Abstract
Waldenstrom’s macroglobulinemia (WM) is a rare type of malignant B-cell lymphoma. The main feature of WM is elevated serum monoclonal immunoglobulin M, similar to multiple myeloma (MM). Unlike in MM, the rarity of destructive bone lesions in WM has been repeatedly emphasized. We report a unique case of WM with a vertebral compression fracture as the first symptom. This case highlights that the presence or absence of bone destruction may not clearly distinguish between WM and MM. The possibility of WM should be considered in patients with vertebral fracture and destruction as the first presentation. Performing vertebral bone marrow aspiration biopsy during percutaneous vertebroplasty is a convenient and effective method to assist in the diagnosis of WM.
Keywords: Waldenstrom’s macroglobulinemia, multiple myeloma, B-cell lymphoma, bone destruction, vertebral compression fracture, case report
Introduction
Waldenstrom’s macroglobulinemia (WM) is a rare malignant B-cell lymphoma that accounts for only 1% to 2% of hematological tumors and has an incidence of three cases per million people per year in the United States. 1 Because of the relatively low prevalence of WM in Asia, it has not been extensively studied. 2 The clinical manifestations of WM include thrombocytopenia, hepatosplenomegaly, lymphadenopathy, and hyperviscosity syndrome. However, most patients are either asymptomatic or only anemic, with few symptoms of bone-destroying reactions.3–4
The causes of vertebral compression fractures (VCFs) in elderly patients include trauma, osteoporosis, spinal infections, spinal tumors, and multiple myeloma (MM). The clinical symptoms of MM are caused by pathological fractures or severe osteoporosis at the site of osteolytic lesions. 5 Unlike in MM, the rarity of destructive bone lesions in WM has been repeatedly emphasized.6–7 Therefore, patients with VCFs as the first manifestation will not be initially diagnosed with WM.
This study reports the case of a 72-year-old man with a reduced albumin-to-globulin (A/G) ratio and VCF at L1 detected by magnetic resonance imaging (MRI). Initially, the patient was misdiagnosed with MM or an osteoporotic vertebral compression fracture (OVCF). After L1 percutaneous vertebroplasty (PVP), he was diagnosed with WM. Misdiagnosis and/or missed diagnosis delays the treatment of patients and increase the complication rate, mortality rate, and cost of hospitalization. This report aims to improve the understanding of bone destruction in patients with WM.
Case report
A 72-year-old male patient was unable to walk because of pain in his left inner thigh for 1 month requiring opioid treatment. He had no history of trauma and/or tumors. His complete blood cell count showed a low hemoglobin level (98 g/L, normal range: 120–160 g/L) and a high erythrocyte sedimentation rate (47 mm/h, normal range: <20 mm/h). His albumin and globulin levels were normal, but his A/G ratio was decreased (1.2, normal range: 1.5–2.5). The bone mineral density (BMD) T-score of the lumbar spine was −2.7. Preoperative X-ray (Figure 1), computed tomography (CT) (Figure 2), and MRI (Figure 3) results showed an L1 VCF and bone destruction. Considering the possibility of vertebral body destruction due to MM or an OVCF, PVP was performed (Figure 4), along with a vertebral bone marrow biopsy.
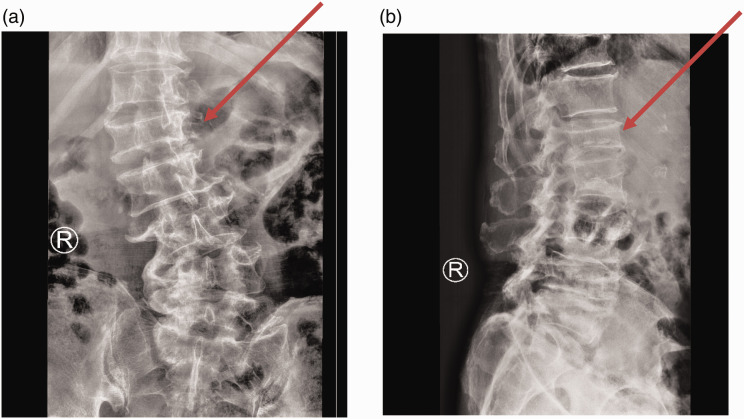
Figure 1.
X-ray imaging analysis of the patient reported in this case. Preoperative X-ray of the lumbar spine (panel a: anterior-posterior view; panel b: lateral view) showing compression of the L1 vertebra and lumbar scoliosis (red arrows), suggesting a compression fracture of the L1 vertebra.
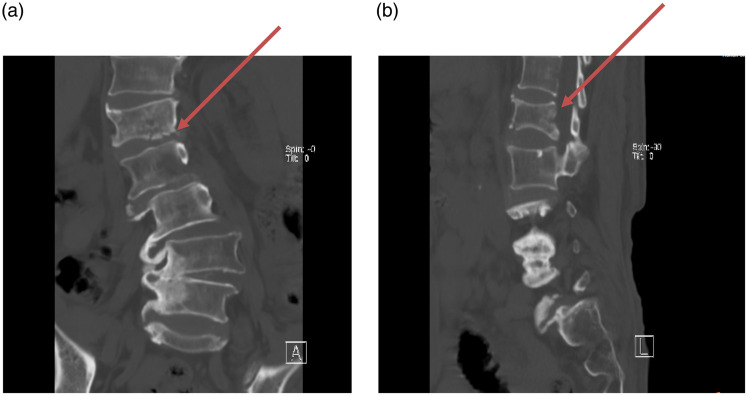
Figure 2.
Computed tomography (CT) imaging. (a) A coronal CT scan showing a fracture of the lower edge of the L1 vertebral body (red arrow) and (b) A sagittal CT scan revealing a compression fracture of the vertebral body (red arrow).
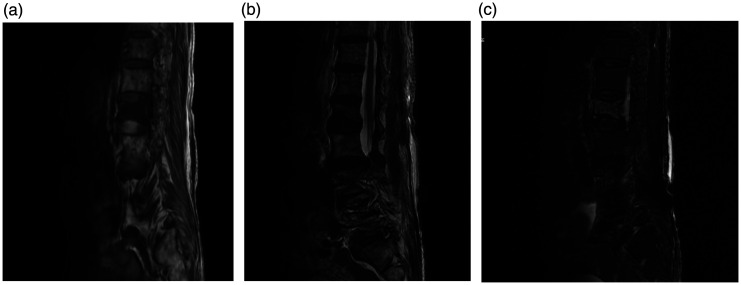
Figure 3.
Magnetic resonance imaging (MRI) analysis. (a) T1-weighted, (b) T2-weighted, and (c) fat-suppressed sagittal MRI suggesting new compression fractures at the L1 level.
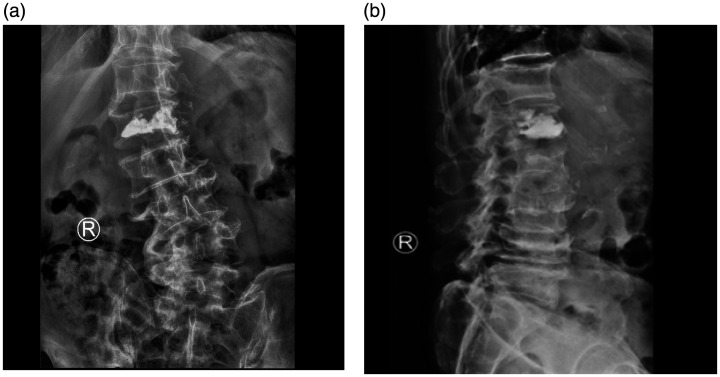
Figure 4.
X-ray imaging after percutaneous vertebroplasty (PVP). (a) Anterior-posterior and (b) lateral postoperative lumbar X-ray images showing good bone cement filling after L1 PVP.
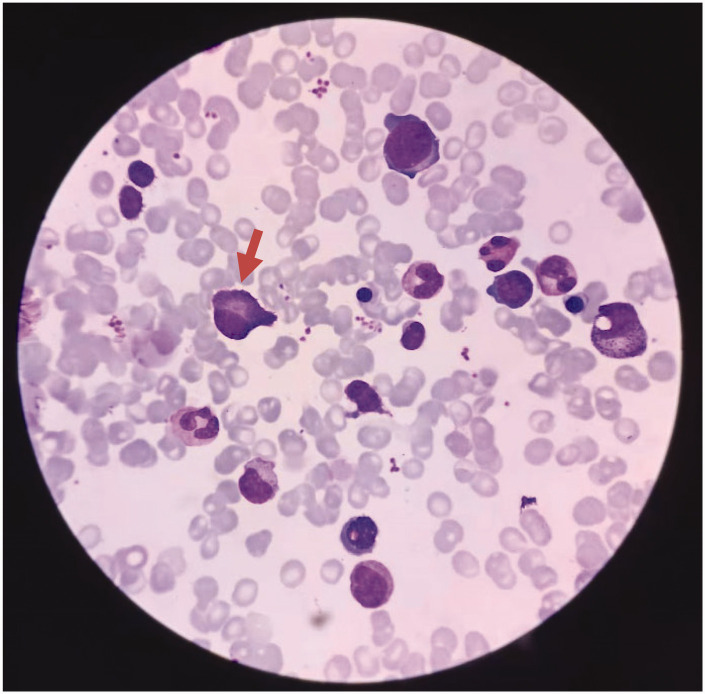
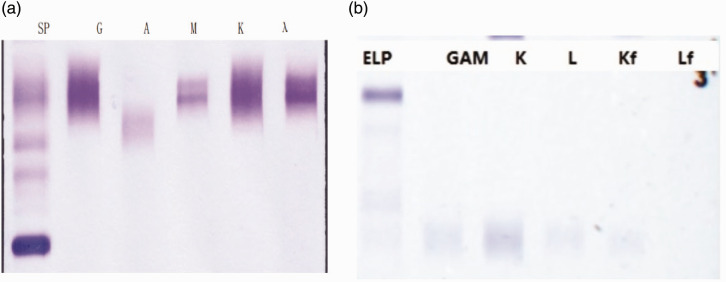
The bone marrow biopsy showed atypical plasma cells, active bone marrow hyperplasia, and trilineage hematopoiesis (Figure 5). Flow cytometry analysis showed no obvious evidence of plasma cell-related quantitative or immunophenotypic abnormalities, and approximately 16% of CD5-negative CD10-negative mature monoclonal B lymphocytes exhibited an abnormal immunophenotype. This finding was consistent with CD5-negative and CD10-negative mature B-cell lymphoma involving the bone marrow (mature monoclonal B lymphocytes account for approximately 10% of the cells). Molecular biological tests showed that the patient had an MYD88 (L265P) mutation, with a mutation rate of 0.77%. No monoclonal rearrangements were detected in the IgH gene (fragment analysis). Monoclonal rearrangements were detected in the T cell receptor gamma (TCRγ) gene (Vγ9–Jγ1.3/2.3 interval of TCRγ). Serum immunofixation electrophoresis analysis confirmed that the monoclonal immunoglobulin was IgM-λ, and immunoglobulin quantification was performed (Figure 6). His IgM level was 6.17 g/L (normal: 0.40–2.3 g/L) (KingMed Diagnostics, Guangzhou, China).
Figure 5.
Bone marrow biopsy showing atypical plasma cells. Red arrow indicates atypical plasma cells.
Figure 6.
Abnormal immunoglobulinemia diagnosis report. (a) Serum immunofixation electrophoresis results showing that the monoclonal immunoglobulin was IgM-λ and (b) Bence–Jones protein electrophoresis showing no abnormal monoclonal bands in any of the lanes, for a negative overall result.
SP, serum protein electrophoresis; ELP elastin-type polypeptide; GAM, immunoglobulin G, A, and M.
According to these findings (the presence of at least 10% plasmacytoid lymphoma cells in the bone marrow, the MYD88 (L265P) mutation, and an elevated serum monoclonal IgM level 8 ), the diagnosis of MM was overruled, and a diagnosis of WM was made. The patient’s International Prognostic System for WM score was determined to be medium risk. 9 The treatment team discussed the treatment plan with the patient and his son, and the patient decided not to pursue additional treatment.
Discussion
WM is a rare indolent mature B-cell lymphoma that accounts for only 1% to 2% of hematological tumors. 1 It is common in elderly male patients in Europe and the United States and is rarely reported by Asians. Patients with WM can be asymptomatic, or IgM deposition and systemic symptoms can occur because of tumor cell infiltration, but there is no bone destruction response.10,11 MM is characterized by the proliferation of plasma cells in the bone marrow and the excessive production of monoclonal immunoglobulin in the serum. 12 Although both MM and WM are characterized by monoclonal gammopathy, they are different hematological diseases and are difficult to distinguish clinically. 9 Differentiating between these two diagnoses is important because their treatment method and prognosis vary greatly. WM is an incurable malignant disease. Advanced age, the male sex, anemia, and pancytopenia are associated with a poor prognosis. Delaying treatment worsens the prognosis and increases the mortality rate of patients with this disease.10,13
Causes of VCFs in elderly patients include primary osteoporosis, spinal infections, tumors, and MM. Patients with OVCFs often have a minor trauma history and short disease course. Typical MRI examinations show a bright fracture line near the endplate, and typical symptoms include exercise-induced pain. 14 The immunophenotype of patients with MM is high CD38 and CD138 expression, negative CD19, CD20, and CD45 expression, and absent MYD88 gene expression, which is often accompanied by bone destruction and hypercalcemia. 15 Unlike in MM, the rarity of bone destruction in WM has been repeatedly emphasized.6–7 These characteristics were previously considered to be reliable markers for distinguishing MM from WM. 16 However, some case reports of bone-destroying lesions in patients with WM challenge this claim and confirm that there is indeed bone involvement in WM.17,18
The main clinical manifestation in our patient was pain in his inner left thigh. Laboratory tests revealed anemia and a reduced A/G ratio. His lumbar spine BMD examination showed osteoporosis. The CT and MRI examinations showed an L1 vertebral body fracture and bone destruction. PVP, vertebral bone marrow biopsy, molecular biology tests, and serum immunofixation electrophoresis analysis confirmed that the patient had WM. According to the previous clinical model, with no obvious history of trauma, elderly patients with VCFs will initially be considered to have MM or OVCFs instead of WM. In this case, our initial misdiagnosis may have been affected by the patient’s known vertebral body destruction. The presence or absence of bone destruction may not allow clinicians to clearly distinguish WM from MM. The diagnosis of WM should also be considered in patients with vertebral fracture and destruction as the first presentation.
Macroglobulinemia with bone destruction was first reported in 1958. 19 The first report of a patient with WM combined with VCF was in 1968. The patient’s X-ray showed changes and pathology that were consistent with multiple VCFs. The autopsy examination of the patient revealed that the vertebral bone marrow was almost completely replaced by plasma cells, accompanied by obvious bone destruction. 20 Because this situation is extremely rare, there are currently no management guidelines for patients with WM combined with VCFs in the relevant case reports. After an in-depth review of the literature, we examined the possible mechanisms underlying WM with bone destruction. First, an elevated serum IgM level may play a key role. Many patients with an elevated serum IgM level show the common clinical features of WM, but the same protein abnormalities may also cause some MM-specific clinical features, such as bone destruction. 20 Patients with MM produce an osteoclast-activating factor that stimulates osteoclasts, leading to local bone resorption around the myeloma lesion, and suppresses local osteoblast activity, resulting in a high serum calcium level. 21 In our case, the patient had a normal serum calcium level; thus, the osteoclast-activating factor may not be present. In addition, WM often affects patients with breast cancer, and bone destruction may be caused by the metastasis of this type of cancer. 22 Our intraoperative pathological biopsy results did not reveal evidence of breast cancer. Our patient did not undergo further examinations because the patient did not give consent. The mechanism of bone destruction in patients with WM needs to be revealed by more in-depth investigations in the future.
After consultation with the hematologist, we performed a bone marrow aspiration biopsy of the fractured vertebra during PVP instead of performing a bone marrow aspiration biopsy of the patient’s anterior superior iliac spine, posterior superior iliac spine, or sternum to avoid unnecessary surgical trauma. 23 We believe that performing a vertebral bone marrow biopsy during PVP is a simple and effective method to prevent a misdiagnosis or a missed diagnosis in patients with VCFs after considering the differential diagnoses for hematological diseases, although this involves additional costs.
Conclusion
We report the unique case of an elderly patient who presented with a lumbar VCF, underwent PVP and was diagnosed with WM. This report conforms to the CARE guidelines. 24 The presence or absence of bone destruction may not clearly distinguish between WM and MM. The possibility of WM should be considered in patients with vertebral fracture and destruction as the first presentation. Performing vertebral bone marrow aspiration biopsy during PVP is a convenient and effective method to assist in the diagnosis of WM.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_03000605221096161 for Does Waldenstrom’s macroglobulinemia also cause bone destruction? A rare case report by Jun-Ming Lin, Xiao-Jun Yuan, Lu Zhang, Guang Li, Xin-rong Gan and Wen-Hua Xu in Journal of International Medical Research
Footnotes
Declaration of conflicting interest: The authors declare that they have no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Department of Health Commission of Jiangxi Provincial, China (No. 20177494) and the Department of Science and Technology Program of Yichun City, Jiangxi Province, China (No. JXYC2020KSA003).
ORCID iD: Jun-Ming Lin https://orcid.org/0000-0002-0431-4627
Ethics statement
This study was approved by the Medical Ethics Committee of Yichun People’s Hospital (No:2021303). The patient provided written informed consent to publish the data in this study.
References
- 1.Awad AK, Elbadawy MA, Boury M, et al. Simple headache revealed a rare lymphoma: Waldenstrom macroglobulinemia with unique markers: a case report and review of the literature[J]. J Egypt Natl Canc Inst 2022; 34: 10. [DOI] [PubMed] [Google Scholar]
- 2.Li J, Zhang R, Gu F, et al. Optical coherence tomography angiography characteristics in Waldenstrom macroglobulinemia retinopathy: A case report[J]. World J Clin Cases 2020; 8: 243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dumas G, Gabarre P, Bigé N, et al. Hyperviscosity syndrome[J]. Intensive Care Med 2015; 44: 1151–1152. [DOI] [PubMed] [Google Scholar]
- 4.Gertz MA. Waldenström macroglobulinemia: 2019 update on diagnosis, risk stratification, and management[J]. Am J Hematol 2019; 94: 266–276. [DOI] [PubMed] [Google Scholar]
- 5.Giuliani N, Ferretti M, Bolzoni M, et al. Increased osteocyte death in multiple myeloma patients: role in myeloma-induced osteoclast formation[J]. Leukemia 2012; 26: 1391–1401. [DOI] [PubMed] [Google Scholar]
- 6.Waldenström J. Macroglobulinemia[J]. Adv Metab Disord 1965; 2: 115–158. [DOI] [PubMed] [Google Scholar]
- 7.Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes[J]. Blood 2009; 114: 937–951. [DOI] [PubMed] [Google Scholar]
- 8.Owen RG, Treon SP, Al-Katib A, et al. Clinicopathological definition of Waldenstrom’s macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom's Macroglobulinemia[J]. Semin Oncol 2003; 30: 110–115. [DOI] [PubMed] [Google Scholar]
- 9.Morel P, Duhamel A, Gobbi P, et al. International prognostic scoring system for Waldenstrom macroglobulinemia[J]. Blood 2009; 113: 4163–4170. [DOI] [PubMed] [Google Scholar]
- 10.Nekooghadam SM, Bozorgmehr R, Safavi-Naini S. Acrocyanosis and Progressive Skin Necrosis as Manifestation of Waldenstrom Macroglobulinemia Associated With Type I Cryoglobulinemia: A Case Report[J]. Int J Low Extrem Wounds 2021: 153473462110269. [DOI] [PubMed] [Google Scholar]
- 11.Gertz MA. Waldenström macroglobulinemia: 2015 update on diagnosis, risk stratification, and management[J]. Am J Hematol 2015; 90: 346–354. [DOI] [PubMed] [Google Scholar]
- 12.Arnulf B, Lecourt S, Soulier J, et al. Phenotypic and functional characterization of bone marrow mesenchymal stem cells derived from patients with multiple myeloma[J]. Leukemia 2007; 21: 158–163. [DOI] [PubMed] [Google Scholar]
- 13.Dimopoulos MA, Panayiotidis P, Moulopoulos LA, et al. Waldenstrom’s Macroglobulinemia: Clinical Features, Complications, and Management. J Clin Oncol 2000; 18: 214–226. [DOI] [PubMed] [Google Scholar]
- 14.Schupfner R, Stoevelaar HJ, Blattert T, et al. Treatment of Osteoporotic Vertebral Compression Fractures: Applicability of Appropriateness Criteria in Clinical Practice[J]. Pain physician 2016; 19: E113–E120. [PubMed] [Google Scholar]
- 15.Luque R, Brieva JA, Moreno A, et al. Normal and clonal B lineage cells can be distinguished by their differential expression of B cell antigens and adhesion molecules in peripheral blood from multiple myeloma (MM) patients—diagnostic and clinical implications[J]. Clin Exp Immunol 1998; 112: 410–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schuster SR, Rajkumar SV, Dispenzieri A, et al. IgM multiple myeloma: disease definition, prognosis, and differentiation from Waldenstrom's macroglobulinemia.[J]. Am J Hematol 2010; 85: 853–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pujani M, Kushwaha S, Sethi N, et al. Waldenstrom’s macroglobulinemia presenting with lytic bone lesions: a rare presentation[J]. Blood Res 2013; 48: 230–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schlesinger N, Neustadter L, Schumacher HR. Lytic Bone Lesions as a Prominent Feature in Waldenstrom’s Macroglobulinemia.[J]. J Clin Rheumatol 2000; 6: 150–153. [DOI] [PubMed] [Google Scholar]
- 19.Droste R. [Histiomonocytic reticulosis with macroglobulinemia & bone destruction[J]. Medizinische 1958; 3: 792–794. [PubMed] [Google Scholar]
- 20.Welton J, Walker SR, Sharp GC, et al. Macroglobulinemia with bone destruction[J]. Am J Med 1968; 44: 280–288. [DOI] [PubMed] [Google Scholar]
- 21.Marks MA, Tow DE, Jay M. Bone scanning in Waldenstrom's macroglobulinemia[J]. J Nucl Med 1986; 26: 1412–1414. [PubMed] [Google Scholar]
- 22.Waldenström J. Clinical Diagnosis and Biochemical Findings in Material of 296 Sera with M-type, Narrow, γ Globulins[J]. Acta Medica Scandinavica 2010; 170: 110–119. [Google Scholar]
- 23.Hernández-García MT, Hernández-Nieto L, Pérez-González E, et al. Bone marrow trephine biopsy: anterior superior iliac spine versus posterior superior iliac spine[J]. Clin Lab Haematol 2010; 15: 15–19. [DOI] [PubMed] [Google Scholar]
- 24.Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case reporting guideline development[J]. J Med Case Rep 2013; 10: 381–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_03000605221096161 for Does Waldenstrom’s macroglobulinemia also cause bone destruction? A rare case report by Jun-Ming Lin, Xiao-Jun Yuan, Lu Zhang, Guang Li, Xin-rong Gan and Wen-Hua Xu in Journal of International Medical Research