Abstract
Introduction:
Over-the-counter cannabidiol (CBD) products have seen unprecedented recent growth in the United Kingdom. However, analysis of these predominantly unregulated products from other countries tells us that they are often mislabeled or contain unlabeled and potentially dangerous chemicals. Thus, the aim of the present study was to analyze CBD oils available in the United Kingdom.
Materials and Methods:
Phytocannabinoids, residual solvent, and heavy metals were measured blinded in 29 widely available CBD products by an independent testing facility using high-performance liquid chromatography with diode-array detection for cannabinoids, Headspace-gas chromatography-flame ionization detector and gas chromatography–mass spectrometry for residual solvents, and inductively coupled plasma–mass spectrometry for heavy metals.
Results:
The mean advertised CBD content was 4.5%, and the actual mean measured CBD content of products was 3.2% (p=0.053, Mann–Whitney test). Only 11/29 (38%) products were within 10% of the advertised CBD content. Fifty five percent of products had measurable levels of the controlled substances Δ9-tetrahydrocannabinol (mean content 0.04%) or cannabinol (mean content 0.01%), as well as most other phytocannabinoid compounds including cannabidiolic acid (CBDA), cannabidivarin (CBDV), and cannabidivarin acid (CBDVA). Detectable levels of N-pentane, ethanol, isopropanol, heptane, lead, and arsenic were found in many of the CBD products, but these were within acceptable levels.
Conclusions:
As demonstrated in other countries, the quality of over-the-counter CBD products in the United Kingdom can be substandard, particularly with regard to CBD content, and often contains levels of controlled substances. We recommend that these products be more strictly regulated for consumer welfare.
Keywords: cannabidiol, over the counter, quality, phytocannabinoid
Introduction
Cannabidiol (CBD) is one of the major constituents of the Cannabis sativa plant of increasing interest due to its broad range of therapeutic properties coupled with a favorable safety and tolerability profile.1,2 The analgesic, anti-inflammatory, antioxidant, anxiolytic, anticonvulsant, and antitumoral effects of CBD are mediated through multiple molecular targets including cannabinoid binding receptor 1 (CB1), cannabinoid binding receptor 2 (CB2) (both generally thought to be indirect), serotonin receptors, opioid receptors, adenosine receptors, orphan receptors such as G protein-coupled receptor 55 (GPR55), GPR18, GPR3, GPR6, and GPR12, peroxisome proliferating activated receptors, transient receptor potential channels, as well as transporters and enzymes.3–6 Clinically, CBD is being investigated in phase 2 and 3 trials in diverse areas including schizophrenia, drug dependency, tumor reduction, pain conditions, and post-traumatic stress disorder. A pure plant-derived CBD product, Epidiolex®, recently became the first Food and Drug Administration (FDA)- and European Medicines Agency (EMEA)-approved CBD medicine, indicated for use in childhood Lennox–Gastaut or Dravet syndrome.
The recent change in UK legislation has not resulted in many prescriptions for cannabis-based medicinal products (CBMPs) within the National Health Service (NHS). However, the public recognition of the medical value of cannabis, coupled with many high-profile stories of patients receiving significant relief with CBMPs, has created huge patient demand for these products. Because of the inability of patients to access CBMPs through their health care providers, they are increasingly turning to over-the-counter cannabis-based products. Since CBD is not a controlled drug in the United Kingdom under the Misuse of Drugs Act or subsequent regulations, CBD-based products have thus become widely available, primarily in an oil format (often called cannabis or hemp oils), although also in many food and beverage products. To remain legally possessed, these products must not contain the psychoactive and controlled chemicals Δ9-tetrahydrocannabinol (THC) or cannabinol (CBN). A recent survey in the United States suggests that 62% of CBD consumers are seeking relief of particular symptoms or medical conditions, with the remaining using CBD as a general wellness product.7 The main medical reasons people use CBD is for pain relief, as a sleep aid, and to reduce anxiety and depression.7 In the United Kingdom, recent market research conducted by the Centre for Medicinal Cannabis suggests that there are 1.3 million regular CBD users.
Studies from other countries have cast doubt over the quality of over-the-counter CBD products. In the United States, a study of 84 CBD products found that only 31% of products tested were accurately labeled for CBD content (within 10% of advertised content), and THC was detected in 18 of the samples with a mean level of 0.45% (i.e., above regulated levels).8 A study in the Netherlands showed that only 5 of 46 products were within 10% of the labeled CBD content, and the percent deviation ranged from 0% to 92%.9 A similar study in Italy showed that of 14 CBD oils tested, only 5 were within 10% of the CBD labeled content.10 Twelve of the 14 CBD products in this study contained THC, although mostly below 0.2%. CBN was also detected in most samples, which is relevant for the United Kingdom, where CBN is still a schedule 1 substance. As CBN is an artifact formed by THC oxidation, these data also suggest that there may have been higher levels of THC present in these products at some point.
Bonn-Miller et al.8 found that CBD vape products were the worst for mislabeling of the 84 CBD products they tested. Another US study looked at nine different CBD e-liquid products from a single manufacturer and found that two of the products had THC and four contained a potent CB1 receptor agonist called 5-fluoro MDMB-PINACA (5F-ADB) and one contained dextromethorphan from the morphinan class of medications.11 In Utah, between 2017 and 2018, there were 52 cases of people who reported adverse reactions in products labeled as CBD (73% of these were vape products) that were inconsistent with CBD consumption, including seizures, vomiting, confusion, and hallucinations.12 Nine products were found to contain a potent synthetic cannabinoid agonist (4-cyano CUMYL-BUTINACA) but no CBD. Fifteen of the people who experienced these adverse reactions were using for medical reasons, which is especially worrying for vulnerable populations.
Based on the increasing CBD consumption in the United Kingdom, and the knowledge that these unregulated products are at best misleading and at worse, pose a health risk, the aim of the present study was to perform detailed analysis of over-the-counter CBD oil products in the United Kingdom. We chose oil products as this is the most common form of delivery for UK consumers and profiled the full phytocannabinoid content of products, as well as other potential contaminants including heavy metals and residual solvents for a fuller safety profile.
Materials and Methods
Twenty-nine of the most popular CBD products available online and on the high street, from 27 different suppliers were chosen for independent testing of cannabinoid content, heavy metals, and residual solvents. All testing was completed in duplicate by a single laboratory (PhytoVista Laboratories Ltd) who were blinded to the source/supplier of the product. For cannabinoid sample preparation, 200 mg of sample was weighed into a 25-mL volumetric flask. The sample was dissolved and diluted with isopropyl alcohol before sonicating, diluting to volume and filtered if required. For residual solvent sample preparation, 200 mg of sample was weighed into a 20-mL headspace vial. Five milliliters of dimethylformamide (headspace grade) was added and the contents of the vial mixed.
For the detection of phytocannabinoids, high-performance liquid chromatography with diode-array detection was used (see Supplementary Fig. S1 for an example chromatogram). For method validation, linearity was calculated across the working range of the method, which shows good correlation with R2 values of 0.998 or above for each analyte. Precision was measured based on 30 replicate analyses across the linear range, giving a percent relative standard deviation (%RSD) of <1 for major components and 8.8 for minor components. Separation was performed on a Waters CORTECS C18 column (4.6 mm×150 mm, 2.7 μm particle size) with a column oven temperature of 54°C, using water and methanol for mobile phase A and B, respectively, both with 0.1%v/v trifluoroacetic acid as modifier. A flow rate of 0.7 mL/min was used, with initial conditions of 79%B with a 5-min hold, followed by a linear gradient to 89.5% at 10 min, then to 100%B over 1 min with a 5-min hold. Data were collected at a wavelength of 226 nm and quantified against reference standards supplied by Cerilliant (Texas). Samples were tested in duplicate with an acceptance criterion of 2% RSD between duplicates. A quality control CBD oil sample was run with each analytical run, with a recovery criterion of 98%–102%. Limits of quantification for THC and CBN have been determined as 0.006 and 0.003%w/w, respectively.
For the detection of class 1 heavy metals, aliquots of homogenized test sample were digested in a mixture of nitric acid and hydrochloric acid using a high-pressure microwave system. Quantification was performed by inductively coupled plasma–mass spectrometry with collision cell employing helium as collision gas. Quality checks included blanks, spikes, and certified reference material. All data were corrected for reagent blank and spike recovery. The reporting limit was calculated from 10×standard deviation (SD) of reagent blank values adjusted for dilution and sample weight.
For the detection of residual solvents, samples were tested by gas chromatography-flame ionization detector with headspace sampler. The chromatographic column used was a DB-624 (with dimensions of 30 m×0.32 mm, 1.8 μm film thickness) utilizing hydrogen as carrier gas. Chromatographic conditions used are detailed in the USP467 residual solvents method. The headspace used an oven temperature of 80°C, with an equilibration time of 15 min and a loop equilibration time of 0.2 min. The injection time used was 0.15 min. The presence of detected solvents was confirmed by gas chromatography–mass spectrometry with electron ionization, using direct injection. The MS was operated in SIM mode, monitoring for three ions present from the analyte of interest.
Product-blinded data were collected, analyzed, and figures generated using GraphPad Prism software. Where appropriate, CBD data (nonparametric) were compared by the Mann–Whitney test. Data are presented as scatterplots with mean±SD.
Results
CBD content
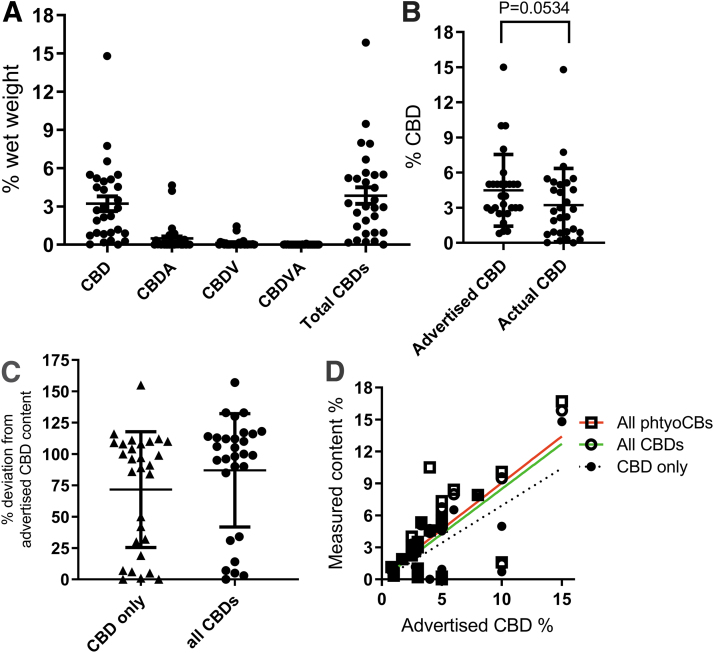
The advertised versus the actual measured CBD (and other phytocannabinoid) content of the 29 tested products is presented in Supplementary Table S1 and Figure 1. The mean advertised CBD content was 4.5%, and the actual mean measured CBD content of product was 3.2% (p=0.0534, Mann–Whitney test, Fig. 1B). Only 11/29 (38%) products were within 10% of the advertised CBD content, and 10/29 (34%) products had <50% of the advertised CBD content. One product had 0% CBD (Supplementary Table S1). The percent deviation from the advertised CBD content ranged from 0% to 155% and averaged at 76% (Fig. 1C and Supplementary Table S1). This was improved if all CBD-derived compounds (i.e., cannabidiolic acid [CBDA], cannabidivarin [CBDV], and cannabidivarin acid [CBDVA]) were included and added together (although it is inappropriate to do so, suppliers may have done so), increasing the mean % CBD content (of all CBD-related compounds) to 3.8% (cf. 3.2%). There was a better correlation between the total CBD content (i.e., all CBD-derived compounds, R2=0.5520) or the total phytocannabinoid content (R2=0.4818) with the advertised CBD content than there was with the actual CBD content (R2=0.4534) of products (Fig. 1D).
FIG. 1.
CBD measurements in 29 commercially available CBD oils. Products were tested for CBD-derived compounds including CBD, CBDA, CBDV, and CBDA (A) and the actual versus the advertised mean content compared (Mann–Whitney test, B). (C) The % deviation of products compared with the advertised CBD content looking at CBD only and if all CBD compounds (CBD, CBDA, CBDV, and CBDVA) were considered. (D) The correlation of the advertised CBD with the total phytocannabinoid content (red), total CBD content (green), or CBD only content (black). Data are presented as scatterplots with mean±SD. CBD, cannabidiol; CBDA, cannabidiolic acid; CBDV, cannabidivarin; CBDVA, cannabidivarin acid; SD, standard deviation.
Other phytocannabinoids
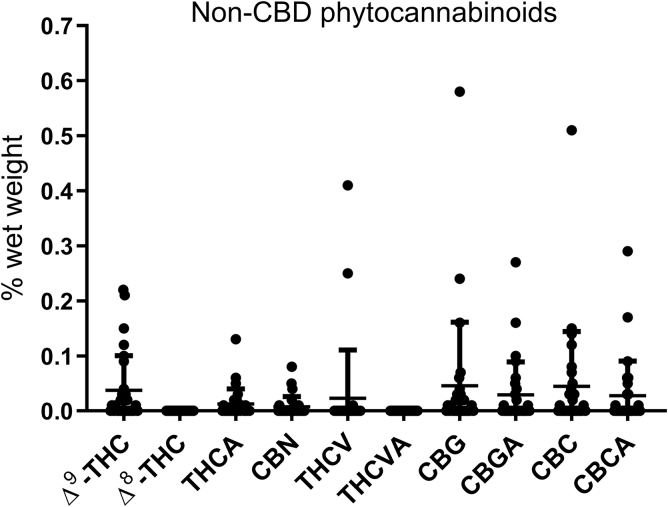
Ten percent of the total cannabinoid content of products was other non-CBD phytocannabinoids, including THC, tetrahydrocannabinolic acid (THCA), tetrahydrocannabivarin (THCV), cannabinol (CBN), cannabigerol (CBG), cannabigerolic acid (CBGA) and cannabichromene (CBC) (Supplementary Table S1 and Fig. 2). The average ratio of CBD:other phytocannabinoids was 15:1 (range 0–114:1). Of these phytocannabinoids, the highest levels measured were of CBG (mean content 0.05%) and CBC (mean content 0.04%). Fifty-five percent of products had measurable levels of THC (mean content of those with measurable levels is 0.07%) or CBN (mean content of those with measurable levels is 0.04%) and are thus technically illegal to be possessed within the United Kingdom, which has zero tolerance for any controlled substances within CBD. The range of THC content was 0%–0.22%, and the range of CBN content was 0%–0.12%.
FIG. 2.
Phytocannabinoid measurements in 29 commercially available CBD oils. Products were tested for phytocannabinoids other than CBD including the controlled phytocannabinoids, THC or CBN. Data are presented as a scatterplot with mean±SD. CBN, cannabinol; THC, Δ9-tetrahydrocannabinol.
Residual solvents and heavy metals
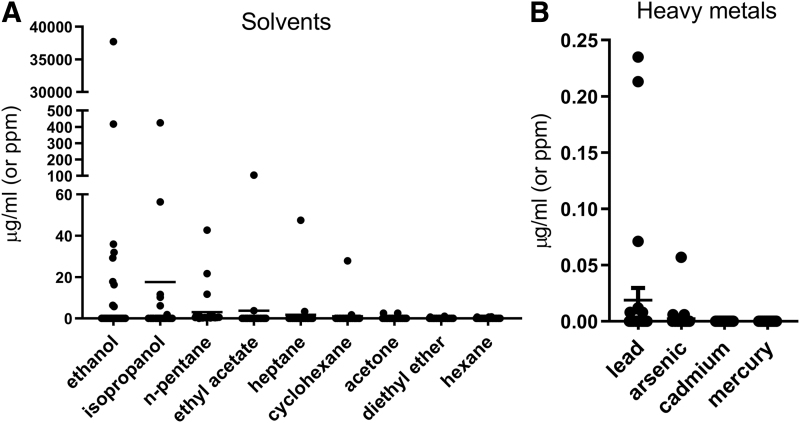
Our analysis included for the first time the range of solvents (Fig. 3A) and heavy metals (Fig. 3B) measured in UK CBD products (Supplementary Table S2). Three of the 29 products had levels of n-pentane above 1.5 ppm (11.7, 21.7, and 42.7 ppm). One product had 37,704 ppm of ethanol, and one product had an ethyl acetate level of 103.8 ppm. Isopropanol levels of 56.4 and 424.3 ppm were found in two products, and heptane (47.5 ppm) was found in another. Cyclohexane was found in one product (27.9 ppm). Small quantities of lead (0.01–0.24 ppm) and arsenic (0.01–0.06 ppm) were detected in some products. Cadmium and mercury were not detected in any of the samples.
FIG. 3.
Solvent (A) and heavy metal (B) content (expressed in μg/mL or ppm) in the 29 commercially available CBD oils analyzed in this study. Data are presented as scatterplots with mean±SD.
Discussion
Based on the knowledge that the quality of over-the-counter CBD products has often been found to be substandard in other countries, coupled with the large increase in consumer use of over-the-counter CBD products, we undertook an independent blinded analysis of 29 CBD oil products available in the United Kingdom. Our results are similar to those that have been reported elsewhere, that is, the majority of products did not contain the amount of CBD advertised on the labeling; the variation from the advertised CBD content ranged from 0 to 155%. Additionally, more than half of the products contained the controlled substances THC and CBN. These data add to the accumulating evidence that there needs to be increased regulation of CBD products to protect consumers.
Our analysis found that only 38% of products were within 10% of the advertised CBD content and 34% of products had 50% or less of the advertised CBD content. This is very similar to what has been observed in the United States (31% of products tested were within 10%8), the Netherlands (11% of products were within 10%9), and in Italy (36% of products were within 10%10). In the United Kingdom, patients are increasingly turning to over-the-counter cannabis-based products for relief for self-treatable symptoms.7 However, over-the-counter CBD is also used for relief from more life-threatening disorders such as childhood epilepsy because of the current inability to access CBD easily through the NHS. The fact that so many available CBD products do not contain the advertised amount of CBD means that vulnerable consumers will not be receiving the “dose” of CBD that they think they are getting, probably leading to reduced or even no efficacy of the products. This is particularly important considering that the doses in over-the-counter products are already lower than used in CBD products being developed as registered medicines,13 and because of the poor bioavailability of CBD.14 Negative experiences with CBD products will also lead to a reduced public perception of the usefulness of CBD products.
Currently in the United Kingdom, it is illegal for CBD products to contain any controlled drug substances, which include THC and CBN. Our study showed that 55% of products had measurable levels of THC or CBN. THC was also detected in 21% of US products8 and in most products in Italy.10 This has several implications for consumers. There could be intoxicating effects of THC or side effects that are attributable to THC, such as loss of memory, hallucinations, or paranoia. However, this is unlikely given the very low levels of THC in these products. However, the presence of THC in CBD products could cause a consumer to test positively for cannabis use (which is based on THC metabolites), which could have significant consequences for professionals, or those with sports careers (CBD is increasing being used as an aid to recovery in sports).
Almost all of the CBD products that were tested contained other CBD-related products including CBDA (mean 0.62%), CBDV (0.13%), and CBDVA (0.01%). The biological effects of these CBD-related compounds have overlapping features to that observed to CBD, sometimes even with greater potency than CBD. For example, CBDV has antiepileptic properties in rats,15 and CBDA is a potent anti-nausea and anti-anxiety agent in rats.16 All the CBD products also contained non-CBD phytocannabinoids. In fact, 10% of the total cannabinoid content of products was non-CBD phytocannabinoids, including THC, THCA, THCV, CBN, CBG, CBGA, and CBC. Of these, the highest contents were of CBG (0.05%) and CBC (0.04%). Animal studies suggest that both CBG and CBC have potential therapeutic benefits in cachexia,17 neuroprotection,18,19 inflammation,20 and cancer.21 Thus, the beneficial effects of over-the-counter CBD products may be partly due to the additive or synergistic effects, or drug–drug interactions, of other phytocannabinoids within these products. For example, this has been demonstrated with a combination of CBD and CBN in a pain model.22 However, it should also be considered that we do not know the impact (with regard to safety or efficacy) of human consumption of larger amounts of these phytocannabinoids than would normally be present in the whole plant.
Our analysis included for the first time the range of solvents and heavy metals in UK CBD products. The percentage of solvents and heavy metals in all products was below the permitted daily dose levels in pharmaceutical products according to the International Council for Harmonisation guidelines, although above food limit safety levels. Thus, our data suggest that the range of solvents and heavy metals tested are not of concern in these UK products, at least of those tested in this analysis.
In conclusion, our data add to the accumulating evidence that over-the-counter CBD products are often mislabeled with respect to the total CBD content and contain many phytocannabinoids that are not always labeled, including controlled drugs. This has significant implications on vulnerable consumers using these products with respect to efficacy, side effects, and drug testing.
Supplementary Material
Acknowledgments
We would like to thank the Centre for Medicinal Cannabis (CMC) for funding the purchase of the CBD products and the independent testing. We would also like to thank PhytoVista Laboratories for providing the analytical testing at discounted rates and its assistance in the description and analysis of the technical laboratory data of this report.
Abbreviations Used
- CB1
cannabinoid binding receptor 1
- CBD
cannabidiol
- CBDA
cannabidiolic acid
- CBDV
cannabidivarin
- CBDVA
cannabidivarin acid
- CBMPs
cannabis-based medicinal products
- CBN
cannabinol
- EMEA
European Medicines Agency
- FDA
Food and Drug Administration
- GPR55
G protein-coupled receptor 55
- RSD
relative standard deviation
- SD
standard deviation
- THC
Δ9-tetrahydrocannabinol
Author Disclosure Statement
S.E.O. is a scientific advisor for Artelo Biosciences, CBDScience Group, Dragonfly Biosciences, and FSD Pharma, but none of these companies was involved in the funding, analysis, or interpretation of these data. A.S.Y. is a scientific advisor for Artelo Biosciences and Dragonfly Biosciences, but neither of these companies was involved in the funding, analysis, or interpretation of these data.
Funding Information
The study was funded by the CMC. PhytoVista Laboratories provided the analytical testing at discounted rates.
Supplementary Material
Cite this article as: Liebling JP, Clarkson NJ, Gibbs BW, Yates AS, O'Sullivan SE (2022) An analysis of over-the-counter cannabidiol products in the United Kingdom, Cannabis and Cannabinoid Research 7:2, 207–213, DOI: 10.1089/can.2019.0078.
References
- 1. Huestis MA, Solimini R, Pichini S, et al. Cannabidiol adverse effects and toxicity. Curr Neuropharmacol. 2019;17:974–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Crippa JA, Guimarães FS, Campos AC, et al. Translational investigation of the therapeutic potential of cannabidiol (CBD): toward a new age. Front Immunol. 2018;9:2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ibeas Bih C, Chen T, Nunn AV, et al. Molecular targets of cannabidiol in neurological disorders. Neurotherapeutics. 2015;12:699–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pistis M, O'Sullivan SE. The role of nuclear hormone receptors in cannabinoid function. Adv Pharmacol. 2017;80:291–328. [DOI] [PubMed] [Google Scholar]
- 5. Muller C, Morales P, Reggio PH. Cannabinoid ligands targeting TRP channels. Front Mol Neurosci. 2018;11:487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Laun AS, Shrader SH, Brown KJ, et al. GPR3, GPR6, and GPR12 as novel molecular targets: their biological functions and interaction with cannabidiol. Acta Pharmacol Sin. 2019;40:300–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Corroon J, Phillips JA. A cross-sectional study of cannabidiol users. Cannabis Cannabinoid Res. 2018;3:152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bonn-Miller MO, Loflin MJE, Thomas BF, et al. Labeling accuracy of cannabidiol extracts sold online. JAMA 2017;318:1708–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hazekamp A. The trouble with CBD oil. Medical Cannabis and Cannabinoids. 2018;1:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pavlovic R, Nenna G, Calvi L, et al. Quality traits of “Cannabidiol Oils”: cannabinoids content, terpene fingerprint and oxidation stability of European commercially available preparations. Molecules. 2018;23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Poklis JL, Mulder HA, Peace MR. The unexpected identification of the cannabimimetic, 5F-ADB, and dextromethorphan in commercially available cannabidiol e-liquids. Forensic Sci Int. 2019;294:e25–e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Horth RZ, Crouch B, Horowitz BZ, et al. Notes from the field: acute poisonings from a synthetic cannabinoid sold as cannabidiol—Utah, 2017–2018. MMWR Morb Mortal Wkly Rep. 2018;67:587–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Millar SA, Stone NL, Bellman ZD, et al. A systematic review of cannabidiol dosing in clinical populations. Br J Clin Pharmacol. 2019;85:1888–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Millar SA, Stone NL, Yates AS, et al. A systematic review on the pharmacokinetics of cannabidiol in humans. Front Pharmacol. 2018;9:1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Amada N, Yamasaki Y, Williams CM, et al. Cannabidivarin (CBDV) suppresses pentylenetetrazole (PTZ)-induced increases in epilepsy-related gene expression. PeerJ. 2013;1:e214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rock EM, Connolly C, Limebeer CL, et al. Effect of combined oral doses of delta(9)-tetrahydrocannabinol (THC) and cannabidiolic acid (CBDA) on acute and anticipatory nausea in rat models. Psychopharmacology (Berl). 2016;233:3353–3360. [DOI] [PubMed] [Google Scholar]
- 17. Brierley DI, Harman JR, Giallourou N, et al. Chemotherapy-induced cachexia dysregulates hypothalamic and systemic lipoamines and is attenuated by cannabigerol. J Cachexia Sarcopenia Muscle. 2019;10:844–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rodriguez-Cueto C, Santos-García I, García-Toscano L, et al. Neuroprotective effects of the cannabigerol quinone derivative VCE-003.2 in SOD1(G93A) transgenic mice, an experimental model of amyotrophic lateral sclerosis. Biochem Pharmacol. 2018;157:217–226. [DOI] [PubMed] [Google Scholar]
- 19. Aguareles J, Paraíso-Luna J, Palomares B, et al. Oral administration of the cannabigerol derivative VCE-003.2 promotes subventricular zone neurogenesis and protects against mutant huntingtin-induced neurodegeneration. Transl Neurodegener. 2019;8:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Romano B, Borrelli F, Fasolino I, et al. The cannabinoid TRPA1 agonist cannabichromene inhibits nitric oxide production in macrophages and ameliorates murine colitis. Br J Pharmacol. 2013;169:213–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Borrelli F, Pagano E, Romano B, et al. Colon carcinogenesis is inhibited by the TRPM8 antagonist cannabigerol, a Cannabis-derived non-psychotropic cannabinoid. Carcinogenesis. 2014;35:2787–2797. [DOI] [PubMed] [Google Scholar]
- 22. Wong H, Cairns BE. Cannabidiol, cannabinol and their combinations act as peripheral analgesics in a rat model of myofascial pain. Arch Oral Biol. 2019;104:33–39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.