Summary
Background
Childhood traumatic events are risk factors for psychopathology, but large-scale studies of how childhood traumatic events relate to mental health and cognition in adulthood, and how the brain is involved, are needed.
Methods
The associations between childhood traumatic events (such as abuse and neglect, and defined by the ‘Childhood Trauma’ questions in the UK Biobank database) and brain functional connectivity, mental health problems, and cognitive performance were investigated by a univariate correlation analysis with 19,535 participants aged 45–79 from the UK Biobank dataset. The results were replicated with 17,747 independent participants in the second release from the same dataset.
Findings
Childhood traumatic events were significantly associated with mental health problems in adulthood including anxiety (r=0.19, p<1.0 × 10−323), depression (r=0.21, p<1.0 × 10−323), and self-harm (r=0.24, p<1.0 × 10−323), and with adult cognitive performance including fluid intelligence (r=−0.05, p=2.8 × 10−10) and prospective memory (r=−0.04, p=6.8 × 10−8). Functional connectivities of the medial and lateral temporal cortex, the precuneus, the medial orbitofrontal cortex; and the superior, middle and inferior prefrontal cortex extending back to precentral regions were negatively correlated with the childhood traumatic events (FDR corrected, p<0.01). These lower functional connectivities significantly mediated the associations between childhood traumatic events and addiction, anxiety, depression and well-being (all p<1.0 × 10−3), and cognitive performance. The association between childhood traumatic events and behavioural measures and functional connectivity were confirmed in a replication with different participants in the second release of the UK Biobank dataset.
Interpretation
Childhood traumatic events are strongly associated with adult mental health problems mediated by brain functional connectivities in brain areas involved in executive function, emotion, face processing, and memory. This understanding may help with prevention and treatment.
Funding
Funding was provided by the National Key R&D Program of China (No. 2018YFC1312900 and No. 2019YFA0709502).
Keywords: Childhood traumatic events, Functional connectivity, Mental health, Cognition, Orbitofrontal cortex
Research in context.
Evidence before this study
Childhood traumatic events (such as abuse and neglect) have been shown to be risk factors for psychopathology, but large-scale studies of how childhood traumatic events relate to mental health and cognition in mature adulthood, and how the brain is involved, are needed.
Added value of this study
In a very large cohort of N=151,009 participants aged 45–79 from the UK Biobank dataset it was shown that childhood traumatic events were significantly associated with mental health problems assessed in adulthood including anxiety, depression, and self-harm; and with cognitive performance including fluid intelligence and prospective memory. Analysis of neuroimaging in 19,535 participants showed that functional connectivities of the temporal cortex, the precuneus, the medial orbitofrontal cortex, and the prefrontal cortex were negatively correlated with the childhood traumatic events scores. These lower functional connectivities significantly mediated the association between childhood traumatic events and addiction, anxiety, depression and well-being, and cognitive performance. The association between childhood traumatic events and behavioural measures and functional connectivity were confirmed in a replication with 17,747 independent participants.
Implications of all the available evidence
Childhood traumatic events such as abuse and neglect are correlated (r=approximately 0.2) with mental health problems present in adulthood. The associations are still present in individuals aged 45–79. There is evidence that these associations are related to brain functional connectivities in brain areas involved in executive function, emotion, face processing, and memory. This is an association study, and the underlying causes are not yet clear. Knowledge of this association is likely to be helpful not only in understanding some mental health problems, but is also relevant to treatment and also prevention.
Alt-text: Unlabelled box
Introduction
Childhood traumatic events are an established risk factor for psychopathology. Children who receive sexual, physical or emotional abuse, or physical and emotional neglect may have associated long-lasting mental and physical differences. The association of an individual's childhood traumatic events and adult psychopathology, and how brain differences relate to that, is an important topic and needs more research with large sample sizes.1, 2, 3 Meta-analyses show that childhood traumatic events are associated with a 2 to 3-fold increase in the risk of psychosis.4, 5, 6 Further, childhood traumatic events are associated not only with mental disorders, including depression,7,8 but also with and physical ill-health, including non-communicable diseases.9,10 In particular, depression is commonly observed among individuals reporting exposure to stressful life events and childhood traumatic events.2,11, 12, 13, 14, 15, 16, 17, 18 In addition, there is evidence that childhood traumatic events are associated with lower measures of cognitive performance in adults.19,20
There is evidence that childhood traumatic events are associated with different volumes and functional connectivities of for example the prefrontal cortex and limbic brain regions.3,21, 22, 23, 24, 25, 26, 27, 28 Further, longitudinal studies have indicated that smaller prefrontal cortex volume associated with childhood traumatic events is linked to later lower cognitive measure scores29 and poorer illness courses.30 However, the brain regions associated with the different associations between childhood traumatic events, and mental health and cognitive measures, need more exploration to be better understood. Indeed, substantial heterogeneity in effects and their sizes has been observed across studies.31 Methodological issues, such as small sample sizes, cross-sectional data, variation in how childhood traumatic events and psychotic experiences were assessed, and the extent of adjustment for potential confounds, could contribute to this heterogeneity.
To achieve a greater understanding of the underlying neural pathways, large population samples are likely to be important. Most of the previous studies have focused on the underlying brain structures associated with childhood traumatic events, not on brain function.23,26,29,30 In addition, the studies described above investigated the relationship between childhood traumatic events and the mental health problems of adolescents or young adults, not the long-lasting associations between childhood traumatic events and mental health in mature adulthood to assess whether the associations are persistent.
The study described here has three main objectives. First, we aimed to assess the association between childhood traumatic events and a wide range of mental health problems and cognitive performance in later life using large scale data from 151,009 participants (with the exact number varying for different behaviors) from the UK Biobank dataset. Second, we aimed to examine the association between childhood traumatic events and brain functional connectivity and to test whether functional connectivity mediates the association between childhood traumatic events and mental health problems in later adulthood. Third, we aimed to test whether the results could be replicated using an independent dataset with 17,747 participants. The approach used was to apply univariate correlation analyses to measure whether there were correlations between the ‘Childhood Trauma’ measures in the UK Biobank and a range of measures of mental health and cognitive performance in adulthood available in the UK Biobank database. That approach was followed by analyses of the functional connectivities in the brain that were associated the childhood traumatic events and with the different measures of mental health and cognitive performance. It is noted that this is an association investigation, rather than an intervention study aiming to investigate causality.
Methods
(Full details of the Methods are provided in the Supplementary Material.)
Participants and data preprocessing
The participants, data, and fMRI preprocessing were from the UK Biobank.32,33 The demographic characteristics of participants who were included in this investigation are summarized in Table S1.
The multi-modal imaging data were collected using a standard Siemens Skyra 3T (see Supplementary Material). The resting-state functional brain imaging data used were from 22,331 participants from release 1. After quality controls and removing some participants without data on childhood traumatic events, 19,535 participants remained in the neuroimaging analysis,32 80% of whom were Caucasian. Further detail is that 681 of the neuroimaging participants reported having ‘had sexual intercourse with someone of the same sex’, and the results were similar if these were not included in the analysis. The data pre-processing performed by the UK Biobank included careful correction for spatial and gradient distortions and head motion etc as described in the Supplementary Material. The imaging site was regressed out in the neuroimaging analysis to control for any possible differences between scanner and site.
Ethics
The UK Biobank received ethical approval from the North West Multi-centre Research Ethics Committee in the UK (REC reference 11/NW/0382). The present analyses were conducted under UK Biobank application number 19542. Written informed consent was obtained from each subject according to the Declaration of Helsinki.
Construction of the whole-brain functional network
After pre-processing, the cortical gray matter was parcellated into 228 regions of interest using the Shen atlas,34 which was modified to incorporate a better parcellation of the orbitofrontal cortex as described in the Supplementary Material. The Shen atlas was used here for two main reasons. First, the Shen atlas was developed based on functional connectivity, which may be useful when investigating findings using functional connectivity.35,36 Second, the Shen atlas does have more brain regions than the AAL2 atlas.37 The cerebellum was not included in this study as the interest of this study focuses on the cerebrum based on the previous literature on childhood trauma and mental health. Because the Shen atlas areas do not have individual names, we show the mapping of Shen atlas areas to the AAL2 (automated anatomical labelling version 2) atlas37 areas in Table S5, and use these names when referring to particular brain areas and their functional connectivity. Then the time series were extracted in each region of interest in the atlas by determining the mean of the signals of all voxels within that region. Pearson cross-correlations between all pairs of regional blood oxygen level-dependent signals were calculated for each participant, followed by z transformation to improve normality, and the whole-brain functional connectivity network (228 × 228 regions with 25,878 edges which are functional connectivity links) was constructed.
Childhood traumatic event score
The childhood traumatic event score was calculated based on five questions related to childhood traumatic events available in the UK Biobank dataset, which are as follows: (field ID question)
20487 Felt hated by family member as a child
20488 Physically abused by family as a child
20489 Felt loved as a child
20490 Sexually molested as a child
20491 Someone to take to doctor when needed as a child.
The answers to these five questions are encoded in the same way: a score from 0 to 4 represents the answer from ‘never true’ to ‘very often true’ (data-coding 532 in the UK Biobank data field), which is a categorical scale, in the UK Biobank dataset, hence no normalization was needed. The childhood traumatic events score describes the severity of the traumatic events of each participant as a child. To ensure that a higher score in the questions was related to a higher traumatic score, the scores of questions 20489 and 20491 were reversed, and then the childhood traumatic event score was calculated as a sum of the scores on these 5 questions.
Association of childhood traumatic events with mental health problems and cognitive performance including statistics
The correlations between the childhood traumatic events scores and mental health problems were measured to examine whether childhood traumatic events are associated with mental health problems later in life. To be specific, a partial correlation was performed between mental health problems scores and the childhood traumatic events scores with age, gender, body mass index, education, Townsend social deprivation index, alcohol use, and tobacco use regressed out. The mental health problems scores included nine aspects: addiction, anxiety, cannabis use, depression, mania, mental distress, unusual and psychotic experiences, self-harm, and wellbeing, which are categories specified under the mental health tab of the online questionnaires in the UK Biobank dataset (https://biobank.ctsu.ox.ac.uk/crystal/label.cgi?id=136). For each category, a mean score of all the questions for that category was calculated as an overall score for that category of mental health problems. The correlation was also measured between the childhood traumatic event score and cognitive performance including fluid intelligence, numeric memory and prospective memory. The score of each category of cognitive performance was the score of single questions recommended by the UK Biobank dataset instead of the calculated sum scores of all measures under each category. To replicate the result, in the main analysis, all participants with available behavioral data except for the participants who were included in the second release of the dataset were included (around 151,009 participants, with the exact number varying for different behaviors). For the data in the second release of the UK Biobank dataset, we performed replication with these 17,747 independent participants, which aims to examine whether consistent results are obtained with an independent data group.
The functional connectivities related to the childhood trauma events score, mental health problems, and cognitive performance were also investigated. In order to calculate the similarities of the brain regions related to the different measures, the Hadamard product (an element-wise vector product to measure similarity across participants) was calculated between the 228 brain regions with significantly different functional connectivity for the childhood traumatic event scores, the mental health problems, and the cognitive performance scores, with the detailed procedure described in the Supplementary Material.
Association between the functional connectivities with childhood traumatic events including statistics
A Spearman correlation was performed to assess the association between whole-brain functional connectivity and the childhood traumatic event scores across all participants. In more detail, a partial correlation was performed between functional connectivities and the childhood traumatic scores with 9 confounding variables regressed out including age, gender, body mass index, education, Townsend index, alcohol use, tobacco use, scanner site information and head motion (mean framewise displacement). To take into account multiple comparisons, false discovery rate (FDR) correction was used to identify the functional connectivity links significantly associated with the childhood traumatic event score. To analyse the robustness of the results, the same analyses were performed with 17,747 independent participants in the second release of the UK Biobank dataset. The maximum number of participants with data available were used, given the importance of large sample sizes in brain wide association analyses.38,39
Mediation analysis and structural equation modelling including statistics
Mediation analyses were conducted to interrogate the relationship between the childhood traumatic events, related brain imaging variables, mental health problems, and cognitive performance as described in the Supplementary Material. The mediation analysis was performed using the Mediation Toolbox developed by Tor Wager (https://github.com/canlab/MediationToolbox), with a 1,000 bias-corrected bootstrap sample for significance testing, which has been used in many neuroimaging studies.40, 41, 42 Age, gender, body mass index, education, Townsend index, alcohol use, tobacco use, site information and head motion (only for functional connectivities) were regressed out as covariances in the mediation analyses.
Structural equation modelling (SEM) was used to further test the role of the functional connectivities in the relationship between the childhood traumatic events scores, mental health problems, and cognitive performance scores in groups modelled by latent variables. Four latent variables were estimated in this model including childhood traumatic events, mental health problems, cognitive performance, and functional connectivity strength. A major advantage of the use of latent variables is control of measurement error, which can artificially reduce the relationship between measured variables in standard univariate analyses.43 Calculations of SEM were performed with the Lavaan toolbox in Matlab,44 with full details in the Supplementary Material.
Role of funders
The Funders had no role in the study design, data collection, data analyses, interpretation, or writing of the report.
Results
Association between childhood traumatic events, and mental health and cognitive performance
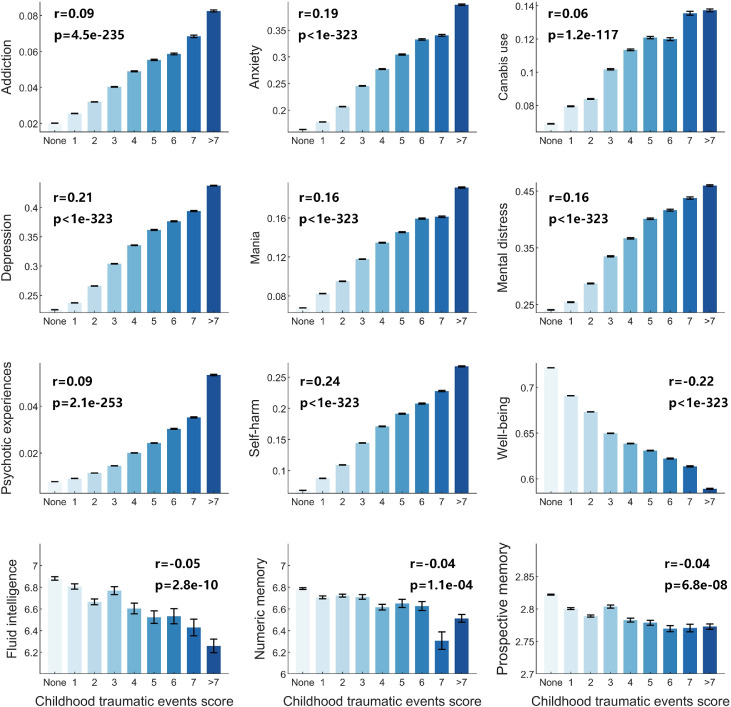
Association analysis was performed between childhood traumatic events and mental health problems (N=151,009). All the mental health problems were positively correlated with the childhood traumatic events (Figure 1), while well-being was negatively correlated (all p<1.0 × 10−200, Figure 1). Of special interest, childhood traumatic events were associated with anxiety at r = 0.19 (p<1.0 × 10−323), with depression at r = 0.21 (p<1.0 × 10−323), with self-harm at r = 0.24 (p<1.0 × 10−323), and with well-being at r = −0.22 (p<1.0 × 10−323). Cognitive performance including fluid intelligence, numeric memory, and prospective memory were also found to be negatively correlated with childhood traumatic events (all p<1.0 × 10−3, Figure 1). Fluid intelligence was the most significantly correlated cognitive performance with childhood traumatic events with r=−0.05 (p=2.8 × 10−10). All these correlations remained very significant after Bonferroni correction for multiple comparisons. In these analyses, there were 10,329 females and 9206 males, and significant effects were found when these were separated into female and male subgroups (Table S7).
Figure 1.
Mental health problems and cognitive performance are all related to childhood traumatic events. They include addiction, anxiety, cannabis use, depression, mania, mental distress, psychotic experiences, self-harm, well-being, fluid intelligence, numeric memory and prospective memory. The correlation coefficient r values of each mental health problem with childhood traumatic events are shown in each subplot. The x-axis indicates the childhood traumatic events score, and the y-axis indicates the mental health or cognitive measure score.
Functional connectivities correlated with childhood traumatic events
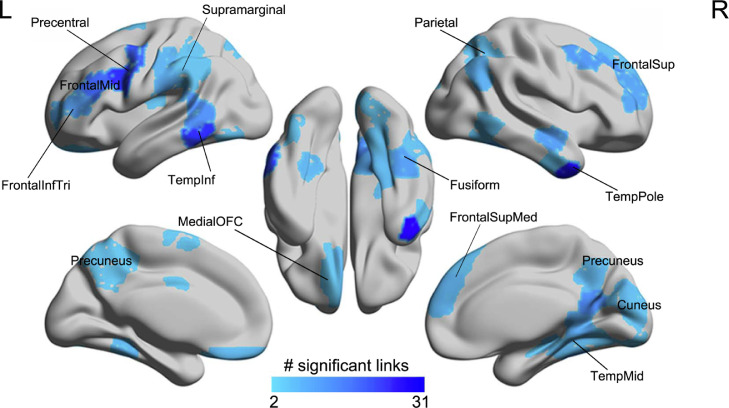
Next, the association between childhood traumatic events and brain functional connectivity was investigated with the first release of neuroimaging data of the UK Biobank dataset (N=19,535). 194 functional connectivities were significantly negatively correlated with the childhood traumatic event score (p<0.01, FDR corrected, Figure 2). The brain regions related to these significant links included the prefrontal cortex (superior, middle, and inferior frontal gyri, and superior medial part, extending back to precentral areas), the temporal cortex (lateral and medial), the precuneus, the fusiform gyrus, and the medial orbitofrontal cortex (Figure 2). A full list of the 194 significant FC links is shown in Table S2. In these analyses there were 10,329 females, and when separated into subgroups, the correlations of the functional connectivities with the childhood traumatic event score remained significant in the females after FDR correction for multiple comparisons, but not in the males, though the overall pattern of brain regions involved was similar in females and males (Figure S7).
Figure 2.
Correlation of the functional connectivity links with childhood traumatic events (p<0.01, FDR corrected). The numbers of links for each brain region in the Shen atlas showing a significant negative correlation with the childhood traumatic events score are shown.
Common brain regions correlated with both childhood traumatic events and mental health problems and cognitive performance
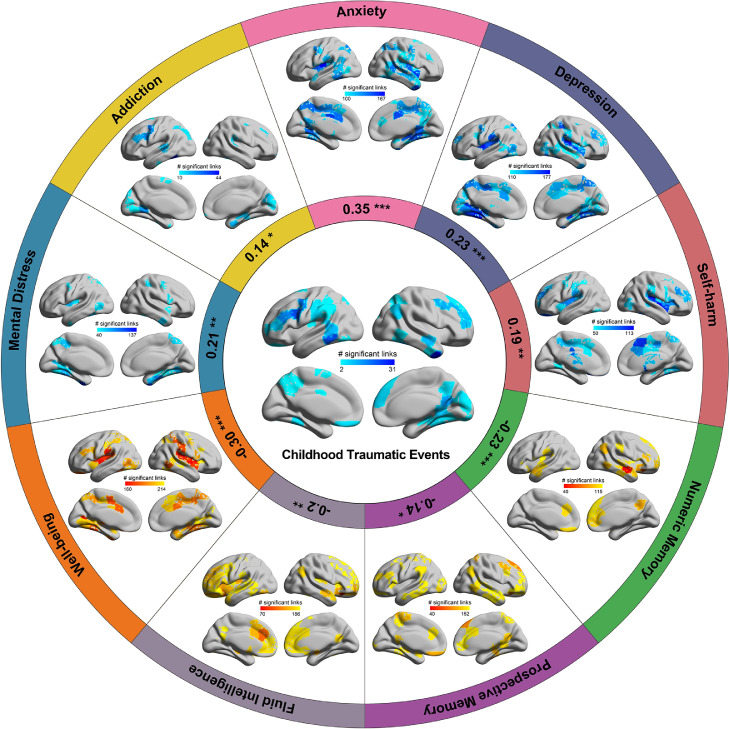
In addition to the association between childhood traumatic events and behavioral measures, we also found correlations between the functional connectivity significantly correlated with childhood traumatic events and the functional connectivity correlated with mental health problems and cognitive performance, especially for anxiety (r=0.35, p=8.3 × 10−8), depression (r=0.23, p=5.1 × 10−4), well-being (r=−0.30, p=3.8 × 10−6) and numeric memory (r=−0.23, p=5.6 × 10−4) (Figure 3). For example, functional connectivities of areas in the precentral cortical areas often extending to the prefrontal cortex were correlated with the childhood traumatic event score and with addiction, depression, mental distress, well-being, prospective memory, and fluid intelligence (Figure 3, and Table S6). The middle temporal gyrus was a common area for the childhood traumatic event score, anxiety, depression, well-being, numeric memory, prospective memory, fluid intelligence, and childhood traumatic events (Figure 3, and Table S6). The superior medial prefrontal area was common for childhood traumatic events and numeric memory. (The utility of this is that it suggests that impairments in numeric memory in those with childhood traumatic events may be related to reduced functional connectivity in the superior medial prefrontal cortex.) The hippocampus/paraHippocampal gyrus was common between childhood traumatic events and addiction, anxiety, mental distress, well-being, and numeric memory (Figure 3 and Table S6).
Figure 3.
Correlation of the functional connectivity links with mental health problems and cognitive performance (p<0.05, FDR corrected). The numbers of links that are different in different brain regions for different mental health problems and cognitive performance are shown. In the center, brain regions with reduced functional connectivity correlated with the childhood traumatic event score are shown to provide a reference for the other measures. The coefficients shown in the inner circle are the correlation r value of the mean correlation coefficient of significant links in all brain regions between the childhood traumatic event score and mental health problems and cognitive performance (* p<0.05, ** p<0.01, ***p<0.001).
The brain regions with Hadamard products showing the common brain regions with functional connectivities correlated with both childhood traumatic events and mental health problems/cognitive performances are shown in Figs. S2-S6 and Table S6. The inferior temporal gyrus and the middle temporal gyrus, and some prefrontal cortical areas including the medial orbitofrontal cortex, were found common between the childhood traumatic events and cognitive measures including numeric memory and prospective memory, and fluid intelligence (in the top 10 highest Hadamard products of all brain regions shown in Table S6; and Figs. S5 and S6). The brain areas with high Hadamard products for childhood traumatic events and mental health measures such as depression, addiction, mental distress, well-being, and anxiety, included the precentral cortical areas extending into the prefrontal cortex, the medial orbitofrontal cortex, precuneus, and fusiform gyrus (Table S6 and Figures S2-S4).
Mediation analysis and structural equation modelling
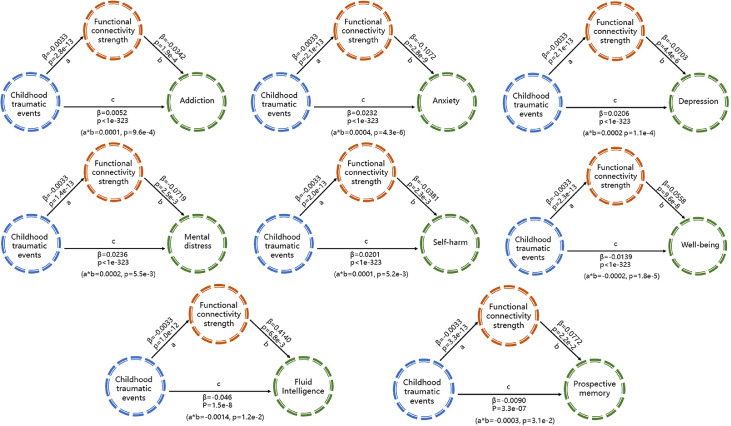
The functional connectivities that were significantly correlated with childhood traumatic events (Figure 2) significantly mediated the relationship between the childhood traumatic events and mental health problems including addiction (1.9% of the variance explained), anxiety (1.3% explained), depression (1.0% explained) and well-being (1.4% explained) (all p<10−3) (Figure 4). The interpretation is that the functional connectivities correlated with childhood traumatic events significantly mediate the association between childhood traumatic events and addiction, anxiety, depression and well-being. Similar results were also found for the cognitive performance measures including fluid intelligence (3.0% explained), and prospective memory (3.3% explained) (p<0.05) (Figure 4). The interpretation is that the functional connectivities correlated with childhood traumatic events significantly mediate the association between childhood traumatic events and fluid intelligence and prospective memory. A full list of the mediation results for all measures of mental health problems and cognitive performance is in Table S3.
Figure 4.
Mediation analyses between the childhood traumatic events, the mental health problems and cognitive measures with a mean strength of functional connectivity links which were significantly correlated with the childhood traumatic events as a mediator. Path a: relationship between childhood traumatic events and functional connectivity links; Path b: the relationship between functional connectivity links and mental health problems/cognitive measures; Path c: the relationship between childhood traumatic events and mental health problems/cognitive measures; Path a*b represents an indirect path which is the relationship between childhood traumatic events and mental health problems/cognitive measures that is mediated by the mean strength of the functional connectivity links which were significantly correlated with the childhood traumatic events. Path a*b indicates the extent to which taking the functional connectivity strength into account can explain the effect of the childhood traumtic events on mental health and cognitive measures inlcuding: addiction (1.9% of the variance explained), anxiety (1.3% of the variance explained), depression (1.0% of the variance explained), mental distress (0.8% of the variance explained), self-harm (0.5% of the variance explained), well-being (1.4% of the variance explained), fluid intelligence (3.0% of the variance explained), and prospective memory (3.3% of the variance explained).
Structural equation modelling was performed to measure the multivariate relationship between childhood traumatic events, mental health problems, cognitive performance, and the functional connectivity in the frontal lobe, the temporal lobe, the precuneus, etc (CFI=0.988, RMSEA=0.038), and as shown in Figure 5, all the paths in the model were significant at p<0.001. The functional connectivities that were significantly correlated with childhood traumatic events had a significant association with mental health problems (β=−0.04, p<0.001) and with cognitive performance (β=0.05, p<0.001), suggesting that childhood traumatic events and the associated brain changes related to those events may contribute to the mental health problems and cognitive performance differences.
Figure 5.
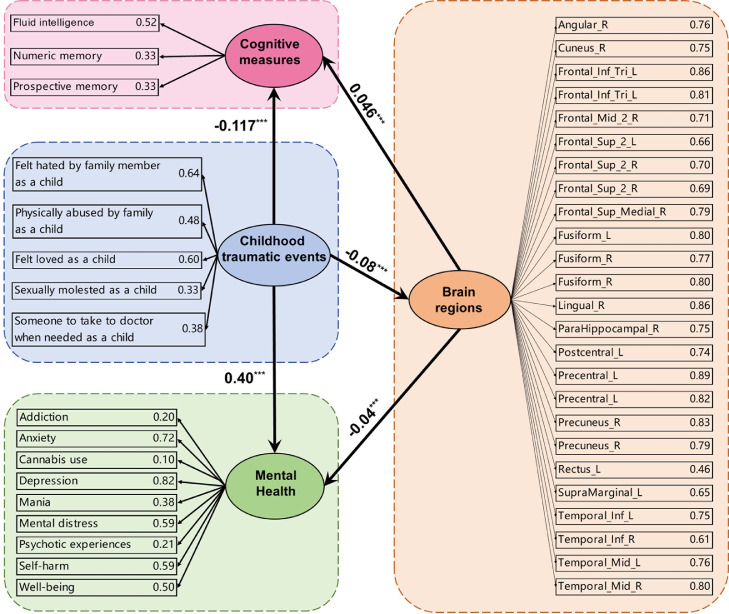
Structural equation model: for the brain regions indicated with functional connectivity of some links associated with the childhood traumatic events score, the correlation of the significant links with the childhood traumatic events score was entered into a structural equation model with the behavioral measures indicated and grouped together to form latent variables. The numbers in bold font show the standardized beta coefficients involving the latent variables. All paths between the latent variables were significant at p < 0.001 (indicated by 3 stars). After the Brain Regions, the functional connectivity between the significant links for that brain area with the childhood traumatic events score is shown. The structural equation model used here enables the different measures for cognition to be grouped, and similarly for the childhood traumatic events and mental health measures, so that relations between the grouped variables can be measured. The direction of the effects to be tested was as shown by the arrows, and was based on a priori hypotheses.
As structural equation modelling can be used to examine relationships between constructs, for example between mental health and brain function, an additional structural equation modelling analysis was performed in which the brain systems were treated as a single construct, instead of as separate brain regions. To perform this analysis, a mean value across all functional connectivities that were significantly correlated with childhood traumatic events was calculated. This is a useful single construct, for a very similar set of brain regions are associated with the mental health and cognitive problems associated with prolonged nausea and vomiting of the mother in pregnancy,45 with conflict in the family environment,46 and with a young age of the mother.47 This SEM model was implemented with childhood traumatic events, mental health, cognitive measures, and the mean Functional Connectivity value in one regression model. It was found that all paths between the latent variables in this new model were significant at p < 0.001. For example, the childhood traumatic events had a significant association with the mean functional connectivity values (β=−0.08, p<0.001), with mental health problems (β=0.40, p<0.001) and with cognitive performance (β=−0.12, p<0.001). The mean functional connectivity values had a significant association with mental health problems (β=−0.04, p<0.001) and with cognitive performance (β=0.04, p<0.001). This further analysis indicates that taking a mean value across all significant functional connectivities as an indicator of the whole group of relevant brain factors still results in the paths between the latent variables in the SEM model remaining significant.
Replication with an independent data group
Replication was performed with the second release of neuroimaging data from the UK Biobank (N=17,747). First, the behavioral associations between childhood traumatic events, and mental health problems and cognitive performance were well-replicated, including anxiety (r=0.19, p=3.8 × 10−93), depression (r=0.19, p=3.2 × 10−95), self-harm (r=0.25, p=2.5 × 10−155), well-being (r=−0.22, p=1.9 × 10−126), fluid intelligence (r=−0.03, p=1.4 × 10−3), and numeric memory (r=−0.03, p=5.3 × 10−3) (Table S4). Second, the correlation between childhood traumatic events and functional connectivity was well-replicated (Figure S1). For example, areas with many links correlated with childhood trauma in the second release include prefrontal cortical areas (superior, mid and inferior frontal and medial superior frontal), the middle and inferior temporal cortex and temporal pole, and the orbitofrontal cortex including the posterior and lateral orbitofrontal cortex (Figure S1A). It was found that the correlation of the association pattern of functional connectivity with childhood traumatic events between the first and second released data group was r=0.40, p < 10−323 (Figure S1B). In addition, the mediation analyses were replicated with the second release of the UK Biobank data. The functional connectivities used in this replication were selected as in the main mediation analysis as those significantly correlated with childhood traumatic events (Figure 2). In the replication, this group of functional connectivities significantly mediated the relationship between the childhood traumatic events and mental health problems including anxiety (0.7% of the variance explained), depression (0.4% explained), self-harm (0.5% explained), and well-being (0.9% explained) (all p<0.05). A full list of the mediation results for all measures of mental health problems and cognitive performance is provided in Table S8. The replication analyses thus provide evidence that the associations between childhood traumatic events, mental health problems and functional connectivity, and the mediation models described above, can be confirmed with a dataset with independent participants.
Discussion
This is the first study with a large sample size from the UK Biobank to examine the association between childhood traumatic events, and in adulthood brain functional connectivity, mental health problems, and cognitive performance. A strong correlation was shown between childhood traumatic events and mental health problems, cognitive performance, and low functional connectivity involving some brain regions. The main brain regions with functional connectivity negatively correlated with the childhood traumatic events scores included the prefrontal cortex (superior, middle, inferior and medial parts, extending to precentral areas), the medial and lateral temporal cortex, the precuneus, the fusiform gyrus, and the medial orbitofrontal cortex. The results were well replicated with an independent data group with the correlation between the two correlation matrices with childhood traumatic events r=0.4, p<10−323. The low functional connectivities of these brain regions were shown to significantly mediate the association between childhood traumatic events, and adult mental health problems including addiction, anxiety, depression, and well-being (all p<10−3).
A range of mental health problems including addiction, anxiety, cannabis use, depression, mania, mental distress, psychotic experiences, self-harm, and well-being in later life were found to be positively significantly associated with childhood traumatic events (p<1 × 10−117) (Figure 1). Brain regions for which functional connectivity was associated with childhood traumatic events and mental health problems included the middle temporal gyrus, the medial orbitofrontal cortex and in the second release lateral orbitofrontal cortex, the prefrontal and precentral cortex, and the precuneus (Figure 3 and Figs S2-S4). The middle temporal gyrus, which was common between anxiety and childhood traumatic events, is implicated in facial emotional expression and face movements, and theory of mind.48, 49, 50, 51, 52, 53 The association between the middle temporal gyrus and childhood traumatic events suggests that childhood traumatic events are associated with differences in the neural mechanisms for detecting face emotional expression and in the theory of mind,51 which may relate to emotional problems such as anxiety, depression, and mental distress in later adulthood. The medial orbitofrontal cortex is involved in reward value and hence in emotion, and decreases in functional connectivity here are associated with depression.54, 55, 56 The lateral orbitofrontal cortex is involved in changing behavior to non-reward and punishment, and reduced functional connectivity here may be associated with rule-breaking and impulsive types of behavior.54, 55, 56 The functional connectivity of the fusiform gyrus was found to be significantly associated with childhood traumatic events and mental health measures including addiction, anxiety, depression, mental distress, and well-being. The fusiform gyrus is implicated in face processing.57,58 The precentral cortex and areas extending anteriorly into the lateral prefrontal cortex, which were significantly associated with childhood traumatic events and the associated problems, contain mirror neurons which are implicated in observational learning.59,60 Impairment in this type of learning may be related to the cognitive and mental problems associated with childhood traumatic events. Executive function, implemented in the prefrontal cortex,61,62 may also be involved. The precuneus is a medial parietal cortex region implicated in the sense of self, autobiographical memory, and spatial function.63,64 The low functional connectivity of the precuneus suggests that childhood traumatic events are associated with a reduced or low sense of self. In addition, a low functional connectivity of the precuneus with some cortical areas is associated with depression.65, 66, 67 The lower connectivity of the precuneus reported here in the participants with childhood traumatic events may be related to depression, and contribute particularly to the low self-esteem in depression.55
Cognitive performance measures including fluid intelligence, numeric memory and prospective memory were also found to be negatively correlated with childhood traumatic events (p<0.001) (Figure 1). Brain regions associated with childhood traumatic events and lower cognitive performance included the superior medial prefrontal cortex, and the middle frontal gyrus (Figure 3). The superior medial prefrontal area was related to both childhood traumatic events and numeric memory, and is involved in planning / executive function68; and numerical information is represented and processed by regions of the prefrontal and posterior parietal lobes.69, 70, 71
The different brain regions for which the functional connectivity is associated with the different mental health and cognitive performance measures related to childhood traumatic events as just discussed are brought out in Figure 3. Figure 3 does show that there are different brain regions associated with some of the different mental health and cognitive measures that are associated with childhood traumatic events. These brain regions each have different functions, and interpreting the findings at the level of the computations performed by different brain regions53 and their connectivity72,73 seems to be appropriate, for what is shown in Figure 3 does not appear to reflect the rather broadly defined ‘default mode’, ‘salience’ etc networks.74
The mediation analyses provided evidence that the lower functional connectivities related to childhood traumatic events mediated the relationship between the childhood traumatic events and the mental health problems including addiction (1.9% variance explained), anxiety (1.3% variance explained), depression (1.0% variance explained) and well-being (1.4% variance explained) (all p<10−3) (Figure 4); and the cognitive measures including fluid intelligence (3.0% variance explained), and prospective memory (3.3% variance explained) (p<0.05). Although the percentage of variance explained by the functional connectivity links in the relationship between the childhood traumatic events and mental health problems was not high, it was significant at p<0.001. This evidence does show that functional connectivity differences present in adulthood after childhood traumatic events are significantly related to mental health problems found in adulthood. The SEM analysis was performed to measure the associations between the grouped variables for Cognitive Effects, Mental health problems, and brain regions for the measures associated with childhood traumatic events in one regression model. In this modelling, the mean functional connectivity strengths of the brain regions that were significantly correlated with the childhood traumatic events score were also significantly associated with mental health problems (β=−0.04, p<0.001) and cognitive performance (β=0.05, p<0.001), suggesting that childhood traumatic events and consequent brain changes related to that may contribute to the mental health problems and cognitive performance measured in later life (Figure 5).
The effect sizes for the main effects being investigated in this research are not very small, as shown by the findings that childhood traumatic events were significantly associated with mental health problems in adulthood including anxiety (r=0.19, p<1.0 × 10-323), depression (r=0.21, p<1.0 × 10-323), and self-harm (r=0.24, p<1.0 × 10-323). The effect sizes are smaller for the correlations with individual brain regions, but the context is that a number of brain regions have connectivity associated with childhood traumatic events. It is commonly observed that large effect sizes are associated with small sample sizes, and that the effect sizes generally become smaller, and better estimates of the real effect size, when the sample size is increased.39 Part of the advantage of the large sample size utilized here is that it is likely to provide a robust estimate of the real effect size. It is further noted that it can be important in medical practice to know about effects even if they are small in size, for across a large population this could make a difference. However, as noted above, the effect sizes being considered here are not very small.
Because this is an association study, causality cannot be directly addressed. One possibility is that childhood traumatic events produce brain differences, behavioral problems, and cognitive differences. Another possibility is that the general socio-economic environment during childhood made a contribution. However, we did regress out as a covariate of no interest the Townsend index, which measures a set of socio-economic variables. We did find that the Townsend index was significantly associated with the childhood traumatic events score (r=0.08, p=5.3 × 10−200). However, the association matrix between childhood traumatic events and functional connectivity with and without regression out of the Townsend index had a high correlation (r=0.9994, p<1 × 10−323), providing evidence that factors relating to the Townsend index were not crucial for the associations between childhood traumatic events and functional connectivity described here. In addition, the association between childhood traumatic events and other factors that might contribute to brain differences, behavioral problems, and cognitive differences were tested, including BMI (r=0.06, p=2.8 × 10−103), smoking (r=0.09, p=3.8 × 10−284), and drinking (r=−0.03, p=7.7 × 10−30). (These variables were regressed out of all the analyses described here.) Another factor might have been that some children, for genetic or possibly other reasons, were more likely to have traumatic events. The present findings do though make it clear that there are significant and long-lasting associations between childhood traumatic events, brain functional differences, and behavioral and cognitive problems.
It is interesting and remarkable that a rather similar constellation of mental health and cognitive problems involving similar brain regions to those described here are associated in children with prolonged nausea and vomiting of the mother in pregnancy,45 with conflict in the family environment46 and with a young age of the mother.47 Whether these similar associations reflect the vulnerability of some brain regions during development, or other factors, is not yet known.
Strengths of the present investigation are the large sample size (over 20,000 participants), which leads to robust findings; a focus on brain functional differences related to childhood traumatic events instead of structure as in many other studies; replication with a large independent sample group in the same dataset (around 20,000 participants); comprehensive analysis of the long-lasting relation into mature adulthood between childhood traumatic events and a range of mental health problems and lower cognitive performance; and the mediation analysis, which links the findings to recent advances in understanding brain mechanisms associated with childhood traumatic events and mental health problems.
In summary, this investigation provides evidence in a very large population that childhood traumatic events are associated with many mental health problems in mature adulthood including anxiety, depression, and well-being; that lower functional connectivity involving the prefrontal cortex (superior, middle, inferior and medial parts, and extending back to precentral regions), the temporal cortex (lateral and medial), the precuneus, and the medial orbitofrontal cortex, which are involved in executive function, face processing, emotion, and memory were associated with childhood traumatic events; and that the low functional connectivities mediated the correlation between the childhood traumatic events and mental health problems including addiction, anxiety, depression and self-harm, and cognitive measures including fluid intelligence and prospective memory. These advances have implications for the understanding, care and treatment of people who previously have had childhood traumatic events, for these results indicate which functions may benefit from treatment given the insight from knowing the brain regions involved. These advances in our understanding also highlight the association between childhood trauma, brain connectivity, mental health, and cognitive function, and may also therefore have implications for prevention.
Data sharing statement
The data analyzed are available from the UK Biobank (https://biobank.ctsu.ox.ac.uk).
Contributors
Zhuo Wan: Conceptualization, Investigation, Data curation, Formal analysis, Methodology, Software, Writing - original draft, Writing - review & editing.
Edmund T. Rolls: Conceptualization, Investigation, Methodology, Supervision, Validation, Writing - original draft, Writing - review & editing.
Wei Cheng: Conceptualization, Data curation, Methodology, Software, Writing - review & editing.
Jianfeng Feng: Conceptualization, Funding acquisition. Drs Wan and Cheng verified the underlying data. All authors read and approved the final version of the manuscript.
Declaration of interests
The authors report no competing interests.
Acknowledgments
Dr. Wei Zhang provided advice on the structural equation modelling. This study utilized the UK Biobank Resource under application number 19542. We would like to thank all the participants and researchers from the UK Biobank. J.F. was supported by National Key R&D Program of China (No. 2018YFC1312900 and No. 2019YFA0709502), Shanghai Municipal Science and Technology Major Project (No. 2018SHZDZX01), ZJ Lab, Shanghai Center for Brain Science and Brain-Inspired Technology, and the 111 Project (No. B18015). W. C. was supported by grants from the National Natural Sciences Foundation of China (No. 82071997) and the Shanghai Rising-Star Program (No. 21QA1408700).
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2022.104002.
Contributor Information
Edmund T. Rolls, Email: Edmund.Rolls@oxcns.org.
Wei Cheng, Email: wcheng@fudan.edu.cn.
Appendix. Supplementary materials
References
- 1.Susser E, Widom CS. Still searching for lost truths about the bitter sorrows of childhood. Schizophr Bull. 2012;38:672–675. doi: 10.1093/schbul/sbs074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baldwin JR, Reuben A, Newbury JB, Danese A. Agreement between prospective and retrospective measures of childhood maltreatment: a systematic review and meta-analysis. JAMA Psychiatry. 2019;76:584–593. doi: 10.1001/jamapsychiatry.2019.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teicher MH, Samson JA. Annual research review: enduring neurobiological effects of childhood abuse and neglect. J Child Psychol Psychiatry. 2016;57:241–266. doi: 10.1111/jcpp.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trotta A, Murray RM, Fisher HL. The impact of childhood adversity on the persistence of psychotic symptoms: a systematic review and meta-analysis. Psychol Med. 2015;45:2481–2498. doi: 10.1017/S0033291715000574. [DOI] [PubMed] [Google Scholar]
- 5.van Dam DS, van der Ven E, Velthorst E, Selten JP, Morgan C, de Haan L. Childhood bullying and the association with psychosis in non-clinical and clinical samples: a review and meta-analysis. Psychol Med. 2012;42:2463–2474. doi: 10.1017/S0033291712000360. [DOI] [PubMed] [Google Scholar]
- 6.Varese F, Smeets F, Drukker M, et al. Childhood adversities increase the risk of psychosis: a meta-analysis of patient-control, prospective- and cross-sectional cohort studies. Schizophr Bull. 2012;38:661–671. doi: 10.1093/schbul/sbs050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McLaughlin KA, Koenen KC, Bromet EJ, et al. Childhood adversities and post-traumatic stress disorder: evidence for stress sensitisation in the World Mental Health Surveys. Br J Psychiatry. 2017;211:280–288. doi: 10.1192/bjp.bp.116.197640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Copeland WE, Shanahan L, Hinesley J, et al. Association of childhood trauma exposure with adult psychiatric disorders and functional outcomes. JAMA Netw Open. 2018;1 doi: 10.1001/jamanetworkopen.2018.4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Basu A, McLaughlin KA, Misra S, Koenen KC. Childhood maltreatment and health impact: the examples of cardiovascular disease and Type 2 diabetes mellitus in adults. Clin Psychol (New York) 2017;24:125–139. doi: 10.1111/cpsp.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suglia SF, Koenen KC, Boynton-Jarrett R, et al. Childhood and adolescent adversity and cardiometabolic outcomes: a scientific statement from the American heart association. Circulation. 2018;137:e15–e28. doi: 10.1161/CIR.0000000000000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green JG, McLaughlin KA, Berglund PA, et al. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: associations with first onset of DSM-IV disorders. Arch Gen Psychiatry. 2010;67:113–123. doi: 10.1001/archgenpsychiatry.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nanni V, Uher R, Danese A. Childhood maltreatment predicts unfavorable course of illness and treatment outcome in depression: a meta-analysis. Am J Psychiatry. 2012;169:141–151. doi: 10.1176/appi.ajp.2011.11020335. [DOI] [PubMed] [Google Scholar]
- 13.Kessler RC. The effects of stressful life events on depression. Annu Rev Psychol. 1997;48:191–214. doi: 10.1146/annurev.psych.48.1.191. [DOI] [PubMed] [Google Scholar]
- 14.McLaughlin KA, Conron KJ, Koenen KC, Gilman SE. Childhood adversity, adult stressful life events, and risk of past-year psychiatric disorder: a test of the stress sensitization hypothesis in a population-based sample of adults. Psychol Med. 2010;40:1647–1658. doi: 10.1017/S0033291709992121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rolls ET . Oxford University Press; 2018. The Brain, Emotion, and Depression. [Google Scholar]
- 16.Kessler RC, Davis CG, Kendler KS. Childhood adversity and adult psychiatric disorder in the US national comorbidity survey. Psychol Med. 1997;27:1101–1119. doi: 10.1017/s0033291797005588. [DOI] [PubMed] [Google Scholar]
- 17.Collishaw S, Pickles A, Messer J, Rutter M, Shearer C, Maughan B. Resilience to adult psychopathology following childhood maltreatment: evidence from a community sample. Child Abuse Negl. 2007;31:211–229. doi: 10.1016/j.chiabu.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry. 1999;156:837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- 19.Petkus AJ, Lenze EJ, Butters MA, Twamley EW, Wetherell JL. Childhood trauma is associated with poorer cognitive performance in older adults. J Clin Psychiatry. 2018;79 doi: 10.4088/JCP.16m11021. 16m11021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Velikonja T, Velthorst E, Zinberg J, et al. Childhood trauma and cognitive functioning in individuals at clinical high risk (CHR) for psychosis. Dev Psychopathol. 2021;33:53–64. doi: 10.1017/S095457941900155X. [DOI] [PubMed] [Google Scholar]
- 21.Marusak HA, Thomason ME, Peters C, Zundel C, Elrahal F, Rabinak CA. You say 'prefrontal cortex' and I say 'anterior cingulate': meta-analysis of spatial overlap in amygdala-to-prefrontal connectivity and internalizing symptomology. Transl Psychiatry. 2016;6:e944. doi: 10.1038/tp.2016.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shonkoff JP, Garner AS, Committee on psychosocial aspects of C, Family H, committee on early childhood A, Dependent C, et al. (2012): the lifelong effects of early childhood adversity and toxic stress. Pediatrics. 129:e232-246. [DOI] [PubMed]
- 23.Gheorghe DA, Li C, Gallacher J, Bauermeister S. Associations of perceived adverse lifetime experiences with brain structure in UK Biobank participants. J Child Psychol Psychiatry. 2021;62:822–830. doi: 10.1111/jcpp.13298. [DOI] [PubMed] [Google Scholar]
- 24.Bolsinger J, Seifritz E, Kleim B, Manoliu A. Neuroimaging correlates of resilience to traumatic events-A comprehensive review. Front Psychiatry. 2018;9:693. doi: 10.3389/fpsyt.2018.00693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whittle S, Yap MB, Yucel M, et al. Maternal responses to adolescent positive affect are associated with adolescents' reward neuroanatomy. Soc Cogn Affect Neurosci. 2009;4:247–256. doi: 10.1093/scan/nsp012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morey RA, Haswell CC, Hooper SR, De Bellis MD. Amygdala, hippocampus, and ventral medial prefrontal cortex volumes differ in maltreated youth with and without chronic posttraumatic stress disorder. Neuropsychopharmacology. 2016;41:791–801. doi: 10.1038/npp.2015.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yeo BT, Krienen FM, Sepulcre J, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tottenham N. Social scaffolding of human amygdala-mPFCcircuit development. Soc Neurosci. 2015;10:489–499. doi: 10.1080/17470919.2015.1087424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanson JL, Chung MK, Avants BB, et al. Structural variations in prefrontal cortex mediate the relationship between early childhood stress and spatial working memory. J Neurosci. 2012;32:7917–7925. doi: 10.1523/JNEUROSCI.0307-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frodl T, Reinhold E, Koutsouleris N, et al. Childhood stress, serotonin transporter gene and brain structures in major depression. Neuropsychopharmacology. 2010;35:1383–1390. doi: 10.1038/npp.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gibson LE, Alloy LB, Ellman LM. Trauma and the psychosis spectrum: a review of symptom specificity and explanatory mechanisms. Clin Psychol Rev. 2016;49:92–105. doi: 10.1016/j.cpr.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller KL, Alfaro-Almagro F, Bangerter NK, et al. Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat Neurosci. 2016;19:1523–1536. doi: 10.1038/nn.4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alfaro-Almagro F, Jenkinson M, Bangerter NK, et al. Image processing and quality control for the first 10,000 brain imaging datasets from UK Biobank. Neuroimage. 2018;166:400–424. doi: 10.1016/j.neuroimage.2017.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen X, Tokoglu F, Papademetris X, Constable RT. Groupwise whole-brain parcellation from resting-state fMRI data for network node identification. Neuroimage. 2013;82:403–415. doi: 10.1016/j.neuroimage.2013.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenberg MD, Finn ES, Scheinost D, et al. A neuromarker of sustained attention from whole-brain functional connectivity. Nat Neurosci. 2016;19:165–171. doi: 10.1038/nn.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Finn ES, Shen X, Scheinost D, et al. Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nat Neurosci. 2015;18:1664–1671. doi: 10.1038/nn.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rolls ET, Joliot M, Tzourio-Mazoyer N. Implementation of a new parcellation of the orbitofrontal cortex in the automated anatomical labeling atlas. Neuroimage. 2015;122:1–5. doi: 10.1016/j.neuroimage.2015.07.075. [DOI] [PubMed] [Google Scholar]
- 38.Cheng W, Rolls ET, Qiu J, et al. Medial reward and lateral non-reward orbitofrontal cortex circuits change in opposite directions in depression. Brain. 2016;139:3296–3309. doi: 10.1093/brain/aww255. [DOI] [PubMed] [Google Scholar]
- 39.Marek S, Tervo-Clemmens B, Calabro FJ, et al. Reproducible brain-wide association studies require thousands of individuals. Nature. 2022;603:654–660. doi: 10.1038/s41586-022-04492-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wager TD, Waugh CE, Lindquist M, Noll DC, Fredrickson BL, Taylor SF. Brain mediators of cardiovascular responses to social threat: part I: reciprocal dorsal and ventral sub-regions of the medial prefrontal cortex and heart-rate reactivity. Neuroimage. 2009;47:821–835. doi: 10.1016/j.neuroimage.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lim SL, Padmala S, Pessoa L. Segregating the significant from the mundane on a moment-to-moment basis via direct and indirect amygdala contributions. Proc Natl Acad Sci USA. 2009;106:16841–16846. doi: 10.1073/pnas.0904551106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McDonald RP. Structural equations with latent variables. J Am Stat Assoc. 1990;85:1175–1177. [Google Scholar]
- 44.Rosseel Y. Lavaan: An R package for structural equation modeling and more. Version 0.5–12 (BETA) J Stat Softw. 2012;48:1–36. [Google Scholar]
- 45.Wang H, Rolls ET, Du X, et al. Severe nausea and vomiting in pregnancy: psychiatric and cognitive problems, and brain structure in children. BMC Med. 2020;18:228. doi: 10.1186/s12916-020-01701-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gong W, Rolls ET, Du J, Feng J, Cheng W. Brain structure is linked to the association between family environment and behavioral problems in children in the ABCD study. Nat Commun. 2021;12:3769. doi: 10.1038/s41467-021-23994-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Du J, Rolls ET, Gong W, et al. Association between parental age, brain structure, and behavioral and cognitive problems in children. Mol Psychiatry. 2021 doi: 10.1038/s41380-021-01325-5. doi: 10/1038/s41380-41021-01325-41385. [DOI] [PubMed] [Google Scholar]
- 48.Hasselmo ME, Rolls ET, Baylis GC. The role of expression and identity in the face-selective responses of neurons in the temporal visual cortex of the monkey. Behav Brain Res. 1989;32:203–218. doi: 10.1016/s0166-4328(89)80054-3. [DOI] [PubMed] [Google Scholar]
- 49.Critchley H, Daly E, Phillips M, et al. Explicit and implicit neural mechanisms for processing of social information from facial expressions: a functional magnetic resonance imaging study. Hum Brain Mapp. 2000;9:93–105. doi: 10.1002/(SICI)1097-0193(200002)9:2<93::AID-HBM4>3.0.CO;2-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hein G, Knight RT. Superior temporal sulcus–It's my area: or is it? J Cogn Neurosci. 2008;20:2125–2136. doi: 10.1162/jocn.2008.20148. [DOI] [PubMed] [Google Scholar]
- 51.Cheng W, Rolls ET, Gu H, Zhang J, Feng J. Autism: reduced connectivity between cortical areas involved in face expression, theory of mind, and the sense of self. Brain. 2015;138:1382–1393. doi: 10.1093/brain/awv051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheng W, Rolls ET, Zhang J, et al. Functional connectivity decreases in autism in emotion, self, and face circuits identified by Knowledge-based Enrichment Analysis. Neuroimage. 2017;148:169–178. doi: 10.1016/j.neuroimage.2016.12.068. [DOI] [PubMed] [Google Scholar]
- 53.Rolls ET. Oxford University Press; Oxford: 2021. Brain Computations: What and How. [Google Scholar]
- 54.Rolls ET. The orbitofrontal cortex and emotion in health and disease, including depression. Neuropsychologia. 2019;128:14–43. doi: 10.1016/j.neuropsychologia.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 55.Rolls ET, Cheng W, Feng J. The orbitofrontal cortex: reward, emotion and depression. Brain Commun. 2020;2:fcaa196. doi: 10.1093/braincomms/fcaa196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rolls ET. Oxford University Press; Oxford: 2019. The Orbitofrontal Cortex. [Google Scholar]
- 57.Grill-Spector K, Weiner KS, Gomez J, Stigliani A, Natu VS. The functional neuroanatomy of face perception: from brain measurements to deep neural networks. Interface Focus. 2018;8 doi: 10.1098/rsfs.2018.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schirmer A, Adolphs R. Emotion perception from face, voice, and touch: comparisons and convergence. Trends Cogn Sci. 2017;21:216–228. doi: 10.1016/j.tics.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- 60.Rizzolatti G. Confounding the origin and function of mirror neurons. Behav Brain Sci. 2014;37:218–219. doi: 10.1017/S0140525X13002471. [DOI] [PubMed] [Google Scholar]
- 61.Shallice T, Cipolotti L. The prefrontal cortex and neurological impairments of active thought. Annu Rev Psychol. 2018;69:157–180. doi: 10.1146/annurev-psych-010416-044123. [DOI] [PubMed] [Google Scholar]
- 62.Cristofori I, Cohen-Zimerman S, Grafman J. Executive functions. Handb Clin Neurol. 2019;163:197–219. doi: 10.1016/B978-0-12-804281-6.00011-2. [DOI] [PubMed] [Google Scholar]
- 63.Freton M, Lemogne C, Bergouignan L, Delaveau P, Lehericy S, Fossati P. The eye of the self: precuneus volume and visual perspective during autobiographical memory retrieval. Brain Struct Funct. 2014;219:959–968. doi: 10.1007/s00429-013-0546-2. [DOI] [PubMed] [Google Scholar]
- 64.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 65.Cheng W, Rolls ET, Qiu J, et al. Functional connectivity of the precuneus in unmedicated patients with depression. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3:1040–1049. doi: 10.1016/j.bpsc.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 66.Peng D, Liddle EB, Iwabuchi SJ, et al. Dissociated large-scale functional connectivity networks of the precuneus in medication-naive first-episode depression. Psychiatry Res. 2015;232:250–256. doi: 10.1016/j.pscychresns.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 67.Schreiner MW, Klimes-Dougan B, Cullen KR. Neural correlates of suicidality in adolescents with major depression: resting-state functional connectivity of the precuneus and posterior cingulate cortex. Suicide Life Threat Behav. 2019;49:899–913. doi: 10.1111/sltb.12471. [DOI] [PubMed] [Google Scholar]
- 68.Mizuhara H, Yamaguchi Y. Human cortical circuits for central executive function emerge by theta phase synchronization. Neuroimage. 2007;36:232–244. doi: 10.1016/j.neuroimage.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 69.Nieder A, Dehaene S. Representation of number in the brain. Annu Rev Neurosci. 2009;32:185–208. doi: 10.1146/annurev.neuro.051508.135550. [DOI] [PubMed] [Google Scholar]
- 70.Wang L, Uhrig L, Jarraya B, Dehaene S. Representation of numerical and sequential patterns in macaque and human brains. Curr Biol. 2015;25:1966–1974. doi: 10.1016/j.cub.2015.06.035. [DOI] [PubMed] [Google Scholar]
- 71.Sheridan MA, McLaughlin KA. Dimensions of early experience and neural development: deprivation and threat. Trends Cogn Sci. 2014;18:580–585. doi: 10.1016/j.tics.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rolls ET, Deco G, Huang CC, Feng J. The effective connectivity of the human hippocampal memory system. Cereb Cortex. 2022 doi: 10.1093/cercor/bhab442. [DOI] [PubMed] [Google Scholar]
- 73.Rolls ET, Deco G, Huang CC, Feng J. The human orbitofrontal cortex, vmPFC, and anterior cingulate cortex effective connectome: emotion, memory, and action. Cereb Cortex. 2022 doi: 10.1093/cercor/bhac070. [DOI] [PubMed] [Google Scholar]
- 74.Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.