Abstract
X-linked acrogigantism (X-LAG) is the most severe form of pituitary gigantism and is characterized by aggressive growth hormone (GH)-secreting pituitary tumors that occur in early childhood. X-LAG is associated with chromosome Xq26.3 duplications (the X-LAG locus typically includes VGLL1, CD40LG, ARHGEF6, RBMX, and GPR101) that lead to massive pituitary tumoral expression of GPR101, a novel regulator of GH secretion. The mechanism by which the duplications lead to marked pituitary misexpression of GPR101 alone was previously unclear. Using Hi-C and 4C-seq, we characterized the normal chromatin structure at the X-LAG locus. We showed that GPR101 is located within a topologically associating domain (TAD) delineated by a tissue-invariant border that separates it from centromeric genes and regulatory sequences. Next, using 4C-seq with GPR101, RBMX, and VGLL1 viewpoints, we showed that the duplications in multiple X-LAG-affected individuals led to ectopic interactions that crossed the invariant TAD border, indicating the existence of a similar and consistent mechanism of neo-TAD formation in X-LAG. We then identified several pituitary active cis-regulatory elements (CREs) within the neo-TAD and demonstrated in vitro that one of them significantly enhanced reporter gene expression. At the same time, we showed that the GPR101 promoter permits the incorporation of new regulatory information. Our results indicate that X-LAG is a TADopathy of the endocrine system in which Xq26.3 duplications disrupt the local chromatin architecture forming a neo-TAD. Rewiring GPR101-enhancer interaction within the new regulatory unit is likely to cause the high levels of aberrant expression of GPR101 in pituitary tumors caused by X-LAG.
Keywords: GPR101, TAD, X-LAG, enhancers, pituitary, gigantism, growth, tumor
Introduction
The correct expression of genes in time and space is mediated predominantly by noncoding cis-regulatory elements (CREs), known as promoters and enhancers, and by trans-acting factors, such as transcription factors (TFs). Promoters are located near the transcription start site (TSS) of a gene, while enhancers can be located >1 Mb away.1 Enhancers increase gene expression by physically interacting with their target promoters via looping of DNA, an interaction that is mediated by tissue-specific TFs. The specificity of enhancer-promoter interactions is achieved, in part, by compartmentalization of the genome into discrete regulatory units termed topologically associating domains (TADs).2, 3, 4 TADs are typically megabase-sized chromatin domains with high levels of internal interaction that are separated from each other by regions of low interaction (TAD borders). TAD borders are enriched for the DNA-binding factor CTCF that acts together with other architectural proteins to establish chromatin interactions and TADs in the human genome.5, 6, 7 Consistent with this proposed role in genome organization, TAD border positions are generally stable across cell types and species.2,8,9 TADs function as a dynamic scaffold for enhancer-promoter DNA loops, guiding them spatially and facilitating interactions. Disruption of normal TAD organization by genomic rearrangements such as deletions or duplications can expose promoters to external ectopic enhancers (a mechanism called enhancer adoption or hijacking) and can lead to the formation of a new TAD (neo-TAD).10,11,12 Indeed, experimental introduction of TAD border elements outside of their normal genomic context can induce chromatin reorganization and TAD disruption.13 This rewiring of enhancer-promoter interactions has been implicated as a novel pathogenic mechanism, although few disease phenotypes (“TADopathies”) have been described to date.10, 14, 15
X-linked acrogigantism (X-LAG [MIM: 300942]) is a rare disease that leads to large, treatment-resistant growth hormone (GH)-secreting pituitary tumors in neonates and toddlers.16, 17, 18 Persons with X-LAG have tandem, non-recurrent, constitutive (in females) or somatic mosaic (in males) microduplications (average size approximately 600 kb) on chromosome Xq26.3 that invariably include GPR101 (MIM: 300393) encoding an orphan G protein-coupled receptor (GPCR).14,17, 18, 19 Marked upregulation of GPR101 at the RNA and protein level is a hallmark of pituitary tumors in X-LAG-affected individuals.16 Experimental studies have highlighted an important role for GPR101 in promoting pituitary GH and prolactin secretion due to potent constitutive activity via Gs and Gq/11.19 Other genes commonly duplicated alongside GPR101 at the X-LAG locus are VGLL1 (MIM: 300583), CD40LG (MIM: 300386), ARHGEF6 (MIM: 300267), and RBMX (MIM: 300199), but their expression is unaltered in pituitary tumors in X-LAG.16,17
In this study, we aimed to unravel the molecular mechanism underlying GPR101 misexpression in X-LAG tumors. We provide strong evidence that the duplication-induced rearrangement of local chromatin creates a neo-TAD, thereby permitting the GPR101 promoter to interact ectopically with nearby CREs.
Material and methods
Subjects
Studies outlined below were performed using genetic samples from six individuals with X-LAG and two unaffected mothers of X-LAG-affected subjects (Table S1). Subjects were recruited under the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health protocol 97-CH-0076 (ClinicalTrials.gov: NCT00001595) and under the University of Liège protocol B707201420418. The Institutional Review Boards of both Centers approved this study, and informed assent/consent was obtained from all the subjects and their parents.
Circular chromosome conformation capture (4C-seq)
3C and 4C library preparation was performed as previously described with modifications described below and elsewhere.20 4C-seq primer sequences, viewpoint fragment coordinates, and corresponding digestion strategies for primary and secondary restriction enzymes are listed in Table S2. Experiments from X-LAG and control samples were performed as singletons.
Cell fixation and nuclei extraction
Approximately 1 × 106 to 2.5 × 106 cells from cultured peripheral blood mononuclear cells (PBMCs from all control subjects and X-LAG-affected individuals S9 and S13) or nucleated cell isolates from peripheral blood samples (X-LAG-affected individuals S2, S6, S7, and S17) were used as input material for 3C library preparation. Trypsinized or disaggregated cells were filtered through a 40 μm cell strainer (Corning, #352340) and pelleted by centrifugation at 500 × g. Cells were fixed in 5 mL of 2% formaldehyde in 10% FCS/PBS and incubated for 10 min at room temperature to cross-link chromatin. The reaction was quenched with glycine at a final concentration of 0.125 M and incubation at room temperature for 5 min. Cells were pelleted by centrifugation for 5 min at 500 × g at 4°C and washed on ice twice with 1× PBS. Cell pellets were either snap frozen in liquid nitrogen or processed for nuclei extraction. For nuclei extraction, fixed cells were resuspended in 2 mL freshly prepared lysis buffer (50 mM Tris [pH 7.5]; 150 mM NaCl; 5 mM EDTA; 0.5% NP-40; 1.15% Triton X-100; 1x Roche Complete protease inhibitors) and incubated for 10 min on ice. Nuclei were pelleted by centrifugation for 5 min, 800 × g at 4°C and washed with 1× PBS. Pelleted nuclei were either snap-frozen in liquid nitrogen or further processed.
Chromatin digestion and proximity ligation for 3C library preparation
Nuclei pellets were resuspended in 100 μL of 0.5% SDS and incubated for 10 min at 62°C, without shaking. 292 μL water and 50 μL 10% Triton X-100 were added to each sample, mixed, and incubated for 15 min at 37°C to quench remaining SDS. 50 μL of 10× restriction enzyme buffer and a total of 400 units of primary restriction enzyme were added to each sample, mixed, and incubated overnight at 37°C with 900 rpm shaking. The restriction enzyme was heat inactivated according to the manufacturer’s instructions. Nuclei were pelleted at 800 × g for 10 min at 4°C and resuspended in 500 μL water. To ligate restriction fragment ends, 500 μL of 2× ligation mix (100 μL of 10× ligation buffer [NEB], 100 μL of 10% Triton X-100, 10 μL of 10 mg/mL BSA, 6.5 μL of T4 DNA ligase [NEB, M0202L], 283.5 μL water) were added to each sample and incubated overnight at 16°C and 800 rpm shaking.
Cross-link reversal and DNA purification
Nuclei were pelleted by centrifugation for 10 min, 800 × g at 4°C and sample volume was reduced to a total of 200 μL. 230 μL of 10 mM Tris HCL (pH 7.5), 20 μL of Proteinase K (10 mg/mL), and 50 μL of 10% SDS were added, mixed by pipetting, and incubated for 30 min at 55°C. Subsequently, 40 μL of 4 M NaCl were added and samples were incubated overnight at 65°C with 700 rpm shaking. Next, 5 μL of RNase A (10 mg/mL) were added, followed by incubation at 37°C for 30 min at 700 rpm. 20 μL Proteinase K (10 mg/mL) were added to the sample and incubated at 55°C for 1–2 h at 700 rpm. DNA was purified by phenol-chloroform extraction. Following DNA precipitation, the dried DNA pellet was reconstituted in 100 μL 10 mM Tris-HCl (pH 7.5). DNA concentration from resulting 3C libraries was measured with Qubit.
Second restriction digestion and intra-molecular ligation for 4C-seq library preparation
3C libraries were digested with secondary restriction enzyme and incubated overnight at 37°C in a 500 μL reaction volume, containing 1× restriction enzyme buffer and 5 units of restriction enzyme per 1 μg DNA. The restriction enzyme was heat-inactivated following the manufacturer’s instructions. Samples were transferred to 50 mL falcon tubes to increase ligation reaction volume with water and to promote intra-molecular ligation events. Samples were ligated overnight at 16°C in a 7 mL ligation reaction, containing 1× ligation buffer, 100 units of T4-DNA ligase (Thermo Fisher Scientific, #EL0011). Samples were further diluted with water to 14 mL. For DNA precipitation, samples were mixed with 1.4 mL 3 M sodium acetate (pH 5.2), 700 μg glycogen, and 35 mL 100% ethanol. Samples were placed at −80°C until solution became viscous. DNA was pelleted by centrifugation at full speed for 1 h at 4°C. The pellet was washed with 70% ethanol and DNA resuspended in 200 μL of 10 mM Tris-HCl (pH 7.5). Samples were subsequently purified using AMPure XP beads (Agencourt, A63881) as follows: 1.8× volume of AMPure beads were added to the sample, mixed by pipetting, and incubated for 10 min at room temperature. Beads were separated on a magnet, and clear supernatant was discarded. Beads were washed twice with 70% EtOH, air-dried for 5 min and DNA was eluted in 100 μL 10 mM Tris-HCl (pH 7.5). DNA concentration of 4C libraries was measured with Qubit.
Inverse 4C PCR and sequencing of 4C-seq libraries
4C libraries were used as templates for inverse PCR using primers designed for the viewpoint digestion fragment (Table S2). Primers were designed as previously described.21 The 5′ ends of primers contained the following partial Illumina TrueSeq adapters, read primer 1: 5′-CTACACGACGCTCTTCCGATCT-3′; primer 2: 5′-CAGACGTGTGCTCTTCCGATCT-3′. Primer pairs were tested for optimal PCR amplification using a template dilution series with 0.5, 1, 2, and 4 ng/μL final concentration in PCR. Optimal template concentration determined by gel electrophoresis corresponded to the highest template concentration producing reproducible banding pattern and amplification in a linear range. PCR conditions in 50 μL (Expand Long Template PCR System, Roche, #11759060001): 1× PCR buffer, 0.2 mM dNTPs, 1 μM primers, 0.075 U/μL DNA polymerase, template as determined. PCR cycler setup: (1) 94°C for 2 min, (2) 94°C for 15 s, (3) 55°C for 1 min, (4) 68°C for 3 min, (5) repeat step (2) 29 times, (6) 68°C for 7 min, (7) 4°C. Parallel PCR reactions were performed to amplify from a total of 1 μg template per 4C library and viewpoint. PCR reactions were pooled and purified using 1.8× volume of AMPure XP beads (Agencourt, A63881), see procedure above.
Finally, 4C-seq libraries were indexed using TruSeq index primers (e.g., NEBNext Index Primer for Illumina [NEB #E7335S] and NEBNext High-Fidelity 2X PCR Master Mix [NEB]). 40 μL PCR reaction, containing 1× NEBNext High-Fidelity PCR Master Mix, 0.75 μM NEBNext Universal Primer, and NEBNext Index Primer, 50 ng of inverse PCR product. PCR cycler setup: (1) 98°C for 60 s; (2) 98°C for 10 s; (3) 65°C for 30 s; (4) 72°C for 30 s; (5) repeat step (2) 7 times; (6) 72°C for 5 min. For each sample, 4 independent PCR reactions were performed then pooled for AMPure beads purification, using 1.0× volume of bead solution. Final 4C-seq libraries were resuspended in 50 μL water, multiplexed, and sequenced using HiSeq technology to produce 50 bp single-end reads and approximately 10 million raw sequencing reads for each viewpoint and sample.
4C-seq data analysis and data visualization
4C-seq data were analyzed as previously described.22 Briefly, sequencing data were demultiplexed and mapped to the human reference genome (GRCh37/hg19). Reads located in restriction fragments flanked by restriction recognition sites of the same enzyme, in fragments smaller than 40 bp or within a window of 10 kb around the viewpoint were filtered out. Mapped reads were then converted to read counts per restriction fragment of the first restriction enzyme. For visualization, all samples were normalized for million mapped reads (reads per million, RPM) for a given genomic region (chrX:135,000,000–137,000,000, hg19) and smoothened using a 10-fragment running window algorithm. To compare interaction profiles of X-LAG subjects to control, subtraction of normalized reads was applied. To reduce variability among control samples, an average profile from three independent control samples was used for subtraction.
Chromatin contact enrichment of candidate enhancers with GPR101 promoter
Normalized read counts for restriction fragments overlapping with candidate enhancer regions (eHTATSF1, chrX:135,577,559–135,581,959; eVGLL1-intronic, chrX:135,625,877–135,641,072; eVGLL1-distal, chrX:135,656,769–135,660,247; ARHGEF6-intronic, chrX:135,846,959–135,851,769; eRBMX, chrX:135,959,959–135,963,959; eAK055694, chrX:135,990,759–135,994,160) were extracted from the GPR101 viewpoint for X-LAG and control samples. Total read count in each candidate region was averaged and normalized by read-covered fragments to correct PCR bias for spurious and high read counts in single fragments. Fold enrichment of chromatin contacts relative to controls (average from three independent control samples) was calculated and plotted on a logarithmic scale.
Hi-C data visualization and TAD coordinates
GM12878 Hi-C data were visualized in the UCSC genome browser (hg19) at 5 kb resolution in Figure 1. The predicted TAD positions for different cell types (Figures 1 and S1) were obtained from the 3D genome browser23 (Table S3). TAD positions were predicted from Hi-C data with the hidden Markov model (HMM) pipeline from Dixon et al.2,23,24 TAD coordinates for all cell types were available in the hg19 genome version, except data for GM12878, which we retrieved in the hg38 genome version and subsequently converted to hg19, using the UCSC liftOver tool.7,24, 25, 26, 27, 28
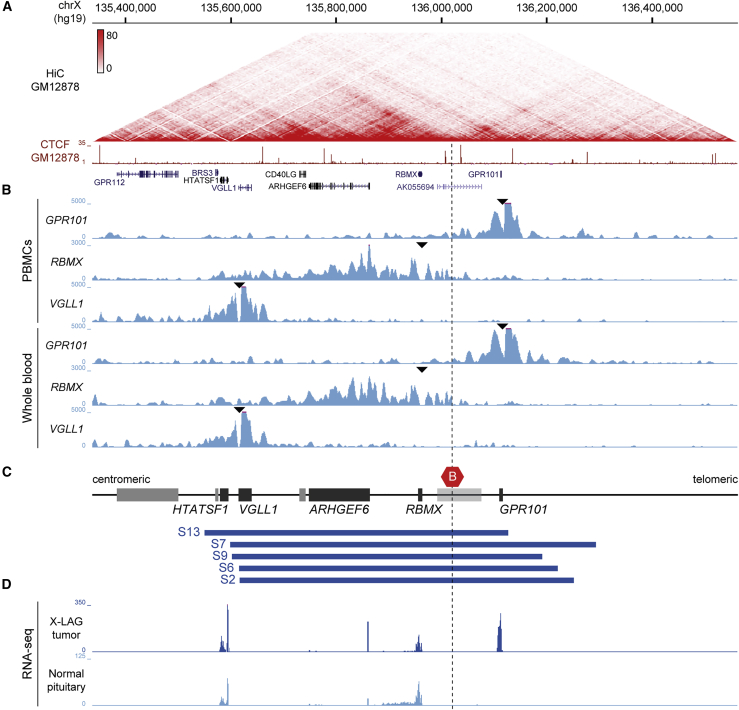
Figure 1.
Chromatin organization at the X-LAG locus
(A) Hi-C data from GM12878 lymphoblastoid cells, visualized in the UCSC genome browser at 5 kb resolution (hg19, chrX:135,336,766–136,561,684), showing TAD organization at the X-LAG locus. The ChIP-seq signal for the transcriptional regulator CTCF from GM12878 lymphoblastoid cells and gene annotation are shown underneath. Frequent chromatin interactions within TADs and prominent loop interactions are delimited by CTCF binding sites.
(B) 4C-seq interaction profiles of control samples with viewpoints (black triangles) in the promoters of GPR101, RBMX, and VGLL1 in samples of peripheral blood mononuclear cells (PBMCs, upper three tracks) and nucleated blood cells from peripheral blood samples (lower three tracks). Prominent chromatin interactions from each promoter are confined to the respective TADs, recapitulating the TAD organization observed by Hi-C.
(C) Schematic representation of the X-LAG locus and genomic positions of five different tandem duplications from subjects with X-LAG (blue boxes). All duplications involve the invariant TAD border (red hexagon) that under normal conditions separates GPR101 and genomic sequences located centromerically, including RBMX and ARHGEF6. For the sake of visualization, genes that were studied in the current experiments are indicated as black boxes while genes not of relevance to the current work are shown in gray boxes. Note that the gene body of the pseudogene AK055694 (light gray box) overlaps with the TAD border but its putative promoter is located centromeric to the TAD border.
(D) Mean RNA expression levels in four X-LAG tumors and three normal pituitaries show consistent upregulation of GPR101 in individuals with X-LAG duplications.
All panels are aligned to have the same start and end genomic coordinates.
CRE annotation
GPR101 promoter annotation was carried out using the Gene2Promoter tool of the Genomatix suite (Genomatix) and the MPromDb software.29 Two promoter sequences were identified with these methods: a proximal 1,101-bp-long region and a distal 1,097-bp-long region (Figure S5). These sequences were then intersected with specific tracks (CpG Islands, H3K27ac, and H3K4me3 marks from ENCODE) retrieved from the UCSC genome browser and the TSS annotations from the FANTOM5 consortium.
The genomic locations of the regions containing candidate enhancers identified by interrogating the H3K27ac ChIP-seq tracks retrieved from NCBI Gene Expression Omnibus (GEO) GSM1119175 and GSM111915230 and the ATAC-seq tracks GEO: GSM357991931 were intersected with the list of regulatory elements retrieved from the genome-wide GeneHancer database.32 This approach allowed the identification of four known putative enhancers sequences: GH0XJ136495, a 4,401-bp-long sequence overlapping the HTASF1 promoter; GH0XJ136764, a 4,811-bp-long sequence located within an intron of ARHGEF6; GH0XJ136878, a 4,001-bp-long sequence overlapping the RBMX promoter; GH0XJ136908, a 3,402-bp-long sequence located within the AK055694 (ENSG00000234062) pseudogene. Two additional candidate enhancers were identified within and downstream of VGLL1 and labeled as eVGLL1-intronic (1,357-bp-long) and eVGLL1-distal (4,101-bp-long), respectively.
Cloning of GPR101 promoter sequences and candidate enhancers into reporter vectors
The two in silico-identified promoter sequences for GPR101 were PCR amplified from the genomic DNA of a healthy control donor and cloned into the pGEM-T-Easy vector (A1360, Promega) using the SacI restriction site. Cloning was performed using the In-Fusion Cloning System (Takara Bio Europe) and the primers listed in Table S2. The primers were designed using the online In-Fusion Cloning Primer Design Tool (Takara Bio Europe). Both SacI fragments were subcloned into a promoter-less luciferase reporter vector (pLightSwitch_Prom, S790005, Switchgear Genomics) using the SacI site located upstream of the start codon of the RenSP (synthetic Renilla luciferase that includes mODC PEST) reporter gene.
The eARHGEF6-intronic (GeneHancer ID: GH0XJ136764) and eVGLL1-intronic fragments were PCR amplified from the genomic DNA of a healthy control donor and cloned into an enhancer reporter vector (pLightSwitch_LR, S990005, Switchgear Genomics) using, respectively, the HindIII or the NheI (5′) and XhoI (3′) sites located upstream of the weak, constitutive herpes simplex virus thymidine kinase (TK) gene promoter. Cloning was performed using the In-Fusion Cloning System (Takara Bio Europe) and the primers are listed in Table S2. The eHTATSF1 (GeneHancer ID: GH0XJ136495), eRBMX (GeneHancer ID: GH0XJ136878), eAK055694 (GeneHancer ID: GHOXJ136908), and eVGLL1-distal fragments were cloned within the same enhancer reporter vector using either the KpnI site (eHTATSF1, eRBMX, eAK055694) or the NheI (5′) and XhoI (3′) sites (eVGLL1-distal). These sequences were synthesized and cloned by GeneWiz Inc. All cloned fragments were verified by Sanger sequencing. The preparations of all plasmid DNAs used in the experiments were checked for integrity by restriction digestion with single or double cutters followed by a run on a 1% agarose gel.
Cell culture
The rat pituitary tumor GH3 cell line and the Human Embryonic Kidney (HEK)-293AD cell line were purchased from ATCC (CCL-82.1 and CRL-1573, respectively). Cells were tested for mycoplasma contamination and tested negative. Cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM, low glucose, pyruvate, no glutamine; GIBCO) supplemented with 10% fetal bovine serum (Gemini Bio Products), 10 mM HEPES, and 1% antibiotic-antimycotic (GIBCO) in a humidified atmosphere at 37°C with 5% CO2.
CRE luciferase reporter assays
GH3 and HEK293AD cells were seeded in 96-well plates (white clear bottom, poly-d-lysine coated, 354651, Corning Biocoat) at a density of 3.2 and 2.5 × 104 cells per well, respectively. After 24 h, cells were transfected with TurboFect (R0531, Thermo Fisher Scientific) according to the manufacturer’s protocol, using 100 ng of each reporter vector. A reporter construct containing the strong, constitutive promoter of the human housekeeping gene beta actin (S717678, Switchgear Genomics) was used in each experiment and served as positive transfection control. At 24 h after transfection, cells were lysed and Renilla luciferase activity measured using the Renilla Luciferase Assay System (E2810, Promega) following the manufacturer’s protocol. All experiments were repeated at least three times with three technical replicates for each construct.
RNA-seq analysis
Each sample was prepared as follows: total RNA was extracted from surgical samples of pituitary tumors in four individuals with X-LAG (three females, one male) and also from three samples of normal adult pituitaries (two males, one female) obtained at autopsy (Cureline Inc.). Total RNA-seq libraries were prepared, libraries were sequenced on an Illumina HiSeq with a 50 bp single- or paired-end protocol and demultiplexed (Zymo Research). Quality of raw data files (fastq) were evaluated using the fastqc (see web resources) and trimmed with Trimmomatic.33 Then, only high-quality reads were aligned to the human reference genome hg19 using Star34 in a two-pass approach. A first STAR pass allowed us to obtain a database of splice junctions, from which we filtered out any non-canonical junctions. A new genome was generated with the splice junctions and a second STAR pass was run. Coverage per sample was computed in 1 kb bins and a per sample upper quartile normalization was performed. The normalized average coverage was then computed for all X-LAG samples in the one hand and for the normal samples on the other. Raw count of reads per gene per sample was obtained using the feature Counts.
As recommended, analyses of differential expression to compare X-LAG and normal pituitary samples gene expression were performed with different analytical packages.35,36 First, we performed an analysis with edgeR and limma as follows: rows with all zero counts and rows with very low counts in at least two samples were removed. Library sizes were then reset in edgeR and a TMM normalization was applied before transforming the counts into log CPM (counts per million) values. A simple linear model matrix with the two conditions as contrast was fit to the data and empirical Bayes statistics were used to identify differentially expressed genes with a robustification against outlier dispersion. This analysis identified 18 differentially expressed genes based on an adjusted p value < 0.05 threshold. The complete list of differentially expressed genes (genes for which a fold change is observed between X-LAG and control samples), is presented in Table S4.
A second differential expression analysis was performed by using HTSeq-count for expression quantification;37 the derived counts files were then analyzed using DESeq2.38 This analysis identified 88 differentially expressed genes based on an adjusted p value < 0.05 threshold. The complete list of differentially expressed genes from this second analysis is also presented in Table S4. A list of common differentially expressed genes was then obtained by intersecting the different analyses’ outputs, using as a cutoff the first 500 genes of each list ranked by p value. This list, comprising 55 genes (Table 1), was subjected to pathway enrichment analysis using Metascape.39
Table 1.
Transcripts of interest differentially expressed in X-LAG versus normal pituitary
| Gene symbol | Description | log2 Fold change | p value | p adjusted |
|---|---|---|---|---|
| GPR101 | G protein-coupled receptor 101 | 12.63436884 | 1.29E−07 | 0.000190613 |
| CBLN1 | cerebellin 1 precursor | 5.512119335 | 0.000563223 | 0.069604995 |
| THBS2 | thrombospondin 2 | 5.308143562 | 2.21E−05 | 0.006548969 |
| SHC3 | SHC adaptor protein 3 | 3.511547641 | 1.30E−05 | 0.006548969 |
| HEPACAM2 | HEPACAM family member 2 | 3.438855732 | 0.001902735 | 0.100777001 |
| ECEL1 | endothelin converting enzyme like 1 | 3.435679699 | 0.000217119 | 0.04024841 |
| OTOS | otospiralin | 3.02397859 | 0.001895077 | 0.100777001 |
| ROBO2 | roundabout guidance receptor 2 | 2.710070311 | 0.001458344 | 0.090601717 |
| PDE3A | phosphodiesterase 3A | 2.661730163 | 1.97E−05 | 0.006548969 |
| FKBP10 | FKBP prolyl isomerase 10 | 2.293691778 | 0.000235806 | 0.041141147 |
| RAB27B | RAB27B, member RAS oncogene family | 2.273319657 | 0.000154602 | 0.035272955 |
| ZNF185 | zinc finger protein 185 with LIM domain | 2.272444372 | 0.00077183 | 0.078939629 |
| ENPP2 | ectonucleotide pyrophosphatase/phosphodiesterase 2 | 2.215979663 | 0.000302407 | 0.049829942 |
| RCN1 | reticulocalbin 1 | 1.79981704 | 0.001001391 | 0.08735662 |
| THY1 | Thy-1 cell surface antigen | 1.693370111 | 0.003678918 | 0.132060483 |
| FAM163A | family with sequence similarity 163 member A | 1.58328952 | 0.015330845 | 0.231996364 |
| MEF2C | myocyte enhancer factor 2C | 1.295394391 | 0.005879336 | 0.169266482 |
| CNTN2 | contactin 2 | 1.257122224 | 0.008331689 | 0.191799297 |
| ACSL6 | acyl-CoA synthetase long chain family member 6 | 1.193527525 | 0.058994577 | 0.356575463 |
| NRXN3 | neurexin 3 | 1.118119659 | 0.003173021 | 0.132060483 |
| ZNF667 | zinc finger protein 667 | 1.085357541 | 0.000197451 | 0.039042708 |
| PTPRK | protein tyrosine phosphatase receptor type K | 1.060081218 | 0.003335589 | 0.132060483 |
| CDH18 | cadherin 18 | 1.035159724 | 0.002714759 | 0.125756193 |
| NCALD | neurocalcin delta | 1.021986775 | 0.025977855 | 0.281559841 |
| AFAP1 | actin filament associated protein 1 | 0.879150352 | 0.004961622 | 0.151713106 |
| JMY | junction mediating and regulatory protein, p53 cofactor | −0.697771242 | 0.00521534 | 0.156249487 |
| IPO7 | importin 7 | −0.703833909 | 0.006282581 | 0.169612007 |
| DENND4A | DENN domain containing 4A | −0.820624498 | 0.00709545 | 0.179872693 |
| ARHGAP26 | Rho GTPase activating protein 26 | −0.858268895 | 0.00593517 | 0.169266482 |
| HTRA1 | HtrA serine peptidase 1 | −0.933790787 | 0.042763447 | 0.326057545 |
| CHD3 | chromodomain helicase DNA binding protein 3 | −0.972833851 | 0.011306594 | 0.224110606 |
| ENAH | ENAH actin regulator | −1.006496792 | 0.000133954 | 0.033108953 |
| PTPRS | protein tyrosine phosphatase receptor type S | −1.078053314 | 0.001090912 | 0.089879005 |
| SYNE2 | spectrin repeat containing nuclear envelope protein 2 | −1.252332472 | 0.001496792 | 0.090601717 |
| ST18 | ST18 C2H2C-type zinc finger transcription factor | −1.257373855 | 0.006370766 | 0.169612007 |
| SLC5A3 | solute carrier family 5 member 3 | −1.27293174 | 0.043192658 | 0.326809756 |
| IGFBP5 | insulin like growth factor binding protein 5 | −1.470642696 | 0.00204994 | 0.104829669 |
| MCOLN3 | mucolipin TRP cation channel 3 | −1.538158909 | 0.008341911 | 0.191799297 |
| A2M | alpha-2-macroglobulin | −1.546266649 | 0.00460635 | 0.148504708 |
| ANXA1 | annexin A1 | −1.554518488 | 0.047250273 | 0.336077478 |
| RAB3B | RAB3B, member RAS oncogene family | −1.559168136 | 0.000489003 | 0.067755285 |
| CACNA1H | calcium voltage-gated channel subunit alpha1 H | −1.615245675 | 0.000484715 | 0.067755285 |
| STEAP4 | STEAP4 metalloreductase | −1.615302685 | 7.62E−05 | 0.020556875 |
| FBXO32 | F-box protein 32 | −1.626663872 | 0.001474508 | 0.090601717 |
| DSP | desmoplakin | −1.805180049 | 8.06E−06 | 0.005978382 |
| SOD2 | superoxide dismutase 2 | −1.853240635 | 0.042101935 | 0.323536447 |
| TGFBR3 | transforming growth factor beta receptor 3 | −1.874227585 | 1.58E−05 | 0.006548969 |
| OBSCN | obscurin, cytoskeletal calmodulin and titin-interacting RhoGEF | −2.04135558 | 0.000920676 | 0.085335122 |
| SORBS1 | sorbin and SH3 domain containing 1 | −2.100457177 | 0.010402284 | 0.220975104 |
| AEBP1 | AE binding protein 1 | −2.318738851 | 0.010430383 | 0.220975104 |
| COL6A6 | collagen type VI alpha 6 chain | −2.618856526 | 0.012027287 | 0.224358063 |
| GPC4 | glypican 4 | −3.013712734 | 0.000545355 | 0.069604995 |
| NPTX2 | neuronal pentraxin 2 | −4.197420142 | 2.05E−05 | 0.006548969 |
| POMC | proopiomelanocortin | −4.876589807 | 3.01E−07 | 0.000297977 |
| LHB | luteinizing hormone subunit beta | −8.869100817 | 4.10E−09 | 1.22E−05 |
55 differentially regulated genes (p value < 0.05) were identified by both analytical methods (edgeR/limma and DESeq2) that were employed to analyze the RNA-seq data. The transcripts are sorted by log2 fold change. EdgeR/limma-derived values are presented in the table.
GTEx
The data used for the analyses described in this manuscript were obtained from the GTEx Portal on 09/30/2021.
Statistical analysis
The graphs presented in Figure 6 were plotted as individual biological replicates with mean ± standard deviation (SD). Data distributions were assessed for approximate normality and differences between experimental groups were analyzed by 1-way ANOVA with Dunnett’s post hoc test or a corresponding non-parametric test, as appropriate. Data were analyzed using GraphPad Prism (GraphPad). p values < 0.05 were considered statistically significant.
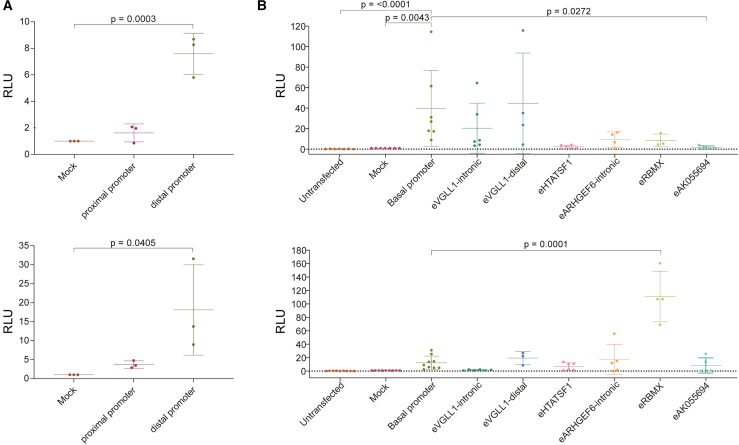
Figure 6.
In vitro characterization of GPR101 promoter and candidate enhancers
(A) Luciferase reporter assay of proximal and distal promoter elements of GPR101 in GH3 (top) and HEK293AD (bottom) cells.
(B) Luciferase reporter assay of candidate pituitary active CREs in GH3 (top) and HEK293AD (bottom) cells. The basal promoter construct does not contain any enhancer sequence.
Data in both panels are plotted as individual biological replicates with mean ± standard deviation (SD). All values are relative to Mock (promoter-less luciferase reporter vector) in both panels. Data distributions were assessed for approximate normality and differences between experimental groups were analyzed by 1-way ANOVA with Dunnett’s post hoc test or corresponding non-parametric test, as appropriate. RLU, relative luciferase units.
Results
Chromatin organization and normal TAD structure at the X-LAG locus on chromosome Xq26.3
Structural variations and tandem duplications can interrupt and rearrange the local TAD organization and thereby interfere with gene regulation. To investigate the potential contribution of TAD disruption to the etiology of X-LAG, we first determined the normal structural organization at the X-LAG locus using Hi-C, a chromosome conformation capture technology that allows for the quantification of genome-wide chromatin contacts. Hi-C data at high resolution in the human lymphoblastoid cell line GM12878 showed that the X-LAG locus is highly structured, forming several TADs that spatially separate the locus (Figure 1A). GPR101 is organized within a TAD extending telomerically and harboring no other nearby genes within the region that is typically duplicated at the X-LAG locus.7 The neighboring genes that lie centromeric to GPR101 (CD40LG, ARHGEF6, RBMX), and that are commonly duplicated alongside GPR101 in X-LAG, are partitioned within distinct TADs. The TAD border separating GPR101 from the centromeric genes is characterized by two CTCF-binding sites located within a 30 kb region. Importantly, comparison of Hi-C data from 21 human tissues, including pluripotent and terminally differentiated cell types, revealed that the spatial separation of the locus by this TAD border is largely tissue invariant (Figure S1 and Table S3). Furthermore, comparison between human and mouse data revealed that the general chromatin structure and invariant TAD border observed at the X-LAG locus is evolutionarily conserved (Figure S2).
Using 4C-seq, we probed chromatin interactions along the X-LAG locus from GPR101, RBMX, and VGLL1 promoters in healthy individuals (Figure 1B). 4C-seq experiments performed in PBMCs and direct cell isolates from peripheral blood samples revealed similar interaction profiles for both sample types. Those profiles resembled the chromatin topology observed from Hi-C data. Chromatin interactions involving the GPR101 promoter were restricted to the telomeric end of the X-LAG locus and separated from chromatin interactions established by the RBMX and the further centromerically located VGLL1 promoters. This topological separation observed with both Hi-C and 4C-seq is consistent with the known cell type- and tissue-specific expression of genes at the locus. RBMX and ARHGEF6, that share the same TAD, show overlapping expression profiles in multiple tissues, including strong expression in the pituitary gland (Figure S3). Pituitary expression, although at a lower level, was also observed for VGLL1 and CD40LG. In contrast, GPR101 exhibits a restricted expression in brain tissues only and is normally not expressed in the adult pituitary gland.40,41 This suggests that, under normal conditions, genes and their CREs at the X-LAG locus are separated into different TADs and that a strong TAD border separates GPR101 from centromeric genes and regulatory sequences.
Overlapping the position of X-LAG-associated tandem duplications with the chromatin structure at the locus revealed that all duplications include the observed strong and tissue-invariant TAD border, GPR101, and genomic sequences centromeric to this TAD border (Figure S4). In this study we will focus on individuals S2, S6, S7, S9, and S13, as shown in Figure 1C.
RNA-seq analysis in X-LAG tumors and normal pituitary tissue further supported an effect on gene expression that was focused on GPR101. As seen in Figure 1D, GPR101 was markedly upregulated while other duplicated genes at the X-LAG locus were unaltered or remained expressed at low levels (mean raw gene counts at the locus are listed in Table S5).
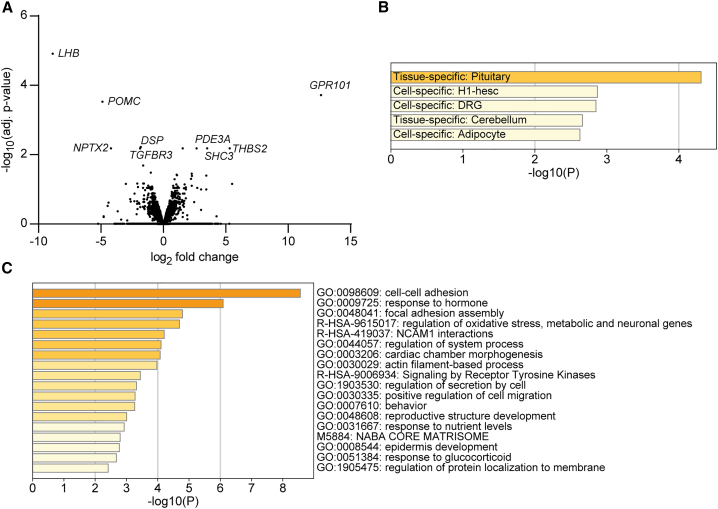
The differentially expressed genes listed in Table 1 show that GPR101 was by far the most significantly dysregulated gene overall in X-LAG tumors versus normal pituitary (>12 log2-fold increase). Interestingly, specific genes for hormones secreted by other pituitary cell types like corticotropes (POMC) and gonadotropes (LHB) were highly downregulated in X-LAG pituitary (Table 1, Figure 2A). This contributed to a strong tissue-specific pituitary signature in terms of significantly disordered pathways (Figure 2B), including individual GO terms such as response to hormone, regulation of secretion by cell, and reproductive structure development (Figure 2C). These RNA-seq data corroborate and expand on the role of GPR101 in the regulation of GH and prolactin secretion that we previously reported in transgenic mice.19 The involvement of other GO pathway terms like cell-cell adhesion and focal adhesion assembly may reflect the molecules and signals involved in the tumorigenic processes in pituitary adenomas from the X-LAG subjects.
Figure 2.
Gene expression analysis in X-LAG tumors versus normal pituitary
Count files from total RNA-seq analyses of four X-LAG tumors and three normal pituitaries were analyzed in parallel using edgeR/limma and DESeq2 methods, which provided highly consistent outputs of differentially expressed genes.
(A) A volcano plot shows the range of significantly up- and downregulated genes, among which GPR101 was the most markedly dysregulated gene overall. The most significantly downregulated genes were those characteristic for pituitary corticotrope (POMC) and gonadotrope (LHB) secretion, reflecting a tumoral process in X-LAG that favored somatotrope development and secretion over other pituitary axes. The most dysregulated genes with the lowest adjusted p values are highlighted. EdgeR/limma-derived values are presented in the plot.
(B) Pituitary was identified as the tissue most significantly affected in a pathway analysis using GO categories from PaGenBase.39,42
(C) The GO terms that were most significantly altered in the RNA-seq dataset were those for cell-cell adhesion and response to hormone; other GO terms related to pituitary or endocrine function included regulation of secretion by cell, reproductive structure development, and response to glucocorticoid.
X-LAG-related chromosome Xq26.3 duplications induce neo-TAD formation and ectopic chromatin interactions
Using 4C-seq, we investigated the effects of chromosome Xq26.3 duplications on TAD structure in PBMCs and nucleated cell isolates from peripheral blood samples collected from six unrelated X-LAG-affected individuals, as compared with normal control subjects. For these studies, the viewpoint for the 4C-seq analysis was placed on the GPR101 promoter region. Across the X-LAG-affected individuals, we demonstrated that the chromosome Xq26.3 duplication led to new interactions that crossed the normal TAD boundary surrounding GPR101. This, in turn, revealed a landscape of ectopic chromatin interactions of GPR101 outside of the normal TAD border (Figure 3).
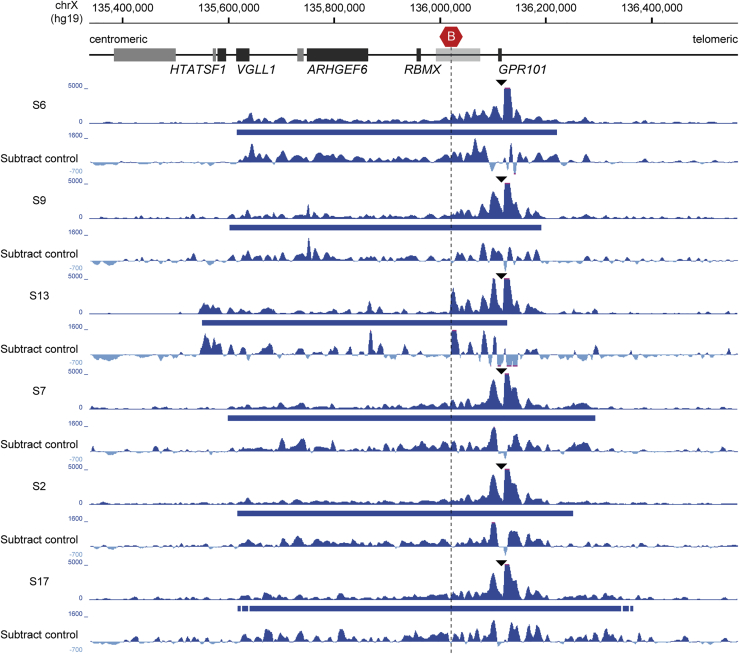
Figure 3.
Ectopic chromatin interactions of GPR101 extend centromerically and cross a TAD border
4C-seq profiles from the GPR101 viewpoint in six different X-LAG-affected individuals. Size and position of each duplication are indicated below each 4C-seq profile (blue bar) and corresponding subtraction profiles to control samples are presented below. GPR101 shows consistent ectopic interactions with regions centromeric of the TAD border. Note that the exact breakpoints for the S17 duplication is not determined.
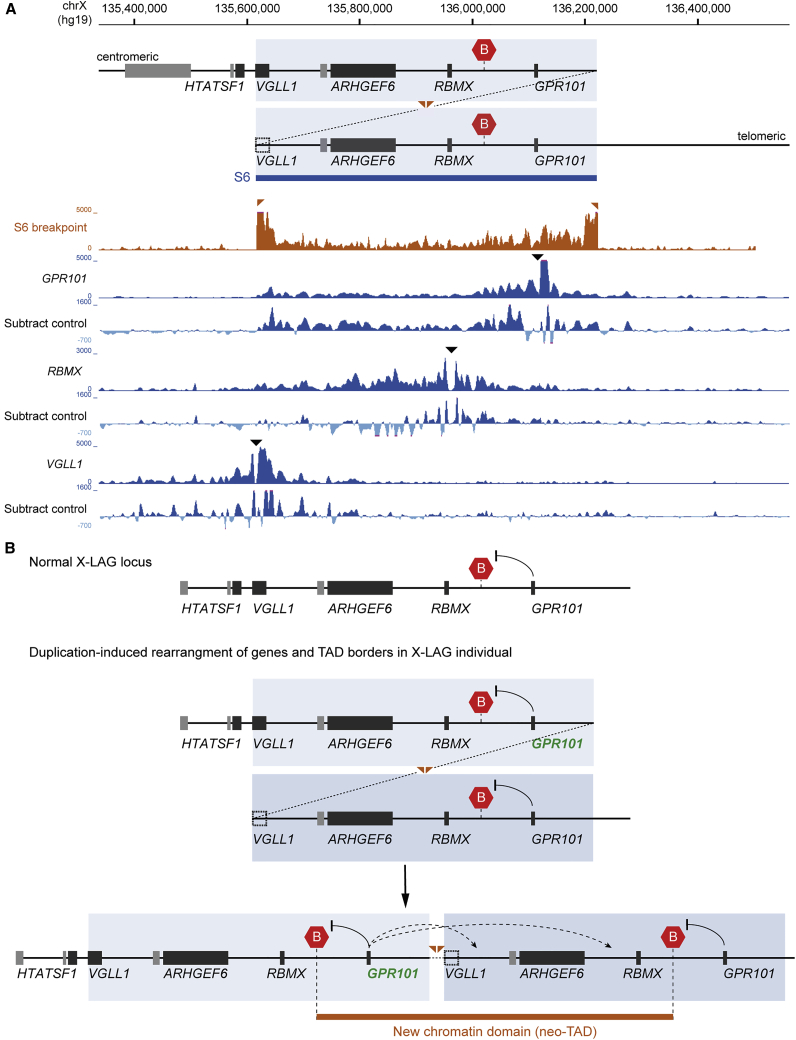
This is illustrated, for example, in subject S6, in whom the duplication creates a unique configuration of genomic sequences around its breakpoint, delineated by the duplicated TAD border (Figure 4A). Using the breakpoint as a unique viewpoint in the subject’s genome, we further demonstrated that the resulting 4C-seq profile showed typical enrichment close to the viewpoint (i.e., the breakpoint) but also frequent chromatin interactions across duplicated sequences that cross the usually invariant normal TAD border. Also, genomic regions outside the duplication showed very low levels of interaction, indicating that the duplicated sequences within the neo-TAD are physically separated. Therefore, in subject S6, GPR101 and RBMX viewpoints within the duplication showed ectopic contacts when compared to control samples (Figure 4A). The GPR101 viewpoint showed additional interactions that extended across the normally invariant TAD border toward the centromeric duplicated sequences, a pattern not observed under normal conditions. The RBMX viewpoint also showed ectopic contacts with genomic regions around GPR101. In contrast, the VGLL1 promoter, which was outside of the duplication in this subject, did not show any abnormal chromatin interactions, confirming that sequences within neo-TADs are spatially insulated. The observed aberrant interaction patterns suggest the formation of a neo-TAD, which rearranges genomic sequences that, under normal conditions, do not interact with each other. Overall, the Hi-C, 4C-seq, and RNA-seq data indicate that the inclusion of one copy of GPR101 within the neo-TAD and the resulting ectopic contacts with potential CREs (Figure 4B) can account for GPR101 upregulation observed in X-LAG pituitary tumors.
Figure 4.
X-LAG duplications create a neo-TAD and ectopic chromatin contacts
(A) 4C-seq experiments in a person harboring a 600 kb tandem duplication (individual S6 reported previously).16 The schematic depicts the S6 duplicated allele with a duplicated TAD border (red hexagons). Size and position of the duplication is indicated by overlap and blue shaded area. The dotted line, connecting the duplicated sequences, illustrates the duplication breakpoint. Note that the duplication breakpoint disrupts VGLL1, excluding the VGLL1 promoter and viewpoint from the duplication. Below, the 4C-seq interaction profile from the unique viewpoint created by the duplication breakpoint is shown (brown track). The breakpoint is flanked by the duplicated TAD border and the 4C-seq profile shows high interaction frequencies with regions restricted to the duplication, indicating that the neo-TAD is spatially insulated. All reads mapped to a wild-type genome (resulting in split viewpoint for duplication breakpoints). Below this, 4C-seq profiles from the GPR101, RBMX, VGLL1 viewpoints and corresponding subtraction profiles to control samples are presented (blue tracks). The viewpoints at GPR101 and RBMX promoters, included in the duplication and neo-TAD, show ectopic chromatin interactions. The VGLL1 promoter viewpoint, excluded from individual S6’s duplication, shows a normal interaction profile.
(B) Model depicting chromatin interactions (arrows) at the X-LAG locus under normal conditions and after duplication-induced rearrangement of genes and the invariant TAD border. Rearranged sequences between the duplicated TAD border creates a new chromatin domain (neo-TAD) that is characterized by ectopic interactions (dotted arrows) and little or no interaction beyond the TAD border.
The 4C-seq data showed consistent effects across all X-LAG samples irrespective of the different spans of the Xq26.3 duplication (Figures 3 and 4). Despite the variable duplication breakpoints, GPR101 showed ectopic interactions that cross the invariant TAD border at the X-LAG locus. These findings strongly suggest that a similar and consistent mechanism of neo-TAD formation exists for all tested duplications in X-LAG.
GPR101 ectopically interacts with candidate pituitary CREs
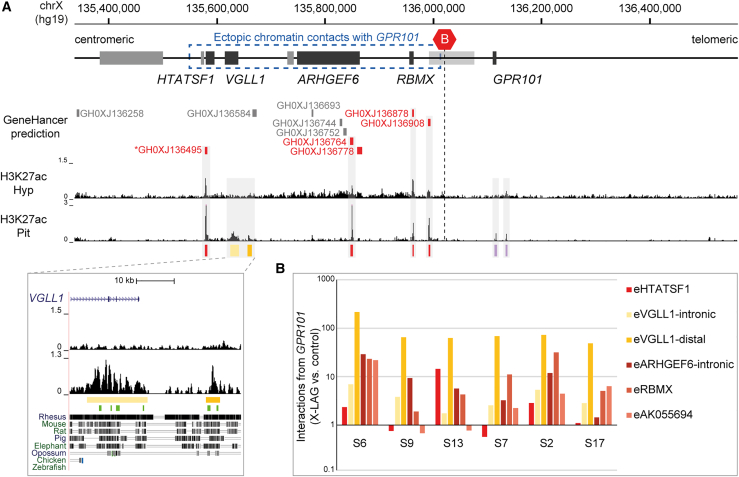
In X-LAG, the newly established interactions harbor potential CRE sequences such as enhancers that could drive the marked overexpression of GPR101 that is seen in the X-LAG pituitary tumors. We searched for CREs predicted in the GeneHancer database and for enhancer-associated histone modifications (H3K27ac) from the human pituitary and hypothalamus to identify those CREs with active chromatin signatures (Figure 5A). The genomic region telomeric to the invariant TAD border and containing GPR101 was largely depleted of predicted CREs and pituitary-specific signals. We identified only two regions showing activity in the pituitary, one in the known promoter of GPR10117,41 and one 21 kb upstream of its start codon. The latter region overlapped with H3K4me3 signals and a CpG island and likely corresponds to a putative far-distal promoter region of GPR101 (Figure S5). In contrast, the region centromeric to the normal invariant TAD border showed multiple predicted CREs and widespread activity in the hypothalamus and pituitary gland, which is consistent with the transcriptional activity of genes in this region (Figure S3). We found four predicted CREs with strong H3K27ac enrichment in the proximal promoter regions of HTATSF1 (MIM: 300346), RBMX, AK055694 (a pseudogene telomeric of RBMX), and in the intron of ARHGEF6 (eARHGEF6-intronic). In addition, and although not predicted by GeneHancer, we identified two CRE candidates that showed a pituitary-specific H3K27ac enrichment in VGLL1 (eVGLL1-intronic) and downstream of VGLL1 (eVGLL1-distal). Interestingly, these enhancer candidates with pituitary-specific signals contain sequences conserved among mammals, and chromatin accessibility assays in mouse pituitary revealed a functional conservation in somatotrope and lactotrope subpopulations of the anterior lobe of the pituitary (Figures 5A and S6). Collectively, this analysis revealed that the genomic region centromeric to the TAD border contains multiple CREs and shows widespread regulatory activity, including in the pituitary gland. These CRE sequences showed increased interaction frequency with the GPR101 promoter in cells from all tested X-LAG-affected individuals (Figure 5B).
Figure 5.
GPR101 ectopically interacts with candidate pituitary CREs in the neo-TAD
(A) X-LAG locus, depicting the region of ectopic chromatin contacts established by GPR101 in X-LAG-affected individuals (blue box). The pituitary (Pit) and hypothalamus (Hyp) H3K27ac ChIP-seq tracks were retrieved from NCBI Gene Expression Omnibus (GEO) GSM1119175 and GSM1119152, respectively.30 Corresponding enriched open chromatin regions identified in mouse somatotropes and lactotropes are indicated by green lines (ATAC-seq tracks retrieved from GEO: GSM3579919).31 Regions with candidate enhancers that ectopically interact with GPR101 are indicated with different saturations of yellow (pituitary-specific CREs) and in red (GeneHancer-predicted CREs). The asterisk preceding the candidate GH0XJ136495 CRE (eHTATSF1) indicates an elite gene association. GPR101 putative promoter regions are indicated in purple.
(B) Enrichment of chromatin contacts from the GPR101 viewpoint with candidate enhancers (X-LAG-affected individuals versus control). Note that the eHTATSF1 CRE is only included in the duplication of subject S13.
The rearrangement of large portions of this pituitary active region and GPR101 within the neo-TAD would favor their spatial proximity and could lead to dysregulation of GPR101. To study this further, we overlapped the position of CREs with the smallest regions of overlap (SROs) of known X-LAG-associated duplications. Interestingly, two out of 29 duplications at the locus (individuals S4 and I) are interrupted, with sequence duplications on either side of the invariant TAD border, defining two SROs, one telomeric and one centromeric to the invariant TAD border (Figure S4).43 The telomeric SRO always contains GPR101 and its known promoter sequence whereas the centromeric SRO includes a pituitary-active region, containing at least the eVGLL1-intronic enhancer. The induced genomic rearrangements of all known X-LAG duplications reposition one copy of GPR101 next to active pituitary regions. This scenario supports the model in which spatial proximity of GPR101 with potential pituitary-active CREs could drive GPR101 dysregulation in X-LAG-affected subjects.
GPR101 is compatible with pituitary and embryonic developmental programs
GPR101 dysregulation in the pituitary by new CREs has as a prerequisite the compatibility of the GPR101 promoter to respond functionally to these new ectopic signals. We, therefore, tested the ability of the GPR101 promoter to activate gene expression in response to pituitary- and embryonic-specific cellular programs. The telomeric SRO overlaps with the known GPR101 promoter and in this region two promoter sequences (defined here as proximal and distal), each overlapping a CpG island, were identified. Their location is corroborated by our previous RNA-seq and 5′-RACE data17,41 and by public CAGE-seq data44 (Figure S5). We functionally tested both promoter elements by cloning these sequences into a promoter-less luciferase reporter vector. We transfected the constructs into rat pituitary tumoral GH- and prolactin-secreting GH3 cells and human embryonic kidney HEK293AD cells, and measured luciferase activity. Both sequences were found to be functional, with the distal promoter showing higher transcriptional activity and potential to drive GPR101 expression in both cell types (Figure 6A).
Next, we tested the ability of CREs located in the centromeric region to drive reporter gene expression in GH3 and HEK293AD cells. We selected the regulatory regions that overlapped GeneHancer-predicted CREs and the therein-identified CREs with pituitary-specific H3K27ac signatures (Figure 5B). We cloned each CRE into a reporter vector containing a minimal promoter and then transfected them into both cell lines. Luciferase activity revealed that GeneHancer-predicted candidates (eHTATSF1, eARHGEF6-intronic, eRBMX, eAK055694) reduced reporter gene expression in GH3 cells while pituitary-specific CREs (eVGLL1-intronic and eVGLL1-distal) maintained basal promoter activity (Figure 6B). However, in embryonic HEK293AD cells the eRBMX element significantly enhanced reporter gene expression (p < 0.0001 versus basal promoter). All the other CREs either maintained basal promoter activity or repressed it (eVGLL1-intronic, Figure 6B).
Taken together, the results revealed that the GPR101 distal promoter is capable of driving gene expression in adult rodent pituitary tumor and human embryonic cellular contexts. Therefore, the GPR101 promoter permits the incorporation of new regulatory information within the neo-TAD. At the same time, CREs located within the centromeric region and that ectopically interact with GPR101 in X-LAG pituitary cells show context-dependent activity patterns. No transcriptional enhancing function for any tested CRE could be scored in GH3 cells. However, the eRBMX element showed transcriptional enhancing activity in the embryonic cellular context of HEK293AD cells. Interestingly, two CREs repressed basal promoter activity in specific cellular contexts.
Discussion
Pituitary gigantism is a rare disorder due to GH-secreting pituitary tumors that leads to dramatic and irreversible skeletal overgrowth and multi-organ pathology, including cardiovascular disease.45 Nearly half of cases of pituitary gigantism are associated with known genetic abnormalities.46 Among these, X-LAG is the most severe form, with large, highly secretory pituitary tumors developing during neonatal life and early childhood. At a genetic level, X-LAG is associated with chromosome Xq26.3 microduplications that invariably include GPR101, leading to extremely elevated GPR101 expression in the pituitary.16 GPR101 increases GH and prolactin production and induces overgrowth via constitutive activation of the G protein α subunits Gs, Gq/11, and G12/13,19 but the mechanism of how a tandem duplication leads to massive overexpression of GPR101 in X-LAG tumors was not explained until now.
Consequences of gene copy-number variations (CNVs) on mRNA expression levels have been studied comprehensively in different species, cell lines, and cells derived from individuals with different types of CNVs. Taken together, these studies concluded that there is an appreciable correlation between mRNA levels and gene copy number.47 However, for individual genes, mRNA levels often deviate from the expected levels. For example, GPR101 is upregulated around 1,000-fold in the pituitary tumors of subjects with X-LAG.16 This observation implies that the phenotypic effect conferred by Xq26.3 duplications in X-LAG is not due simply to increased GPR101 gene dosage. A potential mechanism that could explain GPR101 misexpression in X-LAG tumors is the creation of an abnormal fusion gene, whereby the genomic duplication places the GPR101 protein-coding region under the influence of a strong promoter located close to the breakpoints. However, no fusion events involving GPR101 were detected in X-LAG tumor samples (n = 4, Table S6). Moreover, no other fusion genes shared among the tumors were detected.
Our results, instead, show that Xq26.3 microduplications alter the chromatin configuration and normal TAD organization at the X-LAG locus. These tandem duplications disrupt a tissue-invariant TAD border that normally separates GPR101 from genes and regulatory sequences located centromerically. Tandem duplications that cross TAD borders (inter-TAD duplications) have been shown to rearrange the additional copy number of genes and CREs into new TADs, so-called neo-TADs.10,48 In the current study, we have shown similar re-organization of the chromatin configuration and neo-TAD formation at the X-LAG locus based on several 4C-seq promoter viewpoints and duplication breakpoint analyses. Furthermore, the neo-TAD formation is consistently seen across samples from multiple X-LAG-affected individuals with different microduplication ranges and breakpoints. The rearranged, duplicated genomic sequences ectopically interact within the neo-TAD and these interactions are spatially restricted by the duplicated TAD border. These results strongly suggest that neo-TAD formation (TADopathy) is the genetic pathophysiology behind GPR101 misexpression in X-LAG. As such, the endocrine disease X-LAG joins a limited but growing list of TADopathies involving limb malformation, retinal disease, platelet dysfunction, and cancer.10,12,14,15,48, 49, 50
Within neo-TADs, genes and CREs can interact ectopically, causing aberrant gene expression leading to disease phenotypes. By probing interactions from the GPR101 promoter in cells from multiple X-LAG-affected individuals with different microduplications, we identified consistent patterns of ectopic interactions. Based on CRE predictions and enhancer-associated chromatin marks, we then identified several potential ectopic CREs that are active in the adult pituitary gland.41 These findings lead us to suggest that the new regulatory landscape created by neo-TADs in X-LAG may override the physiological regulation of GPR101 expression. We have previously reported that GPR101 is linked to the maturation of the somatotrope cell population of the pituitary during embryonic development. GPR101 expression then switches off during post-natal development, except possibly for the growth spurt phase occurring during adolescence.41 In contrast, GPR101 is strongly and consistently upregulated in X-LAG pituitary tumors that have been operated at different ages. Subsequent studies in animal models confirmed the crucial contribution of GPR101 misexpression in the hormonal regulation of body growth. Overexpression of GPR101 in the pituitary of transgenic mice leads to GH and prolactin excess and overgrowth,19 while its whole-body loss in zebrafish causes reduced body size by perturbing early embryogenesis.51 These findings indicate that precisely fine-tuning GPR101 expression both temporally (developmental stage) and spatially (specific tissues) is important for proper body growth. In X-LAG, it is conceivable that the exposure of GPR101 to the new regulatory landscape within the neo-TAD maintains active expression at high levels even after embryonic pituitary development is complete. The manifestation of the phenotype as early as the first months of life18,52 underscores the high potency of GPR101 for regulating growth via its permissive role in GH secretion. This may also provide a rationale for the normal localization of GPR101 alone in a TAD, thereby insulating it from nearby CREs. The stability across cell types and evolutionary conservation of the TAD boundary that separates GPR101 from centromeric CREs underscores the tight regulation of the normal GPR101 regulatory unit, as has been reported for other topologically isolated genes with roles in development.53 Stable boundaries are more intolerant of disruption by structural variants than unique ones,54 implying that the X-LAG locus is under strong selection. The severity of the clinical effects of gigantism in X-LAG and its extreme rarity (40 individuals described) combine to support a hypothesis that GPR101 and its surrounding regulatory environment are tightly regulated.
We functionally studied the GPR101 promoter and pituitary CREs using two in vitro models, one a human embryonic cell line (HEK293) to provide species and development stage specificity, and the other an adult rat pituitary tumor cell (GH3) to study tissue specificity. We found that the distal promoter element of GPR101 is active in an embryonic cellular context and is also compatible with adult rat pituitary tumor cells. The CRE located on the promoter region of RBMX (eRBMX) increased basal promoter activity in embryonic cells by about 9-fold. This finding is consistent with the known role of RBMX as a regulator of embryogenesis.55,56
There was no measurable transcriptional enhancing activity for any of the centromeric CREs in adult rat pituitary tumor cells. Interestingly, we also found that some CREs had an unexpected activity as silencers in the cell models, in particular the eAK055694 CRE in pituitary cells. Similar dual enhancer/silencer activities have been reported at other loci and are active in pituitary cells. Those CREs were validated in an analogous experimental setting to ours.57 Interestingly, about a quarter of human candidate silencer elements from one cell type can have dual activity and act as active enhancers in different cell types and chromatin contexts.58 This raises the intriguing possibility that some of our candidate CREs displaying transcriptional silencing in GH3 and HEK293 could actually function as enhancers in the X-LAG-affected subjects’ pituitary cells. Moreover, there has been at least one instance reported in which the relocation of a silencer into a different TAD converts it into an activator of its target gene.59
Our study has several limitations. First, we employed in vitro episomal reporter assays for the functional evaluation of predicted CREs. While episomal vectors are widely used to characterize promoters/enhancers, they inherently suffer from two main disadvantages: (1) their chromatin may have different properties and does not necessarily reflect the endogenous epigenomic chromatin state and, therefore, (2) the intrinsic enhancer activity may not fully recapitulate endogenous target gene expression.60 Second, multiple neighboring enhancers may exert cumulative activity on a single gene regulatory pathway and be part of a so-called multipartite enhancer61 or enhancer chains.62 Our reporter assays evaluated each CRE independently from the others and thus could not measure whether they acted cooperatively or influenced one another. Third, limited biochemical annotations are publicly available to aid in the identification of candidate CREs that are active in the human pituitary gland. The dataset interrogated in this study included few specimens collected postmortem in adult individuals.30 This paucity of data could have led to our missing the identification of other CREs that are active in the pituitary only in specific cell sub-types and/or at specific developmental stages. Fourth, the cell types employed for the assays may have not provided the correct cellular context. The unavailability of either embryonic or adult immortalized human somatotrope cell lines is a known limitation in the field of pituitary research.63 Specific transcription factors or coactivators may be missing or not expressed at the desired levels in the recipient cells we used, thus impairing the activity of the tested CREs.
In conclusion, we show that X-LAG is a TADopathy of the endocrine system in which chromosome Xq26.3 microduplications disrupt the local chromatin architecture forming a neo-TAD that permits ectopic contacts between the promoter of GPR101 and centromeric CREs. Enhancer adoption/hijacking within the new regulatory unit is the likely cause of the extremely high levels of misexpressed pituitary GPR101 and the subsequent tumoral GH hypersecretion that epitomizes X-LAG. Our findings raise the possibility that other unexplained forms of hormonal dysfunction in the endocrine and neuroendocrine systems could be explained by similar TADopathy-based enhancer-gene dysregulation mechanisms.
Acknowledgments
The authors thank Jacques Drouin (Laboratoire de Génétique Moléculaire, Institut de Recherches Cliniques de Montréal, Montreal, QC, Canada) for providing information concerning the ATAC-seq tracks interrogated in this study, Marit W. Vermunt and Menmo P. Creyghton (Hubrecht Institute-KNAW & University Medical Center Utrecht, Utrecht, the Netherlands) for guiding us on the use of the H3K27ac pituitary tracks, Julien Hanson (Laboratory of Molecular Pharmacology, GIGA-Molecular Biology of Diseases, University of Liège, Belgium) for discussions on orphan GPCR function and regulation, and Michela Matteoli (Laboratory of Pharmacology and Brain Pathology, Humanitas Research Hospital – IRCCS, Rozzano, Italy) for scientific guidance. The authors also thank Maria Chiara Zatelli (Section of Endocrinology, Department of Medical Sciences, University of Ferrara, Ferrara, Italy), Giovanna Mantovani (Endocrinology and Diabetology Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Department of Clinical Sciences and Community Health, University of Milan, Milan, Italy), and Catherine Choong (Department of Pediatric Endocrinology and Diabetes, Princess Margaret Hospital for Children, Subiaco, Western Australia, Australia) for providing materials for this study, and thank Lyssikatos Charalampos and Maria de la Luz Sierra (NICHD/NIH) for their help with the collection of biological samples used in this study.
The authors dedicate this paper to the memory of José Luis Gómez-Skarmeta.64,65
The work was supported by the following funding sources: Fondazione Telethon, Italy grant no. GGP20130 (to G.T.); Society for Endocrinology equipment grant (to G.T.); Intramural Research Program, Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD), National Institutes of Health (NIH) Research project Z01-HD008920 (to C.A.S., supporting G.T., F.R.F.); Fonds d’Investissement pour la Recherche Scientifique (FIRS) of the Centre Hospitalier Universitaire de Liège (to A.F.D. and A.B.); the JABBS Foundation, UK (to A.B.); and Novo Nordisk Belgium Educational Grant, Belgium (to A.F.D. and A.B.).
M.F. was funded by the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement (#800396) and a Juan de la Cierva-Formación fellowship from the Spanish Ministry of Science and Innovation (FJC2018-038233-I). G.T. was funded by the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement (#843843). A.F.D. and D.A. were supported by Action de Recherche Concertée (ARC) Grant 17/21-01 from Liège University. D.A. was supported by grants from Télévie (7461117 F, 7454719 F) and the Léon Fredericq Foundation, Belgium.
Declaration of interests
A.B., A.F.D., F.R.F., C.A.S., and G.T. hold a patent on GPR101 and its function (US Patent No. 10,350,273, Treatment of Hormonal Disorders of Growth). C.A.S. holds patents on technologies involving PRKAR1A and related genes causing adrenal, pituitary, and other tumors. In addition, his laboratory has received research funding support by Pfizer Inc. for investigations on growth-hormone producing pituitary adenomas. C.A.S. also has consulted within the last 12 months with Lundbeck Pharmaceuticals and Sync, LLC, and is currently employed by ELPEN Pharmaceuticals. A.B. and A.F.D. have received research funding from Pfizer Inc. and Novo-Nordisk. The authors declare that they have no conflicts of interest with the contents of this article.
Published: February 23, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ajhg.2022.02.002.
Data and code availability
The 4C-seq and RNA-seq datasets we generated in this manuscript have been deposited in the GEO database under the accession code GSE193114.
Web resources
3D Genome Browser, http://3dgenome.org
ClinicalTrials.gov, https://clinicaltrials.gov
FANTOM5 consortium, https://slidebase.binf.ku.dk/human_promoters/
FastQC, https://www.bioinformatics.babraham.ac.uk/projects/fastqc/
GTEx Project, https://www.gtexportal.org/home/
OMIM, https://omim.org/
Primer3, http://bioinfo.ut.ee/primer3-0.4.0/
UCSC Genome Browser, https://genome.ucsc.edu
Supplemental information
References
- 1.Lettice L.A., Heaney S.J., Purdie L.A., Li L., de Beer P., Oostra B.A., Goode D., Elgar G., Hill R.E., de Graaff E. A long-range Shh enhancer regulates expression in the developing limb and fin and is associated with preaxial polydactyly. Hum. Mol. Genet. 2003;12:1725–1735. doi: 10.1093/hmg/ddg180. [DOI] [PubMed] [Google Scholar]
- 2.Dixon J.R., Selvaraj S., Yue F., Kim A., Li Y., Shen Y., Hu M., Liu J.S., Ren B. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature. 2012;485:376–380. doi: 10.1038/nature11082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nora E.P., Lajoie B.R., Schulz E.G., Giorgetti L., Okamoto I., Servant N., Piolot T., van Berkum N.L., Meisig J., Sedat J., et al. Spatial partitioning of the regulatory landscape of the X-inactivation centre. Nature. 2012;485:381–385. doi: 10.1038/nature11049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spielmann M., Lupiáñez D.G., Mundlos S. Structural variation in the 3D genome. Nat. Rev. Genet. 2018;19:453–467. doi: 10.1038/s41576-018-0007-0. [DOI] [PubMed] [Google Scholar]
- 5.Merkenschlager M., Nora E.P. CTCF and Cohesin in Genome Folding and Transcriptional Gene Regulation. Annu. Rev. Genomics Hum. Genet. 2016;17:17–43. doi: 10.1146/annurev-genom-083115-022339. [DOI] [PubMed] [Google Scholar]
- 6.Nora E.P., Goloborodko A., Valton A.L., Gibcus J.H., Uebersohn A., Abdennur N., Dekker J., Mirny L.A., Bruneau B.G. Targeted Degradation of CTCF Decouples Local Insulation of Chromosome Domains from Genomic Compartmentalization. Cell. 2017;169:930–944.e22. doi: 10.1016/j.cell.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rao S.S., Huntley M.H., Durand N.C., Stamenova E.K., Bochkov I.D., Robinson J.T., Sanborn A.L., Machol I., Omer A.D., Lander E.S., Aiden E.L. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell. 2014;159:1665–1680. doi: 10.1016/j.cell.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gómez-Marín C., Tena J.J., Acemel R.D., López-Mayorga M., Naranjo S., de la Calle-Mustienes E., Maeso I., Beccari L., Aneas I., Vielmas E., et al. Evolutionary comparison reveals that diverging CTCF sites are signatures of ancestral topological associating domains borders. Proc. Natl. Acad. Sci. USA. 2015;112:7542–7547. doi: 10.1073/pnas.1505463112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vietri Rudan M., Barrington C., Henderson S., Ernst C., Odom D.T., Tanay A., Hadjur S. Comparative Hi-C reveals that CTCF underlies evolution of chromosomal domain architecture. Cell Rep. 2015;10:1297–1309. doi: 10.1016/j.celrep.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franke M., Ibrahim D.M., Andrey G., Schwarzer W., Heinrich V., Schöpflin R., Kraft K., Kempfer R., Jerković I., Chan W.L., et al. Formation of new chromatin domains determines pathogenicity of genomic duplications. Nature. 2016;538:265–269. doi: 10.1038/nature19800. [DOI] [PubMed] [Google Scholar]
- 11.Lettice L.A., Daniels S., Sweeney E., Venkataraman S., Devenney P.S., Gautier P., Morrison H., Fantes J., Hill R.E., FitzPatrick D.R. Enhancer-adoption as a mechanism of human developmental disease. Hum. Mutat. 2011;32:1492–1499. doi: 10.1002/humu.21615. [DOI] [PubMed] [Google Scholar]
- 12.Weischenfeldt J., Dubash T., Drainas A.P., Mardin B.R., Chen Y., Stütz A.M., Waszak S.M., Bosco G., Halvorsen A.R., Raeder B., et al. Pan-cancer analysis of somatic copy-number alterations implicates IRS4 and IGF2 in enhancer hijacking. Nat. Genet. 2017;49:65–74. doi: 10.1038/ng.3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willemin A., Lopez-Delisle L., Bolt C.C., Gadolini M.L., Duboule D., Rodriguez-Carballo E. Induction of a chromatin boundary in vivo upon insertion of a TAD border. PLoS Genet. 2021;17:e1009691. doi: 10.1371/journal.pgen.1009691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lupiáñez D.G., Kraft K., Heinrich V., Krawitz P., Brancati F., Klopocki E., Horn D., Kayserili H., Opitz J.M., Laxova R., et al. Disruptions of topological chromatin domains cause pathogenic rewiring of gene-enhancer interactions. Cell. 2015;161:1012–1025. doi: 10.1016/j.cell.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang M., Soomro A., Tasneem S., Abatti L.E., Alizada A., Yuan X., Uusküla-Reimand L., Antounians L., Alvi S.A., Paterson A.D., et al. Enhancer-gene rewiring in the pathogenesis of Quebec platelet disorder. Blood. 2020;136:2679–2690. doi: 10.1182/blood.2020005394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trivellin G., Daly A.F., Faucz F.R., Yuan B., Rostomyan L., Larco D.O., Schernthaner-Reiter M.H., Szarek E., Leal L.F., Caberg J.H., et al. Gigantism and acromegaly due to Xq26 microduplications and GPR101 mutation. N. Engl. J. Med. 2014;371:2363–2374. doi: 10.1056/NEJMoa1408028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trivellin G., Faucz F.R., Daly A.F., Beckers A., Stratakis C.A. HEREDITARY ENDOCRINE TUMOURS: CURRENT STATE-OF-THE-ART AND RESEARCH OPPORTUNITIES: GPR101, an orphan GPCR with roles in growth and pituitary tumorigenesis. Endocr. Relat. Cancer. 2020;27:T87–T97. doi: 10.1530/ERC-20-0025. [DOI] [PubMed] [Google Scholar]
- 18.Beckers A., Lodish M.B., Trivellin G., Rostomyan L., Lee M., Faucz F.R., Yuan B., Choong C.S., Caberg J.H., Verrua E., et al. X-linked acrogigantism syndrome: clinical profile and therapeutic responses. Endocr. Relat. Cancer. 2015;22:353–367. doi: 10.1530/ERC-15-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abboud D., Daly A.F., Dupuis N., Bahri M.A., Inoue A., Chevigné A., Ectors F., Plenevaux A., Pirotte B., Beckers A., Hanson J. GPR101 drives growth hormone hypersecretion and gigantism in mice via constitutive activation of Gs and Gq/11. Nat. Commun. 2020;11:4752. doi: 10.1038/s41467-020-18500-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van de Werken H.J., de Vree P.J., Splinter E., Holwerda S.J., Klous P., de Wit E., de Laat W. 4C technology: protocols and data analysis. Methods Enzymol. 2012;513:89–112. doi: 10.1016/B978-0-12-391938-0.00004-5. [DOI] [PubMed] [Google Scholar]
- 21.Splinter E., de Wit E., van de Werken H.J., Klous P., de Laat W. Determining long-range chromatin interactions for selected genomic sites using 4C-seq technology: from fixation to computation. Methods. 2012;58:221–230. doi: 10.1016/j.ymeth.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Noordermeer D., Leleu M., Schorderet P., Joye E., Chabaud F., Duboule D. Temporal dynamics and developmental memory of 3D chromatin architecture at Hox gene loci. eLife. 2014;3:e02557. doi: 10.7554/eLife.02557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y., Song F., Zhang B., Zhang L., Xu J., Kuang D., Li D., Choudhary M.N.K., Li Y., Hu M., et al. The 3D Genome Browser: a web-based browser for visualizing 3D genome organization and long-range chromatin interactions. Genome Biol. 2018;19:151. doi: 10.1186/s13059-018-1519-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dixon J.R., Jung I., Selvaraj S., Shen Y., Antosiewicz-Bourget J.E., Lee A.Y., Ye Z., Kim A., Rajagopal N., Xie W., et al. Chromatin architecture reorganization during stem cell differentiation. Nature. 2015;518:331–336. doi: 10.1038/nature14222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haeussler M., Zweig A.S., Tyner C., Speir M.L., Rosenbloom K.R., Raney B.J., Lee C.M., Lee B.T., Hinrichs A.S., Gonzalez J.N., et al. The UCSC Genome Browser database: 2019 update. Nucleic Acids Res. 2019;47(D1):D853–D858. doi: 10.1093/nar/gky1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hinrichs A.S., Karolchik D., Baertsch R., Barber G.P., Bejerano G., Clawson H., Diekhans M., Furey T.S., Harte R.A., Hsu F., et al. The UCSC Genome Browser Database: update 2006. Nucleic Acids Res. 2006;34:D590–D598. doi: 10.1093/nar/gkj144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leung D., Jung I., Rajagopal N., Schmitt A., Selvaraj S., Lee A.Y., Yen C.A., Lin S., Lin Y., Qiu Y., et al. Integrative analysis of haplotype-resolved epigenomes across human tissues. Nature. 2015;518:350–354. doi: 10.1038/nature14217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmitt A.D., Hu M., Jung I., Xu Z., Qiu Y., Tan C.L., Li Y., Lin S., Lin Y., Barr C.L., Ren B. A Compendium of Chromatin Contact Maps Reveals Spatially Active Regions in the Human Genome. Cell Rep. 2016;17:2042–2059. doi: 10.1016/j.celrep.2016.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta R., Bhattacharyya A., Agosto-Perez F.J., Wickramasinghe P., Davuluri R.V. MPromDb update 2010: an integrated resource for annotation and visualization of mammalian gene promoters and ChIP-seq experimental data. Nucleic Acids Res. 2011;39:D92–D97. doi: 10.1093/nar/gkq1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vermunt M.W., Reinink P., Korving J., de Bruijn E., Creyghton P.M., Basak O., Geeven G., Toonen P.W., Lansu N., Meunier C., et al. Netherlands Brain Bank Large-scale identification of coregulated enhancer networks in the adult human brain. Cell Rep. 2014;9:767–779. doi: 10.1016/j.celrep.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 31.Mayran A., Khetchoumian K., Hariri F., Pastinen T., Gauthier Y., Balsalobre A., Drouin J. Pioneer factor Pax7 deploys a stable enhancer repertoire for specification of cell fate. Nat. Genet. 2018;50:259–269. doi: 10.1038/s41588-017-0035-2. [DOI] [PubMed] [Google Scholar]
- 32.Fishilevich S., Nudel R., Rappaport N., Hadar R., Plaschkes I., Iny Stein T., et al. GeneHancer: genome-wide integration of enhancers and target genes in GeneCards. Database (Oxford) 2017;2017:bax028. doi: 10.1093/database/bax028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bolger A.M., Lohse M., Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dobin A., Davis C.A., Schlesinger F., Drenkow J., Zaleski C., Jha S., Batut P., Chaisson M., Gingeras T.R. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mains R.E., Blaby-Haas C., Rheaume B.A., Eipper B.A. Changes in Corticotrope Gene Expression Upon Increased Expression of Peptidylglycine α-Amidating Monooxygenase. Endocrinology. 2018;159:2621–2639. doi: 10.1210/en.2018-00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soneson C., Delorenzi M. A comparison of methods for differential expression analysis of RNA-seq data. BMC Bioinformatics. 2013;14:91. doi: 10.1186/1471-2105-14-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anders S., Pyl P.T., Huber W. HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31:166–169. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou Y., Zhou B., Pache L., Chang M., Khodabakhshi A.H., Tanaseichuk O., Benner C., Chanda S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019;10:1523. doi: 10.1038/s41467-019-09234-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.GTEx Consortium The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science. 2020;369:1318–1330. doi: 10.1126/science.aaz1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trivellin G., Bjelobaba I., Daly A.F., Larco D.O., Palmeira L., Faucz F.R., Thiry A., Leal L.F., Rostomyan L., Quezado M., et al. Characterization of GPR101 transcript structure and expression patterns. J. Mol. Endocrinol. 2016;57:97–111. doi: 10.1530/JME-16-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pan J.B., Hu S.C., Shi D., Cai M.C., Li Y.B., Zou Q., Ji Z.L. PaGenBase: a pattern gene database for the global and dynamic understanding of gene function. PLoS ONE. 2013;8:e80747. doi: 10.1371/journal.pone.0080747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trivellin G., Hernández-Ramírez L.C., Swan J., Stratakis C.A. An orphan G-protein-coupled receptor causes human gigantism and/or acromegaly: Molecular biology and clinical correlations. Best Pract. Res. Clin. Endocrinol. Metab. 2018;32:125–140. doi: 10.1016/j.beem.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 44.Forrest A.R., Kawaji H., Rehli M., Baillie J.K., de Hoon M.J., Haberle V., Lassmann T., Kulakovskiy I.V., Lizio M., Itoh M., et al. FANTOM Consortium and the RIKEN PMI and CLST (DGT) A promoter-level mammalian expression atlas. Nature. 2014;507:462–470. doi: 10.1038/nature13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beckers A., Petrossians P., Hanson J., Daly A.F. The causes and consequences of pituitary gigantism. Nat. Rev. Endocrinol. 2018;14:705–720. doi: 10.1038/s41574-018-0114-1. [DOI] [PubMed] [Google Scholar]
- 46.Rostomyan L., Daly A.F., Petrossians P., Nachev E., Lila A.R., Lecoq A.L., Lecumberri B., Trivellin G., Salvatori R., Moraitis A.G., et al. Clinical and genetic characterization of pituitary gigantism: an international collaborative study in 208 patients. Endocr. Relat. Cancer. 2015;22:745–757. doi: 10.1530/ERC-15-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weischenfeldt J., Symmons O., Spitz F., Korbel J.O. Phenotypic impact of genomic structural variation: insights from and for human disease. Nat. Rev. Genet. 2013;14:125–138. doi: 10.1038/nrg3373. [DOI] [PubMed] [Google Scholar]
- 48.de Bruijn S.E., Fiorentino A., Ottaviani D., Fanucchi S., Melo U.S., Corral-Serrano J.C., Mulders T., Georgiou M., Rivolta C., Pontikos N., et al. Structural Variants Create New Topological-Associated Domains and Ectopic Retinal Enhancer-Gene Contact in Dominant Retinitis Pigmentosa. Am. J. Hum. Genet. 2020;107:802–814. doi: 10.1016/j.ajhg.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Melo U.S., Schöpflin R., Acuna-Hidalgo R., Mensah M.A., Fischer-Zirnsak B., Holtgrewe M., Klever M.K., Türkmen S., Heinrich V., Pluym I.D., et al. Hi-C Identifies Complex Genomic Rearrangements and TAD-Shuffling in Developmental Diseases. Am. J. Hum. Genet. 2020;106:872–884. doi: 10.1016/j.ajhg.2020.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aavikko M., Kaasinen E., Andersson N., Pentinmikko N., Sulo P., Donner I., Pihlajamaa P., Kuosmanen A., Bramante S., Katainen R., et al. WNT2 activation through proximal germline deletion predisposes to small intestinal neuroendocrine tumors and intestinal adenocarcinomas. Hum. Mol. Genet. 2021;30:2429–2440. doi: 10.1093/hmg/ddab206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trivellin G., Tirosh A., Hernández-Ramírez L.C., Gupta T., Tsai-Morris C.H., Faucz F.R., Burgess H.A., Feldman B., Stratakis C.A. The X-linked acrogigantism-associated gene gpr101 is a regulator of early embryonic development and growth in zebrafish. Mol. Cell. Endocrinol. 2021;520:111091. doi: 10.1016/j.mce.2020.111091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wise-Oringer B.K., Zanazzi G.J., Gordon R.J., Wardlaw S.L., William C., Anyane-Yeboa K., Chung W.K., Kohn B., Wisoff J.H., David R., Oberfield S.E. Familial X-Linked Acrogigantism: Postnatal Outcomes and Tumor Pathology in a Prenatally Diagnosed Infant and His Mother. J. Clin. Endocrinol. Metab. 2019;104:4667–4675. doi: 10.1210/jc.2019-00817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu H.J., Landshammer A., Stamenova E.K., Bolondi A., Kretzmer H., Meissner A., Michor F. Topological isolation of developmental regulators in mammalian genomes. Nat. Commun. 2021;12:4897. doi: 10.1038/s41467-021-24951-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McArthur E., Capra J.A. Topologically associating domain boundaries that are stable across diverse cell types are evolutionarily constrained and enriched for heritability. Am. J. Hum. Genet. 2021;108:269–283. doi: 10.1016/j.ajhg.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dichmann D.S., Fletcher R.B., Harland R.M. Expression cloning in Xenopus identifies RNA-binding proteins as regulators of embryogenesis and Rbmx as necessary for neural and muscle development. Dev. Dyn. 2008;237:1755–1766. doi: 10.1002/dvdy.21590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsend-Ayush E., O’Sullivan L.A., Grützner F.S., Onnebo S.M., Lewis R.S., Delbridge M.L., Marshall Graves J.A., Ward A.C. RBMX gene is essential for brain development in zebrafish. Dev. Dyn. 2005;234:682–688. doi: 10.1002/dvdy.20432. [DOI] [PubMed] [Google Scholar]
- 57.Daly A.Z., Dudley L.A., Peel M.T., Liebhaber S.A., Parker S.C.J., Camper S.A. Multi-omic profiling of pituitary thyrotropic cells and progenitors. BMC Biol. 2021;19:76. doi: 10.1186/s12915-021-01009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Doni Jayavelu N., Jajodia A., Mishra A., Hawkins R.D. Candidate silencer elements for the human and mouse genomes. Nat. Commun. 2020;11:1061. doi: 10.1038/s41467-020-14853-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Galupa R., Nora E.P., Worsley-Hunt R., Picard C., Gard C., van Bemmel J.G., Servant N., Zhan Y., El Marjou F., Johanneau C., et al. A Conserved Noncoding Locus Regulates Random Monoallelic Xist Expression across a Topological Boundary. Mol. Cell. 2020;77:352–367.e8. doi: 10.1016/j.molcel.2019.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gasperini M., Tome J.M., Shendure J. Towards a comprehensive catalogue of validated and target-linked human enhancers. Nat. Rev. Genet. 2020;21:292–310. doi: 10.1038/s41576-019-0209-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Will A.J., Cova G., Osterwalder M., Chan W.L., Wittler L., Brieske N., Heinrich V., de Villartay J.P., Vingron M., Klopocki E., et al. Composition and dosage of a multipartite enhancer cluster control developmental expression of Ihh (Indian hedgehog) Nat. Genet. 2017;49:1539–1545. doi: 10.1038/ng.3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Song W., Sharan R., Ovcharenko I. The first enhancer in an enhancer chain safeguards subsequent enhancer-promoter contacts from a distance. Genome Biol. 2019;20:197. doi: 10.1186/s13059-019-1808-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu Z., Cui W., Zhu D., Gao N., Zhu Y. Common tools for pituitary adenomas research: cell lines and primary cells. Pituitary. 2020;23:182–188. doi: 10.1007/s11102-019-01003-4. [DOI] [PubMed] [Google Scholar]
- 64.Bogdanovic O., Irimia M. José Luis Gómez-Skarmeta (1966–2020) Nat. Genet. 2020;52:1267–1268. [Google Scholar]
- 65.Casares F., Allende M., de Celis J.F., Gónzalez-Reyes A., Martínez-Morales J.R. José Luis Gómez-Skarmeta (1966-2020) Development. 2020;147:147. doi: 10.1242/dev.197996. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The 4C-seq and RNA-seq datasets we generated in this manuscript have been deposited in the GEO database under the accession code GSE193114.