Abstract
Exercise has been shown to benefit brain structure and function, particularly in aging populations. However, the mechanisms by which exercise exerts its effects, especially in humans, are not fully understood. This review argues that one reason for this knowledge gap is that exercise likely operates through multiple levels of mechanisms. Further, the mechanisms of exercise may vary depending on factors such as age and health state. We discuss the state of evidence at each of three levels of analysis (molecular/cellular, brain structure/function, mental states and higher-order behaviors) and highlight consistencies across these levels, inconsistencies within them, and knowledge gaps. Lastly, based on these, we speculate about which mechanisms of exercise may be universal across age groups and populations vs. those that might be distinct to specific age ranges or populations.
Keywords: brain, cognition, exercise, lifespan, physical activity, mechanisms
Challenges of identifying mechanisms of exercise
Exercise unequivocally influences the brain [1–6]. However, fundamental questions remain regarding the effects of exercise on brain health and subsequent behavioral manifestations such as cognitive function. In part, despite important insights from animal models, the mechanisms by which exercise affects the brain remain largely unknown, particularly in humans. But first, what exactly does it mean to refer to mechanisms of exercise?
When discussing mechanisms, we are interested in inferring a path (or paths) by which a behavior or intervention -- in this case, exercise -- produces changes in an endpoint of interest. In this article, we focus primarily on two frameworks that can be used to infer causality and thereby identify mechanisms—one is related to study design, and the other to statistical approach [7,8]. Briefly, in the first framework causal evidence comes from experimental manipulations of exercise behavior, which in humans are referred to as randomized controlled trials (RCT) (see Glossary), or from animal models in which one group of animals is permitted to exercise while another group is treated as a control. Causality is established if the outcome variable (e.g., cognition) changes to a greater extent in the treatment (e.g., exercise training) group relative to the control group.
When random assignment is not possible or the independent (i.e., treatment) variable is not directly manipulated, a statistical framework for causal inference can be used (i.e., statistical mediation). Statistical mediation evaluates pathways between the treatment and outcome variables by examining the roles of several intermediate variables that might lie in the causal path. The intermediate variable is considered a mediator if the coefficient describing the strength of the treatment-outcome relationship through the mediating variable, known as the indirect effect, is statistically significant [9]. In other words, if the indirect effect is significant, the mediator is a viable mechanism by which the independent variable influences the outcome. This approach is most frequently used to infer possible causal relationships from non-experimental study designs, such as longitudinal, observational, and quasi-experimental studies.
Beyond the above strategies to identify mechanisms, we have previously highlighted that there are also multiple levels of mechanisms by which exercise might work [8]. That is, while many of the widely discussed mechanisms of exercise are molecular or cellular in nature and inferred from animal models, exercise-induced changes in cellular and molecular pathways are bound to also initiate changes in more macroscopic properties of the brain (e.g., brain structure and function) and/or in higher-level behaviors or mental states that could in turn independently influence outcomes such as cognition.
Given that exercise affects most, if not all organ systems in the body, it seems likely that its effects on the brain operate via multiple mechanisms, rather than a single one. In particular, it seems logical to assume that different mechanisms could operate across age groups, brain regions, and subject populations. Equally important is that various modes, frequencies, intensities, and durations of exercise might elicit different pathways and thus have differing effects on brain health outcomes. Yet, there are often implicit assumptions about similar mechanisms operating across exercise types, modalities, populations, and brain regions. The primary goal of this paper is not to comprehensively review the literature on exercise mechanisms, as there are already many reviews on this topic [e.g., 2,8,10]. Rather, we aim to highlight consistencies across mechanistic levels, as well as inconsistencies and gaps between them. Through this synthesis, we also speculate about which mechanisms of exercise may be more universal (e.g., across populations or age groups) vs. those that might be distinct to specific contexts. For the purposes of this review, we will rely predominately on studies employing randomized interventions or statistical mediation models, as these are the two frameworks that provide the strongest mechanistic evidence. Moreover, we will focus mostly on aerobic exercise (hereafter ‘exercise’), but will specify when referring to other forms of exercise, such as resistance training.
Exercise and cognitive function
A recent systematic review conducted in part for the 2018 Health and Human Services Physical Activity Guidelines for Americans Advisory Committee, summarized the existing evidence for the effects of exercise interventions on cognitive function across the life span, as well as in clinical disorders [11]. This umbrella review concluded that there is moderately strong evidence that moderate-vigorous exercise leads to improvements in cognition, especially processing speed, memory, and executive function. By far the strongest evidence for the cognitive-enhancing effects of exercise come from studies focusing on two age windows, i.e., children of ages 6–13 and adults over 50 years old, as well as populations with dementia or other cognition-impairing condition (e.g., schizophrenia). Notably, although evidence for the effects of exercise in children and older adults is the strongest, it is still marred by inconsistencies and deficiencies in study design [e.g., see 12]. There is therefore still a need for rigorous and adequately powered RCTs in these groups to more definitively evaluate the effects of exercise on cognition.
There is also emerging evidence that exercise has beneficial effects on cognition in non-neurologic or psychiatric conditions, such as in women treated for breast cancer who experience co-occurring cognitive symptoms and complaints (Box 1). While there are complexities in defining specific age ranges for developmental periods such as adolescence [e.g., see 13], age groups are used to simplify the presentation of the studies discussed here. With that, there are major gaps in our understanding of the effects of exercise on cognition, particularly in early childhood (<6 years old), adolescence (approximate age range of 14–17), and young to middl-eage adulthood (ages 18–50). Work in these areas is emerging [e.g., 14], but there is currently insufficient evidence from studies employing causal designs to support firm conclusions regarding the effects of exercise on cognitive outcomes in these age ranges.
Box 1: Exercise as a treatment for declines in cognition and brain health in non-brain diseases--The case of breast cancer.
Although not considered a neurologic or psychiatric disease, up to 75% of women with breast cancer (BC) experience deteriorations in cognitive function with chemotherapy [69,70]. Given the beneficial role of exercise in counteracting brain aging in both cognitively normal populations and ones with dementia, it is possible that exercise may also provide similar benefits to patients with non-neurologic/psychiatric diseases that involve impaired cognitive function. Emerging work on exercise and BC represents an interesting case study on this topic [71,72]. Cross-sectional and prospective observational studies report improved memory [73] and self-reported cognitive function with aerobic exercise in women with BC [74,75]. In addition, a recent 12-week RCT of aerobic exercise in women with BC (N=87) found that, compared to controls, exercise improved processing speed. No other group differences in subjective or objective cognitive function were observed [76]. Thus, there is promising evidence that aerobic exercise may ameliorate some of the cognitive symptoms in BC patients. However, there is a need for corroborating RCTs of aerobic exercise in this unique patient population.
The effects of exercise on brain health in BC are similarly unclear. In a 24-week RCT in women with early stage breast cancer [77], three months to three years post-adjuvant therapy, improved processing speed (Trail Making Test A) was detected in women who engaged in aerobic exercise (n=10) versus controls (n=9). Functional MRI was performed in a subgroup of this sample when performing a Stroop task. The results indicated that, compared to controls (n = 7), the exercise group (n = 7) had reduced neural activity in the cingulate cortex and superior frontal gyrus with no between-group differences on Stroop performance, which was interpreted as suggesting that less exertion was needed for cognitive performance with exercise [77].
There is reason to expect that exercise may improve cognitive function via pathways similar to those associated with accelerated aging in cancer [76,78]. In a RCT, the authors found improved self-reported cognitive function, reductions in some inflammatory markers (e.g., IFNg, IL-8 and IL1b) and increases in others (e.g., IL-6, IL-10 and sTNFrα) following aerobic plus resistance exercise in adults with non-metastatic cancer [79]. Aerobic plus resistance exercise has also been shown to reduce metabolic syndrome, sarcopenic obesity, insulin, IGF-1, leptin and adiponectin in a 16-week RCT of 100 physically inactive, obese, BC survivors [79]. Finally, self-reported cognitive function frequently coexists with other symptoms commonly experienced by women with BC including fatigue, depressive symptoms, anxiety and pain. Exercise has been demonstrated to reduce fatigue, anxiety, depression, and pain in patients with cancer, suggesting that exercise also may work to improve cognitive function via these more ‘Level 3’ (i.e., psychosocial, see Figure 1) pathways [8,80–83].
What is known about cellular and molecular (‘Level 1’) mechanisms of exercise?
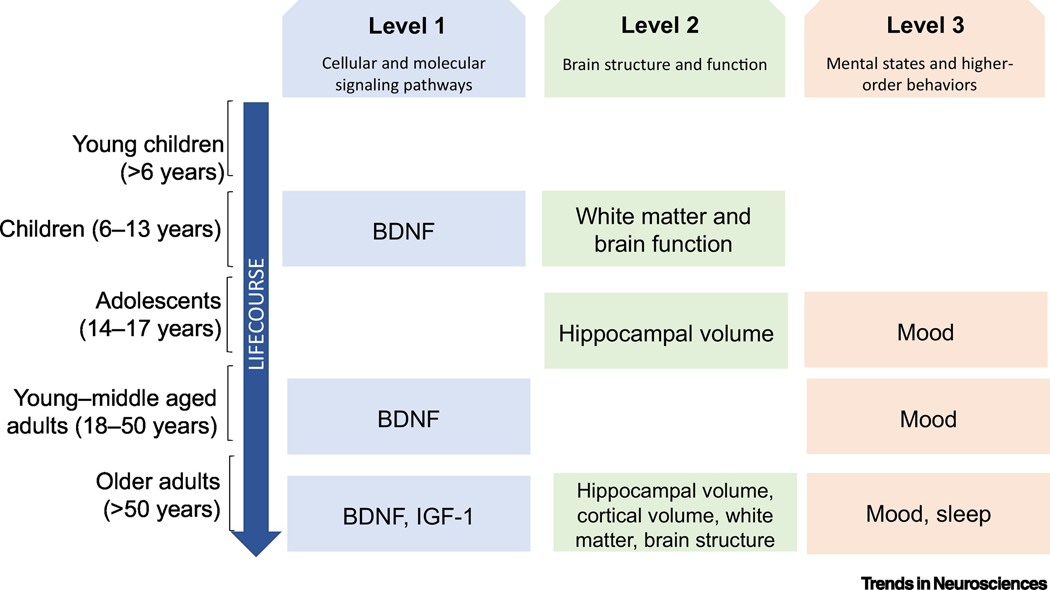
Aerobic exercise induces significant biochemical changes in the brains of animals [2,5 for reviews,10]. Some of the most widely studied molecules in animal models are (1) brain derived neurotrophic factor (BDNF), which initiates a host of downstream effects including long-term potentiation and proliferation of neurons; (2) vascular endothelial growth factor (VEGF) which supports blood vessel survival and growth; and (3) insulin-like growth factor (IGF-1) which influences several neural and angiogenic processes [2]. In humans, most studies on exercise-induced cellular/molecular changes have focused on analytes measurable in the bloodstream or cerebrospinal fluid. For example, meta-analyses and reviews have concluded that there are increases in BDNF after long-term exercise in children, adolescents, younger adults, older adults, Alzheimer’s patients, and those with psychiatric disorders, despite some inconsistency in the findings across individual studies [15,16 for review]. In addition, circulating levels of BDNF in humans statistically mediate exercise-related improvements in executive functioning [17] in adults older than 71 years of age. This pattern of evidence supports the hypothesis that BDNF may be a mechanism of exercise that is conserved across species and age groups in humans (Figure 1, Key Figure).
Figure 1.
Evidence-based mechanisms (only those confirmed by RCTs) of exercise that contribute to its salutary effects on cognition. Evidence is presented across three different levels of analysis and separated by age group to highlight emerging patterns and existing gaps in evidence. While there are complexities in defining specific age ranges for developmental periods such as adolescence, age group is used to simplify the presentation of the studies discussed here. Level 1 refers to cellular and molecular signaling pathways. Level 2 refers to brain structural or functional pathways, and Level 3 refers to psychosocial pathways which are defined as higher-order behaviors or mental states.
In older adults, there is also evidence that IGF-1 levels increase following exercise, although this effect is again somewhat inconsistent across studies [see 18 for a recent meta analysis]. A major open question is whether exercise influences IGF-1 levels across the lifespan, as studies of exercise and IGF-1 in age groups other than older adults are lacking. The evidence linking VEGF and exercise in humans is similarly limited [but see 19].
An overarching limitation of assessing Level 1 mechanisms in humans is that it is notoriously difficult to assess these molecular pathways in vivo. That is, rather than measuring the relevant biomolecules directly from the brain, as is typical in animals, indirect measures of them (i.e., circulating analytes) are often used to infer brain levels in humans. However, there are also non-neuronal sources, kinetics, and roles of these biomolecules in humans [e.g., 20], which introduces an inherent source of error into inferences about their brain levels. Further, these biomolecules could play different roles in childhood vs. older adulthood or across health states [e.g., 21] and this could potentially influence their sensitivity to exercise. For example, it’s possible that older adults or patients deficient in one or more of these biomolecules may experience greater increases in response to exercise compared to groups with normal levels.
What is known about the effects of exercise on brain structure and function (‘Level 2’ mechanisms)?
Gray Matter
The most widely studied brain region in the context of exercise is the hippocampus. This stems from the fact that foundational work on exercise mechanisms was conducted in rodent models and established that exercise promotes neurogenesis (likely in response to increases in aforementioned neurotrophins) in the hippocampus and improves hippocampal-dependent cognitive functions (e.g., learning and memory) in aging animals [10]. While significant questions remain regarding both the similarities and differences between hippocampal neurogenesis in rodents and humans [for discussion see 22,23], many studies on the effects of exercise in humans have focused on gray matter volume of the hippocampus during aging, partly with the goal of assessing the relevance of parallel findings in animal models to humans [24].
While the focus of exercise RCTs to date has been on older adults, a recent meta-analysis reported that (various types of) exercise may attenuate age-related loss of left hippocampal volume including participants ranging from 24–76 years old and ranging from healthy to those diagnosed with a clinical disorder [24]. This net positive effect is notable given the degree of heterogeneity not only in populations, but also in intervention characteristics (e.g., intensity, control groups, duration) across studies included in the meta-analysis. Within older adults, there was mitigation of volume loss for both the left and right hippocampus following exercise. While this could suggest that the effect of exercise might be stronger in this subgroup, the scarcity of studies in other populations makes it difficult to draw a firm conclusion. In fact, for this same reason, in their meta-analysis the authors were unable to examine the effect of exercise in other subgroups. Furthermore, the extent to which hippocampal volumetric changes mediate improvements in cognitive processes in these age groups is still unknown.
Conspicuously missing from the existing literature are RCTs of exercise and hippocampal volume in children and adolescents. RCTs that examine hippocampal volume changes in young and middle age adults are also scarce [but see 25–27]. However, there is cross-sectional evidence in these groups that provides support for a link between exercise and related constructs (e.g., cardiorespiratory fitness), hippocampal volume and cognitive performance in healthy children (9–10 years old), adolescents, young and middle-aged adults (but not yet in very young children) [28–31]. More work is needed to assess whether hippocampal volumetric and functional changes can be detected in the context of RCTs in lesser-studied age groups and what, if any, specific intervention parameters are required to do so.
A common limitation of the existing studies of exercise and hippocampal volume is the lack of consistency in terms of whether studies also concurrently assess behavioral outcomes (i.e., cognitive functioning). Co-assessment of brain volume and cognitive changes would be necessary in order to make a more substantive causal link between exercise, hippocampal volume and cognition. Nonetheless, a net positive effect of exercise on hippocampal volume from existing RCTs, coupled with promising cross-sectional work in less-studied groups lend support to the idea that exercise preserves hippocampal volume and this might be the case across species [e.g., see 32,33 for animal studies], and across age groups and health states (Figure 1). This of course does not rule out the possibility that age or other characteristics could moderate the effect, nor the possibility that that there could be multiple molecular mechanisms underlying the effects of exercise on hippocampal volume [33,34].
Although not as consistently studied as the hippocampus, there is evidence that exercise also affects cortical volume. In population samples of healthy individuals, studies have demonstrated that specific brain regions in the frontal, parietal and temporal cortex are susceptible to the influence of exercise, as denoted by changes in cortical volume and cortical thickness across the lifespan and within different healthy populations [1, see also 35]. However, recent RCTs in clinical populations have been more equivocal [36,37]. Altogether, there is insufficient evidence to conclude whether changes in cortical volume are a universal mechanism underlying exercise-induced cognitive improvements.
The trajectory of neural development across the lifespan is an important factor to consider in relation to the strength of exercise’s effects on regional gray matter volume. Cortical brain regions, particularly the prefrontal cortex, are still rapidly developing in children and adolescents, whereas the hippocampus is (structurally) mostly developed at these ages. Conversely, the hippocampus (along with prefrontal cortex) is often amongst the first regions to show structural atrophy in aging [38]. One could speculate that the effects of exercise are largest in the prefrontal cortex during youth as this region is more developmentally plastic. Given these different developmental influences, one cannot assume that the strength of exercise’s effects in well-studied regions, such as the hippocampus, are the same across the lifespan.
White Matter
A growing number of studies, including several RCTs, have begun to examine the potential effects of exercise on white matter (WM) volume, lesions, and microstructure [39–43]. As with studies of gray matter, studies of WM and exercise have also focused primarily on cognitively-normal older adults [for review see 43]. Although the results of individual studies are mixed, meta-analytical results [43] indicated a small but significant net effect of exercise on higher global WM volume and smaller global volume of WM lesions in older adults. Of note, out of 29 studies included in the meta-analysis, only 2 were RCTs [44,45]. More recent RCTs of exercise and WM in healthy older adults have either found no change in WM structure following exercise [41], or have reported that only certain types of exercise, such as dance [39] or resistance exercise [46] influence WM structure. The fairly inconclusive results from RCTs therefore suggest a need for additional controlled studies on traditional aerobic as well as other types of exercise. In addition, there are likely to be some key boundary conditions [e.g., sex, genetics; see 47] of the effects of exercise on WM in late adulthood.
A systematic review of the effects of exercise in youth reported emerging evidence that exercise benefits WM structure in typically developing children [48]. The effects of exercise on WM structure (e.g., WM integrity) also appear to extend to pediatric patient populations, including those recovering from brain tumors [37,49], although very few RCTs have been conducted in pediatric clinical populations to date.
An overarching limitation of the WM research is that few studies have been randomized interventions. Even fewer have directly examined whether exercise-related increases in WM structure can also be linked to meaningful functional (e.g., cognitive) improvements [but see 42 for a recent example in young adults]. Finally, there is again a dearth of studies in children <6 years old, adolescents, and young to middle-aged adults. Limitations aside, even if changes in WM do turn out to be a universal Level 2 mechanism underlying some of the cognition-enhancing effects of exercise, it is still unlikely that the pathways underlying such changes are consistent across age groups. For example, while changes in health factors such as blood pressure following exercise have been proposed as a mechanism influencing white matter outcomes in middle aged or older adults [e.g., 50], this is a far less likely mechanism in children or patient populations with no vascular pathology. However, this does not rule out the possibility that vascular health changes are closely tied to exercise-related WM changes in older adults, while partly overlapping or completely separate pathways underlie WM changes in other age groups and populations.
Brain Structure and Function
While a less often studied brain outcome in the context of RCTs, several recent reviews provide evidence that changes in brain structure and function mediate some of the effects of exercise on cognition (‘Level 2’ mechanisms) both in preadolescent children and older adults [8,48,51].
Existing studies in older adults have predominately focused on the effects of exercise on the connectivity of large-scale functional networks, such as the default mode network (DMN) [8,51]. Despite much heterogeneity across existing RCTs, some general patterns emerge. For example, exercise generally leads to increased functional coherence within the DMN, as well as increased cross-network specificity across functionally distinct networks [51]. There is also emerging work using EEG in this age group, which demonstrates a decrease in P3b latency following exercise training that is linked to better cognitive performance [52].
In contrast, for children the effects of exercise on brain function have focused predominately on task-evoked activation. Activation changes typically differ in direction depending on the region and task, with conflicting results often reported within the same region (e.g., in the anterior cingulate cortex) across existing studies. The parietal cortex was the only region in which RCTs consistently showed decreased activation during inhibitory task performance coupled with cognitive improvements [48]. This may indicate that exercise improves neural efficiency in childhood. Emerging cross-sectional and RCT work using EEG in children also supports the neural efficiency hypotheses in that improved cognitive performance following exercise is linked to shorter P3b latencies and larger P3b amplitudes [52].
Functional brain changes in response to exercise may be the most uncharacterized of the Level 2 mechanisms, since there are a range of analytical approaches and contexts (e.g., rest vs. task) of functional magnetic resonance imaging that could further compound other overarching limitations, such as the heterogeneous nature of exercise interventions. Further complicating the picture on functional brain mechanisms is the fact that this type of mechanism has been widely unexplored in RCTs or via statistical mediation in populations other than children and healthy older adults [but see 53 for a recent study in individuals with mild cognitive impairment].
Another relevant factor to consider is that exercise-related functional mechanisms could very well differ across age groups. For instance, the DMN and attentional networks are relatively immature in children and may not fully develop until early adulthood [54]. This would likely influence the functional connections most likely to be affected by exercise in younger populations--e.g., one possibility is that exercise training makes these networks more ‘adult-like’ in these age groups. However, convergence in terms of the brain outcomes assessed in exercise interventions across age groups has so far been lacking, so these abovementioned possibilities remain to be tested. A final point to emphasize regarding Level 2 mechanisms is that they are likely to be inter-connected with Level 1 mechanisms (Box 2). Interactions amongst these levels, however, is still an area of emerging research.
Box 2. Convergence between ‘Level 1’ and ‘Level 2’ mechanisms.
The “levels” of mechanism we discuss are not mutually exclusive. For example, the pathways identified using Level 2 and 3 analyses are necessarily invoked by changes at lower levels of analysis, and bidirectional effects, such as feedback loops, are likely to exist between levels. How then might changes in brain structure and function (‘Level 2’ mechanisms) interrelate with cellular and molecular (‘Level 1’) mechanisms?
Some studies in humans have linked volumetric and morphologic brain changes following exercise to the molecular pathways discussed above. For example, changes in peripheral levels of BDNF are correlated with changes in hippocampal volume [84] and changes in BDNF, VEGF, and IGF-1 are correlated with changes in functional connectivity [19] following 12-month exercise interventions in older adults. These findings are consistent with the idea that circulating levels of these molecules may at least partially mediate exercise-induced changes in brain structure and function. However, null effects of exercise on these molecules also exist, albeit following a shorter (3 month) exercise intervention in older adults [85]. Offering a potential explanation for these seemingly disparate results, Voss et al [19] noted that baseline levels of these molecules predicted their degree of change in response to exercise, suggesting that there may be important person- or group-level moderators of their expression. For example, BDNF is dysregulated in normal and pathological aging, and so age itself could be a moderator of the exercise-BDNF-brain relationship [86]. Unfortunately, limited evidence linking BDNF, VEGF, and IGF-1 to exercise-related brain changes exists in younger populations, making it difficult to evaluate this hypothesis across the lifespan. However, age is likely to be an important moderator of molecular-brain mechanisms in younger age groups as well, given that ‘normal’ levels of these molecules fluctuate throughout the lifespan and in response to environmental stimuli for which exposure may vary by age group, such as social interaction, alcohol/drug use, and physical activity [86,87].
What is known about the psychosocial (‘Level 3’) mechanisms of exercise?
Mood
By “psychosocial mechanisms of exercise” we refer to mental states and higher-order behaviors that may be influenced by exercise and thus contribute to some of its salutary effects on brain and cognition. Mood, in particular depressive symptomology, is one likely candidate psychosocial mechanism of exercise [8]. In population samples of healthy individuals, higher levels of objectively measured physical activity are associated with lower levels of depressive symptoms and better cognitive function in children and adolescents (ages 5–17) [55,56], as well as older adults [57]. However, it is important to note that most of the evidence to date in psychologically normal (i.e., not clinically depressed) samples is cross-sectional in nature, and existing RCTs in non-clinically depressed adults rarely examine cognitive outcomes [58,59]. These gaps render any conclusions regarding depressive symptoms as a mechanism of exercise-induced cognitive improvements in psychologically normal samples tenuous.
Most of the causal evidence for mood as a mechanism of exercise comes from studies of patients diagnosed with a mental disorder (e.g., major depression) (Figure 1). A recent meta-analysis of exercise interventions [60] concluded that exercise is an effective additive treatment (compared to treatment-as-usual) to reduce symptoms of depression in adolescents, young adults, and older adults with depressive or psychiatric disorders. Moreover, exercise concurrently improves the cognitive symptoms typical in these disorders, supporting that exercise-induced changes in mood may at least partially underlie exercise-induced improvements in cognitive function in patient populations [60], and there is evidence that this occurs through molecular and structural brain changes (Box 3). It is also possible that exercise-related improvements in cognition partially mediate reductions in depressive symptoms. The mechanisms linking exercise to cognition and depressive symptoms may therefore be bidirectional.
Box 3. Relationships between depressive symptoms, changes in brain structure, and molecular mechanisms of exercise.
There are points of overlap between the potential Level 3 mechanisms (i.e., psychosocial – mental states and higher-order behaviors) and Level 2 mechanisms (i.e., brain structure and function) of exercise. For instance, depression is characterized by structural abnormalities in brain regions including the hippocampus and prefrontal cortex [88]. These are the same regions that show structural plasticity in response to exercise. It may therefore be through volumetric changes to these brain regions that exercise improves symptoms of depression [for reviews see 89,90]. This has led to the idea that lack of exercise may be a risk factor for depression just as depression has long been known to reduce engagement in health behaviors such as exercise, highlighting possible bidirectional relationships between these variables [e.g., 91]. Interestingly, exercise and antidepressant treatments (e.g., medication or electroconvulsive therapy) may alleviate depression through common molecular mechanisms. For example, both antidepressant treatment and electroconvulsive therapies have been shown to increase expression of neurotrophic factors such as BDNF [92]. This provides a possible link between molecular mechanisms of exercise and its psychosocial mechanisms, as expressed for instance in depressive symptoms.
Sleep
Sleep is another candidate psychosocial mechanism of exercise. Sleep outcomes (most often efficiency and duration) improve in middle-aged to older adults following exercise interventions [61,62], although the evidence from intervention studies is inconsistent for children, adolescents and young adults. This is potentially attributable to the heterogeneous methodology and quality of the included studies [61]. In adult patient populations with sleep disruptions (e.g., sleep apnea, insomnia), exercise training also has a positive impact on sleep outcomes [63,64]. While fewer RCTs of exercise and sleep exist in youth patient populations (e.g., pediatric cancer), cross-sectional evidence supports that the positive effects of exercise on sleep extends to these groups as well [65].
Critically, sleep is also known to have restorative effects on brain regions especially sensitive to exercise such as the prefrontal cortex and hippocampus [66], and this may be one of the Level 3 pathways by which exercise-induced improvements in sleep underlie exercise-induced changes in cognitive function. A recent statistical mediation study [67] supports this idea. In a sample of 112 younger (n = 59) and older (n = 53) adults, the authors found that the association between objectively measured physical activity and measures of executive control could be statistically accounted for by sleep efficiency. However, sleep efficiency did not account for the relationship between physical activity and processing speed. Likewise, a correlational study in older adults concluded that physical activity and sleep quality relate to cognitive performance (measured using the Alzheimer’s Disease Assessment Scale) through independent mechanisms after finding no evidence of mediation [68], suggesting some degree of mechanistic specificity of exercise on cognitive processes. Given the central role of the prefrontal cortex in executive control processes, one explanation for this pattern of results is that prefrontal processes may be especially sensitive to exercise due to the effects of efficient sleep on this region. Of course, a major limitation of both studies cited above is that they are cross-sectional in nature. RCTs of exercise measuring both sleep and cognitive outcomes are needed in order to more definitely test sleep as a mechanism of exercise across younger age groups, and to test whether it operates on multiple cognitive domains. For example, it is possible that sleep is only a mediator in populations with poor sleep quality/efficiency as opposed to those with high-quality sleep, but this idea (and others) remains to be tested.
Concluding Remarks
Given that exercise influences multiple organs in the body, it seems likely that there is no single mechanism mediating all of exercise’s effects on the brain and its functions. Further, given the differences in biological processes at play across different age groups and populations, the mechanisms involved in exercise’s influences on the brain are also likely to vary with age and between individuals. Accordingly, we would argue, a limiting factor in understanding the mechanisms by which exercise works has been a tendency to focus on specific age ranges and population samples. Future studies and conceptual models need to consider mechanisms of exercise at multiple levels, and consider mechanisms that might differ depending on the population under investigation, brain region of focus, or parameters of exercise being used (see Outstanding Questions).
Outstanding Questions.
Do the positive effects of exercise on cognitive health, as seen for instance in aging populations and children of ages 6–13, extend to young children (i.e., under 5 years), adolescents, and/or young to middle-aged adults? Are the underlying cellular/molecular, brain, and psychosocial mechanisms the same in these age groups?
Can exercise act as a secondary prevention for mitigating cognitive losses associated with non-neuronal diseases, such as breast cancer?
What is the ideal mode, dose and intensity of exercise necessary to maximize beneficial effects across the lifespan and across different health states?
Does strength training, high-intensity-interval training, or other forms of exercise work through similar or distinct mechanisms compared to aerobic exercise?
What are the characteristics of people most likely to benefit from increasing their exercise levels?
Highlights.
There is evidence that aerobic exercise can affect the brain and cognition through different levels of mechanisms at various points in the lifespan. The strongest evidence comes from children and older adults.
Exercise has significant promise for mitigating some of the cognitive and brain deficits resulting from a variety of neurologic, non-neurologic, and psychiatric conditions.
There is a dire need for more rigorous randomized controlled trials of exercise in lesser studied age groups, especially children under 5, adolescents and young adults.
A common limitation of the existing exercise literature relates to the heterogeneous nature of studies (e.g., in terms of design, duration, and included outcomes assessments)
Acknowledgements
This work was supported by grants P01HL040962, R01AG060741, R01AG053952, R01CA196762, and P30AG024827 to KIE, a grant from the Alicia Koplowitz Foundation and by the Spanish Ministry of Economy and Competitiveness (RTI2018-095284-J-100) to IEC, and a National Health and Medical Research Council Dementia Research Development Fellowship (grant number: GNT1097105) to BB.
Glossary
- Exercise
A type of physical activity that is conducted in a planned and structured manner with the goal of improving fitness.
- Aerobic exercise
A form of physical activity that raises heart rate and has the goal of improving cardiovascular conditioning.
- Indirect effect
A measure from statistical mediation models which is used to evaluate whether a given mediating variable is a viable mechanism by which an independent variable influences an outcome.
- Randomized controlled trial (RCT)
an experimental manipulation in which two (or more) groups would receive equivalent treatment except for the independent variable of interest (e.g., participation in moderate- to vigorous-intensity exercise).
- Statistical mediation
a technique that allows for the evaluation of alternative causal mechanisms between the treatment and outcome variables by examining the roles of intermediate variables that lie in the causal path.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Batouli SAH and Saba V. (2017) At least eighty percent of brain grey matter is modifiable by physical activity: A review study. Behav. Brain Res 332, 204–217 [DOI] [PubMed] [Google Scholar]
- 2.Cotman CW et al. (2007) Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 30, 464–472 [DOI] [PubMed] [Google Scholar]
- 3.Cotman CW and Berchtold NC (2002) Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 25, 295–301 [DOI] [PubMed] [Google Scholar]
- 4.Hillman CH et al. (2008) Be smart, exercise your heart: exercise effects on brain and cognition. Nat. Rev. Neurosci 9, 58–65 [DOI] [PubMed] [Google Scholar]
- 5.van Praag H. (2009) Exercise and the brain: something to chew on. Trends Neurosci. 32, 283–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Voss MW et al. (2011) Exercise, brain, and cognition across the life span. J. Appl. Physiol 111, 1505–1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imai K. (2011) Unpacking the Black Box of Causality: Learning about Causal Mechanisms from Experimental and Observational Studies. Am. Polit. Sci. Rev 105, 765–789 [Google Scholar]
- 8.Stillman CM et al. (2016) Mediators of Physical Activity on Neurocognitive Function: A Review at Multiple Levels of Analysis. Front. Hum. Neurosci 10, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Preacher KJ and Hayes AF (2008) Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav. Res. Methods 40, 879–891 [DOI] [PubMed] [Google Scholar]
- 10.van Praag H. (2008) Neurogenesis and exercise: past and future directions. Neuromolecular Med. 10, 128–140 [DOI] [PubMed] [Google Scholar]
- 11.Erickson KI et al. (2019) Physical Activity, Cognition, and Brain Outcomes: A Review of the 2018 Physical Activity Guidelines. Med. Sci. Sports Exerc 51, 1242–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Young J. et al. (2015) Aerobic exercise to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst. Rev DOI: 10.1002/14651858.CD005381.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Somerville LH (2016) Searching for Signatures of Brain Maturity: What Are We Searching For? Neuron 92, 1164–1167 [DOI] [PubMed] [Google Scholar]
- 14.Carson V. et al. (2016) Systematic review of physical activity and cognitive development in early childhood. J. Sci. Med. Sport 19, 573–578 [DOI] [PubMed] [Google Scholar]
- 15.Mackay CP et al. (2017) The Effect of Aerobic Exercise on Brain-Derived Neurotrophic Factor in People with Neurological Disorders: A Systematic Review and Meta-Analysis. Neural Plast. 2017, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Voss MW et al. (2013) Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn. Sci 17, 525–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leckie RL et al. (2014) BDNF mediates improvements in executive function following a 1-year exercise intervention. Front. Hum. Neurosci 8, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stein AM et al. (2018) Physical exercise, IGF-1 and cognition A systematic review of experimental studies in the elderly. Dement. Neuropsychol 12, 114–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Voss MW et al. (2013) Neurobiological markers of exercise-related brain plasticity in older adults. Brain. Behav. Immun 28, 90–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spadaro O. et al. (2017) IGF1 shapes the macrophage activation in response to immunometabolic challenge. Cell Rep. 19, 225–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skogstrand K. et al. (2019) Reduced neonatal brain-derived neurotrophic factor is associated with autism spectrum disorders. Transl. Psychiatry 9, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snyder JS (2019) Recalibrating the Relevance of Adult Neurogenesis. Trends Neurosci. 42, 164–178 [DOI] [PubMed] [Google Scholar]
- 23.Petrik D. and Encinas JM (2019) Perspective: Of Mice and Men – How Widespread Is Adult Neurogenesis? Front. Neurosci 13, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Firth J. et al. (2018) Effect of aerobic exercise on hippocampal volume in humans: A systematic review and meta-analysis. NeuroImage 166, 230–238 [DOI] [PubMed] [Google Scholar]
- 25.Thomas AG et al. (2016) Multi-modal characterization of rapid anterior hippocampal volume increase associated with aerobic exercise. NeuroImage 131, 162–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wagner G. et al. (2015) Hippocampal structure, metabolism, and inflammatory response after a 6-week intense aerobic exercise in healthy young adults: a controlled trial. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab 35, 1570–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frodl T. (2019) Aerobic exercise increases hippocampal subfield volumes in younger adults and prevents volume decline in the elderly. Brain Imaging Behav. DOI: 10.1007/s11682-019-00088-6 [DOI] [PubMed] [Google Scholar]
- 28.Boots EA et al. (2015) Cardiorespiratory fitness is associated with brain structure, cognition, and mood in a middle-aged cohort at risk for Alzheimer’s disease. Brain Imaging Behav. 9, 639–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chaddock L. et al. (2011) A Review of the Relation of Aerobic Fitness and Physical Activity to Brain Structure and Function in Children. J. Int. Neuropsychol. Soc 17, 975–985 [DOI] [PubMed] [Google Scholar]
- 30.Herting MM and Chu X. (2017) Exercise, Cognition, and the Adolescent Brain. Birth Defects Res. 109, 1672–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whiteman AS et al. (2016) Entorhinal volume, aerobic fitness, and recognition memory in healthy young adults: A voxel-based morphometry study. NeuroImage 126, 229–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cahill LS et al. (2015) MRI-detectable changes in mouse brain structure induced by voluntary exercise. NeuroImage 113, 175–183 [DOI] [PubMed] [Google Scholar]
- 33.Sack M. et al. (2017) Early effects of a high-caloric diet and physical exercise on brain volumetry and behavior: a combined MRI and histology study in mice. Brain Imaging Behav. 11, 1385–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Biedermann S. et al. (2012) In vivo voxel based morphometry: detection of increased hippocampal volume and decreased glutamate levels in exercising mice. NeuroImage 61, 1206–1212 [DOI] [PubMed] [Google Scholar]
- 35.Esteban-Cornejo I. et al. (2018) Commentary: At least eighty percent of brain grey matter is modifiable by physical activity: a review study. Front. Hum. Neurosci 12, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frederiksen KS et al. (2018) A 16-Week Aerobic Exercise Intervention Does Not Affect Hippocampal Volume and Cortical Thickness in Mild to Moderate Alzheimer’s Disease. Front. Aging Neurosci 10, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szulc-Lerch KU et al. (2018) Repairing the brain with physical exercise: Cortical thickness and brain volume increases in long-term pediatric brain tumor survivors in response to a structured exercise intervention. NeuroImage Clin. 18, 972–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fjell AM and Walhovd KB (2010) Structural brain changes in aging: courses, causes and cognitive consequences. Rev. Neurosci 21, 187–221 [DOI] [PubMed] [Google Scholar]
- 39.Burzynska AZ et al. (2017) White Matter Integrity Declined Over 6-Months, but Dance Intervention Improved Integrity of the Fornix of Older Adults. Front. Aging Neurosci 9, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chaddock-Heyman L. et al. (2018) Physical Activity Increases White Matter Microstructure in Children. Front. Neurosci 12, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clark CM et al. (2019) Effect of aerobic exercise on white matter microstructure in the aging brain. Behav. Brain Res 373, 112042 [DOI] [PubMed] [Google Scholar]
- 42.Opel N. et al. (2019) White matter microstructure mediates the association between physical fitness and cognition in healthy, young adults. Sci. Rep 9, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sexton CE et al. (2016) A systematic review of MRI studies examining the relationship between physical fitness and activity and the white matter of the ageing brain. NeuroImage 131, 81–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Colcombe SJ et al. (2006) Aerobic Exercise Training Increases Brain Volume in Aging Humans. J. Gerontol. Ser. A 61, 1166–1170 [DOI] [PubMed] [Google Scholar]
- 45.Voss MW et al. (2013) The influence of aerobic fitness on cerebral white matter integrity and cognitive function in older adults: Results of a one-year exercise intervention. Hum. Brain Mapp 34, 2972–2985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suo C. (2016) Therapeutically relevant structural and functional mechanisms triggered by physical and cognitive exercise. Mol. Psychiatry 21, 1633–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sachdev Perminder S. et al. (2016) White Matter Hyperintensities Are Under Strong Genetic Influence. Stroke 47, 1422–1428 [DOI] [PubMed] [Google Scholar]
- 48.Valkenborghs SR et al. (2019) The Impact of Physical Activity on Brain Structure and Function in Youth: A Systematic Review. Pediatrics 144, [DOI] [PubMed] [Google Scholar]
- 49.Riggs L. et al. (2017) Exercise training for neural recovery in a restricted sample of pediatric brain tumor survivors: a controlled clinical trial with crossover of training versus no training. Neuro-Oncol. 19, 440–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rêgo ML et al. (2019) Physical Exercise for Individuals with Hypertension: It Is Time to Emphasize its Benefits on the Brain and Cognition. Clin. Med. Insights Cardiol 13, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stillman CM et al. (2019) Exercise, Fitness and the Aging Brain: A Review of Functional Connectivity in Aging. Arch. Psychol 3, [Google Scholar]
- 52.Kao S-C et al. (2019) A systematic review of physical activity and cardiorespiratory fitness on P3b. Psychophysiology DOI: 10.1111/psyp.13425 [DOI] [PubMed] [Google Scholar]
- 53.Chirles TJ et al. (2017) Exercise Training and Functional Connectivity Changes in Mild Cognitive Impairment and Healthy Elders. J. Alzheimers Dis. JAD 57, 845–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mak LE et al. (2017) The Default Mode Network in Healthy Individuals: A Systematic Review and Meta-Analysis. Brain Connect. 7, 25–33 [DOI] [PubMed] [Google Scholar]
- 55.Biddle SJH and Asare M. (2011) Physical activity and mental health in children and adolescents: a review of reviews. Br. J. Sports Med 45, 886–895 [DOI] [PubMed] [Google Scholar]
- 56.Poitras VJ et al. (2016) Systematic review of the relationships between objectively measured physical activity and health indicators in school-aged children and youth. Appl. Physiol. Nutr. Metab. Physiol. Appl. Nutr. Metab 41, S197–239 [DOI] [PubMed] [Google Scholar]
- 57.Loprinzi PD (2013) Objectively measured light and moderate-to-vigorous physical activity is associated with lower depression levels among older US adults. Aging Ment. Health 17, 801–805 [DOI] [PubMed] [Google Scholar]
- 58.Adamson BC et al. (2015) Effect of Exercise on Depressive Symptoms in Adults With Neurologic Disorders: A Systematic Review and Meta-Analysis. Arch. Phys. Med. Rehabil 96, 1329–1338 [DOI] [PubMed] [Google Scholar]
- 59.King AC et al. (1993) Effects of differing intensities and formats of 12 months of exercise training on psychological outcomes in older adults. Health Psychol. 12, 292–300 [DOI] [PubMed] [Google Scholar]
- 60.Ashdown-Franks G. et al. (2020) Exercise as Medicine for Mental and Substance Use Disorders: A Meta-review of the Benefits for Neuropsychiatric and Cognitive Outcomes. Sports Med. Auckl. NZ 50, 151–170 [DOI] [PubMed] [Google Scholar]
- 61.Dolezal BA et al. (2017), Interrelationship between Sleep and Exercise: A Systematic Review. , Advances in Preventive Medicine. [Online]. Available: https://www.hindawi.com/journals/apm/2017/1364387/. [Accessed: 02-Mar-2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vanderlinden J. et al. (2020) Effects of physical activity programs on sleep outcomes in older adults: a systematic review. Int. J. Behav. Nutr. Phys. Act 17, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lins-Filho OL et al. (2020) Effect of exercise training on subjective parameters in patients with obstructive sleep apnea: a systematic review and meta-analysis. Sleep Med. 69, 1–7 [DOI] [PubMed] [Google Scholar]
- 64.Yang P-Y et al. (2012) Exercise training improves sleep quality in middle-aged and older adults with sleep problems: a systematic review. J. Physiother 58, 157–163 [DOI] [PubMed] [Google Scholar]
- 65.Orsey AD et al. (2013) Physical activity (PA) and sleep among children and adolescents with cancer. Pediatr. Blood Cancer 60, 1908–1913 [DOI] [PubMed] [Google Scholar]
- 66.Krause AJ et al. (2017) The sleep-deprived human brain. Nat. Rev. Neurosci 18, 404–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wilckens KA et al. (2018) Physical Activity and Cognition: A Mediating Role of Efficient Sleep. Behav. Sleep. Med 16, 569–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Falck RS et al. (2018) The Independent Associations of Physical Activity and Sleep with Cognitive Function in Older Adults. J. Alzheimers Dis. JAD 63, 1469–1484 [DOI] [PubMed] [Google Scholar]
- 69.Ahles TA et al. (2012) Cancer- and Cancer Treatment–Associated Cognitive Change: An Update on the State of the Science. J. Clin. Oncol 30, 3675–3686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Janelsins MC et al. (2014) Prevalence, Mechanisms, and Management of Cancer-Related Cognitive Impairment. Int. Rev. Psychiatry Abingdon Engl 26, 102–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mishra SI et al. (2012) Exercise interventions on health-related quality of life for cancer survivors. Cochrane Database Syst. Rev DOI: 10.1002/14651858.CD007566.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mishra SI et al. (2012) Exercise interventions on health-related quality of life for people with cancer during active treatment. Cochrane Database Syst. Rev DOI: 10.1002/14651858.CD008465.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Crowgey T. et al. (2014) Relationship between exercise behavior, cardiorespiratory fitness, and cognitive function in early breast cancer patients treated with doxorubicin-containing chemotherapy: a pilot study. Appl. Physiol. Nutr. Metab. Physiol. Appl. Nutr. Metab 39, 724–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Myers JS et al. (2015) Potential factors associated with perceived cognitive impairment in breast cancer survivors. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 23, 3219–3228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sprod LK et al. (2012) Exercise and Cancer Treatment Symptoms in 408 Newly Diagnosed Older Cancer Patients. J. Geriatr. Oncol 3, 90–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hartman SJ et al. (2018) Randomized controlled trial of increasing physical activity on objectively measured and self-reported cognitive functioning among breast cancer survivors: The memory & motion study. Cancer 124, 192–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Campbell KL et al. (2018) Effect of aerobic exercise on cancer-associated cognitive impairment: A proof-of-concept RCT. Psychooncology. 27, 53–60 [DOI] [PubMed] [Google Scholar]
- 78.Ross RE et al. (2019) High-Intensity Aerobic Exercise Acutely Increases Brain-derived Neurotrophic Factor. Med. Sci. Sports Exerc DOI: 10.1249/MSS.0000000000001969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mustian KM et al. (2015) EXCAP exercise effects on cognitive impairment and inflammation: A URCC NCORP RCT in 479 cancer patients. J. Clin. Oncol 33, 9504–9504 [Google Scholar]
- 80.Fields J. et al. (2016) Nordic Walking as an Exercise Intervention to Reduce Pain in Women With Aromatase Inhibitor-Associated Arthralgia: A Feasibility Study. J. Pain Symptom Manage 52, 548–559 [DOI] [PubMed] [Google Scholar]
- 81.Galiano-Castillo N. et al. (2016) Telehealth system: A randomized controlled trial evaluating the impact of an internet-based exercise intervention on quality of life, pain, muscle strength, and fatigue in breast cancer survivors. Cancer 122, 3166–3174 [DOI] [PubMed] [Google Scholar]
- 82.Gebruers N. et al. (2019) The effect of training interventions on physical performance, quality of life, and fatigue in patients receiving breast cancer treatment: a systematic review. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 27, 109–122 [DOI] [PubMed] [Google Scholar]
- 83.Irwin ML et al. (2015) Randomized exercise trial of aromatase inhibitor-induced arthralgia in breast cancer survivors. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol 33, 1104–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Erickson KI et al. (2011) Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. U. S. A 108, 3017–3022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Maass A. et al. (2016) Relationships of peripheral IGF-1, VEGF and BDNF levels to exercise-related changes in memory, hippocampal perfusion and volumes in older adults. NeuroImage 131, 142–154 [DOI] [PubMed] [Google Scholar]
- 86.Miranda M. et al. (2019) Brain-Derived Neurotrophic Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain. Front. Cell. Neurosci 13, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bimonte-Nelson HA et al. (2008) Patterns of Neurotrophin Protein Levels in Male and Female Fischer 344 Rats from Adulthood to Senescence: How Young is “Young” and How Old is “Old”? Exp. Aging Res 34, 13–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang F. et al. (2018) Brain structure alterations in depression: Psychoradiological evidence. CNS Neurosci. Ther 24, 994–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gujral S. et al. (2017) Exercise Effects on Depression: Possible Neural Mechanisms. Gen. Hosp. Psychiatry 49, 2–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Polyakova M. et al. (2015) Brain-Derived Neurotrophic Factor and Antidepressive Effect of Electroconvulsive Therapy: Systematic Review and Meta-Analyses of the Preclinical and Clinical Literature. PloS One 10, e0141564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Choi KW et al. (2019) Assessment of Bidirectional Relationships Between Physical Activity and Depression Among Adults: A 2-Sample Mendelian Randomization Study. JAMA Psychiatry 76, 399–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Phillips C. (2017), Brain-Derived Neurotrophic Factor, Depression, and Physical Activity: Making the Neuroplastic Connection. , Neural Plasticity. [Online]. Available: https://www.hindawi.com/journals/np/2017/7260130/. [Accessed: 11-Mar-2020] [DOI] [PMC free article] [PubMed] [Google Scholar]