Significance Statement
Recent research suggests that biomarkers of the TNF pathway (TNFR1 and TNFR2) are associated with worse kidney outcomes. Most of these studies, however, evaluated baseline levels rather than longitudinal changes. In two cohorts (AASK, which enrolled Black people with CKD attributed to hypertension, and VA NEPHRON-D, which enrolled veterans with albuminuric CKD and type 2 diabetes), greater longitudinal increases in serum or plasma TNFR1 and TNFR2 were associated with higher risks of ESKD in AASK and subsequent kidney function decline in VA NEPHRON-D. These associations were independent of baseline biomarker level and kidney function. Longitudinal trajectories in TNFR1 and TNFR2 may ultimately allow improved risk assessment for kidney failure in persons with CKD.
Keywords: AASK (African American Study of Kidney Disease and Hypertension), chronic kidney disease, diabetes mellitus, end-stage kidney disease, renal function decline, chronic inflammation
Abstract
Background
Higher baseline levels of soluble TNF receptors (TNFR1 and TNFR2) have been associated with progressive CKD. Whether longitudinal changes in these biomarkers of inflammation are also associated with worse kidney outcomes has been less studied.
Methods
We evaluated associations of longitudinal changes in TNFR1 and TNFR2 with ESKD in the African American Study of Kidney Disease and Hypertension (AASK; 38% female; 0% diabetes) and kidney function decline (first occurrence of ≥30 ml/min per 1.73 m2 or ≥50% eGFR decline if randomization eGFR ≥60 or <60 ml/min per 1.73 m2, respectively; ESKD) in the Veterans Affairs Nephropathy in Diabetes trial (VA NEPHRON-D; 99% male; 100% diabetes) using Cox models. Biomarkers were measured from samples collected at 0-, 12-, and 24-month visits for AASK (serum) and 0- and 12-month visits for VA NEPHRON-D (plasma). Biomarker slopes (AASK) were estimated using linear mixed-effects models. Covariates included sociodemographic/clinical factors, baseline biomarker level, and kidney function.
Results
There were 129 ESKD events over a median of 7.0 years in AASK (n=418) and 118 kidney function decline events over a median of 1.5 years in VA NEPHRON-D (n=754). In AASK, each 1 SD increase in TNFR1 and TNFR2 slope was associated with 2.98- and 1.87-fold higher risks of ESKD, respectively. In VA NEPHRON-D, each 1 SD increase in TNFR1 and TNFR2 was associated with 3.20- and 1.43-fold higher risks of kidney function decline, respectively.
Conclusions
Among individuals with and without diabetes, longitudinal increases in TNFR1 and TNFR2 were each associated with progressive CKD, independent of initial biomarker level and kidney function.
CKD is an urgent public health issue that afflicts an estimated 37 million adults in the United States.1–4 Diagnosis and classification of CKD are primarily on the basis of GFR and urine albumin-creatinine ratio (UACR), with more severe “G” and “A” stages being associated with increased morbidity and mortality.5–7 Creatinine and cystatin C are the most common biomarkers used in the estimation of GFR; however, both have non-GFR determinants. Moreover, albuminuria is not a feature of all types of kidney diseases and, among patients with albuminuria, the within-person variability of UACR is high.8 Additional biomarkers are therefore needed in the study of progressive CKD.9
The soluble TNF receptors 1 and 2 (TNFR1 and TNFR2) are promising biomarkers of inflammation, a process that is essential to the pathogenesis of many types of kidney disease. In multiple cohorts, higher baseline levels of TNFR1 and TNFR2 have been associated with increased risk of incident CKD,10,11 progressive CKD,11,12 and ESKD.12–14 We previously reported in the African American Study and Kidney Disease and Hypertension (AASK) and Veterans Affairs Nephropathy in Diabetes (VA NEPHRON-D) trial that each two-fold higher baseline concentration of serum/plasma TNFR1 and TNFR2 was independently associated with 2.0–3.7-fold greater risks of ESKD12 and kidney function decline,11 respectively. Changes in these biomarkers may therefore help to identify individuals at greatest risk of disease progression.
In this study, we evaluated whether longitudinal changes in TNFR1 and TNFR2 were associated with subsequent risk of ESKD (AASK) and kidney function decline (VA NEPHRON-D). We also considered the effects of trial interventions (BP control and drug) on TNFR1 and TNFR2 changes.
Methods
AASK Study Population
AASK was a multicenter, 3 × 2 factorial trial that randomized self-reported Black adults with CKD attributed to hypertension to BP drug (ramipril, metoprolol, or amlodipine) and BP goal (MAP ≤92 mmHg or MAP 102–107 mmHg).15 At the end of the trial phase, 691 participants who had not yet developed ESKD continued with the cohort phase, during which they received ramipril and had a common BP goal of <140/90 mmHg (lowered to <130/80 mmHg after 2004).16 Inclusion criteria for the trial included an age of 18–70 years, diastolic BP >95 mmHg, and GFR of 20–65 ml/min per 1.73 m2 as measured by 125I-iothalamate clearance. Major exclusion criteria included history of diabetes mellitus, urine protein-creatinine ratio (UPCR) >2.5 g/g Cr, malignant or secondary hypertension, serious systemic disease, congestive heart failure, or contraindication to one of the study drugs.15 Informed consent was obtained from participants and protocols for both phases were approved by institutional review boards at each study site. Further details regarding the AASK study design have been reported.15,16
Among the 1094 AASK trial participants, 500 had stored serum samples from the 0-month visit, of whom 435 had at least one additional sample available from the 12- and/or 24-month visits (74.5% with samples at all three time points; 16.3% with samples at 0- and 12-month visit; 9.2% with samples at 0 and 24 months). In our primary analyses, wherein follow-up started at the 24-month visit, we excluded 17 participants who developed ESKD or died within the first 24 months of the trial, resulting in a final study population of 418 participants (Supplemental Figure 1).
VA NEPHRON-D Study Population
VA NEPHRON-D was a multicenter, double-blinded trial of veterans, aged ≥18 years, with type 2 diabetes and CKD G2–3b/A3 (eGFR 30 to <90 ml/min per 1.73 m2 as estimated by the four-variable Modification of Diet in Renal Disease formula plus a UACR ≥300 mg/g).,17,24 Data on race and sex were on the basis of self-report and collected as part of the original trial protocol. If more than one race was reported, then the first response was used. Participants were randomized to receive either losartan monotherapy or losartan in combination with lisinopril, and followed for subsequent kidney function decline. Major exclusion criteria included suspected nondiabetic kidney disease, serum potassium >5.5 mmol/L, or need for sodium polystyrene sulfonate, hemoglobin A1c >10.5%, BP >180/90 mmHg, or a history of intolerance to one of the study drugs. All participants provided informed consent and protocols were approved by the institutional review board of each study site.17
Among the 1448 VA NEPHRON-D trial participants, 759 had stored plasma samples available from both the 0- and 12-month visits. The start of follow-up for our analyses was the 12-month visit so we excluded five participants who developed kidney function decline before this time point. Our final study population consisted of 754 participants (Supplemental Figure 1).
Biomarker Measurements
Biomarkers were measured on a 2-plex plate using the Mesoscale Discovery Platform (Meso Scale Diagnostics; Rockville, Maryland) from stored serum samples collected at the 0-, 12-, and 24-month visits of the AASK trial, and plasma samples collected at the 0- and 12-month visits of VA NEPHRON-D. To detect any potential run-to-run bias, two quality control samples were assayed in duplicate on every plate. Biomarker levels for each quality control sample were tracked via Levy-Jennings plots and run acceptance was on the basis of adherence to Westgard 22s and 13s quality control rules. In AASK, interassay coefficients of variation, on the basis of 6% duplicate samples, were 3.33% for TNFR1 and 2.96% for TNFR2. In VA NEPHRON-D, measurements for the 12-month visit were completed in two batches, and a lot-to-lot bridging experiment was conducted to ensure consistent values across batches. For the bridging experiment, a linear regression model was fitted and applied to the second batch of measurements. The interassay coefficients of variation were 16.7% for TNFR1 and 10.8% for TNFR2.
Outcomes
For AASK, the primary outcome was incident ESKD, defined as initiation of chronic dialysis or kidney transplantation. For VA NEPHRON-D, the outcome of interest was kidney function decline, defined as the first occurrence of an absolute eGFR decrease of ≥30 ml/min per 1.73 m2 if eGFR at randomization was ≥60 ml/min per 1.73 m2, a relative decrease in eGFR by ≥50% if eGFR at randomization was <60 ml/min per 1.73 m2 or ESKD (i.e., initiation of maintenance dialysis, kidney transplant, or eGFR <15 ml/min per 1.73 m2).
Covariates
In AASK, GFR was directly measured by 125I iothalamate clearance at baseline (twice), 3 months, 6 months, and every 6 months thereafter. BP was measured using a Hawksley random zero sphygmomanometer with participants in a seated position and after ≥5 minutes of rest. The last two of three BP readings were then averaged.15 UPCR was measured from 24-hour urine collections at a central laboratory using the pyrogallol red method (for protein) and modified Jaffe reaction (for creatinine).18–20 Genotyping for the APOL1 G1 (rs73885319, rs60910145) and G2 (rs71785313) risk variants had previously been performed using ABI Taqman assays.21 APOL1 high-risk status was defined as having two copies of the risk variants, whereas low-risk status was defined as having one or no copy.21–23 Global ancestry was on the basis of 140 ancestry informative markers and estimated using ANCESTRYMAP.21
In VA NEPHRON-D, serum creatinine was measured every 3 months by the Siemens Dimension RxL chemistry system at a central laboratory (University of Maryland; Baltimore, Maryland), and eGFR was estimated using the four-variable Modification of Diet in Renal Disease formula for isotope dilution mass spectrometry traceable creatinine.17,24 BP was measured with participants in the seated position after ≥5 minutes of rest. Two readings were taken at intervals ≥1 minute apart and the average was used. If there was a >5 mmHg difference (systolic and/or diastolic) between the first two readings, a third reading was obtained and the median used. Hemoglobin A1c and UACR were measured locally.17
Statistical Analyses
AASK
We compared baseline characteristics by tertiles of biomarker slopes using linear, logistic, and multinomial logistic regression. Slopes of TNFR1 and TNFR2 were determined by first natural log-transforming each biomarker to achieve a more normal distribution. We then constructed linear mixed-effects models with the log-transformed biomarker as the dependent variable and time in years as the independent variable, using an unstructured covariance with maximum likelihood estimation and allowing for random intercepts and random slopes. Distributions of biomarker slopes and baseline biomarker levels by outcome of ESKD were visually compared with kernel density plots. To investigate the association of biomarker slopes with time to ESKD (using data from both the trial and cohort phases), a hierarchy of Cox proportional hazards models was used: (1) unadjusted; (2) adjusted for baseline (i.e., 0-month visit) natural log-transformed biomarker; (3) further adjusted for baseline sociodemographic and clinical factors (i.e., age, sex, systolic BP, body mass index, and current smoking); and (4) further adjusted for baseline kidney function measures (i.e., GFR and natural log-transformed UPCR), which we considered as the primary model. In sensitivity analyses, two additional models were constructed: (1) the primary model further adjusted for GFR slope; and (2) the primary model further adjusted for randomized BP drug and randomized BP goal. Annual GFR slope from 0 to 24 months of the AASK trial was estimated via linear mixed-effects models with random intercepts and random slopes. In these analyses, biomarker levels, biomarker slopes, and GFR slope were scaled to per SD change to facilitate comparison across variables. Effect modification by APOL1 high-risk status was explored by introducing an interaction term between APOL1 risk status and biomarker slope in the primary model. We then used Harrell’s C Statistic (to evaluate whether biomarker slope provided incremental discrimination for the risk of ESKD), calibration plots (to assess observed versus predicted probabilities within predicted deciles of risk for ESKD), and Brier scores (to quantify the calibration of these predictions) to compare the following models: (1) clinical model alone; (2) clinical model + baseline biomarker; (3) clinical model + baseline biomarker + biomarker slope. We also repeated the primary model accounting for the competing risk of death by the method of Fine and Gray.25
In additional analyses, we considered categories of biomarker change. The four categories (persistently low, decreasing, increasing, and persistently elevated) were defined by whether biomarker levels at the 0- and 24-month (or 12 months, if not available) visits were > or ≤ the 75th percentile for the biomarker level at 0 months. For example, the 75th percentile for TNFR1 at 0 months was 3722 pg/ml. If a participant’s TNFR1 value was below this threshold at both 0 and 24 months, then they would be categorized as persistently low. Conversely, if a participant’s TNFR1 value at 0 months was below 3722 pg/ml but value at 24 months was above 3722 pg/ml, then they would be categorized as increasing. Associations of these categories of biomarker change with incident ESKD were assessed by Cox proportional hazards models.
Finally, we evaluated whether randomized treatment groups were associated with biomarker change over time. For these analyses, linear regression models were used with randomized BP goal or drug as the exposure of interest and TNFR1 or TNFR2 slope as the outcome. Covariates included natural log-transformed biomarker, age, sex, systolic BP, body mass index, current smoking, GFR, and natural log-transformed UPCR at 0 months.
Analyses were performed using Stata 15.1 software (StataCorp LLC; College Station, Texas).
VA NEPHRON-D
Baseline characteristics were compared by linear, logistic, and multinomial logistic regression. Biomarker change was determined by first natural log-transforming each biomarker measurement, then calculating the absolute difference between the two visits (i.e., ln[12-month biomarker] − ln[0-month biomarker]), and finally scaling to per SD change. Distributions of biomarker change and baseline biomarker levels by outcome of kidney function decline were assessed using kernel density plots. Similar to our approach in AASK, we constructed a series of Cox proportional hazards models to evaluate the association of biomarker change with time to kidney function decline, but with a few differences: (1) on the basis of evidence of nonlinearity of TNFR1 and TNFR2 change with the outcome, a spline with knot at 0 was introduced to the models for both biomarkers; (2) we adjusted for the additional confounders of race and hemoglobin A1c; (3) we adjusted for estimated rather than measured GFR and for natural log-transformed UACR rather than UPCR; and (4) in sensitivity analyses, we adjusted for absolute eGFR change from 0 to 12 months, rather than GFR slope. Hemoglobin A1c and eGFR change were not available for seven and 29 participants, respectively: we performed complete case analysis. We also compared models using Harrell’s C Statistic, calibration plots, and Brier scores. In sensitivity analyses, we considered ESKD as a secondary outcome and death as a competing risk.
As in AASK, we considered categories of TNFR1 and TNFR2 change as exposures of interest. We also evaluated associations of the randomized intervention (losartan + lisinopril versus losartan monotherapy) with biomarker change, using linear regression models and adjusting for natural log-transformed biomarker, age, sex, race, systolic BP, body mass index, smoking, hemoglobin A1c, eGFR, and natural log-transformed UACR at 0 months. Analyses were performed using SAS version 9.4 (SAS Institute) and STATA 17 (StataCorp LLC; College Station, Texas).
Results
AASK
Baseline Characteristics
Among the 418 AASK trial participants included in our primary analyses, the mean age was 54 years and 38% were female. At baseline, participants in the highest tertile of TNFR1 slope were younger, had lower mean GFR, higher median biomarker level, and higher median UPCR compared with lower tertiles. Similar trends were noted when comparing tertiles of TNFR2 slope, with the additional observation that the highest tertile also had a higher mean systolic BP (Table 1). Among the 307 participants with genotyping for APOL1, a higher proportion of individuals with the high-risk genotype were in the top tertile of TNFR1 and TNFR2 slopes (P=0.002 and P=0.002, respectively). Participants included in our primary analyses were less likely to smoke compared with those who were excluded (n=676: 659 missing biospecimens and 17 with events before 24 months; Supplemental Table 1).
Table 1.
Baseline characteristics by tertiles of TNFR1 and TNFR2 slopes in AASK
| Characteristic | Tertile 1 (n=140) | Tertile 2 (n=139) | Tertile 3 (n=139) | P Value |
|---|---|---|---|---|
| TNFR1 slope | ||||
| Range, % change per year | −10.5%–0.3% | 0.4%–6.3% | 6.4%–57.6% | — |
| Age, yrs | 55.3±10.1 | 55.6±9.6 | 51.5±11.8 | 0.003 |
| Female | 54 (39%) | 55 (40%) | 51 (37%) | 0.75 |
| European ancestry, % | 14.4±12.4 | 17.4±13.5 | 17.3±12.4 | 0.11 |
| APOL1 | ||||
| Low risk | 84 (83%) | 81 (79%) | 66 (64%) | 0.002 |
| High risk | 17 (17%) | 22 (21%) | 37 (36%) | |
| Systolic BP, mmHg | 148±24 | 152±26 | 153±24 | 0.08 |
| Body mass index, kg/m2 | 30.5±6.1 | 31.0±6.4 | 31.5±7.1 | 0.19 |
| Smoking | 33 (24%) | 38 (27%) | 35 (25%) | 0.76 |
| GFR, ml/min per 1.73 m2 | 51.3±9.8 | 46.7±11.1 | 38.6±12.3 | <0.001 |
| UPCR, g/g Cr | 0.04 (0.02, 0.10) | 0.06 (0.03, 0.26) | 0.27 (0.09, 0.81) | <0.001 |
| TNFR1 | ||||
| At 0 months, pg/ml | 2288 (1954, 2961) | 2583 (2086, 3424) | 3509 (2872, 4459) | <0.001 |
| Last available, pg/ml | 2011 (1762, 2504) | 2699 (2322, 3481) | 4497 (3712, 6407) | <0.001 |
| TNFR2 | ||||
| At 0 months, pg/ml | 10288 (8424, 13182) | 11753 (9177, 15623) | 15397 (12621, 20234) | <0.001 |
| Slope, % change per year | −2.41±2.68 | 1.99±2.71 | 9.68±6.14 | <0.001 |
| BP goal | ||||
| MAP ≤92, mmHg | 72 (51%) | 71 (51%) | 68 (49%) | 0.68 |
| MAP 102–107, mmHg | 68 (49%) | 68 (49%) | 71 (51%) | |
| BP drug | ||||
| Ramipril | 58 (41%) | 53 (38%) | 52 (37%) | Ref |
| Metoprolol | 59 (42%) | 59 (42%) | 56 (40%) | 0.83 |
| Amlodipine | 23 (16%) | 27 (19%) | 31 (22%) | 0.22 |
| TNFR2 slope | ||||
| Range, % change per year | −15.6% to -0.3% | −0.3%–4.8% | 4.9%–45.6% | — |
| Age, years | 56.3±9.5 | 53.6±11.0 | 52.6±11.2 | 0.004 |
| Female | 51 (36%) | 54 (39%) | 55 (40%) | 0.59 |
| European ancestry, % | 15.2±12.8 | 16.7±12.6 | 17.1±13.1 | 0.31 |
| APOL1 | ||||
| Low risk | 82 (85%) | 82 (75%) | 67 (66%) | 0.002 |
| High risk | 14 (15%) | 28 (25%) | 34 (34%) | |
| Systolic BP, mmHg | 147±25 | 152±24 | 155±25 | 0.01 |
| Body mass index, kg/m2 | 30.5±6.2 | 30.9±6.3 | 31.7±7.1 | 0.14 |
| Smoking | 34 (24%) | 33 (24%) | 39 (28%) | 0.47 |
| GFR, ml/min per 1.73 m2 | 51.7±9.5 | 45.5±11.0 | 39.3±12.9 | <0.001 |
| UPCR, g/g Cr | 0.04 (0.02, 0.08) | 0.08 (0.03, 0.26) | 0.26 (0.08, 0.78) | <0.001 |
| TNFR1 | ||||
| At 0 months, pg/ml | 2291 (1896, 2978) | 2730 (2157, 3582) | 3343 (2768, 4444) | <0.001 |
| Slope, % change per year | −2.80±3.37 | 2.94±3.44 | 13.48±8.26 | <0.001 |
| TNFR2 | ||||
| At 0 months, pg/ml | 10014 (7909, 13182) | 11986 (9332, 15623) | 14913 (11810, 20579) | <0.001 |
| Last available, pg/ml | 8805 (7218, 10980) | 12289 (10045, 15505) | 19643 (15550, 27734) | <0.001 |
| BP goal | ||||
| MAP ≤92 mmHg | 74 (53%) | 66 (47%) | 71 (51%) | 0.77 |
| MAP 102–107 mmHg | 66 (47%) | 73 (53%) | 68 (49%) | |
| BP drug | ||||
| Ramipril | 59 (42%) | 54 (39%) | 50 (36%) | Ref |
| Metoprolol | 58 (41%) | 55 (40%) | 61 (44%) | 0.42 |
| Amlodipine | 23 (16%) | 30 (22%) | 28 (20%) | 0.29 |
Data presented as mean±SD, number (%), or median (interquartile range). P values presented are for trend. For UPCR, P value was determined from the natural-log transformed value. Data not available for APOL1 risk status and % European ancestry in 111 participants. APOL1, apolipoprotein L1; MAP, mean arterial pressure.
Biomarker Correlations
There was a strong positive correlation between TNFR1 and TNFR2 slopes (Pearson’s correlation [r]=0.92). TNFR1 slope was positively correlated with initial TNFR1 level (r=0.44) and negatively correlated with initial GFR (r=−0.44) and GFR slope (r=−0.68). TNFR2 slope was also positively correlated with initial TNFR2 level (r=0.40) and negatively correlated with initial GFR (r=−0.42) and GFR slope (r=−0.61; Supplemental Figure 2). In total, 75% of participants with a positive TNFR1 slope had a negative GFR slope. Similarly, 73% of participants with a positive TNFR2 slope had a negative GFR slope (Supplemental Table 2).
Biomarker Slopes and ESKD
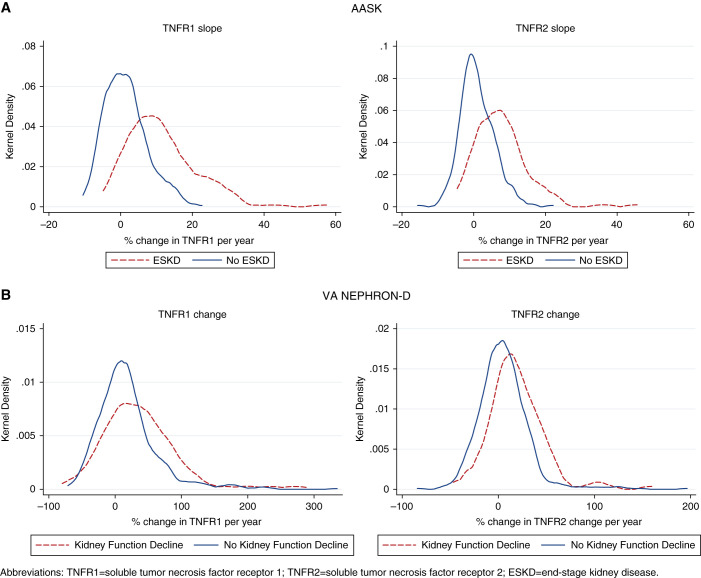
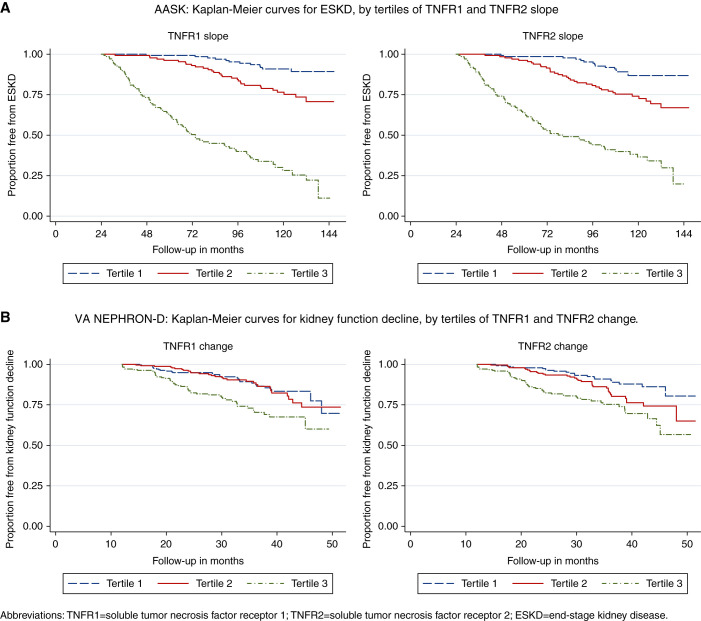
Mean TNFR1 and TNFR2 slopes were a 4.5% and 3.1% change per year, respectively. Over a median follow-up of 7.0 years, 129 (31%) participants developed ESKD. Among participants who developed ESKD, 93% and 88% had positive TNFR1 and TNFR2 slopes over the first 2 years; among participants who did not develop ESKD, the proportion with positive slopes was lower, at 58% and 54% (Figure 1). Overall, participants within higher tertiles of TNFR1 and TNFR2 slopes were more likely to develop ESKD over time (Figure 2). Participants developing ESKD had higher baseline biomarker levels on average (Supplemental Figure 3).
Figure 1.
Distributions of TNFR1 and TNFR2 change by outcome of ESKD in AASK and kidney function decline in VA NEPHRON-D.
Figure 2.
Kaplan–Meier curves for ESKD in AASK and kidney function decline in VA NEPHRON-D, by tertiles of TNFR1 and TNFR2 change. In both cohorts, higher tertiles of TNFR1 and TNFR2 change were associated with greater risk of ESKD and kidney function decline.
In unadjusted analyses, each 1 SD increase in TNFR1 slope was associated with a 3.6-fold greater risk of incident ESKD (hazard ratio [HR], 3.61; 95% confidence interval [95% CI], 3.05 to 4.26). After adjustment for biomarker level, sociodemographic and clinical factors, and kidney function measures at 0 months, the association of TNFR1 slope with ESKD remained statistically significant (HR,2.98; 95% CI, 2.41 to 3.68). For TNFR2 slope, each 1 SD increase was associated with 2.52- and 1.87-fold greater risks of ESKD in unadjusted (95% CI, 2.21 to 2.87) and fully adjusted models (95% CI, 1.57 to 2.23), respectively. In sensitivity analyses, further adjustment for GFR slope or randomized treatment groups did not change overall associations (Table 2). Compared with a clinical model, the addition of baseline biomarker and biomarker slope significantly improved the C Statistic, whereas the addition of baseline biomarker alone did not (Supplemental Table 3). Calibration was relatively similar across models (Supplemental Figure 4). Similar conclusions were obtained when accounting for the competing risk of death (subHR [sHR], 2.66; 95% CI, 2.14 to 3.30 for TNFR1 and sHR, 1.77; 95% CI, 1.45 to 2.16 for TNFR2).
Table 2.
Associations of TNFR1 and TNFR2 slopes, TNFR1 and TNFR2 levels at 0 months, and GFR slope with ESKD in AASK
| Model | Biomarker Slope (per 1 SD increase) | Biomarker Level at t0 (per 1 SD Higher) | GFR Slope (per 1 SD Decrease) | |||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| TNFR1 slope | ||||||
| Unadjusted | 3.61 | 3.05 to 4.26 | — | — | — | — |
| + ln(biomarker) | 3.26 | 2.70 to 3.94 | 2.33 | 1.94 to 2.80 | — | — |
| + age, sex, SBP, BMI, smoking | 3.22 | 2.65 to 3.91 | 2.28 | 1.89 to 2.76 | — | — |
| + GFR, ln(UPCR) | 2.98 | 2.41 to 3.68 | 2.20 | 1.64 to 2.94 | — | — |
| + GFR slope | 2.11 | 1.62 to 2.74 | 1.73 | 1.24 to 2.41 | 2.04 | 1.47 to 2.85 |
| + randomized treatment groups | 2.96 | 2.38 to 3.67 | 2.29 | 1.70 to 3.09 | — | — |
| TNFR2 slope | ||||||
| Unadjusted | 2.52 | 2.21 to 2.87 | — | — | — | — |
| + ln(biomarker) | 2.21 | 1.91 to 2.56 | 2.10 | 1.78 to 2.47 | — | — |
| + age, sex, SBP, BMI, smoking | 2.19 | 1.86 to 2.56 | 2.13 | 1.77 to 2.55 | — | — |
| + GFR, ln(UPCR) | 1.87 | 1.57 to 2.23 | 1.64 | 1.29 to 2.08 | — | — |
| + GFR slope | 1.40 | 1.15 to 1.70 | 1.27 | 0.97 to 1.67 | 2.91 | 2.14 to 3.95 |
| + randomized treatment groups | 1.90 | 1.58 to 2.28 | 1.74 | 1.36 to 2.22 | — | — |
All covariates measured at 0 months except GFR slope, which was measured from 0 to 24 months. n=418 for all models. SD for TNFR1 slope=0.08, TNFR1 at t0=0.37, TNFR2 slope=0.06, TNFR2 at t0=0.40, and GFR slope=3.21. t0=0-month visit; SBP, systolic BP; BMI, body mass index.
When considering categories of biomarker change, participants with decreasing, increasing, and persistently elevated TNFR1 levels were significantly more likely to develop ESKD compared with participants with persistently low TNFR1 levels (HR, 3.48, 6.47, and 7.34, respectively). In addition, participants with increasing and persistently elevated TNFR2 levels had 5.31- and 3.85-fold greater risks of incident ESKD, respectively, compared with participants with persistently low TNFR2 levels (Table 3).
Table 3.
Associations of categories of TNFR1 and TNFR2 change with ESKD in AASK
| Biomarker category | # events/# at risk | Unadjusted | Adjusteda | ||
|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | ||
| TNFR1 | |||||
| Persistently low (low–low) | 29/275 | Ref | Ref | Ref | Ref |
| Decreasing (high–low)b | 7/14 | 5.74 | 2.51 to 13.14 | 3.48 | 1.46 to 8.30 |
| Increasing (low–high) | 26/39 | 13.06 | 7.62 to 22.39 | 6.47 | 3.58 to 11.67 |
| Persistently elevated (high–high) | 67/90 | 17.39 | 11.08 to 27.29 | 7.34 | 4.03 to 13.38 |
| TNFR2 | |||||
| Persistently low (low–low) | 37/272 | Ref | Ref | Ref | Ref |
| Decreasing (high–low)b | 7/21 | 2.69 | 1.20 to 6.05 | 1.67 | 0.74 to 3.77 |
| Increasing (low–high) | 28/42 | 10.54 | 6.38 to 17.41 | 5.31 | 3.11 to 9.06 |
| Persistently elevated (high–high) | 57/83 | 10.94 | 7.16 to 16.72 | 3.85 | 2.29 to 6.45 |
Ref, reference.
Adjusted for age, sex, systolic BP, body mass index, smoking, glomerular filtration rate, and ln(UPCR) at 0 months. Categories defined by whether biomarker levels at 0 and 24 (or 12 if not available) months were > or ≤ the 75th percentile for the biomarker level at 0 months (persistently low: ≤ at both 0 and 24 (or 12 if not available) months; decreasing: > at 0 months and ≤ at 24 (or 12 if not available) months; increasing: ≤ at 0 months and > at 24 (or 12 if not available) months; persistently elevated: > at both 0 and 24 (or 12 if not available) months.
In the adjusted model, when the decreasing category was used as the reference, the increasing and persistently elevated categories were worse for TNFR2 (adjusted HR 3.19 [95% CI, 1.35 to 7.50] and adjusted HR 2.31 [95% CI, 1.00 to 5.34], respectively) but not TNFR1.
Interactions with APOL1 Risk Variants
Among the 307 participants with APOL1 genotyping, APOL1 high-risk status (n=76; 25%) did not modify the association of either TNFR1 or TNFR2 slope with ESKD (P interactions 0.24 and 0.38, respectively).
Randomized Treatment Groups and Biomarker Slopes
The number of participants randomized to each treatment group (goal and drug) was similar across tertiles of TNFR1 and TNFR2 slopes (Table 1). Participants randomized to the amlodipine group had a 1.91% (95% CI, 0.10 to 3.75) higher TNFR1 slope compared with those who were randomized to the ramipril group. In contrast, there was no significant difference in TNFR1 slopes between the metoprolol versus ramipril groups or standard versus intensive therapy. Neither BP drug nor goal were associated with TNFR2 slopes (Table 4).
Table 4.
Associations of randomized treatment groups with TNFR1 and TNFR2 slopes in AASK (n=435) and changes in VA NEPHRON-D (n=759)
| AASK Randomized Treatment Groups | Unadjusted (n=435) | Adjusted for Biomarker Level at t0a (n=435) | Fully Adjusted Modelb (n=435) |
|---|---|---|---|
| % Change (95% CI) | % Change (95% CI) | % Change (95% CI) | |
| TNFR1 slope | |||
| BP goal | |||
| Intensive | Ref | Ref | Ref |
| Standard | −0.41 (−1.98 to 1.19) | −0.65 (−2.02 to 0.73) | −0.68 (−1.98 to 0.63) |
| BP drug | |||
| Ramipril | Ref | Ref | Ref |
| Metoprolol | 1.07 (−0.71 to 2.88) | 1.33 (−0.22 to 2.91) | 1.16 (−0.29 to 2.64) |
| Amlodipine | 1.55 (−0.66 to 3.80) | 1.61 (−0.32 to 3.57) | 1.91 (0.10 to 3.75) |
| TNFR2 slope | |||
| BP goal | |||
| Intensive | Ref | Ref | Ref |
| Standard | −0.27 (−1.45 to 0.92) | −0.39 (−1.45 to 0.67) | −0.38 (−1.37 to 0.62) |
| BP drug | |||
| Ramipril | Ref | Ref | Ref |
| Metoprolol | 0.73 (−0.59 to 2.07) | 0.88 (−0.31 to 2.09) | 0.83 (−0.29 to 1.95) |
| Amlodipine | 0.67 (−0.96 to 2.33) | 0.87 (−0.60 to 2.36) | 0.93 (−0.44, 2.32) |
| VA NEPHRON-D Randomized Treatment Groups | Unadjusted (n=759) | Adjusted for biomarker level at t0a (n=759) | Fully adjusted modelc (n=747) |
| % change (95% CI) | % change (95% CI) | % change (95% CI) | |
| TNFR1 change | |||
| Losartan | Ref | Ref | Ref |
| Losartan + lisinopril | 4.02 (−1.42 to 9.77) | 3.78 (−1.31 to 9.13) | 2.01 (−2.60 to 6.84) |
| TNFR2 change | |||
| Losartan | Ref | Ref | Ref |
| Losartan + lisinopril | 1.24 (−2.32 to 4.93) | 1.21 (−2.32 to 4.85) | 0.78 (−2.63 to 4.31) |
% change determined by exponentiating β coefficients as follows: [eβ-1] x 100. Ref, reference.
Adjusted for ln(biomarker) at 0 months.
Adjusted for ln(biomarker), age, sex, systolic BP, body mass index, smoking, GFR, and ln(UPCR) at 0 months.
Adjusted for ln(biomarker), age, sex, race, systolic BP, body mass index, smoking, hemoglobin A1c, eGFR, and ln(UACR) at 0 months.
VA NEPHRON-D
Baseline Characteristics
Among the 754 VA NEPHRON-D participants included in our study, the mean age was 65 years, and 99% were male. Median UACR was higher among those in the highest tertile of each biomarker change. Mean body mass index was also higher in the highest tertile of TNFR2 change (Table 5). The study population was more likely to smoke and had slightly lower mean systolic BP and median UACR compared with participants who were not included (n=694: 689 with no available biospecimens and five with events before 12 months; Supplemental Table 1).
Table 5.
Baseline characteristics by tertiles of TNFR1 and TNFR2 change in VA NEPHRON-D
| Characteristic | Tertile 1 (n=251) | Tertile 2 (n=252) | Tertile 3 (n=251) | P Value |
|---|---|---|---|---|
| TNFR1 change | ||||
| Range, % change per year | −80% to -0.6% | −0.6%–30% | 30%–335% | — |
| Age, yrs | 64.7±7.5 | 65.1±7.8 | 64.7±7.5 | 0.96 |
| Male | 249 (99%) | 249 (99%) | 250 (100%) | 0.63 |
| Race | ||||
| Black | 54 (22%) | 54 (21%) | 54 (22%) | 0.99 |
| White | 188 (75%) | 189 (75%) | 184 (73%) | |
| Othera | 9 (4%) | 9 (4%) | 13 (5%) | |
| Systolic BP, mmHg | 135±17 | 136±15 | 137±16 | 0.14 |
| Body mass index, kg/m2 | 34.1±6.8 | 34.5±6.3 | 35.1±6.8 | 0.08 |
| Smoking | 50 (20%) | 55 (22%) | 47 (19%) | 0.68 |
| Hemoglobin A1c, % | 7.9±1.3 | 7.8±1.2 | 7.7±1.3 | 0.09 |
| eGFR, ml/min per 1.73 m2 | 56.5±17.9 | 57.2±19.6 | 56.1±19.0 | 0.81 |
| UACR, g/g Cr | 0.68 (0.37–1.37) | 0.76 (0.42, 1.48) | 1.00 (0.51, 2.04) | <0.001 |
| TNFR1 | ||||
| At 0 months, pg/ml | 4815 (3521, 6314) | 3986 (3154, 5529) | 3503 (2446, 4808) | <0.001 |
| At 12 months, pg/ml | 3606 (2718, 4988) | 4541 (3588, 6194) | 5674 (4067, 7798) | <0.001 |
| TNFR2 | ||||
| At 0 months, pg/ml | 11171 (8456, 13755) | 10272 (7942, 13453) | 10143 (7675, 12915) | 0.06 |
| Change, % change per year | −6.68±19.00 | 5.24±19.47 | 23.76±32.78 | <0.001 |
| Randomized group | ||||
| Losartan | 137 (55%) | 124 (49%) | 117 (47%) | 0.19 |
| Losartan + Lisinopril | 114 (45%) | 128 (51%) | 134 (53%) | |
| Tertile 1 (n=251) | Tertile 2 (n=252) | Tertile 3 (n=251) | P value | |
| TNFR2 change | ||||
| Range, % change per year | −84% to -3.8% | −3.8%–14.3% | 14.3%–196% | — |
| Age, years | 64.9±7.5 | 64.6±7.3 | 64.9±7.9 | 0.99 |
| Male | 248 (99%) | 252 (100%) | 248 (99%) | 0.99 |
| Race | ||||
| Black | 50 (20%) | 57 (23%) | 55 (22%) | 0.75 |
| White | 191 (76%) | 182 (72%) | 188 (75%) | |
| Othera | 10 (4%) | 13 (5%) | 8 (3%) | |
| Systolic BP, mmHg | 135±16 | 136±15 | 137±16 | 0.10 |
| Body mass index, kg/m2 | 33.9±6.6 | 34.3±6.4 | 35.5±6.9 | 0.01 |
| Smoking | 54 (22%) | 52 (21%) | 46 (18%) | 0.66 |
| Hemoglobin A1c, % | 7.8±1.3 | 7.7±1.3 | 7.8±1.3 | 0.80 |
| eGFR, ml/min per 1.73 m2 | 55.0±17.5 | 58.5±19.4 | 56.4±19.4 | 0.41 |
| UACR, g/g Cr | 0.66 (0.34, 1.29) | 0.76 (0.46, 1.51) | 1.02 (0.51, 2.20) | <0.001 |
| TNFR1 | ||||
| At 0 months, pg/ml | 4483 (3349, 6006) | 3740 (2908, 5153) | 4086 (3025, 5391) | 0.01 |
| Change, % change per year | −3.14±33.37 | 17.00±35.64 | 50.49±57.35 | <0.001 |
| TNFR2 | ||||
| At 0 months, pg/ml | 11218 (8792, 14347) | 9703 (7648, 12662) | 10273 (7944, 13249) | 0.01 |
| At 12 months, pg/ml | 9108 (7138, 12034) | 10235 (8046, 13319) | 13201 (10239, 18205) | <0.001 |
| Randomized group | ||||
| Losartan | 131 (52%) | 125 (50%) | 122 (49%) | 0.71 |
| Losartan + lisinopril | 120 (48%) | 127 (50%) | 129 (51%) | |
Data presented as mean±SD, number (%), or median (interquartile range). P values presented are for trend. For UACR, P value was determined from natural-log transformed value. Data not available for hemoglobin A1c in seven participants.
Other defined as American Indian or Alaska Native, Asian, or Native Hawaiian or Other Pacific Islander.
Biomarker Correlations
A moderately positive correlation was observed between TNFR1 and TNFR2 change (r=0.51). TNFR1 change was not correlated with initial eGFR (r=−0.03) and had moderately negative correlations with initial TNFR1 level (r=−0.35) and eGFR change (r=−0.25). Similar trends were noted for TNFR2 change (Supplemental Figure 5). Approximately 71% and 72% of participants with an increase in TNFR1 and TNFR2 levels, respectively, had a decrease in eGFR of >1 ml/min per 1.73 m2 per year (Supplemental Table 4).
Biomarker Change and Kidney Function Decline
Mean change in TNFR1 and TNFR2 were 21.4% per year and 7.4% per year, respectively. Over a median follow-up of 1.5 years, 118 (16%) participants developed kidney function decline, of whom 75% had a positive TNFR1 change and 78% had a positive TNFR2 change during the first year of the trial. Among participants who did not develop kidney function decline, 64% and 56% had positive TNFR1 and TNFR2 changes, respectively (Figure 1). Overall, participants within higher tertiles of TNFR1 and TNFR2 changes were more likely to develop kidney function decline over time (Figure 2). Participants developing kidney function decline generally had higher baseline biomarker levels (Supplemental Figure 3).
When TNFR1 change was >0, each 1 SD increase in TNFR1 change was associated with an 81% greater risk of developing subsequent kidney function decline (95% CI, 1.38 to 2.37). This association strengthened to 3.2-fold on further adjustment for biomarker level, sociodemographic and clinical factors, and kidney function measures at 0 months (95% CI, 2.34 to 4.39). In sensitivity analyses, the association of TNFR1 change with kidney function decline was robust to additional adjustment for eGFR change (HR, 2.56; 95% CI, 1.85 to 3.53) and randomized treatment group (HR, 3.21; 95% CI, 2.34 to 4.40). When TNFR1 change was ≤0, each 1 SD increase in TNFR1 change was associated with a 39% lower risk of kidney function decline (HR, 0.61; 95% CI, 0.38 to 0.98) in unadjusted analyses, indicating that more stable levels had lower risk. However, once biomarker level at 0 months was introduced in the model, the association lost statistical significance (Table 6).
Table 6.
Associations of TNFR1 and TNFR2 change, TNFR1 and TNFR2 levels at 0 months, and eGFR change with kidney function decline in VA NEPHRON-D
| Model | N | Biomarker Change (per 1 SD increase) | Biomarker Level at t0 (per 1 SD higher) | eGFR Change (per 1 SD decrease) | |||||
|---|---|---|---|---|---|---|---|---|---|
| When ≤0 | When >0 | ||||||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | ||
| TNFR1 change | |||||||||
| Unadjusted | 754 | 0.61 | 0.38 to 0.98 | 1.81 | 1.38 to 2.37 | — | — | — | — |
| + ln(biomarker) | 754 | 0.63 | 0.39 to 1.03 | 2.86 | 2.17 to 3.76 | 2.34 | 1.91 to 2.87 | — | — |
| + age, sex, race, SBP, BMI, HgbA1c, smoking | 747 | 0.68 | 0.42 to 1.10 | 3.00 | 2.26 to 3.99 | 2.42 | 1.98 to 2.96 | — | — |
| + eGFR, ln(UACR) | 747 | 0.85 | 0.56 to 1.27 | 3.20 | 2.34 to 4.39 | 3.52 | 2.59 to 4.79 | — | — |
| + eGFR change | 718 | 0.71 | 0.47 to 1.08 | 2.56 | 1.85 to 3.53 | 3.21 | 2.34 to 4.40 | 1.94 | 1.54, 2.43 |
| + randomized treatment group | 747 | 0.85 | 0.56 to 1.27 | 3.21 | 2.34 to 4.40 | 3.53 | 2.59 to 4.80 | — | — |
| TNFR2 change | |||||||||
| Unadjusted | 754 | 1.08 | 0.60 to 1.96 | 1.59 | 1.23 to 2.04 | — | — | — | — |
| + ln(biomarker) | 754 | 1.24 | 0.76 to 2.04 | 1.44 | 1.13 to 1.84 | 1.73 | 1.44 to 2.06 | — | — |
| + age, sex, race, SBP, BMI, HgbA1c, smoking | 747 | 1.25 | 0.79 to 1.97 | 1.49 | 1.16 to 1.91 | 1.78 | 1.49 to 2.14 | — | — |
| + eGFR, ln(UACR) | 747 | 1.28 | 0.88 to 1.88 | 1.43 | 1.11 to 1.83 | 1.70 | 1.35 to 2.14 | — | — |
| + eGFR change | 718 | 1.24 | 0.86 to 1.77 | 1.44 | 1.12 to 1.85 | 1.54 | 1.20 to 1.98 | 1.98 | 1.58, 2.48 |
| + randomized treatment group | 747 | 1.29 | 0.88 to 1.88 | 1.42 | 1.11 to 1.83 | 1.70 | 1.36 to 2.14 | — | — |
All covariates measured at 0 months except eGFR change, which was measured from 0 to 12 months. SD for TNFR1 change=0.38, TNFR1 at t0=0.46, TNFR2 change=0.25, TNFR2 at t0 =0.39, and eGFR change=11.5. t0=0-month visit; SBP, systolic BP; BMI, body mass index; HgbA1c, hemoglobin A1c.
When TNFR2 change was >0, each 1 SD increase in TNFR1 change was associated with 59% and 43% greater risks of developing kidney function decline in unadjusted analyses (95% CI, 1.23 to 2.04) and in Model 3 (95% CI, 1.11 to 1.83), respectively. These associations persisted after accounting for eGFR change and randomized treatment group. When TNFR2 change was ≤0, there was no association between TNFR2 change and the outcome in any of the models (Table 6). When comparing models, the addition of baseline and change in either biomarker improved the C statistic compared with a clinical model alone (Supplemental Table 3). Calibration was relatively similar across models (Supplemental Figure 4). In sensitivity analyses, there was a two-fold greater risk of ESKD (n=43 events) for every 1 SD increase in TNFR1 biomarker change (primary model HR, 1.75; 95% CI, 1.08 to 2.82) but not for TNFR2 biomarker change (primary model HR, 1.28; 95% CI, 0.90 to 1.83). Accounting for the competing risk of death did not change overall findings. When TNFR1 or TNFR2 change was >0, the risk of kidney function decline per 1 SD increase was 3.3 (sHR, 3.25; 95% CI 2.19 to 4.82) and 1.5-fold (sHR, 1.47; 95% CI, 1.10 to 1.96) higher, respectively. When TNFR1 or TNFR2 change was ≤0, neither biomarker was associated with kidney function decline (TNFR1 change: sHR, 0.87; 95% CI, 0.62 to 1.22; TNFR2 change: sHR 1.28; 95% CI, 0.92 to 1.79).
When considering categories of biomarker change, participants with decreasing, increasing, or persistently elevated TNFR1 levels and increasing or persistently elevated TNFR2 levels had 3.2–5.2-fold greater risks of developing kidney function decline, compared with participants with persistently low biomarker levels (Table 7).
Table 7.
Associations of categories of TNFR1 and TNFR2 change with kidney function decline in VA NEPHRON-D
| Biomarker Category | # Events/# at Risk | Unadjusted | Adjusteda | ||
|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | ||
| TNFR1 | |||||
| Persistently low (low–low) | 36/454 | Ref | Ref | Ref | Ref |
| Decreasing (high–low)b | 9/45 | 2.81 | 1.35 to 5.85 | 3.83 | 1.70 to 8.60 |
| Increasing (low–high) | 29/112 | 3.61 | 2.18 to 5.97 | 4.39 | 2.41 to 8.00 |
| Persistently elevated (high–high) | 44/143 | 4.43 | 2.84 to 6.93 | 5.17 | 2.77 to 9.65 |
| TNFR2 | |||||
| Persistently low (low–low) | 43/489 | Ref | Ref | Ref | Ref |
| Decreasing (high–low)b | 3/45 | 0.84 | 0.26 to 2.70 | 0.95 | 0.29 to 3.14 |
| Increasing (low–high) | 23/77 | 3.97 | 2.37 to 6.65 | 3.15 | 1.79 to 5.55 |
| Persistently elevated (high–high) | 49/143 | 4.54 | 2.99 to 6.89 | 3.77 | 2.21 to 6.44 |
Ref, reference.
Adjusted for age, sex, race, systolic BP, body mass index, hemoglobin A1c, smoking, eGFR, and ln(UPCR) at 0 months. Data not available for hemoglobin A1c in seven participants. Categories defined by whether biomarker levels at 0 and 12 months were > or ≤ the 75th percentile for the biomarker level at 0 months (persistently low: ≤ at both 0 and 12 months; decreasing: > at 0 months and ≤ at 12 months; increasing: ≤ at 0 months and > at 12 months; persistently elevated: > at both 0 and 12 months).
In the adjusted model, when the decreasing category was used as the reference, the persistently elevated category was significantly worse for TNFR2 (adjusted HR, 3.96; 95% CI, 1.20 to 13.09) but not for TNFR1. There was no significant difference between the decreasing and increasing categories for either biomarker.
Randomized Treatment Groups and Biomarker Change
The number of participants randomized to the combination (losartan plus lisinopril) and monotherapy (losartan only) arms of VA NEPHRON-D were similar across tertiles of TNFR1 and TNFR2 change (Table 5). Across all models, there were no significant differences in TNFR1 or TNFR2 change by randomized intervention (Table 4).
Discussion
In this ancillary study of participants in two clinical trials with baseline moderate to severe CKD, we demonstrated that longitudinal increases in TNFR1 and TNFR2 were independently associated with subsequent worsening of kidney function. In AASK (100% Black participants; 0% diabetes), each 1 SD increase in TNFR1 and TNFR2 slopes was associated with a 1.9–3.0-fold greater risk of ESKD. Despite differences in participant characteristics in VA NEPHRON D (74% White participants; 21% Black participants; 100% with type 2 diabetes), the findings were remarkably similar, with each 1 SD increase in TNFR1 and TNFR2 change being associated with a 1.4–3.2-fold greater risk of kidney function decline. Importantly, the findings in both cohorts were independent of baseline biomarker level and kidney function measures. In additional analyses, we also found that AASK participants randomized to the amlodipine group had a small, but statistically significant, greater increase in TNFR1 slope compared with the ramipril group.
We previously reported that higher baseline levels of TNFR1 and TNFR2 conferred greater risks of ESKD in AASK and kidney function decline in VA NEPHRON-D.11,12 This study shows that longitudinal changes in TNFR1 and TNFR2 are also associated with worse kidney outcomes, similar to data presented from the Canagliflozin Cardiovascular Assessment Study (CANVAS), a study population with type 2 diabetes.26 The approach of adjusting for covariates at the 0-month visit allows for the evaluation of surrogate endpoints, and is consistent with work done in conjunction with the Food and Drug Administration.27,28 The fact that longitudinal increases in TNFR1 and TNFR2 were associated with greater risks of CKD progression, independent of baseline biomarker levels, suggests there is value in repeating biomarker measurements. That results were robust to further adjustment with longitudinal GFR implies that the outcome may be orthogonal to more traditional surrogate endpoints. When we considered categories of biomarker change, participants in both cohorts who had increasing or persistently elevated biomarker levels were more likely to develop ESKD or kidney function decline, compared with participants with persistently low biomarker levels; however, our findings warrant replication in additional cohorts, including those with earlier stages of CKD.
In AASK, we found the amlodipine group had a slightly greater increase in TNFR1 slope compared with the ramipril group. This modest association is in line with the treatment effects of the original AASK trial, which reported a 59% risk reduction in ESKD when comparing ramipril with amlodipine (P<0.001),15 with the amlodipine arm terminated early due to concerns of harm.15,16,29 We did not find an association with TNFR1 or TNFR2 slope/change for intensive BP control (AASK) or combined angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker therapy (VA NEPHRON-D), consistent with the observed null effect of these interventions on clinical trial endpoints.15,17 In the CANVAS trial, canagliflozin was also associated with slower longitudinal increases in TNFR1 and TNFR2 compared with placebo (least squares mean differences of 2.8% for TNFR1 and 1.9% for TNFR2), similar to the effect on the composite kidney outcome.26
The TNF receptors are membrane bound and are activated by the binding of TNF, and on cleavage, are released into circulation as TNFR1 and TNFR2.30,31 With no homology between their intracellular domains, the two receptors are believed to have different signaling pathways and therefore functions.30 For example, the membrane-bound form of TNFR1 (molecular mass 55 kDa) has a death domain, whereas TNFR2 (molecular mass 75 kDa) does not, thus suggesting the former is involved in apoptosis.31,32 Although the exact roles of TNFR1 and TNFR2 are not entirely clear, several mechanisms have been proposed: (1) acting as a buffer when there is an excess of TNF (i.e., “decoy receptor”)30,32; (2) rendering cells less sensitive to the effects of TNF by releasing the extracellular domain30; and (3) prolonging the effects of TNF (i.e., “slow release reservoir”).30 Because inflammation leading to fibrosis is the common pathway for most kidney diseases, there is biologic plausibility for TNFR1 and TNFR2 as biomarkers of CKD progression.
Our study had several strengths. First, we examined two large trials in which data were prospectively and rigorously collected. Second, the cohorts were very different with regards to sex, race, and diabetes status, which speaks to the robustness of our results in persons with established CKD. Third, we examined longitudinal changes in TNFR1 and TNFR2 over time with respect to kidney outcomes. Limitations include the relatively small sample size in AASK, the short duration of follow-up in VA NEPHRON-D, and the measurement of biomarkers at only two timepoints and as two separate lots in VA-NEPHRON-D. The effect size of amlodipine on change in TNFR1 was small and should be interpreted with caution; and neither TNFR1 nor TNFR2 are kidney specific, thus our findings of association should not imply causality. Some participants who developed ESKD had stable TNFR1 and TNFR2 levels, suggesting the use of these biomarkers alone may result in imperfect prediction. The correlation between biomarker change and biomarker levels and biomarker change and kidney function measures in VA NEPHRON-D were lower than expected. These findings may reflect the higher eGFR among VA NEPHRON-D compared with AASK participants. Alternatively, biomarker measurements from VA NEPHRON-D were less precise and only available at two time points. Finally, both AASK and VA NEPHRON-D are older studies (beginning in 1995 and 2008, respectively); thus, they may not be representative of current trends in care and/or kidney event rates. As clinical trials, both were highly selective in recruitment, which may limit the generalizability of our results. That said, the association of TNFR1/TNFR2 with kidney function decline has been demonstrated in several other study populations, including young patients with type 1 diabetes and preserved GFR,10 middle-aged patients with type 2 diabetes and preserved eGFR,11,13,26 and healthy persons with cardiovascular risk factors in the Multi-Ethnic Study of Atherosclerosis.33 Our results, coupled with those from the CANVAS trial, add to the growing body of literature on the potential value of measuring TNFR1 and TNFR2 over time.
In summary, longitudinal increases in TNFR1 and TNFR2 were associated with subsequent worsening of kidney function in two clinical trials of patients with CKD, specifically Black individuals with hypertension-attributed CKD and older adults with type 2 diabetes and albuminuria. Further studies are needed to evaluate the role of longitudinal changes in TNFR1 and TNFR2 as adjuvant or standalone markers of risk in CKD.
Disclosures
C.R. Parikh reports having consultancy agreements with Genfit Biopharmaceutical Company; reports receiving research funding from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), and the National Heart, Lung and Blood Institute (NHLBI); and reports serving on the Data Safety and Monitoring Board of Genfit. C.R. Parikh and S.G. Coca are members of the advisory board of, and own equity in, Renalytix. H. Thiessen Philbrook reports other interests/relationships as Statistical Editor, Kidney360. H.J.L. Heerspink reports consultancy agreements with AbbVie, AstraZeneca, Bayer, Boehringer Ingelheim, CSL Pharma, Chinook, Dimerix Fresenius, Gilead, Janssen, Merck, Mitsubishi Tanabe, Mundi Pharma, NovoNordisk, and Travere Pharmaceuticals; reports receiving research funding from AbbVie, AstraZeneca, Boehringer Ingelheim, and Janssen (grant funding direct to employer); and reports speakers bureau from AstraZeneca. J. Coresh reports consultancy agreements with Healthy.io, and Ultragenyx; reports having an ownership interest in Healthy.io; reports receiving research funding from the National Institutes of Health (NIH), National Kidney Foundation (NKF, which receives industry support); and reports being a scientific advisor or member of Healthy.io, and the NKF. J.H. Ix reports consultancy agreements with Ardelyx, AstraZeneca, Bayer, Jnana, and Sanifit; reports receiving research funding from Baxter International; and reports being a scientific advisor or member of AlphaYoung. L.J. Appel reports receiving honoraria from Wolters Kluwer; and reports other interests/relationships with Bloomberg Philanthropies. L. Fried reports having consultancy agreements with Bayer; reports being on the data safety monitoring boards of 3D Communications, CSL Behring, and Novonordisk; and reports being a consultant for Akebia/Fibrogen. M.M. Estrella reports receiving research funding from Bayer, and Booz Allen Hamilton, Inc.; reports receiving consulting fees from AstraZeneca, Boehringer Ingelheim, Eiland & Bonnin, and Professional Corporation; reports receiving honoraria from the American Kidney Fund, AstraZeneca, Boehringer Ingelheim, and the NKF; and reports being a scientific advisor or member of the CJASN Editorial Board. M.E. Grams reports receiving honoraria from academic institutions for giving grand rounds and American Diabetes Association for reviewing abstracts; reports being a scientific advisor or member for American Journal of Kidney Diseases, CJASN, JASN, NKF Scientific Advisory Board, Kidney Disease: Improving Global Outcomes Executive Committee, United States Renal Data System Scientific Advisory Board; and reports other interests/relationships with the NKF, which in turn receives funding from Abbvie, Relypsa, and Thrasos, among others. M. Shlipak reports consultancy agreements with Cricket Health, Intercept Pharmaceuticals, University of Washington Cardiovascular Health Study, and Veterans Medical; reports receiving research funding from Bayer Pharmaceuticals; reports being a scientific advisor or member of the American Journal of Kidney Diseases, Circulation, JASN, and Journal of the American Society of Nephrology; and has other interests/relationships as a Board Member of the Northern California Institute for Research and Education. P.L. Kimmel reports other interests or relationships as Co-Editor of Chronic Renal Disease Academic Press, Co-Editor of Psychosocial Aspects of Chronic Kidney Disease; and reports receiving Academic Press royalties. S. Coca reports receiving consulting fees from Bayer, Boehringer Ingelheim, CHF Solutions, ProKidney, Relypsa (now Vifor), Renalytix, and Takeda Pharmaceuticals in the past 3 years; reports having consultancy agreements with 3ive, Axon, and Reprieve Cardiovascular; reports having an ownership interest in pulseData; reports receiving research funding from ProKidney, Renalytix, Renal Research Institute, and XORTX; reports having patents and inventions with Renalytix; reports being a scientific advisor or member of Reprieve Cardiovascular; and reports other interests/relationships as Associate Editor for Kidney360, and on the Editorial Boards of CJASN, JASN, and Kidney International. T.K. Chen reports receiving research funding from NIH/NIDDK, and Yale University. Because L. Fried is an editor of the Journal of the American Society of Nephrology, she was not involved in the peer review process for this manuscript. A guest editor oversaw the peer review and decision-making process for this manuscript. All remaining authors have nothing to disclose.
Funding
This work is supported by a Yale University George M. O’Brien Center for Kidney Research Pilot and Feasibility Grant (under NIH/NIDDK grant P30DK079310) and NIH/NIDDK grant K08DK117068 (to T. Chen); NIDDK grant R01DK108803 and NIH/NHLBI grant K24HL155861 (to M. Grams); and NIH/NIDDK grant U01DK106962 (to C. Parikh and S. Coca). Biomarkers were measured by the Translational Research Core of the George M. O’Brien Kidney Center (NIH/NIDDK grant P30DK079310).
Supplementary Material
Acknowledgments
The African American Study of Kidney Disease and Hypertension was conducted by the AASK Investigators and supported by the NIDDK. The data and samples from the AASK trial reported here were supplied by the NIDDK Central Repositories via X01DK118497. This manuscript was not prepared in collaboration with Investigators of the AASK study and does not necessarily reflect the opinions or views of the AASK study, the NIDDK Central Repositories, the NIH, or the NIDDK. The AASK trial and cohort were supported by institutional grants from the NIH and NIDDK (M01 RR-00080, M01 RR-00071, M0100032, P20-RR11145, M01 RR00827, M01 RR00052, 2P20 RR11104, RR029887, DK 2818-02, DK057867, and DK048689), and the following pharmaceutical companies: AstraZeneca, Forest Laboratories, GlaxoSmithKline, King Pharmaceuticals, Pfizer, Pharmacia, and Upjohn. The VA-NEPHRON D trial was supported by the Cooperative Studies Program of the Department of Veterans Affairs Office of Research and Development. The Investigator-Initiated Studies Program of Merck donated the study medications, losartan and lisinopril/placebo, for the VA NEPHRON-D study. CSP# 565 Combination Angiotensin Receptor Blocker and Angiotensin Converting Enzyme Inhibitor for Treatment of Diabetic Nephropathy (VA NEPHRON-D) was conducted and supported by the Department of Veterans Affairs Cooperative Studies Program, Office of Research and Development. This analysis was carried out by the listed authors utilizing data that was specified and obtained under the Data Use Agreement between Department of Veterans Affairs Cooperative Studies Program and Johns Hopkins University, dated June 14, 2019. All statements, opinions, or views are solely of the author(s) and do not reflect official views of VA. The findings reported in this paper do not necessarily reflect the opinions of the NIDDK, the NIH, the Department of Health and Human Services of the government of the United States. We thank the participants of AASK and VA NEPHRON-D.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Contributor Information
Collaborators: CKD Biomarkers Consortium (BioCon), Vasan S. Ramachandran, Joseph Massaro, Clary Clish, Jeffrey Schelling, Michelle Denburg, Susan Furth, Bradley Warady, Joseph Bonventre, Sushrut Waikar, Gearoid McMahon, Venkata Sabbisetti, Josef Coresh, Morgan Grams, Casey Rebholz, Alison Abraham, Adrienne Tin, Chirag Parikh, Jon Klein, Steven Coca, Bart S Ferket, Girish N. Nadkarni, Eugene Rhee, Paul L. Kimmel, Daniel Gossett, Brad Rovin, Michael G. Shlipak, M Sarnak, Andrew S. Levey, Lesley A. Inker, Meredith Foster, Orlando M. Gutiérrez, Joachim Ix, Ruth Dubin, Jesse Seegmiller, Tom Hostetter, Rajat Deo, Harold I. Feldman, Amanda Anderson, Theodore Mifflin, Dawei Xie, Haochang Shou, Shawn Ballard, Krista Whitehead, Heather Collins, Jason Greenberg, and Peter Ganz
Author Contributions
L. Appel, T. Chen, S. Coca, J. Coresh, M. Estrella, M. Grams, C. Parikh, and H. Thiessen Philbrook conceptualized the study; T. Chen and H. Thiessen Philbrook were responsible for the formal analysis; T. Chen, S. Coca, and C. Parikh were responsible for the funding acquisition; W. Obeid and C. Parikh were responsible for the investigation; T. Chen, J. Coresh, M. Estrella, M. Grams, and H. Thiessen Philbrook were responsible for the methodology; T. Chen was responsible for the project administration; M. Grams provided supervision; T. Chen, S. Coca, J. Coresh, L. Fried, M. Grams, H. Heerspink, J. Ix, P. Kimmel, C. Parikh, H. Thiessen Philbrook, and M. Shlipak were responsible for the validation; T. Chen wrote the original draft; and L. Appel, T. Chen, S. Coca, J. Coresh, M. Estrella, L. Fried, M. Grams, H. Heerspink, J. Ix, P. Kimmel, W. Obeid, C. Parikh, H. Thiessen Philbrook, and M. Shlipak reviewed and edited the manuscript.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2021060735/-/DCSupplemental.
Supplemental Table 1. Comparison of AASK and VA NEPHRON-D participants who were included versus excluded from primary analyses.
Supplemental Table 2. Number of ESKD events and participants by subgroups of GFR slope and biomarker slope in AASK.
Supplemental Table 3. Comparison of models by Harrell’s C Statistic and Brier Score in AASK and VA NEPHRON-D.
Supplemental Table 4. Number of kidney function decline events and participants by subgroups of eGFR change and biomarker change in VA NEPHRON-D.
Supplemental Figure 1. Flowchart of study population for primary analyses in (A) AASK and (B) VA NEPHRON-D.
Supplemental Figure 2. Pearson’s correlation coefficients of TNFR1 and TNFR2 slopes with biomarker levels and kidney function measures in AASK.
Supplemental Figure 3. Distributions of baseline TNFR1 and TNFR2 in AASK and VA NEPHRON-D, by outcome.
Supplemental Figure 4. Calibration plots of observed versus predicted 5-year risk for ESKD in AASK and 2-year risk for kidney function decline in VA NEPHRON-D.
Supplemental Figure 5. Pearson’s correlation coefficients of TNFR1 and TNFR2 change with biomarker levels and kidney function measures in VA NEPHRON-D.
CKD Biomarkers Consortium Collaborators: Phase II: Group Members and Affiliations .
References
- 1.Centers for Disease Control and Prevention : Chronic kidney disease in the United States, 2021, Atlanta, GA, US Department of Health and Human Services, Centers for Disease Control and Prevention, 2021 [Google Scholar]
- 2.Chen TK, Knicely DH, Grams ME: Chronic kidney disease diagnosis and management: A review. JAMA 322: 1294–1304, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen TK, Sperati CJ, Thavarajah S, Grams ME: Reducing kidney function decline in patients with CKD: Core curriculum 2021. Am J Kidney Dis 77: 969–983, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.United States Renal Data System : 2020 USRDS Annual Data Report: Epidemiology of kidney disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2020 [Google Scholar]
- 5.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group : KDIGO 2012 Clinical Practice Guideline for the evaluation and management of chronic kidney disease. Available at https://kdigo.org/wp-content/uploads/2017/02/KDIGO_2012_CKD_GL.pdf. Accessed March 11, 2022 [Google Scholar]
- 6.Matsushita K, Coresh J, Sang Y, Chalmers J, Fox C, Guallar E, et al. ; CKD Prognosis Consortium : Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: A collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol 3: 514–525, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coresh J, Turin TC, Matsushita K, Sang Y, Ballew SH, Appel LJ, et al. : Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA 311: 2518–2531, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waikar SS, Rebholz CM, Zheng Z, Hurwitz S, Hsu CY, Feldman HI, et al. ; Chronic Kidney Disease Biomarkers Consortium Investigators : Biological variability of estimated GFR and albuminuria in CKD. Am J Kidney Dis 72: 538–546, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levey AS, Gansevoort RT, Coresh J, Inker LA, Heerspink HL, Grams ME, et al. : Change in albuminuria and GFR as end points for clinical trials in early stages of CKD: A scientific workshop sponsored by the National Kidney Foundation in collaboration with the US Food and Drug Administration and European Medicines Agency. Am J Kidney Dis 75: 84–104, 2020 [DOI] [PubMed] [Google Scholar]
- 10.Gohda T, Niewczas MA, Ficociello LH, Walker WH, Skupien J, Rosetti F, et al. : Circulating TNF receptors 1 and 2 predict stage 3 CKD in type 1 diabetes. J Am Soc Nephrol 23: 516–524, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coca SG, Nadkarni GN, Huang Y, Moledina DG, Rao V, Zhang J, et al. : Plasma biomarkers and kidney function decline in early and established diabetic kidney disease. J Am Soc Nephrol 28: 2786–2793, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen TK, Estrella MM, Appel LJ, Coresh J, Luo S, Reiser J, et al. : Biomarkers of immune activation and incident kidney failure with replacement therapy: Findings from the African American Study of Kidney Disease and Hypertension. Am J Kidney Dis 78: 75–84.e1, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niewczas MA, Gohda T, Skupien J, Smiles AM, Walker WH, Rosetti F, et al. : Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. J Am Soc Nephrol 23: 507–515, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pavkov ME, Nelson RG, Knowler WC, Cheng Y, Krolewski AS, Niewczas MA: Elevation of circulating TNF receptors 1 and 2 increases the risk of end-stage renal disease in American Indians with type 2 diabetes. Kidney Int 87: 812–819, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wright JT Jr, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, et al. ; African American Study of Kidney Disease and Hypertension Study Group : Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: Results from the AASK trial. JAMA 288: 2421–2431, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Appel LJ, Wright JT Jr, Greene T, Agodoa LY, Astor BC, Bakris GL, et al. ; AASK Collaborative Research Group : Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med 363: 918–929, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fried LF, Emanuele N, Zhang JH, Brophy M, Conner TA, Duckworth W, et al. ; VA NEPHRON-D Investigators : Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 369: 1892–1903, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Gassman JJ, Greene T, Wright JT Jr, Agodoa L, Bakris G, Beck GJ, et al. : Design and statistical aspects of the African American Study of Kidney Disease and Hypertension (AASK). J Am Soc Nephrol 14[Suppl 2]: S154–S165, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toto RD, Greene T, Hebert LA, Hiremath L, Lea JP, Lewis JB, et al. ; AASK Collaborative Research Group : Relationship between body mass index and proteinuria in hypertensive nephrosclerosis: Results from the African American Study of Kidney Disease and Hypertension (AASK) cohort. Am J Kidney Dis 56: 896–906, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen TK, Tin A, Peralta CA, Appel LJ, Choi MJ, Lipkowitz MS, et al. : APOL1 risk variants, incident proteinuria, and subsequent eGFR decline in Blacks with hypertension-attributed CKD. Clin J Am Soc Nephrol 12: 1771–1777, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parsa A, Kao WH, Xie D, Astor BC, Li M, Hsu CY, et al. ; AASK Study Investigators; CRIC Study Investigators : APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med 369: 2183–2196, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, et al. : Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 329: 841–845, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tzur S, Rosset S, Shemer R, Yudkovsky G, Selig S, Tarekegn A, et al. : Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum Genet 128: 345–350, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D; Modification of Diet in Renal Disease Study Group : A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Fine JP, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association 94: 496–509, 1999 [Google Scholar]
- 26.Sen T, Li J, Neuen BL, Neal B, Arnott C, Parikh CR, et al. : Effects of the SGLT2 inhibitor canagliflozin on plasma biomarkers TNFR-1, TNFR-2 and KIM-1 in the CANVAS trial. Diabetologia 64: 2147–2158, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grams ME, Sang Y, Ballew SH, Matsushita K, Astor BC, Carrero JJ, et al. : Evaluating glomerular filtration rate slope as a surrogate end point for ESKD in clinical trials: An individual participant meta-analysis of observational data. J Am Soc Nephrol 30: 1746–1755, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inker LA, Heerspink HJL, Tighiouart H, Levey AS, Coresh J, Gansevoort RT, et al. : GFR slope as a surrogate end point for kidney disease progression in clinical trials: A meta-analysis of treatment effects of randomized controlled trials. J Am Soc Nephrol 30: 1735–1745, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agodoa LY, Appel L, Bakris GL, Beck G, Bourgoignie J, Briggs JP, et al. ; African American Study of Kidney Disease and Hypertension (AASK) Study Group : Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: A randomized controlled trial. JAMA 285: 2719–2728, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Bemelmans MHA, van Tits LJH, Buurman WA: Tumor necrosis factor: Function, release and clearance. Crit Rev Immunol 37: 249–259, 2017 [DOI] [PubMed] [Google Scholar]
- 31.Speeckaert MM, Speeckaert R, Laute M, Vanholder R, Delanghe JR: Tumor necrosis factor receptors: Biology and therapeutic potential in kidney diseases. Am J Nephrol 36: 261–270, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Vanamee ES, Faustman DL: Structural principles of tumor necrosis factor superfamily signaling. Sci Signal 11: 11, 2018 [DOI] [PubMed] [Google Scholar]
- 33.Bhatraju PK, Zelnick LR, Shlipak M, Katz R, Kestenbaum B: Association of soluble TNFR-1 concentrations with long-term decline in kidney function: The multi-ethnic study of atherosclerosis. J Am Soc Nephrol 29: 2713–2721, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.