Summary
TIAM Rac1-associated GEF 1 (TIAM1) regulates RAC1 signaling pathways that affect the control of neuronal morphogenesis and neurite outgrowth by modulating the actin cytoskeletal network. To date, TIAM1 has not been associated with a Mendelian disorder. Here, we describe five individuals with bi-allelic TIAM1 missense variants who have developmental delay, intellectual disability, speech delay, and seizures. Bioinformatic analyses demonstrate that these variants are rare and likely pathogenic. We found that the Drosophila ortholog of TIAM1, still life (sif), is expressed in larval and adult central nervous system (CNS) and is mainly expressed in a subset of neurons, but not in glia. Loss of sif reduces the survival rate, and the surviving adults exhibit climbing defects, are prone to severe seizures, and have a short lifespan. The TIAM1 reference (Ref) cDNA partially rescues the sif loss-of-function (LoF) phenotypes. We also assessed the function associated with three TIAM1 variants carried by two of the probands and compared them to the TIAM1 Ref cDNA function in vivo. TIAM1 p.Arg23Cys has reduced rescue ability when compared to TIAM1 Ref, suggesting that it is a partial LoF variant. In ectopic expression studies, both wild-type sif and TIAM1 Ref are toxic, whereas the three variants (p.Leu862Phe, p.Arg23Cys, and p.Gly328Val) show reduced toxicity, suggesting that they are partial LoF variants. In summary, we provide evidence that sif is important for appropriate neural function and that TIAM1 variants observed in the probands are disruptive, thus implicating loss of TIAM1 in neurological phenotypes in humans.
Keywords: TIAM1, Drosophila, developmental delay, intellectual disability, seizures, speech delay, still life, Sif
Graphical abstract
Introduction
Many patients with rare diseases undergo a long and frustrating journey to obtain an accurate diagnosis, often referred to as the diagnostic odyssey. Whole-exome sequencing (WES) and whole-genome sequencing (WGS) are effective approaches to identify diagnoses, as 80% or more of rare diseases are estimated to be caused by genomic abnormalities.1,2 However, these comprehensive sequencing methods also uncover many variants of uncertain significance (VUSs) with unknown clinical impact.3 Drosophila melanogaster allows effective approaches to probe the functional impacts of these variants.4
Here, we argue that bi-allelic loss-of-function (LoF) variants of TIAM Rac1-associated GEF 1 (TIAM1 [MIM: 600687]) cause a disease associated with developmental delay, intellectual disability, speech delay, and seizures. TIAM1 is a guanine nucleotide exchange factor (GEF).5, 6, 7, 8, 9 GEFs are positive regulators of small GTPases that promote their activation. Each individual GEF has a specificity profile,8 and TIAM1 is a Ras-related C3 botulinum toxin substrate 1 (RAC1)-specific GEF.6 RAC1 stimulates signaling pathways that regulate actin cytoskeleton organization, cell movement, differentiation, and proliferation.5
TIAM1 is enriched in the brain.10,11 The rodent ortholog, Tiam1, is also expressed in the brain, is present in dendrites and spines, and is required to maintain proper outgrowth during development.7 When activated by neurotrophins such as brain-derived neurotrophic factor (BDNF), the TRKB receptor binds and activates TIAM1, which in turn activates RAC1, causing morphological changes by increasing neurite outgrowth.12,13 Similarly, when glutamate activates N-methyl-D-aspartate (NMDA) receptors, TIAM1 is also activated to control actin remodeling by inducing RAC1-dependent pathways.7,12 The localization of TIAM1 to spines is regulated by par-3 family cell polarity regulator and thereby controls proper spine formation.14 Mice lacking Tiam1 have simplified dendritic arbors, reduced dendritic spine density, and diminished excitatory synaptic transmission in the dentate gyrus.15 Taken together, TIAM1 controls neuronal morphogenesis and neurite outgrowth by RAC1-dependent actin cytoskeletal remodeling in rodents. However, alterations in TIAM1 have not yet been reported in the Online Mendelian Inheritance in Man (OMIM) database or the literature as causing human disease.16
still life (sif) encodes the ortholog of TIAM1 in Drosophila.10,17 Previous studies showed that loss of sif leads to reduced locomotor activity.18,19 Sif was reported to be localized presynaptically and shown to genetically interact with Fasciclin II, a neural cell-adhesion molecule localized pre- and postsynaptially that controls synaptic growth, stabilization, and presynaptic structural plasticity.19, 20, 21 Its partial loss causes loss of some boutons at neuromuscular junctions.19 Also, Sif is highly enriched in lens-secreting cells in fly eyes and affects the distribution pattern of E-cadherin in pupal eyes.22
Here, we identified bi-allelic TIAM1 missense variants in five individuals from four families with developmental delay, intellectual disability, speech delay, and seizures. Functional studies in Drosophila revealed that the loss of sif reduces the survival rate of flies, and the surviving adult flies have a remarkably reduced lifespan and exhibit severe climbing defects. In addition, sif LoF mutants display severe seizure-like behaviors when stressed. Expression of the human TIAM1 reference (TIAM1 Ref) cDNA partially rescues the lethality of sif LoF mutants, whereas the variants observed in probands behave as partial LoF mutations in different phenotypic assays. Our data support the hypothesis that the variants observed in the probands are the cause of the observed phenotypes.
Material and methods
Human genetics
This study was approved by the institutional review board at Columbia University. Legal guardians of affected individuals provided informed consent. Five individuals, including a pair of monozygotic (MZ) twins, with developmental delay/intellectual disabilities from four independent families had trio clinical exome sequencing.
Exome sequencing
Using genomic DNA from the proband and parents, the exonic regions and flanking splice junctions of the genome were captured using the IDT xGen Exome Research Panel v1.0 (Integrated DNA Technologies, Coralville, IA). Massively parallel (next-generation) sequencing was done on an Illumina sequencer with 100 bp or greater paired-end reads. Reads were aligned to human genome build GRCh37/UCSC hg19 and analyzed for sequence variants using a custom-developed analysis tool. Additional sequencing technology and variant interpretation protocol have been previously described.23 The general assertion criteria for variant classification are publicly available on the GeneDx ClinVar submission page (see web resources). Trio WES of proband 4 and parents was done and analyzed at Chigene, Beijing, China.
Drosophila stocks and maintenance
All fly strains used in this study were generated in house or obtained from the Bloomington Drosophila Stock Center (BDSC). The sifT2A-GAL4 allele was created by conversion of a MiMIC (Minos-mediated integration cassette)24 insertion line, y[1] w[∗]; Mi{y[+mDint2] = MIC}sif[MI02376] (BDSC, 35834) via recombination-mediated cassette exchange (RMCE) as described.25,26 sifT2A-GAL4 LoF mutants were rescued with fly sif cDNA P{w[+mC] = UAS-sif.S}2.1 (BDSC, 9128). For complementation tests, immunostaining, and RNAi assays, see genotypes in Table S1. elav-GAL4, UAS-Empty, and UAS-lacZ were published previously.27,28
Drosophila behavioral assays
For the climbing assay,29 flies were transferred to an empty vial, the flies were tapped to the bottom of the vial, and their climbing ability (negative geotaxis) was measured. Climbing distance was measured (15 cm as maximum). Flies were allowed to climb for 20 s. If some of the fly groups did not show obvious climbing defects, another unbiased method was used by assessing the time for flies to climb vertically to 4 cm.30
For the bang-sensitivity assay,31 flies were transferred to an empty vial and vortexed at maximum speed for 10 s, and the time to recovery to freely moving status (without abnormal falling or flipping) was measured. For the heat shock assay,32 flies were transferred to an empty vial and submerged in a 42°C water bath for 30 s. We measured the percentage of flies that were unable to keep an upright position. We then measured the time to recovery to a freely moving status. In these assays, we record for only 30 s. Flies that do not recover within 30 s are recorded at the 30 s time point even if they require many more seconds or minutes to recover.
For survival rate, the surviving adult flies within 1 day of eclosion were counted, and each genotype was identified to assess Mendelian ratios. For lifespan, freshly eclosed flies were separated and maintained at 18°C, and survival was determined every other day.
Generation of human UAS-TIAM1 transgenic lines
Human UAS-TIAM1 lines were generated as described.33,34 The TIAM1 cDNA clone corresponds to GenBank: NM_001353694, which encodes the full-length TIAM1 isoform 1 and is defined as the reference here. TIAM1 variants were generated by Q5 site-directed mutagenesis (New England Biolabs). Primers for mutagenesis are listed in Table S2. All the human cDNAs were cloned into pGW-UAS-HA.attB plasmid transgenic vector35 and Sanger validated. The vectors were inserted into the VK37 (BDSC #24872) docking site by φC31-mediated transgenesis.36
Immunostaining
Immunostaining of fly larval and adult brains was performed as described.37 Briefly, the dissected samples were fixed with 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) followed by blocking in 0.2% PBS Triton X-100 (PBST) with 5% normal goat serum. Primary antibodies used were rat anti-Drosophila Elav (DSHB: 7E8A10) 1:500 and mouse anti-Drosophila Repo (DSHB: 8D12) 1:50. Secondary antibodies used were goat anti-rat-647 (Jackson ImmunoResearch, 112-605-003) 1:1,000 and goat anti-mouse-488 (Jackson ImmunoResearch, 115-545-062) 1:1,000. Samples were washed three times for 15 min with 0.2% PBST and mounted on a glass slide using Fluoromount-G (SouthernBiotech, 0100-20). Confocal microscopy (Zeiss LSM 880) was used for sample scanning, and images were processed using ImageJ.
Real-time PCR
Real-time PCR was performed as previously described28 with the following changes: All-In-One 5X RT MasterMix (abm, G592), iTaq Universal SYBR Green Master Mix (BioRad, 1725120), and a BioRad C1000 Touch Cycler were used. Primers are listed in Table S2.
Statistical analysis
Statistical analysis was performed using GraphPad software (GraphPad Prism v9.0; GraphPad Software, USA). The statistical analyses of the lifespan assay were with log-rank (Mantel-Cox) and Gehan-Breslow-Wilcoxon tests, and analyses of the other assays were with two-tailed unpaired t tests. Data are represented as mean ± SEM, and n.s. (no significance) indicates p > 0.05; ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001.
Results
Summary of clinical findings
The affected individuals all have neurodevelopmental symptoms, mainly developmental delay, intellectual disability, speech delay, and seizures, and some have autism (supplemental note provides a summary of the clinical data). We first identified MZ twins similarly affected with these phenotypes carrying bi-allelic missense variants in TIAM1 (proband 1). Using GeneMatcher,38 we identified three other individuals with rare bi-allelic missense variants in TIAM1. Comprehensive clinical information including nucleotide changes and pedigrees can be found in Table 1, Figure S1, and supplemental note.
Table 1.
Clinical features of affected individuals with TIAM1 variants
| Proband 1 | MZ twin of proband 1 | Proband 2 | Proband 3 | Proband 4 | |
|---|---|---|---|---|---|
| TIAM1 variant (GenBank: NM_001353694.2) | c.67C>T (p.Arg23Cys); c.2584C>T (p.Leu862Phe) | c.67C>T (p.Arg23Cys); c.2584C>T (p.Leu862Phe) | c.983G>T (p.Gly328Val) | c.4640C>A (p.Ala1547Glu) | c.1144G>C (p.Gly382Arg); c.4016C>T (p.Ala1339Val) |
| Genomic DNA, GRCh38 | 21:31266906 G>A; 21:31187079 G>A | 21:31266906 G>A; 21:31187079 G>A | 21:31252170 C>A | 21:31120504 G>T | 21:31252009 C>G; 21:31130242 G>A |
| CADD score | 29.1; 23.7 | 29.1; 23.7 | 25.4 | 18.1 | 23.9; 26.7 |
| Allele frequency (gnomAD) | 4.04e−4; 3.98e−6 | 4.04e−4; 3.98e−6 | 8.18e−6 | 0 | 5.66e−5; 3.98e−6 |
| Zygosity | compound heterozygous | compound heterozygous | homozygous | homozygous | compound heterozygous |
| Sex | M | M | M | M | F |
| Current age | 35 years | 35 years | 3 years | 7 years | 6 years |
| Developmental delay | + | + | + | + | + |
| Intellectual disability | + | + | N/A | + (severe) | + (severe) |
| Delayed speech | + | + | + | + (non-verbal) | + (severe) |
| Autism | + | + | N/A | N/A | N/A |
| ADD/ADHD | + | + | N/A | N/A | N/A |
| Seizures | + | + | +, complex febrile seizures (abnormal EEGs) | + | + (severe) |
| Brain MRI | N/A | N/A | progressive macrocephaly (secondary to chronic subdural hematoma and extra-axial fluid) | diffuse cerebral atrophy; thin corpus callosum; hypomyelination; deep cortical sulcus | hypothalamic hamartoma |
| Other neurological symptoms | none | impulsive, obsessive behavior | axial hypotonia; appendicular hypertonia | none | N/A |
| Endocrine symptoms | hypothyroidism; Addison’s disease | hypothyroidism | subclinical hypothyroidism | hypomagnesemia | N/A |
| Other | none | none | dysmorphic features; congenital heart defect (atrial and ventricular septal defects); undescended testes | poor growth (difficulty in gaining weight); undescended testis; hirsutism | none |
| Consanguinity | none | none | + | + | none |
MZ, monozygotic; CADD, combined annotation dependent depletion; M, male; N/A, not available; ADD, attention deficit disorder; ADHD, attention deficit hyperactivity disorder; EEG, electroencephalography.
All probands are currently between the ages of 3 and 35 years. In two families (probands 2 and 3), the parents are consanguineous. All probands have neurodevelopmental disabilities with deficits especially noted in speech, including two probands who are non-verbal at 7 years of age. Seizures were reported in five individuals, with onset ranging from 2 months to 13 years of age. Three of the five individuals have abnormal brain MRIs, but the abnormalities are not consistent. Among them, proband 2 exhibited progressive macrocephaly thought to be secondary to chronic subdural hematoma or extra-axial fluid and diffuse cerebral atrophy (Figure 1), but it is unclear if these MRI features are primary to the condition or potentially due to another cause. Proband 2 had additional rare de novo or homozygous variants in ALX4 (MIM: 605420) and CYP27B1 (MIM: 609506), respectively, and proband 3 had rare homozygous variants in PNLIP (MIM: 246600), SP4 (MIM: 600540), WDR75, and ZGRF1 (Table S3), but none of these genes is known to contribute to the brain anatomy or neurocognition.
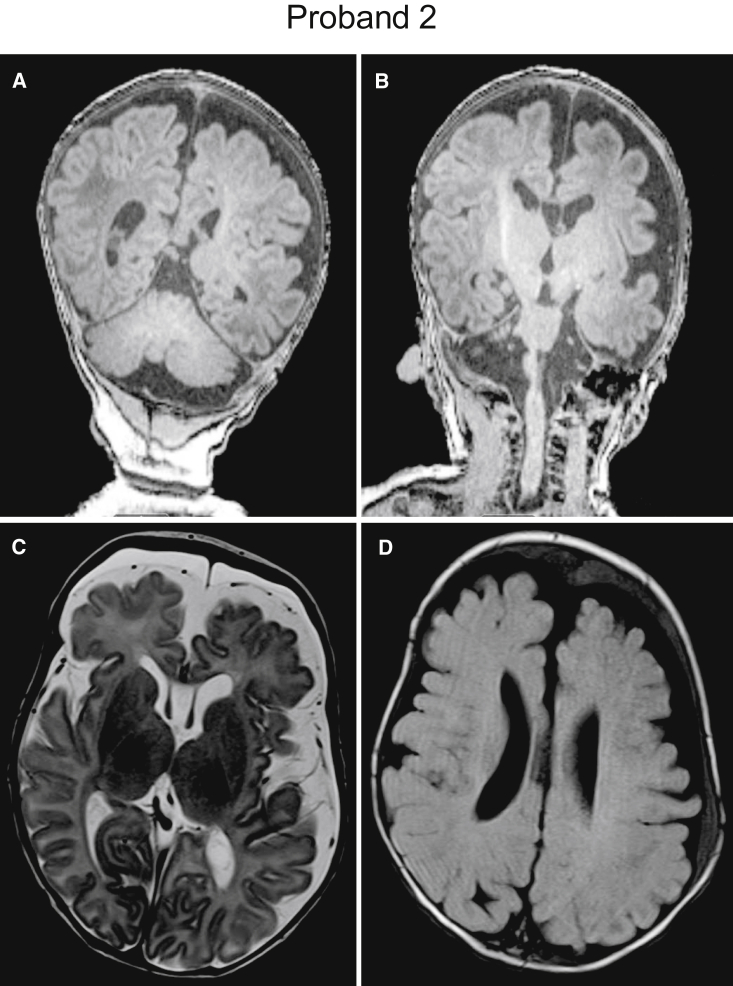
Figure 1.
Brain MRI images of proband 2
(A–D) Brain MRI for proband 2 was obtained to document the acquired macrocephaly (Z ≥ 2) at 14 months of age. 3T coronal T1 images (A and B) show prominence of the extra-axial fluid spaces involving both hemispheres, left greater than right, with prominence of the cortical sulci for age, enlargement of the lateral and third ventricles, and cavum septum pellucidum and cavum vergae. The brain parenchyma items show preservation of the gray-white matter differentiation with a myelination pattern appropriate for age. Axial T2 (C) and T2 FLAIR (D) images demonstrate intact and symmetric subcortical structures without evidence of heterotopia or other migrational defects. Also visible are prominent subarachnoid spaces with superimposed subdural collections most consistent on susceptibility-weighted imaging (not shown) as chronic subdural hemorrhage.
Other medical features observed in one or two probands include hypothyroidism, Addison’s disease, congenital heart disease with septal defects, hypomagnesemia, and difficulty in gaining weight. Hypothyroidism was diagnosed in both MZ twins and required treatment with Synthroid, while an unrelated proband was found to have subclinical hypothyroidism.
To gather information about the variants, we queried MARRVEL (model organism aggregated resources for rare variant exploration, a resource for investigating human gene variants and model organism information).10 The LoF observed/expected (o/e) score for TIAM1 is 0.20, and the probability of being loss-of-function intolerant (pLI) score is 0.96, suggesting that TIAM1 is intolerant to LoF alleles.39 By querying the allele frequency in the Genome Aggregation Database (gnomAD),39 the p.Ala1547Glu (c.4640C>A) variant is absent, while the p.Leu862Phe (c.2584C>T), p.Gly328Val (c.983G>T), p.Gly382Arg (c.1144G>C), and p.Ala1339Val (c.4016C>T) variants are observed in heterozygous individuals at extremely low frequencies (minor allele frequency [MAF] < 0.00006). The p.Arg23Cys (c.67C>T) variant is carried by heterozygous individuals at low frequency (MAF < 0.0005) based on gnomAD. Five of the six variants have combined annotation-dependent depletion (CADD)40,41 scores higher than 20 (Table 1). Taken together, these variants with rare frequencies are likely to be disruptive.
sif is the TIAM1 ortholog of Drosophila
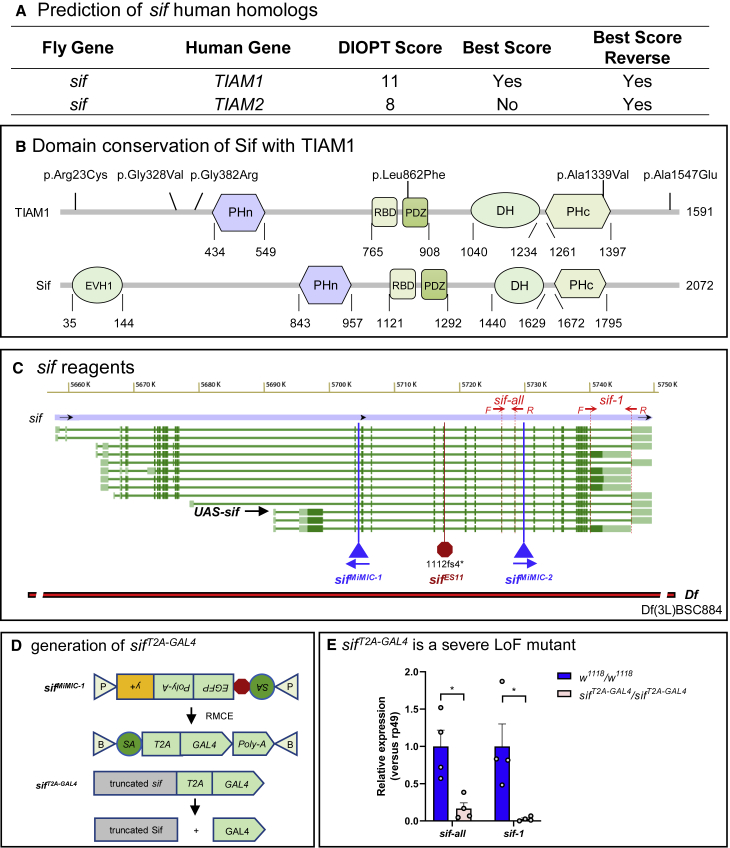
To assess the impact of the TIAM1 variants in vivo, we pursued experiments in Drosophila. The fly ortholog of TIAM1 is sif with a DIOPT (DRSC integrative ortholog prediction tool) score of 11/16.17 sif is also the ortholog of TIAM2 (MIM: 604709) with a DIOPT score of 8/1617 (Figure 2A). Sif and TIAM1 share 31% identity and 46% similarity,17 and nearly all protein domains are shared between the two proteins (Figure 2B). In addition, three of the six TIAM1 variants carried by the probands affect conserved amino acid residues (Figure S2).
Figure 2.
sif is the TIAM1 ortholog in fly
(A) Prediction of sif human homologs using DIOPT (DRSC integrative ortholog prediction tool).
(B) Protein domain structure of TIAM1 and Sif.
(C) Genomic structure of sif locus and reagents used in this study. For detailed information on these reagents, please see Tables S1 and S2.
(D) Schematic of sifT2A-GAL4 generation by RMCE using sifMiMIC-1 (sifMI02376, BDSC #35834). SA, splice acceptor; P, attP; B, attB; y+, yellow+ gene as a marker.
(E) Relative sif mRNA expression levels are lower than 20% in sifT2A-GAL4 mutant larvae when compared to controls (w1118) based on real-time PCR using primers shown in (C). “sif-all” targets all of the 13 sif transcripts, while “sif-1” targets 8 of the 13 transcripts (sif-RA, RB, RC, RI, RL, RM, RN, and RO).
Each sample contains 3–5 L3 larvae. Data are represented as mean ± SEM. Unpaired t tests. ∗p < 0.05.
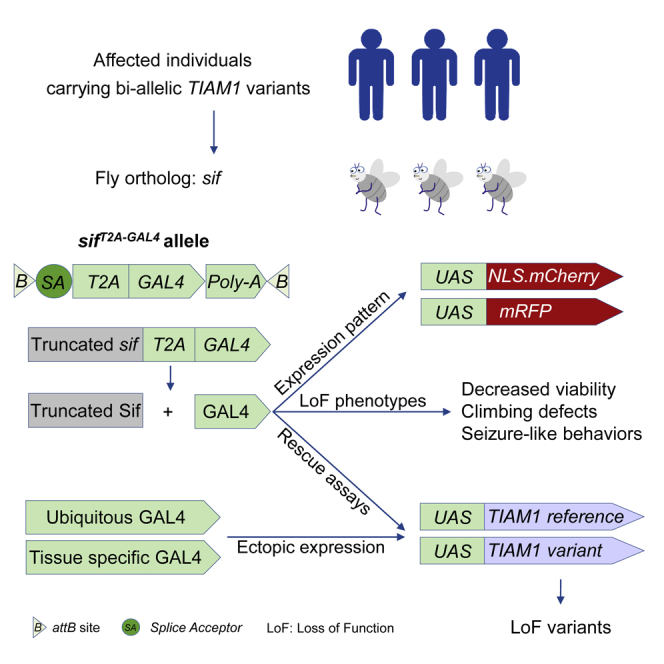
The available sif reagents and tools are shown (Figure 2C and Table S1). They include sifMiMIC-1, a MiMIC insertion in an early intron shared by all transcripts. MiMICs24,26,42 allow us to replace the content between the attP sites with the splice acceptor (SA)-T2A-GAL4-polyA (T2A-GAL4) cassette43 via recombination-mediated cassette exchange (RMCE)25,42 (Figure 2D). The sifT2A-GAL4 allele is predicted to be a null allele or severe LoF allele because the splice acceptor allows the artificial exon to be integrated into the mRNA, and the presence of the poly(A) tail leads to early transcription termination.43 Based on real-time PCR, the total sif transcript levels are severely decreased when compared to wild-type (WT) control levels (w1118) (Figure 2E). Furthermore, the viral T2A sequence allows ribosomal skipping and re-initiation of translation to produce GAL444 (Figure 2D). The GAL4 protein is therefore produced under the endogenous sif regulatory elements, and the sifT2A-GAL4 allele allows us to drive expression of any UAS-cDNA in the same spatial and temporal pattern as the sif gene. The cDNA can be a fluorescent protein-encoding gene to reflect the endogenous expression, a human reference, or proband-specific cDNA to compare rescue ability of the sifT2A-GAL4 mutants and assess if variants carried by the probands affect the protein function.43,45,46
sif is expressed primarily in neurons of the fly CNS
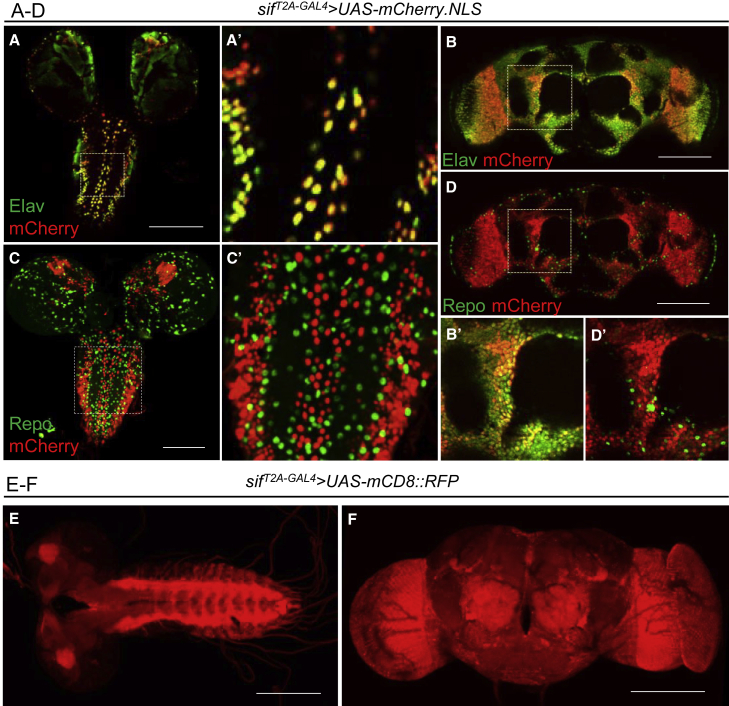
Given that the probands carrying TIAM1 variants have neurological defects and TIAM1 is enriched in the mammalian brain,7 we explored the expression pattern of sif in the fly CNS. To determine the expression pattern, we crossed the sifT2A-GAL4 mutants to UAS-mCherry.NLS (nuclear localized mCherry fluorescent protein) to label the nuclei of the cells that express sif. By co-staining with the nuclear pan-neuronal marker Elav or nuclear pan-glial marker Repo, we found that mCherry (sif) is colocalized with a subset of Elav signals in the developing larval (Figure 3A) and adult CNS (Figure 3B). There is no obvious overlap of mCherry and Repo (glia) in both larval (Figures 3C) and adult brains (Figure 3D). The data are in agreement with the single-cell transcriptome atlas, SCope.47,48 These data indicate that sif is mainly expressed in a subset of neurons but not in glia. To reveal the projections of cells that express sif, we used the sifT2A-GAL4 allele to express UAS-mCD8::RFP (membrane-bound red fluorescent protein). As shown in Figure 3E, third instar larvae exhibit sif expression in the neuropil of the CNS, especially in the central brain and ventral nerve cord. In the adults, sif is enriched in the optic lobe, antennal lobe, and other brain regions (Figure 3F). The expression pattern of sif in the CNS suggested that sif may play a role during development and in adult stages.
Figure 3.
sif is expressed primarily in neurons of the fly CNS
(A–D) The L3 larval CNS (A, C) and adult brains (B, D) of sifT2A-GAL4 allele-driven expression of UAS-mCherry.NLS (nuclear mCherry) were stained with markers for neurons (Elav, A and B) or glia (Repo, C and D). Single-slice confocal images are shown. (A′–D′) Images are from dashed squares of indicated regions to visualize the cellular co-localizations with Elav (A′ and B′) or Repo (C′ and D′). Scale bars: 100 μm. Note that sif is expressed in neurons.
(E and F) The sifT2A-GAL4 allele-driven expression of UAS-mCD8::RFP (membrane-bound RFP) shows that sif is broadly detected in the CNS of L3 larvae (E) and adult flies (F). Scale bars: 100 μm.
Loss of sif impairs viability, and the surviving flies exhibit climbing defects and seizure-like behaviors
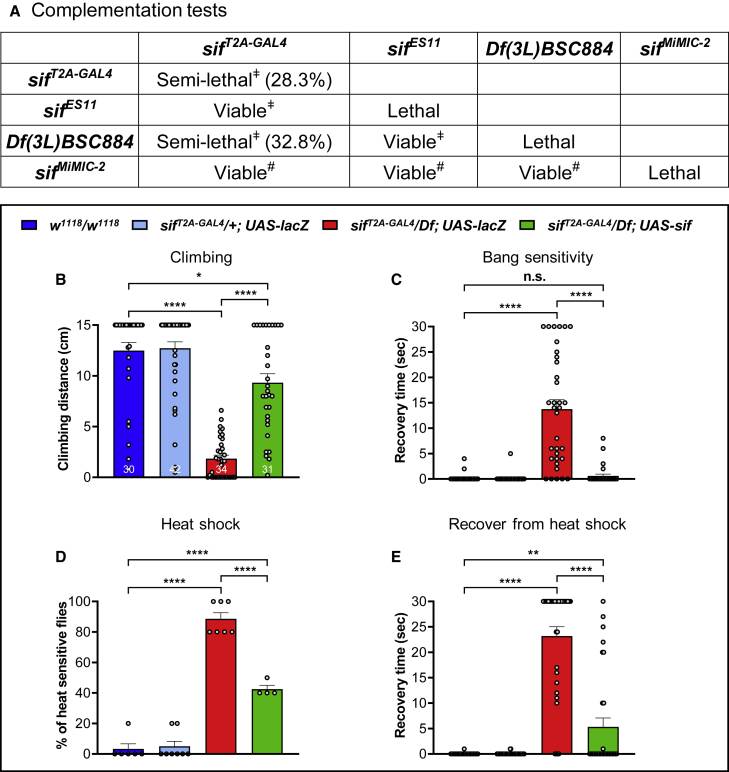
To investigate loss of sif phenotypes in flies, we assessed the survival rate associated with different sif LoF mutant flies. These include a sifES11 allele,19 a truncating sif allele induced by ethyl methanesulfonate; sifMiMIC-2 (Mi{MIC}sifMI07526), a MiMIC inserted in a late common intron of sif transcripts;42 and a deletion that lacks sif, Df(3L)BSC884 (Df). Based on complementation tests shown in Figure 4A, the sifT2A-GAL4 allele is the most severe LoF allele if not a null allele. The sifT2A-GAL4 allele causes semi-lethality, as only ∼28% of the homozygous mutants eclose. The surviving adult flies exhibit very severe climbing defects. A similar number of survivors are observed in trans-heterozygous flies (sifT2A-GAL4/Df) that also exhibit severe climbing defects when they are 3–5 days old (Figures 4A and 4B and Video S1). The sifT2A-GAL4 chromosome is unlikely to carry second-site mutations that affect the survival rate, as the survival rates of sifT2A-GAL4/Df and sifT2A-GAL4/sifT2A-GAL4 are comparable, and both are fully rescued by UAS-sif fly cDNA expression19 (see below). While sifES11/Df flies do not display decreased viability, they do exhibit severe climbing defects (Figure 4A). Finally, sifMiMIC-2/Df flies are viable and exhibit mild climbing defects (Figure 4A). Therefore, the lethality associated with sifES11 and sifMiMIC-2 homozygotes is likely caused by second-site mutations, and these alleles are hypomorphic for sif. These data suggest the allelic series from most severe to less severe is sifT2A-GAL4 > sifES11 > sifMiMIC-2. Note that the climbing defects are significantly rescued in the sifT2A-GAL4/Df flies when GAL4 drives UAS-sif expression (Figure 4B).
Figure 4.
Loss of sif causes semi-lethality, and the surviving flies exhibit climbing defects and seizure-like behaviors
(A) Table summarizing complementation tests of sif LoF mutants. sif LoF mutants are semi-lethal and have severe (“ǂ”) or mild (“#”) climbing defects. The lethality of “sifES11” and “sifMiMIC-2” homozygotes is likely caused by second-site mutations.
(B) sif LoF mutants have severe climbing defects 3–5 days after eclosion. Flies were raised at 22°C.
(C) sif LoF mutants have a bang-sensitive phenotype, which can be rescued by UAS-sif expression.
Flies were raised at 22°C and tested at day 3–5.
(D and E) sif LoF mutants have heat-induced seizure-like behaviors. Flies in vials were immersed in a 42°C water bath for 30 s. The percentage of flies that are unable to keep an upright position in sif LoF mutant group is significantly higher than the control groups (D), and the mutants could not recover 20 s after heat shock (E). The seizure-like behaviors can be rescued by UAS-sif expression.
Flies were raised at 22°C and tested at day 3–5. Data are represented as mean ± SEM. Unpaired t tests. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗∗p < 0.0001; n.s., no significance.
Given that the four probands exhibit seizures, we employed well-established protocols to induce seizure-like behaviors in sif LoF mutants. A seizure is a burst of uncontrolled electrical activity that causes abnormalities in muscle tone and even loss of consciousness. Owing to the similarities at the cellular and subcellular levels between fly and mammalian nervous systems, the fly has been used as a model for human seizures, or epilepsy.49 Seizure-like behaviors in flies are characterized by aberrant posture with wing fluttering, leg twitching, and abdominal muscle contraction.50, 51, 52 Seizures can be induced by mechanical stimulation31 or hyperthermia.32,53 The sif LoF mutants (sifT2A-GAL4/Df and sifT2A-GAL4/sifES11) are extremely sensitive to seizures, as just a few knockdowns of vials containing these flies led to aberrant posture, wing fluttering, and leg twitching in 20%–30% of the flies (Video S1). We therefore tested the flies in a bang-sensitivity assay as well as heat-induced seizure assay. sif LoF mutants (sifT2A-GAL4/Df) show a severe bang-sensitive phenotype and are slow to recover to an upright position after vortexing for 10 s (Figure 4C). Similarly, ∼88% of the mutants show seizure-like behaviors when shifted from room temperature to 42°C for 30 s (Figure 4D), and they require more than 20 s on average to recover to an upright posture (Figure 4E). Again, the seizure-like behaviors are partially but significantly rescued by UAS-sif expression in sifT2A-GAL4/Df flies (Figures 4C–4E), showing that the phenotype is specifically due to loss of sif activity.
Climbing defects and seizure-like behaviors associated with sif LoF are mainly caused by neuronal loss of sif
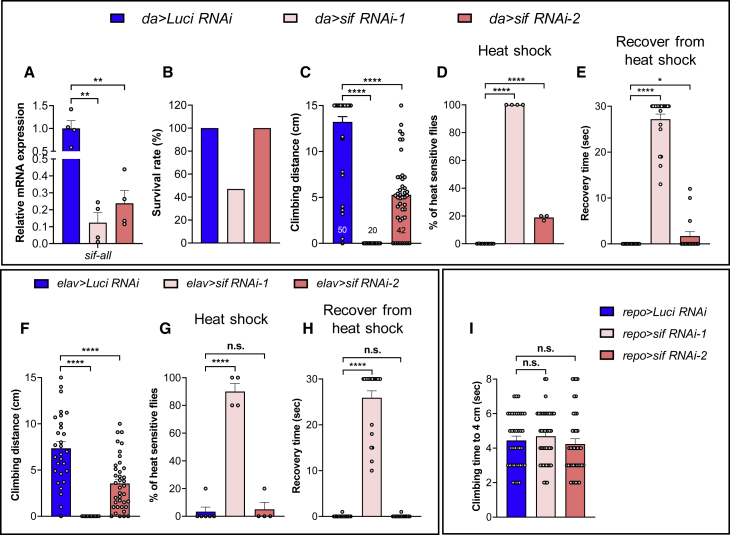
To investigate the role of sif in different cell types, we tested climbing behaviors and seizure-like behaviors using sif RNAi lines driven by tissue-specific GAL4s. Two RNAi lines were used, sif RNAi-1 and sif RNAi-2 (Table S1). When driven by ubiquitous daughterless-GAL4 (da-GAL4), the sif mRNA levels decreased by ∼88% and ∼76%, respectively (Figure 5A). Interestingly, da-GAL4-driven UAS- (da >) sif RNAi-1 cause semi-lethality with a severe drop in survival rate to ∼40% when compared to control flies (da > luciferase RNAi), whereas da > sif RNAi-2 flies are fully viable (Figure 5B). The da > sif RNAi-1 and sif RNAi-2 flies show climbing defects and seizure-like behaviors (Figures 5C–5E and Video S2). da > sif RNAi-1 flies are more severely affected than da > sif RNAi-2, again highlighting a critical role for sif dosage. Since sif is highly enriched in neurons rather than glia in the CNS, we next used neuronal-specific elav-GAL4 to knockdown sif in neurons.54 Neuronal sif knockdowns show phenotypes similar to ubiquitous knockdowns (Figures 5F–5H and Video S3). We also tested glial knockdown of sif using repo-GAL4 as controls: these flies do not show climbing defects (Figure 5I). Together, these data show that the neurological phenotypes, including climbing defects and seizure-like behaviors, of sif LoF mutants are mainly caused by neuronal loss of sif.
Figure 5.
Neurological phenotypes of sif LoF mutants are mainly caused by neuronal loss of sif
(A) Real-time PCR analysis of sif mRNA levels in ubiquitous sif knockdown flies. Each sample contains 3–5 L3 larvae. sif-RNAi-1 causes a higher knockdown efficiency than sif-RNAi-2.
(B) Survival rates of ubiquitous expression of sif RNAi at 25°C. sif knockdown with RNAi-1 causes semi-lethality, while RNAi-2 knockdown does not affect viability, indicating a strong dose dependency.
(C) Ubiquitous sif knockdown causes climbing defects in a dose-dependent manner. Flies were tested at day 3.
(D and E) Ubiquitous sif knockdown causes heat-induced seizure-like behaviors in a dose-dependent manner. The percentage of flies that are unable to keep an upright position in the sif RNAi-1 group is significantly higher than controls (D), and the majority of flies are not able to recover 20 s after heat shock (E). Similar but milder phenotypes were seen in the sif RNAi-2 group. Flies were tested at day 3.
(F) Neuronal sif knockdown causes climbing defects. Flies were allowed to climb for 5 s.
(G and H) Neuronal sif knockdown causes heat-induced seizures. The percentage of flies that are unable to keep an upright position in the sif RNAi-1 group is significantly higher than controls (G), and the majority of flies in the sif RNAi-1 group did not recover in 20 s after heat shock (H). Flies were tested at day 5.
(I) Glial sif knockdown does not cause climbing defects. Flies were tested at day 15.
All the animals were raised at 25°C unless otherwise mentioned. Data are represented as mean ± SEM. Unpaired t tests. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗∗p < 0.0001; n.s., no significance.
Human TIAM1 Ref partially rescues the semi-lethality caused by sif loss, whereas the variants cause a variable rescue
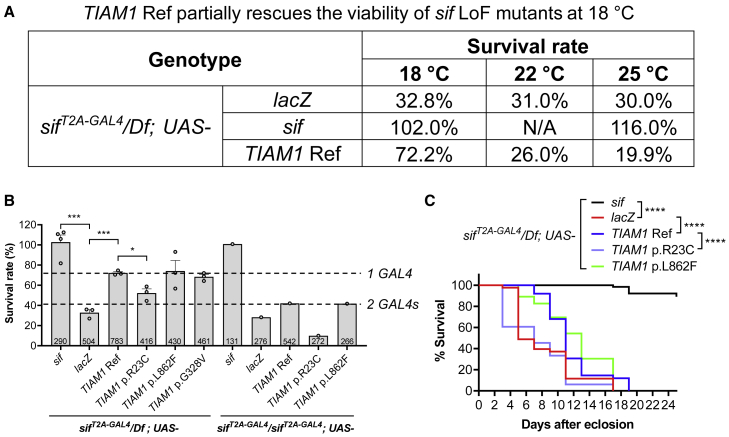
To test whether human TIAM1 can functionally replace the loss of sif, we first examined whether expression of the TIAM1 Ref cDNA rescues the phenotypes of sif LoF mutants. We generated UAS-TIAM1 cDNA transgenic fly lines expressing human TIAM1 Ref, crossed the UAS-TIAM1 cDNA into the sifT2A-GAL4/Df background, and assessed survival rates. At 18°C, the TIAM1 Ref can partially rescue the survival rate of sifT2A-GAL4/Df, from 32.8% to 72.2% (Figure 6A). Since the GAL4 activity increases with temperature,26,55 we also tested the rescue ability of TIAM1 Ref at higher temperatures. However, the rescue ability of TIAM1 Ref decreased to 26.0% at 22°C and 19.9% at 25°C, showing that overexpression of TIAM1 is toxic (Figure 6A). Consistent with this interpretation, when we doubled the copy number of GAL4 at 18°C (sifT2A-GAL4/sifT2A-GAL4), the rescue ability of TIAM1 Ref decreased from 72.2% to 42.1% (Figure 6B). These data indicate that TIAM1 partially replaces the function of sif, but higher expression levels of TIAM1 are toxic. Hence, the toxicity of TIAM1 cDNA overexpression may limit its rescue ability. Furthermore, the TIAM1 Ref and variants cannot rescue the neurological phenotypes associated with the sif LoF mutants (Figures S3A and S3B).
Figure 6.
sif LoF rescue studies show that TIAM1 Ref has partial rescue ability, but the variants have variable rescue abilities
(A) The rescue ability of UAS-TIAM1 Ref decreases with increased temperature. UAS-lacZ was used as a negative control that doesn’t alter the survival rate. Fly UAS-sif was used as a positive control, and it completely rescues the survival rate of sif LoF mutants.
(B) The semi-lethal phenotype of sifT2A-GAL4 mutants can be fully rescued by expression of fly sif cDNA and partially rescued by TIAM1 Ref cDNA. The TIAM1 variant p.Arg23Cys shows reduced rescue ability, while p.Leu862Phe and p.Gly328Val show similar rescue abilities when compared to TIAM1 Ref. Similar but lower rescue abilities were seen in sifT2A-GAL4 homozygous background, suggesting two GAL4 copies cause higher TIAM1-induced toxicity. Flies were raised at 18°C.
(C) sif LoF mutants have significantly shorter lifespans, which can be rescued by UAS-sif and partially rescued by UAS-TIAM1 Ref, while p.Arg23Cys shows reduced rescue ability. Flies were raised and kept at 18°C.
Data are represented as mean ± SEM. Unpaired t tests (B) and log-rank (Mantel-Cox) and Gehan-Breslow-Wilcoxon tests (C). ∗p < 0.05; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001; n.s., no significance.
To investigate the rescue ability of the TIAM1 variants observed in the probands, we generated UAS-TIAM1 cDNA transgenic fly lines expressing human TIAM1 variants in the sifT2A-GAL4/Df background. Since we did not obtain the variant information of proband 3 and 4 until late in the project, we only tested the three variants carried by probands 1 and 2. The TIAM1 p.Arg23Cys variant shows significantly reduced survival rates when compared to TIAM1 Ref (Figure 6B), suggesting that this is a partial LoF variant. The TIAM1 p.Leu862Phe and p.Gly328Val show survival rates comparable to those of the TIAM1 Ref, suggesting that they may be functional proteins. Similar rescue abilities were observed in the lifespan rescue assay (Figure 6C). However, since TIAM1 overexpression is associated with toxicity, it is likely that there are two sources that reduce the viability in the rescue experiments: sif LoF and TIAM1 cDNA overexpression. This affects the interpretation of the data. Since TIAM1 Ref and variants may have different levels of toxicity upon overexpression, the rescue ability of the TIAM1 cDNAs in sif LoF mutants may not be interpreted simply by viability. We therefore explored the function of TIAM1 variants in a variety of overexpression assays in a WT background rather than in a LoF background (see below).
Ectopic expression of WT fly sif or human TIAM1 Ref is toxic, and TIAM1 variants are less toxic
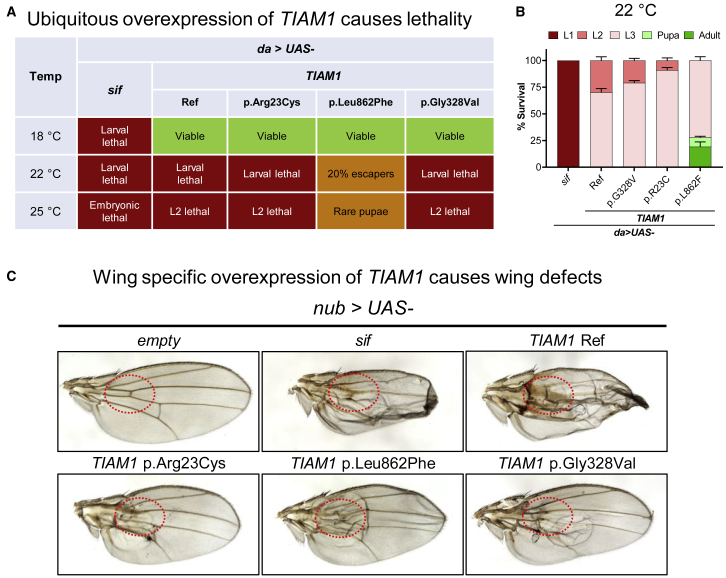
Besides the LoF study, ectopic expression is also a useful tool to identify the function of a certain gene, since ectopic overexpression can sometimes induce phenotypes.56,57 To further investigate the functional consequences of the TIAM1 variants, we overexpressed UAS-TIAM1 in flies using various GAL4 drivers. We also used the UAS-sif transgenic flies for comparison.19 Interestingly, ubiquitous overexpression of sif or TIAM1 using da-GAL4 causes lethality at 22°C and 25°C (Figure 7A, quantified in Figure 7B). Further, wing-specific overexpression of sif or TIAM1 using nub-GAL4 also causes a vein loss as well as blistery wings (Figure 7C, quantified in Figures S4A–S4C). These data indicate that sif and TIAM1 are similarly toxic when overexpressed. We next tested whether the TIAM1 variants alter the observed toxicity. We conducted these overexpression assays at different temperatures as this allows us to assess dose-dependent toxicity effects. At 18°C, the da > TIAM1 Ref flies are viable, and there are no significant viability differences between TIAM1 Ref and variants, suggesting that low expression levels of reference and variants are not or mildly toxic (Figure 7A). At 22°C, da > TIAM1 Ref flies are L2 and L3 lethal. However, the surviving adult flies are observed with p.Leu862Phe, and a larger portion of animals survive into L3 stage carrying the p.Arg23Cys and p.Gly328Val variants than the Ref (Figure 7A, quantified in Figure 7B). At 25°C, da > TIAM1 Ref flies are L2 lethal, but some pupae are observed in the p.Leu862Phe group (Figure 7A). Based on these experiments, the variants from most severe to least severe with respect to toxicity can be ordered as follows: TIAM1 Ref > p.Gly328Val > p.Arg23Cys > p.Leu862Phe (Figure 7B). These data are also consistent with the wing-specific expression assays as documented in Figure 7C and Figures S4A–S4C. It is worth noting that the survival rate of the wing-specific expression at 29°C corroborates the order of this allelic series as well (Figure S4D). Taken together, these ectopic overexpression studies support the hypothesis that p.Arg23Cys, p.Leu862Phe, and p.Gly328Val are partial LoF variants.
Figure 7.
Ectopic expression of WT fly sif or human TIAM1 Ref is toxic, but human TIAM1 variants are less toxic
(A) Table summarizing the lethality phenotypes of ubiquitous overexpression (da-GAL4) of sif, TIAM1 Ref, and variants.
(B) Quantification of survival stages of ubiquitous overexpression of sif, TIAM1 Ref, and variants at 22°C. Note that the variants are less toxic. Data are represented as mean ± SEM.
(C) Representative pictures showing that wing-specific overexpression (nub-GAL4) of sif and TIAM1 Ref causes similar vein loss and blistery wing phenotypes. The defects are highlighted in red dashed circles. Flies were raised and kept at 25°C.
Discussion
Exome or genome sequencing of probands and parents with rare diseases combined with functional investigations in model organisms has led to the discovery of numerous novel Mendelian diseases.2,4,58 Here, we identify TIAM1 as a disease-associated gene. We present five individuals with compound-heterozygous or homozygous coding variants in TIAM1. The main phenotypes observed in the probands are developmental delays, with severe deficits in speech and language, intellectual disability, and seizures. Using Drosophila, we found that loss of sif, the ortholog of TIAM1, causes semi-lethality, and flies that eclose exhibit climbing defects and seizure-like behaviors and have a short lifespan. The sifT2A-GAL4 allele allowed us to determine the expression pattern of the gene and show that sif is expressed in neurons but not in glia. It also allowed us to “humanize” the fly by replacing fly sif with human TIAM1. We show that TIAM1 Ref can partially rescue the survival rate and lifespan but not the climbing defects and seizure-like phenotypes of sif LoF mutants. The lack of rescue is likely due to the toxicity of the overexpression of TIAM1 Ref in flies. Alternatively, the limited rescue ability is due to functional diversity of the human homologs, as there are four other orthologs of sif in humans besides TIAM1.17
The variant pathogenicity prediction algorithm CADD41,59 indicates that these TIAM1 variants are rare and likely pathogenic. Since the TIAM1 variants carried by the probands reported here are bi-allelic, they are predicted to be LoF variants. To test this hypothesis, we assessed three of the six variants in vivo using two-pronged functional assays based on rescue and ectopic-expression experiments. We show that TIAM1 p.Arg23Cys has reduced rescue ability, suggesting that it is a partial LoF variant. TIAM1 p.Leu862Phe and p.Gly328Val show rescue ability comparable to that of TIAM1 Ref and did not allow us to draw a conclusion based on this assay, since TIAM1 cDNAs have different levels of toxicity that also affect viability. Therefore, we used ectopic-expression experiments in a WT background to further test the function of the variants, given that WT sif and TIAM1 Ref induce similar phenotypes, including wing defects, in ectopic-expression assays. Since TIAM1 Ref and the variants are inserted in the same genomic site, we can perform functional analyses of the variants by comparing ectopic-expression-induced phenotypes. The three proband-associated variants exhibit reduced toxicity when compared to TIAM1 Ref. When the Ref causes a toxic phenotype and the variants are less toxic, the variants can typically be classified as LoF variants.1,33,60, 61, 62, 63, 64 Hence, our ectopic-expression data support the hypothesis that TIAM1 p.Arg23Cys, p.Leu862Phe, and p.Gly328Val are partial LoF variants. Taken together, our data suggest that the TIAM1 variants reported here result in a loss of function and are associated with neurodevelopmental phenotypes and seizures.
The bioinformatic data show that the pLI of TIAM1 is high (0.96), and the observed-to-expected ratio of LoF variants for TIAM1 is 0.20.39 However, there are 46 heterozygous individuals with TIAM1 LoF alleles observed in gnomAD, suggesting that loss of one copy of TIAM1 is tolerated. A possible reason why TIAM1 is associated with a high pLI score is that it is a very large gene (>400 kb). We previously also reported an autosomal-recessive disease associated with bi-allelic LoF variants in OXR1,65 a large gene (>400 kb) for which the observed-to-expected ratio of LoF variants is 0.19 and the pLI is 0.84,10 very similar to TIAM1.
Besides TIAM1, sif is also the ortholog of other GEF-encoding genes, including TIAM2, DNMBP (MIM: 611282), ARHGEF37, and ARHGEF38.17 TIAM2 activates RAC1 and controls cell migration in neurons.66 DNMBP is a GEF for cell division control protein 42 that controls the shaping of cell junctions through binding to tight junction protein ZO-1, and knockdown of DNMBP results in a disorganized configuration of cell junctions.67 LoF variants of DNMBP cause infantile-onset cataracts in humans.22 Similarly, sif knockdown in the eye alters the distribution of septate junctions in adjacent cone cells and affects the function of the eye in young flies.22 Finally, ARHGEF37 and ARHGEF38 are Rho GEFs. ARHGEF37 assists dynamin 2 during clathrin-mediated endocytosis,68 while ARHGEF38 is an uncharacterized protein.
Tiam1 knockout mice have decreased spine density, simplified dendritic arbors, and decreased miniature excitatory postsynaptic currents in the hippocampus, but they exhibit only subtle behavior abnormalities,15 which may be due to redundancy of other GEFs. The related TIAM2 shares 37% overall identity and 71% Dbl homology (DH) domain identity with TIAM1.69, 70, 71 The expression levels of Tiam2 correlate with the stages of neuronal morphological development, and Tiam2 knockdown in neurons also causes reduced neurite outgrowth.72,73 TIAM1 promotes the formation and growth of spines and synapses by activating RAC1 signaling pathways that control the actin cytoskeleton. Dysfunction of the neuronal cytoskeleton has been implicated in a variety of diseases, including neurological developmental disorders as well as neurodegenerative diseases.74,75 Moreover, dysregulation of the neuronal cytoskeletal network also contributes to the pathogenesis of epilepsy.76, 77, 78
Previous studies show that sif LoF mutants are viable and have reduced locomotor activity.18,19 In this study, we generated a more severe LoF allele, sifT2A-GAL4, which leads to semi-lethality, a highly reduced lifespan, and a severe sensitivity to seizure-like behaviors, in addition to the climbing defects. It is worth noting that fly mutants with such severe sensitivity to seizures are rarely observed.60,61,79 We also show that these phenotypes are mainly caused by neuronal sif loss based on RNAi knockdown assays. Interestingly, the sif RNAi-1 with ∼12% of the remaining sif transcripts based on real-time PCR show much stronger phenotypes, including semi-lethality, when compared to sif RNAi-2 with ∼24% remaining sif transcripts, indicating a threshold effect of sif expression between these two values, similar to what has been documented for other genes, like flower.80
Finally, we would like to point out the limitations of our study. The number of individuals described is small and some of the phenotypic features differ. Two of the individuals come from consanguineous families, and additional recessive conditions could be contributing to the phenotype. Additionally, the analysis was based on exome sequencing, and noncoding variants were not assessed and could contribute to variation in the phenotype. With additional identified individuals with bi-allelic TIAM1 variants, it should become more obvious which clinical features are core to the condition.
In summary, we find that bi-allelic pathogenic TIAM1 variants are associated with a neurological disorder in humans. We provide functional analysis in flies that supports an LoF model for TIAM1-associated variants. Further studies of the underlying mechanism will be necessary to provide a better understanding of the pathological mechanisms and may provide therapeutic strategies.
Acknowledgments
We thank the probands and families for agreeing to participate in this study. We thank Dr. Xiao Mao for his effort in bringing us into contact with the clinicians and the family in China. We thank Dr. Sjoerd Verkaart for technical support. We thank former and present Bellen and Yamamoto lab members for their discussion and suggestions in this study, particularly Debdeep Dutta, Scott Barish, Mengqi Ma, and Yiming Zheng. We thank Ms. Hongling Pan for the injection of transgenic fly lines. We thank Dr. Michael Wangler for the guidelines for the IRB protocol. We thank the BDSC for numerous stocks. This work was supported by the Howard Hughes Medical Institute (HHMI), the Huffington Foundation, and the Jan and Dan Duncan Neurological Research Institute at Texas Children’s Hospital to H.J.B. Research reported in this publication was also supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health (NIH) under award number P50HD103555 for use of the neurovisualization core facilities. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Further support came from The Office of Research Infrastructure Programs of the NIH under the award numbers R24 OD022005 and R24 OD031447 to H.J.B. P.C.M. is supported by CIHR (MFE-164712) and the Stand by Eli Foundation. H.C. is supported by Warren Alpert Foundation.
Declaration of interests
M.J.G.S. is a salaried employee of GeneDx Inc.
Published: March 2, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ajhg.2022.01.020.
Contributor Information
Wendy K. Chung, Email: wkc15@cumc.columbia.edu.
Hugo J. Bellen, Email: hbellen@bcm.edu.
Data and code availability
This study did not generate datasets. All reagents developed in this study are available upon request. There are restrictions to the availability of sequencing data of the research participants due to privacy and ethical/legal issues.
Web resources
GeneDx ClinVar submission page, https://www.ncbi.nlm.nih.gov/clinvar/submitters/26957/
GeneMatcher, https://genematcher.org/statistics/
MARRVEL, http://www.marrvel.org/
OMIM, https://www.omim.org/
Supplemental information
References
- 1.Splinter K., Adams D.R., Bacino C.A., Bellen H.J., Bernstein J.A., Cheatle-Jarvela A.M., Eng C.M., Esteves C., Gahl W.A., Hamid R., et al. Undiagnosed Diseases Network Effect of Genetic Diagnosis on Patients with Previously Undiagnosed Disease. N. Engl. J. Med. 2018;379:2131–2139. doi: 10.1056/NEJMoa1714458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wangler M.F., Yamamoto S., Chao H.T., Posey J.E., Westerfield M., Postlethwait J., Hieter P., Boycott K.M., Campeau P.M., Bellen H.J., Members of the Undiagnosed Diseases Network (UDN) Model Organisms Facilitate Rare Disease Diagnosis and Therapeutic Research. Genetics. 2017;207:9–27. doi: 10.1534/genetics.117.203067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coventry A., Bull-Otterson L.M., Liu X., Clark A.G., Maxwell T.J., Crosby J., Hixson J.E., Rea T.J., Muzny D.M., Lewis L.R., et al. Deep resequencing reveals excess rare recent variants consistent with explosive population growth. Nat. Commun. 2010;1:131. doi: 10.1038/ncomms1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellen H.J., Wangler M.F., Yamamoto S. The fruit fly at the interface of diagnosis and pathogenic mechanisms of rare and common human diseases. Hum. Mol. Genet. 2019;28(R2):R207–R214. doi: 10.1093/hmg/ddz135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lambert J.M., Lambert Q.T., Reuther G.W., Malliri A., Siderovski D.P., Sondek J., Collard J.G., Der C.J. Tiam1 mediates Ras activation of Rac by a PI(3)K-independent mechanism. Nat. Cell Biol. 2002;4:621–625. doi: 10.1038/ncb833. [DOI] [PubMed] [Google Scholar]
- 6.Leeuwen F.N., Kain H.E., Kammen R.A., Michiels F., Kranenburg O.W., Collard J.G. The guanine nucleotide exchange factor Tiam1 affects neuronal morphology; opposing roles for the small GTPases Rac and Rho. J. Cell Biol. 1997;139:797–807. doi: 10.1083/jcb.139.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tolias K.F., Bikoff J.B., Burette A., Paradis S., Harrar D., Tavazoie S., Weinberg R.J., Greenberg M.E. The Rac1-GEF Tiam1 couples the NMDA receptor to the activity-dependent development of dendritic arbors and spines. Neuron. 2005;45:525–538. doi: 10.1016/j.neuron.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 8.Bos J.L., Rehmann H., Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129:865–877. doi: 10.1016/j.cell.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 9.Tolias K.F., Bikoff J.B., Kane C.G., Tolias C.S., Hu L., Greenberg M.E. The Rac1 guanine nucleotide exchange factor Tiam1 mediates EphB receptor-dependent dendritic spine development. Proc. Natl. Acad. Sci. USA. 2007;104:7265–7270. doi: 10.1073/pnas.0702044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J., Al-Ouran R., Hu Y., Kim S.Y., Wan Y.W., Wangler M.F., Yamamoto S., Chao H.T., Comjean A., Mohr S.E., et al. UDN MARRVEL: Integration of Human and Model Organism Genetic Resources to Facilitate Functional Annotation of the Human Genome. Am. J. Hum. Genet. 2017;100:843–853. doi: 10.1016/j.ajhg.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Consortium G.T., GTEx Consortium Human genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science. 2015;348:648–660. doi: 10.1126/science.1262110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyamoto Y., Yamauchi J., Tanoue A., Wu C., Mobley W.C. TrkB binds and tyrosine-phosphorylates Tiam1, leading to activation of Rac1 and induction of changes in cellular morphology. Proc. Natl. Acad. Sci. USA. 2006;103:10444–10449. doi: 10.1073/pnas.0603914103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shirazi Fard S., Kele J., Vilar M., Paratcha G., Ledda F. Tiam1 as a signaling mediator of nerve growth factor-dependent neurite outgrowth. PLoS ONE. 2010;5:e9647. doi: 10.1371/journal.pone.0009647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang H., Macara I.G. The polarity protein PAR-3 and TIAM1 cooperate in dendritic spine morphogenesis. Nat. Cell Biol. 2006;8:227–237. doi: 10.1038/ncb1368. [DOI] [PubMed] [Google Scholar]
- 15.Cheng J., Scala F., Blanco F.A., Niu S., Firozi K., Keehan L., Mulherkar S., Froudarakis E., Li L., Duman J.G., et al. The Rac-GEF Tiam1 Promotes Dendrite and Synapse Stabilization of Dentate Granule Cells and Restricts Hippocampal-Dependent Memory Functions. J. Neurosci. 2021;41:1191–1206. doi: 10.1523/JNEUROSCI.3271-17.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amberger J.S., Bocchini C.A., Schiettecatte F., Scott A.F., Hamosh A. OMIM.org: Online Mendelian Inheritance in Man (OMIM®), an online catalog of human genes and genetic disorders. Nucleic Acids Res. 2015;43:D789–D798. doi: 10.1093/nar/gku1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu Y., Flockhart I., Vinayagam A., Bergwitz C., Berger B., Perrimon N., Mohr S.E. An integrative approach to ortholog prediction for disease-focused and other functional studies. BMC Bioinformatics. 2011;12:357. doi: 10.1186/1471-2105-12-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sone M., Hoshino M., Suzuki E., Kuroda S., Kaibuchi K., Nakagoshi H., Saigo K., Nabeshima Y., Hama C. Still life, a protein in synaptic terminals of Drosophila homologous to GDP-GTP exchangers. Science. 1997;275:543–547. doi: 10.1126/science.275.5299.543. [DOI] [PubMed] [Google Scholar]
- 19.Sone M., Suzuki E., Hoshino M., Hou D., Kuromi H., Fukata M., Kuroda S., Kaibuchi K., Nabeshima Y., Hama C. Synaptic development is controlled in the periactive zones of Drosophila synapses. Development. 2000;127:4157–4168. doi: 10.1242/dev.127.19.4157. [DOI] [PubMed] [Google Scholar]
- 20.Schuster C.M., Davis G.W., Fetter R.D., Goodman C.S. Genetic dissection of structural and functional components of synaptic plasticity. II. Fasciclin II controls presynaptic structural plasticity. Neuron. 1996;17:655–667. doi: 10.1016/s0896-6273(00)80198-1. [DOI] [PubMed] [Google Scholar]
- 21.Schuster C.M., Davis G.W., Fetter R.D., Goodman C.S. Genetic dissection of structural and functional components of synaptic plasticity. I. Fasciclin II controls synaptic stabilization and growth. Neuron. 1996;17:641–654. doi: 10.1016/s0896-6273(00)80197-x. [DOI] [PubMed] [Google Scholar]
- 22.Ansar M., Chung H.L., Taylor R.L., Nazir A., Imtiaz S., Sarwar M.T., Manousopoulou A., Makrythanasis P., Saeed S., Falconnet E., et al. Bi-allelic Loss-of-Function Variants in DNMBP Cause Infantile Cataracts. Am. J. Hum. Genet. 2018;103:568–578. doi: 10.1016/j.ajhg.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Retterer K., Juusola J., Cho M.T., Vitazka P., Millan F., Gibellini F., Vertino-Bell A., Smaoui N., Neidich J., Monaghan K.G., et al. Clinical application of whole-exome sequencing across clinical indications. Genet. Med. 2016;18:696–704. doi: 10.1038/gim.2015.148. [DOI] [PubMed] [Google Scholar]
- 24.Venken K.J., Schulze K.L., Haelterman N.A., Pan H., He Y., Evans-Holm M., Carlson J.W., Levis R.W., Spradling A.C., Hoskins R.A., Bellen H.J. MiMIC: a highly versatile transposon insertion resource for engineering Drosophila melanogaster genes. Nat. Methods. 2011;8:737–743. doi: 10.1038/nmeth.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diao F., Ironfield H., Luan H., Diao F., Shropshire W.C., Ewer J., Marr E., Potter C.J., Landgraf M., White B.H. Plug-and-play genetic access to drosophila cell types using exchangeable exon cassettes. Cell Rep. 2015;10:1410–1421. doi: 10.1016/j.celrep.2015.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagarkar-Jaiswal S., Lee P.T., Campbell M.E., Chen K., Anguiano-Zarate S., Gutierrez M.C., Busby T., Lin W.W., He Y., Schulze K.L., et al. A library of MiMICs allows tagging of genes and reversible, spatial and temporal knockdown of proteins in Drosophila. eLife. 2015;4 doi: 10.7554/eLife.05338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao C.K., Lin Y.Q., Ly C.V., Ohyama T., Haueter C.M., Moiseenkova-Bell V.Y., Wensel T.G., Bellen H.J. A synaptic vesicle-associated Ca2+ channel promotes endocytosis and couples exocytosis to endocytosis. Cell. 2009;138:947–960. doi: 10.1016/j.cell.2009.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goodman L.D., Cope H., Nil Z., Ravenscroft T.A., Charng W.L., Lu S., Tien A.C., Pfundt R., Koolen D.A., Haaxma C.A., et al. Undiagnosed Diseases Network TNPO2 variants associate with human developmental delays, neurologic deficits, and dysmorphic features and alter TNPO2 activity in Drosophila. Am. J. Hum. Genet. 2021;108:1669–1691. doi: 10.1016/j.ajhg.2021.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madabattula S.T., Strautman J.C., Bysice A.M., O’Sullivan J.A., Androschuk A., Rosenfelt C., Doucet K., Rouleau G., Bolduc F. Quantitative Analysis of Climbing Defects in a Drosophila Model of Neurodegenerative Disorders. J. Vis. Exp. 2015;52741:e52741. doi: 10.3791/52741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hatfield I., Harvey I., Yates E.R., Redd J.R., Reiter L.T., Bridges D. The role of TORC1 in muscle development in Drosophila. Sci. Rep. 2015;5:9676. doi: 10.1038/srep09676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ganetzky B., Wu C.F. Indirect Suppression Involving Behavioral Mutants with Altered Nerve Excitability in DROSOPHILA MELANOGASTER. Genetics. 1982;100:597–614. doi: 10.1093/genetics/100.4.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burg M.G., Wu C.F. Mechanical and temperature stressor-induced seizure-and-paralysis behaviors in Drosophila bang-sensitive mutants. J. Neurogenet. 2012;26:189–197. doi: 10.3109/01677063.2012.690011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ansar M., Chung H.L., Al-Otaibi A., Elagabani M.N., Ravenscroft T.A., Paracha S.A., Scholz R., Abdel Magid T., Sarwar M.T., Shah S.F., et al. Bi-allelic Variants in IQSEC1 Cause Intellectual Disability, Developmental Delay, and Short Stature. Am. J. Hum. Genet. 2019;105:907–920. doi: 10.1016/j.ajhg.2019.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harnish J.M., Deal S.L., Chao H.T., Wangler M.F., Yamamoto S. In Vivo Functional Study of Disease-associated Rare Human Variants Using Drosophila. J. Vis. Exp. 2019;(150) doi: 10.3791/59658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bischof J., Björklund M., Furger E., Schertel C., Taipale J., Basler K. A versatile platform for creating a comprehensive UAS-ORFeome library in Drosophila. Development. 2013;140:2434–2442. doi: 10.1242/dev.088757. [DOI] [PubMed] [Google Scholar]
- 36.Venken K.J., He Y., Hoskins R.A., Bellen H.J. P[acman]: a BAC transgenic platform for targeted insertion of large DNA fragments in D. melanogaster. Science. 2006;314:1747–1751. doi: 10.1126/science.1134426. [DOI] [PubMed] [Google Scholar]
- 37.Dutta D., Briere L.C., Kanca O., Marcogliese P.C., Walker M.A., High F.A., Vanderver A., Krier J., Carmichael N., Callahan C., et al. De novo mutations in TOMM70, a receptor of the mitochondrial import translocase, cause neurological impairment. Hum. Mol. Genet. 2020;29:1568–1579. doi: 10.1093/hmg/ddaa081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sobreira N., Schiettecatte F., Valle D., Hamosh A. GeneMatcher: a matching tool for connecting investigators with an interest in the same gene. Hum. Mutat. 2015;36:928–930. doi: 10.1002/humu.22844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karczewski K.J., Francioli L.C., Tiao G., Cummings B.B., Alföldi J., Wang Q., Collins R.L., Laricchia K.M., Ganna A., Birnbaum D.P., et al. Genome Aggregation Database Consortium The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581:434–443. doi: 10.1038/s41586-020-2308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rentzsch P., Schubach M., Shendure J., Kircher M. CADD-Splice-improving genome-wide variant effect prediction using deep learning-derived splice scores. Genome Med. 2021;13:31. doi: 10.1186/s13073-021-00835-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kircher M., Witten D.M., Jain P., O’Roak B.J., Cooper G.M., Shendure J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat. Genet. 2014;46:310–315. doi: 10.1038/ng.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nagarkar-Jaiswal S., DeLuca S.Z., Lee P.T., Lin W.W., Pan H., Zuo Z., Lv J., Spradling A.C., Bellen H.J. A genetic toolkit for tagging intronic MiMIC containing genes. eLife. 2015;4 doi: 10.7554/eLife.08469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee P.T., Zirin J., Kanca O., Lin W.W., Schulze K.L., Li-Kroeger D., Tao R., Devereaux C., Hu Y., Chung V., et al. A gene-specific T2A-GAL4 library for Drosophila. eLife. 2018;7:e35574. doi: 10.7554/eLife.35574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Diao F., White B.H. A novel approach for directing transgene expression in Drosophila: T2A-Gal4 in-frame fusion. Genetics. 2012;190:1139–1144. doi: 10.1534/genetics.111.136291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chung H.L., Wangler M.F., Marcogliese P.C., Jo J., Ravenscroft T.A., Zuo Z., Duraine L., Sadeghzadeh S., Li-Kroeger D., Schmidt R.E., et al. Members of Undiagnosed Diseases Network Loss- or Gain-of-Function Mutations in ACOX1 Cause Axonal Loss via Different Mechanisms. Neuron. 2020;106:589–606.e6. doi: 10.1016/j.neuron.2020.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marcogliese P.C., Deal S.L., Andrews J., Harnish J.M., Bhavana V.H., Graves H.K., Jangam S., Luo X., Liu N., Bei D., et al. Drosophila functional screening of de novo variants in autism uncovers deleterious variants and facilitates discovery of rare neurodevelopmental diseases. Preprint at bioRxiv. 2021 doi: 10.1101/2020.12.30.424813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davie K., Janssens J., Koldere D., De Waegeneer M., Pech U., Kreft Ł., Aibar S., Makhzami S., Christiaens V., Bravo González-Blas C., et al. A Single-Cell Transcriptome Atlas of the Aging Drosophila Brain. Cell. 2018;174:982–998.e20. doi: 10.1016/j.cell.2018.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li H., Janssens J., De Waegeneer M., Kolluru S.S., Davie K., Gardeux V., Saelens W., David F., Brbić M., Leskovec J., et al. Fly Cell Atlas: a single-cell transcriptomic atlas of the adult fruit fly. Preprint at bioRxiv. 2021 doi: 10.1101/2021.07.04.451050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parker L., Howlett I.C., Rusan Z.M., Tanouye M.A. Seizure and epilepsy: studies of seizure disorders in Drosophila. Int. Rev. Neurobiol. 2011;99:1–21. doi: 10.1016/B978-0-12-387003-2.00001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dare S.S., Merlo E., Rodriguez Curt J., Ekanem P.E., Hu N., Berni J. Drosophila parabss Flies as a Screening Model for Traditional Medicine: Anticonvulsant Effects of Annona senegalensis. Front. Neurol. 2021;11:606919. doi: 10.3389/fneur.2020.606919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parker L., Padilla M., Du Y., Dong K., Tanouye M.A. Drosophila as a model for epilepsy: bss is a gain-of-function mutation in the para sodium channel gene that leads to seizures. Genetics. 2011;187:523–534. doi: 10.1534/genetics.110.123299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saras A., Wu V.V., Brawer H.J., Tanouye M.A. Investigation of Seizure-Susceptibility in a Drosophila melanogaster Model of Human Epilepsy with Optogenetic Stimulation. Genetics. 2017;206:1739–1746. doi: 10.1534/genetics.116.194779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun L., Gilligan J., Staber C., Schutte R.J., Nguyen V., O’Dowd D.K., Reenan R. A knock-in model of human epilepsy in Drosophila reveals a novel cellular mechanism associated with heat-induced seizure. J. Neurosci. 2012;32:14145–14155. doi: 10.1523/JNEUROSCI.2932-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu L., Zhang K., Sandoval H., Yamamoto S., Jaiswal M., Sanz E., Li Z., Hui J., Graham B.H., Quintana A., Bellen H.J. Glial lipid droplets and ROS induced by mitochondrial defects promote neurodegeneration. Cell. 2015;160:177–190. doi: 10.1016/j.cell.2014.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Duffy J.B. GAL4 system in Drosophila: a fly geneticist’s Swiss army knife. Genesis. 2002;34:1–15. doi: 10.1002/gene.10150. [DOI] [PubMed] [Google Scholar]
- 56.Brand A.H., Manoukian A.S., Perrimon N. Ectopic expression in Drosophila. Methods Cell Biol. 1994;44:635–654. doi: 10.1016/s0091-679x(08)60936-x. [DOI] [PubMed] [Google Scholar]
- 57.Wilder E.L. Ectopic expression in Drosophila. Methods Mol. Biol. 2000;137:9–14. doi: 10.1385/1-59259-066-7:9. [DOI] [PubMed] [Google Scholar]
- 58.Baldridge D., Wangler M.F., Bowman A.N., Yamamoto S., Schedl T., Pak S.C., Postlethwait J.H., Shin J., Solnica-Krezel L., Bellen H.J., Westerfield M., Undiagnosed Diseases Network Model organisms contribute to diagnosis and discovery in the undiagnosed diseases network: current state and a future vision. Orphanet J. Rare Dis. 2021;16:206. doi: 10.1186/s13023-021-01839-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rentzsch P., Witten D., Cooper G.M., Shendure J., Kircher M. CADD: predicting the deleteriousness of variants throughout the human genome. Nucleic Acids Res. 2019;47(D1):D886–D894. doi: 10.1093/nar/gky1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kanca O., Andrews J.C., Lee P.T., Patel C., Braddock S.R., Slavotinek A.M., Cohen J.S., Gubbels C.S., Aldinger K.A., Williams J., et al. Undiagnosed Diseases Network De Novo Variants in WDR37 Are Associated with Epilepsy, Colobomas, Dysmorphism, Developmental Delay, Intellectual Disability, and Cerebellar Hypoplasia. Am. J. Hum. Genet. 2019;105:413–424. doi: 10.1016/j.ajhg.2019.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marcogliese P.C., Shashi V., Spillmann R.C., Stong N., Rosenfeld J.A., Koenig M.K., Martínez-Agosto J.A., Herzog M., Chen A.H., Dickson P.I., et al. Program for Undiagnosed Diseases (UD-PrOZA) Undiagnosed Diseases Network IRF2BPL Is Associated with Neurological Phenotypes. Am. J. Hum. Genet. 2018;103:245–260. doi: 10.1016/j.ajhg.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu N., Schoch K., Luo X., Pena L.D.M., Bhavana V.H., Kukolich M.K., Stringer S., Powis Z., Radtke K., Mroske C., et al. Undiagnosed Diseases Network (UDN) Functional variants in TBX2 are associated with a syndromic cardiovascular and skeletal developmental disorder. Hum. Mol. Genet. 2018;27:2454–2465. doi: 10.1093/hmg/ddy146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ravenscroft T.A., Phillips J.B., Fieg E., Bajikar S.S., Peirce J., Wegner J., Luna A.A., Fox E.J., Yan Y.L., Rosenfeld J.A., et al. Undiagnosed Diseases Network Heterozygous loss-of-function variants significantly expand the phenotypes associated with loss of GDF11. Genet. Med. 2021;23:1889–1900. doi: 10.1038/s41436-021-01216-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Uehara T., Sanuki R., Ogura Y., Yokoyama A., Yoshida T., Futagawa H., Yoshihashi H., Yamada M., Suzuki H., Takenouchi T., et al. Recurrent NFIA K125E substitution represents a loss-of-function allele: Sensitive in vitro and in vivo assays for nontruncating alleles. Am. J. Med. Genet. A. 2021;185:2084–2093. doi: 10.1002/ajmg.a.62226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang J., Rousseau J., Kim E., Ehresmann S., Cheng Y.T., Duraine L., Zuo Z., Park Y.J., Li-Kroeger D., Bi W., et al. Loss of Oxidation Resistance 1, OXR1, Is Associated with an Autosomal-Recessive Neurological Disease with Cerebellar Atrophy and Lysosomal Dysfunction. Am. J. Hum. Genet. 2019;105:1237–1253. doi: 10.1016/j.ajhg.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rooney C., White G., Nazgiewicz A., Woodcock S.A., Anderson K.I., Ballestrem C., Malliri A. The Rac activator STEF (Tiam2) regulates cell migration by microtubule-mediated focal adhesion disassembly. EMBO Rep. 2010;11:292–298. doi: 10.1038/embor.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Otani T., Ichii T., Aono S., Takeichi M. Cdc42 GEF Tuba regulates the junctional configuration of simple epithelial cells. J. Cell Biol. 2006;175:135–146. doi: 10.1083/jcb.200605012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Viplav A., Saha T., Huertas J., Selenschik P., Ebrahimkutty M.P., Grill D., Lehrich J., Hentschel A., Biasizzo M., Mengoni S., et al. ArhGEF37 assists dynamin 2 during clathrin-mediated endocytosis. J. Cell Sci. 2019;132:jcs226530. doi: 10.1242/jcs.226530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hoshino M., Sone M., Fukata M., Kuroda S., Kaibuchi K., Nabeshima Y., Hama C. Identification of the stef gene that encodes a novel guanine nucleotide exchange factor specific for Rac1. J. Biol. Chem. 1999;274:17837–17844. doi: 10.1074/jbc.274.25.17837. [DOI] [PubMed] [Google Scholar]
- 70.Chiu C.Y., Leng S., Martin K.A., Kim E., Gorman S., Duhl D.M. Cloning and characterization of T-cell lymphoma invasion and metastasis 2 (TIAM2), a novel guanine nucleotide exchange factor related to TIAM1. Genomics. 1999;61:66–73. doi: 10.1006/geno.1999.5936. [DOI] [PubMed] [Google Scholar]
- 71.Cook D.R., Rossman K.L., Der C.J. Rho guanine nucleotide exchange factors: regulators of Rho GTPase activity in development and disease. Oncogene. 2014;33:4021–4035. doi: 10.1038/onc.2013.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yoshizawa M., Hoshino M., Sone M., Nabeshima Y. Expression of stef, an activator of Rac1, correlates with the stages of neuronal morphological development in the mouse brain. Mech. Dev. 2002;113:65–68. doi: 10.1016/s0925-4773(01)00650-5. [DOI] [PubMed] [Google Scholar]
- 73.Goto A., Hoshino M., Matsuda M., Nakamura T. Phosphorylation of STEF/Tiam2 by protein kinase A is critical for Rac1 activation and neurite outgrowth in dibutyryl cAMP-treated PC12D cells. Mol. Biol. Cell. 2011;22:1780–1790. doi: 10.1091/mbc.E10-09-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Suchowerska A.K., Fath T. Cytoskeletal changes in diseases of the nervous system. Front. Biol. 2014;9:5–17. doi: 10.1007/s11515-014-1290-6. [DOI] [Google Scholar]
- 75.Spence E.F., Soderling S.H. Actin Out: Regulation of the Synaptic Cytoskeleton. J. Biol. Chem. 2015;290:28613–28622. doi: 10.1074/jbc.R115.655118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gambino G., Rizzo V., Giglia G., Ferraro G., Sardo P. Microtubule Dynamics and Neuronal Excitability: Advances on Cytoskeletal Components Implicated in Epileptic Phenomena. Cell. Mol. Neurobiol. 2020 doi: 10.1007/s10571-020-00963-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gardiner J., Marc J. Disruption of normal cytoskeletal dynamics may play a key role in the pathogenesis of epilepsy. Neuroscientist. 2010;16:28–39. doi: 10.1177/1073858409334422. [DOI] [PubMed] [Google Scholar]
- 78.Gavrilovici C., Jiang Y., Kiroski I., Teskey G.C., Rho J.M., Nguyen M.D. Postnatal Role of the Cytoskeleton in Adult Epileptogenesis. Cereb. Cortex Commun. 2020;1:tgaa024. doi: 10.1093/texcom/tgaa024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lin G., Lee P.T., Chen K., Mao D., Tan K.L., Zuo Z., Lin W.W., Wang L., Bellen H.J. Phospholipase PLA2G6, a Parkinsonism-Associated Gene, Affects Vps26 and Vps35, Retromer Function, and Ceramide Levels, Similar to α-Synuclein Gain. Cell Metab. 2018;28:605–618.e6. doi: 10.1016/j.cmet.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 80.Yao C.K., Liu Y.T., Lee I.C., Wang Y.T., Wu P.Y. A Ca2+ channel differentially regulates Clathrin-mediated and activity-dependent bulk endocytosis. PLoS Biol. 2017;15:e2000931. doi: 10.1371/journal.pbio.2000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate datasets. All reagents developed in this study are available upon request. There are restrictions to the availability of sequencing data of the research participants due to privacy and ethical/legal issues.