Abstract
Direct-seeded rice (DSR) seeds are often exposed to multiple environmental stresses in the field, leading to poor emergence, growth and productivity. Appropriate seed priming agents may help to overcome these challenges by ensuring uniform seed germination, and better seedling stand establishment. To examine the effectiveness of sodium selenite (Na-selenite), sodium selenate (Na-selenate), zinc oxide nanoparticles (ZnO-NPs), and their combinations as priming agents for DSR seeds, a controlled pot experiment followed by a field experiment over two consecutive years was conducted on a sandy clay loam soil (Inceptisol) in West Bengal, India. Priming with combinations of all priming agents had advantages over the hydro-priming treatment (control). All the combinations of the three priming agents resulted in the early emergence of seedlings with improved vigour. In the field experiment, all the combinations increased the plant chlorophyll, phenol and protein contents, leaf area index and duration, crop growth rate, uptake of nutrients (N, P, K, B, Zn and Si), and yield of DSR over the control. Our findings suggest that seed priming with the combination of ZnO-NPs, Na-selenite, and Na-selenate could be a viable option for the risk mitigation in DSR.
Subject terms: Biological techniques, Plant sciences, Environmental sciences
Introduction
Rice plays a major role in achieving global food security but the crop now is under several threats, including yield stagnation in major rice-producing regions, negative environmental impacts of the overuse of irrigation water and agrochemicals, high labour requirements which is under short supply, and increasing concerns of loss of natural habitats due to intensive rice cultivation1–3. In particular, traditional puddled-transplanted rice (PTR) requires more irrigation water and labour than direct-seeded rice (DSR)4. Future, production systems should be more productive, cost-effective, and remunerative while minimizing the negative environmental impacts. The DSR could potentially be a viable alternative to PTR due to reduced labour requirement, increased resource (water, nutrient, energy, etc.) use efficiency, and higher returns5,6. Direct seeding can be achieved by sowing on the dry soil either mechanically or manually (Dry DSR), on wet soil through broadcasting (Wet DSR), or in standing water through broadcasting (Water seeding)5,7. Dry DSR could be more advantageous over the other two methods8,9 due to less time required for sowing, thus economizing time and labour, and saving water. Though PTR is the main rice transplanting system in Asia including India, DSR has now been gaining popularity10–13. For example, in China, DSR now covers 28% of the total rice area14, while in Sri Lanka and Malaysia, areas under DSR are > 93% (1.03 M ha) and 95% (0.67 M ha) respectively of their respective total rice area15. In the Indian Punjab alone, DSR was adopted in 0.601 M ha in 202116.
Rapid loss of seed germination and vigor during storage and poor germination and seedling stand establishment in the field is identified as the major factors for the low productivity of DSR in South Asia14,17–20. In DSR when seeds are sown directly in the field, plants are often exposed to multiple environmental stresses, particularly during emergence and early development21–23. These stresses depress the kinetics of many physiological and metabolic processes24 and generate a large number of reactive oxygen species (ROS) in plant cells that trigger lipid peroxidation in membranes25–27. These are followed by damage to biomolecules including proteins, carbohydrates, and DNA26,28, and reduce respiration rate and energy supply to growing plant tissue29. All these processes affect sequentially the germination and seedling establishment, plant stand, growth and resource utilisation, and ultimately the yield of DSR.
The conventional breeding approach to develop varieties for enhanced germination and plant establishment is time-consuming and genetic engineering is also highly controversial albeit with its high potential benefits30. To overcome such difficulties, a large number of abiotic stress-tolerant rice genes have been characterized during the last three decades which are being further exploited for varietal development with higher productivity31,32. In addition to the above methods which are generally costly and time-consuming, seed priming with various priming agents has proven utility in optimising seed vigour and plant physiological processes, which make the plant ready to face multiple stresses in the field more efficiently33–35. Several successful priming agents have been reported in the literature ranging from salts, polyamines, hormones, compatible solutes, and aqueous plant extracts36.
Zinc (Zn), an essential micronutrient, is the only metal that forms a part of the six different classes of enzymes37. It is closely involved in many biochemical and physiological processes38. Seed priming with Zn has shown positive effects on seed vigour, germination, early seedling growth and biomass production, photosynthetic efficiency, and increasing the contents of sugar, total nitrogen, protein, and micronutrients in many crops39–41. In the recent past, biologically synthesised zinc oxide nanoparticles (ZnO-NPs) with their enhanced physical and biochemical characteristics and low environmental toxicity have shown their efficacy as priming agents42,43. Literature indicates that ZnO-NPs have beneficial effects at low concentrations (about 50 ppm) but detrimental effects at concentrations above 500 ppm on plant growth and development44. Recently, extensive work is going on the use of ZnO-NPs in nanooncology and mitigation of its associated toxicity risk on lungs, liver, kidney, pancreas, spleen, stomach, testis, thymus, brain, heart, blood, etc45. Uniformity in seedling emergence and improved growth of rice plants using ZnO-NPs as priming agent was also reported with higher production of antioxidant enzymes against ROS damage46,47.
In plants, selenium (Se) as a constituent of seleno-proteins, has been reported to enhance starch and ATP synthesis48, regulate water status, prevent chlorophyll loss during drought49,50, and delay senescence51. In addition, it regulates redox reactions52,53 and ROS concentration, and consequently lipid peroxidation54. In rice, priming with Na-selenite has been shown to trigger its seed germination55. Subsequent studies also reported the efficacy of rice seed priming with Na-selenate56 and selenite-selenate combination of sodium salt57 with their contributions to the assimilation and storage pathway respectively. Oxyanions of Se can promote the adsorption of Zn and enhance their bioavailability within the plant system by synergy58,59. To our knowledge, there is no literature on the combined application of zinc or nano-zinc with Se as seed priming agents on germination and seedling establishment and growth and yield of DSR.
In response to the above gaps, we hypothesized that (1) the (synergistic) effect of ZnO-NPs and Se as seed priming agents in DSR cultivation influences seed germination, and the emergence of healthy and robust seedlings capable of mitigating environmental stresses; and (2) the synergistic effect could result in higher DSR yield compared to that without the seed priming. To test these hypotheses, a controlled pot experiment and a field experiment were undertaken with different treatments of ZnO-NPs, Na-selenate, and Na-selenite either singly or in their combinations as priming agents in DSR.
Materials and methods
Experimental site
A pot experiment (examined for seed vigour) was conducted followed by field experimentation (examined for plant growth traits and yield) each year (2019 and 2020) on DSR at Central Research Farm of Bidhan Chandra Krishi Viswavidyalaya, West Bengal, India (23° 5.3ʹ N, 83° 5.3ʹ E; subtropical; 9.75 m MSL). The soil was sandy clay loam (sand 64.8%, silt 10.4%, and clay 24.8%) with EC of 0.296 dSm−1 and a pH of 7.360. Initial soil properties of the study site were: 11.2 g kg−1 oxidizable organic carbon61, 315 kg ha−1 available N62, 41.6 kg ha−1 available P63, and 156.4 kg ha-1 available K64. The climate is tropical moist sub-humid, with hot summer, and moderately cold winter. Average maximum and minimum temperatures ranged from 25 to 36 °C in summer and from 10 to 25 °C in winter (Supplementary Figure S1).
Experimental treatments
ZnO-NPs used in this study were with mean hydrodynamic diameter of size distribution (determined by dynamic light scattering technique) below 10 nm, surface charge (measured as zeta potential) of − 5.7 mV and maximum intensity at 1 keV (TEM–EDX) and these biologically synthesized nanoparticles were stable up to 90 days in the aqueous medium65. Eight treatment combinations of ZnO-NPs and Se were selected: T1: Hydropriming with distilled water (Control); T2: Na-selenite at 50 µmol (as Na2SeO3;Sigma-Aldrich USA); T3: Na-selenate at 50 µmol (as Na2SeO4;Sigma-Aldrich USA); T4: Na-selenite at 50 µmol + Na-selenate at 50 µmol; T5: ZnO-NPs at 10 µmol ; T6: ZnO-NPs at 10 µmol + Na-selenite at 50 µmol; T7: ZnO-NPs at 10 µmol + Na-selenate at 50 µmol; and T8: ZnO-NPs at 10 µmol + Na-selenite at 50 µmol + Na-selenate at 50 µmol]. Indica inbred semi-dwarf type medium slender grain early maturing (108–110 d) rice variety Ajit IET 2206666 was used in both pot and field experiments.
Pot experiment
The pot experiment was conducted using a completely randomized design in three replications with factorial arrangements. Twenty-five g of seeds were placed in a 200 ml conical flask containing 125 ml initiator solution as per the priming agent treatments, and aerated distilled water for the control. Seeds were kept for imbibitions for 24 h. The flasks were placed in an incubator [darkness, 27 ± 3 °C and 80% relative humidity] and were shaken once every 6 h. After priming, the seeds were filtered, placed in distilled water for 20 min, and rinsed five times with ultrapure water. Autoclaved glass Petri dishes were lined with double layers of filter paper and were placed on a laboratory bench for air drying for a period of 24 h. Thereafter, 10 ml sterile water was aseptically pipetted into and 100 seeds were placed in each Petri-dish. Each seed soaking treatment was performed in triplicate Petri-dishes, which were kept inside an incubator at 26 ± 0.5 °C under aseptic conditions.
Seeds were sown in plastic pots (15.6 cm height, 18.2 cm top diameter, and 12.5 cm lower diameter), which were filled with air-dried and well-mixed field soil (2000 g) collected from the study site. Seeds were sown uniformly in each pot with soil moisture at field capacity (− 0.03 MPa). Pots were placed in a screen house under the natural condition with a 14/10 h light/dark photoperiod and uniformly irrigated with distilled water as per the requirement to avoid water deficit. The seed emergence was counted daily according to the Association of Official Seed Analysts (AOSA)67 until a constant count was achieved. A seed was considered as “emerged” when the hypocotyl length was ≥ 2 mm. Time taken to 50% emergence of seedlings (E50) was calculated according to the modified formula of Basra et al.68:
| 1 |
where N is the final number of emerged seeds; ni and nj are the cumulative number of seeds emerged by adjacent counts at times ti and tj where ni < N/2 < nj. Mean emergence time (MET), an indicator of the relative emergence of seedlings in a day was calculated according to Ellis and Roberts69:
| 2 |
where n is the number of new emerging seeds on day D (number of days from the beginning of emergence). The emergence index (EI), which is a measure of the percentage and rate of germination, was calculated as described by AOSA70:
| 3 |
Vigour index (VI) was calculated after Wang et al.71.
| 4 |
where Sd is the seedling's dry weight at the end of the test period (7 days), Gt is the number of germinated seeds on day t from the beginning of the test.
Root and shoot lengths were measured 18 days after sowing (DAS) from each treatment. For these, five randomly selected seedlings were oven-dried at 70 °C for 48 h to get the dry biomass of root and shoot, and both the components were summed up to record the total seedling biomass.
Field experiment
The experiment was laid out in a factorial randomized complete block design with three replications. Fields were prepared by cultivating twice using a disc harrow (Unison Exports, Ludhiana, Punjab, India), followed by levelling with a wooden board. Pre-germinated primed seeds were sown manually by a single-row planter having an inclined plane seed metering mechanism at 25 kg ha−1 at 20 cm row-to-row spacing. The field was surface-irrigated immediately after sowing. Soil water potential was monitored with tensiometers installed at 20 cm depth, and the field was irrigated at − 30 kPa potential. Fertilizers were applied as per soil test-based recommendations72 with a basal application of N at 25 kg ha−1, P2O5 at 50 kg ha−1, K2O at 50 kg ha−1, and ZnSO4 at 5 kg ha−1 as urea, single super phosphate and muriate of potash, respectively. Additionally, 75 kg N ha−1 in the form of urea and ammonium sulphate (AS) was applied in two splits: one half at the active tillering stage (urea and AS in equal proportion) and the other half at the panicle initiation stage (only through AS). Weeds were controlled by a pre-emergence herbicide (pendimethalin at 0.75 kg a.i. ha−1) at 2 DAS, followed by a post-emergence herbicide (bispyribac-sodium at 25 g a.i. ha−1) at 20 DAS. Weeds that escaped these treatments were removed manually at 42 DAS. Chloropyriphos at 50 g a.i. ha−1 and propiconazole at 62.5 g a.i. ha−1 was used to control insects and diseases, respectively. Irrigation was withdrawn 15 days before harvest. All agronomic management practices were the same in both years. Grains were harvested at 15–18% grain moisture content.
Growth traits analysis
Plants were collected from 1-m length within a row in each plot at the initiation of tillering (14 DAS), panicle initiation (42 DAS), 50% flowering stage (70 DAS), and physiological maturity (108 DAS) to determine aboveground biomass and green leaf area index (LAI). Green leaves were separated and the area was measured with a leaf area meter (LI-3100, LI-COR, Lincoln, Nebraska, USA). The LAI was expressed as leaf area per unit of the area sampled for each plot. Dry weights of each plant part were determined after oven-drying at 80̊ C to calculate crop growth rate (CGR), net assimilation rate (NAR), and leaf area duration (LAD)73 for vegetative (up to 41 DAS), reproductive (42–69 DAS) and grain filling (70–108 DAS) stages using the following equations:
| 5 |
| 6 |
| 7 |
where W1 and W2 were dry weights of aerial plant parts per unit land area at time t1 and t2, respectively; L1 and L2 were total leaf area of plants per unit land area at time t1 and t2 respectively.
Biochemical analysis of seedlings
Four random rice seedlings from each plot were collected on 18 DAS to determine total soluble phenol compounds74, photosynthetic pigments (chlorophyll “a” and “b”)75, and soluble proteins76.
Nutrient uptake
Plant samples were collected at harvest, oven-dried, and ground. The N content on a dry weight basis was estimated by the Micro-Kjeldahl method77, P content by colorimeter method using vanadomolybdate yellow77, and K content by a flame photometer78. Zinc was analyzed through diacid extract using Perkin-Elmer Atomic Absorption Spectrophotometer. Boron was estimated by dry ashing and azomethine-H methods79, and silica was estimated by the blue silicomolybdic acid method80. The nutrient uptake by grain and straw was expressed on a per hectare basis81.
Yield and yield components
Grain and straw yields were determined at physiological maturity. The harvest index was calculated as the ratio of dry grain yield to total dry biomass yield (both oven-dried at 70 °C). Tiller and panicle densities were determined with a quadrat (0.4 m × 0.5 m) placed randomly in each plot at two locations. At the same time, five plants were selected randomly from each plot to measure the number of filled grains panicle−1 and 1000-grain weight82.
Statistical analyses
All data from pot and field experiments were analysed using a mixed ANOVA model in SAS, considering year and treatment, and their two-way interactions as factors. Treatment adjusted means were separated by using HSD Tukey multiple range test at 5% level of significance83. The coefficient of determination (R2) and correlogram was calculated to assess the degree of association between two variables using the JMP Pro 16.
Results
Pot experiment
Seed germination emergence and vigour, and enzymatic and biochemical activities
Seed priming improved gemination and vigour attributes, although year-to-year differences were evident (Supplementary Table S1). Priming with either Se or ZnO-NPs alone or in combination reduced the time to start of emergence (TSE), time to reach 50% germination (E50), and mean emergence time (MET) compared to the control, while EI and VI parameters improved over the control (Table 1). Percent reduction in TSE (43.3), MET (25.5), and E50 (30.9), and percent increase in EI (66.4) and VI (112.3) were greater with the combined application of ZnO-NPs, Na-selenate, and Na-selenite. Even Na-selenite and ZnO-NPs combination favoured the germination, as evidenced from the germination parameters except for TSE.
Table 1.
Effect of seed priming on seedling emergence and seedling vigour of DSR in a pot experiment (pooled over 2019 and 2020).
| Treatment | TSE (days) | E50 (days) | MET (days) | EI | VI |
|---|---|---|---|---|---|
| T1 | 3.8a | 5.8a | 6.19a | 13.5f | 969f |
| T2 | 3.2b | 5.1bc | 5.26bc | 17.5e | 1290e |
| T3 | 3.3b | 4.9bc | 5.06bc | 20.1c | 1519d |
| T4 | 3.3b | 4.9bcd | 5.15bc | 17.6e | 1590cd |
| T5 | 3.2b | 4.7cd | 5.49b | 18.1de | 1221e |
| T6 | 2.7c | 4.7de | 4.96bc | 19.4cd | 1643c |
| T7 | 2.7c | 4.4e | 4.72c | 20.4bc | 1796b |
| T8 | 2.2d | 4.0f | 4.61c | 22.4a | 2057a |
TSE time to start emergence, E50 time taken to reach to 50% emergence, MET mean emergence time, EI emergence index, VI Vigour index.
T1 hydro priming or control, T2 Na-selenite at 50 µmol, T3 Na-selenate at 50 µmol, T4 Na-selenite at 50 µmol + Na-selenate at 50 µmol, T5 ZnO-NPs at 10 µmol, T6 ZnO-NPs at 10 µmol + Na-selenite at 50 µmol, T7 ZnO-NPs at 10 µmol + Na-selenate at 50 µmol, T8 ZnO-NPs 10 µmol + Na-selenite at 50 µmol + Na-selenate at 50 µmol.
Means not sharing a letter in common differ significantly at 5% probability level by HSD Tukey’s test.
A minimum leachate conductivity of seeds was recorded in the combination of all three priming agents (Supplementary Fig. S2). Na-selenate was more effective in increasing antioxidant enzyme activities compared to Na-selenite, and the priming through the combination of Na-selenite, Na-selenate, and ZnO-NPs resulted in improved biochemical activities in DSR seeds (Supplementary Table S2).
Field experiment
Chlorophyll, phenol, and soluble protein content in rice seedling
Total chlorophyll, phenol, and soluble protein contents in rice seedlings increased through priming of seeds over the control (Table 2). The combination of Na-selenite, Na-selenate, and ZnO-NPs recorded the highest total chlorophyll (5.39 mg g−1), phenol (0.58 mg g−1) and soluble protein (0.37 mg g−1) contents, although comparable with ZnO-NPs + Na-selenate treatment (chlorophyll, phenol and soluble protein were 5.27, 0.57 and 0.35 mg g−1).
Table 2.
Effect of seed priming on total chlorophyll, phenolics, and soluble protein contents (mg g-1 fresh weight) in direct-seeded rice seedlings in a field experiment during 2019 (YI) and 2020 (YII).
| Treatment | Total chlorophyll | Total phenolics | Soluble protein |
|---|---|---|---|
| Year | |||
| YI | 4.56n | 0.55g | 0.28k |
| YII | 4.49o | 0.54g | 0.28k |
| Treatment | |||
| T1 | 5.40h | 0.50f | 0.17j |
| T2 | 5.28h | 0.52ef | 0.25i |
| T3 | 4.68i | 0.54def | 0.30gh |
| T4 | 4.48j | 0.56de | 0.31gh |
| T5 | 4.26k | 0.55de | 0.25i |
| T6 | 4.17k | 0.54def | 0.26hi |
| T7 | 4.09l | 0.56de | 0.35fg |
| T8 | 3.82m | 0.58d | 0.36f |
| Year × treatment | |||
| YIT1 | 3.84g | 0.50c | 0.17e |
| YIT2 | 4.19ef | 0.53abc | 0.26bcd |
| YIT3 | 4.46cd | 0.54abc | 0.30abc |
| YIT4 | 4.75b | 0.56abc | 0.30abc |
| YIT5 | 4.19ef | 0.58ab | 0.25cde |
| YIT6 | 4.28de | 0.55abc | 0.26bcd |
| YIT7 | 5.32a | 0.56abc | 0.36a |
| YIT8 | 5.42a | 0.59a | 0.36a |
| YIIT1 | 3.81g | 0.50c | 0.18de |
| YIIT2 | 3.98fg | 0.51bc | 0.24cde |
| YIIT3 | 4.50cd | 0.54abc | 0.31abc |
| YIIT4 | 4.61bc | 0.55abc | 0.31abc |
| YIIT5 | 4.14ef | 0.52abc | 0.25cde |
| YIIT6 | 4.25e | 0.53abc | 0.27bc |
| YIIT7 | 5.24a | 0.56abc | 0.34ab |
| YIIT8 | 5.38a | 0.57abc | 0.36a |
Means followed by different letters (Tukey ranking) differ significantly at 5% level of significance. Treatments details are given in Table 1.
Plant growth analysis
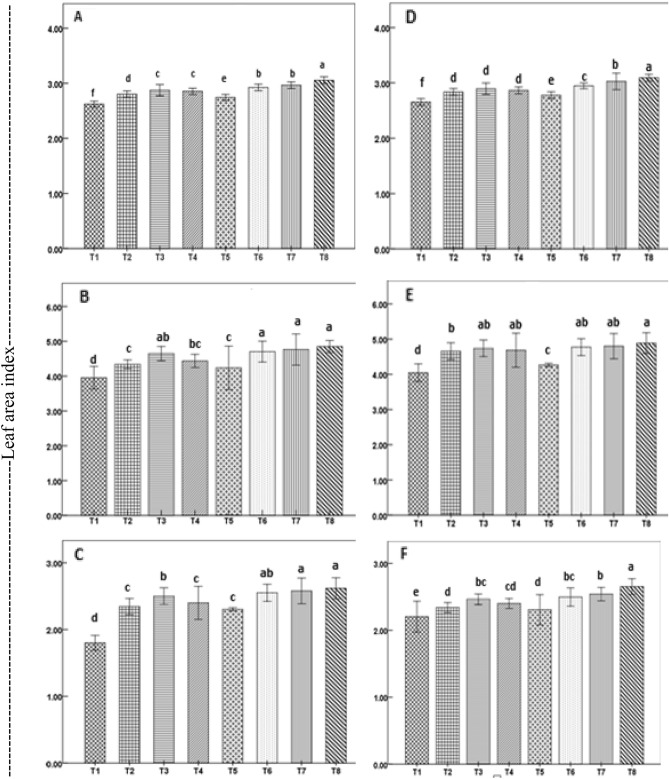
All priming treatments either singly or in combination, affected chlorophyll, phenolics, and soluble protein contents of seedlings, growth behavior, nutrient uptake by plants, and crop yield at harvest (Supplementary Table S3). Year-to-year variation was also noticed in all parameters with a few exceptions (soluble proteins, CGR at the vegetative stage, and NAR at the ripening stage). However, year-treatment interactions were mostly non-significant. The impact of priming of seeds is manifest in plant LAI (Fig. 1). The highest LAI was observed with ZnO-NPs, Na-selenite, and Na-selenate combination in all stages—1.66 (early tillering), 3.50 (panicle initiation), 5.21 (early grain filling), and 3.18 (physiological maturity), with 81%, 93% 56%, and 55% higher, respectively over the control.
Figure 1.
Leaf area index of direct-seeded rice plants during vegetative (A,D), reproductive (B,E), and ripening (C,F) stages as affected by seed priming treatments in a field experiment in 2019 (A–C) and 2020 (D–F). Treatments with different letters (top of bars) represent significant differences (P < 0.05) between treatments. Vertical lines with caps are ± standard error of the mean. Treatments details are given in Table 1.
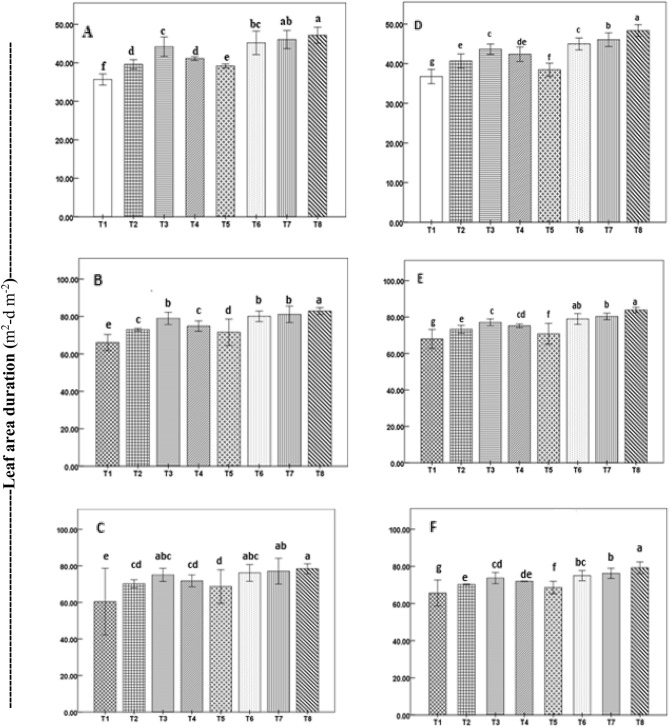
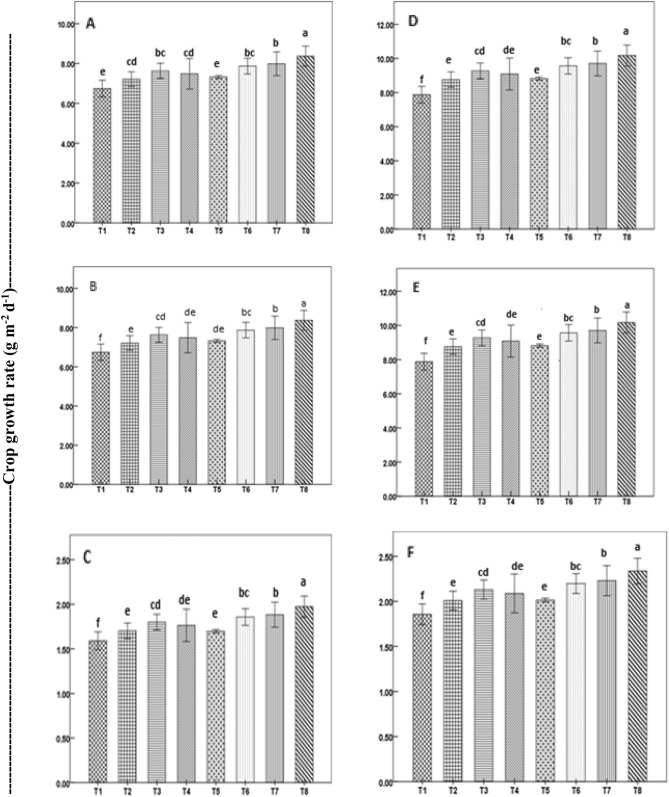
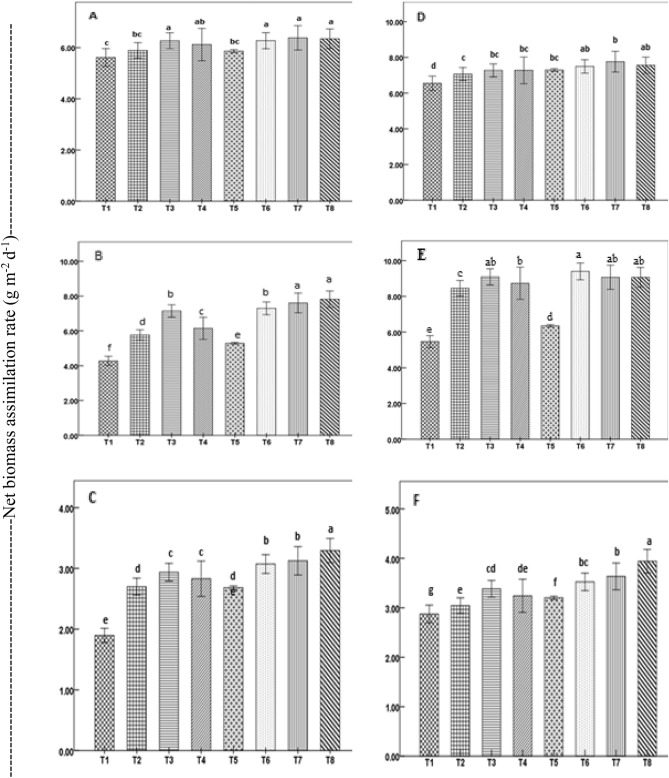
Seed priming impacted other physiological parameters like LAD (Fig. 2), CGR (Fig. 3), and NAR (Fig. 4) during early tillering to panicle initiation stage (14–42 DAS), panicle initiation to completion of pollination (42–70 DAS) and entire grain filling stage (72 DAS-108 DAS).
Figure 2.
Leaf area duration of direct-seeded rice plants during vegetative (A,D), reproductive (B,E), and ripening (C,F) stages as affected by seed priming in a field experiment in 2019 (A–C) and 2020 (D–F). Treatment bars with different letters represent significant differences (P < 0.05) between means. Vertical lines with caps are ± standard error of the mean of treatments. Treatments details are given in Table 1.
Figure 3.
Crop growth rate of direct-seeded rice during vegetative (A,D), reproductive (B,E), and ripening (C,F) stages as affected by seed priming in a field experiment in 2019 (A–C) and 2020 (D–F). Treatment bars with different letters represent significant differences (P < 0.05) between means. Vertical lines with caps are ± standard error of the mean of treatments. Treatments details are given in Table 1.
Figure 4.
Net biomass assimilation rate by direct-seeded rice plants during vegetative (A,D), reproductive (B,E), and ripening (C,F) stages as affected by seed priming in a field experiment in 2019 (A–C) and 2020 (D–F). Treatment bars with different letters represent significant differences (P < 0.05) between means. Vertical lines with caps are ± standard error of the mean of treatments. Treatments details are given in Table 1.
The impact of Na-selenate on LAI and LAD was always greater than that of Na-selenite, and the combined selenite-selenate of sodium salt was even more effective. A similar trend was recorded for CGR except during 42–70 DAS where Na-selenate alone and with Na-selenite had a comparable effect. Seed priming by ZnO-NPs alone or with Na-selenite did not bring a change in either LAI or LAD, although the effect was greater with Na-selenate and was the best when combined with Na-selenite and Na-selenate. Likewise, a combination of ZnO-NPs with Na-selenite and Na-selenate together had the best priming effect on CGR. Overall, improved LAI and LAD with seed priming contributed 9–55% higher CGR and 34–109% higher NAR in comparison to control. The NAR showed a mixed trend, with all treatments behaving similarly at14-42 DAS although all three agents in combination showed less NAR compared to single ZnO-NPs. During 42–70 DAS, ZnO-NPs alone or in combination with both Na-selenite and Na-selenate recorded lower NAR, however, all treatments had a similar effect during 70–108, but higher than the control.
Nutrient uptake by plants
Nutrient uptake (153 kg N ha−1, 48.6 kg P ha−1, 78.7 kg K ha−1, 24.6 kg B ha−1, 321 g Zn ha−1, and 188 kg Si ha−1) was the highest with the combined use of Na-selenite, Na-selenate, and ZnO-NPs (Table 3). The efficacy of Na-selenate was greater in harnessing soil nutrients compared to either Na-selenite or ZnO-NPs.
Table 3.
Effect of seed priming on total plant uptake (kg ha-1) of primary nutrients and micronutrients by direct-seeded rice in a field experimentduring 2019 (YI) and 2020 (YII).
| Treatment | N | P | K | B | Zn | Si |
|---|---|---|---|---|---|---|
| Year | ||||||
| YI | 131o | 38.4k | 68.8l | 21.1m | 276k | 162m |
| YII | 127p | 37.4l | 66.7m | 20.4n | 266l | 157n |
| Treatment | ||||||
| T1 | 109n | 28.9j | 53.8k | 15.8k | 206j | 122l |
| T2 | 119m | 36.4h | 64.2j | 19.9i | 260h | 153j |
| T3 | 128l | 38.4h | 68.5ij | 21i | 275h | 162j |
| T4 | 125lm | 36.5h | 66.9ij | 10i | 261h | 154j |
| T5 | 118m | 31.7i | 67.3ij | 17.4j | 227i | 134k |
| T6 | 137k | 42.5g | 69i | 23.2h | 304g | 178i |
| T7 | 141k | 43.8fg | 73.6h | 24.0g | 313g | 184hi |
| T8 | 153j | 44.9f | 78.7g | 24.6g | 321g | 188h |
| Year × treatment | ||||||
| YIT1 | 121fg | 29.2e | 56.6ef | 16.0f | 208f | 124fg |
| YIT2 | 97i | 36.8c | 51.0f. | 20.2d | 264d | 155d |
| YIT3 | 127efg | 38.9bc | 69.2cd | 21.3cd | 279cd | 120g |
| YIT4 | 129def | 37.3c | 70.0cd | 20.5d | 268d | 165bcd |
| YIT5 | 130def | 32.1de | 78.7a | 17.7ef | 231ef | 158d |
| YIT6 | 132def | 43.1a | 63.4de | 23.6ab | 310ab | 137ef |
| YIT7 | 136cde | 44.5a | 70.1bcd | 24.5ab | 318ab | 180ab |
| YIT8 | 156a | 45.6a | 78.5a | 25.1a | 327a | 187a |
| YIIT1 | 116gh | 28.6e | 64.2d | 15.6f | 204f | 191a |
| YIIT2 | 122fg | 36.0cd | 64.2d | 19.6de | 256de | 151de |
| YIIT3 | 129def | 38.0bc | 67.8cd | 20.7cd | 271cd | 159cd |
| YIIT4 | 121fg | 35.7cd | 63.8d | 19.5de | 254de | 150de |
| YIIT5 | 106hi | 31.3e | 55.9f | 17.1f | 223f | 131fg |
| YIIT6 | 142bcd | 41.9ab | 74.7abc | 22.8bc | 298bc | 176abc |
| YIIT7 | 146abc | 43.2a | 77.1ab | 23.5ab | 308ab | 181ab |
| YIIT8 | 150ab | 44.2a | 78.9a | 24.1ab | 315ab | 185a |
Means followed by different letters (Tukey ranking) differ significantly at 5% level of significance. Treatments detail in Table 1.
Yield attributes and grain and straw yield
Seed priming improved the grain yield in DSR due to an increase in panicle density, grains per panicle, and grain-filling (Table 4). Grain yield ranged from 3.91t ha−1 in the second year with hydropriming treatment to 6.15 t ha−1 in the first year with combined application of ZnO-NPs, Na-selenite, and Na-selenate. Yields were significantly lower in YII than in Y1. Straw yield followed a similar pattern. Application of Na-selenite and Na-selenate with ZnO-NPs registered a 25.9% higher grain yield over the control, compared to 11–13% increase with application of ZnO-NPs with either Na-selenite or Na-selenate. Yield gains in treatment with the combination of all three priming agents could be ascribed to 12.0, 47.1, and 27.8% increase in panicles, filled grains per panicle, and grain filling percentage respectively over the control. Combined application of ZnO-NPS and Na-selenite or ZnO-NPS and Na-selenate also resulted in 7.6–8.7, 32.8–32.9, 22.2–23.6% higher panicles, filled grains per panicle, and grain-filling efficiency respectively over control. However, sole use of Na-selenite, Na-selenate, and ZnO-NPs also contributed 5.5, 6.2, and 5.0% higher grain yield over hydro priming.
Table 4.
Effect of seed priming on yield and yield components of direct-seeded rice in a field experiment during 2019 (YI) and 2020 (YII).
| Treatment | Panicles m−2 | Filled grain panicle−1 | Grain filling percentage | Test weight (g) | Grain yield (t ha−1) | Straw yield (t ha−1) | Harvest index (%) |
|---|---|---|---|---|---|---|---|
| Year | |||||||
| YI | 174j | 114k | 85g | 20.24 | 4.53k | 5.81j | 43.8i |
| YII | 165i | 102j | 86g | 20.22 | 4.22j | 5.57i | 43.1i |
| Treatment | |||||||
| T1 | 184h | 85i | 72f | 20.10 | 4.01i | 5.70g | 41.3h |
| T2 | 188gh | 105h | 84e | 20.40 | 4.23h | 5.68g | 42.6gh |
| T3 | 192g | 110fg | 88e | 20.10 | 4.26h | 5.66g | 42.9fgh |
| T4 | 192g | 110fg | 87e | 20.20 | 4.32h | 5.68g | 43.2fgh |
| T5 | 188gh | 103g | 83e | 20.50 | 4.21h | 5.62gh | 42.8gh |
| T6 | 200ef | 113f | 88e | 20.10 | 4.53g | 5.80fg | 43.9efg |
| T7 | 198f | 113f | 89e | 20.17 | 4.45g | 5.46h | 44.9ef |
| T8 | 206e | 125e | 92d | 20.28 | 5.05f | 5.96f | 45.9e |
| Year × treatment | |||||||
| YIT1 | 189d | 88d | 68c | 20.13 | 4.10e | 5.70e | 40.8d |
| YIT2 | 191c | 112b | 85b | 20.45 | 4.37cd | 5.81c | 42.4bcd |
| YIT3 | 196c | 118b | 90a | 20.11 | 4.41c | 5.75c | 42.4bcd |
| YIT4 | 198bc | 109bc | 85b | 20.17 | 4.49c | 5.91b | 43.2abcd |
| YIT5 | 194c | 115b | 84b | 20.49 | 4.36c | 5.66d | 42.1bcd |
| YIT6 | 205ab | 119b | 91a | 20.04 | 4.70b | 5.92b | 43.5abcd |
| YIT7 | 202ab | 117b | 88b | 20.07 | 4.62b | 5.59b | 44.5abc |
| YIT8 | 209a | 130a | 91a | 20.45 | 5.22a | 6.15a | 45.8a |
| YIIT1 | 179d | 82d | 76c | 20.07 | 3.91e | 5.68c | 41.8cd |
| YIIT2 | 185c | 98c | 84b | 20.35 | 4.08cd | 5.55b | 42.9bcd |
| YIIT3 | 188c | 101bc | 86b | 20.09 | 4.10c | 5.57b | 43.4abcd |
| YIIT4 | 186c | 112b | 88b | 20.23 | 4.14c | 5.44b | 43.2abcd |
| YIIT5 | 182c | 90c | 82b | 20.51 | 4.05d | 5.57b | 43.5abcd |
| YIIT6 | 195b | 106b | 85b | 20.16 | 4.36b | 5.67b | 44.3abc |
| YIIT7 | 194b | 109b | 90a | 20.27 | 4.27b | 5.32b | 45.3ab |
| YIIT8 | 203a | 121a | 93a | 20.11 | 4.87a | 5.77a | 45.9a |
Within a column, means followed by the same letter are not different at the 0.05 level of probability. Treatments detail in Table 1.
Discussion
Seed priming by ZnO-NPs, Na-selenite, and Na-selenate either singly or in combinations, facilitated speedy germination and early vigour of seedlings in the DSR. Priming reduced the time to start emergence as well as the time to reach 50% germination. Primed seeds have shown low MET, a measure of the rate and time-spread of germination84, and enhanced seedling emergence and vigour. There were increases in enzymatic antioxidant functions in seeds necessary to reduce the production of degenerative radicals and therefore, seeds would likely be protected from damage due to environmental stresses during the growing stages. Consequently, seedling length (root and shoot) largely increased at18 DAS (Supplementary Figure S3). All enzymatic activities were positively correlated with seed vigour indices and the seedling growth following seed priming (Supplementary Figure S4). Na-selenate had a clear advantage over Na-selenite when combined with ZnO-NPs. However, the combination of all three priming agents had the maximum impact.
Early vigour of seeds and seedlings is the most desirable trait in field crops to enhance water and nutrient uptake by plants and to provide competitiveness against biotic and abiotic stresses7,85. Due to the chemical priming of seeds, plants used soil nutrients (N, P, K, B, Zn, Si) more efficiently resulting in a higher density of grains per panicle and finally leading to a higher yield in DSR compared to grain imbibition with pure water. Na-selenate improved rice seedling emergence and growth, and soluble carbohydrate and protein contents in plants compared to hydropriming56,86. The ZnO-NPs had similar positive effects on seed germination, seedling growth, and dry biomass in rice87 and wheat88. The internalized ZnO-NPsin primed seeds were several times higher compared to hydropriming which could induce gene expression favouring metabolic activities that enhanced germination performance and seedling vigour87,89. The success with ZnO-NPs may encourage future research on other micronutrients as priming agents87.
Chlorophyll is associated with photosynthetic activity, and hence it is an indicator of vegetative growth and vigour of a plant. Phenolics are secondary metabolites, which promote the adaptation capability of plants during stress. Soluble proteins have diverse roles in promoting growth including osmoregulation in plants under adverse growth environments90. A combination of ZnO-NPs, Na-selenite and Na-selenate priming had the best impact on chlorophyll, phenol and soluble protein contents in rice seedlings, resulting in higher LAI and LAD, and CGR. A combination of Na-selenite and Na-selenate was more effective in promoting the growth of plants (higher LAI, LAD, and CGR) compared to when these were used singly or even when seeds were primed by nano-zinc formulation only. However, the combination of both Na-selenite and Na-selenate with nano-zinc induced the largest changes in growth parameters. These led to enhanced yield-contributing factors such as increased grain-filling efficiency, test weight, and harvest index. The highest DSR yield was, however, obtained with a combination of the three seed priming materials.
Our results established that combinations of Na-selenite, Na-selenate, and ZnO-NPs were potential seed invigoration techniques, and the best results were obtained with all three priming agents in combination. Though responses differed between years, positive impacts on DSR in the field were established. Seeds with high vigour are a proxy of sustainable productivity, especially under adverse conditions, although suitable field studies are lacking. Uniform seedling emergence, and improved crop stand and establishment are major challenges for successful DSR cultivation18. DSR often fails to emerge uniformly due to uneven land preparation leading to imprecise water management in both irrigated and rainfed conditions in both uplands and lowlands, and therefore weed infestations take place heavily91. In particular, seeds fail to germinate due to low oxygen availability in rainfed lowlands owing again to poor water management92. Priming of DSR seeds can give necessary vigour to the plants to sustain better under unfavorable growing conditions. The modified traits in seeds effectively translate into early seedling vigour and agility in growing plants, which is perfectly tuned with increased productivity in the field-grown rice. This small intervention through seed priming will support the rice growers to embrace DSR as a replacement of water- and labour-intensive PTR. Farmers will also get an opportunity to widen the window of sowing of wheat following rice in large tracts of rice–wheat rotation in the Indo-Gangetic Plains of South Asia12.
Conclusion
Both Zn and Se have found their effectiveness in seed-priming. It was observed that ZnO-NPs with both Na-selenite and Na-selenate forms were synergistic in their action, and provided further benefits compared to their single applications. The impact was evaluated in DSR which is often exposed to adverse growing conditions and therefore, not realizing its full potential productivity. Priming of seeds with ZnO-NPs, Na-selenite, and Na-selenate combination resulted in an early seedling emergence due to increased seed vigour, and improved plant growth and productivity in DSR in the field. This will allow sustainable intensification in the vast rice-growing areas in South Asia in general and the rice–wheat system of the Indo-Gangetic Plains of South Asia in particular.
Supplementary Information
Acknowledgements
The corresponding author (Benukar Biswas) acknowledges the fellowship for the first author from a research grant from the Department of Higher Education, Science & Technology and Biotechnology, Government of West Bengal.
Author contributions
S.A.: Data curation; Formal analysis; Investigation, original draft. B.B.: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Supervision; review & editing. D.C.: Review & editing. J.T.: Review & editing. S.P.: Data analysis, Review & editing. J.C.T.: Data analysis, Review & editing. S.B.: Review & editing. A.H.: Editing. S.R.: Editing.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Benukar Biswas, Email: kripahi@yahoo.com.
Debashis Chakraborty, Email: debashisiari@gmail.com.
Jagadish Timsina, Email: timsinaj@hotmail.com.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-11307-4.
References
- 1.Yuan S, et al. Sustainable intensification for a larger global rice bowl. Nat. Commun. 2021;12:2. doi: 10.1038/s41467-020-20340-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yuan S, et al. Southeast Asia must narrow down the yield gap to continue to be a major rice bowl. Nat. Food. 2022;3:217–226. doi: 10.1038/s43016-022-00477-z. [DOI] [PubMed] [Google Scholar]
- 3.Ladha JK, et al. Steady agronomic and genetic interventions are essential for sustaining productivity in intensive rice cropping. Proc. Natl. Acad. Sci. USA. 2021;118:e2110807118. doi: 10.1073/pnas.2110807118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kar I, et al. Productivity trade-off with different water regimes and genotypes of rice under non-puddled conditions in Eastern India. F. Crop. Res. 2018;222:218–229. doi: 10.1016/j.fcr.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chakraborty D, et al. A global analysis of alternative tillage and crop establishment practices for economically and environmentally efficient rice production. Sci. Rep. 2017;7:1–11. doi: 10.1038/s41598-016-0028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang W, et al. The possibility of replacing puddled transplanted flooded rice with dry seeded rice in central China: A review. F. Crop. Res. 2017;214:310–320. doi: 10.1016/j.fcr.2017.09.028. [DOI] [Google Scholar]
- 7.Mahender A, Anandan A, Pradhan SK. Early seedling vigour, an imperative trait for direct-seeded rice: An overview on physio-morphological parameters and molecular markers. Planta. 2015;241:1027–1050. doi: 10.1007/s00425-015-2273-9. [DOI] [PubMed] [Google Scholar]
- 8.Chauhan BS. Weed ecology and weed management strategies for dry-seeded rice in Asia. Weed Technol. 2012;26:1–13. doi: 10.1614/WT-D-11-00105.1. [DOI] [Google Scholar]
- 9.Joshi E, et al. Management of direct seeded rice for enhanced resource—use efficiency. Plant Knowl. J. 2013;2:119–134. [Google Scholar]
- 10.Dhillon BS, et al. Seed priming with potassium nitrate and gibberellic acid enhances the performance of dry direct seeded rice (Oryza sativa L.) in north-western india. Agronomy. 2021;11:2. [Google Scholar]
- 11.Wang W, et al. Effects of different mechanical direct seeding methods on grain yield and lodging resistance of early indica rice in South China. J. Integr. Agric. 2021;20:1204–1215. doi: 10.1016/S2095-3119(20)63191-4. [DOI] [Google Scholar]
- 12.Timsina J, Connor DJ. Productivity and management of rice-wheat cropping systems: Issues and challenges. F. Crop. Res. 2001;69:93–132. doi: 10.1016/S0378-4290(00)00143-X. [DOI] [Google Scholar]
- 13.Chauhan BS, Jabran K, Mahajan G. Rice Production Worldwide. Springer; 2017. [Google Scholar]
- 14.Liu H, et al. Progress and constraints of dry direct-seeded rice in China. J. Food Agric. Environ. 2014;12:465–472. [Google Scholar]
- 15.Kumar, V. & Ladha, J. K. Direct seeding of rice. recent developments and future research needs. Advances in Agronomy vol. 111 (Elsevier Inc., 2011).
- 16.Kamal, N. Direct seeded rice area up in Punjab. The Times of India (2021).
- 17.Qi X, et al. Grain yield and apparent N recovery efficiency of dry direct-seeded rice under different N treatments aimed to reduce soil ammonia volatilization. F. Crop. Res. 2012;134:138–143. doi: 10.1016/j.fcr.2012.05.010. [DOI] [Google Scholar]
- 18.Farooq M, et al. Rice direct seeding: Experiences, challenges and opportunities. Soil Tillage Res. 2011;111:87–98. doi: 10.1016/j.still.2010.10.008. [DOI] [Google Scholar]
- 19.Singh, Y., Singh, G., Johnson, D. & Mortimer, M. Changing from transplanted rice to direct seeding in the rice–wheat cropping system in India. in Rice is Life: Scientific Perspectives for the 21st Century, Tsukuba, Japan: Proceedings of the World Rice Research Conference, 4–7 November 2004, 198–201 (2004).
- 20.Alahakoon AACB, Abeysiriwardena DZ, Gama-Arachchige NS. Low seed moisture and polythene packaging improve storability of seed paddy. J. Stored Prod. Res. 2021;94:101884. doi: 10.1016/j.jspr.2021.101884. [DOI] [Google Scholar]
- 21.Ray S, Vijayan J, Sarkar RK. Germination stage oxygen deficiency (GSOD): An emerging stress in the era of changing trends in climate and rice cultivation practice. Front. Plant Sci. 2016;7:1–4. doi: 10.3389/fpls.2016.00671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaur J, Singh A. Direct seeded rice: Prospects, problems/constraints and Researchable Issues in India. Curr. Agric. Res. J. 2017;5:13–32. doi: 10.12944/CARJ.5.1.03. [DOI] [Google Scholar]
- 23.Rao, A., Wani, S., Ramesha, M. & Ladha, J. Rice production systems. in Rice Production Worldwide (eds. B., C., K., J. & G., M.) 185–205 (Springer, Cham., 2017). doi:10.1007/978-3-319-47516-5.
- 24.Ruelland, E., Vaultier, M. N., Zachowski, A. & Hurry, V. Chapter 2 Cold Signalling and Cold Acclimation in Plants. Advances in Botanical Research vol. 49 (Elesvier Ltd, 2009).
- 25.Guo Z, Ou W, Lu S, Zhong Q. Differential responses of antioxidative system to chilling and drought in four rice cultivars differing in sensitivity. Plant Physiol. Biochem. 2006;44:828–836. doi: 10.1016/j.plaphy.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 26.Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010;48:909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 27.Shu DF, Wang LY, Duan M, Deng YS, Meng QW. Antisense-mediated depletion of tomato chloroplast glutathione reductase enhances susceptibility to chilling stress. Plant Physiol. Biochem. 2011;49:1228–1237. doi: 10.1016/j.plaphy.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 28.Zhang M, Jin ZQ, Zhao J, Zhang G, Wu F. Physiological and biochemical responses to drought stress in cultivated and Tibetan wild barley. Plant Growth Regul. 2015;75:567–574. doi: 10.1007/s10725-014-0022-x. [DOI] [Google Scholar]
- 29.Taylor NL, Day DA, Millar AH. Environmental stress causes oxidative damage to plant mitochondria leading to inhibition of glycine decarboxylase. J. Biol. Chem. 2002;277:42663–42668. doi: 10.1074/jbc.M204761200. [DOI] [PubMed] [Google Scholar]
- 30.Ahmad N, Mukhtar Z. Genetic manipulations in crops: challenges and opportunities. Genomics. 2017;109:494–505. doi: 10.1016/j.ygeno.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Pareek, A., Sopory, S. K., Bohnert, H. J. & Govindjee. Abiotic stress adaptation in plants: physiological, molecular and genomic foundation. Abiotic Stress Adapt. Plants Physiol. Mol. Genomic Found. (2010) doi:10.1007/978-90-481-3112-9.
- 32.Sevanthi, A. M., Prakash, C. & Shanmugavadivel, P. S. Recent progress in rice varietal development for abiotic stress tolerance. Advances in Rice Research for Abiotic Stress Tolerance (Elsevier Inc., 2019). doi:10.1016/B978-0-12-814332-2.00003-4.
- 33.Bałabusta M, Szafrańska K, Posmyk MM. Exogenous melatonin improves antioxidant defense in cucumber seeds (Cucumis sativus L.) germinated under chilling stress. Front. Plant Sci. 2016;7:1–12. doi: 10.3389/fpls.2016.00575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Espanany A, Fallah S, Tadayyon A. Seed priming improves seed germination and reduces oxidative stress in black cumin (Nigella sativa) in presence of cadmium. Ind. Crops Prod. 2016;79:195–204. doi: 10.1016/j.indcrop.2015.11.016. [DOI] [Google Scholar]
- 35.Acharya P, Jayaprakasha GK, Crosby KM, Jifon JL, Patil BS. Nanoparticle-mediated seed priming improves germination, growth, yield, and quality of watermelons (Citrullus lanatus) at multi-locations in Texas. Sci. Rep. 2020;10:1–16. doi: 10.1038/s41598-019-56847-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farooq M, Basra SMA, Wahid A, Khaliq A, Kobayashi N. Rice seed invigoration. In: Lichtfouse E, editor. Sustainable Agriculture Reviews-Book Series. Springer; 2009. [Google Scholar]
- 37.Broadley MR, White PJ, Hammond JP, Zelko I, Lux A. Zinc in plants. New Phytol. 2007;173:677–702. doi: 10.1111/j.1469-8137.2007.01996.x. [DOI] [PubMed] [Google Scholar]
- 38.Li Y, et al. Spatial-temporal analysis of zinc homeostasis reveals the response mechanisms to acute zinc deficiency in Sorghum bicolor. New Phytol. 2013;200:1102–1115. doi: 10.1111/nph.12434. [DOI] [PubMed] [Google Scholar]
- 39.Faizan M, Faraz A, Yusuf M, Khan ST, Hayat S. Zinc oxide nanoparticle-mediated changes in photosynthetic efficiency and antioxidant system of tomato plants. Photosynthetica. 2018;56:678–686. doi: 10.1007/s11099-017-0717-0. [DOI] [Google Scholar]
- 40.Moghaddasi S, et al. Effects of coated and non-coated ZnO nano particles on cucumber seedlings grown in gel chamber. Arch. Agron. Soil Sci. 2017;63:1108–1120. doi: 10.1080/03650340.2016.1256475. [DOI] [Google Scholar]
- 41.Zhang T, et al. Using synchrotron-based approaches to examine the foliar application of ZnSO4 and ZnO nanoparticles for field-grown winter wheat. J. Agric. Food Chem. 2018;66:2572–2579. doi: 10.1021/acs.jafc.7b04153. [DOI] [PubMed] [Google Scholar]
- 42.Wang X, Ding Y, Summers CJ, Wang ZLZ. Large-scale synthesis of six-nanometer-wide ZnO nanobelts xudong. J. Phys. Chem. 2004;108:8773–8777. doi: 10.1021/jp048482e. [DOI] [Google Scholar]
- 43.Sharifi R. Effect of seed priming and foliar application with micronutrients on quality of forage corn (Zea mays) Environ. Exp. Biol. 2016;14:151–156. doi: 10.22364/eeb.14.21. [DOI] [Google Scholar]
- 44.Reddy Pullagurala VL, et al. Finding the conditions for the beneficial use of ZnO nanoparticles towards plants—A review. Environ. Pollut. 2018;241:1175–1181. doi: 10.1016/j.envpol.2018.06.036. [DOI] [PubMed] [Google Scholar]
- 45.Anjum S, et al. Recent advances in zinc oxide nanoparticles (Zno nps) for cancer diagnosis, target drug delivery, and treatment. Cancers. 2021;13:2. doi: 10.3390/cancers13184570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Upadhyaya H, et al. Physiological impact of Zinc nanoparticle on germination of rice (Oryza sativa L) seed. J. Plant Sci. Phytopathol. 2017;1:062–070. doi: 10.29328/journal.jpsp.1001008. [DOI] [Google Scholar]
- 47.Li Y, et al. ZnO nanoparticle-based seed priming modulates early growth and enhances physio-biochemical and metabolic profiles of fragrant rice against cadmium toxicity. J. Nanobiotechnology. 2021;19:1–19. doi: 10.1186/s12951-020-00755-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turakainen M, Hartikainen H, Ekholm P, Seppänen MM. Distribution of selenium in different biochemical fractions and raw darkening degree of potato (Solanum tuberosum L.) tubers supplemented with selenate. J. Agric. Food Chem. 2006;54:8617–8622. doi: 10.1021/jf0613987. [DOI] [PubMed] [Google Scholar]
- 49.Kaklewski K, Nowak J, Ligocki M. Effects of selenium content in green parts of plants on the amount of ATP and ascorbate-glutathione cycle enzyme activity at various growth stages of wheat and oilseed rape. J. Plant Physiol. 2008;165:1011–1022. doi: 10.1016/j.jplph.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 50.Kuznetsov V, Kuznetsov V. Selenium regulates the water status of plants exposed to drought. Dokl. Biol. Sci. 2003;390:266–268. doi: 10.1023/A:1024426104894. [DOI] [PubMed] [Google Scholar]
- 51.Djanaguiraman M, Devi DD, Shanker AK, Sheeba JA, Bangarusamy U. Selenium—An antioxidative protectant in soybean during senescence. Plant Soil. 2005;272:77–86. doi: 10.1007/s11104-004-4039-1. [DOI] [Google Scholar]
- 52.Kong L, Wang M, Bi D. Selenium modulates the activities of antioxidant enzymes, osmotic homeostasis and promotes the growth of sorrel seedlings under salt stress. Plant Growth Regul. 2005;45:155–163. doi: 10.1007/s10725-005-1893-7. [DOI] [Google Scholar]
- 53.Sattar A, et al. Physiological and biochemical attributes of bread wheat (Triticum aestivum L) seedlings are influenced by foliar application of silicon and selenium under water deficit. Acta Physiol. Plant. 2019;41:1–11. doi: 10.1007/s11738-019-2938-2. [DOI] [Google Scholar]
- 54.Sieprawska A, Kornaś A, Filek M. Involvement of selenium in protective mechanisms of plants under environmental stress conditions—Review. Acta Biol. Cracoviensia Ser. Bot. 2015;57:9–20. doi: 10.1515/abcsb-2015-0014. [DOI] [Google Scholar]
- 55.Khaliq A, et al. Seed priming with selenium: Consequences for emergence, seedling growth, and biochemical attributes of rice. Biol. Trace Elem. Res. 2015;166:236–244. doi: 10.1007/s12011-015-0260-4. [DOI] [PubMed] [Google Scholar]
- 56.Du B, et al. Rice seed priming with sodium selenate: Effects on germination, seedling growth, and biochemical attributes. Sci. Rep. 2019;9:1–9. doi: 10.1038/s41598-019-40849-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Versini A, et al. Influence of Se concentrations and species in hydroponic cultures on Se uptake, translocation and assimilation in non-accumulator ryegrass. Plant Physiol. Biochem. 2016;108:372–380. doi: 10.1016/j.plaphy.2016.07.029. [DOI] [PubMed] [Google Scholar]
- 58.Kader M, Lamb DT, Wang L, Megharaj M, Naidu R. Zinc-arsenic interactions in soil: Solubility, toxicity and uptake. Chemosphere. 2017;187:357–367. doi: 10.1016/j.chemosphere.2017.08.093. [DOI] [PubMed] [Google Scholar]
- 59.Xue M, et al. Effects of selenium combined with zinc amendment on zinc fractions and bioavailability in calcareous soil. Ecotoxicol. Environ. Saf. 2020;190:110082. doi: 10.1016/j.ecoenv.2019.110082. [DOI] [PubMed] [Google Scholar]
- 60.Datta SP, SubbaRao A, Ganeshamurthy AN. Effect of electrolytes coupled with variable stirring on soil pH. J. Indian Soc. Soil Sci. 1997;45:185–187. [Google Scholar]
- 61.Walkley A, Black IA. An examination of the degtjarff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. 1934;37:29–38. doi: 10.1097/00010694-193401000-00003. [DOI] [Google Scholar]
- 62.Subbiah BV, Asija GL. A rapid procedure for the determination of available nitrogen in soils. Curr. Sci. 1956;25:259–260. [Google Scholar]
- 63.Olsen SR, Sommers LE. Phosphorus. In: Page AL, editor. Methods of Soil Analysis Part 2 Chemical and Microbiological Properties. ASA-SSSA; 1982. pp. 403–430. [Google Scholar]
- 64.Warncke, D. & Brown, J. R. Potassium and other basic cations Recommended chemical soil test procedures for the North Central Region. vol. 1001 (1998).
- 65.Raliya R, Tarafdar JC. Biosynthesis and characterization of zinc, magnesium and titanium nanoparticles: an eco-friendly approach. Int. Nano Lett. 2014;4:2. doi: 10.1007/s40089-014-0093-8. [DOI] [Google Scholar]
- 66.Sinha SK, Roy A, Satiar S, Bhadra KK. Variety Ajit IET 22066. Indian J. Genet. Plant Breed. 2017;77:583–585. [Google Scholar]
- 67.Ruan S, Xue Q, Tylkowska K. Effects of seed priming on emergence and health of rice (Oryza sativa L.) seeds. Seed Sci. Technol. 2002;30:451–458. [Google Scholar]
- 68.Basra SMA, Farooq M, Tabassum R, Ahmad N. Physiological and biochemical aspects of seed vigor enhancement treatments in fine rice. Seed Sci. Technol. 2005;33:623–628. doi: 10.15258/sst.2005.33.3.09. [DOI] [Google Scholar]
- 69.Ellis RA, Roberts EH. The quantification of ageing and survival in orthodox seeds. Seed Sci. Technol. 1981;9:373–409. [Google Scholar]
- 70.Association of Official Seed Analysts (AOSA) Rules for testing seeds. J. Seed Technol. 1990;12:1–112. [Google Scholar]
- 71.Wang X, Zheng H, Tang Q. Early harvesting improves seed vigour of hybrid rice seeds. Sci. Rep. 2018;8:1–7. doi: 10.1038/s41598-018-29021-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Anonymous. Soil test-based fertilizer recommendations for principal crops and cropping sequences in West Bengal. Department of Agriculture, Government of West Bengal, India. (2008).
- 73.Weraduwage SM, et al. The relationship between leaf area growth and biomass accumulation in Arabidopsis thaliana. Front. Plant Sci. 2015;6:1–21. doi: 10.3389/fpls.2015.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Randhir R, Shetty K. Developmental stimulation of total phenolics and related antioxidant activity in light- and dark-germinated corn by natural elicitors. Process Biochem. 2005;40:1721–1732. doi: 10.1016/j.procbio.2004.06.064. [DOI] [Google Scholar]
- 75.Lichtenthaler HK. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987;148:350–382. doi: 10.1016/0076-6879(87)48036-1. [DOI] [Google Scholar]
- 76.Guy C, Haskell D, Neven L, Klein P, Smelser C. Hydration-state-responsive proteins link cold and drought stress in spinach. Planta. 1992;188:265–270. doi: 10.1007/BF00216823. [DOI] [PubMed] [Google Scholar]
- 77.Jackson ML. Soil Chemical Analysis. Prentice Hall of India Pvt. Ltd.; 1973. [Google Scholar]
- 78.Schindler FV, Woodard HJ, Doolittle JJ. Plant available potassium assessment through chemical prediction methods. Commun. Soil Sci. Plant Anal. 2002;33:1473–1484. doi: 10.1081/CSS-120004295. [DOI] [Google Scholar]
- 79.Bingham FT. Boron. In: Page AL, editor. Methods of Soil Analysis, Part 2 Chemical and Microbiological Properties. ASA-SSSA; 1982. pp. 431–447. [Google Scholar]
- 80.Elliott CL, Snyder GH. Autoclave-induced digestion for the colorimetric determination of silicon in rice straw. J. Agric. Food Chem. 1991;39:1118–1119. doi: 10.1021/jf00006a024. [DOI] [Google Scholar]
- 81.Biswas B, et al. Weed control in transplanted rice with post-emergence herbicides and their effects on subsequent rapeseed in Eastern India. Int. J. Pest Manag. 2020;2:1–13. doi: 10.1080/09670874.2020.1853276. [DOI] [Google Scholar]
- 82.Biswas B, et al. Integrated assessment of cropping systems in the Eastern Indo-Gangetic plain. F. Crop. Res. 2006;99:35–47. doi: 10.1016/j.fcr.2006.03.002. [DOI] [Google Scholar]
- 83.Wang Y, et al. Comparing the performance of approaches for testing the homogeneity of variance assumption in one-factor ANOVA models. Educ. Psychol. Meas. 2017;77:305–329. doi: 10.1177/0013164416645162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bewley JD, Bradford KJ, Hilhorst HWM, Nonogaki H. Seeds: Physiology of Development, Germination and Dormancy. Springer; 2013. [Google Scholar]
- 85.Shi Z, et al. Morphological and physiological factors contributing to early vigor in the elite rice cultivar. Sci. Rep. 2020 doi: 10.1038/s41598-020-71913-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hu F, et al. Seed priming with selenium: Effects on germination, seedling growth, biochemical attributes, and grain yield in rice growing under flooding conditions. Plant Direct. 2022;6:1–10. doi: 10.1002/pld3.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Itroutwar PD, et al. Seaweed-based biogenic ZnO nanoparticles for improving agro-morphological characteristics of rice (Oryza sativa L.) J. Plant Growth Regul. 2020;39:717–728. doi: 10.1007/s00344-019-10012-3. [DOI] [Google Scholar]
- 88.Rai-Kalal P, Jajoo A. Priming with zinc oxide nanoparticles improve germination and photosynthetic performance in wheat. Plant Physiol. Biochem. 2021;160:341–351. doi: 10.1016/j.plaphy.2021.01.032. [DOI] [PubMed] [Google Scholar]
- 89.Sharma D, Afzal S, Singh NK. Nanopriming with phytosynthesized zinc oxide nanoparticles for promoting germination and starch metabolism in rice seeds. J. Biotechnol. 2021;336:64–75. doi: 10.1016/j.jbiotec.2021.06.014. [DOI] [PubMed] [Google Scholar]
- 90.Sadura I, Janeczko A. Physiological and molecular mechanisms of brassinosteroid-induced tolerance to high and low temperature in plants. Biol. Plant. 2018;62:601–616. doi: 10.1007/s10535-018-0805-4. [DOI] [Google Scholar]
- 91.Fukai S, Wade LR. In: Crop Physiology: Case Histories for Major Crops. Sardas V, Calderini D, editors. Academic Press; 2021. pp. 44–97. [Google Scholar]
- 92.Sudhir-Yadav, et al. et al. Growing Rice in Eastern India: new paradigms of risk reduction and improving productivity. In: Mohanty S, et al.et al., editors. The Future Rice Strategy for India. Academic Press; 2017. pp. 221–258. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.