Abstract
Resistant and tolerant bacterial infections lead to billions in healthcare costs and cause hundreds of thousands of deaths each year. The bulk of current antibiotic research efforts focus on molecules which, although novel, are not immune from acquired resistance and seldomly affect tolerant populations. The bacterial SOS response has been implicated in several resistance and tolerance mechanisms, making it an attractive antibiotic target. Using small molecule inhibitors targeting a key step in the deployment of the SOS response, our approach focused on preventing the deployment of mechanisms such as biofilm formation, horizontal gene transfer, and error-prone DNA repair. Herein we report the synthesis and testing of analogs of a triazole-containing tricyclic inhibitor of LexA proteolysis, the key event in the SOS response. Our results hint that our inhibitor’s may function by adopting a b-hairpin conformation, reminiscent of the native cleavage loop of LexA.
Keywords: SOS response, stress response, antibiotic resistance, tolerance, DNA damage
Graphical Abstract
Roughly 36,000 Americans die each year due to antibiotic resistant infections, incurring billions in healthcare costs.1 Previously treatable infections are no longer responsive to typical antibiotics, forcing clinicians to resort to more extreme “last-defense” drugs. Bacterial tolerance poses an additional challenge—these quiescent populations of cells can withstand most antibiotics and are implicated in chronic and recurring infections. This trajectory of resistant and tolerant infections threatens to undo most of the achievements of modern medicine, rendering even simple procedures life-threatening.
Several resistance and tolerance pathways have been connected to the SOS response (a bacterial stress response, Figure 1).2,3 In unstressed bacteria, a repressor called LexA binds to the promoters of DNA damage response genes, maintaining the SOS response in the “off” state. The SOS response is turned “on” in the presence of DNA damage, which can be caused by UV exposure, reactive oxygen species, or antimicrobials. Damaged DNA stalls replication, exposing single-stranded DNA (ssDNA), which then recruits the DNA damage sensor RecA. ssDNA templates the ATP-dependent oligomerization of RecA forming an activated nucleoprotein filament known as RecA*. RecA*, in turn, binds to LexA to induce its self-cleavage. This reaction is presumed to occur due to the RecA*-dependent stabilization of a conformational change in LexA. This conformational change is defined by the formation of a β-turn that positions an internal scissile peptide bond between Ala84-Gly85 adjacent to its internal serine protease active site (Figure 2). LexA autoproteolysis severs the DNA binding domain from the catalytic and dimerization interface-containing protease domain.4 Ultimately, depletion of intact LexA leads to its dissociation from promoters, allowing unrepressed transcription of the SOS response.
Figure 1:

The SOS response
Figure 2:

Crystal structures highlighting the transition from a non-cleavable conformation (red) to a cleavable conformation (green) with the target scissile bond highlighted in yellow. The triazole scaffold may mimic the β-turn adopted by the cleavable conformation, occluding the target residues from the active site.
This SOS response in E. coli includes roughly 40 genes, including low-fidelity DNA polymerases that promote mutagenesis and genes that induce growth arrest, dissemination of mobile gene elements, and resistance determinants.5–12 Given the central role of the response in pathways leading to antibiotic evasion, the SOS response makes for a compelling antimicrobial target. Indeed, interruption of the SOS response with a non-cleavable LexA mutant mitigates acquired resistance to fluoroquinolones.13 Furthermore, attenuation of the SOS response via recA inactivation not only reduces acquired resistance of Escherichia coli to several antibiotics, but also sensitizes the bacteria to some antibiotics, even resensitizing resistant bacteria to fluoroquinolones.14–16
The SOS response has also been connected to biofilm formation.17,18 Biofilms are matrices of cells enveloped in a thick extracellular polymeric substance (EPS) composed of polysaccharides, proteins, nucleic acids, and lipids. The EPS provides bacterial colonies structural stability, enables adhesion to surfaces, facilitates intercellular coordination, and protects resident cells from UV damage, metal toxicity, acid, and dehydration (among other conditions).19–24 LexA represses transcription of fnbB, a fibronectin-binding protein important for adherence to extracellular matrix components and, as a result, biofilm formation.25,26 Indeed, ΔrecA mutants display diminished stress-induced biofilm formation (4x) whereas recA-complemented strains regain biofilm formation ability.17 These studies make the SOS response an attractive target for the abrogation of biofilm formation, among other adaptive responses.
The key step of the SOS response is the RecA-induced autoproteolysis of LexA. Thus far few inhibitors of RecA* activity or filamentation have been studied.27–32 However, RecA is homologous to the fundamental eukaryotic Rad51 recombinase family, rendering it a problematic target. In comparison, LexA inhibitors have been understudied until recently.32–34 We discovered one group of inhibitors—triazole-containing tricycles—in an academic-industry partnership in 2018.33 Previous efforts to increase potency of this scaffold yielded an analog with an improved IC50 (9 ± 1 μM) with activity against both E. coli and Pseudomonas aeruginosa LexA (1). The inhibitors also showed biological activity in a ciprofloxacin-induced mutagenesis assay, supporting the principle that SOS inhibition can suppress acquired antibiotic resistance. Although the exact binding site of the inhibitors is not known, triazole-containing compounds have been used before as β-turn mimetics, which led us to hypothesize that these compounds may be adopting β-turn-like conformations to compete with the cleavage loop within the active site of LexA (Figure 2).35,36 In our prior SAR study, we found isolated changes to either position A or position B in the scaffold of the lead compound could improve potency. We speculated that if the central triazole moiety were indeed positioning A and B more proximate to each other, simultaneous changes in these two regions might be either synergistic or antagonistic.
With this motivation in mind, we planned to further expand our structure-activity relationship (SAR) study by examining the substitutions on the outer rings of the scaffold. Guided by the findings of our first SAR study, we devised an initial panel of analogs (Figure 3). We synthesized 1, the previous lead, as a control. Analogs 2-5 combined the improvements seen in past analogs by melding ring substitutions in position A with the improvement of using an unsubstituted phenyl ring at position B. We designed and synthesized additional analogs decorated with various heteroatoms and electron-withdrawing groups to probe the effect on inhibitory activity. We also postulated that, if effective, analog 11 could serve as a starting point for diazirine photoaffinity probe studies, available in 4 steps.37 These analogs could be easily synthesized using a convergent route that would also enable access to a number of outer ring combinations (Figure 4). First, we combined substituted anilines with 2-bromoacetyl bromide to generate various amides. Then, using either a cyanide or azide nucleophile, we assembled the two fragments before combining them via a base-catalyzed click to form the full scaffold.
Figure 3:

Tricyclic inhibitors of LexA autoproteolysis. A) Small molecule panel inspired by previous lead 1. B) Analogs 2–5 combine the best results from the initial SAR study. C) Proposed expanded library of A and B rings.
Figure 4:

Synthesis of small molecule panel. For full synthesis details, refer to the Supplemental Information.
Initial attempts to perform the base-catalyzed click using past conditions (either sodium ethoxide in ethanol or cesium carbonate in DMSO/water) were unsuccessful.34 Curiously, the only product we were able to isolate was an unexpected dihydropyrrolone (analog 12), confirmed via NMR, HRMS, and X-ray crystallography. Reaction optimization, namely swapping the base and solvent for potassium carbonate and acetonitrile, respectively, provided triazole products in 18–83% yield (full optimization details available in the supporting information).38 We suspect that the change for a less polar solvent favors the desired cycloaddition product, whereas more polar systems might bias the reaction toward ionic transition states.
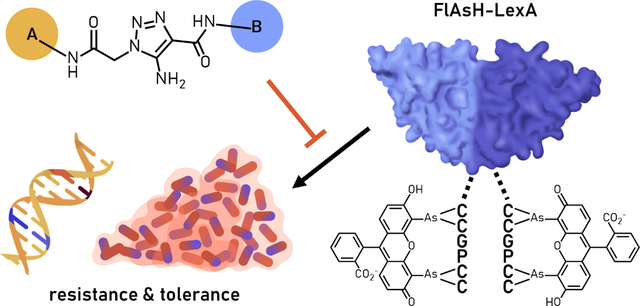
We began by synthesizing analogs varied at the A ring, the activity of which we planned to use to guide a panel of B ring analogs. These analogs were tested using our FlAsH-LexA assay (Figure 5), which utilizes a truncated LexA furnished with a tetracysteine CCPGCC motif bound to the biarsenical fluorophore, FlAsH-EDT2.33 Addition of RecA* triggers LexA proteolysis, releasing a short fluorophore-labelled peptide measured by a corresponding decrease in fluorescence polarization. The addition of LexA inhibitors prevents release of this peptide and maintains fluorescence polarization. Regrettably, our inhibitor analogs displayed no improvement over the previous lead, 1. Only analogs 4 and 10 displayed any appreciable activity. Surprisingly, analogs 2, 3, and 5 were inactive, although their component A and B rings displaying potent activity separately.34
Figure 5:

The FlAsH-LexA assay utilizes a truncated version of LexA in which the DNA-binding N-terminal domain is replaced with a FlAsH-binding tetracysteine tag. Upon RecA*-mediated autoproteolysis, the FlAsH-LexA construct (left) releases a short fluorophore-labeled peptide (right). The rate of proteolysis may then be measured as by a decrease in fluorescence polarization.
Despite no further improvement on our starting compound, our new analogs did offer some added insights. First, in our previous SAR study, we demonstrated that replacement of ortho,meta-dimethyl-phenyl group B with an unsubstituted phenyl ring resulted in ~3-fold inhibitor improvement while replacing the para-ethoxy group with an ortho-methyl on group A resulted in ~2-fold improvement.34 Meanwhile, in our tested compounds here, combining these two improvements resulted in loss of inhibition in our assay (Figure 6, analog 3). The observation that these changes antagonize one another, rather than show additive or synergistic effects, supports the possibility that groups A and B could feasibly interact with another in a manner consistent with β-turn mimicry, attributed to the triazole scaffold. In order to explore this hypothesis, we decided to use GOLD, as part of Cambridge Structural Databases “Discovery” software package, for molecular docking and pose prediction of our best lead compound, compound 1 (Figure S1 and Table S1).42,43 The triazole ring is centered near the target scissile bond with half of the molecule tracking with the backbone of the cleavage loop residues 81–85 in each the top five best performing poses (scored with either GoldScore or ChemScore). However, rather than completing the full β-turn the other half of the compound instead continues deeper into the pocket. Surprisingly, either functional moiety, A or B, can be accommodated on either side within the pocket. This may indicate that simultaneous substitutions at both sites may disrupt the central positioning of the triazole, leading to the observed loss of inhibitory activity. Based on these observations, further rational expansion of the SAR would be imperative in deciphering which of these interactions are indispensable for inhibitor function. As further opportunity for investigation, the observation that analog 10, with alternative para-position functionalization, retains inhibitory activity offers possibilities for generation of reactive probes to further explore binding and mechanism of action. Ongoing work to explore the binding mode and site are essential to further develop this promising lead series for combating the acquisition of antibiotic resistance and is currently under investigation.
Figure 6:

LexA Inhibition measured with our FlAsH-LexA assay. IC50 fits are shown for analogs 1, 4, and 10. Percent inhibition was calculated by normalizing the change in anisotropy relative to the baseline of “uncleaved LexA” condition (mean, (−) ATPγS) and the “maximally cleaved LexA” condition (mean, (+) ATPγS), followed by scaling the data from 0 to 100. This data was then plotted and fit using [Inhibitor] vs. Normalized Response (variable slope) in GraphPad Prism 9.3.1 for Windows. Assay validation can be found in Supplemental Figure S2 and detailed fit statistics can be found in Table S2.
Materials and Methods
Full details available in the Supporting Information.
Supplementary Material
Acknowledgements
We would like to thank John Bacsa for solving the crystal structure of compound 12. Molecular graphics for Figure 2 performed with UCSF ChimeraX,41 developed by the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco, with support from National Institutes of Health R01-GM129325 and the Office of Cyber Infrastructure and Computational Biology, National Institute of Allergy and Infectious Diseases
Funding
This work was supported by the National Institutes of Health (1F31AI152459 to A.V.C.J.), R35-GM119426 (to W.M.W.), and R01-GM127593 (to R.M.K). M.J.C. was supported by T32-AI141393.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- (1).CDC. Antibiotic Resistance Threats in the United States; Centers for Disease Control and Prevention: Atlanta, GA, 2019. 10.15620/cdc:82532. [DOI] [Google Scholar]
- (2).Maslowska KH; Makiela-Dzbenska K; Fijalkowska IJ The SOS System: A Complex and Tightly Regulated Response to DNA Damage. Environ. Mol. Mutagen. 2019, 60 (4), 368–384. 10.1002/em.22267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Baharoglu Z; Mazel D SOS, the Formidable Strategy of Bacteria against Aggressions. FEMS Microbiol. Rev. 2014, 38 (6), 1126–1145. 10.1111/1574-6976.12077. [DOI] [PubMed] [Google Scholar]
- (4).Luo Y; Pfuetzner RA; Mosimann S; Paetzel M; Frey EA; Cherney M; Kim B; Little JW; Strynadka NCJ Crystal Structure of LexA: A Conformational Switch for Regulation of Self-Cleavage. Cell 2001, 106, 585–594. 10.1016/S0092-8674(01)00479-2. [DOI] [PubMed] [Google Scholar]
- (5).Tippin B; Pham P; Goodman MF Error-Prone Replication for Better or Worse. Trends Microbiol. 2004, 12 (6), 288–295. 10.1016/j.tim.2004.04.004. [DOI] [PubMed] [Google Scholar]
- (6).Galhardo RS; Do R; Yamada M; Friedberg EC; Hastings PJ; Nohmi T; Rosenberg SM DinB Upregulation Is the Sole Role of the SOS Response in Stress-Induced Mutagenesis in Escherichia Coli. Genetics 2009, 182, 55–68. 10.1534/genetics.109.100735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Schlacher K; Pham P; Cox MM; Goodman MF Roles of DNA Polymerase V and RecA Protein in SOS Damage-Induced Mutation. Chem. Rev. 2006, 106, 406–419. 10.1021/cr0404951. [DOI] [PubMed] [Google Scholar]
- (8).McKenzie GJ; Harris RS; Lee PL; Rosenberg SM The SOS Response Regulates Adaptive Mutation. Proc. Natl. Acad. Sci. 2000, 97 (12), 6646–6651. 10.1073/pnas.120161797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Dorr T; Lewis K; Vulic M SOS Response Induces Persistence to Fluoroquinolones in Escherichia Coli. PLoS Genet. 2009, 5 (12), e1000760. 10.1371/journal.pgen.1000760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Bojer MS; Wacnik K; Kjelgaard P; Gallay C; Bottomley AL; Cohn MT; Lindahl G; Frees D; Veening J-W; Foster SJ; Ingmer H SosA Inhibits Cell Division in Staphylococcus Aureus in Response to DNA Damage. Mol. Microbiol. 2019. 10.1111/mmi.14350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Beaber JW; Hochhut B; Waldor MK SOS Response Promotes Horizontal Dissemination of Antibiotic Resistance Genes. Nature 2004, 427, 72–74. 10.1038/nature02241. [DOI] [PubMed] [Google Scholar]
- (12).Da Re S; Garnier F; Guérin E; Campoy S; Denis F; Ploy MC The SOS Response Promotes QnrB Quinolone-Resistance Determinant Expression. EMBO Rep. 2009, 10 (8), 929–933. 10.1038/embor.2009.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Cirz RT; Chin JK; Andes DR; De Crécy-Lagard V; Craig WA; Romesberg FE Inhibition of Mutation and Combating the Evolution of Antibiotic Resistance. PLoS Biol. 2005, 3 (6), 1024–1033. 10.1371/journal.pbio.0030176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Thi TD; Lopez E; Rodriguez-Rojas A; Rodriguez-Beltran J; Couce A; Guelfo JR; Castaneda-Garcia A; Blazquez J Effect of RecA Inactivation on Mutagenesis of Escherichia Coli Exposed to Sublethal Concentrations of Antimicrobials. J. Antimicrob. Chemother. 2011, 66, 531–538. 10.1093/jac/dkq496. [DOI] [PubMed] [Google Scholar]
- (15).Mo CY; Manning SA; Roggiani M; Culyba MJ; Samuels AN; Sniegowski PD; Goulian M; Kohli RM Systematically Altering Bacterial SOS Activity under Stress Reveals Therapeutic Strategies for Potentiating Antibiotics. Ther. Prev. 2016, 1 (4), e00163–16. 10.1128/mSphere.00163-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Recacha E; Machuca J; Díaz-Díaz S; García-Duque A; Ramos-Guelfo M; Docobo-Pérez F; Blázquez J; Pascual A; Rodríguez-Martínez JM Suppression of the SOS Response Modifies Spatiotemporal Evolution, Post-Antibiotic Effect, Bacterial Fitness and Biofilm Formation in Quinolone-Resistant Escherichia Coli. J. Antimicrob. Chemother. 2019, 74 (1), 66–73. 10.1093/jac/dky407. [DOI] [PubMed] [Google Scholar]
- (17).Gotoh H; Kasaraneni N; Devineni N; Dallo SF; Weitao T SOS Involvement in Stress-Inducible Biofilm Formation. Biofouling 2010, 26 (5), 603–611. 10.1080/08927014.2010.501895. [DOI] [PubMed] [Google Scholar]
- (18).Walter BM; Cartman ST; Minton NP; Butala M; Rupnik M The SOS Response Master Regulator LexA Is Associated with Sporulation, Motility and Biofilm Formation in Clostridium Difficile. PLoS One 2015, 10 (12), e0144763. 10.1371/journal.pone.0144763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Flemming HC; Wingender J The Biofilm Matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- (20).Espeland EM; Wetzel RG Complexation, Stabilization, and UV Photolysis of Extracellular and Surface-Bound Glucosidase and Alkaline Phosphatase: Implications for Biofilm Microbiota. Microb. Ecol. 2001, 42, 572–585. 10.1007/s00248-001-1023-7. [DOI] [PubMed] [Google Scholar]
- (21).Teitzel GM; Parsek MR Heavy Metal Resistance of Biofilm and Planktonic Pseudomonas Aeruginosa. Appl. Environ. Micrbiology 2003, 69 (4), 2313–2320. 10.1128/AEM.69.4.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).McNeill K; Hamilton IR Acid Tolerance Response of Biofilm Cells of Streptococcus Mutans. FEMS Microbiol. Lett. 2003, 221, 25–30. 10.1016/S0378-1097(03)00164-2. [DOI] [PubMed] [Google Scholar]
- (23).Le Magrex-Debar E; Lemoine J; Gellé MP; Jacquelin LF; Choisy C Evaluation of Biohazards in Dehydrated Biofilms on Foodstuff Packaging. Int. J. Food Microbiol. 2000, 55, 239–243. 10.1016/S0168-1605(00)00177-X. [DOI] [PubMed] [Google Scholar]
- (24).Van Acker H; Van Dijck P; Coenye T Molecular Mechanisms of Antimicrobial Tolerance and Resistance in Bacterial and Fungal Biofilms. Trends Microbiol. 2014, 22 (6), 326–333. 10.1016/j.tim.2014.02.001. [DOI] [PubMed] [Google Scholar]
- (25).Bisognano C; Kelley WL; Estoppey T; Francois P; Schrenzel J; Li D; Lew DP; Hooper DC; Cheung AL; Vaudaux P A RecA-LexA-Dependent Pathway Mediates Ciprofloxacin-Induced Fibronectin Binding in Staphylococcus Aureus. J. Biol. Chem. 2004, 279 (10), 9064–9071. 10.1074/jbc.M309836200. [DOI] [PubMed] [Google Scholar]
- (26).Yeswanth S; Chaudhury A; Sarma PVGK Quantitative Expression Analysis of SpA, FnbA and Rsp Genes in Staphylococcus Aureus: Actively Associated in the Formation of Biofilms. Curr. Microbiol. 2017, 74, 1394–1403. 10.1007/s00284-017-1331-x. [DOI] [PubMed] [Google Scholar]
- (27).Alam MK; Alhhazmi A; Decoteau JF; Luo Y; Geyer CR RecA Inhibitors Potentiate Antibiotic Activity and Block Evolution of Antibiotic Resistance. Cell Chem. Biol. 2016, 23, 381–391. 10.1016/j.chembiol.2016.02.010. [DOI] [PubMed] [Google Scholar]
- (28).Lee AM; Wigle TJ; Singleton SF A Complementary Pair of Rapid Molecular Screening Assays for RecA Activities. Anal. Biochem. 2007, 367, 247–258. 10.1016/j.ab.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Peterson EJR; Janzen WP; Kireev D; Singleton SF High-Throughput Screening for RecA Inhibitors Using a Transcreener Adenosine 5′-O-Diphosphate Assay. Assay Drug Dev. Technol. 2012, 10 (3), 260–268. 10.1089/adt.2011.0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Sexton JZ; Wigle TJ; He Q; Hughes MA; Smith GR; Singleton SF; Williams AL; Yeh L-A Novel Inhibitors of E. Coli RecA ATPase Activity. Curr. Chem. Genomics 2010, 4, 34–42. 10.2174/1875397301004010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Wigle TJ; Lee AM; Singleton SF Conformationally Selective Binding of Nucleotide Analogues to Escherichia Coli RecA: A Ligand-Based Analysis of the RecA ATP Binding Site. Biochemistry 2006, 45, 4502–4513. 10.1021/bi052298h. [DOI] [PubMed] [Google Scholar]
- (32).Bellio P; Di Pietro L; Mancini A; Piovano M; Nicoletti M; Brisdelli F; Tondi D; Cendron L; Franceschini N; Amicosante G; Perilli M; Celenza G SOS Response in Bacteria: Inhibitory Activity of Lichen Secondary Metabolites against Escherichia Coli RecA Protein. Phytomedicine 2017, 29, 11–18. 10.1016/j.phymed.2017.04.001. [DOI] [PubMed] [Google Scholar]
- (33).Mo CY; Culyba MJ; Selwood T; Kubiak M; Hostetler ZM; Jurewicz AJ; Keller PM; Pope AJ; Quinn A; Schneck J; Widdowson KL; Kohli RM Inhibitors of LexA Autoproteolysis and the Bacterial SOS Response Discovered by an Academic − Industry Partnership. ACS Infect. Dis. 2018, 4, 349–359. 10.1021/acsinfecdis.7b00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Selwood T; Larsen BJ; Mo CY; Culyba MJ; Hostetler ZM; Kohli RM; Reitz AB; Baugh SDP Advancement of the 5-Amino-1-(Carbamoylmethyl)-1H-1,2,3-Triazole-4-Carboxamide Scaffold to Disarm the Bacterial SOS Response. Front. Microbiol. 2018, 9 (December). 10.3389/fmicb.2018.02961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Whitby LR; Ando Y; Setola V; Vogt PK; Roth BL; Boger DL Design, Synthesis, and Validation of a β-Turn Mimetic Library Targeting Protein-Protein and Peptide-Receptor Interactions. J. Am. Chem. Soc. 2011, 133, 10184–10194. 10.1021/ja201878v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Oh K; Guan Z A Convergent Synthesis of New β-Turn Mimics by Click Chemistry. Chem. Commun. 2006, 3069–3071. 10.1039/b606185k. [DOI] [PubMed] [Google Scholar]
- (37).Chandrachud PP; Wojtas L; Lopchuk JM Decarboxylative Amination: Diazirines as Single and Double Electrophilic Nitrogen Transfer Reagents. J. Am. Chem. Soc. 2020, 142, 21743–21750. 10.1021/jacs.0c09403. [DOI] [PubMed] [Google Scholar]
- (38).Karmali RA NOVEL COMPOSITIONS AND PROCESSES FOR PREPARING 5-AMINO OR SUBSTITUTED AMINO 1,2,3-TRIAZOLES AND TRIAZOLES OROTATE FORMULATIONS. US 2014/0200247 Al, 2014. [Google Scholar]
- (39).Mo CY; Culyba MJ; Selwood T; Kubiak JM; Hostetler ZM; Jurewicz AJ; Keller PM; Pope AJ; Quinn A; Schneck J; Widdowson KL; Kohli RM Inhibitors of LexA Autoproteolysis and the Bacterial SOS Response Discovered by an Academic-Industry Partnership. ACS Infect. Dis. 2018, 4, 349–359. 10.1021/acsinfecdis.7b00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Hostetler ZM; Cory MB; Jones CM; Petersson EJ; Kohli RM The Kinetic and Molecular Basis for the Interaction of Lexa and Activated Reca Revealed by a Fluorescent Amino Acid Probe. ACS Chem. Biol. 2020, 15, 1127–1133. 10.1021/acschembio.9b00886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Pettersen EF; Goddard TD; Huang CC; Meng EC; Couch GS; Croll TI; Morris JH; Ferrin TE UCSF ChimeraX: Structure Visualization for Researchers, Educators, and Developers. Protein Sci. 2020, 30, 70–82. 10.1002/pro.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Jones G; Willet P; Glen RC; Leach AR; Taylor R Development and Validation of a Genetic Algorithm for Flexible Docking. J. Mol. Biol. 267, 727–748, 1997. 10.1006/jmbi.1996.0897. [DOI] [PubMed] [Google Scholar]
- (43).Groom CR; Bruno IJ; Lightfoot MP; Ward SC The Cambridge Structural Database. Acta Cryst. 2016. B72, 171–179. 10.1107/S2052520616003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


