Abstract
The pathogenesis of inflammatory skin diseases is associated with the abnormal activity of keratinocytes and immune cells infiltrate. Vitamin D3 deficiency can correlate with the increased incidence, severity, and duration of inflammatory skin disorders. The exact mechanism on how vitamin D3 influences inflammatory skin diseases still requires clarification. However, it can be associated with the disturbances in transmembrane glycoprotein - LRP2/megalin, which is implicated in vitamin D3 transport to the cell, and defects in vitamin D-signaling through the nuclear receptors. Therefore, by using immunohistochemistry, we analyzed the expression of LRP2/megalin, VDR, RORα and RORγ in allergic contact dermatitis, lichen simplex chronicus, sarcoidosis and psoriasis in comparison to the normal skin. We observed decreased expression of LRP2/megalin in all inflammatory lesions in comparison to the normal skin. Significant differences were also noticed in VDR, RORα and RORγ levels between inflammatory lesions and normal skin. Our research indicates disturbed expression of LRP2/megalin, VDR, RORα and RORγ in inflammatory skin lesions in comparison to normal skin. Therefore, we suggest that changes in the activity of these proteins may play role in pathogenesis of inflammatory skin disorders. Furthermore, we suggest that LRP2/megalin, VDR, RORα and RORy may serve as targets in therapy of these diseases.
Keywords: vitamin D, nuclear receptors, megalin, sarcoidosis, lichen simplex chronicus, psoriasis, allergic contact dermatitis
Background
The skin serves as a barrier between the environment and the inner part of the organism, and plays a crucial role in the defense against pathogens, chemicals, and/or physical stressors, as well as maintaining the body’s homeostasis1. The skin is also a significant part of immune system2. It is engaged in the development of anti-microbial resistance, autoimmunity and inflammatory skin conditions, allergies or tumor immunity3. The cutaneous immune surveillance includes innate and adaptive immune responses4.
There is increased evidence that the impaired regulation of immune skin functions underlies the pathogenesis of wide range of cutaneous disorders. For example, psoriasis is one of the most commonly diagnosed inflammatory skin diseases5 with worldwide prevalence of about 2%6. This disorder manifests as inflammatory lesions with epidermal hyperplasia and immune cells infiltration7. One commonly used treatment for psoriasis, ultraviolet B phototherapy, inhibits the inflammatory responses, while stimulating vitamin D formation in the skin8. Vitamin D3 is activated by two-step hydroxylation catalyzed by cytochrome P450 enzymes including CYP2R1 and CYP27A1 with final C-1α hydroxylation by CYP27B1, to produce 1,25(OH)2D3, or by sequential hydroxylation of the side chain by CYP11A19, 10. These pathways are also expressed in the skin11, 12 and immune system13.
About 40% of the European population suffer from vitamin D3 deficiency14, which can be linked to autoimmune disorders15. Vitamin D3 has immunomodulatory effects by regulation of T cells16–18 and is implicated in the regulation of both the innate and adaptive immunity16. Several studies have shown a relationship between decreased levels of vitamin D3 and incidence of autoimmune skin disorders19–21, severity of psoriasis22–24 and atopic dermatitis25. In addition, polymorphism in vitamin D receptor (VDR) gene has been implicated in both severity 26 and susceptibility27,28 to psoriasis.
Vitamin D3 is transported by the group-specific component vitamin D binding protein (GC/DBP)29. GC/DBP is a ligand for LRP2/megalin (low density lipoprotein-related protein 2), which is a transmembrane glycoprotein30. LRP2/megalin is involved in reabsorption and further metabolism of chemicals and small proteins, including vitamins transfer proteins31. Previous research has indicated an unambiguous relationship between LRP2/megalin and vitamin D3 endocytosis32 and activation of genomic and non-genomic responses33. LRP2/megalin is implicated in regulation of 25(OH)D uptake by kidneys32. It is therefore likely that there is connection between LRP2/megalin expression and vitamin D in psoriasis development.
Questions addressed
To exert the phenotypic activities, active forms of vitamin D3 must interact with VDR11, 34. More recently, it was reported that retinoic acid-related orphan receptors α and γ (RORα and RORγ, coded by RORA and RORC genes, respectively) are targets for regulation by vitamin D3 hydroxyderivatives34, acting as inverse agonists on RORα and RORγ. RORα and RORγ are expressed both in normal and pathological skin34, 35. RORs are implicated in wide variety of cell functions36, and can play a role in inflammatory skin disorders37. Therefore, to gain an insight into vitamin D endocrine system in inflammatory skin diseases, we analyzed the expression of LRP2/megalin, VDR, RORα and RORγ in psoriasis, allergic contact dermatitis (ACD), lichen simplex chronicus (LSC) and sarcoidosis.
Experimental design
For immunohistochemistry skin samples of ACD, LSC, sarcoidosis, psoriasis and normal skin was included into this study (Table 1). The authors declare that this investigation was carried out following the rules of the Declaration of Helsinki of 1975 (revised in 2008) and this study was approved by the Institutional Review Board of the UAB under IRB-940831016 (OCCC Tissue Procurement CORE Facility) and IRB-00000726 (Title E150427002, Dr. A. Slominski PI). The IRB of the UAB, which gave their permission for conducting this study, waived the requirement to obtain Patients’ informed consent for this research.
Table 1.
Characteristic of normal skin (control) and lesional skin samples.
| Normal skin (control) | Psoriasis | Atopic contact dermatitis | Lichen simplex chronicus | Sarcoidosis | |
|---|---|---|---|---|---|
| Number of cases | 36 | 26 | 10 | 18 | 8 |
| Sex (F/M) | 17/9* | 12/14 | 2/8 | 3/13** | 5/1**** |
| Age (mean [range]) | 44.3 (20–68)* | 52.7 (20–86) | 50.6 (20–62) | 51.1 (34–70) | # |
| Location | * | ** | *** | ||
| Head (forehead, jaw, chin, temple, scalp) | 2 | 1 | 6 | ||
| Neck | 1 | 4 | |||
| Leg | 2 | 4 | 3 | 1 | 1 |
| Arm | 7 | 4 | |||
| Hand | 1 | 1 | |||
| Breast | 6 | 2 | |||
| Back | 1 | 6 | 1 | 1 | 1 |
| Abdomen | 11 | 6 | |||
| Foreskin | 2 | ||||
| Other | 2 | 5 | 2 | ||
| Immunostaining | |||||
| VDR | 34 | 26 | 10 | 17 | 7 |
| LRP2/megalin | 26 | 26 | 10 | 18 | 8 |
| RORα | 33 | 26 | 10 | 18 | 7 |
| RORγ | 31 | 26 | 10 | 18 | 7 |
data available for 26 cases
data available for 16 cases
data available for 6 cases
lack of the data
Number of samples stained for VDR, megalin and RORs differs due to lack of representative of lesional areas in the sections.
The samples were stained for LRP2/megalin, VDR, RORα and RORγ antigens as previously described34, 35, 38, 39. Detailed data are presented in the Supplementary file and Supplementary Table 1. The immunostained sections were assessed using semiquantitative scoring systems using both the percentage of immunoreactive cells (IR) and the staining intensity (SI) from 0 to 3 arbitrary units (A.U.) according to the following formula SQ=mean(IR x SI)/100. Statistical comparison of subgrouped data were performed with GraphPad Prism software (version 5.0; GraphPad Software, La Jolla, CA, USA).
Testing of the expression of VDR, RORα and RORγ in HaCaT keratinocytes was performed using western blot according to the procedure presented in Supplementary file.
The analysis of the expression of VDR, RORα, RORγ and LRP2/megalin on mRNA level followed public genomics data repository (https://www.ebi.ac.uk/gxa/home) and project characterized in 40 (for details see Supplementary file). The comparisons between the psoriatic individuals and healthy controls were performed using t-test with P<0.05 considered to indicate a statistically significant difference.
Results
The expression of all tested markers was detected using immunohistochemistry in skin compartments. VDR showed predominant nuclear immunostaining, RORα and RORγ showed strong nuclear and weak cytoplasmic immunostaining, and LRP2/megalin showed cytoplasmic location. The representative images of immunostained sections are in Fig. 1. The strong nuclear stain vs weak cytoplasmic was further validated by western blot using antibodies against VDR, RORα and RORγ (Supplementary Figure 1).
Figure 1.
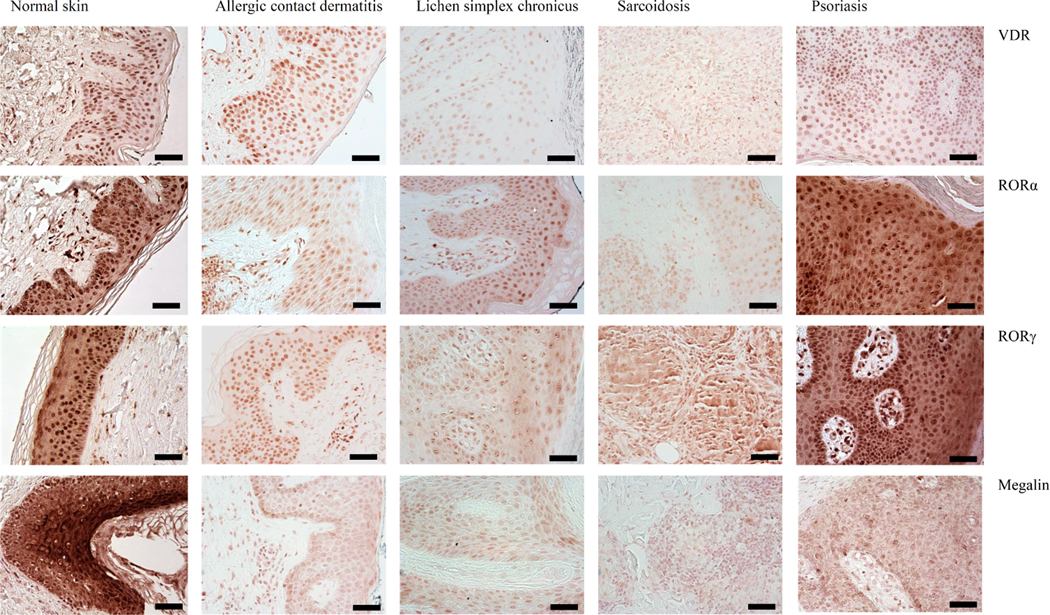
Representative images of immunostaining of VDR, RORα and RORγ and LRP2/megalin in allergic contact dermatitis, lichen simplex chronicus, sarcoidosis and psoriasis. Scale bars: = 50 μm.
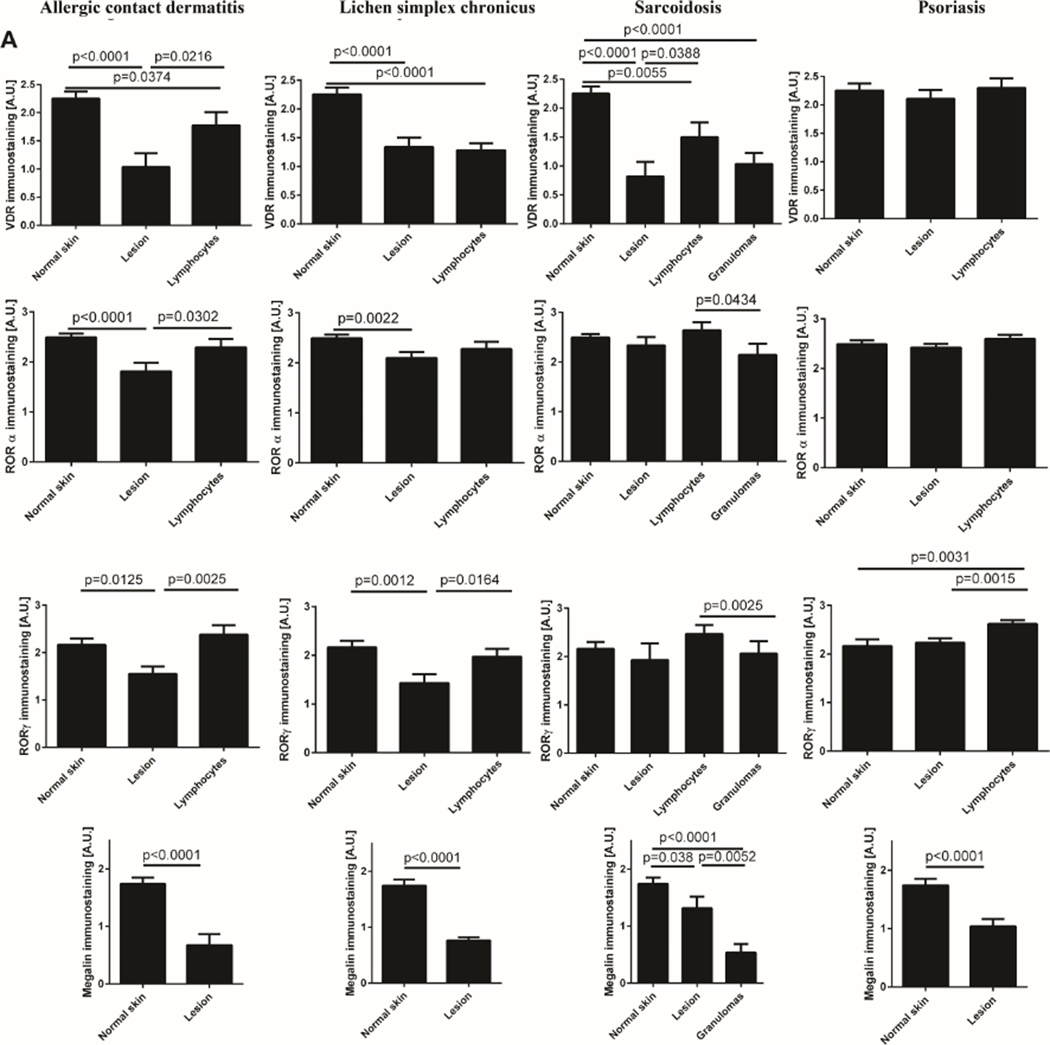
In ACD, the reduced levels of VDR, RORα and RORγ and LRP2/megalin in lesional skin were observed in comparison to normal skin (Fig. 2). The expression levels of analyzed receptors in lymphocytes was higher than in lesional keratinocytes (Fig. 2A).
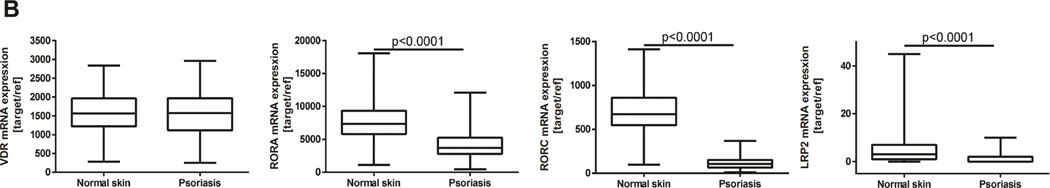
Figure 2.
Expression levels of VDR, RORα and RORγ and LRP2/megalin. A) The expression measured by immunohistochemistry in psoriasis, allergic contact dermatitis, lichen simplex chronicus and sarcoidosis. Statistically significant differences are denoted with p values as determined by Student’s t-test. B). The expression measured by RNA-seq in psoriasis and normal skin (https://www.ebi.ac.uk/gxa/home; 40).
In LSC the lower levels of VDR, RORα, RORγ and LRP2/megalin were found in comparison to normal skin (Fig. 2A). In lymphocytes VDR levels were low and comparable to that observed in lesional epidermis, while RORγ expression was as high as in normal epidermis (Fig. 1).
In sarcoidosis, the expression of RORα and RORγ in lesional skin and lymphocytes was as high as in normal tissue, while in granulomas RORα and RORγ levels were reduced when compared to lymphocytes. VDR and LRP2/megalin levels were significantly reduced in pathological cells and granulomas (Fig. 1). VDR levels in lymphocytes were also reduced in comparison to normal skin.
Levels of VDR and RORα were similar in psoriatic and normal (control) skin (Fig. 2A). RORγ levels in psoriatic keratinocytes were comparable to normal epidermis, being elevated in lymphocytes (Fig. 1 2A). However, LRP2/megalin was significantly reduced in lesional in comparison to the normal skin (Fig. 2A). Analysis of the data from Expression Atlas (https://www.ebi.ac.uk/gxa/home) has shown similar expression of VDR mRNA in psoriasis in comparison to normal skin, with expression of RORA and RORC and LRP2/megalin mRNA being lower in lesional than in normal skin (Fig. 2B). The gene expression pattern for the VDR and LRP2/megalin was consistent with the corresponding protein expression levels.
Conclusions & perspectives
In the present study, we investigated the expression of VDR, RORα and RORγ and LRP2/megalin in inflammatory skin diseases to determine the expression pattern for these receptors and their potential to serve as a predictive targets or markers for vitamin D-based therapies. On the protein level, we observed the reduced expression of LRP2/megalin in all analyzed skin lesions, the reduced RORα and RORγ level in ACD and LCS, the reduced VDR level in lichen simplex chronicus, ACD and sarcoidosis. The immunostaining pattern in all analyzed lesions and normal skin for VDR, RORα and RORγ and LRP2/megalin was similar. Up to now, there is shortage of information on the expression of analyzed proteins in the skin samples of inflammatory cutaneous diseases. To the best of our knowledge, our data is the first time that describes the expression of RORα and LRP2/megalin in inflammatory skin diseases and RORγ in skin samples of these diseases.
Some studies reported that psoriasis patients have reduced vitamin D3 levels24, 41. Vitamin D can regulate the proliferation and growth of keratinocytes17, 42–44, and it was also successfully incorporated as an adjuvant treatment for psoriasis26, 45. The therapeutic action of the active forms of vitamin D is mediated by its receptor, VDR. Additionally, the VDR polymorphism is linked to psoriasis susceptibility 27,28 and susceptibility of psoriatic patients to the vitamin D-based treatment. The reduced VDR levels in psoriatic skin are related to the reduced tight junctions46. It has been suggested that such disturbances can affect the maintenance of skin homeostasis46. VDR polymorphisms can also be considered as genetic risk factors for sarcoidosis47 with no effects of VDR SNPs on severity of sarcoidosis48, oral lichen planus49 and atopic dermatitis50. The expression of VDR in inflammatory skin diseases is altered, but some study showed contradictory data. Kim et al51 observed the gradual decrease of VDR expression from normal skin to atopic dermatitis, from perilesional skin to psoriatic lesion. However, recent study showed that VDR is present in psoriatic skin with its predominantly strong expression52. The differences between these studies could result from the different antibodies used for the VDR detection. This consideration is supported by similar results of our study with the study by Milde et al53, who used 9A7 clone of VDR antibody and showed comparable level of VDR in normal and psoriatic skin.
The expression of RORγ in inflammatory skin diseases is usually reported in peripheral blood samples since RORγt is a crucial receptor for the differentiation of Th17 cells54, that play essential role in the pathogenesis of psoriasis and other chronic inflammatory processes of the skin55. Ecoeur et al56 showed that selective RORγt inhibitor, Cpd A, inhibited Th17 pathway and the production of pro-inflammatory cytokines by T-cells and reduced IL-17-induced responses in keratinocytes. It has been proposed that RORγt can be molecular target for the psoriasis treatment57. In mouse models of atopic dermatitis and acute irritant dermatitis, synthetic RORα/γ inverse agonist - SR1001, exerted anti-inflammatory effects, restored epidermal barrier affecting multiple cell types in the skin58. Our previous study showed that RORγ and RORα are expressed in human skin and can serve as receptors for vitamin D derivatives34. In this study we found that RORα and RORγ are present in inflammatory skin diseases, however their expression was disturbed, suggesting that impaired RORα and RORγ expression can be involved in pathogenesis of inflammation.
LRP2/megalin may be crucial for proper cell development and differentiation59. Its expression was detected in normal skin, hair follicles60 and melanoma cells61. Our data showed the reduced LRP2/megalin levels in lesional cells in comparison to normal skin. Therefore, we suggest that the reduced expression of LRP2/megalin can be involved in pathogenesis of psoriasis and other inflammatory skin disorders through reduction of endocytosis and intracellular vitamin D3 signaling in affected skin cells.
In summary, the expression of VDR, RORα and RORγ and LRP2/megalin is aberrant in inflammatory skin diseases. We suggest that the disturbances in their expression are related to the pathogenesis of ACD, LSC, sarcoidosis and psoriasis. In addition, the presence of these receptors in the skin should allow to target them for vitamin-D based therapies of the inflammatory skin diseases providing a background for future clinical trials.
Supplementary Material
Acknowledgement
Writing of this letter was in part supported by the NIH grants 1R01AR073004, R01AR071189, R21AI149267-01A1 and VA merit 1I01BX004293-01A1 to ATS and the Cancer Center Core grant (P30CA13148).
Footnotes
Competing interests
The authors declare that they have no competing interests.
References
- 1.Slominski AT, Zmijewski MA, Skobowiat C, Zbytek B, Slominski RM, Steketee JD. Sensing the environment: regulation of local and global homeostasis by the skin’s neuroendocrine system. Adv Anat Embryol Cell Biol 2012;212:v, vii, 1–115. doi: 10.1007/978-3-642-19683-6_1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shimada S, Katz SI. The skin as an immunologic organ. Arch Pathol Lab Med 1988;112(3):231–4. [PubMed] [Google Scholar]
- 3.Richmond JM, Harris JE. Immunology and skin in health and disease. Cold Spring Harb Perspect Med 2014;4(12):a015339. doi: 10.1101/cshperspect.a015339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kupper TS, Fuhlbrigge RC. Immune surveillance in the skin: mechanisms and clinical consequences. Nat Rev Immunol 2004;4(3):211–22. doi: 10.1038/nri1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guttman-Yassky E, Nograles KE, Krueger JG. Contrasting pathogenesis of atopic dermatitis and psoriasis--part I: clinical and pathologic concepts. J Allergy Clin Immunol 2011;127(5):1110–8. doi: 10.1016/j.jaci.2011.01.053 [DOI] [PubMed] [Google Scholar]
- 6.Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med 2009;361(5):496–509. doi: 10.1056/NEJMra0804595 [DOI] [PubMed] [Google Scholar]
- 7.Rendon A, Schakel K. Psoriasis Pathogenesis and Treatment. Int J Mol Sci 2019;20(6)doi: 10.3390/ijms20061475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong T, Hsu L, Liao W. Phototherapy in psoriasis: a review of mechanisms of action. J Cutan Med Surg 2013;17(1):6–12. doi: 10.2310/7750.2012.11124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tuckey RC, Cheng CYS, Slominski AT. The serum vitamin D metabolome: What we know and what is still to discover. J Steroid Biochem Mol Biol 2019;186:4–21. doi: 10.1016/j.jsbmb.2018.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slominski AT, Chaiprasongsuk A, Janjetovic Z, et al. Photoprotective Properties of Vitamin D and Lumisterol Hydroxyderivatives. Cell Biochem Biophys 2020;78(2):165–180. doi: 10.1007/s12013-020-00913-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol 2014;21(3):319–29. doi: 10.1016/j.chembiol.2013.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slominski RM, Raman C, Elmets C, Jetten AM, Slominski A, Tuckey RC. The significance of CYP11A1 expression in skin physiology and pathology. Mol Cell Endocrinol 2021;Available online 12 March 2021, 111238:111238. doi: 10.1016/j.mce.2021.111238 [DOI] [PMC free article] [PubMed]
- 13.Slominski RM, Tuckey RC, Manna PR, et al. Extra-adrenal glucocorticoid biosynthesis: implications for autoimmune and inflammatory disorders. Genes Immun 2020;21(3):150–168. doi: 10.1038/s41435-020-0096-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cashman KD, Dowling KG, Skrabakova Z, et al. Vitamin D deficiency in Europe: pandemic? Am J Clin Nutr 2016;103(4):1033–44. doi: 10.3945/ajcn.115.120873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cantorna MT. Mechanisms underlying the effect of vitamin D on the immune system. Proc Nutr Soc 2010;69(3):286–9. doi: 10.1017/S0029665110001722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chambers ES, Hawrylowicz CM. The impact of vitamin D on regulatory T cells. Curr Allergy Asthma Rep 2011;11(1):29–36. doi: 10.1007/s11882-010-0161-8 [DOI] [PubMed] [Google Scholar]
- 17.Gniadecki R, Gajkowska B, Hansen M. 1,25-dihydroxyvitamin D3 stimulates the assembly of adherens junctions in keratinocytes: involvement of protein kinase C. Endocrinology 1997;138(6):2241–8. doi: 10.1210/endo.138.6.5156 [DOI] [PubMed] [Google Scholar]
- 18.Konijeti GG, Arora P, Boylan MR, et al. Vitamin D Supplementation Modulates T Cell-Mediated Immunity in Humans: Results from a Randomized Control Trial. J Clin Endocrinol Metab 2016;101(2):533–8. doi: 10.1210/jc.2015-3599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuang Y, Xiao Y, Fang Z, et al. Association of Serum Vitamin D With Psoriasis and Effect Modification by Central Obesity. Front Med (Lausanne) 2020;7:236. doi: 10.3389/fmed.2020.00236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marahatta S, Agrawal S, Khan S. Study on Serum Vitamin D in Alopecia Areata Patients. J Nepal Health Res Counc 2019;17(1):21–25. doi: 10.33314/jnhrc.1475 [DOI] [PubMed] [Google Scholar]
- 21.Upala S, Sanguankeo A. Low 25-hydroxyvitamin D levels are associated with vitiligo: a systematic review and meta-analysis. Photodermatol Photoimmunol Photomed 2016;32(4):181–90. doi: 10.1111/phpp.12241 [DOI] [PubMed] [Google Scholar]
- 22.Ingram MA, Jones MB, Stonehouse W, et al. Oral vitamin D3 supplementation for chronic plaque psoriasis: a randomized, double-blind, placebo-controlled trial. J Dermatolog Treat 2018;29(7):648–657. doi: 10.1080/09546634.2018.1444728 [DOI] [PubMed] [Google Scholar]
- 23.Adiguna MS, Rusyati LMM, Sudarsa PSS. Correlation of plasma vitamin d receptors with the severity of psoriasis vulgaris. Bali Medical Journal 2020;9(3):668–671. doi:DOI: 10.15562/bmj.v9i3.2013 [DOI] [Google Scholar]
- 24.Filoni A, Vestita M, Congedo M, Giudice G, Tafuri S, Bonamonte D. Association between psoriasis and vitamin D: Duration of disease correlates with decreased vitamin D serum levels: An observational case-control study. Medicine (Baltimore) 2018;97(25):e11185. doi: 10.1097/MD.0000000000011185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raj KAP, Handa S, Narang T, Sachdeva N, Mahajan R. Correlation of serum vitamin D levels with severity of pediatric atopic dermatitis and the impact of vitamin D supplementation on treatment outcomes. J Dermatolog Treat 2020:1–4. doi: 10.1080/09546634.2020.1818677 [DOI] [PubMed]
- 26.Barrea L, Savanelli MC, Di Somma C, et al. Vitamin D and its role in psoriasis: An overview of the dermatologist and nutritionist. Rev Endocr Metab Disord 2017;18(2):195–205. doi: 10.1007/s11154-017-9411-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee YH. Vitamin D receptor ApaI, TaqI, BsmI, and FokI polymorphisms and psoriasis susceptibility: an updated meta-analysis. Clin Exp Dermatol 2019;44(5):498–505. doi: 10.1111/ced.13823 [DOI] [PubMed] [Google Scholar]
- 28.Liu J, Wang W, Liu K, et al. Vitamin D receptor gene polymorphisms are associated with psoriasis susceptibility and the clinical response to calcipotriol in psoriatic patients. Exp Dermatol 2020;29(12):1186–1190. doi: 10.1111/exd.14202 [DOI] [PubMed] [Google Scholar]
- 29.Haddad JG, Matsuoka LY, Hollis BW, Hu YZ, Wortsman J. Human plasma transport of vitamin D after its endogenous synthesis. J Clin Invest 1993;91(6):2552–5. doi: 10.1172/JCI116492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saito A, Pietromonaco S, Loo AK, Farquhar MG. Complete cloning and sequencing of rat gp330/”megalin,” a distinctive member of the low density lipoprotein receptor gene family. Proc Natl Acad Sci U S A 1994;91(21):9725–9. doi: 10.1073/pnas.91.21.9725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Christensen EI, Birn H, Storm T, Weyer K, Nielsen R. Endocytic receptors in the renal proximal tubule. Physiology (Bethesda) 2012;27(4):223–36. doi: 10.1152/physiol.00022.2012 [DOI] [PubMed] [Google Scholar]
- 32.Nykjaer A, Dragun D, Walther D, et al. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell 1999;96(4):507–15. doi: 10.1016/s0092-8674(00)80655-8 [DOI] [PubMed] [Google Scholar]
- 33.Zmijewski MA, Carlberg C. Vitamin D receptor(s): In the nucleus but also at membranes? Exp Dermatol 2020;29(9):876–884. doi: 10.1111/exd.14147 [DOI] [PubMed] [Google Scholar]
- 34.Slominski AT, Kim TK, Takeda Y, et al. RORalpha and ROR gamma are expressed in human skin and serve as receptors for endogenously produced noncalcemic 20-hydroxy- and 20,23-dihydroxyvitamin D. FASEB J 2014;28(7):2775–89. doi: 10.1096/fj.13-242040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brozyna AA, Jozwicki W, Skobowiat C, Jetten A, Slominski AT. RORalpha and RORgamma expression inversely correlates with human melanoma progression. Oncotarget 2016;7(39):63261–63282. doi: 10.18632/oncotarget.11211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jetten AM. Retinoid-related orphan receptors (RORs): critical roles in development, immunity, circadian rhythm, and cellular metabolism. Nucl Recept Signal 2009;7:e003. doi: 10.1621/nrs.07003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jetten AM, Takeda Y, Slominski A, Kang HS. Retinoic acid-related Orphan Receptor gamma (RORgamma): connecting sterol metabolism to regulation of the immune system and autoimmune disease. Curr Opin Toxicol 2018;8:66–80. doi: 10.1016/j.cotox.2018.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brozyna AA, Jozwicki W, Slominski AT. Decreased VDR expression in cutaneous melanomas as marker of tumor progression: new data and analyses. Anticancer Res 2014;34(6):2735–43. [PMC free article] [PubMed] [Google Scholar]
- 39.Jozwicki W, Brozyna AA, Siekiera J, Slominski AT. Expression of Vitamin D Receptor (VDR) Positively Correlates with Survival of Urothelial Bladder Cancer Patients. Int J Mol Sci 2015;16(10):24369–86. doi: 10.3390/ijms161024369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li B, Tsoi LC, Swindell WR, et al. Transcriptome analysis of psoriasis in a large case-control sample: RNA-seq provides insights into disease mechanisms. J Invest Dermatol 2014;134(7):1828–1838. doi: 10.1038/jid.2014.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grassi T, Panico A, Bagordo F, et al. Direct detection of free vitamin D as a tool to assess risk conditions associated with chronic plaque psoriasis. J Prev Med Hyg 2020;61(3):E489–E495. doi: 10.15167/2421-4248/jpmh2020.61.3.1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang JY, Fu T, Lau C, Oh DH, Bikle DD, Asgari MM. Vitamin D in cutaneous carcinogenesis: part II. J Am Acad Dermatol 2012;67(5):817 e1–11; quiz 827–8. doi: 10.1016/j.jaad.2012.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang JY, Fu T, Lau C, Oh DH, Bikle DD, Asgari MM. Vitamin D in cutaneous carcinogenesis: part I. J Am Acad Dermatol 2012;67(5):803 e1–12, quiz 815–6. doi: 10.1016/j.jaad.2012.05.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bikle DD. Vitamin D and the skin: Physiology and pathophysiology. Rev Endocr Metab Disord 2012;13(1):3–19. doi: 10.1007/s11154-011-9194-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Disphanurat W, Viarasilpa W, Chakkavittumrong P, Pongcharoen P. The Clinical Effect of Oral Vitamin D2 Supplementation on Psoriasis: A Double-Blind, Randomized, Placebo-Controlled Study. Dermatol Res Pract 2019;2019:5237642. doi: 10.1155/2019/5237642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Visconti B, Paolino G, Carotti S, et al. Immunohistochemical expression of VDR is associated with reduced integrity of tight junction complex in psoriatic skin. J Eur Acad Dermatol Venereol 2015;29(10):2038–42. doi: 10.1111/jdv.12736 [DOI] [PubMed] [Google Scholar]
- 47.Niimi T, Tomita H, Sato S, et al. Vitamin D receptor gene polymorphism in patients with sarcoidosis. Am J Respir Crit Care Med 1999;160(4):1107–9. doi: 10.1164/ajrccm.160.4.9811096 [DOI] [PubMed] [Google Scholar]
- 48.Stjepanovic MI, Mihailovic-Vucinic V, Spasovski V, et al. Genes and metabolic pathway of sarcoidosis: identification of key players and risk modifiers. Arch Med Sci 2019;15(5):1138–1146. doi: 10.5114/aoms.2018.79682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shen H, Liu Q, Huang P, et al. Vitamin D receptor genetic polymorphisms are associated with oral lichen planus susceptibility in a Chinese Han population. BMC Oral Health 2020;20(1):26. doi: 10.1186/s12903-020-1002-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang L, Zhang S, He C, Wang X. VDR Gene Polymorphisms and Allergic Diseases: Evidence from a Meta-analysis. Immunol Invest 2020;49(1–2):166–177. doi: 10.1080/08820139.2019.1674325 [DOI] [PubMed] [Google Scholar]
- 51.Kim SK, Park S, Lee ES. Toll-like receptors and antimicrobial peptides expressions of psoriasis: correlation with serum vitamin D level. J Korean Med Sci 2010;25(10):1506–12. doi: 10.3346/jkms.2010.25.10.1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chandra R, Roesyanto-Mahadi ID, Yosi A. Pilot study: immunohistochemistry expressions of vitamin D receptor associated with severity of disease in psoriasis patients. Int J Dermatol 2020;59(9):1092–1097. doi: 10.1111/ijd.15018 [DOI] [PubMed] [Google Scholar]
- 53.Milde P, Hauser U, Simon T, et al. Expression of 1,25-dihydroxyvitamin D3 receptors in normal and psoriatic skin. J Invest Dermatol 1991;97(2):230–9. doi: 10.1111/1523-1747.ep12480255 [DOI] [PubMed] [Google Scholar]
- 54.Annunziato F, Cosmi L, Santarlasci V, et al. Phenotypic and functional features of human Th17 cells. J Exp Med 2007;204(8):1849–61. doi: 10.1084/jem.20070663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bergallo M, Accorinti M, Galliano I, et al. Expression of miRNA 155, FOXP3 and ROR gamma, in children with moderate and severe atopic dermatitis. G Ital Dermatol Venereol 2020;155(2):168–172. doi: 10.23736/S0392-0488.17.05707-8 [DOI] [PubMed] [Google Scholar]
- 56.Ecoeur F, Weiss J, Kaupmann K, Hintermann S, Orain D, Guntermann C. Antagonizing Retinoic Acid-Related-Orphan Receptor Gamma Activity Blocks the T Helper 17/Interleukin-17 Pathway Leading to Attenuated Pro-inflammatory Human Keratinocyte and Skin Responses. Front Immunol 2019;10:577. doi: 10.3389/fimmu.2019.00577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tang L, Yang X, Liang Y, Xie H, Dai Z, Zheng G. Transcription Factor Retinoid-Related Orphan Receptor gammat: A Promising Target for the Treatment of Psoriasis. Front Immunol 2018;9:1210. doi: 10.3389/fimmu.2018.01210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dai J, Choo MK, Park JM, Fisher DE. Topical ROR Inverse Agonists Suppress Inflammation in Mouse Models of Atopic Dermatitis and Acute Irritant Dermatitis. J Invest Dermatol 2017;137(12):2523–2531. doi: 10.1016/j.jid.2017.07.819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Akour AA, Kennedy MJ, Gerk P. Receptor-mediated endocytosis across human placenta: emphasis on megalin. Mol Pharm 2013;10(4):1269–78. doi: 10.1021/mp300609c [DOI] [PubMed] [Google Scholar]
- 60.Adly MA. Expression of the carrier protein transthyretin and its receptor megalin in human skin: preliminary findings. Br J Dermatol 2010;162(1):213–5. doi: 10.1111/j.1365-2133.2009.09519.x [DOI] [PubMed] [Google Scholar]
- 61.Andersen RK, Hammer K, Hager H, et al. Melanoma tumors frequently acquire LRP2/megalin expression, which modulates melanoma cell proliferation and survival rates. Pigment Cell Melanoma Res 2015;28(3):267–80. doi: 10.1111/pcmr.12352 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.