Abstract
Introduction:
Recurrent laryngeal nerve (RLN) transection injuries may occur during thyroidectomy and other surgical procedures. Laser nerve welding has been shown to cause less technique-related axonal damage than the traditional suture method. We compared functional adductor results using these two methods of RLN repair.
Method:
Canine hemilarynges underwent pretreatment testing of laryngeal adductor function, followed by RLN transection and repair using KTP laser welding (n=8) or microneural suture (n=16) techniques. Six months later, adductor function was measured again and expressed as a proportion of the pretreatment value.
Results:
The mean laryngeal adductor pressure ratios were 82.4% (95% CI: 72.8 – 92.0%) for the laser repair group and 55.5% (95% CI: 49.4 – 61.6%) for the suture control group, with a difference of 26.9% (95% CI: 15.3 – 38.5%). Both spontaneous and stimulated glottic closure was observed in the laser welding and microsuture repair groups.
Conclusion:
Laser nerve welding resulted in greater strength of adduction than suture repair of an acutely transected RLN. Suture anastomosis may traumatize more axons than the laser. Stronger vocal fold adduction is associated clinically with better protection from aspiration and improved voice outcomes. KTP laser welding should be considered for anastomosis of the RLN and other nerves.
Level of Evidence:
N/A
Introduction
During head and neck surgery, cranial nerve transection is sometimes anticipated1,2 or can occur unintentionally.3,4 Cranial nerve injuries can lead to significant morbidity including facial paralysis,5 dysphagia,6 shoulder disability,7 and aspiration.8 Following complete nerve transection, primary neurorrhaphy with microsuture is the most commonly used repair technique, provided the nerve endings have enough length for primary anastomosis.9,10
While mircosuture neurorrhaphy has better functional recovery compared to no nerve repair,10 primary microsuture repair may impede some axon regeneration.11 This technique requires significant instrumentation of the nerve endings, and the needle and suture material may inadvertently damage axons.11,12 In order to enhance axon regeneration, sutureless repair methods, including laser nerve welding (LNW), have been proposed as alternative methods of nerve coaptation.13–16
LNW uses light energy to “weld” the epineurium of two transected nerve stumps – sealing important growth factors within the repair.11 This technique avoids suture material and is significantly quicker than traditional microsuture repair.13,16 CO2 LNW is the most widely studied laser type in animal studies.17–19 This technique demonstrates minimal scar production histologically, with experiments demonstrating similar effectiveness in nerve regeneration compared to microsuture repair.17 Recently, we completed a pilot study comparing microsuture, CO2, and potassium titanyl phosphate (KTP) LNW in rats and found improved functional recovery using KTP laser, compared to the microsuture repair.13 Given the rats’ small nerve caliber and inherently strong regenerative capacity, however, it is not known how well KTP LNW would translate to human nerve repair.20
This study set out to compare traditional microsuture repair to KTP LNW of the recurrent laryngeal nerve (RLN) using a larger, canine model. Based on the LNW technique, we hypothesized that KTP laser would yield a better functional recovery than microsuture repair. The canine RLN likely experiences similar physiologic stresses as the human RLN, and we were secondarily interested in assessing whether the LNW repair would show any evidence of repair dehiscence.
Methods
Twenty-four canine hemi-laryngeal preparations were used in this pilot study. The effect size and variability were not known, preventing sample size calculation a priori. All canines were purpose-bred, adult, female, socialized mongrel canines between 20 and 25 kg in weight. The animals were housed in a facility approved by the American Association for Accreditation of Laboratory Animal Care. The National Institutes of Health guidelines for animal care were strictly followed. This study was performed in accordance with the PHS Policy on Humane Care and Use of Laboratory Animals, the NIH Guide for the Care and Use of Laboratory Animals, and the Animal Welfare Act (7 U.S.C. et seq.). The animal use protocol was approved by the Institutional Animal Care and Use Committee of Washington University School of Medicine.
Experimental Design
This study compared two types of nerve repair following complete transection of the canine RLN. The nerve was repaired by either microsuture (n=16) or LNW (n=8). Laryngeal adductor pressure (LAP) was measured just prior to nerve transection and 6 months following repair.
Initial Procedure – Baseline LAP and RLN transection/repair
General anesthesia was induced using intravenous thiopental sodium, and 2% isoflurane inhalant was used to maintain anesthesia. A permanent tracheotomy was established for the entirety of the study, as described by Dahm and Paniello.21 The pre-injury laryngeal adductor pressure (LAP) was measured using a previously described method.22,23 Briefly, a Harvard stimulating electrode was placed around the canine RLN, and an inflated endotracheal tube was advanced through the tracheostomy. A pressure transducer was attached. A variable frequency and constant current was applied through the stimulating electrode, causing vocal fold adduction. The stimulating current was adjusted to achieve the maximum pressure. The LAP was derived from the difference between the stimulated adduction and the unstimulated baseline measurement. Measurements were made at various frequencies from 20 Hz to 100 Hz at 10-Hz intervals in a random order. Each set of initial measurements served as a control, which was compared to the measurements 6-months following nerve transection and repair. The endotracheal tube was labeled with each canine’s identification and saved for later use. Next, the RLN was transected approximately 4 cm inferior to the cricothyroid joint. The RLN transection was repaired either by KTP LNW (Group A, n=8) or microsuture repair (Group B, n=16).
Group A: KTP LNW repair
The RLN was transected with straight microsurgical scissors approximately 4 cm inferior to the cricothyroid joint. After transection, the nerve endings separated by several millimeters, and one 9–0 nylon, epineural anchor stitch was placed to re-approximate the nerve stumps.
All KTP nerve welding procedures were performed by the same investigator (NKB). A laser safety-check was performed, and all individuals in the operating room were required to wear proper eye protection. KTP laser was set to 0.5 W and continuous mode. A micromanipulator was used to aim and focus the laser, and the beam diameter was adjusted to approximately half the width of the RLN diameter. LNW was performed the hemi-circumference of the anastomosis most accessible to the surgeon. The micromanipulator joystick was controlled using the surgeon’s dominant hand, while mircoforceps were held with the other hand to precisely position the nerve stumps. The KTP laser was activated using a foot pedal, and 3–5 seconds of continuous laser energy was delivered to the anastomosis. The laser energy caused gradual denaturing of the epineurium. We observed blending of the two nerve endings such that the cleft between the two nerve endings disappeared. The laser beam was slowly advanced along the anastomosis to limit any charring to the epineurium. In some cases, the laser caused the anchor stitch to break. In these cases, the suture was not replaced. Finally, the entire nerve was rotated in order to weld the other half of the anastomosis. The total amount of energy required to weld one RLN was recorded by the laser machine and ranged between 6 and 10 joules.
Group B: Microsuture repair
The RLN was transected with microsurgical scissors approximately 4cm inferior to the cricothyroid joint and immediately repaired. Using an operating microscope, 3 to 4 interrupted, 9–0 nylon epineural sutures were placed.
Terminal procedure
After 6 months, an awake infraglottic exam was digitally recorded through the tracheostomy to document the degree of spontaneous vocal fold motion. The canine was given 1–2 ml of water by mouth to stimulate swallow and glottic closure reflex. The vocal fold motion with swallow was assessed using a numerical scale in all LNW and eight consecutive transection/repair hemi-laryngeal preparations (Table 1). Anesthesia was induced. The RLN was exposed and stimulated laryngeal examination was digitally recorded. LAP measurements were repeated. The anastomosis was examined to ensure that the nerve was intact. The canine was sacrificed. The larynx and RLNs were harvested and preserved. Select RLNs were processed for histology.
Table 1.
Vocal Fold Recovery Rating
| Rating | Vocal Fold Movement |
|---|---|
| 0 | No spontaneous movement |
| 1 | Brief twitch |
| 2 | Slight adduction |
| 3 | Adducts but does not reach midline |
| 4 | Adducts to midline |
| 5 | Adducts and abducts completely |
Statistical Considerations
Each set of baseline (pre-injury) measurements was corrected by the non-stimulated offset constant, then normalized to the 80 Hz value. The 6-month measurements were corrected by the offset and expressed as a percentage of the 80 Hz pre-injury value. The LAP values from 70 – 100 Hz were averaged to give a single adductor measure for each preparation. Mean and standard deviation were reported for normally distributed data. Median and range were reported for ordinal data. Mann-Whitney U-test was used to compare the vocal fold motion recovery between the two groups.
Histology
Nerve samples were immersion-fixed in 10% formalin until further processing. Several small (approximately 2 mm) lengths of nerve from the anastomotic site were rinsed in 0.1M phosphate buffered saline (PBS) and post-fixed in 1% osmium tetroxide for 45 min. The osmium was rinsed free in distilled water and samples were dehydrated through a graded series of acetones, infiltrated with epon-araldite resin, and embedded. Blocks were trimmed and semi-thin sections (2 and 4 um) were collected onto glass slides, counterstained with 1% toluidine blue, and cover-slipped with Permount mounting medium.
Results
All canines in this study completed the six-month experiment. All LNW and microsuture anastomoses were examined and all repairs (n=24, 100.0%) were intact grossly. There were no cases of nerve dehiscence at 6 months.
Laryngeal Adductor Pressure
The mean 6-month LAP measurements are shown (Table 2). The KTP repair (n=8) showed a mean LAP recovery of 82.4% (95% CI: 72.8 – 92.0%). The range of LAP recovery was 67.2% to 100.0%. The microsuture repair (n=16) had a mean LAP recovery of 55.5% (95% CI: 49.4 – 61.6%). The KTP LNW repair demonstrated a higher mean LAP recovery, and the difference was 26.9% (95% CI: 15.3 – 38.5%).
Table 2.
Potassium Titanyl Phosphate Laser Nerve Welding (Group A) Versus Microsuture Repair (Group B) Percent Recovery at 6 Months.
| KTP LNW (Group A) vs microsuture repair (Group B) % recovery at 6-months | ||
|---|---|---|
| Group | N | Percent recovery of baseline |
| A | 8 | 82.4% (95% CI: 72.8 – 92.0%) |
| B | 16 | 55.5% (95% CI: 49.4 – 61.6%) |
| KTP LNW vs microsuture repair % recovery at 6-months | ||
| Group | N | Percent recovery of baseline |
| KTP | 8 | 82.4% (95% CI: 72.8 – 92.0%) |
| Microsuture | 16 | 55.5% (95% CI: 49.4 – 61.6%) |
Vocal Fold Motion Recovery
At 6 months, spontaneous vocal fold motion was assessed and rated. In the LNW group (n=8), the median rating was 3 (range 1–4). In the transection/repair group (n=8), the median rating was 2 (range 1–3). The difference between the two groups was not significant (median difference = 1, 95% CI: 0 – 2, U=22). A histogram tallying the frequency of each rating for both the LNW and transection/repair groups is shown (Figure 1). All rated hemilaryngeal preparations (n=16, 100.0%) experienced some degree of vocal fold motion recovery.
Figure 1:
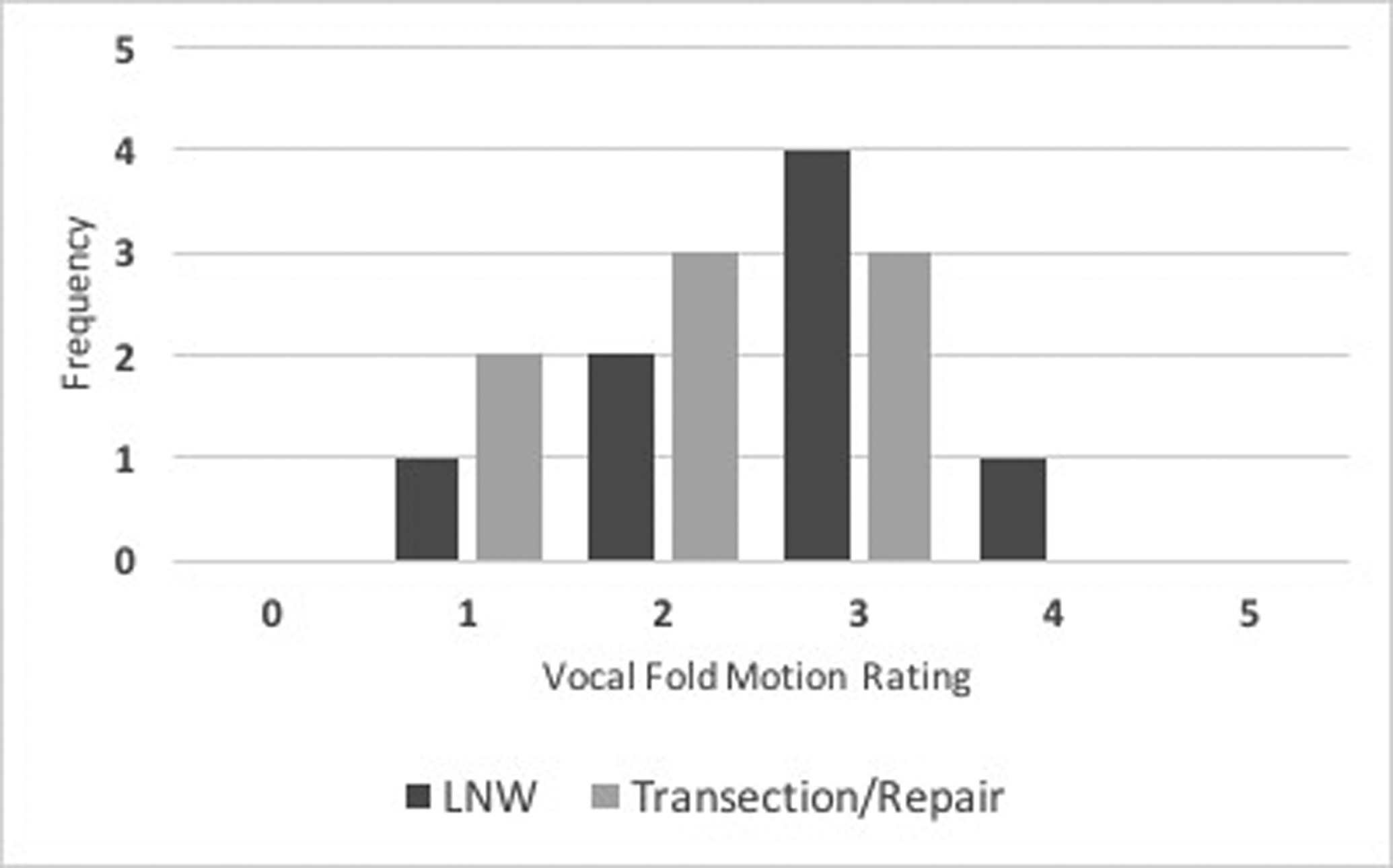
Histogram displaying vocal fold motion recovery at 6 months comparing LNW and transection/repair. The vocal fold motion was scored between 0 and 5.
Stimulated Vocal Fold Adduction
During final measurements at 6 months, the RLN was electrically stimulated and vocal fold adduction was assessed while the canine was under general anesthesia. All canines in the LNW group (n=8, 100.0%) and microsuture group (n=16, 100.0%) demonstrated vocal fold adduction with stimulation.
Histologic Analysis
Select RLN LNW sections (n=3) and microsuture repair (n=3) were assessed by histology. At 6 months, the site of LNW was not grossly obvious. The distal RLN demonstrated a smooth and continuous epineurium, without evidence of a cleft, char, or scar. Thus, the cross-sections excised for histology were approximated based the distance from the cricothyroid joint. Using the described histology protocol, no structural differences between the two groups were identified. All nerve segments showed axonal regeneration. Sample axial nerve section from the LNW is shown (Figure 2).
Figure 2:
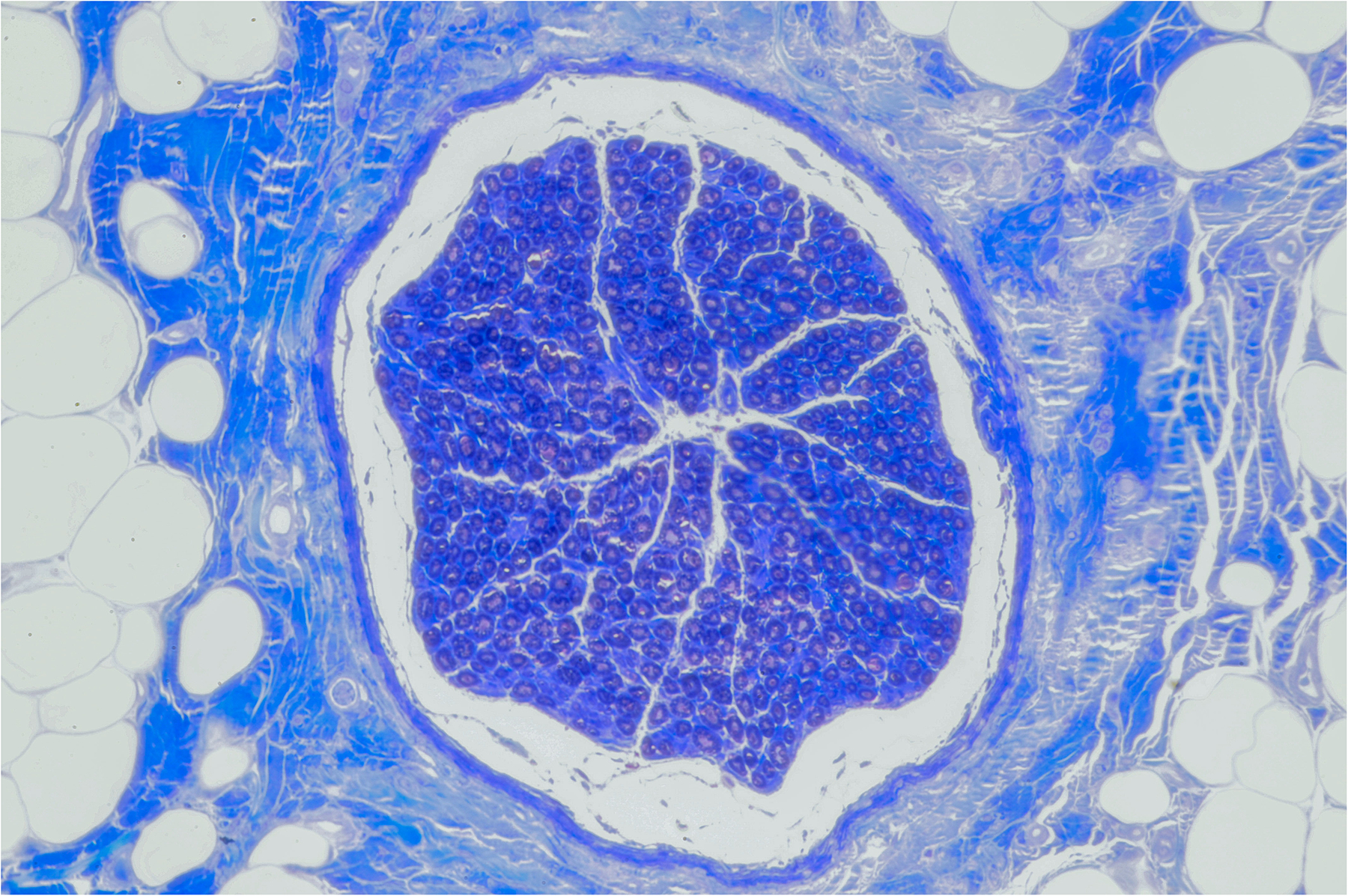
Microscopic RLN cross-section 6 months after KTP LNW (20x objective magnification).
Discussion
In this study, KTP laser promoted better LAP recovery at 6 months compared to microsuture repair. Among the 8 laser anastomoses, the lowest LAP recovery was 65.3% of baseline, which was higher than the mean microsuture repair recovery. When assessing vocal fold movement, KTP laser welding showed a higher median vocal fold motion recovery rating (3 vs 2), although this difference was not significant in this pilot study. In all the assessments of vocal fold movement, the observed movement was only adduction, never abduction. In previous studies, however, a return of spontaneous abduction was observed following milder forms of RLN injury – such as a stretch injury.24,25
Histology was performed on select RLNs in both groups, and axonal regeneration was confirmed in each of these nerve specimens. Previous work in rats,13 comparing LNW and microsuture repair, demonstrated that the axon number did not correlate well with the functional recovery. In this previous study, LNW yielded twice the number of regenerated axons compared to microsuture repair, with only a slight increase in the functional recovery. Since an individual axon can innervate a variable number of motor fibers, the total number of axons may not provide an accurate assessment of functional recovery.
There has been a tremendous amount of research exploring methods to enhance nerve recovery following complete transection.26–30 Strategies include pharmacologic interventions,31 growth factors,30 selective reinnervation inhibitors,32,33 nerve scaffolds,27 stem cells,26,29 and electrical stimulation.28 Many of these regenerative strategies appear experimentally promising and could be mutually applied. With respect to the RLN, adductor and abductor axons are distributed within the nerve.34 With axonal regrowth, synkinetic reinnervation – misguided adductor and abductor regeneration – can occur.35–37 While the LNW technique in the study promoted higher adductor recovery compared to microsuture repair, LNW may have also increased the rate of synkinetic adductor axon reinnervation to abductor muscles. Despite a potentially higher synkinetic rate, LNW promoted greater adductor recovery to overcome additional and opposing abduction.38 This specific feature of the RLN may necessitate regeneration strategies that selectively inhibit synkinetic reinnervation to the abductor muscles.32,33
Within head and neck surgery, specific anatomical locations may make microsuture repair challenging.39 LNW may have a unique role, especially in the repair of nerve injuries in challenging sites like the skull base.40 Laser repair can be accomplished without needing to mobilize large nerve segments and could potentially be performed endoscopically. For small caliber nerves, a hybrid technique consisting of both microsuture and LNW could be employed. The surgeon could place one anchor stitch and spot-weld the remaining epineurium.
One of the main concerns with the LNW technique is comparatively low tensile strength compared to traditional microsuture repair.11,41 LNW has been studied in several smaller animal models, including rats13,42 and rabbits.16,19,43 A major motivation of this study was to assess the rate of dehiscence following laser repair of a larger caliber and lengthier nerve. In the canine, the RLN follows a long, indirect pathway from the brain to the larynx that is similar to the human RLN, and is likely under similar physiologic stresses.44 In this study, there were no cases of nerve dehiscence at 6 months. The LNW repair, however, utilized one anchor stitch, and it is not known whether LNW of the RLN without an epineural stitch would have yielded the same rate of nerve dehiscence.
Since the KTP LNW group anastomosis was not an entirely sutureless technique, this limited the ability to cleanly compare the two repair techniques (LNW vs microsuture). After the canine RLN was cut, the two nerve stumps separated away from each other. An anchor stitch was needed to re-approximate the two nerve stumps, minimizing the tension at the anastomosis during laser welding. Without the stitch, the tensile strength generated by epineural welding was not consistently strong enough to keep the two nerve stumps adherent to one another. Based on previous work in the rat model,13,41 significant tension at the anastomosis led to separation after LNW. It is possible that placement of the anchor stitch impeded some axonal regeneration; however, effective LNW could not reliably be achieved without the initial stitch.
Photochemical tissue bonding (PTB) is a modification to LNW and may be used as a possible method to anchor the anastomosis without the use of microsuture.45,46 This technique uses photosensitive dyes that are activated by light energy to promote protein crosslinking and tissue repair.41,47 PTB alone may have distinct advantages over LNW technique. First, the adhesive light-activated crosslinking promotes high tensile strength at the repair.48,49 O’Neill et al.49 compared PTB using 0.1 wt% rose bengal dye and microsuture repair using harvested porcine brachial arteries. The PTB repaired vessels resisted a significantly higher intraluminal pressure compared to microsuture repair (1100 ± 150 mmHg vs 350 ± 40 mmHg). Second, PTB does not appear to induce thermal tissue injury.48 Lauto et al.48 used chitosan adhesive films with 0.1 wt% rose bengal dye and bonded the chitosan sheets to calf intestine ex vivo. The photochemical reaction was minimally exothermic, and thermocouples at the repair only measured a 6 °C temperature increase upon laser irradiation (26°C to 32°C). As a distinct repair method, PTB may provide the same advantages of LNW while minimizing the risk of post-repair nerve dehiscence and thermal damage to axons. Alternatively, PTB could initially anchor a small portion of the anastomosis. Laser welding could be subsequently performed to seal the remaining epineurium.
While lasers continue to have a greater role in otolaryngology,50,51 utilization of this technique necessitates that a KTP laser is readily available. During certain cases in which there is a possibility of nerve transection, a laser could be pretested and ready for stand-by use. Once the laser is ready, LNW can be performed more quickly than microsuture repair.13,52 In many cases, however, nerve transection may be unanticipated, and the laser may not be available. Without significant benefit in functional nerve recovery, most surgeons would likely continue to use traditional microsuture repair – unless specific anatomic factors warrant its use.
Conclusion
Following complete transection of the canine RLN, KTP LNW with an anchor stitch yielded a better significantly laryngeal adductor recovery, compared to microsuture repair 6 months after injury and repair. Both methods showed spontaneous vocal fold motion recovery at 6 months. There were no cases of nerve separation. In canines, KTP LNW appears to be a viable alternative to the traditional microsuture repair, provided there is an anchor stitch. With greater use of lasers during head and neck surgery, LNW may be a feasible and attractive repair option following complete nerve transection.
Financial support/funding:
Midwest Stone Institute, and National Institutes of Health Grant #: 5T32DC000022
Funding support for this research was provided through the “Development of Clinician/Researchers in Academic ENT” T32 DC000022 from the National Institutes of Deafness and Other Communication Disorders.
Footnotes
Meeting: American Laryngological Association (ALA) meeting, National Harbor, MD, USA. April 18 – 20, 2018.
Author Statement:
The listed authors have no financial disclosures of conflicts of interest.
References:
- 1.Swendseid B et al. Incidence of facial nerve sacrifice in parotidectomy for primary and metastatic malignancies. Oral Oncol. 73, 43–47 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Weisberger EC, Kincaid J & Riteris J Cable Grafting of the Spinal Accessory Nerve After Radical Neck Dissection. Arch. Otolaryngol. Head Neck Surg 124, 377–380 (1998). [DOI] [PubMed] [Google Scholar]
- 3.Chiang F-Y et al. The mechanism of recurrent laryngeal nerve injury during thyroid surgery--the application of intraoperative neuromonitoring. Surgery 143, 743–749 (2008). [DOI] [PubMed] [Google Scholar]
- 4.Visconti G & Salgarello M Anatomical Considerations to Prevent Facial Nerve Injury: Insights on Frontal Branch and Cervicofacial Trunk Nerve Anatomy in SMAS Face Lifts. Plast. Reconstr. Surg 137, 751e–752e (2016). [DOI] [PubMed] [Google Scholar]
- 5.Dulguerov P, Marchal F & Lehmann W Postparotidectomy facial nerve paralysis: Possible etiologic factors and results with routine facial nerve monitoring. The Laryngoscope 109, 754–762 (1999). [DOI] [PubMed] [Google Scholar]
- 6.Bhattacharyya N, Kotz T & Shapiro J Dysphagia and aspiration with unilateral vocal cord immobility: incidence, characterization, and response to surgical treatment. Annals of Otology, Rhinology & Laryngology 111, 672–679 (2002). [DOI] [PubMed] [Google Scholar]
- 7.Eickmeyer SM et al. Quality of Life, Shoulder Range of Motion, and Spinal Accessory Nerve Status in 5-Year Survivors of Head and Neck Cancer. PM&R 6, 1073–1080 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crumley RL Unilateral recurrent laryngeal nerve paralysis. J Voice 8, 79–83 (1994). [DOI] [PubMed] [Google Scholar]
- 9.Bassilios Habre S et al. The Surgical Management of Nerve Gaps: Present and Future. Ann Plast Surg 80, 252 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Chou F-F, Su C-Y, Jeng S-F, Hsu K-L & Lu K-Y Neurorrhaphy of the recurrent laryngeal nerve. Journal of the American College of Surgeons 197, 52–57 (2003). [DOI] [PubMed] [Google Scholar]
- 11.Barton MJ, Morley JW, Stoodley MA, Lauto A & Mahns DA Nerve repair: toward a sutureless approach. Neurosurg Rev 37, 585–595 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Fox IK, Brenner MJ, Johnson PJ, Hunter DA & Mackinnon SE Axonal regeneration and motor neuron survival after microsurgical nerve reconstruction. Microsurgery 32, 552–562 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhatt NK et al. Potassium titanyl phosphate laser welding following complete nerve transection. The Laryngoscope (2016). doi: 10.1002/lary.26383 [DOI] [PubMed] [Google Scholar]
- 14.Menovsky T & Beek JF Carbon dioxide laser-assisted nerve repair: effect of solder and suture material on nerve regeneration in rat sciatic nerve. Microsurgery 23, 109–116 (2003). [DOI] [PubMed] [Google Scholar]
- 15.Korff M et al. An investigation of the potential for laser nerve welding. Otolaryngology - Head and Neck Surgery 106, 345–350 (1992). [DOI] [PubMed] [Google Scholar]
- 16.Bloom JD et al. Laser facial nerve welding in a rabbit model. Arch Facial Plast Surg 14, 52–58 (2012). [DOI] [PubMed] [Google Scholar]
- 17.Menovsky T, Van Den Bergh Weerman M & Beek JF Effect of CO(2)-Milliwatt laser on peripheral nerves: part II. A histological and functional study. Microsurgery 20, 150–155 (2000). [DOI] [PubMed] [Google Scholar]
- 18.Menovsky T, Beek JF & van Gemert MJ CO2 laser nerve welding: optimal laser parameters and the use of solders in vitro. Microsurgery 15, 44–51 (1994). [DOI] [PubMed] [Google Scholar]
- 19.Hwang K, Kim SG & Kim DJ Hypoglossal-facial nerve anastomosis in the rabbits using laser welding. Ann Plast Surg 61, 452–456 (2008). [DOI] [PubMed] [Google Scholar]
- 20.Kaplan HM, Mishra P & Kohn J The overwhelming use of rat models in nerve regeneration research may compromise designs of nerve guidance conduits for humans. J Mater Sci: Mater Med 26, 226 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dahm JD, DAHM J, PANIELLO R & Paniello RC Tracheostomy for long-term laryngeal experimentation☆☆☆★. Otolaryngology - Head and Neck Surgery 118, 376–380 (1998). [DOI] [PubMed] [Google Scholar]
- 22.Paniello RC & West SE Laryngeal adductory pressure as a measure of post-reinnervation synkinesis. Annals of Otology, Rhinology & Laryngology 109, 447–451 (2000). [DOI] [PubMed] [Google Scholar]
- 23.Paniello RC & Bhatt NK Glottic Closing Force Versus Laryngeal Adductory Pressure in the Canine Larynx. Annals of Otology, Rhinology & Laryngology 126, 173–178 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Bhatt NK et al. Compound Motor Action Potential Quantifies Recurrent Laryngeal Nerve Innervation in a Canine Model. Annals of Otology, Rhinology & Laryngology 125, 584–590 (2016). [DOI] [PubMed] [Google Scholar]
- 25.Paniello RC, Park AM, Bhatt NK & Al-Lozi M Recurrent laryngeal nerve recovery patterns assessed by serial electromyography. The Laryngoscope 126, 651–656 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ikeda M et al. Acceleration of peripheral nerve regeneration using nerve conduits in combination with induced pluripotent stem cell technology and a basic fibroblast growth factor drug delivery system. Journal of Biomedical Materials Research Part A 102, 1370–1378 (2013). [DOI] [PubMed] [Google Scholar]
- 27.Niu Y et al. Scaffolds from block polyurethanes based on poly(ɛ-caprolactone) (PCL) and poly(ethylene glycol) (PEG) for peripheral nerve regeneration. Biomaterials 35, 4266–4277 (2014). [DOI] [PubMed] [Google Scholar]
- 28.Willand MP, Nguyen M-A, Borschel GH & Gordon T Electrical Stimulation to Promote Peripheral Nerve Regeneration. Neurorehabilitation and Neural Repair 30, 490–496 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Paniello RC et al. Improved adductor function after canine recurrent laryngeal nerve injury and repair using muscle progenitor cells. The Laryngoscope 110, 279 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sivak WN et al. Delivery of chondroitinase ABC and glial cell line‐derived neurotrophic factor from silk fibroin conduits enhances peripheral nerve regeneration. Journal of Tissue Engineering and Regenerative Medicine 11, 733–742 (2017). [DOI] [PubMed] [Google Scholar]
- 31.Sridharan SS, Rosen CA, Smith LJ, Young VN & Munin MC Timing of nimodipine therapy for the treatment of vocal fold paralysis. The Laryngoscope 125, 186–190 (2015). [DOI] [PubMed] [Google Scholar]
- 32.Park AM, Patil RD & Paniello RC Prevention of post‐traumatic reinnervation with microtubule inhibitors. The Laryngoscope 125, E333–E337 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paniello RC, Bhatt NK & Chernock R Toxicity trial of canine posterior cricoarytenoid intramuscular vincristine injections. The Laryngoscope 111, 844 (2017). [DOI] [PubMed] [Google Scholar]
- 34.Gacek RR, Malmgren LT & Lyon MJ Localization of adductor and abductor motor nerve fibers to the larynx. Annals of Otology, Rhinology & Laryngology 86, 771–776 (1977). [PubMed] [Google Scholar]
- 35.Benjamin B Vocal cord paralysis, synkinesis and vocal fold motion impairment. ANZ J Surg 73, 784–786 (2003). [DOI] [PubMed] [Google Scholar]
- 36.Crumley RL Laryngeal Synkinesis Revisited. Annals of Otology, Rhinology & Laryngology 109, 365–371 (2016). [DOI] [PubMed] [Google Scholar]
- 37.Pei Y-C, Chang W-H, Chuang H-F, Chang C-F & Fang T-J Implications of Synkinesis in Unilateral Vocal Fold Paralysis. Otolaryngol Head Neck Surg 157, 1017–1024 (2017). [DOI] [PubMed] [Google Scholar]
- 38.Paniello RC Synkinesis following recurrent laryngeal nerve injury: A computer simulation. The Laryngoscope 126, 1600–1605 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Janecka IP, Sekhar LN & Sen CN Facial nerve management in cranial base surgery. The Laryngoscope 103, 291–298 (1993). [DOI] [PubMed] [Google Scholar]
- 40.Thieme Medical Publishers, Feldman JS, Farnoosh S, Kellman RM & Tatum SA Skull Base Trauma: Clinical Considerations in Evaluation and Diagnosis and Review of Management Techniques and Surgical Approaches. Seminars in Plastic Surgery 31, 177–188 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhatt NK, Khan TR, Mejias C & Paniello RC Nerve transection repair using laser-activated chitosan in a rat model. The Laryngoscope 127, E253–E257 (2017). [DOI] [PubMed] [Google Scholar]
- 42.Lauto A, Dawes JM, Cushway T, Piper JA & Owen ER Laser nerve repair by solid protein band technique. I: Identification of optimal laser dose, power, and solder surface area. Microsurgery 18, 55–59 (1998). [DOI] [PubMed] [Google Scholar]
- 43.Hwang K, Kim SG, Kim DJ & Lee CH Laser welding of rat’s facial nerve. J Craniofac Surg 16, 1102–1106 (2005). [DOI] [PubMed] [Google Scholar]
- 44.Williams MJ et al. A computational study of the role of the aortic arch in idiopathic unilateral vocal-fold paralysis. J. Appl. Physiol 118, 465–474 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ark M, Cosman PH, Boughton P & Dunstan CR Review: Photochemical Tissue Bonding (PTB) methods for sutureless tissue adhesion. International Journal of Adhesion and Adhesives 71, 87–98 (2016). [Google Scholar]
- 46.Johnson TS et al. Photochemical Tissue Bonding: A Promising Technique for Peripheral Nerve Repair. Journal of Surgical Research 143, 224–229 (2007). [DOI] [PubMed] [Google Scholar]
- 47.Lauto A, Mawad D, Barton M, Piller SC & Longo L Chitosan Adhesive Films for Photochemical Tissue Bonding. in 87–93 (AIP, 2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lauto A et al. Photochemical tissue bonding with chitosan adhesive films. Biomed Eng Online 9, 47 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O’Neill AC et al. Microvascular anastomosis using a photochemical tissue bonding technique. Lasers Surg Med 39, 716–722 (2007). [DOI] [PubMed] [Google Scholar]
- 50.Parker NP Use of the Carbon Dioxide Laser as an Office-Based Surgical Tool: An Old Dog Can Do a New(ish) Trick. JAMA Otolaryngol Head Neck Surg 143, 492–493 (2017). [DOI] [PubMed] [Google Scholar]
- 51.Day AT, Sinha P, Nussenbaum B, Kallogjeri D & Haughey BH Management of primary T1–T4 glottic squamous cell carcinoma by transoral laser microsurgery. The Laryngoscope 127, 597–604 (2017). [DOI] [PubMed] [Google Scholar]
- 52.Huang DTC, Blanks DRHI, Berns DMW & Crumley DRL Laser VS, Suture Nerve Anastomosis. Otolaryngology - Head and Neck Surgery 107, 14–20 (1992). [DOI] [PubMed] [Google Scholar]


