Abstract
Background
Research indicates reduced physical performance from diagnosis into survivorship of pediatric cancer patients. However, there is no systematic information or guideline available on the methods to assess physical performance and function in this population. The purpose was to systematically compile and describe assessments of physical performance and function in patients and survivors of pediatric cancer, including cardiorespiratory fitness, muscle strength, speed, balance, flexibility, functional mobility, gait and motor performance test batteries.
Methods
We searched the databases PubMed, SPORTDiscus, and Cochrane Database and performed abstract and full-text selection of 2619 articles according to the Cochrane Handbook of Systematic Reviews. Information on patients characteristics, assessments, information on validity and reliability, and relevant references was extracted.
Results
In summary, 63 different assessments were found in 149 studies including 11639 participants. Most studies evaluated cardiorespiratory fitness and muscle strength with the majority conducted off treatment. Some outcomes (e.g. speed) and diagnoses (e.g. neuroblastoma) were severely underrepresented. With the exception of gait, leukemia patients represented the largest group of individuals tested.
Conclusions
Insufficient data and patient heterogeneity complicate uniform recommendations for assessments. Our results support researchers and practitioners in selecting appropriate assessment to meet their specific research questions or individual daily practice needs.
Impact
This systematic review includes 149 studies and provides a comprehensive summary of 63 assessments to evaluate cardiorespiratory fitness, muscle strength, speed, balance, flexibility, functional mobility, gait or motor performance test batteries in patients and survivors of pediatric cancer.
We present the most studied fields within the pediatric cancer population, which are cardiorespiratory fitness and muscle strength, off treatment phase, and leukemia patients.
We propose research priorities by identification of subgroups in terms of cancer type, phase of treatment, and outcome of interest that are underrepresented in studies currently available.
Introduction
Age-appropriate healthy physical and functional development of infants, children, and adolescents is an important prerequisite for participation in physical activity and sports representing a major determinant of a long-term active and healthy lifestyle.1 Physical and functional performance of children and adolescents during and after cancer treatment has been the interest of a growing number of studies during past decades. Current literature presents increasing evidence that childhood cancer patients and survivors are challenged by physical performance limitations such as reduced cardiorespiratory fitness, muscle strength, balance, gait, functional mobility, and flexibility/range of motion.2–4 Influencing factors for these impairments might be the cancer itself, side effects of medical therapy, and inactivity during and after treatment.5 Study results demonstrate reduced physical performance shortly after diagnosis,6 during acute treatment,3 and persisting throughout survivorship.7 This is specifically concerning as physical performance limitations are linked to an increased incidence of unemployment and low income.8
At the same time, preliminary exercise intervention studies provide promising results in terms of efficacy to improve physical performance and fitness.9–11 However, evaluation of those positive effects found in research interventions with childhood cancer populations is difficult due to the large number of different physical and functional performance assessments that have been used in pediatric oncology research. An overview of assessments could help future researchers when planning a study on exercise and fitness in child and adolescent cancer patients and survivors. Few attempts have been done to summarize and describe tests performed and used in this population. Grimshaw et al.12 summarized subjective and objective tools to measure physical function and physical activity in the age group 0–18 years with a focus on the evaluation of measurement properties. Another group of researchers13 listed evaluation tools used in childhood cancer physical activity/exercise studies or community-based programs that assess motor performance, physical literacy, well-being, quality of life (QoL), and health behavior, but assessments of physical performance and fitness were excluded. However, no review has predefined the categories of physical and functional performance relevant to health and exercise science14 in order to systematically search and summarize them. Thus, the aim of the present systematic review is to summarize in detail all assessments used to measure cardiorespiratory fitness, muscle strength, speed, balance, flexibility, functional mobility, gait, and motor performance in interventional and non-interventional studies with childhood cancer patients and survivors. This summary is intended to support researchers and therapists in selecting the most appropriate assessments for their individual purposes and needs.
Materials and methods
This paper was written according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA Statement).15
Data sources and searches
We systematically searched PubMed, Cochrane Central Register of Controlled Trials and SPORTDiscus from database inception to 13 February 2020. The search strategy (see Appendix 1) included Medical Subject Headings with terms and text words to identify studies conducted with children, adolescents, or adults during or after childhood cancer treatment who underwent any assessment for either physical or functional performance. In addition, references of relevant reviews and reference lists of included studies were screened. The specific outcomes of interest were assessments for cardiorespiratory fitness, muscle strength, speed, balance, flexibility, functional mobility, gait, and motor performance assessed in test batteries. Motor performance test batteries measuring physical performance provide an important overview of performance levels and motor development and are of great importance in children and adolescents. In pediatric oncology and chronically ill children, they are usually assessing performance of general motor skills. Data on validity and reliability of the included assessments in pediatric cancer patients and survivors were extracted from the included full texts and associated references.
Study selection
After exclusion of duplicates, three teams of two researchers each independently reviewed titles and abstracts of the identified articles. Studies were excluded for the following reasons: (i) less than 75% of the population were diagnosed with cancer <21 years, (ii) the outcome was no measure of either physical or functional performance as defined above, (iii) any non-original articles (e.g. reviews, congress abstracts, commentaries or letters without data), (iv) duplicates that were not identified as such before, (v) studies without description of assessment used (vi) studies/assessment with less than five participants, or (vii) full-texts that were not available in English or German. We included all types of studies and had no restriction in terms of publication date. In case of disagreement between the two reviewers, articles were discussed between these two and if no consensus could be reached, a third reviewer was consulted. After final inclusion of abstracts, the respective full texts were reviewed independently as described above.
Data extraction, synthesis, and analysis
Relevant data from the included full texts were extracted and organized into standardized data tables. During the data extraction process, the following information was extracted from all texts: study citation, characteristics of the study population (sample sizes, age ranges, diagnoses, stage of cancer treatment), assessments used and their measurement properties, and relevant references for further information. In terms of measurement properties, information regarding validity and reliability of the assessment was only extracted if those were evaluated in the childhood cancer study sample. Based on these tables, assessments were sorted into predefined health-related and skill-related categories as defined by the American College of Sports Medicine14 (cardiorespiratory fitness, muscle strength, speed, balance, flexibility). In addition to these main motor domains, functional mobility, gait, and motor performance test batteries which have been identified to be of high relevance for the population of children with cancer3,16,17 and for coping with everyday life and participation with peers18 were included. For each single assessment (e.g. 6-minute walk test (6MWT) in the category cardiorespiratory fitness) all information about the study participants was merged from the studies using this particular assessment. Diagnoses were grouped into categories, i.e. leukemia/lymphoma (as hematological tumors), bone tumor, CNS tumors, and others. This classification was made because individuals after bone tumors or CNS tumors are known to suffer from more severe motor deficits due to the underlying disease.19 In case of insufficient information, study authors were contacted via email. If no answer was received, information was taken from the manuscript as specific as possible.
Results
Literature search results
Figure 1 displays the flow of studies through the review process. After 81 duplicates were removed, 2619 records underwent abstract screening, of which 2295 were excluded and 324 articles were retained for full-text review. Additional 40 articles, identified through reference list screening of reviews and other sources, were then added to full-test screening, resulting in 364 articles. Of those, 215 articles were excluded with reasons and 149 full texts3,6,10,20–165 (see Appendix 2 for detailed study information) were included for data extraction, representing 5.7% of screened abstracts and 40.7% of screened full text articles. Agreement between the reviewers for abstract screening ranged between 82 and 96% and for the full text screening between 84 and 93%.
Fig. 1. PRISMA flow diagram showing the reference selection process.
*Reasons for exclusion: less than 75% of the population were diagnosed with cancer measure of either physical or functional performance (n = 55), any non-original articles (e.g. reviews, congress abstracts, commentaries or letters without data) (n = 31), duplicates that were not identified as such before (n = 10), (v) studies without description of assessment used (n =9), (vi) studies/assessment with less than five participants (n = 11), or (vii) full-texts that were not available in English or German (n = 13).
Study characteristics
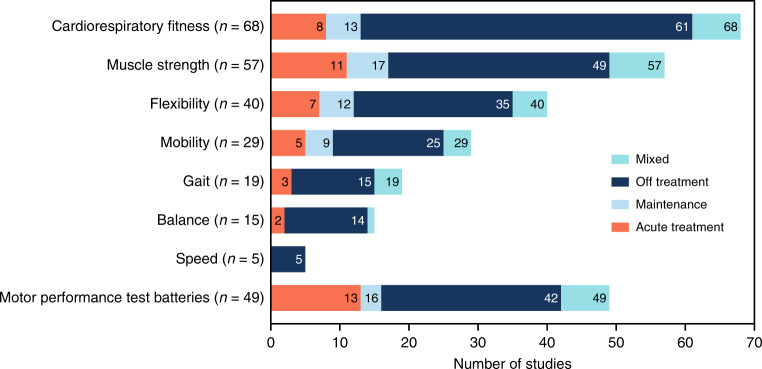
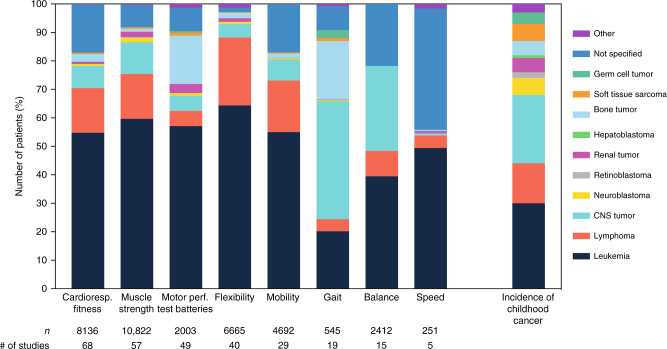
In summary, all 149 studies, describing 63 different assessment methods, included in this systematic review were published between 1984 and 2020. Of those, n = 1 study was published between 1984 and 1990, n = 18 studies between 1991 and 2000, n = 48 studies between 2001 and 2010, and n = 82 studies between 2011 and 2020. The studies included a total number of n = 11,639 participants being treated for childhood cancer and/or having received hematopoietic stem cell transplantation (HSCT). Of these, n = 6295 (54.1%) were diagnosed with leukemia, n = 1408 (12.1%) with lymphoma, n = 1271 (10.9%) with tumors of the central nervous system (CNS), n = 76 (0.7%) with neuroblastoma, n = 12 (0.1%) with retinoblastoma, n = 149 (1.3%) with renal tumor, n = 3 (0.03%) with hepatoblastoma, n = 692 (5.9%) with bone tumor, n = 68 (0.6%) with soft tissue sarcoma, n = 34 (0.3%) with germ cell tumor, and n = 56 (0.5%) with other malignancies. In n = 1575 cases (13.5%) a classification was not possible due to a missing detailed description in the full texts. While 22 studies (14.8%) took place during active cancer treatment, 9 studies (6.0%) were conducted during maintenance therapy and 99 studies (66.4%) after treatment. Nineteen studies (12.8%) included participants during different phases of medical treatment. The age of participants ranged between 1.0 and 68.3 years. Most studies analyzed a parameter of cardiorespiratory fitness, followed by strength, motor performance in test batteries, flexibility, functional mobility, gait and balance. Only five studies evaluated speed (Fig. 2). Considering the incidence of childhood cancer,166 the number of individuals tested in the categories of physical performance and function deviates from the incidence of the tumor type. An overview of the distribution of diagnoses within each category and overall childhood cancer incidence rates are presented in Fig. 3. Some physical performance categories, like cardiorespiratory fitness and muscle strength, were tested in many different types of cancer. However, in all categories, with the exception of gait, individuals with leukemia were over-represented. For gait and motor performance test batteries, the inclusion of bone tumor patients was far above the percentage incidence of bone tumors, whereas in the other six categories, bone tumors as well as other solid tumors were investigated less frequently.
Fig. 2. Number of studies for each outcome measure, indicating the phase of therapy in which the studies were conducted.
Please note: The sum of assessments in Fig. 2 (n = 282) is greater than the number of all included studies (n = 149), because in many studies assessments from several categories were included.
Fig. 3. Distribution of the types of cancer (%) that were included in the assessments of the eight categories of physical performance and function.
The bar on the far right shows the incidence of childhood cancer as a reference. Note: Number of persons tested specified here (e.g. n = 8136 for cardiorespiratory fitness) differs from number of study participants (n = 7936, see section on cardiorespiratory fitness above), because some study participants were tested using several test methods.
Results on methods to assess physical and functional performance
In total, 63 different assessments were used to evaluate at least one of the eight categories of physical performance and/or function. Between 2 and 16 different assessments were used to evaluate one of the eight categories. The largest heterogeneity in assessment type, calculated as the number of assessment types divided by the number of studies, was in gait with 8 different assessments from 19 studies (0.42), balance with 6 different assessments from 15 studies, and speed with 2 different assessments from 5 studies (0.40). To assess motor performance with test batteries, 16 different test batteries were used in 49 studies (0.33). Strength was evaluated with 16 different measures in 57 studies (0.28) and mobility with 5 assessments in 29 studies (0.17). The greatest homogeneity in measurement techniques was for cardiorespiratory fitness (0.10) and flexibility (0.08) (7 and 3 different methods in 68 and 40 studies, respectively). The different methods are summarized in Tables 1–8 with more details in Appendices 3–10.
Table 1.
Summary of study methods assessing cardiorespiratory fitness in pediatric oncology.
| Assessment | No. of studies | Total sample sizea | Type of cancer | Age in years (range) | Phases of treatment | Validityc | Reliabilityc | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leukemia/lymphoma | Bone tumor | CNS tumor | Otherb | During | Maint. | Off | ||||||
| Maximal CPET | 33 | 1170 | ✓ | ✓ | ✓ | ✓ | 3.5–41 | ✓ | ✓ | ✓ | – | – |
| 6MWT | 26 | 6180 | ✓ | ✓ | ✓ | ✓ | 3.5–63.8 | ✓ | ✓ | ✓ | – | – |
| Submaximal CPET | 5 | 457 | ✓ | – | ✓ | ✓ | 7–44.6 | – | – | ✓ | – | – |
| 9MWT | 4 | 154 | ✓ | ✓ | – | ✓ | 4–27 | – | ✓ | ✓ | ✓ | ✓ |
| Wingate anaerobic test | 3 | 58 | ✓ | – | – | ✓ | 7.7–23.8 | – | – | ✓ | – | – |
| 2MWT | 2 | 91 | ✓ | – | ✓ | ✓ | 6–45 | – | ✓ | ✓ | – | – |
| PACER | 2 | 25 | ✓ | – | ✓ | – | 4–18 | – | ✓ | ✓ | – | – |
2MWT 2-minute walk test, 6MWT 6-minute walk test, 9MWT 9-minute walk test, CPET cardiopulmonary exercise test, maint. maintenance treatment, No. number, PACER progressive aerobic cardiovascular endurance run.
aOnly study participants who performed the assessments were counted.
bIncluding other cancer diagnoses and diagnoses that were not clearly specified.
cIf evaluated in a childhood cancer population.
Table 8.
Summary of study methods assessing motor performance in test batteries in pediatric oncology.
| Assessment | No. of studies | Total sample sizea | Type of cancer | Age in years (range) | Phases of treatment | Validityc | Reliabilityc | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leukemia/lymphoma | Bone tumors | CNS tumors | Otherb | active | Maint. | Off | ||||||
| BOT-2 | 10 | 327 | ✓ | ✓ | ✓ | ✓ | 4–22 | ✓ | – | ✓ | – | – |
| BOT-2 SF | 6 | 384 | ✓ | – | – | – | 4–18 | ✓ | ✓ | ✓ | – | – |
| m-ABC | 5 | 283 | ✓ | – | – | ✓ | 4.0–19.3 | ✓ | – | ✓ | – | – |
| BOTMP | 5 | 164 | ✓ | – | – | ✓ | 1.75–25.2 | ✓ | ✓ | ✓ | – | – |
| m-ABC 2 | 5 | 124 | ✓ | ✓ | – | ✓ | 3–18.7 | ✓ | – | ✓ | – | – |
| FMA | 4 | 276 | - | ✓ | – | ✓ | 10.4–42.4 | ✓ | – | ✓ | – | – |
| MOON-test | 4 | 141 | ✓ | ✓ | ✓ | ✓ | 4–23 | ✓ | ✓ | ✓ | – | – |
| DMT 6–18 | 4 | 70 | ✓ | ✓ | ✓ | ✓ | 6–17 | – | ✓ | ✓ | – | – |
| GMFM | 4 | 62 | ✓ | – | – | – | 2–14.6 | ✓ | ✓ | ✓ | ✓ | ✓ |
| MOT 4–6 | 3 | 22 | ✓ | – | – | ✓ | 3.42–5.42 | – | ✓ | ✓ | – | – |
| Lincoln–Oseretzky Motor Development Scale | 1 | 45 | ✓ | – | – | ✓ | 5–14 | – | – | ✓ | – | – |
| FMS | 1 | 26 | ✓ | – | ✓ | ✓ | 5–8 | – | – | ✓ | – | – |
| GMFM – ALL | 1 | 20 | ✓ | – | – | – | 2.8–15.9 | ✓ | ✓ | - | ✓ | ✓ |
| UQAC-UQAM Test Battery | 1 | 20 | ✓ | – | – | – | 9–11 | – | – | ✓ | ✓ | – |
| Physical fitness battery test adapted by alpha-fitness-test-battery | 1 | 18 | ✓ | – | – | – | 7.55 ± 2.43 | – | – | ✓ | – | – |
| FITNESSGRAM | 1 | 10 | ✓ | – | ✓ | ✓ | 14.0–18.0 | ✓ | – | ✓ | – | - |
BOT Bruininks–Oseretsky Test, SF short form, m-ABC Movement Assessment Battery for Children, BOTMP Bruininks–Oseretsky Test of Motor Proficiency, FMA functional mobility assessment, MOON motor performance in pediatric oncology, DMT Deutscher Motorik Test, GMFM gross motor function measure, MOT Motoriktest für Kinder, FMS fundamental movement skills test battery, ALL acute lymphoblastic leukemia, UQAC-UQAM University of Québec in Chicoutimi-University of Québec in Montréal, maint. maintenance treatment, No. number.
aOnly study participants who performed the assessments were counted.
bIncluding other cancer diagnoses and diagnoses that were not clearly specified.
cIf evaluated in a childhood cancer population.
Cardiorespiratory fitness (also referred to as endurance, aerobic fitness, or aerobic capacity) was evaluated by a total of 68 studies including n = 7936 patients/survivors using 7 lab- and field-based assessment methods (Table 1 and Appendix 3). While the most frequently used assessments (maximal cardiopulmonary exercise test (CPET) and 6MWT) were administered in all diagnostic subgroups during all phases of treatment and a very wide age range, no other assessment was applied during treatment. In terms of measurement properties, the 9MWT has shown to be both reliable and valid in the pediatric oncology population.92,94
Muscle strength (i.e. muscular endurance or power) was evaluated in 57 studies including n = 5679 childhood cancer patients and survivors using 16 different laboratory and field-based assessment methods (Table 2 and Appendix 4). Muscle strength was assessed either by laboratory or field tests focusing on the upper and lower extremities as well as several assessments of core and back muscle strength. While leukemia and/or lymphoma patients and off treatment phase were included in all assessments, some researchers included other cancer diagnoses or phases of medical treatment. In addition, a wide range of age groups was assessed. Isokinetic dynamometry, hand-held dynamometry, and repetition maximum tests are the only assessments that have been shown to be reliable with pediatric cancer cohorts.92,96
Table 2.
Summary of study methods assessing muscle strength in pediatric oncology.
| Assessment | No. of studies | Total sample sizea | Type of cancer | Age in years (range) | Phase of treatment | Validityc | Reliabilityc | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leukemia/ lymphoma | Bone tumor | CNS tumor | Otherb | During | Maint. | Off | ||||||
| Grip strength test | 27 | 4451 | ✓ | ✓ | ✓ | ✓ | 3.5–64 | ✓ | ✓ | ✓ | – | – |
| Hand held dynamometry | 17 | 830 | ✓ | ✓ | ✓ | ✓ | 4–58 | ✓ | ✓ | ✓ | – | ✓ |
| Isokinetic fynamometry | 10 | 3718 | ✓ | ✓ | ✓ | ✓ | 10.5–64 | – | ✓ | ✓ | – | ✓ |
| Sit-up test | 10 | 339 | ✓ | ✓ | ✓ | ✓ | 5–62.2 | ✓ | ✓ | ✓ | – | – |
| Push-up test | 6 | 239 | ✓ | ✓ | ✓ | ✓ | 4–62.2 | – | ✓ | ✓ | – | – |
| Manual muscle test | 6 | 165 | ✓ | ✓ | - | ✓ | 2–50+ | ✓ | ✓ | ✓ | – | – |
| Repetition maximum | 6 | 104 | ✓ | ✓ | ✓ | ✓ | 4–23 | ✓ | ✓ | ✓ | – | ✓ |
| Repeated squatting | 5 | 182 | ✓ | – | ✓ | ✓ | 6–30 | – | – | ✓ | – | – |
| Back extension test | 4 | 220 | ✓ | – | – | ✓ | 6–62.2 | – | – | ✓ | – | – |
| Isometric dynamometry | 3 | 143 | ✓ | – | – | ✓ | 5–30 | ✓ | – | ✓ | – | – |
| Chair-stand test | 3 | 100 | ✓ | – | ✓ | ✓ | 3.5–18 | ✓ | – | ✓ | – | – |
| Leg lift test | 2 | 128 | ✓ | – | – | – | 6–30 | – | – | ✓ | – | – |
| Vertical jump | 2 | 92 | ✓ | – | – | ✓ | 16–62.2 | – | – | ✓ | – | – |
| Shoulder lift test | 1 | 21 | ✓ | – | – | – | 16–30 | – | – | ✓ | – | – |
| Standing broad jump | 1 | 18 | ✓ | – | – | – | 7.55 ± 2.43d | – | – | ✓ | – | – |
| Lateral step test | 1 | 12 | ✓ | – | ✓ | – | 6–18 | – | – | ✓ | – | – |
maint. maintenance treatment, No. number.
aOnly study participants who performed the assessments were counted.
bIncluding other cancer diagnoses and diagnoses that were not clearly specified.
cIf evaluated in a childhood cancer population.
dNo information on minimum/maximum.
Speed (ability to perform a movement within a short period of time14) was assessed in five studies using two different assessments, which comprised a total of n = 251 childhood cancer survivors aged between 6 and 30 years (Table 3 and Appendix 5). All testing took place after cessation of treatment. Only field tests, namely shuttle run tests, as the 10 × 5m shuttle run and the 4 × 10 m shuttle run and short distance runs, namely a 60 m run test, were administered. Shuttle run tests were not performed with patients who either present with CNS cancer or bone tumors.
Table 3.
Summary of study methods assessing running speed in pediatric oncology.
| Assessment | No. of studies | Total sample sizea | Type of cancer | Age in years (range) | Phases of treatment | Validityc | Reliabilityc | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leukemia/lymphoma | Bone tumor | CNS tumor | Otherb | During | Maint. | Off | ||||||
| Shuttle run | 3 | 146 | ✓ | – | – | ✓ | 6–30 | – | – | ✓ | – | ✓ |
| 60 m run | 2 | 105 | ✓ | ✓ | ✓ | ✓ | 11 ± 3d | – | – | ✓ | – | – |
maint. maintenance treatment, No. number.
aOnly study participants who performed the assessments were counted.
bIncluding other cancer diagnoses and diagnoses that were not clearly specified.
cIf evaluated in a childhood cancer population.
dNo information on minimum/maximum.
Balance was assessed in 15 studies using six different tests including a total of n = 2412 patients/survivors (Table 4 and Appendix 6). The nature of assessments was based on posturography and non-posturography methods. While posturography was only performed after medical treatment, two studies conducted balance tests during treatment.114,148 Bone tumor patients were only included in one study,103 while CNS cancer cohorts were the population of main interest. In terms of age, a wide spectrum including very young children, as well as older adult survivors of childhood cancer (up to an age of 63 years) were analyzed. No information was available on the validity or reliability of any balance assessment in the pediatric oncology population.
Table 4.
Summary of study methods assessing balance in pediatric oncology.
| Assessment | No. of studies | Total sample sizea | Type of cancer | Age in years (range) | Phases of treatment | Validityc | Reliabilityc | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leukemia/lymphoma | Bone tumor | CNS tumor | Otherb | Active | Maint. | Off | ||||||
| Posturography | ||||||||||||
| SOT on dynamic posturography system (various): | 7 | 1805 | ✓ | – | ✓ | ✓ | 10–63.8 | – | – | ✓ | – | – |
| Balance tests (various) on force platforms | 4 | 292 | ✓ | ✓ | ✓ | ✓ | 4–25.2 | – | – | ✓d | – | – |
| Ultrasound-based motion analysis of postural sway | 1 | 22 | – | – | ✓ | – | 11–39 | – | – | ✓ | – | – |
| Non-posturography | ||||||||||||
| The Berg balance test | 1 | 156 | – | – | ✓ | – | 18–58 | – | – | ✓ | – | – |
| Flamingo balance test | 1 | 75 | ✓ | – | ✓ | ✓ | 11.3 ± 3.1e | ✓ | – | – | – | – |
| Single leg stance | 1 | 62 | ✓ | – | – | – | 1–22 | ✓ | – | – | – | – |
maint. maintenance treatment, No. number, SOT sensory organization test.
aOnly study participants who performed the assessments were counted.
bIncluding other cancer diagnoses and diagnoses that were not clearly specified.
cIf evaluated in a childhood cancer population.
dOne study was performed during inpatient rehabilitation potentially including patients still receiving maintenance treatment.
eNo information on minimum/maximum.
Flexibility was assessed in 40 studies, applying three different test methods which included a total of n = 4309 patients/survivors (Table 5 and Appendix 7). Goniometry, measuring ankle joint range of motion, was performed in most studies including a large number of participants of all ages, with a wide range of diagnoses during all phases of medical treatment. Reliability was analyzed in children with acute lymphoblastic leukemia (ALL) in two studies.92,158 In addition, two other flexibility tests measuring hip flexion and trunk flexibility were performed with leukemia/lymphoma, CNS tumor, and other childhood cancer patients and survivors. However, trunk flexibility assessment was only conducted in one study including (young) adults after childhood cancer treatment62 while the sit and reach test was applied more often with all age groups during all phases of treatment. Measurement properties were not analyzed within the childhood cancer population.
Table 5.
Summary of study methods assessing flexibility in pediatric oncology.
| Assessment | No. of studies | Total sample sizea | Type of cancer | Age in years (range) | Phases of treatment | Validityc | Reliabilityc | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leukemia/lymphoma | Bone tumor | CNS tumor | Otherb | During | Maint. | Off | ||||||
| Goniometry | 33 | 3764 | ✓ | ✓ | ✓ | ✓ | 1–64 | ✓ | ✓ | ✓ | – | ✓ |
| Sit and reach | 12 | 2830 | ✓ | ✓ | ✓ | ✓ | 4–64 | ✓ | ✓ | ✓ | – | – |
| Side-bending | 1 | 71 | ✓ | – | – | ✓ | 18.8–62.2 | – | – | ✓ | – | – |
maint. maintenance treatment, No. number.
aOnly study participants who performed the assessments were counted.
bIncluding other cancer diagnoses and diagnoses that were not clearly specified.
cIf evaluated in a childhood cancer population.
Functional mobility was measured in 29 studies including a total of n = 4421 patients using five different assessment methods (Table 6 and Appendix 8). Of these, the Timed Up and Go Test (TUG) was administered in two ways: covering either a 3 m or a 10 m distance. While the TUG 3 m and the Timed Up and Down Stairs Test (TUDS) were applied within several studies, including various childhood cancer diagnoses during all phases of medical treatment, the TUG 10 m was only used with ALL patients during maintenance and/or off treatment.128,130,131 Two additional functional tests (stand up from bed rest exam and floor to stand performance test) were both administered within one study each during treatment for childhood cancer.79,148 Only the TUG 3 m was performed with older (up to age 64 years) adult survivors of childhood cancer, while all other assessments were conducted with children, adolescents, and young adults. The TUG 3 m demonstrated high validity and reliability96 while the TUDS and TUG 10 m both have shown to be reliable.131
Table 6.
Summary of study methods assessing functional mobility in pediatric oncology.
| Assessment | No. of studies | Total sample sizea | Type of cancer | Age in years (range) | Phases of treatment | Validityc | Reliabilityc | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leukemia/lymphoma | Bone tumors | CNS tumors | Otherb | active | Maint. | Off | ||||||
| TUG 3 m | 25 | 4,283 | ✓ | ✓ | ✓ | ✓ | 3.5–64 | ✓ | ✓ | ✓ | ✓ | ✓ |
| TUDS | 13 | 314 | ✓ | ✓ | – | ✓ | 3.5–27 | ✓ | ✓ | ✓ | – | ✓ |
| TUG 10 m | 3 | 22 | ✓ | – | – | – | 4–16 | – | ✓ | ✓ | – | ✓ |
| Floor to stand performance | 1 | 62 | ✓ | – | – | – | 1–22 | ✓ | – | – | – | – |
| Stand up from bed rest exam | 1 | 11 | ✓ | – | – | ✓ | 3.5–15 | ✓ | – | – | – | – |
maint. maintenance treatment, No. number, TUDS timed up and down stairs test, TUG timed up and go test.
aOnly study participants who performed the assessments were counted.
bIncluding other cancer diagnoses and diagnoses that were not clearly specified.
cIf evaluated in a childhood cancer population.
Gait analyses was carried out in 19 studies, using eight different methods including a total of n = 545 patients/survivors (Table 7 and Appendix 9). A wide variety of systems were used to assess gait in childhood cancer populations. While few studies used video-recording, partly in combination with force platforms and sometimes electromyography (EMG) measurements, single studies used specific systems, visual observation, or a timed walking test. Except for the EMG analysis of gait and visual observation, all systems assessed gait within various groups of childhood cancer diagnoses. However, only two methods (GAITRite and visual observation) were performed during treatment.50,148,150 No information is available on validity and/or reliability of any gait analysis system in the pediatric oncology population.
Table 7.
Summary of study methods assessing gait in pediatric oncology.
| Assessment | No. of studies | Total sample sizea | Type of cancer | Age in years (range) | Phases of treatment | Validityc | Reliabilityc | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leukemia/lymphoma | Bone tumor | CNS tumor | Otherb | During | Maint. | Off | ||||||
| Video-recording and force platforms | 6 | 111 | – | ✓ | ✓ | ✓ | 3–35 | – | – | ✓d | – | – |
| Video-recording | 4 | 210 | – | ✓ | ✓ | ✓ | 4–24 | – | ✓ | ✓e | – | – |
| Video-recording, force plates and EMG | 3 | 67 | ✓ | ✓ | – | ✓ | 5–68.3 | – | ✓ | ✓ | – | – |
| GAITRite | 2 | 58 | ✓ | – | – | ✓ | 5–22 | ✓ | – | – | – | – |
| Visual observation | 1 | 62 | ✓ | – | – | – | 1–22 | ✓ | – | – | – | – |
| 10 m walk test | 1 | 16 | ✓ | – | ✓ | ✓ | 6–19 | – | ✓ | ✓ | – | – |
| Microgate optogait 2D Gait analysis system | 1 | 13 | ✓ | – | ✓ | ✓ | 6–15.8 | – | – | ✓ | – | – |
| EMG analysis (treadmill) | 1 | 8 | – | ✓ | – | – | N/Af | – | – | ✓ | – | – |
EMG electromyographic, maint. maintenance treatment, No. number.
aOnly study participants who performed the assessments were counted.
bIncluding other cancer diagnoses and diagnoses that were not clearly specified.
cIf evaluated in a childhood cancer population.
dOne study did not include this information, but inclusion criteria were at least one year post-surgery and completed adjuvant treatment program (without radiotherapy).
eOne study did not include this information, but stated 1–24 months after surgery.
f(age at surgery: 5–19 years and time since surgery 13–54 months).
Motor performance test batteries were assessed in 49 studies using 16 different motor test batteries and included a total of 1955 participants (Table 8 and Appendix 10). Most tests were applied in leukemia/lymphoma cohorts after medical treatment. Except for the Functional Mobility Assessment (FMA), which was used in survivors up to age 42 years, all motor test batteries are designed for children and adolescents. Considering all three Bruininks–Oseretsky Test (BOT) versions (BOTMP, BOT-2, BOT-2 SF), the BOT and MOON-Test (Motor performance in pediatric oncology) are the only motor performance test batteries evaluated for feasibility in all diagnosis groups and during all phases of cancer treatment and with young adults. The Gross Motor Function Measure (GFMF) and GFMF-ALL Test Battery are the only assessments that have been evaluated in terms of measurement properties in pediatric oncology populations,160 while the University of Québec in Chicoutimi-University of Québec in Montréal (UQAC-UQAM) has been validated using the Jackknife method.85
Discussion
This systematic review summarizes the available studies assessing physical performance and function in pediatric cancer patients and survivors. Based on the included 149 studies with 11,639 participants and 63 different assessment tools, we found important characteristics of the distribution and characteristics of the assessments (Table 9). The majority of studies (45.6%) assessing physical or functional performance evaluated cardiorespiratory fitness as an outcome. The 68 studies testing for cardiorespiratory fitness using seven different assessment tools highlight a high homogeneity in the choice of methods. Flexibility was also frequently examined with very uniform assessments. In contrast, muscle strength tests and motor performance batteries have also been evaluated in a high number of studies (57 resp. 49), although with enormous variation in assessment tools. Therefore, the idea of harmonizing physical and functional performance assessments arises to improve comparability of study results. However, harmonization does not seem appropriate nor reasonable across all pediatric cancer types, age groups, treatment phases, and research questions.
Table 9.
Summary of the main findings.
| Main findings are… |
|---|
| 1. Physical function and performance were mostly evaluated after medical treatment. |
| 2. Leukemia patients formed the most examined group while solid tumors were less studied. |
| 3. Cardiorespiratory fitness and muscle strength were the physical outcomes of main interest. |
| 4. Assessments with the highest number of participants were |
| • 6 MWT (n = 6180 in 26 studies) |
| • Grip strength (n = 4451 in 27 studies) |
| • TUG 3 m (n = 4283 in 25 studies). |
| 5. Most assessments have not been evaluated for validity and reliability in pediatric cancer populations. |
6MWT 6-minute walk test, TUG timed up and go test.
Speed as a physical performance measure has rarely been evaluated. It can be hypothesized that speed, assessed via shuttle run or other running tests, is difficult to assess during cancer treatment, because children are in a reduced overall condition during cancer treatment. In addition, the health benefits of speed for children and young people appear to be less prominent in the literature than cardiorespiratory fitness and muscle strength167 and are therefore less focused in children with and after cancer.
In terms of treatment phase, most studies (66%) have been conducted after cessation of cancer therapy with childhood cancer survivors. The evaluation of persistent physical limitations is of great importance, as they may be limiting to working ability and participation.168 Nevertheless, a continuous monitoring of physical performance should be carried out from the time of diagnosis in order to detect physical limitations at an early stage and prevent further deterioration in a sense of early rehabilitation. At the same time, assessment of physical performance from diagnosis onward is important to determine the need for structured exercise. However, since physical fitness, medical side effects, and motivation vary considerably over the course of the therapy, and are dependent on age, diagnoses, and cancer stage, assessment tools evaluating physical performance and function in children with cancer have to fulfill many requirements. To be feasible and safe, different assessments might be chosen according to different groups of patients.
In terms of sample size, eight tests should be highlighted as they were performed by more than 1000 children each, namely grip and isokinetic dynamometry (muscle strength), 6MWT and maximum CPET (cardiorespiratory fitness), goniometry and sit and reach (flexibility), TUG 3 (functional mobility), and SOT (balance). Of those, the 6MWT (n = 6180), grip strength (n = 4451), and TUG 3 m (n = 4283) were the tests with the greatest number of participants. This fact suggests that those outcomes are of specific interest in pediatric oncology as scientists and clinicians seem specifically concerned about their patients’ ability to perform everyday activities, since functional mobility as well as walking capacity measured with the 6MWT are considered important prerequisites to perform physically activities of everyday life.46,169,170
Concerning the motor test batteries, geographical differences are noticeable. It can be assumed that countries use tests for which reference values of healthy kindergarten and schoolchildren are available. Especially for younger children motor performance test batteries seem to be appropriate to generate an overview of age-related motor development in comparison with age-related reference values. However, generating a database with reference values for children during and after cancer treatment could be helpful to evaluate skills in the context of cancer treatment.
Measurement properties such as validity or reliability of assessments are rarely available for the population of pediatric cancer patients or survivors. With reference to the period covered by this search, only the 9MWT, TUG 3 m, GMFM, and GMFM-ALL have been tested for validity and reliability, while others (hand held dynamometry, isokinetic dynamometry, repetition maximum test, shuttle run, gonimetry, TUDS, TUG 10 m, and UQAC-UQAM test battery) have been tested for reliability within this population. In addition, it might be useful to examine the quality criteria for children with chronic conditions in general since various chronic health conditions are present during/after pediatric cancer treatment ranging from endocrinological to orthopedic and psychosocial problems.171 Moreover, information on quality criteria assessed with healthy children may also be helpful while choosing appropriate assessments. Cooperation of interdisciplinary professional societies and scientists could contribute to a joint evaluation of the quality criteria for chronically ill and disabled children.
Overall, due to the variety of assessments used and the small cohorts often found in childhood cancer studies, the significance of studies on physical and functional performance is very limited. Furthermore, it is hypothesized that children with severe physical limitations have been excluded from many tests that have originally been developed for healthy children.
Limitations and strength
The strength of this article lies in the comprehensive research, systematic elaboration, and overview of all methods used to test physical performance in the context of pediatric oncology. The inclusion of interventional as well as observational studies allows a complete listing and a clear focus on the assessment tools. Although the lack of a rating and recommendation of the assessments seems to be a weakness, this evaluation was deliberately avoided. Recommendations based on single studies with large sample sizes, personal experiences, or geographical preferences appear inappropriate, as the objective presentation of the assessments were the primary aim. Instead, the results enable researchers and practitioners to select methods from this paper that correspond to their individual research questions or everyday practice and inform researchers about urgent research questions. The elaboration of recommendations and contraindications for the individual therapy phases, cancer types, and study settings as well as the evaluation of the quality criteria in the target group of children with cancer should be the subject of further research in the context of an international consensus. Methodological limitations include that no distinction has been made between diagnosis subgroups (i.e. myeloid and lymphoblastic leukemia) due to lack of detailed descriptions within studies included. The reader should be aware that the frequency with which an assessment is used does not allow any direct conclusions to be drawn about the quality and suitability of the assessment. Rather, the publication date of the assessment, national availability, and translations, as well as material and costs may also have an influence.
Clinical implication
This systematic review identified 149 studies assessing any category of physical or functional performance in childhood cancer patients or survivors. However, the evidence for the effectiveness of interventions to improve aspects of physical performance is very limited.9,172 This might be related to the methodological quality of intervention studies. However, using standardized tools to assess physical and functional performance in defined subgroups of pediatric cancer patients and survivors (i.e. children with ALL during treatment) would enable meta-analyses of single cohorts and overall improve the significance of studies. Apart from clinical research, clinicians, exercise physiologists, and physiotherapists may choose assessment tools presented here with regard to their individual needs and objectives.
Future research
Future research should focus on evaluating the measurement properties of methods in pediatric cancer populations and children with other chronic diseases. In addition, building an international recommendation statement for assessments in smaller subgroups of pediatric cancer patients and survivors could be a valuable contribution to the current knowledge. Another important step is to generate a database with standard values of children and adolescents suffering from cancer. This could help to compare measures from research and clinical work with children with other chronic conditions, identify impairments and react with early interventions to improve cancer treatment and decrease negative side effects. To expand existing knowledge about leukemia patients to other diagnoses, cancer types like neuroblastoma, retinoblastoma, renal tumors, or soft tissue sarcoma should be tested for physical performance limitations to evaluate their special needs. Furthermore, acute and maintenance treatment phases are less studied but might be of special interest to prevent physical performance deconditioning. And finally, since survivors of childhood cancer can experience very heterogeneous late sequelae, a transferability of the test applications to children with heart or lung diseases, metabolic diseases, or other chronic conditions is conceivable and should be verified in future research projects.
Supplementary information
Acknowledgements
The authors are a subgroup of the Network ActiveOncoKids. This network is committed to promoting physical activity for children and young people with cancer. The network members had the opportunity to give feedback and contribute ideas during meetings and were thus partly involved in the development of this work. This work was in parts supported by the Deutsche Forschungsgemeinschaft (DFG). The DFG had no role in the design and conduct of the study.
Author contributions
R.S., J.D., and M.G. conceptualized and designed the study, collected data, carried out the analyses, interpreted the data, drafted the initial manuscript, and revised the manuscript. S.R., T.-C.W., and C.C.-V. participated in data collection and data extraction and reviewed and revised the manuscript. S.V.K., K.G.E., V.O., C.S.R., A.S.-M., S.S., A.-M.T., J.W., and P.W. participated in the conceptualization of the study, provided critical feedback throughout data collection and extraction, and critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Competing interests
The authors declare no competing interests.
Consent statement
No patient consent required.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Regine Söntgerath, Julia Däggelmann
Supplementary information
The online version contains supplementary material available at 10.1038/s41390-021-01523-5.
References
- 1.Janssen ILAG. Systematic review of the health benefits of physical activity and fitness in school-aged children and youth. Int. J. Behav. Nutr. Phys. Act. 2010;7:40. doi: 10.1186/1479-5868-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ness KK, et al. Physical performance limitations in the Childhood Cancer Survivor Study cohort. J. Clin. Oncol. 2009;27:2382–2389. doi: 10.1200/JCO.2008.21.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Götte M, Kesting SV, Winter CC, Rosenbaum D, Boos J. Motor performance in children and adolescents with cancer at the end of acute treatment phase. Eur. J. Pediatr. 2015;174:791–799. doi: 10.1007/s00431-014-2460-x. [DOI] [PubMed] [Google Scholar]
- 4.Söntgerath R, Eckert K. Impairments of lower extremity muscle strength and balance in childhood cancer patients and survivors: a systematic review. Pediatr. Hematol. Oncol. 2015;32:585–612. doi: 10.3109/08880018.2015.1079756. [DOI] [PubMed] [Google Scholar]
- 5.Götte M, Taraks S, Boos J. Sports in pediatric oncology: the role(s) of physical activity for children with cancer. J. Pediatr. Hematol. Oncol. 2014;36:85–90. doi: 10.1097/MPH.0000000000000101. [DOI] [PubMed] [Google Scholar]
- 6.Deisenroth A, et al. Muscle strength and quality of life in patients with childhood cancer at early phase of primary treatment. Pediatr. Hematol. Oncol. 2016;33:393–407. doi: 10.1080/08880018.2016.1219796. [DOI] [PubMed] [Google Scholar]
- 7.Phillips NS, et al. Physical fitness and neurocognitive outcomes in adult survivors of childhood acute lymphoblastic leukemia: a report from the St. Jude Lifetime cohort. Cancer. 2020;126:640–648. doi: 10.1002/cncr.32510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ness KK, et al. The impact of limitations in physical, executive, and emotional function on health-related quality of life among adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Arch. Phys. Med. Rehabil. 2008;89:128–136. doi: 10.1016/j.apmr.2007.08.123. [DOI] [PubMed] [Google Scholar]
- 9.Braam KI, et al. Physical exercise training interventions for children and young adults during and after treatment for childhood cancer. Cochrane Database Syst. Rev. 2016;3:CD008796. doi: 10.1002/14651858.CD008796.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Senn-Malashonak A, et al. Psychophysical effects of an exercise therapy during pediatric stem cell transplantation: a randomized controlled trial. Bone Marrow Transplant. 2019;54:1827–1835. doi: 10.1038/s41409-019-0535-z. [DOI] [PubMed] [Google Scholar]
- 11.Stössel S, et al. Benefits of exercise training for children and adolescents undergoing cancer treatment: results from the randomized controlled MUCKI Trial. Front. Pediatr. 2020;8:243. doi: 10.3389/fped.2020.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grimshaw SL, Taylor NF, Mechinaud F, Shields N. Assessment of physical function in children with cancer: a systematic review. Pediatr. Blood Cancer. 2018;65:e27369. doi: 10.1002/pbc.27369. [DOI] [PubMed] [Google Scholar]
- 13.Shank J, et al. Evaluation tools for physical activity programs for childhood cancer: a scoping review. J. Pediatr. Oncol. Nurs. 2020;37:163–179. doi: 10.1177/1043454219891987. [DOI] [PubMed] [Google Scholar]
- 14.American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription 10th edn. (Wolters Kluwer, 2017). [DOI] [PubMed]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wacker K, Tanner L, Ovans J, Mason J, Gilchrist L. Improving functional mobility in children and adolescents undergoing treatment for non-central nervous system cancers: a systematic review. PM R. 2017;9:S385–S397. doi: 10.1016/j.pmrj.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Beulertz J, et al. Limitations in ankle dorsiflexion range of motion, gait, and walking efficiency in childhood cancer survivors. Cancer Nurs. 2016;39:117–124. doi: 10.1097/NCC.0000000000000256. [DOI] [PubMed] [Google Scholar]
- 18.Keiser T, et al. Short-term consequences of pediatric anti-cancer treatment regarding blood pressure, motor performance, physical activity and reintegration into sports structures. Front. Pediatr. 2020;8:463. doi: 10.3389/fped.2020.00463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rueegg CS, et al. Physical performance limitations in adolescent and adult survivors of childhood cancer and their siblings. PLoS ONE. 2012;7:e47944. doi: 10.1371/journal.pone.0047944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akyay A, Olcay L, Sezer N, Atay Sönmez Ç. Muscle strength, motor performance, cardiac and muscle biomarkers in detection of muscle side effects during and after acute lymphoblastic leukemia treatment in children. J. Pediatr. Hematol. Oncol. 2014;36:594–598. doi: 10.1097/MPH.0000000000000067. [DOI] [PubMed] [Google Scholar]
- 21.Bastian AJ, Mink JW, Kaufman BA, Thach WT. Posterior vermal split syndrome. Ann. Neurol. 1998;44:601–610. doi: 10.1002/ana.410440405. [DOI] [PubMed] [Google Scholar]
- 22.Bell W, et al. Perception of effort at low and moderate intensity exercise in survivors of childhood acute lymphoblastic leukaemia. Ann. Hum. Biol. 2006;33:357–371. doi: 10.1080/03014460600687382. [DOI] [PubMed] [Google Scholar]
- 23.Benedetti MG, et al. How much clinical and functional impairment do children treated with knee rotationplasty experience in adulthood? Clin. Orthop. Relat. Res. 2016;474:995–1004. doi: 10.1007/s11999-016-4691-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beulertz J, Bloch W, Prokop A, Baumann FT. Specific deficit analyses in motor performance and quality of life of pediatric cancer patients—a cross-sectional pilot study. Pediatr. Hematol. Oncol. 2013;30:336–347. doi: 10.3109/08880018.2013.776155. [DOI] [PubMed] [Google Scholar]
- 25.Beulertz J, et al. Limitations in ankle dorsiflexion range of motion, gait, and walking efficiency in childhood cancer survivors. Cancer Nurs. 2016;39:117–124. doi: 10.1097/NCC.0000000000000256. [DOI] [PubMed] [Google Scholar]
- 26.Beulertz J, et al. Effects of a 6-month, group-based, therapeutic exercise program for childhood cancer outpatients on motor performance, level of activity, and quality of life. Pediatr. Blood Cancer. 2016;63:127–132. doi: 10.1002/pbc.25640. [DOI] [PubMed] [Google Scholar]
- 27.Bianco A, et al. Evaluation of fitness levels of children with a diagnosis of acute leukemia and lymphoma after completion of chemotherapy and autologous hematopoietic stem cell transplantation. Cancer Med. 2014;3:385–389. doi: 10.1002/cam4.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Black P, Gutjahr P, Stopfkuchen H. Physical performance in long-term survivors of acute leukaemia in childhood. Eur. J. Pediatr. 1998;157:464–467. doi: 10.1007/s004310050854. [DOI] [PubMed] [Google Scholar]
- 29.Braam KI, et al. Cardiorespiratory fitness and physical activity in children with cancer. Support Care Cancer. 2016;24:2259–2268. doi: 10.1007/s00520-015-2993-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Braam KI, et al. Effects of a combined physical and psychosocial training for children with cancer: a randomized controlled trial. BMC Cancer. 2018;18:1289. doi: 10.1186/s12885-018-5181-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Braam KI, et al. Application of the steep ramp test for aerobic fitness testing in children with cancer. Eur. J. Phys. Rehabil. Med. 2015;51:547–555. [PubMed] [Google Scholar]
- 32.Brinkman TM, et al. Attainment of functional and social independence in adult survivors of pediatric cns tumors: a report from the St Jude Lifetime Cohort Study. J. Clin. Oncol. 2018;36:2762–2769. doi: 10.1200/JCO.2018.77.9454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carty CP, Bennett MB, Dickinson IC, Steadman P. Assessment of kinematic and kinetic patterns following limb salvage procedures for bone sarcoma. Gait Posture. 2009;30:547–551. doi: 10.1016/j.gaitpost.2009.08.234. [DOI] [PubMed] [Google Scholar]
- 34.Carty CP, Dickinson IC, Watts MC, Crawford RW, Steadman P. Impairment and disability following limb salvage procedures for bone sarcoma. Knee. 2009;16:405–408. doi: 10.1016/j.knee.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 35.Corr AM, et al. Feasibility and functional outcomes of children and adolescents undergoing preoperative chemotherapy prior to a limb-sparing procedure or amputation. Rehabil. Oncol. 2017;35:38–45. [PMC free article] [PubMed] [Google Scholar]
- 36.Cortés-Reyes É, Escobar-Zabala P, González-García L. The effect of game-based exercise on infant acute lymphocytic leukaemia patients. Rev. Fac. Med. 2013;61:349–355. [Google Scholar]
- 37.Cox, C. L. et al. Modifying bone mineral density, physical function, and quality of life in children with acute lymphoblastic leukemia. Pediatr. Blood Cancer10.1002/pbc.26929 (2018). [DOI] [PMC free article] [PubMed]
- 38.Däggelmann J, et al. Einfluss einer vierwöchigen familienorientierten Rehabilitation auf die motorische Leistungsfähigkeit, Lebensqualität und Fatigue bei krebskranken Kindern und gesunden Geschwistern. Rehabilitation (Stuttg.). 2017;56:119–126. doi: 10.1055/s-0043-103064. [DOI] [PubMed] [Google Scholar]
- 39.Davis EE, Pitchford NJ, Jaspan T, McArthur D, Walker D. Development of cognitive and motor function following cerebellar tumour injury sustained in early childhood. Cortex. 2010;46:919–932. doi: 10.1016/j.cortex.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 40.De Caro E, et al. Exercise capacity in apparently healthy survivors of cancer. Arch. Dis. Child. 2006;91:47–51. doi: 10.1136/adc.2004.071241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DeFeo BM, et al. Long-term functional outcomes among childhood survivors of cancer who have a history of osteonecrosis. Phys. Ther. 2020;100:509–522. doi: 10.1093/ptj/pzz176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dubnov-Raz G, et al. Changes in fitness are associated with changes in body composition and bone health in children after cancer. Acta Paediatr. 2015;104:1055–1061. doi: 10.1111/apa.13052. [DOI] [PubMed] [Google Scholar]
- 43.Ehrhardt, M. J. et al. Late outcomes of adult survivors of childhood non-Hodgkin lymphoma: a report from the St. Jude Lifetime Cohort Study. Pediatr. Blood Cancer10.1002/pbc.26338 (2017). [DOI] [PMC free article] [PubMed]
- 44.Esbenshade AJ, et al. Feasibility and initial effectiveness of home exercise during maintenance therapy for childhood acute lymphoblastic leukemia. Pediatr. Phys. Ther. 2014;26:301–307. doi: 10.1097/PEP.0000000000000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fiorillo A, Rinaldi M, Foggia L. Gait analysis in children treated by surgery followed by adjuvant therapy for posterior fossa tumors. Acta Neurol. Belg. 2010;110:306–310. [PubMed] [Google Scholar]
- 46.Fiuza-Luces C, et al. Exercise intervention in pediatric patients with solid tumors: The Physical Activity in Pediatric Cancer Trial. Med. Sci. Sports Exerc. 2017;49:223–230. doi: 10.1249/MSS.0000000000001094. [DOI] [PubMed] [Google Scholar]
- 47.Fuchs, B., Kotajarvi, B. R., Kaufman, K. R. & Sim, F. H. Functional outcome of patients with rotationplasty about the knee. Clin. Orthop. Relat. Res. 52–58, 10.1097/01.blo.0000093896.12372.c1 (2003). [DOI] [PubMed]
- 48.Galea V, Wright MJ, Barr RD. Measurement of balance in survivors of acute lymphoblastic leukemia in childhood. Gait Posture. 2004;19:1–10. doi: 10.1016/s0966-6362(03)00014-6. [DOI] [PubMed] [Google Scholar]
- 49.Gerber LH, et al. Functional outcomes and life satisfaction in long-term survivors of pediatric sarcomas. Arch. Phys. Med. Rehabil. 2006;87:1611–1617. doi: 10.1016/j.apmr.2006.08.341. [DOI] [PubMed] [Google Scholar]
- 50.Gilchrist L, Tanner L. Gait patterns in children with cancer and vincristine neuropathy. Pediatr. Phys. Ther. 2016;28:16–22. doi: 10.1097/PEP.0000000000000208. [DOI] [PubMed] [Google Scholar]
- 51.Gilchrist LS, Tanner LR. Short-term recovery of balance control: association with chemotherapy-induced peripheral neuropathy in pediatric oncology. Pediatr. Phys. Ther. 2018;30:119–124. doi: 10.1097/PEP.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 52.Gilliam MB, et al. A pilot study evaluation of a web-based token economy to increase adherence with a community-based exercise intervention in child and adolescent cancer survivors. Rehabil. Oncol. 2011;29:16–22. [Google Scholar]
- 53.Ginsberg JP, et al. A comparative analysis of functional outcomes in adolescents and young adults with lower-extremity bone sarcoma. Pediatr. Blood Cancer. 2007;49:964–969. doi: 10.1002/pbc.21018. [DOI] [PubMed] [Google Scholar]
- 54.Gohar SF, Comito M, Price J, Marchese V. Feasibility and parent satisfaction of a physical therapy intervention program for children with acute lymphoblastic leukemia in the first 6 months of medical treatment. Pediatr. Blood Cancer. 2011;56:799–804. doi: 10.1002/pbc.22713. [DOI] [PubMed] [Google Scholar]
- 55.Götte M, et al. MOON-test—determination of motor performance in the pediatric oncology. Klin. Padiatr. 2013;225:133–137. doi: 10.1055/s-0033-1343411. [DOI] [PubMed] [Google Scholar]
- 56.Götte M, Kesting SV, Gerss J, Rosenbaum D, Boos J. Feasibility and effects of a home-based intervention using activity trackers on achievement of individual goals, quality of life and motor performance in patients with paediatric cancer. BMJ Open Sport Exerc. Med. 2018;4:e000322. doi: 10.1136/bmjsem-2017-000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hamari L, et al. The effect of an active video game intervention on physical activity, motor performance, and fatigue in children with cancer: a randomized controlled trial. BMC Res. Notes. 2019;12:784. doi: 10.1186/s13104-019-4821-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harten G, et al. Slight impairment of psychomotor skills in children after treatment of acute lymphoblastic leukemia. Eur. J. Pediatr. 1984;142:189–197. doi: 10.1007/BF00442447. [DOI] [PubMed] [Google Scholar]
- 59.Hartman A, et al. A randomized trial investigating an exercise program to prevent reduction of bone mineral density and impairment of motor performance during treatment for childhood acute lymphoblastic leukemia. Pediatr. Blood Cancer. 2009;53:64–71. doi: 10.1002/pbc.21942. [DOI] [PubMed] [Google Scholar]
- 60.Hartman A, et al. Polymorphisms in genes involved in vincristine pharmacokinetics or pharmacodynamics are not related to impaired motor performance in children with leukemia. Leuk. Res. 2010;34:154–159. doi: 10.1016/j.leukres.2009.04.027. [DOI] [PubMed] [Google Scholar]
- 61.Hartman A, Hop W, Takken T, Pieters R, van den Heuvel-Eibrink M. Motor performance and functional exercise capacity in survivors of pediatric acute lymphoblastic leukemia. Pediatr. Blood Cancer. 2013;60:494–499. doi: 10.1002/pbc.24243. [DOI] [PubMed] [Google Scholar]
- 62.Hartman, A. et al. Health-related fitness in very long-term survivors of childhood cancer: a cross-sectional study. Pediatr. Blood Cancer. 10.1002/pbc.26907 (2018). [DOI] [PubMed]
- 63.Hartman A, van den Bos C, Stijnen T, Pieters R. Decrease in motor performance in children with cancer is independent of the cumulative dose of vincristine. Cancer. 2006;106:1395–1401. doi: 10.1002/cncr.21706. [DOI] [PubMed] [Google Scholar]
- 64.Hartman A, van den Bos C, Stijnen T, Pieters R. Decrease in peripheral muscle strength and ankle dorsiflexion as long-term side effects of treatment for childhood cancer. Pediatr. Blood Cancer. 2008;50:833–837. doi: 10.1002/pbc.21325. [DOI] [PubMed] [Google Scholar]
- 65.Hauser M, Gibson B, Wilson N. Diagnosis of anthracycline-induced late cardiomyopathy by exercise-spiroergometry and stress-echocardiography. Eur. J. Pediatr. 2001;160:607–610. doi: 10.1007/s004310100830. [DOI] [PubMed] [Google Scholar]
- 66.Henderson ER, et al. Outcome of lower-limb preservation with an expandable endoprosthesis after bone tumor resection in children. J. Bone Jt. Surg. Am. 2012;94:537–547. doi: 10.2106/JBJS.I.01575. [DOI] [PubMed] [Google Scholar]
- 67.Hillmann, A. et al. Rotationsplasty Type B IIIa according to Winkelmann: electromyography and gait analysis. Clin. Orthop. Relat. Res. 384, 224–231 (2001). [DOI] [PubMed]
- 68.Hillmann A, et al. Electromyographic and gait analysis of forty-three patients after rotationsplasty. J. Bone Jt. Surg. Am. 2000;82:187–196. doi: 10.2106/00004623-200002000-00004. [DOI] [PubMed] [Google Scholar]
- 69.Hoffman MC, et al. Deficits in physical function among young childhood cancer survivors. J. Clin. Oncol. 2013;31:2799–2805. doi: 10.1200/JCO.2012.47.8081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hogarty AN, et al. Longitudinal evaluation of cardiopulmonary performance during exercise after bone marrow transplantation in children. J. Pediatr. 2000;136:311–317. doi: 10.1067/mpd.2000.103444. [DOI] [PubMed] [Google Scholar]
- 71.Hovi L, Era P, Rautonen J, Siimes MA. Impaired muscle strength in female adolescents and young adults surviving leukemia in childhood. Cancer. 1993;72:276–281. doi: 10.1002/1097-0142(19930701)72:1<276::aid-cncr2820720148>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 72.Hovi L, et al. Suboptimal long-term physical performance in children and young adults after pediatric allo-SCT. Bone Marrow Transplant. 2010;45:738–745. doi: 10.1038/bmt.2009.221. [DOI] [PubMed] [Google Scholar]
- 73.Howell CR, et al. Randomized web-based physical activity intervention in adolescent survivors of childhood cancer. Pediatr. Blood Cancer. 2018;65:e27216. doi: 10.1002/pbc.27216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hung SH, et al. Associating physical activity levels with motor performance and physical function in childhood survivors of acute lymphoblastic leukemia. Physiother. Can. 2017;69:57–64. doi: 10.3138/ptc.2015-67LHC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Järvelä LS, et al. Physical activity and fitness in adolescent and young adult long-term survivors of childhood acute lymphoblastic leukaemia. J. Cancer Surviv. 2010;4:339–345. doi: 10.1007/s11764-010-0131-0. [DOI] [PubMed] [Google Scholar]
- 76.Jenney MEM, Faragher EB, Morris Jones PH, Woodcock A. Lung function and exercise capacity in survivors of childhood leukaemia. Med. Pediatr. Oncol. 1995;24:222–230. doi: 10.1002/mpo.2950240403. [DOI] [PubMed] [Google Scholar]
- 77.Johnson D, et al. Cardiovascular responses to dynamic submaximal exercise in children previously treated with anthracycline. Am. Heart J. 1997;133:169–173. doi: 10.1016/s0002-8703(97)70205-9. [DOI] [PubMed] [Google Scholar]
- 78.Joyce ED, et al. Association of muscle strength and bone mineral density in adult survivors of childhood acute lymphoblastic leukemia. Arch. Phys. Med. Rehabil. 2011;92:873–879. doi: 10.1016/j.apmr.2010.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kabak VY, Duger T, Uckan Cetinkaya D. Investigation of the effects of an exercise program on physical functions and activities of daily life in pediatric hematopoietic stem cell transplantation. Pediatr. Blood Cancer. 2016;63:1643–1648. doi: 10.1002/pbc.26038. [DOI] [PubMed] [Google Scholar]
- 80.Kandula T, et al. Chemotherapy-induced peripheral neuropathy in long-term survivors of childhood cancer: clinical, neurophysiological, functional, and patient-reported outcomes. JAMA Neurol. 2018;75:980–988. doi: 10.1001/jamaneurol.2018.0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Keats MR, Culos-Reed SN. A community-based physical activity program for adolescents with cancer (Project TREK) J. Pediatr. Hematol. Oncol. 2008;30:272–280. doi: 10.1097/MPH.0b013e318162c476. [DOI] [PubMed] [Google Scholar]
- 82.Kesting SV, Götte M, Seidel CC, Rosenbaum D, Boos J. Motor performance after treatment for pediatric bone tumors. J. Pediatr. Hematol. Oncol. 2015;37:509–514. doi: 10.1097/MPH.0000000000000396. [DOI] [PubMed] [Google Scholar]
- 83.Konczak J, Schoch B, Dimitrova A, Gizewski E, Timmann D. Functional recovery of children and adolescents after cerebellar tumour resection. Brain. 2005;128:1428–1441. doi: 10.1093/brain/awh385. [DOI] [PubMed] [Google Scholar]
- 84.Lam KKW, et al. An integrated experiential training programme with coaching to promote physical activity, and reduce fatigue among children with cancer: a randomised controlled trial. Patient Educ. Couns. 2018;101:1947–1956. doi: 10.1016/j.pec.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 85.Leone M, et al. Assessment of gross motor skills and phenotype profile in children 9-11 years of age in survivors of acute lymphoblastic leukemia. Pediatr. Blood Cancer. 2014;61:46–52. doi: 10.1002/pbc.24731. [DOI] [PubMed] [Google Scholar]
- 86.Long, T. M. et al. Fitness, body composition and vascular health in adolescent and young adult survivors of paediatric brain cancer and cranial radiotherapy. Int. J. Adolesc. Med. Health.10.1515/ijamh-2017-0082 (2017). [DOI] [PubMed]
- 87.Long TM, et al. Exercise training improves vascular function and secondary health measures in survivors of pediatric oncology related cerebral insult. PLoS ONE. 2018;13:e0201449. doi: 10.1371/journal.pone.0201449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Luca de CR, et al. Gross and fine motor skills in children treated for acute lymphoblastic leukaemia. Dev. Neurorehabil. 2013;16:180–187. doi: 10.3109/17518423.2013.771221. [DOI] [PubMed] [Google Scholar]
- 89.Malicka, I., Kowaluk, A. & Woźniewski M. Does daily physical activity level determine the physical efficiency of children after treatment of leukemia? Int. J. Environ. Res. Public Health10.3390/ijerph17010307 (2020). [DOI] [PMC free article] [PubMed]
- 90.Malicka, I. et al. Physical fitness of school-age children after cancer treatment. Int. J. Environ. Res. Public Health. 10.3390/ijerph16081436 (2019). [DOI] [PMC free article] [PubMed]
- 91.Manchola-González, J. D. et al. Effects of a home-exercise programme in childhood survivors of acute lymphoblastic leukaemia on physical fitness and physical functioning: results of a randomised clinical trial. Support Care Cancer10.1007/s00520-019-05131-2 (2019). [DOI] [PubMed]
- 92.Marchese VG, Chiarello LA, Lange BJ. Effects of physical therapy intervention for children with acute lymphoblastic leukemia. Pediatr. Blood Cancer. 2004;42:127–133. doi: 10.1002/pbc.10481. [DOI] [PubMed] [Google Scholar]
- 93.Marchese VG, Ogle S, Womer RB, Dormans J, Ginsberg JP. An examination of outcome measures to assess functional mobility in childhood survivors of osteosarcoma. Pediatr. Blood Cancer. 2004;42:41–45. doi: 10.1002/pbc.10462. [DOI] [PubMed] [Google Scholar]
- 94.Marchese VG, et al. Assessing functional mobility in survivors of lower-extremity sarcoma: reliability and validity of a new assessment tool. Pediatr. Blood Cancer. 2007;49:183–189. doi: 10.1002/pbc.20932. [DOI] [PubMed] [Google Scholar]
- 95.Marchese VG, et al. Relationships among range of motion, functional mobility, and quality of life in children and adolescents after limb-sparing surgery for lower-extremity sarcoma. Pediatr. Phys. Ther. 2006;18:238–244. doi: 10.1097/01.pep.0000232620.42407.9f. [DOI] [PubMed] [Google Scholar]
- 96.Marchese VG, Chiarello LA, Lange BJ. Strength and functional mobility in children with acute lymphoblastic leukemia. Med. Pediatr. Oncol. 2003;40:230–232. doi: 10.1002/mpo.10266. [DOI] [PubMed] [Google Scholar]
- 97.Marchese VG, et al. Relationships among severity of osteonecrosis, pain, range of motion, and functional mobility in children, adolescents, and young adults with acute lymphoblastic leukemia. Phys. Ther. 2008;88:341–350. doi: 10.2522/ptj.20070108. [DOI] [PubMed] [Google Scholar]
- 98.Matthys D, et al. Gender difference in aerobic capacity in adolescents after cure from malignant disease in childhood. Acta Paediatr. 1993;82:459–462. doi: 10.1111/j.1651-2227.1993.tb12722.x. [DOI] [PubMed] [Google Scholar]
- 99.McKenzie DC, Coutts KD, Rogers PC, Jespersen DK, Pretula A. Aerobic and anaerobic capacities of children and adolescents successfully treated for solid tumors. Clin. Exerc. Physiol. 2000;2:39–42. [Google Scholar]
- 100.Mitchell WG, et al. Opsoclonus-ataxia caused by childhood neuroblastoma: developmental and neurologic sequelae. Pediatrics. 2002;109:86–98. [PubMed] [Google Scholar]
- 101.Mitra Varedi, et al. Peripheral neuropathy, sensory processing, and balance in survivors of acute lymphoblastic leukemia. J. Clin. Oncol. 2018;36:2315–2322. doi: 10.1200/JCO.2017.76.7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Moyer-Mileur LJ, Ransdell L, Bruggers CS. Fitness of children with standard-risk acute lymphoblastic leukemia during maintenance therapy: response to a home-based exercise and nutrition program. J. Pediatr. Hematol. Oncol. 2009;31:259–266. doi: 10.1097/MPH.0b013e3181978fd4. [DOI] [PubMed] [Google Scholar]
- 103.Müller C, Rosenbaum D, Krauth KA. Prospective evaluation of postural control and gait in pediatric patients with cancer after a 4-week inpatient rehabilitation program. Am. J. Phys. Med. Rehabil. 2017;96:646–653. doi: 10.1097/PHM.0000000000000729. [DOI] [PubMed] [Google Scholar]
- 104.Muratt MD, et al. Strength capacity in young patients who are receiving maintenance therapy for acute lymphoblastic leukemia: a case-control study. Clinics. 2011;66:1277–1281. doi: 10.1590/S1807-59322011000700026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nama N, et al. Vincristine-induced peripheral neurotoxicity: a prospective cohort. Pediatr. Hematol. Oncol. 2020;37:15–28. doi: 10.1080/08880018.2019.1677832. [DOI] [PubMed] [Google Scholar]
- 106.Naumann FL, et al. Assessment of fundamental movement skills in childhood cancer patients. Pediatr. Blood Cancer. 2015;62:2211–2215. doi: 10.1002/pbc.25676. [DOI] [PubMed] [Google Scholar]
- 107.Ness KK, et al. Body composition, muscle strength deficits and mobility limitations in adult survivors of childhood acute lymphoblastic leukemia. Pediatr. Blood Cancer. 2007;49:975–981. doi: 10.1002/pbc.21091. [DOI] [PubMed] [Google Scholar]
- 108.Ness KK, et al. Energy balance and fitness in adult survivors of childhood acute lymphoblastic leukemia. Blood. 2015;125:3411–3419. doi: 10.1182/blood-2015-01-621680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ness KK, et al. Neuromuscular impairments in adult survivors of childhood acute lymphoblastic leukemia: associations with physical performance and chemotherapy doses. Cancer. 2012;118:828–838. doi: 10.1002/cncr.26337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ness KK, et al. Chemotherapy-related neuropathic symptoms and functional impairment in adult survivors of extracranial solid tumors of childhood: results from the St. Jude Lifetime Cohort Study. Arch. Phys. Med. Rehabil. 2013;94:1451–1457. doi: 10.1016/j.apmr.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ness KK, et al. Skeletal, neuromuscular and fitness impairments among children with newly diagnosed acute lymphoblastic leukemia. Leuk. Lymphoma. 2015;56:1004–1011. doi: 10.3109/10428194.2014.944519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ness KK, et al. Physical performance limitations among adult survivors of childhood brain tumors. Cancer. 2010;116:3034–3044. doi: 10.1002/cncr.25051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ness KK, et al. A comparison of function after limb salvage with non-invasive expandable or modular prostheses in children. Eur. J. Cancer. 2014;50:3212–3220. doi: 10.1016/j.ejca.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nielsen MKF, et al. Testing physical function in children undergoing intense cancer treatment-a RESPECT feasibility study. Pediatr. Blood Cancer. 2018;65:e27100. doi: 10.1002/pbc.27100. [DOI] [PubMed] [Google Scholar]
- 115.Oschwald V, et al. Limited walking abilities and impaired ankle dorsiflexion function in children after intense cancer treatment. Klin. Padiatr. 2019;231:142–149. doi: 10.1055/a-0804-1899. [DOI] [PubMed] [Google Scholar]
- 116.Oswald KA, Bo J. Motor functioning and associated cognitive outcomes in pediatric survivors of acute lymphoblastic leukemia. Child Neuropsychol. 2020;26:597–611. doi: 10.1080/09297049.2019.1676406. [DOI] [PubMed] [Google Scholar]
- 117.Ovans JA, Hooke MC, Bendel AE, Tanner LR. Physical therapist coaching to improve physical activity in children with brain tumors: a pilot study. Pediatr. Phys. Ther. 2018;30:310–317. doi: 10.1097/PEP.0000000000000531. [DOI] [PubMed] [Google Scholar]
- 118.Papalia H, et al. Metabolic response to exercise in childhood brain tumor survivors: a pilot controlled study. Pediatr. Blood Cancer. 2020;67:e28053. doi: 10.1002/pbc.28053. [DOI] [PubMed] [Google Scholar]
- 119.Pesenti S, et al. Knee function after limb salvage surgery for malignant bone tumor: comparison of megaprosthesis and distal femur allograft with epiphysis sparing. Int. Orthop. 2018;42:427–436. doi: 10.1007/s00264-017-3608-x. [DOI] [PubMed] [Google Scholar]
- 120.Pihkala J, et al. Cardiopulmonary evaluation of exercise tolerance after chest irradiation and anticancer chemotherapy in children and adolescents. Pediatrics. 1995;95:722–726. [PubMed] [Google Scholar]
- 121.Piscione PJ, et al. Exercise training improves physical function and fitness in long-term paediatric brain tumour survivors treated with cranial irradiation. Eur. J. Cancer. 2017;80:63–72. doi: 10.1016/j.ejca.2017.04.020. [DOI] [PubMed] [Google Scholar]
- 122.Piscione PJ, Bouffet E, Mabbott DJ, Shams I, Kulkarni AV. Physical functioning in pediatric survivors of childhood posterior fossa brain tumors. Neuro-Oncol. 2014;16:147–155. doi: 10.1093/neuonc/not138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ramchandren S, et al. Peripheral neuropathy in survivors of childhood acute lymphoblastic leukemia. J. Peripher. Nerv. Syst. 2009;14:184–189. doi: 10.1111/j.1529-8027.2009.00230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Reinders-Messelink H, et al. Motor performance of children during treatment for acute lymphoblastic leukemia. Med. Pediatr. Oncol. 1999;33:545–550. doi: 10.1002/(sici)1096-911x(199912)33:6<545::aid-mpo4>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 125.Riggs L, et al. Exercise training for neural recovery in a restricted sample of pediatric brain tumor survivors: a controlled clinical trial with crossover of training versus no training. Neuro-Oncol. 2017;19:440–450. doi: 10.1093/neuonc/now177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rosenhagen A, et al. Implementation of structured physical activity in the pediatric stem cell transplantation. Klin. Padiatr. 2011;223:147–151. doi: 10.1055/s-0031-1271782. [DOI] [PubMed] [Google Scholar]
- 127.Sabel M, et al. Active video gaming improves body coordination in survivors of childhood brain tumours. Disabil. Rehabil. 2016;38:2073–2084. doi: 10.3109/09638288.2015.1116619. [DOI] [PubMed] [Google Scholar]
- 128.San Juan AF, et al. Functional capacity of children with leukemia. Int. J. Sports Med. 2008;29:163–167. doi: 10.1055/s-2007-964908. [DOI] [PubMed] [Google Scholar]
- 129.San Juan AF, et al. Benefits of intrahospital exercise training after pediatric bone marrow transplantation. Int. J. Sports Med. 2008;29:439–446. doi: 10.1055/s-2007-965571. [DOI] [PubMed] [Google Scholar]
- 130.San Juan AF, et al. Effects of an intrahospital exercise program intervention for children with leukemia. Med. Sci. Sports Exerc. 2007;39:13–21. doi: 10.1249/01.mss.0000240326.54147.fc. [DOI] [PubMed] [Google Scholar]
- 131.San Juan AF, et al. Early-phase adaptations to intrahospital training in strength and functional mobility of children with leukemia. J. Strength Cond. Res. 2007;21:173–177. doi: 10.1519/00124278-200702000-00031. [DOI] [PubMed] [Google Scholar]
- 132.Schoch B, et al. Impact of surgery and adjuvant therapy on balance function in children and adolescents with cerebellar tumors. Neuropediatrics. 2006;37:350–358. doi: 10.1055/s-2007-964904. [DOI] [PubMed] [Google Scholar]
- 133.Schoch B, Hogan A, Gizewski ER, Timmann D, Konczak J. Balance control in sitting and standing in children and young adults with benign cerebellar tumors. Cerebellum. 2010;9:324–335. doi: 10.1007/s12311-010-0165-x. [DOI] [PubMed] [Google Scholar]
- 134.Schoenmakers MAGC, et al. Muscle strength and functional ability in children during and after treatment for acute lymphoblastic leukemia or T-cell Non-Hodgkin lymphoma: a pilot study. Cancer Ther. 2006;4:241–248. [Google Scholar]
- 135.Segerer FJ, Biko J, Reiners C, Wirth C, Hebestreit H. Exercise. Pediatr. Exerc. Sci. 2017;29:361–370. doi: 10.1123/pes.2016-0189. [DOI] [PubMed] [Google Scholar]
- 136.Shore S, Shepard RJ. Immune response to exercise in children treated for cancer. J. Sports Med. Phys. Fit. 1999;39:240–243. [PubMed] [Google Scholar]
- 137.Slater ME, et al. Physical activity, fitness, and cardiometabolic risk factors in adult survivors of childhood cancer with a history of hematopoietic cell transplantation. Biol. Blood Marrow Transplant. 2015;21:1278–1283. doi: 10.1016/j.bbmt.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Smith WA, et al. Measured versus self-reported physical function in adult survivors of childhood cancer. Med. Sci. Sports Exerc. 2014;46:211–218. doi: 10.1249/MSS.0b013e3182a65c73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Smith, W. A. et al. Exercise training in childhood cancer survivors with subclinical cardiomyopathy who were treated with anthracyclines. Pediatr. Blood Cancer10.1002/pbc.24850 (2013). [DOI] [PMC free article] [PubMed]
- 140.Steenhoff JRM, Daanen HAM, Taminiau AHM. Functional analysis of patients who have had a modified Van Nes Rotationplasty. J. Bone Jt. Surg. Am. 1993;75:1451–1456. doi: 10.2106/00004623-199310000-00005. [DOI] [PubMed] [Google Scholar]
- 141.Su H-L, Wu L-M, Chiou S-S, Lin P-C, Liao Y-M. Assessment of the effects of walking as an exercise intervention for children and adolescents with cancer: a feasibility study. Eur. J. Oncol. Nurs. 2018;37:29–34. doi: 10.1016/j.ejon.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 142.Syczewska M, Dembowska-Bagińska B, Perek-Polnik M, Kalinowska M, Perek D. Postural sway in children and young adults, survivors of CNS tumours. Adv. Med. Sci. 2008;53:256–262. doi: 10.2478/v10039-008-0031-y. [DOI] [PubMed] [Google Scholar]
- 143.Syczewska M, Dembowska-Baginska B, Perek-Polnik M, Perek D. Functional status of children after treatment for a malignant tumour of the CNS: a preliminary report. Gait Posture. 2006;23:206–210. doi: 10.1016/j.gaitpost.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 144.Syczewska M, Dembowska-Bagińska B, Perek-Polnik M, Kalinowska M, Perek D. Gait pathology assessed with Gillette Gait Index in patients after CNS tumour treatment. Gait Posture. 2010;32:358–362. doi: 10.1016/j.gaitpost.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 145.Takken T, et al. Development, feasibility and efficacy of a community-based exercise training program in pediatric cancer survivors. Psychooncology. 2009;18:440–448. doi: 10.1002/pon.1484. [DOI] [PubMed] [Google Scholar]
- 146.Talvensaari KK, Jämsen A, Vanharanta H, Lanning M. Decreased isokinetic trunk muscle strength and performance in long-term survivors of childhood malignancies: correlation with hormonal defects. Arch. Phys. Med. Rehabil. 1995;76:983–988. doi: 10.1016/s0003-9993(95)81033-1. [DOI] [PubMed] [Google Scholar]
- 147.Tanir MK, Kuguoglu S. Impact of exercise on lower activity levels in children with acute lymphoblastic leukemia: a randomized controlled trial from Turkey. Rehabil. Nurs. 2013;38:48–59. doi: 10.1002/rnj.58. [DOI] [PubMed] [Google Scholar]
- 148.Tanner L, Sencer S, Hooke MC. The Stoplight Program: a proactive physical therapy intervention for children with acute lymphoblastic leukemia. J. Pediatr. Oncol. Nurs. 2017;34:347–357. doi: 10.1177/1043454217698093. [DOI] [PubMed] [Google Scholar]
- 149.Tanner LR, Hooke MC. Improving body function and minimizing activity limitations in pediatric leukemia survivors: the lasting impact of the Stoplight Program. Pediatr. Blood Cancer. 2019;66:e27596. doi: 10.1002/pbc.27596. [DOI] [PubMed] [Google Scholar]
- 150.Tanner LR, Hooke MC, Hinshon S, Hansen CR. Effect of an ankle foot orthosis intervention for children with non-central nervous system cancers: a pilot study. Pediatr. Phys. Ther. 2015;27:425–431. doi: 10.1097/PEP.0000000000000180. [DOI] [PubMed] [Google Scholar]
- 151.Taskinen M, Kurimo M, Kanerva J, Hovi L. Physical performance of nontransplanted childhood ALL survivors is comparable to healthy controls. J. Pediatr. Hematol. Oncol. 2013;35:276–280. doi: 10.1097/MPH.0b013e3182830ffa. [DOI] [PubMed] [Google Scholar]
- 152.Tay, C. G. et al. Vincristine-induced peripheral neuropathy in survivors of childhood acute lymphoblastic leukaemia. Pediatr. Blood Cancer10.1002/pbc.26471 (2017). [DOI] [PubMed]
- 153.Turner-Gomes SO, et al. Cardiorespiratory status after treatment for acute lymphoblastic leukemia. Med Pediatr. Oncol. 1996;26:160–165. doi: 10.1002/(SICI)1096-911X(199603)26:3<160::AID-MPO3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 154.van Brussel M, et al. Physical function and fitness in long-term survivors of childhood leukaemia. Pediatr. Rehabil. 2006;9:267–274. doi: 10.1080/13638490500523150. [DOI] [PubMed] [Google Scholar]
- 155.Wallek, S. et al. Impact of the initial fitness level on the effects of a structured exercise therapy during pediatric stem cell transplantation. Pediatr. Blood Cancer10.1002/pbc.26851. [DOI] [PubMed]
- 156.Warner JT, Bell W, Webb DKH, Gregory JW. Relationship between cardiopulmonary response to exercise and adiposity in survivors of childhood malignancy. Arch. Dis. Child. 1997;76:298–303. doi: 10.1136/adc.76.4.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wiernikowski JT, et al. Alendronate for steroid-induced osteopenia in children with acute lymphoblastic leukaemia or non-Hodgkin’s lymphoma: results of a pilot study. J. Oncol. Pharm. Pract. 2005;11:51–56. doi: 10.1191/1078155205jp145oa. [DOI] [PubMed] [Google Scholar]
- 158.Wright MJ, Hanna SE, Halton JM, Barr RD. Maintenance of ankle range of motion in children treated for acute lymphoblastic leukemia. Pediatr. Phys. Ther. 2003;15:146–152. doi: 10.1097/01.PEP.0000083122.74062.1B. [DOI] [PubMed] [Google Scholar]
- 159.Wright MJ, Twose DM, Gorter JW. Gait characteristics of children and youth with chemotherapy induced peripheral neuropathy following treatment for acute lymphoblastic leukemia. Gait Posture. 2017;58:139–145. doi: 10.1016/j.gaitpost.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 160.Wright MJFSM. Adaption and psychometric properties of the gross motor function measure for children receiving treatment for acute lymphoblastic leukemia. Rehabil. Oncol. 2007;25:14–20. [Google Scholar]
- 161.Wright MJ, Galea V, Barr RD. Proficiency of balance in children and youth who have had acute lymphoblastic leukemia. Phys. Ther. 2005;85:782–790. [PubMed] [Google Scholar]
- 162.Wright MJ, Halton JM, Barr RD. Limitation of ankle range of motion in survivors of acute lymphoblastic leukemia: a cross-sectional study. Med. Pediatr. Oncol. 1999;32:279–82. doi: 10.1002/(sici)1096-911x(199904)32:4<279::aid-mpo7>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 163.Wright MJ, Halton JM, Martin RF, Barr RD. Long-term gross motor performance following treatment for acute lymphoblastic leukemia. Med. Pediatr. Oncol. 1998;31:86–90. doi: 10.1002/(sici)1096-911x(199808)31:2<86::aid-mpo7>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 164.Zaccara A, et al. Gait analysis in patients operated on for sacrococcygeal teratoma. J. Pediatr. Surg. 2004;39:947–952. doi: 10.1016/j.jpedsurg.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 165.Wurz A, Chamorro-Vina C, Guilcher GMT, Schulte F, Culos-Reed SN. The feasibility and benefits of a 12-week yoga intervention for pediatric cancer out-patients. Pediatr. Blood Cancer. 2014;61:1828–1834. doi: 10.1002/pbc.25096. [DOI] [PubMed] [Google Scholar]
- 166.Kaatsch, P., Grabow, D. & Spix, C. (Eds.). German Childhood Cancer Registry—Annual Report 2017 (1980–2016) (Institute of Medical Biostatistics, Epidemiology and Informatics (IMBEI) at the University Medical Center of the Johannes Gutenberg University Mainz, 2018).
- 167.Smith JJ, et al. The health benefits of muscular fitness for children and adolescents: a systematic review and meta-analysis. Sports Med. 2014;44:1209–1223. doi: 10.1007/s40279-014-0196-4. [DOI] [PubMed] [Google Scholar]
- 168.Stone DS, Ganz PA, Pavlish C, Robbins WA. Young adult cancer survivors and work: a systematic review. J. Cancer Surviv. 2017;11:765–781. doi: 10.1007/s11764-017-0614-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Solway S, Brooks D, Lacasse Y, Thomas S. A qualitative systematic overview of the measurement properties of functional walk tests used in the cardiorespiratory domain. Chest. 2001;119:256–270. doi: 10.1378/chest.119.1.256. [DOI] [PubMed] [Google Scholar]
- 170.Wurz A, Brunet J. The effects of physical activity on health and quality of life in adolescent cancer survivors: a systematic review. JMIR Cancer. 2016;2:e6. doi: 10.2196/cancer.5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Vuotto SC, et al. Impact of chronic disease on emotional distress in adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Cancer. 2017;123:521–528. doi: 10.1002/cncr.30348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Coombs A, Schilperoort H, Sargent B. The effect of exercise and motor interventions on physical activity and motor outcomes during and after medical intervention for children and adolescents with acute lymphoblastic leukemia: a systematic review. Crit. Rev. Oncol. Hematol. 2020;152:103004. doi: 10.1016/j.critrevonc.2020.103004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.