Abstract
Nonalcoholic steatohepatitis (NASH), as a severe form of nonalcoholic fatty liver disease (NAFLD), is characterized by liver steatosis, inflammation, hepatocellular injury and different degrees of fibrosis. The pathogenesis of NASH is complex and multifactorial, obesity and type 2 diabetes mellitus (T2DM) have been implicated as major risk factors. Glucagon-like peptide-1 receptor (GLP-1R) is one of the most successful drug targets of T2DM and obesity, and its peptidic ligands have been proposed as potential therapeutic agents for NASH. In this article we provide an overview of the pathophysiology and management of NASH, with a special focus on the pharmacological effects and possible mechanisms of GLP-1 mimetics in treating NAFLD/NASH, including dual and triple agonists at GLP-1R, glucose-dependent insulinotropic polypeptide receptor or glucagon receptor.
Keywords: NAFLD, NASH, GLP-1R, GLP-1 mimetics, dual agonists, triple agonists, GPCR
Introduction
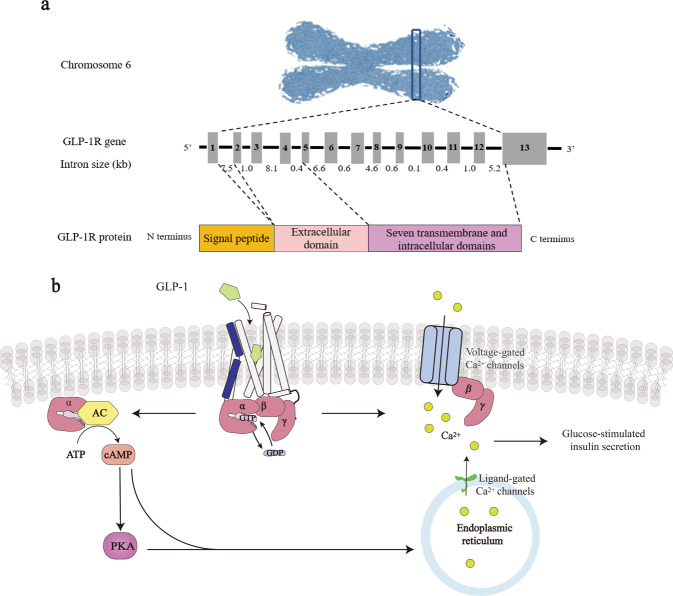
Glucagon-like peptide-1 receptor (GLP-1R) belongs to the class B1 family of G protein-coupled receptors (GPCRs) with 7-transmembrane spanning plus an extracellular N-terminal domain (ECD) (Fig. 1a). The human GLP-1R gene is located on chromosome 6 (6p21) and the receptor is particularly abundant in β-cells in pancreatic islets but can also be found in the lung, kidney, stomach, heart, intestine, retina, adipose, and multiple regions of the central nervous system (CNS) [1, 2]. Upon ligand stimulation, GLP-1R couples to Gαs, leading to activation of multiple downstream signaling pathways [3]. It is functionally linked to adenylyl cyclase (AC) via Gαs to accumulate the second messenger cyclic adenosine monophosphate (cAMP) and activates protein kinase A [4]. Through ligand-gated calcium channel on the endoplasmic reticulum (ER) or voltage-dependent Ca2+ channels, GLP-1R increases the intracellular Ca2+ concentrations to potentiate glucose-dependent insulin secretion [5, 6] (Fig. 1b). The endogenous ligand, GLP-1, is capable of promoting insulin secretion, reducing food intake and slowing gastric emptying [7, 8]. NASH is the pathological progression of NAFLD which stems from abnormal and significant accumulation of fat in the liver and associates with high burden of metabolic comorbidities, such as obesity, insulin resistance (IR), and type 2 diabetes mellitus (T2DM). Therefore, GLP-1R has the potential to become a drug target for NASH.
Fig. 1. Genome organization of the human GLP-1R gene and GLP-1R signaling.
a The GLP-1R gene is located in chromosome 6 (6p21) and contains 13 exons interrupted by introns of varying lengths (0.4–8.1 kb). It encodes a protein of 463 amino acids consisting of signal peptide, ECD, and transmembrane domain (TMD). b Upon GLP-1 binding, the receptor recruits G protein trimers and converts guanosine triphosphate (GTP) to guanosine diphosphate (GDP) enabling Gα to dissociate and activate AC by converting ATP to cAMP. Release of stored calcium ions by the endoplasmic reticulum will follow to increase glucose-stimulated insulin secretion.
Patients with NAFLD will be diagnosed as NASH with the symptoms such as hepatic steatosis, lobular inflammation, hepatocellular ballooning, or other signs of hepatocyte injury, including pericellular fibrosis. If it is not controlled, the condition of some patients with NASH may progress and leads to cirrhosis and hepatocellular carcinoma. The incidence of liver cirrhosis in NASH patients is as high as 15%–25% within 10–15 years [9]. Cardiovascular diseases, malignant tumors, and decompensated liver cirrhosis are common causes of death among NASH patients. It was reported that the global prevalence rate of NAFLD is 25.24% (95% CI: 22.10–28.65) and that of NASH is estimated between 1.5% and 6.45%, according to the fact that 7%–30% of NAFLD patients who underwent voluntary liver biopsies had NASH [10].
Since NASH is more common in obese patients, especially those combined with IR, high triglycerides and/or T2DM, early management of fat accumulation as well as inflammation has become the mainstream strategy for NASH treatment. This review intends to give an overview of the effects of GLP-1 mimetics on the pathogenesis of NAFLD/NASH and their therapeutic potential. Possible mechanisms of action and current status of this approach are also discussed.
Pathophysiology of NASH
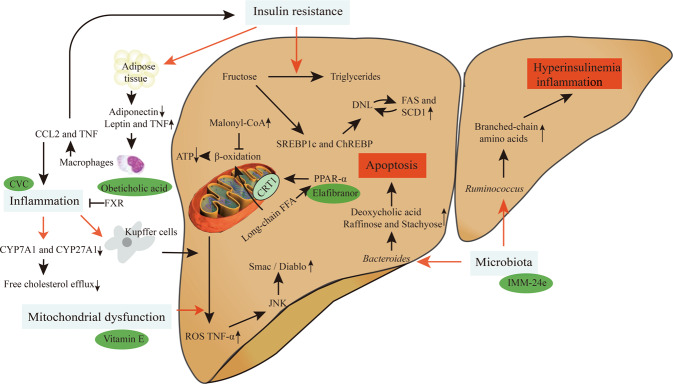
Pathogenesis of NASH is complex and multifactorial (Fig. 2). Genetic predisposition such as single-nucleotide polymorphisms were found to be associated with NASH. For instance, rs738409 C > G encoding I148M protein variant of PNPLA3 [11] causes a loss of acylglycerol hydrolase function leading to impaired lipid metabolism [12]. TM6SF2, a Golgi transmembrane protein, is a lipid transporter. Its variant rs58542926 C > T encoding TM6SF2 E167K was shown to reduce lipoprotein and apolipoprotein B (APOB) levels and impair very low-density lipoprotein (VLDL) secretion with consequent triglycerides (TG) accumulation and heightened susceptibility to hepatic fat, NASH and fibrosis [13, 14].
Fig. 2. The pathophysiology of NASH.
There are four main molecular mechanisms of NASH: (i) Under insulin resistance, fructose could be converted to triglycerides thereby facilitating DNL. Insulin resistance in adipose tissue can cause adipokine distribution disorder and induce liver inflammation; (ii) Mitochondrial dysfunction leads to excessive production of ROS and TNF-α to trigger JNK, which induces the release of pro-apoptotic Smac/Diablo protein; (iii) Two characteristics of NASH are the production of foamy KCs and cholesterol crystals in liver cells; (iv) Bacteroides are independently related to NASH while Ruminococcus is independently related to clinically obvious fibrosis.
Liver lipids come from dietary intake, plasma free fatty acid (FFA) accumulation, and hepatic de novo lipogenesis (DNL). Many contemporary diets are rich of saturated fats and simple sugars, such as sucrose and fructose [15]. DNL of the liver is most commonly produced by carbohydrate catabolism. In addition to glucose, which provides carbon units for DNL, fructose can also drive DNL as an adipogenic substrate. In fact, fructose is increasingly used as a sweetener in corn syrup [16]. Unlike glucose, fructose bypasses the rate-limiting step of glycolysis catalyzed by phosphofructokinase in the liver [17] thereby supplying more substrates for DNL. Fructose can be converted into TG under conditions of IR. In this metabolic process, fructose directly stimulates sterol regulatory element-binding protein (SREBP1c) and carbohydrate response element-binding protein (ChREBP), the major transcription regulators of DNL, and subsequently increases the levels of all DNL-associated enzymes such as fatty acid synthase (FAS) and stearoyl-CoA desaturase 1 (SCD1) [18]. Fructose also decreases mitochondrial adenosine triphosphate (ATP) production by inhibiting mitochondrial β-oxidation via increased malonyl-CoA levels—a passage to abnormal liver ATP homeostasis that is linked to NASH [19].
Mitochondrial dysfunction (particularly respiratory chain deficiency) is widely recognized as a major factor in the pathogenesis of NASH [20]. Activation by long-chain FFA of peroxisome proliferators activate receptor α (PPARα) can increase the expression of carnitine palmitoyltransferase-1 (CPT-1) on the outer mitochondrial membrane, through which long-chain FFA can enter the mitochondria for β-oxidation [21]. Memon and co-workers demonstrated that in three different obesity and a large number of steatosis gene models, including ob/ob and db/db mice, the expression of PPARα in the liver compensatorily increases [22]. However, this basal increase may not be sufficient to remove excess lipids. Mitochondrial dysfunction not only destroys liver fat balance but also produces an excessive amount of reactive oxygen species (ROS), leading to lipid peroxidation and release of highly reactive aldehyde derivatives (such as malondialdehyde). This will worsen lipid peroxidation and overproduce cytokines such as tumor necrosis factor-α (TNF-α), transforming growth factor-β (TGF-β) and Fas ligand. Fat accumulation, ROS and TNF-α can activate c-Jun N-terminal kinase (JNK) in the liver to release pro-apoptotic Smac/Diablo protein by mitochondria [23].
NASH is characterized by liver steatosis, inflammation, hepatocellular injury, and different degrees of fibrosis. The triggers of liver inflammation in the development from NAFLD to NASH may come from other organs such as the adipose tissue (AT) and gut, or may be induced by the liver itself, for instance, lipotoxicity, innate immune responses, cell death pathways, mitochondrial dysfunction and ER stress. Visceral AT (VAT), in particular, is correlated with inflammation and fibrosis in human NAFLD. IR in VAT can cause adipokine distribution disorder (low adiponectin, high leptin and TNF-α levels), making macrophages to secrete chemokines and cytokines such as C-C chemokine 2 (CCL2) and TNF-α to induce liver inflammation and IR [24]. Two characteristics of NASH are the production of foamy Kupffer cells (KCs) and cholesterol crystals in liver cells. Danger-associated molecular patterns, such as ATP, can activate KCs and lead to ROS formation, the severity of which is related to the number of neutrophils and the degree of liver damage in NASH patients [25]. Reduction of cytochrome P450 family 7 subfamily A member 1 (CYP7A1) and cytochrome P450 family 27 subfamily A member 1 (CYP27A1), two enzymes responsible for cholesterol metabolism, is a common feature of patients with NAFLD/NASH resulting in a decreased free cholesterol efflux [26]. Recently developed farnesoid X receptor (FXR) knockout (KO) mice were shown to have the histological characteristics of NASH: increased steatosis, expression of TNF-α and toll-like receptor 4 (TLR-4), with inflammation and fibrosis [27].
An association between enteric malnutrition and NASH was proposed not long ago [28]. It was reported that Bacteroides is independently related to NASH: The abundance of Bacteroides is positively correlated with the content of deoxycholic acid, raffinose, and stachyose (containing glucose and fructose) in feces. Among them, deoxycholic acid induces rat liver apoptosis and its level is increased in the liver of NASH patients [29]. Another microbiota, Ruminococcus, is independently related to clinically obvious fibrosis [28]. Its presence was enhanced in formula-fed infant rhesus monkeys accompanied by elevated branched-chain amino acids, hyperinsulinemia, and signs of serum inflammatory biomarkers [30]. Intestinal bacteria can hydrolyze choline (a cell membrane component of lipid transport to form dimethylamine and trimethylamine in the liver) and metabolize it into methylamine, a toxic compound that causes inflammation and liver damage [31]. Abnormal flora could also induce TG accumulation and promote NASH to reduce choline and increase methylamine [32].
Management of NASH
Accumulation of fat in the liver occurs within the physio-pathological progression from a healthy liver to NAFLD. Patients with NAFLD will be diagnosed as NASH with the symptoms such as hepatic steatosis, lobular inflammation, hepatocellular ballooning or other signs of hepatocyte injury, including pericellular fibrosis. Currently, there is no approved drug therapy for NASH. Based on medical demands, various treatment options were tried aimed at preventing, delaying, and reversing the progress of NASH. Efforts were also made to improve clinical outcomes, such as reducing liver cirrhosis occurrence and its complications, decreasing the need for liver transplantation and improving quality of life. The first line of treatment for NAFLD and NASH is weight loss, done through a combination of calorie reduction, exercise, bariatric surgery, and healthy dietary composition such as decreasing fructose consumption. A threshold of 7% in weight loss is set to elicit histological improvement (reduction of fat and inflammation in the liver) [33].
Albeit not developed for NASH, drug intervention has also been attempted. The first regimen targets oxidative stress with therapeutics such as vitamin E (a free radical scavenger, which efficacy has been established in NASH [34]) and elafibranor (PPAR α/δ agonist, responsible for fatty acid oxidation). The second type of drugs target inflammation and hepatic fibrosis. Obeticholic acid is a synthetic variant of natural bile acid chenodeoxycholic acid (an FXR agonist). Selonsertib inhibits apoptosis signal-regulated kinase 1 thereby preventing hepatocytes from apoptosis and fibrosis [34]. Cenicriviroc is a C–C motif chemokine receptor-2/5 antagonist developed to treat inflammation [35]. The third strategy targets intestinal microbiomes. IMM-24e can reduce the liver exposure to gut-derived bacterial products and lipopolysaccharide. The fourth therapeutics all have antidiabetic properties. Considering that obesity and T2DM are major risk factors for NASH, treatment options that impact body weight and glucose control have been explored. GLP-1R agonist and sodium-glucose cotransporter-2 inhibitor (SGLT2) inhibitors approved for diabetes also show early efficacy in NASH [36, 37]. For example, while cotadutide (a dual agonist at GLP-1R and glucagon receptor, GCGR) reduces body weight, inhibits food intake and maintains glucose homeostasis through GLP-1R, its action on the liver to decrease lipid content, drive glycogen flux and improve mitochondrial function are mediated via the GCGR [38], which is abundantly expressed by hepatocytes [39]. Activation of GCGR results in increased expression of essential gluconeogenic genes and modulation of sugar metabolism related enzymatic activities to increase hepatic glucose production (HGP) [40]. Previous studies showed that chronic GCGR activation reduced body weight and reversed hepatic steatosis in diet-induced obese (DIO) mice [41, 42]. Besides, antagonists of an emerging key modulator of lipid metabolism, glucose-dependent insulinotropic polypeptide (GIP) have been shown to reverse AT, liver, and muscle TG accumulation, and improve associated metabolic disturbances induced by a high-fat diet (HFD) [43].
GLP-1 mimetics
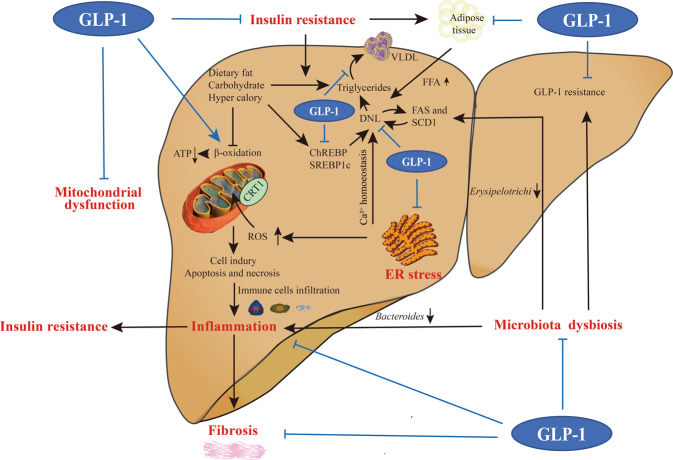
GLP-1 mimetics, commonly described as regulators of glucolipid metabolism, also display positive effects on oxidative stress, inflammatory response, energy expenditure, and microbiota regulation (Fig. 3), and have been proposed as a promising therapeutic target in NAFLD and NASH.
Fig. 3. Possible involvement of GLP-1 signaling in NASH.
The benefits of GLP-1 mimetics include: (i) Alleviation of insulin resistance via insulinotropic action and insulin signal enhancement, both in the liver and in peripheral tissues; (ii) Suppression of DNL, triglycerides synthesis and subsequent VLDL particle production in the liver; (iii) Balancing mitochondria biogenesis and function, decreasing ROS formation and increasing hepatic anti-oxidant capacity; (iv) Participating in Ca2+ homeostasis and reducing factors associated with ER stress; (v) Anti-inflammatory and anti-fibrosis actions; and (vi) Restoring microbiota dysbiosis and reversing the state of GLP-1 resistance.
Effect on glucolipid metabolism
As the largest organ in the body, liver plays a vital role in metabolic homeostasis. One of its functions is nutrient storage and conversion, which is primarily regulated by insulin [44]. Postprandially, increased insulin production promotes liver, muscle and AT to uptake glucose, FFAs and amino acids for further assimilation. This anabolic reaction is dependent on the coordination of intracellular signaling cascade involving insulin receptor substrate 1, phosphoinositide 3-kinase [45], phosphatidylinositol (3,4,5)-triphosphate, Akt (also known as protein kinase B) and phosphoinositide-dependent kinase-1 (PDK-1) to regulate gluconeogenesis and DNL [46]. In the fasting state, a combination of decreased insulin and increased glucagon concentrations promotes HGP to meet the metabolic demands of peripheral tissues.
Gastrointestinal hormones, particularly GLP-1, are involved in glucose homeostasis. GLP-1 exerts insulinotropic action through GLP-1R. Some studies showed that hepatic GLP-1R expression was significantly downregulated in NAFLD patients [47]. In addition, dipeptidyl peptidase-4 (DPP-4) that metabolizes GLP-1 was upregulated in the liver of patients ill with NAFLD [48]. These observations point to a potential mechanism of GLP-1 insensitivity among NAFLD patients. Since there is still controversy about the presence [49, 50] or absence [51, 52] of GLP-1R within hepatocytes, the direct or indirect role of GLP-1 and its mimetics on hepatic glucolipid metabolism remains to be fully elucidated.
Effect on insulin resistance
NASH mirrors several metabolic disorders and IR is one of the key risk factors in NAFLD, the early stage of NASH. The role of GLP-1R agonism in insulin sensitization has been reported. For example, exendin-4 stimulates the phosphorylation of key effectors in the insulin signaling pathway including PDK-1, Akt and protein kinase C (PKC) [50]. Similar results were obtained in HFD fed rats in which the peptide also increased PPARγ expression thereby reducing JNK phosphorylation to sensitize insulin action indirectly [53]. In adipose and muscle tissues, GLP-1 or its mimetics enhanced glucose metabolism. It was found that (i) GLP-1 could promote fatty acid synthesis in rat AT [54]; (ii) exenatide induced glucose transporter type 4 translocation to the plasma membrane facilitating glucose uptake in rat L6 skeletal muscle myotubes [55]; (iii) liraglutide was able to reverse IR induced by HFD [56]; and (iv) GLP-1R overexpression ob/ob mice had improved insulin sensitivity and decreased HGP [57].
Liver IR further exacerbates lipid accumulation in hepatocytes, the major pathological characterization in NASH that disturbs systemic metabolism. GLP-1 mitigates glycaemia, thereby inhibiting ChREBP, whose elevation is definitely related to increased TG levels [58]. SREBP1c is another de novo lipogenic factor associated with hepatic lipid accumulation and IR. It is upregulated by excess glucose and induced by palmitic acid that could be reversed following exendin-4 treatment [59]. Apart from improving IR, participation in lipid metabolism by GLP-1 mimetics renders them a potential therapy for NASH. On the one hand, they alter gene expression profiles of DNL, TG synthesis and β-oxidation, such as SCD1, acetyl-CoA carboxylase [60], FAS [58], CPT-1α, fasn and dgat1 [61]. On the other hand, they are capable of inducing lipolysis [49, 62], browning AT [63], and differentiating pre-adipocytes [64, 65]. In addition, exendin-4 was shown to decrease apoB-100 synthesis leading to decreased VLDL production [66]. Likewise, liraglutide was able to inhibit the expression of pro-hypercholesterolemic factor proprotein convertase subtilisin/kexin type 9 [67], pointing to possible involvement GLP-1R in cholesterol homeostasis.
Effect on mitochondria dysfunction
It is known that IR is related to mitochondria abnormalities [68]. Hepatocyte mitochondria orchestrate energy metabolism via β-oxidation, ATP synthesis, tricarboxylic acid cycle, and reformation [69]. However, this is malfunctioned in animal models and patients with NASH resulting in excessive ROS production [68, 70]. The prolonged oxidative stress aggravates DNA damage, cellular injury, and accumulation of apoptotic and necrotic hepatocytes thereby triggering inflammatory responses. Exenatide and liraglutide exert remarkable anti-oxidative effects through JNK signaling pathway or upregulation of protective anti-oxidative enzymes [53, 71].
Effect on endoplasmic reticulum stress
ER stress is one of the critical contributors during the progression of NAFLD to NASH. Elevated ER stress led to hepatocyte death and concomitant liver injury [72]. GLP-1R agonists benefited NAFLD individuals via alleviating ER stress responses probably by suppressing apoptosis associated with ER-resident protein 46 upregulation and diminishing liver injury [73]. Another crucial molecule in ER stress is Sirtuin 1 (SIRT1), an upstream regulator of AMP-activated protein kinase (AMPK) in hepatocytes. Its involvement in energy homeostasis and mitochondria regulation (e.g., reduction of mitochondrial ROS production) is well-known. The link between SIRT1-mediated deacetylation and increased expression of molecular chaperones HSP70 and HSP40 could mitigate hepatic ER stress. The fact that exenatide attenuates fatty liver partially through activation of SIRT1 [74] supports the rationale of GLP-1-based NASH therapy.
ER Ca2+ homeostasis is necessary in liver metabolism. It is influenced by hormone-initiated Ca2+ release and the entry of Ca2+ through store-operated Ca2+ channel [75]. Lipid accumulation is associated with inhibition of this channel via a PKC-dependent mechanism. The balance is broken when NAFLD evolves to NASH. Exendin-4 inhibited lipid accumulation in steatotic hepatocytes induced by palmitate plus BTP-2 (a store-operated Ca2+ channel blocker) and reversed impaired Ca2+ signaling [76].
Effect on liver inflammation
NAFLD progression shares several features of metabolic syndrome, such as macrophage polarization and pro-inflammatory immune infiltration. Both animal models and patients with NASH present systemic inflammatory responses, involving ubiquitously enhanced NF-κB signaling and upregulated inflammatory cytokines, such as IL-6, IL-1β, and TNF-α [77, 78]. Remarkably, each of these indicators contributes to more severe liver IR and metabolic disorder. Although GLP-1R agonists are initially prescribed for glucose and weight control, their anti-inflammatory effects have become increasingly attractive. It was reported that GLP-1 analogs inhibited human invariant natural killer T cells to secrete cytokine and promoted cAMP-response element-binding protein driven expression of anti-inflammatory genes such as IL-10 [79]. Similarly, intestinal intraepithelial lymphocyte suppressed the expression of inflammatory cytokines IL-2, IL-17A, interferon γ, and TNF-α through GLP-1R signaling [80]. Glp-1r−/− mice accepting bone marrow transplantation from wild-type counterparts had restored intestinal mucosa function and normalized cytokine expression profiles [80, 81]. GLP-1 treatment also attenuated inflammatory response accompanied by amelioration of disease phenotypes in stroke and atherosclerotic lesions in mice [81, 82]. Taken together, accumulating evidence demonstrate a role of GLP-1R agonists in immunomodulation, supporting their potential application in NASH therapy.
KCs have been implicated in steatosis and insulin signaling impairment, partially due to elevated TNF-α concentrations [83]. TNF-α directly impairs proglucagon mRNA transcription and GLP-1 secretion from the enteroendocrine L-cell via JNK and NF-κB signaling [71, 84], which could be mitigated by treatment with exenatide [53] or liraglutide [71]. Meanwhile, exogenous GLP-1 lowered pro-inflammatory macrophage (M1) infiltration in AT of ob/ob mice, along with suppressed TNF-α, IL-6, and MCP-1 mRNA levels and reduced NF-κB activity [85]. GLP-1 also induced macrophage polarization towards the anti-inflammatory phenotype (M2) through STAT3 activation, reduction of adaptive T cell responses, elevation of M2 macrophage-related molecules including IL-10, CD163, and CD204, and eventual alleviation of inflammatory reaction [85].
Ceramide, an intermediate metabolite of sphingolipid, is also taking part in the pathogenesis of NAFLD via crosstalk with inflammatory mediators. It increases TNF-α secretion and the resultant liver inflammation further promotes ceramide production in hepatocytes [86]. Therapeutic intervention with GLP-1R agonist could achieve a concomitant reduction in ceramide species and complications in the NASH as demonstrated by a recent study [61].
DPP-4 inhibitors are capable of enhancing GLP-1R signaling and exerting anti-inflammatory effects. TLRs are multiprotein complexes that recognize pathogen-associated molecular patterns which can activate an inflammatory response and drive the progression of liver injury [87]. Their stimulation triggers intracellular signaling cascade involving NF-κB and inflammasomes, leading to releases of IL-1β and IL-18 related to inflammation and cell death [88]. NLR family pyrin domain containing 3 (NLRP3) is one of inflammasomes closely associated with the progression from NAFLD to NASH [87, 88]. DPP-4 inhibitor sitagliptin decreased NLRP3, IL-1β, and TLR-4 gene expression, and avoided NLRP3 activation indirectly [89]. Another DPP-4 inhibitor linagliptin was able to promote macrophage polarization to M2 phenotype, consistent with decreased IL-6, IL-1β, TNF-α, and NF-κB gene expression [90]. However, the effects of DPP-4 inhibitors were not verified in human clinical trials due to small sample size, lack of histological assessment and a short follow-up duration [91].
Effect on liver fibrosis
A long-standing pro-inflammatory state would provoke liver fibrosis, a non-physiological scarring process characterized by the net accumulation of extracellular matrix, compromising liver architecture and then driving cirrhosis even primary liver cancer or hepatocarcinoma ultimately. Both experimental models and clinical cases determined some key steps responsible for fibrosis, including (i) upregulation of TGF-β, the master regulator of fibrosis initiation [92]; (ii) increase of several pro-fibrogenic markers like tissue inhibitor of metalloproteinase-1, serpin family E member 1 and matrix metalloproteinases (MMPs), as well as proliferative markers such as TNF-α and TGF-β [92]; and (iii) activation of hepatic stellate cells (HSCs) into migratory and proliferative phenotype [61, 93]. Strikingly, GLP-1 based therapy also exhibits the ability to suppress liver fibrosis and reduce NASH progression. Indeed, GLP-1 mimetics were able to decrease the expression of TNF-α, MMP-9, and MMP-13 in T2DM patients [94] and suppress fibrotic phenotypes in a mouse NASH model [95]. Increased pro-fibrogenic regulators and markers following the methionine choline-deficient (MCD) diet exposure was alleviated or fully normalized by liraglutide administration [61]. Liraglutide was also shown to directly inactivate cirrhotic HSCs in vitro, albeit its exact role remains to be elucidated [96].
Effect on the gut-liver axis
The effects of gut dysbiosis on liver damage and obesity were first reported in 2006 [97]. Lean rodents receiving gut microbiota transplantation from their obese counterparts acquired the same metabolic alterations as the donors, including more complex liver lipid profiles and enhanced lipid deposition in adipocytes [97]. Detailed mechanisms linking gut microbiota to several metabolic disorders, including NASH were subsequently proposed that gut microbiota could (i) regulate incretin (GLP-1, peptide YY, and GIP) release thereby indirectly influencing glucose metabolism, gut motility, and energy expenditure [98, 99]; (ii) suppress the activity of angiopoietin like 4, a lipoprotein lipase inhibitor associated with a higher lipid accumulation in the liver [100]; (iii) bring out bacteria-derived products, such as short-chain fatty acids (SCFAs) acting as substrates for liver gluconeogenesis and DNL [99]; and (iv) improve barrier function to prevent translocation of bacterial or bacteria-derived products into the portal circulation [101, 102].
Patients with NAFLD have altered composition of specific gut microbiota compared with healthy controls, such as Bacteroidetes, Prevotella, and Porphyromonas [28, 103]. NASH patients with fibrosis stage F ≥2 had less proportion of Prevotella than patients with F0/1 fibrosis stage. Metagenomic profile analysis indicates that fibrosis stage F ≥2 is mostly related to carbohydrate, lipid and amino acid metabolism [28, 103]. Therefore, NAFLD severity is associated with gut dysbiosis and a change in metabolic function of the gut microbiota.
Specific gut microbiota dysbiosis is also responsible for GLP-1 resistance. Dysbacteriosis was found to be linked with an impaired GLP-1R expression and GLP-1 signaling of enteric neurons in HFD-fed mice, thereby preventing the gut-brain-periphery axis activation for the control of insulin secretion and gastric emptying [104]. Besides, metabolites of intestinal microorganism can strongly regulate host metabolism. Bacterial products such as SCFAs, hydrogen sulfide (H2S) and indole could stimulate GLP-1 release directly or indirectly, exerting an insulin-sensitizing effect on metabolism [102]. In rodent models, sodium butyrate (NaB) was able to slow down the progression of NASH via enhanced GLP-1 secretion [47].
It is interesting that liraglutide treatment caused a significant change in overall composition and relative proportion of weight-relevant bacteria species in HFD-fed mice, including a reduction of Proteobacteria and an increase of Akkermansia muciniphila [105]. Liraglutide also restored the balance between Bacteroides and Erysipelotrichi decreased upon MCD-diet exposure [61]. An adequate presence of these two phylotypes is necessary in the microbiota due to their roles in inflammation and liver fat metabolism [32, 106]. It is worthy of noting that the observation of Proteobacteria and Akkermansia muciniphila in liraglutide-treated HFD-fed mice was not reproduced in MCD-diet fed mice. This inconsistency may be attributable to different dietary pattern and genetic differences in the mouse strain used. Technical limitations in culturing anaerobic bacteria and the absence of selective antibiotics might have also hampered the determination of gut microbiota composition.
Effect on the nervous system
Neuronal effects of GLP-1 were observed in the 1980’s [107]. Subsequent studies revealed that the neuroendocrine signaling mediated by GLP-1R is one of the key mechanisms in energy homeostasis [108] and satiety regulation [109], although there is some confusion in the way of native GLP-1 activates GLP-1R in the CNS (due to a very short half-life of the peptide) which seems to be different from long-acting GLP-1R agonists. According to a recent review by Drucker and colleagues [110], peripherally administered GLP-1 analogs appear to induce receptor activation either in the hypothalamus and hindbrain, both areas with incomplete blood-brain barrier, or on vagal afferents to transmit the signal to the hindbrain which then projects to other key feeding areas in the brain. In fact, intracerebrovascular (i.c.v.) injection of GLP-1 in HFD-fed mice enhanced hepatic insulin signaling via Akt action and hypothalamic AMPK suppression, accompanied by elevations in insulin secretion, improved glucose tolerance and decreased hepatic TG accumulation [111]. Alternatively, knockdown of GLP-1R in dorsomedial hypothalamus resulted in effects that collectively contributed to IR including body weight gain, reduction in energy expenditure, hepatic steatosis, increased plasma TG and elevated liver specific DNL [112].
Of note is that these effects are somehow independent of food consumption. For instance, exendin-4 showed a profound efficacy on peripheral lipid metabolism after acute or repeated i.c.v. administration in cholesterol-fed hamsters, in connection with decreased SREBP1c, increased CYP7A1, and elevated LDL receptor expression [113]. Liraglutide could trigger brown AT thermogenesis and adipocyte browning through hypothalamic ventromedial nucleus (VMH) stimulation which was blunted by AMPK activation. The increased energy expenditure led to weight loss that was also independent of anorexigenic actions [108].
In the peripheral nervous system, GLP-1R locates in the vagal nerve which plays a seminal role in regulation of feeding behavior and metabolic homeostasis. Several studies have suggested that GLP-1 can signal through a neural circuit originating in the hepatic-portal area. Intraportal GLP-1 was able to evoke a powerful neuronal-mediated insulinotropic effect directly through the hepatoportal-pancreatic vagal reflex pathways [114]. Vagotomized animals exhibited rising circulating GLP-1 levels, accounting for metabolic benefits by inhibition of key regulators in hepatic DNL [59]. They also lost the response to peripheral exendin-4 treatment in lipid regulation compared to sham operation littermates, implicating the involvement of parasympathetic system in GLP-1R signaling [115]. Notably, emerging evidence has demonstrated that the vagal nerve suppresses systemic inflammatory response. The synthesis of TNF, HMGB1, and other cytokines were significantly attenuated after vague nerve stimulation in vivo thereby protecting against endotoxemia [116].
Target profile
GLP-1R belongs to class B1 GPCR family [117] and a member of the glucagon receptor subfamily along with GCGR, glucagon-like peptide-2 receptor, growth hormone–releasing hormone receptor, secretin receptor, and glucose-dependent insulinotropic polypeptide receptor (GIPR) [117]. In general, their molecular structures are similar, consisting of two domains: a large ECD at the N terminus and a TMD. Both are indispensable for receptor activation. The ways in which the endogenous ligands bind to and activate the corresponding receptor in this family are comparable. It is accepted that peptide ligands bind to class B1 GPCRs in two steps: the C-terminal region of peptide primarily binds to the ECD via hydrophobic interactions followed by insertion of the N-terminal region into the orthosteric site located in the TMD core causing conformational changes, G protein coupling and downstream signaling [118, 119] (Fig. 4).
Fig. 4. Two-domain binding model for class B1 GPCRs.
Step 1, the C terminus of peptide binds to the ECD of the receptor. This interaction facilitates step 2: insertion of the peptide N terminus to the ligand-binding pocket at the TMD core.
Besides the benefits of GLP-1R agonists in the treatment of NASH, studies have also shown that synergistic actions of dual or triple agonists at GLP-1R, GCGR, and/or GIPR could slow down the process of NASH more effectively [120]. Agonism at GCGR attenuates symptoms of metabolic diseases by increasing energy expenditure and reducing liver fat. However, activation of GCGR also elevates HGP, thus diminishing the glycemic control effect of GLP-1 [121]. Combination of both resulted in a profound therapeutic superiority in reducing body weight, hyperglycemia and hepatic lipids beyond GLP-1R agonist alone [38, 122]. Additional glycemic control efficacy rendered by GIPR activation decreased the inherent hyperglycemic risk of GCGR action [123, 124]. Consequently, there are ongoing preclinical and clinical studies exploring the therapeutic advantages of GLP-1R/GIPR dual and GLP-1R/GCGR/GIPR triple agonists in the management of NAFLD and NASH [125–128].
Therapeutic potential of GLP-1R agonists
The effect of GLP-1 analogs on enhancing insulin sensitivity, decreasing hepatic steatosis, and improving liver histology have been observed in mice [53, 60, 66, 74, 129–131]. Based on the molecular structures, these analogs can be divided into two categories: exendin-like whose amino acid sequence has low homology with human GLP-1, such as exenatide and lixisenatide, as well as GLP-1-like sharing high amino acid sequence homology with peptidic mimetics, such as liraglutide and semaglutide. Major clinical trials of these GLP-1 mimetics are summarized as below and relevant information of each trial is given in Table 1.
Table 1.
Major clinical trials of GLP-1 mimetics in patients with NAFLD/NASH.
| Drug | Stage | Subject | Number of participants and dose per intervention | Duration | Principal efficacy | Reference |
|---|---|---|---|---|---|---|
| Exenatide | Phase 4 | Obesity, NAFLD and T2DM | n = 30, exenatide + insulin glargine n = 30, insulin aspart + insulin glargine | 12 weeks | Levels of the hepatic injury biomarkers ALT, AST, and GGT were significantly decreased in two groups and exenatide’s effects were greater. | [134] |
| Phase 4 | Ectopic fat stores | n = 22, exenatide n = 22, reference treatment according to French guidelines | 26 weeks | Exenatide reduced liver fat content and epicardial fat in patients with T2DM. | [135] | |
| Phase 4 | NAFLD and T2DM | n = 38, exenatide n = 38, insulin glargine | 24 weeks | Exenatide showed better effects on reducing body weight, visceral fat area, liver enzymes, FIB-4, PPG, and LDL-C. | [136] | |
| Liraglutide | Phase 2 | Biopsy-proven NASH | n = 26, 1.8 mg/day liraglutide n = 26, placebo | 48 weeks | Liraglutide improved hepatic steatosis and reduced histological damage. | [37] |
| Phase 4 | NAFLD and T2DM | n = 24, 1.8 mg/day liraglutide n = 27, 100 mg/day sitagliptin n = 24, 0.2 IU/kg insulin glargine | 26 weeks | Liraglutide and sitagliptin improved IHL; liraglutide significantly reduced liver fat content, while insulin glargine did not. | [138] | |
| Phase 4 | NAFLD and T2DM | n = 31, 1000 mg twice daily metformin n = 31, 120 mg/day gliclazide n = 31, 1.8 mg/day liraglutide | 24 weeks | Liraglutide group had slightly better results than metformin group in decreasing ALT, AST, IHF and weight. | [137] | |
| Semaglutide | Phase 2 | Biopsy-proven NASH | n = 80, 0.1 mg semaglutide n = 78, 0.2 mg semaglutide n = 82, 0. 4 mg semaglutide n = 80, placebo | 72 weeks | Treatment with semaglutide resulted in a significant higher percentage of patients with NASH resolution than placebo. | [139] |
| Tirzepatide (TZP) | Phase 2 | T2DM | n = 52, 1 mg TZP n = 55, 5 mg TZP n = 51, 10 mg TZP n = 53, 15 mg TZP n = 54, 1.5 mg dulaglutide n = 51, placebo | 26 weeks | Higher tirzepatide doses significantly decreased NASH-related biomarkers and increased adiponectin in patients with T2DM. | [144] |
FIB-4 fibrosis 4 score, IHL intrahepatic lipid.
Exenatide
Exenatide, also known as exendin-4, shares 53% sequence homology with GLP-1. It is isolated from lizard saliva, functionally similar to GLP-1 and resistant to DPP-4 degradation [132]. Apart from glucoregulatory activities, exenatide treatment was shown to improve lipid homeostasis, reduce body weight [133], improve IR and decrease hepatic steatosis [60]. One clinical trial enrolling 60 patients with T2DM and NAFLD showed that exenatide had a better effect of weight loss compared with intensive insulin. After 12-week treatment, levels of some hepatic injury biomarkers, such as alanine aminotransferase (ALT), aspartate aminotransferase (AST), and γ-glutamyl transpeptidase (GGT), were significantly lower than that in the control group indicative of hepatic protection [134]. Another study involving 44 patients not only confirmed the weight loss effect of exenatide over placebo but also showed a significant reduction in liver fat content (LFC) [135]. Recently, a randomized comparative trial demonstrated that both exenatide and insulin glargine had the ability of reducing LFC in addition to that of blood glucose control [136]. Compared with insulin glargine, exenatide treatment resulted in better reduction in VAT, subcutaneous AT, liver enzymes, weight, postprandial plasma glucose (PPG), and low-density lipoprotein-cholesterol (LDL-C) [136].
Liraglutide
Among all GLP-1R agonists, the efficacy of liraglutide in NAFLD and NASH therapies is extensively investigated. The well-known study “Liraglutide Efficacy and Action in NASH (LEAN trial)” is a double-blinded, randomized and placebo-controlled phase 2 trial [37]. The effect of liraglutide treatment in 52 biopsy-proven NASH patients was evaluated. After 48-week treatment, 9/23 (39%) patients in the liraglutide group had resolution of NASH without worsening fibrosis, while the number of patients who had histological improvement in the placebo group is only 2/22 (9%). Compared to the placebo group (8/22, 36%), patients who demonstrated progression of fibrosis is also fewer in the liraglutide group (2/23, 9%).
A systematic and comparative evaluation of changes in intrahepatic fat (IHF) among T2DM patients with NAFLD receiving liraglutide, gliclazide, or metformin monotherapy was conducted [137]. After 24-week of treatment, liraglutide group showed a greater IHF reduction than gliclazide and metformin groups; liraglutide and metformin monotherapy also had better effects on ameliorating liver aminotransferase levels, alleviating hyperglycemia, and reducing body weight and waistline than gliclazide. A similar comparative trial found that liraglutide treatment caused significant weight loss and LFC reduction, but weight loss in insulin glargine group was not evident [138].
Semaglutide
Semaglutide has a long half-life and is administered once a week. A 72-week, double-blinded phase 2 trial involving 320 patients with biopsy-confirmed NASH and liver fibrosis of stages F1, F2, and F3 showed that the percentage of patients who achieved resolution of NASH without worsening of fibrosis after treatment was significantly higher in semaglutide group (0.4 mg) than in the placebo group (59% vs. 17%), accompanied by reductions in inflammatory biomarker levels and in histologically assessed lobular inflammation. However, with reference to an improvement of at least one fibrosis stage with no worsening of NASH, there existed no significant difference between the two groups (43% vs. 33%) [139]. This observation is consistent with a previous preclinical study showing that semaglutide reduced liver inflammation through mechanisms unrelated to weight loss [140].
Dual agonists
GLP-1R/GCGR dual agonism can effectively counterbalance the hyperglycemic effects of glucagon and in the meanwhile relieve metabolic disorders. This phenomenon was first discovered in DIO mice, in which treatment with a dual agonist displayed a better efficacy than GLP-1R agonist alone in reducing body weight, hepatic fat, and blood glucose [141, 142]. Treatment of T2DM patients with cotadutide, a dual GLP-1R/GCGR agonist, reduced glycaemia, body weight, and hepatic steatosis [38]. Wild-type and Glp-1r−/− mice were administered cotadutide, liraglutide or g1437 (GCGR agonist), respectively, or co-administered liraglutide and g1437. After 14-day treatment, the data of weight loss, intraperitoneal glucose tolerance test, and plasma leptin (a fat mass biomarker) suggest that the effects of cotadutide on weight loss and glucose homeostasis are dependent on GLP-1R, whereas improvement in hepatic lipid content is mediated by GCGR [38]. In a physiologically relevant mouse model, cotadutide also exhibited a superior efficacy in alleviating NASH [38].
A dual agonist targeting GLP-1R and fibroblast growth factor 21 (FGF21) receptor has shown its therapeutic potential for T2DM and NASH [143]. FGF21 is a secreted endocrine factor that regulates glucose and lipid metabolism. This type of dual agonists displayed potent and sustained effects on glucose lowering, weight loss, and lipid profile improvement [143]. In diabetic db/db mice, GLP-1/FGF21 dual agonists had superior ability to reduce glucose than FGF21 and GLP-1 alone [143]. IR ob/ob mice fed with HFD for 6 weeks developed NASH according to nonalcoholic fatty liver disease score; dual agonist GLP-1-Fc-FGF21 D1, Fc-FGF21 S1 and dulaglutide significantly reduced liver steatosis, inflammation and hepatocellular ballooning; GLP-1-Fc-FGF21 D1 also exhibited greater improvement than GLP-1 or FGF21 [143].
GIP is another incretin and can enhance the metabolic effects of GLP-1 via complementary or synergistic actions. Treatment with tirzepatide (a GLP-1R/GIPR dual agonist) improved insulin response and glycemic control compared with GLP-1R agonist alone [144]. In a phase 2 clinical trial, tirzepatide significantly reduced HbA1c and body weight of T2DM patients in 26 weeks [144]. Based on the weight loss findings, the effects of tirzepatide on biomarkers of NASH and liver fibrosis among 316 T2DM patients were explored as a phase 2 trial [123]. Once a week subcutaneous tirzepatide (1, 5, 10, or 15 mg), dulaglutide (1.5 mg), or placebo was given for 26 weeks. Tirzepatide treatment (10 mg) significantly decreased serum keratin-18 (K-18) level by 135.2 unit/L compared with the placebo group, which may be clinically meaningful for the treatment of NASH. In a previous trial, reduction of 150 unit/L in K-18 level was associated with a greater chance of NASH resolution [145]. However, compared with dulaglutide group, the higher doses of tirzepatide did not demonstrate advantages in improving K-18, procollagen III (PRO-C3) and adiponectin levels. Although this work has limitations because it is a post hoc analysis, the NASH biomarker data along with the weight loss findings warrant further evaluation of tirzepatide in patients with NASH. More clinical trials that assess liver histology are thus required.
In summary, with the globalization of obesity and metabolic disorders, NAFLD has become the most common cause of chronic liver diseases. NASH as a progressive form of NAFLD usually results in cirrhosis and hepatocellular cancer. Presently, there is no approved drug for these diseases. However, GLP-1 mimetics, including GLP-1R agonists and DPP-4 inhibitors used to treat T2DM and obesity have been shown to be efficacious in mitigating NAFLD and possibly in preventing NASH. Apart from weight loss and glycemic control, GLP-1 mimetics also improved liver histology in animal models and some human studies. There exist laboratory data supporting the rationale of treating NASH with GLP-1 mimetics, showing positive effects on insulin sensitivity, lipid metabolism, glucose transport, oxidative stress, inflammatory response, and energy expenditure. Unfortunately, most of them were derived from murine models and some conclusions are either controversial or inconsistent between rodent and human studies. In order to objectively present the cited work, we summarized the basic information of NASH-related references in two tables describing the main findings in rodents (Supplementary Table S1) and in humans (Supplementary Table S2, including one monkey study). It remains unclear if the observed effects of GLP-1 are originated from its direct action since the presence of GLP-1R in insulin-targeted tissues such as liver, AT and muscle has yet to be confirmed. Therefore, suitable animal models as well as long-term and well-designed clinical trials are required to advance this import filed of research to a new height.
Supplementary information
Acknowledgements
This work was partially supported by National Natural Science Foundation of China 81872915 (MWW), 82073904 (MWW), 81773792 (DHY), 81973373 (DHY), and 21704064 (QTZ); National Science & Technology Major Project of China–Key New Drug Creation and Manufacturing Program 2018ZX09735–001 (MWW) and 2018ZX09711002–002–005 (DHY); the National Key Basic Research Program of China 2018YFA0507000 (MWW); Science and Technology Commission of Shanghai Municipality 18431907100 (MWW); Novo Nordisk-CAS Research Fund grant NNCAS-2017–1-CC (DHY) and SA-SIBS Scholarship Program (DHY).
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Yan Chen, Ying-na Xu, Chen-yu Ye, Wen-bo Feng
Contributor Information
De-hua Yang, Email: dhyang@simm.ac.cn.
Ming-wei Wang, Email: mwwang@simm.ac.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41401-021-00836-9.
References
- 1.Stoffel M, Espinosa R, Le Beau MM, Bell GI. Human glucagon-like peptide-1 receptor gene. Diabetes. 1993;42:1215–8. doi: 10.2337/diab.42.8.1215. [DOI] [PubMed] [Google Scholar]
- 2.Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol. 1999;403:261–80. doi: 10.1002/(SICI)1096-9861(19990111)403:2<261::AID-CNE8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 3.Montrose-Rafizadeh C, Avdonin P, Garant MJ, Rodgers BD, Kole S, Yang H, et al. Pancreatic glucagon-like peptide-1 receptor couples to multiple G proteins and activates mitogen-activated protein kinase pathways in Chinese hamster ovary cells. Endocrinology. 1999;140:1132–40. doi: 10.1210/endo.140.3.6550. [DOI] [PubMed] [Google Scholar]
- 4.Drucker DJ, Philippe J, Mojsov S, Chick WL, Habener JF. Glucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line. Proc Natl Acad Sci USA. 1987;84:3434–8. doi: 10.1073/pnas.84.10.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu M, Wheeler MB, Leng XH, Boyd AE., 3rd The role of the free cytosolic calcium level in beta-cell signal transduction by gastric inhibitory polypeptide and glucagon-like peptide I (7-37) Endocrinology. 1993;132:94–100. doi: 10.1210/endo.132.1.8380389. [DOI] [PubMed] [Google Scholar]
- 6.Holz GGT, Leech CA, Habener JF. Activation of a cAMP-regulated Ca2+-signaling pathway in pancreatic beta-cells by the insulinotropic hormone glucagon-like peptide-1. J Biol Chem. 1995;270:17749–57. doi: 10.1074/jbc.270.30.17749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Willms B, Werner J, Holst JJ, Orskov C, Creutzfeldt W, Nauck MA. Gastric emptying, glucose responses, and insulin secretion after a liquid test meal: effects of exogenous glucagon-like peptide-1 (GLP-1)-(7-36) amide in type 2 (noninsulin-dependent) diabetic patients. J Clin Endocrinol Metab. 1996;81:327–32. doi: 10.1210/jcem.81.1.8550773. [DOI] [PubMed] [Google Scholar]
- 8.Monami M, Dicembrini I, Marchionni N, Rotella CM, Mannucci E. Effects of glucagon-like peptide-1 receptor agonists on body weight: a meta-analysis. Exp Diabetes Res. 2012;2012:672658. doi: 10.1155/2012/672658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, et al. Charatcharoenwitthaya P, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149:389–97. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 11.Valenti L, Al-Serri A, Daly AK, Galmozzi E, Rametta R, Dongiovanni P, et al. Homozygosity for the patatin-like phospholipase-3/adiponutrin I148M polymorphism influences liver fibrosis in patients with nonalcoholic fatty liver disease. Hepatology. 2010;51:1209–17. doi: 10.1002/hep.23622. [DOI] [PubMed] [Google Scholar]
- 12.BasuRay S, Wang Y, Smagris E, Cohen JC, Hobbs HH. Accumulation of PNPLA3 on lipid droplets is the basis of associated hepatic steatosis. Proc Natl Acad Sci USA. 2019;116:9521–6. doi: 10.1073/pnas.1901974116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kozlitina J, Smagris E, Stender S, Nordestgaard BG, Zhou HH, Tybjaerg-Hansen A, et al. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2014;46:352–6. doi: 10.1038/ng.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prill S, Caddeo A, Baselli G, Jamialahmadi O, Dongiovanni P, Rametta R, et al. The TM6SF2 E167K genetic variant induces lipid biosynthesis and reduces apolipoprotein B secretion in human hepatic 3D spheroids. Sci Rep. 2019;9:11585. doi: 10.1038/s41598-019-47737-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 2004;79:537–43. doi: 10.1093/ajcn/79.4.537. [DOI] [PubMed] [Google Scholar]
- 16.Craig GG, Knox KW. Letter: the use of high-fructose corn syrup as a sweetener. Aust Dent J. 2010;20:414. doi: 10.1111/j.1834-7819.1975.tb04394.x. [DOI] [PubMed] [Google Scholar]
- 17.Tappy L, Le KA. Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev. 2010;90:23–46. doi: 10.1152/physrev.00019.2009. [DOI] [PubMed] [Google Scholar]
- 18.Garbow JR, Doherty JM, Schugar RC, Travers S, Weber ML, Wentz AE, et al. Hepatic steatosis, inflammation, and ER stress in mice maintained long term on a very low-carbohydrate ketogenic diet. Am J Physiol Gastrointest Liver Physiol. 2011;300:G956–67. doi: 10.1152/ajpgi.00539.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cortez-Pinto H, Chatham J, Chacko VP, Arnold C, Rashid A, Diehl AM. Alterations in liver ATP homeostasis in human nonalcoholic steatohepatitis: a pilot study. JAMA. 1999;282:1659–64. doi: 10.1001/jama.282.17.1659. [DOI] [PubMed] [Google Scholar]
- 20.Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, et al. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–92. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 21.Anne M, Tian BL, Bilder G. A potent PPARa agonist stimulates mitochondrial fatty acid beta-oxidation in liver and skeletal muscle. Am J Physiol Endocrinol Metab. 2001;280:E270–9. doi: 10.1152/ajpendo.2001.280.2.E270. [DOI] [PubMed] [Google Scholar]
- 22.Memon RA, Tecott LH, Nonogaki K, Beigneux A, Moser AH, Grunfeld C, et al. Up-regulation of peroxisome proliferator-activated receptors (PPARα) and PPARγ messenger ribonucleic acid expression in the liver in murine obesity: troglitazone induces expression of PPARγ-responsive adipose tissue-specific genes in the liver of obese diabetic mice. Endocrinology. 2000;141:4021–31. doi: 10.1210/endo.141.11.7771. [DOI] [PubMed] [Google Scholar]
- 23.Deng Y, Ren X, Yang L, Lin Y, Wu X. A JNK-dependent pathway is required for TNFa-induced apoptosis. Cell. 2003;115:61–70. doi: 10.1016/S0092-8674(03)00757-8. [DOI] [PubMed] [Google Scholar]
- 24.Schäffler A, Schölmerich J, Büchler C. Mechanisms of disease: adipocytokines and visceral adipose tissue-emerging role in nonalcoholic fatty liver disease. Nat Clin Pr. Gastroenterol Hepatol. 2005;2:103–11. doi: 10.1038/ncpgasthep0186. [DOI] [PubMed] [Google Scholar]
- 25.Mihm S. Danger-associated molecular patterns (DAMPs): molecular triggers for sterile inflammation in the liver. Int J Mol Sci. 2018;19:3104. doi: 10.3390/ijms19103104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caligiuri A, Gentilini A, Marra F. Molecular pathogenesis of NASH. Int J Mol Sci. 2016;17:1575. doi: 10.3390/ijms17091575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102:731–44. doi: 10.1016/S0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 28.Boursier J, Mueller O, Barret M, Machado M, Fizanne L, Araujo-Perez F, et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology. 2016;63:764–75. doi: 10.1002/hep.28356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aranha MM, Cortez-Pinto H, Costa A, da Silva IB, Camilo ME, de Moura MC, et al. Bile acid levels are increased in the liver of patients with steatohepatitis. Eur J Gastroenterol Hepatol. 2008;20:519–25. doi: 10.1097/MEG.0b013e3282f4710a. [DOI] [PubMed] [Google Scholar]
- 30.O'Sullivan A, He X, McNiven EM, Haggarty NW, Lonnerdal B, Slupsky CM, et al. Early diet impacts infant rhesus gut microbiome, immunity, and metabolism. J Proteome Res. 2013;12:2833–45. doi: 10.1021/pr4001702. [DOI] [PubMed] [Google Scholar]
- 31.Zeisel SH, Wishnok JS, Blusztajn JK. Formation of methylamines from ingested choline and lecithin. J Pharmacol Exp Ther. 1983;225:320–4. [PubMed] [Google Scholar]
- 32.Spencer MD, Hamp TJ, Reid RW, Fischer LM, Zeisel SH, Fodor AA. Association between composition of the human gastrointestinal microbiome and development of fatty liver with choline deficiency. Gastroenterology. 2011;140:976–86. doi: 10.1053/j.gastro.2010.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149:367–78. doi: 10.1053/j.gastro.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Brenner C, Galluzzi L, Kepp O, Kroemer G. Decoding cell death signals in liver inflammation. J Hepatol. 2013;59:583–94. doi: 10.1016/j.jhep.2013.03.033. [DOI] [PubMed] [Google Scholar]
- 35.Tacke F. Cenicriviroc for the treatment of non-alcoholic steatohepatitis and liver fibrosis. Expert Opin Investig Drugs. 2018;27:301–11. doi: 10.1080/13543784.2018.1442436. [DOI] [PubMed] [Google Scholar]
- 36.Takeda A, Irahara A, Nakano A, Takata E, Koketsu Y, Kimata K, et al. The improvement of the hepatic histological findings in a patient with non-alcoholic steatohepatitis with type 2 diabetes after the administration of the sodiumglucose cotransporter 2 inhibitor Ipragliflozin. Intern Med. 2017;56:2739–44. doi: 10.2169/internalmedicine.8754-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679–90. doi: 10.1016/S0140-6736(15)00803-X. [DOI] [PubMed] [Google Scholar]
- 38.Boland ML, Laker RC, Mather K, Nawrocki A, Oldham S, Boland BB, et al. Resolution of NASH and hepatic fibrosis by the GLP-1R/GCGR dual-agonist Cotadutide via modulating mitochondrial function and lipogenesis. Nat Metab. 2020;2:413–31. doi: 10.1038/s42255-020-0209-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burcelin R, Li J, Charron MJ. Cloning and sequence analysis of the murine glucagon receptor-encoding gene. Gene. 1995;164:305–10. doi: 10.1016/0378-1119(95)00472-I. [DOI] [PubMed] [Google Scholar]
- 40.Kimura T, Pydi SP, Pham J, Tanaka N. Metabolic functions of G protein-coupled receptors in hepatocytes-potential applications for diabetes and NAFLD. Biomolecules. 2020;10:1445. doi: 10.3390/biom10101445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim T, Nason S, Holleman C, Pepin M, Wilson L, Berryhill TF, et al. Glucagon receptor signaling regulates energy metabolism via hepatic farnesoid X receptor and fibroblast growth factor 21. Diabetes. 2018;67:1773–82. doi: 10.2337/db17-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nason SR, Kim T, Antipenko JP, Finan B, DiMarchi R, Hunter CS, et al. Glucagon-receptor signaling reverses hepatic steatosis independent of leptin receptor expression. Endocrinology. 2020;161:bqz013. doi: 10.1210/endocr/bqz013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McClean PL, Irwin N, Cassidy RS, Holst JJ, Gault VA, Flatt PR. GIP receptor antagonism reverses obesity, insulin resistance, and associated metabolic disturbances induced in mice by prolonged consumption of high-fat diet. Am J Physiol Endocrinol Metab. 2007;293:E1746–55. doi: 10.1152/ajpendo.00460.2007. [DOI] [PubMed] [Google Scholar]
- 44.Ding HR, Wang JL, Ren HZ, Shi XL. Lipometabolism and glycometabolism in liver diseases. Biomed Res Int. 2018;2018:1287127. doi: 10.1155/2018/1287127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saad MJ, Folli F, Kahn JA, Kahn CR. Modulation of insulin receptor, insulin receptor substrate-1, and phosphatidylinositol 3-kinase in liver and muscle of dexamethasone-treated rats. J Clin Invest. 1993;92:2065–72. doi: 10.1172/JCI116803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Niswender KD. Basal insulin: physiology, pharmacology, and clinical implications. Postgrad Med. 2011;123:17–26. doi: 10.3810/pgm.2011.07.2300. [DOI] [PubMed] [Google Scholar]
- 47.Zhou D, Chen YW, Zhao ZH, Yang RX, Xin FZ, Liu XL, et al. Sodium butyrate reduces high-fat diet-induced non-alcoholic steatohepatitis through upregulation of hepatic GLP-1R expression. Exp Mol Med. 2018;50:1–12. doi: 10.1038/s12276-018-0183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miyazaki M, Kato M, Tanaka K, Tanaka M, Kohjima M, Nakamura K, et al. Increased hepatic expression of dipeptidyl peptidase-4 in non-alcoholic fatty liver disease and its association with insulin resistance and glucose metabolism. Mol Med Rep. 2012;5:729–33. doi: 10.3892/mmr.2011.707. [DOI] [PubMed] [Google Scholar]
- 49.Gupta NA, Kolachala VL, Jiang R, Abramowsky C, Romero R, Fifadara N, et al. The glucagon-like peptide-1 receptor agonist Exendin 4 has a protective role in ischemic injury of lean and steatotic liver by inhibiting cell death and stimulating lipolysis. Am J Pathol. 2012;181:1693–701. doi: 10.1016/j.ajpath.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gupta NA, Mells J, Dunham RM, Grakoui A, Handy J, Saxena NK, et al. Glucagon-like peptide-1 receptor is present on human hepatocytes and has a direct role in decreasing hepatic steatosis in vitro by modulating elements of the insulin signaling pathway. Hepatology. 2010;51:1584–92. doi: 10.1002/hep.23569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Panjwani N, Mulvihill EE, Longuet C, Yusta B, Campbell JE, Brown TJ, et al. GLP-1 receptor activation indirectly reduces hepatic lipid accumulation but does not attenuate development of atherosclerosis in diabetic male ApoE(-/-) mice. Endocrinology. 2013;154:127–39. doi: 10.1210/en.2012-1937. [DOI] [PubMed] [Google Scholar]
- 52.Pyke C, Heller RS, Kirk RK, Orskov C, Reedtz-Runge S, Kaastrup P, et al. GLP-1 receptor localization in monkey and human tissue: novel distribution revealed with extensively validated monoclonal antibody. Endocrinology. 2014;155:1280–90. doi: 10.1210/en.2013-1934. [DOI] [PubMed] [Google Scholar]
- 53.Svegliati-Baroni G, Saccomanno S, Rychlicki C, Agostinelli L, De Minicis S, Candelaresi C, et al. Glucagon-like peptide-1 receptor activation stimulates hepatic lipid oxidation and restores hepatic signalling alteration induced by a high-fat diet in nonalcoholic steatohepatitis. Liver Int. 2011;31:1285–97. doi: 10.1111/j.1478-3231.2011.02462.x. [DOI] [PubMed] [Google Scholar]
- 54.Oben J, Morgan L, Fletcher J, Marks V. Effect of the entero-pancreatic hormones, gastric inhibitory polypeptide and glucagon-like polypeptide-1(7-36) amide, on fatty acid synthesis in explants of rat adipose tissue. J Endocrinol. 1991;130:267–72. doi: 10.1677/joe.0.1300267. [DOI] [PubMed] [Google Scholar]
- 55.Andreozzi F, Raciti GA, Nigro C, Mannino GC, Procopio T, Davalli AM, et al. The GLP-1 receptor agonists exenatide and liraglutide activate Glucose transport by an AMPK-dependent mechanism. J Transl Med. 2016;14:229. doi: 10.1186/s12967-016-0985-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li L, Miao Z, Liu R, Yang M, Liu H, Yang G. Liraglutide prevents hypoadiponectinemia-induced insulin resistance and alterations of gene expression involved in glucose and lipid metabolism. Mol Med. 2011;17:1168–78. doi: 10.2119/molmed.2011.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee YS, Shin S, Shigihara T, Hahm E, Liu MJ, Han J, et al. Glucagon-like peptide-1 gene therapy in obese diabetic mice results in long-term cure of diabetes by improving insulin sensitivity and reducing hepatic gluconeogenesis. Diabetes. 2007;56:1671–9. doi: 10.2337/db06-1182. [DOI] [PubMed] [Google Scholar]
- 58.Iizuka K, Takao K, Kato T, Horikawa Y, Takeda J. ChREBP reciprocally regulates liver and plasma triacylglycerol levels in different manners. Nutrients. 2018;10:1699. doi: 10.3390/nu10111699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khound R, Taher J, Baker C, Adeli K, Su Q. GLP-1 elicits an intrinsic gut-liver metabolic signal to ameliorate diet-induced VLDL overproduction and insulin resistance. Arterioscler Thromb Vasc Biol. 2017;37:2252–9. doi: 10.1161/ATVBAHA.117.310251. [DOI] [PubMed] [Google Scholar]
- 60.Ding X, Saxena NK, Lin S, Gupta NA, Anania FA. Exendin-4, a glucagon-like protein-1 (GLP-1) receptor agonist, reverses hepatic steatosis in ob/ob mice. Hepatology. 2006;43:173–81. doi: 10.1002/hep.21006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Somm E, Montandon SA, Loizides-Mangold U, Gaia N, Lazarevic V, De Vito C, et al. The GLP-1R agonist liraglutide limits hepatic lipotoxicity and inflammatory response in mice fed a methionine-choline deficient diet. Transl Res. 2021;227:75–88. doi: 10.1016/j.trsl.2020.07.008. [DOI] [PubMed] [Google Scholar]
- 62.Sancho V, Trigo MV, Martin-Duce A, Gonz Lez N, Acitores A, Arnes L, et al. Effect of GLP-1 on D-glucose transport, lipolysis and lipogenesis in adipocytes of obese subjects. Int J Mol Med. 2006;17:1133–7. [PubMed] [Google Scholar]
- 63.Wan Y, Bao X, Huang J, Zhang X, Liu W, Cui Q, et al. Novel GLP-1 analog supaglutide reduces HFD-induced obesity associated with increased Ucp-1 in white adipose tissue in mice. Front Physiol. 2017;8:294. doi: 10.3389/fphys.2017.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen J, Zhao H, Ma X, Zhang Y, Lu S, Wang Y, et al. GLP-1/GLP-1R signaling in regulation of adipocyte differentiation and lipogenesis. Cell Physiol Biochem. 2017;42:1165–76. doi: 10.1159/000478872. [DOI] [PubMed] [Google Scholar]
- 65.Challa TD, Beaton N, Arnold M, Rudofsky G, Langhans W, Wolfrum C. Regulation of adipocyte formation by GLP-1/GLP-1R signaling. J Biol Chem. 2012;287:6421–30. doi: 10.1074/jbc.M111.310342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Parlevliet ET, Wang Y, Geerling JJ, Schroder-Van der Elst JP, Picha K, O’Neil K, et al. GLP-1 receptor activation inhibits VLDL production and reverses hepatic steatosis by decreasing hepatic lipogenesis in high-fat-fed APOE*3-Leiden mice. PLoS One. 2012;7:e49152. doi: 10.1371/journal.pone.0049152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang SH, Xu RX, Cui CJ, Wang Y, Du Y, Chen ZG, et al. Liraglutide downregulates hepatic LDL receptor and PCSK9 expression in HepG2 cells and db/db mice through a HNF-1a dependent mechanism. Cardiovasc Diabetol. 2018;17:48. doi: 10.1186/s12933-018-0689-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koliaki C, Szendroedi J, Kaul K, Jelenik T, Nowotny P, Jankowiak F, et al. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab. 2015;21:739–46. doi: 10.1016/j.cmet.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 69.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sunny NE, Bril F, Cusi K. Mitochondrial adaptation in nonalcoholic fatty liver disease: novel mechanisms and treatment strategies. Trends Endocrinol Metab. 2017;28:250–60. doi: 10.1016/j.tem.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 71.Zhang L, Yang M, Ren H, Hu H, Boden G, Li L, et al. GLP-1 analogue prevents NAFLD in ApoE KO mice with diet and Acrp30 knockdown by inhibiting c-JNK. Liver Int. 2013;33:794–804. doi: 10.1111/liv.12120. [DOI] [PubMed] [Google Scholar]
- 72.Wang D, Wei Y, Pagliassotti MJ. Saturated fatty acids promote endoplasmic reticulum stress and liver injury in rats with hepatic steatosis. Endocrinology. 2006;147:943–51. doi: 10.1210/en.2005-0570. [DOI] [PubMed] [Google Scholar]
- 73.Ao N, Yang J, Wang X, Du J. Glucagon-like peptide-1 preserves non-alcoholic fatty liver disease through inhibition of the endoplasmic reticulum stressassociated pathway. Hepatol Res. 2016;46:343–53. doi: 10.1111/hepr.12551. [DOI] [PubMed] [Google Scholar]
- 74.Lee J, Hong SW, Chae SW, Kim DH, Choi JH, Bae JC, et al. Exendin-4 improves steatohepatitis by increasing Sirt1 expression in high-fat diet-induced obese C57BL/6J mice. PLoS One. 2012;7:e31394. doi: 10.1371/journal.pone.0031394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wilson CH, Ali ES, Scrimgeour N, Martin AM, Hua J, Tallis GA, et al. Steatosis inhibits liver cell store-operated Ca2+ entry and reduces ER Ca2+ through a protein kinase C-dependent mechanism. Biochem J. 2015;466:379–90. doi: 10.1042/BJ20140881. [DOI] [PubMed] [Google Scholar]
- 76.Ali ES, Hua J, Wilson CH, Tallis GA, Zhou FH, Rychkov GY, et al. The glucagon-like peptide-1 analogue exendin-4 reverses impaired intracellular Ca2+ signalling in steatotic hepatocytes. Biochim Biophys Acta. 2016;1863:2135–46. doi: 10.1016/j.bbamcr.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 77.Wolf MJ, Adili A, Piotrowitz K, Abdullah Z, Boege Y, Stemmer K, et al. Metabolic activation of intrahepatic CD8+ T cells and NKT cells causes nonalcoholic steatohepatitis and liver cancer via cross-talk with hepatocytes. Cancer Cell. 2014;26:549–64. doi: 10.1016/j.ccell.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 78.Hogan AE, Gaoatswe G, Lynch L, Corrigan MA, Woods C, O'Connell J, et al. Glucagon-like peptide 1 analogue therapy directly modulates innate immunemediated inflammation in individuals with type 2 diabetes mellitus. Diabetologia. 2014;57:781–4. doi: 10.1007/s00125-013-3145-0. [DOI] [PubMed] [Google Scholar]
- 79.Hogan AE, Tobin AM, Ahern T, Corrigan MA, Gaoatswe G, Jackson R, et al. Glucagon-like peptide-1 (GLP-1) and the regulation of human invariant natural killer T cells: lessons from obesity, diabetes and psoriasis. Diabetologia. 2011;54:2745–54. doi: 10.1007/s00125-011-2232-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yusta B, Baggio LL, Koehler J, Holland D, Cao X, Pinnell LJ, et al. GLP-1R agonists modulate enteric immune responses through the intestinal intraepithelial lymphocyte GLP-1R. Diabetes. 2015;64:2537–49. doi: 10.2337/db14-1577. [DOI] [PubMed] [Google Scholar]
- 81.Nagashima M, Watanabe T, Terasaki M, Tomoyasu M, Nohtomi K, Kim-Kaneyama J, et al. Native incretins prevent the development of atherosclerotic lesions in apolipoprotein E knockout mice. Diabetologia. 2011;54:2649–59. doi: 10.1007/s00125-011-2241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim S, Jeong J, Jung HS, Kim B, Kim YE, Lim DS, et al. Anti-inflammatory effect of glucagon like peptide-1 receptor agonist, exendin-4, through modulation of IB1/JIP1 expression and JNK signaling in stroke. Exp Neurobiol. 2017;26:227–39. doi: 10.5607/en.2017.26.4.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huang W, Metlakunta A, Dedousis N, Zhang P, Sipula I, Dube JJ, et al. Depletion of liver Kupffer cells prevents the development of diet-induced hepatic steatosis and insulin resistance. Diabetes. 2010;59:347–57. doi: 10.2337/db09-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gagnon J, Sauve M, Zhao W, Stacey HM, Wiber SC, Bolz SS, et al. Chronic exposure to TNFα impairs secretion of glucagon-like peptide-1. Endocrinology. 2015;156:3950–60. doi: 10.1210/en.2015-1361. [DOI] [PubMed] [Google Scholar]
- 85.Lee YS, Park MS, Choung JS, Kim SS, Oh HH, Choi CS, et al. Glucagon-like peptide-1 inhibits adipose tissue macrophage infiltration and inflammation in an obese mouse model of diabetes. Diabetologia. 2012;55:2456–68. doi: 10.1007/s00125-012-2592-3. [DOI] [PubMed] [Google Scholar]
- 86.Longato L, Tong M, Wands JR, de la Monte SM. High fat diet induced hepatic steatosis and insulin resistance: Role of dysregulated ceramide metabolism. Hepatol Res. 2012;42:412–27. doi: 10.1111/j.1872-034X.2011.00934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Heymann F, Tacke F. Immunology in the liver-from homeostasis to disease. Nat Rev Gastroenterol Hepatol. 2016;13:88–110. doi: 10.1038/nrgastro.2015.200. [DOI] [PubMed] [Google Scholar]
- 88.Szabo G, Iracheta-Vellve A. Inflammasome activation in the liver: Focus on alcoholic and non-alcoholic steatohepatitis. Clin Res Hepatol Gastroenterol. 2015;39:S18–23. doi: 10.1016/j.clinre.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 89.Dai Y, Dai D, Wang X, Ding Z, Mehta JL. DPP-4 inhibitors repress NLRP3 inflammasome and interleukin-1beta via GLP-1 receptor in macrophages through protein kinase C pathway. Cardiovasc Drugs Ther. 2014;28:425–32. doi: 10.1007/s10557-014-6539-4. [DOI] [PubMed] [Google Scholar]
- 90.Zhuge F, Ni Y, Nagashimada M, Nagata N, Xu L, Mukaida N, et al. DPP-4 Inhibition by linagliptin attenuates obesity-related inflammation and insulin resistance by regulating M1/M2 macrophage polarization. Diabetes. 2016;65:2966–79. doi: 10.2337/db16-0317. [DOI] [PubMed] [Google Scholar]
- 91.Sumida Y, Yoneda M, Tokushige K, Kawanaka M, Fujii H, Yoneda M, et al. Antidiabetic therapy in the treatment of nonalcoholic steatohepatitis. Int J Mol Sci. 2020;21:1907. doi: 10.3390/ijms21061907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Trautwein C, Friedman SL, Schuppan D, Pinzani M. Hepatic fibrosis: Concept to treatment. J Hepatol. 2015;62:S15–24. doi: 10.1016/j.jhep.2015.02.039. [DOI] [PubMed] [Google Scholar]
- 93.Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14:397–411. doi: 10.1038/nrgastro.2017.38. [DOI] [PubMed] [Google Scholar]
- 94.Balestrieri ML, Rizzo MR, Barbieri M, Paolisso P, D'Onofrio N, Giovane A, et al. Sirtuin 6 expression and inflammatory activity in diabetic atherosclerotic plaques: effects of incretin treatment. Diabetes. 2015;64:1395–406. doi: 10.2337/db14-1149. [DOI] [PubMed] [Google Scholar]
- 95.Zhou M, Mok MT, Sun H, Chan AW, Huang Y, Cheng AS, et al. The anti-diabetic drug exenatide, a glucagon-like peptide-1 receptor agonist, counteracts hepatocarcinogenesis through cAMP-PKA-EGFR-STAT3 axis. Oncogene. 2017;36:4135–49. doi: 10.1038/onc.2017.38. [DOI] [PubMed] [Google Scholar]
- 96.de Mesquita FC, Guixe-Muntet S, Fernandez-Iglesias A, Maeso-Diaz R, Vila S, Hide D, et al. Liraglutide improves liver microvascular dysfunction in cirrhosis: evidence from translational studies. Sci Rep. 2017;7:3255. doi: 10.1038/s41598-017-02866-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–31. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 98.Lin HV, Frassetto A, Kowalik EJ, Jr, Nawrocki AR, Lu MM, Kosinski JR, et al. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS One. 2012;7:e35240. doi: 10.1371/journal.pone.0035240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54:2325–40. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Georgiadi A, Lichtenstein L, Degenhardt T, Boekschoten MV, van Bilsen M, Desvergne B, et al. Induction of cardiac Angptl4 by dietary fatty acids is mediated by peroxisome proliferator-activated receptor beta/delta and protects against fatty acid-induced oxidative stress. Circ Res. 2010;106:1712–21. doi: 10.1161/CIRCRESAHA.110.217380. [DOI] [PubMed] [Google Scholar]
- 101.Pendyala S, Walker JM, Holt PR. A high-fat diet is associated with endotoxemia that originates from the gut. Gastroenterology. 2012;142:1100–01.e2. doi: 10.1053/j.gastro.2012.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14:141–53. doi: 10.1038/nri3608. [DOI] [PubMed] [Google Scholar]
- 103.Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD, et al. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013;57:601–9. doi: 10.1002/hep.26093. [DOI] [PubMed] [Google Scholar]
- 104.Grasset E, Puel A, Charpentier J, Collet X, Christensen JE, Terce F, et al. A specific gut microbiota dysbiosis of type 2 diabetic mice induces GLP-1 resistance through an enteric NO-dependent and gut-brain axis mechanism. Cell Metab. 2017;25:1075–90. doi: 10.1016/j.cmet.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 105.Moreira GV, Azevedo FF, Ribeiro LM, Santos A, Guadagnini D, Gama P, et al. Liraglutide modulates gut microbiota and reduces NAFLD in obese mice. J Nutr Biochem. 2018;62:143–54. doi: 10.1016/j.jnutbio.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 106.Neff CP, Rhodes ME, Arnolds KL, Collins CB, Donnelly J, Nusbacher N, et al. Diverse intestinal bacteria contain putative zwitterionic capsular polysaccharides with anti-inflammatory properties. Cell Host Microbe. 2016;20:535–47. doi: 10.1016/j.chom.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hoosein NM, Gurd RS. Human glucagon-like peptide-1 and peptide-2 activate rat-brain adenylate-cyclase. FEBS Lett. 1984;178:83–6. doi: 10.1016/0014-5793(84)81245-4. [DOI] [PubMed] [Google Scholar]
- 108.Beiroa D, Imbernon M, Gallego R, Senra A, Herranz D, Villarroya F, et al. GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes. 2014;63:3346–58. doi: 10.2337/db14-0302. [DOI] [PubMed] [Google Scholar]
- 109.Renner E, Puskas N, Dobolyi A, Palkovits M. Glucagon-like peptide-1 of brainstem origin activates dorsomedial hypothalamic neurons in satiated rats. Peptides. 2012;35:14–22. doi: 10.1016/j.peptides.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 110.Müller TD, Finan B, Bloom SR, D'Alessio D, Drucker DJ, Flatt PR, et al. Glucagon-like peptide 1 (GLP-1) Mol Metab. 2019;30:72–130. doi: 10.1016/j.molmet.2019.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Burmeister MA, Ferre T, Ayala JE, King EM, Holt RM, Ayala JE. Acute activation of central GLP-1 receptors enhances hepatic insulin action and insulin secretion in high-fat-fed, insulin resistant mice. Am J Physiol Endocrinol Metab. 2012;302:E334–43. doi: 10.1152/ajpendo.00409.2011. [DOI] [PubMed] [Google Scholar]
- 112.Lee SJ, Sanchez-Watts G, Krieger JP, Pignalosa A, Norell PN, Cortella A, et al. Loss of dorsomedial hypothalamic GLP-1 signaling reduces BAT thermogenesis and increases adiposity. Mol Metab. 2018;11:33–46. doi: 10.1016/j.molmet.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Patel V, Joharapurkar AA, Kshirsagar SG, Patel KN, Bahekar R, Shah G, et al. Central GLP-1 receptor activation improves cholesterol metabolism partially independent of its effect on food intake. Can J Physiol Pharmacol. 2016;94:161–7. doi: 10.1139/cjpp-2014-0457. [DOI] [PubMed] [Google Scholar]
- 114.Nishizawa M, Nakabayashi H, Uehara K, Nakagawa A, Uchida K, Koya D. Intraportal GLP-1 stimulates insulin secretion predominantly through the hepatoportal-pancreatic vagal reflex pathways. Am J Physiol Endocrinol Metab. 2013;305:E376–87. doi: 10.1152/ajpendo.00565.2012. [DOI] [PubMed] [Google Scholar]
- 115.Taher J, Baker CL, Cuizon C, Masoudpour H, Zhang R, Farr S, et al. GLP-1 receptor agonism ameliorates hepatic VLDL overproduction and de novo lipogenesis in insulin resistance. Mol Metab. 2014;3:823–33. doi: 10.1016/j.molmet.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–62. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 117.Lagerström MC, Schiöth HB. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat Rev Drug Discov. 2008;7:339–57. doi: 10.1038/nrd2518. [DOI] [PubMed] [Google Scholar]
- 118.Hoare SR. Mechanisms of peptide and nonpeptide ligand binding to Class B G- protein-coupled receptors. Drug Discov Today. 2005;10:417–27. doi: 10.1016/S1359-6446(05)03370-2. [DOI] [PubMed] [Google Scholar]
- 119.Bergwitz C, Gardella TJ, Flannery MR, Potts JT, Jr, Kronenberg HM, Goldring SR, et al. Full activation of chimeric receptors by hybrids between parathyroid hormone and calcitonin. Evidence for a common pattern of ligand-receptor interaction. J Biol Chem. 1996;271:26469–72. doi: 10.1074/jbc.271.43.26469. [DOI] [PubMed] [Google Scholar]
- 120.Svegliati-Baroni G, Patrício B, Lioci G, Macedo MP, Gastaldelli A. Gut-pancreasliver axis as a target for treatment of NAFLD/NASH. Int J Mol Sci. 2020;21:5820. doi: 10.3390/ijms21165820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tan TM, Field BC, McCullough KA, Troke RC, Chambers ES, Salem V, et al. Coadministration of glucagon-like peptide-1 during glucagon infusion in humans results in increased energy expenditure and amelioration of hyperglycemia. Diabetes. 2013;62:1131–8. doi: 10.2337/db12-0797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sanchez-Garrido MA, Brandt SJ, Clemmensen C, Muller TD, DiMarchi RD, Tschop MH. GLP-1/glucagon receptor co-agonism for treatment of obesity. Diabetologia. 2017;60:1851–61. doi: 10.1007/s00125-017-4354-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hartman ML, Sanyal AJ, Loomba R, Wilson JM, Nikooienejad A, Bray R, et al. Effects of novel dual GIP and GLP-1 receptor agonist tirzepatide on biomarkers of nonalcoholic steatohepatitis in patients with type 2 diabetes. Diabetes Care. 2020;43:1352–5. doi: 10.2337/dc19-1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Takeda Y, Ikeda R, Kondo T. Incretin as a novel treatment strategy for NAFLD/NASH. J Pharmacol Soc Jpn. 2016;136:573–7. doi: 10.1248/yakushi.15-00264-2. [DOI] [PubMed] [Google Scholar]
- 125.Finan B, Yang B, Ottaway N, Smiley DL, Ma T, Clemmensen C, et al. A rationally designed monomeric peptide triagonist corrects obesity and diabetes in rodents. Nat Med. 2015;21:27–36. doi: 10.1038/nm.3761. [DOI] [PubMed] [Google Scholar]
- 126.Khajavi N, Biebermann H, Tschöp M, DiMarchi R. Treatment of diabetes and obesity by rationally designed peptide agonists functioning at multiple metabolic receptors. Endocr Dev. 2017;32:165–82. doi: 10.1159/000475737. [DOI] [PubMed] [Google Scholar]
- 127.Kannt A, Madsen AN, Kammermeier C, Elvert R, Klöckener T, Bossart M, et al. Incretin combination therapy for the treatment of non-alcoholic steatohepatitis. Diabetes Obes Metab. 2020;22:1328–38. doi: 10.1111/dom.14035. [DOI] [PubMed] [Google Scholar]
- 128.Finan B, Ma T, Ottaway N, Müller TD, Habegger KM, Heppner KM, et al. Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans. Sci Transl Med. 2013;5:209ra151. doi: 10.1126/scitranslmed.3007218. [DOI] [PubMed] [Google Scholar]
- 129.Trevaskis JL, Griffin PS, Wittmer C, Neuschwander-Tetri BA, Brunt EM, Dolman CS, et al. Glucagon-like peptide-1 receptor agonism improves metabolic, biochemical, and histopathological indices of nonalcoholic steatohepatitis in mice. Am J Physiol Gastrointest Liver Physiol. 2012;302:G762–72. doi: 10.1152/ajpgi.00476.2011. [DOI] [PubMed] [Google Scholar]
- 130.Yamamoto T, Nakade Y, Yamauchi T, Kobayashi Y, Ishii N, Ohashi T, et al. Glucagon-like peptide-1 analogue prevents nonalcoholic steatohepatitis in nonobese mice. World J Gastroenterol. 2016;22:2512–23. doi: 10.3748/wjg.v22.i8.2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bouchi R, Nakano Y, Fukuda T, Takeuchi T, Murakami M, Minami I, et al. Reduction of visceral fat by liraglutide is associated with ameliorations of hepatic steatosis, albuminuria, and micro-inflammation in type 2 diabetic patients with insulin treatment: a randomized control trial. Endocr J. 2017;64:269–81. doi: 10.1507/endocrj.EJ16-0449. [DOI] [PubMed] [Google Scholar]
- 132.Davidson MB, Bate G. Kirkpatrick P. Exenatide. Nat Rev Drug Discov. 2005;4:713–4. doi: 10.1038/nrd1828. [DOI] [PubMed] [Google Scholar]
- 133.Tushuizen ME, Bunck MC, Pouwels PJ, van Waesberghe JH, Diamant M, Heine RJ. Incretin mimetics as a novel therapeutic option for hepatic steatosis. Liver Int. 2006;26:1015–7. doi: 10.1111/j.1478-3231.2006.01315.x. [DOI] [PubMed] [Google Scholar]
- 134.Shao N, Kuang HY, Hao M, Gao XY, Lin WJ, Zou W. Benefits of exenatide on obesity and non-alcoholic fatty liver disease with elevated liver enzymes in patients with type 2 diabetes. Diabetes Metab Res Rev. 2014;30:521–9. doi: 10.1002/dmrr.2561. [DOI] [PubMed] [Google Scholar]
- 135.Dutour A, Abdesselam I, Ancel P, Kober F, Mrad G, Darmon P, et al. Exenatide decreases liver fat content and epicardial adipose tissue in patients with obesity and type 2 diabetes: a prospective randomized clinical trial using magnetic resonance imaging and spectroscopy. Diabetes Obes Metab. 2016;18:882–91. doi: 10.1111/dom.12680. [DOI] [PubMed] [Google Scholar]
- 136.Liu L, Yan H, Xia M, Zhao L, Lv M, Zhao N, et al. Efficacy of exenatide and insulin glargine on nonalcoholic fatty liver disease in patients with type 2 diabetes. Diabetes Metab Res Rev. 2020;36:e3292. doi: 10.1002/dmrr.3292. [DOI] [PubMed] [Google Scholar]
- 137.Feng W, Gao C, Bi Y, Wu M, Li P, Shen S, et al. Randomized trial comparing the effects of gliclazide, liraglutide, and metformin on diabetes with non-alcoholic fatty liver disease. J Diabetes. 2017;9:800–9. doi: 10.1111/1753-0407.12555. [DOI] [PubMed] [Google Scholar]
- 138.Yan J, Yao B, Kuang H, Yang X, Huang Q, Hong T, et al. Liraglutide, sitagliptin, and insulin glargine added to metformin: the effect on body weight and intrahepatic lipid in patients with type 2 diabetes mellitus and nonalcoholic fatty liver disease. Hepatology. 2019;69:2414–26. doi: 10.1002/hep.30320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Newsome PN, Buchholtz K, Cusi K, Linder M, Okanoue T, Ratziu V, et al. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med. 2021;384:1113–24. doi: 10.1056/NEJMoa2028395. [DOI] [PubMed] [Google Scholar]
- 140.Rakipovski G, Rolin B, Nohr J, Klewe I, Frederiksen KS, Augustin R, et al. The GLP-1 analogs liraglutide and semaglutide reduce atherosclerosis in ApoE−/− and LDLr−/− mice by a mechanism that includes inflammatory pathways. JACC Basic Transl Sci. 2018;3:844–57. doi: 10.1016/j.jacbts.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Day JW, Ottaway N, Patterson JT, Gelfanov V, Smiley D, Gidda J, et al. A new glucagon and GLP-1 co-agonist eliminates obesity in rodents. Nat Chem Biol. 2009;5:749–57. doi: 10.1038/nchembio.209. [DOI] [PubMed] [Google Scholar]
- 142.Pocai A, Carrington PE, Adams JR, Wright M, Eiermann G, Zhu L, et al. Glucagon-like peptide 1/glucagon receptor dual agonism reverses obesity in mice. Diabetes. 2009;58:2258–66. doi: 10.2337/db09-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pan Q, Lin S, Li Y, Liu L, Li X, Gao X, et al. A novel GLP-1 and FGF21 dual agonist has therapeutic potential for diabetes and non-alcoholic steatohepatitis. EBio-Medicine. 2021;63:103202. doi: 10.1016/j.ebiom.2020.103202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Frias JP, Nauck MA, Van J, Kutner ME, Cui X, Benson C, et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparatorcontrolled phase 2 trial. Lancet. 2018;392:2180–93. doi: 10.1016/S0140-6736(18)32260-8. [DOI] [PubMed] [Google Scholar]
- 145.Vuppalanchi R, Jain AK, Deppe R, Yates K, Comerford M, Masuoka HC, et al. Relationship between changes in serum levels of keratin 18 and changes in liver histology in children and adults with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2014;12:2121–30. doi: 10.1016/j.cgh.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.