Abstract
Background and Objectives
Epilepsy is defined by the occurrence of multiple unprovoked seizures, but quality of life (QOL) in people with epilepsy is determined by multiple factors, in which psychiatric comorbid conditions play a pivotal role. Therefore, understanding the interplay between comorbid conditions and QOL across epilepsy phenotypes is an important step toward improved outcomes. Here, we report the impact of QOL across distinct epilepsy phenotypes in a cohort of post-9/11 veterans with high rates of traumatic brain injury (TBI).
Methods
This observational cohort study from the Veterans Health Administration included post-9/11 veterans with epilepsy. A process integrating an epilepsy identification algorithm, chart abstraction, and self-reported measures was used to classify patients into 1 of 4 groups: (1) epilepsy controlled with medications, (2) drug-resistant epilepsy (DRE), (3) posttraumatic epilepsy (PTE), or (4) drug-resistant PTE (PT-DRE). Summary scores for 6 QOL measures were compared across the groups after adjustment for age, sex, and number of comorbid conditions.
Results
A total of 529 survey respondents with epilepsy were included in the analysis: 249 controls (i.e., epilepsy without DRE or PTE), 124 with DRE, 86 with PTE, and 70 with PT-DRE. DRE was more common in those with PTE compared with those with nontraumatic epilepsy (45% vs 33%, odds ratio 1.6 [95% CI 1.1–2.4], p = 0.01). Patients with PTE and PT-DRE had significantly more comorbid conditions in health records than those with nontraumatic epilepsy. Those with both PTE and DRE reported the lowest QOL across all 6 measures, and this persisted after adjustment for comorbid conditions and in further linear analyses.
Discussion
Among those with PTE, DRE prevalence was significantly higher than prevalence of nontraumatic epilepsies. PTE was also associated with higher burden of comorbidity and worse overall QOL compared to nontraumatic epilepsies. People with PTE are distinctly vulnerable to the comorbid conditions associated with TBI and epilepsy. This at-risk group should be the focus of future studies aimed at elucidating the factors associated with adverse health outcomes and developing antiepileptogenic therapies.
Epilepsy is a life-altering condition defined by the propensity for unprovoked seizures, but there are important physical and neuropsychiatric manifestations of epilepsy not captured by this definition. Epilepsy is characterized by a diverse range of etiologies,1 comorbid conditions,2 and structural foci.3 Recent studies have shown that these distinctions are critical for understanding health impacts4 because physical and neuropsychiatric effects of epilepsy can affect outcomes more than seizures.5 For example, we recently identified that epilepsy resulting from traumatic brain injury (TBI), i.e., posttraumatic epilepsy (PTE) is associated with a previously unreported “double hit” on quality of life (QOL).6 Just as PTE may have additional QOL impacts, other considerations such as response to antiseizure medications (ASMs) can alter physical and psychosocial well-being in specific ways.7-9 For example, patients with drug-resistant epilepsy (DRE) experience higher rates of physical injury and death, cognitive impairment, and higher prevalence of psychiatric symptoms compared to those without DRE. However, little is known about how DRE, PTE, and other physical/neuropsychiatric conditions may interplay and mediate QOL. A deeper understanding of the factors mediating QOL in PTE is crucial to advance efforts to identify individuals at greatest risk for poor QOL and to identify effective interventions to mitigate/prevent poor health status and QOL.
QOL is a complex construct that involves a person's assessment of their life in the context of their value systems and culture and is influenced by personal standards, expectations, goals, and concerns.10 Assessments of QOL can be generic or specific. For example, epilepsy-specific measures are used to quantify the impact of medications, seizure worry, mood, and functional status among persons with epilepsy, but these measures may not address the broader complexity of QOL. Likewise, generic measures such as the RAND Short Form-36/12 provide a broader picture of QOL (e.g., physical/social function, role social/emotional, mental health, vitality, general health, pain) but may not be as sensitive to QOL in specific populations (e.g., veterans with history of brain injury). Both epilepsy-specific and generic QOL measures have been used to demonstrate that more seizures, more comorbid conditions (especially depression/anxiety), more ASMs, and longer duration of epilepsy significantly lower QOL.11-15 However, prior work has not evaluated the relative effects of PTE or DRE across generic and epilepsy/TBI–specific QOL measures, controlling for other characteristics associated with TBI, epilepsy, and common mental health comorbid conditions. Thus, research using multimodal measures of QOL may provide new insight into the impact of epilepsy, PTE, and DRE on QOL while controlling for other potential confounders. Because prior research has not compared QOL among groups with PTE and our prior work in this cohort found that PTE is associated with lower scores on QOL measures, we hypothesized that both PTE and DRE would be associated with significantly lower scores across all QOL measure types compared to epilepsy-positive controls who are well controlled on medical therapy. We also hypothesized that those with posttraumatic DRE (PT-DRE) would have the lowest QOL scores of all groups due to the double impact of both PTE and DRE.
Methods
The goal of this observational cohort study was to assess the impact of epilepsy, TBI, and DRE on QOL in a highly comorbid cohort of post-9/11 veterans.6 Because the parent study examined a complex cohort with/without TBI and/or epilepsy, we elected to measure a wide range of QOL measures that were epilepsy specific (Quality of Life in Epilepsy Inventory 10 [QOLIE-10]), TBI specific (Quality of Life in Brain Injury Overall Scale [QOLIBRI]), and generic (Veterans RAND-12 [VR-12]). In addition, acquiring multimodal measures of QOL in parallel allowed us to explore how these diverse measures overlap and to explore their relative sensitivities in a highly comorbid population.
Cohort Development
This study used national data from the Veterans Health Administration (VHA) for post-9/11 deployed veterans who entered VHA care between October 1, 2001, and September 30, 2014 (fiscal year [FY]02–FY14) and who received ≥2 years of VHA care (inpatient, outpatient, or pharmacy) before September 30, 2015. As part of a larger study examining the impact of epilepsy and mild TBI (mTBI) on health outcomes, we identified veterans with TBI and/or epilepsy using VHA health system data for subsequent survey administration.6
From this cohort, we then identified veterans who met criteria for epilepsy using an algorithm validated with a positive predictive value of 0.92 to 0.96. A complete description of the algorithm is detailed elsewhere.16-19 Briefly, the algorithm requires ICD-9-CM diagnosis code(s) indicative of epilepsy (345) or convulsion (780.39) and subsequent/concomitant use of ASM. We included individuals whose only ASM was gabapentin/pregabalin only if they had a 345 (epilepsy specific) diagnosis. Next, we conducted medical chart abstraction to validate epilepsy status and excluded cases of definite/probable psychogenic nonepileptic seizures, seizures associated with drug/alcohol exposure, and unspecified convulsions. Only individuals treated with ASMs for epilepsy according to documentation (as assessed January 2017–August 2017) were classified as having epilepsy.
Health System Data
Data sources included (1) TBI severity identified from the Chronic Effects of Neurotrauma Consortium epidemiology study20; (2) inpatient and outpatient data from the national VHA data repository in Austin, TX; (3) pharmacy data from the national pharmacy benefits management outpatient dataset; and (4) mortality data from the VHA vital status files. Sociodemographic data were extracted from VHA inpatient/outpatient records. We also identified clinical conditions common in post-9/11 veterans, including posttraumatic stress disorder (PTSD), depression, anxiety, substance use disorder, bipolar disorder, back pain, neck pain, headache, tinnitus, homelessness, and suicidal ideation/attempt using ICD-9-CM/ICD-10-CM algorithms in VHA data through FY18. We merged these data sources using an encrypted identifier to create a longitudinal record and identified epilepsy status using data from FY02 to FY15.
Survey Administration
As described in prior work,6 surveys measuring lifetime TBI history, PTE screening, indicators of DRE, and measures of health status and QOL were administered to the cohort between September 2017 and February 2019 (receipt date cutoff, July 31, 2019). Mailed invitation packets included a study information sheet and a link to the online survey. If no response was received within 4 weeks, a follow-up invitation (information sheet, the online link, a paper survey) was mailed with a business reply envelope, and if another 4 weeks passed without response, a final reminder with a link to the online survey was mailed. Survey responders received a $50 gift card. The response rate for the cohort who were classified with epilepsy was 30% (n = 623); we included only those responders who self-reported use of ASM at any time after epilepsy diagnosis (n = 529). An analysis of responders and nonresponders found that responders were slightly more likely to be married, college educated, and female but otherwise of similar age, employment, and race/ethnicity as nonresponders.
Standard Protocol Approvals, Registrations, and Patient Consents
Approval with a waiver of informed/written consent was provided by institutional review boards from the University of Utah, the University of Texas Health Science Center at San Antonio, and the Department of Defense Human Research Protection Office.
Independent Variable Definition: Study Groups
We used survey data to define our study groups based on TBI exposure/severity, timing of epilepsy in relation to TBI, and indicators of DRE.
Self-reported lifetime history of TBI was estimated with the self-administered Ohio State University TBI (OSU-TBI). The OSU-TBI is a measure adapted from the highly reliable OSU-TBI Interview.21 This measure was validated to identify the most serious lifetime TBI exposure using self-reported head injury and experience and duration of loss of consciousness (LOC). While it identifies experience of alteration of consciousness (AOC) and post-traumatic amnesia (PTA), it does not identify duration of either. Thus, we classified the most severe lifetime TBI according to duration of LOC: LOC ≤30 minutes was classified as mTBI, and LOC >30 minutes was classified as moderate/severe TBI. TBI with LOC of unknown duration (n = 2) and presence of AOC or PTA without LOC (n = 67) were classified as TBI of unclassified severity and were excluded from this analysis because duration of AOC/PTA was not available from the self-administered OSU-TBI assessment used in this survey.
The Post-Traumatic Epilepsy Screening form22 is a common data element from the National Institutes of Neurologic Disorders and Stroke for epilepsy that asks about history of seizures and the relationship between those seizures/events and head injury. Participants who indicated seizures starting/persisting ≥7 days after TBI were classified as having PTE.
Indicators of DRE were extracted from the Personal Impact of Epilepsy Scale23 identified individuals with continued seizures within the past year, the number of different ASMs taken ever (≥2), and the number of different, current ASMs (≥2).
Classification of Study Groups
Posttraumatic epilepsy classification required 1 of the following criteria: moderate/severe TBI before or proximal to the date of epilepsy diagnosis or self-reported TBI of any severity, including mTBI, with late posttraumatic seizures (i.e., occurring >7 days after injury). Separately, DRE classification required the following 2 criteria to be met in line with the International League Against Epilepsy24 definition of multiple (current or prior) treatments not resulting in seizure freedom: active, recurrent seizures over the last 12 months and current or prior treatment with ≥2 ASMs. With these 2 definitions, each survey responder was classified as having (1) epilepsy (PTE- and DRE-negative controls), (2) DRE (DRE but not PTE), (3) PTE (PTE but not DRE), and (4) PT-DRE (both PTE and DRE criteria).
Survey Measures
The QOLIE-10 is a 10-item QOL measure designed for persons with epilepsy that includes the impact of medication effects, mental health, role functioning, and seizure worry. Mean QOLIE-10 scores were calculated as the sum of scores for all answered questions divided by the number of items answered.25 In contrast to all other QOL measures used in this study, higher QOLIE-10 scores indicate lower QOL.
QOLIBRI is a 6-item measure that assesses satisfaction with cognition, self, autonomy, social relationships, emotions, and physical health problems after TBI to create an overall QOL score.26 Responses to each item range from 1 (not at all) to 5 (very). The sum of item scores and number of items answered are used to calculate a percentage scale, with 0 representing the lowest possible QOL and 100 indicating the best possible QOL.
VR-12 measures physical and emotional health status and has (in the Short Form-36) previously been able to distinguish27-29 differences between patients with and those without epilepsy.30 Prior work using this sample found that mental (MCS) and physical (PCS) component scores were better for veterans with no TBI and veterans without epilepsy.6 We used the MCS and PCS from the VR-12 as dependent variables in this analysis. The Short Form-6D items of the VR-12 were used to calculate quality-adjusted life-years (QALY) and quality-adjusted life expectancy (QALE). QALY is a measure of disease burden for which perfect health is given a value of 1 and death is assigned a value of 0.31 QALY values are determined by measuring people's willingness to trade years of their life in return for hypothetical improvements to their health state.32 These methods have been extended so that QALY can be indirectly calculated from the reweighting of other QOL instruments.33 In this work, we inferred QALY scores from each responders Short Form-6D items using stacked probit model weights calculated for the US population.33,34 QALE is a summary measure of population health that encompasses multiple health domains and length of life.35 QALE provides a measure of life expectancy with QALY score adjustment and was calculated from QALY scores and mortality estimates based on VHA data,36 accounting for each responder's age and sex.
From the above 3 measures, we extracted 6 QOL measures in an effort to capture both a broad view of QOL (i.e., MCS, PCS, QALY, QALE derived from VR-12) and disease-specific aspects of QOL (i.e., QOLIE-10, QOLIBRI). QOLIBRI was chosen because the cohort was upsampled for TBI, and QOLIE-10 was chosen to limit the overall time required to complete the survey. QALY/QALE assessment was derived from a validated algorithm for data from the VR-12 and is not a direct utility analysis focused on epilepsy alone. Overall, we estimate that the survey took between 15 and 20 minutes for participants to complete.
Covariates
Sociodemographic characteristics derived from VHA data included age and sex. Information on education, employment, and discharge status of medically retired was obtained from the survey.
Diagnosed health conditions were identified from ICD-9/10 codes in VHA and Department of Defense health system data. Conditions included the 53 most prevalent mental (e.g., depression, PTSD) and physical (e.g., back pain, lung disease) health conditions in the cohort. For each person, we created a count of unique diagnoses before survey administration.
Statistical Analysis
Cross-group demographic and clinical characteristics were compared by use of the χ2 statistic at a significance of p < 0.05. Differences in percentage DRE and PTE status across self-reported lifetime history of TBI severity groups were assessed with z tests. The total number of comorbid medical conditions for each veteran was calculated as the sum of the 53 most common conditions diagnosed in their VHA record. Six measures of QOL (QOLIE-10, QOLIBRI, MCS, PCS, QALY, QALE) were calculated from each survey, adjusting for responder age, sex, and number of comorbid conditions. We did not adjust for number of ASMs because we expected treatment differences across groups. Adjusted QOL scores across the 4 groups were compared with a Kruskal-Wallis H test (omnibus). All analyses were scripted in Python 3.8.
Data Availability
VHA privacy and information security allow sharing of deidentified data on completion of appropriate and approved data-sharing agreements. Data from this study are also available in the Federal Interagency TBI Registry.
Results
Cohort Description
Among the 529 veterans with epilepsy, study groups were predominantly epilepsy (PTE- and DRE-negative controls, n = 249, 47%) and DRE (but not PTE, n = 124, 24%), followed by PTE (but not DRE, n = 86, 16%) and PT-DRE (both PTE and DRE criteria, n = 70, 13%). No participants in the epilepsy control or DRE groups had moderate or severe TBI, whereas 28 of 86 (32.6%) and 25 of 70 (35.7%) reported moderate or severe TBI in the PTE and PT-DRE groups, respectively. In comparison, 104 of 249 (41.7%) of the epilepsy control group, 56 of 124 (45.2%) of the DRE group, 58 of 86 (67.4%) of PTE group, and 45 of 70 (64.3%) of PT-DRE reported mTBI. Participants with mTBI in the epilepsy control and DRE groups were not considered to have PTE because they indicated that seizures occurred before or within 7 days of TBI, which would not be sufficient for a diagnosis of PTE.
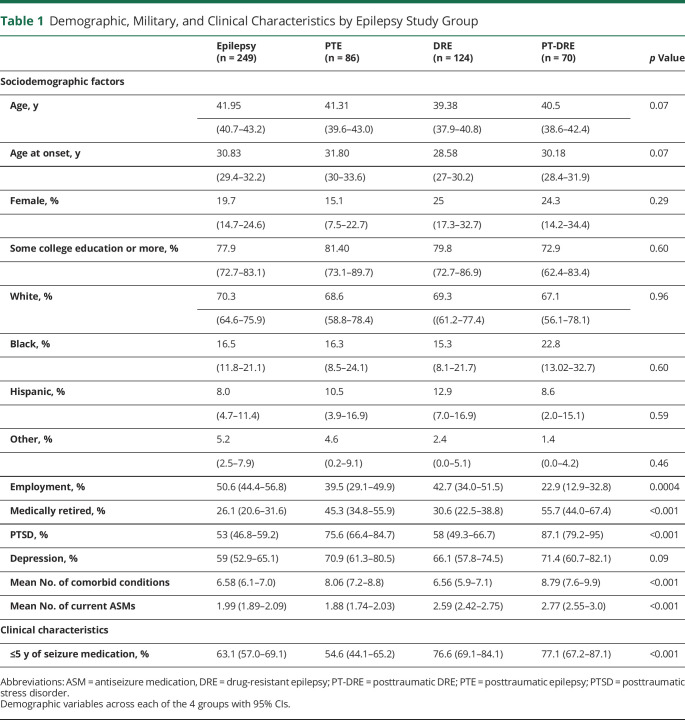
All demographics were statistically similar across groups at the time of survey (Table 1). The groups differed significantly across social and military measures (p < 0.001), with the PTE groups (i.e., PTE and PT-DRE) reporting significantly lower rates of productive employment, higher rates of medical retirement, and greater combat exposure than the non-PTE groups.
Table 1.
Demographic, Military, and Clinical Characteristics by Epilepsy Study Group
Association of TBI With Drug Resistance
Consistent with prior studies, the prevalence of DRE in those with nontraumatic epilepsy was 33% (124 of 373). Among those with PTE, the prevalence of DRE (i.e., PT-DRE) was 45% (70 of 156). The odds ratio (OR) for DRE among those with PTE was 1.6 (95% CI 1.1–2.4, p = 0.01). The percentage of persons with epilepsy with drug resistance also increased with TBI severity. Compared to the no TBI group, both the mild (OR 1.33 [95% CI 0.91–1.94]) and moderate/severe (OR 1.9 [95% CI 1.03–3.51]) TBI groups showed higher odds of DRE. As expected, those with DRE (either DRE or PT-DRE) reported taking more concurrent ASMs and reported more physical and psychological adverse effects from ASMs.
Comorbid Conditions and Medication Use
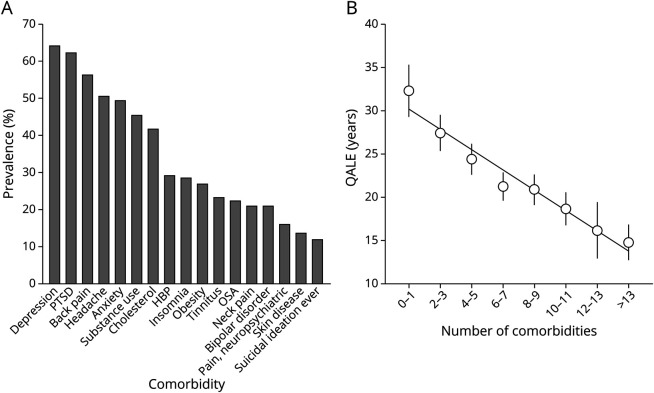
A comprehensive view of the burden of comorbid conditions across the entire cohort is shown in Figure 1A. Several comorbid conditions occurred at rates exceeding 50%, including depression, PTSD, back pain, and headache. Important differences in comorbid conditions between groups are shown in Table 1. The number of comorbid conditions was significantly different across groups (p < 0.001), and those with PTE reported the most comorbid conditions. PTSD and depression were more common in those with PTE (PTE and PT-DRE vs epilepsy and DRE). Figure 1B shows the mean QALE score binned as a function of the total number of comorbid conditions present in health records. The slope indicates that QALE dropped by >1 year per additional comorbidity. Similar trends were observed for all QOL measures as a function of number of comorbid conditions.
Figure 1. Comorbid Conditions: Prevalence and Outcomes.
Comorbid prevalence (A) and relationship (B) with quality-adjusted life expectancy (QALE) are shown. HBP = hypertension; OSA = obstructive sleep apnea; PTSD = posttraumatic stress disorder.
Quality of Life
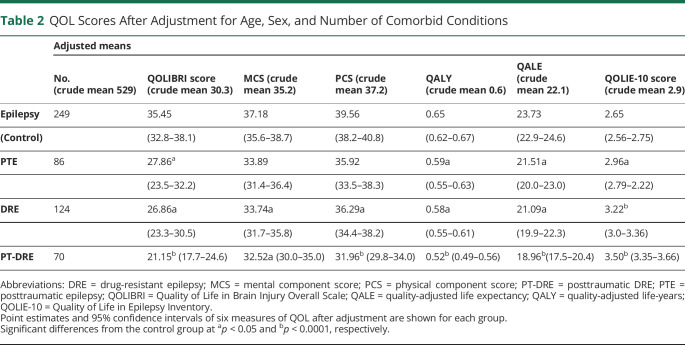
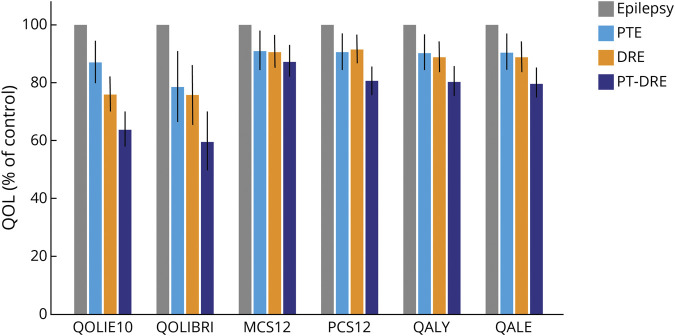
The PTE and DRE groups reported lower QOL overall compared to controls across the 6 measures, while the PT-DRE group consistently showed the lowest QOL across all 6 measures (Table 2). Figure 2 shows the percentage reduction in QOL score relative to epilepsy controls (using the control mean as a reference at 100%). To provide some intuition about the underlying differences in QOL scores, Figure 3 shows a radial breakout of the mean scores for the 10 items of the QOLIE-10 for each group. Higher scores indicate higher levels of impairment, and the group means are presented as a percentage of the maximum response for each item. Overall, those with PT-DRE reported the highest level of impairment across all items, while epilepsy controls reported the least. The largest intergroup differences were observed in memory, social functioning, driving concerns, medication side effects, seizure worry, and work.
Table 2.
QOL Scores After Adjustment for Age, Sex, and Number of Comorbid Conditions
Figure 2. Group QOL Scores Relative to Epilepsy Controls.
Percentage reduction in quality of life (QOL) for the posttraumatic epilepsy (PTE), drug-resistant epilepsy (DRE), and posttraumatic DRE (PT-DRE) groups is shown referenced to mean epilepsy control QOL scores (100%). MCS12 = mental component score; PCS12 = physical component score; QALE = quality-adjusted life expectancy; QALY = quality-adjusted life-years, QOLIBRI = Quality of Life in Brain Injury Overall Scale; QOLIE-10 = Quality of Life in Epilepsy Inventory.
Figure 3. QOLIE-10 Item Breakout Across Epilepsy Subgroups.
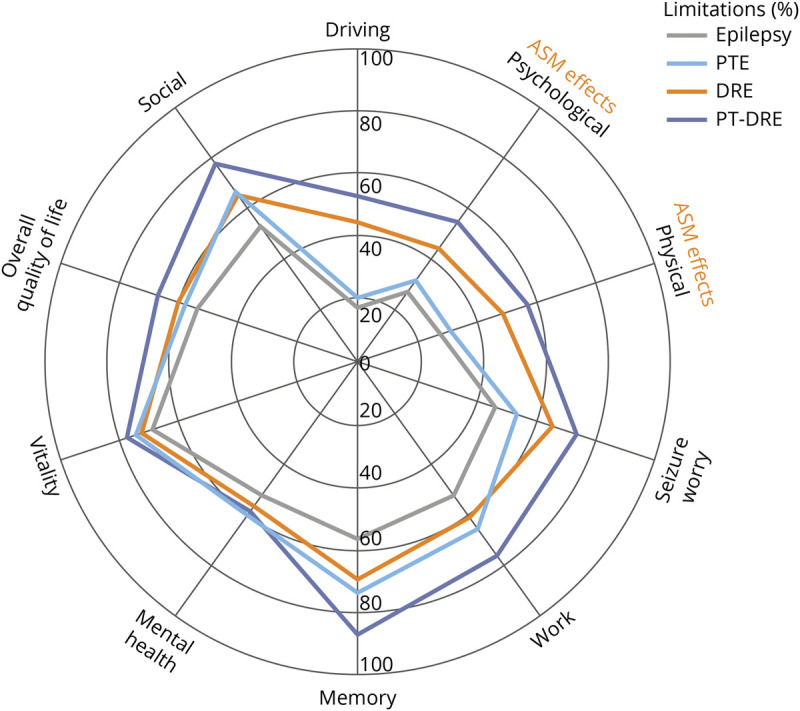
The 4 group means for each of the 10 individual items of the Quality of Life in Epilepsy Inventory (QOLIE-10) are shown along each line. Higher percentages indicate high limitations, and 100% indicates the maximum reportable score for each item. CIs are intentionally excluded to enhance visualization. Caution is advised in drawing conclusions from single item differences across groups. ASM = antiseizure medication; DRE = drug-resistant epilepsy; PTE = posttraumatic epilepsy; PT-DRE = posttraumatic DRE.
Discussion
This study is the first to compare QOL among people with epilepsy, PTE, and DRE. We did so using measures that allow a comprehensive and multifaceted overview of QOL, including overall physical and mental health status and QOL (VR-12: PCS, MCS, QALY, QALE), brain-related QOL (QOLIBRI), and epilepsy-specific QOL (QOLIE-10) without adding significant response burden. A key finding of this study is that DRE is significantly more common (OR 1.6 [95% CI 1.1–2.4]) in veterans with PTE compared to those with nontraumatic epilepsies. Furthermore, there was a substantial burden of comorbidity across the entire cohort, with the PTE and PT-DRE groups having particularly high rates of medical comorbidity in health records. Compared to epilepsy controls, PTE and DRE were both independently associated with lower QOL assessed with a broad range of specific and generic measures, and this persisted even after adjustment for medical comorbid conditions. Item-level analysis indicated that memory and work limitations may preferentially drive PTE QOL loss, whereas the DRE groups indicated problems primarily with social functioning, driving concerns, medication side effects, and seizure worry. Taken together, these results suggest that compared to individuals with nontraumatic epilepsies, those with PTE are less likely to achieve seizure freedom with ASMs and have a greater burden of comorbidity, both of which are associated with lower QOL.
This study examined the prevalence of DRE in a cohort of patients systematically screened for lifetime history of TBI. It is important to note that the rate of DRE in those with nontraumatic epilepsies is in agreement with prior studies.37,38 Although it is generally accepted that symptomatic etiology of epilepsy (e.g., TBI) conveys a higher risk of drug resistance, the evidence for this is mixed. For example, a study of 780 patients with newly diagnosed epilepsy with follow-up over a 20-year period, including 75 patients with TBI39 reported that the OR for DRE among those with TBI etiology was 2.73 (95% CI 1.59–4.69). Conversely, in a hospital-based adult observational study (n = 2,200) examining factors associated with drug resistance, the existence of MRI signs of gliosis (from either TBI or miscellaneous nonstroke causes) did not emerge as a risk factor in multivariate analysis.40 However, these studies did not systematically screen for lifetime history of TBI or stratify patients according to TBI injury severity. Thus, our study is the first to assess DRE risk in a cohort systematically screened for TBI across the injury severity spectrum (with individuals with mTBI making up more than half of our PTE sample). The results of this study suggest a predisposition to DRE among those with PTE and support the need for mechanistic studies exploring the biological basis for this relationship.
There was a large burden of comorbidity across all groups (comorbid conditions per group ≈6.6–8.8). In comparison, the mean number of comorbid conditions among post-9/11 veterans without TBI or epilepsy was 5.0, and in epilepsy-negative veterans with a history of TBI, it was still higher, at ≈5.9.6 Most individuals with mTBI experience complete recovery within 3 months of injury; however, ≈15% experience ongoing emotional, cognitive, or physical symptoms with high rates of depression, PTSD, and chronic pain.41,42 Much of the literature describing the burden of physical and neuropsychiatric complications in PTE comes from those affected by more severe TBI (i.e., moderate, severe, or penetrating TBI),43,44 whereas in this study, more than half of veterans in the PTE-only group reported mTBIs. Therefore, this study indicates that comorbidity rates remain high for those with PTE across the full spectrum of TBI severity. Providers caring for people with epilepsy specifically PTE and PT-DRE should be aware that comorbid conditions, including behavioral health issues and pain, affect QOL and consider an interdisciplinary approach to care with both behavioral health and pain management.
Both drug resistance and TBI etiology were associated with incremental effects on all 6 measures of QOL, and this persisted after adjustment for age, sex, and comorbid conditions. The incremental effects were observed in both epilepsy-specific and generic QOL measures, which is in keeping with prior studies11-14 in which comorbid conditions such as depression and anxiety were important drivers of QOL, in some cases even more so than seizure-related factors. This study demonstrated that DRE and PTE can overlap and affect QOL differences in unique ways. In practice, there is often skepticism as to whether preceding mTBI(s) are relevant to a patient's epilepsy; however, the findings of this study support a relationship among mTBI and epilepsy, drug resistance, increased burden of medical comorbidity, and poor QOL.
The response rate for this study was 30%, which is higher than most recent population-based research surveys of post-9/11 veterans. Of the 3,062 veterans identified and validated as having epilepsy in our chart abstraction, we contacted 2,185, including all women with epilepsy. Comparison of responders and nonresponders to the survey revealed that responders were generally representative of the larger nonresponding group. Therefore, our findings are largely representative of the broader population of post-9/11 veterans with epilepsy. They are not, however, representative of veterans from previous eras.
Another limitation of this work is the potential for ascertainment bias for epilepsy.6 To minimize this, we used a robust epilepsy identification algorithm and chart review of medical records for all patients with epilepsy identified by the algorithm to minimize the likelihood of misclassifying those with provoked seizures and nonepileptic events as having epilepsy. Unfortunately, given that most of the data are collected via survey, we were not able to reliably determine whether ASMs were appropriately dosed as required in the International League Against Epilepsy definition of DRE. An additional limitation is the overrepresentation of TBI in this cohort. mTBI rates are high in veteran populations,45 and mTBI was expected to be overrepresented due to the parent study design, which sampled on the basis of mTBI status.6 This resulted in a disproportionate number of patients with mTBI in the epilepsy and DRE groups. On account of the adverse effect of TBI on QOL, overrepresentation of mTBI in the nontraumatic epilepsy groups may actually diminish the true magnitude of the difference in QOL between those with PTE and nontraumatic epilepsy. Our analysis did not consider the effect of TBI injury severity on QOL, but in our prior work, we showed that increasing TBI injury severity was associated with worse outcomes.6 In this analysis,6 we also demonstrated that survey responders and nonresponders had similar characteristics, thus mitigating the risk that survey responders represented a distinct group. Last, given that the cohort is derived from those deployed to support combat operations on active military service, these results may not be generalizable to the civilian population. Future work will characterize this cohort with a greater degree of clinical granularity, which will further increase our understanding of the diverse clinical phenotype of PTE.
This study of post-9/11 veterans with epilepsy and TBI found that DRE is significantly more common (OR 1.6, p = 0.01) for those with PTE than for those with nontraumatic epilepsies. All epilepsy subgroups reported substantial comorbidity and QOL impairment, but the PT-DRE group reported the worst outcomes across all 6 QOL assessments. The findings from this study represent an initial attempt to define the diverse clinical phenotype of PTE. This at-risk group should be the focus of future studies aimed at elucidating the mechanistic basis of drug resistance, adverse health outcomes, and directionality of these relationships.
Glossary
- AOC
alteration of consciousness
- ASM
antiseizure medication
- DRE
drug-resistant epilepsy
- FY
fiscal year
- ICD-9-CM
International Classification of Diseases, 9th revision, clinical modification
- ICD-10
International Classification of Diseases, 10th revision
- LOC
loss of consciousness
- mTBI
mild TBI
- MCS
mental component score
- OR
odds ratio
- OSU-TBI
Ohio State University TBI
- PCS
physical component score
- PT-DRE
posttraumatic drug-resistant epilepsy
- PTA
posttraumatic amnesia
- PTE
posttraumatic epilepsy
- PTSD
posttraumatic stress disorder
- QALE
quality-adjusted life expectancy
- QALY
quality-adjusted life-years
- QOL
quality of life
- QOLIBRI
Quality of Life in Brain Injury Overall Scale
- QOLIE-10
Quality of Life in Epilepsy Inventory
- TBI
traumatic brain injury
- VHA
Veterans Health Administration
- VR-12
Veterans RAND-12
Appendix. Authors
Study Funding
This study was funded by the Congressionally Directed Medical Research Program, Epilepsy Research Program, project W81XWH-16-2-0046. Dr. Gugger reports receiving salary support from the National Institute of Neurologic Disorders and Stroke (T32NS091006) and the American Epilepsy Society/Citizens United for Research in Epilepsy (Research and Training Fellowship for Clinicians). Dr. Pugh also received funding from VA Health Services Research and Development Service grant (RCS 17-297). Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the views of the US government, the Department of Defense, or the US Department of Veterans Affairs; no official endorsement should be inferred.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Bhalla D, Godet B, Druet-Cabanac M, Preux PM. Etiologies of epilepsy: a comprehensive review. Expert Rev Neurother. 2011;11(6):861-876. [DOI] [PubMed] [Google Scholar]
- 2.Keezer MR, Sisodiya SM, Sander JW. Comorbidities of epilepsy: current concepts and future perspectives. Lancet Neurol. 2016;15(1):106-115. [DOI] [PubMed] [Google Scholar]
- 3.Wang C, Sun W, Zhang J, et al. An electric-field-responsive paramagnetic contrast agent enhances the visualization of epileptic foci in mouse models of drug-resistant epilepsy. Nat Biomed Eng. 2020;5(3):278-289. [DOI] [PubMed] [Google Scholar]
- 4.Sarmast S, Abdullahi A, Jahan N. Current classification of seizures and epilepsies: scope, limitations and recommendations for future action. Cureus. 2020;12(9):e10549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnstone B, Malpas C, Velakoulis D, Kwan P, O'Brien T. Psychiatric symptoms are the strongest predictors of quality of life in patients with drug-resistant epilepsy or psychogenic nonepileptic seizures. Epilepsy Behav. 2021;117:107861. [DOI] [PubMed] [Google Scholar]
- 6.Pugh M, Kennedy E, Gugger JJ, et al. . The military injuries—understanding post-traumatic epilepsy study: understanding relationships among lifetime TBI history, epilepsy, and quality of life. J Neurotrauma. 2021;38(20):2841-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silva B, Canas-Simião H, Cordeiro S, Velosa A, Oliveira-Maia AJ, Barahona-Corrêa JB. Determinants of quality of life in patients with drug-resistant focal epilepsy. Epilepsy Behav. 2019;100:106525. [DOI] [PubMed] [Google Scholar]
- 8.Celiker Uslu S, Yuksel B, Tekin B, Sariahmetoglu H, Atakli D. Cognitive impairment and drug responsiveness in mesial temporal lobe epilepsy. Epilepsy Behav. 2019;90:162-167. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt D. Drug treatment of epilepsy: options and limitations. Epilepsy Behav. 2009;15(1):56-65. [DOI] [PubMed] [Google Scholar]
- 10.The World Health Organization Quality of Life Assessment (WHOQOL): position paper from the World Health Organization. Soc Sci Med. 1995;41(10):1403-1409. [DOI] [PubMed] [Google Scholar]
- 11.Cramer JA, Blum D, Reed M, Fanning K. The influence of comorbid depression on quality of life for people with epilepsy. Epilepsy Behav. 2003;4(5):515-521. [DOI] [PubMed] [Google Scholar]
- 12.Johnson EK, Jones JE, Seidenberg M, Hermann BP. The relative impact of anxiety, depression, and clinical seizure features on health-related quality of life in epilepsy. Epilepsia. 2004;45(5):544-550. [DOI] [PubMed] [Google Scholar]
- 13.van Elst L, Trimble M, Boylan L, Labovitz D, Flint L, Devinsky O. Depression but not seizure frequency predicts quality of life in treatment-resistant epilepsy. Neurology. 2004;63(5):942-943. [DOI] [PubMed] [Google Scholar]
- 14.Loring DW, Meador KJ, Lee GP. Determinants of quality of life in epilepsy. Epilepsy Behav. 2004;5(6):976-980. [DOI] [PubMed] [Google Scholar]
- 15.Zeber JE, Copeland LA, Amuan M, Cramer JA, Pugh MJ. The role of comorbid psychiatric conditions in health status in epilepsy. Epilepsy Behav. 2007;10(4):539-546. [DOI] [PubMed] [Google Scholar]
- 16.Pugh MJ, Knoefel JE, Mortensen EM, Amuan ME, Berlowitz DR, Van Cott AC. New-onset epilepsy risk factors in older veterans. J Am Geriatr Soc. 2009;57(2):237-242. [DOI] [PubMed] [Google Scholar]
- 17.Pugh MJ, Parko K. Research using archival health care data: let the buyer beware. Epilepsia. 2015;56(2):321-322. [DOI] [PubMed] [Google Scholar]
- 18.Pugh MJ, Van Cott AC, Cramer JA, et al. Trends in antiepileptic drug prescribing for older patients with new-onset epilepsy: 2000-2004. Neurology. 2008;70(22 pt 22):2171-2178. [DOI] [PubMed] [Google Scholar]
- 19.Pugh M, Van Cott A, Amuan M, et al. . Epilepsy among Iraq and Afghanistan war veterans–United States, 2002-2015. MMWR Morb Mortal Wkly Rep. 2016;65(44):1224-1227. [DOI] [PubMed] [Google Scholar]
- 20.Swan AA, Nelson JT, Pogoda TK, Amuan ME, Akin FW, Pugh MJ. Sensory dysfunction and traumatic brain injury severity among deployed post-9/11 veterans: a Chronic Effects of Neurotrauma Consortium study. Brain Inj. 2018;32(10):1197-1207. [DOI] [PubMed] [Google Scholar]
- 21.Corrigan JD, Bogner J. Initial reliability and validity of the Ohio State University TBI identification method. J Head Trauma Rehabil. 2007;22(6):318-329. [DOI] [PubMed] [Google Scholar]
- 22.CDE detailed report: NINDS common data elements.Accessed April 28, 2021. commondataelements.ninds.nih.gov/cde_detailed_report/24295/Assessing%20Comorbidities/Outcomes%20and%20End%20Points/Epilepsy/Post-Traumatic%20Epilepsy%20Screening%20Form
- 23.Sirven J. What is in a number? The Personal Impact of Epilepsy Scale (PIES). Epilepsy Behav. 2015;42:138-139. [DOI] [PubMed] [Google Scholar]
- 24.Kwan P, Arzimanoglou A, Berg A, et al. . Definition of drug resistant epilepsy: consensus proposal by the ad hoc task force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2009;51(6):1069-1077. [DOI] [PubMed] [Google Scholar]
- 25.Sajatovic M, Tatsuoka C, Welter E, et al. Correlates of quality of life among individuals with epilepsy enrolled in self-management research: from the US Centers for Disease Control and Prevention Managing Epilepsy Well Network. Epilepsy Behav. 2017;69:177-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.von Steinbuechel N, Wilson L, Gibbons H, et al. . QOLIBRI overall scale: a brief index of health-related quality of life after traumatic brain injury. J Neurol Neurosurg Psychiatry. 2012;83(11):1041-1047. [DOI] [PubMed] [Google Scholar]
- 27.Selim AJ, Rogers W, Fleishman JA, et al. Updated U.S. population standard for the Veterans RAND 12-Item Health Survey (VR-12). Qual Life Res. 2009;18(1):43-52. [DOI] [PubMed] [Google Scholar]
- 28.Selim AJ, Rogers W, Qian SX, Brazier J, Kazis LE. A preference-based measure of health: the VR-6D derived from the veterans RAND 12-Item Health Survey. Qual Life Res. 2011;20(8):1337-1347. [DOI] [PubMed] [Google Scholar]
- 29.Selim A, Rogers W, Qian S, Rothendler JA, Kent EE, Kazis LE. A new algorithm to build bridges between two patient-reported health outcome instruments: the MOS SF-36® and the VR-12 Health Survey. Qual Life Res. 2018;27(8):2195-2206. [DOI] [PubMed] [Google Scholar]
- 30.Pugh MJ, Copeland LA, Zeber JE, et al. The impact of epilepsy on health status among younger and older adults. Epilepsia. 2005;46(11):1820-1827. [DOI] [PubMed] [Google Scholar]
- 31.Bravo Vergel Y, Sculpher M. Quality-adjusted life years. Pract Neurol. 2008;8(3):175-182. [DOI] [PubMed] [Google Scholar]
- 32.Arnold D, Girling A, Stevens A, Lilford R. Comparison of direct and indirect methods of estimating health state utilities for resource allocation: review and empirical analysis. BMJ. 2009;339(3):b2688. [DOI] [PubMed] [Google Scholar]
- 33.Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ. 2002;21(2):271-292. [DOI] [PubMed] [Google Scholar]
- 34.Craig BM, Pickard AS, Stolk E, Brazier JE. US valuation of the SF-6D. Med Decis Making. 2013;33(6):793-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gheorghe M, Wubulihasimu P, Peters F, Nusselder W, Van Baal PH. Health inequalities in the Netherlands: trends in quality-adjusted life expectancy (QALE) by educational level. Eur J Public Health. 2016;26(5):794-799. [DOI] [PubMed] [Google Scholar]
- 36.Mortality rates and life expectancy of veterans.Accessed April 28, 2021. va.gov/vetdata/docs/SpecialReports/Mortality_study_USVETS_2015_1980_2014.pdf
- 37.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342(5):314-319. [DOI] [PubMed] [Google Scholar]
- 38.Chen Z, Brodie M, Liew D, Kwan P. Treatment outcomes in patients with newly diagnosed epilepsy treated with established and new antiepileptic drugs. JAMA Neurol. 2018;75(3):279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hitiris N, Mohanraj R, Norrie J, Sills GJ, Brodie MJ. Predictors of pharmacoresistant epilepsy. Epilepsy Res. 2007;75(2-3):192-196. [DOI] [PubMed] [Google Scholar]
- 40.Semah F, Picot M, Adam C, et al. . Is the underlying cause of epilepsy a major prognostic factor for recurrence? Neurology. 1998;51(5):1256-1262. [DOI] [PubMed] [Google Scholar]
- 41.Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA. Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N Engl J Med. 2008;358(5):453-463. [DOI] [PubMed] [Google Scholar]
- 42.Pugh MJ, Swan AA, Amuan ME, et al. Deployment, suicide, and overdose among comorbidity phenotypes following mild traumatic brain injury: a retrospective cohort study from the Chronic Effects of Neurotrauma Consortium. PLoS One. 2019;14(9):e0222674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bushnik T, Englander J, Wright J, Kolakowsky-Hayner S. Traumatic brain injury with and without late posttraumatic seizures. J Head Trauma Rehabil. 2012;27(6):E36–E44. [DOI] [PubMed] [Google Scholar]
- 44.Juengst SB, Wagner AK, Ritter AC, et al. Post-traumatic epilepsy associations with mental health outcomes in the first two years after moderate to severe TBI: a TBI Model Systems analysis. Epilepsy Behav. 2017;73:240-246. [DOI] [PubMed] [Google Scholar]
- 45.Traumatic Brain Injury Center of Excellence, Military Health System. Accessed April 28, 2021. https://www.health.mil/Military-Health-Topics/Centers-of-Excellence/Traumatic-Brain-Injury-Center-of-Excellence/DOD-TBI-Worldwide-Numbers [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
VHA privacy and information security allow sharing of deidentified data on completion of appropriate and approved data-sharing agreements. Data from this study are also available in the Federal Interagency TBI Registry.