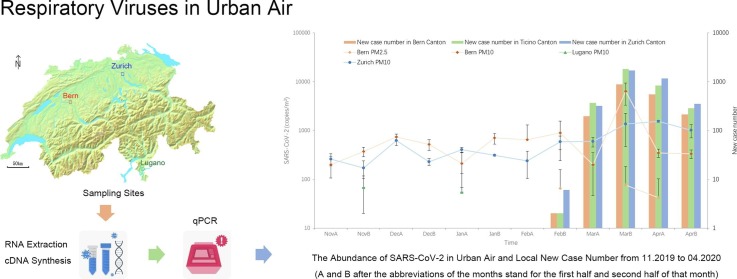
Graphical abstract
Keywords: SARS-CoV-2, COVID-19, Airborne respiratory virus, Bioaerosol, Air pollution, Surveillance
Abstract
Caused by the SARS-CoV-2 virus, Coronavirus disease 2019 (COVID-19) has been affecting the world since the end of 2019. While virus-laden particles have been commonly detected and studied in the aerosol samples from indoor healthcare settings, studies are scarce on air surveillance of the virus in outdoor non-healthcare environments, including the correlations between SARS-CoV-2 and other respiratory viruses, between viruses and environmental factors, and between viruses and human behavior changes due to the public health measures against COVID-19. Therefore, in this study, we collected airborne particulate matter (PM) samples from November 2019 to April 2020 in Bern, Lugano, and Zurich. Among 14 detected viruses, influenza A, HCoV-NL63, HCoV-HKU1, and HCoV-229E were abundant in air. SARS-CoV-2 and enterovirus were moderately common, while the remaining viruses occurred only in low concentrations. SARS-CoV-2 was detected in PM10 (PM below 10 µm) samples of Bern and Zurich, and PM2.5 (PM below 2.5 µm) samples of Bern which exhibited a concentration positively correlated with the local COVID-19 case number. The concentration was also correlated with the concentration of enterovirus which raised the concern of coinfection. The estimated COVID-19 infection risks of an hour exposure at these two sites were generally low but still cannot be neglected. Our study demonstrated the potential functionality of outdoor air surveillance of airborne respiratory viruses, especially at transportation hubs and traffic arteries.
1. Introduction
It has been more than two years since the onset of the Coronavirus Disease 2019 (COVID-19) caused by an enveloped, single-stranded, positive-sense RNA betacoronavirus, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) first being reported in Wuhan, China in December 2019. Unfortunately, even with the rapidly developed vaccines and drugs, most parts of the world are still facing severe risks evidenced by the infection wave in the spring of 2022 mainly caused by Omicron variants. The severity of this pandemic has far exceeded the other two large-scale epidemics caused by severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East Respiratory Syndrome coronavirus (MERS-CoV) in the last two decades.
The possible transmission paths for viral respiratory infectious diseases between humans are mainly through direct (person-to-person) and indirect (via fomites) contact (Morawska and Cao 2020). In addition, respiratory viruses could be present in particulate matter (PM), with aerosols suggested as the third transmission mechanism (Setti et al. 2020a). Notably, 239 scientists from 32 countries wrote an open letter to the World Health Organization (WHO) emphasizing the importance of preventing the airborne transmission of COVID-19 (Morawska and Milton 2020).
Thus, surveillance of virus-laden aerosols is suitable for monitoring this path and assessing qualitative and quantitative molecular epidemiological information on population exposure to SARS-CoV-2 (Anand et al. 2021). So far, many studies have detected the presence of SARS-CoV-2 RNA in indoor aerosols, mainly in hospitals (Ang et al., 2022, Baboli et al., 2021, Barbieri et al., 2021, Bazzazpour et al., 2021, Cheng et al., 2020, Chia et al., 2020, Ding et al., 2021, Dumont-Leblond et al., 2020, Feng et al., 2021, Ghaffari et al., 2021, Guo et al., 2020, Habibi et al., 2021, Hemati et al., 2021, Kenarkoohi et al., 2020, Lednicky et al., 2020a, Lednicky et al., 2020b; Liu et al., 2020, Lopez et al., 2021, Nor et al., 2021, Passos et al., 2021, Razzini et al., 2020, Stern et al., 2021a, Stern et al., 2021b; Tan et al., 2020, Yarahmadi et al., 2021, Zhou et al., 2021), as well as in residential rooms (Nannu Shankar et al. 2022), transportation (Hadei et al., 2021, Lednicky et al., 2021, Moreno et al., 2021) and other public indoor places (Hadei et al. 2021). Some further detected the presence of SARS-CoV-2 nucleocapsids (Krambrich et al. 2021) or even viable viruses (Lednicky et al., 2020a, Lednicky et al., 2021). However, there are much fewer studies focusing on outdoor aerosols (Chirizzi et al., 2021, Dunker et al., 2021, Kayalar et al., 2021, Linillos-Pradillo et al., 2021, Passos et al., 2021, Pivato et al., 2021, Senatore et al., 2021, Setti et al., 2020b), and even fewer which successfully detected the viral RNA (Kayalar et al., 2021, Liu et al., 2020, Setti et al., 2020b, Stern et al., 2021a). This is understandable due to the rapid dilution in outdoor air, and more complex and variable environmental conditions. However, the lack of outdoor studies has left several gaps in our understating of the behaviors of aerosolized viruses and their interactions with the surrounding environment:
First of all, most outdoor studies only focused on the viral RNA detection in the air outside hospitals (Kayalar et al., 2021, Liu et al., 2020, Stern et al., 2021a). The viral concentrations away from hospitals are still largely unknown. How the concentration of SARS-CoV-2 in air is influenced by human activities, such as the rising of infection cases, public health measures and lockdowns, also needs to be studied.
Secondly, the relationship between SARS-CoV-2 and other respiratory viruses in aerosols is also largely unknown. So far, coinfections have been reported between SARS-CoV-2 and influenza A (Dadashi et al. 2021), influenza B (Dadashi et al. 2021), human parainfluenza virus (HPIV1) 1 (Boschiero et al. 2022), HPIV2 (Boschiero et al. 2022), HPIV3 (Boschiero et al. 2022), HPIV4 (Rodriguez et al. 2020), human coronaviruses (HCoV) NL63 (Pigny et al. 2021), HCoV-OC43 (Boschiero et al. 2022), HCoV-HKU1 (Boschiero et al. 2022), HCoV-229E (Lau et al. 2021), human respiratory syncytial virus (HRSV) (Jiang et al. 2020), human metapneumovirus (HMPV) (Jiang et al., 2020, Pigny et al., 2021), adenovirus (Pigny et al. 2021), and picornaviruses which include rhinovirus (Boschiero et al., 2022, De Francesco et al., 2021, Pigny et al., 2021) and enterovirus (Boschiero et al. 2022). Moreover, lockdowns and other measures including mass masking and universal hygiene also seriously impacted other infectious respiratory diseases causing a significant decline of infections during the pandemic (Chiapinotto et al., 2021, Hsieh et al., 2020). Incidence numbers of influenza A and B, HMPV, HPIV, HRSV and other HCoV decreased or disappeared during the pandemic (De Francesco et al., 2021, Diesner-Treiber et al., 2021). Due to the control measures, seasonal epidemics of influenza, HRSV and HMPV either ended earlier (Kuitunen, 2021, Redlberger-Fritz et al., 2021), were delayed (Delestrain et al., 2021, Williams et al., 2021) or even prevented (Kuitunen 2021). In contrast, the case number of adenovirus did not decrease significantly (De Francesco et al. 2021), while Rhinovirus cases were commonly observed to be increased together with SARS-CoV-2 cases (De Francesco et al., 2021, Haapanen et al., 2021, Redlberger-Fritz et al., 2021). These two along with enterovirus and HCoV-NL63 were also commonly observed in children in 2020–2021 winter (Diesner-Treiber et al. 2021). Thus, it is of interest to investigate if their concentrations in air followed the same trends as in patients, which may imply the role of aerosol transmission of these viruses.
Lastly, outdoor air conditions including wind speed, temperature, humidity, UV radiation, seasonal allergens such as pollens and spores and air pollutants such as PM, sulfur dioxides (SO2), ozone (O3) and nitrogen oxides (NOx) may influence the survival of the airborne viruses. Though the impacts of temperature, humidity and sunlight on aerosolized viruses, COVID-19 cases and mortality have been studied (Ahlawat et al., 2020, Dabisch et al., 2021; Huang et al., 2020, Schuit et al., 2020) and better air quality due to less emissions during the lockdowns has been observed worldwide (Grange et al., 2021, Wang et al., 2022), the research regarding the effect of seasonal allergens and air pollutants has only focused on the latter two parameters, not on aerosolized virus. For example, one study observed that a high airborne pollen concentration was associated with an increased SARS-CoV-2 infection rate (Damialis et al. 2021), and more studies found an association between the exposure to poor air quality and COVID-19 incidence or mortality around the world including in Austria (Hutter et al. 2020), China (Pansini and Fornacca, 2021, van der Valk, 2021, Wang et al., 2020), France (Ogen, 2020, Pansini and Fornacca, 2021), Germany (Ogen 2020), India (Kulkarni et al., 2021, Rathod and Beig, 2021), Iran (Pansini and Fornacca, 2021, van der Valk, 2021), Italy (Coccia, 2020, Dragone et al., 2021, Ogen, 2020, Pansini and Fornacca, 2021, van der Valk, 2021), Peru (Vasquez-Apestegui et al., 2021, Wannaz et al., 2021), Singapore(Lorenzo et al. 2021), Spain ( Marquès et al., 2021, Ogen, 2020, Zoran et al., 2021), UK (Pansini and Fornacca 2021), and the US (Gujral and Sinha, 2021, Pansini and Fornacca, 2021, Razzaq et al., 2020, van der Valk, 2021, Meo et al., 2021). This association was also found to apply to other airborne viruses, such as influenza (Chen et al., 2018, Chen et al., 2017, Croft et al., 2019, Xu et al., 2013), measles (Peng et al. 2020), HRSV (Ye et al. 2016), rhinovirus (Rodrigues et al. 2019) and SARS-CoV (Kan et al. 2005). To explain these statistical findings, many hypothesized mechanisms have been proposed: pollutant exposure may alter viral entry and surface levels of attachment receptors, regulate pathogen-sensing mechanisms, influence viral replication and antiviral interferon production, interfere with mitochondrial function and autophagy, impact macrophage and natural killer cell function, alter adaptive immune responses, enhance related immunopathology including virus-induced tissue damage and inflammation(Woodby et al. 2021), and exacerbate comorbidities, such as hypertension, diabetes, and coronary artery diseases(Sharma and Balyan 2020). Another potential but controversial mechanism is that the particulate matter can act as a ‘carrier’ for viral droplet nuclei, facilitating viral survival in the air, promoting atmospheric transport, and causing further spread of viral infection (Ma et al., 2017, Sedlmaier et al., 2009, Sørensen et al., 2000; Zhao et al. 2019b). Some studies have indicated that the absorption to particulate matter prolongs viral infectivity (Cruz-Sanchez et al., 2013, Gerba and Stagg, 1979). However, the conjugation to particles may inhibit syncytia formation and infection rate (Cruz-Sanchez et al. 2013), and pollutants such as NO2 and O3 are common disinfectants which can inactivate viruses (Jiang et al., 2019, Murray et al., 2008, Shomali et al., 2015, Tseng and Li, 2006).
To fill these gaps, a field study is urgently needed to reveal the relationships between aerosolized SARS-CoV-2 and human activities, between SARS-CoV-2 and other airborne viruses, and between aerosolized viruses and air pollutants. In addition, we would also like to evaluate the infection risk induced by exposure to these respiratory viruses in aerosol outdoors. Thus, in this study, we collected aerosol samples from three sites in different environmental settings in Switzerland before and during the first wave of the COVID-19 pandemic, and measured concentrations of 16 different respiratory viruses all of which can cause influenza-like symptoms, lower respiratory infections and cardiopulmonary complications (Ackerson et al., 2019, Shi et al., 2017, Troeger et al., 2017), with quantitative polymerase chain reaction (qPCR), trying to improve understanding of the above questions.
2. Materials and methods
2.1. Sample collection and pretreatment
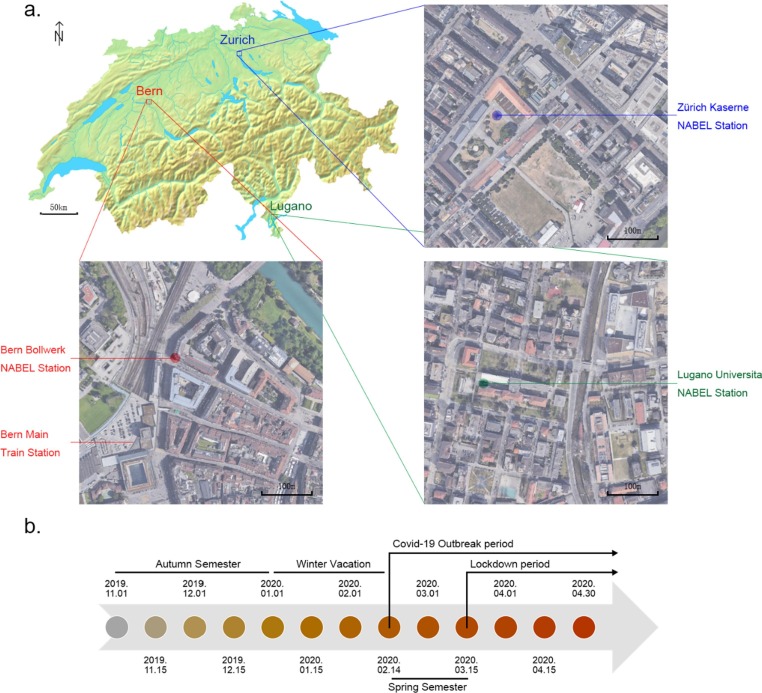
Fine particle samples, which have diameters that are 2.5 μm and smaller (PM2.5) and coarse particle samples, which have diameters that are 10 μm and smaller (PM10) were collected from three sites of the Swiss National Air Pollution Monitoring Network (NABEL) (Fig. 1 ) from November 2019 to April 2020. The three sites were Bern-Bollwerk (a curbside site next to Bern main train station), Lugano –Università (a site on the university campus), Zürich Kaserne (a courtyard site in the city center, 500 m away from Zurich main train station, mainly surrounded by residential areas with high population density). The detailed sampling procedure has been described in previous NABEL studies (Gianini et al., 2012, Hueglin et al., 2005). Briefly speaking, aerosol samples were collected every 4th day for PM2.5, and every 2nd day for PM10 on quartz fiber filters (Pallflex Tissuquartz QAT-2500-Up, Ø = 150 mm) on a 24-hour basis using high-volume samplers (Digitel DA-80H, 30 m3/h flow rate) equipped with respective PM size selective inlets and stored at − 80 ℃ before further analysis.
Fig. 1.
The sampling locations (a) and time periods (b).
With a punch of 25 mm in diameter, 8 pieces were taken from every filter, treated with 750 μL 75% ethanol to deactivate potential viruses and stored at −20 ℃ before further analysis. We pooled seven random pieces from every half month together. They were cut into fragments, put into a 15 ml centrifuge tube with 3 ml phosphate-buffered saline buffer (PBS), vortexed for 30 min and centrifuged for 10 min to extract aliquots.
2.2. RNA extraction, cDNA synthesis and real-time quantitative PCR
RNA was extracted into a 140 μL aliquot for every pooled sample with QIAamp viral RNA Mini kit (Mo Bio, Qiagen, Germany) according to the manufacturer’s protocol. We used 8 μL extracted RNA for cDNA synthesis with SuperScript™ III First-Strand Synthesis SuperMix for qRT-PCR (Thermo Fisher Scientific, USA) according to manufacturer’s protocol. The quality and quantity of the extracted DNA were determined by gel electrophoresis and an Infinite® 200 PRO plate reader (TECAN, Switzerland).
The presence of viral RNA in the samples was first detected by PCR. All PCR products were used for making standard plasmids used in qPCR, except for SARS-CoV-2 and HCoV-229E, of which standard plasmids were made from PCR products of SARS-CoV-2-positive clinical samples and lab cultures of HCoV-229E (ATCCVR-740TM, USA) as detailed in our previous study(Tao et al. 2022). The PCR reaction mixture was 20 μL, containing 1 μL of DNA template, 14.92 μL of ddH2O, while the volumes of other components were proportional according to the protocol of Taq DNA Polymerase, recombinant (Life Technologies, Thermo Fisher, USA). The primers of 16 target respiratory viruses and primer melting temperatures (Tm) are listed in SI. 1 Table 1. The PCR products were run on 2% agarose gel electrophoresis to detect the presence of the target genes. The products were purified with a QIAquick®gel extraction kit (Qiagen, Germany). The purified genes were cloned into E.coli JM109 with pGEM-T Easy vector system (Promega, USA). Positive clones were randomly selected by blue-white screening method, and then cultivated and checked by PCR. The plasmids were extracted with a Qiaprep spin miniprep kit (Qiagen, Germany) to serve as the standard plasmids for qPCR. The concentration of the extracted plasmids was quantified by Infinite® 200 PRO plate reader (TECAN, Switzerland).
All target genes were quantified by qPCR on a CFX96 Touch™Real-Time PCR Detection System (BioRad, USA) using SYBR Green I approach. The reaction mixture of qPCR was 10 μL, containing 5 μL SsoAdvanced Universal SYBR Green supermix (BioRad, USA), 0.25 μL of each primer, 0.5 μL of template and 4 μL of ddH2O. The primers and Tm values were the same as for PCR (SI. 1 Table 1). Purity of the qPCR products was checked using the melting curve method. All measurements were conducted in triplicates. The amount of each target gene was calculated based on the corresponding standard curve which was set up with tenfold serial dilution with the above-mentioned plasmids carrying the corresponding genes.
2.3. The acquisition of environmental information and statistical analyses
The data of environmental factors including O3, nitrogen dioxide (NO2), SO2, carbon monoxide (CO), PM10, PM2.5, elemental carbon (EC) in PM2.5, particle number concentration (PNC), non-methane volatile organic compounds (NMVOC), NOx, temperature (T), precipitation (PREC) and radiation (RAD) of the studied period were downloaded from the Swiss Federal office of the Environment (FOEN)’s website (FOEN. 2021). Daily new case numbers of the studied cantons and date were downloaded from the Swiss Federal office of Public Health (FOPH)’s website (FOPH. 2021). Current population of the studied cantons were downloaded from the Swiss Federal Statistical office (FSO)’s website (FSO. 2021). The related figures, Fig. 3 and SI. 1 Fig. 1 were drawn with Microsoft Excel 2016. The average values, standard deviations of all data including both environmental factors and viral abundances, and the linear regression of the standard curve were determined with Microsoft Excel 2016. All data were added 1 and then logarithmized for normalization.
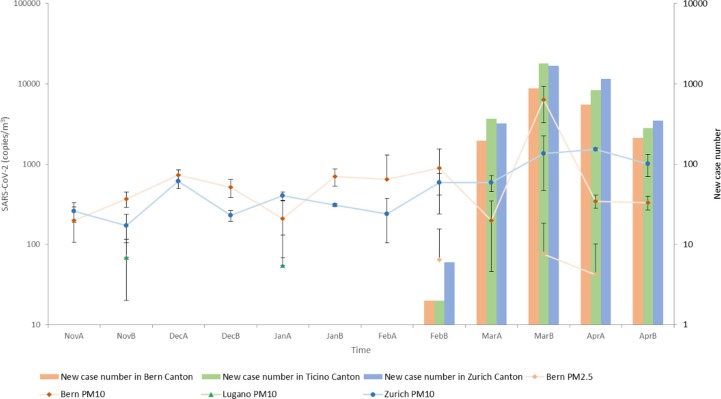
Fig. 3.
The abundance of SARS-CoV-2 and new case number in the local cantons (A and B after the abbreviations of the months stand for the first half and second half of that month).
Shapiro-Wilk test was performed as the normality test of all qPCR results of different cities, PM fractions and collection periods (Fig. 1). For the ones which passed the normality test, Paired Student’s t test was used to assess the abundance differences between PM2.5 and PM10. Otherwise, randomization test on matched samples was performed. Friedman test and its post-hoc analysis were used to examine the abundance differences among the three cities and of different viruses. To evaluate the temporal abundance difference, randomization test was used for the ones which passed the normality test; for the others, F test was firstly used to determine the homogeneity of the variances, and independent two-sample Student’s T Test with equal or unequal variance assumption was used accordingly. All F tests and Student’s T tests were performed in Microsoft Excel 2016, while other tests were performed in Rstudio (v3.6.0) with the ‘stats’ and the’PMCMR’ package (RStudio Team, 2018, Pohlert, 2014).
All figures except Fig. 1, Fig. 3 and SI. 1 Fig. 1 were drawn with Rstudio (v3.6.0)(RStudioTeam 2018). The heat map Fig. 2 was drawn with the ‘pheatmap’ package (Kolde 2010). The principal component analysis (PCA) analysis was conducted with the ‘ggplot2’ (Wickham 2011) and the ‘ggord’ package (Beck 2017). A correlation between the abundances of two target genes or environmental factors was considered statistically robust if Spearman's correlation coefficient (r) was > 0.3 and the P-value was < 0.05. The robust pairwise correlations of the target genes formed their co-occurrence networks using the ‘psych’ (Revelle 2018) and ‘igraph’ package (Csardi and Nepusz, 2006).
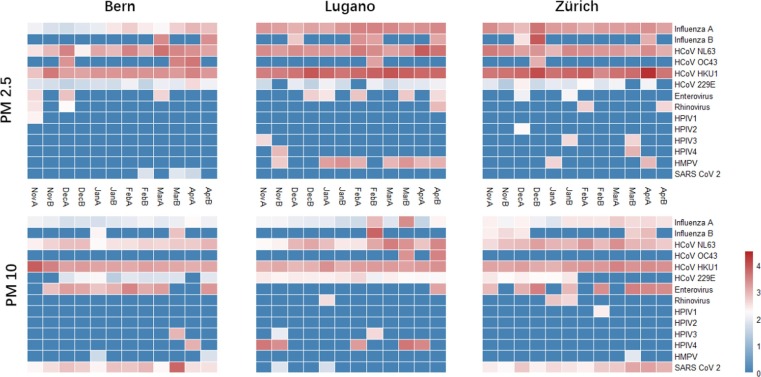
Fig. 2.
The absolute abundance of viruses in all air samples (log10(copies/m3 + 1), A and B after the abbreviations of the months stand for the first half and second half of that month).
2.4. Risk assessment based on a dose–response model
A Beta-Poisson model developed for SARS-CoV, HCoV-229E and influenza A (Watanabe et al. 2012, 2010) was used for the infection risk assessment in this study. The model is expressed as:
where p(d) is the infection risk at a dose of d and parameters N 50 and α in the beta-Poisson model are the median dose to trigger the response and the exponential fitting parameter based on the experiments challenging humans or mice with SARS-CoV (Watanabe et al. 2010), HCoV-229E (Watanabe et al. 2010), and influenza A (Watanabe et al. 2012)(SI. 1 Table. 2). The dose d was estimated by.
where Nresp is the total number of respirations (20 times per minute), Vtidal is the tidal volume (500 ml per respiration), λ is the average viable rate of the virus based on previous field studies ( Ang et al., 2022, Dumont-Leblond et al., 2020, Lednicky et al., 2020a, Lednicky et al., 2020b, Lednicky et al., 2021; Nannu Shankar et al., 2022, Xie et al., 2020), listed in SI. 1 Table. 2. cair,i(t) is the airborne virus concentration carried by the particles in size bin i. There were only 2 size bins, one for PM10-2.5 and one for PM2.5 in this study, due to the limitation of the sampling. The virus dose was converted from the measured viral RNA concentration by using conversion factors: β1 (copies per Plaque Forming Unit (PFU)) and β2 (50% Tissue Culture Infectious Dose (TCID50) per PFU) with average values from previous studies(Kim et al., 2020, Peduru Hewa et al., 2009, Sampath et al., 2005, Thompson and Bennett, 2017), listed in SI. 1 Table. 2. Due to the fact that SARS-CoV-2 was frequently detected in PM10 of Zurich and Bern before the outbreak, we hypothesized that certain background interference in these samples existed and the interference was not detected in Lugano samples. Thus, to estimate the infection risk of SARS-CoV-2, we used the differences between the measured concentrations after the outbreak and the average concentrations before the outbreak to eliminate the potential background environmental interference. In this study, according to these viruses’ phylogeny, all the parameters of SARS-CoV were used for SARS-CoV-2, HCoV-229E for all other coronaviruses, influenza A for both influenza A and B, except that λ for SARS-CoV was used as λ for all HCoVs.
fdep is the deposition fraction of particles in size bin i in the human respiratory tract. It was calculated using the deposition model for pathogenic bioaerosols(Bair, 1995, Guha et al., 2014), with the following assumptions: 1) the studied bioaerosols fitted a polydisperse distribution(Guha et al. 2014) with the activity median aerodynamic diameter(AMAD) assumed as 2.5 μm according to previous field studies(Menut et al., 2016, Rivas et al., 2020); 2) the viral loads of the particles were proportional to their surface.
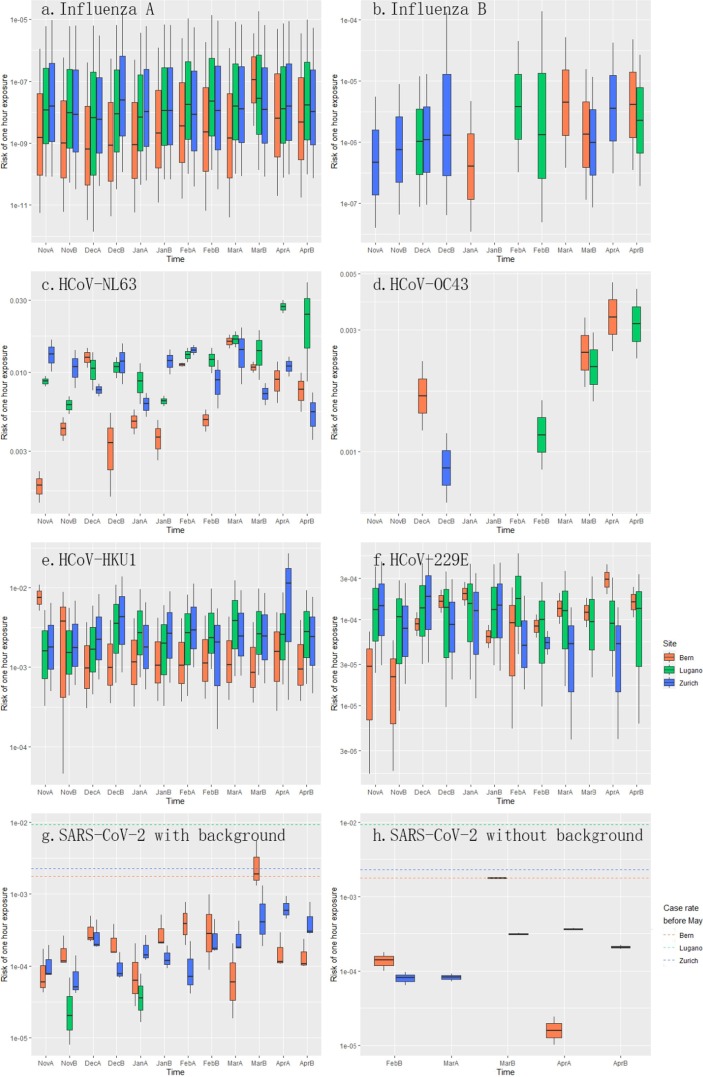
The box plot in Fig. 7 was drawn with the ‘ggplot2′ package (Wickham 2011).
Fig. 7.
Infection risk of different respiratory viruses through aerosol transmission from 1 h of exposure (a. Influenza A, b. Influenza B, c. HCoV-NL63, d. HCoV-OC43, e. HCoV-HKU1, f. HCoV-229E, g. SARS-CoV-2 with background interference, h. SARS-CoV-2 without background interference. A and B after the abbreviations of the months stand for the first half and second half of that month.).
3. Results
3.1. The abundance variations of airborne viruses in three cities and the co-occurrence network of the viruses
Among all 16 target viruses, only HRSVA and HRSVB were not detected (Fig. 2). Influenza A, HCoV-NL63, and HCoV-HKU1 were the ones which passed the normality test during most periods (SI. 1 Table. 3). These three along with HCoV-229E were significantly more abundant than the others, with concentrations expressed as log10(copies/m3 + 1) higher than 1.5 (Fig. 2, SI. 1 Table. 4). Enterovirus and SARS-CoV-2 were the medium ones, with concentrations expressed as log10(copies/m3 + 1) higher than 1 but lower than 1.5 (Fig. 2, SI. 1 Table. 4). The rest ones were the rare ones, occasionally detected generally with low abundances (Fig. 2).
Among the PM10 samples, a peak of SARS-CoV-2 abundance was detected in Bern in late March right after the lockdown coinciding with a peak of new COVID cases (Fig. 3 ), while a significant increase was observed in Zurich after the outbreak which decreased slowly after the lockdown (SI. 2 Table. 1). SARS-CoV-2 abundances in PM10 in Bern and Zurich were significantly higher than the ones in Lugano (SI. 2 Table. 5). Among the PM2.5 samples, SARS-CoV-2 was only detected in Bern after the outbreak (SI. 2 Table. 1).
For the abundant group, most measurements showed significant increases after the outbreak (SI. 2 Table. 1). A possible explanation would be that the SARS-CoV-2 outbreak coincided with the seasonal epidemics of these viruses, for example, the influenza case number did peak in the middle of February in 2020(FOPH; Cabecinhas et al. 2021). In addition, more frequent outdoor activities due to the warm weather after March could also be a factor. One exception was HCoV-229E, which showed significant decreases in Lugano’s PM10 and in Zurich’s both PM2.5 and PM10. Another exception was influenza A showing a significant decrease in PM10 in Lugano after the winter vacation started (SI. 2 Table. 1). For other viruses in the medium group, enterovirus was only observed to have a significant increase in PM10 in Zurich after the lockdown (SI. 2 Table. 1). In the rare group, most viruses did not change significantly during the studied period (SI. 2 Table. 1).
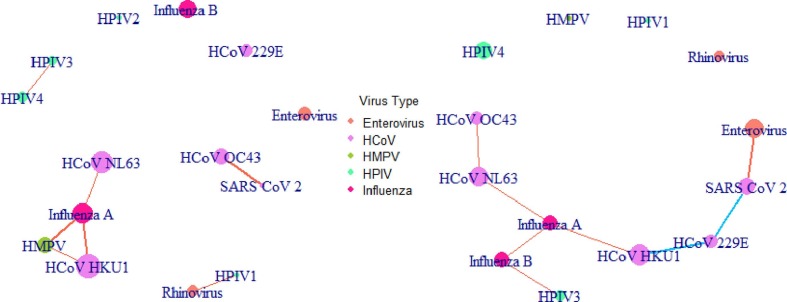
In PM2.5, only positive correlations were detected between viruses (Fig. 4 , SI. 1 Table. 6–7). There were four groups, the biggest one included HMPV, influenza A, HCoV-NL63 and HCoV-HKU1. SARS-CoV-2 was positively correlated with HCoV-OC43 (Fig. 4, SI. 1 Table. 6–7). None of those correlations could be found in PM10 apart from the positive connections between influenza A, HCoV-NL63 and HCoV-HKU1 (Fig. 4, SI. 1 Table. 8–9). In PM10, several positive correlations were found forming a group including influenza A and B, HCoV-NL63, -OC43 and -HKU1 as well as HPIV3. HCoV-229E was negatively correlated to both HCoV-HKU1 and SARS-CoV-2. Finally, SARS-CoV-2 was positively correlated with enterovirus (Fig. 4, SI. 1 Table. 8–9).
Fig. 4.
The co-occurrence network of the airborne viruses in PM2.5 (left) and PM10 (right) (orange lines stand for positive correlation, blue lines for negative correlation, the widths of the lines indicate the relative size of the coefficient, and the sizes of the circles show the relative viral abundances). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2. The redundancy analysis (RDA) of the viruses, new COVID-19 cases and the environmental factors and their co-occurrence network
Among all the environmental factors and new COVID-19 cases, only the concentration of O3, radiation and temperature increased from winter to spring which coincided with the rise of new COVID-19 cases (SI. 1 Fig. 1). Most other environmental factors decreased, except PM2.5, PM10 and precipitation which remained stable (SI. 1 Fig. 1). These trends were generally similar to the ones in other years (FOEN. 2021).
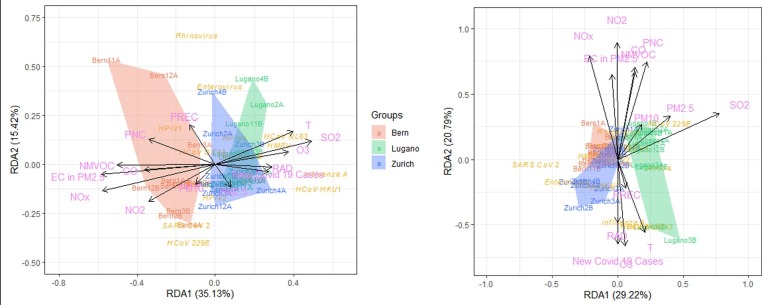
The RDA analysis (Fig. 5 ) showed that the viruses in PM2.5 in Bern were more heavily impacted by pollutants like NO2, NOx, CO, elemental carbon (EC) in PM2.5, particle number concentration (PNC), and non-methane volatile organic compounds (NMVOC), while in Lugano they were more affected by PM2.5, SO2 and O3. O3, radiation (RAD), temperature and new COVID-19 cases pointed to a similar direction in the analyses of viruses in both PM2.5 and PM10. These four factors pointed to the similar direction as SO2 did in the analysis of PM2.5 in Fig. 5, suggesting they have similar influences on the airborne viruses, while in the analysis of PM10 it was precipitation pointed to the similar direction with these four (Fig. 5). This suggests environmental factors influenced the viruses differently in PM2.5 and PM10. Noticeably, in Fig. 5, Bern’s early March sample was almost on the arrow of precipitation. Since PM were negatively correlated with heavy precipitation (Zhao et al., 2020, Zheng et al., 2019, Zhou et al., 2020), it is understandable that SARS-CoV-2 was not detected in this PM2.5 sample and extremely low in the respective PM10 samples, in contrast to Bern’s late February, late March and early April samples.
Fig. 5.
The RDA analysis of all target airborne viruses in PM2.5 (left)and PM10 (right) and environmental factors in three cities (A and B after the abbreviations of the months stand for the first half and second half of that month).
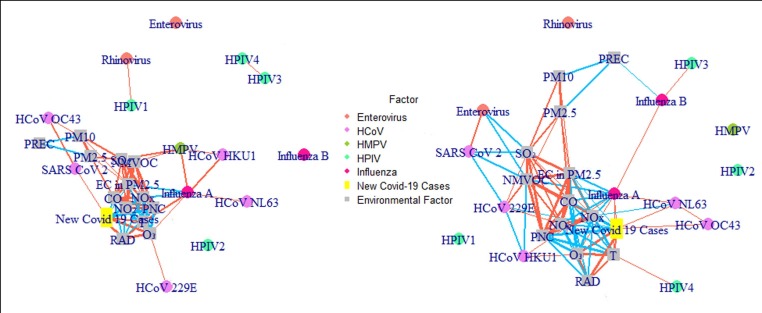
In the co-occurrence network (Fig. 6 , SI. 1 Table. 10–13), only influenza A behaved similarly in both PM2.5 and PM10. In both PM fractions, it was positively correlated with temperature and O3. In PM2.5 SARS-CoV-2 was positively correlated with daily new COVID-19 cases, while this correlation did not exist in PM10 for which daily new COVID-19 cases were correlated with influenza A, HCoV-NL63 and HCoV-OC43 instead. In PM10 SARS-CoV-2 and enterovirus were found to be negatively correlated with SO2 and NMVOC.
Fig. 6.
The Co-occurrence network of the airborne viruses and environmental factors in PM2.5 (left) and PM10 (right) (orange lines stand for positive correlation, blue lines stand for negative correlation, the widths of the lines stand for the relative size of the coefficient, and the sizes of the circles stand for the relative concentrations). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.3. Infection risk of different respiratory viruses through aerosol transmission
Fig. 7 shows the infection risks of different influenza and HCoV viruses through aerosol transmission from 1 h of exposure. Although influenza A was more abundant than influenza B and several HCoVs, its infection risk was similar to influenza B, around 10-8, and much lower than HCoVs (Fig. 7). For HCoVs, HCoV-NL63, HCoV-OC43 and HCoV-HKU1′s infection risks were around 10-3, while HCoV-229E’s was lower, around 10-4 similar to SARS-CoV-2′s (Fig. 7). Yet, in late March, the infection risk of SARS-CoV-2 in Bern was 1.913‰ (Fig. 7g), which was higher than the total COVID case rate before May in Canton Bern (1.769‰). After the potential environmental interference was excluded by subtracting the average measured concentration before the outbreak from the other data, all risks decreased (Fig. 7h) and coincidentally, the infection risk in Bern in late March dropped to 1.769‰, the same as the total case rate in the Canton Bern before May.
4. Discussion
4.1. Influencing factors on the detection and monitoring of SARS-CoV-2 in air
There have been several attempts to detect SARS-CoV-2 viral RNA in outdoor aerosol previously (Chirizzi et al., 2021, Dunker et al., 2021, Kayalar et al., 2021, Linillos-Pradillo et al., 2021, Liu et al., 2020, Passos et al., 2021, Pivato et al., 2021, Setti et al., 2020a, Setti et al., 2020b; Stern et al. 2021a), but only four succeeded, and three of them mainly sampled outside hospitals (Kayalar et al., 2021, Liu et al., 2020, Stern et al., 2021a). Yet, our results along with two previous studies suggested the possibility of the existence of airborne SARS-CoV-2 in urban sites (Kayalar et al., 2021, Setti et al., 2020b). One possible reason for these successful detections was high-flowrate sampling for an extended time. Most of the failed attempts sampled either with a flowrate much lower than 500 L/min (Chirizzi et al., 2021, Dunker et al., 2021, Habibi et al., 2021, Pivato et al., 2021) or for periods much shorter than 24 h (Passos et al. 2021). Two other reasons are the sampling location and timing.
Among our sampling locations, SARS-CoV-2 was rarely detected in Lugano, though it was even more heavily plagued by the pandemic in the first wave than the other two cities. And the possible reason is the sampling site was a university campus. Lugano university acted quickly in the beginning of the pandemic by enforcing social distance, hygiene policies and even shutting down (Isobel 2020). And young people were not the main population group being infected in the first wave (FOPH, 2021). These premises made the air at university campuses less likely to contain SARS-CoV-2 at that time, which may explain the failed detections of several studies (Chirizzi et al., 2021, Dunker et al., 2021), including ours and another study which sampled with 500 L/min for almost 1 day (Linillos-Pradillo et al. 2021). In contrast, SARS-CoV-2 was most frequently detected in Bern, which was a roadside sampling site close to the train station with heavy passenger flow. It was also the only site where SARS-CoV-2 was detected in PM2.5. Fine particles are known to have the tendency to agglomerate into large particles. Thus, even though hospital studies showed that patients shed both fine and coarse particles containing SARS-CoV-2 (Liu et al., 2020, Nor et al., 2021, Stern et al., 2021a), our results showed the absence of SARS-CoV-2 in PM2.5 in all samples from Zurich and some samples from Bern despite its existence in respective PM10. Compared to a residential area, more people with diverse health statuses and contact traces congregate at a traffic artery increasing the possibility of sampling from patients’ emissions. This agrees with a previous study showing that urban roadsides with heavy traffic were more likely to be the hotspots (Kayalar et al. 2021) where the viruses could be from reported potential indoor sources, such as vehicles, trains and stations (Hadei et al., 2021, Lednicky et al., 2021, Moreno et al., 2021). Since the viruses can be quickly dispersed in outdoor air, the risk of outdoor infection by ambient PM in other parts of the cities could be much lower than the one at the hotspots like our sampling site near the traffic hub in Bern.
Noticeably, the positive SARS-CoV-2 signals in PM2.5 in Bern were found right after the outbreak, in late February, late March and early April (Fig. 3) and SARS-CoV-2′s concentration in PM2.5 was positively correlated with the new COVID-19 cases (Fig. 3), highlighting the importance of the timing. In Zurich, SARS-CoV-2′s concentration in PM10 rose significantly after the outbreak, and there was a slight decrease one month after the lockdown (Fig. 3). In contrast, a sharp decrease of SARS-CoV-2′s concentration in PM10 was detected in Bern half a month after the lockdown, synchronized with the decrease of the local new case number. This suggests, the lockdown was generally effective in reducing the ambient SARS-CoV-2 concentration, which may explain that some studies did not detect SARS-CoV-2 in PM since they sampled during the local lockdown (Chirizzi et al., 2021, Linillos-Pradillo et al., 2021). Our results also suggest that lockdowns have a more instant impact on areas with heavy traffic such as the Bern sampling site, a transportation hub, than on residential areas like the Zurich sampling site.
The temporal variations of meteorological factors and pollutants should also be taken into consideration for the timing of airborne virus detection and monitoring. For example, precipitation likely caused the absence of SARS-CoV-2 in PM2.5 and low concentration in PM10 in Bern in early March. Thus, PM could be a carrier of the virus. Yet, the capacity was limited, since there were no positive correlations between SARS-CoV-2 and either PM2.5 or PM10. Therefore, our findings do not support the assumption that the positive correlation between high COVID-19 incidence number or mortality and heavy air pollution was caused directly by more PM carrying more SARS-CoV-2. The correlation is more likely due to polluted air making individuals more vulnerable when exposed to the viruses (Sharma and Balyan, 2020, Woodby et al., 2021).
Another important factor could be the sampling and preservation methods. We utilized the PM samples which were normally collected to provide common air quality parameters such as PM2.5 and PM10. This approach took advantage of the existing infrastructures of air quality monitoring networks and provided added values to the collected PM samples. A recent study suggested the recovery was higher for samples with short or even no preservation (Licen et al. 2022). The recovery from Polytetrafluorethylen(PTFE) filters was also higher and easier and could be further enhanced by an elution buffer containing up to 40% of fetal calf serum (Robotto et al. 2021). This might also be a reason that very few studies succeeded in SARS-CoV-2 viral RNA detection in outdoor air and for those successful ones including ours, the actual abundances could be higher than the reported values (Kayalar et al., 2021, Liu et al., 2020, Setti et al., 2020b, Stern et al., 2021a). In addition, the sampling and preservation methods to test the viability of airborne SARS-CoV-2 have not been fully explored yet. To our knowledge, only two studies successfully detected viable SARS-CoV-2 in air with Sioutas Personal Cascade Impactor Sampler (PCIS) using PTFE filters (Lednicky et al. 2021) and Viable Virus Aerosol Sampler (VIVAS) using High-Efficiency Particulate Air (HEPA) filters (Lednicky et al. 2020a),. Therefore, it is still urgent to explore, optimize and standardize the possible sampling and preservation methods for airborne SARS-CoV-2 in the future.
4.2. Influencing factors on the detection and monitoring of other viruses in air
The influencing factors on the aerosol concentrations of other respiratory viruses include the sampling location, timing and the characteristics of the viruses as well.
Different respiratory viruses surely have different characteristics which influence their aerosol concentrations. Influenza A in air has been commonly compared with influenza B in different environments in previous studies (Coleman et al., 2018, Shiu et al., 2020, Xie et al., 2020, Yadana et al., 2019, Zhao et al., 2019a), with which our study agrees on that influenza A was generally more frequently detected and more abundant in air than influenza B. HCoVs were much less frequently found and less abundant in air compared to influenza A in previous studies, while HRSVs’ concentrations varied in different environments (Coleman et al., 2018, Xie et al., 2020, Yadana et al., 2019), In our study, we failed to detect HRSVs, and some HCoVs like HCoV-NL63, HCoV-HKU1, and HCoV-229E were frequently detected with high abundances, while HCoV-OC43 was rarely found, which could be caused by different sampling locations.
On the topic of sampling locations, as our sampling site of Lugano was a university campus, we noticed a significant decrease of influenza A in PM10 between the 2019 semester and the winter vacation, which did not exist on other sites. This could be caused by the change of human activity, especially student presence between the two time periods. Thus, monitoring airborne viruses on campus during vacation is less efficient.
The sampling timing had a marginal impact on rare viruses because their significant temporal variations were rarely detected as mentioned before, but a strong one on the abundant viruses. Though some studies reported that seasonal epidemics of respiratory viruses in some countries ended earlier in 2020 (Redlberger-Fritz et al. 2021) or were delayed (Delestrain et al., 2021, Williams et al., 2021) or absent in 2021 (Diesner-Treiber et al., 2021, Kuitunen, 2021), our study found significant peaks of influenza A in air in the spring of 2020 which agreed with other studies demonstrating that the seasonality of influenza A still existed in 2020 (SI. 2 Table. 1) in Switzerland (FOPH, 2019; Cabecinhas et al. 2021), similar as the situations in other places around the world (Chiapinotto et al., 2021, De Francesco et al., 2021, Hsieh et al., 2020, Kuitunen, 2021). Similarly, significant spring peaks of three other abundant viruses were also detected in our study (SI. 2 Table. 1).
Just like SARS-CoV-2, these viruses were rarely found to be correlated to environmental factors or air pollutants. Even for certain air pollutants like O3, which was positively correlated with influenza A in both PM2.5 and PM10, HCoV-229E in PM2.5 and HCoV-HKU1 in PM10 (Fig. 6, SI. 1 Table. 10–13), the underlying mechanism could be very complicated, such as its collinearity with the sunny weather and more outdoor activities and emissions of local people in such weather.
4.3. The necessity of monitoring airborne viruses
During this pandemic, sewage surveillance of SARS-CoV-2 has been developed as a monitoring system for the local COVID-19 prevalence and even the emerging of new variants (Ahmed et al., 2020, Medema et al., 2020; Smyth et al., 2022, Wu et al., 2020). Air surveillance is comparably rare, due to the influencing factors mentioned above, but surely air surveillance of SARS-CoV-2 has its own merits. First, according to our findings, the concentrations in PM2.5 can approximately represent local COVID-19 prevalence with the right choice of the sampling site. Moreover, it is possible to estimate the infection risk for local population, which is not applicable for sewage surveillance. Our estimation is generally rough, because of the following reasons: there were other factors we did not include, such as different particle surface area distributions, the variation of respiration frequency and tidal volume of different population groups and their different activities; some estimated parameters were not generated from human studies (Watanabe et al. 2010); the viable rate of SARS-CoV-2 was mainly estimated from hospital studies, which could be much lower in outdoor air. Thus, the estimation can certainly be improved by more experiments, for example testing the viable rate of SARS-CoV-2 in outdoor air directly. Our estimation did reasonably and roughly show that although the outdoor infection risks were generally low comparing to the local case rate, they should not be neglected in certain hotspots since they were not lower than the estimated risks for indoor areas with only one infected individual shedding viruses (Zhang et al., 2021, Zhang and Wang, 2021). Thus, the air of these hotspots such as transportation hubs like our sampling site in Bern should be monitored for the public safety.
For other viruses, monitoring them in air can give us information about their seasonal or non-seasonal prevalence among a certain population, such as influenza A among students or elderly. Moreover, potential risks for coinfections could be monitored, especially since these respiratory viruses we studied share similar transmission routes. Regardless of the frequency, all the abundant viruses we studied have been found to coinfect withSARS-CoV-2 (Boschiero et al., 2022, Dadashi et al., 2021, Jiang et al., 2020, Lau et al., 2021, Pigny et al., 2021, Rodriguez et al., 2020). Nevertheless, most of them were not correlated with SARS-CoV-2 directly in our results (Fig. 4, SI. 1 Table. 6–9). However, among the two viruses which were positively correlated with SARS-CoV-2 (Fig. 4, SI. 1 Table. 6–9), enterovirus is of concern. Enterovirus is one of the most common picornaviruses along with rhinovirus belonging to the same genus. They usually cause mild symptoms for adults, but are very contagious and could be dangerous for small children and cause epidemics among them. The correlation between enterovirus and SARS-CoV-2 found in our result resonated with the fact that their coinfection has been recognized (Boschiero et al., 2022, Pigny et al., 2021) and the cases of its relative rhinovirus were commonly observed to increase with SARS-CoV-2 cases (De Francesco et al., 2021, Haapanen et al., 2021, Redlberger-Fritz et al., 2021), though its correlation with SARS-CoV-2 in air was not detected. In addition, a preprint suggests that the new Omicron variant could have gained a unique insertion mutation from HCoV-229E from a co-infected individual, which is very alarming (Venkatakrishnan et al. 2021). Although more evidence is needed for this and a negative correlation between HCoV-229E and SARS-CoV-2 was detected in our study (Fig. 4, SI. 1 Table. 8–9), the rare cases of SARS-CoV-2 coinfected with HCoV-229E have been reported (Boschiero et al., 2022, Lau et al., 2021), suggesting similar rare coinfections between SARS-CoV-2 and other viruses should not be overlooked. After all, the possibility of the emergence of a more virulent and contagious variant of either SARS-CoV-2 or other virus through this way should not be neglected, in addition to the concern that the coinfections with other viruses such as adenovirus and influenza may enhance SARS-CoV-2 infectivity, aggravate the symptoms and even increase mortality(Bai et al., 2021, Guan et al., 2021, Swets et al., 2022). Clearly, monitoring these viruses in air may give us an early warning.
5. Conclusion
It is possible to monitor airborne viruses including SARS-CoV-2 in ambient PMs. Although there are a lot of influencing factors and uncertain mechanisms in need of further study, we showed that our current monitoring results were able to represent approximately the prevalence of the disease and estimate the infection risks to some level. Therefore, air surveillance in hotspots has the potential to become an essential tool during this and future pandemics and epidemics of other respiratory viruses.
Funding sources
This study was supported by the National Research Program (NRP 78 Covid-19, 198258) of the Swiss National Science Foundation (SNSF).
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The Swiss Federal Human Research Act exempts the use of anonymized leftover specimens for research from ethical approval.
The authors declare no competing interests.
CRediT authorship contribution statement
Yile Tao: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Writing – original draft, Visualization. Xiaole Zhang: Methodology, Software, Validation, Formal analysis, Writing – review & editing. Guangyu Qiu: Resources, Writing – review & editing, Funding acquisition. Martin Spillmann: Methodology, Validation, Investigation, Writing – review & editing. Zheng Ji: Conceptualization, Methodology, Investigation, Writing – review & editing. Jing Wang: Conceptualization, Validation, Resources, Writing – review & editing, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We acknowledge the PM samples from the NABEL network jointly operated by FOEN and Empa. Zheng Ji and Yile Tao acknowledge the support by China Scholarship Council (No. 201906875037 & 201706010410).
Handling Editor: Adrian Covaci
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2022.107266.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- FOEN. Data query NABEL. https://www.bafu.admin.ch/bafu/en/home/topics/air/state/data/data-query-nabel.html; 2021 (accessed November 8, 2021).
- FOPH. Bericht zur Grippesaison 2019/2020. http://meldesysteme.bagapps.ch/sentinella/publikationen/2020%20Saisonbericht%20Grippe%202019_2020_d.pdf; 2022 (accessed January 17, 2022).
- FOPH. COVID-19 Switzerland. https://www.covid19.admin.ch/en/epidemiologic/case; 2021 (accessed November 8, 2021).
- FSO. Portraits of the cantons. https://www.bfs.admin.ch/bfs/en/home/statistics/regional-statistics/regional-portraits-key-figures/cantons.html; 2021 (accessed November 8, 2021).
- Ackerson B., Tseng H.F., Sy L.S., Solano Z., Slezak J., Luo Y.i., Fischetti C.A., Shinde V. Severe morbidity and mortality associated with respiratory syncytial virus versus influenza infection in hospitalized older adults. Clin. Infect. Dis. 2019;69(2):197–203. doi: 10.1093/cid/ciy991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlawat A., Wiedensohler A., Mishra S.K. An overview on the role of relative humidity in airborne transmission of SARS-CoV-2 in indoor environments. Aerosol Air Qual. Res. 2020;20(9):1856–1861. [Google Scholar]
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O'Brien J.W., Choi P.M., Kitajima M., Simpson S.L., Li J., Tscharke B., Verhagen R., Smith W.J.M., Zaugg J., Dierens L., Hugenholtz P., Thomas K.V., Mueller J.F. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728:138764. doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand U., Adelodun B., Pivato A., Suresh S., Indari O., Jakhmola S., Jha H.C., Jha P.K., Tripathi V., Di Maria F. A review of the presence of SARS-CoV-2 RNA in wastewater and airborne particulates and its use for virus spreading surveillance. Environ. Res. 2021;196:110929. doi: 10.1016/j.envres.2021.110929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang A.XY., Luhung I., Ahidjo B.A., Drautz‐Moses D.I., Tambyah P.A., Mok C.K., Lau K.JX., Tham S.M., Chu J.J.H., Allen D.M., Schuster S.C. Airborne SARS-CoV-2 surveillance in hospital environment using high-flowrate air samplers and its comparison to surface sampling. Indoor Air. 2022;32(1) doi: 10.1111/ina.v32.110.1111/ina.12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baboli Z., Neisi N., Babaei A.A., Ahmadi M., Sorooshian A., Birgani Y.T., Goudarzi G. On the airborne transmission of SARS-CoV-2 and relationship with indoor conditions at a hospital. Atmos. Environ. 2021;261:118563. doi: 10.1016/j.atmosenv.2021.118563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai L., Zhao Y., Dong J., Liang S., Guo M., Liu X., Wang X., Huang Z., Sun X., Zhang Z., Dong L., Liu Q., Zheng Y., Niu D., Xiang M., Song K., Ye J., Zheng W., Tang Z., Tang M., Zhou Y.u., Shen C., Dai M., Zhou L.i., Chen Y.u., Yan H., Lan K.e., Xu K.e. Coinfection with influenza A virus enhances SARS-CoV-2 infectivity. Cell Res. 2021;31(4):395–403. doi: 10.1038/s41422-021-00473-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bair W.J. The ICRP human respiratory-tract model for radiological protection. Radiat. Prot. Dosimet. 1995;60(4):307–310. [Google Scholar]
- Barbieri P., Zupin L., Licen S., Torboli V., Semeraro S., Cozzutto S., Palmisani J., Di Gilio A., de Gennaro G., Fontana F., Omiciuolo C., Pallavicini A., Ruscio M., Crovella S. Molecular detection of SARS-CoV-2 from indoor air samples in environmental monitoring needs adequate temporal coverage and infectivity assessment. Environ. Res. 2021;198:111200. doi: 10.1016/j.envres.2021.111200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzazpour S., Rahmatinia M., Mohebbi S.R., Hadei M., Shahsavani A., Hopke P.K., Houshmand B., Raeisi A., Jafari A.J., Yarahmadi M., Farhadi M., Hasanzadeh V., Kermani M., Vaziri M.H., Tanhaei M., Zali M.R., Alipour M.R. The detection of SARS-CoV-2 RNA in indoor air of dental clinics during the COVID-19 pandemic. Environ. Sci. Pollut. Res. Int. 2021 doi: 10.1007/s11356-021-15607-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck, M.W. ggord: Ordination Plots with ggplot2. R package version 1.0.0. https://zenodo.org/badge/latestdoi/35334615; 2017.
- Boschiero M.N., Duarte A., Palamim C.V.C., Alvarez A.E., Mauch R.M., Marson F.A.L. Frequency of respiratory pathogens other than SARS-CoV-2 detected COVID-19. Diagn. Microbiol. Infect. Dis. 2022;102 doi: 10.1016/j.diagmicrobio.2021.115576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabecinhas, A.R.G.; Boquete-Suter, P.; Kaiser, L. Influenza virus surveillance in Switzerland Season 2019–2020. https://www.hug.ch/sites/interhug/files/structures/laboratoire_de_virologie/final_report_nrci_2019_2020.pdf; 2021 (accessed January 17, 2022).
- Chen C.W.S., Hsieh Y.-H., Su H.-C., Wu J.J. Causality test of ambient fine particles and human influenza in Taiwan: Age group-specific disparity and geographic heterogeneity. Environ. Int. 2018;111:354–361. doi: 10.1016/j.envint.2017.10.011. [DOI] [PubMed] [Google Scholar]
- Chen G., Zhang W., Li S., Zhang Y., Williams G., Huxley R., Ren H., Cao W., Guo Y. The impact of ambient fine particles on influenza transmission and the modification effects of temperature in China: a multi-city study. Environ. Int. 2017;98:82–88. doi: 10.1016/j.envint.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng V.C.C., Wong S.-C., Chen J.H.K., Yip C.C.Y., Chuang V.W.M., Tsang O.T.Y., Sridhar S., Chan J.F.W., Ho P.-L., Yuen K.-Y. Escalating infection control response to the rapidly evolving epidemiology of the coronavirus disease 2019 (COVID-19) due to SARS-CoV-2 in Hong Kong. Infect. Control Hosp. Epidemiol. 2020;41(5):493–498. doi: 10.1017/ice.2020.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia P.Y., Coleman K.K., Tan Y.K., Ong S.W.X., Gum M., Lau S.K., Lim X.F., Lim A.S., Sutjipto S., Lee P.H., Son T.T., Young B.E., Milton D.K., Gray G.C., Schuster S., Barkham T., De P.P., Vasoo S., Chan M., Ang B.S.P., Tan B.H., Leo Y.-S., Ng O.-T., Wong M.S.Y., Marimuthu K. Singapore novel coronavirus outbreak research, T. Detection of air and surface contamination by SARS-CoV-2 in hospital rooms of infected patients. Nat. Commun. 2020;11(1) doi: 10.1038/s41467-020-16670-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiapinotto S., Sarria E.E., Mocelin H.T., Lima J.A.B., Mattiello R., Fischer G.B. Impact of non-pharmacological initiatives for COVID-19 on hospital admissions due to pediatric acute respiratory illnesses. Paediatr. Respir. Rev. 2021;39:3–8. doi: 10.1016/j.prrv.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirizzi D., Conte M., Feltracco M., Dinoi A., Gregoris E., Barbaro E., La Bella G., Ciccarese G., La Salandra G., Gambaro A., Contini D. SARS-CoV-2 concentrations and virus-laden aerosol size distributions in outdoor air in north and south of Italy. Environ. Int. 2021;146:106255. doi: 10.1016/j.envint.2020.106255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia M. Factors determining the diffusion of COVID-19 and suggested strategy to prevent future accelerated viral infectivity similar to COVID. Sci. Total Environ. 2020;729:138474. doi: 10.1016/j.scitotenv.2020.138474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman K.K., Nguyen T.T., Yadana S., Hansen-Estruch C., Lindsley W.G., Gray G.C. Bioaerosol sampling for respiratory viruses in Singapore's mass rapid transit network. Sci. Rep. 2018;8:17476. doi: 10.1038/s41598-018-35896-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft D.P., Zhang W.J., Lin S., Thurston S.W., Hopke P.K., Masiol M., Squizzato S., van Wijngaarden E., Utell M.J., Rich D.Q. The association between respiratory infection and air pollution in the setting of air quality policy and economic change. Ann. Am. Thorac. Soc. 2019;16:321–330. doi: 10.1513/AnnalsATS.201810-691OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Sanchez T.M., Haddrell A.E., Hackett T.L., Singhera G.K., Marchant D., Lekivetz R., Meredith A., Horne D., Knight D.A., van Eeden S.F., Bai T.R., Hegele R.G., Dorscheid D.R., Agnes G.R. Formation of a stable mimic of ambient particulate matter containing viable infectious respiratory syncytial virus and its dry-deposition directly onto cell cultures. Anal. Chem. 2013;85(2):898–906. doi: 10.1021/ac302174y. [DOI] [PubMed] [Google Scholar]
- Csardi G., Nepusz T. The igraph software package for complex network research. Int. J. Complex Syst. 2006:1695. [Google Scholar]
- Dabisch P., Schuit M., Herzog A., Beck K., Wood S., Krause M., Miller D., Weaver W., Freeburger D., Hooper I., Green B., Williams G., Holland B., Bohannon J., Wahl V., Yolitz J., Hevey M., Ratnesar-Shumate S. The influence of temperature, humidity, and simulated sunlight on the infectivity of SARS-CoV-2 in aerosols. Aerosol Sci. Technol. 2021;55(2):142–153. doi: 10.1080/02786826.2020.1829536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadashi M., Khaleghnejad S., Abedi Elkhichi P., Goudarzi M., Goudarzi H., Taghavi A., Vaezjalali M., Hajikhani B. COVID-19 and influenza co-infection: a systematic review and meta-analysis. Front. Med. 2021;8 doi: 10.3389/fmed.2021.681469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damialis, A.; Gilles, S.; Sofiev, M.; Sofieva, V.; Kolek, F.; Bayr, D.; Plaza, M.P.; Leier-Wirtz, V.; Kaschuba, S.; Ziska, L.H.; Bielory, L.; Makra, L.; Del Mar Trigo, M.; group, C.-P.s.; Traidl-Hoffmann, C. Higher airborne pollen concentrations correlated with increased SARS-CoV-2 infection rates, as evidenced from 31 countries across the globe. Proc Natl Acad Sci U S A 2021;118 (12):e2019034118. [DOI] [PMC free article] [PubMed]
- De Francesco M.A., Pollara C., Gargiulo F., Giacomelli M., Caruso A. Circulation of respiratory viruses in hospitalized adults before and during the COVID-19 pandemic in Brescia, Italy: a retrospective study. Int. J. Environ. Res. Public Health. 2021;18(18):9525. doi: 10.3390/ijerph18189525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delestrain C., Danis K., Hau I., Behillil S., Billard M.-N., Krajten L., Cohen R., Bont L., Epaud R. Impact of COVID-19 social distancing on viral infection in France: a delayed outbreak of RSV. Pediatr. Pulmonol. 2021;56(12):3669–3673. doi: 10.1002/ppul.25644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diesner-Treiber S.C., Voitl P., Voitl J.J.M., Langer K., Kuzio U., Riepl A., Patel P., Mühl-Riegler A., Mühl B. Respiratory infections in children during a Covid-19 pandemic winter. Front. Pediatr. 2021;9 doi: 10.3389/fped.2021.740785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Z., Qian H., Xu B., Huang Y., Miao T.e., Yen H.-L., Xiao S., Cui L., Wu X., Shao W., Song Y., Sha L.i., Zhou L., Xu Y., Zhu B., Li Y. Toilets dominate environmental detection of severe acute respiratory syndrome coronavirus 2 in a hospital. Sci. Total Environ. 2021;753:141710. doi: 10.1016/j.scitotenv.2020.141710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragone R., Licciardi G., Grasso G., Del Gaudio C., Chanussot J. Analysis of the chemical and physical environmental aspects that promoted the spread of SARS-CoV-2 in the Lombard area. int. J. Environ. Res. Public Health. 2021;18(3):1226. doi: 10.3390/ijerph18031226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont-Leblond N., Veillette M., Mubareka S., Yip L., Longtin Y., Jouvet P., Paquet Bolduc B., Godbout S., Kobinger G., McGeer A., Mikszewski A., Duchaine C. Low incidence of airborne SARS-CoV-2 in acute care hospital rooms with optimized ventilation. Emerg. Microbes. Infect. 2020;9(1):2597–2605. doi: 10.1080/22221751.2020.1850184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunker S., Hornick T., Szczepankiewicz G., Maier M., Bastl M., Bumberger J., Treudler R., Liebert U.G., Simon J.-C. No SARS-CoV-2 detected in air samples (pollen and particulate matter) in Leipzig during the first spread. Sci. Total Environ. 2021;755:142881. doi: 10.1016/j.scitotenv.2020.142881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng B., Xu K., Gu S., Zheng S., Zou Q., Xu Y., Yu L., Lou F., Yu F., Jin T., Li Y., Sheng J., Yen H.-L., Zhong Z., Wei J., Chen Y.u. Multi-route transmission potential of SARS-CoV-2 in healthcare facilities. J. Hazard. Mater. 2021;402:123771. doi: 10.1016/j.jhazmat.2020.123771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerba C.P., Stagg C.H. Protection of viruses during disinfection by adsorption to particulate matter. J. Water Pollut. Con. F. 1979;51:414–416. [PubMed] [Google Scholar]
- Ghaffari H.R., Farshidi H., Alipour V., Dindarloo K., Azad M.H., Jamalidoust M., Madani A., Aghamolaei T., Hashemi Y., Fazlzadeh M., Fakhri Y. Detection of SARS-CoV-2 in the indoor air of intensive care unit (ICU) for severe COVID-19 patients and its surroundings: considering the role of environmental conditions. Environ. Sci. Pollut. Res. Int. 2021 doi: 10.1007/s11356-021-16010-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianini M.F.D., Gehrig R., Fischer A., Ulrich A., Wichser A., Hueglin C. Chemical composition of PM10 in Switzerland: An analysis for 2008/2009 and changes since 1998/1999. Atmos. Environ. 2012;54:97–106. [Google Scholar]
- Grange S.K., Lee J.D., Drysdale W.S., Lewis A.C., Hueglin C., Emmenegger L., Carslaw D.C. COVID-19 lockdowns highlight a risk of increasing ozone pollution in European urban areas. Atmos. Chem. Phys. 2021;21(5):4169–4185. [Google Scholar]
- Guan Z., Chen C., Li Y.T., Yan D.Y., Zhang X.B., Jiang D.X., Yang S.G., Li L.J. Impact of coinfection With SARS-CoV-2 and influenza on disease severity: a systematic review and meta-analysis. Front. Public Health. 2021;9 doi: 10.3389/fpubh.2021.773130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha S., Hariharan P., Myers M.R. Enhancement of ICRP's lung deposition model for pathogenic bioaerosols. Aerosol Sci. Technol. 2014;48(12):1226–1235. [Google Scholar]
- Gujral H., Sinha A. Association between exposure to airborne pollutants and COVID-19 in Los Angeles, United States with ensemble-based dynamic emission model. Environ. Res. 2021;194:110704. doi: 10.1016/j.envres.2020.110704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z.-D., Wang Z.-Y., Zhang S.-F., Li X., Li L., Li C., Cui Y., Fu R.-B., Dong Y.-Z., Chi X.-Y., Zhang M.-Y., Liu K., Cao C., Liu B., Zhang K.e., Gao Y.-W., Lu B., Chen W. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerg. Infect. Dis. 2020;26(7):1583–1591. doi: 10.3201/eid2607.200885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haapanen M., Renko M., Artama M., Kuitunen I. The impact of the lockdown and the re-opening of schools and day cares on the epidemiology of SARS-CoV-2 and other respiratory infections in children - a nationwide register study in Finland. EClinicalMedicine. 2021;34:100807. doi: 10.1016/j.eclinm.2021.100807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habibi N., Uddin S., Al‐Salameen F., Al‐Amad S., Kumar V., Al‐Otaibi M., Razzack N.A., Shajan A., Shirshikar F. SARS-CoV-2, other respiratory viruses and bacteria in aerosols: Report from Kuwait's hospitals. Indoor Air. 2021;31(6):1815–1825. doi: 10.1111/ina.12871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadei M., Mohebbi S.R., Hopke P.K., Shahsavani A., Bazzazpour S., Alipour M., Jafari A.J., Bandpey A.M., Zali A., Yarahmadi M., Farhadi M., Rahmatinia M., Hasanzadeh V., Nazari S.S.H., Asadzadeh-Aghdaei H., Tanhaei M., Zali M.R., Kermani M., Vaziri M.H., Chobineh H. Presence of SARS-CoV-2 in the air of public places and transportation. Atmospheric Pollut. Res. 2021;12(3):302–306. doi: 10.1016/j.apr.2020.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemati S., Mobini G.R., Heidari M., Rahmani F., Soleymani Babadi A., Farhadkhani M., Nourmoradi H., Raeisi A., Ahmadi A., Khodabakhshi A., Sadeghi M., Bagheri M., Validi M., Taghipour S., Mohammadi-Moghadam F. Simultaneous monitoring of SARS-CoV-2, bacteria, and fungi in indoor air of hospital: a study on Hajar Hospital in Shahrekord, Iran. Environ. Sci. Pollut. Res. Int. 2021;28(32):43792–43802. doi: 10.1007/s11356-021-13628-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh C.C., Lin C.H., Wang W.Y.C., Pauleen D.J., Chen J.V. The outcome and implications of public precautionary measures in taiwan-declining respiratory disease cases in the COVID-19 pandemic. Int. J. Environ. Res. Public Health. 2020;17(13):4877. doi: 10.3390/ijerph17134877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z., Huang J., Gu Q., Du P., Liang H., Dong Q. Optimal temperature zone for the dispersal of COVID-19. Sci. Total Environ. 2020;736:139487. doi: 10.1016/j.scitotenv.2020.139487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hueglin C., Gehrig R., Baltensperger U., Gysel M., Monn C., Vonmont H. Chemical characterisation of PM2.5, PM10 and coarse particles at urban, near-city and rural sites in Switzerland. Atmos. Environ. 2005;39(4):637–651. [Google Scholar]
- Hutter H.P., Poteser M., Moshammer H., Lemmerer K., Mayer M., Weitensfelder L., Wallner P., Kundi M. Air pollution is associated with COVID-19 incidence and mortality in Vienna, Austria. Int. J. Environ. Res. Public Health. 2020;17(24):9275. doi: 10.3390/ijerph17249275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isobel, L. How one Swiss university in a hard-hit region coped with Covid-19. https://www.swissinfo.ch/eng/studying-in-ticino_how-a-swiss-university-has-coped-with-covid-19/45806502; 2020 (accessed January 5, 2021).
- Jiang H.J., Chen N., Shen Z.Q., Yin J., Qiu Z.G., Miao J., Yang Z.W., Shi D.Y., Wang H.R., Wang X.W., Li J.W., Yang D., Jin M. Inactivation of poliovirus by ozone and the impact of ozone on the viral genome. Biomed. Environ. Sci. 2019;32:324–333. doi: 10.3967/bes2019.044. [DOI] [PubMed] [Google Scholar]
- Jiang S., Liu P., Xiong G.e., Yang Z., Wang M., Li Y., Yu X.-J. Coinfection of SARS-CoV-2 and multiple respiratory pathogens in children. Clin. Chem. Lab. Med. 2020;58(7):1160–1161. doi: 10.1515/cclm-2020-0434. [DOI] [PubMed] [Google Scholar]
- Kan H.D., Chen B.H., Fu C.F., Yu S.Z., Mu L.N. Relationship between ambient air pollution and daily mortality of SARS in Beijing. Biomed. Environ. Sci. 2005;18:1–4. [PubMed] [Google Scholar]
- Kayalar Ö., Arı A., Babuççu G., Konyalılar N., Doğan Ö., Can F., Şahin Ü.A., Gaga E.O., Levent Kuzu S., Arı P.E., Odabaşı M., Taşdemir Y., Sıddık Cindoruk S., Esen F., Sakın E., Çalışkan B., Tecer L.H., Fıçıcı M., Altın A., Onat B., Ayvaz C., Uzun B., Saral A., Döğeroğlu T., Malkoç S., Üzmez Ö.Ö., Kunt F., Aydın S., Kara M., Yaman B., Doğan G., Olgun B., Dokumacı E.N., Güllü G., Uzunpınar E.S., Bayram H. Existence of SARS-CoV-2 RNA on ambient particulate matter samples: A nationwide study in Turkey. Sci. Total Environ. 2021;789:147976. doi: 10.1016/j.scitotenv.2021.147976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenarkoohi A., Noorimotlagh Z., Falahi S., Amarloei A., Mirzaee S.A., Pakzad I., Bastani E. Hospital indoor air quality monitoring for the detection of SARS-CoV-2 (COVID-19) virus. Sci. Total Environ. 2020;748:141324. doi: 10.1016/j.scitotenv.2020.141324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.R., An S., Hwang J. An integrated system of air sampling and simultaneous enrichment for rapid biosensing of airborne coronavirus and influenza virus. Biosens. Bioelectron. 2020;170:112656. doi: 10.1016/j.bios.2020.112656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolde, R. pheatmap: Pretty Heatmaps. https://CRAN.R-project.org/package=pheatmap: R Package Version 1.0.12; 2010.
- Krambrich J., Akaberi D., Ling J., Hoffman T., Svensson L., Hagbom M., Lundkvist A. SARS-CoV-2 in hospital indoor environments is predominantly non-infectious. J. Virol. 2021;18:109. doi: 10.1186/s12985-021-01556-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuitunen I. Influenza season 2020–2021 did not begin in Finland despite the looser social restrictions during the second wave of COVID-19: A nationwide register study. J. Med. Virol. 2021;93(9):5626–5629. doi: 10.1002/jmv.27048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni H., Khandait H., Narlawar U.W., Rathod P., Mamtani M. Independent association of meteorological characteristics with initial spread of Covid-19 in India. Sci. Total Environ. 2021;764:142801. doi: 10.1016/j.scitotenv.2020.142801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau S.K.P., Lung D.C., Wong E.Y.M., Aw-Yong K.L., Wong A.C.P., Luk H.K.H., Li K.S.M., Fung J., Chan T.T.Y., Tang J.Y.M., Zhu L., Yip C.C.Y., Wong S.C.Y., Lee R.A., Tsang O.T.Y., Yuen K.-Y., Woo P.C.Y., Rasmussen A.L. Molecular Evolution of Human Coronavirus 229E in Hong Kong and a Fatal COVID-19 Case Involving Coinfection with a Novel Human Coronavirus 229E Genogroup. Msphere. 2021;6(1) doi: 10.1128/mSphere.00819-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lednicky J.A., Lauzardo M., Alam M.M., Elbadry M.A., Stephenson C.J., Gibson J.C., Morris J.G. Isolation of SARS-CoV-2 from the air in a car driven by a COVID patient with mild illness. Int. J. Infect. Dis. 2021;108:212–216. doi: 10.1016/j.ijid.2021.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lednicky J.A., Lauzardo M., Fan Z.H., Jutla A., Tilly T.B., Gangwar M., Usmani M., Shankar S.N., Mohamed K., Eiguren-Fernandez A., Stephenson C.J., Alam M.M., Elbadry M.A., Loeb J.C., Subramaniam K., Waltzek T.B., Cherabuddi K., Morris J.G., Wu C.-Y. Viable SARS-CoV-2 in the air of a hospital room with COVID-19 patients. Int J Infect Dis. 2020;100:476–482. doi: 10.1016/j.ijid.2020.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lednicky J.A., Shankar S.N., Elbadry M.A., Gibson J.C., Alam M.M., Stephenson C.J., Eiguren-Fernandez A., Morris J.G., Mavian C.N., Salemi M., Clugston J.R., Wu C.-Y. Collection of SARS-CoV-2 Virus from the Air of a Clinic within a University Student Health Care Center and Analyses of the Viral Genomic Sequence. Aerosol Air Qual. Res. 2020;20(6):1167–1171. doi: 10.4209/aaqr.2020.02.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licen S., Zupin L., Martello L., Torboli V., Semeraro S., Gardossi A.L., Greco E., Fontana F., Crovella S., Ruscio M., Palmisani J., Di Gilio A., Piscitelli P., Pallavicini A., Barbieri P. SARS-CoV-2 RNA Recovery from Air Sampled on Quartz Fiber Filters: A Matter of Sample Preservation? Atmosphere. 2022;13:340. [Google Scholar]
- Linillos-Pradillo B., Rancan L., Ramiro E.D., Vara E., Artíñano B., Arias J. Determination of SARS-CoV-2 RNA in different particulate matter size fractions of outdoor air samples in Madrid during the lockdown. Environ. Res. 2021;195:110863. doi: 10.1016/j.envres.2021.110863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Ning Z., Chen Y.u., Guo M., Liu Y., Gali N.K., Sun L.i., Duan Y., Cai J., Westerdahl D., Liu X., Xu K.e., Ho K.-F., Kan H., Fu Q., Lan K.e. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020;582(7813):557–560. doi: 10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- Lopez J.H., Romo A.S., Molina D.C., Hernandez G.A., Cureno A.B.G., Acosta M.A., Gaxiola C.A.A., Felix M.J.S., Galvan T.G. Detection of Sars-Cov-2 in the air of two hospitals in Hermosillo, Sonora, Mexico, utilizing a low-cost environmental monitoring system. Int. J. Infect. Dis. 2021;102:478–482. doi: 10.1016/j.ijid.2020.10.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo J.S.L., Tam W.W.S., Seow W.J. Association between air quality, meteorological factors and COVID-19 infection case numbers. Environ. Res. 2021;197:111024. doi: 10.1016/j.envres.2021.111024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Zhou J., Yang S., Zhao Y., Zheng X. Assessment for the impact of dust events on measles incidence in western China. Atmos. Environ. 2017;157:1–9. [Google Scholar]
- Marquès M., Rovira J., Nadal M., Domingo J.L. Effects of air pollution on the potential transmission and mortality of COVID-19: A preliminary case-study in Tarragona Province (Catalonia, Spain) Environ. Res. 2021;192:110315. doi: 10.1016/j.envres.2020.110315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-Coronavirus-2 RNA in Sewage and Correlation with Reported COVID-19 Prevalence in the Early Stage of the Epidemic in The Netherlands. Environ. Sci. Technol. Lett. 2020;7(7):511–516. doi: 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- Menut L., Siour G., Mailler S., Couvidat F., Bessagnet B. Observations and regional modeling of aerosol optical properties, speciation and size distribution over Northern Africa and western Europe. Atmos. Chem. Phys. 2016;16(20):12961–12982. [Google Scholar]
- Meo S.A., Abukhalaf A.A., Alessa O.M., Alarifi A.S., Sami W., Klonoff D.C. Effect of Environmental Pollutants PM2.5, CO, NO2, and O3 on the Incidence and Mortality of SARS-CoV-2 Infection in Five Regions of the USA. Int. J. Environ. Res. Public Health. 2021;18:7810. doi: 10.3390/ijerph18157810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawska L., Cao J. Airborne transmission of SARS-CoV-2: The world should face the reality. Environ. Int. 2020;139:105730. doi: 10.1016/j.envint.2020.105730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawska L., Milton D.K. It Is Time to Address Airborne Transmission of Coronavirus Disease 2019 (COVID-19) Clin. Infect. Dis. 2020;71:2311–2313. doi: 10.1093/cid/ciaa939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno T., Pintó R.M., Bosch A., Moreno N., Alastuey A., Minguillón M.C., Anfruns-Estrada E., Guix S., Fuentes C., Buonanno G., Stabile L., Morawska L., Querol X. Tracing surface and airborne SARS-CoV-2 RNA inside public buses and subway trains. Environ. Int. 2021;147:106326. doi: 10.1016/j.envint.2020.106326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B.K., Ohmine S., Tomer D.P., Jensen K.J., Johnson F.B., Kirsi J.J., Robison R.A., O’Neill K.L. Virion disruption by ozone-mediated reactive oxygen species. J. Virol. Methods. 2008;153(1):74–77. doi: 10.1016/j.jviromet.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Nannu Shankar S., Witanachchi C.T., Morea A.F., Lednicky J.A., Loeb J.C., Alam M.M., Fan Z.H., Eiguren-Fernandez A., Wu C.-Y. SARS-CoV-2 in residential rooms of two self-isolating persons with COVID-19. J. Aerosol Sci. 2022;159:105870. doi: 10.1016/j.jaerosci.2021.105870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nor N.S.M., Yip C.W., Ibrahim N., Jaafar M.H., Rashid Z.Z., Mustafa N., Hamid H.H.A., Chandru K., Latif M.T., Saw P.E., Lin C.Y., Alhasa K.M., Hashim J.H., Nadzir M.S.M. Particulate matter (PM2.5) as a potential SARS-CoV-2 carrier. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-81935-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogen Y. Assessing nitrogen dioxide (NO2) levels as a contributing factor to coronavirus (COVID-19) fatality. Sci. Total Environ. 2020;726:138605. doi: 10.1016/j.scitotenv.2020.138605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pansini R., Fornacca D. Early Spread of COVID-19 in the Air-Polluted Regions of Eight Severely Affected Countries. Atmosphere. 2021;12(9):795. [Google Scholar]
- Passos R.G., Silveira M.B., Abrahão J.S. Exploratory assessment of the occurrence of SARS-CoV-2 in aerosols in hospital facilities and public spaces of a metropolitan center in Brazil. Environ. Res. 2021;195:110808. doi: 10.1016/j.envres.2021.110808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peduru Hewa T.M., Tannock G.A., Mainwaring D.E., Harrison S., Fecondo J.V. The detection of influenza A and B viruses in clinical specimens using a quartz crystal microbalance. J. Virol. Methods. 2009;162(1-2):14–21. doi: 10.1016/j.jviromet.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L.u., Zhao X., Tao Y., Mi S., Huang J.u., Zhang Q. The effects of air pollution and meteorological factors on measles cases in Lanzhou, China. Environ. Sci. Pollut. R. 2020;27(12):13524–13533. doi: 10.1007/s11356-020-07903-4. [DOI] [PubMed] [Google Scholar]
- Pigny F., Wagner N., Rohr M., Mamin A., Cherpillod P., Posfay-Barbe K.M., Kaiser L., Eckerle I., L'Huillier A.G., Geneva Pediatric C.G. Viral co-infections among SARS-CoV-2-infected children and infected adult household contacts. Eur. J. Pediatr. 2021;180:1991–1995. doi: 10.1007/s00431-021-03947-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivato A., Amoruso I., Formenton G., Di Maria F., Bonato T., Vanin S., Marion A., Baldovin T. Evaluating the presence of SARS-CoV-2 RNA in the particulate matters during the peak of COVID-19 in Padua, northern Italy. Sci. Total Environ. 2021;784:147129. doi: 10.1016/j.scitotenv.2021.147129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlert, T., 2014. The Pairwise Multiple Comparison of Mean Ranks Package (PMCMR). R package. URL https://CRAN.R-project.org/package=PMCMR.
- Rathod A., Beig G. Impact of biomass induced black carbon particles in cascading COVID-19. Urban Clim. 2021;38:100913. doi: 10.1016/j.uclim.2021.100913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzaq A., Sharif A., Aziz N., Irfan M., Jermsittiparsert K. Asymmetric link between environmental pollution and COVID-19 in the top ten affected states of US: A novel estimations from quantile-on-quantile approach. Environ. Res. 2020;191:110189. doi: 10.1016/j.envres.2020.110189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzini K., Castrica M., Menchetti L., Maggi L., Negroni L., Orfeo N.V., Pizzoccheri A., Stocco M., Muttini S., Balzaretti C.M. SARS-CoV-2 RNA detection in the air and on surfaces in the COVID-19 ward of a hospital in Milan. Italy. Sci Total Environ. 2020;742:140540. doi: 10.1016/j.scitotenv.2020.140540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redlberger-Fritz M., Kundi M., Aberle S.W., Puchhammer-Stöckl E. Significant impact of nationwide SARS-CoV-2 lockdown measures on the circulation of other respiratory virus infections in Austria. J. Clin. Virol. 2021;137:104795. doi: 10.1016/j.jcv.2021.104795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revelle W. psych: Procedures for Psychological, Psychometric, and Personality Research. R Package Version. 2018;1:8. https://CRAN.R-project.org/package=psych: [Google Scholar]
- Rivas I., Beddows D.C.S., Amato F., Green D.C., Järvi L., Hueglin C., Reche C., Timonen H., Fuller G.W., Niemi J.V., Pérez N., Aurela M., Hopke P.K., Alastuey A., Kulmala M., Harrison R.M., Querol X., Kelly F.J. Source apportionment of particle number size distribution in urban background and traffic stations in four European cities. Environ. Int. 2020;135:105345. doi: 10.1016/j.envint.2019.105345. [DOI] [PubMed] [Google Scholar]
- Robotto A., Civra A., Quaglino P., Polato D., Brizio E., Lembo D. SARS-CoV-2 airborne transmission: A validated sampling and analytical method. Environ. Res. 2021;200:111783. doi: 10.1016/j.envres.2021.111783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues A.F., Santos A.M., Ferreira A.M., Marino R., Barreira M.E., Cabeda J.M. Year-Long Rhinovirus Infection is Influenced by Atmospheric Conditions, Outdoor Air Virus Presence, and Immune System-Related Genetic Polymorphisms. Food. Environ. Virol. 2019;11(4):340–349. doi: 10.1007/s12560-019-09397-x. [DOI] [PubMed] [Google Scholar]
- Rodriguez J.A., Rubio-Gomez H., Roa A.A., Miller N., Eckardt P.A. Co-Infection with SARS-COV-2 and Parainfluenza in a young adult patient with pneumonia: Case Report. Idcases. 2020;20:e00762. doi: 10.1016/j.idcr.2020.e00762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RStudio Team . RStudio, Inc.; Boston, MA: 2018. RStudio: Integrated Development for R.http://www.rstudio.com/ URL. [Google Scholar]
- Sampath R., Hofstadler S.A., Blyn L.B., Eshoo M.W., Hall T.A., Massire C., Levene H.M., Hannis J.C., Harrell P.M., Neuman B., Buchmeier M.J., Jiang Y., Ranken R., Drader J.J., Samant V., Griffey R.H., McNeil J.A., Crooke S.T., Ecker D.J. Rapid identification of emerging pathogens: Coronavirus. Emerging Infect Dis. 2005;11(3):373–379. doi: 10.3201/eid1103.040629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuit M., Ratnesar-Shumate S., Yolitz J., Williams G., Weaver W., Green B., Miller D., Krause M., Beck K., Wood S., Holland B., Bohannon J., Freeburger D., Hooper I., Biryukov J., Altamura L.A., Wahl V., Hevey M., Dabisch P. Airborne SARS-CoV-2 Is Rapidly Inactivated by Simulated Sunlight. J. Infect. Dis. 2020;222(4):564–571. doi: 10.1093/infdis/jiaa334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlmaier N., Hoppenheidt K., Krist H., Lehmann S., Lang H., Büttner M. Generation of avian influenza virus (AIV) contaminated fecal fine particulate matter (PM2.5): Genome and infectivity detection and calculation of immission. Vet. Microbiol. 2009;139(1-2):156–164. doi: 10.1016/j.vetmic.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Senatore V., Zarra T., Buonerba A., Choo K.H., Hasan S.W., Korshin G., Li C.W., Ksibi M., Belgiorno V., Naddeo V. Indoor versus outdoor transmission of SARS-COV-2: environmental factors in virus spread and underestimated sources of risk. Euro-Mediterr. J. Environ. Integr. 2021;6:30. doi: 10.1007/s41207-021-00243-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setti L., Passarini F., De Gennaro G., Barbieri P., Licen S., Perrone M.G., Piazzalunga A., Borelli M., Palmisani J., Di Gilio A., Rizzo E., Colao A., Piscitelli P., Miani A. Potential role of particulate matter in the spreading of COVID-19 in Northern Italy: first observational study based on initial epidemic diffusion. Bmj Open. 2020;10(9):e039338. doi: 10.1136/bmjopen-2020-039338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setti L., Passarini F., De Gennaro G., Barbieri P., Perrone M.G., Borelli M., Palmisani J., Di Gilio A., Torboli V., Fontana F., Clemente L., Pallavicini A., Ruscio M., Piscitelli P., Miani A. SARS-Cov-2RNA found on particulate matter of Bergamo in Northern Italy: First evidence. Environ. Res. 2020;188:109754. doi: 10.1016/j.envres.2020.109754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma ArunKumar, Balyan P. Air pollution and COVID-19: Is the connect worth its weight? Indian J. Public Health. 2020;64(6):132. doi: 10.4103/ijph.IJPH_466_20. [DOI] [PubMed] [Google Scholar]
- Shi T., McAllister D.A., O'Brien K.L., Simoes E.A.F., Madhi S.A., Gessner B.D., Polack F.P., Balsells E., Acacio S., Aguayo C., Alassani I., Ali A., Antonio M., Awasthi S., Awori J.O., Azziz-Baumgartner E., Baggett H.C., Baillie V.L., Balmaseda A., Barahona A., Basnet S., Bassat Q., Basualdo W., Bigogo G., Bont L., Breiman R.F., Brooks W.A., Broor S., Bruce N., Bruden D., Buchy P., Campbell S., Carosone-Link P., Chadha M., Chipeta J., Chou M., Clara W., Cohen C., de Cuellar E., Dang D.A., Dash-yandag B., Deloria-Knoll M., Dherani M., Eap T., Ebruke B.E., Echavarria M., Emediato C.C.D.L., Fasce R.A., Feikin D.R., Feng L.Z., Gentile A., Gordon A., Goswami D., Goyet S., Groome M., Halasa N., Hirve S., Homaira N., Howie S.R.C., Jara J., Jroundi I., Kartasasmita C.B., Khuri-Bulos N., Kotloff K.L., Krishnan A., Libster R., Lopez O., Lucero M.G., Lucion F., Lupisan S.P., Marcone D.N., McCracken J.P., Mejia M., Moisi J.C., Montgomery J.M., Moore D.P., Moraleda C., Moyes J., Munywoki P., Mutyara K., Nicol M.P., Nokes D.J., Nymadawa P., Oliveira M.T.D., Oshitani H., Pandey N., Paranhos-Baccala G., Phillips L.N., Picot V.S., Rahman M., Rakoto-Andrianarivelo M., Rasmussen Z.A., Rath B.A., Robinson A., Romero C., Russomando G., Salimi V., Sawatwong P., Scheltema N., Schweiger B., Scott J.A.G., Seidenberg P., Shen K.L., Singleton R., Sotomayor V., Strand T.A., Sutanto A., Sylla M., Tapia M.D., Thamthitiwat S., Thomas E.D., Tokarz R., Turner C., Venter M., Waicharoen S., Wang J.W., Watthanaworawit W., Yoshida L.M., Yu H.J., Zar H.J., Campbell H., Nair H., Network R.G.E. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet. 2017;390:946–958. doi: 10.1016/S0140-6736(17)30938-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu E.Y.C., Huang W., Ye D., Xie Y., Mo J., Li Y., Cowling B.J., Yang Z., Leung N.H.L. Frequent recovery of influenza A but not influenza B virus RNA in aerosols in pediatric patient rooms. Indoor Air. 2020;30(5):805–815. doi: 10.1111/ina.12669. [DOI] [PubMed] [Google Scholar]
- Shomali M., Opie D., Avasthi T., Trilling A., Wu M.-H. Nitrogen Dioxide Sterilization in Low-Resource Environments: A Feasibility Study. PLoS ONE. 2015;10(6):e0130043. doi: 10.1371/journal.pone.0130043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth D.S., Trujillo M., Gregory D.A., Cheung K., Gao A., Graham M., Guan Y., Guldenpfennig C., Hoxie I., Kannoly S., Kubota N., Lyddon T.D., Markman M., Rushford C., San K.M., Sompanya G., Spagnolo F., Suarez R., Teixeiro E., Daniels M., Johnson M.C., Dennehy J.J. Tracking cryptic SARS-CoV-2 lineages detected in NYC wastewater. Nat. Commun. 2022;13:635. doi: 10.1038/s41467-022-28246-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen J.H., Mackay D.K.J., Jensen C.Ø., Donaldson A.I. An integrated model to predict the atmospheric spread of foot-and-mouth disease virus. Epidemiol. Infect. 2000;124(3):577–590. doi: 10.1017/s095026889900401x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern R.A., Al-Hemoud A., Alahmad B., Koutrakis P. Levels and particle size distribution of airborne SARS-CoV-2 at a healthcare facility in Kuwait. Sci. Total Environ. 2021;782:146799. doi: 10.1016/j.scitotenv.2021.146799. [DOI] [Google Scholar]
- Stern R.A., Koutrakis P., Martins M.A.G., Lemos B., Dowd S.E., Sunderland E.M., Garshick E. Characterization of hospital airborne SARS-CoV-2. Resp Res. 2021;22:73. doi: 10.1186/s12931-021-01637-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swets M.C., Russell C.D., Harrison E.M., Docherty A.B., Lone N., Girvan M., Hardwick H.E., Visser L.G., Openshaw P.J.M., Groeneveld G.H., Semple M.G., Baillie J.K. SARS-CoV-2 co-infection with influenza viruses, respiratory syncytial virus, or adenoviruses. Lancet. 2022;399(10334):1463–1464. doi: 10.1016/S0140-6736(22)00383-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y., Yue Y., Qiu G., Ji Z., Spillman M., Gai Z., Chen Q., Bielecki M., Huber M., Trkola A., Wang Q., Cao J., Wang J. Comparison of analytical sensitivity and efficiency for SARS-CoV-2 primer sets by TaqMan-based and SYBR Green-based RT-qPCR. Appl. Microbiol. Biotechnol. 2022;106(5-6):2207–2218. doi: 10.1007/s00253-022-11822-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L.i., Ma B., Lai X., Han L., Cao P., Zhang J., Fu J., Zhou Q., Wei S., Wang Z., Peng W., Yang L., Zhang X. Air and surface contamination by SARS-CoV-2 virus in a tertiary hospital in Wuhan, China. Int. J. Infect. Dis. 2020;99:3–7. doi: 10.1016/j.ijid.2020.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson K.-A., Bennett A.M. Persistence of influenza on surfaces. J. Hosp. Infect. 2017;95(2):194–199. doi: 10.1016/j.jhin.2016.12.003. [DOI] [PubMed] [Google Scholar]
- Troeger C., Forouzanfar M., Rao P.C., Khalil I., Brown A., Swartz S., Fullman N., Mosser J., Thompson R.L., Reiner R.C., Abajobir A., Alam N., Alemayohu M.A., Amare A.T., Antonio C.A., Asayesh H., Avokpaho E., Barac A., Beshir M.A., Boneya D.J., Brauer M., Dandona L., Dandona R., Fitchett J.R.A., Gebrehiwot T.T., Hailu G.B., Hotez P.J., Kasaeian A., Khoja T., Kissoon N., Knibbs L., Kumar G.A., Rai R.K., Abd El Razek H.M., Mohammed M.S.K., Nielson K., Oren E., Osman A., Patton G., Qorbani M., Roba H.S., Sartorius B., Savic M., Shigematsu M., Sykes B., Swaminathan S., Topor-Madry R., Ukwaja K., Werdecker A., Yonemoto N., Zaki M.E., Lim S.S., Naghavi M., Vos T., Hay S.I., Murray C.J.L., Mokdad A.H., Collaborators G.L. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory tract infections in 195 countries: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect. Dis. 2017;17:1133–1161. doi: 10.1016/S1473-3099(17)30396-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng C.-C., Li C.-S. Ozone for inactivation of aerosolized bacteriophages. Aerosol Sci. Technol. 2006;40(9):683–689. [Google Scholar]
- van der Valk J.P.M. The Interplay Between Air Pollution and Coronavirus Disease (COVID-19) Veen J., editor. J. Occup. Environ. Med. 2021;63:e163–e167. doi: 10.1097/JOM.0000000000002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez-Apestegui B.V., Parras-Garrido E., Tapia V., Paz-Aparicio V.M., Rojas J.P., Sanchez-Ccoyllo O.R., Gonzales G.F. Association between air pollution in Lima and the high incidence of COVID-19: findings from a post hoc analysis. BMC Public Health. 2021;21(1):1161. doi: 10.1186/s12889-021-11232-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatakrishnan, A.J., Anand, P., Lenehan, P.J., Suratekar, R., Raghunathan, B., Niesen, M.J.M., Soundararajan, V., 2021 Omicron variant of SARS-CoV-2 harbors a unique insertion mutation of putative viral or human genomic origin. https://osf.io/f7txy (preprint, accessed December 6, 2021).
- Wang B., Liu J., Li Y., Fu S., Xu X., Li L., Zhou J., Liu X., He X., Yan J., Shi Y., Niu J., Yang Y., Li Y., Luo B., Zhang K. Airborne particulate matter, population mobility and COVID-19: a multi-city study in China. BMC Public Health. 2020;20:1585. doi: 10.1186/s12889-020-09669-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wu R., Liu L., Yuan Y., Liu ChenGuang, Hang Ho S.S., Ren H., Wang Q., Lv Y., Yan M., Cao J. Differential health and economic impacts from the COVID-19 lockdown between the developed and developing countries: Perspective on air pollution. Environ. Pollut. 2022;293:118544. doi: 10.1016/j.envpol.2021.118544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wannaz E.D., Larrea Valdivia A.E., Reyes Larico J.A., Salcedo Peña J., Valenzuela Huillca C. PM10 correlates with COVID-19 infections 15 days later in Arequipa, Peru. Environ. Sci. Pollut. R. 2021;28(29):39648–39654. doi: 10.1007/s11356-021-13408-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Bartrand T.A., Omura T., Haas C.N. Dose-response assessment for influenza A virus based on data sets of infection with its live attenuated reassortants. Risk Anal. 2012;32(3):555–565. doi: 10.1111/j.1539-6924.2011.01680.x. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Bartrand T.A., Weir M.H., Omura T., Haas C.N. Development of a dose-response model for SARS coronavirus. Risk Anal. 2010;30(7):1129–1138. doi: 10.1111/j.1539-6924.2010.01427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H. ggplot2. WIRES. 2011;3(2):180–185. [Google Scholar]
- Williams, T.C., Sinha, I., Barr, I.G., Zambon, M., 2021. Transmission of paediatric respiratory syncytial virus and influenza in the wake of the COVID-19 pandemic. Euro. Surveill. 26 (29). pii = 2100186. [DOI] [PMC free article] [PubMed]
- Woodby B., Arnold M.M., Valacchi G. SARS-CoV-2 infection, COVID-19 pathogenesis, and exposure to air pollution: What is the connection? Ann. N. Y. Acad. Sci. 2021;1486:15–38. doi: 10.1111/nyas.14512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Zhang J., Xiao A., Gu X., Lee W.L., Armas F., Kauffman K., Hanage W., Matus M., Ghaeli N., Endo N., Duvallet C., Poyet M., Moniz K., Washburne A.D., Erickson T.B., Chai P.R., Thompson J., Alm E.J., Gilbert J.A. SARS-CoV-2 Titers in Wastewater Are Higher than Expected from Clinically Confirmed Cases. Msystems. 2020;5(4) doi: 10.1128/mSystems.00614-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C., Lau E.H.Y., Yoshida T., Yu H., Wang X., Wu H., Wei J., Cowling B., Peiris M., Li Y., Yen H.L. Detection of Influenza and Other Respiratory Viruses in Air Sampled From a University Campus: A Longitudinal Study. Clin. Infect. Dis. 2020;70:850–858. doi: 10.1093/cid/ciz296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Hu W., Williams G., Clements A.C.A., Kan H., Tong S. Air pollution, temperature and pediatric influenza in Brisbane, Australia. Environ. Int. 2013;59:384–388. doi: 10.1016/j.envint.2013.06.022. [DOI] [PubMed] [Google Scholar]
- Yadana S., Coleman K.K., Nguyen T.T., Hansen-Estruch C., Kalimuddin S., Thoon K.C., Low J.G.H., Gray G.C. Monitoring for airborne respiratory viruses in a general pediatric ward in Singapore. J. Public Health Res. 2019;8:100–103. doi: 10.4081/jphr.2019.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarahmadi R., Bokharaei-Salim F., Soleimani-Alyar S., Moridi P., Moradi-Moghaddam O., Niakan-Lahiji M., Darvishi M.-M., Golmahammadi S., Mousavi S.A.J., Ebrahimi H., Ashtarinezad A., Farshad A.-A., Jonidi-Jafari A., Kiani S.J., Garshasbi S., Mehrzadi S. Occupational exposure of health care personnel to SARS-CoV-2 particles in the intensive care unit of Tehran hospital. Int. J. Environ. Sci. Technol. 2021;18(12):3739–3746. doi: 10.1007/s13762-020-03095-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q., Fu J.-F., Mao J.-H., Shang S.-Q. Haze is a risk factor contributing to the rapid spread of respiratory syncytial virus in children. Environ. Sci. Pollut. R. 2016;23(20):20178–20185. doi: 10.1007/s11356-016-7228-6. [DOI] [PubMed] [Google Scholar]
- Zhang X., Ji Z., Yue Y., Liu H., Wang J. Infection Risk Assessment of COVID-19 through Aerosol Transmission: a Case Study of South China Seafood Market. Environ. Sci. Technol. 2021;55(7):4123–4133. doi: 10.1021/acs.est.0c02895. [DOI] [PubMed] [Google Scholar]
- Zhang X., Wang J. Dose-response Relation Deduced for Coronaviruses From Coronavirus Disease 2019, Severe Acute Respiratory Syndrome, and Middle East Respiratory Syndrome: Meta-analysis Results and its Application for Infection Risk Assessment of Aerosol Transmission. Clin. Infect. Dis. 2021;73(1):e241–e245. doi: 10.1093/cid/ciaa1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Nie W., Zhou C., Cheng M., Wang C., Liu Y., Li J., Qian Y., Ma X., Zhang L., Li L., Hu K. Airborne Transmission of Influenza Virus in a Hospital of Qinhuangdao During 2017–2018 Flu Season. Food Environ Virol. 2019;11(4):427–439. doi: 10.1007/s12560-019-09404-1. [DOI] [PubMed] [Google Scholar]
- Zhao X., Sun Y., Zhao C., Jiang H. Impact of Precipitation with Different Intensity on PM2.5 over Typical Regions of China. Atmosphere. 2020;11:906. [Google Scholar]
- Zhao Y., Richardson B., Takle E., Chai L.L., Schmitt D., Xin H.W. Airborne transmission may have played a role in the spread of 2015 highly pathogenic avian influenza outbreaks in the United States. Sci. Rep. 2019;9:11755. doi: 10.1038/s41598-019-47788-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z., Xu G., Li Q., Chen C., Li J. Effect of precipitation on reducing atmospheric pollutant over Beijing. Atmospheric Pollut Res. 2019;10(5):1443–1453. [Google Scholar]
- Zhou L., Yao M., Zhang X., Hu B., Li X., Chen H., Zhang L.u., Liu Y., Du M., Sun B., Jiang Y., Zhou K., Hong J., Yu N.a., Ding Z., Xu Y., Hu M., Morawska L., Grinshpun S.A., Biswas P., Flagan R.C., Zhu B., Liu W., Zhang Y. Breath-, air- and surface-borne SARS-CoV-2 in hospitals. J. Aerosol Sci. 2021;152:105693. doi: 10.1016/j.jaerosci.2020.105693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Yue Y., Bai Y., Zhang L. Effects of Rainfall on PM2.5 and PM10 in the Middle Reaches of the Yangtze River. Adv. Meteorol. 2020;2020:1–10. [Google Scholar]
- Zoran M.A., Savastru R.S., Savastru D.M., Tautan M.N., Baschir L.A., Tenciu D.V. Exploring the linkage between seasonality of environmental factors and COVID-19 waves in Madrid, Spain. Process Saf. Environ. Prot. 2021;152:583–600. doi: 10.1016/j.psep.2021.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.