Abstract
A growing body of epidemiological and research data has associated neurotropic viruses with accelerated brain aging and increased risk of neurodegenerative disorders. Many viruses replicate optimally in senescent cells, as they offer a hospitable microenvironment with persistently elevated cytosolic calcium, abundant intracellular iron, and low interferon type I. As cell-cell fusion is a major driver of cellular senescence, many viruses have developed the ability to promote this phenotype by forming syncytia. Cell-cell fusion is associated with immunosuppression mediated by phosphatidylserine externalization that enable viruses to evade host defenses. In hosts, virus-induced immune dysfunction and premature cellular senescence may predispose to neurodegenerative disorders. This concept is supported by novel studies that found postinfectious cognitive dysfunction in several viral illnesses, including human immunodeficiency virus-1, herpes simplex virus-1, and SARS-CoV-2. Virus-induced pathological syncytia may provide a unified framework for conceptualizing neuronal cell cycle reentry, aneuploidy, somatic mosaicism, viral spreading of pathological Tau and elimination of viable synapses and neurons by neurotoxic astrocytes and microglia. In this narrative review, we take a closer look at cell-cell fusion and vesicular merger in the pathogenesis of neurodegenerative disorders. We present a “decentralized” information processing model that conceptualizes neurodegeneration as a systemic illness, triggered by cytoskeletal pathology. We also discuss strategies for reversing cell-cell fusion, including, TMEM16F inhibitors, calcium channel blockers, senolytics, and tubulin stabilizing agents. Finally, going beyond neurodegeneration, we examine the potential benefit of harnessing fusion as a therapeutic strategy in regenerative medicine.
Keywords: fusion, HERVs, cellular senescence, virus, syncytia
Graphical Abstract.
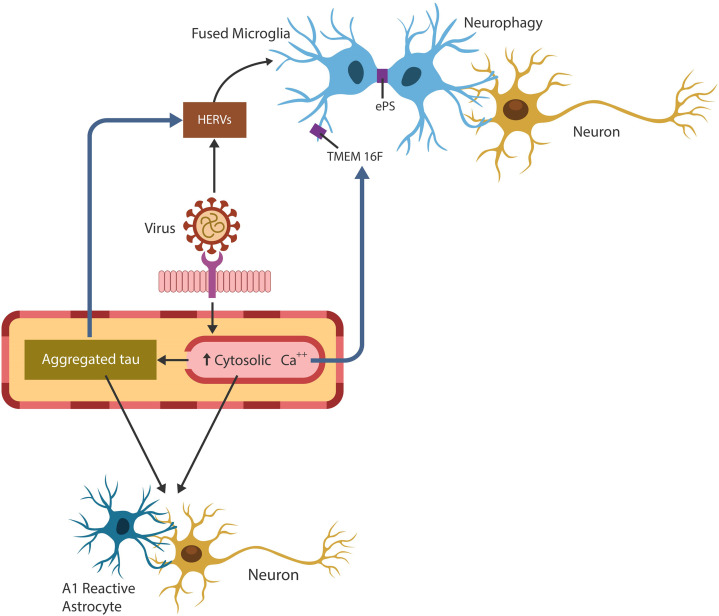
Exogenous viruses hijack human physiological fusogens to generate cellular senescence and immunosuppression, conditions favorable for pathogens’ undetected entry and replication. Pathological cell-cell fusion is initiated by viral arginine motif that drills pores in host plasma cell membranes. The host responds to cellular injury by upregulating cytosolic Ca2+, Tau hyperphosphorylation, TMEM16F activation and phosphatidylserine externalization (ePS). These changes may predispose to neurodegeneration as brain cell-cell fusion results in 1. conversion of supportive to neurotoxic astrocytes, 2. HERVs activation and 3. microglial aberrant phagocytosis of viable neurons (neurophagy) and synapses (synaptophagy).
Highlights
Enveloped viruses induce cell-cell fusion and syncytia formation to increase infectivity and evade detection.
In hosts, fusion promotes immunosuppression and cellular senescence that may contribute to neurodegeneration by several mechanisms:
Viruses spread pathological Tau throughout the extracellular space.
Virus-upregulated cytosolic calcium drives Tau hyperphosphorylation and conversion of trophic into neurotoxic astrocytes.
Virus-activated HERVs promote microglial fusion and aberrant phagocytosis of healthy synapses and neurons.
Neurons with pathological Tau expose phosphatidylserine on the cell surface, facilitating fusion or apoptosis.
Viruses may disrupt cognition by altering host cellular cytoskeleton, especially the microtubules.
Introduction
Cell-cell fusion is a physiological or pathological process in which two or more cells merge their plasma membranes and share the cytoplasm and nuclei, forming syncytia. Under normal circumstances, cell-cell fusion plays a major role in the merger of trophoblasts, gametes, myoblasts, and immune cells (Brukman et al., 2019; Zhang et al., 2020). In addition, physiological syncytia contribute to wound healing and nerve repair (Losick et al., 2013; Neumann et al., 2019). Interestingly, fusogens are derived from viral fossils embedded in human DNA, that can be expressed under pathological circumstances, such as infection with exogenous viruses (Sapir et al., 2008; Balestrieri et al., 2021). In this regard, placental fusogen syncyctin-2 induces immunosuppression necessary for maternal acceptance of the allogeneic fetus, while syncytin-1 triggers placental senescence and immune activation to initiate labor (Cox and Redman, 2017; Gal et al., 2019; Lokossou et al., 2020; Roberts et al., 2021). Viral hijacking of these particular fusogens augments infection by disabling host immune defenses and establishing a virus-friendly environment marked by elevated cytosolic calcium, abundant intracellular iron, and low interferon type I (Martin and Bernard, 2018; Frisch and MacFawn, 2020; Lynch et al., 2021). As cell-cell fusion is a major trigger of cellular senescence, viruses may have developed the ability to exploit host fusogens, promoting premature aging (Gal et al., 2019). Indeed, epidemiological and research data have associated neurotropic viruses with accelerated brain aging and neurodegenerative disorders (Mavrikaki et al., 2021; Sait et al., 2021; Filgueira et al., 2021; Dowd and McKernan, 2021). Along these lines, herpes simplex virus 1 (HSV-1) and human herpesvirus 6 (HHV-6) have been associated with Alzheimer’s disease (AD) and multiple sclerosis (MS) respectively, while human immunodeficiency virus type 1 (HIV-1) contributes to HIV-associated neurocognitive disorders (HAND, 18-19). In addition, cell-cell fusion is associated with the externalization of phosphatidylserine (PS) on the cell surface, a marker of immunosuppression that enables stealthy viral ingress into host cells (Birge et al., 2016). In addition, infection with SARS-CoV-2, the etiologic agent of COVID-19, was accompanied by various cognitive sequelae, linking this virus to neurodegenerative pathology (Paniz-Mondolfi et al., 2020; Wang et al., 2021; Kandemirli et al., 2021; Frontera et al., 2022).
The concept of brain cell syncytia and multinucleation is not new. In the 19th century, Camillo Golgi and Ramón y Cajal were debating whether neurons comprised separate entities or functioned as a brain-wide syncytium (Kiyoshi and Zhou, 2019). In the 20th century, electron microscopy confirmed both viewpoints as under normal circumstances neurons are individual cells, while astrocytes form functional syncytia (Ma et al., 2016). Pathologically however, neurons can fuse with each other or the neighboring cells, forming syncytia. For example, neuron-neuron fusion was documented in aging brains, AD, and MS, linking syncytia to neurodegenerative pathology (Kemp et al.; Hornik et al., 2014). Recent studies in Caenorhabditis elegans (C. elegans) reported fusogen-mediated neuron-neuron and neuron-glia mergers, suggesting that syncytia formation may drive neurocognition (Alexander et al., 2014; Giordano-Santini et al., 2020). Moreover, multinucleated neurons in the supraoptic nucleus were demonstrated in patients with pneumonia, a disease often associated with respiratory viruses, likely implicating virus-usurped host fusogens in this pathology (Ishunina et al., 2000). Fusion of bone marrow cells with Purkinje neurons have been documented by both clinical and preclinical studies, indicating that peripheral and brain cells can merge (Alvarez-Dolado et al., 2003). Furthermore, fused Purkinje neurons were demonstrated in patients with Friedreich’s ataxia, a genetic neurodegenerative movement disorder associated with cellular senescence, calcium (Ca2+), and iron dyshomeostasis (Bolinches-Amorós et al., 2014; Llorens et al., 2019). Interestingly, antiretroviral drug etravirine was found beneficial to patients with Friedreich’s ataxia, perhaps suggesting that the disease may be exacerbated by the activation of human endogenous retroviruses (HERVs) (Alfedi et al., 2019; Lynch et al., 2019).
Tauopathies are neurodegenerative disorders marked by the accumulation of Tau-associated neurofibrillary tangles (NFTs) and cognitive deficits directly correlated with the synaptic and neuronal loss (Giannakopoulos et al., 2003; Dejanovic et al., 2018). Under normal circumstances, the Tau protein is associated with microtubule stabilization and comprises the cellular cytoskeleton of many cell types, including the neurons (Hervy and Bicout, 2019). Pathological Tau (pTau) is hyperphosphorylated and drives neurodegenerative disorders by disrupting both microtubular networks and axonal transport (Millecamps and Julien, 2013; Salvadores et al., 2020).
Many neurotropic viruses, including influenza and SARS-CoV-2, induce cellular senescence and age-related pathology by exploiting the host cellular cytoskeleton and its constituent microtubules (Moujaber et al., 2019; Simpson and Yamauchi, 2020; Wen et al., 2020). Indeed, preclinical studies have found that the envelope (E) of SARS-CoV-1 virus can upregulate intracellular Ca2+ by usurping the host Ca2+ channels in the endoplasmic reticulum Golgi intermediate compartment (ERGIC, 45). As a result, it was suggested that the SARS-CoV-2 spike protein binds Ca2+, facilitating viral infection (Saurav et al., 2021). As Ca2+ is an established regulator of neuronal plasticity, learning, and memory, it is not surprising that Ca2+ dyshomeostasis can promote tauopathies (Vega et al., 2008; Zündorf and Reiser, 2011). Indeed, clinical and preclinical studies have shown that increased intracellular Ca2+ can lead to Tau hyperphosphorylation (Etcheberrigaray et al., 1998; Zempel et al., 2010; Cao et al., 2019). Moreover, recent studies have linked upregulated cytosolic Ca2+ to ferroptosis, an iron-dependent cell death, encountered in AD and other tauopathies (Ashraf and So, 2020; Pedrera et al., 2021; Wang et al., 2022). Although COVID-19 has been associated with hyperinflammatory responses and hypoxia, both of which can upregulate cytosolic Ca2+, this can also be accomplished by virus-induced cellular senescence (Izquierdo et al., 2014; Martin and Bernard, 2018; Danta, 2021; Wicher et al., 2021). In fact, hypoxia may compensate for virus-mediated premature aging, while the accumulation of senescent cells can trigger autoimmune responses (Leontieva et al., 2012; Fukushima et al., 2018; van Vliet et al., 2021).
In this paper, we take the position that virus-induced cellular senescence predisposes to neurodegeneration by upregulating intracellular Ca2+ and iron, increasing ferroptosis and pTau-mediated neuronal loss. In return, this pathology may alter glial homeostasis, contributing to neuronal loss by neurotoxic astrocytes and microglia (Liddelow et al., 2017; Zhang et al., 2020). In this regard, phenomena previously associated with both neurodegeneration and viral infections, including neuronal cycle reentry, aneuploidy, hyperploid DNA, and somatic mosaicism, may be explained by fusion-mediated multinucleation (Knight and Robertson, 2004; Dove et al., 2006; Mosch et al., 2007; Lopes et al., 2009; Arendt, 2012; Miller et al., 2021).
In our previous work, we elaborated on the connection between the SARS-CoV-2 virus and cellular senescence, a subject that will not be discussed in detail here, however, as senescence was associated with microtubular reorganization, the virus may contribute to neurodegeneration by promoting Tau hyperphosphorylation (Ramani et al., 2020; Sfera et al., 2021; Sfera et al., 2021; Pratt et al., 2021) Indeed, pTau, genome destabilization, and HERV activation, may promote neurotoxic astrocytes and microglia, phenomena previously linked to neuronal and synaptic loss (Hornik et al., 2014; Brelstaff et al., 2018; Dittmar et al., 2021; Sokolova et al., 2021). In addition, our focus will be on the cell-cell fusion with less emphasis on virus-host merger, a topic that exceeds the purpose of this review.
In the following sections, we take a closer look at the virus-mediated cell-cell fusion and vesicular merger in the pathogenesis of neurodegenerative disorders. We present a “decentralized” information processing model that conceptualizes neurodegeneration as a systemic illness, triggered by cytoskeletal pathology. We also discuss potential strategies for preventing cellular senescence and immunosuppression, including TMEM16F inhibitors, calcium channel blockers, senolytics, and tubulin stabilizing agents. In addition, we examine the possibility of harnessing fusion as a treatment strategy in regenerative medicine.
Physiological and Pathological Cell-Cell Fusion
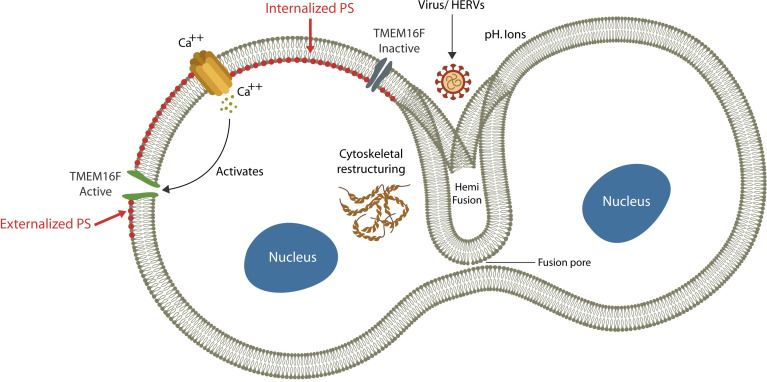
Fusion or merging two biological membranes and their lipid bilayers, is a complex process that requires cellular proximity, bridging the outer leaflets of apposing plasma membranes, and the formation of a “stalk”. Subsequently, a hemifusion diaphragm is generated, followed by fusion pore enlargement that ultimately coalesces the two compartments (Akimov et al., 2014) ( Figure 1 ). This process requires a shift in the membrane structural asymmetry, including exposing phosphatidylserine (ePS) in the exoplasmic leaflet, a move that triggers immunosuppression (Birge et al., 2016) ( Figure 1 ).
Figure 1.
Exogenous viruses and HERVs can hijack physiological fusogens, fusing host cells. The fusion process is comprised of: (1) cytosolic Ca2+ upregulation (via extracellular compartment uptake or endoplasmic reticulum release), (2) TMEM16F activation and (3) PS externalization (ePS). Cells undergo fusion or elimination, depending on extracellular pH. Viruses benefit from ePS and elevated cytosolic Ca2+ as the former induces immunosuppression and the later cellular senescence.
Under normal circumstances, the membrane structural asymmetry is maintained by ATP-dependent transporters, including “floppase” (outward transport), “flippase” (inward transport) and “scramblase” (bi-directional transport) that keep phospholipids in their proper leaflet (Pomorski and Menon, 2016). Under normal circumstances PS is localized on the cytoplasmic side and is not usually externalized, except when the cell is damaged and ready for elimination or fusion (Whitlock and Chernomordik, 2021). As both processes are associated with immunosuppression, they facilitate stealthy viral ingress, promoting infection (Birge et al., 2016). Indeed, ePS induces immunosuppression, while upregulated cytosolic Ca2+ is a major driver of cellular senescence (Martin and Bernard, 2018; Zöphel et al., 2020; Wicher et al., 2021). Cells with exposed PS can undergo either fusion or elimination, probably depending on the extracellular pH; an acidic environment promotes fusion, while an alkaline pH favors phagocytosis (Zöphel et al., 2020; Whitlock and Chernomordik, 2021). Indeed, establishing an alkaline extracellular environment is believed to inhibit cell-cell fusion and lower the SARS-CoV-2 infection (Lardner, 2001).
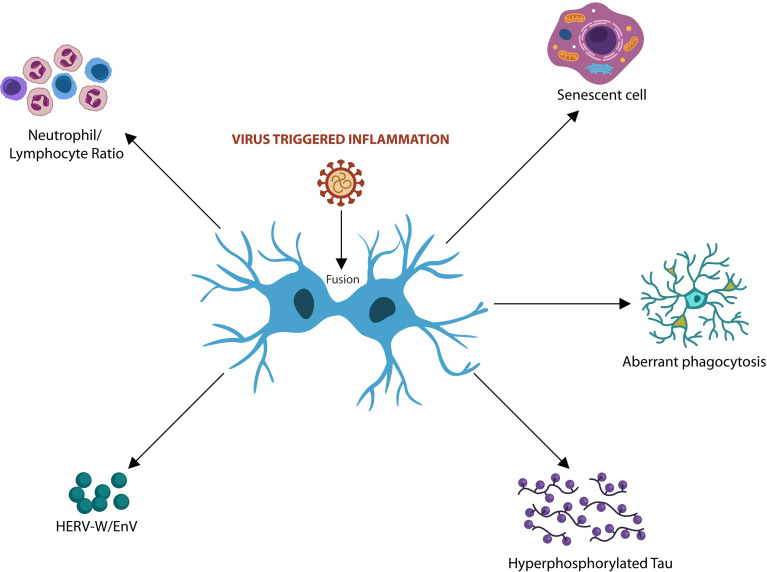
Placental fusogen syncyctin-2 induces immunosuppression, while syncytin-1, a molecule with superantigen properties, triggers cellular senescence and inflammation, probably to facilitate delivery (Cox and Redman, 2017; Gal et al., 2019; Lokossou et al., 2020; Roberts et al., 2021). By exploiting both fusogens, viruses can manipulate host immunity, inducing immunosuppression and/or immune senescence and exhaustion. At first glance, immunosuppression and cellular senescence appear to be opposites as the former inhibits while the later activates immunity. However, both processes contribute to host “immune failure” as the proinflammatory senescence-associated secretory phenotype (SASP) continuously challenges the immune system into exhaustion. For example, HIV-1 can suppress immunity directly by inducing lymphopenia or indirectly by igniting a senescence-mediated prolonged inflammatory response that causes exhaustion (Appay and Sauce, 2008; Fenwick et al., 2019). Moreover, the SARS-CoV-2 virus was associated with both lymphopenia and inflammation, demonstrating its capability to inhibit host immunity by both mechanisms (Huang and Pranata, 2020) ( Figure 2 ).
Figure 2.
Virus-induced fusion and syncytia formation trigger host cellular senescence and immunosuppression associated with tauopathies. Elevated neutrophil/lymphocyte ratio (NLR) and premature senescence were associated with both viral infections and AD. Virus-activated HERVs and Tau hyperphosphorylation were linked to neurodegeneration. Cell-cell fusion drives aberrant microglia and neurotoxic astrocytes that often engulf viable neurons and synapses, contributing to neurodegeneration.
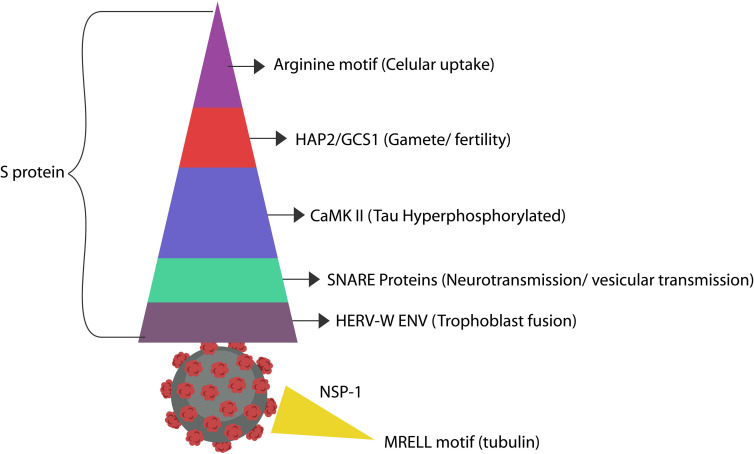
Several viruses, including Zika, promote host immunosuppression by hijacking HAP2/GCS1, a physiological fusogen associated with gamete fusion and zygote formation, disrupting these processes (Polack et al., 2005; Liu et al, 2015; Valansi et al., 2017). In addition, the established fusogenic pathogen, respiratory syncytial virus (RSV), enters host cells via a HAP2/GCS1-like cysteine-rich region, lowering host immunity (Bertrand et al., 2013; Fédry et al., 2017). As the S antigen of SARS-CoV-2 virus contains many cysteine-rich repeats, it may be easily recognized by HAP2/GCS1, triggering infertility (Puthenveetil et al., 2021) ( Figure 3 and Table 2 ). Moreover, the HIV-1 trans-activator of transcription (Tat) protein contains cysteine-rich regions, suggesting HAP2/GCS1 exploitation (Bertrand et al., 2013).
Figure 3.
The SARS-CoV-2 antigens display molecular mimicry with numerous host proteins, some of which affect cognition (Yapici-Eser et al., 2021). Arginine and Ca2+ likely convert MAP-Tau to pTau, promoting neurodegeneration. As pTau-containing neurons externalize PS, they may be eliminated by microglia prematurely, outlining another neurodegeneration mechanism (Brelstaff et al., 2018). In addition, pTau perforates cell membranes, likely explaining the mechanism of PS externalization (Lasagna-Reeves et al., 2014). A recent virtual-screening study found that the S protein of the SARS-CoV-2 virus expresses a CaMKII-like system that contributes to the hyperphosphorylation of Tau protein (Wenzhong and Hualan, 2021). However, even in the absence of CaMKII, upregulated cytosolic Ca2+ can promote Tau hyperphosphorylation (Cao et al., 2019). The cysteine-rich motif in the S antigen resembles the extracellular region of HAP2/GCS1, the fusogen involved in gamete merger. SNAP-25 and SNAP-29 are SNARE proteins in charge of organelle fusion and exocytosis. SNAP-25 drives exocytosis in the CNS, while SNAP-29 orchestrates the fusion of autophagosomes with lysosomes (Kádková et al., 2019). These proteins are likely mimicked by the S antigen repeats (Arora et al., 2017; Mastrodonato et al., 2018). In addition, the SARS-CoV-2 protein ORF3a inhibits the fusion of autophagosomes with lysosomes, suggesting molecular mimicry with SNAP-29 protein (Miao et al., 2021). The SARS-CoV-2 S protein was demonstrated to activate HERV-W-ENV gene in T lymphocytes, a protein associated with Alzheimer’s disease (Balestrieri et al., 2021; Licastro and Porcellini, 2021). Another case of molecular mimicry was found between the SARS-CoV-2 NSP-1 antigen and host tubulin, likely connecting this virus to dysfunctional information processing (Sobhy, 2021).
Table 2.
Viruses induce immunosuppression and cellular senescence by expressing molecular motifs that mimic physiological fusogens.
| Neurotropic viruses | Targets | References |
|---|---|---|
| HIV-1 Tat antigen | α- and β-tubulins | (Sayed et al., 2020) |
| HSV-1 | Microtubular network | (García-Navas et al., 2021) |
| Dengue | Microtubular network | (Martí i Líndez and Reith, 2021) |
| Zika | β-tubulin | (Munder, 2009) |
| SARS-CoV-2 MRELL motif | Tubulins/Microtubular network | (Simpson and Yamauchi, 2020) |
The cell-cell fusion triggered by these repeats may contribute to pathology, including neurodegeneration.
Aside from peptides, physiological fusogens are also comprised of amino acids, fusion-associated small transmembrane (FAST) proteins, and chemical agents, including dextran sulfate, Ca2+, and sodium nitrate (Goujon et al., 2015; Abdou and Henderson, 2019; Chan et al., 2021). As opposed to neurons, astrocytes generate physiological syncytia and share their cytoplasm through gap junctions formed by connexin 30 or 43 (Xing et al., 2019). In response to cytosolic Ca2+, astrocytes release gliotransmitters, maintaining network homeostasis (Guerra-Gomes et al., 2018). Connexin 43 (Cx43), implicated in both AD and HAND, likely plays a major role in the pathogenesis of neurodegenerative disorders via neurotoxic astrocytosis (Kajiwara et al., 2018). Indeed, novel studies have associated Cx43 with both HIV-1 antigen Tat and pTau, connecting these proteins to HAND and neurodegeneration (Berman et al., 2016; Fuglewicz et al., 2017).
Taken together, exogenous viruses hijack host physiological fusogens to lower antiviral immunity and induce cellular senescence, conditions favorable for viral entry and thriving. In susceptible hosts, these processes may predispose to neurodegenerative disorders by several mechanisms, including pathological Tau dissemination, upregulated cytosolic Ca2+ driving Tau hyperphosphorylation, conversion of trophic into neurotoxic glia, Tau-mediated ePS and cytoskeletal dysfunction.
Cell-Cell Fusion in Neuropsychiatric Disorders
The connection between intracellular pathogens and neuropsychiatric symptoms has been known for a long time. Malaria, toxoplasmosis, and lately Porphyromonas gingivalis have been associated with psychopathology, while several viruses were linked to neurodegeneration and developmental disabilities (Henry et al., 2010; Lima et al., 2021; Olsen, 2021; Chemparthy et al., 2021). Along these lines, the 1918 influenza pandemic and the more recent H5N1 epidemic were associated with Parkinson’s disease and autism (Maurizi, 2010; Shuid et al., 2021). In addition, offspring of mothers pregnant during the 1964 rubella epidemic were more likely to develop autism and schizophrenia in adulthood compared to the general population (Brown et al., 2000). However, the question begging for an answer is: can viruses disrupt host cognition directly?
Virus-Induced Neurocognitive Dysfunction, Direct Mechanisms
Several modalities of unmediated viral interference with host neurocognitive brain areas were recently described, including pTau dissemination, anti-pTau antibodies, molecular mimicry with cellular cytoskeleton, and direct viral invasion of host cognitive centers.
Several viruses, including SARS-CoV-2, were demonstrated to spread pTau throughout the brain extracellular space, directly promoting neurodegenerative pathology (Liu et al., 2021).
A recent COVID-19 study found that pTau could elicit autoantibodies, exacerbating end-organ damage, probably including the brain (Magalhães et al., 2021). Along these lines, earlier preclinical studies have reported that tauopathies can be initiated by anti-pTau autoantibodies (Yanamandra et al., 2017).
Several viruses mimic host microtubular proteins, altering cognition at the cytoskeletal level. For example, HIV-1 imitates microtubular end-binding protein 1 (EB1), disrupting cognition directly (Naghavi, 2021). Other viruses, including Influenza A and Zika, exploit the host microtubular network, while SARS-CoV-2 may accomplish the same via a tubulin-like MREL motif located in the NSP1 protein (Simpson and Yamauchi, 2020; Sobhy, 2021).
The SARS-CoV-2 virus can directly invade the brain cognitive centers, inducing Alzheimer’s-like neuropathology (Shen et al., 2022).
The S antigen of SARS-CoV-2 may contain a Ca²+/calmodulin-dependent protein kinase II (CaMKII) motif that could alter the microtubules directly (Wenzhong and Hualan, 2021)(please see the “Fusion by Calcium” section).
Virus-Induced Neurocognitive Dysfunction, Indirect Mechanisms
Aside from the direct effect of viral proteins on microtubules or their constituent, tubulin, viruses can lead to pTau accumulation indirectly via:
Cellular senescence
HERV activation
Cytosolic Ca2+ and iron upregulation,
Neurotoxic glia, and
Vesicular trafficking (Nieto-Torres et al., 2014; Simpson and Yamauchi, 2020; Wen et al., 2020; Spotorno et al., 2020).
Extra and intracellular vesicular trafficking are discussed below, while the other mechanisms are described in the following sections.
SNARE Proteins and Vesicular Trafficking
The soluble NSF Attachment Receptor (SNARE) belongs to a superfamily of fusogenic molecules that mediate the merger of intracellular and extracellular vesicles (EVs), including those participating in the formation of immunological synapse (the interface between the T cells and antigen-presenting cells) (Das et al., 2004; Koike and Jahn, 2019). Vesicular fusion machinery is composed of v and t-SNAREs and their components, the synaptosomal associated protein 25 and 29 (SNAP25)(SNAP-29) highly expressed in neurons (Arora et al., 2017; Mastrodonato et al., 2018) ( Figure 3 and Table 1 ).
Table 1.
Physiological fusogens exploited by endogenous or exogenous viruses via molecular mimicry.
| PHYSIOLOGICAL FUSOGENS | VIRUS | FUNCTION | REFERENCES |
|---|---|---|---|
| Syncytin-1 | Influenza A/HERV-W | Senescence/inflammation/labor | (Moujaber et al., 2019; Simpson and Yamauchi, 2020; Wen et al., 2020) |
| Syncytin-2 | HERV-FRD | Trophoblast fusion/placental exosomes | (Cox and Redman, 2017; Gal et al., 2019; Lokossou et al., 2020; Roberts et al., 2021) |
| HAP2/GCS1 | Zika virus | Gamete fusion | (Valansi et al., 2017) |
| Arginine | SARS-CoV-2 and Influenza-A | Myoblast/vesicle fusion | (Das et al., 2004; Koike and Jahn, 2019; Gong et al., 2021) |
| SNAP25/SNAP-29 | SARS-CoV-2 | Fusion intracellular vesicles | (Yapici-Eser et al., 2021) |
| Ca2+ | SARS-CoV-2/HIV | Cell-cell/vesicle fusion | (Martin and Bernard, 2018; Zöphel et al., 2020; Wicher et al., 2021) |
| MAP-Tau | Herpes simplex virus/HIV Tat protein | Microtubule stabilization/fusion | (Hibbard and Sandri-Goldin, 1995; Saylor et al., 2016; Itzhaki, 2017) |
| TMEM16F | SARS-CoV-2 | Trophoblast fusion | (Arora et al., 2017; Kajiwara et al., 2018; Mastrodonato et al., 2018) |
| Tubulin | influenza A virus | Trophoblast fusion/Cx43 | (Simpson and Yamauchi, 2020; Sobhy, 2021) |
A recent protein-protein interaction (PPI) study reported that the SARS-CoV-2 mimics SNAP25 and SNAP-29, exploiting vesicular transport to egress host cells (Ghosh et al., 2020; Yapici-Eser et al., 2021). These SNARE proteins, characterized by coiled-coil homology domains, facilitate synaptic transmission, memory, and long-term potentiation (LTP), indicating a direct link between viruses and memory (Hou et al., 2006). The SARS-CoV-2 viral protein ORF3a promotes lysosomal exocytosis by inhibiting SNAP-29-mediated fusion of autophagosome and autolysosome (Pan et al., 2005; Barberis et al., 2021; Chen et al., 2021) ( Figure 3 ).
Cell-Cell Fusion and Tau Hyperphosphorylation
Recent studies have associated virus-induced cell-cell fusion with the accumulation and dissemination of pTau throughout the brain extracellular compartment (Miao et al., 2021; Liu et al., 2021). Indeed, exogenous viruses were demonstrated to spread pTau, probably explaining the previously noted prion-like properties of this protein (Lasagna-Reeves et al., 2014; Brunello et al., 2020). Moreover, viruses may exploit pTau for its pore-forming qualities and syncytia formation however, pierced cell membranes may enable extracellular dissemination (d’Errico and Meyer Luehman, 2020). Along these lines, a recent brain organoid study reported abnormal Tau after infection with SARS-CoV-2, suggesting virus-mediated MAP-Tau to pTau conversion (Ramani et al., 2020). Other studies have implicated arginine in the transformation of MAP-Tau into pTau, linking guanidinium side-chains to tauopathies (Walrant et al., 2017). Moreover, virus-mediated Tau hyperphosphorylation was documented in HIV, Influenza A and COVID-19, connecting these viral infections to tauopathies (Brown et al., 2014; Cao et al., 2019).
Recent studies have found that pTau promotes neuronal cell cycle reentry and the subsequent aneuploidy, hyperploidy, and somatic mosaicism documented in both viral infections and neurodegenerative disorders (Knight and Robertson, 2004; Dove et al., 2006; Mosch et al., 2007; Lopes et al., 2009; Arendt, 2012; Miller et al., 2021). Indeed, other novel studies have revealed that pTau can destabilize the genome, activating transposable element (TE), a hallmark of cancer and neurodegeneration (Grundman et al., 2021). In addition, exogenous viruses and pTau can activate HERVs, promoting inflammation and infection (Licastro and Porcellini, 2021). Furthermore, extracellular pTau was demonstrated to alter αV/β1 integrin, converting trophic into neurotoxic astrocytes, emphasizing another neurodegeneration-inducing mechanism (Wang and Ye, 2021).
Aside from its well-established role in neuronal cells, MAP-Tau contributes to the pathophysiology of placenta; it is upregulated in normal pregnancies and lowered in pre-eclampsia (Bergman et al., 2018; Lederer et al., 2020). As viruses disrupt host immunity by converting MAP-Tau to pTau, placental function is likely altered. For example, phosphorylated Tau231, is an early biomarker of pre-eclampsia, linking viruses to reproductive pathology (Brown, 1999; Cheng et al., 2021).
Taken together, viruses promote pTau formation and dissemination to generate cell membrane pores, cell-cell fusion, senescence and immune dysfunction. In this regard, pTau accumulation may predispose to both neurodegeneration and placental pathology.
Cell-Cell Fusion and Information Processing
Elegant studies in artificially fused unicellular microorganisms demonstrated transfer of learned behavior from one cell to the other, suggesting that rudimentary memory may be stored in the cytoskeletal proteins (Vogel and Dussutour, 2016). In addition, information transfer was detected after fusing two bacteria of different species, indicating that microtubules and tubulin, recently identified in microbes, could participate in this process (Pilhofer et al., 2011; Charubin et al., 2020). Interestingly, human tissues, such as the skeletal muscle, fascia and blood cells may process and store information, further implicating microtubules and tubulin in cognition (Moore and Cao, 2008; Tozzi, 2014; Snijders et al., 2020). In this regard, acquisition of donor personality traits, was documented after cardiac transplants, suggesting that information processing and storing may be a decentralized, blockchain phenomenon (Liester, 2020). Indeed, earlier studies have linked cognition to tubulin and tubulin inhibiting chemotherapy with dysfunctional memory (Craddock et al., 2012; Tuszynski et al., 2020; Kalra et al., 2020). With the same token, treatment with colchicine, a microtubule-disassembling drug, disrupts cognition, further connecting tubulin to information processing (Dent, 2017; Chaldakov, 2018; Sordillo and Sordillo, 2020). Interestingly, tubulin interacts directly with Cx43, the gap junction molecule involved in astrocytic syncytia, suggesting a role in the homeostasis of these cells (Giepmans et al., 2001). Along these lines, tubulin loss in C. elegans was connected to neurodegeneration and dysfunctional neurotransmission (Kraemer et al., 2003).
Recent studies have associated several viruses, including HIV-1, HSV-1, Dengue and Zika, with cognitive dysfunction, suggesting that pathogens can exploit host microtubular networks (Naghavi and Walsh, 2017; Dharan and Campbell, 2018). On the other hand, microtubule-stabilizing agents (MSAs) have demonstrated antiviral and neuroprotective effects, emphasizing a potential therapeutic strategy (Sirakanyan et al., 2021).
Recent studies have shown that microtubules can generate action potential-like electrical oscillations, connecting these proteins to the higher brain functions such as memory and consciousness (Ballatore et al., 2012). Indeed, the recent concepts, “cellular consciousness” and “molecular brains” suggest that cognition and information processing may occur at the cellular level (Baluška et al., 2021; Timsit and Grégoire, 2021). Along these lines, neuronal ribosomal proteins and tubulin were showed to form CNS-like circuits with computation power, indicating the possibility of subcellular information processing (Poirot and Timsit, 2016; Chudinova et al., 2019; Timsit and Bennequin, 2019; Fusco et al., 2021). As viruses usurp host ribosomes to replicate, cognition is likely affected, probably explaining the lethargy of “sickness behavior” observed during viral infection (Li, 2019). Moreover, cytoskeletal proteins were recently linked to inflammation and immunogenicity, suggesting that viruses can induce both immunosuppression and immune activation via cytoskeletal manipulation (Moulding et al., 2013; Mostowy and Shenoy, 2015; Tur-Gracia and Martinez-Quiles, 2021). Along these lines, maternal immunological tolerance of the fetus vs. inflammation and preeclampsia may be determined by the viral hijacking of cytoskeleton (Harmon et al., 2016; Wang et al., 2019). Interestingly, the role of the immune system was recently expanded to include information processing and awareness, linking immunity to cognitive processes (Cohen and Efroni, 2019). In addition, proinflammatory cytokines and T cells were found to promote LTP, learning and memory, supporting the concept of immune-based cognition (Bourgognon and Cavanagh, 2020). Indeed, it was hypothesized that “brain superautoantigens”, such as syncytin-1, could drive learning and memory, implicating paleoviruses in human cognitive processes (Nataf, 2017; Mao et al., 2021). Indeed, the viral capsid, activity-regulated cytoskeleton-associated protein Arc, believed to have descended from an ancestral virus, plays a key role in synaptic plasticity, memory and learning (Pastuzyn et al., 2018). Another example, the HERV-K HML-2 (HK2) virus was associated with drug addiction, suggesting that ancient viruses could alter the behavior of modern humans (Karamitros et al., 2018).
Cell-Cell Fusion, the Molecular Actors
Cell-cell fusion is initiated by the viral arginine motif and its interaction with host cell membrane, Ca2+, TMEM16F, PS, and the cytoskeletal proteins (Whitlock and Chernomordik, 2021) ( Figure 1 ). In the following sections, we will introduce the major cell-cell and vesicular fusion players as well as the pathology associated with arginase and arginine, Ca2+ and TMEM16F.
Fusion-induced cellular changes may have body-wide repercussions as they may trigger immunosuppression, cellular senescence, HERV activation, hyperphosphorylated Tau and dysfunctional glia ( Figure 2 ).
Arginase and Nitric Oxide Depletion
Several viruses were found to upregulate host neutrophil/lymphocyte ratio (NLR), by promoting lymphopenia, associated with both COVID-19 critical illness and AD (Kuyumcu et al., 2012; Sayed et al., 2020; Prozan et al., 2021). Elevated NLR likely reflects arginase upregulation and the subsequent depletion of arginine and nitric oxide (NO).
Human neutrophils express high amounts of arginase, therefore upregulation of these cells may cause lymphopenia via decreased arginine and NO (García-Navas et al., 2021; Martí i Líndez and Reith, 2021). Indeed, upregulated neutrophils and arginase in HIV-1 infection were shown to deplete arginine that in turn disrupts B and T cell-mediated immunity (Mistry et al., 2001; Munder, 2009; Márquez-Coello et al., 2021). In addition, low arginine-to-ornithine ratio was documented in COVID-19 critical illness, suggesting that the virus hijacks arginase to lower host immunity (Rees et al., 2021). Since older individuals with hypertension and obesity display upregulated arginase, arginine depletion may explain the unfavorable COVID-19 prognosis in this population (Peyton et al., 2018; Moretto et al., 2019). Moreover, the viral arginine motif may mimic the elevated levels of this amino acid, upregulating host arginase by feedback. This mechanism may explain the beneficial effects of arginase inhibitors in both neurodegenerative disorders and viral illnesses (Toque et al., 2013).
Taken together, NLR elevation in severe viral illnesses and AD likely reflects arginase upregulation. Arginase inhibitors may comprise a new therapeutic strategy for both viral illness and neurodegeneration (Ovsepian and O’Leary, 2018). (Please see section “Arginine: The Fusion Confusion”).
Arginine, the Universal “Hole Puncher”
Amino acids are active participants in viral infection and replication and are often exploited by these pathogens (Melano et al., 2021). Arginine, an amino acid with guanidinium side chains, upregulates cytosolic Ca2+ by release from the endoplasmic reticulum (ER) and/or gating through N-methyl-D-aspartate receptor (NMDAR) and glutamate AMPA receptors (AMPAR) (Cunha et al., 2015). Previous studies have established that arginine promotes cell-cell fusion, suggesting that viruses exploit this amino acid for entering host cells (Allolio et al., 2018; Nyenhuis et al., 2021).
Under normal circumstances, arginine functions as a physiological fusogen that facilitates the merger of myoblasts as well as the fusion of intra and extracellular vesicles in the CNS (Das et al., 2004; Koike and Jahn, 2019; Gong et al., 2021). The positively charged guanidinium side chains, a unique characteristic of this amino acid, pierce cell membranes, forming pores that may facilitate both viral uptake and pTau egress (Walrant et al., 2017; Nyenhuis et al., 2021). Along these lines, a novel study linked arginine side chains to pTau, via guanidinium π interactions, suggesting that this amino acid plays a major role in tauopathies (Ferrari et al., 2020). Indeed, guanidinium pore-forming properties have helped the development of pharmacological vehicles for intracellular drug delivery, indicating that viruses exploit arginine for its side chains (Wender et al., 2008; Wexselblatt et al., 2014; Trujillo et al., 2015). For example, arginine residues demonstrated in the HIV Tat protein, suggest that viruses hijack guanidinium “keys” to enter host cells (Calnan et al., 1991).
The SARS-CoV-2 virus contains 25 arginine residues, [two in the spike (S) protein and ten in the nucleoprotein (N)], indicating that it is highly fusogenic (Al-Motawa et al., 2020). Indeed, the S protein of SARS-CoV-2 contains a polybasic cleavage motif “Proline-Arginine-Arginine-Alanine (PRRA)” that upregulates host Ca2+ and activates TMEM16F, forming syncytia and lowering lymphocyte levels (Argañaraz et al., 2020; Lin et al., 2021; Zheng et al., 2021). In addition, arginine-induced Ca2+ dysregulation was demonstrated in infection with HSV-1 as well as in tauopathies, linking viruses to neurodegeneration once more (Hibbard and Sandri-Goldin, 1995). Moreover, the SARS-CoV-2 arginine motif activates TMEM16F, a physiological placental fusogen that was implicated in the aberrant glial phagocytosis of neurons and synapses, emphasizing a novel arginine-linked neurodegeneration mechanism (Lin et al., 2021). Furthermore, as arginine-rich peptides were reported to induce cytotoxicity in AD, viral arginine may be the missing link between cell-cell fusion and neurodegenerative disorders (Mamsa and Meloni, 2021).
At the epigenetic level, arginine alters RNA methylation via methyltransferase like 14 (METTL14) interaction with N6-methyladenosine (m6A), a mechanism documented in COVID-19 pathogenesis (Li et al., 2021; Zhang et al., 2021). Interestingly, a dysfunctional m6A methylome was recently reported in tauopathies, implicating RNA methylation in these disorders (Jiang et al., 2021).
Taken together, viral arginine plays a major role in hijacking the host fusion machinery, causing immune dysfunction and cellular senescence that likely predispose to neurodegenerative disorders.
TMEM16F, an Enigmatic Scramblase
The Ca2+ dependent phospholipid scramblase, TMEM16F, alters the structural asymmetry of cell membranes by flipping PS from the cytoplasmic into the exoplasmic leaflet (Younan et al., 2018; Shlomovitz et al., 2019). This marks the cell for either fusion or elimination as microglia can “interpret” ePS as a “fuse me” or “eat me” signal (Whitlock and Chernomordik, 2021). These new findings suggest that the cell-cell merger may protect against aberrant phagocytosis (Kemp et al.; Neher et al., 2012). Indeed, as aging was associated with an increased number of fused neurons, syncytia formation may be neuroprotective by averting the premature elimination of neuronal cells (Kemp et al.; Hornik et al., 2014; Giordano-Santini et al., 2020).
TMEM16F is a physiological fusogen that under normal circumstances mediates the fusion of trophoblasts, indicating that viral overactivation of this protein may trigger placental pathology (Zhang et al., 2020). In the brain, TMEM16F is expressed primarily in neurons and microglia, suggesting that viruses may trigger pathological cell-cell fusion and aberrant microglial behavior (Zhang et al., 2020; Brown, 2021). For example, pathological multinucleated microglia with increased phagocytic capacity were documented in AD, suggesting that these cells can eliminate viable neurons (Kemp et al.; Hornik et al., 2014; Gillispie et al., 2021). Indeed, several neurotropic viruses, including HIV-1 and HSV-1 were demonstrated to fuse microglia, connecting them to neurodegeneration (Borrajo López et al., 2021). In addition, fused microglia were shown to comprise major HIV reservoirs, suggesting that SARS-CoV-2 may also avoid elimination by dwelling in these cells (Wallet et al., 2019). Moreover, as activated microglia can spread pTau and convert trophic to neurotoxic astrocytes, they may contribute to neurodegeneration (Perea et al., 2018; Hopp et al., 2018). Furthermore, as HERV-W ENV, encoding for syncytin-1, induces microglial fusion, it is likely that both exogenous and endogenous viruses may predispose to neurodegenerative pathology (Perron et al., 2005).
Fusion by Calcium
Increased cytosolic Ca2+ is a major trigger of cellular senescence and many enveloped viruses hijack this ion to generate a replication-friendly environment (Dimitrov et al., 1993; Chen et al., 2019). Ca2+ is a second messenger that under normal circumstances regulates many cellular processes, including fusion, phagocytosis, and vesicular transport as well as the synaptic plasticity in the CNS (Ahluwalia et al., 2001; Nunes and Demaurex, 2010; Mateos-Aparicio and Rodríguez-Moreno, 2020). In the immune system, Ca2+ regulates the immunological synapse, lymphocyte proliferation, differentiation, and apoptosis, suggesting that viral exploitation of Ca2+ signaling can disrupt immunity (Oh-hora and Rao, 2008; Quintana et al., 2011; Pinto et al., 2015). In addition, Ca2+ drives LTP, learning, memory, and information processing via its associated proteins, including CaMKII, mitogen-activated protein kinase/extracellular signal-regulated kinase ½ (MAPK/ERKs), and calcium homeostasis modulator 1(CALHM1) (Marambaud et al., 2009).
New studies have shown that CaMKII phosphorylates the virion-like memory protein, Arc, promoting LTP and plasticity, implicating this kinase in neurodegeneration (Zhang et al., 2019). In addition, CaMKII interacts with NMDA receptors in the postsynaptic neurons, further contributing to memory and learning (Lisman et al., 2012). Recent data show that CaMKII can alter T cell responses, indicating that viral hijacking of this protein promotes infection (Trebak and Kinet, 2019). In addition, virus-disrupted Ca2+ homeostasis can lead to synaptic loss, protein misfolding and neurodegeneration (Woods and Padmanabhan, 2012; Popugaeva and Bezprozvanny, 2014; Mazzorana et al., 2016).
Viruses, including SARS-CoV-2, HIV-1 and HSV-1, were shown to usurp Ca2+ and CaMKII, impairing plasticity, learning and memory, linking these pathogens to the neurodegenerative disorders (Gupta et al., 2010; Chen et al., 2020). A new in silico study revealed a CaMKII motif in the S antigen of SARS-CoV-2, suggesting that this virus may disrupt cognition directly (Wenzhong and Hualan, 2021) ( Figure 3 and Table 2 ). Interestingly, CaMKII inhibitors were reported to possess antiviral and antipsychotic properties, further emphasizing the role of Ca2+ signaling in these pathologies (Sałaciak et al., 2021). Ca2+ and tubulin also participates in the functioning of the immune synapse, therefore viral exploitation of this protein may disrupt host immunity (Hui and Upadhyaya, 2017; Sferra et al., 2020).
Taken together, Ca2+ signaling is crucial for the functioning of both the neurological and immunological synapses, indicating that plasticity and immune function are highly intertwined, therefore viral exploitation of the immune system may induce CNS pathology.
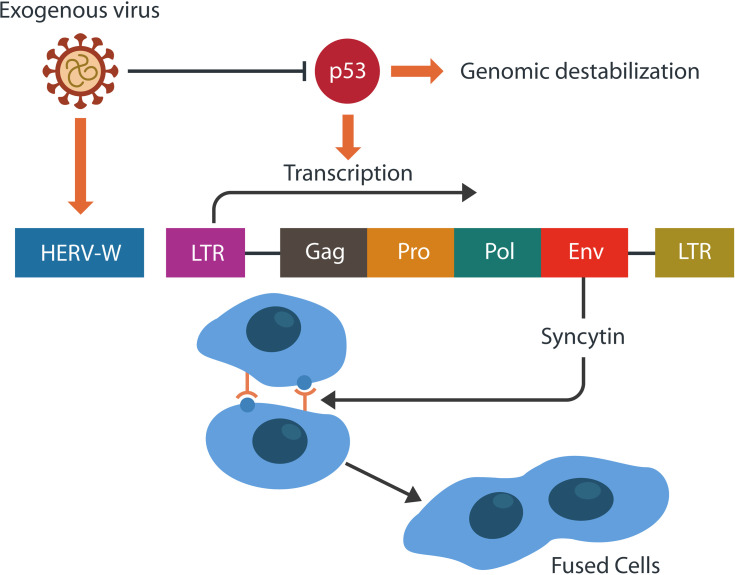
HERVs and Reverse Transcriptase
HERVs comprise about 8% of the human genome that under normal circumstances is epigenetically silenced. At times, some HERV genes may be physiologically or pathologically activated and expressed (Küry et al., 2018). HERVs usually contain one or two long terminal repeats (LTRs), gag (group-specific antigen), pol (polymerase), and env (envelope) genes that encode for proteins, such as syncytin-1 (Mao et al., 2021; Römer, 2021) ( Figure 4 ). Age and disease, including exogenous viral infections, may alter genomic methylation, activating the HERV genes (Sultana et al., 2018; Geis and Goff, 2020). Under normal circumstances, HERV-W ENV gene, encoding for syncitin-1, participates in the physiology of placenta but abnormal activation may trigger pathological cell-cell fusion in many organs, including the brain (Dupressoir et al., 2005; Zhuang et al., 2014).
Figure 4.
HERVs may retain gag, pol, env genes, and one or two long terminal repeats (LTRs). Env gene encodes for syncytin-1, a physiological placental fusogen. The activity-regulated cytoskeleton-associated protein (Arc) resembles the gag gene and encodes for a retroviral capsid. Viruses often inhibit p53, the tumor suppressor, destabilizing the genome. This in return, facilitates HERV-W transcription and the formation of pathological syncytia.
For example, decreased levels of syncytin-1 were associated with pre-eclampsia, while increased expression of this protein was documented in schizophrenia, bipolar disorder and AD (Perron et al., 2013). Indeed, HERV activation plays a major role in several neurodegenerative disorders, including AD, MS, and amyotrophic lateral sclerosis (ALS), suggesting that paleoviruses can trigger contemporary pathology (Cox and Redman, 2017; Lokossou et al., 2020; Roberts et al., 2021; Garcia-Montojo and Nath, 2021).
The SARS-CoV-2 virus was shown to activate HERV-W ENV, suggesting that it could acquire a reverse transcriptase (Zhang et al., 2020; Danta, 2021). Indeed, some COVID-19 patients continue to test positive for viral RNA long time after the recovery, suggesting that SARS-CoV-2 is retrotranscribed or that the virus “hides” in reservoirs, such as microglia and macrophages (Parry et al., 2021). Others have argued against the reverse transcriptase hypothesis, arguing that the SARS-CoV-2 life cycle does not require DNA integration (Yin et al., 2021). However, as the virus can activate HERVs, it may integrate in the genome in tandem with TEs (Jones et al., 2013). For example, SARS-CoV-2 may be hijacked by the Long Interspersed Nuclear Element 1 (LINE-1) retrotransposon and integrated into the DNA without a reverse transcriptase (Singh and Bharara Singh, 2020). Indeed, the Tat antigen of HIV-1 was demonstrated to directly activate LINE-1, indicating that viruses could destabilize the genome (Guo et al., 2018; Zauli et al., 2020; Ramirez et al., 2022).
Several studies found that viruses, including SARS-CoV-2, usurp p53, the tumor suppressor, activating TEs, predisposing to neurodegeneration (Aloi et al., 2015) ( Figure 4 ). In addition, preclinical studies have demonstrated pTau-activated TEs, suggesting a different virus-induced neurodegeneration mechanism (Jayadev et al., 2011). Other recent studies connected the loss of p53 to the aberrant microglial phagocytosis, suggesting that damaged DNA can trigger this phenotype, eliminating viable neurons and synapses (Sola et al., 2020; Farmer et al., 2020). Furthermore, as p53 and MAP-Tau maintain genomic stability in tandem, viral manipulation of either protein could activate TEs (Wylie et al., 2016)
A recent preclinical study found that N6-methyladenosine (m6A RNA) can suppress the expression of HERV, suggesting that exogenous viruses may activate HERVs by usurping this epigenetic mechanism (Balestrieri et al., 2021). Indeed, many viruses, including HIV-1, HSV-1 and SARS-CoV-2, were demonstrated to exploit m6A, increasing the risk of TEs mobilization (Tirumuru et al., 2016; Imam et al., 2020; Chelmicki et al., 2021). This is significant as m6A functions as the epigenetic reader of the heterogeneous nuclear ribonucleoprotein A2/B1 (HNRNPA2B1), a molecule disrupted by pTau and implicated in neurodegeneration (Liu et al., 2021; Jiang et al., 2021).
Taken together, HERVs activation by exogenous viruses can cause inflammation as well as p53 and m6A inhibition, predisposing to TE mobilization and neurodegeneration (Dai et al., 2018).
Harnessing Fusion
Reoviruses are nonenveloped viruses that encode for FAST proteins, inducing cell-cell, but not virus-cell, fusion. For this reason, FAST proteins are excellent tools for harnessing fusion for the treatment of various pathologies, including cancer (Del Papa et al., 2021). In this regard, FAST-containing oncolytic viruses were shown to decrease tumor growth by inducing tumor cell fusion and senescence (Jeon and Jung, 2022). FAST proteins p10, p14, and p15 are promising candidates for cancer gene therapy, but their role in viral infections and neurodegeneration is currently unknown (Del Papa et al., 2021; Brown and Fisher, 2021).
Cell-cell fusion comprises a valuable tool for reprograming fully differentiated human cells into pluripotent ones that can help heal damaged tissues (Pralong et al., 2006; Dörnen et al., 2020). For example, bone marrow-derived stem cells (BMSCs) can restore tissue homeostasis by adopting the properties of those cells (Tan et al., 2021). Regenerative medicine can also repair damaged tissues via the highly fusogenic Sendai virus that converts human cells into induced pluripotent stem cells (human iPSCs) (Nakanishi and Otsu, 2012). Moreover, fusion induced with electric pulses or polyethylene glycol (PEG) was demonstrated to promote nerve repair, including the functional restoration of severed axon (Rems et al., 2013; Greenfield, 2018; Neumann et al., 2019).
Treatment Strategies for Pathological Cell-Cell Fusion
Arginine: The Fusion Confusion
Viruses hijack arginine for its guanidinium pore-forming properties. The post-fusion upregulation of cytosolic Ca2+ and CaMKII promotes excessive phosphorylation of neuronal nitric oxide synthase (nNOS), increasing NO and peroxynitrite (ONOO−) (Zhou et al., 2016). Although under normal circumstances NO is neuroprotective, peroxynitrite accumulation was associated with neurodegenerative disorders and COVID-19 critical illness (Paris et al., 1998).
Novel SARS-CoV-2 studies have revealed a paradox: both arginine supplementation and depletion has proved beneficial to some COVID-19 patients, opening a debate on the best treatment strategy (Dominic et al., 2021; Grimes et al., 2021). These contradictory findings can be reconciled to some extent as arginine may be both helpful and detrimental depending on the amount of arginase expression. Increased arginase depletes both arginine and NO, predisposing to COVID-19 critical illness and neurodegeneration (Derakhshani et al., 2021; Dean et al., 2021). Therefore, patients with elevated arginase levels, would likely benefit from NO supplementation (but not arginine as this may upregulate the ornithine/urea pathway) (Lotz et al., 2021; Fang et al., 2021). On the other hand, NO and/or arginine supplementation may be detrimental to patients with excessive peroxynitrite as it may upregulate the oxidative stress (Nguyen et al., 2016). For this reason, arginase inhibitors (augment NO and lower peroxynitrite), may be a better therapeutic strategy than either arginine depletion or supplementation (Clemente et al., 2020).
Natural arginase inhibitors, diamino and α-amino acids, as well as flavonoid compounds, such as the plant extract (2S)-5,2′5′-trihydroxy-7,8-dimethoxy flavanone may be beneficial for patients with COVID-19 and neurodegenerative disorders (Girard-Thernier et al., 2015; Minozzo et al., 2018; Clemente et al., 2020; Arraki et al., 2021; Li et al., 2021). Synthetic arginase inhibitors are broad-spectrum anthelmintics, including imidazothiazoles and their derivatives levamisole, oxazolopyridine, azabenzimidazole, found to possess antiviral, anticancer, and anti-AD properties (Al-Horani and Kar, 2020; Weiss et al., 2021). Imidazothiazoles have not been adequately studied but are promising as panviral and neuroprotective agents.
Calcium Channel Blockers
Recent studies have reported that calcium channel blockers (CCBs) can ameliorate COVID-19 pathology, decreasing morbidity and mortality (Straus et al., 2021). Drugs, including amlodipine, nifedipine, nimodipine, memantine were demonstrated efficacious against SARS-CoV-2 virus and AD, indicating a related pathogenesis (Nimmrich and Eckert, 2013; Solaimanzadeh, 2020)
The natural CCB compounds, bisbenzylisoquinoline alkaloid, neferine and its analogs liensinine and isoliensinine, inhibit Ca2+ mediated cell-cell fusion, suggesting restoration of host antiviral immunity (Minozzo et al., 2018). Another natural compound, Artemisia annua extract, artemisinin, blocks several voltage-gated ion channels, including NMDA, indicating potential antiviral and anti-neurodegenerative properties (Qiao et al., 2007). Indeed, studies in rodents and cultured human neurons found that artemisinin ameliorated neurodegenerative pathology, emphasizing the role of dysfunctional Ca2+ signaling in these conditions (Zhao et al., 2020).
Several studies found that muscarinic acetylcholine receptors (mAChR) antagonists lower cytosolic Ca2+, averting the conversion of trophic into neurotoxic astrocytes, suggesting a role in neurodegenerative disorders (Takata et al., 2011). Indeed, M1 and M3 muscarinic receptor antagonists were shown to reverse the cocaine-induced astrocytic neurotoxicity, emphasizing their neuroprotective effects (Garcia et al., 2015; Calcutt et al., 2017).
TMEM16F Inhibitors
TMEM16F inhibitors, an important class of cell-cell fusion blockers, include a variety of agents, ranging from anthelmintic drugs to psychotropics and anticancer compounds. Recent studies have suggested that viruses and malignancies invade human cells via similar pathways, emphasizing that antiviral and anticancer drugs are related. For example, Ivermectin, a macrolide anthelmintic with antiviral properties is also an effective tumor suppressor, suggesting similar action mechanisms (Formiga et al., 2021; Tang et al., 2021) ( Table 3 ).
Table 3.
Potential anti-fusion therapeutic strategies.
| COMPOUND | REFERENCES |
|---|---|
| Natural arginase inhibitors | |
| diamino acids | (Tirumuru et al., 2016) |
| α-amino acids S Clemente G | (Tirumuru et al., 2016) |
| 2S)-5,2′5′-trihydroxy-7,8-dimethoxy flavanone | (Tirumuru et al., 2016) |
| Synthetic arginase inhibitors | |
| Imidazothiazoles: levamisole, oxazolopyridine, azabenzimidazole | (Liu et al., 2021; Jiang et al., 2021) |
| Calcium channel blockers | |
| amlodipine, nifedipine, nimodipine, memantine | (Del Papa et al., 2021; Jeon and Jung, 2022) |
| bisbenzylisoquinoline alkaloid | (Brown and Fisher, 2021) |
| neferine, liensinine, isoliensinine | (Brown and Fisher, 2021) |
| artemisinin | (Dörnen et al., 2020) |
| TMEM16F inhibitors | |
| Ivermectin | (Rems et al., 2013; Zhou et al., 2016; Greenfield, 2018) |
| Niclosamide, nitazoxanide, hexachlorophene and dichlorophen | (Hou et al., 2006) |
| Trifluoperazine | (Hou et al., 2006) |
| serotonin reuptake inhibitors (SSRIs) | (Hou et al., 2006) |
| epigallocatechin gallate | (Lotz et al., 2021) |
| Senolytic drugs | |
| hydroxychloroquine | (Minozzo et al., 2018) |
| azithromycin, minocycline and roxithromycin | (Al-Horani and Kar, 2020; Weiss et al., 2021) |
| quercetin | (Minozzo et al., 2018) |
| senolytic vaccine | (Formiga et al., 2021) |
| antibody–drug conjugates | (Tang et al., 2021) |
| Microtubule stabilizing agents | |
| TPI-287 (discontinued) | (Malashkevich et al., 2010) |
| Davunetide (discontinued) | (Malashkevich et al., 2010) |
| CNDR-51549 | (Li et al., 2020) |
| CNDR-51555 | (Li et al., 2020) |
| CNDR-51657 | (Du et al., 2012) |
| Sabizabulin | (Millington-Burgess and Harper, 2021) |
| Taccalonolides | (Ousingsawat et al., 2018) |
| Lithium | (Sargiacomo et al., 2020) |
The COVID-19 pandemic drew attention to the connection between pathogens, cancer, and neuropsychiatric disorders, suggesting the possibility of common treatment strategies (Xu et al., 2020). For example, TMEM16F inhibitor niclosamide and its analogs nitazoxanide, hexachlorophene and dichlorophen, present with intriguing anthelmintic, anticancer, and anti-ALS properties, indicating similar pathogenesis (Peng et al., 2021). Indeed, as these agents target S100A4, a protein involved in schizophrenia and inhibited by the phenothiazine class of antipsychotic drugs, a common pathogenetic mechanism is being highlighted (Malashkevich et al., 2010; D’Ambrosi et al., 2021). In addition, since S100A4 has also been implicated in tumorigenesis, it may be the common denominator between viral illness, cancer, and neuropsychiatric disorders (Fei et al., 2017).
Recent in silico studies, have shown that several psychotropic drugs, including trifluoperazine and serotonin reuptake inhibitors (SSRIs) block TMEM-16F, explaining their antiviral and anti-syncytial properties (Cavaliere et al., 2019). This is significant, as SSRIs were demonstrated to delay the conversion of mild cognitive impairment (MCI) to AD, further connecting TMEM-16F to neurodegenerative disorders (Bartels et al., 2018).
Natural TMEM16F inhibitors, including the polyphenol Epigallocatechin gallate, have antiviral, anti-neurodegenerative, and anti-cancer properties, emphasizing once more a common action mechanism (Du et al., 2012; Li et al., 2020; Millington-Burgess and Harper, 2021). Another polyphenol, tannic acid, may or may not downregulate TMEM16F as two different studies found conflicting results, indicating that more research is needed in this area (Ousingsawat et al., 2018; Le et al., 2020).
Senolytic Drugs
Several senolytic drugs with established antiviral properties, including hydroxychloroquine and related agents, lower β- galactosidase, a well-known senescence marker, indicating efficacy against virus-induced senescence (Van Gool et al., 2001). Interestingly, large observational studies showed that hydroxychloroquine may ameliorate AD symptoms, suggesting that senescent cell clearance may be a useful strategy against neurodegenerative disorders (Sargiacomo et al., 2020; Lai et al., 2021). Other senolytic agents with antiviral properties, such as azithromycin, minocycline and roxithromycin, were deemed salutary to COVID-19 patients as they selectively eliminate senescent and virus-infected cells (Forloni et al., 2001; Ozsvari et al., 2018; Osorio et al., 2019). Indeed, several senolytic antibiotics, including tetracyclines, have demonstrated anti-neurodegenerative properties in preclinical studies, emphasizing the link between viruses and neurodegeneration (Diomede et al., 2010; Di Pierro et al., 2021).
The natural senolytic agent, quercetin, an effective antiviral and anti-neurodegeneration compound, is currently in clinical trials for COVID-19 (Khan et al., 2019; Islam et al., 2021)(NCT05037240). Quercetin was found to preempt the development neuronal damage as well as to possess anticancer and anti-inflammatory properties (Vafadar et al., 2020).
A novel senolytic vaccine, recently tested in progeroid mice, may usher a new era in senolytic interventions as it opens the possibility of preventing the development of neurodegenerative disorders, viral infections and possibly cancer (Yoshida et al., 2020; Suda et al., 2021). Furthermore, an antibody–drug conjugate against a membrane senescence marker was demonstrated to clear senescent and virus-infected cells, emphasizing a new senolytic strategy (Poblocka et al., 2021).
Microtubule Stabilizing Agents
MSAs are compounds that attach to the microtubules, preventing their disassembly. Most drugs targeting microtubules are anticancer agents that may also possess anti-neurodegenerative and antiviral effects (Hung and Fu, 2017; Fernandez-Valenzuela et al., 2020; Tsai et al., 2020; Sirakanyan et al., 2021). Many of these drugs demonstrated beneficial effects in animal models, however those tested in humans are few and include TPI-287 and NAP (Davunetide CP201), an intranasal neuropeptide (NAPVSIPQ)(NAP) (NCT01966666) (Gozes, 2020). These MSAs have not reached the clinic, however, activity-dependent neuroprotective protein (ADNP), derived from NAP, remains a potential hope and is scheduled for future clinical trials (Varidaki et al., 2018; Al-Horani and Kar, 2020; Santiago-Mujika et al., 2021). A recent addition to MSAs, sabizabulin, is currently in clinical trials as an antiviral drug, suggesting possible benefit in tauopathies (NCT04388826) (Malebari et al., 2021).
Natural MSA compounds, CNDR-51549 and CNDR51555 (US patent: US20170173016 A1) were found to cross the blood brain barrier, indicating potential benefit in tauopathies (Kovalevich et al., 2016). Another compound, CNDR-51657, was demonstrated to downregulate the hyperphosphorylated Tau, suggesting a preventive potential (Zhang et al., 2018). Another natural MSA compound and Tacca extract, taccalonolides, may benefit AD patients by augmenting tubulin polymerization, reversing the effect of pTau (Murru et al., 2020; Chen et al., 2021). Interestingly, studies from the 1970s observed that lithium was a MSA, raising interesting questions about its established antiviral and neuroprotective properties (Matsunaga et al., 2015; Murru et al., 2020; Chen et al., 2021). Indeed, lithium reverses pTau-induced astrocytic senescence and enhances T-cell function, suggesting senolytic properties (Bhattacharyya and Wolff, 1976; Kucharz et al., 1988; Olson et al., 2019; Viel et al., 2020). This is significant, as lithium can reverse the virus-induced damage of tubulin, a key molecule in T cell activation (Kopf and Kiermaier, 2021).
Taken together, MSAs, many of which are plant extracts, are interesting compounds that require further research as antiviral and neuroprotective agents (Garcia-Montojo and Nath, 2021).
Conclusions
Viruses augment infectivity by fusing host cells into multinucleated hybrid entities that engender cellular senescence, immunosuppression or immune exhaustion that may predispose to neurodegenerative disorders. The study of physiological and pathological syncytia has emphasized the role of arginine, calcium signaling, TMEM16F and the cytoskeleton in synaptic plasticity, memory, and cognition. These novel findings are likely to contribute to the development of new therapeutic strategies not only for neuropsychiatric conditions but also for cancer and viral infections.
A better understanding of physiological fusogens, their properties and function, will lead to more focused interventions against autoimmune and placental disorders. Adequate HERV inhibition will likely improve the outcome of antiviral and antitumor drugs, opening the possibility of neurodegenerative disorders prevention. This is illustrated by the novel recombinant anti-HERV-W ENV antibody (GNbAC1), currently in clinical trials for MS, and a promising antiviral agent (NCT01639300) (Diebold and Derfuss, 2019; Singh and Bharara Singh, 2020). The same may be true of arginase inhibitors, MSA, and TMEM16F inhibitors, drugs with multiple therapeutic targets.
At present, the study of cell-cell fusion is in its infancy therefore, a better understanding of the molecular underpinnings of syncytia formation would shed light on cellular uptake of pathogens and oncogenes, opening novel avenues for preventive care.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Abdou S. A., Henderson P. W. (2019). Fusogens: Chemical Agents That Can Rapidly Restore Function After Nerve Injury. J. Surg. Res. 233, 36–40. doi: 10.1016/j.jss.2018.07.013 [DOI] [PubMed] [Google Scholar]
- Ahluwalia J. P., Topp J. D., Weirather K., Zimmerman M., Stamnes M. (2001). A Role for Calcium in Stabilizing Transport Vesicle Coats. J. Biol. Chem. 276 (36), 34148–34155. doi: 10.1074/jbc.M105398200 [DOI] [PubMed] [Google Scholar]
- Akimov S. A., Molotkovsky R. J., Galimzyanov T. R., Radaev A. V., Shilova L. A., Kuzmin P. I., et al. (2014). Model of Membrane Fusion: Continuous Transition to Fusion Pore With Regard of Hydrophobic and Hydration Interactions. Biochem. Moscow Suppl. Ser. A. 8, 153–161. doi: 10.1134/S1990747814010024 [DOI] [Google Scholar]
- Alexander A. G., Marfil V., Li C. (2014). Use of Caenorhabditis Elegans as a Model to Study Alzheimer’s Disease and Other Neurodegenerative Diseases. Front. Genet. 5, 279. doi: 10.3389/fgene.2014.00279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfedi G., Luffarelli R., Condò I., Pedini G., Mannucci L., Massaro D. S., et al. (2019). Drug Repositioning Screening Identifies Etravirine as a Potential Therapeutic for Friedreich’s Ataxia. Mov. Disord. 34 (3), 323–334. doi: 10.1002/mds.27604 [DOI] [PubMed] [Google Scholar]
- Al-Horani R. A., Kar S. (2020). Potential Anti-SARS-CoV-2 Therapeutics That Target the Post-Entry Stages of the Viral Life Cycle: A Comprehensive Review. Viruses 12 (10), 1092. doi: 10.3390/v12101092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allolio C., Magarkar A., Jurkiewicz P., Baxová K., Javanainen M., Mason P. E., et al. (2018). Arginine-Rich Cell-Penetrating Peptides Induce Membrane Multilamellarity and Subsequently Enter via Formation of a Fusion Pore. Proc. Natl. Acad. Sci. U. S. A. 115 (47), 11923–11928. doi: 10.1073/pnas.1811520115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Motawa M. S., Abbas H., Wijten P., de la Fuente A., Xue M., Rabbani N., et al. (2020). Vulnerabilities of the SARS-CoV-2 Virus to Proteotoxicity-Opportunity for Repurposed Chemotherapy of COVID-19 Infection. Front. Pharmacol. 11, 585408. doi: 10.3389/fphar.2020.585408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloi M. S., Su W., Garden G. A. (2015). The P53 Transcriptional Network Influences Microglia Behavior and Neuroinflammation. Crit. Rev. Immunol. 35 (5), 401–415. doi: 10.1615/critrevimmunol.v35.i5.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Dolado M., Pardal R., Garcia-Verdugo J. M., Fike J. R., Lee H. O., Pfeffer K., et al. (2003). Fusion of Bone-Marrow-Derived Cells With Purkinje Neurons, Cardiomyocytes and Hepatocytes. Nature 425 (6961), 968–973. doi: 10.1038/nature02069 [DOI] [PubMed] [Google Scholar]
- Appay V., Sauce D. (2008). Immune Activation and Inflammation in HIV-1 Infection: Causes and Consequences. J. Pathol. 214 (2), 231–241. doi: 10.1002/path.2276 [DOI] [PubMed] [Google Scholar]
- Arendt T. (2012). Cell Cycle Activation and Aneuploid Neurons in Alzheimer’s Disease. Mol. Neurobiol. 46 (1), 125–135. doi: 10.1007/s12035-012-8262-0 [DOI] [PubMed] [Google Scholar]
- Argañaraz G. A., Palmeira J. F., Argañaraz E. R. (2020). Phosphatidylserine Inside Out: A Possible Underlying Mechanism in the Inflammation and Coagulation Abnormalities of COVID-19. Cell Commun. Signal 18, 190. doi: 10.1186/s12964-020-00687-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora S., Saarloos I., Kooistra R., van de Bospoort R., Verhage M., Toonen R. F. (2017). SNAP-25 Gene Family Members Differentially Support Secretory Vesicle Fusion. J. Cell Sci. 130 (11), 1877–1889. doi: 10.1242/jcs.201889 [DOI] [PubMed] [Google Scholar]
- Arraki K., Totoson P., Decendit A., Zedet A., Maroilley J., Badoc A., et al. (2021). Mammalian Arginase Inhibitory Activity of Methanolic Extracts and Isolated Compounds From Cyperus Species. Molecules 26 (6), 1694. doi: 10.3390/molecules26061694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf A., So P. W. (2020). Spotlight on Ferroptosis: Iron-Dependent Cell Death in Alzheimer’s Disease. Front. Aging Neurosci. 12, 196. doi: 10.3389/fnagi.2020.00196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balestrieri E., Minutolo A., Petrone V., Fanelli M., Iannetta M., Malagnino V., et al. (2021). Evidence of the Pathogenic HERV-W Envelope Expression in T Lymphocytes in Association With the Respiratory Outcome of COVID-19 Patients. EBioMedicine 66, 103341. doi: 10.1016/j.ebiom.2021.103341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballatore C., Brunden K. R., Huryn D. M., Trojanowski J. Q., Lee V. M., Smith A. B., 3rd (2012). Microtubule Stabilizing Agents as Potential Treatment for Alzheimer’s Disease and Related Neurodegenerative Tauopathies. J. Med. Chem. 55 (21), 8979–8996. doi: 10.1021/jm301079z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluška F., Miller W. B., Jr, Reber A. S. (2021). Biomolecular Basis of Cellular Consciousness via Subcellular Nanobrains. Int. J. Mol. Sci. 22 (5), 2545. doi: 10.3390/ijms22052545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberis E., Vanella V. V., Falasca M., Caneapero V., Cappellano G., Raineri D., et al. (2021). Circulating Exosomes Are Strongly Involved in SARS-CoV-2 Infection. Front. Mol. Biosci. 8, 632290. doi: 10.3389/fmolb.2021.632290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels C., Wagner M., Wolfsgruber S., Ehrenreich H., Schneider A. (2018). Alzheimer’s Disease Neuroimaging Initiative. Impact of SSRI Therapy on Risk of Conversion From Mild Cognitive Impairment to Alzheimer’s Dementia in Individuals With Previous Depression. Am. J. Psychiatry 175 (3), 232–241. doi: 10.1176/appi.ajp.2017.17040404 [DOI] [PubMed] [Google Scholar]
- Bergman L., Zetterberg H., Kaihola H., Hagberg H., Blennow K., Åkerud H. (2018). Blood-Based Cerebral Biomarkers in Preeclampsia: Plasma Concentrations of NfL, Tau, S100B and NSE During Pregnancy in Women Who Later Develop Preeclampsia - A Nested Case Control Study. PloS One 13 (5), e0196025. doi: 10.1371/journal.pone.0196025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman J. W., Carvallo L., Buckner C. M., Luers A., Prevedel L., Bennett M. V., et al. (2016). HIV-Tat Alters Connexin43 Expression and Trafficking in Human Astrocytes: Role in NeuroAIDS. J. Neuroinflamm. 13 (1), 54. doi: 10.1186/s12974-016-0510-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand S. J., Aksenova M. V., Mactutus C. F., Booze R. M. (2013). HIV-1 Tat Protein Variants: Critical Role for the Cysteine Region in Synaptodendritic Injury. Exp. Neurol. 248, 228–235. doi: 10.1016/j.expneurol.2013.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya B., Wolff J. (1976). Stabilization of Microtubules by Lithium Ion. Biochem. Biophys. Res. Commun. 73 (2), 383–390. doi: 10.1016/0006-291x(76)90719-1 [DOI] [PubMed] [Google Scholar]
- Birge R. B., Boeltz S., Kumar S., Carlson J., Wanderley J., Calianese D., et al. (2016). Phosphatidylserine Is a Global Immunosuppressive Signal in Efferocytosis, Infectious Disease, and Cancer. Cell Death Differ. 23, 962–978. doi: 10.1038/cdd.2016.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolinches-Amorós A., Mollá B., Pla-Martín D., Palau F., González-Cabo P. (2014). Mitochondrial Dysfunction Induced by Frataxin Deficiency Is Associated With Cellular Senescence and Abnormal Calcium Metabolism. Front. Cell Neurosci. 8, 124. doi: 10.3389/fncel.2014.00124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrajo López A., Penedo M. A., Rivera-Baltanas T., Pérez-Rodríguez D., Alonso-Crespo D., Fernández-Pereira C., et al. (2021). Microglia: The Real Foe in HIV-1-Associated Neurocognitive Disorders? Biomed. 9 (8), 925. doi: 10.3390/biomedicines9080925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgognon J. M., Cavanagh J. (2020). The Role of Cytokines in Modulating Learning and Memory and Brain Plasticity. Brain Neurosci. Adv. 4, 2398212820979802. doi: 10.1177/2398212820979802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brelstaff J., Tolkovsky A. M., Ghetti B., Goedert M., Spillantini M. G. (2018). Living Neurons With Tau Filaments Aberrantly Expose Phosphatidylserine and Are Phagocytosed by Microglia. Cell Rep. 24 (8), 1939–1948.e4. doi: 10.1016/j.celrep.2018.07.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. W. (1999). Pre-Eclampsia: A Mistake of Trophoblastic Cells for Tumour Cells? Med. Hypotheses 53 (2), 124–126. doi: 10.1054/mehy.1998.0729 [DOI] [PubMed] [Google Scholar]
- Brown G. C. (2021). Neuronal Loss After Stroke Due to Microglial Phagocytosis of Stressed Neurons. Int. J. Mol. Sci. 22, 13442. doi: 10.3390/ijms222413442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. S., Cohen P., Greenwald S., Susser E. (2000). Nonaffective Psychosis After Prenatal Exposure to Rubella. Am. J. Psychiatry 157 (3), 438–443. doi: 10.1176/appi.ajp.157.3.438 [DOI] [PubMed] [Google Scholar]
- Brown K. E., Fisher A. G. (2021). Reprogramming Lineage Identity Through Cell-Cell Fusion. Curr. Opin. Genet. Dev. 70, 15–23. doi: 10.1016/j.gde.2021.04.004 [DOI] [PubMed] [Google Scholar]
- Brown L. A., Scarola J., Smith A. J., Sanberg P. R., Tan J., Giunta B. (2014). The Role of Tau Protein in HIV-Associated Neurocognitive Disorders. Mol. Neurodegener. 9, 40. doi: 10.1186/1750-1326-9-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brukman N. G., Uygur B., Podbilewicz B., Chernomordik L. V. (2019). How Cells Fuse. J. Cell Biol. 218 (5), 1436–1451. doi: 10.1083/jcb.201901017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunello C. A., Merezhko M., Uronen R. L., Huttunen H. J. (2020). Mechanisms of Secretion and Spreading of Pathological Tau Protein. Cell Mol. Life Sci. 77 (9), 1721–1744. doi: 10.1007/s00018-019-03349-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcutt N. A., Smith D. R., Frizzi K., Sabbir M. G., Chowdhury S. K., Mixcoatl-Zecuatl T., et al. (2017). Selective Antagonism of Muscarinic Receptors Is Neuroprotective in Peripheral Neuropathy. J. Clin. Invest. 127 (2), 608–622. doi: 10.1172/JCI88321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calnan B. J., Biancalana S., Hudson D., Frankel A. D. (1991). Analysis of Arginine-Rich Peptides From the HIV Tat Protein Reveals Unusual Features of RNA-Protein Recognition. Genes Dev. 5 (2), 201–210. doi: 10.1101/gad.5.2.201 [DOI] [PubMed] [Google Scholar]
- Cao L. L., Guan P. P., Liang Y. Y., Huang X. S., Wang P. (2019). Calcium Ions Stimulate the Hyperphosphorylation of Tau by Activating Microsomal Prostaglandin E Synthase 1. Front. Aging Neurosci. 11, 108. doi: 10.3389/fnagi.2019.00108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaliere F., Fornarelli A., Bertan F., Russo R., Marsal-Cots A., Morrone L. A., et al. (2019). The Tricyclic Antidepressant Clomipramine Inhibits Neuronal Autophagic Flux. Sci. Rep. 9, 4881. doi: 10.1038/s41598-019-40887-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaldakov G. N. (2018). Colchicine, a Microtubule-Disassembling Drug, in the Therapy of Cardiovascular Diseases. Cell Biol. Int. 42 (8), 1079–1084. doi: 10.1002/cbin.10988 [DOI] [PubMed] [Google Scholar]
- Chan K. M. C., Arthur A. L., Morstein J., Jin M., Bhat A., Schlesinger D., et al. (2021). Evolutionarily Related Small Viral Fusogens Hijack Distinct But Modular Actin Nucleation Pathways to Drive Cell-Cell Fusion. Proc. Natl. Acad. Sci. U. S. A. 118 (1), e2007526118. doi: 10.1073/pnas.2007526118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charubin K., Modla S., Caplan J. L., Papoutsakis E. T. (2020). Interspecies Microbial Fusion and Large-Scale Exchange of Cytoplasmic Proteins and RNA in a Syntrophic Clostridium Coculture. mBio 11 (5), e02030-20. doi: 10.1128/mBio.02030-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelmicki T., Roger E., Teissandier A., Dura M., Bonneville L., Rucli S., et al. (2021). M6a RNA Methylation Regulates the Fate of Endogenous Retroviruses. Nature 591 (7849), 312–316. doi: 10.1038/s41586-020-03135-1 [DOI] [PubMed] [Google Scholar]
- Chemparthy D. T., Kannan M., Gordon L., Buch S., Sil S. (2021). Alzheimer’s-Like Pathology at the Crossroads of HIV-Associated Neurological Disorders. Vaccines (Basel). 9 (8), 930. doi: 10.3390/vaccines9080930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., Zheng Q., Sun L., Ji M., Li Y., Deng H., et al. (2021). ORF3a of SARS-CoV-2 Promotes Lysosomal Exocytosis-Mediated Viral Egress. Dev. Cell 56 (23), 3250–3263.e5. doi: 10.1016/j.devcel.2021.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. C., Simanjuntak Y., Chu L. W., Ping Y. H., Lee Y. L., Lin Y. L., et al. (2020). Benzenesulfonamide Derivatives as Calcium/Calmodulin-Dependent Protein Kinase Inhibitors and Antiviral Agents Against Dengue and Zika Virus Infections. J. Med. Chem. 63 (3), 1313–1327. doi: 10.1021/acs.jmedchem.9b01779 [DOI] [PubMed] [Google Scholar]
- Chen X., Cao R., Zhong W. (2019). Host Calcium Channels and Pumps in Viral Infections. Cells 9 (1), 94. doi: 10.3390/cells9010094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Winstead A., Yu H., Peng J. (2021). Taccalonolides: A Novel Class of Microtubule-Stabilizing Anticancer Agents. Cancers (Basel). 13 (4), 920. doi: 10.3390/cancers13040920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S., Banerjee S., Daiello L. A., Nakashima A., Jash S., Huang Z., et al. (2021). Novel Blood Test for Early Biomarkers of Preeclampsia and Alzheimer’s Disease. Sci. Rep. 11 (1), 15934. doi: 10.1038/s41598-021-95611-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudinova E. M., Brodsky I. B., Nadezhdina E. S. (2019). On the Interaction of Ribosomal Protein RPL22e With Microtubules. Cell Biol. Int. 43 (7), 749–759. doi: 10.1002/cbin.11141 [DOI] [PubMed] [Google Scholar]
- Clemente G. S., van Waarde A F., Antunes I., Dömling A., H Elsinga P. (2020). Arginase as a Potential Biomarker of Disease Progression: A Molecular Imaging Perspective. Int. J. Mol. Sci. 21 (15), 5291. doi: 10.3390/ijms21155291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I. R., Efroni S. (2019). The Immune System Computes the State of the Body: Crowd Wisdom, Machine Learning, and Immune Cell Reference Repertoires Help Manage Inflammation. Front. Immunol. 10, 10. doi: 10.3389/fimmu.2019.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox L. S., Redman C. (2017). The Role of Cellular Senescence in Ageing of the Placenta. Placenta 52, 139–145. doi: 10.1016/j.placenta.2017.01.116 [DOI] [PubMed] [Google Scholar]
- Craddock T. J., Tuszynski J. A., Hameroff S. (2012). Cytoskeletal Signaling: Is Memory Encoded in Microtubule Lattices by CaMKII Phosphorylation? PloS Comput. Biol. 8 (3), e1002421. doi: 10.1371/journal.pcbi.1002421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha M. P., Pazini F. L., Ludka F. K., Rosa J. M., Oliveira Á, Budni J., et al. (2015). The Modulation of NMDA Receptors and L-Arginine/Nitric Oxide Pathway Is Implicated in the Anti-Immobility Effect of Creatine in the Tail Suspension Test. Amino Acids 47 (4), 795–811. doi: 10.1007/s00726-014-1910-0 [DOI] [PubMed] [Google Scholar]
- D’Ambrosi N., Milani M., Apolloni S. (2021). S100A4 in the Physiology and Pathology of the Central and Peripheral Nervous System. Cells 10 (4), 798. doi: 10.3390/cells10040798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Errico P., Meyer-Luehmann M. (2020). Mechanisms of Pathogenic Tau and Aβ Protein Spreading in Alzheimer’s Disease. Front. Aging Neurosci. 12, 265. doi: 10.3389/fnagi.2020.00265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai D., Wang H., Zhu L., Jin H., Wang X. (2018). N6-Methyladenosine Links RNA Metabolism to Cancer Progression. Cell Death Dis. 9, 124. doi: 10.1038/s41419-017-0129-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danta C. C. (2021). SARS-CoV-2, Hypoxia, and Calcium Signaling: The Consequences and Therapeutic Options. ACS Pharmacol. Transl. Sci. 4 (1), 400–402. doi: 10.1021/acsptsci.0c00219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das V., Nal B., Dujeancourt A., Thoulouze M. I., Galli T., Roux P., et al. (2004). Activation-Induced Polarized Recycling Targets T Cell Antigen Receptors to the Immunological Synapse; Involvement of SNARE Complexes. Immunity 20 (5), 577–588. doi: 10.1016/s1074-7613(04)00106-2 [DOI] [PubMed] [Google Scholar]
- Dean M. J., Ochoa J. B., Sanchez-Pino M. D., Zabaleta J., Garai J., Del Valle L., et al. (2021). Severe COVID-19 Is Characterized by an Impaired Type I Interferon Response and Elevated Levels of Arginase Producing Granulocytic Myeloid Derived Suppressor Cells. Front. Immunol. 12, 695972. doi: 10.3389/fimmu.2021.695972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejanovic B., Huntley M. A., De Mazière A., Meilandt W. J., Wu T., Srinivasan K., et al. (2018). Changes in the Synaptic Proteome in Tauopathy and Rescue of Tau-Induced Synapse Loss by C1q Antibodies. Neuron 100 (6), 1322–1336.e7. doi: 10.1016/j.neuron.2018.10.014 [DOI] [PubMed] [Google Scholar]
- Del Papa J., Clarkin R. G., Parks R. J. (2021). Use of Cell Fusion Proteins to Enhance Adenoviral Vector Efficacy as an Anti-Cancer Therapeutic. Cancer Gene Ther. 28 (7-8), 745–756. doi: 10.1038/s41417-020-0192-9 [DOI] [PubMed] [Google Scholar]
- Dent E. W. (2017). Of Microtubules and Memory: Implications for Microtubule Dynamics in Dendrites and Spines. Mol. Biol. Cell 28 (1), 1–8. doi: 10.1091/mbc.E15-11-0769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derakhshani A., Hemmat N., Asadzadeh Z., Ghaseminia M., Shadbad M. A., Jadideslam G., et al. (2021). Arginase 1 (Arg1) as an Up-Regulated Gene in COVID-19 Patients: A Promising Marker in COVID-19 Immunopathy. J. Clin. Med. 10 (5), 1051. doi: 10.3390/jcm10051051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharan A., Campbell E. M. (2018). Role of Microtubules and Microtubule-Associated Proteins in HIV-1 Infection. J. Virol. 92 (16), e00085-18. doi: 10.1128/JVI.00085-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diebold M., Derfuss T. (2019). The Monoclonal Antibody GNbAC1: Targeting Human Endogenous Retroviruses in Multiple Sclerosis. Ther. Adv. Neurol. Disord. 12, 1756286419833574. doi: 10.1177/1756286419833574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov D. S., Broder C. C., Berger E. A., Blumenthal R. (1993). Calcium Ions Are Required for Cell Fusion Mediated by the CD4-Human Immunodeficiency Virus Type 1 Envelope Glycoprotein Interaction. J. Virol. 67 (3), 1647–1652. doi: 10.1128/JVI.67.3.1647-1652.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diomede L., Cassata G., Fiordaliso F., Salio M., Ami D., Natalello A., et al. (2010). Tetracycline and Its Analogues Protect Caenorhabditis Elegans From βamyloid-Induced Toxicity by Targeting Oligomers. Neurobiol. Dis. 40, 424–431. doi: 10.1016/j.nbd.2010.07.002 [DOI] [PubMed] [Google Scholar]
- Di Pierro F., Iqtadar S., Khan A., Mumtaz S. U., Chaudhry M. M., Bertuccioli A., et al. (2021). Potential Clinical Benefits of Quercetin in the Early Stage of COVID-19: Results of a Second, Pilot, Randomized, Controlled and Open-Label Clinical Trial. Int. J. Gen. Med. 14, 2807–2816. doi: 10.2147/IJGM.S318949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmar T., Weiler J., Luo T., Hass R. (2021). Cell-Cell Fusion Mediated by Viruses and HERV-Derived Fusogens in Cancer Initiation and Progression. Cancers (Basel). 13 (21), 5363. doi: 10.3390/cancers13215363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominic P., Ahmad J., Bhandari R., Pardue S., Solorzano J., Jaisingh K., et al. (2021). Decreased Availability of Nitric Oxide and Hydrogen Sulfide Is a Hallmark of COVID-19. Redox Biol. 43, 101982. doi: 10.1016/j.redox.2021.101982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörnen J., Sieler M., Weiler J., Keil S., Dittmar T. (2020). Cell Fusion-Mediated Tissue Regeneration as an Inducer of Polyploidy and Aneuploidy. Int. J. Mol. Sci. 21 (5), 1811. doi: 10.3390/ijms21051811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dove B., Brooks G., Bicknell K., Wurm T., Hiscox J. A. (2006). Cell Cycle Perturbations Induced by Infection With the Coronavirus Infectious Bronchitis Virus and Their Effect on Virus Replication. J. Virol. 80, 4147–4156. doi: 10.1128/JVI.80.8.4147-4156.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd E., McKernan D. P. (2021). Back to the Future: Lessons From Past Viral Infections and the Link With Parkinson’s Disease. Neuronal Signal 5 (1), NS20200051. doi: 10.1042/NS20200051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupressoir A., Marceau G., Vernochet C., Bénit L., Kanellopoulos C., Sapin V., et al. (2005). Syncytin-A and Syncytin-B, Two Fusogenic Placenta-Specific Murine Envelope Genes of Retroviral Origin Conserved in Muridae. Proc. Natl. Acad. Sci. U. S. A. 102 (3), 725–730. doi: 10.1073/pnas.0406509102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du G. J., Zhang Z., Wen X. D., Yu C., Calway T., Yuan C. S., et al. (2012). Epigallocatechin Gallate (EGCG) Is the Most Effective Cancer Chemopreventive Polyphenol in Green Tea. Nutrients 4 (11), 1679–1691. doi: 10.3390/nu4111679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etcheberrigaray R., Hirashima N., Nee L., Prince J., Govoni S., Racchi M., et al. (1998). Calcium Responses in Fibroblasts From Asymptomatic Members of Alzheimer’s Disease Families. Neurobiol. Dis. 5 (1), 37–45. doi: 10.1006/nbdi.1998.0176 [DOI] [PubMed] [Google Scholar]
- Fang W., Jiang J., Su L., Shu T., Liu H., Lai S., et al. (2021). The Role of NO in COVID-19 and Potential Therapeutic Strategies. Free Radic. Biol. Med. 163, 153–162. doi: 10.1016/j.freeradbiomed.2020.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer K. M., Ghag G., Puangmalai N., Montalbano M., Bhatt N., Kayed R. (2020). P53 Aggregation, Interactions With Tau, and Impaired DNA Damage Response in Alzheimer’s Disease. Acta Neuropathol. Commun. 8, 132. doi: 10.1186/s40478-020-01012-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fédry J., Liu Y., Péhau-Arnaudet G., Pei J., Li W., Tortorici M. A., et al. (2017). The Ancient Gamete Fusogen HAP2 Is a Eukaryotic Class II Fusion Protein. Cell 168 (5), 904–915.e10. doi: 10.1016/j.cell.2017.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei F., Qu J., Zhang M., Li Y., Zhang S. (2017). S100A4 in Cancer Progression and Metastasis: A Systematic Review. Oncotarget 8 (42), 73219–73239. doi: 10.18632/oncotarget.18016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick C., Joo V., Jacquier P., Noto A., Banga R., Perreau M., et al. (2019). T-Cell Exhaustion in HIV Infection. Immunol. Rev. 292 (1), 149–163. doi: 10.1111/imr.12823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Valenzuela J. J., Sanchez-Varo R., Muñoz-Castro C., De Castro V., Sanchez-Mejias E., Navarro V., et al. (2020). Enhancing Microtubule Stabilization Rescues Cognitive Deficits and Ameliorates Pathological Phenotype in an Amyloidogenic Alzheimer’s Disease Model. Sci. Rep. 10 (1), 14776. doi: 10.1038/s41598-020-71767-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari L., Stucchi R., Konstantoulea K., van de Kamp G., Kos R., Geerts W. J. C., et al. (2020). Arginine π-Stacking Drives Binding to Fibrils of the Alzheimer Protein Tau. Nat. Commun. 11 (1), 571. doi: 10.1038/s41467-019-13745-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filgueira L., Larionov A., Lannes N. (2021). The Influence of Virus Infection on Microglia and Accelerated Brain Aging. Cells 10 (7), 1836. doi: 10.3390/cells10071836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forloni G., Colombo L., Girola L., Tagliavini F., Salmona M. (2001). Anti-Amyloidogenic Activity of Tetracyclines: Studies In Vitro . FEBS Lett. 487 (3), 404–407. doi: 10.1016/s0014-5793(00)02380-2 [DOI] [PubMed] [Google Scholar]
- Formiga F. R., Leblanc R., de Souza Rebouças J., Farias L. P., de Oliveira R. N., Pena L. (2021). Ivermectin: An Award-Winning Drug With Expected Antiviral Activity Against COVID-19. J. Control Release 329, 758–761. doi: 10.1016/j.jconrel.2020.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch S. M., MacFawn I. P. (2020). Type I Interferons and Related Pathways in Cell Senescence. Aging Cell. 19 (10), e13234. doi: 10.1111/acel.13234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frontera J. A., Boutajangout A., Masurkar A. V., Betensky R. A., Ge Y., Vedvyas A., et al. (2022). Comparison of Serum Neurodegenerative Biomarkers Among Hospitalized COVID-19 Patients Versus Non-COVID Subjects With Normal Cognition, Mild Cognitive Impairment, or Alzheimer’s Dementia. Alzheimers Dement. doi: 10.1002/alz.12556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuglewicz A. J., Piotrowski P., Stodolak A. (2017). Relationship Between Toxoplasmosis and Schizophrenia: A Review. Adv. Clin. Exp. Med. 26 (6), 1031–1036. doi: 10.17219/acem/61435 [DOI] [PubMed] [Google Scholar]
- Fukushima Y., Minato N., Hattori M. (2018). The Impact of Senescence-Associated T Cells on Immunosenescence and Age-Related Disorders. Inflammation Regener. 38, 24. doi: 10.1186/s41232-018-0082-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusco C. M., Desch K., Dörrbaum A. R., Wang M., Staab A., Chan I. C. W., et al. (2021). Neuronal Ribosomes Exhibit Dynamic and Context-Dependent Exchange of Ribosomal Proteins. Nat. Commun. 12 (1), 6127. doi: 10.1038/s41467-021-26365-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal H., Lysenko M., Stroganov S., Vadai E., Youssef S. A., Tzadikevitch‐Geffen K., et al. (2019). Molecular Pathways of Senescence Regulate Placental Structure and Function [Published Orrection Appears in EMBO J. 2020 Aug 3;39(15):E105972]. EMBO J. 38 (18), e100849. doi: 10.15252/embj.2018100849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia R. C., Dati L. M., Torres L. H., da Silva M. A., Udo M. S., Abdalla F. M., et al. (2015). M1 and M3 Muscarinic Receptors may Play a Role in the Neurotoxicity of Anhydroecgonine Methyl Ester, a Cocaine Pyrolysis Product. Sci. Rep. 5, 17555. doi: 10.1038/srep17555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Montojo M., Nath A. (2021). HERV-W Envelope Expression in Blood Leukocytes as a Marker of Disease Severity of COVID-19. EBioMedicine 67, 103363. doi: 10.1016/j.ebiom.2021.103363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Navas R., Gajate C., Mollinedo F. (2021). Neutrophils Drive Endoplasmic Reticulum Stress-Mediated Apoptosis in Cancer Cells Through Arginase-1 Release. Sci. Rep. 11, 12574. doi: 10.1038/s41598-021-91947-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geis F. K., Goff S. P. (2020). Silencing and Transcriptional Regulation of Endogenous Retroviruses: An Overview. Viruses 12 (8), 884. doi: 10.3390/v12080884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S., Dellibovi-Ragheb T. A., Kerviel A., Pak E., Qiu Q., Fisher M., et al. (2020). β-Coronaviruses Use Lysosomes for Egress Instead of the Biosynthetic Secretory Pathway. Cell 183 (6), 1520–1535.e14. doi: 10.1016/j.cell.2020.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakopoulos P., Herrmann F. R., Bussière T., Bouras C., Kövari E., Perl D. P., et al. (2003). Tangle and Neuron Numbers, But Not Amyloid Load, Predict Cognitive Status in Alzheimer’s Disease. Neurology 60 (9), 1495–1500. doi: 10.1212/01.wnl.0000063311.58879.01 [DOI] [PubMed] [Google Scholar]
- Giepmans B. N., Verlaan I., Moolenaar W. H. (2001). Connexin-43 Interactions With ZO-1 and Alpha- and Beta-Tubulin. Cell Commun. Adhes 8 (4-6), 219–223. doi: 10.3109/15419060109080727 [DOI] [PubMed] [Google Scholar]
- Gillispie G. J., Sah E., Krishnamurthy S., Ahmidouch M. Y., Zhang B., Orr M. E. (2021). Evidence of the Cellular Senescence Stress Response in Mitotically Active Brain Cells-Implications for Cancer and Neurodegeneration. Life (Basel) 11 (2), 153. doi: 10.3390/life11020153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano-Santini R., Kaulich E., Galbraith K. M., Ritchie F. K., Wang W., Li Z., et al. (2020). Fusogen-Mediated Neuron-Neuron Fusion Disrupts Neural Circuit Connectivity and Alters Animal Behavior. Proc. Natl. Acad. Sci. U. S. A. 117 (37), 23054–23065. doi: 10.1073/pnas.1919063117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard-Thernier C., Pham T. N., Demougeot C. (2015). The Promise of Plant-Derived Substances as Inhibitors of Arginase. Mini Rev. Med. Chem. 15 (10), 798–808. doi: 10.2174/1389557515666150511153852 [DOI] [PubMed] [Google Scholar]
- Gong L., Zhang X., Qiu K., He L., Wang Y., Yin J. (2021). Arginine Promotes Myogenic Differentiation and Myotube Formation Through the Elevation of Cytoplasmic Calcium Concentration. Anim. Nutr. 7 (4), 1115–1123. doi: 10.1016/j.aninu.2021.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goujon C., Greenbury R. A., Papaioannou S., Doyle T., Malim M. H. (2015). A Triple-Arginine Motif in the Amino-Terminal Domain and Oligomerization Are Required for HIV-1 Inhibition by Human MX2. J. Virol. 89 (8), 4676–4680. doi: 10.1128/JVI.00169-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozes I. (2020). The ADNP Syndrome and CP201 (NAP) Potential and Hope. Front. Neurol. 11, 608444. doi: 10.3389/fneur.2020.608444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield E. A. (2018). Polyethylene Glycol Fusion for Hybridoma Production. Cold Spring Harb. Protoc. 2018 (3). doi: 10.1101/pdb.prot103176 [DOI] [PubMed] [Google Scholar]
- Grimes J. M., Khan S., Badeaux M., Rao R. M., Rowlinson S. W., Carvajal R. D. (2021). Arginine Depletion as a Therapeutic Approach for Patients With COVID-19. Int. J. Infect. Dis. 102, 566–570. doi: 10.1016/j.ijid.2020.10.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundman J., Spencer B., Sarsoza F., Rissman R. A. (2021). Transcriptome Analyses Reveal Tau Isoform-Driven Changes in Transposable Element and Gene Expression. PloS One 16 (9), e0251611. doi: 10.1371/journal.pone.0251611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra-Gomes S., Sousa N., Pinto L., Oliveira J. F. (2018). Functional Roles of Astrocyte Calcium Elevations: From Synapses to Behavior. Front. Cell Neurosci. 11, 427. doi: 10.3389/fncel.2017.00427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C., Jeong H. H., Hsieh Y. C., Klein H. U., Bennett D. A., De Jager P. L., et al. (2018). Tau Activates Transposable Elements in Alzheimer’s Disease. Cell Rep. 23 (10), 2874–2880. doi: 10.1016/j.celrep.2018.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. G., Kelly K. M., Helke K. L., Queen S. E., Karper J. M., Dorsey J. L., et al. (2010). HIV and SIV Induce Alterations in CNS CaMKII Expression and Activation: A Potential Mechanism for Cognitive Impairment. Am. J. Pathol. 176 (6), 2776–2784. doi: 10.2353/ajpath.2010.090809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon A. C., Cornelius D. C., Amaral L. M., Faulkner J. L., Cunningham M. W., Jr, Wallace K., et al. (2016). The Role of Inflammation in the Pathology of Preeclampsia. Clin. Sci. (Lond) 130 (6), 409–419. doi: 10.1042/CS20150702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry J., Smeyne R. J., Jang H., Miller B., Okun M. S. (2010). Parkinsonism and Neurological Manifestations of Influenza Throughout the 20th and 21st Centuries. Parkinsonism Relat. Disord. 16 (9), 566–571. doi: 10.1016/j.parkreldis.2010.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervy J., Bicout D. J. (2019). Dynamical Decoration of Stabilized-Microtubules by Tau-Proteins. Sci. Rep. 9 (1), 12473. doi: 10.1038/s41598-019-48790-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbard M. K., Sandri-Goldin R. M. (1995). Arginine-Rich Regions Succeeding the Nuclear Localization Region of the Herpes Simplex Virus Type 1 Regulatory Protein ICP27 Are Required for Efficient Nuclear Localization and Late Gene Expression. J. Virol. 69 (8), 4656–4667. doi: 10.1128/JVI.69.8.4656-4667.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp S. C., Lin Y., Oakley D., Roe A. D., DeVos S. L., Hanlon D., et al. (2018). The Role of Microglia in Processing and Spreading of Bioactive Tau Seeds in Alzheimer’s Disease. J. Neuroinflamm. doi: 10.1186/s12974-018-1309-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornik T. C., Neniskyte U., Brown G. C. (2014). Inflammation Induces Multinucleation of Microglia via PKC Inhibition of Cytokinesis, Generating Highly Phagocytic Multinucleated Giant Cells. J. Neurochem. 128 (5), 650–661. doi: 10.1111/jnc.12477 [DOI] [PubMed] [Google Scholar]
- Hou Q. L., Gao X., Lu Q., Zhang X. H., Tu Y. Y., Jin M. L., et al. (2006). SNAP-25 in Hippocampal CA3 Region Is Required for Long-Term Memory Formation. Biochem. Biophys. Res. Commun. 347 (4), 955–962. doi: 10.1016/j.bbrc.2006.06.184 [DOI] [PubMed] [Google Scholar]
- Huang I., Pranata R. (2020). Lymphopenia in Severe Coronavirus Disease-2019 (COVID-19): Systematic Review and Meta-Analysis. J. Intensive Care 8, 36. doi: 10.1186/s40560-020-00453-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui K. L., Upadhyaya A. (2017). Dynamic Microtubules Regulate Cellular Contractility During T-Cell Activation. Proc. Natl. Acad. Sci. U. S. A. 114 (21), E4175–E4183. doi: 10.1073/pnas.1614291114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung S. Y., Fu W. M. (2017). Drug Candidates in Clinical Trials for Alzheimer’s Disease. J. BioMed. Sci. 24 (1), 47. doi: 10.1186/s12929-017-0355-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imam H., Kim G. W., Siddiqui A. (2020). Epitranscriptomic(N6-Methyladenosine) Modification of Viral RNA and Virus-Host Interactions. Front. Cell Infect. Microbiol. 10, 584283. doi: 10.3389/fcimb.2020.584283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishunina T. A., Unmehopa U. A., Dolzhikov A. A., Swaab D. F. (2000). Multinucleated Arginine-Vasopressin Neurons in the Human Supraoptic Nucleus: A Hallmark of Pulmonary Pathology. Neuroendocrinology 72 (5), 318–326. doi: 10.1159/000054600 [DOI] [PubMed] [Google Scholar]
- Islam M. S., Quispe C., Hossain R., Islam M. T., Al-Harrasi A., Al-Rawahi A., et al. (2021). Neuropharmacological Effects of Quercetin: A Literature-Based Review. Front. Pharmacol. 12, 665031. doi: 10.3389/fphar.2021.665031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzhaki R. F. (2017). Herpes Simplex Virus Type 1 and Alzheimer’s Disease: Possible Mechanisms and Signposts. FASEB J. 31 (8), 3216–3226. doi: 10.1096/fj.201700360 [DOI] [PubMed] [Google Scholar]
- Izquierdo J. H., Bonilla-Abadía F., Cañas C. A., Tobón G. J. (2014). Calcium, Channels, Intracellular Signaling and Autoimmunity. Reumatol. Clin. 10 (1), 43–47. doi: 10.1016/j.reuma.2013.05.008 [DOI] [PubMed] [Google Scholar]
- Jayadev S., Nesser N. K., Hopkins S., Myers S. J., Case A., Lee R. J., et al. (2011). Transcription Factor P53 Influences Microglial Activation Phenotype. Glia 59 (10), 1402–1413. doi: 10.1002/glia.21178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon Y. H., Jung Y. T. (2022). Production of a Replicating Retroviral Vector Expressing Reovirus Fast Protein for Cancer Gene Therapy. J. Virol. Methods 299, 114332. doi: 10.1016/j.jviromet.2021.114332 [DOI] [PubMed] [Google Scholar]
- Jiang L., Lin W., Zhang C., Ash P. E. A., Verma M., Kwan J., et al. (2021). Interaction of Tau With HNRNPA2B1 and N6-Methyladenosine RNA Mediates the Progression of Tauopathy. Mol. Cell. 81 (20), 4209–4227.e12. doi: 10.1016/j.molcel.2021.07.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. B., Song H., Xu Y., Garrison K. E., Buzdin A. A., Anwar N., et al. (2013). LINE-1 Retrotransposable Element DNA Accumulates in HIV-1-Infected Cells. J. Virol. 87 (24), 13307–13320. doi: 10.1128/JVI.02257-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kádková A., Radecke J., Sørensen J. B. (2019). The SNAP-25 Protein Family. Neuroscience 420, 50–71. doi: 10.1016/j.neuroscience.2018.09.020 [DOI] [PubMed] [Google Scholar]
- Kajiwara Y., Wang E., Wang M., Sin W. C., Brennand K. J., Schadt E., et al. (2018). GJA1 (Connexin43) Is a Key Regulator of Alzheimer’s Disease Pathogenesis. Acta Neuropathol. Commun. 6 (1), 144. doi: 10.1186/s40478-018-0642-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra A. P., Patel S. D., Bhuiyan A. F., Preto J., Scheuer K. G., Mohammed U., et al. (2020). Investigation of the Electrical Properties of Microtubule Ensembles Under Cell-Like Conditions. Nanomater. (Basel). 10 (2), 265. doi: 10.3390/nano10020265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandemirli S. G., Altundag A., Yildirim D., Tekcan Sanli D. E., Saatci O. (2021). Olfactory Bulb MRI and Paranasal Sinus CT Findings in Persistent COVID-19 Anosmia. Acad. Radiol. 28 (1), 28–35. doi: 10.1016/j.acra.2020.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamitros T., Hurst T., Marchi E., Karamichali E., Georgopoulou U., Mentis A., et al. (2018). Human Endogenous Retrovirus-K HML-2 Integration Within RASGRF2 Is Associated With Intravenous Drug Abuse and Modulates Transcription in a Cell-Line Model. Proc. Natl. Acad. Sci. U. S. A. 115 (41), 10434–10439. doi: 10.1073/pnas.1811940115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp K., Wilkins A., Scolding N. (2014). Cell Fusion in the Brain: Two Cells Forward, One Cell Back. Acta Neuropathol. 128 (5), 629–638. doi: 10.1007/s00401-014-1303-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan H., Ullah H., Aschner M., Cheang W. S., Akkol E. K. (2019). Neuroprotective Effects of Quercetin in Alzheimer’s Disease. Biomolecules 10 (1), 59. doi: 10.3390/biom10010059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyoshi C. M., Zhou M. (2019). Astrocyte Syncytium: A Functional Reticular System in the Brain. Neural Regener. Res. 14 (4), 595–596. doi: 10.4103/1673-5374.247462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight J. S., Robertson E. S. (2004). Epstein-Barr Virus Nuclear Antigen 3C Regulates Cyclin A/p27 Complexes and Enhances Cyclin A-Dependent Kinase Activity. J. Virol. 78, 1981–1991. doi: 10.1128/JVI.78.4.1981-1991.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike S., Jahn R. (2019). SNAREs Define Targeting Specificity of Trafficking Vesicles by Combinatorial Interaction With Tethering Factors. Nat. Commun. 10, 1608. doi: 10.1038/s41467-019-09617-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopf A., Kiermaier E. (2021). Dynamic Microtubule Arrays in Leukocytes and Their Role in Cell Migration and Immune Synapse Formation. Front. Cell Dev. Biol. 9, 635511. doi: 10.3389/fcell.2021.635511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalevich J., Cornec A. S., Yao Y., James M., Crowe A., Lee V. M., et al. (2016). Characterization of Brain–Penetrant Pyrimidine–Containing Molecules With Differential Microtubule–Stabilizing Activities Developed as Potential Therapeutic Agents for Alzheimer’s Disease and Related Tauopathies. J. Pharmacol. Exp. Ther. 357, 432–450. doi: 10.1124/jpet.115.231175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer B. C., Zhang B., Leverenz J. B., Thomas J. H., Trojanowski J. Q., Schellenberg G. D. (2003). Neurodegeneration and Defective Neurotransmission in a Caenorhabditis Elegans Model of Tauopathy. Proc. Natl. Acad. Sci. U. S. A. 100 (17), 9980–9985. doi: 10.1073/pnas.1533448100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharz E. J., Sierakowski S., Staite N. D., Goodwin J. S. (1988). Mechanism of Lithium-Induced Augmentation of T-Cell Proliferation. Int. J. Immunopharmacol. 10 (3), 253–259. doi: 10.1016/0192-0561(88)90056-2 [DOI] [PubMed] [Google Scholar]
- Küry P., Nath A., Créange A., Dolei A., Marche P., Gold J., et al. (2018). Human Endogenous Retroviruses in Neurological Diseases. Trends Mol. Med. 24 (4), 379–394. doi: 10.1016/j.molmed.2018.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuyumcu M. E., Yesil Y., Oztürk Z. A., Kizilarslanoğlu C., Etgül S., Halil M., et al. (2012). The Evaluation of Neutrophil-Lymphocyte Ratio in Alzheimer’s Disease. Dement. Geriatr. Cognit. Disord. 34 (2), 69–74. doi: 10.1159/000341583 [DOI] [PubMed] [Google Scholar]
- Lai S. W., Kuo Y. H., Liao K. F. (2021). Chronic Hydroxychloroquine Exposure and the Risk of Alzheimer’s Disease. Ann. Rheum Dis. 80 (7), e105. doi: 10.1136/annrheumdis-2019-216173 [DOI] [PubMed] [Google Scholar]
- Lardner A. (2001). The Effects of Extracellular pH on Immune Function. J. Leukoc. Biol. 69 (4), 522–530. doi: 10.1189/jlb.69.4.522 [DOI] [PubMed] [Google Scholar]
- Lasagna-Reeves C. A., Sengupta U., Castillo-Carranza D., Gerson J. E., Guerrero-Munoz M., Troncoso J. C., et al. (2014). The Formation of Tau Pore-Like Structures Is Prevalent and Cell Specific: Possible Implications for the Disease Phenotypes. Acta Neuropathol. Commun. 2, 56. doi: 10.1186/2051-5960-2-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederer W., Schaffenrath H., Alomar-Dominguez C., Thaler J., Fantin R., Dostal L., et al. (2020). Cerebrospinal Beta-Amyloid Peptides (1-40) and (1-42) in Severe Preeclampsia and HELLP Syndrome – a Pilot Study. Sci. Rep. 10, 5783. doi: 10.1038/s41598-020-62805-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le T., Le S. C., Zhang Y., Liang P., Yang H. (2020). Evidence That Polyphenols do Not Inhibit the Phospholipid Scramblase TMEM16F. J. Biol. Chem. 295 (35), 12537–12544. doi: 10.1074/jbc.AC120.014872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leontieva O. V., Natarajan V., Demidenko Z. N., Burdelya L. G., Gudkov A. V., Blagosklonny M. V. (2012). Hypoxia Suppresses Conversion From Proliferative Arrest to Cellular Senescence. Proc. Natl. Acad. Sci. U. S. A. 109 (33), 13314–13318. doi: 10.1073/pnas.1205690109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S. (2019). Regulation of Ribosomal Proteins on Viral Infection. Cells 8 (5), 508. doi: 10.3390/cells8050508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licastro F., Porcellini E. (2021). Activation of Endogenous Retrovirus, Brain Infections and Environmental Insults in Neurodegeneration and Alzheimer’s Disease. Int. J. Mol. Sci. 22 (14), 7263. doi: 10.3390/ijms22147263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow S., Guttenplan K., Clarke L., Bennett F. C., Bohlen C. J., Schirmer L., et al. (2017). Neurotoxic Reactive Astrocytes Are Induced by Activated Microglia. Nature 541, 481–487. doi: 10.1038/nature21029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liester M. B. (2020). Personality Changes Following Heart Transplantation: The Role of Cellular Memory. Med. Hypotheses 135, 109468. doi: 10.1016/j.mehy.2019.109468 [DOI] [PubMed] [Google Scholar]
- Li N., Hui H., Bray B., Gonzalez G. M., Zeller M., Anderson K. G., et al. (2021). METTL3 Regulates Viral M6a RNA Modification and Host Cell Innate Immune Responses During SARS-CoV-2 Infection. Cell Rep. 35 (6), 109091. doi: 10.1016/j.celrep.2021.109091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima M. N., Freitas R. J. R. X., Passos B. A. B. R., Darze A. M. G., Castro-Faria-Neto H. C., Maron-Gutierrez T. (2021). Neurovascular Interactions in Malaria. Neuroimmunomodulation 28 (3), 108–117. doi: 10.1159/000515557 [DOI] [PubMed] [Google Scholar]
- Lin L., Li Q., Wang Y., Shi Y. (2021). Syncytia Formation During SARS-CoV-2 Lung Infection: A Disastrous Unity to Eliminate Lymphocytes. Cell Death Differ. 28, 2019–2021. doi: 10.1038/s41418-021-00795-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J., Yasuda R., Raghavachari S. (2012). Mechanisms of CaMKII Action in Long-Term Potentiation. Nat. Rev. Neurosci. 13, 169–182. doi: 10.1038/nrn3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Song D., Wang S., Dai Y., Zhou J., Gu J. (2020). Antiviral Effect of Epigallocatechin Gallate via Impairing Porcine Circovirus Type 2 Attachment to Host Cell Receptor. Viruses 12 (2), 176. doi: 10.3390/v12020176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Hossinger A., Heumüller S. E., Hornberger A., Buravlova O., Konstantoulea K., et al. (2021). Highly Efficient Intercellular Spreading of Protein Misfolding Mediated by Viral Ligand-Receptor Interactions. Nat. Commun. 12 (1), 5739. doi: 10.1038/s41467-021-25855-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Pei J., Grishin N., Snell W. J. (2015). The Cytoplasmic Domain of the Gamete Membrane Fusion Protein HAP2 Targets the Protein to the Fusion Site in Chlamydomonas and Regulates the Fusion Reaction. Development 142 (5), 962–971. doi: 10.1242/dev.118844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Xu Y. P., Li K., et al. (2021). The M6a Methylome of SARS-CoV-2 in Host Cells. Cell Res. 31, 404–414. doi: 10.1038/s41422-020-00465-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Zhang H., Lyons T. W., Lu M., Achab A., Pu Q., et al. (2021). Comprehensive Strategies to Bicyclic Prolines: Applications in the Synthesis of Potent Arginase Inhibitors. ACS Med. Chem. Lett. 12 (11), 1678–1688. doi: 10.1021/acsmedchemlett.1c00258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorens J. V., Soriano S., Calap-Quintana P., Gonzalez-Cabo P., Moltó M. D. (2019). The Role of Iron in Friedreich’s Ataxia: Insights From Studies in Human Tissues and Cellular and Animal Models. Front. Neurosci. 13, 75. doi: 10.3389/fnins.2019.00075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokossou A. G., Toudic C., Nguyen P. T., Elisseeff X., Vargas A., Rassart É, et al. (2020). Endogenous Retrovirus-Encoded Syncytin-2 Contributes to Exosome-Mediated Immunosuppression of T Cells†. Biol. Reprod. 102 (1), 185–198. doi: 10.1093/biolre/ioz124 [DOI] [PubMed] [Google Scholar]
- Lopes J. P., Oliveira C. R., Agostinho P. (2009). Cell Cycle Re-Entry in Alzheimer’s Disease: A Major Neuropathological Characteristic? Curr. Alzheimer Res. 6 (3), 205–212. doi: 10.2174/156720509788486590 [DOI] [PubMed] [Google Scholar]
- Losick V. P., Fox D. T., Spradling A. C. (2013). Polyploidization and Cell Fusion Contribute to Wound Healing in the Adult Drosophila Epithelium. Curr. Biol. 23 (22), 2224–2232. doi: 10.1016/j.cub.2013.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotz C., Muellenbach R. M., Meybohm P., Mutlak H., Lepper P. M., Rolfes C. B., et al. (2021). Effects of Inhaled Nitric Oxide in COVID-19-Induced ARDS - Is It Worthwhile? Acta Anaesthesiol. Scand. 65 (5), 629–632. doi: 10.1111/aas.13757 [DOI] [PubMed] [Google Scholar]
- Lynch S. M., Guo G., Gibson D. S., Bjourson A. J., Rai T. S. (2021). Role of Senescence and Aging in SARS-CoV-2 Infection and COVID-19 Disease. Cells 10, 3367. doi: 10.3390/cells10123367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch D. R., Schadt K., Kichula E. (2019). Etravirine in Friedreich’s Ataxia: Lessons From HIV? Mov. Disord. 34 (3), 305–306. doi: 10.1002/mds.27605 [DOI] [PubMed] [Google Scholar]
- Ma B., Buckalew R., Du Y., Kiyoshi C. M., Alford C. C., Wang W., et al. (2016). Gap Junction Coupling Confers Isopotentiality on Astrocyte Syncytium. Glia 64 (2), 214–226. doi: 10.1002/glia.22924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhães A. D., Emmenegger M., De Cecco E., Carta M., Karl Frontzek K., Chincisan A., et al. (2021). Large-Scale Seroepidemiology Identifies a Nephro-Vascular Syndrome Associated With Autoimmune Reactivity to Tau. MedRxiv. doi: 10.1101/2021.11.24.21266833 [DOI] [Google Scholar]
- Malashkevich V. N., Dulyaninova N. G., Ramagopal U. A., Liriano M. A., Varney K. M., Knight D., et al. (2010). Phenothiazines Inhibit S100A4 Function by Inducing Protein Oligomerization. Proc. Natl. Acad. Sci. U. S. A. 107 (19), 8605–8610. doi: 10.1073/pnas.0913660107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malebari A. M., Wang S., Greene T. F., O’Boyle N. M., Fayne D., Khan M. F. (2021). Synthesis and Antiproliferative Evaluation of 3-Chloroazetidin-2-Ones With Antimitotic Activity: Heterocyclic Bridged Analogues of Combretastatin A-4. Pharmaceut. (Basel). 14 (11), 1119. doi: 10.3390/ph14111119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamsa S. S. A., Meloni B. P. (2021). Arginine and Arginine-Rich Peptides as Modulators of Protein Aggregation and Cytotoxicity Associated With Alzheimer’s Disease. Front. Mol. Neurosci. 14, 759729. doi: 10.3389/fnmol.2021.759729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J., Zhang Q., Cong Y. S. (2021). Human Endogenous Retroviruses in Development and Disease. Comput. Struct. Biotechnol. J. 19, 5978–5986. doi: 10.1016/j.csbj.2021.10.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marambaud P., Dreses-Werringloer U., Vingtdeux V. (2009). Calcium Signaling in Neurodegeneration. Mol. Neurodegener. 4, 20. doi: 10.1186/1750-1326-4-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Márquez-Coello M., Ruiz-Sánchez C., Martín-Aspas A., Fernández Gutiérrez Del Álamo C., Illanes-Álvarez F., Cuesta-Sancho S., et al. (2021). Neutrophil Expression of T and B Immunomodulatory Molecules in HIV Infection. Front. Immunol. 12, 670966. doi: 10.3389/fimmu.2021.670966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martí i Líndez A. A., Reith W. (2021). Arginine-Dependent Immune Responses. Cell. Mol. Life Sci. 78, 5303–5324. doi: 10.1007/s00018-021-03828-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin N., Bernard D. (2018). Calcium Signaling and Cellular Senescence. Cell Calcium 70, 16–23. doi: 10.1016/j.ceca.2017.04.001 [DOI] [PubMed] [Google Scholar]
- Mastrodonato V., Morelli E., Vaccari T. (2018). How to Use a Multipurpose SNARE: The Emerging Role of Snap29 in Cellular Health. Cell Stress 2 (4), 72–81. doi: 10.15698/cst2018.04.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateos-Aparicio P., Rodríguez-Moreno A. (2020). Calcium Dynamics and Synaptic Plasticity. Adv. Exp. Med. Biol. 1131, 965–984. doi: 10.1007/978-3-030-12457-1_38 [DOI] [PubMed] [Google Scholar]
- Matsunaga S., Kishi T., Annas P., Basun H., Hampel H., Iwata N. (2015). Lithium as a Treatment for Alzheimer’s Disease: A Systematic Review and Meta-Analysis. J. Alzheimers Dis. 48 (2), 403–410. doi: 10.3233/JAD-150437 [DOI] [PubMed] [Google Scholar]
- Maurizi C. P. (2010). Influenza Caused Epidemic Encephalitis (Encephalitis Lethargica): The Circumstantial Evidence and a Challenge to the Nonbelievers. Med. Hypotheses 74 (5), 798–801. doi: 10.1016/j.mehy.2009.12.012 [DOI] [PubMed] [Google Scholar]
- Mavrikaki M., Lee J. D., Solomon I. H., Slack F. J. (2021). Severe COVID-19 Induces Molecular Signatures of Aging in the Human Brain. medRxiv 24, 2021.11.24.21266779. doi: 10.1101/2021.11.24.21266779 [DOI] [PubMed] [Google Scholar]
- Mazzorana M., Hussain R., Sorensen T. (2016). Ca-Dependent Folding of Human Calumenin. PloS One 11 (3), e0151547. doi: 10.1371/journal.pone.0151547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melano I., Kuo L. L., Lo Y. C., Sung P. W., Tien N., Su W. C. (2021). Effects of Basic Amino Acids and Their Derivatives on SARS-CoV-2 and Influenza-A Virus Infection. Viruses 13 (7), 1301. doi: 10.3390/v13071301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao G., Zhao H., Li Y., Ji M., Chen Y., Shi Y., et al. (2021). ORF3a of the COVID-19 Virus SARS-CoV-2 Blocks HOPS Complex-Mediated Assembly of the SNARE Complex Required for Autolysosome Formation. Dev. Cell. 56 (4), 427–442.e5. doi: 10.1016/j.devcel.2020.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millecamps S., Julien J. P. (2013). Axonal Transport Deficits and Neurodegenerative Diseases. Nat. Rev. Neurosci. 14 (3), 161–176. doi: 10.1038/nrn3380 [DOI] [PubMed] [Google Scholar]
- Miller M. B., Reed H. C., Walsh C. A. (2021). Brain Somatic Mutation in Aging and Alzheimer’s Disease. Annu. Rev. Genomics Hum. Genet. 22, 239–256. doi: 10.1146/annurev-genom-121520-081242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millington-Burgess S. L., Harper M. T. (2021). Epigallocatechin Gallate Inhibits Release of Extracellular Vesicles From Platelets Without Inhibiting Phosphatidylserine Exposure. Sci. Rep. 11, 17678. doi: 10.1038/s41598-021-97212-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minozzo B. R., Fernandes D., Beltrame F. L. (2018). Phenolic Compounds as Arginase Inhibitors: New Insights Regarding Endothelial Dysfunction Treatment. Planta Med. 84 (5), 277–295. doi: 10.1055/s-0044-100398 [DOI] [PubMed] [Google Scholar]
- Mistry S. K., Zheng M., Rouse B. T., Morris S. M., Jr. (2001). Induction of Arginases I and II in Cornea During Herpes Simplex Virus Infection. Virus Res. 73 (2), 177–182. doi: 10.1016/s0168-1702(00)00243-4 [DOI] [PubMed] [Google Scholar]
- Moore C. I., Cao R. (2008). The Hemo-Neural Hypothesis: On the Role of Blood Flow in Information Processing. J. Neurophysiol. 99 (5), 2035–2047. doi: 10.1152/jn.01366.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretto J., Girard C., Demougeot C. (2019). The Role of Arginase in Aging: A Systematic Review. Exp. Gerontol. 116, 54–73. doi: 10.1016/j.exger.2018.12.011 [DOI] [PubMed] [Google Scholar]
- Mosch B., Morawski M., Mittag A., Lenz D., Tarnok A., Arendt T. (2007). Aneuploidy and DNA Replication in the Normal Human Brain and Alzheimer’s Disease. J. Neurosci. 27 (26), 6859–6867. doi: 10.1523/JNEUROSCI.0379-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostowy S., Shenoy A. R. (2015). The Cytoskeleton in Cell-Autonomous Immunity: Structural Determinants of Host Defence. Nat. Rev. Immunol. 15 (9), 559–573. doi: 10.1038/nri3877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moujaber O., Fishbein F., Omran N., Liang Y., Colmegna I., Presley J. F., et al. (2019). Cellular Senescence Is Associated With Reorganization of the Microtubule Cytoskeleton. Cell Mol. Life Sci. 76 (6), 1169–1183. doi: 10.1007/s00018-018-2999-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulding D. A., Record J., Malinova D., Thrasher A. J. (2013). Actin Cytoskeletal Defects in Immunodeficiency. Immunol. Rev. 256 (1), 282–299. doi: 10.1111/imr.12114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munder M. (2009). Arginase: An Emerging Key Player in the Mammalian Immune System. Br. J. Pharmacol. 158 (3), 638–651. doi: 10.1111/j.1476-5381.2009.00291.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murru A., Manchia M., Hajek T., Nielsen R. E., Rybakowski J. K., Sani G., et al. (2020). Lithium’s Antiviral Effects: A Potential Drug for CoViD-19 Disease? Int. J. Bipolar Disord. 8 (1), 21. doi: 10.1186/s40345-020-00191-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naghavi M. H. (2021). HIV-1 Capsid Exploitation of the Host Microtubule Cytoskeleton During Early Infection. Retrovirology 18, 19. doi: 10.1186/s12977-021-00563-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naghavi M. H., Walsh D. (2017). Microtubule Regulation and Function During Virus Infection. J. Virol. 91 (16), e00538-17. doi: 10.1128/JVI.00538-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi M., Otsu M. (2012). Development of Sendai Virus Vectors and Their Potential Applications in Gene Therapy and Regenerative Medicine. Curr. Gene Ther. 12 (5), 410–416. doi: 10.2174/156652312802762518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nataf S. (2017). Autoimmunity as a Driving Force of Cognitive Evolution. Front. Neurosci. 11, 582. doi: 10.3389/fnins.2017.00582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher J. J., Neniskyte U., Brown G. C. (2012). Primary Phagocytosis of Neurons by Inflamed Microglia: Potential Roles in Neurodegeneration. Front. Pharmacol. 3, 27. doi: 10.3389/fphar.2012.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann B., Linton C., Giordano-Santini R., Hilliard M. A. (2019). Axonal Fusion: An Alternative and Efficient Mechanism of Nerve Repair. Prog. Neurobiol. 173, 88–101. doi: 10.1016/j.pneurobio.2018.11.004 [DOI] [PubMed] [Google Scholar]
- Nguyen M. C., Park J. T., Jeon Y. G., Jeon B. H., Hoe K. L., Kim Y. M., et al. (2016). Arginase Inhibition Restores Peroxynitrite-Induced Endothelial Dysfunction via L-Arginine-Dependent Endothelial Nitric Oxide Synthase Phosphorylation. Yonsei Med. J. 57 (6), 1329–1338. doi: 10.3349/ymj.2016.57.6.1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto-Torres J. L., DeDiego M. L., Verdiá-Báguena C., Jimenez-Guardeño J. M., Regla-Nava J. A., Fernandez-Delgado R., et al. (2014). Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Ion Channel Activity Promotes Virus Fitness and Pathogenesis. PloS Pathog. 10 (5), e1004077. doi: 10.1371/journal.ppat.1004077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmrich V., Eckert A. (2013). Calcium Channel Blockers and Dementia. Br. J. Pharmacol. 169 (6), 1203–1210. doi: 10.1111/bph.12240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes P., Demaurex N. (2010). The Role of Calcium Signaling in Phagocytosis. J. Leukoc. Biol. 88 (1), 57–68. doi: 10.1189/jlb.0110028 [DOI] [PubMed] [Google Scholar]
- Nyenhuis S. B., Karandikar N., Kiessling V., Kreutzberger A. J. B., Thapa A., Liang B., et al. (2021). Conserved Arginine Residues in Synaptotagmin 1 Regulate Fusion Pore Expansion Through Membrane Contact. Nat. Commun. 12, 761. doi: 10.1038/s41467-021-21090-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh-hora M., Rao A. (2008). Calcium Signaling in Lymphocytes. Curr. Opin. Immunol. 20 (3), 250–258. doi: 10.1016/j.coi.2008.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen I. (2021). Porphyromonas Gingivalis May Seek the Alzheimer’s Disease Brain to Acquire Iron From Its Surplus. J. Alzheimers Dis. Rep. 5 (1), 79–86. doi: 10.3233/ADR-200272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson A., Hussong S. A., Kayed R., Galvan V. (2019). TAU-INDUCED ASTROCYTE SENESCENCE: A NOVEL MECHANISM FOR NEURONAL DYSFUNCTION IN Alzheimer’s DISEASE. Innov Aging 3 (Suppl 1), S91–S92. doi: 10.1093/geroni/igz038.348 [DOI] [Google Scholar]
- Osorio C., Kanukuntla T., Diaz E., Jafri N., Cummings M., Sfera A. (2019). The Post-Amyloid Era in Alzheimer’s Disease: Trust Your Gut Feeling. Front. Aging Neurosci. 11, 143. doi: 10.3389/fnagi.2019.00143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ousingsawat J., Wanitchakool P., Schreiber R., Kunzelmann K. (2018). Contribution of TMEM16F to Pyroptotic Cell Death. Cell Death Dis. 9, 300. doi: 10.1038/s41419-018-0373-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovsepian S. V., O’Leary V. B. (2018). Can Arginase Inhibitors Be the Answer to Therapeutic Challenges in Alzheimer’s Disease? Neurotherapeutics 15 (4), 1032–1035. doi: 10.1007/s13311-018-0668-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozsvari B., Nuttall J. R., Sotgia F., Lisanti M. P. (2018). Azithromycin and Roxithromycin Define a New Family of "Senolytic" Drugs That Target Senescent Human Fibroblasts. Aging (Albany NY) 10 (11), 3294–3307. doi: 10.18632/aging.101633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan P. Y., Cai Q., Lin L., Lu P. H., Duan S., Sheng Z. H. (2005). SNAP-29-Mediated Modulation of Synaptic Transmission in Cultured Hippocampal Neurons. J. Biol. Chem. 280 (27), 25769–25779. doi: 10.1074/jbc.M502356200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paniz-Mondolfi A., Bryce C., Grimes Z., Gordon R. E., Reidy J., Lednicky J., et al. (2020). Central Nervous System Involvement by Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2). J. Med. Virol. 92 (7), 699–702. doi: 10.1002/jmv.25915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris D., Parker T. A., Town T., Suo Z., Fang C., Humphrey J., et al. (1998). Role of Peroxynitrite in the Vasoactive and Cytotoxic Effects of Alzheimer’s Beta-Amyloid1-40 Peptide. Exp. Neurol. 152 (1), 116–122. doi: 10.1006/exnr.1998.6828 [DOI] [PubMed] [Google Scholar]
- Parry R., Gifford R. J., Lytras S., Ray S. C., Coin L. J. M. (2021). No Evidence of SARS-CoV-2 Reverse Transcription and Integration as the Origin of Chimeric Transcripts in Patient Tissues. Proc. Natl. Acad. Sci. U. S. A. 118 (33), e2109066118. doi: 10.1073/pnas.2109066118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastuzyn E. D., Day C. E., Kearns R. B., Kyrke-Smith M., Taibi A. V., McCormick J., et al. (2018). The Neuronal Gene Arc Encodes a Repurposed Retrotransposon Gag Protein That Mediates Intercellular RNA Transfer. Cell 172 (1-2), 275–288.e18. doi: 10.1016/j.cell.2017.12.024 Erratum in: Cell. 2018 Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrera L., Espiritu R. A., Ros U., Weber J., Schmitt A., Stroh J., et al. (2021). Ferroptotic Pores Induce Ca2+ Fluxes and ESCRT-III Activation to Modulate Cell Death Kinetics. Cell Death Differ. 28, 1644–1657. doi: 10.1038/s41418-020-00691-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X. C., Zhang M., Meng Y. Y., Liang Y. F., Wang Y. Y., Liu X. Q., et al. (2021). Cell−cell Fusion as an Important Mechanism of Tumor Metastasis (Review). Oncol. Rep. 46 (1), 145. doi: 10.3892/or.2021.8096 [DOI] [PubMed] [Google Scholar]
- Perea J. R., Llorens-Martín M., Ávila J., Bolós M. (2018). The Role of Microglia in the Spread of Tau: Relevance for Tauopathies. Front. Cell Neurosci. 12, 172. doi: 10.3389/fncel.2018.00172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perron H., Lazarini F., Ruprecht K., Péchoux-Longin C., Seilhean D., Sazdovitch V., et al. (2005). Human Endogenous Retrovirus (HERV)-W ENV and GAG Proteins: Physiological Expression in Human Brain and Pathophysiological Modulation in Multiple Sclerosis Lesions. J. Neurovirol. 11 (1), 23–33. doi: 10.1080/13550280590901741 [DOI] [PubMed] [Google Scholar]
- Peyton K. J., Liu X. M., Shebib A. R., Johnson F. K., Johnson R. A., Durante W. (2018). Arginase Inhibition Prevents the Development of Hypertension and Improves Insulin Resistance in Obese Rats. Amino Acids 50 (6), 747–754. doi: 10.1007/s00726-018-2567-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilhofer M., Ladinsky M. S., McDowall A. W., Petroni G., Jensen G. J. (2011). Microtubules in Bacteria: Ancient Tubulins Build a Five-Protofilament Homolog of the Eukaryotic Cytoskeleton. PloS Biol. 9 (12), e1001213. doi: 10.1371/journal.pbio.1001213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto M. C., Kihara A. H., Goulart V. A., Tonelli F. M., Gomes K. N., Ulrich H., et al. (2015). Calcium Signaling and Cell Proliferation. Cell Signal. 27 (11), 2139–2149. doi: 10.1016/j.cellsig.2015.08.006 [DOI] [PubMed] [Google Scholar]
- Poblocka M., Bassey A. L., Smith V. M., Falcicchio M., Manso A. S., Althubiti M., et al. (2021). Targeted Clearance of Senescent Cells Using an Antibody-Drug Conjugate Against a Specific Membrane Marker. Sci. Rep. 11, 20358. doi: 10.1038/s41598-021-99852-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirot O., Timsit Y. (2016). Neuron-Like Networks Between Ribosomal Proteins Within the Ribosome. Sci. Rep. 6, 26485. doi: 10.1038/srep26485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack F. P., Irusta P. M., Hoffman S. J., Schiatti M. P., Melendi G. A., Delgado M. F., et al. (2005). The Cysteine-Rich Region of Respiratory Syncytial Virus Attachment Protein Inhibits Innate Immunity Elicited by the Virus and Endotoxin. Proc. Natl. Acad. Sci. U. S. A. 102 (25), 8996–9001. doi: 10.1073/pnas.0409478102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomorski T. G., Menon A. K. (2016). Lipid Somersaults: Uncovering the Mechanisms of Protein-Mediated Lipid Flipping. Prog. Lipid Res. 64, 69–84. doi: 10.1016/j.plipres.2016.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popugaeva E., Bezprozvanny I. (2014). Can the Calcium Hypothesis Explain Synaptic Loss in Alzheimer’s Disease? Neurodegener. Dis. 13 (2-3), 139–141. doi: 10.1159/000354778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pralong D., Trounson A. O., Verma P. J. (2006). Cell Fusion for Reprogramming Pluripotency: Toward Elimination of the Pluripotent Genome. Stem Cell Rev. 2 (4), 331–340. doi: 10.1007/BF02698060 [DOI] [PubMed] [Google Scholar]
- Pratt J., Lester E., Parker R. (2021). Could SARS-CoV-2 Cause Tauopathy? Lancet Neurol. 20 (7), 506. doi: 10.1016/S1474-4422(21)00168-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prozan L., Shusterman E., Ablin J., Mitelpunkt A., Weiss-Meilik A., Adler A., et al. (2021). Prognostic Value of Neutrophil-to-Lymphocyte Ratio in COVID-19 Compared With Influenza and Respiratory Syncytial Virus Infection. Sci. Rep. 11, 21519. doi: 10.1038/s41598-021-00927-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthenveetil R., Lun C. M., Murphy R. E., et al. (2021). S-Acylation of SARS-CoV-2 Spike Protein: Mechanistic Dissection, In Vitro Reconstitution and Role in Viral Infectivity. J. Biol. Chem. 297 (4), 101112. doi: 10.1016/j.jbc.2021.101112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao G., Li S., Yang B., Li B. (2007). Inhibitory Effects of Artemisinin on Voltage-Gated Ion Channels in Intact Nodose Ganglion Neurones of Adult Rats. Basic Clin. Pharmacol. Toxicol. 100 (4), 217–224. doi: 10.1111/j.1742-7843.2006.00009.x [DOI] [PubMed] [Google Scholar]
- Quintana A., Pasche M., Junker C., Al-Ansary D., Rieger H., Kummerow C., et al. (2011). Calcium Microdomains at the Immunological Synapse: How ORAI Channels, Mitochondria and Calcium Pumps Generate Local Calcium Signals for Efficient T-Cell Activation. EMBO J. 30 (19), 3895–3912. doi: 10.1038/emboj.2011.289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramani A., Müller L., Ostermann P. N., Gabriel E., Abida-Islam P., Müller-Schiffmann A., et al. (2020). SARS-CoV-2 Targets Neurons of 3D Human Brain Organoids. EMBO J. 39 (20), e106230. doi: 10.15252/embj.2020106230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez P., Zuniga G., Sun W., Beckmann A., Ochoa E., DeVos S. L., et al. (2022). Pathogenic Tau Accelerates Aging-Associated Activation of Transposable Elements in the Mouse Central Nervous System. Prog. Neurobiol. 208, 102181. doi: 10.1016/j.pneurobio.2021.102181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees C. A., Rostad C. A., Mantus G., Anderson E. J., Chahroudi A., Jaggi P., et al. (2021). Altered Amino Acid Profile in Patients With SARS-CoV-2 Infection. Proc. Natl. Acad. Sci. U. S. A. 118 (25), e2101708118. doi: 10.1073/pnas.2101708118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rems L., Ušaj M., Kandušer M., Reberšek M., Miklavčič D., Pucihar G.. (2013). Cell Electrofusion Using Nanosecond Electric Pulses. Sci. Rep. 3, 3382. doi: 10.1038/srep03382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R. M., Ezashi T., Schulz L. C., Sugimoto J., Schust D. J., Khan T., et al. (2021). Syncytins Expressed in Human Placental Trophoblast. Placenta, 113:8–11314. doi: 10.1016/j.placenta.2021.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römer C. (2021). Viruses and Endogenous Retroviruses as Roots for Neuroinflammation and Neurodegenerative Diseases. Front. Neurosci. 15, 648629. doi: 10.3389/fnins.2021.648629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sait A., Angeli C., Doig A. J., Day P. J. R. (2021). Viral Involvement in Alzheimer’s Disease. ACS Chem. Neurosci. 12 (7), 1049–1060. doi: 10.1021/acschemneuro.0c00719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sałaciak K., Koszałka A., Zmudzka E., Pytka K. (2021). The ˙calcium/Calmodulin-DependentKinases II and IV as Therapeutic Targets in Neurodegenerative and Neuropsychiatric Disorders. Int. J. Mol. Sci. 22, 4307. doi: 10.3390/ijms22094307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvadores N., Gerónimo-Olvera C., Court F. A. (2020). Axonal Degeneration in AD: The Contribution of Aβ and Tau. Front. Aging Neurosci. 12, 581767. doi: 10.3389/fnagi.2020.581767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago-Mujika E., Luthi-Carter R., Giorgini F., Kalaria R. N., Mukaetova-Ladinska E. B. (2021). Tubulin and Tubulin Posttranslational Modifications in Alzheimer’s Disease and Vascular Dementia. Front. Aging Neurosci. 13, 730107. doi: 10.3389/fnagi.2021.730107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapir A., Avinoam O., Podbilewicz B., Chernomordik L. V. (2008). Viral and Developmental Cell Fusion Mechanisms: Conservation and Divergence. Dev. Cell 14 (1), 11–21. doi: 10.1016/j.devcel.2007.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargiacomo C., Sotgia F., Lisanti M. P. (2020). COVID-19 and Chronological Aging: Senolytics and Other Anti-Aging Drugs for the Treatment or Prevention of Corona Virus Infection? Aging (Albany NY) 12 (8), 6511–6517. doi: 10.18632/aging.103001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saurav S., Tanwar J., Ahuja K., Motiani R. K. (2021). Dysregulation of Host Cell Calcium Signaling During Viral Infections: Emerging Paradigm With High Clinical Relevance. Mol. Aspects Med. 81, 101004. doi: 10.1016/j.mam.2021.101004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayed A., Bahbah E. I., Kamel S., Barreto G. E., Ashraf G. M., Elfil M. (2020). The Neutrophil-to-Lymphocyte Ratio in Alzheimer’s Disease: Current Understanding and Potential Applications. J. Neuroimmunol. 349, 577398. doi: 10.1016/j.jneuroim.2020.577398 [DOI] [PubMed] [Google Scholar]
- Saylor D., Dickens A. M., Sacktor N., Haughey N., Slusher B., Pletnikov M., et al. (2016). HIV-Associated Neurocognitive Disorder–Pathogenesis and Prospects for Treatment [Published Correction Appears in Nat Rev Neurol. 2016 May;12(5):309]. Nat. Rev. Neurol. 12 (4), 234–248. doi: 10.1038/nrneurol.2016.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sfera A., Osorio C., Maguire G., Rahman L., Afzaal J., Cummings M., et al. (2021). COVID-19, Ferrosenescence and Neurodegeneration, a Mini-Review. Prog. Neuropsychopharmacol. Biol. Psychiatry 109, 110230. doi: 10.1016/j.pnpbp.2020.110230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sfera A., Osorio C., Zapata Martín Del Campo C. M., Pereida S., Maurer S., Maldonado J. C., et al. (2021). Endothelial Senescence and Chronic Fatigue Syndrome, a COVID-19 Based Hypothesis. Front. Cell Neurosci. 15, 673217. doi: 10.3389/fncel.2021.673217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sferra A., Nicita F., Bertini E. (2020). Microtubule Dysfunction: A Common Feature of Neurodegenerative Diseases. Int. J. Mol. Sci. 21 (19), 7354. doi: 10.3390/ijms21197354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W.-B., Logue J., Yang P., Baracco L., Elahi M., Reece A., et al. (2022). SARS-CoV-2 Invades Cognitive Centers of the Brain and Induces Alzheimer’s-Like Neuropathology. bioRxiv 2022.01.31.478476. doi: 10.1101/2022.01.31.478476 [DOI] [Google Scholar]
- Shlomovitz I., Speir M., Gerlic M. (2019). Flipping the Dogma – Phosphatidylserine in Non-Apoptotic Cell Death. Cell Commun. Signal 17, 139. doi: 10.1186/s12964-019-0437-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuid A. N., Jayusman P. A., Shuid N., Ismail J., Kamal N., Mohamed I. N. (2021). Association Between Viral Infections and Risk of Autistic Disorder: An Overview. Int. J. Environ. Res. Public Health 18, 2817. doi: 10.3390/ijerph18062817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson C., Yamauchi Y. (2020). Microtubules in Influenza Virus Entry and Egress. Viruses 12 (1), 117. doi: 10.3390/v12010117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N., Bharara Singh A. (2020). S2 Subunit of SARS-Ncov-2 Interacts With Tumor Suppressor Protein P53 and BRCA: An in Silico Study. Transl. Oncol. 13 (10), 100814. doi: 10.1016/j.tranon.2020.100814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirakanyan S., Arabyan E., Hakobyan A., Hakobyan T., Chilingaryan G., Sahakyan H., et al. (2021). A New Microtubule-Stabilizing Agent Shows Potent Antiviral Effects Against African Swine Fever Virus With No Cytotoxicity. Emerg. Microbes Infect. 10 (1), 783–796. doi: 10.1080/22221751.2021.1902751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijders T., Aussieker T., Holwerda A., Parise G., van Loon L. J. C., Verdijk L. B. (2020). The Concept of Skeletal Muscle Memory: Evidence From Animal and Human Studies. Acta Physiol. (Oxf) 229 (3), e13465. doi: 10.1111/apha.13465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobhy H. (2021). The Potential Functions of Protein Domains During COVID Infection: An Analysis and a Review. COVID 1, 384–393. doi: 10.3390/covid1010032 [DOI] [Google Scholar]
- Sokolova D., Childs T., Hong S. (2021). Insight Into the Role of Phosphatidylserine in Complement-Mediated Synapse Loss in Alzheimer’s Disease. Fac. Rev. 10, 19. doi: 10.12703/r/10-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solaimanzadeh I. (2020). Nifedipine and Amlodipine Are Associated With Improved Mortality and Decreased Risk for Intubation and Mechanical Ventilation in Elderly Patients Hospitalized for COVID-19. Cureus 12 (5), e8069. doi: 10.7759/cureus.8069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sola M., Magrin C., Pedrioli G., Pinton S., Salvadè A., Papin S., et al. (2020). Tau Affects P53 Function and Cell Fate During the DNA Damage Response. Commun. Biol. 3, 245. doi: 10.1038/s42003-020-0975-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sordillo P. P., Sordillo L. A. (2020). The Mystery of Chemotherapy Brain: Kynurenines, Tubulin and Biophoton Release. Anticancer Res. 40 (3), 1189–1200. doi: 10.21873/anticanres.14061 [DOI] [PubMed] [Google Scholar]
- Spotorno N., Acosta-Cabronero J., Stomrud E., Lampinen B., Strandberg O. T., van Westen D., et al. (2020). Relationship Between Cortical Iron and Tau Aggregation in Alzheimer’s Disease. Brain 143 (5), 1341–1349. doi: 10.1093/brain/awaa089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus M. R., Bidon M. K., Tang T., Jaimes J. A., Whittaker G. R., Daniel S. (2021). Inhibitors of L-Type Calcium Channels Show Therapeutic Potential for Treating SARS-CoV-2 Infections by Preventing Virus Entry and Spread. ACS Infect. Dis. 7 (10), 2807–2815. doi: 10.1021/acsinfecdis.1c00023 [DOI] [PubMed] [Google Scholar]
- Suda M., Shimizu I., Katsuumi G., Yoshida Y., Hayashi Y., Ikegami R., et al. (2021). Senolytic Vaccination Improves Normal and Pathological Age-Related Phenotypes and Increases Lifespan in Progeroid Mice. Nat. Aging. 1, 1117–1126. doi: 10.1038/s43587-021-00151-2 [DOI] [PubMed] [Google Scholar]
- Sultana Z., Maiti K., Dedman L., Smith R. (2018). Is There a Role for Placental Senescence in the Genesis of Obstetric Complications and Fetal Growth Restriction? Am. J. Obstet. Gynecol. 218 (2S), S762–S773. doi: 10.1016/j.ajog.2017.11.567 [DOI] [PubMed] [Google Scholar]
- Takata N., Mishima T., Hisatsune C., Nagai T., Ebisui E., Mikoshiba K., et al. (2011). Astrocyte Calcium Signaling Transforms Cholinergic Modulation to Cortical Plasticity In Vivo . J. Neurosci. 31 (49), 18155–18165. doi: 10.1523/JNEUROSCI.5289-11.2011 Erratum in: J Neurosci. 2012 Aug 29;32(35):12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M., Hu X., Wang Y., Yao X., Zhang W., Yu C., et al. (2021). Ivermectin, a Potential Anticancer Drug Derived From an Antiparasitic Drug. Pharmacol. Res. 163, 105207. doi: 10.1016/j.phrs.2020.105207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan G. W., Kondo T., Imamura K., Suga M., Enami T., Nagahashi A., et al. (2021). Simple Derivation of Skeletal Muscle From Human Pluripotent Stem Cells Using Temperature-Sensitive Sendai Virus Vector. J. Cell Mol. Med. 25 (20), 9586–9596. doi: 10.1111/jcmm.16899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timsit Y., Bennequin D. (2019). Nervous-Like Circuits in the Ribosome Facts, Hypotheses and Perspectives. Int. J. Mol. Sci. 20 (12), 2911. doi: 10.3390/ijms20122911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timsit Y., Grégoire S. P. (2021). Towards the Idea of Molecular Brains. Int. J. Mol. Sci. 22 (21), 11868. doi: 10.3390/ijms222111868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirumuru N., Zhao B. S., Lu W., Lu Z., He C., Wu L. (2016). N(6)-Methyladenosine of HIV-1 RNA Regulates Viral Infection and HIV-1 Gag Protein Expression. Elife 5, e15528. doi: 10.7554/eLife.15528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toque H. A., Kenia P., Nunes K. P, Roja M., Bhatta A., Yao L., et al. (2013). Arginase 1 Mediates Increased Blood Pressure and Contributes to Vascular Endothelial Dysfunction in Deoxycorticosterone Acetate-Salt Hypertension. Front. Immunol. 4, 219. doi: 10.3389/fimmu.2013.00219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozzi P. (2014). Does Fascia Hold Memories? J. Bodyw. Mov. Ther. 18 (2), 259–265. doi: 10.1016/j.jbmt.2013.11.010 [DOI] [PubMed] [Google Scholar]
- Trebak M., Kinet J. P. (2019). Calcium Signalling in T Cells. Nat. Rev. Immunol. 19 (3), 154–169. doi: 10.1038/s41577-018-0110-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo C., Rodriguez-Sanz A. A., Rozas I. (2015). Aromatic Amino Acids-Guanidinium Complexes Through Cation-π Interactions. Molecules 20 (5), 9214–9228. doi: 10.3390/molecules20059214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai R. M., Miller Z., Koestler M., Rojas J. C., Ljubenkov P. A., Rosen H. J., et al. (2020). Reactions to Multiple Ascending Doses of the Microtubule Stabilizer TPI-287 in Patients With Alzheimer Disease, Progressive Supranuclear Palsy, and Corticobasal Syndrome: A Randomized Clinical Trial. JAMA Neurol. 77 (2), 215–224. doi: 10.1001/jamaneurol.2019.3812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tur-Gracia S., Martinez-Quiles N. (2021). Emerging Functions of Cytoskeletal Proteins in Immune Diseases. J. Cell Sci. 134 (3), jcs253534. doi: 10.1242/jcs.253534 [DOI] [PubMed] [Google Scholar]
- Tuszynski J. A., Friesen D., Freedman H., Sbitnev V. I., Kim H., Santelices I., et al. (2020). Microtubules as Sub-Cellular Memristors. Sci. Rep. 10, 2108. doi: 10.1038/s41598-020-58820-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vafadar A., Shabaninejad Z., Movahedpour A., Fallahi F., Taghavipour M., Ghasemi Y., et al. (2020). Quercetin and Cancer: New Insights Into Its Therapeutic Effects on Ovarian Cancer Cells. Cell Biosci. 10, 32. doi: 10.1186/s13578-020-00397-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valansi C., Moi D., Leikina E., Matveev E., Graña M., Chernomordik L. V., et al. (2017). Arabidopsis HAP2/GCS1 Is a Gamete Fusion Protein Homologous to Somatic and Viral Fusogens. J. Cell Biol. 216 (3), 571–581. doi: 10.1083/jcb.201610093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gool W. A., Weinstein H. C., Scheltens P., Walstra G. J., Scheltens P. K. (2001). Effect of Hydroxychloroquine on Progression of Dementia in Early Alzheimer’s Disease: An 18-Month Randomised, Double-Blind, Placebo-Controlled Study. Lancet 358, 455–460. doi: 10.1016/S0140-6736(01)05623-9 [DOI] [PubMed] [Google Scholar]
- van Vliet T., Varela-Eirin M., Wang B., Borghesan M., Brandenburg S. M., Franzin R., et al. (2021). Physiological Hypoxia Restrains the Senescence-Associated Secretory Phenotype via AMPK-Mediated mTOR Suppression. Mol. Cell. 81 (9), 2041–2052.e6. doi: 10.1016/j.molcel.2021.03.018 [DOI] [PubMed] [Google Scholar]
- Varidaki A., Hong Y., Coffey E. T. (2018). Repositioning Microtubule Stabilizing Drugs for Brain Disorders. Front. Cell Neurosci. 12, 226. doi: 10.3389/fncel.2018.00226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega I. E., Traverso E. E., Ferrer-Acosta Y., Matos E., Colon M., Gonzalez J., et al. (2008). A Novel Calcium-Binding Protein Is Associated With Tau Proteins in Tauopathy. J. Neurochem. 106 (1), 96–106. doi: 10.1111/j.1471-4159.2008.05339.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viel T., Chinta S., Rane A., Chamoli M., Buck H., Andersen J. (2020). Microdose Lithium Reduces Cellular Senescence in Human Astrocytes - a Potential Pharmacotherapy for COVID-19? Aging (Albany NY) 12 (11), 10035–10040. doi: 10.18632/aging.103449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel D., Dussutour A. (2016). Direct Transfer of Learned Behaviour via Cell Fusion in Non-Neural Organisms. Proc. Biol. Sci. 283 (1845), 20162382. doi: 10.1098/rspb.2016.2382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallet C., De Rovere M., Van Assche J., Daouad F., De Wit S., Gautier V., et al. (2019). Microglial Cells: The Main HIV-1 Reservoir in the Brain. Front. Cell. Infect. Microbiol. 9, 362. doi: 10.3389/fcimb.2019.00362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walrant A., Cardon S., Burlina F., Sagan S. (2017). Membrane Crossing and Membranotropic Activity of Cell-Penetrating Peptides: Dangerous Liaisons? Acc Chem. Res. 50 (12), 2968–2975. doi: 10.1021/acs.accounts.7b00455 [DOI] [PubMed] [Google Scholar]
- Wang C., Zhang M., Garcia G., Jr, Tian E., Cui Q., Chen X., et al. (2021). ApoE-Isoform-Dependent SARS-CoV-2 Neurotropism and Cellular Response. Cell Stem Cell. 28 (2), 331–342.e5. doi: 10.1016/j.stem.2020.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Ye Y. (2021). Filamentous Recombinant Human Tau Activates Primary Astrocytes via an Integrin Receptor Complex. Nat. Commun. 12, 95. doi: 10.1038/s41467-020-20322-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., Yu R., Zhu C., Lin H. Y., Lu X., Wang H. (2019). Tubulin Detyrosination Promotes Human Trophoblast Syncytium Formation. J. Mol. Cell Biol. 11 (11), 967–978. doi: 10.1093/jmcb/mjz084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Jiang Y., Liu Y., Liu Q., Sun H., Mei M., et al. (2022). Ferroptosis Promotes Microtubule-Associated Protein Tau Aggregation via GSK-3β Activation and Proteasome Inhibition. Mol. Neurobiol. 59, 1486–1501. doi: 10.1007/s12035-022-02731-8 [DOI] [PubMed] [Google Scholar]
- Weiss A., Touret F., Baronti C., Gilles M., Hoen B., Nougairède A., et al. (2021). Niclosamide Shows Strong Antiviral Activity in a Human Airway Model of SARS-CoV-2 Infection and a Conserved Potency Against the Alpha (B.1.1.7), Beta (B.1.351) and Delta Variant (B.1.617.2). PloS One 16 (12), e0260958. doi: 10.1371/journal.pone.0260958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wender P. A., Galliher W. C., Goun E. A., Jones L. R., Pillow T. H. (2008). The Design of Guanidinium-Rich Transporters and Their Internalization Mechanisms. Adv. Drug Delivery Rev. 60 (4-5), 452–472. doi: 10.1016/j.addr.2007.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Z., Zhang Y., Lin Z., Shi K., Jiu Y. (2020). Cytoskeleton-A Crucial Key in Host Cell for Coronavirus Infection. J. Mol. Cell Biol. 12 (12), 968–979. doi: 10.1093/jmcb/mjaa042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzhong L., Hualan L. (2021). COVID-19: The CaMKII-Like System of S Protein Drives Membrane Fusion and Induces Syncytial Multinucleated Giant Cells. Immunol. Res. 69 (6), 496–519. doi: 10.1007/s12026-021-09224-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexselblatt E., Esko J. D., Tor Y. (2014). On Guanidinium and Cellular Uptake. J. Org. Chem. 79 (15), 6766–6774. doi: 10.1021/jo501101s [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock J. M., Chernomordik L. V. (2021). Flagging Fusion: Phosphatidylserine Signaling in Cell-Cell Fusion. J. Biol. Chem. 296, 100411. doi: 10.1016/j.jbc.2021.100411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicher S. A., Roos B. B., Teske J. J., Fang Y. H., Pabelick C., Prakash Y. S. (2021). Aging Increases Senescence, Calcium Signaling, and Extracellular Matrix Deposition in Human Airway Smooth Muscle. PloS One 16 (7), e0254710. doi: 10.1371/journal.pone.0254710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods N. K., Padmanabhan J. (2012). Neuronal Calcium Signaling and Alzheimer’s Disease. Adv. Exp. Med. Biol. 740, 1193–1217. doi: 10.1007/978-94-007-2888-2_54 [DOI] [PubMed] [Google Scholar]
- Wylie A., Jones A. E., Abrams J. M. (2016). P53 in the Game of Transposons. Bioessays 38 (11), 1111–1116. doi: 10.1002/bies.201600115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing L., Yang T., Cui S., Chen G. (2019). Connexin Hemichannels in Astrocytes: Role in CNS Disorders. Front. Mol. Neurosci. 12, 23. doi: 10.3389/fnmol.2019.00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Shi P. Y., Li H., Zhou J. (2020). Broad Spectrum Antiviral Agent Niclosamide and Its Therapeutic Potential. ACS Infect. Dis. 6 (5), 909–915. doi: 10.1021/acsinfecdis.0c00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanamandra K., Patel T. K., Jiang H., Schindler S., Ulrich J. D., Boxer A. L., et al. (2017). Anti-Tau Antibody Administration Increases Plasma Tau in Transgenic Mice and Patients With Tauopathy. Sci. Transl. Med. 9 (386), eaal2029. doi: 10.1126/scitranslmed.aal2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yapici-Eser H., Koroglu Y. E., Oztop-Cakmak O., Keskin O., Gursoy A., Gursoy-Ozdemir Y. (2021). Neuropsychiatric Symptoms of COVID-19 Explained by SARS-CoV-2 Proteins’ Mimicry of Human Protein Interactions. Front. Hum. Neurosci. 15, 656313. doi: 10.3389/fnhum.2021.656313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y., Liu X. Z., He X., Zhou L. Q. (2021). Exogenous Coronavirus Interacts With Endogenous Retrotransposon in Human Cells. Front. Cell. Infect. Microbiol. 11, 609160. doi: 10.3389/fcimb.2021.609160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Nakagami H., Hayashi H., Ikeda Y., Sun J., Tenma A., et al. (2020). The CD153 Vaccine Is a Senotherapeutic Option for Preventing the Accumulation of Senescent T Cells in Mice. Nat. Commun. 11 (1), 2482. doi: 10.1038/s41467-020-16347-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younan P., Iampietro M., Santos R. I., Ramanathan P., Popov V. L., Bukreyev A. (2018). Role of Transmembrane Protein 16F in the Incorporation of Phosphatidylserine Into Budding Ebola Virus Virions [Published Correction Appears in J Infect Dis. 2019 Jan 29;219(4):677]. J. Infect. Dis. 218 (suppl_5), S335–S345. doi: 10.1093/infdis/jiy485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zauli G., Tisato V., Secchiero P. (2020). Rationale for Considering Oral Idasanutlin as a Therapeutic Option for COVID-19 Patients. Front. Pharmacol. 11, 1156. doi: 10.3389/fphar.2020.01156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zempel H., Thies E., Mandelkow E., Mandelkow E. M. (2010). Abeta Oligomers Cause Localized Ca(2+) Elevation, Missorting of Endogenous Tau Into Dendrites, Tau Phosphorylation, and Destruction of Microtubules and Spines. J. Neurosci. 30 (36), 11938–11950. doi: 10.1523/JNEUROSCI.2357-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Yao Y., Cornec A. S., Oukoloff K., James M. J., Koivula P., et al. (2018). A Brain-Penetrant Triazolopyrimidine Enhances Microtubule-Stability, Reduces Axonal Dysfunction and Decreases Tau Pathology in a Mouse Tauopathy Model. Mol. Neurodegener. 13 (1), 59. doi: 10.1186/s13024-018-0291-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Velmeshev D., Hashimoto K., Huang Y. H., Hofmann J. W., Shi X., et al. (2020). Neurotoxic Microglia Promote TDP-43 Proteinopathy in Progranulin Deficiency. Nature 588, 459–465. doi: 10.1038/s41586-020-2709-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Richards A., Khalil A., Wogram E., Ma H., Young R. A., et al. (2020). SARS-CoV-2 RNA Reverse-Transcribed and Integrated Into the Human Genome. bioRxiv 13, 2020.12.12.422516. doi: 10.1101/2020.12.12.422516 [DOI] [Google Scholar]
- Zhang W., Chuang Y. A., Na Y., Ye Z., Yang L., Lin R., et al. (2019). Arc Oligomerization Is Regulated by CaMKII Phosphorylation of the GAG Domain: An Essential Mechanism for Plasticity and Memory Formation. Mol. Cell. 75 (1), 13–25.e5. doi: 10.1016/j.molcel.2019.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Hao H., Ma L., Zhang Y., Hu X., Chen Z., et al. (2021). Methyltransferase-Like 3 Modulates Severe Acute Respiratory Syndrome Coronavirus-2 RNA N6-Methyladenosine Modification and Replication. mBio 12 (4), e0106721. doi: 10.1128/mBio.01067-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Le T., Grabau R., Mohseni Z., Kim H., Natale D. R., et al. (2020). TMEM16F Phospholipid Scramblase Mediates Trophoblast Fusion and Placental Development. Sci. Adv. 6 (19), eaba0310. doi: 10.1126/sciadv.aba0310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Li H., Li X., Wu J., Xue T., Wu J., et al. (2020). TMEM16F Aggravates Neuronal Loss by Mediating Microglial Phagocytosis of Neurons in a Rat Experimental Cerebral Ischemia and Reperfusion Model. Front. Immunol. 11, 1144. doi: 10.3389/fimmu.2020.01144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Li S., Gaur U., Zheng W. (2020). Artemisinin Improved Neuronal Functions in Alzheimer’s Disease Animal Model 3xtg Mice and Neuronal Cells via Stimulating the ERK/CREB Signaling Pathway. Aging Dis. 11 (4), 801–819. doi: 10.14336/AD.2019.0813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Zhou L. L., Su Y., Sun Q. (2021). Cell Fusion in the Pathogenesis of COVID-19. Military Med. Res. 8, 68. doi: 10.1186/s40779-021-00348-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C., Ramaswamy S., Johnson D. E, Vitturi D. A., Schopfer F. J., Freeman B. A., et al. (2016). Novel Roles for Peroxynitrite in Angiotensin II and CaMKII Signaling. Sci. Rep. 6, 23416. doi: 10.1038/srep23416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X. W., Li J., Brost B. C., Xia X. Y., Chen H. B., Wang C. X., et al. (2014). Decreased Expression and Altered Methylation of Syncytin-1 Gene in Human Placentas Associated With Preeclampsia. Curr. Pharm. Des. 20 (11), 1796–1802. doi: 10.2174/13816128113199990541 [DOI] [PubMed] [Google Scholar]
- Zöphel D., Hof C., Lis A. (2020). Altered Ca2+ Homeostasis in Immune Cells During Aging: Role of Ion Channels. Int. J. Mol. Sci. 22 (1), 110. doi: 10.3390/ijms22010110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zündorf G., Reiser G. (2011). Calcium Dysregulation and Homeostasis of Neural Calcium in the Molecular Mechanisms of Neurodegenerative Diseases Provide Multiple Targets for Neuroprotection. Antioxid. Redox Signal 14 (7), 1275–1288. doi: 10.1089/ars.2010.3359 [DOI] [PMC free article] [PubMed] [Google Scholar]