Abstract
Objectives
The analytical sensitivity of high-sensitivity cardiac troponin T (hsTnT) assays has enabled rapid myocardial infarction rule-out algorithms for emergency department (ED) presentations. Few studies have analyzed the real-world impact of hsTnT algorithms on outcomes and operations.
Methods
Comparison of ED length of stay (LOS) and 30-day outcomes (return to ED, inpatient admission, and mortality) for patients presenting with chest pain during 2 separate 208-day periods using a 0/1-hour hsTnT-enabled algorithm or fourth-generation TnT.
Results
Discharge, 30-day readmission, and 30-day mortality rates were not significantly different with fourth-generation TnT vs hsTnT. Thirty-day return rates were significantly decreased with hsTnT (17.4% vs 14.9%; P < .01). For encounters with TnT measured at least twice and resulting in discharge, median ED LOS decreased by 61 minutes with the use of hsTnT (488 vs 427 minutes; P < .0001). Median time between first and second TnT results decreased by 82 minutes with hsTnT (202 vs 120 minutes; P < .0001), suggesting that the 0/1-hour algorithm was incompletely adopted.
Conclusions
Implementation of the hsTnT algorithm was associated with decreased 30-day return rates and decreased ED LOS for a subset of patients, despite incomplete adoption of the 0/1-hour algorithm.
Keywords: Troponin, Sensitive, Acute myocardial infarction, Cardiac biomarkers
Key Points.
There are concerns that high-sensitivity (hs) troponin T (TnT) assays would lead to an increase in myocardial infarction diagnosis and resource utilization without substantial patient benefit.
Patient outcomes, discharge rates, and length of stay (LOS) in the emergency department (ED) were either unchanged or improved after adoption of an hsTnT assay.
Median time between first and second hsTnT was 120 minutes, suggesting a substantial opportunity to further reduce ED LOS when using a 0/1-hour rule-out algorithm.
INTRODUCTION
Measuring cardiac troponin I and T (TnT) concentrations, along with clinical assessment and the electrocardiogram, are cornerstones of the early diagnosis of acute myocardial infarction (AMI).1,2 Successive generations of troponin assays have improved analytical performance with the intention of providing a tool for faster and more accurate diagnosis of AMI.3,4 The latest generation of high-sensitivity (hs) troponin assays can enable detection of troponin concentrations at more than 10-fold lower levels compared with the previous generation of troponin assays, enabling detection of elevated cardiac troponins earlier in the time course of AMI.5,6 The improved analytical performance of the hs assays has facilitated the development of rapid rule-in and rule-out algorithms based on serial troponin measurements as early as 0 to 1 hour (0/1h).7 Evaluations of the implementation of hs troponin-based algorithms in clinical trials have demonstrated their potential for clinical and operational benefits.8,9
Despite the promising data supporting the adoption of hs troponin-based algorithms, concerns have been raised about the potential for increased frequency of false-positive findings of elevated troponin without acute ischemia, leading to inappropriate and potentially harmful increases in hospital admissions and cardiovascular procedures for low- to moderate-risk patients.4,10,11 Additionally, the majority of data demonstrating the advantages of hs troponin-enabled algorithm implementation have been generated in the setting of controlled clinical trials; these findings may not predict the outcomes after implementation in a real-world setting, such as the emergency department (ED) of an academic medical center, with a diverse patient population cared for by providers (including trainees) hailing from multiple specialties.4
University of California, San Diego Health is an urban academic medical center with 2 main hospital campuses that provide tertiary-level care in multiple specialties. This study described the operational impact of a phased transition from use of a fourth-generation cardiac TnT assay to an hs fifth-generation TnT (hsTnT) assay with a 0/1h AMI rule-out protocol.
MATERIALS AND METHODS
0/1h Algorithm Implementation
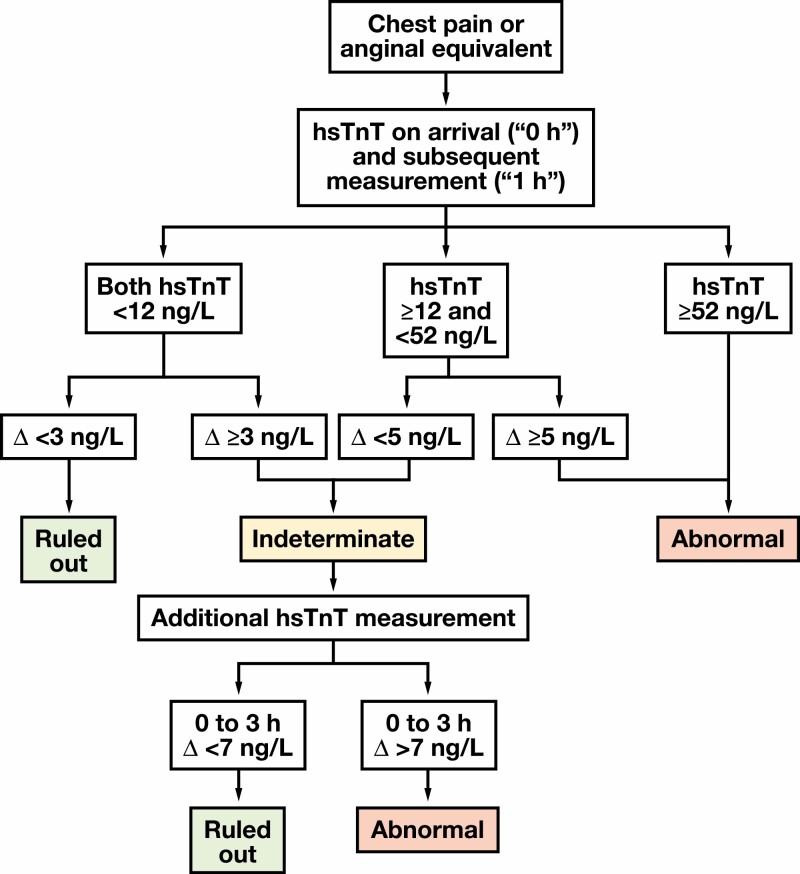
Our institution implemented the hsTnT assay on December 6, 2017. To facilitate clinical familiarity with the reference range and other differences between hsTnT and the fourth-generation TnT assays, both tests were run and reported for each specimen when a troponin was ordered until March 2018. Fourth-generation results were no longer resulted after March 2018; then, all clinical decisions were based on the hsTnT assay. In May 2018, the adoption of a 0/1h algorithm was encouraged FIGURE 1. The algorithm is based on that proposed by the European Society of Cardiology, with modifications based on local expert input.7,9 Trainees and faculty were educated on the algorithm, and in July 2018 clinicians were expected to use the 0/1h algorithm, as appropriate.
FIGURE 1.
Chest pain algorithm: emergency department (ED) 0/1-hour algorithm using high-sensitivity troponin T (hsTnT) testing to rule out myocardial infarction implemented in March 2018. Triage and subsequent care decisions for patients presenting to the ED with chest pain or equivalent based on hsTnT results. Δ = difference between subsequent hsTnT results.
Two 5-month time phases were included in this study. Phase 1 was from July 1 to December 5, 2017, when the fourth-generation troponin assay was being used at our institution. Use of the fourth-generation assay was at the discretion of the clinical care team; no institutional clinical guidance was provided. Phase 2 was from July 1 to December 5, 2018, approximately 7 months after the hsTnT assay had been implemented. These time frames were chosen because they represented routine practice with the fourth-generation assay and routine practice after implementation and training on the hsTnT assay combined with a 0/1h rule-out protocol.
Patient Encounters
Outcomes were calculated for all unique patient encounters during the time frames described. A Consolidated Standards of Reporting Trials (CONSORT) diagram of included patient encounters is available in Supplemental Figure 1 (all supplemental materials can be found at American Journal of Clinical Pathology online). The primary study population consisted of all ED encounters between July 1 and December 5 of 2017 and 2018 with a troponin result while the patient was in the ED (ie, after arrival and before admission or discharge) with a complaint of chest pain. Encounters with the phrase “chest pain” and without the phrases “denies chest pain,” “denies any chest pain,” “negative chest pain,” “absence of chest pain,” and “chest pain (−)” were included in this primary “chest pain” group. Manual audit of 100 charts demonstrated that these inclusion and exclusion criteria accurately captured patients presenting with chest pain. A “Troponin Resulted” group consisted of all ED encounters during the study period with any troponin result while the patient was in the ED. Finally, as a sensitivity analysis, an “Emergency Department Encounters” group consisted of all ED encounters during the study period.
This study was approved by the facility’s institutional review board (IRB), with a waiver of informed consent (IRB protocol No. 181656XL).
Troponin Assays
The fourth-generation TnT was performed using the Elecsys Troponin T (Roche Diagnostics). The hsTnT was performed using the Elecsys Troponin T Gen 5 STAT assay (Roche Diagnostics). Performance characteristics of the hsTnT assay have been described previously.12 Both assays were run on the cobas e 601 or e 602 platform (Roche Diagnostics). All results were acquired during routine clinical care for patients at our institution. Performance characteristics and turnaround times for both fourth-generation and hsTnT assays are shown in Supplemental Table 1 and Table 2.
Outcomes
Primary outcomes assessed included admission rate, 30-day ED return rate, 30-day readmission rate, and 30-day mortality rate. Readmission was defined as an inpatient admission within 30 days of the original encounter regardless of whether the patient was admitted during the original encounter. Encounters resulting in patient transfer to another facility or leaving against medical advice were excluded from this study (273 encounters were excluded in phase 1 and 272 encounters were excluded in phase 2).
Data Analysis
Clinical and laboratory data were acquired through a structured query of the institution’s EPIC and Emergency Department Operations databases, which contained clinical and operational data. Manual audit of 100 charts was performed to ensure data accuracy.
Demographics and outcomes were compared between phase 1 and phase 2 for the primary “chest pain” study population. As a sensitivity analysis, we analyzed the time to ED disposition (discharge or admission) for all patients during these time periods. Continuous variables are presented as means (standard deviation), or medians (interquartile range) if nonnormally distributed. Categorical variables are presented as No. (%). Student t tests, Wilcoxon rank sum tests, and χ 2 tests were used, as appropriate, to test for statistical significance in differences between groups. Data analysis was performed with R studio (rstudio.com). For all analyses, 2-sided P < .05 was considered statistically significant.
RESULTS
The total number of ED visits (as calculated by the Emergency Severity Index [ESI]) was similar between phases, but patient severity (as calculated by the ESI)13 was significantly higher during the hsTnT phase TABLE 1. The number of hospital-admitted patients being cared for in the ED (“ED inpatients”) was approximately doubled during the hsTnT phase, indicating the presence of increased hospital capacity challenges leading to a slowed flow of patients out of the ED and into the hospital after admission TABLE 1.
TABLE 1.
Overall Study Populations in Phase 1 vs Phase 2
| Fourth Generation | hsTnT | PValue | |
|---|---|---|---|
| Total ED visits, No. | 40,175 | 39,898 | NA |
| ESI scorea, mean (SD) | 3.07 (0.57) | 3.02 (0.59) | <.001 |
| ED inpatients, No. (%) | 1,192 (3.0) | 2,342 (5.9) | <.001 |
| Visits with TnT result in ED, No. (%) | 6,319 (15.7) | 6,678 (16.7) | <.001 |
| TnT results in ED, No. (%) | <.0001 | ||
| 1 | 4,645 (78.7) | 3,392 (53.9) | |
| ≥2 | 1,257 (21.3) | 2,900 (46.1) |
ED, emergency department; ESI, Emergency Severity Index; hsTnT, high-sensitivity troponin T; NA, not applicable; SD, standard deviation; TnT, troponin T.
aThe ESI is a 5-level triage algorithm that provides stratification of patients based on acuity from 1 (most urgent) to 5 (least urgent).13
The hsTnT algorithm is shown in FIGURE 1. Briefly, the algorithm uses the difference between an initial hsTnT result and subsequent hsTnT drawn approximately 1 hour after the initial sample to rule out AMI for patients presenting with chest pain or anginal equivalent. ED providers and staff were educated on the algorithm before its implementation. Algorithm use was left to the discretion of individual physicians. Among all ED encounters, implementation of the hsTnT assay coincided with relatively more encounters with troponin results (15.7% vs 16.7%; P < .001; TABLE 1) and more encounters with multiple (≥2) troponin results (21.3% vs 46.1%; P < .0001; TABLE 1).
Impact of the hsTnT-Enabled Rule-Out Algorithm on Patients Presenting With Chest Pain
Demographics of the primary “chest pain” study population are shown in TABLE 2. Patient demographics were not substantially different between phases. Significantly more chest pain patients received multiple troponin results while in the ED in phase 2 after implementation of the hsTnT algorithm compared with phase 1 (48% vs 25%; P < .001; TABLE 2; histogram in Supplemental Figure 2).
TABLE 2.
Demographics of Chest Pain Study Populations in Phase 1 vs Phase 2
| Fourth Generation | hsTnT | P Value | |
|---|---|---|---|
| (n = 3,641) | (n = 4,203) | ||
| Age, mean (SD), y | 59 (16) | 59 (15) | .99 |
| BMI, mean (SD), kg/m2 | 28.3 (7.5) | 27.9 (7.3) | .04 |
| Male sex, No. (%) | 1,968 (54.1) | 2,294 (54.6) | .66 |
| Ethnicity, No. (%) | |||
| American Indian | 8 (0.2) | 22 (0.5) | .05 |
| Asian | 272 (7.5) | 299 (7.1) | .58 |
| Black | 521 (14.3) | 625 (14.9) | .50 |
| Pacific Islander | 21 (0.6) | 33 (0.8) | .33 |
| Other/mixed | 888 (24.4) | 991 (23.6) | .42 |
| Unknown | 9 (0.2) | 24 (0.6) | .42 |
| White | 1,922 (52.8) | 2,208 (52.5) | .85 |
| Medical history (ICD-10 code), No. (%) | |||
| Hypertension (I10) | 454 (35.4) | 546 (37.5) | .29 |
| Chronic ischemic heart disease (I25) | 292 (22.8) | 258 (17.7) | <.01 |
| AF (I48) | 277 (21.6) | 386 (26.5) | <.01 |
| DM (E11) | 264 (20.6) | 270 (18.5) | .19 |
| Hyperlipidemia (E78) | 139 (10.9) | 181 (12.4) | .22 |
| COPD (J44) | 166 (13.0) | 183 (12.6) | .80 |
| MI (I21) | 93 (7.3) | 91 (6.2) | .33 |
| CKD (N18) | 185 (14.4) | 213 (14.6) | .94 |
| TnT results in ED, No. (%) | <.001 | ||
| 1 | 2,749 (75.5) | 2,175 (51.7) | |
| ≥2 | 892 (24.5) | 2,028 (48.3) |
AF, atrial fibrillation; BMI, body mass index; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; ED, emergency department; hsTnT, high-sensitivity troponin T; ICD-10, International Classification of Diseases, Tenth Revision; MI, myocardial infarction; SD, standard deviation.
Discharge rates from the ED were not significantly different in phase 1 compared with phase 2 (67% vs 64%, respectively; P = .55; TABLE 3). Thirty-day return rates (ie, presentation to the ED within 30 days of discharge) were significantly decreased with hsTnT (15% from 17%; P < .01; TABLE 3). Thirty-day readmission rates (ie, inpatient admission after presentation to the ED) were not changed between phases (3.4% fourth generation vs 3.0% hsTnT; P = .48; TABLE 3). Similarly, 30-day mortality rates were not different between phases (0.6% fourth generation vs 0.4% hsTnT; P = .28; TABLE 3).
TABLE 3.
Outcomes
| Fourth Generation | hs | P Value | ||
|---|---|---|---|---|
| (n = 3,641) | (n = 4,203) | |||
| Disposition, No. (%) | Discharged | 2,346 (64.4) | 2,796 (66.5) | .55 |
| Admitted | 1,295 (35.6) | 1,407 (33.5) | ||
| 30-d return, No. (%) | Overall | 635 (17.4) | 625 (14.9) | <.01 |
| Of discharged | 400 (17.1) | 411 (14.7) | .02 | |
| Of admitted | 235 (18.1) | 214 (15.2) | .04 | |
| 30-d readmission, No. (%) | Overall | 122 (3.4) | 128 (3.0) | .48 |
| Of discharged | 0 (0) | 0 (0) | .99 | |
| Of admitted | 122 (9.4) | 128 (9.1) | .82 | |
| 30-d mortality, No. (%) | Overall | 23 (0.6) | 18 (0.4) | .28 |
| Of discharged | 6 (0.3) | 3 (0.1) | .36 | |
| Of admitted | 17 (3.9) | 15 (3.3) | .73 | |
| Single TnT result only | Fourth generation | hs | ||
| (n = 2,749) | (n = 2,175) | |||
| Disposition, No. (%) | Discharged | 1,771 (64.4) | 1,548 (71.1) | <.0001 |
| Admitted | 978 (35.6) | 627 (28.8) | ||
| Multiple TnT results | Fourth generation | hs | ||
| (n = 892) | (n = 2,028) | |||
| Disposition, No. (%) | Discharged | 575 (64.4) | 1,248 (61.5) | .14 |
| Admitted | 317 (35.5) | 780 (38.5) |
hs, high sensitivity; TnT, troponin T.
Overall mean length of stay (LOS) in the ED for chest pain patients was shorter for the fourth-generation phase (408 minutes fourth generation vs 425 minutes hsTnT; P < .0001; Supplemental Figure 3). For encounters leading to admission, ED LOS was substantially shorter for the fourth-generation phase (472 minutes vs 557 minutes; P < .0001; Supplemental Figure 4). In contrast, for encounters leading to discharge, overall ED LOS among patients with chest pain did not differ between phases (364 minutes vs 366 minutes; P = .4; Supplemental Figure 4).
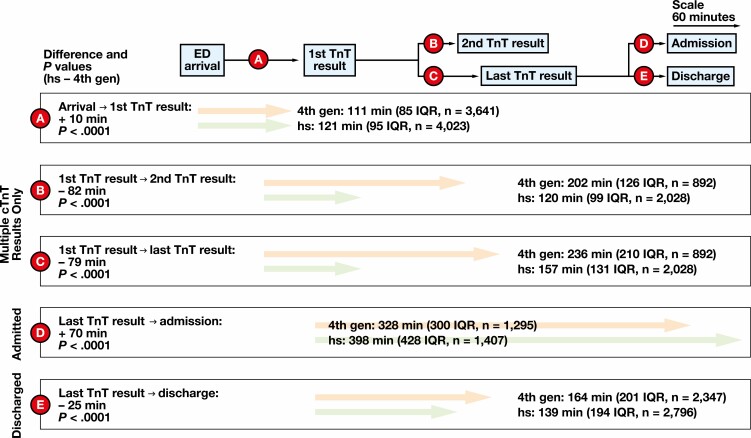
To understand the drivers of ED LOS for hsTnT, we deconstructed the ED visit into distinct time frames for admitted and discharged patients in each phase FIGURE 2. The first time frame is between patient arrival at the ED and the first troponin result. The time from ED presentation to first TnT result increased by 10 minutes during the hsTnT phase (111 minutes fourth generation vs 121 minutes hsTnT; P < .0001; time frame “A” in FIGURE 2, Supplemental Figure 5). The quickest time from ED arrival to first TnT result was similar between phases (36 minutes fourth generation vs 40 minutes hsTnT; Supplemental Figure 5). Laboratory turnaround time from collection to result was not significantly different between phases (77 minutes fourth generation vs 80 minutes hsTnT; P = .17; Supplemental Table 2). The next time frame was between first and second TnT result in the ED, which was analyzed only for those patients who had more than 1 TnT result in the ED. This time frame was reduced by 82 minutes in the hsTnT phase (202 minutes fourth generation vs 120 minutes hsTnT; P < .0001; time frame “B” in FIGURE 2, Supplemental Figure 6). We also analyzed the time frame between first and last TnT result in the ED, which was reduced by 79 minutes in the hsTnT phase (236 minutes fourth generation vs 157 minutes hsTnT; P < .0001; time frame “C” in FIGURE 2, Supplemental Figure 7). The last time frame assessed was between the last TnT result and departure from the ED. This time frame increased by 70 minutes for admitted patients in the hsTnT phase (328 minutes fourth generation vs 398 minutes hsTnT; P < .0001; time frame “C” for “Admitted only” in FIGURE 2) and decreased by 25 minutes for discharged patients in the hsTnT phase (328 minutes fourth generation vs 398 minutes hsTnT; P < .0001; time frame “C” for “Discharged only” in FIGURE 2).
FIGURE 2.
Time frame components for emergency department (ED) length of stay based on arrival, troponin result times, and departure from the ED comparing fourth-generation (4th gen) and high sensitivity (hs) troponin T (TnT) phases. Elapsed time from ED arrival to first troponin result (A). Elapsed time from first troponin result to second troponin result for encounters with 2 or more results (B). Elapsed time from first troponin result to last troponin result for encounters with 2 or more results (C). Elapsed time from last troponin result to ED departure for encounters leading to hospital admission (D) or hospital discharge (E). P values derived from Wilcoxon test. cTNT, troponin T, cardiac form; IQR, interquartile range.
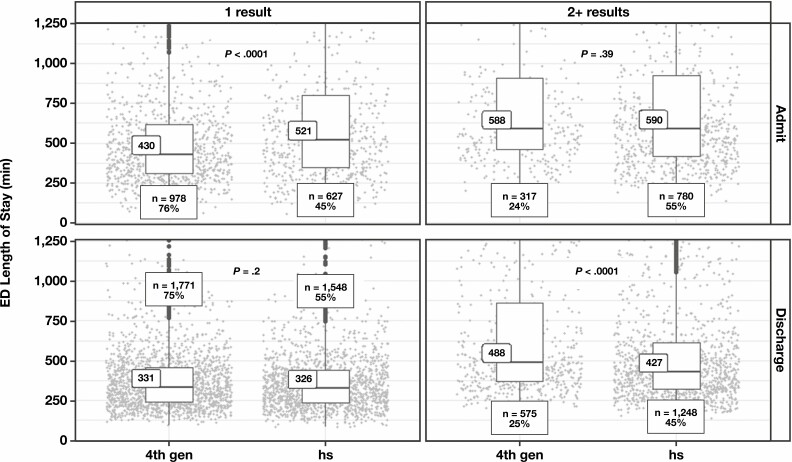
We further segmented ED LOS results by both disposition (admission vs discharge) and by single vs multiple troponin results because we were interested in determining how hsTnT implementation affected time to discharge in patients where providers had a sufficient index of suspicion to order 2 or more troponin tests FIGURE 3. Assessment of an 0/1h hsTnT chest pain algorithm may be less relevant in patients with only a single troponin ordered because it either represents patients with a low pretest probability of MI or those with chest pain that occurred more than 6 hours before presentation. As expected, regardless of subsequent admission or discharge and regardless of study phase, the median ED LOS was longer for encounters with multiple TnT results than for those with single results; as previously described, a substantially greater proportion of visits during the hsTnT phase had multiple TnT results. As shown in FIGURE 3, however, patients who had at least 2 troponin results were discharged 61 minutes faster during the hsTnT phase compared with during the fourth-generation TnT phase.
FIGURE 3.
Length of stay in the emergency department (ED) for encounters with a single troponin result (left) or multiple troponin results (right) stratified by admission (top) or discharge (bottom). Box plot indicates median and interquartile ranges. Individual data points are shown as gray circles. P values derived from Wilcoxon test. 4th gen, fourth generation; hs, high sensitivity.
DISCUSSION
Evidence for the utility of a 0/1h algorithm enabled by hsTnT is robust.14,15 Previous studies to evaluate the implementation of hs cardiac Tn and associated accelerated clinical algorithms have shown clinical improvements after implementation without accompanying increases in hospital admissions or other measures of resource utilization.8,9 A single prospective trial in the United States also demonstrated a clinical advantage to hsTnT use without assessing impact on resource utilization.16 To date, multiple observational studies have shown that implementation of hsTnT did not increase the rate of cardiology consultations, hospital admissions, or coronary angiography and was associated with a decrease in stress tests performed, but these studies were all performed outside the United States.17-20 Two large observational studies in the United States showed mixed results on the impact of hsTnT implementation.21,22
Despite the improved analytical performance of hsTnT and randomized controlled trial evidence demonstrating clinical benefit, questions remain as to how clinicians use the test in a real-world setting. It has been hypothesized that in the absence of operational controls present during a clinical trial, physicians might struggle to manage the increased number of patients with elevated troponins detectable with hsTnT assays compared with that previously detectable by fourth-generation TnT assays.3,4,23-25 The concern is that the growth in numbers of patients with low levels of detectable myocardial injury would subsequently result in increased hospital admissions leading to increased cost, patient inconvenience, and potential patient harm. To address this concern, we analyzed outcomes for all patients with “chest pain” as a chief complaint, as documented by ED physicians. We chose this patient population because the 0/1h rule-out algorithm is designed for patients presenting with symptoms concerning for acute coronary syndrome.
Our analysis was enabled by structured data extracted from an electronic health record followed by manual data quality assurance. Our data-driven approach enabled a large sample size and broad inclusion criteria.
Our data enabled us to investigate the impact of changing from a contemporary to an hs troponin assay as well as changing to an accelerated 0/1h diagnostic protocol for the evaluation of suspected acute coronary syndrome. Specifically, we have investigated the impact on patient disposition, clinical outcomes, and LOS in the ED. Despite the algorithm requiring serial hsTnT measurements, a substantial number of chest pain encounters have only a single hsTnT result, suggesting that many patients had either a late presentation of chest pain or were deemed by the treating clinician to have a low probability of acute coronary syndrome, thus rendering the algorithm irrelevant. For patients with chest pain, implementation of the algorithm was associated with a shortened time to discharge from the ED and a decrease in 30-day return rates (from 17% to 15%). No change was observed in admission rates, 30-day readmission rates, or 30-day mortality rates. Although in the overall ED population ED LOS was longer after implementation of the 0/1h algorithm, further analysis showed that the increased ED LOS was the result of a combination of factors independent of implementing hsTnT, including increased severity of patient symptoms in the ED, slowed flow of hospital admissions, and an increased proportion of the ED-inpatient population. Importantly, we show that the hsTnT-enabled 0/1h rule-out facilitated faster patient discharge. This faster discharge rate was more pronounced in patients in whom troponin was measured at least twice and resulted in patients being discharged on average 61 minutes faster when the hsTnT-enabled 0/1h algorithm was used FIGURE 3. Faster discharge times appear to be driven primarily by clinical comfort with decreased time elapsed between serial troponin measurements because lab turnaround time did not differ significantly between phases.
Our findings demonstrate that implementation of hsTnT and the enabled 0/1h rule-out algorithm for patients with chest pain did not significantly change admission rates nor most patient outcomes, but it did lead to shortened ED LOS for patients discharged and a lower 30-day return rate. Overall, our findings are similar to other real-world studies of hsTnT use in the United States and highlight the importance of reporting real-world data in the face of many clinical trials showing positive outcomes.21,22 As expected, real-world use of hsTnT differs outside the context of a clinical trial. Differences may result from a variety of factors, including changes in perceived medicolegal risk; removal of observer bias; and variation in patient, provider, and institutional characteristics. For innovative diagnostics, such as hs troponin, studying real-world effects is important to ensure that all patients benefit from the potential value and costs of novel tests.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Michael Marshall and Wendy Zhu for assistance with data extraction. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Funding
The project described herein was partially supported by the National Institutes of Health, Grant UL1TR001442 of CTSA funding.
Disclosure: L.B.D. has been a consultant for Quidel, Roche, and Siemens. R.L.F. has received consulting, speaking, and research funding from Roche.
REFERENCES
- 1. Apple FS, Wu AH, Jaffe AS. European Society of Cardiology and American College of Cardiology guidelines for redefinition of myocardial infarction: how to use existing assays clinically and for clinical trials. Am Heart J. 2002;144:981-986. [DOI] [PubMed] [Google Scholar]
- 2. Alpert JS, Thygesen K, Antman E, et al. . Myocardial infarction redefined—a consensus document of the Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000;36:959-969. [DOI] [PubMed] [Google Scholar]
- 3. de Lemos JA, Morrow DA, deFilippi CR. Highly sensitive troponin assays and the cardiology community: a love/hate relationship? Clin Chem. 2011;57:826-829. [DOI] [PubMed] [Google Scholar]
- 4. Omland T. Sensitive cardiac troponin assays: sense and sensibility. Eur Heart J. 2012;33:944-946. [DOI] [PubMed] [Google Scholar]
- 5. Ford I, Shah AS, Zhang R, et al. . High-sensitivity cardiac troponin, statin therapy, and risk of coronary heart disease. J Am Coll Cardiol. 2016;68:2719-2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Twerenbold R, Boeddinghaus J, Nestelberger T, et al. . Clinical use of high-sensitivity cardiac troponin in patients with suspected myocardial infarction. J Am Coll Cardiol. 2017;70:996-1012. [DOI] [PubMed] [Google Scholar]
- 7. Roffi M, Patrono C, Collet JP, et al. ; ESC Scientific Document Group. . 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting Without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:267-315. [DOI] [PubMed] [Google Scholar]
- 8. Twerenbold R, Neumann JT, Sörensen NA, et al. . Prospective validation of the 0/1-h algorithm for early diagnosis of myocardial infarction. J Am Coll Cardiol. 2018;72:620-632. [DOI] [PubMed] [Google Scholar]
- 9. Mueller C, Giannitsis E, Christ M, et al. ; TRAPID-AMI Investigators. . Multicenter evaluation of a 0-hour/1-hour algorithm in the diagnosis of myocardial infarction with high-sensitivity cardiac troponin T. Ann Emerg Med. 2016;68:76-87.e4. [DOI] [PubMed] [Google Scholar]
- 10. Ferraro S, Corona S, Lavarra F, et al. . Troponin T measured with highly sensitive assay (hsTnT) on admission does not reflect infarct size in ST-elevation myocardial infarction patients receiving primary percutaneous coronary intervention. Clin Chem Lab Med. 2015;53:e173-e174. [DOI] [PubMed] [Google Scholar]
- 11. Jesse RL. On the relative value of an assay versus that of a test: a history of troponin for the diagnosis of myocardial infarction. J Am Coll Cardiol. 2010;55:2125-2128. [DOI] [PubMed] [Google Scholar]
- 12. Fitzgerald RL, Hollander JE, Peacock WF, et al. . Analytical performance evaluation of the Elecsys® Troponin T Gen 5 STAT assay. Clin Chim Acta. 2019;495:522-528. [DOI] [PubMed] [Google Scholar]
- 13. Wuerz RC, Milne LW, Eitel DR, et al. . Reliability and validity of a new five-level triage instrument. Acad Emerg Med. 2000;7:236-242. [DOI] [PubMed] [Google Scholar]
- 14. Chiang CH, Chiang CH, Lee GH, et al. . Safety and efficacy of the European Society of Cardiology 0/1-hour algorithm for diagnosis of myocardial infarction: systematic review and meta-analysis. Heart. 2020;106:985-991. [DOI] [PubMed] [Google Scholar]
- 15. Arslan M, Dedic A, Boersma E, et al. . Serial high-sensitivity cardiac troponin T measurements to rule out acute myocardial infarction and a single high baseline measurement for swift rule-in: a systematic review and meta-analysis. Eur Heart J Acute Cardiovasc Care. 2020;9:14-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Peacock WF, Baumann BM, Bruton D, et al. . Efficacy of high-sensitivity troponin T in identifying very-low-risk patients with possible acute coronary syndrome. JAMA Cardiol. 2018;3:104-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dolci A, Braga F, Valente C, et al. . Impact of implementation of the high-sensitivity cardiac troponin T assay in a university hospital setting. Clin Chem. 2011;57:1211-1212. [DOI] [PubMed] [Google Scholar]
- 18. Crowder KR, Jones TD, Lang ES, et al. . The impact of high-sensitivity troponin implementation on hospital operations and patient outcomes in 3 tertiary care centers. Am J Emerg Med. 2015;33:1790-1794. [DOI] [PubMed] [Google Scholar]
- 19. Twerenbold R, Jaeger C, Rubini Gimenez M, et al. . Impact of high-sensitivity cardiac troponin on use of coronary angiography, cardiac stress testing, and time to discharge in suspected acute myocardial infarction. Eur Heart J. 2016;37:3324-3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eggers KM, Lindahl B. Application of cardiac troponin in cardiovascular diseases other than acute coronary syndrome. Clin Chem. 2017;63:223-235. [DOI] [PubMed] [Google Scholar]
- 21. Ola O, Akula A, De Michieli L, et al. . Clinical impact of high-sensitivity cardiac troponin T implementation in the community. J Am Coll Cardiol. 2021;77:3160-3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ganguli I, Cui J, Thakore N, et al. . Downstream cascades of care following high-sensitivity troponin test implementation. J Am Coll Cardiol. 2021;77:3171-3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ho JE. High-sensitivity troponin in the general population: time for a new normal? J Am Coll Cardiol. 2017;70:569-571. [DOI] [PubMed] [Google Scholar]
- 24. Wildi K, Twerenbold R, Jaeger C, et al. . Clinical impact of the 2010-2012 low-end shift of high-sensitivity cardiac troponin T. Eur Heart J Acute Cardiovasc Care. 2016;5:399-408. [DOI] [PubMed] [Google Scholar]
- 25. Wildi K, Boeddinghaus J, Nestelberger T, et al. ; APACE investigators. . Comparison of fourteen rule-out strategies for acute myocardial infarction. Int J Cardiol. 2019;283:41-47. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.