalized rehabilitation program for obese kidney transplant recipients. Transpl Proc. 2014. Dec;46(10):3431–7. [DOI] [PubMed] [Google Scholar]
- 40.Wickerson L, Rozenberg D, Janaudis-Ferreira T, Deliva R, Lo V, Beauchamp G, et al. Physical rehabilitation for lung transplant candidates and recipients: An evidence-informed clinical approach. World J Transpl. 2016. Sep 24;6(3):517–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. 2013;1–150. [DOI] [PubMed]
- 42.Inker LA, Astor BC, Fox CH, Isakova T, Lash JP, Peralta CA, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. 2014. May;63(5):713–35. [DOI] [PubMed] [Google Scholar]
- 43.Armstrong T, Bull F. Development of the World Health Organization Global Physical Activity Questionnaire (GPAQ). J Public Health. 2006. Apr;14(2):66–70. [Google Scholar]
- 44.Strath SJ, Kaminsky LA, Ainsworth BE, Ekelund U, Freedson PS, Gary RA, et al. Guide to the Assessment of Physical Activity: Clinical and Research Applications: A Scientific Statement From the American Heart Association. Circulation. 2013. Nov 12;128(20):2259–79. [DOI] [PubMed] [Google Scholar]
- 45.American Thoracic Society. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. American Journal of Respiratory and Critical Care Medicine; 2015. [DOI] [PubMed] [Google Scholar]
- 46.Jessie Jones C, Roberta E Rikli, Beam William C.. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults [Internet]. Research Quarterly for Exercise and Sport; 1999. Available from: https://www.semanticscholar.org/paper/A-30-s-chair-stand-test-as-a-measure-of-lower-body-Jones-Rikli/2be926f0f014ce4bb4ac4488c29784660a682570 [DOI] [PubMed] [Google Scholar]
- 47.Lesinski M, Hortobágyi T, Muehlbauer T, Gollhofer A, Granacher U. Effects of Balance Training on Balance Performance in Healthy Older Adults: A Systematic Review and Meta-analysis. Sports Med Auckl NZ. 2015. Dec;45(12):1721–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Springer BA, Marin R, Cyhan T, Roberts H, Gill NW. Normative Values for the Unipedal Stance Test with Eyes Open and Closed. J Geriatr Phys Ther [Internet]. 2007;30(1). Available from: https://journals.lww.com/jgpt/Fulltext/2007/04000/Normative_Values_for_the_Unipedal_Stance_Test_with.3.aspx [DOI] [PubMed] [Google Scholar]
- 49.Briggs Randall C, Gossman Marilyn R, Birch Robert, Drews Judith E, Shaddeau Shirley A. Balance Performance Among Noninstitutionalized Elderly Women. Phys Ther. 1989. Sep 1;69(9):748–56. [DOI] [PubMed] [Google Scholar]
- 50.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–81. [PubMed] [Google Scholar]
- 51.Lyden K, Keadle SK, Staudenmayer J, Freedson PS. The activPALTM Accurately Classifies Activity Intensity Categories in Healthy Adults. Med Sci Sports Exerc. 2017. May;49(5):1022–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kozey-Keadle S, Libertine A, Lyden K, Staudenmayer J, Freedson PS. Validation of wearable monitors for assessing sedentary behavior. Med Sci Sports Exerc. 2011. Aug;43(8):1561–7. [DOI] [PubMed] [Google Scholar]
- 53.Greater Metropolitan Clinical Task Force (Renal Service Network). Evidence based practice guidelines for the nutritional management of adult renal transplant recipients. Sydney, Australia; 2008. [Google Scholar]
- 54.Ikizler TA, Burrowes JD, Byham-Gray LD, Campbell KL, Carrero J-J, Chan W, et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am J Kidney Dis. 2020. Sep;76(3):S1–107. [DOI] [PubMed] [Google Scholar]
- 55.Gallegos Danielle, Millichamp Anna. HACC food service client cultural food preferences assessment tool [Internet]. Queensland University of Technology; 2012. [cited 2021 Jul 23]. Available from: https://eprints.qut.edu.au/55544/8/HACC_FoodServiceClient_CulturalFoodPreferencesAssessmentTool.pdf [Google Scholar]
- 56.Swan WI, Vivanti A, Hakel-Smith NA, Hotson B, Orrevall Y, Trostler N, et al. Nutrition Care Process and Model Update: Toward Realizing People-Centered Care and Outcomes Management. J Acad Nutr Diet. 2017. Dec;117(12):2003–14. [DOI] [PubMed] [Google Scholar]
- 57.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009. Apr;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Drake C, Zhang Y, Chaiyachati KH, Polsky D. The Limitations of Poor Broadband Internet Access for Telemedicine Use in Rural America: An Observational Study. Ann Intern Med. 2019;epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 59.Struminger BB, Arora S. Leveraging Telehealth to Improve Health Care Access in Rural America: It Takes More Than Bandwidth. Ann Intern Med. 2019;epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 60.Attkisson CC, Larsen DL, Hargreaves WA, LeVois M, Nguyen TD, Roberts RE, et al. Client Satisfaction Questionnaire-8 (CSQ-8). In: American Psychiatric Association Task Force for the Handbook of Psychiatric Measures; John Rush A, editor. Handbook of psychiatric measures. Washington, DC: American Psychiatric Publishing; 2007. p. 176–8. [Google Scholar]
- 61.Brooke John. SUS - A quick and dirty usability scale. In: Jordan PW, Thomas B, Weerdmeester B, McClelland IL, editors. Usability Evaluation In Industry. Bristol, PA: Taylor and Francis; 1996. p. 189–94. [Google Scholar]
- 62.Berendsen BA, Hendriks MR, Meijer K, Plasqui G, Schaper NC, Savelberg HH. Which activity monitor to use? Validity, reproducibility and user friendliness of three activity monitors. BMC Public Health. 2014. Dec;14(1):749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Health Measures. PROMIS (Patient-Reported Outcomes Measurement Information System). 2020. [Google Scholar]
- 64.Flythe JE, Hilliard TS, Ikeler K, Keller S, Gipson DS, Grandinetti AC, et al. Toward Patient-Centered Innovation. Concept Framew Patient-Rep Outcome Meas Transform Kidney Replace Devices. 2020;CJN.00110120.
- 65.Hays RD, Spritzer KL, Schalet BD, Cella D. PROMIS®−29 v2.0 profile physical and mental health summary scores. Qual Life Res. 2018. Jul 1;27(7):1885–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tang E, Ekundayo O, Peipert JD, Edwards N, Bansal A, Richardson C, et al. Validation of the Patient-Reported Outcomes Measurement Information System (PROMIS)-57 and −29 item short forms among kidney transplant recipients. Qual Life Res. 2019. Mar;28(3):815–27. [DOI] [PubMed] [Google Scholar]
- 67.Verberne WR, Das-Gupta Z, Allegretti AS, Bart HAJ, van Biesen W, Garcia-Garcia G, et al. Development of an International Standard Set of Value-Based Outcome Measures for Patients With Chronic Kidney Disease: A Report of the International Consortium for Health Outcomes Measurement (ICHOM) CKD Working Group. Am J Kidney Dis. 2019. Mar;73(3):372–84. [DOI] [PubMed] [Google Scholar]
- 68.Whiteneck GG, Charlifue SW, Gerhart KA, Overholser JD, Richardson GN. Quantifying handicap: A new measure of long-term rehabilitation outcomes. Arch Phys Med Rehabil. 1992;73:519. [PubMed] [Google Scholar]
- 69.Sander AM, Pappadis MR, Davis LC, Clark AN, Evans G, Struchen MA, et al. Relationship of race/ethnicity and income to community integration following traumatic brain injury: Investigation in a non-rehabilitation trauma sample. Neuro Rehabil. 2009;24(1):15. [DOI] [PubMed] [Google Scholar]
- 70.Mays VM, Ponce NA, Washington DL, Cochran SD. Classification of race and ethnicity: Implications for public health. Annu Rev Public Health. 2003;24:83–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jassal SV, Schaubel DE, Fenton SSA. Baseline comorbidity in kidney transplant recipients: A comparison of comorbidity indices. Transplantation. 2005;46(1):136–42. [DOI] [PubMed] [Google Scholar]
- 72.Tkachuk Gregg A., Harris Cheryl A.. Psychometric Properties of the Tampa Scale for Kinesiophobia-11 (TSK-11). J Pain. 2012;13(10):970–7. [DOI] [PubMed] [Google Scholar]
- 73.Pearlin L, Schooler C. The structure of coping. J Health Soc Behav. 1978;19:2–21. [PubMed] [Google Scholar]
- 74.Dew MA, DiMartini AF, De Vito Dabbs A, Zomak R, De Geest S, Dobbels F, et al. Adherence to the medical regimen during the first two years after lung transplantation. Transplantation. 2008;85(2):193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Myaskovsky L, Switzer GE, Dew MA, Shapiro R, Unruh M, Ford AF, et al. Understanding Race and Culture in Living Donor Kidney Transplantation. NIDDK; 2009. [Google Scholar]
- 76.Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Effectiveness-implementation hybrid designs: Combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care. 2012;50(3):217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Damschroder LJ, Hagedorn HJ. A guiding framework and approach for implementation research in substance use disorders treatment. Psychol Addict Behav J Soc Psychol Addict Behav. 2011;25(2):194–205. [DOI] [PubMed] [Google Scholar]
- 78.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019. Jul;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moore CG, Carter RE, Nietert PJ, Stewart PW. Recommendations for Planning Pilot Studies in Clinical and Translational Research. Clin Transl Sci. 2011;4:332–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.SocioCultural Research Consultants, LLC. Dedoose Version 9.0.17, web application for managing, analyzing, and presenting qualitative and mixed method research data. 2021. Available from: www.dedoose.com.
- 81.Miller W, Crabtree BF. Primary care research: a multi typology and qualitative road map. In: Crabtree BF, Miller WL, editors. Doing Qualitative Research. London: Sage Press; 1992. [Google Scholar]
- 82.Painter PL, Hector L, Ray K, Lynes L, Paul SM, Dodd M, et al. Effects of exercise training on coronary heart disease risk factors in renal transplant recipients. Am J Kidney Dis. 2003;42(2):362–9. [DOI] [PubMed] [Google Scholar]
- 83.O’Connor EM, Koufaki P, Mercer TH, Lindup H, Nugent E, Goldsmith D, et al. Long-term pulse wave velocity outcomes with aerobic and resistance training in kidney transplant recipients - A pilot randomised controlled trial. PLoS One. 2017;12(2):e0171063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wesselman H, Ford CG, Leyva Y, Li X, Chang C-CH, Dew MA, et al. Social Determinants of Health and Race Disparities in Kidney Transplant. Clin J Am Soc Nephrol [Internet]. 2021. Jan 28 [cited 2021 Jan 28]; Available from: https://cjasn.asnjournals.org/content/early/2021/01/27/CJN.04860420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Myaskovsky L, Kendall K, Li X, Chang CCH, Pleis JR, Croswell E, et al. Unexpected race and ethnicity differences in the U.S. National Veterans Affairs Kidney Transplant Program. Transplantation. 2019;103(12):2701–14. [DOI] [PMC free article] [PubMed] [Google Scholar]


