Abstract
In rodents, a single injection of lipopolysaccharide (LPS) during gestation causes chemical and functional abnormalities in the offspring. These effects may involve changes in the kynurenine pathway (KP) of tryptophan degradation and may provide insights into the pathophysiology of psychiatric diseases. Using CD1 mice, we examined acute and long-term effects of prenatal LPS treatment on the levels of kynurenine and its neuroactive downstream products kynurenic acid (KYNA), 3-hydroxykynurenine (3-HK) and quinolinic acid. To this end, LPS (100 μg/kg, i.p.) was administered on gestational day 15, and KP metabolites were measured 4 and 24 h later or in adulthood. After 4 h, kynurenine, KYNA and 3-HK levels were elevated in the fetal brain, 3-HK and KYNA levels were increased in the maternal plasma, and kynurenine was increased in the maternal brain, whereas no changes were seen in the placenta. These effects were less prominent after 24 h, and prenatal LPS did not affect the basal levels of KP metabolites in the forebrain of adult animals. In addition, a second LPS injection (1 mg/kg) in adulthood in the offspring of prenatally saline- and LPS-treated mice caused a similar elevation in 3-HK levels in both groups after 24 h, but the effect was significantly more pronounced in male mice. Thus, acute immune activation during pregnancy has only short-lasting effects on KP metabolism and does not cause cerebral KP metabolites to be disproportionally affected by a second immune challenge in adulthood. However, prenatal KYNA elevations still contribute to functional abnormalities in the offspring.
Keywords: Development, 3-Hydroxykynurenine, Kynurenic acid, Schizophrenia
Introduction
Adverse events during pregnancy have significant effects on brain development and can in turn lead to pathological consequences in the offspring (Debnath et al., 2015; Haddad et al., 2020; Seidman et al., 2000; Stolp et al., 2012). More specifically, epidemiological studies suggest that prenatal exposure to infectious agents can be linked to an increased risk to develop a variety of psychiatric disorders, including depression and schizophrenia (Borrell et al., 2002; Brown, 2012; Conway & Brown, 2019; Kirkbride et al., 2012; Rapoport et al., 2005; Stower, 2019). This concept is supported by a substantial number of experiments in rodents, which showed that maternal immune activation leads to several neurochemical and behavioral abnormalities in the adult offspring, which are similar to those observed in patients (Meyer & Feldon, 2010, 2012; Ozawa et al., 2006; Romero et al., 2007; Smith et al., 2007). One of the most widely used experimental approaches in this respect involves maternal exposure to the prototypical endotoxin lipopolysaccharide (LPS), which triggers an immune response by interacting with Toll-like receptors of the innate immune system (Janssens & Beyaert, 2003; Park & Lee, 2013). This treatment results in distinct deficits in the progeny later in life, including decreased hippocampal neurogenesis, dysfunctional synaptic transmission, and a number of behavioral abnormalities, including cognitive impairments (Chlodzinska et al., 2011; Coyle et al., 2009; Depino, 2015; Escobar et al., 2011; Fernandez de Cossio et al., 2017; Lin & Wang, 2014). Converging evidence suggests a causal relationship between the prenatal bacterial infection and the deficits seen in the adult offspring. However, in spite of their possible relevance for the pathophysiology of major psychiatric diseases, the mechanisms underlying the untoward long-term consequences of maternal LPS administration have not been clarified so far.
Activation of the kynurenine pathway (KP) of tryptophan degradation may be a significant factor in this context. In particular, in adult animals, the initial, rate-limiting enzyme of the KP, indoleamine-2,3-dioxygenase (IDO), is readily induced by LPS and other inflammatory stimuli (Lestage et al., 2002; O’Connor et al., 2009), resulting in an increased conversion of tryptophan to kynurenine (Figure 1). Downstream, the KP contains several neuroactive metabolites, including the free radical generator 3-hydroxykynurenine (3-HK), the NMDA receptor agonist quinolinic acid (QUIN), and, in a competing branch, kynurenic acid (KYNA), an antagonist of α7 nicotinic acetylcholine (α7nACh) and NMDA receptor function (Schwarcz & Stone, 2017). Focusing on KYNA because of its ability to affect these two receptors, which are both believed to be critically involved in the pathophysiology of psychiatric disorders (Lakhan et al., 2013; Olincy & Freedman, 2012), a series of studies demonstrated that even moderate increases in the brain levels of this metabolite cause disease-relevant cognitive deficits in animals (Pocivavsek et al., 2016). Notably, abnormal prenatal increases in KYNA levels, which can be produced experimentally in rodents by administering kynurenine or a kynurenine 3-monooxygenase (KMO) inhibitor to the dam during the last week of gestation (cf. Figure 1), lead to chemical, structural and electrophysiological abnormalities reminiscent of psychiatric disorders in the adult offspring (Alexander et al., 2013; Forrest, Khalil, Pisar, Darlington, et al., 2013; Khalil et al., 2014; Pershing et al., 2015; Pisar et al., 2014; Pocivavsek et al., 2014). Interestingly, these long-term effects include functionally relevant increases in KYNA levels in the adult brain (Pershing et al., 2016; Pocivavsek et al., 2019; Pocivavsek et al., 2014). In view of the fact that the concentration of KYNA is significantly elevated in the brain and cerebrospinal fluid of persons with schizophrenia (Erhardt et al., 2001; Linderholm et al., 2012; Sathyasaikumar et al., 2011; Schwarcz et al., 2001), these findings jointly raised the possibility that an impairment in KP metabolism during the prenatal period may play a role in the emergence of psychiatric symptoms later in life (Notarangelo & Pocivavsek, 2017).
Figure 1:
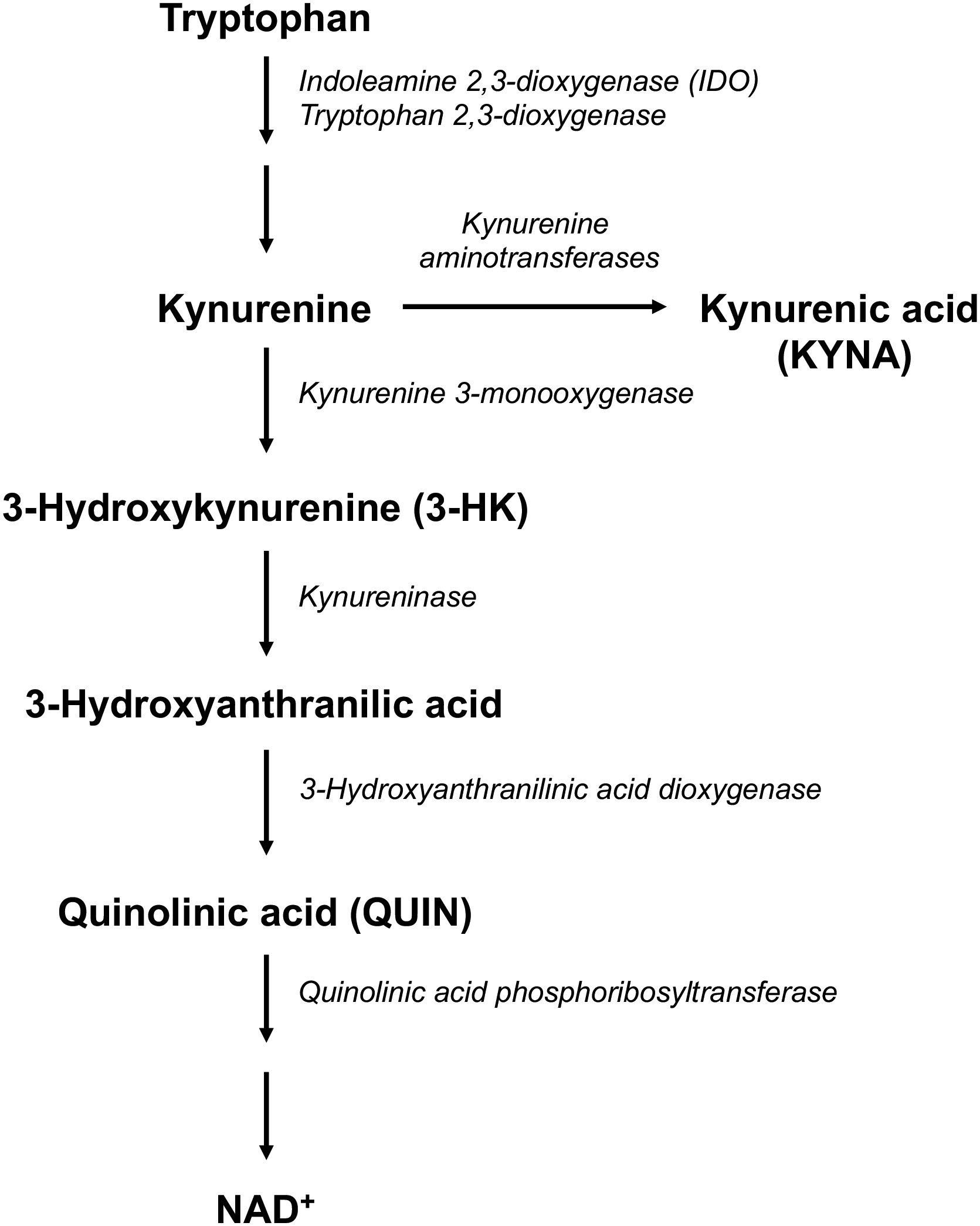
The kynurenine pathway of tryptophan degradation
Despite the fact that both prenatal LPS administration and KYNA up-regulation cause deficits on mature animals, and that the acute consequences of these two interventions during pregnancy have been described individually, the possible relationship between prenatal LPS exposure and KP metabolism has not been directly examined so far. This is particularly relevant as the regulation of the KP differs both qualitatively and quantitatively at various stages of development (Gramsbergen et al., 1997; Notarangelo et al., 2019; Notarangelo & Pocivavsek, 2017).
Because of its possible translational significance, the present study was designed to address this question by examining the short- and long-term effects of a single prenatal injection of LPS on KP metabolism in mice. Using a dose known to raise cytokine levels while minimizing the risk of preterm delivery and maternal mortality (Fricke et al., 2018), we administered 0.1 mg/kg LPS intraperitoneally (i.p.) on gestational day (GD) 15. Since activation of the immune system later in life is also considered to play a role in the pathophysiology of major psychiatric disorders including depression and schizophrenia (Benros et al., 2011; Dantzer et al., 2008), the offspring of prenatally treated animals received an additional LPS injection in adulthood. In all animals, the levels of pivotal KP metabolites were analyzed both during pregnancy and in adulthood.
Materials and Methods
Chemicals
Kynurenic acid (KYNA), 3-hydroxy-DL-kynurenine (3-HK), quinolinic acid (QUIN), [2H6]L-kynurenine, pentafluoropropionic anhydride and 2,2,3,3,3-pentafluoro-1-propanol were purchased from Sigma-Aldrich (St. Louis, MO, USA). L-Kynurenine sulfate (“kynurenine”; purity: 99.4%) was obtained from Sai Advantium (Hyderabad, India). [2H3]Quinolinic acid was purchased from Synfine Research (Richmond Hill, Ontario, Canada). LPS from E. coli (L-3129, serotype 0127.B8) was obtained from Sigma-Aldrich (St. Louis, MO, USA).
All other chemicals were obtained from various commercial suppliers and were of the highest available purity.
Mice
Pregnant CD-1 mice (2–3 month-old; gestational age: 2 days) were obtained from Charles River Laboratories (Frederick, MD, USA) and were individually housed upon arrival. All experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Maryland School of Medicine. Mice were maintained on a 12 h light/dark cycle in a temperature-controlled room with ad libitum access to food and water. For all prenatal studies, 2–4 embryos per litter were used, and the data were expressed as averages of each litter. For studies in adult animals, only one male and one female mouse was used for each experimental condition.
Lipopolysaccharide injection
Pregnant dams were injected i.p. with 0.1 mg/kg LPS on GD 15. Control mice received an i.p. injection of a sterile saline solution. To evaluate the acute consequences of LPS, dams (n = 3–4 per group) were euthanized using carbon dioxide either 4 or 24 h later, and maternal brain (“forebrain”: whole brain without cerebellum) and plasma (collected in EDTA-containing tubes and centrifuged at 6,000 × g for 10 min) as well as placenta and fetal brain were collected and stored at −80°C until analysis. To investigate long-term effects, separate dams were left undisturbed after the prenatal administration of LPS or saline (n = 7–8 per group), and male and female offspring were weaned on postnatal day (PND) 21. At PND 60, mice were then injected i.p. with either LPS (0.1 or 1 mg/kg, i.p.) or saline and euthanized using carbon dioxide 24 h after the treatment. Forebrains of these animals were rapidly collected and stored at −80°C until analysis.
Kynurenic acid and 3-hydroxykynurenine measurement
Ultrapure water was used for all tissue homogenizations and dilutions. Fetal brain tissue was homogenized by sonication (1:10, w/v), and the homogenate was further diluted for the measurement of 3-HK (1:20 final). Placenta was homogenized (1:10, w/v) and then diluted further (1:20 final for 3-HK, 1:100 final for KYNA). Maternal and offspring brain tissue (whole forebrain) was sonicated (1:5, w/v), and maternal plasma was diluted (1:2, v/v for 3-HK and 1:10, v/v for KYNA).
Twenty-five μl of 6% perchloric acid were added to 100 μl of each sample, and precipitated proteins were removed by centrifugation (16,000 × g, 10 min). For KYNA determination, 20 μl of the resulting supernatant were injected onto a 3 μm C18 reverse phase HPLC column (100 mm × 4 mm; Dr. Maisch GmbH, Ammerbuch, Germany), using a mobile phase containing 50 mM sodium acetate and acetonitrile (3% for brain and 6% for placenta and plasma; pH adjusted to 6.2 with glacial acetic acid) at a flow rate of 0.5 ml/min. Zinc acetate (0.5 M; not pH adjusted), was delivered post-column by a peristaltic pump (Dionex AXP, Thermo Fisher, Waltham, MA, USA) at a flow rate of 0.1 ml/min. In the eluate, KYNA was detected fluorimetrically (excitation: 344 nm, emission: 398 nm; S200a fluorescence detector; Perkin Elmer, Waltham, MA, USA).
For 3-HK determination, 20 μl of the supernatant were applied to a 3 μm HPLC column (HR-80; 80 mm × 4.6 mm; ESA, Chelmsford, MA, USA), using a mobile phase consisting of 1.5 % acetonitrile, 0.9 % triethylamine, 0.59 % phosphoric acid, 0.27 mM EDTA and 8.9 mM sodium heptane sulfonic acid, and a flow rate of 0.5 ml/min. In the eluate, 3-HK was detected electrochemically using a HTEC 500 detector (Eicom Corp., San Diego, CA; oxidation potential: +0.5 V).
Kynurenine and quinolinic acid measurement
To measure kynurenine and QUIN in tissue, the original homogenates were further diluted (v/v) in 0.1% ascorbic acid (1:20 final for maternal and offspring brain, 1:50 final for fetal brain, 1:100 final for placenta). For the determination of QUIN in maternal plasma, samples were diluted in 0.1% ascorbic acid (1:10, v/v). Fifty μl of an internal standard mix ([2H3]quinolinic acid, [2H6]L-kynurenine) were added to 50 μl of the samples, and proteins were precipitated with 50 μl of acetone. After centrifugation (13,700 × g, 5 min), 50 μl of methanol:chloroform (20:50) were added to the supernatant, and the samples were centrifuged (13,700 × g, 10 min). The upper layer was added to a glass tube and dried down for 90 min. The samples were then derivatized with 120 μl of 2,2,3,3,3-pentafluoro-1-propanol and 130 μl of pentafluoropropionic anhydride at 75°C for 30 min, dried down and reconstituted in 50 μl of ethyl acetate. One μl was injected in the GC/MS (Notarangelo et al., 2012).
For determination of kynurenine in maternal plasma, 25 μl of 6% perchloric acid were added to 100 μl of the sample. Precipitated proteins were removed by centrifugation (16,000 × g, 10 min), and 20 μl of the resulting supernatant were injected to a 3 μm C18 reverse phase HPLC column (100 mm × 4 mm; Dr. Maisch GmbH), using a mobile phase containing 50 mM sodium acetate and 6% acetonitrile (pH adjusted to 6.2 with glacial acetic acid) at a flow rate of 0.5 ml/min. Zinc acetate (0.5 M; not pH adjusted), was delivered post column by a peristaltic pump (Dionex AXP, Thermo Fisher) at a flow rate of 0.1 ml/min. In the eluate, kynurenine was detected fluorimetrically (excitation: 365 nm, emission: 480 nm; S200a fluorescence detector; Perkin Elmer).
Sex determination
Embryonic tissue was retained for determination of sex by genotyping, using primers specific to Jarid1 (5′-CTGAAGCTTTTGGCTTTGAG-3′ and 5′-CCGCTGCCAAATTCTTTGG-3′; Invitrogen, Carlsbad, CA, USA) as previously described (Clapcote & Roder, 2005). One or two male and female embryos per litter were used for the analyses.
Protein determination
Protein was determined according to Lowry et al. (Lowry et al., 1951), using bovine serum albumin as a standard.
Statistical analysis
All results are expressed as the mean ± SEM. Statistical analyses were performed with Graphpad Prism 9 (San Diego, CA, USA) and two-way ANOVA followed by Bonferroni’s post-hoc test was used to determine significance in all experiments. A p value of <0.05 was considered significant.
Results
Acute effect of prenatal LPS treatment on maternal brain and plasma
To evaluate the effect of moderate immune activation on KP metabolites during pregnancy, mice received a single injection of 0.1 mg/kg LPS on GD15. Assessed 24 h later, this treatment did not influence maternal body weight or the number of embryos (data not shown), confirming that the dose of the endotoxin used does not affect pregnancy (Chlodzinska et al., 2011).
Kynurenine, KYNA, 3-HK and QUIN were analyzed in the maternal brain 4 and 24 h after LPS treatment (Figure 2). Kynurenine levels increased after 4 h (12.8 ± 0.3 vs. 7.4 ± 0.3 pmol/mg protein in saline-treated mice; p < 0.05) but returned to control levels after 24 h (main effect of treatment: F(1,10) = 5.81, p < 0.05; interaction: F(1,10) = 6.17, p < 0.05). No significant changes in the maternal brain levels of 3-HK, KYNA or QUIN were observed at either time point, though the elevation in 3-HK (from 536.8 ± 29.9 in control mice to 901.1 ± 157.6 fmol/mg protein) 4 h following LPS administration approached statistical significance (p = 0.07).
Figure 2:
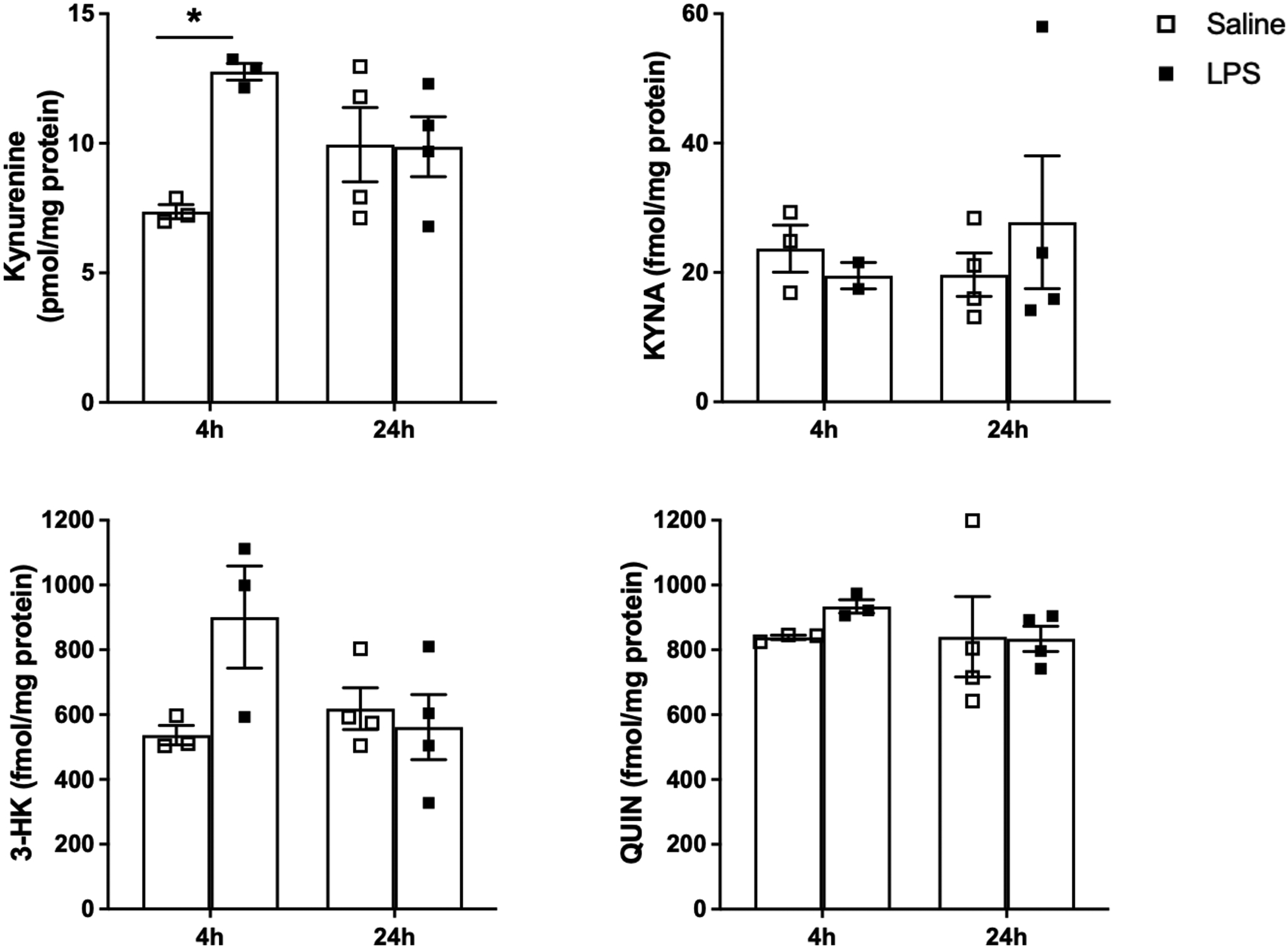
KP metabolite levels in the maternal brain 4 and 24 h after saline or LPS administration (0.1 mg/kg, i.p.) on GD15. Data are the mean ± SEM (n = 3–4). * p < 0.05 (two-way Anova, followed by Bonferroni’s post-hoc test).
LPS treatment induced no significant changes in kynurenine levels in maternal plasma after either 4 or 24 h. In contrast, we observed significant increases in the circulating levels of both KYNA and 3-HK after 4 h (p < 0.05). Although the concentration of both metabolites tended to remain higher than endogenous levels after 24 h (main effect of treatment: F(1,10) = 10.54, p < 0.01 for KYNA; F(1,10) = 9.65, p < 0.05 for 3-HK), neither effect reached statistical significance after post-hoc analysis. No significant changes in QUIN levels were observed in maternal plasma at either timepoint (Figure 3).
Figure 3:
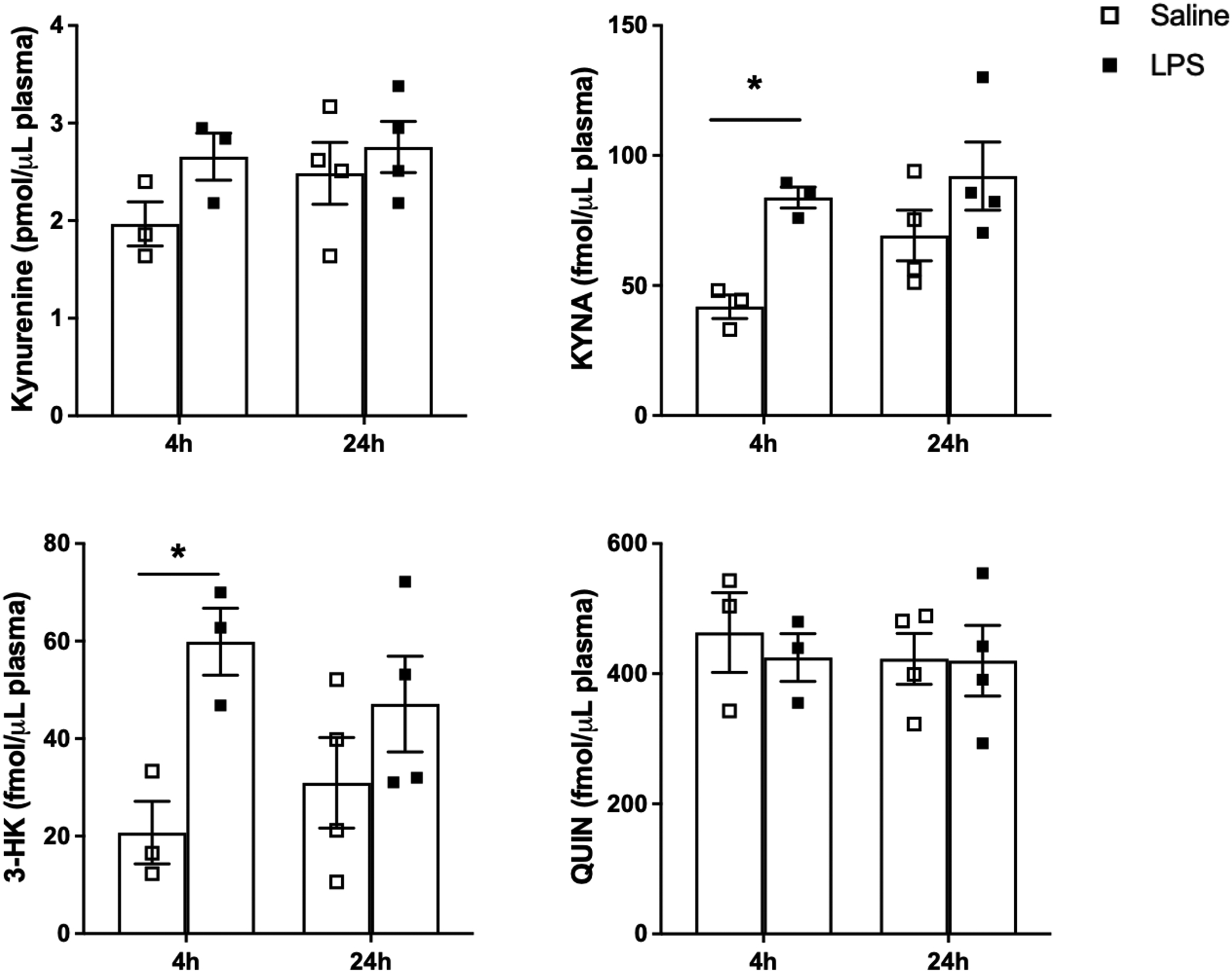
KP metabolite levels in the maternal plasma 4 and 24 h after saline or LPS administration (0.1 mg/kg, i.p.) on GD15. Data are the mean ± SEM (n = 3–4). * p < 0.05 (two-way Anova, followed by Bonferroni’s post-hoc test).
Acute effects of prenatal LPS treatment on placenta and fetal brain
Prenatal LPS administration on GD 15 did not cause significant changes in the levels of kynurenine, KYNA, 3-HK or QUIN after 4 or 24 h in the placenta (Suppl. Figure 1).
However, prenatal LPS treatment affected KP metabolism in the fetal brain (Figure 4). Compared to the control group, the tissue levels of kynurenine were elevated after 4 h (p < 0.01). A trend in the same direction was also seen at 24 h, although the effect did not reach statistical significance after post-hoc analysis (main effect of treatment: F(1,10) = 17.47, p < 0.01). The levels of KYNA and 3-HK, too, increased 4 h after the administration of LPS (p < 0.01 and p<0.05, respectively), and trended back toward endogenous levels by 24 h (main effect of treatment: F(1,10) = 22.90, p < 0.001 for KYNA; F(1,10) = 15.19, p < 0.01 for 3-HK). No significant changes in QUIN levels were observed at either time point. Notably, prenatal LPS induced similar changes in the brain of male and female embryos (Suppl. Figure 2).
Figure 4:
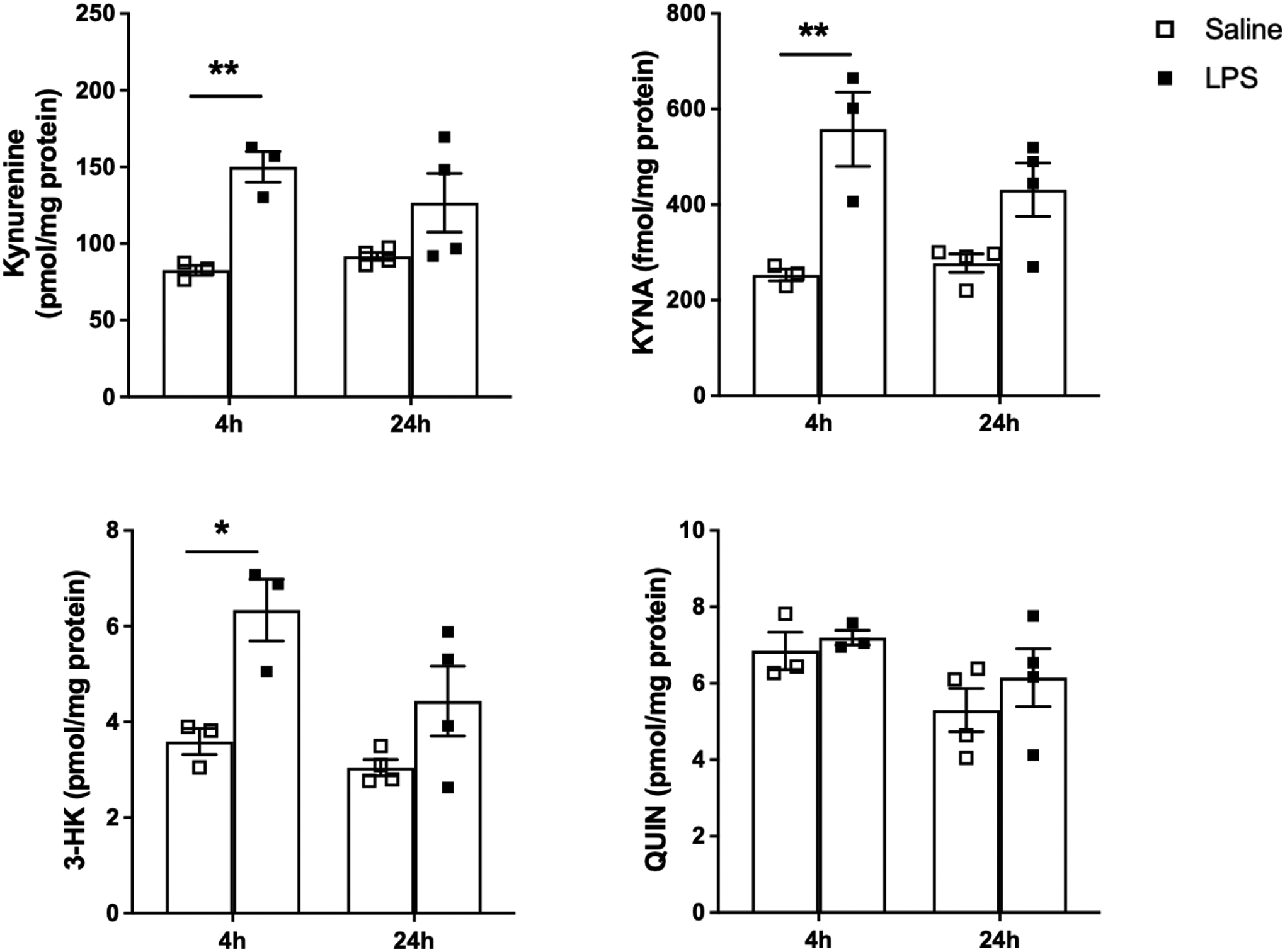
KP metabolite levels in the fetal brain 4 and 24 h after saline or LPS administration (0.1 mg/kg, i.p.) on GD15. Data are the mean ± SEM (n = 3–4). * p < 0.05, ** p < 0.01 (two-way Anova, followed by Bonferroni’s post-hoc test).
Long-term effects of prenatal LPS treatment and effects of an additional LPS challenge in adulthood
Body weight
The long-term effects of prenatal LPS administration were studied in separate cohorts of animals. Compared to control dams, treatment with 0.1 mg/kg LPS on GD 15 did not induce significant changes in maternal body weight until birth or alter the number of pups (data not shown). Moreover, prenatal LPS treatment did not affect the body weight of the offspring at PND 1, 21 or 56 (data not shown).
To investigate if prenatal treatment altered the response to an acute LPS challenge in adulthood, we administered 0.1 or 1 mg/kg LPS to offspring of dams treated prenatally with saline (“prenatal saline”) or LPS (“prenatal LPS”). Irrespective of prenatal treatments, we observed a significant decrease in body weight in both male and female mice receiving 1 mg/kg LPS 24 h later (p < 0.0001 and p < 0.01, respectively; Two-way Anova followed by Bonferroni’s post-hoc test). The lower dose of LPS (0.1 mg/kg) did not affect body weight in either group (data not shown).
KP metabolites in the brain
No significant differences in the basal brain tissue levels of kynurenine, KYNA, 3-HK or QUIN were seen between either male or female offspring of dams which had received saline or LPS injections on GD15 (Figures 5 and 6). Analysis of the effect of an i.p. injection of LPS in adulthood revealed a main effect of treatment (F(2,18) = 4.65, p < 0.05 for males; F(2,24) = 6.15, p < 0.01 for females), but no significant changes after post-hoc analysis in the tissue levels of kynurenine 24 h following the injection of either 0.1 or 1 mg/kg LPS. KYNA and QUIN levels remained unaffected 24 h after the acute administration of either dose of LPS. In the same tissues, 3-HK levels were found to increase significantly after the administration of 1 mg/kg (but not after 0.1 mg/kg) LPS in male (p < 0.001 and p < 0.0001) and female (both p < 0.0001) offspring of both “prenatal saline” and “prenatal LPS” animals. Interestingly, however, while no sex differences were observed for kynurenine, KYNA and QUIN, the acute LPS challenge in adulthood raised 3-HK levels more in the brain of male than in female offspring of both “prenatal saline” and “prenatal LPS” dams (p < 0.001 and p < 0.0001, respectively). Importantly, no significant differences in the levels of any of the KP metabolites were seen between adult offspring of “prenatal saline” and “prenatal LPS” dams after the administration of either dose of LPS in adulthood.
Figure 5:
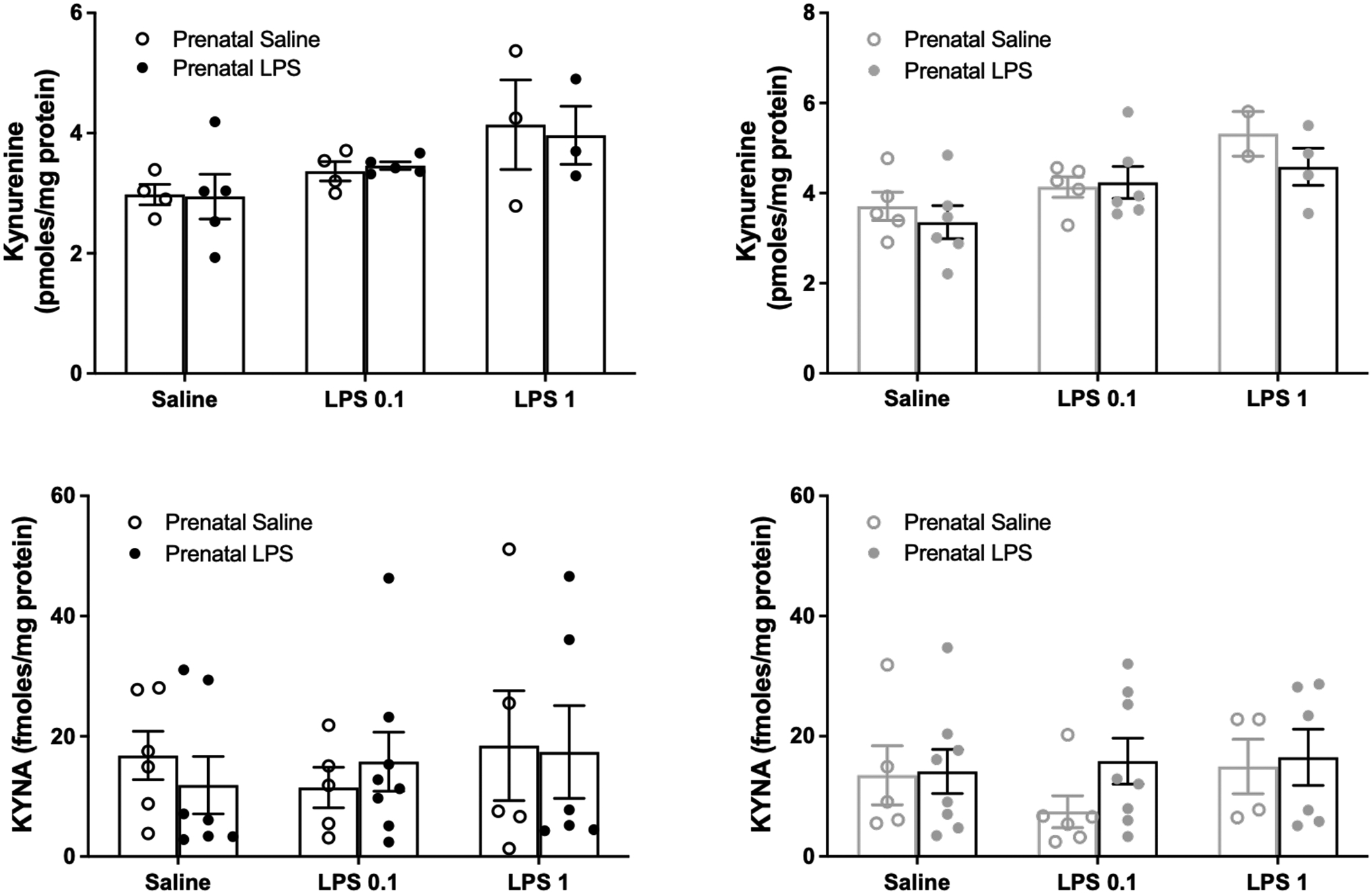
Brain kynurenine and KYNA levels in male (black) and female (grey) offspring of dams treated prenatally with saline or LPS 24 h after an injection of saline or LPS (0.1 or 1 mg/kg i.p.) in adulthood. See text for experimental details. Data are the mean ± SEM (n = 3–8).
Figure 6:
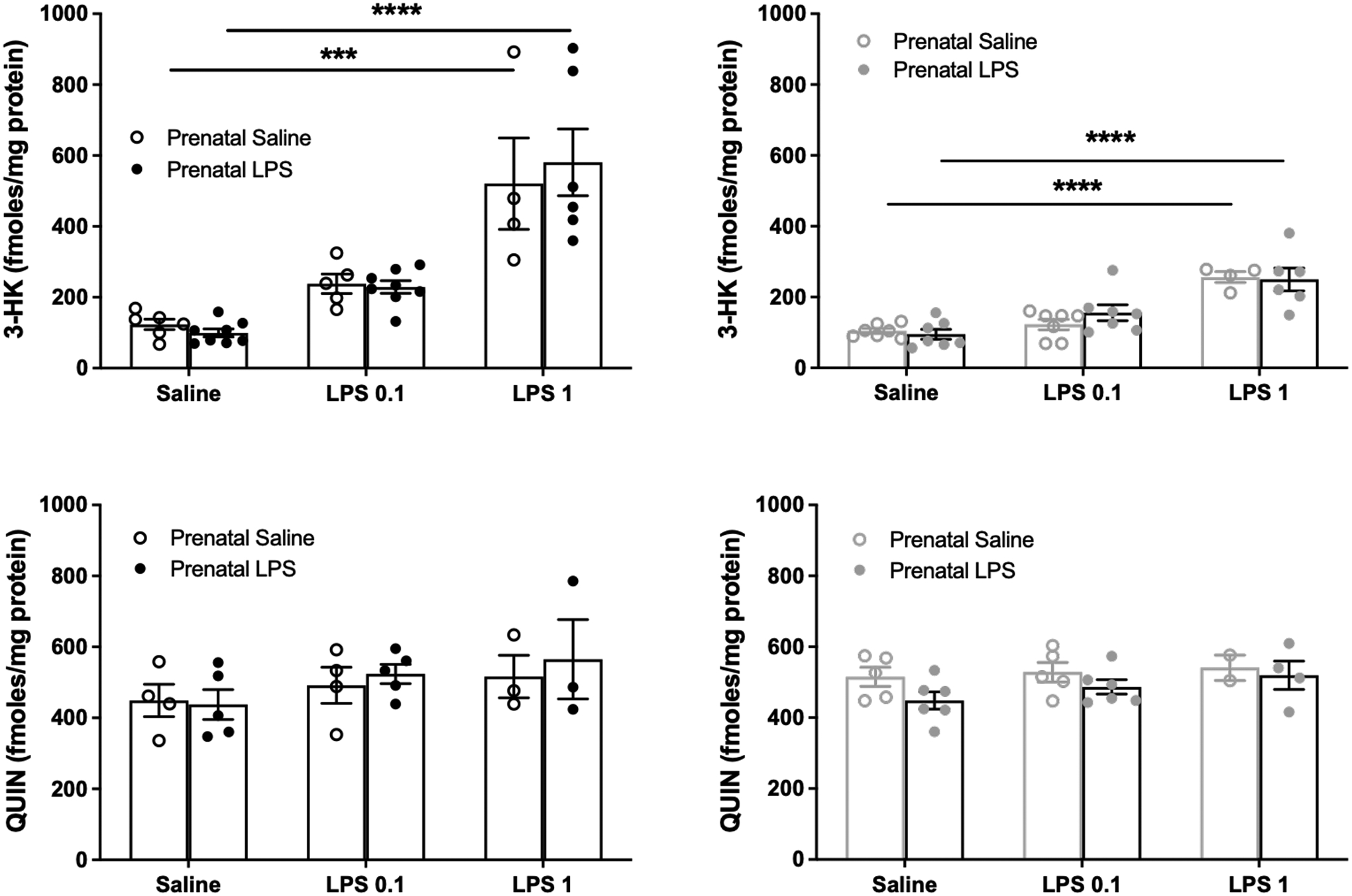
Brain 3-HK and QUIN levels in male (black) and female (gray) offspring of dams treated prenatally with saline or LPS 24 h after an injection of saline or LPS (0.1 or 1 mg/kg i.p.) in adulthood. See text for experimental details. Data are the mean ± SEM (n = 3–8). *** p < 0.001, **** p < 0.0001 (two-way Anova, followed by Bonferroni’s post-hoc test).
Discussion
In light of increasing evidence supporting a role of the KP in the pathophysiology of schizophrenia and other psychiatric diseases (Bryleva & Brundin, 2017; Erhardt et al., 2017; Ogyu et al., 2018; Schwarcz et al., 2012; Zavitsanou et al., 2014) and the consensus that both prenatal infections and immune activation later in life are major risk factors in these disorders (Borrell et al., 2002; Brown, 2012; Conway & Brown, 2019; Kirkbride et al., 2012; Rapoport et al., 2005; Stower, 2019), the present study was designed to provide translationally relevant new insights, using mice as the experimental animals. By measuring the levels of the key KP metabolites kynurenine, KYNA, 3-HK and QUIN, we first examined the short- and long-term effects of a single prenatal injection of the immunogen LPS on KP metabolism, and then investigated whether an additional LPS injection in the offspring of these prenatally treated animals has disproportionate, and possibly sex-specific, acute effects on cerebral KP metabolism. Applied at doses which are widely used in preclinical studies (Chlodzinska et al., 2011; Fricke et al., 2018), a single injection of LPS during pregnancy induced transient increases in the levels of kynurenine, KYNA and 3-HK – but not QUIN - in the fetal brain. However, this prenatal LPS treatment did not affect basal KP metabolite levels and did not influence the response to a second LPS injection in the brain of the adult offspring, possibly due to a blunted immune response to the repeated stimulus, as previously observed (Clark, Notarangelo, et al., 2019). Interestingly, an acute challenge with LPS (1 mg/kg) in adulthood raised cerebral 3-HK levels significantly more in male than in female mice.
In experimental animals, systemic application of LPS or other immunostimulants rapidly promotes the formation of cytokines, which in turn activate IDO activity (Campbell et al., 2014; Williams et al., 2017). In the present study, this effect likely accounted for the prompt, LPS-induced increase in cerebral kynurenine levels, and the rise in 3-HK and KYNA concentrations in the plasma, in the pregnant mouse following a single systemic injection of 0.1 mg/kg LPS on GD15. As the plasma levels of kynurenine, which readily enters the fetus from the maternal circulation (Goeden et al., 2017), did not change significantly in response to LPS, since KYNA does not cross the placental barrier (Goeden et al., 2017), and KP metabolites were not affected in the placenta (despite containing various KP enzymes (Manuelpillai et al., 2005; Murthi et al., 2017; Suzuki et al., 2001), the observed increase in the levels of these two metabolites in the fetal brain was probably due to local events within the fetus. Although controversial (Brown et al., 2019; Fricke et al., 2018), this may have involved the transfer of LPS itself into the embryo or, more likely, the trans-placental influx of LPS-induced maternal cytokines and the subsequent stimulation of fetal KP metabolism (Oskvig et al., 2012; Simoes et al., 2018; Williams et al., 2017). This would also explain the observed increase in 3-HK levels in the fetal brain, though enhanced influx from the maternal blood, where 3-HK was elevated following the LPS treatment, may have played a role as well (Goeden et al., 2017). The molecular dynamics of these processes, and the finding that QUIN levels in the fetal brain remained unaffected by LPS, clearly need to be elaborated in greater detail, keeping in mind qualitative differences in the regulation of cerebral KP metabolism at different stages of early development and lifespan (Gramsbergen et al., 1997; Notarangelo et al., 2019; Notarangelo & Pocivavsek, 2017; Walker et al., 1999). Moreover, brain cytokines were not measured in this study and further experiments are needed to clarify their specific relationship with KP metabolism activation and behavior.
In line with previous studies in mice, we observed a significant increase in brain 3-HK levels, but no changes in KYNA, after a single administration of LPS in adulthood (Larkin et al., 2016; Larsson et al., 2016; Walker et al., 2013). Moreover, as in the fetal brain, and possibly due to its rapid conversion to NAD+ (Moffett et al., 2020), LPS treatment did not raise brain QUIN levels acutely in the adult animals, as also previously reported (Clark, Notarangelo, et al., 2019). However, in contrast to other reports, and possibly related to strain differences, the serotype of the LPS used, and/or the fact that only a single timepoint (24 h) following LPS administration was examined (Migale et al., 2015; Murakami & Saito, 2013; Parrott et al., 2016; Piirsalu et al., 2020), only a non-significant trend toward elevated kynurenine levels was detected. Also of possible relevance in this context, the effect of systemically applied LPS on KP metabolites differs at various doses and between brain regions (Parrott et al., 2016; Tao et al., 2020). These variables should be evaluated to fully characterize the role of altered KP metabolism on cognitive function and kept in mind in the design of follow-up studies exploring the role of KP-related redox processes (Gonzalez Esquivel et al., 2017) and the formation of the neuroactive downstream KP metabolites xanthurenic acid and cinnabarinic acid (Fazio et al., 2017), which were not examined in the present study. Moreover, genetic vulnerability should be considered (Beggiato et al., 2018), and repeated or chronic immune activation either during pregnancy or later in life, too, may have functionally relevant, detrimental impacts on brain KP metabolism (Larsson et al., 2016; Saito et al., 1992).
These considerations could also be relevant for explaining the significantly larger increase in brain 3-HK levels that we observed in male compared to female animals. Though sex differences in LPS-induced immune responses and behavior have been documented in rodents (Cai et al., 2016; Chlodzinska et al., 2011; Foley et al., 2015; Kuo, 2016), it is worth noting that links to cerebral KP metabolism have so far been predominantly examined in males (Larkin et al., 2016; Larsson et al., 2016; Tao et al., 2020; Walker et al., 2013), and that greater effects of immune activation in male mice (Cai et al., 2016; Kuo, 2016) may be related to the fact that females are more resilient due to the modulating effect of estrogen on cytokine gene expression (Dimayuga et al., 2005).
Our study design was based on – and is in line with – an extensive body of literature separately linking immune changes or abnormal KP metabolism during the prenatal period with adverse consequences in adulthood (Haddad et al., 2020; Notarangelo & Pocivavsek, 2017). Specifically, and in accordance with the popular “two-hit” hypothesis of psychiatric disorders (Maynard et al., 2001), our goal here was to evaluate the relationship between these two phenomena by focusing on acute immune-stimulations and their short-term effects on KP metabolism. In this context, we were especially interested in a possible role of KYNA, an established neuromodulator, which can inhibit α7nACh and NMDA receptor function in the adult brain (Pocivavsek et al., 2016) and has been shown to significantly affect progenitor cell proliferation, differentiation, and survival of human cortical cells (Bagasrawala et al., 2016). Thus, acute prenatal elevation in KYNA levels can apparently affect normal brain development and, consequently, influence behavior later in life. Notably, even the normal, i.e. endogenous, brain concentration of KYNA, like that of several other KP metabolites, is substantially higher prenatally than postnatally (Beal et al., 1992; Beggiato et al., 2018; Cannazza et al., 2001; Ceresoli-Borroni & Schwarcz, 2001; Walker et al., 1999), and the fetal brain produces more KYNA from kynurenine than the maternal brain under ex vivo conditions (Notarangelo et al., 2019). Of special interest in the context of dysfunctions triggered by prenatal immune activation, the α7nACh receptor agonist choline (Albuquerque et al., 1998; Alkondon et al., 1999; Fayuk & Yakel, 2004), possibly by counteracting the adverse consequences of enhanced KYNA inhibition of this receptor, attenuates the undesirable long-term effects of maternal immune activation on anxiety- and cognitive-related behaviors in adulthood (Wu et al., 2015). Finally, and further supporting a physiological role of endogenous KYNA in brain development, KYNA’s synthesizing enzyme kynurenine aminotransferase II (Figure 1) is highly expressed in the germinal zones (Csillik et al., 2002; Song et al., 2018) and mediates oligodendrogenesis as well as cell proliferation in the subventricular zone (Clark, Mou, et al., 2019).
Like acute prenatal immune activation (see Introduction), experimentally induced increases in the levels of KP metabolites in the fetal brain have been consistently shown to be associated with dysfunctions later in life. These impairments, which include abnormalities in synaptic transmission, imbalanced neurotransmitter functions and several translationally relevant behavioral deficits (Alexander et al., 2013; Forrest, Khalil, Pisar, Darlington, et al., 2013; Forrest, Khalil, Pisar, McNair, et al., 2013; Khalil et al., 2014; Pershing et al., 2015; Pisar et al., 2014; Pocivavsek et al., 2014), may increase the risk of the offspring for developing major psychiatric disorders, including depression and schizophrenia.
The present results show that brief, transient maternal immune activation, while stimulating KP metabolism in the fetal brain significantly and rapidly, does not cause long-lasting changes in cerebral KP metabolism or disproportionate acute vulnerability of cerebral KP metabolism to a second immune challenge. Similar conclusions were recently drawn from experiments using prenatal treatment with poly I:C, a classic experimental tool for studying translationally relevant long-term effects of immune dysfunctions during pregnancy (Clark, Notarangelo, et al., 2019; Estes et al., 2020; Haddad et al., 2020).
Supplementary Material
Supplemental Figure 1: KP metabolite levels in the placenta 4 and 24 h after saline or LPS administration (0.1 mg/kg, i.p.) on GD15. Data are the mean ± SEM (n = 3–4).
Supplemental Figure 2: KP metabolite levels in the fetal brain of male and female embryos 4 and 24 h after saline or LPS administration (0.1 mg/kg, i.p.) on GD15. Data are the mean ± SEM (n = 2–4). * p < 0.05, ** p < 0.01 (Two-way Anova, followed by Bonferroni’s post-hoc test).
Acknowledgements
We thank Kevin Wons and Ashley Holmes for excellent technical assistance. This work was supported by USPHS grant P50 MH103222 (Conte Center for Translational Mental Health Research)
Abbreviations:
- GD
Gestational day
- 3-HK
3-Hydroxykynurenine
- IDO
Indoleamine-2,3-dioxygenase
- KP
Kynurenine pathway
- KYNA
Kynurenic acid
- LPS
Lipopolysaccharide
- α7nACh
α7 Nicotinic acetylcholine
- NMDA
N-Methyl-D-aspartate
- PND
Postnatal day
- QUIN
Quinolinic acid
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Data Availability Statement
Data will be made available upon reasonable request to the corresponding author.
References
- Albuquerque EX, Pereira EF, Braga MF, & Alkondon M (1998). Contribution of nicotinic receptors to the function of synapses in the central nervous system: the action of choline as a selective agonist of alpha 7 receptors. J Physiol Paris, 92(3–4), 309–316. 10.1016/s0928-4257(98)80039-9 [DOI] [PubMed] [Google Scholar]
- Alexander KS, Pocivavsek A, Wu HQ, Pershing ML, Schwarcz R, & Bruno JP (2013). Early developmental elevations of brain kynurenic acid impair cognitive flexibility in adults: reversal with galantamine. Neuroscience, 238, 19–28. 10.1016/j.neuroscience.2013.01.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Eisenberg HM, & Albuquerque EX (1999). Choline and selective antagonists identify two subtypes of nicotinic acetylcholine receptors that modulate GABA release from CA1 interneurons in rat hippocampal slices. J Neurosci, 19(7), 2693–2705. https://www.ncbi.nlm.nih.gov/pubmed/10087082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagasrawala I, Zecevic N, & Radonjic NV (2016). N-Methyl D-Aspartate Receptor Antagonist Kynurenic Acid Affects Human Cortical Development. Front Neurosci, 10, 435. 10.3389/fnins.2016.00435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal MF, Swartz KJ, & Isacson O (1992). Developmental changes in brain kynurenic acid concentrations. Brain Res Dev Brain Res, 68(1), 136–139. 10.1016/0165-3806(92)90256-v [DOI] [PubMed] [Google Scholar]
- Beggiato S, Notarangelo FM, Sathyasaikumar KV, Giorgini F, & Schwarcz R (2018). Maternal genotype determines kynurenic acid levels in the fetal brain: Implications for the pathophysiology of schizophrenia. J Psychopharmacol, 32(11), 1223–1232. 10.1177/0269881118805492 [DOI] [PubMed] [Google Scholar]
- Benros ME, Nielsen PR, Nordentoft M, Eaton WW, Dalton SO, & Mortensen PB (2011). Autoimmune diseases and severe infections as risk factors for schizophrenia: a 30-year population-based register study. Am J Psychiatry, 168(12), 1303–1310. 10.1176/appi.ajp.2011.11030516 [DOI] [PubMed] [Google Scholar]
- Borrell J, Vela JM, Arevalo-Martin A, Molina-Holgado E, & Guaza C (2002). Prenatal immune challenge disrupts sensorimotor gating in adult rats. Implications for the etiopathogenesis of schizophrenia. Neuropsychopharmacology, 26(2), 204–215. 10.1016/S0893-133X(01)00360-8 [DOI] [PubMed] [Google Scholar]
- Brown AG, Maubert ME, Anton L, Heiser LM, & Elovitz MA (2019). The tracking of lipopolysaccharide through the feto-maternal compartment and the involvement of maternal TLR4 in inflammation-induced fetal brain injury. Am J Reprod Immunol, 82(6), e13189. 10.1111/aji.13189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS (2012). Epidemiologic studies of exposure to prenatal infection and risk of schizophrenia and autism. Dev Neurobiol, 72(10), 1272–1276. 10.1002/dneu.22024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryleva EY, & Brundin L (2017). Suicidality and Activation of the Kynurenine Pathway of Tryptophan Metabolism. Curr Top Behav Neurosci, 31, 269–284. 10.1007/7854_2016_5 [DOI] [PubMed] [Google Scholar]
- Cai KC, van Mil S, Murray E, Mallet JF, Matar C, & Ismail N (2016). Age and sex differences in immune response following LPS treatment in mice. Brain Behav Immun, 58, 327–337. 10.1016/j.bbi.2016.08.002 [DOI] [PubMed] [Google Scholar]
- Campbell BM, Charych E, Lee AW, & Moller T (2014). Kynurenines in CNS disease: regulation by inflammatory cytokines. Front Neurosci, 8, 12. 10.3389/fnins.2014.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannazza G, Chiarugi A, Parenti C, Zanoli P, & Baraldi M (2001). Changes in kynurenic, anthranilic, and quinolinic acid concentrations in rat brain tissue during development. Neurochem Res, 26(5), 511–514. 10.1023/a:1010960812204 [DOI] [PubMed] [Google Scholar]
- Ceresoli-Borroni G, & Schwarcz R (2001). Neonatal asphyxia in rats: acute effects on cerebral kynurenine metabolism. Pediatr Res, 50(2), 231–235. 10.1203/00006450-200108000-00011 [DOI] [PubMed] [Google Scholar]
- Chlodzinska N, Gajerska M, Bartkowska K, Turlejski K, & Djavadian RL (2011). Lipopolysaccharide injected to pregnant mice affects behavior of their offspring in adulthood. Acta Neurobiol Exp (Wars), 71(4), 519–527. https://www.ncbi.nlm.nih.gov/pubmed/22237497 [DOI] [PubMed] [Google Scholar]
- Clapcote SJ, & Roder JC (2005). Simplex PCR assay for sex determination in mice. Biotechniques, 38(5), 702, 704,, 706. 10.2144/05385BM05 [DOI] [PubMed] [Google Scholar]
- Clark SM, Mou TC, Laniyan A, Chandra R, & Tonelli LH (2019). RNAi inhibition of KAT II during postnatal development alters cell generation in the subventricular zone and oliodendrogenesis in the corpus callosum. Soc Neurosci Abstr(457.10). [Google Scholar]
- Clark SM, Notarangelo FM, Li X, Chen S, Schwarcz R, & Tonelli LH (2019). Maternal immune activation in rats blunts brain cytokine and kynurenine pathway responses to a second immune challenge in early adulthood. Prog Neuropsychopharmacol Biol Psychiatry, 89, 286–294. 10.1016/j.pnpbp.2018.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway F, & Brown AS (2019). Maternal Immune Activation and Related Factors in the Risk of Offspring Psychiatric Disorders. Front Psychiatry, 10, 430. 10.3389/fpsyt.2019.00430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle P, Tran N, Fung JN, Summers BL, & Rofe AM (2009). Maternal dietary zinc supplementation prevents aberrant behaviour in an object recognition task in mice offspring exposed to LPS in early pregnancy. Behav Brain Res, 197(1), 210–218. 10.1016/j.bbr.2008.08.022 [DOI] [PubMed] [Google Scholar]
- Csillik AE, Okuno E, Csillik B, Knyihar E, & Vecsei L (2002). Expression of kynurenine aminotransferase in the subplate of the rat and its possible role in the regulation of programmed cell death. Cereb Cortex, 12(11), 1193–1201. 10.1093/cercor/12.11.1193 [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, & Kelley KW (2008). From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci, 9(1), 46–56. 10.1038/nrn2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnath M, Venkatasubramanian G, & Berk M (2015). Fetal programming of schizophrenia: select mechanisms. Neurosci Biobehav Rev, 49, 90–104. 10.1016/j.neubiorev.2014.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depino AM (2015). Early prenatal exposure to LPS results in anxiety- and depression-related behaviors in adulthood. Neuroscience, 299, 56–65. 10.1016/j.neuroscience.2015.04.065 [DOI] [PubMed] [Google Scholar]
- Dimayuga FO, Reed JL, Carnero GA, Wang C, Dimayuga ER, Dimayuga VM, Perger A, Wilson ME, Keller JN, & Bruce-Keller AJ (2005). Estrogen and brain inflammation: effects on microglial expression of MHC, costimulatory molecules and cytokines. J Neuroimmunol, 161(1–2), 123–136. 10.1016/j.jneuroim.2004.12.016 [DOI] [PubMed] [Google Scholar]
- Erhardt S, Blennow K, Nordin C, Skogh E, Lindstrom LH, & Engberg G (2001). Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci Lett, 313(1–2), 96–98. 10.1016/s0304-3940(01)02242-x [DOI] [PubMed] [Google Scholar]
- Erhardt S, Schwieler L, Imbeault S, & Engberg G (2017). The kynurenine pathway in schizophrenia and bipolar disorder. Neuropharmacology, 112(Pt B), 297–306. 10.1016/j.neuropharm.2016.05.020 [DOI] [PubMed] [Google Scholar]
- Escobar M, Crouzin N, Cavalier M, Quentin J, Roussel J, Lante F, Batista-Novais AR, Cohen-Solal C, De Jesus Ferreira MC, Guiramand J, Barbanel G, & Vignes M (2011). Early, time-dependent disturbances of hippocampal synaptic transmission and plasticity after in utero immune challenge. Biol Psychiatry, 70(10), 992–999. 10.1016/j.biopsych.2011.01.009 [DOI] [PubMed] [Google Scholar]
- Estes ML, Prendergast K, MacMahon JA, Cameron S, Aboubechara JP, Farrelly K, Sell GL, Haapanen L, Schauer JD, Horta A, Shaffer IC, Le CT, Kincheloe GN, Tan DJ, van der List D, Bauman MD, Carter CS, Van de Water J, & McAllister AK (2020). Baseline immunoreactivity before pregnancy and poly(I:C) dose combine to dictate susceptibility and resilience of offspring to maternal immune activation. Brain Behav Immun, 88, 619–630. 10.1016/j.bbi.2020.04.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayuk D, & Yakel JL (2004). Regulation of nicotinic acetylcholine receptor channel function by acetylcholinesterase inhibitors in rat hippocampal CA1 interneurons. Mol Pharmacol, 66(3), 658–666. 10.1124/mol.104.000042 [DOI] [PubMed] [Google Scholar]
- Fazio F, Lionetto L, Curto M, Iacovelli L, Copeland CS, Neale SA, Bruno V, Battaglia G, Salt TE, & Nicoletti F (2017). Cinnabarinic acid and xanthurenic acid: Two kynurenine metabolites that interact with metabotropic glutamate receptors. Neuropharmacology, 112(Pt B), 365–372. 10.1016/j.neuropharm.2016.06.020 [DOI] [PubMed] [Google Scholar]
- Fernandez de Cossio L, Guzman A, van der Veldt S, & Luheshi GN (2017). Prenatal infection leads to ASD-like behavior and altered synaptic pruning in the mouse offspring. Brain Behav Immun, 63, 88–98. 10.1016/j.bbi.2016.09.028 [DOI] [PubMed] [Google Scholar]
- Foley KA, MacFabe DF, Kavaliers M, & Ossenkopp KP (2015). Sexually dimorphic effects of prenatal exposure to lipopolysaccharide, and prenatal and postnatal exposure to propionic acid, on acoustic startle response and prepulse inhibition in adolescent rats: relevance to autism spectrum disorders. Behav Brain Res, 278, 244–256. 10.1016/j.bbr.2014.09.032 [DOI] [PubMed] [Google Scholar]
- Forrest CM, Khalil OS, Pisar M, Darlington LG, & Stone TW (2013). Prenatal inhibition of the tryptophan-kynurenine pathway alters synaptic plasticity and protein expression in the rat hippocampus. Brain Res, 1504, 1–15. 10.1016/j.brainres.2013.01.031 [DOI] [PubMed] [Google Scholar]
- Forrest CM, Khalil OS, Pisar M, McNair K, Kornisiuk E, Snitcofsky M, Gonzalez N, Jerusalinsky D, Darlington LG, & Stone TW (2013). Changes in synaptic transmission and protein expression in the brains of adult offspring after prenatal inhibition of the kynurenine pathway. Neuroscience, 254, 241–259. 10.1016/j.neuroscience.2013.09.034 [DOI] [PubMed] [Google Scholar]
- Fricke EM, Elgin TG, Gong H, Reese J, Gibson-Corley KN, Weiss RM, Zimmerman K, Bowdler NC, Kalantera KM, Mills DA, Underwood MA, & McElroy SJ (2018). Lipopolysaccharide-induced maternal inflammation induces direct placental injury without alteration in placental blood flow and induces a secondary fetal intestinal injury that persists into adulthood. Am J Reprod Immunol, 79(5), e12816. 10.1111/aji.12816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeden N, Notarangelo FM, Pocivavsek A, Beggiato S, Bonnin A, & Schwarcz R (2017). Prenatal Dynamics of Kynurenine Pathway Metabolism in Mice: Focus on Kynurenic Acid. Dev Neurosci, 39(6), 519–528. 10.1159/000481168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez Esquivel D, Ramirez-Ortega D, Pineda B, Castro N, Rios C, & Perez de la Cruz V. (2017). Kynurenine pathway metabolites and enzymes involved in redox reactions. Neuropharmacology, 112(Pt B), 331–345. 10.1016/j.neuropharm.2016.03.013 [DOI] [PubMed] [Google Scholar]
- Gramsbergen JB, Hodgkins PS, Rassoulpour A, Turski WA, Guidetti P, & Schwarcz R (1997). Brain-specific modulation of kynurenic acid synthesis in the rat. J Neurochem, 69(1), 290–298. 10.1046/j.1471-4159.1997.69010290.x [DOI] [PubMed] [Google Scholar]
- Haddad FL, Patel SV, & Schmid S (2020). Maternal Immune Activation by Poly I:C as a preclinical Model for Neurodevelopmental Disorders: A focus on Autism and Schizophrenia. Neurosci Biobehav Rev, 113, 546–567. 10.1016/j.neubiorev.2020.04.012 [DOI] [PubMed] [Google Scholar]
- Janssens S, & Beyaert R (2003). Role of Toll-like receptors in pathogen recognition. Clin Microbiol Rev, 16(4), 637–646. 10.1128/cmr.16.4.637-646.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil OS, Pisar M, Forrest CM, Vincenten MC, Darlington LG, & Stone TW (2014). Prenatal inhibition of the kynurenine pathway leads to structural changes in the hippocampus of adult rat offspring. Eur J Neurosci, 39(10), 1558–1571. 10.1111/ejn.12535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkbride JB, Susser E, Kundakovic M, Kresovich JK, Davey Smith G, & Relton CL (2012). Prenatal nutrition, epigenetics and schizophrenia risk: can we test causal effects? Epigenomics, 4(3), 303–315. 10.2217/epi.12.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo SM (2016). Gender Difference in Bacteria Endotoxin-Induced Inflammatory and Anorexic Responses. PLoS One, 11(9), e0162971. 10.1371/journal.pone.0162971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhan SE, Caro M, & Hadzimichalis N (2013). NMDA Receptor Activity in Neuropsychiatric Disorders. Front Psychiatry, 4, 52. 10.3389/fpsyt.2013.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin PB, Sathyasaikumar KV, Notarangelo FM, Funakoshi H, Nakamura T, Schwarcz R, & Muchowski PJ (2016). Tryptophan 2,3-dioxygenase and indoleamine 2,3-dioxygenase 1 make separate, tissue-specific contributions to basal and inflammation-induced kynurenine pathway metabolism in mice. Biochim Biophys Acta, 1860(11 Pt A), 2345–2354. 10.1016/j.bbagen.2016.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson MK, Faka A, Bhat M, Imbeault S, Goiny M, Orhan F, Oliveros A, Stahl S, Liu XC, Choi DS, Sandberg K, Engberg G, Schwieler L, & Erhardt S (2016). Repeated LPS Injection Induces Distinct Changes in the Kynurenine Pathway in Mice. Neurochem Res, 41(9), 2243–2255. 10.1007/s11064-016-1939-4 [DOI] [PubMed] [Google Scholar]
- Lestage J, Verrier D, Palin K, & Dantzer R (2002). The enzyme indoleamine 2,3-dioxygenase is induced in the mouse brain in response to peripheral administration of lipopolysaccharide and superantigen. Brain Behav Immun, 16(5), 596–601. 10.1016/s0889-1591(02)00014-4 [DOI] [PubMed] [Google Scholar]
- Lin YL, & Wang S (2014). Prenatal lipopolysaccharide exposure increases depression-like behaviors and reduces hippocampal neurogenesis in adult rats. Behav Brain Res, 259, 24–34. 10.1016/j.bbr.2013.10.034 [DOI] [PubMed] [Google Scholar]
- Linderholm KR, Skogh E, Olsson SK, Dahl ML, Holtze M, Engberg G, Samuelsson M, & Erhardt S (2012). Increased levels of kynurenine and kynurenic acid in the CSF of patients with schizophrenia. Schizophr Bull, 38(3), 426–432. 10.1093/schbul/sbq086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, & Randall RJ (1951). Protein measurement with the Folin phenol reagent. J Biol Chem, 193(1), 265–275. https://www.ncbi.nlm.nih.gov/pubmed/14907713 [PubMed] [Google Scholar]
- Manuelpillai U, Ligam P, Smythe G, Wallace EM, Hirst J, & Walker DW (2005). Identification of kynurenine pathway enzyme mRNAs and metabolites in human placenta: up-regulation by inflammatory stimuli and with clinical infection. Am J Obstet Gynecol, 192(1), 280–288. 10.1016/j.ajog.2004.06.090 [DOI] [PubMed] [Google Scholar]
- Maynard TM, Sikich L, Lieberman JA, & LaMantia AS (2001). Neural development, cell-cell signaling, and the “two-hit” hypothesis of schizophrenia. Schizophr Bull, 27(3), 457–476. 10.1093/oxfordjournals.schbul.a006887 [DOI] [PubMed] [Google Scholar]
- Meyer U, & Feldon J (2010). Epidemiology-driven neurodevelopmental animal models of schizophrenia. Prog Neurobiol, 90(3), 285–326. 10.1016/j.pneurobio.2009.10.018 [DOI] [PubMed] [Google Scholar]
- Meyer U, & Feldon J (2012). To poly(I:C) or not to poly(I:C): advancing preclinical schizophrenia research through the use of prenatal immune activation models. Neuropharmacology, 62(3), 1308–1321. 10.1016/j.neuropharm.2011.01.009 [DOI] [PubMed] [Google Scholar]
- Migale R, Herbert BR, Lee YS, Sykes L, Waddington SN, Peebles D, Hagberg H, Johnson MR, Bennett PR, & MacIntyre DA (2015). Specific Lipopolysaccharide Serotypes Induce Differential Maternal and Neonatal Inflammatory Responses in a Murine Model of Preterm Labor. Am J Pathol, 185(9), 2390–2401. 10.1016/j.ajpath.2015.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett JR, Arun P, Puthillathu N, Vengilote R, Ives JA, Badawy AA, & Namboodiri AM (2020). Quinolinate as a Marker for Kynurenine Metabolite Formation and the Unresolved Question of NAD(+) Synthesis During Inflammation and Infection. Front Immunol, 11, 31. 10.3389/fimmu.2020.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y, & Saito K (2013). Species and cell types difference in tryptophan metabolism. Int J Tryptophan Res, 6(Suppl 1), 47–54. 10.4137/IJTR.S11558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthi P, Wallace EM, & Walker DW (2017). Altered placental tryptophan metabolic pathway in human fetal growth restriction. Placenta, 52, 62–70. 10.1016/j.placenta.2017.02.013 [DOI] [PubMed] [Google Scholar]
- Notarangelo FM, Beggiato S, & Schwarcz R (2019). Assessment of Prenatal Kynurenine Metabolism Using Tissue Slices: Focus on the Neosynthesis of Kynurenic Acid in Mice. Dev Neurosci, 41(1–2), 102–111. 10.1159/000499736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notarangelo FM, & Pocivavsek A (2017). Elevated kynurenine pathway metabolism during neurodevelopment: Implications for brain and behavior. Neuropharmacology, 112(Pt B), 275–285. 10.1016/j.neuropharm.2016.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notarangelo FM, Wu HQ, Macherone A, Graham DR, & Schwarcz R (2012). Gas chromatography/tandem mass spectrometry detection of extracellular kynurenine and related metabolites in normal and lesioned rat brain. Anal Biochem, 421(2), 573–581. 10.1016/j.ab.2011.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N, Kelley KW, & Dantzer R (2009). Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry, 14(5), 511–522. 10.1038/sj.mp.4002148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogyu K, Kubo K, Noda Y, Iwata Y, Tsugawa S, Omura Y, Wada M, Tarumi R, Plitman E, Moriguchi S, Miyazaki T, Uchida H, Graff-Guerrero A, Mimura M, & Nakajima S (2018). Kynurenine pathway in depression: A systematic review and meta-analysis. Neurosci Biobehav Rev, 90, 16–25. 10.1016/j.neubiorev.2018.03.023 [DOI] [PubMed] [Google Scholar]
- Olincy A, & Freedman R (2012). Nicotinic mechanisms in the treatment of psychotic disorders: a focus on the alpha7 nicotinic receptor. Handb Exp Pharmacol(213), 211–232. 10.1007/978-3-642-25758-2_8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskvig DB, Elkahloun AG, Johnson KR, Phillips TM, & Herkenham M (2012). Maternal immune activation by LPS selectively alters specific gene expression profiles of interneuron migration and oxidative stress in the fetus without triggering a fetal immune response. Brain Behav Immun, 26(4), 623–634. 10.1016/j.bbi.2012.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa K, Hashimoto K, Kishimoto T, Shimizu E, Ishikura H, & Iyo M (2006). Immune activation during pregnancy in mice leads to dopaminergic hyperfunction and cognitive impairment in the offspring: a neurodevelopmental animal model of schizophrenia. Biol Psychiatry, 59(6), 546–554. 10.1016/j.biopsych.2005.07.031 [DOI] [PubMed] [Google Scholar]
- Park BS, & Lee JO (2013). Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp Mol Med, 45, e66. 10.1038/emm.2013.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott JM, Redus L, & O’Connor JC (2016). Kynurenine metabolic balance is disrupted in the hippocampus following peripheral lipopolysaccharide challenge. J Neuroinflammation, 13(1), 124. 10.1186/s12974-016-0590-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pershing ML, Bortz DM, Pocivavsek A, Fredericks PJ, Jorgensen CV, Vunck SA, Leuner B, Schwarcz R, & Bruno JP (2015). Elevated levels of kynurenic acid during gestation produce neurochemical, morphological, and cognitive deficits in adulthood: implications for schizophrenia. Neuropharmacology, 90, 33–41. 10.1016/j.neuropharm.2014.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pershing ML, Phenis D, Valentini V, Pocivavsek A, Lindquist DH, Schwarcz R, & Bruno JP (2016). Prenatal kynurenine exposure in rats: age-dependent changes in NMDA receptor expression and conditioned fear responding. Psychopharmacology (Berl), 233(21–22), 3725–3735. 10.1007/s00213-016-4404-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piirsalu M, Taalberg E, Lillevali K, Tian L, Zilmer M, & Vasar E (2020). Treatment With Lipopolysaccharide Induces Distinct Changes in Metabolite Profile and Body Weight in 129Sv and Bl6 Mouse Strains. Front Pharmacol, 11, 371. 10.3389/fphar.2020.00371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisar M, Forrest CM, Khalil OS, McNair K, Vincenten MC, Qasem S, Darlington LG, & Stone TW (2014). Modified neocortical and cerebellar protein expression and morphology in adult rats following prenatal inhibition of the kynurenine pathway. Brain Res, 1576, 1–17. 10.1016/j.brainres.2014.06.016 [DOI] [PubMed] [Google Scholar]
- Pocivavsek A, Elmer GI, & Schwarcz R (2019). Inhibition of kynurenine aminotransferase II attenuates hippocampus-dependent memory deficit in adult rats treated prenatally with kynurenine. Hippocampus, 29(2), 73–77. 10.1002/hipo.23040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocivavsek A, Notarangelo FM, Wu HQ, Bruno JP, & Schwarcz R (2016). Astrocytes as pharmacological targets in the treatment of schizophrenia: Focus on kynurenic acid. Modeling the Psychopathological Dimensions of Schizophrenia. San Diego, CA, USA: Elsevier, 423–443. 10.1016/B978-0-12-800981-9.00025-0 [DOI] [Google Scholar]
- Pocivavsek A, Thomas MA, Elmer GI, Bruno JP, & Schwarcz R (2014). Continuous kynurenine administration during the prenatal period, but not during adolescence, causes learning and memory deficits in adult rats. Psychopharmacology (Berl), 231(14), 2799–2809. 10.1007/s00213-014-3452-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport JL, Addington AM, Frangou S, & Psych MR (2005). The neurodevelopmental model of schizophrenia: update 2005. Mol Psychiatry, 10(5), 434–449. 10.1038/sj.mp.4001642 [DOI] [PubMed] [Google Scholar]
- Romero E, Ali C, Molina-Holgado E, Castellano B, Guaza C, & Borrell J (2007). Neurobehavioral and immunological consequences of prenatal immune activation in rats. Influence of antipsychotics. Neuropsychopharmacology, 32(8), 1791–1804. 10.1038/sj.npp.1301292 [DOI] [PubMed] [Google Scholar]
- Saito K, Markey SP, & Heyes MP (1992). Effects of immune activation on quinolinic acid and neuroactive kynurenines in the mouse. Neuroscience, 51(1), 25–39. 10.1016/0306-4522(92)90467-g [DOI] [PubMed] [Google Scholar]
- Sathyasaikumar KV, Stachowski EK, Wonodi I, Roberts RC, Rassoulpour A, McMahon RP, & Schwarcz R (2011). Impaired kynurenine pathway metabolism in the prefrontal cortex of individuals with schizophrenia. Schizophr Bull, 37(6), 1147–1156. 10.1093/schbul/sbq112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarcz R, Bruno JP, Muchowski PJ, & Wu HQ (2012). Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci, 13(7), 465–477. 10.1038/nrn3257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarcz R, Rassoulpour A, Wu HQ, Medoff D, Tamminga CA, & Roberts RC (2001). Increased cortical kynurenate content in schizophrenia. Biol Psychiatry, 50(7), 521–530. 10.1016/s0006-3223(01)01078-2 [DOI] [PubMed] [Google Scholar]
- Schwarcz R, & Stone TW (2017). The kynurenine pathway and the brain: Challenges, controversies and promises. Neuropharmacology, 112(Pt B), 237–247. 10.1016/j.neuropharm.2016.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman LJ, Buka SL, Goldstein JM, Horton NJ, Rieder RO, & Tsuang MT (2000). The relationship of prenatal and perinatal complications to cognitive functioning at age 7 in the New England Cohorts of the National Collaborative Perinatal Project. Schizophr Bull, 26(2), 309–321. 10.1093/oxfordjournals.schbul.a033455 [DOI] [PubMed] [Google Scholar]
- Simoes LR, Sangiogo G, Tashiro MH, Generoso JS, Faller CJ, Dominguini D, Mastella GA, Scaini G, Giridharan VV, Michels M, Florentino D, Petronilho F, Reus GZ, Dal-Pizzol F, Zugno AI, & Barichello T (2018). Maternal immune activation induced by lipopolysaccharide triggers immune response in pregnant mother and fetus, and induces behavioral impairment in adult rats. J Psychiatr Res, 100, 71–83. 10.1016/j.jpsychires.2018.02.007 [DOI] [PubMed] [Google Scholar]
- Smith SE, Li J, Garbett K, Mirnics K, & Patterson PH (2007). Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci, 27(40), 10695–10702. 10.1523/JNEUROSCI.2178-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Clark SM, Vaughn CN, Nicholson JD, Murphy KJ, Mou TM, Schwarcz R, Hoffman GE, & Tonelli LH (2018). Quantitative Analysis of Kynurenine Aminotransferase II in the Adult Rat Brain Reveals High Expression in Proliferative Zones and Corpus Callosum. Neuroscience, 369, 1–14. 10.1016/j.neuroscience.2017.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolp H, Neuhaus A, Sundramoorthi R, & Molnar Z (2012). The Long and the Short of it: Gene and Environment Interactions During Early Cortical Development and Consequences for Long-Term Neurological Disease. Front Psychiatry, 3, 50. 10.3389/fpsyt.2012.00050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stower H (2019). Maternal infection linked to psychiatric disorders. Nat Med, 25(4), 541. 10.1038/s41591-019-0430-6 [DOI] [PubMed] [Google Scholar]
- Suzuki S, Tone S, Takikawa O, Kubo T, Kohno I, & Minatogawa Y (2001). Expression of indoleamine 2,3-dioxygenase and tryptophan 2,3-dioxygenase in early concepti. Biochem J, 355(Pt 2), 425–429. 10.1042/0264-6021:3550425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao X, Yan M, Wang L, Zhou Y, Wang Z, Xia T, Liu X, Pan R, & Chang Q (2020). Homeostasis Imbalance of Microglia and Astrocytes Leads to Alteration in the Metabolites of the Kynurenine Pathway in LPS-Induced Depressive-Like Mice. Int J Mol Sci, 21(4). 10.3390/ijms21041460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AK, Budac DP, Bisulco S, Lee AW, Smith RA, Beenders B, Kelley KW, & Dantzer R (2013). NMDA receptor blockade by ketamine abrogates lipopolysaccharide-induced depressive-like behavior in C57BL/6J mice. Neuropsychopharmacology, 38(9), 1609–1616. 10.1038/npp.2013.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DW, Curtis B, Lacey B, & Nitsos I (1999). Kynurenic acid in brain and cerebrospinal fluid of fetal, newborn, and adult sheep and effects of placental embolization. Pediatr Res, 45(6), 820–826. 10.1203/00006450-199906000-00007 [DOI] [PubMed] [Google Scholar]
- Williams M, Zhang Z, Nance E, Drewes JL, Lesniak WG, Singh S, Chugani DC, Rangaramanujam K, Graham DR, & Kannan S (2017). Maternal Inflammation Results in Altered Tryptophan Metabolism in Rabbit Placenta and Fetal Brain. Dev Neurosci, 39(5), 399–412. 10.1159/000471509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WL, Adams CE, Stevens KE, Chow KH, Freedman R, & Patterson PH (2015). The interaction between maternal immune activation and alpha 7 nicotinic acetylcholine receptor in regulating behaviors in the offspring. Brain Behav Immun, 46, 192–202. 10.1016/j.bbi.2015.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavitsanou K, Lim CK, Purves-Tyson T, Karl T, Kassiou M, Banister SD, Guillemin GJ, & Weickert CS (2014). Effect of maternal immune activation on the kynurenine pathway in preadolescent rat offspring and on MK801-induced hyperlocomotion in adulthood: amelioration by COX-2 inhibition. Brain Behav Immun, 41, 173–181. 10.1016/j.bbi.2014.05.011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: KP metabolite levels in the placenta 4 and 24 h after saline or LPS administration (0.1 mg/kg, i.p.) on GD15. Data are the mean ± SEM (n = 3–4).
Supplemental Figure 2: KP metabolite levels in the fetal brain of male and female embryos 4 and 24 h after saline or LPS administration (0.1 mg/kg, i.p.) on GD15. Data are the mean ± SEM (n = 2–4). * p < 0.05, ** p < 0.01 (Two-way Anova, followed by Bonferroni’s post-hoc test).
Data Availability Statement
Data will be made available upon reasonable request to the corresponding author.


