This genetic association study investigates the genetic liability of psychiatric and immune disorders using population-scale data.
Key Points
Questions
What does knowledge of the human genome reveal about directional associations between psychiatric and immune-related disorders, as well as the role of hypothesized confounding or mediating factors?
Findings
In this genetic association study of 44 significant psychiatric-immune genetic correlations, bidirectional 2-sample and multivariable mendelian randomization identified 7 associations of psychiatric liability with increased immune-related liability after sensitivity testing and adjustment for genetic outcomes of other factors.
Meaning
Results of this genetic association study suggest that the predominant associations underlying large-scale epidemiologic patterns originate from psychiatric exposure variables, although there were also strong associations with correlated risk factors, the influence of which cannot be fully excluded.
Abstract
Importance
Certain psychiatric and immune-related disorders are reciprocal risk factors. However, the nature of these associations is unclear.
Objective
To characterize the pleiotropy between psychiatric and immune-related traits, as well as risk factors of hypothesized relevance.
Design, Setting, and Participants
This genetic association study was conducted from July 10, 2020, to January 15, 2022. Analyses used genome-wide association (GWA) statistics related to 14 psychiatric traits; 13 immune-related phenotypes, ie, allergic, autoimmune, and inflammatory disorders; and 15 risk factors related to health-related behaviors, social determinants of health, and stress response. Genetically correlated psychiatric-immune pairs were assessed using 2-sample mendelian randomization (MR) with sensitivity analyses and multivariable adjustment for genetic associations of third variables. False discovery rate correction (Q value < .05) was applied for each analysis.
Exposures
Genetic associations.
Main Outcomes and Measures
Genetic correlations and MR association estimates with SEs and P values. A data-driven approach was used that did not test a priori planned hypotheses.
Results
A total of 44 genetically correlated psychiatric-immune pairs were identified, including 31 positive correlations (most consistently involving asthma, Crohn disease, hypothyroidism, and ulcerative colitis) and 13 negative correlations (most consistently involving allergic rhinitis and type 1 diabetes). Correlations with third variables were especially strong for psychiatric phenotypes. MR identified 7 associations of psychiatric phenotypes on immune-related phenotypes that were robust to multivariable adjustment, including the positive association of (1) the psychiatric cross-disorder phenotype with asthma (odds ratio [OR], 1.04; 95% CI, 1.02-1.06), Crohn disease (OR, 1.09; 95% CI, 1.05-1.14), and ulcerative colitis (OR, 1.09; 95% CI, 1.05-1.14); (2) major depression with asthma (OR, 1.25; 95% CI, 1.13-1.37); (3) schizophrenia with Crohn disease (OR, 1.12; 95% CI, 1.05-1.18) and ulcerative colitis (OR, 1.14; 95% CI, 1.07-1.21); and a negative association of risk tolerance with allergic rhinitis (OR, 0.77; 95% CI, 0.67-0.92).
Conclusions and Relevance
Results of this genetic association study suggest that genetic liability for psychiatric disorders was associated with liability for several immune disorders, suggesting that vertical pleiotropy related to behavioral traits (or correlated third variables) contributes to clinical associations observed in population-scale data.
Introduction
Large-scale prospective and retrospective studies found that certain immune-related disorders increase the risk for psychiatric disorders, and vice versa.1,2,3,4 It is challenging to elucidate the basis for these associations within population-scale data, in part owing to unmeasured confounding or mediating factors. Characterization of shared psychiatric-immune genetic liability may provide epidemiologic insights. Several prior studies using genome-wide association study (GWAS) data found shared liability between the major psychoses and immune-related bowel and biliary disorders5,6,7 and between depression and asthma.8,9
Shared genetic liability can be explained by multiple coexisting mechanisms. Examples of horizonal pleiotropy include scenarios in which genetic factors act through a common biological mechanism to partially influence both disorders or in which genetic proximity links 2 distinct mechanisms. Vertical pleiotropy involves 1 phenotype exerting a direct effect on the other. Multiple lines of evidence identified mechanisms through which immunologic cells, signaling, and effector molecules have the potential to exert effects on behavioral traits.10,11 By contrast, there is also evidence that acute and chronic stress may contribute to the onset and exacerbation of symptoms and histopathological correlates for several immune-related disorders12,13,14; these may be partially transmitted through disturbances in autonomic, neuroendocrine, and inflammatory signaling.15,16
Leveraging 2-sample mendelian randomization (MR),17 several studies reported directional effects for pairs of psychiatric and immune-related phenotypes,5,8,9 including positive effects of schizophrenia on systemic lupus erythematosus and immune-mediated bowel and biliary diseases5 and positive effects of major depression and alcohol use on asthma.8,9 On the other hand, several MR studies found nonsignificant effects pertaining to certain psychiatric-immune comorbidities.18,19,20,21 Another possibility is that shared liability is influenced by a correlated third variable with associations with one or both phenotypes. Many risk factors are associated with both psychiatric and immune-related disorders, briefly reviewed within the eMethods of Supplement 1. For risk factors that have well-powered GWAS data and significant heritability, multivariable MR (MVMR) can be used to assess directional associations while adjusting for genetic associations with other risk factors.22 Accordingly, we performed a comprehensive assessment of the genetic inference associations linking psychiatric and immune-related phenotypes while adjusting for the association of other factors hypothesized to exert confounding or mediating associations. Additionally, we characterized relevant loci, genes, tissues, cell types, and biological functions related to the observed psychiatric-immune associations.
Methods
Data Selection
This genetic association study was conducted from July 10, 2020, to January 15, 2022. To find GWA summary statistics, we searched the published literature (PubMed, Scopus), data repositories (dbGaP, GWAS ATLAS [https://atlas.ctglab.nl], GWAS catalogue [https://www.ebi.ac.uk/gwas], opentargets.org), and the Psychiatric Genomics Consortium (PGC) website (https://www.med.unc.edu/pgc/). We sought data from studies with the largest available European ancestry owing to the limited availability of well-powered GWA data from other ancestry groups. Detailed methods for this study are summarized in eFigure 1 in Supplement 1. We used deidentified GWAS statistics and were granted exemption from obtaining informed consent by the Yale University institutional review board. For all data, we removed the extended major histocompatibility complex region, and single nucleotide variations (SNVs) were filtered based on a minor allele frequency of 1% or greater within phase 3 of the 1000 Genomes European reference panel and, when available, an imputation imputation quality score of 0.9 or greater. We used the linkage disequilibrium score regression (LDSC) method using default quality control settings to estimate the SNV heritability coefficient (h2) and retained those with an heritability z score greater than 4.23 Phenotype selection is described in the eMethods in Supplement 1. The final set included 14 psychiatric and 13 immune-related phenotypes (Table and eTable 1 in Supplement 2). To investigate shared liability across the psychopathology, we included GWAS statistics derived from the PGC cross disorder (PGC-CD) analysis.25 Based on a literature search, we included a representative, but not exhaustive, set of 15 risk factors that could possibly confound or mediate observed associations (Table). For simplicity, we refer to these as third-variable risk factors, and we discuss the rationale in the eMethods in Supplement 1. We investigated neuroticism as a proxy relevant to the stress response, based on associations with negative affective states, stress biomarkers,26 multiple psychiatric disorders,27 and physical health problems.28 We also included thyrotropin29 for associations involving hypothyroidism.
Table. Investigated Genome-Wide Association Study Data Sets.
| Phenotype name | Sample size | Quality control + SNV | Heritability (SE) | Heritability z score | PubMed Identification No. |
|---|---|---|---|---|---|
| Attention-deficit/ hyperactivity disorder | 53 293 | 1098642 | 0.37 (0.02) | 15.4 | 30478444 |
| Anorexia nervosaa | 72 517 | 1094901 | 0.18 (0.01) | 15.2 | 31308545 |
| Bipolar disorder | 51 710 | 1098642 | 0.35 (0.02) | 13.6 | 31043756 |
| Cross disordera | 107 785 | 1094901 | 0.18 (0.01) | 19.9 | 31835028 |
| Cannabis use disordera | 357 806 | 1109803 | 0.02 (0) | 25.7 | 33096046 |
| Generalized anxiety disordera,b | 175 163 | 1094901 | 0.06 (0) | 10.8 | 31906708 |
| Major depressive disorder | 500 199 | 1156949 | 0.06 (0) | 25.0 | 30718901 |
| Neuroticism, personalitya,b | 168 105 | 1168849 | 0.09 (0.01) | 14.3 | 29292387 |
| Obsessive compulsive disordera | 9725 | 1163150 | 0.35 (0.05) | 7.4 | 28761083 |
| Opioid use disorder | 82 707 | 644417 | 0.05 (0.01) | 6.1 | 32492095 |
| Problematic alcohol use | 453 563 | 651122 | 0.07 (0) | 19.8 | 32451486 |
| Posttraumatic stress disordera | 174 659 | 1169663 | 0.02 (0) | 5.5 | 31594949 |
| Risk tolerance, personalityb | 315 894 | 1177066 | 0.11 (0) | 26.4 | 30643258 |
| Schizophrenia | 105 318 | 1143915 | 0.41 (0.01) | 29.5 | 29483656 |
| Tourette syndrome | 14 307 | 1160750 | 0.35 (0.04) | 8.4 | 30818990 |
| Allergic rhinitis | 289 307 | 1129988 | 0.03 (0) | 9.6 | 31427789 |
| Asthma | 385 822 | 1129988 | 0.05 (0) | 11.5 | 31427789 |
| Atopic dermatitis | 40 835 | 1165234 | 0.07 (0.02) | 4.3 | 26482879 |
| Celiac diseasea | 15 283 | 279852 | 0.31 (0.05) | 6.3 | 20190752 |
| Crohn disease | 40 226 | 1144031 | 0.42 (0.04) | 9.5 | 28067908 |
| Hypothyroidism | 244 890 | 1137440 | 0.04 (0) | 10.4 | 31427789 |
| Primary biliary cholangitis | 13 239 | 1026464 | 0.38 (0.06) | 6.4 | 26394269 |
| Primary sclerosing cholangitisa | 14 890 | 730668 | 0.24 (0.05) | 4.6 | 27992413 |
| Rheumatoid arthritis | 58 284 | 1169105 | 0.14 (0.02) | 8.0 | 24390342 |
| Systemic lupus erythematosus | 23 210 | 1171324 | 0.25 (0.04) | 5.7 | 26502338 |
| Type 1 diabetes | 26 890 | 872453 | 0.17 (0.03) | 5.3 | 21980299 |
| Ulcerative colitis | 45 975 | 1144091 | 0.25 (0.02) | 10.8 | 28067908 |
| Vitiligo | 44 266 | 1088792 | 0.18 (0.02) | 8.6 | 27723757 |
| Body mass index | 806 834 | 1182634 | 0.19 (0.01) | 36.9 | 30239722 |
| Cigarettes/d | 263 954 | 1172680 | 0.07 (0.01) | 10.6 | 30643251 |
| Cognitive processing | 257 828 | 1167777 | 0.19 (0.01) | 31.0 | 30038396 |
| Drinks/wk, alcoholic | 537 349 | 1172598 | 0.05 (0) | 32.1 | 30643251 |
| Educational attainment | 766 345 | 1172989 | 0.11 (0) | 41.0 | 30038396 |
| Exercise frequency | 367 908 | 1129988 | 0.04 (0) | 18.0 | 31427789 |
| Heart rate | 361 411 | 1115092 | 0.13 (0.01) | 17.4 | 31427789 |
| Heart rate increase with exercise | 58 818 | 1150892 | 0.03 (0) | 9.5 | 29497042 |
| Heart rate recovery after exercise | 58 818 | 1150892 | 0.03 (0) | 8.8 | 29497042 |
| Heart rate variability | 28 122 | 1010511 | 0.11 (0.02) | 4.8 | 28613276 |
| Income | 332 594 | 1129988 | 0.06 (0) | 20.0 | 31427789 |
| Sleep duration | 446 118 | 1167152 | 0.07 (0) | 23.0 | 30846698 |
| Neighborhood social deprivation | 420 035 | 1158094 | 0.03 (0) | 19.3 | 27818178 |
| Social interaction frequency | 383 941 | 1129988 | 0.03 (0) | 16.4 | 31427789 |
| Thyrotropin | 119 715 | 1165231 | 0.11 (0.02) | 6.9 | 32769997 |
Abbreviation: SNV, single nucleotide variation.
For these data sets, there were less than 15 independent loci available at a threshold of P < 5 × 10−8; therefore, the threshold for mendelian randomization instrument SNV selection was relaxed to P < 1 × 10−5.
Three continuous phenotypes: the personality trait of neuroticism, another dispositional trait reflecting risk tolerance, and generalized anxiety disorder, were assessed based on the Generalized Anxiety Disorder 2-item score,24 a 2-item self-report assessment of the frequency of worrying and related physical sensations.
Genetic Correlations via LDSC
LDSC30 was used with default settings on SNVs to assess pair-wise genetic correlations between immune-related and psychiatric phenotypes and with the third variables (eMethods in Supplement 1) resulting in a total of 587 tests. A false discovery rate (FDR) approach was chosen for multiple testing correction in light of the correlated nature of the observations.31 Genetically correlated pairs (FDR Q value < .05) were interrogated with bidirectional and MVMR as described. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization (STROBE-MR) reporting guidelines.32
Mendelian Randomization
We reviewed the assumptions and limitations of MR methods within the eMethods in Supplement 1. Briefly, the software package TwoSampleMR33 was used within the R computing environment to define genetic instruments using a genome-wide threshold (P < 5 × 10−8), but this threshold was relaxed (P < 1 × 10−5) in instances with less than 15 independent loci. We removed possible outlier instruments. We primarily evaluated inverse variance–weighted models, but we also verified associations in Egger models where relevant (eTable 3 in Supplement 4). We performed single SNV, heterogeneity, and leave-one-out sensitivity, and we also repeated analyses with the software package mr.raps,34 which provides additional adjustment for weak instrument and polygenic phenotypes. In rare instances, we observed statistical evidence of sample overlap among our significant associations, and we re-evaluated the outcomes using the MRlap package, which incorporates additional adjustment based on the LDSC covariance intercept.35 We used the MVMR package22 to reassess psychiatric-immune associations after adjustment for each individual third variable.
Characterization of Loci
Enrichment analyses are described in detail within the eMethods in Supplement 1. Briefly, for the 6 positive MR outcomes (FDR Q value < .05 with consistent sensitivity and MVMR results), we identified relevant instrument loci (ie, single SNV MR uncorrected P < .05), mapped their coordinates, and merged overlapping loci of the same exposure category (ie, psychiatric or immune). We used the Multimarker Analysis of GenoMic Annotation implemented in Functional Mapping and Annotation (FUMA)36 to identify nearby genes, 10-kB window (enrichment FDR Q value < .05). We subsequently tested for enrichment among the various sets of ontologies accessed through the GENE2FUNC analysis.37
For 6 psychiatric-immune pairs, we performed a 2-sided meta-analysis identifying loci with concordant and discordant directional associations.38 We used FUMA’s SNP2GENE process to assess each subset for evidence of enrichment among 54 tissues (GTEx, version 8, database [GTEx Portal]) and various ontological sets. We also used FUMA’s cell-type enrichment analysis (selecting all available human single-cell RNA-seq reference data set),37,39 and we performed Multimarker Analysis of GenoMic Annotation–based gene-set enrichment analysis (default settings). FDR correction was applied for each reference database. Two-tailed z tests was used to assess significant differences in enrichment score between respective concordant and discordant SNV subsets.
Results
Genetic Correlations and Factor Analysis
A total of 44 genetically correlated psychiatric-immune pairs were identified (FDR Q value < .05) (Figure 1), involving 13 psychiatric and 12 immune-related phenotypes. A total of 31 positive genetic correlations were observed, most consistently involving asthma, Crohn disease, hypothyroidism, and ulcerative colitis (UC) and primary biliary cholangitis in relation to multiple psychiatric traits, including depression, anxiety, the major psychoses, and substance use disorders. Positive correlations with hypothyroidism appeared to exclude the major psychoses. Conversely, we observed 13 exclusively negative genetic correlations in relation to allergic rhinitis (AR), primary sclerosing cholangitis, and type 1 diabetes. We also observed 115 significant correlations between some of the third variables and psychiatric phenotypes, with a Pearson genetic correlation coefficient, rg, (SE) of 0.22 (0.15) and 48 involving the immune-related phenotypes, with a Pearson rg (SE) of 0.13 (0.05) (eFigure 2 in Supplement 1 and eTable 2 in Supplement 3).
Figure 1. Genetic Correlations Among Psychiatric and Immune-Related Phenotypes.
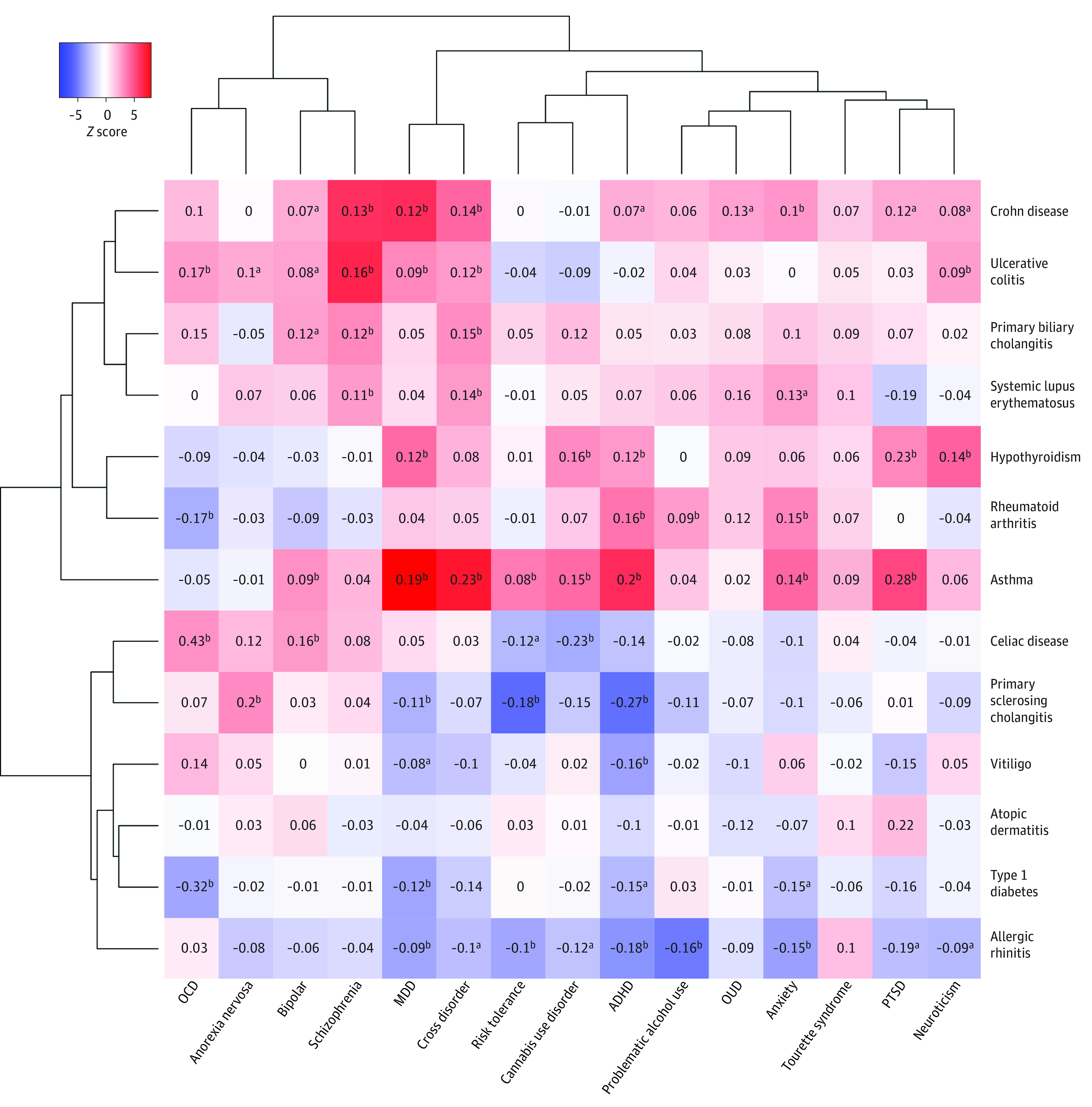
Genetic correlations between psychiatric (x-axis) and immune-related (y-axis) phenotypes with correlation z score reflected in the intensity (red, positive; blue, negative) colors. Phenotypes are clustered hierarchically along both axes based on z scores. Full results, including correlations with covariate phenotypes, are provided in eFigure 2 in Supplement 1 and eTable 2 in Supplement 3. ADHD indicates attention-deficit/hyperactivity disorder; MDD, major depressive disorder; OCD, obsessive-compulsive disorder; OUD, opioid use disorder; PTSD, posttraumatic stress disorder.
aDenotes uncorrected P < .05.
bDenotes false discovery rate Q score < .05.
Mendelian Randomization
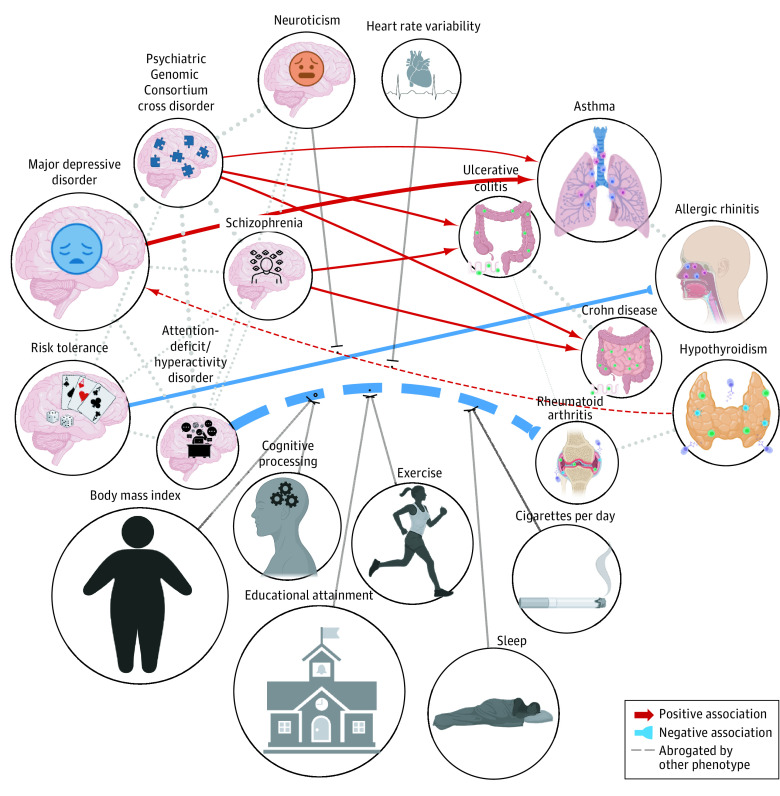
Bidirectional MR analyses revealed 9 associations surviving multiple testing correction (FDR Q value < .05) (Figure 2 and eTable 3 in Supplement 4), including attention-deficit/hyperactivity disorder (ADHD) with rheumatoid arthritis (RA; odds ratio [OR], 0.12; 95% CI, 0-0.23; P = 3.3 × 10−3; FDR Q value = 4.1 × 10−2); PGC-CD with asthma (OR, 1.04; 95% CI, 1.02-1.06; P = 5.0 × 10−4; FDR Q value = 8.0 × 10−3), PGC-CD with Crohn disease (OR, 1.09; 95% CI, 1.05-1.14; P = 3.7 × 10−3; FDR Q value = 7.4 × 10−3), PGC-CD with UC (OR, 1.09; 95% CI, 1.05-1.14; P = 1.9 × 10−5; FDR Q value = 9.4 × 10−4), hypothyroidism with major depressive disorder (MDD; OR, 1.03; 95% CI, 1.01-1.05; P = 1.4 × 10−3; FDR Q value = 2.3 × 10−2), MDD with asthma (OR, 1.25; 95% CI, 1.13-1.37; P = 3.0 × 10−5; FDR Q value = 1.0 × 10−3), risk tolerance with AR (OR, 0.77; 95% CI, 0.67-0.92; P = 6.8 × 10−3; FDR Q value = 8.0 × 10−3), and schizophrenia with Crohn disease (OR, 1.12; 95% CI, 1.05-1.18; P = 5.6 × 10−5; FDR Q value = 1.9 × 10−3), and UC (OR, 1.14; 95% CI, 1.07-1.21; P = 1.4 × 10−3; FDR Q value = 1.0 × 10−5). Six associations still showed significant heterogeneity among instrument SNVs despite the removal of outliers (eTable 3 in Supplement 4). Eight of the associations were consistent using mr.raps and were robust in leave-one-out analyses, with the exception of ADHD with RA. ADHD was observed to have low instrument quality (eTable 3 in Supplement 4). Seven of the outcomes remained significant with third-variable associations (eFigure 3 in Supplement 1 and eTable 3 in Supplement 4). The association of ADHD with RA was attenuated by 8 different risk factors (Figure 2). Notably, body mass index, cognitive processing, and educational attainment showed evidence for association with both RA and ADHD, and cigarettes per day, and neuroticism showed associations with ADHD (eTable 3 in Supplement 4). Additionally, an association of hypothyroidism with MDD was attenuated with the inclusion of heart rate variability, or neuroticism, although we did not observe evidence supporting their associations with hypothyroidism or MDD (eTable 3 in Supplement 4). We observed positive LDSC intercepts for 3 of the robust MR outcomes (eTable 2 in Supplement 3), suggestive of sample overlap, but the MRlap adjustment for nonindependent samples produced MR associations that were not significantly different for PGC-CD on Crohn disease and on UC, and they showed a significantly more positive association of MDD with asthma (eTable 3 in Supplement 4). All MVMR associations are reported in eTable 3 in Supplement 4.
Figure 2. Mendelian Randomization (MR) Associations Linking Psychiatric and Immune-Related Traits After Adjustment for Other Risk Factors.
MR associations between psychiatric and immune-related phenotypes that survived multiple test correction (false discovery rate Q score < .05). Node size is proportional to the genome-wide association study sample size. Images used were licensed or used with permission from multiple sources, including BioRender, Artist: Vectorwin, Alamy.com; Artist: Gan Khoon Lay, Iconfinder.com.
Characterization of MR Loci
Among the 6 robust positive MR associations of psychiatric traits with immunologic traits, we identified 82 nonoverlapping loci with single SNV associations (uncorrected P < .05) (eTable 4 in Supplement 5), including 9 with that disposed toward both asthma and inflammatory bowel disorders (IBDs; ie, Crohn disease and UC). Twenty-eight of 82 loci (34.1%) overlapped with instrument SNVs of a third variable, including educational attainment (13 loci) and body mass index (10 loci), with the others overlapping with 3 or fewer loci each. The remaining 54 loci were significantly enriched (FDR Q value < .05) for genes upregulated during late midterm prenatal brain development (P = 9.7 × 10−7; FDR Q value = 4.1 × 10−3), annotations related to prior GWAS of intellectual (eg, cognitive processing and educational attainment, P = 4.9 × 10−7; FDR Q value = 8.9 × 10−5), behavioral (eg, mood instability, P = 3.7 × 10−9; FDR Q value = 7.4 × 10−5), substance use (eg, smoking status, P = 8.7 × 10−5; FDR Q value = .01), and anthropomorphic phenotypes (eg, body mass index–adjusted waist-hip ratio, P = 1.9 × 10−8; FDR Q value = 6.9 × 10−6). We observed biological terms related to granulocyte counts (P = 2.3 × 10−13; FDR Q value = 1.4 × 10−10), cell adhesion (P = 2.3 × 10−11; FDR Q value = 1.7 × 10−7), genes gained in glioma (P = 4.8 × 10−8; FDR Q value = 8.1 × 10−5), calcium ion binding (P = 1.5 × 10−5; Q value = .03), and genes downregulated in cytomegalovirus infection (P = 1.0 × 10−5; FDR Q value = .01). These themes were also observed in the subset of 40 exposure loci corresponding to IBD outcome phenotypes, but they were less represented among the 17 loci associated with an outcome of asthma (eTable 5 in Supplement 6). Minimal enrichment was detected among genes enriched among SNVs mediating the negative association of risk tolerance with AR (eTable 5 in Supplement 6).
GWAS Meta-analysis and Enrichment
Two-sided meta-analyses for the phenotype pairs with robust MR associations are depicted with Miami plots (eFigure 4 in Supplement 1) and lists of GWS loci and nearby genes (10 kB window) are provided (eTable 6 in Supplement 7). Tissue-level analyses among concordant SNV subsets implicated multiple brain regions, especially cerebellar (PGC-CD and Crohn disease, β, 0.06; P = 2.9 × 10−6; FDR Q value = 9.7 × 10−5) and cortical tissues (PGC-CD and Crohn disease, β, 0.06; P = 5.4 × 10−6; FDR Q value = 9.7 × 10−5) but also identified whole blood (PGC-CD and asthma, β, 0.04; P = 1.8 × 10−4; FDR Q value = .01) and spleen (β, 0.05; P = 8.8 × 10−4; FDR Q value = .02). Stronger enrichment was generally observed in discordant SNV subsets, eg, cerebellum PGC-CD–asthma concordant (β, −0.02; P = .86; FDR Q value = 0.95) vs discordant (β, 0.06; P = 2.5 × 10−5; FDR Q value = 3.3 × 10−3) (eFigure 5 in Supplement 1 and eTable 7 in Supplement 8). Alternatively, cell type enrichment was stronger among the concordant SNV subsets, with evidence for independent associations for excitatory and inhibitory neurons of adult medial temporal gyrus (eg, PGC-CD and UC, RORB/COL22A1+ P = 1.9 × 10−4; FDR Q value = 9.8 × 10−3 and PGC-CD and Crohn disease, LAMP5/DBP+ P = 1.3 × 10−3; FDR Q value = .02), inhibitory neurons of the lateral geniculate (eg, MDD and asthma, LAMP5+ P = 1.3 × 10−4; FDR Q value = .04), fetal populations of astrocytes (eg, PGC-CD and asthma, P = 4.1 × 10−7; FDR Q value = 1.2 × 10−4) and quiescent cortical cells (eg, PGC-CD and Crohn disease, P = 1.0 × 10−5; FDR Q value = 7.6 × 10−4) (eFigure 5 in Supplement 1 and eTable 7 in Supplement 8). Gene-set analysis identified more enriched terms among discordant-direction SNVs. Among concordant-direction SNVs, particularly those from the PGC-CD and Crohn disease meta-analysis), we observed significant enrichment for terms relating to stem cell proliferation (β, 0.78; P = 2.2 × 10−5; FDR Q value = .02), neurogenesis (β, 0.13; P = 7.5 × 10−7; FDR Q value = 2.9 × 10−3), postsynapse (β, 0.26; P = 4.3 × 10−5; FDR Q value = 8.2 × 10−3), γ-aminobutyric acid–mediated (GABAergic) signaling (β, 0.84; P = 2.2 × 10−5; FDR Q value = .02), neuronal (β, 0.12; P = 1.2 × 10−5; FDR Q value = .01), T-cell differentiation (β, 1.16; P = 2.8 × 10−5; FDR Q value = .02), transcription factors regulated by MECP2 (β, 2.80; P = 7.5 × 10−7; FDR Q value = 2.9 × 10−3), and transcriptomal effects of ribosomal 6 kinase (β, 1.79; P = 7.2 × 10−5; FDR Q value = .04) and β-catenin (β, 0.16; P = 7.0 × 10−5; FDR Q value = .05) (Figure 3 and eTable 7 in Supplement 8).
Figure 3. Gene-Set Enrichment Analyses of Concordant and Discordant Meta-analysis Results.
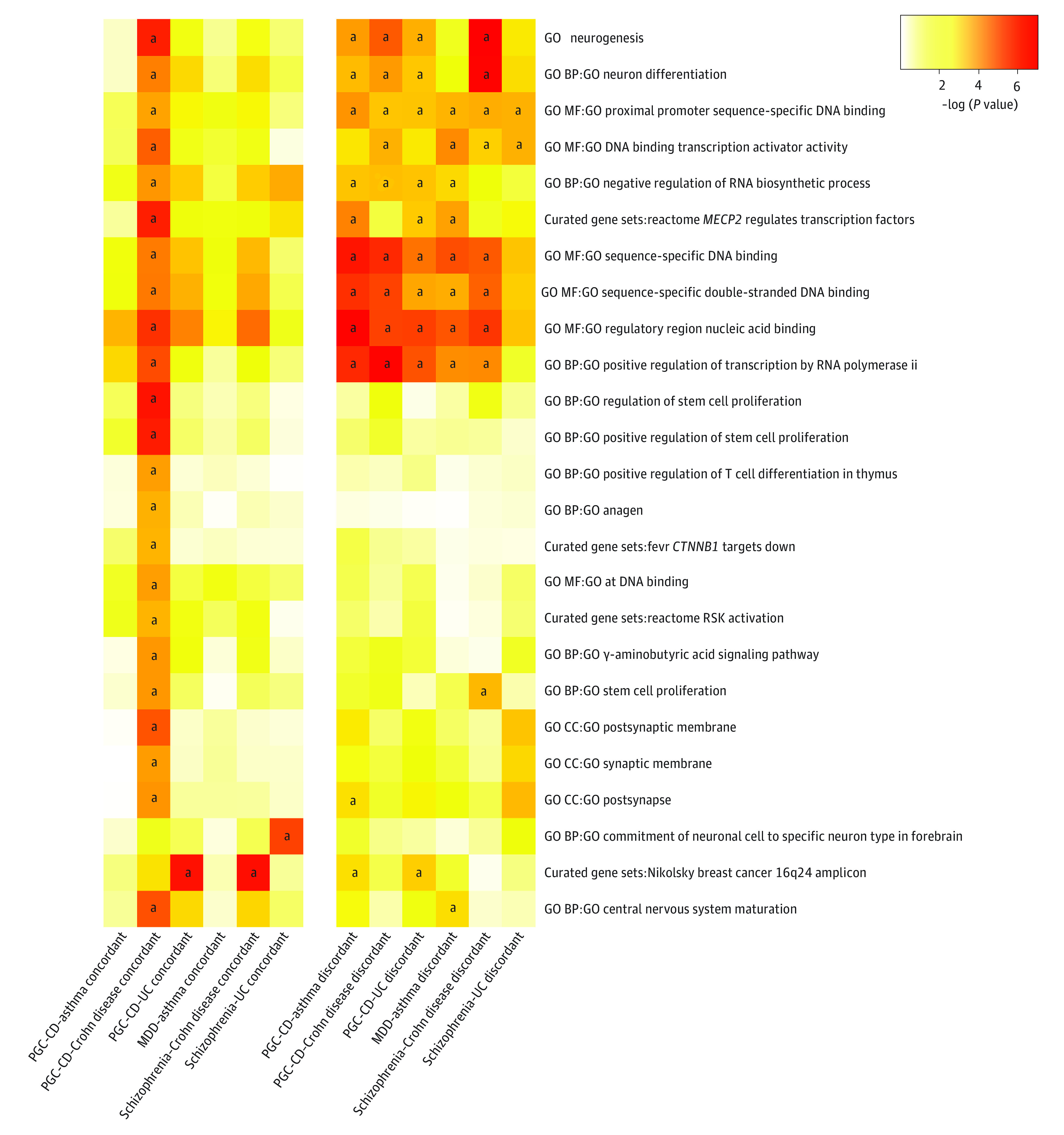
Heatmap depictions of Functional Mapping and Annotation (FUMA) gene set enrichment results performed on the concordant and discordant single nucleotide variation (SNV) subsets generated from cross-trait meta-analysis. Color intensity reflects −log10(enrichment P value). There were no significant differences in enrichment associations between respective concordant and discordant SNV sets. We display annotations with significant enrichment in at least 1 concordant SNV subset, and the rows are hierarchically clustered based on −log10(P value). The full names of gene sets (eTable 7 in Supplement 8) can be searched within the Molecular Signature Database for detailed descriptions. BP indicates biological process; CC, cellular component; CD, cross disorder; GO, gene ontology; MDD, major depressive disorder; MF, molecular function; PGC, Psychiatric Genomic Consortium; RSK, ribosomal 6 kinase; UC, ulcerative colitis.
aFalse discovery rate Q score < .05.
Discussion
In this genetic association study, we quantified the genome-wide shared genetic liability incorporating a range of psychiatric and immunologic disorders and relevant risk factors.5,9,40 We observed a predominance of positive psychiatric-immune correlations, particularly involving asthma, Crohn disease, UC, and primary biliary cholangitis, in relation to multiple psychiatric disorders, most consistently involving MDD, schizophrenia, and PGC-CD (Figure 1). Conversely, other immune-related disorders, eg, AR, primary sclerosing cholangitis, and type 1 diabetes, had predominantly negative correlations with psychiatric phenotypes. Taken together, these data show different patterns in the shared liability across psychiatric and immune traits.
Another novel contribution of this study was the comprehensive characterization of genetic exposure-outcome associations between psychiatric and immune disorders while adjusting for third variables. We showed an association of MDD with asthma consistent with an epidemiologic meta-analysis which found stronger evidence that MDD influences subsequent risk for incident asthma, compared with the reverse.1 Relevant explanations may include depression-related differences in the experience of asthma symptoms leading to increased diagnosis or effects of chronic stress on helper T cell type 1 and type 2 activation contributing to asthma pathophysiology.41 With respect to IBDs, a large-scale study found that MDD was a risk factor for subsequent diagnosis of Crohn disease,42 although others found support for opposite temporal patterning.2,43 Bidirectional mechanisms have been proposed for this association, including the effect of stress with microbial dysbiosis, gut permeability, and microvascular injury.44,45 Although there has been mixed support for clinical associations between schizophrenia and IBDs,2,46,47,48,49,50 we extend previously published genetic inference findings by demonstrating an association for the PGC-CD phenotype.5 We found no prior publications examining epidemiological or genetic associations between risk tolerance and AR.
Our genetic findings suggest that the predominant directional associations between psychiatric and immune traits are behavioral in origin or driven by correlated factors. Additionally, several aspects of our findings suggest general, rather than phenotype-specific mechanisms, including multiple findings driven by PGC-CD liability, reflecting genetic associations across 8 psychiatric disorders25 and the identification of instrument loci associated with both asthma and IBDs. Among our significant MR findings, the most influential third variables included cognitive processing, educational attainment, heart rate variability, and income (eTable 5 in Supplement 6, eFigures 3 and 4 in Supplement 1). Heart rate variability indexes cardiac autonomic tone and individual differences are associated with traits like emotion regulation and executive function,51 suggesting that stress responses may partially mediate some psychiatric-immune associations.
Previous MR studies found positive associations of genetically determined inflammatory biomarkers and cell counts with liability to psychiatric disorders.52,53,54,55,56,57,58,59,60 Unfortunately, only a subset of these evaluated bidirectional associations, and most did not investigate possible third-variable possible confounding or mediating factors. Additionally, immune-related disease phenotypes may relate to psychiatric disorders in a different way than circulating cytokine levels, which are typically assessed in unaffected samples or population-based cohorts.
The psychiatric risk loci mediating positive MR outcomes were enriched for genes involved in neutrophil functions, cell adhesion, calcium-binding, and genes downregulated cytomegalovirus infection (eDiscussion in Supplement 1). Loci with concordant psychiatric-immune associations were statistically more likely to be expressed in brain tissues, particularly cortex and cerebellum, as compared with blood and lymphoid tissue (eFigure 5A in Supplement 1). Concordant loci were also enriched for transcriptomic profiles of fetal astrocytes and subpopulations of cortical excitatory and inhibitory neurons (eFigure 5B in Supplement 1). Functional gene-set analysis showed that discordant and concordant loci were both enriched for terms related to neurogenesis, neural differentiation, synapses, and GABAergic signaling (Figure 3). Taken together, biological characterizations of the loci comprising significant genetic correlations and MR outcomes converge to indicate stronger enrichment for brain- and behavior-related mechanisms in comparison with peripheral pathways.
Limitations
We must caution against overinterpretation owing to study limitations. The phenotypes in our study were highly polygenic with instruments that explain relatively low SNV heritability. Although we used several approaches designed to cope with pertinent biases, our observed associations or discrepancies with other work may still arise from unadjusted confounding. Our ability to account for third-variable associations was necessarily limited by available GWAS data and our selection strategy. Additionally, we adjusted for the effect of each third variable separately, and we observed that more saturated models quickly lost statistical power. Future MVMR studies could explore data reduction with a set of genetically correlated risk factors. Another limitation was that our current findings were based on GWAS data sets generated from individuals of European descent and may not generalize to other ancestry groups. More diverse cohorts are needed to investigate psychiatric-immune associations across worldwide populations.
Conclusions
In this genetic association study, our comprehensive genetically informed inference analyses identified several robust associations of psychiatric phenotypes with immune-related phenotypes, but not vice versa, suggesting the predominant directional associations underlying large-scale epidemiological associations may be behavioral in origin. However, we cannot completely exclude contributions from unmeasured or inadequately adjusted risk factors and other sources of bias.
eMethods.
eDiscussion.
eReferences.
eFigure 1. Flow Chart of the Study Design and Analysis Plan
eFigure 2. Genetic Correlations Among Psychiatric Disorders and Immune-Related Traits and Other Risk Factors
eFigure 3. MVMR Effects After Adjustment for Other Risk Factors
eFigure 4. Miami Plots Depicting Concordant and Discordant Effects Identified via 2-Sided Meta-Analysis of Psychiatric-Immune Phenotype Pairs
eFigure 5. Tissue and Cell Type Enrichment Analyses of Loci With Concordant and Discordant Effects Within the Psychiatric-Immune Associations Identified
eTable 1.
eTable 2.
eTable 3.
eTable 4.
eTable 5.
eTable 6.
eTable 7.
References
- 1.Gao YH, Zhao HS, Zhang FR, et al. The relationship between depression and asthma: a meta-analysis of prospective studies. PLoS One. 2015;10(7):e0132424. doi: 10.1371/journal.pone.0132424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ludvigsson JF, Olén O, Larsson H, et al. Association between inflammatory bowel disease and psychiatric morbidity and suicide: a Swedish nationwide population-based cohort study with sibling comparisons. J Crohns Colitis. 2021;15(11):1824-1836. doi: 10.1093/ecco-jcc/jjab039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jeppesen R, Benros ME. Autoimmune diseases and psychotic disorders. Front Psychiatry. 2019;10(MAR):131. doi: 10.3389/fpsyt.2019.00131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersson NW, Gustafsson LN, Okkels N, et al. Depression and the risk of autoimmune disease: a nationally representative, prospective longitudinal study. Psychol Med. 2015;45(16):3559-3569. doi: 10.1017/S0033291715001488 [DOI] [PubMed] [Google Scholar]
- 5.Tylee DS, Sun J, Hess JL, et al. ; 23 and Me Research Team; Inflammation Working Group of the CHARGE Consortium; METASTROKE Consortium of the International Stroke Genetics Consortium; Netherlands Twin Registry; neuroCHARGE Working Group; Obsessive-Compulsive and Tourette Syndrome Working Group of the Psychiatric Genomics Consortium . Genetic correlations among psychiatric and immune-related phenotypes based on genome-wide association data. Am J Med Genet B Neuropsychiatr Genet. 2018;177(7):641-657. doi: 10.1002/ajmg.b.32652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pouget JG, Han B, Wu Y, et al. ; Schizophrenia Working Group of the Psychiatric Genomics Consortium . Cross-disorder analysis of schizophrenia and 19 immune-mediated diseases identifies shared genetic risk. Hum Mol Genet. 2019;28(20):3498-3513. doi: 10.1093/hmg/ddz145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu Q, Li B, Ou D, et al. A powerful approach to estimating annotation-stratified genetic covariance via GWAS summary statistics. Am J Hum Genet. 2017;101(6):939-964. doi: 10.1016/j.ajhg.2017.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao H, Li S, Baranova A, Zhang F. Shared genetic liability between major depressive disorder and atopic diseases. Front Immunol. 2021;12:665160. doi: 10.3389/fimmu.2021.665160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu Z, Zhu X, Liu CL, et al. Shared genetics of asthma and mental health disorders: a large-scale genome-wide cross-trait analysis. Eur Respir J. 2019;54(6):1901507. doi: 10.1183/13993003.01507-2019 [DOI] [PubMed] [Google Scholar]
- 10.Dantzer R. Neuroimmune interactions: from the brain to the immune system and vice versa. Physiol Rev. 2018;98(1):477-504. doi: 10.1152/physrev.00039.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tay TL, Béchade C, D’Andrea I, et al. Microglia gone rogue: impacts on psychiatric disorders across the life span. Front Mol Neurosci. 2018;10:421. doi: 10.3389/fnmol.2017.00421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhabhar FS. Effects of stress on immune function: the good, the bad, and the beautiful. Immunol Res. 2014;58(2-3):193-210. doi: 10.1007/s12026-014-8517-0 [DOI] [PubMed] [Google Scholar]
- 13.Gong Y, Niu W, Tang Y, et al. Aggravated mucosal and immune damage in a mouse model of ulcerative colitis with stress. Exp Ther Med. 2019;17(3):2341-2348. doi: 10.3892/etm.2019.7162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amano H, Negishi I, Akiyama H, Ishikawa O. Psychological stress can trigger atopic dermatitis in NC/Nga mice: an inhibitory effect of corticotropin-releasing factor. Neuropsychopharmacology. 2008;33(3):566-573. doi: 10.1038/sj.npp.1301435 [DOI] [PubMed] [Google Scholar]
- 15.Stojanovich L, Marisavljevich D. Stress as a trigger of autoimmune disease. Autoimmun Rev. 2008;7(3):209-213. doi: 10.1016/j.autrev.2007.11.007 [DOI] [PubMed] [Google Scholar]
- 16.Ilchmann-Diounou H, Menard S. Psychological stress, intestinal barrier dysfunctions, and autoimmune disorders: an overview. Front Immunol. 2020;11:1823. doi: 10.3389/fimmu.2020.01823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burgess S, Davey Smith G, Davies NM, et al. Guidelines for performing mendelian randomization investigations. Wellcome Open Res. 2020;4:186. doi: 10.12688/wellcomeopenres.15555.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang X, Zhu Z, Manouchehrinia A, Olsson T, Alfredsson L, Kockum I. Alcohol consumption and risk of common autoimmune inflammatory diseases—evidence from a large-scale genetic analysis totaling 1 million individuals. Front Genet. 2021;12:687745. doi: 10.3389/fgene.2021.687745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaheen SO, Rutterford C, Zuccolo L, et al. Prenatal alcohol exposure and childhood atopic disease: a mendelian randomization approach. J Allergy Clin Immunol. 2014;133(1):225-32.e1, 5. doi: 10.1016/j.jaci.2013.04.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Budu-Aggrey A, Joyce S, Davies NM, et al. Investigating the causal relationship between allergic disease and mental health. Clin Exp Allergy. 2021;51(11):1449-1458. doi: 10.1111/cea.14010 [DOI] [PubMed] [Google Scholar]
- 21.Ly A, Leppert B, Rai D, Jones H, Dardani C, Stergiakouli E. Genetic liability to rheumatoid arthritis on autism and autistic traits: polygenic risk score and Mendelian randomization analyses. Transl Psychiatry. 2022;12(1):18. doi: 10.1038/s41398-021-01772-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanderson E, Spiller W, Bowden J. Testing and correcting for weak and pleiotropic instruments in 2-sample multivariable mendelian randomization. Stat Med. 2021;40(25):5434-5452. doi: 10.1002/sim.9133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bulik-Sullivan B, Finucane HK, Anttila V, et al. ; ReproGen Consortium; Psychiatric Genomics Consortium; Genetic Consortium for Anorexia Nervosa of the Wellcome Trust Case Control Consortium 3 . An atlas of genetic correlations across human diseases and traits. Nat Genet. 2015;47(11):1236-1241. doi: 10.1038/ng.3406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kroenke K, Spitzer RL, Williams JBW, Löwe B. The patient health questionnaire somatic, anxiety, and depressive symptom scales: a systematic review. Gen Hosp Psychiatry. 2010;32(4):345-359. doi: 10.1016/j.genhosppsych.2010.03.006 [DOI] [PubMed] [Google Scholar]
- 25.Lee PH, Anttila V, Won H, et al. ; Cross-Disorder Group of the Psychiatric Genomics Consortium. Electronic address: plee0@mgh.harvard.edu; Cross-Disorder Group of the Psychiatric Genomics Consortium . Genomic relationships, novel loci, and pleiotropic mechanisms across 8 psychiatric disorders. Cell. 2019;179(7):1469-1482.e11. doi: 10.1016/j.cell.2019.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oswald LM, Zandi P, Nestadt G, Potash JB, Kalaydjian AE, Wand GS. Relationship between cortisol responses to stress and personality. Neuropsychopharmacology. 2006;31(7):1583-1591. doi: 10.1038/sj.npp.1301012 [DOI] [PubMed] [Google Scholar]
- 27.Jeronimus BF, Kotov R, Riese H, Ormel J. Neuroticism’s prospective association with mental disorders halves after adjustment for baseline symptoms and psychiatric history, but the adjusted association hardly decays with time: a meta-analysis on 59 longitudinal/prospective studies with 443 313 participants. Psychol Med. 2016;46(14):2883-2906. doi: 10.1017/S0033291716001653 [DOI] [PubMed] [Google Scholar]
- 28.Lahey BB. Public health significance of neuroticism. Am Psychol. 2009;64(4):241-256. doi: 10.1037/a0015309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou W, Brumpton B, Kabil O, et al. GWAS of thyroid stimulating hormone highlights pleiotropic effects and inverse association with thyroid cancer. Nat Commun. 2020;11(1):3981. doi: 10.1038/s41467-020-17718-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bulik-Sullivan BK, Loh P-R, Finucane HK, et al. ; Schizophrenia Working Group of the Psychiatric Genomics Consortium . LD score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet. 2015;47(3):291-295. doi: 10.1038/ng.3211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korthauer K, Kimes PK, Duvallet C, et al. A practical guide to methods controlling false discoveries in computational biology. Genome Biol. 2019;20(1):118. doi: 10.1186/s13059-019-1716-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skrivankova VW, Richmond RCR, Woolf BAR, et al. Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization: the STROBE-MR statement. JAMA. 2021;326(16):1614-1621. doi: 10.1001/jama.2021.18236 [DOI] [PubMed] [Google Scholar]
- 33.Hemani G, Tilling K, Smith GD. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet. 2017;13(11):e1007081. doi: 10.1371/journal.pgen.1007081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao Q, Wang J, Hemani G, Bowden J, Small DS. Statistical inference in 2-sample summary-data mendelian randomization using robust-adjusted profile score. Ann Stat. 2020;48(3):1742-1769. doi: 10.1214/19-AOS1866 [DOI] [Google Scholar]
- 35.Mounier N, Kutalik Z. Bias correction for inverse variance weighting mendelian randomization. bioRxiv. Preprint posted online December 18, 2021. doi: 10.1101/2021.03.26.437168 [DOI] [PubMed]
- 36.de Leeuw CA, Mooij JM, Heskes T, Posthuma D. MAGMA: generalized gene-set analysis of GWAS data. PLoS Comput Biol. 2015;11(4):e1004219. doi: 10.1371/journal.pcbi.1004219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watanabe K, Taskesen E, van Bochoven A, Posthuma D. Functional mapping and annotation of genetic associations with FUMA. Nat Commun. 2017;8(1):1826. doi: 10.1038/s41467-017-01261-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhattacharjee S, Rajaraman P, Jacobs KB, et al. ; GliomaScan Consortium . A subset-based approach improves power and interpretation for the combined analysis of genetic association studies of heterogeneous traits. Am J Hum Genet. 2012;90(5):821-835. doi: 10.1016/j.ajhg.2012.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watanabe K, Umićević Mirkov M, de Leeuw CA, van den Heuvel MP, Posthuma D. Genetic mapping of cell type specificity for complex traits. Nat Commun. 2019;10(1):3222. doi: 10.1038/s41467-019-11181-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Q, Yang C, Gelernter J, Zhao H. Pervasive pleiotropy between psychiatric disorders and immune disorders revealed by integrative analysis of multiple GWAS. Hum Genet. 2015;134(11-12):1195-1209. doi: 10.1007/s00439-015-1596-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kewalramani A, Bollinger ME, Postolache TT. Asthma and mood disorders. Int J Child Health Hum Dev. 2008;1(2):115-123. [PMC free article] [PubMed] [Google Scholar]
- 42.Ananthakrishnan AN, Khalili H, Pan A, et al. Association between depressive symptoms and incidence of Crohn disease and ulcerative colitis: results from the Nurses’ Health Study. Clin Gastroenterol Hepatol. 2013;11(1):57-62. doi: 10.1016/j.cgh.2012.08.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bernstein CN, Hitchon CA, Walld R, et al. ; CIHR Team in Defining the Burden and Managing the Effects of Psychiatric Comorbidity in Chronic Immunoinflammatory Disease . Increased burden of psychiatric disorders in inflammatory bowel disease. Inflamm Bowel Dis. 2019;25(2):360-368. doi: 10.1093/ibd/izy235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mawdsley JE, Rampton DS. Psychological stress in IBD: new insights into pathogenic and therapeutic implications. Gut. 2005;54(10):1481-1491. doi: 10.1136/gut.2005.064261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keefer L, Kane SV. Considering the bidirectional pathways between depression and IBD: recommendations for comprehensive IBD care. Gastroenterol Hepatol (N Y). 2017;13(3):164-169. [PMC free article] [PubMed] [Google Scholar]
- 46.Marrie RA, Walld R, Bolton JM, et al. ; CIHR Team in Defining the Burden and Managing the Effects of Psychiatric Comorbidity in Chronic Immunoinflammatory Disease . Rising incidence of psychiatric disorders before diagnosis of immune-mediated inflammatory disease. Epidemiol Psychiatr Sci. 2019;28(3):333-342. doi: 10.1017/S2045796017000579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nielsen PR, Benros ME, Dalsgaard S. Associations between autoimmune diseases and attention-deficit/hyperactivity disorder: a nationwide study. J Am Acad Child Adolesc Psychiatry. 2017;56(3):234-240.e1. doi: 10.1016/j.jaac.2016.12.010 [DOI] [PubMed] [Google Scholar]
- 48.Cullen AE, Holmes S, Pollak TA, et al. Associations between nonneurological autoimmune disorders and psychosis: a meta-analysis. Biol Psychiatry. 2019;85(1):35-48. doi: 10.1016/j.biopsych.2018.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marrie RA, Walld R, Bolton JM, et al. ; CIHR Team in Defining the Burden and Managing the Effects of Psychiatric Comorbidity in Chronic Immunoinflammatory Disease . Increased incidence of psychiatric disorders in immune-mediated inflammatory disease. J Psychosom Res. 2017;101:17-23. doi: 10.1016/j.jpsychores.2017.07.015 [DOI] [PubMed] [Google Scholar]
- 50.Benros ME, Nielsen PR, Nordentoft M, Eaton WW, Dalton SO, Mortensen PB. Autoimmune diseases and severe infections as risk factors for schizophrenia: a 30-year population-based register study. Am J Psychiatry. 2011;168(12):1303-1310. doi: 10.1176/appi.ajp.2011.11030516 [DOI] [PubMed] [Google Scholar]
- 51.Kim HG, Cheon EJ, Bai DS, Lee YH, Koo BH. Stress and heart rate variability: a meta-analysis and review of the literature. Psychiatry Investig. 2018;15(3):235-245. doi: 10.30773/pi.2017.08.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reay WR, Kiltschewskij DJ, Geaghan MP, et al. Genetic estimates of correlation and causality between blood-based biomarkers and psychiatric disorders. medRxiv. Preprint posted online May 14, 2021. doi: 10.1101/2021.05.11.21257061 [DOI] [PMC free article] [PubMed]
- 53.Muniz Carvalho C, Wendt FR, Maihofer AX, et al. Dissecting the genetic association of C-reactive protein with PTSD, traumatic events, and social support. Neuropsychopharmacology. 2021;46(6):1071-1077. doi: 10.1038/s41386-020-0655-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hartwig FP, Davies NM, Hemani G, Davey Smith G. Two-sample mendelian randomization: avoiding the downsides of a powerful, widely applicable but potentially fallible technique. Int J Epidemiol. 2016;45(6):1717-1726. doi: 10.1093/ije/dyx028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ye Z, Kappelmann N, Moser S, et al. Role of inflammation in depression and anxiety: tests for disorder specificity, linearity and potential causality of association in the UK Biobank. EClinicalMedicine. 2021;38:100992. doi: 10.1016/j.eclinm.2021.100992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kappelmann N, Arloth J, Georgakis MK, et al. Dissecting the association between inflammation, metabolic dysregulation, and specific depressive symptoms: a genetic correlation and 2-sample mendelian randomization study. JAMA Psychiatry. 2021;78(2):161-170. doi: 10.1001/jamapsychiatry.2020.3436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kelly KM, Smith JA, Mezuk B. Depression and interleukin-6 signaling: a mendelian randomization study. Brain Behav Immun. 2021;95:106-114. doi: 10.1016/j.bbi.2021.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sealock JM, Lee YH, Moscati A, et al. Use of the PsycheMERGE Network to investigate the association between depression polygenic scores and white blood cell count. JAMA Psychiatry. 2021;78(12):1365-1374. doi: 10.1001/jamapsychiatry.2021.2959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prins BP, Abbasi A, Wong A, et al. PAGE Consortium; International Stroke Genetics Consortium; Systemic Sclerosis Consortium; et al. Investigating the causal relationship of C-reactive protein with 32 complex somatic and psychiatric outcomes: a large-scale cross-consortium mendelian randomization study. PLoS Med. 2016;13(6):e1001976. doi: 10.1371/journal.pmed.1001976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Perry BI, Upthegrove R, Kappelmann N, Jones PB, Burgess S, Khandaker GM. Associations of immunological proteins/traits with schizophrenia, major depression, and bipolar disorder: a bidirectional 2-sample mendelian randomization study. Brain Behav Immun. 2021;97:176-185. doi: 10.1016/j.bbi.2021.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eDiscussion.
eReferences.
eFigure 1. Flow Chart of the Study Design and Analysis Plan
eFigure 2. Genetic Correlations Among Psychiatric Disorders and Immune-Related Traits and Other Risk Factors
eFigure 3. MVMR Effects After Adjustment for Other Risk Factors
eFigure 4. Miami Plots Depicting Concordant and Discordant Effects Identified via 2-Sided Meta-Analysis of Psychiatric-Immune Phenotype Pairs
eFigure 5. Tissue and Cell Type Enrichment Analyses of Loci With Concordant and Discordant Effects Within the Psychiatric-Immune Associations Identified
eTable 1.
eTable 2.
eTable 3.
eTable 4.
eTable 5.
eTable 6.
eTable 7.