Abstract
Activity in the mesolimbic dopamine (DA) pathway is known to have a role in reward processing and related behaviors. The mesolimbic DA response to reward has been well-examined, while the response to aversive or negative stimuli has been studied to a lesser extent and produced inconclusive results. However, a brief increase in the DA concentration in terminals during nociceptive activation has become an established but not well-characterized phenomenon. Consequently, the interpretation of the significance of this neurochemical response is still elusive. The present study was designed to further explore these increases in subsecond DA dynamics triggered by negative stimuli using voltammetry in anesthetized rats. Our experiments revealed that repeated exposure to a tail pinch resulted in more efficacious DA release in rat nucleus accumbens. This fact may suggest a protective nature of immediate DA efflux. Furthermore, a sensitized DA response to a neutral stimulus, such as a touch, was discovered following several noxious pinches, while a touch applied before these pinches did not trigger DA release. Finally, it was found that the pinch-evoked DA efflux was significantly decreased by ethanol acutely administrated at an analgesic dose. Taken together, these results support the hypothesis that subsecond DA release in the nucleus accumbens may serve as an endogenous antinociceptive signal.
Keywords: Voltammetry, nucleus accumbens, pain, dopamine, ethanol
Graphical Abstract
INTRODUCTION
The perception that rewarding and aversive or negative stimuli as well as their predictors are different may suggest opposite electrophysiological and neurochemical responses triggered by those incentives in vertebrates. Dopamine (DA) signaling in the nucleus accumbens is involved in the integration of sensory information and the initiation of an action following diverse stimuli. In fact, earlier studies have demonstrated that mesolimbic DA transmission is enhanced by rewarding stimuli and inhibited, or at least unresponsive, under the influence of negative stimuli.1,2 This resulted in the hypothesis that the increase in accumbal DA release is exclusively associated with reward-related incentives.1,3,4 However, more recent evidence obtained from fast-scan cyclic voltammetry (FSCV) studies indicated that DA release can be both decreased and increased by negative stimuli.5–8 In fact, FSCV is well-suited to explore real-time alterations in extracellular DA concentrations in relatively small brain areas and subregions without overlap.9–12 For example, the increase in the frequency of DA transients was observed in an intruder rat during the interaction with an aggressive resident rat in a social defeat paradigm.5 Furthermore, subsecond DA release was consistently increased upon presentation of the warning signal in a manner that reliably predicted successful punishment avoidance, while the release was decreased at the same cue during escape responses.13 In anesthetized rat experiments, a tail pinch evoked DA efflux in the nucleus accumbens core, and the increase was time-locked to the negative stimulus.6 However, DA release in the nucleus accumbens shell revealed differential responses to the same stimulus, including a monophasic decrease, a monophasic increase with some delay,6,7 and a biphasic response with an initial decrease and subsequent increase.7 Electrophysiological findings in anesthetized14 and freely moving rodents,15,16 which support subregional differences, are in agreement with neurochemical results. Therefore, a brief increase in the DA concentration in terminals during nociceptive activation in an anesthetized preparation is an established, but not well characterized, phenomenon. For example, it is unknown whether the DA response is unchanged, blunted, or enhanced following rapid replication of these stimuli. How do substances with DA-ergic mechanisms and analgesic properties alter the stimulus-triggered DA efflux? The answers to this and other related questions could help shed light on the role of DA signaling in aversive conditions, which is still unclear.
Therefore, the present study was designed to further explore increases in DA release in the nucleus accumbens core under effect of repetitive negative stimuli. We chose to focus on the core subregion because only a monophasic increase in DA concentration was previously revealed in response to a 3-s tail pinch in this brain region.6 First, we explored real-time DA dynamics following repeated exposures to this aversive stimulus. Second, the acute effects of ethanol on tail pinch-induced DA efflux were studied.
RESULTS AND DISCUSSION
FSCV was used to measure DA release in rat nucleus accumbens in response to repetitive tail pinches. Importantly, anesthetized preparations allowed us to avoid confounding associative learning and avoiding behaviors, which take place in awake animals and certainly affect DA transmission.8,17 Therefore, we could focus on fast DA alterations associated with the initial perception and descending modulation of nociceptive signals. Notably, nociceptive activation does not always induce averseness or painful sensation, and alternatively, these feelings can exist without nociceptive input.18
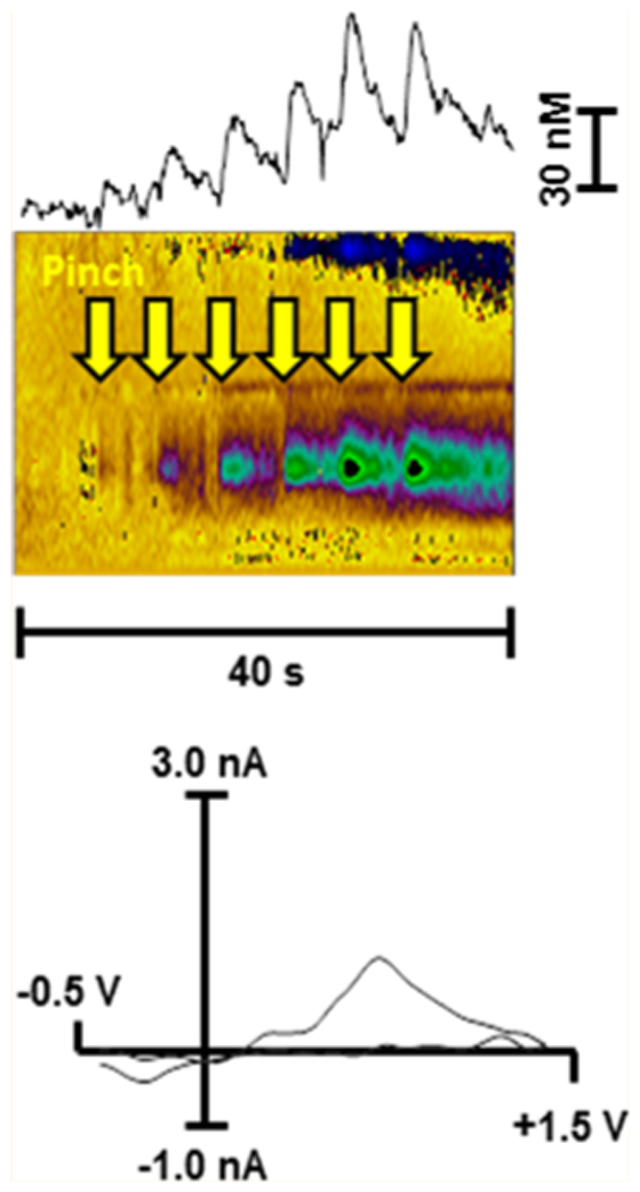
As it was previously observed,6 a tail pinch reliably evoked accumbal DA release that was time-locked to the stimulus. The increase in extracellular DA was sustained after consequent pinches without any weakening of the efflux (Figure 1). Moreover, the following pinch resulted in further enhancement of DA release, reaching higher concentrations (repeated measures ANOVA; F(1.037, 4.150) = 8.15 p = 0.04, n = 5 rats) (Figure 2a). However, the analysis revealed no main effect of time on the delay of DA response to the nociceptive stimulus (repeated measures ANOVA; F(1.326, 5.305) = 0.59) (Figure 2b). It is important to note that no drugs were used to improve DA recordings as it was previously performed for the evaluation of DA signaling in the nucleus accumbens shell in response to an identical stimulus.7 Therefore, observed phasic transients at these concentrations should have physiological importance.
Figure 1.
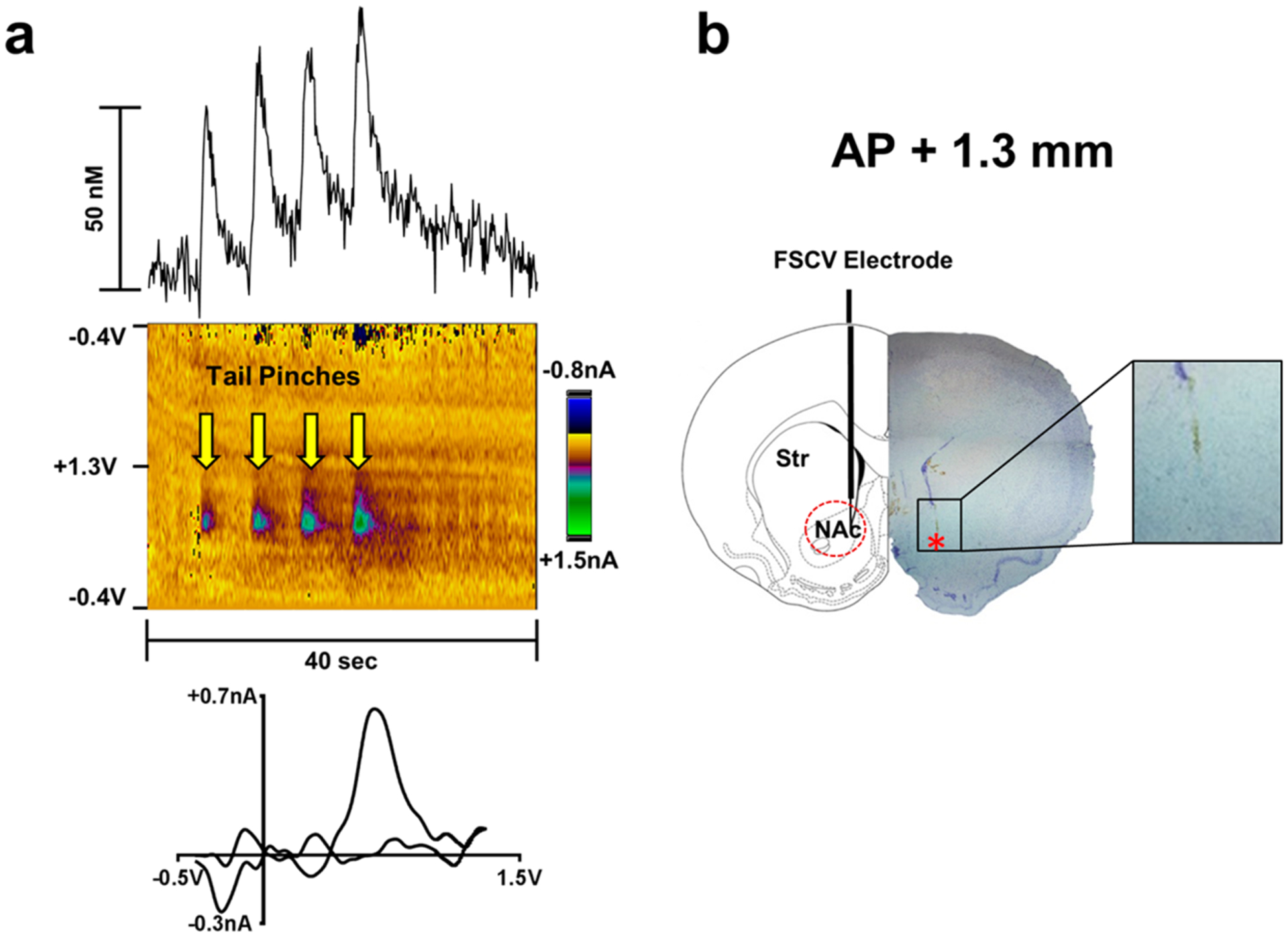
(a) Changes in extracellular DA concentration measured by FSCV in rat nucleus accumbens core in response to successive tail pinches (upper panels). Color plot topographically depicts the voltammetric data, with time on the x-axis, applied scan potential on the y-axis, and background-subtracted faradaic current shown on the z-axis in pseudocolor (middle panels). Representative background-subtracted voltammogram showing characteristic oxidation and reduction peak potentials (~ +0.6 V and ~ −0.2 V, respectively) that identifies DA (lower panels). Anesthetized rats received a tail pinch every 5 s for a total of 4 pinches, while voltammetric recordings were performed over a 40 s time period. Yellow arrows indicate pinch onset. (b) Location of FSCV recordings in rat brain. On the left is a schematic drawing of a coronal slice (AP + 1.3 mm from bregma) from a rat brain atlas (Paxinos and Watson). The diagram simulates the location of the FSCV electrode that was revealed by histological analysis (right side). The dashed red circle indicates where the detecting part of electrodes was localized. On the right side is a photograph of a corresponding section where the electrode track is visible (red star indicates end of the electrode). The inset shows a magnification of the area where the detecting part of the electrode was located.
Figure 2.
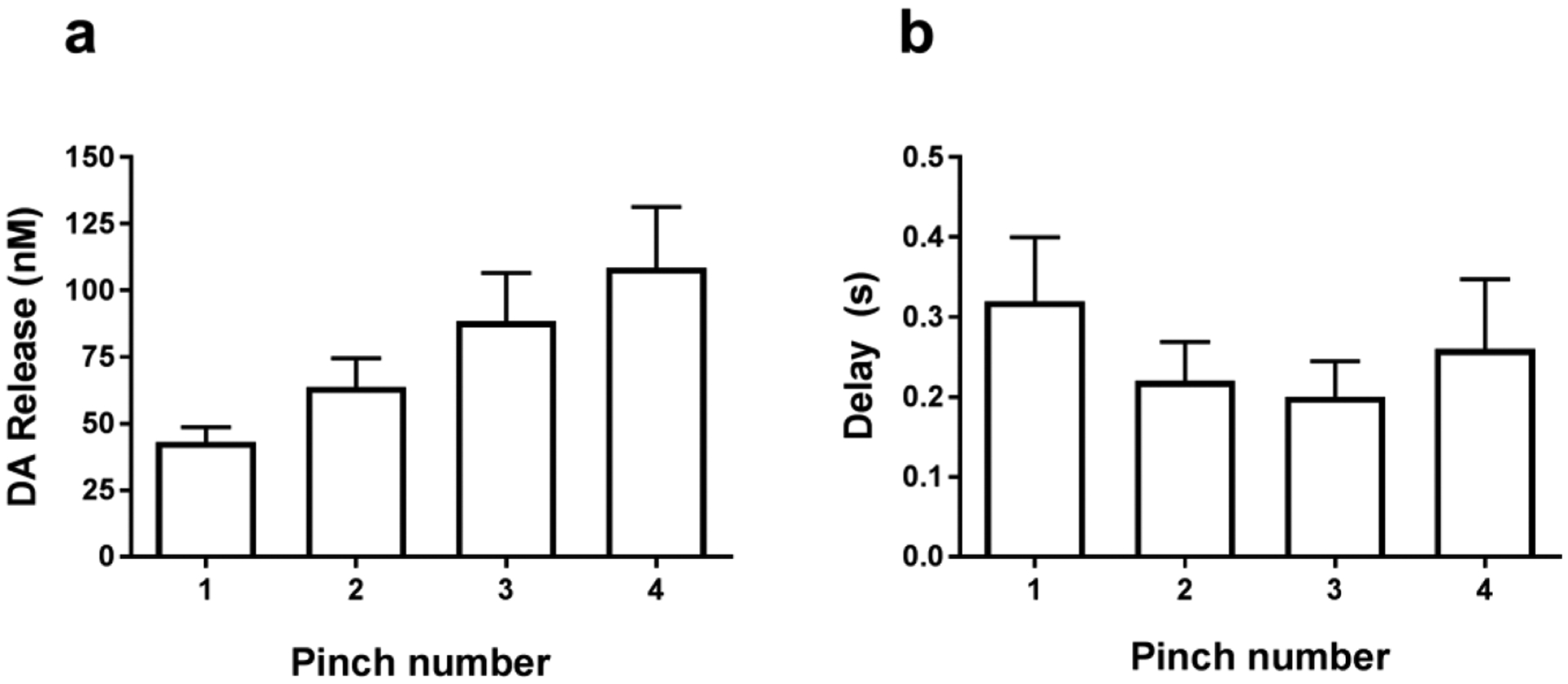
(a) Effects of repetitive tail pinch administration on the maximal amplitude of DA release in rat nucleus accumbens core. (b) The time between detected DA release and onset of the tail pinch during multiple pinch exposure. Pinches were performed with 5 s intervals. Data are presented as means ± SEM of five rats. Pinches were performed at the same location on the tail. There was a significant difference in the amplitude of DA efflux (one-way repeated measures ANOVA; P < 0.05), while no significant changes in the time between detected DA release and onset of the tail pinch were found (P > 0.05).
Unexpectedly, we revealed that a gentle tail touch could also trigger DA release in the same manner as a noxious pinch. However, the DA response to the touch became visible only following several (at least three) subsequent pinches (Figure 3). Moreover, the DA concentrations, which were evoked by these dissimilar incentives, were not significantly different during the final trial (paired t test; t(4) = 1.332, p = 0.25, n = 5) (Figure 3b). No difference was revealed in the delay of DA release onset between the pinch and touch exposure (paired t test; t(4) = 0.6667, p = 0.54, 0.28 ± 0.04 vs 0.24 ± 0.07 s). Because rats were anesthetized, it is unlikely that a touch could work as a cue, which would predict a negative incentive; however, this possibility cannot be completely ruled out. More likely, some sensitization could take place, and therefore, DA release was triggered by weaker inducement, such as a tail touch. Perhaps, minimal tissue damage on the rat’s tail may result in nociceptor activation and possibly sensitization under current circumstances. In fact, a lowered response threshold for an acutely applied stimulus can be observed in the absence of ongoing pain.19 For example, in lightly sunburned skin, a normally innocuous heat stimulus is felt as burning pain.20 Thus, allodynia can be developed following recurring exposure to a noxious stimulus, leading to innocuous stimuli eliciting the same response. Additionally, the repeated exposure to a painful stimulus can result in central nervous system alterations, enabling a quicker and more efficacious response when a possibly harmful action is initiated again. Both of these mechanisms may serve to generate protective responses aimed at preventing further tissue damage and, perhaps more importantly, suppress pain perception. The latter mechanism is essential for survival because this conserved mechanism of pain modulation allows organisms to engage in adequate defensive responses.19,21 Therefore, perhaps, the anesthesia cannot completely overcome this evolutionarily preserved nociceptive input. Our data provide support for this notion.
Figure 3.
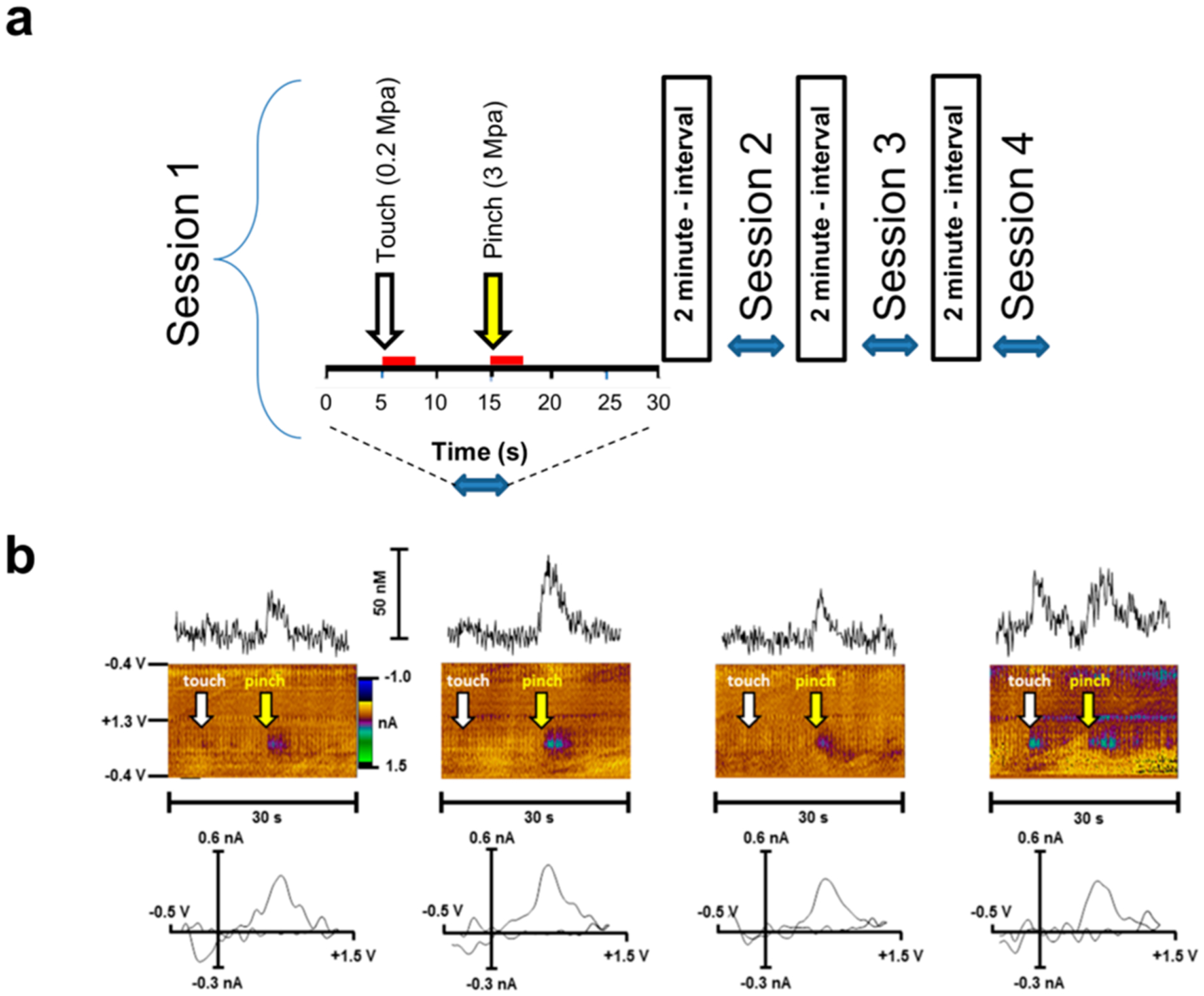
(a) A schematic representation of the experiment with consecutive administration of tail pinches followed by tail touch. During a 30 s recording session, a tail touch was performed at 5 s (white arrow) for a duration of 3 s (red bar), and then a tail pinch (yellow arrow) was performed at 15 s for the same duration. The same procedure was repeated 3 more times with a 2 min interval between each. (b) Representative voltammetry data on DA measures during consecutive tail touch and pinch sessions. Changes in extracellular DA concentration versus time (upper panels) measured by FSCV in rat nucleus accumbens core of a single rat. Color plots topographically depict the voltammetric data with time on the x-axis, applied scan potential on the y-axis, and background-subtracted faradaic current shown on the z-axis in pseudocolor (middle panels). Representative background-subtracted voltammograms showing characteristic oxidation and reduction peak potentials (~ +0.6 V and ~ −0.2 V, respectively) that identify DA (lower panels).The tail pinches and touches were conducted at the same location on the tail for each trial. The tail touch elicited a similar DA response as the aversive stimulus after repeated exposure to the pinch. White arrows indicate touch onset, while yellow arrows indicate pinch onset.
The conception that mesolimbic DA transmission plays an important role in pain control is relatively well-accepted.22,23 Remarkably, pleasurable stimuli, which trigger subsecond DA increase in the nucleus accumbens, may induce significant analgesic effects.24,25 In agreement with these findings, increased affective pain ratings were revealed after dietary DA depletion in humans.26 Interestingly, the initial support for this idea came from observations that some drugs which increase tonic DA concentration also have analgesic actions in animal models.23 However, some recent studies did not find effects of DA-ergic manipulations on a variety of pain tests, opposing a simplistic view of DA as an antinociceptive mediator.27 Therefore, the increased accumbal DA in response to an acute painful stimulus was alternatively interpreted as a signal that triggers avoidance behavior.27 In fact, in our experimental condition, it was impossible to initiate escaping actions because the animals were under anesthesia. Furthermore, in sharp contrast to previous studies, which were focused on prolonged changes in DA release measured by microdialysis during persistent or tonic pain,23 transient DA response induced by the stimulus was explored in the present experiments. This fundamental difference should be taken into account when interpreting and comparing the current results with the above-mentioned studies.
Alcohol has robust analgesic properties28 as well as established effects on accumbal DA.10,29–34 Therefore, we hypothesize that if the detected phasic DA release is acting as an antinociceptive signal, alcohol should reduce the tail pinch-triggered efflux. First, we confirmed that the maximal DA response to the stimulus can be quite stable when a 10 min interval was kept between pinches (Figure 4a). The present data are in line with the earlier study, where unchanging amplitudes of DA efflux were observed with pinches performed every 2–3 min.6 Expectedly, no changes in the pinch-evoked DA were found following saline administration, while the selective D2 DA autoreceptor antagonist raclopride (2 mg/kg, i.p.) significantly altered measured signal (F(2,12) = 28.18; p < 0.0001) with dynamics, which were previously established after an electrical stimulation of the VTA.35 Dunnett’s multiple comparisons test found that raclopride was significantly different than the saline group (p < 0.001). Importantly, the observed drug effect confirms that the substance detected during nociceptive activation is DA and not norepinephrine. Therefore, the stability of the tail pinch-evoked DA signal and its predictable pharmacological responsiveness allowed us to reliably evaluate the changes in DA dynamics under the effect of ethanol. These experiments revealed that the DA efflux elicited by tail pinch was extremely sensitive to administration of ethanol (2 g/kg, i.p.) (Figure 4b). A two-way repeated measures ANOVA calculated a main effect of the drug (p < 0.0001) and a significant interaction (F(16,96) = 7.332; p < 0.0001). Dunnett’s multiple comparisons test found that ethanol (p < 0.05) was significantly different than the saline group.
Figure 4.
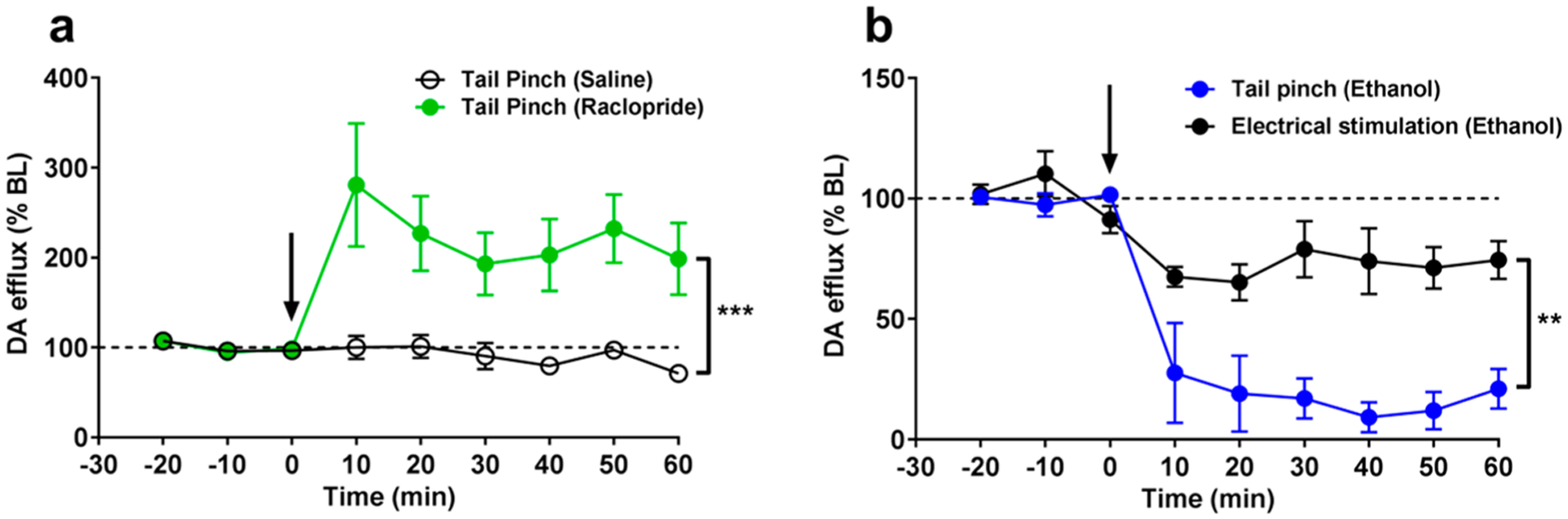
(a) Effects of raclopride and saline on tail pinch induced DA release in rat nucleus accumbens. (b) The comparison of ethanol effects on accumbal DA release evoked by the electrical stimulation of the VTA and tail pinch exposure. Anesthetized rats received a tail pinch or electrical stimulation of the VTA every 10 min until a stable baseline of DA release was established (at least 3 recordings with no more than 10% variance). Immediately following the last baseline recording, an intraperitoneal injection of saline, 2 g/kg ethanol, or 2 mg/kg raclopride was given. DA response was recorded for at least 1 h post injection. Data are presented as mean ± SEM n = 5 rats for each tail pinch group and n = 4 rats for electrical stimulation. **p < 0.01, ***p < 0.001.
It should be highlighted that the acute effects of ethanol on DA release were intensively explored by FSCV in vivo during the last two decades, and these results and their interpretations are controversial to some extent. The most consistent findings are that ethanol decreases terminal DA release induced by electrical stimulation of cell body regions.10,29–32 Consequently, the observed reduction in the pinch-evoked DA release could be just a consequence of the pharmacological action of ethanol on evoked DA release, which may be totally independent from its analgesic effect. However, the direct comparison of ethanol-induced changes in DA levels, which were elevated by VTA stimulation versus tail pinch (Figure 4b), supports the assumption that the analgesia can be partly accountable for the observed decrease in the negative stimulus-triggered DA release. In fact, ethanol more effectively suppressed DA concentrations after the pinch than after the electrical stimulation (F(1,7) = 27.3; p < 0.01). These pharmacological results support the idea that phasic DA release may act as an endogenous antinociceptive signal because an analgesic dramatically diminished this neurochemical response. This is not necessarily contradictory to the role of DA in avoidance behavior.27 Perhaps different cell populations are responsible for these two separate actions.
In summary, the main findings of this study fit well with the possible antinociceptive role of subsecond DA release in the nucleus accumbens core in response to negative stimuli. Therefore, along with the well-established role of mesolimbic DA release in reward and motivation,36 more evidence is available regarding the involvement of this neurotransmitter in mechanisms of nociceptive activity.37,38 The experimental paradigm used here offers new possibilities to further explore the dopaminergic component of these mechanisms with subsecond time resolution.
METHODS
Animals.
Naïve 2.5 month Sprague–Dawley rats were housed on a 12/12 h light/dark cycle with food and water available ad libitum. Animal handling and all procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All protocols were approved by the Wake Forest University School of Medicine Institutional Animal Care and Use Committee.
FSCV.
Rats were anesthetized with urethane (1.5 g/kg, i.p.) and secured in a stereotaxic frame. Two holes were drilled into the skull for electrode placement for the experiment with tail pinch exposure. A carbon fiber recording electrode was lowered into the nucleus accumbens core (AP + 1.3, ML + 1.3, from bregma, DV −6.8 to −7.2 mm; 100–150 μm exposed fiber tip, 7 μm diameter, Goodfellow, Oakdale, PA, United States) and an Ag/AgCl reference electrode was placed in the contralateral hemisphere. A third hole was drilled for the stimulating electrode and was inserted into the VTA (AP + 5.6, ML + 1.0, DV −7.4 to −7.7 mm) for the study of ethanol effects on electrically evoked DA efflux. The electrodes were connected to a voltammetric amplifier, and voltammetric recordings were taken at the carbon fiber electrode by applying a triangular waveform (−0.4 to +1.3 and back to −0.4 V vs Ag/AgCl, 400 V/s). DA was identified with background-subtracted cyclic voltammograms and characterized by oxidation and reduction peaks at ~ +0.6 and ~ −0.2 V, respectively (vs Ag/AgCl reference).
Tail pinches were conducted as previously described.6,7 Soft rubber gloves were used to minimize tissue damage and electrical noise artifacts. Importantly, the tail pinches and touches were conducted at the same location on the tail for each trial. The tail of the rat was held between the thumb and index finger and the pinch or the touch was performed for 2–3 s, approximately 1 cm from the posterior tip of the tail with pressure (P) of 3.12 ± 0.62 and 0.21 ± 0.15 MPa, respectively. P was calculated by measuring the contact area between the fingers and the tail and by a measurement of the applied force using a Pasco CI-6537 Force Sensor (Roseville, CA, United States). When pinches (4) were administered, intervals between applied stimuli were 5 s. There were no visible reactions, such as ear or whisker twitching, to the touch or tail pinches. When the administration of pinches was combined with neutral touches, neutral stimuli were performed 10 s prior to noxious stimulations. This combination was repeated 4 times with 2 min intervals to observe DA release after the touch (Figure 3a). For pharmacological experiments with the tail pinch, saline or raclopride (2 mg/kg, i.p.) or 20% ethanol (2 g/kg, i.p.) were injected after a stable baseline DA response to tail pinch was established (at least 3 measures with no more than 10% variance), and recordings were taken every 10 min for 1 h. The scheme for the experiment, where the effect of ethanol on electrically evoked DA release was explored, was the same as with the tail pinch experiment. The VTA stimulation (2 s, 50 Hz, 80–130 μA) was adjusted to induce DA efflux with an amplitude, which was equivalent to that observed with a tail pinch. The predrug values for the pinch- and electrically evoked DA release were 49 ± 5 and 46 ± 3 nM, respectively.
Data were digitized and stored on a computer. Following an experiment, the carbon fiber recording electrodes were calibrated in vitro using a flow injection analysis system with a known concentration of DA (1 μM) and performed in triplicate. The voltammetric current was measured at the peak oxidation potential and averaged to calculate a calibration factor. This factor was used to normalize the in vivo recordings of DA signals.
Histological Verification of Electrode Placement.
Electrode placements in the nucleus accumbens core were confirmed as previously described.6 Rats were perfused transcardially with a 0.9% saline wash followed by 10% buffered formalin fixative. Brains were rapidly removed, placed into 10% buffered formalin, and refrigerated overnight. They were then put through a series of refrigerated sucrose solutions (10, 20, or 30% in 0.1 M PB, pH 7.4) and sliced at 20 μm on a Leica M3050S cryostat. Slices were wet mounted from 1× PBS and stained with cresyl violet, and images were taken using Neurolucida 64-bit software (MBF Biosciences) with an Olympus BX51 microscope at 2× magnification to verify electrode location.
Statistical Analysis.
Data were analyzed using GraphPad Prism (GraphPad Software version 7.04, San Diego, CA, United States). Two-way and one-way repeated measure ANOVAs and paired t tests were conducted to determine statistical significance. Data are presented as a mean ± SEM, and the criterion for significance was set at p < 0.05.
ACKNOWLEDGMENTS
We would like to thank Joanne Konstantopoulos for help in preparing this manuscript.
Funding
The research for this study was funded by NIH grants AA022449, P50 AA026117-01 (E.A.B.), T32AA007565 (A.L.D.), the Tab Williams Family Endowment Fund (E.A.B.), and by the Russian Science Foundation Grant 14-50-00069 (M.A.M. and R.R.G.).
Footnotes
The authors declare no competing financial interest.
REFERENCES
- (1).Schultz W (1998) Predictive Reward Signal of Dopamine Neurons. J. Neurophysiol 80, 1–27. [DOI] [PubMed] [Google Scholar]
- (2).Ungless MA, Magill PJ, and Bolam JP (2004) Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science (Washington, DC, U. S.) 303, 2040–2042. [DOI] [PubMed] [Google Scholar]
- (3).Ungless MA (2004) Dopamine: the salient issue. Trends Neurosci. 27, 702–706. [DOI] [PubMed] [Google Scholar]
- (4).Montague PR, Hyman SE, and Cohen JD (2004) Computational roles for dopamine in behavioural control. Nature 431, 760–767. [DOI] [PubMed] [Google Scholar]
- (5).Anstrom KK, Miczek KA, and Budygin EA (2009) Increased phasic dopamine signaling in the mesolimbic pathway during social defeat in rats. Neuroscience 161, 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Budygin EA, Park J, Bass CE, Grinevich VP, Bonin KD, and Wightman RM (2012) Aversive stimulus differentially triggers subsecond dopamine release in reward regions. Neuroscience 201, 331–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Park J, Bucher ES, Budygin EA, and Wightman RM (2015) Norepinephrine and dopamine transmission in 2 limbic regions differentially respond to acute noxious stimulation. Pain 156, 318–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Wenzel JM, Rauscher NA, Cheer JF, and Oleson EB (2015) A role for phasic dopamine release within the nucleus accumbens in encoding aversion: a review of the neurochemical literature. ACS Chem. Neurosci 6, 16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Mateo Y, Budygin EA, Morgan D, Roberts DC, and Jones SR (2004) Fast onset of dopamine uptake inhibition by intravenous cocaine. Eur. J. Neurosci 20, 2838–2842. [DOI] [PubMed] [Google Scholar]
- (10).Jones SR, Mathews TA, and Budygin EA (2006) Effect of moderate ethanol dose on dopamine uptake in rat nucleus accumbens in vivo. Synapse (Hoboken, NJ, U. S.) 60, 251–255. [DOI] [PubMed] [Google Scholar]
- (11).Bass CE, Grinevich VP, Gioia D, Day-Brown JD, Bonin KD, Stuber GD, Weiner JL, and Budygin EA (2013) Optogenetic stimulation of VTA dopamine neurons reveals that tonic but not phasic patterns of dopamine transmission reduce ethanol self-administration. Front. Behav. Neurosci 7, 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Oleson EB, Talluri S, Childers SR, Smith JE, Roberts DC, Bonin KD, and Budygin EA (2009) Dopamine uptake changes associated with cocaine self-administration. Neuropsychophar-macology 34, 1174–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Oleson EB, Gentry RN, Chioma VC, and Cheer JF (2012) Subsecond dopamine release in the nucleus accumbens predicts conditioned punishment and its successful avoidance. J. Neurosci 32, 14804–14808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Brischoux F, Chakraborty S, Brierley DI, and Ungless MA (2009) Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc. Natl. Acad. Sci. U. S. A 106, 4894–4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Zweifel LS, Fadok JP, Argilli E, Garelick MG, Jones GL, Dickerson TM, Allen JM, Mizumori SJ, Bonci A, and Palmiter RD (2011) Activation of dopamine neurons is critical for aversive conditioning and prevention of generalized anxiety. Nat. Neurosci 14, 620–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Wang DV, and Tsien JZ (2011) Convergent processing of both positive and negative motivational signals by the VTA dopamine neuronal populations. PLoS One 6, e17047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Phillips PE, Robinson DL, Stuber GD, Carelli RM, and Wightman RM (2002) Real-time measurements of phasic changes in extracellular dopamine concentration in freely moving rats by fast-scan cyclic voltammetry. Methods Mol. Med 79, 443–464. [DOI] [PubMed] [Google Scholar]
- (18).Lee MC, and Tracey I (2010) Unravelling the mystery of pain, suffering, and relief with brain imaging. Curr. Pain Headache Rep 14, 124–131. [DOI] [PubMed] [Google Scholar]
- (19).Porreca F, and Navratilova E (2017) Reward, motivation, and emotion of pain and its relief. Pain 158, S43–s49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Bishop T, Marchand F, Young AR, Lewin GR, and McMahon SB (2010) Ultraviolet-B-induced mechanical hyper-algesia: A role for peripheral sensitisation. Pain 150, 141–152. [DOI] [PubMed] [Google Scholar]
- (21).Navratilova E, and Porreca F (2014) Reward and motivation in pain and pain relief. Nat. Neurosci 17, 1304–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Wood PB (2006) Mesolimbic dopaminergic mechanisms and pain control. Pain 120, 230–234. [DOI] [PubMed] [Google Scholar]
- (23).Altier N, and Stewart J (1999) The role of dopamine in the nucleus accumbens in analgesia. Life Sci. 65, 2269–2287. [DOI] [PubMed] [Google Scholar]
- (24).Gear RW, Aley KO, and Levine JD (1999) Pain-induced analgesia mediated by mesolimbic reward circuits. J. Neurosci 19, 7175–7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Reboucas EC, Segato EN, Kishi R, Freitas RL, Savoldi M, Morato S, and Coimbra NC (2005) Effect of the blockade of mu1-opioid and 5HT2A-serotonergic/alpha1-noradrenergic receptors on sweet-substance-induced analgesia. Psychopharmacology 179, 349–355. [DOI] [PubMed] [Google Scholar]
- (26).Tiemann L, Heitmann H, Schulz E, Baumkotter J, and Ploner M (2014) Dopamine precursor depletion influences pain affect rather than pain sensation. PLoS One 9, e96167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Taylor AM, Becker S, Schweinhardt P, and Cahill C (2016) Mesolimbic dopamine signaling in acute and chronic pain: implications for motivation, analgesia, and addiction. Pain 157, 1194–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Thompson T, Oram C, Correll CU, Tsermentseli S, and Stubbs B (2017) Analgesic Effects of Alcohol: A Systematic Review and Meta-Analysis of Controlled Experimental Studies in Healthy Participants. J. Pain 18, 499–510. [DOI] [PubMed] [Google Scholar]
- (29).Budygin EA, Phillips PE, Robinson DL, Kennedy AP, Gainetdinov RR, and Wightman RM (2001) Effect of acute ethanol on striatal dopamine neurotransmission in ambulatory rats. J. Pharmacol. Exp. Ther 297, 27–34. [PubMed] [Google Scholar]
- (30).Budygin EA, Phillips PE, Wightman RM, and Jones SR (2001) Terminal effects of ethanol on dopamine dynamics in rat nucleus accumbens: an in vitro voltammetric study. Synapse (Hoboken, NJ, U. S.) 42, 77–79. [DOI] [PubMed] [Google Scholar]
- (31).Robinson DL, Volz TJ, Schenk JO, and Wightman RM (2005) Acute ethanol decreases dopamine transporter velocity in rat striatum: in vivo and in vitro electrochemical measurements. Alcohol.: Clin. Exp. Res 29, 746–755. [DOI] [PubMed] [Google Scholar]
- (32).Mathews TA, John CE, Lapa GB, Budygin EA, and Jones SR (2006) No role of the dopamine transporter in acute ethanol effects on striatal dopamine dynamics. Synapse (Hoboken, NJ, U. S.) 60, 288–294. [DOI] [PubMed] [Google Scholar]
- (33).Yim HJ, and Gonzales RA (2000) Ethanol-induced increases in dopamine extracellular concentration in rat nucleus accumbens are accounted for by increased release and not uptake inhibition. Alcohol (N. Y., NY, U. S.) 22, 107–115. [DOI] [PubMed] [Google Scholar]
- (34).Yim HJ, Schallert T, Randall PK, and Gonzales RA (1998) Comparison of local and systemic ethanol effects on extracellular dopamine concentration in rat nucleus accumbens by microdialysis. Alcohol.: Clin. Exp. Res 22, 367–374. [PubMed] [Google Scholar]
- (35).Fox ME, Mikhailova MA, Bass CE, Takmakov P, Gainetdinov RR, Budygin EA, and Wightman RM (2016) Cross-hemispheric dopamine projections have functional significance. Proc. Natl. Acad. Sci. U. S. A 113, 6985–6990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Berridge KC, and Kringelbach ML (2015) Pleasure systems in the brain. Neuron 86, 646–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Baliki MN, and Apkarian AV (2015) Nociception, Pain, Negative Moods, and Behavior Selection. Neuron 87, 474–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Tobaldini G, Sardi NF, Guilhen VA, and Fischer L (2018) Pain Inhibits Pain: an Ascending-Descending Pain Modulation Pathway Linking Mesolimbic and Classical Descending Mechanisms. Mol. Neurobiol, DOI: 10.1007/s12035-018-1116-7. [DOI] [PubMed] [Google Scholar]


