Abstract
Psychiatric and medical comorbidities are common among adults in the United States. Due to the complex interplay between medical and psychiatric illness, comorbidities result in substantial disparities in morbidity, mortality, and health care costs. There is, thus, both an ethical and fiscal imperative to develop care management programs to address the needs of individuals with comorbid conditions. Although there is substantial evidence supporting the use of care management for improving health outcomes for patients with chronic diseases, the majority of interventions described in the literature are condition-specific. Given the prevalence of comorbidities, the authors of this article reviewed the literature and drew on their clinical expertise to guide the development of future multimorbidity care management programs. Their review yielded one study of multimorbidity care management and two studies of multimorbidity collaborative care. The authors supplemented their findings by describing three key pillars of effective care management, as well as specific interventions to offer patients based on their psychiatric diagnoses and illness severity. The authors proposed short-, medium-, and long-term indicators to measure and track the impact of care management programs on disparities in care. Future studies are needed to identify which elements of existing multimorbidity collaborative care models are active ingredients, as well as which of the suggested supplemental interventions offer the greatest value.
Keywords: behavioral health, care management, chronic disease, comorbid conditions, health disparities
Background
Psychiatric and medical comorbidities are extremely common among adults in the United States, with 68% of those with mental disorders also having medical conditions and 29% of those with medical conditions also having mental disorders.1 Due to the complex interplay between medical and psychiatric illness, comorbidities result in substantial health disparities.
People with comorbid conditions tend to have more severe symptoms and greater functional impairment than the general population.1 Further, these patients have a 2- to 4-fold elevated risk of premature mortality, typically due to cardiovascular disease, and greater health care costs.2–4 Bearing these facts in mind, there is an ethical and fiscal imperative to develop interventions that address the needs of individuals with comorbid medical and psychiatric conditions (hereafter referred to as “comorbidities”), to reduce both health disparities and care costs.
One evidence-based approach to improving the quality of care and health outcomes for people with chronic diseases is care management. Care managers support patients through assessment, care planning, and care coordination.5 One specific model of care that uses care managers and has shown promise in addressing the needs of patients with psychiatric illness is collaborative care. This model is a team-based approach that emphasizes using care managers to empower patients to manage their own illnesses; provide decision support tools to ensure measurement-guided, evidence-based care; coordinate the flow of information between various members of a patient's care team; and employ population health strategies, such as patient registries, to track outcomes over time.6 These interventions are guided by case reviews between the care manager and a consulting mental health specialist (typically a psychiatrist).
Although there is substantial evidence supporting the effectiveness of models of care that utilize care managers, including for reducing disparities among racial and ethnic minorities,7 the majority of interventions described in the literature are disease-specific. Given the prevalence of comorbidities in the general population, single-disease care management programs may be less practical to implement and less efficient for improving outcomes than programs that target multiple conditions at once.
For this reason, the authors of this article reviewed the literature on care management models that target both medical and psychiatric outcomes with the goal of enhancing an existing insurance-based care management program for people with comorbidities. The findings of this review are summarized in the next section. Given the relative paucity of literature on interventions that measure both medical and psychiatric outcomes, the authors supplemented their review by outlining a conceptual framework for the development of care management programs serving individuals with mental and physical comorbidities.
Although the framework described below was initially created to serve the needs of Medicare-eligible beneficiaries, the authors believe these recommendations are broadly applicable and important considerations when developing any care management program that aims at improving health equity for individuals with medical and psychiatric comorbidities.
Evidence-Based Models
The authors reviewed the literature to identify studies of care management programs that aimed at improving medical and psychiatric outcomes for patients with comorbidities. Although there were numerous studies examining the impact of such programs on mental health outcomes6 or non-specific scores of physical well-being or functional status,8,9 there were relatively few that measured outcomes for both medical and psychiatric conditions.
Of the studies that did measure both sets of outcomes, the interventions fell predominantly into one of two categories: traditional care management programs and collaborative care interventions.
With regard to traditional care management, the authors identified one randomized controlled trial10 that followed two pilot studies,11,12 all of which were rated as “fair” in quality in a systematic review of care management interventions targeting multimorbidity.13 This randomized controlled trial examined whether integrating depression and diabetes care management improved adherence to medication regimens, as well as outcomes for both depression and diabetes.
The trial included 180 participants who were treated at a single community-based primary care practice in West Philadelphia. The intervention utilized two research coordinators (one bachelors- and one masters-level) as care managers. The care managers received training on pharmacotherapy for depression and diabetes, as well as on culturally competent evidence-based strategies for quickly building rapport.
The role of the care managers was to help patients recognize depression in the context of their medical illness, explain the rationale for treatment with medications, monitor medication adherence, and help address medication side effects. In addition, medication adherence was tracked by using electronic devices that registered when pill bottles were opened. Over the 3-month study period, the care managers met with study participants three times in-person for 30 minutes, and two times by phone for 15 minutes. Baseline demographic and clinical features did not differ between the two study arms.
At the 3-month follow-up, the trial found statistically significant improvements in medication adherence, depression scores, and diabetes outcomes for participants in the intervention group as compared with the usual care group. Although the limitations of this study include questions about generalizability, restriction to patients with comorbid depression and diabetes, and a short follow-up window, strengths include its low level of resource intensiveness (given no involvement from a mental health specialist) and its inclusion of majority African American study participants, who have long suffered from health disparities.14
With regard to the collaborative care model, the first of two studies was TEAMcare,15 which was rated as “good” quality in the aforementioned systematic review of multimorbidity care management programs. The TEAMcare study was an offshoot of the Pathways study (a program aimed at improving depression and diabetes outcomes through enhanced depression care16). The creators of Pathways hypothesized that un- or under-treated depression interfered with self-care behaviors, and that reductions in depressive symptoms would lead to improvements in diet, exercise, and medication adherence, which, in turn, would result in improvements in diabetes outcomes.
However, the Pathways study ultimately found that depression care management alone did not result in improved diabetes outcomes. As a result, TEAMcare was developed to test whether a primary-care based care management program that simultaneously targeted depression and common comorbidities—specifically, diabetes, hypertension, and hyperlipidemia—could improve outcomes across these diagnoses.
TEAMcare enrolled 214 patients (mean age 57) from 14 primary care clinics in Western Washington. The intervention used nurse care managers who received a 2-day course in depression care, with emphasis on diagnosis, treatment, and specific therapeutic interventions, including motivational interviewing and behavioral activation. They also received a refresher course in active management of the three medical conditions listed earlier.
The nurse care managers led structured visits with patients every 2 to 4 weeks, focusing on disease self-management, development of individualized care plans, careful monitoring of outcome measures (such as hemoglobin A1c for diabetes), and relentless adjustment of interventions to achieve the desired health outcomes, also known as “treat-to-target.” The care managers also maintained a patient registry that was reviewed in weekly supervision with a psychiatrist, primary care physician, and psychologist, all with an eye to patients who were new or not responding to treatment as expected.
Over the course of the 12-month study, intervention group participants experienced greater improvements in the composite primary outcome measure—which included SCL-20, hemoglobin A1c, low-density lipoprotein, and systolic blood pressure—than control group participants.15 Intervention group participants also experienced greater improvements in secondary measures, such as quality of life and satisfaction with care. Further, in a 2-year follow-up study assessing cost-effectiveness, the intervention group had a nearly $600 reduction in outpatient costs, even after accounting for the costs of the intervention.17
Thus, TEAMcare demonstrated the cost-effectiveness of a primary care-based program in which one medically trained nurse care manager had regular access to a consulting mental health specialist and simultaneously addressed patients' psychiatric and medical gaps in care.
The second collaborative care study, COMPASS, was a dissemination project that included a much larger sample size (n = 3609) across a broader range of settings.18,19 COMPASS included 18 medical groups spanning 172 primary care and multi-specialty clinics, with between 51 and 684 participants each, across 8 states (in a mix of rural, suburban, and urban settings).
Although the intervention offered in COMPASS was modeled directly after TEAMcare, it did allow for some customization to suit local needs and constraints. For instance, treat-to-target protocols were adapted to regional prescribing practices and care managers were recruited from a variety of backgrounds and specialties. Some offered blended in-person and phone-based care management, whereas others were exclusively phone-based.
Finally, some opted to add members to their systematic case review teams, such as pharmacists, social workers, and diabetes educators. As with TEAMcare, COMPASS was associated with clinically and statistically significant improvements in both psychiatric and medical outcomes. Outcomes did not vary based on the training or background of the care managers.
Given the relative paucity of data, combined with the great need to serve patients with comorbid medical and psychiatric conditions, the authors of this article supplemented the earlier cited review with a conceptual framework that includes “General considerations” and “Specific interventions” to keep in mind when developing care management programs for this patient population.
General Considerations
The considerations that follow are informed by the current literature review and by clinical expertise among the authors. The first consideration is whether to match patients with one or two care managers to address their medical and psychiatric disorders. The authors strongly recommend pairing each patient with a single care manager. Doing so will reduce the risk of miscommunication and duplication of services, thereby leading to more efficient care.
It will also foster a sense among patients that their assigned care manager is their “go-to” person, which will likely breed trust and cut down on confusion. Finally, and perhaps most importantly, assigning a single care manager will allow programs to be nimble enough to address patients' care gaps in the order that is most important to them, which is pivotal to upholding the principle of patient autonomy.
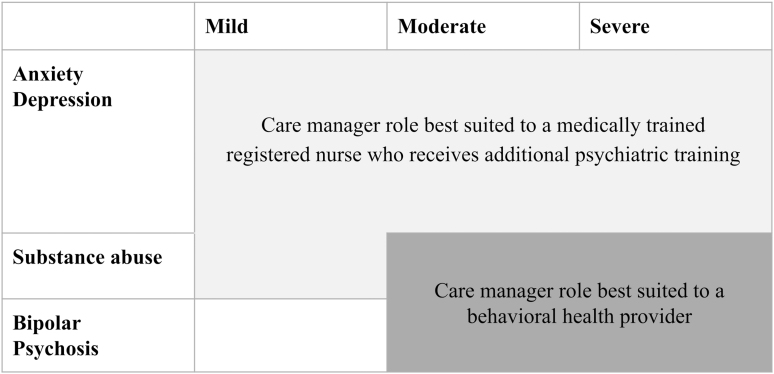
The second consideration is what qualifications a care manager must have to serve in this role (Fig. 1). All else being equal, this should be determined by a patient's primary psychiatric diagnosis. For individuals with mild to moderate anxiety, depression, and substance use disorders (SUDs), registered nurses with competence in assessing clinical status and medication adherence and tolerability would ideally serve in the role of care manager.
FIG. 1.
Recommended qualifications for care manager, stratified by patient's primary psychiatric disorder.
Alternatively, for patients with serious and persistent mental illness (such as bipolar or schizophrenia) or severe mood, anxiety, or SUDs, care managers with behavioral health expertise (such as a social worker or licensed mental health counselor) would be better suited for the role.6 Even more important than the specialization of care managers,20 however, is ensuring (1) access to adequate training and continuing education, (2) an even distribution of complex cases across care managers to reduce burnout and foster a sense of efficacy, and (3) consistent supervision that includes case reviews with a mental health specialist,21 primary care provider, and clinical pharmacist.22,23
Of note, the provision of regular supervision with specialists has previously been identified as an active ingredient in the collaborative care model.24,25 Ideally, this supervision should focus on planning interventions for new patients, problem solving for patients not responding to interventions as expected, gathering input on medication adjustments and management of side effects, and getting guidance on how to approach challenging patient encounters.
If possible, programs should also offer as-needed supervision of care managers with medical specialists to consult on patients with rare or complex conditions. This additional supervision could be conducted in groups to facilitate ongoing education, mutual support, and efficient use of specialist time.
The third consideration in the design of a care management program is how to prioritize among gaps in care for individuals with disparities across multiple health conditions. In general, the authors recommend following a patient's lead, as doing so will enhance buy-in to a process that will likely require behavior change and may even lead to a virtuous cycle in which making progress on one goal leads to increased confidence in one's ability to make progress on another.
Further, honoring individual preferences about which gaps to tackle first will allow care managers to focus on behavioral medicine principles that cut across diseases, such as disease self-monitoring,26 medication adherence,10,27 healthy diet,28 and increased physical activity,27,28 rather than on the specifics of any one condition. In rare cases where members do not have a preference or are unable to communicate a clear preference, behavioral health needs should be addressed before physical needs in patients with severe pathology, such as bipolar affective disorder and schizophrenia.
For all other patients, the authors agree with the approach taken in the studies identified in the literature review, which was to simultaneously address patients' behavioral health needs and common medical risk factors for downstream events, namely hypertension, hyperlipidemia, diabetes, and tobacco use.
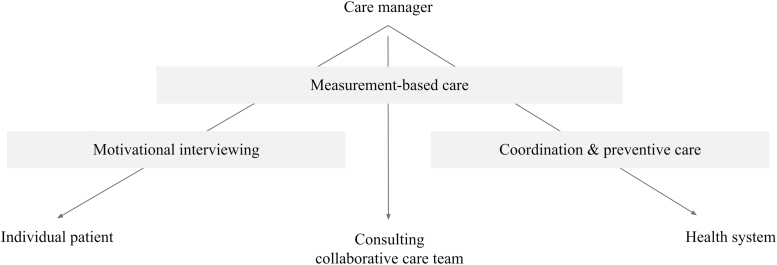
The final consideration is what to include in the training of care managers. The authors argue that training is critical and should focus on three key pillars of effective care management (Fig. 2), which are discussed next in detail:
FIG. 2.
Three key pillars of effective care management.
-
1.
Measurement-based care
-
2.
Motivational interviewing
-
3.
Care coordination
Measurement-based care (MBC) is “the systematic administration of symptom rating scales and use of the results to drive clinical decision making.” Although MBC has been more widely adopted in medical than psychiatric models of care, there is good evidence to support its use in mental health care. A systematic review of 51 studies provided ample evidence that MBC, when conducted frequently and synced with care, led to improved mental health outcomes as compared with usual care.29
The MBC can be used to systematically identify patients for interventions, which has been identified as an active ingredient of collaborative care models in multiple studies.20,25 Further, MBC can guide conversations (1) with patients about how treatment is progressing and to motivate change, (2) when reviewing caseloads with the entire care management team, especially to help in identifying people not responding to treatment as expected, and (3) to frame treatment recommendations that care managers make to patients' individual physicians and other care providers.
Finally, although the purpose of MBC is to guide the treatment of individual patients, an added benefit is that the data collected can also be used to assess the impact of care management programs more broadly, which is discussed in more detail under the Measuring Impact section.
Motivational interviewing (MI) is a therapeutic technique that aims at resolving ambivalence by tapping into a person's individual reasons for behavior change.30 Although MI was originally developed for the treatment of SUDs, there is now robust literature supporting its use in a range of applications, such as weight loss, blood pressure control, cholesterol control, and treatment adherence. A meta-analysis of MI revealed that encounters as brief as 15 minutes can lead to significant outcomes, and that more encounters are associated with a greater impact.30 This same analysis revealed that outcomes did not depend on the level of training of the person performing MI. By implementing MI, care managers can help patients identify personalized treatment goals, improve treatment engagement, and make healthy lifestyle choices.
Finally, poor communication between medical and mental health care systems results in health disparities for individuals with comorbid conditions.31 Thus, coordinating care by ensuring the timely flow of information between medical and mental health providers regarding upcoming appointment times, recent visits, and medication changes is highly valuable. To bring care coordination to the next level, care managers should focus on prevention.
At its most basic, this means ensuring access to preventive services such as immunizations and cancer screenings, which people with comorbidities have historically received at lower rates than the general population.32,33 Further, this involves monitoring for downstream complications of psychiatric medications, such as metabolic syndrome, which result in morbidity, mortality, and high cost care.34 Finally, care teams should work to prevent polypharmacy, which results in not only decreased medication adherence but also drug–drug interactions that lead to medication toxicity, delirium, falls, urinary incontinence, and avoidable admissions.35
Specific Interventions
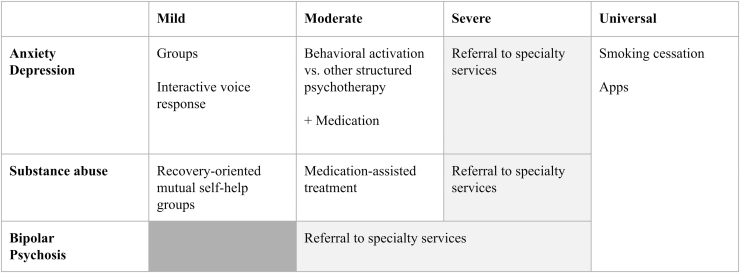
In this section, the authors recommend specific interventions that have the potential to improve outcomes and reduce disparities if incorporated into care management programs for people with medical and psychiatric comorbidities. Some of these interventions have universal applicability, whereas others should be tailored to an individual's psychiatric diagnosis and illness severity (Fig. 3). The list given next, which includes evidence-based, high value interventions, is not exhaustive. Further, the boundaries delineated between the sub-populations of patients listed are not rigid.
FIG. 3.
Recommended evidence-based interventions, stratified by psychiatric diagnosis and illness severity.
Universal interventions
The authors strongly recommend that any care management program serving individuals with comorbidities include an evidence-based cigarette smoking cessation intervention. Tobacco use is the leading preventable cause of death and a risk factor for some of the most common conditions among individuals with medical and psychiatric comorbidities.36 Smoking is more prevalent among people with psychiatric disorders than for people in the general population, thereby contributing to health disparities.37 The majority of cigarette smokers want to quit, but they need support to do so.38
Effective smoking cessation treatments exist, and people who succeed in quitting experience improvements in their mood and anxiety, regardless of whether they have an underlying psychiatric illness.39,40 Finally, smoking cessation programs are an excellent return on investment. Studies estimate that they cost just $0.50 per person-month to operate,41 result in $9800 lifetime savings,42 and can pay for themselves in as little as 3 years.43
All eligible patients should also be offered a curated list of health apps. Two systematic reviews of health apps for anxiety and depression, respectively, show early evidence of efficacy in alleviating symptoms.44,45 Although the available data are less compelling for apps to help with weight management and substance use, the authors of this article have worked with patients who have responded to these sorts of apps. Given the low-cost, if not free, nature of health apps, there is potential benefit and little risk in making a variety of health apps broadly available.
Interventions for mild anxiety and depression
Many cases of mild anxiety and depression are the product of social isolation,46 a problem that has become increasingly prevalent in the context of the COVID-19 pandemic.47 Certain groups—such as immigrants, elderly individuals, and victims of abuse—are at a particularly high risk of loneliness, resulting in mood and anxiety problems.46 Prior studies have observed that social deprivation can pose as great a threat to both mental and physical health as obesity and smoking.48
For this reason, the authors believe that offering interventions that increase social connectedness to patients whose primary behavioral health need is mild anxiety or depression is a worthwhile investment. One such intervention is group therapy, which is cost-effective, has evidence across a range of psychiatric disorders, and may offer particular benefit for patients who are homebound, such as elderly individuals with physical disabilities.49
Another possible intervention is interactive voice response (IVR) technology, a robocaller that can adapt its messages based on patient feedback and can offer supportive messages, track and encourage adherence to treatment, and triage to human support if needed or requested.50 Despite being minimally resource intensive, IVR technology is sufficiently lifelike that patients report feeling cared for and cheered up by IVR calls, with corresponding improvements in depression measures.51
Interventions for moderate anxiety and depression
For patients with moderate anxiety and depression, a combination of brief therapeutic interventions carried out by the care manager, referral to structured psychotherapy with a specialist, and medication management should be offered based on patient preference.
Regarding therapeutic modalities, care managers could offer behavioral activation, an evidence-based intervention that discourages avoidance behaviors and encourages engagement in pleasurable activities. Studies of behavioral activation reveal evidence of equal efficacy when compared with cognitive behavioral therapy (CBT), typically considered the gold standard therapeutic intervention for anxiety and depression, even when administered by junior mental health workers.52
Alternatively, if organizations are unable to train care managers in behavioral activation, care managers could instead assist with referrals to structured psychotherapies, which have been identified as an active ingredient in the collaborative care model.20 Evidence-based therapies include cognitive interventions (such as CBT or mindfulness-based stress reduction), skills-based interventions (such as problem-solving therapy or pain management), and exposure therapy.53,54
Regarding medications for anxiety or depression, care management programs could either use an existing algorithm—such as the one in TEAMcare, Harvard South Shore's Psychopharmacology Algorithm Project,55 or the Texas Medication Algorithm Project56—or develop treatment protocols that reflect local prescribing practices. Regardless of the medications selected, the algorithms should utilize the treat-to-target approach previously described, with the goal of finding the lowest effective dose to achieve symptom remission.
Interventions for mild SUDs
For patients with mild SUDs, care managers can provide education about recovery-oriented mutual self-help groups, such as Alcoholics Anonymous (AA), Narcotics Anonymous (NA), and Self-Management and Recovery Training (SMART) Recovery. A Cochrane Review of 27 studies with more than 10,000 participants concluded that AA and other similar programs were at least as effective, if not more effective, than other established treatments for SUDs, such as CBT.57 This review further concluded that these programs produced substantial health care cost savings among people with alcohol use disorder. With more than 60,000 meetings of varying shapes and sizes in the United States, care managers should focus on helping patients identify meetings that are a good fit and problem-solving barriers to attendance.
Interventions for moderate SUDs
Patients with moderate SUDs should be offered the same education about mutual self-help groups as described earlier and should also be offered referral to medication-assisted treatment (MAT). MAT is typically available through specialty addiction or pain clinics, or through primary care offices with specially licensed providers (sometimes referred to as office-based addiction treatment, or OBAT). MAT should be offered to this group not only because there is clear evidence that it saves lives, but also because MAT has been shown to decrease the need for detoxes and treatment of medical sequelae of SUDs, such as HIV or hepatitis, all of which add to rising health care costs.58,59
Interventions for severe psychiatric conditions
For patients with severe pathology—including bipolar disorder, psychosis, and severe depression, anxiety, or SUDs—care managers should prioritize referral to specialty care in psychiatry and then focus their attention on engagement in care, medication adherence, and careful monitoring for medication-related toxicity.
Measuring Impact
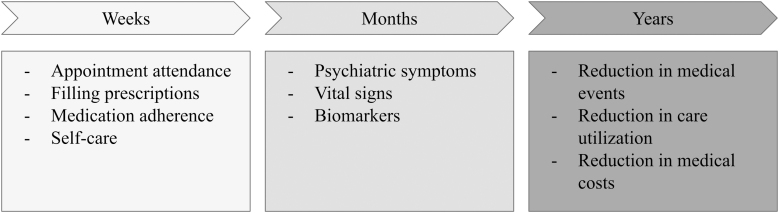
To determine whether the aforementioned interventions are having the desired effect on gaps in care for individuals with medical and psychiatric comorbidities, the authors recommend tracking the following short-, medium-, and long-term indicators (Fig. 4).
FIG. 4.
Indicators to measure the impact of care management programs.
On the order of weeks, one might observe changes in no-shows, prescription fill rates, medication adherence, and other health behaviors such as diet and exercise (“short-term indicators”). These changes could be measured by using a combination of data from billing, pharmacies, self-report, and laboratories. Insurers or health systems might also consider covering the cost of electronic pill boxes and wearables such as pedometers, which—with patient consent—may be able to transmit data directly to care providers or medical records.
On the order of months, one might observe changes in psychiatric symptoms, vital signs, and biomarkers of medical illness (“medium-term indicators”).15 These changes could be tracked through a combination of patient-reported outcome measures, vital sign measurement, and laboratory data. Insurers or health systems might consider covering the cost of durable medical equipment, such as glucose and blood pressure monitors, which can aid patients in disease self-management and may also be able to transmit data to care providers or systems.
Some of the most important outcomes—such as reductions in medical events, care needs, and health care spending—could take one or more years to observe a difference (“long-term indicators”). For instance, the TEAMcare model demonstrated reductions in outpatient care utilization and spending over the course of two years.17 Similarly, a study of collaborative care for bipolar disorder revealed reductions in care utilization after two years and reductions in medical spending emerged over the course of three .60
Finally, one study comparing usual care with a team-based care model in which care managers assisted with patient outreach and care coordination found reduced rates of primary care visits, ambulatory care sensitive visits, emergency department visits, and hospital admissions among patients in the intervention arm over the 3-year study period.61
Although health care systems with limited resources at their disposal might be reluctant to invest in comprehensive care management programs that could take years to demonstrate impact and cost savings, it is worth noting that medium-term indicators such as those listed earlier can provide insight into whether downstream impact is likely to occur.
For instance, in a UK study of a 1-year diabetes intervention with more than 4000 participants, post-trial monitoring revealed sustained risk reductions in diabetes-related end point, microvascular disease, myocardial infarction, and all-cause mortality 10 years after the study ended, even though between-group differences in hemoglobin A1c were lost after the first year.62
With this finding in mind, it stands to reason that if care management interventions can lead to improvements in vital signs, biomarkers, treatment engagement, and health behaviors that persist for more than a year, the resulting health improvements and reductions in care costs would be even greater.
Moving Forward
In conclusion, many adults in the United States have comorbid medical and psychiatric conditions, and these individuals suffer from disparities in morbidity, mortality, and social and occupational functioning. Care management programs have the potential to address gaps in care and improve outcomes for individuals with comorbidities. Despite ample evidence for disease-specific care management programs, the evidence for multimorbidity care management is limited. The review described in this article yielded one study of traditional care management and two of collaborative care programs that simultaneously targeted medical and psychiatric outcomes.
Given the relative dearth of literature on multimorbidity care management, the authors supplemented their review by outlining the pillars of effective care management, which include measurement-based care, motivational interviewing, and care coordination. They also made recommendations regarding specific interventions that could be offered based on an individual's psychiatric diagnosis and illness severity.
As the recommendations outlined here were originally intended to enhance an existing insurance-based care management program, the suggested interventions were limited to what could reasonably be offered by care managers who might not be directly embedded in a patient's primary care team.
Future studies should focus on how to effectively implement and disseminate the core features of existing multimorbidity care management models. They should also build upon prior mental health-focused studies examining which components of the interventions cited earlier offer the greatest value, in what contexts, and for which sub-populations.20,25 Finally, they should evaluate the relative impact of care management programs for individuals with comorbid medical and psychiatric conditions as compared with the direct provision of mental health services in the primary care setting (ie, integrated care). Only once these questions are answered will we truly be able to make progress on providing equitable, high-quality care to individuals with medical and psychiatric comorbidities.
Acknowledgments
The authors wish to thank Nick Patel, PharmD, PhD, for his important role in liaising between Humana, Inc. and Cambridge Health Alliance.
Author Disclosure Statement
Ms. Holland reports employment with Humana, Inc. Dr. Ruble and Dr. Parsons report employment and equity holdings with Humana, Inc. All other authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
Funding Information
This study was supported by funding from Humana, Inc. (grant number 1971660). The sponsors were involved in study design and article review. The sponsors had no role in the conduct of the study, including data collection, management, analysis, and interpretation, or the decision to submit the article for publication.
References
- 1. Druss BG, Walker ER. Mental disorders and medical comorbidity. Synth Proj Res Synth Rep 2011;21:1–26. [PubMed] [Google Scholar]
- 2. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis 2006;3:A42. [PMC free article] [PubMed] [Google Scholar]
- 3. Eaton WW, Martins SS, Nestadt G, Bienvenu OJ, Clarke D, Alexandre P. The burden of mental disorders. Epidemiol Rev 2008;30:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Felker B, Yazel JJ, Short D. Mortality and medical comorbidity among psychiatric patients: a review. Psychiatr Serv 1996;47:1356–1363. [DOI] [PubMed] [Google Scholar]
- 5. National Academy of Certified Care Managers: Content Domains and Care Manager Tasks. https://www.naccm.net/wp-content/uploads/sites/52/2014/01/NACCM-Core-functionsContent-Domains_Revised2017_11.3.pdf Accessed February 24, 2022.
- 6. Archer J, Bower P, Gilbody S, et al. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev 2012;10:Cd006525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hu J, Wu T, Damodaran S, Tabb KM, Bauer A, Huang H. The effectiveness of collaborative care on depression outcomes for racial/ethnic minority populations in primary care: a systematic review. Psychosomatics 2020;61:632–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Coventry P, Lovell K, Dickens C, et al. Integrated primary care for patients with mental and physical multimorbidity: cluster randomised controlled trial of collaborative care for patients with depression comorbid with diabetes or cardiovascular disease. BMJ 2015;350:h638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Camacho EM, Davies LM, Hann M, et al. Long-term clinical and cost-effectiveness of collaborative care (versus usual care) for people with mental-physical multimorbidity: cluster-randomised trial. Br J Psychiatry 2018;213:456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bogner HR, Morales KH, de Vries HF, Cappola AR. Integrated management of type 2 diabetes mellitus and depression treatment to improve medication adherence: a randomized controlled trial. Ann Fam Med 2012;10:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bogner HR, de Vries HF. Integration of depression and hypertension treatment: a pilot, randomized controlled trial. Ann Fam Med 2008;6:295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bogner HR, de Vries HF. Integrating type 2 diabetes mellitus and depression treatment among African Americans: a randomized controlled pilot trial. Diabetes Educ 2010;36:284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baker JM, Grant RW, Gopalan A. A systematic review of care management interventions targeting multimorbidity and high care utilization. BMC Health Serv Res 2018;18:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alegría M, Alvarez K, Ishikawa RZ, DiMarzio K, McPeck S. Removing obstacles to eliminating racial and ethnic disparities in behavioral health care. Health Aff (Millwood) 2016;35:991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Katon WJ, Lin EH, Von Korff M, et al. Collaborative care for patients with depression and chronic illnesses. N Engl J Med 2010;363:2611–2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Katon WJ, Von Korff M, Lin EH, et al. The Pathways Study: a randomized trial of collaborative care in patients with diabetes and depression. Arch Gen Psychiatry 2004;61:1042–1049. [DOI] [PubMed] [Google Scholar]
- 17. Katon W, Russo J, Lin EH, et al. Cost-effectiveness of a multicondition collaborative care intervention: a randomized controlled trial. Arch Gen Psychiatry 2012;69:506–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Coleman KJ, Magnan S, Neely C, et al. The COMPASS initiative: description of a nationwide collaborative approach to the care of patients with depression and diabetes and/or cardiovascular disease. Gen Hosp Psychiatry 2017;44:69–76. [DOI] [PubMed] [Google Scholar]
- 19. Rossom RC, Solberg LI, Magnan S, et al. Impact of a national collaborative care initiative for patients with depression and diabetes or cardiovascular disease. Gen Hosp Psychiatry 2017;44:77–85. [DOI] [PubMed] [Google Scholar]
- 20. Coventry PA, Hudson JL, Kontopantelis E, et al. Characteristics of effective collaborative care for treatment of depression: a systematic review and meta-regression of 74 randomised controlled trials. PLoS One 2014;9:e108114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bauer AM, Williams MD, Ratzliff A, Unützer J. Best practices for systematic case review in Collaborative Care. Psychiatr Serv 2019;70:1064–1067. [DOI] [PubMed] [Google Scholar]
- 22. Chisholm-Burns MA, Kim Lee J, Spivey CA, et al. US pharmacists' effect as team members on patient care: systematic review and meta-analyses. Med Care 2010;48:923–933. [DOI] [PubMed] [Google Scholar]
- 23. Matzke GR, Moczygemba LR, Williams KJ, Czar MJ, Lee WT. Impact of a pharmacist-physician collaborative care model on patient outcomes and health services utilization. Am J Health Syst Pharm 2018;75:1039–1047. [DOI] [PubMed] [Google Scholar]
- 24. Bao Y, Druss BG, Jung HY, Chan YF, Unützer J. Unpacking collaborative care for depression: examining two essential tasks for implementation. Psychiatr Serv 2016;67:418–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bower P, Gilbody S, Richards D, Fletcher J, Sutton A. Collaborative care for depression in primary care. Making sense of a complex intervention: systematic review and meta-regression. Br J Psychiatry 2006;189:484–493. [DOI] [PubMed] [Google Scholar]
- 26. Lorig KR, Holman H. Self-management education: history, definition, outcomes, and mechanisms. Ann Behav Med 2003;26:1–7. [DOI] [PubMed] [Google Scholar]
- 27. Funderburk JS, Shepardson RL, Wray J, et al. Behavioral medicine interventions for adult primary care settings: a review. Fam Syst Health 2018;36:368–399. [DOI] [PubMed] [Google Scholar]
- 28. Kahan S, Wilson DK, Sweeney AM. The role of behavioral medicine in the treatment of obesity in primary care. Med Clin North Am 2018;102:125–133. [DOI] [PubMed] [Google Scholar]
- 29. Fortney JC, Unützer J, Wrenn G, et al. A tipping point for measurement-based care. Psychiatr Serv 2017;68:179–188. [DOI] [PubMed] [Google Scholar]
- 30. Rubak S, Sandbaek A, Lauritzen T, Christensen B. Motivational interviewing: a systematic review and meta-analysis. Br J Gen Pract 2005;55:305–312. [PMC free article] [PubMed] [Google Scholar]
- 31. Institute of Medicine Committee on Crossing the Quality Chasm: Adaptation to Mental Health and Addictive Disorders. The National Academies Collection: Reports funded by National Institutes of Health. Improving the Quality of Health Care for Mental and Substance-Use Conditions: Quality Chasm Series. Washington, DC: National Academy of Sciences (US), 2006. [Google Scholar]
- 32. Druss BG, Rosenheck RA, Desai MM, Perlin JB. Quality of preventive medical care for patients with mental disorders. Med Care 2002;40:129–136. [DOI] [PubMed] [Google Scholar]
- 33. Mitchell AJ, Malone D, Doebbeling CC. Quality of medical care for people with and without comorbid mental illness and substance misuse: systematic review of comparative studies. Br J Psychiatry 2009;194:491–499. [DOI] [PubMed] [Google Scholar]
- 34. Muench J, Hamer AM. Adverse effects of antipsychotic medications. Am Fam Physician 2010;81:617–622. [PubMed] [Google Scholar]
- 35. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf 2014;13:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Health Effects of Cigarette Smoking: Centers for Disease Control and Prevention. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/health_effects/effects_cig_smoking/index.htm Accessed February 24, 2022.
- 37. Cook BL, Wayne GF, Kafali EN, Liu Z, Shu C, Flores M. Trends in smoking among adults with mental illness and association between mental health treatment and smoking cessation. JAMA 2014;311:172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Treating tobacco use and dependence: 2008 update U.S.. Public Health Service Clinical Practice Guideline executive summary. Respir Care 2008;53:1217–1222. [PubMed] [Google Scholar]
- 39. Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet 2016;387:2507–2520. [DOI] [PubMed] [Google Scholar]
- 40. Taylor G, McNeill A, Girling A, Farley A, Lindson-Hawley N, Aveyard P. Change in mental health after smoking cessation: systematic review and meta-analysis. BMJ 2014;348:g1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fitch K, Iwasaki K, Pyenson B.. Covering smoking cessation as a health benefit: a case for employers. New York, NY: Milliman, Inc., 2005. [Google Scholar]
- 42. Solberg LI, Maciosek MV, Edwards NM, Khanchandani HS, Goodman MJ. Repeated tobacco-use screening and intervention in clinical practice: health impact and cost effectiveness. Am J Prev Med 2006;31:62–71. [DOI] [PubMed] [Google Scholar]
- 43. Report: The Business Case for Coverage of Tobacco Cessation. https://www.uams.edu/coph/reports/smokefree_toolkit/Media/Other%20Helpful%20Resources/Business%20Case_Actuarial%20Analysis%20of%20Smoking%20and%20Cessation.pdf Accessed February 24, 2022.
- 44. Firth J, Torous J, Nicholas J, et al. The efficacy of smartphone-based mental health interventions for depressive symptoms: a meta-analysis of randomized controlled trials. World Psychiatry 2017;16:287–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Firth J, Torous J, Nicholas J, Carney R, Rosenbaum S, Sarris J. Can smartphone mental health interventions reduce symptoms of anxiety? A meta-analysis of randomized controlled trials. J Affect Disord 2017;218:15–22. [DOI] [PubMed] [Google Scholar]
- 46. Mushtaq R, Shoib S, Shah T, Mushtaq S. Relationship between loneliness, psychiatric disorders and physical health? A review on the psychological aspects of loneliness. J Clin Diagn Res 2014;8:We01–We04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pancani L, Marinucci M, Aureli N, Riva P. Forced social isolation and mental health: a study on 1,006 Italians under COVID-19 lockdown. Front Psychol 2021;12:663799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: a meta-analytic review. PLoS Med 2010;7:e1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Janis RA, Burlingame GM, Svien H, Jensen J, Lundgreen R. Group therapy for mood disorders: a meta-analysis. Psychother Res 2021;31:369–385. [DOI] [PubMed] [Google Scholar]
- 50. Rigotti NA, Tindle HA, Regan S, et al. A Post-discharge smoking-cessation intervention for hospital patients: helping Hand 2 Randomized Clinical Trial. Am J Prev Med 2016;51:597–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Janevic MR, Aruquipa Yujra AC, Marinec N, et al. Feasibility of an interactive voice response system for monitoring depressive symptoms in a lower-middle income Latin American country. Int J Ment Health Syst 2016;10:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Richards DA, Ekers D, McMillan D, et al. Cost and Outcome of Behavioural Activation versus Cognitive Behavioural Therapy for Depression (COBRA): a randomised, controlled, non-inferiority trial. Lancet 2016;388:871–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jonassaint CR, Gibbs P, Belnap BH, Karp JF, Abebe KK, Rollman BL. Engagement and outcomes for a computerised cognitive-behavioural therapy intervention for anxiety and depression in African Americans. BJPsych Open 2017;3:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sawchuk CN, Craner JR, Berg SL, et al. Initial outcomes of a real-world multi-site primary care psychotherapy program. Gen Hosp Psychiatry 2018;54:5–11. [DOI] [PubMed] [Google Scholar]
- 55. Giakoumatos CI, Osser D. The Psychopharmacology Algorithm Project at the Harvard South Shore Program: an update on unipolar nonpsychotic depression. Harv Rev Psychiatry 2019;27:33–52. [DOI] [PubMed] [Google Scholar]
- 56. Miller AL, Chiles JA, Chiles JK, Crismon ML, Rush AJ, Shon SP. The Texas Medication Algorithm Project (TMAP) schizophrenia algorithms. J Clin Psychiatry 1999;60:649–657. [DOI] [PubMed] [Google Scholar]
- 57. Kelly JF, Humphreys K, Ferri M. Alcoholics Anonymous and other 12-step programs for alcohol use disorder. Cochrane Database Syst Rev 2020;3:Cd012880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Larochelle MR, Bernson D, Land T, et al. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: a cohort study. Ann Intern Med 2018;169:137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Medication-Assisted Treatment (MAT). https://www.samhsa.gov/medication-assisted-treatment Accessed February 24, 2022.
- 60. Bauer MS, McBride L, Williford WO, et al. Collaborative care for bipolar disorder: part II. Impact on clinical outcome, function, and costs. Psychiatr Serv 2006;57:937–945. [DOI] [PubMed] [Google Scholar]
- 61. Reiss-Brennan B, Brunisholz KD, Dredge C, et al. Association of integrated team-based care with health care quality, utilization, and cost. J 2016;316:826–834. [DOI] [PubMed] [Google Scholar]
- 62. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–1589. [DOI] [PubMed] [Google Scholar]