Abstract
Giving and receiving touch are some of the most important social stimuli we exchange in daily life. By touching someone, we can communicate various types of information. Previous studies have also demonstrated that interpersonal touch may affect our altruistic behavior. A classic study showed that customers give bigger tips when they are lightly touched by a waitress, which has been called the Midas touch effect. Numerous studies reported similar effects of touch on different kinds of helping or prosocial behaviors. Here, we aim to examine the neural underpinnings of this effect by employing a functional magnetic resonance imaging approach. While lying in the scanner, participants played different rounds of the dictator game, a measure of prosocial behavior. Before each round, participants were touched (or not touched in the control condition) by an experimenter. We found that touching the hand increased the likeliness to behave prosocial (but not the general liking of control stimuli), thereby confirming the Midas touch effect. The effect was predicted by activity in the primary somatosensory cortex, indicating that the somatosensory cortex here plays a causal role in prosocial behavior. We conclude that the tactile modality in social life may be much more important than previously thought.
Keywords: tactile, social, altruistic, social neuroscience, somatosensory cortex, fMRI
Introduction
In the Metamorphoses, Ovid tells us the legend of King Midas. Midas was granted a wish by Dionysus, and he asked him that whatever he touched should be changed into gold. First, Midas was very happy with his new gift. Every stone or twig he touched turned to gold. However, when he wanted to eat or drink something, food and wine also transformed to gold. Moreover, when Midas touched his daughter, she turned into gold. Now, Midas understood that this gift was a bane. Without a normal sense of touch, Midas was threatened to die from starvation.
While this legend illustrates the importance of a proper sense of touch (among other topics), brain research has long neglected the tactile modality. Although touch may be one of the first senses we develop, its implications are still poorly understood. Touch not only gives us information about the physical nature of our close environment but also is important for our social life. For example, we touch someone in order to comfort or soothe him or her, start a romantic relationship, reassure friendship or try to relieve pain (e.g. Reddan et al., 2020). Touch may be the most elemental form of communication, which even simplest invertebrates have.
It has also been shown that interpersonal touch may affect our prosocial behavior (Crusco and Wetzel, 1984). The perhaps most popular experiment demonstrating this social power of touch is the now classic study on tipping behavior in a restaurant. Waitresses were instructed to briefly touch the customers on the shoulder or the palm of the hand (or did not touch them at all) when they went to get change. They found that customers who were touched became more generous. No effects were found for the type of touch or the gender of the customer. This effect has been named the Midas touch effect, since touching here seems to directly increase the amount of money for the waitress (Crusco and Wetzel, 1984).
Several studies replicated the effect (Stephen and Zweigenhaft, 1986; Hornik, 1992; Lynn et al., 1998), also for different countries (Guéguen and Jacob, 2005). The general link between touch and prosocial behavior was also confirmed by numerous similar field studies, demonstrating that people become more helpful, compliant, generous or unselfish after having been touched (e.g. Kleinke, 1977; Smith et al., 1982; Goldman et al., 1985; Hornik, 1992). For example, touch led subjects return money that they supposed to have lost in a phone booth (Brockner et al., 1982), increased compliance when being asked to watch for a large and very excited dog while its owner shops (Gueguen and Fischer-Lokou, 2002) and even let the bus driver allow free rides (Gueguen and Fischer-Lokou, 2003). Furthermore, it has been shown that touch affects people’s sense of security and leads them to increased financial risk taking (Levav and Argo, 2010).
Although the Midas touch has often been replicated, it is still poorly understood why touch increases the likeliness of unselfish actions. Some recent studies addressed this question in a laboratory context. Koppel et al. (2017) examined the effects of affective touch applied on the forearm (by a soft brush) on trust and prosocial behavior, which was measured using different kinds of economic decision games. The authors hypothesized that pleasant touch may increase the release of oxytocin, which then affects prosocial behavior. The study revealed no effects of touch on prosocial decisions or trust (Koppel et al., 2017). Rosenberger et al. also applied touch to the forearm and hypothesized that touch specifically related to C-tactile (CT) fibers may explain the Midas touch effect. In contrast to myelinated fibers, CT fibers are unmyelinated and represent a slow touch system, projecting to the insular cortex. The authors did not find that stimulating CT fibers increases prosocial behavior in an economic game (Rosenberger et al., 2018). Spapé et al. investigated the virtual Midas touch effect by using an electroencephalography (EEG) approach. In contrast to Koppel et al. (2017) and Rosenberger et al. (2018), they applied touch not on the forearm but to the palm of the hand. Spapé et al. found that tactile messages in an ultimatum game made it more likely for participants to accept offers. Thus, the authors could successfully replicate the Midas touch effect in a laboratory context. These effects were accompanied by a late positive effect, which the authors relate to the P3 component. Given that this component is known to be linked to memory processes, the authors suggest that the Midas touch relies on memory effects (Spape et al., 2015). Taken together, the attempts to replicate the Midas touch effect in a laboratory context resulted in mixed results.
Hence, although the Midas touch has often been replicated in field studies, it is still poorly understood why touch increases the likeliness of unselfish actions and what the underlying neural substrates of this effect are. Although an important role of the CT fibers and the insula for affective touch has been demonstrated (Olausson et al., 2010), it has also been reported that affective touch activates the primary somatosensory cortex (SI). For example, Gazzola et al. demonstrated that SI discriminates affective significance in social touch by manipulating the information of being touched by a male or a female (in fact, the toucher was always a female) (Gazzola et al., 2012). Thus, by decoupling the affective significance of affective touch from its cutaneous sensory properties, the authors demonstrated that SI is linked to affective touch. Traditionally, it was assumed that in particular the insula (together with anterior cingulate cortex (ACC) and orbitofrontal cortex (OFC)) processes emotional touch, whereas SI was supposed to play only an indirect role (for example, after signals first have been processed in the insula) and related solely to the recognition of the sensory properties of tactile stimuli. In contrast to this understanding, Gazzola et al. demonstrated that SI was directly engaged in the discrimination of affective touch independent of the insula. The authors concluded that sensory and affective properties of touch may be processed by partially overlapping neural mechanisms.
This view is also supported by other recent studies showing that SI is not only reflecting simple bottom-up mechanisms. For example, it has been reported that the mere observation of (painful or not) touch (in the absence of real touch on the own body) can activate SI (e.g. Blakemore et al., 2005). This vicarious activation in SI has been linked to empathy (Gazzola et al., 2006; Banissy et al., 2009; Schaefer et al., 2012; Peled-Avron et al., 2016; Riečanský and Lamm, 2019). Many researchers have shown that empathy is important to motivate prosocial behavior (e.g. Zaki, 2017). Moreover, imaging studies on empathy suggest a causal role of the somatosensory cortices in prosocial behavior (Gallo et al., 2018).
Considering these studies pointing to an involvement of the somatosensory cortices in social (affective) touch and prosocial behavior, we hypothesized that touch based on the somatosensory cortex activity might serve as the basis of the Midas touch effect. The somatosensory system covers more than 10% of the cortical surfaces, engaging a widespread cortical network including not only SI but also SII (Ferretti et al., 2007; Avanzini et al., 2016). Previous research suggested a ventral stream linked to recognition and perception of tactile stimuli, similar to the visual or auditory domain. This ventral stream is described to originate from SI, passing SII and terminating in the insula (Dijkerman and de Haan, 2007; Preusser et al., 2015). Thus, we assumed that somatosensory cortices are engaged in the Midas touch. This is also supported by the study of Kirsch et al., which demonstrated that affiliative emotions such as social support are elicited by gentle touch irrespective of CT optimality (Kirsch et al., 2018).
To test the hypothesis of a role for the somatosensory cortex in the Midas touch, we here examined the neural underpinnings of this effect by employing a functional magnetic resonance imaging (fMRI) approach. Participants were asked to participate in an economic decision game, the dictator game, which is used to measure prosocial behavior. Before each round of the game, the subjects were touched by an experimenter (or by a rubber hand or not touched as control conditions) to test whether human interpersonal touch leads to increased prosocial behavior. To control for a general positive bias due to touch, we also included control items, which subjects had to evaluate.
Since we hypothesized that pleasant touch leads to the Midas effect, we applied tactile stimulation by relatively slow gentle touch on the palm of the hand (about 3–4 cm/s). Previous studies have shown that slow gentle touch can be linked to feelings of intimacy and social support (Morrison et al., 2011; Kirsch et al., 2018). Given that previous studies failed to replicate the Midas touch when applying touch to the arm, we decided to touch the palm of the hand, which has been shown to elicit Midas touch effects (Spape et al., 2015). In everyday life, we often receive touch to the palm of the hand by others, for example when shaking hands to say hello or asking for apology. Previous studies have shown that slow gentle touch not only to the forearm but also to the palm of the hand can be related to feelings of social support and intimacy (Kirsch et al., 2018).
We hypothesized a role of the somatosensory cortex (SI and SII) in the Midas touch. Given the known role of the insula in linking cognitive and affective processing (Burton and Sinclair, 2000; Craig, 2009) and its role in the network of somatosensory activation pattern (Dijkerman and de Haan, 2007; Ferretti et al., 2007; Preusser et al., 2015; Avanzini et al., 2016), we also examined a possible contribution of this brain region for the Midas touch effect.
Materials and methods
Participants
In total, 27 people (16 females) with a mean age of 22 years (±2.96) took part in the study. All participants gave written informed consent to the study, which adhered to the Declaration of Helsinki and was approved by the local human subjects’ committee. Participants were right-handed native German volunteers and had no neurological or psychiatric history.
Procedure
We used a cover story and told the participants that they would perform two separate and independent experiments in the fMRI scanner, the first one linked to an examination of tactile processing and the second one to an investigation of neural correlates of behavior in an economic game. All participants were naive to the real aim of the study. At the end of the experiment, participants were debriefed and probed for suspicions about the experiment’s true purpose.
In our experimental design we operationalized the touch factor by three conditions. Touch was either done by the hand of an experimenter (real touch condition), by a rubber hand (rubber touch condition) or was omitted (no touch condition). We then measured the prosocial behavior in the dictator game. Furthermore, we added a control task in which the participants were asked to assess various products.
In the real touch condition, the participants were touched by an experimenter on their right hand for about 10 s. During this time window, the experimenter caressed gently the palm of the participant’s hand several times at a speed of about 3–4 cm/s. In the rubber hand touch condition, the experimenter proceeded in an analogous way, but here a rubber hand of the same size as a real hand was used (control condition). The act of touching in this rubber touch condition was applied in a similar way to that in the real touch condition. In the no touch condition, we presented no tactile or other stimuli.
After each touching, the participants had to evaluate how pleasant it felt to them (2 s). To do so, they had to respond with their left hand by using a key with four buttons (ranging from +2 to −2) (Likert scale from 1 to 4, 1 = not at all pleasant, 4 = very pleasant). Participants were explained that they could rate their responses from moderate (inner buttons) to extreme (outer buttons).
Then, a new screen showed up and the dictator game started. The dictator game is an economic game similar to the ultimatum game. The classic ultimatum game addresses cooperation behavior by playing games in which a certain good (usually money) has to be distributed between two players. Previous research has shown that responders confronted with unfair offers are willing to punish the proposer’s behavior (Güth et al., 1982; Sanfey et al., 2003). The task in the dictator game is similar. The player has to distribute a certain amount of money between himself and a responder, but here, the responder has no chance to punish the proposer. Therefore, the proposer can act as a dictator, without worrying that the responder may be upset about the proposer’s behavior. Surprisingly and in contrast to economic rational choice models, participants do not always strictly prefer more money to less money, but often forgo money to help others (Guala and Mittone, 2010). In the current experiment, participants were told that they will play with a certain player (e.g. player number 12), who they were never going to meet.
We used binary dictator games similar to Brocklebank et al. (Charness and Rabin, 2002; Brocklebank et al., 2011). In each game, the participants had to divide 15 Euro between themselves and another person. In these games, they could choose one of two given pairs of payoffs, one that favors the dictator and one that is in favor for the other player. For example, one game could propose two options: either to keep 7.80 Euro and give 7.20 Euro (selfish decision) or to keep 7.20 and give 7.80 to the other player (prosocial decision). The games differed with respect to the potential decision conflict. Participants had to press buttons with the left hand to choose the one or the other option. Use of right and left buttons were randomized over the games. Participants were told that 30% of the games would be selected at random and that they would be paid proportionately by crediting test person hours.
After 12 s, a new screen showed up asking the participants to rate the following product on a four-point scale (Likert scale, 1 = completely undesirable, 4 = completely desirable). Subsequently, a picture of a product was depicted. Stimuli depicted various products, e.g. a chocolate bar, and were of comparable attractiveness (Denke et al., 2016). This task was used to test if the Midas touch effect would extend to a theoretically unrelated measure. Finally, there was a break of 12 s before the next trial started (see Figure 1).
Fig. 1.

Design of experimental task. See text for details.
The experiment consisted of four runs, each including 15 trials. Participants were allowed to take short breaks between the runs. Each run included all conditions, which were presented in a randomized order. Subjects participated in a total of 60 dictator games. Previous fMRI studies on these kinds of economic games used similar or a smaller number of trials (e.g. Sanfey et al., 2003; Wei et al., 2017; Speer and Boksem, 2019). Half of the games followed after the touch conditions; the other half followed after the assessment of products. Hence, the position of product and game stimuli varied in a random way. Tactile stimuli as well as dictator games were presented in a blocked design. Total duration of the experiment was about 45 min.
All stimuli were presented on a visual display inside the scanner using LCD glasses. Foam cushions were placed tightly around the side of the subject’s head to minimize head motion. Participants were made familiar with the task before entering the scanner. Participants were not told who was going to touch them inside the scanner (e.g. a male or a female experimenter).
FMRI data acquisition and analysis
We measured BOLD responses using T2-weighted echo-planar images (TR = 2 s, TE = 35 ms, flip angle = 80°, FOV = 224 mm, number of slices = 32, voxel size = 3.125 × 3.125 mm) on a 3 T Siemens Tim Trio scanner (Siemens, Germany). High-resolution T1-weighted structural images were acquired using an MP-RAGE sequence prior the functional runs for anatomic reference (TR = 1650 ms, TE = 5 ms).
FMRI data were analyzed using the Statistical Parametric Mapping Software (SPM12, Wellcome Department of Imaging Neuroscience, University College London, London, UK). The fMRI images were motion corrected (realigned), normalized into a standard anatomical space (MNI, Montreal Neurological Institute template, isotropic 3 mm voxels), and smoothed with an 8 mm FWHM Gaussian kernel (Schaefer et al., 2018) (no slice timing). Functional volumes were realigned to the mean volume to control for motion artifacts. For each participant, translational movement parameters never exceeded 3 mm. Scans would have been discarded when surpassing these values. Furthermore, we used high-pass temporal filtering with a cutoff of 128 s to remove low-frequency drifts in signal.
We then computed statistical parametric maps using multiple regressions with the hemodynamic response function modeled in SPM. In the first step, we examined data at the individual subject level (fixed effects model). In the second step, the resulting parameter estimates for each regressor at each voxel went into a second-level analysis (random effects model) (e.g. Schaefer et al., 2018). To examine brain responses when the subjects received touch, we calculated statistical contrasts for receiving touch (real touch and rubber hand touch) relative to the no touch condition. We then calculated correlations of peak activations in the somatosensory brain areas with behavioral responses. To analyze the brain regions during the dictator game, we examined the activity while the participants made prosocial (or egoistic, respectively) decisions. We then performed statistical contrasts (t-tests) to compare brain activation during prosocial decisions in the dictator game when touched with a real hand (relative to no touch) and when touched with a rubber hand (relative to no touch). To determine reduced activation during decisions in the dictator game, we also investigated brain responses during the resting period (fixation baseline) relative to the time window of prosocial decisions. At last, to examine brain responses in the control task, we analyzed the time window while subjects evaluated the desirability of various products. Brain activation for ratings depending on touch conditions was analyzed in an analogous way.
We report regions that survived correction for multiple comparisons over the whole brain (at P < 0.05, familywise (FWE) correction). Anatomical interpretation of the functional imaging results was performed by using the SPM anatomy toolbox.
Behavioral responses were tested by in a similar way both for decisions in the dictator game and ratings of the products. Although non-CT fiber processing of touch may not particularly be prone to variables such as the liking of touch, we added this variable (results of the participant’s rating) and also sex as covariates to control for possible confounding effects (Crusco and Wetzel, 1984). Furthermore, participant’s responses (prosocial behavior) were also used to test for possible correlations (Pearson) with the parameter estimates for voxels in activated brain regions (maximum peaks in SI, SII and insula).
Results
Behavioral results
Four participants (two females) were excluded prior to data analysis due to technical reasons (loss of behavioral data). None of the participants reported any suspicions with respect to our experimental hypotheses.
Participants rated touch applied by the real hand to be more pleasant than touch by the rubber hand, as expected (real hand: 3.32 ± 0.64, rubber hand: 2.15 ± 0.77; t(22) = 5.26, P < 0.001, Cohen’s d = 1.10). During the dictator game, the participants decided in 52.98 ± 11.21% of the games to behave in a non-selfish, altruistic way and not to keep the biggest part of money on their own.
To investigate whether touch influences prosocial behavior, we calculated an ANCOVA with the factor touch (real touch, rubber hand touch, no touch) and covariates (sex and how much they rated touch positively) for prosocial behavior (number of altruistic decisions in dictator games). Results showed a significant effect for touch (F (2,40) = 3.42, P = 0.04, partial eta2 = 0.15).
The results demonstrate that participants decided more often altruistic when touched with a real hand (18.67 ± 4.83% of all decisions) compared with the no touch condition (16.66 ± 4.15%; t(22) = 2.36, P = 0.01, Cohen’s d = 0.49). Touch applied by a rubber hand also resulted in a higher frequency of prosocial behavior relative to the no touch condition but revealed only a trend for significance (rubber hand: 18.22 ± 4.76%; t(22) = 1.44, P = 0.08, Cohen’s d = 0.30; see Figure 2). Comparing rubber hand and real hand touch showed no differences (t(22) = −0.46, P > 0.10). Thus, the likeliness of altruistic behavior seems to be higher when participants were intentionally touched, thereby confirming the Midas touch effect.
Fig. 2.

Participants’ mean decisions (+standard errors) in dictator games (in % of all decisions). When participants were touched by the hand of the experimenter, they decided more often to give the biggest part of money to someone else, thus they acted prosocial (compared to no touch). Touching the participants by a rubber hand revealed similar results but showed only a trend for significance. Participants decided in about half of the dictator games in a prosocial way (52.98%).
Correlations between ratings of the participants (liking of the touch) and prosocial behavior in the dictator game revealed no significant correlations (P > 0.10).
Analysis of the ratings of the control products showed no effects (analogue ANCOVA with factor touch; depending variable number of liked items; F(2,40) = 1.07, P > 0.10). Thus, results suggest that touch increases the likeliness of altruistic behavior but does not make individuals assessing any other stimuli in general more positive.
FMRI results: brain responses while receiving touch
Brain responses while receiving touch applied by a real hand showed activation in left sensorimotor cortex, premotor brain areas, inferior frontal gyrus, anterior insula and other areas, as expected. Similar results were found for touch given by a rubber hand (real hand relative to no touch, rubber hand to no touch; FWE corrected, P < 0.05, see Figure 3).
Fig. 3.
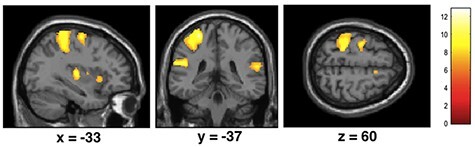
Statistical maps showing brain activation while participants were receiving touch by the hand of the experimenter or by a rubber hand (relative to no touch). Areas of significant fMRI signal change are shown as color overlays on the T1-MNI reference brain (FWE corrected at P < 0.05).
We then examined the relationship between peak activations in somatosensory brain regions during receiving touch by a hand and later prosocial behavior in the dictator game. To do so, Pearson’s correlations of the signal change in SI (and SII) (after real or rubber hand touch relative to no touch) during actually receiving touch with the number of subsequent prosocial decisions (difference in number of prosocial decisions after real or rubber hand touch relative to no touch) were computed.
Results showed that prosocial behavior could be predicted by activations in SI even before participants actually decided (SI: r = 0.43, P = 0.02; left SII: r = 0.34, P = 0.06, right SII: r = 0.42, P = 0.02). Figure 4 displays the scatterplot of the somatosensory activity while receiving touch (touch by a hand relative to no touch) and the increase of prosocial behavior in the real hand touch condition (prosocial behavior in the real hand touch condition relative to prosocial behavior without touch). The figure demonstrates that activation in SI and bilateral SII predicted the increase of the later prosocial decisions in the touch conditions, thereby demonstrating the Midas touch effect and supporting the view of a causal role of the somatosensory cortices (SI, SII) for prosocial decisions. For somatosensory activation during rubber hand stimulation, we also found positive correlations for SI but not for SII (SI: r = 0.49, P = 0.009; left SII: r = 0.05, P > 0.10, right SII: r = 0.10, P > 0.10). To test whether this correlation in SI is stronger for the rubber hand than for real hand touch, we statistically compared the correlation coefficients. We found no significant result (P > 0.10).
Fig. 4.

Brain activation while participants received touch by the hand of the experimenter (FWE corrected at P < 0.05). Brain activity in somatosensory regions predicted the increase of prosocial behavior when touched by the experimenter’s hand relative to no touch. (A) Activation in left SI. Scatterplot of this activity correlated significantly with the prosocial behavior. (B) Same for left SII. (C) Same for right SII.
When calculating analogue correlations for egoistic behavior, we found that brain activity in SI predicted subsequent selfish behavior in a negative way (real hand touch, SI: r = −0.26, P = 0.12; rubber hand touch, SI: r = −0.47, P = 0.01). Similar relationships were found for SII (real hand touch, right SII: r = −0.13, P = 0.28, left SII: r = −0.24, P = 0.14; rubber hand touch, right SII: r = −0.08, P = 0.35, left SII: r = −0.07, P = 0.36).
In addition, we tested whether insula peak activations elicited by touch predicted later altruistic behavior in the dictator game. We found significant correlations for right but not for left anterior insula (real hand, right insula: r = 0.40, P = 0.03; left insula, r = 0.07, P > 0.10.; rubber hand, right insula: r = 0.27, P = 0.10, left insula, r = 0.18, P > 0.10.). Insula and somatosensory (SI, SII) activations were not correlated with touch ratings of the participants (P > 0.10.).
FMRI results: brain responses during dictator games
Brain responses while participants acted prosocial relative to selfish behavior during the dictator game (irrespective of touching the participant) showed activation of clusters in particular in the right temporoparietal junction area, middle temporal gyrus and SI (FWE corrected, P < 0.05).
We then examined brain activations while participants played the dictator games and decided for the altruistic options. An ANOVA with the factor touch (real hand touch, rubber hand touch, no touch) revealed activation in bilateral sensorimotor cortices (bilateral SI, bilateral SII, bilateral precentral gyrus, right supramarginal gyrus). Even with a lenient threshold of P < 0.005 (uncorrected) no other brain regions were engaged (see Figure 5). We then calculated statistical contrasts between the conditions. For the comparison real hand touch relative to no touch, we did not find any suprathreshold voxels. In contrast, the opposite comparison between no touch relative to real hand touch revealed activation in SI (at P < 0.05, FWE corrected) that predicted prosocial behavior (r = 0.59, P = 0.003). Similarly, we did not find activation for the comparison rubber hand touch relative to no touch, but for the contrast, no touch relative to rubber hand touch (at P < 0.05, FWE corrected), for which we found again an engagement of SI that was associated with prosocial responses (r = 0.46, P = 0.03) (see Table 1 and Figure 6). No other brain areas showed activation.
Fig. 5.

Statistical maps showing brain activation while participants decided in a prosocial way during the dictator game (ANOVA). Results demonstrate activation of bilateral somatosensory cortices (SI and SII) and precentral gyri. No other brain regions were active even with a lenient threshold of P < 0.05 (uncorrected).
Table 1.
Results of random effects analysis for brain responses when participants performed the dictator game (whole brain, P < 0.05, FWE corrected)
| Contrast | Brain region | Peak MNI location (x, y, z) | Peak z-value | Number of voxels | |
|---|---|---|---|---|---|
| Prosocial behavior | Real hand touch > no touch | ||||
| Rubber hand touch > no touch | |||||
| No touch > real hand touch | Left SI | −42, −34, 54 | 5.51 | 91 | |
| No touch > rubber hand touch | Left SI | −30, −36, 50 | 5.19 | 15 | |
| Left SII (central/parietal operculum) | −50, −24, 22 | 5.65 | 24 | ||
| Right SI | 54, −14, 36 | 4.80 | 14 | ||
| Right cerebellum | 26, −56, −28 | 4.99 | 8 | ||
| Selfish behavior | Real hand touch > no touch | ||||
| Rubber hand touch > no touch | |||||
| No touch > real hand touch | |||||
| No touch > rubber hand touch |
Fig. 6.

Statistical maps showing brain activation while participants decided in a prosocial way during the dictator game. (A) Figure displays the contrast no touch relative to real hand touch. Results revealed significant activation of left SI for this contrast, which means that SI was deactivated here. No other areas were activated. The opposite contrast real hand touch relative to no touch revealed no significant activation (FWE corrected at P < 0.05). (B) Scatterplots of BOLD response in SI for the contrast no touch relative to real hand touch and increase of prosocial decisions for real hand touch (relative to no touch condition). Results show a significant relationship between SI activity and behavioral response (prosocial decisions). (C and D) Same for the contrast no touch relative to rubber hand touch (FWE corrected at P < 0.05).
Analogue analysis for egoistic decisions demonstrated no significant brain activations with respect to the different touch conditions (FWE corrected, P < 0.05).
Thus, when prosocial decisions were triggered by a touched hand, the somatosensory cortex seems to be less activated during the dictator games. To further examine this relationship, we analyzed brain responses during prosocial decisions (with real hand touch, rubber touch, no touch) compared with brain activity during baseline (instead of the time window of the dictator game with no touch). We focused on the contrast baseline relative to dictator game to analyze brain areas that are less activated during the dictator game. Results showed less activation (compared with baseline) in the angular gyrus, medial frontal cortex and hippocampus whenever the participants decided to act altruistic (baseline relative to each of the three conditions). Thus, these brain areas seem to be suppressed when participants decided in a prosocial way. However, when participants decided to behave prosocial because of the touch of the experimenter (baseline relative to real hand touch or rubber hand touch), additionally SI and SII (parietal operculum) were suppressed during the dictator game (see Table 2).
Table 2.
Results of random effects analysis for brain responses during baseline activation compared with prosocial decisions in dictator game. Displayed are activations surviving peak level correction at P < 0.05, FWE corrected (threshold of P < 0.005 used to define the clusters, small volume correction (SVC, spheres of 10 mm radius) (L = left hemisphere, R = right hemisphere; in brackets: P < 0.001, uncorrected)
| Contrast | Brain region | Peak MNI location (x, y, z) | Peak z-value | Number of voxels | |
|---|---|---|---|---|---|
| Prosocial behavior | Baseline > real hand touch | Left SI Left hippocampus |
−40, −24, 66 −24, −12, −20 |
3.10 4.58 |
16 114 |
| Left par. operculum | 2, 46, 16 | 3.12 | 35 | ||
| Right ACC | 2, 26, −4 | 3.94 | 62 | ||
| Left temporal pole | −36, 2, −28 | 3.37 | 55 | ||
| Left angular gyrus | −42, −80, 34 | 4.13 | 41 | ||
| Right hippocampus | 24, −10, −20 | 3.62 | 28 | ||
| Right STG | 66, −42, 18 | 3.41 | 31 | ||
| Right mid. front. cortex | −56, −34, 18 | 3.32 | 47 | ||
| Baseline > rubber hand touch | Left SI Left angular gyrus |
−38, −24, 68 −48, −74, 34 |
3.46 4.56 |
103 96 |
|
| Right hippocampus | 26, −12, −20 | 3.84 | 88 | ||
| Left hippocampus | −22, −14, −20 | 3.79 | 110 | ||
| Left ACC | −2, 28, −4 | 3.98 | 64 | ||
| Left par. operculum | 66, −42, 16 | 3.35 | 16 | ||
| Right mid. front. cortex | 0, 44, −16 | 3.42 | 79 | ||
| Right angular gyrus | 52, −66, 28 | 4.46 | 33 | ||
| Left temporal pole | −36, 4, −28 | 3.65 | 40 | ||
| Right fusiform gyrus | 40, −18, −22 | 3.47 | 26 | ||
| Right STG | −56, −30, 18 | 3.03 | 57 | ||
| (left orbital gyr.) | −28, 36, −10 | 2.99 | 8 | ||
| (left parahippocampal gy.) | −24, −34, −16 | 2.82 | 9 | ||
| (left precentral gyrus) | −34, −12, 54 | 2.77 | 7 | ||
| (left precuneus) | −8, −56, 14 | 2.71 | 5 | ||
| Baseline > no touch | Left angular gyrus | −42, −80, 34 | 4.67 | 82 | |
| Right mid. front. cortex | −2, 46, −16 | 3.91 | 137 | ||
| Left hippocampus | −24, −12, −20 | 2.88 | 12 | ||
| (right hippocampus) | 24, −10, 18 | 3.21 | 13 | ||
| (left precuneus) | −8, −56, 20 | 2.99 | 17 | ||
| (left STG) | −54, −10, −18 | 2.97 | 32 |
A correlation between BOLD responses in SI during actual receiving touch and brain deactivation in SI during deciding altruistic in the dictator games revealed significant relationships (hand touch: r = 0.47, P = 0.01, rubber hand touch: r = 0.48, P = 0.01), suggesting that those participants who showed a strong activation in SI during actual touch were also the subjects who demonstrated strong deactivation in SI during prosocial decisions in the dictator game.
FMRI results: brain responses during rating of control pictures
At last, we examined brain activations while participants rated various pictures. When contrasting brain activation during evaluation of these pictures for the touch condition (applied by a real or a rubber hand) relative to the no touch condition, we did not find any significant brain activation. Furthermore, the opposite contrast (no touch relative to touch by a rubber hand or a real hand) also failed to show significant activations (FWE corrected, P < 0.05).
Discussion
The Midas touch effect describes how interpersonal touch affects prosocial behavior. While numerous behavioral studies provided support for this effect, the underlying neural substrates remain unclear. Here, we investigated the Midas touch effect by employing an fMRI approach. Participants were touched by a hand while playing dictator games. Results showed that touch led individuals to behave in a more prosocial way. This effect was associated with an involvement of somatosensory cortices. SI activity during receiving touch predicted subsequent prosocial behavior (relative to no touch and relative to baseline).
Our behavioral results could replicate the Midas touch effect in an experimental laboratory context. Touching our participants with the hand or even with a rubber hand made our participants to be more altruistic and generous. This is in line with a recent EEG study pointing to a relationship between touch and prosocial behavior by examining nonverbal communication on fairness perceptions (Spape et al., 2015). In contrast, other previous studies failed to replicate the Midas touch in a laboratory context. For example, Rosenberger et al. examined whether the Midas touch effects relies on an activation of CT fibers, which are known to be in particular important for interpersonal caress and similar interactions (Rosenberger et al., 2018). The authors could not replicate the Midas touch effect when touching the forearms, which are known to process touch by CT fibers (similarly Koppel et al., 2017). In contrast to the study by Rosenberger, our study (as well as the study by Spapé et al.) touched the participants on the glabrous skin of the hand, portions of the body surface that are not related to CT-fiber processing. Using this experimental paradigm, we could replicate the Midas touch effect.
Previous behavioral studies on the Midas touch reported effects when touched on different body sites. For example, Gueguen reported that touch applied to the forearm raised the compliance to answer a questionnaire (Guéguen, 2002). In the original work by Crusco and Wetzel the participants were touched either on the hand or on the shoulder. The authors found effects on generosity, but no differences with respect for the touched body sites (Crusco and Wetzel, 1984). Together with the results of our study it seems that the Midas touch effect is at least not solely based on CT-fiber processing, since somatosensory processing of touch on the glabrous skin (e.g. the hand) demonstrates the Midas touch effect.
Our results also demonstrate that even touch not related to a stimulation of CT fibers is felt pleasant by our participants. This is line with a recent study showing that slowly touching the palm of the hand can be linked to feelings of intimacy and social support (Kirsch et al., 2018). Furthermore, Gazzola et al. (2012) reported that SI discriminates affective touch beyond the engagement of CT fibers. Thus, our results suggest that processing in SI may also include affective aspects of touch, which are unlikely to be explained simply by indirect effects of an insula, ACC or OFC activation.
It is remarkable that we found Midas touch effects not only for skin-to-skin touch (real hand) but also for rubber hand touch (but statistics showed only a trend for significance). Thus, although skin-to-skin touch was perceived as more pleasant, even mechanic touch by a rubber hand seem to cause prosocial behavior. Previous studies demonstrated similar effects. For example, Schirmer et al. confirmed our findings. Although not directly testing the Midas touch effect, the authors found that touch on the forearm not only by a friend but also by a tactile device enhanced cognitive and emotional processing (Schirmer et al., 2011). Thus, the authors are in line with our results by demonstrating that also mechanical touch may have similar effects, pointing to the role of perception of intention when feeling touch.
The present study showed that the magnitude of somatosensory activations (SI and SII) during touch predicted the strength of the Midas touch effect (the prosocial behavior after touch). This positive relationship demonstrates that the Midas touch effect is based in particular on somatosensory brain areas. When examining brain responses during altruistic behavior in the dictator game, we found no activations but a deactivation of SI for touch (real or a rubber hand) relative to no touch before. Suppression or deactivation may be an important property of cortical processing (Sanzeni et al., 2020). Suppression of the somatosensory processing in SI has been reported before (Blankenburg et al., 2003). For example, during regular touch processing, the ipsilateral SI is known to be suppressed, while the contralateral SI is activated (Tal et al., 2017). However, the physiological origin of those negative BOLD responses are still controversial discussed (Klingner et al., 2015). Previous studies suggested that deactivation of somatosensory cortices can be interpreted in terms of decreased neuronal activity (Mayhew et al., 2016). Further analysis of our data revealed that the deactivation correlated significantly with activation of SI during actual touch. Based on this relationship one could interpret the deactivation of the somatosensory cortices as a possible rebound mechanism in the somaosensory cortices. Previous studies have observed suppression and rebound in particular with respect to the 20 Hz brain rhythm over the sensorimotor cortex, which is used to evaluate the functional state of the sensorimotor cortex, e.g. after stroke (e.g. Parkes et al., 2006; Illman et al., 2020). This suppression or rebound may reflect reduced excitability of the sensorimotor cortex after somatosensory activation. Another explanation points to the view that often ‘alternative courses of action and thoughts have to be inhibited to allow the emergence of goal-directed behavior’ (Bari and Robbins, 2013). Previous studies found support for this position (Taskin et al., 2008). Since the somatosensory cortices have also been linked to self–other distinctions (Bari and Robbins, 2013; Boehme et al., 2019) and to bodily self-consciousness (Martuzzi et al., 2015), the suppression of brain activity here might foster altruistic behavior in the dictator games (which is defined in not keeping the biggest part of money on our own but to give it away to the other player).
We also found that brain activity in the right insula elicited by touch predicted later prosocial behavior. The insula has dense reciprocal connections with SII, which in turn sends feedback projections to SI (Augustine, 1996; Nomi et al., 2016), and has been related to the ventral pathway of somatosensory perception (Dijkerman and de Haan, 2007; Preusser et al., 2015). We argue that the insula is here engaged due to its role in the pathway of somatosensory processing. Further research is needed to describe its contribution to the Midas touch effect more in detail.
It has to be noted that the perceived liking of the touch was not correlated with behavioral responses or brain activation in somatosensory or insula brain regions. Thus, the liking of touch does not seem to explain the prosocial touch. This is also in line with our results that more artificial touch by a rubber hand showed similar results than touch provided by a real hand.
Several limitations of this study have to be noted. First, in our sample, the gender of particpants was not balanced. Although the present study did not directly address romantic feelings, it cannot be excluded that gender affected the perception of skin-to-skin touch. In addition, the gender of the experimenter who applied the touch should be controlled, too (Gazzola et al., 2012). Second, we here applied touch only to the palm of the hand. Future studies should include touch to the forearm to compare more systematically the effect of somatosensory and CT-fiber-based processing. Third, we here mesaured prosocial behavior by using the dictator game. There are numerous ways of measuring prosocial behavior, each with a different focus. Further studies should replicate our findings using other approaches to measure prosocial actions.
What are the implications of our findings? Although previous studies already suggested how the Midas touch effect can be used to influence tipping behavior or convince people to help, other implications also seem to be important and possible. For example, the effect may help in situations, in which participants need to work in close relationships. In these situations, touch and our somatosensory cortices might be important to help each other and provide a productive and positive atmosphere. Thus, touch can help us to to increase commitment and maintain good social interrelationships. Furthermore, the effect may also help in the opposite behavior. Psychopathic behavior of indidviduals might be avoided or limited by a touching hand. Further research is needed to examine these speculations, but it seems to be justified to focus more strongly on the long neglected sense of touch.
Contributor Information
Michael Schaefer, Medical School Berlin, Berlin 14197, Germany.
Anja Kühnel, Medical School Berlin, Berlin 14197, Germany.
Franziska Rumpel, Otto-von-Guericke Business School Magdeburg, Magdeburg 39106, Germany.
Matti Gärtner, Medical School Berlin, Berlin 14197, Germany; Charité – Universitätsmedizin Berlin, Berlin 12200, Germany.
Author contributions
M.S., M.G. and F.R. conceived and designed the experiment. M.S., M.G. and A.K. performed the experiments. M.S. and M.G. analyzed the data. M.S. and M.G. wrote the paper.
Conflict of interest
The authors declare no competing financial interests.
References
- Augustine J.R. (1996). Circuitry and functional aspects of the insular lobe in primates including humans. Brain Research Reviews, 22(3), 229–44. [DOI] [PubMed] [Google Scholar]
- Avanzini P., Abdollahi R.O., Sartori I., et al. (2016). Four-dimensional maps of the human somatosensory system. Proceedings of the National Academy of Sciences of the United States of America, 113(13), E1936–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banissy M.J., Walsh V., Ward J. (2009). Enhanced sensory perception in synaesthesia. Experimental Brain Research, 196(4), 565–71. [DOI] [PubMed] [Google Scholar]
- Bari A., Robbins T.W. (2013). Inhibition and impulsivity: behavioral and neural basis of response control. Progress in Neurobiology, 108, 44–79. [DOI] [PubMed] [Google Scholar]
- Blakemore S.-J., Bristow D., Bird G., Frith C., Ward J. (2005). Somatosensory activations during the observation of touch and a case of vision–touch synaesthesia. Brain, 128(7), 1571–83. [DOI] [PubMed] [Google Scholar]
- Blankenburg F., Taskin B., Ruben J., et al. (2003). Imperceptible stimuli and sensory processing impediment. Science, 299(5614), 1864. [DOI] [PubMed] [Google Scholar]
- Boehme R., Hauser S., Gerling G.J., Heilig M., Olausson H. (2019). Distinction of self-produced touch and social touch at cortical and spinal cord levels. Proceedings of the National Academy of Sciences of the United States of America, 116(6), 2290–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklebank S., Lewis G.J., Bates T.C. (2011). Personality accounts for stable preferences and expectations across a range of simple games. Personality and Individual Differences, 51, 881–6. [Google Scholar]
- Brockner J., Pressman B., Cabitt J., Moran P. (1982). Nonverbal intimacy, sex, and compliance: a field study. Journal of Nonverbal Behavior, 6, 253–8. [Google Scholar]
- Burton H., Sinclair R.J. (2000). Attending to and remembering tactile stimuli: a review of brain imaging data and single-neuron responses. Journal of Clinical Neurophysiology, 17(6), 575–91. [DOI] [PubMed] [Google Scholar]
- Charness G., Rabin M. (2002). Understanding social preferences with simple tests. Quarterly Journal of Economics, 117, 817–69. [Google Scholar]
- Craig A.D. (2009). How do you feel — now? The anterior insula and human awareness. Nature Reviews Neuroscience, 10(1), 59–70. [DOI] [PubMed] [Google Scholar]
- Crusco A.H., Wetzel C.G. (1984). The Midas touch. The effecs of interpersonal touch on restaurant tipping. Personality & Social Psychology Bulletin, 10, 512–7. [Google Scholar]
- Denke C., Rotte M., Heinze H.-J., Schaefer M. (2016). Lying and the subsequent desire for toothpaste: activity in the somatosensory cortex predicts embodiment of the moral-purity metaphor. Cerebral Cortex, 26(2), 477–84. [DOI] [PubMed] [Google Scholar]
- Dijkerman H.C., de Haan E.H.F. (2007). Somatosensory processes subserving perception and action. Behavioral and Brain Sciences, 30(2), 189–39. [DOI] [PubMed] [Google Scholar]
- Ferretti A., Babiloni C., Arienzo D., et al. (2007). Cortical brain responses during passive nonpainful median nerve stimulation at low frequencies (0.5–4 Hz): an fMRI study. Human Brain Mapping, 28(7), 645–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo S., Paracampo R., Muller-Pinzler L., et al. (2018). The causal role of the somatosensory cortex in prosocial behaviour. eLife, 7, e32740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzola V., Aziz-Zadeh L., Keysers C. (2006). Empathy and the somatotopic auditory mirror system in humans. Current Biology, 16(18), 1824–9. [DOI] [PubMed] [Google Scholar]
- Gazzola V., Spezio M.L., Etzel J.A., Castelli F., Adolphs R., Keysers C. (2012). Primary somatosensory cortex discriminates affective significance in social touch. Proceedings of the National Academy of Sciences of the United States of America, 109(25), E1657–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman M., Kiyohara O., Pfannensteil D. (1985). Interpersonal touch, social labeling, and the foot-in-the-door effect. The Journal of Social Psychology, 125, 143–7. [Google Scholar]
- Guala F., Mittone L. (2010). Paradigmatic experiments: the dictator game. The Journal of Socio-Economics, 39, 578–84. [Google Scholar]
- Guéguen N. (2002). Touch, awareness of touch, and compliance with a request. Perceptual and Motor Skills, 95(2), 355–60. [DOI] [PubMed] [Google Scholar]
- Guéguen N., Fischer-Lokou J. (2002). An evaluation of touch on a large request: a field setting. Psychological Reports, 90(1), 267–9. [DOI] [PubMed] [Google Scholar]
- Guéguen N., Fischer-Lokou J. (2003). Tactile contact and spontaneous help: an evaluation in a natural setting. The Journal of Social Psychology, 143(6), 785–7. [DOI] [PubMed] [Google Scholar]
- Guéguen N., Jacob C. (2005). The effect of touch on tipping: an evaluation in a French bar. International Journal of Hospitality Management, 24, 295–9. [Google Scholar]
- Güth W., Schmittberger R., Schwarze B. (1982). An experimental analysis of ultimatum bargaining. Journal of Economic Behavior & Organization, 3, 367–88. [Google Scholar]
- Hornik J. (1992). Tactile stimulation and consumer response. Journal of Consumer Research, 19, 449–58. [Google Scholar]
- Illman M., Laaksonen K., Liljeström M., Jousmäki V., Piitulainen H., Forss N. (2020). Comparing MEG and EEG in detecting the∼20-Hz rhythm modulation to tactile and proprioceptive stimulation. NeuroImage, 215, 116804. [DOI] [PubMed] [Google Scholar]
- Kirsch L.P., Krahé C., Blom N., et al. (2018). Reading the mind in the touch: neurophysiological specificity in the communication of emotions by touch. Neuropsychologia, 116(Pt A), 136–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinke C. (1977). Compliance to requests made by gazing and touching experimenters in field settings. Journal of Experimental Social Psychology, 13, 218–23. [Google Scholar]
- Klingner C.M., Brodoehl S., Witte O.W. (2015). The importance of the negative blood-oxygenation-level-dependent (BOLD) response in the somatosensory cortex. Reviews in the Neurosciences, 26(6), 647–53. [DOI] [PubMed] [Google Scholar]
- Koppel L., Andersson D., Morrison I., Västfjäll D., Tinghög G. (2017). The (null) effect of affective touch on betrayal aversion, altruism, and risk taking. Frontiers in Behavioral Neuroscience, 11, 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levav J., Argo J.J. (2010). Physical contact and financial risk taking. Psychological Science, 21(6), 804–10. [DOI] [PubMed] [Google Scholar]
- Lynn M., Le J.-M., Sherwyn D. (1998). Reach out and touch your customers. Cornell Hotel and Restaurant Administration Quarterly, 39, 60–5. [Google Scholar]
- Martuzzi R., van der Zwaag W., Dieguez S., Serino A., Gruetter R., Blanke O. (2015). Distinct contributions of Brodmann areas 1 and 2 to body ownership. Social Cognitive and Affective Neuroscience, 10(11), 1449–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhew S.D., Mullinger K.J., Ostwald D., et al. (2016). Global signal modulation of single-trial fMRI response variability: effect on positive vs negative BOLD response relationship. NeuroImage, 133, 62–74. [DOI] [PubMed] [Google Scholar]
- Morrison I., Björnsdotter M., Olausson H. (2011). Vicarious responses to social touch in posterior insular cortex are tuned to pleasant caressing speeds. Journal of Neuroscience, 31(26), 9554–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomi J.S., Farrant K., Damaraju E., Rachakonda S., Calhoun V.D., Uddin L.Q. (2016). Dynamic functional network connectivity reveals unique and overlapping profiles of insula subdivisions. Human Brain Mapping, 37(5), 1770–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olausson H., Wessberg J., Morrison I., McGlone F., Vallbo Å. (2010). The neurophysiology of unmyelinated tactile afferents. Neuroscience and Biobehavioral Reviews, 34(2), 185–91. [DOI] [PubMed] [Google Scholar]
- Parkes L.M., Bastiaansen M.C.M., Norris D.G. (2006). Combining EEG and fMRI to investigate the post-movement beta rebound. NeuroImage, 29(3), 685–96. [DOI] [PubMed] [Google Scholar]
- Peled-Avron L., Levy-Gigi E., Richter-Levin G., Korem N., Shamay-Tsoory S.G. (2016). The role of empathy in the neural responses to observed human social touch. Cognitive, Affective & Behavioral Neuroscience, 16(5), 802–13. [DOI] [PubMed] [Google Scholar]
- Preusser S., Thiel S.D., Rook C., et al. (2015). The perception of touch and the ventral somatosensory pathway. Brain, 138(3), 540–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddan M.C., Young H., Falkner J., López-Solà M., Wager T.D. (2020). Touch and social support influence interpersonal synchrony and pain. Social Cognitive and Affective Neuroscience, 15(10), 1064–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riečanský I., Lamm C. (2019). The role of sensorimotor processes in pain empathy. Brain Topography, 32(6), 965–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberger L.A., Ree A., Eisenegger C., Sailer U. (2018). Slow touch targeting CT-fibres does not increase prosocial behaviour in economic laboratory tasks. Scientific Reports, 8(1), 7700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanfey A.G., Rilling J.K., Aronson J.A., Nystrom L.E., Cohen J.D. (2003). The neural basis of economic decision-making in the ultimatum game. Science, 300, 1755–8. [DOI] [PubMed] [Google Scholar]
- Sanzeni A., Akitake B., Goldbach H.C., Leedy C.E., Brunel N., Histed M.H. (2020). Inhibition stabilization is a widespread property of cortical networks. eLife, 9, e54875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer M., Heinze H.-J., Rotte M. (2012). Embodied empathy for tactile events: interindividual differences and vicarious somatosensory responses during touch observation. NeuroImage, 60(2), 952–7. [DOI] [PubMed] [Google Scholar]
- Schaefer M., Cherkasskiy L., Denke C., et al. (2018). Incidental haptic sensations influence judgment of crimes. Scientific Reports, 8(1), 6039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmer A., Teh K.S., Wang S., et al. (2011). Squeeze me, but don’t tease me: human and mechanical touch enhance visual attention and emotion discrimination. Social Neuroscience, 6(3), 219–30. [DOI] [PubMed] [Google Scholar]
- Smith D.E., Gier J.A., Willis F.N. (1982). Interpersonal touch and compliance with a marketing request. Basic and Applied Social Psychology, 3, 35–8. [Google Scholar]
- Spapé M.M., Hoggan E.E., Jacucci G., Ravaja N. (2015). The meaning of the virtual Midas touch: an ERP study in economic decision making. Psychophysiology, 52(3), 378–87. [DOI] [PubMed] [Google Scholar]
- Speer S.P.H., Boksem M.A.S. (2019). Decoding fairness motivations from multivariate brain activity patterns. Social Cognitive and Affective Neuroscience, 14(11), 1197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen R.Z., Zweigenhaft R.L. (1986). The effect on tipping of a waitress touching male and female customers. The Journal of Social Psychology, 126, 141–2. [Google Scholar]
- Tal Z., Geva R., Amedi A. (2017). Positive and negative somatotopic BOLD responses in contralateral versus ipsilateral penfield homunculus. Cerebral Cortex, 27(2), 962–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taskin B., Holtze S., Krause T., Villringer A. (2008). Inhibitory impact of subliminal electrical finger stimulation on SI representation and perceptual sensitivity of an adjacent finger. NeuroImage, 39(3), 1307–13. [DOI] [PubMed] [Google Scholar]
- Wei Z., Zhao Z., Zheng Y. (2017). The neural basis of social influence in a dictator decision. Frontiers in Psychology, 8, 2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J. (2017). Moving beyond stereotypes of empathy. Trends in Cognitive Sciences, 21(2), 59–60. [DOI] [PubMed] [Google Scholar]


