Abstract
Objective
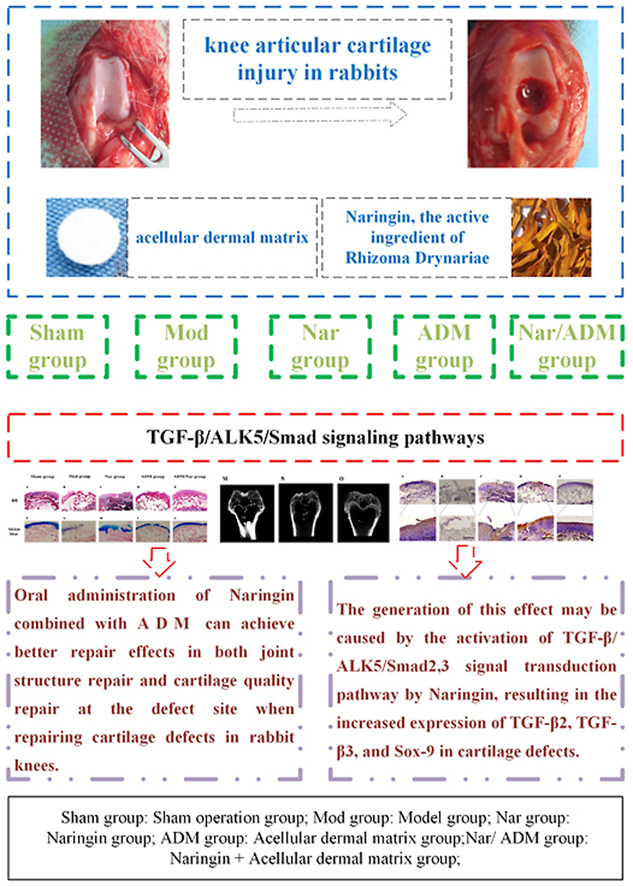
Based on the expression changes in the TGF-β/ALK5/Smad2/3 signal transduction pathway, the repair of cartilage injury in the rabbit knee joint was investigated and evaluated by oral administration of naringin in combination with acellular dermal matrix implantation.
Methods
First, twenty New Zealand white rabbits were randomly divided into five groups: a sham operation group (Sham group), a model group (Mod group), a naringin group (Nar group), an acellular dermal matrix group (ADM group), a naringin + acellular dermal matrix group (Nar/ADM group). After the 12th week, the repaired tissues were assessed for histomorphology and repair content of the repaired site by observing the morphological characteristics of articular cartilage. The International Cartilage Repair Society (ICRS)'s macroscopic evaluation of the cartilage repair scale and the quantitative scoring repair effect of the modified O'Driscoll grading system were used as evaluation criteria. In addition, the structure of the rabbit knee joint was evaluated by micro-CT scan, histological staining (H & E staining, Alcian blue staining, Safranin-O staining) and immunohistochemical staining (TGF-β2 immunostaining, TGF-β3 immunostaining, Sox-9 immunostaining).
Results
① The observation of the repair morphology of joint defect tissues showed that the repair effects of the Nar and ADM groups were better than that of the Mod group, and the repair effect of Nar/ADM group was the best (P < 0.05). ② Quantitative scoring of joint defect tissue showed that the Nar/ADM group had the best repair efficacy in the quantitative scores of the above two scales compared with the other groups (P < 0.05). ③ Micro-CT scan showed that the ADM group had obvious repair of the defect structure, while the ADM/Nar group had blurred repair boundaries, and the layers of cartilage and subchondral bone were clear. ④ Histological staining (H & E staining, Alcian blue stain, Safranin-O staining) showed that the ADM group had a better effect on the repair of joint structure at the joint defect, the Nar group had a better effect on the repair of cartilage quality at the joint defect, and the ADM/Nar group had satisfactory results in both of the above aspects. ⑤ Immunohistochemical staining (TGF-β2 immunostaining, TGF-β3 immunostaining, Sox-9 immunostaining) revealed that the Nar group showed more abundant expression of the above proteins in articular cartilage defects than the Mod and ADM groups and that the Nar/ADM groups showed extensive TGF-β2, TGF-β3 and Sox-9 protein expression, with uniform expression and smooth distribution.
Conclusions
Oral administration of naringin, the active ingredient of Rhizoma Drynariae, combined with acellular dermal matrix can achieve better repair effects in both joint structure repair and cartilage quality repair at the defect site when repairing cartilage defects in rabbit knees, and the generation of this effect may be caused by the activation of the TGF-β/ALK5/Smad2/3 signal transduction pathway by naringin, resulting in the increased expression of TGF-β2, TGF-β3, and Sox-9 in cartilage defects.
The Translational Potential of this Article
Naringin combined with acellular dermal matrix can facilitate the repair of osteochondral defects and has potential for application in osteochondral tissue engineering.
Keywords: Naringin, Cartilage defect, Repair, Acellular dermal matrix, Transforming growth factor-β
Abbreviations: Sham group sham operation group, Mod group model group; Nar group Naringin group, ADM group Acellular dermal matrix group; Nar/ ADM group Naringin + Acellular dermal matrix group, ICRS International Cartilage Repair Society
Graphical abstract
The Translational Potential of this Article: Naringin combined with acellular dermal matrix can facilitate the repair of osteochondral defects and has potential for application in osteochondral tissue engineering.
1. Introduction
Arthritis is a chronic disease characterized by articular cartilage injury, which mainly occurs in the knee joint, with joint stiffness, pain and motor dysfunction as the main clinical manifestations [1]. With the gradual increase in the aging population, the incidence of the disease is gradually increasing and has received increasing attention and research. Cartilage injury of the knee is the main pathological feature of knee osteoarthritis [2]. Articular cartilage is composed of chondrocytes and cartilage extracellular matrix, with the chondrocytes distributed in the cartilage extracellular matrix [3]. Articular cartilage is a highly differentiated tissue in which there is a lack of blood, lymphatic vessels, and nerves; thus, articular cartilage becomes damaged, it has a very poor ability to repair itself [4].
Acellular dermal matrix is a scaffold structure that can be used to repair cartilage defects. Anlun Ma applied intercalary stem cells combined with acellular dermal matrix to repair articular cartilage defects and achieved satisfactory repair results [5]. The acellular dermal matrix scaffold material used in this study was a gift from the bone bank of Beijing Jishuitan Hospital (Fourth Clinical Medical College of Peking University). Acellular dermal matrix is made from the back skin of newborn calves through decellularization, structural remodeling, cross-linking and surface modification (Patent No.: ZL 201110099363.1). This material has the characteristics of good structure, high porosity, good histocompatibility and cell adhesiveness, and the expression level of type II collagen in cells is not significantly different and can be used as a repair material for cartilage defects [6].
Based on the in-depth study of plant derivatives as a source of new active molecules, it has been found that many molecules can obtain good therapeutic effects for various diseases, including skeletal tissues [7]. At present, naringin has received special attention in the study of plant derivatives for the treatment of knee osteoarthritis. Su Youxin's study found that naringin can not only promote the proliferation of chondrocytes, but also protect the function of chondrocytes [8]. Naringin is an important natural flavonoid and one of the main active ingredients of Rhizoma Drynariae [9]. From the perspective of TCM (traditional Chinese medicine), the key pathogenesis of knee osteoarthritis is kidney essence deficiency. As a good medicine in orthopedics and traumatology, Rhizoma Drynariae tonifies the kidney, strengthens bone, and relieves pain. Deficiencies of essence and Qi in the kidney leads to the loss of bone marrow, which leads to deficiencies of essence and blood and the loss of muscle and bone, ultimately leading to muscle, bone and joint disease. Naringin (an effective component of Rhizoma Drynariae) is relevant to the pathogenesis of the disease and has potential for the treatment of articular cartilage injury in knee osteoarthritis. Previous studies by our group have found that naringin can promote the repair of cartilage injury in rabbit knee joints by activating the TGF-β superfamily signaling pathway [10].
TGF-β is a broad family of growth factors; its related cytokines regulate cell proliferation, differentiation, apoptosis, tissue homeostasis and regeneration and are among the signaling factors with the ability to maintain chondrocyte phenotype and articular cartilage homeostasis [11]. The TGF-β/Smad signaling pathway is closely related to the occurrence and development of osteoarthritis and also plays a key role in the repair process of cartilage injury [12]. Further studies have shown that the TGF-β/ALK5/Smad2/3 signal transmission pathway is an important component of the TGF-β/Smad signaling pathway, which plays important roles in maintaining articular cartilage homeostasis and repairing articular cartilage defects [13].
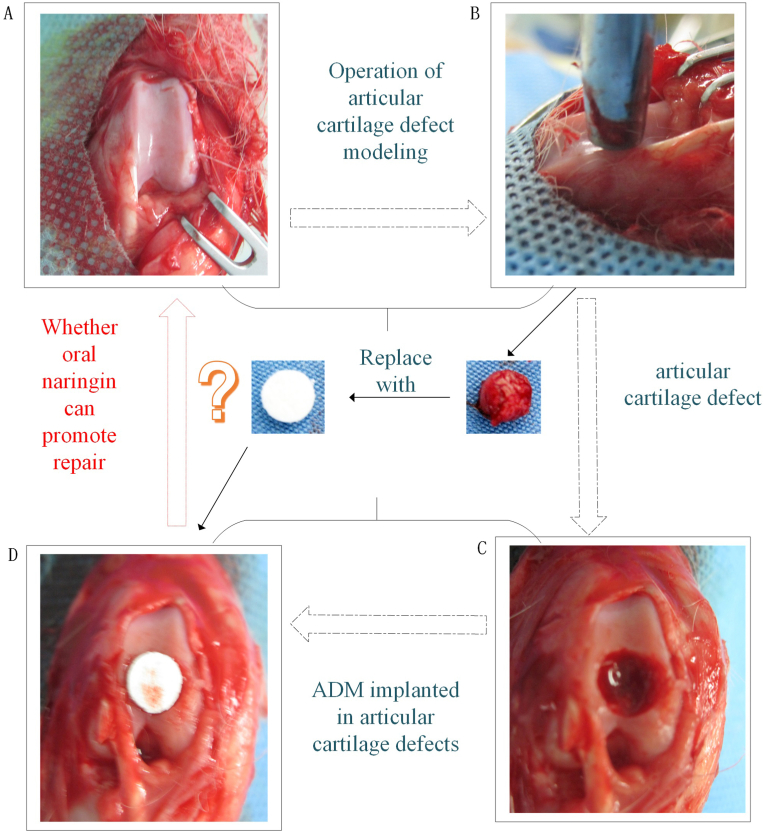
At present, there is no report on naringin combined with acellular dermal matrix for the repair of articular cartilage defects based on the changes in the expression of TGF-β2, TGF-β3, and Sox-9, relying on the TGF-β/ALK5/Smad2/3 signal transmission pathway. Therefore, we designed the topic of this research. In this study, we applied the active ingredient of Rhizoma Drynariae, oral naringin, combined with acellular dermal matrix implantation to the defect to study the repair effects of the above methods on rabbit knee cartilage injury. In addition to observing the morphological characteristics of articular cartilage, the ICRS macroscopic evaluation of the cartilage repair scale and the quantitative scoring repair effect of the modified O'Driscoll grading system, micro-CT scanning images, histological staining and immunohistochemical staining were also evaluated. The repair quality and characteristics of cartilage damage were studied in different dimensions. Emphasis was placed on observing the expression levels of TGF-β2, TGF-β3, and Sox-9 at the site of injury during the repair process, and the possible mechanism of naringin drugs combined with acellular dermal matrix for the repair of cartilage defects in rabbit knee joints was explored in depth to provide an effective method and application basis for the treatment of knee osteoarthritis (Fig. 1).
Fig. 1.
Flowchart of the study. Sham group: Sham operation group; Mod group: Model group: Nar group: Naringin group; ADM group: Acellular dermal matrix group; Nar/ ADM group: Naringin + Acellular dermal matrix group.
2. Materials and methods
2.1. Experimental animals
Twenty New Zealand white rabbits, 3 months old, male, weighing approximately 2.5 kg before surgery were used in this study. Each animal was housed in a separate cage, which allowed free movement and free access to food and water. The housing environment was maintained on a 12-h alternating light–dark cycle with temperature controlled between 21 °C and 25 °C and relative humidity between 40% and 70%. Before the start of the experiment, all animals were acclimatized for at least 7 days. Animal care and experiments were performed according to the guidelines for the Care and Use of Laboratory Animals released by the United States National Institutes of Health. This study was approved by the Animal Ethics Review Committee of the Institute of Basic Theories of Chinese Medicine, China Academy of Chinese Medical Sciences (approval number: 201706058, Beijing, China).
2.2. Animal model
New Zealand white rabbits were anesthetized with 3% pentobarbital sodium (30 mg/kg) by ear vein injection. Then, rabbit knee joints were disinfected and incised layer by layer (skin, subcutaneous tissue, muscle, joint capsule). After exposing the knee joints, a corneal trephine (Beijing North Sanyou Medical Devices Co., Ltd.) was applied to drill holes from the femoral condyle to the subchondral bone at the trochlear articular surface, with a diameter of 5 mm and a depth of 3 mm. The defect site was filled according to the experimental protocol, and the patella was reduced postoperatively and sutured layer by layer. All rabbits were allowed to move their knees freely in the cage without restriction and were observed daily for clinical symptoms. Throughout the experiment, none of the animals were excluded due to abnormal clinical manifestations. Rabbits in each group were sacrificed in the 12th week after surgery using anesthesia (Fig. 2).
Fig. 2.
Animal model preparation, The articular cartilage defect was replaced with acellular dermal matrix, Naringin is taken orally ----- The flow chart. (A) Full exposure of joint surface; (B) Drill holes from the femoral condyle to the subchondral bone at the trochlear articular surface;(C) Articular cartilage defect, a diameter of 5mm and a depth of 3mm;(D) Implant acellular dermal matrix into the articular cartilage defect.
2.3. Acellular dermal matrix preparation
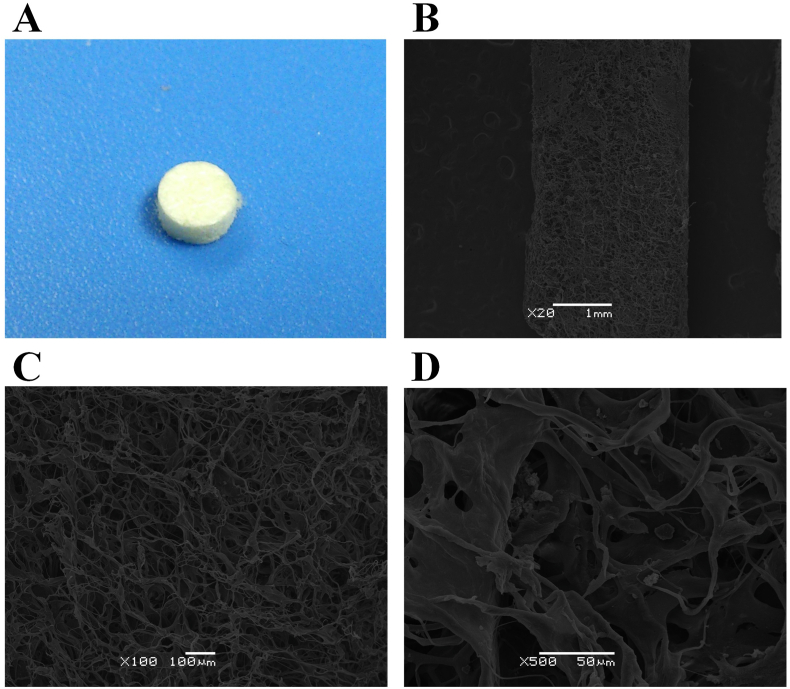
The acellular dermal matrix was a gift from the bone bank of Beijing Jishuitan Hospital (the Fourth Clinical Medical College of Peking University) and was made into the same shape as the articular cartilage defect using a corneal trephine (Fig. 3).
Fig. 3.
Acellular dermal matrix A. Appearance; B. C. D. Electron microscope (different resolution).
2.4. Preparation of the naringin decoction
The naringin solution was prepared with 10 g of naringin and 100 ml of deionized water. The gavage concentration (calculated on the basis of the weight of naringin) was 0.0084 g/kg per day. According to the patient's oral Chinese medicine, a Rhizoma Drynariae dose conversion was performed.
2.5. Grouping of experimental animals and intervention methods
Twenty New Zealand rabbits were randomly divided into five groups (four in each group, eight knees, cartilage defects of both knee joints were modeled in each animal except the sham operation group). The sham operation group (Sham group): the capsule was incised and sutured without treatment of the knee joint; The model group (Mod group): after successful modeling of articular cartilage defects, deionized water was intragastrically administered daily; The Naringin group (Nar group): after successful modeling of articular cartilage defects, Naringin decoction was intragastrically administered daily; The Acellular dermal matrix group (ADM group): after successful modeling of articular cartilage defects, acellular dermal matrix was immediately implanted at the joint defects and deionized water was intragastrically administered daily; The Naringin + Acellular dermal matrix group (Nar/ADM group): after successful modeling of articular cartilage defects, acellular dermal matrix was immediately implanted at the joint defects, and naringin decoction was intragastrically administered every day (Fig. 2).
2.6. Repair scale assessment method
To order to examine the repair structure of the rabbit knee joint, arthrotomy was performed using the same surgical approach as at the time of modeling, and the complete bilateral knee joints were removed. The International Cartilage Repair Society (ICRS)'s macroscopic evaluation of cartilage repair scale was used to evaluate the degrees of articular cartilage repair in the different groups. The main parameters to assess the application were degree of defect repair, integration to the border zone, macroscopic appearance, and overall repair assessment. The levels of articular cartilage repair in the different groups were evaluated using a modified O'Driscoll grading system. The main parameters to assess the application were nature of the predominant tissue (cellular morphology, Safranin-O staining of the matrix), structural characteristics (Surface regularity, Structural integrity, Thickness, Bonding to the adjacent cartilage), and freedom from cellular changes indicating degeneration (Hypocellularity, Chondrocyte clustering, Freedom from degenerative changes in the adjacent cartilage). All specimens were independently identified by two professionals with intermediate titles. The investigators who performed the evaluations were blind to the treatment.
2.7. Micro-CT scan examination
To examine the structure of the rabbit knee joint, micro-CT examination was performed on the rabbit knee joint, focusing on joint structure repair at the defect repair site. The Micro-CT was provided by the Institute of Laboratory Animal Sciences, CAMS & PUMC (Inveon CT, SIEMENS, Munich, Germany). The specific test parameters were as follows: voltage 70 kV, current 142 A, exposure time 1475 ms, 1-mm aluminum filter, 0.5° rotation steps, and 18 μm resolution. All data sets were segmented with a local threshold algorithm. The obtained projection of the image used the modified Feldkamp back projection for reconstruction. The separation of the cortex and bone was performed with built-in software.
2.8. Assessments of histological staining and immunohistochemical staining
The specimens were fixed in 10% neutral formalin at room temperature for 24 h. For decalcification the specimens were placed in EDTA-buffered solution (Beijing Zhongshan Jinqiao Biotechnology Co. Ltd) for 4 weeks. The decalcifying solution was changed twice per week. The articular cartilage of the repaired area was cut out after obtaining satisfactory decalcification (achieved when the syringe needle punctured the tissue without resistance and the tissue was soft and elastic) and was trimmed into a sheet with a thickness of 1.5 mm. Freshly prepared 4% methanol was used for fixation for 48 h at room temperature, followed by paraffin embedding and slicing. The specimens were cut into 4-μm thick slices for histological and immunohistochemical staining, including H&E staining (HE Staining Kit, Beijing Solarbio Science & Technology Co., Ltd.), Alcian blue staining (Alcian Blue Stain Kit, Beijing Solarbio Science & Technology Co., Ltd.), Safranin-O staining (Safranin O-Fast Green FCF Cartilage Stain Kit, Beijing Solarbio Science & Technology Co., Ltd.), TGF-β2 immunostaining (rabbit anti-TGF-beta 2 antibody, Beijing Bioss Biotechnology Co., Ltd.), TGF-β3 immunostaining (rabbit anti-TGF-beta 3 antibody, Beijing Bioss Biotechnology Co., Ltd.), and Sox-9 immunostaining (rabbit anti-SOX9 antibody, Beijing Bioss Biotechnology Co., Ltd.).
Histological staining: ① H & E staining: Slices were incubated in xylene for 15 min twice at room temperature and dehy-drated with gradient ethanol. Subsequently, the sections were stained with hematoxylin (HE staining kit; Beijing Solarbio Science & Technology Co., Ltd.) for 5 min. Following differentiation in 1% hydrochloric acid alcohol for 2 s, the sections were incubated in ammonia water for 2 min, and stained with eosin for 1 min. Finally, the sections were dehydrated, cleared and mounted with neutral resin. ② Alcian blue staining: The sections were stained with Alcian Blue Stain (Alcian Blue Stain Kit, Beijing Solarbio Science & Technology Co., Ltd.) for 30 min, washed with running water for 5 min, dehydrated with graded ethanol, and vitrified with dimethylbenzene. Then, the pieces were sealed with neutral gum. ③ Safranin-O staining: Safranin O-Fast Green staining (Safranine O-Fast Green FCF cartilage stain kit; Beijing Solarbio Science & Technology Co., Ltd.) was performed to observe the morphology of cartilage tissues under a light microscope. Paraffin-embedded slices were dewaxed in xylene at room temperature for 10 min and rehydrated with absolute alcohol for 5 min, 95% alcohol for 5 min and 80% alcohol for 5 min prior to being washed with distilled water for 2 min. Sections were subsequently stained with hematoxylin at 20 °C for 3 min before being washed three times with distilled water. Hydrochloric acid (1%) and ethanol were used for 15 s at 20 °C to differentiate the slices. The slices were washed three times with distilled water and immersed in a 0.02% Fast Green solution at 20 °C for 3 min. The stained slices were washed in 1% glacial acetic acid and stained in 0.1% Safranin O at 20 °C for 3 min.
Immunohistochemical staining: After dewaxing and rehydration, slides were immersed in ethylenediaminetetraacetic acid solution and boiled in an electric pressure cooker (2 min) for antigen retrieval. Cooling was performed at room temperature. The sections were incubated with 3% H2O2 (Beijing Zhongshan Jinqiao Biotechnology Co. Ltd) to block endogenous peroxidase for 10 min at room temperature before staining. An antigen retrieval step in 10 mM citrate buffer (pH 6.0) for 10 min was performed. Sections were blocked using normal goat serum (Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China) at 37 °C for 30 min and were then incubated with immunostaining. ① TGF-β2 immunostaining (rabbit anti-TGF-β2 antibody, Beijing Bioss Biotechnology Co., Ltd.) was performed to evaluate the expression and secretion of TGF-β2. Slices were incubated with rabbit anti-TGF-β2 antibody (rabbit anti-TGF-β2 antibody, Beijing Bioss Biotechnology Co., Ltd.; cat. no. bs-20412R; 1:300) at 37 °C for 2 h, followed by incubation with horseradish peroxidase conjugated goat anti-rabbit/mouse secondary antibody (1:1; cat. no. PV6000; Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.) at room temperature for 30 min ② TGF-β3 immunostaining (rabbit anti-TGF-β3 antibody, Beijing Bioss Biotechnology Co., Ltd.) was performed to evaluate the expression and secretion of TGF-β3. Slices were incubated with rabbit anti-TGF-β3 antibody (rabbit anti-TGF-β3 antibody, Beijing Bioss Biotechnology Co., Ltd.; cat. no. bs-1910R; 1:300) at 37 °C for 2 h, followed by incubation with horseradish peroxidase-conjugated goat anti-rabbit/mouse secondary antibody (1:1; cat. no. PV6000; Beijing Zhongshan Jinqiao Biotechnology Co., Ltd) at room temperature for 30 min ③ Sox-9 immunostaining (rabbit anti-SOX9 antibody; Beijing Bioss Biotechnology Co., Ltd.) was performed to evaluate the expression and secretion of SOX-9. Slices were incubated with rabbit anti-SOX9 antibody (rabbit anti-SOX9 antibody; Beijing Bioss Biotechnology Co., Ltd.; cat. no. bs-10725R; 1:200) at 37 °C for 2 h, followed by incubation with horseradish peroxidase-conjugated goat anti-rabbit/mouse secondary antibody (1:1; PV6000; Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.) at room temperature for 20 min.
Images of stained sections were recorded using an optical microscope (Nikon Eclipse 600; Nikon Corporation) mounted with a digital camera (Nikon DXM1200F; Nikon Corporation), and all staining was performed according to the manufacturer's instructions.
2.9. Statistical analysis
SPSS19.0 software package (IBM Corp.) was used for statistical analysis. Data were presented as the mean value ± standard deviation. All statistical inferences were subjected to two-sided tests. P < 0.05 was considered to indicate a statistically significant difference. The confidence interval of parameter estimation was 95%. All the data of each group met the normal distribution. Application of Levene's test to assess the equality of variances. Statistical differences were analyzed by one-way analysis of variance followed by Tukey's test.
3. Results
3.1. Morphological observation of repaired tissue at joint defects
For the Mod group, significant depressions were observed in the cartilage defects, some of which were filled with fibrous tissue and had more pronounced defect boundaries with well-defined borders (Fig. 4B and G). For the Nar group, the repair height was lower than the level of the surrounding cartilage, the surface was not smooth, and some small depressions could be found, which converged in the center of the defect and were clearly demarcated from the surrounding cartilage, and the residual fissures were still observed in the central part of the defect (Fig. 4C and H); For the ADM group, the repair height was essentially the same as the level of the surrounding cartilage, the newly formed tissue was white osteoid tissue, the interface between the repaired cartilage and the surrounding cartilage could be observed, and its surface was less smooth (Fig. 4D and I). For the ADM/Nar group, the new tissue was bright, smooth, tough, elastic, flush with the adjacent articular surface, and presented the same white color as the surrounding cartilage tissue, and the repair boundary was essentially not observed (Fig. 4E and J).
Fig. 4.
Gross appearance and assessment results of articular cartilage defects in the rabbit models at 12 weeks; A-E The appearance of the defect repaired when the knee was taken (5 groups); F-G Defect repair appearance taken during measurement of knee index(5 groups); K-O Defect repair image taken during micro-CT examination of knee joint(5 groups); P The modified O'Driscoll grading system statistical analysis (∗P<0.05); Q The International Cartilage Repair Society macroscopic evaluation of cartilage repair statistical analysis(∗P<0.05); Sham group: Sham operation group; Mod group: Model group; Nar group: Naringin group; ADM group: Acellular dermal matrix group; Nar/ ADM group: Naringin + Acellular dermal matrix group.
3.2. Micro-CT scanning observation of repair tissue in joint defects
Micro-CT scan findings were generally consistent with the morphologic observations. For the Mod group, the cavity area of the defect was larger, a clear defect was left, and no obvious repair was observed (Fig. 4L). For the Nar group, there were residual defects at the defect site, but they were smaller than those in the Mod group, in which more dense tissues were repaired (Fig. 4K). For the ADM group, the repair of the defect was smooth, and the defect was essentially filled with new tissue, but there was a clear repair boundary with the normal tissue (Fig. 4N). For the ADM/Nar group, the repair of the defect was obvious, flush with the surface of the adjacent articular surface, the new tissue was closely combined with the normal tissue around the defect, the density of the repaired tissue was similar to that of the normal tissue, and the repair boundary was blurred, yet the layers of cartilage and subchondral bone were clear (Fig. 4O).
3.3. Comparison of the international cartilage repair society (ICRS)'s macroscopic evaluation of cartilage repair scale and the modified O'Driscoll grading system scores
Two professionals quantitatively assessed each sample according to scale-related items. The repair contents of main concern included Degree of defect repair, Integration to border zone, Macroscopic appearance, overall repair assessment, nature of the predominant tissue (cellular morphology, Safranin-O staining of the matrix), structural characteristics (surface regularity, Structural integrity, Thickness, Bonding to the adjacent cartilage), and freedom from cellular changes indicating degeneration (hypocellularity, chondrocyte clustering, and freedom from degenerative changes in adjacent cartilage). The scores of the above two scales in each group were statistically analyzed, and the results showed that from the score point of view, the Nar group was superior to the Mod group, the ADM/Nar group was superior to the AMD group, the Nar group was superior to the Mod group, and the ADM/Nar group was superior to the Nar group. In conclusion, the ADM/Nar group had the best repair effect compared with the other four groups. The differences in the above groups were statistically significant (P < 0.05) (Fig. 4P and Q).
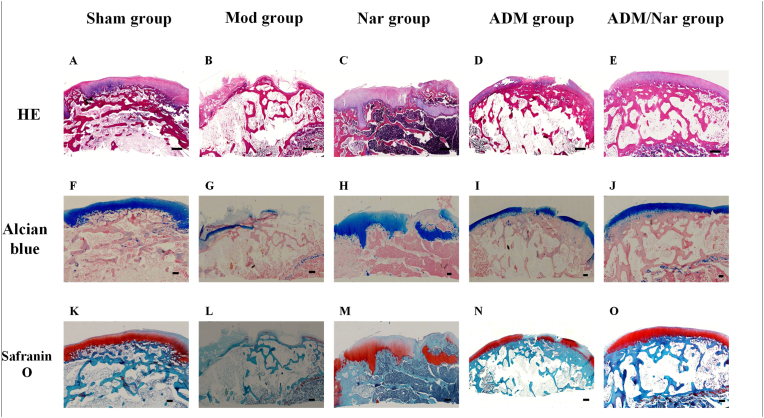
3.4. Histological staining observation of repaired tissues at joint defects
H & E staining: For the Mod group, there was no repair on the surface of the cartilage defect, a large amount of irregular fibrous tissue was observed, the boundary of the defect was obvious, new cartilage was not observed, the tidal line was blurred, and mild erosion was observed on the surface of the defect (Fig. 5B). For the Nar group, obvious repaired cartilage was observed at the cartilage defect, and the chondrocyte morphology was satisfactory, but the chondrocyte level was not clear, and a clear boundary was observed with the surrounding normal tissue (Fig. 5C). For the ADM group, the cartilage defect repair tissue was well connected with the surrounding normal tissue, yet the boundary was still visible, the surface was less smooth, a little hyaline cartilage-like tissue could be covered on the scaffold surface, it was filled with uniform fibrous tissue, the chondrocytes were disorganized, and the tidal line was visible but discontinuous (Fig. 5D). For the ADM/Nar group, the new tissue was well integrated with the surrounding normal tissue, showing hyaline cartilage-like tissue regeneration, uniform arrangement of chondrocytes, smooth surface, blurred boundary, an obvious tidal line between the cartilage layer and the subchondral bone layer, and new cartilage (Fig. 5E).
Fig. 5.
Histological findings of the repair tissue at articular cartilage defect sites (n=8 knees/group). Scale bars represent 200 μm. ®H & E staining at 12 weeks in the ( A) Sham group, (B ) Mod group, (C) Nar group, (D) ADM group and (E) ADM/Nar group. Alcian blue staining at 12 weeks in the (F) Sham group, (G) Mod group, (H) Nar group, (I) ADM group and ( J) ADM/Nar group. Safranin O staining at 12 weeks in the (K) Sham group, (L) Mod group, (M) Nar group, (N) ADM group and (O) ADM/Nar group. Sham group: Sham operation group; Mod group: Model group;Nar group: Naringin group; ADM group: Acellular dermal matrix group; Nar/ ADM group: Naringin + Acellular dermal matrix group.
Alcian blue staining: For the Mod group, the defect was obvious, the repair of cartilage matrix was not seen, the cartilage matrix was ruptured and severely lost, and the articular surface was incomplete (Fig. 5G). For the Nar group, significant cartilage matrix repair was observed at the defect, with darker staining in the deep part of the joint and lighter color and uneven distribution in the superficial part of the joint (Fig. 5H). For the ADM group, discontinuous cartilage matrix expression was observed with uneven color, yet the repair was denser and the articular surface was smoother (Fig. 5I). For the ADM/Nar group, the cartilage matrix repair was continuous, the proteoglycan content was abundant, the hyaline cartilage layer was uniform, the cartilage matrix proteoglycan staining positive areas were balanced, the articular surface was continuous, and the staining was uniform (Fig. 5J).
Safranin-O staining: For the Mod group, the cartilage defect surface showed no red color, indicating that there was no new cartilage matrix, and the chondrocytes were empty of staining (Fig. 5L). For the Nar group, obvious new cartilage tissue was observed, but the distribution was not uniform and not smooth with the defect surface (Fig. 5M). For the ADM group, a small amount of red color and poor continuity were observed, indicating the occurrence of cartilage tissue repair, yet the amount was limited (Fig. 5N). For the ADM/Nar group, a large number of red areas connected to each other were observed, indicating the generation of new cartilage tissue, deep staining of the cartilage surface, obvious new chondrocytes and dense cartilage tissue in the repaired area, close combination of new tissue with the surrounding normal tissue without gaps, the new cartilage was similar to normal tissue in shape and thickness, and the repair boundary of the defect was no longer obvious (Fig. 5O).
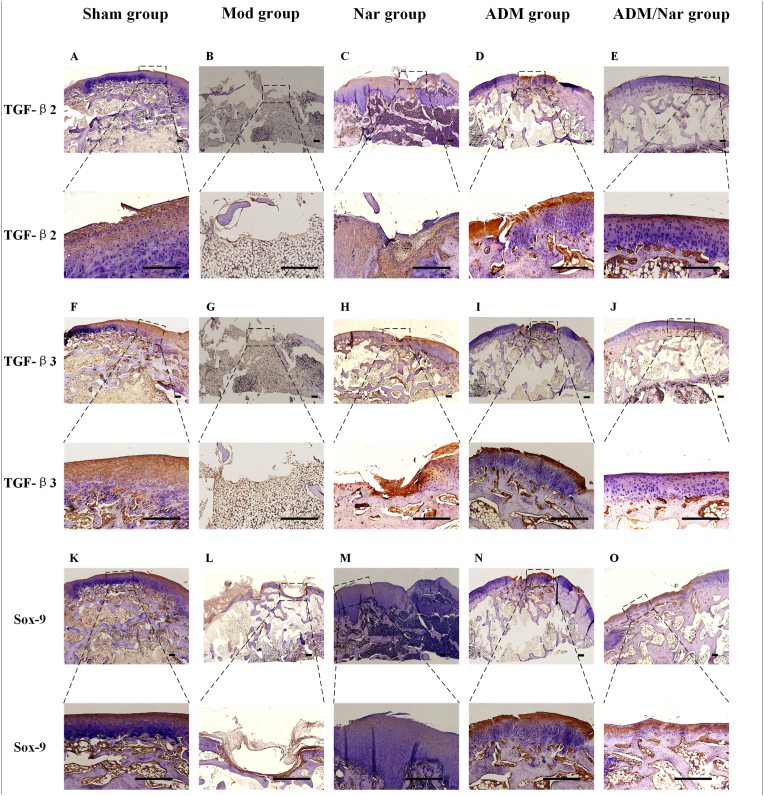
3.5. Immunohistochemical staining of repaired tissue at joint defects
Staining for TGF-β2, TGF-β3 and Sox-9: For the Mod group, expression of the above proteins was not observed in the defect (Fig. 6B, G and L). For the Nar group, significantly expressed TGF-β2, TGF-β3, and Sox-9 proteins were observed at the defect, and the expressed proteins were relatively uniform after magnified observation, yet a clear boundary was observed between them and normal tissues (Fig. 6C, H and M). For the ADM group, although expression of the above proteins was observed, the number of secretions was less than that in the Nar group, and a more satisfactory repair structure was observed after magnifying observation (Fig. 6D, I and N). For the ADM/Nar group, extensive TGF-β2, TGF-β3, and Sox-9 protein expression was observed, which was similar to the surrounding normal tissues. After magnified observation, the expression of the above proteins was uniform and smoothly distributed on the joint surface, and the chondrocytes were evenly arranged with a clear structure, similar to the normal tissues in morphology and thickness (Fig. 6E, J and O).
Fig. 6.
Immunohistological findings of the repair tissue at articular cartilage defect sites (11=8 knees/group). Scale bars represent 200 μm. ①TGF-β 2 immunostaining at 12 weeks in the (A) Sham group. (B) Mod group. (C) Nar group, (D) ADM group and (E) ADM/Nar group. ②TGF-β 3 immunostaining at 12 weeks in the (F) Sham group, (G) Mod group, (H) Nar group, (I) ADM group and (J) ADM/Nar group. ③Sox-9 immunostaining at 12 weeks in the (K) Sham group, (L) Mod group, (M) Nar group, (N) ADM group and (O) ADM/Nar group. Sham group: Sham operation group; Mod group: Model group: Nar group: Naringin group; ADM group: Acellular dermal matrix group;Nar/ ADM group: Naringin + Acellular dermal matrix group.
4. Discussion
The main pathological manifestations of osteoarthritis include articular cartilage injury, subchondral bone remodeling, and osteophyte formation, which can accumulate throughout the joint [14]. Among them, articular cartilage injury is a typical pathological change. Articular cartilage has the function of bearing gravity load and providing a lubricated articular surface. Chondrocytes were mainly located in the cartilage lacunae of the extracellular matrix. Chondrocytes produce and maintain extracellular matrix and can synthesize type II collagen and proteoglycan. The extracellular matrix is mainly composed of type II collagen and proteoglycan. Knee osteoarthritis is closely related to the abnormal condition of cartilage [15]. Knee cartilage injury is one of the important pathophysiological mechanisms of knee osteoarthritis. Cartilage injury can lead to the reduction of joint space, which will lead to friction between bone surfaces, resulting in pain and limited joint activity [16]. Articular cartilage injury is the core feature of knee osteoarthritis [17].
Articular cartilage lacks direct nutrition and chondrocytes with effective migration ability; thus, its self-repair ability is very weak. In the process of repair, both chondrocyte repair and extracellular matrix repair are important for restoring joint function. Due to the lack of undifferentiated chondrocytes in articular cartilage defects, chondrocytes themselves are enclosed in a dense and firm solid matrix of collagen glycoprotein molecules; therefore, implantation of tissue engineering scaffold materials has a positive effect on the repair of structures. Many researchers have performed in-depth studies of cell-free biological scaffolds, which are composed of extracellular matrix with varying degrees of structural collagen and carbohydrate preservation. The reattachment, migration, differentiation and proliferation of this scaffold provide a perfect natural environment [18]. Acellular biological matrices have been applied in the repair of a variety of tissues (e.g., myocardium, skin cartilage) [19] and are especially widely used in the repair of articular cartilage injuries [20].
The acellular dermal matrix material used in this study allowed the differentiated intercellular cells of bone tissue to migrate to the defect site, and the repair of cartilage defects was performed according to the specific morphology of the scaffold material. This acellular dermal matrix material has been patented to completely preserve the basement membrane on the surface of the papillary dermis and provide favorable support for tissue repair. The main component of this material is collagen, which has some mechanical strength, good biocompatibility and plasticity. Because of its high porosity, it facilitates both the attachment and ingrowth of cells, as well as the penetration of nutrients and the excretion of metabolites.
Natural resources rich in naringin have been found to have significant advantages for biomedical applications [21]. Rhizoma Drynariae is a traditional Chinese medicine that has the effect of “strengthening kidney and bone”. It is widely used in the treatment of a variety of bone and joint diseases and has a good clinical effect. Naringin is one of the main active ingredients of Rhizoma Drynariae, which has the effect of treating cartilage injury caused by osteoarthritis [22]. Huang Junbo et al. applied naringin combined with sustained-release microspheres to repair cartilage damage in rabbits, and the cartilage defect was repaired well [23]. Yunpeng Zhao et al. found that naringin can slow down the catabolism of chondrocytes, reduce the degradation of cartilage matrix [24], and induce the differentiation of bone marrow stem cells into chondrocytes [25]. These studies highlight the potential of naringin in the treatment of cartilage injury, which can help repair the cartilage injury site and restore it to a better state.
Micro-CT provides high-resolution ultrastructural imaging of bone and cortical bone, producing accurate and clear three-dimensional anatomical images for small animal studies. It can distinguish changes in the bone tissue structure and is used to evaluate the morphological characteristics of the structure. The repair of bone structure at articular cartilage defects is very important for the recovery of joint function. The repair of bone structure at ADM defects is satisfactory, indicating that acellular dermal matrix material plays an important role in the repair of bone structure below the injury. The change in subchondral bone in osteoarthritis will lead to cartilage loss, while cartilage injury will have a negative impact on subchondral bone [26]. The repair of subchondral bone is the basis of cartilage repair. The Nar/ADM group showed that the best bone repair occurred at articular cartilage defects. The ADM group was the second, indicating that acellular dermal matrix material plays an important role in bone repair.
HE staining was used to observe the morphological structure of the tissue and the morphological changes of chondrocytes. Safranin-O was combined with chondroitin sulfate and keratan sulfate, and the distribution and content of proteoglycans in the extracellular matrix could be observed. Alcian blue staining showed blue staining in the sulfate-rich cartilage lacunae. In the ADM group, the repaired flat articular surface could be seen in the defect, its relationship with the surrounding tissue boundary appeared to be fused, and the boundary with the surrounding cartilage was blurred. The ADM group had satisfactory repair of the joint structure at the defect, but the repair quality of chondrocytes and cartilage extracellular matrix was poor. In the Nar group, resecreted proteoglycan expression was observed, and the cartilage quality repair was satisfactory, though the structural repair was not satisfactory. The Nar/ADM group achieved satisfactory results in both joint structure repair and cartilage quality repair at the defect site.
The TGF-β/Smad signaling pathway is closely related to the development of osteoarthritis [27]. Abnormal changes in this signaling pathway play a key role in the process of cartilage degeneration [28], which can lead to the loss of cartilage phenotype [29] and is significantly associated with the pathological changes of osteoarthritis [30]. TGF-β exerts its biological effects mainly through the TGF-β/ALK5/Smad2/3 signaling pathway, which acts mainly by binding to the type I receptor ALK5 on the cell surface and activating Smad2/3 phosphorylation [31], which can inhibit chondrocyte terminal differentiation, thereby preventing articular cartilage matrix calcification and vascular invasion [32]. During the transduction of this pathway, TGF-β is an upstream signaling protein that has been found to be involved in the synthesis and degradation of articular cartilage in recent studies [33].
There are a total of three isoforms of TGF-β, TGF-β1, TGF-β2, and TGF-β3, and chondrocytes respond to all three isoforms and participate in chondrogenesis [34]. TGF-β protein can induce stem cell aggregation and chondrogenic differentiation [35], inhibit chondrocyte terminal differentiation, block chondrocyte matrix calcification and vascular ingrowth, and thus maintain the integrity of the cartilage extracellular matrix. This protein is the initiating signal of the TGF-β/ALK5/Smad2/3 signal transmission pathway. At present, there have been in-depth studies on TGF-β1. TGF-β1 can stimulate cartilage formation, the growth of articular chondrocytes and cartilage repair [36], which are essential for maintaining cartilage formation and differentiation [37]. TGF-β1 can induce chondrocytes to form intercellular communication through gap junctions [38] and can promote the proliferation of chondrocytes [39]. TGF-β1 can promote the maintenance of the phenotype of healthy articular chondrocytes by upregulating key glycolytic factors [40]. Though studies on TGF-β2 and TGF-β3 are still scarce, this study used TGF-β2 and TGF-β3 as the main indicators for in-depth study.
TGF-β2 can play important roles in the growth and differentiation of a variety of cells. Interstitial cells can be induced by TGF-β2, which converts them into chondrocytes, and the effect of promoting chondrogenesis may be achieved by prompting SMAD2 phosphorylation [41]. TGF-β3 has the ability to induce chondrocyte proliferation. In addition, it regulates chondrocyte differentiation and extracellular matrix synthesis. TGF-β3 can change from type I collagen to type II collagen in advance of TGF-β1-induced chondrogenic differentiation [42]. TGF-β3 can induce chondrogenic differentiation of cells, increase extrachondral matrix components, and synthesize glycosaminoglycan sulfate [43]. Through the observation of TGF-β2 and TGF-β3, it was found that the above factors were significantly highly expressed in the repaired tissues of knee cartilage defects in the Nar/ADM and Nar groups. However, the expression of TGF-β2 and TGF-β3 was not obvious in the Sham and Mod groups, indicating that naringin could promote the protein expression of TGF-β2 and TGF-β3 and then promote the repair of articular cartilage defects.
The TGF-β/ALK5/Smad2/3 signaling pathway can promote cartilage development by regulating the expression of Sox-related genes. Sox-9, one of the key transcription factors that maintains chondrocyte phenotype and cartilage homeostasis [44], plays a key role in chondrogenesis [45] and can be regarded as a downstream factor of the TGF-β/ALK5/Smad2/3 signal transmission pathway. It is closely related to the extracellular matrix metabolism of articular cartilage, and Sox-9 promotes the transcription of the type II collagen gene by activating the 48-bp cartilage enhancer located in the first intron within the type II collagen gene [46], inducing Sox-9 to initiate chondrogenesis [47]. Cartilage Sox-9 gene-deficient mice fail to form cartilage, while human Sox-9 gene mutations cause cartilage dysplasia, resulting in trunk dysplasia [48]. In the presence of naringenin (Nar group, Nar/ADM group), Sox-9 expression was significantly higher in the repaired tissues of knee cartilage defects. Combined with the increased expression of TGF-β2 and TGF-β3 described above, it can be speculated that naringin plays a role in the repair of articular cartilage defects by promoting TGF-β protein expression, which in turn activates the TGF-β/ALK5/Smad2/3 signal transmission pathway, resulting in high expression of Sox-9.
5. Outlook
The TGF-β/ALK5/Smad2/3 signaling pathway is a part of the TGF-β/Smad signaling pathway, and the following work can also be considered in future in-depth studies: (1) What is the initiating factor of the activation of the TGF-β/Smad signaling pathway by naringin? (2) Does naringin have a role in enhancing the recruitment of mesenchymal stem cells in the bone marrow below the articular cartilage defect? (3) When cell experiments are performed, different concentrations of naringin should be studied to determine the different effects on chondrocytes; (4) In the treatment of knee osteoarthritis with naringin in patients with the syndrome of Liver and Kidney Deficiency, does the activation of TGF-β/ALK5/Smad2/3 signal transduction pathway play a key role in the curative effect? (5) In the process of defining naringin's effect on the directional differentiation of bone marrow mesenchymal stem cells, the specific mechanism of action and the characteristics of the TGF-β/ALK5/Smad2/3 signal transduction pathway should be elucidated.
6. Conclusions
Oral administration of naringin, the active ingredient of Rhizoma Drynariae, and implantation of acellular dermal matrix into joint defects can repair cartilage defects in rabbit knee joints. Among them, naringin has a better effect on the repair of cartilage quality at the defect, acellular dermal matrix has a better effect on the repair of joint structure at the defect, and the combined application can achieve the optimal repair effect. On the basis of acellular dermal matrix providing better structural repair, the appearance of this repair effect may be associated with naringin activating the TGF-β/ALK5/Smad2/3 signal transmission pathway, with the result that TGF-β2, TGF-β3, and Sox-9 are more highly expressed in cartilage defects (Fig. 7).
Fig. 7.
Naringin promote the quality repair of cartilage defects, and acellular dermal matrix promote the structure repair of cartilage defects, and satisfactory repair effect can be obtained by combined application, This repair effect may be associated with naringin activating TGF-β/ALK5/Smad signaling pathways, resulting in increased expression of TGF-β2, TGF-β3 and SOX9 in cartilage defects.
Funding
This study was supported by the Fundamental Research Funds for the Central Universities (grant nos. 2018-JYBZZ-JS091, 2015-JYB-JSMS058), General Program of National Natural Science Foundation of China (grant nos. 81874475);
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request;
Ethics approval
The animal experiments were approved by the Science and Technology Department of Beijing University of Chinese Medicine (Beijing, China) and the Animal Ethics Review Committee of the Institute of Basic Theories of Chinese Medicine, Chinese Academy of Chinese Medical Sciences (Beijing, China) approval no. 201706058.
Consent for publication
Consent for publication was obtained from all authors.
Declaration of competing interest
The authors have no conflicts of interest to disclose in relation to this article.
Acknowledgements
The authors would like to thank Prof. Lei Sun (the bone bank of Beijing Jishuitan Hospital (the Fourth Clinical Medical College of Peking University, Beijing, China) for technical assistance with this study.
References
- 1.Martel-Pelletier J., Barr A.J., Cicuttini F.M., Conaghan P.G., Cooper C., Goldring M.B., et al. Osteoarthritis. Nat Rev Dis Primers. 2016;2:16072. doi: 10.1038/nrdp.2016.72. [DOI] [PubMed] [Google Scholar]
- 2.Jiang Y., Tuan R.S. Origin and function of cartilage stem/progenitor cells in osteoarthritis. Nat Rev Rheumatol. 2015;11(4):206–212. doi: 10.1038/nrrheum.2014.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sophia Fox A.J., Bedi A., Rodeo S.A. The basic science of articular cartilage: structure, composition, and function. Sport Health. 2009;1(6):461–468. doi: 10.1177/1941738109350438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armiento A.R., Stoddart M.J., Alini M., Eglin D. Biomaterials for articular cartilage tissue engineering: learning from biology. Acta Biomater. 2018;65:1–20. doi: 10.1016/j.actbio.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 5.Ma A., Jiang L., Song L., Hu Y., Dun H., Daloze P., et al. Reconstruction of cartilage with clonal mesenchymal stem cell-acellular dermal matrix in cartilage defect model in nonhuman primates. Int Immunopharm. 2013;16(3):399–408. doi: 10.1016/j.intimp.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Qi H., Jie Y., Chen L., Jiang L., Gao X., Sun L. [Preparation of acellular dermal matrix as a kind of scaffold for cartilage tissue engineering and its biocompatibility] Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2014;28(6):768–772. [chi] [PubMed] [Google Scholar]
- 7.Che C.T., Wong M.S., Lam C.W. Natural products from Chinese medicines with potential benefits to bone health. Molecules. 2016;21(3):239. doi: 10.3390/molecules21030239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su Y.X., Yan H., Chen B.J., Zahn Q., Wang Y.R., Lu M.L., et al. [Effect of naringin of Drynaria Rhizome, a Chinese medical component of Zhuanggu Jianxi Recipe containing serum on caveolin-p38MAPK signal pathway in IL-1beta induced rabbit degenerated chondrocytes] Zhongguo Zhong Xi Yi Jie He Za Zhi. 2014;34(12):1492–1498. [PubMed] [Google Scholar]
- 9.Zobeiri M., Belwal T., Parvizi F., Naseri R., Farzaei M.H., Nabavi S.F., et al. Naringenin and its nano-formulations for fatty liver: cellular modes of action and clinical perspective. Curr Pharmaceut Biotechnol. 2018;19(3):196–205. doi: 10.2174/1389201019666180514170122. [DOI] [PubMed] [Google Scholar]
- 10.Ye C., Chen J., Qu Y., Liu H., Yan J., Lu Y., et al. Naringin and bone marrow mesenchymal stem cells repair articular cartilage defects in rabbit knees through the transforming growth factor-β superfamily signaling pathway. Exp Ther Med. 2020;20(5):59. doi: 10.3892/etm.2020.9187. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krstic J., Trivanovic D., Obradovic H., Kukolj T., Bugarski D., Santibanez J.F. Regulation of mesenchymal stem cell differentiation by transforming growth factor beta superfamily. Curr Protein Pept Sci. 2018;19(12):1138–1154. doi: 10.2174/1389203718666171117103418. [DOI] [PubMed] [Google Scholar]
- 12.Zhang G.M., Zhang G.M., Gu B. Serum transforming growth factor beta1 level for knee osteoarthritis diagnosis. Clin Chim Acta. 2017;474:136. doi: 10.1016/j.cca.2017.09.017. [DOI] [PubMed] [Google Scholar]
- 13.Van Caam A., Madej W., Garcia de Vinuesa A., Goumans M.J., Ten Dijke P., Blaney Davidson E., et al. TGFβ1-induced SMAD2/3 and SMAD1/5 phosphorylation are both ALK5-kinase-dependent in primary chondrocytes and mediated by TAK1 kinase activity. Arthritis Res Ther. 2017;19(1):112. doi: 10.1186/s13075-017-1302-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robinson W.H., Lepus C.M., Wang Q., Raghu H., Mao R., Lindstrom T.M., et al. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016;12(10):580–592. doi: 10.1038/nrrheum.2016.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou Q., Cai Y., Jiang Y., Lin X. Exosomes in osteoarthritis and cartilage injury: advanced development and potential therapeutic strategies. Int J Biol Sci. 2020;16(11):1811–1820. doi: 10.7150/ijbs.41637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masson A.O., Krawetz R.J. Understanding cartilage protection in OA and injury: a spectrum of possibilities. BMC Muscoskel Disord. 2020;21(1):432. doi: 10.1186/s12891-020-03363-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He Y., Li Z., Alexander P.G., Ocasio-Nieves B.D., Yocum L., Lin H., et al. Pathogenesis of osteoarthritis: risk factors, regulatory pathways in chondrocytes, and experimental models. Biology. 2020;9(8):194. doi: 10.3390/biology9080194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hodde J. Naturally occurring scaffolds for soft tissue repair and regeneration. Tissue Eng. 2002;8(2):295–308. doi: 10.1089/107632702753725058. [DOI] [PubMed] [Google Scholar]
- 19.Sarig U., Au-Yeung G.C., Wang Y., Bronshtein T., Dahan N., Boey F.Y., et al. Thick acellular heart extracellular matrix with inherent vasculature: a potential platform for myocardial tissue regeneration. Tissue Eng. 2012;18(19–20):2125–2137. doi: 10.1089/ten.tea.2011.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye K., Traianedes K., Choong P.F., Myers D.E. Chondrogenesis of human infrapatellar fat pad stem cells on acellular dermal matrix. Front Surg. 2016;3:3. doi: 10.3389/fsurg.2016.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bharti S., Rani N., Krishnamurthy B., Arya D.S. Preclinical evidence for the pharmacological actions of naringin: a review. Planta Med. 2014;80(6):437–451. doi: 10.1055/s-0034-1368351. [DOI] [PubMed] [Google Scholar]
- 22.Xu Q., Zhang Z.F., Sun W.X. Effect of naringin on monosodium iodoacetate-induced osteoarthritis pain in rats. Med Sci Mon Int Med J Exp Clin Res. 2017;23:3746–3751. doi: 10.12659/MSM.902396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang J.B., Wang S.Y., Zhang X.M., Li G., Ji P.Z., Zhao H.B. [Experimental study on loading naringin composite scaffolds for repairing rabbit osteochondral defects] Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2017;31(4):489–496. doi: 10.7507/1002-1892.201611112. [chi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Y., Li Z., Wang W., Zhang H., Chen J., Su P., et al. Naringin protects against cartilage destruction in osteoarthritis through repression of NF-kappaB signaling pathway. Inflammation. 2016;39(1):385–392. doi: 10.1007/s10753-015-0260-8. [DOI] [PubMed] [Google Scholar]
- 25.Pan M., Li M. [Progressive studies on effects of traditional Chinese medicines on differentiation of human bone mesenchymal stem cells] Zhongguo Zhongyao Zazhi. 2010;35(14):1892–1895. doi: 10.4268/cjcmm20101428. [DOI] [PubMed] [Google Scholar]
- 26.Castaneda S., Roman-Blas J.A., Largo R., Herrero-Beaumont G. Subchondral bone as a key target for osteoarthritis treatment. Biochem Pharmacol. 2012;83(3):315–323. doi: 10.1016/j.bcp.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 27.Wang Q., Tan Q.Y., Xu W., Qi H.B., Chen D., Zhou S., et al. Cartilage-specific deletion of Alk5 gene results in a progressive osteoarthritis-like phenotype in mice. Osteoarthritis Cartilage. 2017;25(11):1868–1879. doi: 10.1016/j.joca.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhai G., Dore J., Rahman P. TGF-beta signal transduction pathways and osteoarthritis. Rheumatol Int. 2015;35(8):1283–1292. doi: 10.1007/s00296-015-3251-z. [DOI] [PubMed] [Google Scholar]
- 29.Hellingman C.A., Davidson E.N., Koevoet W., Vitters E.L., van den Berg W.B., van Osch G.J., et al. Smad signaling determines chondrogenic differentiation of bone-marrow-derived mesenchymal stem cells: inhibition of Smad1/5/8P prevents terminal differentiation and calcification. Tissue Eng. 2011;17(7–8):1157–1167. doi: 10.1089/ten.TEA.2010.0043. [DOI] [PubMed] [Google Scholar]
- 30.Cui Z., Crane J., Xie H., Jin X., Zhen G., Li C., et al. Halofuginone attenuates osteoarthritis by inhibition of TGF-beta activity and H-type vessel formation in subchondral bone. Ann Rheum Dis. 2016;75(9):1714–1721. doi: 10.1136/annrheumdis-2015-207923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Derynck R., Zhang Y.E. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425(6958):577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 32.van der Kraan P.M., Blaney Davidson E.N., Blom A., van den Berg W.B. TGF-beta signaling in chondrocyte terminal differentiation and osteoarthritis: modulation and integration of signaling pathways through receptor-Smads. Osteoarthritis Cartilage. 2009;17(12):1539–1545. doi: 10.1016/j.joca.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 33.van der Kraan P.M. The changing role of TGFbeta in healthy, ageing and osteoarthritic joints. Nat Rev Rheumatol. 2017;13(3):155–163. doi: 10.1038/nrrheum.2016.219. [DOI] [PubMed] [Google Scholar]
- 34.Li Z., Kupcsik L., Yao S.J., Alini M., Stoddart M.J. Mechanical load modulates chondrogenesis of human mesenchymal stem cells through the TGF-beta pathway. J Cell Mol Med. 2010;14(6A):1338–1346. doi: 10.1111/j.1582-4934.2009.00780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Onyekwelu I., Goldring M.B., Hidaka C. Chondrogenesis, joint formation, and articular cartilage regeneration. J Cell Biochem. 2009;107(3):383–392. doi: 10.1002/jcb.22149. [DOI] [PubMed] [Google Scholar]
- 36.Ahn J., Kim S.A., Kim K.W., Oh J.H., Kim S.J. Optimization of TGF-beta1-transduced chondrocytes for cartilage regeneration in a 3D printed knee joint model. PloS One. 2019;14(5) doi: 10.1371/journal.pone.0217601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Y., Wu T., Huang S., Suen C.W., Cheng X., Li J., et al. Sustained release SDF-1alpha/TGF-beta1-loaded silk fibroin-porous gelatin scaffold promotes cartilage repair. ACS Appl Mater Interfaces. 2019;11(16):14608–14618. doi: 10.1021/acsami.9b01532. [DOI] [PubMed] [Google Scholar]
- 38.Wang Q., Zhou C., Li X., Cai L., Zou J., Zhang D., et al. TGF-beta1 promotes gap junctions formation in chondrocytes via Smad3/Smad4 signalling. Cell Prolif. 2019;52(2) doi: 10.1111/cpr.12544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang R., Xu B., Xu H. TGF-beta1 promoted chondrocyte proliferation by regulating Sp1 through MSC-exosomes derived miR-135b. Cell Cycle. 2018;17(24):2756–2765. doi: 10.1080/15384101.2018.1556063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang C., Silverman R.M., Shen J., O'Keefe R.J. Distinct metabolic programs induced by TGF-beta1 and BMP2 in human articular chondrocytes with osteoarthritis. J Orthop Translat. 2018;12:66–73. doi: 10.1016/j.jot.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kalwitz G., Neumann K., Ringe J., Sezer O., Sittinger M., Endres M., et al. Chondrogenic differentiation of human mesenchymal stem cells in micro-masses is impaired by high doses of the chemokine CXCL7. J Tissue Eng Regen Med. 2011;5(1):50–59. doi: 10.1002/term.288. [DOI] [PubMed] [Google Scholar]
- 42.Arikawa T., Matsukawa A., Watanabe K., Sakata K.M., Seki M., Nagayama M., et al. Galectin-9 accelerates transforming growth factor beta3-induced differentiation of human mesenchymal stem cells to chondrocytes. Bone. 2009;44(5):849–857. doi: 10.1016/j.bone.2009.01.365. [DOI] [PubMed] [Google Scholar]
- 43.Dahlin R.L., Ni M., Meretoja V.V., Kasper F.K., Mikos A.G. TGF-beta3-induced chondrogenesis in co-cultures of chondrocytes and mesenchymal stem cells on biodegradable scaffolds. Biomaterials. 2014;35(1):123–132. doi: 10.1016/j.biomaterials.2013.09.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao L., Sheu T.J., Dong Y., Hoak D.M., Zuscik M.J., Schwarz E.M., et al. TAK1 regulates SOX9 expression in chondrocytes and is essential for postnatal development of the growth plate and articular cartilages. J Cell Sci. 2013;126(Pt 24):5704–5713. doi: 10.1242/jcs.135483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi S., Chan A.G., Mercer S., Eckert G.J., Trippel S.B. Endogenous versus exogenous growth factor regulation of articular chondrocytes. J Orthop Res. 2014;32(1):54–60. doi: 10.1002/jor.22444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bell D.M., Leung K.K., Wheatley S.C., Ng L.J., Zhou S., Ling K.W., et al. SOX9 directly regulates the type-II collagen gene. Nat Genet. 1997;16(2):174–178. doi: 10.1038/ng0697-174. [DOI] [PubMed] [Google Scholar]
- 47.Xu T., Xu G., Gu Z., Wu H. Role of endoplasmic reticulum stress pathway in hydrostatic pressure-induced apoptosis in rat mandibular condylar chondrocytes. Mol Cell Biochem. 2017;429(1–2):23–31. doi: 10.1007/s11010-016-2933-5. [DOI] [PubMed] [Google Scholar]
- 48.Akiyama H., Chaboissier M.C., Martin J.F., Schedl A., de Crombrugghe B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002;16(21):2813–2828. doi: 10.1101/gad.1017802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request;