ABSTRACT
The pathways of gametogenesis encompass elaborate cellular specialization accompanied by precise partitioning of the genome content in order to produce fully matured spermatozoa and oocytes. Transcription factors are an important class of molecules that function in gametogenesis to regulate intrinsic gene expression programs, play essential roles in specifying (or determining) germ cell fate and assist in guiding full maturation of germ cells and maintenance of their populations. Moreover, in order to reinforce or redirect cell fate in vitro, it is transcription factors that are most frequently induced, over-expressed or activated. Many reviews have focused on the molecular development and genetics of gametogenesis, in vivo and in vitro, in model organisms and in humans, including several recent comprehensive reviews: here, we focus specifically on the role of transcription factors. Recent advances in stem cell biology and multi-omic studies have enabled deeper investigation into the unique transcriptional mechanisms of human reproductive development. Moreover, as methods continually improve, in vitro differentiation of germ cells can provide the platform for robust gain- and loss-of-function genetic analyses. These analyses are delineating unique and shared human germ cell transcriptional network components that, together with somatic lineage specifiers and pluripotency transcription factors, function in transitions from pluripotent stem cells to gametes. This grand theme review offers additional insight into human infertility and reproductive disorders that are linked predominantly to defects in the transcription factor networks and thus may potentially contribute to the development of novel treatments for infertility.
Keywords: gametogenesis, transcription factors, infertility, germ cell, germ cell tumors, gene mutations, transcriptional profiling, single-cell RNA-sequencing, pluripotent stem cells, in vitro differentiation
Introduction
Human embryo development, like that of other organisms, is characterized by a series of cell-fate transitions from one cell type to another, starting from pluripotent stem cells (PSCs) and progressively specifying different lineages including extra-embryonic tissues, germ cell and somatic cell lineages. The primordial germ cells (PGCs) arise early in development as a small group of embryonic cells that will ultimately give rise to sperm and oocytes, and pass on genetic information to subsequent generations (Waters and Trainer, 1996; Donovan, 1998; Tang et al., 2016; Kobayashi and Surani, 2018). The correct functioning of lineage specification is obviously critical; dysfunction during gametogenesis may lead to defects in germ cell development and/or function underlying diverse genetic fertility syndromes (Krausz and Riera-Escamilla, 2018; Xavier et al., 2021). In this review, we use the term ‘specification of cell fate or identity’ in reference to when a cell is committed to differentiate down a specific pathway if left in its normal environment.
Germ cell development is dependent on the regulators of gene expression that function at multiple levels, including transcription factors that orchestrate expression at the transcriptional level by binding to enhancer or promoter regions of target genes. Following embryonic genome activation, a series of transcription factors sequentially regulates the activity of a host of genes involved in cell fate decisions, including PGC specification and migration, sex determination, meiosis and germ cell maturation. Concurrently, developmentally regulated protein expression is also proceeding with coordination by RNA-binding proteins, beginning at fertilization with the translation of maternally inherited mRNA and continuing throughout germ cell development, as evidenced by the number of RNA-binding proteins defined as markers of late stages of germ cell lineages (Clark and Reijo Pera, 2006; Makar and Sasaki, 2020).
PGCs exhibit many properties of classic pluripotent cells, including the property of pluripotency itself, and yet they are committed to the germ cell lineage (Kuijk et al., 2011). The prime example or archetype of a pluripotent cell type, namely embryonic stem cells (ESCs), maintain their undifferentiated state via the activity of a defined set of transcription factors, coordinately regulating those genes necessary for reinforcing the pluripotent state, and suppressing lineage-specific genes that would otherwise drive differentiation (Kim et al., 2008; Niwa, 2009; Ng and Surani, 2011). PGCs appear to employ a subset of members of this set of genes while also adopting a distinct subset or circuitry of transcription factors to define their identity and complete three crucial developmental events: repress somatic programs; reacquire pluripotency; and reprogram genome-wide epigenetics. For example, although human PGCs (hPGCs) are committed to the germ cell lineage, they share expression of a subset of pluripotency genes with human ESCs (hESCs), notably OCT4 (also known as POU5F1, POU class 5 homeobox 1) and NANOG (Kehler et al., 2004; Hoei-Hansen et al., 2005); however, other key pluripotency genes, such as SOX2 (SRY-related HMG box-containing gene 2), are not expressed in hPGCs (Perrett et al., 2008). Co-expression of pluripotency transcription factors, as well as lineage specifiers, distinguishes hPGCs from all other human embryonic cell types as well as mouse PGCs (mPGCs) (Tang et al., 2015). To maintain cell identity, hPGCs likely require a precise regulation/balance of pluripotency-related and lineage-specific transcription factors to repress somatic differentiation and concurrently activate germ cell programs.
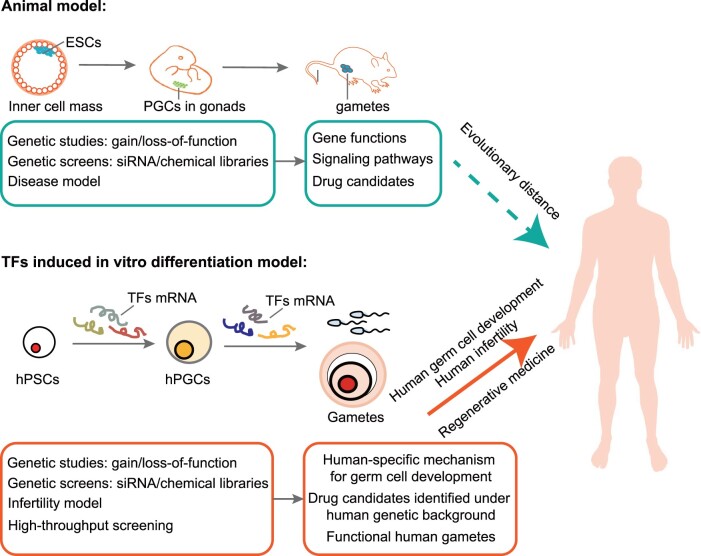
A continuum of in vivo and in vitro models, based on human, mouse and non-human primate cells, has been explored and leveraged to study germ cell development, including the formation of PGCs, and their specification from PSCs or ESCs (Li et al., 2020; Saitou and Hayashi, 2021). While mouse models are extraordinarily useful given their genetic malleability and ability to probe in vivo development of engineered cells, the genetics of germ cell development has both similarities and differences between species (Sasaki et al., 2016; Kojima et al., 2017; Stirparo et al., 2018). For example, efforts to define a core set of transcription factors sufficient for PGC specification have succeeded in driving or even actively directing mouse cells further down the germ cell lineage than what has been achieved in human cell models (Niwa, 2009; Magnúsdóttir et al., 2013). Indeed, it is likely that the microenvironment of the mouse gonad provides as yet undefined signals to induce germ cell differentiation of PGCs; moreover, xenotransplantation and co-culture with somatic cells have provided a superior microenvironment for further development of in vitro-derived PGC-like cells (Dominguez et al., 2014; Durruthy Durruthy et al., 2014; Ramathal et al., 2014). Finally, recent analyses of bona fide germ cells in developing human embryos have provided insight into transcription factor expression as well as their interactions and functions during development (Otte et al., 2017; Wen and Tang, 2019; Estermann and Smith, 2020; Li et al., 2020; La et al., 2021). Further analyses of these experiments are likely to add to our library of transcription factors potentially required for later stages of PGC function and germ cell development.
Methods
PubMed database was used to search articles and reviews with the following main keywords: human gametogenesis; transcription factors; infertility; germ cell; germ cell tumors; infertility; gene mutations; single-cell RNA-sequencing; pluripotent stem cells; in vitro differentiation; and other key terms related to these subjects. The search period included all publications until now (November 2021).
An overview of human gametogenesis
A number of reviews have contrasted, analyzed and discussed gametogenesis across species including humans. Two excellent recent examples are the reviews of Li et al. (2020) and that of Saitou and Hayashi (2021). In these reviews, in vivo and in vitro development are compared and contrasted, and differences between the processes across species are also highlighted. Here, we briefly provide an overview of human gametogenesis that distills details in specification, migration, sex determination and male- and female-specific development and then we focus on transcription factors and their functions and associated pathologies. Recent reviews and this work largely concur on major aspects while providing different content; this is indicative of the field of gametogenesis in vivo and in vitro maturing toward a common set of foundational developmental and genetic principles.
Human germ cell specification
In vivo, hPGCs are first identified in the posterior region of the yolk sac, and begin to migrate to the genital ridge about 4 weeks post-conception (McKay et al., 1953; Motta et al., 1997; Culty, 2009; Leitch et al., 2013). Data from studies in the mouse indicate that signaling via bone morphogenetic proteins (BMPs) released from the extraembryonic ectoderm and proximal endoderm, including BMP4, BMP8b and BMP2, is essential for PGC specification (Lawson et al., 1999; Ohinata et al., 2009). Analysis of human fetal ovary also demonstrates that the expression of BMP2 and BMP4 may regulate the survival and migration of hPGCs (Childs et al., 2010). In addition to BMPs, WNT (Wingless-related integration site) signaling, which is an evolutionarily conserved pathway in embryonic development, is required to activate the expression of many transcription factors that are indispensable in the specification of PGCs (Aramaki et al., 2013). Finally, it is notable that a recent study of non-human primates demonstrates that cynomolgus monkey PGCs (cyPGCs) originate from the dorsal amnion instead of the posterior epiblast as seen in murine development (Sasaki et al., 2016), suggesting the potential for distinct environmental cues for primate PGC specification versus other mammals.
Human germ cell migration
Following specification, hPGCs gradually proliferate as they also gain motility and initiate migration at 4–5 weeks (Pereda et al., 2006; Mamsen et al., 2012; Gomes Fernandes et al., 2018). Despite significant differences in terms of migration rates and distances traveled, PGC migration in all species has conserved elements (Pereda et al., 1998; Richardson and Lehmann, 2010; Grimaldi and Raz, 2020) including: first, the acquisition of motility/initiation of migration; second, directed migration; and third, termination of migration at the developing gonad.
In terms of acquisition of motility and initiation of migration, once PGCs are specified, specific molecular pathways direct the detachment from neighboring cells and the extracellular matrix as a prerequisite to motility. For example, studies in different organisms indicate that downregulation of the cell–cell adhesion protein, E-cadherin, initiates the migration process of PGCs.
Directed migration is regulated by attractive and repulsive cues. Following initiation of migration, PGCs require cues for directionality. PGCs from different organisms migrate along different paths while interacting with diverse cell types and the extracellular matrix. Immunohistochemistry and electron microscopy studies suggest that hPGCs preferentially migrate along autonomic nerve fibers and Schwann cells from the dorsal hind gut mesentery to the developing gonad (Mollgard et al., 2010; Mamsen et al., 2012). The migration is accompanied by a wave of chemical cues expressed by the surrounding somatic cells. Appropriate migration and survival of PGCs are instructed by both an intrinsic transcriptional program and external guidance cues. Stem cell factor, lipids and c-KIT (receptor tyrosine kinase) as well as G protein-coupled receptor signaling are implicated as attractive guidance cues for PGC migration to the genital ridge (Molyneaux et al., 2003; Hoyer et al., 2005). Similar to mouse PGCs (Hayashi et al., 2007; Saitou and Yamaji, 2012), migratory hPGCs maintain a gene expression program characteristic of pluripotency, with sustained expression of pluripotency factors such as OCT4 and NANOG. hPGCs also maintain a broad developmental potential, retaining the capacity for both germ cell and somatic cell differentiation.
Concerning termination of migration at the developing gonad, although there is no evidence for sex-specific differences during PGC migration, once PGCs arrive at the target gonad, motility is lost as the PGCs acquire sex-specific properties to contribute to gonad formation with somatic cells. Studies in mouse PGC development indicate that a change in cell adhesion may play a role in reduced mobility (Bendel-Stenzel et al., 2000; Di Carlo and De Felici, 2000). However, the set of proteins responsible and their precise modes of action have yet to be identified and characterized in full. PGCs that fail to exit the nerve branches at the gonadal site may continue to migrate to other organs, such as the abdomen, adrenal glands, heart, lungs, and central nervous system. If they are not eliminated by apoptosis, these stray germ cells may give rise to germ cell tumors (Mamsen et al., 2012).
Sex determination
Upon arriving at the genital ridge, PGCs interact with somatic cells and form the bipotential gonads. Sex determination of the gonad is a process by which the bipotential gonads differentiate into either testes or ovaries at gestational weeks 6–7 onward (Baker, 1963; Jorgensen et al., 2012). Interestingly, sex determination of germ cells is dependent on external signals from the somatic environment rather than solely on the sex chromosome composition (XX or XY). Studies in mouse models confirmed this mechanism by demonstrating that XY germ cells can develop into oocytes in female chimeric embryos and XX germ cells can develop into prospermatogonia in male chimeric embryos (Ford et al., 1975; Burgoyne et al., 1988; Palmer and Burgoyne, 1991; Patek et al., 1991). In the XX testis, the XX germ cells enter spermatogenesis and become prospermatogonia; however, they are eliminated before differentiation into spermatogonia. In the XY ovary, the XY germ cells enter meiosis and continue to differentiate as the primary oocytes; however, their fertility depends on species, genetic background and causes of sex reversal (Taketo-Hosotani et al., 1989; Heard and Turner, 2011). The developmental fate of the bipotential gonad is dependent on a delicate balance of pro-testis and pro-ovary pathways in the supporting somatic cell lineage. To initiate male differentiation to the testis, the pro-testis pathway, characterized by the SRY (Sex-determining Region on the Y chromosome)-SOX9 (SRY-related HMG bOX-containing gene 9)-FGF9 (Fibroblast Growth Factor 9) gene network, needs to be activated to induce differentiation of the somatic cells into the male-specific Sertoli cells, and simultaneous repression of the ovarian pathway. In females, continuous activation of pro-ovary pathways, characterized by the RSPO1 (R-Spondin1)-WNT4-β-catenin signaling pathway, promotes differentiation of somatic cells to granulosa cells, leading to ovarian development. Once the somatic sex of the gonad is determined, sexual development of the rest of the embryo can progress. In males, the testes produce testosterone and anti-Müllerian hormone (AMH) to induce the formation of other organs in the male reproductive system and promote degeneration of the Müllerian duct. In females, the ovaries produce estrogen, which triggers development of the uterus, oviducts and cervix from the Müllerian duct. In response to somatic sex-determining cues, germ cells in female embryos initiate oogenesis and enter meiosis before birth. In contrast, male germ cells enter a mitotic arrest and do not enter meiosis until after birth.
Female germ cell development
Most of what we know of female germ cell development in vivo derives from studies in mice and rats with similarities observed in human fetal development, as well. After arriving at the genital ridge, female germ cells continue to proliferate through mitotic divisions with incomplete cytokinesis, to form oogonia cysts. In response to retinoic acid signals, oogonia cells then start meiosis and differentiate into primary oocytes (Bowles et al., 2006; Koubova et al., 2006). Meiosis initiates with prophase I stage, which is classically divided into five distinctive sub-stages based on the conformation of chromosomes: leptotene (prophase begins, chromosome start to condense), zygotene (synapsis begins), pachytene (crossing over), diplotene (synapsis ends) and diakinesis (prophase ends, nuclear membrane disintegrates). Primary oocytes arrest at the dictyate stage and become quiescent until sexual maturation. Around this time, the germ cell cyst breaks down, and the majority of oocytes that are not surrounded by somatic cells succumb to apoptosis and/or autophagy (Goldsmith, 1990; Pepling and Spradling, 2001; Escobar et al., 2010). Surviving oocytes are assembled into primordial follicles with pre-granulosa cells; the primordial follicles are the reservoir of germ cells for the entire female reproductive life. At birth in humans, there are approximately 400 000 primordial follicles, and this number gradually declines with age (Block, 1953; Forabosco et al., 1991; Gougeon, 1996). During a woman’s reproductive life, approximately 400 follicles will undergo ovulation. With the onset of puberty, oocyte meiotic maturation is initiated by hormone stimulation, particularly by LH signaling molecules (Mehlmann, 2005). LH releases oocytes from meiotic prophase arrest and induces them to complete the first meiotic division and produce the first polar body. The second meiotic division begins immediately but pauses at metaphase, where the oocyte remains arrested until fertilization. The second meiotic division is triggered by the penetration of the sperm, and the second polar body will be formed at the same time.
Male germ cell development
Upon arriving at the genital ridge of a male embryo, male fetal germ cells (FGCs) will not enter meiosis en masse. Instead, at this stage in normal testis development, somatic cells and FGCs begin to differentiate into seminiferous tubules with germs cells in the center and Sertoli cells at the periphery (Wilhelm et al., 2007). Somatic cells will provide the niche for developing FGCs. Spermatogenesis starts in early puberty, and it is a continuous cellular differentiation process that can be classified into four distinctive stages:
Mitotic proliferation and maturation to generate spermatogonia (SPG). Spermatogonia are composed of three subtypes of cells: Type A (dark) cells (spermatogonia stem cells (SSCs) that do not undergo active mitosis), Type A (pale) cells (SSC that undergo active mitosis and divide to produce Type B cells), and Type B cells, which undergo growth and become spermatocytes.
Two rounds of meiotic division to form haploid spermatocytes (SPC).
Morphological transformation of spherical SPCs to elongated spermatids (SPT), a process also referred to as spermiogenesis.
Final maturation of SPT to spermatozoa and release into the lumen of the seminiferous tubules, with the sperm passing through the epididymis to undergo final maturation (Clermont, 1972).
These four processes are interdependent and regulated by the somatic niche of the seminiferous tubules that is composed of three major cell types: Sertoli, peritubular and Leydig cells. It is estimated that the entire process of human spermatogenesis takes about 74 days (Heller and Clermont, 1964; Amann, 2008).
Intrinsic expression pattern of transcription factors in bona fide developing germ cells
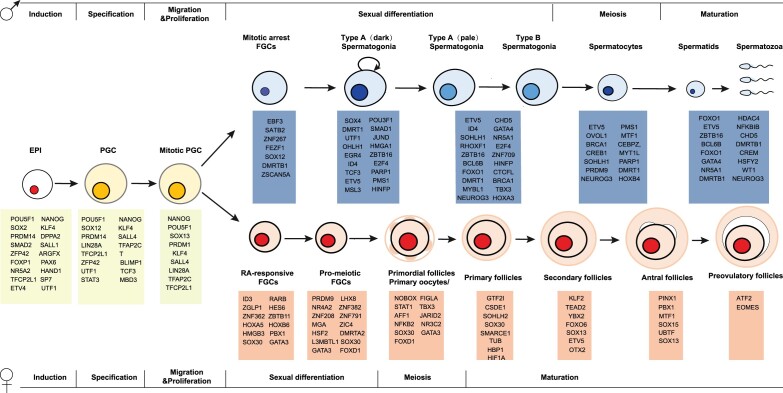
Considering the complexity of the development pathways of germ cells in humans and the relation to the processes outlined above, it is clear that given the rarity of germ cells in developing human embryos and the poor resolution of germ cell isolation methods, it is not possible to profile development and gene expression patterns at all stages. In addition, bulk RNA-seq or microarray analysis cannot resolve the heterogeneity within germ cells, which is essential for understanding the precise trajectory in which development occurs (Raser and O'Shea, 2005; Plass et al., 2018). Transcriptome profiling at the single-cell level (i.e. single-cell RNA-sequencing: scRNA-seq) has been used to overcome this limitation by comprehensively measuring mRNA levels within all individual germ cells at a given developmental stage, and has been applied to diverse biological systems to begin to explore the potential molecular mechanisms for development (Junker and van Oudenaarden, 2014; Raj et al., 2018; Genga et al., 2019; Han et al., 2020). Since 2013, a handful of reports have characterized the transcriptional dynamics during human germ cell development by analyzing human fetal and adult tissues using scRNA-seq. These studies were recently reviewed (Li et al., 2020). Here, we focus on transcription factors that potentially act as master regulators to activate the unique gene expression program for each specific stage of germ cells (Fig. 1; Table I). We note that expression of a gene does not imply function; moreover, it is highly likely that genes are expressed at stages other than those that have been assayed and/or only briefly during development.
Figure 1.
Transcription factors expressed/upregulated in developing human germ cells identified by scRNA-seq. EPI, epiblast; FGC, fetal germ cells; PGC, primordial germ cells; scRNA-seq, single-cell RNA-sequencing.
Table I.
Transcription factors expressed in developing human germ cells, determined by single-cell RNA-sequencing.
| Developmental stage | Cell types analyzed | Transcription factors | Indicated functions of gene products in human reproduction or development | References |
|---|---|---|---|---|
| EPI | Human EPI, hESCs | ESRRB, KLF17, KLF4, KLF5, SOX2, NANOG, ZFP57, FOXP1 |
|
Yan et al. (2013) |
| Human preimplantation Embryos | ESRRB, NANOG, POU5F1, SOX2, PRDM14, NR5A2, TFCP2L1, KLF17, SMAD2, SMAD4, ETV4 |
|
Blakeley et al. (2015) | |
| Human embryonic cells | ARGFX, PRDM14, SOX2, NANOG, KLF17 |
|
Petropoulos et al. (2016) | |
| Human pre-implantation embryos | PRDM14, TFCP2L1, ZFP42, ARGFX, ESRRB, DPPA2 |
|
Stirparo et al. (2018) | |
| Human preimplantation embryos | NANOG, PRDM14, SOX2, SOX21, SALL1, HAND1, SP7, PAX6, UTF1, ELF5 |
|
Zhou et al. (2019) | |
| PGC | Human 4–19 weeks of gestation (WG) PGCs; gonadal somatic cells |
|
|
Guo et al. (2015)
Li et al. (2017) |
| Human prenatal germline cells | SOX17, SOX12, KLF6, LEF1 |
|
Gkountela et al. (2015) | |
| Wk4–Wk9 human embryos | NANOG, OCT4, KLF4, TFCP2L1, T, SOX17, TFAP2C, BLIMP1, UTF1, PRDM14 | KLF2: activates cell transcription | Tang et al. (2015) | |
| Human fetal tissues from first and second trimester | NANOG, POU5F1, SOX4 | Vértesy et al. (2018) | ||
| PCG (cont.) | Prenatal gonads from 4 to 16 weeks post- fertilization | PGC: POU5F1, NANOG, PRDM1, SOX17, TFAP2C | Chitiashvili et al. (2020) | |
| Male | SSEA4+hSSCs and c-KIT+spermatogonia from whole adult human testis |
|
|
Guo et al. (2017) |
| Spermatogenic cells from immature and adult male mice and adult men |
|
RHOXF1: maybe involved in reproductive processes. Modulates expression of target genes encoding proteins involved in processes relevant to spermatogenesis. | Hermann et al. (2018) | |
| Testicular cells from donors with normal spermatogenesis and one with non-obstructive azoospermia (NOA) |
|
OVOL2: plays a critical role in maintaining the identity of epithelial lineages by suppressing epithelial-to mesenchymal transition | Wang et al. (2018) | |
| Testicular cells from there healthy donors: 17,24 and 25 years old |
|
|
Guo et al. (2018) | |
| Testicular samples from obstructive azoospermia or non-obstructive hypergonadotropic azoospermia | SPG: RHOXF1 | RHOXF1: the encoded protein is likely a DNA-binding transcription factor that may play a role in human reproduction. | Laurentino et al., (2019) | |
| Neonatal and adult human testicular cells |
|
|
Sohni et al. (2019) | |
| Pre- and peri-pubertal human testicular samples were obtained from four healthy boys aged 7, 11, 13 and 14 years |
|
PRDM9: mutations in PRDM9 may cause idiopathic infertility in human males. Expressed highest in testis. | Guo et al., (2020) | |
| Human testis tissues from 3 embryonic stages, 3 fetal stages and 1 young infant stage |
|
MSL3: plays a role in chromatin remodeling, in X inactivation and transcriptional regulation. | Guo et al. (2021) | |
| Female | Human 4-26-week fetal germ cells |
|
|
Li et al. (2017) |
| Human fresh ovarian tissues from 7 female donors ranging from 24 to 32 years (yr), with a median age of 28 yr |
|
|
Zhang et al. (2018) | |
| Ovarian cortex samples from 21 patients | Oocytes: FIGLA, PRDM1 | FIGLA: involved in continued oocyte survival as primordial follicles form in the human. | Wagner et al. (2020) | |
| In vivo and In vitro matured human metaphase II (MII) oocytes | Oocytes: FIGLA, SOHLH2 | SOHLH2: involved in follicle development, initiation of primordial follicle growth, primary follicle growth, and germ cell development. | Ye et al. (2020) |
EPI, epiblast; FGC, fetal germ cells; hESCs, human embryonic stem cells; PGC, primordial germ cells; PSC, pluripotent stem cells; SPG, spermatogonia; SSC, spermatogonia stem cells; WNT, Wingless-related integration site.
Transcription factors upregulated in germ cell specification and migratory hPGCs
The gene expression patterns of migrating and mitotic PGCs are similar in male and female germ cells. There is continued expression of transcription factors associated with pluripotency and ESCs, such as POU5F1/OCT4, NANOG and PRDM14 (PR/SET domain 14) (Guo et al., 2015), although at different levels relative to pre-implantation epiblasts (EPI) (Yan et al., 2013; Blakeley et al., 2015; Guo et al., 2015; Petropoulos et al., 2016; Li et al., 2017; Stirparo et al., 2018; Zhou et al., 2019). Concurrently, however, transcription factors that are diagnostic of germline cells, such as PRDM1(PR/SET domain 1) and TFAP2C (Transcription Factor AP-2 gamma), and somatic lineages, such as BRACHYURY (T) and EOMES (eomesodermin), are also expressed in the same cells (Guo et al., 2015; Tang et al., 2015). A recent finding has also demonstrated that during hPGC specification, the classic endodermal transcription factor marker protein, SOX17 (SRY-related HMG box-containing gene 17), is required for hPGC commitment in an in vitro model of hPGC differentiation (Irie et al., 2015). Moreover, scRNA-seq data of human gonadal PGCs in vivo confirmed the presence of SOX17 in early migrating and mitotic PGCs, consistent with its essential role in hPGC function (Guo et al., 2015).
Transcription factors upregulated during male sex determination
To shed light on the critical transcriptional regulation in sex determination, scRNA-seq analyses were performed on both germ cells and their gonadal niche cells in multiple studies (Guo et al., 2015; Li et al., 2017; ChitiashviLi et al., 2020; Guo et al., 2021; Zhao et al., 2021). Male sex determination initiates with activation of the Y chromosome-specific transcription factor, SRY, a dominant determinant for testis differentiation (Berta et al., 1990; Gubbay et al., 1990; Koopman et al., 1990; Sinclair et al., 1990; Kashimada and Koopman, 2010). Transcription factors that regulate SRY function and have been shown likely to be required for male sex determination include WT1(Wilms’ tumor gene), NR5A1(nuclear receptor subfamily 5 group A member 1), GATA4(GATA binding protein 4), FOG2(FOG family member 2) and CBX2 (chromobox2) (Sekido and Lovell-Badge, 2008). Once SRY is activated, it acts by upregulating the expression of SOX9, which then activates a cascade including AMH, prostaglandins and steroidogenic genes, to promote complete organogenesis of the testis in humans and suppress the pro-ovary pathways (Koopman, 2001; Kozhukhar, 2012). SRY is also a direct target of the WT1. WT1 is a zinc finger containing DNA-binding protein that activates the expression of SRY in the initial sex determination process in humans (Shimamura et al., 1997; Hossain and Saunders, 2001; Matsuzawa-Watanabe et al., 2003). Other transcription factors that are essential for early testis differentiation include NR5A1, a highly conserved nuclear receptor transcription factor that interacts with SRY to regulate SOX9 expression during the differentiation of Sertoli cells (Sekido and Lovell-Badge, 2008; Rotgers et al., 2018; Stevant and Nef, 2019). GATA4, a zinc finger transcription factor, also cooperatively interacts with NR5A1 to regulate downstream genes critical for testis differentiation (Viger et al., 2008). Similarly, FOG2, a zinc finger cofactor, is suggested to be involved in testis determination through interaction with Gata4, potentially by modulating the activity of GATA4, and regulating the expression of SRY and SOX9 (Zaytouni et al., 2011). CBX2, a component of the polycomb group (PcG) complex of regulatory proteins, has been reported to act in testis determination by activating the expression of NR5A1 and SRY and repressing genes involved in fetal ovarian development (Biason-Lauber et al., 2009). Transcription factors belonging to the doublesex and mab-3 related transcription factor (DMRT) family, including DMRT1, 2 and 3, are found to be evolutionarily conserved sex-determining transcription factors. DMRT1 is a male-specific transcription factor gene which functions at multiple stages during male germ cell and Sertoli cell development to support spermatogonial development by antagonizing FOXL2 (forkhead box L2) activity and repressing the oogenesis program (Matson et al., 2011). Mouse models found that DMRT1-mutant mice fail to develop functional testes, and continued expression of DMRT1 is necessary to prevent female reprogramming in the postnatal testis (Matson et al., 2011). Mutation of DMRT transcription factors causes abnormal testicular formation and feminization (Ottolenghi and McElreavey, 2000).
Transcription factors upregulated during female sex determination
Female sex determination is regulated by transcription factors associated with RSPO1-WNT4-β-catenin signaling pathways. FOXL2 is considered a gatekeeper transcription factor for ovarian identity (Uhlenhaut et al., 2009; Pannetier and Pailhoux, 2010) and promotes ovary development by blocking testis development through transcriptional repression of SOX9 (Crisponi et al., 2001; De Baere et al., 2002; Udar et al., 2003; Nallathambi et al., 2007; Hersmus et al., 2008; Shah et al., 2009; Auguste et al., 2011). Consistent with its critical role in ovarian cell function, somatic mutations in FOXL2 are found in nearly all cases of adult granulosa cell tumors of the ovary (Jamieson and Fuller, 2012). Other genes, such as NR5A1, may regulate anti-testis gene expression in the ovary; in 46, XX individuals, NR5A1 synergizes with β-catenin to upregulate the expression of anti-testis genes (e.g. DAX1/NR0B1(Dosage-sensitive sex reversal-Adrenal hypoplasia congenita critical region on the X chromosome, gene 1)) and possibly pro-ovarian genes (Gummow et al., 2003; Hossain and Saunders, 2003; Jordan et al., 2003; Mizusaki et al., 2003).
Transcription factors upregulated in male germ cell development
Upon arriving at the genital ridge of a male embryo, germ cells arrest mitotically and transcription factors involved in cell cycle arrest, such as EBF3 (EBF transcription factor 3), are specifically upregulated (Guo et al., 2015). Several groups have profiled the transcriptional trajectory across the entire spectrum of human adult spermatogenesis. Transcription factors mainly involved in repressing gene expression (e.g. E2F4 (E2F transcription factor 4), HMGA1 (high mobility group AT-hook 1)) are enriched in SSCs, consistent with their slow proliferation rate. After progressing to the differentiating SPG, cell cycle activation-associated genes, such as KIT and KI67, are significantly upregulated to ensure active proliferation and differentiation. Later, transcription factors involved in meiotic sex chromosome inactivation, homolog synapsis and meiotic recombination, such as OVOL1 (Ovo like transcription repressor 1), SOHLH1 (spermatogenesis and oogenesis specific basic helix-loop-helix 1) and DMRT1, are upregulated to initiate the meiotic gene expression program (Guo et al., 2017, 2018; Hermann et al., 2018; Wang et al., 2018; Sohni et al., 2019). As SPC complete their differentiation into SPT, nearly all these transcription factors are downregulated as the overall level of transcription gradually declines (Wang et al, 2018), with the exception of CHD5 (chromodomain helicase DNA binding protein 5), which is highly enriched in early SPT (Wang et al., 2018). This is probably because of its involvement in the process of condensation of spermatid chromatin by regulating histone hyperacetylation and the replacement of histones by transition proteins in chromatin (Li et al., 2014).
Transcription factors upregulated in female germ cell development
After arriving at the genital ridge, female germ cells rapidly lose expression of pluripotency transcription factors, for example, POU5F1/OCT4 (Rajpert-De Meyts et al., 2004; Stoop et al., 2005). Oogonia cells then undergo three sequential stages instructed by stage-specific transcription factors to generate fertilization-competent oocytes: the retinoic acid-responsive stage, the meiotic prophase stage and the folliculogenesis stage. Li et al. provided a thorough study to identify master transcription factors for germ cell development in the fetal stage (Li et al., 2017) using the ARACNe (algorithm for the reconstruction of accurate cellular networks) algorithm. ARACNe identifies master regulators of development by correlation of expression of transcription factors and their target genes across various cell types. Their analyses indicate that ZNF208 (ZiNc Finger protein 208), YBX1 (Y-BoX-binding protein 1) and ZNF791 might be critical for the female mitotic phase, whereas HES6 (HES family BHLH transcription factor 6), MAEL (Maelstrom spermatogenic transposon silencer 6), ZGLP1 (Zinc finger GATA-Like protein 1), ZNF362, ZBTB11 (ZiNc Finger and BTB domain containing 11), HOXA5 (HomeobOX A5), HOXB6, HMGB3 (High Mobility Group box3) and PBX1 (PBX homeobox 1) are the potential transcriptional regulators in the retinoic acid-responsive phase. Meiotic recombination transcription factor proteins LHX8 (LIM homeobox 8), together with NR4A2, ZNF382, MGA (MAX dimerization protein), RLF (RLF zinc finger), ZIC4 (Zic Family Member 4), PAXBP1 (PAX3 and PAX7 binding protein), HSF2 (heat shock transcription factor 2), DMRTA2 (DMRT like family A2) and L3MBTL1 (L3MBTL histone methyl-lysine binding protein 1), are implicated in shaping the gene expression program for meiosis in the meiotic prophase (Guo et al., 2015). Then cells start to express master transcriptional regulators, such as NOBOX (NOBOX oogenesis homeobox) and FIGLA (factor in germline alpha, also known as FIGLα or FIGα), to initiate the unique transcription network for folliculogenesis (Li et al., 2017; Wagner et al., 2020; Ye et al., 2020). Human folliculogenesis is a complex process comprising five key stages (primordial, primary, secondary, antral and preovulatory follicles). The development of follicles is considered to be associated with highly dynamic transcriptional regulation (Aquila and De Amicis, 2014). Zhang et al., explored the dynamic transcriptomes of the human oocyte, together with the neighboring granulosa cells across the entire process of follicular development, and identified potential master transcription factors for each stage using the ARACNe algorithm (Zhang et al., 2018). Interestingly, once cells begin follicular development, the DNA methyltransferases DNMT1, DNMT3A and DNMT3B are highly expressed at all stages of oocyte development, suggesting that maintaining a high level of DNA methylation is essential for oocyte maturation.
Transcription factor mutations associated with human infertility
Despite enormous progress in human reproductive physiology, the underlying causes of diverse reproductive diseases, especially infertility, remains obscure. However, whole-exon sequencing or whole-genome sequencing analyses has identified thousands of gene mutations or variants that may be related to human infertility. These results suggest that most human reproductive diseases that were previously categorized as idiopathic may be of genetic origin. We have summarized mutations that were identified within transcription factors associated with human reproductive diseases in Table II.
Table II.
Transcription factor mutations reported to be associated with human infertility.
| Disease | Associated transcription factors | Description | References |
|---|---|---|---|
| Disorders of sex development | |||
| Swyer syndrome | SRY | Mutations in the SRY gene are the cause of 15% to 20% of cases of Swyer syndrome. | Arboleda et al. (2014); Baxter and Vilain (2013) |
| Sex reversal |
|
Copy number variants or mutations in the regulatory regions of the genes lead to human sex reversal. | |
| Denys-Drash syndrome | WT1 | Heterozygous mutations in the zinc finger domain of WT1 gene cause Denys-Drash syndrome. | Pelletier et al. (1991) |
| Frasier syndrome | WT1 | A mutation in a splice donor site in WT1 leads to Frasier syndrome. | Klamt et al. (1998); |
| Gonadal dysgenesis |
|
Mutations in these transcription factors are associated with gonadal dysgenesis. | El-Khairi and Achermann (2012) |
| Cryptorchidism | HOXD13, SOX2, ESR1, NR5A1, ZNF214, ZNF215, ARX | Single gene mutations are associated with cryptorchidism. | Tannour-Louet et al. (2010) |
| Male infertility | |||
| NOA | DMRT1, PRDM9, ESR2, AR, KDM5D, NR0B1, NR5A1, SOX9, NPAS2, PGR | The paper screened OMIM database and identified genes related to human male infertility- and NOA - | Wang et al. (2018) |
| NOA | SOHLH1 | SOHLH1 mutations are associated with loss of testicular reproductive capacity. | Nakamura et al. (2017) |
| NOA | SOX8 | SOX8 mutations were found at increased frequency in oligozoospermic men as compared with fertile/normospermic control populations. | |
| SCOS, MA | YBX1, YBX2 | YBX1 and YBX2 protein was markedly downregulated in SCOS and MA samples. | Alikhani et al. (2017) |
| Female infertility | |||
| POI* | FOXL2 | Foxl2 appears predominantly in the ovary and was first identified as mutated in a syndrome involving risk of POI. | |
| POI* | LHX8 | Preferentially expressed in germ cells and critical for mammalian oogenesis. | Qin et al. (2007) |
| POI* | FOXO3A, FOXO1A | Potentially causal mutations for POI. | |
| POI* | FIGLA | Two plausible mutations in the FIGLA gene were identified among 100 POI cases (2%), whereas none were present among 304 ethnically matched controls. | Zhao et al. (2008) |
| POI* | AIRE | Mutations in AIRE gene are likely cause polyglandular syndrome, which is associated with POI. | McLaren et al. (2003) |
| POI* | NOBOX | Homeobox mutation causes POI. | Qin et al. (2007) |
| POI* | SALL4 | Two novel variants (c.541G>A (p. Val181Met) and c. 2449A>G (p. Thr817Ala)) might be POI-associated gene variants. | Wang et al. (2009) |
| POI* | WT1 | Two novel heterozygous mutations p. P126S and p. R370H were identified to be involved in POI. | Wang et al. (2015) |
| POI* | ESR1 | ESR1 gene variants are associated with both age at natural menopause and premature ovarian failure. | |
| POI | TP63 | The combination of TP63 and BMP15 alterations contributes to the ovarian dysgenesis and early onset POI. | Bestetti et al. (2021) |
| POI | LHX8, NOBOX, FOXL2, SOHLH1, FIGLA | Combined functional and bibliographic analyses identified several novel or recurrent deleterious heterozygous mutations in POI patients. | Bouilly et al. (2016) |
MA, maturation arrest; NOA, non-obstructive azoospermia; POI, premature ovarian insufficiency (also known as premature ovarian failure); SCOS, Sertoli cell-only syndrome.
Transcription factor mutations associated with disorders of sex development
Disorders of sex development (DSD) are defined as congenital conditions with a mismatch between sex chromosomes and gonadal/anatomical sex. DSD are generally classified into three categories: Sex chromosomes DSD; 46, XX DSD; and 46, XY DSD. Sex chromosome DSDs include 45, X Turner Syndrome, 47, XX Y Klinefelter Syndrome and 45,X/46,XY gonadal dysgenesis.
46,XX DSD includes disorders of ovarian development and disorders of the synthesis of congenital adrenal hyperplasia. 46,XY DSD includes disorders of testicular development, defects in testosterone biosynthesis, and impaired testosterone action (Lee et al., 2006). The estimated frequency of DSD is approximately 1 in 2000–5500 newborns (Hughes et al., 2007), and the frequency is as high as 1:200 to 1:300 if all genital congenital anomalies, including cryptorchidism and hypospadias, are considered (Nordenvall et al., 2014). Genetic screening has identified many gene mutations associated with DSD, accounting for nearly 50% of the causality of cases; a few of the mutations are found in transcription factors, as described below.
SRY is the founding member of the SOX class of transcription factors, several of which play critical roles at multiple stages of germ cell development, including SOX8 (Portnoi et al., 2018), SOX9 (Vining et al., 2021) and SOX17 (Irie et al., 2015; Sybirna et al., 2019). DSD are most commonly associated with mutations in SRY gene or malfunction of the SRY protein (McElreavy et al., 1992). For example, mutations in the SRY gene are the cause of 15–20% of cases of Swyer syndrome, which is characterized by failure in the development of the sex glands (Baxter and Vilain, 2013; Arboleda et al., 2014). Mutations within the DNA-binding HMG-domain of SRY often lead to gonadal dysgenesis (McElreavey and Fellous, 1999).
SOX9 is a direct target of SRY and is essential for Sertoli cell development in testis formation. Copy number variants or mutation in non-coding regulatory regions upstream of the SOX9 gene lead to human sex reversal, including XY male to female DSD and XX female to male (Vetro et al., 2015; Gonen et al., 2018; Croft et al., 2018a,b).
SOX3 (SRY-related HMG box-containing gene 3) is a gene closely related to SRY and SOX9. Loss-of-function mutations of SOX3 gene are linked with mental retardation and growth hormone deficiency (Raymond et al., 1999; Laumonnier et al., 2002). De novo duplication of SOX3 gene or its upstream regulatory region has been reported in DSD 46, XX male sex reversal (Sutton et al., 2011; Moalem et al., 2012; Haines et al., 2015; Vetro et al., 2015; Grinspon et al., 2016).
WT1 is a zinc finger transcription factor known to be associated with kidney cancer. Heterozygous mutations in the zinc finger domain of WT1 gene cause Denys-Drash syndrome, characterized by renal failure and 46, XY gonadal dysgenesis. A mutation in a splice donor site in WT1, which results in the loss of a specific isoform of WT1, leads to Frasier syndrome, which is characterized by 46, XY gonadal dysgenesis (Pelletier et al., 1991; Klamt et al., 1998; Hossain and Saunders, 2001).
NR0B1/DAX1 (nuclear receptor subfamily 0, group B, member 1/DSS-AHC critical region of the X chromosome, gene1) encodes an orphan nuclear receptor. Duplication of DAX1 has been reported to be associated with 46, XY DSD (Baumstark et al., 1996; Sanlaville et al., 2004).
NR5A1 is associated with a wide range of reproductive anomalies, including 46, XY gonadal dysgenesis (El-Khairi and Achermann, 2012).
GATA4 is often linked to congenital heart defects. However, a recent study identified a familial case of a heterozygous mutation in the conserved N-terminal zinc finger domain of GATA4. Three of the family members present 46, XY DSD (Lourenco et al., 2011). A 35-kb deletion downstream of GATA4 was also discovered in a 46, XY complete gonadal dysgenesis patient with no evidence of heart disease (White et al., 2011).
FOG2 is suggested, by human sequencing analysis, to play roles in testis determination. Two cases of 46, XY gonadal dysgenesis, are reported to bear translocations that included the FOG2 locus on chromosome 8 (Finelli et al., 2007; Tan et al., 2012). Missense mutations in the FOG2 gene are also identified in two independent cases of 46, XY gonadal dysgenesis (Bashamboo et al., 2014).
CBX2 Presence of 46, XY gonadal dysgenesis in a girl is reported to be associated with loss-of-function mutations in the human CBX2 gene (Biason-Lauber et al., 2009).
DMRT1/2 deletion of chromosome 9 (9p), which contains DMRT1 and DMRT2 genes, is associated with 46, XY DSD. It is suggested that gonadal dysgenesis may result from the combined hemizygosity of DMRT1 and DMRT2 (Raymond et al., 1999; Ledig et al., 2012; Buonocore et al., 2019).
Transcription factor mutations associated with male infertility
Many male infertility syndromes result from large chromosomal deletions, translocations or aneuploidies, often involving the sex chromosomes. Klinefelter syndrome (karyotype: 47, XXY) is the most common chromosomal aberration, detected in up to 14% of infertile males with azoospermia. Characterization of deletions in the Y chromosome, which lead to male infertility, allowed identification of the founding member of the DAZ (deleted in azoospermia) family of RNA-binding genes required for spermatogenesis (Reijo et al., 1995). More recently, many genetic infertility syndromes have been associated with single-gene mutations, some of which are mentioned above in the context of their role in germ cell development. While mutations in any individual gene contribute to a small number of infertility cases, the overall importance of transcriptional regulation in the appropriate development of germ cell lineages is underscored by the number of these syndromes that are characterized by transcription factor mutations.
DMRT1 is infrequently mutated or deleted in patients with nonobstructive azoospermia (NOA) (Lopes et al., 2013; Tewes et al., 2014), defined as no sperm in the ejaculate owing to failure of spermatogenesis and the most severe form of male infertility.
DAX1/NR0B1 DAX1 mutations cause X-linked adrenal hypoplasia congenita and hypogonadotropic hypogonadism (Muscatelli et al., 1994; Zanaria et al., 1994; Jadhav et al., 2011), human syndromes which are characterized by hormonal imbalances leading to azoospermia. DAX1 mutations have also been identified in sporadic cases of NOA, with pathogenic mutations leading to impaired function of the protein (Wang et al., 2018).
NR5A1 regulates a large number of steroidogenic enzymes and other genes critical for male germ cell development. Mutations in NR5A1 are associated with several male infertility syndromes including cryptorchidism (Tannour-Louet et al., 2010), which is a condition in which one or both of the testes fail to descend from the abdomen into the scrotum. Characterization of the NR5A1 gene in infertile males found missense mutations in 1–4% of men with azoospermia to severe oligozoospermia. Oligozoospermia is characterized by low sperm count, usually defined as fewer than 15 million sperm per millilitre of semen.
SOHLH1 encodes a germ cell-specific transcription factor acting in both males and females that is required for spermatogonia differentiation, spermatocyte production and correct testis morphology in mouse models (Ballow et al., 2006; Barrios et al., 2012; Suzuki et al., 2012; Rossi, 2013; Toyoda et al., 2014), as well as oogenesis (Pangas et al., 2006; Toyoda et al., 2014; Shin et al., 2017). Mutations that are found in a subset of patients with NOA impair the transcriptional activity of SOHLH1 (Choi et al., 2010; Nakamura et al., 2017), likely contributing to the defect in normal spermatogenesis in these patients.
HSF2 encodes a testis-specific transcription factor required for spermatogenesis and seminiferous tubule formation in male mice (Wang et al., 2003, 2004). An investigation of HSF2 in patients with idiopathic azoospermia identified deleterious mutations in less than 1% of patients. However, one of these mutations caused not only loss-of-function of the transcriptional activity of the protein, but also a dominant-negative effect on the wild-type allele, underscoring a precise requirement for this pathway in spermatogenesis (Mou et al., 2013).
TAF4B (TATA box-binding protein-associated factor 4B) is predominantly expressed in the testis relative to other organs in the body. A non-sense mutation that results in truncated TAF4B proteins is identified as a disease locus in two unrelated consanguineous families suffering from azoospermia and oligozoospermia (Ayhan et al., 2014). The truncated protein has reduced DNA binding activity and weakened interaction with TAF12, which is essential for DNA binding at the core promoters of a subset of genes (Gazit et al., 2009).
ZMYND15 (zinc finger MYND-Type containing protein 15) acts as a histone deacetylase-dependent transcriptional repressor essential for spermiogenesis and male fertility. A mutation that leads to premature termination of the protein is associated with azoospermia. The truncated domain of the protein is implicated in signal transduction (Yan et al., 2010).
Transcription factor mutations associated with female infertility
There is growing evidence that genetic mutations are present in as many as 10% of female infertility conditions, including ovulatory disorders (e.g. Kallmann syndrome), chromosomal abnormalities (e.g. Turner’s syndrome), endometriosis, pelvic adhesions, tubal abnormalities and hyperprolactinemia. We summarize mutations in transcription factors that are associated with a small subset of female infertility conditions, including premature ovarian insufficiency (POI), also known as premature or primary ovarian failure, (characterized by a loss of ovarian function before the age of 40 years), and uterine leiomyomata, a benign smooth muscle tumor in the uterus.
FOXL2 is one of several forkhead domain-containing transcription factor genes involved in female germ cell development (Gersak et al., 2004). It is expressed in ovarian follicular and stromal cells and acts as a lineage-determining regulator of ovarian differentiation. FOXL2 was first identified as containing the causative mutation in blepharophimosis, ptosis and epicanthus inversus syndrome, a facial development syndrome characterized by POI (Crisponi et al., 2001). Subsequently, FOXL2 mutations have been identified in other female infertility syndromes, including sporadic cases of POI (Harris et al., 2002; De Baere et al., 2005; Nallathambi et al., 2007).
FIGLA is a female-specific transcription factor that acts early in oocyte development to initiate the expression of key genes required for folliculogenesis (Li et al., 2017; Wagner et al., 2020; Ye et al., 2020). FIGLA is a germ cell-specific basic helix-loop-helix transcription factor required for follicle formation in mice (Soyal et al., 2000; Hu et al., 2010). Studies of women with POIhave identified mutations in FIGLA, which disrupt its interaction with transcriptional co-regulators (Zhao et al., 2008; Bouilly et al., 2016).
NOBOX is a homeodomain-containing transcription factor which has also been shown to be required for folliculogenesis and oocyte-specific gene expression in mouse models (Rajkovic et al., 2004). Mutations of NOBOX have been found in up to 6% of sporadic cases of POI in women. The resulting amino acid substitutions in the homeodomain or transactivation domain lead to impaired transcriptional activity (Qin et al., 2007; Bouilly et al., 2016).
NR5A1 is essential for both male and female germ cell development. Mutations in NR5A1 are associated with POI (Philibert et al., 2010).
SALL4 (SAL-like 4) encodes a putative zinc finger transcription factor that plays an important role in the maintenance of pluripotent stem cells and the development of oocytes. A genetic study focused on Chinese women with non-syndromic POIhas identified two probable gene mutations associated with the occurrence of POI (Wang et al., 2009).
FOXO1A/3A (forkhead box o1A/3A) is expressed in the ovary and thought to play roles in ovarian development. Causal mutations were identified in POI patients, although the pathological role is yet undetermined (Watkins et al., 2006).
MED12 (medicator complex subunit 12) is a well-known causal gene for uterine leiomyomas. Approximately 60% of patients with uterine leiomyomas have somatic MED12 mutations in some form, including missense, insertion and deletion. Most of the mutations are localized to exon 2 of the MED12 gene, suggesting that this domain is the major functional domain contributing to the genesis of uterine leiomyomas (Halder et al., 2015; Heinonen et al., 2017; Ajabnoor et al., 2018).
Transcription factors as diagnostic markers for germ cell tumors
Human germ cell tumors (GCTs) are neoplasms presenting in the gonads, primarily in the testes. The transcriptome of GCTs is highly similar to authentic FGCs; thus, GCT cell lines are frequently used as a model to study the function of FGCs (Irie et al., 2015). GCTs can be broadly categorized into seminoma and non-seminomatous GCTs (Oosterhuis and Looijenga, 2005; Vasdev et al., 2013). Seminoma GCTs grow and spread more slowly and are sensitive to chemotherapy and/or radiation therapy. Non-seminomatous GCTs are divided into four subtypes: embryonal carcinoma, yolk sac carcinoma, choriocarcinoma and teratoma. Compared with seminoma, non-seminomatous GCTs are very variable in phenotype and prognosis. Non-seminomatous GCTs tend to grow faster, have an earlier mean age at the time of diagnosis, and have a lower 5-year survival rate (Litchfield et al., 2016; Costa et al., 2017; Shen et al., 2018). Identification of molecular signatures to differentiate subtypes of GCTs is therefore crucial for determining prognostication and subtype-based selection of treatment. Thus, a number of studies have been conducted to identify signature genes for each subtype, and transcription factors are promising to be useful as distinct biomarkers for different categories of GCTs (Alagaratnam et al., 2011; Litchfield et al., 2017).
GCTs are thought to originate from FGCs since pluripotency transcription factors are highly expressed in the precursor lesion of GCTs. Master transcription factors for pluripotency, namely OCT4, NANOG, SOX2 and LIN28 (Lin-28 homolog A), are key markers of certain types of GCTs, implicating their roles in maintenance of these malignant cells in the growth of this tumor (Skakkebaek, 1972, 2002; Looijenga et al., 2003; Cheng et al., 2004; Hart et al., 2005; Hoei-Hansen et al., 2005; Cheng et al., 2007; West et al., 2009; Gillis et al., 2011). Clinically, these pluripotency factors are emerging as diagnostic markers for both testicular and ovarian GCTs (Gillis et al., 2011). Immunohistochemistry studies in primary samples have suggested OCT4 and NANOG as sensitive and specific markers for identifying GCTs (Jones et al., 2004; de Jong et al., 2005; Richie, 2005; de Jong and Looijenga, 2006; Jung et al., 2006). However, these two transcription factors alone do not provide the specificity necessary to distinguish between seminomatous and non-seminomatous tumors (Ulbright and Young, 2005). Recent gene expression profiling and immunohistochemistry analyses have suggested that the combination of expression patterns of multiple transcription factors may serve as a feature to differentiate seminomatous and subtypes of non-seminomatous GCTs (Santagata et al., 2007). For example, seminomas are found to be positive for OCT4 and NANOG and negative for SOX2, whereas embryonal carcinomas are positive for all three pluripotency markers. Besides pluripotency transcription factors, other crucial transcriptional regulators of FGC development are also indicated as diagnostic markers for GCTs. For example, the expression pattern of SOX17, a critical regulator of hPGC specification, can also distinguish seminoma from embryonal carcinoma when combined with SOX2 (Nonaka, 2009). Immunohistochemistry of TFAP2C, another essential transcription factor for germ cell development, has also been evaluated for the diagnosis of multiple subtypes of GCTs (Pauls et al., 2005).
Core transcriptional network for hPGC specification identified by in vitro differentiation
Although both mutations linked to infertility and gene expression in various stages of human germ cell development contribute to identification of genes that act at specific stages of development, functional analysis is necessary to validate their developmental roles and pinpoint underlying mechanisms. Recent developments in stem cell biology and gene editing provide an opportunity to recapitulate human germ cell development in vitro and to functionally dissect genetic requirements. A key step in developing in vitro gametogenesis is identifying and characterizing genetic determinants in a robust model for germ cell specification. While it is clear that in vitro-derived germ cells lack important characteristics of authentic FGCs (notably, the ability to efficiently develop into gametes in vitro), in vitro gametogenesis provides a viable system to explore the core transcriptional machinery for germ cell specification.
Strategies to recapitulate human germ cell development in vitro
Numerous studies have contributed to protocols for directing germ cell differentiation from hPSCs, starting from both hESCs and human induced pluripotent stem cells (hiPSCs). Currently, human primordial germ cell-like cells (hPGCLCs) can be induced using rationally designed cocktails of growth factors and small molecules that have emerged over the years (Kee et al., 2006, 2009; Easley et al., 2012; Irie et al., 2015; Sasaki et al., 2015; Sugawa et al., 2015; Jung et al., 2017; Yamashiro et al., 2018; Murase et al., 2020), or induced by ectopic expression of genes associated with germ cell development, especially transcription factors (Kee et al., 2009; Qiu et al., 2013; Yu et al., 2014; Irie et al., 2015; Medrano et al., 2016; Panula et al., 2016; Jung et al., 2017; Fang et al., 2018; Kojima et al., 2021). To induce maturation and more advanced differentiation in vitro, hPGCLCs are often co-cultured with somatic cells (Park et al., 2009; Lin et al., 2014; Yamashiro et al., 2018). In addition, xenotransplantation assays have been extensively applied to promote in vivo maturation of in vitro-derived germ cells. Two recent review articles have summarized progress and strategies of in vitro gametogenesis (Li et al., 2020; Saitou and Hayashi, 2021).
Identification of transcription factors involved in hPGCLC specification by genetic studies
Delineation of the conditions and factors required to promote in vitro PGC differentiation have set the stage for genetic studies that can probe the function and hierarchies of transcription factors during hPGC specification (Fig. 2). Several transcription factors which are usually involved in lineage specification during embryogenesis have been reported to be repurposed in PGCs to form a specific transcriptional network that may act to safeguard human germ cell fate by maintaining pluripotent status while repressing differentiation. In response to WNT (Kojima et al., 2017) and ACTIVIN signals, the mesoderm specifier EOMES activates SOX17, an endoderm specifier, which in turn upregulates PRDM1. Deletion of EOMES in hPSCs significantly impacts their competence toward hPGCLC differentiation (Chen et al., 2017; Kojima et al., 2017). SOX17 can also be induced directly by BMP signaling to activate germ cell programs: conversely, SOX17-null hESCs cannot undergo hPGCLC specification (Irie et al., 2015; Tang et al., 2015). The trophoblast marker GATA3 is an immediate effector of the BMP pathway and regulates SOX17 and TFAP2C. Accordingly, GATA3 null mutations significantly decreased hPGCLC induction efficiency in response to BMP signals (Kojima et al., 2021). PRDM1 is a transcriptional repressor that acts as one of the key signature genes for germ cell fate (Ohinata et al., 2005; Irie et al., 2015; Kobayashi et al., 2017). PRDM1 function is tightly controlled by multiple transcription factors to repress somatic differentiation during the process of hPGCLC specification; in PRDM1-deficient or PRDM1-knockdown cells, germline differentiation potential is significantly impaired, and somatic lineage genes are de-repressed (Lin et al., 2014; Sasaki et al., 2015). Part of the role of PRDM1 protein is to suppress SOX2 expression and consequently inhibit neuronal differentiation directly. A study that examined hPGCLC specification in TFAP2C−/− cells found that TFAP2C acts upstream of PRDM1 and plays a dominant role in repressing somatic programs in hPGCLCs (Kojima et al., 2017). Another study used single-cell sequencing in TFAP2C−/− cells during hPGCLC specification and confirmed that TFAP2C functions upstream of both PRDM1 and SOX17, acting to prevent cells from adopting somatic fates and thus safeguard germ cell fate (Chen et al., 2019).
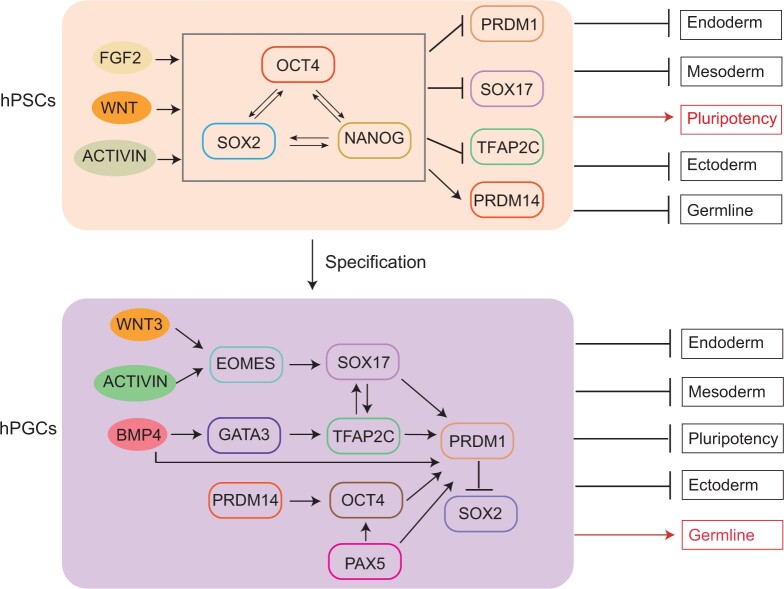
Figure 2.
The core transcriptional network in human pluripotent stem cells and primordial germ cells. Arrows with pointed tips represent activation, and arrows with vertical line tips represent inhibition. In pluripotent stem cells, FGF2, WNT and ACTIVIN signaling pathways are essential to activate the gene expression program for pluripotency. In response to the signals, OCT4, SOX2 and NANOG are activated and form a core transcriptional network that suppresses the somatic and germline gene expression program. Once human pluripotent stem cells (hPSCs) start to differentiate toward germline, WNT3, ACTIVIN and BMP4 signals activate EOMES and GATA3, which then activate the expression of a few transcription factors essential for germ cell development, including SOX17, TFPAP2C and PRDM1. Moderate expression of pluripotency transcription factor OCT4 is also critical for human germ cell development. Upon differentiation, the expression of OCT4 is gradually reduced, and the expression of its functional partner in hPSCs, SOX2, is diminished. Instead, OCT4 partners with PAX5 in human primordial germ cells (hPGCs) to activate the expression of PRDM1.
In addition to transcription factors that act in specifying lineages in development, pluripotency transcription factors are important in germ cell development. One of the unique features of hPGCs compared with other cell types of the body during development is that they share with hPSCs the expression of several pluripotency genes, including a pluripotency master regulator OCT4/POU5F1. In both mouse and human embryo development, OCT4 is initially expressed in all blastomeres of the embryo; subsequently, expression is restricted to the pluripotent stem cells of the inner cell mass. During gastrulation OCT4 level is maintained in epiblast cells and after gastrulation OCT4 expression is confined exclusively to germ cells (Scholer et al., 1990; Scholer, 1991; Yeom et al., 1996; Nichols et al., 1998; Pesce and Scholer, 2001). Mouse embryos depleted of OCT4 fail to form an inner cell mass and the cells are committed to the trophoblast lineage (Nichols et al., 1998); However, conditional knock out of OCT4 in mouse PGCs leads to apoptosis of PGCs rather than cell fate change to the trophectodermal lineage (Kehler et al., 2004), suggesting that OCT4 is playing distinct roles in these two distinct cell types. To dissect the roles of OCT4 in hPSCs and hPGCs, Fang et al. (2018) compared genome-wide binding of OCT4 in hPSCs and hPGCs (the latter from human fetal testis samples). They discovered that OCT4 repressed neuronal differentiation in both hPSCs and hPGCs, while it regulated a unique set of genes during germ cell differentiation by switching partners from SOX2 to PAX5 (paired box 5). In hPSCs, OCT4 and SOX2 interact and form a protein complex to cooperatively bind and regulate target genes in order to activate or maintain pluripotency (Herr and Cleary, 1995; Nichols et al., 1998; Wegner, 1999; Niwa et al., 2000; Avilion et al., 2003). As hPSCs begin to differentiate toward a germline fate, the expression of SOX2 is diminished and OCT4 switches functional partners to PAX5 as germ cells are specified. The PAX5-OCT4 complex functions in activation of PRDM1 expression and other genes implicated in PGC specification. PAX5 null mutations have significantly reduced PRDM1 expression and impaired germ cell potential in hPSC xenotransplants in vivo. Hence, the PAX5-OCT4-PRDM1 proteins function as a genetic switch in the transition from a pluripotent state to germline (Fang et al., 2018). PRDM14, another human pluripotency gene, is also critical for the acquisition and maintenance of the hPGCLC-competent state (Sybirna et al., 2020); it functions to activate OCT4 expression and to upregulate PRDM1 (Chia et al., 2010). Loss of PRDM14 function results in significantly reduced efficiency of in vitro differentiation and an aberrant transcriptome of the resultant hPGCLCs (Sybirna et al., 2020).
Overexpression of SOX17 leads to the generation of hPGCLCs without BMP induction, suggesting that SOX17 is at the top of the transcriptional hierarchy for hPGCLC specification and is sufficient for human germ cell fate acquisition (Irie et al., 2015). Although a major role of SOX17 is to activate TFAP2C and PRDM1, forced expression of TFAP2C could not generate hPGCLCs, even in conjunction with PRDM1 overexpression (Kobayashi et al., 2017). These results suggest that while TFAP2C is indispensable for hPGCLC specification, it is insufficient on its own for germline induction. Recent work reported that the GATA family of transcription factors (GATA2/3), combined with SOX17 and TFAP2C, act as a minimum requirement to replace BMP signaling and confer germ cell fate on incipient mesoderm-like cells (iMeLCs) (Kojima et al., 2021). In total, these genetic studies of hPGCs in vitro begin to allow the construction of a network of transcription factors that are involved in hPGC specification and maturation (Fig. 2).
Murine and human germ cells are characterized by evolutionarily distinct transcriptional networks
Prior to recent advances in stem cell biology and sequencing technologies, our understanding of germ cell development relied almost solely on animal models. However, germline commitment occurs within a limited window of embryo development, when the morphology of embryos and the timing of germ line specification diverges significantly between different species, including mice and humans (Sybirna et al., 2019) (Fig. 3). Notably, mouse embryos develop as an egg cylinder, and mouse PGCs (mPGCs) are clustered in the proximal epiblast around the time of primitive streak formation (Tam and Behringer, 1997; Anderson et al., 2000; McLaren, 2003). Human embryos present as a bilaminar disc and, based on the studies in non-human primates, hPGCs probably arise prior to primitive streak formation from the dorsal amnion, which is physically separate from the posterior epiblast (Behringer et al., 2000; Rossant, 2015; Sasaki et al., 2016; Kobayashi et al., 2017). Given such major developmental differences in terms of timing, shape and cell origin, it is not surprising that the intrinsic transcriptional network required for PGC emergence also has divergent components and functions. In mPGCs, the Prdm1, Tfap2c and Prdm14 proteins constitute a core transcriptional network that is essential for PGC specification in vivo (Ohinata et al., 2005; Yamaji et al., 2008; Weber et al., 2010) and sufficient to induce germ cell fate in vitro (Magnúsdóttir et al., 2013; Nakaki et al., 2013). In contrast, the expression of PRDM14 in humans is strongly downregulated from hPSCs to hPGCs (Sugawa et al., 2015), and SOX17 has instead emerged as the critical determinant for hPGC specification (Irie et al., 2015). Pluripotency genes OCT4 and NANOG are re-expressed in both mPGCs (Murakami et al., 2016) and hPGCs (Guo et al., 2015; Tang et al., 2015), and SOX2 is absent in hPGCs (Lin et al., 2014) although it is required for mouse PGC survival and proliferation (Campolo et al., 2013). In addition, the mesoderm specifier gene T/Brachyury is essential for robust activation of Prdm1 and Prdm14 in mPGCs (Aramaki et al., 2013) but this role is replaced by another mesoderm gene, EOMES, in hPGCs (Chen et al., 2017; Kojima et al., 2017). The co-expression of pluripotency genes and lineage specifier genes persists to the sex determination stage when PGCs differentiate to spermatogonia and oogonia in both mice and humans.
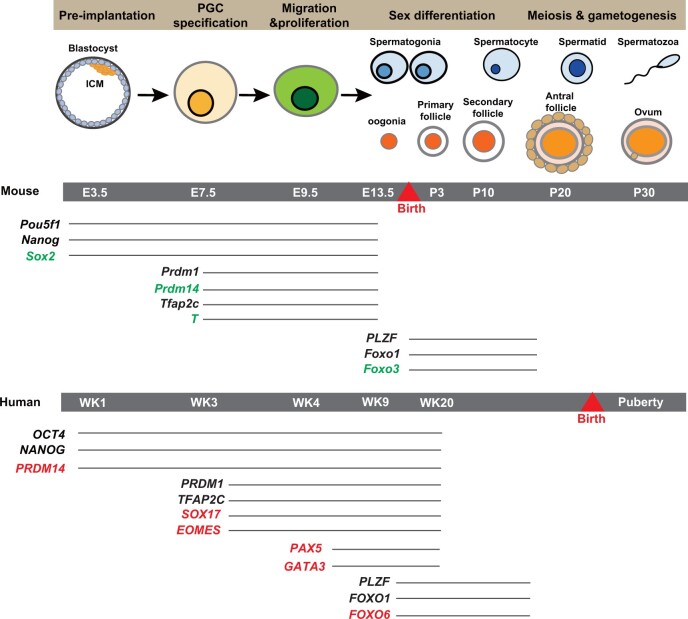
Figure 3.
Differences in transcription factor expression between murine and human germ cells. E, embryonic day; ICM, inner cell mass; WK, week. Green marks the genes specifically expressed in mouse germ cell development, and red marks the genes specifically expressed in human germ cell development.
The expression of pluripotency genes also differs during germ cell development in mice and humans. In male mice, expression of OCT4 persists as cell fate transits from PGCs to the undifferentiated SPG stage, and expression is downregulated once cells enter meiosis (Pesce et al., 1998; Tadokoro et al., 2002). However, OCT4 expression, specifically isoform OCT4A (translated from transcript variant 1), is more restricted during male development in humans, being confined to hPSCs and hPGCs. In female mice, the expression of OCT4 is downregulated by the onset of meiotic prophase and then re-activated after birth in oocytes within primary follicles and at the onset of folliculogenesis (Pesce et al., 1998; Anderson et al., 2007). In humans, the number of OCT4 positive cells peaks by gestational week 8 and diminishes after week 9, as oogonia enter meiosis (Kerr et al., 2008). NANOG has a similar expression pattern to OCT4 (Hoei-Hansen et al., 2005). Once FGCs arrive at the gonads and progress toward meiosis, pluripotency-related transcription factors undergo significant downregulation and are diminished in all adult reproductive tissues.
The FOXO subclass of the forkhead box transcription factors are key regulators of mouse reproduction (Brosens et al., 2009). FOXO3 is required to suppress primordial follicle activation in females as FOXO3-null female mice display age-dependent infertility (Castrillon et al., 2003; Hosaka et al., 2004), and FOXO1 is essential for SSCs maintenance and the initiation of spermatogenesis in males (Goertz et al., 2011). While FOXO1 expression in spermatogonia and granulosa cells is conserved between humans and mice (Richards et al., 2002; Liu et al., 2013), FOXO3 is not expressed in primordial oocytes in humans (Tarnawa et al., 2013), suggesting that other members of the FOXO transcription factor family may replace its function. FOXO6, which has been identified as an upregulated gene in human oocytes by scRNA-seq, may be a potential candidate for this substitution of FOXO3 function.
A unique transcriptional network defines human germ cells
The maintenance of cell identity in FGCs requires the repression of somatic lineages in concert with the activation of germ cell programs (Figs 2 and 4). Of note, human FGCs are defined by a unique transcriptional network that comprises germ cell-specific genes together with somatic lineage specifiers and pluripotency genes. How these transcription factors, which are master regulators for various cell types, function differently from their canonical roles in driving germ cell fate is an intriguing and fundamental question in the field of human germ cell developmental genetics. One hypothesis is that transcription factors work in different protein complexes to perform cell-type-specific roles. In support of this, Fang et al. (2018) observed that OCT4 switches partners from SOX2 in hPSCs to PAX5 and PRDM1 proteins in human FGCs (Fang et al., 2018). While continuing to repress differentiation toward ectoderm, importantly in human FGCs, OCT4 shifts its binding from pluripotency-related genes to germline-specific genes to activate germ cell fate, coincident with the switch in activity of the respective OCT4 complexes.
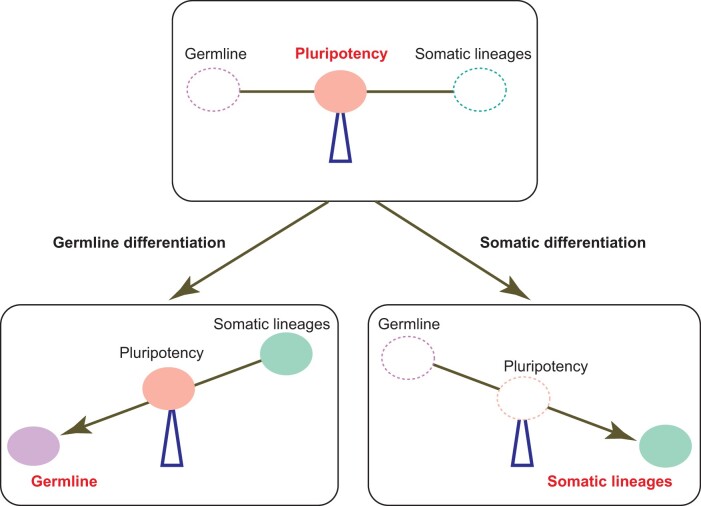
Figure 4.
Proposed model for intrinsic transcription forces that drive human germline and somatic differentiation. Solid ovals represent gene expression programs that are activated, and dotted ovals represent gene expression programs that are silenced.
An alternate but not necessarily mutually exclusive hypothesis is that the delicate balance of different transcription factors defines germ cells. Lineage specifiers that belong to the three germ layers and trophectoderm (SOX17, EOMES, PAX5 and GATAs), as well as pluripotency proteins (OCT4, NANOG), are all expressed in human FGCs and function as drivers of the germ cell lineage, as demonstrated by diverse functional studies. Thus, these master regulators of differentiation and pluripotency must be regulated to modulate their canonical functions (Fig. 4). For example, while the SOX17 gene encodes a classical endoderm specifier and plays a critical role in normal hPGC specification, over-expression of SOX17 beyond a PGC-competent window favors the expression of endoderm genes rather than direction toward a germ cell fate (Kobayashi et al., 2017). In response to hPGC specification, the dosage and actions of these transcription factors must be balanced so that cell identity extends beyond pluripotency but is not co-opted toward any specific somatic lineages. It is likely that other germ cell determinants are activated to inhibit further somatic lineage differentiation and reinforce commitment of cells to the germline. For example, as a transcriptional repressor, PRDM1 activity in hPGCs represses expression of somatic lineage genes (Irie et al., 2015; Sasaki et al., 2015). In support of this, a recent study demonstrated that high-dosage overexpression of SOX17 in hPSCs leads to aberrant expression of endoderm markers, which could be rescued by simultaneously providing a comparable dose of PRDM1 protein (Kobayashi et al., 2017). Given the importance of PRDM1 in hPGCs, its activation may be safeguarded by multiple transcription factors to assure its appropriate expression to maintain germ cell identity.
The gap between hPGCLCs and bona fide hPGCs and beyond
Despite advances in our knowledge of transcription factor function in PGC specification, in vitro-derived hPGCLCs do not progress further down the germ cell lineage efficiently and do not readily enter or complete meiosis to produce functional germ cells. Accordingly, gene expression analysis of in vivo hPGCs from developing human embryos has revealed clear differences with hPGCLCs (Table III) (Gkountela et al., 2013, 2015; Tang et al., 2015; Chen et al., 2018; Sybirna et al., 2020). Most notably, late-stage hPGC markers, such as DAZL (deleted in azoospermia), VASA/DDX4 (DEAD-box helicase 4), and PIWIL1 (Piwi like RNA-mediated gene silencing 1), are not activated in hPGCLC models (Irie et al., 2015) suggesting a lack of activation of necessary transcription factors to induce the transcriptional program of later germ cell stages in vitro. Indeed, a time-course analysis of early- versus late-gestation cyPGCs found that the gene expression signature of hPGCLCs is more similar to early-stage PGCs than later-gestation PGCs, which have been most commonly profiled from human samples (Sasaki et al., 2016). Several transcription factors enriched in expression in ‘late’ cyPGCs, such as RNF17 (ring finger protein 17) and KRBOX1 (KRAB Box domain containing 1), lack expression in hPGCLCs, and are known to function in late-stage germ cell development.
Table III.
Transcription factors reported to be expressed in bona fide hPGCs and in vitro derived hPGCLCs.
|
Bona fide hPGCs |
In vitro derived hPGCLCs |
Functions in reproduction based on Mouse Genome Informatics (MGI) | ||||
|---|---|---|---|---|---|---|
| Gkountela et al. (2013) | Gkountela et al. (2015) | Chen et al. (2018) | Sasaki et al. (2015) | Chen et al. (2018) | Sybirna et al. (2020) | |
| ALX4 | ALX4 | ALX4 | ALX4 | Male sterility | ||
| BNC1 | BNC1 | BNC1 | BNC1 | Required for testis development | ||
| CDX1 | CDX1 | CDX1 | CDX1 | |||
| DLX5 | DLX5 | DLX5 | ||||
| DMRT1 | DMRT1 | DMRT1 | DMRT1 | Male sterility, disorganized seminiferous tubules | ||
| DMRTC2 | DMRTC2 | Male sterility | ||||
| EMX2 | EMX2 | EMX2 | Bipotential gonad marker | |||
| ESX1 | ESX1 | ESX1 | Role in spermatogenesis | |||
| GATA2/3/4 | GATA2/3/4 | GATA2/3/4 | ||||
| HOXA2 | HOXA2 | |||||
| HOXA3/4/5/7/9 | HOXA3/4/5/7/9 | HOXA3/4/5/7/9 | ||||
| HOXB3/4/5 | HOXB3/4/5 | |||||
| HOXB7 | HOXB7 | |||||
| HOXC4 | HOXC4 | HOXC4 | ||||
| HOXC9 | HOXC9 | |||||
| HOXD3 | HOXD3 | |||||
| HOXD9 | HOXD9 | HOXD9 | ||||
| IRX1 | IRX1 | IRX1 | ||||
| IRX4 | IRX4 | |||||
| IRX6 | IRX6 | IRX6 | ||||
| KLF2 | KLF2 | |||||
| KLF4 | KLF4 | KLF4 | ||||
| LHX1 | LHX1 | |||||
| LHX2 | LHX2 | |||||
| MSX2 | MSX2 | MSX2 | ||||
| NR2F2 | NR2F2 | NR2F2 | ||||
| OSR2 | OSR2 | |||||
| PAX5 | PAX5 | PGCs development | ||||
| PAX8 | PAX8 | PAX8 | Male infertility | |||
| RNF17 | RNF17 | RNF17 | RNF17 | Testis specific; regulates piRNA maturation | ||
| RUNX3 | RUNX3 | RUNX3 | ||||
| SIX1 | SIX1 | |||||
| SOX15 | SOX15 | SOX15 | ||||
| SOX17 | SOX17 | SOX17 | ||||
| T | T | T | ||||
| TBX2 | TBX2 | |||||
| TBX3 | TBX3 | TBX3 | TBX3 | |||
| TBX5 | TBX5 | |||||
| TCL1A | TCL1A | |||||
| TFAP2C | TFAP2C | TFAP2C | ||||
| TFCP2L1 | TFCP2L1 | TFCP2L1 | TFCP2L1 | TFCP2L1 | ||
| TLX2 | TLX2 | |||||
| ZEB1 | ZEB1 | |||||
PiRNA, Piwi-interacting RNA.
The wealth of expression data now available from hPGCs in vivo, as well as different models of hPGCLC generation, might enable the unbiased identification of new transcription factors that may drive hPGC specification beyond the current state achievable in vitro (Gkountela et al., 2015; Chen et al., 2018). We have identified transcription factors whose expression is induced in hPGCs or hPGCLCs and found several notable transcription factor classes present in vivo that are not activated in vitro (Table III). For example, DMRT-family transcription factors are known to play a role in sex determination in many organisms, and both DMRT1 and DMRTC2 are found in hPGCs (Guo et al., 2015), but are not expressed in hPGCLCs. Consistent with their well-known role in embryonic development, many homeobox-containing genes, including both classic Hox cluster transcription factors as well as other homeodomain-containing transcription factors, such as ALX4 (ALX homeobox 4), EMX1/2 (empty spiracles homeobox 1/2), ESX1 (ESX homeobox 1), SIX1/2 (Sineoculis homeobox homolog 1/2) and LHX2/8 (LIM-homeodomain protein 2/8), are present in hPGCs but not hPGCLCs. This enrichment of homeodomain transcription factors is somewhat surprising given that repression of homeobox genes is a well-known feature of developing PGCs, so this may reflect contamination from somatic cells. Finally, some members of other transcription factor classes, such as PAX and SOX-domain transcription factors, are active in in vivo but not in vitro models of PGCs. Different gene expression profiles could be linked to different developmental timing (or different development trajectories between in vitro differentiation or in vivo development) or aberrant gene expression. It is likely that future efforts to derive germ cell-like cells in vitro will require the expression or activation of transcriptional programs of one or more of these transcription factors, either by exogenous gene introduction or identification of upstream signaling pathways, which can be activated by the addition of extracellular factors.
In addition to identification of stage-specific gene expression profiles, it is equally important to compare the epigenetic status of in vitro derived germ cells with that of bona fide germ cells. Global erasure of DNA methylation, with the exception of some repetitive elements, is the hallmark of in vivo hPGC development (Leitch et al., 2013; Irie et al., 2015). Several transcription factors are involved in the epigenetic modeling of hPGCs. PRDM1 and SOX17 function in maintaining the epigenetic program of 5-methylcytosine (5mC) erasure from week 4 to week 9. Partial erasure of 5mC and enrichment of 5hmC is also observed in the in vitro differentiation system. In hPGCLCs, loss of PRDM1 inhibits the initiation of DNA demethylation while the expression of SOX17 activates PRDM1, which then sustains the epigenetic program toward global 5mC erasure (Tang et al., 2015). Comparisons of the epigenome of in vitro derived hPGCLCs to that in vivo provides instruction on the progress of generation of germ cells.
Studies incorporating xenotransplantation
Previous research in multiple different stem-cell based experimental systems has demonstrated that cell and tissue transplantation is the gold standard for testing cell identity and function (Hanna et al., 2007; Nelson et al., 2009; Weissman, 2012; Takahashi and Yamanaka, 2013). As noted in a recent review, transplantation may provide the first ability to demonstrate the feasibility of generating PGCLC-derived, fully mature gametes in primates, including humans (Saitou and Hayashi, 2021). Three types of studies have been reported with different transplantation strategies and are summarized below.
In the first studies, whole slices of testicular tissue were transplanted from non-human primates (Macaca fascicularis) to a subcutaneous region of the back or side of male nude mice (Liu et al., 2016). The results indicated that xenotransplanted testis tissue from young mammals, including mice and monkeys, is capable of undergoing full and complete spermatogenesis in xenografts. Moreover, monkey xenografts to nude mice were capable of generating sperm capable of producing monkey offspring when sperm derived from the xenografts testis tissues from juvenile wild-type (WT) and transgenic cynomolgus monkeys (M. fascicularis) were used for assisted reproduction. These results may inform future strategies for in vitro gametogenesis.
Second, in an elegant set of proof-of-concept experiments, Rhesus macaque testicular tissue that was cryopreserved as an experimental validation of fertility preservation for prepubertal human patients was used in autologous transplantation (Fayomi et al., 2019). Prepubertal testicular tissue that had been cryopreserved was autologously grafted under the back skin and scrotal skin of castrated pubertal R. macaque monkeys. Results indicated that mature sperm were produced that were capable of fertilizing R. macaque oocytes and culminating in live birth. This suggests that in non-human primates there is the capability for full gametogenesis, from spermatogonia to mature sperm, through autologous grafting.
Third, in studies reported in several manuscripts, the ability of germ cells to engraft was used to assess validity and quantitative and qualitative aspects of in vitro gametogenesis. In these studies, the ability of hPGCLCs to engraft in mouse seminiferous tubules following differentiation in vitro was tested (Dominguez et al., 2014; Durruthy-Durruthy et al., 2014; Ramathal et al., 2014; Medrano et al., 2016; Fang et al., 2018). Results demonstrated that the ability to differentiate and produce engraftable germ cells was dependent on the genetic composition of the parental iPSC lines used to produce PGCLCs. Thus, PGCLCs differentiated from iPSC lines with Y chromosome deletions that are linked to poor or no spermatogenesis were shown to have limited engraftment relative to PGCLCs without genetic abnormalities derived from fertile men; similar results were also observed with PGCLCs produced from lines with numerical sex chromosome abnormalities (Dominguez et al., 2014; Durruthy-Durruthy et al., 2014; Ramathal et al., 2014). In contrast, over-expression of key germ cell-specific genes was associated with increased engraftment (Medrano et al., 2016; Panula et al., 2016; Fang et al., 2018). Furthermore, these studies documented the migration of PGCLCs to the basement membrane of spermatogenic tubules and expression of spermatogonial markers. As expected, however, complete spermatogenesis was not observed owing to the evolutionary distance between mice and humans. Instead, as reported previously, in transplantation with human SSC the germ cells migrate to the seminiferous tubule basement membrane and proliferate to form chains and patches of spermatogonia that persist in the long term but do not appear to initiate or complete meiosis (Nagano et al., 2002; Hermann et al., 2010; Dovey et al., 2013).
Conclusion and future perspectives
The identification and functional characterization of transcription factors implicated in human germ cell development may not only increase our understanding of the fundamental genetic basis of pluripotency and heredity, but also may help to dissect the causative mechanisms of germline diseases and provide potential strategies for treatment (Fig. 5). With recent advances in stem cell biology and genetic and epigenetic profiling, there is the ability to compare and contrast data across different species, including human-specific aspects of germ cell development. Transcription factors that are specifically expressed or upregulated at critical developmental stages have the potential to act as master regulators of germ cell development. In addition to methods such as scRNA-seq that provide cell type-specific transcriptome information, rapid development of other single-cell ‘multi-omics’ technologies will integrate various components of genomic and epigenomic information, including DNA methylation, histone modification, chromatin accessibility, RNA expression and protein abundance within the same cell, enabling an in-depth understanding of gene regulatory mechanisms. Furthermore, advances in single-molecule imaging allow us to track the dynamic processes of development within a single cell at high spatial and temporal resolution. Together with these new technologies, we can develop a more comprehensive set of hypotheses to test our understanding of cell fate decisions, identity and the function of cells in normal germ cell development, physiology and disease. Knowledge of key transcription factors required for directed stem cell differentiation promises to provide additional tools to directly probe the function of putative master regulatory transcription factors in germ cell development through gain and loss-of-function studies.
Figure 5.
Improving human reproduction research through transcription factor-induced in vitro differentiation models. ESC, embryonic stem cell
Successes in modeling mouse germ cell differentiation and gamete formation have thus far translated to human systems only in part, indicating gaps in knowledge of human-specific factors involved in mature germ cell development. Current methods of human germ cell differentiation in vitro still remain inefficient, asynchronous, and prone to variability, with a majority of the initial pluripotent cells differentiating toward various somatic lineages spontaneously. The efficiency of hPGCLC progression to more mature germ cells is also still very low (Tang et al., 2016). Developing multiple, testable strategies to differentiate hPSCs toward functional oogonia/spermatogonia efficiently is essential to model the entire germline development and diseases.
In recent years, the biomedical community has witnessed the development of iPSC technology, which spawned a new era of controlling cell fate by modulating master transcription factors (Takahashi and Yamanaka, 2006; Takahashi et al., 2007). The successes in mouse germ cell differentiation enhance the prospect of transcription factor-induced differentiation of hESCs to hPGCs, and beyond. However, multiple transcriptome analyses have revealed that human germ cells acquire a unique transcriptional network, which is distinct from that of mouse germ cells and from that of any other human cell type. The germ cell transcriptome is composed of a surprising combination of core germ cell-specific genes, somatic lineage specifiers, and pluripotency genes. We and others have hypothesized that these lineage specifiers cooperate with the pluripotency networks to act as the earliest molecular switch in the developmental transition from PSCs to the germ cell lineage (Fig. 4). Counteracting the activity of lineage specifiers, and continued transcription by pluripotency proteins, synergistically represses somatic lineages and activates germ cell programs to maintain germ cell identity. The identification and overexpression of these master transcription factors of human germ cells in hPSCs may enable faithful and efficient in vitro production of bona fide human germ cells and contribute to diagnosis and applications in human germ cell pathologies.
Numerous protocols over the years have attempted to mimic the spatial component of embryonic development by stimulation of differentiating cells in 3D aggregates. However, these methods can expose cells at different positions in the aggregates to varying levels of extracellular signals, leading to heterogeneity in differentiation. The ability to maintain cells in a uniform monolayer and induce cell fate change by overexpression of transcription factors could enhance differentiation efficiency while improving final purity. More importantly, this strategy would reduce the complexity and variability of current protocols and enhance the widespread availability of germ cell research, as well as reproducibility, between laboratories. Simplifying and increasing the efficiency of differentiation of germ cells will also allow previously challenging studies, such as high-throughput drug discovery and genetic screening assays, thereby accelerating our understanding of human germ cell biology, and expediting infertility research.
Finally, the ability to culture hPSCs and hPGCs indefinitely in vitro, in addition to providing an excellent model for germ cell research, could permit the repair of genetic defects in germ cell development by gene editing tools, for example, clustered regularly interspaced short palindromic repeats (CRISPR) technology. Note that in vitro hPSC differentiation-based methods or disease-modeling studies cannot diminish the importance of animal disease models for studying disease mechanisms or the downstream characterization and validation of putative drugs. However, the use of in vitro hPSC differentiation-based studies is providing additional tools for the identification of human-specific genes and pathways of development, and thereby accelerate the overall drug development process to treat human reproductive disease.
Data availability
The data underlying this article will be shared with all reasonable requests to the corresponding author.
Authors’ roles
All authors contributed to the identification and critical evaluation of the relevant literature and reviewed all sections and critical discussion of the manuscript. F.F., P.J.I. and R.A.R.P. proposed the outlines, made the figures and drafted the main text. N.X., L.L. and L.D. performed the literature search and prepared the data for all the tables.
Funding
The National Institute of Health (1R01HD096026); the National Natural Science Foundation of China (32070830); and the Fundamental Research Funds from University of Science and Technology of China (WK9110000141, YD9100002007).
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- Ajabnoor GMA, Mohammed NA, Banaganapalli B, Abdullah LS, Bondagji ON, Mansouri N, Sahly NN, Vaidyanathan V, Bondagji N, Elango R. et al. Expanded somatic mutation spectrum of MED12 gene in uterine leiomyomas of Saudi Arabian women. Front Genet 2018;552:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagaratnam S, Lind GE, Kraggerud SM, Lothe RA, Skotheim RI.. The testicular germ cell tumour transcriptome. Int J Androl 2011;34:e133. [DOI] [PubMed] [Google Scholar]
- Alikhani M, , MirzaeiM, , SabbaghianM, , ParsamatinP, , KaramzadehR, , AdibS, , SodeifiN, , GilaniMAS, , Zabet-MoghaddamM, , Parker L. et al. Quantitative proteomic analysis of human testis reveals system-wide molecular and cellular pathways associated with non-obstructive azoospermia. J Proteomics 2017;162:141–154. [DOI] [PubMed] [Google Scholar]
- Amann RP. The cycle of the seminiferous epithelium in humans: a need to revisit? J Androl 2008;29:469–487. [DOI] [PubMed] [Google Scholar]
- Anderson R, Copeland TK, Scholer H, Heasman J, Wylie C.. The onset of germ cell migration in the mouse embryo. Mech Dev 2000;91:61–68. [DOI] [PubMed] [Google Scholar]
- Anderson RA, Fulton N, Cowan G, Coutts S, Saunders PT.. Conserved and divergent patterns of expression of DAZL, VASA and OCT4 in the germ cells of the human fetal ovary and testis. BMC Dev Biol 2007;7:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aquila S, De Amicis F.. Steroid receptors and their ligands: effects on male gamete functions. Exp Cell Res 2014;328:303–313. [DOI] [PubMed] [Google Scholar]
- Aramaki S, Hayashi K, Kurimoto K, Ohta H, Yabuta Y, Iwanari H, Mochizuki Y, Hamakubo T, Kato Y, Shirahige K. et al. A mesodermal factor, T, specifies mouse germ cell fate by directly activating germline determinants. Dev Cell 2013;27:516–529. [DOI] [PubMed] [Google Scholar]
- Arboleda VA, Sandberg DE, Vilain E.. DSDs: genetics, underlying pathologies and psychosexual differentiation. Nat Rev Endocrinol 2014;10:603–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auguste A, Chassot AA, Gregoire EP, Renault L, Pannetier M, Treier M, Pailhoux E, Chaboissier MC.. Loss of R-spondin1 and Foxl2 amplifies female-to-male sex reversal in XX mice. Sex Dev 2011;5:304–317. [DOI] [PubMed] [Google Scholar]
- Avilion AA, Nicolis SK, Pevny LH, Perez L, Vivian N, Lovell-Badge R.. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev 2003;17:126–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayhan O, Balkan M, Guven A, Hazan R, Atar M, Tok A, Tolun A.. Truncating mutations in TAF4B and ZMYND15 causing recessive azoospermia. J Med Genet 2014;51:239–244. [DOI] [PubMed] [Google Scholar]
- Baker TG. A quantitative and cytological study of germ cells in human ovaries. Proc R Soc Lond B Biol Sci 1963;158:417–433. [DOI] [PubMed] [Google Scholar]
- Ballow D, Meistrich ML, Matzuk M, Rajkovic A.. Sohlh1 is essential for spermatogonial differentiation. Dev Biol 2006;294:161–167. [DOI] [PubMed] [Google Scholar]
- Barrios F, Filipponi D, Campolo F, Gori M, Bramucci F, Pellegrini M, Ottolenghi S, Rossi P, Jannini EA, Dolci S.. SOHLH1 and SOHLH2 control Kit expression during postnatal male germ cell development. J Cell Sci 2012;125:1455–1464. [DOI] [PubMed] [Google Scholar]
- Bashamboo A, Brauner R, Bignon-Topalovic J, Lortat-Jacob S, Karageorgou V, Lourenco D, Guffanti A, McElreavey K.. Mutations in the FOG2/ZFPM2 gene are associated with anomalies of human testis determination. Hum Mol Genet 2014;23:3657–3665. [DOI] [PubMed] [Google Scholar]
- Baumstark A, Barbi G, Djalali M, Geerkens C, Mitulla B, Mattfeldt T, de Almeida JC, Vargas FR, Llerena Junior JC, Vogel W. et al. Xp-duplications with and without sex reversal. Hum Genet 1996;97:79–86. [DOI] [PubMed] [Google Scholar]
- Baxter RM, Vilain E.. Translational genetics for diagnosis of human disorders of sex development. Annu Rev Genomics Hum Genet 2013;14:371–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behringer RR, Wakamiya M, Tsang TE, Tam PP.. A flattened mouse embryo: leveling the playing field. Genesis 2000;28:23–30. [DOI] [PubMed] [Google Scholar]
- Bendel-Stenzel MR, Gomperts M, Anderson R, Heasman J, Wylie C.. The role of cadherins during primordial germ cell migration and early gonad formation in the mouse. Mech Dev 2000;91:143–152. [DOI] [PubMed] [Google Scholar]
- Berta P, Hawkins JR, Sinclair AH, Taylor A, Griffiths BL, Goodfellow PN, Fellous M.. Genetic evidence equating SRY and the testis-determining factor. Nature 1990;348:448–450. [DOI] [PubMed] [Google Scholar]
- Bestetti I, , BarbieriC, , SironiA, , SpecchiaV, , YatsenkoSA, , De DonnoMD, , CasliniC, , GentiliniD, , CrippaM, , Larizza L. et al. Targeted whole exome sequencing and Drosophila modelling to unveil the molecular basis of primary ovarian insufficiency. Hum Reprod 2021;36:2975–2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biason-Lauber A, Konrad D, Meyer M, DeBeaufort C, Schoenle EJ.. Ovaries and female phenotype in a girl with 46,XY karyotype and mutations in the CBX2 gene. Am J Hum Genet 2009;84:658–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakeley P, Fogarty NM, del Valle I, Wamaitha SE, Hu TX, Elder K, Snell P, Christie L, Robson P, Niakan KK.. Defining the three cell lineages of the human blastocyst by single-cell RNA-seq. Development 2015;142:3151–3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block E. A quantitative morphological investigation of the follicular system in newborn female infants. Acta Anat (Basel) 1953;17:201–206. [DOI] [PubMed] [Google Scholar]
- Bouilly J, Beau I, Barraud S, Bernard V, Azibi K, Fagart J, Fèvre A, Todeschini AL, Veitia RA, Beldjord C. et al. Identification of multiple gene mutations accounts for a new genetic architecture of primary ovarian insufficiency. J Clin Endocrinol Metab 2016;101:4541–4550. [DOI] [PubMed] [Google Scholar]
- Bowles J, Knight D, Smith C, Wilhelm D, Richman J, Mamiya S, Yashiro K, Chawengsaksophak K, Wilson MJ, Rossant J. et al. Retinoid signaling determines germ cell fate in mice. Science 2006;312:596–600. [DOI] [PubMed] [Google Scholar]
- Brosens JJ, Wilson MS, Lam EW.. FOXO transcription factors: from cell fate decisions to regulation of human female reproduction. Adv Exp Med Biol 2009;665:227–241. [DOI] [PubMed] [Google Scholar]
- Buonocore F, Clifford-Mobley O, King TFJ, Striglioni N, Man E, Suntharalingham JP, Del Valle I, Lin L, Lagos CF, Rumsby G. et al. Next-generation sequencing reveals novel genetic variants (SRY, DMRT1, NR5A1, DHH, DHX37) in adults with 46,XY DSD. J Endocr Soc 2019;3:2341–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne PS, Buehr M, Koopman P, Rossant J, McLaren A.. Cell-autonomous action of the testis-determining gene: Sertoli cells are exclusively XY in XX––XY chimaeric mouse testes. Development 1988;102:443–450. [DOI] [PubMed] [Google Scholar]
- Campolo F, Gori M, Favaro R, Nicolis S, Pellegrini M, Botti F, Rossi P, Jannini EA, Dolci S.. Essential role of Sox2 for the establishment and maintenance of the germ cell line. Stem Cells 2013;31:1408–1421. [DOI] [PubMed] [Google Scholar]
- Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA.. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science 2003;301:215–218. [DOI] [PubMed] [Google Scholar]
- Chen D, Liu W, Lukianchikov A, Hancock GV, Zimmerman J, Lowe MG, Kim R, Galic Z, Irie N, Surani MA. et al. Germline competency of human embryonic stem cells depends on eomesodermin. Biol Reprod 2017;97:850–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Liu W, Zimmerman J, Pastor WA, Kim R, Hosohama L, Ho J, Aslanyan M, Gell JJ, Jacobsen SE. et al. The TFAP2C-gegulated OCT4 naive enhancer is involved in human germline formation. Cell Rep 2018;25:3591–3602.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Sun N, Hou L, Kim R, Faith J, Aslanyan M, Tao Y, Zheng Y, Fu J, Liu W. et al. Human primordial germ cells are specified from lineage-primed progenitors. Cell Rep 2019;29:4568–4582.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Sung MT, Cossu-Rocca P, Jones TD, MacLennan GT, De Jong J, Lopez-Beltran A, Montironi R, Looijenga LH.. OCT4: biological functions and clinical applications as a marker of germ cell neoplasia. J Pathol 2007;211:1–9. [DOI] [PubMed] [Google Scholar]
- Cheng L, Thomas A, Roth LM, Zheng W, Michael H, Karim FW.. OCT4: a novel biomarker for dysgerminoma of the ovary. Am J Surg Pathol 2004;28:1341–1346. [DOI] [PubMed] [Google Scholar]
- Chia NY, Chan YS, Feng B, Lu X, Orlov YL, Moreau D, Kumar P, Yang L, Jiang J, Lau MS. et al. A genome-wide RNAi screen reveals determinants of human embryonic stem cell identity. Nature 2010;468:316–320. [DOI] [PubMed] [Google Scholar]
- Childs AJ, Kinnell HL, Collins CS, Hogg K, Bayne RA, Green SJ, McNeilly AS, Anderson RA.. BMP signaling in the human fetal ovary is developmentally regulated and promotes primordial germ cell apoptosis. Stem Cells 2010;28:1368–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitiashvili T, Dror I, Kim R, Hsu FM, Chaudhari R, Pandolfi E, Chen D, Liebscher S, Schenke-Layland K, Plath K. et al. Female human primordial germ cells display X-chromosome dosage compensation despite the absence of X-inactivation. Nat Cell Biol 2020;22:1436–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y, Jeon S, Choi M, Lee MH, Park M, Lee DR, Jun KY, Kwon Y, Lee OH, Song SH. et al. Mutations in SOHLH1 gene associate with nonobstructive azoospermia. Hum Mutat 2010;31:788–793. [DOI] [PubMed] [Google Scholar]
- Clark AT, Reijo Pera RA.. Modeling human germ cell development with embryonic stem cells. Regen Med 2006;1:85–93. [DOI] [PubMed] [Google Scholar]
- Clermont Y. Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonial renewal. Physiol Rev 1972;52:198–236. [DOI] [PubMed] [Google Scholar]
- Costa AL, Lobo J, Jeronimo C, Henrique R.. The epigenetics of testicular germ cell tumors: looking for novel disease biomarkers. Epigenomics 2017;9:155–169. [DOI] [PubMed] [Google Scholar]
- Crisponi L, Deiana M, Loi A, Chiappe F, Uda M, Amati P, Bisceglia L, Zelante L, Nagaraja R, Porcu S. et al. The putative forkhead transcription factor FOXL2 is mutated in blepharophimosis/ptosis/epicanthus inversus syndrome. Nat Genet 2001;27:159–166. [DOI] [PubMed] [Google Scholar]
- Croft B, Ohnesorg T, Hewitt J, Bowles J, Quinn A, Tan J, Corbin V, Pelosi E, van den Bergen J, Sreenivasan R. et al. Human sex reversal is caused by duplication or deletion of core enhancers upstream of SOX9. Nat Commun 2018a;9:5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft B, Ohnesorg T, Sinclair AH.. The role of copy number variants in disorders of sex development. Sex Dev 2018b;12:19–29. [DOI] [PubMed] [Google Scholar]
- Culty M. Gonocytes, the forgotten cells of the germ cell lineage. Birth Defects Res C Embryo Today 2009;87:1–26. [DOI] [PubMed] [Google Scholar]
- De Baere E, Copelli S, Caburet S, Laissue P, Beysen D, Christin-Maitre S, Bouchard P, Veitia R, Fellous M.. Premature ovarian failure and forkhead transcription factor FOXL2: blepharophimosis-ptosis-epicanthus inversus syndrome and ovarian dysfunction. Pediatr Endocrinol Rev 2005;2:653–660. [PubMed] [Google Scholar]
- De Baere E, Lemercier B, Christin-Maitre S, Durval D, Messiaen L, Fellous M, Veitia R.. FOXL2 mutation screening in a large panel of POF patients and XX males. J Med Genet 2002;39:e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong J, Looijenga LH.. Stem cell marker OCT3/4 in tumor biology and germ cell tumor diagnostics: history and future. Crit Rev Oncog 2006;12:171–203. [DOI] [PubMed] [Google Scholar]
- de Jong J, Stoop H, Dohle GR, Bangma CH, Kliffen M, van Esser JW, van den Bent M, Kros JM, Oosterhuis JW, Looijenga LH.. Diagnostic value of OCT3/4 for pre-invasive and invasive testicular germ cell tumours. J Pathol 2005;206:242–249. [DOI] [PubMed] [Google Scholar]
- Di Carlo A, De Felici M.. A role for E-cadherin in mouse primordial germ cell development. Dev Biol 2000;226:209–219. [DOI] [PubMed] [Google Scholar]
- Dominguez AA, Chiang HR, Sukhwani M, Orwig KE, Reijo Pera RA.. Human germ cell formation in xenotransplants of induced pluripotent stem cells carrying X chromosome aneuploidies. Sci Rep 2014;4:6432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan PJ. The germ cell–the mother of all stem cells. Int J Dev Biol 1998;42:1043–1050. [PubMed] [Google Scholar]
- Dovey SL, Valli H, Hermann BP, Sukhwani M, Donohue J, Castro CA, Chu T, Sanfilippo JS, Orwig KE.. Eliminating malignantcontamination from therapeutic human spermatogonial stem cells. J Clin Invest 2013;123:1833–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durruthy-Durruthy J, Ramathal C, Sukhwani M, Fang F, Cui J, Orwig KE, Reijo Pera RA.. Fate of induced pluripotent stem cells following transplantation to murine seminiferous tubules. Hum Mol Genet 2014;23:3071–3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easley CAt, Phillips BT, McGuire MM, Barringer JM, Valli H, Hermann BP, Simerly CR, Rajkovic A, Miki T, Orwig KE. et al. Direct differentiation of human pluripotent stem cells into haploid spermatogenic cells. Cell Rep 2012;2:440–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Khairi R, Achermann JC.. Steroidogenic factor-1 and human disease. Semin Reprod Med 2012;30:374–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar ML, Echeverria OM, Sanchez-Sanchez L, Mendez C, Pedernera E, Vazquez-Nin GH.. Analysis of different cell death processes of prepubertal rat oocytes in vitro. Apoptosis 2010;15:511–526. [DOI] [PubMed] [Google Scholar]
- Estermann MA, Smith CA.. Applying single-cell analysis to gonadogenesis and DSDs (disorders/differences of sex development). Int J Mol Sci 2020;21:6614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang F, Angulo B, Xia N, Sukhwani M, Wang Z, Carey CC, Mazurie A, Cui J, Wilkinson R, Wiedenheft B. et al. A PAX5-OCT4-PRDM1 developmental switch specifies human primordial germ cells. Nat Cell Biol 2018;20:655–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayomi AP, , PetersK, , SukhwaniM, , Valli-PulaskiH, , ShettyG, , MeistrichML, , HouserL, , RobertsonN, , RobertsV, , Ramsey C. et al. Autologous grafting of cryopreserved prepubertal rhesus testis produces sperm and offspring. Science 2019;363:1314–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finelli P, Pincelli AI, Russo S, Bonati MT, Recalcati MP, Masciadri M, Giardino D, Cavagnini F, Larizza L.. Disruption of friend of GATA 2 gene (FOG-2) by a de novo t(8;10) chromosomal translocation is associated with heart defects and gonadal dysgenesis. Clin Genet 2007;71:195–204. [DOI] [PubMed] [Google Scholar]
- Forabosco A, Sforza C, De Pol A, Vizzotto L, Marzona L, Ferrario VF.. Morphometric study of the human neonatal ovary. Anat Rec 1991;231:201–208. [DOI] [PubMed] [Google Scholar]
- Ford CE, Evans EP, Burtenshaw MD, Clegg HM, Tuffrey M, Barnes RD.. A functional ‘sex-reversed’ oocyte in the mouse. Proc R Soc Lond B Biol Sci 1975;190:187–197. [DOI] [PubMed] [Google Scholar]
- Gazit K, Moshonov S, Elfakess R, Sharon M, Mengus G, Davidson I, Dikstein R.. TAF4/4b x TAF12 displays a unique mode of DNA binding and is required for core promoter function of a subset of genes. J Biol Chem 2009;284:26286–26296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genga RMJ, Kernfeld EM, Parsi KM, Parsons TJ, Ziller MJ, Maehr R.. Single-cell RNA-sequencing-based CRISPRi screening resolves molecular drivers of early human endoderm development. Cell Rep 2019;27:708–718.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gersak K, Harris SE, Smale WJ, Shelling AN.. A novel 30 bp deletion in the FOXL2 gene in a phenotypically normal woman with primary amenorrhoea: case report. Hum Reprod 2004;19:2767–2770. [DOI] [PubMed] [Google Scholar]
- Gillis AJ, Stoop H, Biermann K, van Gurp RJ, Swartzman E, Cribbes S, Ferlinz A, Shannon M, Oosterhuis JW, Looijenga LH.. Expression and interdependencies of pluripotency factors LIN28, OCT3/4, NANOG and SOX2 in human testicular germ cells and tumours of the testis. Int J Androl 2011;34:e160–e174. [DOI] [PubMed] [Google Scholar]
- Gkountela S, Li Z, Vincent JJ, Zhang KX, Chen A, Pellegrini M, Clark AT.. The ontogeny of cKIT+ human primordial germ cells proves to be a resource for human germ line reprogramming, imprint erasure and in vitro differentiation. Nat Cell Biol 2013;15:113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gkountela S, Zhang KX, Shafiq TA, Liao WW, Hargan-Calvopina J, Chen PY, Clark AT.. DNA demethylation dynamics in the human prenatal germline. Cell 2015;161:1425–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goertz MJ, Wu Z, Gallardo TD, Hamra FK, Castrillon DH.. Foxo1 is required in mouse spermatogonial stem cells for their maintenance and the initiation of spermatogenesis. J Clin Invest 2011;121:3456–3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith MF. Future surgery: minimal invasion. JAMA 1990;264:2723–2723. [PubMed] [Google Scholar]
- Gomes Fernandes M, Bialecka M, Salvatori DCF, Chuva de Sousa Lopes SM.. Characterization of migratory primordial germ cells in the aorta-gonad-mesonephros of a 4.5-week-old human embryo: a toolbox to evaluate in vitro early gametogenesis. Mol Hum Reprod 2018;24:233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonen N, Futtner CR, Wood S, Garcia-Moreno SA, Salamone IM, Samson SC, Sekido R, Poulat F, Maatouk DM, Lovell-Badge R.. Sex reversal following deletion of a single distal enhancer of Sox9. Science 2018;360:1469–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev 1996;17:121–155. [DOI] [PubMed] [Google Scholar]
- Grimaldi C, Raz E.. Germ cell migration: Evolutionary issues and current understanding. Semin Cell Dev Biol 2020;100:152–159. [DOI] [PubMed] [Google Scholar]
- Grinspon RP, Nevado J, Mori Alvarez M. D L Á, del Rey G, Castera R, Venara M, Chiesa A, Podestá M, Lapunzina P, Rey RA.. 46,XX ovotesticular DSD associated with a SOX3 gene duplication in a SRY-negative boy. Clin Endocrinol 2016;85:673–675. [DOI] [PubMed] [Google Scholar]
- Gubbay J, Collignon J, Koopman P, Capel B, Economou A, Munsterberg A, Vivian N, Goodfellow P, Lovell-Badge R.. A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature 1990;346:245–250. [DOI] [PubMed] [Google Scholar]
- Gummow BM, Winnay JN, Hammer GD.. Convergence of Wnt signaling and steroidogenic factor-1 (SF-1) on transcription of the rat inhibin alpha gene. J Biol Chem 2003;278:26572–26579. [DOI] [PubMed] [Google Scholar]
- Guo J, , NieX, , GieblerM, , MlcochovaH, , WangY, , GrowEJ, , KimR, , TharmalingamM, , MatilionyteG, , Lindskog C. et al. The Dynamic Transcriptional Cell Atlas of Testis Development during Human Puberty. Cell Stem Cell 2020;26:262–276.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F, Yan L, Guo H, Li L, Hu B, Zhao Y, Yong J, Hu Y, Wang X, Wei Y. et al. The Transcriptome and DNA methylome landscapes of human primordial germ cells. Cell 2015;161:1437–1452. [DOI] [PubMed] [Google Scholar]
- Guo J, Grow EJ, Mlcochova H, Maher GJ, Lindskog C, Nie X, Guo Y, Takei Y, Yun J, Cai L. et al. The adult human testis transcriptional cell atlas. Cell Res 2018;28:1141–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Grow EJ, Yi C, Mlcochova H, Maher GJ, Lindskog C, Murphy PJ, Wike CL, Carrell DT, Goriely A. et al. Chromatin and single-cell RNA-Seq profiling reveal dynamic signaling and metabolic transitions during human spermatogonial stem cell development. Cell Stem Cell 2017;21:533–546.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Sosa E, Chitiashvili T, Nie X, Rojas EJ, Oliver E, Plath K, Hotaling JM, Stukenborg J-B, Clark AT. et al. Single-cell analysis of the developing human testis reveals somatic niche cell specification and fetal germline stem cell establishment. Cell Stem Cell 2021;28:764–778.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines B, Hughes J, Corbett M, Shaw M, Innes J, Patel L, Gecz J, Clayton-Smith J, Thomas P.. Interchromosomal insertional translocation at Xq26.3 alters SOX3 expression in an individual with XX male sex reversal. J Clin Endocrinol Metab 2015;100:E815–820. [DOI] [PubMed] [Google Scholar]
- Halder S.K., Laknaur A., Miller J., Layman L.C., Diamond M., Al-Hendy A.. Novel MED12 gene somatic mutations in women from the Southern United States with symptomatic uterine fibroids. Mol Genet Genomics 2015;290:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Zhou Z, Fei L, Sun H, Wang R, Chen Y, Chen H, Wang J, Tang H, Ge W. et al. Construction of a human cell landscape at single-cell level. Nature 2020;581:303–309. [DOI] [PubMed] [Google Scholar]
- Hanna J, Wernig M, Markoulaki S, Sun CW, Meissner A, Cassady JP, Beard C, Brambrink T, Wu LC, Townes TM. et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science 2007;318:1920–1923. [DOI] [PubMed] [Google Scholar]
- Harris SE, Chand AL, Winship IM, Gersak K, Aittomäki K, Shelling AN.. Identification of novel mutations in FOXL2 associated with premature ovarian failure. Mol Hum Reprod 2002;8:729–733. [DOI] [PubMed] [Google Scholar]
- Hart AH, Hartley L, Parker K, Ibrahim M, Looijenga LH, Pauchnik M, Chow CW, Robb L.. The pluripotency homeobox gene NANOG is expressed in human germ cell tumors. Cancer 2005;104:2092–2098. [DOI] [PubMed] [Google Scholar]
- Hayashi K, de Sousa Lopes SM, Surani MA.. Germ cell specification in mice. Science 2007;316:394–396. [DOI] [PubMed] [Google Scholar]
- Heard E, Turner J.. Function of the sex chromosomes in mammalian fertility. Cold Spring Harb Perspect Biol 2011;3:a002675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinonen HR, Pasanen A, Heikinheimo O, Tanskanen T, Palin K, Tolvanen J, Vahteristo P, Sjoberg J, Pitkanen E, Butzow R. et al. Multiple clinical characteristics separate MED12-mutation-positive and -negative uterine leiomyomas. Sci Rep 2017;7:1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller CH, Clermont Y.. Kinetics of the germinal epithelium in man. Recent Prog Horm Res 1964;20:545–575. [PubMed] [Google Scholar]
- Hermann BP, Cheng K, Singh A, Roa-De L, Cruz L, Mutoji KN, Chen IC, Gildersleeve H, Lehle JD, Mayo M, Westernströer . et al. The mammalian spermatogenesis single-cell transcriptome, from spermatogonial stem cells to spermatids. Cell Rep 2018;25:1650–1667.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann BP, Sukhwani M, Hansel MC, Orwig KE.. Spermatogonial stem cells in higher primates: are there differences from those in rodents? Reproduction 2010;139:479–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr W, Cleary MA.. The POU domain: versatility in transcriptional regulation by a flexible two-in-one DNA-binding domain. Genes Dev 1995;9:1679–1693. [DOI] [PubMed] [Google Scholar]
- Hersmus R, Kalfa N, de Leeuw B, Stoop H, Oosterhuis JW, de Krijger R, Wolffenbuttel KP, Drop SL, Veitia RA, Fellous M. et al. FOXL2 and SOX9 as parameters of female and male gonadal differentiation in patients with various forms of disorders of sex development (DSD). J Pathol 2008;215:31–38. [DOI] [PubMed] [Google Scholar]
- Hoei-Hansen CE, Almstrup K, Nielsen JE, Brask Sonne S, Graem N, Skakkebaek NE, Leffers H, Rajpert-De Meyts E.. Stem cell pluripotency factor NANOG is expressed in human fetal gonocytes, testicular carcinoma in situ and germ cell tumours. Histopathology 2005;47:48–56. [DOI] [PubMed] [Google Scholar]
- Hosaka T, Biggs WH 3rd, Tieu D, Boyer AD, Varki NM, Cavenee WK, Arden KC.. Disruption of forkhead transcription factor (FOXO) family members in mice reveals their functional diversification. Proc Natl Acad Sci U S A 2004;101:2975–2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain A, Saunders GF.. The human sex-determining gene SRY is a direct target of WT1. J Biol Chem 2001;276:16817–16823. [DOI] [PubMed] [Google Scholar]
- Hossain A, Saunders GF.. Synergistic cooperation between the beta-catenin signaling pathway and steroidogenic factor 1 in the activation of the Mullerian inhibiting substance type II receptor. J Biol Chem 2003;278:26511–26516. [DOI] [PubMed] [Google Scholar]
- Hoyer PE, Byskov AG, Mollgard K.. Stem cell factor and c-Kit in human primordial germ cells and fetal ovaries. Mol Cell Endocrinol 2005;234:1–10. [DOI] [PubMed] [Google Scholar]
- Hu W, Gauthier L, Baibakov B, Jimenez-Movilla M, Dean J.. FIGLA, a basic helix-loop-helix transcription factor, balances sexually dimorphic gene expression in postnatal oocytes. Mol Cell Biol 2010;30:3661–3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes IA, Nihoul-Fekete C, Thomas B, Cohen-Kettenis PT.. Consequences of the ESPE/LWPES guidelines for diagnosis and treatment of disorders of sex development. Best Pract Res Clin Endocrinol Metab 2007;21:351–365. [DOI] [PubMed] [Google Scholar]
- Irie N, Weinberger L, Tang WW, Kobayashi T, Viukov S, Manor YS, Dietmann S, Hanna JH, Surani MA.. SOX17 is a critical specifier of human primordial germ cell fate. Cell 2015;160:253–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav U, Harris RM, Jameson JL.. Hypogonadotropic hypogonadism in subjects with DAX1 mutations. Mol Cell Endocrinol 2011;346:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson S, Fuller PJ.. Molecular pathogenesis of granulosa cell tumors of the ovary. Endocr Rev 2012;33:109–144. [DOI] [PubMed] [Google Scholar]
- Jones TD, Ulbright TM, Eble JN, Baldridge LA, Cheng L.. OCT4 staining in testicular tumors: a sensitive and specific marker for seminoma and embryonal carcinoma. Am J Surg Pathol 2004;28:935–940. [DOI] [PubMed] [Google Scholar]
- Jordan BK, Shen JH, Olaso R, Ingraham HA, Vilain E.. Wnt4 overexpression disrupts normal testicular vasculature and inhibits testosterone synthesis by repressing steroidogenic factor 1/beta-catenin synergy. Proc Natl Acad Sci U S A 2003;100:10866–10871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen A, Nielsen JE, Blomberg Jensen M, Graem N, Rajpert-De Meyts E.. Analysis of meiosis regulators in human gonads: a sexually dimorphic spatio-temporal expression pattern suggests involvement of DMRT1 in meiotic entry. Mol Hum Reprod 2012;18:523–534. [DOI] [PubMed] [Google Scholar]
- Jung D, Xiong J, Ye M, Qin X, Li L, Cheng S, Luo M, Peng J, Dong J, Tang F. et al. In vitro differentiation of human embryonic stem cells into ovarian follicle-like cells. Nat Commun 2017;8:15680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung SM, Chu PH, Shiu TF, Wu HH, Kuo TT, Chu JJ, Lin PJ.. Expression of OCT4 in the primary germ cell tumors and thymoma in the mediastinum. Appl Immunohistochem Mol Morphol 2006;14:273–275. [DOI] [PubMed] [Google Scholar]
- Junker JP, van Oudenaarden A.. Every cell is special: genome-wide studies add a new dimension to single-cell biology. Cell 2014;157:8–11. [DOI] [PubMed] [Google Scholar]
- Kashimada K, Koopman P.. Sry: the master switch in mammalian sex determination. Development 2010;137:3921–3930. [DOI] [PubMed] [Google Scholar]
- Kee K, Angeles VT, Flores M, Nguyen HN, Reijo Pera RA.. Human DAZL, DAZ and BOULE genes modulate primordial germ-cell and haploid gamete formation. Nature 2009;462:222–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee K, Gonsalves JM, Clark AT, Pera RA.. Bone morphogenetic proteins induce germ cell differentiation from human embryonic stem cells. Stem Cells Dev 2006;15:831–837. [DOI] [PubMed] [Google Scholar]
- Kehler J, Tolkunova E, Koschorz B, Pesce M, Gentile L, Boiani M, Lomeli H, Nagy A, McLaughlin KJ, Scholer HR. et al. Oct4 is required for primordial germ cell survival. EMBO Rep 2004;5:1078–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr CL, Hill CM, Blumenthal PD, Gearhart JD.. Expression of pluripotent stem cell markers in the human fetal ovary. Hum Reprod 2008;23:589–599. [DOI] [PubMed] [Google Scholar]
- Kim J, Chu J, Shen X, Wang J, Orkin SH.. An extended transcriptional network for pluripotency of embryonic stem cells. Cell 2008;132:1049–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klamt B, Koziell A, Poulat F, Wieacker P, Scambler P, Berta P, Gessler M.. Frasier syndrome is caused by defective alternative splicing of WT1 leading to an altered ratio of WT1 +/-KTS splice isoforms. Hum Mol Genet 1998;7:709–714. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Surani MA.. On the origin of the human germline. Development 2018;145:dev150433. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Zhang H, Tang WWC, Irie N, Withey S, Klisch D, Sybirna A, Dietmann S, Contreras DA, Webb R. et al. Principles of early human development and germ cell program from conserved model systems. Nature 2017;546:416–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima Y, Sasaki K, Yokobayashi S, Sakai Y, Nakamura T, Yabuta Y, Nakaki F, Nagaoka S, Woltjen K, Hotta A. et al. Evolutionarily distinctive transcriptional and signaling programs drive human germ cell lineage specification from pluripotent stem cells. Cell Stem Cell 2017;21:517–532.e15. [DOI] [PubMed] [Google Scholar]
- Kojima Y, Yamashiro C, Murase Y, Yabuta Y, Okamoto I, Iwatani C, Tsuchiya H, Nakaya M, Tsukiyama T, Nakamura T. et al. GATA transcription factors, SOX17 and TFAP2C, drive the human germ-cell specification program. Life Sci Alliance 2021;4:e202000974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman P. Sry, Sox9 and mammalian sex determination. EXS 2001;25–56. [DOI] [PubMed] [Google Scholar]
- Koopman P, Munsterberg A, Capel B, Vivian N, Lovell-Badge R.. Expression of a candidate sex-determining gene during mouse testis differentiation. Nature 1990;348:450–452. [DOI] [PubMed] [Google Scholar]
- Koubova J, Menke DB, Zhou Q, Capel B, Griswold MD, Page DC.. Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc Natl Acad Sci U S A 2006;103:2474–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozhukhar VG. SRY and SOX9: the main genetic factors of mammalian sex determination. Tsitologiia 2012;54:390–404. [PubMed] [Google Scholar]
- Krausz C, Riera-Escamilla A.. Genetics of male infertility. Nat Rev Urol 2018;15:369–384. [DOI] [PubMed] [Google Scholar]
- Kuijk EW, Chuva de Sousa Lopes SM, Geijsen N, Macklon N, Roelen BA.. The different shades of mammalian pluripotent stem cells. Hum Reprod Update 2011;17:254–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La H, Yoo H, Lee EJ, Thang NX, Choi HJ, Oh J, Park JH, Hong K.. Insights from the applications of single-cell transcriptomic analysis in germ cell development and reproductive medicine. Int J Mol Sci 2021;22:823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhal B, , LaissueP, , ElghèzalH, , Fellous M.. [Genetic analysis of premature ovarian failure: role of forkhead and TGF-beta genes]. Gynecol Obstet Fertil 2008;36:862–871. [DOI] [PubMed] [Google Scholar]
- Laumonnier F, Ronce N, Hamel BC, Thomas P, Lespinasse J, Raynaud M, Paringaux C, Van Bokhoven H, Kalscheuer V, Fryns JP. et al. Transcription factor SOX3 is involved in X-linked mental retardation with growth hormone deficiency. Am J Hum Genet 2002;71:1450–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurentino S, , HeckmannL, , Di PersioS, , LiX, , Meyer Zu HörsteG, , WistubaJ, , CremersJ-F, , GromollJ, , KlieschS, , Schlatt S. et al. High-resolution analysis of germ cells from men with sex chromosomal aneuploidies reveals normal transcriptome but impaired imprinting. Clin Epigenetics 2019;11:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson KA, Dunn NR, Roelen BA, Zeinstra LM, Davis AM, Wright CV, Korving JP, Hogan BL.. Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev 1999;13:424–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledig S, Hiort O, Wunsch L, Wieacker P.. Partial deletion of DMRT1 causes 46,XY ovotesticular disorder of sexual development. Eur J Endocrinol 2012;167:119–124. [DOI] [PubMed] [Google Scholar]
- Lee PA, Houk CP, Ahmed SF, Hughes IA, International Consensus Conference on Intersex organized by the Lawson Wilkins Pediatric Endocrine Society and the European Society for Paediatric Endocrinology. Consensus statement on management of intersex disorders. International Consensus Conference on Intersex. Pediatrics 2006;118:e488–e500. [DOI] [PubMed] [Google Scholar]
- Leitch HG, Tang WW, Surani MA.. Primordial germ-cell development and epigenetic reprogramming in mammals. Curr Top Dev Biol 2013;104:149–187. [DOI] [PubMed] [Google Scholar]
- Li L, Dong J, Yan L, Yong J, Liu X, Hu Y, Fan X, Wu X, Guo H, Wang X. et al. Single-cell RNA-seq analysis maps development of human germline cells and gonadal niche interactions. Cell Stem Cell 2017;20:858–873.e4. [DOI] [PubMed] [Google Scholar]
- Li L, Yang R, Yin C, Kee K.. Studying human reproductive biology through single-cell analysis and in vitro differentiation of stem cells into germ cell-like cells. Hum Reprod Update 2020;26:670–688. [DOI] [PubMed] [Google Scholar]
- Li W, Wu J, Kim SY, Zhao M, Hearn SA, Zhang MQ, Meistrich ML, Mills AA.. Chd5 orchestrates chromatin remodelling during sperm development. Nat Commun 2014;5:3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin IY, Chiu FL, Yeang CH, Chen HF, Chuang CY, Yang SY, Hou PS, Sintupisut N, Ho HN, Kuo HC. et al. Suppression of the SOX2 neural effector gene by PRDM1 promotes human germ cell fate in embryonic stem cells. Stem Cell Reports 2014;2:189–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litchfield K, Levy M, Huddart RA, Shipley J, Turnbull C.. The genomic landscape of testicular germ cell tumours: from susceptibility to treatment. Nat Rev Urol 2016;13:409–419. [DOI] [PubMed] [Google Scholar]
- Litchfield K, Levy M, Orlando G, Loveday C, Law PJ, Migliorini G, Holroyd A, Broderick P, Karlsson R, Haugen TB. et al. Identification of 19 new risk loci and potential regulatory mechanisms influencing susceptibility to testicular germ cell tumor. Nat Genet 2017;49:1133–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Castrillon DH, Zhou W, Richards JS.. FOXO1/3 depletion in granulosa cells alters follicle growth, death and regulation of pituitary FSH. Mol Endocrinol 2013;27:238–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Nie YH, Zhang CC, Cai YJ, Wang Y, Lu HP, Li YZ, Cheng C, Qiu ZL, Sun Q.. Generation of macaques with sperm derived from juvenile monkey testicular xenografts. Cell Res 2016;26:139–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looijenga LH, Stoop H, de Leeuw HP, de Gouveia Brazao CA, Gillis AJ, van Roozendaal KE, van Zoelen EJ, Weber RF, Wolffenbuttel KP, van Dekken H. et al. POU5F1 (OCT3/4) identifies cells with pluripotent potential in human germ cell tumors. Cancer Res 2003;63:2244–2250. [PubMed] [Google Scholar]
- Lopes AM, Aston KI, Thompson E, Carvalho F, Gonçalves J, Huang N, Matthiesen R, Noordam MJ, Quintela I, Ramu A. et al. Human spermatogenic failure purges deleterious mutation load from the autosomes and both sex chromosomes, including the gene DMRT1. PLoS Genet 2013;9:e1003349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourenco D, Brauner R, Rybczynska M, Nihoul-Fekete C, McElreavey K, Bashamboo A.. Loss-of-function mutation in GATA4 causes anomalies of human testicular development. Proc Natl Acad Sci USA 2011;108:1597–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnúsdóttir E, Dietmann S, Murakami K, Günesdogan U, Tang F, Bao S, Diamanti E, Lao K, Gottgens B, Surani M.. A tripartite transcription factor network regulates primordial germ cell specification in mice. Nat Cell Biol 2013;15:905–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makar K, Sasaki K.. Roadmap of germline development and in vitro gametogenesis from pluripotent stem cells. Andrology 2020;8:842–851. [DOI] [PubMed] [Google Scholar]
- Mamsen LS, Brochner CB, Byskov AG, Mollgard K.. The migration and loss of human primordial germ stem cells from the hind gut epithelium towards the gonadal ridge. Int J Dev Biol 2012;56:771–778. [DOI] [PubMed] [Google Scholar]
- Matson CK, Murphy MW, Sarver AL, Griswold MD, Bardwell VJ, Zarkower D.. DMRT1 prevents female reprogramming in the postnatal mammalian testis. Nature 2011;476:101–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa-Watanabe Y, Inoue J, Semba K.. Transcriptional activity of testis-determining factor SRY is modulated by the Wilms' tumor 1 gene product, WT1. Oncogene 2003;22:7900–7904. [DOI] [PubMed] [Google Scholar]
- McKay DG, Hertig AT, Adams EC, Danziger S.. Histochemical observations on the germ cells of human embryos. Anat Rec 1953;117:201–219. [DOI] [PubMed] [Google Scholar]
- McElreavey K, Fellous M.. Sex determination and the Y chromosome. Am J Med Genet 1999;89:176–185. [DOI] [PubMed] [Google Scholar]
- McElreavy K, Vilain E, Abbas N, Costa JM, Souleyreau N, Kucheria K, Boucekkine C, Thibaud E, Brauner R, Flamant F.. XY sex reversal associated with a deletion 5' to the SRY "HMG box" in the testis-determining region. Proc Natl Acad Sci U S A 1992;89:11016–11020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren A. Primordial germ cells in the mouse. Dev Biol 2003;262:1–15. [DOI] [PubMed] [Google Scholar]
- Medrano JV, Martinez-Arroyo AM, Miguez JM, Moreno I, Martinez S, Quinonero A, Diaz-Gimeno P, Marques-Mari AI, Pellicer A, Remohi J. et al. Human somatic cells subjected to genetic induction with six germ line-related factors display meiotic germ cell-like features. Sci Rep 2016;6:24956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlmann LM. Stops and starts in mammalian oocytes: recent advances in understanding the regulation of meiotic arrest and oocyte maturation. Reproduction 2005;130:791–799. [DOI] [PubMed] [Google Scholar]
- Mizusaki H, Kawabe K, Mukai T, Ariyoshi E, Kasahara M, Yoshioka H, Swain A, Morohashi K.. Dax-1 (dosage-sensitive sex reversal-adrenal hypoplasia congenita critical region on the X chromosome, gene 1) gene transcription is regulated by wnt4 in the female developing gonad. Mol Endocrinol 2003;17:507–519. [DOI] [PubMed] [Google Scholar]
- Moalem S, Babul-Hirji R, Stavropolous DJ, Wherrett D, Bagli DJ, Thomas P, Chitayat D.. XX male sex reversal with genital abnormalities associated with a de novo SOX3 gene duplication. Am J Med Genet A 2012;158A:1759–1764. [DOI] [PubMed] [Google Scholar]
- Mollgard K, Jespersen A, Lutterodt MC, Yding Andersen C, Hoyer PE, Byskov AG.. Human primordial germ cells migrate along nerve fibers and Schwann cells from the dorsal hind gut mesentery to the gonadal ridge. Mol Hum Reprod 2010;16:621–631. [DOI] [PubMed] [Google Scholar]
- Molyneaux KA, Zinszner H, Kunwar PS, Schaible K, Stebler J, Sunshine MJ, O'Brien W, Raz E, Littman D, Wylie C. et al. The chemokine SDF1/CXCL12 and its receptor CXCR4 regulate mouse germ cell migration and survival. Development 2003;130:4279–4286. [DOI] [PubMed] [Google Scholar]
- Motta PM, Makabe S, Nottola SA.. The ultrastructure of human reproduction. I. The natural history of the female germ cell: origin, migration and differentiation inside the developing ovary. Hum Reprod Update 1997;3:281–295. [DOI] [PubMed] [Google Scholar]
- Mou L, Wang Y, Li H, Huang Y, Jiang T, Huang W, Li Z, Chen J, Xie J, Liu Y. et al. A dominant-negative mutation of HSF2 associated with idiopathic azoospermia. Hum Genet 2013;132:159–165. [DOI] [PubMed] [Google Scholar]
- Murakami K, Günesdogan U, Zylicz JJ, Tang WWC, Sengupta R, Kobayashi T, Kim S, Butler R, Dietmann S, Surani MA.. NANOG alone induces germ cells in primed epiblast in vitro by activation of enhancers. Nature 2016;529:403–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase Y, Yabuta Y, Ohta H, Yamashiro C, Nakamura T, Yamamoto T, Saitou M.. Long-term expansion with germline potential of human primordial germ cell-like cells in vitro. Embo J 2020;39:e104929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatelli F, Strom TM, Walker AP, Zanaria E, Récan D, Meindl A, Bardoni B, Guioli S, Zehetner G, Rabl W.. Mutations in the DAX-1 gene give rise to both X-linked adrenal hypoplasia congenita and hypogonadotropic hypogonadism. Nature 1994;372:672–676. [DOI] [PubMed] [Google Scholar]
- Nagano M, Patrizio P, Brinster RL.. Long-term survival of human spermatogonial stem cells in mouse testes. Fertil Steril 2002;78:1225–1233. [DOI] [PubMed] [Google Scholar]
- Nakaki F, Hayashi K, Ohta H, Kurimoto K, Yabuta Y, Saitou M.. Induction of mouse germ-cell fate by transcription factors in vitro. Nature 2013;501:222–226. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Miyado M, Saito K, Katsumi M, Nakamura A, Kobori Y, Tanaka Y, Ishikawa H, Yoshida A, Okada H. et al. Next-generation sequencing for patients with non-obstructive azoospermia: implications for significant roles of monogenic/oligogenic mutations. Andrology 2017;5:824–831. [DOI] [PubMed] [Google Scholar]
- Nallathambi J, Moumné L, De Baere E, Beysen D, Usha K, Sundaresan P, Veitia RA.. A novel polyalanine expansion in FOXL2: the first evidence for a recessive form of the blepharophimosis syndrome (BPES) associated with ovarian dysfunction. Hum Genet 2007;121:107–112. [DOI] [PubMed] [Google Scholar]
- Nelson TJ, Martinez-Fernandez A, Yamada S, Perez-Terzic C, Ikeda Y, Terzic A.. Repair of acute myocardial infarction by human stemness factors induced pluripotent stem cells. Circulation 2009;120:408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng HH, Surani MA.. The transcriptional and signalling networks of pluripotency. Nat Cell Biol 2011;13:490–496. [DOI] [PubMed] [Google Scholar]
- Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, Scholer H, Smith A.. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell 1998;95:379–391. [DOI] [PubMed] [Google Scholar]
- Niwa H. Transcription factor network governing cellular pluripotency. Rinsho Ketsueki 2009;50:1524–1530. [PubMed] [Google Scholar]
- Niwa H, Miyazaki J, Smith AG.. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet 2000;24:372–376. [DOI] [PubMed] [Google Scholar]
- Nonaka D. Differential expression of SOX2 and SOX17 in testicular germ cell tumors. Am J Clin Pathol 2009;131:731–736. [DOI] [PubMed] [Google Scholar]
- Nordenvall AS, Frisen L, Nordenstrom A, Lichtenstein P, Nordenskjold A.. Population based nationwide study of hypospadias in Sweden, 1973 to 2009: incidence and risk factors. J Urol 2014;191:783–789. [DOI] [PubMed] [Google Scholar]
- Ohinata Y, Ohta H, Shigeta M, Yamanaka K, Wakayama T, Saitou M.. A signaling principle for the specification of the germ cell lineage in mice. Cell 2009;137:571–584. [DOI] [PubMed] [Google Scholar]
- Ohinata Y, Payer B, O'Carroll D, Ancelin K, Ono Y, Sano M, Barton SC, Obukhanych T, Nussenzweig M, Tarakhovsky A. et al. Blimp1 is a critical determinant of the germ cell lineage in mice. Nature 2005;436:207–213. [DOI] [PubMed] [Google Scholar]
- Oosterhuis JW, Looijenga LH.. Testicular germ-cell tumours in a broader perspective. Nat Rev Cancer 2005;5:210–222. [DOI] [PubMed] [Google Scholar]
- Otte J, Wruck W, Adjaye J.. New insights into human primordial germ cells and early embryonic development from single-cell analysis. FEBS Lett 2017;591:2226–2240. [DOI] [PubMed] [Google Scholar]
- Ottolenghi C, McElreavey K.. Deletions of 9p and the quest for a conserved mechanism of sex determination. Mol Genet Metab 2000;71:397–404. [DOI] [PubMed] [Google Scholar]
- Palmer SJ, Burgoyne PS.. In situ analysis of fetal, prepuberal and adult XX––XY chimaeric mouse testes: Sertoli cells are predominantly, but not exclusively, XY. Development 1991;112:265–268. [DOI] [PubMed] [Google Scholar]
- Pangas SA, Choi Y, Ballow DJ, Zhao Y, Westphal H, Matzuk MM, Rajkovic A.. Oogenesis requires germ cell-specific transcriptional regulators Sohlh1 and Lhx8. Proc Natl Acad Sci U S A 2006;103:8090–8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannetier M, Pailhoux E.. FOXL2, the gatekeeper of ovarian identity. Med Sci (Paris) 2010;26:470–473. [DOI] [PubMed] [Google Scholar]
- Panula S, Reda A, Stukenborg JB, Ramathal C, Sukhwani M, Albalushi H, Edsgard D, Nakamura M, Soder O, Orwig KE. et al. Over-expression of NANOS3 and DAZL in human embryonic stem cells. PLoS One 2016;11:e0165268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TS, Galic Z, Conway AE, Lindgren A, van Handel BJ, Magnusson M, Richter L, Teitell MA, Mikkola HK, Lowry WE. et al. Derivation of primordial germ cells from human embryonic and induced pluripotent stem cells is significantly improved by coculture with human fetal gonadal cells. Stem Cells 2009;27:783–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patek CE, Kerr JB, Gosden RG, Jones KW, Hardy K, Muggleton-Harris AL, Handyside AH, Whittingham DG, Hooper ML.. Sex chimaerism, fertility and sex determination in the mouse. Development 1991;113:311–325. [DOI] [PubMed] [Google Scholar]
- Pauls K, Jager R, Weber S, Wardelmann E, Koch A, Buttner R, Schorle H.. Transcription factor AP-2gamma, a novel marker of gonocytes and seminomatous germ cell tumors. Int J Cancer 2005;115:470–477. [DOI] [PubMed] [Google Scholar]
- Pelletier J, Bruening W, Kashtan CE, Mauer SM, Manivel JC, Striegel JE, Houghton DC, Junien C, Habib R, Fouser L.. Germline mutations in the Wilms' tumor suppressor gene are associated with abnormal urogenital development in Denys-Drash syndrome. Cell 1991;67:437–447. [DOI] [PubMed] [Google Scholar]
- Pepling ME, Spradling AC.. Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev Biol 2001;234:339–351. [DOI] [PubMed] [Google Scholar]
- Pereda J, Zorn T, Soto-Suazo M.. Migration of human and mouse primordial germ cells and colonization of the developing ovary: an ultrastructural and cytochemical study. Microsc Res Tech 2006;69:386–395. [DOI] [PubMed] [Google Scholar]
- Pereda J, Zorn TM, Soto M, Motta PM.. Morphological and cytochemical study of extracellular matrix during the migratory phase of human and mouse primordial germ cells. Ital J Anat Embryol 1998;103:41–50. [PubMed] [Google Scholar]
- Perrett RM, Turnpenny L, Eckert JJ, O'Shea M, Sonne SB, Cameron IT, Wilson DI, Rajpert-De Meyts E, Hanley NA.. The early human germ cell lineage does not express SOX2 during in vivo development or upon in vitro culture. Biol Reprod 2008;78:852–858. [DOI] [PubMed] [Google Scholar]
- Pesce M, Scholer HR.. Oct-4: gatekeeper in the beginnings of mammalian development. Stem Cells 2001;19:271–278. [DOI] [PubMed] [Google Scholar]
- Pesce M, Wang X, Wolgemuth DJ, Scholer H.. Differential expression of the Oct-4 transcription factor during mouse germ cell differentiation. Mech Dev 1998;71:89–98. [DOI] [PubMed] [Google Scholar]
- Petropoulos S, Edsgärd D, Reinius B, Deng Q, Panula SP, Codeluppi S, Plaza Reyes A, Linnarsson S, Sandberg R, Lanner F.. Single-cell RNA-seq reveals lineage and X chromosome dynamics in human preimplantation embryos. Cell 2016;165:1012–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philibert P, Leprieur E, Zenaty D, Thibaud E, Polak M, Frances AM, Lespinasse J, Raingeard I, Servant N, Audran F. et al. Steroidogenic factor-1 (SF-1) gene mutation as a frequent cause of primary amenorrhea in 46,XY female adolescents with low testosterone concentration. Reprod Biol Endocrinol 2010;8:28–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plass M, Solana J, Wolf FA, Ayoub S, Misios A, Glazar P, Obermayer B, Theis FJ, Kocks C, Rajewsky N.. Cell type atlas and lineage tree of a whole complex animal by single-cell transcriptomics. Science 2018;360:eaaq1723. [DOI] [PubMed] [Google Scholar]
- Portnoi MF, Dumargne MC, Rojo S, Witchel SF, Duncan AJ, Eozenou C, Bignon-Topalovic J, Yatsenko SA, Rajkovic A, Reyes-Mugica M. et al. Mutations involving the SRY-related gene SOX8 are associated with a spectrum of human reproductive anomalies. Hum Mol Genet 2018;27:1228–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, , SunM, , YouL, , WeiD, , SunJ, , LiangX, , ZhangB, , JiangH, , XuJ, , Chen Z-J.. ESR1, HK3 and BRSK1 gene variants are associated with both age at natural menopause and premature ovarian failure. Orphanet J Rare Dis 2012;7:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Choi Y, Zhao H, Simpson JL, Chen ZJ, Rajkovic A.. NOBOX homeobox mutation causes premature ovarian failure. Am J Hum Genet 2007;81:576–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu P, Bai Y, Pan S, Li W, Liu W, Hua J.. Gender depended potentiality of differentiation of human umbilical cord mesenchymal stem cells into oocyte-like cells in vitro. Cell Biochem Funct 2013;31:365–373. [DOI] [PubMed] [Google Scholar]
- Raj B, Wagner DE, McKenna A, Pandey S, Klein AM, Shendure J, Gagnon JA, Schier AF.. Simultaneous single-cell profiling of lineages and cell types in the vertebrate brain. Nat Biotechnol 2018;36:442–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkovic A, Pangas SA, Ballow D, Suzumori N, Matzuk MM.. NOBOX deficiency disrupts early folliculogenesis and oocyte-specific gene expression. Science 2004;305:1157–1159. [DOI] [PubMed] [Google Scholar]
- Rajpert-De Meyts E, Hanstein R, Jorgensen N, Graem N, Vogt PH, Skakkebaek NE.. Developmental expression of POU5F1 (OCT-3/4) in normal and dysgenetic human gonads. Hum Reprod 2004;19:1338–1344. [DOI] [PubMed] [Google Scholar]
- Ramathal C, Durruthy-Durruthy J, Sukhwani M, Arakaki JE, Turek PJ, Orwig KE, Reijo Pera RA.. Fate of iPSCs derived from azoospermic and fertile men following xenotransplantation to murine seminiferous tubules. Cell Rep 2014;7:1284–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raser JM, O'Shea EK.. Noise in gene expression: origins, consequences, and control. Science 2005;309:2010–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond CS, Parker ED, Kettlewell JR, Brown LG, Page DC, Kusz K, Jaruzelska J, Reinberg Y, Flejter WL, Bardwell VJ. et al. A region of human chromosome 9p required for testis development contains two genes related to known sexual regulators. Hum Mol Genet 1999;8:989–996. [DOI] [PubMed] [Google Scholar]
- Reijo R, Lee TY, Salo P, Alagappan R, Brown LG, Rosenberg M, Rozen S, Jaffe T, Straus D, Hovatta O.. Diverse spermatogenic defects in humans caused by Y chromosome deletions encompassing a novel RNA-binding protein gene. Nat Genet 1995;10:383–393. [DOI] [PubMed] [Google Scholar]
- Richards JS, Sharma SC, Falender AE, Lo YH.. Expression of FKHR, FKHRL1, and AFX genes in the rodent ovary: evidence for regulation by IGF-I, estrogen, and the gonadotropins. Mol Endocrinol 2002;16:580–599. [DOI] [PubMed] [Google Scholar]
- Richardson BE, Lehmann R.. Mechanisms guiding primordial germ cell migration: strategies from different organisms. Nat Rev Mol Cell Biol 2010;11:37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richie JP. OCT4 staining in testicular tumors. A sensitive and specific marker for seminoma and embryonal carcinoma. J Urol 2005;174:569–570. [DOI] [PubMed] [Google Scholar]
- Rossant J. Mouse and human blastocyst-derived stem cells: vive les differences. Development 2015;142:9–12. [DOI] [PubMed] [Google Scholar]
- Rossi P. Transcriptional control of KIT gene expression during germ cell development. Int J Dev Biol 2013;57:179–184. [DOI] [PubMed] [Google Scholar]
- Rotgers E, Jorgensen A, Yao HH.. At the crossroads of fate-somatic cell lineage specification in the fetal gonad. Endocr Rev 2018;39:739–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou M, Hayashi K.. Mammalian in vitro gametogenesis. Science 2021;374:eaaz6830. [DOI] [PubMed] [Google Scholar]
- Saitou M, Yamaji M.. Primordial germ cells in mice. Cold Spring Harb Perspect Biol 2012;4:a008375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanlaville D, Vialard F, Thepot F, Vue-Droy L, Ardalan A, Nizard P, Corre A, Devauchelle B, Martin-Denavit T, Nouchy M. et al. Functional disomy of Xp including duplication of DAX1 gene with sex reversal due to t(X;Y)(p21.2;p11.3). Am J Med Genet A 2004;128A:325–330. [DOI] [PubMed] [Google Scholar]
- Santagata S, Ligon KL, Hornick JL.. Embryonic stem cell transcription factor signatures in the diagnosis of primary and metastatic germ cell tumors. Am J Surg Pathol 2007;31:836–845. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Nakamura T, Okamoto I, Yabuta Y, Iwatani C, Tsuchiya H, Seita Y, Nakamura S, Shiraki N, Takakuwa T. et al. The germ cell fate of cynomolgus monkeys is specified in the nascent amnion. Dev Cell 2016;39:169–185. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Yokobayashi S, Nakamura T, Okamoto I, Yabuta Y, Kurimoto K, Ohta H, Moritoki Y, Iwatani C, Tsuchiya H. et al. Robust in vitro induction of human germ cell fate from pluripotent stem cells. Cell Stem Cell 2015;17:178–194. [DOI] [PubMed] [Google Scholar]
- Schlessinger D, , Garcia-OrtizJ-E, , ForaboscoA, , UdaM, , CrisponiL, , Pelosi E.. Determination and stability of gonadal sex. J Androl 2010;31:16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholer HR. Octamania: the POU factors in murine development. Trends Genet 1991;7:323–329. [DOI] [PubMed] [Google Scholar]
- Scholer HR, Ruppert S, Suzuki N, Chowdhury K, Gruss P.. New type of POU domain in germ line-specific protein Oct-4. Nature 1990;344:435–439. [DOI] [PubMed] [Google Scholar]
- Sekido R, Lovell-Badge R.. Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature 2008;453:930–934. [DOI] [PubMed] [Google Scholar]
- Shah SP, Kobel M, Senz J, Morin RD, Clarke BA, Wiegand KC, Leung G, Zayed A, Mehl E, Kalloger SE. et al. Mutation of FOXL2 in granulosa-cell tumors of the ovary. N Engl J Med 2009;360:2719–2729. [DOI] [PubMed] [Google Scholar]
- Shen H, Shih J, Hollern DP, Wang L, Bowlby R, Tickoo SK, Thorsson V, Mungall AJ, Newton Y, Hegde AM. et al. Integrated molecular characterization of testicular germ cell tumors. Cell Rep 2018;23:3392–3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura R, Fraizer GC, Trapman J, Lau Yf C, Saunders GF.. The Wilms' tumor gene WT1 can regulate genes involved in sex determination and differentiation: SRY, Mullerian-inhibiting substance, and the androgen receptor. Clin Cancer Res 1997;3:2571–2580. [PubMed] [Google Scholar]
- Shin YH, Ren Y, Suzuki H, Golnoski KJ, Ahn HW, Mico V, Rajkovic A.. Transcription factors SOHLH1 and SOHLH2 coordinate oocyte differentiation without affecting meiosis I. J Clin Invest 2017;127:2106–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair AH, Berta P, Palmer MS, Hawkins JR, Griffiths BL, Smith MJ, Foster JW, Frischauf AM, Lovell-Badge R, Goodfellow PN.. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature 1990;346:240–244. [DOI] [PubMed] [Google Scholar]
- Skakkebaek NE. Possible carcinoma-in-situ of the testis. Lancet 1972;2:516–517. [DOI] [PubMed] [Google Scholar]
- Skakkebaek NE. Carcinoma in situ of the testis: frequency and relationship to invasive germ cell tumours in infertile men. Histopathology 2002;41:5–18. [PubMed] [Google Scholar]
- Sohni A, Tan K, Song HW, Burow D, de Rooij DG, Laurent L, Hsieh TC, Rabah R, Hammoud SS, Vicini E. et al. The Neonatal and adult human testis defined at the single-cell level. Cell Rep 2019;26:1501–1517.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyal SM, Amleh A, Dean J.. FIGalpha, a germ cell-specific transcription factor required for ovarian follicle formation. Development 2000;127:4645–4654. [DOI] [PubMed] [Google Scholar]
- Stevant I, Nef S.. Genetic control of gonadal sex determination and development. Trends Genet 2019;35:346–358. [DOI] [PubMed] [Google Scholar]
- Stirparo GG, Boroviak T, Guo G, Nichols J, Smith A, Bertone P.. Integrated analysis of single-cell embryo data yields a unified transcriptome signature for the human pre-implantation epiblast. Development 2018;145:dev158501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoop H, Honecker F, Cools M, de Krijger R, Bokemeyer C, Looijenga LH.. Differentiation and development of human female germ cells during prenatal gonadogenesis: an immunohistochemical study. Hum Reprod 2005;20:1466–1476. [DOI] [PubMed] [Google Scholar]
- Sugawa F, Araúzo-Bravo MJ, Yoon J, Kim KP, Aramaki S, Wu G, Stehling M, Psathaki OE, Hübner K, Schöler HR.. Human primordial germ cell commitment in vitro associates with a unique PRDM14 expression profile. Embo J 2015;34:1009–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton E, Hughes J, White S, Sekido R, Tan J, Arboleda V, Rogers N, Knower K, Rowley L, Eyre H. et al. Identification of SOX3 as an XX male sex reversal gene in mice and humans. J Clin Invest 2011;121:328–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Ahn HW, Chu T, Bowden W, Gassei K, Orwig K, Rajkovic A.. SOHLH1 and SOHLH2 coordinate spermatogonial differentiation. Dev Biol 2012;361:301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sybirna A, Tang WWC, Pierson Smela M, Dietmann S, Gruhn WH, Brosh R, Surani MA.. A critical role of PRDM14 in human primordial germ cell fate revealed by inducible degrons. Nat Commun 2020;11:1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sybirna A, Wong FCK, Surani MA.. Genetic basis for primordial germ cells specification in mouse and human: Conserved and divergent roles of PRDM and SOX transcription factors. Curr Top Dev Biol 2019;135:35–89. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S.. Induced pluripotent stem cells in medicine and biology. Development 2013;140:2457–2461. [DOI] [PubMed] [Google Scholar]
- Tadokoro Y, Yomogida K, Ohta H, Tohda A, Nishimune Y.. Homeostatic regulation of germinal stem cell proliferation by the GDNF/FSH pathway. Mech Dev 2002;113:29–39. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S.. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007;131:861–872. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S, Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:1. 663–676. [DOI] [PubMed] [Google Scholar]
- Taketo-Hosotani T, Nishioka Y, Nagamine CM, Villalpando I, Merchant-Larios H, Development and fertility of ovaries in the B6.YDOM sex-reversed female mouse. Development 1989;107:95–105. [DOI] [PubMed] [Google Scholar]
- Tam PP, Behringer RR, Mouse gastrulation: the formation of a mammalian body plan. Mech Dev 1997;68:6. 3–25. [DOI] [PubMed] [Google Scholar]
- Tan ZP, Huang C, Xu ZB, Yang JF, Yang YF, Novel ZFPM2/FOG2 variants in patients with double outlet right ventricle. Clin Genet 2012;82:8. 466–471. [DOI] [PubMed] [Google Scholar]
- Tang WW, Dietmann S, Irie N, Leitch HG, Floros VI, Bradshaw CR, Hackett JA, Chinnery PF, Surani MA, A unique gene regulatory network resets the human germline epigenome for development. Cell 2015;161:1. 1453–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang WW, Kobayashi T, Irie N, Dietmann S, Surani MA, Specification and epigenetic programming of the human germ line. Nat Rev Genet 2016;17:1. 585–600. [DOI] [PubMed] [Google Scholar]
- Tannour-Louet M, Han S, Corbett ST, Louet JF, Yatsenko S, Meyers L, Shaw CA, Kang SH, Cheung SW, Lamb DJ.. Identification of de novo copy number variants associated with human disorders of sexual development. PLoS One 2010;5:e15392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarnawa ED, Baker MD, Aloisio GM, Carr BR, Castrillon DH, Gonadal expression of Foxo1, but not Foxo3, is conserved in diverse mammalian species. Biol Reprod 2013;88. 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewes AC, Ledig S, Tüttelmann F, Kliesch S, Wieacker P.. DMRT1 mutations are rarely associated with male infertility. Fertil Steril 2014;102:816–820.e13. [DOI] [PubMed] [Google Scholar]
- Toyoda S, Yoshimura T, Mizuta J, Miyazaki J.. Auto-regulation of the Sohlh1 gene by the SOHLH2/SOHLH1/SP1 complex: implications for early spermatogenesis and oogenesis. PLoS One 2014;9:e101681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udar N, Yellore V, Chalukya M, Yelchits S, Silva-Garcia R, Small K, BPES Consortium. Comparative analysis of the FOXL2 gene and characterization of mutations in BPES patients. Hum Mutat 2003;22:222–228. [DOI] [PubMed] [Google Scholar]
- Uhlenhaut NH, Jakob S, Anlag K, Eisenberger T, Sekido R, Kress J, Treier A-C, Klugmann C, Klasen C, Holter NI. et al. Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell 2009;139:1130–1142. [DOI] [PubMed] [Google Scholar]
- Ulbright TM, Young RH.. Seminoma with tubular, microcystic, and related patterns: a study of 28 cases of unusual morphologic variants that often cause confusion with yolk sac tumor. Am J Surg Pathol 2005;29:500–505. [DOI] [PubMed] [Google Scholar]
- Vasdev N, Moon A, Thorpe AC.. Classification, epidemiology and therapies for testicular germ cell tumours. Int J Dev Biol 2013;57:133–139. [DOI] [PubMed] [Google Scholar]
- Vetro A, Dehghani MR, Kraoua L, Giorda R, Beri S, Cardarelli L, Merico M, Manolakos E, Parada-Bustamante A, Castro A. et al. Testis development in the absence of SRY: chromosomal rearrangements at SOX9 and SOX3. Eur J Hum Genet 2015;23:1025–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vértesy ÁArindrarto W, Roost MS, Reinius B, Torrens-Juaneda V, Bialecka M, Moustakas, I, Ariyurek Y, Kuijk E, Mei H, Sandberg R, van Oudenaaden A, Chuva de Sousa Lopes SM. Parental haplotype-specific single-cell transcriptomics reveal incomplete epigenetic reprogramming in human female germ cells. Nature Comm 2018;9:1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viger RS, Guittot SM, Anttonen M, Wilson DB, Heikinheimo M.. Role of the GATA family of transcription factors in endocrine development, function, and disease. Mol Endocrinol 2008;22:781–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vining B, Ming Z, Bagheri-Fam S, Harley V.. Diverse regulation but conserved function: SOX9 in vertebrate sex determination. Genes (Basel) 2021;12:486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M, Yoshihara M, Douagi I, Damdimopoulos A, Panula S, Petropoulos S, Lu H, Pettersson K, Palm K, Katayama S. et al. Single-cell analysis of human ovarian cortex identifies distinct cell populations but no oogonial stem cells. Nat Commun 2020;11:1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, , ChenH, , QinY, , ShiZ, , ZhaoX, , XuJ, , MaB, , Chen Z-J.. Risks associated with premature ovarian failure in Han Chinese women. Reprod Biomed Online 2015;30:401–407. [DOI] [PubMed] [Google Scholar]
- Wang B, Li L, Ni F, Song J, Wang J, Mu Y, Ma X, Cao Y.. Mutational analysis of SAL-Like 4 (SALL4) in Han Chinese women with premature ovarian failure. Mol Hum Reprod 2009;15:557–562. [DOI] [PubMed] [Google Scholar]
- Wang G, Ying Z, Jin X, Tu N, Zhang Y, Phillips M, Moskophidis D, Mivechi NF.. Essential requirement for both hsf1 and hsf2 transcriptional activity in spermatogenesis and male fertility. Genesis 2004;38:66–80. [DOI] [PubMed] [Google Scholar]
- Wang G, Zhang J, Moskophidis D, Mivechi NF.. Targeted disruption of the heat shock transcription factor (hsf)-2 gene results in increased embryonic lethality, neuronal defects, and reduced spermatogenesis. Genesis 2003;36:48–61. [DOI] [PubMed] [Google Scholar]
- Wang M, Liu X, Chang G, Chen Y, An G, Yan L, Gao S, Xu Y, Cui Y, Dong J. et al. Single-cell RNA sequencing analysis reveals sequential cell fate transition during human spermatogenesis. Cell Stem Cell 2018;23:599–614.e4. [DOI] [PubMed] [Google Scholar]
- Waters BL, Trainer TD.. Development of the human fetal testis. Pediatr Pathol Lab Med 1996;16:9–23. [PubMed] [Google Scholar]
- Watkins WJ, Umbers AJ, Woad KJ, Harris SE, Winship IM, Gersak K, Shelling AN.. Mutational screening of FOXO3A and FOXO1A in women with premature ovarian failure. Fertil Steril 2006;86:1518–1521. [DOI] [PubMed] [Google Scholar]
- Weber S, Eckert D, Nettersheim D, Gillis AJ, Schäfer S, Kuckenberg P, Ehlermann J, Werling U, Biermann K, Looijenga LH. et al. Critical function of AP-2 gamma/TCFAP2C in mouse embryonic germ cell maintenance. Biol Reprod 2010;82:214–223. [DOI] [PubMed] [Google Scholar]
- Weel AEAM. Estrogen Receptor Polymorphism Predicts the Onset of Natural and Surgical Menopause. Journal of Clinical Endocrinology & Metabolism 1999;84:3146–3150. [DOI] [PubMed] [Google Scholar]
- Wegner M. From head to toes: the multiple facets of Sox proteins. Nucleic Acids Res 1999;27:1409–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman I. (2012). Stem cell therapies could change medicine. If they get the chance. Cell Stem Cell 2012: 10; 663–665. [DOI] [PubMed] [Google Scholar]
- Wen L, Tang F.. Human germline cell development: from the perspective of single-cell sequencing. Mol Cell 2019;76:320–328. [DOI] [PubMed] [Google Scholar]
- West JA, Viswanathan SR, Yabuuchi A, Cunniff K, Takeuchi A, Park IH, Sero JE, Zhu H, Perez-Atayde A, Frazier AL. et al. A role for Lin28 in primordial germ-cell development and germ-cell malignancy. Nature 2009;460:909–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White S, Ohnesorg T, Notini A, Roeszler K, Hewitt J, Daggag H, Smith C, Turbitt E, Gustin S, van den Bergen J. et al. Copy number variation in patients with disorders of sex development due to 46,XY gonadal dysgenesis. PLoS One 2011;6:e17793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm D, Palmer S, Koopman P.. Sex determination and gonadal development in mammals. Physiol Rev 2007;87:1–28. [DOI] [PubMed] [Google Scholar]
- Xavier MJ, Salas-Huetos A, Oud MS, Aston KI, Veltman JA.. Disease gene discovery in male infertility: past, present and future. Hum Genet 2021;140:7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaji M, Seki Y, Kurimoto K, Yabuta Y, Yuasa M, Shigeta M, Yamanaka K, Ohinata Y, Saitou M.. Critical function of Prdm14 for the establishment of the germ cell lineage in mice. Nat Genet 2008;40:1016–1022. [DOI] [PubMed] [Google Scholar]
- Yamashiro C, Sasaki K, Yabuta Y, Kojima Y, Nakamura T, Okamoto I, Yokobayashi S, Murase Y, Ishikura Y, Shirane K. et al. Generation of human oogonia from induced pluripotent stem cells in vitro. Science 2018;362:356–360. [DOI] [PubMed] [Google Scholar]
- Yan L, Yang M, Guo H, Yang L, Wu J, Li R, Liu P, Lian Y, Zheng X, Yan J. et al. Single-cell RNA-seq profiling of human preimplantation embryos and embryonic stem cells. Nat Struct Mol Biol 2013;20:1131–1139. [DOI] [PubMed] [Google Scholar]
- Yan W, Si Y, Slaymaker S, Li J, Zheng H, Young DL, Aslanian A, Saunders L, Verdin E, Charo IF. Zmynd15 encodes a histone deacetylase-dependent transcriptional repressor essential for spermiogenesis and male fertility. J Biol Chem 2010;285:31418–31426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye M, Yang ZY, Zhang Y, Xing YX, Xie QG, Zhou JH, Wang L, Xie W, Kee K, Chian RC.. Single-cell multiomic analysis of in vivo and in vitro matured human oocytes. Hum Reprod 2020;35:886–900. [DOI] [PubMed] [Google Scholar]
- Yeom YI, Fuhrmann G, Ovitt CE, Brehm A, Ohbo K, Gross M, Hubner K, Scholer HR.. Germline regulatory element of Oct-4 specific for the totipotent cycle of embryonal cells. Development 1996;122:881–894. [DOI] [PubMed] [Google Scholar]
- Yu X, Wang N, Qiang R, Wan Q, Qin M, Chen S., Wang H.. Human amniotic fluid stem cells possess the potential to differentiate into primordial follicle oocytes in vitro. Biol Reprod 2014;90:73. [DOI] [PubMed] [Google Scholar]
- Zanaria E, Muscatelli F, Bardoni B, Strom TM, Guioli S, Guo W, Lalli E, Moser C, Walker AP, McCabe ER.. An unusual member of the nuclear hormone receptor superfamily responsible for X-linked adrenal hypoplasia congenita. Nature 1994;372:635–641. [DOI] [PubMed] [Google Scholar]
- Zaytouni T, Efimenko EE, Tevosian SG.. GATA transcription factors in the developing reproductive system. Adv Genet 2011;76:93–134. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Yan Z, Qin Q, Nisenblat V, Chang HM, Yu Y, Wang T, Lu C, Yang M, Yang S. et al. Transcriptome landscape of human folliculogenesis reveals oocyte and granulosa cell interactions. Mol Cell 2018;72:1021–1034.e4. [DOI] [PubMed] [Google Scholar]
- Zhao H, Chen ZJ, Qin Y, Shi Y, Wang S, Choi Y, Simpson JL, Rajkovic A.. Transcription factor FIGLA is mutated in patients with premature ovarian failure. Am J Hum Genet 2008;82:1342–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Yao C, Xing X, Jing T, Li P, Zhu Z, Yang C, Zhai J, Tian R, Chen H. et al. Author Correction: Single-cell analysis of developing and azoospermia human testicles reveals central role of Sertoli cells. Nat Commun 2021;12:3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Wang R, Yuan P, Ren Y, Mao Y, Li R, Lian Y, Li J, Wen L, Yan L. et al. Reconstituting the transcriptome and DNA methylome landscapes of human implantation. Nature 2019;572:660–664. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared with all reasonable requests to the corresponding author.