Abstract
Blacks are at higher risk of developing cognitive impairment with age than non-Hispanic Whites, yet most brain morphometry and cognition research is performed with White samples or with mixed samples that control for race or compare across racial groups. A deeper understanding of the within-group variability in associations between brain structure and cognitive decline in Blacks is critically important for designing appropriate outcomes for clinical trials, predicting adverse outcomes, and developing interventions to preserve cognitive function, but no studies have examined these associations longitudinally within Blacks. We performed deformation-based morphometry (DBM) in 376 older Black participants without dementia and examined associations of DBM with cognitive level and decline for global cognition and five cognitive domains. After correcting for widespread age-associated effects, there remained regions with less tissue and more cerebrospinal fluid (CSF) associated with level and rate of decline in global cognition, memory, and perceptual speed. Further study is needed to examine the moderators of these associations, identify adverse outcomes predicted by brain morphometry, and deepen knowledge of underlying biological mechanisms.
Keywords: Aging, cognition, deformation-based morphometry, neuroimaging, African-American, Black race
1. Introduction
Blacks have a higher prevalence (e.g., Gurland et al., 1999; Mehta and Yeo, 2017) and incidence (e.g., Mayeda et al., 2016; Tang et al., 2001; Weuve et al., 2018) of dementia than non-Hispanic Whites, yet little is known about the link between brain structure and cognitive decline in Blacks. A deeper understanding of within-group variability in associations between brain structure and cognitive decline is critically important for the design of appropriate clinical trial outcomes and interventions to effectively preserve cognitive function in Blacks, but most structural brain imaging-cognition studies are performed with White or mostly White samples (e.g., DeCarli et al., 2005; Fleischman et al., 2013; Raz et al., 1998), or with mixed-race samples (e.g., Aggarwal et al., 2010; Brickman et al., 2008; Brickman et al., 2012; Carmichael et al., 2012; DeCarli et al., 2008; Fletcher et al., 2017; Gavett et al., 2018; Liu et al., 2015; McDonough, 2017; Mungas et al., 2009; Zahodne, Manly et al., 2015; Zahodne, Wall et al., 2015). While race comparison studies are critical to understanding racial disparities in dementia, they often assume that the same processes are being measured across racial groups even though sociocultural and health experiences are quite different (Barnes and Bennett, 2014; Carmichael and Newton, 2019; McDonough, 2017, Whitfield et al., 2008). In order to design interventions that will ultimately reduce racial disparities in dementia, it is important to acknowledge that these differences do exist across race and can influence important outcomes, and, as a complementary approach to mixed-race studies, also examine associations within race. To date, however, there have been only few studies of structural brain imaging-cognition associations reported within Blacks, and all examine cross-sectional associations of atrophy and cognition in samples with medical conditions such as diabetes (Hughes et al., 2018; Hsu et al., 2018; Whitlow et al., 2015), hypertension (Waldron-Perrine et al., 2018), depressive symptoms (Meyer et al., 2019) and Alzheimer’s dementia (AD; Meier et al., 2012; Sencakova et al., 2001).
What is critically needed to move forward in the identification of brain morphometric indices that can signal risk of cognitive decline in Blacks is a longitudinal study of the associations between brain structure and repeated measures of cognition that can separate level and change, using a comprehensive battery of tests that measure cognitive ability globally and in multiple cognitive domains, within a large sample of older Black individuals without dementia. To accomplish this goal, we used deformation-based morphometry (DBM; Ashburner et al., 1998; Gaser et al., 2001; Hua et al., 2008; Sarro et al., 2016) to map brain structural characteristics associated with age and cognition at first MRI and rate of subsequent cognitive decline. Given the dearth of brain imaging studies published within Blacks, we chose to start with the hypothesis-free, voxel-based DBM approach to examine fundamental brain structure-cognition associations. A total of 376 older Blacks without dementia, recruited from three large epidemiological studies of aging and dementia, participated in the study.
2. Materials and Methods
2.1. Participants
The sample comprised persons who signed written consent to participate in one of three epidemiological cohorts studies of aging and dementia: Minority Aging Research Study (MARS, Barnes et al., 2012), Rush Alzheimer’s Disease Center African American Clinical Core (RADC AA Core, Barnes et al., 2012), and the Rush Memory and Aging Project (MAP, Bennett et al., 2018). 3 Tesla neuroimaging was added to these parent studies in 2012, 2012, and 2009 respectively. All Rush Alzheimer’s Disease Center (RADC) parent cohort studies use the same recruitment strategy and the same team of recruiters (as well as trainers, testers and other study staff). The strategy involves recruitment from community-based settings including churches, senior housing facilities, retirement communities, social groups that cater to older Blacks, and social service centers following a presentation by study staff. In the presentation, all details of the study are described, including the annual cognitive and neurological evaluations and blood draw, and written information is provided so that participants can discuss the project with family members. Potential participants are asked to complete a form describing their level of interest in the study, and later, those who expressed interest are contacted to arrange a time to discuss the study in more detail and review and sign the consent forms. Once enrolled and tested, participants are introduced to other sub-studies, including neuroimaging, and if interested, are given the opportunity to schedule a brain scan. All studies were approved by an Institutional Review Board of Rush University Medical Center and were performed in accordance with the ethical standards set forth in the 1964 Declaration of Helsinki and its later amendments.
Eligibility for the sample required the participant to 1) self-identify as Black, 2) have successfully completed at least one MRI acquisition yielding data that passed quality-control, 3) have completed cognitive assessment close to the first such MRI session with at least one subsequent follow-up assessment, and 4) have no history of dementia at the first MRI session. At the time of analysis, 1262 Black participants of the parent projects had completed first cognitive testing, with 1134 alive and active in the studies since the start of 3T MRI imaging. Of these, 458 were ineligible (specific exclusions or no consent), and 407 were scanned. Scans for 14 participants failed QC and, because we process scans in batches for efficiency, processing for 18 more scans was pending. This resulted in 386 scans with approved DBM data. Ten participants were excluded with a diagnosis of dementia based on standard clinical evaluation and NINCDS/ADRDA diagnostic criteria (McKhann et al., 1984), leaving a final sample of 376 participants (MARS=232, RADC AA Core=120, MAP=24). There were a total of 1612 assessments across the 376 participants (an average of about 4.3 assessments per participant) with a mean interval between assessments of 13.6 months (SD 4.4).
2.2. Clinical and Cognitive Evaluation
Each participant underwent annual cognitive assessment on a battery of 18 individual tests that has been shown to be race- and time-invariant (Barnes et al., 2016). Individual tests were used to form a composite score of global cognition as well as composite scores of five different cognitive domains. These five cognitive domains were originally formed based on conceptual areas of interest supported by principal-components factor analysis (Bennett et al., 2018; Wilson et al., 2002). Episodic memory was measured using seven tests: CERAD Word List Memory, Recall, and Recognition, immediate and delayed recall of the East Boston Story and Story A from Logical Memory. Semantic memory was measured using two tests: 15-item version of the Boston Naming Test and Verbal Fluency which involves naming animals and fruits/vegetables in 1-minute epochs. Working memory was measured using three tests: Digit Span Forward, Digit Span Backward and Digit Ordering. Perceptual speed was measured with four tests: the oral version of the Symbol Digit Modalities Test, Stroop Reading and Stroop Interference Tests, and Number Comparison. Visuospatial ability was assessed with two tests: 15-item version of Judgment of Line Orientation and 17-item version of Standard Progressive Matrices. To form composites, raw scores on individual tests were converted to z scores by subtracting the mean and dividing by the standard deviation for all participants in the parent studies at enrollment, and then the average z score among tests in a given domain was computed. A global cognitive score was formed by averaging z scores of all tests.
2.3. Neuroimaging
2.3.1. Image acquisition
T1-weighted data were collected on a 3 Tesla (T) Philips MRI scanner (for 279 persons) and a 3T Siemens scanner (for 97 persons) using a 3D magnetization prepared rapid acquisition gradient echo (MPRAGE) sequence. The parameters on the 3T Philips scanner were TR=8 ms, TE=3.7 ms, TI=955 ms, flip-angle=8°, field of view=240 mm x 228 mm, 181 slices, acquired voxel size=1×1×1 mm3, and an acceleration factor of 2. The parameters on the 3T Siemens scanner were TR=2300 ms, TE=2.98 ms, TI=900 ms, flip-angle=9°, field of view=256 mm x 256 mm, 176 slices, acquired voxel size=1×1×1 mm3, and an acceleration factor of 2.
2.3.2. Pre-processing
Each participant’s T1-weighted images were corrected for bias field inhomogeneity using the N4 algorithm (Tustison et al., 2010) and were skull-stripped using HD-BET (Isensee et al., 2019) The skull-stripped images were non-linearly registered to the T1-weighted template of the Multi-channel Illinois Institute of Technology and Rush university Aging (MIITRA) atlas (Ridwan et al., 2021) using ANTs (Avants et al., 2008). The MIITRA atlas is an older adult atlas based on 222 community dwelling non- demented older adults (65–95 years of age, male: female = 1:1), and the T1-weighted template of the MIITRA atlas was selected as a reference in the present study because when compared to 19 other popular templates it was shown to (a) provide higher inter- subject spatial normalization accuracy for older adult data, (b) allowed detection of smaller inter- group morphometric differences compared to other standardized templates, (c) had similar performance to study- specific templates, and (d) was highly representative of the older adult brain (Ridwan et al., 2021). The determinant of the Jacobian of the transformation was calculated in each voxel, and the resulting images were smoothed by a Gaussian filter with 4mm FWHM. These maps were used for the DBM analyses.
2.4. Statistical Approach
2.4.1. Cognitive Analyses
Cognitive data at first MRI for global cognition and five cognitive domains were used in the voxel-based DBM models. The time between baseline cognitive evaluation to MRI was 7.3 months (SD 4.3). For the analysis of cognitive change in voxel-based models, person-specific slopes were generated using linear-mixed effects models programmed in SAS 9.4 for Linux. Fixed effects were years from image, covariates and their interactions with time. Random effects for level and slope were included.
2.4.2. DBM Analyses
Voxel-wise linear regression was used to test the association between the deformations shown in the smoothed maps of the Jacobian determinant of each participant with global cognition, controlling for age, sex, years of education, and scanner. The same analysis was repeated for each of the five cognitive domains. The analysis was conducted using FSL PALM (Winkler et al., 2015), assuming different variances across scanners and using two exchangeability blocks (one per scanner; i.e. permutations occurred only between participants imaged on the same scanner). Statistical inference was based on 500 permutations of the data, and tail approximation was used to accelerate the analysis (Winkler et al., 2016). Associations were considered significant at p<0.05, Family Wise Error (FWE) corrected. The Threshold-Free Cluster Enhancement (TFCE) method was used to define clusters of significance. The regression models were then repeated controlling for demographics, scanner, and, in separate models, self-report of three common health risk factors in Blacks (hypertension, diabetes, smoking history).
3. Results
3.1. Descriptives
Participant demographics and cognitive scores at first MRI are shown in Table 1. Of the 376 persons in the analytic sample, 82% were female, with a mean age at the time of scan of 76 years (SD = 6 years, range 62–93 years) and a mean education of 15 years (SD = 3 years). On a combined measure of vascular risk factors (hypertension, diabetes, smoking history), 37%, 38% and 11% percent of the sample reported one, two or three risk factors, respectively. Diabetes was reported by 28% of the sample and hypertension was reported by 79% of the sample. Means for cognitive standard scores ranged from −0.148 (visuospatial ability) to 0.291 (episodic memory). A score of 0.123 on global cognition means that this group of persons demonstrated slightly better performance overall on the cognitive tests than the combined parent studies.
Table 1.
Descriptive statistics for demographic characteristics and cognitive domains at first MRI (N = 376)
| Measure | Mean | SD |
|---|---|---|
| Age (years) | 75.94 | 6.32 |
| Education (years) | 15.25 | 3.22 |
| # Female (%) | 309/376 (82.2%) | |
| Global Cognition | 0.123 | 0.527 |
| Episodic Memory | 0.291 | 0.631 |
| Semantic Memory | 0.040 | 0.752 |
| Working Memory | −0.012 | 0.760 |
| Perceptual Speed | 0.139 | 0.861 |
| Visuospatial Ability | −0.148 | 0.769 |
3.2. DBM, Age, and Cognitive Level
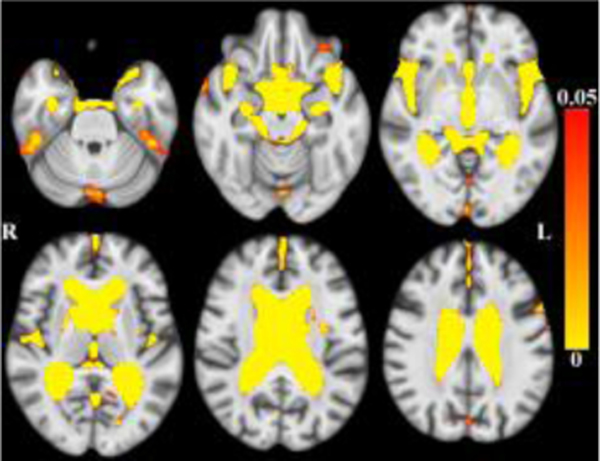
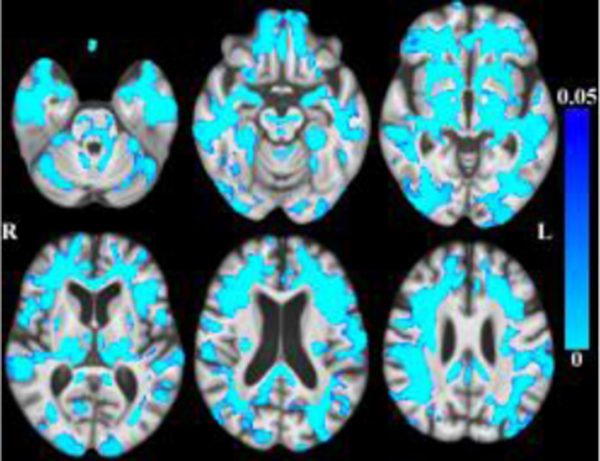
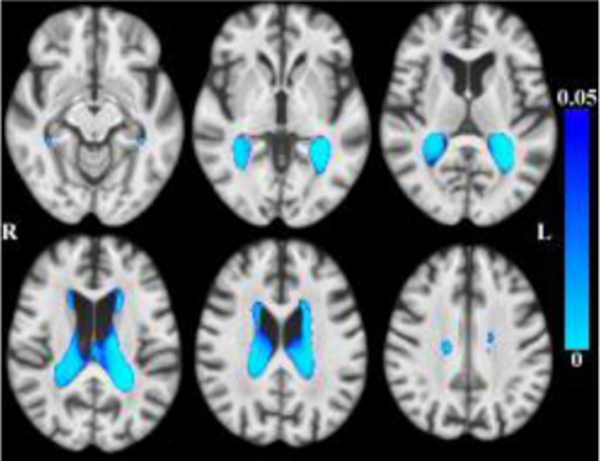
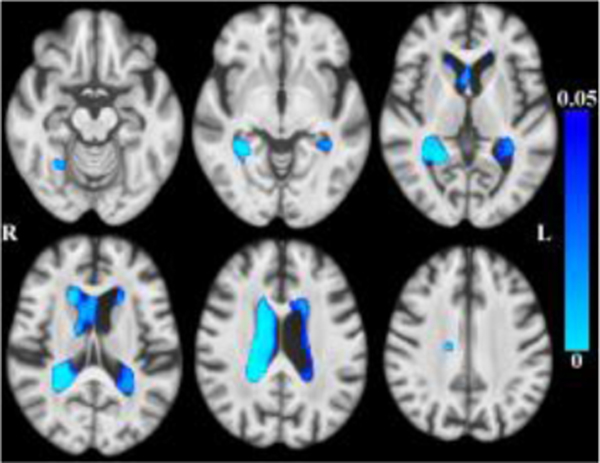
Globally, there was less tissue and more CSF associated with older age within the age range of this sample. (Figures 1–2 here).
Figure 1.
Maps of p-values (warm color scale) showing more CSF associated with age (p<0.05) overlaid on the MIITRA T1-weighted template (grayscale).
Figure 2.
Maps of p-values (cool color scale) showing less tissue associated with age (p<0.05) overlaid on the MIITRA T1-weighted template (grayscale).
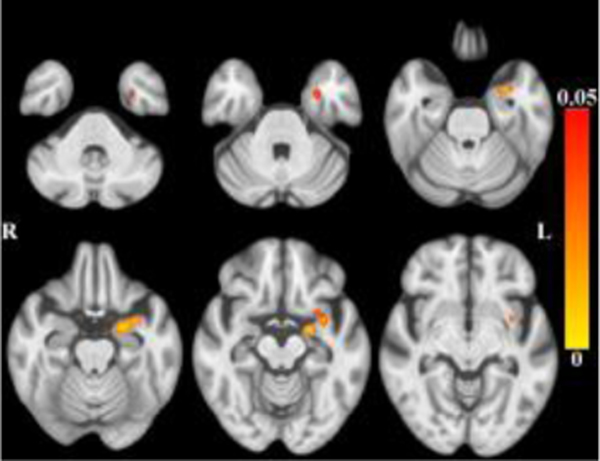
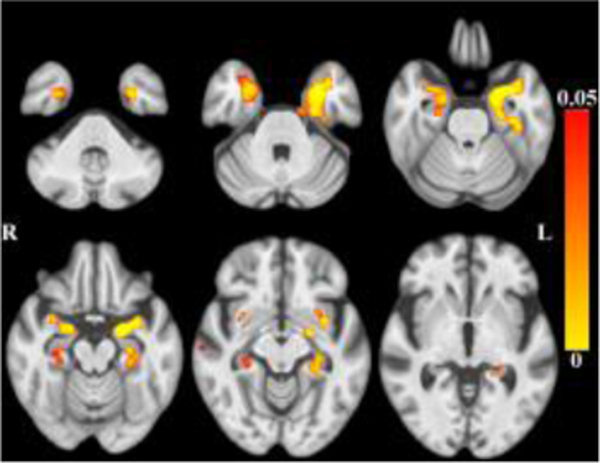
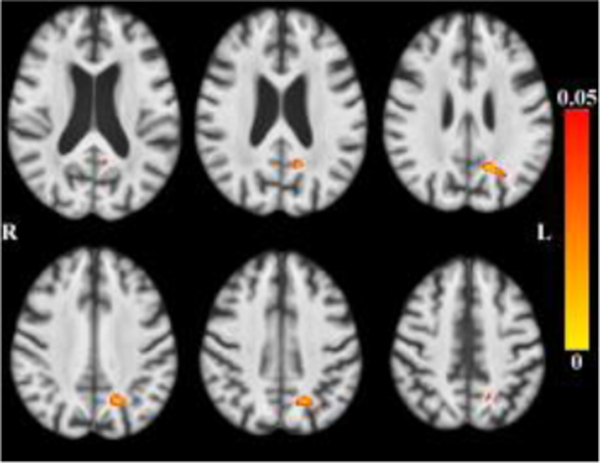
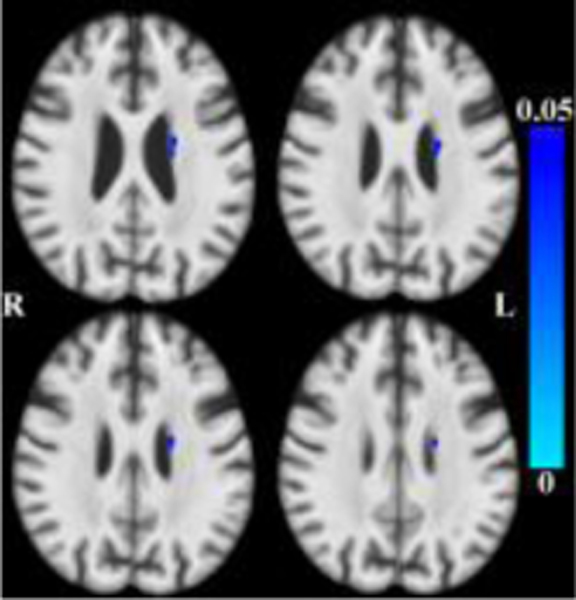
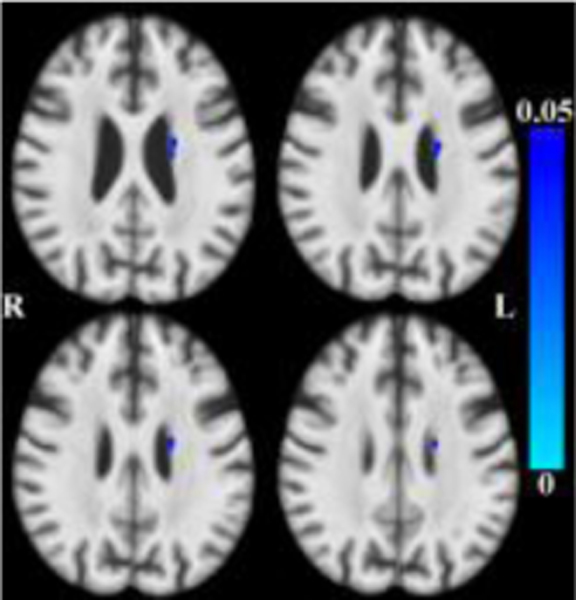
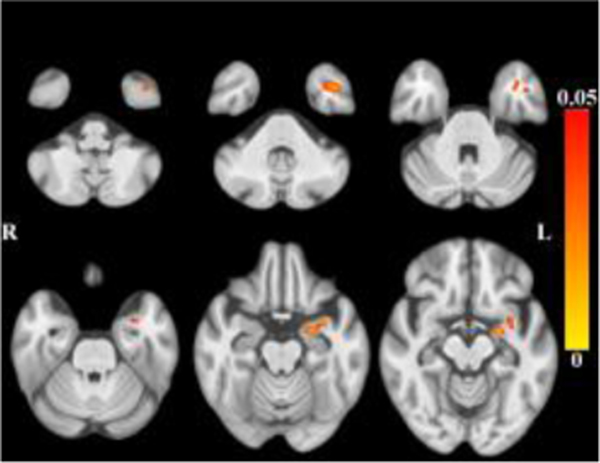
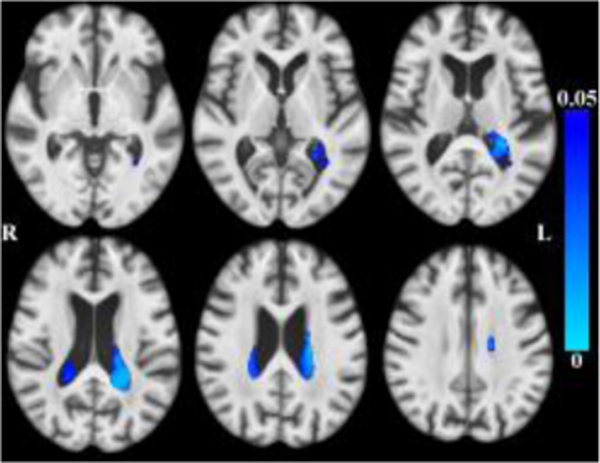
After accounting for the effects of age (and other covariates as described), less tissue in left-hemisphere entorhinal cortex, amygdala, and insular white matter (Figure 3) was associated with lower global cognition. Bilaterally, less tissue in hippocampi, entorhinal cortices, amygdala and insular white matter was associated with lower episodic memory (Figure 4). Less tissue in left-hemisphere posterior cingulate, precuneus and superior parietal white matter (Figure 5), and more CSF (Figure 6), were associated with slower perceptual speed (Figures 3–6 here). There were no associations of brain structure with level of semantic memory, working memory or visuospatial ability.
Figure 3.
Maps of p-values (warm color scale) showing less tissue associated with lower global cognitive level (p<0.05) overlaid on the MIITRA T1-weighted template (grayscale).
Figure 4.
Maps of p-values (warm color scale) showing less tissue associated with lower episodic memory level (p<0.05) overlaid on the MIITRA T1-weighted template (grayscale).
Figure 5.
Maps of p-values (warm color scale) showing less tissue associated with lower perceptual speed level (p<0.05) overlaid on the MIITRA T1-weighted template (grayscale).
Figure 6.
Maps of p-values (cool color scale) showing more CSF associated with lower perceptual speed level (p<0.05) overlaid on the MIITRA T1-weighted template (grayscale).
3.3. DBM and Cognitive Decline
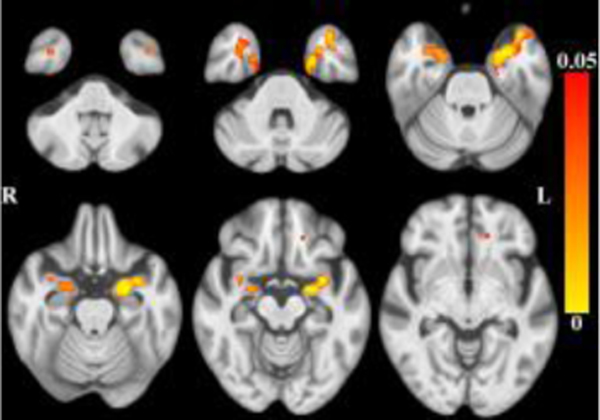
The results for longitudinal mixed-effects models are shown in Table 2. Decline occurred in global cognition and in three of the five cognitive domains: episodic memory, semantic memory, and perceptual speed. There was less tissue and more CSF associated with decline in each of these three domains after controlling for the effects of age (and other covariates as described). There was less tissue bilaterally in the amygdala, the white matter of the anterior cingulate, superior frontal, rostral middle frontal regions, and the splenium (Figure 7), and more CSF bilaterally (Figure 8) associated with faster global cognitive decline. Less tissue in left-hemisphere amygdala, parahippocampal gray and white matter, and insular white matter (Figure 9), was associated with faster decline in episodic memory. CSF was not associated with decline in episodic memory. Less tissue bilaterally in entorhinal cortices, parahippocampal gray and white matter, and anterior cingulate/orbitofrontal white matter (Figure 10), and more CSF (Figure 11), were associated with faster decline in semantic memory. More CSF in the left-hemisphere was associated with faster decline in perceptual speed (Figure 12), but there were no associated tissue differences. (Place Figures 7–12 here). To test the robustness of these results, we repeated the longitudinal mixed models excluding baseline data. The correlation between change scores from models with and without baseline data was .998.
Table 2.
Estimates of level and rate of change by cognitive domain
| Global Cognition |
Episodic Memory |
Semantic Memory |
Working Memory |
Perceptual Speed |
Visuospatial Ability |
|
|---|---|---|---|---|---|---|
| Level SE p-value |
0.146 0.028 <0.0001 |
0.317 0.037 <0.0001 |
0.076 0.038 0.0464 |
−0.044 0.040 0.2830 |
0.163 0.049 <0.0001 |
−0.107 0.040 0.0082 |
| Rate of change SE p-value |
−0.017 0.006 0.0072 |
−0.020 0.007 0.0031 |
−0.047 0.008 <0.0001 |
0.019 0.008 0.0199 |
−0.054 0.008 <0.0001 |
0.007 0.0007 0.3452 |
Level is mean at first MRI. Rate of change is units/year for reference participant.
Figure 7.
Maps of p-values (warm color scale) showing less tissue associated with faster global cognitive decline (p<0.05) overlaid on the MIITRA T1-weighted template (grayscale).
Figure 8.
Maps of p-values (cool color scale) showing more CSF associated with faster global cognitive decline (p<0.05) overlaid on the MIITRA T1-weighted template (grayscale).
Figure 9.
Maps of p-values (warm color scale) showing less tissue associated with faster episodic memory decline (p<0.05) overlaid on the MIITRA T1-weighted template (grayscale).
Figure 10.
Maps of p-values (warm color scale) showing less tissue associated with faster semantic memory decline (p<0.05) overlaid on the MIITRA T1-weighted template (grayscale).
Figure 11.
Maps of p-values (cool color scale) showing more CSF associated with faster semantic memory decline (p<0.05) overlaid on the MIITRA T1-weighted template (grayscale).
Figure 12.
Maps of p-values (cool color scale) showing more CSF associated with faster perceptual speed decline (p<0.05) overlaid on the MIITRA T1-weighted template (grayscale).
3.4. DBM and Health Risk Factors
When adjusting the DBM models for vascular risk factors using the combined measure (along with other covariates), there was less tissue in two very small regions of anterior corpus callosum and more CSF near the left hippocampus (data not shown). Neither of these small regions overlapped with regions shown to be associated with cognition or cognitive decline in the previous models. No differences in tissue or CSF were associated with presence of hypertension or diabetes when examined in separate models.
4. Discussion
The results of this study that used deformation-based morphometry to examine associations between brain structure and cognition in a large sample of older Blacks without dementia showed less tissue and more CSF related to age, but when models were corrected for age, there remained a number of regions with less tissue and more CSF that were associated with cognitive level and with rate of cognitive decline globally, in memory (episodic and semantic), and in perceptual speed.
4.1. Global cognition
Less tissue in temporal lobe regions (entorhinal cortex, amygdala, insular white matter) was associated with both lower level of global cognition and faster global cognitive decline. This finding is consistent with the baseline brain volume-cognitive slope results of the tensor-based morphometry studies reported by Fletcher et al. (2018) and Gavett et al. (2018) using diverse samples. Fletcher et al. (2018) also examined simultaneous brain volume change-cognitive slope associations and found that temporal lobe volume change accounted for incrementally more of the variance in global cognitive slope than global gray volume change. Gavett et al. (2018) also found this incremental power for temporal lobe volume change to predict global cognitive slope, but the finding was only observed in Whites. The current study did not examine change in brain volume with change in cognition and this finding of incremental temporal regional change predicting cognitive change warrants further study within a large sample of older Blacks without dementia.
Integrity of white matter is critical for maintaining cognition in aging (e.g., Boyle et al 2016; Tosto et al., 2014) and, in this study, less tissue in anterior cingulate/superior frontal/rostral midfrontal white matter in the frontal lobe and the splenium of the corpus callosum was additionally associated with faster global cognitive decline. Much of what is known about the relationship between white matter integrity and cognition in aging and dementia (Filley and Fields, 2016), particularly in Black (Meier et al., 2015) or diverse samples (Brickman et al., 2008; Brickman et al., 2012; Carmichael et al., 2012; DeCarli et al., 2008; Gavett et al., 2018; Liu et al., 2015; Stavitsky et al., 2010; Zahodne, Manly et al., 2015), is based on the measurement of white matter hyperintensity (WMH) volume. White matter hyperintensities are thought to reflect varied instantiations of small vessel disease (Wardlaw, Valdes-Hernandez and Munoz-Maniega, 2015) and WMH volume is known to increase with age (e.g., Brickman et al., 2008; Morris et al., 2009) and be greater in Blacks than Whites (e.g., Brickman, et al., 2008; Zahodne, Manly et al., 2015, but see Liu et al., 2015), particularly in the face of cardiovascular disease (Nyquist et al., 2014), hypertension (e.g., Gottesman, 2010; Waldron-Perrine et al., 2018) and diabetes (e.g., Hughes et al., Hsu et al., 2018; Whitlow et al., 2015). White matter volume, as measured in the current study, and WMH volume are macrostructural measures of white matter integrity that have been shown to be inversely related in studies of aging and AD (Capizzano et al., 2004; Schmidt et al., 2005; Vangberg, Eikenes and Haberg, 2019). The results of this study showed that regional frontal-lobe white matter volume is associated with cognitive decline within older Blacks without dementia, supporting and extending the cross-sectional findings of Meier et al., 2012, who found associations between regional frontal-lobe WMH burden, memory, MCI and AD within older Blacks.
It is interesting that less tissue in the splenium was associated with faster global cognitive decline in this study. This association is plausible given the findings of less tissue in the temporal-lobe regions whose fibers pass through the caudal corpus callosum and less tissue in the frontal-lobe regions whose fibers pass through the rostral corpus callosum (Park et al., 2008). Decreasing fractional anisotropy, a measure of white matter microstructural integrity, in the splenium has been associated with progression and severity of Alzheimer’s dementia in diffusion-tensor imaging studies (e.g., Acosta-Cabronero et al., 2012), and tumors of the splenium have been shown to cause marked impairments in memory and visual perception (Rudge and Warringon, 1991). Thus, integrity of this region warrants further study as a candidate for predicting global cognitive decline within older Blacks.
4.2. Memory
Episodic and semantic memory are long-term, declarative memory processes known to rely heavily on the integrity of temporal lobe regions (e.g., Fleischman et al., 2005; Squire and Dede, 2015). Episodic memory, or memory for recent events that occur within a specific spatiotemporal context (Fleischman and Gabrieli, 1999; Tulving, 1983), is known to depend on the integrity of mesial-temporal structures, particularly the hippocampus (Fleischman, Leurgans et al., 2013; Fleischman, Yu et al., 2013; Golomb et al., 1993; Hackert et al., 2002; Tulving and Markowitsch, 1998). In this study, less gray matter tissue in the temporal lobe, including the hippocampus, was associated with lower episodic memory. This is an important finding because, although the cross-sectional association between episodic memory and hippocampal volume is a common finding in the general aging and AD literature, in the few studies that have examined the association specifically in Black samples, the finding is inconsistent. For example, Sencakova et al. (2001) found the association, but only when their groups of AD patients and healthy older participants were collapsed; there were trends toward the association in the patient group, but not in the healthy participants. Brickman et al. (2008) did not find the association in any of their race/ethnicity groups, including Blacks, and, given that hippocampal volume loss is thought to occur early in a trajectory toward AD (e.g., Locascio et al., 1995; Welsh et al., 1992), they attributed the null effect to the exclusion of participants with formal diagnoses of dementia from their sample. In their diverse sample of non-demented participants, Zahodne, Manly et al. (2015) reported that smaller hippocampal volume predicted lower episodic memory scores, but only for non-Hispanic Whites. DeCarli et al. (2008), in their examination of brain structure-cognition associations in a diverse sample (White, Black and Hispanic), found that greater hippocampal volume was associated with better episodic memory for each group with no interaction for race/ethnicity. The findings of the current study suggest that less hippocampal tissue in non-demented, older Blacks, is associated cross-sectionally with lower episodic memory.
Although less tissue in the hippocampus was cross-sectionally associated with episodic memory in this study, the finding did not hold for decline in episodic memory. This is consistent with the finding of Carmichael et al. (2012) who showed in a diverse sample (that did not include interaction effects for race/ethnicity) that hippocampal volume was related to baseline cognitive function (including episodic memory) but did not predict change in cognitive function. In the current study, regions of temporal lobe outside of the hippocampus (parahippocampus, amygdala, insular white matter) predicted faster decline in episodic memory. This finding lends support to previous studies in diverse samples that found that temporal lobe volume had incremental power to predict cognitive decline (Fletcher et al., 2018; Gavett et al., 2018), and, like the associations found with global cognitive decline in this study, extends that finding to particular regions within the temporal lobe that may be critical for predicting episodic memory decline within Blacks. Overall, the current findings for episodic memory suggest that hippocampal volume may be useful for cross-sectional studies aimed at gauging the effects of lifestyle exposures or health conditions on episodic memory in Blacks, but it may not be useful for predicting rate of decline. Rather, less tissue in regions within the temporal lobe, but outside of the hippocampus, may be more suitable markers of faster episodic memory decline within Blacks.
Brain structure was not associated with level semantic memory, the long-term repository of context-free memories (Fleischman and Gabrieli, 1999; Tulving, 1983), suggesting that it is a poor proxy for cross-sectional status of brain structure within Blacks. However, less tissue in parahippocampal cortices and the entorhinal cortex, but not hippocampus, in the temporal lobe, as well as less tissue in the anterior cingulate and orbital white matter in the frontal lobe were associated with faster semantic memory decline. Indeed, the semantic memory domain used in this study includes a category fluency task which, in addition to drawing on temporal-lobe semantic resources, engages (perhaps to a lesser extent; Henry & Crawford, 2004), frontal-lobe executive resources. The finding within Blacks of less tissue in regions of both the temporal and the frontal lobes associated with semantic memory/executive function decline, in contrast to solely the temporal lobe for episodic memory decline, is consistent with the observation in the general literature that semantic memory/executive function engages a larger neural network than episodic memory (e.g., Patterson, Nestor and Rogers, 2007; Peelle et al., 2014), and that atrophy in regions comprising this semantic memory/executive function network may signal an increased risk of adverse outcomes including risk or progression of dementia (e.g., Welsh, 1992).
4.3. Perceptual Speed
White matter integrity (Filley and Fields, 2016) has been linked to perceptual speed in studies of aging in both mostly White (e.g., Arfanakis et al., 2020; Arvanitakis et al., 2016; Boyle et al. 2016; Marquine et al., 2010), and diverse (Zahodne, Manly et al., 2015) samples. In the Zahodne, Manly et al. (2015) study, non-demented Blacks had a greater whole-brain volume of WMH and worse cross-sectional performance on speeded cognitive tasks than Whites. In the current study of non-demented Blacks, less white matter tissue was also associated with slower perceptual speed level, and these associations were localized to superior and medial-inferior parietal lobe regions that are known to support integration of sensory input (Murray et al 2010) and perceptual speed (Laukka et al., 2015).
Parietal white matter integrity has been examined in association with several adverse outcomes in aging. Meier et al. (2012) showed that parietal (and frontal) WMH burden was associated with diagnoses of MCI and AD in an older, all-Black sample. In diverse samples, Brickman et al. (2012) showed that parietal WMH burden predicted incident dementia and Dhamoon et al. (2017) showed that parietal WMH, localized to the left-hemisphere, predicted faster functional decline. In this study, regions of less tissue in parietal white matter that were associated with slower level of perceptual speed were limited to the left-hemisphere. Future studies are needed that test the predictive power of asymmetric parietal white matter integrity for important adverse outcomes within older Blacks.
4.4. Strengths and Limitations
Although this study is the first that we know of that has examined brain structure associations with cognitive decline in a large sample of older Blacks recruited from epidemiological studies of aging and dementia, it has some limitations. First, although we did adjust in secondary models for three health risk factors that are common in Blacks, we did not examine important potential race-relevant sociocultural moderators, including but not limited to, quality of education. The optimal approach to generating useful biomarkers for the design and implementation of clinical trials and interventions for Blacks is to, first, identify relationships between brain integrity and cognitive decline within Blacks; next, determine the factors that influence these relationships; and finally, more deeply examine the biological mechanisms underlying the relationships. In this study, we focus on the first step. Future studies within Blacks will examine race-relevant risk and protective moderators of the reported associations, test the power of the identified morphometric indices to predict adverse outcomes, and finally, examine more fully the complexity of the biological mechanisms in play. Second, the participants in this study reside in the Chicagoland area and are mostly women with an education beyond high school, thus the results may not generalize to the US population of older, community-dwelling Blacks. Third, given the associations between global cognitive ability and domain-specific ability, the two cannot be cleanly separated, even in a study such as this which included a large number of assessments. Fourth, this study did not test simultaneous change in brain morphometry and cognition; local tissue and CSF were measured at study baseline and associated with longitudinal change in cognition. We will have the opportunity to study simultaneous change in future studies because the cohorts from which recruitment of this sample took place acquire longitudinal scans. Finally, one imaging approach, deformation-based morphometry, was used to measure brain integrity. In future studies, we will be able to add other measures to the analysis such as WMH burden, diffusion-weighted imaging, quantitative susceptibility mapping (QSM), and resting-state fMRI, which may yield a more in-depth understanding of underlying biological mechanisms.
4.5. Conclusions
The results of this study identified several morphometric indices linked to cognitive level and decline within older Blacks that could be useful in clinical trial, epidemiological, and interventional studies. Of particular note, less hippocampal tissue was related to lower episodic memory, but not decline, suggesting that it is an appropriate marker of cognitive status in Blacks, whereas less non-hippocampal temporal lobe tissue appears to be a more useful gauge of the rate of cognitive decline when measured by either episodic memory or semantic memory tests or a global cognitive summary score. When less tissue in the temporal-lobe is accompanied by less tissue in the frontal-lobe, this may suggest that the rate of cognitive decline has gone beyond early changes in episodic memory to involving the wider neural networks that support other cognitive domains such as semantic memory and executive function. This is the first epidemiological study of associations between brain structure and cognitive decline accomplished in a large sample of solely older Blacks without dementia and future studies are needed to build upon these findings.
Highlights.
Identification of race-relevant biomarkers is critically needed
Examined within-Black variability in associations of DBM to cognitive decline
Hippocampal contraction related to lower episodic memory but not decline
Non-hippocampal temporal & and frontal contractions predict cognitive decline
Parietal WM & splenium contractions predict cognitive level & decline respectively
Acknowledgments
This work was supported by National Institute on Aging grants R01AG56405, R01AG22018, P30AG10161, R01AG17917, R01AG064233, R01AG052200, R01AG055430, R01AG062711, K01AG064044; National Institute of Neurological Disorders and Stroke UH3NS100599; and by the Illinois Department of Public Health. We are indebted to the altruism of the participants of MARS, RADC AA Core, and MAP. We thank Dominika Drupka for statistical analyses. More information regarding obtaining MARS, RADC AA Core and/or MAP data for research use can be found at the RADC Research Resource Sharing Hub (www.radc.rush.edu).
Footnotes
Disclosure statement
The authors report no disclosures
CRediT authorship contribution statement
Debra A. Fleischman: Conceptualization, Formal analysis, Investigation, Writing - original draft. Konstantios Arfanakis: Conceptualization, Methodology, Formal analysis, Investigation, Writing - original draft, Funding Acquisition. Sue E. Leurgans: Formal analysis, Methodology, Investigation, Writing - original draft. Shengwei Zhang: Methodology, Visualization, Formal analysis - review & editing. Victoria N. Poole: Writing - review & editing, Funding Acquisition. S. Duke Han: Writing - review & editing, Funding Acquisition. Lei Yu: Methodology, Writing - review & editing. Melissa Lamar: Writing - review & editing, Funding Acquisition. Namhee Kim: Writing - review & editing. David A. Bennett: Writing - review & editing, Funding Acquisition. Lisa L. Barnes: Conceptualization, Formal analysis, Investigation, Writing - original draft, Funding Acquisition
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acosta-Cabronero J, Alley S, Williams GB, Pengas G, Nestor PJ. Diffusion tensor metrics as biomarkers in Alzheimer’s disease. PLoS One 2012;7:e49072. doi: 10.1371/journal.pone.0049072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal NT, Wilson RS, Bienias JL, DeJager PL, Bennett DA, Evans DA, DeCarli C. The association of magnetic resonance imaging measures with cognitive function in a biracial population sample. Arch Neurol 2010;67:475–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arfanakis K, Evia AM, Leurgans SE, Cardosoa LFC, Kulkarnia A, Alqama N, Lopesa LF, Vieiraa D, Bennett DA, Schneider JA. Neuropathologic correlates of white matter hyperintensities in a community-based cohort of older adults. J Alzheimers Dis 2020; 73:333–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Hutton C., Frackowiak R, Johnsrude I, Price C, Friston K. Identifying global anatomical differences: deformation-based morphometry. Hum Brain Mapp 1998;6:348–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal 2008;12:26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes LL and Bennett DA. Alzheimer’s disease in African Americans: Risk factors and challenges for the future. Health Aff 2014;33:580–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes LL, Shah RC, Aggarwal NT, Bennett DA, Schneider JA. The Minority Aging Research Study: Ongoing efforts to obtain brain donation in African Americans without dementia. Curr Alzheimer Res 2012;9:734–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes LL, Yumoto F, Capuano A, Wilson RS, Bennett DA, Tractenberg RE. Examination of the factor structure of a global cognitive battery across race and time. J Int Neuropsychol Soc 2016;22:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Buchman AS, Boyle P, Barnes LL, Wilson RS, Schneider JA. Religious Orders Study and Rush Memory and Aging Project. J Alzheimers Dis 2018;64:S161–S189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Wilson RS, Boyle PA, Buchman A, Schneide JA Relation of neuropathology to cognition in persons without cognitive impairment. Ann. Neurol 2012b; 72:599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birns J, Morris R, Jarosz J, Markus H, Kalra L. Ethnic differences in the cerebrovascular impact of hypertension. Cerebrovasc Dis 2008;25:408–16. [DOI] [PubMed] [Google Scholar]
- Boyle PA, Yu L, Fleischman DA, Leurgans S, Yang J, Wilson RS, Schneider JA, Arvanitakis Z, Arfanakis K, Bennett DA. White matter hyperintensities, incident mild cognitive impairment and cognitive decline in old age. Ann Clin Trans Neurol 2016;3:791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman AM, Provenzano FA, Muraskin J, Manly JJ, Blum S, Apa Z, Stern Y, Brown TR, Luchsinger JA, Mayeux R. Regional white matter hyperintensity volume, not hippocampal atrophy, predicts incident Alzheimer’s disease in the community. Arch Neurol 2012;69:1621–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman AM, Schupf N, Manly JJ, Luchsinger JA, Andrews H, Tang MX, Reitz C, Small SA, Mayeux R, DeCarli C, Brown TR. Brain morphology in older African Americans, Caribbean Hispanics, and Whites from Northern Manhattan. Arch Neurol 2008;65:1053–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael O, Mungas D, Beckett L, Harvey D, Tomaszewski FS, Reed J, Miller J, DeCarli C. MRI predictors of cognitive change in a diverse and carefully characterized elderly population. Neurobiol Aging 2012;33:83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael O and Newton R. Brain MRI findings related to Alzheimer’s disease in older African American adults. Prog Mo Biol Trans Sci 2019;165:3–23. [DOI] [PubMed] [Google Scholar]
- Capizzano AA, Acion Bekinschtein T, Furman M, Gomila H, Martinez A, Mizrahi R, Starkstein SE. White matter hyperintensities are significantly associated with cortical atrophy in Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2004;75:822–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhamoon MS, Cheung Y-K, Bagci A, Alperin N, Sacco RL, Elkind MSV, Wright CB. Differential effect of left vs. right white matter hyperintensity burden on functional decline: The Northern Manhattan Study. Front Aging Neurosci 2017; 9:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCarli C, Massaro J, Harvey D, Hald J, Tullberg M, Au R, Beiser A, D’Agostino R, Wolf PA. Measures of brain morphology and infarction in the Framingham Heart Study: Establishing what is normal. Neurobiol Aging 2005;26:491–510. [DOI] [PubMed] [Google Scholar]
- DeCarli C, Reed BR, Jagust W, Martinez O, Ortega M, Mungas D. Brain behavior relationships among African Americans, Whites, and Hispanics. Alzheimer Dis Assoc Disord 2008;22: 382–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filley CM and Field RD. White matter and cognition: Making the connection. J Neurophysiol 2016;116:2093–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischman DA and Gabrieli J. Long-term memory in Alzheimer’s Disease. Curr Opin Neurobiol 1999;9:240–44. [DOI] [PubMed] [Google Scholar]
- Fleischman DA, Leurgans S, Arfanakis K, Arvanitakis Z, Barnes LL, Boyl PA, Han SD, Bennett DA. Gray-matter macrostructure in cognitively healthy older persons: Associations with age and cognition. Brain Struct Funct 2013;219: 2029–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischman DA, Yu L, Arfanakis K, Han SD, Barnes LL, Arvanitakis Z, Boyle PA, Bennett DA. Faster cognitive decline in years prior to MR imaging is associated with smaller hippocampal volumes in cognitively healthy older persons. Front Ag Neurosci 2013; 5: doi: 10.3389/fnagi.2013.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischman DA, Wilson RS, Gabrieli JD, Schneider JA, Bienias JL, Bennett DA. Implicit memory and Alzheimer’s disease pathology. Brain 2005;128: 2006–15. [DOI] [PubMed] [Google Scholar]
- Fletcher E, Gavett B, Harvey D, Farias ST, Olichney J, Beckett L, DeCarli C, Mungas D. Brain volume change and cognitive trajectories in aging. Neuropsychology 2018;32:436–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavett B, Fletcher E, Harvey D Farias ST, Olichney J, Beckett L, DeCarli C, Mungas D. Ethnoracial differences in brain structure change and cognitive change. Neuropsychology 2018;32:529–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaser C, Nenadic I, Buchsbaum BR, Hazlett EA, Buchsbaum MS. Deformation-based morphometry and its relation to conventional volumetry of brain lateral ventricles in MRI. Neuroimage. 2001. Jun;13(6 Pt 1):1140–5. doi: 10.1006/nimg.2001.0771. [DOI] [PubMed] [Google Scholar]
- Golomb J. De Leon MJ, Kluger A, George AE, Tarshish C, Ferris SH. Hippocampal atrophy in normal aging: an association with recent memory impairment. Arch Neurol 1993;50:967–73. [DOI] [PubMed] [Google Scholar]
- Gottesman RF, Coresh J, Catellier DJ, Sharrett AR, Rose KM, Coker LH, Shibata DK,Knopman DS, Jack CR, Mosley TH Jr. Blood pressure and white-matter disease progression in a biethnic cohort: Atherosclerosis Risk in Communities (ARIC) study. Stroke 2010;41:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurland BJ, Wilder DE, Lantigua R, Stern Y, Chen J, Killeffer EH, Mayeux R. Rates of dementia in three ethnoracial groups. Int J Geriatr Psychiatry 1999;14:481–93. [PubMed] [Google Scholar]
- Hackert VH, den Heijer T, Oudkerk M, Koudstaal PJ, Hofman A, Breteler MM. Hippocampal head size associated with verbal memory performance in nondemented elderly. Neuroimage 2002;17:1365–72. [DOI] [PubMed] [Google Scholar]
- Henry JD, Crawford JR. A meta-analytic review of verbal fluency performance following focal cortical lesions. Neuropsychology 2004;18:284–295. [DOI] [PubMed] [Google Scholar]
- Hua X, Leow AD, Parikshak N, Lee S, Chiang MC, Toga AW, Jack CR Jr, Weiner MW, Thompson PM; Alzheimer’s Disease Neuroimaging Initiative. Tensor-based morphometry as a neuroimaging biomarker for Alzheimer’s disease: an MRI study of 676 AD, MCI, and normal subjects. Neuroimage. 2008. Nov 15;43(3):458–69. doi: 10.1016/j.neuroimage.2008.07.013. Epub 2008 Jul 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu F-C, Sink KM, Hugenschmidt CE, Williamson JD, Hughes TM, Palmer N, Xu J, Smith C, Wagner BC, Whitlow CT, Bowden DW, Maldjian JA, Divers J, Freedman BI. Cerebral structure and cognitive performance in African Americans and European Americans with Type 2 diabetes. J Gerontol: Series A 2018;73:407–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes TM, Sink KM, Williamson JD, Hugenschmidt CE, Wagner BC, Whitlow CT, Xu J, Smith SC, Launer LJ, Barzilay JI, Ismail-Beigi F, Bryan RN, Hsu F-C, Bowden DW, Maldjian JA, Divers J, Freedman BI, on behalf of AA-DHS MIND and ACCORD MIND Investigators. J Diabetes Complications 2018;32, 916–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isensee F, Schell M, Pflueger I, Brugnara G, Bonekamp D, Neuberger U, Wick A, Schlemmer HP, Heiland S, Wick W, Bendszus M, Maier-Hein KH, Kickingereder P. Automated brain extraction of multisequence MRI using artificial neural networks. Hum Brain Mapp 2019;40.17:4952–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laukka EJ, Lovden M, Kalpouzos G, Papenberg G, Keller L, Graff C, Li T-Q, Fratiglioni L, Backman L. Microstructural white matter properties mediate the association between APOE and perceptual speed in very old persons without dementia. PLoS One 2015;10;e0134766. DOI: 10.1371/journal.pone.0134766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Allen B, Lopez O, Aizenstein H, Boudreau R, Newan A, Yaffe K., Kritchevsky S, Launer L, Satterfield S, Simonsick E, Rosano C. Racial differences in gray matter integrity by diffusion tensor in black and white octogenarians. Curr Alzheimer Res 2015;12:648–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquine MJ, Attix DK, Goldstein LB, Samsa GP, Payne ME, Chelune GJ, Steffens DC. Differential patterns of cognitive decline in anterior and posterior white matter hyperintensity progression. Stroke 2010;41:1946–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough I M. Beta-amyloid and cortical thickness reveal racial disparities in preclinical Alzheimer’s disease. NeuroImage Clin 2017;16:659–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan E. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984;34:939–44. [DOI] [PubMed] [Google Scholar]
- Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement 2016;12:216–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta KM and Yeo GW. Systematic review of dementia prevalence and incidence in United States race/ethnic populations. Alzheimers Dement 2017;13:72–83. [DOI] [PubMed] [Google Scholar]
- Meyer CS, Schreiner PJ, Lim K, Battapady H, Launer LJ. Depressive symptomatology, racial discrimination experience, and brain tissue volumes observed on magnetric resonance imaging: The CARDIA Study. Am J Epidemiol 2019;188:656–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris Z, Whiteley WN, Longstreth WT Jr, Weber F, Lee YC, Tsushima Y, Alphs H, Ladd SC, Warlow C, Wardlaw JM, Al-Shahi Salman R. Incidental findings on brain magnetic imaging: systematic review and metaanalysis. BMJ 2009;339:b3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungas D, Reed BR, Farias ST, DeCarli C. Age and education effects on relationships of cognitive test scores with brain structure in demographically diverse older persons. Psychol Aging 2009;24:116–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray ME, Senjem ML, Petersen RC, Hollman JT, Weigand SD, Knopman DS, Ferman TJ, Dickson DW, Jack CR. Functional impact of white matter hyperintensities in cognitively normal elderly. Arch Neurol 2010;67:1379–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyquist PA, Bilgel MS, Gottesman R, Yanek LR, Moy TF, Becker LC, Cuzzocreo J, Prince J, Yousem DM, Becker DM, Kral BG, Vaidya D. Extreme deep white matter hyperintensity volumes are associated with African American race. Cerebrovasc Dis 2014;37:244–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H-G, Kim JJ, Lee S-K, Seok JH, Chun J, Kim DI, Lee JD. Corpus callosal connection mapping using cortical gray matter parcellation and DT-MRI. Hum Brain Mapp 2008;29: 503–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson K, Nestor P, Rogers T. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat Rev Neurosci 2007;8:976–87. [DOI] [PubMed] [Google Scholar]
- Peelle JE, Powers J, Cook PA, Smith EE, Grossman M. Frontotemporal neural systems supporting semantic processing in Alzheimer’s disease. Cogn Affect Behav Neurosci 2014;14:7–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon FM, Head DP, DuPuis JH, Acker JD. Neuroanatomical correlates of cognitive aging: Evidence from structural magnetic resonance imaging. Neuropsychology 1998;12:95–114. [DOI] [PubMed] [Google Scholar]
- Ridwan AR, Niaz MR, Wu Y, Qi X, Zhang S, Kontzialis M, Javierre-Petit C, Tazwar M; Alzheimer’s Disease Neuroimaging Initiative, Bennett DA, Yang Y, Arfanakis K. Development and evaluation of a high performance T1-weighted brain template for use in studies on older adults. Hum Brain Mapp 2021;42:1758–1776. Jan 15. doi: 10.1002/hbm.25327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudge P and Warrington EK. Selective impairment of memory and visual perception in splenial tumours. Brain 1991;114:349–60. [DOI] [PubMed] [Google Scholar]
- Sarro L, Senjem ML, Lundt ES, Przybelski SA, Lesnick TG, Graff-Radford J, Boeve BF, Lowe VJ, Ferman TJ, Knopman DS, Comi G, Filippi M, Petersen RC, Jack CR Jr, Kantarci K. Amyloid-β deposition and regional grey matter atrophy rates in dementia with Lewy bodies. Brain. 2016. Oct;139(Pt 10):2740–2750. doi: 10.1093/brain/aww193. Epub 2016 Jul 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R, Ropele S, Enzinger C, Petrovic K, Smith S, Schmidt H, Matthews PM, Fazekas F. White matter lesion progression, brain atrophy, and cognitive decline: The Austrian Stroke Prevention Study. Ann Neurol 2005;58:610–16. [DOI] [PubMed] [Google Scholar]
- Sencakova D, Graff-Radford NR, Willis FB, Lucas JA, Parfitt F, Cha RH, O’Brien PC, Petersen RC, Jack C. Hippocampal atrophy correlates with clinical features of Alzheimer disease in African Americans. Archives of Neurology 2001;58:1593–97. [DOI] [PubMed] [Google Scholar]
- Squire LR and Dede AJ. Conscious and unconscious memory systems. Cold Spring Harb Perspect Biol 2015;7:doi: 10.1101/cshperspect.a021667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang MX, Cross P, Andrews H, Jacobs DM, Small S, Bell K, Merchant C, Lantigua R, Costa R, Stern Y, Mayeux R. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology 2001;56:49–56. [DOI] [PubMed] [Google Scholar]
- Tosto G, Zimmerman ME, Carmichael OT, Brickman AM, Alzheimer’s Disease Neuroimaging Initiative. Predicting aggressive decline in mild cognitive impairment: the importance of white matter hyperintensities. JAMA Neurol 2014;71:872–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. Elements of Episodic Memory. London: Oxford University Press; 1983. [Google Scholar]
- Tustison NJ. Avants BB, Cook PA, Zherg Y, Egan A, Yushkevich PA, Gee JC. N4ITK: improved N3 bias correction. IEEE Trans Med Imaging 2010;29.6:1310–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vangberg TR, Eikenes L, Haberg AK. The effect of white matter hyperintensities on regional brain volumes and white matter microstructure, a population-based study in HUNT. NeuroImage 2019;203: doi. 10.1016/j.neuroimage.2019.116158. [DOI] [PubMed] [Google Scholar]
- Waldron-Perrine B, Kisser JE, Brody A, Haacke EM, Dawood R, Millis S, Levy P. MRI and neuropsychological correlates in African Americans with hypertension and left ventricular hypertrophy. Am J Hypertens 2018;31:865–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw JM, Valdes-Hernandez MC, Munoz-Maniega S. What are white matter hyperintensities made of? Relevance to vascular cognitive impairment. J Am Heart Assoc 2015; 4:e001140 doi: 10.1161/JAHA.114.001140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weuve J, Barnes LL, Mendes de Leon CF, Rajan KB, Beck T, Aggarwal NT, Hebert LE, Bennett DA, Wilson RS, Evans DA. Cognitive aging in Black and White Americans: Cognition, cognitive decline, and incidence of Alzheimer Disease dementia. Epidemiology 2018;29:151–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield KE, Allaire JC, Belue R, Edward CL. Are comparisons the answer to understanding behavioral aspects of aging in racial and ethnic groups? J Gerontol Psychol Sci 2008;63B:P301–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlow CT, Sink KM, Divers J, Smith SC, Xu J, Palmer ND, Hugenschmidt CE, Williamson JD, Bowen DW, Freedman BI, Maldjian JA. Effects of type 2 diabetes on brain structure and cognitive function: African American-Diabetes Heart Study MIND. Am J Radiol 2015;361648–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Beckett LA, Barnes LL, Schneider JA, Bach J, Evans DA, Bennett DA. Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging 2002;17:179–93. [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model Neuroimage 2014; 92:381–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Webster MA, Vidaurre D, Nichols TE, Smith SM. Multi-level block permutation. Neuroimage 2015;123:253–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Douaud G, Nichols TE, Smith SM. Faster permutation inference in brain imaging. Neuroimage 2016;141:502–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahodne LB, Manly JJ, Narkhede A, Griffith EY, DeCarli C, Schupf NS, Mayeux R, Brickman AM. Structural MRI predictors of late-life cognition differ across African Americans, Hispanics, and Whites. Curr Alzheimer Res 2015;12:632–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahodne LB, Wall MM, Schupf N, Mayeux R, Manly JJ, Stern Y, Brickman AM. Late-life memory trajectories in relation to incident dementia and regional brain atrophy. J Neurol 2015;262:2484–90. [DOI] [PMC free article] [PubMed] [Google Scholar]