Abstract
Gatifloxacin, an 8-methoxyfluoroquinolone, was found to be two- to fourfold more active against wild-type Staphylococcus aureus ISP794 than its desmethoxy derivative, AM-1121, and ciprofloxacin, another desmethoxy fluoroquinolone. Single grlBA mutations caused two- to fourfold increases in the MIC of gatifloxacin, and a single gyrase mutation was silent. Double mutations in gyrA and grlA or grlB caused a 32-fold increase in the MIC of gatifloxacin, in contrast to a 128-fold increase for ciprofloxacin and AM-1121. Overexpression of the NorA efflux pump had minimal effect on the MIC of gatifloxacin. The bactericidal activity of the three quinolones at four times the MIC differed only for a double mutant, with gatifloxacin exhibiting a killing pattern similar to that for ISP794, whereas ciprofloxacin and AM-1121 failed to show any killing. With gatifloxacin, selection of resistant mutants at twice the MIC was 100- to 1,000-fold less frequent than with the comparison quinolones, and mutants could rarely be selected at four times the MIC. The limit resistance in ISP74 was 512 times the MIC of gatifloxacin and 1,024 times the MICs of ciprofloxacin and AM-1121. Novel mutations in topoisomerase IV were selected in five of the six single-step mutants, three of which were shown to cause quinolone resistance by genetic studies. In conclusion, topoisomerase IV is the primary target of gatifloxacin. In contrast to comparison quinolones, mutations in both topoisomerase IV and gyrase are required for resistance to gatifloxacin by clinical breakpoints and do not abolish bactericidal effect, further supporting the benefit of the 8-methoxy substituent in gatifloxacin.
Quinolones are known to interact with two essential bacterial enzymes, DNA gyrase and topoisomerase IV, in initiating their bactericidal activity. Understanding of these interactions has classically relied on studies of genetically manipulated drug-resistant mutants to define primary and secondary drug targets and to estimate the range of resistance mutations that may affect drug activity. With increasing use of quinolones for treatment of infections caused by gram-positive bacteria and with established quinolone resistance in some gram-positive species, particularly methicillin-resistant strains of Staphylococcus aureus (44), evaluation of the drug target preferences, the nature of resistance mutations, and resistance selection frequency may be valuable in identifying the determinants of drug action and predicting the risk of resistance in clinical settings.
Fluoroquinolone resistance mutations have been localized to specific regions of the parC and parE genes (grlA and grlB in S. aureus) encoding topoisomerase IV and the gyrA and gyrB genes encoding DNA gyrase, most commonly in the 5′ region of parC or gyrA and less commonly in the midportions of parE and gyrB (9, 10, 12, 18, 28, 43). This clustering of mutations has defined the quinolone resistance-determining regions (QRDRs) of these genes that, in the case of gyrA and parC, are in proximity to the apparent enzyme active site and are thought likely to constitute a domain at which quinolones interact directly with the enzyme-DNA complex (3, 18). Thus, an analysis of mutations in drug-target genes selected with older and newer quinolones may provide insight into distinct features of their target interactions that may be important for improved potency of the newer agents and in defining the effects of already established resistance mutations on drug action (27, 40, 55).
Quinolones differ in their primary and secondary drug targets, as defined by genetic tests, in gram-negative and -positive bacteria, with DNA gyrase being the primary target in gram-negative bacteria and topoisomerase IV the primary target of some but not all quinolones in gram-positive bacteria. In Streptococcus pneumoniae, in particular, sparfloxacin (34), gatifloxacin (13), gemifloxacin (16), clinafloxacin (35, 36), and in some cases moxifloxacin (46) have been reported to differ from the usual pattern and to have DNA gyrase as their primary target.
Gatifloxacin [8-methoxy-7-(3-methylpiperazinyl)] is a recently marketed fluoroquinolone with enhanced activity against gram-positive bacteria that has been approved for treatment of patients with infections of the respiratory and genitourinary tracts, including those caused by S. pneumoniae and S. aureus. Gatifloxacin contains a methoxy substituent at position 8 of the quinolone ring that has been associated in some bacteria with increased bacteriostatic and bactericidal activity as well as decreased selection of resistant mutants (7, 27, 53, 54). Although gatifloxacin has been studied in S. pneumoniae (6, 13, 17, 21, 22, 33, 38) and Escherichia coli (27), there have been few studies of gatifloxacin action and resistance in S. aureus (14, 15, 22). We thus undertook to define the effects of established resistance mechanisms on gatifloxacin activity, to characterize gatifloxacin-selected mutants, and to compare the bactericidal activity of gatifloxacin with its desmethoxy derivative AM-1121. We have identified a role for the 8-methoxy substituent in potency against S. aureus that is proportionally represented in its bactericidal activity. Notably, the methoxy substituent appears to contribute differentially to quinolone bactericidal activity in the presence of mutations in genes encoding subunits of both target enzymes. Two or more mutations were necessary to generate MICs that approached achievable serum drug concentrations. Furthermore, three novel grlA mutations (Lys23Asn, Ala176Gly, and Thr177Ile) and two novel grlB mutations (Pro25His and Pro451Gln), in addition to a gyrA mutation (Gly106Asp) which has only been reported in a clinical strain, have been selected. Two of the grlA mutations and one of the grlB mutations were shown by genetic studies to cause an increase in the MICs of gatifloxacin and other quinolones.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used are listed in Table 1. E. coli strains were grown in Luria-Bertani medium, and S. aureus strains were grown in brain heart infusion medium. All strains were grown at 37°C except the S. aureus strains carrying the thermosensitive shuttle plasmids derived from pCL52.1, which were grown at 30°C. Spectinomycin was used at a concentration of 50 μg/ml, and tetracycline was used at a concentration of 5 μg/ml, except during the integration of the plasmid into the chromosome, when it was used at a concentration of 3 μg/ml.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotypea | Source or reference |
|---|---|---|
| Strains | ||
| S. aureus | ||
| ISP794 | 8325 pig-131 | 41 |
| MT5 | 8325 nov (gyrB142) hisG15 pig-131 | 11, 45 |
| SS1 | 8325 pig-131 nov (gyrB142) gyrA | 2 |
| MT5224c2 | 8325 nov (gyrB142) hisG15 pig-131 grlA552 | 31 |
| MT5224c3 | 8325 nov (gyrB142) hisG15 pig-131 grlA547 | 45 |
| MT5224c4 | 8325 nov (gyrB142) hisG15 pig-131 grlA542 | 45 |
| MT5224c9 | 8325 nov (gyrB142) hisG15 pig-131 grlB543 | 45 |
| MT23142 | 8325 pig-131 flqB Ω(chr::Tn916)1108 | 31 |
| MT52184 | 8325 nov (gyrB142) hisG15 pig-131 grlA545 | 45 |
| EN1252a | 8325 nov (gyrB142) hisG15 pig-131 grlA542 gyrA Ω1051 (Erm) Nov+ | 2 |
| MT1222 | 8325 pig-131 grlA553 flqB gyrA | 45 |
| RN4220 | 8325-4, r− | 24 |
| EN6 | RN4220 nov-142 gyrA | 31 |
| EN14 | RN4220 grlB543Ω(chr::Tn917lac)2 gyrB142 gyrA | 12 |
| EN20 | RN4220 grlA542Ω(chr::Tn917lac)2 | 31 |
| EN9 | RN4220 grlA543Ω(chr::Tn917lac)2 gyrB142 gyrA | 31 |
| G1 | ISP794 grlA557 (Lys23Asn) | This study; spontaneous Gatrb |
| G2 | ISP794 grlA558 (Ala116Glu) | This study; spontaneous Gatr |
| G4 | ISP794 grlA559 (Ala176Gly) | This study; spontaneous Gatr |
| GB | ISP794 grlB560 (Pro25His) | This study; spontaneous Gatr |
| G13 | ISP794 grlA561 (Asp69Tyr) | This study; spontaneous Gatr |
| G21 | ISP794 grlA562 (Pro451Gln) | This study; spontaneous Gatr |
| DIG1 | ISP794 grlA557 | This study; allelic exchange mutant |
| DIGB | ISP794 grlB560 | This study; allelic exchange mutant |
| GS4 | ISP794 grlA (Ser80Phe) (Thr177Ile) gyrA (Ser84Leu) | This study; serial passage mutant |
| GS8 | ISP794 grlA (Ser80Phe) gyrA (Ser84Leu) | This study; serial passage mutant |
| 2GB | ISP794 grlB (Pro25His) | This study; serial passage mutant |
| 3GB | ISP794 grlB (Pro25His) gyrA (Glu88Gly) flqB (ATCA insertion) | This study; serial passage mutant |
| 4GB | ISP794 grlB (Pro25His) gyrA (Glu88Gly) flqB (ATCA insertion) | This study; serial passage mutant |
| 5GB | ISP794 grlA (Glu84Lys) grlB (Pro25His) gyrA (Glu88Gly) flqB (ATCA insertion) | This study; serial passage mutant |
| 6GB1 | ISP794 grlA (Glu84Lys) grlB (Pro25His) gyrA (Glu88Gly) flqB (ATCA insertion) | This study; serial passage mutant |
| 6GB2 | ISP794 grlA (Glu84Lys) grlB (Pro25His) gyrA (Glu88Gly) (Gly106Asp) flqB (ATCA insertion) | This study; serial passage mutant |
| ISP2133 | 8325 pig-131 trp-489 Ω(chr::Tn917lac)2 | 45 |
| ISP2134 | 8325 pig-131 trp-489 Ω(chr::Tn917lac)1 | 45 |
| ISP225 | Ps55 | 45 |
| E. coli | ||
| DH5α | F− φ80dlacZΔM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 phoA hsdR17 (rK−mK−) supE44λ− thi-1 gyrA96 relA1 | GIBCO-BRL |
| Plasmids | ||
| PGEM3-zf(+) | 3,199-bp cloning vector, Apr | Promega |
| pCL52.1 | 8,100-bp plasmid containing the replicon of pGB2, Spr (E. coli), and temperature-sensitive replicon of pE194, Tcr | 25 |
Abbreviations: Ap, ampicillin; Sp, spectinomycin; Tc, tetracycline.
Gatr gatifloxacin resistance.
Drug susceptibility determinations.
Gatifloxacin and AM-1121 were kindly provided by Bristol-Myers Squibb Co., and ciprofloxacin was provided by Bayer Corp. Nalidixic acid, novobiocin, and ethidium bromide were purchased from Sigma Chemical Co. (St. Louis, Mo.). MICs were determined on Trypticase soy agar containing serial twofold dilutions of antibiotics. The effects of reserpine and verapamil on quinolone susceptibility were determined by broth dilution in Penassay broth supplemented with 0.4% glucose (30). Reserpine was used at a concentration of 20 μg/ml, and verapamil was used at a concentration of 25 μg/ml. Incubations were done at 37°C, and growth was scored at 24 and 48 h. MICs of nalidixic acid were used to screen for gyrA mutations, MICs of novobiocin to screen for grlA or grlB mutations (12), and MICs of ethidium bromide to screen for NorA overexpression (23).
Bactericidal activity in wild-type and mutant strains.
The bactericidal effects of four times the MICs of ciprofloxacin, gatifloxacin, and AM-1121 were compared by measuring viable colony counts at intervals after drug exposure for the wild-type strain ISP794, its grlA mutant MT5224c4, and a grlA gyrA double mutant (EN1252a), as previously described (31). Cells were grown to mid-logarithmic phase (optical density at 600 nm, 0.3) at 37°C, with vigorous shaking. Quinolones were added, and incubation was continued. Samples were taken immediately before adding the quinolones and at hourly intervals for up to 5 h and were plated on brain heart infusion agar after appropriate dilutions were made in normal saline. The number of CFU was determined after 24 and 48 h, and time-kill curves were plotted after normalization of the values with the starting colony counts. Time-kill curves for the wild-type S. aureus ISP794 were also determined in the presence of gatifloxacin or AM-1121 at 2, 8, and 16 times their respective MICs. Experiments were repeated at least three times.
Frequency of selection of mutants.
Overnight cultures of S. aureus ISP794 were concentrated in normal saline, and mutants were selected by plating serial dilutions of these samples on brain heart infusion agar containing ciprofloxacin, gatifloxacin, or AM-1121 at two, four, and eight times the MIC of each drug. At least two plates of each antibiotic concentration were used for each dilution plated, and the experiment was repeated at least three times. Selection plate contents were incubated at 37°C. Mutation frequencies were calculated as the ratio of the number of resistant colonies at 48 h to the number of cells inoculated.
Limit resistance.
S. aureus ISP794 was serially passaged on brain heart infusion agar containing twofold-increasing concentrations of gatifloxacin to define the highest level of resistance achievable.
Sequence analysis.
Chromosomal DNA from various single-step- and multiple-step-derived mutants of S. aureus ISP794 were used as templates. PCR for the QRDRs of grlA, grlB, gyrA, and gyrB and the promoter region of norA was performed using Vent DNA polymerase (New England BioLabs) (annealing temperature of 55°C). PCR amplification for the QRDR of grlA was performed using primers 5′-AGTTGAAGATGAAGTGCGTT-3′ (5′ nucleotide at position 2196 in the sequence published by Yamagishi et al. [50]) and 5′-ACCTTGAATAATACCACCAGT-3′ (5′ nucleotide at position 3041) to encompass codons 157 and 176, which have previously been shown to cause quinolone resistance (20). DNA sequencing of the PCR products was performed with the ABI Fluorescent System and Taq dye terminators (Qiagen). For strains in which no mutation was found in these regions, the complete grlA, grlB, gyrA, and gyrB genes were amplified by PCR and sequenced by the same method.
DNA transformation.
For transformation in S. aureus, high-molecular-weight DNA was prepared by the method of Stahl and Pattee (41), and cells were made competent for transformation as described by Lindberg et al. (26), except that the original inoculum was grown in Trypticase soy broth at 35°C overnight in a shaking water bath. Bacteriophage Φ55, maintained on strain ISP225, was used to render the S. aureus strains competent for transformation.
Correlation of resistance phenotype and genotype in genetic crosses.
Ten resistant and 10 susceptible transformants in which DNA from ISP2133 was used to transform mutants G1, G4, and GB were selected for PCR amplification and either sequencing or restriction fragment length analysis of the relevant region of grlA or grlB. PCR primers used for amplification of the grlA fragment encompassing the mutation in G1 were 5′-AGTTGAAGATGAAGTGCGTT-3′ (5′ nucleotide at position 2196 in the sequence published by Yamagishi et al. [50]) and 5′-ACCATTGGTTCGAGTGTCGT-3′ (5′ nucleotide at position 2839). The primers used to amplify the fragment encompassing the mutation in G4 were 5′-GCTGAAGAGTTATTACGTGA-3′ (5′ nucleotide at position 2757) and 5′-TGGACGACCATCACTAATAGCGA-3′ (5′ nucleotide at position 3389). The primers used to amplify the grlB fragment encompassing the mutation in GB were 5′-CGCCGATAAGATAACTTAGTAG-3′ (5′ nucleotide at position 137) and 5′-GCTCTTGACGCTCTTTACCAC-3′ (5′ nucleotide at position 1036). An annealing temperature of 55°C and an extension time of 45 s were used for the grlA fragments, and an annealing temperature of 58°C and an extension time of 1 min were used for the grlB fragment. The grlA (Ala176Gly) mutation in mutant G4 is marked by loss of the BstUI restriction site, and the grlB (Pro25His) mutation in mutant GB is marked by loss of the BstNI restriction site within the PCR fragments. For studies with these mutants, restriction patterns were examined on agarose gels. For the mutant G1, DNA sequencing of the PCR products was performed to determine the presence or absence of the mutation in resistant and susceptible transformants.
Cloning and allelic exchange.
PCR primers and conditions for amplification of the internal fragments (1,312 bp) of grlB and grlA from mutants G1 and G4 have previously been published (20). A 652-bp internal fragment of grlB together with the 378-bp upstream region was amplified by PCR with the upstream primer containing an engineered EcoRI site (5′-ATGTTACCAAGAATTCTGC-3′ [position 7]) and the downstream primer containing an engineered BamHI site (5′-GCTCTGGATCCTCTTTACCAC-3′ [position 1036]), using an annealing temperature of 56°C and an extension time of 1 min 10 s. PCR products were ligated into the EcoRI and BamHI sites of pGEM3-zf(+), and the recombinant plasmids were electroporated into E. coli DH5α. The insert for each mutant was sequenced from the plasmid to verify that no new mutation was introduced during PCR amplification. The inserts were ligated into pCL52.1, a thermosensitive shuttle vector, following restriction and purification by gel extraction, and the plasmid was again electroporated into E. coli DH5α. The resultant plasmids were then electroporated first into S. aureus RN4220 and then into ISP794, as previously described (32). Allelic exchange was performed as described previously (20). Briefly, one colony from the resultant strains was incubated at 42°C for 24 h in the presence of 3 μg of tetracycline per ml of brain heart infusion broth. It was then plated on brain heart infusion agar with the same tetracycline concentration and was incubated at 42°C for 36 to 48 h to isolate single colonies with the integrated plasmid. A single colony was then incubated in brain heart infusion broth at 30°C for 48 h to allow excision of the integrated plasmid from the chromosome. The resultant colonies were screened for tetracycline susceptibility and quinolone resistance. MICs of ciprofloxacin and gatifloxacin for tetracycline-susceptible, quinolone-resistant colonies were determined, and the presence of the expected mutation was verified by direct DNA sequencing of the PCR product of the appropriate region amplified from chromosomal DNA.
RESULTS
Activities against genetically defined mutants.
The activities of ciprofloxacin, gatifloxacin, and AM-1121 against genetically defined mutants of S. aureus are shown in Table 2. Ciprofloxacin and AM-1121 had similar activities. In contrast, gatifloxacin was two- to fourfold more active against wild-type S. aureus ISP794 and the restriction-deficient strain RN4220 than were either ciprofloxacin or AM-1121. Except for a two- to fourfold increase in mutants of RN4220 with gatifloxacin, MICs of all three quinolones increased four- to eightfold in various grlA and grlB mutants with altered subunits of topoisomerase IV. A single mutation in gyrA of DNA gyrase had no effect or at most a twofold effect on MICs of these quinolones, but in double mutants with gyrA and either grlA or grlB mutations, the MICs of gatifloxacin increased 32- to 64-fold (to 4.0 μg/ml), while the MICs of ciprofloxacin and AM-1121 increased 128- to 256-fold (to 32 μg/ml). Overexpression of the NorA efflux pump in an flqB mutant caused a two- to fourfold increase in MICs of gatifloxacin and a four- to eightfold increase in the MICs of ciprofloxacin and AM-1121.
TABLE 2.
Activity of ciprofloxacin, gatifloxacin, and AM-1121 against genetically defined strains of S. aureus
| Strain | Relevant genotype | MICs (μg/ml) of:
|
||
|---|---|---|---|---|
| Ciprofloxacin | Gatifloxacin | AM-1121 | ||
| ISP794 | Wild type (parent) | 0.125–0.25 | 0.064–0.125 | 0.125–0.25 |
| MT5 | gyrB142 | 0.25 | 0.125 | 0.25 |
| SS1 | gyrA (S84L) | 0.25 | 0.125 | 0.25 |
| MT5224c2 | grlA (A116P) | 1.0 | 0.25 | 1.0 |
| MT5224c3 | grlA (A116E) | 1.0 | 0.5 | 1.0 |
| MT5224c4 | grlA (S80F) | 1.0 | 0.25 | 1.0 |
| MT5224c9 | grlB (N470D) | 1.0 | 0.25 | 1.0 |
| MT23142 | flqB (NorA overexpression) | 1.0 | 0.25 | 1.0 |
| MT52184 | gyrB142 grlA (A116P) | 4.0 | 1.0 | 4.0 |
| EN1252a | grlA (S80F) gyrA (S84L) | 32 | 4.0 | 32 |
| MT1222 | grlA (A116E) gyrA (S84L) flqB, other | 32 | 4.0 | 32 |
| RN4220 | 8325-4, r−, parent | 0.5 | 0.25 | 0.5 |
| EN6 | gyrA (S84L) | 0.5 | 0.25 | 0.5 |
| EN14 | grlB (N470D) gyrA | 4.0 | 1.0 | 4.0 |
| EN20 | grlA (S80F) | 4.0 | 0.5 | 2.0 |
| EN9 | grlA (S80F) gyrA (S84L) | 16–32 | 4.0 | 32 |
Bactericidal activity.
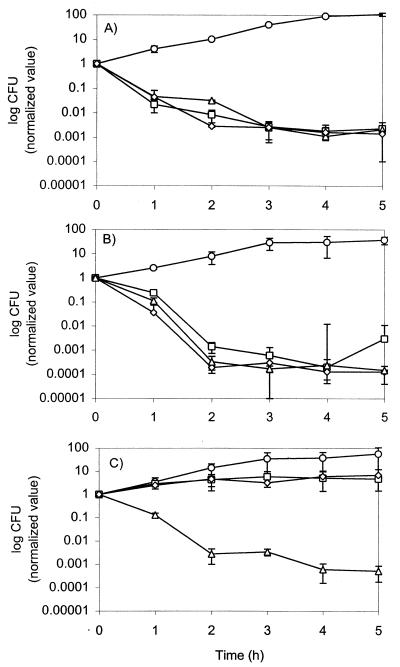
Ciprofloxacin, gatifloxacin, and AM-1121 showed rapid bactericidal activities characteristic of quinolones. In order to normalize for differences in potency (presumably reflecting interaction with enzyme-DNA complexes [4]), we evaluated normalized bactericidal activity at four times the MIC. At these concentrations, gatifloxacin produced a 3-log decrease at the end of 5 h for the wild-type strain ISP794, its grlA mutant MT5224c4, and grlA gyrA double mutant EN1252a. In contrast, ciprofloxacin and AM-1121 produced no killing of the grlA gyrA double mutant while exhibiting a similar effect for the other two strains (Fig. 1).
FIG. 1.
Normalized viable counts of S. aureus strains in the presence of four times the MIC of ciprofloxacin, gatifloxacin, or AM-1121 and the absence of any quinolone. Quinolones were added to cells grown to mid-log phase and were incubated at 37°C with shaking for 5 h. Colony counting was done by plating appropriate dilutions of cultures on drug-free media before the addition of the quinolones and at hourly intervals for 5 h. (A) ISP794, wild-type S. aureus; (B) MT5224c4, grlA (Ser80Phe); (C) EN1252a, grlA (Ser80Phe) gyrA (Ser84Leu). No antibiotic (○); four times the MIC of ciprofloxacin (□), four times the MIC of gatifloxacin (▵), four times the MIC of AM-1121 (⋄).
When the bactericidal effects of gatifloxacin and AM-1121 for the wild-type strain were compared, no significant difference was observed at 2, 4, 8, and 16 times the MIC of each antibiotic (data not shown). Thus, the difference in bactericidal activity between AM-1121 and gatifloxacin was manifest in the presence of dual mutations in grlA and gyrA.
Frequency of selection of mutants.
The frequencies of selection of single-step-resistant mutants with the three quinolones are shown in Table 3. At twice the MIC (0.25 μg/ml for gatifloxacin and 0.5 μg/ml for ciprofloxacin and AM-1121), the selection frequency was lower with gatifloxacin than with ciprofloxacin and AM-1121, and at four times the MIC, mutants could rarely be selected with gatifloxacin. All single-step mutants were selected at 0.25 μg of gatifloxacin/ml (twice the MIC), except G4, which was selected at 0.5 μg/ml (four times the MIC) and GB, which was selected at 0.125 μg/ml (MIC). In contrast, mutants were readily selected at four times the MIC with both ciprofloxacin and AM-1121. At eight times the MIC, mutants could still be selected with ciprofloxacin, although not with AM-1121.
TABLE 3.
Frequency of selection of resistant mutantsa
| Strain | Selecting drug concn expressed as:
|
Frequency of mutation for:
|
|||
|---|---|---|---|---|---|
| μg/ml | Factor of MIC (CIP/GAT/AM-1121) | CIP | GAT | AM-1121 | |
| ISP794 | 0.25 | 1/2/1 | ND | 1.0 × 10−6–7.3 × 10−8 | ND |
| 0.5 | 2/4/2 | 1.5 × 10−5–2.8 × 10−6 | 2.8 × 10−9–<4.5 × 10−11 | 1.8 × 10−5–4.5 × 10−6 | |
| 1.0 | 4/8/4 | 3.0 × 10−9–6.1 × 10−8 | <2.8 × 10−9–<4.5 × 10−11 | 4.3 × 10−8 | |
| 2.0 | 8/16/8 | 2.8 × 10−9–<4.5 × 10−11 | ND | <4.8 × 10−10–<5.6 × 10−10 | |
Abbreviations: CIP, ciprofloxacin; GAT, gatifloxacin. ND, not determined.
Limit resistance.
Stepwise selection on twofold-increasing concentrations of gatifloxacin was used to determine the highest level of resistance achievable with each fluoroquinolone. The maximum increase in resistance of strain ISP794 was 256- to 512-fold for gatifloxacin (up to 32 μg/ml) and 1,024-fold for ciprofloxacin and AM-1121 (up to 256 μg/ml).
Characterization of gatifloxacin-selected mutants.
In single-step mutants selected with gatifloxacin, the MICs of ciprofloxacin and gatifloxacin increased four- to eightfold (Table 4). The MICs of novobiocin (used to screen grlA or grlB mutations), nalidixic acid (to screen gyrA mutations), and ethidium bromide (to screen for NorA overexpression) did not change or showed a twofold change. In the serial passage mutants, MICs of novobiocin decreased fourfold, MICs of nalidixic acid increased twofold, and MICs of ethidium bromide did not change or increased twofold in one mutant. In five of the six single-step mutants, novel mutations either in or outside the QRDR of either subunit of topoisomerase IV were identified; in only one single-step mutant, G2, a previously reported mutation in grlA was found (encoding Ala116Glu). In another mutant, G13, a novel mutation was found in the QRDR of grlA, encoding Asp69Tyr. This mutation was previously shown to cause quinolone resistance in a premafloxacin-selected first-step mutant (20). Novel mutations outside the QRDR of grlA in two single-step mutants (Lys23Asn in mutant G1 and Ala176Gly in G4) and a novel mutation at the 5′ end of grlB in one single-step mutant (Pro25His) were found. A single-step mutant encoded another grlB mutation, Pro451Gln. For mutants selected by serial passage, the QRDRs of grlA, gyrA, grlB, and gyrB from the most resistant mutants were sequenced (GS4 and GS8), and commonly occurring mutations in grlA (Ser80Phe) and gyrA (Ser84Leu) were found together with a novel second grlA mutation (Thr177Ile) in GS4 (Table 4). In another set of serial passage mutants, all the selected mutants were studied to identify the order of appearance of the mutations. The MIC of gatifloxacin for the first mutant (GB) selected at 0.125 μg/ml was 0.5 μg/ml. Since the strains were serially passaged on twofold-increasing concentrations of gatifloxacin before the determination of MICs, the first-step mutant GB readily grew on 0.25 μg of gatifloxacin/ml as strain 2GB. The first mutation to appear, showing the primary target of gatifloxacin in this set of serial passage mutants, was identified to be a novel mutation in the grlB subunit of topoisomerase IV (Pro25His). This mutation was followed by a common mutation in gyrA (Glu88Gly) and a novel mutation, insertion of ATCA between the 99th and 100th bp upstream of the start codon of norA in the norA promoter region right after the −10 consensus sequence in strain 3GB selected at 1.0 μg of gatifloxacin/ml. The strain selected at 0.5 μg/ml was not available, so we could not determine the order of occurrence of these two mutations. In strain 4GB selected at 2 μg of gatifloxacin/ml, another mutation outside the QRDR of either topoisomerase IV or topoisomerase II or the promoter region of NorA is probably present to cause a two- to fourfold increase in the MICs of the three fluoroquinolones. Strain 5GB selected at 4 μg of gatifloxacin/ml has an additional mutation in the QRDR of topoisomerase IV (in grlA, encoding Glu84Lys) (Table 5). The appearance of the mutation upstream of norA for 3GB and subsequent mutants was associated with an increase in the MIC of ethidium bromide, a known NorA substrate. In addition, the MIC of gatifloxacin for mutant 3GB was reduced twofold in the presence of NorA inhibitors reserpine or verapamil, whereas these inhibitors had no effect on the MIC of gatifloxacin for the wild-type parent strain or mutant GB (grlB) (data not shown). In one of the last-step mutants (6GB2) selected at 8 μg/ml, an additional mutation in the QRDR of gyrA, Gly106Asp, was found together with the Glu88Gly mutation, whereas in the other last-step mutant (6GB1), the mutation causing increased resistance must again lie either outside the QRDRs of the topoisomerases or the promoter region of NorA (Table 5).
TABLE 4.
Characteristics of mutants of S. aureus ISP794 selected with gatifloxacinc
| Strain or mutant | MIC (μg/ml) of:
|
Mutation(s)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CIP | GAT | AM-1121 | NOV | NAL | EB | grlA | gyrA | grlB | gyrB | norAb | |
| Strain | |||||||||||
| ISP794 | 0.125–0.25 | 0.064–0.125 | 0.125–0.25 | 0.064 | 64 | 2.0–4.0 | |||||
| Single-step mutants | |||||||||||
| G1 | 0.5–1.0 | 0.25–0.5 | 0.5–10 | 0.064 | 128 | 2.0 | Lys23Asn | None | None | None | |
| G2 | 1.0–2.0 | 0.5 | 1.0 | 0.032 | 128 | 2.0 | Ala116Glua | Nonea | Nonea | Nonea | |
| G4 | 2.0 | 0.5–1.0 | 2.0 | 0.064 | 128 | 2.0 | Ala176Gly | None | None | None | None |
| G13 | 1.0 | 0.25–0.5 | 1.0 | 0.064 | 64–128 | 2.0 | Asp69Tyra | Nonea | Nonea | Nonea | |
| GB | 1.0 | 0.5 | 1.0 | 0.032 | 128 | 4.0 | None | None | Pro25His | None | None |
| G21 | 1.0–2.0 | 1.0 | 2.0 | 0.064 | 256 | 2.0 | None | None | Pro451Gln | None | None |
| Serial passage mutants | |||||||||||
| GS4 | 64 | 8–16 | 64 | 0.016 | 256 | 2.0 | Ser80Phea Thr177Ilea | Ser84Leua | |||
| GS8 | 64 | 32 | 64 | 0.016 | 256 | 2.0 | Ser80Phea | Ser84Leua | |||
QRDR.
Promoter region of norA.
GAT, gatifloxacin; CIP, ciprofloxacin; NOV, novobiocin; NAL, nalidixic acid; EB, ethidium bromide.
TABLE 5.
Stepwise selection of serial passage mutants with gatifloxacine
| Strain or mutationf | MIC (μg/ml) of:
|
Mutation
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CIP | GAT | AM-1121 | NOV | NAL | EB | grlA | grlB | gyrA | gyrB | norAc | |
| ISP794 | 0.25 | 0.064–0.125 | 0.125–0.25 | 0.064–0.128 | 64–128 | 2–4 | |||||
| GB | 1.0 | 0.5 | 1.0 | 0.032 | 128 | 4 | None | Pro25His | None | None | None |
| 2GB | 1.0 | 0.5 | 1.0 | 0.032 | 128 | 4 | Nonea | Pro25Hisb | Nonea | Nonea | Nonea |
| 3GB | 16 | 2 | 8 | 0.064 | 64 | 32–64 | None | Pro25His | Glu88Glya | Nonea | ATCA insertiond |
| 4GB | 32 | 4–8 | 16 | 0.064 | 64 | 32 | None | Pro25His | Glu88Glya | Nonea | ATCA insertiond |
| 5GB | 256 | 8 | 256 | 0.128 | 64 | 32 | Glu84Lysa | Pro25Hisab | Glu88Glya | Nonea | ATCA insertiond |
| 6GB1 | 256 | 32 | 256 | 0.064 | 64 | 32 | Glu84Lysa | Pro25Hisab | Glu88Gly | None | ATCA insertiond |
| 6GB2 | 256 | 16 | 256 | 0.064 | 64 | 32 | Glu84Lysa | Pro25Hisab | Glu88Glya | Nonea | ATCA insertiond |
| Gly106Aspa | |||||||||||
QRDR.
Region of mutation.
Promoter region of NorA.
Insertion is between the 99th and 100th bp upstream of the ATG start codon of norA.
Abbreviations: GAT, gatifloxacin, CIP, ciprofloxacin; NOV, novobiocin; NAL, nalidixic acid; EB, ethidium bromide.
ISP794, wild-type; GB, single-step mutant selected with gatifloxacin. Other mutants are derivatives of GB selected serially on increasing concentrations of gatifloxacin.
Genetic linkage of quinolone resistance.
To define the relationship of the novel mutations in the single-step mutants G1, G4, and GB to quinolone resistance, we performed genetic crosses using transformation of high-molecular weight DNA. Tn917 transposon insertions, Ω(chr::Tn917lac)2 and Ω(chr::Tn917lac)1, encoding erythromycin resistance, have been previously shown to be linked to grlA and grlB (31). For incross experiments, high-molecular-weight chromosomal DNA from strain ISP2133, Ω(chr::Tn917lac)2 grlA+ grlB+, and from ISP2134, Ω(chr::Tn917lac)1 grlA+ grlB+, was used to transform single-step mutants, selecting for erythromycin resistance. These linkage results were similar to those previously reported for other grlA mutants (20, 31). Since the linkage is also dependent on the fragment size of the chromosomal DNA preparation, linkage of MT5224c4 (grlA Ser80Phe) was also performed with the same DNA preparations and shown to be similar (Table 6). To demonstrate the sufficiency of the mutations for resistance, we performed outcross experiments in which ISP794 (grlA+ grlB+) was transformed with high-molecular-weight chromosomal DNA from a resistant transformant of G1 and GB obtained in the incross experiment (Table 7). In the outcross experiments, the linkages of the resistance loci to Ω(chr::Tn917lac)2 were similar to those reported for known grlBA resistance mutations (20, 31).
TABLE 6.
Genetic linkage of grlA and grlB mutations and resistance by transformation: incross of grlBA+
| Donor characteristics
|
Recipient characteristics
|
MIC (μg/ml) for recipient
|
MIC (μg/ml) for susceptible transformants
|
% Susceptible transformants (no. of susceptible transformants/no. of total transformants) | ||||
|---|---|---|---|---|---|---|---|---|
| Strain | Genotype | Strain | Genotype | GAT | CIP | GAT | CIP | |
| ISP2133 | trp-489 Ω(chr::Tn917lac)2 | G1 | grlA (Lys23Asn) | 0.25–0.5 | 0.5–1.0 | 0.125 | 0.25 | 21 (15/70) |
| G4 | grlA (Ala176Gly) | 0.5–1.0 | 2.0 | 0.25 | 0.5 | 20 (21/107) | ||
| GB | grlB (Pro25His) | 0.5–1.0 | 1.0–2.0 | 0.125 | 0.25 | 30 (31/104) | ||
| MT5224c4 | grlA (Ser80Phe) | 0.032 | 1.0 | 0.008 | 0.25 | 21 (8/38) | ||
| ISP2134 | thrB494 Ω(chr::Tn917lac)1 | G1 | grlA (Lys23Asn) | 0.25–0.5 | 0.5–1.0 | 0.125 | 0.25 | 15 (8/52) |
| G4 | grlA (Ala176Gly) | 0.5–1.0 | 2.0 | 0.25 | 0.5 | 11 (12/113) | ||
| GB | grlB (Pro25His) | 0.5–1.0 | 1.0–2.0 | 0.125 | 0.25 | 11 (12/108) | ||
| MT5224c4 | grlA (Ser80Phe) | 0.032 | 1.0 | 0.008 | 0.25 | 5 (8/171) | ||
GAT, gatifloxacin; CIP, ciprofloxacin.
TABLE 7.
Genetic linkage of grlA and grlB mutations and resistance by transformation: outcross of mutationsa
| Donor strainb | Genotype | MIC (μg/ml) for resistant transformants
|
% Resistant transformants (no. of resistant transformants/no. of total transformants) | |
|---|---|---|---|---|
| GAT | CIP | |||
| G1332 | grlA (Lys23Asn) Ω(chr::Tn917lac)2 | 0.25 | 0.5 | 22 (23/105) |
| GB331 | grlB (Pro25His) Ω(chr::Tn917lac)2 | 0.5 | 1.0 | 37 (22/60) |
Abbreviations: GAT, gatifloxacin; CIP, ciprofloxacin.
Recipient ISP794 grlA+ grlB+ (MIC of GAT, 0.064 μg/ml; MIC of CIP, 0.125 μg/ml).
Correlations of resistance phenotype and genotype.
The mutation encoding Pro25His (CATGG) in grlB in mutant GB eliminates the BstNI restriction site (CC↓A or TGG), and the mutation encoding Ala176Gly (TAC GGG) in grlA in G4 eliminates the BstUI restriction site (CG↓CG) (mutations are shown in boldface, and vertical arrows show the restriction enzyme cutting site). The PCR products encompassing nucleotides 137 to 1036 of grlB of 10 resistant outcross transformants of ISP794 transformed with high-molecular-weight chromosomal DNA from G1, as well as the original G1 mutant, yielded a single band of 900 bp following BstNI digestion, whereas 10 susceptible transformants yielded the expected two bands of 580 and 320 bp. Similarly, PCR products of nucleotides 2757 to 3389 of grlA+ of 10 resistant incross transformants of G4 with ISP2133, as well as the G4 mutant, exhibited a single band of 633 bp following digestion with BstUI, in contrast to the wild-type ISP794 and 10 susceptible mutants, all of which yielded two bands of 488 and 145 bp when cut with BstUI. Direct DNA sequencing of the PCR products of grlA from G1 revealed the presence and absence of the Lys23Asn mutation in 10 resistant and 10 susceptible outcross transformants, respectively. Thus, presence of the mutations strongly correlates with the resistance phenotypes.
Confirmation of role of novel mutations in resistance by allelic exchange.
Tetracycline-susceptible, ciprofloxacin-resistant colonies resulting from the allelic exchange experiments were tested for the presence of the expected mutation and for the MICs of ciprofloxacin and gatifloxacin in relation to the original mutants. The MICs of ciprofloxacin (1.0 μg/ml) and gatifloxacin (0.25 to 0.5 μg/ml) were the same for the original and the allele exchange mutants containing the Lys23Asn mutation in grlA (G1 and DIG1). For the grlB mutation, Pro25His, the MIC of ciprofloxacin (1.0 μg/ml) was the same for the original mutant GB and the allelic exchange mutants DIGB, whereas the MIC of gatifloxacin was slightly lower for the allelic exchange mutants (0.25 versus 0.5 μg/ml). Direct DNA sequencing of the PCR products of chromosomal DNA showed the presence of the expected grlA and grlB mutations in allelic exchange mutants DIG1 and DIGB, respectively. For the mutant G4 containing the Ala176Gly mutation, no quinolone-resistant allelic exchange mutant was identified out of the 650 tetracycline-susceptible colonies screened.
DISCUSSION
In previous studies, gatifloxacin has been reported to be 2- to 16-fold more active than ciprofloxacin against staphylococci (19, 47). We found gatifloxacin to be two- to fourfold more active than ciprofloxacin and AM-1121 against wild-type S. aureus but to have progressively greater differential potency against grlA or grlB single mutants (fourfold) and grlA gyrA double mutants (eightfold). Thus, in comparing AM-1121 and gatifloxacin, which differ only in the absence and presence of an 8-methoxy group, respectively, this moiety at the 8 position appears to exert its greatest influence in reducing the effect of common resistance mutations on drug activity. Previously, gatifloxacin has also been shown to exhibit more normalized bacteriostatic activity than ciprofloxacin and AM-1121 against first-step gyrase mutants of E. coli, as well as facilitating activity against the second drug target in E. coli, topoisomerase IV (27). These differences together with its increased potency result in MICs of gatifloxacin (0.25 μg/ml) that remain substantially below achievable peak concentrations in serum (∼4 μg/ml) for first-step mutants (37, 49). Serum drug concentrations exceeding the MIC of first-step mutants suggests that such mutants will be unlikely to be selected since their growth will be inhibited. Only for grlA gyrA double mutants does the MIC of gatifloxacin approximate peak serum drug concentrations.
In addition, overexpression of the NorA efflux pump appears to have a minimal effect on the activity of gatifloxacin. This information, along with the absence of resistance to ethidium bromide and the absence of mutations in the regulatory region of norA in gatifloxacin-selected single-step mutants, suggests that gatifloxacin is a poor substrate for NorA and that mutations causing overexpression of efflux pumps probably appear only after mutations in topoisomerases II and IV. The only mutation in the regulatory region of norA identified in this study was a novel insertional mutation following the −10 consensus sequence, the effect of which on quinolone resistance was demonstrated by the decrease in the MICs of the quinolones with the addition of reserpine or verapamil. The structure of gatifloxacin suggests that it has greater hydrophobicity than ciprofloxacin, a factor that has been associated with reduced effects of NorA expression on drug activity (42).
Gatifloxacin, ciprofloxacin, and AM-1121 exhibited similar bactericidal activity at concentrations fourfold that of their respective MICs for the wild-type strain ISP794 as well as for the grlA mutant MT5224c4. Zhao et al. have reported another C-8-methoxyquinolone to be five times more lethal than its C-8-H counterpart against a wild-type strain of S. aureus when the 99% lethal dose was measured and 10 times more lethal when the 50% lethal dose was measured for a grlA mutant (53). The reason why we did not see this difference might be related to our normalization for MICs. For our S. aureus mutant with dual mutations in grlA and gyrA, however, gatifloxacin exhibited bactericidal activity with a 1,000-fold reduction in viable counts, whereas neither AM-1121 nor ciprofloxacin exhibited bactericidal activity against this mutant. A similar pattern was also seen in E. coli, in which gatifloxacin was more lethal than ciprofloxacin and AM-1121 at 10 times the MIC at which 99% of the isolates tested are inhibited, for most gyrA and parC double mutants (27). In mycobacteria, which have only one quinolone target on the chromosome (topoisomerase II), gatifloxacin was also found to be more lethal, especially against first-step mutants (7, 52). Thus, when gatifloxacin and AM-1121 are compared in S. aureus, E. coli, and mycobacteria, the presence of the methoxy substituent appears to contribute to a retention of bactericidal activity when both drug target enzymes are altered.
The frequency of selection of resistant mutants also differs for 8-methoxyquinolones and desmethoxyquinolones. Moxifloxacin, another 8-methoxyquinolone, was shown to select resistant mutants less frequently than its 8-chlorine analogue (5). Dong et al. have shown that the C-8-methoxy group lowers the concentration required to prevent mutants from being recovered (8). We also found that at four times the MIC, mutants of ISP794 could only rarely be selected with gatifloxacin, although they were readily selected with both ciprofloxacin and AM-1121. Although AM-1121-selected mutants were not analyzed as extensively as gatifloxacin-selected mutants, sequencing of grlA and grlB regions that would have detected three of six novel mutations only identified a novel mutation (grlB Pro451Gln) in one of eight mutants. Also, for the grlA gyrA double mutant, mutant selection was low at twice the MIC and was undetectable at four times the MIC of gatifloxacin (data not shown). Dong et al. have reported that in S. aureus, with selection using low concentrations of ciprofloxacin, mutations have not been found in the QRDRs of some mutants (8). The same observation has been made for Mycobacterium smegmatis after usage of both ciprofloxacin and PD161148, another C-8-methoxyfluoroquinolone selection, showing the importance of antibiotic concentration in allelic diversity (55). The reason why we found so many novel mutations outside the QRDRs of topoisomerase IV (grlA or grlB) in our single-step mutants instead of the most frequent ones in the QRDR might be explained by our having to select most of our mutants at twice the MIC due to the low frequency of selection of resistant mutants. However the mutant selected at four times the MIC, G4, also had a novel mutation.
Sindelar et al. have defined mutant prevention concentration as the minimum concentration of an antibiotic that allows no mutant to be selected when more than 1010 cells are plated on drug-containing agar, and they hypothesized that when antibiotic concentration in tissues is maintained above the mutant prevention concentration, frequency of selection of resistant mutants would be very low (27, 40). The low frequencies that we determined even at twice the MIC of gatifloxacin for both wild-type and resistant S. aureus mutants might indicate that unless concentrations of gatifloxacin in serum fall to near-MIC levels, wild-type or first-step mutants of S. aureus will not be expected to acquire resistance. However, in infections with already highly resistant strains with multiple mutations for which the MIC of gatifloxacin is already near the peak serum drug level, such as would be common with clinical isolates of methicillin-resistant S. aureus (29, 39), selection of resistant mutants would be more probable.
Mutations in the S. aureus GrlA subunit previously shown by genetic studies to cause quinolone resistance include amino acid changes at positions 80, 84, and 116, with mutations at positions 80 and 84 reported most frequently (reviewed in reference 18). Other mutations at positions 41, 45, 48, and 81 have also been reported in resistant clinical isolates but were not themselves shown to contribute to the resistance phenotype. Resistance mutations in E. coli GyrA cluster in a region between amino acids 67 and 106, leading to its designation as the QRDR (51). Ser80 of wild-type S. aureus GrlA is homologous to Ser83 of wild-type E. coli GyrA, a position in which an amino acid change has been shown to cause reduced binding of norfloxacin to the gyrase-DNA complex (48). Thus, mutations in the QRDR have been thought to cause enzyme alterations that destabilize quinolone binding, and the QRDR has been suggested to constitute a site of quinolone binding. In the X-ray crystallographic structure of the related enzymes Saccharomyces cerevisiae topoisomerase II and E. coli gyrase (1, 3), these commonly occurring mutations are located on α-helix 4. Interestingly, we found the previously reported Ala116Glu mutation in only one of the first-step mutants selected with gatifloxacin.
The novel Lys23Asn mutation in grlA in mutant G1 is localized on α-helix 1 and is not a highly conserved amino acid in either E. coli gyrase or in yeast topoisomerase II. No other mutation in this domain has been reported in genetically defined mutants, but we have shown by allelic exchange experiments that this mutation is responsible for resistance.
The mutation Asp69Tyr in grlA in mutant G13 has also been selected with premafloxacin, another quinolone with an 8-methoxy substituent, and was shown too to be responsible for resistance by allelic exchange experiments (20). This same mutation has been reported in a resistant clinical isolate of Mycoplasma hominis (18). Amino acid 69 is localized on α-helix 3, which forms the head dimer by packing side by side with the α-helix 3 of the other subunit (3).
The novel Ala176Gly mutation identified in mutant G4 is outside the QRDR and is conserved and predicted to be localized on β strand 7 based on the E. coli GyrA structure (Ala-179) (3). A serine is found at this position in yeast topoisomerase II. Ala176 of GrlA is in the breakage reunion domain based on E. coli DNA gyrase, and flanking amino acids Gly173 and Ile174 (β strand 6 in E. coli and yeast topoisomerase II) and Gly177, Thr180, Ile182, and Pro183 (which encompass staggered β strands 7 of yeast and E. coli enzymes) are conserved in all three enzyme structures. Ala176 is predicted to be part of a hairpin structure between these two β strands, which are proposed to be involved in contacts with DNA. DNA contacts are predicted to be made with the hairpin between β strands 6 and 7 (3), and thus, it is possible that Ala176Gly mutation affects the ability of the mutant enzyme to form complexes with DNA, thereby creating a lesser number of enzyme-DNA complexes to be targeted and trapped on DNA by interaction with quinolone.
Ala176Gly is a conservative amino acid substitution, and thus, it is somewhat surprising that it is responsible for the resistance phenotype. We sequenced, however, the entire grlA, grlB, gyrA, and gyrB genes in the mutant carrying this mutation and found no other mutations. Furthermore, incross genetic crosses provided a strong correlation of the resistance phenotype with the mutation. In addition, we have selected an Ala176Thr mutation with premafloxacin that maps similarly in genetic crosses and that was proven to be responsible for resistance (20). In allelic exchange experiments for the Ala176Gly mutations, however, we were unable to identify resistant clones among 650 screened. Thus, we cannot confirm definitively the role of this mutation in the resistance phenotype. Interestingly, we found a Thr177Ile (corresponding to Thr180 in E. coli gyrA) mutation in grlA in one of our serial passage mutants in addition to a conventional Ser80Phe mutation. We do not know which mutation appeared first in this mutant, but the contribution of this mutation to resistance might also be due to its effect on enzyme-DNA contact, as suggested above.
The mutations reported to date in grlB have been in the midportions of grlB, possibly altering the intrinsic catalytic efficiency of the enzyme (12). We report for the first time a mutation at the 5′ end of grlB, Pro25His, which causes quinolone resistance. For this mutant, unlike the Asn470Asp mutant, which was about eight times more hypersusceptible to coumarins than the wild-type strain, the MIC of novobiocin decreased only two- to fourfold. The means by which this mutation causes resistance is yet to be determined.
The mutation Pro451Gln in grlB is near the previously reported grlB mutations and has also been identified, in addition to a Pro451His mutation in genetically defined single-step mutants selected with ciprofloxacin and AM-1121 (D. Ince and D. C. Hooper, data not shown). A Pro→Ser mutation at this codon has previously been reported in a clinical strain of S. aureus (18). Pro451 is localized on the β3 sheet in yeast topoisomerase II and is a highly conserved region in E. coli gyrB and grlB and yeast topoisomerase II, as well as being conserved between the grlB (parE) subunits of S. aureus and S. pneumoniae (12).
In two serial passage mutants (GS4 and GS8) in which the QRDRs of grlBA and gyrBA of only the last-step mutants were sequenced, conventional mutations in the QRDRs were found, in addition to the finding of a novel grlA mutation at codon 177. However, the sequencing of the last-step mutants did not show us the order of selection of mutations. Therefore, in another set of serial passage mutants, when all mutants were sequenced, topoisomerase IV was found to be the first target of gatifloxacin, with a novel mutation occurring first (Pro25His in grlB) followed in sequence by a conventional gyrA mutation, a novel norA promoter region mutation, another conventional grlA mutation, and then in one of the last-step mutants, another gyrA mutation (Gly106Asp) in the QRDR. This latter mutation has been reported previously in a clinical S. aureus isolate (18). Thus, the primary target of gatifloxacin seems to be topoisomerase IV. When topoisomerase IV becomes resistant, then topoisomerase II mutations follow, being followed by topoisomerase IV and then topoisomerase II mutations. These data, together with the previously published data on selection of mutants with premafloxacin (20), suggest that 8-methoxyquinolones, although still selecting topoisomerase IV mutations first in S. aureus, select for mutants in a way different from earlier quinolones such as ciprofloxacin.
In summary, gatifloxacin, a new 8-methoxyquinolone, has increased potency and bactericidal activity that can in part be attributed to the 8-methoxy substituent, as previously shown for other 8-methoxyquinolones such as moxifloxacin (5), premafloxacin (20), and PD161148 (7, 52, 54). Dual mutations appear to be required to reach MICs of gatifloxacin that approach peak serum drug concentrations in humans. The primary target of gatifloxacin is topoisomerase IV, but novel mutations selected with gatifloxacin suggest that its interactions with topoisomerase IV may differ from those of earlier quinolones. The identification of these novel mutations further suggests that the QRDRs for both grlA and grlB need to be expanded. Additional studies will be needed to determine the means by which the novel mutations effect resistance at the molecular level.
ACKNOWLEDGMENTS
We thank C. Y. Lee for providing plasmid pCL52.1.
This work was supported in part by a grant from the Public Health Service, National Institutes of Health (R01 AI23983 to D.C.H.) and a grant from Bristol-Myers Squibb Co. (to D.C.H.).
REFERENCES
- 1.Berger J M, Gamblin S J, Harrison S C, Wang J C. Structure and mechanism of DNA topoisomerase II. Nature. 1996;379:225–232. doi: 10.1038/379225a0. [DOI] [PubMed] [Google Scholar]
- 2.Bisognano C, Vaudaux P E, Lew D P, Ng E Y W, Hooper D C. Increased expression of fibronectin-binding proteins by fluoroquinolone-resistant Staphylococcus aureus exposed to subinhibitory levels of ciprofloxacin. Antimicrob Agents Chemother. 1997;41:906–913. doi: 10.1128/aac.41.5.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cabral J H, Jackson A P, Smith C V, Shikotra N, Maxwell A, Liddington R C. Crystal structure of the breakage-reunion domain of DNA gyrase. Nature. 1997;388:903–906. doi: 10.1038/42294. [DOI] [PubMed] [Google Scholar]
- 4.Chen C R, Malik M, Snyder M, Drlica K. DNA gyrase and topoisomerase IV on the bacterial chromosome: quinolone-induced DNA cleavage. J Mol Biol. 1996;258:627–637. doi: 10.1006/jmbi.1996.0274. [DOI] [PubMed] [Google Scholar]
- 5.Dalhoff A. Comparative in vitro and in vivo activity of the C-8 methoxy quinolone moxifloxacin and the C-8 chlorine quinolone BAY y 3118. Clin Infect Dis. 2001;32(Suppl. 1):S16–S22. doi: 10.1086/319371. [DOI] [PubMed] [Google Scholar]
- 6.Doern G V, Pfaller M A, Erwin M E, Brueggemann A B, Jones R N. The prevalence of fluoroquinolone resistance among clinically significant respiratory tract isolates of Streptococcus pneumoniae in the United States and Canada—1997 results from the SENTRY Antimicrobial Surveillance Program. Diagn Microbiol Infect Dis. 1998;32:313–316. doi: 10.1016/s0732-8893(98)00081-9. [DOI] [PubMed] [Google Scholar]
- 7.Dong Y Z, Xu C, Zhao X L, Domagala J, Drlica K. Fluoroquinolone action against mycobacteria: effects of C-8 substituents on growth, survival, and resistance. Antimicrob Agents Chemother. 1998;42:2978–2984. doi: 10.1128/aac.42.11.2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong Y Z, Zhao X L, Domagala J, Drlica K. Effect of fluoroquinolone concentration on selection of resistant mutants of Mycobacterium bovis BCG and Staphylococcus aureus. Antimicrob Agents Chemother. 1999;43:1756–1758. doi: 10.1128/aac.43.7.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrero L, Cameron B, Crouzet J. Analysis of gyrA and grlA mutations in stepwise-selected ciprofloxacin-resistant mutants of Staphylococcus aureus. Antimicrob Agents Chemother. 1995;39:1554–1558. doi: 10.1128/aac.39.7.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrero L, Cameron B, Manse B, Lagneaux D, Crouzet J, Famechon A, Blanche F. Cloning and primary structure of Staphylococcus aureus DNA topoisomerase IV: a primary target of fluoroquinolones. Mol Microbiol. 1994;13:641–653. doi: 10.1111/j.1365-2958.1994.tb00458.x. [DOI] [PubMed] [Google Scholar]
- 11.Fournier B, Hooper D C. Effects of mutations in GrlA of topoisomerase IV from Staphylococcus aureus on quinolone and coumarin activity. Antimicrob Agents Chemother. 1998;42:2109–2112. doi: 10.1128/aac.42.8.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fournier B, Hooper D C. Mutations in topoisomerase IV and DNA gyrase of Staphylococcus aureus: novel pleiotropic effects on quinolone and coumarin activity. Antimicrob Agents Chemother. 1998;42:121–128. doi: 10.1128/aac.42.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukuda H, Hiramatsu K. Primary targets of fluoroquinolones in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1999;43:410–412. doi: 10.1128/aac.43.2.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukuda H, Hori S, Hiramatsu K. Antibacterial activity of gatifloxacin (AM-1155, CG5501, BMS-206584), a newly developed fluoroquinolone, against sequentially acquired quinolone-resistant mutants and the norA transformant of Staphylococcus aureus. Antimicrob Agents Chemother. 1998;42:1917–1922. doi: 10.1128/aac.42.8.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fung-Tomc J, Minassian B, Kolek B, Washo T, Huczko E, Bonner D. In vitro antibacterial spectrum of a new broad-spectrum 8-methoxy fluoroquinolone, gatifloxacin. J Antimicrob Chemother. 2000;45:437–446. doi: 10.1093/jac/45.4.437. [DOI] [PubMed] [Google Scholar]
- 16.Heaton V J, Ambler J E, Fisher L M. Potent antipneumococcal activity of gemifloxacin is associated with dual targeting of gyrase and topoisomerase IV, an in vivo target preference for gyrase, and enhanced stabilization of cleavable complexes in vitro. Antimicrob Agents Chemother. 2000;44:3112–3117. doi: 10.1128/aac.44.11.3112-3117.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoellman D B, Lin G R, Jacobs M R, Appelbaum P C. Anti-pneumococcal activity of gatifloxacin compared with other quinolone and non-quinolone agents. J Antimicrob Chemother. 1999;43:645–649. doi: 10.1093/jac/43.5.645. [DOI] [PubMed] [Google Scholar]
- 18.Hooper D C. Mechanisms of quinolone resistance. Drug Resist Updates. 1999;2:38–55. doi: 10.1054/drup.1998.0068. [DOI] [PubMed] [Google Scholar]
- 19.Hosaka M, Yasue T, Fukuda H, Tomizawa H, Aoyama H, Hirai K. In vitro and in vivo antibacterial activities of AM-1155: a new 6-fluoro-8-methoxy quinolone. Antimicrob Agents Chemother. 1992;36:2108–2117. doi: 10.1128/aac.36.10.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ince D, Hooper D C. Mechanisms and frequency of resistance to premafloxacin in Staphylococcus aureus: novel mutations suggest novel drug-target interactions. Antimicrob Agents Chemother. 2000;44:3344–3350. doi: 10.1128/aac.44.12.3344-3350.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones M E, Sahm D F, Martin N, Scheuring S, Heisig P, Thornsberry C, Köhrer K, Schmitz F-J. Prevalence of gyrA, gyrB, parC, and parE mutations in clinical isolates of Streptococcus pneumoniae with decreased susceptibilities to different fluoroquinolones and originating from worldwide surveillance studies during the 1997-1998 respiratory season. Antimicrob Agents Chemother. 2000;44:462–466. doi: 10.1128/aac.44.2.462-466.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones R N, Beach M L, Pfaller M A, Doern G V. Antimicrobial activity of gatifloxacin tested against 1676 strains of ciprofloxacin-resistant gram-positive cocci isolated from patient infections in North and South America. Diagn Microbiol Infect Dis. 1998;32:247–252. doi: 10.1016/s0732-8893(98)00101-1. [DOI] [PubMed] [Google Scholar]
- 23.Kaatz G W, Seo S M. Inducible NorA-mediated multidrug resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1995;39:2650–2655. doi: 10.1128/aac.39.12.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kreiswirth B N, Lofdahl S, Betley M J, O'Reilly M, Schlievert P M, Bergdoll M S, Novick R P. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature. 1983;305:709–712. doi: 10.1038/305709a0. [DOI] [PubMed] [Google Scholar]
- 25.Lin W S, Cunneen T, Lee C Y. Sequence analysis and molecular characterization of genes required for the biosynthesis of type 1 capsular polysaccharide in Staphylococcus aureus. J Bacteriol. 1994;176:7005–7016. doi: 10.1128/jb.176.22.7005-7016.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindberg M, Sjostrom J E, Johansson T. Transformation of chromosomal and plasmid characters in Staphylococcus aureus. J Bacteriol. 1972;109:844–847. doi: 10.1128/jb.109.2.844-847.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu T, Zhao X L, Drlica K. Gatifloxacin activity against quinolone-resistant gyrase: allele-specific enhancement of bacteriostatic and bactericidal activities by the C-8-methoxy group. Antimicrob Agents Chemother. 1999;43:2969–2974. doi: 10.1128/aac.43.12.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Margerrison E E, Hopewell R, Fisher L M. Nucleotide sequence of the Staphylococcus aureus gyrB-gyrA locus encoding the DNA gyrase A and B proteins. J Bacteriol. 1992;174:1596–1603. doi: 10.1128/jb.174.5.1596-1603.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Milatovic D, Schmitz F J, Brisse S, Verhoef J, Fluit A C. In vitro activities of sitafloxacin (DU-6859a) and six other fluoroquinolones against 8,796 clinical bacterial isolates. Antimicrob Agents Chemother. 2000;44:1102–1107. doi: 10.1128/aac.44.4.1102-1107.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ng E Y, Trucksis M, Hooper D C. Quinolone resistance mediated by norA: physiologic characterization and relationship to flqB, a quinolone resistance locus on the Staphylococcus aureus chromosome. Antimicrob Agents Chemother. 1994;38:1345–1355. doi: 10.1128/aac.38.6.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ng E Y, Trucksis M, Hooper D C. Quinolone resistance mutations in topoisomerase IV: relationship of the flqA locus and genetic evidence that topoisomerase IV is the primary target and DNA gyrase the secondary target of fluoroquinolones in Staphylococcus aureus. Antimicrob Agents Chemother. 1996;40:1881–1888. doi: 10.1128/aac.40.8.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Novick R P. Genetic systems in staphylococci. Methods Enzymol. 1991;204:587–636. doi: 10.1016/0076-6879(91)04029-n. [DOI] [PubMed] [Google Scholar]
- 33.Odland B A, Jones R N, Verhoef A, Fluit A, Beach M L SENTRY Antimicrobial Surveillance Group. Antimicrobial activity of gatifloxacin (AM-1155, CG5501), and four other fluoroquinolones tested against 2,284 recent clinical strains of Streptococcus pneumoniae from Europe, Latin America, Canada, and the United States. Diagn Microbiol Infect Dis. 1999;34:315–320. doi: 10.1016/s0732-8893(99)00037-1. [DOI] [PubMed] [Google Scholar]
- 34.Pan X S, Fisher L M. Targeting of DNA gyrase in Streptococcus pneumoniae by sparfloxacin: selective targeting of gyrase or topoisomerase IV by quinolones. Antimicrob Agents Chemother. 1997;41:471–474. doi: 10.1128/aac.41.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pan X S, Fisher L M. DNA gyrase and topoisomerase IV are dual targets of clinafloxacin action in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1998;42:2810–2816. doi: 10.1128/aac.42.11.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pan X S, Fisher L M. Streptococcus pneumoniae DNA gyrase and topoisomerase IV: overexpression, purification, and differential inhibition by fluoroquinolones. Antimicrob Agents Chemother. 1999;43:1129–1136. doi: 10.1128/aac.43.5.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perry C M, Balfour J A, Lamb H M. Gatifloxacin. Drugs. 1999;58:683–696. doi: 10.2165/00003495-199958040-00010. [DOI] [PubMed] [Google Scholar]
- 38.Piddock L J, Johnson M, Ricci V, Hill S L. Activities of new fluoroquinolones against fluoroquinolone-resistant pathogens of the lower respiratory tract. Antimicrob Agents Chemother. 1998;42:2956–2960. doi: 10.1128/aac.42.11.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmitz F J, Jones M E, Hofmann B, Hansen B, Scheuring S, Lückefahr M F A, Verhoef J, Hadding U, Heinz H P, Köhrer K. Characterization of grlA, grlB, gyrA, and gyrB mutations in 116 unrelated isolates of Staphylococcus aureus and effects of mutations on ciprofloxacin MIC. Antimicrob Agents Chemother. 1998;42:1249–1252. doi: 10.1128/aac.42.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sindelar G, Zhao X, Liew A, Dong Y, Lu T, Zhou J, Domagala J, Drlica K. Mutant prevention concentration as a measure of fluoroquinolone potency against mycobacteria. Antimicrob Agents Chemother. 2000;44:3337–3343. doi: 10.1128/aac.44.12.3337-3343.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stahl M L, Pattee P A. Confirmation of protoplast fusion-derived linkages in Staphylococcus aureus by transformation with protoplast DNA. J Bacteriol. 1983;154:406–412. doi: 10.1128/jb.154.1.406-412.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takenouchi T, Tabata F, Iwata Y, Hanzawa H, Sugawara M, Ohya S. Hydrophilicity of quinolones is not an exclusive factor for decreased activity in efflux-mediated resistant mutants of Staphylococcus aureus. Antimicrob Agents Chemother. 1996;40:1835–1842. doi: 10.1128/aac.40.8.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanaka M, Onodera Y, Uchida Y, Sato K. Quinolone resistance mutations in the GrlB protein of Staphylococcus aureus. Antimicrob Agents Chemother. 1998;42:3044–3046. doi: 10.1128/aac.42.11.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trucksis M, Hooper D C, Wolfson J S. Emerging resistance to fluoroquinolones in staphylococci: an alert. Ann Intern Med. 1991;114:424–426. doi: 10.7326/0003-4819-114-5-424. [DOI] [PubMed] [Google Scholar]
- 45.Trucksis M, Wolfson J S, Hooper D C. A novel locus conferring fluoroquinolone resistance in Staphylococcus aureus. J Bacteriol. 1991;173:5854–5860. doi: 10.1128/jb.173.18.5854-5860.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Varon E, Janoir C, Kitzis M D, Gutmann L. ParC and GyrA may be interchangeable initial targets of some fluoroquinolones in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1999;43:302–306. doi: 10.1128/aac.43.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wakabayashi E, Mitsuhashi S. In vitro antibacterial activity of AM-1155: a novel 6-fluoro-8-methoxy quinolone. Antimicrob Agents Chemother. 1994;38:594–601. doi: 10.1128/aac.38.3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Willmott C J, Maxwell A. A single point mutation in the DNA gyrase A protein greatly reduces binding of fluoroquinolones to the gyrase-DNA complex. Antimicrob Agents Chemother. 1993;37:126–127. doi: 10.1128/aac.37.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wise R, Andrews J M, Ashby J P, Marshall J. A study to determine the pharmacokinetics and inflammatory fluid penetration of gatifloxacin following a single oral dose. J Antimicrob Chemother. 1999;44:701–704. doi: 10.1093/jac/44.5.701. [DOI] [PubMed] [Google Scholar]
- 50.Yamagishi J I, Kojima T, Oyamada Y, Fujimoto K, Hattori H, Nakamura S, Inoue M. Alterations in the DNA topoisomerase IV grlA gene responsible for quinolone resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1996;40:1157–1163. doi: 10.1128/aac.40.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoshida H, Bogaki M, Nakamura M, Nakamura S. Quinolone resistance-determining region in the DNA gyrase gyrA gene of Escherichia coli. Antimicrob Agents Chemother. 1990;34:1271–1272. doi: 10.1128/aac.34.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao B Y, Pine R, Domagala J, Drlica K. Fluoroquinolone action against clinical isolates of Mycobacterium tuberculosis: effects of a C-8 methoxyl group on survival in liquid media and in human macrophages. Antimicrob Agents Chemother. 1999;43:661–666. doi: 10.1128/aac.43.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao X L, Wang J Y, Xu C, Dong Y Z, Zhou J F, Domagala J, Drlica K. Killing of Staphylococcus aureus by C-8-methoxy fluoroquinolones. Antimicrob Agents Chemother. 1998;42:956–958. doi: 10.1128/aac.42.4.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao X L, Xu C, Domagala J, Drlica K. DNA topoisomerase targets of the fluoroquinolones: a strategy for avoiding bacterial resistance. Proc Natl Acad Sci USA. 1997;94:13991–13996. doi: 10.1073/pnas.94.25.13991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou J F, Dong Y H, Zhao X L, Lee S W, Amin A, Ramaswamy S, Domagala A, Musser J M, Drlica K. Selection of antibiotic-resistant bacterial mutants: allelic diversity among fluoroquinolone-resistant mutations. J Infect Dis. 2000;182:517–525. doi: 10.1086/315708. [DOI] [PubMed] [Google Scholar]