Abstract
Background:
Advanced endoscopic technologies led to significant progress in the definition of endoscopic remission of ulcerative colitis (UC) and correlate better with histological changes, compared with standard endoscopy. However, while studies have assessed the diagnostic accuracy of endoscope technologies individually, there are currently limited data comparing between technologies. As such, the aim of this systematic review was to pool data from the existing literature and compare the correlations between endoscopy and histologic disease activity scores across endoscope technologies.
Methods:
We searched PubMed and Embase until February 2021 for eligible studies reporting the correlation between endoscopy and histology activity scores in UC. Studies were grouped by endoscope technology as standard-definition white light (SD-WLE), high-definition white light (HD-WLE) or electronic virtual chromoendoscopy (VCE) and comparisons made between these groups.
Results:
A total of N = 27 studies were identified, of which N = 12 were included in a meta-analysis of correlations between endoscopic and histological activity scores. Combining these studies identified considerable heterogeneity (I2: 89–93%) and returned a pooled correlation coefficient (ρ) for the SD-WLE group of 0.74, which did not differ significantly from HD-WLE (ρ: 0.65, p = 0.521) or VCE (ρ: 0.70, p = 0.801). In addition, N = 4 studies reported the accuracy of endoscopic activity scores on WLE and VCE to diagnose histological remission. Pooling these found significantly higher accuracy for VCE, compared with WLE [risk ratio: 1.13, 95% confidence interval (CI): 1.07–1.19, p < 0.001].
Conclusion:
Activity scores assessed using endoscopy are strongly correlated with activity on histology regardless of endoscopic technology. VCE seems to be more accurate in predicting histological remission than WLE. However, given the heterogeneity between the included studies, head-to-head trials are warranted to confirm these findings.
Keywords: electronic virtual chromoendoscopy, endoscopic remission, histological remission, ulcerative colitis, white light endoscopy
Introduction
Endoscopy represents the most reliable and widely used approach to objectively assess disease activity and endoscopic remission in ulcerative colitis (UC). 1 Endoscopic remission has emerged as a crucial goal to target in the management of UC, as it is associated with improved patient outcomes, including sustained clinical remission, resection-free survival and a reduction in the risk of colorectal cancer.2–4 For several decades, endoscopic remission has been defined using standard-definition white light endoscopy (SD-WLE), but growing data have underlined limitations in the evaluation of mucosal and vascular patterns using this technique. Moreover, the differences between quiescent and mild disease activity are often difficult to distinguish, and subtle inflammation can persist when endoscopic remission is assessed by white light endoscopy (WLE).5,6 As a result, approximately 18–24% of patients with endoscopic remission of UC still have some degree of histologic inflammation. 7 It is unclear whether this gap between endoscopic and histological remission is due to the subjectiveness of endoscopic interpretation, which could be improved with training or limitations in technology.
In light of this, the aim of treatment is increasingly moving to be the achievement of histological remission, rather than the endoscopic remission/improvement that has been the goal in the past. This is particularly the case in UC, where histological remission is a better predictor of clinical outcomes, such as corticosteroid-free remission, risk of relapse and hospitalization rates.8,9
Recently, there have been improvements in the field of WLE, with the introduction of high-definition endoscopes (HD-WLE). In addition, methods of advanced optical diagnosis with electronic virtual chromoendoscopy (VCE) have also been developed, such as narrow band imaging (NBI, Olympus, Japan), optical enhancement i-SCAN (i-SCAN-OE, Pentax, Japan), Linked Colour Image (LCI, Fujifilm) and blue laser image (BLI, Fujifilm, Japan). These new technologies have led to significant progress by providing better quality imaging to use in the assessment of endoscopic healing5,6 (Figure 1). As a result, these advanced endoscopic technologies are increasingly being adopted in routine clinical practice.5,6,10 However, although previous studies have assessed the accuracy of these technologies in diagnosing histological remission, 8 there are limited data making direct comparisons of diagnostic accuracy between the different technologies (i.e. SD-WLE versus HD-WLE versus VCE).
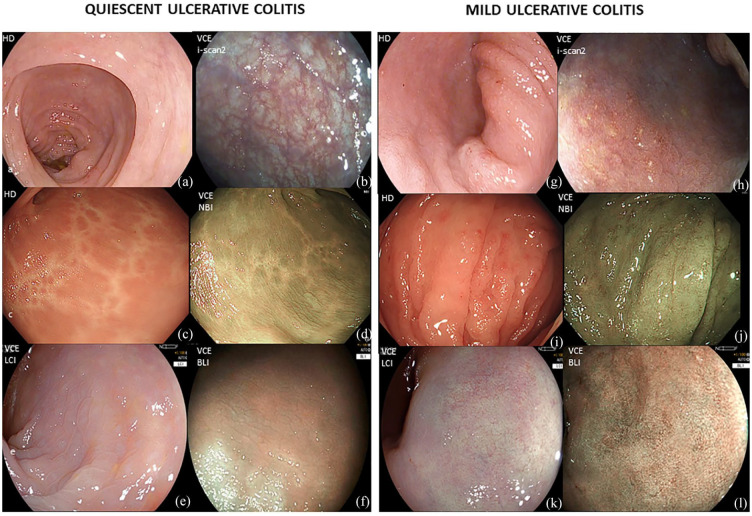
Figure 1.
(a–f) Quiescent ulcerative colitis (UC) assessed by (a) HD-white light endoscopy; (b) i-SCAN modes 2; (c) HD-white light endoscopy; (d) Narrow banding imaging (NBI); (e) Linked colour imaging (LCI); (f) Blue light imaging (BLI). (g–l) Mild inflammation assessed by (g) HD-white light endoscopy; (h) i-SCAN modes 2; (i) HD-white light endoscopy; (j) NBI; (k) LCI; (l) BLI.
Accordingly, the rationale of this systematic review and meta-analysis was to investigate the strength of the correlation between disease activity assessed on histology and endoscopy, to compare this between different endoscopic technologies and to determine the most suitable endoscopic modality for assessing disease activity in UC. We further investigated the diagnostic accuracy of endoscopic techniques, with respect to histological remission in UC.
Methods
Study design
This was a systematic review and meta-analysis of studies that assessed endoscopic and histological remission in UC using disease activity scores and reported either correlation or predictive accuracy data. Randomized controlled studies and prospective or retrospective cohort studies were included. Endoscopic techniques included SD-WLE, HD-WLE and VCE, with the latter comprising methods, including NBI, i-SCAN, LCI and BLI. Studies using other technologies, including artificial intelligence (AI), confocal laser endomicroscopy (CLE) and endocytoscopy (EC), were also included. The systematic review was prospectively registered (PROSPERO ID CRD42020202295) and complies with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.11,12 Ethical approval was not required for this systematic review.
Search criteria
A literature search using PubMed and Embase databases from inception until February 2021 was undertaken by four independent reviewers (O.M.N., Y.S., N.L. and R.C.). The search used the following terms: ‘Inflammatory bowel diseases’ OR ‘ulcerative colitis’ AND ‘mucosal healing’ OR ‘remission’ OR ‘endoscopic remission’ OR ‘no activity’ OR ‘no inflammation’ AND ‘histological remission’ OR ‘histological healing’ OR ‘endoscopic activity’ OR ‘inflammation’ AND ‘high definition’ OR ‘white light endoscopy’ OR ‘NBI’ OR ‘i-SCAN’ OR ‘LCI’ OR ‘standard definition’ OR ‘endocytoscopy’ OR ‘confocal laser endomicroscopy’ OR ‘artificial intelligence’. We selected randomized controlled trials, and prospective or retrospective cohort studies, and excluded duplications, abstracts, studies enrolling patients aged < 18 years, without histology assessment and studies enrolling patients with Crohn’s disease (CD). In addition, studies were also excluded if they did not provide adequate data for the determination of correlation between, or accuracy of, endoscopic versus histological activity. We did not consider articles in languages other than English. Any discrepancies were resolved through consensus among the reviewers.
Data extraction
For each study, we collected data for the following parameters: author, journal, country, year of publication, study design, total number of patients, endoscopic techniques and the activity scores used to define endoscopic and histological remission. The type of correlation coefficient used to quantify the association between the endoscopic and histological activity scores and the resulting statistics were then collected for each study. In addition, for those studies that assessed the ability of endoscopy to diagnose histological remission, the definition of histological remission used, along with the percentage accuracy, was extracted.
Risk of bias for the included studies was assessed using the QUADAS-2 tool, which comprised four domains: patient selection, index test (i.e. endoscopic activity score), reference standard (i.e. histological activity score), and flow and timing. 13 Risk of bias was rated low if patient selection was clear and avoided inappropriate exclusions; if endoscopic and histological scores were a reasonable choice, and applied correctly, with researchers blinded to histology when performing endoscopic scoring (and vice versa) and if exclusions of patients after the commencement of the study were avoided. In addition, any potential conflicts of interest were noted.
Statistical methods
Correlation coefficients were pooled using the ‘metacor’ package in R. This applies a Fisher’s Z-transformation to the coefficients, then estimates the variance and pools studies using a DerSimonian–Laird random-effects meta-analysis model. To compare between pairs of endoscopy technologies, the Z-transformed coefficients from each study were then entered into a random-effects meta-regression model, with the endoscopy technology used as a binary covariate. The first analysis pooled those studies reporting either Spearman’s ρ or Pearson’s r coefficients. Studies reporting Kendall’s τ were analysed separately since the values of this statistic tend to be smaller than those for ρ/r. 14
The accuracy of endoscopic techniques, relative to histology, was then pooled across studies. The majority of included studies had reported results for both WLE and VCE on the same set of cases. As such, a paired analysis was performed for these studies, where the risk ratio for VCE versus WLE was calculated for each study and pooled using a random-effects Mantel-Haenszel model. As a sensitivity analysis, an unpaired analysis was also performed, which additionally allowed those studies that only assessed a single endoscopic technique to be included. This used a meta-regression model to pool the log-odds across studies and compare these between WLE and VCE. Analyses were performed using R version 4.0.5, Stata 14 (College Station, TX: StataCorp LP.) and Review Manager 5.3, and p < 0.05 was classified as statistically significant throughout.
Results
Characteristics of studies
The search strategy yielded a total of 593 potential articles, of which 91 remained after initial screening. Bibliographic review of these articles identified a further nine studies, which were additionally considered, resulting in 100 free-text articles being assessed for eligibility. Of these, a total of 27 studies met the inclusion criteria of the study and were included in the qualitative analysis (Figure 2). Details of these included studies are reported in Tables 1 and 2. Dates of publication ranged from 2010 to 2021, and the studies comprised a total of N = 4257 pairs of endoscopic and histological activity assessments.
Figure 2.
PRISMA flow diagram.
Table 1.
Characteristics of studies included in meta-analysis.
| Study (year) | Study design | Country | N | Endoscope technology | Activity scores | Endoscopy versus histology | |||
|---|---|---|---|---|---|---|---|---|---|
| WLE | Advanced | Endoscopic | Histological | Correlation coefficient | Accuracy assessed a | ||||
| Fluxá et al. 15 | Prospective | Chile | 91 | SD | – | MES | Geboes | Spearman’s | – |
| Frieri et al. 16 | Prospective | Italy | 52 | SD | – | MES | Gupta | Spearman’s | – |
| Irani et al. 17 | Prospective | UK | 125 | SD | – | UCEIS | NI, RHI | Spearman’s | – |
| Lemmens et al. 18 | Retrospective | Belgium | 263 | NA b | – | MES | Geboes | Kendall’s | – |
| Lobatón et al. 19 | Prospective | Belgium | 96 | HD | – | MES | Geboes | Spearman’s | – |
| Osterman et al. 20 | Prospective | USA | 101 | HD | – | MES | Total Riley | Spearman’s | – |
| Rosenberg et al. 21 | Prospective | USA | 103 | HD | – | MES | Geboes | Spearman’s | – |
| Simsek et al. 22 | Retrospective | Turkey | 109 | NA b | – | EAI | HSS | Kendall’s | Yes |
| Kim et al. 23 | Retrospective | Korea | 82 | HD | – | MES | Geboes | Spearman’s | – |
| Iacucci et al. 24 | Retrospective | Canada | 78 | – | i-SCAN | i-SCAN | NYMS | Spearman’s | – |
| Iacucci et al. 25 | Retrospective | Multicentre | 160 | HD | i-SCAN | MES, PICaSSO | RHI | Kendall’s | Yes |
| Iacucci et al. 26 | Prospective | Canada | 41 | HD | i-SCAN | MES, i-SCAN-OE | RHI | Spearman’s | Yes |
| Iacucci et al. 27 | Prospective | Canada | 82 | HD | i-SCAN | MES, PICaSSO | RHI | – c | Yes |
| Iacucci et al. 28 | Prospective | Multicentre | 302 | HD | i-SCAN | MES, PICaSSO | RHI | Pearson’s | Yes |
| Honzawa et al. 29 | Retrospective | Japan | 15 | HD | i-SCAN | MES | Geboes | Kendall’s | – |
| Trivedi et al. 30 | Retrospective | Multicentre | 72 | HD | i-SCAN | PICaSSO | NYMS | Spearman’s | – |
| Kanmura et al. 31 | Prospective | Japan | 73 | HD | LCI | MES | Geboes | Spearman’s | Yes |
EAI, Rachmilewitz endoscopic activity index; HD, high definition; HSS, Harpaz histopathological activity scoring system; MES, Mayo Endoscopic Score; NI, Nancy Index; NYMS, New York Mount Sinai Score; PICaSSO, Paddington International virtual ChromoendoScopy ScOre; RHI, Robarts Histopathology Index; SD, standard definition; UCEIS, Ulcerative Colitis Endoscopic Index of Severity; WLE, White light endoscopy.
Indicates those studies that assessed the predictive accuracy of a dichotomized endoscopic activity score to predict the presence of activity on histology.
The study stated that WLE was used, but it was unclear whether this was SD or HD.
The study was designed specifically to assess the ability of endoscopy to identify patients in clinical remission, and so they did not report a correlation coefficient.
Table 2.
Characteristics of studies only included in qualitative review.
| Study | Study design | Country | Endoscope technology | Activity scores | Endoscopy versus histology | Reason for exclusion b | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | WLE | Advanced | Endoscopic | Histological | Correlation coefficient | Accuracy assessed a | ||||
| Shah et al. 32 | Prospective | India | 96 | SD-WLE | – | MES | Geboes | Kappa | – | A) N/A Correlation |
| Christensen et al. 33 | Retrospective | USA | 646 | SD-WLE | – | MES | Modified Riley |
Kappa | – | A) N/A Correlation |
| Uchiyama et al. 34 | Prospective | Japan | 52 | HD-WLE | LCI | MES | Matts’ histopathological grade | – | – | B) No Correlation or Accuracy |
| Nakazato et al. 35 | Retrospective | Japan | 64 | HD-WLE | EC | MES | Geboes | – | Yes | C) Technology |
| Hundorfean et al. 36 | Prospective | Germany | 23 | HD-WLE | CLE | MES | eMHS | Pearson’s | – | C) Technology |
| Karstensen et al. 37 | Prospective | Denmark | 29 | HD-WLE | CLE | MES | Several CLE parameters | Spearman’s | – | C) Technology |
| Li et al. 38 | Prospective | China | 73 | HD-WLE | CLE | MES | Several CLE parameters | Spearman’s | – | C) Technology |
| Bossuyt et al. 39 | Prospective | Belgium/ Japan | 29 | HD-WLE | AI (RD) | Not reported | RHI | Spearman’s | – | C) Technology |
| Maeda et al. 40 | Retrospective | Japan | 525 c | HD-WLE | AI using EC | MES | Geboes | – | Yes | C) Technology |
| Takenaka et al. 41 | Prospective | Japan | 875 | SD/HD-WLE | AI | UCEIS | Geboes | Kappa | Yes | C) Technology |
HD, High definition; SD, Standard definition; WLE, White light endoscopy.
Indicates those studies that assessed the predictive accuracy of a dichotomized endoscopic activity score to predict the presence of activity on histology.
The reason that the study was excluded from the meta-analysis, classified as (A) the correlation coefficient reported (kappa) could not be pooled in meta-analysis; (B) no correlation coefficient or accuracy assessment was reported or (C) too few studies assessed the endoscope technology for meta-analysis to be possible.
N represents the number of segments assessed.
Risk of bias
Risk of bias assessment (Supplementary Table 1) found potential patient selection bias in several studies, with eight studies16,19–21,28,29,34,35 only including patients in clinical/endoscopic remission, two37,39 only including those in clinical exacerbation and one 38 only including those referred for routine surveillance. Assessment of the index test and reference standard found that the majority of studies did not explicitly state whether the researcher performing the histological assessment had been blinded to the result of the endoscopic activity score or vice versa. In addition, one study 26 used activity scores that had not been validated. Risk of bias due to flow and timing was minimal, with a single study not performing biopsies on all included patients. 40 Finally, five studies25,27,30,34,42 reported receiving support from manufacturers, which could have resulted in conflicts of interest.
Studies included in quantitative review
Of the 27 studies that met inclusion criteria of the review, 10 studies32–41 were excluded from the quantitative review (Table 2). Of these, two32,33 reported correlations between endoscopic and histological activity scores using Kappa statistics, which could not be readily pooled, with a further study 34 not reporting either a correlation coefficient or a measure of diagnostic accuracy. The remaining seven studies35–41 used endoscope technologies that were assessed by too few studies to meaningfully pool the results (EC, CLE and AI); hence, these were analysed descriptively in the qualitative review.
The 17 studies included in the qualitative review are reported in Table 1. Of these, 12 studies15–17,19–24,26,28,30,31 reported the correlation between endoscopic and histological scores using either Spearman’s ρ or Pearson’s r coefficients, whilst four studies18,22,25,29 used Kendall’s τ coefficients. In addition, six studies22,25–28,31 reported the accuracy of endoscopic technologies for the prediction of the presence of remission on histology.
Spearman’s ρ correlations between endoscopic and histological activity scores
Of the 1215–17,19–21,23,24,26,28,30,31 studies reporting associations between endoscopic and histological activity scores using ρ or r coefficients, 3 studies28,30,31 reported outcomes for two different endoscope technologies, giving 15 records for analysis (Table 3). Of these, Kanmura et al. 31 stated that the correlation between endoscopic and histologic activity scores when using HD-WLE was nonsignificant but did not report the corresponding coefficient; hence, this study was excluded from the analysis of HD-WLE. The remaining studies were then divided by the endoscopic technology used, namely SD-WLE (three studies,15–17 N = 268), HD-WLE (six studies,19–21,23,28,30 N = 756) and VCE (five studies,24,26,28,30,31 N = 566).
Table 3.
Comparison of correlations between endoscopic and histological scores by endoscope technology.
| Study | Technology | Endoscopic score | Histological score | N | Correlation coefficient (95% CI) |
|---|---|---|---|---|---|
| Standard white light (ρ) | |||||
| Fluxá et al. 15 | SD-WLE | MES | Geboes | 91 | 0.67 (0.54–0.77) |
| Frieri et al. 16 | SD-WLE | MES | Gupta | 52 | 0.61 (0.40–0.76) |
| Irani et al. 17 | SD-WLE | UCEIS | RHI | 125 | 0.86 (0.81–0.90) |
| Pooled SD-WLE (ρ) | N = 268; I2 = 89% | 0.74 (0.52–0.87) | |||
| High-definition white light (ρ) | |||||
| Rosenberg et al. 21 | HD-WLE | MES | Geboes | 103 | 0.65 (0.52–0.75) |
| Kim et al. 23 | HD-WLE | MES | Geboes | 82 | 0.77 (0.66–0.85) |
| Lobatón et al. 19 | HD-WLE | MES | Geboes | 96 | 0.27 (0.07–0.45) |
| Kanmura et al. 31 | HD-WLE | MES | Geboes | 73 | ns b |
| Trivedi et al. 30 | HD-WLE | MES | NYMS | 72 | 0.88 (0.81–0.92) |
| Osterman et al. 20 | HD-WLE | MES | Total Riley | 101 | 0.35 (0.17–0.51) |
| Iacucci et al. 28 | HD-WLE | MES | RHI | 302 | 0.68 (0.61–0.74) a |
| Pooled HD-WLE (ρ) | N = 756 b ; I 2 = 93% | 0.65 (0.45–0.78) | |||
| VCE (ρ) | |||||
| Iacucci et al. 24 | i-SCAN | i-SCAN | NYMS | 78 | 0.65 (0.50–0.76) |
| Iacucci et al. 26 | i-SCAN | i-SCAN-OE | RHI | 41 | 0.61 (0.37–0.77) |
| Trivedi et al. 30 | i-SCAN | PICaSSO c | NYMS | 72 | 0.90 (0.84–0.94) c |
| Kanmura et al. 31 | LCI | LCI-a | Geboes | 73 | 0.36 (0.14–0.54) |
| Iacucci et al. 28 | i-SCAN | PICaSSO | RHI | 302 | 0.77 (0.72–0.81) a |
| Pooled VCE (ρ) | N = 566; I 2 = 91% | 0.70 (0.50–0.83) | |||
| White light (τ) | |||||
| Lemmens et al. 18 | WLE | MES | Geboes | 263 | 0.48 (0.38–0.57) |
| Simsek et al. 22 | WLE | EAI | HSS | 109 | 0.27 (0.09–0.44) |
| Iacucci et al. 25 | HD-WLE | MES | RHI | 160 | 0.62 (0.51–0.71) |
| Honzawa et al. 29 | HD-WLE | MES | Geboes | 15 | 0.54 (0.03–0.82) |
| Pooled WLE (τ) | N = 547; I 2 = 76% | 0.48 (0.32–0.62) | |||
| VCE (τ) | |||||
| Iacucci et al. 25 | i-SCAN | PICaSSO | RHI | 160 | 0.53 (0.42–0.63) |
| Pooled VCE (τ) | N = 160; I 2 = N/A | 0.53 (0.42–0.63) | |||
EAI, Rachmilewitz endoscopic activity index; MES, Mayo Endoscopic Score; ns, nonsignificant; NYMS, New York Mount Sinai Score; PICaSSO, Paddington International virtual ChromoendoScopy ScOre; RHI, Robarts Histopathology Index; UCEIS, Ulcerative Colitis Endoscopic Index of Severity.
Correlations are between the endoscopic and histological scores and are reported as Spearman’s (ρ) or Kendall’s (τ) coefficients, unless stated otherwise. Confidence intervals (CIs) for individual studies were calculated based on the correlation coefficient and sample size. Pooled correlation coefficients are from DerSimonian–Laird random-effect meta-analysis models, as described in the statistical methods. Analyses were performed separately for Spearman’s and Kendall’s correlation coefficients.
Pearson’s r correlation coefficient.
The study reported the correlation to be nonsignificant, but did not report a coefficient; hence, this was excluded from the meta-analysis and was not included in the total N.
The study assessed the mucosal and vascular scores separately; hence, the mean of the coefficients was used for analysis.
The bold aimed to highlight the results of pooled correlation of the studies.
The studies of WLE almost exclusively used the Mayo Endoscopic Subscore (MES), which quantifies disease activity on a four-point scale from ‘normal/inactive’ to ‘severe’. 43 The remaining WLE study 17 used the Ulcerative Colitis Endoscopic Index of Severity (UCEIS), which scores the vascular pattern, bleeding and erosions/ulcers on separate Likert-type scales and adds these together to give a score in the range 0–8. 44 Studies of VCE generally used i-SCAN, with the PICaSSO score to quantify activity on endoscopy. The latter score assesses the mucosal and vascular architecture separately, with each being quantified using Likert-type scales on four domains, which are added to yield an overall score in the range 0–9 for mucosal architecture and 0–6 for vascular pattern.25,28,30 One study 30 using the PICaSSO score reported correlations with the mucosal and vascular scores separately, rather than for the overall score; the mean of the correlation coefficients for these two scores was used in this meta-analysis.
The histological activity scores used by the included studies were more variable, with the most common being the Geboes score, which classifies the degree of inflammation in a 6-point scale from ‘structural change only’ to ‘erosions/ulcers’. 45 The Robarts Histopathology Index (RHI) was also frequently used, particularly in the VCE studies. This quantifies the involvement of epithelial neutrophils, increase in lamina propria neutrophils, degree of chronic inflammatory cell infiltrate and severity of erosion/ulceration on separate Likert-type scales (range: 0–3), which are then combined with a weighted sum, to give a score in the range 0–33. 46
Pooling the correlation coefficients across studies found considerable heterogeneity, with I2 statistics ranging from 89% to 93% [Figure 3(a)]. The pooled correlation coefficients between endoscopic and histological scores did not differ significantly between SD-WLE and HD-WLE (0.74 versus 0.65, p = 0.521). The pooled correlation coefficient for VCE was 0.70, which was not found to differ significantly from either SD-WLE (p = 0.801) or HD-WLE (p = 0.674).
Figure 3.

(a) Forest plot of correlation coefficients by scope technology. Further details of the studies are reported in Table 3. (b) Forest plot of endoscopic score accuracy by scope technology. Further details of the studies are reported in Table 4.
Kendall’s τ correlations between endoscopic and histological scores
A further four18,22,25,29 studies reported correlations between endoscopic and histological activity scores using Kendall’s τ coefficients, which were analysed separately (Table 3). All of these studies used WLE for endoscopy, with one study additionally reporting outcomes for VCE. 25 Combining the studies of WLE returned a pooled Kendall’s correlation coefficient of 0.48, with considerable heterogeneity detected (I2 = 76%). The single study 25 of VCE reported a Kendall’s τ of 0.53 – this did not differ significantly from the pooled total for WLE (p = 0.768)
Accuracy of endoscopic scores versus histological remission
A total of six22,25–28,31 studies either reported the accuracy of endoscopy for the prediction of histological remission or gave sufficient data for this to be calculated (Table 4). Of these, two studies22,25 only reported accuracy data for one of the endoscope technologies and so were not included in the main analysis. The remaining four26–28,31 studies that reported data for both WLE and VCE assessed a total of N = 498 cases. The histological scores used were either RHI or Geboes, while endoscopic scores were either MES or WLE-b for WLE, with either PICaSSO or i-SCAN-OE used for i-SCAN and LCI-b for LCI. Pooling these studies found the accuracy of endoscopy in the prediction of histological remission to be significantly higher for VCE than for WLE [p < 0.001, Figure 3(b)], with a risk ratio of 1.13 (95% CI: 1.07–1.19). This effect was consistent across studies, with negligible heterogeneity detected (I2 = 0%).
Table 4.
Comparison of endoscopic score accuracy to predict histological remission between WLE and VCE.
| Study | N | Endoscopic score (cut-off) | Histological score (cut-off) |
Accuracy | Risk ratio (95% CI) |
||
|---|---|---|---|---|---|---|---|
| WLE | VCE | WLE | VCE | ||||
| Simsek et al. 22 | 109 | EAI (⩽3) | - | HSS (0) | 84/109 (77%) b | - | N/A |
| Iacucci et al. 25 | 160 | - | PICaSSO (N/A a ) | RHI (N/A a ) | - | 115 c /160 (72%) | N/A |
| Iacucci et al. 26 | 41 | MES (NR) | i-SCAN OE (NR) | RHI (NR) | 22 c /41 (54%) | 28 c /41 (68%) | 1.27 (0.89–1.81) |
| Kanmura et al. 31 | 73 | WLE-b (<38.5) | LCI-b (<19.9) | Geboes (⩽2) | 45 c /73 (62%) | 47 c /73 (64%) | 1.04 (0.81–1.34) |
| Iacucci et al. 27 | 82 | MES (0) | PICaSSO (⩽4) | RHI (⩽3) | 70 c /82 (85%) | 75 c /82 (91%) | 1.07 (0.96–1.20) |
| Iacucci et al. 28 | 302 | MES (0) | PICaSSO total (⩽3) | RHI (⩽3 d ) | 239 c /302 (79%) | 275 c /302 (91%) | 1.15 (1.08–1.23) |
| Pooled Difference | N = 498 pairs; I 2 = 0% | 1.13 (1.07–1.19) | |||||
N/A, not applicable; NR, not reported.
Studies were pooled using a random-effects Mantel-Haenszel model.
No cut-off was used, with accuracy instead representing the proportion of times that a regression model with the endoscopic score as a covariate correctly predicted the histological score.
Accuracy was not directly reported by the study but was calculated based on tabulated data.
Numerators were not explicitly stated by the study hence were estimated from the denominator and the percentage accuracy.
Plus absence of neutrophils.
A sensitivity analysis then was repeated using all six studies,22,25–28,31 using a meta-regression approach that did not account for the pairing. This analysis of 1265 cases returned a pooled odds ratio for VCE versus WLE of 1.48 (95% CI: 0.58–3.77), which was equivalent to a risk ratio of 1.10. However, due to considerable heterogeneity (I2 = 89%), this was not found to be statistically significant (p = 0.417).
In addition to assessing overall accuracy, the sensitivity and specificity of endoscopy for the prediction of histological remission were also assessed. None of the studies explicitly reported the underlying numerators and denominators for these statistics; however, there was sufficient information to derive these values for three studies.27,28,31 Pooling these studies found the sensitivity for the prediction of histological remission to be significantly greater in VCE compared with WLE (risk ratio: 1.19; 95% CI: 1.06–1.34; p = 0.004), with no significant difference in specificity (risk ratio: 0.96; 95% CI: 0.89–1.05, p = 0.39). Further details of this analysis are reported in Supplementary Table 2.
Qualitative review
Overall, 10 studies32–41 were excluded from the meta-analysis, most commonly due to using heterogeneous endoscopic technologies, including AI studies [using different techniques, such as EC and red density (RD) and those using CLE]. The AI studies reported good correlation 39 and high accuracy rates40,41 for the evaluation of histological inflammation and remission in UC. CLE studies36–38 reported comparably high accuracy rates of over 90% in the diagnosis of histological remission and had similar performance to histology for predicting disease relapse.
Discussion
Over the last few years, there has been an increasing interest in striving to achieve the more ambitious goal of histologic remission, rather than endoscopic remission in UC. A recent meta-analysis has shown that the persistence of histological activity is associated with twofold increase in relapse in patients with UC, 47 posing the idea that histological healing could be included in the definition of ‘deep remission’. Nevertheless, STRIDE-II, 48 an update on the selection of therapeutic targets in IBD, did not include histological remission as a formal treatment target. Consistent with this, a recent consensus expert panel convened by the European Crohn’s and Colitis Organization (ECCO) 49 established that there is currently limited evidence that histological activity in patients with UC who are in endoscopic remission has an impact on the need for treatment escalation or biologic therapy. 48 However, the combination of histologic and endoscopic improvement has been proposed to be the ultimate endpoint, with the achievement of histo-endoscopic mucosal healing being more strongly associated with clinical remission, reduced risk of relapse and corticosteroid-free remission in the long-term, compared with either histologic or endoscopic improvement alone. 50 Thus, in recent clinical trials, a composite endoscopic-histologic outcome has been used as a target to assess response to therapy and will continue to be explored.50–52
Past attempts to correlate endoscopic activity with histology have shown variable results. The likely reason is that conventional WLE is not explicitly designed to evaluate endoscopic remission and, consequently, the magnitude of subtle patchy inflammation with mucosal and vascular changes can be easily underestimated using WLE. Discrepancy between endoscopic and histological assessment of remission may also be explained by random sampling heterogeneity, patchiness of healing, lack of standardizing biopsy collection protocols, lack of harmonization of endoscopic and histological activity scores, and inconsistency in the thresholds deemed to be indicative of histologic response. 53
The advent of new endoscopic technologies has facilitated the characterization of mucosal and vascular features, which may give assessments of disease activity that are closer to those produced by histology. 6 Thus, we aimed to determine whether advanced endoscopic technologies correlate better with histology than WLE and to examine the diagnostic accuracy of VCE versus WLE in predicting histological remission. In our meta-analysis and synthesis of data from 17 studies, we report a few key observations. We found a strong correlation between endoscopy and histology activity scores for both WLE and VCE, although no significant difference between endoscopic technologies was observed. However, when specifically assessing the ability of endoscopy to diagnose histological remission, VCE was found to be significantly superior to WLE, particularly with respect to sensitivity. The disparity between the findings of analyses of correlation and diagnostic accuracy may indicate that, while VCE does not improve the ability to quantify the exact magnitude of disease activity, compared with WLE, it is superior at differentiating between patients in histological exacerbation versus histological remission. The systematic review also identified additional studies using more novel technologies, including AI and CLE. While formal meta-analysis of these studies was not possible, qualitative review suggested that these technologies also showed strong correlation with histological assessments. These results provide the basis for implementing modern endoscopic tools to replace the use of random tissue samples with targeted biopsies in the assessment of disease activity.
Our study has several strengths. To our knowledge, this is the first meta-analysis to compare VCE with SD-WLE and HD-WLE for assessing correlations between endoscopic activity scores, and both histological activity and remission. Furthermore, we included the studies using the most recent VCE endoscopic technologies and scoring systems. 28 However, we acknowledge certain limitations of this work, chief among which was the heterogeneity in the designs and reporting of the included studies. The included studies used a variety of different endoscopic and histological activity scores and compared between these using a range of different correlation coefficients (e.g. Spearman’s ρ, Pearson’s r, Kendall’s τ, and kappa). If we had only performed analyses strictly comparing like-with-like, then it would not have been possible to perform any meaningful analysis, as this would result in a large number of subgroups with insufficient within-group sample sizes. Consequently, compromises had to be made, in which studies using different activity scores were combined in the same analysis. In addition, the VCE platforms were combined into a single group for analysis, although we acknowledge that nuances are likely to exist between the different manufacturer platforms. Combining the activity scores and VCE platforms in this way was likely a contributor to the considerable heterogeneity observed when pooling the correlation coefficients, which will have resulted in lower statistical power. In addition, bias assessment indicated that several of the studies had selected only those patients who were either in clinical remission or clinical exacerbation. It has previously been demonstrated that correlation between endoscopy and histology is stronger in the extremes of disease activity (e.g. MES 0 and MES 3).18,23 As such, by only including patients in these extremes, the findings of these studies may not have been generalizable to the UC population as a whole, which may have further exacerbated the observed heterogeneity. Finally, even with the more lenient criteria used when selecting studies for inclusion in the meta-analysis models, it was not possible to meaningfully include some studies in the primary analysis (e.g. those reporting Kendall’s τ or Kappa coefficients); hence, the findings of these studies were not incorporated into the pooled totals.
In conclusion, in this first systematic review and meta-analysis of all currently available studies correlating endoscopic and histological activity, we found that both VCE and WLE are strongly correlated with histology, and that VCE offers better diagnostic accuracy for the evaluation of histological remission in UC. In this era, where treatment goals are being redefined, our findings give some evidence to support the wider adoption of VCE in routine practice of UC endoscopy, as the technology is standard in all of the current generation of endoscopes. However, due to the current significant heterogeneity, further studies are warranted to validate these findings and allow for more accurate comparisons between endoscopic techniques. In particular, high-quality head-to-head studies comparing between endoscopic techniques, either using paired assessments or randomizing cases to the different technique, would be preferable, as these would minimize the impact of between-centre variability. In addition, further research into the optimal endoscopic and histological activity scores would be useful, with a view to standardize the scores used in both clinical practice allowing uniform reporting to predict outcomes.
Supplemental Material
Supplemental material, sj-docx-1-tag-10.1177_17562848221092594 for Advanced technology for assessment of endoscopic and histological activity in ulcerative colitis: a systematic review and meta-analysis by Olga Maria Nardone, Yifat Snir, James Hodson, Rosanna Cannatelli, Nunzia Labarile, Keith Siau, Cesare Hassan, Henit Yanai, Iris Dotan, Subrata Ghosh and Marietta Iacucci in Therapeutic Advances in Gastroenterology
Footnotes
Author contribution(s): Olga Maria Nardone: Conceptualization; Formal analysis; Investigation; Writing – original draft; Writing – review & editing.
Yifat Snir: Conceptualization; Formal analysis; Investigation; Writing – original draft; Writing – review & editing.
James Hodson: Formal analysis; Investigation; Methodology; Writing – original draft; Writing – review & editing.
Rosanna Cannatelli: Investigation.
Nunzia Labarile: Investigation.
Keith Siau: Investigation.
Cesare Hassan: Data curation; Supervision; Validation.
Henit Yanai: Data curation; Supervision; Validation.
Iris Dotan: Data curation; Supervision; Validation.
Subrata Ghosh: Conceptualization; Methodology; Supervision; Validation; Visualization; Writing – review & editing.
Marietta Iacucci: Conceptualization; Methodology; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing.
ORCID iDs: Olga Maria Nardone  https://orcid.org/0000-0002-9554-4785
https://orcid.org/0000-0002-9554-4785
Nunzia Labarile  https://orcid.org/0000-0002-8512-7726
https://orcid.org/0000-0002-8512-7726
Marietta Iacucci  https://orcid.org/0000-0002-3142-9550
https://orcid.org/0000-0002-3142-9550
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: M.I. is part-funded by the National Institute for Health Research (NIHR) Birmingham Biomedical Research Centre. The views expressed are those of the author(s) and not necessarily those of the National Health Service (NHS), the NIHR or the Department of Health.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Olga Maria Nardone, Institute of Immunology and Immunotherapy, University of Birmingham, Birmingham, UK.
Yifat Snir, Division of Gastroenterology, Rabin Medical Center, Beilinson Campus, Petach-Tikva, Israel; Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel.
James Hodson, Institute for of Translational Medicine, Queen Elizabeth Hospital, UHBFT, Birmingham, UK; Department of Health Informatics, University Hospitals Birmingham NHS Foundation Trust, Birmingham, UK.
Rosanna Cannatelli, Institute of Immunology and Immunotherapy, University of Birmingham, Birmingham, UK.
Nunzia Labarile, Institute of Immunology and Immunotherapy, University of Birmingham, Birmingham, UK.
Keith Siau, Institute for Translational Medicine, Queen Elizabeth Hospital, UHBFT, Birmingham, UK; Department of Gastroenterology, Royal Cornwall Hospitals NHS Trust, Truro, UK.
Cesare Hassan, Department of Gastroenterology, Nuovo Regina Margherita Hospital, Roma, Italy.
Henit Yanai, Division of Gastroenterology, Rabin Medical Center, Beilinson Campus, Petach-Tikva, Israel; Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel.
Iris Dotan, Division of Gastroenterology, Rabin Medical Center, Beilinson Campus, Petach-Tikva, Israel; Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel.
Subrata Ghosh, Institute of Immunology and Immunotherapy, University of Birmingham, Birmingham, UK; NIHR/Wellcome Trust Clinical Research Facility, University Hospitals Birmingham NHS Trust, University of Birmingham, Birmingham, UK; NIHR Birmingham Biomedical Research Centre, University of Birmingham and University Hospitals Birmingham NHS Foundation Trust, Birmingham, UK; APC Microbiome Ireland, College of Medicine and Health, University College Cork, Cork, Ireland.
Marietta Iacucci, Institute of Translational Medicine, Heritage Building for Research and Development, University Hospitals Birmingham NHS Foundation Trust, Birmingham B15 2TT, UK; Institute of Immunology and Immunotherapy, University of Birmingham, Birmingham, UK; NIHR/Wellcome Trust Clinical Research Facility, University Hospitals Birmingham NHS Trust, University of Birmingham, Birmingham, UK; NIHR Birmingham Biomedical Research Centre, University of Birmingham and University Hospitals Birmingham NHS Foundation Trust, Birmingham, UK; Department of Gastroenterology, University of Calgary, Calgary, AB, Canada.
References
- 1. Dulai PS, Levesque BG, Feagan BG, et al. Assessment of mucosal healing in inflammatory bowel disease: review. Gastrointest Endosc 2015; 82: 246–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol 2015; 110: 1324–1338. [DOI] [PubMed] [Google Scholar]
- 3. Barreiro-de Acosta M, Vallejo N, de la Iglesia D, et al. Evaluation of the risk of relapse in ulcerative colitis according to the degree of mucosal healing (Mayo 0 vs 1): a longitudinal cohort study. J Crohns Colitis 2016; 10: 13–19. [DOI] [PubMed] [Google Scholar]
- 4. Shah SC, Colombel JF, Sands BE, et al. Systematic review with meta-analysis: mucosal healing is associated with improved long-term outcomes in Crohn’s disease. Aliment Pharmacol Ther 2016; 43: 317–333. [DOI] [PubMed] [Google Scholar]
- 5. Iacucci M, Furfaro F, Matsumoto T, et al. Advanced endoscopic techniques in the assessment of inflammatory bowel disease: new technology, new era. Gut 2019; 68: 562–572. [DOI] [PubMed] [Google Scholar]
- 6. Nardone OM, Cannatelli R, Zardo D, et al. Can advanced endoscopic techniques for assessment of mucosal inflammation and healing approximate histology in inflammatory bowel disease? Therap Adv Gastroenterol 2019; 12: 1756284819863015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Park S, Abdi T, Gentry M, et al. Histological disease activity as a predictor of clinical relapse among patients with ulcerative colitis: systematic review and meta-analysis. Am J Gastroenterol 2016; 111: 1692–1701. [DOI] [PubMed] [Google Scholar]
- 8. Yoon H, Jangi S, Dulai PS, et al. Incremental benefit of achieving endoscopic and histologic remission in patients with ulcerative colitis: a systematic review and meta-analysis. Gastroenterology 2020; 159: 1262–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bryant RV, Burger DC, Delo J, et al. Beyond endoscopic mucosal healing in UC: histological remission better predicts corticosteroid use and hospitalisation over 6 years of follow-up. Gut 2016; 65: 408–414. [DOI] [PubMed] [Google Scholar]
- 10. Solitano V, D’Amico F, Allocca M, et al. Rediscovering histology: what is new in endoscopy for inflammatory bowel disease? Therap Adv Gastroenterol 2021; 14: 17562848211005692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009; 151: 264–269, W64. [DOI] [PubMed] [Google Scholar]
- 12. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015; 4: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011; 155: 529–536. [DOI] [PubMed] [Google Scholar]
- 14. Genest PCC. Spearman’s ρ is larger than kendall’s τ for positively dependent random variables. J Nonparametr Stat 1993; 2: 183–194. [Google Scholar]
- 15. Fluxá D, Simian D, Flores L, et al. Clinical, endoscopic and histological correlation and measures of association in ulcerative colitis. J Dig Dis 2017; 18: 634–641. [DOI] [PubMed] [Google Scholar]
- 16. Frieri G, Galletti B, Di Ruscio M, et al. The prognostic value of histology in ulcerative colitis in clinical remission with mesalazine. Therap Adv Gastroenterol 2017; 10: 749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Irani NR, Wang LM, Collins GS, et al. Correlation between endoscopic and histological activity in ulcerative colitis using validated indices. J Crohns Colitis 2018; 12: 1151–1157. [DOI] [PubMed] [Google Scholar]
- 18. Lemmens B, Arijs I, Van Assche G, et al. Correlation between the endoscopic and histologic score in assessing the activity of ulcerative colitis. Inflamm Bowel Dis 2013; 19: 1194–1201. [DOI] [PubMed] [Google Scholar]
- 19. Lobatón T, Bessissow T, Ruiz-Cerulla A, et al. Prognostic value of histological activity in patients with ulcerative colitis in deep remission: a prospective multicenter study. United European Gastroenterol J 2018; 6: 765–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Osterman MT, Scott FI, Fogt FF, et al. Endoscopic and histological assessment, correlation, and relapse in clinically quiescent ulcerative colitis (MARQUEE). Inflamm Bowel Dis 2021; 27: 207–214. [DOI] [PubMed] [Google Scholar]
- 21. Rosenberg L, Lawlor GO, Zenlea T, et al. Predictors of endoscopic inflammation in patients with ulcerative colitis in clinical remission. Inflamm Bowel Dis 2013; 19: 779–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Simsek HD, Basyigit S, Aktas B, et al. Assessment of the correlation between endoscopic activity and histological activity in ulcerative colitis patients. Med Princ Pract 2016; 25: 378–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim DB, Lee KM, Lee JM, et al. Correlation between histological activity and endoscopic, clinical, and serologic activities in patients with ulcerative colitis. Gastroenterol Res Pract 2016; 2016: 5832051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Iacucci M, Fort Gasia M, Hassan C, et al. Complete mucosal healing defined by endoscopic Mayo subscore still demonstrates abnormalities by novel high definition colonoscopy and refined histological gradings. Endoscopy 2015; 47: 726–734. [DOI] [PubMed] [Google Scholar]
- 25. Iacucci M, Daperno M, Lazarev M, et al. Development and reliability of the new endoscopic virtual chromoendoscopy score: the PICaSSO (Paddington International Virtual ChromoendoScopy ScOre) in ulcerative colitis. Gastrointest Endosc 2017; 86: 1118–1127.e5. [DOI] [PubMed] [Google Scholar]
- 26. Iacucci M, Kiesslich R, Gui X, et al. Beyond white light: optical enhancement in conjunction with magnification colonoscopy for the assessment of mucosal healing in ulcerative colitis. Endoscopy 2017; 49: 553–559. [DOI] [PubMed] [Google Scholar]
- 27. Iacucci M, Cannatelli R, Gui X, et al. Assessment of endoscopic healing by using advanced technologies reflects histological healing in ulcerative colitis. J Crohns Colitis 2020; 14: 1282–1289. [DOI] [PubMed] [Google Scholar]
- 28. Iacucci M, Smith SCL, Bazarova A, et al. An international multicenter real-life prospective study of electronic chromoendoscopy score PICaSSO in ulcerative colitis. Gastroenterology 2021; 160: 1558–1569.e8. [DOI] [PubMed] [Google Scholar]
- 29. Honzawa Y, Matsuura M, Higuchi H, et al. A novel endoscopic imaging system for quantitative evaluation of colonic mucosal inflammation in patients with quiescent ulcerative colitis. Endosc Int Open 2020; 8: E41–E49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Trivedi PJ, Kiesslich R, Hodson J, et al. The Paddington International Virtual Chromoendoscopy Score in ulcerative colitis exhibits very good inter-rater agreement after computerized module training: a multicenter study across academic and community practice (with video). Gastrointest Endosc 2018; 88: 95–106.e2. [DOI] [PubMed] [Google Scholar]
- 31. Kanmura S, Hamamoto H, Tanaka A, et al. Diagnostic utility of linked color imaging in the evaluation of colonic mucosal inflammation in ulcerative colitis: a pilot study. Endosc Int Open 2019; 7: E937–E943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shah J, Dutta U, Das A, et al. Relationship between Mayo endoscopic score and histological scores in ulcerative colitis: a prospective study. JGH Open 2020; 4: 382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Christensen B, Hanauer SB, Erlich J, et al. Histologic normalization occurs in ulcerative colitis and is associated with improved clinical outcomes. Clin Gastroenterol Hepatol 2017; 15: 1557–1564.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Uchiyama K, Takagi T, Kashiwagi S, et al. Assessment of endoscopic mucosal healing of ulcerative colitis using linked colour imaging, a novel endoscopic enhancement system. J Crohns Colitis 2017; 11: 963–969. [DOI] [PubMed] [Google Scholar]
- 35. Nakazato Y, Naganuma M, Sugimoto S, et al. Endocytoscopy can be used to assess histological healing in ulcerative colitis. Endoscopy 2017; 49: 560–563. [DOI] [PubMed] [Google Scholar]
- 36. Hundorfean G, Chiriac MT, Mihai S, et al. Development and validation of a confocal laser endomicroscopy-based score for in vivo assessment of mucosal healing in ulcerative colitis patients. Inflamm Bowel Dis 2017; 24: 35–44. [DOI] [PubMed] [Google Scholar]
- 37. Karstensen JG, Sӑftoiu A, Brynskov J, et al. Confocal laser endomicroscopy in ulcerative colitis: a longitudinal study of endomicroscopic changes and response to medical therapy (with videos). Gastrointest Endosc 2016; 84: 279–286.e1. [DOI] [PubMed] [Google Scholar]
- 38. Li CQ, Xie XJ, Yu T, et al. Classification of inflammation activity in ulcerative colitis by confocal laser endomicroscopy. Am J Gastroenterol 2010; 105: 1391–1396. [DOI] [PubMed] [Google Scholar]
- 39. Bossuyt P, Nakase H, Vermeire S, et al. Automatic, computer-aided determination of endoscopic and histological inflammation in patients with mild to moderate ulcerative colitis based on red density. Gut 2020; 69: 1778–1786. [DOI] [PubMed] [Google Scholar]
- 40. Maeda Y, Kudo SE, Mori Y, et al. Fully automated diagnostic system with artificial intelligence using endocytoscopy to identify the presence of histologic inflammation associated with ulcerative colitis (with video). Gastrointest Endosc 2019; 89: 408–415. [DOI] [PubMed] [Google Scholar]
- 41. Takenaka K, Ohtsuka K, Fujii T, et al. Development and validation of a deep neural network for accurate evaluation of endoscopic images from patients with ulcerative colitis. Gastroenterology 2020; 158: 2150–2157. [DOI] [PubMed] [Google Scholar]
- 42. Bossuyt P, Bisschops R, Vermeire S, et al. Variability in the distribution of histological disease activity in the colon of patients with ulcerative colitis. J Crohns Colitis 2021; 15: 603–608. [DOI] [PubMed] [Google Scholar]
- 43. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med 1987; 317: 1625–1629. [DOI] [PubMed] [Google Scholar]
- 44. Travis SP, Schnell D, Krzeski P, et al. Developing an instrument to assess the endoscopic severity of ulcerative colitis: the Ulcerative Colitis Endoscopic Index of Severity (UCEIS). Gut 2012; 61: 535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Geboes K, Riddell R, Ost A, et al. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut 2000; 47: 404–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mosli MH, Feagan BG, Zou G, et al. Development and validation of a histological index for UC. Gut 2017; 66: 50–58. [DOI] [PubMed] [Google Scholar]
- 47. Gupta A, Yu A, Peyrin-Biroulet L, et al. Treat to target: the role of histologic healing in inflammatory bowel diseases: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2021; 19: 1800–1813.e4. [DOI] [PubMed] [Google Scholar]
- 48. Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the international organization for the study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology 2021; 160: 1570–1583. [DOI] [PubMed] [Google Scholar]
- 49. Magro F, Doherty G, Peyrin-Biroulet L, et al. ECCO position paper: harmonization of the approach to ulcerative colitis histopathology. J Crohns Colitis 2020; 14: 1503–1511. [DOI] [PubMed] [Google Scholar]
- 50. Li K, Marano C, Zhang H, et al. Relationship between combined histologic and endoscopic endpoints and efficacy of ustekinumab treatment in patients with ulcerative colitis. Gastroenterology 2020; 159: 2052–2064. [DOI] [PubMed] [Google Scholar]
- 51. Nardone OM, Ghosh S, Iacucci M. Endoscopy and histology in inflammatory bowel diseases patients: complementary or alternatives? – author’s reply. United European Gastroenterol J. Epub ahead of print 3 March 2022. DOI: 10.1002/ueg2.12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nardone OM, Bazarova A, Bhandari P, et al. PICaSSO virtual electronic chromendoscopy accurately reflects combined endoscopic and histological assessment for prediction of clinical outcomes in ulcerative colitis. United European Gastroenterol J 2022; 10: 147–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ma C, Sedano R, Almradi A, et al. An international consensus to standardize integration of histopathology in ulcerative colitis clinical trials. Gastroenterology 2021; 160: 2291–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tag-10.1177_17562848221092594 for Advanced technology for assessment of endoscopic and histological activity in ulcerative colitis: a systematic review and meta-analysis by Olga Maria Nardone, Yifat Snir, James Hodson, Rosanna Cannatelli, Nunzia Labarile, Keith Siau, Cesare Hassan, Henit Yanai, Iris Dotan, Subrata Ghosh and Marietta Iacucci in Therapeutic Advances in Gastroenterology