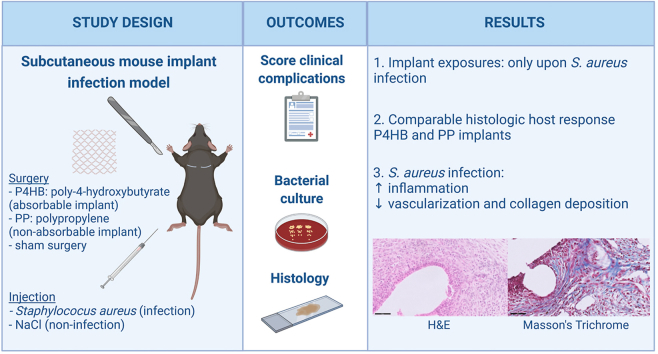
Abstract
Polypropylene (PP) implants for the vaginal surgical correction of pelvic organ prolapse (POP) are known for adverse events, like vaginal or visceral exposures. It is hypothesized that this is a result of a prolonged inflammatory response. One of the triggering factors of prolonged inflammation might be bacterial contamination. A possible solution might lie in an absorbable biomaterial, which provides initial mechanical support while being gradually replaced by the host tissue.
With this study we aimed to compare the host response, in a subcutaneous mouse implant infection model, to delayed absorbable poly-4-hydroxybutyrate (P4HB) and a latest generation PP implant. By comparing non-infected to Staphylococcus aureus infected mice, we assessed how bacterial contamination affects the host response and its role in the development of complications. Further, we included sham surgery as a control, mimicking the wound response in native tissue repair.
Despite the higher surface area of the P4HB implants, the clearance of infection was similarly delayed in the presence of a P4HB or PP implant, as compared to sham. Further, the host response towards P4HB and PP was quite comparable, yet collagen deposition was significantly increased around infected P4HB implants at early time points. Adverse event rates were similar, though implant exposures were only seen in infected mice and more often with PP (11.1%) than P4HB implants (5.6%). Infected mice overall had significantly higher levels of infiltration of inflammatory cells and lower levels of vascularization and collagen deposition compared to non-infected mice. Thus, for both P4HB and PP, bacterial contamination negatively affected mesh integration by increased inflammation and an increased adverse event rate. Altogether, our results from this subcutaneous mouse implant infection study suggest that P4HB could be a promising degradable alternative to PP, warranting further research to study its potential as a new surgical solution for women with POP.
Keywords: Pelvic floor, Pelvic organ prolapse (POP), Poly-4-hydroxybutyrate (P4HB), Polypropylene (PP), Mesh, Biomaterial-associated infection
Graphical abstract
Highlights
-
•
Absorbable poly-4-hydroxybutyrate (P4HB) is studied as a novel pelvic floor implant.
-
•
Comparable host response to P4HB and polypropylene in a subcutaneous mouse implant infection model.
-
•
Implant exposures exclusively occurred upon Staphylococcus aureus infection.
-
•
Exposures occurred less with P4HB (5.6%) compared to polypropylene (11.1%).
-
•
S. aureus infection increased inflammation and deranged the host response.
1. Introduction
Pelvic organ prolapse (POP) is the descent of one of the pelvic organs into the vagina or beyond. It can cause a bothersome sensation of bulging and negatively affects micturition, defecation, and sexual functioning, resulting in a decreased quality of life [1]. It is a prevalent disorder as the incidence is as high as 40% [2,3] and the overall life-time risk for prolapse surgery is around 10% [4]. Surgical repair using the patients' own pelvic support tissues, known as native tissue repair (NTR), has disappointing results with reoperation rates of up to 17–29% [[4], [5], [6]]. This may be explained by the usage of the patients’ own – already weakened – connective tissue to restore the pelvic floor. Based on the success in abdominal hernia repair, synthetic permanent implants for transvaginal POP surgery were introduced as an alternative. While publications on synthetic mesh use for POP date back to the 1990s, there was a rise in publications by the end of the 20th and beginning of 21st century [7]. The regulatory framework at that time (510(k)) did not require large clinical trials demonstrating efficacy and safety, prior to introduction in clinical practice [8]. While polypropylene (PP) surgical mesh implants have been tolerated well for certain applications (e.g. ventral hernia), it became clear that they can induce a prolonged inflammatory response in the vagina, which can eventually lead to adverse events [9]. The fact that mesh-related complications can have disastrous effects that are sometimes irreversible, resulted in several FDA activities over the past years with worldwide implications [8,10]. In January 2012, the FDA ordered manufacturers of transvaginal mesh products for POP to conduct post-market surveillance under Section 522 of the Federal food, drug and cosmetic act (”522 Studies”) [11]. In January 2016, the FDA issued a final order to reclassify mesh devices from class II to class III, requiring extensive clinical studies and manufacturers to submit a premarket approval (PMA) application henceforth [11,12]. While the first postmarket surveillance study has demonstrated similar effectiveness and safety of a latest generation PP mesh as compared to NTR, in 2021 the FDA stated that these implants do not have a favorable benefit-risk profile [11]. All this, has led to highly restricted use of transvaginal implants [13] and withdrawal of some products from the market [14,15].
With an aging population, suboptimal results of NTR and restricted use of current synthetic implants, there is an unmet need for alternative materials that can be used in pelvic floor surgery to accommodate the urgent need among patients. As recommended by the Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR) of the European Union in their final opinion published in 2015, further research should focus on novel materials, particularly degradable implants [16]. Besides the necessity to evaluate new biomaterials in vivo before human use, there is a demand to study additional variables influencing the host response, such as bacterial contamination [17]. While inflammation has been seen in completely sterile settings without infection, it is thought that a prolonged inflammatory response might be triggered by bacterial contamination and subsequent subclinical infection. Yet, more basic research is required to understand the underlying mechanism and to verify the relationship between infection and clinical complications [18]. As the vagina is a so-called ‘clean-contaminated site’, meaning that despite infection prophylaxes bacteria naturally reside in the vagina [19], bacterial contamination during implantation seems unavoidable [20].
Degradable materials should give initial mechanical support while providing a scaffold for native tissue ingrowth leading to their gradual replacement by functional tissue. Furthermore, degradable materials might be less susceptible to infection as compared to non-degradable materials, as bacteria might struggle to adhere to a degrading or eroding surface [21]. Poly-4-hydroxybutyrate (P4HB) is a delayed absorbable material and has shown promising results in the clinical field of ventral hernia repair [22]. Besides, we have demonstrated that P4HB implants have more favorable characteristics in vitro compared to PP implants, with respect to higher vaginal fibroblast proliferation and collagen deposition [23]. In addition, P4HB has mechanical properties compatible with the vaginal tissue [23] and is less susceptible to bacterial biofilm formation [24]. In vivo, P4HB showed a more favorable host response as compared to PP and comparable load bearing capacity [25]. Despite its degradable character and significant resorption one year after implantation, P4HB implants have demonstrated to provide sufficient mechanical strength [26].
In this study, we aimed to assess the host response to absorbable P4HB implants in a subcutaneous mouse model and to compare these results to the host response in current surgical alternatives: either a sham surgery mimicking the wound healing response in NTR or a non-absorbable PP implant. Furthermore, we aimed to study the effect of bacterial contamination on the host response and its role in the development of local complications by introducing Staphylococcus aureus, a common vaginal commensal with pathogenic properties [19], and an important player in many other biomaterial-associated infections [27]. To evaluate the host response, we (1) monitored mice for the development of local complications, (2) assessed the host bacterial clearance capacity in the presence of P4HB and PP implants and (3) performed detailed histological analyses of acute and chronic inflammation and tissue remodeling.
2. Materials and methods
2.1. Animals and ethics
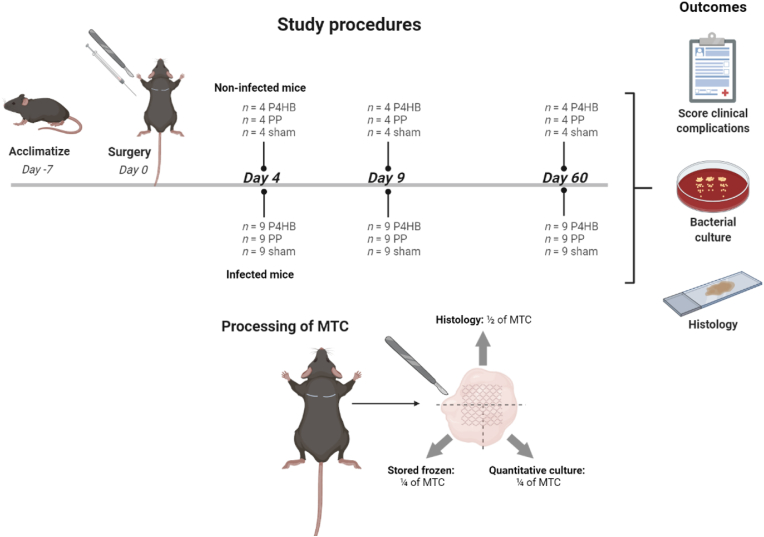
For this study, we used an established subcutaneous biomaterial-associated infection mouse model [28,29] and we adhered to the ARRIVE guidelines 2.0 (Animal Research: Reporting In Vivo Experiments) during preparation, execution, and analyses of the experiment [30] and complied to the ARRIVE Essential 10 checklist [31]. The Animal Welfare Body of the Amsterdam UMC (location AMC) approved the protocol (study number: DMB19-8484-1-01). Experiments were performed in accordance with the European directive 2010/63/EU for animal experiments and the Dutch Experiments on Animals Act (Wod). We studied two latest generation light-weight monofilament macroporous implants: P4HB Diamond (49.7 g/m2 with a pore size of 2.22 mm2) manufactured and provided by Tepha Inc. and PP Restorelle (18.9 g/m2 with a pore size of 3.10 mm2) manufactured and provided by Coloplast [24] (Fig. S1). Specific pathogen-free C57BL/6 J OlaHsd immune competent female mice (Envigo) were used, approximately aged 8–10 weeks and weighing 17–20 g. Mice received two subcutaneous segments of P4HB meshes (“P4HB mice”) or PP meshes (“PP mice”) or underwent sham surgery on two sites (“sham mice”). Further, the host response in a sterile setting (“non-infected mice”) was compared to the response in case of S. aureus bacterial contamination (“infected mice”). Group sizes for the non-infected groups were based on our previous research (n = 4 mice). For the infected groups a power calculation was performed, taking into account a variation of 20% and <5% unexpected animal loss, the group size was set at 9 mice [28,29,32]. In total, 117 mice were randomly divided over the different groups of 4 mice with 2 non-infected wounds/implants each (n = 8 samples per group) and groups of 9 mice with 2 infected wounds/implants each (n = 18 samples per group) (Fig. 1). Randomization was performed by a computer-generated randomization list [33]. All mice were acclimatized for at least 7 days before surgery and were provided with sterile food and water ad libitum.
Fig. 1.
Study procedure and experimental groups In total, 117 mice were randomly divided over 18 experimental groups: P4HB, PP and sham non-infection at 4, 9 and 60 days (all n = 4 mice with 8 samples per group) and P4HB, PP and sham infection at 4, 9 and 60 days (all n = 9 mice with 18 samples per group). Mice were scored for clinical complications and at sacrifice the mesh tissue complex (MTC) was cut into 3 parts for further analysis: half was used for histology and a quarter was used for quantitative culture of bacteria and storage of the lysate. The remaining quarter was used for storage for future RNA analysis.
2.2. Bacterial inoculum preparation
S. aureus ATCC 49230 is a clinical isolate and has a biofilm forming capacity [24,29,34]. Bacteria were stored at −80 °C and re-cultured on a blood agar plate overnight at 37 °C. From a single colony, a mid-logarithmic culture was obtained in tryptic soy broth (TSB; BD Difco) and diluted in saline to an inoculum suspension containing 4 × 107 colony forming units (CFU)/ml, based on the optical density at 620 nm.
2.3. Surgical procedure
Thirty minutes prior to surgery the mice received a subcutaneous injection with 0.05 mg/kg buprenorphine (Vetergesic, Multidose 0.3 mg/ml, Ecuphar) for pain relief. The mice were anesthetized with 1.5–2% isoflurane in oxygen and the back of the mice was shaved and disinfected with 70% ethanol. Working under sterile conditions in a laminar flow cabinet, two 0.5 cm horizontal incisions were made, 1 cm lateral to the spine at the level of the scapulae. With blunt cover glass forceps a subcutaneous pocket was created and a 10 × 7 mm implant was inserted at both sides in the implant groups. In the sham mice the same pockets were created with the forceps, but no biomaterial was implanted. The incisions were closed with Vicryl 6.0 sutures (Ethicon), followed by injection of 25 μl saline (non-infected groups) or 25 μl saline containing 1 × 106 CFU S. aureus bacteria (infected groups) along the implant (Stepper, repetitive dispensing pipette, Tridak). Mice were housed alone in individually ventilated cages until complete wound closure for a maximum of one week, thereafter mice were housed in groups of two or three. In the first week after surgery mice were weighed on a daily basis and after this first week on indication. Mice were scored for adverse events per implant site after 4 and 7 days and prior to sacrifice and investigators were blinded for the groups. Clinical signs of infection were defined as local redness, swelling, edema and/or pus formation per implant side. Implant exposure was defined as an implant erosion through the intact skin, so adjacent to the incision area, or through a fully healed surgical incision.
2.4. Sample collection
Mice were anesthetized, as described above for implantation, and the mesh-tissue complex (MTC) was harvested after 4, 9 or 60 days. The MTC was cut into 3 parts for further analysis (Fig. 1). Half of the MTC was used for histology and one quarter of the MTC was used for quantitative culture of bacteria and the remaining homogenate was stored to allow further analyses. The remaining quarter of the MTC was stored in RNA stabilization solution (RNAlater, Invitrogen) and incubated overnight at 4 °C. After removal of the RNA stabilization solution, the sample was stored at – 80 °C to allow future RNA analysis.
2.5. Bacterial quantification
Samples were collected in 2 ml tubes containing 0.5 ml saline with 5 sterilized zirconium beads (Ø 2 mm, BioSpec Products) and stored on ice. To neutralize mouse endogenous antimicrobial peptide (AMP) activity, 50 μl of a 0.05% (v/v) sodium polyanethole sulfonate (SPS; Sigma) solution was added to the samples [35]. Due to technical issues, no SPS was added to samples from the P4HB, PP and sham non-infected mice at 9 days and samples from the sham infected mice at 9 days. Samples were homogenized with 3 cycles of 30 s at 7000 rpm with 30 s cooling on ice in between the cycles (MagNA Lyser, Roche). Of this homogenate, 100 μl was ten-fold serially diluted in phosphate buffered saline (PBS) and five-fold 10 μl aliquots of the sample and the dilutions were spotted on blood agar plates. After overnight incubation, colonies were counted and converted to log CFU per homogenized biopsy. Morphologically distinct colonies were selected for species determination (MALDI Biotyper, Sirius System, Bruker) to rule out contamination. In case of bacterial contamination other than S. aureus, these colonies were not included in the CFU counts.
2.6. Histology
MTC samples were immediately fixed in 4% paraformaldehyde and stored at 4 °C. After 24 h, the samples were transferred to 70% ethanol which was refreshed after 2 h, and samples were stored at 4 °C until further processing. The fixed MTCs were dehydrated using an automatic tissue processor (ASP300, Leica), embedded in a clean filtered paraffin mixture (Paraplast, Leica) and cut in 5 μm sections (RM2255, Leica). After deparaffinization and rehydration of the sections, staining was performed with Mayer's Hematoxylin and Eosin (H&E) and Masson's Trichrome (MT). Slides were scanned using the IntelliSite UltraFast Scanner (Philips Digital Pathology Solutions). Semi-quantitative assessments were performed at a 40 × magnification by two independent researchers blinded to the different groups, using an ordinal grading scale as previously described [36,37]. Five randomly chosen fields per slide were selected at the mesh tissue interface. Vascularization and foreign body giant cells (FBGCs) were scored on a scale of 0–3 (0 = none, 1 = 1 to 5 vessels/FBGCs, 2 = 6 to 10 vessels/FBGCs, 3 = more than 10 vessels/FBGCs) in the H&E staining. Infiltration of inflammatory cells (predominant polymorphonuclear cells) was scored in the H&E staining and collagen formation in the MT staining on a scale of 0–3 based on the appearance (0 = none, 1 = mild, 2 = moderate, 3 = severe/abundant) (Fig. S2). All histological scores were averaged, in case the difference was >0.5, a senior researcher was consulted for a third opinion.
2.7. Statistical analysis
Adverse event rates were reported as frequencies and groups were compared in SPSS statistics version 26.0 (IBM) using a Chi-square test. All other analyses were performed in GraphPad Prism version 9.1.0 for Windows (GraphPad Software). Weights were reported as mean with standard deviation and compared with an unpaired t-test. Bacterial quantification and histological data were reported as median with interquartile range (IQR), quartile 1 and 3. Two sample comparisons were made using a Mann-Whitney-U test and multiple sample comparisons were made using the Kruskal-Wallis test. In case of a significant difference, the Kruskal-Wallis test was followed by a Dunn's post-hoc test to correct for multiple testing. A p-value of <0.05 was considered to indicate a statistically significant difference. Graphs were created in GraphPad Prism.
3. Results
3.1. Post-operative recovery and weights
All mice survived the surgical procedure. Three mice from the infected groups had to be terminated before the end of the study due to severe discomfort or excessive weight loss (>15% weight loss as compared to the highest weight) and were left out of the bacterial quantification and histologic evaluation. One mouse from the P4HB 4 day group was sacrificed after two days because of 17% weight loss and signs of discomfort, one mouse from the PP 60 day group after 8 days because of a severe mesh exposure and corresponding discomfort, and one mouse from the P4HB 60 day group after 52 days because of 11% weight loss combined with signs of discomfort.
The mean weight of the animals at baseline was 19.35 ± 0.86 g (Fig. S3). On average, mice from all groups lost weight in the first 24 h after surgery. The group mean percentage of weight change compared to the weight at baseline was calculated on day 1 and 4, and day 7 and 60 (if applicable). At day 1, infected mice on average lost significantly more weight than non-infected mice (−7.7% vs. −2.8%, p < 0.001) and infected mice with implants lost significantly more weight than infected sham mice (P4HB −9.1% vs. sham −5.5% p < 0.001 and PP -8.5% vs. sham −5.5% p = 0.002). There were no significant differences between the P4HB and PP mice. On day 4, the weight of most animals was again above baseline and increased during the course of the study.
3.2. Macroscopic observations
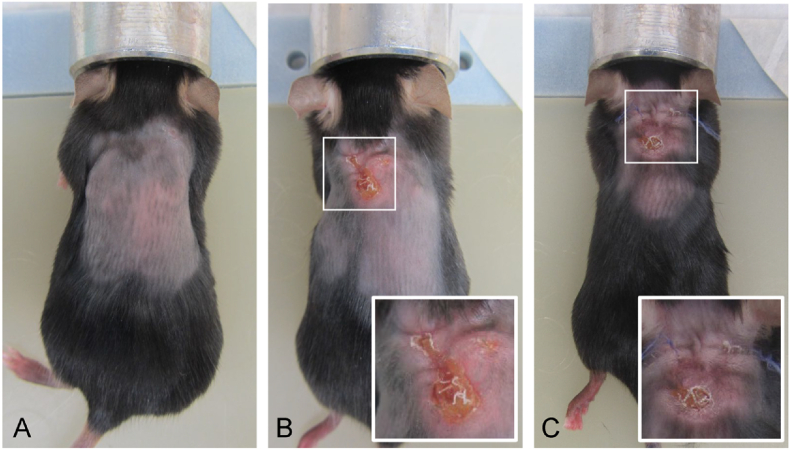
All mice were scored for clinical signs of infection and implant exposure (as defined in methods), for each implant site (Table 1). Since we also observed (unexpected) implant erosions through the original incision site before healing of the surgical wound, these were scored separately and defined as ‘incisional erosion’ (Fig. 2C). Only mice from the infected groups demonstrated clinical signs of infection within the first 14 days at one or both of their implant sites, i.e. 9.3% (5/54) of the implants of the P4HB mice and 5.6% (3/54) of those the PP mice (not significantly different (NS)) compared to 1.9% (1/54) of the sham surgery sites in the sham mice (NS). Mesh exposures, defined as mesh exposures through the intact skin (adjacent to the surgical incision or through a fully healed wound) (Fig. 2B), only occurred in mice with bacterial infection: in 5.6% (3/54) of the implants of the P4HB mice compared to 11.1% (6/54) of those of the PP mice (NS). Incisional erosions were seen twice as often with the implants of infected mice (P4HB: 20.4% (11/54): PP: 22.2% (12/54)) as compared with the implants of non-infected mice (P4HB: 12.5% (3/24); PP: 8.3% (2/24)), but the frequencies were not significantly different between P4HB and PP implants.
Table 1.
Incidence of adverse events (macroscopic morphology) The incidence is reported per implant or sham site, each mouse had two implants or sham sites. We included the three mice which had to be terminated before the end of the experiment, to not underestimate the results. NA = non-applicable.
| Non-infection |
Infection |
|||||
|---|---|---|---|---|---|---|
| Infection | Exposure | Incisional erosion | Infection | Exposure | Incisional erosion | |
| P4HB 4 d | 0/8 | 0/8 | 0/8 | 5/18 | 0/18 | 0/18 |
| P4HB 9 d | 0/8 | 0/8 | 1/8 | 0/18 | 1/18 | 3/18 |
| P4HB 60 d | 0/8 | 0/8 | 2/8 | 0/18 | 2/18 | 8/18 |
| Total P4HB | 0/24 | 0/24 | 3/24 | 5/54 | 3/54 | 11/54 |
| PP 4 d | 0/8 | 0/8 | 0/8 | 0/18 | 0/18 | 0/18 |
| PP 9 d | 0/8 | 0/8 | 0/8 | 3/18 | 1/18 | 5/18 |
| PP 60 d | 0/8 | 0/8 | 2/8 | 0/18 | 5/18 | 7/18 |
| Total PP | 0/24 | 0/24 | 2/24 | 3/54 | 6/54 | 12/54 |
| Sham 4 d | 0/8 | NA | NA | 1/18 | NA | NA |
| Sham 9 d | 0/8 | NA | NA | 0/18 | NA | NA |
| Sham 60 d | 0/8 | NA | NA | 0/18 | NA | NA |
| Total Sham | 0/24 | NA | NA | 1/54 | NA | NA |
Fig. 2.
Mesh exposure and incisional erosion Mice at 9 days after implantation. (A) P4HB infection: example of normal wound healing, (B) P4HB infection: mesh exposure, this was seen earliest after 7 days, (C) PP infection: besides a mesh exposure, on both sides incisional erosions were observed.
3.3. Bacterial quantification
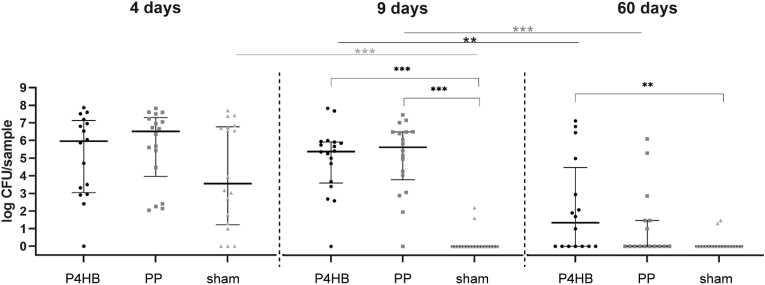
All colonies were identified as S. aureus, except single colonies on 5 out of 232 bacterial culture plates, which were either identified as Enterococcus gallinarum or Enterococcus hirae. These counts were left out of the analysis. Both species are intestinal microbiota, E. gallinarum in humans and mice [38] and E. hirae in different animals [39] and in this case were presumably part of the mouse microflora. The median log numbers of CFU per implant (log CFU) for all non-infection groups was 0 (IQR = 0-0). In all infected mice, after 4 days the log CFU were high but not significantly different between the P4HB, PP and sham mice (Fig. 3). Only in sham mice a rapid and significant clearance of infection was observed as the log CFU after 9 days decreased to undetectable levels (p < 0.001), and was significantly lower than in the P4HB and PP mice (both p < 0.001). Infections were resolving after 60 days in both the P4HB (p = 0.005) and PP mice (p < 0.001), with only for the P4HB mice the number of bacteria (1.35 log CFU (0–4.48)) remaining significantly higher than for the sham mice (0 log CFU (0-0)) (p = 0.002). There were no statistically significant differences in log CFU retrieved from the P4HB and PP mice at any of the three time points (Fig. 3). As mentioned in the methods section, no SPS was added to the samples of the P4HB, PP and sham non-infected mice and sham infected mice at 9 days. This could possibly have yielded an underestimation of the true bacterial numbers in situ due to the residual AMP activity of the homogenates.
Fig. 3.
Bacterial quantification of the mesh-tissue complexBacterial quantification of the mesh-tissue complex on day 4, 9 and 60 for the three infection groups (P4HB, PP and sham). Data are represented as median log CFU/sample with interquartile ranges. The brackets represent the comparisons between P4HB, PP and sham at the given time point. The straight lines represent the comparisons between the different time points for the given implant or sham group.
The non-infection groups all had a median of 0 and are therefore not shown in this figure.∗∗p < 0.01, ∗∗∗p < 0.001.
In the majority (65%) of the mice with adverse events, the numbers of CFU retrieved from affected implants had log CFU higher than the median of their groups (Table S1), especially in case of signs of infection (8/9 mice) and exposure (7/8 evaluable mice (one mouse with an exposure could not be evaluated because of premature termination due to the humane endpoint of severe mesh exposure)).
3.4. Histological evaluation
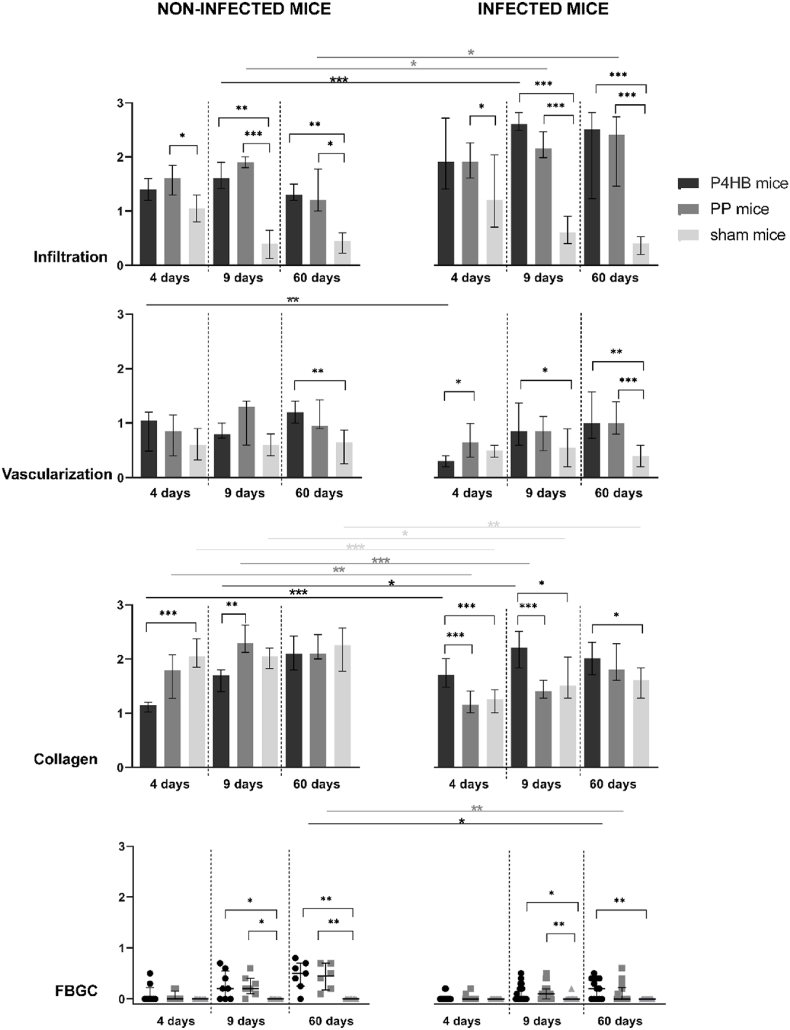
3.4.1. Host response in sham mice
To understand the normal wound healing response and influence of bacterial contamination, we included sham groups (no implant) without and with infection. Of all groups, infiltration of inflammatory cells was the lowest in the non-infected sham mice and decreased over time (Fig. 4). In sham mice, vascularization was also rather low but constant over time. Neither infiltration nor vascularization changed under the influence of bacterial contamination in the sham mice. Collagen deposition at day 4 was highest in the non-infected sham mice and was significantly reduced due to the infection at day 4 (p < 0.001), 9 (p = 0.04), and 60 (p = 0.009). As was to be expected, FBGCs were low in sham mice. Thus the inflammatory response in the sham mice was mild and rapidly resolved to wound healing, in both the presence and absence of infection (Fig. 5).
Fig. 4.
Histological evaluation of non-infected and infected miceHistological scoring of infiltration of inflammatory cells, vascularization, collagen deposition and foreign body giant cell (FBGC) formation, results are reported as median and interquartile ranges. The brackets represent the comparisons between P4HB, PP and sham at the given time point. The straight lines represent the comparisons between non-infected and infected mice, for that given time point and implant group. Only significant difference are shown. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
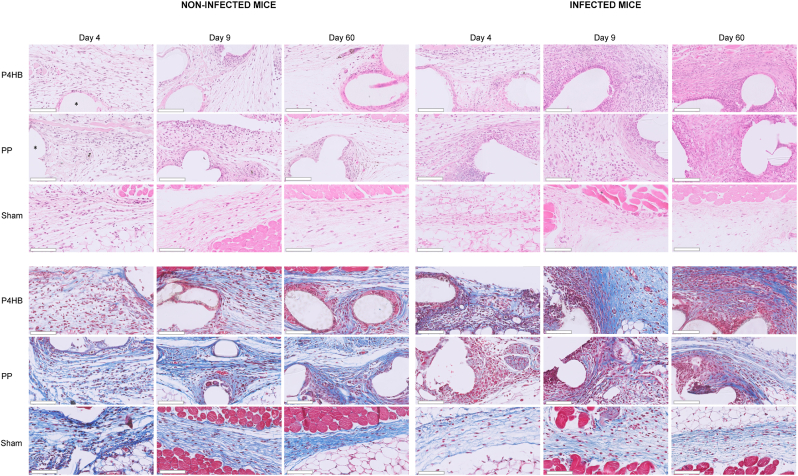
Fig. 5.
Histological sections of non-infected and infected miceHistological sections of mice with P4HB or PP implants or sham surgery, over time for both the non-infected groups (left) and infected groups (right). Infiltration of inflammatory cells (Hematoxylin and Eosin, upper panel) was lower in the sham mice than in the P4HB and PP mice, no differences were found between P4HB and PP. An increase in infiltration was seen in the P4HB and PP mice by S. aureus infection. Collagen deposition (Masson's Trichrome, lower panel) in P4HB mice increased by infection while it decreased in the PP and sham mice.The asterisk shows an example of the mesh. The scale bar in the lower left corner represents 100 μm.
3.4.2. Host response in the P4HB and PP implanted mice in absence of bacterial infection
When compared to the sham mice, non-infected mice with implants showed significantly higher levels of infiltration of inflammatory cells: in the PP mice at day 4 (p = 0.03), and in the P4HB as well as in the PP mice at day 9 (p = 0.008 and p < 0.001, respectively) and day 60 (p = 0.001 and p = 0.01, respectively) (Fig. 4, left). Vascularization was significantly higher in the P4HB mice as compared to the sham mice at day 60 (p = 0.007) and early collagen deposition at day 4 was significantly lower (p < 0.001). FBGC formation was increased in both the P4HB and PP mice at day 9 and 60. So, the presence of an implant induced a higher and prolonged inflammatory response and delayed wound healing. No significant differences were found between the P4HB and PP mice, except for less collagen deposition in the P4HB mice at day 9 (p = 0.005), which did not remain until day 60. So the tissue response to both materials over time was similar, with a potential temporary delay in the progression of wound healing around P4HB (Fig. 5).
3.4.3. Host response in the P4HB and PP implanted mice in the presence of S. aureus infection
In the S. aureus infected groups, infiltration was higher in the P4HB and PP mice as compared to the infected sham mice: at day 4 in the PP mice (p = 0.04) and at 9 and 60 days in both the P4HB and PP mice (all p < 0.001) (Fig. 4, right). Vascularization was significantly higher in the P4HB mice at day 9 (p = 0.04) and in both the P4HB and PP mice at day 60 (p = 0.001 and p < 0.001, respectively). Collagen deposition was significantly higher in the infected P4HB mice than in the infected sham mice at all time points (4 days p < 0.001, 9 days p = 0.01 and 60 days p = 0.04). Finally, also FBGC formation was increased in the P4HB and PP infected groups at 9 and 60 days. When comparing the P4HB to the PP infected mice, vascularization was significantly higher in the PP mice as compared to the P4HB mice at day 4 (p = 0.01). Further, collagen deposition was higher in the P4HB mice as compared to the PP mice at both day 4 and day 9 (both p < 0.001).
In general, S. aureus infection caused a significant increase in infiltration of inflammatory cells and a decrease in vascularization and FBGC formation when comping the infected mice to the non-infected mice (Fig. 4). Collagen deposition was affected by infection in all groups, but differed depending on the biomaterial implanted. A decrease in collagen deposition upon infection was seen for the PP mice at day 4 (p = 0.008) and day 9 (p < 0.001), while in the P4HB mice an increase was seen at these time points, day 4 (p < 0.001) and day 9 (p = 0.01) (Fig. 5).
4. Discussion
Our study showed that adverse events were as common in mice with P4HB as in mice with PP implants. However, implant exposures only occurred in mice with S. aureus infection and more often in the PP mice (11.1% of implants) than in the P4HB mice (5.6% of implants). In the presence of a P4HB or PP implant, S. aureus clearance was similarly delayed relative to the clearance in the sham mice. Further, there were minor differences in the different phases of the host response as observed in histology between P4HB and PP, but these differences were transient and did not exist anymore at 60 days after implantation.
The delayed clearance of S. aureus in our P4HB and PP mice demonstrates that P4HB and PP do not form an exception to the general contention that a foreign body predisposes for infection. This is likely due to a combination of adherence of bacteria to the biomaterial and subsequent biofilm formation, and reduced efficacy or evasion of the local immune response [40]. No significant differences in the log CFU retrieved from the P4HB and PP implants were observed at any of the time points investigated. Interestingly, P4HB implants were thicker, had smaller pores and a higher areal density than the PP implants, designed to induce an appropriate initial host response to compensate for the degradation of P4HB on the long term. Although this larger P4HB implant surface might have provided the bacteria more surface to adhere to and form a biofilm, this apparently did not lead to higher levels of bacterial colonization than on PP. In contrast to our mouse study, results in a subcutaneous rat model showed a higher resistance of P4HB to S. aureus bacterial challenge as compared to PP [41]. This contrasting finding may be related to the different time point (14 days), the different animal species and differences in bacterial strain and inoculum concentrations used, but also to the use of P4HB and PP construct designs differing from the ones used in our study.
Extensive histological analysis revealed higher levels of inflammatory cell infiltration, vascularization, and FBGC formation in mice with a biomaterial as compared to sham mice, confirming previous observations [[42], [43], [44], [45], [46]]. It is known that denser implants with smaller pores induce a more pronounced or prolonged inflammatory response as compared to lighter implants with larger pores [47]. Although both types of constructs are light-weight, macroporous and monofilament, the P4HB construct studied had less beneficial physical characteristics than the PP construct, i.e. thicker fibers, smaller pore size and higher density. Despite this, inflammation and vascularization in the respective mice did not significantly differ. Previous research in other animal models showed varying results. In a sheep model P4HB demonstrated increased inflammation and decreased vascularization as compared to PP at 60 and 180 days [25], whereas in a rabbit model no significant differences were found after 3 and 9 months for inflammation, neovascularization and elastin and collagen deposition around both materials [48].
However, in the presence of S. aureus infection of the implant, we observed increased vascularization, starting earlier around PP than around P4HB implants. As the differences resolved over time, already at day 9, most likely these are not of clinical relevance. Whereas collagen deposition in the non-infected P4HB mice was delayed compared to non-infected PP mice, in the infected P4HB mice collagen deposition was significantly higher at 4 and 9 days than in the infected PP mice. This difference was the result of an increase in collagen deposition observed in P4HB mice after S. aureus infection, while a decrease in collagen deposition was seen in PP mice. S. aureus infection can cause upregulation of matrix-metalloproteinase 2 (MMP2), thereby impairing collagen formation and eventually compromising the biomechanical properties of the tissue [49]. Even though our culture results did not show significant differences, P4HB has shown to be less susceptible to S. aureus adhesion [24]. However, no significant differences remained after 60 days, indicating that neither P4HB or PP, nor S. aureus infection differentially influenced the final tissue regeneration.
Women with a vaginal exposure of the mesh often have positive bacteriological cultures [50]. However, the question is whether the contaminating bacteria are the cause of the exposure or secondary to the exposure. Our study suggests that bacterial contamination increases the risk to develop implant exposure, since exposures only occurred in the mice challenged with S. aureus. Also, adverse events were more common in mice with the highest bacterial counts at explantation. Such correlation between contamination and adverse events has also been observed in an abdominal hernia mesh rat model, where increased contraction of the MTC was observed after contamination with 106 CFU Escherichia coli [51]. Moreover, our infected mice overall had significantly higher scores for infiltration of inflammatory cells and lower scores for vascularization and collagen deposition, except infected P4HB mice. After implantation, bacteria might win ‘the race for the surface’ which may lead to a decrease in host cell attachment [52]. This negatively affects mesh integration and acceptance, ultimately leading to complications such as mesh exposure. While infections were (non-significantly) more common in P4HB mice, implant exposures were more common in PP mice. One explanation may lie in the biomechanical properties of both materials, as P4HB is more biomechanically compliant as it has mechanical properties close to native tissue and a lower stiffness than PP [25]. The higher stiffness of PP might create friction between the implant and its surrounding tissue, which could make particularly infected tissue more vulnerable to implant exposures.
Mice are not a generally used model in POP research, because implants cannot be easily tested functionally. However, the subcutaneous mouse implant model is a well-recognized biomaterial-interaction and infection model, allowing comparison of the host response and susceptibility to infection of different biomaterials [28,[53], [54], [55]]. Like all animal models, this model has some limitations. Firstly, the influence of mechanical loading and its effect on the host response could not be taken into account as in vaginal models. Because of the small size of the implants, we could not assess the mechanical properties of explanted MTC. Secondly, due the delayed degradable nature of P4HB the follow-up of 60 days was too short to study full implant degradation. Degradation of P4HB is reflected by a steady loss of molecular weight without a reduction in fiber size. However, in vivo by 60 days P4HB has demonstrated a molecular weight decrease of 28% [25]. Thirdly, we used relatively high doses and a monoculture of S. aureus bacteria whereas the vaginal flora in women is multimicrobial and also contains non-pathogenic commensals. And finally, the host response is also dependent on the site of implantation because this response has been shown to significantly differ in the vagina as compared to the abdomen [46], so this should be taken into account when interpreting these results.
Considering that ultimately P4HB will be fully degraded, our results are promising as under the influence of infection collagen deposition around P4HB was not negatively affected. However, no significant differences remained compared to PP after 60 days and this model is not valid to study anatomical nor biomechanical outcomes. To assess the possibilities, future studies should focus on the long-term host response to P4HB in larger animal models, e.g. sheep [[56], [57], [58]], including the effect of mesh degradation and mechanical properties. Finally, as bacterial contamination significantly affects the host response, we would recommend future studies to take into account the effect of the vaginal microflora on mesh acceptance and integration.
5. Conclusion
This study indicates an increased level of inflammation and a delayed progression of the foreign body response due to S. aureus infection. This supports the hypothesis that bacterial contamination negatively affects mesh integration and contributes to the development of local complications such as implant exposure. Interestingly, early collagen deposition was not affected by infection around P4HB implants, as opposed to collagen deposition around PP. Further, despite the higher surface area of P4HB, only minor differences were found between the host response to P4HB and PP. As we compared P4HB to the latest generation PP implant (a monofilament, lightweight, macroporous implant), P4HB could be a promising fully absorbable alternative for the correction of POP. However, longer follow-up studies in a large (vaginal) animal model encountering mechanical loads and native microbial flora, should be performed prior to introducing vaginal P4HB implants clinically in women.
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Credit author statement
Verhorstert KWJ: Conceptualization, Methodology, Formal analysis, Investigation, Writing – original draft, Visualization. Riool M: Methodology, Investigation, Writing – review & editing, Project administration. Bulten T: Investigation. Guler Z: Conceptualization, Methodology, Validation, Investigation, Writing – review & editing, Supervision, Funding acquisition. de Boer L: Investigation, Writing – review & editing. Roovers JPWR: Conceptualization, Writing – review & editing, Supervisions, Funding acquisition. Zaat SA: Conceptualization, Methodology, Writing – original draft, Supervision.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: J.P.W.R Roovers reports financial support and equipment, drugs, or supplies were provided by Tepha Inc. and Coloplast.
Acknowledgements
This study was supported by an unrestricted research grant provided by Tepha, Inc. They had no influence on the design and conduct of the study, interpretation of the data and were informed about the results only after study completion. The implants were provided by Tepha Inc. and Coloplast. The work of M.R and L.d.B was supported by the research program of DPI, project NEWPOL, SuperActive and of the Dutch Technology Foundation STW (Project Nr. 14.326; Craniosafe).
We would like to thank the animal care takers of the Animal Research Institute AMC (ARIA) for their assistance during the conduct of the experiments and dedicated work; the animal welfare officers dr. Henriëtte Griffioen and dr. Wouter Florijn (Dept. of Animal Welfare, Amsterdam UMC, Amsterdam, The Netherlands) for their informative input into our study; Prof. dr. S. Florquin (Dept. of Pathology, Amsterdam UMC, Amsterdam, The Netherlands) for her expertise during histological evaluations and to Tepha Inc. and Coloplast for providing the pelvic floor implants.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mtbio.2022.100268.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Fritel X., et al. Symptomatic pelvic organ prolapse at midlife, quality of life, and risk factors. Obstet. Gynecol. 2009;113(3):609–616. doi: 10.1097/AOG.0b013e3181985312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hendrix S.L., et al. Pelvic organ prolapse in the Women's Health Initiative: gravity and gravidity. Am. J. Obstet. Gynecol. 2002;186(6):1160–1166. doi: 10.1067/mob.2002.123819. [DOI] [PubMed] [Google Scholar]
- 3.Nygaard I., et al. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008;300(11):1311–1316. doi: 10.1001/jama.300.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdel-Fattah M., et al. Primary and repeat surgical treatment for female pelvic organ prolapse and incontinence in parous women in the UK: a register linkage study. BMJ Open. 2011;1(2):e000206. doi: 10.1136/bmjopen-2011-000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denman M.A., et al. Reoperation 10 Years after surgically managed pelvic organ prolapse and urinary incontinence. Am. J. Obstet. Gynecol. 2008;198(5):555.e1–555.e5. doi: 10.1016/j.ajog.2008.01.051. [DOI] [PubMed] [Google Scholar]
- 6.Olsen A.L., et al. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet. Gynecol. 1997;89(4):501–506. doi: 10.1016/S0029-7844(97)00058-6. [DOI] [PubMed] [Google Scholar]
- 7.Dällenbach P. To mesh or not to mesh: a review of pelvic organ reconstructive surgery. Int J Womens Health. 2015;7:331–343. doi: 10.2147/IJWH.S71236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mangir N., et al. Landmarks in vaginal mesh development: polypropylene mesh for treatment of SUI and POP. Nat. Rev. Urol. 2019;16(11):675–689. doi: 10.1038/s41585-019-0230-2. [DOI] [PubMed] [Google Scholar]
- 9.Gigliobianco G., et al. Biomaterials for pelvic floor reconstructive surgery: how can we do better? BioMed Res. Int. 2015;2015:1–20. doi: 10.1155/2015/968087. 968087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ng-Stollmann N., et al. The international discussion and the new regulations concerning transvaginal mesh implants in pelvic organ prolapse surgery. Int Urogynecol J. 2020;31(10):1997–2002. doi: 10.1007/s00192-020-04407-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.(FDA), U.S.F.a.D.A . 2021. FDA's Activities: Urogynecologic Surgical Mesh.https://www.fda.gov/medical-devices/urogynecologic-surgical-mesh-implants/fdas-activities-urogynecologic-surgical-mesh 09/02/2021 [cited 2022 22-03-2022]; Available from: [Google Scholar]
- 12.FDA . 2016. Obstetrical and Gynecological Devices; Reclassification of Surgical Mesh for Transvaginal Pelvic Organ Prolapse Repair.https://www.federalregister.gov/documents/2016/01/05/2015-33165/obstetrical-and-gynecological-devices-reclassification-of-surgical-mesh-for-transvaginal-pelvic [cited 2022 08-04-2022]; Available from: [PubMed] [Google Scholar]
- 13.NVOG, Standpunt . 2020. Transvaginale Mesh Zorg in Nederland; p. 3. Nederlandse Vereniging voor Obstetrie & Gynaecologie: Utrecht. [Google Scholar]
- 14.FDA . 2019. Urogynecologic Surgical Mesh Implants.https://www.fda.gov/medical-devices/implants-and-prosthetics/urogynecologic-surgical-mesh-implants [cited 2019 23-05-2019]; Available from: [Google Scholar]
- 15.Medsafe . 2018. New Zealand Medicines and Medical Devices Safety Authority. Surgical Mesh Implants: Regulatory action on Surgical Mesh Products.https://www.medsafe.govt.nz/hot/alerts/UrogynaecologicaSurgicalMeshImplants.asp 28-06-2018 [cited 2021 21-04-2021]; Available from: [Google Scholar]
- 16.Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR). Opinion on the Safety of Surgical Meshes Used in Urogynecological Surgery 2015, Scientific Committees.
- 17.Slack M., et al. A standardized description of graft-containing meshes and recommended steps before the introduction of medical devices for prolapse surgery. Consensus of the 2nd IUGA Grafts Roundtable: optimizing safety and appropriateness of graft use in transvaginal pelvic reconstructive surgery. Int Urogynecol J. 2012;23(Suppl 1):S15–S26. doi: 10.1007/s00192-012-1678-2. [DOI] [PubMed] [Google Scholar]
- 18.Mangir N., et al. Complications related to use of mesh implants in surgical treatment of stress urinary incontinence and pelvic organ prolapse: infection or inflammation? World J. Urol. 2019;38:73–80. doi: 10.1007/s00345-019-02679-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Culligan P., et al. Bacterial colony counts during vaginal surgery. Infect. Dis. Obstet. Gynecol. 2003;11(3):161–165. doi: 10.1080/10647440300025515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vollebregt A., Troelstra A., van der Vaart C.H. Bacterial colonisation of collagen-coated polypropylene vaginal mesh: are additional intraoperative sterility procedures useful? Int. UrogynEcol. J. Pelvic Floor Dysfunct. 2009;20(11):1345–1351. doi: 10.1007/s00192-009-0951-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daghighi S., et al. Infection resistance of degradable versus non-degradable biomaterials: an assessment of the potential mechanisms. Biomaterials. 2013;34(33):8013–8017. doi: 10.1016/j.biomaterials.2013.07.044. [DOI] [PubMed] [Google Scholar]
- 22.Mellia J.A., et al. Outcomes of poly-4-hydroxybutyrate mesh in ventral hernia repair: a systematic review and pooled analysis. Plast Reconstr Surg Glob Open. 2020;8(12):e3158. doi: 10.1097/GOX.0000000000003158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diedrich C.M., et al. Fully absorbable poly-4-hydroxybutyrate implants exhibit more favorable cell-matrix interactions than polypropylene. Mater Sci Eng C Mater Biol Appl. 2021;120:111702. doi: 10.1016/j.msec.2020.111702. [DOI] [PubMed] [Google Scholar]
- 24.Verhorstert K.W.J., et al. In vitro bacterial adhesion and biofilm formation on fully absorbable poly-4-hydroxybutyrate and nonabsorbable polypropylene pelvic floor implants. ACS Appl. Mater. Interfaces. 2020;12(48):53646–53653. doi: 10.1021/acsami.0c14668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diedrich C.M., et al. Bjog; 2021. Evaluation of the Short-Term Host Response and Biomechanics of an Absorbable Poly-4-Hydroxybutyrate Scaffold in a Sheep Model Following Vaginal Implantation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deeken C.R., Matthews B.D. Characterization of the mechanical strength, resorption properties, and histologic characteristics of a fully absorbable material (Poly-4-hydroxybutyrate-PHASIX mesh) in a porcine model of hernia repair. ISRN Surg. 2013:1–12. doi: 10.1155/2013/238067. 2013: p. 238067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arciola C.R., Campoccia D., Montanaro L. Implant infections: adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018;16(7):397–409. doi: 10.1038/s41579-018-0019-y. [DOI] [PubMed] [Google Scholar]
- 28.Riool M., et al. Staphylococcus epidermidis originating from titanium implants infects surrounding tissue and immune cells. Acta Biomater. 2014;10(12):5202–5212. doi: 10.1016/j.actbio.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 29.Riool M., et al. A chlorhexidine-releasing epoxy-based coating on titanium implants prevents Staphylococcus aureus experimental biomaterial-associated infection. Eur. Cell. Mater. 2017;33:143–157. doi: 10.22203/eCM.v033a11. [DOI] [PubMed] [Google Scholar]
- 30.Percie du Sert N., et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. J. Physiol. 2020;105:1459–1466. doi: 10.1113/EP088870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.ARRIVE . 2021. The ARRIVE Essential 10: Compliance Questionnaire; p. 2. [Google Scholar]
- 32.van Zutphen, L.F.M., Chapter 12 Design of animal experiments, in Principles of Laboratory Animal Science - Revised Edition. Elsevier: Amsterdam. p. 229.
- 33.RANDOM.ORG Random Sequence Generator. 1998-2021. https://www.random.org/sequences/ [cited 2020; Available from:
- 34.Li D., et al. Quantitative mouse model of implant-associated osteomyelitis and the kinetics of microbial growth, osteolysis, and humoral immunity. J. Orthop. Res. 2008;26(1):96–105. doi: 10.1002/jor.20452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dijksteel G.S., et al. SPS-neutralization in tissue samples for efficacy testing of antimicrobial peptides. BMC Infect. Dis. 2019;19(1):1093. doi: 10.1186/s12879-019-4700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keane T.J., et al. Preparation and characterization of a biologic scaffold from esophageal mucosa. Biomaterials. 2013;34(28):6729–6737. doi: 10.1016/j.biomaterials.2013.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hympanova L., et al. Eur Urol Focus; 2018. Assessment of Electrospun and Ultra-lightweight Polypropylene Meshes in the Sheep Model for Vaginal Surgery. [DOI] [PubMed] [Google Scholar]
- 38.Manfredo Vieira S., et al. Translocation of a gut pathobiont drives autoimmunity in mice and humans. Science. 2018;359(6380):1156–1161. doi: 10.1126/science.aar7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Devriese L.A., et al. Characterization and identification of Enterococcus species isolated from the intestines of animals. Int. J. Syst. Bacteriol. 1987;37(3):3. [Google Scholar]
- 40.Moriarty F., Zaat S.A., Busscher H.J. 1 ed. Springer; 2012. Biomaterials Associated Infection: Immunological Aspects and Antimicrobial Strategies. [Google Scholar]
- 41.Pineda Molina C., et al. 4-Hydroxybutyrate promotes endogenous antimicrobial peptide expression in macrophages. Tissue Eng. 2019;25(9–10):693–706. doi: 10.1089/ten.TEA.2018.0377. [DOI] [PubMed] [Google Scholar]
- 42.Hympánová L., et al. Assessment of electrospun and ultra-lightweight polypropylene meshes in the sheep model for vaginal surgery. Eur. Urol. Focus. 2020;6(1):190–198. doi: 10.1016/j.euf.2018.07.024. [DOI] [PubMed] [Google Scholar]
- 43.Huffaker R.K., et al. Histologic response of porcine collagen-coated and uncoated polypropylene grafts in a rabbit vagina model. Am. J. Obstet. Gynecol. 2008;198(5):582.e1–582.e7. doi: 10.1016/j.ajog.2007.12.029. [DOI] [PubMed] [Google Scholar]
- 44.Lo T.S., et al. The immunohistochemical and urodynamic evaluation towards the collagen-coated and non-coated polypropylene meshes implanted in the pelvic wall of the rats. Sci. Rep. 2016;6:38960. doi: 10.1038/srep38960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elci G.G., et al. Histopathological and mechanical properties of different meshes in a rat model of pelvic prolapse surgery. E. J. Med. 2017;22(2):45–52. [Google Scholar]
- 46.Verhorstert K.W.J., et al. Animal experimental research assessing urogynecologic surgical mesh implants: outcome measures describing the host response, a systematic review and meta-analysis. Neurourol. Urodyn. 2021;40(5):1107–1119. doi: 10.1002/nau.24677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patel H., Ostergard D.R., Sternschuss G. Polypropylene mesh and the host response. Int Urogynecol J. 2012;23(6):669–679. doi: 10.1007/s00192-012-1718-y. [DOI] [PubMed] [Google Scholar]
- 48.O’Shaughnessy D., et al. Evaluation of the histological and biomechanical properties of poly-4-hydroxybutyrate scaffold for pelvic organ prolapse, compared with polypropylene mesh in a rabbit model. Int Urogynecol J. 2021 doi: 10.1113/EP088870. [DOI] [PubMed] [Google Scholar]
- 49.Roy S., et al. Staphylococcus aureus biofilm infection compromises wound healing by causing deficiencies in granulation tissue collagen. Ann. Surg. 2020;271(6):1174–1185. doi: 10.1097/SLA.0000000000003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mellano E.M., et al. The role of chronic mesh infection in delayed-onset vaginal mesh complications or recurrent urinary tract infections: results from explanted mesh cultures. Female Pelvic Med. Reconstr. Surg. 2016;22(3):166–171. doi: 10.1097/SPV.0000000000000246. [DOI] [PubMed] [Google Scholar]
- 51.Mamy L., et al. Correlation between shrinkage and infection of implanted synthetic meshes using an animal model of mesh infection. Int Urogynecol J. 2011;22(1):47–52. doi: 10.1007/s00192-010-1245-7. [DOI] [PubMed] [Google Scholar]
- 52.Busscher H.J., et al. Biomaterial-associated infection: locating the finish line in the race for the surface. Sci. Transl. Med. 2012;4(153):153rv10. doi: 10.1126/scitranslmed.3004528. [DOI] [PubMed] [Google Scholar]
- 53.Guillaume O., et al. A critical review of the in vitro and in vivo models for the evaluation of anti-infective meshes. Hernia. 2018;22(6):961–974. doi: 10.1007/s10029-018-1807-z. [DOI] [PubMed] [Google Scholar]
- 54.Boelens J.J., et al. Subcutaneous abscess formation around catheters induced by viable and nonviable Staphylococcus epidermidis as well as by small amounts of bacterial cell wall components. J. Biomed. Mater. Res. 2000;50(4):546–556. doi: 10.1002/(sici)1097-4636(20000615)50:4<546::aid-jbm10>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 55.Christensen G.D., et al. Experimental foreign body infections in mice challenged with slime-producing Staphylococcus epidermidis. Infect. Immun. 1983;40(1):407–410. doi: 10.1128/iai.40.1.407-410.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Urbankova I., et al. Transvaginal mesh insertion in the ovine model. JoVE. 2017;125(7):27. doi: 10.3791/55706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aghaei-Ghareh-Bolagh B., et al. A novel tropoelastin-based resorbable surgical mesh for pelvic organ prolapse repair. Mater. Today Biol. 2020;8:100081. doi: 10.1016/j.mtbio.2020.100081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lai C., et al. Development of a cellulose-based prosthetic mesh for pelvic organ prolapse treatment: in vivo long-term evaluation in an Ewe vagina model. Mater. Today Biol. 2021;12:100172. doi: 10.1016/j.mtbio.2021.100172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.