Abstract
Regulatory T cells (Tregs) are fundamentally important for maintaining systemic immune homeostasis and are also required for immune tolerance at the maternal–fetal interface during pregnancy. Recent studies have suggested that epigenetic regulation is critically involved in Treg development and function. However, the role of H3K36me has not yet been investigated. Here, we found that the H3K36me2 methyltransferase Nsd2 was highly expressed in Tregs. Although loss of Nsd2 did not impair systemic Treg development or function, the level of Tregs at the maternal–fetal interface was significantly decreased in pregnant Nsd2 conditional knockout mice. Consequently, maternal–fetal immune tolerance was disrupted in the absence of Nsd2 in Tregs, and the pregnant mice showed severe fetal loss. Mechanistically, Nsd2 was found to upregulate CXCR4 expression via H3K36me2 modification to promote Treg cell recruitment into the decidua and suppress the anti-fetal immune response. Overall, our data identified Nsd2 as a critical epigenetic regulator of Treg recruitment for maternal–fetal tolerance.
Keywords: Regulatory T cell, Cell migration, Immune tolerance
Subject terms: Regulatory T cells, Lymphocyte differentiation
Introduction
As a major suppressive subset of CD4+ T cells, Foxp3+ regulatory T cells (Tregs) play an important role in the maintenance of immune tolerance [1–4]. Accumulating evidence has revealed that epigenetic mechanisms critically regulate the differentiation and function of T-cell subsets, including Tregs. For instance, Ezh2, which is the methyltransferase that catalyzes histone H3K27 trimethylation (H3K27me3), is required for the stabilization of Treg signature genes, especially when cells are in an activated status. Mice with Ezh2-deficient Tregs spontaneously develop autoimmune diseases [5]. Another well-studied epigenetic regulator in Tregs is MLL4, which catalyzes histone H3K4 monomethylation (H3K4me1) at the locus encoding Foxp3 to promote Treg cell differentiation [6]. Recently, H3K36 methylation was found to be an abundant modification in the genome, to be associated with actively transcribed genes and to play important roles in biological processes such as cell differentiation and tumorigenesis [7, 8]. Our previous study showed that the H3K36 dimethyltransferase Nsd2 is critical in the germinal center B-cell response, acting by regulating both B-cell-follicular dendritic cell interactions and follicular helper T-cell differentiation [9, 10]. However, the roles of H3K36 modification in Treg development and function have not yet been defined.
In addition to preventing autoimmunity, Tregs play a role in pregnancy and are suggested to be involved in establishing maternal–fetal immune tolerance and thus preventing immune rejection reactions against fetal alloantigens [11–21]. Upon blastocyst implantation, dramatic morphological and biochemical remodeling occurs in uterine endometrial stromal cells, which differentiate into decidual stromal cells [12]. This process is called decidualization and generates the maternal–fetal interface between the uterine mucosa and the extraembryonic tissues of the conceptus to support the development of the embryo. During this process, multiple maternal immune cells, including natural killer (NK) cells and Tregs, also populate the decidua to support fetal growth and suppress the anti-fetal immune response [13]. Decidual Tregs dramatically increase during pregnancy, and the frequency of Tregs in the decidua is higher than that in peripheral lymphoid organs [12, 19]. Clinical studies have revealed a strong association between a reduced Treg frequency and pregnancy-related diseases, such as recurrent spontaneous pregnancy loss and preeclampsia [22–28]. Studies performed with a mouse model have demonstrated that both thymic natural Tregs and peripheral induced Tregs are required for successful pregnancy [26, 29–31]. Moreover, Treg cells undergo systemic expansion during pregnancy [32]. Importantly, to suppress the anti-fetal immune response, Tregs must be recruited to the local maternal–fetal interface, which is the decidua. A previous study reported that the chemokine receptor CCR5 is involved in the accumulation of Tregs, especially activated Tregs, in the gravid uterus [33]. Adoptive transfer experiments showed that CCR5-deficient Tregs migrated into the uterus less efficiently than their wild-type (WT) counterparts. However, under physiological conditions, CCR5 loss did not result in a marked reduction in Tregs in the uterus, and no fetal loss was observed [33], suggesting that there are other redundant chemokines involved in this process. Subsequent studies indicated that the CCR7-CCL19 and CXCR4-CXCL12 axes might facilitate Treg recruitment [34, 35], but genetic evidence is lacking. Therefore, further investigations are needed to understand the mechanisms underlying Treg recruitment to the maternal–fetal interface.
Here, we found that the H3K36 dimethyltransferase Nsd2 was highly expressed in Tregs. Nsd2 expression was dispensable for Treg development and function. However, we found that during pregnancy, Nsd2 deficiency in Tregs resulted in a low frequency of Tregs at the maternal–fetal interface and fetal loss. This reduction in Tregs observed in the absence of Nsd2 was largely due to CXCR4 downregulation. Our study revealed a critical Nsd2-dependent epigenetic regulatory mechanism for Treg recruitment to the maternal–fetal interface and provided genetic evidence for the role of CXCR4-mediated Treg migration into the decidua during pregnancy.
Results
The H3K36 dimethyltransferase Nsd2 is dispensable for Treg differentiation and function
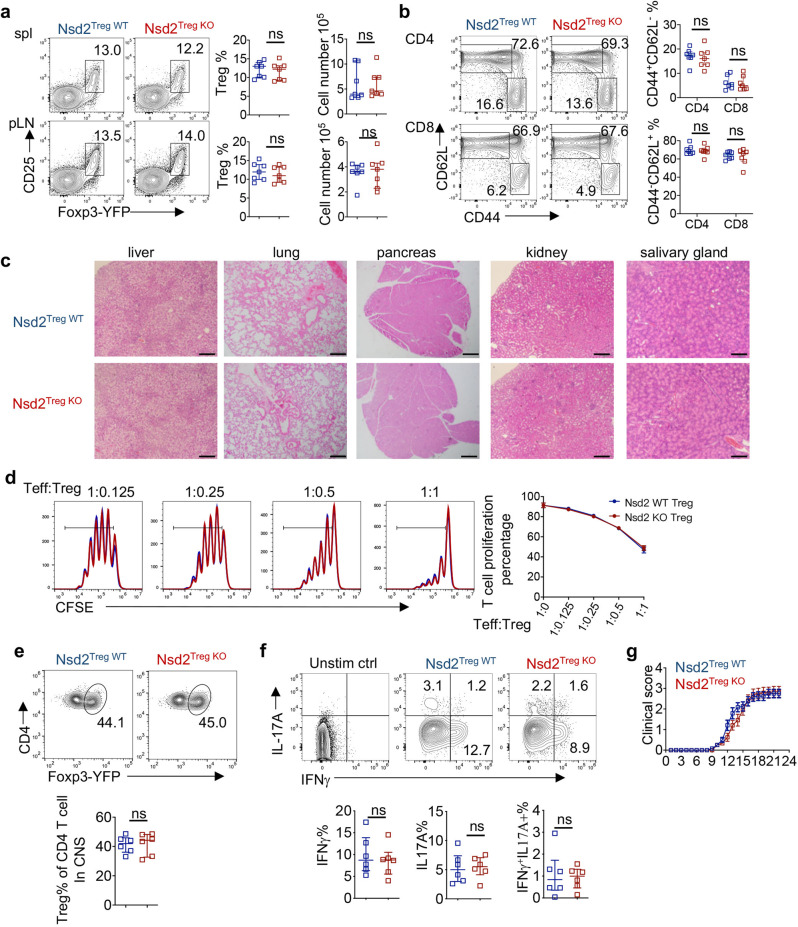
To explore the potential role of H3K36 modification in Tregs, we initially analyzed the expression of all 8 known H3K36-related methyltransferases and found that Nsd2 was the only enzyme that was upregulated in Tregs compared to conventional T cells (Supplementary Fig. 1a, b). Therefore, Treg-specific Nsd2 conditional knockout (KO) mice were generated by crossing mice with loxP-flanked Nsd2 alleles with FoxP3YFP-Cre mice (Nsd2fl/fl FoxP3YFP-Cre mice were designated Nsd2Treg KO mice, and Nsd2+/+ FoxP3YFP-Cre mice were designated Nsd2Treg WT mice). In adult mice, the Treg frequency and cell number in peripheral lymphoid tissues were not altered (Fig. 1a). The T-cell activation status assessed by CD44 and CD62L staining was also normal in the absence of Nsd2 (Fig. 1b). We then aged the mice to 4 months old and found no abnormal infiltration of immune cells into various organs in Nsd2Treg KO mice (Fig. 1c). An in vitro suppression experiment was then performed to assess Treg function. As shown in Fig. 1d, Nsd2 KO Tregs displayed a suppressive capacity similar to that of WT Tregs, suggesting that Nsd2 is dispensable for the suppressive function of Tregs. These data suggested that Treg-expressed Nsd2 is not required for Treg development or function or for the maintenance of systemic immune homeostasis.
Fig. 1.
Nsd2 is dispensable for Treg development and function. a Flow cytometric analysis of the Treg (CD4+CD25+YFP+) percentage and number in the spleen (Spl) and peripheral lymph nodes (pLNs, pooled from inguinal and axillary LNs) of 8-week-old Nsd2Treg WT and Nsd2Treg KO mice. n = 7. b Flow cytometric analysis of the frequencies of naïve (CD44loCD62Lhi) and memory-like (CD44hiCD62Llo) CD4+ and CD8+ T cells in total splenocytes from 8-week-old Nsd2Treg WT and Nsd2Treg KO mice. n = 7. c Representative images of hematoxylin and eosin staining of the indicated tissues from Nsd2Treg WT and Nsd2Treg KO mice at the age of 4 months. Scale bars, 200 µm. d In vitro Treg suppression assay measuring the percentage of divided CD4+ T cells (Teff) by CFSE dilution after coculture for 3 days with the indicated ratio of Teff/Treg cells. Data are representative of two experiments. e Flow cytometric analysis of YFP+ Tregs among CD4+ cells infiltrating the CNS of Nsd2Treg WT and Nsd2Treg KO mice. n = 6. f Flow cytometric analysis of IFNγ+, IL-17A+, and IFNγ+IL-17A+ cells among CD4+ cells infiltrating the CNS of Nsd2Treg WT and Nsd2Treg KO mice. n = 6. g The clinical severity of EAE in Nsd2Treg WT and Nsd2Treg KO mice was monitored for 22 days after immunization with the MOG peptide. n = 12. ns not significant
To further investigate whether Nsd2 is required for Treg function in inflammatory settings, Nsd2Treg KO and control mice were treated to establish experimental autoimmune encephalomyelitis (EAE), an inducible mouse model of multiple sclerosis. Flow cytometric analysis of immune cells from the central nervous system showed that the Nsd2Treg KO mice harbored Tregs at a frequency comparable to that of the control Nsd2Treg WT mice (Fig. 1e). The effector T-cell subsets identified by staining for the cytokines IL-17A and IFNγ were also not altered by Nsd2 loss (Fig. 1f). Accordingly, Nsd2Treg KO mice exhibited disease scores similar to those of Nsd2Treg WT mice (Fig. 1g). Overall, although highly expressed in Tregs, Nsd2 is dispensable for Treg development and immunosuppressive function under both homeostatic conditions and inflammatory conditions.
Nsd2 deficiency results in reduced Tregs at the maternal–fetal interface and in fetal resorption
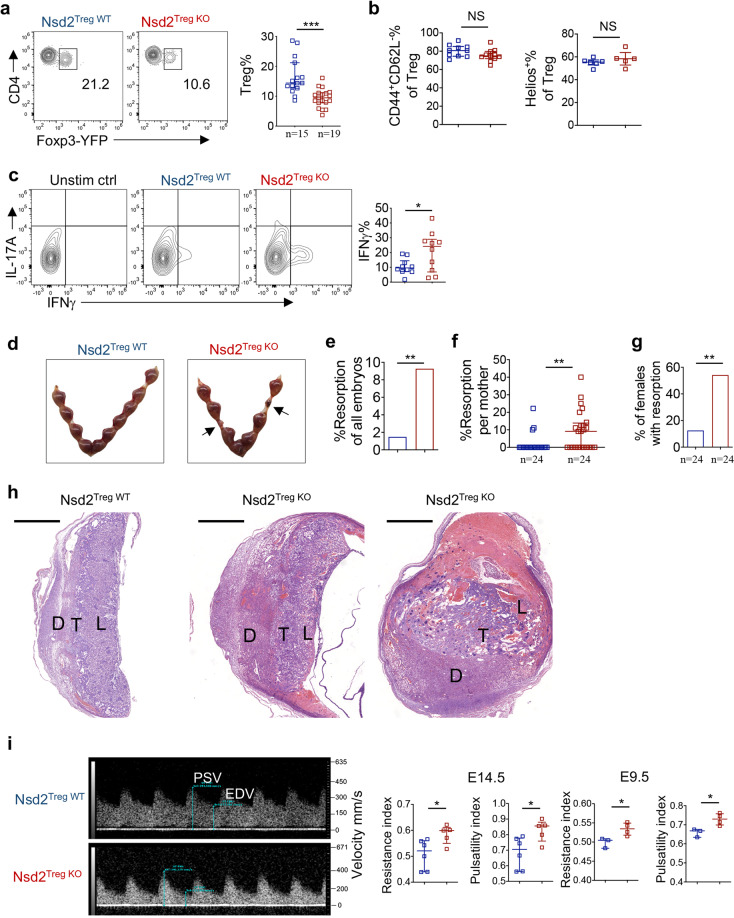
By suppressing the anti-fetal immune response in the decidua, Tregs play a vital role in immune tolerance during pregnancy. We therefore explored whether Treg-expressed Nsd2 is involved in maternal–fetal tolerance. Consistent with previous reports, systemic Tregs (splenic Tregs) significantly expanded during pregnancy (Supplementary Fig. 2a). Nsd2 WT and KO Tregs expanded to a similar level, suggesting that Nsd2 was dispensable for systemic Treg expansion during pregnancy (Supplementary Fig. 2a). However, to our surprise, following mating with male mice, pregnant Nsd2Treg KO mice displayed significantly less accumulation of Tregs in the decidua than pregnant female Nsd2Treg WT mice (Fig. 2a). The Treg frequencies in peripheral LNs, uterus-draining LNs and blood were unaffected by Nsd2 deficiency (Supplementary Fig. 2b). The decidual Treg subset composition was not affected by Nsd2 deficiency, suggesting that all Treg subsets were similarly reduced in the Nsd2Treg KO decidua (Fig. 2b). Accordingly, more IFNγ-expressing T helper 1 (Th1) cells were detected in the Nsd2Treg KO decidua, indicating immune tolerance failure (Fig. 2c). As a consequence, we observed increased fetal resorption in female Nsd2Treg KO mice compared with WT littermate controls (Fig. 2d, e). The resorption rate per pregnant female and the incidence of fetal resorption were also significantly increased (Fig. 2f, g). Notably, although the fetal resorption rate was also significantly increased in Nsd2Treg KO mice, 90% of the fetuses remained alive in the absence of Nsd2, suggesting that the remaining Treg cells and other decidual cells still contributed to immune tolerance. The extent of fetal loss in Nsd2Treg KO mice was similar to that observed in previously reported settings in which peripheral Tregs were selectively ablated [30]. Histological examination of placental tissues clearly revealed a more congested and widened spongiotrophoblast, necrotic labyrinth, hemorrhage and necrosis or thrombosis in decidual vessels in female Nsd2Treg KO mice (Fig. 2h). Immunohistochemical staining revealed dramatically increased neutrophil infiltration in the Nsd2TregKO decidua (Supplementary Fig. 3). A previous study suggested that fetal abortion and local inflammation affect the hemodynamics of the uterine artery [36]. Therefore, we performed ultrasound biomicroscopy analysis of pregnant mice and found that both the uterine artery resistance index and the pulsatility index were significantly increased in Nsd2Treg KO mice (Fig. 2i). Overall, Nsd2 deficiency selectively reduced the Treg frequency in the decidua and resulted in fetal resorption without impacting systemic Treg development or function.
Fig. 2.
Nsd2 deficiency results in reduced Tregs at the maternal–fetal interface and in fetal resorption. a Representative flow cytometric analysis of YFP+ Treg cells in the decidua of Nsd2Treg WT and Nsd2Treg KO mice previously mated with male BALB/c mice on Days E13.5–E14.5. b Percentage of CD44+CD62Llow and Helios+ Tregs determined by flow cytometry in the decidua of Nsd2Treg WT and Nsd2Treg KO mice previously mated with male BALB/c mice on Days E13.5–E14.5. c Flow cytometric analysis of IFNγ and IL-17A expression in decidual CD4+ T cells. n = 10. d Representative image of resorption of allogeneic embryos in the uterus of Nsd2Treg WT and Nsd2Treg KO mice on Day E14.5. Arrows indicate resorptions. e Percentage of resorbed embryos in all Nsd2Treg WT and Nsd2Treg KO pregnancies resulting from matings with male BALB/c mice. Two-sided Fisher’s exact test was used to assess the significance. f Percentage of resorption observed in individual mothers with the indicated genotype. g Incidence of pregnancies with at least one resorption. Two-sided Fisher’s exact test was used to assess the significance. h Histopathological evaluation of placentas from female Nsd2Treg WT (left) and Nsd2Treg KO (right, two examples) mice mated with male BALB/c mice; low-magnification survey of representative H&E-stained sections of placenta. D decidua, T trophoblast, L labyrinths. Scale bars, 1000 mm. i Representative Doppler image of the uterine artery (left, E14.5), which was used to determine the uterine artery resistance and pulsatility indices (right) of Nsd2Treg WT and Nsd2Treg KO female mice on Days E14.5 and E9.5. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001
Nsd2 promotes Treg recruitment into the decidua by regulating CXCR4 expression
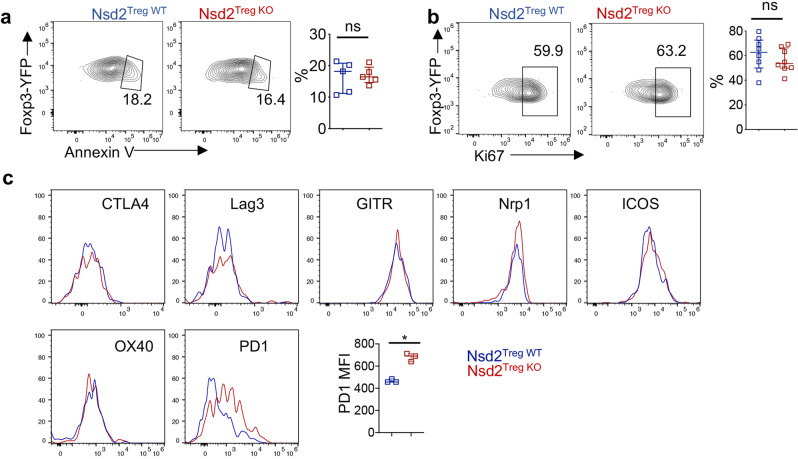
We then investigated the mechanism underlying the reduction in the decidua upon Nsd2 deletion. Given that systemic Treg development and function were not affected, we asked whether local Treg maintenance in the decidua was impaired by Nsd2 deficiency. Therefore, Treg expansion and apoptosis were examined by flow cytometry. Surprisingly, neither the expansion nor apoptosis of Tregs in the decidua was affected by Nsd2 deficiency (Fig. 3a, b). We also assessed the expression of Treg function-associated cell-surface markers and found that most of these markers were expressed at normal levels on decidual Tregs; however, PD-1 displayed a slight increase in the absence of Nsd2 (Fig. 3c). Thus, these data suggest that the local fitness of Tregs in the decidua was not affected by Nsd2 loss.
Fig. 3.
Nsd2 deficiency did not affect the apoptosis or proliferation of decidual Tregs. a Cell-surface staining for Annexin V on decidual Tregs isolated from Nsd2Treg WT and Nsd2Treg KO mice. n = 5. b Flow cytometric analysis of Ki67 expression in decidual Tregs from Nsd2Treg WT and Nsd2Treg KO mice. n = 8. c Histogram overlay of CTLA-4, LAG-3, GITR, Nrp1, ICOS, OX40 and PD-1 expression and plots of the PD-1 (n = 3) mean fluorescence intensity (MFI) of decidual Treg cells from Nsd2Treg WT and Nsd2Treg KO mice. ***P ≤ 0.001; ns not significant
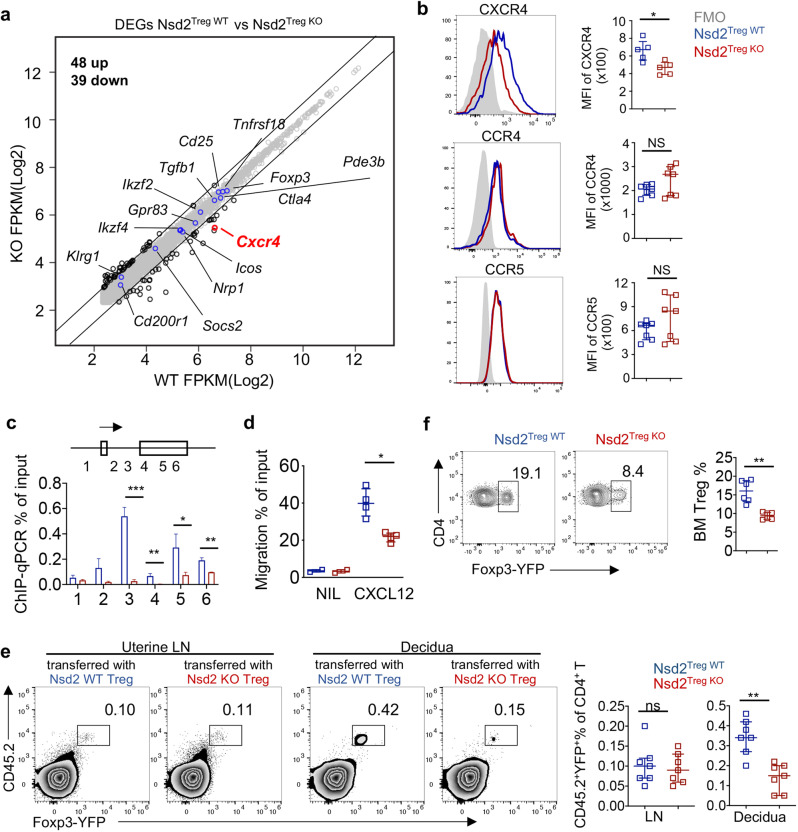
To further explore how Nsd2 deficiency reduced Treg abundance specifically in the decidua, we performed mRNA sequencing (RNA-seq) analysis of Nsd2-deficient and WT Treg cells. Consistent with the fact that overall Treg development and function were normal, only a limited number of differentially expressed genes were detected between Nsd2 KO and WT Treg cells, specifically, 48 upregulated genes and 39 downregulated genes. (Fig. 4a). Most of the functionally relevant Treg genes exhibited normal levels of transcription. Notably, the mRNA level of Cxcr4, the gene encoding the chemokine receptor CXCR4, was significantly decreased in Nsd2 KO Tregs (Fig. 4a). Flow cytometry staining verified the reduced expression of CXCR4 at the protein level (Fig. 4b). Nsd2 loss did not affect the expression levels of CCR4 and CCR5, which have been previously reported to be involved in Treg recruitment into the decidua (Fig. 4b). Nsd2 catalyzes the dimethylation of histone H3 at lysine 36, which is a permissive mark and thus positively influences gene transcription outcomes [7, 8]. Therefore, we performed chromatin immunoprecipitation qPCR (ChIP-qPCR) to analyze H3K36me2 modification of the Cxcr4 locus. We observed significantly reduced H3K36me2 levels in Nsd2-deficient Tregs, suggesting that Nsd2 might directly regulate CXCR4 expression by catalyzing H3K36me2 (Fig. 4c). To assess whether the reduction in CXCR4 in Nsd2 Tregs had functional consequences, an in vitro Transwell migration assay was performed, and we found that Nsd2-deficient Tregs migrated toward CXCL12, the ligand for CXCR4, less efficiently than their WT counterparts (Fig. 4d). A previous study showed that the CXCL12-CXCR4 axis is critical for NK-cell recruitment into the decidua and that trophoblast cells express high levels of CXCL12 [37–39]. It has also been reported that the CXCL12-CXCR4 axis may regulate Treg accumulation at the maternal–fetal interface, although genetic evidence is still lacking [34]. Therefore, ex vivo adoptive transfer experiments were performed to directly examine whether Nsd2 deficiency impairs Treg recruitment into the decidua. We transferred Nsd2 KO or WT Treg cells into congenically distinct pregnant recipient mice and analyzed the distributions of these donor-derived Tregs in lymphoid tissues and the decidua. As shown in Fig. 4e, Nsd2 KO and WT Tregs were distributed at similar frequencies in the uterus-draining lymphoid nodes. In contrast, there was a dramatic reduction in the number of Nsd2 KO Tregs in the decidua, suggesting a severe defect in Nsd2 KO Treg recruitment to the maternal–fetal interface (Fig. 4e). Collectively, these data demonstrated that Nsd2 is required for Treg migration into the decidua to control maternal–fetal immune tolerance during pregnancy, potentially by regulating CXCR4 expression.
Fig. 4.
Nsd2 promotes Treg recruitment into the decidua by regulating CXCR4 expression. a RNA-seq analysis of CD25+ YFP+ Treg cells from Nsd2Treg WT and Nsd2Treg KO mice. The examples of functionally relevant Treg genes without significant differential expression are labeled with blue dots. b Flow cytometric analysis of CXCR4, CCR4, and CCR5 expression in splenic Tregs. n = 5–7. c ChIP-qPCR for H3K36me2 modifications on the CXCR4 locus. Primers specific for the six indicated regions were used for the analysis. d Transwell migration assay performed with Tregs from Nsd2Treg WT and Nsd2Treg KO mice of the indicated genotype in response to medium alone (nil) and 1 µg/ml SDF, shown as the percentage of input cells. e A total of 2 × 106 CD25+YFP+ Tregs from Nsd2Treg WT or Nsd2Treg KO mice were adoptively transferred into pregnant CD45.1+ BoyJ mice on Day E12.5. The cell percentages of transferred Tregs in the uterine-draining lymph nodes and decidua were examined by flow cytometry 48 h after injection. n = 7. f Flow cytometric analysis of the Treg (CD4+YFP+) percentage in the bone marrow (BM) of 8-week-old Nsd2Treg WT and Nsd2Treg KO mice. n = 7. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ns not significant
It has been reported that CXCR4 is critical for Treg migration into the bone marrow [40]. To corroborate our finding that Nsd2 regulates CXCR4 expression, we also analyzed Tregs in the bone marrow and found that the frequency of Tregs was significantly decreased in the bone marrow of Nsd2Treg KO mice compared to that of WT mice, which further supported our finding (Fig. 4f).
Treg-specific CXCR4 conditional KO mice displayed decidual Treg reduction and fetal abortion phenotypes similar to those of Nsd2Treg KO mice
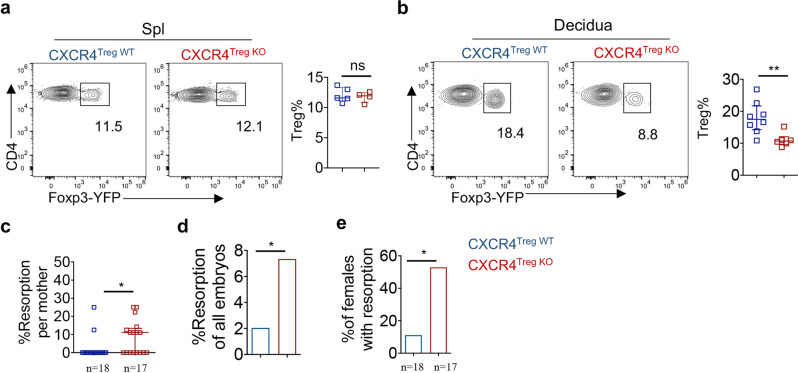
Although a previous study indicated that CXCR4 is potentially important for Treg migration into the decidua, supportive genetic evidence is lacking [34]. Therefore, we constructed Treg-specific CXCR4 conditional KO mice by crossing Cxcr4fl/fl mice with Foxp3-Cre-YFP mice (designated CXCR4Treg KO mice). Following mating with male BALB/c mice, the Treg frequency was significantly reduced in the decidua of pregnant female CXCR4Treg KO mice compared to that of pregnant female WT mice, whereas the splenic Treg frequency was not altered at all (Fig. 5a, b). Correspondingly, pregnant CXCR4Treg KO mice displayed increased fetal loss compared with that of pregnant WT control mice (Fig. 5c–e). Therefore, CXCR4Treg KO mice phenocopied Nsd2Treg KO mice in terms of decidual Treg frequency reduction and fetal loss, further suggesting that Nsd2 regulates CXCR4 expression to promote Treg recruitment to the maternal–fetal interface.
Fig. 5.
Treg-specific CXCR4 conditional KO mice displayed decidual Treg reduction and fetal abortion phenotypes similar to those of Nsd2Treg KO mice. a Flow cytometric analysis of the Treg (CD4+YFP+) percentage and number in the spleen (Spl) of 8-week-old CXCR4Treg WT (n = 5) and CXCR4Treg KO mice (n = 4). b Representative flow cytometric analysis of YFP+ Treg cells in the decidua of CXCR4Treg WT and CXCR4Treg KO mice previously mated with BALB/c males on Days E13.5–E14.5. n = 6–8. c Percentage of resorption observed in individual mothers with the indicated genotype. d Percentage of resorbed embryos in all CXCR4Treg WT and CXCR4Treg KO pregnancies resulting from mating with male BALB/c mice. Two-sided Fisher’s exact test was used to assess the significance. e Incidence of pregnancies with at least one resorption. Two-sided Fisher’s exact test was used to assess the significance. *P ≤ 0.05; **P ≤ 0.01; ns not significant
Nsd2 promotes Treg migration into the decidua primarily by regulating CXCR4 expression
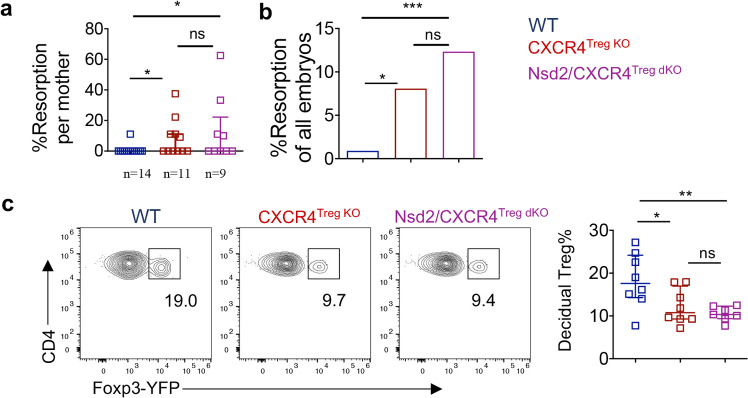
The above data suggest that Nsd2 can enhance Treg migration into the decidua by regulating CXCR4 expression. However, whether CXCR4 regulation is the predominant function of Nsd2 in Tregs remains to be further investigated. To address this question, we crossed Nsd2 conditional KO mice (Nsd2fl/flFoxP3YFP-Cre) with Cxcr4fl/fl mice to obtain Nsd2/CXCR4 double KO mice (designated Nsd2/CXCR4Treg dko mice). We speculated that if CXCR4 regulation is the primary function of Nsd2 in Tregs, Nsd2 deficiency would not cause further fetal loss in the setting of CXCR4 loss. Indeed, following mating with male BALB/c mice, Nsd2/CXCR4 double KO mice did not display more severe fetal loss than Cxcr4 single-gene KO mice but rather a similar fetal absorption phenotype (Fig. 6a, b). This was also the case for the reduced Treg level phenotype in the decidua—Nsd2/CXCR4 double deficiency resulted in a similar reduction in Tregs in the decidua compared to CXCR4 deficiency alone (Fig. 6c). Therefore, these data suggest that Nsd2 promotes Treg migration into the decidua primarily by regulating CXCR4 expression, although we cannot completely exclude the involvement of other minor mechanisms.
Fig. 6.
Nsd2 promotes Treg migration into the decidua primarily by regulating CXCR4 expression. a Percentage of resorption observed in individual WT, CXCR4Treg KO, and Nsd2/CXCR4Treg dKO pregnancies resulting from mating with male BALB/c mice. b Percentage of resorbed embryos in all WT, CXCR4Treg KO, and Nsd2/CXCR4Treg dKO pregnancies resulting from mating with male BALB/c mice. Two-sided Fisher’s exact test was used to assess the significance. c Representative flow cytometric analysis of YFP+ Tregs in the decidua and analysis of the decidua of female WT, CXCR4Treg KO and Nsd2/CXCR4Treg dKO mice mated with male BALB/c mice and analyzed on Days E13.5–E14.5. n = 8. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ns not significant
Discussion
The epigenetic regulation of Treg development and function remains incompletely understood. Here, we investigated the role of Nsd2-mediated H3K36me2 modification in Tregs. Although upregulated in Tregs compared to conventional T cells, Nsd2 was not required for Treg development or function in lymphoid organs and was thus dispensable for systemic immune homeostasis. However, following mating with allogeneic male mice, Nsd2-deficient Tregs failed to be efficiently recruited to the maternal–fetal interface in pregnant mice, and thus, local immune tolerance was disrupted. Mechanistically, we found that Nsd2 promoted the expression of the chemokine receptor CXCR4 to facilitate Treg migration into the decidua, where the CXCR4 ligand CXCL12 is abundantly expressed, as reported. Using conditional KO mice, we provided genetic evidence for the role of CXCR4 in Treg recruitment to the maternal–fetal interface and its epigenetic regulatory mechanism involving the H3K36me2 methyltransferase Nsd2. Clinically, the CXCR4 expression level is negatively associated with miscarriage, and women with spontaneous miscarriage have lower CXCR4 expression at the maternal–fetal interface [41]. Our data are consistent with this observation and provide mechanistic insight. It will be important to assess whether the CXCR4 reduction in spontaneous miscarriage patients leads to a local Treg decrease.
Because of the fundamental importance of immune suppression, Tregs have been proposed to be used in immunotherapies for immune-related diseases. For example, a number of clinical trials evaluating Treg adoptive transfer in kidney and liver transplantation have been initiated, with the ultimate goal of achieving a drug-free tolerance status [42, 43]. Treg therapy was also reported to be effective in clinical studies of type 1 diabetes mellitus [44]. In terms of pregnancy-related diseases, such as recurrent spontaneous pregnancy loss, although no clinical studies have been performed yet, much work has been done with mouse pregnancy models. Adoptive transfer of Tregs efficiently prevented fetal rejection in an abortion-prone mouse model [34, 45, 46]. Even for pathogen infection-induced pregnancy disorders, Treg transfer exhibited a strong beneficial effect [47]. These mouse studies provide a strong rationale for pursuing clinical applications in pregnancy diseases in the future. However, as a cell-based therapy, the clinical translation of Treg adoptive transfer is complicated and challenging. For example, to suppress the anti-fetal immune response, infused Tregs need to be efficiently recruited locally to the maternal–fetal interface. Our finding that the H3K36me2 methyltransferase Nsd2 regulates Treg migration provides a potential strategy to facilitate Treg accumulation at the maternal–fetal interface. As an epigenetic enzyme, Nsd2 can easily be therapeutically targeted with small molecules. It is therefore feasible to design and obtain Nsd2-specific agonizts and use them in combination with Treg adoptive transfer therapy for pregnancy-associated disease therapy.
Materials and methods
Mice
Nsd2fl/fl mice were previously described [10]. Cxcr4fl/fl mice, Foxp3cre-YFP transgenic mice and B6-CD45.1 (Ptprca Pepcb/BoyJ) mice were obtained from the Jackson Laboratory. BALB/c male mice were obtained from the Animal Core Facility of Nanjing Medical University. Mice were maintained in a specific pathogen-free environment, and animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee of Nanjing Medical University.
Timed mating
Two female mice were set up in the afternoon with individual BALB/c males. Females were checked daily for the presence of a vaginal plug in the morning, and plugged females were separated from males; the day of plug detection was considered Day E0.5. Plugged females were analyzed for resorbed fetuses and flow cytometry at E14.5.
Cell preparations
Thymocyte, splenocyte, and LN cell suspensions were prepared by mashing the organs through 70-µm cell strainers. Decidual cells were isolated as described previously, with modifications [48, 49]. In brief, fresh decidual samples were washed extensively in PBS with 100 IU/mL penicillin/streptomycin and then minced into small pieces. Decidual lymphocytes were digested at 37 °C in 0.5 mg/mL collagenase type IV (Worthington Biochemical Corporation) and 0.1 mg/mL DNase I (Sigma-Aldrich) in RPMI 1640 medium for 40 min. The suspensions were strained through nylon mesh and separated on a 40–80% Percoll density gradient (GE Healthcare) after centrifugation at 1200 × g for 25 min at room temperature.
Flow cytometry and cell sorting
For surface staining, cells were incubated with the indicated antibodies on ice for 30 min and washed with staining buffer (2% FBS in PBS with 0.1% NaN3 and 50 mM EDTA). The following antibodies were used for flow cytometry: anti-CD4 (GK1.5), anti-IL-17A (TC11-18H10), anti-CD44 (IM7), anti-CD45.2 (104), anti-CD8a (53-6.7), anti-CTLA4 (UC10-4B9), anti-CXCR4 (L276F12), anti-CCR4 (2G12), anti-CCR5 (4B12), anti-CCR7 (HM-CCR5), anti-Foxp3 (FJK-16s), anti-GITR (DTA-1), anti-ICOS (7E.17G9), anti-IFN-γ (XMG1.2), anti-Ki67 (16A8), anti-LAG-3 (C9B7W), anti-OX-40 (OX-86), anti-PD-1 (RMP1-30), anti-CD25 (PC61.5), anti-CD62L (MEL-14), and anti-Neuropilin-1 (3DS304M). For cytokine-producing T-cell analysis, cells were stimulated for 4 h with phorbol myristate acetate (PMA; 50 ng/ml; Sigma-Aldrich) and ionomycin (500 ng/ml; Sigma-Aldrich) in the presence of GolgiPlug (BD Biosciences). Cells were stained for surface markers and then fixed and permeabilized with Cytofix/Cytoperm solution (BD Biosciences) before intracellular staining. Data were acquired on a Beckman Cytoflex and analyzed with FlowJo 10 software. Cell sorting was performed with a BD Aria Fusion. The sorting efficiency was tested, and only cells with high purity (>98%) were used for the experiments.
Migration assays
Migration assays were performed as previously described [50]. Briefly, dLN lymphocytes were incubated in RPMI 1640 with 0.5% fatty acid-free BSA (Sigma-Aldrich), 1% penicillin/streptomycin, and 10 mM HEPES for 1 h at 37 °C to promote the resensitization of chemokine receptors. Then, the cells were plated onto transwell inserts placed in 24-well tissue culture plates (Corning) containing the indicated concentrations of stromal cell–derived factor 1 (SDF/CXCL12) in migration medium. Controls without SDF (null) or without the Transwell insert (input) were also studied. Plates were then incubated at 37 °C in 5% CO2 for 3 h, followed by enumeration of migrated cells by flow cytometry.
Western blot analysis
For western blot analysis, 1 × 106 sorted CD4+ naïve T cells or Treg cells were lysed in 100 µl of SDS loading buffer. Nonspecific binding to membranes was blocked by 5% milk in PBST (PBS with 0.05% Tween 20) for 1 h at room temperature, and then membranes were incubated with primary antibodies diluted in 5% BSA in PBS overnight at 4 °C with constant rotation. Then, the membranes were incubated with secondary antibodies diluted in PBST for 1 h at room temperature. Chemiluminescence was detected using Immobilon Western Chemiluminescent HRP Substrate (Millipore).
In vitro suppression assay
A total of 5 × 104 CFSE-labeled naïve CD4+ T cells were cocultured with different numbers of CD4+ CD25+YFP+ Treg cells in the presence of 1 × 105 mitomycin-treated splenocytes and 2 μg/ml anti-CD3 in round-bottom 96-well plates for 3 days. The proliferation of labeled naïve T cells was determined by flow cytometry.
Adoptive Treg cell transfer
CD4+CD25+YFP+ Treg cells were purified using a BD Aria Fusion cell sorter. Sorted Treg cells were activated for 24 h with anti-CD3 (5 µg/ml; 145-2C11; Bio-X-Cell) plus anti-CD28 (5 µg/ml; 37.51; Bio-X-Cell) at a concentration of 106 cells/ml in RPMI supplemented with 10% FBS, nonessential amino acids, sodium pyruvate, L-glutamine, HEPES, and β-mercaptoethanol. A total of 1 × 106 activated cells were injected i.v. into pregnant BoyJ mice at E12.5. Forty-eight hours later, the dams were euthanized, and the decidua and LNs were collected. Leukocyte isolation and immunophenotyping of Tregs were performed to determine the proportion of adoptively transferred Tregs present in each tissue.
EAE model
The EAE mouse model was established as previously described. Eight-week-old mice were immunized subcutaneously with 200 ml of emulsified CFA and 200 mg of MOG35–55 peptide (MEVGWYRSPFSRVVHLYRNGK) and received intravenous injections of 200 ng of pertussis toxin at the time of immunization, which was repeated 48 h later. Mice were assigned scores daily on a scale of 0–5 in a double-blinded manner with the following criteria: 0, no disease; 1, tail paralysis; 2, wobbly gait; 3, hindlimb paralysis; 4, forelimb paralysis; and 5, moribund or dead. Gradations of 0.5 were assigned to mice exhibiting signs that fell between two of the scores listed above.
RNA-seq
Total RNA from 106 splenic regulatory T cells was sorted for RNA extraction and library construction at RiboBio Co., Ltd. on an Illumina HiSeq 2500 platform and a 50-bp single-end module. Each sample combined a mixture of RNA from 3 mice of each genotype. Sequence reads were mapped to the mm10 reference genome using HISAT2 with default settings. Differential gene expression analysis was performed using the Bioconductor DESeq2 package with a threshold of RPKM of 5 and fold change of 1.5. Genes with reads per kilobase of transcript per million mapped reads were included for analysis. Raw data files and processed files were deposited in the Gene Expression Omnibus public database (GSE179489).
ChIP-qPCR assay
Briefly, sorted CD4+CD25+YFP+ Treg cells were cross-linked in 1% formaldehyde for 5 min at room temperature, quenched with 0.125 M glycine for 5 min at room temperature and lysed in 0.2% SDS with a protease inhibitor tablet for 10 min on ice. Genomic DNA was then fragmented by sonicating. For immunoprecipitation, anti-dimethyl histone H3 (07-274; Millipore) was used. DNA purification was performed using the ChIP DNA Clean & Concentrator (ZYMO Research) according to the manufacturer’s instructions.
The sequences of the CXCR4 chip-qPCR primer sets were as follows:
Forward 15′-CATCTTAGTGTTCTGCGTG-3′
Reverse 15′-ATTTCATAAGCACATAGGG-3′
Forward 25′-TAGGAGTGCGGTTCTGA-3′
Reverse 25′-GCTGCTCCATCCCAAA-3′
Forward 35′-TCGCTTAGGGAGGGTT-3′
Reverse 35′-CGACCAACACACAGCG-3′
Forward 45′-CCATCTACTTCATCATCT-3′
Reverse 45′-AAAGAGGAGGTCAGCCAC-3′
Forward 55′-GGCAGTCTATGTGGGCGT-3′
Reverse 55′-AACACCACCATCCACA-3′
Forward 65′-TAATGGTGGGTCTCGTC-3′
Reverse 65′-CAGCAGGCAAAGAAAGC-3′
Statistical analyses
Unless otherwise noted, statistical analysis was performed using the Mann–Whitney U test for individual biological replicates in Prism 9 (GraphPad). Fisher’s exact test was used to assess the significance of the ratios of healthy and resorbed fetuses. The data in the figures are displayed as the median with interquartile range (IQR). P values are denoted in figures by *P < 0.05; **P < 0.01; ***P < 0.001.
Supplementary information
Acknowledgements
We thank Dr. Haiming Wei and Dr. Bingqing Fu (University of Science and Technology of China) for technical help with the decidual cell preparation and invaluable discussion.
Author contributions
LZ, YC, and XW conceptualized the project, designed the experimental approaches, analyzed the data, and prepared the paper. LZ, XL, YY, YW, and JW performed most of the experiments.
Funding
This study was supported by the National Key R&D Program of China (2018YFC1003900), the National Natural Science Foundation of China (Grant Number 82001653 to LZ and 31970828 to XW) and Jiangsu Outstanding Young Investigator Program (BK20200030).
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Le Zhang, Xuehui Long.
Contributor Information
Yun Chen, Email: chenyun@njmu.edu.cn.
Xiaoming Wang, Email: xmwang@njmu.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41423-022-00849-2.
References
- 1.Josefowicz SZ, Lu L-F, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–64. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakaguchi S, Mikami N, Wing JB, Tanaka A, Ichiyama K, Ohkura N. Regulatory T cells and human disease. Annu Rev Immunol. 2020;38:541–66. doi: 10.1146/annurev-immunol-042718-041717. [DOI] [PubMed] [Google Scholar]
- 3.Savage PA, Klawon DEJ, Miller CH. Regulatory T cell development. Annu Rev Immunol. 2020;38:421–53. doi: 10.1146/annurev-immunol-100219-020937. [DOI] [PubMed] [Google Scholar]
- 4.Feuerer M, Hill JA, Mathis D, Benoist C. Foxp3+ regulatory T cells: differentiation, specification, subphenotypes. Nat Immunol. 2009;10:689–95. doi: 10.1038/ni.1760. [DOI] [PubMed] [Google Scholar]
- 5.DuPage M, Chopra G, Quiros J, Rosenthal WL, Morar MM, Holohan D, et al. The chromatin-modifying enzyme Ezh2 is critical for the maintenance of regulatory T cell identity after activation. Immunity. 2015;42:227–38. doi: 10.1016/j.immuni.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Placek K, Hu G, Cui K, Zhang D, Ding Y, Lee JE, et al. MLL4 prepares the enhancer landscape for Foxp3 induction via chromatin looping. Nat Immunol. 2017;18:1035–45. doi: 10.1038/ni.3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Husmann D, Gozani O. Histone lysine methyltransferases in biology and disease. Nat Struct Mol Biol. 2019;26:880–9. doi: 10.1038/s41594-019-0298-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagner EJ, Carpenter PB. Understanding the language of Lys36 methylation at histone H3. Nat Rev Mol Cell Biol. 2012;13:115–26. doi: 10.1038/nrm3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J, Li N, Yin Y, Zheng N, Min M, Lin B, et al. Methyltransferase Nsd2 ensures germinal center selection by promoting adhesive interactions between B cells and follicular dendritic cells. Cell Rep. 2018;25:3393–404.e6. doi: 10.1016/j.celrep.2018.11.096. [DOI] [PubMed] [Google Scholar]
- 10.Long X, Zhang L, Zhang Y, Min M, Lin B, Chen J, et al. Histone methyltransferase Nsd2 is required for follicular helper T cell differentiation. J Exp Med. 2019;217:e20190832. doi: 10.1084/jem.20190832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deshmukh H, Way SS. Immunological basis for recurrent fetal loss and pregnancy complications. Annu Rev Pathol. 2019;14:185–210. doi: 10.1146/annurev-pathmechdis-012418-012743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erlebacher A. Immunology of the maternal-fetal interface. Annu Rev Immunol. 2013;31:387–411. doi: 10.1146/annurev-immunol-032712-100003. [DOI] [PubMed] [Google Scholar]
- 13.PrabhuDas M, Bonney E, Caron K, Dey S, Erlebacher A, Fazleabas A, et al. Immune mechanisms at the maternal-fetal interface: perspectives and challenges. Nat Immunol. 2015;16:328–34. doi: 10.1038/ni.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mor G, Aldo P, Alvero AB. The unique immunological and microbial aspects of pregnancy. Nat Rev Immunol. 2017;17:469–82. doi: 10.1038/nri.2017.64. [DOI] [PubMed] [Google Scholar]
- 15.Arck PC, Hecher K. Fetomaternal immune cross-talk and its consequences for maternal and offspring’s health. Nat Med. 2013;19:548–56. doi: 10.1038/nm.3160. [DOI] [PubMed] [Google Scholar]
- 16.Zenclussen AC. CD4(+)CD25+ T regulatory cells in murine pregnancy. J Reprod Immunol. 2005;65:101–10. doi: 10.1016/j.jri.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5:266–71. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- 18.Morita K, Tsuda S, Kobayashi E, Hamana H, Tsuda K, Shima T, et al. Analysis of TCR repertoire and PD-1 expression in decidual and peripheral CD8(+) T cells reveals distinct immune mechanisms in miscarriage and preeclampsia. Front Immunol. 2020;11:1082. doi: 10.3389/fimmu.2020.01082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robertson SA, Care AS, Moldenhauer LM. Regulatory T cells in embryo implantation and the immune response to pregnancy. J Clin Investig. 2018;128:4224–35. doi: 10.1172/JCI122182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sasaki Y. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol Hum Reprod. 2004;10:347–53. doi: 10.1093/molehr/gah044. [DOI] [PubMed] [Google Scholar]
- 21.Tsuda S, Zhang X, Hamana H, Shima T, Ushijima A, Tsuda K, et al. Clonally expanded decidual effector regulatory T cells increase in late gestation of normal pregnancy, but not in preeclampsia, in humans. Front Immunol. 2018;9:1934. doi: 10.3389/fimmu.2018.01934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munoz-Suano A, Hamilton AB, Betz AG. Gimme shelter: the immune system during pregnancy. Immunological Rev. 2011;241:20–38. doi: 10.1111/j.1600-065X.2011.01002.x. [DOI] [PubMed] [Google Scholar]
- 23.Winger EE, Reed JL. Low circulating CD4+ CD25+ Foxp3+ T regulatory cell levels predict miscarriage risk in newly pregnant women with a history of failure. Am J Reprod Immunol. 2011;66:320–8. doi: 10.1111/j.1600-0897.2011.00992.x. [DOI] [PubMed] [Google Scholar]
- 24.Toldi G, Saito S, Shima T, Halmos A, Veresh Z, Vásárhelyi B, et al. The frequency of peripheral blood CD4+ CD25high FoxP3+ and CD4+ CD25− FoxP3+ regulatory T cells in normal pregnancy and pre-eclampsia. Am J Reprod Immunol. 2012;68:175–80. doi: 10.1111/j.1600-0897.2012.01145.x. [DOI] [PubMed] [Google Scholar]
- 25.Saito S, Nakashima A, Shima T, Ito M. REVIEW ARTICLE: Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am J Reprod Immunol. 2010;63:601–10. doi: 10.1111/j.1600-0897.2010.00852.x. [DOI] [PubMed] [Google Scholar]
- 26.Inada K, Shima T, Ito M, Ushijima A, Saito S. Helios-positive functional regulatory T cells are decreased in decidua of miscarriage cases with normal fetal chromosomal content. J Reprod Immunol. 2015;107:10–9. doi: 10.1016/j.jri.2014.09.053. [DOI] [PubMed] [Google Scholar]
- 27.Sasaki Y, Darmochwal-Kolarz D, Suzuki D, Sakai M, Ito M, Shima T, et al. Proportion of peripheral blood and decidual CD4(+) CD25(bright) regulatory T cells in pre-eclampsia. Clin Exp Immunol. 2007;149:139–45. doi: 10.1111/j.1365-2249.2007.03397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santner-Nanan B, Peek MJ, Khanam R, Richarts L, Zhu E, Fazekas de St Groth B, et al. Systemic increase in the ratio between Foxp3+ and IL-17-producing CD4+ T cells in healthy pregnancy but not in preeclampsia. J Immunol. 2009;183:7023–30. doi: 10.4049/jimmunol.0901154. [DOI] [PubMed] [Google Scholar]
- 29.Rowe JH, Ertelt JM, Xin L, Way SS. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature. 2012;490:102–6. doi: 10.1038/nature11462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Samstein RM, Josefowicz SZ, Arvey A, Treuting PM, Rudensky AY. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell. 2012;150:29–38. doi: 10.1016/j.cell.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kopřivová H, Hájková M, Koucký M, Malíčková K, Holáň V, Krulová M. Kinetics of Helios(+) and Helios(-) T regulatory cell subsets in the circulation of healthy pregnant women. Scand J Immunol. 2019;89:e12754. doi: 10.1111/sji.12754. [DOI] [PubMed] [Google Scholar]
- 32.Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5:266–71. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- 33.Kallikourdis M, Andersen KG, Welch KA, Betz AG. Alloantigen-enhanced accumulation of CCR5+ ‘effector’ regulatory T cells in the gravid uterus. Proc Natl Acad Sci USA. 2007;104:594–9. doi: 10.1073/pnas.0604268104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin Y, Xu L, Jin H, Zhong Y, Di J, Lin Q. CXCL12 enhances exogenous CD4+CD25+ T cell migration and prevents embryo loss in non-obese diabetic mice. Fertil Steril. 2009;91:2687–96. doi: 10.1016/j.fertnstert.2008.01.109. [DOI] [PubMed] [Google Scholar]
- 35.Guerin LR, Moldenhauer LM, Prins JR, Bromfield JJ, Hayball JD, Robertson SA. Seminal fluid regulates accumulation of FOXP3+ regulatory T cells in the preimplantation mouse uterus through expanding the FOXP3+ cell pool and CCL19-mediated recruitment1. Biol Reprod. 2011;85:397–408. doi: 10.1095/biolreprod.110.088591. [DOI] [PubMed] [Google Scholar]
- 36.Care AS, Bourque SL, Morton JS, Hjartarson EP, Robertson SA, Davidge ST. Reduction in regulatory T cells in early pregnancy causes uterine artery dysfunction in mice. Hypertension. 2018;72:177–87. doi: 10.1161/HYPERTENSIONAHA.118.10858. [DOI] [PubMed] [Google Scholar]
- 37.Hanna J, Wald O, Goldman-Wohl D, Prus D, Markel G, Gazit R, et al. CXCL12 expression by invasive trophoblasts induces the specific migration of CD16- human natural killer cells. Blood. 2003;102:1569–77. doi: 10.1182/blood-2003-02-0517. [DOI] [PubMed] [Google Scholar]
- 38.Maghazachi AA. Role of chemokines in the biology of natural killer cells. Curr Top Microbiol Immunol. 2010;341:37–58. doi: 10.1007/82_2010_20. [DOI] [PubMed] [Google Scholar]
- 39.Wu X, Jin LP, Yuan MM, Zhu Y, Wang MY, Li DJ. Human first-trimester trophoblast cells recruit CD56brightCD16- NK cells into decidua by way of expressing and secreting of CXCL12/stromal cell-derived factor 1. J Immunol. 2005;175:61–8. doi: 10.4049/jimmunol.175.1.61. [DOI] [PubMed] [Google Scholar]
- 40.Hirata Y, Furuhashi K, Ishii H, Li HW, Pinho S, Ding L, et al. CD150(high) bone marrow tregs maintain hematopoietic stem cell quiescence and immune privilege via adenosine. Cell Stem Cell. 2018;22:445–53.e. doi: 10.1016/j.stem.2018.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Das A, Agrawal NR, Zangmo R, Roy KK, Singh K, Bala R. Comparison of expression of chemokine receptor 4 in maternal decidua and chorionic Villi in women with spontaneous miscarriages and women opting for termination of viable pregnancies. J Hum Reprod Sci. 2021;14:68–72. doi: 10.4103/jhrs.JHRS_64_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang Q, Vincenti F. Transplant trials with Tregs: perils and promises. J Clin Investig. 2017;127:2505–12. doi: 10.1172/JCI90598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sánchez-Fueyo A, Whitehouse G, Grageda N, Cramp ME, Lim TY, Romano M, et al. Applicability, safety, and biological activity of regulatory T cell therapy in liver transplantation. Am J Transplant. 2020;20:1125–36. doi: 10.1111/ajt.15700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marek-Trzonkowska N, Mysliwiec M, Dobyszuk A, Grabowska M, Techmanska I, Juscinska J, et al. Administration of CD4+CD25highCD127- regulatory T cells preserves β-cell function in type 1 diabetes in children. Diabetes Care. 2012;35:1817–20. doi: 10.2337/dc12-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zenclussen AC, Gerlof K, Zenclussen ML, Sollwedel A, Bertoja AZ, Ritter T, et al. Abnormal T-cell reactivity against paternal antigens in spontaneous abortion: adoptive transfer of pregnancy-induced CD4+CD25+ T Regulatory cells prevents fetal rejection in a murine abortion model. Am J Pathol. 2005;166:811–22. doi: 10.1016/S0002-9440(10)62302-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Woidacki K, Meyer N, Schumacher A, Goldschmidt A, Maurer M, Zenclussen AC. Transfer of regulatory T cells into abortion-prone mice promotes the expansion of uterine mast cells and normalizes early pregnancy angiogenesis. Sci Rep. 2015;5:13938. doi: 10.1038/srep13938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu Y, Zhao M, Xu X, Liu X, Zhang H, Jiang Y, et al. Adoptive transfer of Treg cells counters adverse effects of Toxoplasma gondii infection on pregnancy. J Infect Dis. 2014;210:1435–43. doi: 10.1093/infdis/jiu265. [DOI] [PubMed] [Google Scholar]
- 48.Arenas-Hernandez M, Sanchez-Rodriguez EN, Mial TN, Robertson SA, Gomez-Lopez N. Isolation of leukocytes from the murine tissues at the maternal-fetal interface. J Vis Ex. 2015;99:e52866. doi: 10.3791/52866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fu B, Li X, Sun R, Tong X, Ling B, Tian Z, et al. Natural killer cells promote immune tolerance by regulating inflammatory TH17 cells at the human maternal-fetal interface. Proc Natl Acad Sci USA. 2013;110:E231–40. doi: 10.1073/pnas.1206322110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Allen CDC, Ansel KM, Low C, Lesley R, Tamamura H, Fujii N, et al. Germinal center dark and light zone organization is mediated by CXCR4 and CXCR5. Nat Immunol. 2004;5:943–52. doi: 10.1038/ni1100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.